-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaLarge Inverted Duplications in the Human Genome Form via a Fold-Back Mechanism
Inverted duplications are a common type of copy number variation (CNV) in germline and somatic genomes. Large duplications that include many genes can lead to both neurodevelopmental phenotypes in children and gene amplifications in tumors. There are several models for inverted duplication formation, most of which include a dicentric chromosome intermediate followed by breakage-fusion-bridge (BFB) cycles, but the mechanisms that give rise to the inverted dicentric chromosome in most inverted duplications remain unknown. Here we have combined high-resolution array CGH, custom sequence capture, next-generation sequencing, and long-range PCR to analyze the breakpoints of 50 nonrecurrent inverted duplications in patients with intellectual disability, autism, and congenital anomalies. For half of the rearrangements in our study, we sequenced at least one breakpoint junction. Sequence analysis of breakpoint junctions reveals a normal-copy disomic spacer between inverted and non-inverted copies of the duplication. Further, short inverted sequences are present at the boundary of the disomic spacer and the inverted duplication. These data support a mechanism of inverted duplication formation whereby a chromosome with a double-strand break intrastrand pairs with itself to form a “fold-back” intermediate that, after DNA replication, produces a dicentric inverted chromosome with a disomic spacer corresponding to the site of the fold-back loop. This process can lead to inverted duplications adjacent to terminal deletions, inverted duplications juxtaposed to translocations, and inverted duplication ring chromosomes.
Published in the journal: . PLoS Genet 10(1): e32767. doi:10.1371/journal.pgen.1004139
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004139Summary
Inverted duplications are a common type of copy number variation (CNV) in germline and somatic genomes. Large duplications that include many genes can lead to both neurodevelopmental phenotypes in children and gene amplifications in tumors. There are several models for inverted duplication formation, most of which include a dicentric chromosome intermediate followed by breakage-fusion-bridge (BFB) cycles, but the mechanisms that give rise to the inverted dicentric chromosome in most inverted duplications remain unknown. Here we have combined high-resolution array CGH, custom sequence capture, next-generation sequencing, and long-range PCR to analyze the breakpoints of 50 nonrecurrent inverted duplications in patients with intellectual disability, autism, and congenital anomalies. For half of the rearrangements in our study, we sequenced at least one breakpoint junction. Sequence analysis of breakpoint junctions reveals a normal-copy disomic spacer between inverted and non-inverted copies of the duplication. Further, short inverted sequences are present at the boundary of the disomic spacer and the inverted duplication. These data support a mechanism of inverted duplication formation whereby a chromosome with a double-strand break intrastrand pairs with itself to form a “fold-back” intermediate that, after DNA replication, produces a dicentric inverted chromosome with a disomic spacer corresponding to the site of the fold-back loop. This process can lead to inverted duplications adjacent to terminal deletions, inverted duplications juxtaposed to translocations, and inverted duplication ring chromosomes.
Introduction
Inverted duplications adjacent to terminal deletions are a relatively common copy number variation (CNV) first identified by chromosome banding [1]. With the rise in clinical array testing, such rearrangements are now recognized more often by the characteristic copy number gain adjacent to a terminal loss detected via microarray [2], [3]. Inverted duplications adjacent to terminal deletions have been described on nearly every chromosome end and, depending on the genes involved, can lead to a range of clinical phenotypes, including developmental delay, intellectual disability, autism, and birth defects [2], [4], [5], [6], [7], [8]. Moreover, large inverted duplications are a source of oncogene amplification in cancer genomes [9], [10], [11], [12], [13]. Large inverted duplications adjacent to deletions are also present in bacteria, yeast, protozoa, and worm genomes [14], [15], [16], [17], [18], [19], [20], [21] and are therefore a major cause of genomic imbalance in many cell types.
Several models are proposed to explain the formation of inverted duplications adjacent to terminal deletions in the human genome, and most include a dicentric chromosome step, as first described by McClinock [22]. One mechanism relies on homologous recombination (HR) between segmental duplications and is based on the inverted duplication and terminal deletion of the short arm of human chromosome 8. This recurrent rearrangement is always maternal in origin and occurs when normal and inverted homologous chromosomes 8 recombine during meiosis I [23], [24]. Recombination between highly identical inverted segmental duplications on 8p produces a dicentric chromosome and an acentric fragment. The acentric fragment is usually lost, but the dicentric chromosome may be recovered after breakage between the two centromeres and addition of a new telomere. This results in a chromosome with a 7.0-Mb terminal deletion, 5.5-Mb intervening normal copy region, and a proximal inverted duplication that varies in size, depending on the location of the dicentric chromosome break.
The mechanisms responsible for other human inverted duplications have remained elusive for a number of reasons. First, most deletion and duplication breakpoints are not recurrent, so the local genomic architecture underlying double-strand breaks does not point to a common rearrangement mechanism. Second, most inverted duplications adjacent to terminal deletions are characterized by array comparative genome hybridization (CGH) and/or fluorescence in situ hybridization (FISH), without sequencing of breakpoint junctions [6], [7]. Thus, conclusions drawn from such examples are missing key data that could shed light on specific DNA repair processes. In those inverted duplication junctions that have been sequenced, there are no obvious segmental duplications to suggest non-allelic homologous recombination (NAHR) [4], [5]. Thus, some other mechanism likely explains these nonrecurrent chromosome rearrangements, which make up the largest fraction of human inverted duplications.
The timing of inverted duplication formation is another important open question when considering rearrangement mechanism. Most constitutional (non-tumor) inverted duplications are present in a non-mosaic state, consistent with an event that occurred during meiosis or mitosis of the early embryo [2], [4], [6], [7]. Rare mosaic inverted duplications support a mitotic origin for inverted duplication formation [25], [26], and models for both meiotic and mitotic processes have been proposed [6], [9]. Some of the most striking evidence for mitotic inverted duplication formation comes from copy number studies of human blastomeres. CNV analyses of single cells from the same embryo have revealed inverted duplication chromosomes and their reciprocal terminal deletion products, consistent with a mitotic embryonic origin for inverted duplications [27], [28].
In this study, we analyzed the largest cohort of naturally occurring human inverted duplications. We fine-mapped the breakpoints of 50 inverted duplications using custom high-resolution array CGH. In 25/50 of the chromosome rearrangements, we sequenced breakpoint junctions via long-range PCR, custom target capture, and next-generation sequencing. Together, these breakpoint data point to a fold-back model of inverted duplication formation.
Results
Inverted duplication cohort
To capture a large collection of inverted duplications, we recruited 50 participants with pathogenic copy number variation (CNV) from Emory University, Signature Genomic Laboratories, and the Chromosome 18 Clinical Research Center. The children in our study carry nonrecurrent chromosome rearrangements that involve hundreds of genes per deletion or duplication, and they exhibit a range of phenotypes from developmental delay and intellectual disability to autism and other neurodevelopmental disorders. CNVs were initially detected via clinical cytogenetics testing, including array CGH, FISH, and/or chromosome banding (Figures 1 and 2). Individuals with inverted duplications adjacent to terminal deletions and their family members were referred to our study.
Fig. 1. Inverted duplication organization. 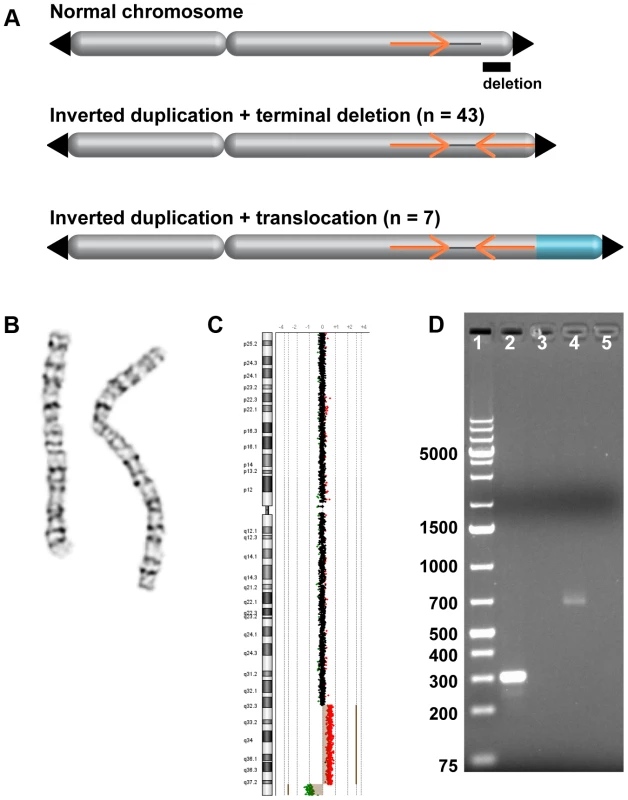
(A) Model of duplicated sequences (orange arrows) separated by disomic spacer sequence (grey line). The end of the inverted duplication may terminate in a telomere (black triangle) or a translocated chromosome (blue). The site of the terminal deletion is shown relative to a normal chromosome. (B) EGL044's inverted duplication of chromosome 2 is detectable by chromosome banding. (C) The 5.8-Mb terminal deletion and 42-Mb inverted duplication of chromosome 2 are detectable by low-resolution array CGH [53]. Note that the 2,047-bp spacer region is not visible. Log2 ratios of oligonucleotide probes are indicated by dots; normal-copy number (black), duplication (red), and deletion (green) regions are shown. (D) PCR of the disomy-inversion junction (lane 2) and the inversion-telomere junction (lane 4) amplifies genomic DNA from EGL044, but not control genomic DNA (lanes 3 and 5). Lane 1 is GeneRuler 1 kb Plus DNA ladder (Thermo Scientific Fermentas #SM1333). Fig. 2. FISH analysis of inverted duplication translocation chromosomes. 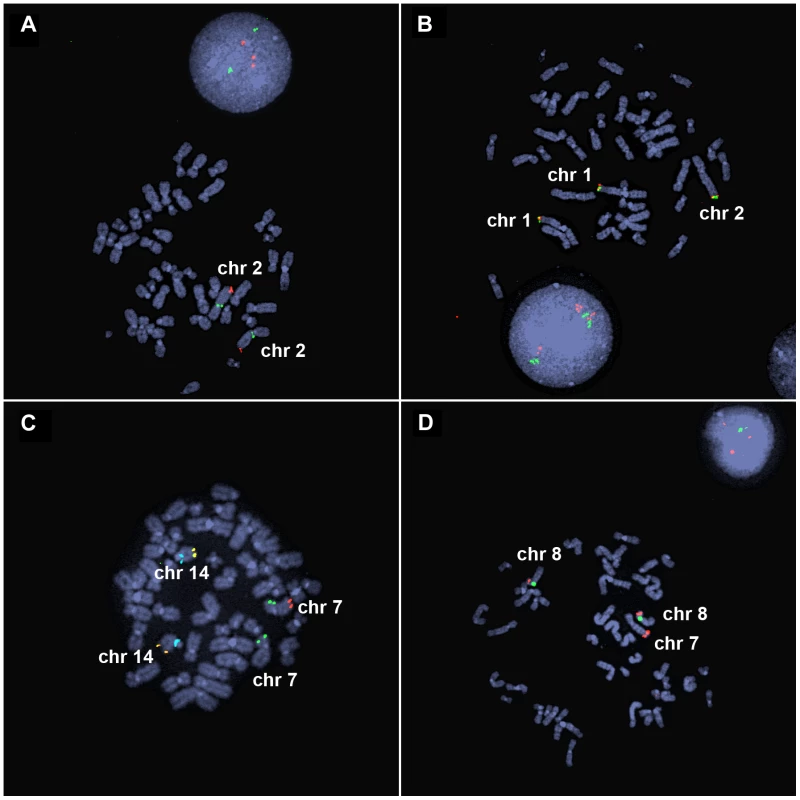
(A) EGL398's 3.1-Mb duplication of 2q37 is visible by interphase FISH. BAC probes RP11-206J15 (red) and RP11-1415N13 (green) hybridize to the duplicated and control regions on chromosome 2, respectively. Three red signals in the interphase nucleus indicate a duplication of chromosome 2q37. (B) BAC probes RP11-798H13 (red) and RP11-380E2 (green) hybridize to the ends of the normal chromosomes 1p and the end of the inverted duplication translocation chromosome in EGL398. (C) EGL399's terminal deletion of 7q is detected as loss of a red signal. Vysis ToTelVysion mix 7 (Abbott Molecular, #05J05-001) probes hybridize to the ends of chromosomes 7p (green), 7q (red), and 14q (yellow). The blue signals correspond to a control probe that hybridizes to chromosome 14q11. (D) BAC RP11-341D4 (red) hybridizes to the normal chromosomes 8p and the translocation of 8p on EGL399's inverted duplication translocation between chromosomes 7 and 8. The green signal corresponds to alpha satellite from the centromere of chromosome 8. Forty-three subjects had a rearranged chromosome with a terminal loss and an adjacent gain, consistent with a simple inverted duplication adjacent to a terminal deletion. Seven had a terminal deletion adjacent to a duplication, plus a gain of another chromosome end, which when analyzed by FISH, turned out to be an unbalanced translocation juxtaposed to the inverted duplication (Figure 2). Parental samples were provided for 26/50 of the subjects in our study. Chromosome analysis and FISH revealed that 25/26 of inverted duplications were not present in a balanced or unbalanced form in either parent (Table S1). In one family (EGL396), the same inverted duplication was inherited from a similarly affected mother. Thus, most inverted duplications arise de novo.
The parental origin of the inverted duplication can shed light on the mechanism of chromosome rearrangement. To this end, we analyzed microsatellites in the deleted and duplicated regions from nine subjects and their parents (Table S2). In seven families there were sufficient informative markers to determine that the duplication and deletion were paternally inherited and that the duplication allele originated from the same chromosome as the deletion. For the families of 18q-119c and EGL106, only the mothers were genotyped. Microsatellites were consistent with a duplication of the paternal allele and retention of the maternal allele in the deleted region. These data support an intrachromosomal origin for inverted duplications that arose on the same allele as the original locus, rather than a duplication from the homologous chromosome.
Breakpoint mapping and sequencing
To refine deletion and duplication breakpoints, we fine-mapped CNVs with custom high-resolution microarrays (Figure 3). Oligonucleotide probes on the custom arrays are spaced one per ∼200 basepairs (bp), which in most cases resolve chromosome breakpoints to ∼1 kilobase (kb). However, repeat-rich regions and assembly gaps can limit array design, leading to poor probe coverage at some breakpoints. We identified deletion, duplication, and translocation breakpoints via array CGH as previously described [29]. Based on breakpoints predicted from our high-resolution array data, we designed long-range PCR, inverse PCR, SureSelect target enrichment, and next-generation sequencing experiments to sequence across breakpoint junctions (Table 1 and Table S1).
Fig. 3. High-resolution array CGH identifies spacers. 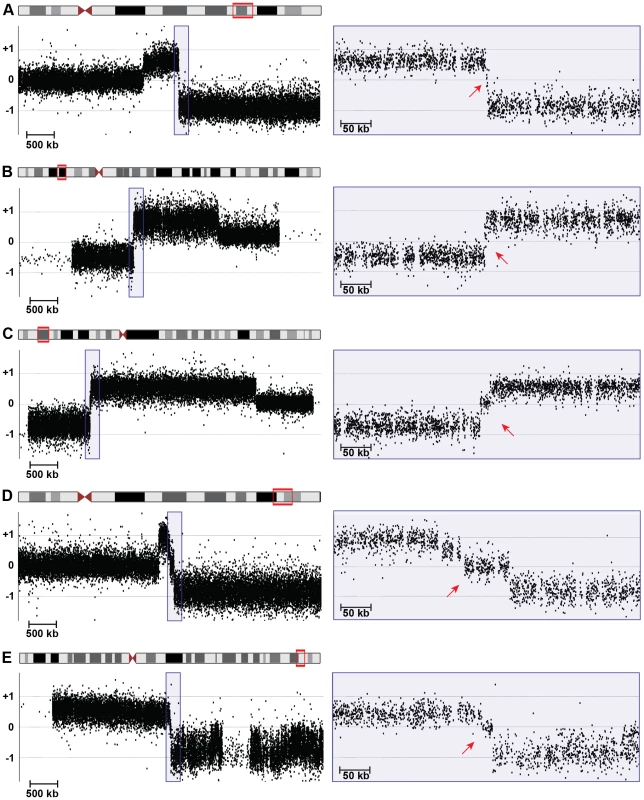
5-Mb (left) and 400-kb (right) views of high-resolution array CGH data from (A) 18q-6c, (B) EGL106, (C) EGL104, (D) 18q-233c, and (E) SG_Tel_010 show 1,866-bp, 3,138-bp, 14,779-bp, 70,466-bp, and 14,779-bp spacers, respectively. Log2 ratios of probe signal intensity are shown as black dots. Boxed region on the left is expanded on the right. Red arrows point out disomic spacer regions between deleted and duplicated segments. Spacer sizes were determined by sequencing breakpoint junctions in (A)–(D), whereas the spacer in (E) was sized using breakpoints determined by array CGH only. Tab. 1. Sequenced breakpoint junctions. 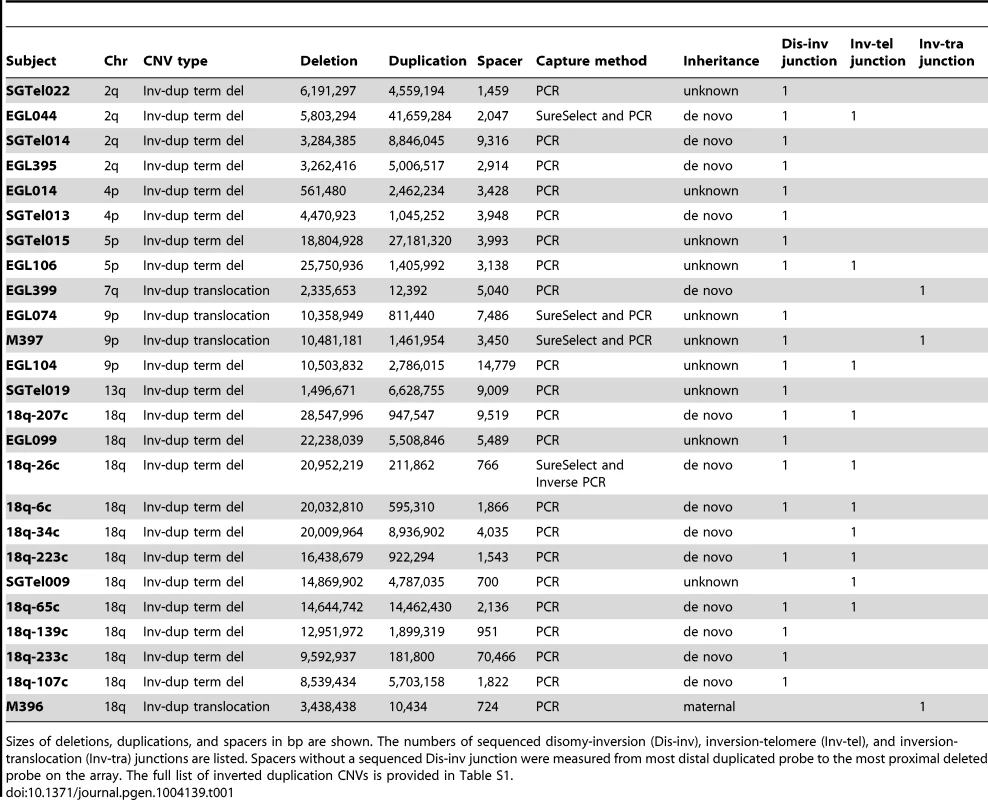
Sizes of deletions, duplications, and spacers in bp are shown. The numbers of sequenced disomy-inversion (Dis-inv), inversion-telomere (Inv-tel), and inversion-translocation (Inv-tra) junctions are listed. Spacers without a sequenced Dis-inv junction were measured from most distal duplicated probe to the most proximal deleted probe on the array. The full list of inverted duplication CNVs is provided in Table S1. Starting with breakpoints identified by high-resolution array, we designed PCR experiments to amplify 68 junctions [29], [30] (Figure 4 and Table S1). In some cases, there was not enough DNA to try multiple junction sequencing strategies. For other junctions that failed long-range and/or inverse PCR conditions, we performed targeted sequence capture with custom SureSelect libraries designed for our breakpoint regions of interest, followed by next-generation sequencing (see Methods). Of the 10 patient samples included in our SureSelect experiments, junctions from EGL044, EGL074, and M397 had sufficient paired-end and/or split read coverage to infer breakpoint structure, which we confirmed by Sanger sequencing.
Fig. 4. Inverted duplication junctions. 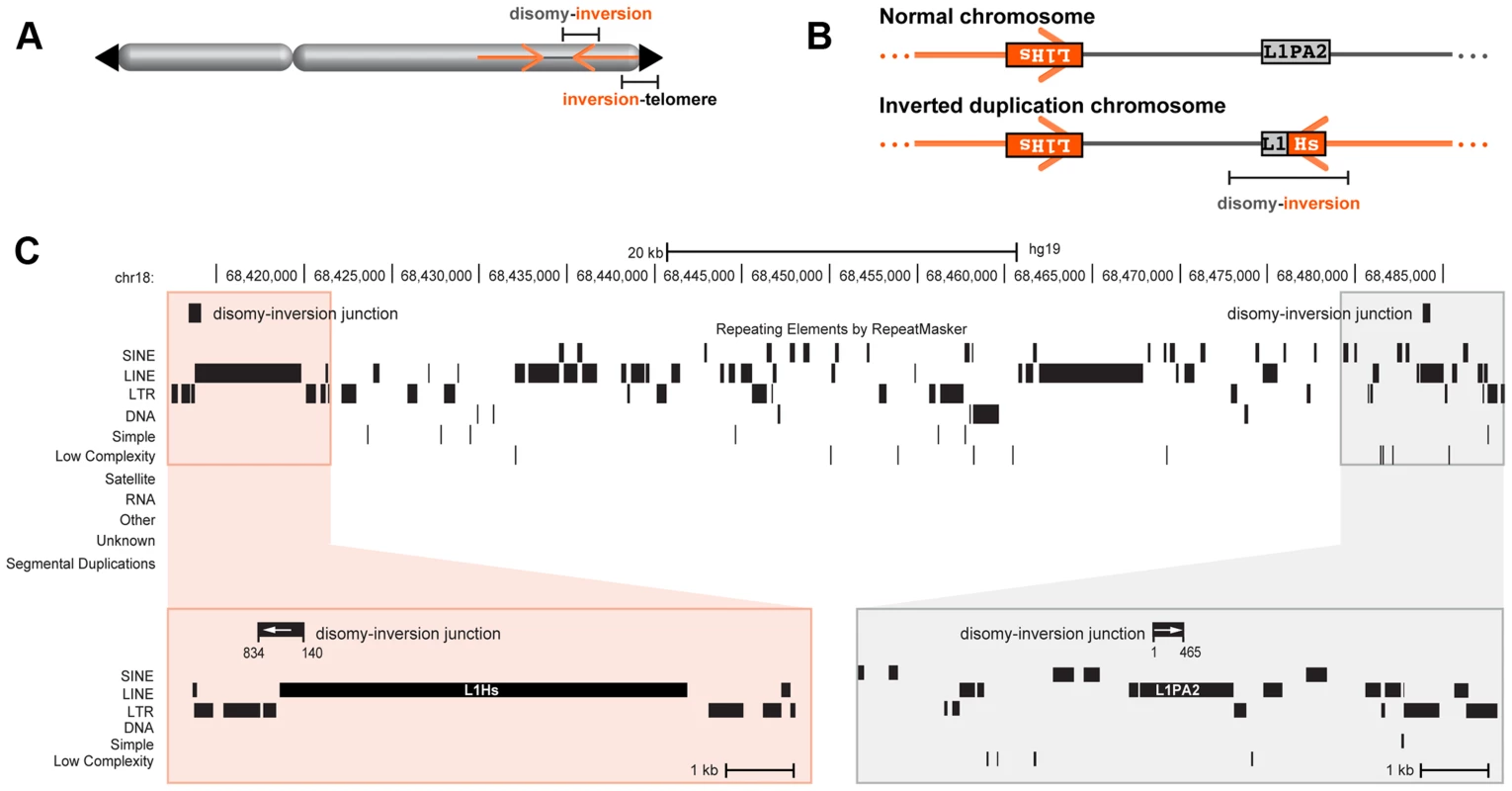
(A) Location of disomy-inversion and inversion-telomere junctions in an inverted duplication terminal deletion chromosome. (B) 18q-233c's disomy-inversion junction spans a hybrid LINE made up of L1PA2 and L1Hs elements. On a normal chromosome 18, these elements are positioned in opposite orientation. (C) Local genomic context of 18q-233c's spacer and breakpoints relative to the reference genome assembly. The distal end of the disomic spacer (grey box) includes the L1PA2, and the proximal region corresponding to the beginning of the inverted duplication (orange box) includes the L1Hs. The disomy-inversion junction sequence (black rectangles with white arrows) aligns to the distal end of the spacer (positions 1–465 of the junction) and the start of the inverted duplication (positions 140–834 of the junction). Interspersed repeats are shown as black rectangles. No segmental duplications are present in the breakpoint regions. Simple inverted duplications adjacent to terminal deletions have two breakpoint junctions: one from the non-inverted part of the chromosome to the start of the inverted duplication (disomy-inversion) and one from the end of the inverted duplication to the new telomere (inversion-telomere). Similarly, inverted duplications with unbalanced translocations have one disomy-inversion junction and one junction between the inverted duplication and the translocated chromosome (inversion-translocation). In both types of rearrangement, the terminal deletion corresponds to the region distal of the duplication (Figure 1). In total, we sequenced across 21 disomy-inversion junctions from 19 simple inverted duplications and two inverted duplications adjacent to translocations. We also sequenced 10 inversion-telomere junctions and three inversion-translocation junctions. All 34 of these sequenced junctions are present in the patient with the chromosome rearrangement, but not in a control genome, consistent with patient-specific junctions (Figure 1D). We aligned junction sequences to the human reference genome assembly to analyze the transitions across breakpoints and detect regions of microhomology and/or short inversions and insertions at junctions (Figure S1).
Inverted duplication organization
Analysis of breakpoint junctions can point to mechanisms of chromosome rearrangement and modes of DNA repair. Remarkably, in all 21 sequenced disomy-inversion junctions, we found a short “spacer” region between the non-inverted and inverted segments (Figures 3 and 5). This region is 766–70,466 bp long (median = 3,428 bp) and is not duplicated; rather, it has a normal disomic copy number in the subject's genome. Since 20 out of 21 disomic spacers are less than 15 kb, it is not surprising that they were not detected by routine cytogenetics testing (Figure 1). Spacers that were not sequenced have a median size of 3,568 bp, as determined by array CGH (Table S1). Detection and analysis of spacers provide important clues to the mechanism of inverted duplications.
Fig. 5. Characterization of spacers. 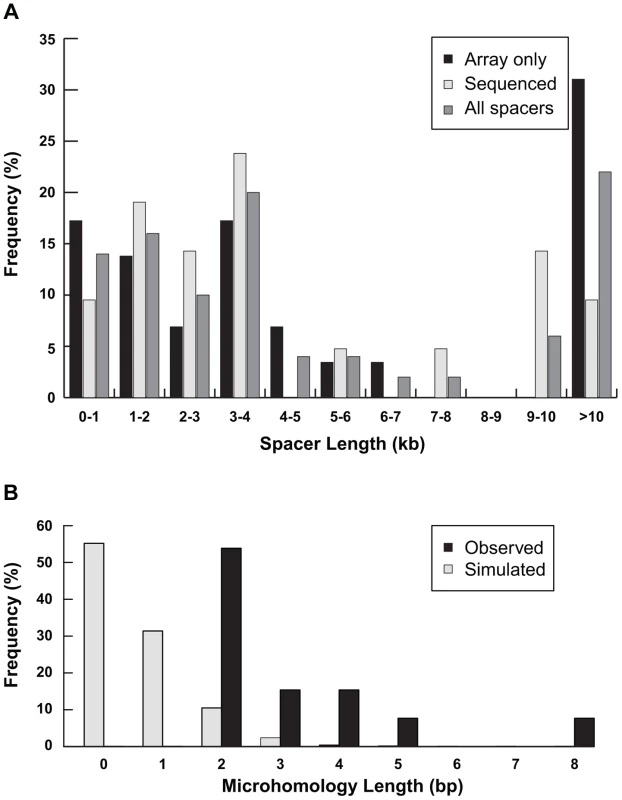
(A) Distribution of lengths of spacers measured by high-resolution array CGH only (n = 29) or junction sequencing (n = 21) are plotted separately. The distribution of all 50 spacer lengths is also shown. (B) The amount of inverted microhomology observed at 13 sequenced disomy-inverted duplication junctions—2 bp (n = 7), 3 bp (n = 2), 4 bp (n = 2), 5 bp (n = 1), or 8 bp (n = 1)—are shown relative to the microhomology detected for 1,000 simulated spacers (see Methods). Previous studies from cancer genomes and model systems support a fold-back mechanism of duplication formation [9], [16], [17], [18], [20], [31]. In this scenario, an initial double-strand break (DSB) deletes the end of a chromosome, leaving an unprotected end without a telomere. DNA from this free end could resect, fold back on itself, and pair with a more proximal region of the chromosome, especially if the two regions share homologous sequence oriented in the reverse complement. If the fold-back mechanism is responsible for the inverted duplications in our study, we would expect to find direct sequence homology between the distal end of the disomic spacer and the start of the inverted duplication. When aligned to the normal reference genome, the breakpoint junction would share inverted homology between the distal end of the disomic spacer and the distal end of the region that is duplicated.
Analysis of the disomy-inversion junctions revealed sequence homologies between the end of the disomic spacer and the start of the inverted duplication. In three out of 21 sequenced junctions, homologous LINE or SINE repeats are present at the edges of the disomic spacer and the inverted duplication (Figure S2). Analysis of EGL104's disomy-inversion junction revealed 296 bp of sequence homology between 90% identical AluY elements that lie in opposite orientation as positioned in the reference genome. SGTel014's junction crosses an AluSx1 at the end of the disomic spacer to an AluSq2 in the duplicated segment; the Alus are 82% identical over 296 bp. LINE elements flank 18q-233c's disomy-inversion junction in which a L1PA2 element at the end of the disomic spacer transitions to a L1Hs that is 95% identical across 330 bp at the junction (Figure 4). In all three of these rearrangements, the disomy-inversion transition occurs at homologous sites within the repetitive element, creating a hybrid repeat in the same orientation at the breakpoint junction.
Shorter inverted microhomologies are present in 13 of the remaining 18 disomy-inverted duplication junctions (Figure S1). The other five disomy-inversion junctions contain sequence insertions at the breakpoints. To determine whether the amount of inverted microhomology is greater than expected by chance, we simulated 1,000 spacers in the human genome. We counted the number of bp shared between the distal end of the spacer and the reverse complement of the other end of the sequence, representing the start of the inverted duplication (see Methods). Simulated spacers have 0–4 bp of microhomology, with no microhomology at 55% of all simulated junctions. On the other hand, the 13 sequenced disomy-inversion junctions had 2–8 bp of inverted microhomology (Figure 5B). Microhomology of greater than or equal to 2 bp is enriched at sequenced spacer junctions compared to simulated junctions (p = 2.7×10−12). Together, these data suggest that short inverted sequences at disomy-inversion junctions are an important feature of human inverted duplications.
Complex rearrangements
Although most of the breakpoint junctions we sequenced were simple, transitioning from disomy to inversion, inversion to telomere, or inversion to translocation, five rearrangements had additional sequence inserted and/or inverted at the breakpoint junctions. EGL106 and 18q-6c had insertions at the inversion-telomere junctions of chromosomes 5p and 18q, respectively. Analysis of EGL106's inversion-telomere junction revealed a 22-bp insertion between the telomere and the inverted duplication. This sequence is identical to part of the inverted duplication, ∼600 bp from the end of the duplicated sequence (chr5 : 27,159,451–27,159,471). Interestingly, the inserted sequence is in the opposite orientation to that seen in the inverted duplication (Figure 6A). 18q-6c's inversion-telomere junction also has a small insertion that could be the product of replication slippage. Six basepairs of local junction sequence (TTTTTG) is inserted in the same orientation as the end of the inverted duplication (Figure S1).
Fig. 6. Complex junctions from EGL106 and 18q-65c. 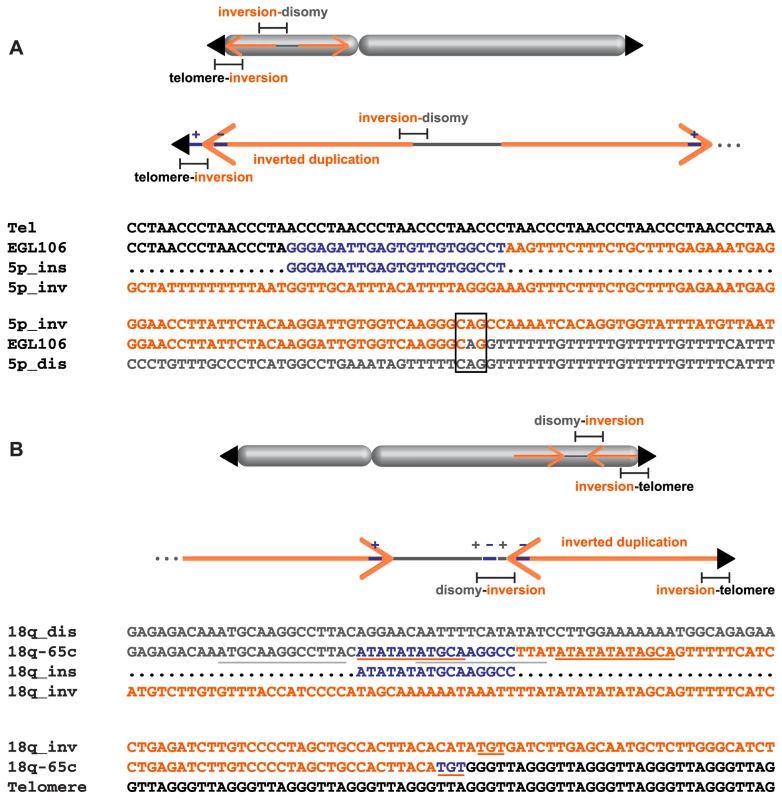
Insertion orientation (+/−) is indicated relative to the reference genome. (A) Alignment of telomere (black), inverted duplication (orange), inserted sequence (blue), and junction sequence (EGL106) from the telomere-inversion junction is shown above. The inverted duplication, disomic sequence (grey), and inversion-disomy junction sequence (EGL106) alignment is shown below. Microhomology at the junction is boxed. (B) Above, disomic, inserted, and inverted duplication sequences are aligned to the disomy-inversion junction sequence (18q-65c). Below, inverted duplication and telomere sequences are aligned to the inversion-telomere junction sequence (18q-65c). Inserted sequences and their neighboring homologous sequences are underlined. SGTel015's disomy-inversion junction has a 4-bp insertion derived from the disomic side of the breakpoint. “CAAA” was inserted in the direct orientation between the inverted duplication of 5p and the disomic segment (Figure S1). 18q-65c's disomy-inversion junction also contains a short, 16-bp insertion: the first 11 bp are identical to sequence only a few bp away at the start of the inverted duplication, and the last 10 bp of the insertion are identical to nearby disomic sequence (Figure 6B). At the center of the 16-bp insertion, there are five bp (ATGCA) shared between both sides of the junction. Both halves of the insertion are in the same orientation relative to the disomic and inverted duplication segments. Insertions of local DNA sequence at breakpoints could occur via template slippage events [32], [33].
SGTel022's disomy-inversion junction contains a 70-bp insertion that lacks homology to nearby sequence on chromosome 2q (Figure S1). We aligned this sequence to the reference human genome using BLAT [34] and found all 70 bp to be mitochondrial in origin. The top alignment is homologous to positions 6513–6582 of the human mitochondrial genome, with all 70 bp aligning with 100% identity. The second-best alignment is homologous to a nuclear sequence of mitochondrial origin (numt) located on chromosome 1p that shares 97.2% sequence identity across 69 bp of the insertion sequence. Greater sequence homology to the mitochondrial genome than to existing numts is consistent with a new mitochondrial insertion that occurred at the time of inverted duplication formation [35]. A similar mitochondrial insertion has been described at the breakpoint of a balanced translocation between chromosomes 9 and 11 [36]. Like most mitochondrial insertions in primate genomes [37], the 70-bp insertion in SGTel022's junction lacks microhomology to the insertion site.
In addition to these five complex junctions, three out of 34 sequenced breakpoint junctions contain 1–3 bp of inserted sequence (Figure S1). Given the short insertion size, we cannot infer the origin of the inserted material. Most insertions are derived from the rearranged chromosome, usually within 1 kb of the breakpoint junction.
Discussion
Sequence analyses of 34 breakpoint junctions in this study support a fold-back model of inverted duplication formation (Figure 7). We propose that an initial DSB generates a terminal deletion, then 5′-3′ resection of the free chromosome end creates a 3′ overhang that can intrastrand pair with itself, most often at a site of inverted sequence homology. DNA synthesis fills in the resected gap, creating a monocentric fold-back chromosome. Slippage during synthesis would produce templated insertions, derived from regions near the breakpoint [33]. Insertions could also arise via nonhomologous end-joining (NHEJ) or alternative NHEJ (alt-NHEJ) processes [38], especially for non-local insertions like the mitochondrial sequence in SGTel022. Insertions of 1–372 bp have been described in other inverted duplication breakpoints [9].
Fig. 7. Fold-back model of inverted duplication formation. 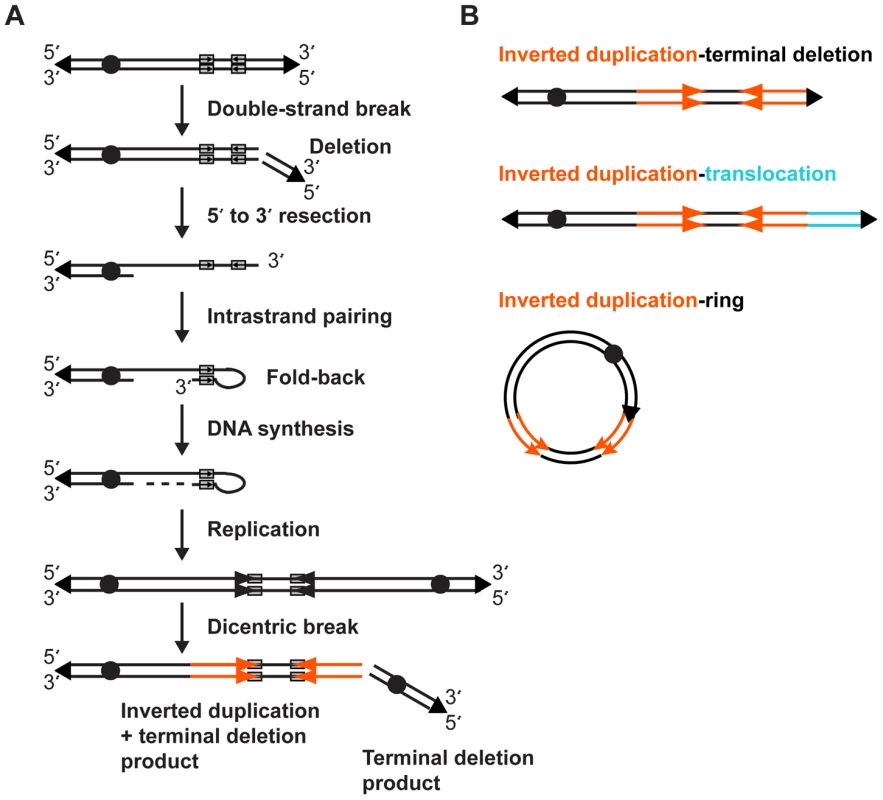
(A) 5′ and 3′ strands of the chromosome with telomeres (triangles) and centromere (circle) are shown. Short inverted sequences (grey rectangles with arrows) lie adjacent to the terminal deletion breakpoint. The inverted duplication mechanism occurs as described in the Discussion. The resulting inverted duplication is indicated by orange arrows. (B) After a breakage-fusion-bridge cycle, the inverted duplication chromosome may be repaired as a terminal deletion, translocation, or ring chromosome. After DNA replication, the dicentric chromosome has a short disomic spacer in between the inverted sides of the chromosome, corresponding to the fold-back loop region. Such a dicentric chromosome is unstable during cell division, and after the BFB cycle(s), a second DSB between the two centromeres gives rise to two monocentric chromosomes: one with a terminal deletion and one with an inverted duplication plus a terminal deletion. The simple terminal deletion could acquire a new telomere or translocate with another free end; in either case there is no sign of the inverted duplication process in this chromosomal product. Terminal deletions are a relatively common type of CNV [29], and many could be formed through such a dicentric intermediate.
After dicentric breakage, there are at least three possible outcomes for the inverted duplication product (Figure 7B). Addition of a new telomere would produce a simple inverted duplication adjacent to a terminal deletion. End-joining between the free end of the inverted duplication and another chromosome would give rise to an inverted duplication translocation chromosome. Finally, fusion of the inverted duplication end and the other arm of the chromosome would produce a ring chromosome that harbors an inverted duplication. Though we did not analyze this type of chromosome rearrangement in this study, inverted duplication ring chromosomes consistent with this model have been reported [39], [40], [41]. Genotype analysis of inverted duplication ring chromosomes demonstrates the rings are derived from a single chromatid end, as predicted by our model, and not via a mechanism that requires recombination between homologous chromosomes [40]. All of these outcomes occur after a dicentric chromosome intermediate, so they may be subject to additional BFB cycles, resulting in additional copy number changes.
Telomere addition may occur through end-joining or through de novo synthesis of telomere repeats at the site of the DSB. Other terminal deletion telomere junctions include microhomology in some cases, and insertions in others [4], [29], [42], [43]. Similarly, three out of ten inversion-telomere junctions in our study had inserted sequences, and five junctions had 1–4 bp of microhomology with the (TTAGGG)n repeat (Figure S1).
The length of the fold-back loop will depend on the amount of DNA resection and the distance to the inverted sequence. In mammalian systems of induced DSBs, DNA resection is up to 1.3 kb; however, only rearrangements that preserve selectable markers are recovered, so those with greater resection lengths would be missed [33], [44]. Most of the disomic spacers described here are a few kb in size, within the range of DNA resection in other studies (Table 1 and Figure 5A). The amount of inverted homology required for intrastrand pairing at the fold-back loop is unknown. In our inverted duplications, we find 13 disomy-inversion junctions with 2–8 bp of microhomology, and three cases of ∼300 bp of sequence homology. Experimental inverted duplication systems have found similar lengths of inverted homology at breakpoint junctions. Tanaka et al. used 229-bp inverted repeats to stimulate inverted duplication formation in Chinese hamster ovary cells. Sequencing revealed “several nucleotides” of inverted microhomology at the breakpoint junctions [9]. In a yeast model of inverted duplication formation, as little as 4–6 bp of inverted homology was sufficient for fold-back [18]. Although short microhomologies are not sufficient to induce DSBs, they are likely to be important for intrastrand fold-back after DSBs.
We propose that the first step of duplication formation is a DSB that generates the terminal deletion. This exposes a free chromosome end that can intrastrand pair with itself to produce the characteristic inverted duplication and disomic spacer structure we observe in all junctions. Recently, Mizuno and colleagues described a HR-dependent mechanism of inverted duplication in fission yeast that does not require an initial DSB [45]. In this process, replication forks stalled at a replication-terminator sequence invade a nearby DNA strand at a site of inverted homology via NAHR. Resolution of the Holliday junction can produce dicentric chromosomes with inverted duplications and terminal deletions. Thus, it is possible that some human inverted duplications are initiated by replication fork stalling, rather than by a DSB. Fork stalling and template switching (FoSTeS) has been implicated in other complex breakpoints in the human genome that involve insertions and inversions [30], [46], [47], [48], [49], [50], [51], [52], and this process could explain complex junctions like those in 18q-65c and EGL106. However, in the fission yeast system, 150–1,200 bp of inverted homology was required for strand invasion [45]. The 2–8 bp of inverted microhomology we find at most inverted duplication junctions is not sufficient for NAHR, but it is possible that the ∼300 bp of homology between inverted Alus or LINEs could be involved in HR-dependent strand invasion, similar to results from Mizuno et al.
Some have proposed a “U-type” exchange mechanism for human inverted duplication formation [6], [7]. In this model, pre-meiotic DSBs on sister chromatids of the same chromosome fuse to form a symmetric U-type structure. This dicentric chromosome is susceptible to breakage-fusion-bridge cycles, generating an inverted duplication chromosome and a terminal deletion chromosome. A key feature of this model is the absence of a disomic spacer between the inverted regions at the site of sister chromatid fusion. Lower-resolution studies will miss short disomic spacers, leading to the conclusion that U-type exchange is a common mechanism of inverted duplication formation [6], [7]. It is worth noting that we sequenced the disomy-inversion junctions of three subjects who were also included in the lower-resolution Rowe et al. (2009) study. EGL014 (Rowe 0152), EGL395 (Rowe 2998), and M397 (Rowe 9218) junctions have 3,428-bp, 2,914-bp, and 3,450-bp disomic spacers, respectively, which were not detected by the previous study [6]. This is not surprising since these samples were originally analyzed using arrays with probes spaced one every ∼75 kb [6], [53]. These discrepancies highlight the importance of sequencing breakpoint junctions when investigating chromosome rearrangement mechanisms.
Though we applied multiple experimental strategies to capture breakpoint junctions, some of the most complex junctions may have escaped detection due to large insertions or inversions that are difficult to infer from structural variation data. This is a common problem with CNV breakpoint studies, especially for those that include chromosome duplications [30], [54], [55], [56]. It is possible that segmental duplications at breakpoint junctions could have complicated junction sequencing; however, only one inverted duplication, SG_Tel_018, had a breakpoint near a segmental duplication. This segmental duplication is unlikely to be involved in SG_Tel_018's inverted duplication of chromosome 4q since the homology is shared between chromosomes 4 and 9, rather than the two regions of chromosome 4 involved in the rearrangement.
We were able to sequence half (34/68) of the attempted breakpoint junctions in our cohort (Table S1). This success was largely due to the integration of copy number data (high-resolution array CGH), DNA sequence analysis (PCR, SureSelect, NGS), and chromosomal localization of deletions and duplications (chromosome banding, FISH). Studies that rely on just one of these approaches will likely misinterpret chromosome rearrangements and confirm fewer breakpoint junctions. For example, M396's chromosome rearrangement was originally identified as an unbalanced translocation between chromosomes 10 and 18 by low-resolution array CGH and FISH, but high-resolution array CGH and sequencing of the inversion-translocation breakpoint revealed a 10-kb inverted duplication of chromosome 18 adjacent to the translocated segment from chromosome 10, consistent with an inverted duplication translocation chromosome. It is also important to point out that junction sequencing is dependent on the amount of DNA available for multiple sequencing strategies; for 22 inverted duplications, we exhausted the DNA sample (Table S1).
Microsatellite analysis of nine inverted duplications determined that the duplicated segment is always derived from the same chromosome as the original locus, not from the homologous chromosome. This indicates that the duplication arose through an intrachromosomal event, and points to intrastrand pairing within a sister chromatid. Other studies have also reported intrachromosomal inverted duplications [8], [40]. Copy number analyses of human blastomeres have revealed terminal deletions and duplications adjacent to terminal deletions involving the same chromosome end in different cells from the same embryo, consistent with the expected chromosomal products of our model [27], [28]. Furthermore, rare mosaic inverted duplication chromosomes have been described in lymphocytes and amniotic fluid [25], [26]. These data support a mitotic origin for nonrecurrent inverted duplications adjacent to terminal deletions. This is similar to the case for nonrecurrent CNVs that may be induced in mitosis by experimental conditions of replication stress [30], [57]. On the other hand, recurrent inverted duplications mediated by NAHR, such as the inv dup(8), likely originate during meiosis when homologous recombination occurs [23], [24]. Analysis of other recurrent chromosome rearrangements has shown that NAHR-mediated events are meiotic in origin [58].
All nine of the inverted duplications we analyzed for parent of origin occurred on a paternal allele. This is likely due in part to the paternal bias in rearrangements of chromosome 18. Heard et al. (2009) reported that 95/109 (87%) of de novo 18q deletions, duplications, and translocations are paternally derived [59]. Six of our inverted duplications arose on chromosome 18q and were part of the Heard study, two inverted duplications arose on chromosome 2q, and one occurred on chromosome 5p. Other studies have described maternal and paternal origins of inverted duplications [4], [8], [60], [61], [62], [63], [64], [65]. These data argue against a parent-of-origin bias for inverted duplications overall.
Inverted duplications almost always occur de novo. In our cohort, 25/26 inverted duplications were not present in parents in either a balanced or unbalanced state. Other studies of inverted duplications find similar inheritance patterns [6], [7]. Furthermore, analysis of human blastomeres detects inverted duplications with terminal deletions as new events in the developing embryo [27], [28]. Together, these data suggest that the inverted duplication and terminal deletion occur in a single step, rather than as a progression from a balanced rearrangement in an unaffected parent to unbalanced inheritance in an affected child. This is an important finding when considering recurrence risk for inverted duplication formation in genetic counseling.
Our large-scale breakpoint analysis has determined the genomic structure and CNV formation mechanism for human inverted duplications. Disomic spacers between inverted regions point to a fold-back step, and short inverted sequences at breakpoint boundaries are consistent with fold-back looping that occurs after the DSB and DNA resection steps of the chromosome rearrangement. Complex breakpoints may arise via template insertions during DNA synthesis or via alt-NHEJ. These data support a fold-back mechanism for nonrecurrent inverted duplications.
Materials and Methods
Ethics statement
We received peripheral blood and/or DNA samples from subjects with pathogenic CNVs and their parents. Samples were ascertained from the Emory Genetics Laboratory (EGL), Signature Genomic Laboratories (SG), the Chromosome 18 Clinical Research Center (18q-), and the Martin laboratory (M). See Table S1 for details. This study was approved by the Emory University Institutional Review Board.
High-resolution array CGH
Chromosome rearrangements were originally analyzed in clinical cytogenetics laboratories with different array CGH platforms, subtelomeric FISH assays, and/or G-banded chromosome analysis. Array CGH results were confirmed by chromosome analysis or FISH in diagnostic laboratories using standard methodologies. We confirmed all chromosome rearrangements via custom high-resolution array CGH.
We designed custom 60k CGH arrays with oligonucleotide probes targeted to previously identified breakpoints with a mean probe spacing of one probe per 200 bp. Oligonucleotide arrays were designed with Agilent's eArray program (https://earray.chem.agilent.com/earray/). Custom array designs (AMADID numbers) are listed in Table S1. DNA extraction from peripheral blood and cell lines, microarray hybridization, array scanning, and breakpoint analysis were performed as described previously [29]. Array CGH data have been submitted to the NCBI Gene Expression Omnibus (GEO) database under accession number GSE45395 (http://www.ncbi.nlm.nih.gov/geo/).
SureSelect and NGS
We designed SureSelect libraries to target the 40 kb flanking CNV breakpoints mapped by high-resolution array CGH. SureSelect target enrichment baits were designed using the “Bait Tiling” option in eArray. 120-bp baits were tiled with 3x coverage, 20-bp allowable overlap, and a centered design strategy. Electronic Library ID (ELID) #0349851 targeted breakpoint regions from 18q-186c and M397; ELID #0368031 targeted breakpoint regions from 18q-26c, 18q-119c, 18q-62c, M396, EGL044, EGL398, EGL399, and EGL074 (Table S1).
SureSelect capture and Illumina HiSeq sequencing were performed at Hudson Alpha Genomic Services Lab (http://www.hudsonalpha.org/gsl/). After NGS, we aligned 100-bp paired-end reads from fastq files to the GRC37/hg19 reference genome using Burrows-Wheeler Alignment (BWA) tool 0.5.9 [66] and identified misaligned pairs using the SAMTools 0.1.18 filter function [67]. Paired-end reads that aligned to the reference genome too far apart, too close together, in the wrong orientation/genome order, or to different chromosomes were clustered to predict structural variation, as described [68]. We identified split reads using CIGAR scores of the aligned reads and inspected junctions manually using Integrative Genomics Viewer (IGV) [69]. Using this approach, we successfully captured M397's inversion-translocation breakpoint, EGL074's disomy-inversion junction, and EGL044's disomy-inversion and inversion-telomere junctions. These junctions were confirmed by PCR and Sanger sequencing. Sequence data from SureSelect experiments have been deposited at the Sequence Read Archive (https://submit.ncbi.nlm.nih.gov/) under accession number SRP032751.
Breakpoint amplification and sequencing
We performed long-range PCR to amplify breakpoint junctions inferred from high-resolution array CGH following conditions described previously [29]. PCR primers are listed in Table S3. We optimized reactions by adjusting the MgCl concentration (1 mM–3 mM) or by adding Betaine (0.7–2.0 M), DMSO (1–10%), and/or Tween 20 (0.5–2%). We designed PCR primers to cross the two sides of the inverted duplication junction (including the disomic spacer), the disomy-inversion junction, the inversion-telomere junction, and the inversion-translocation junction, as appropriate. For inversion-telomere junctions, we designed a primer complementary to the inverted duplication side of the junction and paired this primer with one of two telomere primers, 5′-CCCTAACCCTAACCCTAACCCTAACCCTAA-3′ or 5′-TATGGATCCCTAACCCTGACCCTAACCC-3′ [42].
The disomy-inversion junction from 18q-26C was amplified via inverse PCR. A BsrDI restriction site is located ∼2 kb proximal to the distal end of the duplication, but is absent from the predicted spacer region. Genomic DNA (5 µg) from 18q-26C and a normal control was digested following the manufacturer's protocol (NEB #R0574S; 1 h at 65°C, 20 min at 80°C, and store at 4°C). Digested DNA was purified with a QIAquick Purification Kit (#28106) following the manufacturer's protocol. Blunt ends were created using T4 DNA Polymerase (NEB #M0203S) in 1X NEBuffer 2, supplemented with 100 µg/ml BSA and 100 µM dNTPs in a 50-µl reaction incubated 15 min at 12°C. The reaction was stopped with 1 µl of 0.5 M EDTA heated to 75°C for 20 min. Blunt-end fragments (≤50 ng DNA per 20 µl ligation reaction) were circularized and ligated with T4 DNA Ligase (Quick Ligation Kit, NEB #M2200L) for 5 min at room temperature. We performed PCR on circularized template DNA using outward-facing primers and standard PCR conditions.
PCR-amplified junctions were Sanger sequenced (Beckman Coulter Genomics, Danvers, MA). We aligned DNA sequences to the human genome reference assembly (GRC37/hg19) using the BLAT tool [34] on the UCSC Genome Browser (http://genome.ucsc.edu/). Disomy-inversion junctions from 18q-233c, SGTel014, and EGL104 aligned to interspersed repeats (Figure S2). Other junction sequences are described in Figure S1. Breakpoint junction sequences have been submitted to GenBank under project number 1611902. Accession numbers are listed in Table S1.
Microhomology simulation
To estimate the amount of inverted microhomology expected by chance at disomy-inversion breakpoints, we simulated 1,000 spacers in the human genome. We used the random number function and a custom Perl script to generate sequence coordinates for sequences less than 70,466 bp long (maximum sequenced spacer length) and within 5.5 Mb from the chromosome end (median terminal deletion size) from random chromosomes. Disomic spacers in the simulated dataset are between 811 bp and 70.5 kb long (mean = 36.1 kb). We downloaded each disomic spacer sequence from the Ensembl database and counted the bp of microhomology between the 3′ end of the spacer and the reverse complement of the 5′ end, allowing for zero mismatches using Perl regular expressions. The frequency of 0–8 bp of simulated inverted microhomology compared to observed microhomology is shown in Figure 5B.
To compute an empirical p-value based on these simulations, we first noted that 134 of 1,000 simulations had microhomology of ≥2 bp (the minimum-sized microhomology in the 13 disomy-inversion junctions). We then used simple combinatorics to count 1) the number of different 13-junction groups that could be formed from 134 simulated junctions, and 2) the number of different 13-junction groups possible from 1,000 simulated junctions. We computed our empirical p-value as the ratio of these values: ; this value is a simulation-based estimate of the proportion of 13-junction groups that would have ≥2 bp of microhomology for all 13 junctions by chance alone.
Microsatellite analysis
Microsatellite markers within the deleted and duplicated regions were selected from the UniSTS database (http://www.ncbi.nlm.nih.gov/unists; Table S2). We used the Type-it Microsatellite PCR Kit (Qiagen, Valencia, CA) and primers labeled with 6-carboxyfluorescine (6-FAM) or hexachloro-fluorescein (HEX) (Integrated DNA Technologies, Coralville, Iowa). Amplification was performed in 25-µl volumes with 50 ng of DNA template and 0.2 µM of each primer in a multiplexed reaction. The PCR cycles were 95°C for 5 min, then 26 cycles at 95°C for 30 s, 58°C for 90 s, 72°C for 30 s, with a final extension of 60°C for 60 min. We ran amplicons on a 16-capillary Applied Biosystems 3130XL Genetic Analyzer with a GeneScan 500 size standard. GeneMarker Software v1.95 (Soft Genetics, LLC, State College, PA) was used to size the alleles to the nearest bp and determine peak heights.
Supporting Information
Zdroje
1. WeleberRG, VermaRS, KimberlingWJ, FiegerHGJr, lubsHA (1976) Duplication-deficiency of the short arm of chromosome 8 following artificial insemination. Annales de genetique 19 : 241–247.
2. ZuffardiO, BonagliaM, CicconeR, GiordaR (2009) Inverted duplications deletions: underdiagnosed rearrangements?? Clinical genetics 75 : 505–513.
3. Rudd MK (2011) Structural variation in subtelomeres. In: Feuk L, editor. Genomic Structural Variants: Methods and Protocols. New York: Springer Science+Business Media, LLC.
4. BallifBC, YuW, ShawCA, KashorkCD, ShafferLG (2003) Monosomy 1p36 breakpoint junctions suggest pre-meiotic breakage-fusion-bridge cycles are involved in generating terminal deletions. Hum Mol Genet 12 : 2153–2165.
5. BonagliaMC, GiordaR, MassagliA, GalluzziR, CicconeR, et al. (2009) A familial inverted duplication/deletion of 2p25.1–25.3 provides new clues on the genesis of inverted duplications. European journal of human genetics : EJHG 17 : 179–186.
6. RoweLR, LeeJY, RectorL, KaminskyEB, BrothmanAR, et al. (2009) U-type exchange is the most frequent mechanism for inverted duplication with terminal deletion rearrangements. J Med Genet 46 : 694–702.
7. YuS, GrafWD (2010) Telomere capture as a frequent mechanism for stabilization of the terminal chromosomal deletion associated with inverted duplication. Cytogenetic and genome research 129 : 265–274.
8. Vera-CarbonellA, Lopez-ExpositoI, BafalliuJA, Ballesta-MartinezM, GloverG, et al. (2010) Molecular characterization of a new patient with a non-recurrent inv dup del 2q and review of the mechanisms for this rearrangement. American journal of medical genetics Part A 152A: 2670–2680.
9. TanakaH, CaoY, BergstromDA, KooperbergC, TapscottSJ, et al. (2007) Intrastrand annealing leads to the formation of a large DNA palindrome and determines the boundaries of genomic amplification in human cancer. Mol Cell Biol 27 : 1993–2002.
10. StephensPJ, McBrideDJ, LinML, VarelaI, PleasanceED, et al. (2009) Complex landscapes of somatic rearrangement in human breast cancer genomes. Nature 462 : 1005–1010.
11. CampbellPJ, YachidaS, MudieLJ, StephensPJ, PleasanceED, et al. (2010) The patterns and dynamics of genomic instability in metastatic pancreatic cancer. Nature 467 : 1109–1113.
12. StephensPJ, GreenmanCD, FuB, YangF, BignellGR, et al. (2011) Massive genomic rearrangement acquired in a single catastrophic event during cancer development. Cell 144 : 27–40.
13. GuenthoerJ, DiedeSJ, TanakaH, ChaiX, HsuL, et al. (2012) Assessment of palindromes as platforms for DNA amplification in breast cancer. Genome research 22 : 232–245.
14. OuelletteM, HettemaE, WustD, Fase-FowlerF, BorstP (1991) Direct and inverted DNA repeats associated with P-glycoprotein gene amplification in drug resistant Leishmania. The EMBO journal 10 : 1009–1016.
15. ButlerDK, YasudaLE, YaoMC (1995) An intramolecular recombination mechanism for the formation of the rRNA gene palindrome of Tetrahymena thermophila. Molecular and cellular biology 15 : 7117–7126.
16. QinZ, CohenSN (2000) Long palindromes formed in Streptomyces by nonrecombinational intra-strand annealing. Genes & development 14 : 1789–1796.
17. LinCT, LinWH, LyuYL, Whang-PengJ (2001) Inverted repeats as genetic elements for promoting DNA inverted duplication: implications in gene amplification. Nucleic Acids Research 29 : 3529–3538.
18. RattrayAJ, ShaferBK, NeelamB, StrathernJN (2005) A mechanism of palindromic gene amplification in Saccharomyces cerevisiae. Genes Dev 19 : 1390–1399.
19. AdmireA, ShanksL, DanzlN, WangM, WeierU, et al. (2006) Cycles of chromosome instability are associated with a fragile site and are increased by defects in DNA replication and checkpoint controls in yeast. Genes & development 20 : 159–173.
20. NarayananV, MieczkowskiPA, KimHM, PetesTD, LobachevKS (2006) The pattern of gene amplification is determined by the chromosomal location of hairpin-capped breaks. Cell 125 : 1283–1296.
21. LowdenMR, FlibotteS, MoermanDG, AhmedS (2011) DNA synthesis generates terminal duplications that seal end-to-end chromosome fusions. Science 332 : 468–471.
22. McClintockB (1939) The Behavior in Successive Nuclear Divisions of a Chromosome Broken at Meiosis. Proceedings of the National Academy of Sciences of the United States of America 25 : 405–416.
23. FloridiaG, PiantanidaM, MinelliA, DellavecchiaC, BonagliaC, et al. (1996) The same molecular mechanism at the maternal meiosis I produces mono - and dicentric 8p duplications. American journal of human genetics 58 : 785–796.
24. GiglioS, BromanKW, MatsumotoN, CalvariV, GimelliG, et al. (2001) Olfactory receptor-gene clusters, genomic-inversion polymorphisms, and common chromosome rearrangements. Am J Hum Genet 68 : 874–883.
25. PramparoT, GiglioS, GregatoG, de GregoriM, PatricelliMG, et al. (2004) Inverted duplications: how many of them are mosaic? European journal of human genetics : EJHG 12 : 713–717.
26. DanielA, St HeapsL, SylvesterD, DiazS, PetersG (2008) Two mosaic terminal inverted duplications arising post-zygotically: Evidence for possible formation of neo-telomeres. Cell & chromosome 7 : 1.
27. VannesteE, VoetT, Le CaignecC, AmpeM, KoningsP, et al. (2009) Chromosome instability is common in human cleavage-stage embryos. Nat Med 15 : 577–583.
28. VoetT, VannesteE, Van der AaN, MelotteC, JackmaertS, et al. (2011) Breakage-fusion-bridge cycles leading to inv dup del occur in human cleavage stage embryos. Human mutation 32 : 783–793.
29. LuoY, HermetzKE, JacksonJM, MulleJG, DoddA, et al. (2011) Diverse mutational mechanisms cause pathogenic subtelomeric rearrangements. Human molecular genetics 20 : 3769–3778.
30. ArltMF, MulleJG, SchaibleyVM, RaglandRL, DurkinSG, et al. (2009) Replication stress induces genome-wide copy number changes in human cells that resemble polymorphic and pathogenic variants. Am J Hum Genet 84 : 339–350.
31. OkunoY, HahnPJ, GilbertDM (2004) Structure of a palindromic amplicon junction implicates microhomology-mediated end joining as a mechanism of sister chromatid fusion during gene amplification. Nucleic acids research 32 : 749–756.
32. Nick McElhinnySA, HavenerJM, Garcia-DiazM, JuarezR, BebenekK, et al. (2005) A gradient of template dependence defines distinct biological roles for family X polymerases in nonhomologous end joining. Molecular cell 19 : 357–366.
33. SimsekD, JasinM (2010) Alternative end-joining is suppressed by the canonical NHEJ component Xrcc4-ligase IV during chromosomal translocation formation. Nature structural & molecular biology 17 : 410–416.
34. KentWJ (2002) BLAT–the BLAST-like alignment tool. Genome Res 12 : 656–664.
35. Hazkani-CovoE, ZellerRM, MartinW (2010) Molecular poltergeists: mitochondrial DNA copies (numts) in sequenced nuclear genomes. PLoS genetics 6: e1000834.
36. Willett-BrozickJE, SavulSA, RicheyLE, BaysalBE (2001) Germ line insertion of mtDNA at the breakpoint junction of a reciprocal constitutional translocation. Human genetics 109 : 216–223.
37. Hazkani-CovoE, CovoS (2008) Numt-mediated double-strand break repair mitigates deletions during primate genome evolution. PLoS genetics 4: e1000237.
38. YuAM, McVeyM (2010) Synthesis-dependent microhomology-mediated end joining accounts for multiple types of repair junctions. Nucleic acids research 38 : 5706–5717.
39. RossiE, RiegelM, MessaJ, GimelliS, MaraschioP, et al. (2008) Duplications in addition to terminal deletions are present in a proportion of ring chromosomes: clues to the mechanisms of formation. Journal of medical genetics 45 : 147–154.
40. MurmannAE, ConradDF, MashekH, CurtisCA, NicolaeRI, et al. (2009) Inverted duplications on acentric markers: mechanism of formation. Human molecular genetics 18 : 2241–2256.
41. GuilhermeRS, MeloniVF, KimCA, PellegrinoR, TakenoSS, et al. (2011) Mechanisms of ring chromosome formation, ring instability and clinical consequences. BMC medical genetics 12 : 171.
42. FlintJ, CraddockCF, VillegasA, BentleyDP, WilliamsHJ, et al. (1994) Healing of broken human chromosomes by the addition of telomeric repeats. Am J Hum Genet 55 : 505–512.
43. YatsenkoSA, BrundageEK, RoneyEK, CheungSW, ChinaultAC, et al. (2009) Molecular mechanisms for subtelomeric rearrangements associated with the 9q34.3 microdeletion syndrome. Hum Mol Genet 18 : 1924–1936.
44. RichardsonC, JasinM (2000) Frequent chromosomal translocations induced by DNA double-strand breaks. Nature 405 : 697–700.
45. MizunoK, MiyabeI, SchalbetterSA, CarrAM, MurrayJM (2013) Recombination-restarted replication makes inverted chromosome fusions at inverted repeats. Nature 493 : 246–249.
46. LeeJA, CarvalhoCM, LupskiJR (2007) A DNA replication mechanism for generating nonrecurrent rearrangements associated with genomic disorders. Cell 131 : 1235–1247.
47. ZhangF, KhajaviM, ConnollyAM, TowneCF, BatishSD, et al. (2009) The DNA replication FoSTeS/MMBIR mechanism can generate genomic, genic and exonic complex rearrangements in humans. Nat Genet 41 : 849–853.
48. HastingsPJ, IraG, LupskiJR (2009) A microhomology-mediated break-induced replication model for the origin of human copy number variation. PLoS genetics 5: e1000327.
49. SobreiraNL, GnanakkanV, WalshM, MarosyB, WohlerE, et al. (2011) Characterization of complex chromosomal rearrangements by targeted capture and next-generation sequencing. Genome research 21 : 1720–1727.
50. CarvalhoCM, RamockiMB, PehlivanD, FrancoLM, Gonzaga-JaureguiC, et al. (2011) Inverted genomic segments and complex triplication rearrangements are mediated by inverted repeats in the human genome. Nature genetics 43 : 1074–1081.
51. ChiangC, JacobsenJC, ErnstC, HanscomC, HeilbutA, et al. (2012) Complex reorganization and predominant non-homologous repair following chromosomal breakage in karyotypically balanced germline rearrangements and transgenic integration. Nature genetics 44 : 390–S391, 390-397, S391.
52. AnkalaA, KohnJN, HegdeA, MekaA, EphremCL, et al. (2012) Aberrant firing of replication origins potentially explains intragenic nonrecurrent rearrangements within genes, including the human DMD gene. Genome research 22 : 25–34.
53. BaldwinEL, LeeJY, BlakeDM, BunkeBP, AlexanderCR, et al. (2008) Enhanced detection of clinically relevant genomic imbalances using a targeted plus whole genome oligonucleotide microarray. Genet Med 10 : 415–429.
54. PerryGH, Ben-DorA, TsalenkoA, SampasN, Rodriguez-RevengaL, et al. (2008) The fine-scale and complex architecture of human copy-number variation. Am J Hum Genet 82 : 685–695.
55. ConradDF, BirdC, BlackburneB, LindsayS, MamanovaL, et al. (2010) Mutation spectrum revealed by breakpoint sequencing of human germline CNVs. Nat Genet 42 : 385–391.
56. MillsRE, WalterK, StewartC, HandsakerRE, ChenK, et al. (2011) Mapping copy number variation by population-scale genome sequencing. Nature 470 : 59–65.
57. ArltMF, WilsonTE, GloverTW (2012) Replication stress and mechanisms of CNV formation. Current opinion in genetics & development 22 : 204–210.
58. TurnerDJ, MirettiM, RajanD, FieglerH, CarterNP, et al. (2008) Germline rates of de novo meiotic deletions and duplications causing several genomic disorders. Nat Genet 40 : 90–95.
59. HeardPL, CarterEM, CrandallAC, SeboldC, HaleDE, et al. (2009) High resolution genomic analysis of 18q - using oligo-microarray comparative genomic hybridization (aCGH). Am J Med Genet A 149A: 1431–1437.
60. BonagliaMC, GiordaR, PoggiG, RaggiME, RossiE, et al. (2000) Inverted duplications are recurrent rearrangements always associated with a distal deletion: description of a new case involving 2q. Eur J Hum Genet 8 : 597–603.
61. KotzotD, MartinezMJ, BagciG, BasaranS, BaumerA, et al. (2000) Parental origin and mechanisms of formation of cytogenetically recognisable de novo direct and inverted duplications. Journal of medical genetics 37 : 281–286.
62. CotterPD, KaffeS, LiL, GershinIF, HirschhornK (2001) Loss of subtelomeric sequence associated with a terminal inversion duplication of the short arm of chromosome 4. Am J Med Genet 102 : 76–80.
63. ChenCP, ChernSR, LinSP, LinCC, LiYC, et al. (2005) A paternally derived inverted duplication of distal 14q with a terminal 14q deletion. American journal of medical genetics Part A 139A: 146–150.
64. CuscoI, del CampoM, VilardellM, GonzalezE, GenerB, et al. (2008) Array-CGH in patients with Kabuki-like phenotype: identification of two patients with complex rearrangements including 2q37 deletions and no other recurrent aberration. BMC medical genetics 9 : 27.
65. ManolakosE, SifakisS, SotiriouS, PeitsidisP, EleftheriadesM, et al. (2012) Prenatal detection of an inverted duplication deletion in the long arm of chromosome 1 in a fetus with increased nuchal translucency. Molecular cytogenetic analysis and review of the literature. Clinical dysmorphology 21 : 101–105.
66. LiH, DurbinR (2009) Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25 : 1754–1760.
67. LiH, HandsakerB, WysokerA, FennellT, RuanJ, et al. (2009) The Sequence Alignment/Map format and SAMtools. Bioinformatics 25 : 2078–2079.
68. NgCK, CookeSL, HoweK, NewmanS, XianJ, et al. (2012) The role of tandem duplicator phenotype in tumour evolution in high-grade serous ovarian cancer. The Journal of pathology 226 : 703–712.
69. RobinsonJT, ThorvaldsdottirH, WincklerW, GuttmanM, LanderES, et al. (2011) Integrative genomics viewer. Nature biotechnology 29 : 24–26.
Štítky
Genetika Reprodukční medicína
Článek Unwrapping BacteriaČlánek A Chaperone-Assisted Degradation Pathway Targets Kinetochore Proteins to Ensure Genome StabilityČlánek The Candidate Splicing Factor Sfswap Regulates Growth and Patterning of Inner Ear Sensory OrgansČlánek The SPF27 Homologue Num1 Connects Splicing and Kinesin 1-Dependent Cytoplasmic Trafficking inČlánek Down-Regulation of eIF4GII by miR-520c-3p Represses Diffuse Large B Cell Lymphoma DevelopmentČlánek Meta-Analysis Identifies Gene-by-Environment Interactions as Demonstrated in a Study of 4,965 MiceČlánek High Risk Population Isolate Reveals Low Frequency Variants Predisposing to Intracranial Aneurysms
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2014 Číslo 1
-
Všechny články tohoto čísla
- How Much Is That in Dog Years? The Advent of Canine Population Genomics
- The Sense and Sensibility of Strand Exchange in Recombination Homeostasis
- Unwrapping Bacteria
- DNA Methylation Changes Separate Allergic Patients from Healthy Controls and May Reflect Altered CD4 T-Cell Population Structure
- Evidence for Mito-Nuclear and Sex-Linked Reproductive Barriers between the Hybrid Italian Sparrow and Its Parent Species
- Translation Enhancing ACA Motifs and Their Silencing by a Bacterial Small Regulatory RNA
- Relationship Estimation from Whole-Genome Sequence Data
- Genetic Models of Apoptosis-Induced Proliferation Decipher Activation of JNK and Identify a Requirement of EGFR Signaling for Tissue Regenerative Responses in
- ComEA Is Essential for the Transfer of External DNA into the Periplasm in Naturally Transformable Cells
- Loss and Recovery of Genetic Diversity in Adapting Populations of HIV
- Bioelectric Signaling Regulates Size in Zebrafish Fins
- Defining NELF-E RNA Binding in HIV-1 and Promoter-Proximal Pause Regions
- Loss of Histone H3 Methylation at Lysine 4 Triggers Apoptosis in
- Cell-Cycle Dependent Expression of a Translocation-Mediated Fusion Oncogene Mediates Checkpoint Adaptation in Rhabdomyosarcoma
- How a Retrotransposon Exploits the Plant's Heat Stress Response for Its Activation
- A Nonsense Mutation in Encoding a Nondescript Transmembrane Protein Causes Idiopathic Male Subfertility in Cattle
- Deletion of a Conserved -Element in the Locus Highlights the Role of Acute Histone Acetylation in Modulating Inducible Gene Transcription
- Developmental Link between Sex and Nutrition; Regulates Sex-Specific Mandible Growth via Juvenile Hormone Signaling in Stag Beetles
- PP2A/B55 and Fcp1 Regulate Greatwall and Ensa Dephosphorylation during Mitotic Exit
- Differential Effects of Collagen Prolyl 3-Hydroxylation on Skeletal Tissues
- Comprehensive Functional Annotation of 77 Prostate Cancer Risk Loci
- Evolution of Chloroplast Transcript Processing in and Its Chromerid Algal Relatives
- A Chaperone-Assisted Degradation Pathway Targets Kinetochore Proteins to Ensure Genome Stability
- New MicroRNAs in —Birth, Death and Cycles of Adaptive Evolution
- A Genome-Wide Screen for Bacterial Envelope Biogenesis Mutants Identifies a Novel Factor Involved in Cell Wall Precursor Metabolism
- FGFR1-Frs2/3 Signalling Maintains Sensory Progenitors during Inner Ear Hair Cell Formation
- Regulation of Synaptic /Neuroligin Abundance by the /Nrf Stress Response Pathway Protects against Oxidative Stress
- Intrasubtype Reassortments Cause Adaptive Amino Acid Replacements in H3N2 Influenza Genes
- Molecular Specificity, Convergence and Constraint Shape Adaptive Evolution in Nutrient-Poor Environments
- WNT7B Promotes Bone Formation in part through mTORC1
- Natural Selection Reduced Diversity on Human Y Chromosomes
- In-Vivo Quantitative Proteomics Reveals a Key Contribution of Post-Transcriptional Mechanisms to the Circadian Regulation of Liver Metabolism
- The Candidate Splicing Factor Sfswap Regulates Growth and Patterning of Inner Ear Sensory Organs
- The Acid Phosphatase-Encoding Gene Contributes to Soybean Tolerance to Low-Phosphorus Stress
- p53 and TAp63 Promote Keratinocyte Proliferation and Differentiation in Breeding Tubercles of the Zebrafish
- Affects Plant Architecture by Regulating Local Auxin Biosynthesis
- The SET Domain Proteins SUVH2 and SUVH9 Are Required for Pol V Occupancy at RNA-Directed DNA Methylation Loci
- Down-Regulation of Rad51 Activity during Meiosis in Yeast Prevents Competition with Dmc1 for Repair of Double-Strand Breaks
- Multi-tissue Analysis of Co-expression Networks by Higher-Order Generalized Singular Value Decomposition Identifies Functionally Coherent Transcriptional Modules
- A Neurotoxic Glycerophosphocholine Impacts PtdIns-4, 5-Bisphosphate and TORC2 Signaling by Altering Ceramide Biosynthesis in Yeast
- Subtle Changes in Motif Positioning Cause Tissue-Specific Effects on Robustness of an Enhancer's Activity
- C/EBPα Is Required for Long-Term Self-Renewal and Lineage Priming of Hematopoietic Stem Cells and for the Maintenance of Epigenetic Configurations in Multipotent Progenitors
- The SPF27 Homologue Num1 Connects Splicing and Kinesin 1-Dependent Cytoplasmic Trafficking in
- Down-Regulation of eIF4GII by miR-520c-3p Represses Diffuse Large B Cell Lymphoma Development
- Genome Sequencing Highlights the Dynamic Early History of Dogs
- Re-sequencing Expands Our Understanding of the Phenotypic Impact of Variants at GWAS Loci
- Meta-Analysis Identifies Gene-by-Environment Interactions as Demonstrated in a Study of 4,965 Mice
- , a -Antisense Gene of , Encodes a Evolved Protein That Inhibits GSK3β Resulting in the Stabilization of MYCN in Human Neuroblastomas
- A Transcription Factor Is Wound-Induced at the Planarian Midline and Required for Anterior Pole Regeneration
- A Comprehensive tRNA Deletion Library Unravels the Genetic Architecture of the tRNA Pool
- A PNPase Dependent CRISPR System in
- Genomic Confirmation of Hybridisation and Recent Inbreeding in a Vector-Isolated Population
- Zinc Finger Transcription Factors Displaced SREBP Proteins as the Major Sterol Regulators during Saccharomycotina Evolution
- GATA6 Is a Crucial Regulator of Shh in the Limb Bud
- Tissue Specific Roles for the Ribosome Biogenesis Factor Wdr43 in Zebrafish Development
- A Cell Cycle and Nutritional Checkpoint Controlling Bacterial Surface Adhesion
- High Risk Population Isolate Reveals Low Frequency Variants Predisposing to Intracranial Aneurysms
- E3 Ubiquitin Ligase CHIP and NBR1-Mediated Selective Autophagy Protect Additively against Proteotoxicity in Plant Stress Responses
- Evolutionary Rate Covariation Identifies New Members of a Protein Network Required for Female Post-Mating Responses
- 3′ Untranslated Regions Mediate Transcriptional Interference between Convergent Genes Both Locally and Ectopically in
- Single Nucleus Genome Sequencing Reveals High Similarity among Nuclei of an Endomycorrhizal Fungus
- Metabolic QTL Analysis Links Chloroquine Resistance in to Impaired Hemoglobin Catabolism
- Notch Controls Cell Adhesion in the Drosophila Eye
- AL PHD-PRC1 Complexes Promote Seed Germination through H3K4me3-to-H3K27me3 Chromatin State Switch in Repression of Seed Developmental Genes
- Genomes Reveal Evolution of Microalgal Oleaginous Traits
- Large Inverted Duplications in the Human Genome Form via a Fold-Back Mechanism
- Variation in Genome-Wide Levels of Meiotic Recombination Is Established at the Onset of Prophase in Mammalian Males
- Age, Gender, and Cancer but Not Neurodegenerative and Cardiovascular Diseases Strongly Modulate Systemic Effect of the Apolipoprotein E4 Allele on Lifespan
- Lifespan Extension Conferred by Endoplasmic Reticulum Secretory Pathway Deficiency Requires Induction of the Unfolded Protein Response
- Is Non-Homologous End-Joining Really an Inherently Error-Prone Process?
- Vestigialization of an Allosteric Switch: Genetic and Structural Mechanisms for the Evolution of Constitutive Activity in a Steroid Hormone Receptor
- Functional Divergence and Evolutionary Turnover in Mammalian Phosphoproteomes
- A 660-Kb Deletion with Antagonistic Effects on Fertility and Milk Production Segregates at High Frequency in Nordic Red Cattle: Additional Evidence for the Common Occurrence of Balancing Selection in Livestock
- Comparative Evolutionary and Developmental Dynamics of the Cotton () Fiber Transcriptome
- The Transcription Factor BcLTF1 Regulates Virulence and Light Responses in the Necrotrophic Plant Pathogen
- Crossover Patterning by the Beam-Film Model: Analysis and Implications
- Single Cell Genomics: Advances and Future Perspectives
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- GATA6 Is a Crucial Regulator of Shh in the Limb Bud
- Large Inverted Duplications in the Human Genome Form via a Fold-Back Mechanism
- Differential Effects of Collagen Prolyl 3-Hydroxylation on Skeletal Tissues
- Affects Plant Architecture by Regulating Local Auxin Biosynthesis
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



