-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaIntracranial Aneurysm Risk Locus 5q23.2 Is Associated with Elevated Systolic Blood Pressure
Although genome-wide association studies (GWAS) have identified hundreds of complex trait loci, the pathomechanisms of most remain elusive. Studying the genetics of risk factors predisposing to disease is an attractive approach to identify targets for functional studies. Intracranial aneurysms (IA) are rupture-prone pouches at cerebral artery branching sites. IA is a complex disease for which GWAS have identified five loci with strong association and a further 14 loci with suggestive association. To decipher potential underlying disease mechanisms, we tested whether there are IA loci that convey their effect through elevating blood pressure (BP), a strong risk factor of IA. We performed a meta-analysis of four population-based Finnish cohorts (nFIN = 11 266) not selected for IA, to assess the association of previously identified IA candidate loci (n = 19) with BP. We defined systolic BP (SBP), diastolic BP, mean arterial pressure, and pulse pressure as quantitative outcome variables. The most significant result was further tested for association in the ICBP-GWAS cohort of 200 000 individuals. We found that the suggestive IA locus at 5q23.2 in PRDM6 was significantly associated with SBP in individuals of European descent (pFIN = 3.01E-05, pICBP-GWAS = 0.0007, pALL = 8.13E-07). The risk allele of IA was associated with higher SBP. PRDM6 encodes a protein predominantly expressed in vascular smooth muscle cells. Our study connects a complex disease (IA) locus with a common risk factor for the disease (SBP). We hypothesize that common variants in PRDM6 can contribute to altered vascular wall structure, hence increasing SBP and predisposing to IA. True positive associations often fail to reach genome-wide significance in GWAS. Our findings show that analysis of traditional risk factors as intermediate phenotypes is an effective tool for deciphering hidden heritability. Further, we demonstrate that common disease loci identified in a population isolate may bear wider significance.
Published in the journal: . PLoS Genet 8(3): e32767. doi:10.1371/journal.pgen.1002563
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002563Summary
Although genome-wide association studies (GWAS) have identified hundreds of complex trait loci, the pathomechanisms of most remain elusive. Studying the genetics of risk factors predisposing to disease is an attractive approach to identify targets for functional studies. Intracranial aneurysms (IA) are rupture-prone pouches at cerebral artery branching sites. IA is a complex disease for which GWAS have identified five loci with strong association and a further 14 loci with suggestive association. To decipher potential underlying disease mechanisms, we tested whether there are IA loci that convey their effect through elevating blood pressure (BP), a strong risk factor of IA. We performed a meta-analysis of four population-based Finnish cohorts (nFIN = 11 266) not selected for IA, to assess the association of previously identified IA candidate loci (n = 19) with BP. We defined systolic BP (SBP), diastolic BP, mean arterial pressure, and pulse pressure as quantitative outcome variables. The most significant result was further tested for association in the ICBP-GWAS cohort of 200 000 individuals. We found that the suggestive IA locus at 5q23.2 in PRDM6 was significantly associated with SBP in individuals of European descent (pFIN = 3.01E-05, pICBP-GWAS = 0.0007, pALL = 8.13E-07). The risk allele of IA was associated with higher SBP. PRDM6 encodes a protein predominantly expressed in vascular smooth muscle cells. Our study connects a complex disease (IA) locus with a common risk factor for the disease (SBP). We hypothesize that common variants in PRDM6 can contribute to altered vascular wall structure, hence increasing SBP and predisposing to IA. True positive associations often fail to reach genome-wide significance in GWAS. Our findings show that analysis of traditional risk factors as intermediate phenotypes is an effective tool for deciphering hidden heritability. Further, we demonstrate that common disease loci identified in a population isolate may bear wider significance.
Introduction
Intracranial aneurysms (IA) are berry-shaped pouches at the branching sites of cerebral arteries. 2–5% of the world population is estimated to harbor IA [1]. Most IA go unnoticed during one's lifetime. However, when they become symptomatic, it is usually due to rupture, causing subarachnoid hemorrhage (SAH). SAH is devastating intracranial bleeding, and half of those with SAH die within a year [2], [3]. SAH affects the working age population, with a median age of 55 [4]. Its incidence in Finland is 19/100 000/year [5], [6], triple than that of the rest of the world. The reason for this higher than average incidence is unknown. Aneurysmal SAH places a heavy burden on society both emotionally and financially. The strongest known non-modifiable risk factor of SAH is family history of the disease, and the strongest modifiable risk factors are smoking, excessive alcohol intake, and hypertension [7]. An important step in tackling SAH is to understand why IAs develop.
Our understanding of the environmental and genetic background of IA formation is limited. Positive family history of IA or SAH, older age and female sex increase the risk of developing IA [1]. Of the general cardiovascular risk factors, smoking has been shown to increase the risk of IA formation [8], and high blood pressure has long been speculated to do so [9]. The high, often undocumented, prevalence of high blood pressure in the control populations is likely the reason why it frequently fails to reach statistical significance as an IA risk factor [1]. Chronic hypertension may contribute to IA formation by imposing constantly high shear stress on vascular walls [9].
Multiple factors, such as familial aggregation of the disease, make a genetic contribution likely to the risk of IA. A minority of IAs show familial aggregation (under 10%) [7]. Linkage studies in IA families have highlighted numerous genetic regions and a recent exome sequencing study identified coding mutations in familial thoracic aortic aneurysm with intracranial aneurysm [10]. However, the majority of IA is sporadic. Sporadic IA is a complex disease and no gene with a certain role has been identified yet. Recent genome-wide association studies (GWAS) [11], [12] involving Finnish IA patients, have attempted to decipher the complex genetic background of IA. From these studies, five loci emerged with strong association to IA (p<5E-07, posterior probability of association –PPA>0.5), with the highest statistical significance at 9p21.3, a risk locus of multiple cardiovascular diseases. Further 14 loci exhibited suggestive association to IA (0.1≤PPA<0.5).
Despite the success of GWAS in identifying IA susceptibility loci, the pathomechanism by which they contribute to IA formation remains elusive. We hypothesize that hypertension, a strong modifiable risk factor of IA, may possess an overlapping genetic background with IA. To test this hypothesis, we analyzed the IA loci so far identified, in well-characterized population-based cohorts consisting of more than 210 000 individuals with blood pressure measurements.
Results
41 SNPs from 19 independent IA loci [13] were first analyzed for association with blood pressure in the national Health 2000 survey (H2000) [14] discovery cohort of 1581 individuals without blood pressure lowering medication (Table S1). We adjusted the analysis for age and gender (ROBUST model). The most significant association (p<0.1) were observed at 2q33.1 with diastolic blood pressure (DBP) and with mean arterial pressure (MAP), at 4q31.23 and 19q13.12 with DBP, and at 5q23.2 with systolic blood pressure (SBP), DBP, and MAP. We did not detect association with pulse pressure (PP) (Table S1). Next, we wanted to analyze the independence of the association signals observed. We tested all 19 loci SNPs adjusting for further factors known to affect blood pressure, namely smoking habits, alcohol consumption, and body mass index (BMI) (ADVANCED model). There was no tendency of association with DBP at 4q31.23 and 19q13.12 with the ADVANCED model. The strength of the association decreased for the four SNPs at the 2q33.1 locus for SBP, but increased marginally for DBP, and MAP. At 5q23.2 the strength of association increased substantially for most blood pressure measurements, such as SBP, DBP, and MAP (Table 1 and Table S2) for all three SNPs tested. The IA risk alleles at 5q23.2 were associated with elevated blood pressure.
Tab. 1. 2q33.1 and 5q23.2 loci cohort-wise ADVANCED model effect estimates and meta-analysis results with systolic blood pressure (SBP).$ 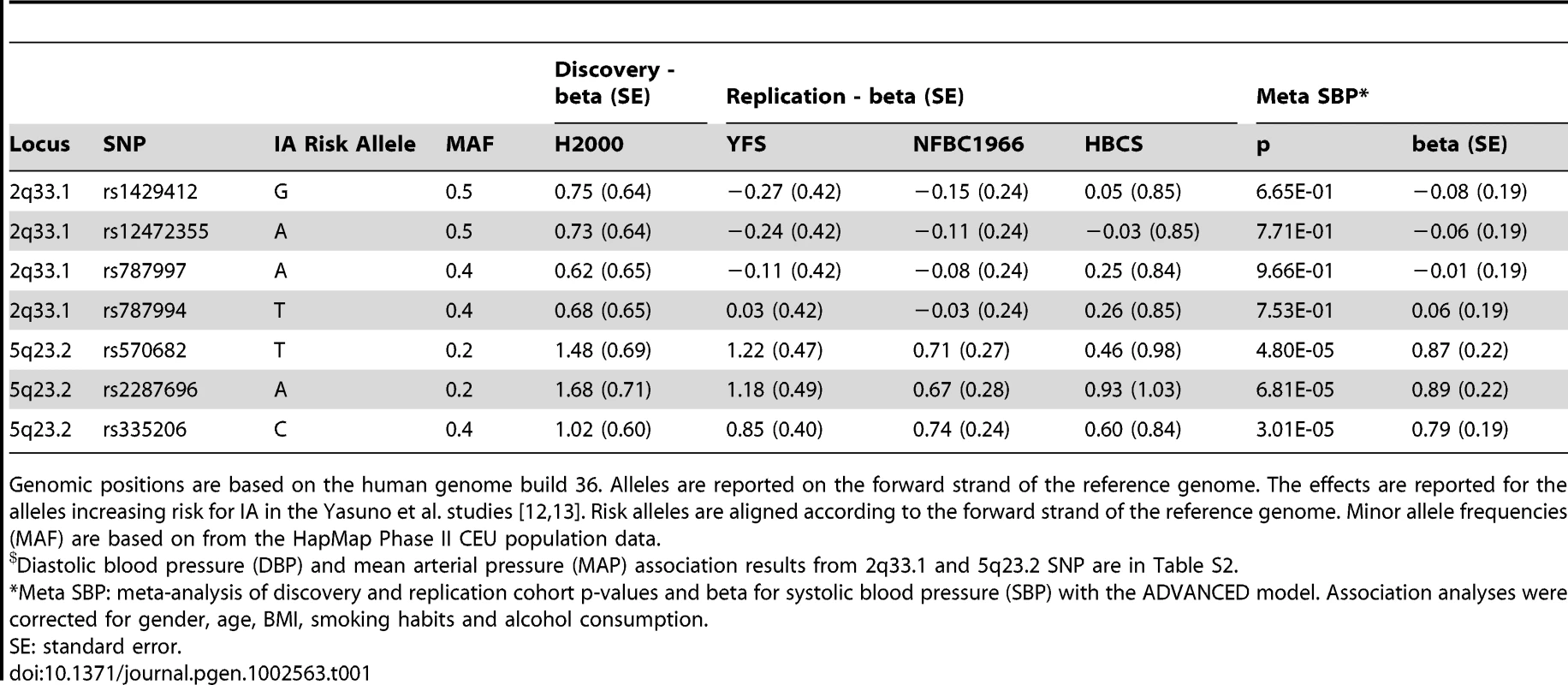
Genomic positions are based on the human genome build 36. Alleles are reported on the forward strand of the reference genome. The effects are reported for the alleles increasing risk for IA in the Yasuno et al. studies [12], [13]. Risk alleles are aligned according to the forward strand of the reference genome. Minor allele frequencies (MAF) are based on from the HapMap Phase II CEU population data. To confirm the initial association signals at the 2q33.1 and 5q23.2 loci observed in the H2000 discovery cohort, we tested them for association with blood pressure in three additional population-based cohorts from Finland. SNPs at 2q33.1 failed to show significant association with DBP and MAP in any of the replication cohorts (the Cardiovascular Risk in Young Finns Study-YFS [15], [16], the Northern Finland Birth Cohort 1966-NFBC1966 [17], and the Helsinki Birth Cohort Study-HBCS [18]). When the results were combined from all cohorts in a fixed effect meta-analysis, they remained non-significant (Table 2). At 5q23.2 SNPs showed significant association with SBP in YFS and NFBC1966 (Table 1). In HBCS, although consistent in the direction of the effect, the association remained suggestive. When the results were combined from all cohorts in a fixed effect meta-analysis, we detected significant association with SBP at 5q23.2 (prs570682 = 4.80E-05, prs2287696 = 6.81E-05, prs335206 = 3.01E-05) (Table 1). Comparing the mean SBP of the study participants stratified for their 5q.23.2 genotypes indicated a positive correlation between the number of risk alleles and higher SBP for all three SNPs tested (Figure 1). Study participants homozygous for the risk allele (C, in the case of rs335206), had on average 1.3 Hgmm higher SBP compared to those who were homozygous for the protective allele, and 0.9 Hgmm higher than those with the heterozygous genotype. This effect size is comparable to those of most blood pressure loci identified by The International Consortium for Blood Pressure Genome-wide Association Studies (ICBP-GWAS) consortium [19]. The observed linear effect of risk allele count is strongly suggestive of a true association. Association at 5q23.2 with DBP (prs570682 = 0.02, prs2287696 = 0.04, prs335206 = 0.03) and MAP (prs570682 = 0.0007, prs2287696 = 0.0010, prs335206 = 0.0004) showed a reduction of significance when results were combined from all cohorts (Table S2).
Fig. 1. Cohort-wise effects of risk allele count on SBP. 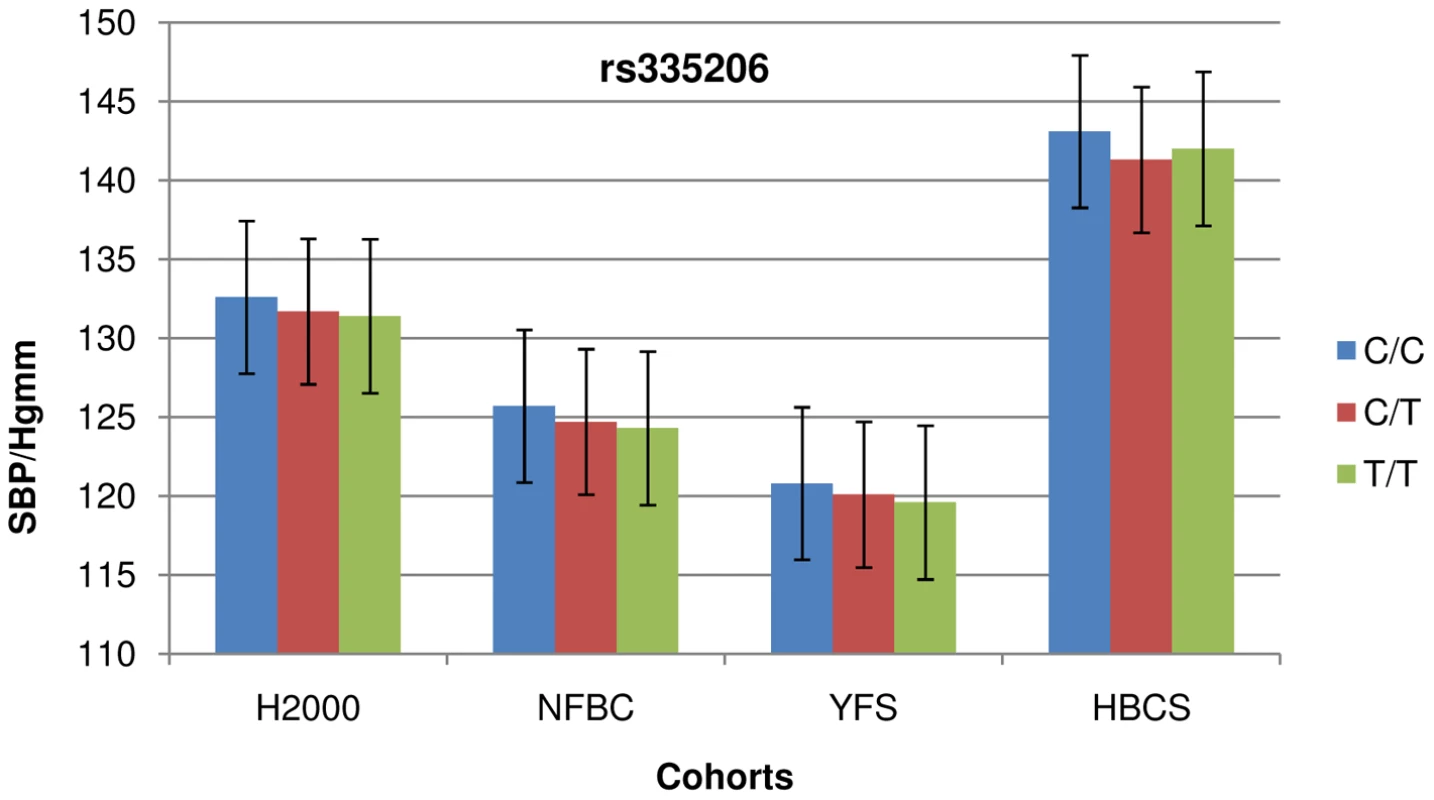
The higher median age in HBCS is reflected as higher systolic blood pressure (SBP) and less consistent association. Error bars show standard error. Tab. 2. Summary of leading SNPs from the 19 loci showing strong or suggestive association with IA in a multinational GWAS containing Finnish patients [12]. ![Summary of leading SNPs from the 19 loci showing strong or suggestive association with IA in a multinational GWAS containing Finnish patients <em class="ref">[12]</em>.](https://www.prolekarniky.cz/media/cache/resolve/media_object_image_small/media/image/ea45034b037574e921b64863038d4ff3.png)
Association results with intracranial aneurysm (IA) by Yasuno and colleagues are followed by our meta-analysis association results with systolic blood pressure (SBP), with the ROBUST and the ADVANCED models, respectively. To test whether the association at the 5q23.2 locus is unique to the Finnish cohorts, we attempted to replicate the association with the three SNPs in the multinational cohort ICBP-GWAS [19]. All three SNPs showed significant association with SBP (prs570682 = 0.0065, prs2287696 = 0.00079, prs335206 = 0.0014) in the ICBP-GWAS cohort of 200 000 individuals of European descent. The risk allele for elevated SBP in the ICBP-GWAS cohort was the same as in our meta-analysis of four Finnish population-based cohorts. When the results from the four Finnish cohorts were combined with the ICBP-GWAS results in a fixed effect meta-analysis, the strength of the association increased with all three SNPs tested (Table 3). The strongest association was observed with rs2287696 (pALL = 8.13E-07). This suggests that the variant at 5q23.2 is a common risk factor present in multiple populations of European descent. Further loci or results for DBP or MAP were not tested for association in ICBP-GWAS, since they failed to show significant association in our replication cohorts.
Tab. 3. Meta-analysis results of 5q23.2 SNPs with systolic blood pressure from all four Finnish cohorts and ICBP-GWAS combined. 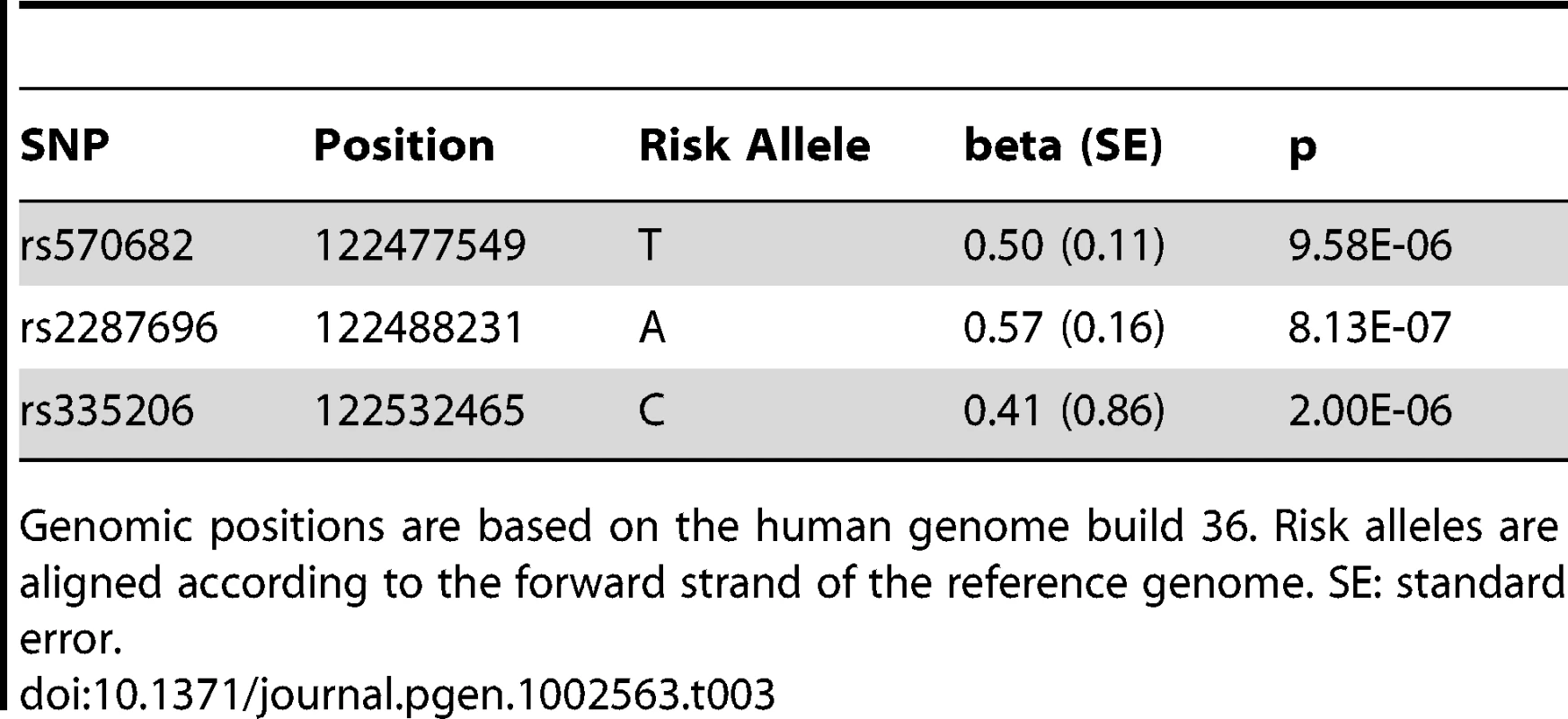
Genomic positions are based on the human genome build 36. Risk alleles are aligned according to the forward strand of the reference genome. SE: standard error. All three tested SNPs at 5q23.2 reside in intronic regions of the gene PR domain containing 6 (short form: PRDM6) and showed comparable p-values. To further explore the associated region in an attempt to pinpoint the causative variant, we examined all 1000 Genomes variants around PRDM6 in the four Finnish cohorts (Figure 2). The strongest association was observed with rs163189 (p = 6.12E-06) near rs570682 and rs2287696, in the second intron, where the most significantly associated SNPs clustered. All five of the strongest associated SNPs are located within a 4.7 kb region at 122.4 MB (Human genome build 36), surrounding a Sterol regulatory element binding transcription factor 1 (SREBP1) binding site (Figure 2) [20].
Fig. 2. Association with SBP around PRDM6: Meta-analysis results from the four Finnish cohorts. 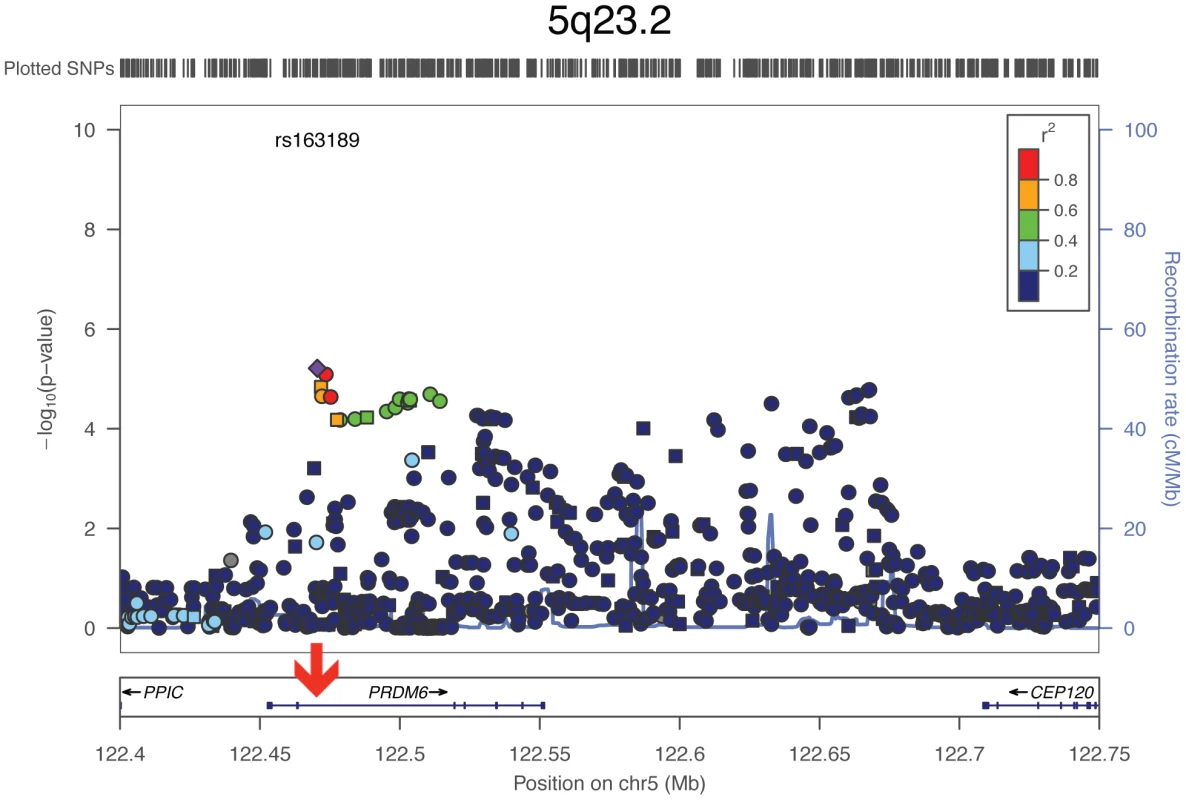
Square shapes represent genotyped SNPs, circles imputed ones. Red arrow depicts the SREBP1 binding site. For each SNP, the symbol color indicates the SNP's pairwise linkage disequilibrium with the most significant SNP, rs163189, measured as r2. SNPs for which no recombination data is available from the 1000 Genomes June 2010 CEU panel are in grey. Discussion
Hypertension, a leading cardiovascular risk factor, is a strong modifiable risk factor for IA and its deadly rupture. Our study establishes a genetic link between elevated SBP and IA formation. Further, we demonstrate the benefits of using population isolates for mapping complex disease loci valid in multiple populations.
5q23.2 was identified as a suggestive IA risk locus by Yasuno and colleagues [13] in a multinational GWAS including Finnish IA patients. The strength of the association at 5q23.2 in their study mainly came from the Finnish cohort (Figure S1). However, albeit weaker, association to IA at 5q23.2 was observable in all cohorts tested by Yasuno and colleagues. In the two tier approach we applied, the suggestive aneurysmal locus at 5q23.2 showed robust association to blood pressure traits in three cohorts (namely the discovery cohort H2000, and the replication cohorts NFBC1966 and YFS). The trend of the effect was the same while the association remained suggestive with blood pressure traits in the HBCS. HBCS participants' average age was higher (61 years) than that of the rest (36 years) (Figure S2). With age, the relative contribution of genetic predisposition and lifestyle may change, potentially accounting for the less significant association in HBCS.
In our meta-analysis of candidate loci the most significant association was observed at 5q23.2 in PRDM6. Although an association can be observed throughout the whole gene, fine-mapping of the region with 1000 Genomes variants revealed the focus of association to be within a 4.7 kb region in the second intron (Figure 2). PRDM6 encodes an epigenetic modulator of transcription with roles in endothelial [21] and vascular smooth muscle cells (SMC) [22]. PRDM6 has a critical role in arterial wall SMC, where it is predominantly expressed. PRDM6 participates in the phenotypic switch between proliferative and differentiating vascular SMC phenotypes [22]; when active, PRDM6 inhibits differentiation and promotes proliferation. Excess vascular SMC proliferation is an important pathomechanism in hypertension, and it exacerbates the vascular wall remodeling often seen in IA [23], [24]. When vascular SMCs re-enter the cell cycle to proliferate, they lose their contractile qualities. Distinct from extracranial arteries, cerebral arteries lack an external elastic lamina and the adventitia is weakly developed, making them inflexible, and less resistant to stress [25]. It is possible that when SMC proliferation further stiffens cerebral arteries, they become incapable of adjusting to shear stress, and give way to IA formation. This is a plausible explanation to why the intracranial manifestation of a supposedly generalized vasculopathy can be so distinct. Intriguingly, excessive vascular SMC proliferation is part of the pathomechanism of the strongest common IA risk locus at 9p21.3 [26]. However, to test possible causality, examination of whether the risk variant at 5q23.2 is associated with higher PRDM6 activity is necessary. Although the causative variant remains elusive, we succeeded in narrowing down the associated region markedly. The 4.7 kb region showing the strongest association harbors a SREBP1 binding site. SREBP1 is a transcription factor governing cellular lipid biosynthesis. Highlighting its biological significance in vascular traits, non-synonymous mutations in SREBP1 cause spontaneous hypertension in rats [27]. It is possible that common variants facilitate SREBP1 binding, and thus, as shown by Zhou and colleagues [28], cause vascular SMC proliferation. We propose that this effect is conveyed by PRDM6 activation.
Although both the location and the function of the gene highlight PRDM6 as a likely candidate, it is not the only plausible gene near the association signal. Centrosomal protein of 120 KD (short form: Cep120) is just downstream from the region of association (Figure 2). Cep120 is a centrosomal protein with preferentially high expression in neuronal progenitors during development [29]. Cep120 could contribute to IA risk by causing perturbation in the neurovascular niche.
This is the first study establishing a shared genetic background at 5q23.2 for IA and its important risk factor, high blood pressure. However, both IA [30], [31] and hypertension [32] have shown linkage to 5q23.2 in previous studies. Resequencing the genomic region in families that previously showed linkage to 5q23.2 might reveal penetrant variants causing familial IA or severe high blood pressure, or possibly both. Notably, Vasan and colleagues [33] found that rs17470137, less than 8 kb downstream from PRDM6, is associated with aortic root size, a feasible proxy of blood pressure [34].
GWAS are designed to identify associations, they do not prove causality. Deep resequencing of the associated region may improve the fine mapping and guide closer to the causative variant, or even uncover it, although resequencing efforts of GWAS regions have had limited success [35]. A further limitation of our study is that we were unable to address whether the identified risk variant at 5q23.2 increases the risk of developing IA as a consequence of elevated SBP (causality between high SBP and IA) or whether the variant modifies vessel wall structure in a way that elevates SBP and increases IA risk as a pleiotropic effect (Figure S3). A study conducted in a cohort characterized both for IA and blood pressure would likely be a more suitable way of addressing this question. Unfortunately, to the best of our knowledge, such a large-scale cohort does not currently exist. The identified risk variant, however, is unlikely to confer its effect solely by increasing blood pressure, as leading hypertension risk loci fail to show association with IA (data not shown). Yet, the mechanical effect of elevated BP on the vessel wall, likely exacerbates IA formation. The significance of the association identified in our study awaits confirmation in other ethnicities.
To further decipher the genetics of IA, it is important to test if genetic links can be established between IA and other strong risk factors, such as smoking and alcohol consumption. In conclusion, our results highlight the link between IA and blood pressure.
Materials and Methods
Study Subjects
Four Finnish population-based cohorts were included in our study (Table 4). These cohorts were not characterized for IA. We utilized genome-wide genotyped participants with available blood pressure data, excluding those on blood pressure medication and those for whom blood pressure medication data was not available (nexcluded = 1373). In our two tier approach, the discovery cohort (ndiscovery = 1581) was a subsample of the H2000 [14]. The H2000 study was carried out in several regions of Finland from fall 2000 to spring 2001, and was designed to provide information on the health of the Finnish population. A subset of this cohort, consisting of metabolic syndrome cases and matched controls, was genotyped and utilized in this analysis. The replication cohort (nreplication = 8312) consisted of the YFS (n = 1874) [15], [16], the NFBC1966 (n = 5361) [17], and the HBCS (n = 1077) [18]. YFS participants were recruited from all around Finland for a large follow-up study on cardiovascular risk factors in young individuals in 1980. Clinical data are from the follow-up at age 27 performed in 2007. NFBC1966 comprises individuals born in 1966 in the two northernmost provinces of Finland (Oulu and Lapland). Clinical examinations took place at the follow-up at age 31 in 1997. The HBCS participants were recruited from the Helsinki region. The study examines the impact of fetal environmental factors on childhood and adult life. Clinical examinations took place during 2001–2004. HBCS participants had the highest average age (Figure S2).
Tab. 4. Summary of cohort characteristics. 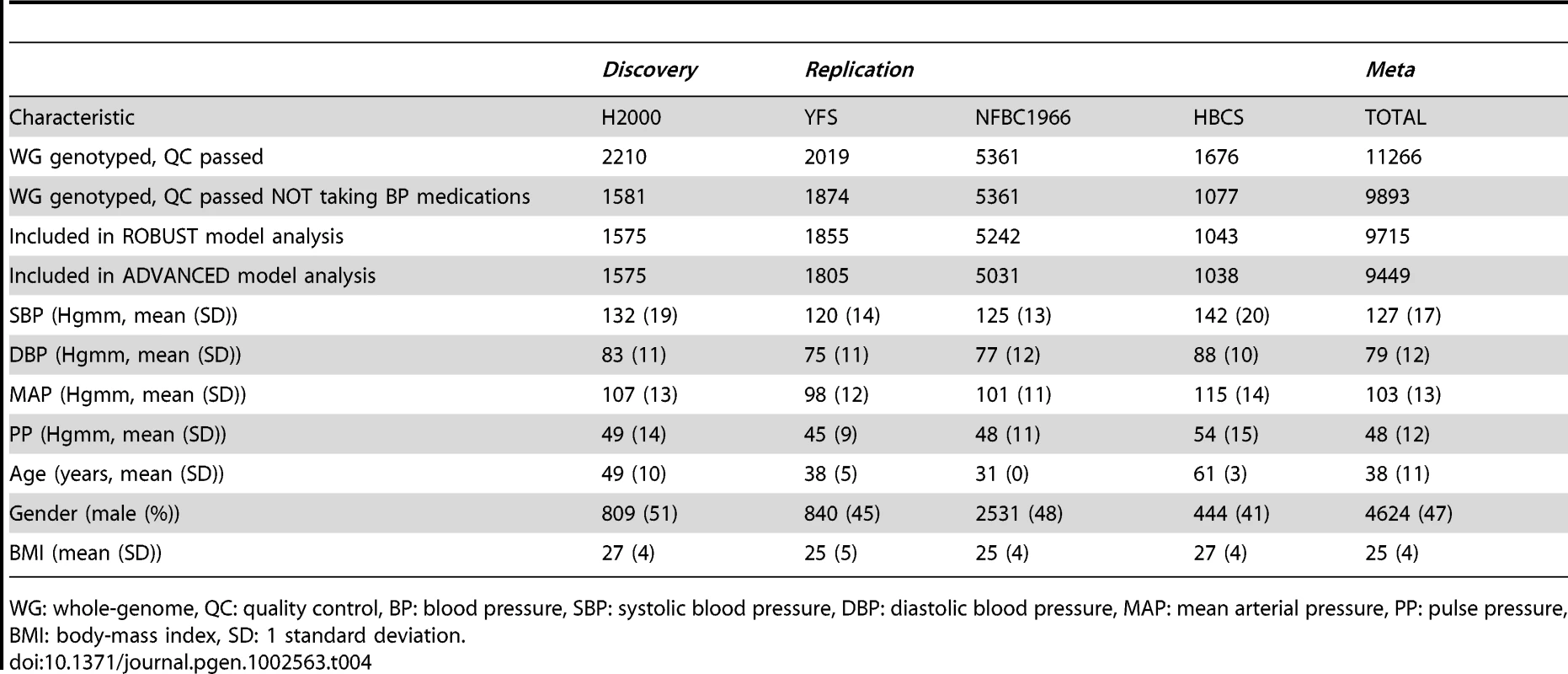
WG: whole-genome, QC: quality control, BP: blood pressure, SBP: systolic blood pressure, DBP: diastolic blood pressure, MAP: mean arterial pressure, PP: pulse pressure, BMI: body-mass index, SD: 1 standard deviation. The ICBP-GWAS represents a union of numerous prior blood pressure GWAS consortia to create a discovery meta-analysis of over 200 000 individuals of European ancestry. NFBC1966 is part of the ICBP-GWAS; however, this overlap does not represent a significant risk for bias, due to the small relative contribution of NFBC1966 to the ICBP-GWAS results.
Genotyping and Imputation
All Finnish cohorts were genotyped using Illumina arrays (Illumina Inc. San Diego, CA, USA): Illumina Infinium HD Human610-Quad BeadChip for H2000, Illumina HumanCNV370-Duo BeadChip for NFBC1966, and Illumina Human670K custom BeadChip for YFS and HBCS. For SNPs to be successfully genotyped, a per individual and per marker success rate minimum of 95% was defined as default. 36 out of 41 candidate SNPs were successfully genotyped in all cohorts. For SNPs with no directly genotyped data available, we imputed genotypes with MACH [36] using HapMap CEU from Phase II as the reference panel (further referred to as HM2 imputed data). If a SNP was not present in HM2 imputed data, we used genotypes imputed with IMPUTEv2 using the 1000 Genomes pilot data CEU panel (August 2009 haplotypes) combined with HapMap Phase 3 (Public Release #2) haplotypes as the reference panel [37], extended with Finnish specific HapMap Phase 3 haplotypes [38] (further referred to as 1000G+HM3 imputed data). All missing genotypes were imputed, so the number of individuals included in the analyses for each SNP is the same and equals the final number (Table 4).
IA Loci Association Analysis with Blood Pressure and Meta-Analysis of Results
Candidate loci were selected based on IA GWAS results [13]. Loci associated with IA with PPA≥0.1 were included (Table 2). PPA was calculated as described by Yasuno et al [12]. Briefly, a uniform prior probability of association of 1/10 000 was assumed for all SNPs and used to provide a probabilistic measure of evidence. We tested 41 SNPs from 19 independent loci. We defined SBP, DBP, MAP, and PP as quantitative outcome variables. MAP was counted as the average of SBP and DBP ((SBP+DBP)/2) and PP as the difference of the two (SBP-DBP). We tested all 19 loci in the discovery cohort (H2000), and those showing suggestive association (uncorrected p<0.1) with any outcome variable were tested in the replication cohorts (YFS, NFBC1966, and HBCS). Association analyses with an additive genetic model were performed with ProbABEL [39] for HM2 imputed data, and with SNPTESTv2 [40], [41] for 1000G+HM3 imputed data. The analyses were adjusted for age and gender (ROBUST model), or for age, gender, smoking habits, alcohol consumption, and BMI (ADVANCED model). Additionally, in the metabolic syndrome case-control subset of the H2000 cohort we corrected for case-control status in both models. Population stratification was corrected for by calculating principal components from genome-wide SNP data and including significant principal components in the association models as covariates. Association results were combined in a fixed effect meta-analysis with MetABEL [39] for HM2 imputed data, and with METAv1.2 [42] for 1000G+HM3 imputed data. The best result at 5q23.2 in PRDM6 was tested for association in the ICBP-GWAS [19] cohort of 200 000 individuals of European descent. In the ICBP-GWAS association with SBP was tested by linear regression assuming an additive model and correcting for age, age-squared and BMI. To test the per-allele effect size of risk alleles on blood pressure, we calculated the mean blood pressure for the three genotypic states for the three 5q23.2 SNPs using Plink v1.07 [43]. Results were plotted using the Microsoft Excel charts function.
To further investigate the strongest associated locus, we analyzed all 1000 Genomes variants, with minor allele frequency greater than 1%, in and around PRDM6. We took uncertainty of imputation into account by using the maximum likelihood estimates of the reference allele counts as genotypes (these estimates may be fractional and range from 0 to 2). Fine mapping of the 5q23.2 region was performed with 1000G+HM3 imputed data. Results were plotted with LocusZoom [44].
Supporting Information
Zdroje
1. VlakMHAlgraABrandenburgRRinkelGJ 2011 Prevalence of unruptured intracranial aneurysms, with emphasis on sex, age, comorbidity, country, and time period: a systematic review and meta-analysis. Lancet Neurol 10 626 636
2. NieuwkampDJSetzLEAlgraALinnFHde RooijNK 2009 Changes in case fatality of aneurysmal subarachnoid haemorrhage over time, according to age, sex, and region: a meta-analysis. Lancet Neurol 8 635 642
3. van GijnJKerrRSRinkelGJ 2007 Subarachnoid haemorrhage. Lancet 369 306 318
4. HopJWRinkelGJAlgraAvan GijnJ 1997 Case-fatality rates and functional outcome after subarachnoid hemorrhage: a systematic review. Stroke 28 660 664
5. SartiCTuomilehtoJSalomaaVSiveniusJKaarsaloE 1991 Epidemiology of subarachnoid hemorrhage in Finland from 1983 to 1985. Stroke 22 848 853
6. de RooijNKLinnFHvan der PlasJAAlgraARinkelGJ 2007 Incidence of subarachnoid haemorrhage: a systematic review with emphasis on region, age, gender and time trends. J Neurol Neurosurg Psychiatry 78 1365 1372
7. RuigrokYMRinkelGJWijmengaC 2005 Genetics of intracranial aneurysms. Lancet Neurol 4 179 189
8. JuvelaS 2002 Natural history of unruptured intracranial aneurysms: risks for aneurysm formation, growth, and rupture. Acta Neurochir Suppl 82 27 30
9. InciSSpetzlerRF 2000 Intracranial aneurysms and arterial hypertension: a review and hypothesis. Surg Neurol 53 530 540; discussion 540–532
10. RegaladoESGuoDCVillamizarCAvidanNGilchristD 2011 Exome Sequencing Identifies SMAD3 Mutations as a Cause of Familial Thoracic Aortic Aneurysm and Dissection With Intracranial and Other Arterial Aneurysms. Circ Res
11. BilguvarKYasunoKNiemelaMRuigrokYMvon Und Zu FraunbergM 2008 Susceptibility loci for intracranial aneurysm in European and Japanese populations. Nat Genet 40 1472 1477
12. YasunoKBilguvarKBijlengaPLowSKKrischekB 2010 Genome-wide association study of intracranial aneurysm identifies three new risk loci. Nat Genet 42 420 425
13. YasunoKBakirciogluMLowSKBilguvarKGaalE 2011 Common variant near the endothelin receptor type A (EDNRA) gene is associated with intracranial aneurysm risk. Proc Natl Acad Sci U S A 108 49 19707 12
14. Health 2000 THL - National Institute for Health and Welfare. (Accessed 30.8.2010, at http://www.terveys2000.fi/indexe.html)
15. The Cardiovascular Risk in Young Finns Study. 2008. (Accessed 30.8.2010, at http://vanha.med.utu.fi/cardio/youngfinnsstudy/index.html)
16. RaitakariOTJuonalaMRonnemaaTKeltikangas-JarvinenLRasanenL 2008 Cohort profile: the cardiovascular risk in Young Finns Study. Int J Epidemiol 37 1220 1226
17. Northern Finland Birth Cohort Studies, NFBC. FACULTY OF MEDICINE, Institute of Health Sciences, University of Oulu. (Accessed 30.8.2010, at http://kelo.oulu.fi/NFBC/content.htm)
18. IDEFIX Study. National Institute for Health and Welfare, 2009. (Accessed 30.8.2010, at http://www.ktl.fi/portal/5481)
19. EhretGBMunroePBRiceKMBochudMJohnsonAD 2011 Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature 478 103 109
20. BirneyEStamatoyannopoulosJADuttaAGuigoRGingerasTR 2007 Identification and analysis of functional elements in 1% of the human genome by the ENCODE pilot project. Nature 447 799 816
21. WuYFergusonJE3rdWangHKelleyRRenR 2008 PRDM6 is enriched in vascular precursors during development and inhibits endothelial cell proliferation, survival, and differentiation. J Mol Cell Cardiol 44 47 58
22. DavisCAHaberlandMArnoldMASutherlandLBMcDonaldOG 2006 PRISM/PRDM6, a transcriptional repressor that promotes the proliferative gene program in smooth muscle cells. Mol Cell Biol 26 2626 2636
23. HasslerO 1961 Morphological studies on the large cerebral arteries, with reference to the aetiology of subarachnoid haemorrhage. Acta Psychiatr Scand Suppl 154 1 145
24. StehbensWE 1960 Focal intimal proliferation in the cerebral arteries. Am J Pathol 36 289 301
25. NystroemSH 1963 Development of Intracranial Aneurysms as Revealed by Electron Microscopy. J Neurosurg 20 329 337
26. ViselAZhuYMayDAfzalVGongE 2010 Targeted deletion of the 9p21 non-coding coronary artery disease risk interval in mice. Nature 464 409 412
27. PravenecMJansaPKostkaVZidekVKrenV 2001 Identification of a mutation in ADD1/SREBP-1 in the spontaneously hypertensive rat. Mamm Genome 12 295 298
28. ZhouRHPesantSCohnHIEckhartAD 2008 Enhanced sterol response element-binding protein in postintervention restenotic blood vessels plays an important role in vascular smooth muscle proliferation. Life Sci 82 174 181
29. XieZMoyLYSanadaKZhouYBuchmanJJ 2007 Cep120 and TACCs control interkinetic nuclear migration and the neural progenitor pool. Neuron 56 79 93
30. Santiago-SimTDepalmaSRJuKLMcDonoughBSeidmanCE 2009 Genomewide linkage in a large Caucasian family maps a new locus for intracranial aneurysms to chromosome 13q. Stroke 40 S57 60
31. OndaHKasuyaHYoneyamaTTakakuraKHoriT 2001 Genomewide-linkage and haplotype-association studies map intracranial aneurysm to chromosome 7q11. Am J Hum Genet 69 804 819
32. LiuWZhaoWChaseGA 2004 Genome scan meta-analysis for hypertension. Am J Hypertens 17 1100 1106
33. VasanRSGlazerNLFelixJFLiebWWildPS 2009 Genetic variants associated with cardiac structure and function: a meta-analysis and replication of genome-wide association data. JAMA 302 168 178
34. KimMRomanMJCavalliniMCSchwartzJEPickeringTG 1996 Effect of hypertension on aortic root size and prevalence of aortic regurgitation. Hypertension 28 47 52
35. RivasMABeaudoinMGardetAStevensCSharmaY 2011 Deep resequencing of GWAS loci identifies independent rare variants associated with inflammatory bowel disease. Nat Genet 43 1066 1073
36. ScottLJMohlkeKLBonnycastleLLWillerCJLiY 2007 A genome-wide association study of type 2 diabetes in Finns detects multiple susceptibility variants. Science 316 1341 1345
37. HowieBNDonnellyPMarchiniJ 2009 A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLoS Genet 5 e1000529 doi:10.1371/journal.pgen.1000529
38. SurakkaIKristianssonKAnttilaVInouyeMBarnesC 2010 Founder population-specific HapMap panel increases power in GWA studies through improved imputation accuracy and CNV tagging. Genome Res 20 1344 1351
39. AulchenkoYSStruchalinMVvan DuijnCM 2010 ProbABEL package for genome-wide association analysis of imputed data. BMC Bioinformatics 11 134
40. MarchiniJHowieB 2010 Genotype imputation for genome-wide association studies. Nat Rev Genet 11 499 511
41. MarchiniJHowieBMyersSMcVeanGDonnellyP 2007 A new multipoint method for genome-wide association studies by imputation of genotypes. Nat Genet 39 906 913
42. LiuJZTozziFWaterworthDMPillaiSGMugliaP 2010 Meta-analysis and imputation refines the association of 15q25 with smoking quantity. Nat Genet 42 436 440
43. PurcellSNealeBTodd-BrownKThomasLFerreiraMA 2007 PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 81 559 575
44. PruimRJWelchRPSannaSTeslovichTMChinesPS 2010 LocusZoom: regional visualization of genome-wide association scan results. Bioinformatics 26 2336 2337
Štítky
Genetika Reprodukční medicína
Článek Physiological Notch Signaling Maintains Bone Homeostasis via RBPjk and Hey Upstream of NFATc1Článek Intronic -Regulatory Modules Mediate Tissue-Specific and Microbial Control of / TranscriptionČlánek Probing the Informational and Regulatory Plasticity of a Transcription Factor DNA–Binding DomainČlánek Repression of Germline RNAi Pathways in Somatic Cells by Retinoblastoma Pathway Chromatin ComplexesČlánek An Alu Element–Associated Hypermethylation Variant of the Gene Is Associated with Childhood ObesityČlánek Three Essential Ribonucleases—RNase Y, J1, and III—Control the Abundance of a Majority of mRNAsČlánek Genomic Tools for Evolution and Conservation in the Chimpanzee: Is a Genetically Distinct Population
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 3
-
Všechny články tohoto čísla
- Comprehensive Research Synopsis and Systematic Meta-Analyses in Parkinson's Disease Genetics: The PDGene Database
- Genomic Analysis of the Hydrocarbon-Producing, Cellulolytic, Endophytic Fungus
- Networks of Neuronal Genes Affected by Common and Rare Variants in Autism Spectrum Disorders
- Akirin Links Twist-Regulated Transcription with the Brahma Chromatin Remodeling Complex during Embryogenesis
- Too Much Cleavage of Cyclin E Promotes Breast Tumorigenesis
- Imprinted Genes … and the Number Is?
- Genetic Architecture of Highly Complex Chemical Resistance Traits across Four Yeast Strains
- Exploring the Complexity of the HIV-1 Fitness Landscape
- MNS1 Is Essential for Spermiogenesis and Motile Ciliary Functions in Mice
- A Fundamental Regulatory Mechanism Operating through OmpR and DNA Topology Controls Expression of Pathogenicity Islands SPI-1 and SPI-2
- Evidence for Positive Selection on a Number of MicroRNA Regulatory Interactions during Recent Human Evolution
- Variation in Modifies Risk of Neonatal Intestinal Obstruction in Cystic Fibrosis
- PIF4–Mediated Activation of Expression Integrates Temperature into the Auxin Pathway in Regulating Hypocotyl Growth
- Critical Evaluation of Imprinted Gene Expression by RNA–Seq: A New Perspective
- A Meta-Analysis and Genome-Wide Association Study of Platelet Count and Mean Platelet Volume in African Americans
- Mouse Genetics Suggests Cell-Context Dependency for Myc-Regulated Metabolic Enzymes during Tumorigenesis
- Transcriptional Control in Cardiac Progenitors: Tbx1 Interacts with the BAF Chromatin Remodeling Complex and Regulates
- Synthetic Lethality of Cohesins with PARPs and Replication Fork Mediators
- APOBEC3G-Induced Hypermutation of Human Immunodeficiency Virus Type-1 Is Typically a Discrete “All or Nothing” Phenomenon
- Interpreting Meta-Analyses of Genome-Wide Association Studies
- Error-Prone ZW Pairing and No Evidence for Meiotic Sex Chromosome Inactivation in the Chicken Germ Line
- -Dependent Chemosensory Functions Contribute to Courtship Behavior in
- Diverse Forms of Splicing Are Part of an Evolving Autoregulatory Circuit
- Phenotypic Plasticity of the Drosophila Transcriptome
- Physiological Notch Signaling Maintains Bone Homeostasis via RBPjk and Hey Upstream of NFATc1
- Precocious Metamorphosis in the Juvenile Hormone–Deficient Mutant of the Silkworm,
- Igf1r Signaling Is Indispensable for Preimplantation Development and Is Activated via a Novel Function of E-cadherin
- Accurate Prediction of Inducible Transcription Factor Binding Intensities In Vivo
- Mitochondrial Oxidative Stress Alters a Pathway in Strongly Resembling That of Bile Acid Biosynthesis and Secretion in Vertebrates
- Mammalian Neurogenesis Requires Treacle-Plk1 for Precise Control of Spindle Orientation, Mitotic Progression, and Maintenance of Neural Progenitor Cells
- Tcf7 Is an Important Regulator of the Switch of Self-Renewal and Differentiation in a Multipotential Hematopoietic Cell Line
- REST–Mediated Recruitment of Polycomb Repressor Complexes in Mammalian Cells
- Intronic -Regulatory Modules Mediate Tissue-Specific and Microbial Control of / Transcription
- Age-Dependent Brain Gene Expression and Copy Number Anomalies in Autism Suggest Distinct Pathological Processes at Young Versus Mature Ages
- A Genome-Wide Association Study Identifies Variants Underlying the Shade Avoidance Response
- -by- Regulatory Divergence Causes the Asymmetric Lethal Effects of an Ancestral Hybrid Incompatibility Gene
- Genome-Wide Association and Functional Follow-Up Reveals New Loci for Kidney Function
- A Natural System of Chromosome Transfer in
- Cell Size and the Initiation of DNA Replication in Bacteria
- Probing the Informational and Regulatory Plasticity of a Transcription Factor DNA–Binding Domain
- Repression of Germline RNAi Pathways in Somatic Cells by Retinoblastoma Pathway Chromatin Complexes
- Temporal Transcriptional Profiling of Somatic and Germ Cells Reveals Biased Lineage Priming of Sexual Fate in the Fetal Mouse Gonad
- Rapid Analysis of Genome Rearrangements by Multiplex Ligation–Dependent Probe Amplification
- Metabolic Profiling of a Mapping Population Exposes New Insights in the Regulation of Seed Metabolism and Seed, Fruit, and Plant Relations
- The Atypical Calpains: Evolutionary Analyses and Roles in Cellular Degeneration
- The Silkworm Coming of Age—Early
- Development of a Panel of Genome-Wide Ancestry Informative Markers to Study Admixture Throughout the Americas
- Balanced Codon Usage Optimizes Eukaryotic Translational Efficiency
- The Min System and Nucleoid Occlusion Are Not Required for Identifying the Division Site in but Ensure Its Efficient Utilization
- Neurobeachin, a Regulator of Synaptic Protein Targeting, Is Associated with Body Fat Mass and Feeding Behavior in Mice and Body-Mass Index in Humans
- Statistical Analysis of Readthrough Levels for Nonsense Mutations in Mammalian Cells Reveals a Major Determinant of Response to Gentamicin
- Gene Reactivation by 5-Aza-2′-Deoxycytidine–Induced Demethylation Requires SRCAP–Mediated H2A.Z Insertion to Establish Nucleosome Depleted Regions
- The miR-35-41 Family of MicroRNAs Regulates RNAi Sensitivity in
- Genetic Basis of Hidden Phenotypic Variation Revealed by Increased Translational Readthrough in Yeast
- An Alu Element–Associated Hypermethylation Variant of the Gene Is Associated with Childhood Obesity
- Modelling Human Regulatory Variation in Mouse: Finding the Function in Genome-Wide Association Studies and Whole-Genome Sequencing
- Novel Loci for Adiponectin Levels and Their Influence on Type 2 Diabetes and Metabolic Traits: A Multi-Ethnic Meta-Analysis of 45,891 Individuals
- Polycomb-Like 3 Promotes Polycomb Repressive Complex 2 Binding to CpG Islands and Embryonic Stem Cell Self-Renewal
- Insulin/IGF-1 and Hypoxia Signaling Act in Concert to Regulate Iron Homeostasis in
- EMF1 and PRC2 Cooperate to Repress Key Regulators of Arabidopsis Development
- Three Essential Ribonucleases—RNase Y, J1, and III—Control the Abundance of a Majority of mRNAs
- Contrasted Patterns of Molecular Evolution in Dominant and Recessive Self-Incompatibility Haplotypes in
- A Machine Learning Approach for Identifying Novel Cell Type–Specific Transcriptional Regulators of Myogenesis
- Genomic Tools for Evolution and Conservation in the Chimpanzee: Is a Genetically Distinct Population
- Nos2 Inactivation Promotes the Development of Medulloblastoma in Mice by Deregulation of Gap43–Dependent Granule Cell Precursor Migration
- Intracranial Aneurysm Risk Locus 5q23.2 Is Associated with Elevated Systolic Blood Pressure
- Heritability and Genetic Correlations Explained by Common SNPs for Metabolic Syndrome Traits
- A Genome-Wide Association Study of Nephrolithiasis in the Japanese Population Identifies Novel Susceptible Loci at 5q35.3, 7p14.3, and 13q14.1
- DNA Damage in Nijmegen Breakage Syndrome Cells Leads to PARP Hyperactivation and Increased Oxidative Stress
- DNA Resection at Chromosome Breaks Promotes Genome Stability by Constraining Non-Allelic Homologous Recombination
- Genetic Analysis of Floral Symmetry in Van Gogh's Sunflowers Reveals Independent Recruitment of Genes in the Asteraceae
- A Splice Site Variant in the Bovine Gene Compromises Growth and Regulation of the Inflammatory Response
- Promoter Nucleosome Organization Shapes the Evolution of Gene Expression
- The Nucleoside Diphosphate Kinase Gene Acts as Quantitative Trait Locus Promoting Non-Mendelian Inheritance
- The Ciliogenic Transcription Factor RFX3 Regulates Early Midline Distribution of Guidepost Neurons Required for Corpus Callosum Development
- Phosphorylation of the RNA–Binding Protein HOW by MAPK/ERK Enhances Its Dimerization and Activity
- A Genome-Wide Scan of Ashkenazi Jewish Crohn's Disease Suggests Novel Susceptibility Loci
- Parkinson's Disease–Associated Kinase PINK1 Regulates Miro Protein Level and Axonal Transport of Mitochondria
- LMW-E/CDK2 Deregulates Acinar Morphogenesis, Induces Tumorigenesis, and Associates with the Activated b-Raf-ERK1/2-mTOR Pathway in Breast Cancer Patients
- Mapping the Hsp90 Genetic Interaction Network in Reveals Environmental Contingency and Rewired Circuitry
- Autoregulation of the Noncoding RNA Gene
- The Human Pancreatic Islet Transcriptome: Expression of Candidate Genes for Type 1 Diabetes and the Impact of Pro-Inflammatory Cytokines
- Spo0A∼P Imposes a Temporal Gate for the Bimodal Expression of Competence in
- Antagonistic Regulation of Apoptosis and Differentiation by the Cut Transcription Factor Represents a Tumor-Suppressing Mechanism in
- A Downstream CpG Island Controls Transcript Initiation and Elongation and the Methylation State of the Imprinted Macro ncRNA Promoter
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- PIF4–Mediated Activation of Expression Integrates Temperature into the Auxin Pathway in Regulating Hypocotyl Growth
- Metabolic Profiling of a Mapping Population Exposes New Insights in the Regulation of Seed Metabolism and Seed, Fruit, and Plant Relations
- A Splice Site Variant in the Bovine Gene Compromises Growth and Regulation of the Inflammatory Response
- Comprehensive Research Synopsis and Systematic Meta-Analyses in Parkinson's Disease Genetics: The PDGene Database
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



