-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaA Fundamental Regulatory Mechanism Operating through OmpR and DNA Topology Controls Expression of Pathogenicity Islands SPI-1 and SPI-2
DNA topology has fundamental control over the ability of transcription factors to access their target DNA sites at gene promoters. However, the influence of DNA topology on protein–DNA and protein–protein interactions is poorly understood. For example, relaxation of DNA supercoiling strongly induces the well-studied pathogenicity gene ssrA (also called spiR) in Salmonella enterica, but neither the mechanism nor the proteins involved are known. We have found that relaxation of DNA supercoiling induces expression of the Salmonella pathogenicity island (SPI)-2 regulator ssrA as well as the SPI-1 regulator hilC through a mechanism that requires the two-component regulator OmpR-EnvZ. Additionally, the ompR promoter is autoregulated in the same fashion. Conversely, the SPI-1 regulator hilD is induced by DNA relaxation but is repressed by OmpR. Relaxation of DNA supercoiling caused an increase in OmpR binding to DNA and a concomitant decrease in binding by the nucleoid-associated protein FIS. The reciprocal occupancy of DNA by OmpR and FIS was not due to antagonism between these transcription factors, but was instead a more intrinsic response to altered DNA topology. Surprisingly, DNA relaxation had no detectable effect on the binding of the global repressor H-NS. These results reveal the underlying molecular mechanism that primes SPI genes for rapid induction at the onset of host invasion. Additionally, our results reveal novel features of the archetypal two-component regulator OmpR. OmpR binding to relaxed DNA appears to generate a locally supercoiled state, which may assist promoter activation by relocating supercoiling stress-induced destabilization of DNA strands. Much has been made of the mechanisms that have evolved to regulate horizontally-acquired genes such as SPIs, but parallels among the ssrA, hilC, and ompR promoters illustrate that a fundamental form of regulation based on DNA topology coordinates the expression of these genes regardless of their origins.
Published in the journal: . PLoS Genet 8(3): e32767. doi:10.1371/journal.pgen.1002615
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002615Summary
DNA topology has fundamental control over the ability of transcription factors to access their target DNA sites at gene promoters. However, the influence of DNA topology on protein–DNA and protein–protein interactions is poorly understood. For example, relaxation of DNA supercoiling strongly induces the well-studied pathogenicity gene ssrA (also called spiR) in Salmonella enterica, but neither the mechanism nor the proteins involved are known. We have found that relaxation of DNA supercoiling induces expression of the Salmonella pathogenicity island (SPI)-2 regulator ssrA as well as the SPI-1 regulator hilC through a mechanism that requires the two-component regulator OmpR-EnvZ. Additionally, the ompR promoter is autoregulated in the same fashion. Conversely, the SPI-1 regulator hilD is induced by DNA relaxation but is repressed by OmpR. Relaxation of DNA supercoiling caused an increase in OmpR binding to DNA and a concomitant decrease in binding by the nucleoid-associated protein FIS. The reciprocal occupancy of DNA by OmpR and FIS was not due to antagonism between these transcription factors, but was instead a more intrinsic response to altered DNA topology. Surprisingly, DNA relaxation had no detectable effect on the binding of the global repressor H-NS. These results reveal the underlying molecular mechanism that primes SPI genes for rapid induction at the onset of host invasion. Additionally, our results reveal novel features of the archetypal two-component regulator OmpR. OmpR binding to relaxed DNA appears to generate a locally supercoiled state, which may assist promoter activation by relocating supercoiling stress-induced destabilization of DNA strands. Much has been made of the mechanisms that have evolved to regulate horizontally-acquired genes such as SPIs, but parallels among the ssrA, hilC, and ompR promoters illustrate that a fundamental form of regulation based on DNA topology coordinates the expression of these genes regardless of their origins.
Introduction
Salmonella enterica is a facultative intracellular pathogen of the mammalian gut. After passing through the diverse environments of the stomach and digestive tract, S. enterica can invade host epithelial cells to gain access to internal tissues where it can persist inside macrophage [1]. The Salmonella pathogenicity islands 1 and 2 (SPI-1 and SPI-2) encode type three secretion systems (T3SS) and effector proteins that enable the manipulation and invasion of host tissues [2], [3]. SPI-1 genes are expressed primarily in the intestine during the early stages of invasion, followed by a decrease in SPI-1 expression and an increase in SPI-2 expression inside epithelial cells, and finally SPI-2 expression predominates once S. enterica has crossed the epithelium and resides in macrophage vacuoles [4]. Despite this apparently reciprocal pattern of expression over the course of invasion, both gene islands are co-regulated by many of the same global regulatory proteins. For example, SPI-1 and SPI-2 genes are strongly repressed by the nucleoid-associated protein H-NS, a highly-abundant protein that blocks and traps RNA polymerase at gene promoters by forming repressive nucleoprotein complexes [5], [6]. SPI-1 and SPI-2 also share the transcriptional activators FIS and OmpR. FIS is required for full activation of both SPI-1 and SPI-2 genes in laboratory conditions [7], and Δfis mutants are attenuated for virulence in mice [8] and show reduced survival in macrophage [9]. OmpR is a well-characterized direct transcriptional activator of the SPI-2 ssrAB promoter [10], and ΔompR mutants are attenuated [11], but the role of OmpR in SPI-1 gene expression has remained ambiguous [2]. It has been recently discovered that together OmpR and FIS drive low-level transcription of SPI-2 in the intestinal lumen, an environment classically thought to be the exclusive domain of SPI-1 [12].
By regulating expression of SPI-encoded transcription factors, H-NS, FIS, OmpR, and other global regulators sit atop a hierarchical network that integrates diverse environmental and physiological cues. SPI-encoded transcription factors fine-tune these global inputs to control precisely the dosage of T3SS and effector protein production [13]. SPI-1 encodes four AraC-like transcription factors: HilA, HilC, HilD, InvF. Through a complex feedback and feedforward mechanism, HilC and HilD control their own and each other's transcription, and together activate transcription of hilA [14], [15], [16] (Figure 1A). HilA in turn activates invF and the genes encoding the T3SS and effector proteins [17]. Additionally, there is crosstalk between SPI-1 and SPI-2 through which HilD induces expression of ssrAB [18] (Figure 1A). Unlike SPI-1, SPI-2 encodes a single cognate regulator. Here, an unidentified signal causes the sensor kinase SsrA to phosphorylate the DNA binding protein SsrB, which in turn activates transcription of SPI-2 T3SS and effector genes [3].
Fig. 1. Transcriptional control of SPI-1 and SPI-2 gene expression. 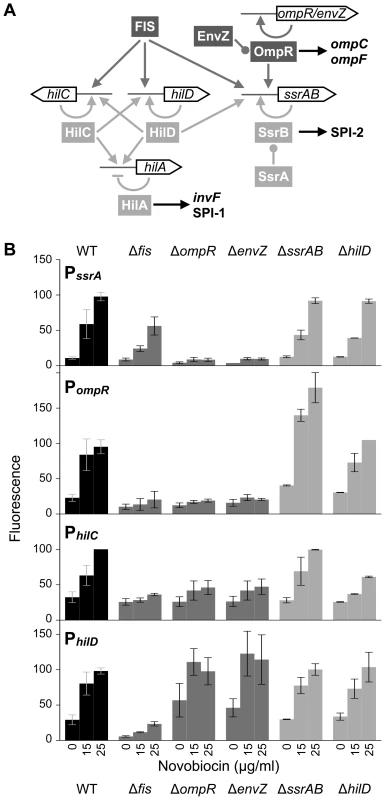
A) Schematic of the regulatory connections at the gene promoters described in the text. Global regulators (dark grey) and local SPI-encoded regulators (light grey) are highlighted. Arrows indicate a positive regulatory effect, perpendicular bars indicate a repressive regulatory effect, and rounded bars indicate activation by phosphorylation. B) Expression of the PssrA:gfp, PompR:gfp, PhilC:gfp, and PhilD:gfp transcriptional reporter fusion in response to increasing concentrations of novobiocin (0, 15, and 25 µg/ml) in different genetic backgrounds. Fluorescence values are percentages relative to wild type cells (WT) treated with 25 µg/ml novobiocin; variation in WT values at 25 µg/ml novobiocin indicate differences between replicate experiments conducted on the same day. The mean and standard deviation of 3 to 10 biological replicates are plotted. SPI-1 and SPI-2 are among the best-studied genetic systems in bacteriology, yet their complex regulation has meant that the mechanisms that integrate the myriad of regulatory signals have remained enigmatic. Even less clear are the contributions made by DNA topology to the interactions and architecture of the nucleoprotein complexes that form at SPI promoters. Several lines of evidence implicate altered DNA supercoiling in coordinating SPI gene expression during invasion. The invA gene in SPI-1, which encodes an effector protein, is repressed by relaxed DNA supercoiling [19]. Conversely, ssrA expression is induced by relaxation of DNA supercoiling [9]. S. enterica DNA is highly supercoiled in low oxygen environments but is more relaxed in oxygenated conditions, and this may reflect the DNA supercoiling dynamics that occur as S. enterica approaches the aerobic region immediately adjacent to the intestinal epithelium [20], [21]. In tissue culture, S. enterica DNA supercoiling appears to remain static in epithelial cells but is dynamic when the bacterium resides inside macrophage [9], which demonstrates the complexity of S. enterica's interactions with host environments. Our investigation of the links between environment, DNA supercoiling, and gene expression has uncovered a fundamental mechanism of SPI-1 and SPI-2 regulation in which relaxation of DNA supercoiling recruits OmpR to the ssrA, hilC, and hilD promoters, and this level of control functions independently of the fine-tuning effected by SPI-encoded transcription factors.
Results
Induction of SPI genes by DNA relaxation requires specific transcription factors
The ssrAB promoter (PssrA) is induced by novobiocin, an aminocoumarin antibiotic that specifically inhibits the DNA supercoiling activity of the DNA gyrase subunit B (GyrB) (Figure 1B). In contrast, the SPI-2 T3SS and effector gene promoters, ssaB-E, sseA-G, ssaG-L, and ssaM-R, are only very slightly induced by altered DNA topology and the presumed increase in SsrA and SsrB concentrations brought about by novobiocin treatment (Figure S1A). Thus, the ability of DNA relaxation to activate SPI-2 is channeled through the cognate SsrA/B two-component regulator. PssrA induction was reduced in cells lacking FIS, a master regulator of DNA supercoiling (Figure 1B), possibly because novobiocin has a reduced effect on DNA supercoiling in Δfis mutants compared to wild type cells [20]. Unlike FIS, OmpR and its phospo-donor EnvZ were both absolutely required for induction of PssrA, suggesting that relaxed DNA supercoiling alone cannot activate PssrA in the absence of OmpR's ability to recruit RNAP. The requirement for EnvZ indicates that OmpR must be phosphorylated in order to stimulate these promoters, and also indicates that other phospho-donors do not activate OmpR in these conditions.
The SPI-encoded regulators HilD and SsrA/B played no detectable role in PssrA induction. The alternate sigma factor RpoS is better at transcribing relaxed DNA than is the primary housekeeping sigma factor RpoD [22], and the elevated level of RpoS during stationary phase correlates with ssrA expression in standard laboratory conditions, but deletion of rpoS did not reduce PssrA induction by novobiocin (Figure S1B).
Previous experiments in S. enterica have shown that the ompR-envZ promoter (PompR) is induced by high concentrations of novobiocin at late stages of growth, and that OmpR is an auto-regulator of this induction [23]. We found that PompR is also activated by low concentrations of novobiocin during exponential growth, and FIS and OmpR-EnvZ contribute to this induction (Figure 1B). Deletion of hilD did not affect PompR induction. However, PompR activity was unexpectedly elevated in the ΔssrA/B mutant, suggesting that SsrA/B may directly or indirectly regulate ompR expression.
Because SPI-1 and SPI-2 genes are usually observed to have inverse expression patterns, we expected SPI-1 genes to be insensitive or repressed by DNA relaxation. We tested the effects of novobiocin treatment on expression of the master regulators hilA, hilC, and hilD, and were surprised to find that both the hilC and hilD promoters (PhilC and PhilD) were induced by DNA relaxation (Figure 1B). PhilA was insensitive to DNA relaxation (Figure S1A), suggesting that the inducing signal is limited to PhilC and PhilD (Figure 1A). Like PssrA, PhilC required both FIS and OmpR-EnvZ for induction; yet unlike PssrA, the absence of FIS was not compensated by increasing concentrations of novobiocin. Consistent with its role as a transcriptional activator, HilD was required for full activation of PhilC (Figure 1B). SsrA/B did not contribute to PhilC induction. PhilD was unique among the four promoters in having higher expression in the absence of ompR and envZ, but it nevertheless required FIS for full activation (Figure 1B). PhilD induction was unaffected by the absence of SsrA/B or HilD. Quantitative PCR measurement of ssrA, ompR, hilC, hilD, and hilA mRNA levels confirmed the results obtained from the reporter gene fusions (Figure S1C).
OmpR requires DNA relaxation to stimulate transcription
Having found that OmpR and relaxed DNA supercoiling work in concert to stimulate transcription from PssrA, PhilC, and PompR, we wished to test the relative contributions of OmpR and DNA topology to promoter function. To this end, the ompR-envZ operon (ompB) was cloned under the control of the arabinose-inducible PBAD promoter in a ΔompB mutant. PssrA expression increased only very slightly when ompB was overexpressed (0.2% arabinose) in the absence of DNA relaxation (Figure 2). In contrast, DNA relaxation in the complete absence of OmpR (empty pBAD vector) had a stimulatory effect on PssrA; this activation was higher in cells carrying pBADompB, likely due to leaky transcription of ompB in the absence of arabinose. The combination of ompB over-expression and DNA relaxation had the strongest stimulatory effect, confirming that OmpR and DNA relaxation work in concert to activate PssrA. The combination of ompB over-expression and DNA relaxation also resulted in maximal expression of PhilC and PompR, however the effect was more subtle for PhilC (Figure 2). Consistent with the results presented in Figure 1B, PhilD was repressed by ompB expression, and this repression occurred in both the absence and presence of DNA relaxation. Repression of PhilD was strongest at the lower concentration of novobiocin (15 µg/ml), raising the possibility that a high degree of DNA relaxation reduces repression by OmpR, perhaps through elevated HilC levels brought about by DNA relaxation (Figure 1A).
Fig. 2. The relative contributions of OmpR and DNA relaxation to promoter activity. 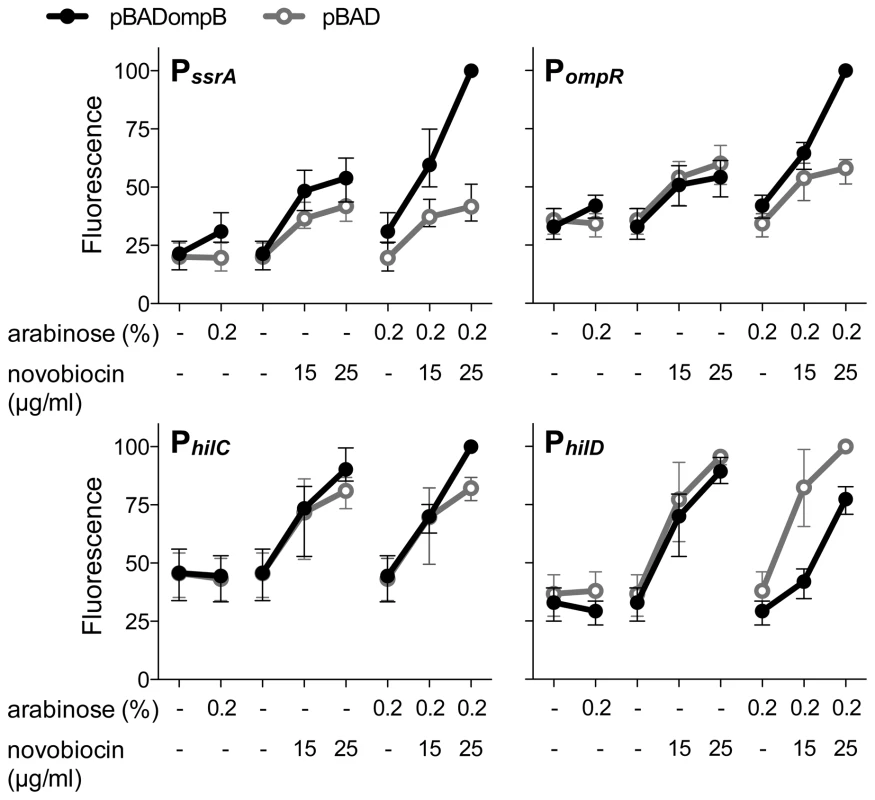
GFP production from transcriptional reporter fusions was measured in ΔompB mutant cells carrying either an arabinose-inducible ompR-envZ operon (pBADompB) or the empty pBAD vector. The mean and range of expression from 3 biological replicates is plotted as in Figure 1B. OmpR binding to SPI-1 promoters
The control of hilC and hilD expression by OmpR suggested that OmpR may regulate these genes through direct interactions. Electrophoretic mobility shift (bandshift) assays confirmed that OmpR binds specifically to both PhilC and PhilD, with OmpR demonstrating an affinity for PhilC similar to that for the positive control PompC (Figure 3A). A negative control bait DNA (kan) was not bound specifically by OmpR at the concentrations tested. In these equilibrium binding assays, the rapid appearance of OmpR-DNA complexes over a small range of protein concentrations was evidence of cooperative OmpR binding to the bait DNA. Moreover, OmpR-DNA complexes demonstrated slower migration at higher OmpR concentrations, indicating that multiple OmpR molecules were bound to a single bait DNA molecule. Cooperative DNA binding is a feature common among NAPs — like FIS and H-NS — that bind with low-specificity to multiple proximal DNA sites [24], [25]. Indeed, OmpR monomers are thought to first bind cooperatively to form a nucleating dimer that recruits additional OmpR dimers in a cooperative fashion [26].
Fig. 3. OmpR binding to SPI-1 gene promoters. 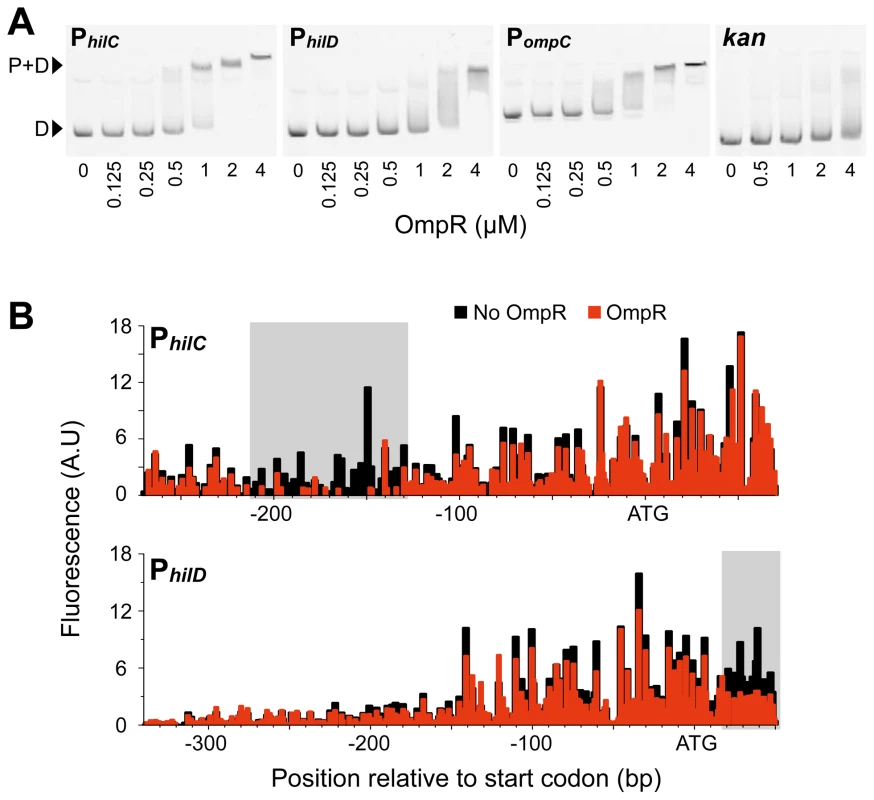
A) Bandshifts showing OmpR binding to PhilC and PhilD, as well as to the PompC positive control and the kan gene negative control. D, free DNA; P+D, protein-DNA complexes. B) Footprinting of OmpR binding to PhilC and PhilD using end-labelled linear DNA fragments. The size and quantity of 6-FAM-labelled digestion products were measured using a capillary electrophoresis DNA sequencing instrument. DNase footprinting revealed that PhilC and PhilD each have a single region protected by OmpR (Figure 3B). The PhilC region bound by OmpR is located over 100 bp upstream of the hilC start codon, consistent with OmpR's function as a transcriptional activator of this promoter. Conversely, the OmpR-protected region of PhilD is downstream of the hilD start codon, where OmpR binding is likely to have a repressive effect on hilD transcription.
Promoter occupancy
If OmpR function is enhanced by relaxation of DNA supercoiling, does DNA relaxation result in increased DNA binding by OmpR? This was tested by quantifying OmpR binding to gene promoters in vivo using chromatin immuno-precipitation (ChIP). A fusion of the 3×-Flag epitope tag to the C-terminus of OmpR was used for these experiments. The epitope tag added 22 amino acids adjacent to OmpR's DNA-binding domain and created a new ribosome binding site for the envZ open reading frame; nonetheless, cells with OmpR:Flag showed only a slight reduction in promoter activation by novobiocin (Figure S1C). Alternatively, a 3×Flag tag at the N-terminus of OmpR could not be used because it generated a ΔompR phenotype at promoters (not shown).
Novobiocin treatment caused a significant increase in OmpR occupancy at PssrA, PompR, PhilC, and PhilD (Figure 4A). Increased promoter occupancy was due solely to a change in binding activity as OmpR levels were observed to decrease after novobiocin treatment (Figure 4B). Because OmpR requires DNA relaxation for it to be fully active at SPI promoters (Figure 2), we predicted that OmpR is an ineffective antagonist of H-NS binding and thus requires novobiocin-induced changes in DNA topology to assist in H-NS displacement. Our H-NS ChIP results confirm earlier studies that have found H-NS to occupy SPI promoters, but demonstrates a low affinity for PompR [5], [27] (Figure 4A). Surprisingly, at all four promoters H-NS abundance was not affected by DNA relaxation nor by increased OmpR binding. It is important to note however that while ChIP quantifies protein abundance at genomic regions at a resolution around 500 bp, ChIP does not resolve changes to higher-order protein complexes if protein abundance remains constant. Therefore, we cannot rule out that although H-NS is not displaced, promoter activity may increase because H-NS oligomers are restructured by OmpR binding as well as by changes in DNA topology.
Fig. 4. OmpR, FIS, and H-NS binding to promoter DNA. 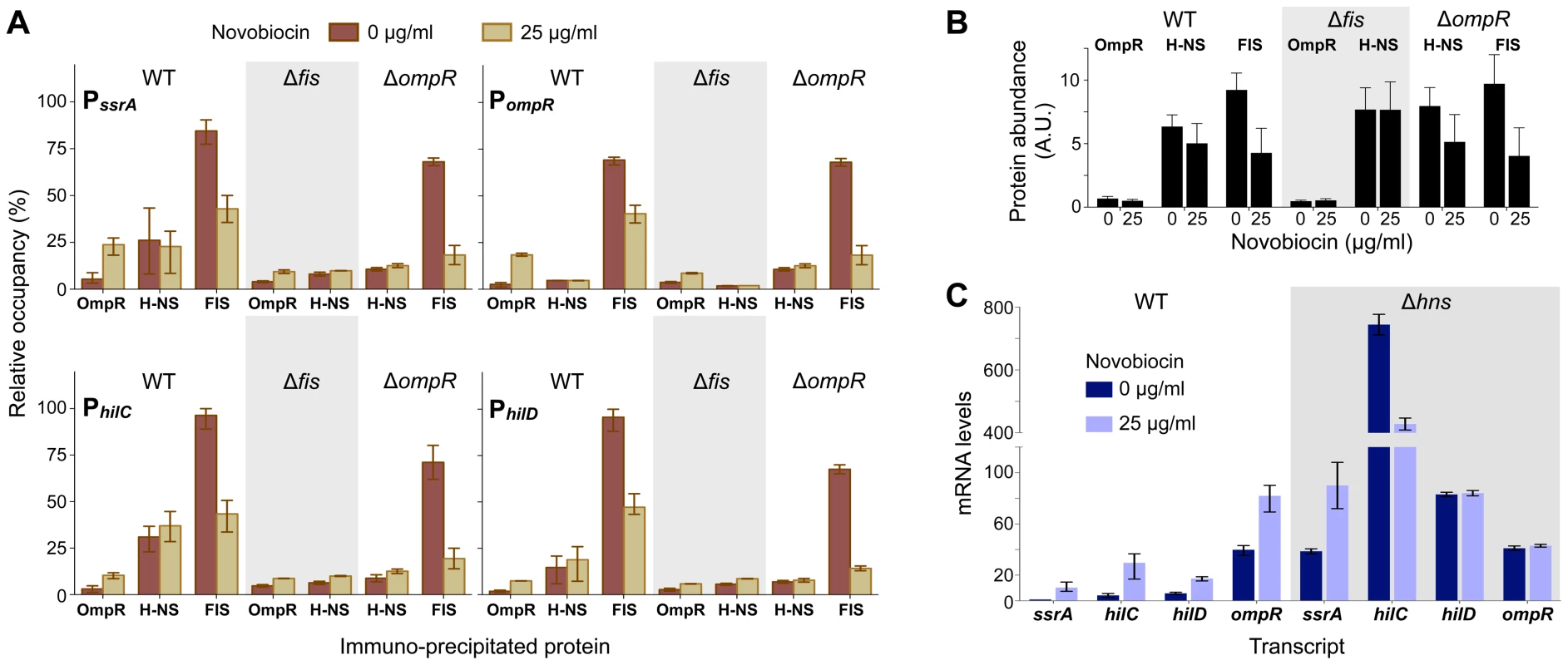
A) Quantification of protein binding to promoter DNA by immuno-precipitation 40 minutes after addition of novobiocin. The quantity of precipitated DNA is expressed as a percentage of maximal precipitation of the PhilC locus by FIS in wild type cells. The mean and range from 3 biological replicates is plotted. B) Protein levels in wild type and mutant cells 40 minutes after addition of novobiocin. Protein abundance was quantified by western blot analysis; the mean and standard deviation of three biological replicates is plotted. C) Quantitative PCR measurement of gene transcript levels in wild type and Δhns mutant cells before and 40 minutes after addition of novobiocin. The mean and range of mRNA levels (expressed relative to ssrA in wild type cells at 0 µg/ml novobiocin) in three (wild type) and two (Δhns) biological replicates are plotted. Chromatin immuno-precipitation of FIS fused to a 8×Myc epitope tag has been used previously to examine genome-wide FIS binding in E. coli [28]. We constructed an identical FIS:Myc fusion protein in S. enterica and used this to measure FIS occupancy of gene promoters in our experimental conditions. This revealed a high abundance of FIS at the SPI promoters, with slightly less FIS bound to PompR (Figure 4A). At all loci tested, FIS occupancy decreased when cells were treated with novobiocin. This reduced FIS occupancy can be explained mostly by the ∼50% decrease in FIS levels in novobiocin-treated cells (Figure 4B). Although FIS contributes to transcriptional activation of these promoters, the finding that transcriptional activation occurs even when FIS is depleted suggests that FIS may act in part through its global control of DNA topology. Because FIS transitions from a filamentous DNA-binding mode to an ordered dimer as its concentration decreases [24], it is also possible that the depletion of FIS coupled with changes in DNA topology restructures FIS complexes into forms that favour transcription activation.
We next tested whether the decrease in promoter activity observed in a Δfis mutant (Figure 1B) was due to a reduced ability by OmpR to access gene promoters. The ChIP data suggest that both OmpR and H-NS have less access to promoter DNA in a Δfis mutant (Figure 4A). We have previously found that the Δfis mutant is resistant to relaxation of DNA supercoiling by novobiocin [20]. It may be that H-NS and OmpR require DNA relaxation to gain full access to PssrA, PompR, PhilC, and PhilD, and the degree of relaxation is too modest in the Δfis mutant. Nevertheless, novobiocin treatment caused a small increase in OmpR occupancy in Δfis mutants, indicating that OmpR binding does not absolutely require the topological constraints imposed on DNA by FIS binding.
The same experiment was conducted in a ΔompR mutant. In the absence of OmpR, novobiocin treatment caused a reduction in FIS binding (Figure 4A), again consistent with a reduction in FIS levels in these cells (Figure 4B). Although less H-NS bound to SPI promoters in the ΔompR mutant, significantly more H-NS bound to PompR, suggesting that OmpR is an effective H-NS antagonist at its own promoter. Surprisingly, the reduced H-NS levels observed in the ΔompR mutant (Figure 4B), along with the further reduction in H-NS levels upon novobiocin treatment, implicates OmpR as a regulator of hns expression.
Regulation in a Δhns mutant
Because DNA relaxation does not appear to displace H-NS from gene promoters, we tested how removing H-NS from the system affects promoter function. Although all four test promoters had a similar pattern of induction by DNA relaxation in wild type cells, contrasting responses were observed in the absence of H-NS. As expected, all three SPI promoters were strongly upregulated (20 to 200-fold) in the Δhns mutant (Figure 4C). In the absence of H-NS, ssrA was induced, hilC was repressed, and hilD was unaffected by DNA relaxation. These contrasting responses may result from the different and complex regulatory inputs acting at each promoter, and further confirm that promoter induction by DNA relaxation is not due simply to antagonism of H-NS repression.
Transcriptional output from PompR was the same in wild type and Δhns mutant cells in normal growth conditions (Figure 4C). This finding that H-NS does not repress PompR is consistent with the low affinity of H-NS for this promoter (Figure 4A). Surprisingly though, PompR was not induced by novobiocin in the Δhns mutant, which may be indirectly caused by the highly pleiotropic effects of the Δhns mutation.
OmpR constrains a supercoiled-like state
To determine how DNA supercoiling affects OmpR affinity for DNA, we used primer extension to resolve DNase footprints on supercoiled and linear DNA templates. This approach can also determine if OmpR binds to different target sites depending on DNA supercoiling state, thus PompR was used as the target DNA in this set of experiments because it has multiple, clearly delineated OmpR binding sites [23]. The grey filled boxes in Figure 5A highlight the regions of PompR protected from DNase I digestion by OmpR. Most protection in the absence of supercoiling (linear DNA) was observed at a 60 bp region, OmpR-2, with lesser protection of regions on either side. Protected regions were assigned numbers to correspond with the OmpR sites identified previously by Bang et al. [23] (horizontal, dark-grey lines). Unlike Bang et al. [23], we analyzed OmpR binding to the full PompR intergenic region to resolve a more promoter-distal site, OmpR-4. DNase I digestion of an end-labeled linear template confirmed that OmpR-2 is the primary site of OmpR binding to PompR (Figure S2A).
Fig. 5. OmpR binding to DNA at different supercoiling levels. 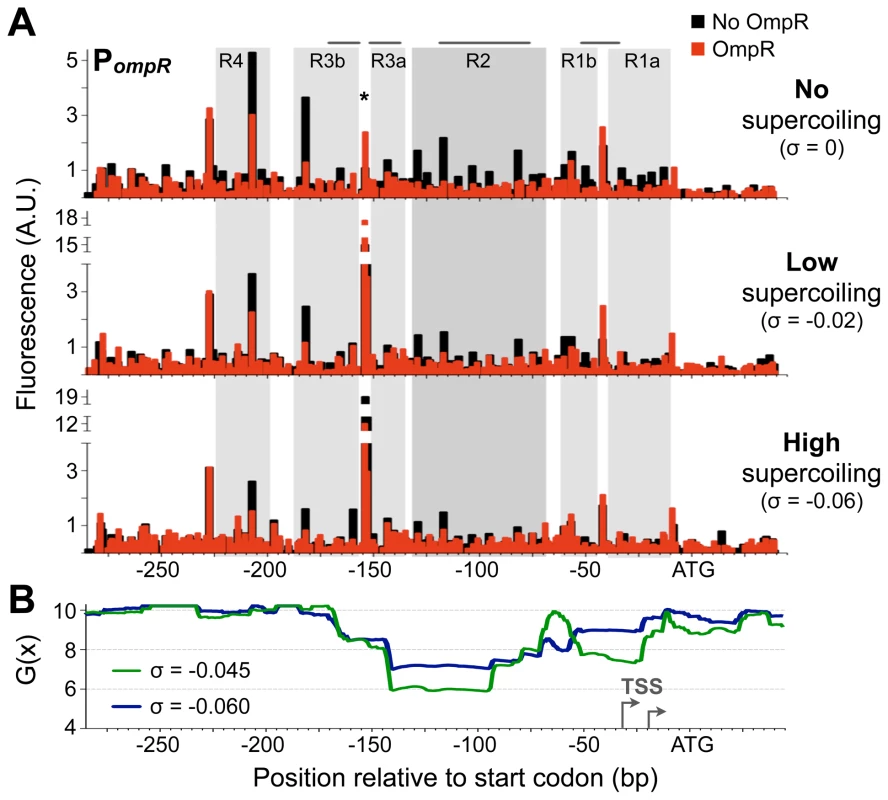
A) Quantification of OmpR binding to PompR in the plasmid pZec-PompR at various supercoiling states using primer extension DNase I footprinting. The size and quantity of 6-FAM-labelled primer extension products were measured using a capillary electrophoresis DNA sequencing instrument. The approximate superhelical density (σ) of DNA in binding reactions is indicated. Horizontal grey lines above the footprints show where Bang et al. [23] observed OmpR footprinting; note that they measured binding to a limited region of the promoter. Grey-filled background highlights OmpR-protected regions, with darker grey highlighting the OmpR-2 site. The asterisk indicates the hypersensitive site at position −154. B) SIDD profile of PompR in pZec-PompR at two superhelical densities. To detect long range effects of DNA supercoiling, WebSIDD [32] uses a default 5 kbp window that slides by 500 bp, thus each base pair is considered 10 times and the G(x) is calculated by weighting the effects of proximal bases. G(x) is expressed in kcal/mol/bp. Grey arrows indicate transcription start sites (TSS), mapped in [23]. As supercoiling levels increased, protection by OmpR appeared to decrease (Figure 5A), giving the impression that OmpR binds DNA better at lower supercoiling levels. However, this result was caused by a decrease in DNase I cutting of unprotected supercoiled DNA, perhaps due to DNA compaction and the loss of B-DNA conformation at higher superhelical densities. The amount of DNase I digestion in the presence of OmpR was consistent regardless of the superhelical density of PompR DNA. Thus, OmpR may reduce DNase I digestion across the entire promoter region by constraining a supercoiled-like state in DNA, as has been observed for FIS and H-NS [29], [30].
DNA positions that are hypersensitive to endonuclease cutting offer additional insight into changes in DNA topology. For example, positions −207 and −182 became less sensitive whereas positions −154 and −9 became increasingly sensitive to DNase I digestion as supercoiling increased. Position −154 (marked with an asterisk) is particularly intriguing because it was ultra-hypersensitive to DNase I digestion when DNA was supercoiled. When DNA was fully relaxed, OmpR binding greatly enhanced DNase I cutting at position −154, supporting a model in which OmpR binding creates DNA structures similar to those induced by negative DNA supercoiling.
DNA supercoiling exerts torsional stress that weakens base pairing, and so reduces the amount of energy needed for DNA melting and transcription initiation. This is referred to as stress-induced duplex destabilization (SIDD), and the energy required for strand separation at each base pair in a specific sequence, G(x), can be predicted for different superhelical densities [31]. Stable base pairs have G(x) values around 10, whereas lower values indicate positions prone to SIDD. We used WebSIDD [32] to predict the stability of PompR DNA at the approximate superhelical densities observed during exponential growth (σ = −0.06) and after treatment with 15 µg/ml novobiocin (σ = −0.045). The G(x) profiles of PompR at both superhelical densities revealed a highly destabilized region ranging from positions −70 to −160, with a weakly destabilized region (−20 to −55) encompassing the ompR transcription start sites (Figure 5B). This analysis makes the counterintuitive prediction that PompR becomes increasingly destabilized as DNA relaxes, which is nevertheless consistent with the observed gene activation in these conditions (Figure 1B). DNA upstream of position −170 is highly stable, indicating that the destabilization effect is specific and concentrated at the main binding site used by OmpR. The primary OmpR binding sites, OmpR-1 and OmpR-2, cover most of the highly destabilized regions, raising the possibility that OmpR binding transmits the destabilizing force to the adjacent RNA polymerase binding site where DNA strand separation can assist in transcription initiation. A similar SIDD-transmission function has been characterized for FIS and IHF [33].
Novobiocin treatment caused the same degree of DNA relaxation in ΔompR mutant cells as in wild type cells (Figure S2B). This suggests that promoter DNA experiences the same stress-induced strand destabilization in both mutant and wild type, indicating that reduced promoter activation in the ΔompR mutant (Figure 1B) is due to the absence of OmpR binding, not an altered degree of DNA relaxation. In other words, DNA strand destabilization caused by DNA supercoiling is insufficient for PompR activation in the absence of OmpR binding.
Discussion
S. enterica traverses various extracellular and intracellular environments during infection of host tissues, thus it requires genetic programs capable of balancing shifting requirements for the T3SS and effector proteins that mediate the invasion process. Two recent studies unexpectedly discovered that SPI-2 genes are expressed in the mouse intestinal lumen prior to cellular invasion, leading to the hypotheses that SPI-2 is either important for colonization of the intestine or requires priming before intracellular invasion [12], [34]. SPI-2 expression during growth in rich medium, which roughly mimics conditions in the intestinal lumen, requires OmpR and FIS but is independent of SsrB, SlyA, and PhoP [12], [18]. Here we describe a fundamental mechanism that activates both SPI-2 and SPI-1 promoters through changes in DNA topology, and this mechanism depends on OmpR and FIS but is independent of SsrB and HilD. It is intriguing that PssrA induction does not require FIS in culture conditions that mimic the vacuolar environment [12], nor is FIS required for PssrA induction when DNA is highly relaxed [20]. These findings support a model in which fine-tuning of SPI gene expression by factors such as SsrB, HilD, SlyA, and PhoP may occur primarily in the vacuolar environment.
Although OmpR-EnvZ is the archetypal two component signal transduction pathway, the environmental stimulus of EnvZ kinase activity remains unclear [35], and this stimulus appears to differ between E. coli and S. enterica [36]. It is perhaps for this reason that a role for OmpR in the regulation of SPI-1 has been enigmatic. Previous studies have found no effect or only weak effects from deletion of ompR or envZ. It was initially proposed that OmpR-EnvZ directly controls hilC expression [37], but others have favoured a model in which OmpR somehow acts post-transcriptionally through HilD protein function [2]. Here we provide evidence that under conditions of relaxed DNA supercoiling, OmpR binds directly to both the hilC and hilD promoters where it activates the former and represses the latter.
There is a growing body of evidence that in addition to the classic role of OmpR as a site-specific transcription factor that activates gene expression through RNAP recruitment, it also exhibits NAP-like features and functions. Because OmpR makes few specific contacts with DNA, it demonstrates an affinity for non-specific DNA [38], [39]. The preferred OmpR target sites, which are highly degenerate at the sequence level, may serve as nucleating points for cooperative recruitment of additional OmpR molecules. Nucleation and cooperative DNA binding can explain the broad regions of OmpR protection observed at PhilC (Figure 3B), PompR (Figure 5A), and PssrA [10]. Additionally, our ChIP data revealed OmpR binding to regions not predicted to be specific targets (Figure S2C). This is similar to the ChIP survey of cAMP receptor protein (CRP) targets in E. coli which revealed a high background of CRP binding across the entire chromosome [40]. CRP binding to thousands of weak sites lead the authors to propose that this archetypal transcription factor should also be considered as a chromosome-structuring protein. An additional interesting parallel between OmpR and CRP is that both are calculated to have similar cellular concentrations: ∼3,000 CRP and ∼3,500 OmpR molecules/cell [41], [42]. Thus, both OmpR and CRP may represent a class of NAP-like DNA-binding proteins whose high abundance allows them to have a broad influence on chromosome shape and function, yet whose modulation through allosteric effectors generates titratable DNA-binding modes that preferentially target specific promoters.
The discovery that DNA relaxation results in increased OmpR binding to DNA in vivo presents an intriguing model in which this mechanism is complementary to phosphorylation of OmpR by EnvZ as a means to stimulate OmpR-DNA binding. Thus, phosphorylated OmpR may be recruited to promoters by DNA relaxation. Future global analysis of OmpR binding to the S. enterica chromosome will shed light on the relative contributions of phosphorylation and DNA relaxation to OmpR-DNA interactions.
Whole-genome analysis of the transcriptional consequences of DNA relaxation in E. coli revealed that relaxation-induced promoters are significantly more A+T-rich than are uninduced promoters [43]. Because H-NS preferentially binds to regions of high A+T content, relaxation-induced promoters are very likely to be H-NS repressed. In E. coli, the OmpR targets PompR and PompC were induced whereas PompF was repressed by novobiocin [43], and we found the same response in S. enterica (Figure S2D). This shared response of OmpR-regulated genes to novobiocin in E. coli and S. enterica coupled with the proposed ability of DNA relaxation to weaken H-NS repression hints at an evolutionarily conserved gene regulatory mechanism that predates horizontal acquisition of SPI-1 and SPI-2 by Salmonella.
Transcriptional activators of SPI genes (HilC, HilD, SsrB, and SlyA) function in large part through displacement of H-NS from SPI promoters [16], [44]. Members of the AraC-like protein family, which includes HilC and HilD, have a well-documented ability to displace H-NS [45]. SsrB, a member of the NarL protein family, activates transcription by displacing H-NS but does not appear able to break H-NS bridges [44]. OmpR and SlyA are winged-helix DNA binding proteins. Like OmpR, SlyA relieves H-NS repression without displacing H-NS [46]; SlyA also generates regions of DNase I hypersensitivity, thus may have a topological restructuring mode that contributes to breaking H-NS bridges [46]. However, unlike OmpR, SlyA relies on activators such as PhoP to recruit RNAP. Variable modes of H-NS antagonism — from anti-polymerization by HilD, HilC, and SsrB to anti-bridging by OmpR and SlyA — may represent a gate-keeper mechanism that selects which of the numerous regulators known to act at SPI gene promoters are allowed access to their target DNA sites, thus fine-tuning transcriptional output.
Materials and Methods
Strains
Salmonella enterica serovar Typhimurium strain SL1344 was used for all experiments. Detailed descriptions of mutant strains used in this study are provided in Table S1. E. coli XL-1 blue was used for all cloning steps.
Mutant construction
Strains and plasmids used in this study are listed in Table S1. To generate S. enterica mutants, the kanamycin resistance cassette was PCR amplified from pKD4 [47] using primers listed in Table S2, which were designed to replace only open reading frames. Because the ompR and envZ open reading frames overlap, special care was taken to preserve open reading frames when constructing deletion and epitope-fusion mutations.
PCR amplicons were spin column purified then transformed into electrocompetent S. enterica SL1344 containing the Red helper plasmid pKD46 as previously described [47], [48]. Mutations were transduced into a fresh SL1344 background by bacteriophage P22 generalized transduction [49], then were confirmed by DNA sequencing.
Site-directed mutagenesis of the ompB locus cloned in pUC18 was carried out using the QuikChange II kit (Stratagene) and primers listed in Table S2, following the manufacturer's protocol.
Cloning
Transcriptional reporter fusions were constructed by cloning gene promoters in pZep and pZec vectors, which contain a promoterless gfp+ gene [20], [50]. The cat gene was removed from pZep to generate pZec so that transcriptional reporter fusions could be cloned in cells containing pBAD. The removal of the cat gene from pZep to generate pZec had no effect on expression of cloned promoters (not shown). Data presented in Figure 1 and Figure 2 is from cells carrying pZec reporter plasmids.
pZep or and pZec plasmids were digested with SmaI and XbaI, spin column purified using the HiYield PCR DNA Fragment Extraction Kit (RBC Bioscience), and dephosphorylated with Antarctic Phosphatase (New England Biolabs). Gene promoter sequences were PCR amplified using the Phusion DNA polymerase (NEB) and primers listed in Table S2. SmaI and XbaI digested amplicons were spin column purified, then ligated to pZep or pZec by T4 ligase (Roche). The exception was the ompR promoter region which was digested with NotI and XbaI before cloning into similarly digested vector.
The ompB locus along with its native ribosome binding site was PCR amplified using primers listed in Table S2, followed by PstI and SacI digestion and spin column purification. PstI and SacI digested pBAD33 was gel purified, de-phosphorylated by Antarctic Phosphatase (NEB), then ligated to the digested PCR amplicon. The effects of inducible ompB on promoter function were assessed by measuring GFP levels in cells carrying both pBADompB and pZec transcriptional reporter clones. Arabinose and novobiocin were added to cultures at the concentrations indicated in Figure 2.
Culture conditions and novobiocin treatment
Cells were cultured in a shaking waterbath at 37°C in LB (1% tryptone and 0.5% yeast extract) without any NaCl added. Cells used for gfp reporter fusion experiments were cultured in 4 ml of LB in glass tubes (interior diameter 14 mm) shaking at 200 RPM whereas cells used for ChIP and quantitative PCR experiments were cultured in 55 ml of LB in 250 ml glass flasks shaking at 140 RPM. Previous studies testing the effects of novobiocin on gene expression in S. enterica have used high concentrations of novobiocin (25–150 µg/ml) in cells transitioning from late exponential to stationary phase physiology [9], [23], thus introducing additional variables arising from growth phase transitions. To ensure a steady state of growth, we conducted all experiments using cells that had been growing exponentially for more than six doublings at low cell density (OD600 less than 0.3). In addition, we used low concentrations of novobiocin (15–25 µg/ml) to minimize effects on growth rate. Cells were fixed after 3 hrs of continued growth at low density in the presence or absence of novobiocin (final OD600 0.1–0.25), as in [20].
Quantitative PCR measurement of gene expression
Total RNA was isolated from cultures using the SV Total RNA Isolation System (Promega) and purity and quality was assessed by electrophoresis in 1% agarose (1×TAE). For each sample, 5 µg total RNA was DNase treated in a 50-µl reaction using the Turbo DNA-free kit (AMBION), and cDNA templates were synthesized by random priming 0.5 µg RNA in a 20 µl reaction using the GoScrip Reverse Transcription System (Promega). Quantitative PCR (qPCR) primers are listed in Table S2. PCR reactions were carried out in duplicate with each primer set on an ABI 7500 Sequence Detection System (Applied Biosystems) using FastStart SYBR Green Master with ROX (Roche). Standard curves were included in every qPCR run; standard curves were generated for each primer set using five serial tenfold dilutions of S. enterica chromosomal DNA.
Chromatin immuno-precipitation (ChIP)
ChIP was conducted as previously described [27]. Two ChIP replicates were performed using a strain containing both the ompR:flag and fis:myc epitope fusions, allowing for simultaneous precipitation of OmpR:Flag, Fis:Myc, and H-NS from the same biological sample. One ChIP replicate was conducted for each strain carrying a single epitope tag (ompR:flag or fis:myc). ChIP results overlapped between the double and single fusion strains, indicating that the epitope-tagged proteins did not negatively affect nucleoprotein interactions when combined. Precipitated DNA was quantified by quantitative PCR using primers listed in Table S2.
Western blot analysis
Cells were pelleted and resuspended in 1× Laemmli buffer (4% SDS, 20% glycerol, 10% 2-mercaptoethanol, 0.004% bromophenol blue, 0.125 M Tris HCl, pH 6.8) and denatured at 100°C for 5 min. Samples were electrophoresed on 15% polyacrylamide SDS gels. Gels and nitrocellulose membranes were equilibrated in transfer buffer (25 mM Tris HCl, 192 mM glycine, 0.02% SDS, 20% methanol) and proteins were transferred to membranes at 150 V for 90 min using a Trans-blot (BioRad) apparatus packed in ice. Membranes were blocked overnight at 4°C in 5% non-fat powdered milk in PBS (137 mM NaCl, 12 mM Phosphate, 2.7 mM KCl, pH 7.4), followed by incubation at room temperature for 2 hr with rocking in primary antibodies diluted as follows: 1/100,000 anti-DnaK mAb rabbit (Enzo Life Sciences), 1/10,000 anti-FLAG mAb rabbit (Sigma), 1/10,000 anti-Myc mAb rabbit (Sigma), and 1/5,000 anti-H-NS polyclonal mouse [5]. Blots were washed thoroughly and probed with horseradish peroxidase-linked anti-rabbit and anti-mouse antibodies (Millipore) diluted 1/5,000 in PBS (1% blocking agent) for 1 hr at room temperature with rocking, followed by thorough washing. Blots were incubated in ECL reagent (Pierce) for 1 min, and bands were visualized using an ImageQuant LAS 4000 scanner (GE Healthcare) then quantified using ImageJ v1.43 (National Institutes of Health, U.S.A.). Probing for all proteins (DnaK, FIS:Myc, H-NS, and OmpR:Flag) simultaneously on the same blot allowed for protein quantities to be normalized to the internal standard (DnaK) and expressed relative to one another. Each cell sample was run on three independent western blots to improve the accuracy of quantification. Thus, the protein abundance value for each biological replicate is the average value from three replicate blots.
OmpR purification
The OmpR D55E mutation creates a constitutively active protein by mimicking phosphorylation [51]. OmpR(D55E) with a C-terminal His-tag was purified and used in bandshifts and DNase I footprinting. BL21 cells carrying the pET21-ompR(D55E) plasmid were grown in L broth (0.5% NaCl; 100 µg/ml carbenicillin) and ompR(D55E) expression was induced at OD600 0.5 with 1 mM IPTG. Cells were harvested after 4.5 hr by centrifugation and the pellet were frozen overnight at −20°. Native OmpR(D55E)-His was purified as follows: the pellet was resuspended in lysis buffer (50 mM sodium phosphate, 300 mM sodium chloride, 10 mM imidazole), then treated with 1 mg/ml lysozyme for 30 min at 24°C followed by sonication on ice. Insoluble material was removed by centrifugation at 10,000 g for 25 min and the supernatant was then incubated with nickel-nitriloacetic acid agarose beads for 1 hr at 4°C with gentle rocking. The agarose beads were loaded in a column and washed twice with four column volumes of wash buffer (50 mM NaH2PO4, 300 mM NaCl, 20 mM imidazole, pH 8.0), and protein was collected in elution buffer (50 mM NaH2PO4, 300 mM NaCl, 250 mM imidazole, pH 8.0). Purified protein was desalted with Nanosep 3K Omega membranes (Pall) at 4°C, then resuspended in storage buffer (20% glycerol, 40 mM Tris, 200 mM KCl) and stored at −80°. OmpR(D55E) purity was assessed on Coomassie stained SDS-PAGE gels, and concentration was quantified by both the Bradford assay and by comparison to protein standards on Coomassie stained SDS-PAGE gels.
Bandshifts
Bait DNA was PCR amplified using the primers pZec.6FAM.R (labeled with a 5′ 6-FAM fluorophore) and pZec.confirm.F (Table S2), from pZec promoter clones. Amplicons were spin column purified then used as bait DNA in bandshifts. OmpR-DNA binding reactions (10 µl) contained 0.2× TBE (89 mM Tris, 89 mM borate, 2 mM EDTA (pH 8.3), 40 µg/µl poly(dI-dC) DNA, and 40 nM bait DNA. Reactions were incubated at room temperature for 15 min before being loaded onto a running polyacrylamide gel (30∶1 acrylamide/bisacrylamide, 0.2× TBE, 2% glycerol) with 0.2× TBE running buffer. After electrophoresis for 40 min at 100 V, 6-FAM-labeled DNA was visualized using a Typhoon scanner (GE Healthcare).
DNase I footprinting of OmpR binding to PCR-amplified gene promoters
Bait DNA was prepared as for bandshifts using either the primer sets pZec.6FAM.F and pZec.confirm.R (top strand) or pZec.6FAM.R and pZec.confirm.F (bottom strand). DNase I footprinting reactions were conducted in 15 µl reaction volumes containing 1× DNase I buffer (Roche)(40 mM Tris-HCl, 10 mM NaCl, 6 mM MgCl2, 1 mM CaCl2; pH 7.9), 0.01 mM dithiothreitol, 100 ng/µl BSA, 50 nM bait DNA, and 5 µM OmpR(D55E)-His. OmpR-DNA binding was allowed to equilibrate at 37°C for 15 minutes, then 1 µl (0.015 units) of pre-warmed DNase I was added and mixed gently, then incubated at 37°C for 10 minutes. Reactions were stopped by addition of 2 µl EDTA (100 mM) followed by vigorous vortex mixing and heat denaturation at 95°C for 10 min. Digestion products were desalted using MicroSpin G-50 columns (GE Healthcare) and were analyzed on an ABI 3130 Genetic Analyzer along with GeneScan 500-LIZ size standards (Applied Biosystems).
DNase I footprinting of OmpR binding to supercoiled gene promoters
Plasmids with varying degrees of superhelical density were generated as follows: pZec-PompR was purified from E. coli CSH50 at different topological states by growing cells overnight in 25 ml L (0% NaCl) in a well-aerated 250 ml glass flask (low supercoiling) or overnight in 6 ml L (0.5% NaCl) in 14 mm diameter glass culture tubes (high supercoiling). Topoisomers at the desired topological state were purified after separation on a 1% agarose gel containing 2.5 µg/ml chloroquine. The average superhelical density of each purified plasmid pool was determined by calculating the linking difference between the dominant topoisomer and fully relaxed DNA [52]. A 1% agarose gel containing 25 µg/ml chloroquine was used to improve the resolution of topoisomers in the low supercoiling sample; in these conditions, more relaxed DNA migrates faster through the gel. To generate a plasmid pool that lacked supercoiling, pZec-ompR was digested with XhoI, which cuts 500 bp away from the cloned promoter.
Because nicking of plasmid DNA by DNase I will allow DNA supercoils to relax, footprinting reactions were treated with DNase I for no more than 1 minute to reduce the time in which nicked plasmids could lose their topology. In these reactions, 1 µl (0.15 units) of DNase I was added to 15 µl OmpR-DNA binding reactions containing 10 µM OmpR and 1.5 nM bait DNA. OmpR-DNA binding was equilibrated as above.
Primer extension was conducted using the primer pZec.6FAM.F and Thermo Sequenase polymerase (USB) with the following thermocycle: 95°C for 30 sec, 53°C for 30 sec, and 72°C for 90 sec, repeated 50 times. Extension reactions contained 0.4 nM of nicked plasmid template. Amplification products were desalted using MicroSpin G-50 columns (GE Healthcare) and were analyzed on an ABI 3130 Genetic Analyzer along with GeneScan 500-LIZ size standards (Applied Biosystems). To compare samples, each was normalized to have the same total fluorescence signal across the DNA region being analysed.
Supporting Information
Zdroje
1. HaragaAOhlsonMBMillerSI 2008 Salmonellae interplay with host cells. Nat Rev Microbiol 6 53 66
2. EllermeierJRSlauchJM 2007 Adaptation to the host environment: regulation of the SPI1 type III secretion system in Salmonella enterica serovar Typhimurium. Curr Opin Microbiol 10 24 29
3. FassEGroismanEA 2009 Control of Salmonella pathogenicity island-2 gene expression. Curr Opin Microbiol 12 199 204
4. HautefortIThompsonAEriksson-YgbergSParkerMLLucchiniS 2008 During infection of epithelial cells Salmonella enterica serovar Typhimurium undergoes a time-dependent transcriptional adaptation that results in simultaneous expression of three type 3 secretion systems. Cell Microbiol 10 958 984
5. LucchiniSRowleyGGoldbergMDHurdDHarrisonM 2006 H-NS mediates the silencing of laterally acquired genes in bacteria. PLoS Pathog 2 e81 doi:10.1371/journal.ppat.0020081
6. OshimaTIshikawaSKurokawaKAibaHOgasawaraN 2006 Escherichia coli histone-like protein H-NS preferentially binds to horizontally acquired DNA in association with RNA polymerase. DNA Res 13 141 153
7. KellyAGoldbergMDCarrollRKDaninoVHintonJC 2004 A global role for Fis in the transcriptional control of metabolism and type III secretion in Salmonella enterica serovar Typhimurium. Microbiology 150 2037 2053
8. YoonHMcDermottJEPorwollikSMcClellandMHeffronF 2009 Coordinated regulation of virulence during systemic infection of Salmonella enterica serovar Typhimurium. PLoS Pathog 5 e1000306 doi:10.1371/journal.ppat.1000306
9. Ó CróinínTCarrollRKKellyADormanCJ 2006 Roles for DNA supercoiling and the Fis protein in modulating expression of virulence genes during intracellular growth of Salmonella enterica serovar Typhimurium. Mol Microbiol 62 869 882
10. FengXOropezaRKenneyLJ 2003 Dual regulation by phospho-OmpR of ssrA/B gene expression in Salmonella pathogenicity island 2. Mol Microbiol 48 1131 1143
11. DormanCJChatfieldSHigginsCFHaywardCDouganG 1989 Characterization of porin and ompR mutants of a virulent strain of Salmonella typhimurium: ompR mutants are attenuated in vivo. Infect Immun 57 2136 2140
12. OsborneSECoombesBK 2011 Transcriptional priming of Salmonella Pathogenicity Island-2 precedes cellular invasion. PLoS ONE 6 e21648 doi:10.1371/journal.pone.0021648
13. RhenMDormanCJ 2005 Hierarchical gene regulators adapt Salmonella enterica to its host milieus. Int J Med Microbiol 294 487 502
14. EllermeierCDEllermeierJRSlauchJM 2005 HilD, HilC and RtsA constitute a feed forward loop that controls expression of the SPI1 type three secretion system regulator hilA in Salmonella enterica serovar Typhimurium. Mol Microbiol 57 691 705
15. SchechterLMLeeCA 2001 AraC/XylS family members, HilC and HilD, directly bind and derepress the Salmonella typhimurium hilA promoter. Mol Microbiol 40 1289 1299
16. OlekhnovichINKadnerRJ 2002 DNA-binding activities of the HilC and HilD virulence regulatory proteins of Salmonella enterica serovar Typhimurium. J Bacteriol 184 4148 4160
17. BajajVHwangCLeeCA 1995 hilA is a novel ompR/toxR family member that activates the expression of Salmonella typhimurium invasion genes. Mol Microbiol 18 715 727
18. BustamanteVHMartinezLCSantanaFJKnodlerLASteele-MortimerO 2008 HilD-mediated transcriptional cross-talk between SPI-1 and SPI-2. Proc Natl Acad Sci U S A 105 14591 14596
19. GalanJECurtissR3rd 1990 Expression of Salmonella typhimurium genes required for invasion is regulated by changes in DNA supercoiling. Infect Immun 58 1879 1885
20. CameronADStoebelDMDormanCJ 2011 DNA supercoiling is differentially regulated by environmental factors and FIS in Escherichia coli and Salmonella enterica. Mol Microbiol 80 85 101
21. MarteynBWestNPBrowningDFColeJAShawJG 2010 Modulation of Shigella virulence in response to available oxygen in vivo. Nature 465 355 358
22. BordesPConterAMoralesVBouvierJKolbA 2003 DNA supercoiling contributes to disconnect σS accumulation from σS-dependent transcription in Escherichia coli. Mol Microbiol 48 561 571
23. BangISAudiaJPParkYKFosterJW 2002 Autoinduction of the ompR response regulator by acid shock and control of the Salmonella enterica acid tolerance response. Mol Microbiol 44 1235 1250
24. SkokoDYooDBaiHSchnurrBYanJ 2006 Mechanism of chromosome compaction and looping by the Escherichia coli nucleoid protein Fis. J Mol Biol 364 777 798
25. RimskySZuberFBuckleMBucH 2001 A molecular mechanism for the repression of transcription by the H-NS protein. Mol Microbiol 42 1311 1323
26. YoshidaTQinLEggerLAInouyeM 2006 Transcription regulation of ompF and ompC by a single transcription factor, OmpR. J Biol Chem 281 17114 17123
27. DillonSCCameronADHokampKLucchiniSHintonJC 2010 Genome-wide analysis of the H-NS and Sfh regulatory networks in Salmonella Typhimurium identifies a plasmid-encoded transcription silencing mechanism. Mol Microbiol 76 1250 1265
28. ChoBKKnightEMBarrettCLPalssonBO 2008 Genome-wide analysis of Fis binding in Escherichia coli indicates a causative role for A-/AT-tracts. Genome Res 18 900 910
29. SchneiderRLurzRLuderGTolksdorfCTraversA 2001 An architectural role of the Escherichia coli chromatin protein FIS in organising DNA. Nucleic Acids Res 29 5107 5114
30. TupperAEOwen-HughesTAUsseryDWSantosDSFergusonDJ 1994 The chromatin-associated protein H-NS alters DNA topology in vitro. EMBO J 13 258 268
31. WangHNoordewierMBenhamCJ 2004 Stress-induced DNA duplex destabilization (SIDD) in the E. coli genome: SIDD sites are closely associated with promoters. Genome Res 14 1575 1584
32. BiCBenhamCJ 2004 WebSIDD: server for predicting stress-induced duplex destabilized (SIDD) sites in superhelical DNA. Bioinformatics 20 1477 1479
33. HatfieldGWBenhamCJ 2002 DNA topology-mediated control of global gene expression in Escherichia coli. Annu Rev Genet 36 175 203
34. BrownNFVallanceBACoombesBKValdezYCoburnBA 2005 Salmonella pathogenicity island 2 is expressed prior to penetrating the intestine. PLoS Pathog 1 e32 doi:10.1371/journal.ppat.0010032
35. BatchelorEGoulianM 2006 Imaging OmpR localization in Escherichia coli. Mol Microbiol 59 1767 1778
36. Martinez-FloresICanoRBustamanteVHCalvaEPuenteJL 1999 The ompB operon partially determines differential expression of OmpC in Salmonella typhi and Escherichia coli. J Bacteriol 181 556 562
37. LucasRLLeeCA 2001 Roles of hilC and hilD in regulation of hilA expression in Salmonella enterica serovar Typhimurium. J Bacteriol 183 2733 2745
38. HeadCGTardyAKenneyLJ 1998 Relative binding affinities of OmpR and OmpR-phosphate at the ompF and ompC regulatory sites. J Mol Biol 281 857 870
39. RheeJEShengWMorganLKNoletRLiaoX 2008 Amino acids important for DNA recognition by the response regulator OmpR. J Biol Chem 283 8664 8677
40. GraingerDCHurdDHarrisonMHoldstockJBusbySJ 2005 Studies of the distribution of Escherichia coli cAMP-receptor protein and RNA polymerase along the E. coli chromosome. Proc Natl Acad Sci U S A 102 17693 17698
41. BergOGvon HippelPH 1988 Selection of DNA binding sites by regulatory proteins. II. The binding specificity of cyclic AMP receptor protein to recognition sites. J Mol Biol 200 709 723
42. CaiSJInouyeM 2002 EnvZ-OmpR interaction and osmoregulation in Escherichia coli. J Biol Chem 277 24155 24161
43. PeterBJArsuagaJBreierAMKhodurskyABBrownPO 2004 Genomic transcriptional response to loss of chromosomal supercoiling in Escherichia coli. Genome Biol 5 R87
44. WalthersDLiYLiuYAnandGYanJ 2011 Salmonella enterica response regulator SsrB relieves H-NS silencing by displacing H-NS bound in polymerization mode and directly activates transcription. J Biol Chem 286 1895 1902
45. EganSM 2002 Growing repertoire of AraC/XylS activators. J Bacteriol 184 5529 5532
46. PerezJCLatifiTGroismanEA 2008 Overcoming H-NS-mediated transcriptional silencing of horizontally acquired genes by the PhoP and SlyA proteins in Salmonella enterica. J Biol Chem 283 10773 10783
47. DatsenkoKAWannerBL 2000 One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A 97 6640 6645
48. UzzauSFigueroa-BossiNRubinoSBossiL 2001 Epitope tagging of chromosomal genes in Salmonella. Proc Natl Acad Sci U S A 98 15264 15269
49. SternbergNLMaurerR 1991 Bacteriophage-mediated generalized transduction in Escherichia coli and Salmonella typhimurium. Methods Enzymol 204 18 43
50. HautefortIProencaMJHintonJC 2003 Single-copy green fluorescent protein gene fusions allow accurate measurement of Salmonella gene expression in vitro and during infection of mammalian cells. Appl Environ Microbiol 69 7480 7491
51. LanCYIgoMM 1998 Differential expression of the OmpF and OmpC porin proteins in Escherichia coli K-12 depends upon the level of active OmpR. J Bacteriol 180 171 174
52. RichardsonSMHigginsCFLilleyDM 1984 The genetic control of DNA supercoiling in Salmonella typhimurium. EMBO J 3 1745 1752
Štítky
Genetika Reprodukční medicína
Článek Physiological Notch Signaling Maintains Bone Homeostasis via RBPjk and Hey Upstream of NFATc1Článek Intronic -Regulatory Modules Mediate Tissue-Specific and Microbial Control of / TranscriptionČlánek Probing the Informational and Regulatory Plasticity of a Transcription Factor DNA–Binding DomainČlánek Repression of Germline RNAi Pathways in Somatic Cells by Retinoblastoma Pathway Chromatin ComplexesČlánek An Alu Element–Associated Hypermethylation Variant of the Gene Is Associated with Childhood ObesityČlánek Three Essential Ribonucleases—RNase Y, J1, and III—Control the Abundance of a Majority of mRNAsČlánek Genomic Tools for Evolution and Conservation in the Chimpanzee: Is a Genetically Distinct Population
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 3
-
Všechny články tohoto čísla
- Comprehensive Research Synopsis and Systematic Meta-Analyses in Parkinson's Disease Genetics: The PDGene Database
- Genomic Analysis of the Hydrocarbon-Producing, Cellulolytic, Endophytic Fungus
- Networks of Neuronal Genes Affected by Common and Rare Variants in Autism Spectrum Disorders
- Akirin Links Twist-Regulated Transcription with the Brahma Chromatin Remodeling Complex during Embryogenesis
- Too Much Cleavage of Cyclin E Promotes Breast Tumorigenesis
- Imprinted Genes … and the Number Is?
- Genetic Architecture of Highly Complex Chemical Resistance Traits across Four Yeast Strains
- Exploring the Complexity of the HIV-1 Fitness Landscape
- MNS1 Is Essential for Spermiogenesis and Motile Ciliary Functions in Mice
- A Fundamental Regulatory Mechanism Operating through OmpR and DNA Topology Controls Expression of Pathogenicity Islands SPI-1 and SPI-2
- Evidence for Positive Selection on a Number of MicroRNA Regulatory Interactions during Recent Human Evolution
- Variation in Modifies Risk of Neonatal Intestinal Obstruction in Cystic Fibrosis
- PIF4–Mediated Activation of Expression Integrates Temperature into the Auxin Pathway in Regulating Hypocotyl Growth
- Critical Evaluation of Imprinted Gene Expression by RNA–Seq: A New Perspective
- A Meta-Analysis and Genome-Wide Association Study of Platelet Count and Mean Platelet Volume in African Americans
- Mouse Genetics Suggests Cell-Context Dependency for Myc-Regulated Metabolic Enzymes during Tumorigenesis
- Transcriptional Control in Cardiac Progenitors: Tbx1 Interacts with the BAF Chromatin Remodeling Complex and Regulates
- Synthetic Lethality of Cohesins with PARPs and Replication Fork Mediators
- APOBEC3G-Induced Hypermutation of Human Immunodeficiency Virus Type-1 Is Typically a Discrete “All or Nothing” Phenomenon
- Interpreting Meta-Analyses of Genome-Wide Association Studies
- Error-Prone ZW Pairing and No Evidence for Meiotic Sex Chromosome Inactivation in the Chicken Germ Line
- -Dependent Chemosensory Functions Contribute to Courtship Behavior in
- Diverse Forms of Splicing Are Part of an Evolving Autoregulatory Circuit
- Phenotypic Plasticity of the Drosophila Transcriptome
- Physiological Notch Signaling Maintains Bone Homeostasis via RBPjk and Hey Upstream of NFATc1
- Precocious Metamorphosis in the Juvenile Hormone–Deficient Mutant of the Silkworm,
- Igf1r Signaling Is Indispensable for Preimplantation Development and Is Activated via a Novel Function of E-cadherin
- Accurate Prediction of Inducible Transcription Factor Binding Intensities In Vivo
- Mitochondrial Oxidative Stress Alters a Pathway in Strongly Resembling That of Bile Acid Biosynthesis and Secretion in Vertebrates
- Mammalian Neurogenesis Requires Treacle-Plk1 for Precise Control of Spindle Orientation, Mitotic Progression, and Maintenance of Neural Progenitor Cells
- Tcf7 Is an Important Regulator of the Switch of Self-Renewal and Differentiation in a Multipotential Hematopoietic Cell Line
- REST–Mediated Recruitment of Polycomb Repressor Complexes in Mammalian Cells
- Intronic -Regulatory Modules Mediate Tissue-Specific and Microbial Control of / Transcription
- Age-Dependent Brain Gene Expression and Copy Number Anomalies in Autism Suggest Distinct Pathological Processes at Young Versus Mature Ages
- A Genome-Wide Association Study Identifies Variants Underlying the Shade Avoidance Response
- -by- Regulatory Divergence Causes the Asymmetric Lethal Effects of an Ancestral Hybrid Incompatibility Gene
- Genome-Wide Association and Functional Follow-Up Reveals New Loci for Kidney Function
- A Natural System of Chromosome Transfer in
- Cell Size and the Initiation of DNA Replication in Bacteria
- Probing the Informational and Regulatory Plasticity of a Transcription Factor DNA–Binding Domain
- Repression of Germline RNAi Pathways in Somatic Cells by Retinoblastoma Pathway Chromatin Complexes
- Temporal Transcriptional Profiling of Somatic and Germ Cells Reveals Biased Lineage Priming of Sexual Fate in the Fetal Mouse Gonad
- Rapid Analysis of Genome Rearrangements by Multiplex Ligation–Dependent Probe Amplification
- Metabolic Profiling of a Mapping Population Exposes New Insights in the Regulation of Seed Metabolism and Seed, Fruit, and Plant Relations
- The Atypical Calpains: Evolutionary Analyses and Roles in Cellular Degeneration
- The Silkworm Coming of Age—Early
- Development of a Panel of Genome-Wide Ancestry Informative Markers to Study Admixture Throughout the Americas
- Balanced Codon Usage Optimizes Eukaryotic Translational Efficiency
- The Min System and Nucleoid Occlusion Are Not Required for Identifying the Division Site in but Ensure Its Efficient Utilization
- Neurobeachin, a Regulator of Synaptic Protein Targeting, Is Associated with Body Fat Mass and Feeding Behavior in Mice and Body-Mass Index in Humans
- Statistical Analysis of Readthrough Levels for Nonsense Mutations in Mammalian Cells Reveals a Major Determinant of Response to Gentamicin
- Gene Reactivation by 5-Aza-2′-Deoxycytidine–Induced Demethylation Requires SRCAP–Mediated H2A.Z Insertion to Establish Nucleosome Depleted Regions
- The miR-35-41 Family of MicroRNAs Regulates RNAi Sensitivity in
- Genetic Basis of Hidden Phenotypic Variation Revealed by Increased Translational Readthrough in Yeast
- An Alu Element–Associated Hypermethylation Variant of the Gene Is Associated with Childhood Obesity
- Modelling Human Regulatory Variation in Mouse: Finding the Function in Genome-Wide Association Studies and Whole-Genome Sequencing
- Novel Loci for Adiponectin Levels and Their Influence on Type 2 Diabetes and Metabolic Traits: A Multi-Ethnic Meta-Analysis of 45,891 Individuals
- Polycomb-Like 3 Promotes Polycomb Repressive Complex 2 Binding to CpG Islands and Embryonic Stem Cell Self-Renewal
- Insulin/IGF-1 and Hypoxia Signaling Act in Concert to Regulate Iron Homeostasis in
- EMF1 and PRC2 Cooperate to Repress Key Regulators of Arabidopsis Development
- Three Essential Ribonucleases—RNase Y, J1, and III—Control the Abundance of a Majority of mRNAs
- Contrasted Patterns of Molecular Evolution in Dominant and Recessive Self-Incompatibility Haplotypes in
- A Machine Learning Approach for Identifying Novel Cell Type–Specific Transcriptional Regulators of Myogenesis
- Genomic Tools for Evolution and Conservation in the Chimpanzee: Is a Genetically Distinct Population
- Nos2 Inactivation Promotes the Development of Medulloblastoma in Mice by Deregulation of Gap43–Dependent Granule Cell Precursor Migration
- Intracranial Aneurysm Risk Locus 5q23.2 Is Associated with Elevated Systolic Blood Pressure
- Heritability and Genetic Correlations Explained by Common SNPs for Metabolic Syndrome Traits
- A Genome-Wide Association Study of Nephrolithiasis in the Japanese Population Identifies Novel Susceptible Loci at 5q35.3, 7p14.3, and 13q14.1
- DNA Damage in Nijmegen Breakage Syndrome Cells Leads to PARP Hyperactivation and Increased Oxidative Stress
- DNA Resection at Chromosome Breaks Promotes Genome Stability by Constraining Non-Allelic Homologous Recombination
- Genetic Analysis of Floral Symmetry in Van Gogh's Sunflowers Reveals Independent Recruitment of Genes in the Asteraceae
- A Splice Site Variant in the Bovine Gene Compromises Growth and Regulation of the Inflammatory Response
- Promoter Nucleosome Organization Shapes the Evolution of Gene Expression
- The Nucleoside Diphosphate Kinase Gene Acts as Quantitative Trait Locus Promoting Non-Mendelian Inheritance
- The Ciliogenic Transcription Factor RFX3 Regulates Early Midline Distribution of Guidepost Neurons Required for Corpus Callosum Development
- Phosphorylation of the RNA–Binding Protein HOW by MAPK/ERK Enhances Its Dimerization and Activity
- A Genome-Wide Scan of Ashkenazi Jewish Crohn's Disease Suggests Novel Susceptibility Loci
- Parkinson's Disease–Associated Kinase PINK1 Regulates Miro Protein Level and Axonal Transport of Mitochondria
- LMW-E/CDK2 Deregulates Acinar Morphogenesis, Induces Tumorigenesis, and Associates with the Activated b-Raf-ERK1/2-mTOR Pathway in Breast Cancer Patients
- Mapping the Hsp90 Genetic Interaction Network in Reveals Environmental Contingency and Rewired Circuitry
- Autoregulation of the Noncoding RNA Gene
- The Human Pancreatic Islet Transcriptome: Expression of Candidate Genes for Type 1 Diabetes and the Impact of Pro-Inflammatory Cytokines
- Spo0A∼P Imposes a Temporal Gate for the Bimodal Expression of Competence in
- Antagonistic Regulation of Apoptosis and Differentiation by the Cut Transcription Factor Represents a Tumor-Suppressing Mechanism in
- A Downstream CpG Island Controls Transcript Initiation and Elongation and the Methylation State of the Imprinted Macro ncRNA Promoter
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- PIF4–Mediated Activation of Expression Integrates Temperature into the Auxin Pathway in Regulating Hypocotyl Growth
- Metabolic Profiling of a Mapping Population Exposes New Insights in the Regulation of Seed Metabolism and Seed, Fruit, and Plant Relations
- A Splice Site Variant in the Bovine Gene Compromises Growth and Regulation of the Inflammatory Response
- Comprehensive Research Synopsis and Systematic Meta-Analyses in Parkinson's Disease Genetics: The PDGene Database
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



