-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaRNAi–Based Functional Profiling of Loci from Blood Lipid Genome-Wide Association Studies Identifies Genes with Cholesterol-Regulatory Function
Genome-wide association studies (GWAS) are powerful tools to unravel genomic loci associated with common traits and complex human disease. However, GWAS only rarely reveal information on the exact genetic elements and pathogenic events underlying an association. In order to extract functional information from genomic data, strategies for systematic follow-up studies on a phenotypic level are required. Here we address these limitations by applying RNA interference (RNAi) to analyze 133 candidate genes within 56 loci identified by GWAS as associated with blood lipid levels, coronary artery disease, and/or myocardial infarction for a function in regulating cholesterol levels in cells. Knockdown of a surprisingly high number (41%) of trait-associated genes affected low-density lipoprotein (LDL) internalization and/or cellular levels of free cholesterol. Our data further show that individual GWAS loci may contain more than one gene with cholesterol-regulatory functions. Using a set of secondary assays we demonstrate for a number of genes without previously known lipid-regulatory roles (e.g. CXCL12, FAM174A, PAFAH1B1, SEZ6L, TBL2, WDR12) that knockdown correlates with altered LDL–receptor levels and/or that overexpression as GFP–tagged fusion proteins inversely modifies cellular cholesterol levels. By providing strong evidence for disease-relevant functions of lipid trait-associated genes, our study demonstrates that quantitative, cell-based RNAi is a scalable strategy for a systematic, unbiased detection of functional effectors within GWAS loci.
Published in the journal: . PLoS Genet 9(2): e32767. doi:10.1371/journal.pgen.1003338
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003338Summary
Genome-wide association studies (GWAS) are powerful tools to unravel genomic loci associated with common traits and complex human disease. However, GWAS only rarely reveal information on the exact genetic elements and pathogenic events underlying an association. In order to extract functional information from genomic data, strategies for systematic follow-up studies on a phenotypic level are required. Here we address these limitations by applying RNA interference (RNAi) to analyze 133 candidate genes within 56 loci identified by GWAS as associated with blood lipid levels, coronary artery disease, and/or myocardial infarction for a function in regulating cholesterol levels in cells. Knockdown of a surprisingly high number (41%) of trait-associated genes affected low-density lipoprotein (LDL) internalization and/or cellular levels of free cholesterol. Our data further show that individual GWAS loci may contain more than one gene with cholesterol-regulatory functions. Using a set of secondary assays we demonstrate for a number of genes without previously known lipid-regulatory roles (e.g. CXCL12, FAM174A, PAFAH1B1, SEZ6L, TBL2, WDR12) that knockdown correlates with altered LDL–receptor levels and/or that overexpression as GFP–tagged fusion proteins inversely modifies cellular cholesterol levels. By providing strong evidence for disease-relevant functions of lipid trait-associated genes, our study demonstrates that quantitative, cell-based RNAi is a scalable strategy for a systematic, unbiased detection of functional effectors within GWAS loci.
Introduction
To date more than 120 genomic loci have been tightly linked to variation in blood lipid levels (low-density lipoprotein (LDL), high-density lipoprotein (HDL), total cholesterol (TC), triglycerides (TG)), susceptibility to coronary artery disease (CAD) and/or myocardial infarction (MI) in more than 23 published large-scale GWAS [1]–[23]. These loci contain 15 out of 18 genes in which variants cause monogenic lipid disorders and further genes with previously defined roles in lipid metabolism [17], supporting the assumption that GWAS enrich for genes with a functional importance on the associated trait [17], [24]–[26]. For the majority of associated loci, however, genes with a function in regulating blood lipid levels or with relevance to CAD/MI have yet to be identified.
Several recent examples show that non-coding variants within associated loci affect the expression of nearby genes, suggesting that cis-regulatory effects on functionally relevant proteins constitute a major trait determinant [17], [24]–[28]. On the level of gene transcripts, such dominant-negative regulatory effects can be closely mimicked by RNAi. RNAi also permits to evaluate the functional consequences of gene knockdown in vitro and in vivo [29] and has previously enabled us to unravel regulators of cholesterol metabolism from a subset of sterol-regulated genes [30]. This was performed using a strategy that relies on the knockdown of candidate genes in tissue culture cells using siRNA-arrays [30]–[32] and the quantification of how this impacts on two major determinants of blood lipid levels: cellular levels of free cholesterol (FC) and the efficiency of LDL-uptake into cells [30].
Results/Discussion
Here we applied this technology with the aim to identify candidate genes within trait-associated loci with a conserved lipid-regulatory function in cells. For this, we functionally analyzed 56 of the 64 genomic loci that were reported until 2009 as associated with lipid traits and/or CAD/MI for genes with a role in cellular cholesterol homeostasis (Figure 1A; Table S1; see Materials and Methods for details). For 38 of the 56 loci all protein-coding genes within ±50 kb of the respective lead SNPs were analyzed, with up to 16 genes at the 19p12 locus. The 18 remaining loci were represented by candidate genes close to the lead SNPs (Table S2). We followed a two-step screening-approach: First, a core gene set of 109 genes was analyzed (“GWAS1”). Promising loci from this gene set were then complemented by additional genes and experimentally re-evaluated (“GWAS2”) (see Materials and Methods). In total, we profiled 133 candidate genes out of which 93 genes had not previously been functionally linked to lipid metabolism (Table S3). Each gene was profiled with 3–5 independent siRNAs, resulting in a total of 534 gene-specific siRNAs tested (Table S4). Uptake of fluorescently-labeled LDL and free perinuclear cholesterol (FC) within siRNA-transfected cells was determined using high-content automated microscopy as described [30] (see Figure S1 and Materials and Methods for how specificity of filipin to reliably detect free cholesterol was assured). For siRNAs analyzed in both, GWAS1 and GWAS2 screens (n = 86), findings correlated well (e.g., Pearson's correlations for the parameter “total cellular intensity” were 0.81 for DiI-LDL uptake and 0.71 for FC) (Table S5), proposing that the results obtained are reproducible and specific. SiRNA-mediated knockdown of multiple known and novel regulators resulted in a consistent increase or reduction of LDL-uptake, FC, or an altered distribution of relevant sub-cellular organelles as signs of perturbed cellular lipid homeostasis (Figure 1B, 1C).
Fig. 1. Functional profiling of lipid-trait/CAD/MI associated genes by cell-based RNAi. 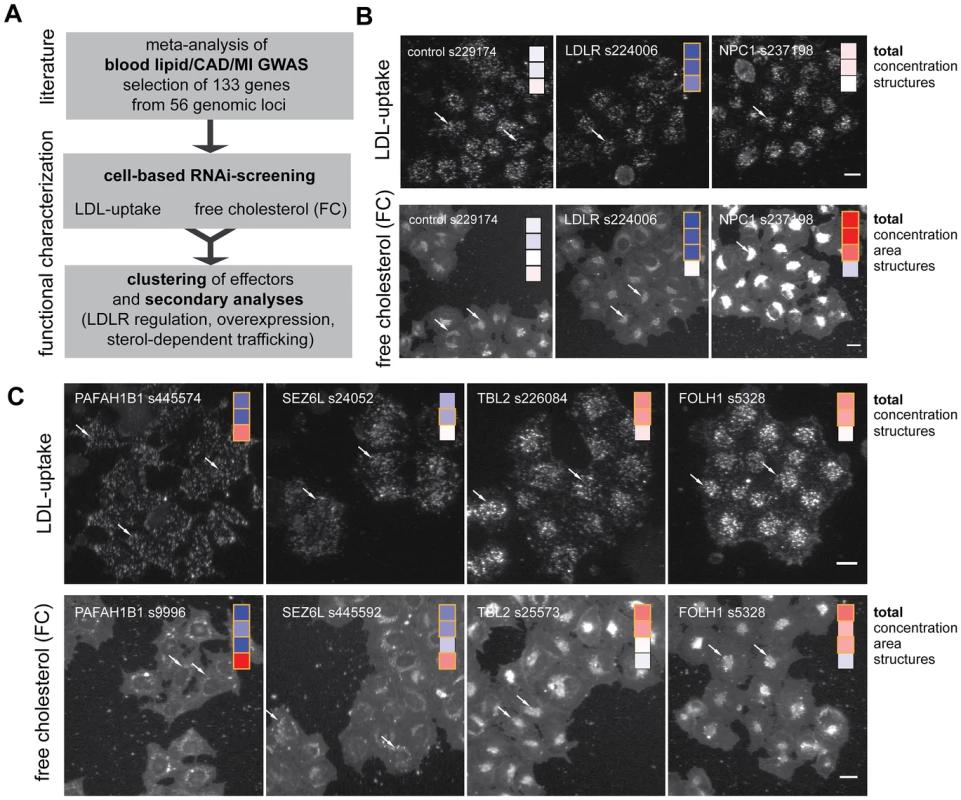
(A) Workflow of this study. (B,C) Profiling of lipid-trait associated genes for a cholesterol-regulating function in cells was performed by monitoring LDL-uptake (upper panels) and free perinuclear cholesterol (FC; lower panels) in siRNA-knockdown cells (for details, see [30]). Shown are automatically acquired images of Hela-Kyoto cells cultured and reverse siRNA transfected on cell microarrays for 48 h with control siRNAs (B) or indicated siRNAs targeting selected candidate genes increasing (red) or decreasing (blue) typical cellular phenotypes (C; see Figure 2 and Materials and Methods for details). Arrows denote selected compartments representative for respective heatmaps (see text). Bars = 20 µm. For an objective and quantitative evaluation of our results we developed an automated pipeline for multi-parametric image analysis (Figure S2 and Materials and Methods). From each cell, three (for LDL-uptake) or four (for FC) parameters were measured per siRNA-transfected cell and scored according to effect size (Figure 2A, Figure S3, Table S4 and Materials and Methods). Knockdown of 55 (41%) of the 133 candidate genes tested significantly affected the parameter “total cellular intensity” with two independent siRNAs in at least one of the two screening assays, suggesting these genes as functional effectors on cellular LDL-uptake, FC or both (Table 1, Table S4). This suggested an unexpected high number of effectors, as the typical hit rate of most reported siRNA-screens with unbiased gene sets ranges from 1–6% [32]–[36]. Even in our recent siRNA-screen on a gene set enriched for sterol-regulated genes and known lipid regulators using the same assays as applied here only 25% of the genes scored as effectors [30]. However, of the 63 siRNAs that scored as effectors in GWAS1 and were re-analyzed in validation screens, 30 siRNAs met our stringent statistical criteria also in GWAS2, resulting in a validation rate of 48% (Table S4). Our findings thus strongly support the hypothesis that GWAS enrich for functional regulators of the underlying trait or pathogenic process. They further support previous assumptions that a large proportion of the genes uncovered by GWAS also have a conserved role in tissue culture cells [17], [24], [26].
Fig. 2. Multiparametric analysis and clustering of functional effector genes. 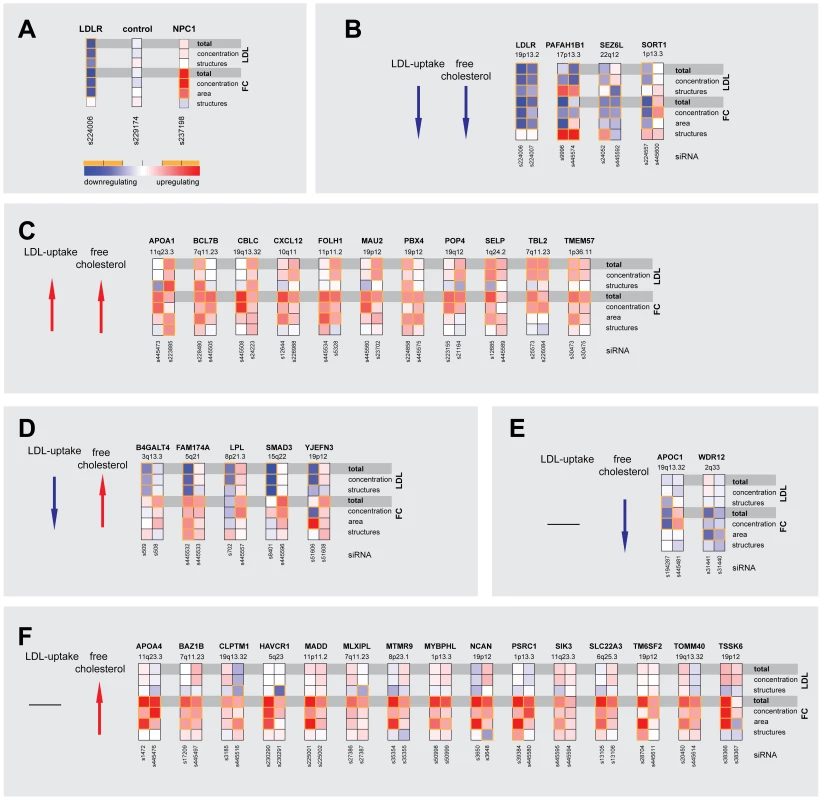
(A) Functional consequences upon knockdown of each candidate gene (using 3–5 different siRNAs/gene) were quantified from microscopic images with regard to seven phenotypic parameters: total cellular LDL-signal; LDL concentration and number of cellular structures; total free cholesterol (FC) signal; and FC concentration, area and number of cellular structures. Shown are heatmaps for 37 out of 55 most pronounced functional effector genes that according to parameter “total cellular intensity” (“total”) of the two strongest effector siRNAs/gene were clustered into five distinct functional groups (B–F) (see Figure S2 and Table S4 for comprehensive datasets). Phenotypes (red, increasing; blue, decreasing) meeting statistical criteria as described in Materials and Methods are framed in orange. Tab. 1. Strongest functional effectors in RNAi screens. 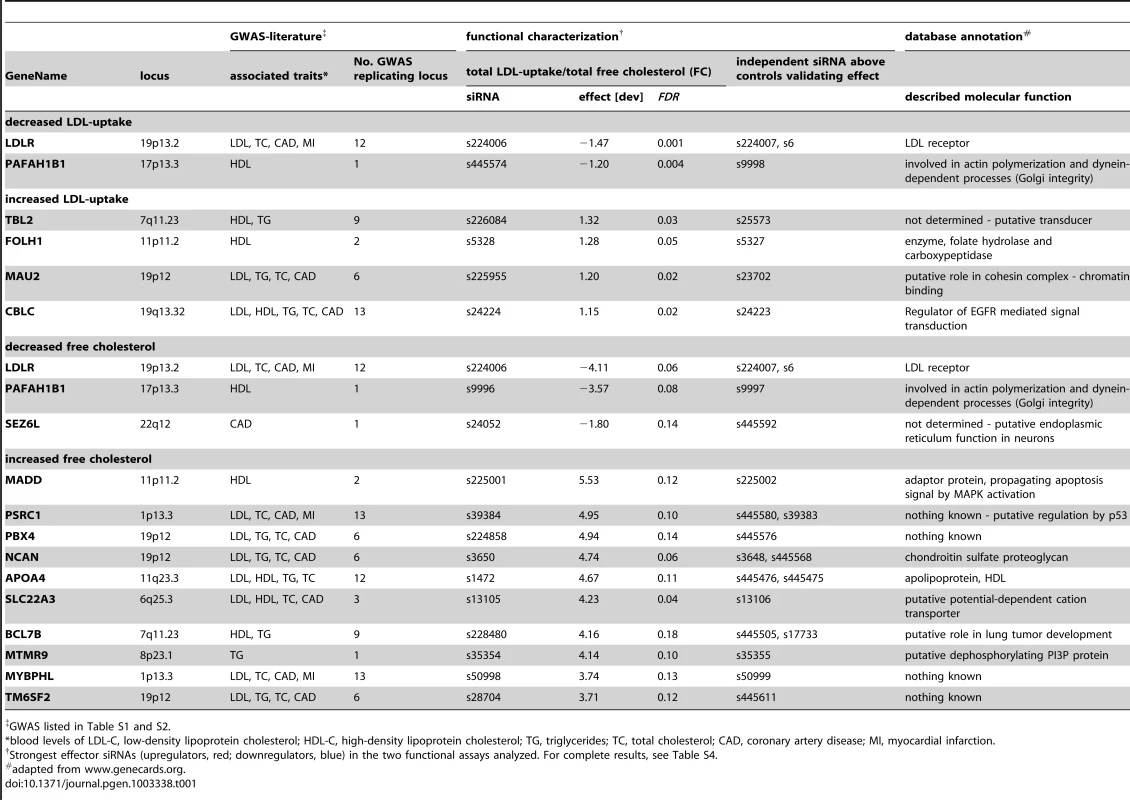
GWAS listed in Table S1 and S2. 55 genes that scored as the most pronounced functional effectors on total LDL-signal and/or cellular FC-levels were selected for further analyses (Tables S3, S6, S7, S8). According to phenotypic fingerprints of the two strongest effector siRNAs/gene (Figure S3 and Materials and Methods), 37 of these genes were tentatively clustered into five distinct functional groups (Figure 2). For 15 genes, direction of functional effects in both screening assays positively correlated (Figure 2B, 2C). Knockdown of 17 genes consistently impacted on FC without obvious effects on LDL-uptake (Figure 2E, 2F), while for 5 genes effects on FC were inversely directed to those observed for LDL-uptake (Figure 2D). For several known effectors, our results were consistent with a priori knowledge on the respective genes. For instance, it was recently elegantly demonstrated that altered expression of SORT1 at the 1p13.3 locus inversely correlates with serum LDL [24]. Consistently, one siRNA targeting SORT1 induced a strong reduction in FC and also tended to inhibit LDL-uptake, thereby corroborating further that SORT1 is a key-player in cellular cholesterol homeostasis [24]. Several examples demonstrate that this is most certainly true also for other genes among our effectors that had not previously been linked to lipid metabolism. For instance, two GWAS report association of the WDR12 locus (2q33) with CAD/MI [10], [16], while a demonstration that this locus is associated with lipid traits is so far missing. However, siRNAs targeting this gene consistently reduced FC, making a lipid-regulatory role for WDR12 highly likely.
Correlation analysis of the multi-parametric datasets enabled us to hypothesize by which mechanisms some of the previously uncharacterized effectors could possibly impact on cellular lipid homeostasis (Figure 2, Table S5 and Materials and Methods). For instance, a higher number of LDL-positive endosomes and a scattering of FC-retaining organelles upon knockdown of PAFAH1B1 (Figure 1C) is consistent with a role for this gene in the organization of endosomal membranes [37] and may be a sign of impaired LDL-internalization and/or transport within the endo-/lysosomal system. Effectors such as TBL2 on the other hand are likely to exert more direct lipid-regulatory functions as knockdown of this gene increased LDL-concentration within endosomes and FC-load, but subcellular structures remained largely unaffected (Figure 1C).
The identification of genes with relevance for lipid traits and/or CAD/MI from GWAS is complicated by the fact that many lead SNPs locate to gene rich regions [7], [24]. We therefore assessed whether for selected GWAS loci our unbiased approach could help prioritizing functional effectors among several possible candidate genes in such loci. Indeed, in six of the 30 loci for which more than one candidate gene/locus was functionally analyzed, our results suggested one prominent effector gene. Most surprisingly, in 9 of these 30 loci knockdown of more than one gene per locus affected cellular cholesterol homeostasis (Figure 3). For instance, of the 8 genes analyzed at the 7q11.23 locus (Figure 3D) not only MLXIPL as the most likely candidate to explain association with TG [8], but also five other genes scored as significantly increasing FC, among them TBL2, knockdown of which also induced the strongest observed stimulation of LDL-uptake. Similar observations for novel effectors in addition to genes with well-characterized lipid-regulatory functions were made for loci 1p36.11, 11q23.3 or 12q24.11 among others (Figure 3). Furthermore, while none of the 16 candidate genes at the 19p12 locus was previously ascribed a lipid-regulatory function, ten scored as effectors with two independent siRNAs in at least one of the two functional assays, which might reflect the substantial pleiotropy at this locus in six different GWAS [1], [6], [7], [17], [20], [21] (Table S1).
Fig. 3. Comparison of multiparametric datasets for neighboring genes within lipid-trait-associated loci. 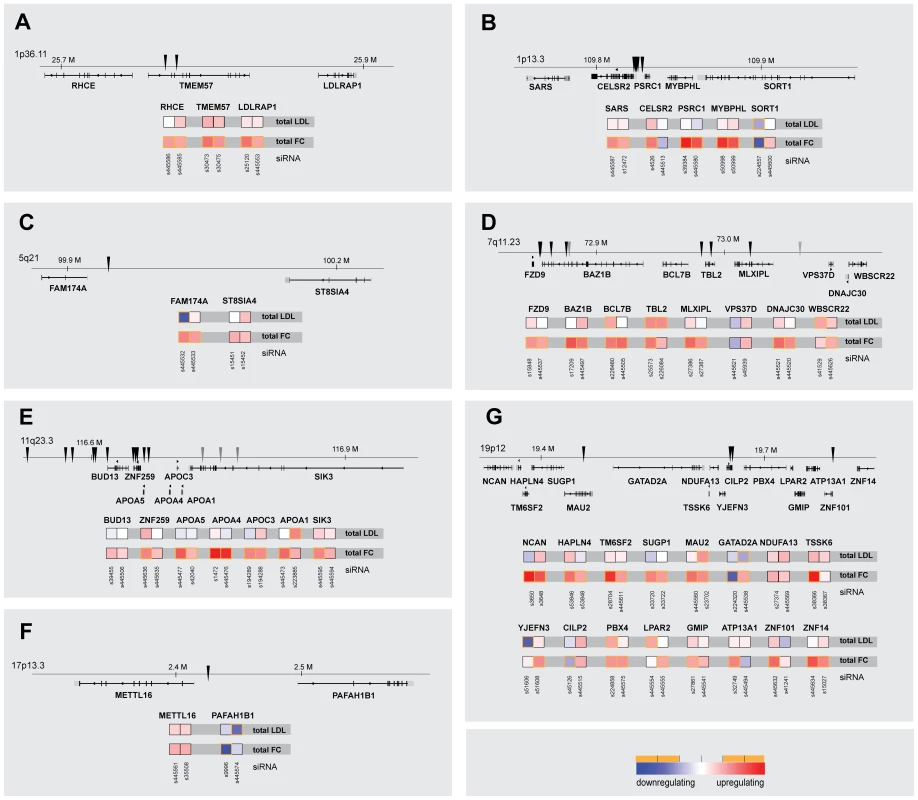
Shown are parameters “total cellular intensity” (“total”) of the two strongest effector siRNAs/gene and relative genomic position of lead SNPs (arrowheads) for seven (A–G) selected lipid-trait/CAD/MI loci in which multiple neighboring candidate genes (±50 kB up-/downstream of lead SNP) were functionally analyzed (see Figure S2 and Table S4 for comprehensive datasets). Phenotypes (red, increasing; blue, decreasing) meeting statistical criteria as described in Materials and Methods are framed in orange. In order to gain initial insight into the mechanisms how newly identified effectors could functionally contribute to cholesterol homeostasis, we validated data from our RNAi-screens with a set of secondary assays (Table 2; Figure 4; Figures S4, S5, S6, S7; Tables S6, S7, S8; and Materials and Methods). For instance, an enzymatic assay (see Materials and Methods) applied under screening conditions showed a considerably lower dynamic range to detect changes in cellular cholesterol levels than our image-based approach using filipin. However, results from both approaches correlated well (R2 = 0.48, p<10−6), and biochemical analyses corroborated six candidate genes (C12orf43, GATAD2A, SEZ6L, SORT1, TOMM40, TSSK6) as cholesterol regulators. Likewise, 18 out of 70 effector-siRNAs tested (26%) were also above thresholds when FC was measured from HuH7 liver cells and two candidate genes (BAZ1B, HAVCR1) could be validated with two independent siRNAs also in this cell model (Figure S4, Table S7). Interestingly, despite similar knockdown efficiencies at the protein level (Figure S4C), phenotypic changes upon knockdown of individual effectors were in general less pronounced in HuH7 compared to Hela cells (Figure S4D). One explanation why only a low number of candidate genes could be confirmed in HuH7 cells could be a reduced sensitivity of the filipin assay to monitor changes in free cholesterol in this cell line. Alternatively, liver cells could have mechanisms that compensate in parts the knockdown of specific candidate genes tested that are absent or less effective in Hela cells. One way to identify such compensatory mechanisms in the future could be double knockdown experiments where the candidate genes identified in this work in Hela cells would be knocked-down in combination with putative genes accounting for the compensatory mechanisms in HuH7 cells. We further assessed whether knockdown of effector genes affected mRNA and protein levels of LDLR, a major determinant of blood LDL-levels [38]. Remarkably, for 19 out of 35 effector genes tested at least one siRNA also affected LDLR expression, either on the mRNA level (3 siRNAs), protein level (15 siRNAs) or both (CETP). In several instances LDLR levels and phenotypic effects on LDL-uptake and/or FC positively correlated. For instance, impaired LDL-uptake upon knockdown of B4GALT4 and PAFAH1B1 could be directly caused by a lack of LDLR, as siRNAs targeting these genes also reduced LDLR-protein levels. Perturbed levels of LDLR-mRNA or protein on the other hand are consistent with increased or decreased FC upon knockdown of CXCL12, TSSK6 or WDR12. These results support a role for these effectors on LDLR and suggest a mechanism how variants affecting expression and/or function of these genes may impact on lipid traits and/or CAD/MI risk. No direct correlation between LDLR levels and cellular phenotype was observed for most other effectors. Such results may be explained by compensatory cellular mechanisms that tightly control LDLR at the transcriptional and post-transcriptional level [39], [40]. Alternatively, they could as well hint at yet unknown mechanisms and pathways that contribute to control blood lipid levels independent of LDLR, which await clarification in further studies.
Fig. 4. Impact on FC levels and subcellular localization of GFP–tagged candidate genes. 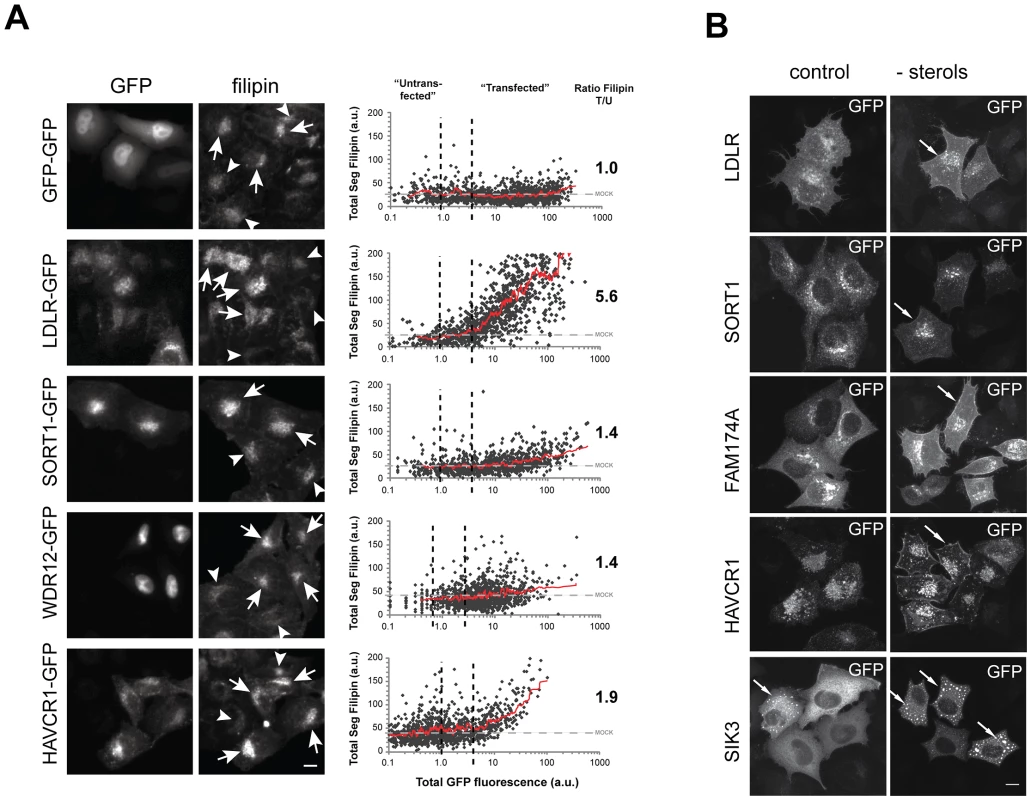
(A) cDNAs encoding for indicated candidate genes linked to GFP were transiently expressed in Hela-Kyoto cells and impact on cellular FC levels was analyzed (see Materials and Methods, Figure S6 and Table S8 for comprehensive datasets). Arrows denote “transfected”, arrowheads “untransfected” cells. See Materials and Methods for definition of threshholds (dashed lines in graphs).Graphs depict total segmental filipin signal plotted against total cellular intensities in the GFP-channel. Each dot reflects one individual cell, trend lines are given in red. Numbers indicate mean ratios of FC in GFP-positive relative to non-expressing cells within the identical dish (n = 3–4 experiments). (B) Maximal projections of confocal stacks showing representative GFP-cDNA expressing cells under control and sterol-depleted conditions (see Materials and Methods). Arrows denote cellular compartments with increased signals upon sterol-depletion. Bars = 10 µm. Tab. 2. Selected results of secondary assays.* 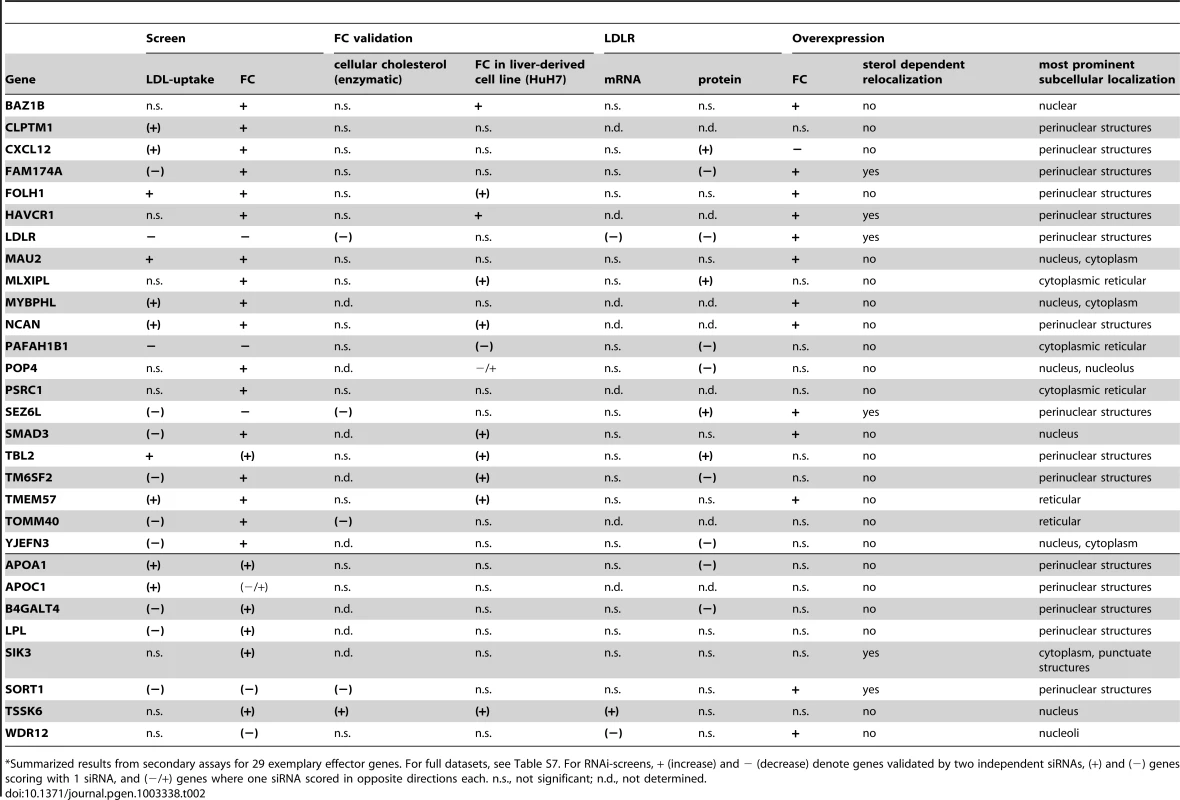
Summarized results from secondary assays for 29 exemplary effector genes. For full datasets, see Table S7. For RNAi-screens, + (increase) and − (decrease) denote genes validated by two independent siRNAs, (+) and (−) genes scoring with 1 siRNA, and (−/+) genes where one siRNA scored in opposite directions each. n.s., not significant; n.d., not determined. 29 promising effector genes were further followed up in systematic overexpression experiments. For this, GFP-tagged proteins were transiently transfected into Hela cells and cellular FC levels upon candidate gene overexpression were quantified (see Materials and Methods). Under our experimental settings, overexpression of 14 of the 29 candidates tested significantly increased or reduced FC levels (Figure 4A, Table 2, Table S8). For four candidates (CXCL12, SEZ6L, SORT1, WDR12) overexpression and knockdown shifted FC levels into opposite directions, thereby confirming levels of these genes as critical for maintenance of cellular cholesterol homeostasis. It is highly likely that future, more tailored studies will corroborate overexpression also of further candidates from our gene set as regulating cellular cholesterol that were here missed due to the screening format chosen. For instance, overexpression of CXCL12, a secretory cytokine highly associated with CAD/MI [41], significantly reduced FC (as opposed to increased FC and LDL-uptake in CXCL12 knockdown cells) in the whole cell population, but not individual GFP-expressing cells, indicating that CXCL12 might exert its cholesterol-modulating functions in trans, via being secreted (Table S8). Interestingly, candidates such as BAZ1B or HAVCR1 strongly increased FC in a concentration-dependent manner, similar to the effect obtained by knockdown of these genes. One explanation for these apparently contradictory results could be that such genes achieve their functions by interplay with other factors in known or yet unknown lipid-regulatory pathways. Under that scenario, knockdown of individual candidates would also affect the function of their interactors and thereby result in complex phenotypic readouts. Likewise, elevated levels of GFP-tagged versions of these genes could affect complex formation and function (e.g. due to the GFP-tagging of the proteins) and thus result in similar effects on cholesterol homeostasis as observed by their knockdown. Further work will be necessary to identify the cellular mechanisms to support these hypotheses. Confocal imaging revealed that many of the GFP-tagged proteins analyzed here localize to subcellular organelles of known relevance to cellular lipid homeostasis (Figure S6). Interestingly, however, several effectors were enriched in organelles without previous lipid-relevant functions, e.g. nucleoli (WDR12). Moreover, for six candidates (FAM174A, HAVCR1, LDLR, SEZ6L, SIK3, SORT1) we observed a re-localization to alternative organelles when cholesterol-levels were reduced (Figure 4B), which is a feature also of other crucial regulators of cellular cholesterol homeostasis [30], [41].
Taken together, our study demonstrates the potency of RNAi combined with systematic follow-up analyses to identify and profile functionally relevant effector genes within GWAS loci in an objective and unbiased manner. Several independent studies on individual candidate genes are well in line with some of the findings described here. For instance, it was recently shown that overexpression and knockdown of TBL2 inversely modulates cellular cholesterol in HEK293 and bladder cancer cells [42]. Likewise, SIK3-deficient mice have low levels of serum HDL and total cholesterol, but under a lipid-rich diet cholesterol accumulates in mouse livers [43]. Although systematic studies in mammalian cells have contributed significantly to our understanding of human lipid biology and disease [44], future work using e.g. suitable animal models will be necessary to test the in vivo roles in cholesterol metabolism of the candidate genes identified in the cell-based work here. As cell-based RNAi is scalable up to the whole genome [32], [45], its potential to complement genomic data with information on the functional significance of trait-associated candidate genes and sequence variants is considerable. Together with functional studies in animal models and more thorough approaches for cellular and biochemical profiling of candidates as we have initiated here, systematic cell-based functional analyses may thus emerge as a key technology for the detection of genes and pathways underlying a biological trait or disease process.
Materials and Methods
Cells and reagents
Hela-Kyoto cells are a strongly adherent Hela isolate (gift from S. Narumiya, Kyoto University, Japan) also used in previous siRNA-screens [30], [32]. HuH7 cells were obtained from the Japanese Collection of Research Bioresources. Filipin III (Sigma) was prepared as a 1 mg/ml stock-solution in di-methyl-formamide. DRAQ5 (Biostatus), ER-tracker Blue/White DPX (Molecular Probes), Fugene 6 (Roche). Lipofectamine 2000 (Invitrogen), 2-hydroxy-propyl-beta-cyclodextrin (HPCD) (Sigma), DiI-LDL (Inivtrogen) and Benzonase (Novagen) were purchased from the respective suppliers.
RNAi screening
SiRNA selection, production of transfected siRNA microarrays, and functional assays
56 of the 64 genomic loci that until 2009 were reported as associated with lipid traits and/or CAD/MI in 23 large-scale GWAS 1–7,9–23,30 were considered for RNAi-screening (Table S1). Eight published loci were omitted from analysis either due to the absence of annotated genes in the vicinity of the lead SNPs (2q36.3 [14], 4q12 [19], 9p24.3 [19], 15q14 [13]) or because of annotation discrepancies of published genomic positions relative to the current human reference genome GRCh37.p6, (Ensembl 66, February 2012) (1q25.2 [19], 1q43 [22], 9q31.3 [19], 20q13.12 [12]). Out of the 56 selected loci, 40 have since been replicated as highly associated (p<7×10−6) in at least a second independent GWAS (Table S1). For 36 loci association with more than one of the six traits analyzed (LDL-C, HDL-C, TC, TG, CAD, MI) was observed (Table S1, Table S2). Based on proximity to the lead SNP given in the literature, 106 candidate genes were selected from the 56 GWAS-loci for two primary RNAi-screens (“GWAS1”). For follow-up screens (“GWAS2”), 17 loci were complemented with 27 additional candidate genes so that for 38 loci all protein-coding genes within ±50 kbp of all reported lead-SNPs were covered. This resulted in a total of 133 genes analyzed in this study (Table S3).
Knockdown of each of the 133 candidate genes was performed in Hela-Kyoto cells cultured at 37°C/5%CO2 in DMEM/2 mM L-glutamine(Sigma)/10%FCS(PAA) and transfected with 21 nt SilencerSelect siRNAs from Applied Biosystems for 48 h. For GWAS1, each gene was silenced by three independent pre-designed siRNAs. For candidate genes that were re-analyzed in GWAS2 (n = 76), two siRNAs targeting the coding region were complemented by two additional siRNAs directed against the untranslated regions (UTR) of a respective target-mRNA (Table S4). SiRNAs against the UTR were designed using the BLOCK-iT RNAi Designer tool (https://rnaidesigner.invitrogen.com/rnaiexpress/). All siRNA sequences were mapped to the human reference genome GRCh37 (Ensembl 66, February 2012) using the in-house software tool bluegecko (J.K. Hériché, unpublished). This provided information on how many protein-coding transcripts per gene were targeted by an individual siRNA (Table S4) and allowed us to identify unspecific siRNAs that targeted also alternative human mRNAs, showed a mismatch to the reference sequence of the respective target gene or were directed against transcripts not anymore considered as protein coding. Based on these results, 24 siRNAs were thus excluded from further analysis (including all siRNAs targeting APOC2, SLC35G5 and LPAL2; Table S4).
Glass-bottomed chambered cell culture slides and 384-well plates coated with siRNAs for solid phase reverse-transfection of cells were produced as described [31], [46]. Of the 384 positions, 16 contained non-silencing control siRNA (s229174), three (GWAS1) or eight (GWAS2) positions, respectively, transfection mix without siRNA (mock), four positions INCENP-siRNA (to control for transfection efficiency [32]), eight positions siRNAs targeting LDLR (s237197 in GWAS1, s224006 in GWAS2) as a positive control for LDL-uptake, and eight positions siRNAs targeting NPC1 (s237198) as a positive control for free cholesterol (FC). Assays to monitor cellular LDL-uptake and FC as well as image acquisition were performed as described using an Olympus IX81 automated microscope and an UPlanSApo 10×/NA 0.40 objective [30]. FC was analyzed from 384-well plates in three (GWAS1) or four (GWAS2) biological replicas with four images per well. For this, 48 h before analysis 900 Hela-Kyoto cells/siRNA-coated 384-well were seeded using a cell seeder. HuH7-cells were seeded at a density of 500 cells/siRNA-coated 384-well and cultivated for 72 hrs before analysis. LDL-uptake was analyzed from cell arrays with ten (GWAS1) or eight (GWAS2) biological replicas by seeding 105 Hela-Kyoto cells per array 48 h before analysis.
Analysis of RNAi screens
All 21,494 images from GWAS1 and GWAS2 were visually quality controlled in order to exclude out of focus and otherwise not analyzable images (e.g. due to aberrant cell density or dust particles). This resulted in 20,078 images (93%) for further analysis comprising a total of ∼2.5×106 cells with a median of 835 cells per siRNA for LDL-uptake and of 2778 cells per siRNA for FC.
Automated image analysis was performed using the open source software Cellprofiler (http://www.cellprofiler.org [47]). In brief, areas of single cells were approximated by stepwise dilation of masks generated from images of DRAQ5-stained cell nuclei (for FC) or propagating the nuclear masks on the dpx (GWAS1) or DRAQ5 (GWAS2) channels (for LDL-uptake). Using a project-specific module (MorphoQuant), filipin (for FC) or DiI-signal (for LDL-uptake) was quantified from masks representing intracellular areas that were determined by local adaptive thresholding according to pre-defined parameters for size and shape (Figure S1). From each cell, three (for LDL-uptake) or four (for FC) parameters were quantified reflecting (i.) total signal intensity above local background within these masks/cell (“total”), (ii.) mean signal intensity above local background within these masks per cell (“concentration”), (iii.) number of masks per cell (“structures”) and (iv.) total area covered by masks per cell (“area”) for FC. Means were calculated from all cells per image and different images from the same biological replicate were averaged. Then, for each siRNA/biological replicate a “deviation value” was calculated (deviationreplicate,siRNA) by subtracting from the mean signal of a respective siRNA the mean signal of the 16 negative control siRNAs of the identical replica and division by twice the error of these controls (1,2). With this, the “deviation value” is calculated as a z-score, but instead of the standard deviation twice the absolute error was used, which takes into account variation in signal intensities. This allowed for normalization and taking into account the variation of negative controls. Deviation values of the negative controls were controlled for normal distribution by qqplot and Shapiro-Wilk test for each parameter. To antagonize plate effects on the 384-well plates, parameters for siRNAs localizing to wells at the edge of the plate (region 1), those neighboring these wells (region 2) and the rest of the plate (region 3) were normalized to controls within the respective region. To define the functional effect of a respective siRNA from 3 to 10 biological replicas, deviation values from all biological replicas were averaged (3).
SiRNAs were defined as effectors in an assay when the mean deviation value for a respective siRNA on the parameter “total signal intensity within masks/cell” (“total”) was above or below the mean of any of the negative controls (comprising 16 negative control siRNAs and three to nine mock transfection controls) ± 3-times the standard error of the mean (the standard deviation of all negative control values divided by the square root of the number of biological replicates). Identical criteria were applied to identify siRNAs exceeding thresholds (framed orange in Figures and colored in Table S4) for the other parameters analyzed. When treating the negative controls as candidate siRNAs, none of the up to 16 control siRNAs or three (GWAS1) to nine (GWAS2) mock controls passed these thresholds in none of the screens. A gene was considered a hit if these thresholds were exceeded by two independent siRNAs.To test for a possible interdependence of the parameters measured, pairwise Pearson's correlation values between the seven listed plus two additional parameters/assay (reflecting number and area of cells) were calculated across the entire dataset (comprising 557 independent siRNAs) (Table S5). For instance, in both assays the parameters reflecting “total” and “concentration” positively correlated. This is well in line with observations for epidermal-growth factor (EGF) and transferrin (TF) endocytosis [45] and corroborates that the uptake of LDL into cells underlies similar basic mechanisms as do alternative endocytic markers [38]. The parameter “total” did not strongly correlate with with number of Cells (nCells) or cell area (AreaCell), indicating its robustness to variation in cell density and size. Conversely, all seven parameters used for quantifications strongly correlated between GWAS1 and GWAS2-screens, reflecting a high reproducibility of individual results.
Secondary analyses
Quantification of cellular cholesterol
Filipin was recently reported to under certain conditions also bind to GM1 as well as further membrane lipids [48]. In order to assure specificity of filipin to accurately reflect free cholesterol under the experimental settings used here (Figure S1), SphingoStrips (Echelon Biosciences) and MembraneLipidStrips (Echelon Biosciences) representing 26 different lipids were washed for 5 min in PBS before staining for 30 min with 50 µg/ul filipin III in PBS. Then, strips were washed three times with PBS and illuminated with UV-light for readout. Cholesterol was the only lipid detected under these conditions, demonstrating the high specificity of filipin III. Moreover, addition of 1 µM GM1 for 15 min to the medium of Hela-Kyoto cells cultured in 6-well dishes prior to fixation doubled the levels of cell-associated GM1 (as detected by the GM1-stain cholera toxin AF-568), but did not affect total perinuclear or cell surface-associated filipin signals. Conversely, sterol-depletion by culture in DMEM/2 mM L-glutamine(Sigma)/0.5%BSA for 16 h followed by exposure for 45 min to 1% (w/v) HPCD [30] strongly reduced filipin intensities without changing cellular cholera toxin signals. These results suggested that a possible cross-reactivity of filipin with GM1 or other abundant membrane lipids is negligible under our experimental settings (Figure S1).
For enzymatic determination of cellular cholesterol, the Amplex Red Cholesterol Assay Kit (Life Technologies) was used according to manufacturer's instructions. Hela-Kyoto cells were seeded at a density of 3000 cells/well on 96-well plates (Nunc) coated with siRNAs as described above. 48 h post transfection, cells were washed three times with room-temperature PBS and 50 µl 1× reaction buffer was added (100 mM potassium phosphate, 50 mM NaCl, 50 mM cholic acid, 0.1% Triton-X-100), followed by 50 µl/well of Amplex Red solution (300 µM Amplex Red, 2 U/ml horse-radish peroxidase, 2 U/ml cholesterol oxidase in 1× reaction buffer). Upon 45 min at 37°C plates were analyzed in a Tecan2 plate reader at excitation wavelengths 535–555 nm and emission wavelengths 590–610 nm. SiRNAs were considered as effectors on cellular cholesterol if means from 3–6 experimental replicas deviated >2 standard deviations from the mean of negative controls (control siRNA and mock) and showed a p<0.05 in two two-sided Student's t-tests against both, control siRNA and mock transfection controls.
FC determination from HuH7-cells
FC levels from HuH7 liver cells were determined as described above. However, to accommodate for considerable morphological heterogeneity between the cells of one experiment, quantitation was limited to cells that were visually assessed as correctly segmented. Selection of 150–600 cells/siRNA from three independent biological replicates was assisted by a customized ImageJ plugin (C. Tischer, unpublished). SiRNAs were considered as effectors if ≥50 cells/experiment could be quantified, mean deviation values were outside a pre-defined confidence interval (mean ± 2-times the standard error of the mean of both negative controls) and a two-sided Student's t-test was p<0.05 in at least two biological replicas.
Analysis of LDL–receptor regulation
For analysis of LDLR mRNA and protein levels, Hela-Kyoto cells seeded at a density of 3·104 cells/well and cultured in DMEM/2 mM L-glutamine/10% FCS were reverse siRNA-transfected for 48 h on 96-well plates (Nunc). The mRNA was extracted and cDNAs obtained by using the Cells-to-CT kit (Applied Biosystems #4402955). QPCR was performed in triplicates from 3–4 biological replicates/siRNA with the following primers: LDLR fwd 5′-AGTGTGACCGGGAATATGACT-3′ rev 5′-CCGCTGTGACACTTGAACTT-3′; GAPDH fwd 5′-CATGAGAAGTATGACAACAGCCT-3′ rev 5′-AGTCCTTCCACGATACCAAAGT-3′; ACTB fwd 5′-CGCGAGAAGATGACCCAGAT-3′ rev 5′-TCACCGGAGTCCATCACGAT-3′. For each siRNA the fold change (2−ΔΔCT) of LDLR-mRNA was normalized to that of ACTB and GAPDH (Figure S5 and Table S6). SiRNAs were considered as differentially regulating LDLR-mRNA levels if levels deviated >2 standard deviations from the mean of control (untreated, mock) cells (n = 16; STDEVLDLR/GAPDH = 0.21; STDEVLDLR/ACTB = 0.20) and four two-sided Student's t-tests against both untreated and mock transfection controls of both housekeeping genes (ACTB, GAPDH) resulted in a p<0.01.
For Western Blot, siRNA-transfected cells were lysed in 40 µl SDS-loading buffer and subjected to immunoblotting with α-LDLR (Cayman Chemicals) and α-tubulin (Neomarkers) antibodies. Integrated density of bands was normalized to the average integrated density of bands from untreated and control siRNA treated samples on the identical SDS-gel and absolute LDLR protein signal as well as LDLR normalized to tubulin (LDLR/tub) was measured (Figure S6 and Table S6). SiRNAs were considered as differentially regulating LDLR-protein levels if levels deviated >2 standard deviations from the mean of control (untreated, control siRNA) cells (n = 34; STDEVLDLR = 0.13; STDEVLDLR/tub = 0.18) and four two-sided Student's t-tests against both untreated and control siRNA treated controls of both normalization controls (LDLR, LDLR/tub) resulted in a p<0.01.
GFP–cDNA overexpression and analysis
For 29 candidate genes, sequence-verified human cDNA-clones carboxy-terminally linked to EGFP were obtained (SourceBiosciences) (Table S8). Hela-Kyoto cells were seeded at a density of 8×105 onto glass coverslips in 12-well plates (Nunc), cultured in DMEM/2 mM L-glutamine/10% FCS for 16 h at 37°C/5% CO2, and fluid-phase transfected with 2 µg cDNA/well with Lipofectamine2000 (Invitrogen) according to manufacturer's instructions. 24 h post transfection cells were fixed and FC was analyzed as described above using a Cellprofiler pipeline together with a customized ImageJ plugin (C. Tischer, unpublished). Additionally, integrated densities from cell masks in the GFP-channel were recorded. For each construct, ∼500 cells were randomly selected from 3–4 replica experiments and non-GFP expressing (“untransfected”) cells determined as showing GFP-signal intensities <97% of mock transfected cells. Conversely, GFP-cDNA expressing (“transfected”) cells were defined as showing cellular GFP-signals >4-fold above the upper threshold of “untransfected” cells. For each cell, total segmental filipin intensities were determined relative to cellular GFP-levels (Figure 4, graphs). For each candidate gene and the ratio of filipin signal in “transfected” relative to mock-transfected, as well of “transfected” relative to “untransfected” cells in the same dish was calculated as means from all experimental replicas. Effects were considered as significant when a two-tailed Student's t-test resulted in p-values <0.01 in 2 (*) or 3–4 (***) experimental replicas (Table S8).
For determination of subcellular localizations (Figure S6), Hela-Kyoto cells were seeded at a density of 4×105 cells/well onto glass coverslips in 24-well plates (Nunc), cultured in DMEM/2 mM L-glutamine/10% FCS for 16 h at 37°C/5% CO2, and fluid-phase transfected with 1 µg cDNA/well Fugene 6 (Roche) or 2 µg cDNA/well Lipofectamine2000 (Invitrogen) according to manufacturer's instructions. For depletion of cellular sterols, medium was exchanged 12 h post transfection for DMEM/2 mM L-glutamine/0.5% lipoprotein-depleted serum (LDS). After 16 h, 1%(w/v) HPCD was added to cells for an additional 3 h as described [30]. Images were acquired on a Zeiss LSM780 confocal microscope using a 63×/NA 1.4 oil objective.
Supporting Information
Zdroje
1. AulchenkoYS, RipattiS, LindqvistI, BoomsmaD, HeidIM, et al. (2009) Loci influencing lipid levels and coronary heart disease risk in 16 European population cohorts. Nat Genet 41 : 47–55.
2. BurkhardtR, KennyEE, LoweJK, BirkelandA, JosowitzR, et al. (2008) Common SNPs in HMGCR in micronesians and whites associated with LDL-cholesterol levels affect alternative splicing of exon13. Arterioscler Thromb Vasc Biol 28 : 2078–2084.
3. ChasmanDI, PareG, MoraS, HopewellJC, PelosoG, et al. (2009) Forty-three loci associated with plasma lipoprotein size, concentration, and cholesterol content in genome-wide analysis. PLoS Genet 5: e1000730 doi:10.1371/journal.pgen.1000730..
4. ErdmannJ, GrosshennigA, BraundPS, KonigIR, HengstenbergC, et al. (2009) New susceptibility locus for coronary artery disease on chromosome 3q22.3. Nat Genet 41 : 280–282.
5. HelgadottirA, ThorleifssonG, ManolescuA, GretarsdottirS, BlondalT, et al. (2007) A common variant on chromosome 9p21 affects the risk of myocardial infarction. Science 316 : 1491–1493.
6. KathiresanS, MelanderO, GuiducciC, SurtiA, BurttNP, et al. (2008) Six new loci associated with blood low-density lipoprotein cholesterol, high-density lipoprotein cholesterol or triglycerides in humans. Nat Genet 40 : 189–197.
7. KathiresanS, WillerCJ, PelosoGM, DemissieS, MusunuruK, et al. (2009) Common variants at 30 loci contribute to polygenic dyslipidemia. Nat Genet 41 : 56–65.
8. KoonerJS, ChambersJC, Aguilar-SalinasCA, HindsDA, HydeCL, et al. (2008) Genome-wide scan identifies variation in MLXIPL associated with plasma triglycerides. Nat Genet 40 : 149–151.
9. McPhersonR, PertsemlidisA, KavaslarN, StewartA, RobertsR, et al. (2007) A common allele on chromosome 9 associated with coronary heart disease. Science 316 : 1488–1491.
10. KathiresanS, VoightBF, PurcellS, MusunuruK, ArdissinoD, et al. (2009) Genome-wide association of early-onset myocardial infarction with single nucleotide polymorphisms and copy number variants. Nat Genet 41 : 334–341.
11. PollinTI, DamcottCM, ShenH, OttSH, SheltonJ, et al. (2008) A null mutation in human APOC3 confers a favorable plasma lipid profile and apparent cardioprotection. Science 322 : 1702–1705.
12. RidkerPM, PareG, ParkerAN, ZeeRY, MiletichJP, et al. (2009) Polymorphism in the CETP gene region, HDL cholesterol, and risk of future myocardial infarction: Genomewide analysis among 18 245 initially healthy women from the Women's Genome Health Study. Circ Cardiovasc Genet 2 : 26–33.
13. SabattiC, ServiceSK, HartikainenAL, PoutaA, RipattiS, et al. (2009) Genome-wide association analysis of metabolic traits in a birth cohort from a founder population. Nat Genet 41 : 35–46.
14. SamaniNJ, ErdmannJ, HallAS, HengstenbergC, ManginoM, et al. (2007) Genomewide association analysis of coronary artery disease. N Engl J Med 357 : 443–453.
15. SandhuMS, WaterworthDM, DebenhamSL, WheelerE, PapadakisK, et al. (2008) LDL-cholesterol concentrations: a genome-wide association study. Lancet 371 : 483–491.
16. SchunkertH, KonigIR, KathiresanS, ReillyMP, AssimesTL, et al. (2011) Large-scale association analysis identifies 13 new susceptibility loci for coronary artery disease. Nat Genet 43 : 333–338.
17. TeslovichTM, MusunuruK, SmithAV, EdmondsonAC, StylianouIM, et al. (2010) Biological, clinical and population relevance of 95 loci for blood lipids. Nature 466 : 707–713.
18. TregouetDA, KonigIR, ErdmannJ, MunteanuA, BraundPS, et al. (2009) Genome-wide haplotype association study identifies the SLC22A3-LPAL2-LPA gene cluster as a risk locus for coronary artery disease. Nat Genet 41 : 283–285.
19. WallaceC, NewhouseSJ, BraundP, ZhangF, TobinM, et al. (2008) Genome-wide association study identifies genes for biomarkers of cardiovascular disease: serum urate and dyslipidemia. Am J Hum Genet 82 : 139–149.
20. WillerCJ, SannaS, JacksonAU, ScuteriA, BonnycastleLL, et al. (2008) Newly identified loci that influence lipid concentrations and risk of coronary artery disease. Nat Genet 40 : 161–169.
21. WaterworthDM, RickettsSL, SongK, ChenL, ZhaoJH, et al. (2010) Genetic variants influencing circulating lipid levels and risk of coronary artery disease. Arterioscler Thromb Vasc Biol 30 : 2264–2276.
22. The Wellcome Trust Case Control Consortium (2007) Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature 447 : 661–678.
23. SaxenaR, VoightBF, LyssenkoV, BurttNP, de BakkerPI, et al. (2007) Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science 316 : 1331–1336.
24. MusunuruK, StrongA, Frank-KamenetskyM, LeeNE, AhfeldtT, et al. (2010) From noncoding variant to phenotype via SORT1 at the 1p13 cholesterol locus. Nature 466 : 714–719.
25. ManolioTA (2010) Genomewide association studies and assessment of the risk of disease. N Engl J Med 363 : 166–176.
26. LanderES (2011) Initial impact of the sequencing of the human genome. Nature 470 : 187–197.
27. AltshulerD, DalyMJ, LanderES (2008) Genetic mapping in human disease. Science 322 : 881–888.
28. ManolioTA, CollinsFS, CoxNJ, GoldsteinDB, HindorffLA, et al. (2009) Finding the missing heritability of complex diseases. Nature 461 : 747–753.
29. MohrS, BakalC, PerrimonN (2010) Genomic screening with RNAi: results and challenges. Annu Rev Biochem 79 : 37–64.
30. BartzF, KernL, ErzD, ZhuM, GilbertD, et al. (2009) Identification of cholesterol-regulating genes by targeted RNAi screening. Cell Metab 10 : 63–75.
31. ErfleH, NeumannB, LiebelU, RogersP, HeldM, et al. (2007) Reverse transfection on cell arrays for high content screening microscopy. Nat Protoc 2 : 392–399.
32. NeumannB, WalterT, HericheJK, BulkescherJ, ErfleH, et al. (2010) Phenotypic profiling of the human genome by time-lapse microscopy reveals cell division genes. Nature 464 : 721–727.
33. MoreauD, KumarP, WangSC, ChaumetA, ChewSY, et al. (2011) Genome-Wide RNAi Screens Identify Genes Required for Ricin and PE Intoxications. Dev Cell 21 : 231–244.
34. BrassAL, DykxhoornDM, BenitaY, YanN, EngelmanA, et al. (2008) Identification of host proteins required for HIV infection through a functional genomic screen. Science 319 : 921–926.
35. ZhouH, XuM, HuangQ, GatesAT, ZhangXD, et al. (2008) Genome-scale RNAi screen for host factors required for HIV replication. Cell Host Microbe 4 : 495–504.
36. KonigR, ZhouY, EllederD, DiamondTL, BonamyGM, et al. (2008) Global analysis of host-pathogen interactions that regulate early-stage HIV-1 replication. Cell 135 : 49–60.
37. BechlerME, DoodyAM, HaKD, JudsonBL, ChenI, et al. (2011) The phospholipase A2 enzyme complex PAFAH Ib mediates endosomal membrane tubule formation and trafficking. Mol Biol Cell 22 : 2348–2359.
38. GoldsteinJL, BrownMS (2009) The LDL receptor. Arterioscler Thromb Vasc Biol 29 : 431–438.
39. GoldsteinJL, DeBose-BoydRA, BrownMS (2006) Protein sensors for membrane sterols. Cell 124 : 35–46.
40. MoonYA, LiangG, XieX, Frank-KamenetskyM, FitzgeraldK, et al. (2012) The Scap/SREBP pathway is essential for developing diabetic fatty liver and carbohydrate-induced hypertriglyceridemia in animals. Cell Metab 15 : 240–246.
41. MehtaN, LiM, WilliamD, KheraA, DerOhannessianS, et al. (2011) The novel atherosclerosis locus at 10q11 regulates plasma CXCL12 levels. European heart journal 32 : 963–971.
42. FredericksWJ, McGarveyT, WangH, LalP, PuthiyaveettilR, et al. (2011) The bladder tumor suppressor protein TERE1 (UBIAD1) modulates cell cholesterol: implications for tumor progression. DNA Cell Biol 30 : 851–864.
43. UebiT, ItohY, HatanoO, KumagaiA, SanosakaM, et al. (2012) Involvement of SIK3 in Glucose and Lipid Homeostasis in Mice. PLoS ONE 7: e37803 doi:10.1371/journal.pone.0037803..
44. GoldsteinJL, RawsonRB, BrownMS (2002) Mutant mammalian cells as tools to delineate the sterol regulatory element-binding protein pathway for feedback regulation of lipid synthesis. Archives of biochemistry and biophysics 397 : 139–148.
45. CollinetC, StoterM, BradshawCR, SamusikN, RinkJC, et al. (2010) Systems survey of endocytosis by multiparametric image analysis. Nature 464 : 243–249.
46. ErfleH, NeumannB, RogersP, BulkescherJ, EllenbergJ, et al. (2008) Work flow for multiplexing siRNA assays by solid-phase reverse transfection in multiwell plates. J Biomol Screen 13 : 575–580.
47. CarpenterAE, JonesTR, LamprechtMR, ClarkeC, KangIH, et al. (2006) CellProfiler: image analysis software for identifying and quantifying cell phenotypes. Genome Biol 7: R100.
48. ArthurJR, HeineckeKA, SeyfriedTN (2011) Filipin recognizes both GM1 and cholesterol in GM1 gangliosidosis mouse brain. J Lipid Res 52 : 1345–1351.
49. SimpsonJC, JoggerstB, LaketaV, VerissimoF, CetinC, et al. (2012) Genome-wide RNAi screening identifies human proteins with a regulatory function in the early secretory pathway. Nature cell biology 14 : 764–774.
Štítky
Genetika Reprodukční medicína
Článek MSH3 Polymorphisms and Protein Levels Affect CAG Repeat Instability in Huntington's Disease MiceČlánek Yemanuclein and HIRA Cooperate for Assembly of H3.3-Containing Nucleosomes in the Male Pronucleus
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2013 Číslo 2
-
Všechny články tohoto čísla
- Complex Inheritance of Melanoma and Pigmentation of Coat and Skin in Grey Horses
- A Meta-Analysis of Thyroid-Related Traits Reveals Novel Loci and Gender-Specific Differences in the Regulation of Thyroid Function
- Genetic Landscape of Open Chromatin in Yeast
- Deleterious Alleles in the Human Genome Are on Average Younger Than Neutral Alleles of the Same Frequency
- Age-Dependent Transition from Cell-Level to Population-Level Control in Murine Intestinal Homeostasis Revealed by Coalescence Analysis
- Next-Generation Sequencing Identifies the Danforth's Short Tail Mouse Mutation as a Retrotransposon Insertion Affecting Expression
- ImmunoChip Study Implicates Antigen Presentation to T Cells in Narcolepsy
- Massive Mitochondrial Gene Transfer in a Parasitic Flowering Plant Clade
- Comment on “Genomic Hypomethylation in the Human Germline Associates with Selective Structural Mutability in the Human Genome”
- The Prefoldin Bud27 Mediates the Assembly of the Eukaryotic RNA Polymerases in an Rpb5-Dependent Manner
- Genetic Determinants of Trabecular and Cortical Volumetric Bone Mineral Densities and Bone Microstructure
- Encodes a Novel and -Genus-Specific Regulator of Photoperiodic Flowering in Rice
- Only One Isoform of CTP Synthase Forms the Cytoophidium
- Mechanisms Involved in the Functional Divergence of Duplicated GroEL Chaperonins in DK1622
- A Genome-Wide RNAi Screen in Identifies the Nicotinic Acetylcholine Receptor Subunit ACR-7 as an Antipsychotic Drug Target
- Autophagy Induction Is a Tor- and Tp53-Independent Cell Survival Response in a Zebrafish Model of Disrupted Ribosome Biogenesis
- Ancient DNA Reveals Prehistoric Gene-Flow from Siberia in the Complex Human Population History of North East Europe
- Inflammation-Mediated Genetic and Epigenetic Alterations Drive Cancer Development in the Neighboring Epithelium upon Stromal Abrogation of TGF-β Signaling
- MicroRNA-3148 Modulates Allelic Expression of Toll-Like Receptor 7 Variant Associated with Systemic Lupus Erythematosus
- RNAi–Based Functional Profiling of Loci from Blood Lipid Genome-Wide Association Studies Identifies Genes with Cholesterol-Regulatory Function
- CELF Family RNA–Binding Protein UNC-75 Regulates Two Sets of Mutually Exclusive Exons of the Gene in Neuron-Specific Manners in
- Coordination of Chromatid Separation and Spindle Elongation by Antagonistic Activities of Mitotic and S-Phase CDKs
- The Ubiquitin Ligase Subunit Acts in Target Tissue to Restrict Tracheal Terminal Cell Branching and Hypoxic-Induced Gene Expression
- Mitotic Evolution of Shows a Stable Core Genome but Recombination in Antigen Families
- Tysnd1 Deficiency in Mice Interferes with the Peroxisomal Localization of PTS2 Enzymes, Causing Lipid Metabolic Abnormalities and Male Infertility
- A Regulatory Pathway, Ecdysone-Transcription Factor Relish-Cathepsin L, Is Involved in Insect Fat Body Dissociation
- PcG-Mediated Higher-Order Chromatin Structures Modulate Replication Programs at the BX-C
- MSH3 Polymorphisms and Protein Levels Affect CAG Repeat Instability in Huntington's Disease Mice
- JNK-Interacting Protein 3 Mediates the Retrograde Transport of Activated c-Jun N-Terminal Kinase and Lysosomes
- Discovery of a Splicing Regulator Required for Cell Cycle Progression
- Rearrangements of 2.5 Kilobases of Noncoding DNA from the Locus Define Predictive Rules of Genomic -Regulatory Logic
- Admixture Mapping in Lupus Identifies Multiple Functional Variants within IFIH1 Associated with Apoptosis, Inflammation, and Autoantibody Production
- Roles of the Developmental Regulator Homothorax in Limiting Longevity in
- miR-199a-5p Is Upregulated during Fibrogenic Response to Tissue Injury and Mediates TGFbeta-Induced Lung Fibroblast Activation by Targeting Caveolin-1
- A Kinome-Wide RNAi Screen in Glia Reveals That the RIO Kinases Mediate Cell Proliferation and Survival through TORC2-Akt Signaling in Glioblastoma
- Assembly of the Auditory Circuitry by a Genetic Network in the Mouse Brainstem
- SOX2 Co-Occupies Distal Enhancer Elements with Distinct POU Factors in ESCs and NPCs to Specify Cell State
- Retrotransposon Activates Ectopic Expression: A Short Tail
- Confounding by Repetitive Elements and CpG Islands Does Not Explain the Association between Hypomethylation and Genomic Instability
- Cell Reprogramming Requires Silencing of a Core Subset of Polycomb Targets
- Properties and Modeling of GWAS when Complex Disease Risk Is Due to Non-Complementing, Deleterious Mutations in Genes of Large Effect
- Essential Developmental, Genomic Stability, and Tumour Suppressor Functions of the Mouse Orthologue of
- Conditional Inactivation of the DNA Damage Response Gene in Mouse Testis Reveals Separable Roles for Components of the RAD9-RAD1-HUS1 Complex in Meiotic Chromosome Maintenance
- Genome-Wide Analysis Points to Roles for Extracellular Matrix Remodeling, the Visual Cycle, and Neuronal Development in Myopia
- Patterning of Leaf Vein Networks by Convergent Auxin Transport Pathways
- An Evolutionary Perspective on Epistasis and the Missing Heritability
- A Retrotransposon Insertion in the 5′ Regulatory Domain of Ptf1a Results in Ectopic Gene Expression and Multiple Congenital Defects in Danforth's Short Tail Mouse
- The Mub1/Ubr2 Ubiquitin Ligase Complex Regulates the Conserved Dsn1 Kinetochore Protein
- Mutations Can Cause Enamel-Renal Syndrome (ERS)
- Yemanuclein and HIRA Cooperate for Assembly of H3.3-Containing Nucleosomes in the Male Pronucleus
- Hepatocyte Growth Factor, a Determinant of Airspace Homeostasis in the Murine Lung
- ISWI and CHD Chromatin Remodelers Bind Promoters but Act in Gene Bodies
- COM-1 Promotes Homologous Recombination during Meiosis by Antagonizing Ku-Mediated Non-Homologous End Joining
- Control of Multicellular Development by the Physically Interacting Deneddylases DEN1/DenA and COP9 Signalosome
- Antagonism Versus Cooperativity with TALE Cofactors at the Base of the Functional Diversification of Hox Protein Function
- Dynamic Association of NUP98 with the Human Genome
- Ectopic Expression of Induces Spinal Defects, Urogenital Defects, and Anorectal Malformations in Mice
- Regulation of Contributes to the Lineage Potential of Neurogenin3+ Endocrine Precursor Cells in the Pancreas
- Gene-Based Testing of Interactions in Association Studies of Quantitative Traits
- The Amidation Step of Diphthamide Biosynthesis in Yeast Requires , a Gene Identified through Mining the - Interaction Network
- Plant-Symbiotic Fungi as Chemical Engineers: Multi-Genome Analysis of the Clavicipitaceae Reveals Dynamics of Alkaloid Loci
- Genome-Wide Diversity in the Levant Reveals Recent Structuring by Culture
- DNA Methylation Mediated Control of Gene Expression Is Critical for Development of Crown Gall Tumors
- Identification of the SlmA Active Site Responsible for Blocking Bacterial Cytokinetic Ring Assembly over the Chromosome
- Expression of a Novel P22 ORFan Gene Reveals the Phage Carrier State in Typhimurium
- Altered Cohesin Gene Dosage Affects Mammalian Meiotic Chromosome Structure and Behavior
- Quantitative Analysis of Histone Modifications: Formaldehyde Is a Source of Pathological N-Formyllysine That Is Refractory to Histone Deacetylases
- Duplicate Abalone Egg Coat Proteins Bind Sperm Lysin Similarly, but Evolve Oppositely, Consistent with Molecular Mimicry at Fertilization
- Lessons from on the Strengths and Weaknesses of Structured Association Mapping
- DNA–Methylome Analysis of Mouse Intestinal Adenoma Identifies a Tumour-Specific Signature That Is Partly Conserved in Human Colon Cancer
- Transposon Variants and Their Effects on Gene Expression in
- Polygenic Modeling with Bayesian Sparse Linear Mixed Models
- Single Transmembrane Peptide DinQ Modulates Membrane-Dependent Activities
- The JNK Signaling Pathway Activates Expression of Stress Response Genes by Derepressing the Fos/HDAC Repressor Complex
- The Interaction of CtIP and Nbs1 Connects CDK and ATM to Regulate HR–Mediated Double-Strand Break Repair
- Regulation of Metamorphosis by Xenobiotic Response Regulators
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Complex Inheritance of Melanoma and Pigmentation of Coat and Skin in Grey Horses
- Coordination of Chromatid Separation and Spindle Elongation by Antagonistic Activities of Mitotic and S-Phase CDKs
- Autophagy Induction Is a Tor- and Tp53-Independent Cell Survival Response in a Zebrafish Model of Disrupted Ribosome Biogenesis
- Assembly of the Auditory Circuitry by a Genetic Network in the Mouse Brainstem
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání






