-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaHow a Retrotransposon Exploits the Plant's Heat Stress Response for Its Activation
Retrotransposons are major components of plant and animal genomes. They amplify by reverse transcription and reintegration into the host genome but their activity is usually epigenetically silenced. In plants, genomic copies of retrotransposons are typically associated with repressive chromatin modifications installed and maintained by RNA-directed DNA methylation. To escape this tight control, retrotransposons employ various strategies to avoid epigenetic silencing. Here we describe the mechanism developed by ONSEN, an LTR-copia type retrotransposon in Arabidopsis thaliana. ONSEN has acquired a heat-responsive element recognized by plant-derived heat stress defense factors, resulting in transcription and production of full length extrachromosomal DNA under elevated temperatures. Further, the ONSEN promoter is free of CG and CHG sites, and the reduction of DNA methylation at the CHH sites is not sufficient to activate the element. Since dividing cells have a more pronounced heat response, the extrachromosomal ONSEN DNA, capable of reintegrating into the genome, accumulates preferentially in the meristematic tissue of the shoot. The recruitment of a major plant heat shock transcription factor in periods of heat stress exploits the plant's heat stress response to achieve the transposon's activation, making it impossible for the host to respond appropriately to stress without losing control over the invader.
Published in the journal: . PLoS Genet 10(1): e32767. doi:10.1371/journal.pgen.1004115
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004115Summary
Retrotransposons are major components of plant and animal genomes. They amplify by reverse transcription and reintegration into the host genome but their activity is usually epigenetically silenced. In plants, genomic copies of retrotransposons are typically associated with repressive chromatin modifications installed and maintained by RNA-directed DNA methylation. To escape this tight control, retrotransposons employ various strategies to avoid epigenetic silencing. Here we describe the mechanism developed by ONSEN, an LTR-copia type retrotransposon in Arabidopsis thaliana. ONSEN has acquired a heat-responsive element recognized by plant-derived heat stress defense factors, resulting in transcription and production of full length extrachromosomal DNA under elevated temperatures. Further, the ONSEN promoter is free of CG and CHG sites, and the reduction of DNA methylation at the CHH sites is not sufficient to activate the element. Since dividing cells have a more pronounced heat response, the extrachromosomal ONSEN DNA, capable of reintegrating into the genome, accumulates preferentially in the meristematic tissue of the shoot. The recruitment of a major plant heat shock transcription factor in periods of heat stress exploits the plant's heat stress response to achieve the transposon's activation, making it impossible for the host to respond appropriately to stress without losing control over the invader.
Introduction
Transposable elements (TEs) and their host organisms depend on each other for better or for worse. New TE insertions can give rise to deleterious mutations [1] or overall genetic instability [2], but they can also make a positive contribution to gene regulation and adaptation [3], [4]. Host organisms have developed mechanisms to reach a balance between both consequences by suppressing TE activity. Plants have evolved a complex regulatory network of epigenetic silencing that is effective for numerous different TEs. Silent elements are typically associated with high levels of DNA methylation at cytosines in every sequence context (mCG, mCHG, mCHH, where H stands for A, T or C), with methylation at lysine 9 of histone H3 (H3K9me2), and with the presence of 24 nt small interfering RNAs (siRNAs) that guide the RNA-directed DNA methylation (RdDM) machinery in a reinforcing loop, reviewed in [5], [6], [7]. Disruption of DNA methylation patterns can activate transposons, as, for instance, a null mutation of the Arabidopsis maintenance METHYLTRANSFERASE 1 (MET1) activates EVADÉ (EVD), a retrotransposon of the ATCOPIA93 family that can amplify during sexual propagation of the mutant [8]. EVADÉ is also transcriptionally active in plants with hypomethylated DNA due to a lack of the chromatin-remodeling factor DECREASE IN DNA METHYLATION 1 (DDM1). DDM1 mutants and many other plants lacking components of the RdDM pathway activate a specific but partially overlapping subset of TEs, including members of ATCOPIA13, ATCOPIA21, ATGP3 retrotransposon families and VANDAL21 and CACTA DNA transposons [9], [10], [11].
In addition to genetic interference, TEs can be activated by stress. In fact, this was already postulated by the discoverer of TEs [12] who also recognized the important role of these elements for gene regulation. Stress-induced transposon activation was later documented by molecular data in many different hosts, for instance the activation of the Tnt1 element by pathogens in tobacco [13], [14], of the ZmMI1 in maize and the PAL/Tam3 in snapdragon by cold [15], [16], [17], or of the CLCoi1 by wounding or salt stress in lemon [18]. More recently, a Ty1/copia-type long terminal repeat (LTR) retrotransposon family (ATCOPIA78) named ONSEN was found activated by heat stress in Arabidopsis [19], [20]. Surprisingly, without heat stress, ONSEN was not expressed in ddm1 mutants [19] or other mutants lacking RdDM components, in contrast to most other known and potentially functional TEs in Arabidopsis. However, new ONSEN insertions were found in the progeny of heat-stressed plants deficient in small RNA production, and this retrotransposition appears to occur during flower development and before gametogenesis [21]. Higher activation in callus compared to vegetative tissue indicates a possible coupling to active cell cycling [22]. Transcription of ONSEN-related sequences after heat exposure was found in most species of the Brassicaceae [23], indicating a conserved mechanism of activation and control of their spreading.
Here, we provide insight into the initiation of ONSEN activation in Arabidopsis, quantifying transcription, and the formation of extrachromosomal DNA upon extended heat stress. We also determine which of the genomic copies become transcriptionally active. Although the LTR sequences representing the ONSEN promoter are methylated, reduction of the methylation is not sufficient to activate the element. Rather, the LTR has acquired a sequence that is recognized by the plant's heat-responsive transcription factors, thereby coupling ONSEN activation to an important stress defense. Even more cunningly, this response is most pronounced in regions comprising the meristematic zone, providing an enhanced chance for new ONSEN copies to enter the next generation.
Results
Transcriptional activation of ONSEN during heat stress is followed by efficient multiplication of extrachromosomal DNA copies
A. thaliana grows mainly in zones with moderate climates. A temperature of 37°C represents acute and drastic heat stress for this plant, as shown by the quick transcriptional activation of many heat shock factors, [reviewed in 24]. The heat-induced transcriptional activation of retrotransposon ONSEN was also observed above a threshold of 37°C [19], [20], [22], though only after an extended exposure to heat [19], [20]. To determine the kinetics of ONSEN activation in more detail, we monitored the ONSEN expression at several time points during 30 h of heat stress (HS) treatment, keeping the regular long-day light regime (Fig. 1A). This treatment causes substantial growth arrest but is sublethal and allows recovery of the plants upon subsequent transfer to ambient temperature [19]. ONSEN RNA was first quantified by qRT-PCR 6 hours after the onset of heat stress, and its amount continued to increase to the highest level after 24 h, remaining high until 30 h, the end point of the stress treatment (Fig. 1B).
Fig. 1. ONSEN is activated by heat stress. 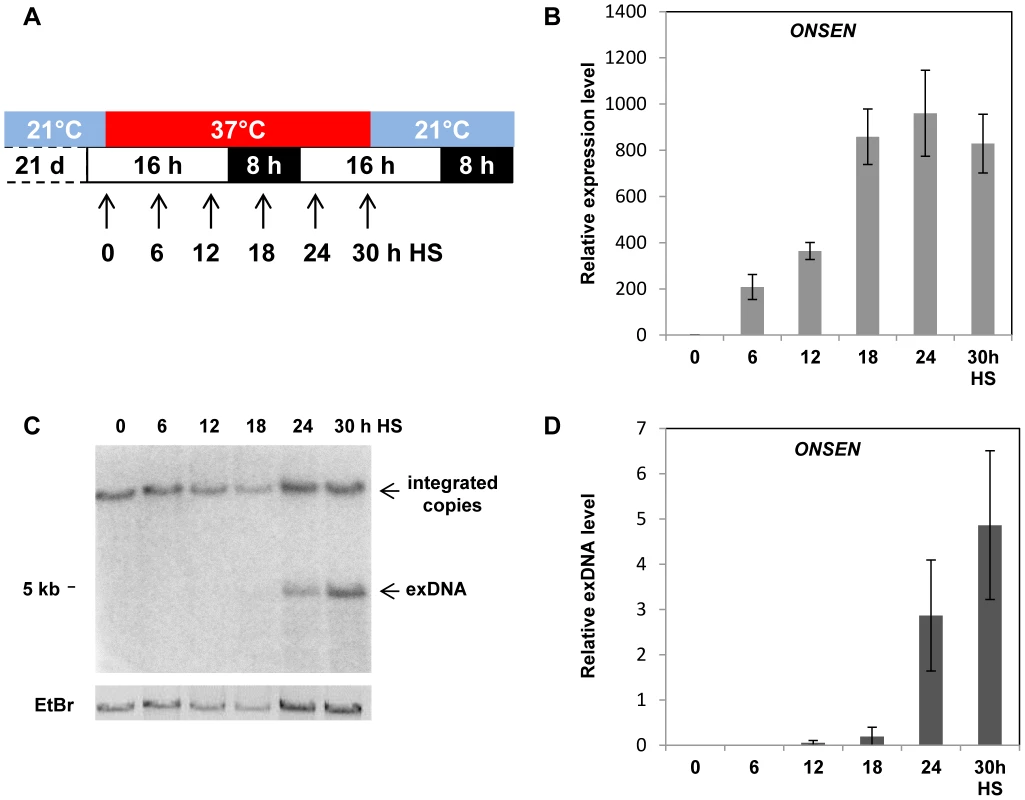
(A) Schematic representation of the experiments. White and black boxes represent light or darkness intervals; blue and red boxes represent periods of standard growth temperature or heat stress, arrows indicate sampling time points. (B) Relative amounts of ONSEN RNA determined by quantitative RT-PCR in three week-old wild type seedlings harvested as indicated in A. Bars represent the ONSEN transcripts in relation to that of AtSAND (equal level in all samples) and normalized to WT at 0 h HS. Error bars correspond to the s.e.m. (n = 3). (C) Southern blot analysis of undigested genomic DNA isolated from the same material as in B and hybridized to an ONSEN-specific probe. Upper and lower arrows indicate integrated and extrachromosomal ONSEN copies, respectively. The EtBr image indicates the loading of genomic DNA. (D) Quantification of ONSEN extrachromosomal DNA based on C. Bars represent the ratio between signal intensities of extrachromosomal and integrated copies determined by densitometry. Error bars correspond to the s.d. (n = 3). Following transcriptional activation, retrotransposons form linear extrachromosomal DNA copies along with circular by-products of replication; these are capable of reintegrating in the genome [25], [26], [27]. To determine the presence and amount of extrachromosomal ONSEN DNA, we performed Southern blot analysis with non-digested DNA from samples collected at the same time points as for the RNA. The single, high molecular weight band hybridizing to the ONSEN probe in the non-heat stressed samples corresponds to ONSEN copies integrated in the genomic DNA (gDNA). An additional band present in later heat stress samples indeed indicated extrachromosomal ONSEN DNA (exDNA), with a size of 5 kb corresponding to the expected full length of the linear element that is capable of reintegrating in the genome (Fig. 1C). To quantify the relative amount of exDNA, we calculated the relative intensity of hybridization signals between exDNA and gDNA in each sample, postulating a fixed number of integrated ONSEN copies. Small amounts of linear exDNA of ONSEN first appeared after 12 hours of heat stress. The maximum was achieved after 30 hours HS, corresponding to approximately five times more than integrated in the genome (Fig. 1D). Therefore, the formation of the linear extrachromosomal ONSEN DNA follows the transcriptional activation during heat stress after approximately 6 hours, likely reflecting the need for a threshold level of RNA and time to complete the reverse transcription. Once started, the process can produce many more additional copies than templates present in the genome.
Most extrachromosomal DNA is derived from younger genomic copies
The family of ONSEN retrotransposons in the Col-0 reference genome consists of eight full length copies distributed over chromosomes 1, 3, and 5 (Supplementary Fig. S1A). To investigate whether all copies contribute to the pool of extrachromosomal DNA under heat stress, we performed a sequencing analysis of isolated exDNA, scoring for element-specific SNPs in the ONSEN coding region that distinguish seven out of eight genomic templates (Supplementary Fig. S1A, Supplementary Table S1). Out of 55 independent clones, 57% could be assigned to three elements (ONSEN 1: At1g11265; ONSEN 2: At3g61330; and ONSEN 3: At5g13205; Fig. 2) that form a subgroup with 100% identical 5′and 3′ LTRs, indicating their evolutionarily recent transposition [21]. Several other sequences (20%) contained additional and different SNPs, so not allowing an unambiguous assignment to genomic templates and supporting the notion that reverse transcription is an error-prone process [28].
Fig. 2. Several ONSEN genomic copies contribute to the extrachromosomal DNA. 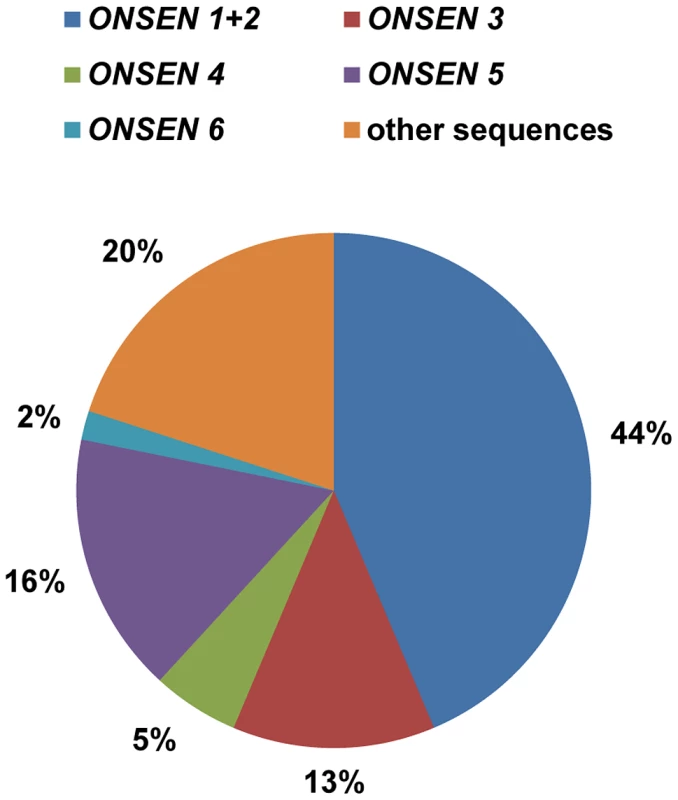
The pie chart indicates the ratio of extrachromosomal ONSEN sequences (n = 55) present after 30 h HS, distinguished by element-specific polymorphisms (color-coded). Polymorphisms are listed in Supplementary Table S1. In spite of the prevalence of the elements with perfect LTRs, three other ONSEN elements from the Col-0 genome contributed 23% to the pool of sequences, indicating that ONSEN 4, 5 and 6 (At1g58140, At1g48710 and At3g59720) are still functional and capable of forming extrachromosomal copies. In contrast, ONSEN 7 and 8 (At1g21945 and At3g32415) were not represented. Interestingly, these two copies are shared between most natural accessions of Arabidopsis [23], and they have acquired the largest number of polymorphisms.
DNA demethylation of the LTR is not sufficient to activate ONSEN but favors its heat stress response
RdDM is the major pathway to restrict transposon mobility by installing DNA methylation, leading to a reinforcing loop that creates a heterochromatic environment. Most RdDM targets have multiple CHG and CHH sequences, which become modified by cooperative action of two different DNA methyltransferases. However, the LTR of active ONSEN copies contains only CHH sites (Supplementary Fig. S1B). To analyze the DNA methylation state at the 5′ LTRs of several ONSEN elements, we performed bisulfite conversion and sequencing before and after heat stress, distinguishing three genomic copies by specific primers in the flanking genomic regions (Supplementary Table S2). At ambient temperature, the two most recently transposed elements (ONSEN 1 and 2) showed relatively high CHH methylation profiles across their 5′LTRs (Fig. 3A, Supplementary Fig. S2A, B, and Supplementary Dataset S1). Strikingly, ONSEN 8 that was not activated had substantially less methylation under normal growth conditions (Fig. 3A, Supplementary Fig. S2C). After heat stress, ONSEN 8 was more methylated throughout the 5′LTR, while ONSEN 1 and 2 lost the modification at several positions (Fig. 3A, Supplementary Fig. S2D–F).
Fig. 3. Loss of CHH DNA methylation at the promoter is not sufficient to activate the element but enhances the response to heat stress. 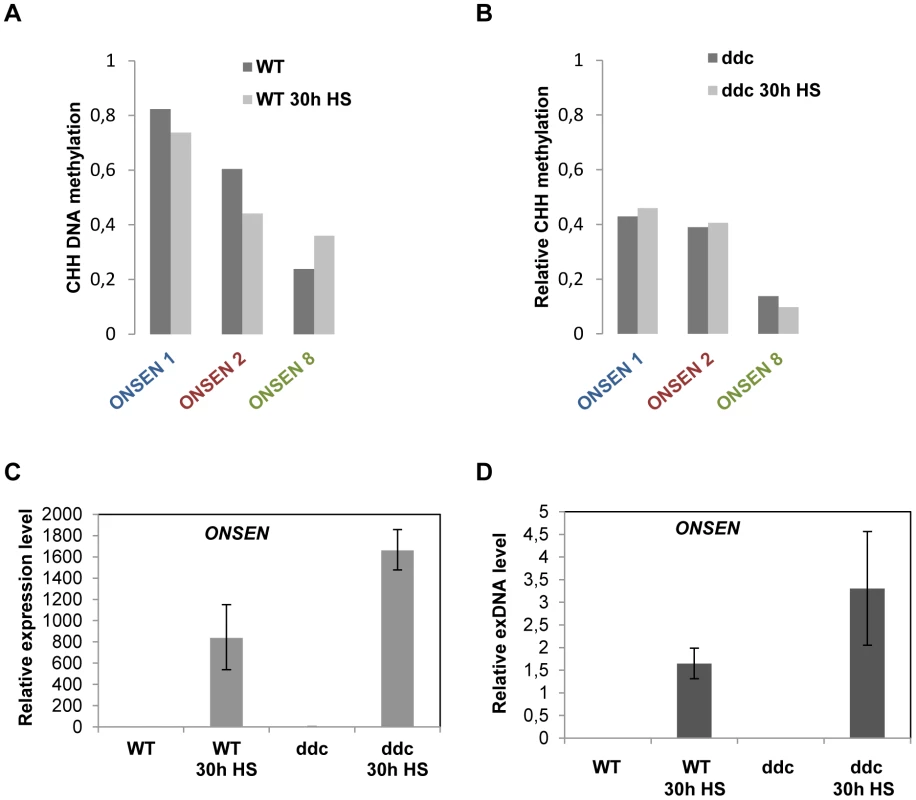
(A) Total CHH methylation at LTRs of ONSEN 1 and 2 (contributing the majority of exDNA) and ONSEN 8 (not activated) in non-stressed and 30 h HS wild type plants. Only CHH positions shared between all three LTRs were considered. CHH methylation profiles along the LTRs and data for individual genomic copies are shown in Supplementary Fig. 2A–F and Supplementary Dataset S1. (B) Total CHH methylation of the same ONSEN LTRs in non-stressed and 30 h HS drm1/drm2/cmt3 triple mutant plants (ddc). Only CHH positions shared between all three LTRs were considered. CHH methylation profiles along the LTRs and data for individual genomic copies are shown in Supplementary Fig. 2G–L and Supplementary Dataset S1. (C) Relative amount of ONSEN RNA determined by quantitative RT-PCR in three week old wild type and ddc mutant plants, non-stressed and after 30 h HS. Bars represent the ONSEN transcripts in relation to that of AtSAND and normalized to WT at 0 h HS. Error bars correspond to the s.e.m. (n = 3). (D) Quantification of ONSEN extrachromosomal DNA from the same samples as in F. Bars represent the ratio between extrachromosomal and integrated copies determined by densitometry after Southern blot analysis. Error bars correspond to the s.d. (n = 3). To investigate whether this erasure of DNA methylation had any impact on the level of ONSEN activation, we repeated RNA and exDNA quantification as well as DNA methylation analysis in the triple mutant ddc (drm1/drm2/cmt3), lacking RdDM-mediated methylation at CHH and CHG sites [29]. As expected, the levels of CHH methylation were significantly reduced at the ONSEN LTRs even in unstressed mutant plants (Fig. 3B, Supplementary Fig. S2G–I, and Supplementary Dataset S1). However, this was not sufficient to trigger any ONSEN transcription or exDNA formation (Fig. 3C, D). After heat stress, the already reduced CHH methylation in the triple mutant was hardly changed (Fig. 3B, Supplementary Fig. S2J–L and Supplementary Dataset S1), but the levels of ONSEN mRNA, as well as its extrachromosomal DNA, were significantly increased after heat stress compared to Col-0 wild type (Fig. 3C, D). Sequencing 47 clones of ONSEN extrachromosomal DNA from ddc mutant plants after heat stress revealed an even more dominant representation of activated ONSEN 1 and 2 than in the wild type (Supplementary Fig. S3). Collectively, these results indicate that reduction of DNA methylation at the retrotransposon's promoter does not per se activate the element but can favor the activation of ONSEN upon heat exposure.
ONSEN activation requires the plant's heat stress response pathway
The strong activation of ONSEN by heat stress conditions, together with the enhanced response in the methylation triple mutant, suggested the dependence on a heat-induced transcription factor whose action could be modulated by presence or absence of the DNA modification. Therefore, we analyzed the ONSEN promoter for cis-regulatory elements and found a heat response element (HRE) with the consensus sequence nTTCnnGAAn in the LTR of all elements (Supplementary Fig. S1C). HREs are bound by heat shock factors (HSFs), which form trimers and thereby induce expression of downstream target genes [30]. Arabidopsis has 21 heat shock factors [31], among which HSFA1 was identified as a main positive regulator in heat-responsive gene expression [32].
To analyze whether HSFA1 mediates the transcriptional activation of the retrotransposon we determined ONSEN RNA and exDNA in hsfa1 mutants. There are 4 genes for HSFA1-type proteins in Arabidopsis [a, b, d, e; 32] with partially redundant functions. We tested all different combinations of triple mutants along with the quadruple mutant (Fig. 4). HSFA1b or d were sufficient to activate ONSEN to comparable levels as in the wild type, and HSFA1a to a lower extent. In contrast, no ONSEN RNA or exDNA was formed after heat stress in the quadruple mutant or in plants with HSFA1e as the only functional HSFA1 factor. Therefore, either HSFA1a, or HSFA1b, or HSFA1d are necessary for heat-induced expression of ONSEN. However, they were unlikely candidates for direct transcriptional activators of ONSEN, since all three genes are constitutively expressed and proposed to initiate a cascade of heat stress-responsive genes only upon additional signals [32].
Fig. 4. Heat shock factors are necessary for ONSEN activation by heat stress. 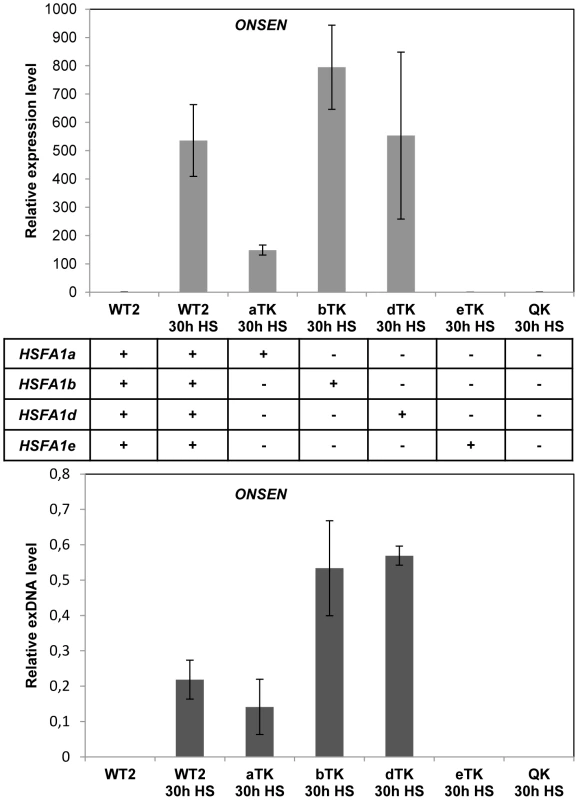
Relative amounts of ONSEN RNA and extrachromosomal DNA prepared from three week-old, non-stressed or 30 h HS treated seedlings of wild type Wassilewskija (WT2, background of the mutants), triple or quadruple mutants lacking three or all functional HSFA1 proteins (A, B, D, E). RNA and exDNA were determined by quantitative RT-PCR and densitometry after Southern blot analysis as described before (n = 3). ONSEN activation requires the plant's heat-induced transcription factor HSFA2
Transcripts of the Arabidopsis heat stress transcription factor HSFA2 are not detectable at ambient temperatures, but the gene is most strongly and stably expressed under heat stress conditions [33]. The protein can be found in leaves after 3 hours of heat stress and is still present after 21 hours of recovery [34]. We quantified the HSFA2 transcript under our heat stress conditions in wild type, hsfa1 triple and quadruple mutants. Interestingly, HSFA2 transcription showed the same dependence on the individual HSFA1 factors as ONSEN, hardly or not at all transcribed upon lack of HSFA1a,b,d or of all four factors (Fig. 5A). This suggested HSFA2 as a candidate for the heat stress factor necessary to activate ONSEN.
Fig. 5. HSFA2 acts downstream of HSFA1 and is able to bind to the ONSEN LTR. 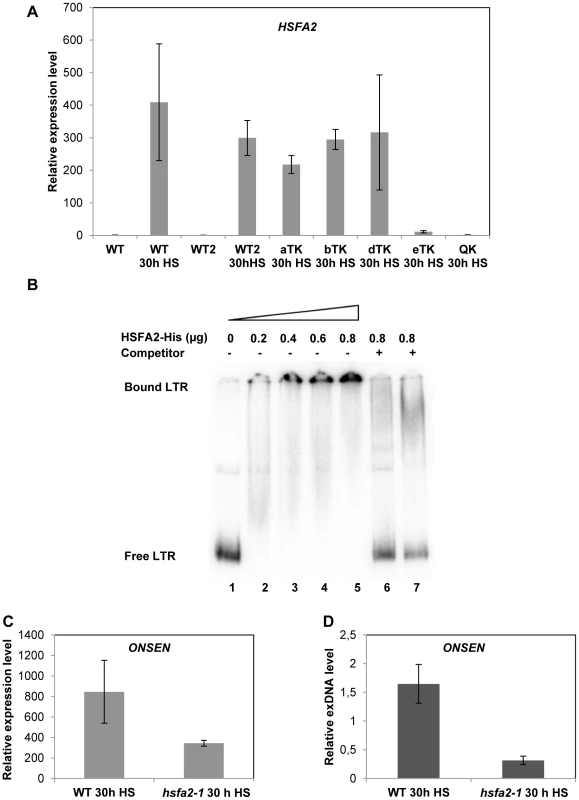
(A) Relative amount of HSFA2 RNA determined by quantitative RT-PCR in three week old WT (Col-0), WT2 (Ws), triple and quadruple HSFA1 mutant seedlings. Bars represent the HSFA2 transcript in relation to AtSAND and normalized to Col-0 WT at 0 h HS. Error bars correspond to the s.e.m. (n = 3). (B) Electrophoretic mobility shift assay (EMSA) with increasing amounts (lanes 1–5) of recombinant HSFA2 protein and constant amounts of radioactively labeled ONSEN LTR fragment derived from ONSEN 1. Competition by unlabeled DNA of the full length LTR in 50-fold excess (lane 6) or the fragment containing only the heat-responsive element (Supplementary Table S2) in a 200-fold excess (lane 7) demonstrate the binding specificity. (C) Relative amount of ONSEN RNA determined by quantitative RT-PCR in wild type and hsfa2-1 mutant plants after 30 h HS. Bars represent the ONSEN transcripts in relation to that of AtSAND and normalized to WT at 0 h HS. Error bars correspond to the s.e.m. (n = 3). (D) Quantification of ONSEN extrachromosomal DNA from the same samples as in C. Bars represent the ratio between extrachromosomal and integrated copies determined by densitometry after Southern blot analysis. Error bars correspond to the s.d. (n = 3). The HSFA2 protein has a highly conserved N-terminal DNA binding domain (DBD) that is required for its binding to HREs, and mutation within the domain or within the HRE block the binding [34] . To investigate whether HSFA2 would bind to the HRE in the ONSEN promoter we performed electrophoretic mobility shift assays (EMSAs) with recombinant Arabidopsis HSFA2 protein and the LTR DNA (Fig. 5B). Indeed, HSFA2 bound to the LTR probe in a concentration-dependent and specific manner: increasing amounts of the protein enhanced the shift (Fig. 5B, lanes 1–5), while pre-incubation with non-labeled LTR fragment inhibited the shift of the labeled probe (Fig. 5B, lane 6). The specificity of HSFA2 binding to the HRE in the LTR could be further supported by successful binding competition with an LTR fragment containing just 51 bp with the complete HRE (Fig. 5B, lane 7).
To further confirm the involvement of HSFA2 in transcriptional activation of ONSEN we quantified RNA and extrachromosomal DNA with and without heat stress in the hsfa2-1 mutant (Fig. 5C, D). ONSEN activation was severely reduced in the mutant, although not to the same low level as in the hsfa1a/b/d triple or the hsfa1a/b/d/e quadruple mutants. This indicates that HSFA2 is a major, but probably not the only heat shock factor involved in the heat-induced activation of ONSEN. However, it is clear that the HRE in its promoter couples the retrotransposon to the heat response pathway of the plant, thereby exploiting an important defense mechanism of its host to prepare for its own amplification.
The heat-responsive LTR of ONSEN is preferentially active in tissues with cell divisions
Transgenic analysis of the HSFA2 promoter activity by fusion with the GUS reporter gene had revealed very low activity under non-stress conditions but high expression under heat stress, in rosette leaves and even more in veins, root tips and root branching points [35]. We asked if the expression pattern of the ONSEN promoter would be similar and constructed a transgene consisting of the full 440 bp LTR upstream of a sequence encoding a GUS-GFP fusion protein (Supplementary Fig. S4A). This construct was introduced into wild type plants, and T4 plants homozygous for a single copy insert of the transgene analyzed for reporter gene expression. Under ambient temperatures, very low GUS activity was detectable in some root cells, while the 30 h heat stress treatment resulted in deep blue staining all over the plants (Fig. 6A–F). Expression starts already shortly after the beginning of the stress, as the first staining in leaf and root tips can be seen as early as 1 h, sometimes also at specific sites such as the leaf tips or emerging root branches, similar to the pattern seen for HSFA2 (Supplementary Fig. S4B, C; [35]). Reporter gene expression later spreads rapidly (Fig. 6G–L). Live imaging of the GFP expression confirms the preferential promoter activity of the LTR in dividing tissues (Supplementary Fig. S4D, E). Correspondingly, the amount of extrachromosomal ONSEN DNA after heat exposure in meristem-enriched tissue exceeds by 8 times that in leaves (Supplementary Fig. S4F), thereby producing a high number of retrotransposon copies ready to reintegrate into the genome of cells that give rise to the next generation. This might represent an especially successful strategy of the element to exploit the plant's own indispensable stress protection mechanism to increase the probability of proliferation.
Fig. 6. The ONSEN LTR is rapidly activated throughout the seedlings. 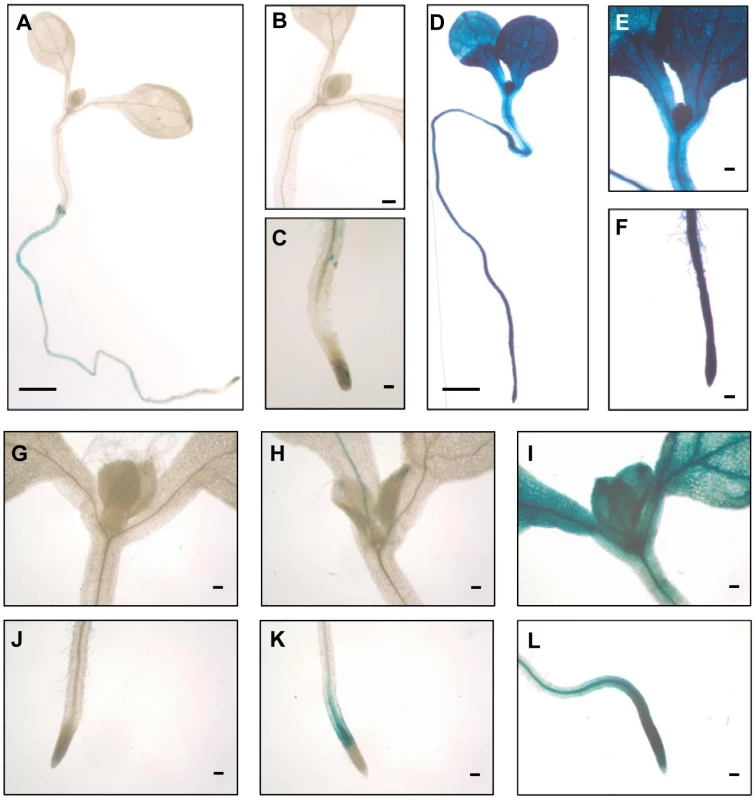
Transcriptional activity of the ONSEN LTR fused to the GUS reporter gene in Col-0 wild type plants in one week-old seedling under non-stressed (A–C) and 30 h heat stress conditions (D–F). Details of shoot (G–I) and root (J–L) images of GUS stained seedlings after 30 min (G, J), 1 h (H, K) and 2 h heat stress (I, L). Scale bars represent 1 mm (A and D) or 0.1 mm (other panels). Discussion
Transposons employ various strategies to gain replication advantage over their hosts in order to produce additional copies. In this study we show that ONSEN exploits a conserved stress defense response to initiate its amplification, in tissue that ultimately produces the germ line, and avoiding stable maintenance of DNA methylation.
Several mobile elements in many organisms are activated upon different environmental cues. Closest to the example described here is the heat responsiveness of Copia elements in Drosophila. While the data on the rate of transposition and their interpretation are controversial [reviewed in 36], there is unquestioned evidence for a correlation between heat-induced expression of a copia type retrotransposon and the presence of heat shock consensus sequences in the promoter [37]. However, in contrast to ONSEN, the Drosophila element is transcribed even without heat stress, the expression increase is faster but much less pronounced, and its promoter lacks at least three more copies of the nGAAn sequence required for high affinity interactions with HSFs [30]. Accumulation of DNA copies of the Drosophila element was not described and is unlikely, since a substantial amount of transcript is needed to generate sufficient Gag and Pol proteins and to serve as template for reverse transcription. In the case of the tobacco Tto1 retrotransposon studied in a heterologous system, Gag protein and linear DNA molecules are synthetized in a ratio of 1400∶1 [38]. Therefore, ONSEN has been (more) successful in acquiring an efficient and specific heat-responsive regulatory element that allows production of extrachromosomal DNA copies by far exceeding the genomic copies.
Extended heat stress conditions can transiently release epigenetic regulation from several genes and transposons [19], [20], and most of these do not have a heat responsive element in their promoter. Some transposons respond equally well to other types of stress [39]. High copy number repeats like TSI and the GUS transgene are effectively activated also in an hsfa2 mutant [19]. The requirement of the HSFA factors suggests that the pronounced ONSEN activation is not just a consequence of a general loss of epigenetic control as for the other elements. At least one of the three genes HSFA1a, b or d is necessary to generate ONSEN transcript and extrachromosomal DNA, and the triple mutant shows severely impaired resistance and viability under heat stress conditions [40]. Expression of HSFA2, the dominant heat shock factor downstream of the HSFA1 complex in Arabidopsis [33] able to bind to the HRE of several target genes [34], is almost completely eliminated in the hsfa1a/b/d triple mutant upon heat exposure, like ONSEN. The specific binding of the HSFA2 protein to the HRE in the ONSEN LTR in vitro demonstrates that ONSEN can recruit this factor, thereby “masking” itself as a heat shock gene and coupling its transcriptional activation to the plants' heat stress response. Reduced but not completely abolished ONSEN transcription in the hsfa2 mutant indicates that it can also acquire other factors from the partially redundant heat-related transcription factor family.
HSFA2 protein is found in leaves, stem, flowers, green siliques and roots of heat-stressed plants [34], and the GUS reporter with the ONSEN LTR shows a similar ubiquitous expression after longer heat exposure. However, the early and strong expression of the ectopic ONSEN GUS fusion construct in tissue with dividing cells resembles the more pronounced expression in the root meristems and root branch points of the HSFA2 promoter [35]. Although an analysis of microarray data for transposon expression under ambient temperatures in different Arabidopsis tissue did not provide evidence for a preferential expression in dividing tissue [41], ONSEN activation upon heat stress was elevated in undifferentiated callus [22], and several mobile elements in maize are preferentially expressed in the shoot apical meristem [42]. This and other evidence has led to the hypothesis of developmental relaxation of TE silencing (DRTS) and suggested that transposons could amplify preferentially in tissues or cells that undergo epigenetic reprogramming in the course of developmental processes [43]. Accordingly, reintegration of ONSEN into new locations occurred frequently in somatic cells during flower formation, although only in epigenetically compromised mutant plants [21]. Therefore, exploiting the higher activity of the heat stress defense in highly dividing tissue for a preferential accumulation of extrachromosomal ONSEN copies might be another optimization strategy of the element to achieve a higher probability of transmission to the next generation. What appears as developmental relaxation might, in some cases at least, be a similarly sophisticated adaptation.
In addition to coupling itself to the plants' stress response and preferentially producing amplicons where propagation chances are high, the sequence of the ONSEN promoter might represent another refinement. Its LTR has a GC content of 28%, not far from the average of 34.7% in the Arabidopsis genome [44], but it does not contain any C in palindromic arrangement between the strands. This is in contrast to LTRs of similar length and composition of other elements that are under the control of the RdDM pathway: EVADÉ [8] (AtCopia93, 406 bp, 37% GC, 5 CG and 6 CHG) and solo-LTR [45] (376 bp, 27%GC, 2 CG, 3 CHG). The absence of CG in the ONSEN promoter circumvents maintenance methylation by MET1, and the lack of any CHG site also precludes modification by CMT3. This, in consequence, interferes with the reinforcing loop in which the histone methyltransferase KRYPTONITE (KYP) installs H3K9me2 [46], thereby strongly impeding the formation of heterochromatin at the ONSEN LTR. The CHH sites in the promoter can only be modified by CMT2 [47] or RdDM, two synergistic pathways that are responsible for substantial methylation of transposable elements in Arabidopsis. While ONSEN was not amplified in non-stressed ddc mutant plants, the activation after heat stress was more pronounced compared to wild type. Since the lack of the RdDM-related methyltransferases DRM1 and DRM2 reduced, but did not totally erase DNA methylation at ONSEN LTRs. the residual CHH methylation by CMT2 could potentially also contribute to the responsiveness of the promoter. However, lack of CMT2 is also not sufficient to completely eliminate CHH methylation at the ONSEN LTRs (Assaf Zemach and Daniel Zilberman, personal communication). This is only obtained in plants lacking both chromatin remodelers DDM1/DRD1 that support CMT2 and RdDM-mediated methylation [47]. However, even upon complete loss of CHH methylation in the double mutant ddm1/drd1, there is very weak activation of ONSEN in non-stressed plants (Assaf Zemach and Daniel Zilberman, personal communication). A more pronounced response of ONSEN to heat stress after partial demethylation may simply indicate better accessibility, as suggested for other regions in Arabidopsis [48].
In spite of the multiple levels at which ONSEN has optimized its transcription and production of extrachromosomal copies, it is an element with only a limited copy number in the Arabidopsis thaliana reference genome, and although other accessions have variable copy numbers [23], we do not know of any ecotype with a substantially larger number of inserts. Therefore, integration of extrachromosomal DNA copies into the plant genome seems to be a limiting factor, although rapid propagation of ONSEN was observed in the progeny of plants compromised in the RdDM pathway [21]. Restricting the chances for successful retrotransposon integration might indicate one efficient counter defense of the plants, the rapid mutation of already integrated copies another. Since retrotransposons usually remain in the genome and LTRs are identical upon insertion, an evolutionary history of degeneration like this can in fact be read from their sequence. The extrachromosomal ONSEN DNA copies were mainly produced from elements with 100% identical LTRs. These evolutionarily young elements are found in very few of the 95 Arabidopsis accessions tested [23], whereas elements not represented in the extrachromosomal fraction have acquired a large number of polymorphisms and are shared between most accessions. In addition to the eight recognizable full length copies, the reference genome has more than 10 sequences annotated as incomplete Copia78 = ONSEN (TAIR database), remnants that indicate previous invasion but sequence degeneration of the element.
The tandem repeat array of the heat-responsive LTRs at the 5′ and 3′ end of ONSEN render any downstream sequence potentially transcribed upon heat stress, and a new insertion indeed conferred heat responsiveness to a neighboring gene [21]. Our finding that this is exerted by integration of the plants' target site for a heat shock transcription factor into the LTR lets us speculate that this is another example of co-evolution between the genomes of host and TE: activation of the element in times of stress might allow the element to propagate but provides at the same time some benefit by rendering additional plant genes stress-responsive, thereby generating genetic diversity as a premise for selection. It resembles other copia-like retrotransposon insertions in Citrus that convey cold induction of the transcription factor Ruby controlling anthocyanin production. This results in the intensive coloration of blood oranges [49] and is just one example that stress-induced transposon-mediated control of plant genes might also be of interest for plant breeders and agriculture.
On the one hand, the acquisition of the plants' heat responsive element by ONSEN masks the retrotransposon as familiar, corresponding to the “wolf-in-sheep's-clothing” strategy [50], and represents a particularly intriguing molecular parasitism in which it is impossible for the plant to respond appropriately to heat stress without the risk of retrotransposon amplification. On the other hand, plants might occasionally benefit from this strategy if their own regulatory element gets moved around upon stress into new insertion sites where it might prove useful. Evolution has the last word.
Materials and Methods
Plant material and growth conditions
The hsfa2-1 [51] and drm1 drm2 cmt3 triple mutant (ddc) [29], [52] in Col-0 background were previously described and obtained from the NASC stock center. hsfa2 has a T-DNA insertion in At2g26150 (SALK_008978). cmt3-11, drm1-2 and drm2-2 are T-DNA insertion mutants in AT1G69770 (SALK 148381), At5g15380 (SALK 021316), and At5g14620 (SALK 150863), respectively. Also the hsfa1 mutants were previously described [32], [53]. hsfa1a (former hsf1-tt1) has an insertion in At4g17750, hsfa1b (former hsf3-tt1) in At5g16820, hsfa1d-1 in At1g32330 (SAIL_410_E01) and hsfa1e in At3g02990 (SALK_094943). Triple (aTK, bTK, dTK, eTK) and quadruple (QK) mutants of HSFA1 generated in Col-0 and Wassilewskija background were a kind gift from Yee-yung Charng.
Plants were sown on germination medium and grown in vitro at 21°C under long-day conditions (16 h light/8 h dark) for 21 days prior to heat stress. For heat stress treatments (HS), plants were transferred to 37°C for 30 h (standard HS conditions) or shorter periods as indicated, starting 3 h after beginning of the light period. GFP and GUS images were taken on 7 day-old seedlings after standard heat stress conditions. Meristem-enriched tissue was prepared from 21 day-old seedlings by manual dissection of shoot tips smaller than 2 mm.
RNA analysis
Total RNA was extracted from whole seedlings using the RNeasy Plant Mini Kit (Qiagen). An additional DNase treatment was performed to remove all extrachromosomal ONSEN DNA in the heat stress samples, prior to cDNA synthesis with random hexamer primers and the RevertAid M-MuLV Reverse Transcriptase (Thermo Scientific).
All qRT-PCR analyses were performed using the Sensi Mix SYBR & Fluorescin Kit (Bioline) and iQ5 equipment (Biorad). Each data point is based on nine PCR reactions deriving from three biological replicates. Expression values were normalized to AtSAND (At2g28390), documented to have equal expression levels under all tested conditions [54], [55].
Quantification of extrachromosomal DNA
Genomic DNA was isolated from whole seedlings, leaves or meristem-enriched tissue using the Illustra DNA Extraction Kit Phytopure (GE Healthcare). Total DNA was separated on 0.8% agarose gel, depurinated for 10 min in 250 mM HCl, denatured in 0.5 M NaOH and 1.5 M NaCl for 30 min, and neutralized in 0.5 M Tris, 1.5 M NaCl and 1 mM EDTA at pH 7.2 for 2×15 min. DNA was blotted onto Hybond N+ membranes (Amersham) with 20× SSC, washed and UV-crosslinked with a Stratalinker (Stratagene).
Hybridization was performed as described [56]. An ONSEN-specific probe (see Supplementary Table 2) was radioactively labelled with 50 µCi of dCT-α-32P (Amersham) using the Rediprime II Random Prime Labelling System (GE Healthcare) and purified via G50 Probequant (Amersham) columns. Signals were detected using Phosphorimager screens (Amersham) and scanned by a Molecular Imager FX (Biorad). Densitometric quantification was performed using Image Lab software on three biological replicates.
Sequencing extrachromosomal DNA
Undigested DNA prepared from whole seedlings was run on a 0.8% agarose gel, and a region around the size range of 5 kb, corresponding to the full length of linear ONSEN copies, was cut out. DNA was purified using the QIAquick Gel Extraction Kit (Qiagen). After a PCR amplification step, using ONSEN-SNPs F and R primers (Supplementary Table S2), the PCR product was purified with the MinElute Gel Extraction Kit (Qiagen) and ligated using the InsTAclone PCR Cloning Kit (Thermo Scientific). Individual clones were Sanger-sequenced.
DNA methylation analyses
Bisulfite conversion of BamHI digested genomic DNA was performed using the Epitect Kit (Qiagen). Purified DNA after conversion was amplified and cloned for Sanger sequencing. Primers are listed in Supplementary Table S2. For each analysis, at least 13 independent clones were Sanger-sequenced. Sequencing data were visualized and the methylation percentage was calculated for each cytosine position using CyMate [57].
Electrophoretic mobility shift assay
The full length of the HSFA2 ORF was amplified by PCR from cDNA (see Supplementary Table S2), cloned in frame with the C-terminal 6× His-tag using NdeI and XhoI sites in the pET-24B vector. The construct was transformed into E. coli strain BL21(RIL) (Novagene) for expression. The bacterially expressed HSFA2-His fusion protein was purified with HisTrap 5 ml columns (GE Healthcare) and ÄKTA purifier (GE Healthcare) and used for EMSA. EMSA was performed as described previously [58]. The LTR sequence was amplified by PCR (see Supplementary Table S2) and the fragment gel-purified prior to labeling. For the competitor assay, complementary oligonucleotides (Supplementary Table S2) were annealed to generate double-stranded DNA. All probes were end-labeled using T4 PNK and (γ-32P)ATP and purified with G50 Probequant columns (Amersham). The binding assay was carried out in a buffer containing 10 mM Tris (pH 7.5), 1 mM EDTA, 0.1 M KCl, 0.1 mM DTT, 5% vol/vol glycerol and 0.01 mg/ml BSA. The binding reaction was incubated at RT for 30 min. The reaction mixtures were separated on 5% non-denaturating polyacrylamide gels in 0.5× TBE buffer at 140 V for 2 h, and the gels were exposed to Phosphoimager screens (Amersham) and scanned by a Molecular Imager FX (Biorad).
Transgene construction and transformation
The 440 bp LTR sequence of ONSEN 1 was cloned into the pENTR2B vector (Invitrogen) using BamHI and EcoRV restriction sites and subsequently into the pBGWFS7 binary vector [59] using the Gateway LR Clonase II Enzyme Mix (Invitrogen). The transgene (Supplementary Figure S4) was introduced into Agrobacterium strain AGL1, which was then used to transform Col-0 plants by floral dipping [60]. After selection of primary transformants by selection with Basta, plants were grown and allowed to self-pollinate. Plants homozygous for a single copy of the transgene (confirmed by Southern blot analysis) were selected and amplified. All experiments were done with plants of the T4 generation.
GUS staining
GUS histochemical staining was performed as described [61].
Supporting Information
Zdroje
1. CallinanPA, BatzerMA (2006) Retrotransposable elements and human disease. Genome Dynamics 1 : 104–115.
2. HedgesDJ, DeiningerPL (2007) Inviting instability: Transposable elements, double-strand breaks, and the maintenance of genome integrity. Mutation Research 616 : 46–59.
3. SinzelleL, IzsvakZ, IvicsZ (2009) Molecular domestication of transposable elements: from detrimental parasites to useful host genes. Cellular and Molecular Life Science 66 : 1073–1093.
4. LischD (2012) How important are transposons for plant evolution? Nature Reviews Genetics 14 : 49–61.
5. LischD (2009) Epigenetic regulation of transposable elements in plants. Annual Reviews in Plant Biology 60 : 43–66.
6. SlotkinRK, MartienssenR (2007) Transposable elements and the epigenetic regulation of the genome. Nature Reviews Genetics 8 : 272–285.
7. ZhangH, ZhuJ-K (2011) RNA-directed DNA methylation. Current Opinion in Plant Biology 14 : 142–147.
8. MirouzeM, ReindersJ, BucherE, NishimuraT, SchneebergerK, et al. (2009) Selective epigenetic control of retrotransposition in Arabidopsis. Nature 461 : 427–430.
9. MiuraA, YonebayashiS, WatanabeK, ToyamaT, ShimadaH, et al. (2001) Mobilization of transposons by a mutation abolishing full DNA methylation in Arabidopsis. Nature 411 : 212–214.
10. TsukaharaS, KobayashiA, KawabeA, MathieuO, MiuraA, et al. (2009) Bursts of retrotransposition reproduced in Arabidopsis. Nature 461 : 423–426.
11. LippmanZ, GendrelAV, BlackM, VaughnMW, DedhiaN, et al. (2004) Role of transposable elements in heterochromatin and epigenetic control. Nature 430 : 471–476.
12. McClintockB (1984) The significance of responses of the genome to challenge. Science 226 : 792–801.
13. GrandbastienMA (1998) Activation of plant retrotransposons under stress conditions. Trends in Plant Science 3 : 181–187.
14. GrandbastienM, AudeonC, BonnivardE, CasacubertaJM, ChalhoubB, et al. (2005) Stress activation and genomic impact of Tnt1 retrotransposons in Solanaceae. Cytogenetic and Genome Research 110 : 229–241.
15. HarrisonBJ, FinchamJRS (1964) Instability at PAL locus in Antirrhinum majus. I. Effects of environment on frequencies of somatic and germinal mutation. Heredity 19 : 237–258.
16. HashidaSN, UchiyamaT, MartinC, KishimaY, SanoY, et al. (2006) The temperature-dependent change in methylation of the Antirrhinum transposon Tam3 is controlled by the activity of its transposase. Plant Cell 18 : 104–118.
17. StewardN, ItoM, YamaguchiY, KoizumiN, SanoH (2002) Periodic DNA methylation in maize nucleosomes and demethylation by environmental stress. Journal of Biological Chemistry 277 : 37741–37746.
18. De FeliceB, WilsonRR, ArgenzianoC, KafantarisI, ConicellaC (2009) A transcriptionally active copia-like retroelement in Citrus limon. Cellular & Molecular Biology Letters 14 : 289–304.
19. PecinkaA, DinhHQ, BaubecT, RosaM, LettnerN, et al. (2010) Epigenetic regulation of repetitive elements is attenuated by prolonged heat stress in Arabidopsis. Plant Cell 22 : 3118–3129.
20. Tittel-ElmerM, BucherE, BrogerL, MathieuO, PaszkowskiJ, et al. (2010) Stress-induced activation of heterochromatic transcription. PLoS Genetics 6: e1001175.
21. ItoH, GaubertH, BucherE, MirouzeM, VaillantI, et al. (2011) An siRNA pathway prevents transgenerational retrotransposition in plants subjected to stress. Nature 472 : 115–119.
22. MatsunagaW, KobayashiA, KatoA, ItoH (2012) The effects of heat induction and the siRNA biogenesis pathway on the transgenerational transposition of ONSEN, a copia-like retrotransposon in Arabidopsis thaliana. Plant Cell Physiology 53 : 824–833.
23. ItoH, YoshidaT, TsukaharaS, KawabeA (2013) Evolution of the ONSEN retrotransposon family activated upon heat stress in Brassicaceae. Gene 518 : 256–261.
24. KotakS, LarkindaleJ, LeeU, von Koskull-DoringP, VierlingE, et al. (2007) Complexity of the heat stress response in plants. Current Opinions in Plant Biology 10 : 310–316.
25. HaveckerER, GaoX, VoytasDF (2004) The diversity of LTR retrotransposons. Genome Biology 5 : 225.
26. HirochikaH, OtsukiH (1995) Extrachromosomal circular forms of the tobacco retrotransposon Tto1. Gene 165 : 229–232.
27. FeuerbachF, DrouaudJ, LucasH (1997) Retrovirus-like end processing of the tobacco Tnt1 retrotransposon linear intermediates of replication. Journal of Virology 71 : 4005–4015.
28. BerkhoutB, DasAT, BeerensN (2001) HIV-1 RNA editing, hypermutation, and error-prone reverse transcription. Science 292 : 7.
29. CaoX, JacobsenSE (2002) Locus-specific control of asymmetric and CpNpG methylation by the DRM and CMT3 methyltransferase genes. Proceedings of the National Academy of Sciences USA 99(Suppl 4): 16491–16498.
30. WuC (1995) Heat shock transcription factors: structure and regulation. Annual Reviews in Cellular and Developmental Biology 11 : 441–469.
31. von Koskull-DöringP, ScharfKD, NoverL (2007) The diversity of plant heat stress transcription factors. Trends in Plant Science 12 : 452–457.
32. YoshidaT, OhamaN, NakajimaJ, KidokoroS, MizoiJ, et al. (2011) Arabidopsis HsfA1 transcription factors function as the main positive regulators in heat shock-responsive gene expression. Molecular Genetics and Genomics 286 : 321–332.
33. BuschW, WunderlichM, SchofflF (2005) Identification of novel heat shock factor-dependent genes and biochemical pathways in Arabidopsis thaliana. Plant Journal 41 : 1–14.
34. SchrammF, GanguliA, KiehlmannE, EnglichG, WalchD, et al. (2006) The heat stress transcription factor HsfA2 serves as a regulatory amplifier of a subset of genes in the heat stress response in Arabidopsis. Plant Molecular Biology 60 : 759–772.
35. NishizawaA, YabutaY, YoshidaE, MarutaT, YoshimuraK, et al. (2006) Arabidopsis heat shock transcription factor A2 as a key regulator in response to several types of environmental stress. Plant Journal 48 : 535–547.
36. Garcia GuerreiroMP (2012) What makes transposable elements move in the Drosophila genome? Heredity 108 : 461–468.
37. StrandDJ, McdonaldJF (1985) Copia Is transcriptionally responsive to environmental stress. Nucleic Acids Research 13 : 4401–4410.
38. TakedaS, SugimotoK, KakutaniT, HirochikaH (2001) Linear DNA intermediates of the Tto1 retrotransposon in Gag particles accumulated in stressed tobacco and Arabidopsis thaliana. Plant Journal 28 : 307–317.
39. Lang-MladekC, PopovaO, KiokK, BerlingerM, RakicB, et al. (2010) Transgenerational inheritance and resetting of stress-induced loss of epigenetic gene silencing in Arabidopsis. Molecular Plant 3 : 594–602.
40. LiuHC, CharngYY (2013) Common and distinct functions of Arabidopsis class A1 and A2 heat shock factors in diverse abiotic stress responses and development. Plant Physiology 163 : 276–290.
41. SlotkinRK, VaughnM, BorgesF, TanurdzicM, BeckerJD, et al. (2009) Epigenetic reprogramming and small RNA silencing of transposable elements in pollen. Cell 136 : 461–472.
42. OhtsuK, SmithMB, EmrichSJ, BorsukLA, ZhouR, et al. (2007) Global gene expression analysis of the shoot apical meristem of maize (Zea mays L.). Plant Journal 52 : 391–404.
43. MartinezG, SlotkinRK (2012) Developmental relaxation of transposable element silencing in plants: functional or byproduct? Current Opinions in Plant Biology 15 : 496–502.
44. Arabidopsis GenomeI (2000) Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature 408 : 796–815.
45. HuettelB, KannoT, DaxingerL, AufsatzW, MatzkeAJ, et al. (2006) Endogenous targets of RNA-directed DNA methylation and Pol IV in Arabidopsis. EMBO Journal 25 : 2828–2836.
46. JacksonJP, LindrothAM, CaoXF, JacobsenSE (2002) Control of CpNpG DNA methylation by the KRYPTONITE histone H3 methyltransferase. Nature 416 : 556–560.
47. ZemachA, KimMY, HsiehPH, Coleman-DerrD, Eshed-WilliamsL, et al. (2013) The Arabidopsis nucleosome remodeler DDM1 allows DNA methyltransferases to access H1-containing heterochromatin. Cell 153 : 193–205.
48. ShuH, WildhaberT, SiretskiyA, GruissemW, HennigL (2012) Distinct modes of DNA accessibility in plant chromatin. Nature Communications 3 : 1281.
49. ButelliE, LicciardelloC, ZhangY, LiuJ, MackayS, et al. (2012) Retrotransposons control fruit-specific, cold-dependent accumulation of anthocyanins in blood oranges. Plant Cell 24 : 1242–1255.
50. LischD, SlotkinRK (2011) Strategies for silencing and escape: the ancient struggle between transposable elements and their hosts. International Reviews in Cellular and Molecular Biology 292 : 119–152.
51. CharngYY, LiuHC, LiuNY, ChiWT, WangCN, et al. (2007) A heat-inducible transcription factor, HsfA2, is required for extension of acquired thermotolerance in Arabidopsis. Plant Physiology 143 : 251–262.
52. HendersonIR, JacobsenSE (2008) Tandem repeats upstream of the Arabidopsis endogene SDC recruit non-CG DNA methylation and initiate siRNA spreading. Genes & Development 22 : 1597–1606.
53. LohmannC, Eggers-SchumacherG, WunderlichM, SchofflF (2004) Two different heat shock transcription factors regulate immediate early expression of stress genes in Arabidopsis. Molecular Genetics and Genomics 271 : 11–21.
54. HruzT, WyssM, DocquierM, PfafflMW, MasanetzS, et al. (2011) RefGenes: identification of reliable and condition specific reference genes for RT-qPCR data normalization. BMC Genomics 12 : 156.
55. CzechowskiT, StittM, AltmannT, UdvardiMK, ScheibleWR (2005) Genome-wide identification and testing of superior reference genes for transcript normalization in Arabidopsis. Plant Physiology 139 : 5–17.
56. ChurchGM, GilbertW (1984) Genomic sequencing. Proceedings of the National Academy of Sciences of the USA 81 : 1991–1995.
57. HetzlJ, FoersterAM, RaidlG, Mittelsten ScheidO (2007) CyMATE: a new tool for methylation analysis of plant genornic DNA after bisulphite sequencing. Plant Journal 51 : 526–536.
58. HellmanLM, FriedMG (2007) Electrophoretic mobility shift assay (EMSA) for detecting protein-nucleic acid interactions. Nature Protocols 2 : 1849–1861.
59. KarimiM, InzeD, DepickerA (2002) GATEWAY((TM)) vectors for Agrobacterium-mediated plant transformation. Trends in Plant Science 7 : 193–195.
60. CloughSJ, BentAF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant Journal 16 : 735–743.
61. PecinkaA, RosaM, SchikoraA, BerlingerM, HirtH, et al. (2009) Transgenerational stress memory is not a general response in Arabidopsis. PLoS One 4: e5202.
Štítky
Genetika Reprodukční medicína
Článek Unwrapping BacteriaČlánek A Chaperone-Assisted Degradation Pathway Targets Kinetochore Proteins to Ensure Genome StabilityČlánek The Candidate Splicing Factor Sfswap Regulates Growth and Patterning of Inner Ear Sensory OrgansČlánek The SPF27 Homologue Num1 Connects Splicing and Kinesin 1-Dependent Cytoplasmic Trafficking inČlánek Down-Regulation of eIF4GII by miR-520c-3p Represses Diffuse Large B Cell Lymphoma DevelopmentČlánek Meta-Analysis Identifies Gene-by-Environment Interactions as Demonstrated in a Study of 4,965 MiceČlánek High Risk Population Isolate Reveals Low Frequency Variants Predisposing to Intracranial Aneurysms
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2014 Číslo 1
-
Všechny články tohoto čísla
- How Much Is That in Dog Years? The Advent of Canine Population Genomics
- The Sense and Sensibility of Strand Exchange in Recombination Homeostasis
- Unwrapping Bacteria
- DNA Methylation Changes Separate Allergic Patients from Healthy Controls and May Reflect Altered CD4 T-Cell Population Structure
- Evidence for Mito-Nuclear and Sex-Linked Reproductive Barriers between the Hybrid Italian Sparrow and Its Parent Species
- Translation Enhancing ACA Motifs and Their Silencing by a Bacterial Small Regulatory RNA
- Relationship Estimation from Whole-Genome Sequence Data
- Genetic Models of Apoptosis-Induced Proliferation Decipher Activation of JNK and Identify a Requirement of EGFR Signaling for Tissue Regenerative Responses in
- ComEA Is Essential for the Transfer of External DNA into the Periplasm in Naturally Transformable Cells
- Loss and Recovery of Genetic Diversity in Adapting Populations of HIV
- Bioelectric Signaling Regulates Size in Zebrafish Fins
- Defining NELF-E RNA Binding in HIV-1 and Promoter-Proximal Pause Regions
- Loss of Histone H3 Methylation at Lysine 4 Triggers Apoptosis in
- Cell-Cycle Dependent Expression of a Translocation-Mediated Fusion Oncogene Mediates Checkpoint Adaptation in Rhabdomyosarcoma
- How a Retrotransposon Exploits the Plant's Heat Stress Response for Its Activation
- A Nonsense Mutation in Encoding a Nondescript Transmembrane Protein Causes Idiopathic Male Subfertility in Cattle
- Deletion of a Conserved -Element in the Locus Highlights the Role of Acute Histone Acetylation in Modulating Inducible Gene Transcription
- Developmental Link between Sex and Nutrition; Regulates Sex-Specific Mandible Growth via Juvenile Hormone Signaling in Stag Beetles
- PP2A/B55 and Fcp1 Regulate Greatwall and Ensa Dephosphorylation during Mitotic Exit
- Differential Effects of Collagen Prolyl 3-Hydroxylation on Skeletal Tissues
- Comprehensive Functional Annotation of 77 Prostate Cancer Risk Loci
- Evolution of Chloroplast Transcript Processing in and Its Chromerid Algal Relatives
- A Chaperone-Assisted Degradation Pathway Targets Kinetochore Proteins to Ensure Genome Stability
- New MicroRNAs in —Birth, Death and Cycles of Adaptive Evolution
- A Genome-Wide Screen for Bacterial Envelope Biogenesis Mutants Identifies a Novel Factor Involved in Cell Wall Precursor Metabolism
- FGFR1-Frs2/3 Signalling Maintains Sensory Progenitors during Inner Ear Hair Cell Formation
- Regulation of Synaptic /Neuroligin Abundance by the /Nrf Stress Response Pathway Protects against Oxidative Stress
- Intrasubtype Reassortments Cause Adaptive Amino Acid Replacements in H3N2 Influenza Genes
- Molecular Specificity, Convergence and Constraint Shape Adaptive Evolution in Nutrient-Poor Environments
- WNT7B Promotes Bone Formation in part through mTORC1
- Natural Selection Reduced Diversity on Human Y Chromosomes
- In-Vivo Quantitative Proteomics Reveals a Key Contribution of Post-Transcriptional Mechanisms to the Circadian Regulation of Liver Metabolism
- The Candidate Splicing Factor Sfswap Regulates Growth and Patterning of Inner Ear Sensory Organs
- The Acid Phosphatase-Encoding Gene Contributes to Soybean Tolerance to Low-Phosphorus Stress
- p53 and TAp63 Promote Keratinocyte Proliferation and Differentiation in Breeding Tubercles of the Zebrafish
- Affects Plant Architecture by Regulating Local Auxin Biosynthesis
- The SET Domain Proteins SUVH2 and SUVH9 Are Required for Pol V Occupancy at RNA-Directed DNA Methylation Loci
- Down-Regulation of Rad51 Activity during Meiosis in Yeast Prevents Competition with Dmc1 for Repair of Double-Strand Breaks
- Multi-tissue Analysis of Co-expression Networks by Higher-Order Generalized Singular Value Decomposition Identifies Functionally Coherent Transcriptional Modules
- A Neurotoxic Glycerophosphocholine Impacts PtdIns-4, 5-Bisphosphate and TORC2 Signaling by Altering Ceramide Biosynthesis in Yeast
- Subtle Changes in Motif Positioning Cause Tissue-Specific Effects on Robustness of an Enhancer's Activity
- C/EBPα Is Required for Long-Term Self-Renewal and Lineage Priming of Hematopoietic Stem Cells and for the Maintenance of Epigenetic Configurations in Multipotent Progenitors
- The SPF27 Homologue Num1 Connects Splicing and Kinesin 1-Dependent Cytoplasmic Trafficking in
- Down-Regulation of eIF4GII by miR-520c-3p Represses Diffuse Large B Cell Lymphoma Development
- Genome Sequencing Highlights the Dynamic Early History of Dogs
- Re-sequencing Expands Our Understanding of the Phenotypic Impact of Variants at GWAS Loci
- Meta-Analysis Identifies Gene-by-Environment Interactions as Demonstrated in a Study of 4,965 Mice
- , a -Antisense Gene of , Encodes a Evolved Protein That Inhibits GSK3β Resulting in the Stabilization of MYCN in Human Neuroblastomas
- A Transcription Factor Is Wound-Induced at the Planarian Midline and Required for Anterior Pole Regeneration
- A Comprehensive tRNA Deletion Library Unravels the Genetic Architecture of the tRNA Pool
- A PNPase Dependent CRISPR System in
- Genomic Confirmation of Hybridisation and Recent Inbreeding in a Vector-Isolated Population
- Zinc Finger Transcription Factors Displaced SREBP Proteins as the Major Sterol Regulators during Saccharomycotina Evolution
- GATA6 Is a Crucial Regulator of Shh in the Limb Bud
- Tissue Specific Roles for the Ribosome Biogenesis Factor Wdr43 in Zebrafish Development
- A Cell Cycle and Nutritional Checkpoint Controlling Bacterial Surface Adhesion
- High Risk Population Isolate Reveals Low Frequency Variants Predisposing to Intracranial Aneurysms
- E3 Ubiquitin Ligase CHIP and NBR1-Mediated Selective Autophagy Protect Additively against Proteotoxicity in Plant Stress Responses
- Evolutionary Rate Covariation Identifies New Members of a Protein Network Required for Female Post-Mating Responses
- 3′ Untranslated Regions Mediate Transcriptional Interference between Convergent Genes Both Locally and Ectopically in
- Single Nucleus Genome Sequencing Reveals High Similarity among Nuclei of an Endomycorrhizal Fungus
- Metabolic QTL Analysis Links Chloroquine Resistance in to Impaired Hemoglobin Catabolism
- Notch Controls Cell Adhesion in the Drosophila Eye
- AL PHD-PRC1 Complexes Promote Seed Germination through H3K4me3-to-H3K27me3 Chromatin State Switch in Repression of Seed Developmental Genes
- Genomes Reveal Evolution of Microalgal Oleaginous Traits
- Large Inverted Duplications in the Human Genome Form via a Fold-Back Mechanism
- Variation in Genome-Wide Levels of Meiotic Recombination Is Established at the Onset of Prophase in Mammalian Males
- Age, Gender, and Cancer but Not Neurodegenerative and Cardiovascular Diseases Strongly Modulate Systemic Effect of the Apolipoprotein E4 Allele on Lifespan
- Lifespan Extension Conferred by Endoplasmic Reticulum Secretory Pathway Deficiency Requires Induction of the Unfolded Protein Response
- Is Non-Homologous End-Joining Really an Inherently Error-Prone Process?
- Vestigialization of an Allosteric Switch: Genetic and Structural Mechanisms for the Evolution of Constitutive Activity in a Steroid Hormone Receptor
- Functional Divergence and Evolutionary Turnover in Mammalian Phosphoproteomes
- A 660-Kb Deletion with Antagonistic Effects on Fertility and Milk Production Segregates at High Frequency in Nordic Red Cattle: Additional Evidence for the Common Occurrence of Balancing Selection in Livestock
- Comparative Evolutionary and Developmental Dynamics of the Cotton () Fiber Transcriptome
- The Transcription Factor BcLTF1 Regulates Virulence and Light Responses in the Necrotrophic Plant Pathogen
- Crossover Patterning by the Beam-Film Model: Analysis and Implications
- Single Cell Genomics: Advances and Future Perspectives
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- GATA6 Is a Crucial Regulator of Shh in the Limb Bud
- Large Inverted Duplications in the Human Genome Form via a Fold-Back Mechanism
- Differential Effects of Collagen Prolyl 3-Hydroxylation on Skeletal Tissues
- Affects Plant Architecture by Regulating Local Auxin Biosynthesis
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



