-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaA Genome-Wide Association Study Identifies Variants Underlying the Shade Avoidance Response
Shade avoidance is an ecologically and molecularly well-understood set of plant developmental responses that occur when the ratio of red to far-red light (R∶FR) is reduced as a result of foliar shade. Here, a genome-wide association study (GWAS) in Arabidopsis thaliana was used to identify variants underlying one of these responses: increased hypocotyl elongation. Four hypocotyl phenotypes were included in the study, including height in high R∶FR conditions (simulated sun), height in low R∶FR conditions (simulated shade), and two different indices of the response of height to low R∶FR. GWAS results showed that variation in these traits is controlled by many loci of small to moderate effect. A known PHYC variant contributing to hypocotyl height variation was identified and lists of significantly associated genes were enriched in a priori candidates, suggesting that this GWAS was capable of generating meaningful results. Using metadata such as expression data, GO terms, and other annotation, we were also able to identify variants in candidate de novo genes. Patterns of significance among our four phenotypes allowed us to categorize associations into three groups: those that affected hypocotyl height without influencing shade avoidance, those that affected shade avoidance in a height-dependent fashion, and those that exerted specific control over shade avoidance. This grouping allowed for the development of explicit hypotheses about the genetics underlying shade avoidance variation. Additionally, the response to shade did not exhibit any marked geographic distribution, suggesting that variation in low R∶FR–induced hypocotyl elongation may represent a response to local conditions.
Published in the journal: . PLoS Genet 8(3): e32767. doi:10.1371/journal.pgen.1002589
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002589Summary
Shade avoidance is an ecologically and molecularly well-understood set of plant developmental responses that occur when the ratio of red to far-red light (R∶FR) is reduced as a result of foliar shade. Here, a genome-wide association study (GWAS) in Arabidopsis thaliana was used to identify variants underlying one of these responses: increased hypocotyl elongation. Four hypocotyl phenotypes were included in the study, including height in high R∶FR conditions (simulated sun), height in low R∶FR conditions (simulated shade), and two different indices of the response of height to low R∶FR. GWAS results showed that variation in these traits is controlled by many loci of small to moderate effect. A known PHYC variant contributing to hypocotyl height variation was identified and lists of significantly associated genes were enriched in a priori candidates, suggesting that this GWAS was capable of generating meaningful results. Using metadata such as expression data, GO terms, and other annotation, we were also able to identify variants in candidate de novo genes. Patterns of significance among our four phenotypes allowed us to categorize associations into three groups: those that affected hypocotyl height without influencing shade avoidance, those that affected shade avoidance in a height-dependent fashion, and those that exerted specific control over shade avoidance. This grouping allowed for the development of explicit hypotheses about the genetics underlying shade avoidance variation. Additionally, the response to shade did not exhibit any marked geographic distribution, suggesting that variation in low R∶FR–induced hypocotyl elongation may represent a response to local conditions.
Introduction
Since plants are sessile organisms that rely on the harvest of light to fulfill their energy requirements, the ability to monitor the ambient light environment has been key to their evolutionary success. Faced with the challenge of modulating their development to best suit changing light environments, plants have evolved a sophisticated array of photoreceptors to comprehensively survey both light quality and quantity [1]. These photoreceptors are integrated into key developmental pathways, allowing for efficient optimization of development. One well-studied case in which changes in light quality elicit specific developmental responses is shade avoidance [2].
When sunlight is intercepted by a plant canopy, plant pigments absorb light in the red and blue portions of the spectrum to use as energy for photosynthesis, while far-red light passes through the canopy relatively unimpeded. As a result, the ratio of R∶FR light is reduced in canopy shade or when neighboring plants are present. Plants sense this change in light quality primarily through type II (light stable) phytochromes and initiate a suite of plastic developmental responses known as shade avoidance [2]. These responses include elongation of plant organs, including hypocotyls, internodes, and petioles, increased leaf angle, and acceleration of flowering. In natural plant communities, the shade avoidance response has long been the subject of ecological genetic studies. Initially, researchers observed that the phytochrome-mediated response to low R∶FR in species originating from shaded environments was lower than that of species from open environments [3]. Dudley and Schmitt [4] observed a similar pattern between populations of a single species, Impatiens capensis. A subsequent physiological manipulation study of this species confirmed that in natural populations, shade avoidance elongation responses are indeed an example of adaptive plasticity [4], while a genetic manipulation study of transgenic tobacco and Brassica demonstrated that this adaptive plasticity could be phytochrome-mediated [5]. Interestingly, R∶FR-mediated shade avoidance elongation has also been shown to be adaptive in the model plant Arabidopsis thaliana [6], and in a survey of 105 Arabidopsis accessions, Botto and Smith observed considerable natural variation in hypocotyl elongation in response to low R∶FR [7]. Therefore, evolutionary and ecological genetics studies of shade avoidance present an opportunity to use the extensive genetic resources of Arabidopsis to investigate an adaptive trait.
Shade avoidance is also relevant to agricultural settings, since high planting densities can create low R∶FR conditions, triggering shade avoidance and thereby decreasing yield [8]. As a result, extensive studies of the molecular nature of shade avoidance have been undertaken, particularly in Arabidopsis. In this species, light stable phytochromes, especially phytochrome B, initiate shade avoidance [2]. In response to a reduction in R∶FR, these proteins undergo a conformational change to the inactive (Pr) state. Through mechanisms that are as yet unclear, but that most likely involve interactions with the transcription factors PIF4 and PIF5 [9], this conformational change triggers the upregulation of a suite of transcription factors, including HFR1, ATHB-2, ATHB-4, PIL1, PAR1, and PAR2 [10]–[12]. This upregulation ultimately leads to hypocotyl elongation through increased synthesis and modulated signaling of plant hormones including auxin, gibberellic acid, brassinosteroids, cytokinins, and ethylene [13]. Of these hormones, the involvement of auxin and gibberellic acid (GA) is best supported. Genes controlling both auxin synthesis (TAA1 and YUCCAs) [14], [15] as well as auxin transport (BIG, PIN3) [16], [17] have been shown to play roles in low R∶FR-mediated elongation. Additionally, genes encoding two auxin-responsive proteins, IAA19 and IAA29, are upregulated in response to shade [10], [18]. The importance of GA signaling in shade avoidance is evidenced not only by the low R∶FR-induced upregulation of two gibberellic acid (GA) synthesis genes, GA20ox1 and GA20ox2 [19], but also by the role of the DELLA proteins. These negative regulators of PIF activity are degraded as a result of increased GA synthesis under low R∶FR conditions and therefore serve as integrators of light and hormone signaling [20]. Although a completely unified understanding of the shade avoidance pathway remains elusive, the reasonably well-understood molecular nature of the shade avoidance response is another reason why this phenotype is well-suited for studies of natural variation that seek to uncover the genetic control of adaptive traits.
In fact, quantitative genetics studies of Arabidopsis natural variation have been successful in identifying genetic variation in the phytochrome B-mediated signaling pathway. QTL studies have identified natural variants of PHYB and ELF3 that impart a difference in light sensitivity [21]–[23], while researchers taking a candidate gene approach have identified alleles of both PHYD and PIF4 that contribute to shade avoidance variation [24], [25]. These studies, however, have been somewhat limited in scope, as QTL analyses with recombinant inbred lines can only assess the variation present between the parental accessions, while candidate gene approaches rely entirely on previous knowledge about the pathway in question. Studying shade avoidance responses using a genome-wide association study (GWAS), therefore, expands upon this work in two ways. First, by examining genetic variation in many accessions simultaneously, GWAS not only tests more genetic variation than the QTL approach, it also emphasizes variation that is more likely to be broadly important in natural populations. Secondly, the use of high-density genome-wide SNPs in GWAS not only allows for truly de novo candidate gene discovery, but also enables a comprehensive view of genetic architecture of the traits in question. The goal of this study was to capitalize upon these strengths of GWAS, combined with the strategy of representing the shade avoidance response as a genotype by environment (GxE) interaction, to identify genetic variants underlying natural variation in shade avoidance.
Results/Discussion
Measurement of Natural Variation
To assess the extent of natural variation in the hypocotyl response to shade, 180 Arabidopsis thaliana accessions (Table S1) were grown in both simulated sun (high R∶FR) and simulated shade (low R∶FR) conditions. This set of accessions not only included samples covering the broad range of Arabidopsis throughout the world, but also incorporated focused subsampling from Sweden to improve our ability to detect local adaptation. As expected, when all accessions were considered together, hypocotyls of seedings grown in low R∶FR were taller than those of seedlings grown in high R∶FR (t-test P-value2.2e-16) (Figure 1A).
Fig. 1. Hypocotyl height phenotypes. 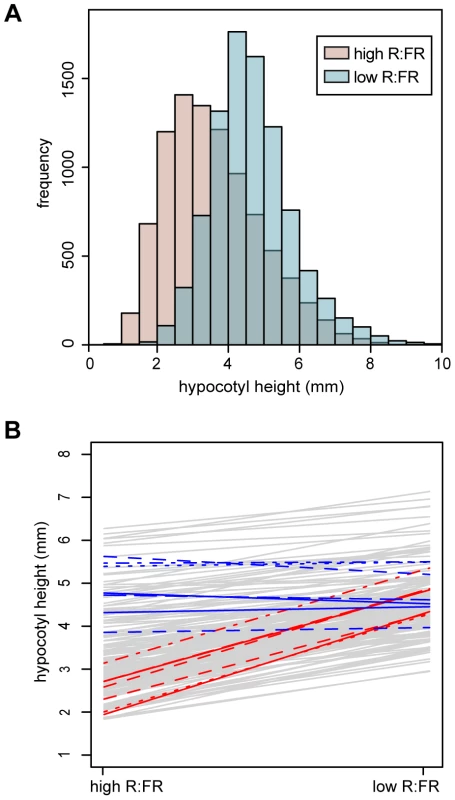
(A) Histograms of hypocotyl height for seedings grown under high R∶FR (pink) or low R∶FR (blue) treatments. (B) Hypocotyl height reaction norms of 180 Arabidopsis accessions. Reaction norms for the seven highest-responding accessions (in descending order: 9057, 8242, 6929, 6009, 6914, 6968, 8231) are plotted with red lines, while reaction norms for the seven lowest-responding accession (in ascending order: 6928, 8304, 7515, 6943, 8395, 6916, 8337) are plotted in blue. The three lowest-responding accessions showed a slight negative response to low R∶FR (−0.41, −0.25, and −0.11 millimeters). Next we asked if phenotypic variation among the accessions could be due to genetic variation. Significant differences in hypocotyl height among the accessions were observed in both light conditions (P-value2.2e-16). Broad-sense heritability of hypocotyl height was 0.54 for the high R∶FR - and 0.44 for the low R∶FR-treated seedlings. Variation in the shade avoidance response among the accessions was assessed by fitting a mixed linear model that included genetic (accession), environment (light treatment), and GxE (accession×light) components. Because the experiment was repeated with chamber-swapping, we also included an experiment effect in the model. All variables in the model were significant (P-value2.2e-16, Table 1), indicating significant differences in the shade avoidance response among accessions. Similarly, the genetic correlation between environments was 0.78, revealing that at least part of the genetic control of hypocotyl height varied between the two environments [26], [27]. The fitted values for hypocotyl height in high R∶FR, hypocotyl height in low R∶FR, and response to low R∶FR for each genotype were extracted from the full mixed-effects model for subsequent GWAS analysis (Figure S1).
Tab. 1. Parameters from the phenotype mixed effects model. 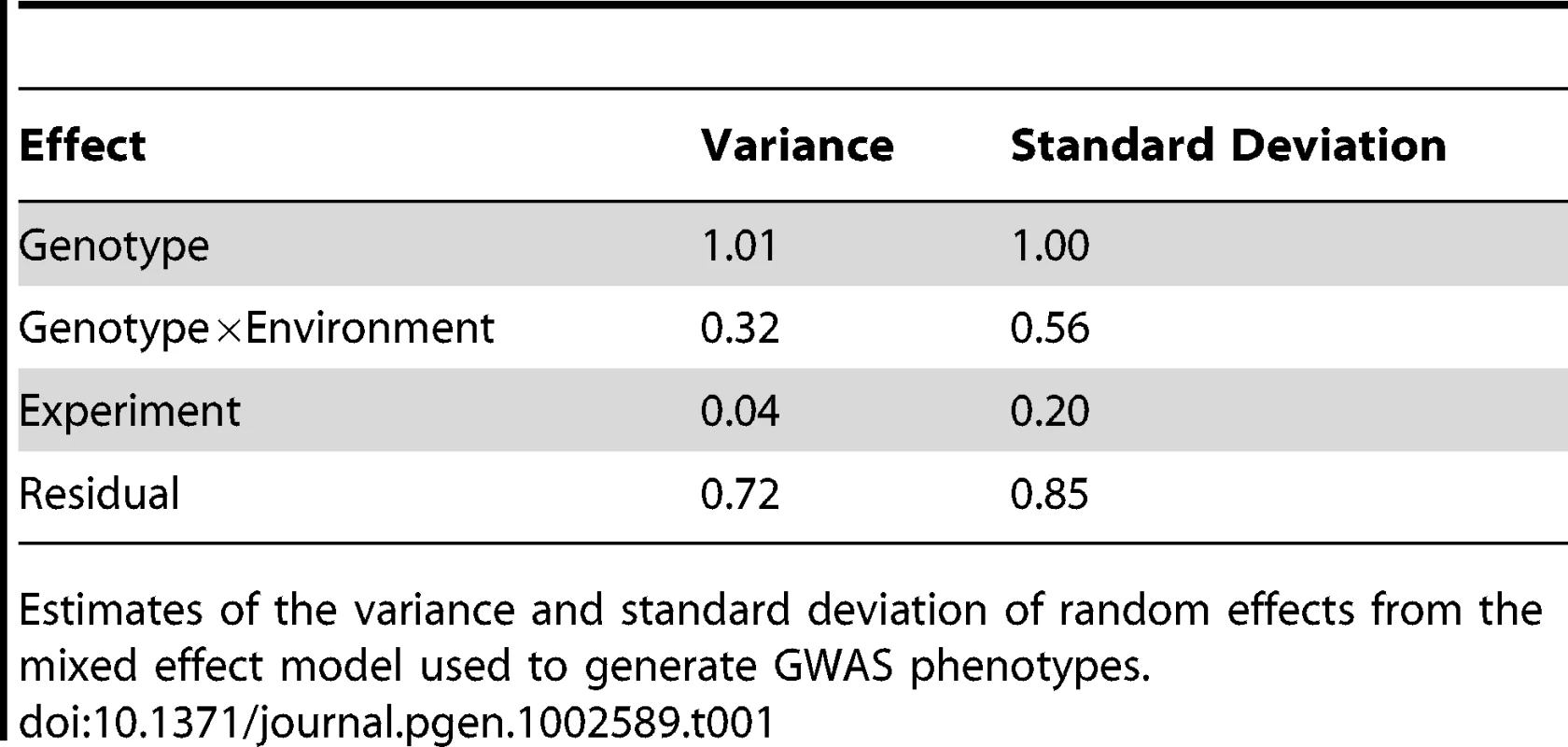
Estimates of the variance and standard deviation of random effects from the mixed effect model used to generate GWAS phenotypes. To explore patterns in variation among these phenotypes, a reaction norm plot was generated from the modeled phenotypic values (Figure 1B). The accessions that were most responsive to low R∶FR tended to have shorter hypocotyls in high R∶FR, while the least-responsive accessions tended to be taller in high R∶FR. In order to assess these relationships more thoroughly, we examined correlations between the phenotypes (Figure 2A–2C). The high R∶FR and low R∶FR phenotypes were highly positively correlated (P-value2.2e-16, r = 0.85) and the high R∶FR and response phenotypes were strongly negatively correlated (P-value2.2e-16, r = −0.67). The correlation between low R∶FR and response was also significant, although this correlation was much weaker (P-value = 0.016, r = −0.18). These results suggest that much of the variation in the shade avoidance response, as well in hypocotyl height in shade, could be attributed to variation in hypocotyl height in sun conditions, with tall accessions responding less strongly to reduced R∶FR. This relationship, however, was not absolute; analysis of the residuals from a regression of response against height in high R∶FR revealed that the some accessions responded more or less strongly than predicted (Figure 2D). In order to capture this “corrected” variation in response, we used these residuals as a fourth phenotype. Unlike the response phenotype, this corrected response phenotype is significantly correlated with height in low R∶FR (P-value = 5.8e-14, r = 0.52) and response to low R∶FR (P-value = 2.2e-16, r = 0.75) without being significantly correlated with height in high R∶FR (P-value = 1, r = 4.6e-16) (Figure S2). Therefore, the inclusion of this corrected phenotype in our analysis permitted the differentiation of genetic variants that specifically underlie variation in low R∶FR mediated elongation from alleles that underlie general elongation variation.
Fig. 2. Relationships between phenotypes. 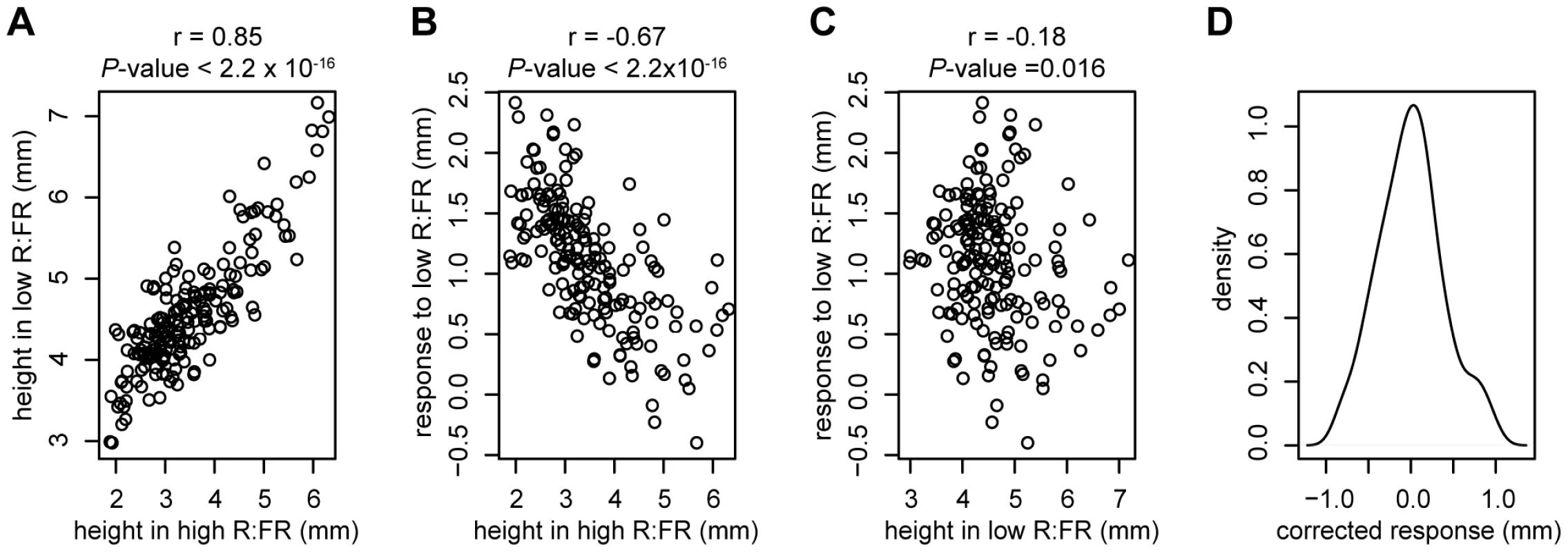
Correlations between height in high R∶FR and height in low R∶FR (A), height in high R∶FR and response to low R∶FR (B), and height in low R∶FR and response to low R∶FR (C). The kernel density plot in panel D shows the distribution of the corrected response phenotype (residuals from a regression of response to low R∶FR against height in high R∶FR). Previous studies have found negative correlations between hypocotyl height and latitude of accession origin in European Arabidopsis accessions [28]–[30], suggesting that this natural variation in light sensitivity could be a result of adaptation to the north-south gradient in ambient light intensity. Since the population structure of Arabidopsis in Europe is best thought of as isolation-by-distance [31], confounding due to population structure is a risk when using geographically-correlated phenotypes in GWAS. To test whether phenotypes measured for this study were correlated with latitude, and therefore potentially at risk of population structure problems in GWAS analysis, we examined the relationships between these phenotypes and the latitude of origin for European accessions. Both hypocotyl height in high R∶FR and response to low R∶FR were significantly correlated with latitude (P-value = 0.0001 and P-value0.0001, respectively)(Figure 3). Hypocotyl height in low R∶FR was also correlated, but with lower significance (P-value = 0.021). Although these correlations were not particularly strong (r = −0.32, 0.32, and −0.19, respectively), we still concluded that a population structure correction was necessary in our GWAS study. Interestingly, the corrected response phenotype was not significantly correlated with geography (P-value = 0.09), although the ratio of R∶FR light decreases with latitude [32]. This result suggests that variation in the corrected shade avoidance response, if adaptive, might not be due to the same selective pressure responsible for the more generalized differences in light sensitivity seen in Arabidopsis. An interesting possibility is that shade avoidance is locally adaptive and is therefore driven more by local variation in plant community composition than by larger-scale patterns of R∶FR. Evidence of local adaptation has been found in Arabidopsis [33], and the idea that shade avoidance is locally adaptive would be consistent with the adaptive population-level variation in shade-induced elongation seen in both Impatiens capensis [34] and Abutilon theophrasti [35].
Fig. 3. Correlations between phenotypes and latitude. 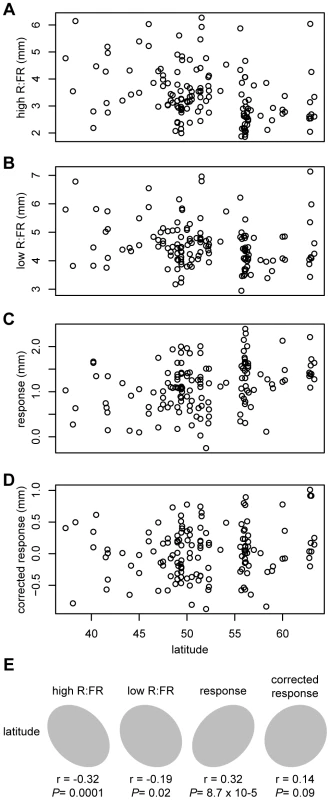
Relationships between phenotypic values and latitude of accession origin for European accessions (A–D) and the correlation ellipses for these comparisons (E). Association Mapping
To uncover the genotypic variation underlying these shade avoidance traits, the significance of associations between phenotypes and the approximately 210,000 genome-wide SNP markers from Atwell et al. [36] was evaluated using both linear mixed model (EMMA) [37] and non-parametric (Kruskal-Wallis) approaches. Genotype information was not available for twelve of the accessions phenotyped for shade avoidance, bringing the total number of accessions used for association testing to 168 (Table S1). When the results of these tests were plotted as genome scans (Figure 4), significant SNPs, some arranged in distinct peaks, were visible for all phenotypes using both association methods. These scans show many peaks of moderate significance rather than the single dominant peak seen in GWAS studies of sodium accumulation and response to bacterial elicitors [36], [38]. This result suggests that variation mapped here is polygenic, as might be expected for an environmentally-sensitive developmental trait (for other examples, see [36]). Comparing across phenotypes, the differences between the significance and location of these peaks allude to differences in the genetic variation underlying the observed variation in the four traits. Differences in peak number and significance were also seen between the two statistical methods, with a greater number of more highly significant peaks seen in the Kruskal-Wallis (KW) scans. The Kruskal-Wallis test includes no correction for population structure, resulting in inflated P-values genomewide for all phenotypes, while the use of a kinship matrix to correct for population structure, as implemented in the EMMA method, reduced this P-value inflation (Figure S3). Although the Kruskal-Wallis method results in more false positives than the EMMA method, it does have two advantages. First, as a non-parametric test, it is more robust than EMMA. Secondly, since it includes no correction for population structure, the KW method presents no risk of P-value overcorrection when applied to traits that are correlated with population structure. Comparisons between the KW and EMMA P-values for all SNPs (Figure S4) show that while some SNPs were considered significant in both EMMA and KW tests, the vast majority of SNPs were significant in either one test or the other, most likely for the reasons mentioned above. Therefore, we felt that it was important to consider associations made with both EMMA and KW, keeping the limitations and advantages of both tests in mind.
Fig. 4. Manhattan plots of GWAS results. 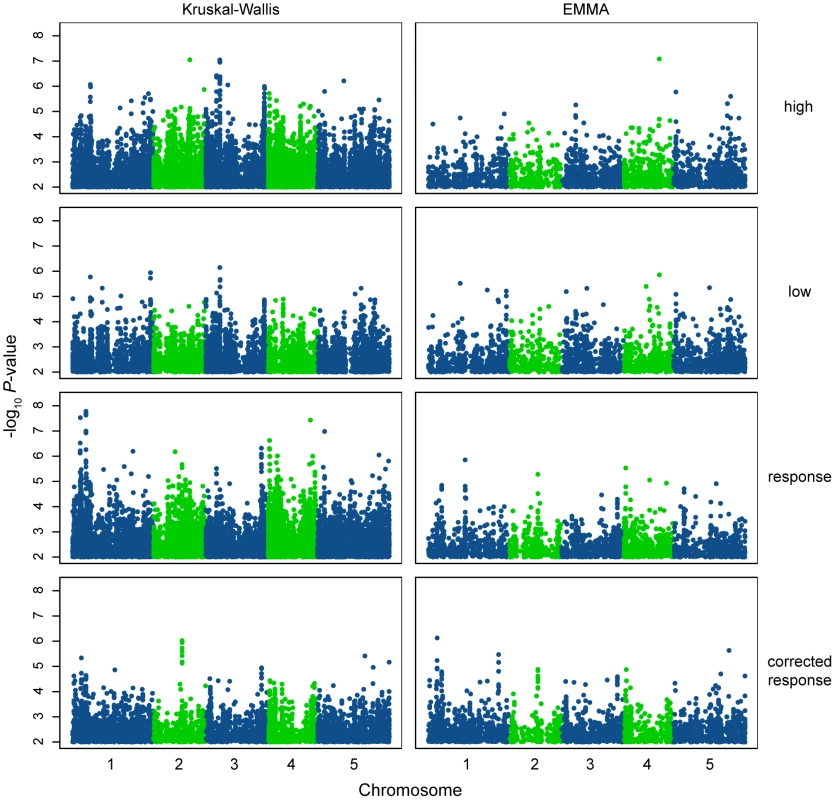
Genome-wide distribution of the −log10 P-values of SNP/phenotype associations using the Kruskal-Wallis (left panels) and EMMA (right panels) methods. For clarity, only SNPs with a −log10P-value = 2 are shown. Out of 214548 SNPs assayed, 11102, 6864, 11616, and 5301 SNPs are represented in the Kruskal-Wallis panels (top to bottom) while 2538, 2698, 2399, and 2802 SNPs are represented in the EMMA panels (top to bottom). SNPs are accurately plotted according to their position along the appropriate chromosome. Plotting colors alternate between blue and green in order to facilitate the visualization of individual chromosomes. To assess whether genome-wide associations were a result of “true” signal rather than noise, associations in the genomic region +/−20 kb around the genes PHYC and PHYB were examined. Natural genetic variation in both of these photoreceptors has previously been shown to underlie variation in hypocotyl elongation in white and red light [21], [30], [39]. Indeed, significant SNPs in linkage disequilibrium with both PHYC and PHYB were identified using the KW method (maximum −log10 P-value in a +/−20 kb window around the genes = 4.83 and 5.09, respectively; Figures S5 and S6) and the predicted effect directions of these SNPs were consistent with those of the polymorphisms identified from previous work (data not shown). However, these SNPs were not identified as significant in EMMA tests (although their P-values appeared elevated in comparison to surrounding SNPs). Given the geographical distribution of the high R∶FR phenotype, which broadly mirrors population structure [40], it is possible that these tests were overcorrected in EMMA, resulting in false negatives. Alternatively, it is possible that these associations truly are false positives caused by the confounding effects of population structure. To distinguish between these two possibilities, we genotyped our panel of accessions for the previously-identified PHYC and PHYB variants and tested associations between these variants and the high R∶FR phenotype. The PHYC causative variant was significantly associated with hypocotyl height in high R∶FR using KW yet not with EMMA (Table 2), supporting the hypothesis that EMMA overcorrected a true association. On the other hand, none of the PHYB polymorphisms that we tested were significant using either association method (Table 2).
Tab. 2. Associations with previously-identified SNPs in PHYC and PHYB. 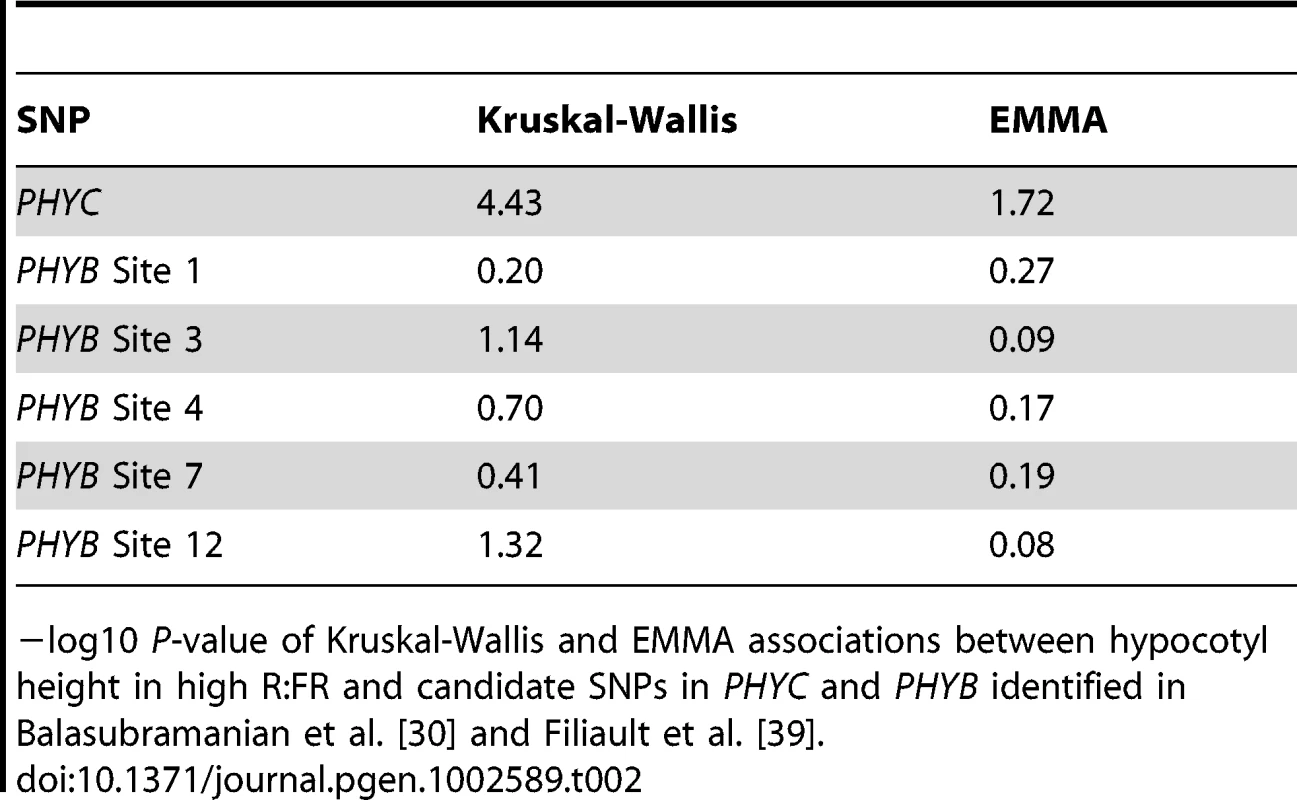
−log10 P-value of Kruskal-Wallis and EMMA associations between hypocotyl height in high R∶FR and candidate SNPs in PHYC and PHYB identified in Balasubramanian et al. [30] and Filiault et al. [39]. There are a variety of possible explanations for this negative result. First, although statistical tests identified polymorphism three as the most significant of the SNPs tested in Filiault et al. [39], the effect of this specific polymorphism was not functionally verified; it is possible that an alternate SNP is the true causative SNP and would show a significant association if tested. A second possibility is that the genome-wide KW association, while true, is not the same association that was identified in Filiault et al. [39], either due to a polymorphism that does not segregate in the parental accession used in Borevitz et al. [21] or due to differences in light treatment between the experiments. Finally, the GWAS PHYB association peak could truly be a false positive. Although the difference between the Ler and Cvi alleles of PHYB was significant in a QTL study and has been verified in transgenics [21], [39], it is possible that this difference does not contribute significantly to hypocotyl height either in a broader population sample or under our study conditions. Although additional work is needed to understand these PHYB results, the identification of an experimentally verified natural variant of PHYC is evidence that the GWAS is identifying true signal in our data set.
Identification of A Priori Candidate Genes
With the GWAS successfully identifying at least one known natural variant, we next looked for novel genetic variation underlying our phenotypes. The first strategy was to focus on a list of a priori genes whose specific roles in vegetative shade avoidance responses have been experimentally confirmed (Table 3). A gene was considered significantly associated with a phenotype if at least one SNP in the genomic region +/−20 kb around the gene had a P-value of 0.0001. The number of SNPs passing this cutoff is provided in Table S2 and detailed descriptions of the individual SNPs used to call a priori genes significant can be found in Tables S3 and S4. These criteria were applied to all TAIR9-annotated genes to generate a significant gene list for each combination of phenotype and association method. Fisher exact tests were then used to determine whether the resulting lists were enriched in a priori genes. None of the gene lists generated from KW results showed significant enrichment, although associations with a priori genes were found for all four phenotypes (Table 3). EMMA results, on the other hand, were significantly enriched in a priori candidate genes for both high and low R∶FR phenotypes (P-value0.016 and P-value0.005). Given the P-value inflation observed when using non-population-structure-corrected KW tests, we expected more false positives with these tests than when using EMMA, potentially explaining the disparity in enrichment P-values. Regardless, these results suggested that our GWAS results represented biologically relevant associations rather than noise.
Tab. 3. Associations with a priori candidate genes. 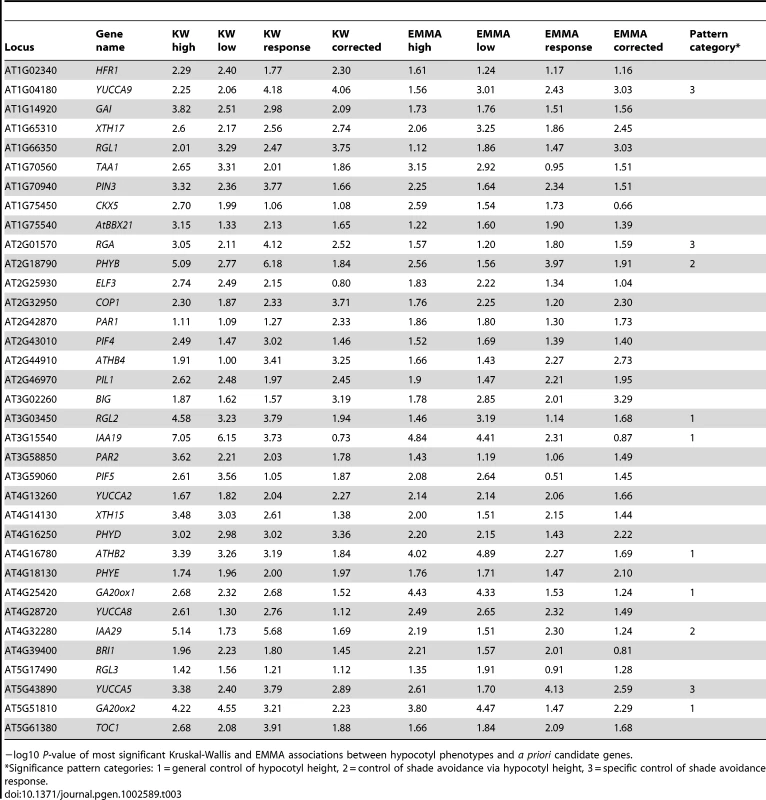
−log10 P-value of most significant Kruskal-Wallis and EMMA associations between hypocotyl phenotypes and a priori candidate genes. Next, the individual a priori candidate gene associations were examined in more detail, with the goal of looking for patterns in the genetic control of phenotypic variation. When the significance of both KW and EMMA associations across the four phenotypes was considered, candidate genes seemed to fall into three main patterns (Table 3 and Figure S7, row1), which corresponded to the phenotypic correlation patterns observed in Figure 2 and Figure S2. The first pattern consisted of genes associated with hypocotyl height under high and/or low R∶FR conditions without showing significant associations with response or corrected response phenotypes. These genes could be responsible for variation in general elongation without causing variation in shade avoidance. Even though a priori genes were chosen specifically as known shade avoidance loci, five of the ten significantly-associated genes fell into this generalist category. The functions of these five genes are quite diverse; GA20ox1 and GA20ox2 are involved in gibberellic acid (GA) biosynthesis [19], IAA19 is part of the auxin signaling pathway [41], RGL2 encodes a DELLA protein involved in the integration of the GA and light signaling pathways [42], and ATHB2 is a transcription factor involved in phytochrome B signaling [43]. Notably, while all five genes have been shown to be upregulated in response to low R∶FR, their expression under high R∶FR conditions has also been demonstrated [11], [18], [19], suggesting a mechanism whereby variation in these genes could potentially underlie variation in elongation in a more general fashion.
The other two patterns of significance observed involved either the response or the corrected response to low R∶FR. The first of these patterns was association with both height in high R∶FR and response to low R∶FR without a significant association with the corrected R∶FR response. Candidate genes which fit this pattern might underlie variation in the shade avoidance response primarily by controlling hypocotyl height in sun conditions, reflecting the high inverse correlation between these two phenotypes (Figure 2). Two a priori candidate genes fell into this category: the auxin-responsive transcription factor IAA29, which has been shown to be responsive to both red and far-red light [44], and the photoreceptor PHYB, the primary photoreceptor involved in sensing the changes in R∶FR that initiate shade avoidance [2]. Genes specifically affecting the shade avoidance response would be predicted to fall into the final pattern of significance: significant association with response to low R∶FR and/or corrected response to low R∶FR without a significant association with high R∶FR. Three a priori genes YUCCA5, YUCCA9, and RGA1 exhibited significance patterns consistent with this third group of genetic control. YUCCA5 and YUCCA9 are involved in auxin biosynthesis [15], while RGA1 is another member of the five-gene DELLA family discussed above [20].
One candidate gene that was not significantly associated with our phenotypes was ELF3. Although natural variation between the Bayreuth and Shahdara alleles of ELF3 has been shown to underlie variation in shade avoidance between these two accessions [22], [23], we found no evidence of associations with this variant in our data. This result is perhaps not unexpected as the polymorphism presumed to cause reduced response to shade in the Shahdara accession seems to be rare [23], a condition which would result in very little power to detect this polymorphism in GWAS. Overall, however, the strategy of using an a priori gene list was useful one for two reasons. First, significant enrichment of a priori genes lends additional support to the hypothesis that the GWAS is indeed identifying true positives. Second, the resulting lists of significantly-associated a priori genes and their corresponding significance pattern groups can easily be used to generate specific testable hypotheses about both the identity and molecular nature of natural variants.
Identification of De Novo Candidate Genes
Our final goal was to look beyond our a priori gene list to find de novo candidates. As in the a priori analysis, genes +/−20 kb of a significant SNP were considered significant, but for de novo discovery, a more stringent P-value cutoff was instituted for KW tests (P-value0.00001). This cutoff P-value was chosen to be slightly lower than that of the association between height in high R∶FR and PHYC, since this association was considered confirmed. For EMMA tests, a cutoff that resulted in a similar number of significant genes for both EMMA and KW tests was chosen (P-value0.0001). SNPs with a minor allele frequency 0.1 were also removed from the analysis, since these SNPs can produce misleading results in EMMA tests; this filter reduced the number of SNPs considered from 210 k to 170 k. The number of SNPs matching these criteria is provided in Table S2 and detailed descriptions of the individual SNPs used to call de novo genes significant can be found in Datasets S1 and S2. Applying these selection criteria, we identified significant SNPs for all phenotypes (Table S2), defining 1709 genes as significant. As in the a priori gene analysis, genes identified using the de novo criteria were easily separable into the same three significance pattern groups (Figure S7, row 2). Of the unique genes identified, 192 were significant for both KW and EMMA. Although genes that were significant in both KW and EMMA were considered particularly interesting, all associated genes were included when looking for de novo candidates.
To sort through this gene list, we took advantage of metadata to help identify possible de novo candidate genes. First, microarray data from previously-published experiments [10], [14] was reanalyzed to generate a list of genes differentially regulated in response to low R∶FR treatment. Secondly, the biological process GO terms and other annotation for all the genes on the list were retrieved. No significant enrichment either for differential regulation or for specific GO terms was seen in this list, nor was any GO term significantly different either between EMMA and KW de novo candidate gene lists or among the lists of candidate genes for the four different phenotypes (data not shown). This lack of GO term enrichment is not surprising given both the incomplete nature of the GO resource and the presumed low ratio of causative to non-causative genes in the analysis, resulting both from the +/−20 kb window used for candidate gene identification and from lack of population structure correction in KW tests. Both differential regulation and GO terms were, however, used to manually parse through this comprehensive de novo gene list to identify potentially interesting candidates. This selection process reduced the de novo candidate list to 53 genes which were subsequently easily assigned to the three significance pattern groups established in the a priori analysis (Figure S7, third row). The resulting de novo gene list is in Table S5.
Although this list of candidate genes included many a priori genes, we were able to identify truly de novo candidates, as well. From the filtered list of 53 de novo genes, 28 genes fell into the first significance group pattern: genes responsible for general hypocotyl height variation. Here, two genomic regions stood out as being significant in both KW and EMMA tests. The first region contained IAA19, which had been found as an a priori candidate gene, and the second region contained CGA1. This low R∶FR-regulated GATA family transcription factor functions downstream of the DELLAs to control elongation growth, and its expression is increased in pif-family (PHYTOCHROME INTERACTING FACTOR) knockout plants. This regulation seems to be direct, since an element of the CGA1 promoter co-immunoprecipitates with PIF3 [45]. Given this de novo association, as well as those of two DELLAs (RGA and RGL2) seen in the a priori analysis, it seems that modulating the integration of light and GA signals could be a common mechanism for generating general hypocotyl height variation in natural populations.
Genetic variants of the loci in the second significance pattern group are hypothesized to cause hypocotyl height-dependent variation in shade avoidance. Two of the seven de novo genes in this group, PHYB and IAA29, had also been analyzed as a priori genes. The only group two gene to be significant in both EMMA and KW tests, however, was a locus near, yet not in the same +/−20 kb window as, IAA29. This gene is ATH1 (AT4G32980), a homeobox transcription factor implicated in photomorphogenesis [46] that has also been shown to be involved in stem growth and shoot apical meristem maintenance in older plants, as well [47], [48].
Significance group three contains genes with variants that potentially influenceshade avoidance in a specific manner. Interestingly, many group three de novo genes seemed to be involved in phytochrome A signaling. PHYA and PIF3, a transcription factor that interacts directly with both phyA and phyB, are separated only by about 16 kb in the Arabidopsis genome. A significantly-associated SNP fell into this interval, making both of these genes potential de novo candidates. Two additional genes involved in phyA signaling, ATNAP2/ABCI21 and FRS11 [44], [49] were also significant. A fifth gene, PP5Pa, a proposed inorganic pyrophosphatase, initially seemed an unlikely candidate despite being differentially-regulated in response to low R∶FR and being significant in both EMMA and KW tests. However, transcription of this gene has been shown to be under the control of FAR1 [50], a transcription factor in the phytochrome A signaling pathway that is involved in the nuclear accumulation of phyA [51]. Altogether, five of 18 group three de novo candidate genes are involved in phyA signaling, suggesting that variation in this pathway could be responsible for at least some of the observed variation in the shade avoidance response. This phenomenon could be explained by the light-labile nature of phyA itself. Although phytochrome A is rapidly degraded in red light, it becomes more stable as the ratio of R∶FR decreases, allowing increased signaling through the phyA pathway and a concomitant inhibition of elongation growth in shade conditions [52].
Variants in both PHYA and PHYB were associated with variation in shade avoidance, yet the association/phenotype significance patterns seemed to suggest that PHYB variation affected shade avoidance in a strictly height-dependent way, while PHYA control was more specific for the response itself. We decided, therefore, to ask whether these two variants exerted independent effects on our phenotypes. Two-way ANOVAs with the most significant PHYB and PHYA SNPs as factors found no significant interaction term for any of the four phenotypes used in this study (data not shown), and the loci seemed to be acting additively (Figure S8). These results indicate that genetic variants linked to PHYA and PHYB were exerting independent control over shade avoidance. T-tests between the means of the allelic variants of both SNPs showed effect sizes and P-values that were consistent with the patterns seen in the GWAS (Table S6). The notion that PHYA and PHYB act independently in shade avoidance is in agreement with a microarray study of shade avoidance using phyB and phyAB mutants which identified a number of shade-responsive genes under independent control of PHYA [53]. Again, however, PIF3 and PHYA are situated quite nearby each other in the genome, so the SNP identified as significant here could be a marker for variation in either gene. Nonetheless, this particular association is one of many promising targets for which validation of these GWAS results using crosses and functional studies seems warranted.
Finally, intrigued by the result that variation in PHYA/PIF3 seemed to underlie specific shade avoidance variation, we asked whether the PHYA/PIF3 variant could shed light on the idea that the shade avoidance response could be locally adaptive. We decided to focus on Swedish accessions, since 43 of the 168 accessions used for the GWAS study originated from Sweden. When the phenotypes of these accessions were plotted on a map (Figure S9A–S9D), no obvious geographic patterns could be seen and in fact, none of the phenotypes were significantly correlated with latitude (data not shown). On the contrary, phenotypes from accessions in very close proximity to one another often had quite disparate phenotypes, especially for the response and corrected response phenotypes. This observation is consistent with the population-level phenotypic variation that could result from adaptation to local R∶FR conditions. The allelic distribution of the most significant PHYA/PIF3 SNP showed a similar pattern of local co-existence, with the exception of the 14 most northern accessions which all carried the same variant (Figure S9E). T-tests for differences between the mean phenotypes of the two alleles were performed for all four phenotypes (Figure S9F). These tests indicated that just as in the main set of accessions, the PHYA/PIF3 variant had a specific effect on the shade avoidance response in the Swedish subset. Given that Arabidopsis exhibits isolation by distance [31], we cannot rule out the possibility that these associations are false positives due to population structure, especially since the most northern accessions all carry the same allele of the SNP under consideration. However, if the variation in shade avoidance that has been measured in this study is indeed adaptive, then the evidence presented here is a solid starting point for further exploration of hypothetical local adaptation in shade avoidance in Swedish Arabidopsis populations.
Conclusions
We performed a genome-wide association study (GWAS) to look for genetic variants underlying four phenotypes: hypocotyl height in both high and low R∶FR, the response of hypocotyl height to shade, and the response to shade corrected for hypocotyl height. Rather than the few peaks of large effect size seen in some earlier published Arabidopsis GWAS, our results showed many peaks with small to moderate effect sizes. Instead of representing a shortcoming with the study or method, these results suggest that variation in the shade avoidance response is complex trait that is controlled by many genetic variants. Through analysis of known variants, a priori candidate genes lists, and metadata-enabled de novo candidate discovery, we were able to identify genetic variants associated with shade avoidance phenotypes. One goal of future work will be to verify these associations in lines that minimize confounding due to population structure, such as F2 populations or recombinant inbred lines. A second goal will be to identify and characterize causative polymorphisms through functional molecular work.
While previous GWAS studies in Arabidopsis have found environment-dependent associations [54]–[56], the results of the study described here emphasize the strength of explicitly incorporating GxE interactions into the GWAS approach. First, our study design enabled the identification of genetic variants specifically underlying the response to low R∶FR. As many aspects of plant development and physiology are intrinsically environmentally-sensitive, an improved understanding of genotype-by-environment interactions will be a key part of exploring the genotype-phenotype map for these traits. As statistical methods and mapping designs improve [57], our power to examine these interactions will only continue to grow.
The second benefit of our study design is that the results serve as a springboard to ecological and population genetics studies exploring the evolutionary relevance of environmental responses. For example, in this study, the observation that the shade avoidance response is not correlated with latitude lead us to hypothesize that the response to low R∶FR is locally adaptive. Our subsequent GWAS identified variants that were specifically associated with the shade avoidance response, suggesting a set of experiments that can be performed to explore this hypothesis. First, our candidate variant list can be used in designing physiological and/or genetic manipulations to assess whether this variation in shade avoidance is an example of adaptive plasticity [58], [59]. Second, the resequencing of hundreds of Arabidopsis accessions [60] will provide a powerful resource to look for genomic evidence of selection around candidate SNPs. Finally, if the variants identified in our GWAS are adaptive, it would be interesting to understand the scale of this adaptation. Since little information about habitat or ecology was collected at the time of accession sampling, this work would require returning to the field, assessing the light environment in field sites and taking new population samples. If our candidate SNPs are responsible for local adaptation, then population-level differences in the frequency of these variants should correspond with local differences in the R∶FR ratio. This suite of experiments, which has the potential to shed light on the genetics of phenotypic plasticity, is made possible by the specific nature of the candidate SNP lists generated as a result of the incorporation of genotype by environment interactions into GWAS, indicating that this strategy promises to be a useful tool in furthering our understanding of evolution and natural variation.
Materials and Methods
Plant Culture and Measurement
180 Arabidopsis thaliana accessions (Table S1) were phenotyped. Seeds were gas sterilized, plated on 0.5× MSMO with 0.7% agar, and stratified for four days in the dark at 4C. Plates were then moved to two LED chambers with constant light conditions set to 34 E/m2/s red light and 7 E/m2/s blue light. After 24 hours, far-red light was added to bring the red-to-far-red ratio (R∶FR) to 2. After an additional 24 hours, non-germinated seeds were marked and excluded from further analysis in order to minimize hypocotyl height variation due solely to variation in germination time. 24 hours after this marking, the R∶FR ratio in one chamber was lowered to 0.5 and plants were grown for an additional 4 days. Seedings were harvested to transparencies and scanned to .jpg files. Hypocotyl height was measured from these images using Image J [61]. A completely randomized design of two repetitions of 20 plants each per treatment was used. The experiment was repeated with a reversal of the R∶FR treatment assignments for the two chambers.
Phenotype Modeling
This, and all subsequent analyses were done using the R statistical programming language [62]. Phenotypes were modeled with the following mixed linear model using the lme4 package [63]:where HYP is hypocotyl height, ENV is light treatment (high or low R∶FR), GEN is genotype (accession), EXP is experimental repeat, GEN*ENV is the genotype by environment interaction, and e is the error. ENV is fitted as a fixed effect while all other variables are fitted as random effects. Significance of each model term was assessed using the anova.lm method implemented in R. The predicted effects for hypocotyl height in high and low R∶FR, as well as for response to R∶FR were extracted and used as phenotypes in GWAS analysis.
To determine heritability of hypocotyl height, the following model was fit for both high and low R∶FR data:where HYP is hypocotyl height, GEN is genotype (accession), EXP is experimental repeat, and e is the error, with all variables fitted as random effects. Heritability was then calculated as the genotypic variance divided by the total variance.
Genetic correlation across high and low R∶FR environments was calculated as in Falconer and MacKay [64] using variance estimates from the above models.
Genome-Wide Associations
All analyses were done in R [62]. Two methods were used to perform association tests between modeled phenotypes and the genome-wide SNP data from Atwell et al. [36]. The first method was EMMA, the mixed linear model approach with a K matrix as populations structure correction as outlined in Kang et al. [37], and the second method was a Kruskal-Wallis test. Linkage disequilibrium was calculated using the genetics package [65]. Phytochrome B and C genotyping was done as in Balasubramanian et al. [30] and Filiault et al. [39], with results in Table S7.
Enrichment Analysis
Genes differentially regulated in response to R∶FR treatment were determined by reanalyzing data from Sessa et al. [10] and Tao et al. [14] using the limma package [66] in Bioconductor [67]. A false discovery rate (FDR) cutoff of 0.1 was used for determining gene significance. GO annotation and other annotation was taken from the org.At.tairGO package [68] in Bioconductor [67]. Enrichment for a priori genes and for R∶FR differentially-regulated genes was assessed with a Fisher's exact test. The GOstat program [69] with default settings, an FDR of 0.05, and the TAIR GO database was used to look for overrepresentation of GO terms in candidate gene lists.
Supporting Information
Zdroje
1. KamiCLorrainSHornitschekPFankhauserC 2010 Light-regulated plant growth and development. Current Topics in Developmental Biology 91 29 66
2. FranklinKA 2008 Shade avoidance. New Phytol 179 930 944
3. MorganDCSmithH 1979 A systematic relationship between phytochrome-controlled development and species habitat, for plants grown in simulated natural radiation. Planta 145 253 258
4. DudleySASchmittJ 1996 Testing the adaptive plasticity hypothesis: density-dependent selection on manipulated stem length in Impatiens capensis. The American Naturalist 147 445 465
5. SchmittJMcCormacACSmithH 1995 A Test of the Adaptive Plasticity Hypothesis Using Transgenic and Mutant Plants Disabled in Phytochrome-Mediated Elongation Responses to Neighbors. The American Naturalist 146 937 953
6. DornLAPyleEHSchmittJ 2000 Plasticity to light cues and resources in Arabidopsis thaliana: testing for adaptive value and costs. Evolution 54 1982 94
7. BottoJFSmithH 2002 Differential genetic variation in adaptive strategies to a common environmental signal in Arabidopsis accessions: phytochrome-mediated shade avoidance. Plant, Cell & Environment 25 53 63
8. KebromBrutnell 2007 The molecular analysis of the shade avoidance syndrome in the grasses has begun. J Exp Bot 58 3079 3089
9. HornitschekPLorrainSZoeteVMichielinOFankhauserC 2009 Inhibition of the shade avoidance response by formation of non-DNA binding bHLH heterodimers. EMBO J 28 3893 3902
10. SessaGCarabelliMSassiMCiolfiAPossentiM 2005 A dynamic balance between gene activation and repression regulates the shade avoidance response in Arabidopsis. Genes Dev 19 2811 5
11. CarabelliMSessaGBaimaSMorelliGRubertiI 1993 The Arabidopsis Athb-2 and -4 genes are strongly induced by far-red-rich light. Plant J 4 469 479
12. SalterMGFranklinKAWhitelamGC 2003 Gating of the rapid shade-avoidance response by the circadian clock in plants. Nature 426 680 683
13. StammPKumarPP 2010 The phytohormone signal network regulating elongation growth during shade avoidance. J Exp Bot 61 2889 903
14. TaoYFerrerJLLLjungKPojerFHongF 2008 Rapid synthesis of auxin via a new tryptophan-dependent pathway is required for shade avoidance in plants. Cell 133 164 176
15. WonCShenXMashiguchiKZhengZDaiX 2011 Conversion of tryptophan to indole-3-acetic acid by TRYPTOPHAN AMINOTRANSFERASES OF ARABIDOPSIS and YUCCAs in Arabidopsis. Proc Natl Acad Sci U S A 108 18518 18523
16. KanyukaKPraekeltUFranklinKABillinghamOEHooleyR 2003 Mutations in the huge Arabidopsis gene BIG affect a range of hormone and light responses. Plant J 35 57 70
17. KeuskampDPollmannSVoesenekLPeetersAPierikR 2010 Auxin transport through PINFORMED 3 (PIN3) controls shade avoidance and fitness during competition. Proc Natl Acad Sci U S A 107 22740
18. KozukaTKobayashiJHoriguchiGDemuraTSakakibaraH 2010 Involvement of auxin and brassinosteroid in the regulation of petiole elongation under the shade. Plant Physiol 153 1608 18
19. HisamatsuTKingRWHelliwellCAKoshiokaM 2005 The involvement of gibberellin 20-oxidase genes in phytochrome-regulated petiole elongation of Arabidopsis. Plant Physiol 138 1106 1116
20. Djakovic-PetrovicTde WitMVoesenekLACJPierikR 2007 DELLA protein function in growth responses to canopy signals. Plant J 51 117 26
21. BorevitzJOMaloofJNLutesJDabiTRedfernJL 2002 Quantitative trait loci controlling light and hormone response in two accessions of Arabidopsis thaliana. Genetics 160 683 96
22. ColuccioMPSanchezSEKasulinLYanovskyMJBottoJF 2010 Genetic mapping of natural variation in a shade avoidance response: ELF3 is the candidate gene for a QTL in hypocotyl growth regulation. J Exp Bot 62 167 176
23. Jiménez-GómezJMWallaceADMaloofJN 2010 Network analysis identifies ELF3 as a QTL for the shade avoidance response in Arabidopsis. PLoS Genet 6 e1001100 doi:10.1371/journal.pgen.1001100
24. AukermanMJHirschfeldMWesterLWeaverMClackT 1997 A deletion in the PHYD gene of the Arabidopsis Wassilewskija ecotype defines a role for phytochrome D in red/far-red light sensing. Plant Cell 9 1317 1326
25. BrockMTMaloofJNWeinigC 2010 Genes underlying quantitative variation in ecologically important traits: PIF4 (phytochrome interacting factor 4) is associated with variation in internode length, owering time, and fruit set in Arabidopsis thaliana. Mol Ecol 19 1187 1199
26. ViaSLandeR 1987 Evolution of genetic variability in a spatially heterogeneous environment: effects of genotype-environment interaction. Genetical research 49 147 56
27. ScheinerSM 1993 Genetics and Evolution of Phenotypic Plasticity. Annual Review of Ecology and Systematics 24 35 68
28. MaloofJNBorevitzJODabiTLutesJNehringRB 2001 Natural variation in light sensitivity of Arabidopsis. Nat Genet 29 441 446
29. StenøienHKFensterCBKuittinenHSavolainenO 2002 Quantifying latitudinal clines to light responses in natural populations of Arabidopsis thaliana (Brassicaceae). American Journal of Botany 89 1604 8
30. BalasubramanianSSureshkumarSAgrawalMMichaelTPWessingerC 2006 The PHYTOCHROME C photoreceptor gene mediates natural variation in owering and growth responses of Arabidopsis thaliana. Nat Genet 38 711 715
31. PlattAHortonMHuangYSLiYAnastasioAE 2010 The scale of population structure in Arabidopsis thaliana. PLoS Genet 6 e1000843 doi:10.1371/journal.pgen.1000843
32. SmithH 1982 Light quality, photopercetion, and plant strategy. Annual Review of Plant Physiology 33 481 518
33. Fournier-LevelAKorteACooperMDNordborgMSchmittJ 2011 A Map of Local Adaptation in Arabidopsis thaliana. Science 334 86 89
34. DudleySASchmittJ 1995 Genetic Differentiation in Morphological Responses to Simulated Foliage Shade between Populations of Impatiens capensis from Open and Woodland Sites. Functional Ecology 9 655 666
35. WeinigC 2000 Plasticity versus canalization: population di_erences in the timing of shadeavoidance responses. Evolution Int J Org Evolution 54 441 451
36. AtwellSHuangYSVilhjálmssonBJWillemsGHortonM 2010 Genome-wide association study of 107 phenotypes in Arabidopsis thaliana inbred lines. Nature 465 627 631
37. KangHMZaitlenNAWadeCMKirbyAHeckermanD 2008 Efficient control of population structure in model organism association mapping. Genetics 178 1709 23
38. BaxterIBrazeltonJNYuDHuangYSLahnerB 2010 A Coastal Cline in Sodium Accumulation in Arabidopsis thaliana Is Driven by Natural Variation of the Sodium Transporter AtHKT1;1. PLoS Genet 6 e1001193 doi:10.1371/journal.pgen.1001193
39. FiliaultDLWessingerCADinnenyJRLutesJBorevitzJO 2008 Amino acid polymorphisms in Arabidopsis phytochrome B cause differential responses to light. Proc Natl Acad Sci U S A 105 3157 3162
40. NordborgMHuTTIshinoYJhaveriJToomajianC 2005 The pattern of polymorphism in Arabidopsis thaliana. PLoS Biol 3 e196 doi:10.1371/journal.pbio.0030196
41. TatematsuKKumagaiSMutoHSatoAWatahikiMK 2004 MASSUGU2 encodes Aux/IAA19, an auxin-regulated protein that functions together with the transcriptional activator NPH4/ARF7 to regulate differential growth responses of hypocotyl and formation of lateral roots in Arabidopsis thaliana. The Plant Cell 16 379 93
42. FengSMartinezCGusmaroliGWangYZhouJ 2008 Coordinated regulation of Arabidopsis thaliana development by light and gibberellins. Nature 451 475 9
43. SteindlerCMatteucciASessaGWeimarTOhgishiM 1999 Shade avoidance responses are mediated by the ATHB-2 HD-zip protein, a negative regulator of gene expression. Development 126 4235 45
44. TeppermanJMHwangYSQuailPH 2006 phyA dominates in transduction of red-light signals to rapidly responding genes at the initiation of Arabidopsis seedling de-etiolation. Plant J 48 728 42
45. RichterRBehringerCMüllerIKSchwechheimerC 2010 The GATA-type transcription factors GNC and GNL/CGA1 repress gibberellin signaling downstream from DELLA proteins and PHYTOCHROME-INTERACTING FACTORS. Genes Dev 24 2093 104
46. QuaedvliegNDockxJRookFWeisbeekPSmeekensS 1995 The homeobox gene ATH1 of Arabidopsis is derepressed in the photomorphogenic mutants cop1 and det1. Plant Cell 7 117 29
47. Gómez-MenaCde FolterSCostaMMRAngenentGCSablowskiR 2005 Transcriptional program controlled by the oral homeotic gene AGAMOUS during early organogenesis. Development 132 429 38
48. RutjensBBaoDvan Eck-StoutenEBrandMSmeekensS 2009 Shoot apical meristem function in Arabidopsis requires the combined activities of three BEL1-like homeodomain proteins. Plant J 58 641 54
49. LinRWangH 2004 Arabidopsis FHY3/FAR1 gene family and distinct roles of its members in light control of Arabidopsis development. Plant Physiol 136 4010 22
50. HudsonMELischDRQuailPH 2003 The FHY3 and FAR1 genes encode transposase-related proteins involved in regulation of gene expression by the phytochrome A-signaling pathway. Plant J 34 453 71
51. LinRDingLCasolaCRipollDRFeschotteC 2007 Transposase-derived transcription factors regulate light signaling in Arabidopsis. Science 318 1302 5
52. FranklinKAQuailPH 2010 Phytochrome functions in Arabidopsis development. Journal of experimental botany 61 11 24
53. DevlinPFYanovskyMJKaySA 2003 A genomic analysis of the shade avoidance response in Arabidopsis. Plant Physiol 133 1617 29
54. ChanEKFRoweHCHansenBGKliebensteinDJ 2010 The Complex Genetic Architecture of the Metabolome. PLoS Genet 6 e1001198 doi:10.1371/journal.pgen.1001198
55. BrachiBFaureNHortonMFlahauwEVazquezA 2010 Linkage and association mapping of Arabidopsis thaliana owering time in nature. PLoS Genet 6 e1000940 doi:10.1371/journal.pgen.1000940
56. LiYHuangYBergelsonJNordborgMBorevitzJO 2010 Association mapping of local climatesensitive quantitative trait loci in Arabidopsis thaliana. Proc Natl Acad Sci U S A 107 21199 204
57. BrachiBMorrisGPBorevitzJO 2011 Genome-wide association studies in plants: the missing heritability is in the field. Genome Biology 12 232
58. SchmittJDudleySAPigliucciM 1999 Manipulative Approaches to Testing Adaptive Plasticity: PhytochromeMediated ShadeAvoidance Responses in Plants. The American Naturalist 154 S43 S54
59. SchmittJStinchcombeJRHeschelMSHuberH 2003 The adaptive evolution of plasticity: phytochrome-mediated shade avoidance responses. Integrative and Comparative Biology 43 459 69
60. CaoJSchneebergerKOssowskiSGüntherTBenderS 2011 Whole-genome sequencing of multiple Arabidopsis thaliana populations. Nature Genetics 43 956 963
61. RasbandW 1997–2011 ImageJ. U. S. National Institutes of Health, Bethesda, Maryland, USA, http://imagej.nih.gov/ij/edition
62. R Development Core Team 2010 R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria
63. BatesDMaechlerM 2010 lme4: Linear mixed-effects models using S4 classes
64. FalconerDSMackayTFC 1996 Introduction to Quantitative Genetics (4th Edition) Prentice Hall
65. WarnesGLeischFManM with contributions from Gregor Gorjanc 2008 genetics: Population Genetics
66. SmythGK 2005 Limma: linear models for microarray data. GentlemanRCareyVDudoitSR IrizarryWH Bioinformatics and Computational Biology Solutions using R and Bioconductor New York Springer 397 420
67. GentlemanRCCareyVJBatesDM 2004 Bioconductor: Open software development for computational biology and bioinformatics. Genome Biology 5 R80
68. CarlsonMFalconSPagesHLiN org.At.tair.db: Genome wide annotation for Arabidopsis
69. BeissbarthTSpeedTP 2004 GOstat: find statistically overrepresented Gene Ontologies within a group of genes. Bioinformatics 20 1464 5
Štítky
Genetika Reprodukční medicína
Článek Physiological Notch Signaling Maintains Bone Homeostasis via RBPjk and Hey Upstream of NFATc1Článek Intronic -Regulatory Modules Mediate Tissue-Specific and Microbial Control of / TranscriptionČlánek Probing the Informational and Regulatory Plasticity of a Transcription Factor DNA–Binding DomainČlánek Repression of Germline RNAi Pathways in Somatic Cells by Retinoblastoma Pathway Chromatin ComplexesČlánek An Alu Element–Associated Hypermethylation Variant of the Gene Is Associated with Childhood ObesityČlánek Three Essential Ribonucleases—RNase Y, J1, and III—Control the Abundance of a Majority of mRNAsČlánek Genomic Tools for Evolution and Conservation in the Chimpanzee: Is a Genetically Distinct Population
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 3
-
Všechny články tohoto čísla
- Comprehensive Research Synopsis and Systematic Meta-Analyses in Parkinson's Disease Genetics: The PDGene Database
- Genomic Analysis of the Hydrocarbon-Producing, Cellulolytic, Endophytic Fungus
- Networks of Neuronal Genes Affected by Common and Rare Variants in Autism Spectrum Disorders
- Akirin Links Twist-Regulated Transcription with the Brahma Chromatin Remodeling Complex during Embryogenesis
- Too Much Cleavage of Cyclin E Promotes Breast Tumorigenesis
- Imprinted Genes … and the Number Is?
- Genetic Architecture of Highly Complex Chemical Resistance Traits across Four Yeast Strains
- Exploring the Complexity of the HIV-1 Fitness Landscape
- MNS1 Is Essential for Spermiogenesis and Motile Ciliary Functions in Mice
- A Fundamental Regulatory Mechanism Operating through OmpR and DNA Topology Controls Expression of Pathogenicity Islands SPI-1 and SPI-2
- Evidence for Positive Selection on a Number of MicroRNA Regulatory Interactions during Recent Human Evolution
- Variation in Modifies Risk of Neonatal Intestinal Obstruction in Cystic Fibrosis
- PIF4–Mediated Activation of Expression Integrates Temperature into the Auxin Pathway in Regulating Hypocotyl Growth
- Critical Evaluation of Imprinted Gene Expression by RNA–Seq: A New Perspective
- A Meta-Analysis and Genome-Wide Association Study of Platelet Count and Mean Platelet Volume in African Americans
- Mouse Genetics Suggests Cell-Context Dependency for Myc-Regulated Metabolic Enzymes during Tumorigenesis
- Transcriptional Control in Cardiac Progenitors: Tbx1 Interacts with the BAF Chromatin Remodeling Complex and Regulates
- Synthetic Lethality of Cohesins with PARPs and Replication Fork Mediators
- APOBEC3G-Induced Hypermutation of Human Immunodeficiency Virus Type-1 Is Typically a Discrete “All or Nothing” Phenomenon
- Interpreting Meta-Analyses of Genome-Wide Association Studies
- Error-Prone ZW Pairing and No Evidence for Meiotic Sex Chromosome Inactivation in the Chicken Germ Line
- -Dependent Chemosensory Functions Contribute to Courtship Behavior in
- Diverse Forms of Splicing Are Part of an Evolving Autoregulatory Circuit
- Phenotypic Plasticity of the Drosophila Transcriptome
- Physiological Notch Signaling Maintains Bone Homeostasis via RBPjk and Hey Upstream of NFATc1
- Precocious Metamorphosis in the Juvenile Hormone–Deficient Mutant of the Silkworm,
- Igf1r Signaling Is Indispensable for Preimplantation Development and Is Activated via a Novel Function of E-cadherin
- Accurate Prediction of Inducible Transcription Factor Binding Intensities In Vivo
- Mitochondrial Oxidative Stress Alters a Pathway in Strongly Resembling That of Bile Acid Biosynthesis and Secretion in Vertebrates
- Mammalian Neurogenesis Requires Treacle-Plk1 for Precise Control of Spindle Orientation, Mitotic Progression, and Maintenance of Neural Progenitor Cells
- Tcf7 Is an Important Regulator of the Switch of Self-Renewal and Differentiation in a Multipotential Hematopoietic Cell Line
- REST–Mediated Recruitment of Polycomb Repressor Complexes in Mammalian Cells
- Intronic -Regulatory Modules Mediate Tissue-Specific and Microbial Control of / Transcription
- Age-Dependent Brain Gene Expression and Copy Number Anomalies in Autism Suggest Distinct Pathological Processes at Young Versus Mature Ages
- A Genome-Wide Association Study Identifies Variants Underlying the Shade Avoidance Response
- -by- Regulatory Divergence Causes the Asymmetric Lethal Effects of an Ancestral Hybrid Incompatibility Gene
- Genome-Wide Association and Functional Follow-Up Reveals New Loci for Kidney Function
- A Natural System of Chromosome Transfer in
- Cell Size and the Initiation of DNA Replication in Bacteria
- Probing the Informational and Regulatory Plasticity of a Transcription Factor DNA–Binding Domain
- Repression of Germline RNAi Pathways in Somatic Cells by Retinoblastoma Pathway Chromatin Complexes
- Temporal Transcriptional Profiling of Somatic and Germ Cells Reveals Biased Lineage Priming of Sexual Fate in the Fetal Mouse Gonad
- Rapid Analysis of Genome Rearrangements by Multiplex Ligation–Dependent Probe Amplification
- Metabolic Profiling of a Mapping Population Exposes New Insights in the Regulation of Seed Metabolism and Seed, Fruit, and Plant Relations
- The Atypical Calpains: Evolutionary Analyses and Roles in Cellular Degeneration
- The Silkworm Coming of Age—Early
- Development of a Panel of Genome-Wide Ancestry Informative Markers to Study Admixture Throughout the Americas
- Balanced Codon Usage Optimizes Eukaryotic Translational Efficiency
- The Min System and Nucleoid Occlusion Are Not Required for Identifying the Division Site in but Ensure Its Efficient Utilization
- Neurobeachin, a Regulator of Synaptic Protein Targeting, Is Associated with Body Fat Mass and Feeding Behavior in Mice and Body-Mass Index in Humans
- Statistical Analysis of Readthrough Levels for Nonsense Mutations in Mammalian Cells Reveals a Major Determinant of Response to Gentamicin
- Gene Reactivation by 5-Aza-2′-Deoxycytidine–Induced Demethylation Requires SRCAP–Mediated H2A.Z Insertion to Establish Nucleosome Depleted Regions
- The miR-35-41 Family of MicroRNAs Regulates RNAi Sensitivity in
- Genetic Basis of Hidden Phenotypic Variation Revealed by Increased Translational Readthrough in Yeast
- An Alu Element–Associated Hypermethylation Variant of the Gene Is Associated with Childhood Obesity
- Modelling Human Regulatory Variation in Mouse: Finding the Function in Genome-Wide Association Studies and Whole-Genome Sequencing
- Novel Loci for Adiponectin Levels and Their Influence on Type 2 Diabetes and Metabolic Traits: A Multi-Ethnic Meta-Analysis of 45,891 Individuals
- Polycomb-Like 3 Promotes Polycomb Repressive Complex 2 Binding to CpG Islands and Embryonic Stem Cell Self-Renewal
- Insulin/IGF-1 and Hypoxia Signaling Act in Concert to Regulate Iron Homeostasis in
- EMF1 and PRC2 Cooperate to Repress Key Regulators of Arabidopsis Development
- Three Essential Ribonucleases—RNase Y, J1, and III—Control the Abundance of a Majority of mRNAs
- Contrasted Patterns of Molecular Evolution in Dominant and Recessive Self-Incompatibility Haplotypes in
- A Machine Learning Approach for Identifying Novel Cell Type–Specific Transcriptional Regulators of Myogenesis
- Genomic Tools for Evolution and Conservation in the Chimpanzee: Is a Genetically Distinct Population
- Nos2 Inactivation Promotes the Development of Medulloblastoma in Mice by Deregulation of Gap43–Dependent Granule Cell Precursor Migration
- Intracranial Aneurysm Risk Locus 5q23.2 Is Associated with Elevated Systolic Blood Pressure
- Heritability and Genetic Correlations Explained by Common SNPs for Metabolic Syndrome Traits
- A Genome-Wide Association Study of Nephrolithiasis in the Japanese Population Identifies Novel Susceptible Loci at 5q35.3, 7p14.3, and 13q14.1
- DNA Damage in Nijmegen Breakage Syndrome Cells Leads to PARP Hyperactivation and Increased Oxidative Stress
- DNA Resection at Chromosome Breaks Promotes Genome Stability by Constraining Non-Allelic Homologous Recombination
- Genetic Analysis of Floral Symmetry in Van Gogh's Sunflowers Reveals Independent Recruitment of Genes in the Asteraceae
- A Splice Site Variant in the Bovine Gene Compromises Growth and Regulation of the Inflammatory Response
- Promoter Nucleosome Organization Shapes the Evolution of Gene Expression
- The Nucleoside Diphosphate Kinase Gene Acts as Quantitative Trait Locus Promoting Non-Mendelian Inheritance
- The Ciliogenic Transcription Factor RFX3 Regulates Early Midline Distribution of Guidepost Neurons Required for Corpus Callosum Development
- Phosphorylation of the RNA–Binding Protein HOW by MAPK/ERK Enhances Its Dimerization and Activity
- A Genome-Wide Scan of Ashkenazi Jewish Crohn's Disease Suggests Novel Susceptibility Loci
- Parkinson's Disease–Associated Kinase PINK1 Regulates Miro Protein Level and Axonal Transport of Mitochondria
- LMW-E/CDK2 Deregulates Acinar Morphogenesis, Induces Tumorigenesis, and Associates with the Activated b-Raf-ERK1/2-mTOR Pathway in Breast Cancer Patients
- Mapping the Hsp90 Genetic Interaction Network in Reveals Environmental Contingency and Rewired Circuitry
- Autoregulation of the Noncoding RNA Gene
- The Human Pancreatic Islet Transcriptome: Expression of Candidate Genes for Type 1 Diabetes and the Impact of Pro-Inflammatory Cytokines
- Spo0A∼P Imposes a Temporal Gate for the Bimodal Expression of Competence in
- Antagonistic Regulation of Apoptosis and Differentiation by the Cut Transcription Factor Represents a Tumor-Suppressing Mechanism in
- A Downstream CpG Island Controls Transcript Initiation and Elongation and the Methylation State of the Imprinted Macro ncRNA Promoter
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- PIF4–Mediated Activation of Expression Integrates Temperature into the Auxin Pathway in Regulating Hypocotyl Growth
- Metabolic Profiling of a Mapping Population Exposes New Insights in the Regulation of Seed Metabolism and Seed, Fruit, and Plant Relations
- A Splice Site Variant in the Bovine Gene Compromises Growth and Regulation of the Inflammatory Response
- Comprehensive Research Synopsis and Systematic Meta-Analyses in Parkinson's Disease Genetics: The PDGene Database
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



