-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaFast Evolution from Precast Bricks: Genomics of Young Freshwater Populations of Threespine Stickleback
Adaptation to novel environments is a keystone of evolution. There is only a handful of natural and experimental systems in which the process of adaptation has been studied in detail, and each studied system brings its own surprises with regard to the number of loci involved, dynamics of adaptation, extent of interactions between loci and of parallelism between different adapting populations. The threespine stickleback is an excellent model organism for evolutionary studies. Marine-derived freshwater populations of this species have consistently acquired a specific set of morphological, physiological and behavioral traits allowing them to reside in freshwater for their whole lifespan. Previous studies identified several genomic regions responsible for this adaptation. Here, using whole-genome sequencing, we compare the allele frequencies at such regions in four derived freshwater populations of known ages: two natural, and two artificially established in 1978. Knowledge of population ages allows us to infer the strength of selection that acted at these loci. Adaptation of threespine stickleback to freshwater is typically fast, and is driven by strong selection favoring pre-existing alleles that are likely present in the ancestral marine population at low frequencies; however, some of the adaptation may also be due to young population-specific alleles.
Published in the journal: . PLoS Genet 10(10): e32767. doi:10.1371/journal.pgen.1004696
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004696Summary
Adaptation to novel environments is a keystone of evolution. There is only a handful of natural and experimental systems in which the process of adaptation has been studied in detail, and each studied system brings its own surprises with regard to the number of loci involved, dynamics of adaptation, extent of interactions between loci and of parallelism between different adapting populations. The threespine stickleback is an excellent model organism for evolutionary studies. Marine-derived freshwater populations of this species have consistently acquired a specific set of morphological, physiological and behavioral traits allowing them to reside in freshwater for their whole lifespan. Previous studies identified several genomic regions responsible for this adaptation. Here, using whole-genome sequencing, we compare the allele frequencies at such regions in four derived freshwater populations of known ages: two natural, and two artificially established in 1978. Knowledge of population ages allows us to infer the strength of selection that acted at these loci. Adaptation of threespine stickleback to freshwater is typically fast, and is driven by strong selection favoring pre-existing alleles that are likely present in the ancestral marine population at low frequencies; however, some of the adaptation may also be due to young population-specific alleles.
Introduction
Studies of adaptation in nature are complicated by the typically long timescales at which evolution proceeds, and therefore are rather rare (e.g. [1]–[3]). Positive selection, the hallmark of adaptation, can be inferred from patterns of divergence and/or polymorphism in genome comparisons. While experimental evolution coupled with searches for patterns consistent with positive selection is becoming an accepted tool for “real time” studies of adaptation in microbes [4], [5], it is rarely possible to use genomic data to observe the adaptation process in higher animals such as vertebrates [6], [7]. Furthermore, the mechanisms of adaptation at the genomic level are still poorly understood [8]–[10].
The study of the genomics of adaptation has experienced a recent upheaval since the advent of population-level next-generation sequencing, which enables identification of selected loci and detailed studies of divergence and polymorphism within them in a wide range of model systems [11]–[13]. The data reveal that the number of loci responsible for adaptation, the ratio of coding and regulatory changes, the proportions of parallel to non-parallel genetic changes vary between systems [13]–[15]. The reasons for such variation are still unclear, making further genomic studies of adaptation a priority.
Threespine stickleback (Gasterosteus aculeatus) has become a widely used model organism for studying adaptation and speciation [16], [17]. The species is very variable, and is represented by a number of morphs [18], [19]. The ancestral populations of G. aculeatus likely lived in the sea, and colonization of new freshwater habitats, followed by evolution of freshwater populations, occurred repeatedly all over the Northern Hemisphere. While fish from marine populations utilize freshwater lakes and streams only as temporary spawning grounds, thousands of isolated freshwater resident populations have been independently established, and they have diverged in morphological, physiological and behavioral traits allowing them to survive in the freshwater for their entire lifespan. Independent origin of freshwater populations of G. aculeatus in different locations in the Northern Hemisphere provides an opportunity to study adaptive evolution under similar environments [15], [20]–[24]. Much of this adaptive evolution has been shown to be parallel, involving repeated recruitment for adaptation at different freshwater populations of the same pre-existing alleles that are presumably carried at low frequencies by marine populations. However, some of the adaptations are specific to individual populations [15],[22]; the relative importance of adaptations by new mutations vs. standing variation, and of population-specific vs. parallel adaptations, is not known.
Freshwater and marine forms of G. aculeatus possess a number of phenotypic differences. One of the most obvious is their armor plates: while the marine form has a complete set of lateral plates covering their body from pelvic girdle to the caudal peduncle, there are usually just a few lateral plates in the freshwater form [25],[26]. Genetic differences responsible for the number of the armor plates have been identified, pointing to the EDA gene on chromosome IV [27]. Later, sequencing of the G. aculeatus genome (available at http://genome.ucsc.edu) facilitated studying the genetic basis of the differences between the two forms, and several large genomic regions with high concentrations of nucleotide substitutions between the forms were found by comparing individuals from marine and freshwater habitats in a RAD-Seq analysis [22]. A recent study of G. aculeatus from Atlantic and Pacific basins used whole genome sequencing to reveal more than two hundred small genetic regions throughout the stickleback genome that differ between the forms [15].
Populations of G. aculeatus adapted to freshwater inhabit lakes and streams that originated after the retreat of the Pleistocene glaciers, indicating that adaptation can be fast [16]. Although rather different phenotypically, the freshwater and the marine forms often can hybridize and produce fertile offspring [18],[28],[29]. However, in some populations, there can also be a nearly-complete reproductive isolation in natural habitats between freshwater residential populations and anadromous marine forms spawning in the same lake [30],[31]. Reproductive isolation is mediated by phenotypic traits [32], and generally, there is not a clear cut relationship between the age of freshwater populations and reproductive isolation between marine and freshwater morphs.
Studies of G. aculeatus in the White Sea and its basin were initiated by Valery Ziuganov in the 1970s [33],[34]. The upper boundary for the age of the marine population in the White Sea is 15,000–18,000 years, because earlier, this area was covered in ice sheets during the Last Glacial Maximum [35]. After the end of the glaciation, the White Sea region experienced eustatic raising, giving rise to a unique system of young lakes as bays gradually separated from the sea by lift of the coast [36]. The rate of this process, which is still ongoing, has been estimated as 3.8 mm/year [37]; this allows inferring the age of a freshwater population from the elevation of the lake above sea level. Furthermore, in 1978, Ziuganov established several independent artificial sticklebacks populations in abandoned mica and spar quarries filled with ground water, by seeding each quarry with controlled numbers of marine and freshwater individuals [33]. Sampling these populations in 2011 allowed us to study two evolutionary trajectories with known points of departure. Thus, the availability of a wide range of young lakes of known ages in the White Sea basin provides an opportunity to trace the dynamics of adaptation to freshwater environments.
Here, we use whole-genome sequencing to study eight populations of G. aculeatus from the White Sea basin, including two artificial populations. We aimed to detect the genetic differences between the ancestral marine and the derived freshwater populations, and to measure the rate of adaptation, and the strength of positive selection which drives it, at divergent genomic loci. Whole-genome comparisons of multiple artificial and natural derived populations allow detailed analysis of selection acting simultaneously at multiple loci. Using multiple populations of different ages allows studying the process of adaptation at these loci at a range of time points, from tens to hundreds of years, and the uniformity of the process of selection. Finally, whole-genome analysis reveals the detailed patterns of divergence and polymorphism within the selected loci and in their vicinity.
Results
Genetic differences between the marine and the freshwater populations
We searched for the genetic markers of differences between the ancestral population of G. aculeatus in the White Sea and the derived freshwater populations in its vicinity. To identify such markers, we compared the genome sequences of two samples of marine individuals with two samples of freshwater individuals (Figure 1, Table 1). Phenotypically marine individuals were collected in Nilma bay and among the anadromous (marine) fish in Lake Ershovskoye where they came to spawn. Phenotypically freshwater individuals were collected from Lake Lobaneshskoye on the Island Velikiy and Lake Mashinnoye on the mainland. Their ages since desalination, inferred from their current elevations above the sea level, are ∼600 and ∼700 years, respectively [37]. By using these two freshwater populations of independent origins which are the oldest in the area, we aimed to identify those genetic changes that occurred in parallel in both freshwater populations, and therefore likely include sites responsible for adaptation of G. aculeatus to freshwater.
Fig. 1. Map showing the locations of populations studied. 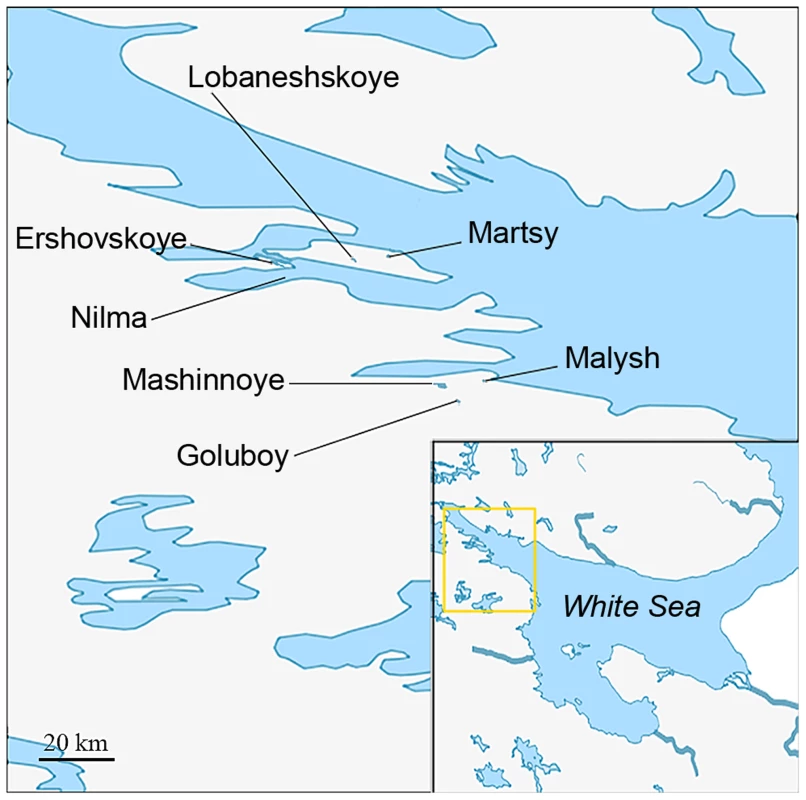
Please see Table 1 for description of sampling sites. Tab. 1. Sampling sites and library characteristics. 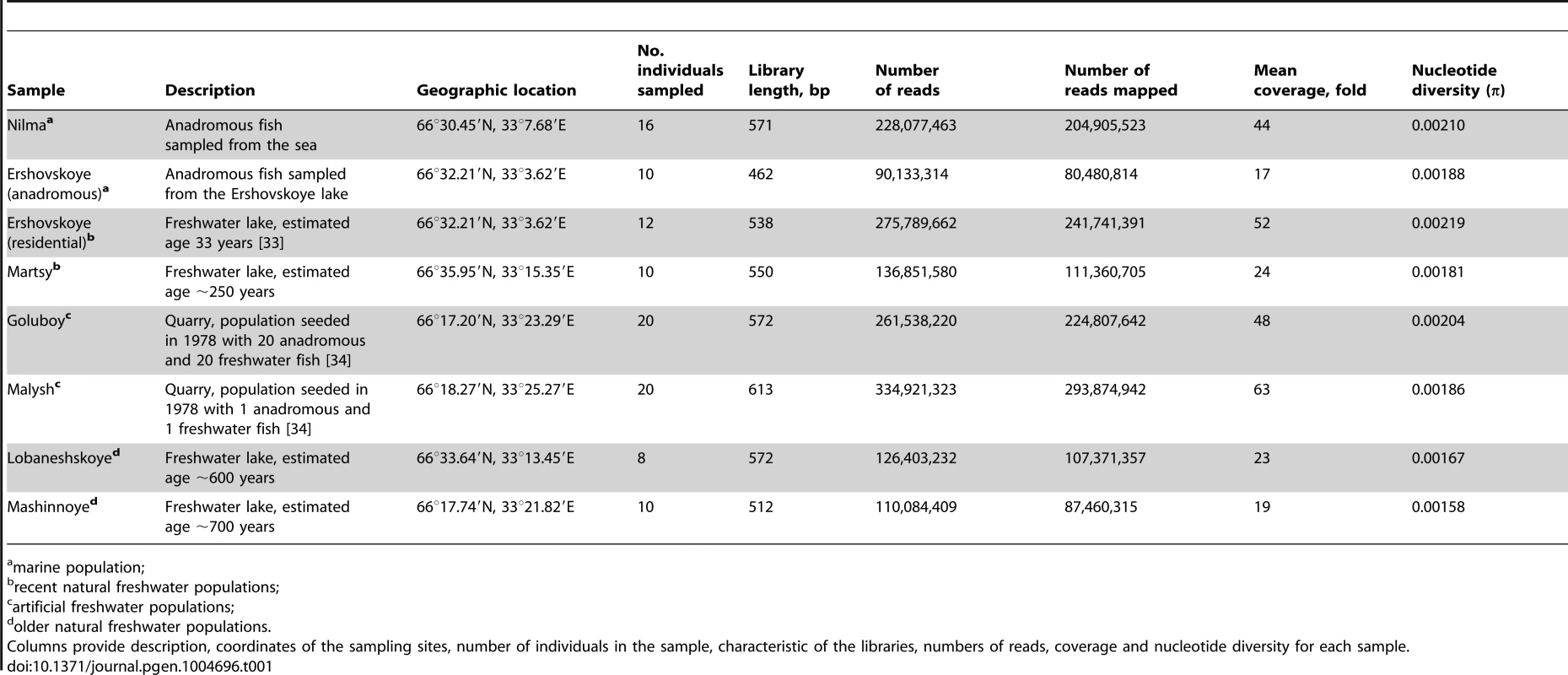
marine population; We estimated allele frequencies from pooled samples of individuals; these allele frequencies were confirmed using allele-specific PCR for specific loci (see below). We defined “marker” single-nucleotide polymorphisms (SNPs) as polymorphic nucleotide sites where both marine samples contained a particular allele at frequencies above 80%, while both freshwater samples contained another allele at frequencies above 80% (“strong criterion”) or above 50% (“weak criterion”). For comparison, we also identified marker SNPs according to the strong criterion using only one marine-freshwater pair of populations (Nilma vs. Mashinnoye).
Identified marker SNPs were distributed unevenly along the reference genome, clearly consisting of dense aggregations (“divergence islands”, DIs; [11],[15]) of markers in short genomic regions. A strong (weak) DI was defined as a continuous region where each 10 Kb window carried at least 10 strong (20 weak) markers, after merging any two such regions that are closer than 40 Kb to each other, because recombination is not likely to occur on such short distance [38],[39]. This definition, which seems to describe our data well (Figure 2), leads to a smaller number of wider DIs than the definitions used in [15] (see Methods for an alternative approach). Among the 6,107 marker SNPs obtained under the strong criterion, 5,801 (95.0%) were concentrated in DIs. By overlapping the strong and the weak criteria, we identified 19 DIs, which were located on ten out of the 21 G. aculeatus chromosomes (Figure 2) and covered a total of 3,301,948 nucleotides, or 0.74% of the genome.
Fig. 2. Distribution of SNPs that distinguish the marine and freshwater populations along the G. aculeatus genome. 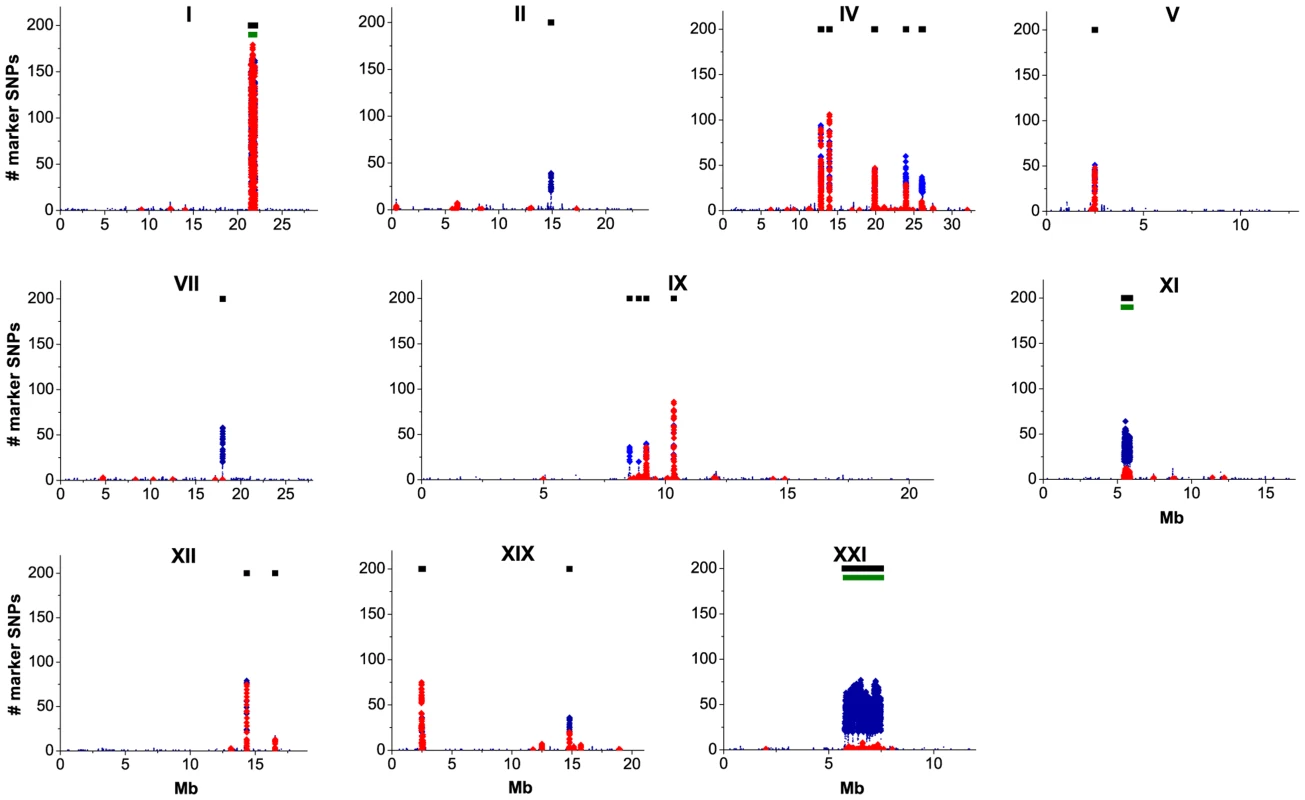
For each chromosome carrying a DI of marker SNPs, the horizontal axis shows the position along the chromosome in Mb and the vertical axis shows, for 10 Kb frames, the numbers of marker SNPs under the strong (red) or weak (blue) criterion. To reduce clutter, only every 1000th frame is displayed, and the numbers of weak marker SNPs below 20 are represented by small dots. Black bars identify DIs of marker SNPs, and green bars at chromosomes I, XI and XXI underlie known inverted genome segments. In the majority of the DIs, the number of weak markers is only slightly above the number of strong markers, indicating that the freshwater-specific alleles have usually reached the frequency of 80% in both lake populations. Two exceptions are DIs IV-5 and XI-1 (i.e., the fifth DI on the 4th chromosome, and the first DI on the 11th chromosome), which, although identifiable both by the strong and the weak criteria, contains twenty times as many weak markers as strong markers. Furthermore, five DIs (II-1, VII-1, IX-1, IX-2 and XXI-1) could be identified only by the weak criterion, indicating that the frequency of freshwater-specific alleles in one or both freshwater populations is below 0.8.
The overall nucleotide diversity within the two freshwater samples was reduced by 22% relative to that in the two marine samples (Table 1), consistent with lower effective sizes of freshwater populations and/or moderate bottlenecks in the course of their origin. Both strong and weak DIs in marine population carried higher levels of nucleotide diversity (0.0049 and 0.0034, respectively), compared to the genomic background (0.0020). In the freshwater populations, diversity was reduced in the strong DIs (0.0012), but elevated in the weak DIs (0.0048), compared to the genomic background (0.0016).
Seventeen out of the 19 detected DIs overlap protein-coding genes, for a total of 170 genes (Table 2, Table S1); among the 285 marker SNPs identified under the strong marker criteria and covered by these genes, 139 were nonsynonymous, while 146 were synonymous (Table 3). The ratio of nonsynonymous to synonymous marker SNPs was much higher than that within non-marker SNPs in protein-coding genes segregating within the marine population (290 nonsynonymous to 528 synonymous; Table 3), implying positive selection favoring the preferential fixation of amino-acid changing marker SNPs between marine and freshwater populations [40]. However, 7 of the 17 DIs do not include any nonsynonymous marker SNPs. The remaining two DIs do not overlap any known protein-coding or miRNA genes. The DIs encompass genes that might affect several traits responsible for phenotypic difference between the marine and freshwater forms, including the well-known EDA gene responsible for body armor ([27],[41], DI IV-1), as well as genes likely to be important for adaptation to freshwater through their effects on osmoregulation, immunity, or morphology: Na+/K+ transporting ATPase (ATP1A1 [42], DI I-1), neurotransmitter and hormone binding (SULT4A1 [43], DI IV-4), and immunity response to viral infection (NLRC5 [44], DI XIX-1). Other genes might be involved in several important aspects of metabolism and behavior (INHA [45], DI I-1), responsible for growth and development of nerve cells (SLITRK2 [46], DI IV-5), adhesion and differentiation of nerve cells (CTNNA2 [47], DI IX-3), calcium/phosphate homeostasis (STC2 [48], DI IV-2), and mediation of functions in the central and peripheral nervous systems (HTR3A [49], DI V-1).
Tab. 2. 19 DIs of marker SNPs distinguishing marine and freshwater populations. 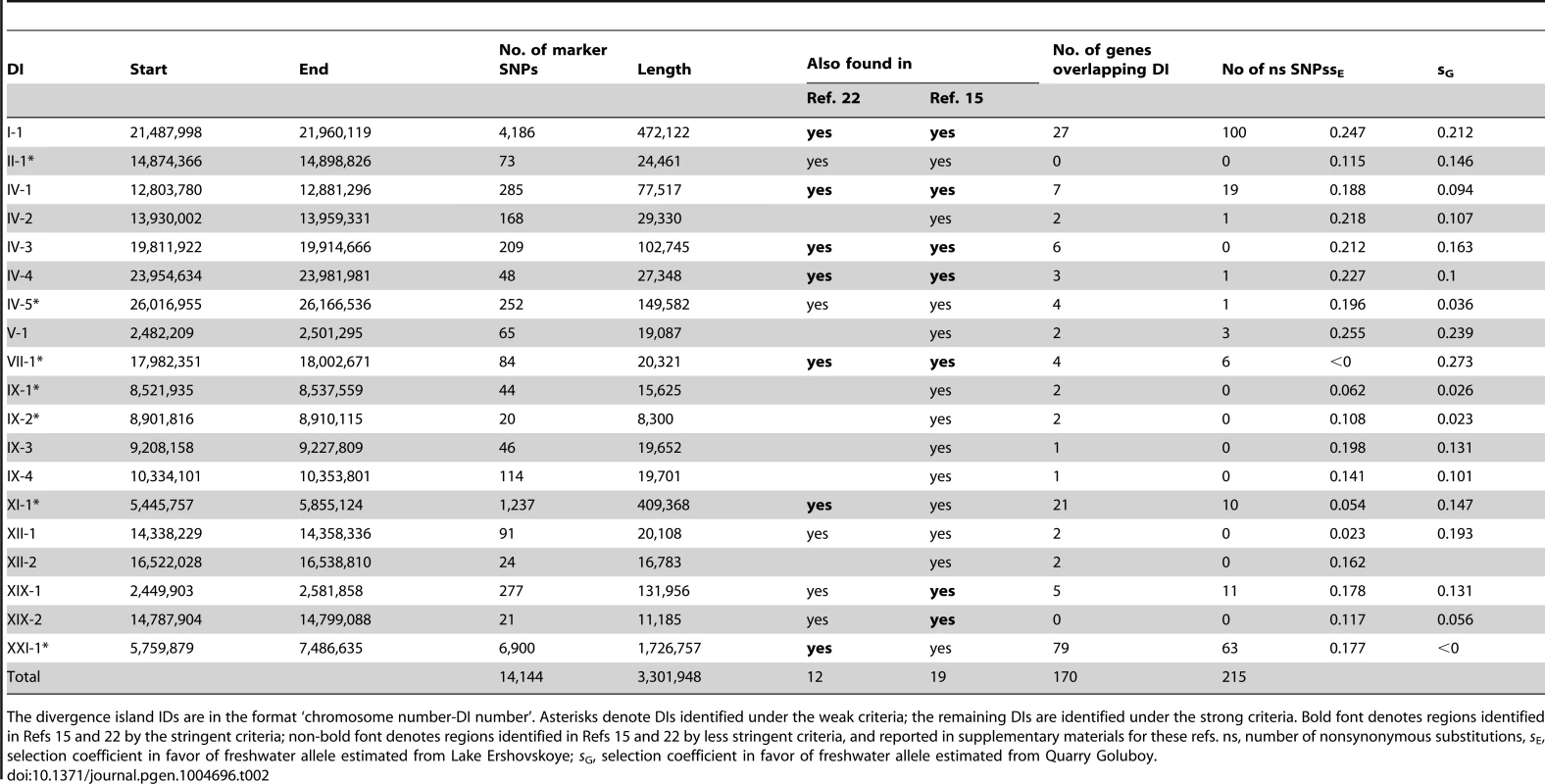
The divergence island IDs are in the format ‘chromosome number-DI number’. Asterisks denote DIs identified under the weak criteria; the remaining DIs are identified under the strong criteria. Bold font denotes regions identified in Refs 15 and 22 by the stringent criteria; non-bold font denotes regions identified in Refs 15 and 22 by less stringent criteria, and reported in supplementary materials for these refs. ns, number of nonsynonymous substitutions, sE, selection coefficient in favor of freshwater allele estimated from Lake Ershovskoye; sG, selection coefficient in favor of freshwater allele estimated from Quarry Goluboy. Tab. 3. SNPs in protein-coding genes. 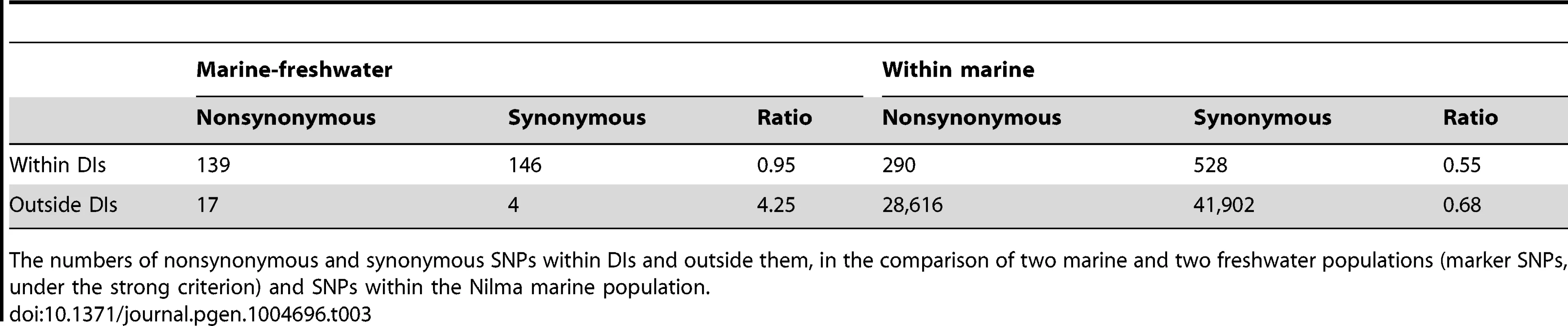
The numbers of nonsynonymous and synonymous SNPs within DIs and outside them, in the comparison of two marine and two freshwater populations (marker SNPs, under the strong criterion) and SNPs within the Nilma marine population. Only 5.0% of marker SNPs identified under the strong marker criteria were not located within any of the DIs. This amounted to a total of 306 marker SNPs, located on 19 out of the 21 chromosomes. 21 of the 306 SNPs were located within protein-coding regions; of these 21 SNPs, 17 were amino-acid changing, while only 4 were synonymous. Again, this ratio of nonsynonymous to synonymous substitutions is higher than that observed within a single (marine) population (28,616 and 41,902, respectively; Table 3), consistent with positive selection favoring amino acid-changing mutations even outside the DIs. Notably, among the 12 genes that carried amino-acid changes, a number could be plausibly held responsible for adaptation and speciation in G. aculeatus. For example, GCNT3 gene, which plays an important role in biosynthesis of mucin which is used for nest building [50], carries 4 marker SNPs, all nonsynonymous. MUC-like gene on the chromosome II carries 2 non-synonymous substitutions. Two nonsynonymous non-DI marker SNPs are positioned within 300 bp of the EPX-like gene on chromosome XIII; EPX is a gene contributing to the activity of eosinophils which are responsible for lysis of parasites [51]. Another nonsynonymous SNP is in INSR gene on the chromosome III; this gene is known to play a crucial role in early development and growth, and in the development of the neural system [52],[53].
Dynamics of adaptation of G. aculeatus to freshwater
In addition to lakes Mashinnoye and Lobaneshskoye, we also sampled G. aculeatus from four bodies of freshwater of more recent origin: quarries Goluboy and Malysh, and lakes Martsy and Ershovskoye (Table 1), all located near the White Sea. Populations in Goluboy and Malysh were established by Ziuganov in 1978 in isolated pools that developed in quarries after they had been abandoned, but were, before the start of the experiments, devoid of fish; therefore, they both were 34 years old at the time of sampling. The Quarry Goluboy population (area ∼70,000 m2, carrying capacity over 1,000 fish) was started from 20 marine (10 females and 10 males) and 20 freshwater (10 females and 10 males) individuals. The Quarry Malysh population (∼75 m2, carrying capacity about 100 fish, but the number of reproducing males may be limited by the very low number of nesting sites) was started from 1 marine female and 1 freshwater male [34]. Founding marine individuals were taken from the White Sea, and founding freshwater individuals were taken from Lake Mashinnoye. Lakes Martsy and Ershovskoye originated through isolation of marine bays due to the steady glacio-isostatic rise of the coast at the rate of ∼3.8 mm per year [37]. The age of the freshwater population in Lake Martsy can be estimated as ∼250 years, because the surface of the lake is currently at about 1 meter above the sea level. As recently as in 1978, Lake Ershovskoye (now ∼14 cm above spring-tide level) was a typical meromictic lake, inhabited by only anadromous fish with the typical marine phenotype [33]. This lake became fresh soon afterwards, and now contains an abundant residential population, which can be easily distinguished from anadromous individuals both morphologically and by a different parasite load (Schistocephalus solidus are dominant in residential individuals, and nematodes in anadromous individuals [33]); therefore, we estimate the age of Lake Ershovskoye also as 34 years. Thus, at these four bodies of water, the adaptation of G. aculeatus populations to freshwater is likely to still be ongoing.
In all four young populations, frequencies of freshwater alleles at marker SNPs within DIs have been increasing rapidly (Figure 3, see Table S2 for allele frequencies data on all populations). For five of the DIs, the estimates of allele frequency obtained by Illumina sequencing of DNA pools were also validated by genotyping individual fish from each population with allele-specific primers (Table S3, Table S4). These increases imply that in each of the freshwater populations, selection favors the identified freshwater alleles. The initial frequencies of freshwater alleles in natural lakes Ershovskoye and Martsy were likely the same as the frequency observed in the marine population, i.e., ∼0.1 (Table S2). In the two quarry populations, the initial frequencies were assumed to equal 0.5. The mean frequency of freshwater alleles over all DIs in the artificial populations was 0.56 at Quarry Malysh, and 0.73 at Quarry Goluboy (Figure 3B). A lower average and a higher variance of freshwater allele frequencies at Quarry Malysh population are likely due to its small effective population size, and therefore, stronger genetic drift. The frequencies of freshwater alleles in Lake Martsy are higher than in Lake Ershovskoye, consistent with the former being older than the latter.
Fig. 3. Mean frequencies of freshwater alleles at marker SNPs within identified DIs, at freshwater populations of different ages. 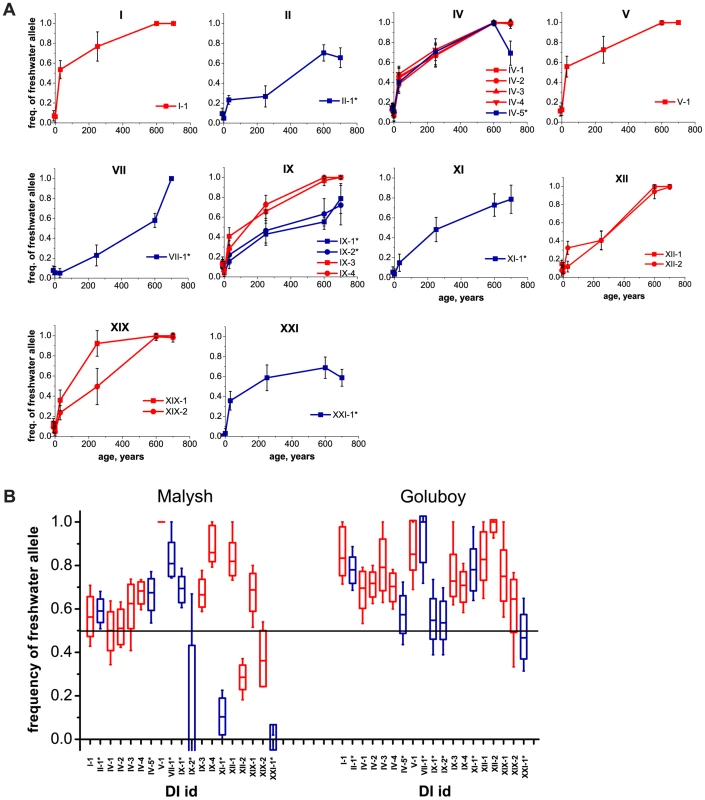
(A) Natural populations. The horizontal axis shows the approximate ages of populations, ranging (left to right) from two marine populations (∼0 years) to recent (∼34 and ∼250 years) and older lake populations (∼600 and ∼700 years). Whiskers, standard deviation. (B) Experimental populations. For each of the DIs of marker SNPs, the assumed initial 50% frequency of freshwater alleles (black line) and their current frequencies are shown for two experimental populations: quarries Malysh (left) and Goluboy (right), each started in 1978. Dashes, boxes and whiskers correspond to the median, standard deviation, and 5th and 95th percentiles, respectively. Red, DIs identified under the strong criterion; blue, DIs identified under the weak criterion. Freshwater alleles also increased in frequency at the marker SNPs located outside DIs. This was observed for all chromosomes in the two natural populations, except chromosomes XIV and XVI, each carrying only one marker SNP (Figure S1A); and for most of the chromosomes in the Quarry Goluboy artificial population (Figure S1B). Overall, however, the marker SNPs located within DIs have reached substantially higher frequencies in all freshwater populations, compared with the marker SNPs outside DIs (Figure 4). An increase of the frequency of freshwater alleles was also observed when only one marine-freshwater pair of populations (Nilma vs. Mashinnoye), rather than two pairs, was used to define the marker SNPs, although it was even less pronounced (Figure S3).
Fig. 4. Comparison of mean frequencies of freshwater alleles at marker SNPs within and outside of identified DIs, at freshwater populations of different ages. 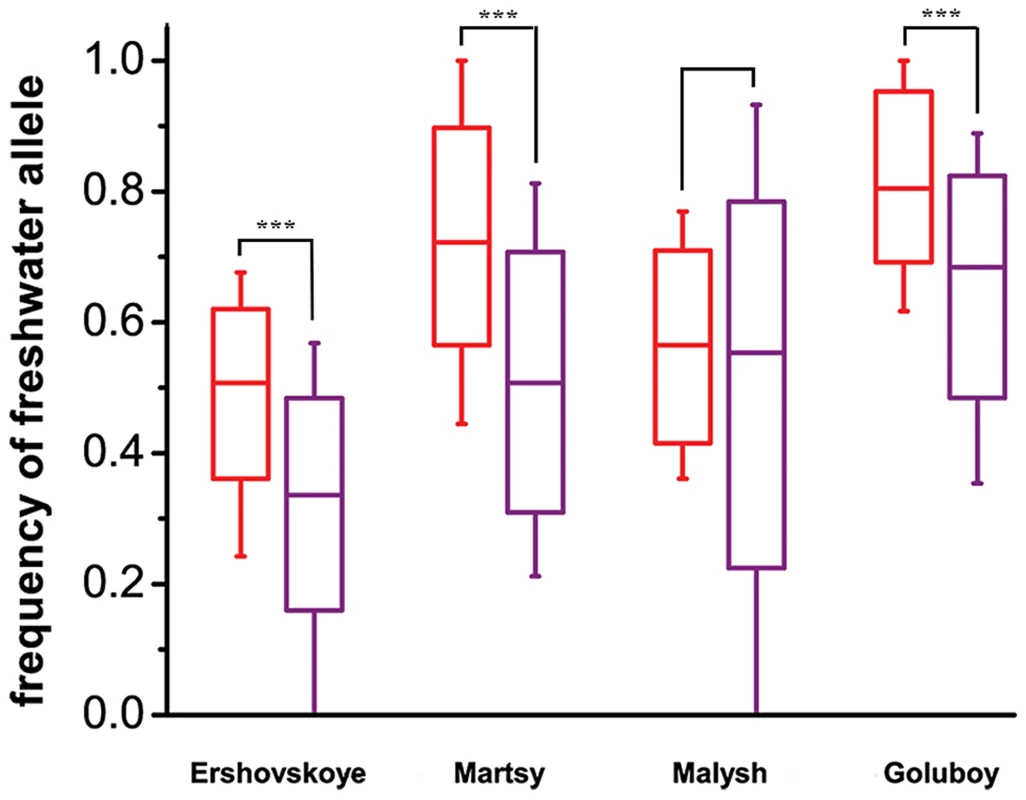
Each two boxes correspond to the four young freshwater populations (anadromous from Lake Ershovskoye, Lake Martsy, Quarry Malysh, Quarry Goluboy). Statistical analysis was performed with two-tailed nonparametric Mann–Whitney U test. P values of <0.001 are designated with three (***) asterisks. Dashes, boxes and whiskers correspond to the median, standard deviation, and 5th and 95th percentiles, respectively. Red, marker SNPs (under the strong criterion) located within clumps; purple, marker SNPs (under the strong criterion) located outside clumps. Strength of selection responsible for adaptation
Knowing the rate of increase of an allele frequency makes it possible to estimate the strength of positive selection favoring this allele [54]. Because the age of the Lake Martsy is known only approximately, we cannot reliably estimate the rate of allele frequency change in it, while for the Lake Ershovskoye, the age of the population is known rather precisely. Furthermore, among the two artificial populations, selection in Quarry Goluboy was more pronounced than in Quarry Malysh, probably due to a stronger contribution of drift in the latter (see above). Therefore, for estimation of selection strengths, we used Lake Ershovskoye and Quarry Goluboy populations. We made such estimates assuming that the generation time of G. aculeatus is two years ([1] and our data; see Methods). We also made the simplifying assumptions that selection remained constant, and that freshwater alleles possess intermediate dominance (h = 0.5), as has been recently shown to be true, in particular, for most skeletal quantitative trait loci (QTL) in G. aculeatus [55].
We estimated the selection coefficient s for each DI (no estimates were made for non-DI marker SNPs, as estimates based on individual SNPs are unlikely to be robust). Only one selection coefficient s was ascribed to a DI, on the basis of the average frequency of freshwater marker alleles in it. As the allele frequencies within a DI are non-independent, the strength of selection cannot be estimated with precision, and the estimates for individual DIs should be treated with caution; still, this analysis allows us to appreciate the selection in effect.
Overall, estimated values of s were high: out of the 19 DIs, fifteen in Lake Ershovskoye, and twelve in Quarry Goluboy, had s>0.1 (Table 2, Figure 5). The selection coefficients estimated for each DI were not significantly correlated between the two freshwater populations (Figure 5; Spearman's rho = 0.30, p = 0.27), possibly due to the low number of DIs and high variance in estimation of s for individual DIs; however, the mean values of s inferred from Lake Ershovskoye (s = 0.16) and Quarry Goluboy (s = 0.13) were rather similar. In particular, DI IV-1, carrying the EDA allele, experienced s = 0.19 in Lake Ershovskoye, and s = 0.09 in Quarry Goluboy, consistent with the previously reported data on dynamics of armor phenotype over an even shorter time period [1]. The mean shift in allele frequency observed in Lake Ershovskoye was the largest in DI V-1, where freshwater allele frequency has changed from 0.1 to 0.56, corresponding to s = 0.255. This DI is centered on several nonsynonymous substitutions in gene HTR3A, a subunit of serotonin ligand-gated ion channel receptor with a wide spectrum of physiological functions. The close second was DI I-1 (sE = 0.247, sG = 0.212), a 470 kb long chromosomal inversion overlapping 27 genes, including the ATP1A1 gene which encodes a well-studied Na+/K+ transporting ATPase; the differences between the freshwater and the marine allele of this gene include 6 amino acid substitutions, and may be responsible for the differences in osmoregulation between the two forms [56]. In Quarry Goluboy, the most radical change in allele frequency was observed at DI XII-2 (the freshwater allele has reached near-fixation here, so s is hard to estimate). This region overlaps the upstream region of the gene OVGP1 (estrogen-dependent oviduct protein or mucin-9). This gene is involved in reproduction [57], and therefore is a likely target for positive selection; whether the strong selection associated with formation of freshwater phenotype is associated with arising reproductive isolation between the two forms should be the subject of a further study.
Fig. 5. Selection coefficients at DIs estimated from Lake Ershovskoye and Quarry Goluboy. 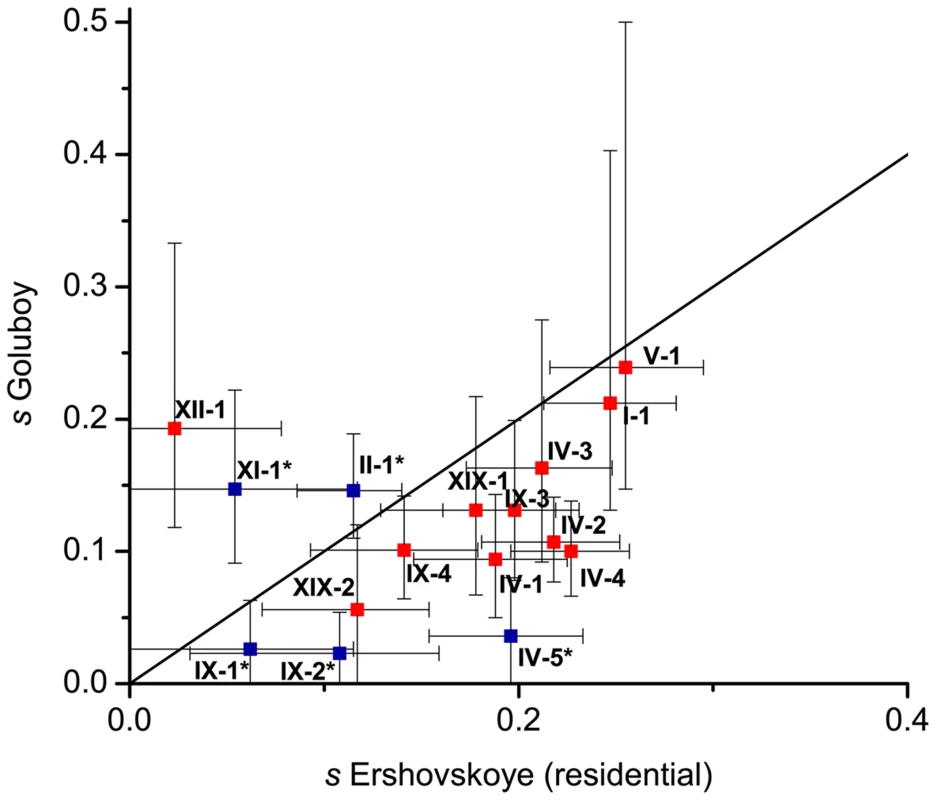
Red, DIs identified under the strong criterion; blue, DIs identified under the weak criterion. Three DIs are not shown: VII-1 and XXI-1, which had inferred s below 0 in one of the populations; and XII-2, for which s at Quarry Goluboy could not be inferred reliably due to near-fixation of the freshwater allele (Table 2). Whiskers correspond to values of s inferred from mean±st. dev. of allele frequency change. Discussion
We have identified more than 18,000 marker SNPs that distinguish the two freshwater populations of G. aculeatus from the White Sea basin from the ancestral marine population. The great majority of these markers cluster within 19 short genomic regions, or DIs (Figure 2). All the DIs we found overlap with regions found in previous studies [15],[22], indicating a substantial degree of genetic parallelism in the origin of geographically distant freshwater populations and supporting the significance of the corresponding genomic regions for the process of adaptation.
Out of the 19 DIs, 12 overlap the regions reported both in [22] and [15] as responsible for adaptation to freshwater (Table 2), and the remaining 7 overlap loci reported in [15] only. Out of the 81 top-scoring regions described in [15], 71 regions are located within our DIs. 7 out of our 19 DIs (I-1, IV-1, IV-3, IV-4, VII-1, XIX-1, and XIX-2) overlap the top-scoring regions identified in [15] by both SOM/HMM and CSS analyses, and 12 more overlap the regions identified in [15] by less stringent criteria.
Extensive overlap between the adaptive regions described here for the White Sea populations and the ones reported from other regions confirms the previously described widespread parallelism in stickleback evolution [15],[22],[24],[27],[58]. A caveat is that both our study and the previous studies are specifically focused on finding genomic signatures of parallel evolution, and it is hard to infer the extent of parallelism from them directly. When only one, rather than two, marine-freshwater pairs of populations is used to identify marker SNPs, their number increases radically, likely due to addition of some true loci of population-specific positive selection, but also due to an increased number of false positives (Figure S3). Future studies involving many populations, and comparing loci involved in adaptation in some of the populations but not in others, are necessary to reveal the exact extent of parallelism.
The aggregated distribution of marker SNPs in the genome is probably determined by recombination patterns. For each DI, the observed increase of allele frequencies could be driven by positive selection favoring the “freshwater” allele at only one crucial polymorphic site, accompanied by hitch-hiking at surrounding neutral SNPs [59]. Alternatively, several distinct adaptive loci may be clustered together in regions of low recombination, with selection acting simultaneously at several such loci [15],[55]. Both these explanations are consistent with the fact that the three longest DIs found in our study all correspond to known inversions (DIs I-1, XI-1, XXI-1; Figure 2), i.e., the regions where recombination is minimal and hitch-hiking is most pronounced. In any case, our data do not imply that freshwater alleles at all the markers within a DI are favored by selection, even when a DI encompasses several protein-coding genes; the vast majority of the marker SNPs are probably neutral, and get fixed between the marine and freshwater populations due to hitch-hiking. Still, among the SNPs within the protein-coding genes, the nonsynonymous-to-synonymous ratio is higher for marker SNPs than for SNPs segregating within the ancestral marine population (Table 3), consistent with positive selection favoring marine-freshwater divergence at nonsynonymous sites [40]. Nine out of the 19 DIs do not contain any genes with nonsynonymous substitutions between freshwater and marine populations (Table 2), suggesting that evolution of regulatory mechanisms played a major role in the process of adaptation [15],[60],[61].
We observed a gradual increase of freshwater allele frequencies inside DIs (Figure 3 and Table S2) by exploring two young lake populations as well as two artificial populations established in 1978 from equal numbers of founder individuals of different forms. The selection coefficients estimated from the rates of increase of freshwater allele frequencies in Quarry Goluboy and Lake Ershovskoye are generally high (mean s = 0.13 and 0.16, respectively), implying rapid adaptation of G. aculeatus to the lacustrine environment (Table 2). The selection coefficient inferred for the EDA allele (s = 0.19 and 0.09) is somewhat lower than that inferred previously from short-term experimental populations (s∼0.5 [6]). Outside sticklebacks, the observed range of selection coefficients is comparable to that acting in the course of adaptation of Biston betularia peppered moth butterflies to predation (0.05–0.16 [2]), or estimated for the lactose-persistence allele in humans (0.014–0.19 [62]), and exceeds those estimated for PDYN promoter (<0.01 [63]) or genes involved in pigmentation (0.02–0.10, [64]) in humans. The variance of the frequencies of freshwater alleles among DIs is larger in the Quarry Malysh population than in the Quarry Goluboy population. The population of Malysh is much smaller than that of Goluboy and, therefore, this difference is possibly due to stronger genetic drift [65] in Malysh.
Within-DI densities of marker SNPs are rather high, and the marine and freshwater haplotypes differ from each other at over 1% of nucleotide sites within some DIs. Because most of marker SNPs are probably selectively neutral by themselves, this implies that these haplotypes diverged ∼106 generations ago, assuming the mutation rate of 10−8 per nucleotide site per generation [66]. Such high divergence times are obviously inconsistent with de novo origin of freshwater alleles in each of the freshwater populations. Instead, they are consistent with repeated recruitment of the same ancient alleles in the course of establishment of different freshwater populations.
Only 5.0% of the marker SNPs identified under the strong marker criteria is located outside DIs. Some of these markers are in fact clustered, although they do not form DIs under our formal criteria. For example, at chromosome XIII, which carries no DIs, 11 out of the 18 markers are located within 0.5 Mb from each other. Notably, these marker SNPs also have the nonsynonymous-to-synonymous ratio substantially exceeding that in non-marker SNPs segregating within the marine population (Table 3). The fraction of nonsynonymous SNPs among coding marker SNPs is even higher outside DIs (17 out of 21) than within DIs (139 out of 285; Fisher's test, two-tailed P = 0.0056), suggesting that the fraction of marker SNPs under selection may be even higher outside than within DIs. Arguably, the differences in allelic frequencies of non-DI SNPs could be due to hitch-hiking with the DI marker SNPs; however, we see no difference in the rate of increase when the non-DI marker is located on a chromosome with a DI vs. without a DI (Figure S1A – natural populations; Figure S1B – artificial populations), and for markers at chromosomes with DIs, there is little correlation with the distance from the DI (Figure S2 – two natural lakes), suggesting that at least some of the non-island markers are also targets for selection. Plausibly, these SNPs are young and not yet surrounded by as many adjacent neutral hitchhikers accompanying them; the higher fraction of selected SNPs among them is therefore as expected. Still, each individual marker SNP located outside DIs is probably more likely to be indeed neutral, and to result from genetic drift and/or sampling bias, than a DI. Indeed, although the non-DI marker SNPs also increase their frequency in freshwater, this increase is less pronounced than within DIs (Figure 4), probably due to a higher fraction of neutral loci and/or weaker selection in the former.
7 out of the 19 detected DIs are “weak”. Analysis of such DIs reveals a number of patterns. In some of the weak DIs (VII-1 and IV-5, Figure 3A), the frequencies of freshwater alleles are close to 80%, but do not reach this threshold in either of the two populations from lakes Mashinnoye and Lobaneshskoye, so that the strong marker criteria do not hold. In others, although the frequencies of freshwater alleles seemingly increased gradually with the age of the population, they have not reached 80% in both lakes, perhaps due to weakness of selection favoring them (DIs II-1, IX-1, IX-2, and XI-1). In still others, freshwater alleles rapidly reach rather high frequencies in the young population, but these frequencies remain at the same level when the population's age increases (DI XXI-1, Figure 3A). The latter scenario could conceivably be due to the some form of balancing selection, perhaps due to interactions between the genes linked within the long inversion which constitutes DI XXI-1.
According to the “transporter hypothesis” [23], freshwater alleles are constantly present at low frequencies in the marine population, probably due to rare emigration from freshwater populations, and are recruited when a new freshwater population is established. The fact that alleles recruited in different freshwater populations tend to coincide, and that freshwater and marine haplotypes are highly divergent within DIs, support this hypothesis. Thus, a sort of balancing selection acts on the sites directly involved in adaptation to freshwater at the level of the global metapopulation [67] of G. aculeatus, keeping freshwater alleles from extinction. This metapopulation consists of the ancestral anadromous marine population and many derived residential freshwater populations. While the individual derived populations are often short-lived, the metapopulation has probably existed during much of the history of the species.
Although the ancestral alleles favored in the sea and the derived alleles favored in freshwater have coexisted for a long time, they have had only occasional opportunities to be separated by recombination from adjacent neutral polymorphisms. Indeed, this recombination can happen only in new residential populations, before fixation of alleles favored in freshwater together with linked neutral markers around them, or during the presumably short periods of time when such alleles exist in the sea before being recruited for formation of a new residential population. Thus, the width of a DI must be determined by the strength of selection favoring the freshwater alleles in freshwater populations and disfavoring them in the sea, by the recombination rate, and by the number of generations between the time when the freshwater allele has escaped from one freshwater population into the sea and the time when it has become recruited in another emerging lake residential population. Nucleotide diversity within strong DIs in our freshwater populations is somewhat lower than in the marine population, but not radically, indicating that adaptation to each freshwater lake has involved soft, rather than hard, selective sweeps [68]. Indeed, soft sweeps involve recruitment of multiple simultaneous sweeping haplotypes, and thus do not lead to a significant reduction in the nucleotide diversity around the selected site. Soft sweeps are likely when the sweeping alleles arise from pre-existing genetic variation rather than de novo mutations, and thus the lack of major reduction in diversity also supports the transporter hypothesis [23]. When a freshwater allele is brought into a new lake population by several individuals, nucleotide diversity is expected to increase on both sides of the DIs due to unequal length of freshwater DIs in founder individuals [69]; however, we see no such effect, probably due to the young age of our populations.
The very high evolutionary rate observed at several of the DIs during transition from marine to residential form could be attributed to pre-existing genomic regions, recruited from the standing variation of the marine population. Such “precast bricks” allow emerging freshwater population of sticklebacks to build rapidly a phenotype adapted to various challenges (salinity, parasites, energy metabolism, etc.) which it faces in the new environment. Plausibly, this form of evolution may be widespread beyond the stickleback model. Rapid emergence of parallel well-differentiated autochthonous flocks in the genus Eubosmina (Cladocera: Crustacea) in European lakes [70], flocks of Labeobarbus (Cyprinidae: Teleostei) in lakes and rivers of Ethiopia [71],[72], and genus Salvelinus (Salmonidae: Teleostei) [73],[74] could be a few out of many examples of this kind of evolution from precast bricks, during which new adaptive phenotypes are repeatedly created by rearrangement of ancient genetic elements, which were formed during earlier adaptive radiations and retained in ancestral population as standing variation.
Materials and Methods
Collection of samples and ethics statement
Fish were collected in June–August 2011 by scoop-net or landing-net, anaesthetized and euthanized with a tricaine methane sulphonate solution (MS222), and then immediately fixed in 96% ethanol on site. Fish euthanasia was conducted under the supervision of the Ethics Committee for Animal Research of the Koltzov Institute of Developmental Biology Russian Academy of Sciences. Location of lakes and quarries, estimated age of population, and sample size are presented in Table 1.
Genome sequencing
Total genomic DNA was extracted from each individual using Wizard genomic DNA purification kit (Promega). Prior to library preparation, DNA samples of between 8 and 20 (Table 1) fish from the same population were pooled in equal proportions. Resulting pooled DNA samples were processed as described in the TruSeq DNA Sample Preparation Guide (Illumina). Library lengths were estimated using 2100 Bioanalyzer (Agilent). Libraries were quantified using fluorimetry with Qubit (Invitrogen) and real-time PCR (primers I-qPCR-1.1: AATGATACGGCGACCACCGAGAT and I-qPCR-2.1: CAAGCAGAAGACGGCATACGA) and diluted to final concentration of 9 pM. Diluted libraries were clustered on a paired-end flow cell using cBot instrument and sequenced using HiSeq2000 sequencer with TruSeq SBS Kit v3-HS (Illumina), read length 101 from each end. Sequences for each population are available at the NCBI Short Read Archive (http://www.ncbi.nlm.nih.gov/Traces/sra; accession number of the project SRP023197).
Genome mapping and sequence analysis
The reads were mapped onto the reference genome of G. aculeatus downloaded from the UCSC (http://genome.ucsc.edu/) using bwa aln program of the BWA (Burrows-Wheeler Alignment Tool) package (http://bio-bwa.sourceforge.net/). Output was then converted to SAM format using bwa sampe. Next, data were processed with picard (http://picard.sourceforge.net/) in order to remove duplicated reads. We identified SNPs in all populations using program mpileup of the samtools package (http://samtools.sourceforge.net/). For SNP calling, different depth cutoffs were used for different populations due to differences in read coverage among populations: >10 for Nilma, Malysh, Goluboy and residential fish from Ershovskoye, and >5 for Mashinnoye, Lobaneshskoye, Martsy, and anadromous fish from Ershovskoye. To minimize sequencing errors, positions with base qualities lower than 40 within a population were excluded from the analysis. As an alternative approach to SNP calling, we used UnifiedGenotyper program from GATK package (http://www.broadinstitute.org/gatk/) with the same coverage cutoffs. This led to a larger pool of marker SNPs, but similar clustering patterns: all the DIs identified with the program mpileup were observed, as well as several new clusters (Table S5). The patterns of allele frequency dynamics were qualitatively similar under mpileup and GATK SNP calling (Table S6). Overall, a higher proportion of marker SNP were located outside DIs using GATK (Table S5); therefore, we chose to use mpileup for the results in the main text.
Positions of genes were derived from Ensembl database release 72 (http://www.ensembl.org/)
Clustering of SNPs along the genome
To define DIs, we merged any two regions with above-threshold numbers of marker SNPs that were closer than 40 Kb to each other. Generally, this merging procedure described our data well: for example, it prevented splitting several DIs all corresponding to a single known inversion, or division of one DI into several due to the lack of coverage in some regions. Not merging adjacent DIs led to a drastic increase in their number [71]; the qualitative patterns of allele dynamics remain the same (Table S7). This is as expected, because recombination (average recombination rate in threespine stickleback is 3.11 cM/Mb [39]) is not likely to occur between regions located so close to each other (less than 40 Kb) over the considered timescales.
Allele frequencies validation
To validate our estimates of allelic frequencies based on high-throughput sequencing data, we also genotyped each fish used for pooled DNA sample with an allele-specific set of primers for markers located within several of the DIs. For this purpose, we designed 8 allele-specific sets of primers for 7 of the DIs (one DI was genotyped with two sets of primers). Each set consisted of three primers: two allele-specific, and one anchor primer. Additionally, we used previously published primers Stn382 [27] to genotype DI IV-1. Primers, positions of target SNPs, and PCR annealing temperature for each pair of primers are presented in Table S3. Two allele-specific PCR reactions (each with one allele-specific and one common anchor primers) were set for each individual, and the second PCR product was applied in the same well of agarose gel as the first PCR reaction after 5 min of running the gel. Individuals with one or both PCR products were categorized as homo - or heterozygotes, respectively. The obtained allele frequencies matched well those estimated from high-throughput sequencing data (Table S4).
Age of freshwater populations
As a proxy for the time of formation of natural residential populations, we use the time of desalination. Before complete desalination, a lake is meromictic, and contains two water layers – a higher freshwater layer, and a lower saltwater layer, forming a halocline. This halocline prevents proper oxygenation; as a result, the lake becomes anoxic every winter, causing extirpation of residential populations [75].
Estimation of selection coefficients
For each DI, we calculated the average frequency of a freshwater allele over all marker SNPs (Table S2). We estimated generation time using length-cohort analysis, which revealed two cohorts present in each lake: immature one-year old fish, and a second-year class which participated in reproduction. Presence of three year-old and older fish in the lake population was negligible. Therefore, we assumed generation length of two years; reproduction at older ages will lead to underestimation of s. The ages of Goluboy and Malysh populations are known to be 34 years (17 generations). The Ershovskoye freshwater population is known to be 34 years old or younger [33]; we assumed its age to be 34 years (17 generations), and younger age will again lead to underestimation of the true s.
The initial frequencies of freshwater alleles in the two artificial populations, Goluboy and Malysh, were assumed to equal 0.5. In 1978, all fish in Lake Ershovskoye had phenotypic composition similar to that of a typical marine population [33]. We assume that the initial allelic frequencies in the Lake Ershovskoye matched the frequencies in marine populations, i.e., p0∼0.1. Selection coefficient s was calculated from the per generation change in allele frequency under the assumption that this change is driven by selection alone (Eqn. 3.2 in [76], assuming h = 0.5).
Supporting Information
Zdroje
1. BellMA, AguirreWE, BuckNJ (2004) Twelve years of contemporary armor evolution in a threespine stickleback population. Evolution 58 : 814–824.
2. CookLM (2003) The rise and fall of the carbonaria form of the peppered moth. Q Rev Biol 78 : 399–417.
3. GrantPR, GrantBR (2002) Unpredictable evolution in a 30-year study of Darwin's finches. Science 296 : 707–711.
4. KryazhimskiyS, RiceDP, DesaiMM (2012) Population subdivision and adaptation in asexual populations of Saccharomyces cerevisiae. Evolution 66 : 1931–1941.
5. WiserMJ, RibeckN, LenskiRE (2013) Long-term dynamics of adaptation in asexual populations. Science 342 : 1364–1367.
6. BarrettRDH, RogersSM, SchluterD (2008) Natural selection on a major armor gene in threespine stickleback. Science 322 : 255–257.
7. KolbeJJ, LealM, SchoenerTW, SpillerDA, LososJB (2012) Founder effects persist despite adaptive differentiation: a field experiment with lizards. Science 335 : 1086–1089.
8. BarrettRDH, SchluterD (2007) Adaptation from standing genetic variation. Trends in Ecology & Evolution 23 : 38–44.
9. RadwanJ, BabikW (2012) The genomics of adaptation. Proceedings of the Royal Society B: Biological Sciences 279 : 5024–5028.
10. StapleyJ, RegerJ, FeulnerPGD, SmadjaC, GalindoJ, et al. (2010) Adaptation genomics: the next generation. Trends in Ecology & Evolution 25 : 705–712.
11. EllegrenH, SmedsL, BurriR, OlasonPI, BackstromN, et al. (2012) The genomic landscape of species divergence in Ficedula flycatchers. Nature 491 : 756–760.
12. LiuS, Lorenzen ElineD, FumagalliM, LiB, HarrisK, et al. (2014) Population genomics reveal recent speciation and rapid evolutionary adaptation in polar bears. Cell 157 : 785–794.
13. Soria-CarrascoV, GompertZ, ComeaultAA, FarkasTE, ParchmanTL, et al. (2014) Stick insect genomes reveal natural selection's role in parallel speciation. Science 344 : 738–742.
14. ConteGL, ArnegardME, PeichelCL, SchluterD (2012) The probability of genetic parallelism and convergence in natural populations. Proceedings of the Royal Society B: Biological Sciences 279 : 5039–5047.
15. JonesFC, GrabherrMG, ChanYF, RussellP, MauceliE, et al. (2012) The genomic basis of adaptive evolution in threespine sticklebacks. Nature 484 : 55–61.
16. Bell MA, Foster SA (1994) The evolutionary biology of the threespine stickleback. Oxford: Oxford University Press. 571 p.
17. HagenDW, GilbertsonLG (1972) Geographic variation and environmental selection in Gasterosteus aculeatus L. in the Pacific Northwest, America. Evolution 26 : 32–51.
18. HagenDW (1967) Isolating mechanisms in threespine sticklebacks (Gasterosteus). Can J Fish Aquat Sci 24 : 1637–1692.
19. McKinnonJS, RundleHD (2002) Speciation in nature: the threespine stickleback model systems. Trends Ecol Evol 17 : 480–488.
20. BellMA (1995) Intraspecific systematics of Gasterosteus aculeatus populations: implications for behavioral ecology. Behaviour 132 : 15–16.
21. HohenlohePA, BasshamS, CurreyM, CreskoWA (2012) Extensive linkage disequilibrium and parallel adaptive divergence across threespine stickleback genomes. Philos Trans R Soc Lond B Biol Sci 367 : 395–408.
22. HohenlohePA, BasshamS, EtterPD, StifflerN, JohnsonEA, et al. (2010) Population genomics of parallel adaptation in threespine stickleback using sequenced RAD tags. PLoS Genet 6: e1000862.
23. SchluterD, ConteGL (2009) Genetics and ecological speciation. Proc Natl Acad Sci U S A 106 : 9955–9962.
24. ShimadaY, ShikanoT, MerilaJ (2011) A high incidence of selection on physiologically important genes in the three-spined stickleback, Gasterosteus aculeatus. Mol Biol Evol 28 : 181–193.
25. BellMA (2001) Lateral plate evolution in the threespine stickleback: getting nowhere fast. Genetica 112 : 445–461.
26. LoehrJ, LeinonenT, HerczegG, O'HaraRB, MeriläJ (2012) Heritability of asymmetry and lateral plate number in the threespine stickleback. PLoS ONE 7: e39843.
27. ColosimoPF, HosemannKE, BalabhadraS, VillarrealGJr, DicksonM, et al. (2005) Widespread parallel evolution in sticklebacks by repeated fixation of Ectodysplasin alleles. Science 307 : 1928–1933.
28. JonesFC, BrownC, PembertonJM, BraithwaiteVA (2006) Reproductive isolation in a threespine stickleback hybrid zone. J Evol Biol 19 : 1531–1544.
29. FurinCG, von HippelFA, BellMA (2012) Partial reproductive isolation of a recently derived resident-freshwater population of threespine stickleback (Gasterosteus aculeatus) from its putative anadromous ancestor. Evolution 66 : 3277–3286.
30. ZiuganovVV, GolovatjukGJ, SavvaitovaKA, BugaevVF (1987) Genetically isolated sympatric forms of threespine stickleback, Gasterosteus aculeatus, in Lake Azabachije (Kamchatka-peninsula, USSR). Environ Biol Fish 18 : 241–247.
31. KarveAD, von HippelFA, BellMA (2008) Isolation between sympatric anadromous and resident threespine stickleback species in Mud Lake, Alaska. Environ Biol Fishes 81 : 287–296.
32. McKinnonJS, MoriS, BlackmanBK, DavidL, KingsleyDM, et al. (2004) Evidence for ecology's role in speciation. Nature 429 : 294–298.
33. ZiuganovVV (1978) Factors determining morphological differentiation in Gasterosteus aculeatus (Pisces, Gasterosteidae) (in Russian). Zool Zhurn 57 : 1686–1694.
34. ZiuganovVV (1983) Genetics of osteal plate polymorphism and microevolution of threespine stickleback (Gasterosteus aculeatus L.). Theor Appl Genet 65 : 239–246.
35. ClarkPU, DykeAS, ShakunJD, CarlsonAE, ClarkJ, et al. (2009) The Last Glacial Maximum. Science 325 : 710–714.
36. CornerGD, KolkaVV, YevzerovVY, MollerJJ (2001) Postglacial relative sea-level change and stratigraphy of raised coastal basins on the Kola Peninsula, northwest Russia. Glob Planet Change 31 : 153–175.
37. KolkaVV, KorsakovaOP (2005) Application of geological methods for dating of stone labyrinths on the White Sea coast. Proceedings of the MSTU 15 : 349–356.
38. NatriHM, ShikanoT, MeriläJ (2013) Progressive recombination suppression and differentiation in recently evolved neo-sex chromosomes. Molecular Biology and Evolution 30 : 1131–1144.
39. RoestiM, MoserD, BernerD (2013) Recombination in the threespine stickleback genome–patterns and consequences. Molecular Ecology 22 : 3014–3027.
40. McDonaldJH, KreitmanM (1991) Adaptive protein evolution at the Adh locus in Drosophila. Nature 351 : 652–654.
41. AapoTK, AlistairRE, IrmaT, JukkaJ (2004) Nonindependence of mammalian dental characters. Nature 432 : 211–214.
42. KaplanJH (2002) Biochemistry of Na,K-ATPase. Annu Rev Biochem 71 : 511–535.
43. Allali-HassaniA, PanPW, DombrovskiL, NajmanovichR, TempelW, et al. (2007) Structural and Chemical Profiling of the Human Cytosolic Sulfotransferases. PLoS Biol 5: e97.
44. NeerincxA, LautzK, MenningM, KremmerE, ZigrinoP, et al. (2010) A role for the human nucleotide-binding domain, leucine-rich repeat-containing family member NLRC5 in antiviral responses. J Biol Chem 285 : 26223–26232.
45. WeltC, SidisY, KeutmannH, SchneyerA (2002) Activins, inhibins, and follistatins: from endocrinology to signaling. A paradigm for the new millennium. Exp Biol Med (Maywood) 227 : 724–752.
46. ArugaJ, YokotaN, MikoshibaK (2003) Human SLITRK family genes: genomic organization and expression profiling in normal brain and brain tumor tissue. Gene 315 : 87–94.
47. ParkC, FallsW, FingerJH, Longo-GuessCM, AckermanSL (2002) Deletion in Catna2, encoding alpha N-catenin, causes cerebellar and hippocampal lamination defects and impaired startle modulation. Nat Genet 31 : 279–284.
48. WagnerGF, JaworskiEM, HaddadM (1998) Stanniocalcin in the seawater salmon: structure, function, and regulation. Am J Physiol Regul Integr Comp Physiol 274: R1177–R1185.
49. ThompsonAJ, LummisSCR (2006) 5-HT3 receptors. Curr Pharm Des 12 : 3615–3630.
50. KawaharaR, NishidaM (2007) Extensive lineage-specific gene duplication and evolution of the spiggin multi-gene family in stickleback. BMC Evolutionary Biology 7 : 209–221.
51. KlionAD, NutmanTB (2004) The role of eosinophils in host defense against helminth parasites. Journal of Allergy and Clinical Immunology 113 : 30–37.
52. ChiuS-L, ClineHT (2010) Insulin receptor signaling in the development of neuronal structure and function. Neural Development 5 : 1–18.
53. RotherKI, AcciliD (2000) Role of insulin receptors and IGF receptors in growth and development. Pediatric Nephrology 14 : 558–561.
54. Haldane JBS (1924) A mathematical theory of natural and artificial selection. Part 1. Transactions of the Cambridge philosophical society Trinity College, Cambridge. pp. 19–41.
55. MillerCT, GlazerAM, SummersBR, BlackmanBK, NormanAR, et al. (2014) Modular skeletal evolution in sticklebacks is controlled by additive and clustered quantitative trait loci. Genetics 197 : 405–420.
56. McCairnsRJS, BernatchezL (2010) Adaptive divergence between freshwater and marine sticklebacks: insights into the role of phenotypic plasticity from an integrated analysis of candidate gene expression. Evolution 64 : 1029–1047.
57. BuhiWC (2002) Characterization and biological roles of oviduct-specific, oestrogen-dependent glycoprotein. Reproduction 123 : 355–362.
58. DeFaveriJ, ShikanoT, ShimadaY, GotoA, MerilaJ (2011) Global analysis of genes involved in freshwater adaptation in threespine sticklebacks (Gasterosteus aculeatus). Evolution 65 : 1800–1807.
59. SmithJM, HaighJ (2009) The hitch-hiking effect of a favourable gene. Genet Res 23 : 23–35.
60. ChanYF, MarksME, JonesFC, VillarrealGJr, ShapiroMD, et al. (2010) Adaptive evolution of pelvic reduction in sticklebacks by recurrent deletion of a Pitx1 enhancer. Science 327 : 302–305.
61. WrayGA (2007) The evolutionary significance of cis-regulatory mutations. Nat Rev Genet 8 : 206–216.
62. BersaglieriT, SabetiPC, PattersonN, VanderploegT, SchaffnerSF, et al. (2004) Genetic signatures of strong recent positive selection at the lactase gene. Am J Hum Genet 74 : 1111–1120.
63. RockmanMV, HahnMW, SoranzoN, ZimprichF, GoldsteinDB, et al. (2005) Ancient and recent positive selection transformed opioid cis-regulation in humans. PLoS Biol 3: e387.
64. WildeS, TimpsonA, KirsanowK, KaiserE, KayserM, et al. (2014) Direct evidence for positive selection of skin, hair, and eye pigmentation in Europeans during the last 5,000 y. Proc Natl Acad Sci 111 : 4832–4837.
65. LandeR (1976) Natural selection and random genetic drift in phenotypic evolution. Evolution 30 : 314–334.
66. LynchM (2010) Evolution of the mutation rate. Trends Genet 26 : 345–352.
67. LefflerEM, GaoZ, PfeiferS, SegurelL, AutonA, et al. (2013) Multiple instances of ancient balancing selection shared between humans and chimpanzees. Science 339 : 1578–1582.
68. HermissonJ, PenningsPS (2005) Soft sweeps: molecular population genetics of adaptation from standing genetic variation. Genetics 169 : 2335–2352.
69. RoestiM, GavriletsS, HendryAP, SalzburgerW, BernerD (2014) The genomic signature of parallel adaptation from shared genetic variation. Molecular Ecology 23 : 3944–3956.
70. FaustováM, SacherováV, SvenssonJ-E, TaylorDJ (2011) Radiation of european Eubosmina (Cladocera) from Bosmina (E.) longispina –concordance of multipopulation molecular data with paleolimnology. Limnol Oceanogr 56 : 440–450.
71. DimmickWW, BerendzenPB, GolubtsovAS (2001) Genetic comparison of three barbus (Cyprinidae) Morphotypes from the Genale River, Ethiopia. Copeia 2001 : 1123–1129.
72. NagelkerkeLAJ, SibbingFA, BoogaartJGM, LammensEHRR, OsseJWM (1994) The barbs (Barbus spp.) of Lake Tana: a forgotten species flock? Environ Biol Fish 39 : 1–22.
73. Alekseyev SS, Pichugin MY, Samusenok VP. Studies of charrs Salvelinus alpinus complex from Transbaikalia (distribution, diversity and the problem of sympatric forms). Proceedings of the Eighth and Ninth ISACF Workshops on Arctic Char, 1996 and 1998. University of Maine Printing Office Orono. pp. 71–86.
74. SenchukovaAL, PavlovSD, Mel'nikovaMN, MugueNS (2012) Genetic differentiation of chars (Genus Salvelinus) from lake Kronotskoe based on analysis of mitochondrial DNA. J Ichthyol 52 : 389–399.
75. KrasnovaED, PantyulinAN, BelevichTA, VoronovDA, DemidenkoNA, et al. (2013) Multidisciplinary studies of the separating lakes at different stage of isolation from the White Sea performed in March 2012. Oceanology 53 : 639–642.
76. Gillespie JH (2004) Population genetics: a concise guide: Johns Hopkins University Press. 214 p.
Štítky
Genetika Reprodukční medicína
Článek Oligoasthenoteratozoospermia and Infertility in Mice Deficient for miR-34b/c and miR-449 LociČlánek The Kinesin AtPSS1 Promotes Synapsis and is Required for Proper Crossover Distribution in MeiosisČlánek Payoffs, Not Tradeoffs, in the Adaptation of a Virus to Ostensibly Conflicting Selective PressuresČlánek Examination of Prokaryotic Multipartite Genome Evolution through Experimental Genome ReductionČlánek BMP-FGF Signaling Axis Mediates Wnt-Induced Epidermal Stratification in Developing Mammalian SkinČlánek Role of STN1 and DNA Polymerase α in Telomere Stability and Genome-Wide Replication in ArabidopsisČlánek RNA-Processing Protein TDP-43 Regulates FOXO-Dependent Protein Quality Control in Stress ResponseČlánek Integrating Functional Data to Prioritize Causal Variants in Statistical Fine-Mapping StudiesČlánek Salt-Induced Stabilization of EIN3/EIL1 Confers Salinity Tolerance by Deterring ROS Accumulation inČlánek Ethylene-Induced Inhibition of Root Growth Requires Abscisic Acid Function in Rice ( L.) SeedlingsČlánek Metabolic Respiration Induces AMPK- and Ire1p-Dependent Activation of the p38-Type HOG MAPK PathwayČlánek Signature Gene Expression Reveals Novel Clues to the Molecular Mechanisms of Dimorphic Transition inČlánek A Mouse Model Uncovers LKB1 as an UVB-Induced DNA Damage Sensor Mediating CDKN1A (p21) DegradationČlánek Dominant Sequences of Human Major Histocompatibility Complex Conserved Extended Haplotypes from to
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2014 Číslo 10
-
Všechny články tohoto čísla
- An Deletion Is Highly Associated with a Juvenile-Onset Inherited Polyneuropathy in Leonberger and Saint Bernard Dogs
- Licensing of Yeast Centrosome Duplication Requires Phosphoregulation of Sfi1
- Oligoasthenoteratozoospermia and Infertility in Mice Deficient for miR-34b/c and miR-449 Loci
- Basement Membrane and Cell Integrity of Self-Tissues in Maintaining Immunological Tolerance
- The Kinesin AtPSS1 Promotes Synapsis and is Required for Proper Crossover Distribution in Meiosis
- Germline Mutations in Are Associated with Familial Gastric Cancer
- POT1a and Components of CST Engage Telomerase and Regulate Its Activity in
- Controlling Meiotic Recombinational Repair – Specifying the Roles of ZMMs, Sgs1 and Mus81/Mms4 in Crossover Formation
- Payoffs, Not Tradeoffs, in the Adaptation of a Virus to Ostensibly Conflicting Selective Pressures
- FHIT Suppresses Epithelial-Mesenchymal Transition (EMT) and Metastasis in Lung Cancer through Modulation of MicroRNAs
- Genome-Wide Mapping of Yeast RNA Polymerase II Termination
- Examination of Prokaryotic Multipartite Genome Evolution through Experimental Genome Reduction
- White Cells Facilitate Opposite- and Same-Sex Mating of Opaque Cells in
- BMP-FGF Signaling Axis Mediates Wnt-Induced Epidermal Stratification in Developing Mammalian Skin
- Genome-Wide Association Study of CSF Levels of 59 Alzheimer's Disease Candidate Proteins: Significant Associations with Proteins Involved in Amyloid Processing and Inflammation
- COE Loss-of-Function Analysis Reveals a Genetic Program Underlying Maintenance and Regeneration of the Nervous System in Planarians
- Fat-Dachsous Signaling Coordinates Cartilage Differentiation and Polarity during Craniofacial Development
- Identification of Genes Important for Cutaneous Function Revealed by a Large Scale Reverse Genetic Screen in the Mouse
- Sensors at Centrosomes Reveal Determinants of Local Separase Activity
- Genes Integrate and Hedgehog Pathways in the Second Heart Field for Cardiac Septation
- Systematic Dissection of Coding Exons at Single Nucleotide Resolution Supports an Additional Role in Cell-Specific Transcriptional Regulation
- Recovery from an Acute Infection in Requires the GATA Transcription Factor ELT-2
- HIPPO Pathway Members Restrict SOX2 to the Inner Cell Mass Where It Promotes ICM Fates in the Mouse Blastocyst
- Role of and in Development of Abdominal Epithelia Breaks Posterior Prevalence Rule
- The Formation of Endoderm-Derived Taste Sensory Organs Requires a -Dependent Expansion of Embryonic Taste Bud Progenitor Cells
- Role of STN1 and DNA Polymerase α in Telomere Stability and Genome-Wide Replication in Arabidopsis
- Keratin 76 Is Required for Tight Junction Function and Maintenance of the Skin Barrier
- Encodes the Catalytic Subunit of N Alpha-Acetyltransferase that Regulates Development, Metabolism and Adult Lifespan
- Disruption of SUMO-Specific Protease 2 Induces Mitochondria Mediated Neurodegeneration
- Caudal Regulates the Spatiotemporal Dynamics of Pair-Rule Waves in
- It's All in Your Mind: Determining Germ Cell Fate by Neuronal IRE-1 in
- A Conserved Role for Homologs in Protecting Dopaminergic Neurons from Oxidative Stress
- The Master Activator of IncA/C Conjugative Plasmids Stimulates Genomic Islands and Multidrug Resistance Dissemination
- An AGEF-1/Arf GTPase/AP-1 Ensemble Antagonizes LET-23 EGFR Basolateral Localization and Signaling during Vulva Induction
- The Proteomic Landscape of the Suprachiasmatic Nucleus Clock Reveals Large-Scale Coordination of Key Biological Processes
- RNA-Processing Protein TDP-43 Regulates FOXO-Dependent Protein Quality Control in Stress Response
- A Complex Genetic Switch Involving Overlapping Divergent Promoters and DNA Looping Regulates Expression of Conjugation Genes of a Gram-positive Plasmid
- ZTF-8 Interacts with the 9-1-1 Complex and Is Required for DNA Damage Response and Double-Strand Break Repair in the Germline
- Integrating Functional Data to Prioritize Causal Variants in Statistical Fine-Mapping Studies
- Tpz1-Ccq1 and Tpz1-Poz1 Interactions within Fission Yeast Shelterin Modulate Ccq1 Thr93 Phosphorylation and Telomerase Recruitment
- Salt-Induced Stabilization of EIN3/EIL1 Confers Salinity Tolerance by Deterring ROS Accumulation in
- Telomeric (s) in spp. Encode Mediator Subunits That Regulate Distinct Virulence Traits
- Ethylene-Induced Inhibition of Root Growth Requires Abscisic Acid Function in Rice ( L.) Seedlings
- Ancient Expansion of the Hox Cluster in Lepidoptera Generated Four Homeobox Genes Implicated in Extra-Embryonic Tissue Formation
- Mechanism of Suppression of Chromosomal Instability by DNA Polymerase POLQ
- A Mutation in the Mouse Gene Leads to Impaired Hedgehog Signaling
- Keeping mtDNA in Shape between Generations
- Targeted Exon Capture and Sequencing in Sporadic Amyotrophic Lateral Sclerosis
- TIF-IA-Dependent Regulation of Ribosome Synthesis in Muscle Is Required to Maintain Systemic Insulin Signaling and Larval Growth
- At Short Telomeres Tel1 Directs Early Replication and Phosphorylates Rif1
- Evidence of a Bacterial Receptor for Lysozyme: Binding of Lysozyme to the Anti-σ Factor RsiV Controls Activation of the ECF σ Factor σ
- Hsp40s Specify Functions of Hsp104 and Hsp90 Protein Chaperone Machines
- Feeding State, Insulin and NPR-1 Modulate Chemoreceptor Gene Expression via Integration of Sensory and Circuit Inputs
- Functional Interaction between Ribosomal Protein L6 and RbgA during Ribosome Assembly
- Multiple Regulatory Systems Coordinate DNA Replication with Cell Growth in
- Fast Evolution from Precast Bricks: Genomics of Young Freshwater Populations of Threespine Stickleback
- Mmp1 Processing of the PDF Neuropeptide Regulates Circadian Structural Plasticity of Pacemaker Neurons
- The Nuclear Immune Receptor Is Required for -Dependent Constitutive Defense Activation in
- Genetic Modifiers of Neurofibromatosis Type 1-Associated Café-au-Lait Macule Count Identified Using Multi-platform Analysis
- Juvenile Hormone-Receptor Complex Acts on and to Promote Polyploidy and Vitellogenesis in the Migratory Locust
- Uncovering Enhancer Functions Using the α-Globin Locus
- The Analysis of Mutant Alleles of Different Strength Reveals Multiple Functions of Topoisomerase 2 in Regulation of Chromosome Structure
- Metabolic Respiration Induces AMPK- and Ire1p-Dependent Activation of the p38-Type HOG MAPK Pathway
- The Specification and Global Reprogramming of Histone Epigenetic Marks during Gamete Formation and Early Embryo Development in
- The DAF-16 FOXO Transcription Factor Regulates to Modulate Stress Resistance in , Linking Insulin/IGF-1 Signaling to Protein N-Terminal Acetylation
- Genetic Influences on Translation in Yeast
- Analysis of Mutants Defective in the Cdk8 Module of Mediator Reveal Links between Metabolism and Biofilm Formation
- Ribosomal Readthrough at a Short UGA Stop Codon Context Triggers Dual Localization of Metabolic Enzymes in Fungi and Animals
- Gene Duplication Restores the Viability of Δ and Δ Mutants
- Selection on a Variant Associated with Improved Viral Clearance Drives Local, Adaptive Pseudogenization of Interferon Lambda 4 ()
- Break-Induced Replication Requires DNA Damage-Induced Phosphorylation of Pif1 and Leads to Telomere Lengthening
- Dynamic Partnership between TFIIH, PGC-1α and SIRT1 Is Impaired in Trichothiodystrophy
- Signature Gene Expression Reveals Novel Clues to the Molecular Mechanisms of Dimorphic Transition in
- Mutations in Moderate or Severe Intellectual Disability
- Multifaceted Genome Control by Set1 Dependent and Independent of H3K4 Methylation and the Set1C/COMPASS Complex
- A Role for Taiman in Insect Metamorphosis
- The Small RNA Rli27 Regulates a Cell Wall Protein inside Eukaryotic Cells by Targeting a Long 5′-UTR Variant
- MMS Exposure Promotes Increased MtDNA Mutagenesis in the Presence of Replication-Defective Disease-Associated DNA Polymerase γ Variants
- Coexistence and Within-Host Evolution of Diversified Lineages of Hypermutable in Long-term Cystic Fibrosis Infections
- Comprehensive Mapping of the Flagellar Regulatory Network
- Topoisomerase II Is Required for the Proper Separation of Heterochromatic Regions during Female Meiosis
- A Splice Mutation in the Gene Causes High Glycogen Content and Low Meat Quality in Pig Skeletal Muscle
- KDM5 Interacts with Foxo to Modulate Cellular Levels of Oxidative Stress
- H2B Mono-ubiquitylation Facilitates Fork Stalling and Recovery during Replication Stress by Coordinating Rad53 Activation and Chromatin Assembly
- Copy Number Variation in the Horse Genome
- Unifying Genetic Canalization, Genetic Constraint, and Genotype-by-Environment Interaction: QTL by Genomic Background by Environment Interaction of Flowering Time in
- Spinster Homolog 2 () Deficiency Causes Early Onset Progressive Hearing Loss
- Genome-Wide Discovery of Drug-Dependent Human Liver Regulatory Elements
- Developmentally-Regulated Excision of the SPβ Prophage Reconstitutes a Gene Required for Spore Envelope Maturation in
- Protein Phosphatase 4 Promotes Chromosome Pairing and Synapsis, and Contributes to Maintaining Crossover Competence with Increasing Age
- The bHLH-PAS Transcription Factor Dysfusion Regulates Tarsal Joint Formation in Response to Notch Activity during Leg Development
- A Mouse Model Uncovers LKB1 as an UVB-Induced DNA Damage Sensor Mediating CDKN1A (p21) Degradation
- Notch3 Interactome Analysis Identified WWP2 as a Negative Regulator of Notch3 Signaling in Ovarian Cancer
- An Integrated Cell Purification and Genomics Strategy Reveals Multiple Regulators of Pancreas Development
- Dominant Sequences of Human Major Histocompatibility Complex Conserved Extended Haplotypes from to
- The Vesicle Protein SAM-4 Regulates the Processivity of Synaptic Vesicle Transport
- A Gain-of-Function Mutation in Impeded Bone Development through Increasing Expression in DA2B Mice
- Nephronophthisis-Associated Regulates Cell Cycle Progression, Apoptosis and Epithelial-to-Mesenchymal Transition
- Beclin 1 Is Required for Neuron Viability and Regulates Endosome Pathways via the UVRAG-VPS34 Complex
- The Not5 Subunit of the Ccr4-Not Complex Connects Transcription and Translation
- Abnormal Dosage of Ultraconserved Elements Is Highly Disfavored in Healthy Cells but Not Cancer Cells
- Genome-Wide Distribution of RNA-DNA Hybrids Identifies RNase H Targets in tRNA Genes, Retrotransposons and Mitochondria
- The Chromosomal Association of the Smc5/6 Complex Depends on Cohesion and Predicts the Level of Sister Chromatid Entanglement
- Cell-Autonomous Progeroid Changes in Conditional Mouse Models for Repair Endonuclease XPG Deficiency
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- The Master Activator of IncA/C Conjugative Plasmids Stimulates Genomic Islands and Multidrug Resistance Dissemination
- A Splice Mutation in the Gene Causes High Glycogen Content and Low Meat Quality in Pig Skeletal Muscle
- Keratin 76 Is Required for Tight Junction Function and Maintenance of the Skin Barrier
- A Role for Taiman in Insect Metamorphosis
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



