-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaA Transcription Factor Is Wound-Induced at the Planarian Midline and Required for Anterior Pole Regeneration
Planarian regeneration requires positional information to specify the identity of tissues to be replaced as well as pluripotent neoblasts capable of differentiating into new cell types. We found that wounding elicits rapid expression of a gene encoding a Forkhead-family transcription factor, FoxD. Wound-induced FoxD expression is specific to the ventral midline, is regulated by Hedgehog signaling, and is neoblast-independent. FoxD is subsequently expressed within a medial subpopulation of neoblasts at wounds involving head regeneration. Ultimately, FoxD is co-expressed with multiple anterior markers at the anterior pole. Inhibition of FoxD with RNA interference (RNAi) results in the failure to specify neoblasts expressing anterior markers (notum and prep) and in anterior pole formation defects. FoxD(RNAi) animals fail to regenerate a new midline and to properly pattern the anterior blastema, consistent with a role for the anterior pole in organizing pattern of the regenerating head. Our results suggest that wound signaling activates a forkhead transcription factor at the midline and, if the head is absent, FoxD promotes specification of neoblasts at the prior midline for anterior pole regeneration.
Published in the journal: . PLoS Genet 10(1): e32767. doi:10.1371/journal.pgen.1003999
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003999Summary
Planarian regeneration requires positional information to specify the identity of tissues to be replaced as well as pluripotent neoblasts capable of differentiating into new cell types. We found that wounding elicits rapid expression of a gene encoding a Forkhead-family transcription factor, FoxD. Wound-induced FoxD expression is specific to the ventral midline, is regulated by Hedgehog signaling, and is neoblast-independent. FoxD is subsequently expressed within a medial subpopulation of neoblasts at wounds involving head regeneration. Ultimately, FoxD is co-expressed with multiple anterior markers at the anterior pole. Inhibition of FoxD with RNA interference (RNAi) results in the failure to specify neoblasts expressing anterior markers (notum and prep) and in anterior pole formation defects. FoxD(RNAi) animals fail to regenerate a new midline and to properly pattern the anterior blastema, consistent with a role for the anterior pole in organizing pattern of the regenerating head. Our results suggest that wound signaling activates a forkhead transcription factor at the midline and, if the head is absent, FoxD promotes specification of neoblasts at the prior midline for anterior pole regeneration.
Introduction
Planarians can regenerate from nearly any injury, but how missing tissues are recognized and replaced is poorly understood. The adult population of proliferating cells (neoblasts) in Schmidtea mediterranea includes pluripotent stem cells [1] and is responsible for new tissue production in regeneration. New cell production at wounds produces an outgrowth called a blastema, which will replace some of the missing tissues [2]. At the molecular level, injuries trigger a rapid wound response program that includes conserved immediate early genes and patterning factors required for normal regeneration [3]. Wnt and Hedgehog (Hh) signaling pathways instruct the regeneration of the anterior-posterior (AP) axis, whereas the Bmp signaling pathway controls the regeneration of the dorsal-ventral (DV) axis [4]–[13]. Multiple genes required for positional identity control during embryonic development of other organisms, such as Wnt and Bmp signaling ligands, display constitutive regionalized expression in adult planarians and also guide pattern maintenance during tissue turnover [14].
Two distinct regions composed of a small cluster of cells located at the anterior and posterior animal extremities are referred to here as the anterior and posterior planarian poles. The poles are found at the midline of the animal and are subjects of current intense study. The anterior pole expresses notum, a Wnt inhibitor required for head regeneration [8], whereas the posterior pole expresses wnt1 [7], [14]. A number of genes encoding proteins predicted to be involved in signaling pathways that regulate planarian body plan patterning, and that display regional expression in planarian muscle cells have been described [15]. The genes that display these unique attributes will be referred to as position control genes (PCGs) for simplicity of discussion, but it is important to note that patterning phenotypes have not yet been described for many of these genes. PCGs expressed broadly in the planarian head include the candidate Wnt inhibitor, sFRP-1; candidate FGF inhibitors nou darake (ndk) and ndl-4; and a homeodomain transcription factor, prep [6], [7], [14]–[17]. PCGs expressed broadly in the posterior region of the animal include genes encoding additional Wnt ligands, wnt11-1, wnt11-2, and wntP-2/wnt11-5 and the Wnt receptor frizzled-4 [6], [7], [14], [15], [18]; Hox genes are also expressed in the posterior [14]. Several PCGs are expressed at the planarian poles, but have broader expression domains that extend beyond the cluster of notum+ or wnt1+ cells at the animal head and tail tips.
How the poles are formed and the role they have in organizing regeneration of an animal with a properly patterned body plan is poorly understood. Two genes encoding transcription factors of the TALE-class homeodomain family, prep and pbx, are required for regeneration of the expression domains of anterior PCGs and the anterior pole marker notum [17], [19], [20]. pbx [20] and a LIM-homeobox gene (djislet, [21]) are required for regeneration of expression domains of posterior PCGs and the posterior pole marker wnt1. follistatin, which encodes a secreted regulator of TGF-β proteins, is also expressed at the anterior pole and is required for normal head regeneration [22], but strong inhibition of this gene can also result in the absence of blastema formation indicating a broader role of this gene during regeneration [23].
Forkhead-box (Fox) genes are an evolutionary ancient family of winged-helix transcription factors involved in a wide variety of biological processes such as regulation of cell proliferation, growth, and differentiation [24]. During embryogenesis, Fox genes are involved in organogenesis and patterning of several tissues from all three germ layers [25]. Mutations in Fox genes have a profound impact in human disease causing a variety of phenotypes, from eye abnormalities to speech impediments [25]–[27]. Some members of the Fox gene family are expressed in restricted regions of embryos. In Drosophila, genes encoding Forkhead-family proteins, fkh, sloppy paired 1 and 2, and crocodile, are all expressed in the anterior region of the embryo, and are required for midline establishment as well as head patterning [28]–[31]. In amphioxus, FoxQ is expressed at the anterior pole during embryogenesis [32]. In Xenopus, the forkhead family gene, XDF1 is expressed in Spemann's organizer and at later stages in the anterior neural region [33]. In planarians, few Fox genes have been described. In particular, DjFoxD is expressed in few cells at the anterior pole region of the planarian D. japonica [34] and FoxD influences expression of follistatin in planarian heads [22], [34]. Given the potential importance of the planarian anterior pole in organizing head regeneration, we investigated the role of Schmidtea mediterranea FoxD in regeneration.
Results
Co-expression of FoxD with a number of anterior-expressed genes defines the anterior pole
A number of genes have been identified that are expressed in different domains of planarian heads. To provide a molecular definition of the anterior-most end of the planarian head, the anterior pole, we investigated the expression of a number of genes expressed near the planarian head tip. FoxD is expressed in a very small number of cells at the head tip (Fig. 1 and [22], [34]), but the pole and its role(s) are poorly defined; we focused our investigation of the anterior pole on the Schmidtea mediterranea ortholog of DjFoxD, Smed-FoxD, or FoxD in short (Fig. S1). FoxD expression in intact animals was dorsal-biased and most FoxD+ cells also expressed the gene notum. notum is required for the head-versus-tail regeneration decision known as AP regeneration polarity, and encodes a secreted inhibitor of Wnt signaling [8]. Like FoxD, notum expression in uninjured animals is largely restricted to a very small number of cells at the head tip (Fig. 1 and [8]). FoxD+ cells at the head tip also expressed multiple anterior markers (PCGs) that are expressed in AP transcriptional domains extending beyond the FoxD+ cells, including sFRP-1, ndk, ndl-4, and prep, but not sFRP-2 (Fig. 1). We also assessed whether FoxD is co-expressed with two planarian genes expressed at the DV boundary (a lateral domain surrounding the animal at the midpoint of dorsal and ventral surfaces) and/or at the midline (the median plane about which bilateral symmetry is organized): admp, encoding a BMP-family signaling ligand expressed in the ventral midline and at the DV boundary [10], [11] and slit, a conserved midline cue with a prominent role in regulation of axon guidance [35] expressed in the ventral and dorsal planarian midline [35]. admp and slit expression did not substantially coincide with pole cells expressing FoxD; midline slit expression did not reach the anterior-most region where FoxD-expressing cells are found (Fig. 1). We propose a definition for the planarian anterior pole in the mature head as the few cells restricted to the head tip and that co-express the highly restricted FoxD and notum genes together with a set of anterior PCGs, but displaying little expression of DV boundary and midline genes. The cellular basis for formation of these cells and the roles of these cells in regeneration are investigated below.
Fig. 1. FoxD is expressed with multiple other PCGs at the anterior pole. 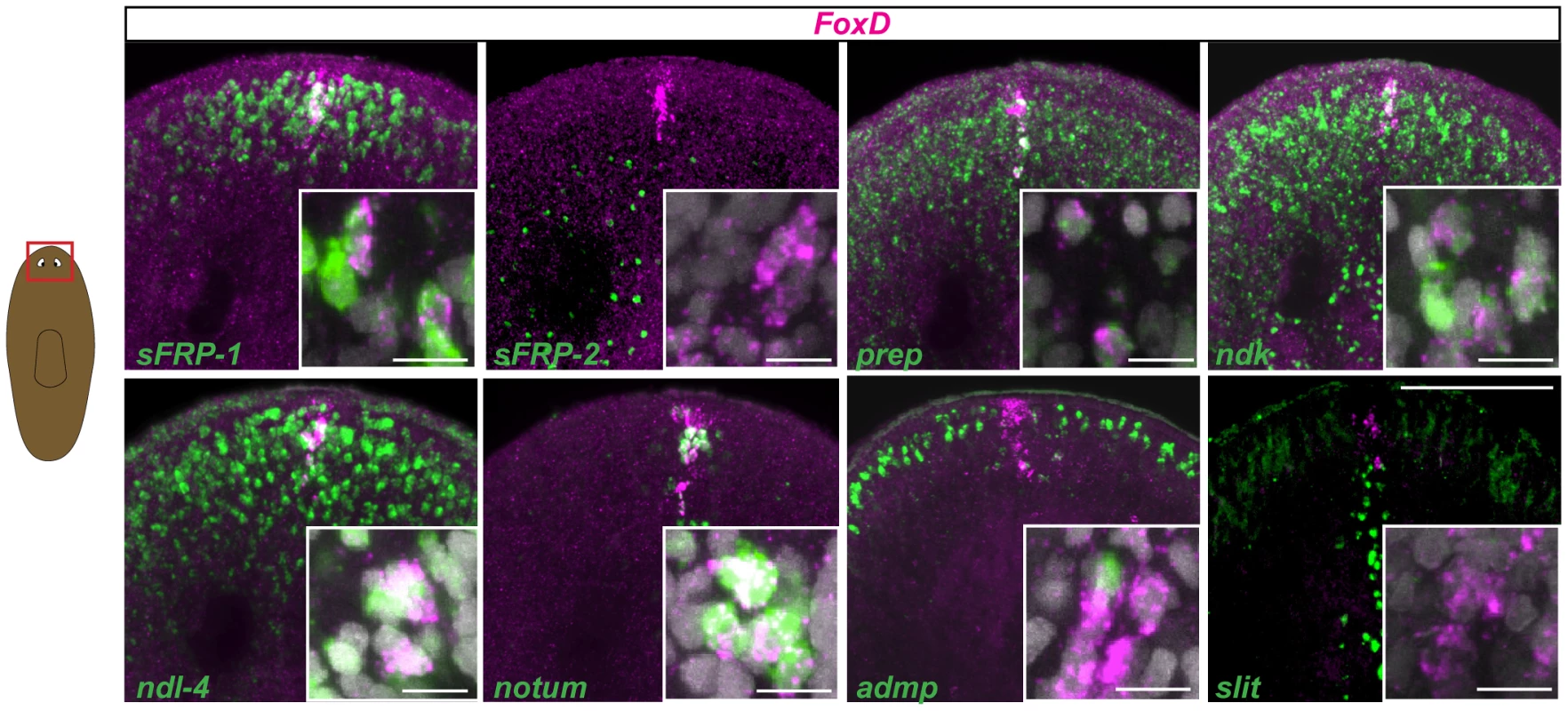
Wild-type intact animals were labeled in double whole-mount fluorescence in situ hybridization (FISH) with FoxD (magenta) and several anterior and midline patterning genes (green). Percentages (mean ± SD) of FoxD cells co-expressing anterior markers are: 56±5% with sFRP1; 66±9% with ndk, 43±9% with ndl-4, 72±19% with prep, and 68±11% with notum; co-expression with the midline genes admp is 2±4%, and slit is 5±8% (n>50 FoxD+ cells examined in each case). Red box in cartoon on the left shows the area depicted. Animals are anterior up; dorsal view. Insets show higher magnification images of co-expressing cells. Images shown are maximal intensity projections. Images are representative of results seen in >6 animals per panel. Scale bars for all panels, 100 µm. Inset scale bars, 10 µm. FoxD expression is wound-induced in the ventral midline
FoxD expression was highly induced by three hours following wounding in subepidermal cells, with expression peaking at approximately six hours after wounding (Fig. 2A). FoxD expression after amputation occurred at both anterior - and posterior-facing wounds, raising the possibility that FoxD is a generically wound-induced gene (Fig. 2A). FoxD expression after amputation was greatly diminished by 18 to 24 hours following injury (Fig. 2B), but increased again between 24 and 48 hours after amputation. At this later time FoxD expression was only observed at anterior-facing wounds that required the formation of a new anterior pole (Fig. 2B and Fig. S2A). Irradiation eliminates neoblasts [36], which comprise the entire population of dividing adult planarian cells; therefore, amputation experiments in irradiated animals can determine whether new gene expression in regeneration occurs in pre-existing cells at wound sites, or requires new cell production. Early wound-induced expression of FoxD was irradiation-insensitive, indicating that it is a transcriptional response in cells present at the time of wounding (Fig. 2B).
Fig. 2. FoxD is wound-induced in the midline. 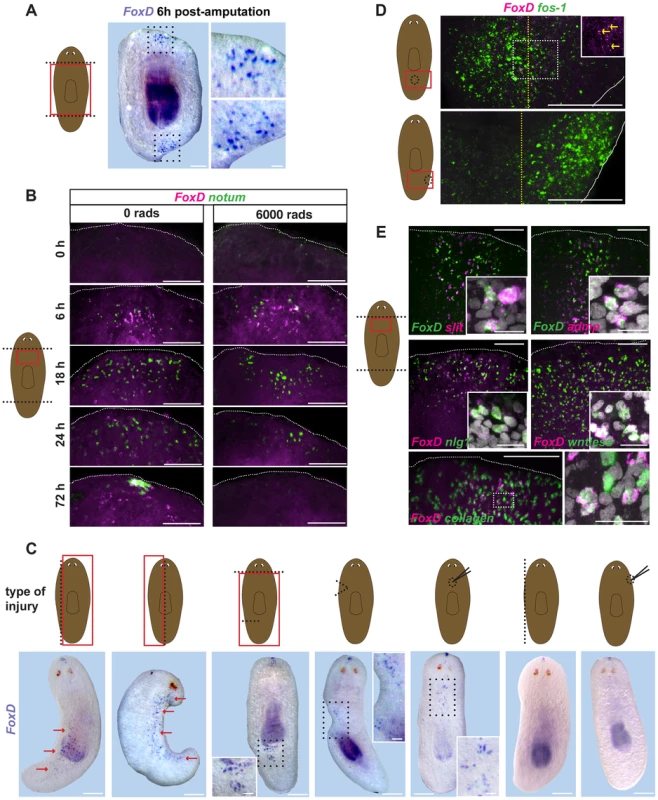
(A) Wild-type animals were transversely amputated, fixed at six hours following wounding, and FoxD (purple) expression was analyzed by in situ hybridization. Red box in cartoon on the left shows the area imaged. All animals showed anterior and posterior FoxD expression at the midline domain following wounding (n>10). Animals are anterior up; ventral view. Area within dotted black box is enlarged in right panels. Scale bar, 500 µm. Right panels, scale bar, 100 µm. (B) Double FISH, notum (green) and FoxD (magenta) in wild-type (0 rads) and lethally irradiated animals (6,000 rads) at different time points following transverse amputation. Red box in cartoon on the left shows the area depicted. Images shown are maximal intensity projections. Images are representative of results seen in n>6 animals per panel. Dorsal view for the 72 hours time point, ventral view for all others. Dotted white line marks the approximate wound boundary. Scale bars, 100 µm. (C) Cartoons show injury types performed in wild-type animals. Dotted black line shows the wound site. Type of wound from left to right: parasagittal, sagittal, incision, wedge, dorsal midline puncture, lateral edge, and lateral puncture. Red box shows the region imaged below. Whole-mount in situ hybridization using FoxD (purple) RNA probe at six hours following wounding of wild-type animals. Red arrows indicate FoxD expression. Dotted black box is enlarged in the inset. Images are representative of results seen in >10 animals per wound type. Animals are anterior up, ventral view. Scale bars, 500 µm. Inset scale bars, 100 µm. (D) Double FISH, FoxD (magenta) and the immediate early gene fos-1 (green) of wounded wild-type animals fixed at six hours following midline (upper panel) and lateral (lower panel) puncture injuries. Cartoon on the left shows type of wound, red box is area imaged. Dotted yellow line depicts the approximate midline, white line depicts the animal edge. Area within dotted white box is shown in the inset for FoxD expression. Yellow arrows point to FoxD-expressing cells at the midline. Images shown are maximal intensity projections. Images are representative of results seen in >10 animals per panel. Anterior is up, ventral view. Scale bars, 100 µm (E) Double FISH, FoxD (green for upper two panels, magenta all other panels) and midline, W2 genes or the muscle gene collagen of wild-type animals fixed six hours following transverse amputation. Percentage (mean ± SD) of FoxD co-expression with slit was 86.3±5.5% (n = 124 FoxD+ cells examined), with collagen was 92.6±4.6% (n = 52 FoxD+ cells examined). Red box shows the area imaged. Anterior wounds are up, ventral view. Insets show higher magnification of co-expressing cells, scale bars are 10 µm. For the double FoxD, collagen FISH, area within dotted white box is shown in the right panel. Images shown are maximal intensity projections. Images are representative of results seen in >5 animals per panel. Dotted white line marks the approximate wound boundary. Scale bars, 100 µm. The large numbers of planarian wound-induced genes described so far are expressed broadly at the wound site [3], [8], [18], [37]. By contrast, wound-induced FoxD expression following amputation was unique: restricted to cells found in the ventral midline (Fig. 2A,B). To better understand the regulation of FoxD expression by wounding, we examined the impacts of several injury types on FoxD expression (Fig. 2C). First, we observed that expression of FoxD was not exclusive to wounds requiring pole regeneration; both parasagittal and sagittal amputations induced FoxD expression in the midline broadly along the wound site (first two cuts from the left, Fig. 2C). Incision into the planarian side with a scalpel, a wound not requiring blastema formation for repair, was sufficient to induce FoxD expression. Strikingly, FoxD was expressed at anterior and posterior areas of the incised wound site, but only within the midline on the ventral side (third cut from left, Fig. 2C). This wound-induced FoxD expression occurred even in the absence of the anterior pole, indicating that local cues rather than signals from the pole control wound-induced expression of FoxD. An even more minor injury, a dorsal puncture with a needle (third panel from right, Fig. 2C), induced FoxD expression in the vicinity of the dorsal puncture, but only ventrally in the midline. All of these wound types impinged upon the midline of the animal, raising the possibility that any midline injury is sufficient to trigger FoxD expression, regardless of whether blastema formation would be required for repair or not. Supporting this hypothesis, two wound types that do not damage the midline (lateral puncture and lateral edge removal; first and second panels from right, Fig. 2C) did not trigger expression of FoxD. These two wounds did, by contrast, elicit normal expression of other wound-induced genes (Fig. 2D).
The wound-induced expression of FoxD occurred within ventral midline cells expressing the midline markers admp and slit (Fig. 2E). Furthermore, FoxD was also co-expressed at wounds with other defined wound-induced genes, such as noggin-like1 (nlg1) [3], [38], wntless [3], [12], [38], notum [8], and wnt1 [37] (Fig. 2E and Fig. S2B). Wound-induced genes that peak in expression around six hours following injury and that are mostly expressed subepidermally at wound sites are known as W2 genes [3]. Although most W2 genes are predicted to be secreted proteins, such as signaling and matrix remodeling factors, our results indicate that the transcription factor FoxD itself belongs in this category. W2 gene expression is induced in muscle cells expressing collagen [15], and most wound-induced FoxD expressing cells (92.5±4.6%) also co-expressed the muscle gene collagen (Fig. 2E). We conclude that FoxD is a wound-induced gene in planarian muscle, but unique among known planarian wound-induced genes with expression occurring only in ventral midline cells and only following injury that impinges on the midline. FoxD also defines a fourth gene (together with wnt1, notum, and follistatin) that is wound-induced and subsequently expressed in either the anterior or posterior pole.
FoxD is expressed in a subset of smedwi-1+ cells during anterior pole regeneration
The second phase of FoxD expression during regeneration, initiating around 24 hours post-amputation, occurred in cells that were coalesced at the anterior pole. This expression phase presented the opportunity to define the cellular steps of anterior pole formation. Irradiated animals did not form an anterior pole and did not express FoxD at 24 to 72 hours following wounding (Fig. 2B), indicating the requirement of neoblasts for this process. FoxD-expressing cells at the regenerating anterior pole at this time also co-expressed multiple anterior PCGs as well as the anterior pole-expressing gene notum (Fig. 3A). follistatin, which is required for anterior regeneration [22], [23], was also co-expressed with FoxD at the regenerating anterior pole at this regeneration stage (Fig. 3A). FoxD expression gradually became restricted with time to fewer cells at the regenerating anterior pole, adopting the same appearance as in intact animals (Figs. 1 and 3A).
Fig. 3. FoxD is expressed in anterior pole progenitors. 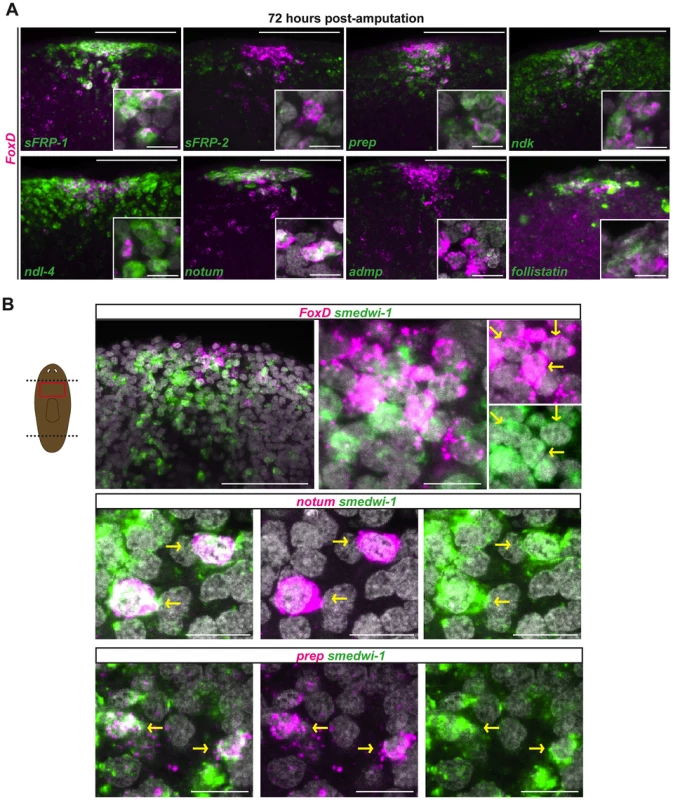
Double FISH in wild-type head blastemas 72 hours after transverse amputation. Red box in cartoon shows the region imaged. Images shown are maximal intensity projections. Images are representative of n>5 animals per panel. Anterior is up, dorsal view. (A) FoxD (magenta) and PCGs (green). Percentages (mean ± SD) of FoxD cells co-expressing anterior patterning genes and anterior pole markers are as follows: 95±2% with sFRP1; 93±3% with ndk; 81±9% with ndl-4; 86±5% with prep; and 74±11% with notum; co-expression with the midline gene admp is 3±5% (n>120 FoxD+ cells examined in each case). Scale bars, 100 µm. Inset shows higher magnification of co-expressing cells, scale bars are 10 µm. (B) FoxD, notum, and prep (magenta), smedwi-1 (green). Percentage (mean ± SD) of FoxD cells co-expressing smedwi-1 was 39.7±13.3% (n = 157 FoxD+ cells examined); percentage of notum cells co-expressing smedwi-1 was 22.9±6.1% (n = 111 notum+ cells examined). Yellow arrows point to double-labeled cells. Scale bars in upper left panel, 100 µm; other panels, 10 µm. The planarian smedwi-1 gene encodes a PIWI family protein and is expressed in all dividing adult planarian cells, marking the neoblast population [1]. We found that some FoxD-expressing cells located at the forming anterior pole co-expressed smedwi-1 in regenerating blastemas 72 hours following amputation (Fig. 3B). In addition to FoxD, expression of the anterior pole gene notum and the anterior-patterning gene prep was also found in smedwi-1-expressing neoblasts at the regenerating anterior pole (Fig. 3B). These data suggest that by three days of regeneration some neoblasts have been specified to produce the new pole cells of the regenerating head, potentially marking the first cellular step in formation of a new anterior pole.
Hh signaling impacts wound-induced FoxD expression
Both Wnt and Hh signaling pathways are required for the head-versus-tail regeneration decision made at planarian amputation planes [4]–[8], [39]. To test whether wound-induced FoxD expression was regulated by these signaling pathways, we inhibited Wnt (β-catenin and APC) and Hh (patched (ptc) and hedgehog (hh)) pathway genes with RNAi and examined FoxD expression six hours following amputation. As controls, we analyzed the numbers of notum - and wnt1-expressing cells in the different RNAi conditions and, as expected from prior reports [5], [8], notum expression was exclusively affected in β-catenin and APC RNAi animals and wnt1 expression was affected following perturbation of the Hh signaling pathway (Figs. 4 and S3A). Perturbation of Wnt signaling did not affect wound-induced FoxD expression (Figs. 4 and S3A). By contrast, ptc(RNAi) animals displayed fewer than normal FoxD-expressing cells at wounds and hh(RNAi) animals had increased numbers of FoxD-expressing cells (Figs. 4 and S3A). patched (ptc) encodes a receptor for Hh that antagonizes pathway output [40]. Hh signaling therefore negatively regulates FoxD induction at the midline following wounding. Because hh impacts wound-induced wnt1 expression oppositely to FoxD [4], [5], this result does not reflect a generic requirement for hh in wound-induced gene activation. FoxD expression at the anterior pole was normal in hh(RNAi) anterior blastemas (Fig. S3B), indicating a specific role for hh in regulating the FoxD wound-induced phase of expression.
Fig. 4. Hh signaling is required for wound-induced FoxD expression. 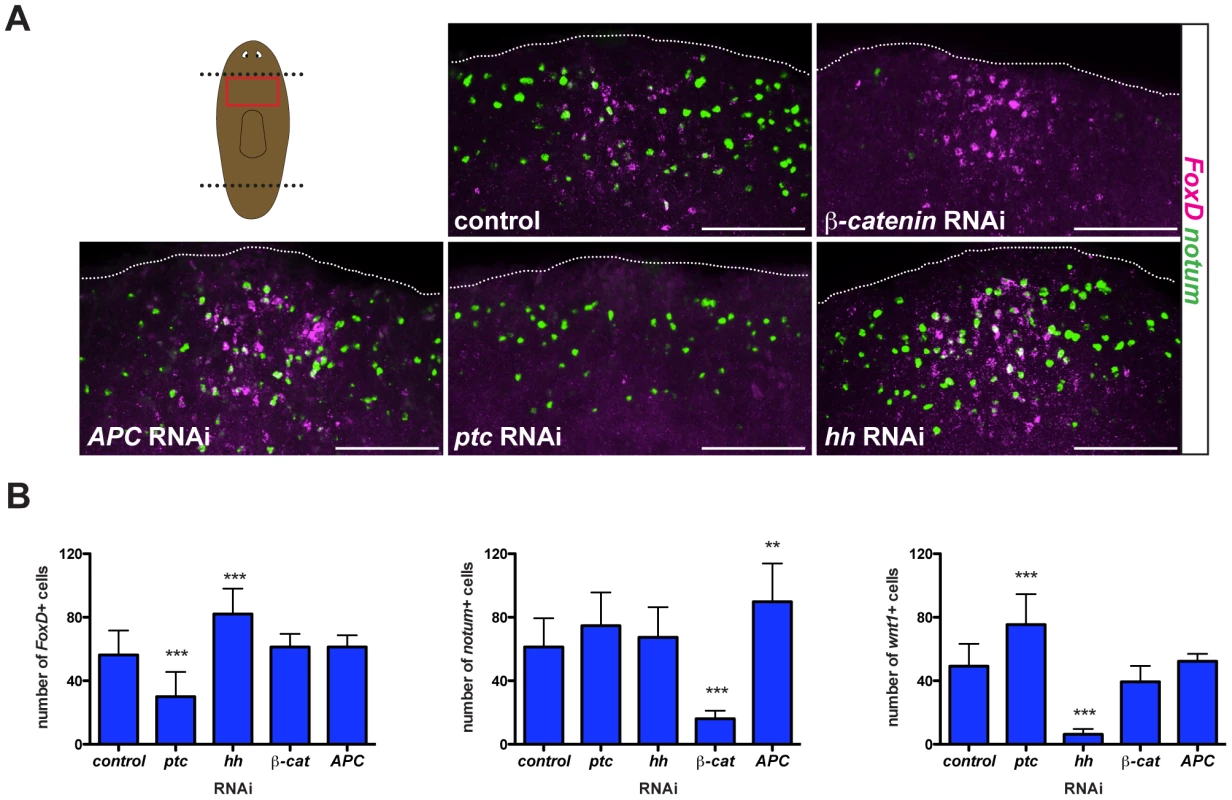
(A) Double FISH; notum (green) and FoxD (magenta) of RNAi fed animals fixed six hours following transverse amputation. Red box in cartoon shows region imaged. Images shown are maximal intensity projections. Dotted white line marks the approximate wound boundary. Anterior wounds are up, ventral view. Scale bars for all panels, 100 µm. (B) Graphs show number of cells expressing FoxD, notum, or wnt1 in the different RNAi conditions. Data are shown as mean ± SD, and analyzed using a one-way ANOVA test; **p<0.01, ***p<0.001, n>10 animals per RNAi condition. ptc(RNAi) animals with a strong phenotype regenerate tails in place of heads, but we found no evidence that FoxD influences the head-versus-tail regeneration choice.
However, ptc(RNAi) animals with a weak phenotype (e.g., cyclopic heads) do resemble FoxD(RNAi) animals [4], [5]. Cyclopic or headless ptc(RNAi) animals showed decreased expression of the anterior PCG sFRP-1 ([5] and Fig. S3D) and decreased expression of the anterior pole marker notum (Fig. S3D), indicating a defect in anterior pole regeneration. Therefore, the defect in FoxD expression might contribute to the ptc(RNAi) phenotype. In vertebrates, Sonic hedgehog (a member of the Hh family) signaling is required for normal forebrain development and midline induction [40], [41]. In planarians, hh is expressed ventrally and medially [4], [5]; the impact on wound-induced expression of FoxD at the midline raises the interesting possibility that hh might also have a role in midline biology in planarians. In ptc(RNAi) animals, midline expression of slit at six hours following amputation was normal (Fig. S3C). 86.3±5.5% of FoxD-expressing cells following wounding co-express the midline gene slit in wild-type animals. Therefore, these results indicate that the reduced wound-induced expression of FoxD in ptc(RNAi) animals is not a consequence of the absence of the midline cells that normally express FoxD.
Disruption of pole formation with FoxD RNAi perturbs anterior regeneration
FoxD(RNAi) animals displayed defective regeneration, with variable blastema size and heads that regenerated either one or no eyes (Fig. 5A). FoxD(RNAi) head blastemas had abnormal anatomy, with medial collapse of cephalic ganglia (labeled with an RNA probe to choline acetyltransferase (chat) [42]), one or no eyes (detected with an anti-ARRESTIN antibody (VC1) [43]), and a slightly abnormal anterior intestine morphology (labeled with an RNA probe to mat [1]) (Fig. 5A). Tail fragments regenerated pharynges (Fig. S4B), demonstrating that some missing tissues can regenerate in FoxD(RNAi) animals. Moreover, intestinal branches were normally regenerated in FoxD(RNAi) tail blastemas (Fig. S4C), indicating that FoxD has a largely specific role in anterior regeneration. Parasagittal thin fragments of FoxD(RNAi) animals also regenerated pharynges (Fig. S6B), further demonstrating that neoblasts can replace missing tissues. However, most of these fragments regenerated only one eye, showed slightly reduced expression of the anterior PCG sFRP1, and regenerated asymmetric cephalic ganglia (Fig. S6B).
Fig. 5. FoxD(RNAi) animals display abnormal anterior pole, head patterning and midline gene expression. 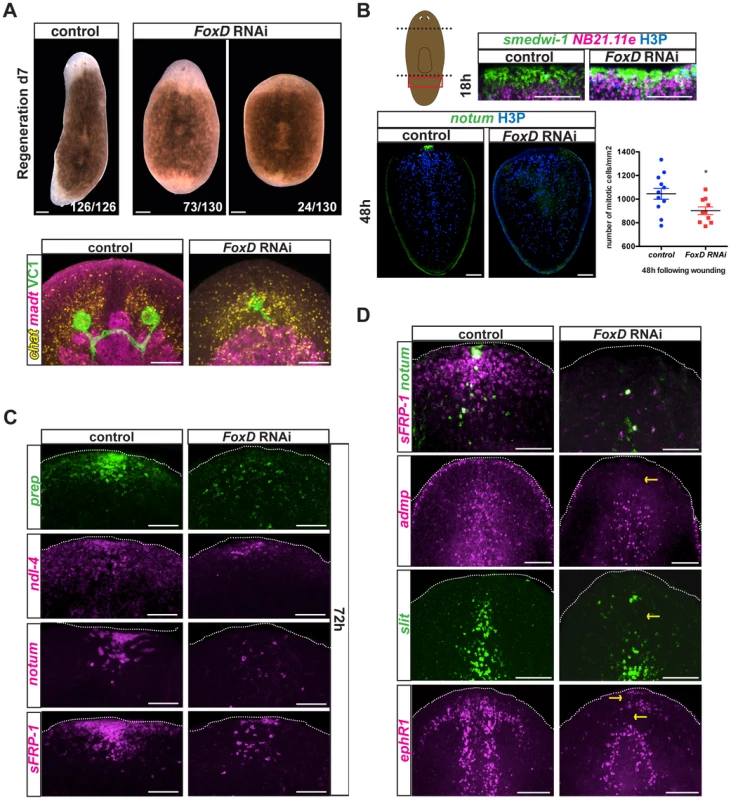
(A) Upper panels: animals injected with FoxD dsRNA regenerated normally sized anterior blastemas with one eye (73/130) or smaller blastemas with no eyes (24/130) seven days following amputation. Scale bars, 200 µm. Lower panels: double FISH and immunostainings using anti-ARRESTIN (VC1) antibody in regenerating FoxD dsRNA injected animals. Defects in head regeneration of FoxD(RNAi) animals were observed; probes used: brain, chat (7/7 animals showed a medially collapsed or a very small brain); intestine, mat (3/7 animals showed some regeneration defect in the anterior blastema). Images shown are maximal intensity projections. Scale bars, 100 µm. Anterior is up, dorsal view. (B) Upper panels: double FISH (smedwi-1 in green, NB.21.11e in magenta) and immunostaining with anti-phospho histone 3 (H3P in blue) of tail fragments fixed at 18 hours following amputation of eight weeks RNAi fed animals. Red box in cartoon on the left shows the area imaged. Lower panels: FISH (notum, green) and immunostaining with anti-H3P (blue) were performed at 48 hours following amputation in regenerating tail fragments of eight weeks RNAi fed animals. Anterior is up, dorsal view. Images shown are maximal intensity projections. Scale bars, 100 µm. Numbers of mitotic cells were counted and normalized by the tail area (mm2) and analyzed using a Student-t-test analysis; *p<0.05, n>10. (C) FoxD dsRNA injected animals displayed defects in anterior pole gene and PCG expression at 72 hours following amputation (sFRP-1, 5/9; ndl-4, 3/4; notum, 10/11; prep, 5/6). Dotted white line marks the approximate animal edge. Images shown are maximal intensity projections. Anterior is up, dorsal view. Scale bars, 50 µm. (D) Single or double FISH in day seven regenerating dsRNA injected animals. Defective expression of the anterior pole gene notum (8/20 no expression, 10/20 decreased expression) and sFRP-1 (9/18 no expression, 7/18 reduced expression) is observed in FoxD(RNAi) animals. Reduced expression of the midline genes slit (6/11 severe defect, 2/11 mild defect), admp (8/10 reduced expression), and ephrin receptor 1, ephR1 (7/14 severe reduction, 3/14 mild reduction) was observed in FoxD(RNAi) animals. Yellow arrows point to missing or aberrant expression. Images shown are maximal intensity projections. Anterior is up, dorsal view for all panels except for admp and slit FISH. Scale bars, 100 µm. Blastema size abnormalities in FoxD(RNAi) animals did not appear to be an overt consequence of a neoblast maintenance defect, because normal mitotic cell numbers were present in FoxD(RNAi) animals at the time of the amputation (0 hours following wounding, Fig. S4A). Following FoxD RNAi, neoblast (smedwi-1-expressing cells) proliferation (6 hours post-amputation, Fig. S4A) and migration (18 hours post-amputation, Fig. 5B, upper panel) in response to wounding were normal. At 48 hours following wounding, neoblasts of regenerating FoxD(RNAi) tail, but not trunk, fragments displayed slightly reduced neoblast proliferation (Fig. 5B and Fig. S4A). Reduced proliferation in FoxD(RNAi) tail fragments persisted five days following wounding (Fig. S4A).
FoxD(RNAi) animals have reduced numbers of follistatin-expressing cells at the anterior pole [22]. At 48 hours following wounding notum+ and notum+ follistatin+ cells were reduced from regenerating FoxD(RNAi) anterior blastemas (Fig. 5B and Fig. S5A). Furthermore, neoblasts expressing notum and prep were also fewer or absent in FoxD(RNAi) animals (Fig. S7), suggesting that FoxD is required for neoblast specification into anterior pole cell progenitors. These observations suggest a role for FoxD in anterior pole regeneration by specifying pole progenitors.
At 72 hours following head amputation, we observed significantly lower or complete absence in expression of the anterior PCGs prep, ndl-4, and sFRP-1, and decreased or no notum-coalesced cells in FoxD(RNAi) animals (Fig. 5C). After seven days of regeneration, FoxD(RNAi) anterior blastemas also showed severe defects in the expression of sFRP-1 and ndl-4, as well as the pole-restricted gene notum (Fig. 5D and Fig. S5B). Together, these results establish a role for FoxD and the anterior pole in head patterning during regeneration.
FoxD(RNAi) animals had normal numbers of notum - and wnt1-expressing cells at six hours following wounding (Fig. 6A), indicating that FoxD does not prevent the wound-induced phase of notum expression during the specification of head regeneration. Furthermore, wound-induced expression of follistatin [22], [23] was normal in FoxD(RNAi) animals (Fig. S5A). We also did not observe ectopic expression of posterior markers (wnt11-2 and wnt1) in regenerating anterior blastemas of FoxD(RNAi) animals (Fig. S4D), demonstrating that the choice to regenerate a head instead of a tail (AP regeneration polarity) was not detectably affected. In addition, we observed normal expression of posterior markers (wnt11-2 and wnt1) in regenerating posterior blastemas, indicating a specific role for FoxD in anterior regeneration (Fig. 6B).
Fig. 6. FoxD(RNAi) animals have normal expression of wound-induced genes and posterior genes. 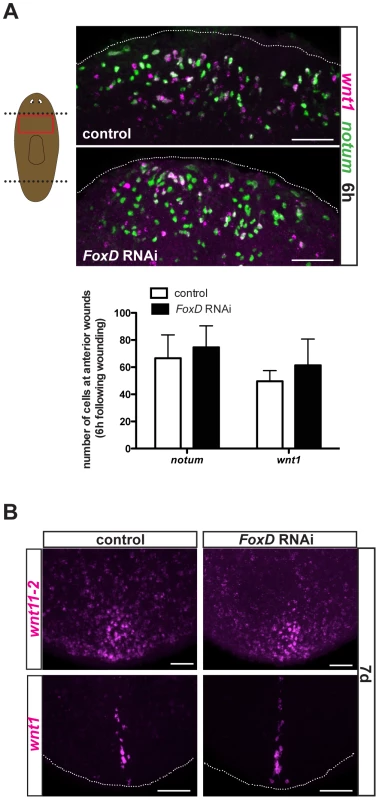
(A) Double FISH using notum (green) and wnt1 (magenta) in trunk fragments of control and FoxD dsRNAi injected animals six hours following amputation. Red box in cartoon on the left shows the area imaged. Graph shows total number of cells expressing notum, or wnt1 in the different RNAi conditions. Data are shown as mean± SD, n>5 animals per group. Anterior is up, ventral view. (B) Posterior blastemas at day seven of regeneration of FoxD dsRNA injected animals are shown; expression of posterior patterning gene wnt11-2 (17/17) and the posterior pole gene wnt1 (14/14) was normal in FoxD(RNAi) animals. Anterior is up, dorsal view. For all panels, images shown are maximal intensity projections. Scale bars, 50 µm. pbx and prep are required for FoxD expression during anterior pole formation
The TALE-homeodomain-encoding pbx and prep genes are required for regeneration and maintenance of anterior PCG expression, including for markers of the anterior pole [17], [19], [20]. Because prep is expressed broadly at the head tip and pbx in most cells of the regenerating head [17], [19], [20], it was not previously possible to determine whether poles promoted anterior PCG expression or vice versa. However, because FoxD expression is restricted to the regenerating pole, the FoxD RNAi phenotype described above suggests the pole is required for anterior PCG expression. This model predicts that pbx and prep might be required for FoxD expression. Wound-induced FoxD expression at six hours following injury was normal in both pbx and prep RNAi animals (Fig. 7A). Similarly, other wound-induced genes (notum and wnt1) are expressed normally in pbx(RNAi) animals [19], [20], indicating that pbx and prep act downstream of wound-induced expression of these genes. By contrast, anterior pole-specific expression of FoxD and notum was completely absent in both pbx and prep RNAi animals 72 hours following head amputation (Fig. 7B). Because both FoxD and notum expression is induced in neoblasts at 72 hours following amputation of wild-type animals, the complete absence of expression of these two genes at this time point suggests a requirement for pbx and prep in the specification of neoblasts into anterior pole progenitors and that this defect might underlie the pbx and prep phenotypes.
Fig. 7. pbx and prep are required for FoxD pole expression, and anterior pole and midline regeneration. 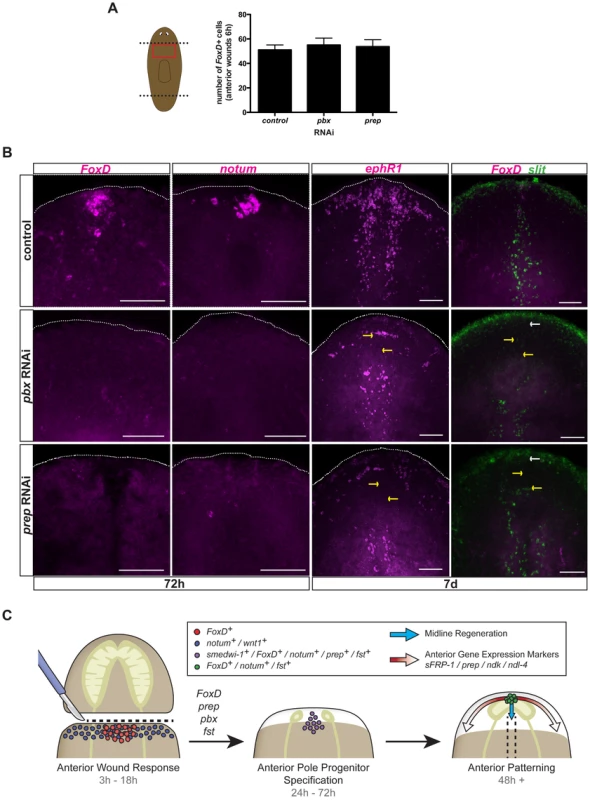
(A) Single FISH for FoxD in trunk fragments of control, pbx, and prep RNAi animals six hours following amputation. Graph shows total number of cells expressing FoxD in the different RNAi conditions. Data are shown as mean ± SD, n>9 animals per group. (B) Single or double FISH in regenerating pbx and prep RNAi animals. No FoxD or notum expression in anterior blastemas at 72 hours (n>5 animals per condition) and no FoxD expression at seven days (n>6 animals per panel) were observed in pbx and prep RNAi animals. No extension of slit expression to the anterior pole (5/5 animals), and defective expression of ephrin receptor 1, ephR1 (n>4 animals per group) was observed in both pbx and prep RNAi animals. Yellow arrows point to missing or aberrant expression of midline genes. White arrows point to absence of FoxD expression at the anterior pole. Dotted white line marks the approximate animal edge. Images shown are maximal intensity projections. Anterior is up, dorsal view except in slit panels. Scale bars, 100 µm. (C) Proposed model for anterior regeneration: Following head amputation, wound-induced genes, like notum and wnt1 (blue dots) are expressed throughout the wound, whereas FoxD is induced in the ventral midline (red dots). Note that wnt1 is also wound-induced at posterior-facing wounds and that the gene follistatin (fst) is also wound induced. Subsequently, specification of anterior pole progenitors occurs within neoblasts. FoxD, prep, pbx, and fst are required for this step. fst RNAi can cause a strong block in regeneration, but under weaker RNAi conditions early expression of notum in this time window was affected [22]. Cells co-expressing the neoblast marker smedwi-1 and the anterior-pole markers FoxD or notum, or the anterior-patterning gene prep are found within the anterior midline (purple dots). In a third phase, once the anterior pole is formed (cells expressing FoxD, notum, and fst; green dots), this pole organizes pattern of head blastemas: the cephalic ganglia, anterior gene expression domains (red arrows), and the regeneration of the midline (blue arrows). FoxD is required for midline regeneration
The restricted, wound-induced expression of FoxD in the midline raises the possibility of a regenerative connection between the midline and the anterior pole. To explore this possibility further, we analyzed the expression pattern of several midline genes, such as slit, admp, and ephR1, in FoxD(RNAi) animals. Indeed, these midline genes were not properly expressed in regenerating FoxD(RNAi) anterior blastemas (Fig. 5D). In addition, parasagittal thin pieces showed reduced slit expression at the anterior midline (Fig. S6B). By contrast, tail blastemas of transversely amputated FoxD(RNAi) animals displayed normal extension of slit-expressing cells to the posterior pole (Fig. S4C), further demonstrating the specific role of FoxD in the biology of the anterior pole.
To further investigate the possible connection of the regenerating anterior pole and the midline, we examined the midline-expressed slit and ephR1 genes in pbx and prep RNAi animals, which are unable to regenerate an anterior pole (Fig. 7B and [19], [20]). pbx(RNAi) animals have been reported to have defective slit expression in anterior and posterior blastemas [19], [20]. As predicted, expression of both slit and ephR1 was defective in anterior blastemas of pbx and prep RNAi animals seven days following head amputation (Fig. 7B). Moreover, the midline expression of ephR1 was defective in all ptc(RNAi) animals showing intermediate phenotypes (cyclopia and headless; and therefore, reduced or absent anterior pole) at seven days following head amputation (Fig. S3D); because of the broad role of ptc it is unknown whether this defect is solely explained by a pole defect in ptc(RNAi) animals. hh(RNAi) animals, which can regenerate a normal anterior pole (Fig. S3B), showed proper expression of the ephR1 gene in anterior blastemas at seven days following amputation (Fig. S3B). Intact FoxD(RNAi) animals undergoing long-term RNAi did not show an obvious phenotype (Fig. S6A); following transverse amputation of these animals, small blastemas with one or no eyes were regenerated, and the anterior pole was rarely formed or very reduced (Fig. S6C, D). In some of these amputated FoxD(RNAi) animals a small cluster of anterior pole cells were regenerated but offset from the midline (n = 5/19 offset, with 14/19 showing medial but severely reduced poles, Fig. S6D). Altogether, our results suggest a role for the anterior pole in organizing the regeneration of the new midline and patterning of the head.
Discussion
The ends of embryos that will become the poles of the primary body axis can be involved in organizing embryonic tissue pattern in many animals. For example, bicoid mRNA in Drosophila is locally translated at the prospective anterior end of the oocyte and controls establishment of AP body pattern [44], [45]. PAR-3/PAR-6/aPKC-3 proteins localize to one side of the C. elegans zygote after fertilization, establishing the anterior embryo end [46]. In neurula-stage Xenopus embryos, Wnt genes regulate AP neurectoderm patterning and are active at the posterior, with Wnt inhibitory genes active at the anterior [47]. Early steps in development, such as asymmetric deposition of maternal determinants in oocytes or events triggered at the sperm entry site can be involved in establishment of polarized regions that impact primary axis pattern formation. In animals capable of whole-body regeneration, by contrast, the anterior and posterior poles must be formed de novo without reliance on events specific for embryogenesis. This problem exemplifies a general challenge faced by all regeneration paradigms: the establishment of pattern in an adult tissue context utilizing initial steps that can differ from the cues accessible to embryos. Planarian pole regeneration presents an ideal setting to explore the mechanistic basis for this process.
FoxD encodes a Forkhead-family transcription factor that functions during planarian regeneration to specify cells at a key signaling center occurring at the intersection of the anterior extreme and the midline. Transcription factors expressed regionally in embryos can promote establishment of signaling domains that control tissue pattern. A classic example is goosecoid, which encodes a homeodomain transcription factor required for expression of many patterning factors at Spemann's organizer in frog embryos [48]. In regeneration, how transcription factors might be suitably activated to organize regeneration of tissue pattern is poorly understood. The early and restricted midline expression of FoxD following wounding, the subsequent expression of FoxD at the anterior pole (which is located at the midline), and the anterior pole and midline regeneration phenotype in FoxD, pbx, and prep RNAi animals suggest that the anterior pole organizes many aspects of head regeneration. We propose the following model for head regeneration (Fig. 7C):
(i) Anterior wound response
In the first phase, the anterior wound response (3–18 hours post-amputation), signaling mechanisms occurring in pre-existing muscle cells at wounds determine whether a new head is to be regenerated. Wound signaling triggers rapid (within ∼6 hours) expression of wnt1 at all wounds [37]. Wnt signaling is selectively inhibited at anterior-facing wounds, involving activation of the notum gene [8]. The mechanism that leads to selectivity in notum activation is unknown. This first phase of head regeneration results in inhibition of Wnt signaling at anterior-facing wounds, whereas Wnt signaling is active at posterior-facing wounds. FoxD is activated at the midline of most wounds during this initial phase of wound-induced wnt1 and notum expression, but our data do not indicate a role for this gene in the decision to regenerate a head-versus-tail.
(ii) Anterior pole progenitor specification
If an anterior-facing wound is not juxtaposed by anterior tissue, a second phase of head regeneration involving anterior pole progenitor specification ensues. This phase (24–72 hours post-amputation) involves formation of a new anterior pole in an emerging blastema. The initial medial FoxD expression presents a candidate mechanism for establishing the location of pole regeneration–at the prior midline. This location of FoxD induction highlights the connection between the midline and pole regeneration as an important area for further investigation. We found that a small cluster of cells at the midline expressing notum, follistatin, and FoxD emerges from neoblasts in this second phase. Neoblasts can be specified to form eyes [48] or protonephridia [49] in regeneration, and here we demonstrate that some neoblasts near the forming anterior pole express FoxD, notum, and prep. We propose that neoblast induction of these genes near the midline at wounds represents an initial cellular step in formation of the cells of the new anterior pole.
(iii) Anterior patterning
In a third phase of head regeneration (∼48 hours+ post-amputation), as the blastema grows substantially, pattern of the head blastema is established. FoxD RNAi severely reduced anterior pole regeneration. Whereas these FoxD(RNAi) anterior blastemas still displayed anterior character (such as the presence of brain cells), the blastemas lacked the normal tissue organization of wild-type head blastemas. FoxD(RNAi) blastemas also had defects in the expression of a number of anterior PCGs with broad anterior expression domains (e.g., prep, sFRP-1). This raises the possibility that the anterior pole is involved in establishing and maintaining more general anterior patterns of gene expression during this third phase of head regeneration. Consistent with this hypothesis, head tissue can also be regenerated in pbx or prep RNAi animals lacking poles, but establishment of anterior patterning gene expression domains is severely affected [17], [19], [20]. Finally, expression domains of genes expressed at the midline were aberrant in FoxD(RNAi) head blastemas. Together, these results suggest that the regenerating anterior pole promotes midline regeneration for proper bilateral patterning of the head and promotes establishment of gene expression domains for AP patterning of the head.
Materials and Methods
Animals and radiation treatment
Asexual Schmidtea mediterranea strain (CIW4) animals starved 7–14 days prior experiments were used. Animals were exposed to a 6,000 rads dose of radiation using a dual Gammacell-40 137 cesium source and amputated three days after irradiation. No vertebrate animals were used in this study, and usage of planarians (invertebrates) is unregulated.
RNAi
Animals were injected with control (the C. elegans gene unc-22), FoxD, or pbx dsRNA. Animals were transversely amputated and trunk pieces injected within 1 hour post-amputation with dsRNA. A booster dsRNA injection of trunk pieces was performed the following day. This procedure (amputation, injection and booster injection) was performed a total of three times, every two to three days. Following the third cycle of amputation and injections, animals were scored for phenotype at 72 hours and seven days post amputation, fixed and analyzed with in situ hybridizations [50] and for many experiments involved azide quenching as described [50].
For RNAi by feeding experiments, dsRNA-expressing bacteria cultures were mixed with 70% liver solution in a 1∶300 ratio to culture volume. β-catenin, APC, and prep RNAi animals have been fed four times (days 0, 4, 7 and 11), and amputated at d12. 72 hours or seven days following amputation, animals were fixed and in situ hybridizations performed. hh and ptc RNAi animals were fed six times every three to four days. Long-term FoxD(RNAi) homeostasis experiments were performed by feeding the animals every three to four days during a ten week-period.
Whole-mount in situ hybridizations and immunostainings
Animals were wounded and fixed at six hours following injury in 4% formaldehyde [50] and nitroblue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate (NBT/BCIP) colorimetric whole-mount in situ hybridizations or fluorescence in situ hybridizations (FISH) were performed as described [50]. For immunostainings, animals were fixed as for in situ hybridizations and then treated as described [51]. A mouse anti-ARRESTIN antibody was kindly provided by Kiyokazu Agata and used in a 1∶5,000 dilution, and an anti-mouse-Alexa conjugated antibody was used in a 1∶500 dilution. For the neoblast wound response assay, RNAi animals were fed during the course of eight weeks every three to four days, then were transversely amputated and trunk or tail fragments were fixed at 0, 6, 18, 48, and 120 hours following wounding. Animals were immunostained using a rabbit anti-phospho histone 3 antibody and an anti-rabbit HRP in a 1∶100 dilution as previously described [52]. Fluorescent images were taken with a Zeiss LSM700 Confocal Microscope. Light images were taken with a Zeiss Discovery Microscope.
Supporting Information
Zdroje
1. WagnerDE, WangIE, ReddienPW (2011) Clonogenic neoblasts are pluripotent adult stem cells that underlie planarian regeneration. Science 332 : 811–816.
2. ReddienPW, Sánchez AlvaradoA (2004) Fundamentals of planarian regeneration. Annu Rev Cell Dev Biol 20 : 725–757.
3. WenemoserD, LapanSW, WilkinsonAW, BellGW, ReddienPW (2012) A molecular wound response program associated with regeneration initiation in planarians. Genes Dev 26 : 988–1002.
4. YazawaS, UmesonoY, HayashiT, TaruiH, AgataK (2009) Planarian Hedgehog/Patched establishes anterior-posterior polarity by regulating Wnt signaling. Proc Natl Acad Sci U S A 106 : 22329–22334.
5. RinkJC, GurleyKA, ElliottSA, Sánchez AlvaradoA (2009) Planarian Hh signaling regulates regeneration polarity and links Hh pathway evolution to cilia. Science 326 : 1406–1410.
6. GurleyKA, RinkJC, Sánchez AlvaradoA (2008) Beta-catenin defines head versus tail identity during planarian regeneration and homeostasis. Science 319 : 323–327.
7. PetersenCP, ReddienPW (2008) Smed-betacatenin-1 is required for anteroposterior blastema polarity in planarian regeneration. Science 319 : 327–330.
8. PetersenCP, ReddienPW (2011) Polarized notum activation at wounds inhibits Wnt function to promote planarian head regeneration. Science 332 : 852–855.
9. ReddienPW, BermangeAL, KiczaAM, Sánchez AlvaradoA (2007) BMP signaling regulates the dorsal planarian midline and is needed for asymmetric regeneration. Development 134 : 4043–4051.
10. GaviñoMA, ReddienPW (2011) A Bmp/Admp regulatory circuit controls maintenance and regeneration of dorsal-ventral polarity in planarians. Curr Biol 21 : 294–299.
11. MolinaMD, NetoA, MaesoI, Gomez-SkarmetaJL, SaloE, et al. (2011) Noggin and noggin-like genes control dorsoventral axis regeneration in planarians. Curr Biol 21 : 300–305.
12. AdellT, SaloE, BoutrosM, BartschererK (2009) Smed-Evi/Wntless is required for beta-catenin-dependent and -independent processes during planarian regeneration. Development 136 : 905–910.
13. IglesiasM, Almuedo-CastilloM, AboobakerAA, SaloE (2011) Early planarian brain regeneration is independent of blastema polarity mediated by the Wnt/beta-catenin pathway. Dev Biol 358 : 68–78.
14. ReddienPW (2011) Constitutive gene expression and the specification of tissue identity in adult planarian biology. Trends Genet 27 : 277–285.
15. WitchleyJN, MayerM, WagnerDE, OwenJH, ReddienPW (2013) Muscle cells provide instructions for planarian regeneration. Cell Rep 4 : 633–641.
16. CebriaF, KobayashiC, UmesonoY, NakazawaM, MinetaK, et al. (2002) FGFR-related gene nou-darake restricts brain tissues to the head region of planarians. Nature 419 : 620–624.
17. FelixDA, AboobakerAA (2010) The TALE class homeobox gene Smed-prep defines the anterior compartment for head regeneration. PLoS Genet 6: e1000915.
18. GurleyKA, ElliottSA, SimakovO, SchmidtHA, HolsteinTW, et al. (2010) Expression of secreted Wnt pathway components reveals unexpected complexity of the planarian amputation response. Dev Biol 347 : 24–39.
19. BlassbergRA, FelixDA, Tejada-RomeroB, AboobakerAA (2013) PBX/extradenticle is required to re-establish axial structures and polarity during planarian regeneration. Development 140 : 730–739.
20. ChenCC, WangIE, ReddienPW (2013) pbx is required for pole and eye regeneration in planarians. Development 140 : 719–729.
21. HayashiT, MotoishiM, YazawaS, ItomiK, TanegashimaC, et al. (2011) A LIM-homeobox gene is required for differentiation of Wnt-expressing cells at the posterior end of the planarian body. Development 138 : 3679–3688.
22. Roberts-GalbraithRH, NewmarkPA (2013) Follistatin antagonizes Activin signaling and acts with Notum to direct planarian head regeneration. Proc Natl Acad Sci U S A 110 : 1363–1368.
23. GaviñoMA, WenemoserD, WangIE, ReddienPW (2013) Tissue absence initiates regeneration through Follistatin-mediated inhibition of Activin signaling. eLiFE 2: e00247.
24. BenayounBA, CaburetS, VeitiaRA (2011) Forkhead transcription factors: key players in health and disease. Trends Genet 27 : 224–232.
25. CarlssonP, MahlapuuM (2002) Forkhead transcription factors: key players in development and metabolism. Dev Biol 250 : 1–23.
26. LehmannOJ, SowdenJC, CarlssonP, JordanT, BhattacharyaSS (2003) Fox's in development and disease. Trends Genet 19 : 339–344.
27. HannenhalliS, KaestnerKH (2009) The evolution of Fox genes and their role in development and disease. Nat Rev Genet 10 : 233–240.
28. WeigelD, JurgensG, KuttnerF, SeifertE, JackleH (1989) The homeotic gene fork head encodes a nuclear protein and is expressed in the terminal regions of the Drosophila embryo. Cell 57 : 645–658.
29. HackerU, KaufmannE, HartmannC, JurgensG, KnochelW, et al. (1995) The Drosophila fork head domain protein crocodile is required for the establishment of head structures. EMBO J 14 : 5306–5317.
30. GrossniklausU, PearsonRK, GehringWJ (1992) The Drosophila sloppy paired locus encodes two proteins involved in segmentation that show homology to mammalian transcription factors. Genes Dev 6 : 1030–1051.
31. CadiganKM, GrossniklausU, GehringWJ (1994) Localized expression of sloppy paired protein maintains the polarity of Drosophila parasegments. Genes Dev 8 : 899–913.
32. YuJK, HollandND, HollandLZ (2003) AmphiFoxQ2, a novel winged helix/forkhead gene, exclusively marks the anterior end of the amphioxus embryo. Dev Genes Evol 213 : 102–105.
33. DirksenML, JamrichM (1992) A novel, activin-inducible, blastopore lip-specific gene of Xenopus laevis contains a fork head DNA-binding domain. Genes Dev 6 : 599–608.
34. KoinumaS, UmesonoY, WatanabeK, AgataK (2003) The expression of planarian brain factor homologs, DjFoxG and DjFoxD. Gene Expr Patterns 3 : 21–27.
35. CebriaF, GuoT, JopekJ, NewmarkPA (2007) Regeneration and maintenance of the planarian midline is regulated by a slit orthologue. Dev Biol 307 : 394–406.
36. DuboisF (1949) Contribution à l'étude de la migration des cellules. de régénération chez les Planaires dulcicoles. Bull Biol Fr Belg 83 : 213–283.
37. PetersenCP, ReddienPW (2009) A wound-induced Wnt expression program controls planarian regeneration polarity. Proc Natl Acad Sci U S A 106 : 17061–17066.
38. OgawaK, IshiharaS, SaitoY, MinetaK, NakazawaM, et al. (2002) Induction of a noggin-like gene by ectopic DV interaction during planarian regeneration. Dev Biol 250 : 59–70.
39. IglesiasM, Gomez-SkarmetaJL, SaloE, AdellT (2008) Silencing of Smed-betacatenin1 generates radial-like hypercephalized planarians. Development 135 : 1215–1221.
40. KalderonD (2000) Transducing the hedgehog signal. Cell 103 : 371–374.
41. MuroneM, RosenthalA, de SauvageFJ (1999) Hedgehog signal transduction: from flies to vertebrates. Exp Cell Res 253 : 25–33.
42. NishimuraK, KitamuraY, TaniguchiT, AgataK (2010) Analysis of motor function modulated by cholinergic neurons in planarian Dugesia japonica. Neuroscience 168 : 18–30.
43. CebriaF, NewmarkPA (2005) Planarian homologs of netrin and netrin receptor are required for proper regeneration of the central nervous system and the maintenance of nervous system architecture. Development 132 : 3691–3703.
44. DrieverW, Nusslein-VolhardC (1988) A gradient of bicoid protein in Drosophila embryos. Cell 54 : 83–93.
45. DrieverW, Nusslein-VolhardC (1988) The bicoid protein determines position in the Drosophila embryo in a concentration-dependent manner. Cell 54 : 95–104.
46. NanceJ (2005) PAR proteins and the establishment of cell polarity during C. elegans development. Bioessays 27 : 126–135.
47. KieckerC, NiehrsC (2001) A morphogen gradient of Wnt/beta-catenin signalling regulates anteroposterior neural patterning in Xenopus. Development 128 : 4189–4201.
48. LapanSW, ReddienPW (2011) dlx and sp6-9 control optic cup regeneration in a prototypic eye. PLoS Genet 7: e1002226.
49. ScimoneML, SrivastavaM, BellGW, ReddienPW (2011) A regulatory program for excretory system regeneration in planarians. Development 138 : 4387–4398.
50. PearsonBJ, EisenhofferGT, GurleyKA, RinkJC, MillerDE, et al. (2009) Formaldehyde-based whole-mount in situ hybridization method for planarians. Dev Dyn 238 : 443–450.
51. NewmarkPA, Sánchez AlvaradoA (2000) Bromodeoxyuridine specifically labels the regenerative stem cells of planarians. Dev Biol 220 : 142–153.
52. ScimoneML, MeiselJ, ReddienPW (2010) The Mi-2-like Smed-CHD4 gene is required for stem cell differentiation in the planarian Schmidtea mediterranea. Development 137 : 1231–1241.
Štítky
Genetika Reprodukční medicína
Článek Unwrapping BacteriaČlánek A Chaperone-Assisted Degradation Pathway Targets Kinetochore Proteins to Ensure Genome StabilityČlánek The Candidate Splicing Factor Sfswap Regulates Growth and Patterning of Inner Ear Sensory OrgansČlánek The SPF27 Homologue Num1 Connects Splicing and Kinesin 1-Dependent Cytoplasmic Trafficking inČlánek Down-Regulation of eIF4GII by miR-520c-3p Represses Diffuse Large B Cell Lymphoma DevelopmentČlánek Meta-Analysis Identifies Gene-by-Environment Interactions as Demonstrated in a Study of 4,965 MiceČlánek High Risk Population Isolate Reveals Low Frequency Variants Predisposing to Intracranial Aneurysms
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2014 Číslo 1
-
Všechny články tohoto čísla
- How Much Is That in Dog Years? The Advent of Canine Population Genomics
- The Sense and Sensibility of Strand Exchange in Recombination Homeostasis
- Unwrapping Bacteria
- DNA Methylation Changes Separate Allergic Patients from Healthy Controls and May Reflect Altered CD4 T-Cell Population Structure
- Evidence for Mito-Nuclear and Sex-Linked Reproductive Barriers between the Hybrid Italian Sparrow and Its Parent Species
- Translation Enhancing ACA Motifs and Their Silencing by a Bacterial Small Regulatory RNA
- Relationship Estimation from Whole-Genome Sequence Data
- Genetic Models of Apoptosis-Induced Proliferation Decipher Activation of JNK and Identify a Requirement of EGFR Signaling for Tissue Regenerative Responses in
- ComEA Is Essential for the Transfer of External DNA into the Periplasm in Naturally Transformable Cells
- Loss and Recovery of Genetic Diversity in Adapting Populations of HIV
- Bioelectric Signaling Regulates Size in Zebrafish Fins
- Defining NELF-E RNA Binding in HIV-1 and Promoter-Proximal Pause Regions
- Loss of Histone H3 Methylation at Lysine 4 Triggers Apoptosis in
- Cell-Cycle Dependent Expression of a Translocation-Mediated Fusion Oncogene Mediates Checkpoint Adaptation in Rhabdomyosarcoma
- How a Retrotransposon Exploits the Plant's Heat Stress Response for Its Activation
- A Nonsense Mutation in Encoding a Nondescript Transmembrane Protein Causes Idiopathic Male Subfertility in Cattle
- Deletion of a Conserved -Element in the Locus Highlights the Role of Acute Histone Acetylation in Modulating Inducible Gene Transcription
- Developmental Link between Sex and Nutrition; Regulates Sex-Specific Mandible Growth via Juvenile Hormone Signaling in Stag Beetles
- PP2A/B55 and Fcp1 Regulate Greatwall and Ensa Dephosphorylation during Mitotic Exit
- Differential Effects of Collagen Prolyl 3-Hydroxylation on Skeletal Tissues
- Comprehensive Functional Annotation of 77 Prostate Cancer Risk Loci
- Evolution of Chloroplast Transcript Processing in and Its Chromerid Algal Relatives
- A Chaperone-Assisted Degradation Pathway Targets Kinetochore Proteins to Ensure Genome Stability
- New MicroRNAs in —Birth, Death and Cycles of Adaptive Evolution
- A Genome-Wide Screen for Bacterial Envelope Biogenesis Mutants Identifies a Novel Factor Involved in Cell Wall Precursor Metabolism
- FGFR1-Frs2/3 Signalling Maintains Sensory Progenitors during Inner Ear Hair Cell Formation
- Regulation of Synaptic /Neuroligin Abundance by the /Nrf Stress Response Pathway Protects against Oxidative Stress
- Intrasubtype Reassortments Cause Adaptive Amino Acid Replacements in H3N2 Influenza Genes
- Molecular Specificity, Convergence and Constraint Shape Adaptive Evolution in Nutrient-Poor Environments
- WNT7B Promotes Bone Formation in part through mTORC1
- Natural Selection Reduced Diversity on Human Y Chromosomes
- In-Vivo Quantitative Proteomics Reveals a Key Contribution of Post-Transcriptional Mechanisms to the Circadian Regulation of Liver Metabolism
- The Candidate Splicing Factor Sfswap Regulates Growth and Patterning of Inner Ear Sensory Organs
- The Acid Phosphatase-Encoding Gene Contributes to Soybean Tolerance to Low-Phosphorus Stress
- p53 and TAp63 Promote Keratinocyte Proliferation and Differentiation in Breeding Tubercles of the Zebrafish
- Affects Plant Architecture by Regulating Local Auxin Biosynthesis
- The SET Domain Proteins SUVH2 and SUVH9 Are Required for Pol V Occupancy at RNA-Directed DNA Methylation Loci
- Down-Regulation of Rad51 Activity during Meiosis in Yeast Prevents Competition with Dmc1 for Repair of Double-Strand Breaks
- Multi-tissue Analysis of Co-expression Networks by Higher-Order Generalized Singular Value Decomposition Identifies Functionally Coherent Transcriptional Modules
- A Neurotoxic Glycerophosphocholine Impacts PtdIns-4, 5-Bisphosphate and TORC2 Signaling by Altering Ceramide Biosynthesis in Yeast
- Subtle Changes in Motif Positioning Cause Tissue-Specific Effects on Robustness of an Enhancer's Activity
- C/EBPα Is Required for Long-Term Self-Renewal and Lineage Priming of Hematopoietic Stem Cells and for the Maintenance of Epigenetic Configurations in Multipotent Progenitors
- The SPF27 Homologue Num1 Connects Splicing and Kinesin 1-Dependent Cytoplasmic Trafficking in
- Down-Regulation of eIF4GII by miR-520c-3p Represses Diffuse Large B Cell Lymphoma Development
- Genome Sequencing Highlights the Dynamic Early History of Dogs
- Re-sequencing Expands Our Understanding of the Phenotypic Impact of Variants at GWAS Loci
- Meta-Analysis Identifies Gene-by-Environment Interactions as Demonstrated in a Study of 4,965 Mice
- , a -Antisense Gene of , Encodes a Evolved Protein That Inhibits GSK3β Resulting in the Stabilization of MYCN in Human Neuroblastomas
- A Transcription Factor Is Wound-Induced at the Planarian Midline and Required for Anterior Pole Regeneration
- A Comprehensive tRNA Deletion Library Unravels the Genetic Architecture of the tRNA Pool
- A PNPase Dependent CRISPR System in
- Genomic Confirmation of Hybridisation and Recent Inbreeding in a Vector-Isolated Population
- Zinc Finger Transcription Factors Displaced SREBP Proteins as the Major Sterol Regulators during Saccharomycotina Evolution
- GATA6 Is a Crucial Regulator of Shh in the Limb Bud
- Tissue Specific Roles for the Ribosome Biogenesis Factor Wdr43 in Zebrafish Development
- A Cell Cycle and Nutritional Checkpoint Controlling Bacterial Surface Adhesion
- High Risk Population Isolate Reveals Low Frequency Variants Predisposing to Intracranial Aneurysms
- E3 Ubiquitin Ligase CHIP and NBR1-Mediated Selective Autophagy Protect Additively against Proteotoxicity in Plant Stress Responses
- Evolutionary Rate Covariation Identifies New Members of a Protein Network Required for Female Post-Mating Responses
- 3′ Untranslated Regions Mediate Transcriptional Interference between Convergent Genes Both Locally and Ectopically in
- Single Nucleus Genome Sequencing Reveals High Similarity among Nuclei of an Endomycorrhizal Fungus
- Metabolic QTL Analysis Links Chloroquine Resistance in to Impaired Hemoglobin Catabolism
- Notch Controls Cell Adhesion in the Drosophila Eye
- AL PHD-PRC1 Complexes Promote Seed Germination through H3K4me3-to-H3K27me3 Chromatin State Switch in Repression of Seed Developmental Genes
- Genomes Reveal Evolution of Microalgal Oleaginous Traits
- Large Inverted Duplications in the Human Genome Form via a Fold-Back Mechanism
- Variation in Genome-Wide Levels of Meiotic Recombination Is Established at the Onset of Prophase in Mammalian Males
- Age, Gender, and Cancer but Not Neurodegenerative and Cardiovascular Diseases Strongly Modulate Systemic Effect of the Apolipoprotein E4 Allele on Lifespan
- Lifespan Extension Conferred by Endoplasmic Reticulum Secretory Pathway Deficiency Requires Induction of the Unfolded Protein Response
- Is Non-Homologous End-Joining Really an Inherently Error-Prone Process?
- Vestigialization of an Allosteric Switch: Genetic and Structural Mechanisms for the Evolution of Constitutive Activity in a Steroid Hormone Receptor
- Functional Divergence and Evolutionary Turnover in Mammalian Phosphoproteomes
- A 660-Kb Deletion with Antagonistic Effects on Fertility and Milk Production Segregates at High Frequency in Nordic Red Cattle: Additional Evidence for the Common Occurrence of Balancing Selection in Livestock
- Comparative Evolutionary and Developmental Dynamics of the Cotton () Fiber Transcriptome
- The Transcription Factor BcLTF1 Regulates Virulence and Light Responses in the Necrotrophic Plant Pathogen
- Crossover Patterning by the Beam-Film Model: Analysis and Implications
- Single Cell Genomics: Advances and Future Perspectives
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- GATA6 Is a Crucial Regulator of Shh in the Limb Bud
- Large Inverted Duplications in the Human Genome Form via a Fold-Back Mechanism
- Differential Effects of Collagen Prolyl 3-Hydroxylation on Skeletal Tissues
- Affects Plant Architecture by Regulating Local Auxin Biosynthesis
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



