-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaGenes Integrate and Hedgehog Pathways in the Second Heart Field for Cardiac Septation
Atrioventricular septal defects (AVSDs) are a common severe class of congenital heart defects. Recent work demonstrates that events in the second heart field (SHF) progenitors, rather than in the heart, drive atrioventricular (AV) septation. Our laboratory has shown that both Hedgehog signaling and the T-box transcription factor, Tbx5, are required in the SHF for AV septation. To understand the molecular underpinnings of the AV septation process we investigated SHF Hedgehog-dependent gene regulatory networks. Transcriptional profiling and chromatin interaction assays identified the Forkhead box transcription factors Foxf1a and Foxf2 as SHF Hedgehog targets. Compound haploinsufficiency for Foxf1a and Foxf2 caused AVSDs in mice, demonstrating the biological relevance of this pathway. We identified a cis-regulatory element at Foxf1a that bound TBX5 and Hedgehog transcriptional regulators GLI1 and GLI3 in-vivo. Furthermore, TBX5 and Gli1 co-activate transcription of the identified cis-regulatory element in-vitro. The enhancer is expressed primarily in the pSHF in-vivo, where Tbx5 and Gli1 expression overlap. Our findings implicate Foxf1a and Foxf2 in AV septation and establish Tbx5 and Hedgehog signaling upstream of Foxf genes in a gene regulatory network for cardiac septation.
Published in the journal: . PLoS Genet 10(10): e32767. doi:10.1371/journal.pgen.1004604
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004604Summary
Atrioventricular septal defects (AVSDs) are a common severe class of congenital heart defects. Recent work demonstrates that events in the second heart field (SHF) progenitors, rather than in the heart, drive atrioventricular (AV) septation. Our laboratory has shown that both Hedgehog signaling and the T-box transcription factor, Tbx5, are required in the SHF for AV septation. To understand the molecular underpinnings of the AV septation process we investigated SHF Hedgehog-dependent gene regulatory networks. Transcriptional profiling and chromatin interaction assays identified the Forkhead box transcription factors Foxf1a and Foxf2 as SHF Hedgehog targets. Compound haploinsufficiency for Foxf1a and Foxf2 caused AVSDs in mice, demonstrating the biological relevance of this pathway. We identified a cis-regulatory element at Foxf1a that bound TBX5 and Hedgehog transcriptional regulators GLI1 and GLI3 in-vivo. Furthermore, TBX5 and Gli1 co-activate transcription of the identified cis-regulatory element in-vitro. The enhancer is expressed primarily in the pSHF in-vivo, where Tbx5 and Gli1 expression overlap. Our findings implicate Foxf1a and Foxf2 in AV septation and establish Tbx5 and Hedgehog signaling upstream of Foxf genes in a gene regulatory network for cardiac septation.
Introduction
Cardiac septation, the morphogenetic process that transitions the looped heart tube into the multi-chambered heart observed in mammals, is complex and often goes awry in Congenital Heart Disease (CHD). Atrioventricular septation is the crucial process that separates the common atrioventricular canal into right and left compartments. Atrioventricular septal defects (AVSDs) are a common severe form of CHD. A novel paradigm for the developmental ontogeny of the atrioventricular septum has recently emerged [1]–[6]. This work describes atrioventricular septation as a process driven by molecular events in second heart field (SHF) cardiac progenitors rather than in the heart itself [1]–[6]. The identification of extracardiac lineages that generate the atrial and atrioventricular septum implies that the search for gene regulatory networks germane to cardiac septation should occur in SHF cardiac progenitors not in the heart itself.
Hedgehog signaling is an essential developmental pathway conserved from flies to man [7], [8]. Mutations in key Hedgehog pathway genes, including ligands such as Sonic hedgehog (Shh; 20423) and downstream signaling cascade member Smoothened (Smo; 319757) cause significant cardiac defects including complete atrioventricular septal defects [9], [10]. Tissue specific knockout of Hedgehog signaling in the SHF recapitulates atrioventricular septal defects [4], [5] and genetic inducible fate mapping showed that the atrial/atrioventricular septum is derived from Hedgehog-receiving SHF cardiac progenitors [5]. These observations laid the groundwork for identifying the Hedgehog-dependent SHF gene regulatory networks essential for atrial septation.
Cardiogenic transcription factor genes Tbx5 (21388), Nkx2.5 (18091) and GATA4 (14463) have been implicated in human atrial septation [11]–[14]. These transcription factors form a complex and can co-activate gene expression [12], [15]–[17]. Tbx5 has been shown to be required in multiple contexts during cardiac development and adult function in mice. Tbx5 is required in the SHF for atrioventricular septation [6], [15], in embryonic cardiomyocytes for proliferation [18], in adult myocardium for contractile function [19], and in the adult cardiac conduction system for cardiac rhythm control [20]. Tbx5 target genes differ significantly between these distinct cellular and temporal contexts [6], [21]. Yet the Tbx5-responsive cis-regulatory elements specific to these cellular contexts and the molecular cues that establish context dependent selectivity remain unknown.
We previously described genetic interactions between Tbx5 and Hedgehog signaling in the SHF for atrioventricular septation in mice [6]. Mice haploinsufficient for both Tbx5 and the obligate Hedgehog signaling receptor gene Smo express AVSDs more frequently than mice haploinsufficient for either gene alone [6]. Furthermore, constitutive Hedgehog signaling in Tbx5-mutant SHF progenitors can rescue atrioventricular septation [6]. These studies predict that Hedgehog-dependent and Tbx5-dependent gene regulatory networks share vital, yet undescribed overlap in the SHF that is necessary for atrioventricular septation.
In this study we attempted to define Hedgehog-dependent SHF gene regulatory networks and identify the molecular basis of the genetic interaction between Hedgehog signaling and Tbx5. We characterized the Hedgehog-dependent SHF gene regulatory networks by in vivo whole genome transcriptional profiling and GLI-chromatin interaction studies. We found that Foxf1a (15227) and Foxf2 (14238) are downstream of Hedgehog signaling in the SHF. Mice haploinsufficient for both Foxf1a and Foxf2 compound heterozygotes have atrial septal defects, demonstrating the biological relevance of these Hedgehog targets. GLI3T (14634) binding data identified a candidate cis-regulatory element upstream of Foxf1a that contained an adjacent Tbx5 binding site. This enhancer binds to GLI1 (14632), GLI3 and TBX5 in the SHF in vivo. In vitro and in vivo analysis demonstrated that this cis-regulatory element integrates Hedgehog signaling with Tbx5 activity and provides strong specific activity in the posterior SHF. This work identifies a novel role for Foxf transcription factors at the intersection of Tbx5 and hedgehog signaling in atrioventricular septation and describes a SHF gene regulatory network for cardiac morphogenesis.
Results
Transcriptional profiling of the posterior SHF in Shh mutants
Progenitor cells for the atrial and atrioventricular septum require Shh signaling in the posterior SHF (pSHF) between embryonic day 8 and embryonic day 10 (E8–E10) to migrate into the heart to form the atrial septum between E9–E11 [4], [5]. To identify the Hedgehog-dependent gene regulatory networks required for this process, we compared transcriptional profiling of the posterior SHF from wild-type and Shh (MGI: 1932461) null embryos at E9.5 to identify differentially expressed transcripts. We isolated the pSHF by microdissection including the dorsal mesenchymal protrusion and closely associated surrounding ventral lateral plate mesenchyme. Our dissection included the attached foregut, but excluded the heart, dorsal lateral plate mesenchyme and neural tube (Figure 1A). RNA was isolated and known Hedgehog-dependent transcripts were evaluated by RT-PCR to verify genotyping prior to whole genome transcriptional profiling. Shh, Ptch1 (19206) and Gli1 all demonstrated significantly reduced expression (p>0.05) in the Shh null samples compared to wild-type micro-dissected samples (Figure 1B). Specifically, Shh was reduced more than 90%, while Ptch1 and Gli1 were each reduced approximately 50%, consistent with significantly reduced Hedgehog signaling in the mutant samples and confirming the genotypic fidelity of the isolated samples.
Fig. 1. Transcriptional profiling of SHF from shh−/− embryos. 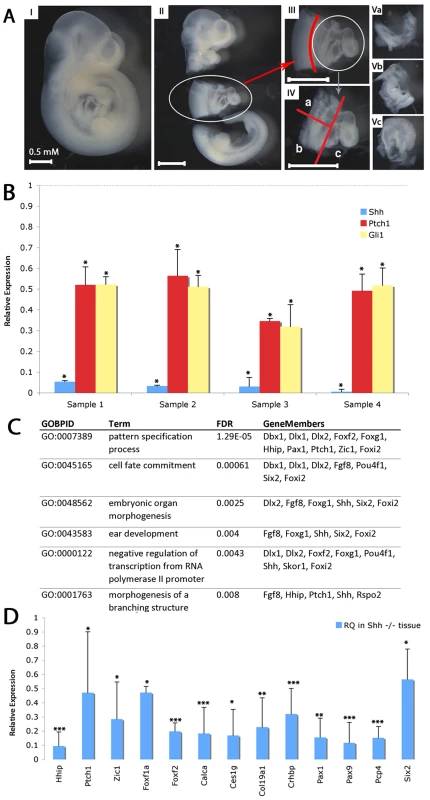
(A) Microdissection for isolation of SHF tissues. E9.5 embryos were isolated (I). Thoracic tissues including the heart were removed from head and tail, kept for genotyping or non-cardiac controls (II). Neural tube was removed (III). SHF tissue was bisected and separated from the heart (IV). Microdissected tissue was kept as anterior SHF (Va), posterior SHF (Vb) or heart (Vc). (B) RT-PCR demonstrates decreased expression of Shh, Gli1 and Ptch1 in shh mutant SHF tissues isolated for transcriptional profiling (C) Gene Ontology biological processes (GOBPs) enriched in the transcriptional profile analysis of SHF tissue from wild-type and Shh mutant embryos identifies developmental terms. (D) 13 genes identified in the transcriptional profile were verified as Shh-dependent using RT-qPCR (relative quantitation, RQ). * indicates p<0.05, ** indicates p<0.01, *** indicates p<0.001. Transcriptional profiling of pSHF samples was performed on Agilent Mouse Whole Genome Arrays. Using a significance threshold with a multi-test adjusted p-value (Q-value) <0.005 and absolute fold change larger than 2, comparing Shh−/− mutant mouse embryos (n = 4) with wild-type embryos (n = 3) identified a differentially expressed 560-gene signature (Table S1). Gene Ontology (GO) enrichment analysis of differentially expressed genes captured known processes disrupted in Hedgehog pathway mutants, such as pattern specification and organ morphogenesis (Figure 1C) [22]. To further identify the best candidates for an experimental validation, 65 genes were computationally evaluated according to more stringent criteria by three statistical tests (non-parameter Wilcox-tested theoretical p<0.15, empirical t-tested FDR<0.1, and absolute fold change>3, Figure S1) on the same data sets. From the Shh down-regulated candidates, we chose 21 targets and validated significant misexpression of 13 by qPCR (p<2e-16, Fisher's Exact test, FET) (Figure 1D). Eight others did not meet criteria for statistically significant misexpression primarily due to large expression variation, possibly related to the presence of non-SHF tissue isolated by our dissection process.
Identification of Hedgehog signaling direct targets in the SHF
To define loci directly downstream of Hedgehog signaling, we analyzed genome-wide chromosomal binding locations of the Hedgehog transcriptional regulator Gli3 in the embryonic SHF by chromatin immunoprecipitation with deep sequencing (ChIP-seq). We performed ChIP using a Cre-inducible flag-tagged Gli3T expression line (RosaGli3TFlag c/c MGI: 3828280) [23] combined with the SHF Cre driver Mef2c-AHF-Cre [24] (MGI: 3639735). The SHF tissue from 50 Mef2cAHF-Cre+; RosaGli3TFlag/+ embryos was micro-dissected and immunoprecipitated using an anti-FlagM2 antibody (Sigma). To verify enrichment of Gli3T bound sequences by immunoprecipitation prior to sequencing, we tested a previously identified Gli3T peak upstream of Ptch1 (Chromosome 13, nucleotides 63577408–63579384, mm9), a known Gli3T-bound cis-regulatory element in the limb [23]. This sequence was 13.7-fold enriched in the SHF IP fraction by ChIP-PCR. We proceeded to sequence the IP library and apply Model-based Analysis for ChIP-Seq (MACS) [25]. We identified 1316 Gli3-bound peaks by comparing 68 million sequence tags in IP to 21 million sequence tags in input (tag size = 36 bps, effective genome size = 2e+9, band width = 200, 2<model fold<200, and p-value cutoff = 1e-05) [25]. From these peaks, we analyzed the distribution of the signal around the peak center and identified a typical distribution, confirming successful sequencing (Figure 2A). The predominant GLI3T peak location from the binding data was intergenic and a considerable distance from the transcriptional start sites. We therefore considered the possibility that genes and up to 100 kbp in both directions from intergenic peaks may fall under control of GLI-mediated cis-regulatory elements, given that enhancers often reside thousands of base pairs away from their target of regulation and act independently of their orientation [26], [27]. We therefore annotated GLI3T-bound regions to all transcription start sites within 100 kbp and to the nearest TSS if it resided outside the 100 kbp window [28], [29]. This consideration resulted in mapping the 1316 peaks to 3296 neighbor genes (Table S2). The enrichment between GLI3T-bound and Shh-dependent genes was significant among approximately 22,000 mouse genes (FET p<0.01, Figure 2B).
Fig. 2. Analysis of ChIP-Seq data and its intersection with transcriptional profiling data. 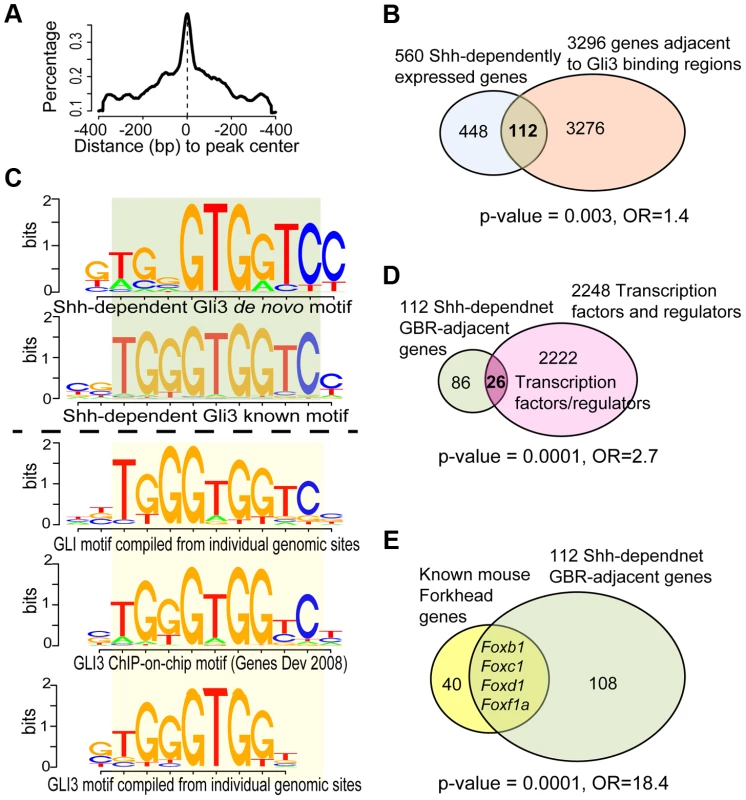
(A) Distribution of ChIP-seq peaks highlighted the modeled GLI3-binding centered in peak regions, using MACS2 software. (B) GLI3 ChIP-seq revealed 1316 peaks defining potential binding sites in the mouse genome. Intersection with shh-dependent transcriptional profiling identified 112 candidate direct Hedgehog-dependent target genes. (C) Summary of de novo and known motifs enriched in shh-dependent GLI3-bound regions (Top 2 sub-panels) compared with similar known GLI motifs from literature and TRANSFAC database (Bottom 3 sub-panels). (D) Among the 112 genes, 26 are transcription factors or regulators of transcription, a significant over-representation. (E) Among the 112 genes, 4 are FOX family transcription factors, a significant over-representation. To define the direct Hedgehog-dependent SHF gene regulatory networks, we intersected the SHF Shh-dependent transcriptional profile signature with the SHF Gli3T chromatin contact results to define candidate Hedgehog-dependent Gli-target genes. This dataset intersection comprised 119 peaks annotated to 112 genes (Figure 2B, Table S3). The enrichment between Gli3T-bound and Shh-dependent genes was significant among ∼22k mouse genes (FET p = 0.003, odds ration = 1.4, Figure 2B). The 119 Shh-dependent Gli3T-bound sites contained significant enrichment of the de novo and known Gli3-binding motif, as derived by ChIP-Chip (CGTGGGTGGTCC) [23] and by computational implication (TRANSFAC database; Figure 2C, bottom panel) [30], [31] at a high degree of significance (p≤1e-10; Figures 2C, top panel).
A significant enrichment of transcription factors was observed in SHF Hedgehog target genes. Transcription factor activity and DNA binding were the two most significant gene-sets over-represented among the 112 Shh-dependent Gli3-bound genes. We directly analyzed our gene set for overrepresentation of transcription factors by searching TRANSFAC version 2013.1 [31] and identified 26 TFs among the 112 unique genes with significant Gli3T-bound peaks (Figure 2D, Table S4), representing a significant enrichment (p = 0.0001, odds ratio = 2.7, Fishers exact test). Specifically, Shh transcriptional profiling and GLI3T chromatin interaction data both identified an enrichment of FOX gene family members, encoding Forkhead transcription factors, identifying FOX genes as potential SHF Hedgehog targets (Figure S2). The set of 112 Shh-dependent Gli3T bound genes included four Fox transcription factors, Foxb1 (64290), Foxc1 (17300), Foxd1 (15229) and Foxf1a, representing a significant enrichment (Figure 2E, p = 0.0001, odds ratio = 18.4).
Foxf1a and Foxf2 are downstream of Shh in the SHF
We investigated the hypothesis that Foxf1a and Foxf2 expression was downstream of Hedgehog signaling in cardiac development. Shh-dependent expression of both genes in the SHF was confirmed by qPCR: Foxf1a expression was reduced by 50% (p = 0.05) and Foxf2 was reduced by 80% in the SHF of Shh−/− versus wild-type controls (p = 0.01) (Figure 1D). In-situ hybridization to evaluate the patterning of expression showed that Foxf1a and Foxf2 were both expressed in the posterior SHF, but not in the heart, in wild-type embryos at E9.5, with Foxf1a expression extending more ventrally than Foxf2 to include the DMP (Figures 3A, A′, E, E′). Mesenchymal expression of both Foxf1a and Foxf2 demonstrated a severe decrement in shh−/− mutant embryos (Figures 3B, B′, F, F′).
Fig. 3. Expression of Foxf1a and Foxf2 in shh−/− and Tbx5+/− mutants embryos at E9.5. 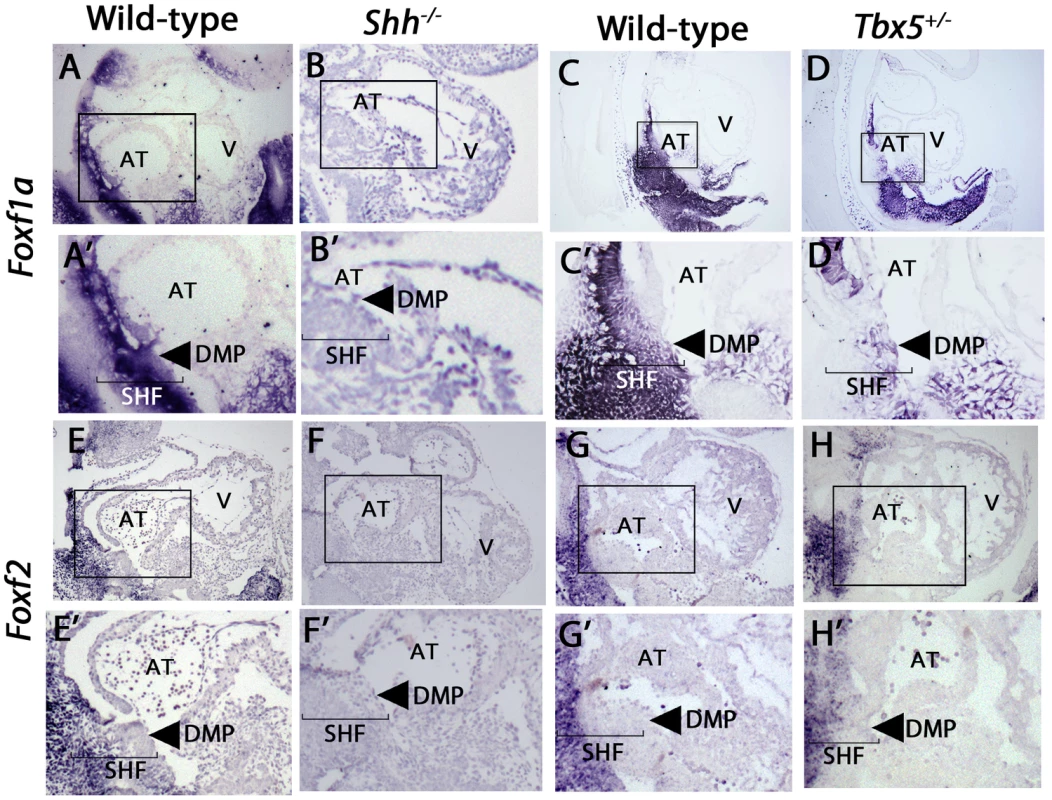
In-situ hybridization demonstrated SHF expression of both Foxf1a and Foxf2, with a loss of SHF expression of Foxf1a in Shh mutants, (A, B) and a near-complete loss of Foxf2 in Shh mutants (E, F). Tbx5 heterozygotes expressed Foxf1a at decreased levels specifically in the posterior SHF tissues (C, D), whereas Foxf2 expression patterns were unchanged (G, H). Arrow: dorsal mesenchymal protrusion tissues in A′–H′, Brackets: SHF mesenchymal tissues. AT: Atrium, V: Ventricles. In a search for common targets between Tbx5 and Hedgehog signaling in the SHF, we tested whether Foxf1a and/or Foxf2 SHF expression was Tbx5-dependent. We performed in situ hybridization for Foxf1a and Foxf2 in Tbx5+/− heterozygous mutant embryos (MGI: 2387850), which demonstrate 40% penetrance of AVSDs [15]. Foxf1a but not Foxf2 expression demonstrated significant reduction in Tbx5 heterozygotes at E9.5. In Tbx5+/− embryos, Foxf1a expression was specifically decreased in the posterior SHF (Figure 3C, C′, D, D′, arrow) in the area of expression overlap with Tbx5 expression [6]. In regions where Foxf1a expression does not overlap with Tbx5 expression, such as the anterior SHF, Foxf1a expression appeared normal (Figure 3D, D′). Foxf2 expression in Tbx5+/− embryos appeared unaltered compared to wild-type embryos (Figure 3G, G′, H, H′). Taken together, this analysis demonstrates that posterior SHF Foxf1a expression was Shh - and Tbx5-dependent whereas Foxf2 pSHF expression was Shh-dependent alone.
Foxf1a and Foxf2 are required for atrioventricular septation
We hypothesized that Foxf1a and Foxf2 were required in a dosage sensitive manner for atrioventricular septation. We analyzed the cardiac anatomy of embryos from an intercross between Foxf1a+/− and Foxf2+/− at E14.5, when cardiac septation is normally complete. Foxf1a+/−; Foxf2+/− double-heterozygote embryos all exhibited atrioventricular septal defects (Figure 4D, D′ asterisk; p = 0.03). Primum-type atrial septal defects, characterized by absence of the dorsal mesenchymal protrusion, were observed in each case (Figure 4D, D′). Additionally, Foxf1a+/−; Foxf2+/− double-heterozygotes displayed larger than normal mesenchymal caps covering the primary atrial septum (Figure 4D′ arrow), an observation in keeping with the known redundant requirement for Foxf1a and Foxf2 in limiting mesenchymal growth in other contexts [32]. Atrial septal defects were never observed in Foxf1a+/− (Figure 4B, B′) or Foxf2+/− (Figure 4C, C′) single-heterozygotes or wildtype control littermate embryos (Figure 4A, A′). We concluded that Foxf1a and Foxf2 are redundantly required for atrioventricular septation.
Fig. 4. Atrioventricular septal defects in Foxf1a+/−; Foxf2+/− compound heterozygote embryos at E14.5. 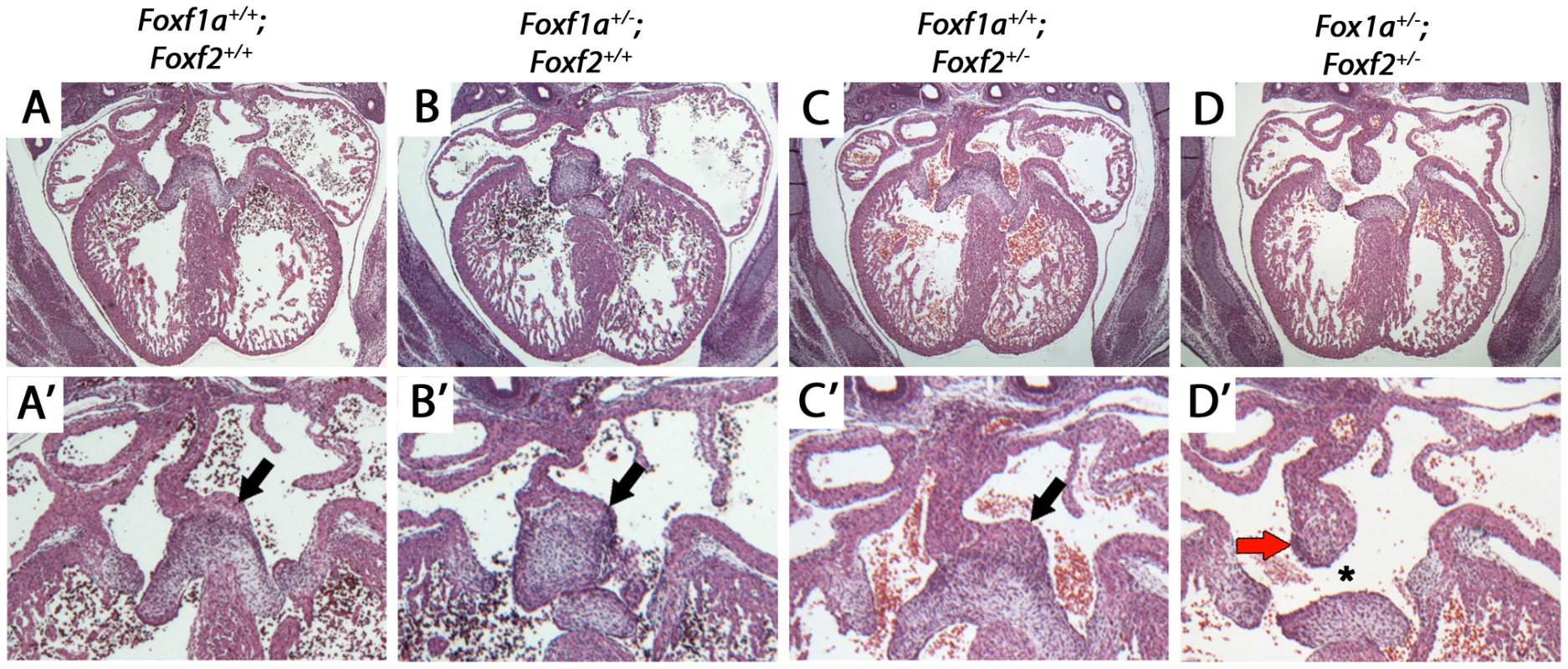
Foxf1a+/−; Foxf2+/− embryos displayed atrial septal defects including absence of the dorsal mesenchymal protrusion (D, D′, black arrows). Compound heterozygotes also displayed expanded mesenchymal cap of primary atrial septum (red arrow) (D, D′). Wild-type (A, A′), Foxf1a+/− (B, B′), and Foxf2+/− embryos (C, C′) showed no atrial septal defects. P-values (Fisher's exact test): Foxf1a+/− (9 embryos) vs wild-type (4 embryos) = 1; Foxf2+/− (2 embryos) vs wild-type = 0.33; Foxf1a+/−; Foxf2+/− (3 embryos) vs wild-type = 0.03. A cis-regulatory element at Foxf1a binds TBX5, GLI1, and GLI3 in vivo
We hypothesized that Foxf1a may represent a direct downstream target of Hedgehog signaling and/or Tbx5 in the SHF. We identified Foxf1a as a candidate direct target based on unbiased interrogation of GLI3T and TBX5 transcription factor chromatin interaction and transcriptional profiling data sets. We intersected our SHF GLI3T ChIP data set (Figure 2B) with a published TBX5 ChIP-seq data set generated from HL-1 cardiomyocytes [33] to define regions with potential co-occupancy of both transcription factors. The intersection of the ChIP-seq datasets identified a single overlapping interaction peak for Gli3T (in the SHF (Figure 2B)) and TBX5 (in HL-1 cardiomyocytes) [33] located approximately 90 kb upstream of the Foxf1a transcription start site (Figure 5A and Figure S3). The Foxf1a transcriptional start site is the closest protein-coding gene to the described peak. The transcriptional start site for a non-coding RNA is located approximately 1.3 kbp upstream of Foxf1a, oriented in the opposite direction [34]. Closer interrogation of the sequence underlying the interaction domains revealed a conserved canonical T-box binding site (AGGTGTGG; chr 8, nucleotides 123,517,714–721, NCBI137/mm9 assembly) and a conserved canonical Gli binding site (GGACCACCCAGC; chr 8, nucleotides 123,517,754–762, NCBI137/mm9 assembly) within 30 base pairs of one another (Figure 5A). We evaluated the sequence information content for these sites from our SHF Gli3 ChIP-seq experiment and found close agreement with published binding sites for Gli3 [23], [33]. This chromatin interaction data in combination with the Tbx5 and Hedgehog signaling-dependent Foxf1a SHF expression (Figure 3) identified this conserved region (mouse chromosome 8, nucleotides 123,517,714–762) as a candidate Foxf1a cis-regulatory element.
Fig. 5. Integration of Hedgehog and Tbx5 activity on an enhancer at Foxf1a. 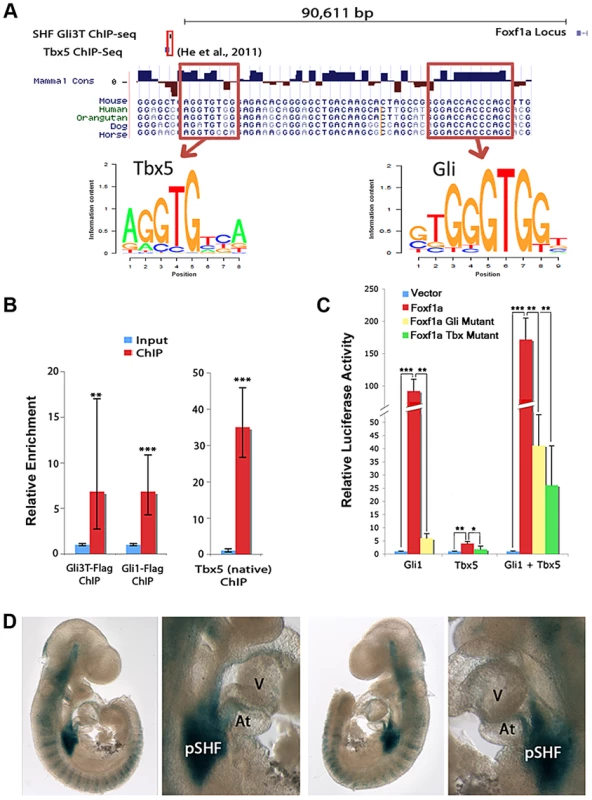
(A) Integration of Hedgehog and Tbx5 activity on an enhancer at Foxf1a. ChIP-seq for GLI3 (Figure 2) and TBX5 [35] identified a candidate Foxf1a enhancer. (B) ChIP-PCR from microdissected pSHF for GLI3, GLI1 and TBX5 demonstrated in vivo binding of each factor to the candidate enhancer. (C) Luciferase assays demonstrated that GLI1 and TBX5 individually and together synergistically activated the enhancer. Activation of enhancer with mutated GLI binding sites was significantly reduced by GLI1; however, synergistic GLI1/TBX5 activity is largely maintained. Activation of enhancer with mutated TBX binding sites was reduced cells transfected with TBX5 alone, but activation in cells transfected with both GLI1 and TBX5 was still relatively high. (D) Representative images of the enhancer activated specific posterior SHF expression of lacZ in transient transgenic embryos at E9.5. Atria: At; Ventricle: V. P-values:, * indicates p<0.05, ** indicates p<0.01, *** indicates p<0.001. We evaluated the binding of TBX5 and the Hedgehog transcriptional regulators GLI1 and GLI3 to the candidate cis-regulatory element at Foxf1a in vivo in the SHF. We evaluated TBX5 binding in vivo by performing ChIP using an anti-TBX5 antibody on the micro-dissected wildtype SHF at E10.5 and observed 35-fold enrichment of the cis-regulatory element in the TBX5-immunoprecipitation fraction compared to the input fraction by qPCR (Figure 5B). We evaluated GLI1 and GLI3T binding in vivo by performing ChIP on the micro-dissected SHF of mice carrying either a Cre-activated flag-tagged Gli3 (RosaGli3TFlag c/c) or Gli1 allele (RosaGli1Flag c/c MGI: 4460761) in concert with the Nkx2.5-Cre (MGI 2654594), broadly expressed cardiac tissues and progenitors. We performed ChIP using an anti-flag antibody on the SHF from R26R-Gli3-flagNkx2.5-Cre/+ or R26R-Gli1-flagNkx2.5-Cre/+ embryos at E10.5 and observed, respectively, 6.8-fold and 7.1-fold enrichment of the Foxf1a cis-regulatory element in the GLI1 - and GLI3T-overexpressing embryos over the input control by qPCR (Figure 5B). We also evaluated two genomic loci between our identified binding site and the Foxf1a transcription start site to determine whether nonspecific pulldown occurred in our ChIP experiments. These loci were not significantly enriched in the IP'd DNA (Figure S4) These results validate in vivo SHF binding of TBX5, GLI1, and GLI3 to the candidate cis-regulatory element at Foxf1a.
A cis-regulatory element at Foxf1a integrates Tbx5 and Hedgehog activity
We hypothesized that the conserved, adjacent, and functional in vivo Gli and Tbx5 binding sites may integrate Tbx5 and Hedgehog activity as a component of a downstream gene regulatory network. We evaluated the activity of TBX5 and GLI1 on the candidate Foxf1a enhancer in vitro. The conserved element was cloned into a pGL4.23 vector containing a minimal promoter driving luciferase as a transcriptional readout and was transfected into HEK293T cells along with expression vectors for Gli1 and/or Tbx5. Co-transfection with the expression vector for Gli1, a Hedgehog-responsive transcriptional activator, provided a 91.9-fold induction of luciferase activity (p = 0.0017). Co-transfection with the expression vector for Tbx5 alone provided a 3.9-fold increase of luciferase activity (p = 0.039). Co-transfection with both Gli1 and Tbx5 expression constructs provided a 171.6-fold increase in luciferase activity (p = 0.00091), demonstrating synergistic activity between these transcriptional co-activators (Figure 5C).
We assessed the requirement of TBX5 and GLI binding sites for transcriptional activation of the enhancer. To assess the requirement of TBX5-dependant transcriptional activation of the enhancer on TBX5 binding sites, a TBX5-mutant enhancer-luciferase construct with the 7 base pair core of 3 canonical TBX binding sites was generated by site-directed mutagenesis. This TBX5-mutant construct eliminated transcriptional activation by TBX5 alone (p = 0.04) and limited transcriptional activation by TBX5 and GLI1 together (p = 0.006) (Figure 5C). A GLI-mutant enhancer-luciferase construct was also constructed with the 8 base pair core of 3 canonical binding sites altered by site-directed mutagenesis (see materials and methods). This GLI-mutant construct profoundly diminished transcriptional activation by GLI1 alone (p = 0.001) (Figure 5C). Interestingly, transcriptional activation by TBX5 and GLI1 on the GLI-mutant enhancer construct was only modestly abrogated luciferase compared to the activity of GLI1 and TBX5 on the wild-type enhancer (p = 0.003).
SHF-specific in vivo expression of the cis-regulatory element at Foxf1a
We hypothesized that the cis-regulatory element at Foxf1a may integrate Hedgehog signaling and Tbx5 activity as a SHF-specific enhancer in vivo. We cloned the Foxf1a genomic region into an Hsp68-LacZ expression construct, whose minimal promoter affords no intrinsic in vivo expression activity [35]. We evaluated the enhancer activity of the Foxf1a genomic fragment by evaluating LacZ expression in transient transgenic mouse embryos at E9.5. The posterior SHF demonstrated strong lacZ expression and was the only anatomic region demonstrating consistent and robust expression in the 8 transgenic embryos genetically positive for LacZ (Figure 5D). Interestingly, the SHF region with the most consistent and robust expression was the area of overlap between Hedgehog signaling and Tbx5 expression [6], including the early dorsal mesenchymal protrusion and surrounding mesenchyme of the posterior SHF (less frequent and intense expression was also observed in other anatomic locations that receive Hedgehog signaling outside of the Tbx5 expression domain, including the anterior SHF (5/8), anterior lateral plate mesoderm (5/8) and somites (2/8) (Figure 5D). These observations, in concert with the in vitro analysis suggested that Hedgehog and Tbx5 act synergistically to provide strong reproducible transcriptional activation of this enhancer in the posterior SHF.
Discussion
Identification of the gene regulatory networks required for atrioventricular septation will be the basis for a mechanistic understanding of AVSDs, a common severe form of CHD. We investigated Hedgehog-dependent networks and implicated Foxf genes for the first time in vertebrate heart development. We examined the overlap between Hedgehog pathways and Tbx5, both known to be integral in the pSHF for atrioventricular septation. Using transcription factor-chromatin interaction data, we identified a cis-regulatory element at Foxf1a that bound both TBX5 and the Hedgehog pathway transcriptional activator GLI1. In vitro analysis of TBX5 and GLI activity on the cis-regulatory element at Foxf1a proved predictive of in vivo biology: this enhancer exhibited strong transcriptional activation only in the pSHF region where Tbx5 expression and Hedgehog signaling intersect. This region is the location of atrial septum progenitors [5], where both Hedgehog signaling and Tbx5 are required for atrioventricular septation (Figure 6) [4]–[6]. These observations provide molecular detail for the genetic interaction between Tbx5 and Hedgehog signaling in atrioventricular septation.
Fig. 6. Model for Hedgehog/Tbx5 interaction. 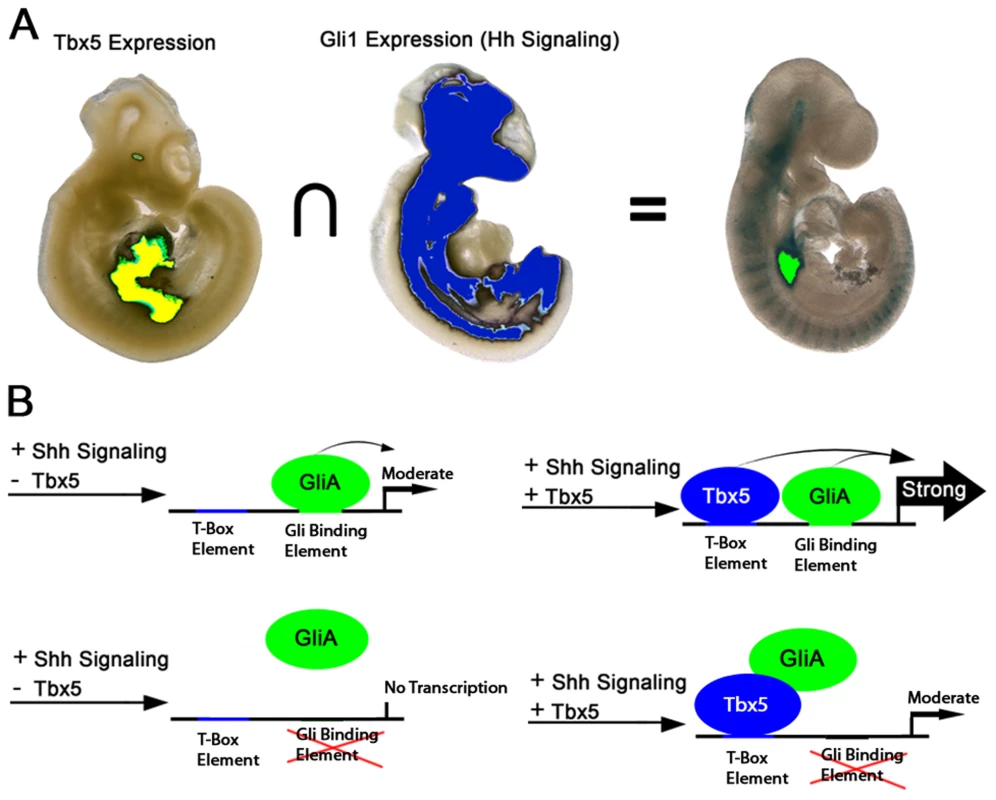
(A) Intersection of Tbx5 expression, restricted to the posterior SHF and heart, and Gli1 expression, broadly expressed in axial mesenchyme and brain but excluded from the heart, is the posterior SHF. Activation of TBX5/GLI1 responsive enhancer is observed principally in the overlap between the Tbx5 and Gli1 expression domains. (B) In the presence of GLI activator (GLIA) alone, the enhancer is weakly active. In the presence of both GLIA and TBX5 is transcription from the enhancer strongly activated. When the GLI binding site is mutated, GLIA alone is insufficient to activate strong expression, but GlLIA may interact with TBX5 to activate expression more strongly than TBX5 alone. Foxf transcription factors are required in the SHF for atrial septation
Our observations identified a requirement for the Forkhead-box transcription factors Foxf1a and Foxf2 in heart development. Compound haploinsufficiency for both Foxf1a and Foxf2 caused an atrial septal defect of the primum type, an atrioventricular septal defect characterized by absence of the dorsal mesenchymal protrusion. Foxf1a and Foxf2 were expressed selectively in the SHF, not in the heart (Figure 3). The requirement for Foxf genes in atrioventricular septation (Figure 4) provided further support for a model of atrioventricular septation as driven by molecular events in SHF cardiac progenitors as opposed to in the heart itself. We found that Foxf1a and Foxf2 are required downstream of Hedgehog signaling in atrioventricular septation, adding cardiac development to the previously described Hedgehog-dependent role for Foxf genes in murine gut development [32], [36]–[37]. Atrioventricular septal defects are also observed in Shh-null mutant embryos [10]. Because Foxf1a and Foxf2 expression were each decreased in the SHF by more than 50% in shh−/− null embryos (Figure 1D), Foxf1a+/−; Foxf2+/− double heterozygote embryos provided a reasonable developmental facsimile of their diminished expression levels in shh−/− embryos. The observation that Foxf1a and Foxf2 compound haploinsufficiency resulted in AVSDs is therefore consistent with the supposition that Foxf1a and Foxf2 are essential components of the Hedgehog-dependent SHF gene regulatory network.
Foxf genes have also been previously implicated in cardiac specification in the ascidian Ciona intestinalis [38], [39]. In ascidians, the single Foxf orthologue lies at the center of a pathway regulating numerous migration-related cellular processes, such as polarity, migration and membrane protrusion in trunk ventral cardiac progenitor cells [38], [39]. Ciona trunk ventral cells with disrupted Foxf activity fail to migrate properly, but still differentiate into cardiac tissue at an improper location. Interestingly, removing Hedgehog signaling from the mouse SHF causes a migration failure of SHF progenitors [4], [5]. Like the Ciona trunk cells without Foxf, SHF cells without Hedgehog responsiveness differentiate into cardiomyocytes, but their altered migration causes AVSDs [5]. Future efforts will determine whether the requirement for Foxf genes in cardiac progenitor migration is a conserved feature of mammalian cardiac development.
A SHF cis-regulatory element integrates Tbx5 and Hedgehog pathways: Building a gene regulatory network for cardiac septation
Genetic interaction and rescue experiments investigating the requirement for Hedgehog signaling and Tbx5 in atrioventricular septation were consistent with Tbx5 acting either in parallel or upstream of Hedgehog signaling in atrioventricular septation [6]. Our interrogation of these pathways on a cis-regulatory element at Foxf1a provides molecular detail for their interaction. We observed that TBX5 and GLI1, the Hedgehog-dependent transcriptional activator, synergistically activated the cis-regulatory element in vitro (Figure 5C) predicting strong activation of expression in areas of overlap between Tbx5 expression and Hedgehog signaling. This prediction held in vivo, where transcriptional activity of the enhancer was strong and reproducible only in the posterior SHF region, where Tbx5 expression and Hedgehog signaling overlap (Figures 5D and 6). This work is consistent and a model describing a SHF-specific gene regulatory network driven by GLI1 and TBX5 and essential for atrioventricular septation (Figure 6). This model provides specific predictions for the logic underlying enhancer choice in the SHF with ramifications for understanding the molecular and biochemical basis of atrioventricular septation and clinical AVSDs.
Materials and Methods
Ethics statement
Mouse experiments were completed according to a protocol reviewed and approved by the Institutional Animal Care and Use Committee of the University of Chicago, in compliance with the USA Public Health Service Policy on Humane Care and Use of Laboratory Animals.
Mouse lines and handling
The Shh− line was obtained from the Jackson laboratory. The Tbx5+/− mice have been previously reported [15]. Foxf1+/− and Foxf2+/− mouse lines were generated in the Kalinichenko lab (Cincinnati Children's Hospital Medical Center) by breeding Foxf1afl/fl and Foxf2fl/fl mice with EIIA-Cre transgenic mice (Jackson Lab). Mef2c-AHF-Cre [24], ROSA26-Gli1 [40] and ROSA26-Gli3T [23] were reported previously.
Dissection techniques
For ChIP, transcriptional profiles and in-situ hybridizations, embryos were dissected in nuclease-free PBS on ice. For SHF microdissection procedures, head tissues anterior to the heart were removed, as were tail tissues posterior to the heart. Portions of these tissues were retained for genotyping if necessary. Neural tube tissues were also removed. The SHF mesenchyme was bisected into anterior and posterior portions when necessary, and then removed from the cardiac tissue (Figure 1A).
Transcriptional profiling
Shh+/+ and Shh−/− embryos were dissected as described above at E9.5. SHF tissues from these embryos were pooled to isolate sufficient amount of RNA for synthesis of labeled cRNA. Transcriptional profiles were performed using Agilent Mouse Whole Genome Arrays mgug4122a.
ChIP
Microdissected SHF tissues were grouped into pools of approximately 50. Tissues were briefly fixed in 1.8% formaldehyde, then washed and homogenized. Sonication was performed with a Misonix 4000 sonicator until the sheared chromatin was approximately 100–300 bp in length. Input control samples were reserved prior to overnight immunoprecipitation with the appropriate antibody bound to magnetic Dynabeads (Invitrogen). Beads were precipitated and washed, the chromatin was eluted, de-crosslinked and purified using a PCR cleanup kit (Qiagen). To determine fold enrichment, qPCR was performed using input controls compared with DNA bound to immunoprecipitated proteins, using primers specific to the site of interest as well as primers to two sites not expected to be enriched.
Gli3-bind peaks and the enriched motifs derived from ChIP-seq
RNA extraction and ChIP-seq
To prepare the ChIP-seq library, chromatin was fixed and sonicated to 300–500 bp fragments, then was immunoprecipitated, eluted, de-crosslinked and column-purified before submission for sequencing. High-throughput sequencing was performed on Illumina Genome Analyzer following the manufacturer's protocols. The raw data was deposited in GEO with an accession number GSE44755.
Binding peaks
Gli3T ChIP-Seq immunoprecipitated product (IP) was compared to the input from SHF tissue dissected from mouse embryos at E9.5. The raw IP sequence reads were first trimmed 8 bps on the left end for two reasons: 1) they showed unexpected low quality and 2) they were 8 bps longer than the input reads. Then we used Bowtie aligner software to map ChIP-seq and control sequencing reads to the mouse reference genome build mm9. The genome-wide locations of Gli3-binding peaks were identified using a model-based analysis of ChIP-seq (MACS) algorithm version 2.
Motif identification
Motifs were identified using HOMER software by the default parameters. Visualization was conducted using R and a local mirror of the UCSC Genome Browser with customized data.
Candidate Gli3-targets annotation
The Gli3-bound sites were first annotated to the mouse mm9 assembly genome by HOMER software to the nearest transcription start sites (TSSs). Additional genes were included following analyses that localized them to the same chromosome within 100 kbp distance to any Gli3T-bound peaks. These genes were annotated using Bioconductor packages biomaRt version 2.14.0 and ChIPpeakAnno version 2.6.0.
Shh-dependent transcriptomic alteration derived from microarray
Data pre-process
Expression of the pooled RNAs (8 Shh−/− pools and 7 Shh+/+ pools) were extracted by Feature Extraction Software (v. 10.5.1.1) available from Agilent, using the default variables. Outlier features on the arrays were flagged by the Bioconductor [41] package Agi4x44PreProcess and were excluded. Raw feature intensities (the meanSignals) were background corrected, varance stabilizing normalization (VSN) normalized and log2 transformed [42]. As has been noted in previous studies, non-expressed probes are merely background noise and thus no longer track the expression of genes, lower expressed probes were filtered to increase true positive on the array [43]. As a result, 11,469 probes (encoding 8,867 out of 20,674 genes on the array) were retained for signature identification that met the following three criteria: 1) Met a minimum criterion of signal quality flagged by Agilent Feature Extraction; 2) Cross-sample expression fell into the top half of the inter-quartile range (IQR); 3) Presented known genes as annotated by Biocondctor package mgug4122a.db version 2.6.3. The raw data was deposited in GEO with an accession number GSE44754.
Data analysis
To identify Shh-dependent gene signature, the whole mouse genome gene-expression was compared between Shh mutant samples and wide-type controls, using R and Bioconductor package samr (unpaired two-class t-statistic, 100 permutations) [44]. The resultant p-values of all genes with at least 2-fold changes (computed by samr) were corrected for multiple testing using the Q-value adjustment. Differentially expressed genes were identified that had a Q-value less than 0.5%. The hypergeometric test for all Gene Ontology biological processes was performed using Bioconductor package GOStats.
Tbx5-dependent microarray transcriptomic data analysis
Data pre-process
Analysis performed was identical to that used to identify Shh-dependent transcriptomic alterations. Briefly, VSN [42] was used to do between arrays normalization, and the resultant expression measurements were log2 transformed. The raw data was deposited in GEO with an accession number GSE43599. A Q-value <0.05 indicated significantly differentially expression.
Candidate Tbx5 and Gli3 co-targets
Tbx5-bound peaks derived from ChIP-seq were recently published using HL-1 cells [33]. These Tbx5 binding sites were annotated by HOMER software to the nearest transcription start sites (TSSs) in the mouse mm9 reference genome. As T-box motif is significantly enriched among the 119 Shh-dependent and Gli3-binding peaks in SHF, we further searched for candidate Tbx5 and Gli3 co-targets, using HOMER software (Figure 5A).
We checked the intersection of transcriptomic altered genes dependent on either Tbx5 or Shh that intersected with Tbx5 and Gli3 bound genes. The list of resultant genes includes those with Shh - or Tbx5-dependent expression on the microarray experiments and being located within 100 kbp distance to a Gli3-bound region derived from the ChIP-seq experiment. These genes were further annotated using Bioconductor package biomaRt version 2.14.0.
Data accession
ChIP-seq and microarray data were deposited in the Gene Expression Omnibus (GEO) database with a super accession number GSE44756.
In-situ hybridization
In-situ hybridization was performed as in Moorman et al. [45] with slight modifications. Specifically, after post-hybridization washes with 50% formamide/2X SSC, specimens were incubated for 30 minutes at 37 degrees C in 20 ug/ml RNase A to remove unbound probe and reduce nonspecific staining. All in-situ hybridization experiments were performed on a minimum of three control and three experimental embryos.
Luciferase assays
Expression vectors for Gli1 and Gli3T were obtained from the Vokes lab. Tbx5 was cloned into the pCDNA 3.1 expression construct [20] Foxf1a fragment was cloned into the pGL4.23 vector (Promega). Expression and reporter vectors were transfected into HEK293T cells using FuGENE (Promega). Cells were cultured for 48 hours after transfection, then lysed and assayed using the Dual-Luciferase Reporter Assay system (Promega).
Transient transgenics
The Foxf1a enhancer and minimal promoter used in the luciferase assays were subcloned from the pENTR vector into the Hsp68-LacZ vector [35] using the Gateway system (Invitrogen). The resulting construct was digested with NotI enzyme to remove the pBlueScript backbone, gel-purified, injected into fertilized mouse eggs at the University of Chicago Transgenics Core Facility and implanted into female mice. Embryos were harvested at E9.5 and stained as described previously [5].
Supporting Information
Zdroje
1. MommersteegMT, SoufanAT, de LangeFJ, van den HoffMJ, AndersonRH, et al. (2006) Two distinct pools of mesenchyme contribute to the development of the atrial septum. Circ Res 99 : 351–3.
2. SnarrBS, O'NealJL, ChintalapudiMR, WirrigEE, PhelpsAL, et al. (2007) Isl1 expression at the venous pole identifies a novel role for the second heart field in cardiac development. Circ Res 101 : 971–4.
3. SnarrBS, WirrigEE, PhelpsAL, TruskTC, WesselsA (2007) A spatiotemporal evaluation of the contribution of the dorsal mesenchymal protrusion to cardiac development. Dev Dyn 236 : 1287–94.
4. GoddeerisMM, RhoS, PetietA, DavenportCL, Johnson, et al. (2008) Intracardiac septation requires hedgehog-dependent cellular contributions from outside the heart. Development 135 : 1887–1895.
5. HoffmannAD, PetersonMA, Friedland-LittleJM, AndersonSA, MoskowitzIP (2009) sonic hedgehog is required in pulmonary endoderm for atrial septation. Development 136 : 1761–1770.
6. XieL, HoffmannAD, Burnicka-TurekO, Friedland-LittleJM, ZhangK, et al. (2012) Tbx5-hedgehog molecular networks are essential in the second heart field for atrial septation. Dev Cell 23 : 280–91.
7. Nüsslein-VolhardC, WieschausE (1980) Mutations affecting segment number and polarity in Drosophila. Nature 287 : 795–801.
8. InghamPW, McMahonAP (2001) Hedgehog signaling in animal development: paradigms and principles. Genes Dev 15 : 3059–87.
9. ZhangXM, Ramalho-SantosM, McMahonAP (2001) Smoothened mutants reveal redundant roles for Shh and Ihh signaling including regulation of L/R symmetry by the mouse node. Cell 106 : 781–92.
10. Washington SmoakI, ByrdNA, Abu-IssaR, GoddeerisMM, AndersonR, et al. (2005) Sonic hedgehog is required for cardiac outflow tract and neural crest cell development. Dev Biol 283 : 357–72.
11. BassonCT, BachinskyDR, LinRC, LeviT, ElkinsJA, et al. (1997) Mutations in human TBX5 [corrected] cause limb and cardiac malformation in Holt-Oram syndrome. Nat Genet 15 : 30–35.
12. GargV, KathiriyaIS, BarnesR, SchlutermanMK, KingIN, et al. (2003) GATA4 mutations cause human congenital heart defects and reveal an interaction with TBX5. Nature 424 : 443–447.
13. LiQY, Newbury-EcobRA, TerrettJA, WilsonDI, CurtisAR, et al. (1997) Holt-Oram syndrome is caused by mutations in TBX5, a member of the Brachyury (T) gene family. Nat Genet 15 : 21–29.
14. SchottJJ, BensonDW, BassonCT, PeaseW, SilberbachGM, et al. (1998) Congenital heart disease caused by mutations in the transcription factor NKX2-5. Science 281 : 108–111.
15. BruneauBG, NemerG, SchmittJP, CharronF, RobitailleL, et al. (2001) A murine model of Holt-Oram syndrome defines roles of the T-box transcription factor Tbx5 in cardiogenesis and disease. Cell 106 : 709–21.
16. HiroiY, KudohS, MonzenK, IkedaY, YazakiY, et al. (2001) Tbx5 associates with Nkx2-5 and synergistically promotes cardiomyocyte differentiation. Nat Genet 28 : 276–280.
17. TakeuchiJK, BruneauBG (2009) Directed transdifferentiation of mouse mesoderm to heart tissue by defined factors. Nature 459 : 708–711.
18. GoetzSC, BrownDD, ConlonFL (2006) TBX5 is required for embryonic cardiac cell cycle progression. Development 133 : 2575–84.
19. ZhuY, GramoliniAO, WalshMA, ZhouYQ, SlorachC, et al. (2008) Tbx5-dependent pathway regulating diastolic function in congenital heart disease. Proc Natl Acad Sci U S A 105 : 5519–24.
20. ArnoldsDE, LiuF, FahrenbachJP, KimGH, SchillingerKJ, et al. (2012) TBX5 drives Scn5a expression to regulate cardiac conduction system function. J Clin Invest 122 : 2509–18.
21. MoriAD, ZhuY, VahoraI, NiemanB, Koshiba-TakeuchiK, et al. (2006) Tbx5-dependent rheostatic control of cardiac gene expression and morphogenesis. Dev Biol 297 : 566–86.
22. FalconS, GentlemanR (2007) Using GOstats to test gene lists for GO term association. Bioinformatics 23 : 257–258.
23. VokesSA, JiH, WongWH, McMahonAP (2008) A genome-scale analysis of the cis-regulatory circuitry underlying sonic hedgehog-mediated patterning of the mammalian limb. Genes Dev 22 : 2651–63.
24. VerziMP, McCulleyDJ, De ValS, DodouE, BlackBL (2005) The right ventricle, outflow tract, and ventricular septum comprise a restricted expression domain within the secondary/anterior heart field. Dev Biol 287 : 134–45.
25. ZhangY, LiuT, MeyerCA, EeckhouteJ, JohnsonDS, et al. (2008) Model-based analysis of ChIP-Seq (MACS). Genome Biol 9: R137.
26. HeintzmanND, RenB (2009) Finding distal regulatory elements in the human genome. Curr Opin Genet Dev 19 : 541–9.
27. ViselA, RubinEM, PennacchioLA (2009) Genomic views of distant-acting enhancers. Nature 461 : 199–205.
28. HusainA, ZhangX, DollMA, StatesJC, BarkerDF, et al. (2007) Functional analysis of the human N-acetyltransferase 1 major promoter: quantitation of tissue expression and identification of critical sequence elements. Drug Metab Dispos 35 : 1649–56.
29. SanyalA, LajoieBR, JainG, DekkerJ (2012) The long-range interaction landscape of gene promoters. Nature 489 : 109–13.
30. HeinzS, BennerC, SpannN, BertolinoE, LinYC, et al. (2010) Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol Cell 38 : 576–589.
31. MatysV, Kel-MargoulisOV, FrickeE, LiebichI, LandS, et al. (2006) TRANSFAC and its module TRANSCompel: transcriptional gene regulation in eukaryotes. Nucleic Acids Res 34: D108–110.
32. OrmestadM, AstorgaJ, LandgrenH, WangT, JohanssonBR, et al. (2006) Foxf1 and Foxf2 control murine gut development by limiting mesenchymal Wnt signaling and promoting extracellular matrix production. Development 133 : 833–43.
33. HeA, KongSW, MaQ, PuWT (2011) Co-occupancy by multiple cardiac transcription factors identifies transcriptional enhancers active in heart. Proc Natl Acad Sci U S A 108 : 5632–7.
34. GroteP, WittlerL, HendrixD, KochF, WährischS, et al. (2013) The tissue-specific lncRNA Fendrr is an essential regulator of heart and body wall development in the mouse. Dev Cell 24 : 206–14.
35. KotharyR, ClapoffS, DarlingS, PerryMD, MoranLA, et al. (1989) Inducible expression of an hsp68-lacZ hybrid gene in transgenic mice. Development 105 : 707–14.
36. MadisonBB, McKennaLB, DolsonD, EpsteinDJ, KaestnerKH (2008) FoxF1 and FoxL1 link hedgehog signaling and the control of epithelial proliferation in the developing stomach and intestine. J Biol Chem 284 : 5936–44.
37. MahlapuuM, EnerbäckS, CarlssonP (2001) Haploinsufficiency of the forkhead gene Foxf1, a target for sonic hedgehog signaling, causes lung and foregut malformations. Development 128 : 2397–406.
38. BehJ, ShiW, LevineM, DavidsonB, ChristiaenL (2007) FoxF is essential for FGF-induced migration of heart progenitor cells in the ascidian Ciona intestinalis. Development 134 : 3297–305.
39. ChristiaenL, DavidsonB, KawashimaT, PowellW, NollaH, et al. (2008) The transcription/migration interface in heart precursors of Ciona intestinalis. Science 320 : 1349–52.
40. VokesSA, JiH, McCuineS, TenzenT, GilesS, et al. (2007) Genomic characterization of Gli-activator targets in sonic hedgehog-mediated neural patterning. Development 134 : 1977–89.
41. GentlemanRC, CareyVJ, BatesDM, BolstadB, DettlingM, et al. (2004) Bioconductor: open software development for computational biology and bioinformatics. Genome Biol 5: R80.
42. HuberW, von HeydebreckA, SültmannH, PoustkaA, VingronM (2002) Variance stabilization applied to microarray data calibration and to the quantification of differential expression. Bioinformatics 18 Suppl 1: S96–104.
43. LangerW, SohlerF, LederG, BeckmannG, SeidelH, et al. (2010) Exon array analysis using re-defined probe sets results in reliable identification of alternatively spliced genes in non-small cell lung cancer. BMC Genomics 11 : 676.
44. TusherVG, TibshiraniR, ChuG (2001) Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci U S A 98 : 5116–5121.
45. MoormanAF, HouwelingAC, de BoerPA, ChristoffelsVM (2001) Sensitive nonradioactive detection of mRNA in tissue sections: novel application of the whole-mount in situ hybridization protocol. J Histochem Cytochem 49 : 1–8.
Štítky
Genetika Reprodukční medicína
Článek Oligoasthenoteratozoospermia and Infertility in Mice Deficient for miR-34b/c and miR-449 LociČlánek The Kinesin AtPSS1 Promotes Synapsis and is Required for Proper Crossover Distribution in MeiosisČlánek Payoffs, Not Tradeoffs, in the Adaptation of a Virus to Ostensibly Conflicting Selective PressuresČlánek Examination of Prokaryotic Multipartite Genome Evolution through Experimental Genome ReductionČlánek BMP-FGF Signaling Axis Mediates Wnt-Induced Epidermal Stratification in Developing Mammalian SkinČlánek Role of STN1 and DNA Polymerase α in Telomere Stability and Genome-Wide Replication in ArabidopsisČlánek RNA-Processing Protein TDP-43 Regulates FOXO-Dependent Protein Quality Control in Stress ResponseČlánek Integrating Functional Data to Prioritize Causal Variants in Statistical Fine-Mapping StudiesČlánek Salt-Induced Stabilization of EIN3/EIL1 Confers Salinity Tolerance by Deterring ROS Accumulation inČlánek Ethylene-Induced Inhibition of Root Growth Requires Abscisic Acid Function in Rice ( L.) SeedlingsČlánek Metabolic Respiration Induces AMPK- and Ire1p-Dependent Activation of the p38-Type HOG MAPK PathwayČlánek Signature Gene Expression Reveals Novel Clues to the Molecular Mechanisms of Dimorphic Transition inČlánek A Mouse Model Uncovers LKB1 as an UVB-Induced DNA Damage Sensor Mediating CDKN1A (p21) DegradationČlánek Dominant Sequences of Human Major Histocompatibility Complex Conserved Extended Haplotypes from to
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2014 Číslo 10
-
Všechny články tohoto čísla
- An Deletion Is Highly Associated with a Juvenile-Onset Inherited Polyneuropathy in Leonberger and Saint Bernard Dogs
- Licensing of Yeast Centrosome Duplication Requires Phosphoregulation of Sfi1
- Oligoasthenoteratozoospermia and Infertility in Mice Deficient for miR-34b/c and miR-449 Loci
- Basement Membrane and Cell Integrity of Self-Tissues in Maintaining Immunological Tolerance
- The Kinesin AtPSS1 Promotes Synapsis and is Required for Proper Crossover Distribution in Meiosis
- Germline Mutations in Are Associated with Familial Gastric Cancer
- POT1a and Components of CST Engage Telomerase and Regulate Its Activity in
- Controlling Meiotic Recombinational Repair – Specifying the Roles of ZMMs, Sgs1 and Mus81/Mms4 in Crossover Formation
- Payoffs, Not Tradeoffs, in the Adaptation of a Virus to Ostensibly Conflicting Selective Pressures
- FHIT Suppresses Epithelial-Mesenchymal Transition (EMT) and Metastasis in Lung Cancer through Modulation of MicroRNAs
- Genome-Wide Mapping of Yeast RNA Polymerase II Termination
- Examination of Prokaryotic Multipartite Genome Evolution through Experimental Genome Reduction
- White Cells Facilitate Opposite- and Same-Sex Mating of Opaque Cells in
- BMP-FGF Signaling Axis Mediates Wnt-Induced Epidermal Stratification in Developing Mammalian Skin
- Genome-Wide Association Study of CSF Levels of 59 Alzheimer's Disease Candidate Proteins: Significant Associations with Proteins Involved in Amyloid Processing and Inflammation
- COE Loss-of-Function Analysis Reveals a Genetic Program Underlying Maintenance and Regeneration of the Nervous System in Planarians
- Fat-Dachsous Signaling Coordinates Cartilage Differentiation and Polarity during Craniofacial Development
- Identification of Genes Important for Cutaneous Function Revealed by a Large Scale Reverse Genetic Screen in the Mouse
- Sensors at Centrosomes Reveal Determinants of Local Separase Activity
- Genes Integrate and Hedgehog Pathways in the Second Heart Field for Cardiac Septation
- Systematic Dissection of Coding Exons at Single Nucleotide Resolution Supports an Additional Role in Cell-Specific Transcriptional Regulation
- Recovery from an Acute Infection in Requires the GATA Transcription Factor ELT-2
- HIPPO Pathway Members Restrict SOX2 to the Inner Cell Mass Where It Promotes ICM Fates in the Mouse Blastocyst
- Role of and in Development of Abdominal Epithelia Breaks Posterior Prevalence Rule
- The Formation of Endoderm-Derived Taste Sensory Organs Requires a -Dependent Expansion of Embryonic Taste Bud Progenitor Cells
- Role of STN1 and DNA Polymerase α in Telomere Stability and Genome-Wide Replication in Arabidopsis
- Keratin 76 Is Required for Tight Junction Function and Maintenance of the Skin Barrier
- Encodes the Catalytic Subunit of N Alpha-Acetyltransferase that Regulates Development, Metabolism and Adult Lifespan
- Disruption of SUMO-Specific Protease 2 Induces Mitochondria Mediated Neurodegeneration
- Caudal Regulates the Spatiotemporal Dynamics of Pair-Rule Waves in
- It's All in Your Mind: Determining Germ Cell Fate by Neuronal IRE-1 in
- A Conserved Role for Homologs in Protecting Dopaminergic Neurons from Oxidative Stress
- The Master Activator of IncA/C Conjugative Plasmids Stimulates Genomic Islands and Multidrug Resistance Dissemination
- An AGEF-1/Arf GTPase/AP-1 Ensemble Antagonizes LET-23 EGFR Basolateral Localization and Signaling during Vulva Induction
- The Proteomic Landscape of the Suprachiasmatic Nucleus Clock Reveals Large-Scale Coordination of Key Biological Processes
- RNA-Processing Protein TDP-43 Regulates FOXO-Dependent Protein Quality Control in Stress Response
- A Complex Genetic Switch Involving Overlapping Divergent Promoters and DNA Looping Regulates Expression of Conjugation Genes of a Gram-positive Plasmid
- ZTF-8 Interacts with the 9-1-1 Complex and Is Required for DNA Damage Response and Double-Strand Break Repair in the Germline
- Integrating Functional Data to Prioritize Causal Variants in Statistical Fine-Mapping Studies
- Tpz1-Ccq1 and Tpz1-Poz1 Interactions within Fission Yeast Shelterin Modulate Ccq1 Thr93 Phosphorylation and Telomerase Recruitment
- Salt-Induced Stabilization of EIN3/EIL1 Confers Salinity Tolerance by Deterring ROS Accumulation in
- Telomeric (s) in spp. Encode Mediator Subunits That Regulate Distinct Virulence Traits
- Ethylene-Induced Inhibition of Root Growth Requires Abscisic Acid Function in Rice ( L.) Seedlings
- Ancient Expansion of the Hox Cluster in Lepidoptera Generated Four Homeobox Genes Implicated in Extra-Embryonic Tissue Formation
- Mechanism of Suppression of Chromosomal Instability by DNA Polymerase POLQ
- A Mutation in the Mouse Gene Leads to Impaired Hedgehog Signaling
- Keeping mtDNA in Shape between Generations
- Targeted Exon Capture and Sequencing in Sporadic Amyotrophic Lateral Sclerosis
- TIF-IA-Dependent Regulation of Ribosome Synthesis in Muscle Is Required to Maintain Systemic Insulin Signaling and Larval Growth
- At Short Telomeres Tel1 Directs Early Replication and Phosphorylates Rif1
- Evidence of a Bacterial Receptor for Lysozyme: Binding of Lysozyme to the Anti-σ Factor RsiV Controls Activation of the ECF σ Factor σ
- Hsp40s Specify Functions of Hsp104 and Hsp90 Protein Chaperone Machines
- Feeding State, Insulin and NPR-1 Modulate Chemoreceptor Gene Expression via Integration of Sensory and Circuit Inputs
- Functional Interaction between Ribosomal Protein L6 and RbgA during Ribosome Assembly
- Multiple Regulatory Systems Coordinate DNA Replication with Cell Growth in
- Fast Evolution from Precast Bricks: Genomics of Young Freshwater Populations of Threespine Stickleback
- Mmp1 Processing of the PDF Neuropeptide Regulates Circadian Structural Plasticity of Pacemaker Neurons
- The Nuclear Immune Receptor Is Required for -Dependent Constitutive Defense Activation in
- Genetic Modifiers of Neurofibromatosis Type 1-Associated Café-au-Lait Macule Count Identified Using Multi-platform Analysis
- Juvenile Hormone-Receptor Complex Acts on and to Promote Polyploidy and Vitellogenesis in the Migratory Locust
- Uncovering Enhancer Functions Using the α-Globin Locus
- The Analysis of Mutant Alleles of Different Strength Reveals Multiple Functions of Topoisomerase 2 in Regulation of Chromosome Structure
- Metabolic Respiration Induces AMPK- and Ire1p-Dependent Activation of the p38-Type HOG MAPK Pathway
- The Specification and Global Reprogramming of Histone Epigenetic Marks during Gamete Formation and Early Embryo Development in
- The DAF-16 FOXO Transcription Factor Regulates to Modulate Stress Resistance in , Linking Insulin/IGF-1 Signaling to Protein N-Terminal Acetylation
- Genetic Influences on Translation in Yeast
- Analysis of Mutants Defective in the Cdk8 Module of Mediator Reveal Links between Metabolism and Biofilm Formation
- Ribosomal Readthrough at a Short UGA Stop Codon Context Triggers Dual Localization of Metabolic Enzymes in Fungi and Animals
- Gene Duplication Restores the Viability of Δ and Δ Mutants
- Selection on a Variant Associated with Improved Viral Clearance Drives Local, Adaptive Pseudogenization of Interferon Lambda 4 ()
- Break-Induced Replication Requires DNA Damage-Induced Phosphorylation of Pif1 and Leads to Telomere Lengthening
- Dynamic Partnership between TFIIH, PGC-1α and SIRT1 Is Impaired in Trichothiodystrophy
- Signature Gene Expression Reveals Novel Clues to the Molecular Mechanisms of Dimorphic Transition in
- Mutations in Moderate or Severe Intellectual Disability
- Multifaceted Genome Control by Set1 Dependent and Independent of H3K4 Methylation and the Set1C/COMPASS Complex
- A Role for Taiman in Insect Metamorphosis
- The Small RNA Rli27 Regulates a Cell Wall Protein inside Eukaryotic Cells by Targeting a Long 5′-UTR Variant
- MMS Exposure Promotes Increased MtDNA Mutagenesis in the Presence of Replication-Defective Disease-Associated DNA Polymerase γ Variants
- Coexistence and Within-Host Evolution of Diversified Lineages of Hypermutable in Long-term Cystic Fibrosis Infections
- Comprehensive Mapping of the Flagellar Regulatory Network
- Topoisomerase II Is Required for the Proper Separation of Heterochromatic Regions during Female Meiosis
- A Splice Mutation in the Gene Causes High Glycogen Content and Low Meat Quality in Pig Skeletal Muscle
- KDM5 Interacts with Foxo to Modulate Cellular Levels of Oxidative Stress
- H2B Mono-ubiquitylation Facilitates Fork Stalling and Recovery during Replication Stress by Coordinating Rad53 Activation and Chromatin Assembly
- Copy Number Variation in the Horse Genome
- Unifying Genetic Canalization, Genetic Constraint, and Genotype-by-Environment Interaction: QTL by Genomic Background by Environment Interaction of Flowering Time in
- Spinster Homolog 2 () Deficiency Causes Early Onset Progressive Hearing Loss
- Genome-Wide Discovery of Drug-Dependent Human Liver Regulatory Elements
- Developmentally-Regulated Excision of the SPβ Prophage Reconstitutes a Gene Required for Spore Envelope Maturation in
- Protein Phosphatase 4 Promotes Chromosome Pairing and Synapsis, and Contributes to Maintaining Crossover Competence with Increasing Age
- The bHLH-PAS Transcription Factor Dysfusion Regulates Tarsal Joint Formation in Response to Notch Activity during Leg Development
- A Mouse Model Uncovers LKB1 as an UVB-Induced DNA Damage Sensor Mediating CDKN1A (p21) Degradation
- Notch3 Interactome Analysis Identified WWP2 as a Negative Regulator of Notch3 Signaling in Ovarian Cancer
- An Integrated Cell Purification and Genomics Strategy Reveals Multiple Regulators of Pancreas Development
- Dominant Sequences of Human Major Histocompatibility Complex Conserved Extended Haplotypes from to
- The Vesicle Protein SAM-4 Regulates the Processivity of Synaptic Vesicle Transport
- A Gain-of-Function Mutation in Impeded Bone Development through Increasing Expression in DA2B Mice
- Nephronophthisis-Associated Regulates Cell Cycle Progression, Apoptosis and Epithelial-to-Mesenchymal Transition
- Beclin 1 Is Required for Neuron Viability and Regulates Endosome Pathways via the UVRAG-VPS34 Complex
- The Not5 Subunit of the Ccr4-Not Complex Connects Transcription and Translation
- Abnormal Dosage of Ultraconserved Elements Is Highly Disfavored in Healthy Cells but Not Cancer Cells
- Genome-Wide Distribution of RNA-DNA Hybrids Identifies RNase H Targets in tRNA Genes, Retrotransposons and Mitochondria
- The Chromosomal Association of the Smc5/6 Complex Depends on Cohesion and Predicts the Level of Sister Chromatid Entanglement
- Cell-Autonomous Progeroid Changes in Conditional Mouse Models for Repair Endonuclease XPG Deficiency
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- The Master Activator of IncA/C Conjugative Plasmids Stimulates Genomic Islands and Multidrug Resistance Dissemination
- A Splice Mutation in the Gene Causes High Glycogen Content and Low Meat Quality in Pig Skeletal Muscle
- Keratin 76 Is Required for Tight Junction Function and Maintenance of the Skin Barrier
- A Role for Taiman in Insect Metamorphosis
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



