-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaIntronic -Regulatory Modules Mediate Tissue-Specific and Microbial Control of / Transcription
The intestinal microbiota enhances dietary energy harvest leading to increased fat storage in adipose tissues. This effect is caused in part by the microbial suppression of intestinal epithelial expression of a circulating inhibitor of lipoprotein lipase called Angiopoietin-like 4 (Angptl4/Fiaf). To define the cis-regulatory mechanisms underlying intestine-specific and microbial control of Angptl4 transcription, we utilized the zebrafish system in which host regulatory DNA can be rapidly analyzed in a live, transparent, and gnotobiotic vertebrate. We found that zebrafish angptl4 is transcribed in multiple tissues including the liver, pancreatic islet, and intestinal epithelium, which is similar to its mammalian homologs. Zebrafish angptl4 is also specifically suppressed in the intestinal epithelium upon colonization with a microbiota. In vivo transgenic reporter assays identified discrete tissue-specific regulatory modules within angptl4 intron 3 sufficient to drive expression in the liver, pancreatic islet β-cells, or intestinal enterocytes. Comparative sequence analyses and heterologous functional assays of angptl4 intron 3 sequences from 12 teleost fish species revealed differential evolution of the islet and intestinal regulatory modules. High-resolution functional mapping and site-directed mutagenesis defined the minimal set of regulatory sequences required for intestinal activity. Strikingly, the microbiota suppressed the transcriptional activity of the intestine-specific regulatory module similar to the endogenous angptl4 gene. These results suggest that the microbiota might regulate host intestinal Angptl4 protein expression and peripheral fat storage by suppressing the activity of an intestine-specific transcriptional enhancer. This study provides a useful paradigm for understanding how microbial signals interact with tissue-specific regulatory networks to control the activity and evolution of host gene transcription.
Published in the journal: . PLoS Genet 8(3): e32767. doi:10.1371/journal.pgen.1002585
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002585Summary
The intestinal microbiota enhances dietary energy harvest leading to increased fat storage in adipose tissues. This effect is caused in part by the microbial suppression of intestinal epithelial expression of a circulating inhibitor of lipoprotein lipase called Angiopoietin-like 4 (Angptl4/Fiaf). To define the cis-regulatory mechanisms underlying intestine-specific and microbial control of Angptl4 transcription, we utilized the zebrafish system in which host regulatory DNA can be rapidly analyzed in a live, transparent, and gnotobiotic vertebrate. We found that zebrafish angptl4 is transcribed in multiple tissues including the liver, pancreatic islet, and intestinal epithelium, which is similar to its mammalian homologs. Zebrafish angptl4 is also specifically suppressed in the intestinal epithelium upon colonization with a microbiota. In vivo transgenic reporter assays identified discrete tissue-specific regulatory modules within angptl4 intron 3 sufficient to drive expression in the liver, pancreatic islet β-cells, or intestinal enterocytes. Comparative sequence analyses and heterologous functional assays of angptl4 intron 3 sequences from 12 teleost fish species revealed differential evolution of the islet and intestinal regulatory modules. High-resolution functional mapping and site-directed mutagenesis defined the minimal set of regulatory sequences required for intestinal activity. Strikingly, the microbiota suppressed the transcriptional activity of the intestine-specific regulatory module similar to the endogenous angptl4 gene. These results suggest that the microbiota might regulate host intestinal Angptl4 protein expression and peripheral fat storage by suppressing the activity of an intestine-specific transcriptional enhancer. This study provides a useful paradigm for understanding how microbial signals interact with tissue-specific regulatory networks to control the activity and evolution of host gene transcription.
Introduction
The vertebrate intestine harbors a dense community of microorganisms (gut microbiota) that exerts a profound influence on distinct aspects of host physiology [1], [2]. The gut microbiota has been identified as a potent environmental factor in a growing number of human diseases, including inflammatory bowel disease [3], antibiotic-associated diarrheas [4], cardiovascular disease [4], and obesity [5]. As a consequence, there is considerable interest in understanding the mechanisms by which this resident microbial community influences health and disease in humans and other animals.
The ability of the microbiota to modify host nutrient metabolism and energy balance is a prominent theme in host-microbe commensalism in the intestine. Recent mechanistic insights into this process have been provided by comparisons between mice reared in the absence of microbes (germ-free or GF) to those colonized with members of the normal microbiota, as well as high-throughput DNA sequencing analysis of the metabolic potential of gut microbial genomes. These approaches have shown that the gut microbiota contributes biochemical activities not encoded in the host genome that enhance digestion of dietary nutrients [6], [7]. The resulting increase in digestive efficiency results in elevated plasma levels of triglyceride (TG)-rich lipoproteins [8], [9]. TG within circulating lipoprotein particles is hydrolyzed through the rate-limiting activity of lipoprotein lipase (LPL) located at the luminal surface of capillaries. TG hydrolysis releases free fatty acids (FFA) for uptake by adjacent tissues for oxidation (e.g., in cardiac and skeletal muscle) or fat storage (e.g., in adipose tissues) [10]. The presence of a gut microbiota also results in a concomitant reduction in intestinal expression of Angiopoietin-like 4 (Angptl4, also called Fiaf, Pgar, and Hfarp) [8], [11], encoding a circulating peptide hormone that acts as a direct inhibitor of LPL activity [12]–[15]. Studies in gnotobiotic mice have indicated that microbial suppression of Angptl4 expression is restricted to the intestinal epithelium and is not observed in other tissues that express Angptl4, such as liver and adipose tissue. This restricted suppression leads to a significant increase in LPL activity and fat storage in adipose tissue of animals colonized with a microbiota, which is an effect abolished in mice lacking Angptl4 [8]. These results have established Angptl4 as a key host factor mediating the microbial regulation of host energy balance and have raised considerable interest in defining the mechanisms underlying the tissue-specific and microbial regulation of Angptl4 expression. The importance of understanding mechanisms regulating Angptl4 production is further underscored by reports suggesting that human ANGPTL4 functions as an important determinant of plasma TG levels [16], [17] and by Angptl4's additional functions in angiogenesis [18], tumor cell survival [19] and metastasis [20], [21], and wound healing [22].
Previous studies have revealed that mammalian Angptl4 expression is subject to complex cell type-specific regulation but the underlying mechanisms remain unclear. Angptl4 mRNA in humans and rodents is expressed in multiple tissues, including adipose tissue, liver, intestinal epithelium, pancreatic islets, and cardiac and skeletal muscle [8], [19], [23]–[26]. Preliminary insights into the trans - and cis-regulatory mechanisms controlling Angptl4 transcription have been provided by analyses in non-intestinal tissues. Members of the peroxisome proliferator-activated receptor (PPAR) family of nuclear receptors (i.e., PPARγ, PPARα, and PPARβ/δ) have been identified as activators of Angptl4 expression in adipose tissue, liver [23], [27], skeletal [28] and cardiac muscle [29], myofibroblasts [30], and colon carcinoma cells [31]. A PPAR-responsive element (element defined as a transcription factor binding site or TFBS) located in the proximal portion of Angptl4 intron 3 has been shown to directly bind different PPAR family members in adipose tissue, liver [27], and myofibroblasts [30]. Additional studies in non-intestinal cell types have identified functional TFBSs for SMAD3 and glucocorticoid receptor in the 5′ distal region and 3′ untranslated region (UTR), respectively [30], [32]. Angptl4 transcription is induced under hypoxic conditions in several non-intestinal cell types by hypoxia-inducible factor 1α (HIF1α) [33], [34]; however, the TFBSs mediating this response have not been identified. These studies support a role for these trans - and cis-regulatory factors in controlling Angptl4 transcription in these cell types, yet the mechanisms underlying the transcription of Angptl4 in other tissues, such as the intestine and pancreatic islet, remain unknown. Moreover, the cis/trans-regulatory mechanisms underlying microbial suppression of Angptl4 transcription in the intestinal epithelium remain undefined.
The zebrafish (Danio rerio) provides unique opportunities to study the transcriptional regulatory programs mediating tissue-specific and the microbial control of vertebrate gene expression. Robust transgenesis methods using the Tol2 transposon system [35], large numbers of offspring, and optical transparency facilitate efficient spatiotemporal analysis of reporters driven by potential DNA regulatory regions in mosaic and stable transgenic animals [36]. The anatomy and physiology of the zebrafish digestive tract are highly similar to mammals, including an intestine, liver, gall bladder, and exocrine and endocrine pancreas [37]–[39]. The intestinal epithelium of the zebrafish displays proximal-distal functional specification and is composed of absorptive enterocytes as well as secretory goblet and enteroendocrine lineages [40], [41]. The zebrafish intestine is colonized by a microbiota shortly after the animals hatch from their protective chorions at 3 days post-fertilization (dpf) [42], [43] and reaches a stage sufficient to support nutrient digestion by 5 dpf [44]. To study the roles of commensal microbes on zebrafish development and physiology, we have developed methods for rearing GF zebrafish and colonizing them with members of the normal zebrafish microbiota [45], [46]. By combining these methods with functional genomic approaches, we identified zebrafish transcripts that display altered expression levels in animals raised GF compared to those colonized with a normal microbiota, including microbial suppression of a zebrafish homolog of mammalian Angptl4 [47]–[49]. The expression pattern of this zebrafish Angptl4 homolog, and the mechanisms underlying the tissue-specific and microbial regulation of its expression, have not been previously described.
These features position the zebrafish as a powerful model for assaying the regulatory potential of DNA involved in mediating cell-specific and microbe-responsive transcriptional events. Previous studies of DNA regulatory potential in the zebrafish system have focused primarily on developmental genes [50]–[54], and it remains unclear if the lessons learned from these analyses [55] will apply to physiologic genes like Angptl4 that are regulated by endogenous as well as exogenous cues. Moreover, a paucity of available genome sequences for teleost species closely related to zebrafish has severely limited prior evolutionary analysis of cis-regulatory sequence and function. Here, we utilize the zebrafish to investigate the cis-regulatory mechanisms governing tissue-specific and microbial control of Angptl4 transcription. We focus our analysis on intestinal and islet expression, where the mechanisms regulating Angptl4 transcription have not been adequately examined. We first uncover distinct intronic cis-regulatory modules (CRM, defined here as a discrete DNA region containing sufficient information to confer a regulatory function) that mediate intestinal and islet expression. Using this information, we reveal that the intestine-specific CRM also responds to microbial stimuli to suppress angptl4 expression. These results provide novel insights into how vertebrates might control the tissue-specific transcription of Angptl4 and constitute an important advance towards understanding how commensal gut microbes regulate gene expression and energy balance in their vertebrate hosts.
Results
Tissue-specific expression of zebrafish angptl4
A comparative sequence analysis revealed that the zebrafish genome encodes a single ortholog of mammalian Angptl4 that displays marked amino acid sequence conservation with other vertebrate homologs (See Text S1, Figure 1A, Figures S1 and S2). We used RNA whole-mount in situ hybridization (WISH) to identify the tissues in which angptl4 is transcribed during zebrafish development. We found that zebrafish angptl4 mRNA is expressed ubiquitously in 1 dpf embryos (Figure 1B) but becomes enriched in specific tissues during post-embryonic stages. Transcripts for angptl4 are enriched in the intestinal epithelium by 4 dpf, shortly after the intestinal tract becomes completely patent (Figure 1C), and become localized to the anterior intestine (segment 1) by 6 dpf (Figure 1D, 1E). Transcripts for angptl4 were also enriched in the pancreatic islet by 8 dpf (Figure 1F) and in the liver by 17 dpf (Figure 1G, 1H). Notably, the intestinal epithelium [8], [11], liver [24], [27], and pancreatic islet [25] in mammals also express Angptl4 mRNA. These data establish that the zebrafish angptl4 ortholog is expressed in a tissue-specific pattern that is conserved across vertebrate lineages and suggest that the underlying transcriptional regulatory mechanisms may also be conserved.
Fig. 1. Tissue-specific expression of zebrafish angptl4 mRNA. 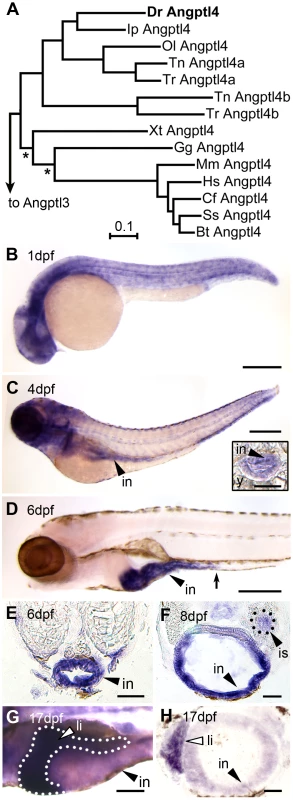
(A) Distance phylogram of Angptl4 protein from zebrafish (Dr, Danio rerio), catfish (Ip, Ictalurus punctatus), medaka (Ol, Oryzias latipes), tetraodon (Tn, Tetraodoan nigroviridis), fugu (Tr, Takifugu rubipres), xenopus (Xt, Xenopus tropicalis), chicken (Gg, Gallus gallus), mouse (Mm, Mus musculus), human (Hs, Homo sapiens), dog (Cf, Canis familiaris), pig (Ss, Sus scrofa), cow (Bt, Bos taurus). All nodes are significant (>700/1000 bootstrap replicates) except those marked with an asterisk (*). Scale bar indicates phylogenetic distance, in number of amino acid substitutions per site. We found that the genomes of zebrafish, channel catfish (Ictaluris punctatus), and medaka (Oryzias latipes) encode a single ortholog of mammalian Angptl4, whereas two pufferfish species (Takifugu rubripes and Tetraodon nigroviridis) encode two Angptl4 paralogs. See also Figure S1. (B–G) Whole-mount in situ hybridization (WISH) using a riboprobe targeting angptl4 mRNA during various stages in zebrafish development reveals dynamic spatiotemporal gene expression patterns. (B) At 1 day post fertilization (dpf) embryos exhibit ubiquitous expression of angptl4. (C–D) By 4 dpf, marked expression is observed in the intestinal epithelium (in, black arrowhead), but by 6 dpf, robust expression becomes largely localized to the intestine (black arrowhead) and pancreatic islet (not shown). The black arrow marks the boundary between the anterior intestine (segment 1) and mid-intestine (segment 2). Scale bars = 500 µm. (E–F) Transverse sections of 6 dpf and 8 dpf animals confirm expression in the intestinal epithelium (E, in, black arrowhead) and pancreatic islet (F, is, black triangle). Scale bars = 50 µm. (G–H) At 17 dpf, strong expression is observed in the liver (li, white arrowhead, dotted line outlines the liver). G, Scale bar = 250 µm; H, Scale bar = 50 µm. Conservation in DNA sequence guides cis-regulatory module discovery
Previous studies have indicated that conservation in non-coding genomic DNA sequence across vertebrate lineages can be a reliable predictor of cis-regulatory DNA regions [56], [57]. We therefore used this approach to discover regulatory regions controlling transcription of angptl4 in the liver, islet, and intestinal epithelium. Mammals and teleost fishes diverged approximately 438–476 million years ago [58], whereas zebrafish (clade Otocephala) diverged from other teleost fishes with currently-available genome sequence [clade Euteleostei; i.e., medaka (Oryzias latipes), stickleback (Gasterosteus aculeatus), fugu (Takifugu rubripes), and tetraodon (Tetraodon nigroviridis)] approximately 230–307 million years ago [59]. We generated multiple-species LAGAN alignments with Vista software using 10 kb of genomic sequence surrounding and including the angptl4 loci from four teleost fishes (zebrafish, medaka, tetraodon, fugu) and three mammals [human (Homo sapiens), dog (Canis familiaris), and mouse (Mus musculus)]. Alignment of teleost and mammalian genomic sequences did not detect regions of primary sequence conservation within angptl4 non-coding regions (>50% over 100 bp; data not shown), suggesting that these alignment methods are not sufficiently sensitive to detect existing non-coding conservation [56] or that the composition and/or location of non-coding regulatory regions are not stringently conserved between these lineages. We therefore separately aligned teleost angptl4 (Figure 2A) and mammalian Angptl4 loci (Figure 2B) and searched for non-coding sequence conservation in each lineage. These alignments revealed that human and zebrafish angptl4 loci both contain 7 conserved exons as well as a concentration of conserved non-coding sequences directly upstream of exon 1 and in intron 3 (Figure 2). Similarities in gene structure and locations of conserved non-coding regions, in addition to conservation in gene expression patterns, support the hypothesis that the regulatory mechanisms of angptl4 transcription may be evolutionarily conserved.
Fig. 2. Multiple-species alignments reveal conservation in angptl4 gene structure and location of conserved non-coding regions. 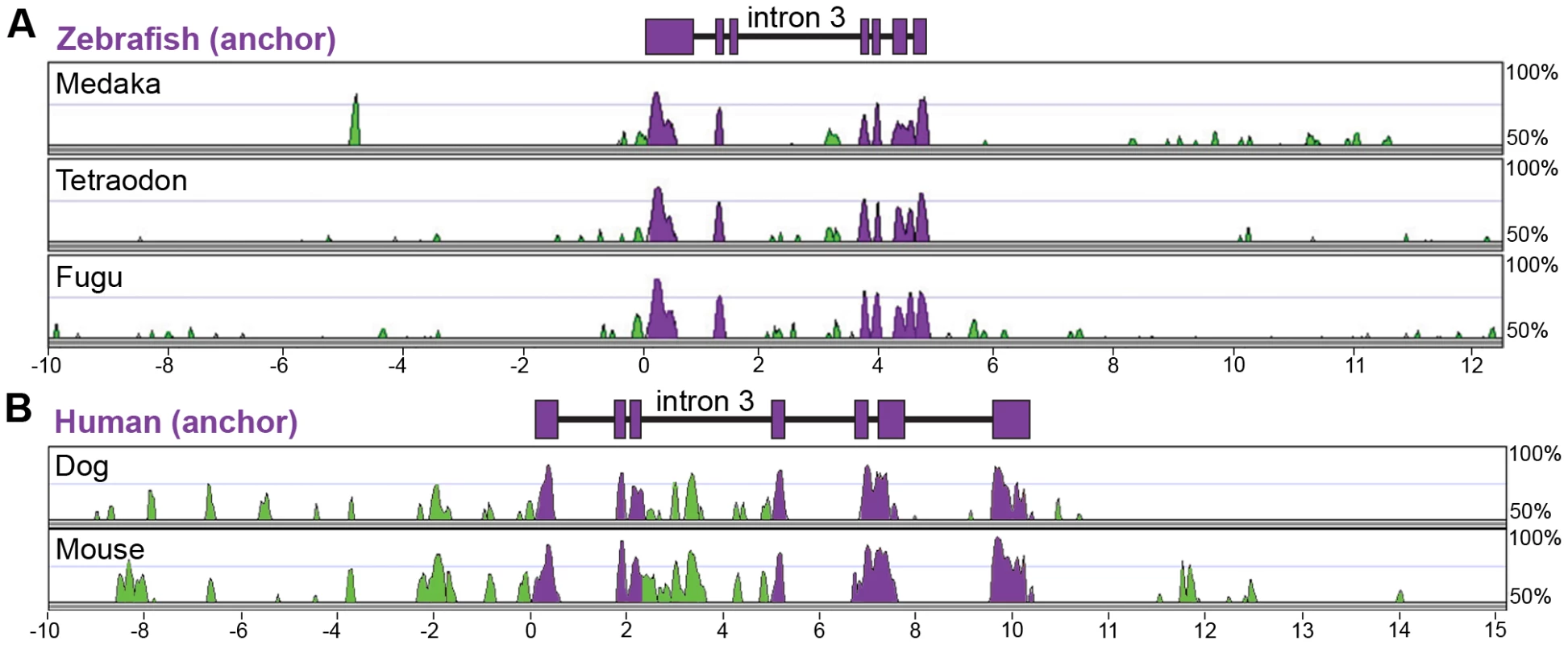
(A) VISTA plot displaying the global pairwise alignment of the zebrafish angptl4 locus with the orthologous medaka, tetraodon, and fugu regions and (B) human ANGPTL4 locus with the orthologous mouse and dog regions. Purple conservation peaks correspond to exonic sequences, and green conservation peaks represent non-coding sequences. The zebrafish and human gene structure are denoted by purple boxes above the corresponding VISTA plot (VISTA parameters: 100 bp sliding window, LAGAN alignment). Note that the concentration of conservation peaks within intron 3 of both teleost and mammalian angptl4 genes. The angptl4 proximal promoter does not recapitulate mRNA expression patterns
We assayed the regulatory potential of DNA upstream and proximal to the zebrafish angptl4 transcription start site (TSS) for the ability to transcribe a reporter in the intestine, liver, and islet. We first employed 5′ rapid amplification of cDNA ends (5′RACE) to determine the location of the TSS (Figure S3B). We identified a single TSS located 89 base pairs (bp) upstream of the translation start site and a canonical TATA box at position −31 bp of the TSS (Figure S3B). Based on this analysis and expressed sequence tag (EST) coverage of the zebrafish angptl4 locus (data not shown), we found no evidence of alternative promoters farther upstream of the defined TSS. Using Tol2 transposon transgenesis, we assayed the regulatory potential of genomic DNA upstream of the zebrafish angptl4 TSS, including the 5′ untranslated region (UTR) (Figure S3A), to drive expression of an enhanced green fluorescent protein (GFP) reporter in 0–7 dpf zebrafish larvae. We found that regulatory DNA within −1 kb, −3.5 kb, or −5.2 kb upstream of the TSS harbors the potential to drive GFP expression in mosaic animals in several tissues including liver at 6 dpf (Figure S3C, S3E). Robust expression in the liver was confirmed in animals harboring stable germ-line incorporation of these transgenes (Figure S3D, S3F). However, these angptl4 upstream regulatory sequences were not sufficient to drive detectable reporter expression in the intestine (Figure S3G) or islet (data not shown). We therefore reasoned that information governing transcription in the intestine and islet must be located distal to the TSS and proximal promoter.
Multiple angptl4 intronic regulatory modules confer tissue-specific transcription
Relatively high levels of DNA sequence conservation in both teleost and mammalian lineages (Figure 2) prompted us to test the 3rd intron of zebrafish angptl4 for transcriptional regulatory potential. We cloned full-length zebrafish angptl4 intron 3 (2,136 bp; designated in3) into a Tol2 transposon reporter vector upstream of a minimal mouse Fos promoter (Mmu.Fos) driving transcription of a GFP or tdTomato reporter. Importantly, the minimal Fos promoter alone is relatively inactive in most tissues and is not sufficient to drive transcription of detectable levels of GFP in the intestine, islet, or liver [50]. Analysis of 6 dpf zebrafish larvae with mosaic expression of the Tg(in3-Mmu.Fos:GFP) transgene disclosed that full-length in3 is sufficient to confer reporter expression in multiple tissues including the liver, muscle, intestine (Figure 3C), and islet (not shown). This expression pattern was confirmed in fish with stable germ-line incorporation of the transgene (Figure 3D). Guided by sequence conservation between zebrafish and medaka (Figure 2A), we assayed serial truncations of in3 for spatial regulatory potential to determine whether reporter transcriptional activity in these distinct tissues is governed by the same CRM or through multiple discrete CRMs, (Figure 3B). The first truncation separated liver expression (1,219 bp, designated in3.1, Figure 3E, 3F) from islet and intestinal expression (701 bp; designated in3.2, Figure 3G, 3H). Further truncation of in3.2 uncoupled islet (387 bp; designated in3.3; Figure 3I, 3J) and intestinal (316 bp; designated in3.4; Figure 3K, 3L) expression. This analysis therefore revealed non-overlapping modules sufficient to confer mosaic and stable reporter expression in the liver, islet, and intestinal epithelium that is consistent with endogenous angptl4 mRNA expression (Figure 1).
Fig. 3. Non-overlapping regulatory modules within angptl4 intron 3 confer liver, islet, and enterocyte-specific reporter expression. 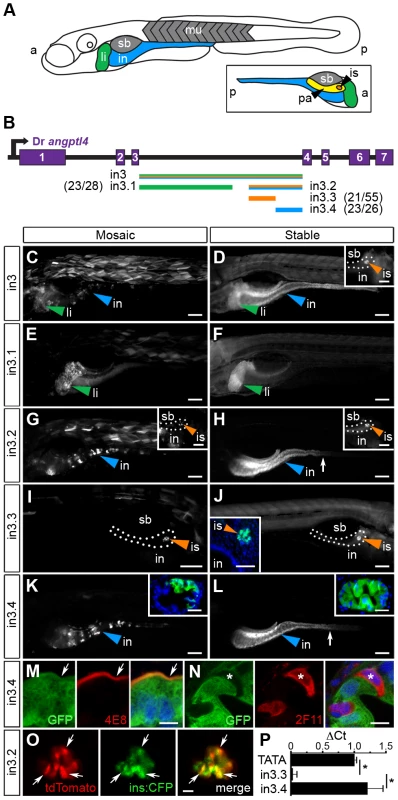
(A) Depiction of the 6 dpf zebrafish showing liver (li, green), intestine (in, blue), swim bladder (sb, grey), and muscle (mu, grey), with the fish oriented anterior (a) to the left and posterior (p) to the right. The opposite orientation reveals the exocrine pancreas (pa, yellow) and islet (is, orange). (B) Scaled schematic of the zebrafish angptl4 locus and non-coding DNA assayed for regulatory potential. Modules are color coded according to the tissues in which they confer expression. Ratios of islet or intestine positive fish versus total fish expressing gfp are shown in parentheses next to truncation labels. (C–N) Representative images of GFP reporter expression in mosaic (column 1) and F1 stable (column 2) animals driven by each non-coding DNA region (rows). Scale bars = 100 µm; li = liver, is = islet, in = intestine, sb = swim bladder. Colored arrowheads indicate tissue with specific reporter expression. (C–D) Full-length intron 3 (in3; 2,136 bp) is sufficient to promote expression of the reporter in the liver, islet (D, inset, scale bar = 50 µm), and intestine. (E–F) Truncation in3.1 (1,219 bp) confers expression in the liver. (G–H) Truncation in3.2 (701 bp) confers expression in both the intestine and islet (H, inset). Inset scale bar = 50 µm. (I–J) Truncation in3.3 (387 bp) confers islet expression. A transverse section (inset, J) reveals islet expression (nuclei stained with DAPI). Inset scale bar = 50 µm. (K–L) Truncation in3.4 (316 bp) confers intestinal expression. Insets in panels K and L contain transverse sections showing expression localized to the intestinal epithelium (nuclei stained with DAPI). Inset scale bar = 25 µm. The dotted lines in panels D, G, H, and I outline the pancreas. The white arrows in panels H, K, and L mark the boundary between the anterior intestine (segment 1) and mid-intestine (segment 2). (M–N) Cells expressing GFP driven by the in3.4 regulatory module colocalize with a marker (4E8, red, white arrow) of the brush border of absorptive enterocytes, but fail to co-localize with marker for secretory cells (2F11, red, asterisk). Nuclei stained with DAPI. Scale bars = 5 µm. (O) Intercross of Tg(in3.2-Mmu.Fos:tdTomato) with β-cell specific reporter line (Tg(ins:CFP-NTR)s892) show colocalization of tdTomato and CFP in the islet. Scale bars = 10 µm. (P) Quantitative PCR shows that the in3.4 module and the angptl4 promoter (TATA box), but not the in3.3 module, are hypersensitive to DNase I cleavage in intestinal epithelial cells isolated from adult zebrafish. Asterisks denote P-value<.01 from unpaired T-tests between TATA box or in3.4 and in3.3 regions. Error bars represent standard deviation from four biological replicates using cells pooled from 3 wild-type adult zebrafish per replicate. We next sought to identify the specific cell types in the intestinal epithelium and pancreatic islet in which modules in3.3 and in3.4 respectively enhance transcription. To define the cell type within the islet in which module in3.3 is active, we utilized a zebrafish transgenic line that drives expression of cyan fluorescent reporter (CFP) specifically in insulin-producing β-cells within the islet (Tg(ins:CFP-NTR)s892) [60]. In vivo imaging of 6 dpf progeny from intercrosses of Tg(ins:CFP-NTR)s892 and Tg(in3.2-Mmu.Fos:tdTomato) adults revealed strong co-localization of CFP and tdTomato (Figure 3O), indicating that the in3.3 module specifically enhances transcription in pancreatic β-cells. Immunofluorescence assays of sectioned 6 dpf zebrafish stably expressing the Tg(in3.4-Mmu.Fos:GFP) transgene revealed that GFP driven by the in3.4 module co-localizes with 4E8-positive absorptive enterocytes (Figure 3M) but not with 2F11-positive secretory cells in the intestinal epithelium (Figure 3N). These data suggest that in3.4 functions as an enterocyte-specific transcriptional regulatory module.
We next tested whether the intestine-specific reporter expression generated by module in3.4 is independent of the Fos minimal promoter, orientation, and proximal position to the TSS. This module is located downstream of the TSS in intron 3 of the endogenous angptl4 gene; however, our synthetic reporter construct positions it upstream of the TSS and the Fos minimal promoter. We therefore cloned in3.4 into a position downstream of GFP in either the forward or inverse orientation under control of either a Fos minimal promoter or the −1 kb angptl4 promoter. Each of these constructs was sufficient to promote robust reporter expression in the anterior intestine of 6 dpf mosaic and stable zebrafish (Figure S4A and data not shown), similar to our observations with in3.4 located in the proximal position (Figure 3K, 3L). These results establish that in3.4 is a bona fide transcriptional enhancer module active in enterocytes in the anterior intestine.
We next used DNase I hypersensitivity to determine if the in3.4 module functions as an intestinal regulatory module in vivo at the endogenous angptl4 locus. To obtain a sufficient number of intestinal epithelial cells for this assay, we analyzed intestines from adult zebrafish. Stable transgenic zebrafish harboring the in3.2 or in3.4 reporter maintain reporter activity in the intestine into adulthood (Figure S4B and data not shown) indicating this module and associated trans-regulators are active in the adult zebrafish intestine. We find that the endogenous angptl4 promoter and in3.4 module, but not the adjacent in3.3 module, are hypersensitive to DNase I cleavage in intestinal epithelial cells isolated from adult zebrafish (Figure 3P). The endogenous in3.4 module is therefore an active regulatory module in the intestinal epithelium, under regulatory control distinct from the adjacent in3.3 module, consistent with our transgenic reporter analysis of this same region. Together, these data reveal extensive transcriptional regulatory potential within intron 3 of zebrafish angptl4 and suggest that distinct intronic modules may mediate spatially restricted transcription of angptl4 in the intestinal epithelium, pancreatic β-cells, and liver.
Evolution of the islet and intestinal regulatory modules
We used comparative genome sequence analysis from 12 teleost fishes and heterologous in vivo reporter assays to explore the evolution of the islet and intestinal regulatory modules. We originally postulated that evolutionary conservation of non-coding sequences could be used to predict the location of cis-regulatory regions controlling spatial and environmental regulation of angptl4 transcription (Figure 2). However, the significant amount of time (approximately 230–307 million years ago) [59] since the divergence between zebrafish (clade Otocephala; order Cypriniformes) and the other teleost fish with available genome sequence (all from clade Clupeocephala, such as medaka) did not permit high-resolution analysis of recent evolution of zebrafish angptl4 regulatory sequences (Figure 2A). We therefore sequenced the intronic region orthologous to in3.2 from 10 additional Ostariophysi species, including 1 from order Siluriformes (channel catfish, Ictalurus punctatus) and 9 other members of order Cypriniformes (Figure 4A). Because genome sequences are not currently available for these species, we took advantage of the intronic location of these regulatory modules by utilizing PCR primers targeting highly conserved sequences in flanking exons 3/4 or intron 3 to clone and sequence these putative regulatory regions. As expected, pairwise alignments of new sequences orthologous to zebrafish in3.2 revealed an inverse relationship between the phylogenetic distance between the two species and module sequence conservation, with the intestinal module diverging more rapidly than the islet module (Figure 4B, Figures S5 and S6). To test the functional consequences of the observed module divergence in these teleost species, we analyzed each module using our zebrafish mosaic transgenic assay for regulatory potential in the intestine and islet. Despite accounts of functional conservation in the absence of primary sequence conservation [50], [61], the non-coding sequence within medaka angptl4 intron 3 orthologous to zebrafish in3.2 (Ol in3.2) failed to drive reporter expression in either the intestine or islet (Figure 4C). Notably, all tested Ostariophysi modules elicited robust reporter expression in the islet (Figure 4C). However, only in3.2 from Cypriniformes species within the Danio monophyletic group (Danio nigrofasciatus, D. choprae, D. feegradei) [62], [63] were sufficient to confer reporter expression in the intestine (Figure 4C) despite marked regions of sequence conservation within the intestinal module in other Cypriniformes species (D. aequipinnatus, C. auratus, C. carpio, P. conchonius). These results reveal differential evolutionary dynamics of the angptl4 intestinal and islet modules and support the hypothesis that high sequence conservation is required for tissue-specific transcription.
Fig. 4. Functional evolution of the islet and intestinal regulatory modules in 12 fish species. 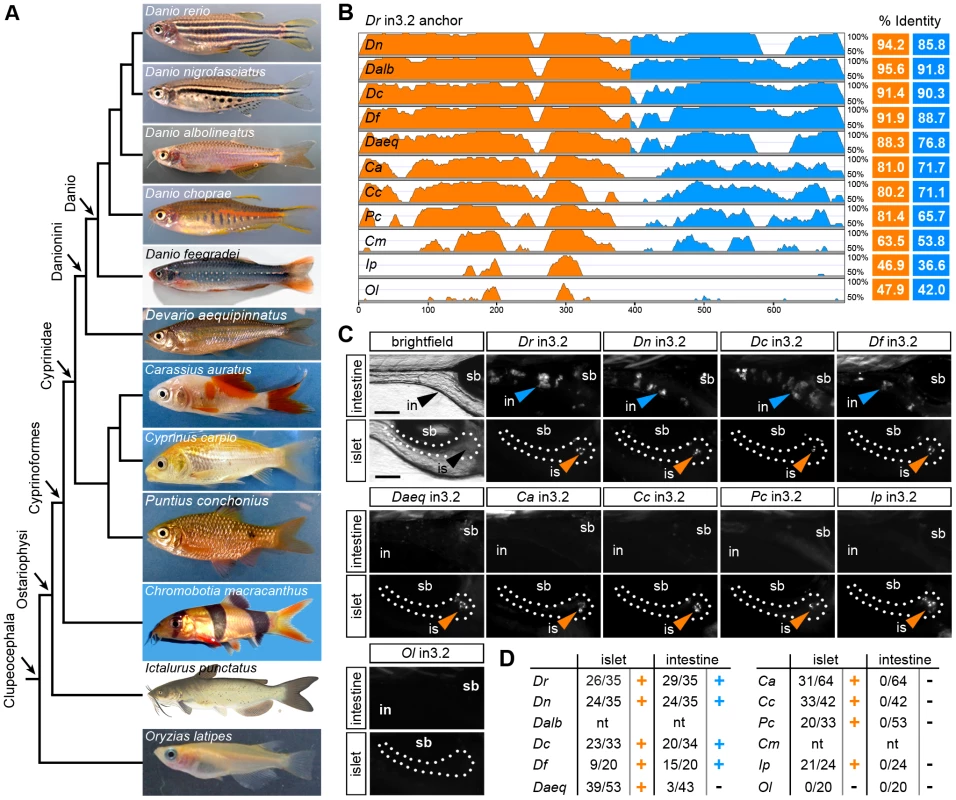
(A) Unscaled phylogram based on information from [58], [59] showing images and relative relationships of 12 fish for which intronic sequences were analyzed. Danio rerio (Dr, zebrafish), Danio nigrofasciatus (Dn), Danio albolineatus (Dalb), Danio choprae (Dc), Danio feegradei (Df), Devario aequipinnatus (Daeq, giant danio), Carassius auratus (Ca, goldfish), Cyprinus carpio (Cc, carp), Puntius conchonius (Pc, rosy barb), Chromobotia macracanthus (Cm, clown loach), Ictalurus punctatus (Ip, channel catfish), Oryzias latipes (Ol, medaka). (B) VISTA plot displaying the global pairwise alignment of orthologous in3.2 regions from each species anchored to zebrafish (Dr) in3.2. Orange peaks correspond to regions in the alignment that correspond to Dr in3.3 (islet module). Blue peaks correspond to regions in the alignment that correspond to Dr in3.4 (intestine module). Percent identity is calculated from pairwise alignments of each module with zebrafish (VISTA parameters: 25 bp sliding window, LAGAN alignment). (C) Representative islet and intestinal images from injections of each orthologous in3.2 module. Orange or blue arrowheads mark positive islet or intestine expression, respectively. The absence of arrowheads denotes negative expression in each tissue. (D) Summary of mosaic expression for each species. Ratios of islet or intestine positive fish versus total fish expressing gfp are shown. Orange or blue (+) denotes that the construct was sufficient to confer expression in the islet or intestine, respectively. Black (−) denotes insufficiency. Note that Dalb and Cm sequences were not tested (nt) in this heterologous functional assay. See also Figures S5 and S6. Truncation mapping of the islet and intestinal regulatory modules in angptl4 intron 3
Guided by our conservation analyses, we next sought to map the boundaries of critical regulatory regions in the zebrafish in3.3 islet and in3.4 intestinal CRMs by creating and testing truncations of these modules. Each truncation construct was injected into embryos and analyzed at 6–7 dpf for mosaic expression in the islet or intestine. These analyses defined a 164 bp region sufficient to confer islet expression (in3.17; Figure 5A, 5B) including a 129 bp region present in all islet-sufficient truncations (Figure 5A). This 129 bp region overlaps with conserved regions identified in our comparative evolutionary analysis (Figure 7A). In silico prediction of transcription factor binding sites in this critical region identified putative binding sites for multiple transcription factors known to be active in pancreatic islets such as Myc [64], [65] and Arnt/HIF1b [66], [67], as well ubiquitously expressed transcription factors with important regulatory roles in β-cells such as USF [68] and CREB/ATF [69] (Figure 7A).
Fig. 5. Truncation mapping of the islet and intestinal regulatory module. 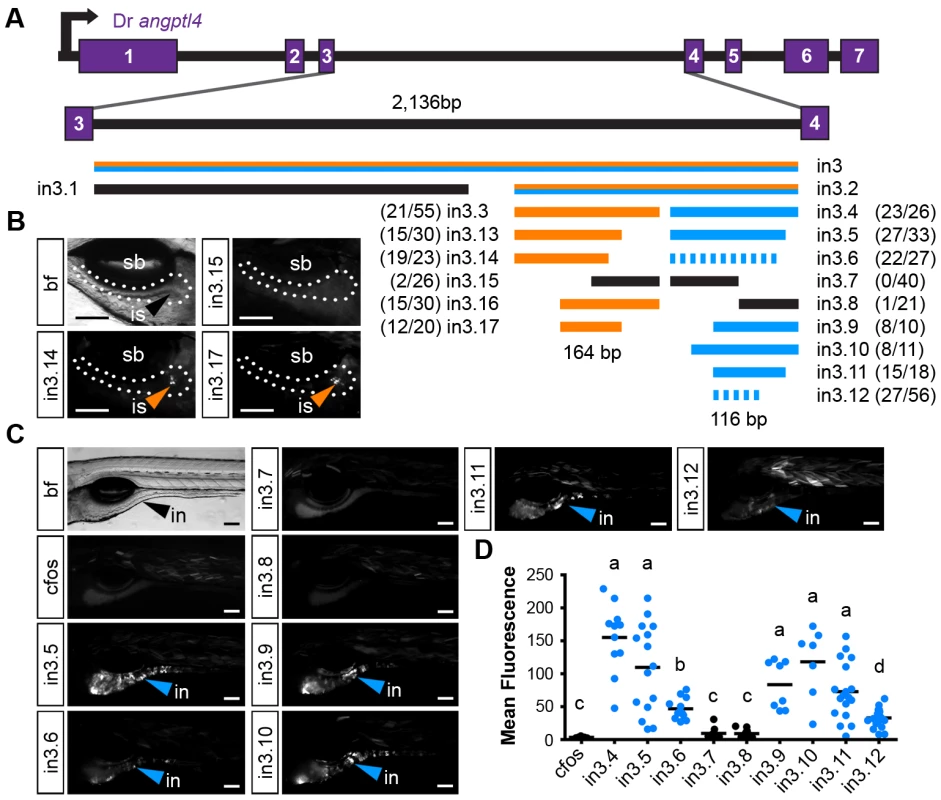
(A) Scaled schematic of the zebrafish angptl4 locus showing annotations of truncations assayed for regulatory potential. Orange lines indicate sufficiency to confer islet expression, blue lines indicate sufficiency to confer intestinal expression, and black lines indicate insufficiency in intestine and islet. Dashed blue lines indicate reduced intestinal expression compared to in3.4. Ratios of islet or intestine positive fish versus total fish expressing gfp are shown in parentheses next to truncation labels. (B) Representative images of islet views from mosaic injected fish of each truncation construct. Orange arrows mark islet expression (is). Scale bars = 100 µm. (C) Representative images of intestinal views from mosaic fish injected with each truncation construct. Blue arrows mark intestinal expression (in). Scale bars = 100 µm. (D) Relative mean intestinal fluorescence within the intestine was quantified in mosaic animals (see Materials and Methods) and plotted per injected fish. Circles represent mean fluorescence averaged for three mosaic patches within one fish, and are colored blue or black to designate truncations that are sufficient or insufficient to confer intestinal expression, respectively. Statistical significance was tested using Kruskal-Wallis one-way analysis of variance (labels: a = P<.001, b = P<.05 vs. Fos; c = P<.001, d = P<.01 vs. in3.4). Scale bars = 100 µm. A distinct 116 bp region (in3.12) was found to be sufficient to confer intestinal expression (Figure 5A, 5C). Notably, the intensity driven by in3.12 in the intestine was lower than other larger truncations of this module that confer strong intestine-specific expression, such as in3.9 and in3.11 (Figure 5C, 5D). The in3.12 truncation therefore represents a minimal intestinal regulatory module that requires additional flanking sequence information to facilitate maximal activity. Intriguingly, the in3.11 truncation, which displays strong intestinal activity, overlaps with two regions of high conservation identified in our comparative evolutionary analysis (Figure 7B), suggesting that specific sequences within these conserved regions may be responsible for mediating intestine-specific enhancer activity. Together, these results define the approximate boundaries of functional regulatory DNA within angptl4 intron 3 required for intestinal and islet transcription.
Site-directed mutagenesis confirms functional motifs within the intestinal module
To complement our comparative genomic and truncation strategies, we used site-directed mutagenesis (SDM) to generate a higher-resolution understanding of the functional DNA motifs required for enterocyte-specific transcription of angptl4. Ten base-pair substitutions were tiled across the region corresponding to in3.11 within the context of the entire in3.4 module, and assayed for competency to drive intestinal transcription (Figure 6A). This analysis revealed two regions of 40 bp and 20 bp that disrupt intestinal reporter expression when mutated (Figure 6B, 6C). DNA adjacent to these regions was not required for intestinal expression, validating the efficacy of the experimental approach. These data support our truncation mapping experiments (Figure 5) by localizing a required region within the in3.12 truncation, as well as a second region within the larger, more active in3.11 truncation. We observed strong overlap between conserved sequences in intestine-positive in3.4 modules identified in our comparative genomic analysis and regions identified by SDM as required for intestinal expression (Figure 7B). Specifically, SDM revealed that regions deleted in Daeq and Dn lineages do not harbor functional motifs required for intestinal expression. Most notably, mutation block 4–7 overlap with the single nucleotide polymorphisms between Devario and Danio species (Figure S6). This region harbors predicted binding sites for transcription factors involved in intestinal epithelial cell biology (Figure 7B; see Discussion) that represent attractive candidates for controlling enterocyte-specific angptl4 transcription.
Fig. 6. Site-directed mutagenesis defines DNA motifs required for intestinal expression. 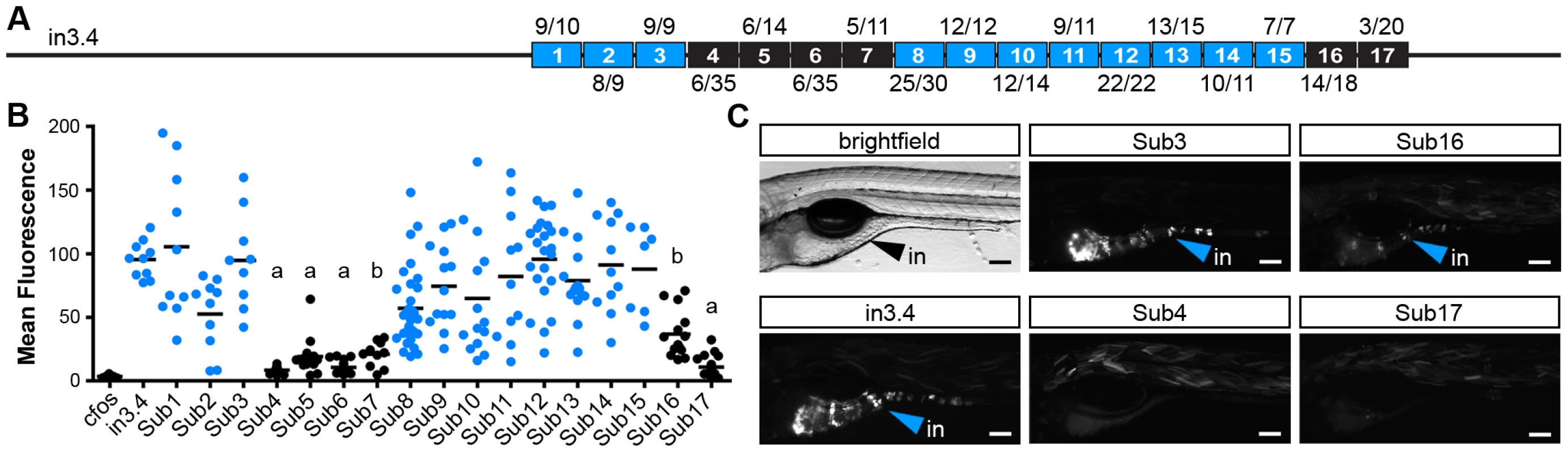
(A) Scaled schematic showing 10 bp substitution blocks tiled across the zebrafish angptl4 in3.11 region within the context of the entire in3.4 intestinal module. Black or blue blocks represent mutations that do or do not significantly alter intestinal expression compared to wild type in3.4, respectively (see below). Ratios of intestine positive fish versus total fish expressing GFP are shown in parentheses above or below substitution block labels. (B) Relative mean intestinal fluorescence was quantified in mosaic animals (see Materials and Methods) and plotted per injected fish. Circles represent mean fluorescence averaged for three mosaic patches within a single fish and are colored blue or black to designate mutations that do or do not confer intestinal expression, respectively. Statistical significance was tested using Kruskal-Wallis one-way analysis of variance (labels: a = P<.01 vs. in3.4, P>.05 vs. Fos; b = P>.05 vs. Fos; unlabeled = P>.05 vs. in3.4, P<.01 vs. Fos). (C) Images from animals with mosaic expression of five representative mutant constructs are shown. Blue arrows indicate intestinal expression (in). Scale bars = 100 µm. Fig. 7. Summary of functional conservation and mapping of islet and intestinal regulatory information. 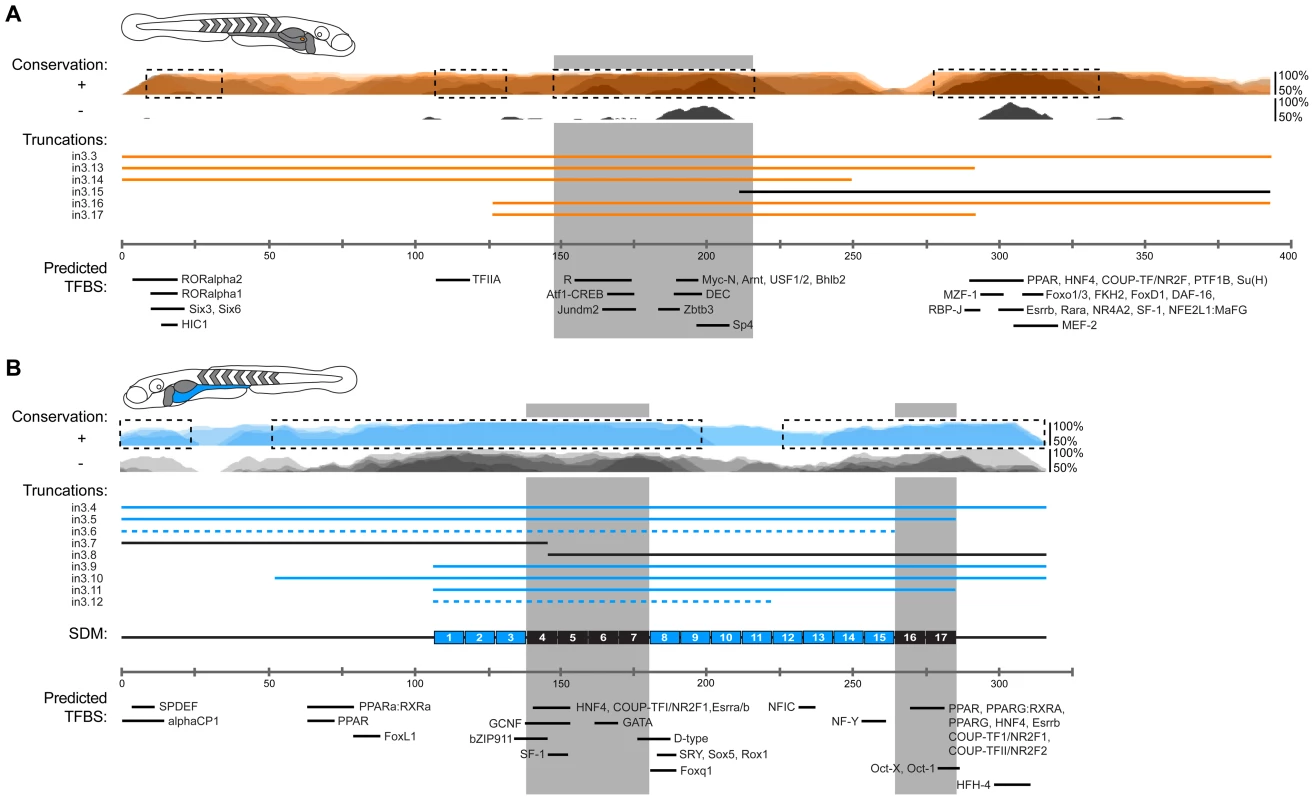
(A) Conservation plots, module truncations, and predicted transcription factor binding sites (TFBS) in islet CRM in3.3 are overlayed and annotated to scale. The grey shaded box represents the region that is present in all positive truncations and has strong conservation in islet-positive species. (B) Conservation plots, module truncations, SDM data, and predicted transcription factor binding sites (TFBS) in intestinal CRM in3.4 are overlayed and annotated to scale. Two grey shaded boxes represent regions that are present in all positive truncations, are required for intestinal expression, and have strong conservation in intestine-positive species. Dotted boxes in panels A and B represent highly conserved regions from each (A) islet-positive or (B) intestine-positive species used to predict common TFBS (see Figures S5 and S6, and Materials and Methods). The in3.4 module recapitulates angptl4 suppression by the microbiota
The presence of commensal gut microbiota in mice results in decreased levels of Angptl4 transcript specifically in the intestinal epithelium, which is thought to lead to increased peripheral fat storage [8]. However, it remained unknown whether this microbe-induced change in transcript levels was due to alterations in transcriptional activity or transcript stability. We speculated that the intestine-specific cis-regulatory module within intron 3 could impart this environmental response in the zebrafish. Our previous comparisons of 6 dpf GF zebrafish to age-matched ex-GF zebrafish colonized since 3 dpf with a normal microbiota (conventionalized or CONVD) indicated that the presence of a microbiota results in reduced angptl4 transcript levels [47]–[49]. To define the cellular origins of this response in zebrafish hosts, we used semi-quantitative WISH assays to reveal marked reduction of angptl4 mRNA in the intestinal epithelium in 6 dpf CONVD zebrafish compared to age-matched GF controls (Figure 8A). These results indicate that intestinal epithelial suppression of angptl4 expression is a conserved response to the microbiota in zebrafish and mammalian hosts.
Fig. 8. The intestinal module in3.4 recapitulates microbial suppression of angptl4. 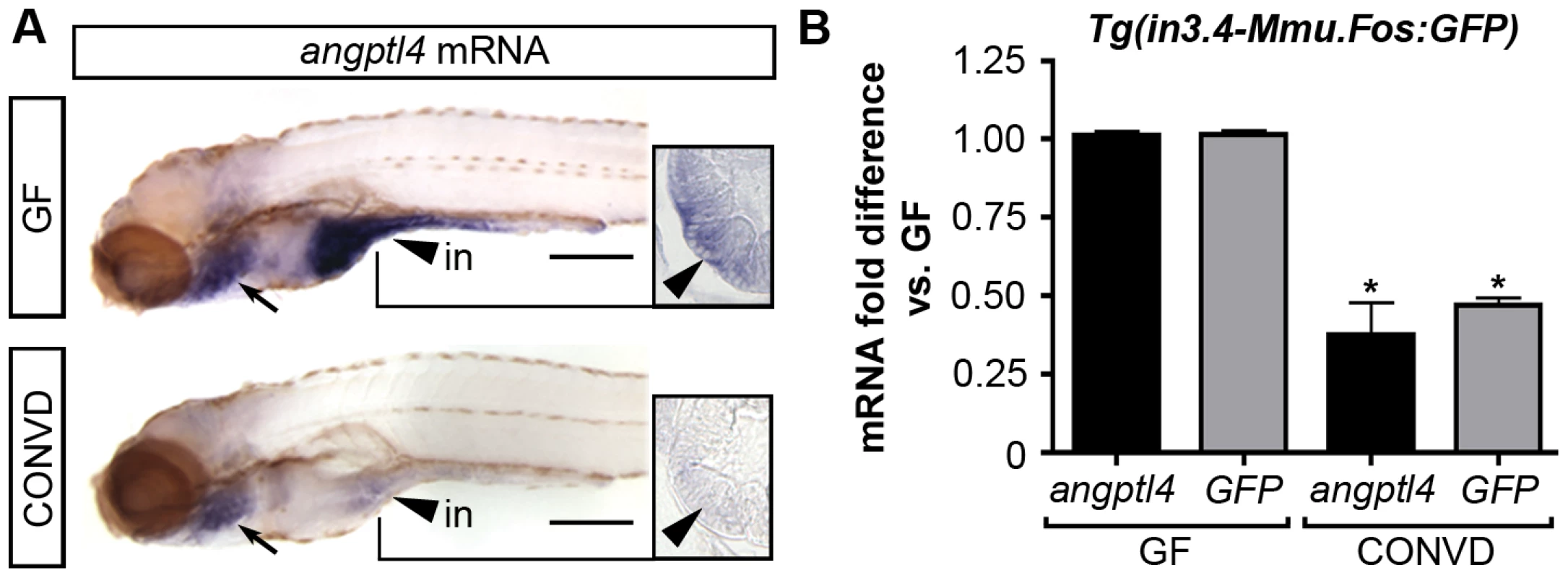
(A) Semi-quantitative whole mount in situ hybridization of angptl4 mRNA in 6 dpf germ-free (GF) and conventionalized (CONVD) animals. Arrowheads mark intestinal expression. Note that the background staining in the gills (arrows) is similar in GF and CONVD fish. Transverse sections show that microbial suppression of angptl4 mRNA is specific to the intestinal epithelium. (B) Quantitative RT-PCR of angptl4 and GFP mRNA levels in 6 dpf GF and CONVD Tg(in3.4-Mmu.Fos:GFP) animals. GF and CONVD animals were derived from the same Tg(in3.4-Mmu.Fos:GFP) stable line. GFP and angptl4 mRNA were normalized to 18S rRNA levels and are shown as fold difference compared to GF controls averaged across 3 experimental replicates ± SEM (2 biological replicate groups of 10 larvae per condition per experiment). Similar results were attained when normalized to ribosomal protein L32 (rpl32) rRNA levels. Asterisks denote P-value<.01 from unpaired T-test between GF and CONVD conditions for each gene. See also Figure S8. We next tested the ability of the zebrafish intestinal CRM in3.4 to mediate the observed microbial suppression of the endogenous angptl4 gene. We reared stable Tg(in3.4-Mmu.Fos:GFP) zebrafish to 6 dpf under GF or CONVD conditions and assayed transcript levels for both GFP reporter and endogenous angptl4 using qRT-PCR. Consistent with our WISH results, endogenous angptl4 transcript levels were significantly and reproducibly reduced in CONVD compared to GF animals (Figure 8B). Strikingly, transcript levels of the GFP reporter gene were similarly reduced in CONVD compared to GF animals (Figure 8B). These observations were confirmed using an independent stable transgenic line, Tg(in3.2-Mmu.Fos:tdT), harboring the in3.2 reporter which includes the in3.4 module (Figure S7). These data identify the angptl4 in3.4 module as a nodal cis-regulatory module that integrates transcriptional regulatory input from intestinal epithelial-specific and microbial factors.
Discussion
Non-overlapping modules confer cell-type specific transcription of angptl4
Transcriptional regulation is a key determinant of gene function in the context of animal development and physiology. Recent biochemical and genetic studies in mouse and humans have identified Angptl4 as a critical hormonal regulator of TG-rich lipoprotein metabolism, angiogenesis, and tumor cell survival and metastasis. An improved understanding of the mechanisms controlling Angptl4 activity levels could therefore lead to new approaches for controlling multiple pathophysiologic processes. Although we have a working understanding of Angptl4's post-translational functions, our current knowledge of the mechanisms underlying Angptl4 transcription in different tissues is relatively limited. Here, we exploited the advantages of the zebrafish model system to examine the regulatory potential of DNA at the angptl4 locus in all cell types simultaneously and within an intact and living vertebrate organism that can be raised under gnotobiotic conditions. We found that the zebrafish angptl4 ortholog is expressed in many of the same tissues and cell types as mammalian Angptl4 (i.e., liver, pancreatic β-cells, and intestinal enterocytes). This finding suggests that the tissue-specific pattern of Angptl4 expression may have been conserved in the last common ancestor of mammalian and teleost lineages and might have important functional consequences on vertebrate physiology.
Our results reveal that transcription of angptl4 in distinct tissues might be governed by independent cis-regulatory mechanisms. This modular design could have important implications for Angptl4 evolution and function. First, tissue-specific CRMs could have allowed independent evolution of CRM sequence structure. Consistent with this notion, we observed evidence of differential evolution of the islet and intestinal modules within teleost fish lineages (Figure 4). Differential selective pressures influencing CRM sequence evolution likely arise from the vastly different cellular contexts and exogenous stimuli of each cell type. Pancreatic β-cells are surrounded by other endocrine and exocrine pancreatic cells as well as vascular endothelial cells, whereas intestinal epithelial cells are exposed to complex and variable contents of the intestinal lumen and to the cells of the underlying lamina propria. Combining the observations that (i) functional conservation of the intestinal module is restricted to Danio species, (ii) transcriptional activity of the intestinal module is sensitive to the microbial status of the intestinal lumen, and (iii) this microbial regulation of angptl4 transcript levels is conserved in mammals, suggests an intriguing possibility that genes expressed in intestinal epithelia exposed to the dynamic and potentially hazardous luminal environment undergo relatively rapid regulatory evolution. Previous studies have suggested that the expression and function of defensin genes within the epithelia of the intestine and other exposed tissues has driven rapid evolution of their coding sequences [70], and our results raise the possibility that similar selective pressures may also affect evolutionary rate of regulatory sequences for angptl4 and potentially other genes. Second, discrete cis-regulatory modules could have led to the independent evolution of Angptl4 synthesis in each respective cell type. This evolution would allow each expressing cell type to independently communicate its physiologic status and environmental exposures systemically by secreting Angptl4 into circulation, and locally by secreting Angptl4 into the extracellular space. The modular organization of these independent tissue-specific CRMs suggests that therapeutic strategies could be developed to control Angptl4 synthesis in specific target tissues without unintended effects on Angptl4 synthesis in other tissues.
Previous studies of CRM evolution in vertebrates and invertebrates have focused primarily on enhancers regulating expression of genes involved in development [50], [61], [71]. These studies revealed that maintenance of regulatory function can be sustained over long evolutionary distances despite marked sequence dissimilarity and turnover of regulatory information. Our work provides a novel example of utilizing genomic DNA sequences from both close and distant relatives to define the evolutionary dynamics of multiple CRMs and marks the first time to our knowledge that such an extensive exploration (i.e., 12 related fish species) was carried out in a vertebrate. We find that transcriptional output generated by both the intestinal and islet modules is maintained through a striking conservation in DNA sequence throughout the entire functional module, with little or no turnover of predicted binding sites. This finding suggests that these modules can comply with the “enhanceosome model” of regulatory information organization, as opposed to the “billboard model,” which accommodates variation in binding site order, orientation, and spacing [72], [73]. However, we detected little non-coding sequence conservation between zebrafish angptl4 and mouse Angptl4 intron 3, and we did not detect islet or intestinal reporter expression in a heterologous assay in which we tested full and truncated versions of mouse introns 3 and 4 in the zebrafish (data not shown). This result suggests either that regulatory information governing islet and intestinal expression of murine Angptl4 is not located within intron 3 or that compensatory cis/trans mutations render murine intron 3 sequences non-functional in the zebrafish. We suspect that rules governing CRM function and evolution are dependent on the distinct nature of the organism, the specific module, and the signals that the module integrates. It therefore remains an intriguing question as to what extent lessons learned from developmental gene regulation are applicable to the evolution of CRMs controlling expression of genes like Angptl4 that function in homeostatic physiology or in response to environmental factors like the microbiota [72].
Analyses of Drosophila genomes have elegantly shown that CRM “discovery power scales with the divergence time and number of species compared” [74], and our results suggest that the same will be true in vertebrate lineages. Moreover, our data underscore the need for more reference genome sequences from phylogenetically diverse fish species, in combination with experimentally tractable fish models such as the zebrafish, to facilitate new insights into vertebrate CRM function and evolution.
The nature of microbial signals regulating intestinal transcription of angptl4
The intestinal microbiota has been identified as an important environmental factor that contributes to host energy storage and obesity, and our results provide critical new insights into how this might be achieved. Previous studies in gnotobiotic mice have shown that the intestinal microbiota regulates fat storage in part by suppressing Angptl4 transcript levels in the epithelium of the small intestine but not in liver or WAT [8], [11]. However, it remained unclear whether these microbe-induced reductions of Angptl4 transcript levels were due to alterations in Angptl4 transcription or mRNA turnover. Furthermore, the molecular basis of the intestinal specificity of this response remained unknown. Our results reveal that zebrafish angptl4 transcript levels are also reduced in the intestinal epithelium in the presence of a microbiota, suggesting that the microbial regulation of angptl4 transcript levels might be an evolutionarily ancient feature of host-microbe commensalism in the vertebrate intestine. Our observation that transcript levels from the in3.4 reporter and the endogenous angptl4 gene respond similarly to microbial colonization strongly suggests that the microbiota regulates angptl4 expression, at least in part, by reducing the transcriptional activity of this enterocyte-specific enhancer module. These results indicate that enterocyte-specific and microbial control of angptl4 transcription is conferred through a shared intronic enhancer.
Future investigation will be required to determine whether microbial regulation of in3.4 activity is achieved by (i) reducing the accessibility of this chromatin region to activating trans-factors, (ii) subverting the expression or activity of activating trans-factors, and/or (iii) inducing expression or activity of repressive trans-factors that function through this module. To distinguish between these models, it will be useful to identify the microbial activity and host transcription factors that regulate angptl4 transcription in the intestinal epithelium. We previously reported that colonization of GF zebrafish with a microbiota harvested from conventionally raised zebrafish or mice resulted in similar suppression of angptl4 transcript levels in the digestive tract [48]. This finding suggests that the microbial factor(s) regulating zebrafish angptl4 transcription is expressed by the ‘native’ zebrafish microbiota and in the ‘non-native’ and compositionally distinct mouse gut microbiota. Previous studies have identified individual microbial species sufficient to regulate angptl4 expression in gnotobiotic zebrafish [47], [48] and mouse hosts [9], [75] as well as in cultured colon cancer cells [31], [76], suggesting that reductionist approaches in these microbial species could be used to define the specific factors they utilize to control expression of angptl4 homologs and other host genes.
Potential transcription factors regulating intestinal expression of angptl4
In this study, we define two minimal regions within the in3.4 CRM that harbor regulatory activity in the intestine and are also conserved within the Danio lineage (Figure 7B). Predicted transcription factor binding sites within these regions intimates potential roles for these factors in regulation of angptl4 tissue-specific transcription and/or microbial suppression. Because sequence-specific transcription factors typically recognize 6–12 bp motifs [77], it is reasonable to assume that multiple factors cooperate to combinatorially regulate intestinal expression through this CRM. The Hnf4 family of fatty acid-regulated nuclear receptors has evolutionarily conserved roles in lipid metabolism [78], [79], and Hnf4a is expressed in the intestinal epithelium of zebrafish [80] and mouse [81]. Similarly, GATA factors 4, 5, and 6 are all expressed in the zebrafish [82], [83] and mouse [84], [85] intestinal epithelium and have proposed roles in regulating epithelial cell differentiation. Notably, C. elegans GATA family member elt-2 has been implicated in mediating intestinal epithelial cell immune responses [86], suggesting that GATA factors could mediate tissue-specific as well as microbial regulatory inputs at angptl4. PPAR family members have been identified as key regulators of mammalian Angptl4 expression in adipocytes and hepatocytes through PPAR responsive elements located in the 5′ portion of human ANGPTL4 intron 3 [27], [30], and zebrafish PPARγ [87] and PPARδ [88] homologs are expressed in the larval intestine. The zebrafish angptl4 locus contains multiple predicted PPRE sites, including several in both the 5′ and 3′ portion of intron 3 [89]. Most notably, a predicted PPRE was detected within the substitution blocks 16/17 in the intestinal enhancer in3.4 (Figure 7B). However, the PPREs within zebrafish angptl4 intron 3 that display the highest sequence homology to the defined human ANGPTL4 intron 3 PPRE mapped outside of minimal regions for either intestinal or islet expression within the 5′ liver module (data not shown). The location of these PPREs in the 5′ region of zebrafish angptl4 intron 3, combined with the fact that the PPREs discovered in human ANGPTL4 are also located in the 5′ portion of intron 3, suggests that the predicted PPREs within the 3′ islet and intestine CRMs of zebrafish angptl4 could represent novel elements for which functional equivalents have not been identified in mammals.
Although these predicted factors represent candidates for controlling intestine-specific regulation of angptl4, databases of predicted TFBSs are incomplete and commonly produce both false-positive and false-negative predictions. Moreover, critical regions identified by SDM might reflect sequences that alter nucleosome positioning or histone modification patterns rather than binding sites for sequence-specific transcription factors. Therefore, we anticipate that unbiased methods for transcription factor discovery will provide the most rigorous approach to an improved understanding of this cis/trans system. The structure-function analysis of the zebrafish in3.4 intestinal enhancer module reported here was designed to identify sequences critical for intestinal activity. It will therefore be interesting to determine whether exogenous microbial inputs are interpreted through the same or distinct motifs within this CRM and how the endogenous trans-acting factors mediating microbial and intestinal regulatory inputs interact to determine transcriptional output.
Materials and Methods
Zebrafish husbandry
All experiments using zebrafish were performed in wild-type TL or Tg(ins:CFP-NTR)s892 [60] strains according to established protocols approved by the Animal Studies Committee at the University of North Carolina at Chapel Hill. New stable transgenic lines genereated in this study are listed in Table S3. Conventionally raised zebrafish were reared and maintained as described [87]. Production, colonization, maintenance, and sterility testing of germ-free zebrafish were performed as described [45], [49].
Protein sequence analysis
Protein sequences from top BlastP hits to human (Homo sapiens, Hs) ANGPTL4 and zebrafish Angptl4 (Danio rerio, Dr) were acquired through NCBI or Ensembl and aligned using MUSCLE with default settings [90]. Amino acids highlighted in black represent identical residues in at least 50% of species, whereas amino acids highlighted in grey represent biochemically similar residues (Boxshade). The cleavage recognition sequence and LPL inhibition domain were annotated using information from previous publications [15], [91]. The boundaries of the fibrinogen domain were annotated using in silico predictions [92], [93]. Gaps in the alignment resulting from poorly annotated sequences were manually curated using primary DNA sequence and in silico translated using ExPASy [94]. The workflow for inferring phylogenetic relationships was performed at http://mobyle.pasteur.fr/cgi-bin/portal.py. A distance matrix was computed using Phylip 3.67 (Protdist, JTT matrix, default settings), and trees were built using the neighbor-joining method. Bootstrap analysis was performed from 1000 replicates. PHYLIP software and the maximum likelihood probability model [95] using default settings were used to confirm the phylogeny inferred using distance methods. See Table S1 for a complete list of protein sequences used in this study.
DNA sequence analysis
Genomic DNA sequences encompassing 10 kb upstream, including, and 10 kb downstream of the Angptl4 locus from Homo sapiens (GRCh37 : 19 : 8419011 : 8449257 : 1), Mus musculus (NCBIM37 : 17 : 33900702 : 33928520:−1), Canis familiaris (BROADD2 : 20 : 55933601 : 55958821 : 1), Danio rerio (Zv9 : 2:23312551 : 23337293), Oryzias latipes (MEDAKA1 : 17 : 6095931 : 6120384 : 1), Takifugu rubripes (FUGU4:scaffold_212 : 367815 : 391593 : 1), and Tetraodon nigroviridis (TETRAODON8 : 15 : 3989265 : 4012887 : 1) were acquired through Ensembl. 10 kb was chosen as a cutoff because of proximity to neighboring gene loci. Genomic DNA sequence encompassing the angptl4 locus from Danio albolineatus was generously provided by David Parichy (Department of Biology, University of Washington). For species without available genomic sequence, angptl4 intron 3 regions were PCR amplified from the relevant genomic DNA using a high-fidelity Taq polymerase (Platinum, Invitrogen) and the primers listed in Table S2. PCR products were cloned into TOPO vector pCR2.1 (Invitrogen) prior to sequencing with M13F primers. An EST corresponding to an angptl4 homolog in Ictalurus punctatus (CK419825) was used to design primers targeting exon 3 and exon 4 for PCR amplification of the full-length intron 3. For Cypriniformes species, ESTs EG548328 (Rutilus rutilus), DT085020 (Pimephales promelas), GH715226 (Pimephales promelas), and AM929131 (Carassius auratus) were aligned and used to design primers targeting highly conserved regions in angptl4 exon 3 and exon 4, which we predicted would function for multiple Cypriniformes species. These primers were used to amplify, clone, and sequence the full-length intron 3 from Cyprinus carpio and Chromobotia macracanthus. Alignment of Cc, Cm, and Dr revealed 100% conservation at the extreme 5′ end of the in3.2 module. We used a forward primer targeting in3.2 in combination with a reverse primer targeting exon 4 for cloning of the remaining Cyprinidae species. The bacterial artificial chromosome golwb118_K01 containing the angptl4 locus from Oryzias latipes was provided by Hiroyo Kaneko (Laboratory of Bioresource, National Institute for Basic Biology, Okazaki, Japan). Carassius auratus, Puntius conchonius, Cyprinus carpio, Devario aequipinnatus, and Chromobotia macracanthus genomic DNA was extracted from the fins of two individuals acquired from commercial suppliers. Genomic DNA from Ictalurus punctatus and Danio species (Danio nigrofasciatus, Danio choprae, Danio feegradei) from one individual were generously provided by Zhanjiang Liu (Department of Fisheries and Allied Aquacultures, Auburn University) and David Parichy (Department of Biology, University of Washington), respectively. Novel angptl4 intron 3 sequences generated in this study were deposited in GenBank with accession numbers JN606312–JN606321. Intronic sequences were aligned in mVISTA using LAGAN [96] and visualized using VISTA conservation plots (100 bp windows Figure 2 and 25 bp windows Figure 4) [97].
Motif and transcription factor binding site (TFBS) predictions
DNA sequences were queried for predicted transcription factor binding sites deposited in TRANSFAC [98] and JASPAR [99] databases using MATCH [100] and TESS [101] programs using default settings. We used a discriminative motif MEME [102] search to discover motifs common to islet-positive or intestine-positive intronic regions, using sequences orthologous to in3.4 or sequences orthologous to in3.3, respectively, as negative selectors. To determine if MEME motifs were unique to islet - or intestine-positive regions, we used MAST [103] to query islet-negative (Ol in.3) or intestine-negative (Daeq, Ca, Cc, Pc, Cm, Ip, Ol in3.4) sequences for islet-positive or intestine-positive MEME motifs, respectively. TOMTOM [104] was used to query MEME hits against TRANSFAC and JASPAR databases.
Whole-mount in situ hybridization assays
In situ hybridization was performed in whole zebrafish as described [87], except that heads and tails were removed from euthanized 17 dpf animals prior to fixation. Sense and anti-sense riboprobes targeting zebrafish angptl4 were generated by digesting plasmid fj89c07 in pBK-CMV (NCBI Accession XM_686956) with NotI (sense) or BamHI (anti-sense), and transcribed in vitro using T3 (sense; Epicentre) or T7 RNA polymerase (anti-sense; Epicentre). Sense riboprobes were used in each experiment as a negative control.
Quantitative reverse transcription PCR assays
Total RNA was extracted from groups of 6 dpf whole zebrafish larvae from 6 dpf zebrafish (10 larvae per group, 2 biological replicate groups per condition per experiment, 2 experimental replicates total) using TRIzol Reagent (Invitrogen) or the Qiagen RNeasy (Qiagen) kit using manufacturer's protocol. qRT-PCR was performed as described [49]. Primers used in qRT-PCR assays are listed in Table S2.
Transcription start site and promoter mapping
ESTs at the zebrafish angptl4 locus were analyzed using UCSC and Ensembl genome browsers. Total RNA was extracted from adult zebrafish intestines and subjected to 5′RACE using the FirstChoice RLM-RACE kit (Ambion), according to the manufacturer's specifications (see Table S2 for primers). Three clones were sequenced and mapped to the zebrafish angptl4 locus.
Reporter construct cloning
All PCR reactions used for cloning were performed with high-fidelity DNA polymerase (PfuTurbo, Stratagene; Phusion, Invitrogen; Platinum Taq, Invitrogen) and TOP10 chemically competent E. coli (Invitrogen). The bacterial artificial chromosome C177A22 containing the zebrafish angptl4 locus was used as the template for all zebrafish angptl4 promoter and intronic PCR amplification and cloning. Mouse BAC (RP24-294G12, CHORI), Medaka BAC (golwb118_K01), and sequenced pCR2.1 clones (Ip, Pc, Cc, Ca, Daeq, Df, Dc, Dn) containing intronic regions orthologous to zebrafish in3.2 from each species were used as source material for cloning in heterologous reporter assays. The plasmid pT2cfosGW [50] was used as the vector backbone for all Tol2 transgenic reporter assays. The Fos minimal promoter and angptl4 5′ upstream regions were PCR amplified and directionally cloned into pT2cfosGW using XhoI and BamHI restriction sites. This step removed both the original Fos promoter and the upstream Gateway site. Of note, we observed significant levels of reporter expression in muscle tissue upon removal of the Gateway cloning site (Figure 5C,D and data not shown). Intronic DNA was cloned upstream of the Fos minimal promoter in pT2cfosGW using Gateway reagents as described [36]. The intronic module in3.4 was non-directionally cloned into Tg(-1kbangptl4:GFP) using the single BglII site located downstream of SV40polyA. A vector (Tg(in3.4-Mmu.Fos:GFP)) containing the angptl4 intronic module in3.4 was used as the source vector for site-directed mutagenesis. To create site-directed substitutions, 50 bp complementary primers containing two 20 bp regions complementary to in3.4, separated by a 10 bp substitution block, were used in circular PCR followed by DpnI treatment to digest methylated parent plasmid. A ClaI restriction site was incorporated into the 10 bp region in order to screen for mutant bacterial colonies. Selection of nucleotide exchange was generally A–C and G–T, except in cases that would create a site amenable to DamI methylation. All plasmids were verified by Sanger dideoxy terminator sequencing. All primers used are listed in Table S2.
Injections, imaging, and reporter quantification
Co-injections of Tol2 plasmid and transposase mRNA were performed as described [36]. Generally, 100–200 zebrafish embryos were injected at the 1–2 cell stage with approximately 69 pg of plasmid DNA at a DNA∶transposase ratio of 1∶2. Injections of each construct were performed with at least two sequence-verified plasmids in two independent experiments. Mosaic expression patterns were quantified as follows: at least 200 fish were visually observed, and at least 10 were scored per construct for positive/negative expression in selected tissues. At least 7–20 fish/construct were imaged at the same magnification and exposure time and densitometric measures were quantified in 8-bit grey scale images using ImageJ software [105]. Three mosaic patches within a given tissue of an imaged fish were quantified for mean fluorescence intensity and averaged. Statistical significance was analyzed using Kruskal-Wallis one-way analysis of variance and Dunn's multiple comparison test using GraphPad Prism software. Injected larvae were raised to adulthood and screened for stable germ-line insertion. Where indicated, patterns identified in mosaic animals were verified in a least two independent stable germ-line insertions (3). In each case, independent pedigrees of the same Tol2 vector displayed the same specific pattern of expression in the intestine, liver, and islet, respectively.
Immunohistochemistry
Staining of fixed and sectioned 6 dpf zebrafish was performed exactly as described [49]. Primary antibodies used in this study were anti-GFP (Rabbit, 1∶500, Invitrogen), 2F11 (mouse, 1∶200), 4E8 (mouse, 1∶200; gifts from Julian Lewis), and secondary antibodies were AF568 (goat anti-mouse, 1∶500, Invitrogen) and AF488 (goat anti-mouse, 1∶500, Invitrogen).
DNase I hypersensitivity
Three intestines were dissected from adult zebrafish at 1 year post-fertilization, splayed, and washed extensively with 1× PBS. Intestines were incubated for 15 minutes on ice in 5 ml of Dissociation Reagent 1 (1× PBS, 30 mM EDTA, 1.5 mM DTT, 1× Complete protease inhibitors; Roche), then transferred to Dissociation Reagent 2 (1× PBS, 30 mM EDTA, 1× Complete protease inhibitors) and shaken at 25°C until epithelial layers were sufficiently sloughed. Epithelial cells were collected, washed in 1× PBS, and re-suspended in 500 microliters of RSB (10 mM Tris-HCl pH 7.4, 10 mM NaCl, 3 mM MgCl2). Cells were gently lysed in 10 ml cold RSB plus 0.075% NP-40 and nuclei pelleted at 500× G at 4°C for 10 minutes. Nuclei were incubated with various concentrations of Dnase I (0–1.5 units, NEB) for 10 minutes at 37°C. Reactions were stopped by adding an equal volume of 2× Lysis Buffer (1% SDS, 200 mM NaCl, 10 mM EDTA, 20 mM Tris pH 7.5, 0.4 mg/ml proteinase K) and incubated overnight at 37°C. Digested DNA was extracted using phenol/cholorform/isoamyl alcohol (Fisher), precipitated with ethanol and sodium acetate, and quantified using a fluorimeter (Qubit, Invitrogen). Quantitative PCR was performed as described above using primers listed in Table S2.
Supporting Information
Zdroje
1. CampJGKantherMSemovaIRawlsJF 2009 Patterns and scales in gastrointestinal microbial ecology. Gastroenterology 136 1989 2002
2. BackhedFLeyRESonnenburgJLPetersonDAGordonJI 2005 Host-bacterial mutualism in the human intestine. Science 307 1915 1920
3. SartorRB 2008 Microbial influences in inflammatory bowel diseases. Gastroenterology 134 577 594
4. WangZKlipfellEBennettBJKoethRLevisonBS 2011 Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 472 57 63
5. LeyRETurnbaughPJKleinSGordonJI 2006 Microbial ecology: human gut microbes associated with obesity. Nature 444 1022 1023
6. FlintHJBayerEARinconMTLamedRWhiteBA 2008 Polysaccharide utilization by gut bacteria: potential for new insights from genomic analysis. Nat Rev Microbiol 6 121 131
7. QinJLiRRaesJArumugamMBurgdorfKS 2010 A human gut microbial gene catalogue established by metagenomic sequencing. Nature 464 59 65
8. BackhedFDingHWangTHooperLVKohGY 2004 The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U S A 101 15718 15723
9. BackhedFManchesterJKSemenkovichCFGordonJI 2007 Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc Natl Acad Sci U S A 104 979 984
10. WangHEckelRH 2009 Lipoprotein lipase: from gene to obesity. Am J Physiol Endocrinol Metab 297 E271 288
11. FleissnerCKHuebelNAbd El-BaryMMLohGKlausS 2010 Absence of intestinal microbiota does not protect mice from diet-induced obesity. Br J Nutr 104 919 929
12. KosterAChaoYBMosiorMFordAGonzalez-DeWhittPA 2005 Transgenic angiopoietin-like (angptl)4 overexpression and targeted disruption of angptl4 and angptl3: regulation of triglyceride metabolism. Endocrinology 146 4943 4950
13. DesaiULeeECChungKGaoCGayJ 2007 Lipid-lowering effects of anti-angiopoietin-like 4 antibody recapitulate the lipid phenotype found in angiopoietin-like 4 knockout mice. Proc Natl Acad Sci U S A 104 11766 11771
14. LeeECDesaiUGololobovGHongSFengX 2009 Identification of a new functional domain in angiopoietin-like 3 (ANGPTL3) and angiopoietin-like 4 (ANGPTL4) involved in binding and inhibition of lipoprotein lipase (LPL). J Biol Chem 284 13735 13745
15. YauMHWangYLamKSZhangJWuD 2009 A highly conserved motif within the NH2-terminal coiled-coil domain of angiopoietin-like protein 4 confers its inhibitory effects on lipoprotein lipase by disrupting the enzyme dimerization. J Biol Chem 284 11942 11952
16. RomeoSPennacchioLAFuYBoerwinkleETybjaerg-HansenA 2007 Population-based resequencing of ANGPTL4 uncovers variations that reduce triglycerides and increase HDL. Nat Genet 39 513 516
17. FolsomARPeacockJMDemerathEBoerwinkleE 2008 Variation in ANGPTL4 and risk of coronary heart disease: the Atherosclerosis Risk in Communities Study. Metabolism 57 1591 1596
18. CazesAGalaupAChomelCBignonMBrechotN 2006 Extracellular matrix-bound angiopoietin-like 4 inhibits endothelial cell adhesion, migration, and sprouting and alters actin cytoskeleton. Circ Res 99 1207 1215
19. ZhuPTanMJHuangRLTanCKChongHC 2011 Angiopoietin-like 4 protein elevates the prosurvival intracellular O2(−):H2O2 ratio and confers anoikis resistance to tumors. Cancer Cell 19 401 415
20. PaduaDZhangXHWangQNadalCGeraldWL 2008 TGFbeta primes breast tumors for lung metastasis seeding through angiopoietin-like 4. Cell 133 66 77
21. GalaupACazesALe JanSPhilippeJConnaultE 2006 Angiopoietin-like 4 prevents metastasis through inhibition of vascular permeability and tumor cell motility and invasiveness. Proc Natl Acad Sci U S A 103 18721 18726
22. GohYYPalMChongHCZhuPTanMJ 2010 Angiopoietin-like 4 interacts with integrins beta1 and beta5 to modulate keratinocyte migration. Am J Pathol 177 2791 2803
23. KerstenSMandardSTanNSEscherPMetzgerD 2000 Characterization of the fasting-induced adipose factor FIAF, a novel peroxisome proliferator-activated receptor target gene. J Biol Chem 275 28488 28493
24. YoonJCChickeringTWRosenEDDussaultBQinY 2000 Peroxisome proliferator-activated receptor gamma target gene encoding a novel angiopoietin-related protein associated with adipose differentiation. Mol Cell Biol 20 5343 5349
25. KutluBBurdickDBaxterDRasschaertJFlamezD 2009 Detailed transcriptome atlas of the pancreatic beta cell. BMC Med Genomics 2 3
26. BikopoulosGda Silva PimentaALeeSCLakeyJRDerSD 2008 Ex vivo transcriptional profiling of human pancreatic islets following chronic exposure to monounsaturated fatty acids. J Endocrinol 196 455 464
27. MandardSZandbergenFTanNSEscherPPatsourisD 2004 The direct peroxisome proliferator-activated receptor target fasting-induced adipose factor (FIAF/PGAR/ANGPTL4) is present in blood plasma as a truncated protein that is increased by fenofibrate treatment. J Biol Chem 279 34411 34420
28. StaigerHHaasCMachannJWernerRWeisserM 2009 Muscle-derived angiopoietin-like protein 4 is induced by fatty acids via peroxisome proliferator-activated receptor (PPAR)-delta and is of metabolic relevance in humans. Diabetes 58 579 589
29. GeorgiadiALichtensteinLDegenhardtTBoekschotenMVvan BilsenM 2010 Induction of cardiac Angptl4 by dietary fatty acids is mediated by peroxisome proliferator-activated receptor beta/delta and protects against fatty acid-induced oxidative stress. Circ Res 106 1712 1721
30. KaddatzKAdhikaryTFinkernagelFMeissnerWMuller-BrusselbachS 2010 Transcriptional profiling identifies functional interactions of TGF beta and PPAR beta/delta signaling: synergistic induction of ANGPTL4 transcription. J Biol Chem 285 29469 29479
31. AronssonLHuangYPariniPKorach-AndreMHakanssonJ 2010 Decreased fat storage by Lactobacillus paracasei is associated with increased levels of angiopoietin-like 4 protein (ANGPTL4). PLoS ONE 5 e13087 doi:10.1371/journal.pone.0013087
32. KoliwadSKKuoTShippLEGrayNEBackhedF 2009 Angiopoietin-like 4 (ANGPTL4, fasting-induced adipose factor) is a direct glucocorticoid receptor target and participates in glucocorticoid-regulated triglyceride metabolism. J Biol Chem 284 25593 25601
33. BelangerAJLuHDateTLiuLXVincentKA 2002 Hypoxia up-regulates expression of peroxisome proliferator-activated receptor gamma angiopoietin-related gene (PGAR) in cardiomyocytes: role of hypoxia inducible factor 1alpha. J Mol Cell Cardiol 34 765 774
34. WangBWoodISTrayhurnP 2007 Dysregulation of the expression and secretion of inflammation-related adipokines by hypoxia in human adipocytes. Pflugers Arch 455 479 492
35. KawakamiK 2004 Transgenesis and gene trap methods in zebrafish by using the Tol2 transposable element. Methods Cell Biol 77 201 222
36. FisherSGriceEAVintonRMBesslingSLUrasakiA 2006 Evaluating the biological relevance of putative enhancers using Tol2 transposon-mediated transgenesis in zebrafish. Nat Protoc 1 1297 1305
37. PackMSolnica-KrezelLMalickiJNeuhaussSCSchierAF 1996 Mutations affecting development of zebrafish digestive organs. Development 123 321 328
38. FieldHADongPDBeisDStainierDY 2003 Formation of the digestive system in zebrafish. II. Pancreas morphogenesis. Dev Biol 261 197 208
39. ChuJSadlerKC 2009 New school in liver development: lessons from zebrafish. Hepatology 50 1656 1663
40. WallaceKNAkhterSSmithEMLorentKPackM 2005 Intestinal growth and differentiation in zebrafish. Mech Dev 122 157 173
41. NgANde Jong-CurtainTAMawdsleyDJWhiteSJShinJ 2005 Formation of the digestive system in zebrafish: III. Intestinal epithelium morphogenesis. Dev Biol 286 114 135
42. BatesJMMittgeEKuhlmanJBadenKNCheesmanSE 2006 Distinct signals from the microbiota promote different aspects of zebrafish gut differentiation. Dev Biol 297 374 386
43. RawlsJFMahowaldMAGoodmanALTrentCMGordonJI 2007 In vivo imaging and genetic analysis link bacterial motility and symbiosis in the zebrafish gut. Proc Natl Acad Sci U S A 104 7622 7627
44. HamaKProvostEBaranowskiTCRubinsteinALAndersonJL 2009 In vivo imaging of zebrafish digestive organ function using multiple quenched fluorescent reporters. Am J Physiol Gastrointest Liver Physiol 296 G445 453
45. PhamLNKantherMSemovaIRawlsJF 2008 Methods for generating and colonizing gnotobiotic zebrafish. Nat Protoc 3 1862 1875
46. Milligan-MyhreKCharetteJRPhennicieRTStephensWZRawlsJF 2011 Study of host-microbe interactions in zebrafish. Methods Cell Biol 105 87 116
47. RawlsJFSamuelBSGordonJI 2004 Gnotobiotic zebrafish reveal evolutionarily conserved responses to the gut microbiota. Proc Natl Acad Sci U S A 101 4596 4601
48. RawlsJFMahowaldMALeyREGordonJI 2006 Reciprocal gut microbiota transplants from zebrafish and mice to germ-free recipients reveal host habitat selection. Cell 127 423 433
49. KantherMSunXMuhlbauerMMackeyLCFlynnEJ3rd 2011 Microbial Colonization Induces Dynamic Temporal and Spatial Patterns of NF-kappaB Activation in the Zebrafish Digestive Tract. Gastroenterology 141 197 207
50. FisherSGriceEAVintonRMBesslingSLMcCallionAS 2006 Conservation of RET regulatory function from human to zebrafish without sequence similarity. Science 312 276 279
51. NavratilovaPFredmanDHawkinsTATurnerKLenhardB 2009 Systematic human/zebrafish comparative identification of cis-regulatory activity around vertebrate developmental transcription factor genes. Dev Biol 327 526 540
52. TsujimuraTHosoyaTKawamuraS 2010 A single enhancer regulating the differential expression of duplicated red-sensitive opsin genes in zebrafish. PLoS Genet 6 e1001245 doi:10.1371/journal.pgen.1001245
53. ChaoCHWangHDYuhCH 2010 Complexity of cis-regulatory organization of six3a during forebrain and eye development in zebrafish. BMC Dev Biol 10 35
54. NgCEYokomizoTYamashitaNCirovicBJinH 2010 A Runx1 intronic enhancer marks hemogenic endothelial cells and hematopoietic stem cells. Stem Cells 28 1869 1881
55. BorokMJTranDAHoMCDrewellRA 2010 Dissecting the regulatory switches of development: lessons from enhancer evolution in Drosophila. Development 137 5 13
56. WassermanWWSandelinA 2004 Applied bioinformatics for the identification of regulatory elements. Nat Rev Genet 5 276 287
57. HaeusslerMJolyJS 2011 When needles look like hay: how to find tissue-specific enhancers in model organism genomes. Dev Biol 350 239 254
58. HedgesS 2009 Vertebrates; HedgesSBKS Oxford University Press
59. PengZDR. HeS 2009 Teleost fishes.; HedgesSBKS Oxford University Press
60. CuradoSAndersonRMJungblutBMummJSchroeterE 2007 Conditional targeted cell ablation in zebrafish: a new tool for regeneration studies. Dev Dyn 236 1025 1035
61. HareEEPetersonBKIyerVNMeierREisenMB 2008 Sepsid even-skipped enhancers are functionally conserved in Drosophila despite lack of sequence conservation. PLoS Genet 4 e1000106 doi:10.1371/journal.pgen.1000106
62. TangKLAgnewMKHirtMVSadoTSchneiderLM 2010 Systematics of the subfamily Danioninae (Teleostei: Cypriniformes: Cyprinidae). Mol Phylogenet Evol 57 189 214
63. QuigleyIKManuelJLRobertsRANuckelsRJHerringtonER 2005 Evolutionary diversification of pigment pattern in Danio fishes: differential fms dependence and stripe loss in D. albolineatus. Development 132 89 104
64. JonasJCLaybuttDRSteilGMTrivediNPertusaJG 2001 High glucose stimulates early response gene c-Myc expression in rat pancreatic beta cells. J Biol Chem 276 35375 35381
65. PascalSMGuiotYPelengarisSKhanMJonasJC 2008 Effects of c-MYC activation on glucose stimulus-secretion coupling events in mouse pancreatic islets. Am J Physiol Endocrinol Metab 295 E92 102
66. GuntonJEKulkarniRNYimSOkadaTHawthorneWJ 2005 Loss of ARNT/HIF1beta mediates altered gene expression and pancreatic-islet dysfunction in human type 2 diabetes. Cell 122 337 349
67. PillaiRHuypensPHuangMSchaeferSSheininT 2011 Aryl hydrocarbon receptor nuclear translocator/hypoxia-inducible factor-1{beta} plays a critical role in maintaining glucose-stimulated anaplerosis and insulin release from pancreatic {beta}-cells. J Biol Chem 286 1014 1024
68. MartinCCSvitekCAOeserJKHendersonESteinR 2003 Upstream stimulatory factor (USF) and neurogenic differentiation/beta-cell E box transactivator 2 (NeuroD/BETA2) contribute to islet-specific glucose-6-phosphatase catalytic-subunit-related protein (IGRP) gene expression. Biochem J 371 675 686
69. HanSIYasudaKKataokaK 2011 ATF2 interacts with beta-cell-enriched transcription factors, MafA, Pdx1, and beta2, and activates insulin gene transcription. J Biol Chem 286 10449 10456
70. SempleCAGautierPTaylorKDorinJR 2006 The changing of the guard: Molecular diversity and rapid evolution of beta-defensins. Mol Divers 10 575 584
71. LevineM 2010 Transcriptional enhancers in animal development and evolution. Curr Biol 20 R754 763
72. Meireles-FilhoACStarkA 2009 Comparative genomics of gene regulation-conservation and divergence of cis-regulatory information. Curr Opin Genet Dev 19 565 570
73. PanneDManiatisTHarrisonSC 2007 An atomic model of the interferon-beta enhanceosome. Cell 129 1111 1123
74. StarkALinMFKheradpourPPedersenJSPartsL 2007 Discovery of functional elements in 12 Drosophila genomes using evolutionary signatures. Nature 450 219 232
75. HooperLVWongMHThelinAHanssonLFalkPG 2001 Molecular analysis of commensal host-microbial relationships in the intestine. Science 291 881 884
76. GrootaertCVan de WieleTVan RoosbroeckIPossemiersSVercoutter-EdouartAS 2011 Bacterial monocultures, propionate, butyrate and H(2) O(2) modulate the expression, secretion and structure of the fasting-induced adipose factor in gut epithelial cell lines. Environ Microbiol 13 1778 1789
77. DavidsonE 2006 The Regulatory Genome: Gene Regulatory Networks In Development And Evolution: Academic Press 304
78. PalankerLTennessenJMLamGThummelCS 2009 Drosophila HNF4 regulates lipid mobilization and beta-oxidation. Cell Metab 9 228 239
79. HayhurstGPLeeYHLambertGWardJMGonzalezFJ 2001 Hepatocyte nuclear factor 4alpha (nuclear receptor 2A1) is essential for maintenance of hepatic gene expression and lipid homeostasis. Mol Cell Biol 21 1393 1403
80. ThisseCThisseB 2008 High-resolution in situ hybridization to whole-mount zebrafish embryos. Nat Protoc 3 59 69
81. VerziMPShinHHeHHSulahianRMeyerCA 2010 Differentiation-specific histone modifications reveal dynamic chromatin interactions and partners for the intestinal transcription factor CDX2. Dev Cell 19 713 726
82. ThisseBPfumioSFürthauerMLoppinBHeyerVDegraveAWoehlRLuxASteffanTCharbonnierXQThisseC 2001 Expression of the zebrafish genome during embryogenesis. ZFIN on-line publication
83. ZengLCarterADChildsSJ 2009 miR-145 directs intestinal maturation in zebrafish. Proc Natl Acad Sci U S A 106 17793 17798
84. DusingMRWigintonDA 2005 Epithelial lineages of the small intestine have unique patterns of GATA expression. J Mol Histol 36 15 24
85. BeulingEBaffour-AwuahNYStapletonKAAronsonBENoahTK 2011 GATA factors regulate proliferation, differentiation, and gene expression in small intestine of mature mice. Gastroenterology 140 1219 1229 e1211-1212
86. ShapiraMHamlinBJRongJChenKRonenM 2006 A conserved role for a GATA transcription factor in regulating epithelial innate immune responses. Proc Natl Acad Sci U S A 103 14086 14091
87. FlynnEJ3rdTrentCMRawlsJF 2009 Ontogeny and nutritional control of adipogenesis in zebrafish (Danio rerio). J Lipid Res 50 1641 1652
88. BertrandSThisseBTavaresRSachsLChaumotA 2007 Unexpected novel relational links uncovered by extensive developmental profiling of nuclear receptor expression. PLoS Genet 3 e188 doi:10.1371/journal.pgen.0030188
89. HeinaniemiMUskiJODegenhardtTCarlbergC 2007 Meta-analysis of primary target genes of peroxisome proliferator-activated receptors. Genome Biol 8 R147
90. EdgarRC 2004 MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinformatics 5 113
91. LeiXShiFBasuDHuqARouthierS 2011 Proteolytic processing of angiopoietin-like protein 4 by proprotein convertases modulates its inhibitory effects on lipoprotein lipase activity. J Biol Chem 286 15747 15756
92. LetunicIDoerksTBorkP 2009 SMART 6: recent updates and new developments. Nucleic Acids Res 37 D229 232
93. FinnRDMistryJTateJCoggillPHegerA 2010 The Pfam protein families database. Nucleic Acids Res 38 D211 222
94. GasteigerEGattikerAHooglandCIvanyiIAppelRD 2003 ExPASy: The proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res 31 3784 3788
95. FelsensteinJ 2005 Using the quantitative genetic threshold model for inferences between and within species. Philos Trans R Soc Lond B Biol Sci 360 1427 1434
96. BrudnoMDoCBCooperGMKimMFDavydovE 2003 LAGAN and Multi-LAGAN: efficient tools for large-scale multiple alignment of genomic DNA. Genome Res 13 721 731
97. FrazerKAPachterLPoliakovARubinEMDubchakI 2004 VISTA: computational tools for comparative genomics. Nucleic Acids Res 32 W273 279
98. MatysVKel-MargoulisOVFrickeELiebichILandS 2006 TRANSFAC and its module TRANSCompel: transcriptional gene regulation in eukaryotes. Nucleic Acids Res 34 D108 110
99. BryneJCValenETangMHMarstrandTWintherO 2008 JASPAR, the open access database of transcription factor-binding profiles: new content and tools in the 2008 update. Nucleic Acids Res 36 D102 106
100. ChekmenevDSHaidCKelAE 2005 P-Match: transcription factor binding site search by combining patterns and weight matrices. Nucleic Acids Res 33 W432 437
101. SchugJOvertonGC 1998 TESS: Transcription Element Search Software on the WWW. http://wwwcbilupennedu/cgi-bin/tess/tess?RQ=WELCOME
102. BaileyTLElkanC 1994 Fitting a mixture model by expectation maximization to discover motifs in biopolymers. Proc Int Conf Intell Syst Mol Biol 2 28 36
103. BaileyTLGribskovM 1998 Combining evidence using p-values: application to sequence homology searches. Bioinformatics 14 48 54
104. GuptaSStamatoyannopoulosJABaileyTLNobleWS 2007 Quantifying similarity between motifs. Genome Biol 8 R24
105. AbramoffMDMagalhaesPJRamSJ 2004 Image Processing with ImageJ. Biophotonics International 11 36 42
Štítky
Genetika Reprodukční medicína
Článek Physiological Notch Signaling Maintains Bone Homeostasis via RBPjk and Hey Upstream of NFATc1Článek Probing the Informational and Regulatory Plasticity of a Transcription Factor DNA–Binding DomainČlánek Repression of Germline RNAi Pathways in Somatic Cells by Retinoblastoma Pathway Chromatin ComplexesČlánek An Alu Element–Associated Hypermethylation Variant of the Gene Is Associated with Childhood ObesityČlánek Three Essential Ribonucleases—RNase Y, J1, and III—Control the Abundance of a Majority of mRNAsČlánek Genomic Tools for Evolution and Conservation in the Chimpanzee: Is a Genetically Distinct Population
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 3
-
Všechny články tohoto čísla
- Comprehensive Research Synopsis and Systematic Meta-Analyses in Parkinson's Disease Genetics: The PDGene Database
- Genomic Analysis of the Hydrocarbon-Producing, Cellulolytic, Endophytic Fungus
- Networks of Neuronal Genes Affected by Common and Rare Variants in Autism Spectrum Disorders
- Akirin Links Twist-Regulated Transcription with the Brahma Chromatin Remodeling Complex during Embryogenesis
- Too Much Cleavage of Cyclin E Promotes Breast Tumorigenesis
- Imprinted Genes … and the Number Is?
- Genetic Architecture of Highly Complex Chemical Resistance Traits across Four Yeast Strains
- Exploring the Complexity of the HIV-1 Fitness Landscape
- MNS1 Is Essential for Spermiogenesis and Motile Ciliary Functions in Mice
- A Fundamental Regulatory Mechanism Operating through OmpR and DNA Topology Controls Expression of Pathogenicity Islands SPI-1 and SPI-2
- Evidence for Positive Selection on a Number of MicroRNA Regulatory Interactions during Recent Human Evolution
- Variation in Modifies Risk of Neonatal Intestinal Obstruction in Cystic Fibrosis
- PIF4–Mediated Activation of Expression Integrates Temperature into the Auxin Pathway in Regulating Hypocotyl Growth
- Critical Evaluation of Imprinted Gene Expression by RNA–Seq: A New Perspective
- A Meta-Analysis and Genome-Wide Association Study of Platelet Count and Mean Platelet Volume in African Americans
- Mouse Genetics Suggests Cell-Context Dependency for Myc-Regulated Metabolic Enzymes during Tumorigenesis
- Transcriptional Control in Cardiac Progenitors: Tbx1 Interacts with the BAF Chromatin Remodeling Complex and Regulates
- Synthetic Lethality of Cohesins with PARPs and Replication Fork Mediators
- APOBEC3G-Induced Hypermutation of Human Immunodeficiency Virus Type-1 Is Typically a Discrete “All or Nothing” Phenomenon
- Interpreting Meta-Analyses of Genome-Wide Association Studies
- Error-Prone ZW Pairing and No Evidence for Meiotic Sex Chromosome Inactivation in the Chicken Germ Line
- -Dependent Chemosensory Functions Contribute to Courtship Behavior in
- Diverse Forms of Splicing Are Part of an Evolving Autoregulatory Circuit
- Phenotypic Plasticity of the Drosophila Transcriptome
- Physiological Notch Signaling Maintains Bone Homeostasis via RBPjk and Hey Upstream of NFATc1
- Precocious Metamorphosis in the Juvenile Hormone–Deficient Mutant of the Silkworm,
- Igf1r Signaling Is Indispensable for Preimplantation Development and Is Activated via a Novel Function of E-cadherin
- Accurate Prediction of Inducible Transcription Factor Binding Intensities In Vivo
- Mitochondrial Oxidative Stress Alters a Pathway in Strongly Resembling That of Bile Acid Biosynthesis and Secretion in Vertebrates
- Mammalian Neurogenesis Requires Treacle-Plk1 for Precise Control of Spindle Orientation, Mitotic Progression, and Maintenance of Neural Progenitor Cells
- Tcf7 Is an Important Regulator of the Switch of Self-Renewal and Differentiation in a Multipotential Hematopoietic Cell Line
- REST–Mediated Recruitment of Polycomb Repressor Complexes in Mammalian Cells
- Intronic -Regulatory Modules Mediate Tissue-Specific and Microbial Control of / Transcription
- Age-Dependent Brain Gene Expression and Copy Number Anomalies in Autism Suggest Distinct Pathological Processes at Young Versus Mature Ages
- A Genome-Wide Association Study Identifies Variants Underlying the Shade Avoidance Response
- -by- Regulatory Divergence Causes the Asymmetric Lethal Effects of an Ancestral Hybrid Incompatibility Gene
- Genome-Wide Association and Functional Follow-Up Reveals New Loci for Kidney Function
- A Natural System of Chromosome Transfer in
- Cell Size and the Initiation of DNA Replication in Bacteria
- Probing the Informational and Regulatory Plasticity of a Transcription Factor DNA–Binding Domain
- Repression of Germline RNAi Pathways in Somatic Cells by Retinoblastoma Pathway Chromatin Complexes
- Temporal Transcriptional Profiling of Somatic and Germ Cells Reveals Biased Lineage Priming of Sexual Fate in the Fetal Mouse Gonad
- Rapid Analysis of Genome Rearrangements by Multiplex Ligation–Dependent Probe Amplification
- Metabolic Profiling of a Mapping Population Exposes New Insights in the Regulation of Seed Metabolism and Seed, Fruit, and Plant Relations
- The Atypical Calpains: Evolutionary Analyses and Roles in Cellular Degeneration
- The Silkworm Coming of Age—Early
- Development of a Panel of Genome-Wide Ancestry Informative Markers to Study Admixture Throughout the Americas
- Balanced Codon Usage Optimizes Eukaryotic Translational Efficiency
- The Min System and Nucleoid Occlusion Are Not Required for Identifying the Division Site in but Ensure Its Efficient Utilization
- Neurobeachin, a Regulator of Synaptic Protein Targeting, Is Associated with Body Fat Mass and Feeding Behavior in Mice and Body-Mass Index in Humans
- Statistical Analysis of Readthrough Levels for Nonsense Mutations in Mammalian Cells Reveals a Major Determinant of Response to Gentamicin
- Gene Reactivation by 5-Aza-2′-Deoxycytidine–Induced Demethylation Requires SRCAP–Mediated H2A.Z Insertion to Establish Nucleosome Depleted Regions
- The miR-35-41 Family of MicroRNAs Regulates RNAi Sensitivity in
- Genetic Basis of Hidden Phenotypic Variation Revealed by Increased Translational Readthrough in Yeast
- An Alu Element–Associated Hypermethylation Variant of the Gene Is Associated with Childhood Obesity
- Modelling Human Regulatory Variation in Mouse: Finding the Function in Genome-Wide Association Studies and Whole-Genome Sequencing
- Novel Loci for Adiponectin Levels and Their Influence on Type 2 Diabetes and Metabolic Traits: A Multi-Ethnic Meta-Analysis of 45,891 Individuals
- Polycomb-Like 3 Promotes Polycomb Repressive Complex 2 Binding to CpG Islands and Embryonic Stem Cell Self-Renewal
- Insulin/IGF-1 and Hypoxia Signaling Act in Concert to Regulate Iron Homeostasis in
- EMF1 and PRC2 Cooperate to Repress Key Regulators of Arabidopsis Development
- Three Essential Ribonucleases—RNase Y, J1, and III—Control the Abundance of a Majority of mRNAs
- Contrasted Patterns of Molecular Evolution in Dominant and Recessive Self-Incompatibility Haplotypes in
- A Machine Learning Approach for Identifying Novel Cell Type–Specific Transcriptional Regulators of Myogenesis
- Genomic Tools for Evolution and Conservation in the Chimpanzee: Is a Genetically Distinct Population
- Nos2 Inactivation Promotes the Development of Medulloblastoma in Mice by Deregulation of Gap43–Dependent Granule Cell Precursor Migration
- Intracranial Aneurysm Risk Locus 5q23.2 Is Associated with Elevated Systolic Blood Pressure
- Heritability and Genetic Correlations Explained by Common SNPs for Metabolic Syndrome Traits
- A Genome-Wide Association Study of Nephrolithiasis in the Japanese Population Identifies Novel Susceptible Loci at 5q35.3, 7p14.3, and 13q14.1
- DNA Damage in Nijmegen Breakage Syndrome Cells Leads to PARP Hyperactivation and Increased Oxidative Stress
- DNA Resection at Chromosome Breaks Promotes Genome Stability by Constraining Non-Allelic Homologous Recombination
- Genetic Analysis of Floral Symmetry in Van Gogh's Sunflowers Reveals Independent Recruitment of Genes in the Asteraceae
- A Splice Site Variant in the Bovine Gene Compromises Growth and Regulation of the Inflammatory Response
- Promoter Nucleosome Organization Shapes the Evolution of Gene Expression
- The Nucleoside Diphosphate Kinase Gene Acts as Quantitative Trait Locus Promoting Non-Mendelian Inheritance
- The Ciliogenic Transcription Factor RFX3 Regulates Early Midline Distribution of Guidepost Neurons Required for Corpus Callosum Development
- Phosphorylation of the RNA–Binding Protein HOW by MAPK/ERK Enhances Its Dimerization and Activity
- A Genome-Wide Scan of Ashkenazi Jewish Crohn's Disease Suggests Novel Susceptibility Loci
- Parkinson's Disease–Associated Kinase PINK1 Regulates Miro Protein Level and Axonal Transport of Mitochondria
- LMW-E/CDK2 Deregulates Acinar Morphogenesis, Induces Tumorigenesis, and Associates with the Activated b-Raf-ERK1/2-mTOR Pathway in Breast Cancer Patients
- Mapping the Hsp90 Genetic Interaction Network in Reveals Environmental Contingency and Rewired Circuitry
- Autoregulation of the Noncoding RNA Gene
- The Human Pancreatic Islet Transcriptome: Expression of Candidate Genes for Type 1 Diabetes and the Impact of Pro-Inflammatory Cytokines
- Spo0A∼P Imposes a Temporal Gate for the Bimodal Expression of Competence in
- Antagonistic Regulation of Apoptosis and Differentiation by the Cut Transcription Factor Represents a Tumor-Suppressing Mechanism in
- A Downstream CpG Island Controls Transcript Initiation and Elongation and the Methylation State of the Imprinted Macro ncRNA Promoter
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- PIF4–Mediated Activation of Expression Integrates Temperature into the Auxin Pathway in Regulating Hypocotyl Growth
- Metabolic Profiling of a Mapping Population Exposes New Insights in the Regulation of Seed Metabolism and Seed, Fruit, and Plant Relations
- A Splice Site Variant in the Bovine Gene Compromises Growth and Regulation of the Inflammatory Response
- Comprehensive Research Synopsis and Systematic Meta-Analyses in Parkinson's Disease Genetics: The PDGene Database
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



