-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaLifespan Extension Conferred by Endoplasmic Reticulum Secretory Pathway Deficiency Requires Induction of the Unfolded Protein Response
Cells respond to accumulation of misfolded proteins in the endoplasmic reticulum (ER) by activating the unfolded protein response (UPR) signaling pathway. The UPR restores ER homeostasis by degrading misfolded proteins, inhibiting translation, and increasing expression of chaperones that enhance ER protein folding capacity. Although ER stress and protein aggregation have been implicated in aging, the role of UPR signaling in regulating lifespan remains unknown. Here we show that deletion of several UPR target genes significantly increases replicative lifespan in yeast. This extended lifespan depends on a functional ER stress sensor protein, Ire1p, and is associated with constitutive activation of upstream UPR signaling. We applied ribosome profiling coupled with next generation sequencing to quantitatively examine translational changes associated with increased UPR activity and identified a set of stress response factors up-regulated in the long-lived mutants. Besides known UPR targets, we uncovered up-regulation of components of the cell wall and genes involved in cell wall biogenesis that confer resistance to multiple stresses. These findings demonstrate that the UPR is an important determinant of lifespan that governs ER stress and identify a signaling network that couples stress resistance to longevity.
Published in the journal: . PLoS Genet 10(1): e32767. doi:10.1371/journal.pgen.1004019
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004019Summary
Cells respond to accumulation of misfolded proteins in the endoplasmic reticulum (ER) by activating the unfolded protein response (UPR) signaling pathway. The UPR restores ER homeostasis by degrading misfolded proteins, inhibiting translation, and increasing expression of chaperones that enhance ER protein folding capacity. Although ER stress and protein aggregation have been implicated in aging, the role of UPR signaling in regulating lifespan remains unknown. Here we show that deletion of several UPR target genes significantly increases replicative lifespan in yeast. This extended lifespan depends on a functional ER stress sensor protein, Ire1p, and is associated with constitutive activation of upstream UPR signaling. We applied ribosome profiling coupled with next generation sequencing to quantitatively examine translational changes associated with increased UPR activity and identified a set of stress response factors up-regulated in the long-lived mutants. Besides known UPR targets, we uncovered up-regulation of components of the cell wall and genes involved in cell wall biogenesis that confer resistance to multiple stresses. These findings demonstrate that the UPR is an important determinant of lifespan that governs ER stress and identify a signaling network that couples stress resistance to longevity.
Introduction
Membrane and secretory proteins fold into their native conformations in the endoplasmic reticulum (ER) assisted by chaperones, thiol-disulfide oxidoreductases and other systems supporting protein post-translational control. Impairments in this complex process cause unfolded proteins to accumulate, provoking ER stress. Adaptation to ER stress is dependent on the unfolded protein response (UPR) signaling pathway that senses accumulation of unfolded proteins in the ER and restores ER homeostasis by (i) temporarily inhibiting protein synthesis, (ii) degrading misfolded or unassembled proteins, and (iii) increasing expression of chaperones and oxidative folding components that facilitate protein folding [1]. However, depending on the severity and timing of ER stress, it may also lead to cell death when adaptive mechanisms fail.
In mammalian cells, the UPR consists of multiple signaling cascades that are activated by three known ER stress sensor proteins, inositol-requiring protein 1 (IRE1), activating transcription factor 6 (ATF6), and double-stranded RNA-activated protein kinase-like ER kinase (PERK) [2]–[4]. Among these signal transducers, only IRE1 is conserved in budding yeast and is solely responsible for the UPR activation in Saccharomyces cerevisiae [5], [6]. Ire1p is an ER-localized transmembrane protein, containing kinase and endoribonuclease (endo-RNase) enzyme activities. Upon activation by ER stress, Ire1p undergoes oligomerization and autophosphorylation [7], [8]. In turn, Ire1p autophosphorylation activates its endo-RNAse domain, which facilitates the excision of an intron and unconventional splicing of HAC1 mRNA in yeast [9]. Spliced HAC1 mRNA codes for a functional transcription factor capable of inducing transcription of genes that enhance ER protein folding capacity and alleviating ER stress [10]. However, Ire1p may have other, Hac1p transcription factor-independent functions. In addition to up-regulation of the UPR target genes, metazoan IRE1 has been implicated in degradation of ER-localized mRNAs through its endonuclease activity [11]–[13]. Such ER-localized mRNA decay occurs during prolonged irremediable ER stress due to higher-order oligomerization and hyperactivation of IRE1 [12]. Thus, IRE1 may play a dual role in cell fate, by both allowing cellular adaptation to increased protein folding load and promoting apoptosis depending on the severity of ER stress.
ER stress and protein misfolding are increasingly recognized as contributing factors to the pathophysiology of age-related diseases and aging [14]. Moreover, studies involving model organisms demonstrate that improved ER stress resistance is often associated with increased lifespan and healthy aging [15]–[18]. However, the role of UPR signaling and individual components of ER stress response in regulating lifespan is not known. In this study, we investigated the contribution of the UPR and its downstream targets, including chaperones, oxidative folding components and components of the ER-associated degradation (ERAD), to aging and examined the mechanism of such regulation in a simple model organism, S. cerevisiae. We demonstrate that modulation of the UPR by genetic means can extend yeast lifespan, and that induction of UPR signaling is required for activation of multiple stress response pathways that drive lifespan extension.
Results
Components of the ER stress response pathway differentially modulate replicative lifespan in S. cerevisiae
Analysis of replicative lifespan, which is defined as the number of times each yeast cell divides before it undergoes senescence, is based on the ability of budding yeast to divide asymmetrically producing distinct mother and daughter cells and is often used as a model of aging in mitotically active cells [19]. To examine the relationship between ER stress response genes and aging, we measured replicative lifespan of mutant S. cerevisiae strains lacking individual components of the UPR and its transcriptional targets. In addition to IRE1 and HAC1, several downstream effector genes were analyzed including chaperones (KAR2), oxidative folding (ERO1, EUG1, MPD1, PDI1) and ER-associated degradation (ERAD) components (DER1, SEL1, HRD1), as well as genes involved in N-linked glycosylation (ALG3, ALG12, DIE2, OST3, OST6) and protein trafficking (BST1) [10], [20]. We found that deletion of either IRE1 or HAC1, two genes that are involved in sensing accumulation of unfolded proteins in the ER, did not affect yeast lifespan (Figure 1A, B). Unexpectedly, many of the downstream UPR target mutants, 9 out of 14, were found to be significantly long-lived compared to experiment-matched control wild-type cells (Figure 1C, D and Table 1). Hereafter, we refer to these mutants as “long-lived ER secretory pathway mutants” or “long-lived UPR target gene deletion mutants”. These data demonstrate that components of the ER stress response pathway may differentially modulate replicative lifespan and are important determinants of longevity in S. cerevisiae.
Fig. 1. ER stress response genes differentially modulate yeast replicative lifespan. 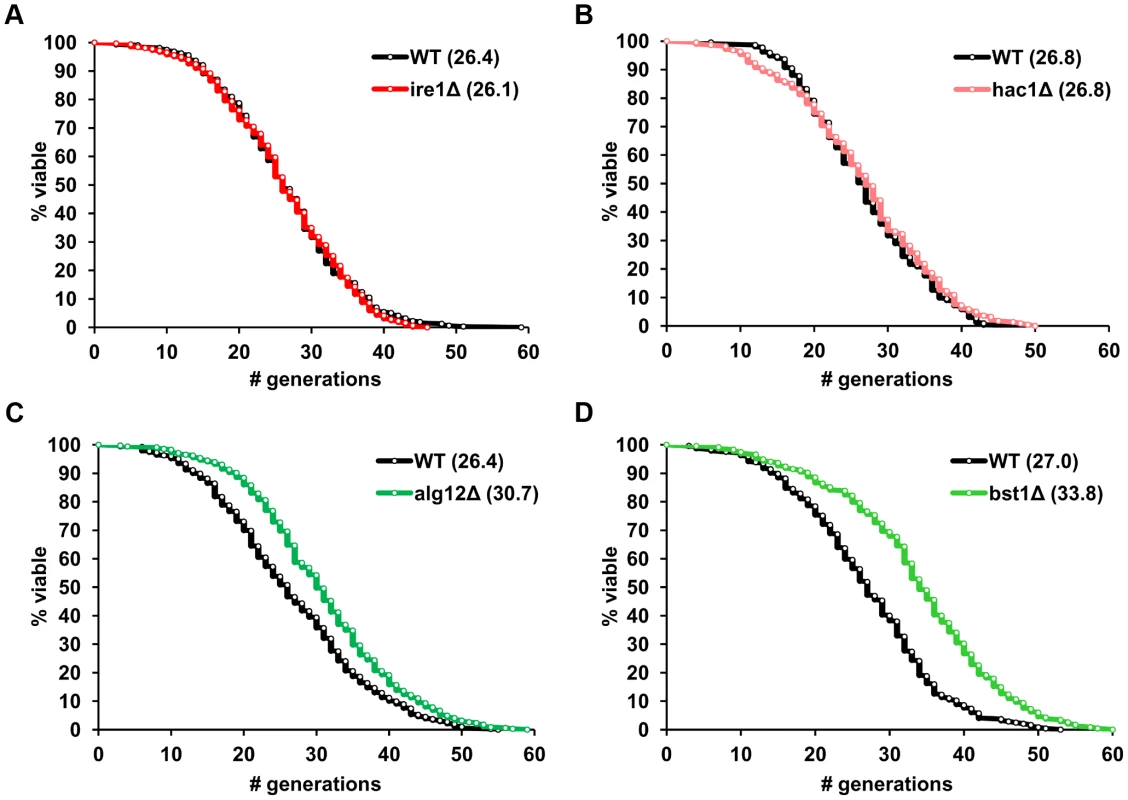
(A–D) Survival curves for ire1Δ, hac1Δ, alg12Δ and bst1Δ deletion strains. Replicative lifespan data for the strains from both the MATa and MATα ORF deletion collections are pooled, and experiment-matched wild-type cells are shown. Mean lifespans are shown in parentheses. Tab. 1. Regulation of lifespan by ER stress response mutants*. 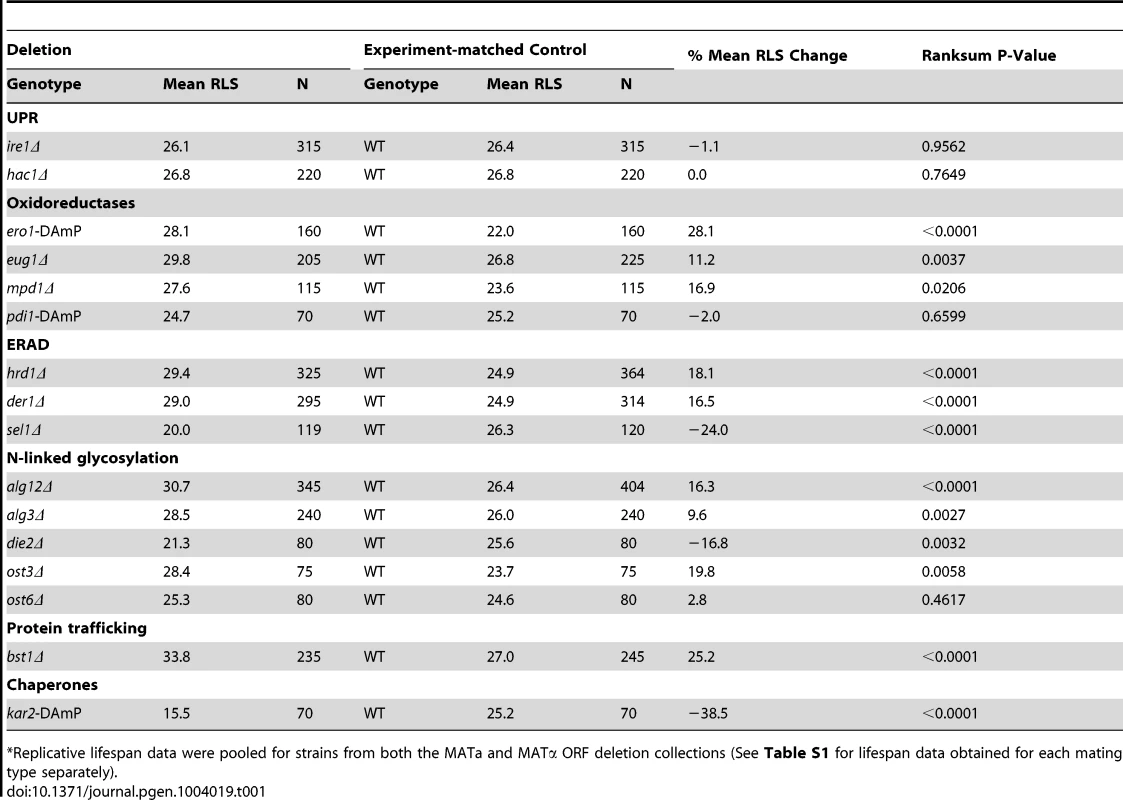
Replicative lifespan data were pooled for strains from both the MATa and MATα ORF deletion collections (See Table S1 for lifespan data obtained for each mating type separately). Lifespan extension in mutants lacking downstream components of the UPR is associated with elevated basal UPR activity
The observation that UPR target gene deletions extend lifespan was counterintuitive, as genes activated by UPR are perceived as protective factors required to restore ER homeostasis, and their deletion might be expected to decrease lifespan. The unexpected and consistent lifespan extension by UPR target gene inactivation may be attributable to hormesis, a phenomenon by which limited stress elicits response mechanisms that protect against similar but higher level stresses associated with aging. To study the molecular mechanisms by which reduced levels of UPR target genes lead to lifespan extension, we focused on two well-characterized genes ALG12 and BST1. Alg12p is an enzyme that catalyzes one of the steps in the synthesis of N-linked glycans [21], whereas Bst1p performs removal of the inositol acyl group required for the quality control of ER to Golgi transport of glycosylphosphatidylinositol-anchored proteins [22].
We hypothesized that deletion of genes downstream of the UPR may lead to constitutive activation of Ire1p and induction of UPR dependent cytoprotective pathways. To test this hypothesis, we analyzed whether the level of UPR activity may correlate with the lifespan in alg12Δ and bst1Δ. Analysis of HAC1 mRNA splicing was used to monitor the level of UPR activity in wild-type cells and corresponding mutants that were grown in the absence or presence of pharmacological ER stressor tunicamycin for 12 h (Figure 2A). In wild-type cells the basal level of UPR activity was very low, as evidenced by the fact that most of the detected HAC1 mRNA (99%) represented the unspliced form and only 1% corresponded to the spliced form. Treatment of wild-type cells with tunicamycin increased the fraction of spliced HAC1 mRNA to 31%. In contrast, deficiency of the UPR transcriptional targets, ALG12 and BST1, was associated with increased basal HAC1 mRNA splicing (7% and 21% of HAC1 mRNA was spliced for alg12Δ and bst1Δ, respectively). These data were also in good agreement with ribosome profiling data (see below), which showed different level of HAC1 translational activation in alg12Δ and bst1Δ mutants. In addition, we confirmed the level of UPR activation by analyzing the expression of Kar2p, an ER chaperone that is induced by UPR, and found increased Kar2p levels in the long-lived mutants, compared to the mutants that do not affect lifespan (Figure S1A). Taken together, our data indicate that lifespan extension conferred by deficiency of UPR components downstream of Ire1p, including ALG12 and BST1, is associated with increased basal UPR activity.
Fig. 2. Extended lifespan in alg12Δ and bst1Δ mutants is dependent on functional Ire1p and Hac1p and is associated with increased basal UPR activity. 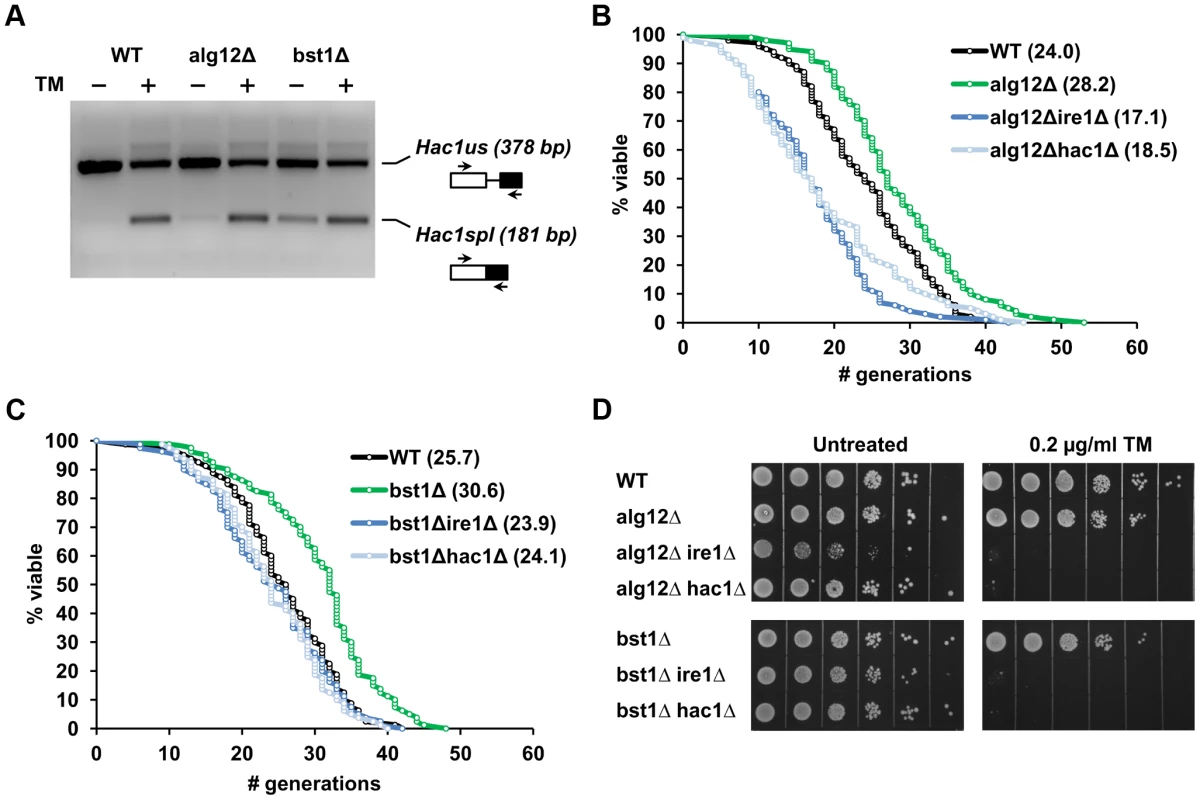
(A) Analysis of HAC1 mRNA splicing in wild-type, alg12Δ and bst1Δ cells treated with or without 1 µg/ml tunicamycin for 1 h. Sliced (spl) and unslpliced (us) HAC1 mRNA were detected by RT-PCR. The image was inverted to negative for better clarity. (B, C) Survival curves for alg12Δ and bst1Δ and the corresponding double mutant strains combining the long-lived gene deletion with either ire1Δ or hac1Δ. (D) Sensitivity of alg12Δ and bst1Δ and the corresponding double mutant strains to ER stress. For each strain 10× serial dilutions of logarithmically growing cells were spotted on agar plates without the drug (untreated) or plates containing 0.2 µg/ml tunicamycin (TM). Pictures were taken after 48 h incubation at 30°C. Deletion of IRE1 and HAC1 prevents lifespan extension in UPR target gene deletion mutants
To address whether lifespan extension in strains lacking UPR target genes is dependent on functional Ire1p (an ER stress sensor) and Hac1p (an ER stress-responsive transcription factor), we generated double mutant strains combining the long-lived alg12Δ and bst1Δ deletions with either ire1Δ or hac1Δ. If high basal UPR activity is required for increased longevity in the ER secretory pathway mutants, one would predict that deletion of either IRE1 or HAC1 should attenuate lifespan extension in these mutants. Consistent with this hypothesis, we observed decreased lifespan in alg12Δire1Δ and alg12Δhac1Δ double mutants compared to alg12Δ (p<0.0001) (Figure 2B and Table S2). Moreover, both double mutants were significantly shorter-lived than the wild-type strain (p<0.0001). Since a single deletion of either IRE1 or HAC1 did not affect yeast replicative lifespan under unstressed conditions (Figure 1), these data suggest an adverse genetic interaction of ALG12 deletion with that of IRE1 or HAC1. We also found that lifespan extension conferred by bst1Δ deletion was significantly reduced in bst1Δire1Δ and bst1Δhac1Δ cells (p<0.0001), and that the corresponding double mutants had lifespan similar to that of wild-type cells (Figure 2C). In contrast, deletion of IRE1 and HAC1 did not significantly change the lifespan of the long-lived strain overexpressing Sir2 (SIR2OE) as well as fob1Δ and tor1Δ deletion mutants (Figure S1B, C, and Table S2). Therefore, these genetic epistasis experiments demonstrate that lifespan extension in the long-lived UPR target gene mutants is dependent on functional Ire1p and the ability to activate ER stress response. Moreover, deletion of UPR target genes extends lifespan by mechanisms distinct from those responsible for the lifespan extension observed under conditions of increased Sir2 activity or reduced mTOR signaling, a genetic mimic of dietary restriction [23].
Elevated basal UPR activity in mutants lacking downstream components of the UPR does not confer resistance to pharmacologically induced ER stress
It is possible that constitutive activation of UPR signaling in the long-lived ER secretory pathway mutants may lead to increased resistance to pharmacologically induced ER stress. To test whether elevated basal UPR signaling may pre-condition cells against stress and increase cellular stress resistance, alg12Δ and bst1Δ strains were analyzed for growth in the presence of tunicamycin. However, both alg12Δ and bst1Δ had decreased resistance to this pharmacological ER stressor (Figure 2D) suggesting that ALG12 and BST1 deficiency puts cells at a disadvantage in the presence of ER stress. Moreover, the double mutant strains combining the long-lived deletions with either ire1Δ or hac1Δ completely abolished the growth of cells in the presence of tunicamycin, similar to ire1Δ and hac1Δ single mutants (Figure S1D). Together, these data indicate that Ire1p and Hac1p are required for lifespan extension in alg12Δ and bst1Δ, but the long lifespan in these mutants cannot be explained solely by increased ER stress resistance, at least as measured by tunicamycin resistance.
Ribosome profiling detects translational control by UPR signaling
To characterize the mechanisms of lifespan extension in the long-lived ER secretory pathway mutants, we examined genome-wide translational changes in response to Ire1p hyperactivation in alg12Δ and bst1Δ mutants using ribosome profiling. Ribosome profiling is based on deep sequencing of ribosome-protected mRNA fragments and provides quantitative data on the translation level of thousands of genes [24]. A key advantage of this method is the much greater sensitivity than that obtained with microarrays as mRNA abundance is not always a good predictor of protein synthesis. When coupled with mRNA-sequencing (RNA-seq), ribosome profiling data can also be used to measure translational regulation by monitoring translation efficiency (TE).
We hypothesized that deletion of ALG12 and BST1 leads to activation of Ire1p and induction of cytoprotective pathways. To test if genes induced by ALG12 and BST1 deficiency are translationally regulated by the UPR, we first defined the list of UPR activated genes by measuring changes in mRNA abundance and protein translation in wild-type cells treated with tunicamycin. In S. cerevisiae, the UPR has been shown to transcriptionally activate ∼380 genes [10]. Many of these genes encode proteins that are components of secretory pathway organelles and are involved in translocation, protein folding, glycosylation, vesicular trafficking, and ERAD. We found that, following 30 min treatment with tunicamycin, translational changes were observed for 170 genes (changed more than 1.5-fold), of which 63 were down-regulated and 107 were up-regulated (Table S3). Genes up-regulated by tunicamycin treatment demonstrated a limited overlap with the genes whose expression was induced by the UPR as shown by microarray analysis [10] (Figure S2A and Table S4). As expected, many of the genes were regulated at the level of transcription, but our analysis also revealed a set of genes for which the scope of translational activation by the UPR was much greater compared to transcriptional induction (Figure S2B). Moreover, measuring translation rates allowed us to examine the relative contribution of translational regulation to both up-regulated and down-regulated changes. At the level of mRNA abundance, there were significantly fewer down-regulated genes (out of 241 genes that changed expression, 220 genes were induced and 21 were repressed) than genes repressed at the translational level (63 genes). The fact that there were more genes whose expression was reduced at the translational level indicates that UPR largely induces genes at the level of transcription, whereas UPR repressed genes are mostly regulated at the level of protein translation.
Deletion of ALG12 and BST1 leads to transcriptional and translational induction of multiple stress response genes
We next used ribosome profiling to detect translational changes in the long-lived alg12Δ and bst1Δ mutants and found enhanced expression of UPR target genes, which correlated with increased HAC1 mRNA splicing and production of Hac1p. We observed ∼3 and 12-fold increase in Hac1p production in alg12Δ and bst1Δ, respectively (Figure 3A, B). In the case of the alg12Δ mutant, more than 1.5-fold increase in protein production was observed for 34 genes, whereas 16 genes were down-regulated (Table S5). Compared to alg12Δ, BST1 deficiency resulted in a much stronger translational regulation. In the bst1Δ mutant, translational changes were observed for 373 genes (52 genes were repressed and 321 genes were induced) (Table S6). As expected, there was a significant overlap with the genes that were up-regulated by tunicamycin treatment (Figure S3). Known UPR targets, including chaperones (KAR2, LHS1, JEM1, SCJ1), oxidoreductases (ERO1, MPD1, EUG1, PDI1) and genes involved in glycosylation (PMT3) and ERAD (ADD37 and HRD1) were among the top hits (Figure 3C and Table S5 and S6). In addition, genes involved in many other ER secretory pathway processes were induced including glycophospatidylinositol anchor synthesis (ERI1, MCD4, GWT1), lipid biogenesis (INO4, SCS3), and vesicular trafficking (MVB12, ERV29). Although many more of the UPR target genes were induced in the bst1Δ mutant compared to alg12Δ, the lower extent of induction in alg12Δ mutant cells can be explained by the lower level of ER stress and reduced HAC1 splicing.
Fig. 3. Deletion of ALG12 and BST1 induce UPR signaling and translation of UPR target genes. 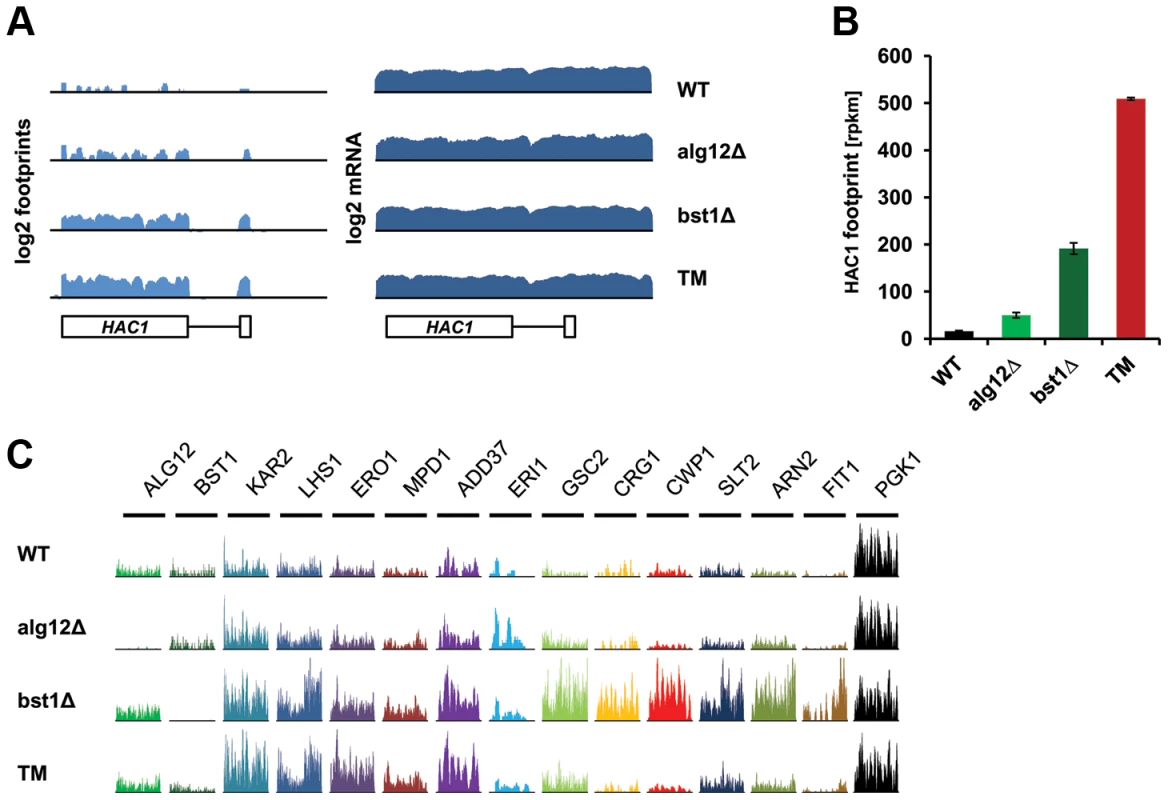
(A) Log2 footprints and mRNA (rpkm) for a region containing HAC1 in untreated wild-type, alg12Δ, bst1Δ, and wild-type cells treated with tunicamycin (TM). (B) Footprint counts (rpkm) for HAC1 in untreated wild-type, alg12Δ, bst1Δ, and wild-type cells treated with tunicamycin (TM). Error bars indicate SEM. Measurements from biological replicates are shown. (C) Ribosome footprint coverage for UPR target genes. The scales of the Y axis, which shows the number of footprint reads, are independent by gene. In addition to genes associated with secretory pathway function, both of the long-lived mutants showed enrichment in genes with functions in mRNA splicing and degradation (CWC21, CWC25, DCS1), iron homeostasis (ARN2, FIT1, FIT3, FTH1, HMX1, TIS11/CTH2), mitochondrial protein quality control and sorting (MGR1, MSP1), as well as multiple stress response pathways (DDR2, HOR7, HLR1, LOT6, TSL1, DOG2, ICT1, SED1, CRG1) [25]–[33].
Similar to the tunicamycin treated cells, the number of genes whose translation was increased in alg12Δ and bst1Δ mutants exceeded the number of down-regulated genes (Figure 4A). To analyze if any of the observed differences can be explained by translational control, we calculated TE for each mRNA, which represents the relative number of footprints normalized to mRNA abundance. A significantly larger fraction of genes whose TE changed more than 1.5-fold had a decreased TE rather than increased (Figure 4B). These data are consistent with the overall down-regulation of protein synthesis during ER stress. However, several genes showed translational activation in alg12Δ and bst1Δ mutants as well as in tunicamycin treated cells. Among these genes, HAC1 and ERI1 were strongly regulated at the level of translation, but were not up-regulated transcriptionally.
Fig. 4. Translational control in the long-lived ER secretory pathway mutants. 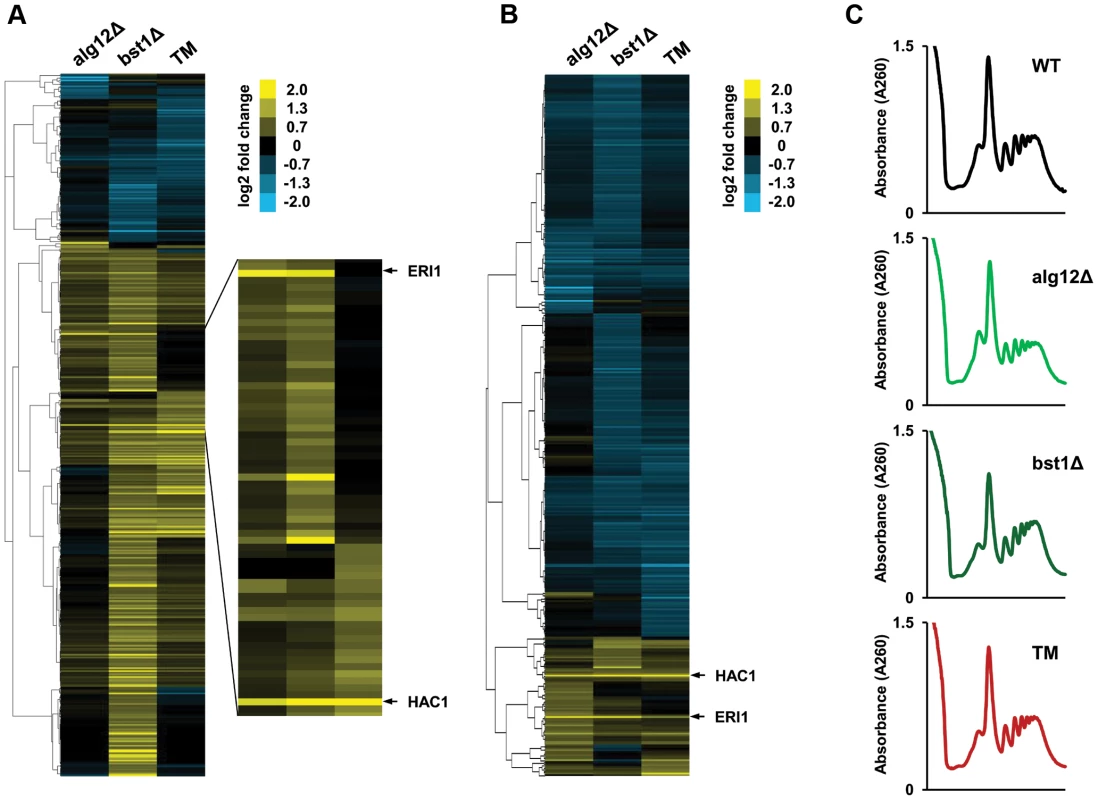
(A) Changes in protein translation in alg12Δ, bst1Δ, and wild-type cells treated with tunicamycin (TM) relative to untreated wild-type cells are shown in log2 scale for all genes that are activated or repressed more than 1.5-fold in at least one of the strains. (B) Cluster analysis of log2 TE changes in alg12Δ, bst1Δ, and TM treated cells relative to untreated wild-type cells. Changes in log2 TE are shown for all genes that showed more than 1.5-fold decrease or increase in TE. (C) Polysome profiles of alg12Δ, bst1Δ, and TM treated cells. Long-lived deletion strains alg12Δ and bst1Δ do not show overall translational suppression. Induction of the cell wall integrity signaling in alg12Δ and bst1Δ
Gene ontology analyses (DAVID) [34] of footprint data revealed that a number of genes up-regulated in the long-lived mutants are involved in cellular response to stress (Figure 5A). A second cluster of genes that were expressed at higher level in alg12Δ and bst1Δ mutants comprises of cell wall components and genes involved in cell wall biogenesis. The induction of cell wall components in the long-lived strains was particularly appealing, as it suggested a link between the UPR, stress resistance and increased longevity. Many of the genes that code for proteins of the cell wall have been implicated in resistance to multiple stressors, and are known to be regulated by the cell wall integrity (CWI) pathway. The CWI pathway responds to cell wall stress through several cell-surface sensors (Wsc1p, Wsc2p, Wsc3p, Mid2p and Mtl1p) [35] that activate a small G protein, Rho1p. Activation of Rho1p triggers a MAPK signaling cascade leading to transcriptional up-regulation of CWI target genes through two transcription factors Rlm1p and SBF (Swi4p/Swi6p). Among other targets, CWI regulates synthesis of β-glucan and biogenesis of cell wall components. Strikingly, among genes that were up-regulated in the long-lived mutants were MID2 stress sensor, SLT2/MPK1 MAPK kinase, and RLM1 transcription factor. We also observed increased expression of Rlm1p transcription factor targets in bst1Δ mutant including genes involved in cell wall biogenesis (β-glucan synthases GSC2/FKS2 and FKS1, chitin synthase CHS3) and multiple cell wall components (BGL2, CIS3, CWP1, CWP2, CRH1, SED1 YLR194C) [36]. To test if the CWI pathway is important for the lifespan extension in alg12Δ and bst1Δ mutants, we tested sensitivity of these strains to calcofluor white and congo red, which are known pharmacological inducers of the cell wall stress. Consistent with activation of the CWI signaling, we observed increased resistance of both alg12Δ and bst1Δ to cell wall stress compared to wild-type strain (Figure 5B). Moreover, induction of the CWI signaling in the long-lived ER secretory pathway mutants was associated with increased resistance to other stresses including heat shock and oxidative stress (Figure S4), providing additional evidence that deletion of ALG12 and BST1 confers multiple stress resistance.
Fig. 5. Deletion of ALG12 and BST1 leads to activation of the CWI signaling pathway. 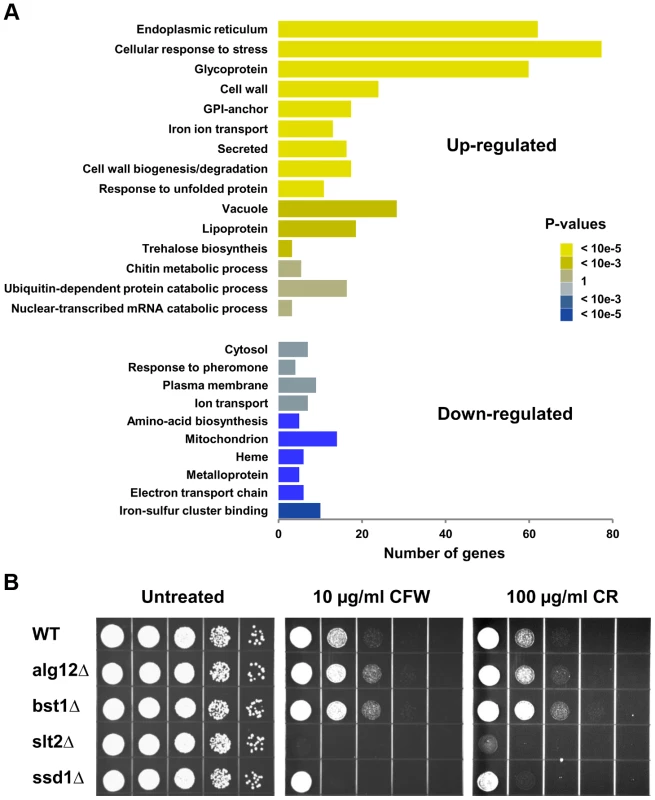
(A) Gene Ontology analysis of differentially regulated genes in bst1Δ. X-axis shows the number of genes in each functional category. (B) Sensitivity of alg12Δ and bst1Δ mutant strains to cell wall stress caused by calcofluor white (CFW) and congo red (CR). For each strain, 10× serial dilutions of logarithmically growing cells were spotted on agar plates containing indicated concentrations of the drugs. Pictures were taken after 48 h incubation at 30°C. We also found strong induction of genes involved in trehalose (TSL1, TPS1, TPS2, NTH1) and chitin (CHS1, CHS7, CRH1, GFA1) synthesis. Increased trehalose and chitin accumulation is a common cell defense strategy that protects cells against a variety of stressful conditions, including heat, acid and cold shock. Another potential target up-regulated in bst1Δ that may mediate lifespan extension is glycerol-3-phosphate dehydrogenase GPD1. Gpd1 catalyzes the production and accumulation of glycerol in response to hyperosmotic stress and acts as an osmoregulator by preventing loss of water and turgor of the cells. Induction of the osmotic stress response and increased glycerol production have been shown to extend yeast replicative lifespan, whereas deletion of GPD1 shortens lifespan even in the absence of osmotic stress [37]. In addition, up-regulation of glycerol biosynthesis genes has been linked to extension of chronological lifespan in Tor1 - and Sch9-deficient mutants [38].
Decreased protein translation has been shown to extend lifespan in a wide range of species, including S. cerevisiae, Caenorhabditis elegans, and Drosophila melanogaster [39], [40]. For example, increased longevity caused by Tor1p inhibition or knockout of Tor1-regulated SCH9 kinase is achieved, at least in part, by reduction in mRNA translation. In addition, decreased protein synthesis caused by deficiency of ribosomal protein subunits often leads to ER stress resistance and increased lifespan [15]. However, overall protein translation was not affected in alg12Δ and bst1Δ mutants (Figure 4C). We also did not observe changes in the expression of antioxidant genes or components of proteasome suggesting that elevated proteasomal capacity and oxidative stress response do not contribute to longevity in alg12Δ and bst1Δ mutants. In addition, we did not observe induction of other stress response transcription factors, including YAP1, SKN7, MSN2 and MSN4.
Taken together, our data demonstrate that lifespan extension conferred by the ER secretory pathway mutants is dependent on functional UPR, and that increased basal UPR signaling may promote longevity in S. cerevisiae through increased expression of multiple stress response genes and activation of the CWI-MAPK pathway.
Discussion
It is commonly accepted that aging is associated with a decline in homeostatic mechanisms that protect organisms from accumulation of senescence factors including aggregated proteins, oxidatively damaged cellular components and toxic metabolites [41]–[43]. Recent studies suggest that cellular capacity to adapt to ER stress may also decline with age [44]. Cells respond to accumulation of misfolded proteins in the ER by activating the UPR signaling pathway that restores ER homeostasis by degrading misfolded proteins, inhibiting translation, and increasing expression of chaperones and oxidative folding components [1]. Although the mechanisms by which cells sense ER stress and activate stress response genes are well studied [45], [46], the role of UPR signaling in aging remains unknown. We have begun to characterize the role of UPR in regulating lifespan in S. cerevisiae. To our surprise, we determined that inactivation of IRE1 and HAC1 that are involved in sensing ER stress in yeast does not affect lifespan under physiological conditions. However, from the analysis of 14 different UPR target gene deletions, at least 9 were found to be significantly long-lived. In addition, we found that extended lifespan in the UPR target gene deletion mutants is associated with increased basal UPR activity. These observations prompted us to hypothesize that deletion of genes downstream of UPR may lead to constitutive activation of Ire1p and increased ER stress resistance. Our data provide evidence that functional Ire1p and transcriptional factor Hac1p are required for lifespan extension by deletion of UPR target genes.
Despite elevated basal UPR activity in alg12Δ and bst1Δ, these strains were not resistant to pharmacologically induced ER stress conferred by tunicamycin. This provides an interesting contrast to another recent study whereby it was found that many ribosomal deletion mutants were resistant to tunicamycin through a HAC1-independent mechanism [15]. In that case, however, tunicamycin resistance did not correlate with lifespan extension. From comparing these studies, it is clear that the long lifespan of alg12Δ and bst1Δ does not come from mitigating ER stress, at least phenocopied by tunicamycin exposure, and that induction of the UPR and associated stress response pathways is more likely to modulate aging through separate mechanisms.
We used ribosome profiling to identify specific pathways and protective mechanisms that contribute to lifespan extension in the long-lived ER secretory pathway mutants at the genome-wide level. Using this method, we identified translational changes in the long-lived mutants alg12Δ and bst1Δ compared to wild-type cells in unstressed conditions. We discovered that ALG12 and BST1 deficiency selectively regulates a subset of genes that belong to only a few functional groups. In addition to activation of UPR target genes, we observed induction of other cytoprotective pathways including general stress response proteins and proteins involved in multidrug resistance. The second most prominent change that occurs in the long-lived ER secretory pathway mutants is cell wall remodeling. Although multiple signaling pathways contribute to remodeling of the cell wall, the regulation of this process is controlled primarily by the mitogen-activated protein kinase (MAPK) Slt2p/Mpk1p via the CWI pathway. Our analysis revealed extensive up-regulation of components of CWI signaling including Slt2p/Mpk1p, Mid2p cell wall stress sensor and Rlm1p transcription factor. Activation of the CWI pathway leads to an increased synthesis of β-glucan and enhanced expression of Rlm1p targets that confer resistance to multiple stresses. Genes up-regulated by CWI signaling have been implicated in the tolerance of S. cerevisiae to a variety of stressors including oxidative stress, heat shock, hypo-osmotic stress, actin depolymerization, high and low pH stress and DNA damage [35]. In addition to the CWI signaling cascade, two Slt2p-independent pathways, which require Mpt5p and Ssd1p, have been shown to regulate integrity of the cell wall and promote longevity in S. cerevisiae [47]. Interestingly, MPT5 and SSD1 encode RNA-binding proteins that have been proposed to post-transcriptionally up-regulate expression of genes involved in cell wall biogenesis by increasing TE and stability of the target mRNAs. We found significant up-regulation of Ssd1 in at least one of the long-lived ER secretory pathway mutants analyzed in our study (bst1Δ). Therefore, we conclude that the lifespan extension in the ER secretory pathway mutants does not result solely from improved protein homeostasis caused by UPR activation, but might also require activation of multiple stress response pathways.
In support of this mechanism, we identified four components of the chitin biosynthesis (CHS1, CHS7, CRH1, GFA1) and four genes involved in the synthesis of trehalose (TSL1, TPS1, TPS2, NTH1). Chitin, β(1,4)-linked N-acetylglucosamin polymer, serves as a structural component of the cell wall and represents about 1–2% of its inner layer polymers. However, during stress, cell wall chitin levels can increase up to 20% [48] making cell tolerant to adverse environmental conditions. In turn, trehalose (α,α-glucose disaccharide) has been implicated in heat shock resistance. In response to thermal stress, accumulation of cytoplasmic trehalose leads to increased osmolarity that protects proteins from denaturation and aggregation [49].
Another possible means by which deletion of UPR transcriptional targets could increase replicative lifespan is by enhancing mitochondrial protein turnover. Two other genes that were up-regulated in bst1Δ mutant include MGR1 and MSP1. MGR1 encodes a component of the mitochondrial inner-membrane iAAA protease complex that functions to degrade misfolded mitochondrial proteins and participates in protein quality control in mitochondria [50], whereas Msp1p is involved in mitochondrial protein sorting [51]. Thus, activation of mitochondrial protein turnover upon ER stress caused by deletion of UPR components may increase longevity in part by influencing mitochondrial function.
Activation of the UPR signaling in the UPR target gene deletion mutants is consistent with previous reports showing hyperactivation of UPR in cells lacking genes involved in the ER secretory pathway function. In yeast, deletion of several components of the ERAD system (DER1, SEL1, HRD1, UBC1 and UBC7) [10], [52] as well as inactivation of the ER chaperone Kar2p [53] have been shown to dramatically induce UPR. More recently, hundreds of single-gene knockouts have been shown to perturb UPR signaling in yeast representing a number of diverse functional groups [20]. It would be important to determine in future studies if these deletion mutants show significant overlap with those affecting longevity and the requirement for increased UPR function in these settings.
Although stress resistance correlates with increased longevity in a variety of model organisms, including yeast [15], [18], worms [54], [55] and fruit flies, the link of UPR signaling pathway, ER stress resistance and longevity remains poorly understood. Interestingly, inactivation of IRE-1 and XBP-1 results in shortened lifespan in C. elegans, and UPR signaling contributes to the increased longevity of daf-2 mutants and in response to dietary restriction [56], [57]. Moreover, overexpression of a constitutively active form of XBP-1 in neurons, but not in other tissues, results in increased ER stress resistance and extends lifespan in worms [58]. However, ubiquitous up-regulation of UPR signaling in the whole animal does not promote longevity despite elevated resistance to ER stressors.
Our data suggest that while increased UPR signaling is an important determinant of lifespan extension, it is not sufficient to confer enhanced ER stress resistance in yeast cells. Instead, we found that the increased longevity in the UPR target gene mutants is associated with induction of multiple stress response programs. Taken together, these data highlight the complexity of organism's response to various stresses and demonstrate interdependencies among multiple longevity pathways.
Materials and Methods
Yeast strains and media
All yeast strains were derived from the parent strains of the haploid yeast ORF knockout collection [59], BY4741 (MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0) and BY4742 (MATα his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0), or the DAmP library [60] (Table S7). Double mutant strains combining the long-lived deletions with either ire1Δ or hac1Δ were prepared by standard PCR-based gene disruption method. The deletion of each ORF was confirmed by PCR with locus-specific primers (Figure S5). All strains were grown at 30°C in complete YPD medium (1.0% yeast extract, 2.0% peptone, and 2.0% glucose).
Replicative lifespan assay
Lifespan assays were carried out as described previously [61]. Analysis of replicative lifespan is based on the ability of budding yeast to divide asymmetrically producing distinct mother and daughter cells. For the replicative lifespan assay, cells were grown on freshly prepared YPD plates for 2 days at 30°C. For each strain, founder cells were plated on agar plates by selecting the newborn daughter cells using micromanipulator. Cells were monitored for cell divisions every 90 min, and subsequent budded daughter cells were separated and removed as they formed. The process continued until cells stopped dividing. Replicative lifespan was calculated as the number of times each mother cell divided before it underwent senescence. Statistical analysis of the lifespan data was performed using a Wilcoxon Rank-Sum test.
HAC1 mRNA splicing
Total RNA was extracted by RiboPure-Yeast Kit (Ambion) according to the manufacturer's instructions. RNA was treated with DNaseI, and first strand cDNA was synthesized using the SuperScript III reverse transcriptase (Invitrogen) with random hexamer primers. For the analysis of HAC1 mRNA slicing, RT-PCR was performed with the following primers that flank the HAC1 intron: 5′-CCGTAGACAACAACAATTTG-3′ and 5′-CATGAAGTGATGAAGAAATC-3′. PCR fragments were resolved on 2% agarose gels, stained with EtBr, and quantified by densitometry.
Spot assays
Resistance of strains to tunicamycin, calcofluor white, and congo red was determined using spot assays. Cells were initially grown in liquid culture without the drugs until OD600 = 0.6, and 10× serial dilutions for each strain were spotted on agar plates containing indicated concentrations of the drugs. The plates were incubated at 30°C, and images were taken 48 h after plating.
Ribosome profiling and mRNA sequencing
Yeast cultures were grown to OD600 = 0.5 in 500 ml of complete YPD medium, and cells were collected by filtering through 0.45 µm filter (Millipore) with glass holder. Pellets were scraped with spatula, flash frozen in liquid nitrogen and stored at −80°C. To pharmacologically induce ER stress, tunicamycin was added into the medium at a final concentration 1 µg/ml, and cells were incubated at 30°C for an additional 30 min. Yeast extracts were prepared by cryogrinding the cell paste with BioSpec cryomill. Aliquots of cell lysates were used for footprint extraction and isolation of total RNA. Preparation of lysates, ribosome fractionation, and construction of footprint and RNA-seq libraries were performed as in [62] with modifications. A detailed description of protocols can be found in the Text S1. Sequencing of footprint and RNA-seq libraries was performed on the Illumina HiSeq2000 platform. Primers used in library preparation are listed in Table S8.
Bioinformatics analyses
Ribosomal footprints and mRNA reads were aligned to the S. cerevisiae genome from the Saccharomyces Genome Database (SGD, http://www.yeastgenome.org/, release number R64-1-1). Sequence alignment was performed by Bowtie software v.0.12.7 [63] allowing two mismatches per read. Custom Perl scripts were used to count reads over features of interests (genes, UTRs etc.), deal with introns, overlaps and highly homologous sequences.
Analyses of differential gene translation and translation efficiency
To analyze differential gene expression and translation we disregarded 100 nt from the 5′-end of each gene therefore avoiding bias caused by the region with elevated footprint density in the vicinity of the ATG start codon. Rpkm (reads per kilobase per million mapped reads) values, which represent the number of reads normalized to gene length and total number of reads, were used as a measure of gene expression. An average rpkm value for two biological replicates was calculated for each gene, and the genes with fewer than 10 rpkm were excluded from further analysis (Figure S6). The gene was considered regulated if its rpkm value changed more than 1.5-fold (0.6 in log2 scale). To calculate TE, footprint rpkm values were divided by mRNA rpkm. Clustering was performed using Cluster 3.0 software [64] and the data were visualized using Java Treeview [65].
Polysome analyses
Polysome profile analysis of an aliquot of cell extracts was performed using sucrose gradients (10–50% wt/wt) in polysome gradient buffer [20 mM TrisHCl (pH 8.0), 140 mM KCl, 5 mM MgCl2, 0.2 g/l cycloheximide, 0.5 mM DTT]. 1 ml of cell lysate containing 50 units (OD260) were loaded on top of the gradients, and sedimented at 35,000 rpm at 4°C in a SW41 Ti rotor (Beckman) for 3 h. Gradients were collected from the top using the Brandel gradient fractionation system and profiles were monitored at 254 nm.
Supporting Information
Zdroje
1. RonD, WalterP (2007) Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol 8 : 519–529.
2. McCrackenAA, BrodskyJL (2003) Evolving questions and paradigm shifts in endoplasmic-reticulum-associated degradation (ERAD). Bioessays 25 : 868–877.
3. HardingHP, ZhangY, BertolottiA, ZengH, RonD (2000) Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol Cell 5 : 897–904.
4. SidrauskiC, WalterP (1997) The transmembrane kinase Ire1p is a site-specific endonuclease that initiates mRNA splicing in the unfolded protein response. Cell 90 : 1031–1039.
5. CoxJS, ShamuCE, WalterP (1993) Transcriptional induction of genes encoding endoplasmic reticulum resident proteins requires a transmembrane protein kinase. Cell 73 : 1197–1206.
6. MoriK, MaW, GethingMJ, SambrookJ (1993) A transmembrane protein with a cdc2+/CDC28-related kinase activity is required for signaling from the ER to the nucleus. Cell 74 : 743–756.
7. AragonT, van AnkenE, PincusD, SerafimovaIM, KorennykhAV, et al. (2009) Messenger RNA targeting to endoplasmic reticulum stress signalling sites. Nature 457 : 736–740.
8. ShamuCE, WalterP (1996) Oligomerization and phosphorylation of the Ire1p kinase during intracellular signaling from the endoplasmic reticulum to the nucleus. Embo J 15 : 3028–3039.
9. LeeKP, DeyM, NeculaiD, CaoC, DeverTE, et al. (2008) Structure of the dual enzyme Ire1 reveals the basis for catalysis and regulation in nonconventional RNA splicing. Cell 132 : 89–100.
10. TraversKJ, PatilCK, WodickaL, LockhartDJ, WeissmanJS, et al. (2000) Functional and genomic analyses reveal an essential coordination between the unfolded protein response and ER-associated degradation. Cell 101 : 249–258.
11. LeeAH, HeidtmanK, HotamisligilGS, GlimcherLH (2011) Dual and opposing roles of the unfolded protein response regulated by IRE1alpha and XBP1 in proinsulin processing and insulin secretion. Proc Natl Acad Sci U S A 108 : 8885–8890.
12. HanD, LernerAG, Vande WalleL, UptonJP, XuW, et al. (2009) IRE1alpha kinase activation modes control alternate endoribonuclease outputs to determine divergent cell fates. Cell 138 : 562–575.
13. HollienJ, WeissmanJS (2006) Decay of endoplasmic reticulum-localized mRNAs during the unfolded protein response. Science 313 : 104–107.
14. SalminenA, KaarnirantaK (2010) ER stress and hormetic regulation of the aging process. Ageing Res Rev 9 : 211–217.
15. SteffenKK, McCormickMA, PhamKM, MacKayVL, DelaneyJR, et al. (2012) Ribosome deficiency protects against ER stress in Saccharomyces cerevisiae. Genetics 191 : 107–118.
16. JohnsonTE, HendersonS, MurakamiS, de CastroE, de CastroSH, et al. (2002) Longevity genes in the nematode Caenorhabditis elegans also mediate increased resistance to stress and prevent disease. J Inherit Metab Dis 25 : 197–206.
17. RionS, KaweckiTJ (2007) Evolutionary biology of starvation resistance: what we have learned from Drosophila. J Evol Biol 20 : 1655–1664.
18. DelaneyJR, AhmedU, ChouA, SimS, CarrD, et al. (2012) Stress profiling of longevity mutants identifies Afg3 as a mitochondrial determinant of cytoplasmic mRNA translation and aging. Aging Cell 12 : 156–166.
19. KaeberleinM (2010) Lessons on longevity from budding yeast. Nature 464 : 513–519.
20. JonikasMC, CollinsSR, DenicV, OhE, QuanEM, et al. (2009) Comprehensive characterization of genes required for protein folding in the endoplasmic reticulum. Science 323 : 1693–1697.
21. BurdaP, JakobCA, BeinhauerJ, HegemannJH, AebiM (1999) Ordered assembly of the asymmetrically branched lipid-linked oligosaccharide in the endoplasmic reticulum is ensured by the substrate specificity of the individual glycosyltransferases. Glycobiology 9 : 617–625.
22. TanakaS, MaedaY, TashimaY, KinoshitaT (2004) Inositol deacylation of glycosylphosphatidylinositol-anchored proteins is mediated by mammalian PGAP1 and yeast Bst1p. J Biol Chem 279 : 14256–14263.
23. KaeberleinM, PowersRW3rd, SteffenKK, WestmanEA, HuD, et al. (2005) Regulation of yeast replicative life span by TOR and Sch9 in response to nutrients. Science 310 : 1193–1196.
24. IngoliaNT, GhaemmaghamiS, NewmanJR, WeissmanJS (2009) Genome-wide analysis in vivo of translation with nucleotide resolution using ribosome profiling. Science 324 : 218–223.
25. GhoshAK, RamakrishnanG, RajasekharanR (2008) YLR099C (ICT1) encodes a soluble Acyl-CoA-dependent lysophosphatidic acid acyltransferase responsible for enhanced phospholipid synthesis on organic solvent stress in Saccharomyces cerevisiae. J Biol Chem 283 : 9768–9775.
26. HirayamaT, MaedaT, SaitoH, ShinozakiK (1995) Cloning and characterization of seven cDNAs for hyperosmolarity-responsive (HOR) genes of Saccharomyces cerevisiae. Mol Gen Genet 249 : 127–138.
27. Martinez-PastorMT, MarchlerG, SchullerC, Marchler-BauerA, RuisH, et al. (1996) The Saccharomyces cerevisiae zinc finger proteins Msn2p and Msn4p are required for transcriptional induction through the stress response element (STRE). Embo J 15 : 2227–2235.
28. PereiraMD, EleutherioEC, PanekAD (2001) Acquisition of tolerance against oxidative damage in Saccharomyces cerevisiae. BMC Microbiol 1 : 11.
29. TsujimotoY, IzawaS, InoueY (2000) Cooperative regulation of DOG2, encoding 2-deoxyglucose-6-phosphate phosphatase, by Snf1 kinase and the high-osmolarity glycerol-mitogen-activated protein kinase cascade in stress responses of Saccharomyces cerevisiae. J Bacteriol 182 : 5121–5126.
30. SollnerS, NebauerR, EhammerH, PremA, DellerS, et al. (2007) Lot6p from Saccharomyces cerevisiae is a FMN-dependent reductase with a potential role in quinone detoxification. Febs J 274 : 1328–1339.
31. ShimoiH, KitagakiH, OhmoriH, IimuraY, ItoK (1998) Sed1p is a major cell wall protein of Saccharomyces cerevisiae in the stationary phase and is involved in lytic enzyme resistance. J Bacteriol 180 : 3381–3387.
32. LissinaE, YoungB, UrbanusML, GuanXL, LowensonJ, et al. (2011) A systems biology approach reveals the role of a novel methyltransferase in response to chemical stress and lipid homeostasis. PLoS Genet 7: e1002332.
33. VerseleM, TheveleinJM (2001) Lre1 affects chitinase expression, trehalose accumulation and heat resistance through inhibition of the Cbk1 protein kinase in Saccharomyces cerevisiae. Mol Microbiol 41 : 1311–1326.
34. Huang daW, ShermanBT, LempickiRA (2009) Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4 : 44–57.
35. LevinDE (2011) Regulation of cell wall biogenesis in Saccharomyces cerevisiae: the cell wall integrity signaling pathway. Genetics 189 : 1145–1175.
36. JungUS, LevinDE (1999) Genome-wide analysis of gene expression regulated by the yeast cell wall integrity signalling pathway. Mol Microbiol 34 : 1049–1057.
37. KaeberleinM, AndalisAA, FinkGR, GuarenteL (2002) High osmolarity extends life span in Saccharomyces cerevisiae by a mechanism related to calorie restriction. Mol Cell Biol 22 : 8056–8066.
38. WeiM, FabrizioP, MadiaF, HuJ, GeH, et al. (2009) Tor1/Sch9-regulated carbon source substitution is as effective as calorie restriction in life span extension. PLoS Genet 5: e1000467.
39. McCormickMA, TsaiSY, KennedyBK (2011) TOR and ageing: a complex pathway for a complex process. Philos Trans R Soc Lond B Biol Sci 366 : 17–27.
40. HansenM, TaubertS, CrawfordD, LibinaN, LeeSJ, et al. (2007) Lifespan extension by conditions that inhibit translation in Caenorhabditis elegans. Aging Cell 6 : 95–110.
41. DavidDC, OllikainenN, TrinidadJC, CaryMP, BurlingameAL, et al. (2010) Widespread protein aggregation as an inherent part of aging in C. elegans. PLoS Biol 8: e1000450.
42. PoonHF, VaishnavRA, GetchellTV, GetchellML, ButterfieldDA (2006) Quantitative proteomics analysis of differential protein expression and oxidative modification of specific proteins in the brains of old mice. Neurobiol Aging 27 : 1010–1019.
43. SquierTC (2001) Oxidative stress and protein aggregation during biological aging. Exp Gerontol 36 : 1539–1550.
44. NaidooN (2009) The endoplasmic reticulum stress response and aging. Rev Neurosci 20 : 23–37.
45. GardnerBM, WalterP (2011) Unfolded proteins are Ire1-activating ligands that directly induce the unfolded protein response. Science 333 : 1891–1894.
46. RutkowskiDT, HegdeRS (2010) Regulation of basal cellular physiology by the homeostatic unfolded protein response. J Cell Biol 189 : 783–794.
47. KaeberleinM, GuarenteL (2002) Saccharomyces cerevisiae MPT5 and SSD1 function in parallel pathways to promote cell wall integrity. Genetics 160 : 83–95.
48. ValdiviesoMH, FerrarioL, VaiM, DuranA, PopoloL (2000) Chitin synthesis in a gas1 mutant of Saccharomyces cerevisiae. J Bacteriol 182 : 4752–4757.
49. SingerMA, LindquistS (1998) Multiple effects of trehalose on protein folding in vitro and in vivo. Mol Cell 1 : 639–648.
50. DunnCD, TamuraY, SesakiH, JensenRE (2008) Mgr3p and Mgr1p are adaptors for the mitochondrial i-AAA protease complex. Mol Biol Cell 19 : 5387–5397.
51. NakaiM, EndoT, HaseT, MatsubaraH (1993) Intramitochondrial protein sorting. Isolation and characterization of the yeast MSP1 gene which belongs to a novel family of putative ATPases. J Biol Chem 268 : 24262–24269.
52. FriedlanderR, JaroschE, UrbanJ, VolkweinC, SommerT (2000) A regulatory link between ER-associated protein degradation and the unfolded-protein response. Nat Cell Biol 2 : 379–384.
53. SchuldinerM, CollinsSR, ThompsonNJ, DenicV, BhamidipatiA, et al. (2005) Exploration of the function and organization of the yeast early secretory pathway through an epistatic miniarray profile. Cell 123 : 507–519.
54. CurranSP, RuvkunG (2007) Lifespan regulation by evolutionarily conserved genes essential for viability. PLoS Genet 3: e56.
55. ShoreDE, CarrCE, RuvkunG (2012) Induction of cytoprotective pathways is central to the extension of lifespan conferred by multiple longevity pathways. PLoS Genet 8: e1002792.
56. ChenD, ThomasEL, KapahiP (2009) HIF-1 modulates dietary restriction-mediated lifespan extension via IRE-1 in Caenorhabditis elegans. PLoS Genet 5: e1000486.
57. Henis-KorenblitS, ZhangP, HansenM, McCormickM, LeeSJ, et al. (2010) Insulin/IGF-1 signaling mutants reprogram ER stress response regulators to promote longevity. Proc Natl Acad Sci U S A 107 : 9730–9735.
58. TaylorRC, DillinA (2013) XBP-1 Is a Cell-Nonautonomous Regulator of Stress Resistance and Longevity. Cell 153 : 1435–1447.
59. GiaeverG, ChuAM, NiL, ConnellyC, RilesL, et al. (2002) Functional profiling of the Saccharomyces cerevisiae genome. Nature 418 : 387–391.
60. BreslowDK, CameronDM, CollinsSR, SchuldinerM, Stewart-OrnsteinJ, et al. (2008) A comprehensive strategy enabling high-resolution functional analysis of the yeast genome. Nat Methods 5 : 711–718.
61. SteffenKK, KennedyBK, KaeberleinM (2009) Measuring replicative life span in the budding yeast. J Vis Exp 28: pii: 1209.
62. GerashchenkoMV, LobanovAV, GladyshevVN (2012) Genome-wide ribosome profiling reveals complex translational regulation in response to oxidative stress. Proc Natl Acad Sci U S A 109 : 17394–17399.
63. LangmeadB, TrapnellC, PopM, SalzbergSL (2009) Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol 10: R25.
64. de HoonMJ, ImotoS, NolanJ, MiyanoS (2004) Open source clustering software. Bioinformatics 20 : 1453–1454.
65. SaldanhaAJ (2004) Java Treeview–extensible visualization of microarray data. Bioinformatics 20 : 3246–3248.
Štítky
Genetika Reprodukční medicína
Článek Unwrapping BacteriaČlánek A Chaperone-Assisted Degradation Pathway Targets Kinetochore Proteins to Ensure Genome StabilityČlánek The Candidate Splicing Factor Sfswap Regulates Growth and Patterning of Inner Ear Sensory OrgansČlánek The SPF27 Homologue Num1 Connects Splicing and Kinesin 1-Dependent Cytoplasmic Trafficking inČlánek Down-Regulation of eIF4GII by miR-520c-3p Represses Diffuse Large B Cell Lymphoma DevelopmentČlánek Meta-Analysis Identifies Gene-by-Environment Interactions as Demonstrated in a Study of 4,965 MiceČlánek High Risk Population Isolate Reveals Low Frequency Variants Predisposing to Intracranial Aneurysms
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2014 Číslo 1
-
Všechny články tohoto čísla
- How Much Is That in Dog Years? The Advent of Canine Population Genomics
- The Sense and Sensibility of Strand Exchange in Recombination Homeostasis
- Unwrapping Bacteria
- DNA Methylation Changes Separate Allergic Patients from Healthy Controls and May Reflect Altered CD4 T-Cell Population Structure
- Evidence for Mito-Nuclear and Sex-Linked Reproductive Barriers between the Hybrid Italian Sparrow and Its Parent Species
- Translation Enhancing ACA Motifs and Their Silencing by a Bacterial Small Regulatory RNA
- Relationship Estimation from Whole-Genome Sequence Data
- Genetic Models of Apoptosis-Induced Proliferation Decipher Activation of JNK and Identify a Requirement of EGFR Signaling for Tissue Regenerative Responses in
- ComEA Is Essential for the Transfer of External DNA into the Periplasm in Naturally Transformable Cells
- Loss and Recovery of Genetic Diversity in Adapting Populations of HIV
- Bioelectric Signaling Regulates Size in Zebrafish Fins
- Defining NELF-E RNA Binding in HIV-1 and Promoter-Proximal Pause Regions
- Loss of Histone H3 Methylation at Lysine 4 Triggers Apoptosis in
- Cell-Cycle Dependent Expression of a Translocation-Mediated Fusion Oncogene Mediates Checkpoint Adaptation in Rhabdomyosarcoma
- How a Retrotransposon Exploits the Plant's Heat Stress Response for Its Activation
- A Nonsense Mutation in Encoding a Nondescript Transmembrane Protein Causes Idiopathic Male Subfertility in Cattle
- Deletion of a Conserved -Element in the Locus Highlights the Role of Acute Histone Acetylation in Modulating Inducible Gene Transcription
- Developmental Link between Sex and Nutrition; Regulates Sex-Specific Mandible Growth via Juvenile Hormone Signaling in Stag Beetles
- PP2A/B55 and Fcp1 Regulate Greatwall and Ensa Dephosphorylation during Mitotic Exit
- Differential Effects of Collagen Prolyl 3-Hydroxylation on Skeletal Tissues
- Comprehensive Functional Annotation of 77 Prostate Cancer Risk Loci
- Evolution of Chloroplast Transcript Processing in and Its Chromerid Algal Relatives
- A Chaperone-Assisted Degradation Pathway Targets Kinetochore Proteins to Ensure Genome Stability
- New MicroRNAs in —Birth, Death and Cycles of Adaptive Evolution
- A Genome-Wide Screen for Bacterial Envelope Biogenesis Mutants Identifies a Novel Factor Involved in Cell Wall Precursor Metabolism
- FGFR1-Frs2/3 Signalling Maintains Sensory Progenitors during Inner Ear Hair Cell Formation
- Regulation of Synaptic /Neuroligin Abundance by the /Nrf Stress Response Pathway Protects against Oxidative Stress
- Intrasubtype Reassortments Cause Adaptive Amino Acid Replacements in H3N2 Influenza Genes
- Molecular Specificity, Convergence and Constraint Shape Adaptive Evolution in Nutrient-Poor Environments
- WNT7B Promotes Bone Formation in part through mTORC1
- Natural Selection Reduced Diversity on Human Y Chromosomes
- In-Vivo Quantitative Proteomics Reveals a Key Contribution of Post-Transcriptional Mechanisms to the Circadian Regulation of Liver Metabolism
- The Candidate Splicing Factor Sfswap Regulates Growth and Patterning of Inner Ear Sensory Organs
- The Acid Phosphatase-Encoding Gene Contributes to Soybean Tolerance to Low-Phosphorus Stress
- p53 and TAp63 Promote Keratinocyte Proliferation and Differentiation in Breeding Tubercles of the Zebrafish
- Affects Plant Architecture by Regulating Local Auxin Biosynthesis
- The SET Domain Proteins SUVH2 and SUVH9 Are Required for Pol V Occupancy at RNA-Directed DNA Methylation Loci
- Down-Regulation of Rad51 Activity during Meiosis in Yeast Prevents Competition with Dmc1 for Repair of Double-Strand Breaks
- Multi-tissue Analysis of Co-expression Networks by Higher-Order Generalized Singular Value Decomposition Identifies Functionally Coherent Transcriptional Modules
- A Neurotoxic Glycerophosphocholine Impacts PtdIns-4, 5-Bisphosphate and TORC2 Signaling by Altering Ceramide Biosynthesis in Yeast
- Subtle Changes in Motif Positioning Cause Tissue-Specific Effects on Robustness of an Enhancer's Activity
- C/EBPα Is Required for Long-Term Self-Renewal and Lineage Priming of Hematopoietic Stem Cells and for the Maintenance of Epigenetic Configurations in Multipotent Progenitors
- The SPF27 Homologue Num1 Connects Splicing and Kinesin 1-Dependent Cytoplasmic Trafficking in
- Down-Regulation of eIF4GII by miR-520c-3p Represses Diffuse Large B Cell Lymphoma Development
- Genome Sequencing Highlights the Dynamic Early History of Dogs
- Re-sequencing Expands Our Understanding of the Phenotypic Impact of Variants at GWAS Loci
- Meta-Analysis Identifies Gene-by-Environment Interactions as Demonstrated in a Study of 4,965 Mice
- , a -Antisense Gene of , Encodes a Evolved Protein That Inhibits GSK3β Resulting in the Stabilization of MYCN in Human Neuroblastomas
- A Transcription Factor Is Wound-Induced at the Planarian Midline and Required for Anterior Pole Regeneration
- A Comprehensive tRNA Deletion Library Unravels the Genetic Architecture of the tRNA Pool
- A PNPase Dependent CRISPR System in
- Genomic Confirmation of Hybridisation and Recent Inbreeding in a Vector-Isolated Population
- Zinc Finger Transcription Factors Displaced SREBP Proteins as the Major Sterol Regulators during Saccharomycotina Evolution
- GATA6 Is a Crucial Regulator of Shh in the Limb Bud
- Tissue Specific Roles for the Ribosome Biogenesis Factor Wdr43 in Zebrafish Development
- A Cell Cycle and Nutritional Checkpoint Controlling Bacterial Surface Adhesion
- High Risk Population Isolate Reveals Low Frequency Variants Predisposing to Intracranial Aneurysms
- E3 Ubiquitin Ligase CHIP and NBR1-Mediated Selective Autophagy Protect Additively against Proteotoxicity in Plant Stress Responses
- Evolutionary Rate Covariation Identifies New Members of a Protein Network Required for Female Post-Mating Responses
- 3′ Untranslated Regions Mediate Transcriptional Interference between Convergent Genes Both Locally and Ectopically in
- Single Nucleus Genome Sequencing Reveals High Similarity among Nuclei of an Endomycorrhizal Fungus
- Metabolic QTL Analysis Links Chloroquine Resistance in to Impaired Hemoglobin Catabolism
- Notch Controls Cell Adhesion in the Drosophila Eye
- AL PHD-PRC1 Complexes Promote Seed Germination through H3K4me3-to-H3K27me3 Chromatin State Switch in Repression of Seed Developmental Genes
- Genomes Reveal Evolution of Microalgal Oleaginous Traits
- Large Inverted Duplications in the Human Genome Form via a Fold-Back Mechanism
- Variation in Genome-Wide Levels of Meiotic Recombination Is Established at the Onset of Prophase in Mammalian Males
- Age, Gender, and Cancer but Not Neurodegenerative and Cardiovascular Diseases Strongly Modulate Systemic Effect of the Apolipoprotein E4 Allele on Lifespan
- Lifespan Extension Conferred by Endoplasmic Reticulum Secretory Pathway Deficiency Requires Induction of the Unfolded Protein Response
- Is Non-Homologous End-Joining Really an Inherently Error-Prone Process?
- Vestigialization of an Allosteric Switch: Genetic and Structural Mechanisms for the Evolution of Constitutive Activity in a Steroid Hormone Receptor
- Functional Divergence and Evolutionary Turnover in Mammalian Phosphoproteomes
- A 660-Kb Deletion with Antagonistic Effects on Fertility and Milk Production Segregates at High Frequency in Nordic Red Cattle: Additional Evidence for the Common Occurrence of Balancing Selection in Livestock
- Comparative Evolutionary and Developmental Dynamics of the Cotton () Fiber Transcriptome
- The Transcription Factor BcLTF1 Regulates Virulence and Light Responses in the Necrotrophic Plant Pathogen
- Crossover Patterning by the Beam-Film Model: Analysis and Implications
- Single Cell Genomics: Advances and Future Perspectives
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- GATA6 Is a Crucial Regulator of Shh in the Limb Bud
- Large Inverted Duplications in the Human Genome Form via a Fold-Back Mechanism
- Differential Effects of Collagen Prolyl 3-Hydroxylation on Skeletal Tissues
- Affects Plant Architecture by Regulating Local Auxin Biosynthesis
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



