-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaNovel Loci for Adiponectin Levels and Their Influence on Type 2 Diabetes and Metabolic Traits: A Multi-Ethnic Meta-Analysis of 45,891 Individuals
Circulating levels of adiponectin, a hormone produced predominantly by adipocytes, are highly heritable and are inversely associated with type 2 diabetes mellitus (T2D) and other metabolic traits. We conducted a meta-analysis of genome-wide association studies in 39,883 individuals of European ancestry to identify genes associated with metabolic disease. We identified 8 novel loci associated with adiponectin levels and confirmed 2 previously reported loci (P = 4.5×10−8–1.2×10−43). Using a novel method to combine data across ethnicities (N = 4,232 African Americans, N = 1,776 Asians, and N = 29,347 Europeans), we identified two additional novel loci. Expression analyses of 436 human adipocyte samples revealed that mRNA levels of 18 genes at candidate regions were associated with adiponectin concentrations after accounting for multiple testing (p<3×10−4). We next developed a multi-SNP genotypic risk score to test the association of adiponectin decreasing risk alleles on metabolic traits and diseases using consortia-level meta-analytic data. This risk score was associated with increased risk of T2D (p = 4.3×10−3, n = 22,044), increased triglycerides (p = 2.6×10−14, n = 93,440), increased waist-to-hip ratio (p = 1.8×10−5, n = 77,167), increased glucose two hours post oral glucose tolerance testing (p = 4.4×10−3, n = 15,234), increased fasting insulin (p = 0.015, n = 48,238), but with lower in HDL-cholesterol concentrations (p = 4.5×10−13, n = 96,748) and decreased BMI (p = 1.4×10−4, n = 121,335). These findings identify novel genetic determinants of adiponectin levels, which, taken together, influence risk of T2D and markers of insulin resistance.
Published in the journal: . PLoS Genet 8(3): e32767. doi:10.1371/journal.pgen.1002607
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002607Summary
Circulating levels of adiponectin, a hormone produced predominantly by adipocytes, are highly heritable and are inversely associated with type 2 diabetes mellitus (T2D) and other metabolic traits. We conducted a meta-analysis of genome-wide association studies in 39,883 individuals of European ancestry to identify genes associated with metabolic disease. We identified 8 novel loci associated with adiponectin levels and confirmed 2 previously reported loci (P = 4.5×10−8–1.2×10−43). Using a novel method to combine data across ethnicities (N = 4,232 African Americans, N = 1,776 Asians, and N = 29,347 Europeans), we identified two additional novel loci. Expression analyses of 436 human adipocyte samples revealed that mRNA levels of 18 genes at candidate regions were associated with adiponectin concentrations after accounting for multiple testing (p<3×10−4). We next developed a multi-SNP genotypic risk score to test the association of adiponectin decreasing risk alleles on metabolic traits and diseases using consortia-level meta-analytic data. This risk score was associated with increased risk of T2D (p = 4.3×10−3, n = 22,044), increased triglycerides (p = 2.6×10−14, n = 93,440), increased waist-to-hip ratio (p = 1.8×10−5, n = 77,167), increased glucose two hours post oral glucose tolerance testing (p = 4.4×10−3, n = 15,234), increased fasting insulin (p = 0.015, n = 48,238), but with lower in HDL-cholesterol concentrations (p = 4.5×10−13, n = 96,748) and decreased BMI (p = 1.4×10−4, n = 121,335). These findings identify novel genetic determinants of adiponectin levels, which, taken together, influence risk of T2D and markers of insulin resistance.
Introduction
Adiponectin is a highly abundant adipocyte-derived plasma protein whose levels correlate inversely with a range of important clinical parameters including blood glucose, indices of insulin resistance, proatherogenic dyslipidemia, and risk of type 2 diabetes (T2D), stroke and coronary artery disease [1], [2], [3], [4]. Collectively these conditions account for most of the burgeoning pandemic of obesity-related morbidity and mortality that poses a severe and global healthcare challenge [5]. Murine studies suggest that adiponectin plays a mediating role in at least some of these obesity-related complications, and although less clearly established in humans, this suggests that understanding the pathophysiology of adiponectin may uncover novel therapeutic targets in major, highly prevalent human disease.[6], [7].
Twins and family studies have revealed moderate to high estimates of heritability (30–70%) for plasma adiponectin levels [8], [9], [10], [11]. However, until recently, few genes associated with adiponectin levels have been identified. Candidate and genome-wide association studies (GWAS) have shown pronounced associations between common polymorphisms in the adiponectin gene (ADIPOQ) and adiponectin levels [12], [13], [14], [15]. A recent meta-analysis of three GWAS for adiponectin levels identified variants in a novel candidate gene, ARL15, that were associated with adiponectin levels, coronary heart disease (CHD), T2D and other metabolic traits [16]. Furthermore, CDH13 and KNG1 genes were found to be associated with adiponectin levels in two studies involving East Asian populations [17], [18]. Although part of the variance explained by the ADIPOQ locus, most of the heritability of adiponectin levels remains unaccounted for. Therefore, we sought to identify novel common variants influencing adiponectin levels and test their association with risk of T2D and related metabolic traits within the framework of a large multi-ethnic consortium of GWAS.
We combined genome-wide association results of 35,355 individuals from three different ethnicities (white Europeans (n = 29,347), African American s(n = 4,232) and East Asians (n = 1,776)), applying a novel meta-analytic method to allow for heterogeneity in allelic effects between populations of different ethnic backgrounds. We next examined whether identified genome-wide significant single nucleotide polymorphisms (SNPs) also associated with expression of their nearest gene in human adipocytes, the main source of adiponectin. Since adiponectin has been associated with T2D, insulin resistance and metabolic traits we next investigated whether a multi-SNP genotypic risk, comprising genome-wide significant SNPs for adiponectin levels, also influenced risk of T2D and related traits measured in the DIAbetes Genetics Replication and Meta-analysis (DIAGRAM+) [19], Meta-Analysis of Glucose and Insulin Related Traits Consortium (MAGIC) [20], Genetic Investigation of ANthropometric measures Traits (GIANT) [21] , Global Lipids Genetic Consortium (GLGC) [22], and Body Fat GWAS consortia [23].
Results
Results of Meta-Analysis of GWAS
The meta-analysis was divided into four phases 1) Discovery phase, which involved cohorts providing GWAS results, 2) In-silico replication phase which included additional GWAS cohorts joining our meta-analysis after the completion of the discovery phase, 3) De-novo genotyping in cohorts without GWAS genotyping and 4) Multi-Ethnic meta-analysis applying a novel method for complex trait mapping using different ethnicities.
Discovery phase in individuals of white European origin
The meta-analysis of sex-combined data from 16 GWAS (n = 29,347) of individuals of white European descent identified ten loci associated with adiponectin levels at p≤5.0×10−8 (Table 1 and Figure 1A and Figure S1, Table S2). These results include the previously described associations with adiponectin at ADIPOQ (rs6810075[T]; ß = 0.06, p-value = 3.60×10−41), KNG1 (rs2062632[T]; ß = 0.05, p-value = 2.52×10−19) on 3q27.3, and CDH13 (rs12922394[T; ß = −0.1, p = 3.16×10−18) on 16q23.3 (Table 1). Furthermore, we identified variants that showed genome-wide significant association in eight novel independent loci including rs9853056 (within the STAB1 gene, rs4282054 (within the NT5DC2 gene), rs13083798 (within the PBRM1 gene), rs1108842 (within the GNL3 gene), rs11235 (within the NEK4 gene), rs2710323 (within the ITIH1 gene), rs3617 (within the ITIH3 gene), and rs2535627 (within 200 Kb of ITIH4 gene) at 3p21.1; rs1597466 (within 1 Mb of TSC22D2 gene) at 3q25.1; rs2980879 (within 1 Mb of TRIB1 gene) at 8q24.13; rs7955516 (within 1.3 Mb PDE3A gene) at 12p12.2; rs601339 (within the GPR109A gene) at 12q24.31; rs6488898 (within the ATP6V0A2 gene), rs7133378 (within the DNAH10 gene), rs7305864 (within the CCDC92 gene), and rs7978610 (within the ZNF664 gene at 12q24.31, which is 1.3 Mb away from GPR109A); rs2925979 (within the CMIP at 16q23.2 gene); and rs731839 (within the PEPD gene) at 19q13.11. (Figure 2A–2E, Table 1).
Fig. 1. Manhattan plots for meta-analyses in the discovery phase. 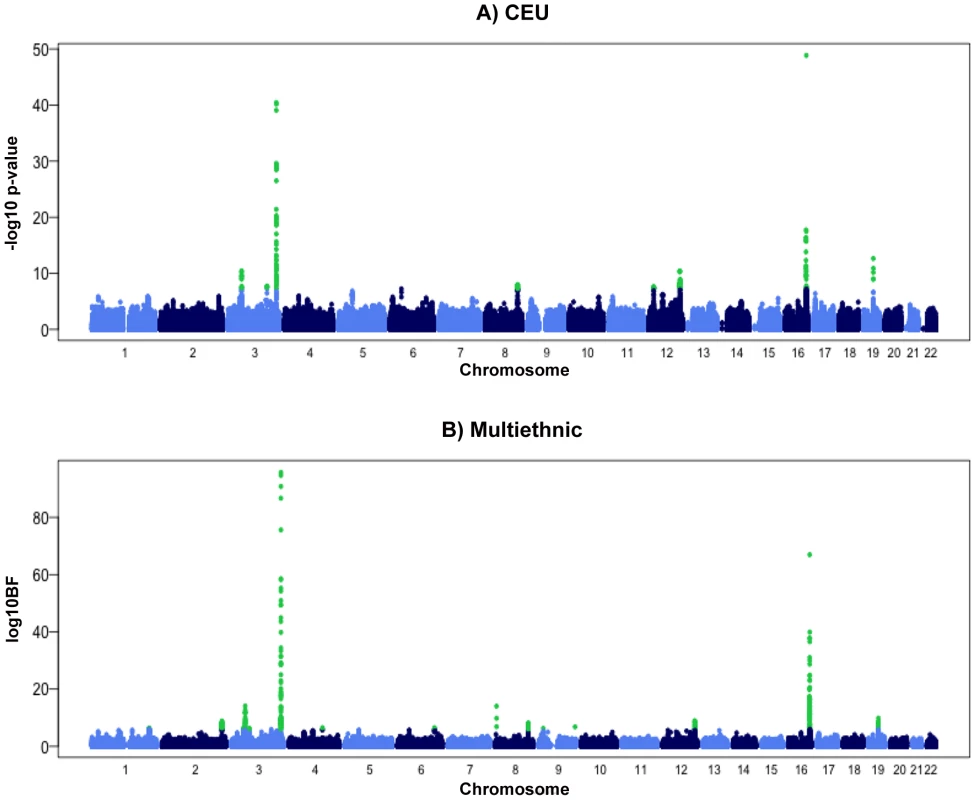
A) Combined sex analysis in European populations, B) Meta-Analysis of Multiple Ethnicities. The Manhattan plots show −Log10 (p-value) measures for association between single nucleotide polymorphisms (SNPs) and chromosomal position. The SNPs that achieved genome-wide significance are highlighted in green. Fig. 2. Regional plots of eight newly discovered genome-wide significant chromosomal regions associated with adiponectin concentrations in European populations. 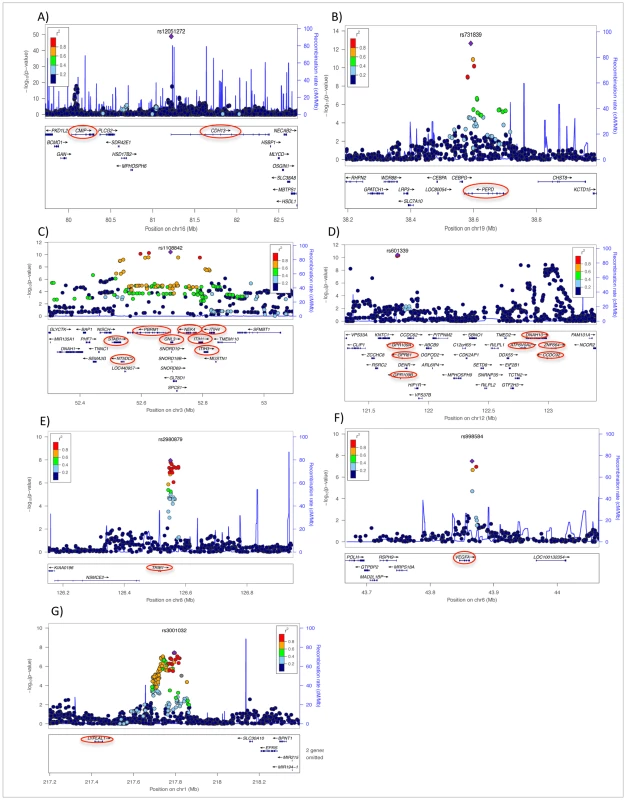
A) chromosome 16q23.2, B) chromosome 19 q13.11 C) Chromosome 3p21.1, D) two loci on chromosome 12q24.31, E) chromosome 8q24.13, F) chromosome 6p21.1, and G) chromosome 1q41. In each panel, purple diamonds indicate the top SNPs, which have the strongest evidence of association. Each circle shows a SNP with a color scale relating the r2 value for that SNP and the top SNP from HapMap CEU. Blue lines indicate estimated recombination rates from HapMap. The bottom panels illustrate the relative position of genes near each locus. Candidate genes are indicated by red ovals. Tab. 1. Lead SNP per Locus for Genome-Wide Significant SNPs Arising from the Sex-Combined Meta-Analysis in European Populations. 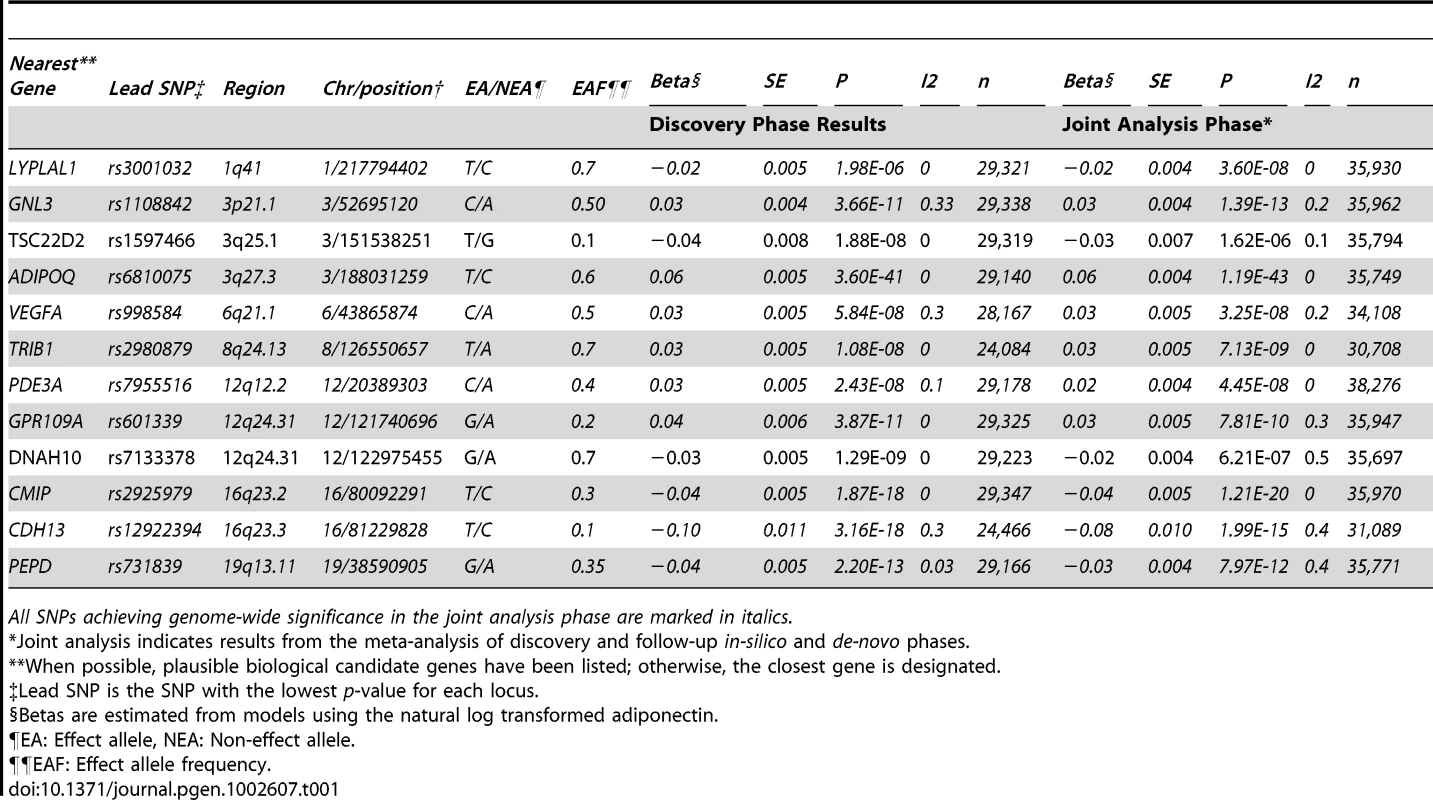
Joint analysis indicates results from the meta-analysis of discovery and follow-up in-silico and de-novo phases. In our analysis a common variant (rs601339, MAF = 0.18, allele G) downstream of the GPR109A gene (the putative niacin receptor) was associated with adiponectin (ß = 0.04, p = 7.94×10−10) and HDL-C (ß = 0.03, p = 5.59×10−7) in the global lipid analysis. In a coincident candidate gene analysis 11 SNPs were typed in GPR109A/B in CoLaus and LOLIPOP cohorts, containing individuals of European descent. A single nominally significant coding SNP R311C (rs7314976, MAF = 0.14) within the GPR109A gene was taken forward for replication and found to be consistently associated with adiponectin in the three cohorts (CoLaus, Fenland and MRC Ely study, n = 8285, p = 4.6×10−8) and HDL-cholesterol (HDL-C) in four cohorts (CoLaus, Fenland, Ely study and Lolipop, n = 18425, p = 2.9×10−8) (Figure S2A, S2B). However R311C and rs601339 were not in linkage disequilibirium with each other (r2 = 0.04). Therefore the two variants represent two independent signals from the same locus but with similar effects on HDL-cholesterol and adiponectin.
In silico follow-up phase
In the in-silico follow-up phase 468 SNPs demonstrating genome-wide significant (p<5×10−8 ) or suggestive (p<5×10−6) association with adiponectin in the discovery phase were tested for association in additional European cohorts. (Table S3). These SNPs were tested in seven additional GWAS datasets (n = 6,623 from NHS, HPFS, HABC, ERF2, LLS, GARP and ARIC studies) and the combined meta-analysis of the discovery and follow-up in-silico GWAS datasets detected additional loci on chromosomes 1q41 near the LYPLAL1 gene (rs3001032, p = 3.6×10−8) and chromosome 6p21.1 near the VEGFA gene (rs998584, p = 5.8×10−12) that reached genome-wide significance. While we confirmed seven loci that had reached genome-wide significance at the discovery stage (Table 1, Figure 2F and 2G, Table S2), two identified loci (3q25.1 and 12q24.31) did not remain genome-wide significant in the joint analysis of discovery and follow-up results.
De novo follow-up phase
Next, in the de-novo genotyping follow-up phase, we genotyped 10 SNPs with suggestive evidence of association (5×10−8<p<5×10−6) from the meta-analysis of the discovery and in-silico follow-up phases in an additional 3,913 individuals. Meta-analyzing the discovery and 2 follow-up stages identified a SNP in ARL15 (rs6450176 [G]; ß = 0.026, p = 5.8×10−8), which was initially described in a previous GWAS for adiponectin levels (Table S3) [16].
Multi-ethnic meta-analysis
To identify loci influencing adiponectin levels in non-European individuals we performed an additional analysis in 4,232 individuals from an African American population and 1,776 individuals from an East Asian population. In the African American populations, only associations at the ADIPOQ locus reached genome-wide significance, while in the East Asian population there was evidence of association at the ADIPOQ and CDH13 loci (Table S4). Subsequently, we performed a meta-analysis that combined all available GWAS including those of white European origin, African American and East Asian ancestry using novel method MANTRA [24]. This analysis identified two additional loci in or near IRS1 gene on 2q36.3 and at 6q24.1 within a gene desert. (Table 2, Figure 1B).
Tab. 2. Genome-Wide Significant SNPs from the Sex-Combined Multi-Ethnic Meta-Analysis. 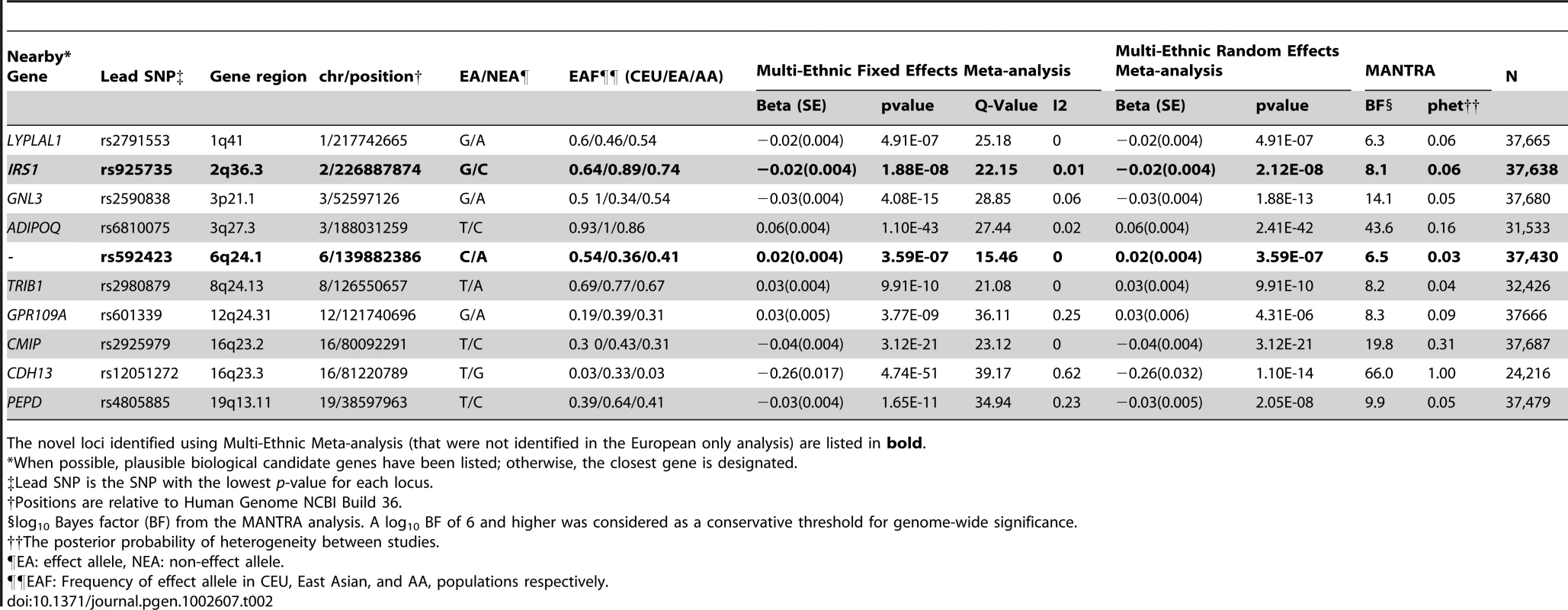
The novel loci identified using Multi-Ethnic Meta-analysis (that were not identified in the European only analysis) are listed in bold. Secondary GWAS analyses
We next performed meta-analysis of the GWAS data in women (n = 16,685) and men (n = 12,662) separately (Figure S2A, S2B, Tables S5 and S6). Although no novel loci reached genome-wide significance in women or men separately, three loci (chromosome 3p, 8 and 12) associated with adiponectin levels in the sex-combined analysis showed evidence of association (p value<5×10−8) in women (Figure S3). Since different assays were used to measure adiponectin levels, we next tested whether stratification by assay rendered similar results and found the results were highly concordant with the combined analysis. GWAS for high molecular weight adiponectin in the CHS study (n = 2,718) identified 2 SNPs in ADIPOQ (rs17300539, p = 3.0×10−16) and CMIP (rs2927307, p = 2.7×10−8). These two genes are located within the loci identified in our discovery meta-analysis of adiponectin levels.
Gene Expression Studies
Through gene expression studies we sought to address two questions: First, are any of the SNPs that were genome-wide significant for adiponectin levels associated with expression of their nearest transcripts (cis-eQTLs) and second, whether mRNA levels of loci identified through the GWAS for adiponectin levels are associated with circulating adiponectin levels. To address the first question, we examined whether SNPs within 1 Mb of the SNPs achieving genome-wide significance in the discovery stage were associated with the expression levels of nearby genes in human adipocytes from 776 participants of the MuTHER Consortium [25]. We identified 74 SNPs in three eQTLs to be associated with the expression of five genes in adipocytes, using an array-wide level of statistical significance for eQTLs (P<5.1×10−5. See Materials and Methods for details). These genes included: NT5DC2 on chromosome 3; CCDC92, GPR109A, and ZNF664 on chromosome12; and PEPD on chromosome 19 (Table 3). The cis-eQTL SNPs often are proxies for the lead SNPs from the GWAS, however, this relationship may also be influenced through mechanisms that are independent from gene expression, such as gene function.
Tab. 3. The Association of Lead Genome-Wide Significant SNPs for Adiponectin with mRNA Levels of Their Nearest Gene. 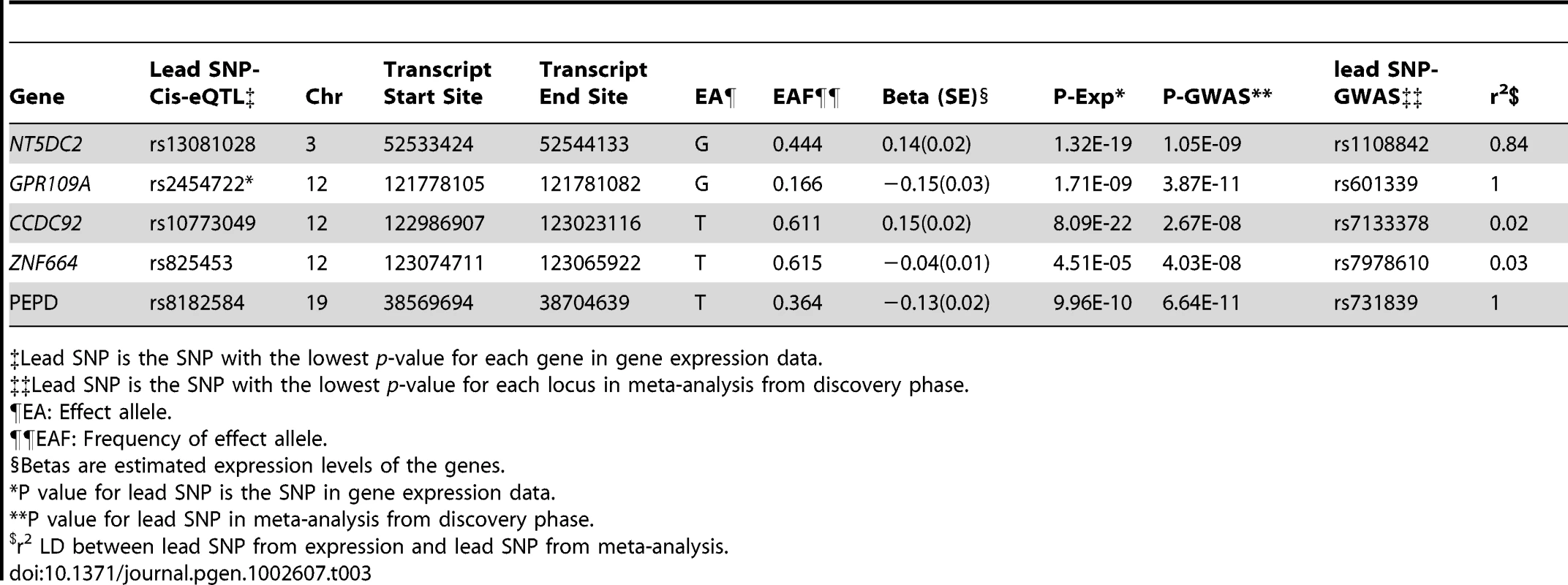
Lead SNP is the SNP with the lowest p-value for each gene in gene expression data. We next identified that mRNA levels of 18 genes arising from six candidate loci were correlated with circulating adiponectin levels (Table 4). Since circulating adiponectin levels may be associated with a surplus of adipocyte transcripts we next tested for enrichment of signal from the candidate loci. There were 133 transcripts in the identified candidate regions, of which 8.2% (11/133) were associated with adiponectin levels at an array-wide level of significance (p<2×10−6), while 7.5% of the 24k probes on the entire array exceeded the same p-value threshold, indicating there was therefore no additional enrichment of signal at these candidate loci.
Tab. 4. The Association of mRNA Levels from Genes in Candidate Loci in Human Adipocytes with Circulating Adiponectin Levels. 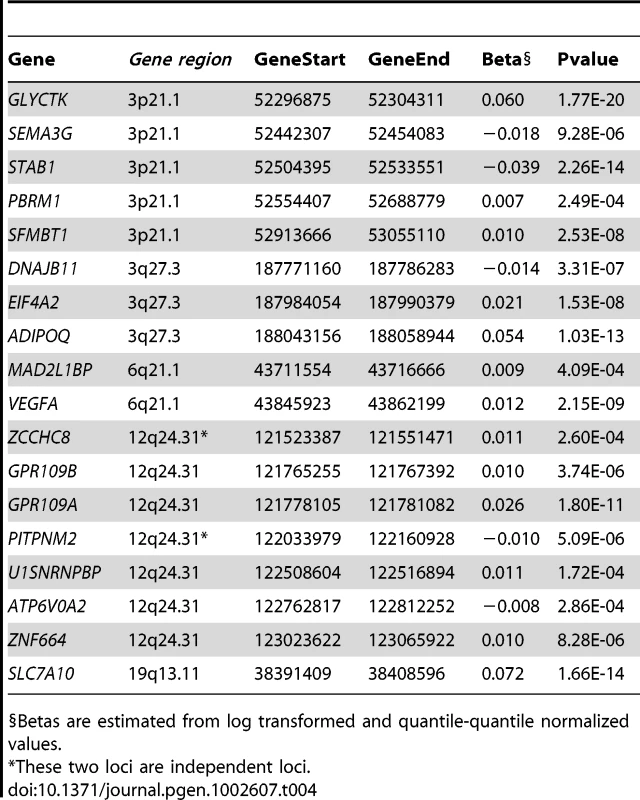
Betas are estimated from log transformed and quantile-quantile normalized values. T2D and Metabolic Traits
Using data from several large-scale GWAS consortia, some of the significantly associated variants identified here demonstrated associations with T2D and its related traits (Table S7A, S7B, S7C, and S7D). Several individual SNPs showed evidence for association with T2D and various metabolic traits after accounting for the number of statistically independent SNPs (p-value threshold of 5×10−4) among the SNPs that were genome-wide significant for adiponectin. These include associations with HDL-C (n = 104 SNPs), triglycerides (TG) (n = 65 SNPs), total cholesterol (TC, n = 12 SNPs), LDL-cholesterol (LDL–C, n = 11 SNPs), and waist-hip ratio (WHR) (n = 65 SNPs) [26]. (However, we note that since sample sizes are different across different consortia power to identify associations is not consistent.) Among these, coding and intronic variants in STAB1 and NT5DC2 genes were associated with WHR and HDL-C, while the variants 1 Mb near TRIB1 were associated with all lipid traits. The coding and intronic variants ariants in the locus on chromosome 12 harboring ZNF664, CCDC92, and DNAH10 showed evidence of association with WHR, HDL-C, and TG. Finally, variants in the PEPD gene were associated with TG.
We next calculated a multi-SNP genotypic risk score based genome-wide significant SNPs from the discovery phase. This multi-SNP genotypic risk score explained 5% of the variance of natural log-transformed adiponectin levels. We then tested the association of this risk score with risk of T2D and metabolic related traits. The multi-SNP genotypic risk score was associated with increased risk for T2D (ß = 0.3, p = 4.3×10−3), where ß is the average additive effect of adiponectin-decreasing risk alleles on the log odds ratio of T2D), increased TG (ß = 0.25, p = 2.6×10−14), increased WHR adjusted for BMI (ß = 0.18, p = 1.8×10−5), increased post-prandial glucose (ß = 0.25, p = 0.01), increased fasting insulin (ß = 0.05, p = 0.01), homeostatic model assessment - insulin resistance (HOMA-IR) (ß = 0.04, p = 0.047), and with lower HDL-C concentrations (ß = −0.24, p = 4.5×10−13) and decreased BMI (ß = −0.16, p = 1.4×10−4). (Table 5).
Tab. 5. Results of Association of Multi-SNP Genotypic Risk Score with Diabetes and Related Traits. 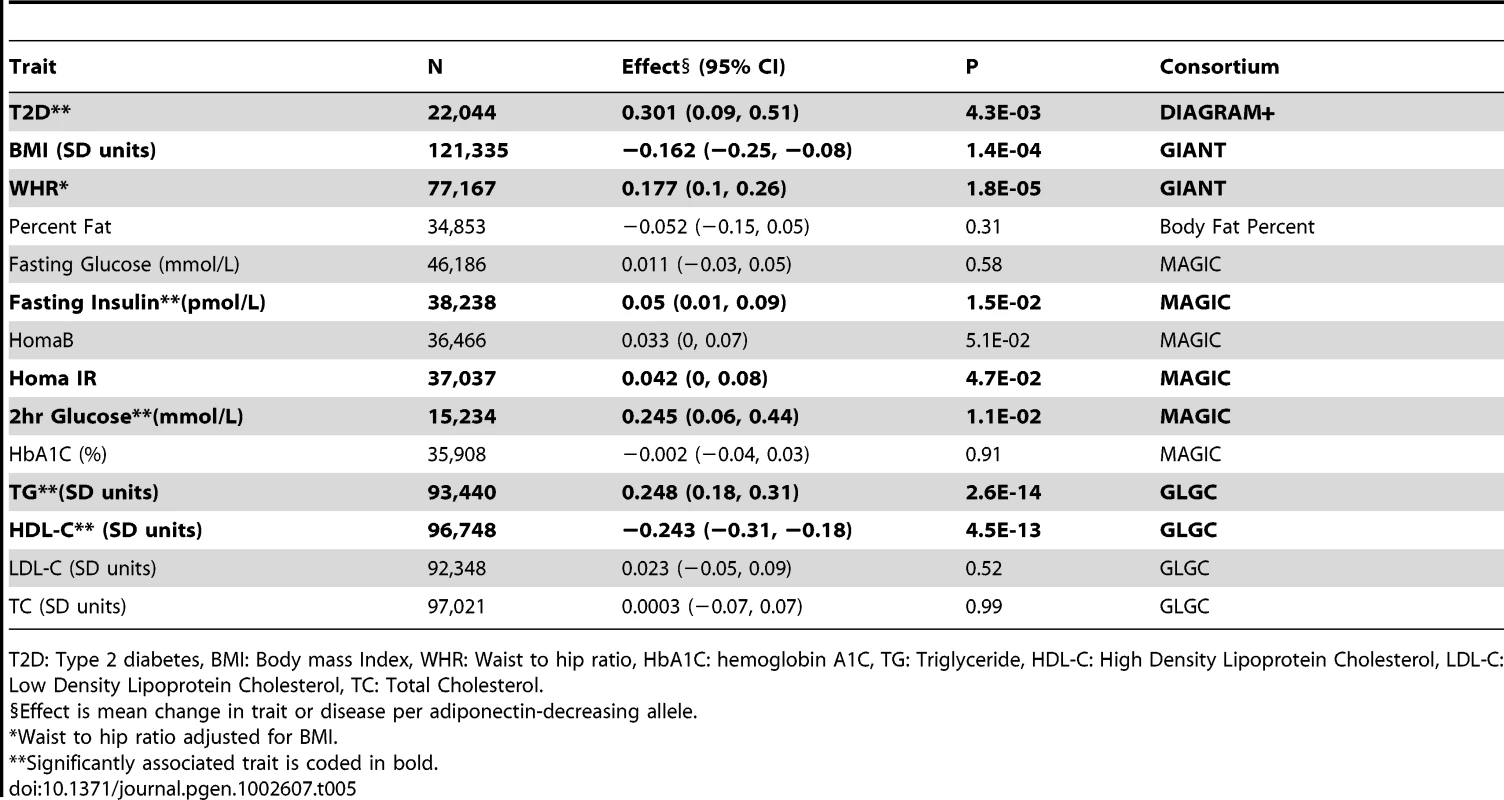
T2D: Type 2 diabetes, BMI: Body mass Index, WHR: Waist to hip ratio, HbA1C: hemoglobin A1C, TG: Triglyceride, HDL-C: High Density Lipoprotein Cholesterol, LDL-C: Low Density Lipoprotein Cholesterol, TC: Total Cholesterol. Discussion
In this comprehensive multi-ethnic analysis of the genetic influences on adiponectin levels and their impact on metabolic traits and T2D, we have identified 10 novel loci and confirmed the associations of variants in the ADIPOQ and CDH13 loci with adiponectin levels. The adiponectin risk alleles were associated with T2D and related metabolic traits such as BMI, WHR, TG, HDL-C, 2-hour glucose, HOMA-IR and fasting insulin. These findings demonstrate that adiponectin, T2D and metabolic syndrome have a shared allelic architecture.
Biological Relevance of the GWAS Loci
In the first step toward understanding the biological relevance of the identified regions, we examined the genes harbored by the associated loci using human disease and animal databases. Although some of the genes in these loci do not have a known function, several signify diverse biological functions.
On chromosome 1, the lead SNP was located 300 kb from the LYPLAL1, a protein that regulates phospholipids on cellular membranes. Independent efforts have also identified this locus in other metabolic/obesity related traits GWAS: first with WHR (rs2605100; r2 = 0.49 [21] and rs4846567; r2 = 0.55 [27] respectively with the lead adiponectin SNP, rs3001032), and more recently with fasting insulin by a joint meta-analysis including the interaction between SNP and BMI (MF Hivert for the MAGIC investigators, personal communication). In the same report by MAGIC, variants near IRS1 (insulin receptor substrate 1) and PEPD (a protein that hydrolyzes dipeptides and tripeptides) have also been associated with fasting insulin at genome wide significant levels, demonstrating a close link between adiponectin regulation and insulin resistance pathways. Moreover, both IRS1 and PEPD have been associated with T2D (IRS1 in DIAGRAM [28] and PEPD in a Japanese population [29]; p = 9.3×10−12 and p = 1.4×10−5, respectively).
The lead SNP at 3p21.1 falls within GNL3 that is located in a genomic region containing many genes which could have potential functions in metabolism. Our data provide evidence that adiponectin levels were correlated with human adipocyte mRNA levels of many genes in this region (GLYCTK, SEMA3G, STAB1, PBRM1, SFMBT1; see Table 4). However, this association does not imply a direct influence of these genes on adiponectin level. Among those genes, STAB1 encodes for stabilin 1, described as an endocytic receptor for advanced glycation end products and may have a function in angiogenesis, lymphocyte homing, cell adhesion, or receptor scavenging for acetylated low-density lipoprotein [30].
Interestingly, several of the genes near lead genome-wide significant SNPs have been implicated in angiogenesis, which might be important for adipose tissue expansion, highlighting the recurring theme of “adipose tissue expandability” in the genetic origins of obesity-related complications [31]. For example, VEGFA is the vascular endothelial growth factor A gene, a known gene in a variety of vascular endothelial cell functions, such as angiogenesis and maintenance of the glomerular endothelium in nephrons [32]. Variants in this gene are also associated with diabetic retinopathy and WHR [27], [33]. Moreover, the product of VEGFA interacts with resveratrol, which has been shown to have a beneficial influence in some metabolic traits, including diabetes [34]. Rodent studies show that resveratrol decreases blood glucose, blood insulin, and glycated hemoglobin, as well as increases insulin sensitivity in animals with hyperglycemia (reviewed in [35]). Resveratrol also inhibits TNF-α-induced reductions in adiponectin levels in 3T3-L1 adipocytes [36]. Furthermore, it has been shown that resveratrol modulates adiponectin expression and improves insulin sensitivity, likely through the inhibition of inflammatory-like response in adipocytes [37]. At this locus, VEGFA mRNA levels in adipocytes were the strongest association with adiponectin levels (Table 4). Also likely involved in vascular biology, TRIB1 encodes a G protein-coupled receptor-induced protein interacting with MAP kinases that regulates proliferation and chemotaxis of vascular smooth muscle cells [38]. TRIB1 expression was shown to be elevated in human atherosclerotic arteries [39]. Several variants (rs2954029, rs2954021, rs17321515; all in moderate LD with our lead SNP) in the TRIB1 gene have been associated with HDL-C, LDL-C and CHD risk in European and Asian populations [22], [40], [41], [42], [43]. These two loci (TRIB1 and VEGFA) argue for the importance of vascular biology in adiponectin regulation as underlined previously by findings of adiponectin levels associated with variants near CDH13 (a receptor for adiponectin expressed by endothelial smooth muscle) [44].
All three homologous genes GPR109A/B/81 located on chromosome 12 are predominantly expressed in adipocytes and mediate antilipolytic effects [45]. Our eQTL results (Table 3) and the correlation between mRNA and adiponectin levels (Table 4) argue strongly for a role of GPR109A at this locus. GPR109A (also known as NIACR1) is a receptor with a high-affinity, concentration-dependent response to nicotinic acid (niacin) [45]. Treatment by niacin increases serum adiponectin levels by up to 94% in obese men with metabolic syndrome in a time - and dose-dependent manner [46]. Functional studies in GPR109A receptor knockout mice demonstrate that niacin increases serum total and HMW adiponectin concentrations and decreases lipolysis following GPR109A receptor activation [47]. Moreover, a recent meta-analysis on cohorts containing extremes of HDL-C provided evidence suggestive of association in GPR109A/B/81 [48].
Finally, variants in ZNF664 have been associated with CHD, HDL-C and TG levels in a large meta-analysis of over 100,000 individuals of European ancestry [22]. The sex heterogeneity observed in this study is comparable to our finding that the more loci associated with adiponectin at genome wide significance level have been shown in female stratified analysis.
Taken together, the loci identified in this large-scale GWAS for adiponectin levels highlight many genes with demonstrated relationships with metabolic disease.
Shared Allelic Architecture of Adiponectin Levels and Metabolic Traits
Using a multi-SNP genotypic risk score we attempted to understand if the allelic architecture of adiponectin levels was shared with T2D and metabolic traits. This risk score was associated with increased risk of T2D and traits associated with insulin resistance and the metabolic syndrome. However, unexpectedly, adiponectin decreasing alleles were associated with a decrease in BMI. In our adiponectin GWAS, BMI was included as a covariate in order to avoid direct identification of obesity SNPs since BMI is strongly related to adiponectin levels [49], [50]. Furthermore, this unexpected direction of effect was entirely explained by SNPs at the ZNF664 and PEPD loci; when these loci were removed from the analysis, the association of the genotypic risk score with BMI disappeared (results not shown). Therefore, adiponectin risk alleles at ZNF664 and PEPD are of considerable interest since they impart deleterious changes on aspects of the metabolic syndrome (increased TC, TG, LDL-C and WHR and decreased HDL-C), but also act to decrease BMI and percent fat.
Our data do not provide direct evidence as to whether the genetic determinants of adiponectin levels influence these traits through adiponectin itself, or through pleiotropic pathways and therefore do not constitute a Mendelian randomization study. These findings provide a note of caution for Mendelian randomization studies, which may be prone to erroneous conclusions if pleiotropic effects of tested variants are not considered. Nonetheless, in aggregate, these results provide strong evidence that the genetic determinants of adiponectin levels are shared with metabolic disease, and in particular, traits related to insulin resistance.
We note that there are several strengths and limitations of this study. Our main findings, identifying genetic determinants of adiponectin levels, are based on the largest meta-analysis to date and include results from three ethnicities. The availability of expression data from human adipose tissue permitted the association of identified SNPs with mRNA levels at candidate genes and, in turn, correlation of these mRNA levels with circulating adiponectin itself. While access to the data from large consortia permitted assessment of the relevance of the identified SNPs to T2D and components of the metabolic syndrome, we note that a subset of the cohorts included in our GWAS were also included in these external consortia. However, we note that even if we assume that all ADIPOGen study participants were included in the external consortia, for cohorts participating in both studies, that the majority of data in these external consortia still arises from study participants not present in ADIPOGen (minimum percent of non-overlapping subects: 86.8%, 85.5%, 86.4% and 82.5% for MAGIC, GLGC, GIANT, and DIAGRAM+ consortia, respectively). Therefore, since a substantial majority of participants are independent between ADIPOGen and these consortia, it is unlikely that our findings demonstrating a shared allelic architecture between adiponectin levels and these traits are spurious.
Further, we suggest that locus, 6q24.1, identified only through multi-ethnic meta-analysis using MANTRA and not confirmed through fixed and random effects meta-analysis, be replicated for confirmation of this finding.
In conclusion, the data presented in this study provide strong evidence of association for 10 novel loci for adiponectin levels. Further analyses confirmed that the level of expression of some of these candidate genes in human adipocytes correlated directly with adiponectin levels. A multi-SNP genotypic risk score, and several of the identified variants, directly influence parameters of the metabolic syndrome and, in particular, markers of insulin resistance. These findings identify novel genetic determinants of adiponectin levels, which, taken together, influence risk of T2D and markers of insulin resistance.
Materials and Methods
Ethical Consideration
All participants provided informed written consent. The research protocol of all studies were reviewed and approved by institutional ethics review committees at the involved institutions.
Study Design
Our study consisted of three stages. First, in the discovery stage we performed a meta-analysis of the GWAS summary statistics of 16 studies involving 29,347 participants of white European origin to detect SNPs that are associated with adiponectin levels. All signals with p<5×10−6 were followed up in seven additional cohorts (n = 6,623) with GWAS data (in-silico phase) that later joined the consortium and then a subset of SNPs (n = 10) by de-novo genotyping in 3,913 additional participants from three cohorts (n = 39,883 for the combined analysis in Europeans). We also performed a multi-ethnic meta-analysis by combining summary statistics from the 16 studies of individuals of white European discovery cohorts (n = 29,347) with those of five cohort studies that included African Americans subjects (n = 4,232) and one East Asian cohort (n = 1,776) to obtain a total 35,355 individuals for the GWAS meta-analysis involving different ethnicities. After identifying variation near two genes of pharmaceutical importance (GPR109A and GPR109B), which encode the putative niacin receptors, we typed additional rare coding and tagging variants in a subset of cohorts. Second, we examined whether the identified SNPs of the first stage also associate with mRNA levels of nearest gene(s) expressed using adipose tissue of 776 European women. We also tested for association between adiponectin levels and mRNA levels of the genes in our candidate loci in adipose tissue of a subgroup of 436 individuals [25]. Third, we calculated a multi-SNP genotypic risk score using genome-wide significant adiponectin-lowering alleles and tested the association of this risk score with T2D and related metabolic traits. Figure 3 shows a flow chart detailing the study design.
Fig. 3. Flow chart of study design. 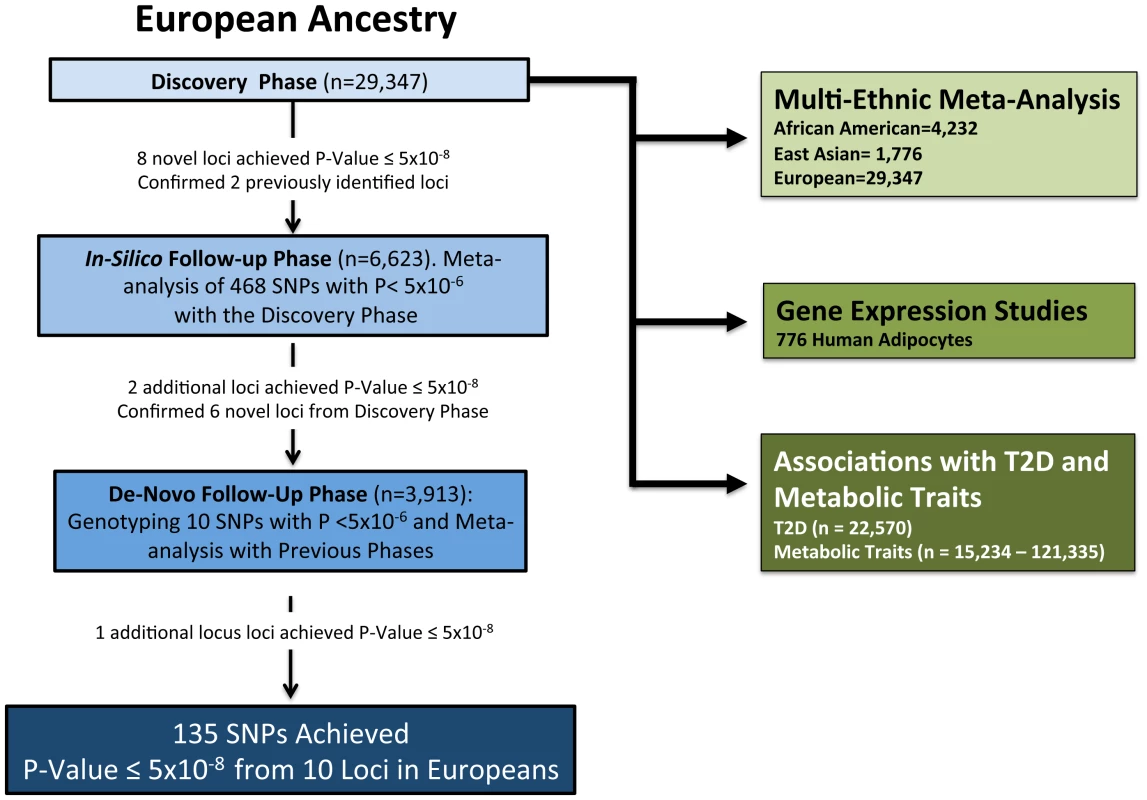
Study Populations
In total, 45,891 individuals from 26 European and 7 non-European cohorts participated in the different phases of this meta-analysis. Participating cohorts were either population-based (n = 23), family-based (n = 4), or case-control (n = 4) studies. The age of participants ranged from 10 to 95 years. Adiponectin levels were measured using ELISA or RIA methods. More details on the study cohorts and adiponectin measurement are presented in the Text S1 and Table S1. In addition, genotyping of four coding and tagging SNPs in the candidate genes, GRP109A and GPR109B, was undertaken in samples from the Lausanne, Lolipop, MRC Ely, and Fenland cohorts.
Genotyping and Imputation
All cohorts were genotyped using commercially available Affymetrix or Illumina genome-wide genotyping arrays. Quality control was performed for each study independently and genotype imputation was carried out using IMPUTE, MACH, BimBam or Beagle with reference to either the Phase II CEU, CEU+YRI, or CHB+JPT+CEU HapMap according to the origin of population. Imputation of East Asian genotypes was undertaken by first masking genotypes of 200 SNPs and then imputing them based on the CEU+CHB+JPT panel from HapMap. This resulted in an allelic concordance rate of ∼96.7%. For the African Americans, a combined CEU+YRI reference panel was created. This panel included SNPs segregating in both CEU and YRI, as well as SNPs segregating in one panel and monomorphic and non-missing in the other (2.74 million SNPs). Due to the overlap of African American individuals on the Affymetrix 6.0 and IBC arrays [51], it was possible to analyze imputation performance at SNPs not genotyped on Affymetrix 6.0. For imputation based on Affymetrix data, the use of the CEU+YRI panel resulted in an allelic concordance rate of ∼95.6% (calculated as 1−0.5 * [imputed_ dosage – chip_dosage]). This rate is comparable to rates calculated for individuals of African descent imputed with the HapMap 2 YRI individuals. Table S1 summarizes the genotyping methods used for each cohort, genotype-calling algorithms, imputation algorithms and exclusion thresholds. SNP-level quality control metrics were applied prior to meta-analysis for each cohort. These were: call rate ≥95%, minor allele frequency (MAF)≥1%, Hardy-Weinberg equilibrium (HWE) p>10−6, and quality measures for imputed SNPs (r2≥0.3, or proper info ≥0.4, for cohorts imputing their data with MACH and IMPUTE, respectively).
Eleven coding and tagging variants in two candidate genes of pharmaceutical importance (GPR109A encoding the niacin receptor and GPR109B) were genotyped in a parallel study in Lausanne, Lolipop, MRC Ely, and Fenland white subjects. Genotyping was performed using a KASPar-On-Demand SNP Genotyping Assay (KBioscience Ltd., Hoddesdon, UK). In Lausanne and Lolipop samples the genotyping assay was carried out on 3.75 ng of genomic DNA in 1 µl 1536-well plate reactions, dispensed with a Meridian, microfluidic dispenser (KBioscience Ltd., Hoddesdon, UK), thermocycled using a Hydrocycler (KBioscience Ltd., Hoddesdon, UK). A Pherastar (BMG GmbH, Germany) was used for end-point detection and Kraken-LIMS (KBioscience Ltd., Hoddesdon, UK) was used for automated allele calling. In MRC Ely and Fenland samples, the genotyping assay was carried out on 10 ng of genomic DNA in 5 µl 384-well plate reactions using a G-Storm GS4 Thermal Cycler (GRI, Rayne, UK). The ABI PRISM 7900HT Sequence Detection System (Applied Biosystems, Warrington, UK) was used for end-point detection and allele calling.
Statistical Analysis
Genome-wide association studies
All cohorts independently tested for the additive genetic association of common (MAF>1%) genotyped and imputed SNPs with natural log transformed adiponectin levels, while adjusting for age, sex, body mass index (BMI), principal components of population stratification and study site (where appropriate), and for family structure in cohorts with family members [49], [50], [52]. The analyses were performed for men and women combined, as well as for men and women separately. The Cardiovascular Health Study cohort (CHS) also provided GWA results for high molecular weight (HMW) adiponectin using the same methods as described above.
Meta-analysis of GWAS
The meta-analysis was performed by two analysts independently each using different methods; inverse variance-weighted methods using both fixed and random effect models available through either the METAL (http://www.sph.umich.edu/csg/abecasis/metal/) or GWAMA version 2.0.5 (http://www.well.ox.ac.uk/gwama/) software packages [53]. Summary statistics were crosschecked to ensure consistency of results. Prior to the meta-analysis, study-specific summary statistics were corrected using genomic control (lambda range = 0.99–1.25) and the overall meta-analytic results were additionally corrected for genomic control (lambda = 1.06). To examine whether associations with adiponectin were sex-specific, we performed meta-analyses for men and women separately. A p-value threshold of 5×10−8 was considered to be genome-wide significant. Ethnicity-specific meta-analyses were performed for white European and non-European populations separately, using the same methods as described above.
Presence of heterogeneity in the meta-analysis was assessed by the I2 statistic and Q-test [54]. Since cohorts measured adiponectin concentrations using either RIA or ELISA methods, we also performed a GWA meta-analysis stratified by the method of measurement to test whether this contributed to heterogeneity.
Follow-up phase
The follow-up phase comprised two stages; in-silico follow-up and de-novo follow-up.
In silico follow-up
468 SNPs with p<5×10−6 from the discovery phase (which includes both genome-wide significant [n = 196, p<5×10−8] and “suggestive” [n = 272, 5×10−8<p<5×10−6] SNPs Table S3) were tested for their association in 6,623 individuals from seven additional cohorts with GWAS data that joined the consortium after the discovery stage had been finalized.
De novo follow-up
We next selected the lead SNP arising from selected loci from the joint analysis of the discovery and in-silico follow-up phase with p-values greater than 5×10−8 but less than 5×10−6 and genotyped 10 SNPs in 3,164 samples from the SAPHIR cohort and an additional subgroup of the KORA cohort. Finally, these same SNPs, or their proxy SNPs (n = 2), were tested for association in the THISEAS cohort (n = 738), which had been genotyped using the Metabochip [55]. Study-level summary statistics from the follow-up phases were meta-analyzed with the data from the discovery phase.
Multi-ethnic meta-analysis
In order to perform a meta-analysis of GWAS data from cohorts of different ethnic backgrounds, we utilized the novel MANTRA (Meta-ANalysis of Trans-ethnic Association studies) software [24]. This method combines GWAS from different ethnic groups by taking advantage of the expected similarity in allelic effects between the most closely related populations. Fixed-effects meta-analysis assumes the allelic effect to be the same in all populations, and cannot account for heterogeneity between ethnic groups. Conversely, random effects meta-analysis assumes that each population has a different underlying allelic effect, however, populations from the same ethnic group would be more homogeneous than those that are more distantly related. To address this challenge we accounted for the expected similarity in allelic effects between the most closely related populations by means of a Bayesian partition model. For each variant, allelic effects and corresponding standard errors are estimated within each population under the assumption of an additive model. Populations are then clustered according to their similarity in terms of relatedness as measured by the mean allele frequency difference at 10,000 independent SNPs, and to their allelic effects at the variant. If all populations are assigned to the same cluster, this is equivalent to a fixed allelic effect across all populations (i.e. no trans-ethnic heterogeneity). The posterior distribution of the allelic effect in each population under the Bayesian partition model is approximated by means of a Monte-Carlo Markov chain algorithm. Evidence in favor of association of the trait with the variant was assessed by means of a Bayes' factor (BF). A log10 BF of 6 or higher is considered a relatively conservative threshold for genome-wide significance. We also performed meta-analysis by using both random and fixed effects models including all ethnicities. Those loci that achieved both a BF>6 in MANTRA and a P-value less than 5×10−7 in multiethnic analysis are presented in Table 2.
Association of Genome-Wide Significant SNPs with Gene Expression (Stage 2)
In order to identify cis-expression quantitative trait loci (cis-eQTLs) and test whether mRNA levels of candidate genes arising from our GWAS were associated with adiponectin levels, we used expression profiles in human adipocytes from the Multiple Tissue Human Expression Resource (MuTHER) Consortium, (856 female twins from the UK) [25]. mRNA expression profiles from subcutaneous fat and genome-wide genotypes were available for 776 individuals and circulating adiponectin levels for 436 of these women. We note that while adiponectin levels were measured at an earlier time point than the fat biopsies, the BMI at time of adipose expression measurement and time of adiponectin measurement was highly correlated (r2 = 0.9).
cis-eQTLs were defined as associations between SNPs and a transcript within 1 Mb of the identified SNP. To correct for multiple testing, we used QVALUE software [56], and estimated that a genome-wide false discovery rate of 1% corresponds to a p-value threshold of 5.06×10−5 (this conservative threshold accounts for all multiple arising from the use of the array, rather than multiple testing arising from assessing only transcripts in the genome-wide significant regions). To test whether mRNA levels of candidate genes identified in the GWAS meta-analysis are associated with circulating adiponectin levels, we applied a Bonferoni corrected threshold of p<3×10−4 (where 3×10−4 = 0.05/133 and 133 was the number of transcripts tested at the candidate loci).
Association of Genome-Wide Significant SNPs with T2D and Metabolic Traits (Stage 3)
The DIAGRAM+ (effective n = 22,044) [19], MAGIC (n = up to 46,186) [20], GLGC (n = up to 97,021) [22], GIANT (n = up to 121,335) [21], and Body Fat GWAS (n = up to 36,625) consortia provided summary statistics for the association of each SNP that was genome-wide significant in the discovery phase. Since 196 SNPs (which were estimated to be equivalent to 96 independent statistical tests due to linkage disequilibrium [LD]) [26] were tested for their association, we employed a Bonferroni-corrected threshold of α = 0.0005 (where 0.0005 = 0.05/96) to define the threshold of association for any individual SNP association with T2D and related traits.
While any individual SNP may demonstrate a relationship with T2D or related traits, it can be more informative to test whether a multi-SNP genotypic risk score is associated with the outcome of interest. In the absence of pleiotropic effects arising from loci other than ADIPOQ, such a multi-SNP genotypic risk score would enable testing of whether adiponectin levels are causally related to risk of T2D or metabolic traits through a Mendelian randomization framework. Since most of the SNPs that we identified to be genome-wide significant for adiponectin levels were not in the ADIPOQ locus, the presence of such pleiotropy precluded a formal Mendelian randomization study. To create a multi-SNP genotypic risk score we implemented a novel method that approximates the average effect of adiponectin decreasing alleles on T2D or related traits. Further, this method allows the use of consortium-level meta-analytic results for a set of SNPs, rather than requiring the re-analysis of individual-level data in each cohort, thereby providing more accurate effects of each allele (due to the larger sample size in the consortium-level meta-analysis). The weighted sum of the individual SNP coefficients leads not only to an estimate of the average combined allelic effect, but also to an approximate estimate of the explained variance (when scaled by the inverse of the total meta-analysis sample size) from a multivariate regression model containing these SNPs.
Specifically, suppose m SNPs have shown association in the discovery phase, and effects are denoted wi. However, suppose that the goal of interest is to estimate the joint effect of these SNPs on an outcome of interest, y. Let j index the individuals in the outcome of interest dataset and letbe a risk score based on the discovery data SNPs, and their associated parameter estimates wi. Therefore, the desired goal is to estimate the parameter in the following equation: in the outcome of interest dataset. The proportion of variance in y explained by the previous equation, (i.e. the R2) attributable to the risk score can be estimated. Standard linear model theory shows that the change in log likelihood is proportional to the R2,If the SNPs are uncorrelated, and if the total percentage of variance explained is small, then the change in log likelihood can be approximated bywhere βi now refers to the effect of SNP i in the outcome data, is the outcome data estimate, and si is the associated standard error estimate. Assuming that this log likelihood difference approximation is maximized with an appropriate value of C, then it can be shown that a can be estimated by:with a standard error estimate ofTherefore, under the assumption of uncorrelated SNPs, their joint effect can be estimated in external data by a weighted mean of the individual SNP effects, weighted by the estimates from the discovery data. All these quantities can be obtained from meta-analysis or summary data, so that individual-level data are not required to obtain these results.
To implement this method, we first selected LD-independent adiponectin associated alleles by LD pruning the set of genome-wide significant adiponectin SNPs from the discovery phase with an LD threshold of r2≤0.05 in the HapMap CEU population, yielding 20 independent LD blocks from the 196 SNPs in Table S2. (We also applied the method using an LD threshold of r2≤0.01 and found no relevant change in results). Since many SNPs from the same independent blocks were associated with adiponectin, we selected the SNP from the LD block that explained the most variance in adiponectin levels. Next, we approximated the effect of the multi-SNP genetic risk score using β and its standard error as derived from the consortium-level meta-analysis in DIAGRAM+, MAGIC, GLGC, GIANT and Body Fat GWAS consortium.
Supporting Information
Zdroje
1. HivertMFSullivanLMFoxCSNathanDMD'AgostinoRBSr 2008 Associations of adiponectin, resistin, and tumor necrosis factor-alpha with insulin resistance. J Clin Endocrinol Metab 93 3165 3172
2. TilgHMoschenAR 2006 Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol 6 772 783
3. PischonTGirmanCJHotamisligilGSRifaiNHuFB 2004 Plasma Adiponectin Levels and Risk of Myocardial Infarction in Men. JAMA: The Journal of the American Medical Association 291 1730 1737
4. LiSShinHJDingELvan DamRM 2009 Adiponectin levels and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA 302 179 188
5. StumvollMGoldsteinBJvan HaeftenTW 2005 Type 2 diabetes: principles of pathogenesis and therapy. Lancet 365 1333 1346
6. NawrockiARRajalaMWTomasEPajvaniUBSahaAK 2006 Mice lacking adiponectin show decreased hepatic insulin sensitivity and reduced responsiveness to peroxisome proliferator-activated receptor gamma agonists. J Biol Chem 281 2654 2660
7. WangYZhouMLamKSXuA 2009 Protective roles of adiponectin in obesity-related fatty liver diseases: mechanisms and therapeutic implications. Arq Bras Endocrinol Metabol 53 201 212
8. ComuzzieAGFunahashiTSonnenbergGMartinLJJacobHJ 2001 The genetic basis of plasma variation in adiponectin, a global endophenotype for obesity and the metabolic syndrome. Journal of Clinical Endocrinology and Metabolism 86 4321 4325
9. VasseurFHelbecqueNDinaCLobbensSDelannoyV 2002 Single-nucleotide polymorphism haplotypes in the both proximal promoter and exon 3 of the APM1 gene modulate adipocyte-secreted adiponectin hormone levels and contribute to the genetic risk for type 2 diabetes in French Caucasians. Hum Mol Genet 11 2607 2614
10. CesariMNarkiewiczKDe ToniRAldighieriEWilliamsCJ 2007 Heritability of plasma adiponectin levels and body mass index in twins. J Clin Endocrinol Metab 92 3082 3088
11. LiuPHJiangYDChenWJChangCCLeeTC 2008 Genetic and environmental influences on adiponectin, leptin, and BMI among adolescents in Taiwan: a multivariate twin/sibling analysis. Twin Res Hum Genet 11 495 504
12. MenzaghiCTrischittaVDoriaA 2007 Genetic influences of adiponectin on insulin resistance, type 2 diabetes, and cardiovascular disease. Diabetes 56 1198 1209
13. HivertMFManningAKMcAteerJBFlorezJCDupuisJ 2008 Common variants in the adiponectin gene (ADIPOQ) associated with plasma adiponectin levels, type 2 diabetes, and diabetes-related quantitative traits: the Framingham Offspring Study. Diabetes 57 3353 3359
14. LingHWaterworthDMStirnadelHAPollinTIBarterPJ 2009 Genome-wide Linkage and Association Analyses to Identify Genes Influencing Adiponectin Levels: The GEMS Study. Obesity (Silver Spring)
15. HeidIMHennemanPHicksACoassinSWinklerT 2010 Clear detection of ADIPOQ locus as the major gene for plasma adiponectin: results of genome-wide association analyses including 4659 European individuals. Atherosclerosis 208 412 420
16. RichardsJBWaterworthDO'RahillySHivertMFLoosRJ 2009 A genome-wide association study reveals variants in ARL15 that influence adiponectin levels. PLoS Genet 5 e1000768 doi:10.1371/journal.pgen.1000768
17. JeeSHSullJWLeeJEShinCParkJ 2010 Adiponectin concentrations: a genome-wide association study. Am J Hum Genet 87 545 552
18. WuYLiYLangeEMCroteau-ChonkaDCKuzawaCW 2010 Genome-wide association study for adiponectin levels in Filipino women identifies CDH13 and a novel uncommon haplotype at KNG1-ADIPOQ. Hum Mol Genet 19 4955 4964
19. VoightBFScottLJSteinthorsdottirVMorrisAPDinaC 2010 Twelve type 2 diabetes susceptibility loci identified through large-scale association analysis. Nat Genet 42 579 589
20. DupuisJLangenbergCProkopenkoISaxenaRSoranzoN 2010 New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat Genet 42 105 116
21. LindgrenCMHeidIMRandallJCLaminaCSteinthorsdottirV 2009 Genome-wide association scan meta-analysis identifies three Loci influencing adiposity and fat distribution. PLoS Genet 5 e1000508 doi:10.1371/journal.pgen.1000508
22. TeslovichTMMusunuruKSmithAVEdmondsonACStylianouIM 2010 Biological, clinical and population relevance of 95 loci for blood lipids. Nature 466 707 713
23. KilpeläinenTOZillikensMCStančákováAFinucaneFMRiedJS 2011 Genetic variation near IRS1 associates with reduced adiposity and an impaired metabolic profile. Nature Genetics In press
24. MorrisAP 2011 Transethnic meta-analysis of genomewide association studies. Genetic epidemiology 35 809 822
25. NicaACPartsLGlassDNisbetJBarrettA 2011 The Architecture of Gene Regulatory Variation across Multiple Human Tissues: The MuTHER Study. PLoS Genet 7 e1002003 doi:10.1371/journal.pgen.1002003
26. NyholtDR 2004 A simple correction for multiple testing for single-nucleotide polymorphisms in linkage disequilibrium with each other. Am J Hum Genet 74 765 769
27. HeidIMJacksonAURandallJCWinklerTWQiL 2010 Meta-analysis identifies 13 new loci associated with waist-hip ratio and reveals sexual dimorphism in the genetic basis of fat distribution. Nat Genet 42 949 960
28. RungJCauchiSAlbrechtsenAShenLRocheleauG 2009 Genetic variant near IRS1 is associated with type 2 diabetes, insulin resistance and hyperinsulinemia. Nat Genet 41 1110 1115
29. TakeuchiFSerizawaMYamamotoKFujisawaTNakashimaE 2009 Confirmation of multiple risk Loci and genetic impacts by a genome-wide association study of type 2 diabetes in the Japanese population. Diabetes 58 1690 1699
30. AdachiHTsujimotoM 2002 FEEL-1, a novel scavenger receptor with in vitro bacteria-binding and angiogenesis-modulating activities. J Biol Chem 277 34264 34270
31. GraySLVidal-PuigAJ 2007 Adipose tissue expandability in the maintenance of metabolic homeostasis. Nutr Rev 65 S7 12
32. EreminaVBaeldeHJQuagginSE 2007 Role of the VEGF–a signaling pathway in the glomerulus: evidence for crosstalk between components of the glomerular filtration barrier. Nephron Physiol 106 p32 37
33. BuraczynskaMKsiazekPBaranowicz-GaszczykIJozwiakL 2007 Association of the VEGF gene polymorphism with diabetic retinopathy in type 2 diabetes patients. Nephrol Dial Transplant 22 827 832
34. HuYSunCYHuangJHongLZhangL 2007 Antimyeloma effects of resveratrol through inhibition of angiogenesis. Chin Med J (Engl) 120 1672 1677
35. SzkudelskiTSzkudelskaK 2011 Anti-diabetic effects of resveratrol. Ann N Y Acad Sci 1215 34 39
36. AhnJLeeHKimSHaT 2007 Resveratrol inhibits TNF-alpha-induced changes of adipokines in 3T3-L1 adipocytes. Biochem Biophys Res Commun 364 972 977
37. KangLHengWYuanABaolinLFangH 2010 Resveratrol modulates adipokine expression and improves insulin sensitivity in adipocytes: Relative to inhibition of inflammatory responses. Biochimie 92 789 796
38. Kiss-TothEBagstaffSMSungHYJozsaVDempseyC 2004 Human tribbles, a protein family controlling mitogen-activated protein kinase cascades. J Biol Chem 279 42703 42708
39. SungHYGuanHCzibulaAKingAREderK 2007 Human tribbles-1 controls proliferation and chemotaxis of smooth muscle cells via MAPK signaling pathways. J Biol Chem 282 18379 18387
40. WaterworthDMRickettsSLSongKChenLZhaoJH 2010 Genetic variants influencing circulating lipid levels and risk of coronary artery disease. Arterioscler Thromb Vasc Biol 30 2264 2276
41. ParkMHKimNLeeJYParkHY 2011 Genetic loci associated with lipid concentrations and cardiovascular risk factors in the Korean population. J Med Genet 48 10 15
42. ChasmanDIPareGMoraSHopewellJCPelosoG 2009 Forty-three loci associated with plasma lipoprotein size, concentration, and cholesterol content in genome-wide analysis. PLoS Genet 5 e1000730 doi:10.1371/journal.pgen.1000730
43. WillerCJSannaSJacksonAUScuteriABonnycastleLL 2008 Newly identified loci that influence lipid concentrations and risk of coronary artery disease. Nat Genet 40 161 169
44. IvanovDPhilippovaMAntropovaJGubaevaFIljinskayaO 2001 Expression of cell adhesion molecule T-cadherin in the human vasculature. Histochemistry and cell biology 115 231 242
45. WiseAFoordSMFraserNJBarnesAAElshourbagyN 2003 Molecular identification of high and low affinity receptors for nicotinic acid. J Biol Chem 278 9869 9874
46. WestphalSBoruckiKTanevaEMakarovaRLuleyC 2006 Adipokines and treatment with niacin. Metabolism 55 1283 1285
47. PlaisanceEPLukasovaMOffermannsSZhangYCaoG 2009 Niacin stimulates adiponectin secretion through the GPR109A receptor. Am J Physiol Endocrinol Metab 296 E549 558
48. EdmondsonACBraundPSStylianouIMKheraAVNelsonCP 2011 Dense Genotyping of Candidate Gene Loci Identifies Variants Associated with High-Density Lipoprotein Cholesterol. Circ Cardiovasc Genet
49. WeyerCFunahashiTTanakaSHottaKMatsuzawaY 2001 Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab 86 1930 1935
50. LindsayRSFunahashiTHansonRLMatsuzawaYTanakaS 2002 Adiponectin and development of type 2 diabetes in the Pima Indian population. The Lancet 360 57 58
51. KeatingBJTischfieldSMurraySSBhangaleTPriceTS 2008 Concept, design and implementation of a cardiovascular gene-centric 50 k SNP array for large-scale genomic association studies. PLoS ONE 3 e3583 doi:10.1371/journal.pone.0003583
52. PriceALPattersonNJPlengeRMWeinblattMEShadickNA 2006 Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet 38 904 909
53. MagiRMorrisAP 2010 GWAMA: software for genome-wide association meta-analysis. BMC Bioinformatics 11 288
54. HigginsJPThompsonSGDeeksJJAltmanDG 2003 Measuring inconsistency in meta-analyses. Bmj 327 557 560
55. TheodorakiEVNikopensiusTSuhorutsenkoJPeppesVFiliP 2010 Fibrinogen beta variants confer protection against coronary artery disease in a Greek case-control study. BMC Med Genet 11 28
56. StoreyJDTibshiraniR 2003 Statistical significance for genomewide studies. Proc Natl Acad Sci U S A 100 9440 9445
Štítky
Genetika Reprodukční medicína
Článek Physiological Notch Signaling Maintains Bone Homeostasis via RBPjk and Hey Upstream of NFATc1Článek Intronic -Regulatory Modules Mediate Tissue-Specific and Microbial Control of / TranscriptionČlánek Probing the Informational and Regulatory Plasticity of a Transcription Factor DNA–Binding DomainČlánek Repression of Germline RNAi Pathways in Somatic Cells by Retinoblastoma Pathway Chromatin ComplexesČlánek An Alu Element–Associated Hypermethylation Variant of the Gene Is Associated with Childhood ObesityČlánek Three Essential Ribonucleases—RNase Y, J1, and III—Control the Abundance of a Majority of mRNAsČlánek Genomic Tools for Evolution and Conservation in the Chimpanzee: Is a Genetically Distinct Population
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 3
-
Všechny články tohoto čísla
- Comprehensive Research Synopsis and Systematic Meta-Analyses in Parkinson's Disease Genetics: The PDGene Database
- Genomic Analysis of the Hydrocarbon-Producing, Cellulolytic, Endophytic Fungus
- Networks of Neuronal Genes Affected by Common and Rare Variants in Autism Spectrum Disorders
- Akirin Links Twist-Regulated Transcription with the Brahma Chromatin Remodeling Complex during Embryogenesis
- Too Much Cleavage of Cyclin E Promotes Breast Tumorigenesis
- Imprinted Genes … and the Number Is?
- Genetic Architecture of Highly Complex Chemical Resistance Traits across Four Yeast Strains
- Exploring the Complexity of the HIV-1 Fitness Landscape
- MNS1 Is Essential for Spermiogenesis and Motile Ciliary Functions in Mice
- A Fundamental Regulatory Mechanism Operating through OmpR and DNA Topology Controls Expression of Pathogenicity Islands SPI-1 and SPI-2
- Evidence for Positive Selection on a Number of MicroRNA Regulatory Interactions during Recent Human Evolution
- Variation in Modifies Risk of Neonatal Intestinal Obstruction in Cystic Fibrosis
- PIF4–Mediated Activation of Expression Integrates Temperature into the Auxin Pathway in Regulating Hypocotyl Growth
- Critical Evaluation of Imprinted Gene Expression by RNA–Seq: A New Perspective
- A Meta-Analysis and Genome-Wide Association Study of Platelet Count and Mean Platelet Volume in African Americans
- Mouse Genetics Suggests Cell-Context Dependency for Myc-Regulated Metabolic Enzymes during Tumorigenesis
- Transcriptional Control in Cardiac Progenitors: Tbx1 Interacts with the BAF Chromatin Remodeling Complex and Regulates
- Synthetic Lethality of Cohesins with PARPs and Replication Fork Mediators
- APOBEC3G-Induced Hypermutation of Human Immunodeficiency Virus Type-1 Is Typically a Discrete “All or Nothing” Phenomenon
- Interpreting Meta-Analyses of Genome-Wide Association Studies
- Error-Prone ZW Pairing and No Evidence for Meiotic Sex Chromosome Inactivation in the Chicken Germ Line
- -Dependent Chemosensory Functions Contribute to Courtship Behavior in
- Diverse Forms of Splicing Are Part of an Evolving Autoregulatory Circuit
- Phenotypic Plasticity of the Drosophila Transcriptome
- Physiological Notch Signaling Maintains Bone Homeostasis via RBPjk and Hey Upstream of NFATc1
- Precocious Metamorphosis in the Juvenile Hormone–Deficient Mutant of the Silkworm,
- Igf1r Signaling Is Indispensable for Preimplantation Development and Is Activated via a Novel Function of E-cadherin
- Accurate Prediction of Inducible Transcription Factor Binding Intensities In Vivo
- Mitochondrial Oxidative Stress Alters a Pathway in Strongly Resembling That of Bile Acid Biosynthesis and Secretion in Vertebrates
- Mammalian Neurogenesis Requires Treacle-Plk1 for Precise Control of Spindle Orientation, Mitotic Progression, and Maintenance of Neural Progenitor Cells
- Tcf7 Is an Important Regulator of the Switch of Self-Renewal and Differentiation in a Multipotential Hematopoietic Cell Line
- REST–Mediated Recruitment of Polycomb Repressor Complexes in Mammalian Cells
- Intronic -Regulatory Modules Mediate Tissue-Specific and Microbial Control of / Transcription
- Age-Dependent Brain Gene Expression and Copy Number Anomalies in Autism Suggest Distinct Pathological Processes at Young Versus Mature Ages
- A Genome-Wide Association Study Identifies Variants Underlying the Shade Avoidance Response
- -by- Regulatory Divergence Causes the Asymmetric Lethal Effects of an Ancestral Hybrid Incompatibility Gene
- Genome-Wide Association and Functional Follow-Up Reveals New Loci for Kidney Function
- A Natural System of Chromosome Transfer in
- Cell Size and the Initiation of DNA Replication in Bacteria
- Probing the Informational and Regulatory Plasticity of a Transcription Factor DNA–Binding Domain
- Repression of Germline RNAi Pathways in Somatic Cells by Retinoblastoma Pathway Chromatin Complexes
- Temporal Transcriptional Profiling of Somatic and Germ Cells Reveals Biased Lineage Priming of Sexual Fate in the Fetal Mouse Gonad
- Rapid Analysis of Genome Rearrangements by Multiplex Ligation–Dependent Probe Amplification
- Metabolic Profiling of a Mapping Population Exposes New Insights in the Regulation of Seed Metabolism and Seed, Fruit, and Plant Relations
- The Atypical Calpains: Evolutionary Analyses and Roles in Cellular Degeneration
- The Silkworm Coming of Age—Early
- Development of a Panel of Genome-Wide Ancestry Informative Markers to Study Admixture Throughout the Americas
- Balanced Codon Usage Optimizes Eukaryotic Translational Efficiency
- The Min System and Nucleoid Occlusion Are Not Required for Identifying the Division Site in but Ensure Its Efficient Utilization
- Neurobeachin, a Regulator of Synaptic Protein Targeting, Is Associated with Body Fat Mass and Feeding Behavior in Mice and Body-Mass Index in Humans
- Statistical Analysis of Readthrough Levels for Nonsense Mutations in Mammalian Cells Reveals a Major Determinant of Response to Gentamicin
- Gene Reactivation by 5-Aza-2′-Deoxycytidine–Induced Demethylation Requires SRCAP–Mediated H2A.Z Insertion to Establish Nucleosome Depleted Regions
- The miR-35-41 Family of MicroRNAs Regulates RNAi Sensitivity in
- Genetic Basis of Hidden Phenotypic Variation Revealed by Increased Translational Readthrough in Yeast
- An Alu Element–Associated Hypermethylation Variant of the Gene Is Associated with Childhood Obesity
- Modelling Human Regulatory Variation in Mouse: Finding the Function in Genome-Wide Association Studies and Whole-Genome Sequencing
- Novel Loci for Adiponectin Levels and Their Influence on Type 2 Diabetes and Metabolic Traits: A Multi-Ethnic Meta-Analysis of 45,891 Individuals
- Polycomb-Like 3 Promotes Polycomb Repressive Complex 2 Binding to CpG Islands and Embryonic Stem Cell Self-Renewal
- Insulin/IGF-1 and Hypoxia Signaling Act in Concert to Regulate Iron Homeostasis in
- EMF1 and PRC2 Cooperate to Repress Key Regulators of Arabidopsis Development
- Three Essential Ribonucleases—RNase Y, J1, and III—Control the Abundance of a Majority of mRNAs
- Contrasted Patterns of Molecular Evolution in Dominant and Recessive Self-Incompatibility Haplotypes in
- A Machine Learning Approach for Identifying Novel Cell Type–Specific Transcriptional Regulators of Myogenesis
- Genomic Tools for Evolution and Conservation in the Chimpanzee: Is a Genetically Distinct Population
- Nos2 Inactivation Promotes the Development of Medulloblastoma in Mice by Deregulation of Gap43–Dependent Granule Cell Precursor Migration
- Intracranial Aneurysm Risk Locus 5q23.2 Is Associated with Elevated Systolic Blood Pressure
- Heritability and Genetic Correlations Explained by Common SNPs for Metabolic Syndrome Traits
- A Genome-Wide Association Study of Nephrolithiasis in the Japanese Population Identifies Novel Susceptible Loci at 5q35.3, 7p14.3, and 13q14.1
- DNA Damage in Nijmegen Breakage Syndrome Cells Leads to PARP Hyperactivation and Increased Oxidative Stress
- DNA Resection at Chromosome Breaks Promotes Genome Stability by Constraining Non-Allelic Homologous Recombination
- Genetic Analysis of Floral Symmetry in Van Gogh's Sunflowers Reveals Independent Recruitment of Genes in the Asteraceae
- A Splice Site Variant in the Bovine Gene Compromises Growth and Regulation of the Inflammatory Response
- Promoter Nucleosome Organization Shapes the Evolution of Gene Expression
- The Nucleoside Diphosphate Kinase Gene Acts as Quantitative Trait Locus Promoting Non-Mendelian Inheritance
- The Ciliogenic Transcription Factor RFX3 Regulates Early Midline Distribution of Guidepost Neurons Required for Corpus Callosum Development
- Phosphorylation of the RNA–Binding Protein HOW by MAPK/ERK Enhances Its Dimerization and Activity
- A Genome-Wide Scan of Ashkenazi Jewish Crohn's Disease Suggests Novel Susceptibility Loci
- Parkinson's Disease–Associated Kinase PINK1 Regulates Miro Protein Level and Axonal Transport of Mitochondria
- LMW-E/CDK2 Deregulates Acinar Morphogenesis, Induces Tumorigenesis, and Associates with the Activated b-Raf-ERK1/2-mTOR Pathway in Breast Cancer Patients
- Mapping the Hsp90 Genetic Interaction Network in Reveals Environmental Contingency and Rewired Circuitry
- Autoregulation of the Noncoding RNA Gene
- The Human Pancreatic Islet Transcriptome: Expression of Candidate Genes for Type 1 Diabetes and the Impact of Pro-Inflammatory Cytokines
- Spo0A∼P Imposes a Temporal Gate for the Bimodal Expression of Competence in
- Antagonistic Regulation of Apoptosis and Differentiation by the Cut Transcription Factor Represents a Tumor-Suppressing Mechanism in
- A Downstream CpG Island Controls Transcript Initiation and Elongation and the Methylation State of the Imprinted Macro ncRNA Promoter
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- PIF4–Mediated Activation of Expression Integrates Temperature into the Auxin Pathway in Regulating Hypocotyl Growth
- Metabolic Profiling of a Mapping Population Exposes New Insights in the Regulation of Seed Metabolism and Seed, Fruit, and Plant Relations
- A Splice Site Variant in the Bovine Gene Compromises Growth and Regulation of the Inflammatory Response
- Comprehensive Research Synopsis and Systematic Meta-Analyses in Parkinson's Disease Genetics: The PDGene Database
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



