-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaTpz1-Ccq1 and Tpz1-Poz1 Interactions within Fission Yeast Shelterin Modulate Ccq1 Thr93 Phosphorylation and Telomerase Recruitment
Proper maintenance of telomeres is essential for maintaining genomic stability, and genomic instability caused by dysfunctional telomeres could lead to accumulation of mutations that may drive tumor formation. Telomere dysfunction has also been linked to premature aging caused by depletion of stem cells. Therefore, it is important to understand how cells ensure proper maintenance of telomeres. Mammalian cells and fission yeast cells utilize an evolutionarily conserved multi-subunit telomere protection complex called shelterin to ensure protection against telomere fusions by DNA repair factors and cell cycle arrest by DNA damage checkpoint kinases. However, previous studies have not yet fully established how protein-protein interactions within the shelterin complex contribute to the regulation of DNA damage checkpoint signaling and telomerase recruitment. By utilizing separation of function mutations that specifically disrupt either Tpz1-Ccq1 or Tpz1-Poz1 interaction within the fission yeast shelterin, we establish that Tpz1-Ccq1 interaction is essential for phosphorylation of Ccq1 by the DNA damage checkpoint kinases Rad3ATR and Tel1ATM that is needed for telomerase recruitment to telomeres, while Tpz1-Poz1 interaction prevents Ccq1 phosphorylation by promoting Poz1 association with telomeres. These findings thus establish for the first time how protein-protein interactions within the shelterin complex modulate checkpoint kinase-dependent phosphorylation essential for telomerase recruitment.
Published in the journal: . PLoS Genet 10(10): e32767. doi:10.1371/journal.pgen.1004708
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004708Summary
Proper maintenance of telomeres is essential for maintaining genomic stability, and genomic instability caused by dysfunctional telomeres could lead to accumulation of mutations that may drive tumor formation. Telomere dysfunction has also been linked to premature aging caused by depletion of stem cells. Therefore, it is important to understand how cells ensure proper maintenance of telomeres. Mammalian cells and fission yeast cells utilize an evolutionarily conserved multi-subunit telomere protection complex called shelterin to ensure protection against telomere fusions by DNA repair factors and cell cycle arrest by DNA damage checkpoint kinases. However, previous studies have not yet fully established how protein-protein interactions within the shelterin complex contribute to the regulation of DNA damage checkpoint signaling and telomerase recruitment. By utilizing separation of function mutations that specifically disrupt either Tpz1-Ccq1 or Tpz1-Poz1 interaction within the fission yeast shelterin, we establish that Tpz1-Ccq1 interaction is essential for phosphorylation of Ccq1 by the DNA damage checkpoint kinases Rad3ATR and Tel1ATM that is needed for telomerase recruitment to telomeres, while Tpz1-Poz1 interaction prevents Ccq1 phosphorylation by promoting Poz1 association with telomeres. These findings thus establish for the first time how protein-protein interactions within the shelterin complex modulate checkpoint kinase-dependent phosphorylation essential for telomerase recruitment.
Introduction
Telomeres are protective nucleoprotein structures found at the natural ends of eukaryotic linear chromosomes [1]. In most eukaryotes, telomeric DNA consists of GT-rich repeat sequences, primarily double-stranded but terminating in a single-stranded 3′ overhang, known as G-tail [1]. Telomerase, a specialized reverse transcriptase, solves the “end-replication problem” by de novo addition of GT-rich repeats to chromosome ends [2], [3]. While its catalytic subunit TERT (Trt1 in fission yeast Schizosaccharomyces pombe, Est2 in budding yeast Saccharomyces cerevisiae, and hTERT in humans) and telomerase RNA (TER1 in S. pombe, TLC1 in S. cerevisiae, and hTR in humans) [4]–[7] are sufficient for generating telomerase activity that can be monitored in vitro [8], [9], additional regulatory subunits are necessary for telomere maintenance in vivo. For example, a regulatory subunit Est1 is required for telomere maintenance in both budding and fission yeasts, as it contributes to efficient recruitment and/or activation of telomerase in late S-phase [10]–[12].
In mammalian cells, the shelterin complex (consisting of TRF1, TRF2, RAP1, TIN2, TPP1 and POT1) ensures stable maintenance of telomeres [1]. While TRF1 and TRF2 specifically recognize and bind to the double-stranded telomeric repeats, POT1 specifically binds to the G-tail [1]. TIN2 and TPP1 are essential for assembly of the shelterin complex, since these two proteins, via TRF1-TIN2, TRF2-TIN2, TIN2-TPP1, and TPP-POT1 interactions, connect the double-stranded DNA (dsDNA) binding proteins TRF1/TRF2 to the single-stranded DNA (ssDNA) binding protein POT1 [13]. On the other hand, a fully assembled shelterin complex might occur only transiently during cell cycle, since TRF1, TRF2 and POT1 show distinct cell cycle-regulated telomere binding patterns [14], and distinct sub-complexes of shelterin components have been identified in cell extracts [15]. In addition, a careful quantitative western blot analysis indicated that protein expression levels of TPP1 and POT1 are significantly lower than TRF1, TRF2, RAP1 and TIN2, suggesting that a majority of shelterin subunits may be assembled only as the TRF1-TRF2-RAP1-TIN2 sub-complex [16].
Previous studies have found that TRF1, along with TIN2, TPP1 and POT1, function as negative regulators of telomerase-dependent telomere elongation [17]–[20], but TIN2, POT1 and TPP1 also play roles in promoting telomere extension by facilitating telomerase recruitment [21]–[25]. The shelterin complex is also important for preventing telomeres from being recognized as broken DNA ends, which can undergo chromosome rearrangements and fusions by various DNA repair proteins, and cause cell cycle arrest mediated by the DNA damage checkpoint kinases ATM and ATR [26]–[28]. Mutations in telomerase and shelterin subunits have been linked to genomic instability and human diseases, highlighting the importance of understanding how the shelterin complex regulates telomerase and DNA damage response factors [29], [30].
Fission yeast Schizosaccharomyces pombe serves as an attractive model for understanding how cells regulate telomere maintenance, since its shelterin complex (composed of Taz1, Rap1, Poz1, Tpz1, Pot1 and Ccq1) (Figure 1A) shares many conserved features with the mammalian shelterin complex [31], [32], and fission yeast cells are highly amenable to genetic and biochemical analyses. Pot1, the ortholog of mammalian POT1, binds directly to the G-tail and protects telomeres against chromosome fusions and Rad3ATR-dependent checkpoint activation [33], [34]. Due to loss of telomere protection, deletions of Pot1 or the Pot1-interacting protein Tpz1 (TPP1 ortholog) lead to immediate cell death for the majority of cells, while rare survivor cells carrying circularized chromosomes can be recovered [31], [33]. Poz1, proposed to be a functional analog of TIN2, forms a bridge between the ssDNA binding protein Pot1 and dsDNA binding protein Taz1 through its interactions with both Tpz1 and the Taz1-interacting protein Rap1 [31]. Much like in mammalian cells, different subunits of the fission yeast shelterin complex also show distinct cell cycle-regulated telomere association patterns [35], [36], suggesting that a commonly drawn fully connected shelterin complex (Figure 1A) might never be formed or formed only transiently during the cell cycle.
Fig. 1. Summary of Tpz1-Ccq1 and Tpz1-Poz1 interaction domains. 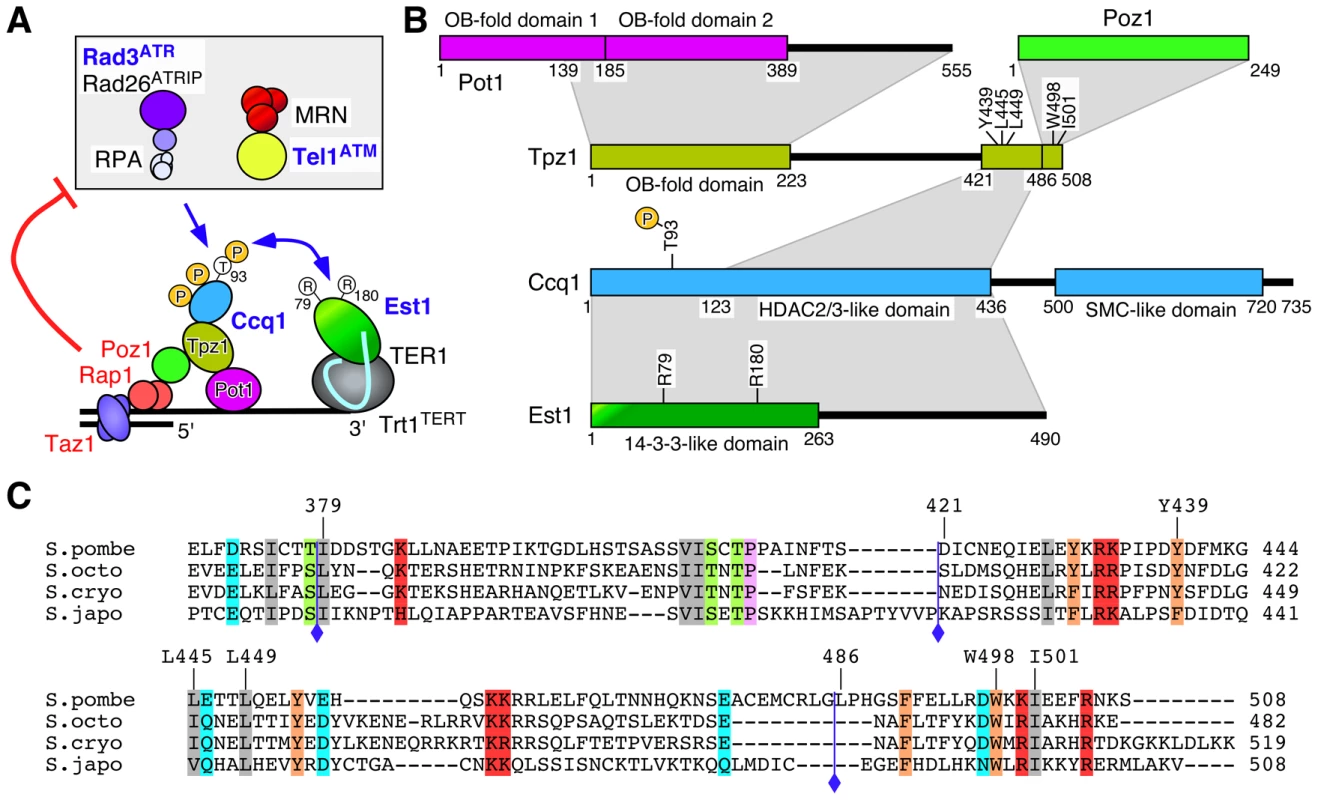
(A) A model of telomere maintenance regulation in fission yeast [12]. Taz1, Rap1 and Poz1 negatively regulate telomere extension by limiting accumulation of Rad3ATR kinase, Ccq1 Thr93 phosphorylation and telomerase recruitment, while recognition of Ccq1 Thr93 phosphorylation by the 14-3-3-like domain of Est1 promotes telomerase recruitment [12]. (B) Summary of protein-protein interaction domains for shelterin and telomerase subunits determined by Y2H assays. The current study identified Tpz1 Y439, L445 and L449 as critical for Tpz1-Ccq1 interaction and Tpz1 W498 and I501 as critical for Tpz1-Poz1 interaction. A previous study has identified Ccq1 T93 and Est1 R79 and R180 as critical for Ccq1-Est1 interaction [12]. (C) Sequence alignment of Tpz1 region responsible for Tpz1-Ccq1 and Tpz1-Poz1 interaction in S. pombe and corresponding regions from three additional Schizosaccharomyces species (S. octosporus, S. cryophilus and S. japonicus). Blue diamonds indicate a previously defined Ccq1/Poz1 binding domain (379–508) [31], and newly defined Ccq1 binding (421–485) and Poz1 binding (486–508) domains. In addition, amino acid residues critical for Tpz1-Ccq1 (Y439, L445 and L449) and Tpz1-Poz1 (W498 and I501) interactions are indicated. Taz1, Rap1, and Poz1 are all important for the negative regulation of telomerase-dependent telomere elongation, as deletion of any of these three proteins causes telomerase-dependent massive elongation of telomeres [31], [37]–[39]. We have previously shown that Taz1, Rap1 and Poz1 are necessary to properly coordinate leading and lagging strand synthesis and to limit accumulation of the DNA damage checkpoint kinase Rad3ATR at telomeres [36]. Since Rad3ATR/Tel1ATM-dependent phosphorylation of Ccq1 at Thr93 is recognized by the 14-3-3-like domain of Est1 to promote Est1-Ccq1 interaction and telomerase recruitment [12], increased accumulation of Rad3ATR in taz1Δ, rap1Δ and poz1Δ cells leads to increased Ccq1 Thr93 phosphorylation and telomerase recruitment [12], [36].
Ccq1 interacts not only with Tpz1 and Est1, but also with the Clr3 subunit of SHREC (Snf2/HDAC-Containing Repressor Complex), a mediator of heterochromatic gene silencing, to facilitate heterochromatin formation at telomeres [31], [40]. While yeast two-hybrid (Y2H) assays have detected Tpz1-Ccq1 and Ccq1-Clr3 interactions and co-immunoprecipitation (co-IP) assays have detected association of Pot1 with Ccq1 and association of Clr3 with Ccq1, co-IP assays failed to detect association of Pot1 with Clr3 [31], suggesting that shelterin and SHREC likely represent two distinct complexes with the shared subunit Ccq1. In addition to its roles as telomerase recruiter and promoter of heterochromatin formation, Ccq1 also acts to repress checkpoint activation and recombination at telomeres [31], [41]. However, previous studies have utilized ccq1Δ strains to examine its roles at telomeres, and thus did not address which of these previously described Ccq1 functions is dependent on the proper formation of Tpz1-Ccq1 interaction.
Poz1, besides functioning as a negative regulator of telomerase recruitment, is also required for heterochromatin formation at telomeres [32], and disruption of Poz1-Rap1 interaction leads to massively elongated telomeres and loss of heterochromatin [42]. However, it remained unclear if Poz1-Tpz1 interaction might play any functional role in this negative regulation of telomerase recruitment or heterochromatin formation at telomeres, as previous studies have utilized only poz1Δ strains [12], [31]. Simultaneous deletion of ccq1 and poz1 genes leads to severe telomere de-protection phenotypes reminiscent of tpz1Δ and pot1Δ cells, suggesting that Ccq1 and Poz1 play redundant roles in allowing Pot1-Tpz1 to protect telomeres against DNA repair and checkpoint proteins [31]. However, it had not been established if Tpz1-Poz1 and Tpz1-Ccq1 interactions are necessary for Poz1 and Ccq1 to protect telomeres, or the presence of these proteins per se are necessary for telomere capping.
Therefore, we have decided to map the domain(s) of Tpz1 that are responsible for dictating Tpz1-Poz1 and Tpz1-Ccq1 interaction, and to generate a series of tpz1 mutations that are specifically defective in Tpz1-Poz1 and/or Tpz1-Ccq1 interaction while retaining Tpz1-Pot1 interaction. Using these separation-of-function mutants of Tpz1, we will establish that Tpz1-Ccq1 interaction is essential for Ccq1 Thr93 phosphorylation and telomerase recruitment, heterochromatin formation, and checkpoint suppression at telomeres, while Tpz1-Poz1 interaction is required for efficient localization of Poz1 at telomeres, heterochromatin formation, and inhibition of Ccq1 Thr93 phosphorylation and telomerase recruitment. Furthermore, we will establish that Tpz1-Poz1 and Tpz1-Ccq1 interactions are redundantly required for telomere protection. These conclusions are in conflict with a recent study that concluded that Tpz1-Ccq1 interaction is not necessary for telomerase recruitment and Tpz1-Poz1 interaction does not contribute to Poz1 recruitment to telomeres [43]. Therefore, we will also discuss contrasting observations between our current study and the previous findings.
Results
Identification of Tpz1-Ccq1 and Tpz1-Poz1 interaction domains within Tpz1
A previous study that discovered the fission yeast shelterin complex also identified amino acids 379–508 of Tpz1 as the domain that mediates both Tpz1-Ccq1 and Tpz1-Poz1 interactions by Y2H assay [31] (Figure 1). In an attempt to identify smaller regions sufficient for mediating either Tpz1-Ccq1 or Tpz1-Poz1 interaction, we further truncated this fragment and found that amino acid residues 421–485 are sufficient for Tpz1-Ccq1 interaction while amino acid residues 486–508 are sufficient for Tpz1-Poz1 interaction by Y2H assay (Figure 2A).
Fig. 2. Determination of domains and amino acid residues critical for mediating Tpz1-Ccq1 and Tpz1-Poz1 interaction in Y2H assays. 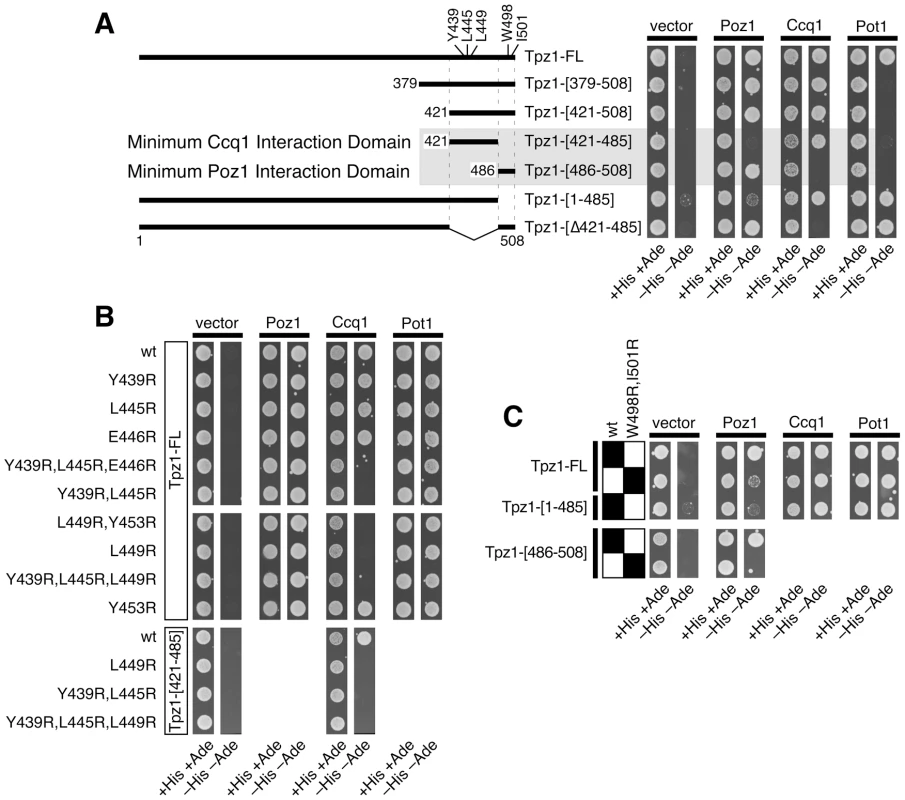
(A) Identification of minimum Tpz1-Ccq1 and Tpz1-Poz1 interaction domains by Y2H assay. A positive interaction is identified by growth on −His −Ade plates. (B) Identification of mutations that specifically disrupt Tpz1-Ccq1 interaction by Y2H assay. Indicated mutations were tested in full length Tpz1 (Tpz1-FL) or the minimum Ccq1 interaction domain construct of Tpz1 (Tpz1-[421–485]). (C) Identification of mutations that specifically disrupt Tpz1-Poz1 interaction by Y2H assay. The effect of W498R,I501R mutation was tested in full length Tpz1 (Tpz1-FL) or the minimum Poz1 interaction domain construct of Tpz1 (Tpz1-[486–508]). Analysis of Tpz1 sequences from four Schizosaccharomyces species [44] identified highly conserved amino acid residues within the minimum Tpz1-Ccq1 and Tpz1-Poz1 interaction domains (Figures 1C and S1A). Therefore, we decided to mutate them to determine if these conserved residues play any role in promoting Tpz1-Ccq1 or Tpz1-Poz1 interaction. For charged residues (Lys, Arg, Asp and Glu), we introduced either a charge swap (Lys/Arg to Glu) or Alanine mutation (Lys/Arg/Asp/Glu to Ala), but failed to find any mutation that disrupt Tpz1-Poz1 or Tpz1-Ccq1 Y2H interaction (Figure S1B). On the other hand, we were able to identify mutations that specifically disrupt either Tpz1-Ccq1 or Tpz1-Poz1 Y2H interaction by changing hydrophobic residues (Tyr/Trp/Leu/Ile) to Arginine (Figure 2B–C).
Within its Ccq1 interaction domain, simultaneous mutations of Tyr439 and Leu445 or a single mutation of Leu449 to Arg disrupted the Tpz1-Ccq1 Y2H interaction without affecting Tpz1-Poz1 and Tpz1-Pot1 interactions (Figure 2B). Within its Poz1 interaction domain, simultaneous mutations of Trp498 and Ile501 to Arg significantly weakened the Tpz1-Poz1 Y2H interaction for full-length Tpz1, and completely eliminated the interaction when introduced into the minimal Poz1-binding domain construct (residues 486–508) (Figure 2C). When mutated to Ala, tpz1-W498A,I501A only mildly affected Tpz1-Poz1 Y2H interaction, and tpz1-Y439A,I445A failed to disrupt Tpz1-Ccq1 Y2H interaction (Figure S2B). While tpz1-L449A mutation also caused disruption of Tpz1-Ccq1 Y2H interaction (Figure S2B), the disruption was more severe for tpz1-L449R than tpz1-L449A (Figure S2C). Since we were interested in generating mutants that fully disrupt Tpz1-Ccq1 or Tpz1-Poz1 interaction, we then decided to focus our efforts on characterizing hydrophobic to Arg mutants rather than Ala mutants, except for tpz1-L449A.
Based on our results from Y2H analysis, we integrated mutant tpz1 alleles into their endogenous locus in fission yeast, and characterized their effects on Tpz1-Ccq1, Tpz1-Poz1 and Tpz1-Pot1 interactions by co-IP (Figures 3 and S3A). As predicted by Y2H assays, we found that Tpz1-L449R, Tpz1-Y439R,L445R and Tpz1-Y439R,L445R,L449R mutant proteins specifically lose Tpz1-Ccq1 interaction but retain Tpz1-Poz1 and Tpz1-Pot1 interactions in fission yeast cells (Figure 3B–D). In addition, co-IP analysis revealed that both L449R and L449A mutations both completely disrupt Tpz1-Ccq1 interaction even when lower salt (60 mM NaCl) than our standard salt (150 mM NaCl) lysis and wash conditions were used (Figure S3A). Furthermore, we found that both Tpz1-W498R,I501R and the C-terminally truncated Tpz1-[1–485] (Figure 3A) specifically disrupt Tpz1-Poz1 interaction without affecting Tpz1-Ccq1 and Tpz1-Pot1 interactions (Figure 3E–G). While all tpz1 mutant alleles showed comparable expression levels of Tpz1 protein and did not affect protein levels of Pot1 or Ccq1 (Figure 3B–G), we found that Poz1 expression levels are reduced in cells expressing Tpz1-W498R,I501R or Tpz1-[1–485], suggesting that Poz1 stability partially depends on its interaction with Tpz1 (Figure 3E). Taken together, our results indicated that two distinct C-terminal regions of Tpz1 mediate its interaction with either Ccq1 or Poz1.
Fig. 3. Co-IP experiments to examine how various Tpz1 mutations affect Tpz1-Poz1, Tpz1-Ccq1 and Tpz1-Pot1 interactions in fission yeast cells. 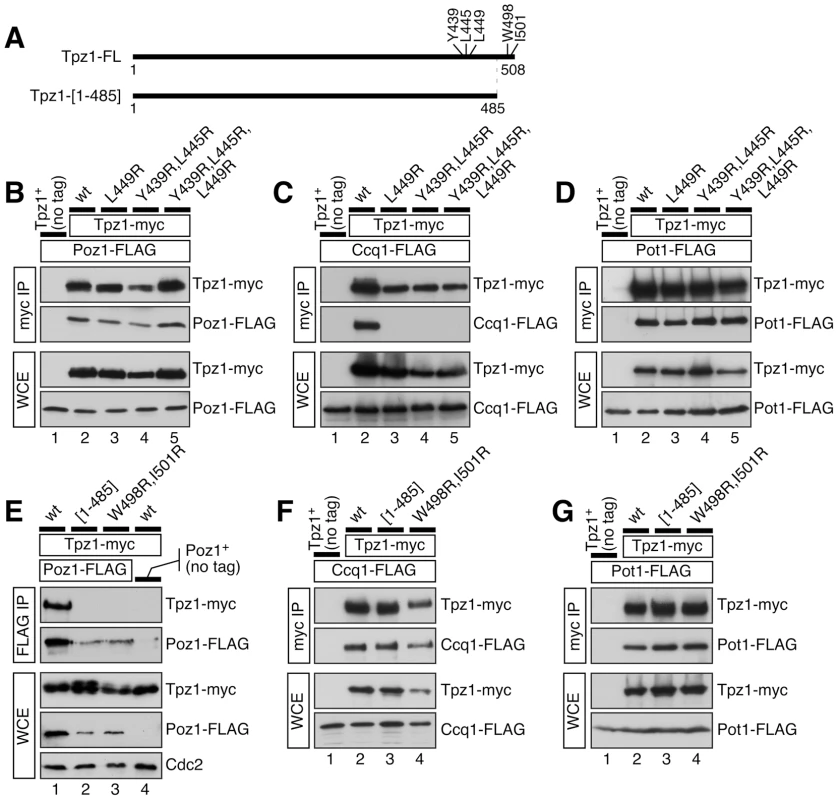
(A) A schematic representation of full length (Tpz1-FL) or C-terminally truncated (Tpz1-[1–485]) Tpz1 constructs used in co-IP experiments. Locations of mutated amino acid residues are also indicated. (B–D) Examination of Tpz1-Poz1 (B), Tpz1-Ccq1 (C) and Tpz1-Pot1 (D) interactions by co-IP for indicated Tpz1-Ccq1 interaction disruption mutants. (E–G) Examination of Tpz1-Poz1 (E), Tpz1-Ccq1 (F) and Tpz1-Pot1 (G) interactions by co-IP for indicated Tpz1-Poz1 interaction disruption mutants. For strains carrying Poz1-FLAG, Cdc2 western blot was included to serve as loading control to show reduced expression of Poz1 in tpz1-[1–485] and tpz1-W498R,I501R backgrounds. Disruption of Tpz1-Ccq1 interaction resembles Ccq1 deletion
Identification of Tpz1 mutants that specifically disrupt either Tpz1-Ccq1 or Tpz1-Poz1 interaction allowed us to investigate the functional significance of these interactions for fission yeast telomere maintenance. For all four Tpz1-Ccq1 interaction mutants tested (tpz1-L449R, tpz1-L449A, tpz1-Y439R,L445R and tpz1-Y439R,L445R,L449R), Southern blot analysis indicated that newly generated haploid tpz1 mutant cells (derived from heterozygous diploid cells) show progressive telomere shortening when restreaked on agar plates, very much like ccq1Δ cells (Figure 4A lanes 6, 7, 9, 10, 12 and 13, and Figure S3B lanes 2–9) [31], [41]. However, colony sizes of tpz1 mutant cells on plates were highly variable, and when smaller colonies were selectively picked to monitor telomere structures, even early generation colonies (restreaked twice) showed very strong signals for a band corresponding to intra-chromosome fusion (I+L), and faint signals for bands corresponding to inter-chromosome fusions (I+M and L+M) on a pulsed-field gel (Figures 4B and S3C). Conversely, when faster growing colonies were chosen for successive restreaks on plates, they maintained short telomeres even after restreaked 11 times (estimated to be 220–275 cell divisions, assuming 20–25 cell divisions per restreak on agar plates) (Figure S4), suggesting that those cells have adapted to the loss of Tpz1-Ccq1 interaction and managed to stably maintain short telomeres. Furthermore, tpz1-L449R or tpz1-L449A cells derived from larger colonies also showed no telomere fusions when analyzed on a pulsed field gel (Figure 4D lane 5, and Figure S3E lanes 2, 3 and 7). Taken together, we thus concluded that the loss of Tpz1-Ccq1 interaction causes heterogeneous phenotypes with either immediate loss of cell viability due to elevated telomere fusions or frequent emergence of cells that manage to maintain short telomeres for many generations.
Fig. 4. Effects of Tpz1-Ccq1 interaction disruption mutations on telomere maintenance. 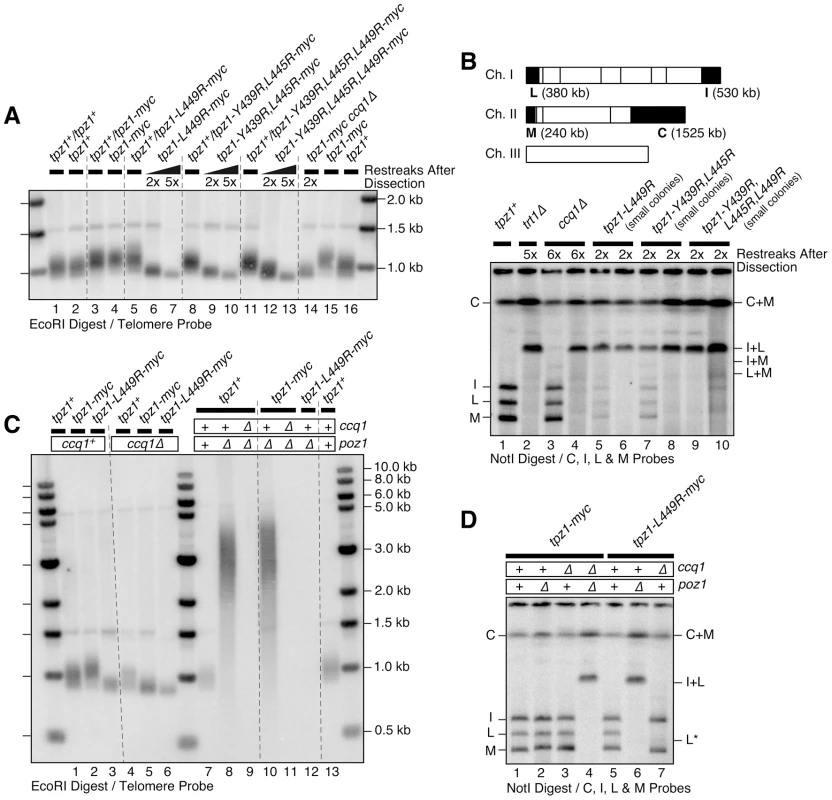
(A) Southern blot analysis of telomere length for indicated Tpz1-Ccq1 interaction disruption mutants. Haploid cells were generated by dissection of spores derived from heterozygous tpz1+/mutated tpz1 diploid cells, and restreaked twice or 5 times on plates prior to preparation of genomic DNA. For each round of restreak, several faster growing colonies were combined and streaked for single colonies on YES plates. (B) Pulsed-field gel analysis of telomere fusions for early generation small colonies of Tpz1-Ccq1 interaction disruption mutants, which showed prominent I+L fusion band as well as much fainter bands for I+M, L+M, I, L and M bands. C and C+M bands could not be distinguished by size. A NotI restriction map of fission yeast chromosomes is shown on top with telomeric fragments from chromosomes I and II marked with black boxes (C, I, L and M). (C) Epistasis analysis for telomere maintenance phenotype of Tpz1-Ccq1 disruption mutants against ccq1Δ or poz1Δ by Southern blot. Cells were restreaked 5 times on plates prior to preparation of genomic DNA to achieve steady state telomere length, except for tpz1-myc ccq1Δ poz1Δ and tpz1-L449R-myc poz1Δ cells where DNA from survivors with circular chromosomes were made after restreaked twice on plates. (D) Epistasis analysis for telomere fusions by pulsed-field gel for indicated combination of tpz1 mutants, ccq1Δ and poz1Δ. For (C–D), samples were prepared from early generation cells after strains were generated by genetic cross of parental haploid strains and dissection of resulting double mutant spores. In liquid culture, tpz1-L449R and ccq1Δ cells showed similar changes in cell growth rate. The slowest cell growth occurred on days 6–8 after haploid colonies derived from heterozygous diploid cells were first inoculated and subsequently serially diluted every 24 hours (Figure S5). Progressive telomere shortening of tpz1-L449R and ccq1Δ cells during days 1–7 correlated with progressive loss of growth, but both cultures eventually generated survivors that recover in growth rate and carry highly elongated and rearranged telomeres (Figure S5C–D). Intensities of telomere signals for tpz1-L449R and ccq1Δ survivor cells were much more intense than for trt1Δ survivor cells (Figure S5B–D), suggesting that elimination of Tpz1-Ccq1 interaction or Ccq1 protein may allow for more efficient telomere elongation by telomere-telomere recombination in survivor cells [41], [45]. The appearance of survivor cells with highly elongated telomeres in liquid culture is reminiscent of the situation in budding yeast, where rare but faster growing budding yeast Type II recombination survivors with long telomeres predominate in liquid culture, and more common but slower growing Type I survivors with short telomeres predominate when selected on agar plates [46], [47].
Double mutant tpz1-L449R ccq1Δ cells grew comparably to tpz1-L449R and ccq1Δ single mutant cells, and Southern blot analysis revealed that tpz1-L449R ccq1Δ double mutant cells exhibit a similar extent of telomere shortening as tpz1-L449R and ccq1Δ single mutant cells (Figure 4C lanes 3, 5 and 6). By contrast, the majority of tpz1-L449R poz1Δ and tpz1-L449A poz1Δ double mutant cells died immediately after they were generated by dissection of spores derived from heterozygous diploid cells, and rare survivor cells had lost their telomeres (Figures 4C and S3D) and carried circular chromosomes (Figures 4D and S3E), much like ccq1Δ poz1Δ double mutant cells. These data supported the notion that disruption of Tpz1-Ccq1 interaction mainly affects the Ccq1-dependent pathway of telomere maintenance.
Much like ccq1Δ cells [41], Tpz1-Ccq1 interaction disruption mutants immediately activated the G2 DNA damage checkpoint, based on the appearance of highly elongated cells and a slow mobility band corresponding to hyper-phosphorylated Chk1 on SDS PAGE (Figure S6). In addition, tpz1-L449R and tpz1-Y439R,L445R cells, much like ccq1Δ cells, failed to repress the his3+ gene inserted adjacent to telomere repeats, suggesting that Tpz1-Ccq1 interaction is essential for heterochromatin formation at telomere/sub-telomere regions (Figure S7A) [48]. Thus, disruption of Tpz1-Ccq1 interaction recapitulated all phenotypes of ccq1Δ cells we have examined, highlighting the importance of this interaction not only for telomerase regulation and telomere protection [12], [31], [41], [49] but also for the SHREC-dependent role of Ccq1 in heterochromatin formation at sub-telomeres [40].
Tpz1-Ccq1 interaction is required for Ccq1 Thr93 phosphorylation and telomerase recruitment
Next, we performed chromatin immunoprecipitation (ChIP) assays to understand how disruption of Tpz1-Ccq1 interaction affects the association of Tpz1, Ccq1 and Trt1TERT with telomeres. We utilized early generation mutant strains, and the presence of telomeres was confirmed by Southern blot analysis (Figure S8A–C). The amount of Tpz1 bound to telomeres was reduced in tpz1 mutant cells which disrupt Tpz1-Ccq1 interaction (tpz1-L449R, tpz1-Y439R,L445R and tpz1-Y439R,L445R,L449R), much like in ccq1Δ cells (Figure 5A). Likewise, Ccq1 association with telomeres was reduced in these mutants, but retained substantial binding (Figure 5B). Furthermore, we found that tpz1-L449A and tpz1-L449R cause comparable reduction in Ccq1 binding at telomeres (Figure S9A). Thus, even though Tpz1 is the only shelterin subunit known to directly interact with Ccq1 [31], we concluded that Ccq1 could still be recruited to telomeres in the absence of detectable Tpz1-Ccq1 interaction, strongly implicating the existence of additional mechanism(s) that allow recruitment of Ccq1 to telomeres. For both Tpz1 and Ccq1, the reduction in telomere binding we observed could be a consequence of having fewer telomeric repeats. On the other hand, previous studies have found that ccq1-T93A cells, despite having short telomeres, do not show reduced Ccq1 association with telomeres [12], [50], suggesting that Tpz1-Ccq1 does indeed contribute to the efficient accumulation of Ccq1 at telomeres.
Fig. 5. Telomere association of Tpz1, Ccq1 and Trt1TERT in Tpz1-Ccq1 interaction mutant cells. 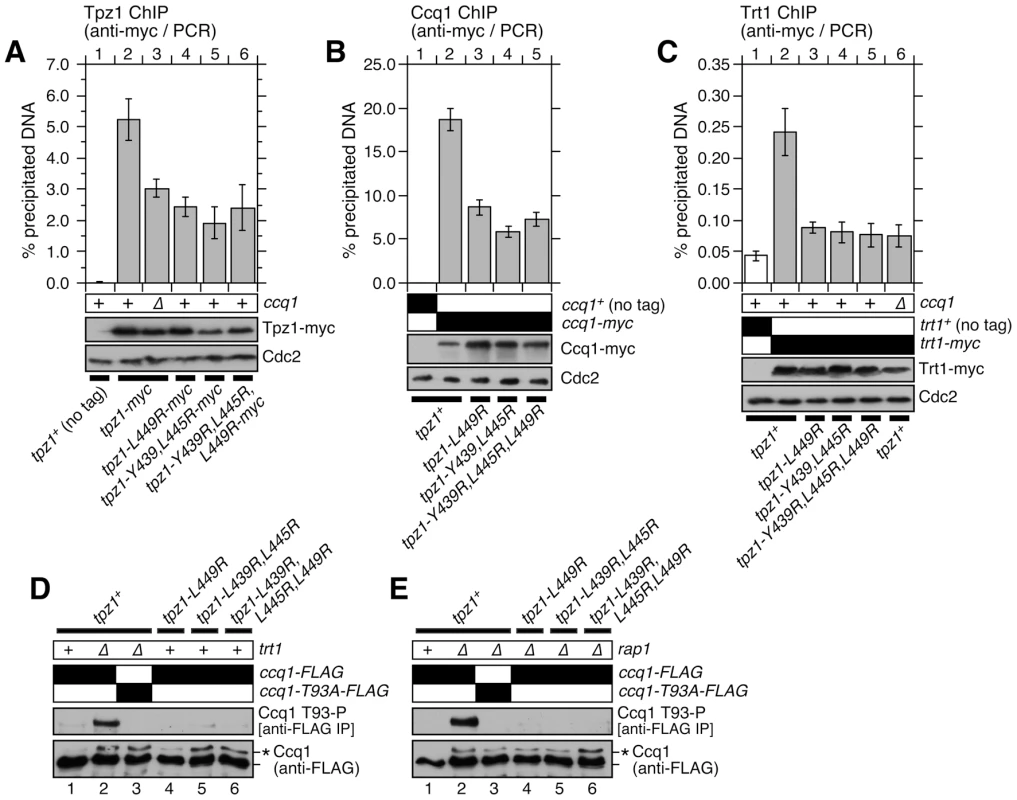
Effects of disrupting Tpz1-Ccq1 interaction on telomere association for (A) Tpz1, (B) Ccq1 and (C) Trt1TERT were monitored by ChIP assays. Error bars represent standard error of the mean from three to nine independent experiments. Statistical analysis of ChIP data by 2-tailed Student's t-test is shown in Table S5. Southern blot analyses of telomere lengths for strains used in ChIP assays are shown in Figure S8A–C. Expression levels of myc-tagged proteins were monitored by anti-myc western blot analysis of whole cell extracts. Anti-Cdc2 blots served as loading control. (D–E) Ccq1 Thr93 phosphorylation, detected by anti-phospho(S/T)Q (Phospho-(Ser/Thr) ATM/ATR substrate antibody in both rap1+ (D) or rap1Δ (E) backgrounds, is abolished in Tpz1-Ccq1 interaction disruption mutants. For anti-FLAG blots, asterisk (*) indicates hyper-phosphorylated form of Ccq1. Ccq1 is required for recruitment of telomerase to telomeres [41], [49]. Thus, we next examined the association of telomerase with telomeres by performing ChIP assays for the catalytic subunit of telomerase Trt1TERT. Despite the fact that substantial amounts of Ccq1 remained bound to telomeres, we found that Trt1TERT binding to telomeres is nearly eliminated in all Tpz1-Ccq1 interaction disruption mutants, much like in ccq1Δ cells (Figures 5C and S9B). Furthermore, we found that Tpz1 fails to co-IP with telomerase RNA TER1 in the absence of Tpz1-Ccq1 interaction (Figure S8D).
Because Rad3ATR/Tel1ATM-dependent Ccq1 Thr93 phosphorylation is required for recruitment of telomerase to telomeres [12], [50], we hypothesized that the loss of telomerase recruitment may be explained by loss of Ccq1 Thr93 phosphorylation. We examined Ccq1 phosphorylation in both rap1+ and rap1Δ backgrounds, since elimination of Rap1 strongly induces Rad3ATR/Tel1ATM-dependent hyper-phosphorylation of Ccq1 at multiple sites including Thr93 [12], allowing us to more robustly determine the effect of disrupting Tpz1-Ccq1 interaction on Ccq1 phosphorylation.
Based on the appearance of a phosphatase sensitive slow mobility band on SDS PAGE, we found that disruption of Tpz1-Ccq1 interaction alone, much like trt1Δ, is sufficient to induce hyper-phosphorylation of Ccq1, due to telomere shortening [12] (Figure 5D bottom panel). Moreover, Ccq1 was still hyper-phosphorylated when Tpz1-Ccq1 interaction was disrupted in rap1Δ cells (Figure 5E bottom panel). By contrast, disruption of Tpz1-Ccq1 interaction completely eliminated Ccq1 Thr93 phosphorylation in both rap1+ and rap1Δ backgrounds (Figure 5D–E top panels). Taken together, we thus concluded that Tpz1-Ccq1 interaction plays an essential role in telomerase recruitment by facilitating Rad3ATR/Tel1ATM-dependent phosphorylation of Ccq1 Thr93. Furthermore, our data indicated that Ccq1 Thr93 phosphorylation is differentially regulated from phosphorylation of other Ccq1 sites and much more dependent on Tpz1-Ccq1 interaction.
Disruption of Tpz1-Poz1 interaction resembles Poz1 deletion
When various truncation mutants of Tpz1, which all expressed well in fission yeast based on western blot analysis (Figure S10A–B), were tested for their effects on telomere maintenance, we found that deletion of the internal Tpz1-Ccq1 interaction domain alone (tpz1-[Δ421–485]) or deletion of both Tpz1-Ccq1 and Tpz1-Poz1 interaction domains (tpz1-[1–420]) result in immediate telomere loss and chromosome circularization (Figure S10C–D). By contrast, deletion of the Tpz1-Poz1 interaction domain alone (tpz1-[1–485]) allowed cells to maintain highly elongated telomeres, much like in poz1Δ cells (Figure 6A lanes 7 and 8, and Figure S10C lane 6).
Fig. 6. Effects of Tpz1-Poz1 interaction disruption mutations on telomere maintenance. 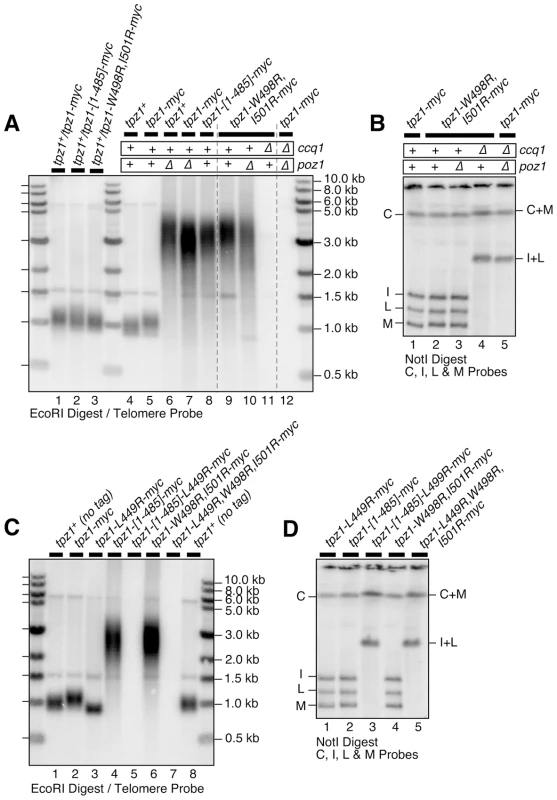
(A) Southern blot analysis and (B) pulsed-field gel analysis for indicated Tpz1-Poz1 interaction disruption mutants. Haploid cells were generated by dissection of spores derived from heterozygous tpz1+/mutated tpz1 diploid cells, and restreaked 5 times on plates prior to preparation of genomic DNA to achieve steady state telomere length, except for tpz1-W499R,I501R poz1Δ and ccq1Δ poz1Δ cells where DNA from survivors with circular chromosomes were made after restreaked twice on plates. For each round of restreak, several faster growing colonies were combined and streaked for single colonies on YES plates. (C) Southern blot analysis and (D) pulsed-field gel analysis for mutants that disrupted either Tpz1-Ccq1 or Tpz1-Poz1 interaction, or both Tpz1-Ccq1 and Tpz1-Poz1 interactions. DNA samples were prepared after restreaked twice on plates. Simultaneous loss of Tpz1-Ccq1 and Tpz1-Poz1 interactions resulted in complete loss of telomeres and circularization of chromosomes, much like in ccq1Δ poz1Δ, tpz1-L449R poz1Δ and tpz1-W498R,I501R ccq1Δ cells. Tpz1 point mutations that disrupted Tpz1-Poz1 interaction (tpz1-W498R,I501R) (Figure 3E) likewise caused telomere elongation comparable to poz1Δ, and telomeres did not show any further elongation in tpz1-W498R,I501R poz1Δ cells (Figure 6A lanes 7, 9 and 10). Furthermore, tpz1-W498R,I501R ccq1Δ cells immediately lost telomeres, as soon as they were germinated from spores derived from heterozygous diploid (tpz1+/tpz1-W498R,I501R ccq1+/ccq1Δ) cells, and survived by circularizing their chromosomes, very much like in ccq1Δ poz1Δ cells (Figure 6A lanes 11 and 12, and Figure 6B lanes 4 and 5). We also observed that cells carrying tpz1 mutants that incorporate disruption mutations for both Tpz1-Ccq1 and Tpz1-Poz1 interactions (tpz1-[1–485]-L449R and tpz1-L449R,W498R,I501R) fail to protect telomeres against fusions, immediately lose viability for the majority of cells, and exclusively generate survivors with circular chromosomes (Figure 6C lanes 5 and 7, and Figure 6D lanes 3 and 5).
Taken together, we thus concluded that telomere length deregulation caused by disrupting Tpz1-Poz1 interaction specifically inactivates Poz1's ability to prevent uncontrolled telomere elongation. Furthermore, we concluded that Tpz1-Poz1 and Tpz1-Ccq1 interactions redundantly provide essential telomere protection functions of Tpz1 [31]. While it remains to be established why Ccq1 and Poz1 are redundantly required to prevent telomere fusions, we can rule out the possibility that they are redundantly required for assembly of the Tpz1-Pot1 complex, since Tpz1-Pot1 interaction detected by co-IP remain intact in ccq1Δ poz1Δ cells (Figure S11D). Furthermore, in a Pot1-dependent in vitro pull down assay for Tpz1 utilizing a magnetic-bead coupled telomeric G-tail oligo, wild-type Tpz1 could still be detected in ccq1Δ poz1Δ cells, and both Tpz1-[1–485]-L449R and Tpz1-L449R,W498R,I501R mutant proteins, which interact with neither Ccq1 nor Poz1, were also detected (Figure S11A–C).
Disruption of Tpz1-Poz1 interaction also allowed expression of the his3+ gene inserted adjacent to telomere repeats (Figure S7B), much like poz1Δ cells [49], suggesting that heterochromatin formation at telomeres also requires Tpz1-Poz1 interaction. However, both tpz1-W498,I501R and tpz1-[1–485] cells grew slower than poz1Δ cells on selective media lacking histidine, suggesting that Poz1, even in the absence of Tpz1-Poz1 interaction, weakly contributes to the formation of heterochromatin at telomeres.
Disruption of Tpz1-Poz1 interaction causes strong reduction in Poz1 binding to telomeres, and increases Ccq1 Thr93 phosphorylation and telomerase recruitment
In order to gain insight into how the disruption of Tpz1-Poz1 interaction affects the association of shelterin subunits and telomerase with telomeres, we next carried out ChIP assays for Tpz1, Ccq1, Poz1 and Trt1TERT. It was necessary to utilize dot blot-based ChIP assays, rather than quantitative real-time PCR-based ChIP assays, since tpz1-W498R,I501R caused massive elongation of telomere repeats (Figures 6A and S12) and thus putting the sub-telomeric annealing sites for our PCR primers too far away from actual telomeric ends [12], [36].
By quantifying hybridization intensities of precipitated and input DNA to a telomeric repeat DNA probe, we first established % DNA that was precipitated by ChIP relative to input (raw % precipitated DNA) (Figure S13). Changes in raw % precipitated DNA values more closely reflect changes in density of a given protein on telomeric repeats, rather than changes in total amount of protein associated per chromosome end. Thus, it became necessary to correct raw % precipitated DNA values for telomere length to better represent changes in amount of protein bound per chromosome end, especially for cells carrying highly elongated telomeres.
To account for changes in telomere length, we first established “telomere length correction factors” for individual strains by measuring changes in telomere/rDNA hybridization intensity ratios compared to wild-type cells (Table S1) [36]. We then established “telomere length corrected” ChIP values by multiplying background subtracted % precipitated DNA values (raw % precipitated DNA from epitope tagged strain – no tag control % precipitated DNA) with the telomere length correction factors, and normalizing them to wild-type ChIP values (plotted as “relative ChIP signal”) [36]. Although not perfect, this adjustment for variations in telomere length allowed us to better estimate changes in amount of protein localized per chromosome end.
Analysis of ChIP data revealed that tpz1-W498R,I501R, poz1Δ and tpz1-W498R,I501R poz1Δ cells show comparable increases in amount of Tpz1 and Ccq1 per chromosome end over wild-type cells when corrected for telomere elongation in these mutant cells (Figure 7A–B). Since single and double mutants for tpz1-W498R,I501R and poz1Δ showed comparable changes in Tpz1 and Ccq1 association with telomeres, these ChIP data further confirmed that the loss of Tpz1-Poz1 interaction solely disrupts Poz1 function at telomeres.
Fig. 7. Telomere association of Tpz1, Ccq1, Poz1 and Trt1TERT in Tpz1-Poz1 interaction mutant cells. 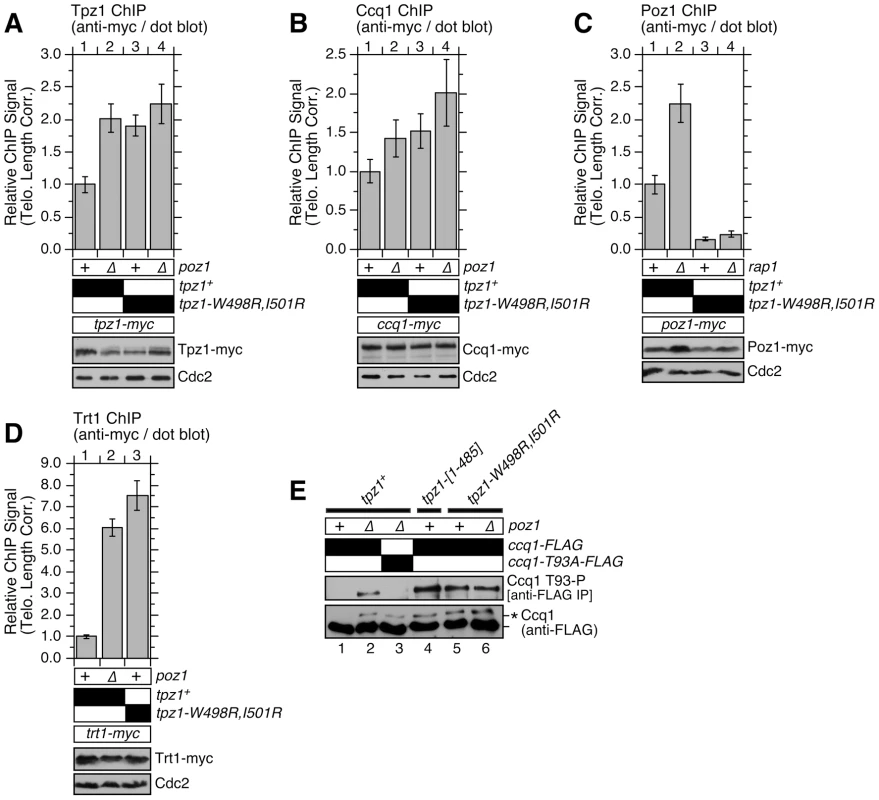
Effects of disrupting Tpz1-Poz1 interaction on telomere association for (A) Tpz1, (B) Ccq1, (C) Poz1 and (D) Trt1TERT were monitored by dot-blot ChIP assays and corrected for changes in telomere length [36]. Error bars represent standard error of the mean from three to eight independent experiments. Statistical analysis of ChIP data by 2-tailed Student's t-test is shown in Table S5. Southern blot analysis of telomere length for strains used in ChIP assays is shown in Figure S12, and raw data for ChIP assays are shown in Figure S13. Expression levels of myc-tagged proteins were monitored by anti-myc western blot analysis of whole cell extracts. Anti-Cdc2 blots served as loading control. (E) Ccq1 Thr93 phosphorylation, detected by anti-phospho(S/T)Q (Phospho-(Ser/Thr) ATM/ATR substrate antibody, is enhanced in mutant cells lacking Tpz1-Poz1 interaction. For anti-FLAG blots, asterisk (*) indicates hyper-phosphorylated form of Ccq1. Further analysis of Poz1 ChIP data indicated that Tpz1-Poz1 interaction is crucial for efficient accumulation of Poz1 at telomeres, as tpz1-W498R,I501R or tpz1-W498R,I501R rap1Δ cells showed a substantial reduction in Poz1 association with telomeres compared to wild-type cells (Figure 7C). By contrast, rap1Δ cells showed an increase in Poz1 association (Figure 7C) [36]. We have previously shown that rap1Δ also causes a comparable increase in telomere association for Tpz1 and Ccq1 [36]. Taken together, we concluded that Poz1 association with telomeres is primarily facilitated by Tpz1-Poz1 interaction, and that Poz1-Rap1 interaction does not play a significant role in association of Poz1 with telomeres.
On the other hand, it should be noted that Poz1 association, although significantly decreased, is not completely eliminated even in tpz1-W498R,I501R rap1Δ cells (Figures 7C and S13C). As noted earlier, we found that the presence of Poz1 protein appears to contribute weakly to the transcriptional repression of the his3+ marker in tpz1-W498R,I501R cells (Figure S7B). Therefore, residual Rap1 - and Tpz1-independent association of Poz1 with telomeres may also be functionally important. Alternatively, since Tpz1-W498R,I501R protein still showed residual interaction with Poz1 in Y2H assay (Figure 2C), it might also retain a residual weak interaction in vivo (not detected by co-IP) that is responsible for its residual localization to telomeres.
Since introduction of tpz1-W498R,I501R or tpz1-[1–485] caused telomere extensions comparable to poz1Δ (Figure 6A), we expected that loss of Tpz1-Poz1 interaction would cause increases in both telomerase association with telomeres and Ccq1 Thr93 phosphorylation, as previously established for poz1Δ cells [12], [36]. Indeed, ChIP assays for the telomerase catalytic subunit Trt1TERT revealed that tpz1-W498R,I501R causes a comparable increase in Trt1TERT binding to telomeres as poz1Δ cells (Figures 7D and S13D). In addition, we found that both tpz1-W498R,I501R and tpz1-[1–485] mutations cause hyper-phosphorylation at Thr93 and other sites of Ccq1 (Figure 7E). Thus, we concluded that Tpz1-Poz1 interaction-dependent recruitment of Poz1 is essential for enforcing a negative regulation on Ccq1 Thr93 phosphorylation-dependent recruitment of telomerase.
Discussion
Tpz1-Ccq1 and Tpz1-Poz1 interactions modulate Ccq1 Thr93 phosphorylation and telomerase recruitment
In this study, we determined amino acid residues within two distinct C-terminal domains of Tpz1 that are responsible for mediating either Tpz1-Ccq1 or Tpz1-Poz1 interaction, and characterized how these interactions individually or in combination affect the ability of the shelterin complex to ensure telomere maintenance and protection in fission yeast. (Key findings are summarized in Figure 8). Our results indicated that disruption of Tpz1-Ccq1 interaction causes telomere phenotypes that are essentially identical to those of ccq1Δ cells (Figures 4, S3, and S5). Cells lacking Tpz1-Ccq1 interaction fail to efficiently recruit telomerase to telomeres, due to loss of Rad3ATR/Tel1ATM kinase-dependent Ccq1 Thr93 phosphorylation (Figure 5C–E), which is essential for promoting Est1-Ccq1 interaction and telomerase recruitment [12] (Figure 8).
Fig. 8. Summary of key findings from the current study. 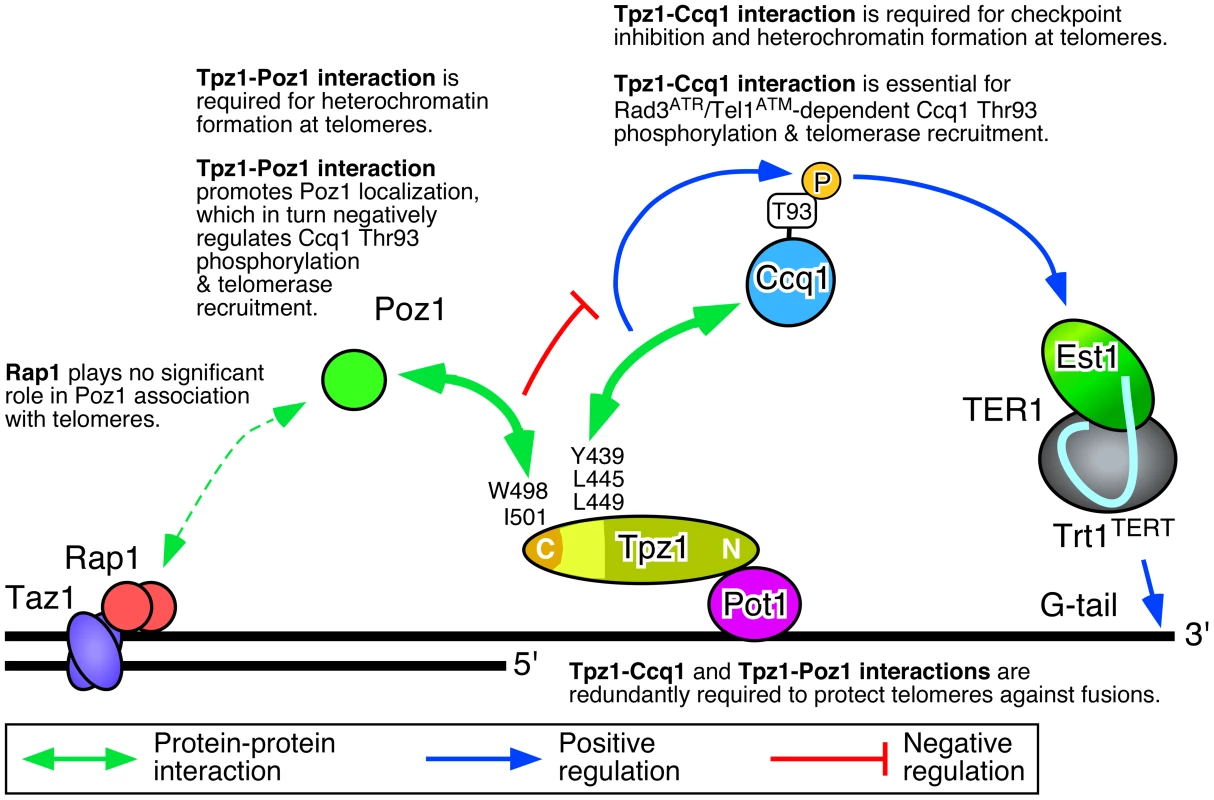
Our current study establish that (1) Tpz1-Ccq1 interaction is essential for Ccq1 Thr93 phosphorylation and telomerase recruitment, (2) Tpz1-Poz1 interaction promotes efficient accumulation of Poz1 to inhibit Ccq1 Thr93 phosphorylation and telomerase recruitment, and (3) Tpz1-Ccq1 and Tpz1-Poz1 interactions are redundantly required for protection against telomere fusions. Although Ccq1 association with telomeres was reduced, significant amounts were still detectable in the absence of Tpz1-Ccq1 interaction (Figure 5B), implicating the existence of an alternative mechanism that allows recruitment of Ccq1 to telomeres. Ccq1 also interacts with the SHREC complex that facilitates heterochromatin formation at telomeres [40] and heterochromatin-dependent recruitment of Ccq1 has been proposed as a mechanism to allow recruitment of Pot1 to protect chromosome ends in HATTI survivor cells that lack telomere repeats at chromosome ends [51]. Thus, it is possible that the SHREC complex is responsible for allowing Ccq1 localization at telomeres in the absence of Tpz1-Ccq1 interaction. On the other hand, we cannot completely rule out the possibility that a weak residual Tpz1-Ccq1 interaction, as detected under less stringent (−His) Y2H assay conditions (Figure S2C) but not by co-IP experiments (Figure 3C), may still contribute to Ccq1 binding at telomeres. In any case, our results established that localization of Ccq1 at telomeres alone is not sufficient for telomerase recruitment to telomeres, due to the fact that Tpz1-Ccq1 interaction is essential for Rad3ATR/Tel1ATM-dependent phosphorylation of Ccq1 Thr93.
Furthermore, we have shown that disruption of Tpz1-Poz1 interaction causes a dramatic reduction in Poz1 association with telomeres (Figure 7C), and results in phenotypes that are essentially identical to those of poz1Δ cells, including strong induction of Ccq1 Thr93 phosphorylation (Figure 7E), enhanced telomerase recruitment (Figure 7D) and massive elongation of telomeres (Figure 6). Since we have previously shown that poz1Δ cells accumulate more RPA (Replication Protein A) and Rad3ATR-Rad26ATRIP kinase complex at telomeres [36], it is likely that increased Ccq1 Thr93 phosphorylation in Tpz1-Poz1 interaction disruption mutant cells is also caused by a failure to limit the accumulation of Rad3ATR kinase.
Consistent with the notion that Tpz1-Ccq1 disruption and Tpz1-Poz1 disruption cause telomere defects nearly identical to ccq1Δ and poz1Δ respectively, we also found that (1) simultaneous disruption of both Tpz1-Ccq1 and Tpz1-Poz1 interaction, (2) combining the Tpz1-Ccq1 disruption mutation with poz1Δ, and (3) combining the Tpz1-Poz1 disruption mutation with ccq1Δ, all cause a strong synergistic loss of telomere protection and immediate telomere fusion phenotype (Figures 4, 6, and S3D–E), much like in ccq1Δ poz1Δ cells [31]. Thus, our current study establishes that Tpz1-Poz1 and Tpz1-Ccq1 interactions redundantly fulfill the essential telomere protection function of the shelterin complex. Taken together, our data from current and previous studies [12], [36] suggest that the negative regulatory function of Tpz1-Poz1 interaction works upstream of Rad3ATR kinase to limit Rad3ATR/Tel1ATM-dependent phosphorylation of Ccq1 Thr93, while Tpz1-Ccq1 interaction works downstream of Rad3ATR kinase to facilitate Ccq1 Thr93 phosphorylation and telomerase recruitment (Figure 8).
While our studies were in progress, another study, which also identified distinct Tpz1 C-terminal domains critical for mediating either Tpz1-Ccq1 or Tpz1-Poz1 interactions, was published [43]. While their results agreed well with our current study for the domains and amino acid residues that promote Tpz1-Ccq1 or Tpz1-Poz1 interaction, our conclusions differ significantly with regard to the functional significance of these interactions for fission yeast telomere regulation.
While their ChIP analysis of a Tpz1-Ccq1 disruption mutant (tpz1-L449A) showed no effect on Ccq1 or Trt1TERT association with telomeres [43], our current findings clearly showed that all Tpz1-Ccq1 interaction mutants (including tpz1-L449A) reduce Ccq1 binding and almost entirely eliminate Trt1TERT recruitment at telomeres (Figure 5B–D). Even more strikingly, they reported that tpz1-L449A poz1Δ cells carry highly elongated telomeres [43], unlike our finding that tpz1-L449A poz1Δ and tpz1-L449R poz1Δ cells immediately lose telomeres and survive by circularizing their chromosomes (Figures 4C–D and S3D–E). Furthermore, their ChIP analysis also indicated that disruption of Tpz1-Poz1 does not affect localization of Poz1 (for tpz1-I501R and tpz1-I501A,R505E) or Trt1TERT (for tpz1-I501R) at telomeres [43], in contrast to our current study, which found that Tpz1-Poz1 interaction promotes Poz1 localization and prevent telomerase recruitment by limiting Ccq1 Thr93 phosphorylation.
Unfortunately, we are not entirely sure why our findings are so different from the recent study, especially with regard to tpz1-L449A where both studies have in theory analyzed the effect of the same single amino acid mutation. A direct comparison of the Tpz1-Poz1 results is more complicated due to the fact that mutant alleles analyzed in two studies are not identical. However, we do note that our Tpz1-Poz1 disruption mutants (tpz1-[1–485] and tpz1-W498R,I501R) appears to destabilize Poz1 (Figure 3E), and behave essentially identical to poz1Δ cells. In contrast, their mutants showed significantly less telomere elongation than poz1Δ cells and did not affect Poz1 stability [43], raising the possibility that their mutants have retained residual Tpz1-Poz1 interaction not detected by their co-IP analysis. Another potential weakness of the previous study was that their ChIP data for Trt1TERT localization was quantified with real-time PCR primers that anneal to the sub-telomeric sequence adjacent to telomeric repeats, even for tpz1-I501A cells, which carry long telomeres [43]. In contrast, we performed dot blot-based Trt1TERT ChIP analysis and corrected for telomere length for tpz1-W498R,I501R cells.
In any case, our results are incompatible with the model proposed by Jun et al. [43], which suggested that Tpz1-Ccq1 interaction works “upstream” (rather than downstream as we propose here) of Tpz1-Poz1 interaction to overcome a “non-extendible” shelterin complex status that is defined by the fully connected Taz1-Rap1-Poz1-Tpz1-Pot1 linkage, based primarily on their observation that tpz1-L449A poz1Δ and tpz1-L449A-I501R cells carry highly elongated telomeres in their hand [43]. Furthermore, it should be noted that their proposed model did not even attempt to explain how the shelterin complex enforces late S-phase specific recruitment of telomerase to telomeres [35], or how it regulates Rad3ATR/Tel1ATM-dependent Ccq1 Thr93 phosphorylation [12], [50] to allow preferential recruitment of telomerase to shorter telomeres (Figure S9B) [36]. In contrast, our current model (Figure 8) [36] provides an explanation for all previous observations [12], [31], [36], [41], [49] with regard to how telomerase association and telomere extensions are controlled by the shelterin complex and Rad3ATR/Tel1ATM kinases in fission yeast. Furthermore, since our detailed cell cycle ChIP analyses have recently identified Poz1 as a critical regulatory factor that promotes the timely arrival of the lagging strand DNA polymerase α at telomeres to limit accumulation of ssDNA and Rad3ATR kinase in late S-phase [36], we suggest that Tpz1-Poz1 interaction-dependent localization of Poz1 to telomeres is required to negatively regulate telomere extension during late-S phase by ensuring proper coordination of leading and lagging strand synthesis at telomeres to limit Ccq1 Thr93 phosphorylation and telomerase recruitment (Figure 8) [36].
Possible implications for regulation of telomere maintenance by the mammalian shelterin complex
While the shelterin complex in mammalian cells has been found to negatively regulate the DNA damage checkpoint kinases ATM and ATR [26], [27], ATM and ATR kinases also contribute to telomere protection and maintenance [52]–[54]. Furthermore, much like in fission yeast, ATM and ATR specifically associate with telomeres during S/G2-phases [55], and lagging strand synthesis at mammalian telomeres is significantly delayed compared to leading strand synthesis [56]–[58]. In addition, mammalian TPP1 (Tpz1 ortholog), in collaboration with TRF1, TIN2 and POT1, has been shown to play both positive and negative roles in extension of telomeres [18], [19], [22]–, much like Tpz1 in fission yeast.
Studies have also found that TIN2 plays a major role in POT1-dependent inhibition of ATR activation at telomeres [60]. Therefore, TIN2-TPP1 interaction (much like Poz1-Tpz1 interaction) is also likely to function upstream of ATR kinase to ensure telomere stability. Since the mammalian shelterin complex subunit TRF1 (much like Taz1) has been found to promote semi-conservative replication of telomeres by replicative DNA polymerases [36], [61], [62], TRF1-TIN2-TPP1 interaction might control accumulation of ATM and ATR kinases at telomeres by regulating ssDNA accumulation at telomeres by coordinating leading and lagging strand synthesis. Thus, our current findings, which established roles of Tpz1-Poz1 and Tpz1-Ccq1 interactions in regulating Rad3ATR/Tel1ATM-dependent Ccq1 Thr93 phosphorylation and telomerase recruitment, may be relevant in understanding how the mammalian shelterin complex can collaborate with DNA damage checkpoint kinases to ensure telomere maintenance.
Materials and Methods
Yeast strains and plasmids
Fission yeast and budding yeast strains were generated and cultured using standard techniques and protocols [63], [64]. Growth analysis of serially diluted liquid cultures was performed as previously described [45] with cells diluted to 4×104 cells/ml every 24 hours in YES (Yeast Extract Supplemented) media [63]. Effects of various tpz1 mutations on telomere heterochromatin formation were monitored by utilizing his3+::tel(1L) strains that carry an integrated his3+ marker adjacent to telomere repeats of chromosome I left arm, as previously described [48].
Fission yeast strains used in this study are listed in Table S2. For ccq1Δ::hphMX, poz1Δ::natMX6, rap1Δ::ura4+, and trt1Δ::his3+, original deletion strains were described previously [4], [38], [49], [65]. For pot1Δ::natMX, the drug resistance marker of pot1Δ::kanMX strain [33] was changed by transformation of a natMX fragment. For tpz1-myc, trt1-myc, poz1-myc, ccq1-myc, ccq1-FLAG, pot1-FLAG and chk1-myc, original tagged strains were described previously [31], [35], [49], [66], [67]. For poz1-FLAG, an epitope tag was introduced by PCR-based method [68]. Various myc-tagged tpz1 mutant alleles were integrated at the endogenous tpz1+ locus using a BsgI-BglI tpz1-myc-kanMX fragment, excised from mutagenized pBS-tpz1-13myc-kanMX plasmids listed in Table S3. Point mutations were introduced by Phusion site-directed mutagenesis (NEB, F-541) or QuikChange Lightning site-directed mutagenesis (Agilent, 210513). Strains carrying various tpz1 mutations without epitope tags were subsequently generated by transforming tpz1-myc mutant strains with a C-terminal tpz1 fragment marked with hphMX within the 3′ untranslated region of the tpz1+ gene.
Yeast two-hybrid (Y2H) assays were performed by mating Saccharomyces cerevisiae MATa (Y2HGold: MATa trp1-901 leu2-3,-112 ura3-52 his3-200 LYS2::GAL1(UAS)-GAL1(TATA)-HIS3 GAL2(UAS)-GAL2(TATA)-ADE2 gal4Δ gal80Δ URA3::MEL1(UAS)-MEL1(TATA)-AUR1-C MEL1) strains harboring GAL4-DBD (DNA-binding domain) plasmids with MATα (Y187: MATα trp1-901 leu2-3,-112 ura3-52 his3-200 ade2-101 gal4Δ gal80Δ met− URA3::GAL1(UAS)-GAL1(TATA)-LacZ MEL1) strains harboring GAL4-AD (activation domain) plasmids, as described in the MATCHMAKER system manual (Clontech). Plasmids used in Y2H assays are listed in Table S4. Positive Y2H interactions were identified by spotting mated cells onto SD−HTL (−His) or SD−HTLA (−His −Ade) plates. To increase selection stringency, 1 mM or 5 mM 3-amino-1,2,4-triazole (3AT) was added to SD−HTL plates.
Co-immunoprecipitation (co-IP) and western blot analysis
Co-IP experiments were performed as previously described [12], [49]. Briefly, cell extracts were prepared in lysis buffer [50 mM Tris pH 8.0, 150 mM NaCl, 10% glycerol, 5 mM EDTA, 0.5% NP40, 50 mM NaF, 1 mM DTT, 1 mM PMSF, 0.2 mM APMSF, 1 mM Na3VO4, ‘Complete’ protease inhibitor cocktail] using glass beads. For low salt co-IP experiment shown in Figure S3, lysis buffer contained 60 mM NaCl instead of 150 mM. Proteins were immunoprecipitated using either monoclonal anti-myc antibody (9B11, Cell Signaling) or monoclonal anti-FLAG antibody (M2-F1804, Sigma), and Dynabeads Protein G (Life Technologies), and washed 3× in lysis buffer. Immunoprecipitated proteins were analyzed by western blot analysis using monoclonal anti-FLAG or anti-myc as primary antibodies. In addition, anti-Cdc2 (y100.4, Abcam) was used to detect Cdc2 in whole cell extract (WCE) to serve as loading control. To detect Ccq1 Thr93 phosphorylation by anti-phospho(S/T)Q (Phospho-(Ser/Thr) ATM/ATR substrate antibody (2851, Cell Signaling), Ccq1 was first immuno-purified from whole cell extract using anti-FLAG antibody as previously described [12]. Either horseradish peroxidase (HRP)-conjugated (goat) anti-mouse (Pierce, 31430) or HRP-conjugated (goat) anti-rabbit (Pierce, 31460) was used as the secondary antibody.
Pulsed-field gel electrophoresis and Southern blot analysis
Pulsed-field gel electrophoresis of NotI-digested chromosomal DNA was separated on a 1% agarose gel as previously described [69] in 0.5xTAE buffer at 14°C, using the CHEF-DR III system (BioRad) at 6 V/cm (200 V) and a pulse time of 60 to 120 sec for 24 hours. For telomere length analysis by Southern blotting, EcoRI-digested genomic DNA was separated on a 1% agarose gel, transferred to nylon membrane, and hybridized to a P32-labeled telomeric DNA probe as previously described [69], [70].
ChIP assay, quantitative PCR and dot blot analysis
ChIP assays were carried out as previously described [36], [49], [70]. Fission yeast cells were crosslinked with 1% formaldehyde for 20 min at room temperature, and then incubated with 125 mM glycine for 5 min at room temperature. Cell extracts were then prepared in lysis buffer [50 mM Hepes-KOH pH 7.5, 140 mM NaCl, 1 mM EDTA, 1% (v/v) Triton X-100, 0.1% (w/v) sodium deoxycholate, 1 mM PMSF, ‘Complete’ protease inhibitor cocktail] using glass beads, and sonicated in an ice bath (Misonix Sonicator 3000 with cup horn device) to obtain ∼500 bp DNA fragments. 1–2 mg lysate was prepared in 200–250 µl lysis buffer and 5 µl was set aside for input. 1 µg of monoclonal anti-Myc (9B11) antibody was added to the ChIP sample and incubated for two hours at 4°C, then 30 µl of Dynabeads Protein G (Life Technologies) were added and incubated for one hour at 4°C. IPs were washed 3× with 1 ml lysis buffer, and DNA was recovered from ChIP and input samples using Chelex 100 as previously described [36], [49], [70].
Quantitative real-time PCR was performed with primers annealing to a region adjacent to the telomeric repeat (jk380 5′-TAT TTC TTT ATT CAA CTT ACC GCA CTT C-3′ and jk381 5′-CAG TAG TGC AGT GTA TTA TGA TAA TTA AAA TGG-3′) [71] utilizing SYBR green to quantify PCR products. % Precipitated DNA values were calculated based on ΔCt between Input and IP samples with the following formula: [% Precipitated DNA] = [100*EΔCt]/[D*R], where E = amplification efficiency of primer pairs, ΔCt = [Ct Input]−[Ct ChIP], D = [dilution factor of ChIP]/[dilution factor of Input] and R = [Sample volume used in IP]/[Sample volume set aside for Input control].
For dot blot-based analysis of ChIP samples, DNA was denatured in 0.4 M NaOH, 10 mM EDTA for 10 min at 100°C, then rapidly cooled on ice, and subsequently blotted onto Hybond XL membrane (GE Healthcare) using a Bio-Dot Microfiltration Apparatus (Bio-Rad), and hybridized to a P32-labeled telomeric DNA probe as previously described [36], [49], [70]. For cells carrying highly elongated telomeres, data were corrected for changes in telomere length as previously described [36] by using correction factors for telomere length, established by measuring the hybridization signal intensity of telomere versus rDNA repeats (telomere/rDNA) (Table S1). “Telomere length corrected” ChIP values were then calculated by multiplying the background subtracted % precipitated DNA values (raw % precipitated DNA – no tag control % precipitated DNA) with the correction factors, and normalized to values from wild-type cells. SEM of telomere length corrected ChIP (SEMQ) was calculated as previously described [36].
Telomere primer pull-down assay
Biotin-DNA primer coated Streptavidin beads were prepared by mixing 30 µl Dynabeads M-280 Streptavidin (Invitrogen, 10 mg/ml) with 100 pmol of biotinylated oligonucleotide primers (C-oligo: 5′ Biotin-CGT AAC CGT AAC CCT GTA ACC TGT AAC CTG TAA CCG TGT AAC C 3′; G-oligo: 5′ Biotin-GGT TAC ACG GTT ACA GGT TAC AGG TTA CAG GGT TAC GGT TAC G 3′) in Binding Buffer BW [5 mM Tris, pH 7.5, 0.5 mM EDTA, 1M NaCl] for 15 min at RT. Beads were then washed 2× with 1 ml BW and resuspended in 30 µl lysis buffer LB [50 mM Tris pH 8.0, 150 mM NaCl, 10% glycerol, 5 mM EDTA, 0.5% NP40, 50 mM NaF, 1 mM DTT, 1 mM PMSF, 0.2 mM APMSF, 1 mM Na3VO4, ‘Complete’ protein inhibitor cocktail]. 2 mg of fission yeast whole cell extract, prepared in LB, was then incubated with 30 µl Biotin-primer coated beads for 2 h at 4°C, washed 3× with 1 ml LB, and finally resuspended in 25 µl 2× Laemmli sample buffer for subsequent western blot analysis.
Supporting Information
Zdroje
1. PalmW, de LangeT (2008) How shelterin protects mammalian telomeres. Annu Rev Genet 42 : 301–334.
2. GilsonE, GeliV (2007) How telomeres are replicated. Nat Rev Mol Cell Biol 8 : 825–838.
3. VerdunRE, KarlsederJ (2007) Replication and protection of telomeres. Nature 447 : 924–931.
4. NakamuraTM, MorinGB, ChapmanKB, WeinrichSL, AndrewsWH, et al. (1997) Telomerase catalytic subunit homologs from fission yeast and human. Science 277 : 955–959.
5. NugentCI, LundbladV (1998) The telomerase reverse transcriptase: components and regulation. Genes Dev 12 : 1073–1085.
6. LeonardiJ, BoxJA, BunchJT, BaumannP (2008) TER1, the RNA subunit of fission yeast telomerase. Nat Struct Mol Biol 15 : 26–33.
7. FengJ, FunkWD, WangSS, WeinrichSL, AvilionAA, et al. (1995) The RNA component of human telomerase. Science 269 : 1236–1241.
8. LingnerJ, CechTR, HughesTR, LundbladV (1997) Three Ever Shorter Telomere (EST) genes are dispensable for in vitro yeast telomerase activity. Proc Natl Acad Sci U S A 94 : 11190–11195.
9. WeinrichSL, PruzanR, MaL, OuelletteM, TesmerVM, et al. (1997) Reconstitution of human telomerase with the template RNA component hTR and the catalytic protein subunit hTRT. Nat Genet 17 : 498–502.
10. ChanA, BouleJB, ZakianVA (2008) Two pathways recruit telomerase to Saccharomyces cerevisiae telomeres. PLoS Genet 4: e1000236.
11. BeerninkHT, MillerK, DeshpandeA, BucherP, CooperJP (2003) Telomere maintenance in fission yeast requires an Est1 ortholog. Curr Biol 13 : 575–580.
12. MoserBA, ChangYT, KostiJ, NakamuraTM (2011) Tel1ATM and Rad3ATR kinases promote Ccq1-Est1 interaction to maintain telomeres in fission yeast. Nat Struct Mol Biol 18 : 1408–1413.
13. O'ConnorMS, SafariA, XinH, LiuD, SongyangZ (2006) A critical role for TPP1 and TIN2 interaction in high-order telomeric complex assembly. Proc Natl Acad Sci U S A 103 : 11874–11879.
14. VerdunRE, CrabbeL, HaggblomC, KarlsederJ (2005) Functional human telomeres are recognized as DNA damage in G2 of the cell cycle. Mol Cell 20 : 551–561.
15. YeJZ, DonigianJR, van OverbeekM, LoayzaD, LuoY, et al. (2004) TIN2 binds TRF1 and TRF2 simultaneously and stabilizes the TRF2 complex on telomeres. J Biol Chem 279 : 47264–47271.
16. TakaiKK, HooperS, BlackwoodS, GandhiR, de LangeT (2010) In vivo stoichiometry of shelterin components. J Biol Chem 285 : 1457–1467.
17. SmogorzewskaA, de LangeT (2004) Regulation of telomerase by telomeric proteins. Annu Rev Biochem 73 : 177–208.
18. HoughtalingBR, CuttonaroL, ChangW, SmithS (2004) A dynamic molecular link between the telomere length regulator TRF1 and the chromosome end protector TRF2. Curr Biol 14 : 1621–1631.
19. LiuD, SafariA, O'ConnorMS, ChanDW, LaegelerA, et al. (2004) PTOP interacts with POT1 and regulates its localization to telomeres. Nat Cell Biol 6 : 673–680.
20. YeJZ, HockemeyerD, KrutchinskyAN, LoayzaD, HooperSM, et al. (2004) POT1-interacting protein PIP1: a telomere length regulator that recruits POT1 to the TIN2/TRF1 complex. Genes Dev 18 : 1649–1654.
21. XinH, LiuD, WanM, SafariA, KimH, et al. (2007) TPP1 is a homologue of ciliate TEBP-β and interacts with POT1 to recruit telomerase. Nature 445 : 559–562.
22. NandakumarJ, BellCF, WeidenfeldI, ZaugAJ, LeinwandLA, et al. (2012) The TEL patch of telomere protein TPP1 mediates telomerase recruitment and processivity. Nature 492 : 285–289.
23. ZhongFL, BatistaLF, FreundA, PechMF, VenteicherAS, et al. (2012) TPP1 OB-fold domain controls telomere maintenance by recruiting telomerase to chromosome ends. Cell 150 : 481–494.
24. AbreuE, AritonovskaE, ReichenbachP, CristofariG, CulpB, et al. (2010) TIN2-tethered TPP1 recruits human telomerase to telomeres in vivo. Mol Cell Biol 30 : 2971–2982.
25. TejeraAM, Stagno d'AlcontresM, ThanasoulaM, MarionRM, MartinezP, et al. (2010) TPP1 is required for TERT recruitment, telomere elongation during nuclear reprogramming, and normal skin development in mice. Dev Cell 18 : 775–789.
26. DenchiEL, de LangeT (2007) Protection of telomeres through independent control of ATM and ATR by TRF2 and POT1. Nature 448 : 1068–1071.
27. SfeirA, de LangeT (2012) Removal of shelterin reveals the telomere end-protection problem. Science 336 : 593–597.
28. GuoX, DengY, LinY, Cosme-BlancoW, ChanS, et al. (2007) Dysfunctional telomeres activate an ATM-ATR-dependent DNA damage response to suppress tumorigenesis. EMBO J 26 : 4709–4719.
29. ArmaniosM, BlackburnEH (2012) The telomere syndromes. Nat Rev Genet 13 : 693–704.
30. LansdorpPM (2009) Telomeres and disease. EMBO J 28 : 2532–2540.
31. MiyoshiT, KanohJ, SaitoM, IshikawaF (2008) Fission yeast Pot1-Tpp1 protects telomeres and regulates telomere length. Science 320 : 1341–1344.
32. MoserBA, NakamuraTM (2009) Protection and replication of telomeres in fission yeast. Biochem Cell Biol 87 : 747–758.
33. BaumannP, CechTR (2001) Pot1, the putative telomere end-binding protein in fission yeast and humans. Science 292 : 1171–1175.
34. PittCW, CooperJP (2010) Pot1 inactivation leads to rampant telomere resection and loss in one cell cycle. Nucleic Acids Res 38 : 6968–6975.
35. MoserBA, SubramanianL, ChangYT, NoguchiC, NoguchiE, et al. (2009) Differential arrival of leading and lagging strand DNA polymerases at fission yeast telomeres. EMBO J 28 : 810–820.
36. ChangYT, MoserBA, NakamuraTM (2013) Fission yeast shelterin regulates DNA Polymerases and Rad3ATR kinase to limit telomere extension. PLoS Genet 9: e1003936.
37. CooperJP, NimmoER, AllshireRC, CechTR (1997) Regulation of telomere length and function by a Myb-domain protein in fission yeast. Nature 385 : 744–747.
38. KanohJ, IshikawaF (2001) spRap1 and spRif1, recruited to telomeres by Taz1, are essential for telomere function in fission yeast. Curr Biol 11 : 1624–1630.
39. ChikashigeY, HiraokaY (2001) Telomere binding of the Rap1 protein is required for meiosis in fission yeast. Curr Biol 11 : 1618–1623.
40. SugiyamaT, CamHP, SugiyamaR, NomaK, ZofallM, et al. (2007) SHREC, an effector complex for heterochromatic transcriptional silencing. Cell 128 : 491–504.
41. TomitaK, CooperJP (2008) Fission yeast Ccq1 is telomerase recruiter and local checkpoint controller. Genes Dev 22 : 3461–3474.
42. FujitaI, TanakaM, KanohJ (2012) Identification of the functional domains of the telomere protein Rap1 in Schizosaccharomyces pombe. PLoS One 7: e49151.
43. JunHI, LiuJ, JeongH, KimJK, QiaoF (2013) Tpz1 controls a telomerase-nonextendible telomeric state and coordinates switching to an extendible state via Ccq1. Genes Dev 27 : 1917–1931.
44. RhindN, ChenZ, YassourM, ThompsonDA, HaasBJ, et al. (2011) Comparative Functional Genomics of the Fission Yeasts. Science 332 : 930–936.
45. SubramanianL, MoserBA, NakamuraTM (2008) Recombination-based telomere maintenance is dependent on Tel1-MRN and Rap1 and inhibited by telomerase, Taz1, and Ku in fission yeast. Mol Cell Biol 28 : 1443–1455.
46. LundbladV, BlackburnEH (1993) An alternative pathway for yeast telomere maintenance rescues est1 − senescence. Cell 73 : 347–360.
47. TengSC, ZakianVA (1999) Telomere-telomere recombination is an efficient bypass pathway for telomere maintenance in Saccharomyces cerevisiae. Mol Cell Biol 19 : 8083–8093.
48. NimmoER, PidouxAL, PerryPE, AllshireRC (1998) Defective meiosis in telomere-silencing mutants of Schizosaccharomyces pombe. Nature 392 : 825–828.
49. MoserBA, SubramanianL, KhairL, ChangYT, NakamuraTM (2009) Fission yeast Tel1ATM and Rad3ATR promote telomere protection and telomerase recruitment. PLoS Genet 5: e1000622.
50. YamazakiH, TarumotoY, IshikawaF (2012) Tel1ATM and Rad3ATR phosphorylate the telomere protein Ccq1 to recruit telomerase and elongate telomeres in fission yeast. Genes Dev 26 : 241–246.
51. JainD, HebdenAK, NakamuraTM, MillerKM, CooperJP (2010) HAATI survivors replace canonical telomeres with blocks of generic heterochromatin. Nature 467 : 223–227.
52. McNeesCJ, TejeraAM, MartinezP, MurgaM, MuleroF, et al. (2010) ATR suppresses telomere fragility and recombination but is dispensable for elongation of short telomeres by telomerase. J Cell Biol 188 : 639–652.
53. HandeMP, BalajeeAS, TchirkovA, Wynshaw-BorisA, LansdorpPM (2001) Extra-chromosomal telomeric DNA in cells from Atm(−/−) mice and patients with ataxia-telangiectasia. Hum Mol Genet 10 : 519–528.
54. MetcalfeJA, ParkhillJ, CampbellL, StaceyM, BiggsP, et al. (1996) Accelerated telomere shortening in ataxia telangiectasia. Nat Genet 13 : 350–353.
55. VerdunRE, KarlsederJ (2006) The DNA damage machinery and homologous recombination pathway act consecutively to protect human telomeres. Cell 127 : 709–720.
56. ZhaoY, SfeirAJ, ZouY, BusemanCM, ChowTT, et al. (2009) Telomere extension occurs at most chromosome ends and is uncoupled from fill-in in human cancer cells. Cell 138 : 463–475.
57. WangF, StewartJA, KasbekC, ZhaoY, WrightWE, et al. (2012) Human CST has independent functions during telomere duplex replication and C-strand fill-in. Cell Rep 2 : 1096–1103.
58. WuP, TakaiH, de LangeT (2012) Telomeric 3′ overhangs derive from resection by Exo1 and Apollo and fill-in by POT1b-associated CST. Cell 150 : 39–52.
59. KimSH, DavalosAR, HeoSJ, RodierF, ZouY, et al. (2008) Telomere dysfunction and cell survival: roles for distinct TIN2-containing complexes. J Cell Biol 181 : 447–460.
60. TakaiKK, KibeT, DonigianJR, FrescasD, de LangeT (2011) Telomere protection by TPP1/POT1 requires tethering to TIN2. Mol Cell 44 : 647–659.
61. SfeirA, KosiyatrakulST, HockemeyerD, MacRaeSL, KarlsederJ, et al. (2009) Mammalian telomeres resemble fragile sites and require TRF1 for efficient replication. Cell 138 : 90–103.
62. MillerKM, RogO, CooperJP (2006) Semi-conservative DNA replication through telomeres requires Taz1. Nature 440 : 824–828.
63. Alfa C, Fantes P, Hyams J, McLoed M, Warbrick E (1993) Experiments with fission yeast. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press.
64. Amberg DC, Burke DJ, Strathern JN (2005) Methods in yeast genetics. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press.
65. KhairL, SubramanianL, MoserBA, NakamuraTM (2010) Roles of heterochromatin and telomere proteins in regulation of fission yeast telomere recombination and telomerase recruitment. J Biol Chem 285 : 5327–5337.
66. WebbCJ, ZakianVA (2008) Identification and characterization of the Schizosaccharomyces pombe TER1 telomerase RNA. Nat Struct Mol Biol 15 : 34–42.
67. Lopez-GironaA, TanakaK, ChenXB, BaberBA, McGowanCH, et al. (2001) Serine-345 is required for Rad3-dependent phosphorylation and function of checkpoint kinase Chk1 in fission yeast. Proc Natl Acad Sci U S A 98 : 11289–11294.
68. BählerJ, WuJQ, LongtineMS, ShahNG, McKenzieA3rd, et al. (1998) Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast 14 : 943–951.
69. NakamuraTM, MoserBA, RussellP (2002) Telomere binding of checkpoint sensor and DNA repair proteins contributes to maintenance of functional fission yeast telomeres. Genetics 161 : 1437–1452.
70. MoserBA, ChangYT, NakamuraTM (2014) Telomere regulation during the cell cycle in fission yeast. Methods Mol Biol 1170 : 411–424.
71. KanohJ, SadaieM, UranoT, IshikawaF (2005) Telomere binding protein Taz1 establishes Swi6 heterochromatin independently of RNAi at telomeres. Curr Biol 15 : 1808–1819.
Štítky
Genetika Reprodukční medicína
Článek Oligoasthenoteratozoospermia and Infertility in Mice Deficient for miR-34b/c and miR-449 LociČlánek The Kinesin AtPSS1 Promotes Synapsis and is Required for Proper Crossover Distribution in MeiosisČlánek Payoffs, Not Tradeoffs, in the Adaptation of a Virus to Ostensibly Conflicting Selective PressuresČlánek Examination of Prokaryotic Multipartite Genome Evolution through Experimental Genome ReductionČlánek BMP-FGF Signaling Axis Mediates Wnt-Induced Epidermal Stratification in Developing Mammalian SkinČlánek Role of STN1 and DNA Polymerase α in Telomere Stability and Genome-Wide Replication in ArabidopsisČlánek RNA-Processing Protein TDP-43 Regulates FOXO-Dependent Protein Quality Control in Stress ResponseČlánek Integrating Functional Data to Prioritize Causal Variants in Statistical Fine-Mapping StudiesČlánek Salt-Induced Stabilization of EIN3/EIL1 Confers Salinity Tolerance by Deterring ROS Accumulation inČlánek Ethylene-Induced Inhibition of Root Growth Requires Abscisic Acid Function in Rice ( L.) SeedlingsČlánek Metabolic Respiration Induces AMPK- and Ire1p-Dependent Activation of the p38-Type HOG MAPK PathwayČlánek Signature Gene Expression Reveals Novel Clues to the Molecular Mechanisms of Dimorphic Transition inČlánek A Mouse Model Uncovers LKB1 as an UVB-Induced DNA Damage Sensor Mediating CDKN1A (p21) DegradationČlánek Dominant Sequences of Human Major Histocompatibility Complex Conserved Extended Haplotypes from to
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2014 Číslo 10
-
Všechny články tohoto čísla
- An Deletion Is Highly Associated with a Juvenile-Onset Inherited Polyneuropathy in Leonberger and Saint Bernard Dogs
- Licensing of Yeast Centrosome Duplication Requires Phosphoregulation of Sfi1
- Oligoasthenoteratozoospermia and Infertility in Mice Deficient for miR-34b/c and miR-449 Loci
- Basement Membrane and Cell Integrity of Self-Tissues in Maintaining Immunological Tolerance
- The Kinesin AtPSS1 Promotes Synapsis and is Required for Proper Crossover Distribution in Meiosis
- Germline Mutations in Are Associated with Familial Gastric Cancer
- POT1a and Components of CST Engage Telomerase and Regulate Its Activity in
- Controlling Meiotic Recombinational Repair – Specifying the Roles of ZMMs, Sgs1 and Mus81/Mms4 in Crossover Formation
- Payoffs, Not Tradeoffs, in the Adaptation of a Virus to Ostensibly Conflicting Selective Pressures
- FHIT Suppresses Epithelial-Mesenchymal Transition (EMT) and Metastasis in Lung Cancer through Modulation of MicroRNAs
- Genome-Wide Mapping of Yeast RNA Polymerase II Termination
- Examination of Prokaryotic Multipartite Genome Evolution through Experimental Genome Reduction
- White Cells Facilitate Opposite- and Same-Sex Mating of Opaque Cells in
- BMP-FGF Signaling Axis Mediates Wnt-Induced Epidermal Stratification in Developing Mammalian Skin
- Genome-Wide Association Study of CSF Levels of 59 Alzheimer's Disease Candidate Proteins: Significant Associations with Proteins Involved in Amyloid Processing and Inflammation
- COE Loss-of-Function Analysis Reveals a Genetic Program Underlying Maintenance and Regeneration of the Nervous System in Planarians
- Fat-Dachsous Signaling Coordinates Cartilage Differentiation and Polarity during Craniofacial Development
- Identification of Genes Important for Cutaneous Function Revealed by a Large Scale Reverse Genetic Screen in the Mouse
- Sensors at Centrosomes Reveal Determinants of Local Separase Activity
- Genes Integrate and Hedgehog Pathways in the Second Heart Field for Cardiac Septation
- Systematic Dissection of Coding Exons at Single Nucleotide Resolution Supports an Additional Role in Cell-Specific Transcriptional Regulation
- Recovery from an Acute Infection in Requires the GATA Transcription Factor ELT-2
- HIPPO Pathway Members Restrict SOX2 to the Inner Cell Mass Where It Promotes ICM Fates in the Mouse Blastocyst
- Role of and in Development of Abdominal Epithelia Breaks Posterior Prevalence Rule
- The Formation of Endoderm-Derived Taste Sensory Organs Requires a -Dependent Expansion of Embryonic Taste Bud Progenitor Cells
- Role of STN1 and DNA Polymerase α in Telomere Stability and Genome-Wide Replication in Arabidopsis
- Keratin 76 Is Required for Tight Junction Function and Maintenance of the Skin Barrier
- Encodes the Catalytic Subunit of N Alpha-Acetyltransferase that Regulates Development, Metabolism and Adult Lifespan
- Disruption of SUMO-Specific Protease 2 Induces Mitochondria Mediated Neurodegeneration
- Caudal Regulates the Spatiotemporal Dynamics of Pair-Rule Waves in
- It's All in Your Mind: Determining Germ Cell Fate by Neuronal IRE-1 in
- A Conserved Role for Homologs in Protecting Dopaminergic Neurons from Oxidative Stress
- The Master Activator of IncA/C Conjugative Plasmids Stimulates Genomic Islands and Multidrug Resistance Dissemination
- An AGEF-1/Arf GTPase/AP-1 Ensemble Antagonizes LET-23 EGFR Basolateral Localization and Signaling during Vulva Induction
- The Proteomic Landscape of the Suprachiasmatic Nucleus Clock Reveals Large-Scale Coordination of Key Biological Processes
- RNA-Processing Protein TDP-43 Regulates FOXO-Dependent Protein Quality Control in Stress Response
- A Complex Genetic Switch Involving Overlapping Divergent Promoters and DNA Looping Regulates Expression of Conjugation Genes of a Gram-positive Plasmid
- ZTF-8 Interacts with the 9-1-1 Complex and Is Required for DNA Damage Response and Double-Strand Break Repair in the Germline
- Integrating Functional Data to Prioritize Causal Variants in Statistical Fine-Mapping Studies
- Tpz1-Ccq1 and Tpz1-Poz1 Interactions within Fission Yeast Shelterin Modulate Ccq1 Thr93 Phosphorylation and Telomerase Recruitment
- Salt-Induced Stabilization of EIN3/EIL1 Confers Salinity Tolerance by Deterring ROS Accumulation in
- Telomeric (s) in spp. Encode Mediator Subunits That Regulate Distinct Virulence Traits
- Ethylene-Induced Inhibition of Root Growth Requires Abscisic Acid Function in Rice ( L.) Seedlings
- Ancient Expansion of the Hox Cluster in Lepidoptera Generated Four Homeobox Genes Implicated in Extra-Embryonic Tissue Formation
- Mechanism of Suppression of Chromosomal Instability by DNA Polymerase POLQ
- A Mutation in the Mouse Gene Leads to Impaired Hedgehog Signaling
- Keeping mtDNA in Shape between Generations
- Targeted Exon Capture and Sequencing in Sporadic Amyotrophic Lateral Sclerosis
- TIF-IA-Dependent Regulation of Ribosome Synthesis in Muscle Is Required to Maintain Systemic Insulin Signaling and Larval Growth
- At Short Telomeres Tel1 Directs Early Replication and Phosphorylates Rif1
- Evidence of a Bacterial Receptor for Lysozyme: Binding of Lysozyme to the Anti-σ Factor RsiV Controls Activation of the ECF σ Factor σ
- Hsp40s Specify Functions of Hsp104 and Hsp90 Protein Chaperone Machines
- Feeding State, Insulin and NPR-1 Modulate Chemoreceptor Gene Expression via Integration of Sensory and Circuit Inputs
- Functional Interaction between Ribosomal Protein L6 and RbgA during Ribosome Assembly
- Multiple Regulatory Systems Coordinate DNA Replication with Cell Growth in
- Fast Evolution from Precast Bricks: Genomics of Young Freshwater Populations of Threespine Stickleback
- Mmp1 Processing of the PDF Neuropeptide Regulates Circadian Structural Plasticity of Pacemaker Neurons
- The Nuclear Immune Receptor Is Required for -Dependent Constitutive Defense Activation in
- Genetic Modifiers of Neurofibromatosis Type 1-Associated Café-au-Lait Macule Count Identified Using Multi-platform Analysis
- Juvenile Hormone-Receptor Complex Acts on and to Promote Polyploidy and Vitellogenesis in the Migratory Locust
- Uncovering Enhancer Functions Using the α-Globin Locus
- The Analysis of Mutant Alleles of Different Strength Reveals Multiple Functions of Topoisomerase 2 in Regulation of Chromosome Structure
- Metabolic Respiration Induces AMPK- and Ire1p-Dependent Activation of the p38-Type HOG MAPK Pathway
- The Specification and Global Reprogramming of Histone Epigenetic Marks during Gamete Formation and Early Embryo Development in
- The DAF-16 FOXO Transcription Factor Regulates to Modulate Stress Resistance in , Linking Insulin/IGF-1 Signaling to Protein N-Terminal Acetylation
- Genetic Influences on Translation in Yeast
- Analysis of Mutants Defective in the Cdk8 Module of Mediator Reveal Links between Metabolism and Biofilm Formation
- Ribosomal Readthrough at a Short UGA Stop Codon Context Triggers Dual Localization of Metabolic Enzymes in Fungi and Animals
- Gene Duplication Restores the Viability of Δ and Δ Mutants
- Selection on a Variant Associated with Improved Viral Clearance Drives Local, Adaptive Pseudogenization of Interferon Lambda 4 ()
- Break-Induced Replication Requires DNA Damage-Induced Phosphorylation of Pif1 and Leads to Telomere Lengthening
- Dynamic Partnership between TFIIH, PGC-1α and SIRT1 Is Impaired in Trichothiodystrophy
- Signature Gene Expression Reveals Novel Clues to the Molecular Mechanisms of Dimorphic Transition in
- Mutations in Moderate or Severe Intellectual Disability
- Multifaceted Genome Control by Set1 Dependent and Independent of H3K4 Methylation and the Set1C/COMPASS Complex
- A Role for Taiman in Insect Metamorphosis
- The Small RNA Rli27 Regulates a Cell Wall Protein inside Eukaryotic Cells by Targeting a Long 5′-UTR Variant
- MMS Exposure Promotes Increased MtDNA Mutagenesis in the Presence of Replication-Defective Disease-Associated DNA Polymerase γ Variants
- Coexistence and Within-Host Evolution of Diversified Lineages of Hypermutable in Long-term Cystic Fibrosis Infections
- Comprehensive Mapping of the Flagellar Regulatory Network
- Topoisomerase II Is Required for the Proper Separation of Heterochromatic Regions during Female Meiosis
- A Splice Mutation in the Gene Causes High Glycogen Content and Low Meat Quality in Pig Skeletal Muscle
- KDM5 Interacts with Foxo to Modulate Cellular Levels of Oxidative Stress
- H2B Mono-ubiquitylation Facilitates Fork Stalling and Recovery during Replication Stress by Coordinating Rad53 Activation and Chromatin Assembly
- Copy Number Variation in the Horse Genome
- Unifying Genetic Canalization, Genetic Constraint, and Genotype-by-Environment Interaction: QTL by Genomic Background by Environment Interaction of Flowering Time in
- Spinster Homolog 2 () Deficiency Causes Early Onset Progressive Hearing Loss
- Genome-Wide Discovery of Drug-Dependent Human Liver Regulatory Elements
- Developmentally-Regulated Excision of the SPβ Prophage Reconstitutes a Gene Required for Spore Envelope Maturation in
- Protein Phosphatase 4 Promotes Chromosome Pairing and Synapsis, and Contributes to Maintaining Crossover Competence with Increasing Age
- The bHLH-PAS Transcription Factor Dysfusion Regulates Tarsal Joint Formation in Response to Notch Activity during Leg Development
- A Mouse Model Uncovers LKB1 as an UVB-Induced DNA Damage Sensor Mediating CDKN1A (p21) Degradation
- Notch3 Interactome Analysis Identified WWP2 as a Negative Regulator of Notch3 Signaling in Ovarian Cancer
- An Integrated Cell Purification and Genomics Strategy Reveals Multiple Regulators of Pancreas Development
- Dominant Sequences of Human Major Histocompatibility Complex Conserved Extended Haplotypes from to
- The Vesicle Protein SAM-4 Regulates the Processivity of Synaptic Vesicle Transport
- A Gain-of-Function Mutation in Impeded Bone Development through Increasing Expression in DA2B Mice
- Nephronophthisis-Associated Regulates Cell Cycle Progression, Apoptosis and Epithelial-to-Mesenchymal Transition
- Beclin 1 Is Required for Neuron Viability and Regulates Endosome Pathways via the UVRAG-VPS34 Complex
- The Not5 Subunit of the Ccr4-Not Complex Connects Transcription and Translation
- Abnormal Dosage of Ultraconserved Elements Is Highly Disfavored in Healthy Cells but Not Cancer Cells
- Genome-Wide Distribution of RNA-DNA Hybrids Identifies RNase H Targets in tRNA Genes, Retrotransposons and Mitochondria
- The Chromosomal Association of the Smc5/6 Complex Depends on Cohesion and Predicts the Level of Sister Chromatid Entanglement
- Cell-Autonomous Progeroid Changes in Conditional Mouse Models for Repair Endonuclease XPG Deficiency
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- The Master Activator of IncA/C Conjugative Plasmids Stimulates Genomic Islands and Multidrug Resistance Dissemination
- A Splice Mutation in the Gene Causes High Glycogen Content and Low Meat Quality in Pig Skeletal Muscle
- Keratin 76 Is Required for Tight Junction Function and Maintenance of the Skin Barrier
- A Role for Taiman in Insect Metamorphosis
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



