-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaGenome-Wide Analysis Points to Roles for Extracellular Matrix Remodeling, the Visual Cycle, and Neuronal Development in Myopia
Myopia, or nearsightedness, is the most common eye disorder, resulting primarily from excess elongation of the eye. The etiology of myopia, although known to be complex, is poorly understood. Here we report the largest ever genome-wide association study (45,771 participants) on myopia in Europeans. We performed a survival analysis on age of myopia onset and identified 22 significant associations (), two of which are replications of earlier associations with refractive error. Ten of the 20 novel associations identified replicate in a separate cohort of 8,323 participants who reported if they had developed myopia before age 10. These 22 associations in total explain 2.9% of the variance in myopia age of onset and point toward a number of different mechanisms behind the development of myopia. One association is in the gene PRSS56, which has previously been linked to abnormally small eyes; one is in a gene that forms part of the extracellular matrix (LAMA2); two are in or near genes involved in the regeneration of 11-cis-retinal (RGR and RDH5); two are near genes known to be involved in the growth and guidance of retinal ganglion cells (ZIC2, SFRP1); and five are in or near genes involved in neuronal signaling or development. These novel findings point toward multiple genetic factors involved in the development of myopia and suggest that complex interactions between extracellular matrix remodeling, neuronal development, and visual signals from the retina may underlie the development of myopia in humans.
Published in the journal: . PLoS Genet 9(2): e32767. doi:10.1371/journal.pgen.1003299
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003299Summary
Myopia, or nearsightedness, is the most common eye disorder, resulting primarily from excess elongation of the eye. The etiology of myopia, although known to be complex, is poorly understood. Here we report the largest ever genome-wide association study (45,771 participants) on myopia in Europeans. We performed a survival analysis on age of myopia onset and identified 22 significant associations (), two of which are replications of earlier associations with refractive error. Ten of the 20 novel associations identified replicate in a separate cohort of 8,323 participants who reported if they had developed myopia before age 10. These 22 associations in total explain 2.9% of the variance in myopia age of onset and point toward a number of different mechanisms behind the development of myopia. One association is in the gene PRSS56, which has previously been linked to abnormally small eyes; one is in a gene that forms part of the extracellular matrix (LAMA2); two are in or near genes involved in the regeneration of 11-cis-retinal (RGR and RDH5); two are near genes known to be involved in the growth and guidance of retinal ganglion cells (ZIC2, SFRP1); and five are in or near genes involved in neuronal signaling or development. These novel findings point toward multiple genetic factors involved in the development of myopia and suggest that complex interactions between extracellular matrix remodeling, neuronal development, and visual signals from the retina may underlie the development of myopia in humans.
Introduction
Myopia, or nearsightedness, is the most common eye disorder worldwide. In the United States, an estimated 30–40% of the adult population has clinically relevant myopia (more severe than −1 diopter), and the prevalence has increased markedly in the last 30 years [1], [2]. Myopia is a refractive error that results primarily from increased axial length of the eye [3]. The increased physical length of the eye relative to optical length causes images to be focused in front of the retina, resulting in blurred distance vision.
The etiology of myopia is multifactorial [3]. Briefly, postnatal eye growth is directed by visual stimuli that evoke a signaling cascade within the eye. This cascade is initiated in the retina and passes through the retinal pigment epithelium (RPE) and choroid to guide remodeling of the sclera (the white outer wall of the globe) (cf. [4], [5]). Animal models implicate these visually-guided alterations of the scleral extracellular matrix in the eventual development of myopia [4], [6].
The human eye grows from an average of 17 mm at birth to 21–22 mm in adulthood [7]. By ages 5–6 only about 2% of children are myopic [7]. Although the eye grows only 0.5 mm through puberty [8], the incidence of myopia increases sevenfold during this time [7], peaking between the ages 9–14 [9]. Myopia developed during childhood or early adolescence generally worsens throughout adolescence and then stabilizes by age 20. Compared to myopia that develops in childhood or adolescence, adult onset myopia tends to be less severe [10]–[12]. The majority of myopia cases are primary and nonsyndromic [3]; however, myopia can arise as a complication of other conditions, such as severe prematurity, cataracts, and keratoconus [13], [14], and is sometimes associated with certain connective tissue disorders, such as Stickler syndrome [15].
Although epidemiological studies have implicated numerous environmental factors in the development of myopia, most notably education, outdoor exposure, reading, and near work [3], it is well established that genetics plays a substantial role. Twin and sibling studies have provided heritability estimates that range from 50% to over 90% [16]–[20]. Children of myopic parents tend to have longer eyes and are at higher than average risk of developing myopia in childhood [21]. Segregation analyses suggest that multiple genes are involved in the development of myopia [22], [23]. To date, there have been seven genome-wide association studies (GWAS) on myopia or related phenotypes (pathological myopia, refractive error, and ocular axial length): two in Europeans [24], [25] and five in Asian populations [26]–[30]. Each of these publications has identified a different single association with myopia. In addition there have been several linkage studies (see [3], [31] for reviews) and an exome sequencing study of severe myopia [32].
In contrast to the previous GWAS that used degree of refractive error as a quantitative dependent measure, we analyzed data for 45,771 individuals from the 23andMe database who reported whether they had been diagnosed with nearsightedness, and if so, at what age. We performed a genome-wide survival analysis on age of onset of myopia, discovering 22 genome-wide significant associations with myopia age of onset, 20 of which are novel. Ten of the novel and one of the previously identified associations replicate in a separate (smaller and more coarsely phenotyped) cohort of 8,323 individuals.
Results/Discussion
Participants reported via web-based questionnaires whether they had been diagnosed with nearsightedness, and if so, at what age. Only those participants who reported onset between five and 30 years of age were included to limit cases of secondary myopia (e.g., myopia due to premature birth or cataracts). Further filtering was performed to limit errors in reporting (see Methods).
All participants were customers of 23andMe and of primarily European ancestry; no pair was related at the level of first cousins or closer. We performed a genome-wide survival analysis using a Cox proportional hazards model on data for 45,771 individuals (“discovery set”). The Cox model assumes that there is an (unknown) baseline probability of developing myopia at every year of age. The model then tests whether each single nucleotide polymorphism (SNP) is associated with a significantly higher or lower probability of developing myopia compared to baseline. The Cox model can be thought of as a generalization of an analysis of myopia age of onset. In contrast to an analysis of age of onset, the Cox model allows for the inclusion of non-myopic controls, resulting in increased statistical power. Analyses controlled for sex and five principal components of genetic ancestry. An additional, non-overlapping set of 8,323 participants who reported on their use of corrective eyewear for nearsightedness before the age of ten were used as a replication set. See Table 1 for characteristics of the two cohorts.
Tab. 1. Cohort statistics. 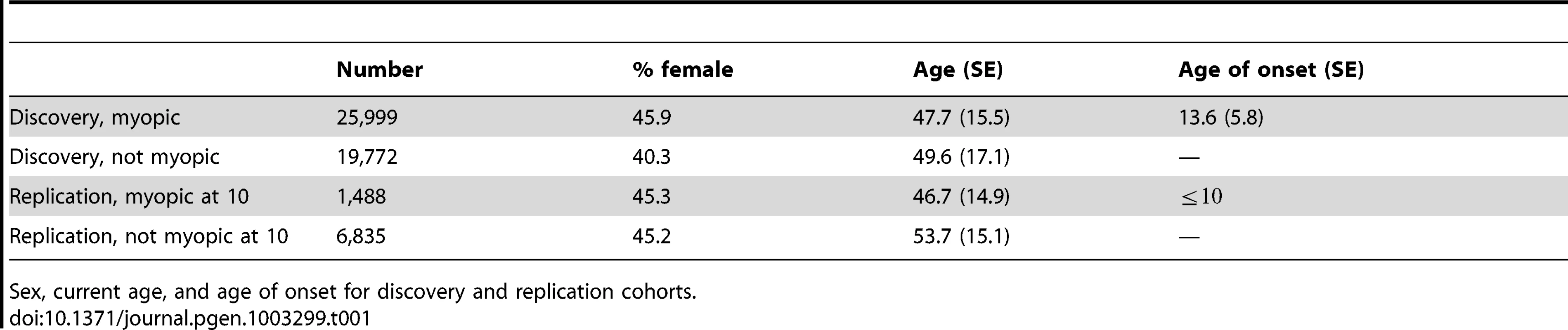
Sex, current age, and age of onset for discovery and replication cohorts. Table 2 shows the top SNPs for all 35 genetic regions associated with myopia with a -value smaller than . All -values from the GWAS have been corrected for the inflation factor of GC = 1.167. A total of 22 of the SNPs cross our threshold for genome-wide significance (, see Figure S1). These 22 include two SNPs previously associated with refractive error in GWAS of European populations: rs524952 near GJD2 and ACTC1 and rs28412916 near RASGRF1 [24], [25], [33]. -values genome-wide are shown in Figure 1; Figure S2 shows the quantile-quantile plot for the analysis. Table S3 shows all SNPs with -values under .
Fig. 1. Negative -values genome wide for myopia. 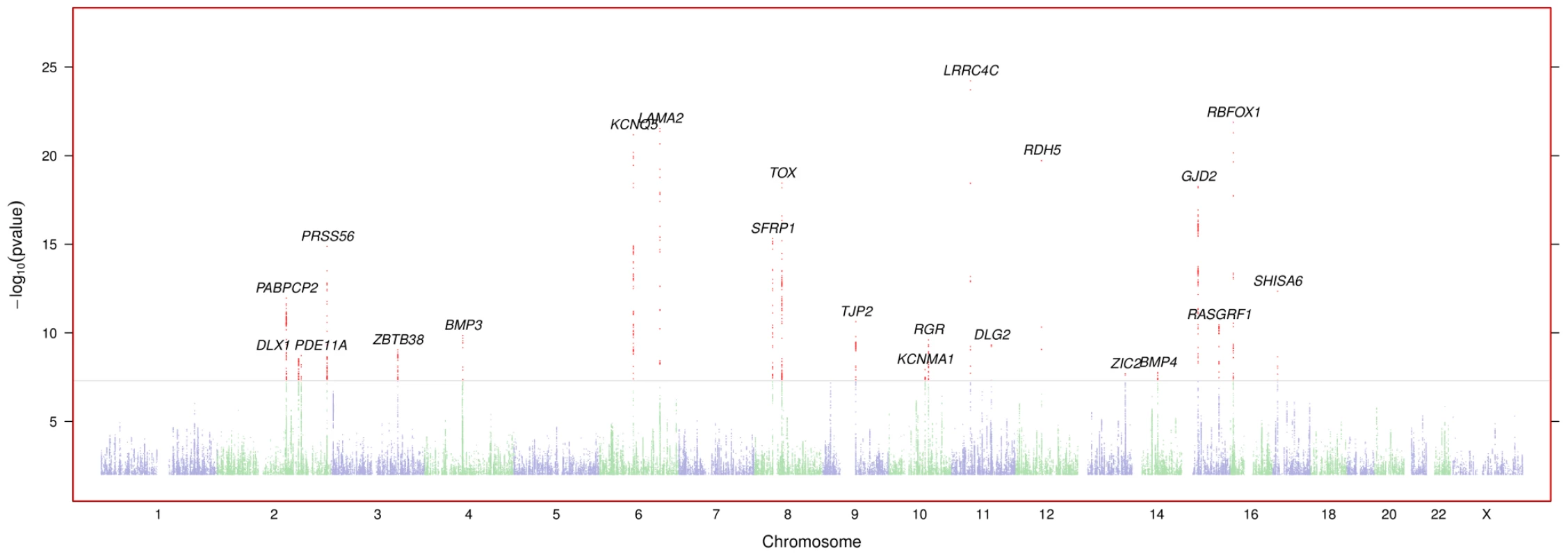
Regions are named with their postulated candidate gene or genes. -values under have been cut off (only the LAMA2 and LRRC4C regions are affected). See Figure S1 for plots in each region with a significant association. Tab. 2. Index SNPs for regions with . 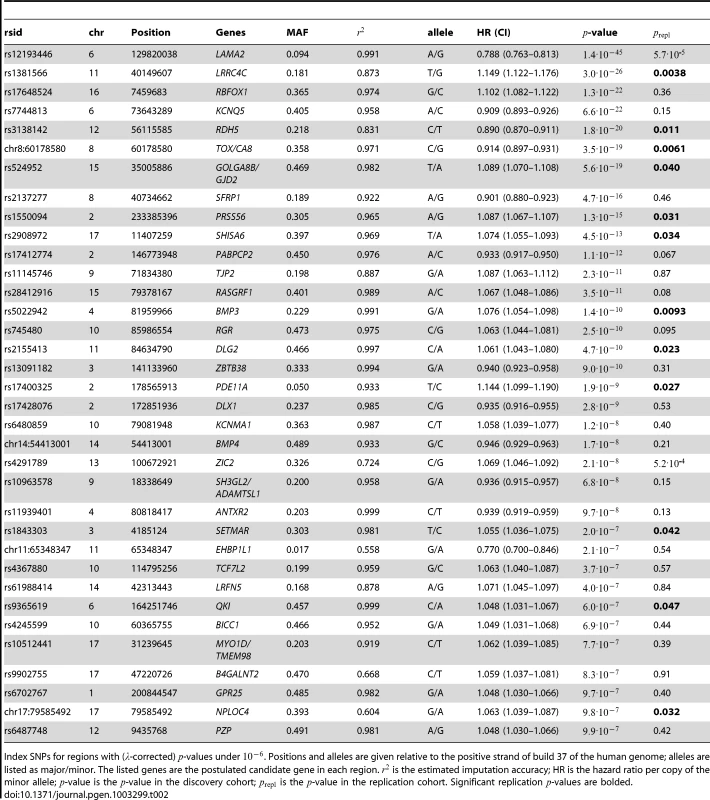
Index SNPs for regions with (-corrected) -values under . Positions and alleles are given relative to the positive strand of build 37 of the human genome; alleles are listed as major/minor. The listed genes are the postulated candidate gene in each region. is the estimated imputation accuracy; HR is the hazard ratio per copy of the minor allele; -value is the -value in the discovery cohort; is the -value in the replication cohort. Significant replication -values are bolded. Of the 22 SNPs significant in the discovery set, 11 were also significant in the replication set (Table 2). Of the 11 SNPs that did not replicate, only two showed different signs between the discovery and replication sets (). Given these results, and considering that the replication set was much smaller than the discovery set and measured age of onset less exactly, we suspect that much of the lack of replication is due to lack of power.
We defined a genetic myopia propensity score as the number of copies of the risk alleles across all 22 SNPs identified via the discovery set. The propensity score showed a strong association with early onset myopia (less than 10 years old) in our replication cohort (, odds ratio 1.075 per risk allele). The top decile of genetic propensity had 1.97 greater odds of developing myopia before the age of 10 than the bottom decile. In a Cox model fit to the discovery set, the propensity score explains 2.9% of the total variance. Note that this estimate may be inflated, as it is calculated on the discovery population. In this model, someone in the 90th percentile of risk (a score of 21.95) is nearly twice as likely to develop myopia by the age of 25 as someone in the 10th percentile of risk (score of 15.01), Figure 2.
Fig. 2. Estimated survival curves by genetic propensity score. 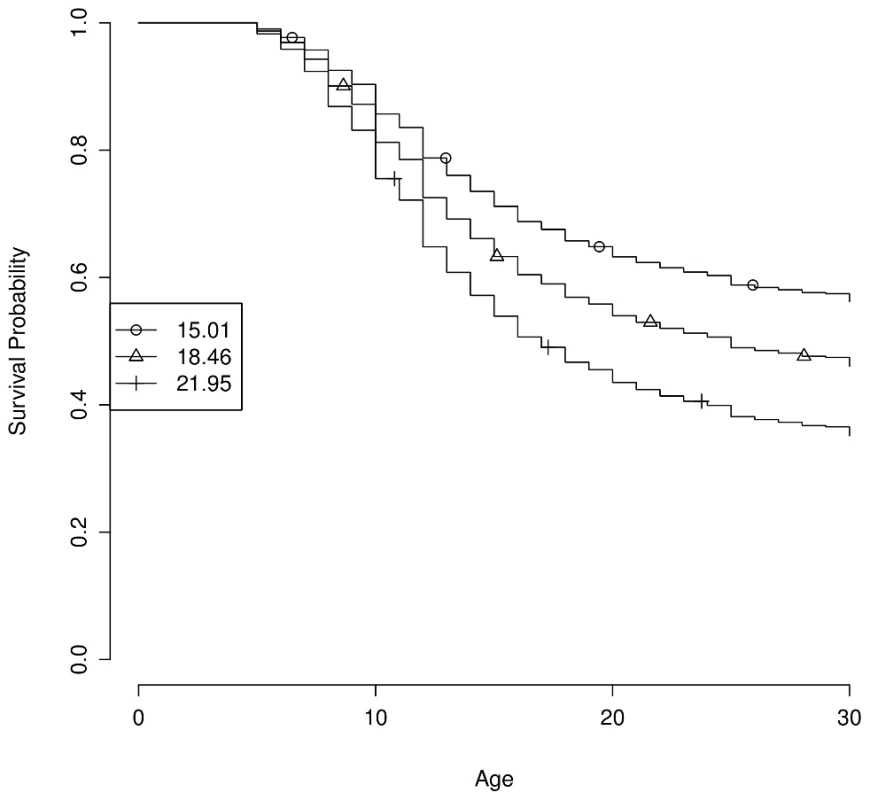
The genetic propensity score is computed as the number of risk alleles across the 22 genome-wide significant SNPs. Curves show estimated survival probability (i.e., the probability of not having developed myopia) by age under the fitted Cox model for the 10th, 50th, and 90th percentiles of scores (15.01, 18.46, and 21.95, respectively). Of the 20 novel associations, many lie in or near genes with direct links to processes related to myopia development. Two of them lie in regions associated with myopia in linkage studies: rs1550094 in PRSS56 (MIM: 609995) [34] and chr14 : 54413001 near BMP4 (MIM: 255500) [35]. Two suggestive associations also are in such regions: rs4245599 in BICC1 (MIM: 612717) [36] and rs9902755 in B4GALNT2 (MIM: 608474) [37]. Below, we briefly sketch out possible connections between these associations and extracellular matrix (ECM) remodeling, the visual cycle, eye and body growth, retinal neuron development, and general neuronal development or signaling.
Extracellular Matrix Remodeling
The strongest association is a SNP in an intron of LAMA2 (laminin, alpha 2 subunit, rs12193446, , hazard ratio (HR) = 0.79). Laminins are extracellular structural proteins that are integral parts of the ECM. Changes in the composition of the ECM of the sclera have been shown to alter the axial length of the eye [5]. Laminins play a role in the development and maintenance of different eye structures [38], [39]. The laminin alpha 2 chain in particular is found in the extraocular muscles during development [38], and may act as an adhesive substrate and possibly a guidance cue for retinal ganglion cell growth cones [40]. We also found a suggestive association related to laminin (rs11939401, , HR = 0.939) approximately 17 kb upstream of ANTXR2 (anthrax toxin receptor 2). ANTXR2 binds laminin and possibly collagen type IV [41] and thus may also be involved in extracellular matrix remodeling.
The Visual Cycle
Two of the novel associations are in or near genes involved in the regeneration of 11-cis-retinal, the light sensitive component of photoreceptors, a process commonly referred to as the visual cycle of the retina. These associations are with rs3138142, , HR = 0.89, in RDH5 (retinol dehydrogenase 5 (11-cis/9-cis)) and rs745480 (, HR = 1.06), a SNP 18 kb upstream of RGR, which encodes the retinal G protein-coupled receptor. The SNP rs3138142 is a synonymous change in RDH5. It has been linked to RDH5 expression [42], [43], and it is part of an Nr2f2 (nuclear receptor subfamily 2, group F, member 2) transcription factor binding motif in mouse [44], [45]. Both RDH5 and RGR play crucial roles in the regeneration of 11-cis retinal in the RPE [46]. Mutations in RDH5 have been linked with fundus albipunctatus, a rare form of congenital stationary night blindness (for a recent review, see [47]) and progressive cone dystrophy [48], and mutations in RGR have been linked with autosomal recessive and autosomal dominant retinitis pigmentosa [49], [50].
We also identified an association within another gene that functions in the RPE: rs7744813 (, HR = 0.91), a SNP in KCNQ5 (potassium voltage-gated channel, KQT-like subfamily, member 5). KCNQ5 encodes a potassium channel found in the RPE and neural retina. These channels are believed to contribute to ion flow across the RPE [51], [52] and to affect the function of cone and rod photoreceptors [52].
Eye and Body Growth
Five of our associations show possible links to eye or overall morphology. The first is a missense mutation in PRSS56 (A224T, rs1550094, , HR = 1.09). Other mutations in PRSS56 have been shown to cause strikingly small eyes with severe decreases in axial length [53]–[55]. Two other associated SNPs are near genes that encode bone morphogenetic proteins: chr14 : 54413001 (, HR = 0.95) near BMP4 (bone morphogenic protein-4), and rs5022942 (, HR = 1.08) in BMP3 (bone morphogenic protein-3). Inherited BMP4 mutations have been associated with syndromic microphthalmia and various eye, brain and digital malformations [56], [57]. Although BMP3 is primarily known for its role in bone development (e.g., it is linked to skeletal defects in humans and skull shapes in dogs [58], [59]), it was found to be uniquely expressed in keratocytes, specialized mesenchymal cells that are important for development of the cornea by producing and maintaining the extracellular matrix of the corneal stroma [60]. One associated SNP, rs13091182 (, HR = 0.94), in ZBTB38 (zinc finger and BTB domain-containing protein 38), is in linkage disequilibrium (LD) with a SNP previously associated with height (rs6763931; ) [61]. The final SNP with a link to morphology is rs17428076 (, HR = 0.94), near DLX1 (homo sapiens distal-less homeobox 1). Disruption of DLX1 has been shown to result in poor optic cup regeneration in planarians and small eyes in mice [62], [63].
Retinal Ganglion Cell Projections
Two of the novel associations are near genes that affect the outgrowth of retinal ganglion neurons during development. The first is rs4291789 (, HR = 1.07), which lies 34 kb downstream of ZIC2 (Zic family member 2). ZIC2 regulates two independent parts of ipsilateral retinal ganglion cell development: axon repulsion at the optic chiasm midline [64], [65], and organization of the axonal projections at their final targets in the brain [66].
The second, rs2137277 (, HR = 0.90), is a variant in ZMAT4 (zinc finger, matrin-type 4). ZMAT4 has no known link to vision, but this variant also lies 385 kb downstream of SFRP1 (secreted frizzled-related protein 1). SFRP1 is involved in the differentiation of the optic cup from the neural retina [67], retinal neurogenesis [68], the development and function of photoreceptor cells [69], [70], and the growth of retinal ganglion cells [71].
Neuronal Signaling and Development
Finally, we found five associations with SNPs in genes involved in neuronal development and signaling, but without a known role in vision development or the vision cycle: in KCNMA1 (potassium large conductance calcium-activated channel, subfamily M, alpha member 1; rs6480859, , HR = 1.06); in RBFOX1 (RNA binding protein, fox-1 homolog; rs17648524, , HR = 1.10); in LRRC4C, leucine rich repeating region containing 4C, also known as NGL-1 (rs1381566, , HR = 1.15); in DLG2 (discs, large homolog 2; rs2155413, , HR = 1.06); and in TJP2 (tight junction protein 2; rs11145746, , HR = 1.09).
KCNMA1 encodes the pore-forming alpha subunit of a MaxiK channel, a family of large conductance, voltage and calcium-sensitive potassium channels involved in the control of smooth muscle and neuronal excitation. RBFOX1 belongs to a family of RNA binding proteins that regulates the alternative splicing of several neuronal transcripts implicated in neuronal development and maturation [72]. LRRC4C encodes a binding partner for netrin G1 and promotes the outgrowth of thalamocortical axons [73]. DLG2 plays a critical role in the formation and regulation of protein scaffolding at postsynaptic sites [74]. TJP2 has been linked with hearing loss: its duplication and subsequent overexpression are found in adult-onset progressive nonsyndromic hearing loss [75].
Conclusion
This study represents the largest GWAS on myopia in Europeans to date. This cohort of 45,771 individuals led to the discovery of 20 novel associations as well as replication of the two previously reported associations in Europeans. Ten of these novel associations replicate in our much smaller replication set of 8,323 individuals. In contrast to the earlier studies that used refractive error as a quantitative outcome, we used a Cox proportional hazards model with age of onset of myopia as our major endpoint. This model yielded greater statistical power than a simple case-control study of myopia. Of the 22 significant SNPs found using this model, all but two had smaller -values when a hazards model was employed, and only 20 would be genome-wide significant using a case-control analysis on the same dataset (Table S1).
The proportional hazards model assumes that the effect of each SNP on myopia risk does not vary by age. When we tested the validity of this assumption for the 22 significant SNPs, only the one in LAMA2 (rs12193446) showed evidence of different effects at different ages (Table S2). While this violation should not lead to overly small -values for this SNP in the GWAS, it does make risk prediction based on these results less straightforward. For example, rs12193446 shows a large effect on myopia hazard at an early age, peaking around 11 years, and then a null or even negative effect on hazard at older ages (Figure S3). This age dependent hazard suggests that different biological processes may affect the development of myopia at different ages.
Our findings further suggest that there may be somewhat different genetic factors underlying myopia age of onset and refractive error. Because adult onset myopia tends to be less severe than myopia developed in childhood or adolescence [10]–[12], age of onset is likely correlated with refractive error, but it is not known how strongly. Many of the associations with myopia age of onset that we found are stronger than the two previously detected associations with refractive error (near GJD2 and near RASGRF1). Notably, the latter association, near RASGRF1, also failed to replicate in a recent meta-analysis [33]. The fact that many of our associations with strong effects on age of onset have not shown up in previous refractive error GWAS implies that some genetic factors may affect the age of onset independent of eventual severity, and that the strength of different genetic associations with myopia may depend on the specific phenotype under study.
We also note that our phenotype was based on participants' reports rather than clinical assessments. Although in theory errors in recall could have affected our results, we expect that the vast majority of people are able to recall when they first wore glasses with at most a few years of error. In fact, a subset of those eligible to be part of our discovery cohort provided age of myopia diagnosis in two independent places (see Methods for details). Out of 1,463 people who reported age of diagnosis in both surveys and met our inclusion criteria (European ancestry, age at diagnosis between five and 30 and less than current age), 96.0% reported ages that differed by at most three years and 97.8% by at most five years.
The five associations previously reported in pathological myopia or refractive error GWAS in Asian populations [26]–[30] show no overlap with the significant or suggestive regions found here. Nor did we find an association with the ZNF644 locus that was identified as the site of high-penetrance, autosomal dominant mutations in Han Chinese families with apparent monogenic inheritance of high-grade myopia [32]. This lack of overlap could result from different genetic factors being involved in myopia across populations. It has been suggested that pathological myopia, which represents less than 2% of cases in the United States [1], has different underlying genetic factors than non-pathological myopia [31].
Our identification of 20 novel genetic associations suggests several novel genetic pathways in the development of human myopia. These findings augment existing research on the development of myopia, which to date has been studied primarily in animal models of artificially induced myopia. Some of the associations are consistent with the current view, based largely on animal models, that a visually-triggered signaling cascade from the retina ultimately guides the scleral remodeling that leads to eye growth, and that the RPE plays a key role in this process [4]. A number of the novel associations point to the potential importance of early neuronal development in the eventual development of myopia, particularly the growth and topographical organization of retinal ganglion cells. These associations suggest that early neuronal development may also contribute to future refractive errors. We expect that these findings will drive new research into the complex etiology of myopia.
Methods
Human Subjects
All participants were drawn from the customer base of 23andMe, Inc., a consumer genetics company. This cohort has been described in detail previously [76], [77]. Participants provided informed consent and participated in the research online, under a protocol approved by the external AAHRPP-accredited IRB, Ethical & Independent Review Services (E&I Review).
Phenotype Data
Participants in the discovery cohort were asked online as part of a medical history questionnaire or an eyesight questionnaire: “Have you ever been diagnosed by a doctor with any of the following vision conditions?: Nearsightedness (near objects are clear, far objects are blurry) (Yes/No/I don't know)”. If they answered “yes”, they were asked, “At what age were you first diagnosed with nearsightedness (near objects are clear, far objects are blurry)? Your best guess is fine.” Those reporting an age of onset either greater than their current age or outside of the range 5–30 were removed from analysis. All participants also reported current age. A total of 4,758 participants reporting age of onset outside of 5–30 and 87 reporting age of onset in the future were removed.
To limit errors in reporting, we excluded from the discovery cohort those who provided discordant answers in the medical history and eyesight questionnaires. We defined discordance as a disagreement in diagnosis or a difference in more than 5 years in age of onset. A total of 92 people with discordant age of onset and 276 with discordant diagnosis were removed. Many of these people would have been filtered out by our other restrictions: only 32 of the 92 with discordant ages of onset would not have been removed for other reasons (mostly because their stated age of onset was not between 5–30), and only 139 of the 276 with discordant diagnoses. These 32 and 139 are out of 1,463 and 2,845 eligible people, respectively, leading us to estimates of 97.8% and 95.1% concordance in age of onset and myopia diagnosis (after the filters mentioned above were applied).
The replication cohort consisted of 8,323 23andMe customers who were not part of the discovery cohort and were not closely related (at 700 cM or greater IBD) to each other or to anyone in the discovery cohort. They provided information on myopia age of onset in one of two ways. 5,265 answered a single question: “Did you wear glasses or other corrective eyewear for nearsightedness before the age of 10? (Yes/No/I'm not sure).” The other 3,058 provided age of onset in the same manner as the discovery cohort. Note that these 3,058 were people that would have been eligible for the discovery cohort, however, they provided data in the time in between our analysis of the discovery and replication cohorts. Their data was converted to the same binary scale as the first group.
Genotyping and Imputation
Participants were genotyped and additional SNP genotypes were imputed against the August 2010 release of the 1000 genomes data as described previously [78]. Briefly, they were genotyped on at least one of three genotyping platforms, two based on the Illumina HumanHap550+ BeadChip, the third based on the Illumina Human OmniExpress+ BeadChip. The platforms included assays for 586,916, 584,942, and 1,008,948 SNPs respectively. Genotypes for a total of 11,914,767 SNPs were imputed in batches of roughly 10,000 individuals, grouped by genotyping platform. Of these, 7,087,609 met our criteria of 0.005 minor allele frequency, average across batches of at least 0.5, and minimum across batches of at least 0.3. (The minimum requirement was added to filter out SNPs that imputed poorly in the batches consisting of the less dense platform.)
Statistical Analysis
In order to minimize population substructure while maximizing statistical power, the study was limited to individuals with European ancestry. Ancestry was inferred from the genome-wide genotype data, and principal component analysis was performed as in [76], [79]. The combined discovery and replication populations were filtered by relatedness to remove participants at a first cousin or closer relationship. More precisely, no two participants shared more than 700 cM of DNA identical by descent (IBD, approximately the lower end of sharing between a pair of first cousins). IBD was calculated using the methods described in [80].
For the survival analysis, let the hazard function be the rate of developing myopia at time . Then the Cox proportional hazards model is
for an arbitrary baseline hazard function and covariates (genotype), (sex), (age), and (projections onto principal components). was coded as a dosage from 0–2 as the estimated number of minor alleles present.For each SNP, we fit a Cox proportional hazards model using R [81] and computed a p-value using a likelihood ratio test for the genotype term. All SNPs with -values under after genomic control correction were considered genome-wide significant. The hazard ratio (HR) reported throughout can be interpreted as the multiplicative change in the rate of onset of myopia per copy of the minor allele (e.g., ). To test the proportional hazards assumption, we tested for independence of the scaled Schoenfeld residuals for each significant SNP and time using cox.zph (Table S2). Replication -values in Table 2 are one-sided -values from a likelihood ratio test for a logistic regression model controlling for age, sex, and five principal components.
For Figure 2, we computed a myopia propensity score for each individual as the (estimated) number of risk alleles among the 22 genome-wide significant SNPs. We then fit a Cox model including that score, sex, and five principal components. To estimate proportion variance explained for this model, we used a pseudo - using likelihoods (similar to the Nagelkerke pseudo for logistic regression). That is, we calculated the variance explained as
where is the null likelihood and the likelihood for the full model. This is one of several methods used to compute variance explained for Cox proportional hazards models [82].Supporting Information
Zdroje
1. VitaleS, SperdutoRD, FerrisFL (2009) Increased prevalence of myopia in the United States between 1971–1972 and 1999–2004. Arch Ophthalmol 127 : 1632–1639.
2. KempenJH, MitchellP, LeeKE, TielschJM, BromanAT, et al. (2004) The prevalence of refractive errors among adults in the United States, Western Europe, and Australia. Arch Ophthalmol 122 : 495–505.
3. WojciechowskiR (2011) Nature and nurture: the complex genetics of myopia and refractive error. Clin Genet 79 : 301–320.
4. RymerJ, WildsoetCF (2005) The role of the retinal pigment epithelium in eye growth regulation and myopia: a review. Vis Neurosci 22 : 251–261.
5. RadaJA, SheltonS, NortonTT (2006) The sclera and myopia. Exp Eye Res 82 : 185–200.
6. WildsoetC, WallmanJ (1995) Choroidal and scleral mechanisms of compensation for spectacle lenses in chicks. Vision Res 35 : 1175–1194.
7. MuttiDO, ZadnikK, AdamsAJ (1996) Myopia. The nature versus nurture debate goes on. Invest Ophthalmol Vis Sci 37 : 952–957.
8. FledeliusHC (1982) Ophthalmic changes from age of 10 to 18 years. A longitudinal study of sequels to low birth weight. IV. Ultrasound oculometry of vitreous and axial length. Acta Ophthalmol (Copenh) 60 : 403–411.
9. FanDS, LamDS, LamRF, LauJT, ChongKS, et al. (2004) Prevalence, incidence, and progression of myopia of school children in Hong Kong. Invest Ophthalmol Vis Sci 45 : 1071–1075.
10. FledeliusHC (1995) Myopia of adult onset. Can analyses be based on patient memory? Acta Ophthalmol Scand 73 : 394–396.
11. IribarrenR, CortinezMF, ChiappeJP (2009) Age of first distance prescription and final myopic refractive error. Ophthalmic Epidemiol 16 : 84–89.
12. IribarrenR, CerrellaMR, ArmestoA, IribarrenG, FornaciariA (2004) Age of lens use onset in a myopic sample of office-workers. Curr Eye Res 28 : 175–180.
13. IsazaG, AroraS (2012) Incidence and severity of retinopathy of prematurity in extremely premature infants. Can J Ophthalmol 47 : 296–300.
14. KennedyRH, BourneWM, DyerJA (1986) A 48-year clinical and epidemiologic study of keratoconus. Am J Ophthalmol 101 : 267–273.
15. RichardsAJ, McNinchA, MartinH, OakhillK, RaiH, et al. (2010) Stickler syndrome and the vitreous phenotype: mutations in COL2A1 and COL11A1. Hum Mutat 31: E1461–1471.
16. HammondCJ, SniederH, GilbertCE, SpectorTD (2001) Genes and environment in refractive error: the twin eye study. Invest Ophthalmol Vis Sci 42 : 1232–1236.
17. LyhneN, SjolieAK, KyvikKO, GreenA (2001) The importance of genes and environment for ocular refraction and its determiners: a population based study among 20–45 year old twins. Br J Ophthalmol 85 : 1470–1476.
18. TeikariJM, O'DonnellJ, KaprioJ, KoskenvuoM (1991) Impact of heredity in myopia. Hum Hered 41 : 151–156.
19. WojciechowskiR, CongdonN, BowieH, MunozB, GilbertD, et al. (2005) Heritability of refractive error and familial aggregation of myopia in an elderly American population. Invest Ophthalmol Vis Sci 46 : 1588–1592.
20. PeetJA, CotchMF, WojciechowskiR, Bailey-WilsonJE, StambolianD (2007) Heritability and familial aggregation of refractive error in the Old Order Amish. Invest Ophthalmol Vis Sci 48 : 4002–4006.
21. GossDA, JacksonTW (1996) Clinical findings before the onset of myopia in youth: 4. Parental history of myopia. Optom Vis Sci 73 : 279–282.
22. KleinAP, DuggalP, LeeKE, KleinR, Bailey-WilsonJE, et al. (2005) Support for polygenic influences on ocular refractive error. Invest Ophthalmol Vis Sci 46 : 442–446.
23. AshtonGC (1985) Segregation analysis of ocular refraction and myopia. Hum Hered 35 : 232–239.
24. SoloukiAM, VerhoevenVJ, van DuijnCM, VerkerkAJ, IkramMK, et al. (2010) A genome-wide association study identifies a susceptibility locus for refractive errors and myopia at 15q14. Nat Genet 42 : 897–901.
25. HysiPG, YoungTL, MackeyDA, AndrewT, Fernandez-MedardeA, et al. (2010) A genome-wide association study for myopia and refractive error identifies a susceptibility locus at 15q25. Nat Genet 42 : 902–905.
26. LiYJ, GohL, KhorCC, FanQ, YuM, et al. (2011) Genome-wide association studies reveal genetic variants in CTNND2 for high myopia in Singapore Chinese. Ophthalmology 118 : 368–375.
27. NakanishiH, YamadaR, GotohN, HayashiH, YamashiroK, et al. (2009) A genome-wide association analysis identified a novel susceptible locus for pathological myopia at 11q24.1. PLoS Genet 5: e1000660.
28. LiZ, QuJ, XuX, ZhouX, ZouH, et al. (2011) A genome-wide association study reveals association between common variants in an intergenic region of 4q25 and high-grade myopia in the Chinese Han population. Hum Mol Genet 20 : 2861–2868.
29. ShiY, QuJ, ZhangD, ZhaoP, ZhangQ, et al. (2011) Genetic variants at 13q12.12 are associated with high myopia in the Han Chinese population. Am J Hum Genet 88 : 805–813.
30. FanQ, BarathiVA, ChengCY, ZhouX, MeguroA, et al. (2012) Genetic variants on chromosome 1q41 influence ocular axial length and high myopia. PLoS Genet 8: e1002753.
31. YoungTL, MetlapallyR, ShayAE (2007) Complex trait genetics of refractive error. Arch Ophthalmol 125 : 38–48.
32. ShiY, LiY, ZhangD, ZhangH, LiY, et al. (2011) Exome sequencing identifies ZNF644 mutations in high myopia. PLoS Genet 7: e1002084.
33. VerhoevenVJ, HysiPG, SawSM, VitartV, MirshahiA, et al. (2012) Large scale international replication and meta-analysis study confirms association of the 15q14 locus with myopia. The CREAM consortium. Hum Genet 131 : 1467–1480.
34. PaluruPC, NallasamyS, DevotoM, RappaportEF, YoungTL (2005) Identification of a novel locus on 2q for autosomal dominant high-grade myopia. Invest Ophthalmol Vis Sci 46 : 2300–2307.
35. YangZ, XiaoX, LiS, ZhangQ (2009) Clinical and linkage study on a consanguineous Chinese family with autosomal recessive high myopia. Mol Vis 15 : 312–318.
36. NallasamyS, PaluruPC, DevotoM, WassermanNF, ZhouJ, et al. (2007) Genetic linkage study of high-grade myopia in a Hutterite population from South Dakota. Mol Vis 13 : 229–236.
37. PaluruP, RonanSM, HeonE, DevotoM, WildenbergSC, et al. (2003) New locus for autosomal dominant high myopia maps to the long arm of chromosome 17. Invest Ophthalmol Vis Sci 44 : 1830–1836.
38. BystromB, VirtanenI, RousselleP, GullbergD, Pedrosa-DomellofF (2006) Distribution of laminins in the developing human eye. Invest Ophthalmol Vis Sci 47 : 777–785.
39. PetersonPE, PowCS, WilsonDB, HendrickxAG (1995) Localisation of glycoproteins and glycosaminoglycans during early eye development in the macaque. J Anat 186 (Pt 1) 31–42.
40. MorissetteN, CarbonettoS (1995) Laminin alpha 2 chain (M chain) is found within the pathway of avian and murine retinal projections. J Neurosci 15 : 8067–8082.
41. BellSE, MavilaA, SalazarR, BaylessKJ, KanagalaS, et al. (2001) Differential gene expression during capillary morphogenesis in 3D collagen matrices: regulated expression of genes involved in basement membrane matrix assembly, cell cycle progression, cellular differentiation and G-protein signaling. J Cell Sci 114 : 2755–2773.
42. VeyrierasJB, KudaravalliS, KimSY, DermitzakisET, GiladY, et al. (2008) High-resolution mapping of expression-QTLs yields insight into human gene regulation. PLoS Genet 4: e1000214.
43. StrangerBE, NicaAC, ForrestMS, DimasA, BirdCP, et al. (2007) Population genomics of human gene expression. Nat Genet 39 : 1217–1224.
44. BadisG, BergerMF, PhilippakisAA, TalukderS, GehrkeAR, et al. (2009) Diversity and complexity in DNA recognition by transcription factors. Science 324 : 1720–1723.
45. BoyleA, HongE, HariharanM, ChengY, ShaubM, et al. (2012) Annotation of functional variation in personal genomes using RegulomeDB. Genome Res 22 : 1790–1797.
46. StraussO (2005) The retinal pigment epithelium in visual function. Physiol Rev 85 : 845–881.
47. WangNK, ChuangLH, LaiCC, ChouCL, ChuHY, et al. (2012) Multimodal fundus imaging in fundus albipunctatus with RDH5 mutation: a newly identified compound heterozygous mutation and review of the literature. Doc Ophthalmol 125 : 51–62.
48. NakamuraM, HottaY, TanikawaA, TerasakiH, MiyakeY (2000) A high association with cone dystrophy in Fundus albipunctatus caused by mutations of the RDH5 gene. Invest Ophthalmol Vis Sci 41 : 3925–3932.
49. WangQ, ChenQ, ZhaoK, WangL, WangL, et al. (2001) Update on the molecular genetics of retinitis pigmentosa. Ophthalmic Genet 22 : 133–154.
50. BernalS, CalafM, Garcia-HoyosM, Garcia-SandovalB, RosellJ, et al. (2003) Study of the involvement of the RGR, CRPB1, and CRB1 genes in the pathogenesis of autosomal recessive retinitis pigmentosa. J Med Genet 40: e89.
51. PattnaikBR, HughesBA (2012) Effects of KCNQ channel modulators on the M-type potassium current in primate retinal pigment epithelium. Am J Physiol, Cell Physiol 302: C821–833.
52. ZhangX, YangD, HughesBA (2011) KCNQ5/K(v)7.5 potassium channel expression and subcellular localization in primate retinal pigment epithelium and neural retina. Am J Physiol, Cell Physiol 301: C1017–1026.
53. NairKS, Hmani-AifaM, AliZ, KearneyAL, Ben SalemS, et al. (2011) Alteration of the serine protease PRSS56 causes angle-closure glaucoma in mice and posterior microphthalmia in humans and mice. Nat Genet 43 : 579–584.
54. OrrA, DubeMP, ZentenoJC, JiangH, AsselinG, et al. (2011) Mutations in a novel serine protease PRSS56 in families with nanophthalmos. Mol Vis 17 : 1850–1861.
55. GalA, RauI, El MatriL, KreienkampHJ, FehrS, et al. (2011) Autosomal-recessive posterior microphthalmos is caused by mutations in PRSS56, a gene encoding a trypsin-like serine protease. Am J Hum Genet 88 : 382–390.
56. ReisLM, TylerRC, SchilterKF, Abdul-RahmanO, InnisJW, et al. (2011) BMP4 loss-of-function mutations in developmental eye disorders including SHORT syndrome. Hum Genet 130 : 495–504.
57. BakraniaP, EfthymiouM, KleinJC, SaltA, BunyanDJ, et al. (2008) Mutations in BMP4 cause eye, brain, and digit developmental anomalies: overlap between the BMP4 and hedgehog signaling pathways. Am J Hum Genet 82 : 304–319.
58. LipskaBS, BrzeskwiniewiczM, WierzbaJ, MorzuchiL, PiotrowskiA, et al. (2011) 8.6 Mb interstitial deletion of chromosome 4q13.3q21.23 in a boy with cognitive impairment, short stature, hearing loss, skeletal abnormalities and facial dysmorphism. Genet Couns 22 : 353–363.
59. SchoenebeckJJ, HutchinsonSA, ByersA, BealeHC, CarringtonB, et al. (2012) Variation of BMP3 contributes to dog breed skull diversity. PLoS Genet 8: e1002849.
60. ScottSG, JunAS, ChakravartiS (2011) Sphere formation from corneal keratocytes and phenotype specific markers. Exp Eye Res 93 : 898–905.
61. GudbjartssonDF, WaltersGB, ThorleifssonG, StefanssonH, HalldorssonBV, et al. (2008) Many sequence variants affecting diversity of adult human height. Nat Genet 40 : 609–615.
62. LapanSW, ReddienPW (2011) dlx and sp6-9 Control optic cup regeneration in a prototypic eye. PLoS Genet 7: e1002226.
63. StoykovaA, FritschR, WaltherC, GrussP (1996) Forebrain patterning defects in Small eye mutant mice. Development 122 : 3453–3465.
64. Garcia-FrigolaC, CarreresMI, VegarC, MasonC, HerreraE (2008) Zic2 promotes axonal divergence at the optic chiasm midline by EphB1-dependent and -independent mechanisms. Development 135 : 1833–1841.
65. HerreraE, BrownL, ArugaJ, RachelRA, DolenG, et al. (2003) Zic2 patterns binocular vision by specifying the uncrossed retinal projection. Cell 114 : 545–557.
66. Garcia-FrigolaC, HerreraE (2010) Zic2 regulates the expression of Sert to modulate eye-specific refinement at the visual targets. EMBO J 29 : 3170–3183.
67. EsteveP, SandonisA, IbanezC, ShimonoA, GuerreroI, et al. (2011) Secreted frizzled-related proteins are required for Wnt/-catenin signalling activation in the vertebrate optic cup. Development 138 : 4179–4184.
68. EsteveP, SandonisA, CardozoM, MalapeiraJ, IbanezC, et al. (2011) SFRPs act as negative modulators of ADAM10 to regulate retinal neurogenesis. Nat Neurosci 14 : 562–569.
69. Garcia-HoyosM, CantalapiedraD, ArroyoC, EsteveP, RodriguezJ, et al. (2004) Evaluation of SFRP1 as a candidate for human retinal dystrophies. Mol Vis 10 : 426–431.
70. EsteveP, TrousseF, RodriguezJ, BovolentaP (2003) SFRP1 modulates retina cell differentiation through a beta-catenin-independent mechanism. J Cell Sci 116 : 2471–2481.
71. RodriguezJ, EsteveP, WeinlC, RuizJM, FerminY, et al. (2005) SFRP1 regulates the growth of retinal ganglion cell axons through the Fz2 receptor. Nat Neurosci 8 : 1301–1309.
72. FogelBL, WexlerE, WahnichA, FriedrichT, VijayendranC, et al. (2012) RBFOX1 regulates both splicing and transcriptional networks in human neuronal development. Hum Mol Genet 21 : 4171–4186.
73. LinJC, HoWH, GurneyA, RosenthalA (2003) The netrin-G1 ligand NGL-1 promotes the outgrowth of thalamocortical axons. Nat Neurosci 6 : 1270–1276.
74. OlivaC, EscobedoP, AstorgaC, MolinaC, SierraltaJ (2012) Role of the MAGUK protein family in synapse formation and function. Dev Neurobiol 72 : 57–72.
75. WalshT, PierceSB, LenzDR, BrownsteinZ, Dagan-RosenfeldO, et al. (2010) Genomic duplication and overexpression of TJP2/ZO-2 leads to altered expression of apoptosis genes in progressive nonsyndromic hearing loss DFNA51. Am J Hum Genet 87 : 101–109.
76. ErikssonN, MacphersonJM, TungJY, HonLS, NaughtonB, et al. (2010) Web-based, participant-driven studies yield novel genetic associations for common traits. PLoS Genet 6: e1000993.
77. TungJY, DoCB, HindsDA, KieferAK, MacphersonJM, et al. (2011) Efficient Replication of over 180 Genetic Associations with Self-Reported Medical Data. PLoS ONE 6: e23473.
78. ErikssonN, BentonGM, DoCB, KieferAK, MountainJL, et al. (2012) Genetic variants associated with breast size also influence breast cancer risk. BMC Med Genet 13 : 53.
79. ErikssonN, TungJY, KieferAK, HindsDA, FranckeU, et al. (2012) Novel associations for hypothyroidism include known autoimmune risk loci. PLoS ONE 7: e34442.
80. HennB, HonL, MacphersonJM, ErikssonN, SaxonovS, et al. (2012) Cryptic distant relatives are common in both isolated and cosmopolitan genetic samples. PLoS ONE 7: e34267.
81. Therneau T (2012) A Package for Survival Analysis in S. R package version 2.36-14.
82. SchemperM, StareJ (1996) Explained variation in survival analysis. Stat Med 15 : 1999–2012.
Štítky
Genetika Reprodukční medicína
Článek MSH3 Polymorphisms and Protein Levels Affect CAG Repeat Instability in Huntington's Disease MiceČlánek Yemanuclein and HIRA Cooperate for Assembly of H3.3-Containing Nucleosomes in the Male Pronucleus
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2013 Číslo 2
-
Všechny články tohoto čísla
- Complex Inheritance of Melanoma and Pigmentation of Coat and Skin in Grey Horses
- A Meta-Analysis of Thyroid-Related Traits Reveals Novel Loci and Gender-Specific Differences in the Regulation of Thyroid Function
- Genetic Landscape of Open Chromatin in Yeast
- Deleterious Alleles in the Human Genome Are on Average Younger Than Neutral Alleles of the Same Frequency
- Age-Dependent Transition from Cell-Level to Population-Level Control in Murine Intestinal Homeostasis Revealed by Coalescence Analysis
- Next-Generation Sequencing Identifies the Danforth's Short Tail Mouse Mutation as a Retrotransposon Insertion Affecting Expression
- ImmunoChip Study Implicates Antigen Presentation to T Cells in Narcolepsy
- Massive Mitochondrial Gene Transfer in a Parasitic Flowering Plant Clade
- Comment on “Genomic Hypomethylation in the Human Germline Associates with Selective Structural Mutability in the Human Genome”
- The Prefoldin Bud27 Mediates the Assembly of the Eukaryotic RNA Polymerases in an Rpb5-Dependent Manner
- Genetic Determinants of Trabecular and Cortical Volumetric Bone Mineral Densities and Bone Microstructure
- Encodes a Novel and -Genus-Specific Regulator of Photoperiodic Flowering in Rice
- Only One Isoform of CTP Synthase Forms the Cytoophidium
- Mechanisms Involved in the Functional Divergence of Duplicated GroEL Chaperonins in DK1622
- A Genome-Wide RNAi Screen in Identifies the Nicotinic Acetylcholine Receptor Subunit ACR-7 as an Antipsychotic Drug Target
- Autophagy Induction Is a Tor- and Tp53-Independent Cell Survival Response in a Zebrafish Model of Disrupted Ribosome Biogenesis
- Ancient DNA Reveals Prehistoric Gene-Flow from Siberia in the Complex Human Population History of North East Europe
- Inflammation-Mediated Genetic and Epigenetic Alterations Drive Cancer Development in the Neighboring Epithelium upon Stromal Abrogation of TGF-β Signaling
- MicroRNA-3148 Modulates Allelic Expression of Toll-Like Receptor 7 Variant Associated with Systemic Lupus Erythematosus
- RNAi–Based Functional Profiling of Loci from Blood Lipid Genome-Wide Association Studies Identifies Genes with Cholesterol-Regulatory Function
- CELF Family RNA–Binding Protein UNC-75 Regulates Two Sets of Mutually Exclusive Exons of the Gene in Neuron-Specific Manners in
- Coordination of Chromatid Separation and Spindle Elongation by Antagonistic Activities of Mitotic and S-Phase CDKs
- The Ubiquitin Ligase Subunit Acts in Target Tissue to Restrict Tracheal Terminal Cell Branching and Hypoxic-Induced Gene Expression
- Mitotic Evolution of Shows a Stable Core Genome but Recombination in Antigen Families
- Tysnd1 Deficiency in Mice Interferes with the Peroxisomal Localization of PTS2 Enzymes, Causing Lipid Metabolic Abnormalities and Male Infertility
- A Regulatory Pathway, Ecdysone-Transcription Factor Relish-Cathepsin L, Is Involved in Insect Fat Body Dissociation
- PcG-Mediated Higher-Order Chromatin Structures Modulate Replication Programs at the BX-C
- MSH3 Polymorphisms and Protein Levels Affect CAG Repeat Instability in Huntington's Disease Mice
- JNK-Interacting Protein 3 Mediates the Retrograde Transport of Activated c-Jun N-Terminal Kinase and Lysosomes
- Discovery of a Splicing Regulator Required for Cell Cycle Progression
- Rearrangements of 2.5 Kilobases of Noncoding DNA from the Locus Define Predictive Rules of Genomic -Regulatory Logic
- Admixture Mapping in Lupus Identifies Multiple Functional Variants within IFIH1 Associated with Apoptosis, Inflammation, and Autoantibody Production
- Roles of the Developmental Regulator Homothorax in Limiting Longevity in
- miR-199a-5p Is Upregulated during Fibrogenic Response to Tissue Injury and Mediates TGFbeta-Induced Lung Fibroblast Activation by Targeting Caveolin-1
- A Kinome-Wide RNAi Screen in Glia Reveals That the RIO Kinases Mediate Cell Proliferation and Survival through TORC2-Akt Signaling in Glioblastoma
- Assembly of the Auditory Circuitry by a Genetic Network in the Mouse Brainstem
- SOX2 Co-Occupies Distal Enhancer Elements with Distinct POU Factors in ESCs and NPCs to Specify Cell State
- Retrotransposon Activates Ectopic Expression: A Short Tail
- Confounding by Repetitive Elements and CpG Islands Does Not Explain the Association between Hypomethylation and Genomic Instability
- Cell Reprogramming Requires Silencing of a Core Subset of Polycomb Targets
- Properties and Modeling of GWAS when Complex Disease Risk Is Due to Non-Complementing, Deleterious Mutations in Genes of Large Effect
- Essential Developmental, Genomic Stability, and Tumour Suppressor Functions of the Mouse Orthologue of
- Conditional Inactivation of the DNA Damage Response Gene in Mouse Testis Reveals Separable Roles for Components of the RAD9-RAD1-HUS1 Complex in Meiotic Chromosome Maintenance
- Genome-Wide Analysis Points to Roles for Extracellular Matrix Remodeling, the Visual Cycle, and Neuronal Development in Myopia
- Patterning of Leaf Vein Networks by Convergent Auxin Transport Pathways
- An Evolutionary Perspective on Epistasis and the Missing Heritability
- A Retrotransposon Insertion in the 5′ Regulatory Domain of Ptf1a Results in Ectopic Gene Expression and Multiple Congenital Defects in Danforth's Short Tail Mouse
- The Mub1/Ubr2 Ubiquitin Ligase Complex Regulates the Conserved Dsn1 Kinetochore Protein
- Mutations Can Cause Enamel-Renal Syndrome (ERS)
- Yemanuclein and HIRA Cooperate for Assembly of H3.3-Containing Nucleosomes in the Male Pronucleus
- Hepatocyte Growth Factor, a Determinant of Airspace Homeostasis in the Murine Lung
- ISWI and CHD Chromatin Remodelers Bind Promoters but Act in Gene Bodies
- COM-1 Promotes Homologous Recombination during Meiosis by Antagonizing Ku-Mediated Non-Homologous End Joining
- Control of Multicellular Development by the Physically Interacting Deneddylases DEN1/DenA and COP9 Signalosome
- Antagonism Versus Cooperativity with TALE Cofactors at the Base of the Functional Diversification of Hox Protein Function
- Dynamic Association of NUP98 with the Human Genome
- Ectopic Expression of Induces Spinal Defects, Urogenital Defects, and Anorectal Malformations in Mice
- Regulation of Contributes to the Lineage Potential of Neurogenin3+ Endocrine Precursor Cells in the Pancreas
- Gene-Based Testing of Interactions in Association Studies of Quantitative Traits
- The Amidation Step of Diphthamide Biosynthesis in Yeast Requires , a Gene Identified through Mining the - Interaction Network
- Plant-Symbiotic Fungi as Chemical Engineers: Multi-Genome Analysis of the Clavicipitaceae Reveals Dynamics of Alkaloid Loci
- Genome-Wide Diversity in the Levant Reveals Recent Structuring by Culture
- DNA Methylation Mediated Control of Gene Expression Is Critical for Development of Crown Gall Tumors
- Identification of the SlmA Active Site Responsible for Blocking Bacterial Cytokinetic Ring Assembly over the Chromosome
- Expression of a Novel P22 ORFan Gene Reveals the Phage Carrier State in Typhimurium
- Altered Cohesin Gene Dosage Affects Mammalian Meiotic Chromosome Structure and Behavior
- Quantitative Analysis of Histone Modifications: Formaldehyde Is a Source of Pathological N-Formyllysine That Is Refractory to Histone Deacetylases
- Duplicate Abalone Egg Coat Proteins Bind Sperm Lysin Similarly, but Evolve Oppositely, Consistent with Molecular Mimicry at Fertilization
- Lessons from on the Strengths and Weaknesses of Structured Association Mapping
- DNA–Methylome Analysis of Mouse Intestinal Adenoma Identifies a Tumour-Specific Signature That Is Partly Conserved in Human Colon Cancer
- Transposon Variants and Their Effects on Gene Expression in
- Polygenic Modeling with Bayesian Sparse Linear Mixed Models
- Single Transmembrane Peptide DinQ Modulates Membrane-Dependent Activities
- The JNK Signaling Pathway Activates Expression of Stress Response Genes by Derepressing the Fos/HDAC Repressor Complex
- The Interaction of CtIP and Nbs1 Connects CDK and ATM to Regulate HR–Mediated Double-Strand Break Repair
- Regulation of Metamorphosis by Xenobiotic Response Regulators
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Complex Inheritance of Melanoma and Pigmentation of Coat and Skin in Grey Horses
- Coordination of Chromatid Separation and Spindle Elongation by Antagonistic Activities of Mitotic and S-Phase CDKs
- Autophagy Induction Is a Tor- and Tp53-Independent Cell Survival Response in a Zebrafish Model of Disrupted Ribosome Biogenesis
- Assembly of the Auditory Circuitry by a Genetic Network in the Mouse Brainstem
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání





