-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaHeritability and Genetic Correlations Explained by Common SNPs for Metabolic Syndrome Traits
We used a bivariate (multivariate) linear mixed-effects model to estimate the narrow-sense heritability (h2) and heritability explained by the common SNPs (hg2) for several metabolic syndrome (MetS) traits and the genetic correlation between pairs of traits for the Atherosclerosis Risk in Communities (ARIC) genome-wide association study (GWAS) population. MetS traits included body-mass index (BMI), waist-to-hip ratio (WHR), systolic blood pressure (SBP), fasting glucose (GLU), fasting insulin (INS), fasting trigylcerides (TG), and fasting high-density lipoprotein (HDL). We found the percentage of h2 accounted for by common SNPs to be 58% of h2 for height, 41% for BMI, 46% for WHR, 30% for GLU, 39% for INS, 34% for TG, 25% for HDL, and 80% for SBP. We confirmed prior reports for height and BMI using the ARIC population and independently in the Framingham Heart Study (FHS) population. We demonstrated that the multivariate model supported large genetic correlations between BMI and WHR and between TG and HDL. We also showed that the genetic correlations between the MetS traits are directly proportional to the phenotypic correlations.
Published in the journal: . PLoS Genet 8(3): e32767. doi:10.1371/journal.pgen.1002637
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002637Summary
We used a bivariate (multivariate) linear mixed-effects model to estimate the narrow-sense heritability (h2) and heritability explained by the common SNPs (hg2) for several metabolic syndrome (MetS) traits and the genetic correlation between pairs of traits for the Atherosclerosis Risk in Communities (ARIC) genome-wide association study (GWAS) population. MetS traits included body-mass index (BMI), waist-to-hip ratio (WHR), systolic blood pressure (SBP), fasting glucose (GLU), fasting insulin (INS), fasting trigylcerides (TG), and fasting high-density lipoprotein (HDL). We found the percentage of h2 accounted for by common SNPs to be 58% of h2 for height, 41% for BMI, 46% for WHR, 30% for GLU, 39% for INS, 34% for TG, 25% for HDL, and 80% for SBP. We confirmed prior reports for height and BMI using the ARIC population and independently in the Framingham Heart Study (FHS) population. We demonstrated that the multivariate model supported large genetic correlations between BMI and WHR and between TG and HDL. We also showed that the genetic correlations between the MetS traits are directly proportional to the phenotypic correlations.
Introduction
Obesity associated traits such as central adiposity, dyslipidemia, hypertension, and insulin resistance are major risk factors for type 2 diabetes and cardiovascular complications [1]. The constellation of these traits has been termed metabolic syndrome (MetS). Understanding the genetic factors underlying these traits and how they are correlated is clinically important. Large-scale genotyping investigations such as genome-wide association studies (GWAS) are useful tools for identifying genetic factors. However, significant genetic variants discovered in GWAS explain only a small proportion of the expected narrow-sense heritability, h2, defined as the ratio of additive genetic variance to phenotypic variance [2]. This discrepancy underlies the debate concerning “missing” genetic factors among the common variants [3], [4].
The main approach of GWAS has been to identify significant single-nucleotide polymorphisms (SNPs) by examining each SNP individually for significance. The h2 attributed to that marker is then given by 2f(1−f)a2, where f is the frequency of the marker and a is the additive effect. To reduce the chance of false positives, a stringent p-value criterion has been adopted (typically p = 5*10−8, based on an adjusted p-value of 0.05 for one-million tests). It has been suggested that this selection criterion is too conservative [5] and that some of the missing heritability may be linked to genetic markers of small effect that fail this stringent cutoff.
Alternatively, the narrow sense heritability explained by the common SNPs, hg2, may be estimated by adapting a linear mixed-effects model [6], [7] that is used to estimate h2. This model decomposes the phenotypic variance into genetic and residual variance components. Usually, the model is applied to related individuals where the genetic relationships are estimated by using family pedigree or genetic markers [8], [9]. Yang et al. [6], [7] pointed out that hg2 could be estimated using genetic relationships obtained from the common SNPs for unrelated individuals. The main assumed difference between hg2 and h2 is due to the difference in linkage disequilibrium (LD) between the common SNP markers and the rest of the genome, with the assumption that closely related individuals would be in greater LD than unrelated individuals. Thus, heritability estimated with the genetic relationships of unrelated individuals is attributed to the common variants while that estimated with genetic relationships of related individuals is attributed to the entire genome. While the method does not identify single variants, it provides the maximum expected variance expected by the set of markers or the relative complement of the set (e.g., common versus rare variants). Recently, it has been shown that a large proportion of h2 is explained by the common single-nucleotide polymorphisms (SNPs) for several traits using this model [6], [7]. Here, we showed that large proportions of the phenotypic variance for several metabolic syndrome (MetS) traits were also captured by the common SNPs. Among these, we validated the height and body-mass index estimates by Yang et al. [6], [7] in independent GWAS populations. We also quantified the genetic correlation between traits explained by the common SNPs.
Results
We estimated h2 and hg2 for height and body-mass index (BMI) in the Framingham Heart Study population (FHS), and height and seven metabolic syndrome traits (MetS) traits: BMI, waist-to-hip ratio (WHR), systolic blood pressure (SBP), fasting glucose (GLU), fasting insulin (INS), fasting triglycerides (TG), and fasting high-density lipoprotein (HDL) in the Atherosclerosis Risk in Communities population (ARIC) (ARIC MetS estimates shown in Table 1). Our base FHS population consisted of 4,240 subjects and our base ARIC population consisted of 8,451 subjects (see Methods and Tables S1 and S2 for a description of the populations). The genetic relationship between pairs of subjects was estimated using 436,126 genome-wide common SNP markers for ARIC and 320,118 SNPs for FHS (see Methods for details).
Tab. 1. h2 and hg2 estimates (ARIC population). 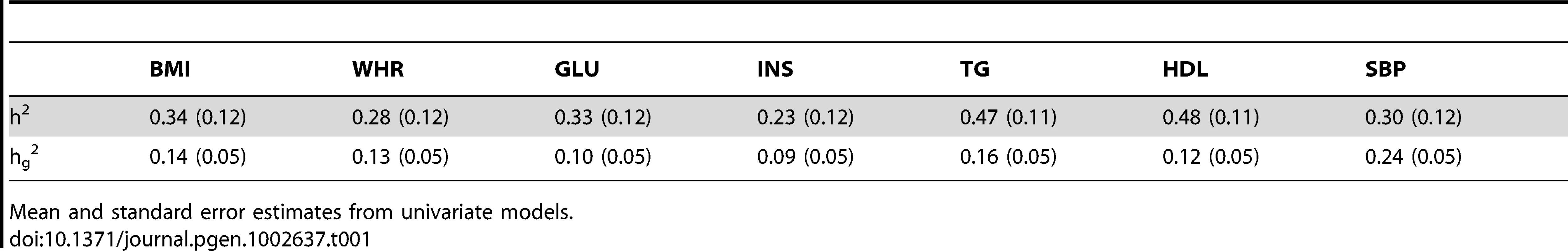
Mean and standard error estimates from univariate models. We first estimated h2 for related individuals with relationships between 0.35 and 0.65, derived empirically from the SNP markers, for height and BMI in the ARIC and FHS populations (see Methods for derivation of the relationship matrix). This resulted in 3,663 subjects (6,706,953 pairs of subjects) for FHS and 530 subjects (140,185 pairs of subjects) for ARIC. We found h2 to be 0.77 (s.e. 0.03) for height and 0.39 (s.e. 0.04) for BMI in FHS, and 0.88 (s.e. 0.09) for height and 0.34 (s.e. 0.12) for BMI in ARIC. The estimated h2 were consistent with values obtained using phenotypic regression (data not shown) and previous results [6], [7], [10], [11].
We then compared these values to estimates for hg2 for unrelated individuals with relationships less than 0.025 (see Methods for derivation of the relationship matrix). This resulted in 1,489 subjects (1,107,816 pairs of subjects) for FHS and 5,647 subjects (31,882,962 pairs of subjects) for ARIC. As mentioned above, hg2 provides an estimate of the heritability explained by common variants because of presumed lesser linkage disequilibrium between the common SNPs and the rest of the genome as compared to related individuals. We found hg2 to be 0.50 (s.e. 0.18) for height and 0.10 (s.e. 0.18) for BMI in FHS, and 0.46 (s.e. 0.05) for height and 0.14 (s.e. 0.05) for BMI in ARIC. These values are consistent with previously estimated values [6], [7]. Using the average across FHS and ARIC estimates, this implied that the common SNPs accounted for approximately 58% of h2 for height and 33% for BMI. To assess whether including more common SNPs would explain more of the h2, we examined how hg2 depended on the number of SNPs. As shown in Figure S1, the mean and standard error of the hg2 estimate for height in the ARIC population appeared to stabilize after approximately 300,000 SNPs.
We then estimated h2 and hg2 for the MetS traits in the ARIC population using the same subjects as above (see Table 1). We validated our h2 estimates by using phenotypic regression between related individuals for some of the traits (data not shown). The median h2 was 0.33, the minimum was 0.23 (INS), and the maximum was 0.48 (HDL). The median hg2 was 0.13, the minimum was 0.09 (INS), and maximum was 0.24 (SBP). Comparing the medians suggested that hg2 explains ∼39% of the h2 for these MetS traits. We found that the common SNPs explained large proportions of the h2: 41% of h2 for BMI, 46% for WHR, 30% for GLU, 39% for INS, 34% for TG, 25% for HDL, and 80% for SBP.
We next estimated the genetic correlations between MetS traits using a bivariate (multivariate) model (see Tables S3 and S4 for covariances). Table 2 shows the genetic and residual correlations for related individuals using bivariate models. The genetic correlation is the additive genetic covariance between traits normalized by the geometric mean of the individual trait genetic variances. The residual correlation is similarly estimated using the residual covariance and variances. For related individuals, we found significant genetic correlations for BMI-WHR, WHR-INS, GLU-INS, INS-TG, and TG-HDL and significant residual correlations between BMI-WHR, BMI-INS, BMI-HDL, WHR-INS, INS-HDL, and TG-HDL. Table 3 shows the genetic and residual correlations for the unrelated individuals. We found significant genetic correlations for BMI-WHR and TG-HDL and significant residual correlations for all of the estimates except SBP-HDL. The genetic correlations for unrelated individuals were proportional to the genetic correlations for related individuals (see Figure S2) with a proportionality constant of 0.44 (s.e. = 0.15 ; two-tail t-distribution p-value with 20 d.f. = 8.2*10−3). The phenotypic correlations between traits were similar for related and unrelated individuals and are shown in Table 4. These values were also consistent with the reported estimates in the National Heart Lung and Blood Institute-Family Heart Study (NHLBI-FHS), which included Framingham Heart Study and ARIC families [11].
Tab. 2. Genetic and residual correlation coefficients between MetS traits in the ARIC population among related individuals from the bivariate REML model. 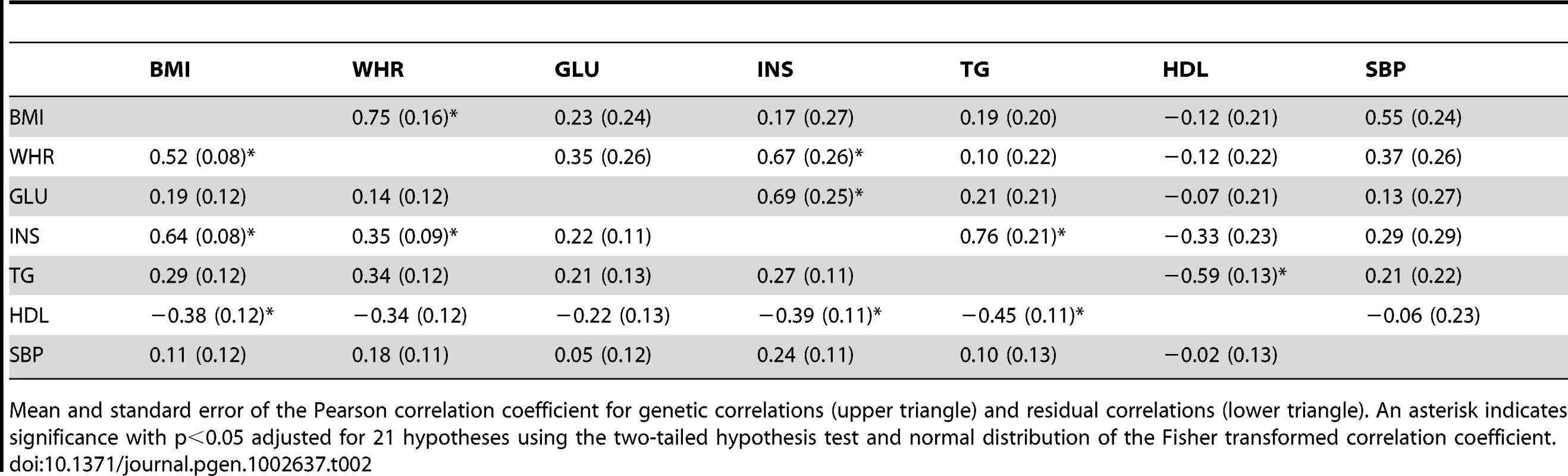
Mean and standard error of the Pearson correlation coefficient for genetic correlations (upper triangle) and residual correlations (lower triangle). An asterisk indicates significance with p<0.05 adjusted for 21 hypotheses using the two-tailed hypothesis test and normal distribution of the Fisher transformed correlation coefficient. Tab. 3. Genetic and residual correlations between MetS traits in the ARIC population among unrelated individuals from the bivariate REML model. 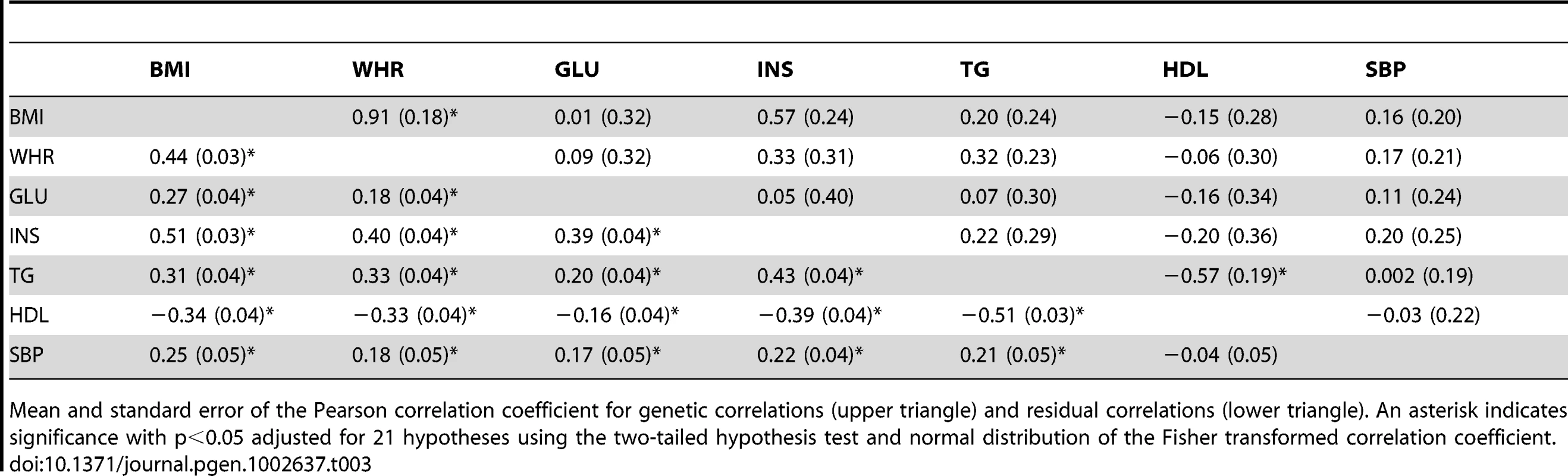
Mean and standard error of the Pearson correlation coefficient for genetic correlations (upper triangle) and residual correlations (lower triangle). An asterisk indicates significance with p<0.05 adjusted for 21 hypotheses using the two-tailed hypothesis test and normal distribution of the Fisher transformed correlation coefficient. Tab. 4. Phenotypic correlation coefficients between MetS traits in the ARIC population. 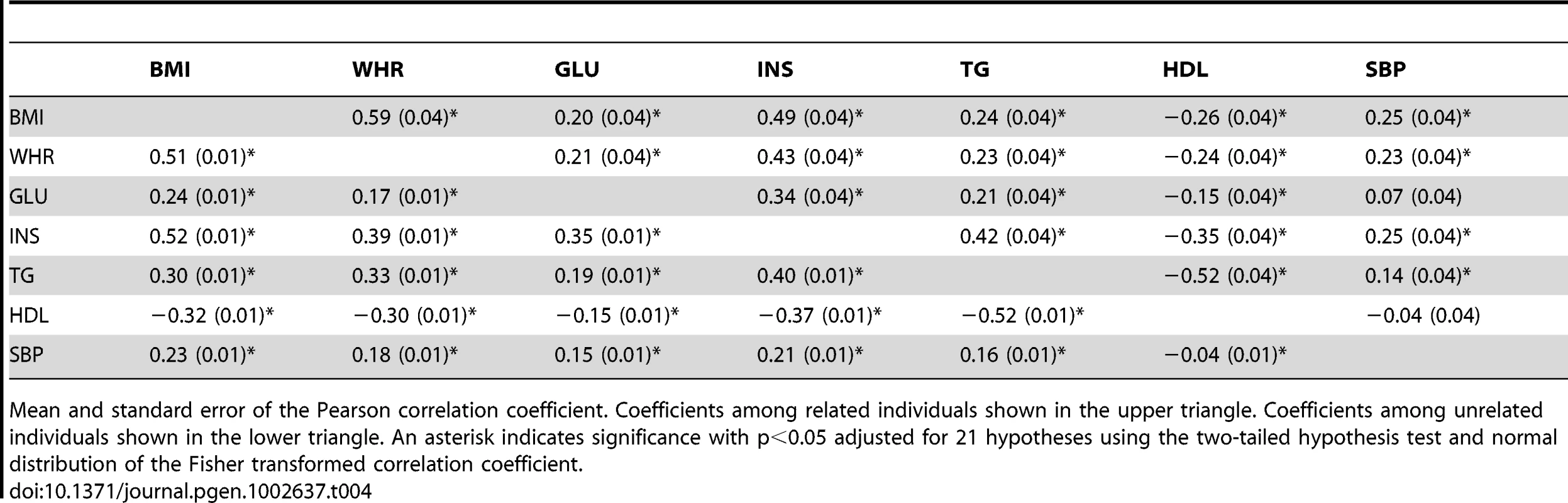
Mean and standard error of the Pearson correlation coefficient. Coefficients among related individuals shown in the upper triangle. Coefficients among unrelated individuals shown in the lower triangle. An asterisk indicates significance with p<0.05 adjusted for 21 hypotheses using the two-tailed hypothesis test and normal distribution of the Fisher transformed correlation coefficient. We validated our genetic correlation estimates using bivariate models for each pair of traits by analyzing all 7 MetS traits simultaneously for the unrelated individuals in a single multivariate model. This 7 trait multivariate model was much more expensive computationally so we used a less stringent convergence rule. The results were similar to the bivariate model (see Table S5 and S6) although the genetic correlation increased and their error decreased for a number of the estimates. In addition to the significant genetic correlations in the bivariate models, we also found the genetic correlation for BMI-INS to be significant in the 7 trait model.
We then examined the relationship between the genetic and phenotypic correlations (see Figure S3). For related individuals, we found that the phenotypic correlations rp were proportional to the genetic correlations rg with a proportionality constant of 1.2 (s.e. = 0.16; two-tail t-distribution p-value with 20 d.f. = 3.1*10−7). For unrelated individuals, we found that the phenotypic correlations were proportional to the genetic correlations with a proportionality constant of 0.85 (s.e. = 0.19 ; two-tail t-distribution p-value with 20 d.f. = 2.3*10−4). The direct proportionality between rp and rg implies that the ratio rg/rp is approximately constant for the MetS traits.
Discussion
We used a recently developed approach to analyzing GWAS data and provided new estimates for the total amount of additive genetic information contained in the common SNPs for MetS traits. The approach uses a linear mixed-effects model to estimate the additive genetic variances and correlations between traits. The model relies on knowing the genetic relationships between the individuals analyzed. Previously, this had been obtained from family pedigrees. Visscher et al. [9] and Yang et al. [6] observed that the genetic relationships could be computed from the GWAS SNPs. They also presumed that the heritability estimated for unrelated individuals with low SNP correlation are explained mainly by these common SNPs because the linkage disequilibrium between the common SNPs and the rest of the genome is weak. This would be in contrast to related individuals with high SNP correlation where linkage disequilibrium is strong. Thus, heritability estimated with the genetic relationships of unrelated individuals is attributed to the common SNPs while that estimated with the related individuals is attributed to the entire genome. This then creates a major distinction between h2 and hg2. We computed both in the same population. However, differences between estimates of h2 and hg2 may also arise due to differences in environmental influences and non-additive genetic effects that may bias the estimates. Provided that these biases are small then the ratio of hg2 to h2 provides an estimate of the proportion of narrow sense heritability captured by the common SNPs.
We confirmed previous findings that a large proportion of h2 is explained by the common SNPs. Our hg2 estimates for height and BMI in two independent analyses (i.e. ARIC and FHS) were consistent with previously reported values [6], [7]. Our h2 estimates for BMI, GLU, INS, TG, HDL, and SBP were similar to the findings of the large family National Heart, Lung, and Blood Institute (NHLBI) Family Heart Study [11], which included Framingham Heart Study and ARIC families. We found that hg2 explained a large proportion of h2 across the MetS traits, and hg2 explained approximately 39% of the h2 for these traits. We estimated that the common SNPs explain 58% of h2 for height, 41% for BMI, 46% for WHR, 30% for GLU, 39% for INS, 34% for TG, 25% for HDL, and 80% for SBP. Our hg2 findings are striking compared to traditional GWAS approaches where significant common SNPs have been shown to explain only 4% of h2 for BMI with 32 SNPs, 11% for GLU with 14 SNPs, 20% for TG with 48 SNPs, 25% for HDL with 60 SNPs, 3% for SBP with 10 SNPs, and 12% for height with 180 SNPs [12]–[16]. Height had the largest absolute hg2, which was consistent with having a large h2. Surprisingly, SBP had the largest proportion of h2 explained by the common SNPs while only a few percent of this has been uncovered by traditional GWAS. However, the standard error of hg2 for SBP was large and reducing this error will be important for further investigation. Conversely, our analysis suggested that the SNP markers already identified for TG and HDL may contain the maximum heritability expected from the common SNPs.
Our analysis of hg2 against the number of SNPs suggested that the mean and standard error of hg2 for height is well estimated by approximately 300,000 markers and that including more markers would have little effect for this trait and perhaps others. The standard error of hg2 also increased with SNP number. This may seem paradoxical but can be explained by recalling that the estimate for hg2 is proportional to the regression coefficient of the square of the phenotype differences versus the genetic relationship (i.e. Haseman-Elston regression) [8]. The standard error of hg2 is thus inversely proportional to the variance of the genetic relationship. Since the latter is estimated from the common SNPs, this variance is expected to decrease as the number of SNPs increases thereby increasing the standard error [6].
Using the bivariate (multivariate) model [17], [18] we estimated the genetic and residual correlations between the MetS traits. Among these, we found that the genetic correlations in related and unrelated individuals for BMI and WHR were significantly different from zero. This is consistent with both traits as indirect measures of body fat and common health risks [19]. Previously, Rice et al., 1994 [20] found significant genetic correlations between BMI and SBP among normotensive nonobese families. This suggested a common genetic etiology to their physiological relationship through hyperinsulinemia resulting in increased renal reabsorption of sodium and sympathetic activation [20]. We found a large genetic correlation among related subjects, although it was not significant because of the large error. This was consistent with the large family study by the NHLBI that did not find a significant genetic correlation [8]. Perusse et al, 1997 [21] argued that cross-trait resemblance between BMI and lipids is mostly environmental. In concordance, we did not find significant genetic correlations between either BMI or WHR and TG and HDL for either related or unrelated individuals (see Table 3 and Table 4) while residual (which includes environmental) correlations were significant for BMI–HDL. We found that the residual covariance accounted for a minimum of 71% (derived from the estimates in Table 4 and Table S3) of the phenotype covariance between BMI or WHR and the lipid measurements for related individuals. Genetic correlations between TG and HDL were also large, which is consistent with their direct physiological relationship [22]. This is also consistent with the findings from a recent GWAS meta-analysis whose results showed that 50% of the significant markers for TG were also significant for HDL (derived from Supplementary Tables 6 and 11 in [16]), and with a genome-wide LOD correlation analysis [23]. While we found some significant genetic correlations among both related and unrelated subjects, the variance was large for these estimates and greater statistical power is needed for better accuracy.
We found that the genetic correlation was directly proportional to the phenotypic correlation, which was an unexpected, empirical finding. Previously, a linear relationship between the correlations was hypothesized by Cheverud for sets of traits with common functions, and shown empirically for a number of traits [8], [24]–[26]. While this finding is interesting from an evolutionary genetics perspective, it may also serve a useful purpose in the maximum likelihood computation of the linear mixed-effects model by providing initial genetic correlation (i.e. covariance) estimates based on the phenotypic correlations.
In summary, we provided evidence that the common SNPs explain large proportions of the variance for several MetS traits in agreement with previous findings for some of these traits [6], [7]. This is consistent with the original premise of GWAS that a large proportion of phenotypic variation for common traits may be due to common variants [27]. However, an amendment to this premise is that it is likely to be many common variants with small effect. This is supported by recent meta-analyses with larger sample sizes that have identified more associated common SNPs. This approach can serve as a first approximation of the total heritability expected from common SNPs given a genome-wide set of markers and requires fewer subjects to achieve significant results. We also found genetic associations that will be useful for single gene and systems biology studies. Future studies with greater power will provide estimates for weaker multivariate genetic associations and provide greater precision for the estimates presented here.
Methods
ARIC population and GWAS data
Our main study population was the Atherosclerosis Risk In Communities (ARIC) population. The ARIC population consists of a large sample of unrelated individuals and some families across North America. The population was recruited from four centers across the United States: Forsyth County, North Carolina; Jackson, Mississippi; Minneapolis, Minnesota; and Washington County, Maryland. For this study, we restricted our analysis to the European-American group. The population was recruited in 1987 from the general population consisting of subjects aged 45 to 64 years. The ARIC population consisted of 8,451 subjects.
Quality control and genotype calls for common SNPs were evaluated previously for ARIC using the Affymetrix Human SNP Array 6.0. We selected bilallelic autosomal markers based on the following criteria: missingness <0.05, Hardy-Weinberg equilibrium (p<10−6) and minor allele frequency >0.05. Subjects with missingness >0.05 were removed. This resulted in 436,126 retained markers.
Quality control measurements from dbGAP (GENEVA ARIC Project Quality Control Report Sept 22, 2009) indicate significant population stratification between self-identified white (European-ancestory kind group) and black populations when projected onto HapMap components. Furthermore, principal-components analysis of the European-ancestory group by dbGAP showed that no component explained more than 0.1% of the population variance. For this study we only analyzed the European-ancestory group and treated it as a single population.
ARIC phenotypes were adjusted for age, sex, and study center. Only single measurements from visit 1 were used for these subjects. We only used subjects with negative diabetes status and with genotype and phenotype information for all traits. This resulted in 8,451 subjects. We standardized all the traits. We first log-transformed BMI, glucose, insulin, triglycerides, HDL, and systolic blood pressure. All laboratory measurements are under fasting conditions. Population trait statistics are in Table S1.
Framingham Heart Study (FHS) population
We estimated h2 and hg2 for height and BMI in the Framingham Heart Study population (FHS). The FHS population is a large multi-generational dataset that started in 1948 in Framingham, Massachusetts in the United States. It consists of a number of ethnicities predominantly from the United Kingdom, Ireland, Italy, and Western Europe [28]. Markers were screened similarly to ARIC and we also removed any SNPs that did not overlap with the ARIC set, which results in 320,118 SNPs. We used principal components analysis of the linkage disequilibrium (LD) pruned genetic relationship matrix to identify components with variance >0.1%. LD pruning was as in the ARIC 2009 report. This resulted in 73,432 retained SNPs. We found three significant components that were then used as covariates in the REML model. For consistency with ARIC, we restricted the age range at time of exam to 45 to 65 years and randomly selected a single measurement in the case of multiple measurements. Phenotypes were adjusted for age, sex, and generation prior to the REML estimation and standardized. We first log-transformed BMI. Population trait statistics are in Table S2. Our base FHS population consisted of 4,240 subjects.
h2 estimates using common SNP estimated relationship
We determined h2 using the linear mixed-effects model (see derivation below) and related individuals defined as genomic relatedness between 0.35 and 0.65. We assume that the common SNPs are in greater linkage disequilbrium among related individuals and, as such, can be used to estimate the total additive-genetic variance across the allele spectrum as suggested by Visscher et al., 2006 [9]. We constrained the relationship matrix to have at least one related pair per subject. This was done by pruning the entire population relationship matrix by randomly selecting a row and removing the row and its corresponding column if no genomic covariance in the row was between the cutoff values. For all pairs, including unrelated individuals, we used their empirically defined relationship. This resulted in 530 individuals being selected for analysis in ARIC and 3,663 individuals in FHS.
h2 was estimated with h2 = varg/(varg+vare), where varg and vare are the genetic and residual variance components estimated by the REML model using related individuals. The error was estimated from the inverse Fisher Information (see linear mixed-effects model below) and propagated using a first-order Taylor expansion.
Common SNP linear mixed-effects model estimate of hg2
We used the linear mixed-effects model and only unrelated individuals to estimate the additive-genetic variance attributable to the common SNPs (hg2). Unrelated individuals were defined as subjects with maximum genomic correlation of <0.025. The genomic relationship matrix was then produced as above based on this cutoff. The cutoff was taken from Yang et al. 2010 [6] and is less than the expected coefficient of relatedness between 2nd cousins. For these estimates we used the same group of 5,647 unrelated individuals for all estimates in ARIC and 1,489 individuals in FHS. hg2 was estimated as hg2 = varg/(varg+vare), where varg and vare are the genetic and residual variance components estimated by the REML model using unrelated individuals. The standard error was estimated as above. The height hg2 versus SNP number analyses were performed over allele frequency range of 0.05 to 0.5 in order of increasing and decreasing frequency.
Correlations
The genetic correlation (rg) is defined as , where (varg(ti)) is the additive genetic variance of trait i and covariance (covg(ti,tj)) is the additive genetic covariance between the traits. The variances and covariances are estimated directly in the multivariate linear mixed-effects model. The error was computed from the estimated errors of the variances and covariance using a first-order Taylor expansion. The residual and phenotypic correlations were analogously defined. Phenotype correlations and error were estimated by linear regression of the standardized phenotypes.
Proportionality constants
The mean and errors for proportionality constants between the genetic and phenotypic correlations were determined by randomly sampling over the distributions of the parameter estimates (i.e. Monte Carlo method) assuming that the error around the mean parameter estimate was normally distributed and that the parameters were independent. We then fit a linear function with the y-intercept fixed at 0 (after first confirming that it was not significantly different from zero).
Significance testing
We assessed significance for correlation coefficients (r) using the standardized Fisher transformed estimate of r: arctan(r)/arctan(s.e.(r)). We estimated the two-tailed p-value from a normal distribution and significance was determined by p<0.05 and Bonferroni corrected for 21 hypotheses.
Significance for regression coefficient () was estimated using the standardized coefficient . We estimated the two-tailed p-value from a t-distribution and 20 degrees of freedom and significance was determined by p<0.05.
Preprocessing of SNPs and phenotypes was done using PLINK [29] (v1.07,http://pngu.mgh.harvard.edu/purcell/plink/) and MATLAB (2010b, MathWorks, Natick, MA). REML optimization was executed using software written in MATLAB.
Bivariate (multivariate) linear mixed-effects linear model
We considered the following multivariate linear mixed-effects model for m individuals, n loci and t traits [6]–[8], [17], [18], [30]:where yi is a m×1 vector of trait i for m individuals, Xi is an m×s fixed effects matrix for trait i, vi is a s×1 vector of fixed effects parameters for trait i, Z is an m×n matrix of standardized genotypes, ui is an n×1 vector of random effects for trait i satisfying ui∼N(0,G) and ei is an m×1 vector of residual effects satisfying ei∼N(0,R), with matrix blocks Gij = covgijIn and Rij = coveijIm and Il is the l×l identity matrix. This model can be used for single or multiple traits. For two traits, it is called a bivariate model. The model is identical to that used by [6], [7], [17].
We considered only bi-allelic SNPs in Hardy-Weinberg equilibrium. Denote the minor allele by q and the major allele by Q. Let the minor allele frequency at locus i have frequency pi. We assign a value of 2 for genotype qq, 1 for genotype qQ and 0 for genotype QQ. The Hardy-Weinberg mean frequency for the genotype at locus i is 2pi and the variance is 2pi(1−pi). The standardized genotype entries have values of (2−2pi)/(2pi(1−2pi))1/2 for qq, (1−2pi)/(2pi(1−2pi))1/2 for qQ, and −2pi/(2pi(1−2pi))1/2 for the QQ genotype.
The log of the likelihood function is given bywhere the covariance matrix can be expressed as a tensor product with m×m blocks V−1ij and A is the genetic relationship matrix. Following Yang et al. [6], we used a modified covariance matrix for A, , where the diagonals of A are computed using the formulaWe use the restricted maximum likelihood (REML) approach [8] where the gradients of the log likelihood are given bywhere Iij is a tm×tm dimensional matrix with zero entries except for a m×m identity matrix at block location i, j, and , where .
We solved the REML equations using an EM algorithm [8], which was given byfor iteration k+1 in terms of iteration k. We iterated until the rate of change of the log likelihood function was less than about 10−4. We also checked that the rate of change of the square of the covariance predictions was less than 10−8. We checked our results against the software developed by Yang et al. (GCTA) [31] for the univariate model.
For the multivariate model, we transformed to a coordinate system where the covariance matrices were diagonal [8] to speed up the computation. Let zj be the set of phenotypes for individual j. We used the canonical transformation such that and . Q can be computed from the formula where , (S is the matrix of left eigenvectors of GR−1). The transformed genetic covariances are given by and the residual covariances are It. Each step consisted of taking a single step with the univariate EM algorithm for the transformed additive genetic and residual variance followed by a transformation back to the original coordinates. We iterated until the maximum of the magnitudes of the components of the gradient of the log likelihood function was less than approximately .
In our computations, we used both the direct EM algorithm and the canonically transformed algorithm because even though the transformed algorithm was in principle faster, it sometimes had poor convergence properties if the initial guess was not sufficiently close to the maximum likelihood value. We ensured that both give the same results. For computational efficiency, the results shown are computed from the bivariate model for the different trait pairs. We confirmed our results with a multivariate model that included all traits.
Our error estimates were given by the inverse of the Fisher information matrix F, which we computed by evaluating the Hessian of the log likelihood at the maximum likelihood predictions. F is a t(t+1)×t(t+1) dimensional matrix with rows corresponding to the genetic and residual variances and covariances (where covij was set equal to covji) and with block elements (that are not all contiguous) given byfor and .
Supporting Information
Zdroje
1. PermuttMAWassonJCoxN 2005 Genetic epidemiology of diabetes. J Clin Invest 115 1431 1439
2. VisscherPMHillWGWrayNR 2008 Heritability in the genomics era–concepts and misconceptions. Nat Rev Genet 9 255 266
3. GibsonG 2010 Hints of hidden heritability in GWAS. Nat Genet 42 558 560
4. MaherB 2008 Personal genomes: The case of the missing heritability. Nature 456 18 21
5. PearsonTAManolioTA 2008 How to interpret a genome-wide association study. JAMA 299 1335 1344
6. YangJBenyaminBMcEvoyBPGordonSHendersAK 2010 Common SNPs explain a large proportion of the heritability for human height. Nat Genet 42 565 569
7. YangJManolioTAPasqualeLRBoerwinkleECaporasoN 2011 Genome partitioning of genetic variation for complex traits using common SNPs. Nat Genet 43 519 525
8. LynchMWalshB 1998 Genetics and analysis of quantitative traits Sunderland, Mass. Sinauer xvi, 980 p.
9. VisscherPMMedlandSEFerreiraMAMorleyKIZhuG 2006 Assumption-free estimation of heritability from genome-wide identity-by-descent sharing between full siblings. PLoS Genet 2 e41 doi:10.1371/journal.pgen.0020041
10. CoadySAJaquishCEFabsitzRRLarsonMGCupplesLA 2002 Genetic variability of adult body mass index: a longitudinal assessment in framingham families. Obes Res 10 675 681
11. TangWHongYProvinceMARichSSHopkinsPN 2006 Familial clustering for features of the metabolic syndrome. Diabetes Care 29 631
12. DupuisJLangenbergCProkopenkoISaxenaRSoranzoN 2010 New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat Genet 42 105 116
13. Lango AllenHEstradaKLettreGBerndtSIWeedonMN 2010 Hundreds of variants clustered in genomic loci and biological pathways affect human height. Nature 467 832 838
14. LevyDEhretGBRiceKVerwoertGCLaunerLJ 2009 Genome-wide association study of blood pressure and hypertension. Nat Genet 41 677 687
15. SpeliotesEKWillerCJBerndtSIMondaKLThorleifssonG 2010 Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat Genet 42 937 948
16. TeslovichTMMusunuruKSmithAVEdmondsonACStylianouIM 2010 Biological, clinical and population relevance of 95 loci for blood lipids. Nature 466 707 713
17. DearyIJYangJDaviesGHarrisSETenesaA 2012 Genetic contributions to stability and change in intelligence from childhood to old age. Nature
18. LeeSHWrayNRGoddardMEVisscherPM 2011 Estimating missing heritability for disease from genome-wide association studies. Am J Hum Genet 88 294 305
19. CzernichowSKengneAPStamatakisEHamerMBattyGD 2011 Body mass index, waist circumference and waist-hip ratio: which is the better discriminator of cardiovascular disease mortality risk?: evidence from an individual-participant meta-analysis of 82 864 participants from nine cohort studies. Obes Rev 12 680 687
20. RiceTProvinceMPerusseLBouchardCRaoDC 1994 Cross-trait familial resemblance for body fat and blood pressure: familial correlations in the Quebec Family Study. Am J Hum Genet 55 1019 1029
21. PerusseLRiceTDespresJPBergeronJProvinceMA 1997 Familial resemblance of plasma lipids, lipoproteins and postheparin lipoprotein and hepatic lipases in the HERITAGE Family Study. Arterioscler Thromb Vasc Biol 17 3263 3269
22. FraynKN 2010 Metabolic Regulation: A Human Perspective (Frayn, Metabolic Regulation) Wiley-Blackwell 384
23. MartinLJNorthKEDyerTBlangeroJComuzzieAG 2003 Phenotypic, genetic, and genome-wide structure in the metabolic syndrome. BMC Genet 4 Suppl 1 S95
24. CheverudJM 1988 A comparison of genetic and phenotypic correlations. Evolution 958 968
25. CheverudJM 1982 Relationships among ontogenetic, static, and evolutionary allometry. Am J Phys Anthropol 59 139 149
26. RoffDA 1995 The estimation of genetic correlations from phenotypic correlations: a test of Cheverud's conjecture. Heredity 74 481 490
27. LanderES 2011 Initial impact of the sequencing of the human genome. Nature 470 187 197
28. GovindarajuDRCupplesLAKannelWBO'DonnellCJAtwoodLD 2008 Genetics of the Framingham Heart Study population. Adv Genet 62 33 65
29. PurcellSNealeBTodd-BrownKThomasLFerreiraMA 2007 PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 81 559 575
30. PriceALHelgasonAThorleifssonGMcCarrollSAKongA 2011 Single-tissue and cross-tissue heritability of gene expression via identity-by-descent in related or unrelated individuals. PLoS Genet 7 e1001317 doi:10.1371/journal.pgen.1001317
31. YangJLeeSHGoddardMEVisscherPM 2010 GCTA: a tool for genome-wide complex trait analysis. The American Journal of Human Genetics
Štítky
Genetika Reprodukční medicína
Článek Physiological Notch Signaling Maintains Bone Homeostasis via RBPjk and Hey Upstream of NFATc1Článek Intronic -Regulatory Modules Mediate Tissue-Specific and Microbial Control of / TranscriptionČlánek Probing the Informational and Regulatory Plasticity of a Transcription Factor DNA–Binding DomainČlánek Repression of Germline RNAi Pathways in Somatic Cells by Retinoblastoma Pathway Chromatin ComplexesČlánek An Alu Element–Associated Hypermethylation Variant of the Gene Is Associated with Childhood ObesityČlánek Three Essential Ribonucleases—RNase Y, J1, and III—Control the Abundance of a Majority of mRNAsČlánek Genomic Tools for Evolution and Conservation in the Chimpanzee: Is a Genetically Distinct Population
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 3
-
Všechny články tohoto čísla
- Comprehensive Research Synopsis and Systematic Meta-Analyses in Parkinson's Disease Genetics: The PDGene Database
- Genomic Analysis of the Hydrocarbon-Producing, Cellulolytic, Endophytic Fungus
- Networks of Neuronal Genes Affected by Common and Rare Variants in Autism Spectrum Disorders
- Akirin Links Twist-Regulated Transcription with the Brahma Chromatin Remodeling Complex during Embryogenesis
- Too Much Cleavage of Cyclin E Promotes Breast Tumorigenesis
- Imprinted Genes … and the Number Is?
- Genetic Architecture of Highly Complex Chemical Resistance Traits across Four Yeast Strains
- Exploring the Complexity of the HIV-1 Fitness Landscape
- MNS1 Is Essential for Spermiogenesis and Motile Ciliary Functions in Mice
- A Fundamental Regulatory Mechanism Operating through OmpR and DNA Topology Controls Expression of Pathogenicity Islands SPI-1 and SPI-2
- Evidence for Positive Selection on a Number of MicroRNA Regulatory Interactions during Recent Human Evolution
- Variation in Modifies Risk of Neonatal Intestinal Obstruction in Cystic Fibrosis
- PIF4–Mediated Activation of Expression Integrates Temperature into the Auxin Pathway in Regulating Hypocotyl Growth
- Critical Evaluation of Imprinted Gene Expression by RNA–Seq: A New Perspective
- A Meta-Analysis and Genome-Wide Association Study of Platelet Count and Mean Platelet Volume in African Americans
- Mouse Genetics Suggests Cell-Context Dependency for Myc-Regulated Metabolic Enzymes during Tumorigenesis
- Transcriptional Control in Cardiac Progenitors: Tbx1 Interacts with the BAF Chromatin Remodeling Complex and Regulates
- Synthetic Lethality of Cohesins with PARPs and Replication Fork Mediators
- APOBEC3G-Induced Hypermutation of Human Immunodeficiency Virus Type-1 Is Typically a Discrete “All or Nothing” Phenomenon
- Interpreting Meta-Analyses of Genome-Wide Association Studies
- Error-Prone ZW Pairing and No Evidence for Meiotic Sex Chromosome Inactivation in the Chicken Germ Line
- -Dependent Chemosensory Functions Contribute to Courtship Behavior in
- Diverse Forms of Splicing Are Part of an Evolving Autoregulatory Circuit
- Phenotypic Plasticity of the Drosophila Transcriptome
- Physiological Notch Signaling Maintains Bone Homeostasis via RBPjk and Hey Upstream of NFATc1
- Precocious Metamorphosis in the Juvenile Hormone–Deficient Mutant of the Silkworm,
- Igf1r Signaling Is Indispensable for Preimplantation Development and Is Activated via a Novel Function of E-cadherin
- Accurate Prediction of Inducible Transcription Factor Binding Intensities In Vivo
- Mitochondrial Oxidative Stress Alters a Pathway in Strongly Resembling That of Bile Acid Biosynthesis and Secretion in Vertebrates
- Mammalian Neurogenesis Requires Treacle-Plk1 for Precise Control of Spindle Orientation, Mitotic Progression, and Maintenance of Neural Progenitor Cells
- Tcf7 Is an Important Regulator of the Switch of Self-Renewal and Differentiation in a Multipotential Hematopoietic Cell Line
- REST–Mediated Recruitment of Polycomb Repressor Complexes in Mammalian Cells
- Intronic -Regulatory Modules Mediate Tissue-Specific and Microbial Control of / Transcription
- Age-Dependent Brain Gene Expression and Copy Number Anomalies in Autism Suggest Distinct Pathological Processes at Young Versus Mature Ages
- A Genome-Wide Association Study Identifies Variants Underlying the Shade Avoidance Response
- -by- Regulatory Divergence Causes the Asymmetric Lethal Effects of an Ancestral Hybrid Incompatibility Gene
- Genome-Wide Association and Functional Follow-Up Reveals New Loci for Kidney Function
- A Natural System of Chromosome Transfer in
- Cell Size and the Initiation of DNA Replication in Bacteria
- Probing the Informational and Regulatory Plasticity of a Transcription Factor DNA–Binding Domain
- Repression of Germline RNAi Pathways in Somatic Cells by Retinoblastoma Pathway Chromatin Complexes
- Temporal Transcriptional Profiling of Somatic and Germ Cells Reveals Biased Lineage Priming of Sexual Fate in the Fetal Mouse Gonad
- Rapid Analysis of Genome Rearrangements by Multiplex Ligation–Dependent Probe Amplification
- Metabolic Profiling of a Mapping Population Exposes New Insights in the Regulation of Seed Metabolism and Seed, Fruit, and Plant Relations
- The Atypical Calpains: Evolutionary Analyses and Roles in Cellular Degeneration
- The Silkworm Coming of Age—Early
- Development of a Panel of Genome-Wide Ancestry Informative Markers to Study Admixture Throughout the Americas
- Balanced Codon Usage Optimizes Eukaryotic Translational Efficiency
- The Min System and Nucleoid Occlusion Are Not Required for Identifying the Division Site in but Ensure Its Efficient Utilization
- Neurobeachin, a Regulator of Synaptic Protein Targeting, Is Associated with Body Fat Mass and Feeding Behavior in Mice and Body-Mass Index in Humans
- Statistical Analysis of Readthrough Levels for Nonsense Mutations in Mammalian Cells Reveals a Major Determinant of Response to Gentamicin
- Gene Reactivation by 5-Aza-2′-Deoxycytidine–Induced Demethylation Requires SRCAP–Mediated H2A.Z Insertion to Establish Nucleosome Depleted Regions
- The miR-35-41 Family of MicroRNAs Regulates RNAi Sensitivity in
- Genetic Basis of Hidden Phenotypic Variation Revealed by Increased Translational Readthrough in Yeast
- An Alu Element–Associated Hypermethylation Variant of the Gene Is Associated with Childhood Obesity
- Modelling Human Regulatory Variation in Mouse: Finding the Function in Genome-Wide Association Studies and Whole-Genome Sequencing
- Novel Loci for Adiponectin Levels and Their Influence on Type 2 Diabetes and Metabolic Traits: A Multi-Ethnic Meta-Analysis of 45,891 Individuals
- Polycomb-Like 3 Promotes Polycomb Repressive Complex 2 Binding to CpG Islands and Embryonic Stem Cell Self-Renewal
- Insulin/IGF-1 and Hypoxia Signaling Act in Concert to Regulate Iron Homeostasis in
- EMF1 and PRC2 Cooperate to Repress Key Regulators of Arabidopsis Development
- Three Essential Ribonucleases—RNase Y, J1, and III—Control the Abundance of a Majority of mRNAs
- Contrasted Patterns of Molecular Evolution in Dominant and Recessive Self-Incompatibility Haplotypes in
- A Machine Learning Approach for Identifying Novel Cell Type–Specific Transcriptional Regulators of Myogenesis
- Genomic Tools for Evolution and Conservation in the Chimpanzee: Is a Genetically Distinct Population
- Nos2 Inactivation Promotes the Development of Medulloblastoma in Mice by Deregulation of Gap43–Dependent Granule Cell Precursor Migration
- Intracranial Aneurysm Risk Locus 5q23.2 Is Associated with Elevated Systolic Blood Pressure
- Heritability and Genetic Correlations Explained by Common SNPs for Metabolic Syndrome Traits
- A Genome-Wide Association Study of Nephrolithiasis in the Japanese Population Identifies Novel Susceptible Loci at 5q35.3, 7p14.3, and 13q14.1
- DNA Damage in Nijmegen Breakage Syndrome Cells Leads to PARP Hyperactivation and Increased Oxidative Stress
- DNA Resection at Chromosome Breaks Promotes Genome Stability by Constraining Non-Allelic Homologous Recombination
- Genetic Analysis of Floral Symmetry in Van Gogh's Sunflowers Reveals Independent Recruitment of Genes in the Asteraceae
- A Splice Site Variant in the Bovine Gene Compromises Growth and Regulation of the Inflammatory Response
- Promoter Nucleosome Organization Shapes the Evolution of Gene Expression
- The Nucleoside Diphosphate Kinase Gene Acts as Quantitative Trait Locus Promoting Non-Mendelian Inheritance
- The Ciliogenic Transcription Factor RFX3 Regulates Early Midline Distribution of Guidepost Neurons Required for Corpus Callosum Development
- Phosphorylation of the RNA–Binding Protein HOW by MAPK/ERK Enhances Its Dimerization and Activity
- A Genome-Wide Scan of Ashkenazi Jewish Crohn's Disease Suggests Novel Susceptibility Loci
- Parkinson's Disease–Associated Kinase PINK1 Regulates Miro Protein Level and Axonal Transport of Mitochondria
- LMW-E/CDK2 Deregulates Acinar Morphogenesis, Induces Tumorigenesis, and Associates with the Activated b-Raf-ERK1/2-mTOR Pathway in Breast Cancer Patients
- Mapping the Hsp90 Genetic Interaction Network in Reveals Environmental Contingency and Rewired Circuitry
- Autoregulation of the Noncoding RNA Gene
- The Human Pancreatic Islet Transcriptome: Expression of Candidate Genes for Type 1 Diabetes and the Impact of Pro-Inflammatory Cytokines
- Spo0A∼P Imposes a Temporal Gate for the Bimodal Expression of Competence in
- Antagonistic Regulation of Apoptosis and Differentiation by the Cut Transcription Factor Represents a Tumor-Suppressing Mechanism in
- A Downstream CpG Island Controls Transcript Initiation and Elongation and the Methylation State of the Imprinted Macro ncRNA Promoter
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- PIF4–Mediated Activation of Expression Integrates Temperature into the Auxin Pathway in Regulating Hypocotyl Growth
- Metabolic Profiling of a Mapping Population Exposes New Insights in the Regulation of Seed Metabolism and Seed, Fruit, and Plant Relations
- A Splice Site Variant in the Bovine Gene Compromises Growth and Regulation of the Inflammatory Response
- Comprehensive Research Synopsis and Systematic Meta-Analyses in Parkinson's Disease Genetics: The PDGene Database
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



