-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaThe Silkworm Coming of Age—Early
article has not abstract
Published in the journal: . PLoS Genet 8(3): e32767. doi:10.1371/journal.pgen.1002591
Category: Perspective
doi: https://doi.org/10.1371/journal.pgen.1002591Summary
article has not abstract
In order to grow, immature insects must periodically synthesize a new cuticle and shed the old one (a process of molting and ecdysis) until they have reached a stage permitting metamorphosis to the reproductive adult. Each molt is induced by a pulse of ecdysteroids, but the nature of the molt is determined by the juvenile hormones (JHs) [1]. These are sesquiterpenoids, synthesized from three isoprene units via the mevalonate pathway and decorated by an epoxide group on one end and a methyl ester on the other [1]. The JHs maintain larval characters, a status quo effect that is lifted when the level of JH drops. A threshold size must be attained for each molt and for metamorphosis to occur, and this size may relate to the limit of oxygen supply by the tracheal system [2], [3]. The number of molts varies among and sometimes within species; it is influenced by nutrition and by environmental and genetic signals. Yet, the mechanisms that “measure size” or “count the larval instars” are overridden by the experimental depletion of JHs. It has long been a tenet of insect endocrinology that removal of the corpora allata (CA), the endocrine glands that produce the JHs, causes precocious metamorphosis [1]. However, bringing the JH titer down experimentally is not as trivial as it may seem.
In this issue, Daimon et al. [4] utilize the genetic resources of the silkworm Bombyx mori to explore why larvae of the dimolting (mod) mutant strain undergo metamorphosis early, giving miniature pupae and adults after just three instars rather than the normal five (Figure 1). They identified this mod trait as a gene for a cytochrome P450 enzyme, CYP15C1, the silkworm ortholog of a previously identified JH epoxidase of the cockroach CA [5]. Daimon et al. show that the mod mutant is a null caused by a deletion that truncates CYP15C1. The mutation can be rescued by transgenic production of the wild type enzyme in the CA using the GAL4-UAS system, or by topical application of a JH agonist. Biochemical experiments confirm that CYP15C1 is a stereospecific epoxidase expressed specifically in the CA throughout postembryonic development.
Fig. 1. Development of the silkworm without juvenile hormones (JHs). 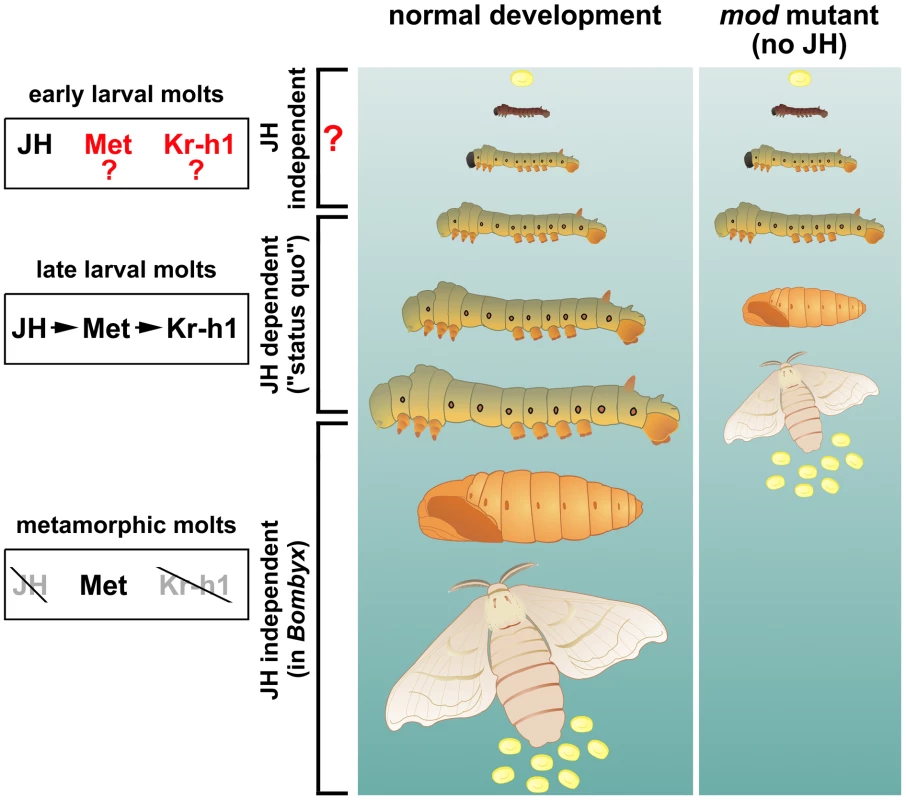
Maintenance of larval characters before the larval-pupal and pupal-adult molts is the classical status quo role of JH. This JH effect is carried out through its receptor Met and the transcription factor Kr-h1. The mod mutants studied by Daimon et al. [4] lack a P450 enzyme (epoxidase) and cannot make functional JH, yet they do not enter metamorphosis until after two larval molts, producing precocious pupae instead of the normal fourth larval instar. This unexpected result suggests that the early larval instars might be insensitive to the lack of JH. Illustration: Martina Hajduskova. The work of Daimon et al. is forceful in its elegance, and it brings a surprise: the CA of mod mutants do not produce the JHs normally needed for the status quo of larval development, yet the mod larvae do not commit to become pupae until the third larval instar (Figure 1). Might this work break the status quo of insect endocrinology? Daimon et al. propose that authentic (epoxidized) JHs are essential for the classic status quo but not in early larval stages (1 and 2) in Bombyx. It turns out that previous work corroborates this statement, and that the morphogenetic function of JHs in early larval instars of insects now requires closer attention.
There are different ways to remove JHs. Removal of the CA (“allatectomy”) is possible in some insects when there is a conjunction of skilled microsurgery with favorable size and anatomy of the glands. Thus, the steady hands and patience of Bounhiol [6] and Fukuda [7] succeeded in obtaining miniature pupae of the silkworm after operating on third-instar larvae, but they could not operate on earlier instars with smaller heads. The discovery of precocenes, plant compounds causing precocious metamorphosis [8], enabled “chemical allatectomy”. However, these compounds are blunt instruments [9] that work well in some insects, but are too toxic or too quickly metabolized in others to elicit precocious metamorphosis. Tarrant and Cupp [10] noted that the first-instar larvae of the true bug Rhodnius prolixus were “quite refractory” to chemical allatectomy with precocene. Pener and his colleagues treated late embryos or newly hatched first-instar larvae of locusts and cockroaches with precocene and saw precocious appearance of adult features after the second larval molt, but not earlier ([11], [12] and papers cited therein). Yet, the early-larval instar CA secrete JH as shown by transplantation experiments or direct measurements [13]. Significantly, constitutive overexpression of a JH esterase using an actin-Gal4 driver caused precocious metamorphosis in the silkworm [14]. The excess JH esterase specifically inactivates circulating JH. Similar to the loss of CYP15C1 in mod mutants, however, overproduction of JH esterase alone was insufficient to force Bombyx to enter metamorphosis before they underwent three larval instars [14].
The observations of Daimon et al. are therefore not unprecedented, but their evidence is compelling. The question of the role of JH during early larval instars and the reason for its status quo function at the organismal level becoming apparent only in later instars is now open. A “competence for metamorphosis” requiring some amount of postembryonic growth has been advanced, but this sounds much like a “dormitive virtue”, and needs to be dissected with current molecular tools. Early experiments such as parabiosis and tissue transplantation indicated an immediate competence for metamorphosis (cited by Wigglesworth [15]), a conflict with the results showing delayed competence that was attributed to the persistence of circulating JH. This is not the case in the silkworm, where either loss of JH production [4] or elimination of active JH [14] both result in precocious metamorphosis, but only after it undergoes a minimum of two larval molts. Clearly, factors other than JHs must be at play during the earliest larval instars.
The silkworm appears to be a good model to study this apparent JH-independent growth of the early larval stages, because in this species JH is essential neither during pupal and adult development, nor for reproduction, as evidenced by the viability of the mod mutants. Additional loci are known to control the number of larval molts in Bombyx [16] and require identification in order to separate JH-dependent from JH-independent effects. Further manipulation of JH synthesis and/or signaling is now possible in Bombyx. Inactivation of another enzyme of JH synthesis, the methyl transferase JHAMT [17], should confirm the results obtained with CYP15C1 and prove that the larval JHs cannot be substituted by their non-epoxidized methyl esters. JHAMT RNAi knockdown causes precocious metamorphosis in the beetle Tribolium castaneum, clearly establishing this enzyme as essential for the maintenance of larval status [18]. It may also become possible to genetically ablate the CA in the silkworm as was done in Drosophila [19], [20]. Manipulation of JH signaling also becomes feasible, as it has recently been shown that insects use a “common core” JH signaling pathway [21] consisting of the bHLH-PAS protein Met [22]–[24] and the transcription factor Kr-h1 [25], [26]. Met emerges as the long-sought JH receptor [27] that controls expression of Kr-h1 to prevent metamorphosis. Responsiveness of Met and Kr-h1 to JH during the earliest larval instars should now be tested. Furthermore, CA cells along with JH appear at or close to dorsal closure in insects, and the role of JH in the transition from embryogenesis to larval stages needs reexamination [28]. With a size propitious for physiological experimentation, a complete genome [29], a thousand races including over 500 mutants, and the tools for targeted gene expression [30] and disruption [31], Bombyx mori brings answers to the open questions within reach. The silkworm as a genetic model for insect physiology has truly come of age.
Zdroje
1. GoodmanWGCussonM 2012 The juvenile hormones. GilbertLI Insect endocrinology Amsterdam Elsevier 310 365
2. NijhoutHF 1975 A threshold size for metamorphosis in the tobacco hornworm, Manduca sexta (L.). Biological Bulletin 149 214 225
3. CallierVNijhoutHF 2011 Control of body size by oxygen supply reveals size-dependent and size-independent mechanisms of molting and metamorphosis. Proc Natl Acad Sci U S A 108 14664 14669
4. DaimonTKozakiTNiwaRKobayashiIFurutaK 2012 Precocious metamorphosis in the juvenile hormone-deficient mutant of the silkworm, Bombyx mori. PLoS Genet 8 e1002486 doi:10.1371/journal.pgen.1002486
5. HelvigCKoenerJFUnnithanGCFeyereisenR 2004 CYP15A1, the cytochrome P450 that catalyzes epoxidation of methyl farnesoate to Juvenile Hormone III in cockroach corpora allata. Proc Natl Acad Sci U S A 101 4024 4029
6. BounhiolJJ 1938 Recherches expérimentales sur le déterminisme de la métamorphose chez les Lépidoptères. Bulletin Biologique de France et de Belgique 24 1 199
7. FukudaS 1944 The hormonal mechanism of larval molting and metamorphosis in the silkworm. J Fac Sci Tokyo Univ Sect IV 6 477 532
8. BowersWSOhtaTCleereJSMarsellaPA 1976 Discovery of insect anti-juvenile hormones in plants. Science 193 542 547
9. FeyereisenRJohnsonGKoenerJFStayBTobeSS 1981 Precocenes as pro-allatocidins in adult female Diploptera punctata: a functional and ultrastructural study. J Insect Physiol 27 855 868
10. TarrantCACuppEW 1978 Morphogenetic effects of precocene II on the immature stages of Rhodnius prolixus. Trans R Soc Trop Med Hyg 72 666 668
11. Aboulafia-BaginskyNPenerMPStaalGB 1984 Chemical allatectomy of late Locusta embryos by a synthetic precocene and its effect on hopper morphogenesis. J Insect Physiol 30 839 852
12. PenerMPDesbergDLazaroviciPReuterCCTsaiLW 1986 The effect of a synthetic precocene on juvenile hormone III titre in late Locusta eggs. J Insect Physiol 32 853 857
13. KikukawaSTobeSS 1986 Juvenile hormone biosynthesis in female larvae of Diploptera punctata and the effect of allatectomy on haemolymph ecdysteroid titre. J Insect Physiol 32 981 986
14. TanATanakaHTamuraTShiotsukiT 2005 Precocious metamorphosis in transgenic silkworms overexpressing juvenile hormone esterase. Proc Natl Acad Sci U S A 102 11751 11756
15. WigglesworthVB 1952 Hormone balance and the control of metamorphosis in Rhodnius prolixus (Hemiptera). J Exp Biol 29 620 631
16. WangHBAliSMMoriyamaMIwanagaMKawasakiH 2012 20-hydroxyecdysone and juvenile hormone analog prevent precocious metamorphosis in recessive trimolter mutants of Bombyx mori. Insect Biochem Mol Biol 42 102 108
17. ShinodaTItoyamaK 2003 Juvenile hormone acid methyltransferase: a key regulatory enzyme for insect metamorphosis. Proc Natl Acad Sci U S A 100 11986 11991
18. MinakuchiCNamikiTYoshiyamaMShinodaT 2008 RNAi-mediated knockdown of juvenile hormone acid O-methyltransferase gene causes precocious metamorphosis in the red flour beetle Tribolium castaneum. FEBS J 275 2919 2931
19. LiuYShengZLiuHWenDHeQ 2009 Juvenile hormone counteracts the bHLH-PAS transcription factors MET and GCE to prevent caspase-dependent programmed cell death in Drosophila. Development 136 2015 2025
20. RiddifordLMTrumanJWMirthCKShenYC 2010 A role for juvenile hormone in the prepupal development of Drosophila melanogaster. Development 137 1117 1126
21. KonopovaBSmykalVJindraM 2011 Common and distinct roles of juvenile hormone signaling genes in metamorphosis of holometabolous and hemimetabolous insects. PLoS ONE 6 e28728 doi:10.1371/journal.pone.0028728
22. AshokMTurnerCWilsonTG 1998 Insect juvenile hormone resistance gene homology with the bHLH-PAS family of transcriptional regulators. Proc Natl Acad Sci U S A 95 2761 2766
23. KonopovaBJindraM 2007 Juvenile hormone resistance gene Methoprene-tolerant controls entry into metamorphosis in the beetle Tribolium castaneum. Proc Natl Acad Sci U S A 104 10488 10493
24. ParthasarathyRTanAPalliSR 2008 bHLH-PAS family transcription factor methoprene-tolerant plays a key role in JH action in preventing the premature development of adult structures during larval-pupal metamorphosis. Mech Dev 125 601 616
25. MinakuchiCNamikiTShinodaT 2009 Krüppel homolog 1, an early juvenile hormone-response gene downstream of Methoprene-tolerant, mediates its anti-metamorphic action in the red flour beetle Tribolium castaneum. Dev Biol 325 341 350
26. MinakuchiCZhouXRiddifordLM 2008 Krüppel homolog 1 (Kr-h1) mediates juvenile hormone action during metamorphosis of Drosophila melanogaster. Mech Dev 125 91 105
27. CharlesJ-PIwemaTEpaVCTakakiKRynesJ 2011 Ligand-binding properties of a juvenile hormone receptor, Methoprene-tolerant. Proc Natl Acad Sci U S A 108 21128 21133
28. KonopovaBZrzavyJ 2005 Ultrastructure, development, and homology of insect embryonic cuticles. J Morphol 264 339 362
29. The International Silkworm Genome Consortium 2008 The genome of a lepidopteran model insect, the silkworm Bombyx mori. Insect Biochem Mol Biol 38 1036 1045
30. UchinoKSezutsuHImamuraMKobayashiITatematsuK 2008 Construction of a piggyBac-based enhancer trap system for the analysis of gene function in silkworm Bombyx mori. Insect Biochem Mol Biol 38 1165 1173
31. TakasuYKobayashiIBeumerKUchinoKSezutsuH 2010 Targeted mutagenesis in the silkworm Bombyx mori using zinc finger nuclease mRNA injection. Insect Biochem Mol Biol 40 759 765
Štítky
Genetika Reprodukční medicína
Článek Physiological Notch Signaling Maintains Bone Homeostasis via RBPjk and Hey Upstream of NFATc1Článek Intronic -Regulatory Modules Mediate Tissue-Specific and Microbial Control of / TranscriptionČlánek Probing the Informational and Regulatory Plasticity of a Transcription Factor DNA–Binding DomainČlánek Repression of Germline RNAi Pathways in Somatic Cells by Retinoblastoma Pathway Chromatin ComplexesČlánek An Alu Element–Associated Hypermethylation Variant of the Gene Is Associated with Childhood ObesityČlánek Three Essential Ribonucleases—RNase Y, J1, and III—Control the Abundance of a Majority of mRNAsČlánek Genomic Tools for Evolution and Conservation in the Chimpanzee: Is a Genetically Distinct Population
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 3
-
Všechny články tohoto čísla
- Comprehensive Research Synopsis and Systematic Meta-Analyses in Parkinson's Disease Genetics: The PDGene Database
- Genomic Analysis of the Hydrocarbon-Producing, Cellulolytic, Endophytic Fungus
- Networks of Neuronal Genes Affected by Common and Rare Variants in Autism Spectrum Disorders
- Akirin Links Twist-Regulated Transcription with the Brahma Chromatin Remodeling Complex during Embryogenesis
- Too Much Cleavage of Cyclin E Promotes Breast Tumorigenesis
- Imprinted Genes … and the Number Is?
- Genetic Architecture of Highly Complex Chemical Resistance Traits across Four Yeast Strains
- Exploring the Complexity of the HIV-1 Fitness Landscape
- MNS1 Is Essential for Spermiogenesis and Motile Ciliary Functions in Mice
- A Fundamental Regulatory Mechanism Operating through OmpR and DNA Topology Controls Expression of Pathogenicity Islands SPI-1 and SPI-2
- Evidence for Positive Selection on a Number of MicroRNA Regulatory Interactions during Recent Human Evolution
- Variation in Modifies Risk of Neonatal Intestinal Obstruction in Cystic Fibrosis
- PIF4–Mediated Activation of Expression Integrates Temperature into the Auxin Pathway in Regulating Hypocotyl Growth
- Critical Evaluation of Imprinted Gene Expression by RNA–Seq: A New Perspective
- A Meta-Analysis and Genome-Wide Association Study of Platelet Count and Mean Platelet Volume in African Americans
- Mouse Genetics Suggests Cell-Context Dependency for Myc-Regulated Metabolic Enzymes during Tumorigenesis
- Transcriptional Control in Cardiac Progenitors: Tbx1 Interacts with the BAF Chromatin Remodeling Complex and Regulates
- Synthetic Lethality of Cohesins with PARPs and Replication Fork Mediators
- APOBEC3G-Induced Hypermutation of Human Immunodeficiency Virus Type-1 Is Typically a Discrete “All or Nothing” Phenomenon
- Interpreting Meta-Analyses of Genome-Wide Association Studies
- Error-Prone ZW Pairing and No Evidence for Meiotic Sex Chromosome Inactivation in the Chicken Germ Line
- -Dependent Chemosensory Functions Contribute to Courtship Behavior in
- Diverse Forms of Splicing Are Part of an Evolving Autoregulatory Circuit
- Phenotypic Plasticity of the Drosophila Transcriptome
- Physiological Notch Signaling Maintains Bone Homeostasis via RBPjk and Hey Upstream of NFATc1
- Precocious Metamorphosis in the Juvenile Hormone–Deficient Mutant of the Silkworm,
- Igf1r Signaling Is Indispensable for Preimplantation Development and Is Activated via a Novel Function of E-cadherin
- Accurate Prediction of Inducible Transcription Factor Binding Intensities In Vivo
- Mitochondrial Oxidative Stress Alters a Pathway in Strongly Resembling That of Bile Acid Biosynthesis and Secretion in Vertebrates
- Mammalian Neurogenesis Requires Treacle-Plk1 for Precise Control of Spindle Orientation, Mitotic Progression, and Maintenance of Neural Progenitor Cells
- Tcf7 Is an Important Regulator of the Switch of Self-Renewal and Differentiation in a Multipotential Hematopoietic Cell Line
- REST–Mediated Recruitment of Polycomb Repressor Complexes in Mammalian Cells
- Intronic -Regulatory Modules Mediate Tissue-Specific and Microbial Control of / Transcription
- Age-Dependent Brain Gene Expression and Copy Number Anomalies in Autism Suggest Distinct Pathological Processes at Young Versus Mature Ages
- A Genome-Wide Association Study Identifies Variants Underlying the Shade Avoidance Response
- -by- Regulatory Divergence Causes the Asymmetric Lethal Effects of an Ancestral Hybrid Incompatibility Gene
- Genome-Wide Association and Functional Follow-Up Reveals New Loci for Kidney Function
- A Natural System of Chromosome Transfer in
- Cell Size and the Initiation of DNA Replication in Bacteria
- Probing the Informational and Regulatory Plasticity of a Transcription Factor DNA–Binding Domain
- Repression of Germline RNAi Pathways in Somatic Cells by Retinoblastoma Pathway Chromatin Complexes
- Temporal Transcriptional Profiling of Somatic and Germ Cells Reveals Biased Lineage Priming of Sexual Fate in the Fetal Mouse Gonad
- Rapid Analysis of Genome Rearrangements by Multiplex Ligation–Dependent Probe Amplification
- Metabolic Profiling of a Mapping Population Exposes New Insights in the Regulation of Seed Metabolism and Seed, Fruit, and Plant Relations
- The Atypical Calpains: Evolutionary Analyses and Roles in Cellular Degeneration
- The Silkworm Coming of Age—Early
- Development of a Panel of Genome-Wide Ancestry Informative Markers to Study Admixture Throughout the Americas
- Balanced Codon Usage Optimizes Eukaryotic Translational Efficiency
- The Min System and Nucleoid Occlusion Are Not Required for Identifying the Division Site in but Ensure Its Efficient Utilization
- Neurobeachin, a Regulator of Synaptic Protein Targeting, Is Associated with Body Fat Mass and Feeding Behavior in Mice and Body-Mass Index in Humans
- Statistical Analysis of Readthrough Levels for Nonsense Mutations in Mammalian Cells Reveals a Major Determinant of Response to Gentamicin
- Gene Reactivation by 5-Aza-2′-Deoxycytidine–Induced Demethylation Requires SRCAP–Mediated H2A.Z Insertion to Establish Nucleosome Depleted Regions
- The miR-35-41 Family of MicroRNAs Regulates RNAi Sensitivity in
- Genetic Basis of Hidden Phenotypic Variation Revealed by Increased Translational Readthrough in Yeast
- An Alu Element–Associated Hypermethylation Variant of the Gene Is Associated with Childhood Obesity
- Modelling Human Regulatory Variation in Mouse: Finding the Function in Genome-Wide Association Studies and Whole-Genome Sequencing
- Novel Loci for Adiponectin Levels and Their Influence on Type 2 Diabetes and Metabolic Traits: A Multi-Ethnic Meta-Analysis of 45,891 Individuals
- Polycomb-Like 3 Promotes Polycomb Repressive Complex 2 Binding to CpG Islands and Embryonic Stem Cell Self-Renewal
- Insulin/IGF-1 and Hypoxia Signaling Act in Concert to Regulate Iron Homeostasis in
- EMF1 and PRC2 Cooperate to Repress Key Regulators of Arabidopsis Development
- Three Essential Ribonucleases—RNase Y, J1, and III—Control the Abundance of a Majority of mRNAs
- Contrasted Patterns of Molecular Evolution in Dominant and Recessive Self-Incompatibility Haplotypes in
- A Machine Learning Approach for Identifying Novel Cell Type–Specific Transcriptional Regulators of Myogenesis
- Genomic Tools for Evolution and Conservation in the Chimpanzee: Is a Genetically Distinct Population
- Nos2 Inactivation Promotes the Development of Medulloblastoma in Mice by Deregulation of Gap43–Dependent Granule Cell Precursor Migration
- Intracranial Aneurysm Risk Locus 5q23.2 Is Associated with Elevated Systolic Blood Pressure
- Heritability and Genetic Correlations Explained by Common SNPs for Metabolic Syndrome Traits
- A Genome-Wide Association Study of Nephrolithiasis in the Japanese Population Identifies Novel Susceptible Loci at 5q35.3, 7p14.3, and 13q14.1
- DNA Damage in Nijmegen Breakage Syndrome Cells Leads to PARP Hyperactivation and Increased Oxidative Stress
- DNA Resection at Chromosome Breaks Promotes Genome Stability by Constraining Non-Allelic Homologous Recombination
- Genetic Analysis of Floral Symmetry in Van Gogh's Sunflowers Reveals Independent Recruitment of Genes in the Asteraceae
- A Splice Site Variant in the Bovine Gene Compromises Growth and Regulation of the Inflammatory Response
- Promoter Nucleosome Organization Shapes the Evolution of Gene Expression
- The Nucleoside Diphosphate Kinase Gene Acts as Quantitative Trait Locus Promoting Non-Mendelian Inheritance
- The Ciliogenic Transcription Factor RFX3 Regulates Early Midline Distribution of Guidepost Neurons Required for Corpus Callosum Development
- Phosphorylation of the RNA–Binding Protein HOW by MAPK/ERK Enhances Its Dimerization and Activity
- A Genome-Wide Scan of Ashkenazi Jewish Crohn's Disease Suggests Novel Susceptibility Loci
- Parkinson's Disease–Associated Kinase PINK1 Regulates Miro Protein Level and Axonal Transport of Mitochondria
- LMW-E/CDK2 Deregulates Acinar Morphogenesis, Induces Tumorigenesis, and Associates with the Activated b-Raf-ERK1/2-mTOR Pathway in Breast Cancer Patients
- Mapping the Hsp90 Genetic Interaction Network in Reveals Environmental Contingency and Rewired Circuitry
- Autoregulation of the Noncoding RNA Gene
- The Human Pancreatic Islet Transcriptome: Expression of Candidate Genes for Type 1 Diabetes and the Impact of Pro-Inflammatory Cytokines
- Spo0A∼P Imposes a Temporal Gate for the Bimodal Expression of Competence in
- Antagonistic Regulation of Apoptosis and Differentiation by the Cut Transcription Factor Represents a Tumor-Suppressing Mechanism in
- A Downstream CpG Island Controls Transcript Initiation and Elongation and the Methylation State of the Imprinted Macro ncRNA Promoter
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- PIF4–Mediated Activation of Expression Integrates Temperature into the Auxin Pathway in Regulating Hypocotyl Growth
- Metabolic Profiling of a Mapping Population Exposes New Insights in the Regulation of Seed Metabolism and Seed, Fruit, and Plant Relations
- A Splice Site Variant in the Bovine Gene Compromises Growth and Regulation of the Inflammatory Response
- Comprehensive Research Synopsis and Systematic Meta-Analyses in Parkinson's Disease Genetics: The PDGene Database
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



