-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaPOT1a and Components of CST Engage Telomerase and Regulate Its Activity in
Telomeres are required to stabilize the ends of linear chromosomes, and thus ensure genome integrity. Telomeric DNA is maintained though the action of both conventional and non-conventional DNA replication mechanisms. To ensure that chromosome ends are fully protected and fully replicated, telomeres dynamically oscillate between a closed (non-extendable) and an open (extendable) conformation throughout the cell cycle. The telomerase reverse transcriptase engages telomeres when they are in an extendable conformation. How this conversion occurs, how telomerase is recruited to the chromosome terminus and how telomerase action is terminated are unanswered questions. Here we provide evidence that POT1a, a telomerase accessory protein from the flowering plant Arabidopsis, helps to convert the telomere into a telomerase-extendable state through dynamic interactions with a critical telomere binding protein complex, and through stimulation of telomerase enzyme activity. The results of this study provide new insight into the regulation of telomeric DNA replication.
Published in the journal: . PLoS Genet 10(10): e32767. doi:10.1371/journal.pgen.1004738
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004738Summary
Telomeres are required to stabilize the ends of linear chromosomes, and thus ensure genome integrity. Telomeric DNA is maintained though the action of both conventional and non-conventional DNA replication mechanisms. To ensure that chromosome ends are fully protected and fully replicated, telomeres dynamically oscillate between a closed (non-extendable) and an open (extendable) conformation throughout the cell cycle. The telomerase reverse transcriptase engages telomeres when they are in an extendable conformation. How this conversion occurs, how telomerase is recruited to the chromosome terminus and how telomerase action is terminated are unanswered questions. Here we provide evidence that POT1a, a telomerase accessory protein from the flowering plant Arabidopsis, helps to convert the telomere into a telomerase-extendable state through dynamic interactions with a critical telomere binding protein complex, and through stimulation of telomerase enzyme activity. The results of this study provide new insight into the regulation of telomeric DNA replication.
Introduction
Eukaryotes face end-protection and end-replication problems due to the linear nature of their chromosomes and the limitations of conventional DNA replication. Telomerase averts these crises using its RNA subunit (TER) as a template to reiteratively synthesize G-rich repeat sequences on the 3′ single-strand extension (G-overhang) of the chromosome terminus. Both the single (ss) and double-strand (ds) portions of the telomere are host to protein complexes that modulate telomerase action and distinguish natural chromosome ends from double-strand breaks [1]–[4].
Telomeres vacillate between a telomerase extendable and a telomerase un-extendable state during the cell cycle [5], [6]. In G1, the G-overhang is sequestered, preventing the DNA terminus from eliciting a damage response, but also preventing telomerase access. In late S/G2 phase, telomerase is recruited to chromosome ends for DNA synthesis. Once telomerase extends the G-rich strand, the C-strand is replicated by DNA Polymerase α/primase [7], [8], followed by terminal DNA processing to create the 3′ G-overhang [9]. The terminus is then sequestered once again. These reactions are highly coordinated, and driven by the exchange of large replication/processing complexes on the G-overhang.
One telomere complex under intensive scrutiny is CST (Cdc13/CTC1, Stn1, Ten1), an RPA-like heterotrimer [10], [11] first identified in budding yeast. Cdc13 anchors CST to ss telomeric DNA via its central oligosaccharide-oligonucleotide binding domain (OB-fold) [12]. Genetic analysis of separation-of-function alleles reveals that Cdc13 maintains genome integrity and regulates telomere maintenance [13], [14]. Stn1 and Ten1 are also essential for telomere integrity, and their association with Cdc13 renders telomeres into an un-extendable state [15]–[17]. However, the CST heterotrimer is not static, and recent data show that Stn1 and Ten1 make contributions distinct from Cdc13 [18]. In addition, phosphorylation of Cdc13 in late S phase shifts the binding preference from Stn1 and Ten1 to the telomerase accessory factor Est1 [19], [20], converting the telomere into an extendable conformation. Est1 is a multifunctional protein that directly binds the TER subunit (Tlc1) as well as Cdc13. This interaction recruits telomerase to the chromosome end [21]–[24]. Consistent with its critical role in telomere maintenance, Est1 deletion causes progressive telomere shortening [25]. Est1 also stimulates the activity of telomerase on telomeric DNA [23], [26] likely through contacts with Cdc13 [27].
Mammalian telomeres are protected by an alternative complex termed shelterin. The six shelterin subunits include TRF1, TRF2, and RAP1, which are tethered to ds telomeric DNA and are bridged by TIN2 and TPP1 to the ss DNA binding protein POT1 [1], [28]. All shelterin components are critical for genome stability, and like budding yeast CST, may shift between sub-complexes during the cell cycle [29]. POT1 inhibits telomerase elongation in vitro by preventing substrate access [30], [31]. In contrast, the POT1-TPP1 heterodimer stimulates telomerase repeat addition processivity (RAP) by promoting substrate association and template translocation during telomerase extension [32]–[34]. In addition, TPP1 appears to directly contact the telomerase catalytic subunit TERT and thereby recruits telomerase to telomeres [35]–[37].
CST also exists in vertebrates and plants, although Cdc13 has been replaced by another large OB-fold containing protein, CTC1 [38]–[41]. In contrast to yeast where CST functions in both end protection and telomeric DNA replication [4], vertebrate CST primarily serves to promote telomere replication by stimulating C-strand fill-in and genome-wide replication rescue [42]–[45]. CTC1 and STN1 directly contact the telomerase activator proteins TPP1/POT1 [32], [46], [47]. Recent studies indicate that human CST negatively regulates telomerase by competing with TPP1/POT1 for telomeric DNA binding and by squelching the stimulation of telomerase RAP by TPP1/POT1 [46]. Thus, the interaction of TPP1/POT1 with CST is proposed to terminate G-strand synthesis by telomerase. While the molecular basis for the dynamic exchange between shelterin, telomerase and CST is unknown, shifting interactions between shelterin constituents [48], [49] prompted through posttranslational modification [20], [37], [50], [51] likely control telomere transactions.
Arabidopsis telomeres represent an intriguing blend of features from yeast and vertebrates. Only a subset of shelterin components can be discerned in plants, and although the Arabidopsis CST complex is structurally analogous to mammalian CST, it appears to play a role in chromosome end protection. Loss of any of the Arabidopsis CST subunits elicit dramatic telomere shortening, increased ss telomeric DNA, and chromosomal fusions [38], [39], [41], culminating in stem cell failure [52]. Notably, TEN1 is detected at a significantly smaller fraction of telomeres than CTC1 [39], [41]. In addition, unlike plants lacking STN1 or CTC1, ten1 mutants have higher levels of telomerase enzyme activity overall, and generate longer telomere repeat arrays in vitro, indicating that TEN1 negatively regulates telomerase activity [41].
Arabidopsis harbors two TER genes encoding RNAs that assemble into different RNP complexes with opposing functions. TER1 is a canonical TER subunit required for telomere maintenance, whereas TER2 negatively regulates telomere synthesis by the TER1 RNP in response to DNA damage [53], [54]. Arabidopsis encodes several telomerase accessory factors, but notably the two Est1-like proteins play no obvious role in telomere maintenance and rather are implicated in the regulation of the meiotic cell cycle [55]. POT1a, one of three A. thaliana POT1 paralogs [56]–[58] exhibits properties reminiscent of Est1. POT1a associates with TER1, and localizes to telomeres in S phase [59]. Moreover, plants lacking POT1a are defective in telomere maintenance, and undergo progressive telomere shortening. In addition, pot1a mutants have reduced telomerase activity in vitro [59]. These findings indicate that POT1a positively regulates telomerase enzyme activity and promotes telomere repeat synthesis on chromosome ends.
In this study, we further explore the role of POT1a. We report that POT1a is not required to recruit telomerase to telomeres, but is required for telomerase to maintain telomere tracts. Our biochemical data indicate that POT1a stimulates telomerase enzyme activity, likely by enhancing its RAP. We further show that POT1a directly contacts STN1 and CTC1 in vitro, and its association with STN1 is mutually exclusive of TEN1-STN1 binding. Finally, we demonstrate that CTC1 and STN1, but not TEN1, interact with enzymatically active telomerase in vivo. These findings suggest a model in which POT1a promotes telomere maintenance by activating telomerase at chromosome ends. The data further suggest that the opposing functions of POT1a and TEN1 in telomerase regulation may contribute to the switch from telomerase extendable to the telomerase un-extendable state.
Results
POT1a is not required for TERT association with chromosome ends
Chromatin immunoprecipitation (ChIP) was used to investigate whether POT1a is needed for telomerase association with telomeres. As expected, the telomerase catalytic subunit TERT [60] could be detected at telomeres in rapidly dividing young wild type seedlings (Fig. 1A). However, there was no significant difference in the level of telomere-bound TERT in pot1a mutants versus wild type (Fig. 1A and C). One possible explanation is that the TERT signal includes telomere-bound TER2 RNP. Since POT1a does not interact with TER2 [54], loss of this protein is not expected to perturb the alternative telomerase RNP. To address this possibility, we generated plants doubly deficient in POT1a and TER2. ChIP assays performed on pot1a ter2 mutants showed the same level of telomere-bound TERT as in wild type plants (Fig. 1A and C). We conclude POT1a is not required for TERT recruitment to telomeres.
Fig. 1. Telomerase associates with telomeres in the absence of POT1a. 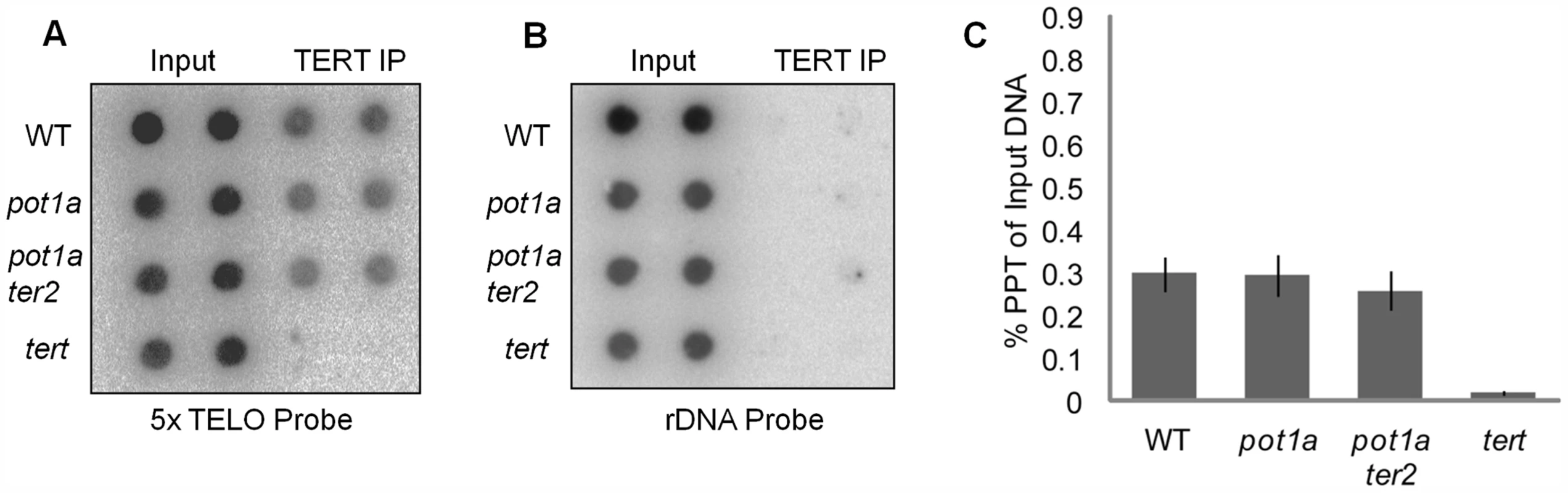
(A) Results of ChIP assays using TERT antibody in wild type, pot1a, pot1a ter2, and tert seedlings. Signal was assessed by dot blot using a telomeric probe. Reactions shown are technical duplicates. (B) Membrane was stripped and re-hybridized with a rDNA oligonucleotide probe. (C) Quantification of TERT ChIP. IP signal is represented as percent precipitation of input DNA. Error bars represent standard error of the mean from three independent biological replicates. POT1a stimulates activity of the TER1 telomerase RNP
If POT1a is not required for telomerase's association with chromosome ends, how does it promote telomere maintenance? One possibility is that POT1a directly modulates telomerase enzyme activity. The conventional telomere repeat amplification protocol (TRAP) assay shows an ∼13 fold decrease in telomerase activity in pot1a relative to wild type extracts [59]. This change in enzyme activity is not due to altered expression of TERT and TER1 transcripts or genes previously shown to inhibit telomerase activity such as TER2 and TEN1 (Fig. S1). Attempts to develop a direct primer extension assay in Arabidopsis have been unsuccessful thus far. To obtain a more accurate gauge of the distribution and quantity of the products of Arabidopsis telomerase, we used a modified version of the TRAP assay, telomerase processivity TRAP (TP-TRAP), developed to provide an indication of mammalian telomerase RAP [41], [61]. Pilot reactions with an oligonucleotide bearing five telomere repeats yielded a discrete band of the expected size (Fig. S2), indicating that the PCR amplification step of TP-TRAP gives a reliable assessment of the length of a telomere repeat array generated in the PCR reaction.
TP-TRAP performed with wild type Arabidopsis extract generated a broad distribution of elongation products, including high molecular weight species corresponding to the addition of at least 15 TTTAGGG repeats (Fig. 2A and C). As expected, extract from ten1 mutants, but not stn1 or ctc1 mutants, generated slightly longer products than wild type (Fig. 2A and S3) [41], supporting the conclusion TEN1 negatively regulates telomerase activity and further that this is a unique property of this CST subunit. The TP-TRAP results for pot1a mutants were markedly different and showed a dramatic reduction in high molecular weight products relative to wild type (Fig. 2C). While standard TRAP assays show a general decrease in telomerase activity in pot1a mutants (Fig. 2B), the TP-TRAP indicated that the defect lies in the production of long arrays of telomere repeats (Fig. 2C). The primer is in vast excess over telomerase in TP-TRAP reactions as in conventional TRAP and the direct primer extension assay. Consequently, long products are unlikely to be generated by telomerase dissociation and rebinding the same primer molecule. The data are consistent with the notion that POT1a stimulates RAP.
Fig. 2. POT1a promotes synthesis of long telomere repeat arrays. 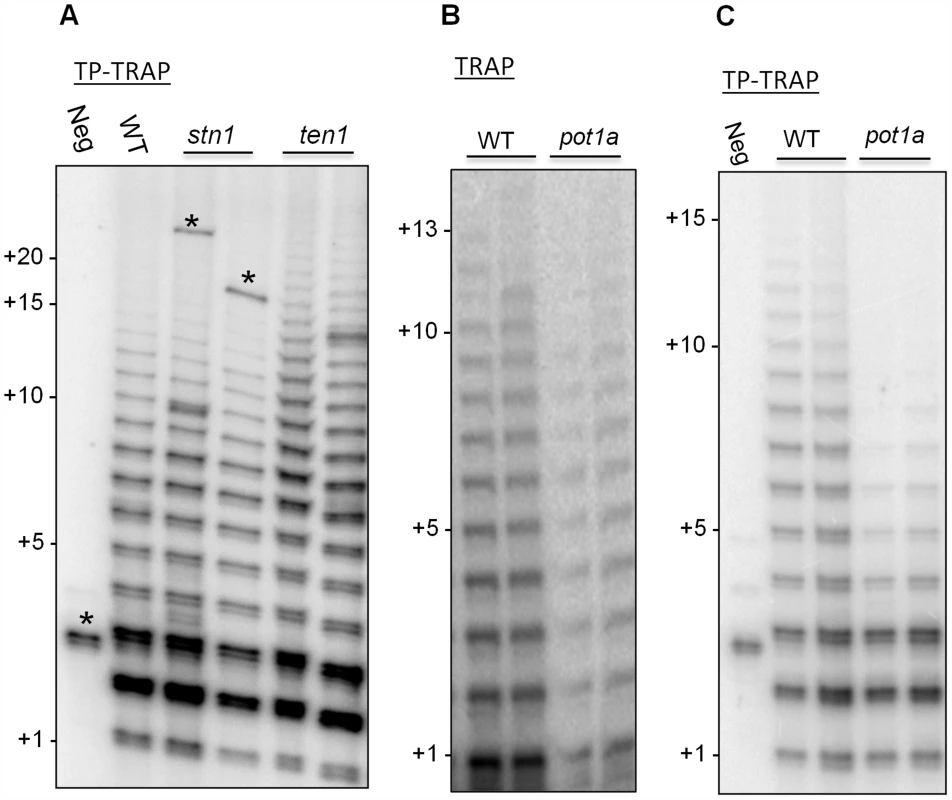
(A) TP-TRAP assay results performed on flower extracts from wild type, and two independent stn1, and ten1 mutants. Negative control is without extract to monitor PCR contamination. Asterisks indicate non-specific amplification products. (B) Conventional TRAP results from wild type and pot1a seedling extracts. Results from two independent seedling extractions are shown. (C) TP-TRAP results for pot1a and wild type seedling extracts. Results from two independent seedling extracts are shown. Negative control is without extract. To determine if the decreased telomerase activity associated with pot1a mutants is specific to the TER1 RNP complex, we performed TP-TRAP on ter2 seedling extracts. The product profiles were nearly identical to wild type (Fig. 3A), indicating the TER1 RNP efficiently synthesizes telomeric DNA in wild type plants. We confirmed that POT1a modulates the TER1 RNP by analyzing pot1a ter2 mutants. Long products were reduced in the double mutants, but not to the same extent as pot1a (Fig. 3A). In agreement with previous results showing that TER2 negatively regulates TER1 RNP [54], quantitative TRAP (qTRAP) revealed a higher level of telomerase activity in ter2 mutants relative to wild type (Fig. 3B), which could explain why the TP-TRAP and qTRAP signal is higher in pot1a ter2 than pot1a (Fig. 3A and B). Since the TER1 RNP is the only functional telomerase complex in pot1a ter2 mutants, the data indicate POT1a distinctly modulates this complex.
Fig. 3. POT1a stimulates telomerase activity of the TER1 RNP. 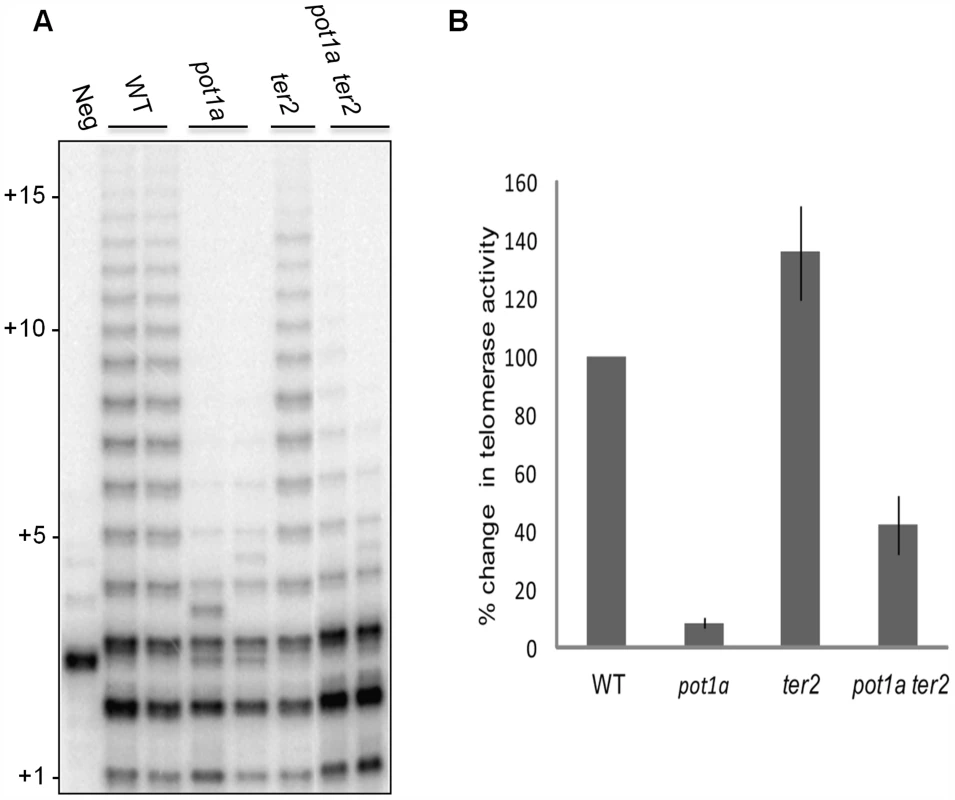
(A) TP-TRAP analysis from two independent biological replicates wild type, pot1a, ter2, and pot1a ter2 mutants. (B) Results of quantitative TRAP (qTRAP). Error bars represent standard error of the mean from three biological replicates. Telomere dysfunction is exacerbated in plants lacking telomerase and STN1 or CTC1
In both yeast and vertebrates, CST plays a key role in controlling G-overhang access to telomerase and DNA Pol-α [4], [7], [46]. To test whether telomerase acts in concert with CST for telomere maintenance, we used a genetic approach. As expected, ctc1 and stn1 mutants exhibited severe morphological aberrancies including irregular phyllotaxy, fasciated stems, and reduced fertility (Fig. 4A and C, and S4A; [38], [39]). These phenotypes were even more pronounced when telomerase was inactivated in stn1 and ctc1 plants (Fig. 4A and S4A). Progeny lacking CTC1/STN1 and TERT were rarely recovered, and when they were, double mutants arrested in a dwarf vegetative state without producing germline tissue (Fig. 4A and Fig. S4A). Telomere length was examined using Terminal Restriction Fragment (TRF) analysis or Primer Extension Telomere Repeat Amplification (PETRA) when insufficient material was available for TRF. Consistent with previous studies [38], [39], stn1 or ctc1 mutants displayed shorter, more heterogeneous telomere tracts than wild type plants. In contrast, while telomeres in tert mutants consisted of a discrete, homogeneous population of bands shorter than wild type (Fig. 4B and Fig. S4B) [62]. The telomeres of plants lacking either CTC1 or STN1 and telomerase were dramatically shorter with some telomeres dipping below the critical threshold of 1 kb (Fig. 4B and Fig. S4B), which triggers telomere fusions [63]. We conclude telomerase is capable of extending telomeres devoid of CTC1 or STN1 to partially alleviate their dysfunction. However, given the very severe telomere deprotection phenotype associated with the loss of CST, these epistasis experiments do not rule out the possibility that STN1 or CTC1 engage telomerase and modulate its activity in vivo.
Fig. 4. POT1a acts with telomerase to partially rescue the telomere dysfunction of stn1 mutants. 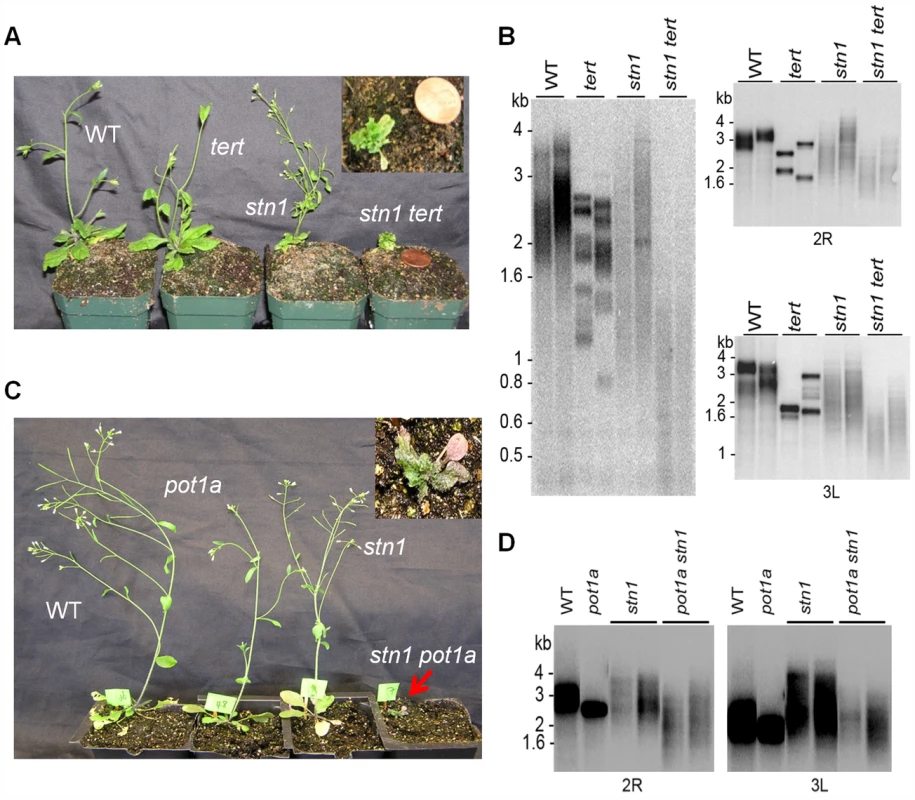
(A) Morphology of wild type, stn1, tert, and stn1 tert double mutants. Telomere length analysis assessed by TRF (B, left panel) and PETRA (B, right panel; D) for the genotypes indicated. In each case, results for two independent plants are shown. For PETRA, telomeres on the right arm of chromosome 2 (2R) or the left arm of chromosome 3 (3L) were analyzed. Wild type controls were segregated from either the stn1 × tert cross (B) or the stn1 × pot1a cross (D). (C) Morphology of wild type, stn1, pot1a, and stn1 pot1a double mutants. To determine if POT1a is required for telomerase to mitigate telomere defects in STN1/CTC1 deficient plants, we evaluated pot1a ctc1 and pot1a stn1 double mutants. We were unable to recover viable pot1a ctc1 mutants. However, stn1 pot1a mutants exhibited similar morphological defects as stn1 tert plants (Fig. 4C). In addition, molecular analysis revealed the same type of telomere aberrations (Fig. 4D). Thus, the absence of POT1a renders stn1 mutants incapable of employing telomerase as a recovery mechanism (Fig. 4B). These findings support the conclusion that POT1a is required to activate telomerase at chromosome ends.
POT1a associates with CTC1 and STN1, but not TEN1 in vitro
Recent studies show that human POT1 and mouse POT1b bind CTC1 and STN1 [46], [47], [64]. Additional contacts between TPP1 and CTC1 and TPP1 and STN1 have been observed [46], [64], [65]. Therefore, we asked if POT1a binds individual CST subunits in vitro via co-immunoprecipitation assays using rabbit reticulocyte lysate (RRL) expressed proteins. We were unable to express intact full length CTC1, and so we employed an amino-terminal deletion construct (CTC1ΔN) that was sufficient to bind STN1 and the DNA Pol α subunit, ICU2 [4], [39]. POT1a was tagged with T7 on its amino terminus and immunoprecipitation (IP) was performed using T7 antibody-conjugated agarose beads. Binding was assessed by the ability of POT1a to co-precipitate 35S-methionine labeled CTC1ΔN, STN1, or TEN1. We detected POT1a binding to CTC1ΔN and STN1, but no interaction between TEN1 and POT1a was observed (Fig. 5A).
Fig. 5. POT1a associates with CTC1 and STN1 in vitro. 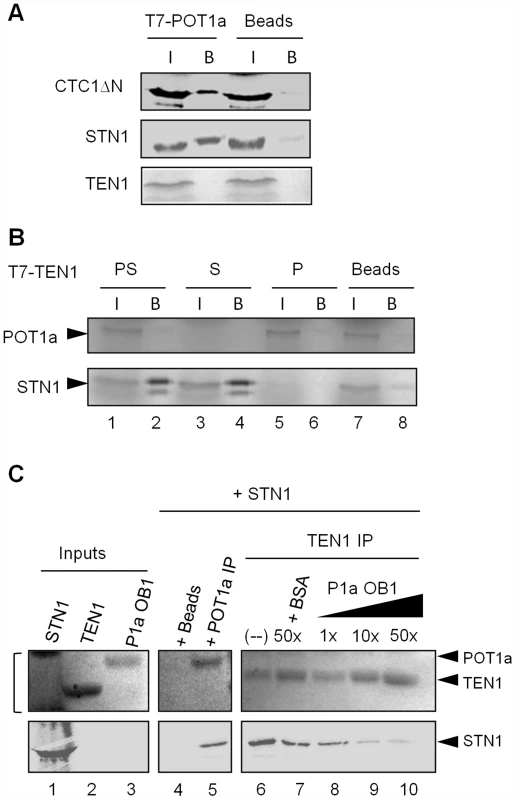
(A) In vitro co-immunoprecipitation (co-IP) results for RRL-expressed T7-tagged POT1a interactions with labeled CTC1ΔN, STN1, and TEN1. Negative control (beads conjugated with T7-tag antibody) was performed without tagged POT1a. (I) denotes protein input, (B) indicates bound protein. (B) Co-IP results for RRL-expressed T7 tagged TEN1 with labeled POT1a (P; lane 6), STN1 (S; lane 4) or both proteins (“PS”, lane 2). The beads control contained no T7 tagged TEN1 (lane 8). (C) In vitro Co-IP competition assay using E. coli-expressed TEN1 and POT1a OB1 detected by coomassie stain, and RRL-expressed 35S methionine labeled STN1 detected by autoradiography. Protein inputs are shown in lanes 1–3. Bracket adjacent to lane 1 denotes non-specific RRL proteins in the STN1 expression reaction (lane 1, top). TEN1 was incubated with STN1 and increasing concentrations of POT1a OB1 (lanes 8–10). 50× BSA was used as a control (lane 7) IP of POT1a was performed independently to verify its interaction with STN1 (lane 5). Beads alone was used to monitor background binding of STN1 protein (lane 4). Since TEN1 and STN1 form a heterodimer, we considered the possibility that POT1a might compete with TEN1 for STN1 binding. We first tested if STN1 can simultaneously bind POT1a and TEN1. TEN1 was T7 tagged, and incubated with labeled STN1 (Fig. 5B, lane 4), POT1a (Fig. 5B, lane 6) or both proteins (Fig. 5B, lane 2) followed by IP. In the reaction containing STN1 and POT1a, only STN1 was detected in the TEN1 IP (Fig. 5B, lane 2). Because TEN1 does not bind POT1a (Fig. 5A and Fig. 5B, lane 6), this result argues that STN1 binding to TEN1 and POT1a is mutually exclusive.
Next, we asked whether POT1a can compete with TEN1 for STN1 binding in vitro. We expressed and purified E. coli TEN1 protein as well as the first OB-fold of POT1a (POT1a OB1), which is sufficient for POT1a-STN1 interaction in vitro (Fig. S5 and Fig. 5C, lane 5). A competition assay was performed by incubating TEN1 with RRL-expressed [35S]-methionine labeled STN1 in the presence of increasing amounts of POT1a OB1. Following TEN1 IP, E. coli-expressed proteins (TEN1 and POT1a OB1) were monitored by coomassie stain (Fig. 5C top) and STN1 by autoradiography (Fig. 5C bottom). As expected, TEN1 pulled down STN1 (Fig 5C, lane 6). At an equal molar ratio of POT1a OB1 to TEN1, the TEN1-STN1 interaction persisted (Fig. 5C, lane 8). However, a ten-fold excess of POT1a OB1 significantly reduced STN1 in the TEN1 IP (Fig. 5C, lane 9). In contrast, 50-fold excess bovine serum albumin did not dislodge STN1 from TEN1 (Fig. 5C lane 7). Because E. coli POT1a OB1 directly binds STN1 (Fig. 5C, lane 5), these data support the conclusion that STN1 binding to POT1a and TEN1 is mutually exclusive. However, because excess POT1a OB1 is required to disrupt the STN1-TEN1 interaction, the data indicate that STN1 has a higher affinity for TEN1 than POT1a OB1.
STN1 and CTC1, but not TEN1, associate with enzymatically active telomerase in vivo
The discovery of in vitro interactions between POT1a with STN1 and CTC1 raised the possibility that these CST components associate with enzymatically active telomerase in vivo (Fig. 6). To test this idea, we generated a STN1 antibody that could be used for IP-TRAP. Western blot analysis confirmed that the antibody specifically recognizes STN1 (Fig. 6B). IP-TRAP using TERT antibody as a control revealed abundant telomerase activity (Fig. 6A). Strikingly, IP-TRAP with STN1 antibody gave a similar result. Western blot analysis verified that STN1 was precipitated in the reaction (Fig. 6B). Telomerase activity was not detected in an IP with pre-immune sera and was removed by RNaseA treatment, indicating that the STN1 interaction with telomerase was specific. Importantly, STN1 protein was present in the TERT IP (Fig. 6B), confirming the association of these molecules in vivo. IP of a transgenic CTC1-CFP protein also pulled down active telomerase as well as POT1a (Fig. S6). These findings indicate that both STN1 and CTC1 are associated with enzymatically active telomerase in vivo.
Fig. 6. STN1, but not TEN1 associates with enzymatically active telomerase in vivo. 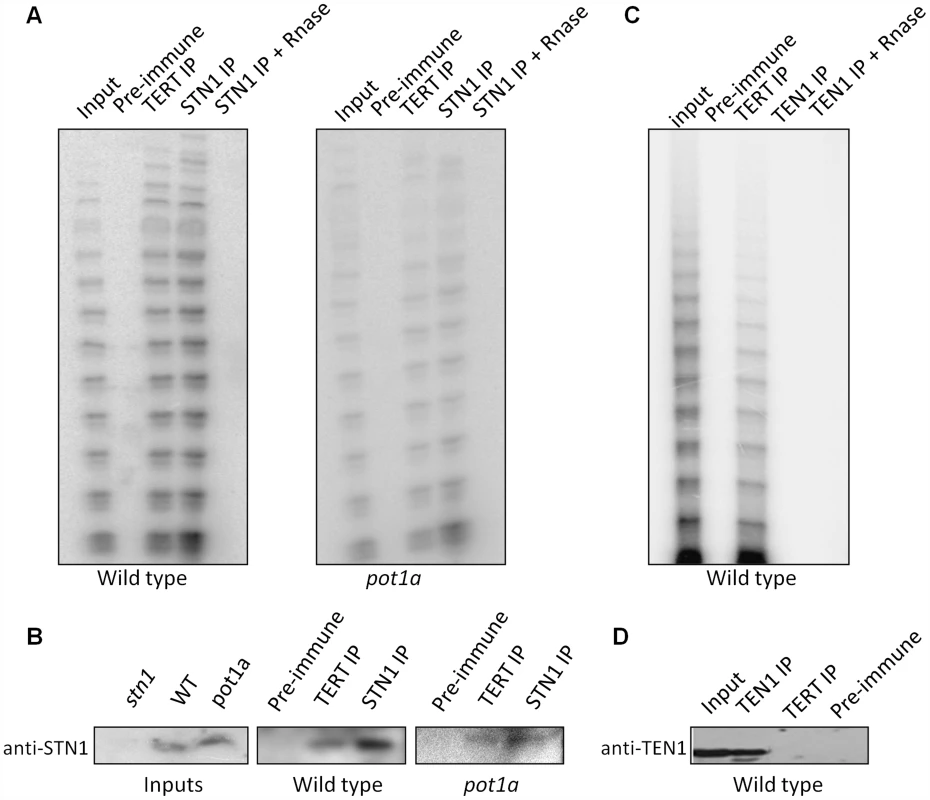
(A) Protein extract from wild type or pot1a seedlings was used for immunoprecipitation with STN1 or TERT antibody. IP samples and extract input were subjected to conventional TRAP (A) or western blot (B) analysis with STN1 antibody. (C) Protein extract from wild type seedlings was used for IP with TEN1 antibody. IP samples and extract input were subjected to conventional TRAP or (D) western blot to monitor for TEN1 protein after IP. We asked if POT1a was essential for the STN1-telomerase interaction by repeating the STN1 IP-TRAP experiment in a pot1a mutant. Telomerase activity and TERT were detected in the STN1 IP of pot1a extracts (Fig. 6A and B). As expected, telomerase activity was visibly decreased in this background ([59]; Fig. 6B). These data indicate that telomerase can associate with STN1 in the absence of POT1a. The data also support the conclusion that POT1a is not necessary for telomerase localization to telomeres, but is required to promote the full activation of telomerase.
Finally, we performed IP-TRAP with our TEN1 antibody to test if TEN1 is associated with active telomerase. In marked contrast to STN1 and CTC1, telomerase activity was not observed in the TEN1 pull down (Fig. 6C). Moreover, TEN1 protein could not be detected in the TERT IP (Fig. 6D). We conclude that TEN1 is not associated with enzymatically active telomerase in vivo, consistent with its role as a negative regulator of telomerase activity.
Discussion
Telomere accessibility to telomerase is tightly regulated during the cell cycle. Whereas aspects of telomerase recruitment are similar in yeast and vertebrates, many questions remain unanswered, in part because the specific proteins that mediate these interactions are not well conserved [29]. In this study, we investigated how the interplay between POT1a and CST in Arabidopsis promotes telomere maintenance. Like the budding yeast recruitment factor Est1 [21], [22], [25], [27], POT1a directly contacts the canonical TER, TER1 [53], and is required for robust telomerase activity in vitro and telomere maintenance in vivo [59]. However, unlike Est1 [66], we found that POT1a is not necessary for the telomere localization of TERT. The TERT interaction with telomeres was also unperturbed in plants doubly deficient in POT1a and TER2, indicating TERT is not tethered to telomeres through the TER2 RNP. How telomerase is recruited to chromosome ends in the absence of POT1a is unclear. In yeast, Ku provides an alternative route for telomerase recruitment in G1 [66]. However, Ku inhibits telomere synthesis in plants [67], [68], and thus this mechanism is not used to dock telomerase at Arabidopsis telomeres. The TRF-like protein AtTRB1 was recently shown to interact with telomeres and to contact TERT, suggesting that it might be involved in telomerase recruitment [69]. Another potential telomerase recruitment factor is HOT1, which stimulates telomerase recruitment in mammals through contacts with telomeric DNA and the telomerase RNP independent of shelterin [70]. Notably, Arabidopsis has a putative HOT1 ortholog, but lacks several of the core shelterin components, including TPP1, which is implicated in recruiting vertebrate telomerase [33], [36].
Although POT1a is not required for telomerase recruitment, it is required for the enzyme to extend telomere tracts in vivo ([59]; this study). Our data indicate POT1a directly stimulates telomerase catalysis. Using a modified version of the TRAP assay to gauge the length of telomerase products, we discovered that POT1a is necessary for the synthesis of long telomere repeat arrays. An attractive model is that POT1a promotes telomerase RAP, as shown for other telomerase-associated OB-fold bearing proteins such as human TPP1 and Tetrahymena Teb1 [32], [35], [71]. However, in the absence of a direct primer extension assay for Arabidopsis telomerase, we cannot exclude the possibility that POT1a affects some other parameter of telomerase enzymology (e.g. nucleotide addition processivity, nucleotide binding affinity or affinity for the DNA primer).
Once telomerase binds the telomere, how is its activity controlled? CST has a central role to play in this regard, but precisely how it interfaces with telomerase and whether this association stimulates or represses telomerase differs in yeast and vertebrates. Our analysis indicates that CST is not required to recruit Arabidopsis telomerase to chromosome ends. We found that telomerase can act on telomeres lacking CTC1 or STN1, partially alleviating the telomere dysfunction and the aberrant morphological defects associated with these mutations. Importantly, telomere extension in CTC1 and STN1 deficient plants is dependent upon POT1a, supporting the conclusion that POT1a is required to promote telomere maintenance.
In mammals, CST interaction with POT1 orthologs is linked to telomerase termination [46] and G-overhang maturation [47]. In contrast, we find that STN1 and CTC1 like POT1a are associated with enzymatically active telomerase in Arabidopsis [59]. Our experiments do not distinguish whether these telomerase interactions occur on or off the telomere. Nevertheless, since CTC1 can be detected at Arabidopsis telomeres even in cells arrested in G1 (Surovtseva et al 2009), we postulate that telomerase associates with CTC1 and STN1 on the G-overhang during S phase to facilitate telomere repeat incorporation (see below).
We found a direct interaction between POT1a with both STN1 and CTC1, but not TEN1 in vitro. Our data indicate that STN1 interaction with POT1a and TEN1 is mutually exclusive. FurthermoreTEN1 unlike STN1 and POT1a is not associated with active telomerase in vivo. These observations are consistent with a role for TEN1 in negative regulation of telomerase enzyme activity [41]. Intriguingly, TEN1 may only transiently associate with Arabidopsis telomeres. CTC1 can be detected at ∼50% of the Arabidopsis chromosome ends [39]. Since only half of the Arabidopsis telomeres carry G-overhangs [72], essentially all of the G-overhangs are bound by CTC1. In contrast, TEN1 can only be detected at 11% of the telomeres [41], implying that it dynamically binds telomeres and does not function exclusively in the context of a trimeric CST complex.
Altogether, our data suggest a model in which POT1a facilitates telomere maintenance in two ways: by promoting the switch from the un-extendable to the extendable state and by stimulating telomerase enzyme activity (Fig. 7). In S phase, telomerase holoenzyme is recruited to the G-overhang through an unknown mechanism. The enzyme associates with CTC1 and STN1 through contacts with POT1a, and POT1a stimulates G-strand synthesis. One attractive hypothesis is that mobilization of POT1a to the chromosome terminus triggers the exchange of the telomerase negative regulator TEN1 from STN1 as part of the switch to the telomerase extendable state. Although our in vitro data indicate that STN1 has a higher affinity for TEN1 than POT1a OB1, additional contacts by other regions of POT1a or between POT1a and CTC1 may stabilize its interaction with STN1. Furthermore, shifting telomerase-CST interactions are likely to be governed by cell cycle specific posttranslational modifications such as those described for yeast Est1 and CST, as well as human TPP1 [19], [20], [37]. Once the G-strand is extended, telomerase action is terminated, perhaps with the assistance of TEN1. This clears the way for conventional replication machinery and processing enzymes to complete telomere replication and return the telomere to its fully protected un-extendable state. Although additional studies are needed to precisely delineate the telomere-telomerase interface and its control during telomere replication, our findings underscore the highly dynamic nature of telomerase-telomere transactions and suggest that modulation of telomerase enzyme activity at the chromosome terminus contributes to the bimodal switch in telomere states.
Fig. 7. A model for telomere replication in Arabidopsis. 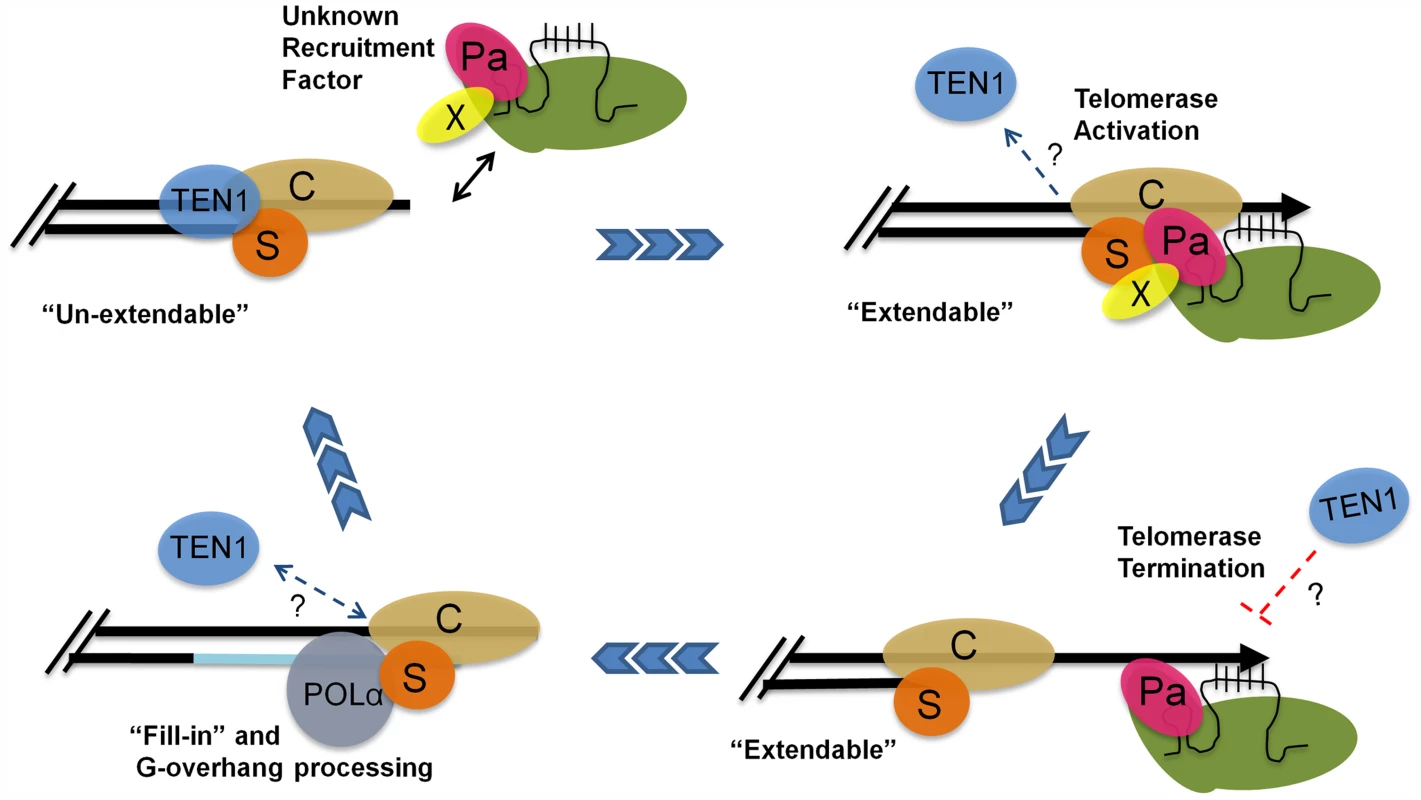
In the un-extendable state, telomeres are bound by the heterotrimeric CST complex. The telomerase RNP is positioned at the chromosome terminus by an unknown recruitment factor (X) during S phase. TEN1 is displaced. POT1a (Pa) contacts STN1 (S) and CTC1 (C) to promote a telomere extendable state. POT1a also stimulates telomerase enzymatic properties. TEN1 represses telomerase activity and thus may help to terminate telomerase action. Telomerase is removed and replaced by POLα for C-strand fill-in and terminal DNA processing. The telomere is then converted into an un-extendable state. Materials and Methods
Plant materials
Plants were housed in growth chambers with a 16 hr photoperiod at 22°C. stn1-1, ctc1-1, tert, pot1a-1 and ten1-3 mutants were used for crosses and genotyped as described [38], [39], [41], [59]. pot1a ter2 crosses were generated from homozygous parents. F1 progeny was planted for selection by genotyping. F3 seedlings were used for ChIP assays and pTRAP. In vivo experiments examining telomerase activity, protein interactions, or gene expression were either performed in juvenile seedlings or flowers, which both exhibit high levels of telomerase activity. For telomere length analysis, wild type controls were segregated from heterozygous parents to ensure that changes reflect mutations in the target genes and not stochastic variation [73].
Chromatin immunoprecipitation
Approximately 4–6 grams of Arabidopsis seven day-old seedlings were used for each genotype. The protocol was adapted from [74] with minor changes. Sonication was performed on ice after crosslinking and nuclei extraction using (Fisher Scientific) with 4 cycles of 15 sec on and 1 min off per sample at 40% amplification. Immunoprecipitation (IP) was performed using rabbit anti-TERT antibody and Protein-A agarose/salmon sperm DNA beads (Millipore). Eluted DNA was subjected to Southern dot blotting using a telomeric [32P] 5′ end-labeled oligonucleotide probe. Stripping and rDNA hybridization performed as previously described using a combination of 5S (5′-TTGCAGAATCCCGTGAACCATCGAGT-3′) and 18S (5′-TGGAGCCTGCGGCTTAATTTGACTCA-3′) rDNA oligo-probes [59]. Quantification was performed on at least three independent biological replicates using Quantity One software (Bio-Rad).
E. coli protein purification
Constructs for E. coli expression of TEN1 and POT1a OB1 were cloned in pET28a vector (Novagen). The POT1a OB1 domain was cloned from the POT1a start codon to residue 158. Four amino acids (SISS) were added to the C-terminus to increase protein solubility. Affinity column purification was achieved using Ni-NTA agarose resin (Qiagen) from BL21 DE3 lysates. Protein was eluted in imidazole buffer and dialyzed overnight. POT1a OB1 was further purified using a Sephadex G-75 (GE Healthcare) size exclusion column. TEN1 and POT1a OB1 protein fractions were analyzed for homogeneity on coomassie stained SDS-PAGE gels and verified by mass spectrometry. Proteins were expressed in rabbit reticulocyte lysate (RRL) (Promega) as indicated according to the manufacturer's instructions with [S35] Met (Perkin-Elmer) to label the protein expressed from pCITE4a, and in some cases pET28a.
Protein interaction assays
POT1a, STN1, TEN1, and CTC1ΔN cDNA were cloned into pET28a (T7-tag fusion) and pCITE4a vectors (Novagen). Details for POT1a OB1, OB1+2, and C-terminus constructs are previously described [53]. Co-IP with the RRL-expressed proteins was performed as described [75]. Competition assays were performed by incubating E. coli TEN1 protein with RRL-expressed STN1, and various amounts of E. coli POT1a OB1 or BSA. Equal loading for STN1 was achieved by evenly dividing a single master mix of RRL-expressed protein among the samples. Pull downs were performed by IP of TEN1 using purified TEN1 antibody [41] and protein-A agarose beads (Pierce). Complexes were washed 10× with buffer W300 [75] and eluted by boiling for 5 min in SDS loading dye. Samples were resolved on 12% SDS-PAGE gels followed by coomassie staining and then dried for analysis by autoradiography.
Protein immunoprecipitation
Extracts from ∼5 grams of wild type and pot1a seedling tissue were prepared as previously described [76] and pre-cleared using protein-A agarose beads (Pierce) with gentle rocking at 4°C for 1 h. IP was performed by adding 15 µg of affinity purified TERT, STN1, TEN1 or anti-GFP (Abcam) antibody (or pre-immune sera) overnight with gentle rocking at 4°C. Anti-rabbit STN1 antibody was raised from E. coli expressed and purified MBP-STN1 antigen. Protein-A agarose beads were added the following day for 2 hrs followed by 5× washes with buffer W300 [75], and 2× washes with buffer TMG [75]. IP samples were left in a final 50∶50 slurry in buffer TMG.
Telomere and telomerase assays
DNA from whole plants was extracted as described [77]. TRF analysis was performed using 50 µg of DNA digested with Tru1I (Fermentas) and hybridized with a [P32] 5′ end–labeled (TTTAGGG)4 probe [76]. Blots were developed using a Pharos FX Plus Molecular Imager (Bio-Rad) and data were analyzed with Quantity One software (Bio-Rad). Primer extension telomere repeat amplification (PETRA) was performed as described [63]. 2 µg of DNA was used per reaction for telomere extension, followed by PCR amplification. PETRA products were separated on an agarose gel and subjected to Southern blotting using the same telomeric probe mentioned above.
Protein for Telomere Repeat Amplification Protocol (TRAP) assays were extracted from 5 day-old seedlings and reactions were conducted as described [76]. TRAP assays on STN1, TEN1, CTC1-CFP, or TERT IP samples were performed by using 1 µl of the final IP slurry. The telomerase processivity TRAP (TP-TRAP) protocol was adapted from [61] and performed as previously described [41]. Briefly, TP-TRAP entails telomerase extension of a substrate primer followed by the first round of PCR with a 1RPgg primer to incorporate a unique sequence tag on telomerase products. A primer complementary to the tagged region (2RP) is added for the second PCR step followed by 33× cycles of PCR. Relative telomerase activity was measured by Quantitative TRAP (qTRAP) via SYBR Geen (Bio-RAD) qPCR after primer extension as discussed [78].
Western blotting
Fifty micrograms of wild type, stn1, and pot1a extracts were used for input samples. IP samples were boiled for 5 min in SDS loading dye. Samples were run on a 12% SDS-PAGE gel followed by protein gel blotting. Proteins were transferred overnight at 4°C onto a polyvinylidene difluoride (PVDF) membrane, followed by 2 hrs of blocking using 6% non-fat dried milk dissolved in 1× TBS-T (50 mM Tris, 150 mM NaCl, 0.1% Tween-20). Rabbit anti-STN1 antibody was diluted 1∶5000 in TBS-T and incubated with the protein blot for 4 hrs followed by 3× washes with TBS-T. Secondary anti-rabbit horseradish peroxidase was diluted 1∶7500 in TBS-T and incubated with the protein blot for 2 hrs, followed by 3× washes with TBS-T. Final detection was performed using an ECL prime protein blotting kit (GE Healthcare). Western blotting was performed as described for CTC1-CFP and POT1a [59] and TEN1 [41].
Quantitative RT-PCR
RNA was extracted from 5 day-old seedlings (Omega Bio-tek) followed by DNase I digestion (Zymogen) for 30 min at room temperature. RNA was phenol: chloroform extracted followed by EtOH precipitation. 1 µg of RNA was reverse transcribed (Quanta Supermix), then diluted 1∶4 using thousand-fold diluted yeast tRNAs. 1 µl of cDNA was used for qRT-PCR using CFX Connect Real-Time System (Bio-Rad) in triplicate. Quantification is from three biological replicates and normalized to wild type for each gene expression.
Supporting Information
Zdroje
1. PalmW, de LangeT (2008) How shelterin protects mammalian telomeres. Annu Rev Genet 42 : 301–334.
2. de LangeT (2009) How telomeres solve the end-protection problem. Science 326 : 948–952.
3. JainD, CooperJP (2010) Telomeric strategies: means to an end. Annu Rev Genet, Vol 44 44 : 243–269.
4. PriceCM, BoltzKA, ChaikenMF, StewartJA, BeilsteinMA, et al. (2010) Evolution of CST function in telomere maintenance. Cell Cycle 9 : 3157–3165.
5. BlackburnEH (2000) Telomere states and cell fates. Nature 408 : 53–56.
6. TeixeiraMT, ArnericM, SperisenP, LingnerJ (2004) Telomere length homeostasis is achieved via a switch between telomerase - extendible and -nonextendible states. Cell 117 : 323–335.
7. QiH, ZakianVA (2000) The Saccharomyces telomere-binding protein Cdc13p interacts with both the catalytic subunit of DNA polymerase alpha and the telomerase-associated est1 protein. Genes Dev 14 : 1777–1788.
8. MoserBA, SubramanianL, ChangYT, NoguchiC, NoguchiE, et al. (2009) Differential arrival of leading and lagging strand DNA polymerases at fission yeast telomeres. EMBO J 28 : 810–820.
9. DaiX, HuangC, BhusariA, SampathiS, SchubertK, et al. (2010) Molecular steps of G-overhang generation at human telomeres and its function in chromosome end protection. EMBO J 29 : 2788–2801.
10. GaoH, CervantesRB, MandellEK, OteroJH, LundbladV (2007) RPA-like proteins mediate yeast telomere function. Nat Struct Mol Biol 14 : 208–214.
11. SunJ, YuEY, YangY, ConferLA, SunSH, et al. (2009) Stn1-Ten1 is an Rpa2-Rpa3-like complex at telomeres. Genes Dev 23 : 2900–2914.
12. Mitton-FryRM, AndersonEM, TheobaldDL, GlustromLW, WuttkeDS (2004) Structural basis for telomeric single-stranded DNA recognition by yeast Cdc13. J Mol Biol 338 : 241–255.
13. GarvikB, CarsonM, HartwellL (1995) Single-stranded-DNA arising at telomeres in Cdc13 mutants may constitute a specificsignal for the Rad9 checkpoint. Mol Cell Biol 15 : 6128–6138.
14. NugentCI, HughesTR, LueNF, LundbladV (1996) Cdc13p: A single-strand telomeric DNA binding protein with a dual role in yeast telomere maintenance. Science 274 : 249–252.
15. GrandinN, DamonC, CharbonneauM (2001) Ten1 functions in telomere end protection and length regulation in association with Stn1 and Cdc13. EMBO J 20 : 1173–1183.
16. GrandinN, ReedSI, CharbonneauM (1997) Stn1, a new Saccharomyces cerevisiae protein, is implicated in telomere size regulation in association with Cdc13. Genes Dev 11 : 512–527.
17. PennockE, BuckleyK, LundbladV (2001) Cdc13 delivers separate complexes to the telomere for end protection and replication. Cell 104 : 387–396.
18. HolsteinEM, ClarkKR, LydallD (2014) Interplay between nonsense-mediated mRNA decay and DNA damage response pathways reveals that Stn1 and Ten1 are the key CST telomere-cap components. Cell Rep 7 : 1259–1269.
19. LiS, MakovetsS, MatsuguchiT, BlethrowJD, ShokatKM, et al. (2009) Cdk1-dependent phosphorylation of Cdc13 coordinates telomere elongation during cell-cycle progression. Cell 136 : 50–61.
20. LiuCC, GopalakrishnanV, PoonLF, YanT, LiS (2014) Cdk1 regulates the temporal recruitment of telomerase and Cdc13-Stn1-Ten1 Complex for telomere replication. Mol and Cell Biol 34 : 57–70.
21. LinJJ, ZakianVA (1995) An in-vitro assay for saccharomyces telomerase requires Est1. Cell 81 : 1127–1135.
22. SteinerBR, HidakaK, FutcherB (1996) Association of the Est1 protein with telomerase activity in yeast. Proc Natl Acad Sci U S A 93 : 2817–2821.
23. EvansSK, LundbladV (1999) Est1 and Cdc13 as comediators of telomerase access. Science 286 : 117–120.
24. WuY, ZakianVA (2011) The telomeric Cdc13 protein interacts directly with the telomerase subunit Est1 to bring it to telomeric DNA ends in vitro. Proc Natl Acad Sci U S A 108 : 20362–20369.
25. LundbladV, SzostakJW (1989) A mutant with a defect in telomere elongation leads to senescence in yeast. Cell 57 : 633–643.
26. TaggartAKP, TengSC, ZakianVA (2002) Est1p as a cell cycle-regulated activator of telomere-bound telomerase. Science 297 : 1023–1026.
27. DeZwaanDC, FreemanBC (2009) The conserved Est1 protein stimulates telomerase DNA extension activity. Proc Natl Acad Sci U S A 106 : 17337–17342.
28. BaumannP, CechTR (2001) Pot1 the putative telomere end-binding protein in fission yeast and humans (vol 292, pg 1171, 2001). Science 293 : 214–214.
29. NandakumarJ, CechTR (2013) Finding the end: recruitment of telomerase to telomeres. Nat Rev Mol Cell Biol 14 : 69–82.
30. LeiM, PodellER, CechTR (2004) Structure of human POT1 bound to telomeric single-stranded DNA provides a model for chromosome end-protection. Nat Struct Mol Biol 11 : 1223–1229.
31. LeiM, ZaugAJ, PodellER, CechTR (2005) Switching human telomerase on and off with hPOT1 protein in vitro. J Biol Chem 280 : 20449–20456.
32. WangF, PodellER, ZaugAJ, YangYT, BaciuP, et al. (2007) The POT1-TPP1 telomere complex is a telomerase processivity factor. Nature 445 : 506–510.
33. XinHW, LiuD, WanM, SafariA, KimH, et al. (2007) TPP1 is a homologue of ciliate TEBP-beta and interacts with POT1 to recruit telomerase. Nature 445 : 559–562.
34. LatrickCM, CechTR (2010) POT1-TPP1 enhances telomerase processivity by slowing primer dissociation and aiding translocation. EMBO J 29 : 924–933.
35. ZaugAJ, PodellER, NandakumarJ, CechTR (2010) Functional interaction between telomere protein TPP1 and telomerase. Genes Dev 24 : 613–622.
36. NandakumarJ, BellCF, WeidenfeldI, ZaugAJ, LeinwandLA, et al. (2012) The TEL patch of telomere protein TPP1 mediates telomerase recruitment and processivity. Nature 492 : 285–289.
37. ZhangY, ChenLY, HanX, XieW, KimH, et al. (2013) Phosphorylation of TPP1 regulates cell cycle-dependent telomerase recruitment. Proc Natl Acad Sci U S A 110 : 5457–5462.
38. SongXY, LeehyK, WarringtonRT, LambJC, SurovtsevaYV, et al. (2008) STN1 protects chromosome ends in Arabidopsis thaliana. Proc Natl Acad Sci U S A 105 : 19815–19820.
39. SurovtsevaYV, ChurikovD, BoltzKA, SongXY, LambJC, et al. (2009) Conserved Telomere Maintenance Component 1 Interacts with STN1 and maintains chromosome ends in higher eukaryotes. Mol Cell 36 : 207–218.
40. MiyakeY, NakamuraM, NabetaniA, ShimamuraS, TamuraM, et al. (2009) RPA-like Mammalian Ctc1-Stn1-Ten1 Complex Binds to Single-Stranded DNA and Protects Telomeres Independently of the Pot1 Pathway. Mol Cell 36 : 193–206.
41. LeehyKA, LeeJR, SongX, RenfrewKB, ShippenDE (2013) MERISTEM DISORGANIZATION1 encodes TEN1, an essential telomere protein that modulates telomerase processivity in Arabidopsis. Plant Cell 25 : 1343–1354.
42. StewartJA, WangF, ChaikenMF, KasbekC, ChastainPD, et al. (2012) Human CST promotes telomere duplex replication and general replication restart after fork stalling. EMBO J 31 : 3537–3549.
43. GuPL, MinJN, WangY, HuangCH, PengT, et al. (2012) CTC1 deletion results in defective telomere replication, leading to catastrophic telomere loss and stem cell exhaustion. EMBO J 31 : 2309–2321.
44. KasbekC, WangF, PriceCM (2013) Human TEN1 maintains telomere integrity and functions in genome-wide replication restart. J Biol Chem 288 : 30139–30150.
45. WangF, StewartJA, KasbekC, ZhaoY, WrightWE, et al. (2012) Human CST has independent functions during telomere duplex replication and C-strand fill-in. Cell Rep 2 : 1096–1103.
46. ChenLY, RedonS, LingnerJ (2012) The human CST complex is a terminator of telomerase activity. Nature 488 : 540–544.
47. WuP, TakaiH, de LangeT (2012) Telomeric 3′ overhangs derive from resection by Exo1 and Apollo and fill-in by POT1b-associated CST. Cell 150 : 39–52.
48. LoayzaD, de LangeT (2003) POT1 as a terminal transducer of TRF1 telomere length control. Nature 423 : 1013–1018.
49. JunHI, LiuJQ, JeongH, KimJK, QiaoF (2013) Tpz1 controls a telomerase-nonextendible telomeric state and coordinates switching to an extendible state via Ccq1. Genes Dev 27 : 1917–1931.
50. GargM, GurungRL, MansoubiS, AhmedJO, DaveA, et al. (2014) Tpz1TPP1 SUMOylation reveals evolutionary conservation of SUMO-dependent Stn1 telomere association. EMBO Rep (8): 871–7.
51. MiyagawaK, LowRS, SantosaV, TsujiH, MoserBA, et al. (2014) SUMOylation regulates telomere length by targeting the shelterin subunit Tpz1(Tpp1) to modulate shelterin-Stn1 interaction in fission yeast. Proc Natl Acad Sci U S A 111 : 5950–5955.
52. HashimuraY, UeguchiC (2011) The Arabidopsis MERISTEM DISORGANIZATION 1 gene is required for the maintenance of stem cells through the reduction of DNA damage. Plant J 68 : 657–669.
53. Cifuentes-RojasC, KannanK, TsengL, ShippenDE (2011) Two RNA subunits and POT1a are components of Arabidopsis telomerase. Proc Natl Acad Sci U S A 108 : 73–78.
54. Cifuentes-RojasC, NelsonADL, BoltzKA, KannanK, SheXT, et al. (2012) An alternative telomerase RNA in Arabidopsis modulates enzyme activity in response to DNA damage. Genes Dev 26 : 2512–2523.
55. RiehsN, AkimchevaS, PuizinaJ, BulankovaP, IdolRA, et al. (2008) Arabidopsis SMG7 protein is required for exit from meiosis. J Cell Sci 121 : 2208–2216.
56. ShakirovEV, SurovtsevaYV, OsbunN, ShippenDE (2005) The Arabidopsis Pot1 and Pot2 proteins function in telomere length homeostasis and chromosome end protection. Mol Cell Biol 25 : 7725–7733.
57. RossignolP, CollierS, BushM, ShawP, DoonanJH (2007) Arabidopsis POT1A interacts with TERT-V(I8), an N-terminal splicing variant of telomerase. J Cell Sci 120 : 3678–3687.
58. KucharM, FajkusJ (2004) Interactions of putative telomere-binding proteins in Arabidopsis thaliana: identification of functional TRF2 homolog in plants. FEBS Lett 578 : 311–315.
59. SurovtsevaYV, ShakirovEV, VespaL, OsbunN, SongXY, et al. (2007) Arabidopsis POT1 associates with the telomerase RNP and is required for telomere maintenance. EMBO J 26 : 3653–3661.
60. FitzgeraldMS, RihaK, GaoF, RenS, McKnightTD, et al. (1999) Disruption of the telomerase catalytic subunit gene from Arabidopsis inactivates telomerase and leads to a slow loss of telomeric DNA. Proc Natl Acad Sci U S A 96 : 14813–14818.
61. SzatmariI, AradiJ (2001) Telomeric repeat amplification, without shortening or lengthening of the telomerase products: a method to analyze the processivity of telomerase enzyme. Nucleic Acids Res 29 : 2E3.
62. RihaK, McKnightTD, GriffingLR, ShippenDE (2001) Living with genome instability: Plant responses to telomere dysfunction. Science 291 : 1797–1800.
63. HeacockM, SpanglerE, RihaK, PuizinaJ, ShippenDE (2004) Molecular analysis of telomere fusions in Arabidopsis: multiple pathways for chromosome end-joining. EMBO J 23 : 2304–2313.
64. ChenLY, MajerskaJ, LingnerJ (2013) Molecular basis of telomere syndrome caused by CTC1 mutations. Genes Dev 27 : 2099–2108.
65. WanM, QinJ, SongyangZ, LiuD (2009) OB fold-containing protein 1 (OBFC1), a human homolog of yeast Stn1, associates with TPP1 and is implicated in telomere length regulation. J Biol Chem 284 : 26725–26731.
66. ChanA, BouleJB, ZakianVA (2008) Two pathways recruit telomerase to Saccharomyces cerevisiae telomeres. PLOS Genetics 4: e1000236.
67. GallegoME, BleuyardJY, Daoudal-CotterellS, JallutN, WhiteCI (2003) Ku80 plays a role in non-homologous recombination but is not required for T-DNA integration in Arabidopsis. Plant J 35 : 557–565.
68. RihaK, WatsonJM, ParkeyJ, ShippenDE (2002) Telomere length deregulation and enhanced sensitivity to genotoxic stress in Arabidopsis mutants deficient in Ku70. EMBO J 21 : 2819–2826.
69. SchrumpfovaPP, VychodilovaI, DvorackovaM, MajerskaJ, DokladalL, et al. (2014) Telomere repeat binding proteins are functional components of Arabidopsis telomeres and interact with telomerase. Plant J 77 : 770–781.
70. KappeiD, ButterF, BendaC, ScheibeM, DraskovicI, et al. (2013) HOT1 is a mammalian direct telomere repeat-binding protein contributing to telomerase recruitment. EMBO J 32 : 1681–1701.
71. MinBS, CollinsK (2009) An RPA-related sequence-specific DNA-binding subunit of telomerase holoenzyme Is required for elongation processivity and telomere maintenance. Mol Cell 36 : 609–619.
72. KazdaA, ZellingerB, RosslerM, DerbovenE, KusendaB, et al. (2012) Chromosome end protection by blunt-ended telomeres. Genes Dev 26 : 1703–1713.
73. ShakirovEV, ShippenDE (2004) Length regulation and dynamics of individual telomere tracts in wild-type Arabidopsis. Plant Cell 16 : 1959–1967.
74. SalehA, Alvarez-VenegasR, AvramovaZ (2008) An efficient chromatin immunoprecipitation (ChIP) protocol for studying histone modifications in Arabidopsis plants. Nat Protoc 3 : 1018–1025.
75. KaramyshevaZN, SurovtsevaYV, VespaL, ShakirovEV, ShippenDE (2004) A C-terminal Myb extension domain defines a novel family of double-strand telomeric DNA-binding proteins in Arabidopsis. J Biol Chem 279 : 47799–47807.
76. FitzgeraldMS, McKnightTD, ShippenDE (1996) Characterization and developmental patterns of telomerase expression in plants. Proc Natl Acad Sci U S A 93 : 14422–14427.
77. CoccioloneSM, ConeKC (1993) Pl-Bh, an anthocyanin regulatory gene of maize that leads to variegated pigmentation. Genetics 135 : 575–588.
78. KannanK, NelsonADL, ShippenDE (2008) Dyskerin is a component of the Arabidopsis telomerase RNP required for telomere maintenance. Mol Cell Biol 28 : 2332–2341.
Štítky
Genetika Reprodukční medicína
Článek Oligoasthenoteratozoospermia and Infertility in Mice Deficient for miR-34b/c and miR-449 LociČlánek The Kinesin AtPSS1 Promotes Synapsis and is Required for Proper Crossover Distribution in MeiosisČlánek Payoffs, Not Tradeoffs, in the Adaptation of a Virus to Ostensibly Conflicting Selective PressuresČlánek Examination of Prokaryotic Multipartite Genome Evolution through Experimental Genome ReductionČlánek BMP-FGF Signaling Axis Mediates Wnt-Induced Epidermal Stratification in Developing Mammalian SkinČlánek Role of STN1 and DNA Polymerase α in Telomere Stability and Genome-Wide Replication in ArabidopsisČlánek RNA-Processing Protein TDP-43 Regulates FOXO-Dependent Protein Quality Control in Stress ResponseČlánek Integrating Functional Data to Prioritize Causal Variants in Statistical Fine-Mapping StudiesČlánek Salt-Induced Stabilization of EIN3/EIL1 Confers Salinity Tolerance by Deterring ROS Accumulation inČlánek Ethylene-Induced Inhibition of Root Growth Requires Abscisic Acid Function in Rice ( L.) SeedlingsČlánek Metabolic Respiration Induces AMPK- and Ire1p-Dependent Activation of the p38-Type HOG MAPK PathwayČlánek Signature Gene Expression Reveals Novel Clues to the Molecular Mechanisms of Dimorphic Transition inČlánek A Mouse Model Uncovers LKB1 as an UVB-Induced DNA Damage Sensor Mediating CDKN1A (p21) DegradationČlánek Dominant Sequences of Human Major Histocompatibility Complex Conserved Extended Haplotypes from to
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2014 Číslo 10
-
Všechny články tohoto čísla
- An Deletion Is Highly Associated with a Juvenile-Onset Inherited Polyneuropathy in Leonberger and Saint Bernard Dogs
- Licensing of Yeast Centrosome Duplication Requires Phosphoregulation of Sfi1
- Oligoasthenoteratozoospermia and Infertility in Mice Deficient for miR-34b/c and miR-449 Loci
- Basement Membrane and Cell Integrity of Self-Tissues in Maintaining Immunological Tolerance
- The Kinesin AtPSS1 Promotes Synapsis and is Required for Proper Crossover Distribution in Meiosis
- Germline Mutations in Are Associated with Familial Gastric Cancer
- POT1a and Components of CST Engage Telomerase and Regulate Its Activity in
- Controlling Meiotic Recombinational Repair – Specifying the Roles of ZMMs, Sgs1 and Mus81/Mms4 in Crossover Formation
- Payoffs, Not Tradeoffs, in the Adaptation of a Virus to Ostensibly Conflicting Selective Pressures
- FHIT Suppresses Epithelial-Mesenchymal Transition (EMT) and Metastasis in Lung Cancer through Modulation of MicroRNAs
- Genome-Wide Mapping of Yeast RNA Polymerase II Termination
- Examination of Prokaryotic Multipartite Genome Evolution through Experimental Genome Reduction
- White Cells Facilitate Opposite- and Same-Sex Mating of Opaque Cells in
- BMP-FGF Signaling Axis Mediates Wnt-Induced Epidermal Stratification in Developing Mammalian Skin
- Genome-Wide Association Study of CSF Levels of 59 Alzheimer's Disease Candidate Proteins: Significant Associations with Proteins Involved in Amyloid Processing and Inflammation
- COE Loss-of-Function Analysis Reveals a Genetic Program Underlying Maintenance and Regeneration of the Nervous System in Planarians
- Fat-Dachsous Signaling Coordinates Cartilage Differentiation and Polarity during Craniofacial Development
- Identification of Genes Important for Cutaneous Function Revealed by a Large Scale Reverse Genetic Screen in the Mouse
- Sensors at Centrosomes Reveal Determinants of Local Separase Activity
- Genes Integrate and Hedgehog Pathways in the Second Heart Field for Cardiac Septation
- Systematic Dissection of Coding Exons at Single Nucleotide Resolution Supports an Additional Role in Cell-Specific Transcriptional Regulation
- Recovery from an Acute Infection in Requires the GATA Transcription Factor ELT-2
- HIPPO Pathway Members Restrict SOX2 to the Inner Cell Mass Where It Promotes ICM Fates in the Mouse Blastocyst
- Role of and in Development of Abdominal Epithelia Breaks Posterior Prevalence Rule
- The Formation of Endoderm-Derived Taste Sensory Organs Requires a -Dependent Expansion of Embryonic Taste Bud Progenitor Cells
- Role of STN1 and DNA Polymerase α in Telomere Stability and Genome-Wide Replication in Arabidopsis
- Keratin 76 Is Required for Tight Junction Function and Maintenance of the Skin Barrier
- Encodes the Catalytic Subunit of N Alpha-Acetyltransferase that Regulates Development, Metabolism and Adult Lifespan
- Disruption of SUMO-Specific Protease 2 Induces Mitochondria Mediated Neurodegeneration
- Caudal Regulates the Spatiotemporal Dynamics of Pair-Rule Waves in
- It's All in Your Mind: Determining Germ Cell Fate by Neuronal IRE-1 in
- A Conserved Role for Homologs in Protecting Dopaminergic Neurons from Oxidative Stress
- The Master Activator of IncA/C Conjugative Plasmids Stimulates Genomic Islands and Multidrug Resistance Dissemination
- An AGEF-1/Arf GTPase/AP-1 Ensemble Antagonizes LET-23 EGFR Basolateral Localization and Signaling during Vulva Induction
- The Proteomic Landscape of the Suprachiasmatic Nucleus Clock Reveals Large-Scale Coordination of Key Biological Processes
- RNA-Processing Protein TDP-43 Regulates FOXO-Dependent Protein Quality Control in Stress Response
- A Complex Genetic Switch Involving Overlapping Divergent Promoters and DNA Looping Regulates Expression of Conjugation Genes of a Gram-positive Plasmid
- ZTF-8 Interacts with the 9-1-1 Complex and Is Required for DNA Damage Response and Double-Strand Break Repair in the Germline
- Integrating Functional Data to Prioritize Causal Variants in Statistical Fine-Mapping Studies
- Tpz1-Ccq1 and Tpz1-Poz1 Interactions within Fission Yeast Shelterin Modulate Ccq1 Thr93 Phosphorylation and Telomerase Recruitment
- Salt-Induced Stabilization of EIN3/EIL1 Confers Salinity Tolerance by Deterring ROS Accumulation in
- Telomeric (s) in spp. Encode Mediator Subunits That Regulate Distinct Virulence Traits
- Ethylene-Induced Inhibition of Root Growth Requires Abscisic Acid Function in Rice ( L.) Seedlings
- Ancient Expansion of the Hox Cluster in Lepidoptera Generated Four Homeobox Genes Implicated in Extra-Embryonic Tissue Formation
- Mechanism of Suppression of Chromosomal Instability by DNA Polymerase POLQ
- A Mutation in the Mouse Gene Leads to Impaired Hedgehog Signaling
- Keeping mtDNA in Shape between Generations
- Targeted Exon Capture and Sequencing in Sporadic Amyotrophic Lateral Sclerosis
- TIF-IA-Dependent Regulation of Ribosome Synthesis in Muscle Is Required to Maintain Systemic Insulin Signaling and Larval Growth
- At Short Telomeres Tel1 Directs Early Replication and Phosphorylates Rif1
- Evidence of a Bacterial Receptor for Lysozyme: Binding of Lysozyme to the Anti-σ Factor RsiV Controls Activation of the ECF σ Factor σ
- Hsp40s Specify Functions of Hsp104 and Hsp90 Protein Chaperone Machines
- Feeding State, Insulin and NPR-1 Modulate Chemoreceptor Gene Expression via Integration of Sensory and Circuit Inputs
- Functional Interaction between Ribosomal Protein L6 and RbgA during Ribosome Assembly
- Multiple Regulatory Systems Coordinate DNA Replication with Cell Growth in
- Fast Evolution from Precast Bricks: Genomics of Young Freshwater Populations of Threespine Stickleback
- Mmp1 Processing of the PDF Neuropeptide Regulates Circadian Structural Plasticity of Pacemaker Neurons
- The Nuclear Immune Receptor Is Required for -Dependent Constitutive Defense Activation in
- Genetic Modifiers of Neurofibromatosis Type 1-Associated Café-au-Lait Macule Count Identified Using Multi-platform Analysis
- Juvenile Hormone-Receptor Complex Acts on and to Promote Polyploidy and Vitellogenesis in the Migratory Locust
- Uncovering Enhancer Functions Using the α-Globin Locus
- The Analysis of Mutant Alleles of Different Strength Reveals Multiple Functions of Topoisomerase 2 in Regulation of Chromosome Structure
- Metabolic Respiration Induces AMPK- and Ire1p-Dependent Activation of the p38-Type HOG MAPK Pathway
- The Specification and Global Reprogramming of Histone Epigenetic Marks during Gamete Formation and Early Embryo Development in
- The DAF-16 FOXO Transcription Factor Regulates to Modulate Stress Resistance in , Linking Insulin/IGF-1 Signaling to Protein N-Terminal Acetylation
- Genetic Influences on Translation in Yeast
- Analysis of Mutants Defective in the Cdk8 Module of Mediator Reveal Links between Metabolism and Biofilm Formation
- Ribosomal Readthrough at a Short UGA Stop Codon Context Triggers Dual Localization of Metabolic Enzymes in Fungi and Animals
- Gene Duplication Restores the Viability of Δ and Δ Mutants
- Selection on a Variant Associated with Improved Viral Clearance Drives Local, Adaptive Pseudogenization of Interferon Lambda 4 ()
- Break-Induced Replication Requires DNA Damage-Induced Phosphorylation of Pif1 and Leads to Telomere Lengthening
- Dynamic Partnership between TFIIH, PGC-1α and SIRT1 Is Impaired in Trichothiodystrophy
- Signature Gene Expression Reveals Novel Clues to the Molecular Mechanisms of Dimorphic Transition in
- Mutations in Moderate or Severe Intellectual Disability
- Multifaceted Genome Control by Set1 Dependent and Independent of H3K4 Methylation and the Set1C/COMPASS Complex
- A Role for Taiman in Insect Metamorphosis
- The Small RNA Rli27 Regulates a Cell Wall Protein inside Eukaryotic Cells by Targeting a Long 5′-UTR Variant
- MMS Exposure Promotes Increased MtDNA Mutagenesis in the Presence of Replication-Defective Disease-Associated DNA Polymerase γ Variants
- Coexistence and Within-Host Evolution of Diversified Lineages of Hypermutable in Long-term Cystic Fibrosis Infections
- Comprehensive Mapping of the Flagellar Regulatory Network
- Topoisomerase II Is Required for the Proper Separation of Heterochromatic Regions during Female Meiosis
- A Splice Mutation in the Gene Causes High Glycogen Content and Low Meat Quality in Pig Skeletal Muscle
- KDM5 Interacts with Foxo to Modulate Cellular Levels of Oxidative Stress
- H2B Mono-ubiquitylation Facilitates Fork Stalling and Recovery during Replication Stress by Coordinating Rad53 Activation and Chromatin Assembly
- Copy Number Variation in the Horse Genome
- Unifying Genetic Canalization, Genetic Constraint, and Genotype-by-Environment Interaction: QTL by Genomic Background by Environment Interaction of Flowering Time in
- Spinster Homolog 2 () Deficiency Causes Early Onset Progressive Hearing Loss
- Genome-Wide Discovery of Drug-Dependent Human Liver Regulatory Elements
- Developmentally-Regulated Excision of the SPβ Prophage Reconstitutes a Gene Required for Spore Envelope Maturation in
- Protein Phosphatase 4 Promotes Chromosome Pairing and Synapsis, and Contributes to Maintaining Crossover Competence with Increasing Age
- The bHLH-PAS Transcription Factor Dysfusion Regulates Tarsal Joint Formation in Response to Notch Activity during Leg Development
- A Mouse Model Uncovers LKB1 as an UVB-Induced DNA Damage Sensor Mediating CDKN1A (p21) Degradation
- Notch3 Interactome Analysis Identified WWP2 as a Negative Regulator of Notch3 Signaling in Ovarian Cancer
- An Integrated Cell Purification and Genomics Strategy Reveals Multiple Regulators of Pancreas Development
- Dominant Sequences of Human Major Histocompatibility Complex Conserved Extended Haplotypes from to
- The Vesicle Protein SAM-4 Regulates the Processivity of Synaptic Vesicle Transport
- A Gain-of-Function Mutation in Impeded Bone Development through Increasing Expression in DA2B Mice
- Nephronophthisis-Associated Regulates Cell Cycle Progression, Apoptosis and Epithelial-to-Mesenchymal Transition
- Beclin 1 Is Required for Neuron Viability and Regulates Endosome Pathways via the UVRAG-VPS34 Complex
- The Not5 Subunit of the Ccr4-Not Complex Connects Transcription and Translation
- Abnormal Dosage of Ultraconserved Elements Is Highly Disfavored in Healthy Cells but Not Cancer Cells
- Genome-Wide Distribution of RNA-DNA Hybrids Identifies RNase H Targets in tRNA Genes, Retrotransposons and Mitochondria
- The Chromosomal Association of the Smc5/6 Complex Depends on Cohesion and Predicts the Level of Sister Chromatid Entanglement
- Cell-Autonomous Progeroid Changes in Conditional Mouse Models for Repair Endonuclease XPG Deficiency
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- The Master Activator of IncA/C Conjugative Plasmids Stimulates Genomic Islands and Multidrug Resistance Dissemination
- A Splice Mutation in the Gene Causes High Glycogen Content and Low Meat Quality in Pig Skeletal Muscle
- Keratin 76 Is Required for Tight Junction Function and Maintenance of the Skin Barrier
- A Role for Taiman in Insect Metamorphosis
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



