-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaRibosomal Readthrough at a Short UGA Stop Codon Context Triggers Dual Localization of Metabolic Enzymes in Fungi and Animals
Eukaryotic organisms use various strategies to generate protein isoforms with different function or intracellular localization from a single gene. While differential splicing of mRNA is the most common mechanism to expand the number of encoded proteins, translational readthrough of stop codons is an alternative strategy to create protein variants with C-terminally extended proteins. Recently, it has been shown that fungi use both alternative splicing and translational readthrough to specify peroxisomal isoforms of glycolytic enzymes. Here we show that stop codon readthrough is also used in the animal kingdom to target important metabolic enzymes to peroxisomes. Interestingly, several of these enzymes have a function in peroxisomal redox homeostasis and energy metabolism. It has been described that termination fidelity is modulated by oxidation of specific ribosomal proteins. This suggests that dual targeting via translational readthrough allows adaptation of peroxisomal metabolism to the oxidative status of the cell.
Published in the journal: . PLoS Genet 10(10): e32767. doi:10.1371/journal.pgen.1004685
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004685Summary
Eukaryotic organisms use various strategies to generate protein isoforms with different function or intracellular localization from a single gene. While differential splicing of mRNA is the most common mechanism to expand the number of encoded proteins, translational readthrough of stop codons is an alternative strategy to create protein variants with C-terminally extended proteins. Recently, it has been shown that fungi use both alternative splicing and translational readthrough to specify peroxisomal isoforms of glycolytic enzymes. Here we show that stop codon readthrough is also used in the animal kingdom to target important metabolic enzymes to peroxisomes. Interestingly, several of these enzymes have a function in peroxisomal redox homeostasis and energy metabolism. It has been described that termination fidelity is modulated by oxidation of specific ribosomal proteins. This suggests that dual targeting via translational readthrough allows adaptation of peroxisomal metabolism to the oxidative status of the cell.
Introduction
Although translation of mRNA usually occurs with high fidelity, different recoding mechanisms exist that alter the amino acid sequence of the resulting polypeptide [1], [2]. Programmed ribosomal frameshifting and stop codon readthrough are commonly used to maximize genomic coding capacity in viruses [3]. Bacteria containing suppressor tRNAs and Saccharomyces cerevisiae strains expressing a non-functional prion form of the eukaryotic release factor 3 display significantly enhanced bypass of stop codons [4], [5]. This results in C-terminal extension of many proteins and increases phenotypic variability. Stop codon readthrough of individual genes was first described for viral replicases in bacteriophage Qβ and tobacco mosaic virus [6]–[9]. In their pro - and eukaryotic hosts, however, only a few cellular genes have been identified, where a biological function could be attributed to readthrough derived extended polypeptides [2], [10], [11], [12]. Although in mammals readthrough derived isoforms have been detected for beta-hemoglobin [13] and for myelin P0 [14], it is unknown whether the derived C-terminal extensions are functionally important. Recently, bioinformatic analysis and ribosome profiling revealed evidence for abundant and regulated bypass of termination in different developmental stages of Drosophila and other metazoa, suggesting a conserved function for translational readthrough in animals [15], [16].
We have recently reported that translational readthrough and alternative splicing are used in fungi to generate peroxisomal isoforms of several glycolytic enzymes [17]. Peroxisomes are single-membrane compartments with a major role in fatty acid degradation [18], [19]. In humans, peroxisomes are essential and peroxisome disorders cause severe syndromes [20]–[22]. The majority of proteins destined for the peroxisomal matrix contain a type 1 peroxisomal targeting signal (PTS1), a short C-terminal motif derived from the prototypical sequence Ser-Lys-Leu (SKL) [23], [24]. Here, we report that translational readthrough at a short conserved stop codon context is used in animals and fungi to generate peroxisomal isoforms of important metabolic enzymes.
Results/Discussion
A UGA stop codon followed by the dinucleotide CU serves as a core element for efficient translational readthrough in fungi
We have previously shown that in the fungus Ustilago maydis, a PTS1 containing isoform of triosephosphate isomerase (Tpi1) is generated by stop codon readthrough (Fig. 1A) [17]. To determine the minimal sequence requirements for efficient readthrough, we fused the reporter gene gfp (green fluorescent protein) at different positions downstream of the tpi1 stop codon (Fig. 1B). Western analysis revealed that three nucleotides (CUA) following the UGA stop codon were sufficient to trigger efficient stop codon readthrough (Fig. 1B). Readthrough was also observed if this sequence motif was inserted between two reporter genes (Fig. 1C). Stop codon identity was found to be important as replacement of UGA by UAG or UAA reduced translational readthrough (Fig. 1C). Mutational analysis revealed that the identity of the first and the second nucleotide following the stop codon mainly determine readthrough efficiency (Fig. 1D). Thus, a UGA stop codon followed by the dinucleotide CU is sufficient to trigger efficient translational readthrough in U. maydis. Readthrough cannot result from incorporation of the non-canonical amino acid selenocysteine at the UGA termination codon since fungi do not contain the required specialized translation machinery [2]. It remains to be elucidated which near-cognate tRNA is required for recoding of the UGA stop codon within the UGA CU context. It has already been shown that tryptophan, arginine, cysteine or serine can be incorporated at UGA stop codons [25], [26]. Aminoglycoside antibiotics inhibit protein biosynthesis by reducing the accuracy of ribosomal translation. They also cause misreading of termination codons and thus enhance the level of ribosomal readthrough [27], [28], [29]. If the aminoglycoside G418 was added to growing U. maydis cells we observed a concentration dependent increase of C-terminally extended fusion proteins, for both Tpi1 and the readthrough reporter construct (Fig. 1E and F).
Fig. 1. Characterization of sequence requirements for translational readthrough in U. maydis. 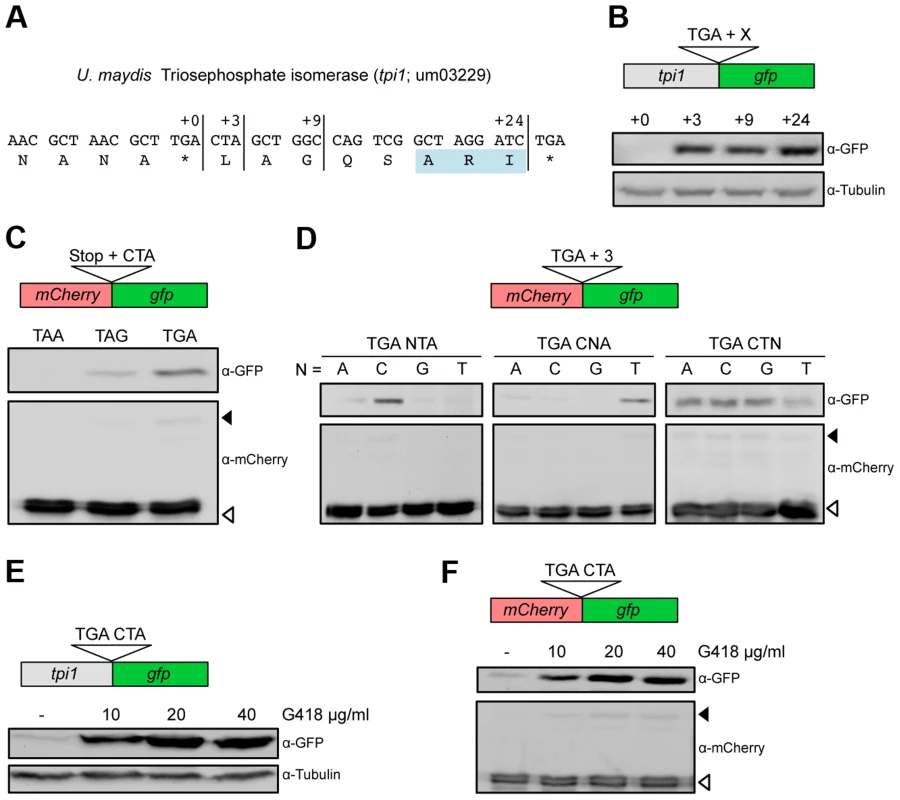
(A) 3′ sequence of the U. maydis tpi1 gene and its translation. PTS1 is highlighted. (B) Tpi1 with indicated C-terminal extensions was fused to GFP and analyzed by Western blot. (C) A reporter construct consisting of mCherry and gfp interrupted by a stop codon was expressed. Stop codon readthrough was analyzed by detection of GFP by Western blot. The termination codon TGA was replaced by TAA or TAG and tested in combination with the downstream sequence CTA for ribosomal readthrough. (D) Nucleotides downstream of TGA were exchanged as indicated and tested as described in (C) for stop codon readthrough. (E) Tpi1 including TGA CTA was fused to GFP. Cells were incubated with different amounts of G418 and analyzed for readthrough by Western blotting. (F) TGA CTA was inserted between mCherry and gfp of the reporter construct. Cells were incubated with different amounts of G418 and analyzed for readthrough by Western blotting. Filled arrows indicate readthrough products while blank arrows indicate products terminated at the first stop codon. To identify additional U. maydis genes coding for proteins with readthrough derived PTS1 motifs we screened all open reading frames ending on TGA that are followed by CT (Tab. S1). In addition to the previously described glycolytic enzymes with cryptic PTS1 motifs [17], we identified D-ribulose-5-phosphate-3-epimerase and an NADH-dependent aldehyde reductase (Fig. 2A). We could demonstrate that the readthrough derived extensions of both proteins trigger peroxisomal targeting in U. maydis (Fig. 2B). While the biological function of the aldehyde reductase is yet unknown, D-ribulose-5-phosphate-3-epimerase is an enzyme of the pentose phosphate pathway. Other components of this pathway have already been described to be located in peroxisomes [30]–[32]. Genome comparison revealed that peroxisomal targeting of both enzymes via ribosomal readthrough at UGA CU occurs also in other fungal species (Fig. 2C).
Fig. 2. Peroxisomal targeting of NADH-dependent aldehyde reductase Art1 and D-ribulose-5-phosphate-3-epimerase Rpe1 is conserved in fungi. 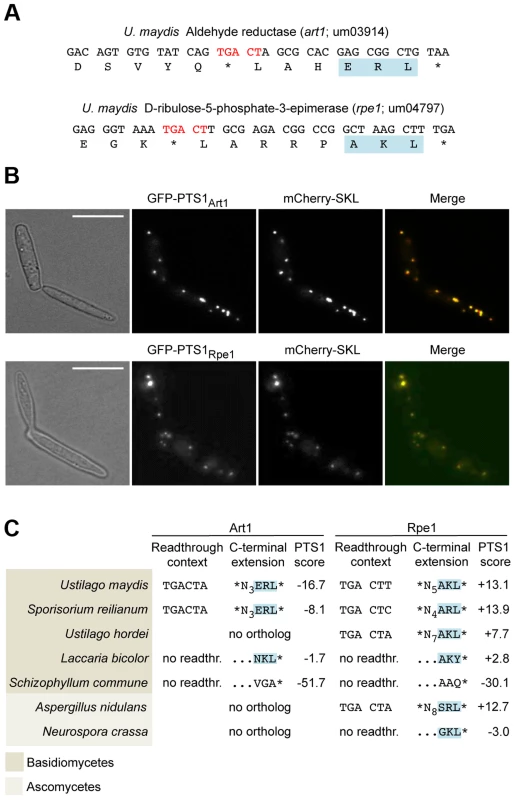
(A) 3′ sequences of U. maydis genes art1 and rpe1 together with their derived polypeptide sequence. PTS1 and readthrough elements are highlighted. All accession numbers are from MIPS Ustilago maydis DataBase (MUMDB). (B) The PTS1 motifs of Art1 and Rpe1 were fused to GFP and co-expressed with the peroxisomal marker mCherry-SKL in U. maydis cells. BF indicates bright field. Scale bars represent 10 µm. (C) Conservation of readthrough derived peroxisomal isoforms of Art1 and Rpe1 orthologs in fungi. Predictions were carried out with a PTS1 predictor [66]. In some organisms the PTS1 is part of the original open reading frame indicated by no readthr. and …NNN*. Translational readthrough at UGA CU in human cells
It has been described previously that nucleotides downstream of the termination codon influence termination efficiency in pro - and eukaryotes [2], [33], [34]. In the mammalian Sindbis virus the motif UGA C is important to trigger stop codon readthrough in vitro [35]. Remarkably, a similar but extended stop codon context was found to suppress the lethal phenotype of a nonsense mutation in a human patient [36]. Indeed, we were able to show that the UGA CU motif identified in U. maydis also triggered efficient readthrough in HeLa cells (Fig. 3A). We observed a similar dependence on the identity of the stop codon and the two downstream nucleotides (Fig. 3A and B). An adenine residue at position +3 further stimulated readthrough efficiency (Fig. 3B). Taken together this suggests that the short sequence motif UGA CU(A) promotes stop codon readthrough in a wider range of species.
Fig. 3. Characterization of sequence requirements for translational readthrough in human cells. 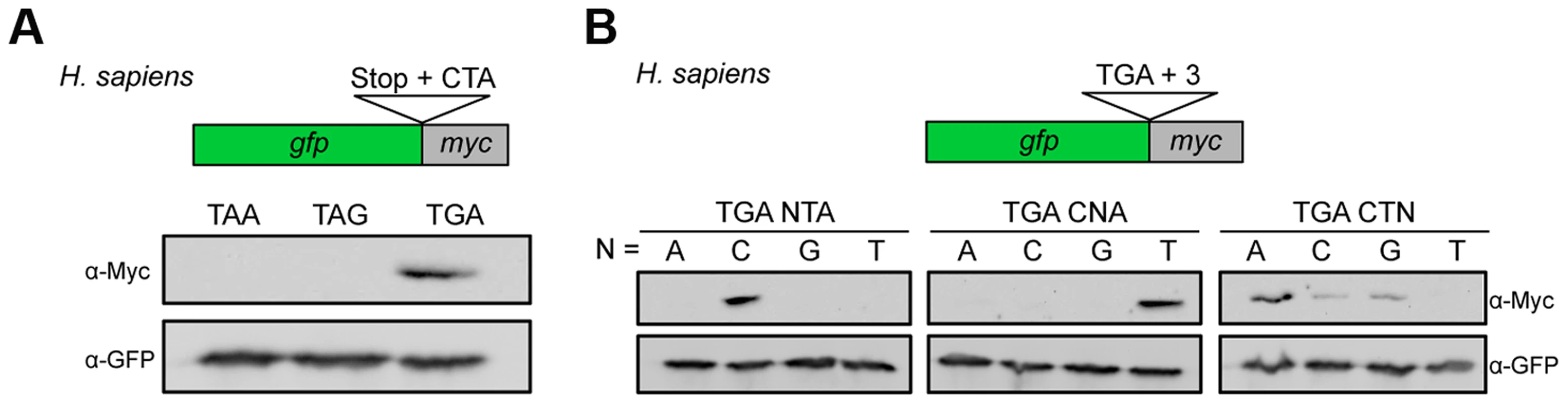
(A) A reporter construct consisting of gfp and myc interrupted by a stop codon was expressed in HeLa cells. Stop codon readthrough was analyzed by detection of the myc-epitope by Western blot. The termination codon TGA was replaced by TAA or TAG and tested in combination with the downstream sequence CTA for ribosomal readthrough. (B) Nucleotides downstream of TGA were exchanged as indicated and tested as described in (A) for stop codon readthrough. Readthrough derived peroxisomal isoforms of human lactate dehydrogenase B (LDHB) and cytosolic malate dehydrogenase (MDH1)
Next, we screened all human protein coding genes ending on TGA CT for potential peroxisomal isoforms generated by stop codon readthrough. To exclude false positives arising from chance occurrence of putative PTS1 motifs, all candidate genes were analyzed for phylogenetic conservation of both stop codon context and readthrough dependent PTS1 (Tab. S2). This analysis revealed two striking candidates, which were characterized in greater detail: cytosolic NAD-dependent malate dehydrogenase 1 (MDH1) (Fig. 4A) and NAD-dependent lactate dehydrogenase B (LDHB) (Fig. 4B). Both enzymes are critical for intracellular redox homeostasis and participate in redox-shuttling across organellar membranes [17], [37]–[39]. Accordingly, these enzymes are generally found in different cellular compartments. Lactate dehydrogenase activity has been detected in rat liver peroxisomes and was found to be required for regeneration of NAD+ [40]. MDH1 was recently identified as a peroxisomal protein in human liver cells [41]. However, the import mechanism of both enzymes is not obvious. Our data suggest that peroxisomal isoforms of MDH1 and LDHB containing a PTS1 motif are generated by stop codon readthrough. We confirmed targeting of the predicted peroxisomal isoforms of MDH1 and LDHB by fluorescence microscopy of GFP fusion proteins in HeLa cells (Fig. S1). Phylogenetic analysis revealed that readthrough dependent peroxisomal localization of LDHB is conserved in eutherian mammals (Fig. 4C). Remarkably, the cryptic PTS1 motif of MDH1 is highly conserved in the animal kingdom and occurs already in the cnidarian Hydra vulgaris (Fig. 4C).
Fig. 4. Translational readthrough derived PTS1 motifs of MDH1 and LDHB are conserved in animals. 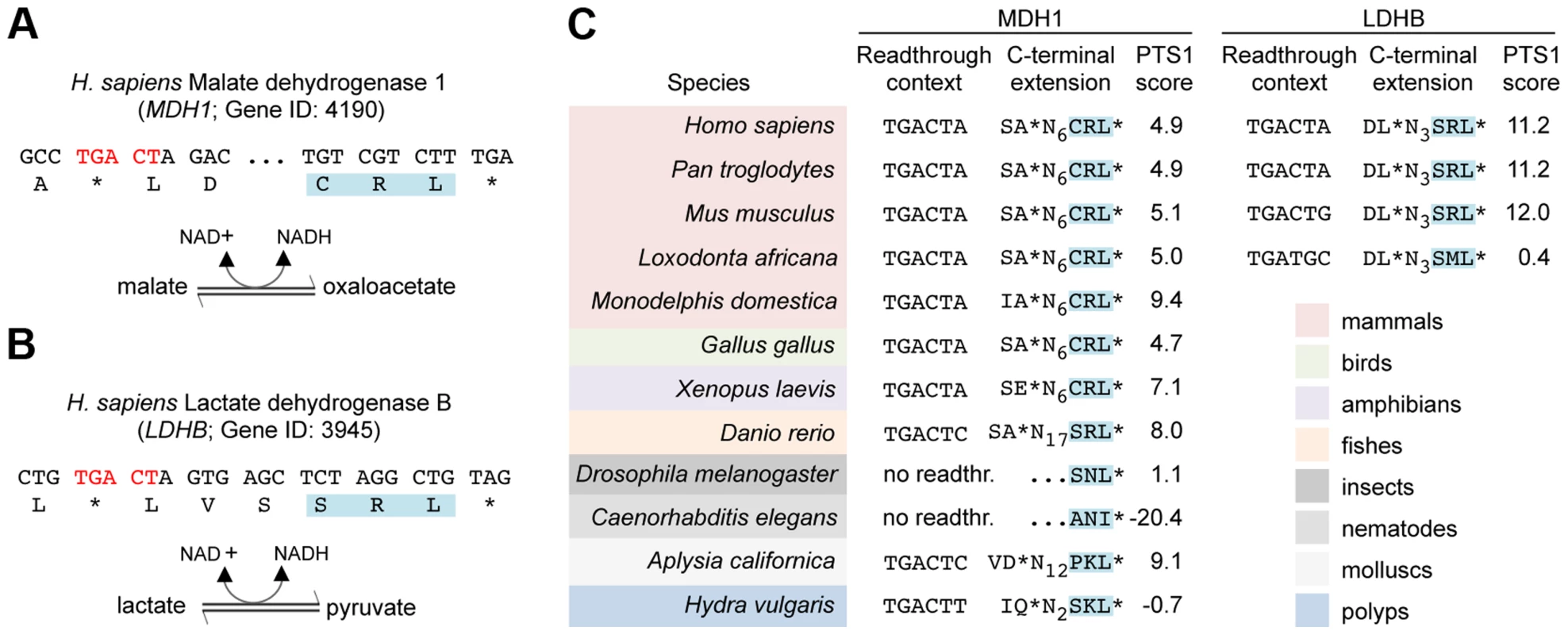
(A) 3′ sequences of the human MDH1 and its translation. The readthrough element and the predicted PTS1 motifs are highlighted. Enzymatic reactions catalyzed by MDH1 is indicated. NCBI Gene IDs is shown. (B) Human LDHB and its translation as in (A). (C) Phylogenetic conservation of readthrough derived MDH1 and LDHB peroxisomal isoforms is indicated by colouring. PTS1 predictions were carried out with PTS1 predictor [66]. PTS1 motifs of MDH1 homologs encoded by the original open reading frame are indicated by no readthr. and …NNN*. Dual targeting of LDHB and MDH1 to peroxisomes and the cytosol in human cells
Ribosome profiling experiments in human foreskin fibroblasts revealed evidence for stop codon readthrough for MDH1 but not for LDHB [16]. We used HeLa cells to address ribosomal readthrough of full-length LDHB harboring both an N-terminal HA-tag and a C-terminal Myc-tag (Fig. 5A and B). The latter epitope is only generated upon ribosomal readthrough. Leaky termination again depended on the identity of both the UGA stop codon and the nucleotides immediately downstream (Fig. 5A and B). Quantification of readthrough revealed only a small fraction (approx. 1%) of C-terminally extended proteins (Fig. 5A). However, this amount is sufficient to yield a similar protein concentration in peroxisomes and the cytosol as peroxisomes comprise only a small fraction (1–2%) of the cellular volume [42]. To further confirm that the C-terminal extensions are generated by stop codon readthrough we analyzed the quantity of the extended LDHB isoform in the presence of the aminoglycoside gentamicin. We detected an increased amount of the extended LDHB isoform corroborating that it is generated by ribosomal readthrough (Fig. 5C).
Fig. 5. Analysis of peroxisomal targeting of LDHB via translational readthrough in HeLa cells. 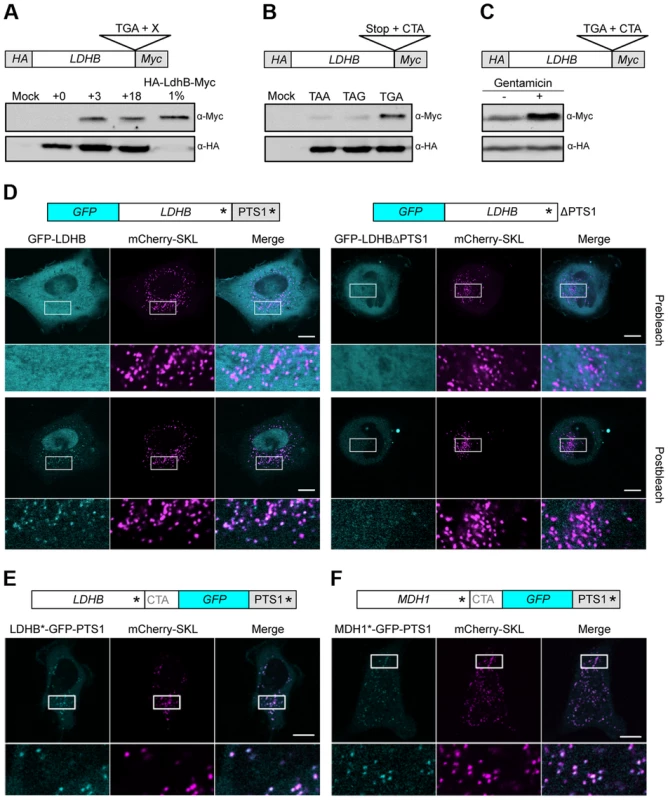
(A) Western blot analysis of LDHB derivatives fused to HA and Myc. (B) The stop codon of the LDHB construct described in (A) was exchanged by TAA and TAG. (C) Western Blot analysis of LDHB fused to HA and Myc in dependence on Gentamicin (+). (D) GFP-LDHB and GFP-LDHBΔPTS1 were co-expressed with the peroxisomal marker mCherry-SKL. (E) LDHB*-GFP-PTS1 was co-expressed with mCherry-SKL. (F) MDH1*-GFP-PTS1 was co-expressed with mCherry-SKL. Asterisks mark the positions of stop codons. PTS1 indicates the peroxisomal targeting signal downstream of the conventional stop codon of LDHB and MDH1, respectively. Intracellular localization was followed by fluorescence microscopy before and after repeated photobleaching. Magnified areas are indicated and shown below the micrographs. Scale bars represent 10 µm. To determine the intracellular localization of MDH1 and LDHB we monitored full-length N-terminal GFP-fusion proteins in HeLa cells by live cell imaging. Strong cytosolic fluorescence prevented direct observation of peroxisomal LDHB and MDH1 isoforms (Fig. 5D and S2). However, depletion of cytosolic GFP-LDHB and GFP-MDH1 by repeated photobleaching [43] unveiled peroxisomal targeting of both enzymes (Fig. 5D and S2). Deletion of the PTS1 containing extension abolished peroxisomal localization demonstrating that intracellular sorting of both enzymes depends on ribosomal readthrough (Fig. 5D and S2).
To confirm these data we inserted GFP between the TGA CTA motif and the PTS1 encoding sequence of either LDHB or MDH1. Microscopic analysis confirmed the presence of readthrough derived GFP fusion proteins that predominantly co-localize with the peroxisomal marker mCherry-SKL (Fig. 5E and F).
Peroxisomal targeting of additional central metabolic enzymes via translational readthrough in animals
We identified additional interesting candidate genes also in other model organisms. D. melanogaster NADP-dependent isocitrate dehydrogenase and Caenorhabditis elegans inorganic pyrophosphatase contain both the stop codon context UGA CU and a predicted PTS1 in the readthrough derived extension. (Fig. 6A, S3A and S3B; Tab. S2). Interestingly, mammalian and fungal orthologs of NADP-dependent isocitrate dehydrogenase contain a PTS1 motif at their regular C-termini (Fig. S3B and S3C) [44]. Peroxisomal localization of NADP-dependent isocitrate dehydrogenase has already been demonstrated in the fungi Aspergillus nidulans and S. cerevisiae [45], [46], [47].
Fig. 6. Inorganic pyrophosphatase is targeted to peroxisomes in different species by various mechanisms. 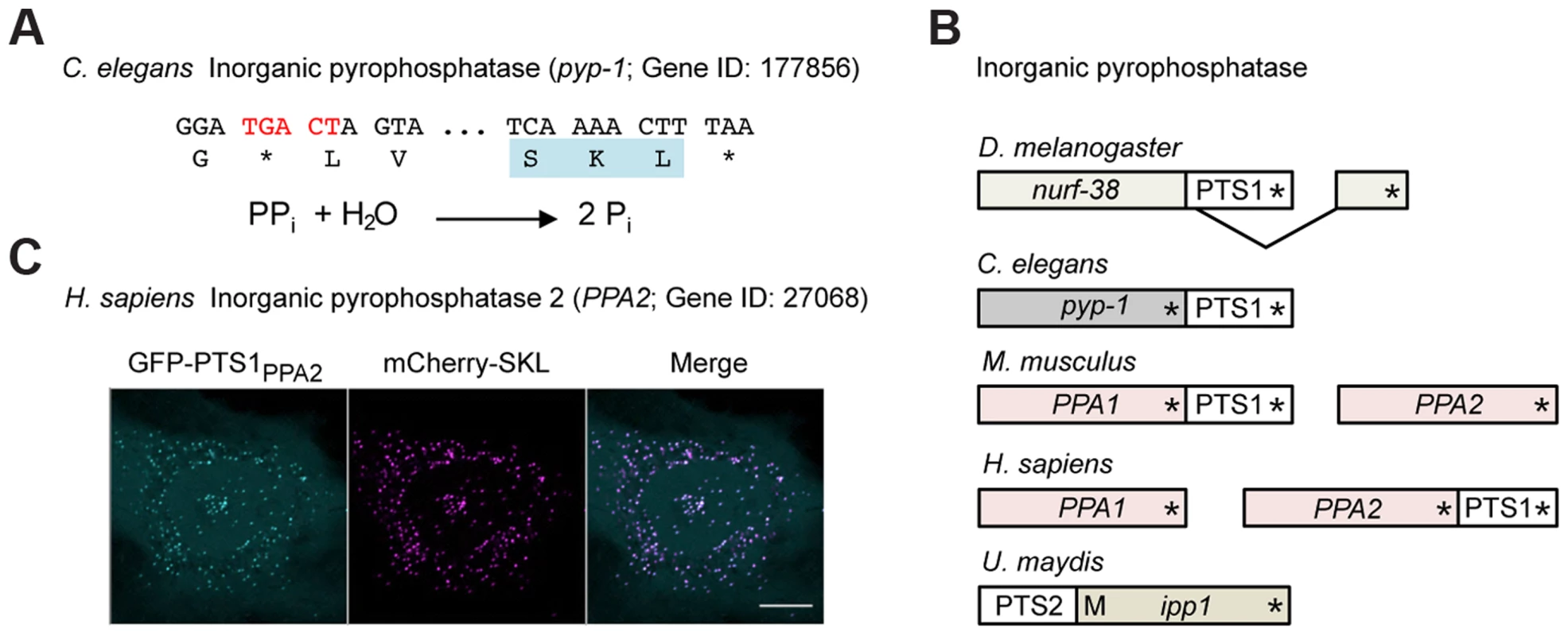
(A) 3′ sequence of the C. elegans pyp-1 gene encoding inorganic pyrophosphatase (Gene ID: 177856) and its translation. The readthrough element and the PTS1 are highlighted. The reaction catalyzed by inorganic pyrophosphatase is shown. (B) Schematic drawings illustrating different mechanisms leading to peroxisomal isoforms of inorganic pyrophosphatase in a variety of species. Asterisks mark the positions of stop codons. M represents an alternative methionine start codon. (C) The readthrough derived PTS1 motif of human PPA2 was fused to GFP and analyzed for peroxisomal localization in HeLa cells. Scale bar represents 10 µm. The predicted peroxisomal targeting of inorganic pyrophosphatase was found to depend on a variety of molecular mechanisms in different species (Fig. 6B). While alternative splicing generates a C-terminal PTS1 in D. melanogaster Nurf-38, the U. maydis Ipp1 enzyme contains an N-terminal PTS2 signal (Fig. 6B). Mammals contain two genes coding for cytosolic and mitochondrial versions of inorganic pyrophosphatase (PPA1 and PPA2). Putative readthrough derived PTS1 motifs could be identified in either of these genes depending on the organism (Fig. 6B and C). Inorganic pyrophosphatase is an essential enzyme that drives the direction of all biochemical reactions in which pyrophosphate is generated, e.g. DNA and RNA polymerization [48]. Activity of this enzyme has already been detected in isolated rat liver peroxisomes, where it might be important for ATP dependent activation of fatty acids [49].
Conclusions
In this study, we have shown that the short readthrough core motif UGA CU(A) is widely used for generation of C-terminally extended proteins. Recently, readthrough derived isoforms of four other mammalian proteins were reported [50]. All corresponding genes contain a TGA CTA motif but readthrough efficiency was found to be further increased by downstream sequences. The presence of a guanine nucleotide at position +4 was of particular importance. We did not test the effect of this residue systematically but noticed that many of the genes identified in our study contain a G at this position (see Fig. 1, 2 and 4). This suggests that the extended motif UGA CUA (G) represents a weak context for termination of translation. While in some cases the amount of readthough product generated by this context alone might be sufficient, higher levels of readthrough require downstream mRNA sequences, which often form secondary structures [51]. Such structural elements might also be involved in efficient readthrough of cellular and viral genes that lack the TGA CT context [1], [3], [16], [17]. A genome wide study in D. melanogaster has shown that in genes subject to readthrough secondary structures occur more often if the corresponding stop codons are TAA or TAG [15]. Both stop codons are known to be less leaky than TGA [52].
Another property of the UGA CU(A) element might be relevant for its function in generating peroxisomal isoforms. Recently, it was shown that hydroxylation of a proline residue in the decoding center of ribosomes regulates translational fidelity in response to oxygen availability [53], [54]. Mutational analysis revealed that hydroxylation decreased termination efficiency if the stop codon was followed by a cytosine residue [37]. Thus, intracellular sorting of enzymes targeted to peroxisomes via translational readthrough at the conserved UGA CU motif is likely to be regulated by oxygen levels, which are critical for peroxisomal β-oxidation [30], [55]. Therefore, we speculate that the use of specific readthrough signals allows differential regulation to modulate protein function in response to physiological conditions.
Materials and Methods
Strains, growth conditions and transformation
Escherichia coli strain TOP10 (Invitrogen) was used for all cloning purposes and amplification of plasmid DNA. All U. maydis strains used and created in this study are derivatives of strain Bub8 [56] and listed in table S3. Liquid cultures of U. maydis were grown either in YEPSlight medium [57] or yeast nitrogen base medium (YNB; Difco) supplemented with 2% glucose at 28°C. E. coli and U. maydis cells were transformed as described previously [56], [58]. To analyze the effect of G418 on readthrough levels cells of the U. maydis strains Bub8 mCherry-TGACTA-GFP and Bub8 TPI+3-GFP were grown overnight and diluted to reach OD600 0,5 after 2 h of growth. At this point 0 µg/ml, 10 µg/ml, 20 µg/ml or 40 µg/ml G418 (Roth) were added to the cultures. After 6 hours the cells were harvested and protein isolation and Western blotting was performed as described below.
Human cell culture
HeLa cells were kindly provided by Lucie Sauerhering (Institute for Virology, Marburg). The cells were maintained in Dulbecco's modified Eagle's medium (DMEM, Life Technologies) supplemented with 10% fetal bovine serum (FBS, Life Technologies) and 1% antibiotics (10.000 units/ml Penicillin, 10.000 µg/ml Streptomycin, Life Technologies) at 37°C, 5% CO2. For transfection, the cells were seeded on 6-well plates and transfected the next day using Lipofectamine2000 (Life Technologies) according to the manufacturer's protocol. 24 h after transfection protein extracts were prepared with RIPA-buffer (50 mM Tris-HCl, pH 7,5; 1% NP-40; 0.25% sodium deoxycholate; 150 mM NaCl; 1 mM EDTA) supplemented with protease inhibitors (Complete, Roche) and phosphatase inhibitors (Phosphatase inhibitor cocktail 2, Sigma-Aldrich). Post nuclear supernatants were stored at −80°C and used for Western blot analysis.
To analyze the effect of aminoglycosides on readthrough levels HeLa cells were transfected as described above. 24 h after transfection the medium was replaced with DMEM supplemented with 10% FBS, 1% Pen/Strep and 800 µg/ml Gentamicin. 48 h after transfection protein isolation and Western blotting was performed as described.
Molecular cloning and DNA procedures
Standard procedures were followed for all DNA manipulations [59]. Plasmids used for expression analysis in U. maydis are derivatives of the plasmid otef-Ala6-MMXN [60]. Oligonucleotides and resulting plasmids are listed in table S4. Genomic DNA from U. maydis was prepared as described previously [61]. Plasmids used for human cell culture are derivatives of plasmids pEGFP-C1 (Clontech), pEGFP-N1 (Clontech), pmCherry-C1 (Clontech) and pcDNA3.1+ (Invitrogen). All DNA-fragments were inserted between the EcoRI and BamHI sites. cDNA clones encoding human LDHB and MDH1 are from Origene. All plasmids were verified by sequencing.
Protein isolation and western blotting
For protein isolation, U. maydis strains were grown to mid-log phase in YEPSlight. 10 ml of cells were centrifuged for 5 min at 4000 rpm. The pellet was resuspended in 200 µl TBS containing 0.1% (v/v) Triton-×100 and 1% protease inhibitor cocktail for use with fungal extracts (Sigma) and supplemented with glass beads. Suspensions were frozen to −80°C and disrupted by 30 min incubation on a vibrax shaker (IKA) at 4°C. Suspensions were centrifuged for 15 min at 12000 rpm at 4°C. Supernatants were quantified by a Bradford assay (Biorad). Preparation of proteins from human cell culture was performed as described (see Human cell culture). For protein detection Western blotting analysis was performed. Proteins were separated by SDS-PAGE and blotted on polyvinylidene difluoride membranes or nitrocellulose membranes. Antibodies used in this study: anti-GFP mouse monoclonal (Santa Cruz), anti-mCherry mouse monoclonal (Abcam), anti-α-Tubulin mouse monoclonal (Calbiochem), anti-HA mouse monoclonal (Sigma Aldrich) and anti-Myc mouse monoclonal (New England Biolabs) and secondary antibody (HRP-conjugated goat anti-mouse, Santa Cruz). Detection was performed with Supersignal West Femto and Supersignal West Pico Chemiluminescent Substrate (Thermo Fisher Scientific) and a ChemoCam Imager (INTAS Science Imaging). Western blots were quantified using ImageJ [62].
Microscopy
U. maydis cells from logarithmically growing cultures (YNB-medium) were placed on agarose cushions and visualized by phase contrast (PC) and epifluorescence microscopy using a Zeiss Axiovert 200 microscope. Images were taken using a cooled CCD camera (Hamamatsu Orca-ER) with an exposure time of 30–300 ms. Image acquisition was performed using Improvision Volocity software and processing was carried out with ImageJ.
For microscopic analysis, HeLa cells were grown on Glass Bottom dishes (Mattek) in DMEM medium supplemented with 10% FCS. Cells were transfected with equal amounts (1 µg of total DNA) of the tested construct and the peroxisomal marker mCherry-SKL using Fugene6 reagent (Promega). Prior to imaging, cells were shifted to Life Cell Imaging buffer (Life Technologies) and kept at 37°C. Life cell imaging and photobleaching experiments were performed using a LSM710 confocal microscope (Zeiss) equipped with a 63×1.4 NA objective and 405, 488 and 561 nm laser lines. For both co-localization and photo-depletion experiments, single image planes with an optical section thickness of 0.7 um were acquired. In order to exclude spurious co-localization by cross-excitation or bleed-through, only a narrow band of emission fluorescence was detected (GFP: Excitation 488 nm, Emission 493–532 nm, mCherry: Excitation 561 nm, Emission 599–708 nm). Using those settings, we did not observe any bleed-through or cross-excitation, even when observing extremely bright samples. To deplete the freely diffusible cytoplasmic pool of the respective GFP fusions, a small region of interest (ROI) was repeatedly bleached using 405 nm and 488 nm laser lines. Loss of fluorescence was monitored using a second ROI at the opposite end of the observed cell. During bleaching, only these two areas of the cell were recorded to minimize photobleaching of the rest of the cell. After complete depletion of the cytoplasmic GFP-fusion pool, as estimated by the lack of further photobleaching in the second ROI, an image of the whole cell was acquired.
Image processing and analysis was performed using ImageJ. Only cropping and linear adjustments of brightness and contrasts were performed. In order to visualize the weak signals after photobleaching, a different scaling of the display range in comparison to the non-bleached images was used; also this adjustment was linear.
Computational analysis
For the identification of C-terminal protein extensions containing a peroxisomal targeting signal the TransTerm [63] database was used to retrieve 3′ flanking sequences from several organisms. The sequences were processed using regular expressions to retain only sequences between the regular stop codon to the next in-frame stop codon. Sequences were translated using Virtual Ribosome [64]. C-terminal tripeptides of the translated sequences were scanned for putative PTS1 motifs using the following regular expression:
(∧\*[∧*]*?([ASTPCE]RL|[SATPCVNG]KL|S[SNH]L|ARI|S[KR]M|[AG]NL|SN[IM]|[SA][KRQ]Y|HHL|[QS][KRQ]F)\*). PTS2 motifs were predicted with TargetSignalPredictor (http://www.peroxisomedb.org). Data analysis was performed with NCBI (National Center for Biotechnology Information) Basic local alignment search tool (Blast) [65], other NCBI resources and GenRE - MIPS Ustilago maydis DataBase.
Supporting Information
Zdroje
1. GestelandRF, AtkinsJF (1996) Recoding: dynamic reprogramming of translation. Annu Rev Biochem 65 : 741–768.
2. NamyO, RoussetJ-P, NapthineS, BrierleyI (2004) Reprogrammed genetic decoding in cellular gene expression. Mol Cell 13 : 157–168.
3. FirthAE, BrierleyI (2012) Non-canonical translation in RNA viruses. J Gen Virol 93 : 1385–1409.
4. MurgolaEJ (1985) tRNA, suppression, and the code. Annu Rev Genet 19 : 57–80.
5. Von Der HaarT, TuiteMF (2007) Regulated translational bypass of stop codons in yeast. Trends Microbiol 15 : 78–86.
6. WeinerAM, WeberK (1971) Natural read-through at the UGA termination signal of Qβ coat protein cistron. Nature new Biol 234 : 206–209.
7. HofstetterH, MonsteinHJ, WeissmannC (1974) The readthrough protein A1 is essential for the formation of viable Q beta particles. Biochim Biophys Acta 374 : 238–251.
8. PelhamHRB (1978) Leaky UAG termination codon in tobacco mosaic virus RNA. Nature 272 : 469–471.
9. SkuzeskiJM, NicholsLM, GestelandRF, AtkinsJF (1991) The signal for a leaky UAG stop codon in several plant viruses includes the two downstream codons. J Mol Biol 218 : 365–373.
10. XueF, CooleyL (1993) Kelch encodes a component of intercellular bridges in Drosophila egg chambers. Cell 72 : 681–693.
11. NamyO, Duchateau-NguyenG, RoussetJP (2002) Translational readthrough of the PDE2 stop codon modulates cAMP levels in Saccharomyces cerevisiae. Mol Microbiol 43 : 641–652.
12. StenebergP, EnglundC, KronhamnJ, WeaverTA, SamakovlisC (1998) Translational readthrough in the hdc mRNA generates a novel branching inhibitor in the Drosophila trachea. Genes Dev 12 : 956–967.
13. GellerAI, RichA (1980) A UGA termination suppression tRNATrp active in rabbit reticulocytes. Nature 283 : 41–46.
14. YamaguchiY, HayashiA, CampagnoniCW, KimuraA, InuzukaT, et al. (2012) L-MPZ, a novel isoform of myelin P0, is produced by stop codon readthrough. J Biol Chem 287 : 17765–17776.
15. JungreisI, LinMF, SpokonyR, ChanCS, NegreN, et al. (2011) Evidence of abundant stop codon readthrough in Drosophila and other metazoa. Genome Res 21 : 2096–2113.
16. DunnJG, FooCK, BelletierNG, GavisER, WeissmanJS (2013) Ribosome profiling reveals pervasive and regulated stop codon readthrough in Drosophila melanogaster. Elife 2: e01179.
17. FreitagJ, AstJ, BölkerM (2012) Cryptic peroxisomal targeting via alternative splicing and stop codon readthrough in fungi. Nature 485 : 522–525.
18. De DuveD (1969) The peroxisome: a new cytoplasmic organelle. Proc R Soc L B Biol Sci 173 : 71–83.
19. PoirierY, AntonenkovVD, GlumoffT, HiltunenJK (2006) Peroxisomal β-oxidation—a metabolic pathway with multiple functions. Biochim Biophys Acta 1763 : 1413–1426.
20. PassargeE, McAdamsAJ (1967) Cerebro-hepato-renal syndrome: A newly recognized hereditary disorder of multiple congenital defects, including sudanophilic leukodystrophy, cirrhosis of the liver, and polycystic kidneys. J Pediatr 71 : 691–702.
21. HeymansHSA, SchutgensRBH, TanR, van den BoschH, BorstP (1983) Severe plasmalogen deficiency in tissues of infants without peroxisomes (Zellweger syndrome). Nature 306 : 69–70.
22. PoulosA, SinghH, PatonB, SharpP, DerwasN (1986) Accumulation and defective β-oxidation of very long chain fatty acids in Zellweger's syndrome, adrenoleukodystrophy and Refsum's disease variants. Clin Genet 29 : 397–408.
23. GouldSJ, KellerGA, HoskenN, WilkinsonJ, SubramaniS (1989) A conserved tripeptide sorts proteins to peroxisomes. J Cell Biol 108 : 1657–1664.
24. RucktäschelR, GirzalskyW, ErdmannR (2011) Protein import machineries of peroxisomes. Biochim Biophys Acta - Biomembr 1808 : 892–900.
25. BlanchetS, CornuD, ArgentiniM, NamyO (2014) New insights into the incorporation of natural suppressor tRNAs at stop codons in Saccharomyces cerevisiae. Nucleic Acids Res 42 : 10061–10072.
26. EswarappaSM, PotdarAA, Koch WJ; FanY, VasuK, et al. (2014) Programmed translational readthrough generates antiangiogenic VEGF-Ax. Cell 157 : 1605–1618.
27. WilhelmJM, PettittSE, JessopJJ (1978) Aminoglycoside antibiotics and eukaryotic protein synthesis: structure–function relationships in the stimulation of misreading with a wheat embryo system. Biochem 17 : 1143–1149.
28. PalmerE, WilhelmJM, ShermanF (1979) Phenotypic suppression of nonsense mutants in yeast by aminoglycoside antibiotics. Nature 277 : 148–150.
29. BurkeJF, MoggAE (1985) Suppression of a nonsense mutation in mammalian cells in vivo by the aminoglycoside antibiotics G-418 and paromomycin. Nucleic Acids Res 13 : 6265–6272.
30. AntonenkovVD (1989) Dehydrogenases of the pentose phosphate pathway in rat liver peroxisomes. Fed Eur Biochem Soc J 183 : 75–82.
31. MeyerT, HölscherC, SchwöppeC, von SchaewenA (2011) Alternative targeting of Arabidopsis plastidic glucose-6-phosphate dehydrogenase G6PD1 involves cysteine-dependent interaction with G6PD4 in the cytosol. Plant J 66 : 745–758.
32. StrijbisK, den BurgJ, F VisserW, den BergM, DistelB (2012) Alternative splicing directs dual localization of Candida albicans 6-phosphogluconate dehydrogenase to cytosol and peroxisomes. FEMS Yeast Res 12 : 61–68.
33. BonettiB, FuL, MoonJ, BedwellDM (1995) The efficiency of translation termination is determined by a synergistic interplay between upstream and downstream sequences in Saccharomyces cerevisiae. J Mol Biol 251 : 334–345.
34. BaranovPV, GestelandRF, AtkinsJF (2002) Recoding: translational bifurcations in gene expression. Gene 286 : 187–201.
35. LiG, RiceCM (1993) The signal for translational readthrough of a UGA codon in Sindbis virus RNA involves a single cytidine residue immediately downstream of the termination codon. J Virol 67 : 5062–5067.
36. PachoF, ZambrunoG, CalabresiV, KiritsiD, SchneiderH (2011) Efficiency of translation termination in humans is highly dependent upon nucleotides in the neighbourhood of a (premature) termination codon. J Med Genet 48 : 640–644.
37. McAlister-HennL, SteffanJS, MinardKI, AndersonSL (1995) Expression and function of a mislocalized form of peroxisomal malate dehydrogenase (MDH3) in yeast. J Biol Chem 270 : 21220–21225.
38. GladdenLB (2004) Lactate metabolism: a new paradigm for the third millennium. J Physiol 558 : 1–12.
39. VisserWF, Van RoermundCWT, IjlstL, WaterhamHR, WandersRJA (2007) Metabolite transport across the peroxisomal membrane. Biochem J 401 : 365–375.
40. BaumgartE, FahimiHD, StichA, VölklA (1996) L-lactate dehydrogenase A - and A3B isoforms are bona fide peroxisomal enzymes in rat liver: Evidence for involvement in intraperoxisomal NADH reoxidation. J Biol Chem 271 : 3846–3855.
41. GronemeyerT, WieseS, OfmanR, BunseC, PawlasM, et al. (2013) The proteome of human liver peroxisomes: Identification of five new peroxisomal constituents by a label-free quantitative proteomics survey. PLoS One 8: e57395.
42. MoodyDE, ReddyJK (1976) Morphometric analysis of the ultrastructural changes in rat liver induced by the peroxisome proliferator SaH 42-348. J Cell Biol 71 : 768–780.
43. BuchC, HuntMC, AlexsonSEH, HallbergE (2009) Localization of peroxisomal matrix proteins by photobleaching. Biochem Biophys Res Commun 388 : 355–359.
44. VisserWF, van RoermundCWT, IjlstL, HellingwerfKJ, WaterhamHR, et al. (2006) First identification of a 2-ketoglutarate/isocitrate transport system in mammalian peroxisomes and its characterization. Biochem Biophys Res Commun 348 : 1224–1231.
45. HenkeB, GirzalskyW, Berteaux-LecellierV, ErdmannR (1998) IDP3 encodes a peroxisomal NADP-dependent isocitrate dehydrogenase required for the beta-oxidation of unsaturated fatty acids. J Biochem 273 : 3702–3711.
46. Van RoermundCW, HettemaEH, KalAJ, van den BergM, TabakHF;, et al. (1998) Peroxisomal beta-oxidation of polyunsaturated fatty acids in Saccharomyces cerevisiae: isocitrate dehydrogenase provides NADPH for reduction of double bonds at even positions. EMBO J 17 : 677–687.
47. SzewczykE, AndrianopoulosA, DavisMA, HynesMJ (2001) A single gene produces mitochondrial, cytoplasmic, and peroxisomal NADP-dependent isocitrate dehydrogenase in Aspergillus nidulans. J Biochem 276 : 37722–37729.
48. Kornberg A (1962) On the metabolic significance of phosphorolytic and pyrophosphorolytic reactions. In: Kasha M, Pullmann B, editors. Horizons in Biochemistry. New York: Academic Press. pp251–264.
49. ShimizuS, OhkumaS (1993) Inorganic pyrophosphatase of clofibrate-induced rat liver peroxisomes. J Biochem 113 : 462–466.
50. LoughranG, ChouMY, IvanovIP, JungreisI, KellisM, et al. (2014) Evidence of stop codon readthrough in four mammalian genes. Nucleic Acids Res 42 : 8928–8938.
51. FirthAE, WillsNM, GestelandRF, AtkinsJF (2011) Stimulation of stop codon readthrough: frequent presence of an extended 3′ RNA structural element. Nucleic Acids Res 39 : 6679–6691.
52. HowardMT, ShirtsBH, PetrosLM, FlaniganKM, GestelandRF, et al. (2000) Sequence specificity of aminoglycoside-induced stop condon readthrough: potential implications for treatment of Duchenne muscular dystrophy. Ann Neurol 48 : 164–169.
53. LoenarzC, SekirnikR, ThalhammerA, GeW, SpivakovskyE, et al. (2014) Hydroxylation of the eukaryotic ribosomal decoding center affects translational accuracy. Proc Natl Acad Sci U S A 111 : 4019–4024.
54. SingletonRS, Liu-YiP, FormentiF, GeW, SekirnikR, et al. (2014) OGFOD1 catalyzes prolyl hydroxylation of RPS23 and is involved in translation control and stress granule formation. Proc Natl Acad Sci U S A 111 : 4031–4036.
55. NordgrenM, WangB, ApanasetsO, FransenM (2013) Peroxisome degradation in mammals: mechanisms of action, recent advances, and perspectives. Front Physiol 4 : 145.
56. SchulzB, BanuettF, DahlM, SchlesingerR, SchäferW, et al. (1990) The b alleles of U. maydis, whose combinations program pathogenic development, code for polypeptides containing a homeodomain-related motif. Cell 60 : 295–306.
57. TsukudaT, CarletonS, FotheringhamS, HollomanWK (1988) Isolation and characterization of an autonomously replicating sequence from Ustilago maydis. Mol Cell Biol 8 : 3703–3709.
58. HanahanD, JesseeJ, BloomFR (1991) Plasmid transformation of Escherichia coli and other bacteria. Methods Enzymol 204 : 63–113.
59. Sambrook J, Fritsch EF, Maniatis T (1989) Molecular Cloning: A Laboratory Manual. Cold Spring Harbor: Cold Spring Harbor Laboratory Press.
60. BöhmerC, BöhmerM, BölkerM, SandrockB (2008) Cdc42 and the Ste20-like kinase Don3 act independently in triggering cytokinesis in Ustilago maydis. J Cell Sci 121 : 143–148.
61. HoffmanCS, WinstonF (1987) A ten-minute DNA preparation from yeast efficiently releases autonomous plasmids for transformaion of Escherichia coli. Gene 57 : 267–272.
62. AbràmoffMD, HospitalsI, MagalhãesPJ, AbràmoffM (2004) Image processing with ImageJ. Biophotonics Int 11 : 36–42.
63. JacobsGH, ChenA, StevensSG, StockwellPA, BlackMA, et al. (2009) Transterm: a database to aid the analysis of regulatory sequences in mRNAs. Nucleic Acids Res 37: D72–D76.
64. WernerssonR (2006) Virtual Ribosome—a comprehensive DNA translation tool with support for integration of sequence feature annotation. Nucleic Acids Res 34: W385–W388.
65. AltschulSF, GishW, MillerW, MyersEW, LipmanDJ (1990) Basic local alignment search tool. J Mol Biol 215 : 403–410.
66. NeubergerG, Maurer-StrohS, EisenhaberB, HartigA (2003) EisenhaberF (2003) Prediction of peroxisomal targeting signal 1 containing proteins from amino acid sequence. J Mol Biol 328 : 581–592.
Štítky
Genetika Reprodukční medicína
Článek Oligoasthenoteratozoospermia and Infertility in Mice Deficient for miR-34b/c and miR-449 LociČlánek The Kinesin AtPSS1 Promotes Synapsis and is Required for Proper Crossover Distribution in MeiosisČlánek Payoffs, Not Tradeoffs, in the Adaptation of a Virus to Ostensibly Conflicting Selective PressuresČlánek Examination of Prokaryotic Multipartite Genome Evolution through Experimental Genome ReductionČlánek BMP-FGF Signaling Axis Mediates Wnt-Induced Epidermal Stratification in Developing Mammalian SkinČlánek Role of STN1 and DNA Polymerase α in Telomere Stability and Genome-Wide Replication in ArabidopsisČlánek RNA-Processing Protein TDP-43 Regulates FOXO-Dependent Protein Quality Control in Stress ResponseČlánek Integrating Functional Data to Prioritize Causal Variants in Statistical Fine-Mapping StudiesČlánek Salt-Induced Stabilization of EIN3/EIL1 Confers Salinity Tolerance by Deterring ROS Accumulation inČlánek Ethylene-Induced Inhibition of Root Growth Requires Abscisic Acid Function in Rice ( L.) SeedlingsČlánek Metabolic Respiration Induces AMPK- and Ire1p-Dependent Activation of the p38-Type HOG MAPK PathwayČlánek Signature Gene Expression Reveals Novel Clues to the Molecular Mechanisms of Dimorphic Transition inČlánek A Mouse Model Uncovers LKB1 as an UVB-Induced DNA Damage Sensor Mediating CDKN1A (p21) DegradationČlánek Dominant Sequences of Human Major Histocompatibility Complex Conserved Extended Haplotypes from to
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2014 Číslo 10
-
Všechny články tohoto čísla
- An Deletion Is Highly Associated with a Juvenile-Onset Inherited Polyneuropathy in Leonberger and Saint Bernard Dogs
- Licensing of Yeast Centrosome Duplication Requires Phosphoregulation of Sfi1
- Oligoasthenoteratozoospermia and Infertility in Mice Deficient for miR-34b/c and miR-449 Loci
- Basement Membrane and Cell Integrity of Self-Tissues in Maintaining Immunological Tolerance
- The Kinesin AtPSS1 Promotes Synapsis and is Required for Proper Crossover Distribution in Meiosis
- Germline Mutations in Are Associated with Familial Gastric Cancer
- POT1a and Components of CST Engage Telomerase and Regulate Its Activity in
- Controlling Meiotic Recombinational Repair – Specifying the Roles of ZMMs, Sgs1 and Mus81/Mms4 in Crossover Formation
- Payoffs, Not Tradeoffs, in the Adaptation of a Virus to Ostensibly Conflicting Selective Pressures
- FHIT Suppresses Epithelial-Mesenchymal Transition (EMT) and Metastasis in Lung Cancer through Modulation of MicroRNAs
- Genome-Wide Mapping of Yeast RNA Polymerase II Termination
- Examination of Prokaryotic Multipartite Genome Evolution through Experimental Genome Reduction
- White Cells Facilitate Opposite- and Same-Sex Mating of Opaque Cells in
- BMP-FGF Signaling Axis Mediates Wnt-Induced Epidermal Stratification in Developing Mammalian Skin
- Genome-Wide Association Study of CSF Levels of 59 Alzheimer's Disease Candidate Proteins: Significant Associations with Proteins Involved in Amyloid Processing and Inflammation
- COE Loss-of-Function Analysis Reveals a Genetic Program Underlying Maintenance and Regeneration of the Nervous System in Planarians
- Fat-Dachsous Signaling Coordinates Cartilage Differentiation and Polarity during Craniofacial Development
- Identification of Genes Important for Cutaneous Function Revealed by a Large Scale Reverse Genetic Screen in the Mouse
- Sensors at Centrosomes Reveal Determinants of Local Separase Activity
- Genes Integrate and Hedgehog Pathways in the Second Heart Field for Cardiac Septation
- Systematic Dissection of Coding Exons at Single Nucleotide Resolution Supports an Additional Role in Cell-Specific Transcriptional Regulation
- Recovery from an Acute Infection in Requires the GATA Transcription Factor ELT-2
- HIPPO Pathway Members Restrict SOX2 to the Inner Cell Mass Where It Promotes ICM Fates in the Mouse Blastocyst
- Role of and in Development of Abdominal Epithelia Breaks Posterior Prevalence Rule
- The Formation of Endoderm-Derived Taste Sensory Organs Requires a -Dependent Expansion of Embryonic Taste Bud Progenitor Cells
- Role of STN1 and DNA Polymerase α in Telomere Stability and Genome-Wide Replication in Arabidopsis
- Keratin 76 Is Required for Tight Junction Function and Maintenance of the Skin Barrier
- Encodes the Catalytic Subunit of N Alpha-Acetyltransferase that Regulates Development, Metabolism and Adult Lifespan
- Disruption of SUMO-Specific Protease 2 Induces Mitochondria Mediated Neurodegeneration
- Caudal Regulates the Spatiotemporal Dynamics of Pair-Rule Waves in
- It's All in Your Mind: Determining Germ Cell Fate by Neuronal IRE-1 in
- A Conserved Role for Homologs in Protecting Dopaminergic Neurons from Oxidative Stress
- The Master Activator of IncA/C Conjugative Plasmids Stimulates Genomic Islands and Multidrug Resistance Dissemination
- An AGEF-1/Arf GTPase/AP-1 Ensemble Antagonizes LET-23 EGFR Basolateral Localization and Signaling during Vulva Induction
- The Proteomic Landscape of the Suprachiasmatic Nucleus Clock Reveals Large-Scale Coordination of Key Biological Processes
- RNA-Processing Protein TDP-43 Regulates FOXO-Dependent Protein Quality Control in Stress Response
- A Complex Genetic Switch Involving Overlapping Divergent Promoters and DNA Looping Regulates Expression of Conjugation Genes of a Gram-positive Plasmid
- ZTF-8 Interacts with the 9-1-1 Complex and Is Required for DNA Damage Response and Double-Strand Break Repair in the Germline
- Integrating Functional Data to Prioritize Causal Variants in Statistical Fine-Mapping Studies
- Tpz1-Ccq1 and Tpz1-Poz1 Interactions within Fission Yeast Shelterin Modulate Ccq1 Thr93 Phosphorylation and Telomerase Recruitment
- Salt-Induced Stabilization of EIN3/EIL1 Confers Salinity Tolerance by Deterring ROS Accumulation in
- Telomeric (s) in spp. Encode Mediator Subunits That Regulate Distinct Virulence Traits
- Ethylene-Induced Inhibition of Root Growth Requires Abscisic Acid Function in Rice ( L.) Seedlings
- Ancient Expansion of the Hox Cluster in Lepidoptera Generated Four Homeobox Genes Implicated in Extra-Embryonic Tissue Formation
- Mechanism of Suppression of Chromosomal Instability by DNA Polymerase POLQ
- A Mutation in the Mouse Gene Leads to Impaired Hedgehog Signaling
- Keeping mtDNA in Shape between Generations
- Targeted Exon Capture and Sequencing in Sporadic Amyotrophic Lateral Sclerosis
- TIF-IA-Dependent Regulation of Ribosome Synthesis in Muscle Is Required to Maintain Systemic Insulin Signaling and Larval Growth
- At Short Telomeres Tel1 Directs Early Replication and Phosphorylates Rif1
- Evidence of a Bacterial Receptor for Lysozyme: Binding of Lysozyme to the Anti-σ Factor RsiV Controls Activation of the ECF σ Factor σ
- Hsp40s Specify Functions of Hsp104 and Hsp90 Protein Chaperone Machines
- Feeding State, Insulin and NPR-1 Modulate Chemoreceptor Gene Expression via Integration of Sensory and Circuit Inputs
- Functional Interaction between Ribosomal Protein L6 and RbgA during Ribosome Assembly
- Multiple Regulatory Systems Coordinate DNA Replication with Cell Growth in
- Fast Evolution from Precast Bricks: Genomics of Young Freshwater Populations of Threespine Stickleback
- Mmp1 Processing of the PDF Neuropeptide Regulates Circadian Structural Plasticity of Pacemaker Neurons
- The Nuclear Immune Receptor Is Required for -Dependent Constitutive Defense Activation in
- Genetic Modifiers of Neurofibromatosis Type 1-Associated Café-au-Lait Macule Count Identified Using Multi-platform Analysis
- Juvenile Hormone-Receptor Complex Acts on and to Promote Polyploidy and Vitellogenesis in the Migratory Locust
- Uncovering Enhancer Functions Using the α-Globin Locus
- The Analysis of Mutant Alleles of Different Strength Reveals Multiple Functions of Topoisomerase 2 in Regulation of Chromosome Structure
- Metabolic Respiration Induces AMPK- and Ire1p-Dependent Activation of the p38-Type HOG MAPK Pathway
- The Specification and Global Reprogramming of Histone Epigenetic Marks during Gamete Formation and Early Embryo Development in
- The DAF-16 FOXO Transcription Factor Regulates to Modulate Stress Resistance in , Linking Insulin/IGF-1 Signaling to Protein N-Terminal Acetylation
- Genetic Influences on Translation in Yeast
- Analysis of Mutants Defective in the Cdk8 Module of Mediator Reveal Links between Metabolism and Biofilm Formation
- Ribosomal Readthrough at a Short UGA Stop Codon Context Triggers Dual Localization of Metabolic Enzymes in Fungi and Animals
- Gene Duplication Restores the Viability of Δ and Δ Mutants
- Selection on a Variant Associated with Improved Viral Clearance Drives Local, Adaptive Pseudogenization of Interferon Lambda 4 ()
- Break-Induced Replication Requires DNA Damage-Induced Phosphorylation of Pif1 and Leads to Telomere Lengthening
- Dynamic Partnership between TFIIH, PGC-1α and SIRT1 Is Impaired in Trichothiodystrophy
- Signature Gene Expression Reveals Novel Clues to the Molecular Mechanisms of Dimorphic Transition in
- Mutations in Moderate or Severe Intellectual Disability
- Multifaceted Genome Control by Set1 Dependent and Independent of H3K4 Methylation and the Set1C/COMPASS Complex
- A Role for Taiman in Insect Metamorphosis
- The Small RNA Rli27 Regulates a Cell Wall Protein inside Eukaryotic Cells by Targeting a Long 5′-UTR Variant
- MMS Exposure Promotes Increased MtDNA Mutagenesis in the Presence of Replication-Defective Disease-Associated DNA Polymerase γ Variants
- Coexistence and Within-Host Evolution of Diversified Lineages of Hypermutable in Long-term Cystic Fibrosis Infections
- Comprehensive Mapping of the Flagellar Regulatory Network
- Topoisomerase II Is Required for the Proper Separation of Heterochromatic Regions during Female Meiosis
- A Splice Mutation in the Gene Causes High Glycogen Content and Low Meat Quality in Pig Skeletal Muscle
- KDM5 Interacts with Foxo to Modulate Cellular Levels of Oxidative Stress
- H2B Mono-ubiquitylation Facilitates Fork Stalling and Recovery during Replication Stress by Coordinating Rad53 Activation and Chromatin Assembly
- Copy Number Variation in the Horse Genome
- Unifying Genetic Canalization, Genetic Constraint, and Genotype-by-Environment Interaction: QTL by Genomic Background by Environment Interaction of Flowering Time in
- Spinster Homolog 2 () Deficiency Causes Early Onset Progressive Hearing Loss
- Genome-Wide Discovery of Drug-Dependent Human Liver Regulatory Elements
- Developmentally-Regulated Excision of the SPβ Prophage Reconstitutes a Gene Required for Spore Envelope Maturation in
- Protein Phosphatase 4 Promotes Chromosome Pairing and Synapsis, and Contributes to Maintaining Crossover Competence with Increasing Age
- The bHLH-PAS Transcription Factor Dysfusion Regulates Tarsal Joint Formation in Response to Notch Activity during Leg Development
- A Mouse Model Uncovers LKB1 as an UVB-Induced DNA Damage Sensor Mediating CDKN1A (p21) Degradation
- Notch3 Interactome Analysis Identified WWP2 as a Negative Regulator of Notch3 Signaling in Ovarian Cancer
- An Integrated Cell Purification and Genomics Strategy Reveals Multiple Regulators of Pancreas Development
- Dominant Sequences of Human Major Histocompatibility Complex Conserved Extended Haplotypes from to
- The Vesicle Protein SAM-4 Regulates the Processivity of Synaptic Vesicle Transport
- A Gain-of-Function Mutation in Impeded Bone Development through Increasing Expression in DA2B Mice
- Nephronophthisis-Associated Regulates Cell Cycle Progression, Apoptosis and Epithelial-to-Mesenchymal Transition
- Beclin 1 Is Required for Neuron Viability and Regulates Endosome Pathways via the UVRAG-VPS34 Complex
- The Not5 Subunit of the Ccr4-Not Complex Connects Transcription and Translation
- Abnormal Dosage of Ultraconserved Elements Is Highly Disfavored in Healthy Cells but Not Cancer Cells
- Genome-Wide Distribution of RNA-DNA Hybrids Identifies RNase H Targets in tRNA Genes, Retrotransposons and Mitochondria
- The Chromosomal Association of the Smc5/6 Complex Depends on Cohesion and Predicts the Level of Sister Chromatid Entanglement
- Cell-Autonomous Progeroid Changes in Conditional Mouse Models for Repair Endonuclease XPG Deficiency
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- The Master Activator of IncA/C Conjugative Plasmids Stimulates Genomic Islands and Multidrug Resistance Dissemination
- A Splice Mutation in the Gene Causes High Glycogen Content and Low Meat Quality in Pig Skeletal Muscle
- Keratin 76 Is Required for Tight Junction Function and Maintenance of the Skin Barrier
- A Role for Taiman in Insect Metamorphosis
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



