-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaThe Nucleoside Diphosphate Kinase Gene Acts as Quantitative Trait Locus Promoting Non-Mendelian Inheritance
The t-haplotype, a variant form of the t-complex region on mouse chromosome 17, acts as selfish genetic element and is transmitted at high frequencies (>95%) from heterozygous (t/+) males to their offspring. This phenotype is termed transmission ratio distortion (TRD) and is caused by the interaction of the t-complex responder (Tcr) with several quantitative trait loci (QTL), the t-complex distorters (Tcd1 to Tcd4), all located within the t-haplotype region. Current data suggest that the distorters collectively impair motility of all sperm derived from t/+ males; t-sperm is rescued by the responder, whereas +-sperm remains partially dysfunctional. Recently we have identified two distorters as regulators of RHO small G proteins. Here we show that the nucleoside diphosphate kinase gene Nme3 acts as a QTL on TRD. Reduction of the Nme3 dosage by gene targeting of the wild-type allele enhanced the transmission rate of the t-haplotype and phenocopied distorter function. Genetic and biochemical analysis showed that the t-allele of Nme3 harbors a mutation (P89S) that compromises enzymatic activity of the protein and genetically acts as a hypomorph. Transgenic overexpression of the Nme3 t-allele reduced t-haplotype transmission, proving it to be a distorter. We propose that the NME3 protein interacts with RHO signaling cascades to impair sperm motility through hyperactivation of SMOK, the wild-type form of the responder. This deleterious effect of the distorters is counter-balanced by the responder, SMOKTcr, a dominant-negative protein kinase exclusively expressed in t-sperm, thus permitting selfish behaviour and preferential transmission of the t-haplotype. In addition, the previously reported association of NME family members with RHO signaling in somatic cell motility and metastasis, in conjunction with our data involving RHO signaling in sperm motility, suggests a functional conservation between mechanisms for motility control in somatic cells and spermatozoa.
Published in the journal: . PLoS Genet 8(3): e32767. doi:10.1371/journal.pgen.1002567
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002567Summary
The t-haplotype, a variant form of the t-complex region on mouse chromosome 17, acts as selfish genetic element and is transmitted at high frequencies (>95%) from heterozygous (t/+) males to their offspring. This phenotype is termed transmission ratio distortion (TRD) and is caused by the interaction of the t-complex responder (Tcr) with several quantitative trait loci (QTL), the t-complex distorters (Tcd1 to Tcd4), all located within the t-haplotype region. Current data suggest that the distorters collectively impair motility of all sperm derived from t/+ males; t-sperm is rescued by the responder, whereas +-sperm remains partially dysfunctional. Recently we have identified two distorters as regulators of RHO small G proteins. Here we show that the nucleoside diphosphate kinase gene Nme3 acts as a QTL on TRD. Reduction of the Nme3 dosage by gene targeting of the wild-type allele enhanced the transmission rate of the t-haplotype and phenocopied distorter function. Genetic and biochemical analysis showed that the t-allele of Nme3 harbors a mutation (P89S) that compromises enzymatic activity of the protein and genetically acts as a hypomorph. Transgenic overexpression of the Nme3 t-allele reduced t-haplotype transmission, proving it to be a distorter. We propose that the NME3 protein interacts with RHO signaling cascades to impair sperm motility through hyperactivation of SMOK, the wild-type form of the responder. This deleterious effect of the distorters is counter-balanced by the responder, SMOKTcr, a dominant-negative protein kinase exclusively expressed in t-sperm, thus permitting selfish behaviour and preferential transmission of the t-haplotype. In addition, the previously reported association of NME family members with RHO signaling in somatic cell motility and metastasis, in conjunction with our data involving RHO signaling in sperm motility, suggests a functional conservation between mechanisms for motility control in somatic cells and spermatozoa.
Introduction
In general, diploid organisms transmit homologous chromosomes at the Mendelian (equal) ratio to their offspring. However, several types of non-Mendelian inheritance have been described, and in mammals a prominent example is transmission ratio distortion (TRD) in the mouse, which is caused by the t-haplotype. The t-haplotype is a variant form of the t-complex, which maps to the centromere-proximal third of chromosome 17. According to evolutionary studies, this haplotype originated more than one million years ago and, due to four large inversions, has since evolved devoid of meiotic exchange with the wild-type t-complex [1]–[3]. The t-haplotype is transmitted at an abnormally high ratio from heterozygous (t/+) males to their offspring [4]. This selective advantage is due to superior swimming behaviour of t-haplotype sperm as compared to +-sperm derived from the same male [5], [6]. t-sperm does not, however, function superiorly to sperm derived from wild-type (+/+) males [7]. The t-haplotype rather encodes several t-complex-distorters (Tcd), which cumulatively affect sperm motility. This deleterious effect is rescued by the t-complex-responder (Tcr), but exclusively in t-haplotype carrying sperm. Thus, only sperm carrying the wild-type t-complex are affected.
The first evidence for how TRD is caused molecularly was obtained following the isolation of Tcr, which was found to encode a mutant, dominant-negative form of Sperm motility kinase 1 (Smok1), termed Smok1Tcr [8]. Expression of wild-type Smok1 and of Smok1Tcr commences in haploid spermatids and, in contrast to other haploid expressed genes, neither their RNA nor their protein products are shared between haploid sperm cells, which are connected in a syncytium [9]. This exceptional behaviour provided a molecular explanation for the exclusive rescue of t-sperm from the deleterious effect of the distorters.
The molecular nature of SMOKTcr revealed that TRD is caused by alterations in a signaling pathway involved in sperm motility, and led to the identification of Tcd genes, which were postulated to act upstream of SMOK1 in this signaling pathway [8]. The first Tcd isolated was Tcd1a, which was identified as a hypermorph of Tagap1, a GTPase activating protein (GAP) and inhibitor of Rho small G proteins [10]. Tcd2 was later shown to encode a hypermorph of Fgd2 (Faciogenital dysplasia 2), a GDP/GTP exchange factor (GEF) and activator of the Rho protein CDC42 [11], [12]. These data established the involvement of Rho signaling in the control of sperm motility and in TRD. Rho G proteins are molecular switches that cycle between an active, GTP-bound, and an inactive, GDP-bound, state. GAPs enhance the hydrolysis of GTP, driving Rho small G proteins into the inactive state, while GEFs enhance the loading of small G proteins with GTP, thus promoting the active state.
Here we show that the nucleoside diphosphate kinase gene Nme3 (protein expressed in non-metastatic cells 3; MGI acc. number 1930182, Ensembl gene ENSMUSG00000073435) acts as a quantitative trait locus in TRD. Group I nucleoside diphosphate kinases such as NME3 function to phosphorylate GDP to GTP, the activator molecule for small G proteins, providing a link between Nme3 and the previously identified Tcd genes. We show that reduction in the Nme3 gene dosage by gene targeting enhances the transmission rate of the t-haplotype, while transgenic over-expression of the t-allele reduces t-haplotype transmission. Genetic and biochemical data demonstrate that the t-allele of Nme3 is a distorter and acts as hypomorph, in contrast to previously identified distorters. Nme3 complements the family of G protein-related factors acting as QTLs in non-Mendelian inheritance.
Results/Discussion
Nme3 Is Expressed in Testis and Is Altered in the t-Haplotype
The identification of the Rho small G protein regulators Tagap1 and Fgd2 as t-complex-distorters within the t-haplotype suggested that more genes involved in G protein signaling which may have a quantitative effect on t-haplotype transmission might be located within this chromosome segment. Therefore, we initiated a search for genes related to Rho signaling within this region of chromosome 17. We identified the gene Nme3, encoding a member of the nucleoside diphosphate kinase (NDK) family, at position 25 Mb from the centromere (Figure 1A). Nme3 belongs to the group I Nmes (Nme1–4) which are all catalytically active and share significant sequence homology [13], [14].
Fig. 1. Nme3 is a Tcd2 candidate. 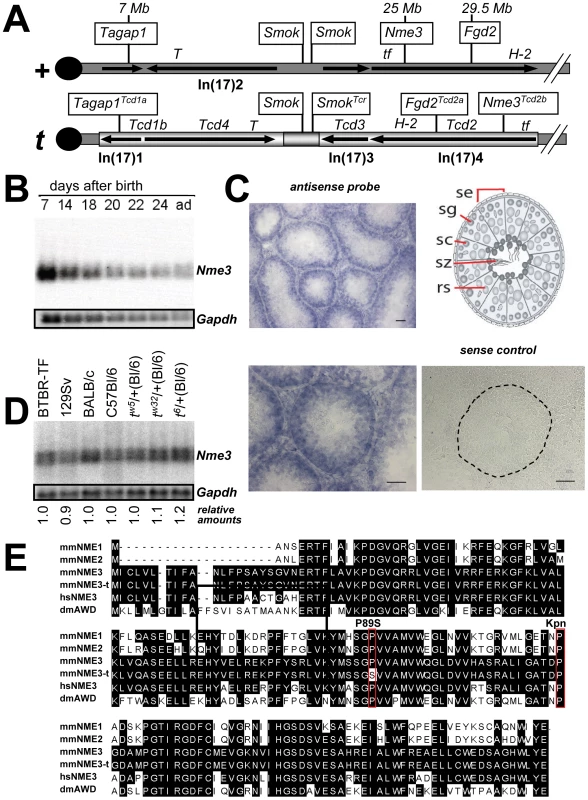
(A) The position of Nme3 in the wild-type t-complex (+) and genetic mapping on tw18 localized it to the Tcd2 region of the t-haplotype (t) (see Figure S1). Symbols of genes verified to be involved in TRD are boxed, and map positions relative to the wild-type chromosome 17 (in Mb) are indicated. Molecularly unknown Tcd loci are also listed along with Chr17 inversions and their relative orientations in both, the wild-type and t-haplotype chromosome, as well as crucial genetic markers (T, tf, H-2). (B) Northern blot analysis of Nme3 transcripts in testes from consecutive post partum stages reflecting the first round of spermatogenesis (P7–P24), and in testes of adult mice (ad). (C) In situ hybridization analysis of Nme3 on testis cryosections from an adult male. Expression is predominant in cells near the basal lamina (dotted line) representing diploid cell types. Schematic view of a seminiferous tubule. (Se) Sertoli cells; (SG) spermatogonia; (SC) spermatocytes; (RS) round spermatids; (SZ) spermatozoa. Scale bar: 50 µm. (D) Northern blot analysis of Nme3 expression in different wild-type strains and t-haplotypes. Quantification of the signals with respect to C57Bl/6 did not reveal significant differences. (E) Amino acid sequence comparison of several NME proteins encoded by Mus musculus (mmNME1 to mmNME3), the t-haplotype (mmNME3-t), Homo sapiens (hsNME3), and Drosophila melanogaster (dmAWD); a conserved proline at position 89 is altered in mmNME3-t (P89S), and a nearby proline residue was described as killer-of-prune mutation (K-pn) in the abnormal-wing-disc (awd) gene of D. melanogaster when mutated to serine (red boxed). In order to qualify as a Tcd candidate, a gene must be expressed in the testis and show variability between t - and wild-type alleles. Northern blot analysis showed expression of Nme3 in testes from the earliest stage after birth tested (7 days) to the adult (Figure 1B). Using in situ hybridization on sections of adult testes, Nme3 transcripts are detectable predominantly in early stages of spermatogenesis, while expression appears to be down-regulated in haploid cells (Figure 1C). However, the expression analysis of round spermatids using microarrays have shown that Nme3 transcripts are also present in spermatids ([15], and EMBL-EBI: Gene Expression Atlas). Thus, Nme3 transcripts apparently persist during spermiogenesis and allow translation of NME3 protein acting later in spermatozoa. In conclusion, Nme3 was found to fulfill the first important criterion for a distorter.
Since the RNA expression level can be a good indicator of a QTL, as shown for the t-alleles of Fgd2 and Tagap1 [10], [11], we analyzed the expression of Nme3 from the t-haplotype allele and compared it to the wild-type alleles from several mouse strains. The expression levels were found to be nearly identical between RNA derived from testes of C57BL/6 or other wild-type strains, and t-haplotype carrying strains (Figure 1D). t6/+ male testes showed marginally higher expression.
Since the analysis of RNA expression level revealed no significant variability between t - and wild-type alleles we examined sequence variation. We isolated cDNA clones by RT-PCR from testicular RNA from several wild-type and t-haplotype carrying strains and from a testis cDNA library prepared from t6/tw5 males. In addition, we analyzed genomic fragments derived from several t-haplotypes. All sequence analyses detected a t-specific C to T transition in the coding sequence of Nme3, a missense mutation resulting in the change of proline to serine at position 89 (P89S; Figure 1E). This mutation was found in all t-haplotypes tested, which carry the t-form of the inversion In(17)4 (tw5, tw32, tw12, t6), but in none of the wild-type strains analyzed (C57BL/6, DBA/2, 129Sv, NMRI) (Figure 1E and data not shown). Therefore, this P89S mutation in Nme3 distinguishes the t-allele from the wild-type allele. All group I NDK enzymes possess almost identical 3-D structures, and the mutation affects a highly conserved amino acid located between alpha-helix α2 and beta-sheet β3 of NME3 [13], [14], which may alter the function of the protein. A similar proline to serine exchange was described in the Killer-of-prune mutation (awdKpn) of the abnormal-wing-disc (awd) gene of D. melanogaster (Figure 1E; [16]). The awdKpn mutation was shown to decrease the nucleoside diphosphate kinase activity substantially with respect to the wild-type awd gene product [17].
The combined data identified the t-allele of Nme3 as a distorter candidate.
According to its position on chromosome 17 it was not clear whether the Nme3 gene is located within the Tcd2 or Tcd3 region. The proximal partial t-haplotype tw18, which extends into inversion In(17)4, carries Tcd3, but not Tcd2, which maps more distally [18], [19]. Therefore, analysis of tw18 allows the assignment of Nme3t to either the Tcd3 or Tcd2 region. Genomic Southern blot analysis complemented by cDNA sequencing demonstrated that tw18 carries the wild-type allele of Nme3 and thus, Nme3t is a Tcd2 candidate (Figure S1 and data not shown).
A Knock-Out Allele of Nme3 Phenocopies a Distorter
Distorter genes act as QTLs in the sense that up - or down-regulation of gene expression and/or activity has a quantitative effect on the phenotype; observed here as TRD. A proven method for testing a possible effect of gene dosage on TRD is to inactivate the wild-type allele by gene targeting, assay the transmission of a t-haplotype from males carrying the knock-out allele on the homologous chromosome, and compare it to control males which carry the wild-type allele. We targeted the Nme3 gene in ES cells by replacing exon 1 and part of exon 2 with a Pgk-Neomycin resistance cassette, generating a null allele (Figure 2A). Successful integration of the targeting construct was verified by Southern blot analysis (Figure 2B). We introduced the targeted allele into the germ line and confirmed by RT-PCR that Nme3 transcripts are lacking in the testes of homozygous-null males (Figure 2C). We then generated males carrying the targeted allele on the wild-type chromosome and the wild-type allele on either of the partial t-haplotypes tw18 or th49. Littermates carrying the wild-type allele on both chromosomes 17, in conjunction with tw18 or th49, served as controls. For each male we determined the number of offspring that inherited the t-haplotype. In both tests the t-haplotype was transmitted at a significantly higher rate (15% and 8% respectively) from males carrying one targeted allele compared to littermates homozygous for the wild-type allele (Table 1). Therefore, a reduction of the gene dosage by half significantly increased the transmission rate of the t-haplotype. These data demonstrate that Nme3 acts as a QTL on t-haplotype inheritance.
Fig. 2. Targeted inactivation of the Nme3 gene. 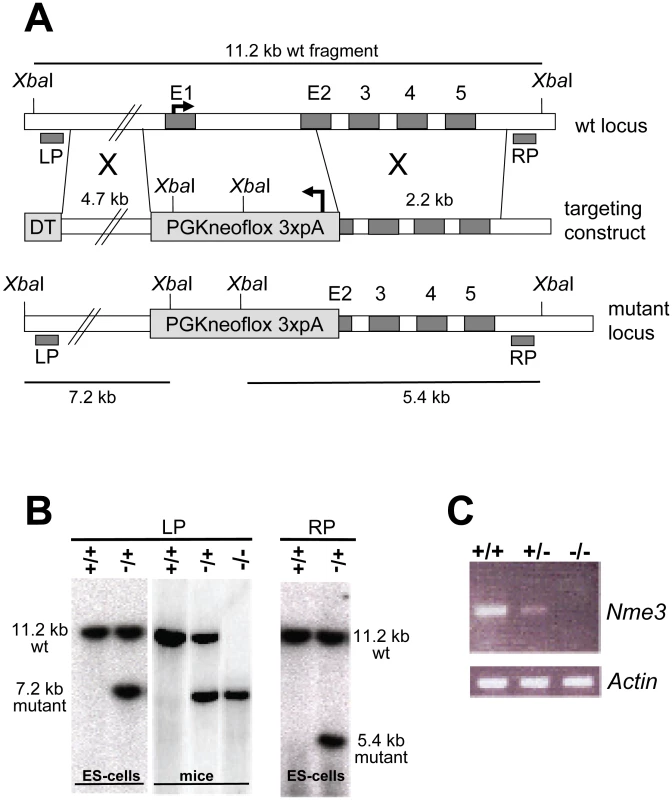
(A) Gene targeting strategy: a neomycin selection cassette was used to replace the entire first and part of the second exon of Nme3. (B) Confirmation of correct homologous recombination by Southern blot analysis of XbaI digested genomic DNA derived from ES-cells or mice. (C) Loss of Nme3 transcripts in homozygous knock-out mice (−/−) as determined by expression analysis using RT-PCR confirms that the targeted allele represents a null mutation. Genomic regions in (A) are not drawn to scale. Abbr.: LP, RP: left or right external probes; DT, diphteria toxin cassette for negative selection; wt, wild-type; +, wild-type allele; −, knock-out allele. Tab. 1. The reduction of the wild-type Nme3 gene dosage and over-expresssion of the Nme3 t-allele have opposite effects on t-haplotype transmission. 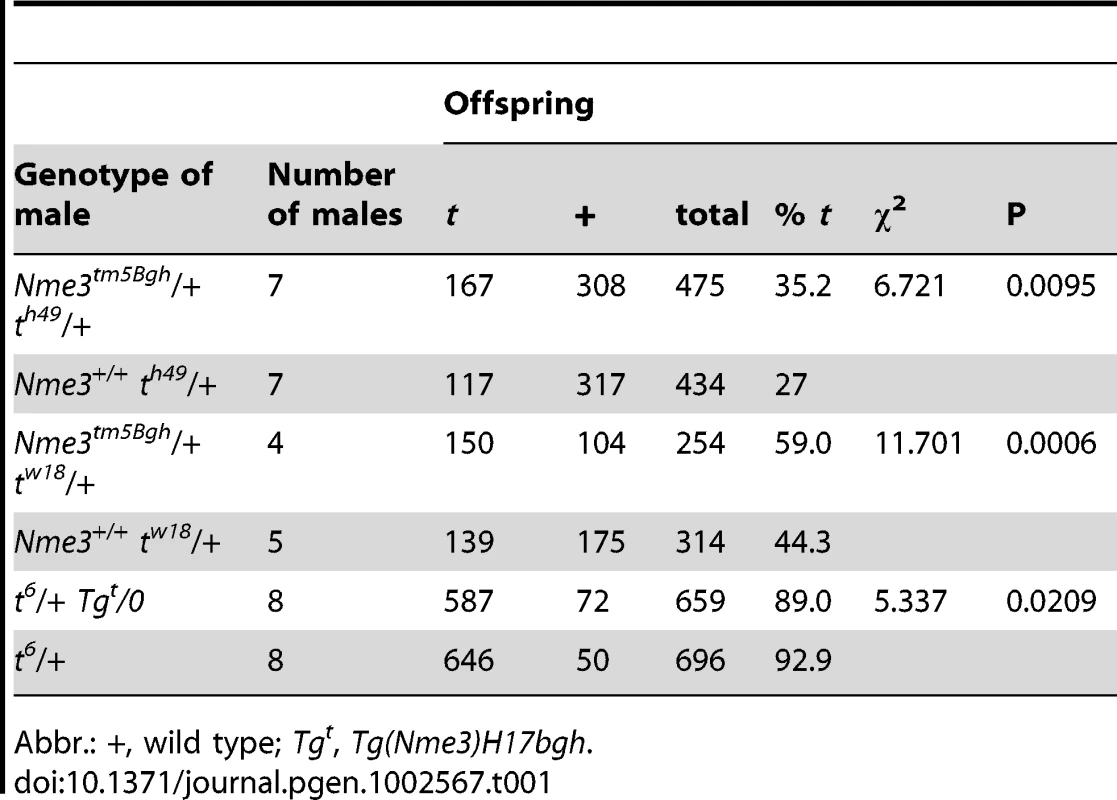
Abbr.: +, wild type; Tgt, Tg(Nme3)H17bgh. The t-Allele of Nme3 Encodes a Hypomorph and Acts as Distorter
Although the genetic inheritance test proved the nature of Nme3 as a QTL, it did not verify that the Nme3 t-allele acts as distorter gene. The latter requires that the t-allele itself alters the overall Nme3 activity in sperm, and causes a statistically significant change in t-haplotype transmission. To determine the mechanisms through which this may happen we first assessed the enzymatic activity of NME3-P89S relative to wild-type NME3.
We produced the enzyme in vitro using a coupled transcription/translation system in rabbit reticulocyte lysate, purified the complexes, and measured the activity in an enzymatic assay. NME3-P89S showed strongly reduced enzymatic activity compared to wild-type (129Sv) NME3 protein (18% of wt activity; Figure 3A).
Fig. 3. The t-allele of Nme3 encodes a hypomorph. 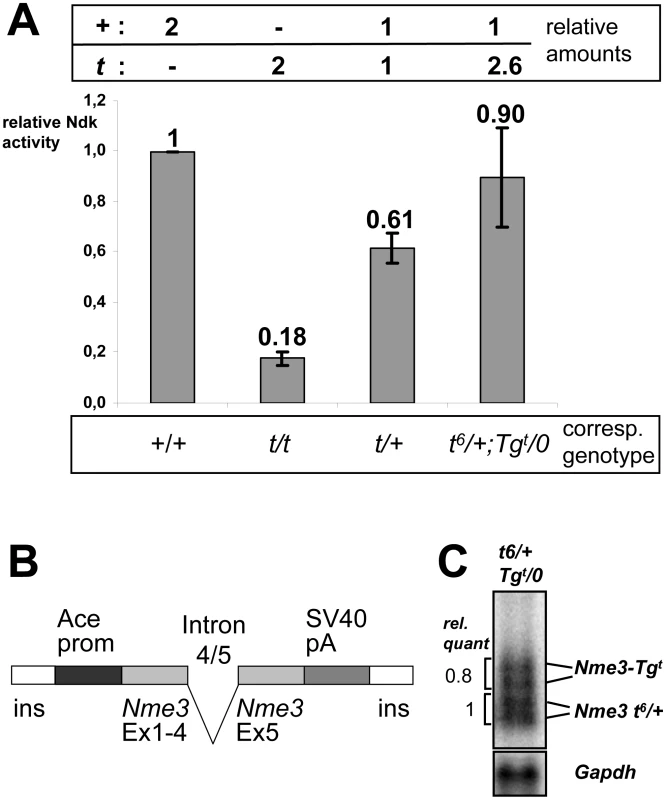
(A) Nucleoside diphosphate kinase activities of wild-type (+) and t-allele (t)-derived proteins or mixtures of both expressed in vitro. The 1∶1 mixture reflects the relative contribution of both alleles to NME3 activity in t/+ heterozygotes, the 1∶2.6 ratio of + and t alleles corresponds to the expression of + and t alleles measured in testes from t6/+;Tgt/0 males (B, C). The columns show the mean of three experiments with the standard deviation. (B) Schematic representation of the transgene construct Tg(Nme3)H17bgh (Tgt) generated for expression of the Nme3 t-allele during spermatogenesis. (C) Expression of the Nme3 t-allele from the transgene construct determined by Northern blot analysis of testis RNA derived from a t6/+;Tgt/0 male. Quantification of endogenous (t6, +) and transgene (Tgt)-derived transcripts revealed a ratio of 1∶0.8. Assuming that the t6 allele contributes 50% of the endogenous Nme3 transcripts, the overall ratio of + to t derived Nme3 transcripts expressed in a t6/+;Tgt/0 male is therefore 1∶2.6. Abbr.: Ace-prom, angiotensin converting enzyme promoter; SV40pA, Simian virus 40 polyadenylation signal fragment; ins, chicken beta globin insulator [33]; Ex, exon; Ndk, nucleoside diphosphate kinase. It is important to note that nucleoside diphosphate kinases function as hexamers. Therefore, in vivo the t-encoded NME3 monomers may form mixed hexamers with the wild-type protein, and in mixed complexes NME3-P89S might function as dominant-negative protein interfering with the function of the wild-type protein. Alternatively, it might form semi-functional complexes with wild-type monomers or have no effect. In order to discriminate between these possible effects, we assayed the enzymatic activity of a 1∶1 mixture of NME3-P89S with wild-type protein, which would reflect the situation in a t/+ male. We combined plasmids encoding the wild-type and the t-allele at an equal ratio prior to in vitro transcription and translation, purified the complexes, and measured their enzymatic activity. The activity dropped to 58% of that of the wild-type protein, suggesting that both the wild-type and the mutant protein contribute to the total enzyme activity (Figure 3A).
In order to assess whether the Nme3 t-allele acts as an antimorph (dominant-negative) or hypomorph (semi-functional) in vivo we took a transgenic approach. If the former were the case, a transgenic construct expressing the t-allele should enhance the transmission rate of a t-haplotype from a t/+ male, since a dosage increase of the t-allele should further reduce endogenous NME3 activity. In contrast, if the t-allele acts as hypomorph the transgene would provide extra NME3 activity to the endogenous gene products and thus the t-haplotype transmission should drop.
We created a transgene construct (Tg(Nme3)H17bgh, abbreviated Tgt) expressing the Nme3t allele in haploid sperm cells using the testis–specific Ace promoter (Figure 3B) [20]. Northern blot analysis and quantification showed that the transgene construct was expressed at approximately 80% of the level of the two endogenous alleles from a t6/+ male (Figure 3C). We generated t6/+ mice carrying one wild-type and one t-allele, along with t6/+ males additionally expressing the transgene. The former should maintain a 1∶1 ratio of NME3 to NME3-P89S, while the latter should produce around 2.6-fold more NME3-P89S than NME3 (one wild-type allele and one t-allele plus approximately 1.6-fold over-expression of the t-allele from the Tgt construct). We determined the transmission rate of t6 from t6/+;Tgt/0 and compared it to t6 transmission from control littermates of the genotype t6/+.
Males (t6/+) expressing one wild-type and one t-allele transmitted the t6-haplotype to 92.8% of their offspring. An increase of the t-allele dosage in hemizygous transgenic males (t6/+;Tgt/0) reduced the t6-transmission to 89% of the offspring (p = 0.02; Table 1), indicating that the t-allele does not act as an antimorph, but as a hypomorph.
Biochemical testing confirmed this conclusion. Increasing the amount of NME3-P89S to 2.6-fold of the wild-type protein (1 part NME3 : 2.6 parts NME3-P89S), which reflects the relative expression of wild-type and t-allele-derived Nme3 RNA in t6/+;Tgt/0 males, significantly increased the enzymatic activity in comparison to the 1∶1 mixture (Figure 3A). Thus, the NME3-P89S protein contributes to the overall enzymatic activity, rather than interfering with activity of the wild-type protein. These biochemical data are consistent with and support the genetic data.
In summary, both data sets identify the t-allele of Nme3 as a hypomorphic allele acting as distorter of t-haplotype transmission.
The ability of the mouse t-haplotype to promote its transmission from t/+ males to a high proportion of their offspring is due to the unusual properties of the responder, SmokTcr, a dominant negative protein kinase which is retained in the haploid sperm cells expressing the gene and able to rescue the impairment of sperm motility caused by the distorters [9]. These latter act as QTLs, which additively contribute to the high transmission rate of the responder. We have previously identified two distorters, which act as hypermorphs, the Rho-GAP Tagap1 and the Rho-GEF Fgd2 [10], [11]. Although the two proteins have antagonistic effects on Rho activity, excess activity of either gene enhances the transmission rate of the t-haplotype from t/+ males. Thus, we proposed that Tagap1 controls a negative regulator and Fgd2 an activator of SMOK, the wild-type form of the responder SMOKTcr [11]. Increased down-regulation of the negative regulator by Tagap1 and increased up-regulation of the activator by Fgd2 both contribute to hyperactivation of SMOK, leading to impairment of sperm motility. This deleterious effect of the distorters is counterbalanced by SMOKTcr, which exclusively rescues t-sperm resulting in TRD (Figure 4).
Fig. 4. Model of the role of NME3 in t-haplotype transmission ratio distortion. 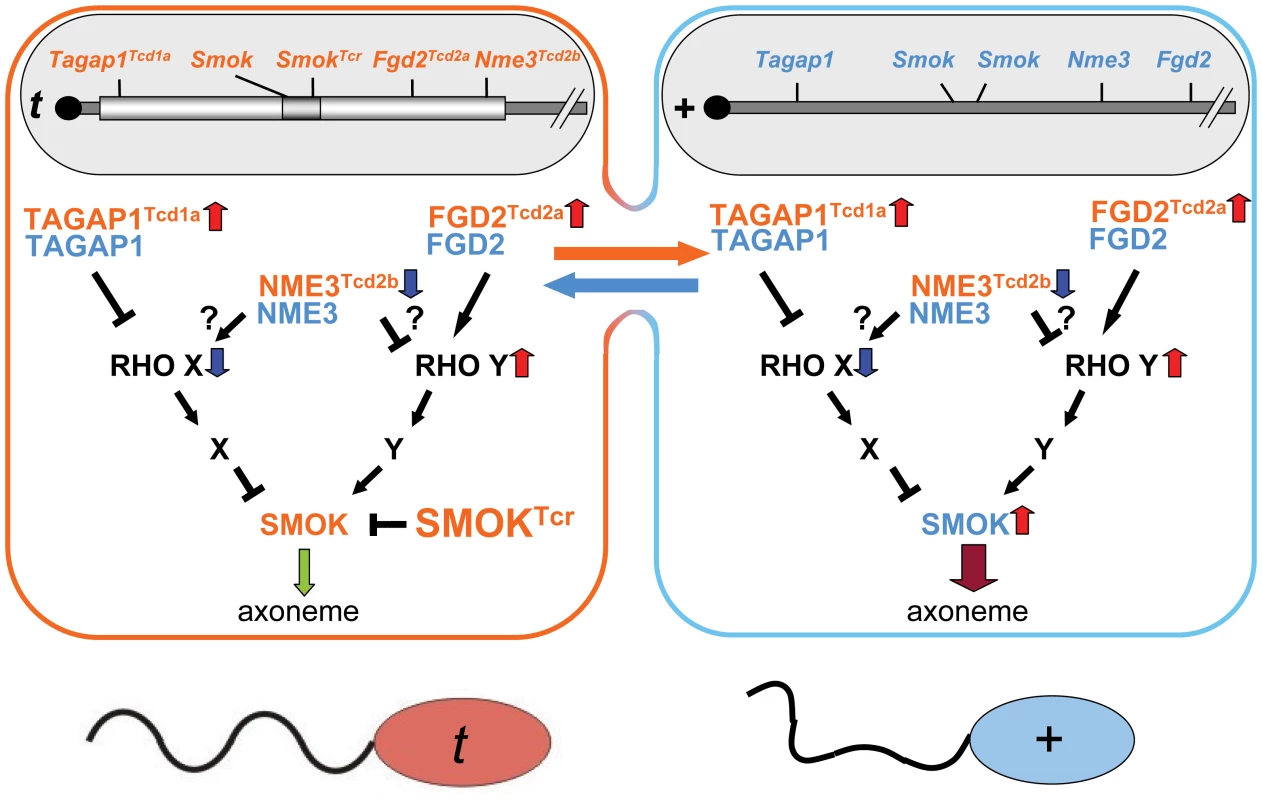
Nme3Tcd2b encodes a hypomorphic allele of Nme3 leading to reduction of NME3 activity in sperm derived from a t/+ male. NME3 may be an activator of the inhibitory Rho signaling pathway or an inhibitor of the activating pathway controlling SMOK activity, or both. NME3Tcd2b thus might synergize with TAGAP1Tcd1a to reduce inhibition and/or with FGD2Tcd2a to enhance activation of SMOK in all sperm. The combined activity of all distorters leads to impairment of sperm motility, which is rescued exclusively in t-sperm by the responder encoding the dominant-negative variant SMOKTCR, resulting in transmission ratio distortion in favor of t-sperm. Red upward pointing arrows indicate up-regulation, blue down-pointing arrows down-regulation; the green down-pointing arrow symbolizes rescued, the dark-red down-pointing arrow impaired flagellar motility. It is not yet clear how Nme3 interacts with the Rho signaling cascades involved in TRD. Recent reports have revealed negative interactions between NMEs and Rho small G protein signaling [21]–[23]. Nme1 can act as a negative regulator of CDC42 by binding to the PH domain of the CDC42-GEF DBL. It can also inhibit CDC42 by direct interaction [21], [22]. Similarly, NME1 has been demonstrated to inhibit RAC1 activity by interacting with the RAC1 activator TIAM1, an effect which is independent of its nucleoside disphosphate kinase activity [23]. It is unknown whether NME3 may function similarly. However, since genetic reduction of Nme3 promotes TRD, it is likely that the NME3 protein is able to activate the inhibitory pathways controlling SMOK activity. Alternatively, it cannot be excluded that NME3 may inhibit the activating pathway or exert both, activating and inhibiting functions. The former effect might be caused by local increase of the GTP concentration promoting activation of Rho, the latter in a manner similar to that observed for NME1 action on CDC42. In any case, the NME-P89S protein expressed by the t-haplotype leads to reduction of total NME3 activity and thus to down-regulation of the inhibitory pathway and/or up-regulation of the activating pathway (Figure 4). The possibility of some other link between NME3 and SMOK, though less likely, cannot be excluded.
Positional mapping of Nme3 within the t-allele places it in the Tcd2 region, demonstrating for the first time a distorter region that conclusively contains several distorter loci (Fgd2 and Nme3). Accordingly, the two distorters in the Tcd2 region are termed Fgd2Tcd2a and Nme3Tcd2b. A previous report had suggested the presence of two distorters in the Tcd1 region, which was confirmed by our data, but isolation of the second distorter in the Tcd1 region has not yet been reported [10], [24], [25].
Various Nme genes are expressed in male germ cells, several of them more specifically and prominently than Nme3 [14], [26]. However, to our knowledge this study is the first providing strong evidence for a role of a group I Nme gene in sperm function. It is conceivable that other Nme genes as well are involved in the control of sperm motility and affect non-Mendelian inheritance, either as enhancers or suppressors of t-haplotype TRD. In this way they may contribute to the high variability of t-haplotype transmission observed in different genetic backgrounds [27]. Also, partial redundancy among Nme genes may complement the loss of Nme3 function, since Nme3−/− males show no gross defects in viability and fertility (this report, data not shown).
The human group I gene NME1 (Nm23-H1) was the first metastasis suppressor gene discovered and has been shown to suppress tumor cell motility in a variety of cancer models [28]. A similar function as an inhibitor of cell motility in a breast cancer cell line has been shown for human NME3 (NM23-H3) [29].
Together with our previous findings implicating Rho signaling in the control of sperm motility, this report provides further evidence for a functional conservation of the signaling networks controlling cell motility in somatic cells and in spermatozoa.
Materials and Methods
Ethics Statement
Animal experiments were approved by the ethics committee of the Regierungspräsidium Freiburg (registration number T-00/28) and the LAGeSo Berlin (registration numbers ZH120 and Reg 0248/03).
Primer oligonucleotides are listed in Table S1.
Transcript Analysis
We amplified Nme3 transcripts by RT-PCR of the Nme3 coding region from mouse testis RNA isolated from different wild-type strains and t-haplotypes (primers Nme3-s and Nme3-as). We isolated the amplicons (622 bp), cloned them into pBS-SK (Stratagene), and sequenced several independent clones for each genotype. We isolated Nme3 clones from a t6/tw5 testes cDNA library by PCR-screening of subpools and colony hybridization [11] using the Nme3 cDNA as a probe and sequenced the library clones as above. We performed Northern blot analysis using the NorthernMax-Gly Kit (Ambion) according to the manufacturer's instructions. For in situ hybridization analysis, DIG-labelled in vitro transcribed antisense RNA corresponding to the coding region of Nme3 (primers Nme3-s and Nme3-as) was hybridized to 10 µm frozen sections as described [30].
Gene Targeting and Transgene Constructs
We targeted the Nme3 locus in CJ7 ES-cells [31] by replacing exon 1 and part of exon 2 (bp 33550 to 33847 in BAC 126c8, accession number AF220294.1) with a Pgk1-neo-polyA (Pgk1-neoflox3xpA) - cassette [11] as depicted in Figure 2A. We included a diphtheria toxin cassette for negative selection. To construct the targeting vector, we isolated the left - and right homology arms (4695 bp and 2180 bp, respectively) of the targeting construct by PCR and cloned both arms on either side of the selection cassette, introducing a SalI site for linearization at the end of the left arm. We electroporated CJ7 ES-cells with the linearized targeting construct and selected, isolated, and analyzed clones according to standard procedures [32]. Correctly targeted ES-clones were identified by Southern hybridization of XbaI digested genomic DNA with the left (5′) probe (LP) and right (3′) probe (RP) (Primers LP-s and LP-as, 1052 bp probe fragment; RP-s and RP-as, 814 bp probe fragment). Both probes detect a 11.206 kb XbaI fragment in wild-type. Upon successful targeting, the LP detects a 7.228 kb fragment and the RP a 5.354 kb fragment. The Nme3t transgenic construct consists of the angiotensin converting enzyme (Ace) spermiogenesis-specific promoter including the transcriptional start site (position −91 to +17) [20] followed by the cDNA of Nme3 exon 1 to 4, and genomic sequence comprising intron 4/5, exon 5 and 141 bp from the 3′ - untranslated region, ending 12 bp upstream of the endogenous polyadenylation (pA) signal, which we replaced by the SV40 pA sequence from pCS2+. We flanked this expression cassette with 2 copies of the chicken beta-globin insulator on both sides [33] (Figure 3B).
Biochemical Assays
We cloned the Nme3 alleles into the pET30c vector (Novagen) in-frame with a 6xHis and S-tag. Since expression of full-length recombinant proteins in E. coli was unsuccessful, we used the constructs for in vitro transcription/translation reactions (IVT) with the TNT T7 Quick Coupled Transcription/Translation System (Promega) according to the manufacturer's instructions. Plasmid concentrations were kept constant relative to the reaction volumes. We purified recombinant NME3 using Ni-NTA agarose (Qiagen) (12.5 µl bed volume/50 µl IVT reaction). We added 0.6 ml Ni-NTA buffer for binding (50 mM NaH2PO4, 30 mM NaCl, 20 mM Imidazole, 1× complete protease inhibitors EDTA-free (Roche), pH 8) and incubated at 4°C for 1.5 h with agitation. Samples were then washed 3× in Ni-NTA-buffer (0.8 ml) and NME3-protein was eluted with 45 µl elution buffer (Ni-NTA buffer containing 250 mM imidazole). For quantification of protein, we loaded 20 µl of eluted protein on a NuPAGE 4–12% Bis-Tris Gel (Invitrogen) and blotted with the I-blot system (Invitrogen) on a PVDF membrane (Millipore). After blocking 1–2 h at RT using 5% skim milk powder in TBS-T/0.2% Tween, we probed the blot with an anti-S-tag antibody (1∶500 dilution, Delta Biolabs) overnight at 4°C, followed by incubation with donkey anti-rabbit HRP-coupled secondary antibody (1∶10000 diluted, Jackson Immunoresearch), and detected the signal with the ECL Advance Western Blotting Detection Kit from GE Healthcare. Densitometry quantification of Western blots was performed using ImageJ software.
We analyzed NME3 protein preparations for nucleoside diphosphate kinase activity using a transphosphorylation assay followed by thin layer chromatography (TLC) essentially as described [34]. We used 20 µl NME3 protein eluate in a reaction mixture containing 10 mM HEPES, 20 mM NaCl and 2 mM MgCl2, 2 mM ATP, 1 mM TDP and 2 mM [γ-32P] ATP. We took 6 µl samples after each 15, 30, and 45 minutes incubation at room temperature and stopped the reaction with 1 µl of 50 mM EDTA (pH 8.0). As a positive control we used 0.1 U of nucleoside 5′-diphosphate kinase from bakers yeast (Sigma Aldrich N0379). Reactions were analyzed by TLC on PEI-cellulose plates (Macherey-Nagel) using 0.75 M KH2PO4 (pH 3.65) as running buffer. We exposed dried TLC plates to phosphorimager screens and quantified with ImageJ. Wild-type NME3 and NME3-P89S protein activities were normalized to total protein levels.
Supporting Information
Zdroje
1. SilverLM 1993 The peculiar journey of a selfish chromosome: mouse t haplotypes and meiotic drive. Trends Genet 9 250 254
2. SchimentiJ 2000 Segregation distortion of mouse t haplotypes the molecular basis emerges. Trends Genet 16 240 243
3. LyonMF 2003 Transmission ratio distortion in mice. Annu Rev Genet 37 393 408
4. ChesleyPDunnLC 1936 The Inheritance of Taillessness (Anury) in the House Mouse. Genetics 21 525 536
5. KatzDFEricksonRPNathansonM 1979 Beat frequency is bimodally distributed in spermatozoa from T/t12 mice. J Exp Zool 210 529 535
6. Olds-ClarkePJohnsonLR 1993 t haplotypes in the mouse compromise sperm flagellar function. Dev Biol 155 14 25
7. Olds-ClarkePPeitzB 1985 Fertility of sperm from t/+ mice: evidence that +-bearing sperm are dysfunctional. Genet Res 47 49 52
8. HerrmannBGKoschorzBWertzKMcLaughlinKJKispertA 1999 A protein kinase encoded by the t complex responder gene causes non-mendelian inheritance. Nature 402 141 146
9. VeronNBauerHWeisseAYLuderGWerberM 2009 Retention of gene products in syncytial spermatids promotes non-Mendelian inheritance as revealed by the t complex responder. Genes Dev 23 2705 2710
10. BauerHWillertJKoschorzBHerrmannBG 2005 The t complex-encoded GTPase-activating protein Tagap1 acts as a transmission ratio distorter in mice. Nat Genet 37 969 973
11. BauerHVeronNWillertJHerrmannBG 2007 The t-complex-encoded guanine nucleotide exchange factor Fgd2 reveals that two opposing signaling pathways promote transmission ratio distortion in the mouse. Genes Dev 21 143 147
12. HuberCMartenssonABokochGMNemazeeDGavinAL 2008 FGD2, a CDC42-specific exchange factor expressed by antigen-presenting cells, localizes to early endosomes and active membrane ruffles. J Biol Chem 283 34002 34012
13. ErentMGoninPCherfilsJTissierPRaschellaG 2001 Structural and catalytic properties and homology modelling of the human nucleoside diphosphate kinase C, product of the DRnm23 gene. Eur J Biochem 268 1972 1981
14. BoissanMDabernatSPeuchantESchlattnerULascuI 2009 The mammalian Nm23/NDPK family: from metastasis control to cilia movement. Mol Cell Biochem
15. ChalmelFRollandADNiederhauser-WiederkehrCChungSSDemouginP 2007 The conserved transcriptome in human and rodent male gametogenesis. Proc Natl Acad Sci U S A 104 8346 8351
16. LascuIChaffotteALimbourg-BouchonBVeronM 1992 A Pro/Ser substitution in nucleoside diphosphate kinase of Drosophila melanogaster (mutation killer of prune) affects stability but not catalytic efficiency of the enzyme. J Biol Chem 267 12775 12781
17. TimmonsLXuJHerspergerGDengXFShearnA 1995 Point mutations in awdKpn which revert the prune/Killer of prune lethal interaction affect conserved residues that are involved in nucleoside diphosphate kinase substrate binding and catalysis. J Biol Chem 270 23021 23030
18. LyonMF 1984 Transmission ratio distortion in mouse t-haplotypes is due to multiple distorter genes acting on a responder locus. Cell 37 621 628
19. BucanMHerrmannBGFrischaufAMBautchVLBodeV 1987 Deletion and duplication of DNA sequences is associated with the embryonic lethal phenotype of the t9 complementation group of the mouse t complex. Genes Dev 1 376 385
20. HowardTBaloghROverbeekPBernsteinKE 1993 Sperm-specific expression of angiotensin-converting enzyme (ACE) is mediated by a 91-base-pair promoter containing a CRE-like element. Mol Cell Biol 13 18 27
21. MurakamiMMenesesPIKnightJSLanKKaulR 2008 Nm23-H1 modulates the activity of the guanine exchange factor Dbl-1. Int J Cancer 123 500 510
22. MurakamiMMenesesPILanKRobertsonES 2008 The suppressor of metastasis Nm23-H1 interacts with the Cdc42 Rho family member and the pleckstrin homology domain of oncoprotein Dbl-1 to suppress cell migration. Cancer Biol Ther 7 677 688
23. OtsukiYTanakaMYoshiiSKawazoeNNakayaK 2001 Tumor metastasis suppressor nm23H1 regulates Rac1 GTPase by interaction with Tiam1. Proc Natl Acad Sci U S A 98 4385 4390
24. LyonMF 1992 Deletion of mouse t-complex distorter-1 produces an effect like that of the t-form of the distorter. Genet Res 59 27 33
25. LyonMF 2005 Elucidating mouse transmission ratio distortion. Nat Genet 37 924 925
26. MunierASerresCKannMLBoissanMLesaffreC 2003 Nm23/NDP kinases in human male germ cells: role in spermiogenesis and sperm motility? Exp Cell Res 289 295 306
27. GummereGRMcCormickPJBennettD 1986 The influence of genetic background and the homologous chromosome 17 on t-haplotype transmission ratio distortion in mice. Genetics 114 235 245
28. McDermottWGBoissanMLacombeMLSteegPSHorakCE 2008 Nm23-H1 homologs suppress tumor cell motility and anchorage independent growth. Clin Exp Metastasis 25 131 138
29. CarinciFArcelliDLo MuzioLFranciosoFValentiniD 2007 Molecular classification of nodal metastasis in primary larynx squamous cell carcinoma. Transl Res 150 233 245
30. BrentAESchweitzerRTabinCJ 2003 A somitic compartment of tendon progenitors. Cell 113 235 248
31. SwiatekPJGridleyT 1993 Perinatal lethality and defects in hindbrain development in mice homozygous for a targeted mutation of the zinc finger gene Krox20. Genes Dev 7 2071 2084
32. Ramirez-SolisRDavisACBradleyA 1993 Gene targeting in embryonic stem cells. Methods Enzymol 225 855 878
33. ChungJHWhiteleyMFelsenfeldG 1993 A 5′ element of the chicken beta-globin domain serves as an insulator in human erythroid cells and protects against position effect in Drosophila. Cell 74 505 514
34. YanoAUmedaMUchimiyaH 1995 Expression of functional proteins of cDNA encoding rice nucleoside diphosphate kinase (NDK) in Escherichia coli and organ-related alteration of NDK activities during rice seed germination (Oryza sativa L.). Plant Mol Biol 27 1053 1058
Štítky
Genetika Reprodukční medicína
Článek Physiological Notch Signaling Maintains Bone Homeostasis via RBPjk and Hey Upstream of NFATc1Článek Intronic -Regulatory Modules Mediate Tissue-Specific and Microbial Control of / TranscriptionČlánek Probing the Informational and Regulatory Plasticity of a Transcription Factor DNA–Binding DomainČlánek Repression of Germline RNAi Pathways in Somatic Cells by Retinoblastoma Pathway Chromatin ComplexesČlánek An Alu Element–Associated Hypermethylation Variant of the Gene Is Associated with Childhood ObesityČlánek Three Essential Ribonucleases—RNase Y, J1, and III—Control the Abundance of a Majority of mRNAsČlánek Genomic Tools for Evolution and Conservation in the Chimpanzee: Is a Genetically Distinct Population
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 3
-
Všechny články tohoto čísla
- Comprehensive Research Synopsis and Systematic Meta-Analyses in Parkinson's Disease Genetics: The PDGene Database
- Genomic Analysis of the Hydrocarbon-Producing, Cellulolytic, Endophytic Fungus
- Networks of Neuronal Genes Affected by Common and Rare Variants in Autism Spectrum Disorders
- Akirin Links Twist-Regulated Transcription with the Brahma Chromatin Remodeling Complex during Embryogenesis
- Too Much Cleavage of Cyclin E Promotes Breast Tumorigenesis
- Imprinted Genes … and the Number Is?
- Genetic Architecture of Highly Complex Chemical Resistance Traits across Four Yeast Strains
- Exploring the Complexity of the HIV-1 Fitness Landscape
- MNS1 Is Essential for Spermiogenesis and Motile Ciliary Functions in Mice
- A Fundamental Regulatory Mechanism Operating through OmpR and DNA Topology Controls Expression of Pathogenicity Islands SPI-1 and SPI-2
- Evidence for Positive Selection on a Number of MicroRNA Regulatory Interactions during Recent Human Evolution
- Variation in Modifies Risk of Neonatal Intestinal Obstruction in Cystic Fibrosis
- PIF4–Mediated Activation of Expression Integrates Temperature into the Auxin Pathway in Regulating Hypocotyl Growth
- Critical Evaluation of Imprinted Gene Expression by RNA–Seq: A New Perspective
- A Meta-Analysis and Genome-Wide Association Study of Platelet Count and Mean Platelet Volume in African Americans
- Mouse Genetics Suggests Cell-Context Dependency for Myc-Regulated Metabolic Enzymes during Tumorigenesis
- Transcriptional Control in Cardiac Progenitors: Tbx1 Interacts with the BAF Chromatin Remodeling Complex and Regulates
- Synthetic Lethality of Cohesins with PARPs and Replication Fork Mediators
- APOBEC3G-Induced Hypermutation of Human Immunodeficiency Virus Type-1 Is Typically a Discrete “All or Nothing” Phenomenon
- Interpreting Meta-Analyses of Genome-Wide Association Studies
- Error-Prone ZW Pairing and No Evidence for Meiotic Sex Chromosome Inactivation in the Chicken Germ Line
- -Dependent Chemosensory Functions Contribute to Courtship Behavior in
- Diverse Forms of Splicing Are Part of an Evolving Autoregulatory Circuit
- Phenotypic Plasticity of the Drosophila Transcriptome
- Physiological Notch Signaling Maintains Bone Homeostasis via RBPjk and Hey Upstream of NFATc1
- Precocious Metamorphosis in the Juvenile Hormone–Deficient Mutant of the Silkworm,
- Igf1r Signaling Is Indispensable for Preimplantation Development and Is Activated via a Novel Function of E-cadherin
- Accurate Prediction of Inducible Transcription Factor Binding Intensities In Vivo
- Mitochondrial Oxidative Stress Alters a Pathway in Strongly Resembling That of Bile Acid Biosynthesis and Secretion in Vertebrates
- Mammalian Neurogenesis Requires Treacle-Plk1 for Precise Control of Spindle Orientation, Mitotic Progression, and Maintenance of Neural Progenitor Cells
- Tcf7 Is an Important Regulator of the Switch of Self-Renewal and Differentiation in a Multipotential Hematopoietic Cell Line
- REST–Mediated Recruitment of Polycomb Repressor Complexes in Mammalian Cells
- Intronic -Regulatory Modules Mediate Tissue-Specific and Microbial Control of / Transcription
- Age-Dependent Brain Gene Expression and Copy Number Anomalies in Autism Suggest Distinct Pathological Processes at Young Versus Mature Ages
- A Genome-Wide Association Study Identifies Variants Underlying the Shade Avoidance Response
- -by- Regulatory Divergence Causes the Asymmetric Lethal Effects of an Ancestral Hybrid Incompatibility Gene
- Genome-Wide Association and Functional Follow-Up Reveals New Loci for Kidney Function
- A Natural System of Chromosome Transfer in
- Cell Size and the Initiation of DNA Replication in Bacteria
- Probing the Informational and Regulatory Plasticity of a Transcription Factor DNA–Binding Domain
- Repression of Germline RNAi Pathways in Somatic Cells by Retinoblastoma Pathway Chromatin Complexes
- Temporal Transcriptional Profiling of Somatic and Germ Cells Reveals Biased Lineage Priming of Sexual Fate in the Fetal Mouse Gonad
- Rapid Analysis of Genome Rearrangements by Multiplex Ligation–Dependent Probe Amplification
- Metabolic Profiling of a Mapping Population Exposes New Insights in the Regulation of Seed Metabolism and Seed, Fruit, and Plant Relations
- The Atypical Calpains: Evolutionary Analyses and Roles in Cellular Degeneration
- The Silkworm Coming of Age—Early
- Development of a Panel of Genome-Wide Ancestry Informative Markers to Study Admixture Throughout the Americas
- Balanced Codon Usage Optimizes Eukaryotic Translational Efficiency
- The Min System and Nucleoid Occlusion Are Not Required for Identifying the Division Site in but Ensure Its Efficient Utilization
- Neurobeachin, a Regulator of Synaptic Protein Targeting, Is Associated with Body Fat Mass and Feeding Behavior in Mice and Body-Mass Index in Humans
- Statistical Analysis of Readthrough Levels for Nonsense Mutations in Mammalian Cells Reveals a Major Determinant of Response to Gentamicin
- Gene Reactivation by 5-Aza-2′-Deoxycytidine–Induced Demethylation Requires SRCAP–Mediated H2A.Z Insertion to Establish Nucleosome Depleted Regions
- The miR-35-41 Family of MicroRNAs Regulates RNAi Sensitivity in
- Genetic Basis of Hidden Phenotypic Variation Revealed by Increased Translational Readthrough in Yeast
- An Alu Element–Associated Hypermethylation Variant of the Gene Is Associated with Childhood Obesity
- Modelling Human Regulatory Variation in Mouse: Finding the Function in Genome-Wide Association Studies and Whole-Genome Sequencing
- Novel Loci for Adiponectin Levels and Their Influence on Type 2 Diabetes and Metabolic Traits: A Multi-Ethnic Meta-Analysis of 45,891 Individuals
- Polycomb-Like 3 Promotes Polycomb Repressive Complex 2 Binding to CpG Islands and Embryonic Stem Cell Self-Renewal
- Insulin/IGF-1 and Hypoxia Signaling Act in Concert to Regulate Iron Homeostasis in
- EMF1 and PRC2 Cooperate to Repress Key Regulators of Arabidopsis Development
- Three Essential Ribonucleases—RNase Y, J1, and III—Control the Abundance of a Majority of mRNAs
- Contrasted Patterns of Molecular Evolution in Dominant and Recessive Self-Incompatibility Haplotypes in
- A Machine Learning Approach for Identifying Novel Cell Type–Specific Transcriptional Regulators of Myogenesis
- Genomic Tools for Evolution and Conservation in the Chimpanzee: Is a Genetically Distinct Population
- Nos2 Inactivation Promotes the Development of Medulloblastoma in Mice by Deregulation of Gap43–Dependent Granule Cell Precursor Migration
- Intracranial Aneurysm Risk Locus 5q23.2 Is Associated with Elevated Systolic Blood Pressure
- Heritability and Genetic Correlations Explained by Common SNPs for Metabolic Syndrome Traits
- A Genome-Wide Association Study of Nephrolithiasis in the Japanese Population Identifies Novel Susceptible Loci at 5q35.3, 7p14.3, and 13q14.1
- DNA Damage in Nijmegen Breakage Syndrome Cells Leads to PARP Hyperactivation and Increased Oxidative Stress
- DNA Resection at Chromosome Breaks Promotes Genome Stability by Constraining Non-Allelic Homologous Recombination
- Genetic Analysis of Floral Symmetry in Van Gogh's Sunflowers Reveals Independent Recruitment of Genes in the Asteraceae
- A Splice Site Variant in the Bovine Gene Compromises Growth and Regulation of the Inflammatory Response
- Promoter Nucleosome Organization Shapes the Evolution of Gene Expression
- The Nucleoside Diphosphate Kinase Gene Acts as Quantitative Trait Locus Promoting Non-Mendelian Inheritance
- The Ciliogenic Transcription Factor RFX3 Regulates Early Midline Distribution of Guidepost Neurons Required for Corpus Callosum Development
- Phosphorylation of the RNA–Binding Protein HOW by MAPK/ERK Enhances Its Dimerization and Activity
- A Genome-Wide Scan of Ashkenazi Jewish Crohn's Disease Suggests Novel Susceptibility Loci
- Parkinson's Disease–Associated Kinase PINK1 Regulates Miro Protein Level and Axonal Transport of Mitochondria
- LMW-E/CDK2 Deregulates Acinar Morphogenesis, Induces Tumorigenesis, and Associates with the Activated b-Raf-ERK1/2-mTOR Pathway in Breast Cancer Patients
- Mapping the Hsp90 Genetic Interaction Network in Reveals Environmental Contingency and Rewired Circuitry
- Autoregulation of the Noncoding RNA Gene
- The Human Pancreatic Islet Transcriptome: Expression of Candidate Genes for Type 1 Diabetes and the Impact of Pro-Inflammatory Cytokines
- Spo0A∼P Imposes a Temporal Gate for the Bimodal Expression of Competence in
- Antagonistic Regulation of Apoptosis and Differentiation by the Cut Transcription Factor Represents a Tumor-Suppressing Mechanism in
- A Downstream CpG Island Controls Transcript Initiation and Elongation and the Methylation State of the Imprinted Macro ncRNA Promoter
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- PIF4–Mediated Activation of Expression Integrates Temperature into the Auxin Pathway in Regulating Hypocotyl Growth
- Metabolic Profiling of a Mapping Population Exposes New Insights in the Regulation of Seed Metabolism and Seed, Fruit, and Plant Relations
- A Splice Site Variant in the Bovine Gene Compromises Growth and Regulation of the Inflammatory Response
- Comprehensive Research Synopsis and Systematic Meta-Analyses in Parkinson's Disease Genetics: The PDGene Database
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



