-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaA Downstream CpG Island Controls Transcript Initiation and Elongation and the Methylation State of the Imprinted Macro ncRNA Promoter
A CpG island (CGI) lies at the 5′ end of the Airn macro non-protein-coding (nc) RNA that represses the flanking Igf2r promoter in cis on paternally inherited chromosomes. In addition to being modified on maternally inherited chromosomes by a DNA methylation imprint, the Airn CGI shows two unusual organization features: its position immediately downstream of the Airn promoter and transcription start site and a series of tandem direct repeats (TDRs) occupying its second half. The physical separation of the Airn promoter from the CGI provides a model to investigate if the CGI plays distinct transcriptional and epigenetic roles. We used homologous recombination to generate embryonic stem cells carrying deletions at the endogenous locus of the entire CGI or just the TDRs. The deleted Airn alleles were analyzed by using an ES cell imprinting model that recapitulates the onset of Igf2r imprinted expression in embryonic development or by using knock-out mice. The results show that the CGI is required for efficient Airn initiation and to maintain the unmethylated state of the Airn promoter, which are both necessary for Igf2r repression on the paternal chromosome. The TDRs occupying the second half of the CGI play a minor role in Airn transcriptional elongation or processivity, but are essential for methylation on the maternal Airn promoter that is necessary for Igf2r to be expressed from this chromosome. Together the data indicate the existence of a class of regulatory CGIs in the mammalian genome that act downstream of the promoter and transcription start.
Published in the journal: . PLoS Genet 8(3): e32767. doi:10.1371/journal.pgen.1002540
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002540Summary
A CpG island (CGI) lies at the 5′ end of the Airn macro non-protein-coding (nc) RNA that represses the flanking Igf2r promoter in cis on paternally inherited chromosomes. In addition to being modified on maternally inherited chromosomes by a DNA methylation imprint, the Airn CGI shows two unusual organization features: its position immediately downstream of the Airn promoter and transcription start site and a series of tandem direct repeats (TDRs) occupying its second half. The physical separation of the Airn promoter from the CGI provides a model to investigate if the CGI plays distinct transcriptional and epigenetic roles. We used homologous recombination to generate embryonic stem cells carrying deletions at the endogenous locus of the entire CGI or just the TDRs. The deleted Airn alleles were analyzed by using an ES cell imprinting model that recapitulates the onset of Igf2r imprinted expression in embryonic development or by using knock-out mice. The results show that the CGI is required for efficient Airn initiation and to maintain the unmethylated state of the Airn promoter, which are both necessary for Igf2r repression on the paternal chromosome. The TDRs occupying the second half of the CGI play a minor role in Airn transcriptional elongation or processivity, but are essential for methylation on the maternal Airn promoter that is necessary for Igf2r to be expressed from this chromosome. Together the data indicate the existence of a class of regulatory CGIs in the mammalian genome that act downstream of the promoter and transcription start.
Introduction
Atypical CpG-rich regions known as CpG islands (CGIs) overlap 60–70% of mammalian transcription start sites [1]. Although most CGIs extend downstream of the transcription start and are therefore partly transcribed, they are considered to have promoter regulatory functions and are often described as ‘CGI promoters’. A recent study used a biochemical purification strategy to identify a large number of novel CGIs not associated with annotated promoters, in the body of coding genes or in intergenic regions [2]. While this could indicate the mammalian genome has many transcripts still to be identified, it is also possible that CGIs have additional functions in addition to promoter regulation.
The best examples of CGIs with additional regulatory functions are those that lie inside imprint control elements (ICE, also known as an imprint control region) [3]. An ICE is a genetically defined region whose epigenetic state controls parental-specific expression of small clusters of genes [4]–[6]. CGIs within an ICE are similar to classic promoter-associated CGIs as they show a CpG density higher than the genome average and lack sequence conservation, even between homologous mouse and human elements [7]. However they differ in several ways [8], [9]. First, their CpG density is less than that of classic promoter-associated CGIs. Second, whereas most promoter-associated CGIs are free of DNA methylation [1], CGIs within ICEs gain DNA methylation during gametogenesis but only in one of the two parental gametes. These modified regions are also known as gametic ‘differentially-methylated-regions’ (gDMRs), since once established in a gamete they are maintained in all somatic cells on the same parental chromosome, while the other parental allele remains methylation-free. In six imprinted clusters the ICE has been shown by deletion experiments that include the CGI, to control repression of all imprinted genes [10]–[15]. Thus, the third distinguishing feature is that an unmethylated ICE can act as a cis-acting long-range repressor of multiple flanking genes. This indicates that CGIs residing in an ICE may be a prototype for a class of cis-regulatory CGIs that may differ from classic promoter-associated CGIs.
Remarkably, the silencing ability of the unmethylated ICE correlates with its action as a promoter or cis-activator of a macro non-protein-coding (nc) RNA (provisionally defined as a ncRNA >200 bp whose function does not depend on processing to smaller RNAs) [16], [17]. Three imprinted macro ncRNAs that play a direct role in imprinted gene silencing i.e., Airn, Kcnq1ot1, and Nespas, have their promoter in the ICE [18]–[20]. These cis-repressor macro ncRNAs therefore contain CGI sequences at their 5′ end that could contribute to their repressor function. The Airn, Kcnq1ot1 and Nespas macro ncRNAs are expressed only from the paternal chromosome and induce paternal-specific silencing of flanking protein-coding genes. Imprinted expression of the flanking protein-coding genes arises because these repressor macro ncRNAs are repressed on the maternal chromosome by an ICE gametic methylation imprint [9], [21], [22]. Maternal gametic methylation imprints depend on expression in growing oocytes of the DNMT3A/B de novo methyltransferases and the DNMT3L cofactor [23], [24]. It has also been shown that transcription across the ICE controlling Nespas ncRNA expression is required for methylation in oocytes [25]. In addition, recent high-throughput analyses show a general link between overlapping transcription and CGI methylation in oocytes [26]. However, there is little information on the relative contribution of DNA elements within the ICE for the methylation state. Tandem direct repeats (TDRs) that show organizational but not sequence conservation, are frequently found in or adjacent to the ICE and have been suggested to guide epigenetic modifications [9], [27], [28]. The TDRs are present on both parental chromosomes but methylation of the ICE restricts expression of the macro ncRNA to one parental chromosome. Thus, it is possible that the TDRs play a role in ICE methylation on one parental chromosome and in the repressor function of the macro ncRNA expressed from the other parental chromosome. However, to date various experiments analysing their function either at the endogenous locus or in a transgene context, have not yet identified a general function for TDRs in imprinted clusters [28].
The Airn macro ncRNA promoter that is embedded in the ICE, lies in intron 2 of the Igf2r gene. Airn overlaps and represses the Igf2r promoter (Figure 1A); in extra-embryonic lineages Airn also represses the non-overlapped Slc22a2 and Slc22a3 genes that lie more than 100 kb upstream of the Airn promoter [19], [29]. Airn is an unusually long 118 kb ncRNA that is transcribed by RNA polymerase II (RNAPII). The majority of nascent Airn transcripts are unspliced and nuclear-localized while the minority that are spliced are exported to the cytoplasm [21]. Splicing suppression, unusual length and gene silencing ability are also features shared with the Kcnq1ot1 macro ncRNA [30]. Previously we have established an ES cell imprinting model that recapitulates the onset of imprinted Igf2r expression in early mouse embryonic development [31] (Figure 1A). Undifferentiated ES cells show bi-allelic but low-level Igf2r expression and Airn is not expressed [31]. Airn expression is initiated during ES cell differentiation and induces imprinted Igf2r expression by blocking up-regulation of the overlapped paternal promoter between days 3–5. The Igf2r promoter, which is also associated with a CGI, gains DNA methylation on the paternal allele after the onset of imprinted expression between days 5–14. However, this somatic methylation mark is not required for repression, as Airn silences Igf2r in mouse embryos lacking DNA methylation [21], [22]. We previously used a deletion/replacement approach in this ES cell imprinting model to identify a 959 bp promoter region immediately upstream of the Airn main transcription start [32]. These experiments demonstrated not only that the promoter lies upstream of the annotated CGI (Figure 1B), but also, that the endogenous Airn promoter does not control the unusual features of the macro ncRNA, as Airn driven by the mouse Pgk1 promoter is indistinguishable from Airn driven by its endogenous promoter [32]. Thus control of the unusual biology of the Airn macro ncRNA lies outside its promoter.
Fig. 1. CpG islands lie upstream and downstream of the transcription start site. 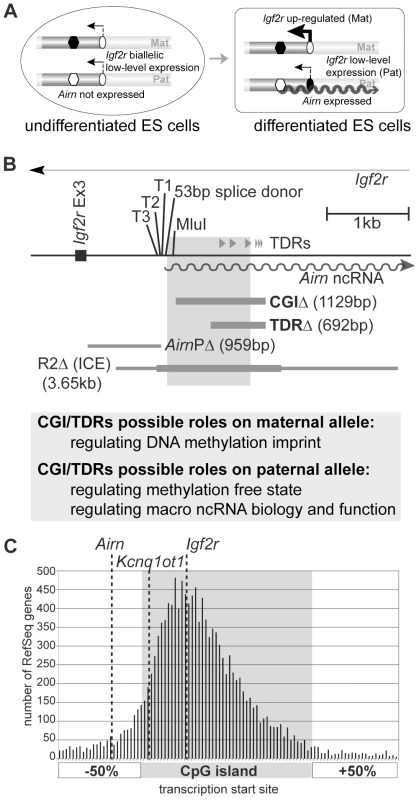
(A) ES cell imprinting model. In undifferentiated ES cells Igf2r is expressed from both chromosomes and Airn is not expressed [31]. The ICE, which contains the Airn promoter, is methylated on the maternal allele (black hexagon) and unmethylated on the paternal allele (white hexagon). The Igf2r promoter is unmethylated on both parental alleles (white oval). During differentiation, Airn (wavy line) is expressed from the unmethylated paternal allele. Igf2r is upregulated up to 20-fold on the maternal allele, but its upregulation on the paternal allele is blocked by Airn expression in cis. The repressed paternal Igf2r promoter gains DNA methylation late in differentiation (black oval). (B) Airn 5′ region. T1,T2,T3: multiple closely-spaced Airn TSSs. 53 bp splice donor: shared by all Airn splice variants. MluI: MluI restriction site. Grey shading: CGI. Grey triangles labelled TDRs: tandem direct repeats. CGIΔ/TDRΔ: 1129/692 bp deletions generated here. AirnPΔ: 959 bp deletion defining Airn promoter [32] that lies upstream of the CGI. R2Δ: 3656 bp deletion defining the imprint control element or ICE [10]. Thicker grey line on R2Δ: maternally methylated region. Possible roles of the CGI and the TDRs on the two parental alleles are listed below. (C) Transcription start sites (TSSs) of mouse RefSeq genes are plotted relative to their CpG island (CGI) and 50% of the CGI length upstream and downstream (see Materials and Methods). Dotted line: TSS of Airn, Kcnq1ot1 and Igf2r. The Airn TSS lies upstream to the CGI in contrast to the majority of TSSs that lie inside of the CGI. Here we use the ES cell imprinting model and also mouse models, to test if the Airn downstream CGI plays a role either on the paternal allele in regulating Airn expression and function and the unmethylated state of the ICE or, on the maternal allele in regulating ICE methylation (Figure 1A, 1B). The Airn downstream CGI contains in its distal half two classes of imperfect TDRs that are each repeated three times, one with a 172–180 bp monomer length and one with a 30–32 bp monomer length (Figure 1B). We used homologous recombination in ES cells to delete a 1129 bp fragment containing the entire CGI and also to delete a 692 bp fragment containing just the TDRs. Both deletions left the Airn promoter and transcription start site (TSS) intact. Analysis of the effects of the deletion on the paternal chromosome that expresses Airn shows that the CGI deletion decreased Airn transcription initiation and strongly reduced transcript elongation, which as predicted from previous analyses [19], led to a loss of its ability to repress Igf2r in cis. The TDR deletion on the paternal chromosome led to a minor defect in transcript processivity that progressively affected the 3′ end of Airn, combined with a minor effect on its repressor function in differentiated ES cells and in mouse tissues. In contrast to the minor role on the paternal chromosome, analysis in mouse embryos of maternal chromosomes carrying the TDR deleted allele shows this element is essential for the DNA methylation imprint. Together these data show the Airn CGI has a dual parental-specific function and is necessary both for Airn biology and function as well as the critical epigenetic modifications that control its imprinted expression.
Results
CGIs can lie up - and downstream of the TSS
We first examined if the position of the Airn CGI that lies downstream of the TSS, represents a rare exception or a common occurrence in the mouse genome (Figure 1C). We asked, for each CGI annotated by the UCSC genome browser (http://genome.ucsc.edu/), if a known protein-coding or non-protein-coding gene taken from the NCBI RNA reference sequences collection (RefSeq), has its TSS within the CGI or in the DNA region representing half the length of the CGI up - or downstream. 57% of all RefSeq genes were associated with a CGI, of which 88.5% including Igf2r, have their TSSs within the body of the CGI (grey shaded area Figure 1C), with the majority lying in the first half. 11.5% of CGI-associated RefSeq genes have their TSS located outside of the annotated CGI, 8.7% of these have their TSS upstream and 2.8% have a TSS downstream of the CGI. Airn represents one of those with their TSS located upstream of the CGI while the Kcnq1ot1 macro ncRNA has its TSS on the 5′ border of the CGI. As the Airn macro ncRNA has its TSS located upstream of the CGI it is possible to distinguish separate functions for the promoter (previously mapped to a 959 bp fragment lying upstream of the Airn-TSS [32]) and the CGI.
Generation of TDR deletion embryonic stem (ES) cells
We first generated a deletion of the TDRs that occupy the second half of the Airn downstream CGI (Figure 1B). We used feeder-dependent D3 ES cells previously modified to contain a single nucleotide polymorphism (SNP) in Igf2r exon 12 that is used to distinguish maternal and paternal Igf2r expression [31]. The SNP modifies the maternal allele, thus this cell line is called S12/+ (note the maternal allele is always written on the left i.e., Mat/Pat). S12/+ cells were used as the wildtype control for all differentiation experiments. We targeted S12/+ cells by homologous recombination to delete a 692 bp region containing all TDRs starting 614 bp downstream of the major Airn-TSS (T1). The selection cassette was inserted 2 kb upstream of the deletion to avoid leaving a loxP site at the deletion, which has been reported to attract DNA methylation [33] and also to minimise potential effects on the Airn promoter region from the transient presence of a selection cassette (Figure S1A). Two independent homologously-targeted clones (named S12/TDRΔ+cas-1 and -2) were verified by Southern blot. The selection cassette was removed by transient transfection with a CRE-recombinase expressing plasmid (Figure S1A, S1B) and cells were subcloned to obtain four cell lines (named S12/TDRΔ-1A/-1B/-2A/-2B). Southern blot analysis showed that all cells were targeted on the paternal allele that carries the unmethylated active ICE and expresses the Airn ncRNA (Figure S1C, S1D). Preferential paternal targeting of the region between the Airn and Igf2r promoters is a feature of this cluster (data not shown). Initial analysis of the deletion was performed in the ES cell imprinting model that we have shown recapitulates the onset of imprinted Igf2r expression in the early embryo [31], [32]. To further validate the ES cell imprinting model and to observe a possible role of the TDRs in ICE methylation on the maternal allele, we deleted the same region in intraspecies 129/B6 A9-ES cells to generate knock-out mice (Figure S2A). The homologously-targeted A9 clone (+/TDRΔ+cas) was injected into blastocysts and the selection cassette removed by mating to a MORE CRE-deleter strain (Figure S2B, S2C) [34].
The TDRs play a role in Airn transcript processivity
It is technically challenging to determine the exact length of macro ncRNAs as they are too long to be resolved on RNA blots. Therefore to test if the TDR deletion changed the length of the Airn transcript, we performed an RNA hybridisation to a genome tiling array. Genome tiling arrays allow the detection of changes in transcript length using approximately 50 bp long probes spaced every 100 bp of single copy genomic DNA. As Airn is only expressed upon differentiation, we differentiated wildtype (S12/+) and two independently derived TDRΔ ES cells (S12/TDRΔ-2A and +/TDRΔ). cDNA prepared from total RNA labelled with one fluorochrome and sonicated genomic DNA labelled with a second fluorochrome, were cohybridized to the tiling array and the relative signal intensity plotted (Figure 2). The displayed 180 kb region contains the overlapping Igf2r and Airn transcripts and can be divided into three parts: the first part is specific to the 3′ end of the Igf2r transcript, the second part is the region of Igf2r/Airn sense/antisense transcriptional overlap and the third part is specific to the 3′ end of the Airn transcript from 28–118 kb. In the ‘Igf2r-specific’ region, comparison of signal intensities does not indicate a difference in Igf2r levels between the wildtype and two TDRΔ ES cell lines, as the overlapping error bars show the technical variation is larger than the biological difference. All three relative signal intensities then show an abrupt increase in the ‘overlap’ region due to the combined signals of Igf2r and Airn. In the ‘Airn-specific’ region, all three signal intensities decline from left to right as previously shown for wildtype Airn [32]. However, in both TDRΔ cell lines compared to wildtype cells, although Airn relative intensity was unchanged from 28–68 kb downstream of the Airn-TSS, it was reduced after 68 kb and absent from 90 kb onwards while wildtype Airn extends 20 kb further (Figure 2 single and double dashed arrows). Thus the TDR deletion on the paternal chromosome has no effect on the first half of Airn but reduces its overall length progressively towards the 3′ end.
Fig. 2. Tandem direct repeats play a role in Airn processivity. 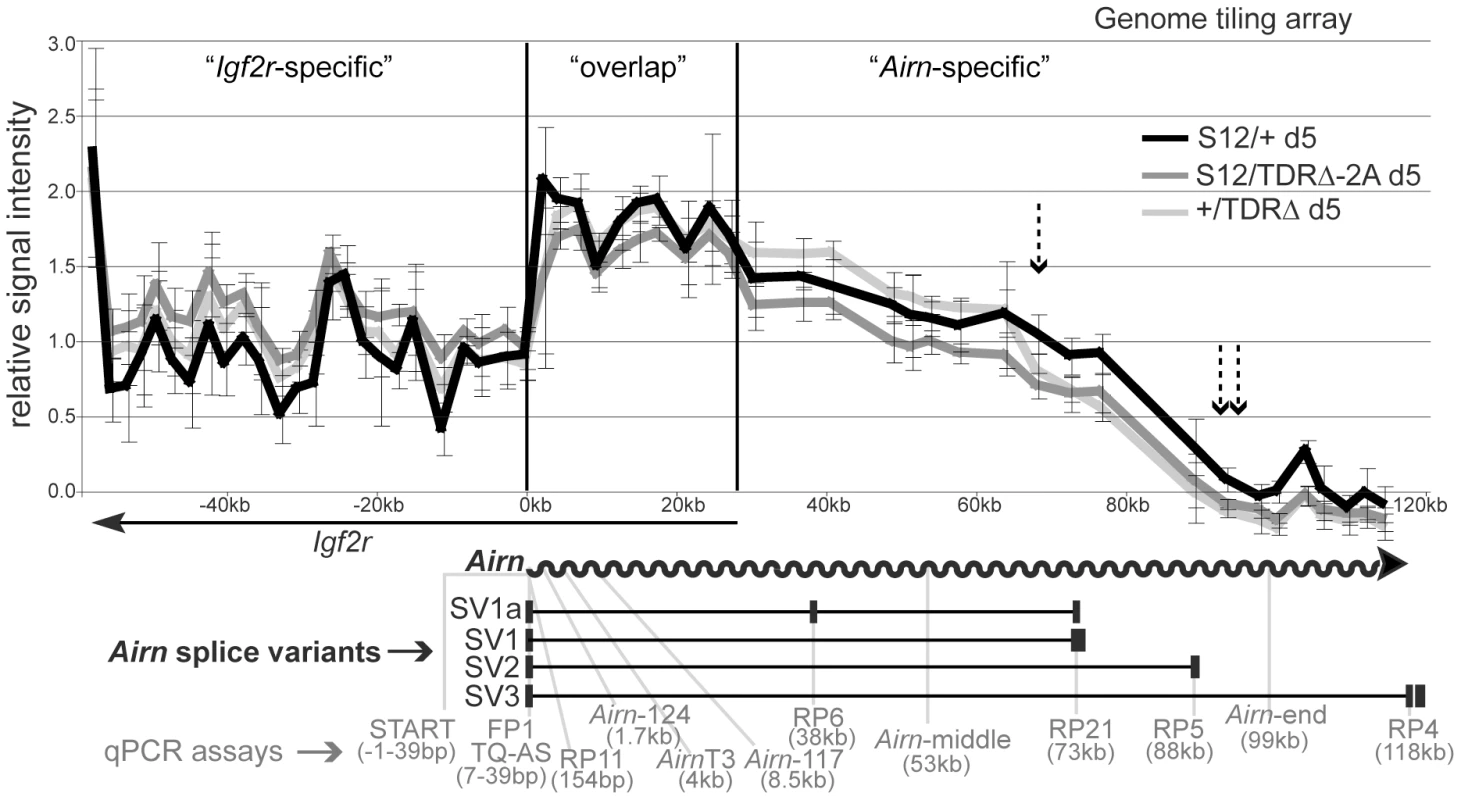
Airn expression by genome tiling array in day 5 differentiated ES cells carrying a paternal wildtype (S12/+) or mutated (S12/TDRΔ-2A and +/TDRΔ) allele. Note the maternal allele is always written on the left side (Mat/Pat). x-axis: basepairs, y-axis: averaged relative signal intensities with standard deviation (see Materials and Methods). Single and double dashed arrows: position after which consistent differences between wildtype and two TDRΔ cell lines are seen. Grey arrow: Airn hybridisation signals are lost after 90 kb in two TDRΔ cell lines. Below: Airn (wavy arrow) and Airn splice variants (black boxes: exons). Grey font: Airn qPCR assays with their distance from Airn-TSS. RP11, RP6, RP21, RP5, RP4 were combined with FP1+TQ-AS. This analysis shows that Airn in TDRΔ cells is reduced after 68 kb and lost after 90 kb. While 95% of Airn transcripts are unspliced, spliced transcripts comprise 23–44% of the steady-state population due to their increased stability [21]. To confirm the shortening of Airn on unspliced and spliced transcripts, we quantified steady-state levels of spliced and unspliced Airn in undifferentiated and differentiated TDRΔ and control ES cells using qPCR assays spread throughout the Airn transcript (Figure 2 map). These qPCR assays allowed us to specifically test if splicing suppression of Airn is affected by the TDR deletion. As previously reported, neither spliced nor unspliced Airn is expressed in undifferentiated (d0) wildtype (S12/+) ES cells but Airn is strongly upregulated during differentiation (d5–d14) [31] (Figure 3A, 3B). Similar kinetic behaviour with some biological variation was found for all four S12/TDRΔ ES cells using four qPCR assays spaced over the first 73 kb of Airn. These were an assay within the first exon of Airn that detects both unspliced and spliced Airn (START, Figure 3A black bars), two assays that detect only unspliced Airn (RP11 at 154 bp and Airn-middle at 53 kb, Figure 3A light and dark grey bars) and, two assays that only detect spliced Airn (RP6 detecting the SV1a splice variant at 38 kb and RP21 detecting the SV1 variant at 73 kb that are the most abundant spliced products, Figure 3B black and light grey bars). At d14, RP5 detecting the SV2 splice variant at 88 kb showed a significant reduction in 2 of 4 TDRΔ clones compared to wildtype (Figure 3B dark grey bars). Two qPCR assays in the 3′ part of Airn showed a significant reduction in all four S12/TDRΔ cells compared to wildtype cells. These were one assay that detects unspliced Airn (Airn-end at 99 kb, Figure 3A white bars) and one assay that detects spliced Airn (RP4 detecting the SV3 splice variant at 118 kb; Figure 3B white bars). Although splice variants that used an exon 2 located after 73 kb were reduced, the TDR deletion did not induce a major shift in spliced versus unspliced Airn transcripts for SV1a and SV1 that end before 73 kb. Thus, the inefficient splicing of Airn is not dependent on sequences in the TDRs. In addition, neither the absence of Airn transcription in undifferentiated ES cells, nor its ability to be upregulated during differentiation depends on TDR sequences.
Fig. 3. Tandem direct repeats regulate the length of Airn. 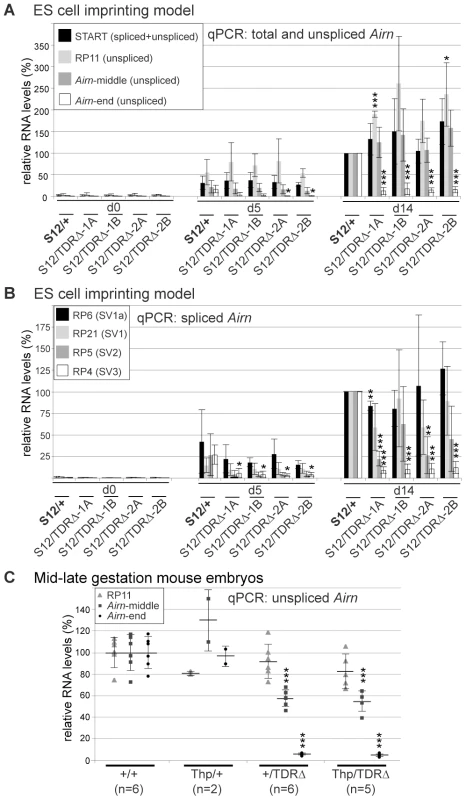
(A) qPCR of total (spliced+unspliced) Airn in S12/+ and four S12/TDRΔ cell lines (1A/1B/2A/2B), in undifferentiated (d0) and day 5 or 14 differentiated ES cells (see Figure 2 map for location of qPCR assays). Relative Airn levels were set to 100% in S12/+ cells at d14. Bars and error bars: mean and standard deviation of three differentiation sets. S12/+ and S12/TDRΔ were compared using an unpaired t-test (*P = 0.1–0.5, **P = 0.001–0.01, ***P<0.001). The data show that Airn steady-state levels are unchanged up to 53 kb but are greatly reduced and lost at the 3′ end. (B) qPCR of spliced Airn in S12/+ and four S12/TDRΔ cell lines (1A/1B/2A/2B), in undifferentiated (d0) and day 5 or 14 differentiated ES cells. Details as in (A). These data show that the TDR deletion does not affect Airn splicing suppression but leads to a shortening at the 3′ end. (C) qPCR of unspliced Airn in 12.5–13.5 dpc mouse embryos confirms the significant loss of Airn steady-state levels at the 3′ end as seen in differentiated ES cells (A,B). Embryos from 3 litters were assayed carrying wildtype (+/+, Thp/+) or TDRΔ (+/TDRΔ, Thp/TDRΔ) paternal alleles. The Thp allele carries a deletion of the entire Igf2r cluster thus only the paternal allele is present. Samples of the same genotype were averaged and the horizontal lines and error bars show mean and standard deviation. Values for individual embryos are plotted as single data points. The number of samples is given below the genotype (n). Relative Airn levels were set to 100% for +/+, all others are displayed relative to it. Samples were compared to +/+ using an unpaired t-test. Details as (A). We also analysed steady-state levels of Airn with or without the TDRs in 12.5–13.5 dpc mouse embryos. We used +/+ and +/TDRΔ embryos and as additional controls, Thp/+ and Thp/TDRΔ embryos. Thp is a hemizygous deletion of the Igf2r cluster allowing the specific analysis of one parental allele [35]. We analysed unspliced Airn with three different qPCR assays at the beginning, middle and end of Airn and found that the mouse data largely recapitulate those from the ES cell imprinting model by showing a progressive length reduction towards the 3′ end of Airn (Figure 3C). The 5′ assay (RP11 at 154 bp, grey triangles) detected similar amounts of Airn from the wildtype and the TDRΔ allele. In contrast to the results obtained from the ES cell imprinting model, one assay in the middle of Airn (Airn-middle at 53 kb, black rectangles) showed a significant reduction to approximately 55% of wildtype levels of Airn from the TDRΔ allele. The end assay, in agreement with the ES cell imprinting model (Airn-end at 99 kb, black circles), detected significant reduction to approximately 5% from the TDRΔ allele compared to the wildtype allele. A similar progressive loss of Airn towards the 3′ end was detected in the extra-embryonic visceral yolk sac (VYS) (Figure S3A). Together, the quantitative analysis of Airn expression supports the conclusion drawn from the genome tiling array, that sequences in the TDR deletion are necessary for full-length Airn.
TDR loss has a minor effect on Igf2r imprinted expression
In addition to the unusual biology that results in a very long unspliced RNA, the other key property of the Airn ncRNA is its cis-silencing ability. We have previously shown that shortening Airn from 118 kb to 3 kb by targeted insertion of a polyA-signal, leads to a loss of repression of the overlapped Igf2r gene and the upstream flanking Slc22a2 and Slc22a3 genes [19]. As TDR deletion led to a 3′ shortening of Airn we first asked if this affected its ability to repress Igf2r. This can be monitored indirectly by the gain of DNA methylation on the paternal Igf2r promoter-associated CGI or, directly by assaying allelic Igf2r expression. We first analysed gain of DNA methylation by methyl-sensitive restriction digestion of genomic DNA from undifferentiated and differentiated wildtype (S12/+) and TDRΔ (S12/TDRΔ) ES cells (Figure 4A). In undifferentiated wildtype ES cells both Igf2r parental alleles are methylation-free as demonstrated by the single 4 kb band. Upon differentiation a 5 kb band indicating a gain of DNA methylation on the paternal allele appears, which increases in strength during differentiation. At d14, quantification from three independent differentiation sets (Figure 4A, Figure S4A) revealed methylated∶unmethylated ratios of 0.75∶1 in wildtype ES cells and a similar ratio in four TDRΔ ES cells. This indicates the gain of DNA methylation on the repressed paternal Igf2r promoter is not dependent on the TDRs.
Fig. 4. TDR absence has a minor effect on paternal Igf2r repression. 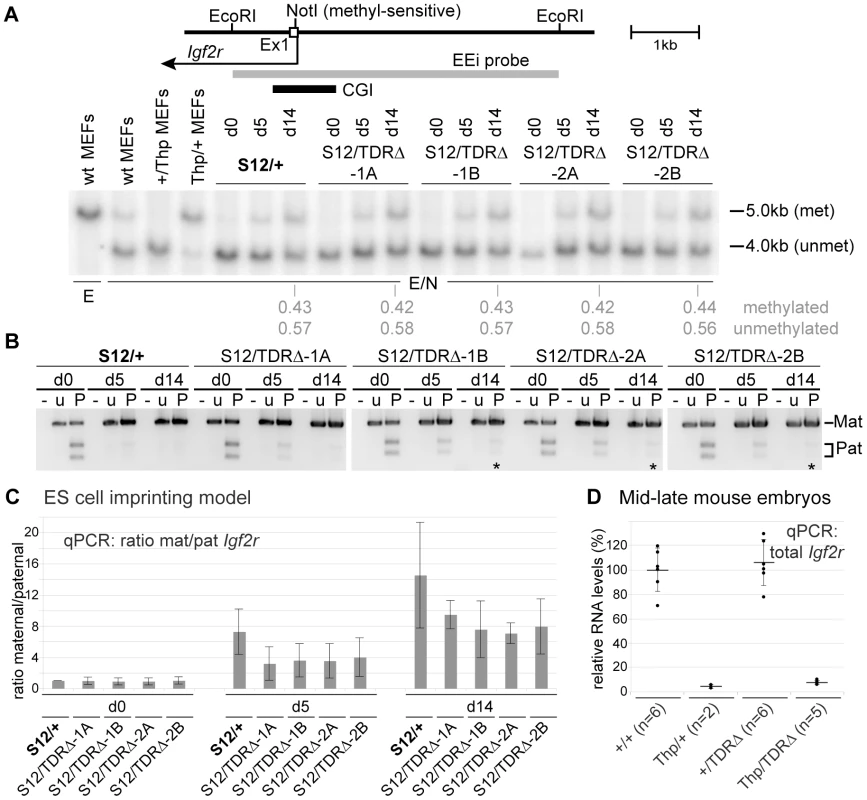
(A) Genomic DNA digested with EcoRI (E) or EcoRI+methyl-sensitive NotI (E/N) hybridised with probe EEi. wt:wildtype, met:methylated, unmet:unmethylated, Thp:deletes the entire Igf2r cluster. Quantification of the methylated/unmethylated hybridisation signal shown below for d14, shows equal gain of DNA methylation on the paternal Igf2r promoter in S12/+ and S12/TDRΔ cells. Figure S4A shows two further differentiation sets. (B) RT-PCR followed by digestion of a paternal-specific PstI site to assay allelic Igf2r expression in ES cells carrying a paternal wild type (S12/+) or mutated (S12/TDRΔ) allele in four targeted clones. Two further differentiation sets are shown in Figure S4B. -: minus RT, u: undigested, P: PstI digested, Mat: maternal, Pat: paternal. Impaired paternal Igf2r repression indicated by the clear presence of two paternal bands at d5 and faint presence at d14 (*) was seen in all four S12/TDRΔ cell lines. (C) Allele-specific qPCR quantifying Igf2r expression using the same SNP as in (B). The mean maternal∶paternal Igf2r expression ratio and standard deviation of three differentiation sets is displayed. As undifferentiated ES cells show biallelic Igf2r expression the ratio was set to 1 in S12/+ d0 cells. Also the S12/TDRΔ cells show biallelic expression in undifferentiated ES cells, as the ratio mat/pat is around 1. During differentiation, the ratio in S12/+ cells increases twofold more compared to S12/TDRΔ cells, indicating a compromised although not statistically significantly impaired imprinted expression of Igf2r in S12/TDRΔ cells. S12/+ and S12/TDRΔ were compared using an unpaired t-test. (D) qPCR of total Igf2r steady-state levels in 12.5–13.5 dpc mouse embryos shows a minor loss of paternal Igf2r repression. Embryos from 3 litters carrying wildtype (+/+, Thp/+) or TDRΔ (+/TDRΔ, Thp/TDRΔ) paternal alleles were assayed and compared using an unpaired t-test. The Thp allele carries a deletion of the entire Igf2r cluster thus only the paternal allele is present. Details as Figure 3C. We next directly analysed Igf2r imprinted expression using the SNP that lies in Igf2r exon 12, 20 kb upstream to the ICE, which can be distinguished by PstI digestion (Figure 4B, Figure S4B). In undifferentiated (d0) wildtype ES cells, PstI digested cDNA results in an undigested maternal (Mat) fragment and two restriction fragments representing the paternal (Pat) allele, indicating biallelic Igf2r expression. As previously described, the paternal-specific fragments are gradually lost during differentiation, which indicates maternally-biased Igf2r imprinted expression [31]. Undifferentiated ES cells with a paternal TDRΔ allele (S12/TDRΔ) also express Igf2r biallelically. However, they differ from wildtype cells by showing reduced paternal Igf2r repression during differentiation, as the two paternal-specific restriction fragments are more visible at d5 and in some cases, remain visible at d14. We quantified this effect on paternal Igf2r repression using a qPCR assay that uses forward primers specific for the two SNP alleles in combination with a common reverse primer. The ratio of the maternal to the paternal allele in undifferentiated wildtype ES cells was set to 1 (Figure 4C), as they were shown previously to express Igf2r biallelically [31]. Wildtype ES cells show a consistent increase in the maternal to paternal Igf2r ratio during differentiation, representing specific upregulation of the maternal allele, with constant low-level paternal expression. Undifferentiated ES cells with a paternal TDRΔ allele (S12/TDRΔ) also show ratios close to 1, indicating biallelic Igf2r expression. S12/TDRΔ cells show an increased maternal∶paternal Igf2r ratio during differentiation, however, this only reached 44–55% at d5 and 49–65% at d14 of the ratio seen in wildtype cells, representing an approximate 2-fold upregulation of the paternal Igf2r allele in TDRΔ cells compared to wildtype (Figure 4C). However, neither the maternal∶paternal Igf2r ratio, nor total Igf2r levels (data not shown) were statistically different in TDRΔ cells compared to wildtype cells. Total Igf2r levels were then analyzed in 12.5–13.5 dpc mouse embryos carrying a paternal TDRΔ (+/TDRΔ, Thp/TDRΔ) or paternal wildtype (+/+, Thp/+) chromosome (Figure 4D). Mean total Igf2r levels in +/+ embryos were set to 100% and +/TDRΔ embryos showed an average of 106%. As the majority of Igf2r transcripts are produced from the maternal wildtype allele that could mask changes on the paternal allele, we analysed embryos carrying a maternal Thp deletion allele that only have the paternal Igf2r allele. The wildtype chromosome in Thp/+ embryos showed 4.5% of levels in +/+ embryos while the TDRΔ chromosome in Thp/TDRΔ embryos showed 7.6% of wild type levels, representing a 1.7-fold upregulation of Igf2r from the paternal TDRΔ allele that was however, not statistically significant. In extra-embryonic tissues, in addition to Igf2r, the Slc22a2 and Slc22a3 genes show Airn-dependent imprinted expression [19]. Analysis of all three genes in VYS shows a similar trend for a modest but not consistently significant loss, of paternal repression upon paternal transmission of the TDRΔ allele (Figure S3B–S3D). Together this indicates a similar trend for a minor loss of imprinted repression of protein-coding genes in both ES cells and mid-late gestation embryos and extra-embryonic tissues, indicating that deletion of the TDRs slightly reduces the repressor efficiency of Airn.
TDRs are required for the regulation of ICE DNA methylation
The Airn CGI is contained within the ICE that carries a gametic DNA methylation imprint on the maternal allele while the paternal allele is free of methylation. This gametic methylation imprint is present in undifferentiated ES cells as they are derived from the inner cell mass of the 3.5 dpc blastocyst [31]. To test if TDR deletion from the paternal allele compromised the methylation-free state of the paternal ICE, we analysed genomic DNA from undifferentiated (d0) and differentiated (d5 and/or d14) S12/+ and S12/TDRΔ ES cells by methyl-sensitive restriction digestion of genomic DNA (Figure 5A). Wildtype (S12/+) undifferentiated and differentiated ES cells both show a 6.2 kb band originating from the methylated maternal allele and a 5.0 kb band from the unmethylated paternal allele. In S12/TDRΔ ES cells, two fragments were similarly present, the 6.2 kb fragment from the wildtype maternal allele and a 4.3 kb fragment from the unmethylated paternal allele that is shortened by the TDR deletion. In addition, a faint but reproducible 5.5 kb band that must originate from a methylated TDRΔ paternal allele (Figure S1) was detected in undifferentiated but not in differentiated S12/TDRΔ ES cells (*Figure 5A). This indicates a transient gain of DNA methylation in undifferentiated S12/TDRΔ ES cells that is lost during differentiation. Figure S5A shows two additional differentiation sets with similar behaviour. To test if low-level DNA methylation on the paternal ICE represents a property of undifferentiated ES cells, rather than a consequence of the TDR deletion, we performed bisulfite sequencing, specifically analysing the paternal allele, in undifferentiated S12/TDRΔ-1A and -2A ES cells and a control ES cell line with the ICE deleted from the maternal allele (R2Δ/+) [36]. Figure 5B, 5C and Figure S5C–S5E show that low-level DNA methylation from 11–14% with extremes ranging from 0–64%, is a general feature of the paternal ICE in undifferentiated ES cells and not a consequence of the TDR deletion. This low-level DNA methylation is however transient as the ICE becomes methylation-free in differentiated ES cells and in differentiated primary embryonic cells (Figure 5A, Figure S5A, S5D, S5E).
Fig. 5. TDRs are required for the regulation of ICE DNA methylation. 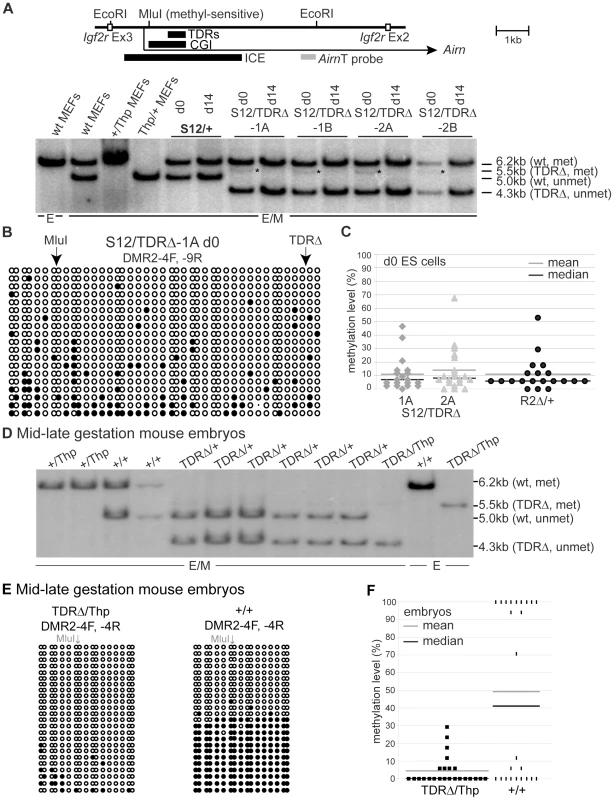
(A) Genomic DNA digested with EcoRI (E) or EcoRI+methyl-sensitive MluI (E/M) and hybridised with probe AirnT. Cells carrying a paternal TDRΔ (S12/TDRΔ) or wildtype (S12/+) allele have a 6.2 kb band from the maternally-methylated plus 4.3 kb or 5.0 kb from the paternally-unmethylated allele. S12/TDRΔ cells additionally show at d0, a faint 5.5 kb band representing a paternal partially methylated allele (*) that is lost by d14 in differentiated cells. (B) Bisulfite sequencing of undifferentiated S12/TDRΔ-1A ES cells. Each row represents one allele, single columns: one CpG, double columns: two neighbouring CpGs. White/black circles: unmethylated/methylated CpGs. Arrows: position of MluI site and TDRΔ deletion. Primers DMR2-4F and -9R specifically amplify the paternal allele in S12/TDRΔ ES cells (details in Figure S5B). Bisulfite sequencing confirms a low level of DNA methylation on the paternal TDRΔ allele at d0 only. (C) Percent methylation levels for two S12/TDRΔ and control R2Δ/+ ES cells. The R2Δ deletion removes the maternal ICE, thus only the wildtype paternal ICE is detected. Each point represents one sequenced clone and shows that low-level DNA methylation is a general feature of the paternal ICE in d0 ES cells and not a consequence of the TDR deletion. (D) DNA blot analysing the MluI methylation status as in (A) for 12.5–13.5 dpc embryos carrying a maternal TDRΔ (TDRΔ/+, TDRΔ/Thp) or wildtype (+/+, +/Thp) allele. The 5.0 kb methylated band is faint or absent in TDRΔ/+ embryos, but present in +/+ embryos (6.2 kb), showing that maternal inheritance of the TDRΔ allele leads to loss of DNA methylation. (E) Bisulfite sequencing of embryonic genomic DNA (details as in B) shows that maternal transmission of the TDR deletion leads to a major loss in DNA methylation. For TDRΔ/Thp only the maternal allele, for +/+ both parental alleles were analysed. (F) Percent methylation level for TDRΔ/Thp and +/+ embryos as in (C). A possible effect of the TDR deletion on the methylated state of the maternal allele cannot be analysed in the ES cell imprinting model, as homologous recombination with the targeting vector replaces the CpG methylated genomic DNA with unmethylated DNA grown in bacteria. We therefore used TDRΔ embryos to analyse DNA methylation of the ICE by methyl-sensitive restriction digestion of genomic DNA. Paternal transmission of the TDRΔ did not affect the maintenance of the unmethylated state, confirming the data obtained from the ES cell imprinting model (data not shown). In contrast, maternal transmission of the TDRΔ led to almost complete loss of the methylated 5.5 kb fragment (detected as 6.2 kb in wildtype mice, Figure 5D). Bisulfite sequencing of mouse embryos carrying a maternal TDRΔ (TDRΔ/+ and TDRΔ/Thp) or a wildtype maternal allele (+/+) confirms that maternal transmission of the TDRΔ allele led to near complete loss of the maternal methylation imprint (Figure 5E, 5F, Figure S5F). The TDRΔ allele showed mean methylation levels of only 4% with extremes ranging from 0–29%. Together these results demonstrate that the TDRs do not play a role in the maintenance of the methylation-free state of the paternal ICE but are essential for methylation on the maternal ICE. Whether the TDRs play a role in the acquisition of the maternal ICE methylation mark in oocytes or its maintenance at later developmental stages was not determined. The loss of maternal ICE methylation in TDRΔ/+ embryos and VYS, resulted in maternal expression of the same progressively shorter Airn transcript with similar ability to repress Igf2r, Slc22a2 and Slc22a3 (Figure S3E–S3J), as shown above for a paternal TDRΔ allele. Neither paternal nor maternal TDR inheritance has an effect on viability or fertility, examining respectively 29 and 65 offspring. In addition TDR homozygotes are obtained in the expected ratio from double heterozygote crosses (i.e., 19 wildtype, 34 heterozygotes and 12 homozygotes were found in 65 offspring). However, although male TDR homozygotes are fertile and produce viable young (16 offspring from 4 litters), female TDR homozygotes show reduced fertility and do not produce viable offspring (10 offspring were obtained from 4 litters but all died within 2 days, indicating a role for Igf2r in the female reproductive tract as noted earlier [37]. We have previously shown that low levels of Igf2r that continue to be expressed from the paternal allele in wildtype embryos (approximately 5%, see Thp/+ in Figure 4D) are not sufficient for viability in the absence of a maternal Igf2r allele [37]. Live born fertile TDRΔ offspring are obtained with Igf2r levels that average 16% (ranging from 11–21%) of wildtype at 12.5–13.5 dpc (see Figure S3F). These crosses contain 129Sv and C57BL/6J genotypes and additional contribution to the survival of TDRΔ/+ mice could come from a mixed genetic background, which was previously shown to influence viability upon loss of maternal Igf2r contribution [37], [38].
Generation of CGI deletion ES cells
The above data shows that the TDRs that lie in the 3′ half of the CGI (Figure 1B), act on the paternal chromosome to control the full-length of Airn and on the maternal chromosome to regulate ICE DNA methylation. To determine if the CGI contains additional elements regulating Airn expression we next removed the entire CGI. The same wildtype parental (S12/+) ES cells were used to delete a 1129 bp fragment, starting 177 bp downstream of the Airn-TSS and ending at the same position as for the TDR deletion (Figure S6A). This deletion left behind 106 bp of the 5′ part of the CGI including the diagnostic MluI site, however the remnant is too small to fit conventional CGI definition criteria [39]. The selection cassette was inserted at the same position as for the TDR deletion to avoid leaving a loxP site at the deletion site. We obtained two homologously-targeted clones that were both targeted on the paternal allele (S12/CGIΔ+cas-1, -2) (Figure S6B, S6C). The selection cassette was removed by transient CRE expression and four ES cell subclones (S12/CGIΔ-1A/-1B/-2A/-2B) were used for analysis in the ES cell imprinting model (Figure 1A, Figure S6B).
The CGI plays a major role in Airn length
To test if the CGI deletion enhanced the shortening of the Airn transcript observed after the TDR deletion, we differentiated S12/+ and S12/CGIΔ-1A ES cells and analysed them by RNA hybridization to genome tiling arrays (Figure 6A). In contrast to the S12/+ cells (and S12/TDRΔ cells in Figure 2), the relative signal intensities from S12/CGIΔ cells did not increase at the transition from the ‘Igf2r-specific’ to the ‘overlap’ region but instead were similar. Since signals in the overlap region are derived from both Igf2r and Airn, this indicated an absence of Airn transcription in this region or a shortening of Airn not resolved on the array. In addition, S12/CGIΔ cells showed a sharp drop in signal intensity at the start of the ‘Airn-specific’ region and signals were not detected after 73 kb downstream of the Airn transcription start (respectively single and double dashed arrows, Figure 6A). This was in contrast to Airn in TDRΔ cells that showed a drop in signal intensity from 68 kb onwards and no signal only after 90 kb (Figure 2). Lastly, higher signal intensities in the Igf2r-specific region in S12/CGIΔ cells compared to wildtype, indicate a gain of bi-allelic Igf2r expression. We also performed strand-specific RNA-Seq and plotted the log2 ratio of the number of reads originating from the forward and reverse strand to obtain an estimate for strand-specific expression in the analysed region (Figure 6A, dotted lines). In S12/+ cells, reads in the ‘Igf2r-specific’ region show specific expression of Igf2r. In the ‘overlap’ region, the ratio then shifts towards the Airn-expressing forward strand, which is even more pronounced in the ‘Airn-specific’ region. In S12/CGIΔ cells, a similar albeit less pronounced shift in the ratio is seen upon transition from the ‘Igf2r-specific’ to the ‘overlap’ region, with a further shift occurring at the transition from the ‘overlap’ to the ‘Airn-specific’ region that is not detected after 73 kb and is reduced compared to S12/+ cells. This confirms in a strand-specific manner, a low level but persistent Airn-expression upon deletion of the CGI. Together, these data indicate that high Airn expression and production of full-length Airn transcripts and as a consequence, the ability to repress Igf2r in cis, are dependent on the CGI.
Fig. 6. The Airn CGI plays a major role in Airn transcription and function. 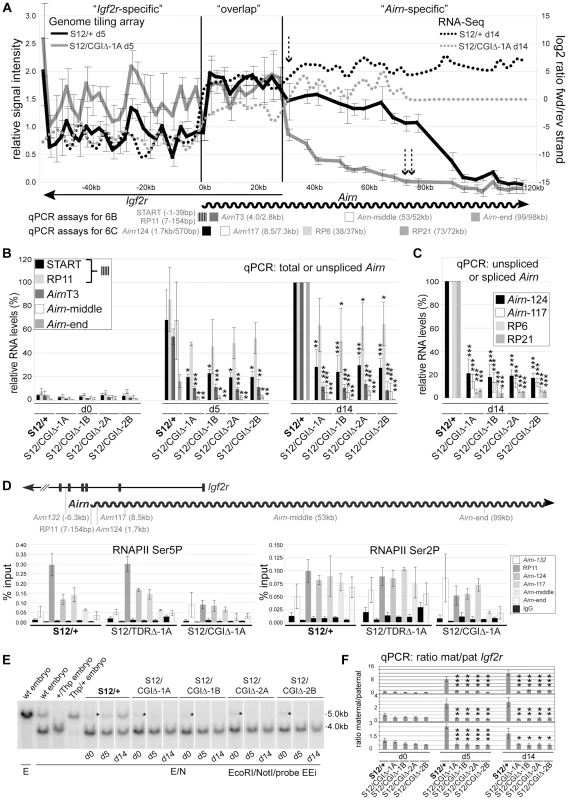
(A) Airn expression by genome-tiling array (left axis) and strand-specific expression analysis by RNA-Seq (right axis) for differentiated S12/+ and S12/CGIΔ-1A cells. Dashed arrows: sharp drop of Airn hybridisation signals in the Airn-specific region (single) and absence after 73 kb (doublet). Below: qPCR assays relative to Airn-TSS with colour code as (B,C). Striped box: overlapping START+RP11 assays. (B) qPCR of total+unspliced Airn in d0/d5/d14 differentiated S12/+ and four S12/CGIΔ clones shows unspliced Airn is reduced by ∼40% at the 5′ end (RP11/154 bp), but when assayed downstream (Airn-middle/53 kb, Airn-end/99 kb) or at positions which include splice variants (START), is reduced by >70% in S12/CGIΔ cells. Shown are mean and standard deviation of three differentiation sets (details as Figure 3A). (C) Airn qPCR in S12/+ and four S12/CGIΔ d14 clones shows that unspliced Airn is reduced by 79–83% at 0.57 kb and ∼85% at 7.3 kb, while spliced Airn reduced by >85%. Shown are mean and standard deviation of three differentiation sets (details as Figure 3A). (D) ChIP for Ser5P/Ser2P RNAPII in S12/+, S12/TDRΔ-1A and S12/CGIΔ-1A d11 cells shows unaffected Airn initiation and elongation (except at Airn-end) in TDRΔ and a sharp RNAPII decrease in the CGIΔ allele. The mean and standard deviation of three technical replicates is shown. Assay Airn-132 controls for background from the overlapping Igf2r transcript, which is 2-fold higher in CGIΔ that fails to repress the paternal Igf2r promoter. Map for qPCR assays as Figure 2. (E) DNA blot analysing methylation of the Igf2r promoter NotI site (see Figure 4A). *methylated fragment in d0 cells originating from feeder-cells. This blot shows that cells carrying a paternal CGIΔ allele contrary to wildtype cells do not gain the methylated 5 kb band on the paternal Igf2r promoter. White lines: indicate the order of samples run on the same gel was changed electronically. (F) qPCR quantifying allelic expression shows absence of Igf2r imprinted expression (Mat∶Pat ratio is close to 1), in four CGIΔ (S12/CGIΔ) cell lines compared to wildtype (S12/+). Three differentiation sets are shown separately due to variability in Mat∶Pat ratios in wildtype controls for each set. Bars represent the mean, error bars the standard deviation of 3 technical replicates (details as Figure 4C). To validate the genome tiling array data we analysed cDNA from undifferentiated and differentiated wildtype (S12/+) and CGIΔ (S12/CGIΔ) ES cells using five qPCR assays spaced along the length of Airn. In undifferentiated ES cells with and without the CGI, Airn expression was mostly absent consistent with the previously observed lack of Airn expression in undifferentiated ES cells [31]. The low level of Airn expression seen in undifferentiated ES cells in Figure 6B represents a small amount of spontaneous differentiation that was similar in wildtype and CGIΔ ES cells. During differentiation Airn was upregulated in wildtype ES cells using all five qPCR assays. In contrast, the CGIΔ ES cells showed consistently less Airn at all analysed positions (Figure 6B). However, the extent of the loss of steady-state levels differed along the length of the transcript. The START assay showed that total unspliced and spliced Airn is reduced to an average of 24–30%. Unspliced Airn detected with RP11 at 154 bp downstream of the TSS showed an average reduction to 50–65%, which was statistically significant in 3 of 4 clones at d14. This difference between total and unspliced Airn is explained by the major loss of the Airn splice variants that require transcription elongation to at least 72 kb (Figure 2), and represent up to 44% of steady-state Airn levels [21]. Unspliced Airn detected with the AirnT3 assay at 2.8 kb from the TSS on the CGIΔ allele showed an average reduction to 8–14% and detection by the Airn-middle assay at 52 kb from the TSS showed reduction to 6–8% (note that distances from the TSS on the CGIΔ allele are reduced by 1129 bp). Finally at Airn-end (98 kb from the Airn-TSS) the reduction was to 0–0.4% of wildtype levels. This indicates that the CGI deletion induced successive loss of Airn with increasing distance from the 5′ end.
To further map the observed shortening of Airn we used two more assays at the 5′ end (Figure 6C). In CGIΔ cells, the Airn-124 assay at 570 bp from the TSS showed an average reduction of Airn steady-state levels to 17–21%, while the Airn-117 assay at 7.3 kb from the TSS showed an average reduction to 13–14%. We also analysed steady-state levels of two Airn splice variants to see if the splicing suppression was altered by the CGI deletion (Figure 6C). The RP6 assay showed the SV1a splice variant is reduced on average to 5–7% of wildtype levels, while the RP21 assay showed the SV1 splice variant is reduced on average to 4–7%. Both these splice variants require transcription elongation to 72 kb (Figure 2). Splicing suppression was therefore not altered after the CGI deletion as the abundance of splice variants decreased in a similar manner as unspliced Airn. Furthermore, the abundance of splice variants from the CGIΔ allele is at most 7% of wildtype levels. As splice variants represent up to 44% of the Airn steady-state population, this indicates that the 24–30% of steady-state levels observed with the START assay (Figure 2B) represent more than 60% of initiating transcripts, confirming the result obtained for the RP11 assay. Together the data shows that Airn full-length elongation is significantly affected by deletion of the CGI with a successive loss of Airn with increasing distance from the 5′ end. However, by analysing RNA steady-state levels by qPCR a more moderate change is seen in transcription initiation such that 50–65% of Airn transcripts elongate at least to 154 bp.
To test if the observed decrease of Airn ncRNA expression was reflected by altered recruitment of RNA polymerase II (RNAPII) we performed chromatin immunoprecipitation using antibodies specifically recognising initiating RNAPII phosphorylated at the Serine 5 (Ser5P) residue of its carboxy-terminal domain (CTD) and elongating RNAPII phosphorylated at Serine 2 (Ser2P) of its CTD. RNAPII occupancy in differentiated S12/+, S12/TDRΔ-1A and S12/CGIΔ-1A ES cells was analysed at five positions along the gene body of Airn as well as in intron 5 of Igf2r to control for overlapping Igf2r transcription (Figure 6D map). Whereas equal amounts of RNAPII Ser5P were found in S12/+ and S12/TDRΔ-1A cells, it was strongly reduced at the Airn 5′ region in S12/CGIΔ-1A cells, indicating reduced Airn transcriptional initiation on the CGIΔ allele (Figure 6D left). For RNAPII Ser2P, S12/+ and S12/TDRΔ-1A showed similar enrichment except for Airn-end, where S12/TDRΔ-1A showed reduced levels. In S12/CGIΔ-1A cells, RNAPII Ser2P levels were increased in intron5 of Igf2r consistent with the increase in Igf2r levels observed in Figure 6A. RNAPII Ser2P levels within the Igf2r/Airn transcriptional overlap were lower compared to the other two cell lines indicating that Airn transcriptional elongation on the CGIΔ allele is strongly reduced (Figure 6D right). An independent RNAPII ChIP experiment showed a similar result (data not shown). Together, the analysis of RNA levels and RNAPII occupancy indicate that the CGI which is localised downstream of the Airn promoter, controls Airn initiation and elongation.
Airn CGI deletion results in biallelic expression of Igf2r
As the majority of Airn transcripts were only between 154–570 bp long (Figure 6C) we tested if Airn produced from the CGIΔ allele was unable to silence Igf2r, as expected from previous experiments that truncated Airn to 3 kb from the TSS [19]. We first analysed the DNA methylation status on the paternal Igf2r promoter-associated CGI, as described in Figure 4A for the TDRΔ. Figure 6E and Figure S7 show that in contrast to wildtype ES cells, all four CGIΔ ES cell lines fail to gain DNA methylation on the paternal Igf2r promoter during differentiation, indicative of biallelic Igf2r expression in these cells. Next, we performed allelic expression analysis of Igf2r using the qPCR assay described above for the TDR deletion in Figure 4C. The results (Figure 6F) show that all differentiated CGIΔ cells displayed an unchanging maternal∶paternal expression ratio during differentiation indicative of biallelic Igf2r expression. This contrasts to wildtype cells that show an increasing ratio of maternal∶paternal Igf2r expression during differentiation indicative of maternally-biased imprinted expression. An absence of Igf2r imprinted expression can also be inferred from the tiling array analysis in Figure 6A where increased Igf2r hybridization signals are seen in the Igf2r-specific region and from the increased RNAPII occupancy in Igf2r intron 5 in Figure 6D. Thus, these results show that Airn transcripts in S12/CGIΔ ES cells, the majority of which had a length of between 154–570 bp are as expected, defective in their ability to silence Igf2r.
Paternal ICE methylation-free state depends on the CGI
Finally, we tested if deletion of the CGI affected the methylation-free state of the Airn promoter region on the normally unmethylated paternal allele. The CGI deletion left behind 106 bp of the 5′ part of the CGI including the diagnostic MluI site analysed for the TDR deletion in Figure 5A. Undifferentiated ES cells with a wildtype paternal allele (S12/+) showed a 6.2 kb maternally methylated and an equally strong 1.1 kb paternally unmethylated band (Figure 7A). Cells with a paternal CGI deletion (S12/CGIΔ) showed a wildtype 6.2 kb maternally-methylated fragment and a 5 kb paternally-methylated band. The size of the paternal fragment is reduced to 1.1 kb when unmethylated. In Figure 7A we also examined CGIΔ cells with (S12/CGIΔ+cas) and without (S12/CGIΔ) the selection cassette, to obtain information from cells that had experienced a short and long culture period since the loss of the CGI. Compared to S12/CGIΔ+cas cells, S12/CGIΔ cells that lack the selection cassette have been an additional 8 passages in culture and show an increased intensity of the 5.0 kb band indicating that DNA methylation increases with passage number. However, in contrast to the TDR deletion shown in Figure 5A, DNA methylation was not lost upon differentiation as indicated by the similar intensity of the 5 kb band in d0 and d14 S12/CGIΔ cells (Figure 7B, S8). Bisulfite sequencing was used to determine the extent of DNA methylation on the CGIΔ allele in undifferentiated ES cells using primers spanning the deletion that specifically amplify the paternal CGIΔ allele (Figure S5B). In two S12/CGIΔ ES cell lines the results show a high level of DNA methylation (Figure 7C left). The % methylation levels were 69–76%, with extremes ranging from 9–100% (Figure 7C right). Taken together, this analysis shows that deletion of the CGI leads to a strong non-reversible gain of DNA methylation in cis, indicating that one major function of the CGI on the paternal allele is to block DNA methylation on the paternal ICE.
Fig. 7. The methylation-free state of the paternal ICE depends on the CGI. 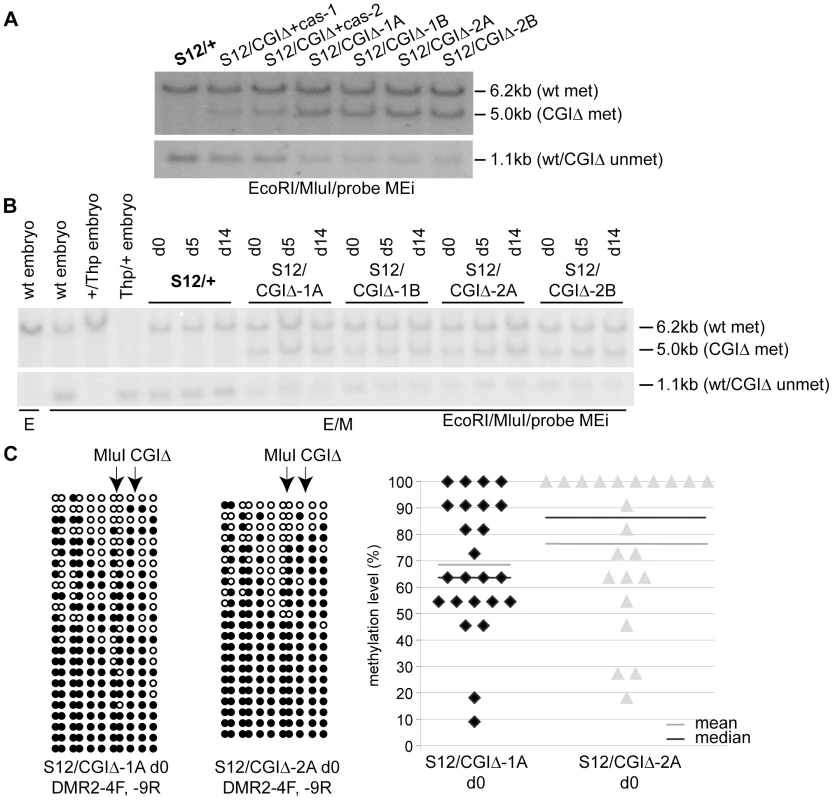
(A) DNA blot assaying methylation of the Airn promoter MluI site as in Figure 5A, in undifferentiated ES cells carrying a paternal CGIΔ or wildtype (+) allele. The 5.0 kb band identified by probe MEi (see Figure 5A map) indicates a gain of methylation on the CGIΔ paternal allele. This band is weaker in cells with lower passage numbers that still retain the selection cassette (S12/CGIΔ+cas-1,-2) compared to cells that have been in culture for 8 more passages (S12/CGIΔ-1A,-1B,-2A,-2B) with a deleted selection cassette. The lower panel confirms this by showing a matching loss of the unmethylated 1.1 kb fragment specific to the paternal allele in cells with a higher passage number. Both panels were from the same blot and the intervening area lacking any hybridisation signal removed. (B) DNA blot as in (A) assaying Airn promoter MluI methylation during ES cell differentiation showing that the level of paternal methylation on the CGIΔ allele in undifferentiated ES (d0) cells (5 kb band) does not change in differentiated d5 and d14 cells. Probe MEi is a 1 kb EcoRI-MluI fragment shown in Figure 5A map. (C) Bisulfite sequencing of two undifferentiated S12/CGIΔ ES cell clones using primers spanning the deletion that specifically amplify the paternal CGIΔ allele, confirms the strong gain of DNA methylation, but also shows that some alleles are more methylated than others (details as Figure 5B). Discussion
This study assessed the possible role played by the Airn CpG island (CGI) and a region of tandem direct repeats (TDRs) on the Airn transcript and the allelic methylation state of the ICE that controls Airn expression. Since these elements lie immediately downstream of the Airn transcription start and thus are present on the Airn transcript, they may also play a role in Airn biology. Our results using two targeted deletions analyzed in an ES cell imprinting model and in knockout mice, show that the CGI regulates both initiation and elongation efficiency of the Airn promoter and is also necessary to maintain the unmethylated state of the paternal ICE. This indicates the existence of a new transcriptional role for CGIs in the mammalian genome acting downstream of the promoter and transcription start site. In contrast, the TDRs that occupy the second half of the CGI play a minor role in Airn transcriptional processivity but are essential for methylation of the maternal ICE that represses the Airn promoter on this chromosome.
The Airn downstream CGI controls its length and thereby its silencing ability
The 1129 bp deletion of the complete Airn downstream CGI had a moderate effect on the most 5′ RNA levels such that two qPCR assays within the first 154 bp downstream of the Airn-TSS detected approximately 50–65% of wildtype levels of the normally 118 kb long Airn ncRNA. Airn transcripts were reduced to ∼14% between 1.7–8.5 kb and to ∼6% between 53–73 kb, while no transcripts were detected at 99 kb. In addition, both initiating and elongating forms of RNAPII were reduced compared to control cells. In contrast the TDR deletion had a minor effect on the length of Airn with transcripts at normal levels for the first two-thirds, but progressively reduced after 68 kb and absent at 99 kb, with the elongating form of RNAPII reduced at the 3′ end of Airn. This indicates that the efficiency of RNAPII to elongate Airn over 118 kb is regulated by the CGI and at least in part, also by the TDRs. Notably, in view of the splicing suppression of wildtype Airn that results in splicing of only 5% of transcripts [21], the production of all four splice variants was decreased in proportion to the unspliced transcripts indicating that neither the CGI nor TDRs cause splicing suppression.
We have previously shown that Airn must be longer than 3 kb and be expressed from a strong promoter, to induce silencing of the overlapped Igf2r promoter [19], [32]. A loss of paternal Igf2r repression after the CGI deletion that shortened the majority of Airn transcripts to less than 0.5% of its normal length is therefore expected, and this deletion was only analysed in the ES cell imprinting model. The TDR deletion although producing normal levels of the Airn transcript that overlap the 28 kb distant Igf2r promoter and elongate up to 90 kb from the Airn-TSS, nevertheless showed a minor loss of paternal Igf2r repression. This was seen as a 1.7–2.0 fold upregulation of the paternal Igf2r allele in differentiated ES cells and in mid-late gestation embryos and VYS, which was not statistically significantly different from paternal repression on wildtype chromosomes. A similar minor increase in paternal steady-state levels was observed for Slc22a2 and Slc22a3 in the VYS. We lack an explanation for this minor effect. It appears not to arise from changed developmental kinetics of Airn expression that were similar in wildtype and TDRΔ differentiating ES cells, but it may reflect changes in RNAPII post-translational modification not detected with current antibodies. Currently it is unknown if the Airn ncRNA or the act of its transcription induce imprinted expression of Igf2r [16], [40]. For the Slc22a3 gene that lies 275 kb downstream of Igf2r and is repressed by Airn only in placenta, Airn was shown to localize to the Slc22a3 promoter and to induce imprinted expression by interacting with the G9A histone methyltransferase. However, imprinted expression of Igf2r was not affected in these studies [41] or in studies eliminating PRC2 activity [42]. The results obtained here do not distinguish between a role for the Airn ncRNA or its transcription, but are in agreement with previous analyses that demonstrated a role for high Airn expression and a length longer than 3 kb to repress Igf2r in cis [21], [32].
The Xist macro ncRNA that induces whole chromosome silencing in female XX mammals has been suggested to share similarities with imprinted repressor ncRNAs such as Airn and Kcnq1ot1 [43], [44]. Notably Xist contains a set of 5′ direct ‘A’ repeats that are essential for Xist to induce chromosome silencing [45]. The Airn TDRs may have served a similar purpose. Here we show that the TDRs are not required for Airn to repress its target genes as despite the minor loss of paternal repression, imprinted expression is present in TDRΔ cells and mice. However, since the TDRs are required for maternal ICE methylation, they are necessary to ensure expression of the maternal Igf2r allele as it has been previously shown that mouse embryos lacking maintenance DNA methylation, repress both parental Igf2r alleles [22]. The imprinted Kcnq1ot1 macro ncRNA shares many features with the Airn ncRNA and its TSS lies on the 5′ border of the CGI (Figure 1C), which contains a series of TDRs that lack sequence conservation with those in Airn [30], [46]. Two overlapping deletions have been used to test the function of this region in the Kcnq1ot1 ncRNA. The first is a 657 bp deletion starting just downstream of the Kcnq1ot1-TSS [18], while the second deletion removed 890 bp and overlapped 40% of the 657 bp deletion [47]. The ability of the deleted Kcnq1ot1 ncRNA to repress flanking genes on the paternal chromosome was found to be unchanged in midgestation embryos for the 657 bp deletion. However, the 890 bp deleted Kcnq1ot1 allele showed a failure to repress some genes in this cluster in a lineage-specific manner that correlated with failure to gain DNA methylation on the derepressed genes. Although the failure to repress flanking genes was attributed to a failure in recruiting DNMT1 due to the lack of the 890 bp region in the ncRNA [47], both Kcnq1ot1 and Airn are able to repress genes in mouse embryos lacking the Dnmt1 gene that are deficient in genomic methylation [29], [48]. The TDR deletion described here resulted in loss of the 3′ part of Airn and a minor loss of paternal repression of protein-coding genes with the paternal Igf2r promoter showing a normal gain of DNA methylation (the Slc22a2 and Slc22a3 genes are repressed in the absence of promoter methylation [49]). A direct comparison between the two imprinted clusters is not possible since although Kcnq1ot1 steady-state levels were unchanged in both the deletion experiments [18], [47], measurements were only made in the first half of the transcript and it is not known if these deletions affected the full-length of Kcnq1ot1.
The Airn downstream CGI controls efficient transcription initiation and elongation
Classic mammalian promoter-associated CGIs extend upstream and downstream of the transcription start of the majority of mouse and human genes and these CGIs are considered to have promoter regulatory functions [1]. The promoter region of a CGI is perceived as the region between the 5′ boundary of the CGI and the TSS [50], although none have been subject to deletion at the endogenous locus and analyzed as described here. Recently, evidence has been accumulating that gene regulation acts not only at the step of RNAPII recruitment by the promoter, but also at later steps of transcription elongation and processing [51]–[53]. The data here show that elements located downstream of the transcription start site are required for RNAPII transcription initiation and elongation and also indicate that CGIs can play a different role to that of the upstream promoter.
Reduced Airn transcript length could be explained by alternative polyadenylation site choice that is often seen in mammalian genes [54]. The Airn ncRNA produces four splice variants, three of which have alternative polyadenylation sites spread over 45 kb (Figure 2) [21]. Although premature polyadenylation could explain progressive Airn shortening in TDRΔ and CGIΔ alleles, we think this unlikely for two reasons. First, the genome tiling array analysis shows Airn shortening is gradual and not stepwise, which would be expected from use of alternative polyadenylation sites. Second, the RT-qPCR data indicate that Airn shortening on CGIΔ alleles occurs within the first 570 bp, which does not contain a known polyadenylation site (http://rulai.cshl.edu/tools/polyadq/polyadq_form.html). Cells with a paternal TDRΔ allele showed similar occupancy of the initiating and elongating forms of RNAPII to wildtype cells, except for the 3′ end of Airn where elongating RNAPII was reduced. As Airn transcription initiation is unchanged in TDRΔ cells with the majority of transcripts longer than 68 kb, this indicates the length of Airn is subject to regulation after the switch between paused and elongated transcription. In cells with a CGIΔ allele however, both initiating and elongating RNAPII were decreased compared to wildtype and TDRΔ cells, although ∼60% of wildtype RNA levels were found at the very 5′ end. This indicates that the deletion of the whole CGI affected the ability of the upstream promoter region not only to elongate but also to efficiently initiate Airn transcription. The finding that both the TDR and CGI deletions induced progressive Airn shortening indicates that cumulative elements distributed throughout the CGI play distinct roles in regulating Airn transcription elongation and processivity.
An obvious feature involved in regulating expression of a CGI associated gene is DNA methylation. Gain of methylation was not seen on the paternal TDRΔ allele, but up to 70% of DNA methylation was gained on the flanking sequences after paternal CGI deletion. However, Airn 5′ levels only showed a moderate change on the CGIΔ allele. As methylation levels showed a high variability between different alleles, ranging from 9–100%, it could be possible that hypomethylated alleles are still able to initiate Airn transcription as detected by RT-qPCR which specifically analyses Airn transcripts, but not by the RNAPII ChIP which might be relatively less sensitive and also suffers from background problems due to increased Igf2r levels in the overlap region. We therefore suggest that this gain of methylation and not loss of the CGI, explains the reduction in Airn transcription initiation shown by the CGIΔ allele. Since most CpG dinucleotides including those in the body of genes, are methylated when they lie outside CGIs [55], it is clear that DNA methylation downstream of promoters does not block transcript elongation of endogenous mammalian genes. Furthermore, as no increase in DNA methylation was observed upon TDR deletion on the paternal allele that also induced shortening of Airn, we can exclude DNA methylation as the cause of the length phenotype. Thus, loss of sequences within the CGI and not gain of DNA methylation correlate with loss of full-length Airn.
The Airn CGI is required to block DNA methylation on its paternal promoter
Deletion of the TDRs removed the 3′ half of the CGI from the paternal ICE but did not change its unmethylated status. Notably, controls used in these experiments allowed us to observe for the first time, a low level of DNA methylation on the wildtype paternal ICE in two different undifferentiated ES cell lines, that was fully reversible upon differentiation and was also absent in differentiated primary embryonic fibroblasts. Although Airn is not expressed in undifferentiated ES cells, the paternal ICE is marked by H3K4me3 [31], [56], which has been shown to block DNMT3L, an essential cofactor for the de novo methylation complex, from binding histone H3 [57]. The existence of low-level DNA methylation at the Airn promoter in undifferentiated cells despite the presence of H3K4me3 indicates either, that high Airn expression induced during differentiation is required in addition to H3K4me3 to fully block DNA methylation or, that DNA methylation modifies a small number of chromosomes in the population that lack H3K4me3 [58]. Deletion of the whole CGI led to a substantial gain of DNA methylation on the paternal allele that was not reversible upon differentiation but was enhanced after removal of the selection cassette. We attribute this enhancement to the longer period in cell culture required to remove the cassette. Thus the CGI deletion shows that one role located in the first half of the island, is to block DNA methylation on the paternal Airn promoter that is 177 bp upstream from the deleted sequences. Transgene reporter experiments have been used to show that SP1 transcription factor binding sites protect a CGI from DNA methylation [59]–[62]. Furthermore a high CpG density also correlates with protection from DNA methylation by recruitment of the CpG-binding protein CFP1, which in turn leads to H3K4me3 via recruitment of the SETD1 histone methyltransferase [58]. As the CGI deletion reduces CpG density considerably and removes three predicted SP1 binding sites [21], this may explain the gain of DNA methylation upon deletion of the CGI.
The 3′ part of the CGI is necessary for the maternally methylated state of the ICE
Deletion of the 3′ half of the CGI that included the TDRs, led to loss of ICE methylation following maternal transmission of the deleted allele. The Airn-TDRs are conserved in human and mouse at an organizational level and in their ability to be methylated on the maternal chromosome only [27], [63]. The conservation of TDRs in the ICE may be explained by the preference of the DNMT3A de novo methyltransferase for an 8–10 bp periodicity in CpG frequency, that is seen in the 12 known maternally-methylated ICE [64]. Previous experiments using multicopy transgenes randomly inserted in the genome have also identified the Airn-TDRs, in particular the three long 172–180 bp monomer repeats, as important for maternal-specific methylation of a hybrid RSVIgmyc imprinted transgene [65]. These experiments also demonstrated a role for the TDRs in maintaining the unmethylated state on paternal transmission. The data reported here that deleted the TDRs from the endogenous Airn CGI, confirm a role for the TDRs in the methylation of the maternal ICE, but do not demonstrate a role in maintaining the unmethylated state of the paternal ICE. The two overlapping 657 and 890 bp deletions cited above for the Kcnq1ot1 downstream CGI [18], [47], were not directly tested for their role in the methylation of the maternal ICE. Indirect evidence that indicates no role for these deleted regions comes from the finding that the maternal transmission of the 890 bp deletion did not lead to derepression of Kcnq1ot1. Together this would indicate that the Airn-TDRs but not the Kcnq1ot1 TDRs, have a function in methylation of the maternal ICE. However two minor caveats could be considered. First, the two overlapping deletions reported from the Kcnq1ot1 downstream CGI might not have removed all necessary sequences and second, these two overlapping deletions left a single loxP site at the site of the deletion, which has been reported to attract DNA methylation [33]. In contrast, the Airn-TDR deletions reported here placed the remaining single loxP site 2 kb upstream from the deletion and we are now able to assign a specific role for the Airn-TDRs in the methylation of the maternal ICE at the endogenous locus.
Together the data presented here show that the CGI lying immediately downstream of the Airn transcription start regulates both the epigenetic and transcription state of its upstream promoter. Classically, with the exception of retrotransposons, RNA polymerase II promoters are viewed as lying upstream of the transcription start [66], [67]. In contrast, the majority of CGIs can be seen in Figure 1C to extend downstream of the transcription start, with some located entirely downstream of the transcription start. The importance of CGIs as regulators of gene expression has been emphasised with the advent of genome-wide studies showing CGIs are not only associated with genes showing tissue-specific and inducible expression but are also present in large numbers as orphan CGIs not associated with annotated promoters [1], [2], [68]. The data here identify a role for the downstream Airn CGI to regulate its epigenetic state and the production of transcripts expressed at sufficiently high levels and of sufficient length to silence flanking target genes. Future work will determine how this regulation is achieved and if these features are shared by CGIs regulating non-imprinted gene expression.
Materials and Methods
Ethics statement
Mice were bred and housed at the Forschungsinstitut für Molekulare Pathologie GmbH, Dr. Bohr-Gasse 7, 1030 Vienna, Austria in strict accordance with national recommendations described in the “IMP/IMBA Common Institutional policy concerning the care and use of live animals” with the permission of the national authorities under Laboratory Animal Facility Permit MA58-0375/2007/4. Blastocyst injections and chimeric mice were prepared under the permit M58/003079/2009/8: Production of Chimeras, Examination of Germline, Examination of Gene Effects in Parents and Successor Generations (Model B). Mouse embryos were obtained after humane killing of pregnant female mice by cervical dislocation by skilled qualified personnel.
Generation of knock-out ES cells and mice
All targeting vectors were generated from a plasmid with a 6.4 kb 129Sv homology region (chr17 : 12931344–12937792/NCBI37-mm9). In the TDRΔ construct a 692 bp SacII-NsiI fragment (chr17 : 12934848–12935543) was deleted. The selection cassette (loxP)-(HSVTk-Neomyocin-SV40polyA)-(HSVTk-ThymidineKinase+polyA)-(loxP) for the ES cell imprinting model and (loxP)-(Pgk1-Neomycin-Pgk1polyA)-(loxP) for blastocyst injection was subcloned into the NheI site at chr17 : 12932836. In the CGIΔ construct the 1129 bp deletion (chr17 : 12934414–12935543) was created by PCR (primers: TGGAACCCTTCCTTTGCGGAATC - TGCATGAGGGTGCCACACTCCT). The selection cassette: (loxP)-(Pgk1-Neomycin-Pgk1polyA)-(loxP) was inserted at the same position as for TDRΔ. Electroporation and neomycin-selection were performed using standard conditions into S12/+ cells (a D3 feeder-dependent 129 ES line previously modified to carry a SNP in Igf2r exon 12 [31]) for the ES cell imprinting model experiments and into the feeder-dependent BL6/129 intraspecies A9 ES cell line for blastocyst injection. The selection cassette in the ES cells used for the ES cell imprinting model was removed by electroporation of the pMC-Cre plasmid leaving a single loxP site 2 kb upstream of each deletion. One A9 ES cell clone carrying the TDRΔ+cas allele was injected into C57BL/6J blastocysts and transferred into pseudo-pregnant recipient mice and one chimeric male mouse was obtained who transmitted the TDRΔ+cas allele. The selection cassette was removed by crossing TDRΔ+cas males with MORE-Cre females. Heterozygous TDRΔ mice were mated with wildtype FVB or FVB with a Thp allele and embryos were isolated at 12.5 dpc or 13.5 dpc. Visceral yolk sacs were isolated as described in [29].
ES cell culture and differentiation
ES cells were grown on irradiated primary mouse embryonic fibroblasts using standard conditions and differentiation induced by feeder-depletion, LIF-withdrawal and 0.27 µM retinoic acid.
RNA and DNA analysis
RNA was isolated using TRIreagent and was DNaseI treated prior to reverse transcription. Realtime qPCR for Taqman assays was as described [21]. SybrGreen assays used 100 nM primers and cycling conditions: 5 min 95°C, 40 cycles: 15 sec 95°C+1 min 60–65°C. Allele-specific qPCR was as described [31] with 5 mM MgCl2. The assay specificity was improved by a mismatch in the primer body. See for primers and probes. All assays were normalised to CyclophilinA. DNA isolation and blots were performed using standard techniques and ImageJ quantified signal intensities. For some blots the contrast was linearly enhanced with Adobe Photoshop. RNA hybridization to genome tiling array was performed by Source BioScience LifeSciences, Berlin, as described [69]. The data were Tukey bi-weight normalised before analysis. Relative signal intensities (normalised to the average signal in the region) of overlapping windows of 9 tiles were averaged and each displayed data point is the average of 20 windows, the standard deviation is displayed as error bars. Two pseudogenes in the region, Au76 and LA41, were removed from the analysis. RNA sequencing:1 µg of total RNA was treated with the RiboZero kit (Epicentre) and two strand-specific RNA-Seq libraries prepared using the ScripSeq kit and two compatible barcodes (Epicentre) according to the manufacturer's protocol. Sequencing and read alignment to the mouse genome (mm9) was as described [70]. The region shown in Figure 6A was divided into non-overlapping 3.2 kb windows, reads mapping to the forward or reverse strand in these windows were counted and the log2 ratio of these counts was calculated and plotted. Windows overlapping Igf2r exons and the Au76 and the LA41 pseudogenes were removed from the plot.
Chromatin immunoprecipitation
Preparation of soluble chromatin and chromatin immunoprecipitation assays were carried out as described [71]. 25 µg of sonicated chromatin were diluted 10-fold and precipitated overnight with the following antibodies: Phospho RNA Pol II (S5) (Bethyl Laboratories A300-655A), Phospho RNA Pol II (S2) (Bethyl Laboratories A300-654A) or rabbit IgG (Invitrogen 10500C) as control. Chromatin antibody complexes were isolated using Protein A magnetic beads (Dynabeads). The extracted DNA was then used for qPCR as described above. A 1∶20 dilution of input DNA was assayed.
Bisulfite sequencing
1 µg genomic DNA from undifferentiated ES cells grown on feeder cells carrying a homozygous ICE deletion or 1 µg genomic DNA from 12.5–13.5 dpc embryos was RNaseA treated, EcoRI digested and Bisulfite converted using the EpiTect Bisulfite Kit (Qiagen). PCR amplification used JumpStart Taq DNA Polymerase (Sigma), primers: DMR2-F4 (GGGGAATTGAGGTAAGTTAGGGTTTT) with DMR2-R4 (TCTTATAACCCAAAAATCTTCACCCTAAC) for wt alleles or DMR2-R9 (AACACCTTCATATACCCCTAAACAC) for TDRΔ and CGIΔ alleles [8], cycle conditions: 94°C 1 min, 40 cycles of 94°C 1 min, 60°C 1 min, 72°C 1 min then 72°C 5 min. PCR fragments were gel-purified, subcloned and plasmid DNA from single colonies sequenced using standard primers. Analysis and sequence quality control used BiQAnalyzer and standard settings [72].
CpG island and transcription start site analysis
Each CGI (UCSC Genome Browser, mm9) with flanking regions (50% of the CGI length upstream and downstream) was divided into 100 equal-sized bins (i.e., parts) and the number of RefSeq genes (USCS mm9) was calculated with a transcription start site in each bin. Bins were summed for all CGIs and plotted using Microsoft Excel.
Statistical analysis
For qPCRs an unpaired t-test was performed using www.graphpad.com/quickcalcs/.
Supporting Information
Zdroje
1. DeatonAMBirdA 2011 CpG islands and the regulation of transcription. Genes & development 25 1010 1022
2. IllingworthRSGruenewald-SchneiderUWebbSKerrARJamesKD 2010 Orphan CpG islands identify numerous conserved promoters in the mammalian genome. PLoS Genet 6 e1001134 doi:10.1371/journal.pgen.1001134
3. SpahnLBarlowDP 2003 An ICE pattern crystallizes. Nat Genet 35 11 12
4. WanLBBartolomeiMS 2008 Regulation of imprinting in clusters: noncoding RNAs versus insulators. Adv Genet 61 207 223
5. ThorvaldsenJLBartolomeiMS 2007 SnapShot: imprinted gene clusters. Cell 130 958
6. Ferguson-SmithAC 2011 Genomic imprinting: the emergence of an epigenetic paradigm. Nature reviews Genetics 12 565 575
7. AntequeraF 2003 Structure, function and evolution of CpG island promoters. Cell Mol Life Sci 60 1647 1658
8. KobayashiHSudaCAbeTKoharaYIkemuraT 2006 Bisulfite sequencing and dinucleotide content analysis of 15 imprinted mouse differentially methylated regions (DMRs): paternally methylated DMRs contain less CpGs than maternally methylated DMRs. Cytogenet Genome Res 113 130 137
9. TomizawaSKobayashiHWatanabeTAndrewsSHataK 2011 Dynamic stage-specific changes in imprinted differentially methylated regions during early mammalian development and prevalence of non-CpG methylation in oocytes. Development 138 811 820
10. WutzASmrzkaOWSchweiferNSchellanderKWagnerEF 1997 Imprinted expression of the Igf2r gene depends on an intronic CpG island. Nature 389 745 749
11. WilliamsonCMTurnerMDBallSTNottinghamWTGlenisterP 2006 Identification of an imprinting control region affecting the expression of all transcripts in the Gnas cluster. Nat Genet 38 350 355
12. ThorvaldsenJLDuranKLBartolomeiMS 1998 Deletion of the H19 differentially methylated domain results in loss of imprinted expression of H19 and Igf2. Genes Dev 12 3693 3702
13. LinSPYoungsonNTakadaSSeitzHReikW 2003 Asymmetric regulation of imprinting on the maternal and paternal chromosomes at the Dlk1-Gtl2 imprinted cluster on mouse chromosome 12. Nat Genet 35 97 102
14. FitzpatrickGVSolowayPDHigginsMJ 2002 Regional loss of imprinting and growth deficiency in mice with a targeted deletion of KvDMR1. Nat Genet 9 9
15. BielinskaBBlaydesSMBuitingKYangTKrajewska-WalasekM 2000 De novo deletions of SNRPN exon 1 in early human and mouse embryos result in a paternal to maternal imprint switch. Nat Genet 25 74 78
16. KoernerMVPaulerFMHuangRBarlowDP 2009 The function of non-coding RNAs in genomic imprinting. Development 136 1771 1783
17. SantoroFBarlowDP 2011 Developmental control of imprinted expression by macro non-coding RNAs. Seminars in cell & developmental biology
18. Mancini-DinardoDSteeleSJLevorseJMIngramRSTilghmanSM 2006 Elongation of the Kcnq1ot1 transcript is required for genomic imprinting of neighboring genes. Genes Dev 20 1268 1282
19. SleutelsFZwartRBarlowDP 2002 The non-coding Air RNA is required for silencing autosomal imprinted genes. Nature 415 810 813
20. WilliamsonCMBallSTDawsonCMehtaSBeecheyCV 2011 Uncoupling antisense-mediated silencing and DNA methylation in the imprinted Gnas cluster. PLoS Genet 7 e1001347 doi:10.1371/journal.pgen.1001347
21. SeidlCIStrickerSHBarlowDP 2006 The imprinted Air ncRNA is an atypical RNAPII transcript that evades splicing and escapes nuclear export. Embo J 25 3565 3575
22. LiEBeardCJaenischR 1993 Role for DNA methylation in genomic imprinting. Nature 366 362 365
23. OoiSKO'DonnellAHBestorTH 2009 Mammalian cytosine methylation at a glance. J Cell Sci 122 2787 2791
24. LiYSasakiH 2011 Genomic imprinting in mammals: its life cycle, molecular mechanisms and reprogramming. Cell Res
25. ChotaliaMSmallwoodSARufNDawsonCLuciferoD 2009 Transcription is required for establishment of germline methylation marks at imprinted genes. Genes Dev 23 105 117
26. SmallwoodSATomizawaSIKruegerFRufNCarliN 2011 Dynamic CpG island methylation landscape in oocytes and preimplantation embryos. Nature genetics
27. NeumannBKubickaPBarlowDP 1995 Characteristics of imprinted genes. Nat Genet 9 12 13
28. WalterJHutterBKhareTPaulsenM 2006 Repetitive elements in imprinted genes. Cytogenet Genome Res 113 109 115
29. HudsonQJSeidlCIKulinskiTMHuangRWarczokKE 2011 Extra-embryonic-specific imprinted expression is restricted to defined lineages in the post-implantation embryo. Developmental biology 353 420 431
30. PandeyRRMondalTMohammadFEnrothSRedrupL 2008 Kcnq1ot1 antisense noncoding RNA mediates lineage-specific transcriptional silencing through chromatin-level regulation. Mol Cell 32 232 246
31. LatosPAStrickerSHSteenpassLPaulerFMHuangR 2009 An in vitro ES cell imprinting model shows that imprinted expression of the Igf2r gene arises from an allele-specific expression bias. Development 136 437 448
32. StrickerSHSteenpassLPaulerFMSantoroFLatosPA 2008 Silencing and transcriptional properties of the imprinted Airn ncRNA are independent of the endogenous promoter. EMBO J 27 3116 3128
33. RassoulzadeganMMaglianoMCuzinF 2002 Transvection effects involving DNA methylation during meiosis in the mouse. EMBO J 21 440 450
34. TallquistMDSorianoP 2000 Epiblast-restricted Cre expression in MORE mice: a tool to distinguish embryonic vs. extra-embryonic gene function. Genesis 26 113 115
35. BarlowDPStogerRHerrmannBGSaitoKSchweiferN 1991 The mouse insulin-like growth factor type-2 receptor is imprinted and closely linked to the Tme locus. Nature 349 84 87
36. WutzATheusslHCDausmanJJaenischRBarlowDP 2001 Non-imprinted Igf2r expression decreases growth and rescues the Tme mutation in mice. Development 128 1881 1887
37. WangZQFungMRBarlowDPWagnerEF 1994 Regulation of embryonic growth and lysosomal targeting by the imprinted Igf2/Mpr gene. Nature 372 464 467
38. ForejtJGregorovaS 1992 Genetic analysis of genomic imprinting: an Imprintor-1 gene controls inactivation of the paternal copy of the mouse Tme locus. Cell 70 443 450
39. IllingworthRSBirdAP 2009 CpG islands–‘a rough guide’. FEBS Lett 583 1713 1720
40. PaulerFMKoernerMVBarlowDP 2007 Silencing by imprinted noncoding RNAs: is transcription the answer? Trends Genet 23 284 292
41. NaganoTMitchellJASanzLAPaulerFMFerguson-SmithAC 2008 The Air noncoding RNA epigenetically silences transcription by targeting G9a to chromatin. Science 322 1717 1720
42. MagerJMontgomeryNDde VillenaFPMagnusonT 2003 Genome imprinting regulated by the mouse Polycomb group protein Eed. Nat Genet 33 502 507
43. LeeJT 2003 Molecular links between X-inactivation and autosomal imprinting: X-inactivation as a driving force for the evolution of imprinting? Curr Biol 13 R242 254
44. ReikWLewisA 2005 Co-evolution of X-chromosome inactivation and imprinting in mammals. Nat Rev Genet 6 403 410
45. WutzARasmussenTPJaenischR 2002 Chromosomal silencing and localization are mediated by different domains of Xist RNA. Nat Genet 30 167 174
46. SmilinichNJDayCDFitzpatrickGVCaldwellGMLossieAC 1999 A maternally methylated CpG island in KvLQT1 is associated with an antisense paternal transcript and loss of imprinting in Beckwith - Wiedemann syndrome. Proc Natl Acad Sci U S A 96 8064 8069
47. MohammadFMondalTGusevaNPandeyGKKanduriC 2010 Kcnq1ot1 noncoding RNA mediates transcriptional gene silencing by interacting with Dnmt1. Development 137 2493 2499
48. GreenKLewisADawsonCDeanWReinhartB 2007 A developmental window of opportunity for imprinted gene silencing mediated by DNA methylation and the Kcnq1ot1 noncoding RNA. Mamm Genome 18 32 42
49. ZwartRSleutelsFWutzASchinkelAHBarlowDP 2001 Bidirectional action of the Igf2r imprint control element on upstream and downstream imprinted genes. Genes Dev 15 2361 2366
50. CuadradoMSacristanMAntequeraF 2001 Species-specific organization of CpG island promoters at mammalian homologous genes. EMBO Rep 2 586 592
51. FudaNJArdehaliMBLisJT 2009 Defining mechanisms that regulate RNA polymerase II transcription in vivo. Nature 461 186 192
52. BrookesEPomboA 2009 Modifications of RNA polymerase II are pivotal in regulating gene expression states. EMBO Rep 10 1213 1219
53. LevineM 2011 Paused RNA polymerase II as a developmental checkpoint. Cell 145 502 511
54. TianBHuJZhangHLutzCS 2005 A large-scale analysis of mRNA polyadenylation of human and mouse genes. Nucleic Acids Res 33 201 212
55. EdwardsJRO'DonnellAHRollinsRAPeckhamHELeeC 2010 Chromatin and sequence features that define the fine and gross structure of genomic methylation patterns. Genome Res
56. MikkelsenTSKuMJaffeDBIssacBLiebermanE 2007 Genome-wide maps of chromatin state in pluripotent and lineage-committed cells. Nature
57. OoiSKQiuCBernsteinELiKJiaD 2007 DNMT3L connects unmethylated lysine 4 of histone H3 to de novo methylation of DNA. Nature 448 714 717
58. ThomsonJPSkenePJSelfridgeJClouaireTGuyJ 2010 CpG islands influence chromatin structure via the CpG-binding protein Cfp1. Nature 464 1082 1086
59. MacleodDCharltonJMullinsJBirdAP 1994 Sp1 sites in the mouse aprt gene promoter are required to prevent methylation of the CpG island. Genes Dev 8 2282 2292
60. BrandeisMFrankDKeshetISiegfriedZMendelsohnM 1994 Sp1 elements protect a CpG island from de novo methylation. Nature 371 435 438
61. DicksonJGowherHStrogantsevRGasznerMHairA 2010 VEZF1 elements mediate protection from DNA methylation. PLoS Genet 6 e1000804 doi:10.1371/journal.pgen.1000804
62. GebhardCBennerCEhrichMSchwarzfischerLSchillingE 2010 General transcription factor binding at CpG islands in normal cells correlates with resistance to de novo DNA methylation in cancer cells. Cancer research 70 1398 1407
63. SmrzkaOWFaeIStogerRKurzbauerRFischerGF 1995 Conservation of a maternal-specific methylation signal at the human IGF2R locus. Hum Mol Genet 4 1945 1952
64. JiaDJurkowskaRZZhangXJeltschAChengX 2007 Structure of Dnmt3a bound to Dnmt3L suggests a model for de novo DNA methylation. Nature 449 248 251
65. ReinhartBPaoloni-GiacobinoAChailletJR 2006 Specific differentially methylated domain sequences direct the maintenance of methylation at imprinted genes. Mol Cell Biol 26 8347 8356
66. GoodrichJATjianR 2010 Unexpected roles for core promoter recognition factors in cell-type-specific transcription and gene regulation. Nat Rev Genet 11 549 558
67. LeeSHChoSYShannonMFFanJRangasamyD 2010 The impact of CpG island on defining transcriptional activation of the mouse L1 retrotransposable elements. PLoS ONE 5 e11353 doi:10.1371/journal.pone.0011353
68. Ramirez-CarrozziVRBraasDBhattDMChengCSHongC 2009 A unifying model for the selective regulation of inducible transcription by CpG islands and nucleosome remodeling. Cell 138 114 128
69. PaulerFMSloaneMAHuangRReghaKKoernerMV 2009 H3K27me3 forms BLOCs over silent genes and intergenic regions and specifies a histone banding pattern on a mouse autosomal chromosome. Genome Res 19 221 233
70. HuangRJaritzMGuenzlPVlatkovicIMSommerA 2011 An RNA-Seq strategy to detect the complete coding and non-coding transcriptome including full-length imprinted macro ncRNAs. PLoS ONE 6 e27288 doi:10.1371/journal.pone.0027288
71. HauserCSchuettengruberBBartlSLaggerGSeiserC 2002 Activation of the mouse histone deacetylase 1 gene by cooperative histone phosphorylation and acetylation. Molecular and cellular biology 22 7820 7830
72. BockCReitherSMikeskaTPaulsenMWalterJ 2005 BiQ Analyzer: visualization and quality control for DNA methylation data from bisulfite sequencing. Bioinformatics 21 4067 4068
Štítky
Genetika Reprodukční medicína
Článek Physiological Notch Signaling Maintains Bone Homeostasis via RBPjk and Hey Upstream of NFATc1Článek Intronic -Regulatory Modules Mediate Tissue-Specific and Microbial Control of / TranscriptionČlánek Probing the Informational and Regulatory Plasticity of a Transcription Factor DNA–Binding DomainČlánek Repression of Germline RNAi Pathways in Somatic Cells by Retinoblastoma Pathway Chromatin ComplexesČlánek An Alu Element–Associated Hypermethylation Variant of the Gene Is Associated with Childhood ObesityČlánek Three Essential Ribonucleases—RNase Y, J1, and III—Control the Abundance of a Majority of mRNAsČlánek Genomic Tools for Evolution and Conservation in the Chimpanzee: Is a Genetically Distinct Population
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 3
-
Všechny články tohoto čísla
- Comprehensive Research Synopsis and Systematic Meta-Analyses in Parkinson's Disease Genetics: The PDGene Database
- Genomic Analysis of the Hydrocarbon-Producing, Cellulolytic, Endophytic Fungus
- Networks of Neuronal Genes Affected by Common and Rare Variants in Autism Spectrum Disorders
- Akirin Links Twist-Regulated Transcription with the Brahma Chromatin Remodeling Complex during Embryogenesis
- Too Much Cleavage of Cyclin E Promotes Breast Tumorigenesis
- Imprinted Genes … and the Number Is?
- Genetic Architecture of Highly Complex Chemical Resistance Traits across Four Yeast Strains
- Exploring the Complexity of the HIV-1 Fitness Landscape
- MNS1 Is Essential for Spermiogenesis and Motile Ciliary Functions in Mice
- A Fundamental Regulatory Mechanism Operating through OmpR and DNA Topology Controls Expression of Pathogenicity Islands SPI-1 and SPI-2
- Evidence for Positive Selection on a Number of MicroRNA Regulatory Interactions during Recent Human Evolution
- Variation in Modifies Risk of Neonatal Intestinal Obstruction in Cystic Fibrosis
- PIF4–Mediated Activation of Expression Integrates Temperature into the Auxin Pathway in Regulating Hypocotyl Growth
- Critical Evaluation of Imprinted Gene Expression by RNA–Seq: A New Perspective
- A Meta-Analysis and Genome-Wide Association Study of Platelet Count and Mean Platelet Volume in African Americans
- Mouse Genetics Suggests Cell-Context Dependency for Myc-Regulated Metabolic Enzymes during Tumorigenesis
- Transcriptional Control in Cardiac Progenitors: Tbx1 Interacts with the BAF Chromatin Remodeling Complex and Regulates
- Synthetic Lethality of Cohesins with PARPs and Replication Fork Mediators
- APOBEC3G-Induced Hypermutation of Human Immunodeficiency Virus Type-1 Is Typically a Discrete “All or Nothing” Phenomenon
- Interpreting Meta-Analyses of Genome-Wide Association Studies
- Error-Prone ZW Pairing and No Evidence for Meiotic Sex Chromosome Inactivation in the Chicken Germ Line
- -Dependent Chemosensory Functions Contribute to Courtship Behavior in
- Diverse Forms of Splicing Are Part of an Evolving Autoregulatory Circuit
- Phenotypic Plasticity of the Drosophila Transcriptome
- Physiological Notch Signaling Maintains Bone Homeostasis via RBPjk and Hey Upstream of NFATc1
- Precocious Metamorphosis in the Juvenile Hormone–Deficient Mutant of the Silkworm,
- Igf1r Signaling Is Indispensable for Preimplantation Development and Is Activated via a Novel Function of E-cadherin
- Accurate Prediction of Inducible Transcription Factor Binding Intensities In Vivo
- Mitochondrial Oxidative Stress Alters a Pathway in Strongly Resembling That of Bile Acid Biosynthesis and Secretion in Vertebrates
- Mammalian Neurogenesis Requires Treacle-Plk1 for Precise Control of Spindle Orientation, Mitotic Progression, and Maintenance of Neural Progenitor Cells
- Tcf7 Is an Important Regulator of the Switch of Self-Renewal and Differentiation in a Multipotential Hematopoietic Cell Line
- REST–Mediated Recruitment of Polycomb Repressor Complexes in Mammalian Cells
- Intronic -Regulatory Modules Mediate Tissue-Specific and Microbial Control of / Transcription
- Age-Dependent Brain Gene Expression and Copy Number Anomalies in Autism Suggest Distinct Pathological Processes at Young Versus Mature Ages
- A Genome-Wide Association Study Identifies Variants Underlying the Shade Avoidance Response
- -by- Regulatory Divergence Causes the Asymmetric Lethal Effects of an Ancestral Hybrid Incompatibility Gene
- Genome-Wide Association and Functional Follow-Up Reveals New Loci for Kidney Function
- A Natural System of Chromosome Transfer in
- Cell Size and the Initiation of DNA Replication in Bacteria
- Probing the Informational and Regulatory Plasticity of a Transcription Factor DNA–Binding Domain
- Repression of Germline RNAi Pathways in Somatic Cells by Retinoblastoma Pathway Chromatin Complexes
- Temporal Transcriptional Profiling of Somatic and Germ Cells Reveals Biased Lineage Priming of Sexual Fate in the Fetal Mouse Gonad
- Rapid Analysis of Genome Rearrangements by Multiplex Ligation–Dependent Probe Amplification
- Metabolic Profiling of a Mapping Population Exposes New Insights in the Regulation of Seed Metabolism and Seed, Fruit, and Plant Relations
- The Atypical Calpains: Evolutionary Analyses and Roles in Cellular Degeneration
- The Silkworm Coming of Age—Early
- Development of a Panel of Genome-Wide Ancestry Informative Markers to Study Admixture Throughout the Americas
- Balanced Codon Usage Optimizes Eukaryotic Translational Efficiency
- The Min System and Nucleoid Occlusion Are Not Required for Identifying the Division Site in but Ensure Its Efficient Utilization
- Neurobeachin, a Regulator of Synaptic Protein Targeting, Is Associated with Body Fat Mass and Feeding Behavior in Mice and Body-Mass Index in Humans
- Statistical Analysis of Readthrough Levels for Nonsense Mutations in Mammalian Cells Reveals a Major Determinant of Response to Gentamicin
- Gene Reactivation by 5-Aza-2′-Deoxycytidine–Induced Demethylation Requires SRCAP–Mediated H2A.Z Insertion to Establish Nucleosome Depleted Regions
- The miR-35-41 Family of MicroRNAs Regulates RNAi Sensitivity in
- Genetic Basis of Hidden Phenotypic Variation Revealed by Increased Translational Readthrough in Yeast
- An Alu Element–Associated Hypermethylation Variant of the Gene Is Associated with Childhood Obesity
- Modelling Human Regulatory Variation in Mouse: Finding the Function in Genome-Wide Association Studies and Whole-Genome Sequencing
- Novel Loci for Adiponectin Levels and Their Influence on Type 2 Diabetes and Metabolic Traits: A Multi-Ethnic Meta-Analysis of 45,891 Individuals
- Polycomb-Like 3 Promotes Polycomb Repressive Complex 2 Binding to CpG Islands and Embryonic Stem Cell Self-Renewal
- Insulin/IGF-1 and Hypoxia Signaling Act in Concert to Regulate Iron Homeostasis in
- EMF1 and PRC2 Cooperate to Repress Key Regulators of Arabidopsis Development
- Three Essential Ribonucleases—RNase Y, J1, and III—Control the Abundance of a Majority of mRNAs
- Contrasted Patterns of Molecular Evolution in Dominant and Recessive Self-Incompatibility Haplotypes in
- A Machine Learning Approach for Identifying Novel Cell Type–Specific Transcriptional Regulators of Myogenesis
- Genomic Tools for Evolution and Conservation in the Chimpanzee: Is a Genetically Distinct Population
- Nos2 Inactivation Promotes the Development of Medulloblastoma in Mice by Deregulation of Gap43–Dependent Granule Cell Precursor Migration
- Intracranial Aneurysm Risk Locus 5q23.2 Is Associated with Elevated Systolic Blood Pressure
- Heritability and Genetic Correlations Explained by Common SNPs for Metabolic Syndrome Traits
- A Genome-Wide Association Study of Nephrolithiasis in the Japanese Population Identifies Novel Susceptible Loci at 5q35.3, 7p14.3, and 13q14.1
- DNA Damage in Nijmegen Breakage Syndrome Cells Leads to PARP Hyperactivation and Increased Oxidative Stress
- DNA Resection at Chromosome Breaks Promotes Genome Stability by Constraining Non-Allelic Homologous Recombination
- Genetic Analysis of Floral Symmetry in Van Gogh's Sunflowers Reveals Independent Recruitment of Genes in the Asteraceae
- A Splice Site Variant in the Bovine Gene Compromises Growth and Regulation of the Inflammatory Response
- Promoter Nucleosome Organization Shapes the Evolution of Gene Expression
- The Nucleoside Diphosphate Kinase Gene Acts as Quantitative Trait Locus Promoting Non-Mendelian Inheritance
- The Ciliogenic Transcription Factor RFX3 Regulates Early Midline Distribution of Guidepost Neurons Required for Corpus Callosum Development
- Phosphorylation of the RNA–Binding Protein HOW by MAPK/ERK Enhances Its Dimerization and Activity
- A Genome-Wide Scan of Ashkenazi Jewish Crohn's Disease Suggests Novel Susceptibility Loci
- Parkinson's Disease–Associated Kinase PINK1 Regulates Miro Protein Level and Axonal Transport of Mitochondria
- LMW-E/CDK2 Deregulates Acinar Morphogenesis, Induces Tumorigenesis, and Associates with the Activated b-Raf-ERK1/2-mTOR Pathway in Breast Cancer Patients
- Mapping the Hsp90 Genetic Interaction Network in Reveals Environmental Contingency and Rewired Circuitry
- Autoregulation of the Noncoding RNA Gene
- The Human Pancreatic Islet Transcriptome: Expression of Candidate Genes for Type 1 Diabetes and the Impact of Pro-Inflammatory Cytokines
- Spo0A∼P Imposes a Temporal Gate for the Bimodal Expression of Competence in
- Antagonistic Regulation of Apoptosis and Differentiation by the Cut Transcription Factor Represents a Tumor-Suppressing Mechanism in
- A Downstream CpG Island Controls Transcript Initiation and Elongation and the Methylation State of the Imprinted Macro ncRNA Promoter
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- PIF4–Mediated Activation of Expression Integrates Temperature into the Auxin Pathway in Regulating Hypocotyl Growth
- Metabolic Profiling of a Mapping Population Exposes New Insights in the Regulation of Seed Metabolism and Seed, Fruit, and Plant Relations
- A Splice Site Variant in the Bovine Gene Compromises Growth and Regulation of the Inflammatory Response
- Comprehensive Research Synopsis and Systematic Meta-Analyses in Parkinson's Disease Genetics: The PDGene Database
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



