-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaMetabolic QTL Analysis Links Chloroquine Resistance in to Impaired Hemoglobin Catabolism
Drug resistant strains of the malaria parasite, Plasmodium falciparum, have rendered chloroquine ineffective throughout much of the world. In parts of Africa and Asia, the coordinated shift from chloroquine to other drugs has resulted in the near disappearance of chloroquine-resistant (CQR) parasites from the population. Currently, there is no molecular explanation for this phenomenon. Herein, we employ metabolic quantitative trait locus mapping (mQTL) to analyze progeny from a genetic cross between chloroquine-susceptible (CQS) and CQR parasites. We identify a family of hemoglobin-derived peptides that are elevated in CQR parasites and show that peptide accumulation, drug resistance, and reduced parasite fitness are all linked in vitro to CQR alleles of the P. falciparum chloroquine resistance transporter (pfcrt). These findings suggest that CQR parasites are less fit because mutations in pfcrt interfere with hemoglobin digestion by the parasite. Moreover, our findings may provide a molecular explanation for the reemergence of CQS parasites in wild populations.
Published in the journal: . PLoS Genet 10(1): e32767. doi:10.1371/journal.pgen.1004085
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004085Summary
Drug resistant strains of the malaria parasite, Plasmodium falciparum, have rendered chloroquine ineffective throughout much of the world. In parts of Africa and Asia, the coordinated shift from chloroquine to other drugs has resulted in the near disappearance of chloroquine-resistant (CQR) parasites from the population. Currently, there is no molecular explanation for this phenomenon. Herein, we employ metabolic quantitative trait locus mapping (mQTL) to analyze progeny from a genetic cross between chloroquine-susceptible (CQS) and CQR parasites. We identify a family of hemoglobin-derived peptides that are elevated in CQR parasites and show that peptide accumulation, drug resistance, and reduced parasite fitness are all linked in vitro to CQR alleles of the P. falciparum chloroquine resistance transporter (pfcrt). These findings suggest that CQR parasites are less fit because mutations in pfcrt interfere with hemoglobin digestion by the parasite. Moreover, our findings may provide a molecular explanation for the reemergence of CQS parasites in wild populations.
Introduction
Drug resistance is a critical issue facing worldwide malaria control. The spread and persistence of chloroquine-resistant (CQR) Plasmodium falciparum has rendered chloroquine, an inexpensive and potent drug, ineffective throughout most of the world [1]. In sub-Saharan Africa [2] and the island of Hainan (China) [3], where CQR parasites formerly accounted for 85–98% of the population, the coordinated cessation of chloroquine treatment resulted in a dramatic reduction (40–100%) in the prevalence of CQR parasites over 10 years. Although the reemergence of chloroquine sensitive (CQS) parasites is a major development with regard to human health, the underlying molecular mechanisms behind this phenomenon are unknown.
The importance of drug resistance to world health has prompted a half century of intensive research into the parasite's mechanisms of resistance [4]. These efforts identified the predominant gene responsible for chloroquine resistance, the P. falciparum chloroquine resistance transporter (pfcrt, pf3D7_0709000) [5]. PfCRT is a multiple pass membrane protein that is localized to the digestive vacuole [5], [6]. Mutations associated with chloroquine resistance have been mapped [7]–[10] and a single polymorphism in the first transmembrane domain (K76T) has been shown to be essential for drug resistance [11]. Recently, the resistant form of PfCRT was found to transport chloroquine under physiologically relevant conditions. Wildtype PfCRT is also assumed to function as a transporter [12], but its native substrates are unclear and the impact of CQR alleles on PfCRT's normal function remains a mystery.
Although mutations in pfcrt are necessary and sufficient to confer chloroquine resistance, several other genes have also been implicated in drug tolerance. The interactions between pfcrt and these other loci, including the P. falciparum multiple drug resistance gene (pfmdr1) [9], [13]–[15] and P. falciparum multiple resistance protein (pfmrp1) [16], are not clearly understood. One possibility is that mutations at secondary loci interact with pfcrt to modulate drug resistance. Alternatively, mutations at other loci may compensate for loss of function associated with CQR forms of PfCRT. Understanding how pfcrt mutations affect parasite physiology is an essential step towards unraveling these polygenic contributions to chloroquine resistance.
Given PfCRT's transmembrane structure, its localization to the digestive vacuole, and its ability to transport chloroquine in vitro, we hypothesized that wildtype PfCRT functions as a transporter. Furthermore, we predicted that mutations in pfcrt, and other CQR-associated genes, alter steady-state metabolite levels in PfCRT-associated pathways. Identifying these phenotypes and linking them to specific genes is difficult because 1) metabolites are often derived from multiple pathways, 2) steady-state levels of compounds can be affected by small perturbations far upstream or downstream of a particular compound and 3) metabolic regulation often involves complex interactions between nonlinear factors, such as covalent modification of enzymes, feedback inhibition, and allosteric regulation.
Quantitative trait locus (QTL) mapping is a powerful tool for unraveling complex metabolic networks and tracing metabolic regulation to specific genes [17], [18]. QTL mapping uses the segregation of alleles across a phenotypically and genetically diverse population to measure the genome-wide contribution of individual alleles to a phenotype (e.g. a metabolite concentration) [19]–[24]. Recently, this approach has been integrated with untargeted metabolomics to study metabolic regulation on a genome-wide scale [17]. This emerging metabolic QTL (mQTL) strategy is of obvious applicability to malariology. However, only three genetic crosses of P. falciparum have ever been completed because of serious logistical challenges [25]. One of these efforts crossed the CQS HB3 parasite clone with the CQR Dd2 clone. The haploid progeny from this cross provide a unique opportunity to investigate the metabolic consequences of drug resistance and the role of compensatory mutations in maintaining metabolic homeostasis.
In this study, we use high resolution mass spectrometry to measure the global metabolic profiles of progeny from the HB3×Dd2 genetic cross. Using mQTL mapping, we identify a family of hemoglobin-derived peptides that accumulate in parasites carrying CQR pfcrt alleles. We show that this phenotype can be recapitulated in transgenic parasite lines in which the native pfcrt gene has been replaced with a recombinant CQR or CQS pfcrt allele. In addition, we show that two independently evolved CQR alleles of pfcrt confer a fitness cost. From these data, we propose that CQR imparts a fitness cost on parasites by disrupting hemoglobin catabolism.
Results
Mapping metabolite levels to genetic loci
We combined genome-wide QTL mapping with mass spectrometry (MS)-based metabolomics to identify genetic loci in P. falciparum that have a significant influence over steady-state metabolite levels. To achieve this, synchronous trophozoite-stage cultures (24 hour post invasion) of the 34 haploid progeny and two parental lines from the HB3×Dd2 genetic were grown using established in vitro methods [26]. Metabolites were harvested from each of the cultures and high-resolution mass data were collected on an LTQ-Orbitrap. For maximum sensitivity, mass data were peak-picked near the noise threshold (minimum signal/noise = 3) and biologically relevant data were identified using a two-stage assignment routine. In the first stage, promising signals in the untargeted mass list (N = 124,020) were identified by QTL analysis and genetic linkages with LOD scores greater than 3 (N = 1,707) were manually curated to remove artifacts and correct for errors in the automated MS data peak picking algorithm. Untargeted mass data are highly redundant because electrospray ionization generates numerous adducts and in-source fragments for each input metabolite. Consequently, we employed a second assignment stage to condense redundant data into a single representative parent mass for each compound. Curated signals (N = 279) were clustered by coelution, signal covariance, and mass difference relative to common adducts/isotopomers/fragments. The most intense signal from each group was designated as the parent mass. A total of 15 signals passed this two-stage filtering routine. Each of these signals had LOD scores above their permutation-established thresholds for genome-wide significance (α = .05), and all but 3 of these signals were significant after Bonferroni correction (α = .05/24) for multiple hypothesis testing (Table 1). Notably, all but one of these 15 signals have LOD scores above the 5% false discovery threshold (LOD = 5.5) established by Q-value analysis for the original unfiltered mass list. Surprisingly, all Bonferroni-compliant signals were linked to a single 22 cM genomic region on chromosome 7 (Figure 1) containing pfcrt, the gene responsible for conferring chloroquine resistance [5].
Fig. 1. Significant genome-wide mQTL hits. 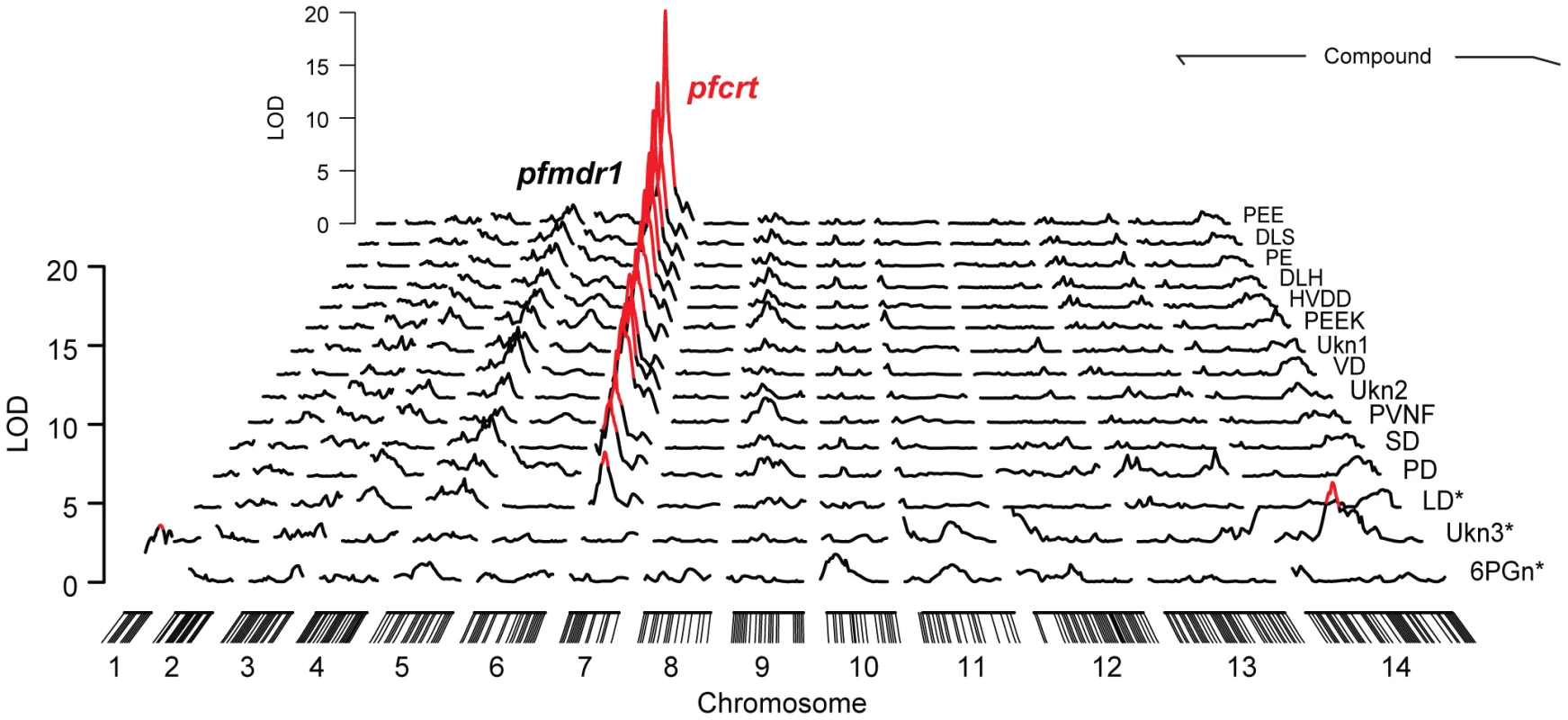
Significance scores were calculated from the natural log of the median compound intensity observed in triplicate pool replicates. All markers showing significant associations (genome-wide α = .05, n = 35) are colored in red and hash marks on the x axis denote the positions of the microsatellite markers. The mean location of the chromosome 7 associated peaks is 22.0 cM, which contains pfcrt and eight other genes. The secondary LOD peak observed on chromosome 5 is consistent with pfmdr1, another CQR-related locus. Peptides are listed by their standard abbreviations, unknowns are abbreviated as Ukn, and 6PGn indicates 6-phosphogluconate. Compounds were identified by ms/ms fragmentation and coelution assays (Figure 2). *All compounds except LD, Ukn3, and 6PGn had significant peak LOD scores after Bonferroni correction for multiple hypothesis testing (α = 0.05/24). Tab. 1. Genetic linkage by QTL and concentrations of metabolites‡ (µM ± s.d.) observed in parasite extracts from chloroquine sensitive (CQS) and resistant (CQR) transgenic parasites. 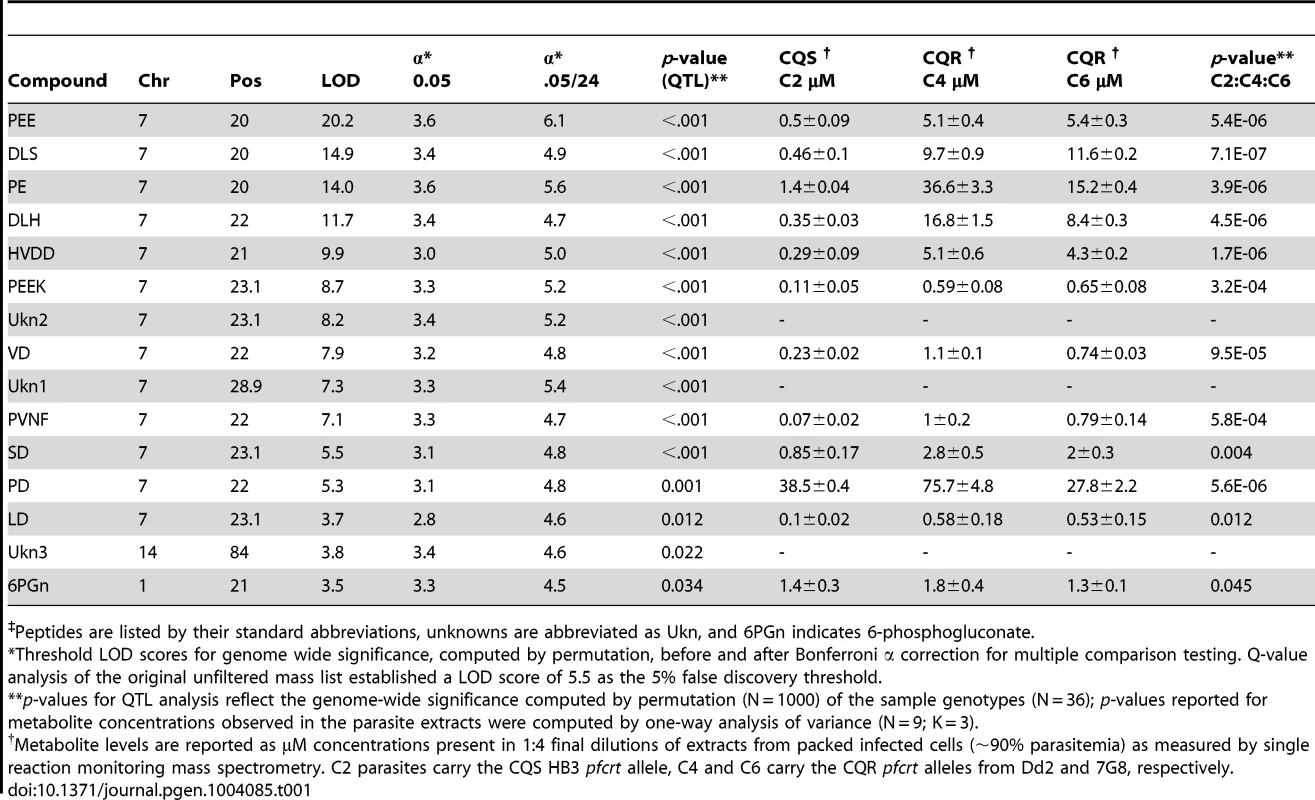
Peptides are listed by their standard abbreviations, unknowns are abbreviated as Ukn, and 6PGn indicates 6-phosphogluconate. Identifying PfCRT-linked metabolites
Tentative metabolite assignments were generated for each of the PfCRT-linked masses by submitting observed signals to the Madison Metabolomics Consortium Database [27] and the Human Metabolome Database [28]. The resulting list of putative IDs was evaluated by analyzing ms/ms fragmentation spectra from enriched parasite extracts (Figure 2). Eleven of the 15 significant compounds (α = .05) had exact masses and fragmentation spectra consistent with small peptides. Peptide assignments were empirically validated by co-elution of the parasite-derived signal with synthetic peptide standards. These experiments were conducted using single reaction monitoring (SRM) mass spectrometry, a robust analytical method for confirming specific metabolite assignments in complex mixtures [29]. Each of the PfCRT-linked peptides co-eluted with their respective synthetic standards. Moreover, the intensity of the parasite-derived signal changed in a concentration dependent manner when standards were added to parasite extracts (Figure 2).
Fig. 2. Strategy for identifying unknown compounds. 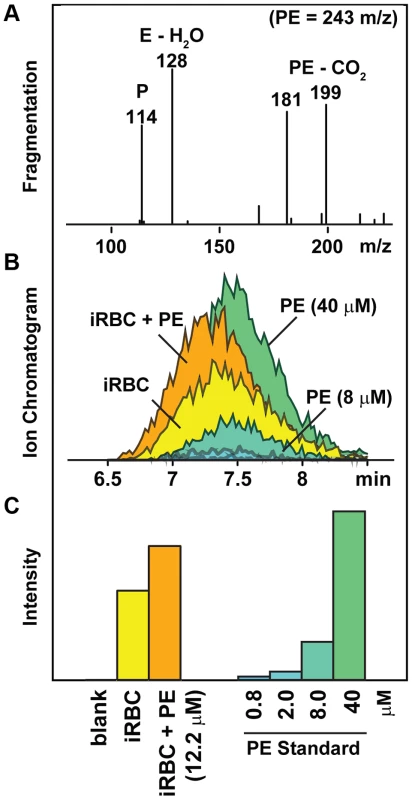
A representative dataset used to identify the dipeptide prolylglutamate (PE) is shown. Putative compound identities were assigned by exact mass matching followed by (A) ms/ms fragmentation analysis of extracts from purified infected red blood cells (iRBC). These assignments were confirmed by (B) chromatographic coelution of the parasite-derived compound with a standard, and (C) a concentration-dependent change in the target compound's intensity when a standard was added to the iRBC extract. Mass fragments are listed by their negative-mode mass (m/z −H+). The 114 m/z fragment shown in panel A was used for identification and quantification of the PE peptide (Table 1). Validating the PfCRT/peptide linkage
QTL analysis demonstrated that the peptide accumulation phenotype observed in CQR parasites maps to a 36 kb locus on chromosome 7 containing pfcrt and eight other genes (Figure 3). To determine if the pfcrt gene is responsible for the peptide accumulation phenotype, we analyzed transgenic parasites in which the native pfcrt gene has been replaced with either a CQS (C2; HB3 allele) or CQR (C4, Dd2 allele; C6, 7G8 allele) variant of pfcrt [11]. The two CQR alleles we tested have distinct evolutionary origins but all of the transgenic parasites share the CQS GC03 background (a progeny clone from the HB3×Dd2 cross). MS analysis demonstrated that parasites carrying either of the CQR pfcrt alleles accumulate peptides over the 48-hour intraerythrocytic life cycle to much higher levels (>32-fold) than parasites carrying the CQS allele (Figure S1). Furthermore, a survey of diverse parasite genotypes showed that all of the parasites that accumulate peptides carry the critical PfCRT-K76T polymorphism that is required for chloroquine resistance [30] (Figure 4).
Fig. 3. Peptide accumulation co-segregates with the pfcrt locus on Chromosome 7. 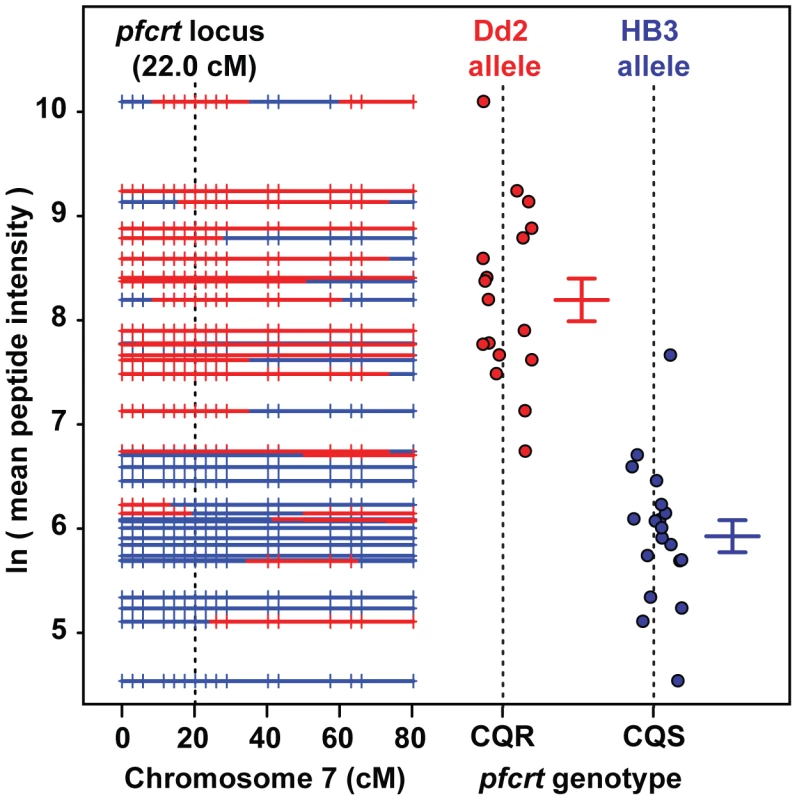
A genetic recombination map is plotted versus the mean pfcrt-linked peptide level observed with each clone. Progeny carrying the Dd2 (red) chloroquine resistant allele at the pfcrt locus show higher levels of peptides than progeny carrying the HB3 chloroquine sensitive allele (blue). The mean peptide intensity used for the y-axis was computed from all of the covariate pfcrt-linked signals (Figure S1). The hash marks shown on each of the chromosomes indicate the positions of microsatellite markers used in this analysis. Fig. 4. Geographically diverse isolates carrying the PfCRT-K76T polymorphism accumulate peptides. 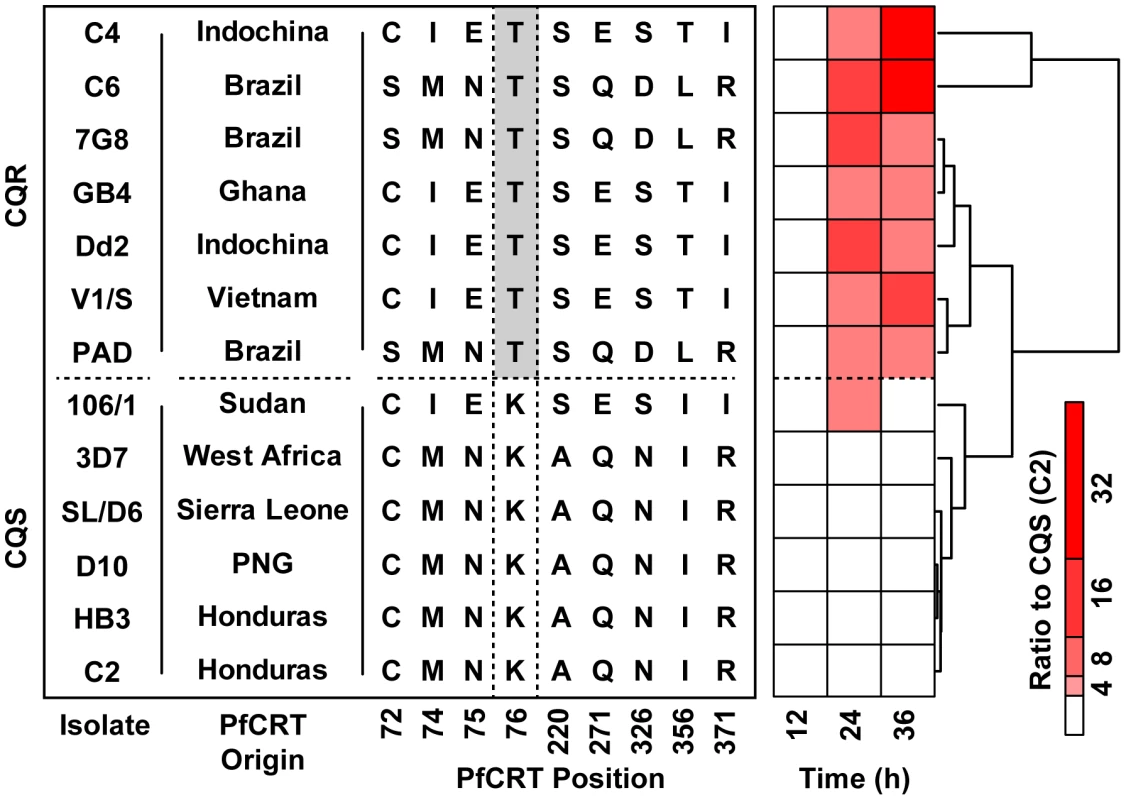
The K76T mutation is necessary to confer chloroquine resistance. The isolate nomenclature, geographical origin of the pfcrt allele, genotype at key residues, and the mean peptide signal observed in each isolate are shown. The mean peptide signal is a composite phenotype reporting the average intensity of 12 pfcrt-linked signals identified by mQTL (see Figure S1 for individual peptide profiles). All signals are expressed as a fold change relative to the maximum corresponding intensity observed in the CQS C2 line. The polymorphisms and origins of these lines were adapted from a previous publication [30]. Peptidomics analysis of parasites
Hemoglobin catabolism is an essential activity that provides amino acids and the physical space parasites need to grow [31]. All of the PfCRT-linked peptides identified by QTL analysis are found in, but not unique to, hemoglobin. Because PfCRT is located on the digestive vacuole membrane, which is the organelle where hemoglobin metabolism occurs, we hypothesized that PfCRT polymorphisms directly affect hemoglobin catabolism. To test this hypothesis, we conducted a comprehensive peptidomics analysis of parasites to monitor PfCRT-related effects on the hemoglobin digestion pathway. Erythrocytes infected with either CQS (C2) or CQR (C4, C6) parasites were purified by Percoll density gradient and endogenous peptides present in parasite extracts were analyzed by high-resolution nanospray LC-MS/MS. This analysis identified 362 endogenous peptides ranging from trimers to 32-mers that exactly correspond to sequences found in either the α or β chain of hemoglobin (Figure 5, Figure S3, and Figure S4). The majority of these peptides (e.g. VHLTPEE) have sequences that are unique to hemoglobin (i.e. have no other exact matches in either the P. falciparum or human genomes) and exist in overlapping clusters of structurally related peptides. The peptide clustering we observed is consistent with the parasite's semi-ordered hemoglobin digestion cascade, which involves protein degradation via a series of proteases (plasmepsins, falcipains, and falcilysin) and aminopeptidases [32]. The boundaries of most of the peptide clusters we observed coincide with established proteolytic cleavage sites [33]. In addition, we observed several sequence breaks that may suggest previously unmapped cut sites (Figure 5). Quantitative analysis identified 87 peptides that show evidence for differential accumulation between CQS and CQR lines (|z-score|>4; Figure 5). These peptides include the mQTL-linked peptides (e.g. PEE and DLS), other structurally related peptides (e.g. VHLTPEEK and HFDLS), and novel classes of peptides that fell below the analytical limit of detection in the original mQTL analysis (e.g. DPENFR in β-hemoglobin).
Fig. 5. CQR isoforms of PfCRT disrupt hemoglobin metabolism. 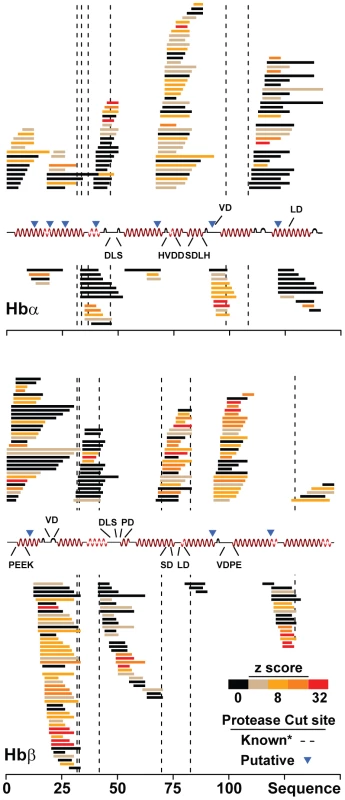
Endogenous peptides present in CQS (C2) versus CQR (C4 or C6) PfCRT allele-exchange parasites lines were identified by LC-MS/MS peptidomics and mapped to their corresponding locations on the α and β hemoglobin primary sequence. Peptides are colored by their mean absolute z-score: mean(|(C4pep-C2pep)/σpep|, |(C6pep-C2pep)/σpep|). The pfcrt-linked sequences that were identified by mQTL are denoted above the hemoglobin secondary structure. *Known protease cut sites depicted here were taken from published sites [33]. The sequence of each peptide is available in Figure S3, Figure S4, and Table S1. Metabolomics analysis of transgenic parasites
We interpret peptide accumulation in CQR parasites as evidence for impaired hemoglobin catabolism. Because hemoglobin catabolism is essential to P. falciparum [31], we anticipated that CQR parasites may have alterations in other metabolic pathways. To determine the degree to which CRQ pfcrt alleles affect metabolic homeostasis, we quantified metabolites present in extracts from density-purified samples of RBCs infected with transgenic CQS (C2) and CQR (C4, C6) allele exchange parasites. Using high resolution HPLC-MS, we quantified 80 metabolites, including representative central carbon metabolites, nucleotides, cofactors, amino acids, and peptides. Surprisingly, the only compounds showing consistent steady-state metabolic differences between the CQS and CQR lines were hemoglobin-derived peptides (Figure S5). These data suggest that altered hemoglobin catabolism is the most significant metabolic consequence of CQR mutations.
Fitness cost of chloroquine resistance
Given the significance of hemoglobin catabolism in the parasite's blood stage development, we hypothesized that CQR-induced peptide-accumulation would be associated with a fitness cost. To test this hypothesis, we conducted long-term competition experiments between CQS and CQR transgenic parasites grown for 70 days in mixed cultures containing either two (C2, C4; C2, C6) or three (C2, C4, C6) allele-exchange parasite lines in the same flask. This experiment differs from previous in vitro studies in our use of the transgenic pfcrt allele exchange parasites, which controls for the polygenic contributions from genetic background, and the long timeframe over which cultures were allowed to compete (∼35 generations). DNA was harvested every 48 hours and the abundance of each pfcrt allele was quantified by Sanger sequencing (Figure S6 and Figure S7). Quantitative DNA sequencing showed that mixed populations of CQS/CQR parasites converted to nearly pure populations of CQS parasites after 70 days (Figure 6). This result was consistent across both Asian (Dd2, C4) and South American (7G8, C6) CQR pfcrt alleles and the outcome was not influenced by the starting ratio of the mixed populations (Figure 6 and Figure S8).
Fig. 6. Chloroquine sensitive alleles outcompete resistant alleles in vitro and in endemic populations. 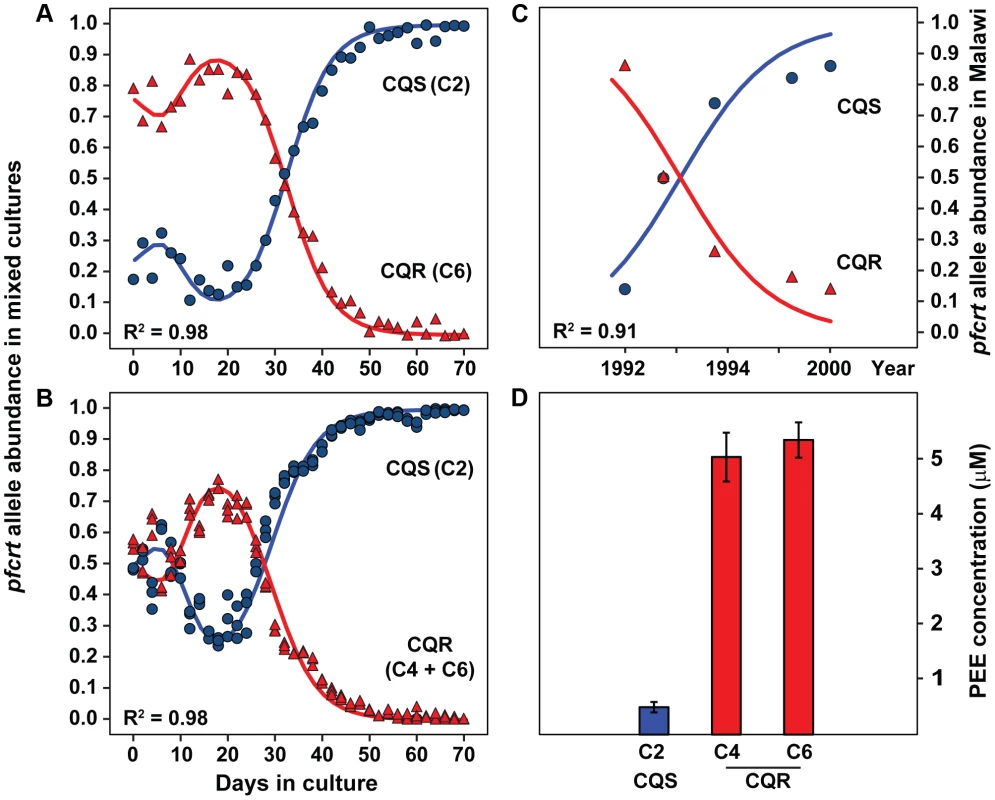
Transgenic parasites carrying either a CQS (C2, HB3) or CQR (Dd2, C4; 7G8, C6) allele of pfcrt were mixed in culture flasks and competed head-to-head for 70 days (∼35 generations) in vitro. (A) Culture flasks were prepared with either 2 (C2, C6) or (B) 3 (C2, C4, C6) transgenic parasite lines and were maintained using standard culturing conditions. DNA was harvested every 48 h and the allelic composition of each flask was determined by quantitative DNA sequencing. The peak CQR DNA abundance at 18 days and the subsequent disappearance of CQR alleles are consistent with an in vitro competition model that allows for differences in lifecycle length and overall fitness (R2 = .98, Δ2 h cycle length and 13% differential fitness). Validation of the quantitative method and a comprehensive panel of the 2-way competition data are provided in Figure S7 and Figure S8. (C) Reemergence of CQS parasites in a wild population of parasites in Malawi following the cessation of chloroquine treatment. The Malawi data were adapted from Kublin et al. [2] and are reproduced with permission from Oxford University Press. The regression line shows the best fit reciprocally-adjusted exponential competition curve. (D) Concentrations of the tripeptide proline-glutamate-glutamate (PEE) in metabolite extracts from red blood cells infected with transgenic parasites carrying either CQS or CQR alleles of pfcrt. Our competition assay showed a transient increase in CQR allele abundance that peaked at ∼20 days. This transient peak is attributable to a difference in cell cycle length between CQR and CQS parasites. Differences in cycle length accumulate across generations and thus progressively offset cycle stages of competing populations. Since parasites amplify their DNA midway through their 48 hour cell cycle, and daughter progeny do not all successfully re-invade, the population with the longer generation-to-generation replication time will have more DNA (but fewer infected cells) when populations are offset across generations (i.e. the leading population is at the ring stage whereas the trailing population is at the schizont stage). To quantify this phenomenon, we constructed a computer model of in vitro P. falciparum competition. Our model showed that a 2 hour difference in cell cycle length and a 13% overall fitness cost per generation can explain both the transient increase in CQR DNA and the subsequent disappearance of CQR alleles from mixed cultures (R2 = .98, Figure 6). This computational assessment is empirically supported by competition experiments involving asynchronous populations of parasites, which showed the same long-term population dynamics but lack the transient increase in CQR allele abundance (Figure S8).
Since CQR parasites are less fit (Figure 6 and Figure S8) and have altered hemoglobin metabolism when compared to CQS parasites (Figure 6), we predicted that CQR parasites would have more difficulty in using hemoglobin-derived amino acids for biomass production. To test this, growth assays were conducted in a modified RPMI medium lacking all amino acids except isoleucine (which is not present in hemoglobin), which forces parasites to use hemoglobin to supply its amino acid needs. In agreement with the literature [31], [34] parasites incubated in isoleucine-only medium grew more slowly than those incubated in rich medium. However, CQR parasites were significantly more impaired than CQS parasites (p = 0.0075, Figure S9), suggesting that CQR-induced fitness changes are linked to hemoglobin catabolism.
Discussion
This study provides four independent lines of evidence linking chloroquine resistance to hemoglobin catabolism: I) mQTL analysis demonstrated that elevated hemoglobin-derived peptides co-segregate with the CQR-encoding pfcrt locus (Figure 1 and Figure 3), II) levels of hemoglobin-derived peptides observed in parasite extracts predict chloroquine susceptibility in genetically diverse parasite strains from around the world (Figure 4), III) genetically identical parasite lines that differ only by CQR versus CQS isoforms of PfCRT recapitulate the peptide phenotypes observed in wild isolates (Figure S1 and Figure S2), and IV) forcing parasites to rely on hemoglobin as an amino acid source affects CQR parasites more severely than CQS parasites. In addition, we show that chloroquine resistant alleles affect the levels of 87 of the 362 observable peptides in the parasite's hemoglobin catabolism pathway (Figure 5). Finally, we demonstrate that the peptide accumulation phenotype is associated with a 2 hour increase in cell cycle duration and a 13% overall fitness cost in transgenic parasites that only differ by their pfcrt allele. Together, these data argue that the significant fitness disadvantage observed in CQR parasites is attributable to impaired hemoglobin metabolism.
These results provide the first molecular explanation for the reemergence of CQS parasites in wild populations following the cessation of chloroquine treatment. Parasites degrade ∼75% of the host cell hemoglobin over the course of their 48 hour intraerythrocytic developmental cycle [35], [36]. Any impairment in the hemoglobin digestion pathway directly affects I) the amino acid pool available for new protein synthesis [32], II) the osmotic stability of infected cells [37], and III) may reduce the number of developing merozoites that can fit within the physical confines of the infected erythrocyte. Any of these mechanisms could account for the increased cycle length and lower generation-to-generation fecundity we observed [37]. Although this is the first report of a metabolic perturbation inherent to CQR alleles of pfcrt, similar fitness-linked phenomena are associated with other drug resistance genes [13], [38]. Epidemiological analysis of the parasite population changes in Malawi and Hainan estimated the fitness cost of CQR to be ∼5% [39], [40]. Our in vitro competition studies support this conclusion and show that the fitness cost may actually be much higher in the absence of compensatory mutations (Figure 6).
While PfCRT isoforms are clearly the main contributor to the peptide-accumulation phenotype, our data also show that genetic background modulates PfCRT's effects on hemoglobin metabolism. The wide distribution of peptide levels observed across the HB3×Dd2 progeny (Figure 3), and the consistent secondary peaks observed in the mQTL analysis (Figure 1), suggest that loci other than PfCRT are contributing to peptide accumulation. Similarly, the CQR allele-exchange parasite lines (C4 and C6) both showed more extreme phenotypes than their respective parental lines (Dd2 and 7G8; Figure 4) despite having identical pfcrt sequences. The PfCRT/hemoglobin catabolism link we describe here, along with our peptidomics approach, provide a framework for investigating compensatory mutations elsewhere in the genome.
Identifying the native function of PfCRT is a subject of considerable interest to the parasitology community. One possible interpretation of the peptide accumulation phenotype is that wildtype PfCRT functions as a peptide transporter and that CQR mutations interfere with this activity [41]. This interpretation is supported by a recent report of glutathione transport in Xenopus oocytes expressing PfCRT [42], and by work in Arabidopsis, which showed that a plant PfCRT homolog mediates glutathione transport [43]. However, the broad diversity of sizes (2–32mers) and physical properties of peptides accumulated by CQR parasites are inconsistent with the relatively narrow range of substrates carried by most peptide transporters [44]. An alternative interpretation of the peptide phenotype is that CQR-associated mutations affect hemoglobin catabolism indirectly by altering the permeability of the digestive vacuole membrane. Resistance mutations in PfCRT, and perhaps other membrane proteins, may cause the digestive vacuole to leak protons [45], glutathione [42], heme, or other osmolytes, which thereby alter the solution conditions of the vacuolar compartment. Given that protease activity can be very sensitive to solution conditions [46], even modest changes in vacuolar conditions could interfere with hemoglobin catabolism by the parasite. Similarly, perturbations in solution conditions may affect protein-protein interaction and thereby disrupt the recently described hemoglobin degradation complex [47].
In conclusion, this study demonstrates that chloroquine resistance, impaired hemoglobin catabolism, and reduced parasite fitness are linked to polymorphisms in PfCRT. This surprising linkage provides a molecular explanation for the reemergence of CQS parasites in Africa and Asia. Our results suggest that co-formulating chloroquine with a P. falciparum protease inhibitor [48] may be an effective strategy for slowing the emergence of resistant parasites.
Materials and Methods
Parasite culture
Synchronous parasites were grown using established methods [26] in RPMI 1640 supplemented with 25 mM HEPES, 100 µM hypoxanthine (all from Sigma), 10 µg/mL gentamycin (Gibco) and 2.5 g/L Albumax II (Gibco). A total of 47 parasite strains were analyzed in this study; these strains include 36 lines from the HB3×Dd2 cross (34 progeny and 2 parental), three pfcrt allele swap lines (C2, C4, and C6) prepared in an isogenic GC03 background [11], and 8 out-group lines (V1/S, PAD, 7G8, GB4, 3D7, D10, SL/D6, and 106/1) used to measure metabolic phenotypes in diverse genetic backgrounds. All cultures were maintained at 5% hematocrit in a 37°C incubator with an atmosphere of 5% CO2, 6% O2, and 89% N2. Cultures were triple synchronized using consecutive treatments with 5% sorbitol at 0, 48, and 56 h of culture. Invasion time was determined by preparing blood smears every two hours starting 34 h after the last sorbitol treatment. The zero time point was designated when cultures reached 95% rings. Samples were harvested at 24 h post invasion for the HB3×Dd2 cross study, 38 h for the parasite enrichment studies, and at several time points throughout the cyclic 48-hour asexual blood stage (12, 24, and 36 h) for the out-group analysis.
Purifying infected cells
To confirm our PfCRT-related phenotypes and improve our analytical sensitivity, erythrocytes infected with late trophozoite-stage parasites (38 h post invasion) were separated from uninfected erythrocytes using an established density gradient method [49]. Briefly, bulk cultures were suspended at 30% hematocrit in RPMI. Cultures were layered over dual Percoll layers (70% lower, 30% upper) diluted in 1× RPMI (final concentration). Samples were centrifuged (2,000× g, 15 min) and the infected erythrocyte layer was collected from the 30%/70% interface. Infected cells were washed with 50 volumes of RPMI, then suspended at 0.4% hematocrit in RPMI. Parasitemias of the purified samples were checked by blood smear and the purified samples were allowed to recover for 4 h in a 37°C incubator prior to metabolite extraction (Text S1 provides a step-by-step protocol).
Metabolite extraction
Our metabolite extraction protocol is adapted from a previously established method [50]. Metabolites were extracted by suspending 50 µL packed cells in 1 mL 4°C 90% methanol. Samples were vortexed and briefly sonicated, if necessary, to disrupt the cell pellet and generate a uniform homogenate. Homogenates were centrifuged (13,000× g, 10 min) and the supernatants were harvested. Samples were stored at −80°C as 90% methanolic extracts until metabolite analysis. Just prior to analysis, extracts were dried under a stream of N2 gas and resuspended in 200 µL H2O (Text S1 provides a step-by-step protocol).
Mass spectrometry
Metabolite extracts were analyzed by high performance liquid chromatography (HPLC) mass spectrometry (MS). The chromatographic conditions used in this study have been described in detail elsewhere [50]. Briefly, metabolites were separated by reverse phase C18 chromatography run over a 50 minute (HB3×Dd2 cross study and coelution assays) or 25 minute gradient (all other studies) using tributylamine as an ion pairing agent. General metabolite analyses were conducted using negative-mode electrospray ionization on a Thermo Scientific LTQ-Orbitrap (HB3×Dd2 cross) or Thermo Exactive (all other studies). Metabolite assignments were validated by single reaction monitoring (SRM) on a Thermo TSQ Quantum Discovery Max triple quad. For peptidomics analysis, aliquots of each metabolite extract were harvested, diluted 1∶4 in a 3% acetonitrile and 0.1% formic acid solution (final concentrations), and analyzed in positive mode on an LTQ-Orbitrap using nanospray from a 120 minute hydrophilic interaction liquid chromatography (HILIC) gradient. Scans were conducted at both low (150–500 m/z) and high (450–1800 m/z) mass ranges to accommodate multiple charge states and MS2 scans were automatically conducted on fragments from each of the top seven signals observed at any given time. Both the original cross dataset and the peptidomic analyses were collected at the Princeton mass spectrometry facility; all other data were collected in house.
QTL analysis
Genome-wide scans were performed using pseudomarker [51] to detect QTLs associated with metabolite levels in the HB3×Dd2 genetic cross. Intensities of mass signals were log-transformed and the median signal for each mass across replicates was used as a phenotype. Batch number was included as an independent covariate [52] to correct for run-to-run changes in MS instrument sensitivity. Genome-wide significance thresholds were determined by permutation testing (n = 1000 permutations) [53] and the strength of each linkage was expressed as a LOD score. We accounted for multiple hypothesis testing using established methods [21]. Briefly, false discovery rates were calculated from p-values using the q-value approach [54]. QTL-based significance scores were used to filter the large untargeted mass list to a manageable subset of putative signals. Final LOD scores and significance thresholds were computed using R/qtl [55] (interval mapping parameters step = 1, n.draws = 64; QTL mapping method = hk). The custom R code (Text S2 and Text S3) and data tables used for this analysis (Table S2 and Table S3) are included in the supplemental materials.
Metabolomics data analysis
Mass data were visualized and analyzed using MAVEN, a freely available software package for MS-based metabolomics [56]. Data were peak picked using a permissive threshold (S/N = 3) and raw LOD scores generated by QTL mapping were then used to identify the most promising subset of signals. The extracted ion chromatogram of each signal with a LOD score greater than 3 was visually inspected and data originating from peak picking errors, thermal noise, elution artifacts, or associated with the void and wash volumes were excluded. Coeluting adducts, fragments, and isotopomers were condensed into their respective parent masses and the intensities for each of the final parent masses were hand-verified to correct for peak picking errors.
Compound identification
A list of potential compound identities was generated by searching the Madison Metabolomics Consortium Database [27] and the Human Metabolome Database [28] for metabolites matching the QTL-identified masses. Putative IDs were evaluated by ms/ms fragmentation analysis. Final compound identities were confirmed by coelution of the parasite-derived compounds with standards purchased from Sigma and the Proteomics Resource Center at Rockefeller University. The final assignment and quantification steps were conducted by single reaction monitoring (SRM) on a triple quadrupole mass spectrometer.
Peptidomics data analysis
Peptide assignments were conducted using a comprehensive hemoglobin digestion library loaded into Mascot proteomics software (Matrix Science). Only assignments with mass defects of less than 10 ppm, matching scores greater than nine, and observable peptide signals in all nine of the extracts (N = 3 per genotype) were included. All assignments based on parent masses that mapped to adducts or fragments of hemoglobin peptides were excluded. A custom MAVEN-format standards library was generated using the Mascot results and the extracted ion chromatogram of each assignment was visually inspected and quantified in MAVEN. Peak intensities for each peptide were compiled and aligned to both the hemoglobin α and β primary sequences using custom software written in R.
Parasite fitness assays
Synchronous cultures of isogenic pfcrt allele exchange parasites (C2, C4, C6) were grown to the late trophozoite phase and magnetically purified from uninfected cells using a MACS column. The parasitemia of each enriched sample was determined by microscopy and cell counts were determined by hemocytometer. Mixed culture flasks containing either two (C2, C4; C2, C6) or three (C2, C4, C6) genotypes were constructed at mixing ratios of 50∶50 (C2/C4, C2/C6), 25∶75 (C2/C6), or 50∶15∶35 (C2, C4, C6). Each two-way competition experiment was run as a single biological replicate whereas the three-way competition was replicated in three independent flasks (established from a single seed culture) run in parallel. The entire experimental procedure was repeated a second time using asynchronous populations of parasites. Flasks were maintained continuously for 70 days under standard culturing conditions. Culture flasks were cut back 1∶10 every 48 h (to ∼0.5% parasitemia) and DNA was harvested from the excess parasites via Saponin lysis (0.1%) followed by genomic DNA isolation (DNeasy kit, Qiagen). The pfcrt allele present in each sample was PCR amplified (primers: CGAGCGTTATAGAGAATTAG, ACAACATCACCGGCTAAGAA). Products were then Sanger sequenced using independent diagnostic primers (GGCTCACGTTTAGGTGGAGG, ACAACATCACCGGCTAAGAA). Sequencing results were analyzed using online tools from Genewiz and allelic abundances in each flask were quantified using diagnostic single nucleotide polymorphisms in PfCRT amino acid positions 74–76, and 98 (C2: ATG AAT AAA AAC, C4: ATT GAA ACA AGC, C6: ATG AAT ACA GAC, Figure S6).
Modeling in vitro competition
Allele frequencies observed in long-term competition experiments were fit using a custom model of P. falciparum in vitro growth. All modeling and regression analyses were conducted using custom software written for the R statistical software environment. Our model makes the following assumptions: 1) long-term changes in allele frequencies follow exponential kinetics, 2) parasite clones can differ with respect to life cycle length, 3) DNA abundance follows a sigmoidal accumulation over the life cycle with peak DNA synthesis occurring mid lifecycle, 4) most of the DNA synthesized in one generation is not amplified in the following generation because not all merozoites successfully reinvade, 5) parasite life cycle synchronicity follows a Gaussian distribution that becomes progressively broader with each generation. Using these assumptions, the relative DNA content expected in mixed culture flasks was modeled for each point in the 70 day competition experiment. Initial differences in allele frequencies were set according to the empirical mixing ratio, then life cycle lengths and exponential growth rates were sampled by grid search. A best-fit multiple regression model was identified by iterative grid searches with progressively finer increments of cycle lengths and growth rates. The custom R code (Text S2 and Text S3) and data table (Table S4) used for this analysis is provided in the supplemental materials.
Supporting Information
Zdroje
1. VestergaardLS, RingwaldP (2007) Responding to the challenge of antimalarial drug resistance by routine monitoring to update national malaria treatment policies. Am J Trop Med Hyg 77 : 153–159.
2. KublinJG, CorteseJF, NjunjuEM, MukadamRA, WirimaJJ, et al. (2003) Reemergence of chloroquine-sensitive plasmodium falciparum malaria after cessation of chloroquine use in malawi. J Infect Dis 187 : 1870–1875.
3. LiuDQ, LiuRJ, RenDX, GaoDQ, ZhangCY, et al. (1995) Changes in the resistance of plasmodium falciparum to chloroquine in hainan, china. Bull World Health Organ 73 : 483–486.
4. BURGESSRW, YOUNGMD (1959) The development of pyrimethamine resistance by plasmodium falciparum. Bull World Health Organ 20 : 37–46.
5. FidockDA, NomuraT, TalleyAK, CooperRA, DzekunovSM, et al. (2000) Mutations in the P. falciparum digestive vacuole transmembrane protein PfCRT and evidence for their role in chloroquine resistance. Mol Cell 6 : 861–871.
6. CooperRA, FerdigMT, SuXZ, UrsosLM, MuJ, et al. (2002) Alternative mutations at position 76 of the vacuolar transmembrane protein PfCRT are associated with chloroquine resistance and unique stereospecific quinine and quinidine responses in plasmodium falciparum. Mol Pharmacol 61 : 35–42.
7. WoottonJC, FengX, FerdigMT, CooperRA, MuJ, et al. (2002) Genetic diversity and chloroquine selective sweeps in plasmodium falciparum. Nature 418 : 320–323.
8. ChenN, KyleDE, PasayC, FowlerEV, BakerJ, et al. (2003) Pfcrt allelic types with two novel amino acid mutations in chloroquine-resistant plasmodium falciparum isolates from the philippines. Antimicrob Agents Chemother 47 : 3500–3505.
9. Best PlummerW, Pinto PereiraLM, CarringtonCV (2004) Pfcrt and pfmdr1 alleles associated with chloroquine resistance in plasmodium falciparum from guyana, south america. Mem Inst Oswaldo Cruz 99 : 389–392.
10. DurrandV, BerryA, SemR, GlaziouP, BeaudouJ, et al. (2004) Variations in the sequence and expression of the plasmodium falciparum chloroquine resistance transporter (pfcrt) and their relationship to chloroquine resistance in vitro. Mol Biochem Parasitol 136 : 273–285.
11. SidhuAB, Verdier-PinardD, FidockDA (2002) Chloroquine resistance in plasmodium falciparum malaria parasites conferred by pfcrt mutations. Science 298 : 210–213.
12. MartinRE, KirkK (2004) The malaria parasite's chloroquine resistance transporter is a member of the drug/metabolite transporter superfamily. Mol Biol Evol 21 : 1938–1949.
13. HaywardR, SalibaKJ, KirkK (2005) pfmdr1 mutations associated with chloroquine resistance incur a fitness cost in plasmodium falciparum. Mol Microbiol 55 : 1285–1295.
14. ReedMB, SalibaKJ, CaruanaSR, KirkK, CowmanAF (2000) Pgh1 modulates sensitivity and resistance to multiple antimalarials in plasmodium falciparum. Nature 403 : 906–909.
15. BrayPG, MartinRE, TilleyL, WardSA, KirkK, et al. (2005) Defining the role of PfCRT in plasmodium falciparum chloroquine resistance. Mol Microbiol 56 : 323–333.
16. RajDK, MuJ, JiangH, KabatJ, SinghS, et al. (2009) Disruption of a plasmodium falciparum multidrug resistance-associated protein (PfMRP) alters its fitness and transport of antimalarial drugs and glutathione. J Biol Chem 284 : 7687–7696.
17. KeurentjesJJ, FuJ, de VosCH, LommenA, HallRD, et al. (2006) The genetics of plant metabolism. Nat Genet 38 : 842–849.
18. KeurentjesJJ (2009) Genetical metabolomics: Closing in on phenotypes. Curr Opin Plant Biol 12 : 223–230.
19. Ranford-CartwrightLC, MwangiJM (2012) Analysis of malaria parasite phenotypes using experimental genetic crosses of plasmodium falciparum. Int J Parasitol 42 : 529–534.
20. SuX, FerdigMT, HuangY, HuynhCQ, LiuA, et al. (1999) A genetic map and recombination parameters of the human malaria parasite plasmodium falciparum. Science 286 : 1351–1353.
21. GonzalesJM, PatelJJ, PonmeeN, JiangL, TanA, et al. (2008) Regulatory hotspots in the malaria parasite genome dictate transcriptional variation. PLoS Biol 6: e238.
22. Reilly AyalaHB, WackerMA, SiwoG, FerdigMT (2010) Quantitative trait loci mapping reveals candidate pathways regulating cell cycle duration in plasmodium falciparum. BMC Genomics 11 : 577.
23. HaytonK, GaurD, LiuA, TakahashiJ, HenschenB, et al. (2008) Erythrocyte binding protein PfRH5 polymorphisms determine species-specific pathways of plasmodium falciparum invasion. Cell Host Microbe 4 : 40–51.
24. PatelJJ, ThackerD, TanJC, PleeterP, CheckleyL, et al. (2010) Chloroquine susceptibility and reversibility in a plasmodium falciparum genetic cross. Mol Microbiol 78 : 770–787.
25. SuX, HaytonK, WellemsTE (2007) Genetic linkage and association analyses for trait mapping in plasmodium falciparum. Nat Rev Genet 8 : 497–506.
26. TragerW, JensenJB (1976) Human malaria parasites in continuous culture. Science 193 : 673–675.
27. CuiQ, LewisIA, HegemanAD, AndersonME, LiJ, et al. (2008) Metabolite identification via the madison metabolomics consortium database. Nat Biotechnol 26 : 162–164.
28. WishartDS, TzurD, KnoxC, EisnerR, GuoAC, et al. (2007) HMDB: The human metabolome database. Nucleic Acids Res 35: D521–6.
29. BajadSU, LuW, KimballEH, YuanJ, PetersonC, et al. (2006) Separation and quantitation of water soluble cellular metabolites by hydrophilic interaction chromatography-tandem mass spectrometry. J Chromatogr A 1125 : 76–88.
30. EckerA, LehaneAM, ClainJ, FidockDA (2012) PfCRT and its role in antimalarial drug resistance. Trends Parasitol 28 : 504–514.
31. LiuJ, IstvanES, GluzmanIY, GrossJ, GoldbergDE (2006) Plasmodium falciparum ensures its amino acid supply with multiple acquisition pathways and redundant proteolytic enzyme systems. Proc Natl Acad Sci U S A 103 : 8840–8845.
32. GoldbergDE (2005) Hemoglobin degradation. Curr Top Microbiol Immunol 295 : 275–291.
33. GluzmanIY, FrancisSE, OksmanA, SmithCE, DuffinKL, et al. (1994) Order and specificity of the plasmodium falciparum hemoglobin degradation pathway. J Clin Invest 93 : 1602–1608.
34. BabbittSE, AltenhofenL, CobboldSA, IstvanES, FennellC, et al. (2012) Plasmodium falciparum responds to amino acid starvation by entering into a hibernatory state. Proc Natl Acad Sci U S A 109: E3278–87.
35. KrugliakM, ZhangJ, GinsburgH (2002) Intraerythrocytic plasmodium falciparum utilizes only a fraction of the amino acids derived from the digestion of host cell cytosol for the biosynthesis of its proteins. Mol Biochem Parasitol 119 : 249–256.
36. LoriaP, MillerS, FoleyM, TilleyL (1999) Inhibition of the peroxidative degradation of haem as the basis of action of chloroquine and other quinoline antimalarials. Biochem J 339 (Pt 2) 363–370.
37. LewVL, TiffertT, GinsburgH (2003) Excess hemoglobin digestion and the osmotic stability of plasmodium falciparum-infected red blood cells. Blood 101 : 4189–4194.
38. PetersJM, ChenN, GattonM, KorsinczkyM, FowlerEV, et al. (2002) Mutations in cytochrome b resulting in atovaquone resistance are associated with loss of fitness in plasmodium falciparum. Antimicrob Agents Chemother 46 : 2435–2441.
39. LauferMK, ThesingPC, EddingtonND, MasongaR, DzinjalamalaFK, et al. (2006) Return of chloroquine antimalarial efficacy in malawi. N Engl J Med 355 : 1959–1966.
40. ChenN, GaoQ, WangS, WangG, GattonM, et al. (2008) No genetic bottleneck in plasmodium falciparum wild-type pfcrt alleles reemerging in hainan island, china, following high-level chloroquine resistance. Antimicrob Agents Chemother 52 : 345.
41. SummersRL, NashMN, MartinRE (2012) Know your enemy: Understanding the role of PfCRT in drug resistance could lead to new antimalarial tactics. Cell Mol Life Sci 69 : 1967–1995.
42. PatzewitzEM, Salcedo-SoraJE, WongEH, SethiaS, StocksPA, et al. (2012) Glutathione transport: A new role for PfCRT in chloroquine resistance. Antioxid Redox Signal 19 (7) 683–95.
43. MaughanSC, PasternakM, CairnsN, KiddleG, BrachT, et al. (2010) Plant homologs of the plasmodium falciparum chloroquine-resistance transporter, PfCRT, are required for glutathione homeostasis and stress responses. Proc Natl Acad Sci U S A 107 : 2331–2336.
44. ChiangCS, StaceyG, TsayYF (2004) Mechanisms and functional properties of two peptide transporters, AtPTR2 and fPTR2. J Biol Chem 279 : 30150–30157.
45. LehaneAM, HaywardR, SalibaKJ, KirkK (2008) A verapamil-sensitive chloroquine-associated H+ leak from the digestive vacuole in chloroquine-resistant malaria parasites. J Cell Sci 121 : 1624–1632.
46. StennickeHR, SalvesenGS (1997) Biochemical characteristics of caspases-3, -6, -7, and -8. J Biol Chem 272 : 25719–25723.
47. ChughM, SundararamanV, KumarS, ReddyVS, SiddiquiWA, et al. (2013) Protein complex directs hemoglobin-to-hemozoin formation in plasmodium falciparum. Proc Natl Acad Sci U S A 110 : 5392–5397.
48. KamkumoRG, NgoutaneAM, TchokouahaLR, FokouPV, MadiesseEA, et al. (2012) Compounds from sorindeia juglandifolia (anacardiaceae) exhibit potent anti-plasmodial activities in vitro and in vivo. Malar J 11 : 382-2875-11-382.
49. DluzewskiAR, LingIT, RangachariK, BatesPA, WilsonRJ (1984) A simple method for isolating viable mature parasites of plasmodium falciparum from cultures. Trans R Soc Trop Med Hyg 78 : 622–624.
50. OlszewskiKL, MorriseyJM, WilinskiD, BurnsJM, VaidyaAB, et al. (2009) Host-parasite interactions revealed by plasmodium falciparum metabolomics. Cell Host Microbe 5 : 191–199.
51. SenS, ChurchillGA (2001) A statistical framework for quantitative trait mapping. Genetics 159 : 371–387.
52. ZeegersM, RijsdijkF, ShamP (2004) Adjusting for covariates in variance components QTL linkage analysis. Behav Genet 34 : 127–133.
53. ChurchillGA, DoergeRW (1994) Empirical threshold values for quantitative trait mapping. Genetics 138 : 963–971.
54. StoreyJD, TibshiraniR (2003) Statistical significance for genomewide studies. Proc Natl Acad Sci U S A 100 : 9440–9445.
55. BromanKW, WuH, SenS, ChurchillGA (2003) R/qtl: QTL mapping in experimental crosses. Bioinformatics 19 : 889–890.
56. ClasquinMF, MelamudE, RabinowitzJD (2012) LC-MS data processing with MAVEN: A metabolomic analysis and visualization engine. Curr Protoc Bioinformatics Chapter 14: Unit14.11.
Štítky
Genetika Reprodukční medicína
Článek Unwrapping BacteriaČlánek A Chaperone-Assisted Degradation Pathway Targets Kinetochore Proteins to Ensure Genome StabilityČlánek The Candidate Splicing Factor Sfswap Regulates Growth and Patterning of Inner Ear Sensory OrgansČlánek The SPF27 Homologue Num1 Connects Splicing and Kinesin 1-Dependent Cytoplasmic Trafficking inČlánek Down-Regulation of eIF4GII by miR-520c-3p Represses Diffuse Large B Cell Lymphoma DevelopmentČlánek Meta-Analysis Identifies Gene-by-Environment Interactions as Demonstrated in a Study of 4,965 MiceČlánek High Risk Population Isolate Reveals Low Frequency Variants Predisposing to Intracranial Aneurysms
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2014 Číslo 1
-
Všechny články tohoto čísla
- How Much Is That in Dog Years? The Advent of Canine Population Genomics
- The Sense and Sensibility of Strand Exchange in Recombination Homeostasis
- Unwrapping Bacteria
- DNA Methylation Changes Separate Allergic Patients from Healthy Controls and May Reflect Altered CD4 T-Cell Population Structure
- Evidence for Mito-Nuclear and Sex-Linked Reproductive Barriers between the Hybrid Italian Sparrow and Its Parent Species
- Translation Enhancing ACA Motifs and Their Silencing by a Bacterial Small Regulatory RNA
- Relationship Estimation from Whole-Genome Sequence Data
- Genetic Models of Apoptosis-Induced Proliferation Decipher Activation of JNK and Identify a Requirement of EGFR Signaling for Tissue Regenerative Responses in
- ComEA Is Essential for the Transfer of External DNA into the Periplasm in Naturally Transformable Cells
- Loss and Recovery of Genetic Diversity in Adapting Populations of HIV
- Bioelectric Signaling Regulates Size in Zebrafish Fins
- Defining NELF-E RNA Binding in HIV-1 and Promoter-Proximal Pause Regions
- Loss of Histone H3 Methylation at Lysine 4 Triggers Apoptosis in
- Cell-Cycle Dependent Expression of a Translocation-Mediated Fusion Oncogene Mediates Checkpoint Adaptation in Rhabdomyosarcoma
- How a Retrotransposon Exploits the Plant's Heat Stress Response for Its Activation
- A Nonsense Mutation in Encoding a Nondescript Transmembrane Protein Causes Idiopathic Male Subfertility in Cattle
- Deletion of a Conserved -Element in the Locus Highlights the Role of Acute Histone Acetylation in Modulating Inducible Gene Transcription
- Developmental Link between Sex and Nutrition; Regulates Sex-Specific Mandible Growth via Juvenile Hormone Signaling in Stag Beetles
- PP2A/B55 and Fcp1 Regulate Greatwall and Ensa Dephosphorylation during Mitotic Exit
- Differential Effects of Collagen Prolyl 3-Hydroxylation on Skeletal Tissues
- Comprehensive Functional Annotation of 77 Prostate Cancer Risk Loci
- Evolution of Chloroplast Transcript Processing in and Its Chromerid Algal Relatives
- A Chaperone-Assisted Degradation Pathway Targets Kinetochore Proteins to Ensure Genome Stability
- New MicroRNAs in —Birth, Death and Cycles of Adaptive Evolution
- A Genome-Wide Screen for Bacterial Envelope Biogenesis Mutants Identifies a Novel Factor Involved in Cell Wall Precursor Metabolism
- FGFR1-Frs2/3 Signalling Maintains Sensory Progenitors during Inner Ear Hair Cell Formation
- Regulation of Synaptic /Neuroligin Abundance by the /Nrf Stress Response Pathway Protects against Oxidative Stress
- Intrasubtype Reassortments Cause Adaptive Amino Acid Replacements in H3N2 Influenza Genes
- Molecular Specificity, Convergence and Constraint Shape Adaptive Evolution in Nutrient-Poor Environments
- WNT7B Promotes Bone Formation in part through mTORC1
- Natural Selection Reduced Diversity on Human Y Chromosomes
- In-Vivo Quantitative Proteomics Reveals a Key Contribution of Post-Transcriptional Mechanisms to the Circadian Regulation of Liver Metabolism
- The Candidate Splicing Factor Sfswap Regulates Growth and Patterning of Inner Ear Sensory Organs
- The Acid Phosphatase-Encoding Gene Contributes to Soybean Tolerance to Low-Phosphorus Stress
- p53 and TAp63 Promote Keratinocyte Proliferation and Differentiation in Breeding Tubercles of the Zebrafish
- Affects Plant Architecture by Regulating Local Auxin Biosynthesis
- The SET Domain Proteins SUVH2 and SUVH9 Are Required for Pol V Occupancy at RNA-Directed DNA Methylation Loci
- Down-Regulation of Rad51 Activity during Meiosis in Yeast Prevents Competition with Dmc1 for Repair of Double-Strand Breaks
- Multi-tissue Analysis of Co-expression Networks by Higher-Order Generalized Singular Value Decomposition Identifies Functionally Coherent Transcriptional Modules
- A Neurotoxic Glycerophosphocholine Impacts PtdIns-4, 5-Bisphosphate and TORC2 Signaling by Altering Ceramide Biosynthesis in Yeast
- Subtle Changes in Motif Positioning Cause Tissue-Specific Effects on Robustness of an Enhancer's Activity
- C/EBPα Is Required for Long-Term Self-Renewal and Lineage Priming of Hematopoietic Stem Cells and for the Maintenance of Epigenetic Configurations in Multipotent Progenitors
- The SPF27 Homologue Num1 Connects Splicing and Kinesin 1-Dependent Cytoplasmic Trafficking in
- Down-Regulation of eIF4GII by miR-520c-3p Represses Diffuse Large B Cell Lymphoma Development
- Genome Sequencing Highlights the Dynamic Early History of Dogs
- Re-sequencing Expands Our Understanding of the Phenotypic Impact of Variants at GWAS Loci
- Meta-Analysis Identifies Gene-by-Environment Interactions as Demonstrated in a Study of 4,965 Mice
- , a -Antisense Gene of , Encodes a Evolved Protein That Inhibits GSK3β Resulting in the Stabilization of MYCN in Human Neuroblastomas
- A Transcription Factor Is Wound-Induced at the Planarian Midline and Required for Anterior Pole Regeneration
- A Comprehensive tRNA Deletion Library Unravels the Genetic Architecture of the tRNA Pool
- A PNPase Dependent CRISPR System in
- Genomic Confirmation of Hybridisation and Recent Inbreeding in a Vector-Isolated Population
- Zinc Finger Transcription Factors Displaced SREBP Proteins as the Major Sterol Regulators during Saccharomycotina Evolution
- GATA6 Is a Crucial Regulator of Shh in the Limb Bud
- Tissue Specific Roles for the Ribosome Biogenesis Factor Wdr43 in Zebrafish Development
- A Cell Cycle and Nutritional Checkpoint Controlling Bacterial Surface Adhesion
- High Risk Population Isolate Reveals Low Frequency Variants Predisposing to Intracranial Aneurysms
- E3 Ubiquitin Ligase CHIP and NBR1-Mediated Selective Autophagy Protect Additively against Proteotoxicity in Plant Stress Responses
- Evolutionary Rate Covariation Identifies New Members of a Protein Network Required for Female Post-Mating Responses
- 3′ Untranslated Regions Mediate Transcriptional Interference between Convergent Genes Both Locally and Ectopically in
- Single Nucleus Genome Sequencing Reveals High Similarity among Nuclei of an Endomycorrhizal Fungus
- Metabolic QTL Analysis Links Chloroquine Resistance in to Impaired Hemoglobin Catabolism
- Notch Controls Cell Adhesion in the Drosophila Eye
- AL PHD-PRC1 Complexes Promote Seed Germination through H3K4me3-to-H3K27me3 Chromatin State Switch in Repression of Seed Developmental Genes
- Genomes Reveal Evolution of Microalgal Oleaginous Traits
- Large Inverted Duplications in the Human Genome Form via a Fold-Back Mechanism
- Variation in Genome-Wide Levels of Meiotic Recombination Is Established at the Onset of Prophase in Mammalian Males
- Age, Gender, and Cancer but Not Neurodegenerative and Cardiovascular Diseases Strongly Modulate Systemic Effect of the Apolipoprotein E4 Allele on Lifespan
- Lifespan Extension Conferred by Endoplasmic Reticulum Secretory Pathway Deficiency Requires Induction of the Unfolded Protein Response
- Is Non-Homologous End-Joining Really an Inherently Error-Prone Process?
- Vestigialization of an Allosteric Switch: Genetic and Structural Mechanisms for the Evolution of Constitutive Activity in a Steroid Hormone Receptor
- Functional Divergence and Evolutionary Turnover in Mammalian Phosphoproteomes
- A 660-Kb Deletion with Antagonistic Effects on Fertility and Milk Production Segregates at High Frequency in Nordic Red Cattle: Additional Evidence for the Common Occurrence of Balancing Selection in Livestock
- Comparative Evolutionary and Developmental Dynamics of the Cotton () Fiber Transcriptome
- The Transcription Factor BcLTF1 Regulates Virulence and Light Responses in the Necrotrophic Plant Pathogen
- Crossover Patterning by the Beam-Film Model: Analysis and Implications
- Single Cell Genomics: Advances and Future Perspectives
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- GATA6 Is a Crucial Regulator of Shh in the Limb Bud
- Large Inverted Duplications in the Human Genome Form via a Fold-Back Mechanism
- Differential Effects of Collagen Prolyl 3-Hydroxylation on Skeletal Tissues
- Affects Plant Architecture by Regulating Local Auxin Biosynthesis
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



