-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaThe SPF27 Homologue Num1 Connects Splicing and Kinesin 1-Dependent Cytoplasmic Trafficking in
The conserved NineTeen protein complex (NTC) is an integral subunit of the spliceosome and required for intron removal during pre-mRNA splicing. The complex associates with the spliceosome and participates in the regulation of conformational changes of core spliceosomal components, stabilizing RNA-RNA - as well as RNA-protein interactions. In addition, the NTC is involved in cell cycle checkpoint control, response to DNA damage, as well as formation and export of mRNP-particles. We have identified the Num1 protein as the homologue of SPF27, one of NTC core components, in the basidiomycetous fungus Ustilago maydis. Num1 is required for polarized growth of the fungal hyphae, and, in line with the described NTC functions, the num1 mutation affects the cell cycle and cell division. The num1 deletion influences splicing in U. maydis on a global scale, as RNA-Seq analysis revealed increased intron retention rates. Surprisingly, we identified in a screen for Num1 interacting proteins not only NTC core components as Prp19 and Cef1, but several proteins with putative functions during vesicle-mediated transport processes. Among others, Num1 interacts with the motor protein Kin1 in the cytoplasm. Similar phenotypes with respect to filamentous and polar growth, vacuolar morphology, as well as the motility of early endosomes corroborate the genetic interaction between Num1 and Kin1. Our data implicate a previously unidentified connection between a component of the splicing machinery and cytoplasmic transport processes. As the num1 deletion also affects cytoplasmic mRNA transport, the protein may constitute a novel functional interconnection between the two disparate processes of splicing and trafficking.
Published in the journal: . PLoS Genet 10(1): e32767. doi:10.1371/journal.pgen.1004046
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004046Summary
The conserved NineTeen protein complex (NTC) is an integral subunit of the spliceosome and required for intron removal during pre-mRNA splicing. The complex associates with the spliceosome and participates in the regulation of conformational changes of core spliceosomal components, stabilizing RNA-RNA - as well as RNA-protein interactions. In addition, the NTC is involved in cell cycle checkpoint control, response to DNA damage, as well as formation and export of mRNP-particles. We have identified the Num1 protein as the homologue of SPF27, one of NTC core components, in the basidiomycetous fungus Ustilago maydis. Num1 is required for polarized growth of the fungal hyphae, and, in line with the described NTC functions, the num1 mutation affects the cell cycle and cell division. The num1 deletion influences splicing in U. maydis on a global scale, as RNA-Seq analysis revealed increased intron retention rates. Surprisingly, we identified in a screen for Num1 interacting proteins not only NTC core components as Prp19 and Cef1, but several proteins with putative functions during vesicle-mediated transport processes. Among others, Num1 interacts with the motor protein Kin1 in the cytoplasm. Similar phenotypes with respect to filamentous and polar growth, vacuolar morphology, as well as the motility of early endosomes corroborate the genetic interaction between Num1 and Kin1. Our data implicate a previously unidentified connection between a component of the splicing machinery and cytoplasmic transport processes. As the num1 deletion also affects cytoplasmic mRNA transport, the protein may constitute a novel functional interconnection between the two disparate processes of splicing and trafficking.
Introduction
The basidiomycete Ustilago maydis is the causal agent of the smut disease on corn (Zea mays). During its life cycle, U. maydis displays yeast-like, haploid cells that divide by budding, and dikaryotic cells that grow as filamentous hyphae. The filamentous stage is initiated by fusion of two yeast-like cells (sporidia) and marks the onset of the biotrophic stage in which the fungus depends on its host plant for propagation. The switch to polarized growth to form the elongated filament is indispensable for the successful infection of the host plant.
The regulatory circuits that underlie the dimorphic switch and concurrent pathogenic development in U. maydis have been well studied within the past years. Similar to other basidiomycetes, a heterodimeric complex of two homeodomain transcription factors, both encoded by the b-mating type locus, represents the major regulatory instance. The two proteins, termed bE and bW in U. maydis, form a functional heterodimer, but only if they originate from different b-alleles (e.g. bE1 and bW2). Hyphae formed upon activation of the b-pathway grow unipolar, but only the tip compartment is filled with cytoplasm and contains the two genetically distinct nuclei, while the distal part of the hypha is composed of empty segments separated by evenly spaced retraction septa. Simultaneously with the switch towards filamentous growth, cells become arrested in the G2 phase of the cell cycle. Only after the penetration of the plant surface, this cell cycle block is released, and the “true” filament with multiple septated compartments is developed [for recent review, see 1].
The bE/bW-heterodimer orchestrates a hierarchic, multi-layered transcriptional network. Only few b-responsive genes are direct targets of the bE/bW heterodimer. The majority of the genes is regulated via the b-dependently induced C2H2 zinc-finger transcription factor Rbf1. Rbf1 is the master regulator for several other transcription factors that coordinate expression of multiple genes associated with cell cycle coordination, morphogenesis and pathogenic development. Deletion of rbf1 prevents the formation of the b-dependent filaments, and ectopic expression of rbf1 is sufficient to induce the dimorphic transition. Thus, rbf1 is both sufficient as well as required for the switch from budding to polarized filamentous growth [2].
Prerequisite for the growth of the filamentous hyphae is the establishment and maintenance of a defined axis of polarity. The filaments expand by polar tip growth, which is dependent on long-distance transport towards the growth cones at the cell apices. This directed transport is facilitated by arrays of polarized microtubules and a highly conserved set of microtubule-dependent kinesin and dynein motor proteins [reviewed in 3]. The cellular cargos that rely on microtubule-based transport include endosomes, peroxisomes and nuclei, but also mRNA, which, as shown recently in U. maydis, is as well instrumental for establishment and maintenance of polarity [4]–[6]. The latter, however, is not a direct microtubule-based cargo, but passively travels on endosomes [7]. In U. maydis, transport is mainly mediated through the concerted action of the plus-end directed UNC104-like Kinesin-3 motor protein Kin3, which moves endosomes in both directions within the cell along an array of antiparallel microtubules, and the minus-end directed dynein motor protein Dyn1/2 [8]–[11]. The minus-end directed Dyn1/2 is particularly important at the poles of filaments, where unipolar microtubules, with their plus-ends directed to the tip, extend from the antiparallel array [9], [12]. The conventional kinesin motor protein Kin1 additionally contributes to the establishment of hyphal morphology by transporting Dynein in the direction of the microtubule plus-ends within the hyphal apex where a loading zone for the retrograde transport processes is established [9], [12]. Other known cargos for the Kin1 motor protein include membranous structures; Kin1 was previously described to be involved in organelle transport [13] and to foster the transport of secretory vesicles into the growing tip [14]. More recently, transport of the fungal-specific class-17 myosin Mcs1 was shown to dependent directly on Kin1. Mcs1 is attached to vesicles and contains an N-terminal myosin motor domain fused to a chitin synthase region. Anterograde trafficking of Mcs1-positive vesicles requires both microtubules and filamentous actin and depends on Myosin-5 and Kinesin-1, which cooperate in delivering vesicles to the sites of exocytosis [15]–.
In an effort to achieve a better understanding of the complex processes required for the establishment and maintenance of the dikaryotic hyphae in U. maydis, we employed a candidate approach with genes that, based on function or phenotype in other systems, were anticipated to be involved in nuclear migration and determination of cell polarity. Here, we describe the molecular characterization of one of these genes, num1. The num1 mutation has originally been identified in the basidiomycete Coprinopsis cinerea in a screen for mutants affected in the nuclear migration during the formation of the heterokaryotic hyphae [18].
We show now that Num1 is a homologue of SPF27, one of the core components of the highly conserved Prp19/CDC5L or NineTeen (NTC) splicing complex [19].
Unexpectedly, we identified the conventional Kinesin 1 motor protein Kin1 [13], [20] to interact with Num1. Similar phenotypes of Δnum1 and Δkin1 hyphae corroborate a functional interconnection between the two proteins. Our data implicate a previously unidentified connection between a component of the splicing machinery and cytoplasmic (Kin1-dependent) transport processes in U. maydis.
Results
Num1, a conserved BCAS2/SPF27-like protein, is required for polar growth and septum formation
In the basidiomycete Coprinopsis cinerea, the num1 gene was identified in a screen for mutants affected in nuclear migration during the initial phase of sexual development [18]. Unlike U. maydis, C. cinerea grows strictly as a filament, and mating is initiated by fusion of two different haploid hyphae harboring compatible alleles of the mating type loci. The dikaryotic filament is then generated upon migration of “donor”-nuclei into the “acceptor”-mycelium. The num1 mutation results in strains that are still able to donate nuclei, but fail to accept nuclei in compatible mating reactions [18]. To examine whether the gene has a conserved function in U. maydis with respect to nuclear migration, we set out to analyze the potential U. maydis homologue. The U. maydis predicted protein Um01682 (MIPS Ustilago maydis Database (MUMDB), http://mips.helmholtz-muenchen.de/genre/proj/ustilago/) exhibits 43% identity and 63% similarity with the C. cinerea Num1 protein, and 33% identity to the human SPF27 protein (Figure S1). SPF27 homologues are found in all eukaryotic clades, with the exception of the Saccharomycetales (Figure S2). SPF27 is part of the Prp19/CDC5L complex, which is an integral component of active spliceosomes and required for intron removal [21]. Similar to the SPF27 homologues, the U. maydis protein harbors a BCAS2 domain (breast carcinoma amplified sequence 2) with a so far unknown function and a classical, basically charged nuclear localization signal (Figure S1) [22].
To determine the function of Num1 in U. maydis, the gene was deleted in the strains FB1, FB2, SG200 and AB31. FB1 (a1 b1) and FB2 (a2 b2) are compatible wild-type strains that, upon mating, form filaments on solid media supplemented with charcoal. SG200 (a1 mfa2 bE1 bW2) forms such filaments without a mating partner, as the strain harbors a compatible combination of the bE1 and bW2 genes [23]. For both FB1/FB2 and SG200, cell division upon filament formation is stalled in axenic culture, and hyphal proliferation depends on the plant host. Polarized growth in axenic culture can be best monitored in strain AB31 (a1 Pcrg1:bE1/Pcrg1:bW2), which harbors a set of compatible bE1 and bW2 genes under control of the arabinose-responsive Pcrg1-promoter [24]. Upon arabinose-induced expression of bE1/bW2, AB31 switches from budding - to polarized growth and forms long filaments reminiscent of those formed after fusion of compatible FB1 and FB2 sporidia [2], [24].
Yeast-like cells of AB31Δnum1 - and SG200Δnum1 showed no obvious phenotype when grown in axenic culture in complete or minimal media (Figure S3). In plant infection assays, the mixture of the compatible strains FB1Δnum1 and FB2Δnum1 as well as SG200Δnum1 showed reduced disease symptoms compared to the respective wild-type strains; both the number of plants with tumors as well as the size of tumors decreased (Figure S3). However, microscopic observation of hyphae after plant infection did not reveal major morphological differences to wild-type strains (Figure S3). The cell-cell fusion of compatible sporidia to generate the dikaryotic hyphae, which can be visualized by the appearance of “fuzzy” colonies on charcoal containing media, is not altered by the num1 mutation (Figure S3). However, the phenotype of conjugation hyphae upon treatment with synthetic pheromone as well as the phenotype of the dikaryotic hyphae showed considerable alterations from wild-type strains. Pheromone treatment of Δnum1 strains led to the formation of branched conjugation tubes that are never observed in wild-type strains. Dikaryotic hyphae of Δnum1 strains appeared thicker, branched, and fused sporidia displayed bipolar growth; in wild-type strains, however, the fusion event is initiated only from a single cell pole (Figures S4 and S5). To analyze the phenotype of the hyphae in more detail, we employed strain AB31 and induced the formation of the filament via the expression of bE1/bW2. Similar to the dikaryotic hyphae, AB31Δnum1 filaments displayed severe alterations. 95% of the filaments were significantly shorter than wild-type AB31 filaments (average length of 68.9 µm compared to 121.7 µm, t-test: p = 6.35×10−46). Filament formation upon b-induction in AB31 is initiated at one of the cell poles of the sporidium; the resulting hyphae grow unbranched and strictly unipolar (Figure 1A). In AB31Δnum1, hyphae generally exhibited an irregular and curved morphology (Figure 1B, Table 1). In 18% of the sporidia, hyphae initiated at both cell poles (bipolar, Figure 1C, Table 1), and 4.4% exhibited a branched morphology (N = 461) (Figure 1D, Table 1). In addition, 21.2% of the AB31Δnum1 filaments had delocalized septa, and 2.4% of the hyphae generated empty compartments at the cell tips (Figure 1E and F, Table 1). Septa in AB31Δnum1 could be visualized by different chitin-stains, as Calcofluor [25], Congo Red [26] or fluorescein-conjugated wheat germ agglutinin (WGA) [27] (Figures 1E and S6; see also Figure S6 legend). However, in contrast to septa in the dikaryotic hyphae, septa in AB31Δnum1 were not always visible in the DIC channel, and staining with fluorescein-WGA revealed extensive chitin accumulations or ring-like chitin structures throughout the hyphae, indicating that these septa or septa-like structures may be structurally different from wild-type septa.
Fig. 1. Num1 is required for polar growth and septum formation. 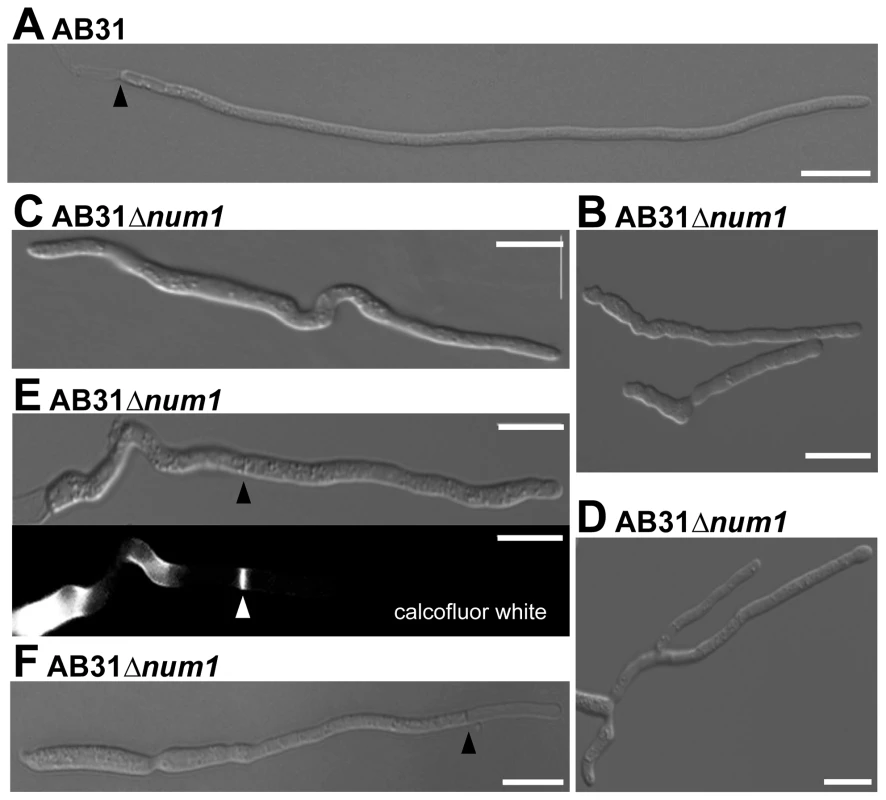
Filament formation of AB31 wild-type and AB31Δnum1 deletion strains was monitored 12–14 hours upon induction of the bE1/bW2 heterodimer. (A) AB31 wild-type cells grow as long, straight filaments; only the tip compartment is filled with cytoplasm, separated from highly vacuolated distal compartments by a septum (arrowhead). (B) AB31Δnum1 hyphae grow shorter and more curved. In AB31Δnum1, bipolar (C) and branched (D) hyphae are observed frequently. (E) Septa of AB31Δnum1 are often formed within the compartment filled with cytoplasm (arrowheads denote delocalized septum; in lower panel, Calcofluor White staining was used to visualize the septum). (F) In approx. 2.5% of hyphae delocalized septa (arrowhead) lead to empty tip compartments. Scale bars: 10 µm. Tab. 1. Quantification of phenotypes in AB31 and AB31Δnum1 12 hours after the induction of the bE/bW-heterodimer. 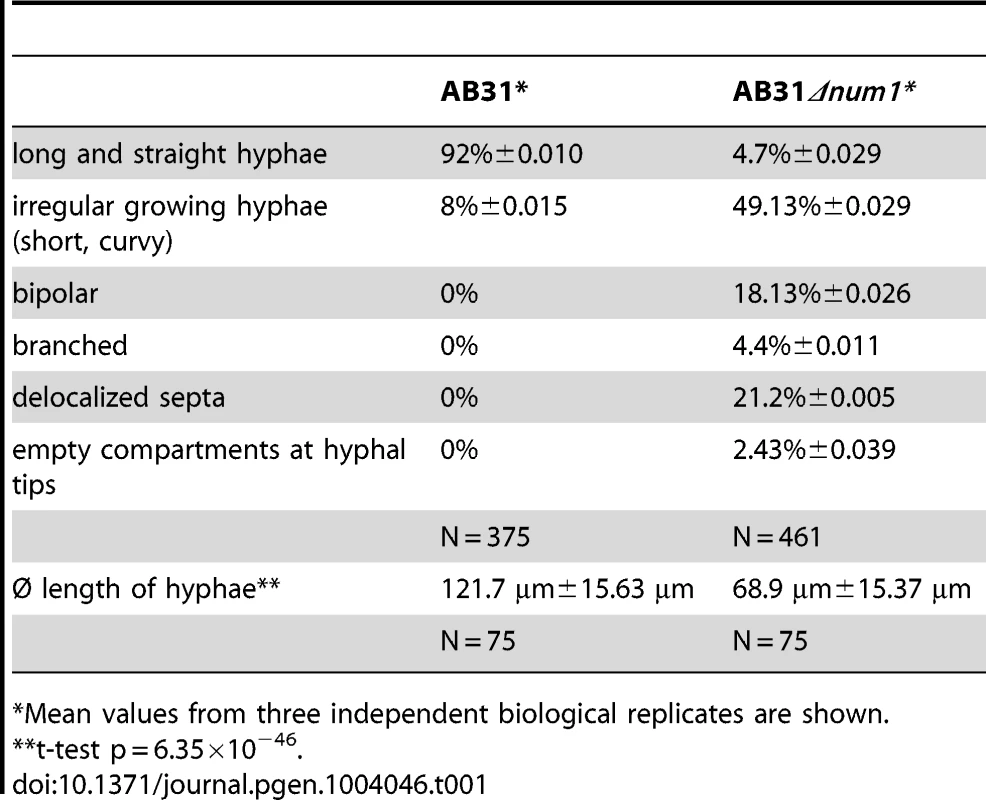
Mean values from three independent biological replicates are shown. To ensure that the observed phenotype is due to the num1 deletion, a construct harboring a num1:eGFP fusion gene under control of the arabinose inducible Pcrg1-promoter was introduced in single copy into the cbx locus. Strain UNK220 (AB31Δnum1 Pcrg1:num1:egfp) was grown in arabinose-containing media to induce both the bE1/bW2 heterodimer as well as the num1:eGFP gene; the induced filaments were indistinguishable from that of the wild-type AB31 strain (Figure S7).
Num1 is a component of the highly conserved spliceosome-associated Prp19/CDC5L-complex
Num1 has striking similarities to the human BCAS2/SPF27 protein that has been identified as one of the four core components (Prp19, PLR1, CDC5L and SPF27) of the Prp19/CDC5L spliceosome-associated complex, commonly termed NineTeen Complex (NTC) (Figure 2A). To assess whether Num1 is a structural component of the putative NTC complex in U. maydis, we employed a directed yeast two-hybrid approach using the full-length Num1 protein as bait as well as full-length and C - and N-terminal versions of the Prp19 (Um10027) and CDC5L-homologues (termed Cef1, Um04411) as prey, respectively. Consistent with the previously identified interactions in S. pombe [28], Num1 interacts with the Prp19 N-terminus as well as the Cef1 C-terminus (Figure 2B). The interaction of Num1 with Prp19 and Cef1 was verified by in vivo co-immunoprecipitation. num1 was replaced by an eGFP-tagged version of the gene in strain AB31 (a2, Pcrg:bW2, bE1), and additionally prp19 or cef1 were exchanged with HA-tagged versions, resulting in strains UNK200 (AB31 num1 : 3×eGFP:hygR, prp19 : 3×HA:cbxR) and UMO8 (AB31 num1 : 3×eGFP:hygR, cef1 : 3×HA:cbxR), respectively. All genes were introduced via homologous recombination into their native loci to ensure native protein levels. All fusion proteins were shown to be functional (Supporting Text S1, Figure S8). By use of an anti-HA-antibody, Num1 : 3eGFP was specifically co-precipitated with both Prp19:HA and Cef1:HA in protein extracts from strains UNK200 and UMO8 (Figure 2C). The interaction of Num1 with Prp19 and Cef1 was further corroborated by their localization. Num1 : 3eGFP and Prp19:RFP or Cef1:RFP were co-expressed in strain AB31, and all proteins co-localized in the nuclei (Figure 2E and F). While for Prp19:RFP and Cef1:RFP the fluorescence signal was exclusively localized in the nucleus, Num1 : 3eGFP was also detected in the cytoplasm (Figure 2D). Transformation of AB31Δnum1 with a num1:eGFP derivative harboring a mutated nuclear localization sequence (KKRK to AAAA) did not lead to complementation of the Δnum1 phenotype, and the fusion protein localized predominantly in the cytoplasm (Figure S7), which implies that nuclear localization of Num1 is required for function of the protein.
Fig. 2. Num1 interacts with components of the Prp19/Cdc5L complex. 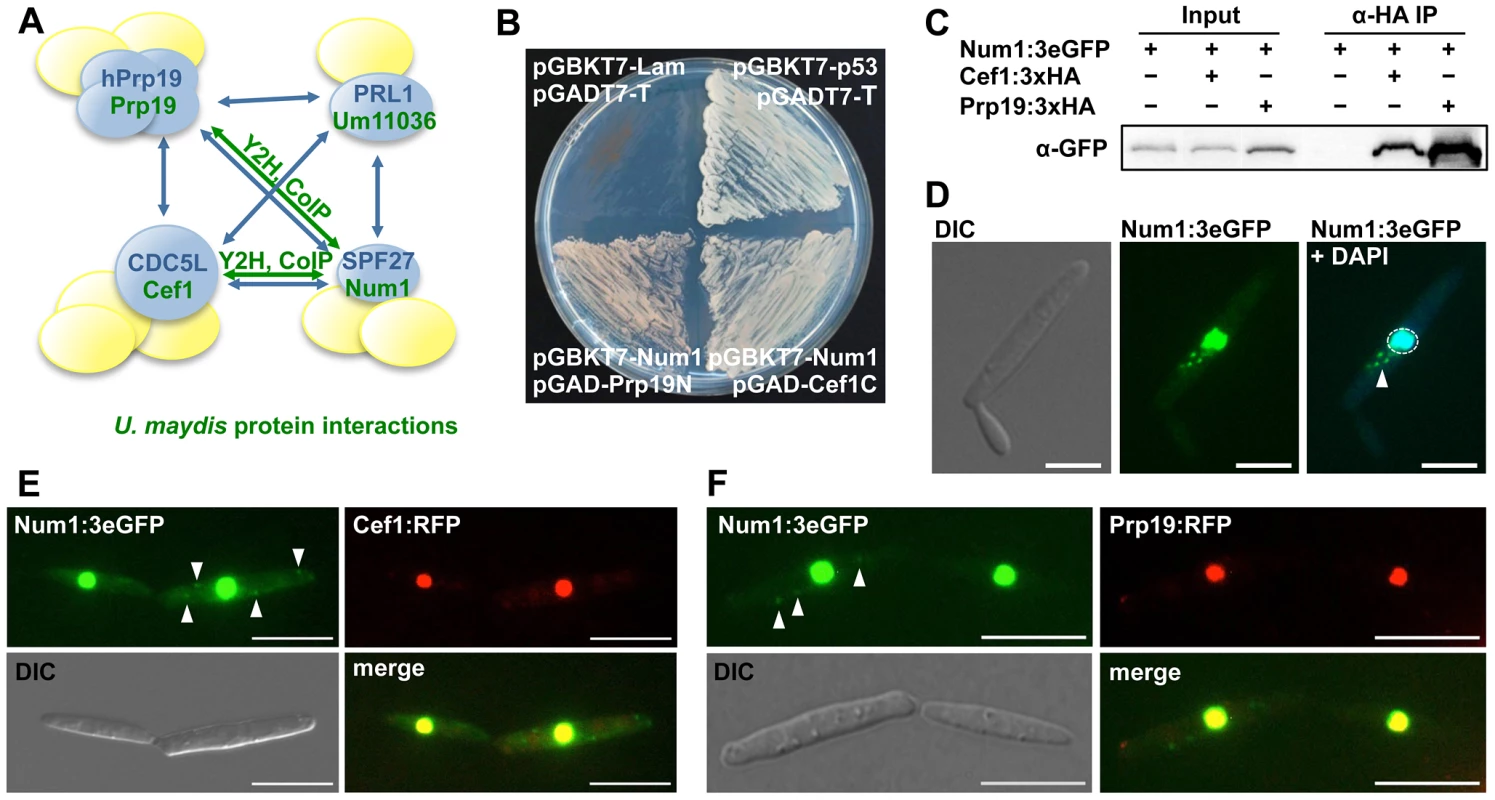
(A) Schematic representation of the protein-protein interactions (blue arrows) within the human hPrp19/CDC5L-complex [21]. Proteins depicted in blue represent the core complex, additional components (yellow) are associated with individual proteins. Green arrows indicate U. maydis protein interactions identified in this study. (B) The Prp19 N-terminus and the Cef1 C-terminus interact with the full length Num1 protein in a yeast two-hybrid analysis. p53/SV40 T-antigen and Lamin/SV40 T-antigen (Clontech) serve as positive and negative control, respectively. (C) In vivo co-immunoprecipitation of HA-tagged Prp19 and Cef1 and 3eGFP-tagged Num1. α-HA coupled agarose beads were used to precipitate Prp19 and Cef1, respectively. The Num1 protein was detected on a Western blot using an α-GFP antibody. The negative control shows no unspecific binding of the Num1 protein to α-HA agarose. (D) Subcellular localization of the Num1:3eGFP fusion protein. In addition to the nuclear localization of Num1 (enclosed by dashed circle), a cytoplasmic distribution to distinct foci can be observed (arrowhead). Scale bar: 5 µm. (E, F) Num1:3eGFP as well as Cef1:RFP and Prp19:RFP were co-expressed in AB31 under control of their native promoters. Num1 co-localizes with both Cef1 and Prp19 in the nuclei. Additional cytoplasmic Num1:3eGFP foci are indicated by arrowheads. Scale bars: 10 µm. In addition to their function in mRNA splicing, NTC and NTC-associated proteins are known to be involved in several other cellular processes. In S. pombe and S. cerevisiae, Cdc5 and Cef1p, respectively, were originally identified as cell division cycle mutants that are required for G2 progression and mitotic entry [29]–[31], and Prp19p has been isolated in S. cerevisiae in a screen for mutants conferring sensitivity to DNA damaging agents [32]. It has been proposed that Prp19p homologues play a direct role in DNA damage repair that is conserved from yeast to human [33]–[38].
To define whether Num1 acts in line with previously known functions of the NTC-complex, we investigated cell cycle regulation and the response to DNA damage in AB31Δnum1. In AB31, the filaments generated after induction of the bE1/bW2 heterodimer are arrested in the G2 phase of the cell cycle, harboring a single haploid nucleus that is positioned in the tip compartment [39]. In filaments of AB31Δnum1, however, deviations from this pattern became evident. 18% of the filaments (N = 100) exhibited two (or more) nuclei within a single hyphal compartment; additionally, compartments devoid of any nuclei were generated, most likely due to the aberrant formation of septa (Figure 3A). In accordance with this observation, FACS analysis of AB31 cells revealed that upon prolonged induction of the bE1/bW2-heterodimer cells with a 2C DNA content accumulated, whereas in AB31Δnum1, an increased number of cells with both 1C and 2C DNA content was observed (Figure S9), indicative for a deregulated cell cycle. Taken together, our results implicate that the num1 deletion affects both cell cycle regulation as well as cell division.
Fig. 3. Num1 influences the cell cycle and cell division as well as the cellular response to DNA damage. 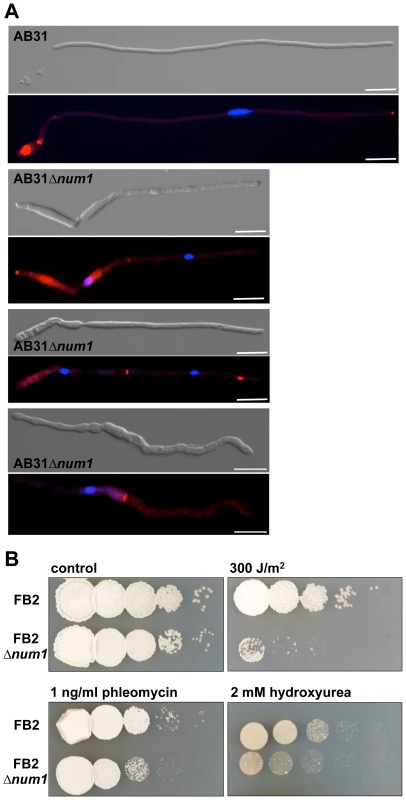
(A) Induction of the bE1/bW2 heterodimer in wild-type cells (AB31) induces a G2 cell cycle arrest, resulting in hyphae with a single nucleus positioned in the tip compartment. In AB31Δnum1, 18% of the hyphae contain more than one nucleus, usually separated by a septum or a septum-like chitin structure, which is not always visible in the DIC-channel (see also Figure S6). In some cases, delocalized septa give rise to compartments that are devoid of any nuclei (bottom panel). Filaments were stained with DAPI and Congo Red, which effectively stains chitin in fungal cell walls [26], to visualize nuclei and septa within the same hypha. Scale bars: 10 µm. (B) Sensitivity of an FB2Δnum1 deletion strain to DNA-damage in comparison to wild-type cells. Serial 10-fold dilutions were spotted on complete medium containing the indicated DNA-damage inducing supplements, or subsequently irradiated with 300 J/m2 UV light (254 nm), respectively. Pictures were taken 2–3 days after incubation at 28°C. Consistent with the function of the NTC in response to DNA damage, we found that Δnum1 cells exhibited increased sensitivity to UV irradiation and DNA-damaging agents. The survival rate upon UV-treatment was reduced approximately by a factor of 10 in AB31Δnum1 (Figure 3B). Similarly, treatment with phleomycin, causing double-strand breaks in DNA [40] or hydroxyurea, which triggers stalling of the replication fork during DNA synthesis [41], led to reduced survival of Δnum1 cells (Figure 3B).
Num1 affects splicing of mRNA on a global scale
To assess whether the Δnum1 mutation affects splicing, we employed next-generation RNA sequencing (RNA-Seq) to determine the transcriptome of strains AB31 and AB31Δnum1. bE1/bW2 expression was induced for eight hours; at this time point, in both strains a comparable quantity of filamentous cells was observed. The expression levels of bW, of the central regulator rbf1 (a direct b-target gene) and of num1 were monitored by quantitative RT-PCR analysis to ensure similar expression levels (Figure S10). For both AB31 and AB31Δnum1, RNA-Seq experiments from three independent biological samples were performed, and sequences were compared to the manually curated U. maydis gene models of the MIPS Ustilago maydis Database (MUMDB). Splicing efficiency was calculated from the normalized read number within introns in relation to the normalized read number of exon regions of a gene (for details, see Materials and Methods). Based on 2142 intron sequences, introns of AB31 genes were spliced with an average efficiency of 0.93 (±0.13); splicing efficiency in AB31Δnum1 was significantly lower (factor −1.44, t-test p = 6.22*10−111), with an average of 0.80 (±0.23). The splicing defect observed by RNA-Seq was verified for a small set of genes by qRT-PCR (Figure S11). In general, we noticed a wide variation in splicing efficiency of different introns in response to the num1-deletion. For 80.2% of the introns (1717 of 2142), the intron retention rate (fraction of RNA in intronic sequences in relation to exonic sequences) in AB31Δnum1 was at least twice as high as in the wild-type strain, indicating that Num1 is required for splicing in general. We noticed, however, that for few introns (83, allowing a 10% threshold) splicing was not affected, while other introns were barely spliced at all in AB31Δnum1. Differences in intron retention rates were specific for discrete introns, and not for particular transcripts, as we observed that in genes with more than one intron separate introns were spliced to different extent (Table S1, examples are shown in Figure S12).
It has been proposed that the function of the NTC with regards to cell cycle regulation or DNA damage repair may result from the decreased splicing efficiency of intron-containing genes involved in recombination, repair, cell cycle or chromosome segregation [42]. We did not observe an enrichment of genes with high intron retention rates in any “category for functional annotation” (FunCat). However, inspection of genes in the FunCats “cell cycle” and “DNA repair” (Table S2) revealed that for individual genes involved in DNA repair splicing is impaired, as, for example, for rad51 (um03290), for the gene for the repair exonuclease Rec1 (um11016), or for mms2 (um10097), involved in the error-free post-replication repair. Similarly, splicing of genes involved in cell cycle control, as cks1 (um03210), encoding a regulatory subunit of cyclin-dependent kinases, or the gene for the cyclin dependent kinase 1 (cdk1, um 10705) is affected in AB31Δnum1 (Table S2, Figure S13).
We have shown before that cell cycle and hyphal development are controlled by a cascade of transcription factors [2]. Intriguingly, although most U. maydis genes contain no introns, both bW and bE carry single introns; rbf1 harbors even four introns. It could be well possible that the observed phenotype of the Δnum1 strains is caused by inefficiently spliced genes of the master regulators for filamentous growth and pathogenic development. In AB31, the bW2-allele is expressed without the intron sequence [24], but we observed that bE1 is indeed spliced less efficiently in AB31Δnum1 (Table S3). However, all direct b-target genes were induced to a similar extent in AB31Δnum1 compared to AB31 (Table S4), which implies that expression of the bE and bW genes in the Δnum1 background is sufficient to provide full functionality of the bE/bW heterodimer.
In accordance with the unaltered functionality of bE/bW, the expression of the direct target gene rbf1, was not altered in AB31Δnum1 (Figure 4C); however, all four introns were spliced less efficiently. Under the assumption that splicing of the introns occurs independently from each other, only in 45% of the mature mRNA in AB31Δnum1 all introns were spliced correctly, compared to 80% in AB31 wild-type cells (Figure 4A, Table S3). The splicing defect in rbf1 was validated via RT-PCR and qRT-PCR analyses (Figure S14), showing additional unspliced variants and increased intron retention, respectively, in AB31Δnum1. Consistently, we observed that the abundance of the Rbf1 protein in AB31Δnum1 is reduced to about 30% of wild-type level (Figure 4B), although qRT-PCR analysis showed that the total level of the rbf1-transcript was not altered (Figure 4C).
Fig. 4. Reduced splicing efficiency of the rbf1-gene leads to impaired function of the Rbf1 master regulator. 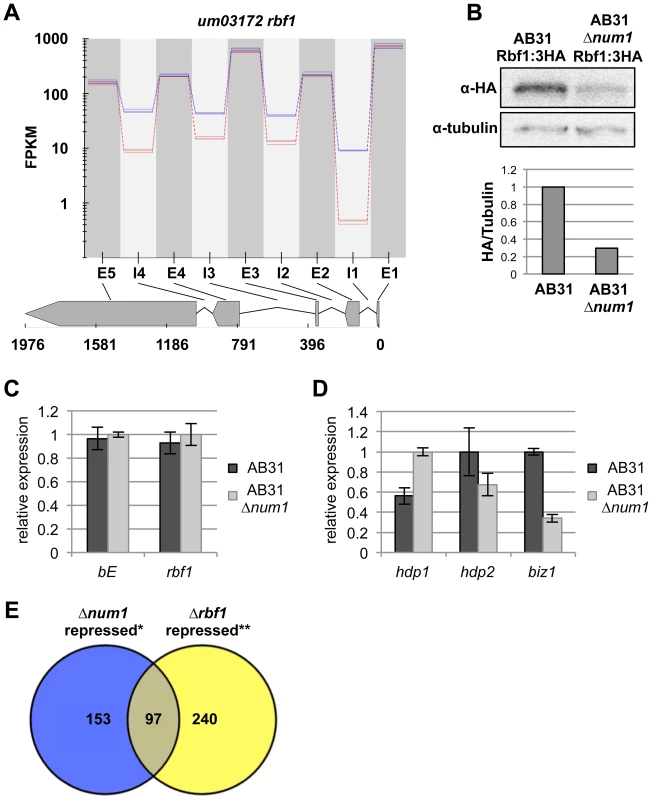
(A) Plot depicting splicing efficiency of the rbf1-gene. Plotted are the FPKM values (fragments per kilobase of sequence per million fragments mapped) across the genomic region indicated (coordinates in nucleotides) of three independent RNA-Seq experiments for AB31 wild-type (blue lines) and AB31Δnum1 (red lines), respectively. Exons (E) and introns (I) are indicated. All four introns show increased intron retention rates in AB31Δnum1. (B) Western analysis showing abundance of Rbf1:3×HA and α-tubulin (loading control) from AB31 wild-type and Δnum1-deletion strains. In AB31Δnum1, Rbf1 is reduced to 30% of wild-type-level (Quantification: ImageJ [109]). (C, D) Gene expression analyses of b- and rbf1-genes (C) as well as rbf1-target genes (D) using qRT-PCR. RNA samples were isolated from strains AB31 and AB31Δnum1 eight hours after induction of the bE1/bW2-heterodimer. Gene expression is shown relative to the highest expression value, using actin and eIF2b for normalization. Shown are the mean values of three biological and two technical replicates. Error bars represent the SD. (E) Venn diagram depicting the total number of genes repressed in AB31Δnum1 and AB31Δrbf1. * RNA-Seq analysis, this study; ** Microarray analysis conducted five hours after induction of the bE1/bW2-heterodimer [2]. Rbf1 controls the expression of biz1, hdp1 and hdp2, encoding transcription factors that are collectively involved in cell cycle regulation, filamentous growth and pathogenic development [43, Scherer, Vranes, Pothiratana and Kämper, unpublished]. In accordance with the reduced Rbf1 levels, RNA-Seq analysis revealed that biz1 as well as hdp2 expression was significantly down-regulated in AB31Δnum1, which was verified by qRT-PCR (Figures 4D and S15, Table S4). hdp1 splicing efficiency was reduced in AB31Δnum1 from 0.83 to 0.14 (Table S3); interestingly, hdp1 RNA-levels were increased about 2-fold, which may argue for a self-regulation of the gene (Figures 4D and S15, Table S5). In total, we observed an at least two-fold down-regulation for 228 genes in AB31Δnum1; intriguingly, 89 of these genes overlap with a set of 351 genes that has been identified as down-regulated in response to a deletion of rbf1 in AB31 (Figure 4E, Table S5, [2]).
As rbf1 is one of the major regulators implicated in filamentous growth and cell cycle regulation, it was tempting to speculate that the reduced splicing efficiency of the gene may be the cause for the observed phenotype in Δnum1-mutants. To test this hypothesis, we exchanged the rbf1 gene with an intron-free derivative in strains AB31 and AB31Δnum1. However, we observed that the intron-free copy of rbf1 is not capable to substitute for the native gene; despite wild-type-like expression levels the strains did not grow filamentous, nor were the known rbf1-target genes induced (Figure S16). Obviously, introns are essential for the expression of the Rbf1-protein. As a consequence, expression of the rbf1 cDNA did not rescue the Δnum1 phenotype either.
In summary, we conclude that Num1 is a structural component of the conserved Prp19/CDC5L complex and fulfills common functions that have previously been associated with this complex, as cell cycle control, cellular response to DNA damage, and splicing. Our data indicate that the observed phenotype in U. maydis hyphae may be at least in part the result of the splicing defect of genes involved in cell cycle control, DNA damage repair and of central components of the regulatory cascade controlling filamentous growth and pathogenic development.
Num1 interacts with the Kin1 motor protein
To further elaborate the molecular function of the Num1 protein, we employed the yeast two-hybrid system. Proteins identified as potential interactors for Num1 are shown in Table S6. Consistent with a putative function of Num1 within the Prp19/CDC5L complex, the CDC5L homologue Cef1 was isolated as an interacting protein.
Unexpectedly, we identified various interactions with proteins involved in cellular transport processes. Um10158 is an adaptin-like protein from clathrin/coatomer-adapter complexes (IPR002553) mediating endocytic protein transport between ER and Golgi. Um03539 and Um11510 contain a BAR-domain (IPR004148) and a BRO1-domain (IPR004328), respectively, and are thus likely involved in intracellular vesicular transport processes and protein targeting to endosomes or the vacuole. The conventional kinesin motor protein Kin1 [formerly named Kin2, 20] functions in long distance trafficking within fungal hyphae as it transports Dynein along the microtubule cytoskeleton towards their plus-ends directed to the hyphal tips [9], [14]. We were not able to generate deletion strains for um10158, indicating that the respective protein might be of essential function. Deletion analysis for um03539 and um11510 in strain AB31 revealed no obvious phenotype with respect to polar growth and vesicular movement (data not shown), indicating that the respective proteins have no function with respect to Num1-associated processes. For kin1, however, it had already been demonstrated that the gene has impact on hyphal morphology [14, see below].
To confirm the interaction of Num1 with Kin1, co-immunoprecipitation analyses were carried out. A full-length Num1:Myc-tagged protein and a Kin1:HA-tagged protein fragment encompassing amino acids 650–968 were generated by coupled in vitro transcription/translation, and Num1:Myc was specifically co-precipitated from mixtures of Kin1:HA with an anti-HA antibody (Figure 5A). By use of the yeast two-hybrid system we mapped the interaction domain of Num1 (encompassing amino acids 67 to 147) with the C-terminal Kin1 domain used in the co-immunoprecipitation, which provides additional evidence for a specific interaction with the Kin1 motor protein (Figure S17). The interaction was further verified in vivo, using U. maydis strain UNK197 that harbors num1 : 3eGFP and kin1:HA fusion genes integrated into their native loci by homologous recombination, respectively. By use of dithiobis[succinimidyl]-propionate (DSP) to crosslink proteins in extracts prior to immunoprecipitation, the Num1 : 3eGFP protein was efficiently co-precipitated with the Kin1:HA protein using anti-HA antibodies (Figure 5B). Without cross-linking of the proteins, only a faint signal was detectable (not shown), suggesting that the interaction is only transient. Taken together, we conclude from our data that Num1 interacts with the motor protein Kin1.
Fig. 5. Num1 physically interacts with the Kin1 motor protein. 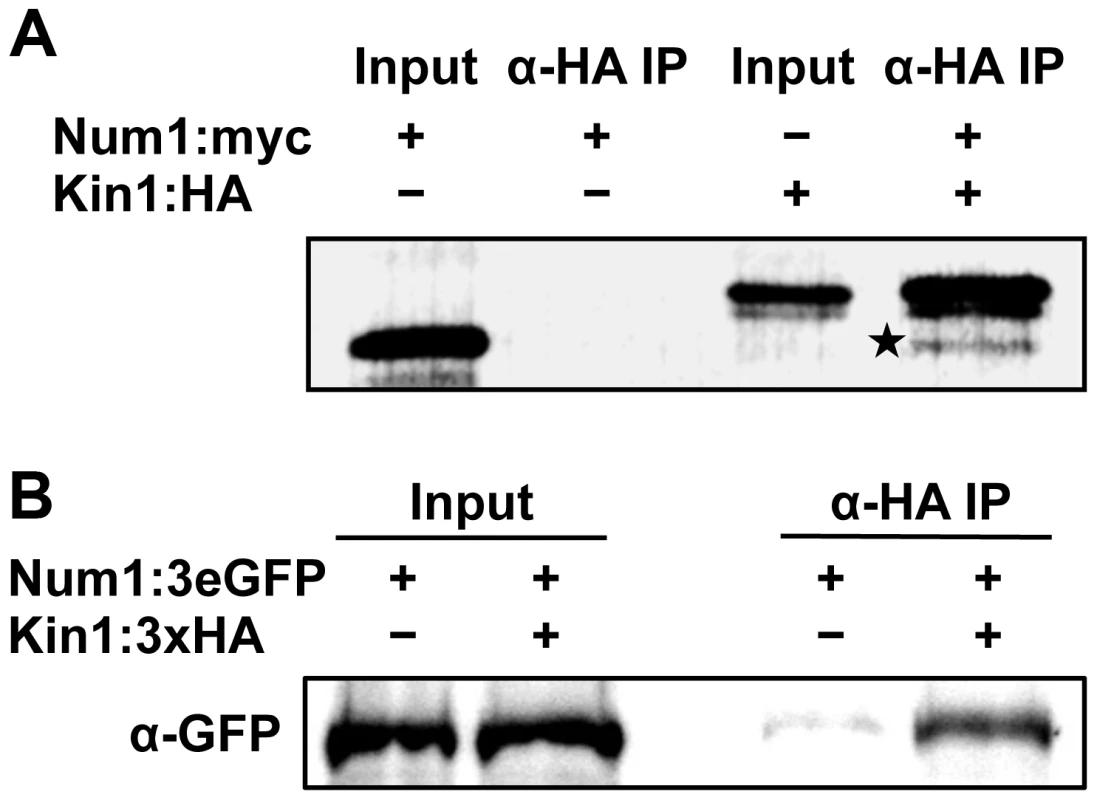
(A) In vitro expression and co-immunoprecipitation of Myc-tagged Num1 and HA-tagged Kin1. Proteins were labeled with biotin during in vitro synthesis, detected with streptavidin-conjugated alkaline phosphatase, and visualized with NBT/BCIP. In the negative control (Num1:Myc), no unspecific binding of Myc-tagged Num1 to the HA-antibody was detected. In contrast, after co-incubation with HA-tagged Kin1, the Myc-tagged Num1 protein was co-immunoprecipitated (indicated by asterisk), demonstrating that the Num1 protein physically interacts with Kin1 in vitro. (B) In vivo co-immunoprecipitation of U. maydis strains expressing HA-tagged Kin1 and 3eGFP-tagged Num1 under control of their endogenous promoters. After crosslinking of proteins using DSP, α-HA coupled agarose beads were used to precipitate Kin1. The Num1 protein was detected on Western blots using an α-GFP antibody. As negative control, a strain expressing only Kin1:3×HA was used. Num1 and the motor protein Kin1 are functionally connected
In addition to the physical interaction, several lines of evidence argue for a functional connection between Num1 and Kin1. Induced expression of bE1/bW2 in a Δkin1 strain leads to short curved hyphae with delocalized septa and the formation of bipolar filaments [14]. Similarly, the induction of hyphal growth in AB31Δnum1 leads to irregular or bipolar filaments with an aberrant insertion of septa (Figure 1, Table 1). In strains deleted for kin1 the number of vacuoles is increased, while size is reduced [13]. We observed a similar phenotype in strains SG200Δnum1 (Figure 6A).
Fig. 6. Num1 and Kin1 are functionally connected. 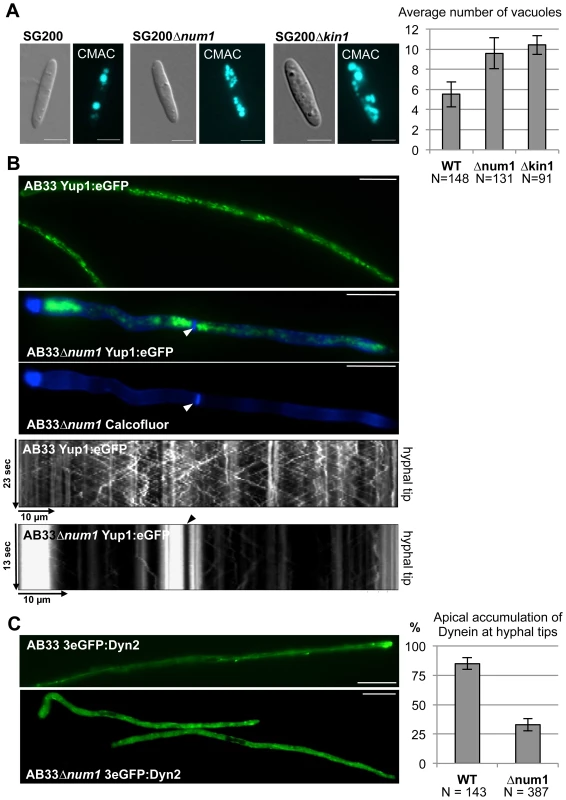
(A) The num1 deletion leads to aberrant vacuole morphology. Vacuoles were visualized by CellTracker Blue (7-amino-4-chloromethyl-coumarin, CMAC) staining. Both num1 and kin1 deletion strains contain more, but smaller vacuoles in comparison to wild-type sporidia. Scale bars: 5 µm. Right panel: Quantification of the average number of vacuoles of strains indicated. N represents the number of individual cells analyzed. Mean values of three independent experiments are shown. (B) The num1 deletion leads to an aberrant distribution of early endosomes. For visualization of early endosomes, a Yup1:eGFP fusion protein [9] was expressed under control of the constitutively active Potef-promoter in strains AB33 and AB33Δnum1, which harbor b-genes under control of the nitrate-inducible Pnar1-promoter. Endosomes were analyzed 14 hours after bE1/bW2-induction. Septa (arrowhead) were visualized by Calcofluor White staining. Yup1-labelled early endosomes accumulate at basal and apical parts of the hyphae as well as around delocalized septa in AB33Δnum1 in contrast to wild-type hyphae. Scale bars: 10 µm. Movies detecting Yup1:eGFP-fluorescence of the depicted hyphae are shown in Videos S1 and S2. Shown below are the corresponding kymographs (time and distance as indicated) reflecting the motility of early endosomes. In AB33 hyphae, early endosomes travel continuously towards the hyphal tip (anterograde) and reverse direction to travel back towards the septum (retrograde). Pausing organelles are reflected by a vertical line. In contrast, endosomal motility is drastically reduced in AB33Δnum1 hyphae containing additional septa, where the majority of the Yup1:eGFP-signal is non-motile. An arrowhead indicates the septum. (C) The localization of Dynein is dependent on Num1. In AB33 filaments 3eGFP:Dyn2 fusion proteins form comet-like motile structures that accumulate at the hyphal tip and from there move into retrograde direction [9]. In filaments of AB33Δnum1 strains the apical accumulation is significantly reduced (p = 0.002); instead a stronger signal is observed in the cytoplasm. Dynein localization was monitored 14 hours after induction of the bE1/bW2 heterodimer. Scale bars: 10 µm. Right panel: Quantification of the apical accumulation of 3eGFP:Dyn2 fusion proteins. N represents the number of hyphae analyzed. Mean values of three independent experiments are shown. To analyze the functional relation between the two proteins further, we generated strains deleted for both genes. Filaments induced in AB31Δnum1/Δkin1 displayed a more severe phenotype with respect to polarized growth, as hyphae exhibited a very irregular, swollen morphology, and also the number of branched hyphae and hyphae with an altered septation pattern was significantly higher than in AB31Δnum1 or AB31Δkin1 (Figure S18). Infection experiments with SG200Δnum1, SG200Δkin1 and SG200Δnum1/Δkin1 deletion strains revealed that the virulence of the double deletion strain is significantly reduced when compared to the respective single deletion strains (Figure S18). The more severe phenotype excludes that both genes are in pure epistatic interaction; besides their function within a common complex, both proteins must fulfill functions that are independent from each other.
Num1 and Kin1 are both required for trafficking of early endosomes
The deletion of kin1 results in a loss of rapid bi-directional trafficking of early endosomes. Early endosomes visualized by a GFP-fusion with the endosomal t-SNARE Yup1 (Yup1:eGFP) [4] accumulate at the hyphal tips of kin1-deletion mutants [9], while in the wild-type situation Yup1:eGFP labeled vesicles shuttle bi-directionally throughout the hyphae along microtubule tracks [4]. The anterograde transport of early endosomes is mediated via the motor protein Kin3 (directed to the microtubule plus-ends at the hyphal tip), whereas the retrograde transport is accomplished by Dynein1/2 [8], [10], . Since the Kin1 motor protein transports Dynein toward the hyphal tips, the accumulation of early endosomes at the tip of Δkin1 hyphae can be explained indirectly by the failure of the Kin1-dependent anterograde transport of Dynein [9].
The deletion of num1 leads to a comparable scenario in the distribution of early endosomes, which appeared to cluster at hyphal tips and the distal pole of the hyphae as well as around the delocalized septa frequently found in Δnum1-hyphae (Figure 6B). Endosomal motility was monitored in strain AB33, in which the b-genes are expressed under control of the nitrogen-responsive Pnar1-promoter [44]. Filamentous growth in AB33 is repressed by ammonium and can be elicited in nitrate-containing media. In AB33Δnum1 filaments, long-distance movement of early endosomes was constrained, and velocity of the vesicles was reduced to a speed of 1.14 µm/sec in AB33Δnum1, in contrast to 1.46 µm/sec in wild-type hyphae (t-test p = 0.005). In AB33Δnum1 filaments with aberrant septa, long-distance trafficking of endosomes was entirely abolished, and only residual movement of vesicles was observed (Table 2, Videos S1 and S2). Next we addressed the question whether the altered movement and positioning of early endosomes in Δnum1 strains may be caused by a distortion of cytoskeletal elements. Neither the arrangement or abundance of microtubules (visualized by an α-tubulin mCherry fusion, Tub1:mCherry) nor of actin patches (visualized by LifeAct:YFP [45]) was altered in the Δnum1 mutant strain (Figure S19). To analyze the orientation of the microtubule cytoskeleton, we used strains expressing an α-tubulin GFP fusion [14] together with the microtubule plus-end marker Peb1, fused to RFP, which localizes to growing microtubule plus-ends [46]. As previously described [46], in wild-type interphase sporidia the majority of microtubule plus-ends is oriented towards the budding cell and the opposing cell pole of the mother cell; in Δnum1 sporidia, the orientation of the microtubule cytoskeleton was not considerably altered (Figure S20 A,B). Similarly, in hyphae the majority of microtubule plus-ends is oriented towards the cell poles, i.e. hyphal tips and basal retraction septa [9]. In Δnum1-mutants, we observed microtubule plus-ends also in close vicinity to the delocalized septa (Figure S20 C–E). Apparently, endosomes accumulate at microtubule plus-ends in num1-deletion mutants, supporting the Kin1-dependent effect on endosomal transport. In some hyphae of the num1-deletion strain we observed the appearance of mitotic spindles, which supports the defect in cell cycle regulation.
Tab. 2. Reduced endosomal motility in num1-deletion strains. 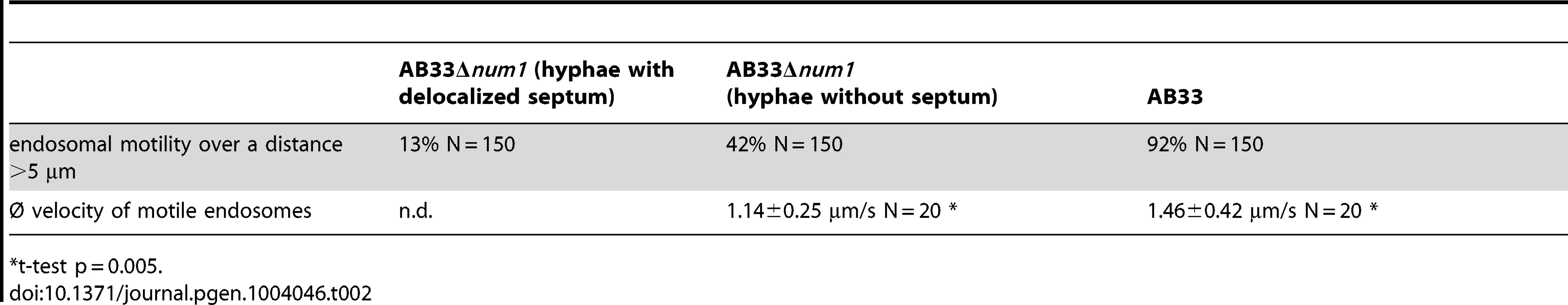
t-test p = 0.005. To determine whether the effect on endosomal trafficking in AB33Δnum1 strains is attributed to an aberrant anterograde transport of Dynein towards the hyphal apex, as it was suggested for Δkin1 mutants, we visualized the subcellular distribution of Dynein using an N-terminal 3eGFP:Dyn2 fusion protein [9]. In wild-type filaments, 3eGFP:Dyn2 fusion proteins form comet-like motile structures that accumulate at microtubule plus-ends in hyphal tips, where they reverse direction and move into retrograde direction [9]. In AB33Δnum1 filaments, however, the number of cells harboring an apical accumulation of 3eGFP:Dyn2 was significantly reduced (t-test p = 0.002); concomitantly, in most cells the 3eGFP:Dyn2 signal in the cytoplasm was higher than in wild-type filaments (Figure 6C).
As Kin1 affects motility of early endosomes only indirectly (via the anterograde transport of Dynein), we set out to analyze the movement of a direct Kin1-cargo. The myosin chitin synthase Mcs1 [15] localizes to Kin1-dependently transported vesicles [17]. It has been described before that 3eGFP:Mcs1 fusion proteins localize to the poles of growing sporidia, where it is secreted and participates in the synthesis of chitin [15]. Δnum1-mutants showed reduced Mcs1-accumulations at the growth region in budding cells, which coincides with the distribution of Mcs1 in kin1-deletion mutants [17] (Figure S21 A,B). In AB33 filaments, 3eGFP:Mcs1 localizes to distinct foci close to the cell membrane and forms a gradient towards the growth zone within the hyphal apex or localizes to the basal retraction septum, i.e. to sites of chitin synthesis. In contrast, in AB33Δnum1 hyphae no tip-directed gradient was obvious and less foci were observed at the cell membrane (Figure S21 C,D), indicating an impaired delivery of Mcs1-containing vesicles. In many cases, Mcs1 was not observed at delocalized septa in Δnum1-mutant hyphae. In summary, U. maydis strains deleted for either num1 or kin1 show similar phenotypes with respect to hyphal morphology and septation, formation of vacuoles, endosomal trafficking as well as the localization of the Kin1-cargos Dynein and Mcs1, which strongly corroborates a functional interrelation of Num1 and Kin1.
Num1 is required for transport of cytoplasmic mRNA particles
Recently, it has been shown that the RNA-binding protein Rrm4 is associated with early endosomes [7]. Rrm4 is required for cytoplasmic transport of mRNAs in U. maydis filaments [47]. We deleted num1 in an AB33 strain expressing an Rrm4:eGFP fusion protein. In the wild-type background vesicles associated with eGFP-labeled Rrm4 shuttled within the hyphae to the distal and proximal ends. In contrast, movement in Δnum1 was reduced, and similar to Yup1-labeled early endosomes, Rrm4-containing particles frequently accumulated in clusters at hyphal tips or around delocalized septa (Figure S22, Videos S3, S4), indicating that num1 is required for cytoplasmic mRNA transport.
Num1 has a functional homologue in the filamentous ascomycete Aspergillus nidulans
To address the function of Num1 in filamentous fungi in general, we deleted a gene encoding a protein with high similarities to Num1 (AN4244, 49% similarity, 30% identity) in the ascomycete Aspergillus nidulans (strain TN02A3). The deletion mutants displayed smaller colonies with a reduced rate of conidiospore formation. Germination of spores was drastically reduced in the ΔAN4244 mutant strains: after eight hours of incubation at 37°C, only 5% of the spores germinated, compared to 98% in the wild-type strain. Similar to the phenotype observed in U. maydis, hyphae from germinating ΔAN4244 conidia were shorter and thicker than those of the wild-type strain. Staining with CellTracker Blue revealed that in the mutant the number of vacuoles was increased, while size was reduced (Figure 7A). In contrast to U. maydis, A. nidulans hyphae have cell compartments with multiple nuclei. In wild-type hyphae, these nuclei are evenly distributed. In ΔAN4244, however, nuclei were often found in clusters (Figure 7B). A similar phenotype has been observed earlier for strains deleted for KinA, the homologue of the U. maydis Kin1 [48]. To test a potential functional relation between AN4244 and KinA in A. nidulans, we analyzed, in analogy to U. maydis, the distribution of Dynein (NudA in A. nidulans). We deleted AN4244 in strain LZ12, which harbors a GFP:NudA fusion protein. Similar as described for the U. maydis hyphae, GFP:NudA was predominantly localized at the tip of hyphae in strain LZ12, while the apical accumulation was reduced in LZ12ΔAN4244 (Figure 7C). Taken together, our data suggest that the A. nidulans AN4244 has a conserved function as the U. maydis protein with respect to polarized growth and intracellular transport processes.
Fig. 7. Deletion of the gene for a Num1-homologue in the ascomycete Aspergillus nidulans leads to a phenotype reminiscent to the Δnum1 phenotype in U. maydis. 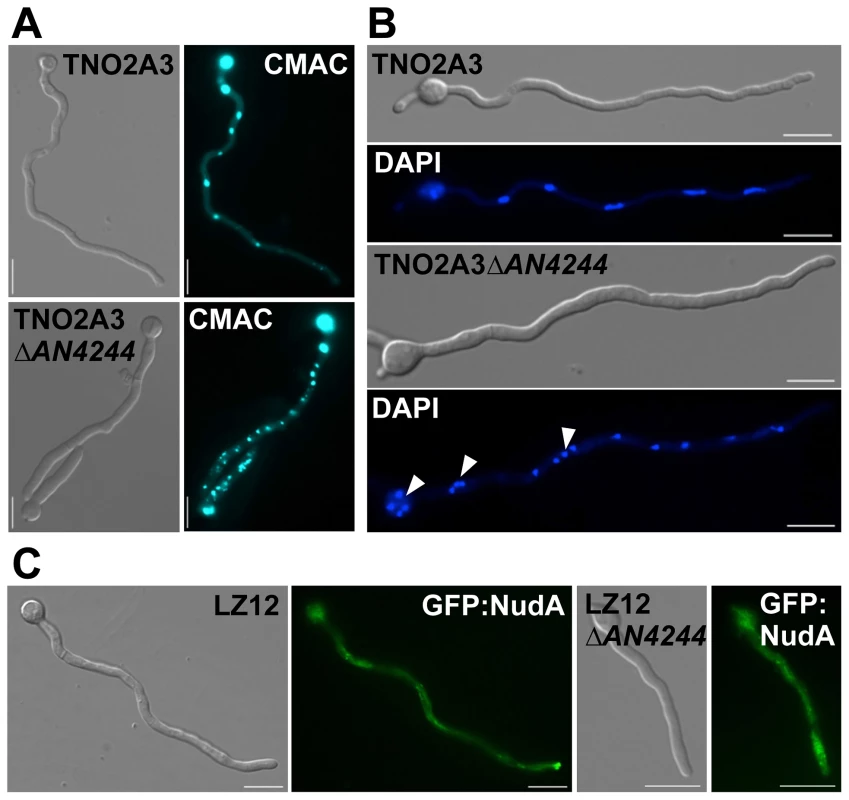
(A) The morphology of vacuoles is affected by the AN4244-deletion. Spore suspensions of a wild-type (TNO2A3) and the AN4244 deletion mutant were incubated for 18 hours at 37°C and the germinated filaments were CellTracker Blue (CMAC)-stained to visualize the vacuoles. Similar to the num1-deletion in U. maydis, the AN4244-deletion in A. nidulans leads to an increased number of vacuoles. (B) Deletion of AN4244 affects nuclear positioning. Spore suspensions of A. nidulans wild-type (TNO2A3) and the AN4244 deletion mutant were incubated for 12 and 18 hours, respectively, at 37°C, and the germinated filaments were DAPI-stained to visualize nuclei. In wild-type filaments, nuclei are evenly spaced, whereas in the mutant nuclei are often found in clusters. (C) The localization of Dynein (NudA) is dependent on AN4244. Spore suspensions of strains LZ12, expressing a GFP:NudA fusion protein, and LZ12ΔAN4244 were incubated for 18 hours at 37°C. In LZ12 the GFP:NudA signal strongly accumulated in tips of germinating hyphae, whereas in mutant filaments, this apical accumulation was reduced; instead a stronger signal was observed in the cytoplasm. Scale bars: 10 µm. Discussion
In this study we have characterized the function of the Num1 protein in U. maydis. We show that Num1 is a functional homologue to the human SPF27 protein, which is an integral component of the conserved spliceosome-associated Prp19/CDC5L-complex (NTC). Deletion of the num1 gene affects polar and filamentous hyphal growth, cell cycle control as well as DNA repair. Consistent with its function as component of the NTC, deletion of num1 results in a genome-wide reduced splicing-activity, which could explain the observed pleiotropic phenotype. In addition we demonstrate a previously unknown function of an NTC-component in the cytoplasm, where Num1 interacts with the Kinesin 1 motor protein. This functional interconnection of Num1 with elements of the cytoskeleton implies additional, yet unknown functions during intracellular trafficking processes.
Num1 is a conserved component of the spliceosome-associated Prp19/CDC5L-complex (NTC)
Num1 shows significant similarities to SPF27, and its interaction with Prp19 and Cef1 is in accordance with the interactions within the NTC complex demonstrated in S. pombe [28], humans [21] and plants [49].
The NTC was initially identified in S. cerevisiae as a complex containing Prp19 and at least seven other components [50], most of which are conserved from yeast to human. The NTC is a non-snRNP (small nuclear ribonucleoprotein particle) multi-protein complex, and as a subunit of the spliceosome it is required for intron removal during pre-mRNA splicing. The complex associates with the spliceosome during or after dissociation of U4 snRNP and participates in the regulation of conformational changes of core spliceosomal components, stabilizing RNA-RNA - as well as RNA-protein interactions [reviewed in 51]. The only known enzymatic function of the NTC is a conserved U-box domain with E3-ubiquitin ligase activity of the Prp19-protein [52], [53]. It was conclusively shown that individual core components of the NTC, as Prp19, Cef1/Cdc5 in S. cerevisiae and S. pombe, or the S. pombe SPF27 homologue Cwf7 independently affect splicing [42], [50], [54]–[59]. However, it is mostly unresolved to which extend single components of the NTC influence splicing of individual introns. We now show that one of the core components of the NTC, the SPF27 homologue Num1, has a global effect on splicing in U. maydis, as retention rates of nearly 80% of all introns were significantly increased in num1-deletion mutants. We observed variations in intron retention between different transcripts in response to the Δnum1 mutation. Similar observations have been made in S. cerevisiae on a genome-wide scale for depletion of individual components of the spliceosome, including the NTC-components Prp19 and Prp17. The resulting splicing defects displayed considerable differences in different mRNAs, indicating that individual splicing factors affect a discrete set of genes [60]. It is well possible that structural alterations of the spliceosome complex after deletion of num1 result in altered recognition/affinity of specific introns, probably caused by their different GC content or secondary structures. Following the conclusions made in S. cerevisiae, we favor the possibility that deletion of num1 leads to an altered integrity of the NTC, resulting in a reduced activity and/or specificity and hence a diminished splicing efficiency. However, we cannot rule out that Num1 may recruit splicing enhancer - or inhibitor-proteins that might affect splicing of only a subset of introns.
Splicing defects can account for pleiotropic phenotypes in num1-deletion mutants
In addition to disturbed polarity we frequently observed delocalized septa in num1-deletion strains; in addition, chitin-staining indicate structural differences to wild-type septa. Formation of septa requires a highly coordinated and dynamic positioning of septins into ring-like structures [61], a process tightly regulated by the small GTPase Cdc42, its guanine exchange factor Don1 and the protein kinase Don3 [62]–[64] as well as by formins or Cdc4, an essential light chain of the type II myosin motor protein [65]. The genes encoding the septins Cdc3 (um10503) and Sep3 (um03449), but also Cdc4 (um11848) display significantly increased intron retention rates (Table S1) and the expression of don3 (um05543) is reduced more than 2-fold in the num1-deletion strain (Table S4), which altogether might rationalize the formation of delocalized septa.
We found that Num1 affects splicing of several genes of the functional class “cell cycle”, including genes for the catalytic and regulatory subunits of cyclin-dependent kinases, for mitotic cyclins as well as for γ-Tubulin (Table S2). Similarly, several genes grouped in the functional class “DNA-repair” (e.g. for the DNA repair recombinase Rad51, the nucleotide excision machinery component Rad1 and the repair exonuclease Rec1) show increased intron retention rates (Table S2). The synergistic effect of several genes with reduced splicing efficiency (and the resulting reduced activity) within the same functional category may be sufficient to explain the effects on the corresponding cellular processes. However, various components of the NTC have been found to be directly involved in DNA damage repair. Initially, the resistance to the interstrand cross-linking reagent psoralen in a S. cerevisiae Prp19 mutant strain [32] has been attributed to the splicing defect of the intron containing RAD14 gene [66]. Subsequently, it has been conclusively shown that the DNA damage phenotype is independent from the splicing defect [42]. In addition, for Prp19 several interaction partners have been identified which belong into the functional classes “cell cycle”, “chromatin structure” and “DNA repair” rather than pre-mRNA processing. For example, the human Prp19/Pso4 directly interacts with terminal deoxynucleotidyl-transferase (TdT), which is involved in DSB repair [67]. The human Cef1-homologue CDC5L interacts with the Werner syndrome protein WRN, a DNA helicase that functions during homologous recombination in response to DNA-damage [35] and also with the non-homologous end-joining (NHEJ) factor DNA PKcs [68].
Thus, it is well possible that also in U. maydis the increased UV-sensitivity of Δnum1 mutants results from a direct function of Num1 in a DNA damage repair pathway. Similarly, disturbed cell cycle and cell division can be explained as indirect effect of a disturbed DNA-damage repair. Interaction of the human CDC5L with the cell cycle checkpoint kinase ATR (“ataxia-telangienctasia and Rad3-related”) was shown to be required for activation of the ATR kinase as well as for further downstream effectors and mediators of ATR function as the checkpoint kinase CHK1 [69]. In U. maydis, the homologues of ATR1 and CHK1 are known to be critical cell cycle regulators in addition to their decisive roles in the DNA-damage response [70], [71].
We have shown previously that cell cycle and polarized growth in U. maydis is regulated by a cascade of transcription factors controlled by the bE/bW-heterodimer [2]. Several of the transcription factors of this b-cascade were found to have increased intron retention rates or a differential expression pattern in AB31Δnum1 and might collectively contribute to the effects on cell cycle control and polar hyphal growth. For example, splicing efficiency of the hdp1 gene, which encodes a homeodomain transcription factor required for filamentous growth and cell cycle control (Pothiratana and Kämper, unpublished data), was reduced from 83% in wild-type to 14% in the num1-deletion strain. More importantly, all four introns of rbf1 are spliced less efficiently in the num1-deletion strain. As a consequence, protein levels of Rbf1, the master regulator for hyphal development, are significantly decreased in Δnum1 strains. In accordance with the reduced Rbf1 protein levels in Δnum1 strains, we observed the down-regulation of rbf1-dependently expressed genes. Among these are the rbf1-dependently expressed genes encoding the transcription factors Biz1 and Hdp2, which were previously described as cell cycle regulators and factors for filamentous growth and pathogenic development [43; Scherer and Kämper, unpublished data]. Conclusively, we can assume that the reduced splicing efficiency of the rbf1 gene and in turn the deregulation of rbf1-dependently expressed genes contributes to hyphal morphology and the deregulated cell cycle in num1-deletion mutants. A direct proof of this hypothesis by replacing the rbf1 open reading frame with a cDNA copy failed. The intron-free copy, although expressed to the same level as the intron-containing copy, was not able to provide wild-type Rbf1 function, even when introduced into wild-type cells. Such a reduced expression of cDNA copies in relation to intron-containing genes has been described frequently before, and in various cases this has been related to direct effects of introns to translational efficiency [reviewed in 72], [73].
Num1 has a specific role in cytoplasmic trafficking processes
Aberrant or incomplete splicing events of the genes encoding motor proteins by themselves would be an explanation for the impaired endosomal trafficking processes observed in num1-deletion strains. However, none of the genes for the major transport-mediating motor proteins (kin1, kin3, dyn1/2) contains introns or show altered expression levels in the Δnum1-mutant. Although we cannot rule out an effect caused by aberrant splicing of genes for unknown components of the transport machinery, we favor a specific role of the Num1 protein in intracellular trafficking processes, based on the observation that (a) Num1 is localized in distinct foci in the cytoplasm, implicating functions beyond its role as a splicing factor, and (b) Num1 interacts with the Kin1 motor protein, which is involved in long-distant transport processes during filamentous growth [9], [12], [14]. The physical interaction of Kin1 and Num1 is in line with the similar phenotypes of the two mutant strains with respect to cell morphology, determination of polarity and septation, and morphology of vacuoles. Δnum1 and likewise Δkin1 hyphae display impaired trafficking of early endosomes and reduced apical localization of Dynein, two interconnected phenotypes, as the transport of early endosomes relies on Dynein and the anterior transport of Dynein depends on Kinesin 1. In addition, the distribution of the direct Kin1 cargo Mcs1 is altered in both Δkin1 and Δnum1 mutants, which argues for a direct connection of Kin1 and Num1. However, we can rule out that Num1 is required for all Kin1-related processes, as (a) both mutations appear not to be epistatic, and (b) the num1 and kin1 mutant strains have overlapping, but not identical phenotypes; for example, haploid Δkin1 strains are defective in mating [20], while Δnum1 strains are not. This is anticipated, as each of the proteins participates in different cellular processes. The conventional kinesin is best known for its function in cellular transport processes. However, conventional kinesin has also been implicated in cell signaling, or to serve as bona fide, static anchor for mRNA–protein complexes or vesicular compartments, or to participate in the phosphorylation of cargo proteins to which it binds [reviewed in 74]. As outlined above, also SPF27 (or the NTC) participates in a wide spectrum of processes that, however, show no overlap with Kin1-dependent processes.
Interaction of SPF27 homologues with conventional kinesin motor proteins is not specific to U. maydis, but appears to be conserved between basidio - and ascomycetes, as in Aspergillus nidulans the phenotype of deletion mutants of the SPF27 homologue AN4244 or the Kin1 homologue KinA [48] are comparable to those observed for U. maydis.
The association of a conventional kinesin motor protein with a component of the spliceosome-associated NTC has not been described so far and implies a novel function for SPF27-homologous proteins. The question is whether the Num1 protein fulfills dual functions, (a) during splicing as an NTC-component in the nucleus and (b) during Kin1-dependent intracellular trafficking processes in the cytoplasm, or whether these two disparate mechanisms are connected by one single function. Discrete cytoplasmic functions may serve to regulate the activity of Kin1, as described in neuronal development for the interaction of Kinesin-1 and JIP3, an interactor of the c-Jun N-terminal kinase [75]. Alternatively, Num1 could function as a scaffold or adapter molecule, providing stability to the motor protein/cargo-complex and/or to the NTC. In both scenarios, deletion of num1 could alter the specificity/activity of Kin1-mediated transport processes, resulting in reduced motility of early endosomes and by that influence polar growth. We have not been able to visualize Num1 proteins moving or co-migrating with Kin1 along microtubule tracks. As GFP-Kin1 shows a strong cytoplasmic background, visualization of Kin1 molecules moving on microtubules has not been achieved yet [14], [76]. Taken into account that the interaction of Kin1 and Num1 appears to be transient, we cannot rule out that Kin1 and Num1 may interact on microtubule tracks.
A possible process that could couple both nuclear and cytoplasmic functions of Num1 is the cytoplasmic transport of mRNAs. In U. maydis, the RNA binding protein Rrm4 was found to be instrumental for bidirectional transport of mRNAs in filaments [47]. Intriguingly, cytoplasmic transport of Rrm4 is dependent on Kin1, Kin3 and Dynein, and recently it was shown that Rrm4 co-localizes with early endosomes [7], [77]. There is a remarkable overlap of the phenotypes of rrm4-, num1 - as well as kin1-deletion mutants with respect to polarized growth [14], [78], which raises the possibility of a concerted function of all three proteins within the same process. Indeed, the motility of early endosomes associated with Rrm4:eGFP fusion proteins is drastically reduced in Δkin1-mutants [7], [14] and, as we show here, also in num1-deletion strains.
In Drosophila oocytes, it has been shown that components of the exon junction complex (EJC) are essential for transport of oskar transcripts [79]. The EJC marks exon/exon-transitions during splicing, remains bound to the processed mRNA and serves as a quality control mechanism for the splicing reaction during translation [reviewed in 80]. Reminiscent to mRNA transport in U. maydis, transport of oskar-mRNA is also dependent on the conventional Kinesin 1 [81].
Unlike the components of the EJC that remain bound to mRNA during nuclear export, the NTC is thought to disassemble after the splicing reaction. However, recently it has been shown in S. cerevisiae that the NTC is required for efficient recruitment of TREX to the transcriptional machinery. This is mediated by the C-terminus of the NTC-component Syf1p, which connects TREX to Rpb1, the largest subunit of RNA-polymerase II [82]. TREX is a conserved protein complex (“transcription export”), which facilitates both transcription elongation as well as mRNA-export processes [83]. Yra1p, a component of the TREX complex, is an RNA-binding protein and interacts with the Mex67/Mtr2-receptor complex, which in turn act together with components of the nuclear pore complex (NPC) to guide the transcripts into the cytoplasm [84]–[86]. Thus, it appears well possible that NTC components remain tethered to the spliced mRNA. In this context it is interesting to note the recently discovered association of Kinesin 1 - and Dynein to fungal nuclear pores, which mediate NPC-motility and are thus required for chromosome organization and nucleo-cytoplasmic transport [76]. This result corroborates a functional coupling between the nuclear transcription machinery and cytoplasmic transport processes in U. maydis.
The major finding of our study is that a component of the conserved spliceosome-associated NTC is involved in cytoplasmic trafficking processes. Due to the interaction of Num1 with the Kin1 and the functional connection to the RNA-binding protein Rrm4, it is tempting to speculate that Num1 could have a function during intracellular mRNA-transport processes. We hypothesize that one of the functions of the Num1-protein is to participate in the coordination of pre-mRNA splicing and the formation and export of mRNP-particles out of the nucleus. The transcripts would be transferred into the cytoplasm where they could be passed on to the microtubule-based transport machinery via the interaction of Num1 with Kin1 motor proteins that were found to be associated with the nuclear pore complex (Figure 8). In this model, additional adapter-proteins that act as connecting proteins between the nuclear splicing machinery and the cytoplasmic NPC-bound motor proteins cannot be excluded and remain to be identified.
Fig. 8. Hypothetical model for function of Num1 coordinating pre-mRNA splicing with nuclear pore complex-dependent export of mRNP-particles and microtubule-based mRNA-transport. 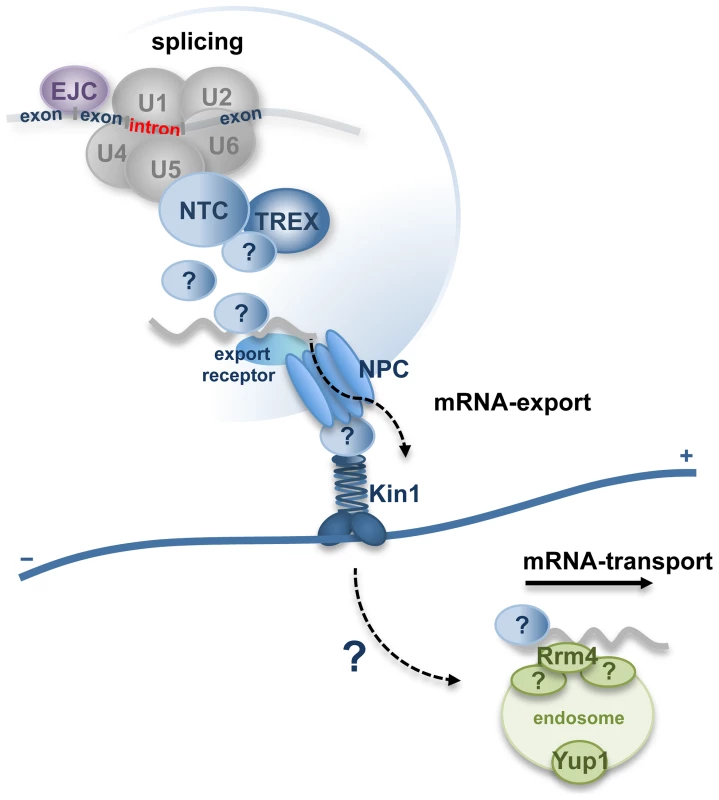
Num1 is a component of the spliceosome-associated Prp19/CDC5L-complex (NTC) and has a global effect on splicing efficiency. NTC-components interact with the TREX (“transcription-coupled export”)-complex [82], which mediates the export of mature transcripts into the cytoplasm via the nuclear pore complex (NPC) [83]–[86]. Adapter proteins connect the mRNA to NPC-bound export receptors. The Kinesin-1 motor protein has been shown to be associated to the NPC and promotes nucleo-cytoplasmic transport processes [76]. mRNAs within the hyphae are distributed via the RNA-binding protein Rrm4, which shuttles associated with early endosomes along the polar microtubule cytoskeleton [7], [77]. Motility of the Rrm4 associated early endosomes depends both on Kin1 [7], [14] and Num1. Proteins labeled with (?) depict yet unidentified adapter-proteins that might function as connectors between the nuclear splicing machinery and the cytoplasmic NPC-bound Kin1. As Num1 is a component of the NTC complex and interacts with Kin1, it might fulfill a function during mRNA export processes, possibly by connecting exported mRNP to the cytoplasmic transport machinery via its interaction with Kin1. Materials and Methods
Strains and growth conditions
Escherichia coli strain TOP10 (Invitrogen) was used for cloning purposes. Growth conditions and media for the cultivation of E. coli followed the protocols described previously [87]. The Saccharomyces cerevisiae strain AH109 (Clontech) was used for yeast two-hybrid interaction studies. S. cerevisiae cells were grown in YPDA complete medium, or on minimal medium (SD) supplemented with the dropout-mix needed for selection, as described in the Clontech Matchmaker™ GAL4 Two-Hybrid System 3 Manual (http://www.clontech.com). Ustilago maydis cells were grown in YEPSL [88], CM (complete medium) supplemented with 1% glucose (CM-G) and 1% arabinose (CM-A), respectively, or NM (nitrate minimal medium) [89] at 28°C. Solid media contained 2% agar. The induction of hyphal growth in AB31 - and AB33 derivatives was done as previously described in [24]. Plate mating assays followed the protocol of [24]. Mating assays in liquid culture for the generation of dikaryotic hyphae were carried out as described in [90]. Pheromone stimulation of U. maydis cells was performed according to the protocol of [91]. Aspergillus nidulans was grown in CM (complex medium) [92] or MM (minimal medium) [93].
All U. maydis and A. nidulans strains used in this study are listed in Tables S7 and S8, respectively.
Plant infections were performed as described in [94]. For infection studies with U. maydis, the maize cultivar Early Golden Bantam was used under controlled conditions in a CLF Plant Climatics GroBank with a 16 h (28°C)/8 h (22°C) day/night rhythm. Disease symptoms were evaluated according to the disease rating criteria published previously [23].
Yeast two-hybrid analyses
Screening for Num1-interacting proteins was performed as previously described [95], using the Matchmaker III system (Clontech). Plasmid pGBKT7-Num1 was generated by PCR amplification of the num1 open reading frame (ORF) from genomic DNA (strain 521 [23]), introducing two incompatible SfiI restriction sites at the 3′ and 5′ ends and subsequent ligation into the respective sites of pBGKT7 (Clontech). Plasmids pGAD-Prp19 and pGAD-Cef1 containing full-length as well as N - and C-terminal variants were generated by PCR amplification using either genomic DNA (for cef1) or a full-length cDNA library (for prp19) and subsequent ligation into pGAD-DS (Dualsystems Biotech) via SfiI sites. Oligonucleotide sequences are given in Table S9.
DNA and RNA procedures
Molecular techniques followed the protocols described in [87]. DNA from U. maydis was isolated according to the protocol given in [96]. Transformation procedures were performed as described in [97]. DNA from A. nidulans was isolated according to [98] and transformations were carried out as described in [99].
For gene deletions in U. maydis, a PCR-based approach [100] was used. Open reading frames were replaced by either hygromycin, carboxin or nourseothricin resistance cassettes. Similarly, for gene deletions in A. nidulans, a pyridoxine auxotrophic marker was used. C-terminal eGFP, RFP or 3×HA gene fusion constructs were generated in U. maydis via homologous recombination following the protocol given in [101], using SfiI cassettes from plasmids pUMA647 (eGFP) [7], pUMA738 [47] (RFP) or pUMa791 or pUMA792 (Feldbrügge, unpublished) for 3×HA fusions. Fusion constructs were subcloned in pCRII TOPO (Invitrogen), PCR-generated linear DNA was used for transformation of U. maydis. Homologous integration of the constructs was verified by Southern analysis. All sequences of oligonucleotides used for PCR are listed in Table S9.
Total RNA was extracted using Trizol reagent (Invitrogen) according to the manufacturer's instructions. RNA samples to be used for real-time qRT-PCR or mRNA-Seq analysis were column purified (RNeasy; Qiagen) and the quality was checked using a Bioanalyzer with an RNA 6000 Nano LabChip kit (Agilent). Quantitative RT-PCR analysis for pcna, rpb3, ubi1, rho3 and rbf1 was conducted with oligonucleotide pairs that specifically detected spliced and unspliced RNA species, respectively: forward primers used for quantification of spliced transcript were specific for exon/intron borders, whereas forward primers for the unspliced transcripts were placed within the intron sequence. Ratios of spliced vs. unspliced RNA of AB31 (wild-type) samples were set to 1.0 and compared to the ratios obtained with the AB31Δnum1. qRT-PCR experiments were done in three independent biological and two technical replicates each and followed the protocols given in [102] and [103].
DNA content was measured by flow cytometry. FACS analyses were performed according to [104] with the exception that 105 cells were harvested for each time point and analyzed with a BD Biosciences FACSVerse flow cytometer using the FACSDiva 6.2 software. For each acquisition, 2*104 events were measured at an average flow rate of 100–500 events per second.
mRNA-sequencing
mRNA was sequenced starting from total RNA isolated from strains AB31 and AB31Δnum1 on an Illumina HiSeq 1000 using the TrueSeq mRNA sequencing kit according to the manufacturer's instructions. Three independent libraries for each of the two strains were generated (Olivier Armant, Insitute for Toxology and Genetics, KIT Campus North), using three independently grown cultures (biological replicates). Each library yielded an average of 60 million paired-end reads of 2×56 bp, which equals about 20×109 bp sequences for each strain. Raw paired-end reads (2×56 bp) were aligned with TopHat 1.3.2 [105] to the Ustilago genome assembly provided by the MIPS Ustilago maydis Database (MUMDB, http://mips.helmholtz-muenchen.de/genre/proj/ustilago). TopHat was supplied with a gtf file derived from MUMDB's p3_t237631_Ust_maydi_20110629.gtf, but not restricted to known transcripts. Aligned reads were counted using custom Python scripts using the pysam library (http://code.google.com/p/pysam/). Gene expression values were derived by counting read pairs that were completely inside the exon regions of MUMDB-genes and differential expression was assessed with DESeq 1.6.1 [106] and BaySeq 1.8.1 [107] and filtered for an adjusted p-value≤0.05 and a more than two fold change.
For comparison and visualization of splicing efficiency, read pairs overlapping the respective intron/exon with their first segment by at least three base pairs were counted and normalized to fragments per kb per million (FPKM). Intron retention was determined by calculating the ratio of introns FPKM to the gene's overall exon FPKM. Exon FPKM included all exons with more than 10 bp. Only genes with an FPKM above 10 were considered. A small number of introns with a retention rate above 0.9 were excluded, based on the assumption that they base on false gene models. Sequencing data was deposited at the EBI ArrayExpress Database (E-MTAB-1300).
Co-immunoprecipitation analysis
For in vitro protein expression and co-immunoprecipitation of Num1 and Kin1, the TNT quick coupled transcription/translation system (Promega) was used according to the manufacturer's protocol. For expression of Myc-tagged Num1 protein, plasmid pGBKT7-Num1 was used and HA-tagged Kin1 protein was generated using the pGAD-Kin1650–968 derivative identified in the yeast two-hybrid screen. All subsequent steps were carried out as described previously [103].
For in vivo co-immunoprecipitation, U. maydis sporidia were grown in 100 ml CM–G to an OD600 of 0.8. Cells were washed once with PBS buffer, resuspended in 1 ml PBS supplemented with Complete proteinase inhibitor cocktail (Roche), frozen in liquid nitrogen and homogenized in a Retsch mill for 10 minutes at 30 Hz. The cell lysate was incubated with 40 µg monoclonal anti-HA coupled agarose (Sigma-Aldrich) on a rotating wheel at 4°C over night. Agarose beads were washed three times in PBS prior to resuspension in 20 µl Laemmli buffer. Samples were boiled for three minutes and separated by SDS-PAGE. For co-immunoprecipitation of Num1 and Kin1, a crosslinking reaction with dithiobis[succinimidylpropionate] (Thermo Fisher) was conducted according to [108] using 300 ml of cells grown in CM-G to an OD600 of 0.8.
Proteins were transferred to PVDF nitrocellulose membranes in a semidry blot chamber. Western blots were probed with monoclonal anti-HA, anti-c-Myc, and anti-GFP (Sigma-Aldrich) antibodies. Horseradish peroxidase–conjugated anti-mouse or anti-rabbit IgG (Promega) was used as secondary antibody, and an ECL system was used for protein detection.
Microscopy, image processing and quantitative analysis
For microscopic analyses, logarithmically growing U. maydis cells were taken from liquid cultures grown in CM-G medium. For the induction of hyphal growth, cells were shifted to CM-A or NM to induce the Pcrg1 - or Pnar1 - promoters, respectively, for 12–14 hours. For microscopy of A. nidulans germlings and young hyphae, MM on cover slips was inoculated with a small amount of spores and incubated for 12–18 hours at 37°C. Cells were then placed on top of a 2% agarose cushion placed on the microscope slide and immediately observed using an Axioimager Z1 microscope equipped with an Axiocam MRm camera (Carl Zeiss). Standard filter sets for DAPI, GFP, CFP and Rhodamine were used for epifluorescence analysis.
Nuclei were stained with DAPI Vectashield H-1200 (Vector Laboratories); chitin was stained with 2 µg/ml Calcofluor White (Sigma-Aldrich) or with Congo Red solution (1 µl/ml; stock: 1 mg/ml in H2O). Congo Red was incubated at 25°C for 10 minutes on a turning wheel and washed twice with CM prior to microscopic analysis. Fungal cell walls in general were stained with 1 mg/ml WGA/Fluorescein (Invitrogen) in PBS. For the visualization of vacuoles, growing cells were incubated in CM supplemented with 10 µg/ml CellTracker Blue (7-amino-4-chloromethyl-coumarin, CMAC) (Invitrogen) for 30 minutes at 28°C, washed twice with CM and then subjected to microscopy. Chlorazole Black E staining was performed according to [24].
Endosome motility was measured in image sequences of 50 frames, taken with an exposure time of 500 msec. Only organelles that were moving for a distance of at least 5 µm were considered. The resulting movies were converted into kymographs using ImageJ software [109]. For quantification of Mcs1 signal intensity, the corrected total cell fluorescence (CTCF) of mid-size growing buds was calculated with ImageJ software (CTCF = integrated density – (area of selected cell×fluorescence of background reading)).
For quantification of the microtubule orientation, cells were grown to mid-log phase in YEPSL medium. Cells were analyzed via 45 sec time-lapse recordings (four frames each 15 sec). Pictures were taken with an exposure time of 90 msec for Tub1-GFP and 300 msec for Peb1:RFP signals. Movement of Peb1:RFP signals was scored at a screen as described in [46]. All image processing, including adjustment of brightness, contrast and gamma-values was performed with the AxioVision and ZEN software (Carl Zeiss), respectively.
Phylogenetic analysis
For comparative phylogenetic analysis of Num1, 65 sequences with the highest similarity to Num1 were obtained by BLASTP analysis. The sequence of Saccharomyces cerevisiae Snt309p was included as outgroup. Sequences were aligned with MAFFT version 6 [110] using the global alignment G-INS-i. A phylogenetic tree was calculated using the minimum linkage clustering method (http://align.bmr.kyushu-u.ac.jp/mafft/online/server/).
Fig Tree 1.3 (http://tree.bio.ed.ac.uk/software/figtree/) was used to visualize the Nexus formats of the MAFFT results.
Accession numbers
Sequence data from this article can be found at the Munich Information Center for Protein Sequences (MIPS) Ustilago maydis database (http://mips. helmholtz-muenchen.de/genre/proj/ustilago/), the Aspergillus genome database (http://www.aspgd.org/) and the National Center for Biotechnology Information (NCBI) database under the following accession numbers: num1 (um01682), XP_757829; prp19 (um10027), XP_756259; cef1 (um04411), XP_760558; rbf1 (um03172), XP_759319; pcna (um05403), XP_761550; rpb3 (um03550), XP_759697; ubi1 (um02440), XP_758587; rho3 (um04070), XP_760217; rad51 (um03290), XP_759437; rec1 (um11016), XP_759527.1; mms2 (um10097), XP_756467.1; cks1 (um03210), XP_759357; cdk1 (um10705), AAP94021.1; hdp1 (um12024), XP_761909.1; hdp2 (um04928), XP_761075; biz1 (um02549), XP_758696; um02704 (related to allantoate permease), XP_758851; um12105 (probable PUP3 – 20S proteasome subunit), XP_756658.1; actin (um11232), XP_762364; eIF2b (um04869), XP_761016; kin1 (um04218), XP_760356; dyn2 (um04372), XP_760519; yup1 (um05406), XP_761553; tub1 (um01221), XP_757368; AN4244, XP_661848.1; nudA (AN0118), XP_657722.1; kinA (AN5343), XP_662947.1.; the gene model for um15049 (related to PUF3 transcript specific regulator of mRNA degradation) is implemented only at MUMDB (http://mips.helmholtz-muenchen.de/genre/proj/ustilago), as the predicted protein is composed of two fragments located on adjacent contig ends and, according to the sequence gap, part of the protein may be absent.
Supporting Information
Zdroje
1. BrefortT, DoehlemannG, Mendoza-MendozaA, ReissmannS, DjameiA, et al. (2009) Ustilago maydis as a Pathogen. Annu Rev Phytopathol 47 : 423–445.
2. HeimelK, SchererM, VranesM, WahlR, PothiratanaC, et al. (2010) The transcription factor Rbf1 is the master regulator for b-mating type controlled pathogenic development in Ustilago maydis. PLoS Pathog 6: e1001035.
3. SteinbergG, Perez-MartinJ (2008) Ustilago maydis, a new fungal model system for cell biology. Trends Cell Biol 18 : 61–67.
4. Wedlich-SöldnerR, BölkerM, KahmannR, SteinbergG (2000) A putative endosomal t-SNARE links exo - and endocytosis in the phytopathogenic fungus Ustilago maydis. EMBO J 19 : 1974–1986.
5. StraubeA, EnardW, BernerA, Wedlich-SöldnerR, KahmannR, et al. (2001) A split motor domain in a cytoplasmic dynein. EMBO J 20 : 5091–5100.
6. VollmeisterE, SchipperK, FeldbrüggeM (2012) Microtubule-dependent mRNA transport in the model microorganism Ustilago maydis. RNA Biol 9 : 261–268.
7. BaumannS, PohlmannT, JungbluthM, BrachmannA, FeldbrüggeM (2012) Kinesin-3 and dynein mediate microtubule-dependent co-transport of mRNPs and endosomes. J Cell Sci 125(Pt 11): 2740–52.
8. Wedlich-SöldnerR, StraubeA, FriedrichMW, SteinbergG (2002) A balance of KIF1A-like kinesin and dynein organizes early endosomes in the fungus Ustilago maydis. EMBO J 21 : 2946–2957.
9. LenzJH, SchuchardtI, StraubeA, SteinbergG (2006) A dynein loading zone for retrograde endosome motility at microtubule plus-ends. EMBO J 25 : 2275–2286.
10. SchusterM, KilaruS, FinkG, CollemareJ, RogerY, et al. (2011) Kinesin-3 and dynein cooperate in long-range retrograde endosome motility along a nonuniform microtubule array. Mol Biol Cell 22 : 3645–3657.
11. SchusterM, LipowskyR, AssmannMA, LenzP, SteinbergG (2011) Transient binding of dynein controls bidirectional long-range motility of early endosomes. Proc Natl Acad Sci U S A 108 : 3618–3623.
12. SchusterM, KilaruS, AshwinP, LinC, SeversNJ, et al. (2011) Controlled and stochastic retention concentrates dynein at microtubule ends to keep endosomes on track. EMBO J 30 : 652–664.
13. SteinbergG, SchliwaM, LehmlerC, BölkerM, KahmannR, et al. (1998) Kinesin from the plant pathogenic fungus Ustilago maydis is involved in vacuole formation and cytoplasmic migration. J Cell Sci 111(Pt 15): 2235–2246.
14. SchuchardtI, AssmannD, ThinesE, SchuberthC, SteinbergG (2005) Myosin-V, Kinesin-1, and Kinesin-3 cooperate in hyphal growth of the fungus Ustilago maydis. Mol Biol Cell 16 : 5191–5201.
15. WeberI, AssmannD, ThinesE, SteinbergG (2006) Polar localizing class V myosin chitin synthases are essential during early plant infection in the plant pathogenic fungus Ustilago maydis. Plant Cell 18 : 225–242.
16. TreitschkeS, DoehlemannG, SchusterM, SteinbergG (2010) The myosin motor domain of fungal chitin synthase V is dispensable for vesicle motility but required for virulence of the maize pathogen Ustilago maydis. Plant Cell 22 : 2476–2494.
17. SchusterM, TreitschkeS, KilaruS, MolloyJ, HarmerNJ, et al. (2012) Myosin-5, kinesin-1 and myosin-17 cooperate in secretion of fungal chitin synthase. EMBO J 31 : 214–227.
18. MakinoR, KamadaT (2004) Isolation and characterization of mutations that affect nuclear migration for dikaryosis in Coprinus cinereus. Curr Genet 45 : 149–156.
19. NeubauerG, KingA, RappsilberJ, CalvioC, WatsonM, et al. (1998) Mass spectrometry and EST-database searching allows characterization of the multi-protein spliceosome complex. Nat Genet 20 : 46–50.
20. LehmlerC, SteinbergG, SnetselaarK, SchliwaM, KahmannR, et al. (1997) Identification of a motor protein required for filamentous growth in Ustilago maydis. EMBO J 16 : 3464–3473.
21. GroteM, WolfE, WillCL, LemmI, AgafonovDE, et al. (2010) Molecular architecture of the human Prp19/CDC5L complex. Mol Cell Biol 30 : 2105–2119.
22. ChelskyD, RalphR, JonakG (1989) Sequence requirements for synthetic peptide-mediated translocation to the nucleus. Mol Cell Biol 9 : 2487–2492.
23. KämperJ, KahmannR, BölkerM, MaLJ, BrefortT, et al. (2006) Insights from the genome of the biotrophic fungal plant pathogen Ustilago maydis. Nature 444 : 97–101.
24. BrachmannA, WeinzierlG, KämperJ, KahmannR (2001) Identification of genes in the bW/bE regulatory cascade in Ustilago maydis. Mol Microbiol 42 : 1047–1063.
25. MitchisonJM, NurseP (1985) Growth in cell length in the fission yeast Schizosaccharomyces pombe. J Cell Sci 75 : 357–376.
26. MatsuokaH, YangH-C, HommaT, NemotoY, YamadaS, et al. (1995) Use of Congo red as a microscopic fluorescence indicator of hyphal growth. Appl Microbiol Biotechnol 43 : 102–108.
27. NagataY, BurgerMM (1974) Wheat germ agglutinin. Molecular characteristics and specificity for sugar binding. J Biol Chem 249 : 3116–3122.
28. OhiMD, GouldKL (2002) Characterization of interactions among the Cef1p-Prp19p-associated splicing complex. Rna 8 : 798–815.
29. NasmythK, NurseP (1981) Cell division cycle mutants altered in DNA replication and mitosis in the fission yeast Schizosaccharomyces pombe. Mol Gen Genetics 182 : 119–124.
30. OhiR, McCollumD, HiraniB, Den HaeseGJ, ZhangX, et al. (1994) The Schizosaccharomyces pombe cdc5+ gene encodes an essential protein with homology to c-Myb. EMBO J 13 : 471–483.
31. BernsteinHS, CoughlinSR (1998) A mammalian homolog of fission yeast Cdc5 regulates G2 progression and mitotic entry. J Biol Chem 273 : 4666–4671.
32. HenriquesJA, MoustacchiE (1980) Isolation and characterization of pso mutants sensitive to photo-addition of psoralen derivatives in Saccharomyces cerevisiae. Genetics 95 : 273–288.
33. GreyM, DüsterhöftA, HenriquesJA, BrendelM (1996) Allelism of PSO4 and PRP19 links pre-mRNA processing with recombination and error-prone DNA repair in Saccharomyces cerevisiae. Nucleic Acids Res 24 : 4009–4014.
34. BrendelM, BonattoD, StraussM, ReversLF, PungartnikC, et al. (2003) Role of PSO genes in repair of DNA damage of Saccharomyces cerevisiae. Mutat Res 544 : 179–193.
35. ZhangN, KaurR, LuX, ShenX, LiL, et al. (2005) The Pso4 mRNA splicing and DNA repair complex interacts with WRN for processing of DNA interstrand cross-links. J Biol Chem 280 : 40559–40567.
36. BeckBD, ParkSJ, LeeYJ, RomanY, HromasRA, et al. (2008) Human Pso4 is a metnase (SETMAR)-binding partner that regulates metnase function in DNA repair. J Biol Chem 283 : 9023–9030.
37. LuX, LegerskiRJ (2007) The Prp19/Pso4 core complex undergoes ubiquitylation and structural alterations in response to DNA damage. Biochem Biophys Res Commun 354 : 968–974.
38. LegerskiRJ (2009) The Pso4 complex splices into the DNA damage response. Cell Cycle 8 : 3448–3449.
39. Garcia-MuseT, SteinbergG, Perez-MartinJ (2003) Pheromone-induced G2 arrest in the phytopathogenic fungus Ustilago maydis. Eukaryot Cell 2 : 494–500.
40. MazzeiT (1984) Chemistry and mechanism of action of bleomycin. Chemioterapia : international journal of the Mediterranean Society of Chemotherapy 3 : 316–319.
41. YarbroJW (1992) Mechanism of action of hydroxyurea. Semin Oncol 19 : 1–10.
42. ReversLF, CardoneJM, BonattoD, SaffiJ, GreyM, et al. (2002) Thermoconditional modulation of the pleiotropic sensitivity phenotype by the Saccharomyces cerevisiae PRP19 mutant allele pso4-1. Nucleic Acids Res 30 : 4993–5003.
43. Flor-ParraI, VranesM, KämperJ, Perez-MartinJ (2006) Biz1, a zinc finger protein required for plant invasion by Ustilago maydis, regulates the levels of a mitotic cyclin. Plant Cell 18 : 2369–2387.
44. BanksGR, SheltonPA, KanugaN, HoldenDW, SpanosA (1993) The Ustilago maydis nar1 gene encoding nitrate reductase activity: sequence and transcriptional regulation. Gene 131 : 69–78.
45. RiedlJ, CrevennaAH, KessenbrockK, YuJH, NeukirchenD, et al. (2008) Lifeact: a versatile marker to visualize F-actin. Nat Methods 5 : 605–607.
46. StraubeA, BrillM, OakleyBR, HorioT, SteinbergG (2003) Microtubule organization requires cell cycle-dependent nucleation at dispersed cytoplasmic sites: polar and perinuclear microtubule organizing centers in the plant pathogen Ustilago maydis. Mol Biol Cell 14 : 642–657.
47. BechtP, KönigJ, FeldbrüggeM (2006) The RNA-binding protein Rrm4 is essential for polarity in Ustilago maydis and shuttles along microtubules. J Cell Sci 119 : 4964–4973.
48. RequenaN, Alberti-SeguiC, WinzenburgE, HornC, SchliwaM, et al. (2001) Genetic evidence for a microtubule-destabilizing effect of conventional kinesin and analysis of its consequences for the control of nuclear distribution in Aspergillus nidulans. Mol Microbiol 42 : 121–132.
49. KonczC, DejongF, VillacortaN, SzakonyiD, KonczZ (2012) The spliceosome-activating complex: molecular mechanisms underlying the function of a pleiotropic regulator. Front Plant Sci 3 : 9.
50. TarnWY, HsuCH, HuangKT, ChenHR, KaoHY, et al. (1994) Functional association of essential splicing factor(s) with PRP19 in a protein complex. EMBO J 13 : 2421–2431.
51. HoggR, McGrailJC, O'KeefeRT (2010) The function of the NineTeen Complex (NTC) in regulating spliceosome conformations and fidelity during pre-mRNA splicing. Biochem Soc Trans 38 : 1110–1115.
52. OhiMD, Vander KooiCW, RosenbergJA, ChazinWJ, GouldKL (2003) Structural insights into the U-box, a domain associated with multi-ubiquitination. Nat Struct Biol 10 : 250–255.
53. OhiMD, Vander KooiCW, RosenbergJA, RenL, HirschJP, et al. (2005) Structural and functional analysis of essential pre-mRNA splicing factor Prp19p. Mol Cell Biol 25 : 451–460.
54. ChengSC, TarnWY, TsaoTY, AbelsonJ (1993) PRP19: a novel spliceosomal component. Mol Cell Biol 13 : 1876–1882.
55. TarnWY, LeeKR, ChengSC (1993) The yeast PRP19 protein is not tightly associated with small nuclear RNAs, but appears to associate with the spliceosome after binding of U2 to the pre-mRNA and prior to formation of the functional spliceosome. Mol Cell Biol 13 : 1883–1891.
56. TarnWY, LeeKR, ChengSC (1993) Yeast precursor mRNA processing protein PRP19 associates with the spliceosome concomitant with or just after dissociation of U4 small nuclear RNA. Proc Natl Acad Sci U S A 90 : 10821–10825.
57. TsaiWY, ChowYT, ChenHR, HuangKT, HongRI, et al. (1999) Cef1p is a component of the Prp19p-associated complex and essential for pre-mRNA splicing. J Biol Chem 274 : 9455–9462.
58. McDonaldWH, OhiR, SmelkovaN, FrendeweyD, GouldKL (1999) Myb-related fission yeast cdc5p is a component of a 40S snRNP-containing complex and is essential for pre-mRNA splicing. Mol Cell Biol 19 : 5352–5362.
59. QueryCC, KonarskaMM (2012) CEF1/CDC5 alleles modulate transitions between catalytic conformations of the spliceosome. Rna 18 : 1001–1013.
60. PleissJA, WhitworthGB, BergkesselM, GuthrieC (2007) Rapid, transcript-specific changes in splicing in response to environmental stress. Mol Cell 27 : 928–937.
61. BöhmerC, BöhmerM, BölkerM, SandrockB (2008) Cdc42 and the Ste20-like kinase Don3 act independently in triggering cytokinesis in Ustilago maydis. J Cell Sci 121 : 143–148.
62. BöhmerC, RippC, BölkerM (2009) The germinal centre kinase Don3 triggers the dynamic rearrangement of higher-order septin structures during cytokinesis in Ustilago maydis. Mol Microbiol 74 : 1484–1496.
63. MahlertM, LevelekiL, HlubekA, SandrockB, BölkerM (2006) Rac1 and Cdc42 regulate hyphal growth and cytokinesis in the dimorphic fungus Ustilago maydis. Mol Microbiol 59 : 567–578.
64. WeinzierlG, LevelekiL, HasselA, KostG, WannerG, et al. (2002) Regulation of cell separation in the dimorphic fungus Ustilago maydis. Mol Microbiol 45 : 219–231.
65. FreitagJ, LanverD, BöhmerC, SchinkKO, BölkerM, et al. (2011) Septation of infectious hyphae is critical for appressoria formation and virulence in the smut fungus Ustilago maydis. PLoS Pathog 7: e1002044.
66. BankmannM, PrakashL, PrakashS (1992) Yeast RAD14 and human xeroderma pigmentosum group A DNA-repair genes encode homologous proteins. Nature 355 : 555–558.
67. MahajanKN, MitchellBS (2003) Role of human Pso4 in mammalian DNA repair and association with terminal deoxynucleotidyl transferase. Proc Natl Acad Sci U S A 100 : 10746–10751.
68. AjuhP, KusterB, PanovK, ZomerdijkJC, MannM, et al. (2000) Functional analysis of the human CDC5L complex and identification of its components by mass spectrometry. EMBO J 19 : 6569–6581.
69. ZhangN, KaurR, AkhterS, LegerskiRJ (2009) Cdc5L interacts with ATR and is required for the S-phase cell-cycle checkpoint. EMBO Rep 10 : 1029–1035.
70. Perez-MartinJ (2009) DNA-damage response in the basidiomycete fungus Ustilago maydis relies in a sole Chk1-like kinase. DNA Repair 8 : 720–731.
71. Perez-MartinJ, de Sena-TomasC (2011) Dikaryotic cell cycle in the phytopathogenic fungus Ustilago maydis is controlled by the DNA damage response cascade. Plant Signal Behav 6 : 1574–1577.
72. ChorevM, CarmelL (2012) The function of introns. Frontiers in genetics 3 : 55.
73. Le HirH, NottA, MooreMJ (2003) How introns influence and enhance eukaryotic gene expression. Trends Biochem Sci 28 : 215–220.
74. MuresanV, MuresanZ (2012) Unconventional functions of microtubule motors. Arch Biochem Biophys 520 : 17–29.
75. SunF, ZhuC, DixitR, CavalliV (2011) Sunday Driver/JIP3 binds kinesin heavy chain directly and enhances its motility. EMBO J 30 : 3416–3429.
76. SteinbergG, SchusterM, TheisenU, KilaruS, ForgeA, et al. (2012) Motor-driven motility of fungal nuclear pores organizes chromosomes and fosters nucleocytoplasmic transport. J Cell Biol 198 : 343–355.
77. KönigJ, BaumannS, KoepkeJ, PohlmannT, ZarnackK, et al. (2009) The fungal RNA-binding protein Rrm4 mediates long-distance transport of ubi1 and rho3 mRNAs. EMBO J 28 : 1855–1866.
78. BechtP, VollmeisterE, FeldbrüggeM (2005) Role for RNA-binding proteins implicated in pathogenic development of Ustilago maydis. Eukaryot Cell 4 : 121–133.
79. ZimyaninVL, BelayaK, PecreauxJ, GilchristMJ, ClarkA, et al. (2008) In vivo imaging of oskar mRNA transport reveals the mechanism of posterior localization. Cell 134 : 843–853.
80. ChangYF, ImamJS, WilkinsonMF (2007) The nonsense-mediated decay RNA surveillance pathway. Annu Rev Biochem 76 : 51–74.
81. BrendzaRP, SerbusLR, SaxtonWM, DuffyJB (2002) Posterior localization of dynein and dorsal-ventral axis formation depend on kinesin in Drosophila oocytes. Curr Biol 12 : 1541–1545.
82. ChanaratS, SeizlM, StrasserK (2011) The Prp19 complex is a novel transcription elongation factor required for TREX occupancy at transcribed genes. Genes Dev 25 : 1147–1158.
83. KatahiraJ, YonedaY (2009) Roles of the TREX complex in nuclear export of mRNA. RNA Biol 6 : 149–152.
84. KatahiraJ, StrasserK, PodtelejnikovA, MannM, JungJU, et al. (1999) The Mex67p-mediated nuclear mRNA export pathway is conserved from yeast to human. EMBO J 18 : 2593–2609.
85. Santos-RosaH, MorenoH, SimosG, SegrefA, FahrenkrogB, et al. (1998) Nuclear mRNA export requires complex formation between Mex67p and Mtr2p at the nuclear pores. Mol Cell Biol 18 : 6826–6838.
86. SegrefA, SharmaK, DoyeV, HellwigA, HuberJ, et al. (1997) Mex67p, a novel factor for nuclear mRNA export, binds to both poly(A)+ RNA and nuclear pores. EMBO J 16 : 3256–3271.
87. Sambrook J, Frisch EF, Maniatis T (1989) Molecular Cloning: A Laboratory Manual. Cold Spring Harbour, New York: Cold Spring Harbour Laboratory Press.
88. TsukudaT, CarletonS, FotheringhamS, HollomanWK (1988) Isolation and characterization of an autonomously replicating sequence from Ustilago maydis. Mol Cell Biol 8 : 3703–3709.
89. Holliday R (1974) Ustilago maydis. In: King RC, editor. Handbook of Genetics. New York, USA: Plenum Press. pp. 575–595.
90. MahlertM, VoglerC, StelterK, HauseG, BasseCW (2009) The a2 mating-type-locus gene lga2 of Ustilago maydis interferes with mitochondrial dynamics and fusion, partially in dependence on a Dnm1-like fission component. J Cell Sci 122 : 2402–2412.
91. MüllerP, WeinzierlG, BrachmannA, FeldbrüggeM, KahmannR (2003) Mating and pathogenic development of the Smut fungus Ustilago maydis are regulated by one mitogen-activated protein kinase cascade. Eukaryot Cell 2 : 1187–1199.
92. PontecorvoG, RoperJA, HemmonsLM, MacdonaldKD, BuftonAW (1953) The genetics of Aspergillus nidulans. Adv Genet 5 : 141–238.
93. Hill TWuK, E (2001) Improved protocols for Aspergillus minimal medium: trace element and minimal medium salt stock solutions. Fungal Genetics Newsletter 48 : 20–21.
94. GillissenB, BergemannJ, SandmannC, SchröerB, BölkerM, et al. (1992) A two-component regulatory system for self/non-self recognition in Ustilago maydis. Cell 68 : 647–657.
95. Mendoza-MendozaA, EskovaA, WeiseC, CzajkowskiR, KahmannR (2009) Hap2 regulates the pheromone response transcription factor prf1 in Ustilago maydis. Mol Microbiol 72 : 683–698.
96. HoffmanCS, WinstonF (1987) A ten-minute DNA preparation from yeast efficiently releases autonomous plasmids for transformation of E. coli. Gene 57 : 267–272.
97. SchulzB, BanuettF, DahlM, SchlesingerR, SchäferW, et al. (1990) The b alleles of U. maydis, whose combinations program pathogenic development, code for polypeptides containing a homeodomain-related motif. Cell 60 : 295–306.
98. TimberlakeWE, MarshallMA (1989) Genetic engineering of filamentous fungi. Science 244 : 1313–1317.
99. YeltonMM, HamerJE, TimberlakeWE (1984) Transformation of Aspergillus nidulans by using a trpC plasmid. Proc Natl Acad Sci U S A 81 : 1470–1474.
100. KämperJ (2004) A PCR-based system for highly efficient generation of gene replacement mutants in Ustilago maydis. Mol Genet Genomics 271 : 103–110.
101. BrachmannA, KönigJ, JuliusC, FeldbrüggeM (2004) A reverse genetic approach for generating gene replacement mutants in Ustilago maydis. Mol Genet Genomics 272 : 216–226.
102. SchererM, HeimelK, StarkeV, KämperJ (2006) The Clp1 protein is required for clamp formation and pathogenic development of Ustilago maydis. Plant Cell 18 : 2388–2401.
103. HeimelK, SchererM, SchulerD, KämperJ (2010) The Ustilago maydis Clp1 protein orchestrates pheromone and b-dependent signaling pathways to coordinate the cell cycle and pathogenic development. Plant Cell 22 : 2908–2922.
104. GarridoE, Perez-MartinJ (2003) The crk1 gene encodes an Ime2-related protein that is required for morphogenesis in the plant pathogen Ustilago maydis. Mol Microbiol 47 : 729–743.
105. TrapnellC, PachterL, SalzbergSL (2009) TopHat: discovering splice junctions with RNA-Seq. Bioinformatics 25 : 1105–1111.
106. AndersS, HuberW (2010) Differential expression analysis for sequence count data. Genome Biol 11: R106.
107. HardcastleTJ, KellyKA (2010) baySeq: empirical Bayesian methods for identifying differential expression in sequence count data. BMC Bioinformatics 11 : 422.
108. LanverD, Mendoza-MendozaA, BrachmannA, KahmannR (2010) Sho1 and Msb2-related proteins regulate appressorium development in the smut fungus Ustilago maydis. Plant Cell 22 : 2085–2101.
109. AbramoffMD, MagalhaesPJ, RamSJ (2004) Image Processing with Image J. Biophotonics International 11 : 36–42.
110. KatohK, TohH (2008) Recent developments in the MAFFT multiple sequence alignment program. Brief Bioinform 9 : 286–298.
Štítky
Genetika Reprodukční medicína
Článek Unwrapping BacteriaČlánek A Chaperone-Assisted Degradation Pathway Targets Kinetochore Proteins to Ensure Genome StabilityČlánek The Candidate Splicing Factor Sfswap Regulates Growth and Patterning of Inner Ear Sensory OrgansČlánek Down-Regulation of eIF4GII by miR-520c-3p Represses Diffuse Large B Cell Lymphoma DevelopmentČlánek Meta-Analysis Identifies Gene-by-Environment Interactions as Demonstrated in a Study of 4,965 MiceČlánek High Risk Population Isolate Reveals Low Frequency Variants Predisposing to Intracranial Aneurysms
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2014 Číslo 1
-
Všechny články tohoto čísla
- How Much Is That in Dog Years? The Advent of Canine Population Genomics
- The Sense and Sensibility of Strand Exchange in Recombination Homeostasis
- Unwrapping Bacteria
- DNA Methylation Changes Separate Allergic Patients from Healthy Controls and May Reflect Altered CD4 T-Cell Population Structure
- Evidence for Mito-Nuclear and Sex-Linked Reproductive Barriers between the Hybrid Italian Sparrow and Its Parent Species
- Translation Enhancing ACA Motifs and Their Silencing by a Bacterial Small Regulatory RNA
- Relationship Estimation from Whole-Genome Sequence Data
- Genetic Models of Apoptosis-Induced Proliferation Decipher Activation of JNK and Identify a Requirement of EGFR Signaling for Tissue Regenerative Responses in
- ComEA Is Essential for the Transfer of External DNA into the Periplasm in Naturally Transformable Cells
- Loss and Recovery of Genetic Diversity in Adapting Populations of HIV
- Bioelectric Signaling Regulates Size in Zebrafish Fins
- Defining NELF-E RNA Binding in HIV-1 and Promoter-Proximal Pause Regions
- Loss of Histone H3 Methylation at Lysine 4 Triggers Apoptosis in
- Cell-Cycle Dependent Expression of a Translocation-Mediated Fusion Oncogene Mediates Checkpoint Adaptation in Rhabdomyosarcoma
- How a Retrotransposon Exploits the Plant's Heat Stress Response for Its Activation
- A Nonsense Mutation in Encoding a Nondescript Transmembrane Protein Causes Idiopathic Male Subfertility in Cattle
- Deletion of a Conserved -Element in the Locus Highlights the Role of Acute Histone Acetylation in Modulating Inducible Gene Transcription
- Developmental Link between Sex and Nutrition; Regulates Sex-Specific Mandible Growth via Juvenile Hormone Signaling in Stag Beetles
- PP2A/B55 and Fcp1 Regulate Greatwall and Ensa Dephosphorylation during Mitotic Exit
- Differential Effects of Collagen Prolyl 3-Hydroxylation on Skeletal Tissues
- Comprehensive Functional Annotation of 77 Prostate Cancer Risk Loci
- Evolution of Chloroplast Transcript Processing in and Its Chromerid Algal Relatives
- A Chaperone-Assisted Degradation Pathway Targets Kinetochore Proteins to Ensure Genome Stability
- New MicroRNAs in —Birth, Death and Cycles of Adaptive Evolution
- A Genome-Wide Screen for Bacterial Envelope Biogenesis Mutants Identifies a Novel Factor Involved in Cell Wall Precursor Metabolism
- FGFR1-Frs2/3 Signalling Maintains Sensory Progenitors during Inner Ear Hair Cell Formation
- Regulation of Synaptic /Neuroligin Abundance by the /Nrf Stress Response Pathway Protects against Oxidative Stress
- Intrasubtype Reassortments Cause Adaptive Amino Acid Replacements in H3N2 Influenza Genes
- Molecular Specificity, Convergence and Constraint Shape Adaptive Evolution in Nutrient-Poor Environments
- WNT7B Promotes Bone Formation in part through mTORC1
- Natural Selection Reduced Diversity on Human Y Chromosomes
- In-Vivo Quantitative Proteomics Reveals a Key Contribution of Post-Transcriptional Mechanisms to the Circadian Regulation of Liver Metabolism
- The Candidate Splicing Factor Sfswap Regulates Growth and Patterning of Inner Ear Sensory Organs
- The Acid Phosphatase-Encoding Gene Contributes to Soybean Tolerance to Low-Phosphorus Stress
- p53 and TAp63 Promote Keratinocyte Proliferation and Differentiation in Breeding Tubercles of the Zebrafish
- Affects Plant Architecture by Regulating Local Auxin Biosynthesis
- The SET Domain Proteins SUVH2 and SUVH9 Are Required for Pol V Occupancy at RNA-Directed DNA Methylation Loci
- Down-Regulation of Rad51 Activity during Meiosis in Yeast Prevents Competition with Dmc1 for Repair of Double-Strand Breaks
- Multi-tissue Analysis of Co-expression Networks by Higher-Order Generalized Singular Value Decomposition Identifies Functionally Coherent Transcriptional Modules
- A Neurotoxic Glycerophosphocholine Impacts PtdIns-4, 5-Bisphosphate and TORC2 Signaling by Altering Ceramide Biosynthesis in Yeast
- Subtle Changes in Motif Positioning Cause Tissue-Specific Effects on Robustness of an Enhancer's Activity
- C/EBPα Is Required for Long-Term Self-Renewal and Lineage Priming of Hematopoietic Stem Cells and for the Maintenance of Epigenetic Configurations in Multipotent Progenitors
- The SPF27 Homologue Num1 Connects Splicing and Kinesin 1-Dependent Cytoplasmic Trafficking in
- Down-Regulation of eIF4GII by miR-520c-3p Represses Diffuse Large B Cell Lymphoma Development
- Genome Sequencing Highlights the Dynamic Early History of Dogs
- Re-sequencing Expands Our Understanding of the Phenotypic Impact of Variants at GWAS Loci
- Meta-Analysis Identifies Gene-by-Environment Interactions as Demonstrated in a Study of 4,965 Mice
- , a -Antisense Gene of , Encodes a Evolved Protein That Inhibits GSK3β Resulting in the Stabilization of MYCN in Human Neuroblastomas
- A Transcription Factor Is Wound-Induced at the Planarian Midline and Required for Anterior Pole Regeneration
- A Comprehensive tRNA Deletion Library Unravels the Genetic Architecture of the tRNA Pool
- A PNPase Dependent CRISPR System in
- Genomic Confirmation of Hybridisation and Recent Inbreeding in a Vector-Isolated Population
- Zinc Finger Transcription Factors Displaced SREBP Proteins as the Major Sterol Regulators during Saccharomycotina Evolution
- GATA6 Is a Crucial Regulator of Shh in the Limb Bud
- Tissue Specific Roles for the Ribosome Biogenesis Factor Wdr43 in Zebrafish Development
- A Cell Cycle and Nutritional Checkpoint Controlling Bacterial Surface Adhesion
- High Risk Population Isolate Reveals Low Frequency Variants Predisposing to Intracranial Aneurysms
- E3 Ubiquitin Ligase CHIP and NBR1-Mediated Selective Autophagy Protect Additively against Proteotoxicity in Plant Stress Responses
- Evolutionary Rate Covariation Identifies New Members of a Protein Network Required for Female Post-Mating Responses
- 3′ Untranslated Regions Mediate Transcriptional Interference between Convergent Genes Both Locally and Ectopically in
- Single Nucleus Genome Sequencing Reveals High Similarity among Nuclei of an Endomycorrhizal Fungus
- Metabolic QTL Analysis Links Chloroquine Resistance in to Impaired Hemoglobin Catabolism
- Notch Controls Cell Adhesion in the Drosophila Eye
- AL PHD-PRC1 Complexes Promote Seed Germination through H3K4me3-to-H3K27me3 Chromatin State Switch in Repression of Seed Developmental Genes
- Genomes Reveal Evolution of Microalgal Oleaginous Traits
- Large Inverted Duplications in the Human Genome Form via a Fold-Back Mechanism
- Variation in Genome-Wide Levels of Meiotic Recombination Is Established at the Onset of Prophase in Mammalian Males
- Age, Gender, and Cancer but Not Neurodegenerative and Cardiovascular Diseases Strongly Modulate Systemic Effect of the Apolipoprotein E4 Allele on Lifespan
- Lifespan Extension Conferred by Endoplasmic Reticulum Secretory Pathway Deficiency Requires Induction of the Unfolded Protein Response
- Is Non-Homologous End-Joining Really an Inherently Error-Prone Process?
- Vestigialization of an Allosteric Switch: Genetic and Structural Mechanisms for the Evolution of Constitutive Activity in a Steroid Hormone Receptor
- Functional Divergence and Evolutionary Turnover in Mammalian Phosphoproteomes
- A 660-Kb Deletion with Antagonistic Effects on Fertility and Milk Production Segregates at High Frequency in Nordic Red Cattle: Additional Evidence for the Common Occurrence of Balancing Selection in Livestock
- Comparative Evolutionary and Developmental Dynamics of the Cotton () Fiber Transcriptome
- The Transcription Factor BcLTF1 Regulates Virulence and Light Responses in the Necrotrophic Plant Pathogen
- Crossover Patterning by the Beam-Film Model: Analysis and Implications
- Single Cell Genomics: Advances and Future Perspectives
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- GATA6 Is a Crucial Regulator of Shh in the Limb Bud
- Large Inverted Duplications in the Human Genome Form via a Fold-Back Mechanism
- Differential Effects of Collagen Prolyl 3-Hydroxylation on Skeletal Tissues
- Affects Plant Architecture by Regulating Local Auxin Biosynthesis
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



