-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaThe Ciliogenic Transcription Factor RFX3 Regulates Early Midline Distribution of Guidepost Neurons Required for Corpus Callosum Development
The corpus callosum (CC) is the major commissure that bridges the cerebral hemispheres. Agenesis of the CC is associated with human ciliopathies, but the origin of this default is unclear. Regulatory Factor X3 (RFX3) is a transcription factor involved in the control of ciliogenesis, and Rfx3–deficient mice show several hallmarks of ciliopathies including left–right asymmetry defects and hydrocephalus. Here we show that Rfx3–deficient mice suffer from CC agenesis associated with a marked disorganisation of guidepost neurons required for axon pathfinding across the midline. Using transplantation assays, we demonstrate that abnormalities of the mutant midline region are primarily responsible for the CC malformation. Conditional genetic inactivation shows that RFX3 is not required in guidepost cells for proper CC formation, but is required before E12.5 for proper patterning of the cortical septal boundary and hence accurate distribution of guidepost neurons at later stages. We observe focused but consistent ectopic expression of Fibroblast growth factor 8 (Fgf8) at the rostro commissural plate associated with a reduced ratio of GLIoma-associated oncogene family zinc finger 3 (GLI3) repressor to activator forms. We demonstrate on brain explant cultures that ectopic FGF8 reproduces the guidepost neuronal defects observed in Rfx3 mutants. This study unravels a crucial role of RFX3 during early brain development by indirectly regulating GLI3 activity, which leads to FGF8 upregulation and ultimately to disturbed distribution of guidepost neurons required for CC morphogenesis. Hence, the RFX3 mutant mouse model brings novel understandings of the mechanisms that underlie CC agenesis in ciliopathies.
Published in the journal: . PLoS Genet 8(3): e32767. doi:10.1371/journal.pgen.1002606
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002606Summary
The corpus callosum (CC) is the major commissure that bridges the cerebral hemispheres. Agenesis of the CC is associated with human ciliopathies, but the origin of this default is unclear. Regulatory Factor X3 (RFX3) is a transcription factor involved in the control of ciliogenesis, and Rfx3–deficient mice show several hallmarks of ciliopathies including left–right asymmetry defects and hydrocephalus. Here we show that Rfx3–deficient mice suffer from CC agenesis associated with a marked disorganisation of guidepost neurons required for axon pathfinding across the midline. Using transplantation assays, we demonstrate that abnormalities of the mutant midline region are primarily responsible for the CC malformation. Conditional genetic inactivation shows that RFX3 is not required in guidepost cells for proper CC formation, but is required before E12.5 for proper patterning of the cortical septal boundary and hence accurate distribution of guidepost neurons at later stages. We observe focused but consistent ectopic expression of Fibroblast growth factor 8 (Fgf8) at the rostro commissural plate associated with a reduced ratio of GLIoma-associated oncogene family zinc finger 3 (GLI3) repressor to activator forms. We demonstrate on brain explant cultures that ectopic FGF8 reproduces the guidepost neuronal defects observed in Rfx3 mutants. This study unravels a crucial role of RFX3 during early brain development by indirectly regulating GLI3 activity, which leads to FGF8 upregulation and ultimately to disturbed distribution of guidepost neurons required for CC morphogenesis. Hence, the RFX3 mutant mouse model brings novel understandings of the mechanisms that underlie CC agenesis in ciliopathies.
Introduction
The Corpus Callosum (CC), the major commissure of the brain, is composed of millions of axons that connect the two brain hemispheres [1], [2]. Malformation of the CC is one of the most frequent brain anomalies found at birth, and may occur in as much as 7/1000 of the total newborn population. The most severe form of CC malformation is its complete absence also called callosal agenesis.
In mouse, callosal axons first start to cross the midline during late gestation at E16.5 [3], [4]. Callosal axons are directed through the Cortical Septal Boundary (CSB) by several guidepost cell populations expressing guidance cues. Glial cell populations were first described to be involved in CC formation in this region [1], . Guidepost glial cells are found at the glial wedge (GW) of the lateral ventricles (initially described as the cortical septal plate [5]), in the induseum griseum (IG) of the medial pallium and in the so-called sling at the CSB [5]–[10]. More recently, GABAergic (γ-aminobutyric acidergic) neurons and glutamatergic neurons that populate transiently the CSB have also been shown to be involved in guiding callosal axons at the midline [11]. These glial and neuronal guidepost populations are also observed in the human foetal CC [2], [12].
In humans, malformations of the CC have been found to be associated with a variety of syndromes [13], [14]. In particular, a reduction or a complete absence of the CC has been found to be associated with several human syndromes recently recognized as ciliopathies [15], [16]. However, it is not known where and at what stage of embryonic development cilia are required for proper CC formation. Several mouse models defective in cilia formation or function have been described in the literature, but only few have been shown to be associated with CC malformations and none of them has so far been used to explore the molecular mechanisms that underlie CC development. One reason is that most mouse mutants for ciliary genes die early during embryogenesis and that the surviving mutants present severe brain malformations that preclude the study of late defects such as CC formation.
RFX transcription factors have been shown to play fundamental roles in the control of ciliogenesis by regulating many genes involved in cilia assembly or function [17]. Rfx3 deficient mouse mutants exhibit several hallmarks of ciliopathies and in particular left-right asymmetry defects and hydrocephalus [18], [19]. We show here that Rfx3 deficient mice also harbour marked defects in CC development leading in most cases to agenesis of the CC. RFX3 is first expressed throughout the anterior neural tube and is then progressively restricted to particular cell populations, particularly at the midline CSB, before and while pioneer callosal axons cross the midline. Rfx3 loss of function leads to a distorted distribution of the neuronal but not of the glial guidepost cell populations that have both been shown to direct callosal axons through the midline. Reciprocal transplant experiments demonstrate that in Rfx3−/− brains, defects of the midline corticoseptal region are indeed responsible for improper crossing of the midline by callosal axons. However, conditional inactivation of Rfx3 at specific time points in corticoseptal cell populations does not lead to CC defects, demonstrating that RFX3 is required early during brain development to pattern the CSB. We show that E12.5 Rfx3 deficient brains present a mild expansion of Fgf8 expression in the rostromedial septum, similar to a Gli3 hypomorphic phenotype. We indeed show that GLI3 processing is altered in Rfx3 deficient brains. Last we show, using organotypic slice cultures, that ectopic FGF8 expression disrupts guidepost neuronal distribution similar to the in vivo defects observed in Rfx3 mutants. Altogether, our data show that loss of function of Rfx3 at early stages of embryonic development is responsible for disturbed GLI3 processing and to small alterations in Fgf8 expression, likely sufficient to induce dramatic aberrations in corticoseptal organization of guidepost neurons and consequently in CC formation. Rfx3 mouse mutants thus appear to be particularly informative for understanding the molecular mechanisms that govern early midline patterning and offers a rare insight into the causes of CC defects in ciliopathies.
Results
Rfx3 mutant mice are acallosal
The consequence of Rfx3 inactivation on CC development was analysed on sections stained with haematoxylin-eosin or immunostained for specific guidance markers of callosal axons: Neuropilin1 (Npn-1) and L1CAM cell adhesion protein (L1) (Figure 1 and Figure S1). At E18.5, callosal axons have crossed the CC midline in wild-type (WT) mice (Figure 1A, 1C, 1E and Figure S1A). In contrast, Rfx3−/ − mice exhibited partial (n = 4/11) to complete agenesis (n = 4/11) of the CC, with few or no callosal axons crossing the midline (Figure 1 and Figure S1). Remaining Rfx3−/ − mice did not exhibit any obvious callosal defects (n = 3/11). In Rfx3−/ − brains, many callosal axons reached the midline but, instead of crossing it, accumulated on both sides of the midline and formed dense axonal bundles called Probst Bundles (PB) (arrowheads, Figure 1 and Figure S1). In some animals there was a relatively mild phenotype in which the two cerebral hemispheres fused correctly, and a few callosal axons still crossed the midline albeit with abnormal trajectories (Figure 1B, 1F and Figure S1B). The most severe phenotype that we observed was a complete agenesis of the CC with no callosal axons crossing the midline. In these Rfx3−/ − mice, the two cerebral hemispheres did not fuse correctly and displayed a large bulge along the inter-hemispheric fissure where callosal axons approach the midline (Figure 1D and Figure S1C, symbol O). Additionally, we observed in these embryos strong defects in the formation of the hippocampal commissure but not of the anterior commissure (Figure 1, Figure S1 and not shown). Thus Rfx3 contributes to the formation of the CC and the hippocampal commissure.
Fig. 1. Abnormal callosal axon pathfinding in Rfx3−/− mice. 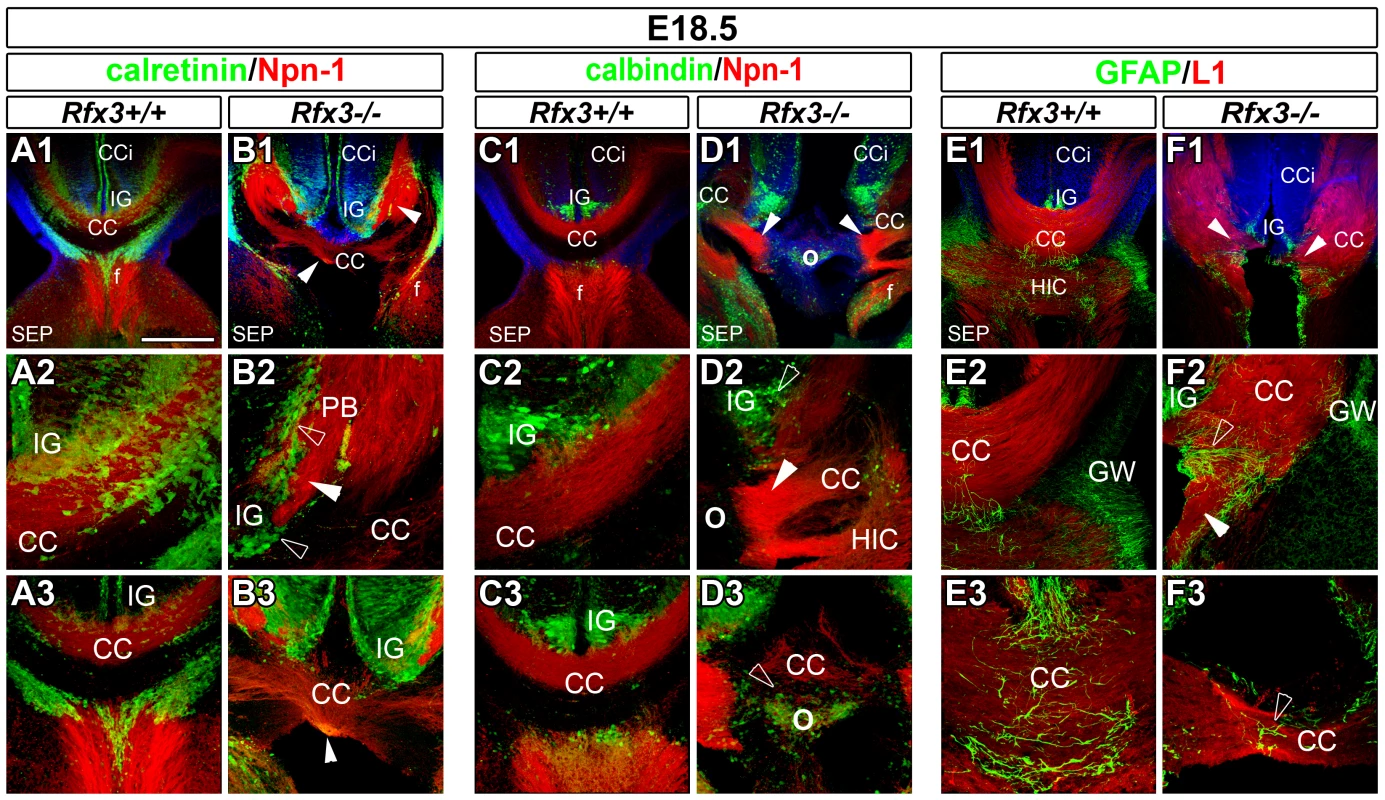
(A–F) Immunohistochemistry for calretinin and Npn-1 (A1–A3 and B1–B3), for calbindin and Npn-1 (C1–C3 and D1–D3), and for GFAP and L1 (E1–E3 and F1–F3) in coronal CC sections from E18.5 WT (A1–A3, C1–C3 and E1–E3) or Rfx3−/− (B1–B3, D1–D3 and F1–F3) mice. A2, B2, C2, D2, E2 and F2 are higher magnifications of the lateral CC seen in A1, B1, C1, D1, E1 and F1, respectively. A3, B3, C3, D3, E3 and F3 are higher magnifications of the medial CC seen in A1, B1, C1, D1, E1 and F1, respectively. (A1–A3, C1–C3 and E1–E3) At E18.5, the hemispheres of WT brains have fused. Callosal fibres (in red) cross the midline and project into the contralateral cortex. (B1–B3, D1–D3 and F1–F3) Aberrant callosal axon bundles are observed in Rfx3−/− embryos (arrowheads). (B1–B3 and F1–F3) While the hemispheres have fused, most of callosal fibres do not cross the midline and form large ectopic bundles on the CC border, reminiscent of Probst bundles (PB). (D1–D3) Some Rfx3−/− embryos exhibit a more severe phenotype with an absence of midline hemispheric fusion and absolutely no callosal axons crossing the midline. In this case, a large bulge is observed along the inter-hemispheric fissure at the location where the callosal axons approach the midline (O). In all mutants, axonal defects are accompanied by cellular mis-positioning through the CC and the IG (open arrowheads). While calretinin+ or calbindin+ neurons, as well as, GFAP+ glia, are still present in the CC and the IG of Rfx3−/− mice, there is a midline disorganization and a lateral shift of these cell populations. Bar = 435 µm in A1, B1, C1, D1, E1, F1; 220 µm in A3, B3, C3, D2, D3, E2, F2 and 110 µm in A2, B2, C2, E3, F3. Expression of RFX3 in glutamatergic neurons of the developing CC and cortex
To understand how RFX3 is involved in CC formation, we analysed Rfx3 mRNA expression in coronal sections of the developing mouse telencephalon prior to and during CC formation. From E8 to E10.5, Rfx3 was uniformly expressed in the entire neuroepithelium (not shown). From E11.5 to E16.5, Rfx3 expression became progressively restricted to specific rostro-caudal levels in the telencephalon (Figure 2 and Figure S2). Rfx3 hybridization signal was strong at the CSB (*) where the CC will form, and in the cingulate cortex (CCi) that contains pioneer callosal projection neurons [3], [4] (Figure 2B–2D). In addition, Rfx3 was expressed in the primordium of the IG and the ventricular zone of the GW at the border of the lateral ventricles (Figure 2B1, 2C1, 2D1–D2). Both regions surround the CSB and are known to be important for CC formation [1], [7], [8]. High Rfx3 expression was also observed at more rostral levels in the retrobulbar region and at caudal levels in the cortical hem (CH), the choroid plexus, the ventral pallium (VP) laterally, as well as, in the preoptic area (POA) (Figure 2B3, 2C3 and 2D2–D3, open arrows). From E16.5 to birth, Rfx3 expression in the rostral telencephalon was restricted to the IG, the GW and the cerebral cortex (Figure S2E, S2G and Figure S3C, S3E to S3H).
Fig. 2. Expression pattern of Rfx3 in the developing mouse telencephalon from E11.5 to E14.5. 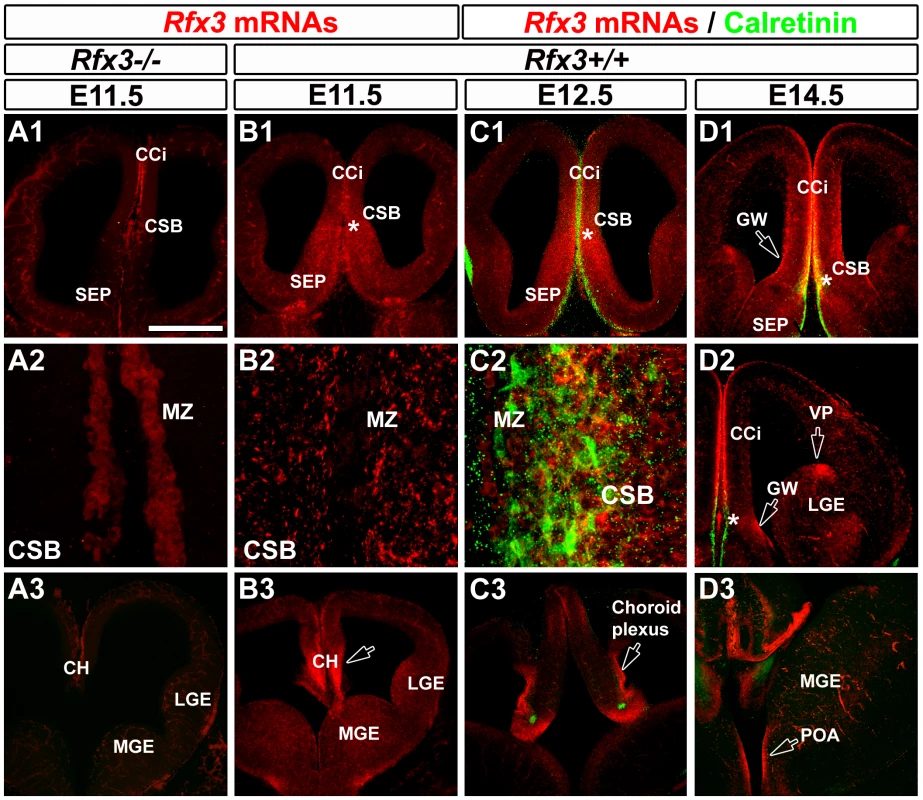
(A–D) In situ hybridization for Rfx3 mRNAs on coronal brain sections of wild type (B1–B3) and Rfx3−/− (A1–A3) embryos at E11.5. In situ hybridizations for Rfx3 (in red) combined with immunohistochemical staining for calretinin (C1–C3 and D1–D3) (in green) on coronal brain sections of wild type embryos at E12.5 (C1–C3) and E14.5 (D1–D3). A1, B1, C1 and D1 are coronal sections at the corticoseptal boundary (CSB, *) level, while A3, B3, C3 and D3 are caudal coronal sections at the level of the cortical hem (CH). A2, B2 and C2 are higher power views of the CSB seen in A1, B1 and C1 respectively. D2 is a lateral view of the telencephalon. (A and B) At E11.5, Rfx3 is strongly expressed in wild type mice throughout the entire neuroepithelium of the CSB, and at more caudal levels in the cortical hem (B1–B3). The Rfx3 hybridization signal is specific since no signal is visible in the same brain area of Rfx3−/− (A1–A3). (C–D) From E12.5 to E14.5, Rfx3 expression is restricted to the cingulate cortex (CCi) that contains pioneer callosally projecting neurons, and throughout the CSB at the midline where the CC will form (C1–C2 to D1–D2). In addition, Rfx3 is detected within the glial wedge (GW, open arrows) and the septum. (C3 to D3) On more caudal sections, Rfx3 mRNAs are expressed in the cortical hem (CH), choroid plexus, ventral pallium (VP) and preoptic area (POA) (open arrows). Bar = 435 µm in A1, A3, B1, B3, C1, C3, D1, D2, D3 and 60 µm in A2, B2, C2. To clarify the nature of the embryonic midline cells expressing RFX3, we performed co-labelling experiments with markers for different cell types. Glutamatergic guidepost neurons colonizing the forming CC express the calcium binding protein calretinin as well as several transcription factors known to promote the glutamatergic fate such as empty spiracles homolog 1 (EMX1) (Figure S3A) and T-box brain transcription factor 1 (TBR1) [11] (Figure S2 and Figure S3A). In the embryonic IG, part of the neurons express calbindin and are also glutamatergic since they express EMX1 (Figure S3B). In addition, GABAergic guidepost neurons can be identified using a GAD67-GFP mouse line in which the green fluorescent protein (GFP) is reliably expressed in GABAergic neurons. Finally, CC guidepost glia of the IG and of the GW can be distinguished by Nestin, Glutamate Aspartate Transporter (GLAST) and Glial Fibrillary Acidic Protein (GFAP) expression [5], [6].
At the CSB, Rfx3 was expressed in glutamatergic guidepost neurons labelled for calretinin, reelin and TBR1 as early as E14.5 (Figure 2D and Figure S2A, S2B and S2D, *). In the IG, Rfx3 mRNA was detected in glutamatergic neurons labelled for the calcium binding protein calbindin (Figure S2F). After E16.5, at the brain level where the cerebral hemispheres have already fused, Rfx3 was no longer expressed by glutamatergic guidepost neurons of the CC white matter (Figure S2E1, arrow). Rfx3 was still expressed by glutamatergic calretinin+ neurons of the marginal zone (MZ) and calbindin+ neurons of the IG (Figure S2E2 and S2G). We observed no co-localization between GAD67-GFP and Rfx3 in neurons from E14.5 to E18.5 (not shown and Figure S3C). Thus, Rfx3-expressing neurons of the corticoseptal region are strictly glutamatergic.
Moreover, Rfx3-positive cells populating the ventricular zone in the GW region from E14.5 to E18.5 are radial glial cells, labelled for Nestin, GFAP and GLAST (Figure S3D–S3F). However, no such overlap can be observed in the IG region, confirming that Rfx3-positive cells are not glial cells in the IG (Figure S3F1).
In addition to Rfx3 expression in the midline, we also observed at E12.5, that Rfx3 mRNA was detected in pioneer calretinin+ glutamatergic cortical neurons of the preplate (Figure 2C). From E13.5 to E16.5, Rfx3 mRNA was found in calretinin+ glutamatergic neurons in all layers of the developing cortex (Figure 2D and Figure S2B–S2E, S3E) and at E18.5 it was found in the projection neurons of the upper cortical layers labelled for Special-AT-rich sequence Binding protein 2 (SATB2) and in those of the lower cortical layers labelled for COUP-TF Interacting Protein 2 (CTIP2) (Figure S3G, S3H). By contrast, we never detected any Rfx3 hybridization signal in the reelin+ Cajal Retzius cells or calbindin+ neurons of the cortical MZ (Figure S2A, S2F).
The presence of Rfx3 transcripts at the midline in the corticoseptal region and in the cerebral cortex is consistent with the importance of this gene in CC formation. Given the large distribution of RFX3 in the embryonic brain, it might contribute to the proper development of the cortex or of midline structures. We, thus, examined if these different regions are affected by Rfx3 inactivation.
RFX3 is not required in callosal axons for proper CC formation
We first analysed the cerebral cortex of Rfx3 mutants. In Rfx3−/− cortex, the laminar distribution of SATB2+ and CUX1+ (Cut-like homeobox 1) callosally projecting neurons [20]–[26] was normal (Figure S4A–S4D). In addition, CTIP2+ cortical layer V and TBR1+ cortical layers V–VI which contain about 20% of the callosally projecting neurons, were similar in mutant and WT brains (Figure S4E–S4H).
To study if RFX3 expression in cortical neurons is necessary for axonal growth in the CC, we investigated whether the targeted inactivation of Rfx3 in pyramidal cortical neurons results in pathfinding defects. Using a Ngn2-CreER driver line, we induced recombination of a Rfx3 floxed allele in neurogenin 2 (NGN2)-derived glutamatergic projection neurons of the cortex by tamoxifen application at E13.5 [27], [28]. While Rfx3 was not any more expressed in the cerebral cortex of Rfx3f/f; Ngn2-CreERtm+/− mice, (compare Figure S4I2 and S4J2), the SATB2+ callosally projecting neurons and the CC still formed normally (n = 7/7; Figure S4J1 and S4L). This result shows that the loss of Rfx3 in cortical pyramidal neurons is not responsible for callosal axon guidance defects.
RFX3 is required for proper distribution of guidepost neurons in the CSB
To determine if RFX3 was required for the development of the CSB we followed the organization of guidepost cells in mutant CSB compared to WT. We first followed the distribution of CC guidepost glutamatergic neurons in Rfx3−/− mice at E18.5, after callosal axons have crossed the midline. Glutamatergic neurons of the CC, labelled for TBR1 and calretinin were shifted laterally, leaving a large portion of the CC devoid of neurons (not shown and Figure 1A and 1B, open arrowheads). Calretinin+ and calbindin+ glutamatergic neurons were both severely disorganized through the IG (Figure 1A–1D, open arrowheads). In addition, a progressive disorganization of CC glial cells was noticed in Rfx3−/− CC regions. The GFAP-positive astrocyte-like cells of the IG and of the midline were disorganized and the curvature of the radial glial processes was increased (Figure 1E and 1F, open arrowheads). Because this disorganization could be a secondary effect of callosal misrouting, we also looked at the distribution of guidepost cells before callosal axons cross the midline.
As early as E14.5, glutamatergic guidepost neurons labelled for reelin, calretinin and TBR1 failed to form a well organized band of neurons at the CSB of Rfx3−/− mice and instead accumulated ectopically on both sides of the midline (Figure 3A–3D; open arrowheads; Figure 4A–4D and not shown). In addition, Reelin+ Cajal Retzius and calretinin+ neurons lost dramatically their tangential distribution in the MZ layer and are more broadly distributed in the cortico-septal region (Figure 3A–3D and Figure 4A–4D). In addition, they lost their fusiform/bipolar shape. For both neuronal populations, given the density of the cells, the number of neurons in the corticoseptal region and the MZ was difficult to quantify. Similarly, from E14.5 to E16.5 glutamatergic neurons labelled for calbindin were mislocalised in the cortical MZ and IG of Rfx3−/− brains (Figure 3E–3F and Figure 4E and 4F). Moreover, some calbindin+ neurons were found to accumulate within the CC white matter (Figure 4E and 4F). The midline neuronal defects were accompanied, at E16.5, by pathfinding errors of pioneer callosal axons that failed to cross the midline and formed ectopic bundles (Figure 4A–4F, white arrowheads).
Fig. 3. Aberrant localization of midline neurons before CC formation at E14.5. 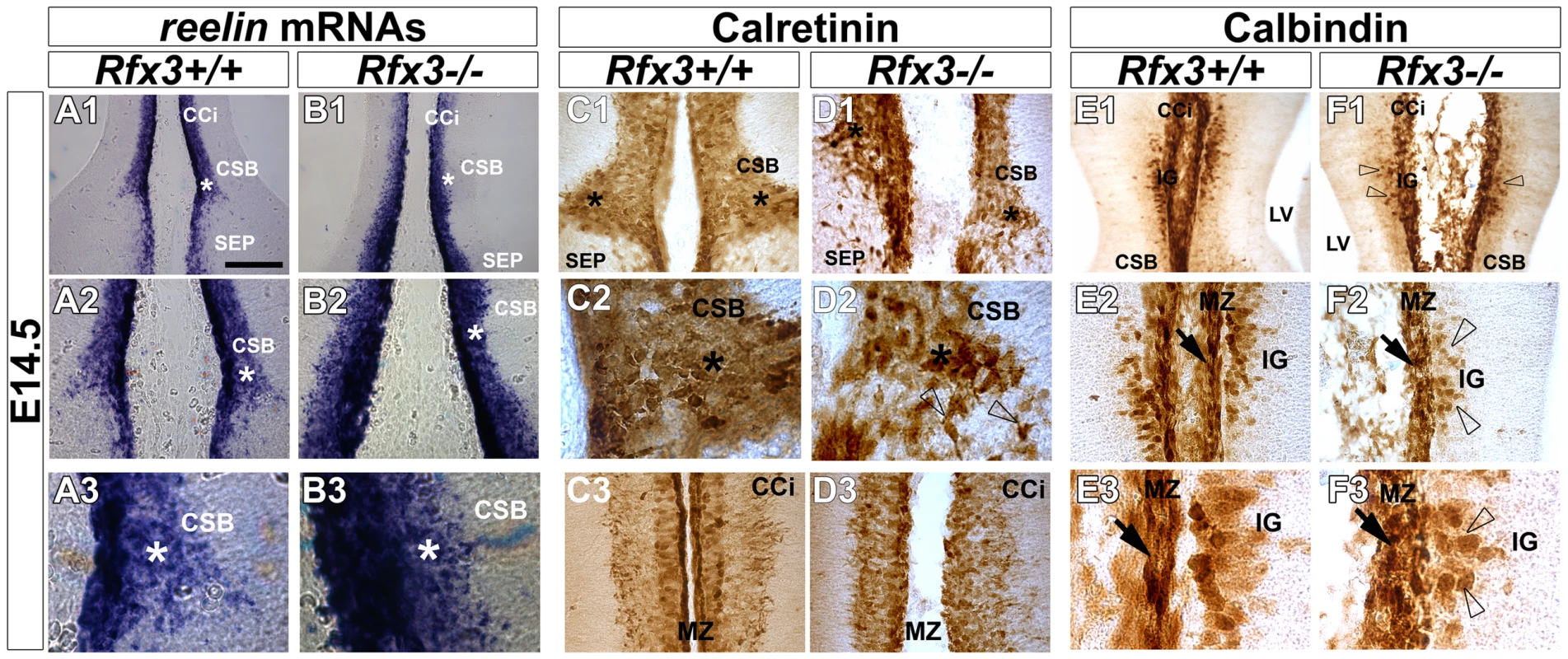
In situ hybridization for reelin mRNAs (A1–A3 and B1–B3) and DAB staining for calretinin (C1–C3 and D1–D3) and calbindin (E1–E3 and F1–F3) on coronal rostromedial slices from E14.5 WT (A1–A3, C1–C3 and E1–E3) and Rfx3−/− (B1–B3, D1–D3 and F1–F3) mice. A2, A3, B2, B3, C2 and D2 are higher power views of the corticoseptal boundary (CSB,*) seen in A1, B1, C1 and D1 respectively. E2, E3, F2 and F3 are higher power views of the induseum griseum (IG) region seen in E1 and F1, respectively. C3 and D3 are higher power views of the cortical marginal zone (MZ) seen in C1 and D1 respectively. (A–F) As early as E14.5, before inter-hemispheric midline fusion occurs, the organization of reelin+ (B1–B3), calretinin+ (D1–D2) and calbindin+ (F1–F2) midline neurons is severely affected in the CSB and the IG regions of the Rfx3−/− mutant (arrows and open arrowheads). In addition, the neurons lose their tangential organization through the Rfx3−/− cortical MZ (B3, D3 and F2–F3; arrows). Bar = 300 µm in A1, B1, E1, F1; 150 µm in A2, B2, C1, C3, D1, D3, E2, F2 and 60 µm in A3, B3, C2, D2, E3, F3. Fig. 4. Abnormal neuron localization and aberrant callosal axon pathfinding at the onset of CC formation. 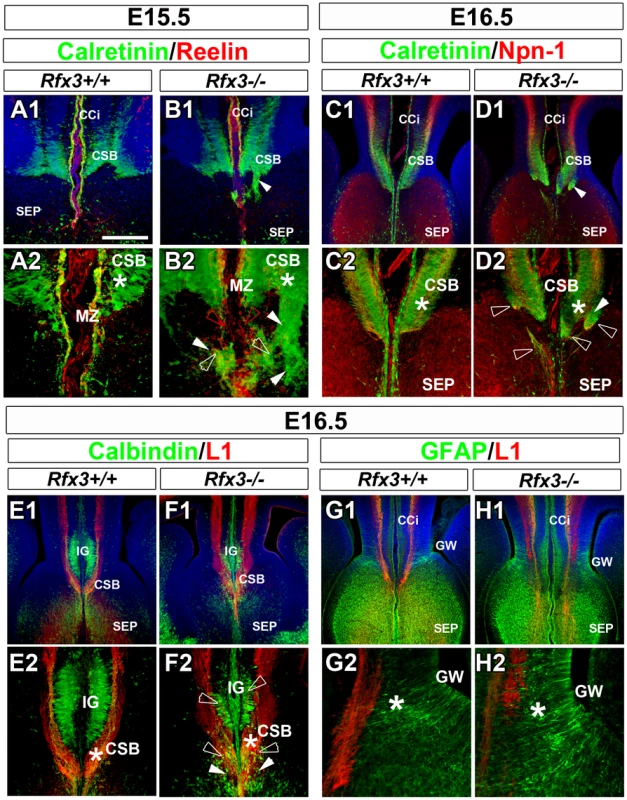
(A–H) Immunohistochemistry for calretinin and reelin (A1–A2 and B1–B2), for calretinin and neuropilin-1 receptor (Npn-1) (C1–C2 and D1–D2), for calbindin and L1 receptor (E1–E2 and F1–F2) and for GFAP and L1 receptor (G1–G2 and H1–H2), in coronal CC sections from WT (A1–A2, C1–C2, E1–E2 and G1–G2) and Rfx3−/− (B1–B2, D1–D2, F1–F2 and H1–H2) mice. A2, B2, C2, D2, E2, F2, G2 and H2 are higher magnifications of the midline seen in A1, B1, C1, D1, E1, F1, G1 and H1. (A1–A2 to D1–D2) From E15.5 to E16.5, calretinin+ guidepost neurons fail to form a well organized band of neurons at the CSB (*) and are dispersed in the septum of Rfx3−/− mice (B2 and D2, white open arrowheads). Reelin+ and calretinin+ neurons loose their tangential organization through the cortical marginal zone (MZ) (compare B2 to A2, red open arrowheads). (E1–E2 and F1–F2) At E16.5, calbindin+ neurons (green) do not organize appropriately within the indusium griseum (IG) and accumulate at the CC midline in Rfx3−/− mice (compare F2 to E2, open arrowheads). (G1–G2 and H1–H2) At E16.5, the organization of GFAP+ glial cell populations within the CC is indistinguishable between WT and Rfx3−/− mice. (A to H) Axonal misrouting of pioneer callosal axons from E15.5 to E16.5. (A1–A2, C1–C2, and E1–E2) In WT brains, pioneer callosal fibres grow within the CSB and reach the midline. (B1–B2, D1–D2 and F1–F2) In Rfx3−/− brains, most callosal fibres form ectopic bundles of axons in the septum (B2 and D2) and the IG (F2) on either side of the midline (white arrowheads). Bar = 435 µm in C1, D1, E1, F1, G1, H1; 220 µm in A1, B1, C2, D2, E2, F2; 110 µm in G2, H2; 60 µm in A2, B2. By contrast, GAD67-expressing GABAergic neurons were properly positioned through the lateral part of the CC at E16.5 in Rfx3 mutant (Figure S5A and S5B). Finally, immunohistochemistry with several markers for astrocytes (nestin, GLAST and GFAP), indicated that the position and organization of the guidepost glial cell populations of the GW and of the midline glial zipper was indistinguishable in WT and Rfx3−/− mice, suggesting that their development is not sensitive to the loss of Rfx3 (Figure 4G–4H and Figure S5).
Altogether, these experiments indicate that RFX3 is necessary for the proper positioning of multiple corticoseptal neuronal populations but not of glial cell populations at the midline early in development.
Abnormal development of the CC midline in Rfx3 mutant mice is responsible for callosal axon pathfinding defects
To verify if CC guidance defaults were caused by altered development in the midline region, we performed transplantation experiments as previously described [11]. Midline structures comprising the CC were transplanted into telencephalic slices at E16.5, using different combinations of wild type and Rfx3−/− embryos (Figure 5). When midline explants from Rfx3+/+ mice were transplanted into Rfx3+/+ slices, DiI-labelled callosal axons crossed the midline (Figure 5A; n = 7 slices with crossing axons out of 10), thereby reproducing the in vivo behavior of callosal axons. By contrast, with Rfx3−/− midline explants transplanted in Rfx3−/− slices, DiI-labelled callosal axons failed to cross the midline (Figure 5B; n = 0 slices with crossing axons out of 3). Similarly, transplantation of midline from Rfx3−/− mice into Rfx3+/+ slices leads to impaired midline crossing of axons (Figure 5C; n = 2 slices with crossing axons out of 7). We then tested whether the transplantation of Rfx3+/+ midline into Rfx3−/− mutant slices could restore correct pathfinding of DiI-labelled Rfx3−/− callosal axons. Remarkably, Rfx3+/+ midline structure restored normal axonal guidance of the majority of Rfx3−/− callosal axons (Figure 5D; n = 5 slices with crossing axons out of 7).
Fig. 5. Midline integrity is necessary for pathfinding by callosal axons. 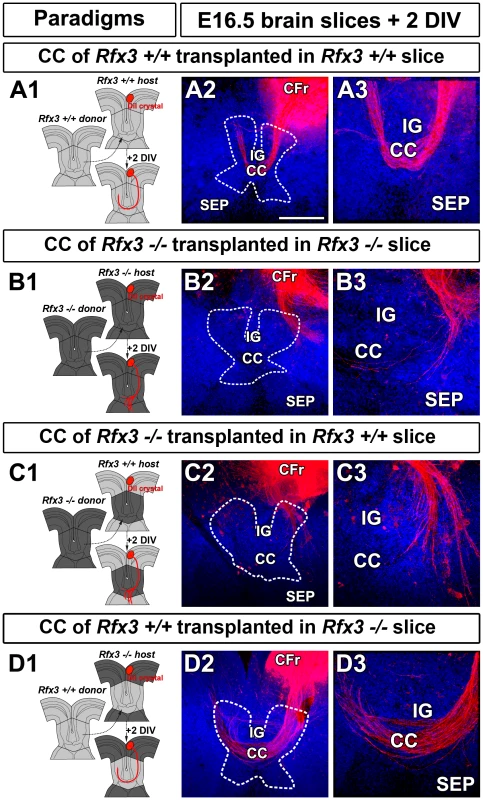
(A1) Experimental paradigm used to confirm the growth of E16.5 Rfx3+/+ control callosal axons in midline structure transplants from Rfx3+/+ control mice. (A2–A3) DiI labeling showing that WT callosal axons grow normally and cross the midline when they are confronted to a WT environment. (B1) Experimental paradigm used to confirm the growth defects of E16.5 Rfx3−/− callosal axons in midline transplants from Rfx3−/− mice. (B2–B3) DiI labeling showing that Rfx3−/− callosal axons are misrouted and do not cross the midline of Rfx3 mutants. (C1) Experimental paradigm used to study the growth defects of E16.5 control Rfx3+/+ callosal axons in transplants of midline structures from Rfx3−/− mice. (C2–C3) DiI labeling showing that WT callosal axons are misrouted and do not cross the midline of Rfx3 mutants. (D1) Experimental paradigm used to test whether the midline integrity is necessary and sufficient to direct the growth of callosal axons. To this end, control Rfx3+/+ midline regions are transplanted in Rfx3−/− slices. (D2–D3) DiI labeling showing the complete restoration of Rfx3−/− callosal axon pathfinding. Dashed lines outline the CC transplant localizations. Brain slices in A2–A3, B2–B3, C2–C3 and D2–D3 were counterstained with Hoechst. Bar = 435 µm in A2, B2, C2, D2 and 220 µm in A3, B3, C3 and D3. Therefore, the misrouting of callosal axons in Rfx3 mutant embryos is due to defects in corticoseptal midline associated structures.
Rfx3 inactivation in midline guidepost cells is not responsible for CC developmental defects
To study if RFX3 expression in guidepost glia or neurons is required for callosal axon growth, we investigated whether the targeted inactivation of Rfx3 in both cell type progenitors might result in cell differentiation defects that could have an impact on axonal guidance. We thus inactivated Rfx3 in GFAP-positive radial glia precursors by mating Rfx3f/f and hGfap-Cre+/− mice [29]. According to Zhuo et al., 2001, these mice start to express Cre recombinase in the forebrain at E13.5 [29]. We observed that while Rfx3 was already inactivated at E15.5 in CC guidepost glia and neurons of Rfx3f/−; hGfap-Cre+/− mice (Figure 6A and 6B), the CC still formed normally (Figure 6C–6F; n = 9/9). This result shows that the loss of Rfx3 in midline neuronal and glial guidepost cells as early as E15.5 is not responsible for CC agenesis.
Fig. 6. Mice carrying a conditional deletion of Rfx3 in guidepost cells have a normal corpus callosum. 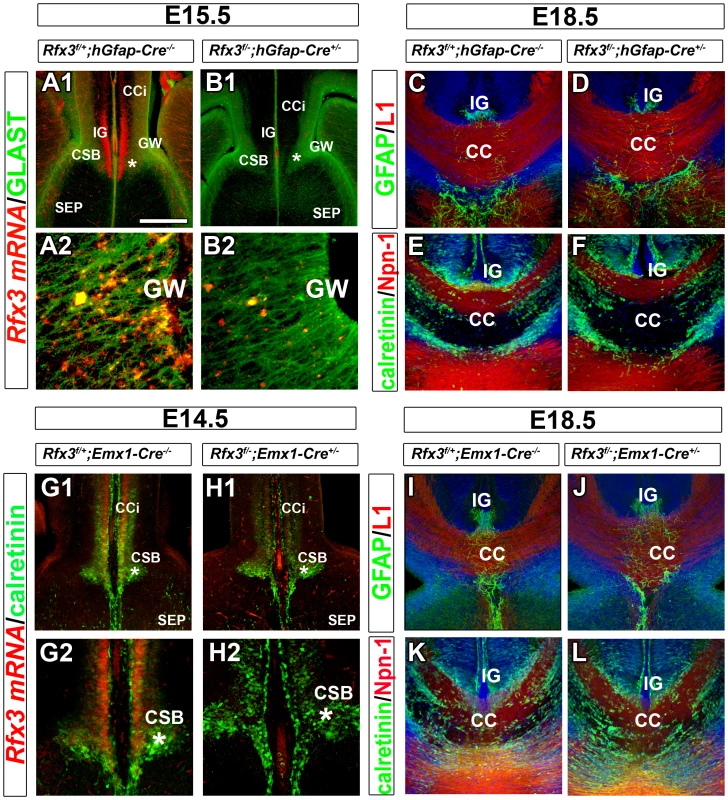
(A–B) Rfx3 mRNA (red) and GLAST protein (green) expression on coronal brain sections of control Rfx3f/+;hGfap-Cre−/− (A1–A2) and Rfx3f/−;hGfap-cre+/− (B1–B2) embryos at E15.5. A2 and B2 are higher magnifications of the glial wedge (GW) seen in A1 and B1, respectively. (A1–A2) In control Rfx3f/+;hGfap-Cre−/− mice, Rfx3 is strongly expressed through the cortex, the induseum griseum (IG) and the CSB, as well as, the GW. (B1–B2) No more Rfx3 mRNA is detected in guidepost glia and neurons of Rfx3f/−;hGfap-Cre+/− CC. (C–F) Immunohistochemistry for GFAP and L1 receptor (C and D) and for calretinin and Npn-1 receptor (E and F) in coronal CC sections from E18.5 control Rfx3f/+;hGfap-Cre−/− (C and E) and Rfx3f/−;hGfap-Cre+/− (D and F) mice. In mice where Rfx3 is inactivated after E14.5 in midline neurons and glia, the CC and callosal axons develop normally. (G–H) Rfx3 mRNA (red) and calretinin protein (green) expression on coronal brain sections of control Rfx3f/+;Emx1-Cre−/− (G1-G2) and Rfx3f/−;Emx1-cre+/− (H1–H2) embryos at E14.5. G2 and H2 are higher magnifications of the corticoseptal boundary (CSB, *) seen in G1 and H1, respectively. (G1–G2) In control Rfx3f/+;Emx1-Cre−/− mice, Rfx3 is strongly expressed through the calretinin+ glutamatergic neurons of the cortex and of the CSB. (H1–H2) In Rfx3f/−;Emx1-Cre+/− brains, no more Rfx3 mRNA is detected in midline glutamatergic guidepost neurons of the CSB. (I–L) Immunohistochemistry for GFAP and L1 (I and J) and for calretinin and Npn-1 (K and L) in coronal CC sections from E18.5 control Rfx3f/+;Emx1-Cre−/− (I and K) and Rfx3f/−;Emx1-Cre+/− (J and L) mice. Rfx3 inactivation after E12.5 in guidepost glutamatergic neurons of the CSB does not affect callosal axon navigation. Bar = 435 µm in A1, B1, G1, H1; 220 µm in C, D, E, F, I, J, K, L; 110 µm in G2, H2 and 40 µm in A2, B2. However, we cannot exclude that RFX3 is needed in the glutamatergic guidepost neurons that invade the CSB region from E12.5 to E14.5. To test this possibility, we induced recombination of Rfx3 floxed allele specifically in guidepost neurons, as early as E12.5, by mating Rfx3f/f and Emx1-Cre+/− mice [30]. Inactivation of Rfx3 in Emx1+ precursors of Rfx3f/−; Emx1-Cre+/− mice led to loss of Rfx3 in all the CSB anlage at E12.5 and in midline postmitotic glutamatergic neurons at E14.5 (Figure 6G and 6H). While Rfx3 was already inactivated at E12.5 in the CSB region of Rfx3f/−;Emx1-Cre+/−, we did not observe any callosal pathfinding defects (Figure 6I–6L; n = 6/6). These results also sustain the conclusion that Rfx3 is not required in CC neurons. Finally, Rfx3 was inactivated in GABAergic neurons originating from the ventral telencephalon by using the Nkx2.1-Cre+/− mice [31]. In accordance with the absence of Rfx3 expression in CC guidepost GABAergic neurons, Rfx3f/−;Nkx2.1-Cre+/− mice did not present any CC defects (not shown).
Taken together, these results demonstrate that callosal pathfinding defects observed in Rfx3−/− mice are not due to a cell autonomous function of RFX3 in CC guidepost cells suggesting a requirement for RFX3 for proper CC development, at early embryonic stages, during midline specification.
Midline specification is affected in Rfx3−/− forebrains
To understand how RFX3 governs CC midline structure formation before E12.5, we looked at early patterning defects that could affect Rfx3−/− brains. Telencephalic patterning relies on the interaction of well-described dorsal, rostral and ventral signalling centres in the forebrain that produce secreted signalling molecules [32]. We first analysed the expression of genes characteristic for the dorsal signalling centres in Rfx3−/− embryos by in situ hybridization. Genetic evidences show that Bone morphogenic 4 (Bmp4) is essential for roof plate formation in the mouse forebrain [33]. BMP4 is expressed in the telencephalic midline at E10.5 and in the entire forebrain midline at E12.5. Moreover, several WNT proteins are expressed in the cortical hem [34] which is crucial for dorsal midline development. However, our analyses did not reveal any differences in Bmp4 and Wnt2b expression between E12.5 WT and Rfx3−/− embryos (n = 2/2, Figure S6A–S6D). Thus no major defects could be observed in dorsal midline markers in Rfx3−/− brains.
The rostral signaling center is specified at E8.5 as the anterior neural ridge (ANR) at the anterior border between the ectoderm and neuroectoderm and will give rise to the commissural plate at later stages. Both the ANR and the commissural plate express FGF8 that has been shown to be important to induce ventral and rostrodorsal cell fates [35]. We determined the telencephalic rostral expression profile of Fgf8 in wild type and Rfx3−/− embryos. As observed in Figure 7, at E12.5, Fgf8 expression was restricted to the commissural plate of wild type embryos. Remarkably, we observed an extension of Fgf8 expression into the rostromedial pallium in Rfx3−/− embryos on both coronal and sagittal sections (n = 6/6, Figure 7A, 7B and Figure S7A, S7B). These data suggest that RFX3 is necessary to restrict Fgf8 expression to the commissural plate. FGF8 has been shown to induce Sprouty2 gene expression which in turns negatively regulates FGF8 signalling [36], [37] and we observed a small expansion of Sprouty2 in the rostromedial pallium consistent with an increase in FGF signalling in the midline (n = 7/7, Figure 7C, 7D and Figure S7C, S7D).
Fig. 7. Disturbed expression of Fgf8 and of the ratio of GLI3 repressor/GLI3 activator forms in Rfx3−/− CSB. 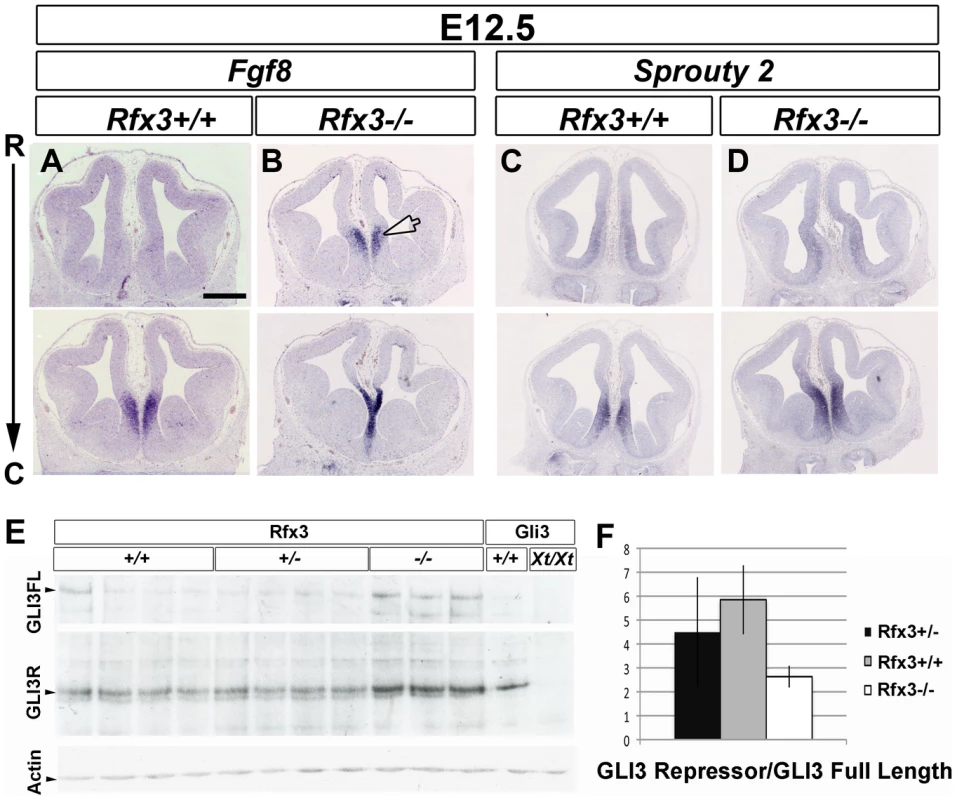
In situ hybridization for Fgf8 (A and B), Sprouty2 (C and D) mRNAs on coronal sections from E12.5 WT (A and C) and Rfx3−/− (B and D) mice at the CSB. Fgf8 expression domains is expanded into the rostromedial pallium in Rfx3−/− embryos. Interestingly, the frontier region between the septum and the cortex is reduced in the mutant compare to WT (arrowhead). In addition, Sprouty2 expression is slightly increased in the Rfx3 mutant. Bar = 500 µm in all figures. (E) Western blot analysis of E13.5 individual forebrains from Rfx3+/+, Rfx3+/− and Rfx3−/− embryos from a same litter. As control, extracts from bodies of Gli3+/+ or Gli3Xt/Xt embryos were included. No GLI3 protein is produced in Gli3Xt mutants allowing the identification of GLI3 specific bands. (F) Quantification of the Western blot shows that the ratio of GLI3 repressor form to the full-length form is reduced in Rfx3 deficient mice compared to heterozygotes and WT mice. Several key transcription factors have been associated with the specification of the commissural plate in mouse, including: SIX3, nuclear factor I/A (NFIa) and EMX1 [38]. We precisely analyzed the expression of these markers but did not observe extensive variations in the expression of Emx1 (n = 3/3), Six3 (n = 3/3), and Nfia (n = 4/4) between WT and Rfx3 mutant mice (Figure S6E–S6H and not shown) suggesting that ectopic FGF8 in Rfx3−/− rostral telencephalon does not induce dramatic changes in the expression of these key transcription factors.
It has been shown that proper Sonic Hedgehog (SHH) signalling is required to maintain FGF8 signaling at the rostral midline. In addition, defects in ciliary proteins lead to defective SHH signaling in many tissues and organs (for review see [39]) and also in the telencephalon [40], [41]. In comparison with Rfx3+/+ embryos, we did not observe any differences in Shh expression in the ventral telencephalon of Rfx3−/− mutants (n = 3/3, Figure S6I and S6J). However, we observed that SHH signalling is likely to be affected since the Shh target genes Patched1 (Ptc1) (n = 3/4) and Gli1 (n = 2/3), were both slightly down-regulated in the Rfx3−/− ventral telencephalon (Figure S6K–S6N, arrows). Taken together, these findings suggest that the up-regulation of Fgf8 expression does not coincide with an up-regulation of SHH signalling in the ventral telencephalon.
Interestingly, it has been shown that Gli3−/− embryos present an abnormal development of the prosencephalic midline with a similar ectopic expansion of Fgf8 expression into the dorsal midline [42]–[44]. Since GLI3 processing has been shown to require cilia [45] and that RFX3 regulates ciliogenesis in several mouse cell types, we hypothesized that the FGF8 expression defects could result from abnormal function of GLI3 in Rfx3−/− embryos. Gli3 mRNA expression did not appear to be affected in Rfx3−/− telencephalon, as observed by in situ hybridization on coronal sections (Figure S6O and S6P). Thus, RFX3 does not seem to act on Gli3 transcription. Gli3 produces two antagonistic protein isoforms: the full-length activator form (GLI3A) and the proteolytic cleaved repressor form (GLI3R) [46]–[48] with the ratio between GLI3A and GLI3R being an important determinant of patterning for various tissues. We thus investigated GLI3 proteolytic processing by western blot in wild type or Rfx3 deficient brains and found that the GLI3R/GLI3A ratio is reduced in mutant brains (Figure 7E and 7F).
These results show that RFX3 is required for the proper specification of the CSB at early stages of embryonic development and that this RFX3 function is likely to be mediated by altered GLI3 processing.
Ectopic FGF8 signalling at the CSB leads to altered organisation of CC guidepost neurons
To study whether ectopic FGF8 signalling could be responsible for the disorganisation of guidepost neurons at the CSB, we performed ex-vivo cultures of brain slices at E12.5 in the presence or absence of ectopic FGF8 (Figure 8). Ectopic FGF8 sources were provided by bath application (Figure 8B) or by implanting FGF8-coated beads into the rostromedial pallium (Figure 8D) where the extension of Fgf8 expression was observed in Rfx3−/− embryos. We followed the distribution of guidepost neurons 2–3 days later by immunostaining for Calretinin. Remarkably, under both conditions, we observed drastic consequences of ectopic FGF8 on guidepost distribution at the midline in treated explants (n = 10/10 after FGF8 bath application and n = 6/6 with FGF8-coated beads) compared to control (n = 11/11 without FGF8 bath application and n = 5/5 with control-coated beads). The observed phenotypes were similar to what is observed in Rfx3−/− brains. The tangential distribution of Calretinin+ neurons through the cortical MZ was severely reduced and neurons were broadly distributed at the CSB and in cortical layers. No MZ could be clearly distinguished. We also noticed a marked thinning of the commissural plate as it was observed in several Rfx3 mutant mice compared to WT mice (Figure 7B and white arrowheads in Figure S6). These results support the hypothesis that FGF8 controls guidepost neuronal distribution at the CSB. Hence, in Rfx3−/− brains, the observed increase in Fgf8 expression can be indirectly responsible at early stages for disturbed guidepost neuron distribution by acting on CSB patterning, but also directly responsible at later stages for the positioning of guidepost neurons at the CSB. Therefore, loss of CC in Rfx3−/− mice likely results from the perturbed processing of GLI3 which controls Fgf8 expression early during development.
Fig. 8. Fgf8 ectopic expression causes severe guidepost neurons mislocalization at the midline. 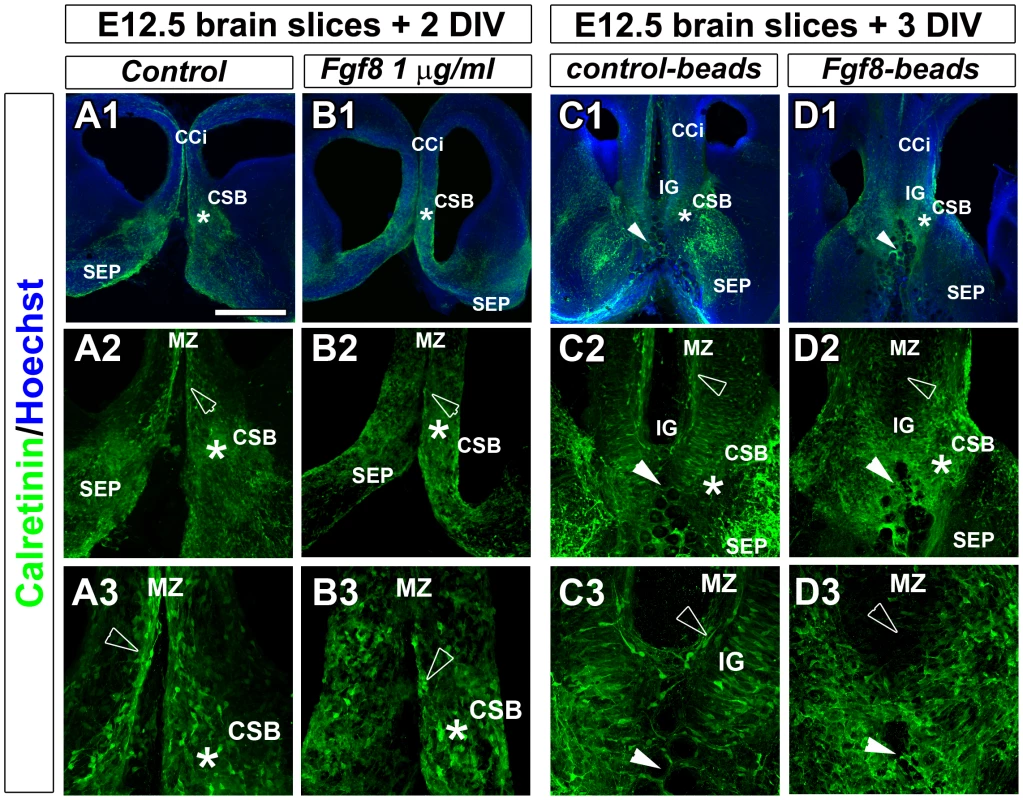
Immunohistochemical staining for calretinin in control (A1–A3, C1–C3) and FGF8-treated (B1–B3, D1–D3) coronal CC organotypic sections. (A2–A3, B2–B3, C2–C3, D1–D3) are higher power views of the CSB region (*) seen in (A1, B1, C1, D1). In control conditions, after BSA bath application (A1–A3) or after implanting BSA-coated beads in the rostromedial pallium (white arrowheads) (C1–C3), neurons labelled with calretinin are properly positioned in cortical layers and at the CSB (*) as it is observed in vivo. By contrast, FGF8 bath application (B1–B3) or FGF8-coated beads implantation in the rostromedial pallium (white arrowheads) (D1–D3) results in the complete disorganization of calretinin-positive neurons that are dispersed in the entire rostromedial pallium. They failed to form a proper structure at the CSB, in the IG and to organize in layers within the cortex. They disappear at the MZ or form aggregates (arrowheads). Bar = 435 µm in A1, B1, C1, D1; 220 µm in A2, B2, C2, D2; 110 µm in A3, B3, C3, D3. Discussion
Rfx3−/− mouse mutant provides a valuable tool for dissecting the relative contributions of early brain patterning mechanisms in the organization of the CSB and in CC formation. We show that Rfx3−/− mice have mild and focused FGF8 over expression that is restricted to the CSB and that severe CC defects coincide with this early patterning anomaly. In turn, this patterning defect is responsible for dramatic changes in the distribution of guidepost neurons but not of glial cells at the corticoseptal midline in Rfx3−/− mice. In addition, our transplantation experiments support the conclusion that proper guidepost neuronal network organization mediated by FGF8 signalling at the midline is essential for proper CC formation.
RFX3 and guidepost cells of the corticoseptal boundary
RFX3 is strongly expressed in corticoseptal guidepost cells from E14.5 to E16.5. However, we did not observe any consequences of Rfx3 loss of function on CC formation by Emx1-Cre or hGFAP-Cre induced Rfx3 inactivation in these cells, suggesting that RFX3 function in guidepost cells is not required for commissural axon formation or that RFX3 function in these cells is masked by a redundant function of other RFX transcription factors. Indeed, 7 RFX proteins are present in the mouse genome and at least one, RFX4, has already been shown to play major functions in brain patterning and to be widely expressed in the telencephalon [49], [50]. However, no precise description of RFX4 expression in guidepost neurons or glial cells have been described and CC callosum defects have not been precisely investigated in RFX4 mutants. Hence, the function of RFX proteins in guidepost cells still remains to be deciphered. Nevertheless, our work shows that RFX3 plays a crucial function in early patterning of the CSB.
Increased FGF8 signalling disturbs distribution of guidepost neurons required for CC formation
We show that RFX3 is required early to restrict FGF8 expression in the telencephalic rostral midline. Our results suggest that a small increase in Fgf8 signalling is sufficient to disorganize the CSB and hence guidepost neuron distribution at early stages of embryonic development. This is supported by in-vivo observations on Rfx3 mutant brains. On the other hand, our ex-vivo explant experiments support the hypothesis that FGF8 also has a direct action on guidepost neuron distribution at later stages. Altogether, these observations show that FGF8 plays a critical function for the distribution of guidepost neurons at the CSB. Previous data in the literature indicated that reducing FGF8 signalling in the telencephalon leads to severe brain phenotypes, and in particular to holoprosencephaly [35], [51], [52] and Fgf8 hypomorphic mutants show corpus callosum defects [51]. Moreover Fgf8 inactivation by Emx1-Cre induced recombination has been shown to induce CC defects at E18.5, suggesting a key role for FGF8 in CC formation [38]. In all these studies, while FGF8 signalling defects were associated with severe alterations of the dorsal signalling centres and possible defects at the CSB, the distribution of guidepost neurons was not examined. In Rfx3−/− mice, unbalanced FGF8 expression in the rostral telencephalon is not associated with alterations of the dorsal signalling centre but is nevertheless sufficient to disturb the distribution of guidepost neurons, leading to the conclusion that FGF8 signalling is primarily responsible for guidepost neuron organization at the CSB and hence CC formation.
Our work also indicates that RFX3 acts on FGF8 signalling by regulating GLI3 activity in the telencephalon. This in agreement with previous works showing that GLI3 acts upstream of FGF8 signalling in the telencephalon. Indeed, in Shh mutants Fgf8 expression is lost, whereas in Gli3 mutants Fgf8 expression is expanded [42]–[44]. Moreover, in Shh; Gli3 double mutants, Fgf8 expression is also expanded suggesting that this expansion occurs independently of Shh in a Gli3 mutant background [44]. Last, loss of Gli3 rescues ventral patterning defects in Shh mutants but not in Fgfr1;Fgfr2 mutants, placing FGF signalling downstream of Gli3 [53]. This also explains why the upregulation of Fgf8 expression likely occurs in Rfx3−/− brains despite an observed downregulation of Shh signalling. Our observations and these data are consistent with the conclusion that Rfx3 acts on GLI3 activity and consequently on FGF8 signalling at the rostral telencephalic midline. Interestingly, our work brings a mechanistic interpretation to the observation that Gli3 mutations have been found in human patients suffering from Acrocallosal syndrome [54]. In addition, hypomorphic Gli3Pdn mutant mice show CC defects [55] with a similar but more severe increase in FGF8 expression in the rostral midline [42]. Consistent with our observations, Gli3Pdn mutants also show strong alterations of guidepost neuronal organization (D. Magnani and T. Theil, unpublished).
Ciliary proteins and corpus callosum defects
In Rfx3−/− brains, we observed a reduced processing of GLI3 in its repressor form and a reduction in SHH signalling in agreement with the already described function of RFX3 in ciliogenesis in various cell types. Indeed, the function of cilia in regulating SHH signalling and GLI3 processing has been well documented in various cell types and organs (for review see [39]). Interestingly, RFX proteins have been shown to control consistently the regulation of several IFT components, dynein retrograde motors and many basal body associated proteins such as MKS1 from C. elegans to mammals [17], [56], [57]. Many of these RFX target genes appear to be associated with overall reduced SHH signalling and reduced GLI3 processing when mutated in mouse. These observations suggest that RFX3 indirectly modulates GLI3 activity and SHH signaling in the anterior telencephalon by regulating the levels of several proteins involved in cilia associated transport and biogenesis.
In humans, several syndromes resulting from mutations in genes encoding ciliary proteins are associated with corpus callosum malformation of various severity [15]. Recently, mutations in the Kif7 gene involved in ciliogenesis and GLI3 processing have been found in human patients suffering from acrocallosal syndrome, characterized by Corpus Callosum and digit malformations [58]. Our work provides a first insight into the cellular mechanisms that are responsible for Corpus Callosum defects following GLI3 processing alterations. Our work demonstrates that small alterations in GLI3 processing is correlated with altered patterning of the CSB and aberrant distribution of guidepost neurons in this region and that this is sufficient to induce midline crossing defects of callosal axons. In mouse, only a few ciliary mutants with altered patterning of the telencephalon have been described. The cobblestone hypomorphic mutation of Ift88, and the ftm, and alien mutants all show severe morphological defects of the brain associated with dorsal ventral patterning defects of the telencephalon [40], [41], [59]. All three mutants are associated with an alteration in GLI3 processing, but with a more severe shift in the balance of GLI3 activator and repressor forms than in Rfx3 mutants. However, the CC was not precisely investigated in these mutants, probably because embryos die too early to allow for an analysis of CC development. Another interesting mutant is a Wnt1-Cre induced Kif3A-deleted mouse that shows craniofacial anomalies due to neural crest migration defects and is associated with agenesis of the CC [60]. Neural crest migration is required for the proper patterning of the telencephalon, in particular by acting on FGF8 rostral patterning centre [61], [62]. The patterning of the telencephalon has not been described in Wnt1-cre; Kif3Aflox/flox mice but it is tempting to hypothesize that dysregulation of FGF8 signalling could be sufficient to mis-pattern the commissural plate in this mutant. Hence, the Rfx3−/− mutant represents the first mouse model establishing a link between proper GLI3 processing and the distribution of guidepost neurons at the CSB for CC formation.
In conclusion, the analysis of Rfx3−/− mice provides strong evidence for the important contribution of corticoseptal neuronal populations in CC formation and for the critical function of Fgf8 signalling in CSB patterning at early stages of CC formation. It provides new understanding of the cellular origins of CC defects in human ciliopathies.
Materials and Methods
Animals
All animal research has been conducted according to relevant national and international guidelines. Rfx3-deficient and floxed mice were generated and genotyped as previously described [18]. GAD67-GFP knock-in mice, hGfap-Cre+/−, Ngn2-creERTM+/−, Emx1-cre+/−, Nkx2.1-cre+/− mice used in this work have been previously described [27], [29]–[31].
Histology
Brains were fixed in 4% PFA/PBS at 4°C until experimentation and then dehydrated in graded series of ethanol (25–100%). Brains were transferred into absolute butanol and substituted for paraffin in graded series of butanol/paraffin solutions. Sections of 10 µm were deparaffinized in Methylcyclohexan, rehydrated and stained with Hematoxylin following standard procedures before mounting in Eukitt.
Immunocytochemistry
Brain embryos were dissected and fixed overnight at 4°C in 4% paraformaldehyde (PFA) (Sigma P6148) in 1×PBS (Invitrogen). Brains were cryoprotected in 30% sucrose and cut in coronal 50 µm-thick frozen sections for staining. Mouse monoclonal antibodies were: Nestin (1/600) (BD bioscience). Rat monoclonal antibodies were: L1 (1/200) (Chemicon, Temecula, CA) and CTIP2 (1/500) (Abcam, Cambridge, UK). Rabbit polyclonal antibodies were: calbindin (1/2500) and calretinin (1/2000) (Swant, Bellinzona, Switzerland); CUX1 (1/200) (Santacruz, Heidelberg, Germany); EMX1 (1/250) (gift form A. Trembleau); GFAP (1/500) (DAKO, Carpinteria, CA); GFP (Molecular Probes, Eugene, OR); SATB2 (1/500) (gift from V. Tarabykin); RFX3 (1/100) [63], TBR1 (1/500) (Abcam, Cambridge, UK). Goat polyclonal antibody was Npn-1 (1/50) (R&D System, Minneapolis, Mn). GLAST guinea-pig polyclonal antibody was (1/2000) (Millipore, Billerica, MA). GFP chicken polyclonal antibody was (1/500) (AVES, Oregon, USA).
Fluorescence immunostaining: The primary antibodies were detected with secondary antibodies coupled with Cy or Alexa (Jackson ImmunoResearch and Molecular Probes; respectively). For RFX3 detection, we used an amplification system with secondary anti-rabbit IgG coupled to biotin (1/250) (Jackson Laboratory) and subsequent revelation with Streptavidin Fluoprobes 547 (Interchim) (1/400). Sections were counterstained with Hoechst 33258 (Molecular Probes), mounted on glass slides and covered in Mowiol 4–88 (Calbiochem, Bad Soden, Germany).
In situ hybridization on cryosections
Embryos were fixed overnight at 4°C in 4% PFA in PBS. Embryos were transferred in PBS/30%sucrose, cryoprotected in PBS/15% Sucrose (Sigma S0389)/7.5% Gelatin (Merck 4078) and frozen at −50°C. Cryosections of 20 µm were collected, thaw-mounted on polylysin coated slides (Miom France). Hybridization was performed as previously described (Niquille et al., 2009). Rfx3 mRNA probe was previously described [18].
In situ hybridization combined with immunocytochemistry
Embryonic brains were treated as described for immunocytochemistry procedure and coronal 100 µm-thick sections were cut using a vibratome (Leica Microsystems). 100 µm free-floating vibratome sections were hybridized with digoxigenin-labeled cRNA probe as described before [64]. To combine in situ hybridization with immunocytochemistry, fast Red (Roche) was used as an alkaline phosphatase fluorescent substrate instead of NBT/BCIP solution. Slides were incubated in Fast Red (Roche) until the appearance of staining in dark chamber at RT. Thereafter, sections were fixed for 15 min in 4% PAF and immunostaining was performed.
Imaging
Fluorescent-immunostained sections were imaged using confocal microscope (Zeiss LSM 510 Meta, Leica SP5 or Zeiss Qasar 710) equipped with 10×, 20×, 40×oil Plan-NEOFLUAR and 63×oil, 100×oil Plan-Apochromat objectives. Fluorophore excitation and scanning were done with an Argon laser 458, 488, 514 nm (blue excitation for GFP and Alexa488), with a HeNe laser 543 nm (green excitation for Alexa 594, CY3 and DiI), with a HeNe laser 633 nm (excitation for Alexa 647 and CY5) and a Diode laser 405 nm (for Hoechst staining). Z-stacks of 10–15 plans were acquired for each CC coronal section in a multitrack mode avoiding crosstalk.
Images processing: all 3D Z stack reconstructions and image processing were performed with Imaris 6.0 software. To create real 3D data sets we used the mode “Surpass”. The colocalization between two fluorochromes was calculated and visualized by creating a yellow channel. Figures were processed in Adobe Photoshop CS2.
Western blot
Western blots were performed following standard procedures. Gli3 6F5 mouse monoclonal antibody was kindly provided by S. Scales [65]. Gli3XT and WT body samples were kindly provided by M. Willaredt and S. Schneider-Maunoury.
Slice culture experiments
We have used our previously developed in vitro model of CC organotypic slices [11]. Embryos were placed in ice cold dissecting medium (MEM Gibco ref 11012-044 with 15 mM glucose and 10 mM Tris pH 7–9). Brains were removed and embedded in 3% low-melting point agarose (In vitrogen). 250 µm thick coronal sections were then cut using a vibrotome filled with cold dissecting medium and slices at the level of the CC were collected in the same medium. CC slices were cultured on Millicell membranes in tissue dishes containing 1 ml of BME/HBSS (Invitrogen) supplemented with glutamine, 5% horse serum, and Pen/Strep [54]. In our slice assay, as in vivo, the callosal axons from dorso-lateral neocortex develop later and their growth cones enter after E16.5 the CC region in successive streams over a period of several days. Our slice assay performed at E16.5 allowed us to study: (1) the function of CSB cells, (2) the outgrowth properties of the majority of callosal axons that are growing through the CC after E16.5 and (3) the effects of transplantations on callosal axon navigations.
The transplantation assay was performed at E16.5 as previously described [11] to analyze the growth of either WT or Rfx3−/− DiI-labelled callosal axons within midline structures of WT or Rfx3−/− slices. Small explants of E16.5 WT or Rfx3−/− midline structure comprising were excised using tungsten needles and transplanted into the midline of WT or Rfx3−/− host slices. After incubation for 48 hours, the slices were fixed and axon trajectories through the various regions were analysed by confocal analysis. For FGF8 bath application: slices of E12.5 brains were cultured as above but FGF8 (FGF-8b isoform, R&D Systems, ref 423-F8) or BSA (control) was added in the culture medium at 1 µg/ml in PBS 1×. After two days of incubation, slices were processed for immunohistochemistry as described above. For FGF8-coated beads experiments: slices of E12.5 brains were cultured as above but FGF8-coated beads or control-coated beads were implanted in the rotromedial pallium. After three days of incubation, slices were processed for immunohistochemistry as described above. To make FGF8-soaked beads, 45 µm polystyrene beads (Polysciences) were rinsed in PBS, the beads were pelleted by 5-min centrifugation at 13,000 rpm and the supernatant was removed. They were incubated in 5 mg/ml heparin for 1 hour at room temperature, rinsed and then incubated with 250 µg/ml mouse FGF8b (R&D Systems) in 0.5% bovine serum albumin (BSA) in PBS overnight at 4°C. Control beads were incubated in 0.5% BSA in PBS. Before implantation, beads were rinsed three times for 10 minutes in PBS.
Atlas and nomenclature
The nomenclature for callosal development is based on the “Atlas of the prenatal mouse brain” [66].
Supporting Information
Zdroje
1. RichardsLJPlachezCRenT 2004 Mechanisms regulating the development of the corpus callosum and its agenesis in mouse and human. Clin Genet 66 276 289
2. PaulLKBrownWSAdolphsRTyszkaJMRichardsLJ 2007 Agenesis of the corpus callosum: genetic, developmental and functional aspects of connectivity. Nat Rev Neurosci 8 287 299
3. OzakiHSWahlstenD 1998 Timing and origin of the first cortical axons to project through the corpus callosum and the subsequent emergence of callosal projection cells in mouse. J Comp Neurol 400 197 206
4. RashBGRichardsLJ 2001 A role for cingulate pioneering axons in the development of the corpus callosum. J Comp Neurol 434 147 157
5. SilverJEdwardsMALevittP 1993 Immunocytochemical demonstration of early appearing astroglial structures that form boundaries and pathways along axon tracts in the fetal brain. J Comp Neurol 328 415 436
6. ShuTPucheACRichardsLJ 2003 Development of midline glial populations at the corticoseptal boundary. J Neurobiol 57 81 94
7. ShuTRichardsLJ 2001 Cortical axon guidance by the glial wedge during the development of the corpus callosum. J Neurosci 21 2749 2758
8. ShuTSundaresanVMcCarthyMMRichardsLJ 2003 Slit2 guides both precrossing and postcrossing callosal axons at the midline in vivo. J Neurosci 23 8176 8184
9. SilverJOgawaMY 1983 Postnatally induced formation of the corpus callosum in acallosal mice on glia-coated cellulose bridges. Science 220 1067 1069
10. SmithKMOhkuboYMaragnoliMERasinMRSchwartzML 2006 Midline radial glia translocation and corpus callosum formation require FGF signaling. Nat Neurosci 9 787 797
11. NiquilleMGarelSMannFHornungJPOtsmaneB 2009 Transient neuronal populations are required to guide callosal axons: a role for semaphorin 3C. PLoS Biol 7 e1000230
12. Jovanov-MilosevicNPetanjekZPetrovicDJudasMKostovicI 2010 Morphology, molecular phenotypes and distribution of neurons in developing human corpus callosum. Eur J Neurosci 32 1423 1432
13. KamnasaranD 2005 Agenesis of the corpus callosum: lessons from humans and mice. Clin Invest Med 28 267 282
14. EngleEC 2010 Human genetic disorders of axon guidance. Cold Spring Harb Perspect Biol 2 a001784
15. BadanoJLMitsumaNBealesPLKatsanisN 2006 The ciliopathies: an emerging class of human genetic disorders. Annu Rev Genomics Hum Genet 7 125 148
16. TobinJLBealesPL 2009 The nonmotile ciliopathies. Genet Med 11 386 402
17. ThomasJMorleLSoulavieFLaurenconASagnolS 2010 Transcriptional control of genes involved in ciliogenesis: a first step in making cilia. Biol Cell 102 499 513
18. BonnafeEToukaMAitLounisABaasDBarrasE 2004 The transcription factor RFX3 directs nodal cilium development and left-right asymmetry specification. Mol Cell Biol 24 4417 4427
19. BaasDMeinielABenadibaCBonnafeEMeinielO 2006 A deficiency in RFX3 causes hydrocephalus associated with abnormal differentiation of ependymal cells. Eur J Neurosci 24 1020 1030
20. AlcamoEAChirivellaLDautzenbergMDobrevaGFarinasI 2008 Satb2 regulates callosal projection neuron identity in the developing cerebral cortex. Neuron 57 364 377
21. BritanovaOde Juan RomeroCCheungAKwanKYSchwarkM 2008 Satb2 is a postmitotic determinant for upper-layer neuron specification in the neocortex. Neuron 57 378 392
22. FameRMMacdonaldJLMacklisJD 2010 Development, specification, and diversity of callosal projection neurons. Trends Neurosci 34 41 50
23. IvyGOAkersRMKillackeyHP 1979 Differential distribution of callosal projection neurons in the neonatal and adult rat. Brain Res 173 532 537
24. LeoneDPSrinivasanKChenBAlcamoEMcConnellSK 2008 The determination of projection neuron identity in the developing cerebral cortex. Curr Opin Neurobiol 18 28 35
25. MolyneauxBJArlottaPFameRMMacDonaldJLMacQuarrieKL 2009 Novel subtype-specific genes identify distinct subpopulations of callosal projection neurons. J Neurosci 29 12343 12354
26. YorkeCHJrCavinessVSJr 1975 Interhemispheric neocortical connections of the corpus callosum in the normal mouse: a study based on anterograde and retrograde methods. J Comp Neurol 164 233 245
27. FodeCMaQCasarosaSAngSLAndersonDJ 2000 A role for neural determination genes in specifying the dorsoventral identity of telencephalic neurons. Genes Dev 14 67 80
28. SchuurmansCArmantONietoMStenmanJMBritzO 2004 Sequential phases of cortical specification involve Neurogenin-dependent and -independent pathways. EMBO J 23 2892 2902
29. ZhuoLTheisMAlvarez-MayaIBrennerMWilleckeK 2001 hGFAP-cre transgenic mice for manipulation of glial and neuronal function in vivo. Genesis 31 85 94
30. GorskiJATalleyTQiuMPuellesLRubensteinJL 2002 Cortical excitatory neurons and glia, but not GABAergic neurons, are produced in the Emx1-expressing lineage. J Neurosci 22 6309 6314
31. XuQTamMAndersonSA 2008 Fate mapping Nkx2.1-lineage cells in the mouse telencephalon. J Comp Neurol 506 16 29
32. HebertJMFishellG 2008 The genetics of early telencephalon patterning: some assembly required. Nat Rev Neurosci 9 678 85
33. FurutaYPistonDWHoganBL 1997 Bone morphogenetic proteins (BMPs) as regulators of dorsal forebrain development. Development 124 2203 2212
34. GroveEAToleSLimonJYipLRagsdaleCW 1998 The hem of the embryonic cerebral cortex is defined by the expression of multiple Wnt genes and is compromised in Gli3-deficient mice. Development 125 2315 2325
35. StormEEGarelSBorelloUHebertJMMartinezS 2006 Dose-dependent functions of Fgf8 in regulating telencephalic patterning centers. Development 133 1831 1844
36. MinowadaGJarvisLAChiCLNeubuserASunX 1999 Vertebrate Sprouty genes are induced by FGF signaling and can cause chondrodysplasia when overexpressed. Development 126 4465 4475
37. MasonIChambersDShamimHWalsheJIrvingC 2000 Regulation and function of FGF8 in patterning of midbrain and anterior hindbrain. Biochem Cell Biol 78 577 584
38. MoldrichRXGobiusIPollakTZhangJRenT 2010 Molecular regulation of the developing commissural plate. J Comp Neurol 518 3645 3661
39. GoetzSCAndersonKV 2010 The primary cilium: a signalling centre during vertebrate development. Nat Rev Genet 11 331 344
40. WillaredtMAHasenpusch-TheilKGardnerHAKitanovicIHirschfeld-WarnekenVC 2008 A crucial role for primary cilia in cortical morphogenesis. J Neurosci 28 12887 12900
41. StottmannRWTranPVTurbe-DoanABeierDR 2009 Ttc21b is required to restrict sonic hedgehog activity in the developing mouse forebrain. Dev Biol 335 166 178
42. KuschelSRutherUTheilT 2003 A disrupted balance between Bmp/Wnt and Fgf signaling underlies the ventralization of the Gli3 mutant telencephalon. Dev Biol 260 484 495
43. TheilTAlvarez-BoladoGWalterARutherU 1999 Gli3 is required for Emx gene expression during dorsal telencephalon development. Development 126 3561 3571
44. AotoKNishimuraTEtoKMotoyamaJ 2002 Mouse GLI3 regulates Fgf8 expression and apoptosis in the developing neural tube, face, and limb bud. Dev Biol 251 320 332
45. MaySRAshiqueAMKarlenMWangBShenY 2005 Loss of the retrograde motor for IFT disrupts localization of Smo to cilia and prevents the expression of both activator and repressor functions of Gli. Dev Biol 287 378 389
46. DaiPAkimaruHTanakaYMaekawaTNakafukuM 1999 Sonic Hedgehog-induced activation of the Gli1 promoter is mediated by GLI3. J Biol Chem 274 8143 8152
47. SasakiHNishizakiYHuiCNakafukuMKondohH 1999 Regulation of Gli2 and Gli3 activities by an amino-terminal repression domain: implication of Gli2 and Gli3 as primary mediators of Shh signaling. Development 126 3915 3924
48. WangBFallonJFBeachyPA 2000 Hedgehog-regulated processing of Gli3 produces an anterior/posterior repressor gradient in the developing vertebrate limb. Cell 100 423 434
49. BlackshearPJGravesJPStumpoDJCobosIRubensteinJL 2003 Graded phenotypic response to partial and complete deficiency of a brain-specific transcript variant of the winged helix transcription factor RFX4. Development 130 4539 4552
50. ZarbalisKMaySRShenYEkkerMRubensteinJL 2004 A focused and efficient genetic screening strategy in the mouse: identification of mutations that disrupt cortical development. PLoS Biol 2 E219
51. GarelSHuffmanKJRubensteinJL 2003 Molecular regionalization of the neocortex is disrupted in Fgf8 hypomorphic mutants. Development 130 1903 1914
52. RosenfeldJABallifBCMartinDMAylsworthASBejjaniBA 2010 Clinical characterization of individuals with deletions of genes in holoprosencephaly pathways by aCGH refines the phenotypic spectrum of HPE. Hum Genet 27 421 440
53. RalluMMacholdRGaianoNCorbinJGMcMahonAP 2002 Dorsoventral patterning is established in the telencephalon of mutants lacking both Gli3 and Hedgehog signaling. Development 129 4963 4974
54. ElsonEPerveenRDonnaiDWallSBlackGC 2002 De novo GLI3 mutation in acrocallosal syndrome: broadening the phenotypic spectrum of GLI3 defects and overlap with murine models. J Med Genet 39 804 806
55. NaruseIKatoKAsanoTSuzukiFKameyamaY 1990 Developmental brain abnormalities accompanied with the retarded production of S-100 beta protein in genetic polydactyly mice. Brain Res Dev Brain Res 51 253 258
56. PiaseckiBPBurghoornJSwobodaP 2010 Regulatory Factor X (RFX)-mediated transcriptional rewiring of ciliary genes in animals. Proc Natl Acad Sci U S A 107 12969 12974
57. ChuJSBaillieDLChenN 2010 Convergent evolution of RFX transcription factors and ciliary genes predated the origin of metazoans. BMC Evol Biol 10 130
58. PutouxAThomasSCoeneKLDavisEEAlanayY 2011 KIF7 mutations cause fetal hydrolethalus and acrocallosal syndromes. Nat Genet 43 601 606
59. VierkottenJDildropRPetersTWangBRutherU 2007 Ftm is a novel basal body protein of cilia involved in Shh signalling. Development 134 2569 2577
60. BrugmannSAAllenNCJamesAWMekonnenZMadanE 2010 A primary cilia-dependent etiology for midline facial disorders. Hum Mol Genet 19 1577 1592
61. CreuzetSEMartinezSLe DouarinNM 2006 The cephalic neural crest exerts a critical effect on forebrain and midbrain development. Proc Natl Acad Sci U S A 103 14033 14038
62. CreuzetSE 2009 Neural crest contribution to forebrain development. Semin Cell Dev Biol 20 751 759
63. ReithWUclaCBarrasEGaudADurandB 1994 RFX1, a transactivator of hepatitis B virus enhancer I, belongs to a novel family of homodimeric and heterodimeric DNA-binding proteins. Mol Cell Biol 14 1230 1244
64. GarelSMarinFMatteiMGVesqueCVincentA 1997 Family of Ebf/Olf-1-related genes potentially involved in neuronal differentiation and regional specification in the central nervous system. DevDyn 210 191 205
65. WenXLaiCKEvangelistaMHongoJAde SauvageFJ 2010 Kinetics of hedgehog-dependent full-length Gli3 accumulation in primary cilia and subsequent degradation. Mol Cell Biol 30 1910 1922
66. SchambraUBLauderJMSilverJ 1992 Atlas of the prenatal mouse brain: Academic Press Inc., 327 p.
Štítky
Genetika Reprodukční medicína
Článek Physiological Notch Signaling Maintains Bone Homeostasis via RBPjk and Hey Upstream of NFATc1Článek Intronic -Regulatory Modules Mediate Tissue-Specific and Microbial Control of / TranscriptionČlánek Probing the Informational and Regulatory Plasticity of a Transcription Factor DNA–Binding DomainČlánek Repression of Germline RNAi Pathways in Somatic Cells by Retinoblastoma Pathway Chromatin ComplexesČlánek An Alu Element–Associated Hypermethylation Variant of the Gene Is Associated with Childhood ObesityČlánek Three Essential Ribonucleases—RNase Y, J1, and III—Control the Abundance of a Majority of mRNAsČlánek Genomic Tools for Evolution and Conservation in the Chimpanzee: Is a Genetically Distinct Population
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 3
-
Všechny články tohoto čísla
- Comprehensive Research Synopsis and Systematic Meta-Analyses in Parkinson's Disease Genetics: The PDGene Database
- Genomic Analysis of the Hydrocarbon-Producing, Cellulolytic, Endophytic Fungus
- Networks of Neuronal Genes Affected by Common and Rare Variants in Autism Spectrum Disorders
- Akirin Links Twist-Regulated Transcription with the Brahma Chromatin Remodeling Complex during Embryogenesis
- Too Much Cleavage of Cyclin E Promotes Breast Tumorigenesis
- Imprinted Genes … and the Number Is?
- Genetic Architecture of Highly Complex Chemical Resistance Traits across Four Yeast Strains
- Exploring the Complexity of the HIV-1 Fitness Landscape
- MNS1 Is Essential for Spermiogenesis and Motile Ciliary Functions in Mice
- A Fundamental Regulatory Mechanism Operating through OmpR and DNA Topology Controls Expression of Pathogenicity Islands SPI-1 and SPI-2
- Evidence for Positive Selection on a Number of MicroRNA Regulatory Interactions during Recent Human Evolution
- Variation in Modifies Risk of Neonatal Intestinal Obstruction in Cystic Fibrosis
- PIF4–Mediated Activation of Expression Integrates Temperature into the Auxin Pathway in Regulating Hypocotyl Growth
- Critical Evaluation of Imprinted Gene Expression by RNA–Seq: A New Perspective
- A Meta-Analysis and Genome-Wide Association Study of Platelet Count and Mean Platelet Volume in African Americans
- Mouse Genetics Suggests Cell-Context Dependency for Myc-Regulated Metabolic Enzymes during Tumorigenesis
- Transcriptional Control in Cardiac Progenitors: Tbx1 Interacts with the BAF Chromatin Remodeling Complex and Regulates
- Synthetic Lethality of Cohesins with PARPs and Replication Fork Mediators
- APOBEC3G-Induced Hypermutation of Human Immunodeficiency Virus Type-1 Is Typically a Discrete “All or Nothing” Phenomenon
- Interpreting Meta-Analyses of Genome-Wide Association Studies
- Error-Prone ZW Pairing and No Evidence for Meiotic Sex Chromosome Inactivation in the Chicken Germ Line
- -Dependent Chemosensory Functions Contribute to Courtship Behavior in
- Diverse Forms of Splicing Are Part of an Evolving Autoregulatory Circuit
- Phenotypic Plasticity of the Drosophila Transcriptome
- Physiological Notch Signaling Maintains Bone Homeostasis via RBPjk and Hey Upstream of NFATc1
- Precocious Metamorphosis in the Juvenile Hormone–Deficient Mutant of the Silkworm,
- Igf1r Signaling Is Indispensable for Preimplantation Development and Is Activated via a Novel Function of E-cadherin
- Accurate Prediction of Inducible Transcription Factor Binding Intensities In Vivo
- Mitochondrial Oxidative Stress Alters a Pathway in Strongly Resembling That of Bile Acid Biosynthesis and Secretion in Vertebrates
- Mammalian Neurogenesis Requires Treacle-Plk1 for Precise Control of Spindle Orientation, Mitotic Progression, and Maintenance of Neural Progenitor Cells
- Tcf7 Is an Important Regulator of the Switch of Self-Renewal and Differentiation in a Multipotential Hematopoietic Cell Line
- REST–Mediated Recruitment of Polycomb Repressor Complexes in Mammalian Cells
- Intronic -Regulatory Modules Mediate Tissue-Specific and Microbial Control of / Transcription
- Age-Dependent Brain Gene Expression and Copy Number Anomalies in Autism Suggest Distinct Pathological Processes at Young Versus Mature Ages
- A Genome-Wide Association Study Identifies Variants Underlying the Shade Avoidance Response
- -by- Regulatory Divergence Causes the Asymmetric Lethal Effects of an Ancestral Hybrid Incompatibility Gene
- Genome-Wide Association and Functional Follow-Up Reveals New Loci for Kidney Function
- A Natural System of Chromosome Transfer in
- Cell Size and the Initiation of DNA Replication in Bacteria
- Probing the Informational and Regulatory Plasticity of a Transcription Factor DNA–Binding Domain
- Repression of Germline RNAi Pathways in Somatic Cells by Retinoblastoma Pathway Chromatin Complexes
- Temporal Transcriptional Profiling of Somatic and Germ Cells Reveals Biased Lineage Priming of Sexual Fate in the Fetal Mouse Gonad
- Rapid Analysis of Genome Rearrangements by Multiplex Ligation–Dependent Probe Amplification
- Metabolic Profiling of a Mapping Population Exposes New Insights in the Regulation of Seed Metabolism and Seed, Fruit, and Plant Relations
- The Atypical Calpains: Evolutionary Analyses and Roles in Cellular Degeneration
- The Silkworm Coming of Age—Early
- Development of a Panel of Genome-Wide Ancestry Informative Markers to Study Admixture Throughout the Americas
- Balanced Codon Usage Optimizes Eukaryotic Translational Efficiency
- The Min System and Nucleoid Occlusion Are Not Required for Identifying the Division Site in but Ensure Its Efficient Utilization
- Neurobeachin, a Regulator of Synaptic Protein Targeting, Is Associated with Body Fat Mass and Feeding Behavior in Mice and Body-Mass Index in Humans
- Statistical Analysis of Readthrough Levels for Nonsense Mutations in Mammalian Cells Reveals a Major Determinant of Response to Gentamicin
- Gene Reactivation by 5-Aza-2′-Deoxycytidine–Induced Demethylation Requires SRCAP–Mediated H2A.Z Insertion to Establish Nucleosome Depleted Regions
- The miR-35-41 Family of MicroRNAs Regulates RNAi Sensitivity in
- Genetic Basis of Hidden Phenotypic Variation Revealed by Increased Translational Readthrough in Yeast
- An Alu Element–Associated Hypermethylation Variant of the Gene Is Associated with Childhood Obesity
- Modelling Human Regulatory Variation in Mouse: Finding the Function in Genome-Wide Association Studies and Whole-Genome Sequencing
- Novel Loci for Adiponectin Levels and Their Influence on Type 2 Diabetes and Metabolic Traits: A Multi-Ethnic Meta-Analysis of 45,891 Individuals
- Polycomb-Like 3 Promotes Polycomb Repressive Complex 2 Binding to CpG Islands and Embryonic Stem Cell Self-Renewal
- Insulin/IGF-1 and Hypoxia Signaling Act in Concert to Regulate Iron Homeostasis in
- EMF1 and PRC2 Cooperate to Repress Key Regulators of Arabidopsis Development
- Three Essential Ribonucleases—RNase Y, J1, and III—Control the Abundance of a Majority of mRNAs
- Contrasted Patterns of Molecular Evolution in Dominant and Recessive Self-Incompatibility Haplotypes in
- A Machine Learning Approach for Identifying Novel Cell Type–Specific Transcriptional Regulators of Myogenesis
- Genomic Tools for Evolution and Conservation in the Chimpanzee: Is a Genetically Distinct Population
- Nos2 Inactivation Promotes the Development of Medulloblastoma in Mice by Deregulation of Gap43–Dependent Granule Cell Precursor Migration
- Intracranial Aneurysm Risk Locus 5q23.2 Is Associated with Elevated Systolic Blood Pressure
- Heritability and Genetic Correlations Explained by Common SNPs for Metabolic Syndrome Traits
- A Genome-Wide Association Study of Nephrolithiasis in the Japanese Population Identifies Novel Susceptible Loci at 5q35.3, 7p14.3, and 13q14.1
- DNA Damage in Nijmegen Breakage Syndrome Cells Leads to PARP Hyperactivation and Increased Oxidative Stress
- DNA Resection at Chromosome Breaks Promotes Genome Stability by Constraining Non-Allelic Homologous Recombination
- Genetic Analysis of Floral Symmetry in Van Gogh's Sunflowers Reveals Independent Recruitment of Genes in the Asteraceae
- A Splice Site Variant in the Bovine Gene Compromises Growth and Regulation of the Inflammatory Response
- Promoter Nucleosome Organization Shapes the Evolution of Gene Expression
- The Nucleoside Diphosphate Kinase Gene Acts as Quantitative Trait Locus Promoting Non-Mendelian Inheritance
- The Ciliogenic Transcription Factor RFX3 Regulates Early Midline Distribution of Guidepost Neurons Required for Corpus Callosum Development
- Phosphorylation of the RNA–Binding Protein HOW by MAPK/ERK Enhances Its Dimerization and Activity
- A Genome-Wide Scan of Ashkenazi Jewish Crohn's Disease Suggests Novel Susceptibility Loci
- Parkinson's Disease–Associated Kinase PINK1 Regulates Miro Protein Level and Axonal Transport of Mitochondria
- LMW-E/CDK2 Deregulates Acinar Morphogenesis, Induces Tumorigenesis, and Associates with the Activated b-Raf-ERK1/2-mTOR Pathway in Breast Cancer Patients
- Mapping the Hsp90 Genetic Interaction Network in Reveals Environmental Contingency and Rewired Circuitry
- Autoregulation of the Noncoding RNA Gene
- The Human Pancreatic Islet Transcriptome: Expression of Candidate Genes for Type 1 Diabetes and the Impact of Pro-Inflammatory Cytokines
- Spo0A∼P Imposes a Temporal Gate for the Bimodal Expression of Competence in
- Antagonistic Regulation of Apoptosis and Differentiation by the Cut Transcription Factor Represents a Tumor-Suppressing Mechanism in
- A Downstream CpG Island Controls Transcript Initiation and Elongation and the Methylation State of the Imprinted Macro ncRNA Promoter
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- PIF4–Mediated Activation of Expression Integrates Temperature into the Auxin Pathway in Regulating Hypocotyl Growth
- Metabolic Profiling of a Mapping Population Exposes New Insights in the Regulation of Seed Metabolism and Seed, Fruit, and Plant Relations
- A Splice Site Variant in the Bovine Gene Compromises Growth and Regulation of the Inflammatory Response
- Comprehensive Research Synopsis and Systematic Meta-Analyses in Parkinson's Disease Genetics: The PDGene Database
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



