-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaDNA–Methylome Analysis of Mouse Intestinal Adenoma Identifies a Tumour-Specific Signature That Is Partly Conserved in Human Colon Cancer
Aberrant CpG methylation is a universal epigenetic trait of cancer cell genomes. However, human cancer samples or cell lines preclude the investigation of epigenetic changes occurring early during tumour development. Here, we have used MeDIP-seq to analyse the DNA methylome of APCMin adenoma as a model for intestinal cancer initiation, and we present a list of more than 13,000 recurring differentially methylated regions (DMRs) characterizing intestinal adenoma of the mouse. We show that Polycomb Repressive Complex (PRC) targets are strongly enriched among hypermethylated DMRs, and several PRC2 components and DNA methyltransferases were up-regulated in adenoma. We further demonstrate by bisulfite pyrosequencing of purified cell populations that the DMR signature arises de novo in adenoma cells rather than by expansion of a pre-existing pattern in intestinal stem cells or undifferentiated crypt cells. We found that epigenetic silencing of tumour suppressors, which occurs frequently in colon cancer, was rare in adenoma. Quite strikingly, we identified a core set of DMRs, which is conserved between mouse adenoma and human colon cancer, thus possibly revealing a global panel of epigenetically modified genes for intestinal tumours. Our data allow a distinction between early conserved epigenetic alterations occurring in intestinal adenoma and late stochastic events promoting colon cancer progression, and may facilitate the selection of more specific clinical epigenetic biomarkers.
Published in the journal: . PLoS Genet 9(2): e32767. doi:10.1371/journal.pgen.1003250
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003250Summary
Aberrant CpG methylation is a universal epigenetic trait of cancer cell genomes. However, human cancer samples or cell lines preclude the investigation of epigenetic changes occurring early during tumour development. Here, we have used MeDIP-seq to analyse the DNA methylome of APCMin adenoma as a model for intestinal cancer initiation, and we present a list of more than 13,000 recurring differentially methylated regions (DMRs) characterizing intestinal adenoma of the mouse. We show that Polycomb Repressive Complex (PRC) targets are strongly enriched among hypermethylated DMRs, and several PRC2 components and DNA methyltransferases were up-regulated in adenoma. We further demonstrate by bisulfite pyrosequencing of purified cell populations that the DMR signature arises de novo in adenoma cells rather than by expansion of a pre-existing pattern in intestinal stem cells or undifferentiated crypt cells. We found that epigenetic silencing of tumour suppressors, which occurs frequently in colon cancer, was rare in adenoma. Quite strikingly, we identified a core set of DMRs, which is conserved between mouse adenoma and human colon cancer, thus possibly revealing a global panel of epigenetically modified genes for intestinal tumours. Our data allow a distinction between early conserved epigenetic alterations occurring in intestinal adenoma and late stochastic events promoting colon cancer progression, and may facilitate the selection of more specific clinical epigenetic biomarkers.
Introduction
Epigenetic mechanisms play critical roles in controlling the cellular transcript repertoire and ultimately cellular phenotypes. The methylation of cytosine bases of CpG dinucleotides, as well as the covalent modification of histone proteins represent major target sites of the protein complexes involved in epigenetic control. Epigenetic histone and DNA codes are established and interpreted in a combinatorial fashion, and both interact in the short-term regulation of gene activity and in the establishment of long-term epigenetic memory, such as heterochromatin formation and gene silencing [1], [2]. The Polycomb Repressive Complexes (PRC1 and PRC2) and Trithorax Group Complexes (TrxG) are major histone modifying protein complexes setting repressive or activating marks, respectively, while CpG methylation patterns are set and propagated by DNA methlytransferases (DNMTs). A major role of the PRC2 complex in development is to tag gene regulatory sequences with a specific tri-methyl mark on lysine 27 of histone 3 (H3K27me3), resulting in short-term transcriptional repression [3]. Moreover, PRC2 complexes can also interact with DNMTs, and initiate long-term silencing of genes via de-novo CpG methylation [4]. By default, non-transformed cells are characterized by high genome-wide CpG methylation, with the exception of CpG islands (CGIs), which are mostly unmethylated [1], [2], [5].
Tumour cells contain aberrant epigenomes [4], [6]–[12]. Tumours are characterized by general genomic hypomethylation of CpGs, while CGIs are hypermethylated [13]. It has been found that histone modification patterns of tumour cells resemble those found in embryonic stem cells and likely guide CpG methylation [14]–[18]. This epigenetic pattern of tumour cells has been associated with uncontrolled PRC2 activity [6], [15], [19]–[21]. As a consequence of the deregulation of epigenetic control mechanisms, many types of cancer display epigenetic silencing of tumour suppressor genes, which interact with genetic mutations in establishing the tumour phenotype.
Intestinal tumours of humans and mice usually initiate via hyperactivation of Wnt/beta-catenin signalling, which is often caused by genetic loss of the tumour suppressor APC [22], [23]. Additional genetic and epigenetic alterations are involved in the gradual loss of tissue homeostasis during tumour progression, among them epigenetic silencing of the tumour suppressor Cdkn2a (coding for a cell cycle and apoptosis control gene), Dkk1 and Sfrp family genes (coding for Wnt antagonists) or Hic1 (coding for a developmental control gene) [24], [25]. How and when colon cancer-specific methylation patterns form is largely unknown. This is in contrast to genetic mutations, of which many have been linked to specific stages of tumour progression [22].
Here we have utilized the APCMin mouse model to characterize early steps of epigenetic modifications in intestinal cancer. APCMin mice form multiple intestinal adenomas following the somatic loss of functional APC, similar to the initiation of a large majority of sporadic human colon cancers and to familial colon cancer syndromes [22], [23]. We report a comprehensive catalogue of differentially methylated regions (DMRs), which form de novo and consistently in APCMin adenoma. Hypermethylated DMRs were found prevalently at sites of PRC2 activity, but silencing of tumour suppressors was rarely observed in adenoma. The comparison to human colon cancer samples identified a core epigenetic map of intestinal cancer DMRs, which is conserved between mice and humans.
Results
MeDIP-seq analysis of APCMin adenomas reveals a large number of DMRs
To investigate DNA methylation patterns that occur shortly after intestinal tumour initiation in the mouse, we compared normal and adenomatous tissues of wildtype C57BL/6J (B6) and isogenic APCMin mice by immunoprecipitation of methylated DNA followed by massively parallel sequencing (MeDIP-seq) [6], [7], [26]. Three intestinal samples of normal B6 mice, as well as three normal intestinal and five adenoma samples of B6-APCMin mice were subjected to MeDIP-seq to generate a total of 5.6×108 36mer reads, which were mapped to the mouse genome [27] (approximately 2×107 uniquely mapped reads per sample; see Figure 1a for summary of samples, and Table S1 for read generation and mapping statistics). To test for efficacy of the immunoprecipitation, we analysed a highly methylated and a CpG-free control region by quantitative PCR (Xist, Csa; [6]), and found a median enrichment of 72-fold (Figure S1). Pairwise Pearson's correlations revealed a high overall similarity between the MeDIP-seq samples (r = 0.88 to 0.93; Table S2).
Fig. 1. Generation and validation of genome-wide CpG methylation maps of APCMin mouse normal and adenoma tissues. 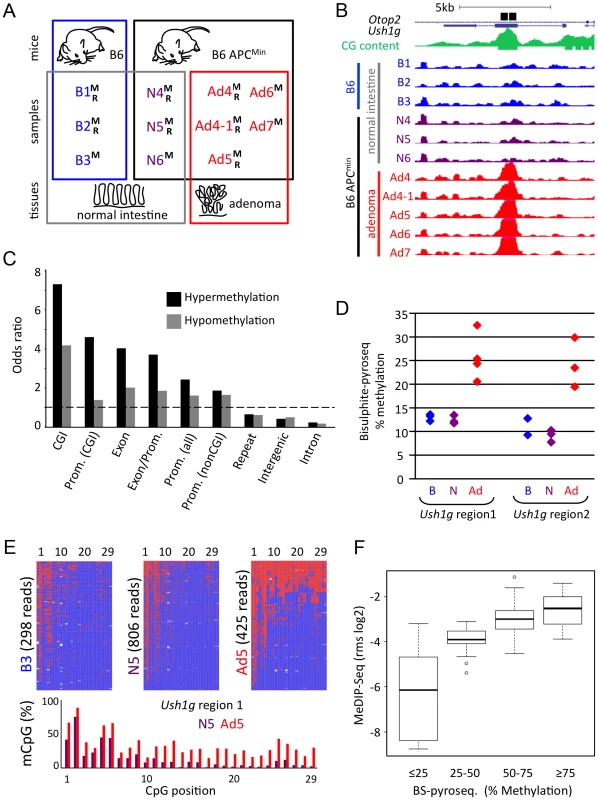
a) Summary of tissue samples used for genome-wide analyses. B6 wildtype (B) and isogenic B6-APCMin (APCMin)mice were employed for MeDIP-seq (M) and RNA-seq (R) of normal intestinal tissue (B, N) and intestinal adenoma (Ad). b) Visualisation of the adenoma-hypermethylated DMR in Ush1g, using the UCSC browser. Maximal height for visualization was set to rpm = 2 for all MeDIP-seq tracks. Black bars, regions that were validated by SIRPH or bisulfite-pyrosequencing (see below, d, e); green, CpG density; blue, purple, red: MeDIP-seq tracks of B6 mouse normal intestine, APCMin mouse normal intestine, and APCMin adenoma, respectively. Mice/samples are numbered consecutively. c) Distribution of DMRs in different subgenomic compartments. Odds ratios (i.e. fraction of experimentally observed DMRs divided by relative size of subgenomic compartment) of hyper- and hypomethylation within CpG islands (CGI), promoters that contain or do not contain CGIs, promoter-to-exon junctions, exons, introns, intergenic and repeat regions are given. Dashed line demarcates over- versus underrepresentation. d)–f) Validation of genome-wide MeDIP-seq data, using bisulfite pyrosequencing methodology d) Validation of DMR within Ush1g by bisulfite pyrosequencing using two samples that were subjected to MeDIP-seq and nine additional samples. Percent Methylation of all CpGs across the complete regions is given, colour code as in b). e) High-resolution graphical reconstruction of bisulfite pyrosequencing results for Ush1g DMR region 1, samples B3, N5, Ad5. Red: Methylated; blue: Unmethylated CpG f) Comparison of MeDIP-seq and bisulfite pyrosequencing data, as shown in Table S4c. y-axis represents MeDIP-seq derived and MEDIPS normalized rms-values (log2 scale) for cross-validated genomic regions from three samples (one sample each B6, APCMin normal, APCMin adenoma). Box plots depict MeDIP rms values for different methylation classes, as defined by bisulfite pyrosequencing. It is of note that MeDIP-seq procedures cannot detect DMRs with constant reliability over the complete genome, and may under-represent repetitive regions and regions with low CpG density. To identify focal methylation differences between normal tissue and adenoma, we calculated read density in overlapping 500 bp windows over the complete genome, and compared the six normal with the five adenoma samples (Wilcoxon P<0.01, mean signal in at least one group >0.25 mapped reads per million, ratio between normal and adenoma >1.33). We identified 13,980 DMRs, of which 5,135 (37%) were hypermethylated and 8,845 (63%) hypomethylated in adenoma (see Figure 1b for a characteristic hypermethylated DMR within Ush1g; Table 1 for DMR summaries; Table S3 for a full list of DMRs).
Tab. 1. Numbers of DMRs identified by MeDIP-seq, and assignment to subgenomic regions. 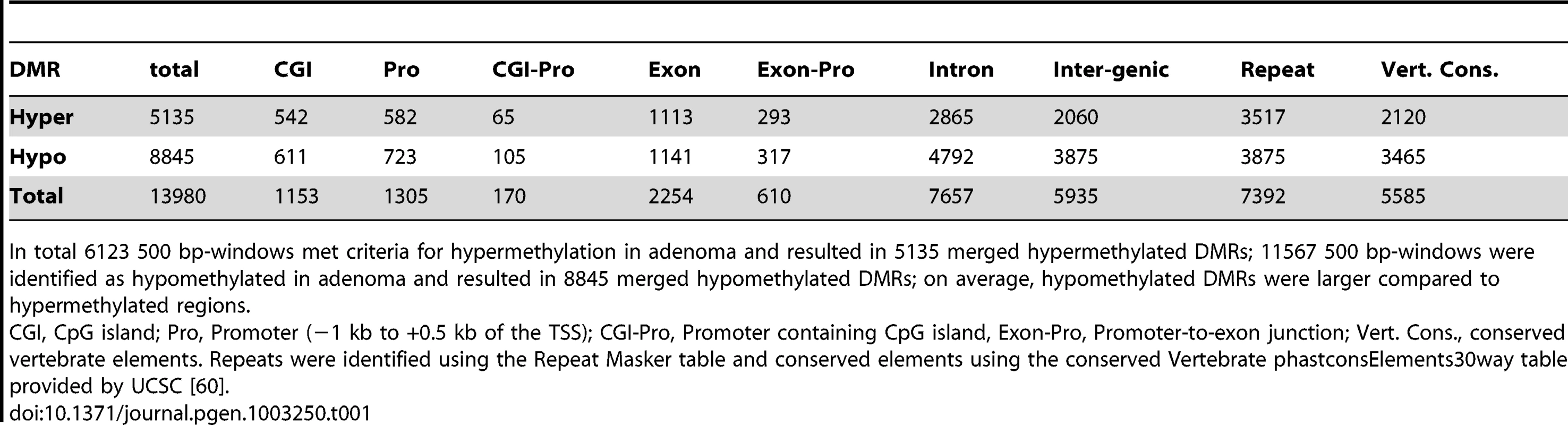
In total 6123 500 bp-windows met criteria for hypermethylation in adenoma and resulted in 5135 merged hypermethylated DMRs; 11567 500 bp-windows were identified as hypomethylated in adenoma and resulted in 8845 merged hypomethylated DMRs; on average, hypomethylated DMRs were larger compared to hypermethylated regions. Using odds ratio calculation, we found that methylation changes were unevenly distributed over the genome. Both hyper - and hypomethylated DMRs were found highly enriched in CpG islands, promoter and exon regions. In contrast, lower frequencies of DMRs were observed in repetitive, intergenic and intronic regions (Figure 1c). In particular, we did not observe general hypomethylation in Line-1 and IAP-repeats, which is in contrast to advanced human colon carcinomas that frequently show defects in the maintenance of repeat methylation [11], [28], [29] (Table S4a). It is however of note that the highest absolute numbers of DMR windows were detected in repetitive, intergenic and intronic regions, which make up the largest fraction of the genome (Table 1, Figure S2). Methylation patterns present at DMRs clearly separated normal intestine from adenoma in hierarchical cluster analyses (Figure S3)
We used two bisulfite-based approaches to validate our genome-wide MeDIP data. Methylation-specific single-nucleotide primer extension followed by HPLC separation (SIRPH, [30]) on seven samples previously used in MeDIP-seq confirmed 24 of 24 tested DMRs, and was consistent with MeDIP-seq data (Median Pearson correlation of r = 0.86; Table S4b). Massively parallel bisulfite pyrosequencing provided high-resolution profiles of individual regions and validated 21 of 21 DMRs on three samples used in the genome-wide study (Pearson correlations of the log2 ratios r = 0.8 to 0.9; Figure 1d–1f, Table S4c, Figure 1d, 1e). Additional bisulfite analysis of nine independent samples that were not used for MeDIP-seq analysis confirmed 18 of the 21 DMRs (Table S4a, cut-off P<0.05; FC>1.33), while three regions displayed variations between individual mice.
We were able to exclude gross copy number changes, which might have occurred in adenoma, by low-coverage genomic DNA sequencing of two normal intestinal samples and one adenoma (Table S1). Overall, these multiple tests validate our genome-wide DMR maps for intestinal adenoma.
DNA hypermethylation in adenomas prevails at Polycomb target genes
It is well established that DNA methylation of gene control regions is accompanied by histone modification. Since we found an enrichment of DMRs in promoters and gene bodies, we examined if adenoma methylation patterns correlated with known Polycomb Repressive Complex (PRC), Trithorax Group (TrxG/MLL) or TET1 binding sites [31]–[34]. Using Gene Set Enrichment Analysis (GSEA [35]), we found that genes previously identified as PRC1/2 targets in mouse embryonic stem cells were enriched among the genes whose promoters were hypermethylated in adenoma (Figure 2a, see Table S5 for gene-centric MeDIP-seq data, and Table S6 for signature genes). A similar correlation was found with mouse orthologues of PRC2 target genes identified in human ES cells, or with target genes of the PRC2 component EED. When we evaluated target genes of TrxG/MLL, no significant correlation with adenoma-specific hypo - or hypermethylation was found (Figure 2a). TET1 complexes have previously been implicated in DNA hypomethylation in tumours [17], however we found no correlation with the occurrence of hypomethylation of promoters in adenoma. These analyses indicate that PRC2 target genes are preferred targets of DNA hypermethylation in mouse intestinal adenoma.
Fig. 2. Hypermethylated DMRs are associated with Polycomb targets. 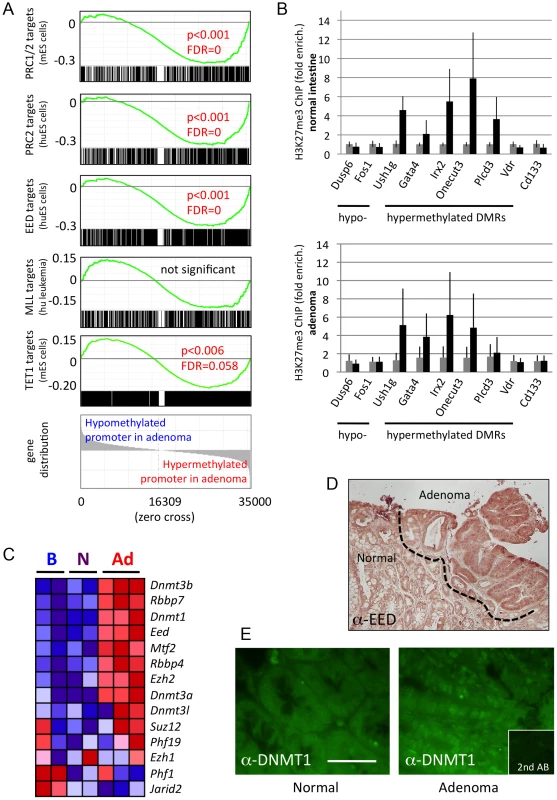
a) Gene Set Enrichment Analysis (GSEA) [35] is used to probe established epigenetic signatures. Mouse genes were ordered by normal (6 samples) versus adenoma (5 samples) promoter methylation (−1,0 to +0,5 kb). Gene signatures comprising PRC1/2 target genes or mouse homologues of human targets of PRC2 complexes, EED targets, MLL targets or TET1 targets were mapped onto the ordered list, and enrichment at the extremes (hypo- or hypermethylation) was assessed. PRC and EED targets were found strongly enriched among hypermethylated promoters, while no enrichment was detected for MLL targets. TET1 targets were found weakly enriched among hypermethylated promoters, probably due to their known association with PRC complexes, and prevalence at CpG-rich sites [34]. Enrichment score graphs (top, green), signature gene distributions (black line graphs, below ES curves), p-values and false discovery rates (FDRs) are given. Significance cut-offs were P<0.05, FDR<0.25. b) Analysis of H3K27me3 marks in chromatin of mouse intestinal epithelium (n = 4 biological replicates) and adenoma (n = 3), using chromatin immunoprecipitation, followed by qPCR. black bars: Immunoprecipitated chromatin, grey: Input chromatin. Error bars give standard deviation. c) Expression of genes coding for PRC2 components or DNA methyl transferases, as determined by RNA-seq. Gene expression is colour-coded: red, high relative expression; blue, low relative expression. d) Immunohistochemical staining of EED in mouse intestine and adenoma. Dotted line demarcates normal intestinal tissue from adenoma. Adenoma contains higher levels of cytoplasmic and nuclear EED protein. e) Immunofluorescence analysis of DNMT1 in a section of normal intestine and adjacent adenoma of the mouse. Adenoma displays distinct nuclear fluorescence for DNMT1. Scale bar is 50 µm. To test directly if hypermethylated DMRs found in adenoma are enriched for the H3K27me3 histone mark set by PRC2, we examined several DMRs in chromatin derived from mouse intestine, by chromatin immunoprecipitation, followed by qPCR. We found that five out of six adenoma-hypermethylated DMRs (associated with the Ush1g, Gata4, Irx2, Onecut3 and Plcd3 genes, but not the one associated with Vdr) displayed an increased H3K27me3 mark in normal intestine and, to a large extent, also in adenoma, and hence were associated with PRC2 activity in the intestine (Figure 2b, and Figure S4). In contrast, two hypomethylated DMRs (Dusp6 and Fos), as well as the stem cell marker gene Cd133 were not enriched for the H3K27me3 mark.
Next we assessed the expression of genes coding for Polycomb complex components and DNA methyltransferases. We found up-regulation of the maintenance DNA methyltransferase gene Dnmt1, the de-novo methyltransferase genes Dnmt3a and Dnmt3b, and of several PRC2 component genes in adenoma compared to normal intestinal tissue (Figure 2c, see also Table S7 for additional gene sets related to epigenetic modification). In addition, immunohistochemical analysis on sections of normal and adenoma tissue found up-regulation of EED and DNMT1 protein in adenoma (Figure 2d, 2e). Taken together, our data suggest a functional correlation between increased DNA methyltransferase and PRC2 expression and CpG hypermethylation in adenoma.
Methylation patterns of adenoma form de novo after cellular transformation
The intestinal epithelium contains proliferative and differentiated cells in different domains (crypts and villi, respectively), and mouse intestinal adenoma is known to originate from intestinal stem cells (ISC) of the crypt ([36], see Figure 3a for a schematic representation of intestinal cell types). ISCs make up only a small fraction of the total tissue. Therefore we wanted to exclude that the CpG methylation signatures of proliferative or stem cells, which might have been masked by prevalent patterns of differentiated cells in normal tissue, match the signatures of adenoma. We obtained tissue samples enriched for villus or crypt cells, and in addition, purified intestinal stem cells by FACS from transgenic mice expressing the fluorescent stem cell-specific Lgr5 reporter [37]. The villus, crypt and ISC preparations were subjected to bisulfite pyrosequencing of eleven specific DMRs, among them Dusp6 and Fos (containing adenoma-hypomethylated DMRs), as well as Ush1g and Vdr (containing adenoma-hypermethylated DMRs). We found minimal methylation differences between stem cells, crypt (proliferating cells) and villus (differentiated cells) at all DMRs examined, and methylation levels in all three types of cell preparations by and large matched those observed in bulk normal tissue. In contrast, the methylation levels found in adenoma were distinct (Figure 3b). Hierarchical clustering further illustrated that the DNA methylation status of the DMRs chosen is quite different in adenoma as compared to normal tissues and cell preparations (Figure 3c). Importantly, these data strongly suggest that the CpG hypo - and hypermethylation patterns of intestinal tumours do not derive from ISCs, but form de-novo and in a recurring manner after tumour initiation following the loss of APC.
Fig. 3. CpG methylation differentiates normal epithelial cell types from adenoma. 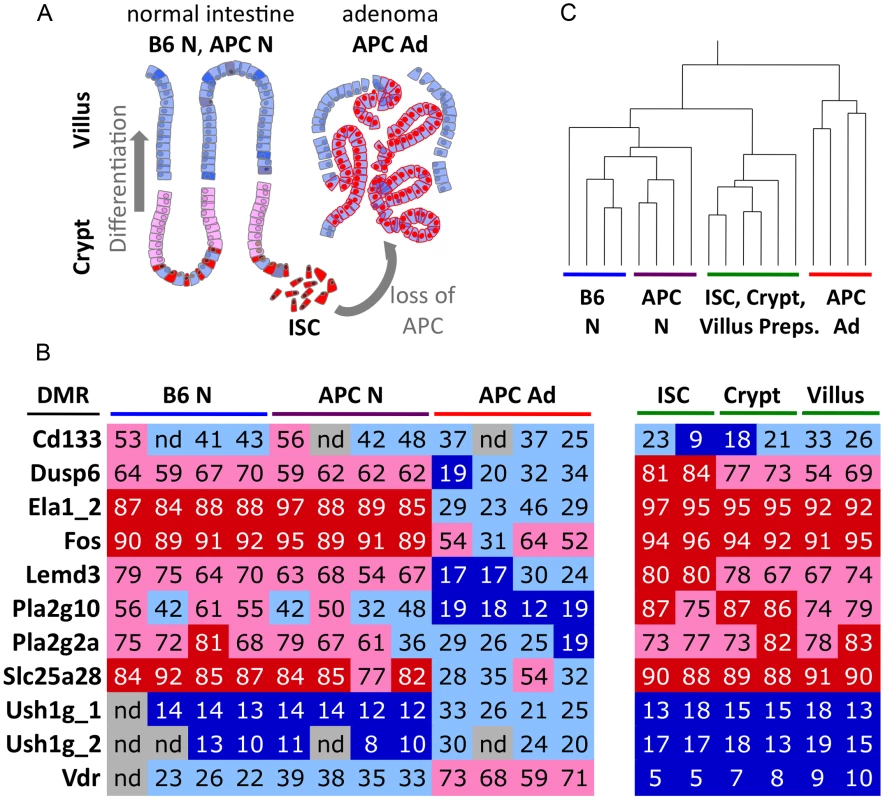
a) Schematic representation of intestinal tissues and cell types. Differentiated villus cells are the prevailing component of bulk normal tissue samples. b) Colour-coded table of CpG methylation analyses of 11 DMRs, using bisulfite pyrosequencing. Cd133 is an intestinal stem cell and cancer stem cell marker. Dusp6 to Slc25a28 represent adenoma hypomethylated DMRs, Ush1g_1 to Vdr represent adenoma hypermethylated DMRs. Percent CpG methylation within the regions are given as numbers and colour-code. Dark blue, <20% CpG methylation; light blue, 20–50% CpG methylation, light red, 50–80% CpG methylation; bright red, >80% methylation. c) Hierarchical clustering of methylation data (as shown in b) separates adenoma from normal tissue and cell preparations. Pearson correlation was employed. Differential promoter methylation and differential gene transcription in adenoma do not correlate extensively
It is generally assumed that promoter hypermethylation causes down-regulation of gene expression, whereas promoter hypomethylation is associated with up-regulation of genes. We asked if and to what extent this assumption holds true. To do so, we evaluated our RNA-seq data, which was derived from the same tissue samples that were used for MeDIP-seq (see Figure 1a for samples; Figure S5, S6 for RNA-seq quality assessment). We evaluated groups of genes that were both, differentially methylated and differentially expressed between normal intestine and adenoma. The number of genes that were hypomethylated at their promoters and up-regulated in adenoma was larger than expected to occur by chance (Figure 4a, see also Table S5, P<2*10−8, hypergeometric distribution). One example is the Pla2g2a gene (also known as Mom1), which is a known modifier of adenoma development [38] (Figure S7). Likewise, promoter hypermethylation correlated for some genes with decreased gene expression, but the overlap between promoter hypermethylated genes and transcriptionally down-regulated genes was not significant (P>0.05; Figure 4a). However, the majority of genes showed no correlation between promoter methylation and transcriptional activity, and some genes even displayed an inverse correlation. For example, Slc9a3 contains a hypermethylated DMR in the promoter, and is strongly activated in adenoma (Figure S8). We also evaluated genes that were differentially methylated in their gene body and differentially expressed, and again found only a small overlap between changes in gene body methylation and gene expression (Figure 4b).
Fig. 4. Differential gene methylation and differential gene transcription in adenoma do not correlate extensively. 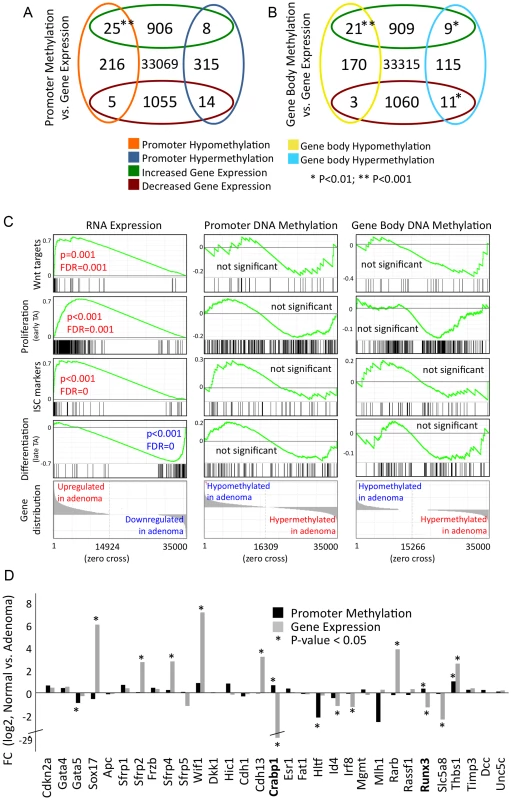
a)–b) Venn diagrams displaying numbers of transcriptionally regulated and differentially methylated genes. Cut-off criteria were FDR<0.001 for transcriptional regulation, and P<0.01 for methylation (calculation using edgeR). a) Genes that display differential methylation of promoter (−1.0 to +0.5 bk relative to transcription start site) b) Genes that display differential gene body methylation. c) GSEA analyses of gene signatures comprising Wnt targets, ISC-, proliferative- and differentiated cell-specific genes. Left panels: analysis of gene activity, as assessed by RNA-seq; middle and right panels: analysis of promoter or gene body methylation. Data is given as described in Figure 2a. d) Relative gene expression and promoter methylation data for 31 selected epigenetically regulated tumour suppressor genes. Fold change between normal and adenoma is given. Crabp1 and Runx3 are both, promoter hypermethylated and transcriptionally down-regulated. Asterisk denotes P<0.05. We asked whether gene signatures, which are representative for key cell types and processes of normal development show a common behaviour in terms of gene expression and methylation patterns in adenoma. Gene activity, as assessed by RNA-seq, differed strongly between normal intestinal tissue and adenoma for all gene signatures analysed (Figure 4c, Figure S6). We found that Wnt target genes [39], along with ISC/crypt progenitor cell and TA (transiently amplifying) cell marker genes were almost uniformly up-regulated in adenoma, while differentiation signature genes were mostly down-regulated [40]–[42] (Figure 4c, see Table S6 for signature genes). Despite the transcriptional co-regulation of the signature genes, we found no consistent or significant trend of differential promoter or gene body methylation within the ISC-, proliferative - and differentiation-associated gene groups (Figure 4c).
Previous research revealed a number of tumour suppressors, which are hypermethylated at their promoters and consequently silenced in carcinoma. We thus assessed the promoter methylation patterns and transcriptional activity of the mouse orthologues of 31 tumour suppressors previously reported to be frequently epigenetically silenced in human colon cancer [12], [24], [25]. In mouse intestinal adenoma, however, we found only two out of 31 tumour suppressors that were both, promoter-hypermethylated and transcriptionally silenced (Figure 4d). These are the retinoic-acid signal transducer Crabp1 and the runt-related transcription factor Runx3, which both control differentiation and apoptosis in gut epithelia [43]–[45].
The combined data show that the correlation of differential promoter methylation and differential gene expression in mouse intestinal adenoma applies only to a small subset of genes, while most genes show an uncoupling of both processes. In particular, epigenetic silencing of tumour suppressors, which is a frequent trait in human colon carcinoma, was found to occur rarely in mouse adenoma.
A core set of APCMin adenoma-specific methylation patterns is conserved in advanced human colon cancer
Mouse intestinal adenoma shares key functional traits and transcriptional programmes with human colon cancer [23], [46]. We therefore asked whether the similarities extend to epigenetic regulation, and consequently, whether the methylation signature of mouse intestinal adenoma could be detected in human colon cancer. To this end, we identified the human orthologues of genes whose promoters are hypo - or hypermethylated in mouse adenoma, and assessed promoter methylation patterns from MeDIP-seq data of 14 advanced human colon cancers of the stages T2–T4 (unpublished data, C. G., M. R. S. et al.). Strikingly, we found that a significant fraction of hyper - or hypomethylated genes identified in mouse adenoma displayed matching promoter hyper - or hypomethylation in human colon cancers (Figure 5a). Among the group of the most consistently hypermethylated genes in both human colon cancer and mouse intestinal adenoma (48 genes), we found Cdh4, Crabp1, Crmp1, Dbc1, Duox1, Grm7, Hand1, Hs3st2, Pcdh17 and Pdpn, which have previously been suggested as cancer biomarkers [15], [47]–[54]. Among the genes, which are consistently promoter-hypomethylated in both mice and humans (54 genes), we identified the matrix metalloproteinase and colon cancer prognostic marker Mmp14, which is also over-expressed in mouse adenoma. Promoter methylation changes in the genes identified here were quite consistent across the panel of the 14 human colon cancers (see Figure 5b for a selection of top eleven hypo - and hypermethylated genes, and Table S8 for complete gene sets).
Fig. 5. A core set of APCMin adenoma-specific CpG methylation patterns is conserved in human colon cancer. 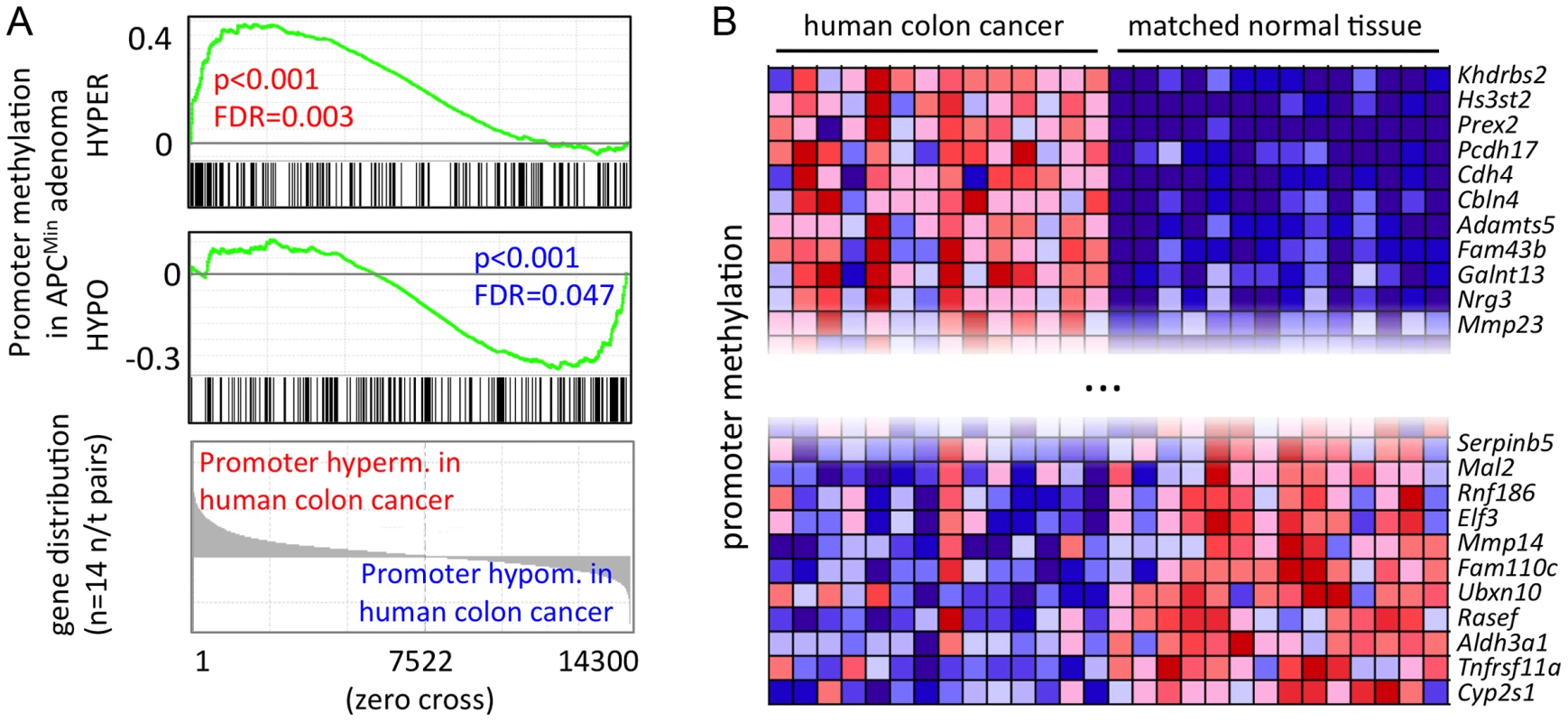
a) GSEA identifies methylation changes of mouse adenoma in human colon cancer. Gene signatures comprise genes with promoter hypo- or hypermethylation in mouse adenoma (see also Figure 4a, Table S5), genes were ordered by directional methylation changes in human colon cancer (normal tissue versus carcinoma). Mouse and human gene homologues were matched using ENSEMBL Biomart (approx. 14300 unique orthologue pairs were identified). b) Promoter hypo- and hypermethlyation is conserved between mouse APCMin adenoma and human colon cancer. Genes were selected from those that are significantly hyper- or hypomethylated in APCMin adenoma. Conserved genes were identified as the core enrichment group of GSEA analysis in a). Figure shows top eleven hypo- and hypermethylated genes in human colon cancer. blue: low relative methylation; red: high relative methylation. Our data define a core methylation signature of intestinal cancer, which is conserved between humans and mice. The comparison of mouse with human data (comprising early lesions in mice and advanced stages of human cancer) indicates that this core epigenetic signature is established early during tumour formation, and is retained when the cancer epigenome is further modified during tumour progression to carcinoma.
Discussion
We report that the formation of APCMin mouse adenoma from normal intestinal tissue is accompanied by characteristic CpG methylation changes in a large number of genomic regions, and that a core set of such DMRs is conserved between mouse adenoma and human colon cancer. The finding of recurring DMRs in independent adenomas is in agreement with an instructive mechanism guiding CpG methylation in tumours, as proposed previously [7], [15]. It has been suggested that gain of CpG methylation in tumour cells correlates with the H3K27me3 mark set by PRC2 activity [15], [55], and our data demonstrating preferential DNA hypermethylation of Polycomb target sites confirm this model. Furthermore, our gene expression data show that PRC2 components are over-expressed in adenoma, and immunohistochemistry confirmed up-regulation of EED protein in adenoma tissue. These observations suggest that enhanced PRC2 activity may be instrumental in the rapid establishment of the adenoma-specific DNA hypermethylation pattern following the loss of APC. Hypomethylation in mouse adenoma was likewise found to occur in a regular pattern, rather than in the form of genome-wide demethylation. We could however not identify correlations between recurring hypomethylation and binding patterns of epigenetic regulators, such as PRC, TrxG or TET complexes.
Previous reports have shown that in human colon cancer tumour suppressors are frequently silenced by promoter methylation, and individual tumours differ in the genes that are silenced [12], [24], [25]. In contrast, we rarely detected tumour suppressors among the recurring DMRs, which are common between individual APCMin adenomas and partly shared between mouse adenoma and human colon cancer. These observations suggest that the variable epigenetic patterns of individual human colon cancers derive by clonal expansion following stochastic methylation changes that confer a growth advantage to individual tumour cells. Since such clonal selection is dependent on rare initiating events, tumour suppressor silencing is not detected in early APCMin adenoma, but frequently and variably with respect to the genes affected in human colon cancer. This different situation also applies to genomic instability [56]. It thus appears likely that most epigenetic changes in tumour suppressor genes represent late steps that arise during adenoma-to-carcinoma progression rather than representing differences between the mouse and human species. Taken together, our combined analyses suggest that the epigenetic deregulation in tumours can be divided into two principal components, an early and fast instructive patterning mechanism, which immediately follows tumour initiation, and late rare stochastic events promoting clonal expansion (for a model, see Figure 6).
Fig. 6. A model for stepwise formation of cancer cell CpG epigenomes. 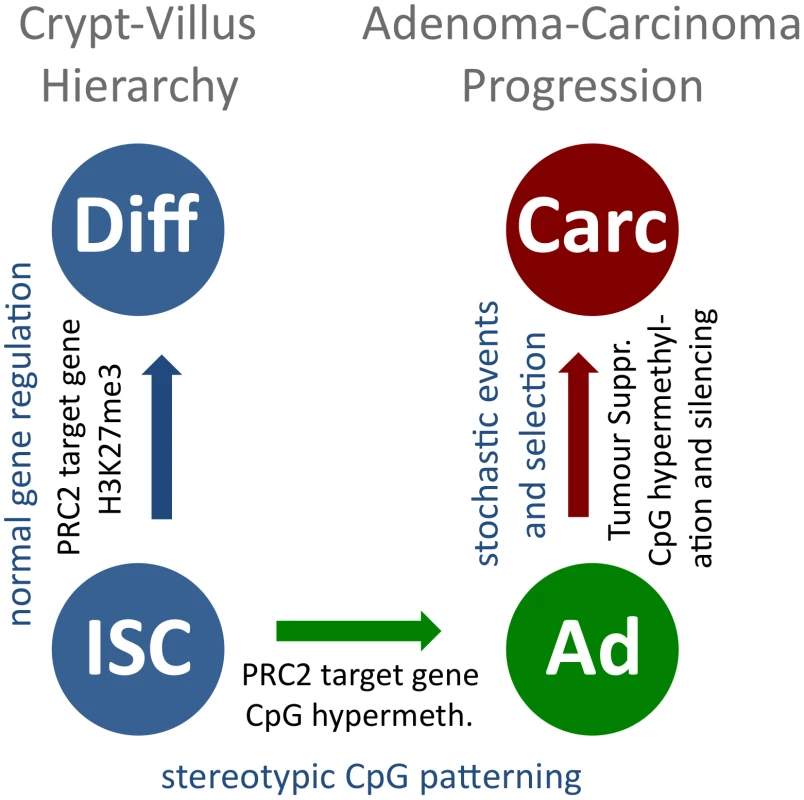
CpG methylation is uniform within the normal cellular hierarchy of the intestine, and PRC2-associated H3K27me3 marks are present in crypt and villus cells (blue, to the left). Upon tumour initiation, recurring CpG methylation patterns form, guided by an instructive mechanism that is linked to PRC2 for hypermethylated sites (blue to green). Further CpG methylation changes occur slowly, probably in a stochastic manner. A fraction of these bestow tumour cells with a selective advantage and are subject to clonal expansion during tumour progression (green to red). We present for the first time DNA methylation data comparing primary normal cell types with transformed tissue derived from the same organ. We observed that the adenoma-specific DNA methylation patterns were distinct from those found in the major cell types of intestinal epithelia, including intestinal stem cells, which give rise to adenoma in the mouse [36]. This indicates that adenoma-specific DMRs are not pre-existing in a cell population comprising a minor fraction of the intestinal tissue, such as ISCs, and simply emerge by expansion of this cell type in adenoma, but are formed de-novo in a recurring and characteristic manner. Our data therefore establish beyond doubt that epigenetic patterns found in tumour cells arise upon transformation, and are thus suitable as specific epigenetic disease markers.
Current epigenomic models assume that DNA hypermethylation, in particular methylation of promoter regions, means gene silencing. We find that this model, with respect to adenoma, holds true only for a minor fraction of genes whose promoters are hypermethylated. Likewise, only a minor fraction of genes with hypomethylated promoters in adenoma are transcriptionally up-regulated. These gene groups are larger than expected to occur by chance, however, the large majority of genes does not follow this simple logic. Overall, changes in promoter methylation do not appear to generally cause altered gene expression. Further studies involving additional control elements such as enhancers and intragenic silencers are required for a deeper understanding of the control of gene expression by epigenetic mechanisms in adenoma.
We identified a core set of recurring DMRs, which is conserved between mouse adenoma and human colon carcinoma. This has important clinical implications, since our finding suggests the existence of a conserved set of DMRs arising early in human colon cancer, which may be universal to a large majority of human intestinal malignancies. This class of biomarkers may be suitable for the discovery of intestinal cancer at an early stage.
We rarely found hypermethylation and silencing of specific tumour suppressors in early adenoma of the mouse. Epigenetic silencing of tumour suppressors by promoter hypermethylation is however frequent in a subset of advanced human tumours. These latter marks may be useful as markers, which may correlate with histopathological features and be suited for distinguishing clinically relevant tumour subtypes.
Materials and Methods
Ethics statement and mouse strains
All mouse work has been conducted according to the relevant national and international guidelines. APCMin mice (genetic background C57BL/6J (B6), Jackson laboratory (www.jax.org, USA)), were maintained by backcrossing to C57BL/6JOlaHsd (Harlan, The Netherlands). Animals were housed at a 12 h/12 h light/dark cycle and fed ad libitum. B6-APCMin/+ (APCMin) and B6-APC+/+ (B6) wild type littermates were dissected at the age of 15 to 19 weeks and individual adenomas and normal intestinal tissue was excised from the ileum.
Histology and cell-based methods
For immunohistochemistry, tissues were fixed in 4% formaldehyde, dehydrated via a graded ethanol series, embedded in paraffin, and sectioned at 4 µm. Anti-EED (1∶50, ARP-38384 Aviva) and anti-DNMT1 (1∶200, Alexis 804-369-C100) primary antibodies, anti-mouse Alexa488 and ImmPRESS secondary antibodies, and the NovaRED substrate kit (Vector) were used. Crypt, villus and intestinal stem cell preparations were prepared as described previously [57]. In short, villus preparations were scraped from the inner intestinal surface after initial washing steps, and crypts were isolated by filtering (70 µm, CellTRICS) after 30 min of PBS/2 mM EDTA incubation. FACS sorting for GFP+ intestinal stem cells was done after TrypLE dissociation of cells, using a FACS Aria system (BD) and a 100 µm nozzle. Crypt and villus tissue preparations were controlled for enrichment by visual inspection.
DNA and RNA methods
In short, DNA and RNA of normal and adenoma intestinal samples were isolated using the Allprep DNA/RNA Mini kit (Qiagen). A DNAse digest of the RNA was performed on the columns according to the manufacturer's instructions. Nucleic acid concentrations were measured with a Nanodrop photometer (Implen, Germany) or Qubit fluorometer (Invitrogen) and quality was assessed on an agarose gel (DNA) or a 2100 Bioanalyzer (Agilent, RNA). MeDIP-seq was adapted from previously published protocols [6], [26], [58]. RNA-seq was performed from 4 µg of total RNA, after depletion for ribosomal RNA using the RiboMinus Eukaryote Kit for RNA-seq (Invitrogen), following a strand-specific protocol [59]. Sequencing was done on 454 GS-FLX for the bisulfite-pyrosequencing and Illumina Genome Analyser (GAIIx) machines for MeDIP-seq and RNA-seq. Extended RNA/DNA material and methods are available online as Text S1.
Bioinformatic analysis
Bioinformatic methods section is available online as Text S1 along with associated Tables S9, S10. MeDIP-seq and RNA-seq data can be accessed in GEO under GSE38983.
Supporting Information
Zdroje
1. MargueronR, ReinbergD (2010) Chromatin structure and the inheritance of epigenetic information. Nat Rev Genet 11 : 285–296 doi:10.1038/nrg2752.
2. CedarH, BergmanY (2009) Linking DNA methylation and histone modification: patterns and paradigms. Nat Rev Genet 10 : 295–304 doi:10.1038/nrg2540.
3. LundAH, van LohuizenM (2004) Polycomb complexes and silencing mechanisms. Curr Opin Cell Biol 16 : 239–246 doi:10.1016/j.ceb.2004.03.010.
4. ViréE, BrennerC, DeplusR, BlanchonL, FragaM, et al. (2006) The Polycomb group protein EZH2 directly controls DNA methylation. Nature 439 : 871–874 doi:10.1038/nature04431.
5. RazinA, ShemerR (1995) DNA methylation in early development. Hum Mol Genet 4 Spec No: 1751–1755.
6. WeberM, DaviesJJ, WittigD, OakeleyEJ, HaaseM, et al. (2005) Chromosome-wide and promoter-specific analyses identify sites of differential DNA methylation in normal and transformed human cells. Nat Genet 37 : 853–862 doi:10.1038/ng1598.
7. KeshetI, SchlesingerY, FarkashS, RandE, HechtM, et al. (2006) Evidence for an instructive mechanism of de novo methylation in cancer cells. Nat Genet 38 : 149–153 doi:10.1038/ng1719.
8. JiaD, JurkowskaRZ, ZhangX, JeltschA, ChengX (2007) Structure of Dnmt3a bound to Dnmt3L suggests a model for de novo DNA methylation. Nature 449 : 248–251 doi:10.1038/nature06146.
9. OoiSKT, QiuC, BernsteinE, LiK, JiaD, et al. (2007) DNMT3L connects unmethylated lysine 4 of histone H3 to de novo methylation of DNA. Nature 448 : 714–717 doi:10.1038/nature05987.
10. JonesPA, BaylinSB (2007) The epigenomics of cancer. Cell 128 : 683–692 doi:10.1016/j.cell.2007.01.029.
11. EstellerM (2008) Epigenetics in cancer. N Engl J Med 358 : 1148–1159 doi:10.1056/NEJMra072067.
12. BaylinSB (2009) Stem cells, cancer, and epigenetics. StemBook 1–14 doi:10.3824/stembook.1.50.1.
13. JonesPA, BaylinSB (2002) The fundamental role of epigenetic events in cancer. Nat Rev Genet 3 : 415–428 doi:10.1038/nrg816.
14. WidschwendterM, FieglH, EgleD, Mueller-HolznerE, SpizzoG, et al. (2007) Epigenetic stem cell signature in cancer. Nat Genet 39 : 157–158 doi:10.1038/ng1941.
15. SchlesingerY, StraussmanR, KeshetI, FarkashS, HechtM, et al. (2007) Polycomb-mediated methylation on Lys27 of histone H3 pre-marks genes for de novo methylation in cancer. Nat Genet 39 : 232–236 doi:10.1038/ng1950.
16. OhmJE, McGarveyKM, YuX, ChengL, SchuebelKE, et al. (2007) A stem cell-like chromatin pattern may predispose tumor suppressor genes to DNA hypermethylation and heritable silencing. Nat Genet 39 : 237–242 doi:10.1038/ng1972.
17. ZhuangJ, JonesA, LeeS-H, NgE, FieglH, et al. (2012) The dynamics and prognostic potential of DNA methylation changes at stem cell gene loci in women's cancer. PLoS Genet 8: e1002517 doi:10.1371/journal.pgen.1002517.
18. EaswaranH, JohnstoneSE, Van NesteL, OhmJ, MosbrugerT, et al. (2012) A DNA hypermethylation module for the stem/progenitor cell signature of cancer. Genome Res 22 : 837–849 doi:10.1101/gr.131169.111.
19. SparmannA, van LohuizenM (2006) Polycomb silencers control cell fate, development and cancer. Nat Rev Cancer 6 : 846–856 doi:10.1038/nrc1991.
20. MillsAA (2010) Throwing the cancer switch: reciprocal roles of polycomb and trithorax proteins. Nat Rev Cancer 10 : 669–682 doi:10.1038/nrc2931.
21. SchuettengruberB, MartinezA-M, IovinoN, CavalliG (2011) Trithorax group proteins: switching genes on and keeping them active. Nat Rev Mol Cell Biol 12 : 799–814 doi:10.1038/nrm3230.
22. FearonER, VogelsteinB (1990) A genetic model for colorectal tumorigenesis. Cell 61 : 759–767.
23. SuLK, KinzlerKW, VogelsteinB, PreisingerAC, MoserAR, et al. (1992) Multiple intestinal neoplasia caused by a mutation in the murine homolog of the APC gene. Science 256 : 668–670.
24. EstellerM (2007) Cancer epigenomics: DNA methylomes and histone-modification maps. Nat Rev Genet 8 : 286–298 doi:10.1038/nrg2005.
25. LaoVV, GradyWM (2011) Epigenetics and colorectal cancer. Nat Rev Gastroenterol Hepatol doi:10.1038/nrgastro.2011.173.
26. DownTA, RakyanVK, TurnerDJ, FlicekP, LiH, et al. (2008) A Bayesian deconvolution strategy for immunoprecipitation-based DNA methylome analysis. Nat Biotechnol 26 : 779–785 doi:10.1038/nbt1414.
27. ChavezL, JozefczukJ, GrimmC, DietrichJ, TimmermannB, et al. (2010) Computational analysis of genome-wide DNA methylation during the differentiation of human embryonic stem cells along the endodermal lineage. Genome Res 20 : 1441–1450 doi:10.1101/gr.110114.110.
28. EstécioMRH, GharibyanV, ShenL, IbrahimAEK, DoshiK, et al. (2007) LINE-1 hypomethylation in cancer is highly variable and inversely correlated with microsatellite instability. PLoS ONE 2: e399 doi:10.1371/journal.pone.0000399.
29. SunamiE, de MaatM, VuA, TurnerRR, HoonDSB (2011) LINE-1 hypomethylation during primary colon cancer progression. PLoS ONE 6: e18884 doi:10.1371/journal.pone.0018884.
30. El-MaarriO (2004) SIRPH analysis: SNuPE with IP-RP-HPLC for quantitative measurements of DNA methylation at specific CpG sites. Methods Mol Biol 287 : 195–205 doi:10.1385/1-59259-828-5 : 195.
31. BoyerLA, PlathK, ZeitlingerJ, BrambrinkT, MedeirosLA, et al. (2006) Polycomb complexes repress developmental regulators in murine embryonic stem cells. Nature 441 : 349–353 doi:10.1038/nature04733.
32. Ben-PorathI, ThomsonMW, CareyVJ, GeR, BellGW, et al. (2008) An embryonic stem cell-like gene expression signature in poorly differentiated aggressive human tumors. Nat Genet 40 : 499–507 doi:10.1038/ng.127.
33. GuentherMG, LawtonLN, RozovskaiaT, FramptonGM, LevineSS, et al. (2008) Aberrant chromatin at genes encoding stem cell regulators in human mixed-lineage leukemia. Genes Dev 22 : 3403–3408 doi:10.1101/gad.1741408.
34. WilliamsK, ChristensenJ, PedersenMT, JohansenJV, CloosPAC, et al. (2011) TET1 and hydroxymethylcytosine in transcription and DNA methylation fidelity. Nature 473 : 343–348 doi:10.1038/nature10066.
35. SubramanianA, TamayoP, MoothaVK, MukherjeeS, EbertBL, et al. (2005) Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA 102 : 15545–15550 doi:10.1073/pnas.0506580102.
36. BarkerN, RidgwayRA, van EsJH, van de WeteringM, BegthelH, et al. (2009) Crypt stem cells as the cells-of-origin of intestinal cancer. Nature 457 : 608–611 doi:10.1038/nature07602.
37. BarkerN, van EsJH, KuipersJ, KujalaP, van den BornM, et al. (2007) Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449 : 1003–1007 doi:10.1038/nature06196.
38. DietrichWF, LanderES, SmithJS, MoserAR, GouldKA, et al. (1993) Genetic identification of Mom-1, a major modifier locus affecting Min-induced intestinal neoplasia in the mouse. Cell 75 : 631–639.
39. HlubekF, BrabletzT, BudcziesJ, PfeifferS, JungA, et al. (2007) Heterogeneous expression of Wnt/beta-catenin target genes within colorectal cancer. Int J Cancer 121 : 1941–1948 doi:10.1002/ijc.22916.
40. SatoT, van EsJH, SnippertHJ, StangeDE, VriesRG, et al. (2010) Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature 469 : 415 doi:10.1038/nature09637.
41. Merlos-SuárezA, BarrigaFM, JungP, IglesiasM, CéspedesMV, et al. (2011) The intestinal stem cell signature identifies colorectal cancer stem cells and predicts disease relapse. Cell Stem Cell 8 : 511–524 doi:10.1016/j.stem.2011.02.020.
42. FarrallAL, RiemerP, LeushackeM, SreekumarA, GrimmC, et al. (2012) Wnt and BMP signals control intestinal adenoma cell fates. Int J Cancer doi:10.1002/ijc.27500.
43. LiQL, ItoK, SakakuraC, FukamachiH, InoueKI, et al. (2002) Causal relationship between the loss of RUNX3 expression and gastric cancer. Cell 109 : 113–124.
44. NishioM, SakakuraC, NagataT, KomiyamaS, MiyashitaA, et al. (2010) RUNX3 promoter methylation in colorectal cancer: its relationship with microsatellite instability and its suitability as a novel serum tumor marker. Anticancer Res 30 : 2673–2682.
45. TanakaK, ImotoI, InoueJ, KozakiK, TsudaH, et al. (2007) Frequent methylation-associated silencing of a candidate tumor-suppressor, CRABP1, in esophageal squamous-cell carcinoma. Oncogene 26 : 6456–6468 doi:10.1038/sj.onc.1210459.
46. GasparC, CardosoJ, FrankenP, MolenaarL, MorreauH, et al. (2008) Cross-species comparison of human and mouse intestinal polyps reveals conserved mechanisms in adenomatous polyposis coli (APC)-driven tumorigenesis. Am J Pathol 172 : 1363–1380 doi:10.2353/ajpath.2008.070851.
47. JinB, YaoB, LiJ-L, FieldsCR, DelmasAL, et al. (2009) DNMT1 and DNMT3B modulate distinct polycomb-mediated histone modifications in colon cancer. Cancer Res 69 : 7412–7421 doi:10.1158/0008-5472.CAN-09-0116.
48. FacklerMJ, UmbrichtCB, WilliamsD, ArganiP, CruzL-A, et al. (2011) Genome-wide methylation analysis identifies genes specific to breast cancer hormone receptor status and risk of recurrence. Cancer Res 71 : 6195–6207 doi:10.1158/0008-5472.CAN-11-1630.
49. PeterzielH, MüllerJ, DannerA, BarbusS, LiuH-K, et al. (2012) Expression of podoplanin in human astrocytic brain tumors is controlled by the PI3K-AKT-AP-1 signaling pathway and promoter methylation. Neuro-oncology 14 : 426–439 doi:10.1093/neuonc/nos055.
50. IzumiH, InoueJ, YokoiS, HosodaH, ShibataT, et al. (2005) Frequent silencing of DBC1 is by genetic or epigenetic mechanisms in non-small cell lung cancers. Hum Mol Genet 14 : 997–1007 doi:10.1093/hmg/ddi092.
51. LuxenS, BelinskySA, KnausUG (2008) Silencing of DUOX NADPH oxidases by promoter hypermethylation in lung cancer. Cancer Res 68 : 1037–1045 doi:10.1158/0008-5472.CAN-07-5782.
52. MiottoE, SabbioniS, VeroneseA, CalinGA, GulliniS, et al. (2004) Frequent aberrant methylation of the CDH4 gene promoter in human colorectal and gastric cancer. Cancer Res 64 : 8156–8159 doi:10.1158/0008-5472.CAN-04-3000.
53. HarukiS, ImotoI, KozakiK-I, MatsuiT, KawachiH, et al. (2010) Frequent silencing of protocadherin 17, a candidate tumour suppressor for esophageal squamous cell carcinoma. Carcinogenesis 31 : 1027–1036 doi:10.1093/carcin/bgq053.
54. LindGE, KleiviK, MelingGI, TeixeiraMR, Thiis-EvensenE, et al. (2006) ADAMTS1, CRABP1, and NR3C1 identified as epigenetically deregulated genes in colorectal tumorigenesis. Cell Oncol 28 : 259–272.
55. MeissnerA, MikkelsenTS, GuH, WernigM, HannaJ, et al. (2008) Genome-scale DNA methylation maps of pluripotent and differentiated cells. Nature 454 : 766–770 doi:10.1038/nature07107.
56. HalbergRB, WaggonerJ, RasmussenK, WhiteA, ClipsonL, et al. (2009) Long-lived Min mice develop advanced intestinal cancers through a genetically conservative pathway. Cancer Res 69 : 5768–5775 doi:10.1158/0008-5472.CAN-09-0446.
57. SatoT, StangeDE, FerranteM, VriesRGJ, van EsJH, et al. (2011) Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett's epithelium. Gastroenterology 141 : 1762–1772 doi:10.1053/j.gastro.2011.07.050.
58. GrimmC, AdjayeJ (2012) Analysis of the methylome of human embryonic stem cells employing methylated DNA immunoprecipitation coupled to next-generation sequencing. Methods Mol Biol 873 : 281–295 doi:10.1007/978-1-61779-794-1_19.
59. ParkhomchukD, BorodinaT, AmstislavskiyV, BanaruM, HallenL, et al. (2009) Transcriptome analysis by strand-specific sequencing of complementary DNA. Nucleic Acids Res 37: e123 doi:10.1093/nar/gkp596.
60. FujitaPA, RheadB, ZweigAS, HinrichsAS, KarolchikD, et al. (2011) The UCSC Genome Browser database: update 2011. Nucleic Acids Res 39: D876–D882 doi:10.1093/nar/gkq963.
Štítky
Genetika Reprodukční medicína
Článek MSH3 Polymorphisms and Protein Levels Affect CAG Repeat Instability in Huntington's Disease MiceČlánek Yemanuclein and HIRA Cooperate for Assembly of H3.3-Containing Nucleosomes in the Male Pronucleus
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2013 Číslo 2
-
Všechny články tohoto čísla
- Complex Inheritance of Melanoma and Pigmentation of Coat and Skin in Grey Horses
- A Meta-Analysis of Thyroid-Related Traits Reveals Novel Loci and Gender-Specific Differences in the Regulation of Thyroid Function
- Genetic Landscape of Open Chromatin in Yeast
- Deleterious Alleles in the Human Genome Are on Average Younger Than Neutral Alleles of the Same Frequency
- Age-Dependent Transition from Cell-Level to Population-Level Control in Murine Intestinal Homeostasis Revealed by Coalescence Analysis
- Next-Generation Sequencing Identifies the Danforth's Short Tail Mouse Mutation as a Retrotransposon Insertion Affecting Expression
- ImmunoChip Study Implicates Antigen Presentation to T Cells in Narcolepsy
- Massive Mitochondrial Gene Transfer in a Parasitic Flowering Plant Clade
- Comment on “Genomic Hypomethylation in the Human Germline Associates with Selective Structural Mutability in the Human Genome”
- The Prefoldin Bud27 Mediates the Assembly of the Eukaryotic RNA Polymerases in an Rpb5-Dependent Manner
- Genetic Determinants of Trabecular and Cortical Volumetric Bone Mineral Densities and Bone Microstructure
- Encodes a Novel and -Genus-Specific Regulator of Photoperiodic Flowering in Rice
- Only One Isoform of CTP Synthase Forms the Cytoophidium
- Mechanisms Involved in the Functional Divergence of Duplicated GroEL Chaperonins in DK1622
- A Genome-Wide RNAi Screen in Identifies the Nicotinic Acetylcholine Receptor Subunit ACR-7 as an Antipsychotic Drug Target
- Autophagy Induction Is a Tor- and Tp53-Independent Cell Survival Response in a Zebrafish Model of Disrupted Ribosome Biogenesis
- Ancient DNA Reveals Prehistoric Gene-Flow from Siberia in the Complex Human Population History of North East Europe
- Inflammation-Mediated Genetic and Epigenetic Alterations Drive Cancer Development in the Neighboring Epithelium upon Stromal Abrogation of TGF-β Signaling
- MicroRNA-3148 Modulates Allelic Expression of Toll-Like Receptor 7 Variant Associated with Systemic Lupus Erythematosus
- RNAi–Based Functional Profiling of Loci from Blood Lipid Genome-Wide Association Studies Identifies Genes with Cholesterol-Regulatory Function
- CELF Family RNA–Binding Protein UNC-75 Regulates Two Sets of Mutually Exclusive Exons of the Gene in Neuron-Specific Manners in
- Coordination of Chromatid Separation and Spindle Elongation by Antagonistic Activities of Mitotic and S-Phase CDKs
- The Ubiquitin Ligase Subunit Acts in Target Tissue to Restrict Tracheal Terminal Cell Branching and Hypoxic-Induced Gene Expression
- Mitotic Evolution of Shows a Stable Core Genome but Recombination in Antigen Families
- Tysnd1 Deficiency in Mice Interferes with the Peroxisomal Localization of PTS2 Enzymes, Causing Lipid Metabolic Abnormalities and Male Infertility
- A Regulatory Pathway, Ecdysone-Transcription Factor Relish-Cathepsin L, Is Involved in Insect Fat Body Dissociation
- PcG-Mediated Higher-Order Chromatin Structures Modulate Replication Programs at the BX-C
- MSH3 Polymorphisms and Protein Levels Affect CAG Repeat Instability in Huntington's Disease Mice
- JNK-Interacting Protein 3 Mediates the Retrograde Transport of Activated c-Jun N-Terminal Kinase and Lysosomes
- Discovery of a Splicing Regulator Required for Cell Cycle Progression
- Rearrangements of 2.5 Kilobases of Noncoding DNA from the Locus Define Predictive Rules of Genomic -Regulatory Logic
- Admixture Mapping in Lupus Identifies Multiple Functional Variants within IFIH1 Associated with Apoptosis, Inflammation, and Autoantibody Production
- Roles of the Developmental Regulator Homothorax in Limiting Longevity in
- miR-199a-5p Is Upregulated during Fibrogenic Response to Tissue Injury and Mediates TGFbeta-Induced Lung Fibroblast Activation by Targeting Caveolin-1
- A Kinome-Wide RNAi Screen in Glia Reveals That the RIO Kinases Mediate Cell Proliferation and Survival through TORC2-Akt Signaling in Glioblastoma
- Assembly of the Auditory Circuitry by a Genetic Network in the Mouse Brainstem
- SOX2 Co-Occupies Distal Enhancer Elements with Distinct POU Factors in ESCs and NPCs to Specify Cell State
- Retrotransposon Activates Ectopic Expression: A Short Tail
- Confounding by Repetitive Elements and CpG Islands Does Not Explain the Association between Hypomethylation and Genomic Instability
- Cell Reprogramming Requires Silencing of a Core Subset of Polycomb Targets
- Properties and Modeling of GWAS when Complex Disease Risk Is Due to Non-Complementing, Deleterious Mutations in Genes of Large Effect
- Essential Developmental, Genomic Stability, and Tumour Suppressor Functions of the Mouse Orthologue of
- Conditional Inactivation of the DNA Damage Response Gene in Mouse Testis Reveals Separable Roles for Components of the RAD9-RAD1-HUS1 Complex in Meiotic Chromosome Maintenance
- Genome-Wide Analysis Points to Roles for Extracellular Matrix Remodeling, the Visual Cycle, and Neuronal Development in Myopia
- Patterning of Leaf Vein Networks by Convergent Auxin Transport Pathways
- An Evolutionary Perspective on Epistasis and the Missing Heritability
- A Retrotransposon Insertion in the 5′ Regulatory Domain of Ptf1a Results in Ectopic Gene Expression and Multiple Congenital Defects in Danforth's Short Tail Mouse
- The Mub1/Ubr2 Ubiquitin Ligase Complex Regulates the Conserved Dsn1 Kinetochore Protein
- Mutations Can Cause Enamel-Renal Syndrome (ERS)
- Yemanuclein and HIRA Cooperate for Assembly of H3.3-Containing Nucleosomes in the Male Pronucleus
- Hepatocyte Growth Factor, a Determinant of Airspace Homeostasis in the Murine Lung
- ISWI and CHD Chromatin Remodelers Bind Promoters but Act in Gene Bodies
- COM-1 Promotes Homologous Recombination during Meiosis by Antagonizing Ku-Mediated Non-Homologous End Joining
- Control of Multicellular Development by the Physically Interacting Deneddylases DEN1/DenA and COP9 Signalosome
- Antagonism Versus Cooperativity with TALE Cofactors at the Base of the Functional Diversification of Hox Protein Function
- Dynamic Association of NUP98 with the Human Genome
- Ectopic Expression of Induces Spinal Defects, Urogenital Defects, and Anorectal Malformations in Mice
- Regulation of Contributes to the Lineage Potential of Neurogenin3+ Endocrine Precursor Cells in the Pancreas
- Gene-Based Testing of Interactions in Association Studies of Quantitative Traits
- The Amidation Step of Diphthamide Biosynthesis in Yeast Requires , a Gene Identified through Mining the - Interaction Network
- Plant-Symbiotic Fungi as Chemical Engineers: Multi-Genome Analysis of the Clavicipitaceae Reveals Dynamics of Alkaloid Loci
- Genome-Wide Diversity in the Levant Reveals Recent Structuring by Culture
- DNA Methylation Mediated Control of Gene Expression Is Critical for Development of Crown Gall Tumors
- Identification of the SlmA Active Site Responsible for Blocking Bacterial Cytokinetic Ring Assembly over the Chromosome
- Expression of a Novel P22 ORFan Gene Reveals the Phage Carrier State in Typhimurium
- Altered Cohesin Gene Dosage Affects Mammalian Meiotic Chromosome Structure and Behavior
- Quantitative Analysis of Histone Modifications: Formaldehyde Is a Source of Pathological N-Formyllysine That Is Refractory to Histone Deacetylases
- Duplicate Abalone Egg Coat Proteins Bind Sperm Lysin Similarly, but Evolve Oppositely, Consistent with Molecular Mimicry at Fertilization
- Lessons from on the Strengths and Weaknesses of Structured Association Mapping
- DNA–Methylome Analysis of Mouse Intestinal Adenoma Identifies a Tumour-Specific Signature That Is Partly Conserved in Human Colon Cancer
- Transposon Variants and Their Effects on Gene Expression in
- Polygenic Modeling with Bayesian Sparse Linear Mixed Models
- Single Transmembrane Peptide DinQ Modulates Membrane-Dependent Activities
- The JNK Signaling Pathway Activates Expression of Stress Response Genes by Derepressing the Fos/HDAC Repressor Complex
- The Interaction of CtIP and Nbs1 Connects CDK and ATM to Regulate HR–Mediated Double-Strand Break Repair
- Regulation of Metamorphosis by Xenobiotic Response Regulators
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Complex Inheritance of Melanoma and Pigmentation of Coat and Skin in Grey Horses
- Coordination of Chromatid Separation and Spindle Elongation by Antagonistic Activities of Mitotic and S-Phase CDKs
- Autophagy Induction Is a Tor- and Tp53-Independent Cell Survival Response in a Zebrafish Model of Disrupted Ribosome Biogenesis
- Assembly of the Auditory Circuitry by a Genetic Network in the Mouse Brainstem
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



