-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaA Genome-Wide Screen for Bacterial Envelope Biogenesis Mutants Identifies a Novel Factor Involved in Cell Wall Precursor Metabolism
The cell envelope of Gram-negative bacteria is a formidable barrier that is difficult for antimicrobial drugs to penetrate. Thus, the list of treatments effective against these organisms is small and with the rise of new resistance mechanisms is shrinking rapidly. New therapies to treat Gram-negative bacterial infections are therefore sorely needed. This goal will be greatly aided by a detailed mechanistic understanding of envelope assembly. Although excellent progress in the identification of essential envelope biogenesis systems has been made in recent years, many aspects of the process remain to be elucidated. We therefore developed a simple, quantitative, and high-throughput assay for mutants with envelope biogenesis defects and used it to screen an ordered single-gene deletion library of Escherichia coli. The screen was robust and correctly identified numerous mutants known to be involved in envelope assembly. Importantly, the screen also implicated 102 genes of unknown function as encoding factors that likely impact envelope biogenesis. As a proof of principle, one of these factors, ElyC (YcbC), was characterized further and shown to play a critical role in the metabolism of the essential lipid carrier used for the biogenesis of cell wall and other bacterial surface polysaccharides. Further analysis of the function of ElyC and other hits identified in our screen is likely to uncover a wealth of new information about the biogenesis of the Gram-negative envelope and the vulnerabilities in the system suitable for drug targeting. Moreover, the screening assay described here should be readily adaptable to other organisms to study the biogenesis of different envelope architectures.
Published in the journal: . PLoS Genet 10(1): e32767. doi:10.1371/journal.pgen.1004056
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004056Summary
The cell envelope of Gram-negative bacteria is a formidable barrier that is difficult for antimicrobial drugs to penetrate. Thus, the list of treatments effective against these organisms is small and with the rise of new resistance mechanisms is shrinking rapidly. New therapies to treat Gram-negative bacterial infections are therefore sorely needed. This goal will be greatly aided by a detailed mechanistic understanding of envelope assembly. Although excellent progress in the identification of essential envelope biogenesis systems has been made in recent years, many aspects of the process remain to be elucidated. We therefore developed a simple, quantitative, and high-throughput assay for mutants with envelope biogenesis defects and used it to screen an ordered single-gene deletion library of Escherichia coli. The screen was robust and correctly identified numerous mutants known to be involved in envelope assembly. Importantly, the screen also implicated 102 genes of unknown function as encoding factors that likely impact envelope biogenesis. As a proof of principle, one of these factors, ElyC (YcbC), was characterized further and shown to play a critical role in the metabolism of the essential lipid carrier used for the biogenesis of cell wall and other bacterial surface polysaccharides. Further analysis of the function of ElyC and other hits identified in our screen is likely to uncover a wealth of new information about the biogenesis of the Gram-negative envelope and the vulnerabilities in the system suitable for drug targeting. Moreover, the screening assay described here should be readily adaptable to other organisms to study the biogenesis of different envelope architectures.
Introduction
The cell envelope of bacteria serves as their interface with the environment. This structure plays an essential role in maintaining cellular integrity and provide protection from external insults. For pathogens, the envelope is the site of first contact with the host and where major pathogenicity determinants such as adhesins and toxin secretion systems assemble [1]–[3]. Uniquely bacterial in origin, cell envelope building blocks are also recognized by host innate immune receptors as signs of invasion [4]. The cell envelope is therefore both a strength and a weakness for pathogenic organisms. Similarly, these structures simultaneously present great challenges and opportunities for therapeutic intervention. Many otherwise effective drugs have difficulty penetrating bacterial envelopes to reach their cellular target [5]. On the other hand, molecules like penicillin and vancomycin that disrupt envelope assembly processes have been some of our most effective antibacterial treatments.
Although envelope composition varies throughout the bacterial domain, the structures are typically complex and multi-layered [6], [7]. The two most well-studied classes of envelopes belong to the Firmicutes and Proteobacteria and are traditionally referred to as being either Gram-positive or Gram-negative, respectively, based on how they normally react to the classic Gram-staining procedure. Gram-positive (monoderm) envelopes are bi-layered structures consisting of a single membrane surrounded by a thick cell wall composed of peptidoglycan (PG) and teichoic acids [7]. The envelopes of Gram-negative bacteria (diderm), on the other hand, have three layers: an inner (cytoplasmic) membrane, an outer membrane, and a thin layer of PG sandwiched between them [7]. Mycobacteria possess another distinct envelope class. In addition to a cell membrane and PG layer, they contain a second polysaccharide layer called the arabinogalactan, which is attached to waxy hydrocarbons called mycolic acids that are thought to form the equivalent of the Gram-negative outer membrane [8].
The outer membrane of Gram-negative proteobacteria provides these organisms with a high intrinsic resistance to antibiotics [9]. Thus, therapeutic options for treating Gram-negative bacterial infections are relatively limited. The problem has worsened significantly in recent years with the emergence of carbapenem-resistant Gram-negative Enterobacteriaceae like Klebsiella pneumoniae and Escherichia coli [10]. It is therefore important that new vulnerabilities in the Gram-negative envelope be identified to serve as targets for antibacterial drugs, or for the development of inhibitors that disrupt the permeability barrier to sensitize resistant organisms to approved therapeutics. Along these lines, tremendous progress has been made in our understanding of Gram-negative envelope assembly over the last two decades [11]. Most of the essential envelope biogenesis systems have now been identified in E. coli and related proteobacterial pathogens including: (i) the Sec system that transports proteins across the inner membrane or inserts them into it, (ii) the Lol system for lipoprotein transport to the outer membrane, (iii) the Bam system for outer membrane beta-barrel protein assembly, (iv) the Lpt system for lipopolysaccharide (LPS) transport to and assembly in the outer membrane, and (v) the penicillin-binding proteins (PBPs) and associated factors that construct the PG layer [7]. What remains unclear is how these different processes are controlled and coordinated with one another so that the envelope grows uniformly and maintains its integrity as it is remodeled. Given that genes coding for envelope proteins constitute roughly one quarter of the E. coli genome, and that over a third of these have an unknown or poorly understood function [12], it is likely that many factors important for modulating envelope assembly remain to be identified. Large-scale genetic methods represent a promising avenue for discovering these factors.
Genetic screens for envelope biogenesis mutants were performed many years ago taking advantage of the release of periplasmic RNase from defective cells, which was detected as a zone of clearing on RNA-containing agar plates [13], [14]. However, these “periplasmic leaky” screens were performed in the pre-genomic era and only identified a small handful of mutants, some of which were never precisely mapped [15]–[18]. We therefore thought that revisiting this genetic approach with our current knowledge and technology would be fruitful for the identification of new factors involved in the biogenesis of the Gram-negative envelope. Rather than rely on the detection of RNase leakage, which requires replica-plating and the use of RNA-containing soft agar overlays [13], [14], we decided to use an old reporter, β-galactosidase (LacZ), in a new way. The β-galactosidase substrate chlorophenyl red-β-D-galactopyranoside (CPRG) fails to penetrate the E. coli envelope and cannot be processed by cytoplasmic LacZ (Figure 1). Because mutants impaired for envelope biogenesis typically either lyse at an elevated frequency to release LacZ into the medium and/or are more permeable to small hydrophobic molecules, we reasoned that they should be readily identifiable based on CPRG hydrolysis and the formation of red colonies on CPRG-containing agar. A preliminary screen of a transposon-mutagenized wild-type E. coli strain proved this to indeed be the case. We then proceeded to systematically screen an ordered E. coli deletion library for mutants with a CPRG+ phenotype, implicating numerous new factors in proper envelope assembly. As a proof of principle, we further analyzed a mutant inactivated for ycbC, which encodes a factor with a highly conserved domain of unknown function (DUF218) [19]. Loss of YcbC function was found to cause a severe growth defect at low temperature accompanied by an elevated frequency of cell lysis resulting from impaired metabolism of the essential lipid precursor required for PG biogenesis. We have therefore renamed this factor ElyC (elevated frequency of lysis) to reflect this phenotype. Further analysis of the function of ElyC and other CPRG+ mutants is likely to uncover a wealth of new information about the biogenesis of the Gram-negative envelope and its control under different environmental conditions. Importantly, the CPRG screen described here should also be transferable to other organisms to study the biogenesis of different envelope architectures and understand how one of the most rapidly evolving features of the bacterial cell adapts to different niches.
Fig. 1. A screen for mutants defective in cell envelope assembly. 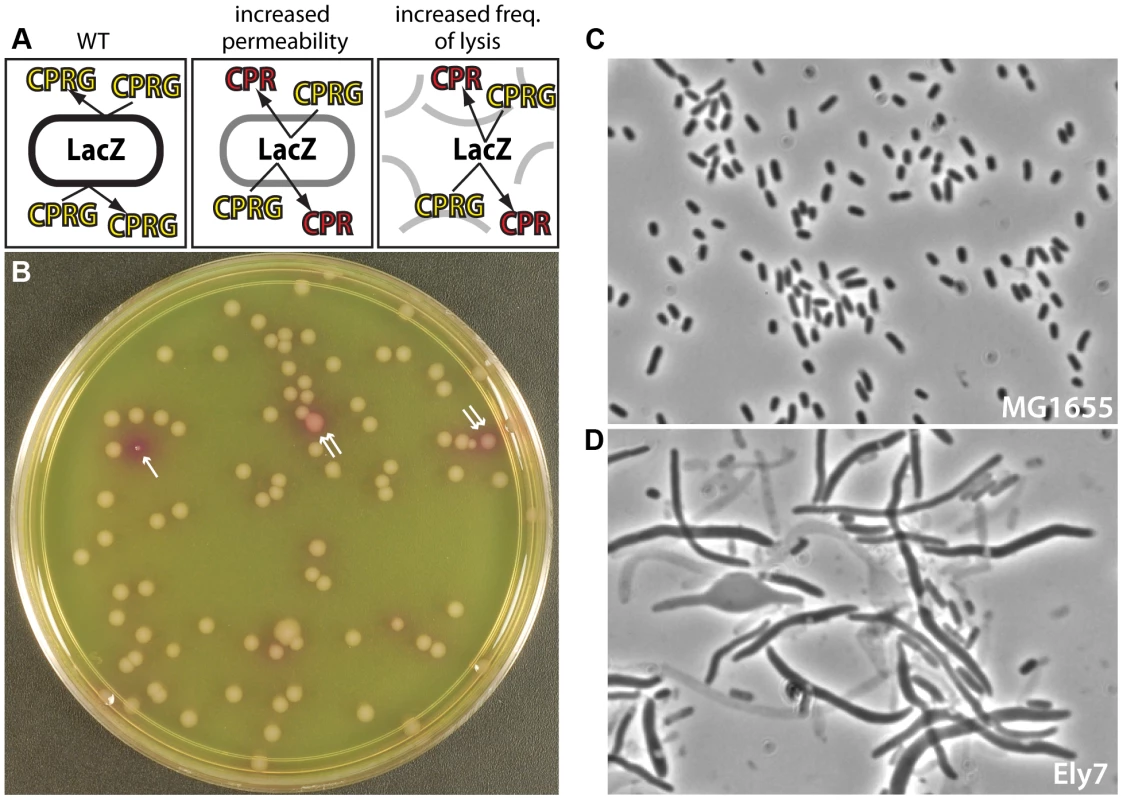
A. Shown is a schematic illustrating the logic of the screen. Wild-type (WT) cells are unable to cleave CPRG because the enzyme (LacZ) is separated from its substrate (CPRG) by the intact cell envelope and thus remain white. Mutants that lyse or have defects in envelope permeability are able to cleave CPRG and turn red. B. Picture of CPRG agar with colonies from an E. coli transposon mutant library. Most colonies are “white”, with occasional red (single arrow) or pink (double arrow) colonies identifying mutants likely to have envelope defects. The particular “red” mutant shown also has a growth defect. The plate was incubated overnight at 30°C. Note that we were unable to carry out the screen at 37°C due to excessive background color development on the CPRG agar. C–D. Micrographs show cells from a colony of WT (MG1655) (C) or an Ely mutant (Ely7) (D) grown on LB agar prepared with 1% NaCl. The transposon in Ely7 was mapped to the nhaA gene coding for a sodium-proton antiporter. The phenotype shown was observed on LB with 1% NaCl, but not on LB lacking added salt (data not shown). Cells were imaged on 1.2% agarose pads with a Nikon 50i microscope with a 100× phase-contrast objective. Results
A high-throughput screen for mutants defective in envelope biogenesis
Our goal was to develop a simple screen for the identification of new factors required for Gram-negative cell envelope biogenesis. We thought that β-galactosidase (LacZ) would be a useful reporter for envelope integrity because the classic protocol for measuring LacZ activity with o-nitrophenyl-β-D-galactopyranoside (ONPG) requires a membrane permeabilization step to allow substrate entry in cells [20]. We therefore reasoned that in the absence of membrane permeabilization, mutants impaired in envelope biogenesis could be identified by their enhanced LacZ activity over normal cells. The synthetic substrate CPRG was chosen over ONPG for screen development because of its increased sensitivity [21] and the red color of its cleavage product, CPR (chlorophenyl red), which we assumed would be easier to detect on LB agar than the yellow ONPG product. Although it is unclear which membrane of the Gram-negative envelope is primarily responsible for preventing CPRG from entering cells, the outer membrane is a good candidate given the hydrophobic nature of the synthetic substrate and the well-known effectiveness of this layer at blocking uptake of other hydrophobic molecules. Thus, mutants capable of processing CPRG may either be defective in the permeability barrier of the outer membrane or possess a defect that results in an elevated frequency of cell lysis promoting the release of LacZ into the medium (Figure 1A).
As an initial test of the screen, we mutagenized wild-type E. coli MG1655, which is Lac+, with the EzTn-Kan transposome (Epicentre) and plated dilutions of the resulting mutant library on LB agar supplemented with CPRG (20 µg/ml) and IPTG (50 µM) to induce the lac operon. Following overnight incubation at 30°C, mutant colonies ranging from pink to dark red with intense halos were observed at a frequency of approximately 1–2% (Figure 1B, and data not shown). These CPRG+ colonies were purified on LB agar prepared with a range of NaCl concentrations (0, 0.5, and 1%) to assess growth of the mutants under different osmotic conditions. Cells from the resulting colonies were visualized by phase contrast microscopy, and mutant strains displaying an elevated frequency of lysis were given the designation Ely. Figure 1D shows an example micrograph of an isolate with a severe Ely phenotype relative to wild-type (Figure 1C). Several mutants, including the example shown in Figure 1D, displayed lysis and/or morphological phenotypes that varied greatly in their magnitude depending on the NaCl concentration of the medium or the growth temperature, thus highlighting the utility of testing the different growth conditions (data not shown).
Transposon insertion sites were mapped in approximately 100 CPRG+ strains, many of which displayed some level of cell lysis in micrographs. A large fraction of the mutants harbored insertions in genes known to encode envelope assembly factors, including: (i) components of the Tol-Pal system [22], [23], (ii) the Tat transport system [24], [25], (iii) PBP1b and its partner LpoB [26], [27], and (iv) EnvC [28]–[30]. Thus, the screen was identifying factors important for envelope assembly as intended. One problem with the transposon mutagenesis approach, however, was that insertions in large genes or operons represented a large fraction of the CPRG+ isolates. For example, over 10% of the isolates had insertions in the tol-pal locus (data not shown). This observation suggested that screening an ordered mutant collection would likely result in a more comprehensive identification of envelope defective mutants than random mutagenesis and screening. We therefore set out to screen a defined mutant library [31] that includes the E. coli knockout (Keio) collection [32] as well as a collection of mutants with hypomorphic alleles of essential genes and mutants lacking genes for small RNAs.
The parent strain background (BW25113) of the ordered mutant library is LacZ−. We thus could not directly use the ordered library for CPRG screening. To convert the library to LacZ+ en masse, we constructed a mobile plasmid, pCB112, encoding lacZ under control of the lactose promoter (Plac). Lawns of a pCB112-containing donor strain were prepared, and the defined mutant collection was then transferred onto the lawns in 384-pin format. After overnight incubation, spots corresponding to the locations of pinned library cells were transferred to agar supplemented with kanamycin (Kan) and chloramphenicol (Cam) to select for exconjugants possessing both the defined mutation (marked by a KanR cassette) and the lacZ plasmid (marked by a CamR cassette). Doubly drug-resistant colonies were then transferred in 384-pin format to LB agar supplemented with IPTG and CPRG to screen for envelope mutants. Based on our experience with the transposon library screen, we decided to screen the ordered mutant set at room temperature and 30°C on media with different NaCl concentrations (0 and 1% NaCl for both temperatures) in order to maximize the number of identified envelope biogenesis factors and potentially identify factors required for adaptation to different temperatures or osmotic conditions.
To identify potential envelope biogenesis mutants, plate images from each growth condition were quantitatively analyzed for red color development using a custom software application called Iris that can quantify several different colony features including color (Kritikos & Typas, unpublished data). The observed CPR color development on the screening plates was found to be directly proportional to incubation time (data not shown). However, because the color diffuses, an early time-point was used for quantification to avoid neighbors of CPRG+ colonies from being scored as hits. An example of a screening plate and corresponding quantification is shown in Figure 2A–B. These panels as well as the CPRG score distributions for all 4 different conditions (Figure 2C and S1) clearly show that the assay is specific (only a small proportion of mutants score positively) and has a wide dynamic range. Following quantification of all plates and setting the CPRG score threshold at 103.7 units, 120–200 CPRG+ mutants were identified in each condition (Table S1), with the majority (∼75%) being condition-specific (Figure 2D). For example, mutants with defects in enterobacterial common antigen (ECA) biogenesis were detected as CPRG+ on LB no NaCl, while mutants defective for outer membrane biogenesis, the Tol-Pal machinery, and colanic acid biosynthesis were scored as positive on LB with 1% NaCl. On the other hand, LPS biosynthesis genes were CPRG+ at low temperature. Surprisingly, mutants involved in homologous recombination were also enriched in LB containing salt. The reason for this is not clear at present. Functional enrichment analysis of Gene Ontology (GO) and KEGG pathways indicated that the terms envelope, membrane, cell-wall, peptidoglycan, and cell surface structure-related were the most statistically significant terms associated with hits from all four datasets (Table S2 and S3). Although many factors involved in outer membrane biogenesis were identified in the screen (Table S1 and S2), it remains unclear whether they were identified due to the loss of the outer membrane barrier function or because mutants defective for these factors lyse at an elevated frequency. In any case, the screen was clearly effective at identifying factors involved in many different aspects of envelope biogenesis/assembly. Importantly, 102 CPRG+ mutants (∼22% of all hits) were in genes coding for proteins of unknown function (Table S4). For more than half of these factors, no significant growth phenotype was observed when the same library was subjected to over 300 different plating conditions [31]. This suggests that the CPRG readout could be a very sensitive and powerful assay to use in larger-scale phenotyping screens for discovering gene function and network architecture in a guilt-by-association manner [31].
Fig. 2. Using the CPRG assay in high-throughput. 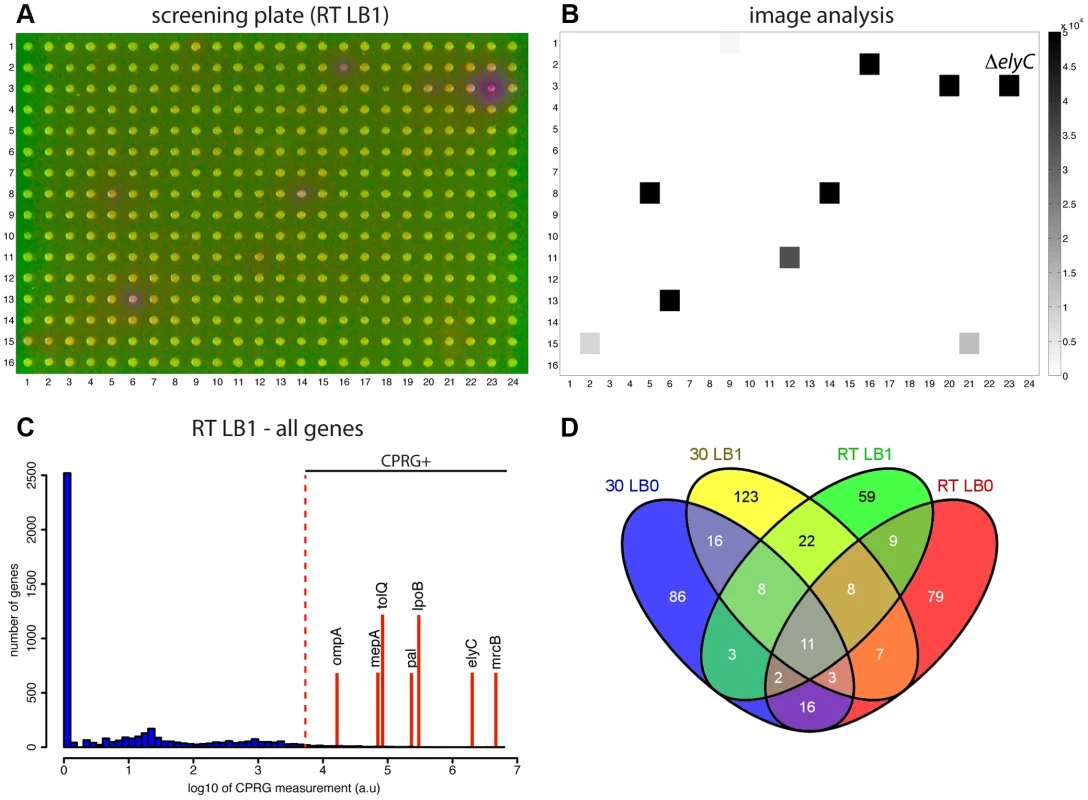
A. Picture of indicator agar (1% NaCl) with pin-transferred cells of the ordered library converted to Lac+ by conjugation. Plate was incubated for 23 hrs at room temperature. B. Output of the image analysis software (Iris) for the plate shown in (A). C. CPRG assay score distribution for the screen carried out at room temperature on agar prepared with 1% NaCl. Positions of genes of interest and/or known importance for envelope integrity are indicated by the red lines. Genes with scores above the cut-off (103.7 units) were designated as CPRG+ hits. D. Venn diagram comparing the hits identified in the different growth conditions. Loss of ElyC function results in cell lysis at low temperature
One of the mutants with the most striking CPRG+ phenotype was a deletion of the gene of unknown function ycbC. Its phenotype was strongest at room temperature, and based on the elevated frequency of lysis observed in cell populations from colonies (not shown), we renamed the gene elyC. The elyC reading frame encodes a protein with two predicted transmembrane domains and a large domain of unknown function (DUF) designated as a DUF218 domain in the Pfam database [19]. Topology predictions indicate that the DUF218 domain of ElyC is likely to be periplasmic (Figure 3A). Interestingly, DUF218 domains are abundant and widely distributed in the bacterial domain [19], but little is known about their biological activity. The CPRG screen indicated a potentially prominent role for ElyC in envelope assembly, so as a proof of principle we initiated a more detailed analysis of its function as well as the function of its paralogues.
Fig. 3. Phenotypes of mutants inactivated for DUF218 factors. 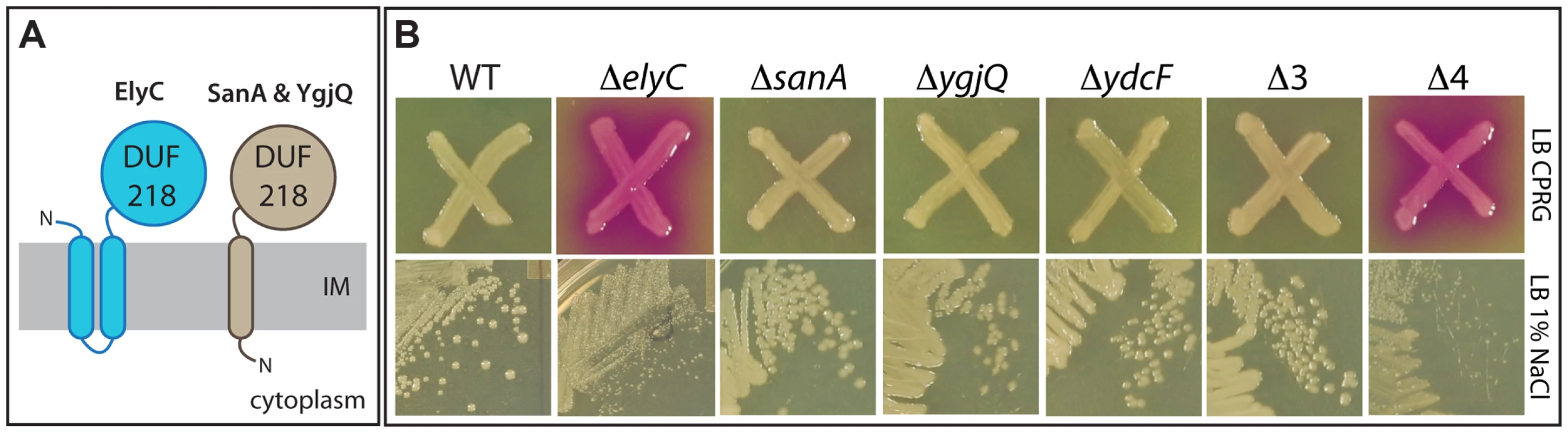
A. Schematic showing the predicted membrane topologies of ElyC and its paralogues SanA and YgjQ. The fourth paralogue, YdcF, is predicted to be cytoplasmic and is not shown. Topology predictions were performed using the TMHMM server (http://www.cbs.dtu.dk/services/TMHMM/). B. CPRG and growth phenotypes of deletion mutants lacking DUF218 factors. Cells of the indicated deletion mutants in an MG1655 strain background were either patched onto CPRG indicator agar (20 µg/ml CPRG and 50 µM IPTG, upper panels) or streaked onto LB agar prepared with 1% NaCl (lower panels). All plates were incubated at room temperature. E. coli encodes four proteins annotated as possessing a DUF218 domain: ElyC, SanA, YgjQ, and YdcF [19]. All of these factors are predicted to be integral membrane proteins with periplasmic domains (Figure 3A) with the exception of YdcF, which is predicted to be cytoplasmic. SanA was originally identified because its overproduction suppressed the vancomycin sensitivity of an uncharacterized E. coli mutant with an envelope permeability defect [33]. The protein was also found to play a role in vancomycin resistance of wild-type cells at high temperature (43°C and above), and the inactivation of its orthologue SfiX in Salmonella typhimurium was found to suppress the cell division defect induced by HisHF overproduction (the HisC pleiotropic response) [33], [34]. These observations suggested a potential yet undefined role for SanA in envelope biogenesis [33], [34]. The crystal structure of the related YdcF protein was reported several years ago, revealing a fold for the DUF218 domain resembling that of the adenine nucleotide alpha hydrolase-like family [35]. It was also reported that YdcF binds S-adenosyl-L-methionine, but the physiological relevance of this observation is not clear [35].
To further investigate the effect of inactivating DUF218 factors, deletion mutations from the Keio collection were transduced to a wild-type (MG1655) strain background and their growth and CPRG phenotypes were assessed. As expected from the screening results, the only single mutation that resulted in a CPRG+ phenotype on indicator agar was ΔelyC (Figure 3B). It was also the only single mutation that caused an observable growth phenotype. Compared to wild-type, the ΔelyC mutant grew normally at 30, 37 and 42°C, but grew poorly and formed very small colonies on LB agar plates incubated at room temperature (Figure 3B). The growth defect at this temperature was most severe on LB agar containing 1% NaCl, and became less pronounced at lower salt concentrations (data not shown). Importantly, the growth and CPRG+ phenotypes of the ΔelyC mutant were corrected by the expression of elyC in trans (see below), indicating that they were indeed the result of ElyC inactivation rather than an effect of the deletion on the expression of nearby genes. None of the other single mutants lacking a DUF218 factor displayed a growth or morphological phenotype on LB agar prepared with 0, 0.5, or 1% NaCl at any incubation temperature tested (room temperature, 30, 37, or 42°C) (data not shown). Moreover, a triple mutant (Δ3) lacking sanA, ygjQ, and ydcF also grew indistinguishably from wild-type under the conditions tested (Figure 3B). We also constructed a quadruple mutant (Δ4) lacking all DUF218 factors. The mutant was viable and displayed growth and CPRG phenotypes that were equivalent to the single elyC deletion (Figure 3B). Thus, inactivating additional DUF218 factors did not exacerbate the ΔelyC growth defect. Moreover, overproduction of other DUF218 factors did not correct the growth phenotypes resulting from ElyC inactivation, suggesting that the functions of the different proteins do not overlap (see below). We conclude that DUF218 factors are not required for viability and that, of the four such proteins produced by E. coli, only ElyC appears to play a significant role in envelope biogenesis as revealed by its low temperature growth and CPRG+ phenotypes.
To investigate the consequence of ElyC inactivation further, we monitored the growth and morphology of a ΔelyC mutant grown in liquid LB medium (1% NaCl) at room temperature. Interestingly, the mutant culture grew as rapidly as wild-type cells until it reached late-exponential phase when culture density abruptly stopped increasing and began a slow decline (Figure 4A). Microscopic analysis revealed that up until the point of growth divergence, the mutant cells had a morphology that was indistinguishable from wild-type cells (data not shown). However, visualization of ΔelyC cells harvested just after the decrease in culture density was observed revealed that the cells lysed via membrane blebs emanating from midcell or the cell quarter positions (Figure 4B and C). This morphological phenotype bears a striking resemblance to cells lysing following treatment with penicillin and other β-lactams [36]–[38], suggesting a potential role for ElyC in PG biogenesis.
Fig. 4. Loss of ElyC function results in lysis. 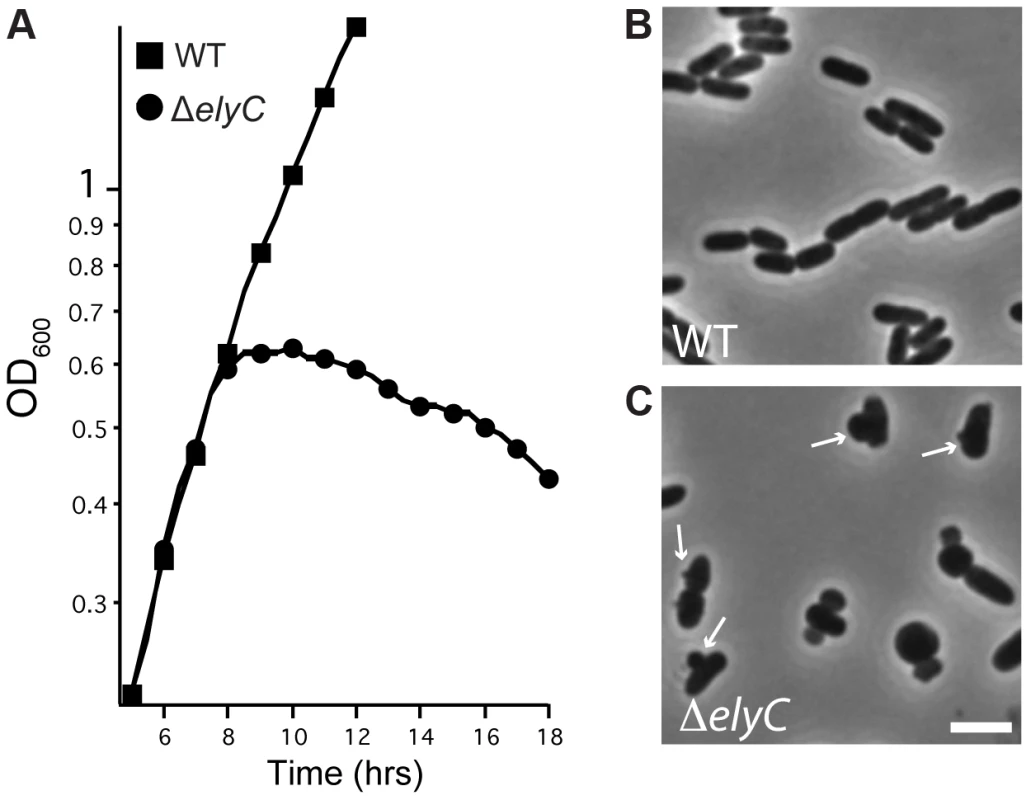
A. Cells of MG1655 [WT] or CB152 [ΔelyC] were grown overnight in LB medium (1% NaCl) at 37°C, diluted 1∶100 and grown at 37°C to an OD600 of approximately 0.4. Cultures were then diluted to an OD600 of 0.04 in LB 1% NaCl and grown at room temperature in a shaking water bath. Cell growth was monitored by following culture OD600. Time is hours after the final inoculation. B–C. At approximately 8 hours post inoculation, cells from the indicated cultures were imaged on 1.2% agarose pads using a Nikon TE2000 microscope with a 100× phase contrast objective. Bar equals 3 microns. ElyC is required for proper cell wall biogenesis
We reasoned that if ElyC is indeed important for PG assembly, its inactivation may be synthetically lethal with deletion mutants lacking PG synthases. Two important PG synthases produced by E. coli are the bifunctional (Class A) PBPs, PBP1a and PBP1b, encoded by the mrcA (ponA) and mrcB (ponB) genes, respectively [39]. These factors possess both peptidoglycan glycosyltransferase (PGT) activity to synthesize the glycan strands of PG and transpeptidase (TP) activity to crosslink the glycan chains via their attached peptide moieties [40]. Cells lacking either of these PBPs are viable, but the simultaneous inactivation of both factors results in rapid lysis and cell death [26], [27], [41], [42]. We were able to construct both ΔelyC ΔmrcA and ΔelyC ΔmrcB double mutants and maintain them at 37°C. However, consistent with a role for ElyC in PG biogenesis at lower temperatures, we found that ΔelyC was synthetically lethal with PBP1b inactivation but not in combination with ΔmrcA when the mutants were grown at room temperature (Figure 5A, data not shown).
Fig. 5. Mutants lacking ElyC have a PG synthesis defect. 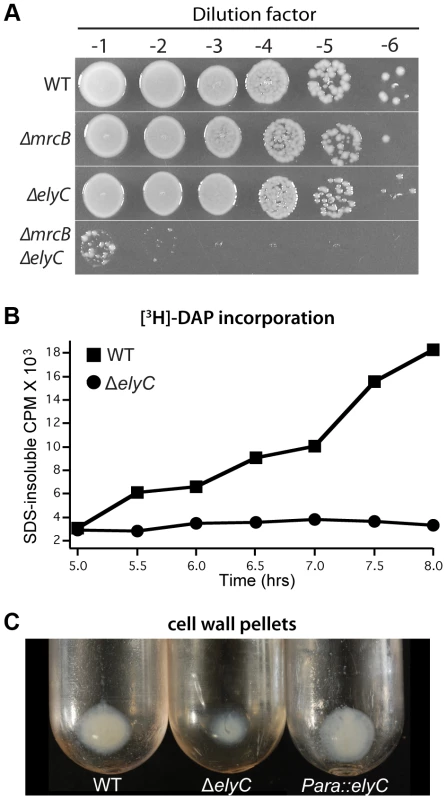
A. Overnight cultures of TB28 [WT], TU122 [ΔmrcB], CB152 [ΔelyC], and CB172 [ΔelyC ΔmrcB] were serially diluted following normalization for culture OD600. Five microliters of each dilution was spotted onto LB agar and plates were incubated for 3 days at room temperature. B. Cultures of CB74 [lysA::Tn10] and CB330 [ΔelyC lysA::Tn10] were grown as in Figure 4A in medium supplemented with [3H]-mDAP. PG synthesis was monitored by the incorporation of radioactivity into SDS-insoluble material. See text for details. C. Cultures (500 ml) of TB28 [WT], CB152 [ΔelyC], and CB152(attλCB118) [ΔelyC (Para::elyC)] were grown at room temperature to an OD600 of 0.5 and cell wall pellets were prepared as described in Methods and Materials. Shown is a picture of the pellets following ultracentrifugation to sediment isolated sacculi. Similar preparations from cultures grown at 37°C showed no observable difference in pellet size (data not shown). Note that the initial pellet following SDS treatment of cells is likely to include material in addition to sacculi. Thus the magnitude of the reduction in pellet size is only a rough measure of the PG synthesis defect in the ΔelyC strain. To directly measure PG synthesis in a mutant lacking ElyC, we metabolically labeled cells with [3H]-meso-diaminopimelic acid (mDAP), an amino acid that is unique to the stem peptide of PG. The PG sacculus is one of the few cellular structures that remains insoluble in a boiling detergent solution (4% SDS). Therefore, upon [3H]-mDAP labeling, PG synthesis can be monitored by withdrawing aliquots of culture at times following label addition, boiling them in 4% SDS, and passing the solution through a 0.22 µm filter [43]. Radioactivity retained on the filter reflects the amount of [3H]-mDAP incorporated into the PG layer. For the labeling experiments, cultures of wild-type and ElyC− cells were subcultured to an OD600 of 0.04 in LB 1% NaCl containing [3H]-mDAP and grown at room temperature. Incorporation of the radiolabel into SDS-insoluble material was then monitored over the time-course shown in Figure 5B. Strikingly, PG synthesis in ElyC− cells appeared to be completely blocked relative to wild-type cells, with the first observable signs of inhibition detected at 5.5 hours of growth at room temperature. Culture growth in this experiment mirrored that shown in Figure 4A such that the growth defect of the ElyC− cells was not apparent until approximately 8 hours post-inoculation, or just over one doubling of the culture following the initial signs of a PG synthesis defect. The observation that growth can continue without PG synthesis for about one mass doubling is consistent with prior results of Prats and de Pedro [44] demonstrating that E. coli cells can grow normally with up to 50% less PG per cell.
Also consistent with a PG synthesis defect in the ElyC− mutant, when PG sacculi were purified from unlabeled room temperature cultures of a ΔelyC strain harvested prior to observable cell lysis, the PG pellet obtained following boiling in 4% SDS was much smaller than the corresponding pellet from wild-type cells (Figure 5C). Pellet size was restored in ΔelyC cells expressing elyC in trans (Figure 5C). We conclude that ElyC− cells have a severe defect in PG biogenesis at low temperatures.
Multicopy suppression of the PG biogenesis defect in ElyC− cells
The PG synthesis pathway takes place in three stages (Figure 6A) [39]. Precursor biogenesis begins in the cytoplasm with the conversion of UDP-N-acetylglucosamine (UDP-GlcNAc) to UDP-N-acetylmuramic acid (UDP-MurNAc) by MurA and MurB. The peptide moiety is then added to UDP-MurNAc by MurC, D, E, and F, ultimately forming UDP-MurNAc-pentapeptide (UDP-MurNAc-pep). In the second, membrane-associated phase, UDP-MurNAc-pep is converted to the precursor lipid-IPG by MraY, which transfers phospho-MurNAc-pep to the lipid carrier undecaprenol-phosphate (Und-P). Und-P is synthesized by the enzyme UppS. Lipid-IIPG is formed by MurG via the addition of GlcNAc to lipid-IPG from UDP-GlcNAc. This final precursor contains the basic monomeric unit of PG, the disaccharide-pentapeptide. Following its production, lipid-IIPG must be flipped to expose the sugar units to the periplasmic space where it can then be polymerized and crosslinked into PG by the PBPs. The identity of the flippase remains controversial [45], [46].
Fig. 6. Suppression of the ElyC− phenotypes. 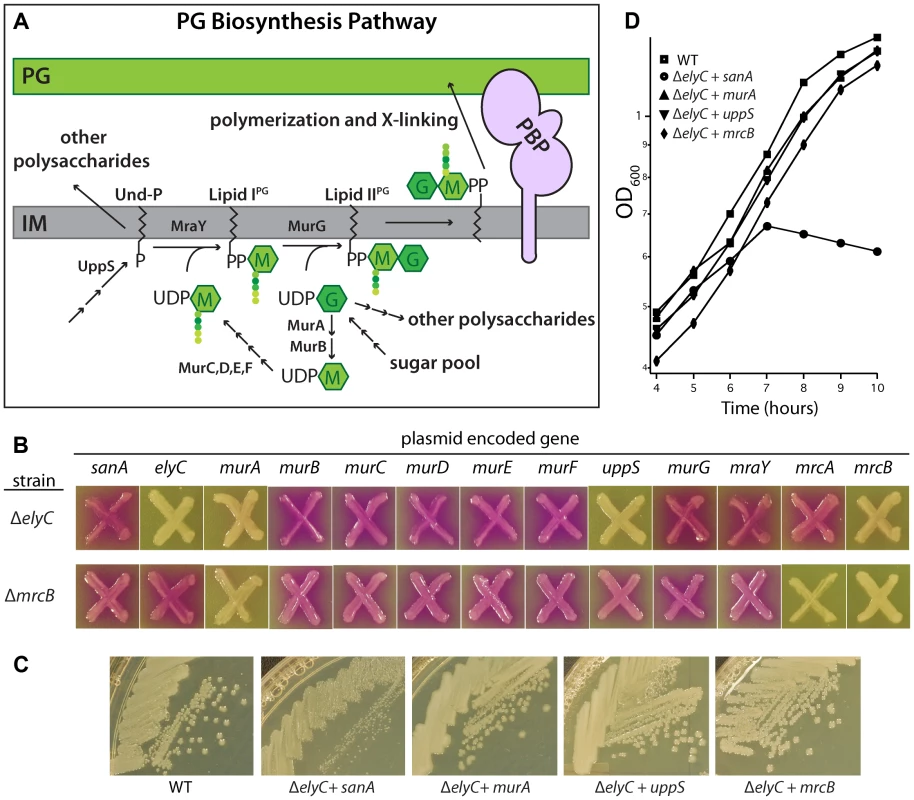
A. Diagram of the PG synthesis pathway. See text for details. M, MurNAc; G, GlcNAc. Colored circles represent amino acids in the PG stem peptide. B. Cells of EM1 [ΔelyC] and CB3 [ΔmrcB] containing multicopy plasmids with the indicated genes were patched onto CPRG indicator agar and grown as described for Figure 3B. Plasmids were selected from an ordered ORF library set [47]. C. A subset of the strains from B was grown on LB (1% NaCl) agar at room temperature and plates were photographed. D. The indicated subset of these strains was also grown in liquid and monitored as described for Figure 4A except that the medium was supplemented with 100 µM IPTG. In B–D, the WT strain did not contain any plasmid. Antibiotics were not included in the plates or in the liquid medium to select for the plasmid. To determine what stage of PG biogenesis might be affected by ElyC inactivation, we took a candidate approach to identify multi-copy suppressors of the ElyC growth and lysis phenotypes. Clones from a multi-copy plasmid ORF library [47] encoding untagged enzymes in the PG synthesis pathway expressed from a ColE1 plasmid under control of the tac promoter were selected and introduced into ΔelyC cells. The CPRG phenotype of the resulting strains was then assessed (Figure 6B). As expected, a plasmid harboring elyC suppressed the CPRG+ phenotype of a ΔelyC mutant. This was not the case for plasmids encoding sanA, the other DUF218 factors, or most of the enzymes involved in PG precursor biogenesis (Figure 6B–C, data not shown). Strikingly, however, overproduction of MurA, UppS, or PBP1b fully suppressed the CPRG+ phenotype of ElyC− cells and restored their growth at room temperature to normal (Figure 6B–D). Interestingly, each of the enzymes with suppression activity functions at a major transition point in PG biogenesis. MurA catalyzes the committed step for PG synthesis [48], UppS is responsible for producing the lipid carrier Und-P, which is likely limiting for the synthesis of lipid-linked precursors [49], and PBP1b performs the final polymerization and crosslinking reactions [40]. Thus, overproduction of these factors may generally increase the flux through the pathway to alleviate the ElyC− defect. To determine the specificity of the observed suppression, we monitored the ability of the plasmid set to correct the CPRG+ phenotype of a mutant lacking the PG synthase PBP1b (Figure 6B). The murA-containing plasmid retained suppressing activity in this background as did the PBP1b-encoding plasmid as expected. Interestingly, overproduction of PBP1a suppressed the CPRG+ phenotype of PBP1b− cells but not that of the ΔelyC mutant (Figure 6B), suggesting that PBP activity is not generally limiting in cells lacking ElyC. On the other hand, UppS overproduction appeared to specifically suppress the loss of ElyC function (Figure 6B–D). We therefore infer that the primary defect in ElyC− cells is likely to be at the level of lipid carrier metabolism.
Genetic interactions between ElyC and the enterobacterial common antigen biogenesis pathway
Further support for a functional role for ElyC in lipid carrier metabolism was uncovered using high-throughput genetic interaction analysis technology (GIANT-coli) [50]. An Hfr donor strain harboring a ΔelyC::CamR allele was crossed with the Keio collection en masse to search for deletion alleles that either suppress the growth defect resulting from the loss of ElyC function at room temperature or exacerbate the ΔelyC phenotype at higher temperatures (30 and 37°C). Interestingly, the analysis identified both positive and negative interactions between ΔelyC and deletions of genes coding for enzymes involved in the biogenesis of enterobacterial common antigen (ECA), a surface polysaccharide produced by all enteric bacteria [51] (Table 1). The ECA polysaccharide is assembled from repeating units of GlcNAc, N-acetyl-D-mannosaminuronic acid (ManNAcA), and 4-acitamido-4,6-dideoxy-D-galactose (Fuc4NAc). It is commonly found as either a cyclic form in the periplasm or linked to phosphatidylglycerol in the outer membrane, but it can also be found attached to lipid A-core in some bacteria [51], [52]. Despite its ubiquity in enterobacteria, the exact function of ECA remains unclear. However, the polymer has been implicated in acid and bile-salt resistance and has been shown to be important for virulence [53]–[55].
Tab. 1. Genetic interactions between elyC and enterobacterial common antigen biosynthesis genes revealed by high-throughput GIANT coli analysis. 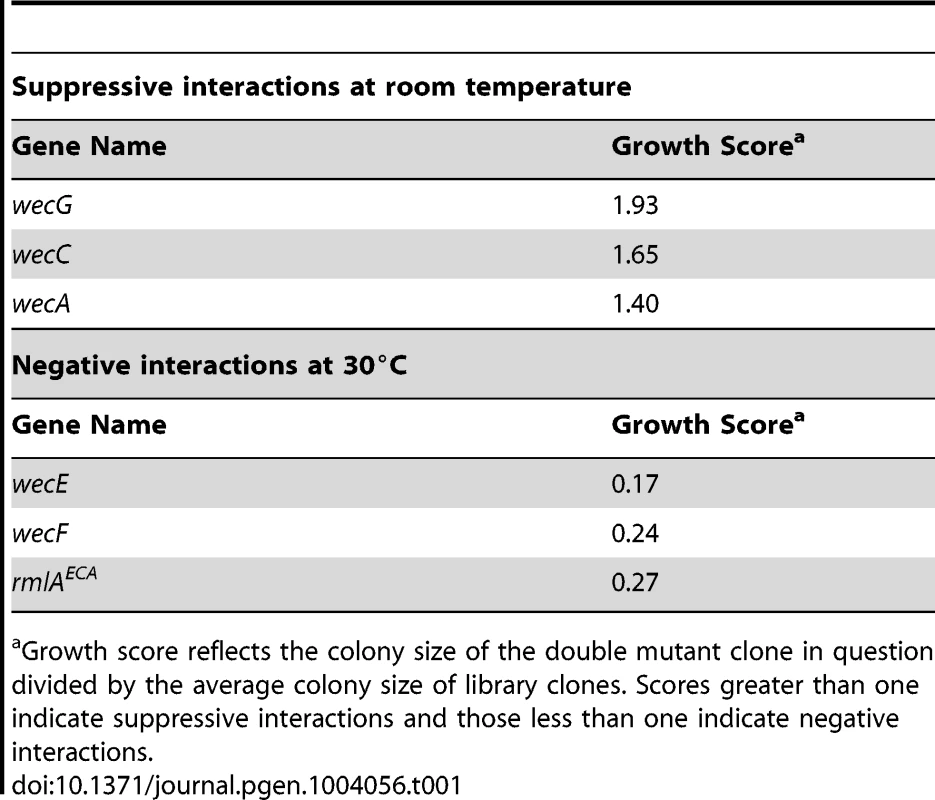
a Growth score reflects the colony size of the double mutant clone in question divided by the average colony size of library clones. Scores greater than one indicate suppressive interactions and those less than one indicate negative interactions. The steps required for the synthesis of the ECA precursor, lipid-IIIECA, have been elucidated (Figure 7A) [56]–[58]. Like PG, its production proceeds via the progressive addition of sugar units to the lipid carrier Und-P. The genetic interaction analysis indicated that blocking lipid-IECA synthesis or its utilization suppressed the growth defect of ΔelyC cells grown at room temperature (Table 1). To confirm this observation, we transduced the ΔwecA::KanR, ΔwecB::KanR, and ΔwecG::KanR alleles from the Keio collection into a ΔelyC strain (MG1655 ΔelyC::frt) and assessed the phenotypes of the resulting transductants. Strikingly, all three Δwec alleles fully suppressed the CPRG+ phenotype and growth defect of ElyC− cells on solid medium, and the ΔwecA::KanR allele was shown to completely rescue the growth defect of ΔelyC cells in liquid medium at room temperature (Figure 7B–C). As opposed to the positive effects of blocks early in the ECA pathway, the genetic interaction analysis suggested that defects in lipid-IIECA utilization adversely impact the growth of ΔelyC mutants. We tested this observation by transducing the ΔrmlAECA::KanR and ΔwecF::KanR alleles from the Keio collection into an ElyC depletion strain CB152(attλCB118) [ΔelyC (Para::elyC)]. The resulting transductants failed to grow at room temperature in the absence of elyC induction with arabinose (Figure 7D), indicating that the simultaneous inactivation of RmlAECA or WecF and ElyC is lethal under these conditions. Interestingly, depletion of ElyC in the ΔrmlAECA or ΔwecF backgrounds also resulted in a plating defect at 37°C (Figure 7D), suggesting that ElyC function is not limited to low temperatures. The negative interaction between ΔelyC and ΔrmlAECA or ΔwecF suggests that the accumulation of the intermediate precursor lipid-IIECA is toxic when ElyC is inactivated. Accordingly, inactivation of WecA suppressed the observed synthetic lethal phenotype displayed by ElyC− RmlAECA− and ElyC− WecF− cells (Figure 7D).
Fig. 7. Genetic interaction between ElyC and the ECA biogenesis pathway. 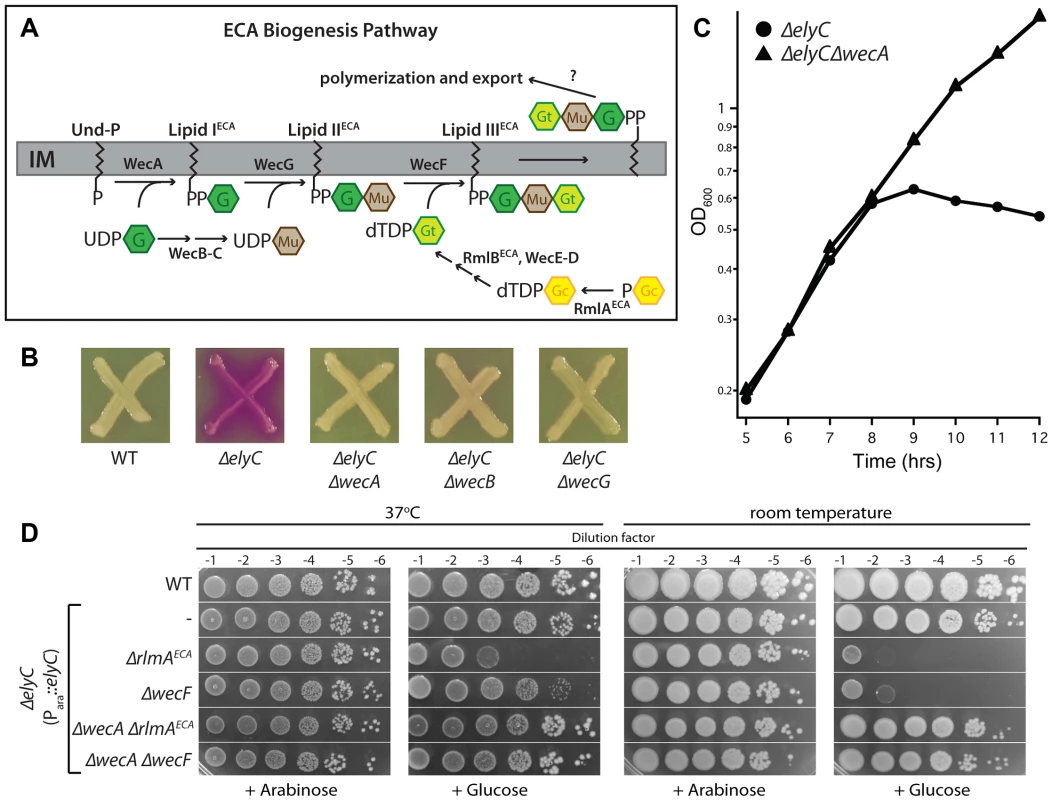
A. Diagram of the ECA precursor synthesis pathway. G, GlcNAc; Mu, ManNAcA; Gt, Fuc4NAc; P-Gc, glucose-1-phosphate. B. Cells of MG1655 [WT], EM9 [ΔelyC], CB329 [ΔelyC ΔwecA], CB337 [ΔelyC ΔwecB], and CB265 [ΔelyC ΔwecG] were patched onto CPRG indicator agar and grown as described for Figure 3B. C. The indicated subset of these strains was also grown in liquid LB 1% NaCl at room temperature and monitored as described for Figure 4A. Cultures of ΔelyC ΔwecB and ΔelyC ΔwecG strains grew as well as the ΔelyC ΔwecA strain (data not shown). D. Overnight cultures of TB28 [WT] or CB152(attλCB118) [ΔelyC (Para::elyC)] and its ΔrlmAECA, ΔwecF, ΔwecA ΔrlmAECA, and ΔwecA ΔwecF derivatives were grown in LB supplemented with 0.2% arabinose and serially diluted following normalization for culture OD600. Five microliters of each dilution was spotted onto LB 1% NaCl agar supplemented with 0.2% arabinose or glucose as indicated and plates were incubated at the indicated temperature. The genetic interactions between elyC and the ECA biosynthesis genes are consistent with the observed suppression of the ElyC− phenotype by the overproduction of the Und-P synthase UppS. Taken together, these findings suggest that in the absence of ElyC, the PG biogenesis pathway is hypersensitive to competition for the lipid carrier from ECA synthesis and potentially the synthesis of other surface polysaccharides that utilize Und-P. In this context, it is the basal level of competition for Und-P due to ECA synthesis that likely results in the baseline growth defect of ElyC− cells. We suspect that this competition is intensified when flux through the ECA pathway is blocked at the stage of lipid-IIECA utilization, resulting in the observed synthetic lethal phenotypes due to the build-up of this precursor and the further depletion of the free Und-P pool. Mutations that lead to a defect in lipid-IECA utilization are likely to be suppressive rather than negative because the WecA reaction is readily reversible. Overall, our genetic analysis of ElyC function is consistent with a model in which it plays an important role in Und-P metabolism, possibly by promoting the efficient utilization of Und-P or Und-P-linked precursors by the PG biogenesis pathway. The example of ElyC thus clearly demonstrates the utility of the high-throughput CPRG screening method in uncovering new factors involved in important aspects of envelope biogenesis.
Discussion
The cell envelope of Gram-negative bacteria is a formidable barrier that is difficult for antimicrobial drugs to penetrate. Thus, the list of treatments effective against these organisms is small and shrinking rapidly with the rise of new resistance mechanisms, especially those possessed by carbapenem-resistant Enterobacteriaceae [59]. To address this problem, input from all levels of the scientific endeavor is required, from the most fundamental to the applied. Key to success in combating resistance will be a detailed mechanistic understanding of the cell envelope assembly process. Great strides have been made towards this goal, particularly in recent years with the identification of the essential machineries required for outer membrane biogenesis [7]. However, many aspects of Gram-negative envelope assembly remain poorly characterized, including the regulatory strategies used to coordinate the construction of the different envelope layers. To shed light on these and other aspects of envelope assembly, we developed the high-throughput screening platform described in this report. A number of factors with previously described roles in envelope assembly were positively identified in our screen, indicating that it works as intended. Moreover, the screen also implicated many genes of currently unknown function as being important for envelope integrity. Further study of these factors is likely to reveal new mechanisms underlying the envelope assembly process, any one of which could serve as a drug target to either block cell growth or render the envelope permeable to approved drugs currently ineffective against Gram-negative organisms.
Our discovery and characterization of ElyC as a new envelope biogenesis factor validates the utility of the CPRG screening approach. ElyC belongs to a broadly conserved family of proteins with the DUF218 domain. Even though a wide range of bacteria encode factors with DUF218 domains [19], the function(s) of these proteins have remained largely mysterious. At higher temperatures, mutants lacking ElyC grow normally. However, when grown at room temperature, ElyC inactivation results in a striking lysis phenotype. Our genetic and physiological studies indicate that cell lysis at low temperatures is due to a severe defect in PG synthesis. Because this phenotype can be overcome by the overproduction of the lipid carrier synthase UppS, we infer that mutants lacking ElyC are impaired in the lipid stages of the pathway. Consistent with this observation, mutations that disrupt the synthesis of the ECA polysaccharide and lead to the accumulation of lipid intermediates in its synthesis were found to be synthetically lethal with an ElyC defect. This phenotype was caused by the accumulation of ECA lipid intermediates and not the loss of ECA production because inactivating the first enzyme in the pathway, WecA, suppressed the synthetic lethality. Surprisingly, we found that a WecA defect also suppressed the baseline CPRG+ phenotype and growth defect of a mutant lacking ElyC alone. This observation suggests that the phenotypes displayed by a ΔelyC mutant are likely to be the result of competition between the PG and ECA synthetic pathways for the lipid carrier Und-P. Competition is likely heightened when the ECA pathway is impaired and its lipid intermediates accumulate [55], thus causing a greater drain on the Und-P pool and the observed synthetic lethal phenotypes.
The connection between ElyC and ECA synthesis provides a likely explanation for the temperature-dependent nature of the phenotypes displayed by a ΔelyC mutant. In the related bacterium Yersinia enterocolitica, it was recently shown that ECA production is higher at 22°C relative to 37°C, and that this correlates with an increase in the expression of the ECA biosynthetic cluster at low temperature [60]. Thus, we suspect that increased ECA synthesis at low temperatures is likely to place additional demands on the lipid carrier pool that reveals the defect in Und-P metabolism resulting from the loss of ElyC function. ECA biogenesis may also increase as cells enter late-exponential phase at room temperature, thus providing a potential explanation for why ΔelyC cells grow normally until this stage of growth. Interestingly, the observed negative genetic interaction between ΔelyC and either ΔrmlAECA or ΔwecF at 37°C suggests that ElyC function is not restricted to low temperatures, but rather its function just becomes more important as the temperature drops.
Although the technical challenges of working with low abundance phospholipids has thus far prevented us from pinpointing the exact function of ElyC, our genetic analysis suggests a potential role for ElyC in lipid-IIPG metabolism. Overproduction of MurA and UppS are both likely to suppress the ElyC defect by enhancing lipid-IIPG synthesis. The former likely does so by increasing the flux through the PG synthesis pathway and the latter by increasing the pool size of Und-P available for the lipid-precursor generating enzymes. We therefore infer that cells defective for ElyC may either have reduced lipid-IIPG levels and/or may not efficiently utilize the lipid-IIPG that is produced. According to this view, overproduction of PBP1b is probably able to suppress the ElyC− defect by providing more synthases to overcome a potentially lower effective lipid-IIPG concentration. If this interpretation is correct, the PG synthesis defect of ElyC− cells may be most apparent when the synthesis of other polysaccharides is induced because ElyC functions to preferentially “funnel” lipid carrier or precursors through the PG pathway. One attractive possibility is that ElyC is part of a mechanism that enhances the affinity of MraY for Und-P so that PG synthesis is the dominant pathway for lipid carrier utilization. Alternatively, ElyC may help the PBPs properly select lipid-IIPG over the many other lipid-linked precursors likely to be present in the cell membrane at times when other extracellular polysaccharides are being produced at high levels. Other functions for ElyC in lipid carrier metabolism are also consistent with the data, including a role in Und-P production or its recycling, and indirect effects are difficult to exclude at this stage. Nevertheless, given the central location of Und-P in the synthetic pathways of all manner of extracellular polysaccharides, any of the aforementioned roles for ElyC has important implications for our understanding of envelope biogenesis. Further study of its function and that of other factors identified in our screen is therefore likely to reveal new and interesting ways to disrupt the envelope assembly process for therapeutic purposes.
An exciting aspect of the CPRG assay is the potential for expanding its use in larger scale chemical genomics analyses. Using only four conditions in the current study, we were able to identify phenotypes for ∼80 mutants of genes of unknown function that were previously unresponsive when using colony size alone as a proxy of fitness in >300 conditions [31]. Although there are aspects of the assay that still need to be optimized before it can be expanded to a large number of growth conditions (color diffusion and timing of color development), its use as a highly sensitive readout for chemical genomics studies holds great promise for uncovering gene function and pathway organization based on the similarity of the response profiles for different mutants. Furthermore, because of its simplicity and use of the widely employed LacZ reporter, the CPRG assay described here should be readily adaptable to other organisms and enable similar high-throughput screens to discover envelope biogenesis factors in bacteria with distinct surface architectures.
Materials and Methods
Media, bacterial strains, and plasmids
Cells were grown in LB [1% tryptone, 0.5% yeast extract, and 0.5% NaCl], unless the salt concentration is specified otherwise (0 or 1% NaCl). Antibiotics were used at 10 (chloramphenicol; Cm), 15 (ampicillin; Amp) or 20 (kanamycin; Kan) µg/ml. Bacterial strains used are listed in Table S5. All E. coli strains used in the reported experiments are derivatives of MG1655. Plasmids used in this study are listed in Table S6. Plasmids from the multicopy ORF library [47] used throughout this study encode untagged proteins expressed from a ColE1-derived vector under control of the tac promoter. See Text S1 for plasmid construction details.
Screening of transposon library for envelope defective mutants
Wild-type MG1655 cells were mutagenized with the EzTn-Kan transposome from Epicentre as described previously [28]. Cells from the resulting library were plated on LB (0.5% NaCl) agar supplemented with CPRG (20 µg/ml) and IPTG (50 µM) to achieve a density of about 100 colonies per plate. After approximately 15–28 hours of growth at 30°C, colonies displaying a red or strong pink CPRG+ phenotype were purified on LB agar prepared with 0, 0.5, and 1% salt and grown at 30°C and 42°C overnight. Cells from colonies of mutants grown under each condition were visualized by phase contrast microscopy. The location of the transposon insertions in about 100 CPRG+ mutants was determined using arbitrarily primed PCR and DNA sequencing as described previously [28].
Screening of the ordered library for CPRG+ mutants
A copy of the previously described ordered E. coli mutant library [31] stored in 96-well format at −80°C was thawed, pinned onto LB-Kan agar plates, and grown overnight at 37°C. The following day the library was condensed to 384-pin format on LB-Kan agar plates using a Singer Rotor robot. After growth overnight, the library was transferred to LB agar plates spread with 100 µl of an overnight culture of JA200/pCB112 [donor strain/Plac::lacZ, CamR]. The resulting mating plates were incubated overnight at 37°C, positions corresponding to the ordered library were transferred to LB-Kan-Cam plates, and the plates were incubated again at 37°C overnight. Finally, the Lac+ exconjugants of the ordered library were transferred to LB agar supplemented with CPRG (20 µg/ml), IPTG (100 µM), and various NaCl concentrations (0, and 1%), and incubated either at room temperature or at 30°C. Plates were monitored through time and were imaged both at the end of vegetative growth (∼12 or 23 hours for the 30°C or room temperature grown cells, respectively), and after their growth plateaued, 7 hours later. Images were analyzed using in-house software (Iris) that segments the image and measures the hue of each pixel in the HSV color space. The score for each mutant is an average of the scores of the 2 clones present in the library for each mutant. CPRG scores for the early time-points were used for the subsequent analysis. Thresholds were set as described in the text and were manually evaluated to minimize false negatives and false positives.
Assessment of phenotypes and multicopy suppression analysis
To monitor the growth of ΔelyC cells in liquid medium, overnight cultures of TB28 [WT] and CB152 [ΔelyC] strains were grown in LB at 37°C. These cultures were diluted 1∶100 in LB 1% NaCl and grown at 37°C to an OD600 of approximately 0.4. Cultures were then diluted to an OD600 of 0.04 in LB and grown at room temperature in a shaking water bath. Measurements of the culture OD600 were then taken every 30–60 minutes.
For viability measurements, overnight cultures were adjusted to an OD600 of 2, serial dilutions from 10−1 to 10−6 were prepared in LB, and 5 µL of each dilution were spotted onto solid medium. Plates were incubated overnight at 37°C or 3 days at room temperature and photographed. To assess the CPRG phenotypes of various strains, single colonies from a freshly streaked LB agar plate were patched in the shape of an X onto LB medium containing 20 µg/ml of CPRG and 50 µM of IPTG. Plates were incubated overnight at room temperature and photographed. For strains containing multicopy plasmids, the CPRG phenotypes were assessed identically except that the concentration of IPTG was increased to 100 µM.
Measurement of PG biogenesis and sacculi preparation
Overnight cultures of CB74 [lysA::Tn10] and CB330 [ΔelyC lysA::Tn10] were grown in LB medium supplemented with 100 µg/ml lysine. A lysA::Tn10 strain background was used to prevent the incorporation of added mDAP into proteins. The overnight cultures were diluted 1∶100 and grown in LB to an OD600 of about 0.4 as above. The cultures were then diluted to an OD600 of 0.04 into LB 1% NaCl supplemented with lysine, methionine and threonine at 100 µg/ml and [3H]-mDAP (American Radiolabeled Chemicals) at 10 µCi/ml. Cultures were grown at room temperature in a shaking water bath and at the time points indicated in Figure 5B, 0.2 ml of culture was withdrawn and added to 0.8 ml of hot (95°C) 5% SDS solution. Samples were incubated at 95°C for an additional 30 minutes, cooled to room temperature, and SDS-insoluble PG material was recovered by filtration essentially as described [61]. Filters were dried and radioactivity detected by scintillation counting using a Microbeta Trilux 1450 LSC from Perkin-Elmer and Ecolite (MP biomedicals) scintillation fluid.
Sacculi pellets were prepared for TB28 [WT], CB152 [ΔelyC], and CB152(attλCB118) [ΔelyC (Para::elyC)]. Overnight cultures were diluted and grown to exponential phase as described above. They were then diluted into 500 ml of LB 1% NaCl and grown at 37°C or room temperature to an OD600 of 0.5. Cultures of CB152(attλCB118) additionally contained 0.2% arabinose. Cells were harvested by centrifugation at 4000× g for 10 minutes at 4°C and the pellet was resuspended in 10 ml of ice-cold phosphate buffered saline. The cell suspension was added drop-wise to 40 ml of boiling SDS 5% solution. The mixtures were boiled for 30 minutes with stirring, and left to cool at room temperature overnight. Sacculi were sedimented by ultracentrifugation at 100,000 g for 1 h at 25°C and the resulting cell pellets were photographed.
High-throughput genetic interaction analysis
The ordered library described above was further condensed to 1536-pin format and transferred to plates spread with 50 µl of a culture of CB157 [Hfr donor, ΔelyC::cat] at an OD600 of 1. The mating plates were incubated overnight and after an intermediate selection against the donor parent, double mutants were selected in LB plates containing both antibiotics [50]. Interactions were scored by directly comparing the growth of the double mutants to that of the KEIO single mutants (Table 1).
Supporting Information
Zdroje
1. HendersonB, NairS, PallasJ, WilliamsMA (2010) Fibronectin: a multidomain host adhesin targeted by bacterial fibronectin-binding proteins. FEMS Microbiol Rev 35 : 147–200 doi:10.1111/j.1574-6976.2010.00243.x
2. ThanassiDG, BliskaJB, ChristiePJ (2012) Surface organelles assembled by secretion systems of Gram-negative bacteria: diversity in structure and function. FEMS Microbiol Rev 36 : 1046–1082 doi:10.1111/j.1574-6976.2012.00342.x
3. AbdallahAM, Gey van PittiusNC, ChampionPAD, CoxJ, LuirinkJ, et al. (2007) Type VII secretion–mycobacteria show the way. Nat Rev Microbiol 5 : 883–891 doi:10.1038/nrmicro1773
4. KinnebrewMA, PamerEG (2011) Innate immune signaling in defense against intestinal microbes. Immunol Rev 245 : 113–131 doi:10.1111/j.1600-065X.2011.01081.x
5. BreidensteinEBM, la Fuente-Núñez deC, HancockREW (2011) Pseudomonas aeruginosa: all roads lead to resistance. Trends in Microbiology 19 : 419–426 doi:10.1016/j.tim.2011.04.005
6. SutcliffeIC (2010) A phylum level perspective on bacterial cell envelope architecture. Trends in Microbiology 18 : 464–470 doi:10.1016/j.tim.2010.06.005
7. SilhavyTJ, KahneD, WalkerS (2010) The bacterial cell envelope. Cold Spring Harb Perspect Biol 2: a000414 doi:10.1101/cshperspect.a000414
8. HettEC, RubinEJ (2008) Bacterial growth and cell division: a mycobacterial perspective. Microbiol Mol Biol Rev 72 : 126–156 tableofcontents. doi:10.1128/MMBR.00028-07
9. DelcourAH (2009) Outer membrane permeability and antibiotic resistance. Biochim Biophys Acta 1794 : 808–816 doi:10.1016/j.bbapap.2008.11.005
10. NordmannP, PoirelL, WalshTR, LivermoreDM (2011) The emerging NDM carbapenemases. Trends in Microbiology 19 : 588–595 doi:10.1016/j.tim.2011.09.005
11. RuizN, KahneD, SilhavyTJ (2006) Advances in understanding bacterial outer-membrane biogenesis. Nat Rev Microbiol 4 : 57–66 doi:10.1038/nrmicro1322
12. HuP, JangaSC, BabuM, Díaz-MejíaJJ, ButlandG, et al. (2009) Global Functional Atlas of Escherichia coli Encompassing Previously Uncharacterized Proteins. PLoS Biol 7: e1000096 doi:10.1371/journal.pbio.1000096
13. LopesJ, GottfriedS, RothfieldL (1972) Leakage of periplasmic enzymes by mutants of Escherichia coli and Salmonella typhimurium: isolation of “periplasmic leaky” mutants. J Bacteriol 109 : 520–525.
14. LazzaroniJ-C, PortalierRC, ATLANDL (1979) Isolation and preliminary characterization of periplasmic-leaky mutants of Escherichia coli K-12. FEMS Microbiol Lett 5 : 411–416 doi:10.1111/j.1574-6968.1979.tb03370.x
15. FungJ, MacAlisterTJ, RothfieldLI (1978) Role of murein lipoprotein in morphogenesis of the bacterial division septum: phenotypic similarity of lkyD and lpo mutants. J Bacteriol 133 : 1467–1471.
16. WeigandRA, RothfieldLI (1976) Genetic and physiological classification of periplasmic-leaky mutants of Salmonella typhimurium. J Bacteriol 125 : 340–345.
17. WeigandRA, VinciKD, RothfieldLI (1976) Morphogenesis of the bacterial division septum: a new class of septation-defective mutants. Proc Natl Acad Sci USA 73 : 1882–1886.
18. LazzaroniJC, PortalierRC (1981) Genetic and biochemical characterization of periplasmic-leaky mutants of Escherichia coli K-12. J Bacteriol 145 : 1351–1358.
19. FinnRD, TateJ, MistryJ, CoggillPC, SammutSJ, et al. (2008) The Pfam protein families database. Nucleic Acids Res 36: D281–D288 doi:10.1093/nar/gkm960
20. Miller J (1972) Experiments in Molecular Genetics. New York: Cold Spring Harbor Laboratory.
21. EusticeDC, FeldmanPA, Colberg-PoleyAM, BuckeryRM, NeubauerRH (1991) A sensitive method for the detection of beta-galactosidase in transfected mammalian cells. BioTechniques 11 : 739–40–742–3.
22. GerdingMA, OgataY, PecoraND, NikiH, de BoerPAJ (2007) The trans-envelope Tol-Pal complex is part of the cell division machinery and required for proper outer-membrane invagination during cell constriction in E. coli. Molecular Microbiology 63 : 1008–1025 doi:10.1111/j.1365-2958.2006.05571.x
23. LloubèsR, CascalesE, WalburgerA, BouveretE, LazdunskiC, et al. (2001) The Tol-Pal proteins of the Escherichia coli cell envelope: an energized system required for outer membrane integrity? Res Microbiol 152 : 523–529.
24. IzeB, StanleyNR, BuchananG, PalmerT (2003) Role of the Escherichia coli Tat pathway in outer membrane integrity. Molecular Microbiology 48 : 1183–1193.
25. BernhardtTG, de BoerPAJ (2003) The Escherichia coli amidase AmiC is a periplasmic septal ring component exported via the twin-arginine transport pathway. Molecular Microbiology 48 : 1171–1182.
26. TypasA, BanzhafM, van den Berg van SaparoeaB, VerheulJ, BiboyJ, et al. (2010) Regulation of peptidoglycan synthesis by outer-membrane proteins. Cell 143 : 1097–1109 doi:10.1016/j.cell.2010.11.038
27. Paradis-BleauC, MarkovskiM, UeharaT, LupoliTJ, WalkerS, et al. (2010) Lipoprotein cofactors located in the outer membrane activate bacterial cell wall polymerases. Cell 143 : 1110–1120 doi:10.1016/j.cell.2010.11.037
28. BernhardtTG, de BoerPAJ (2004) Screening for synthetic lethal mutants in Escherichia coli and identification of EnvC (YibP) as a periplasmic septal ring factor with murein hydrolase activity. Molecular Microbiology 52 : 1255–1269 doi:10.1111/j.1365-2958.2004.04063.x
29. HaraH, NaritaS, KaribianD, ParkJT, YamamotoY, et al. (2002) Identification and characterization of the Escherichia coli envC gene encoding a periplasmic coiled-coil protein with putative peptidase activity. FEMS Microbiol Lett 212 : 229–236.
30. RodolakisA, ThomasP, StarkaJ (1973) Morphological mutants of Escherichia coli. Isolation and ultrastructure of a chain-forming envC mutant. J Gen Microbiol 75 : 409–416.
31. NicholsRJ, SenS, ChooYJ, BeltraoP, ZietekM, et al. (2011) Phenotypic Landscape of a Bacterial Cell. Cell 144 : 143–156 doi:10.1016/j.cell.2010.11.052
32. BabaT, AraT, HasegawaM, TakaiY, OkumuraY, et al. (2006) Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol 2 : 2006.0008 doi:10.1038/msb4100050
33. RidaS, CailletJ, AlixJH (1996) Amplification of a novel gene, sanA, abolishes a vancomycin-sensitive defect in Escherichia coli. J Bacteriol 178 : 94–102.
34. MouslimC, CanoDA, CasadesúsJ (1998) The sfiX, rfe and metN genes of Salmonella typhimurium and their involvement in the His(c) pleiotropic response. Mol Gen Genet 259 : 46–53.
35. ChaoKL, LimK, LehmannC, DoseevaV, HowardAJ, et al. (2008) The Escherichia coli YdcF binds S-adenosyl-L-methionine and adopts an alpha/beta-fold characteristic of nucleotide-utilizing enzymes. Proteins 72 : 506–509 doi:10.1002/prot.22046
36. LederbergJ (1956) Bacterial Protoplasts Induced by Penicillin. Proc Natl Acad Sci USA 42 : 574–577.
37. DonachieWD, BeggKJ (1970) Growth of the bacterial cell. Nature 227 : 1220–1224.
38. SchwarzU, AsmusA, FrankH (1969) Autolytic enzymes and cell division of Escherichia coli. J Mol Biol 41 : 419–429.
39. TypasA, BanzhafM, GrossCA, VollmerW (2012) From the regulation of peptidoglycan synthesis to bacterial growth and morphology. Nat Rev Microbiol 10 : 123–136 doi:10.1038/nrmicro2677
40. SauvageE, KerffF, TerrakM, AyalaJA, CharlierP (2008) The penicillin-binding proteins: structure and role in peptidoglycan biosynthesis. FEMS Microbiol Rev 32 : 234–258 doi:10.1111/j.1574-6976.2008.00105.x
41. YousifSY, Broome-SmithJK, SprattBG (1985) Lysis of Escherichia coli by beta-lactam antibiotics: deletion analysis of the role of penicillin-binding proteins 1A and 1B. J Gen Microbiol 131 : 2839–2845.
42. KatoJ, SuzukiH, HirotaY (1985) Dispensability of either penicillin-binding protein-1a or -1b involved in the essential process for cell elongation in Escherichia coli. Mol Gen Genet 200 : 272–277.
43. WientjesFB, PasE, TaschnerPE, WoldringhCL (1985) Kinetics of uptake and incorporation of meso-diaminopimelic acid in different Escherichia coli strains. J Bacteriol 164 : 331–337.
44. PratsR, de PedroMA (1989) Normal growth and division of Escherichia coli with a reduced amount of murein. J Bacteriol 171 : 3740–3745.
45. RuizN (2008) Bioinformatics identification of MurJ (MviN) as the peptidoglycan lipid II flippase in Escherichia coli. Proc Natl Acad Sci USA 105 : 15553–15557 doi:10.1073/pnas.0808352105
46. MohammadiT, van DamV, SijbrandiR, VernetT, ZapunA, et al. (2011) Identification of FtsW as a transporter of lipid-linked cell wall precursors across the membrane. EMBO J 30 : 1425–1432 doi:10.1038/emboj.2011.61
47. SakaK, TadenumaM, NakadeS, TanakaN, SugawaraH, et al. (2005) A complete set of Escherichia coli open reading frames in mobile plasmids facilitating genetic studies. DNA Res 12 : 63–68.
48. BrownED, VivasEI, WalshCT, KolterR (1995) MurA (MurZ), the enzyme that catalyzes the first committed step in peptidoglycan biosynthesis, is essential in Escherichia coli. J Bacteriol 177 : 4194–4197.
49. BarreteauH, MagnetS, GhachiME, TouzéT, ArthurM, et al. (2009) Quantitative high-performance liquid chromatography analysis of the pool levels of undecaprenyl phosphate and its derivatives in bacterial membranes. Journal of Chromatography B 877 : 213–220 doi:10.1016/j.jchromb.2008.12.010
50. TypasA, NicholsR, SiegeleD, ShalesM, CollinsS, et al. (2008) High-throughput, quantitative analyses of genetic interactions in E. coli. Nat Methods 5 : 787 doi:10.1038/nmeth.1240
51. Rick PD, RP S (1996) Enterobacterial common antigen and capsular polysaccharides. In: FC N, Curtis R III, Ingraham JL, Lin E, Low KB, et al.., editors. Escherichia coli and Salmonella: cellular amd molecular biology. Washington DC: ASM Press. pp. 104–122.
52. ErbelPJA, BarrK, GaoN, GerwigGJ, RickPD, et al. (2003) Identification and biosynthesis of cyclic enterobacterial common antigen in Escherichia coli. J Bacteriol 185 : 1995–2004.
53. Ramos-MoralesF, PrietoAI, BeuzónCR, HoldenDW, CasadesúsJ (2003) Role for Salmonella enterica enterobacterial common antigen in bile resistance and virulence. J Bacteriol 185 : 5328–5332.
54. BaruaS, YamashinoT, HasegawaT, YokoyamaK, ToriiK, et al. (2002) Involvement of surface polysaccharides in the organic acid resistance of Shiga Toxin-producing Escherichia coli O157:H7. Molecular Microbiology 43 : 629–640.
55. DanesePN, OliverGR, BarrK, BowmanGD, RickPD, et al. (1998) Accumulation of the enterobacterial common antigen lipid II biosynthetic intermediate stimulates degP transcription in Escherichia coli. J Bacteriol 180 : 5875–5884.
56. BarrK, Nunes-EdwardsP, RickPD (1989) In vitro synthesis of a lipid-linked trisaccharide involved in synthesis of enterobacterial common antigen. J Bacteriol 171 : 1326–1332.
57. BarrK, RickPD (1987) Biosynthesis of enterobacterial common antigen in Escherichia coli. In vitro synthesis of lipid-linked intermediates. J Biol Chem 262 : 7142–7150.
58. RickPD, MayerH, NeumeyerBA, WolskiS, Bitter-SuermannD (1985) Biosynthesis of enterobacterial common antigen. J Bacteriol 162 : 494–503.
59. McKennaM (2013) Antibiotic resistance: The last resort. Nature 499 : 394–396 doi:10.1038/499394a
60. MuszynskiA, RabsztynK, KnapskaK, DudaKA, Duda-GrychtolKT, et al. (2013) Enterobacterial common antigen and O-specific polysaccharide coexist in the lipopolysaccharide of Yersinia enterocolitica serotype O:3. Microbiology 159 : 1782–1793 doi:10.1099/mic.0.066662-0
61. BernhardtTG, StruckDK, YoungR (2001) The lysis protein E of phi X174 is a specific inhibitor of the MraY-catalyzed step in peptidoglycan synthesis. J Biol Chem 276 : 6093–6097 doi:10.1074/jbc.M007638200
Štítky
Genetika Reprodukční medicína
Článek Unwrapping BacteriaČlánek A Chaperone-Assisted Degradation Pathway Targets Kinetochore Proteins to Ensure Genome StabilityČlánek The Candidate Splicing Factor Sfswap Regulates Growth and Patterning of Inner Ear Sensory OrgansČlánek The SPF27 Homologue Num1 Connects Splicing and Kinesin 1-Dependent Cytoplasmic Trafficking inČlánek Down-Regulation of eIF4GII by miR-520c-3p Represses Diffuse Large B Cell Lymphoma DevelopmentČlánek Meta-Analysis Identifies Gene-by-Environment Interactions as Demonstrated in a Study of 4,965 MiceČlánek High Risk Population Isolate Reveals Low Frequency Variants Predisposing to Intracranial Aneurysms
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2014 Číslo 1
-
Všechny články tohoto čísla
- How Much Is That in Dog Years? The Advent of Canine Population Genomics
- The Sense and Sensibility of Strand Exchange in Recombination Homeostasis
- Unwrapping Bacteria
- DNA Methylation Changes Separate Allergic Patients from Healthy Controls and May Reflect Altered CD4 T-Cell Population Structure
- Evidence for Mito-Nuclear and Sex-Linked Reproductive Barriers between the Hybrid Italian Sparrow and Its Parent Species
- Translation Enhancing ACA Motifs and Their Silencing by a Bacterial Small Regulatory RNA
- Relationship Estimation from Whole-Genome Sequence Data
- Genetic Models of Apoptosis-Induced Proliferation Decipher Activation of JNK and Identify a Requirement of EGFR Signaling for Tissue Regenerative Responses in
- ComEA Is Essential for the Transfer of External DNA into the Periplasm in Naturally Transformable Cells
- Loss and Recovery of Genetic Diversity in Adapting Populations of HIV
- Bioelectric Signaling Regulates Size in Zebrafish Fins
- Defining NELF-E RNA Binding in HIV-1 and Promoter-Proximal Pause Regions
- Loss of Histone H3 Methylation at Lysine 4 Triggers Apoptosis in
- Cell-Cycle Dependent Expression of a Translocation-Mediated Fusion Oncogene Mediates Checkpoint Adaptation in Rhabdomyosarcoma
- How a Retrotransposon Exploits the Plant's Heat Stress Response for Its Activation
- A Nonsense Mutation in Encoding a Nondescript Transmembrane Protein Causes Idiopathic Male Subfertility in Cattle
- Deletion of a Conserved -Element in the Locus Highlights the Role of Acute Histone Acetylation in Modulating Inducible Gene Transcription
- Developmental Link between Sex and Nutrition; Regulates Sex-Specific Mandible Growth via Juvenile Hormone Signaling in Stag Beetles
- PP2A/B55 and Fcp1 Regulate Greatwall and Ensa Dephosphorylation during Mitotic Exit
- Differential Effects of Collagen Prolyl 3-Hydroxylation on Skeletal Tissues
- Comprehensive Functional Annotation of 77 Prostate Cancer Risk Loci
- Evolution of Chloroplast Transcript Processing in and Its Chromerid Algal Relatives
- A Chaperone-Assisted Degradation Pathway Targets Kinetochore Proteins to Ensure Genome Stability
- New MicroRNAs in —Birth, Death and Cycles of Adaptive Evolution
- A Genome-Wide Screen for Bacterial Envelope Biogenesis Mutants Identifies a Novel Factor Involved in Cell Wall Precursor Metabolism
- FGFR1-Frs2/3 Signalling Maintains Sensory Progenitors during Inner Ear Hair Cell Formation
- Regulation of Synaptic /Neuroligin Abundance by the /Nrf Stress Response Pathway Protects against Oxidative Stress
- Intrasubtype Reassortments Cause Adaptive Amino Acid Replacements in H3N2 Influenza Genes
- Molecular Specificity, Convergence and Constraint Shape Adaptive Evolution in Nutrient-Poor Environments
- WNT7B Promotes Bone Formation in part through mTORC1
- Natural Selection Reduced Diversity on Human Y Chromosomes
- In-Vivo Quantitative Proteomics Reveals a Key Contribution of Post-Transcriptional Mechanisms to the Circadian Regulation of Liver Metabolism
- The Candidate Splicing Factor Sfswap Regulates Growth and Patterning of Inner Ear Sensory Organs
- The Acid Phosphatase-Encoding Gene Contributes to Soybean Tolerance to Low-Phosphorus Stress
- p53 and TAp63 Promote Keratinocyte Proliferation and Differentiation in Breeding Tubercles of the Zebrafish
- Affects Plant Architecture by Regulating Local Auxin Biosynthesis
- The SET Domain Proteins SUVH2 and SUVH9 Are Required for Pol V Occupancy at RNA-Directed DNA Methylation Loci
- Down-Regulation of Rad51 Activity during Meiosis in Yeast Prevents Competition with Dmc1 for Repair of Double-Strand Breaks
- Multi-tissue Analysis of Co-expression Networks by Higher-Order Generalized Singular Value Decomposition Identifies Functionally Coherent Transcriptional Modules
- A Neurotoxic Glycerophosphocholine Impacts PtdIns-4, 5-Bisphosphate and TORC2 Signaling by Altering Ceramide Biosynthesis in Yeast
- Subtle Changes in Motif Positioning Cause Tissue-Specific Effects on Robustness of an Enhancer's Activity
- C/EBPα Is Required for Long-Term Self-Renewal and Lineage Priming of Hematopoietic Stem Cells and for the Maintenance of Epigenetic Configurations in Multipotent Progenitors
- The SPF27 Homologue Num1 Connects Splicing and Kinesin 1-Dependent Cytoplasmic Trafficking in
- Down-Regulation of eIF4GII by miR-520c-3p Represses Diffuse Large B Cell Lymphoma Development
- Genome Sequencing Highlights the Dynamic Early History of Dogs
- Re-sequencing Expands Our Understanding of the Phenotypic Impact of Variants at GWAS Loci
- Meta-Analysis Identifies Gene-by-Environment Interactions as Demonstrated in a Study of 4,965 Mice
- , a -Antisense Gene of , Encodes a Evolved Protein That Inhibits GSK3β Resulting in the Stabilization of MYCN in Human Neuroblastomas
- A Transcription Factor Is Wound-Induced at the Planarian Midline and Required for Anterior Pole Regeneration
- A Comprehensive tRNA Deletion Library Unravels the Genetic Architecture of the tRNA Pool
- A PNPase Dependent CRISPR System in
- Genomic Confirmation of Hybridisation and Recent Inbreeding in a Vector-Isolated Population
- Zinc Finger Transcription Factors Displaced SREBP Proteins as the Major Sterol Regulators during Saccharomycotina Evolution
- GATA6 Is a Crucial Regulator of Shh in the Limb Bud
- Tissue Specific Roles for the Ribosome Biogenesis Factor Wdr43 in Zebrafish Development
- A Cell Cycle and Nutritional Checkpoint Controlling Bacterial Surface Adhesion
- High Risk Population Isolate Reveals Low Frequency Variants Predisposing to Intracranial Aneurysms
- E3 Ubiquitin Ligase CHIP and NBR1-Mediated Selective Autophagy Protect Additively against Proteotoxicity in Plant Stress Responses
- Evolutionary Rate Covariation Identifies New Members of a Protein Network Required for Female Post-Mating Responses
- 3′ Untranslated Regions Mediate Transcriptional Interference between Convergent Genes Both Locally and Ectopically in
- Single Nucleus Genome Sequencing Reveals High Similarity among Nuclei of an Endomycorrhizal Fungus
- Metabolic QTL Analysis Links Chloroquine Resistance in to Impaired Hemoglobin Catabolism
- Notch Controls Cell Adhesion in the Drosophila Eye
- AL PHD-PRC1 Complexes Promote Seed Germination through H3K4me3-to-H3K27me3 Chromatin State Switch in Repression of Seed Developmental Genes
- Genomes Reveal Evolution of Microalgal Oleaginous Traits
- Large Inverted Duplications in the Human Genome Form via a Fold-Back Mechanism
- Variation in Genome-Wide Levels of Meiotic Recombination Is Established at the Onset of Prophase in Mammalian Males
- Age, Gender, and Cancer but Not Neurodegenerative and Cardiovascular Diseases Strongly Modulate Systemic Effect of the Apolipoprotein E4 Allele on Lifespan
- Lifespan Extension Conferred by Endoplasmic Reticulum Secretory Pathway Deficiency Requires Induction of the Unfolded Protein Response
- Is Non-Homologous End-Joining Really an Inherently Error-Prone Process?
- Vestigialization of an Allosteric Switch: Genetic and Structural Mechanisms for the Evolution of Constitutive Activity in a Steroid Hormone Receptor
- Functional Divergence and Evolutionary Turnover in Mammalian Phosphoproteomes
- A 660-Kb Deletion with Antagonistic Effects on Fertility and Milk Production Segregates at High Frequency in Nordic Red Cattle: Additional Evidence for the Common Occurrence of Balancing Selection in Livestock
- Comparative Evolutionary and Developmental Dynamics of the Cotton () Fiber Transcriptome
- The Transcription Factor BcLTF1 Regulates Virulence and Light Responses in the Necrotrophic Plant Pathogen
- Crossover Patterning by the Beam-Film Model: Analysis and Implications
- Single Cell Genomics: Advances and Future Perspectives
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- GATA6 Is a Crucial Regulator of Shh in the Limb Bud
- Large Inverted Duplications in the Human Genome Form via a Fold-Back Mechanism
- Differential Effects of Collagen Prolyl 3-Hydroxylation on Skeletal Tissues
- Affects Plant Architecture by Regulating Local Auxin Biosynthesis
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



