-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaLoss and Recovery of Genetic Diversity in Adapting Populations of HIV
The evolution of drug resistance in HIV occurs by the fixation of specific, well-known, drug-resistance mutations, but the underlying population genetic processes are not well understood. By analyzing within-patient longitudinal sequence data, we make four observations that shed a light on the underlying processes and allow us to infer the short-term effective population size of the viral population in a patient. Our first observation is that the evolution of drug resistance usually occurs by the fixation of one drug-resistance mutation at a time, as opposed to several changes simultaneously. Second, we find that these fixation events are accompanied by a reduction in genetic diversity in the region surrounding the fixed drug-resistance mutation, due to the hitchhiking effect. Third, we observe that the fixation of drug-resistance mutations involves both hard and soft selective sweeps. In a hard sweep, a resistance mutation arises in a single viral particle and drives all linked mutations with it when it spreads in the viral population, which dramatically reduces genetic diversity. On the other hand, in a soft sweep, a resistance mutation occurs multiple times on different genetic backgrounds, and the reduction of diversity is weak. Using the frequency of occurrence of hard and soft sweeps we estimate the effective population size of HIV to be ( confidence interval ). This number is much lower than the actual number of infected cells, but much larger than previous population size estimates based on synonymous diversity. We propose several explanations for the observed discrepancies. Finally, our fourth observation is that genetic diversity at non-synonymous sites recovers to its pre-fixation value within 18 months, whereas diversity at synonymous sites remains depressed after this time period. These results improve our understanding of HIV evolution and have potential implications for treatment strategies.
Published in the journal: . PLoS Genet 10(1): e32767. doi:10.1371/journal.pgen.1004000
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004000Summary
The evolution of drug resistance in HIV occurs by the fixation of specific, well-known, drug-resistance mutations, but the underlying population genetic processes are not well understood. By analyzing within-patient longitudinal sequence data, we make four observations that shed a light on the underlying processes and allow us to infer the short-term effective population size of the viral population in a patient. Our first observation is that the evolution of drug resistance usually occurs by the fixation of one drug-resistance mutation at a time, as opposed to several changes simultaneously. Second, we find that these fixation events are accompanied by a reduction in genetic diversity in the region surrounding the fixed drug-resistance mutation, due to the hitchhiking effect. Third, we observe that the fixation of drug-resistance mutations involves both hard and soft selective sweeps. In a hard sweep, a resistance mutation arises in a single viral particle and drives all linked mutations with it when it spreads in the viral population, which dramatically reduces genetic diversity. On the other hand, in a soft sweep, a resistance mutation occurs multiple times on different genetic backgrounds, and the reduction of diversity is weak. Using the frequency of occurrence of hard and soft sweeps we estimate the effective population size of HIV to be ( confidence interval ). This number is much lower than the actual number of infected cells, but much larger than previous population size estimates based on synonymous diversity. We propose several explanations for the observed discrepancies. Finally, our fourth observation is that genetic diversity at non-synonymous sites recovers to its pre-fixation value within 18 months, whereas diversity at synonymous sites remains depressed after this time period. These results improve our understanding of HIV evolution and have potential implications for treatment strategies.
Introduction
Understanding the process of adaptation is one of the basic questions in evolutionary biology. The evolution of drug resistance in pathogens such as HIV is a prime example of adaptation and a major clinical and public health concern because it leads to treatment failures.
The likelihood that a population adapts in response to an environmental challenge, e.g., a viral population in a patient develops resistance in response to a drug treatment, depends, among other things, on the amount of genetic diversity that the population harbors [1], [2]. The amount of genetic diversity in a population depends in turn on the details of the adaptive process. In the classical model of adaptation, a beneficial mutation arises once in a single individual and, as this mutation increases in frequency in the population, other mutations “hitchhike” with it to high frequency [3]. In this process, referred to as a “hard selective sweep”, genetic diversity at linked sites is strongly reduced when the beneficial mutation fixes in the population. However, if the population is sufficiently large, beneficial mutations will occur frequently or will be present in multiple copies prior to the onset of selection. Then, multiple beneficial alleles may replace the wildtype without leading to a significant reduction in diversity. This is called a “soft selective sweep” [4].
We use HIV as a model system to ask the following evolutionary questions. How much do selective sweeps affect genetic diversity at neighboring sites? How often does adaptation occur via soft and hard sweeps? What is the effective population size of HIV relevant for adaptation (later referred to simply as )? How does diversity recover after a selective sweep at synonymous and non-synonymous sites? Three factors make HIV well suited to address these questions: (a) HIV evolves rapidly due to its short replication time and high mutation rate, (b) HIV populations in different patients act as independent replicates of the same evolutionary process, and (c) the main genetic targets of positive selection in HIV during antiviral treatment are known: these targets are drug-resistance mutations which have been well characterized.
From the questions above, the estimation of the short-term effective population size () of HIV is perhaps the most interesting and clinically important. We are interested in the short-term effective population size because it is one of the determinants of the probability that drug resistance evolves in a certain generation. This effective population size is primarily determined by current processes (such as possibly background selection), and much less so by historical processes or events (such as bottlenecks). The question of the effective population size of HIV has been a subject of a heated debate because of enormous discrepancies in the estimates obtained by different methods (see [5] for a review). In an influential 1997 paper, Leigh-Brown used genetic diversity at synonymous sites and estimated the effective size of the viral population to be on the order of [6]. Around the same time, it became clear that the number of active virus-producing cells in the body of an infected person was around [7] which lead to a natural expectation that the viral population should be very large with almost deterministic evolutionary dynamics [8]. However, Frost et al found that frequencies of resistance mutations before the start of treatment varied greatly between patients, which led them to conclude that stochastic effects play an important role in HIV, and the population size must therefore be or smaller [9]. With more data and a more sophisticated linkage disequilibrium-based method Rouzine and Coffin inferred that the population size of the virus must be at least [10]. Finally, a recent study by Maldarelli et al [11] places between and , based on changes in allele frequencies over time.
We estimate of HIV using previously published longitudinal sequence data from several HIV-infected patients under drug treatment [12]. Our approach to estimating is most closely related to the recent estimates done in Drosophila [13], Plasmodium [14], and a chimeric simian-human immunodeficiency virus [15], but differs from these studies in that we use data from several patients for increased statistical power. The main idea of our method is as follows. Since the mode of adaptation (hard versus soft sweeps) depends on the supply of beneficial mutations which in turn depends on the effective size of the population, it is possible to use the observed frequency of hard and soft sweeps to estimate . The key problem is to distinguish hard and soft sweeps in the sequence data. We address this problem by taking advantage of a unique property of codon 103 in the reverse-transcriptase gene, namely that there are two distinct nucleotide changes that convert the (wildtype) amino acid lysine into asparagine, which confers drug resistance. If both of these nucleotide changes are observed in a single sample, a soft sweep must have occurred.
While the effects of selection and demographic processes on the reduction of diversity have been extensively studied, the question of how diversity recovers after perturbations has received little attention. Yet, understanding the dynamics of recovery is important because it determines how long signatures of selective sweep or bottlenecks remain detectable in genetic data. Failure to account for the time it takes for diversity to reach its equilibrium after a perturbation can lead to biases in estimation of population genetic parameters such as [5]. Using longitudinal data we show here that diversity after a selective sweep recovers faster at non-synonymous sites than at synonymous sites.
Results and Discussion
Selective sweeps reduce genetic diversity
We study how the genetic composition of HIV populations in patients treated with a combination of reverse transcriptase (RT) and protease inhibitors changed over the course of about one year (see Ref. [12] and Loss of diversity for details on the data). Out of 118 patients studied in Ref. [12], we selected a subset of 30 patients in which the virus had no resistance mutations at the start of the study but fixed a resistance mutation at one of the later time points. All our analyses were done on this subset of patients (see Loss of diversity for details). We note that in 24 out of 30 patients () we detect fixation of a single resistance mutation within the observation period, even though all patients were treated with multiple drugs (see Suppl Table S1).
Samples taken prior to treatment in these patients show nucleotide diversities of at synonymous sites and at non-synonymous sites. At the time points when we detect fixations of drug-resistance alleles at one or several known sites we observe a substantial decrease in the levels of polymorphism in the 1 Kb region that was sequenced (see Figures 1, 2, 3, and Loss of diversity). Between the last sample before and the first sample after the fixation of the first drug-resistance mutation, viral populations lose of their genetic diversity, measured by the median drop in per-site heterozygosity. This difference is significant, with (all P-values we report are from Mann-Whitney tests). We observe no loss of diversity in control intervals in which no resistance mutation was fixed (see Loss of diversity).
Fig. 1. Soft sweep in patient 058. 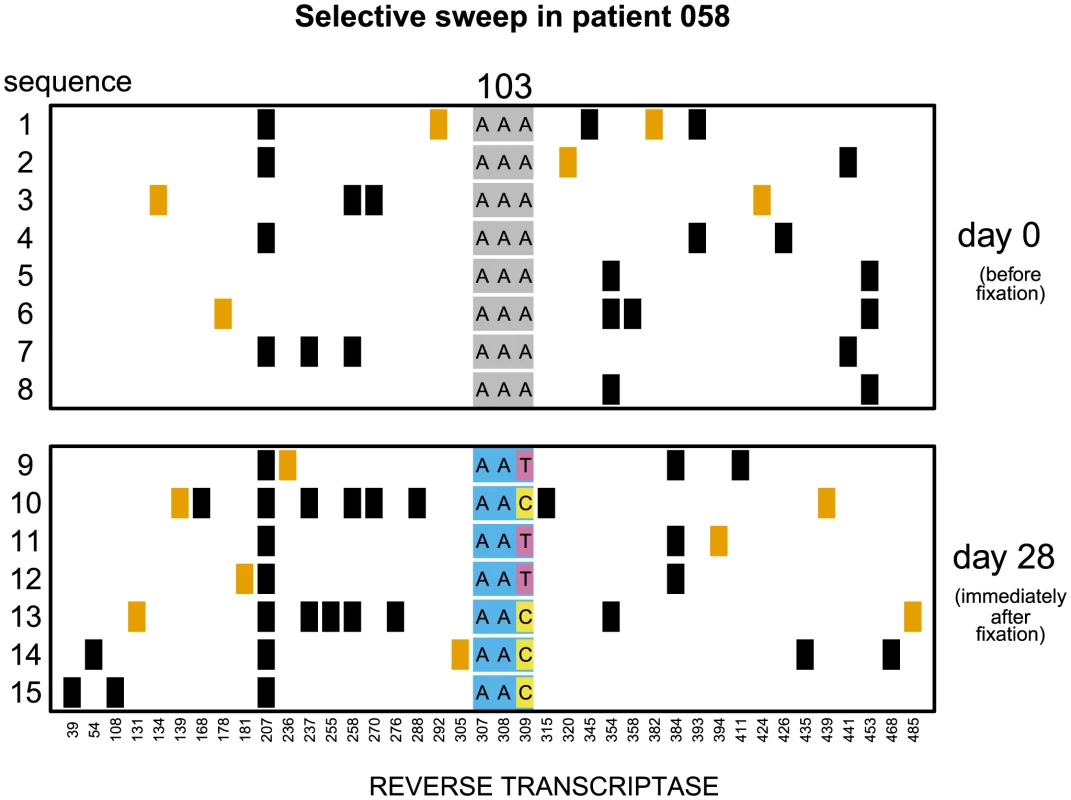
Codon AAA coding for lysine at position 103 was replaced by day 28 with a mixture of codons AAT and AAC both coding for asparagine which confers resistance to NNRTI drugs. Genetic diversity close to the selected site was not significantly reduced. The plot shows only the polymorphic sites among the first 500 basepairs of the reverse transcriptase region. Each row represents a sequenced viral isolate. Each column represents a polymorphic site, with the derived synonymous and non-synonymous polymorphisms shown in black and orange respectively. Codon 103 is shown explicitly. It is grey when coding for lysine (susceptible) and blue when coding for asparagine (resistant). The responsible mutations are colored pink (T) and yellow (C). Fig. 2. Putatively hard sweep in patient 086. 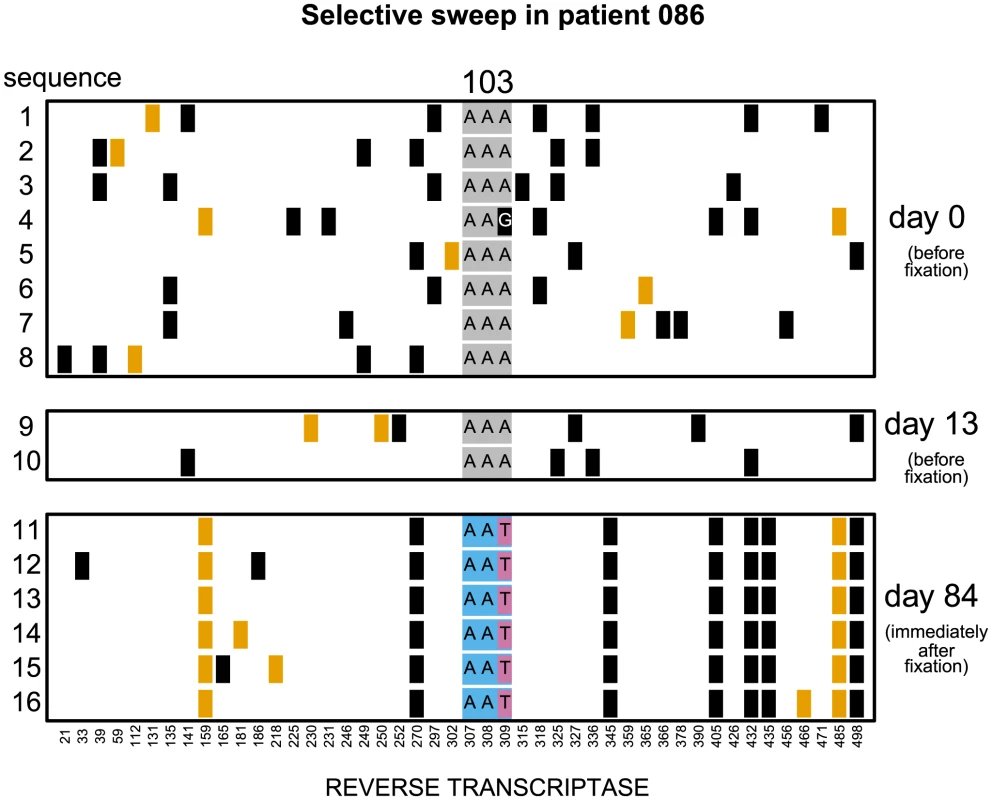
Codon AAA coding at position 103 was replaced by day 84 with codon AAT. Genetic diversity around the selected site was strongly reduced. Notations as in Figure 1. Fig. 3. Nucleotide diversity over time in 30 patients. 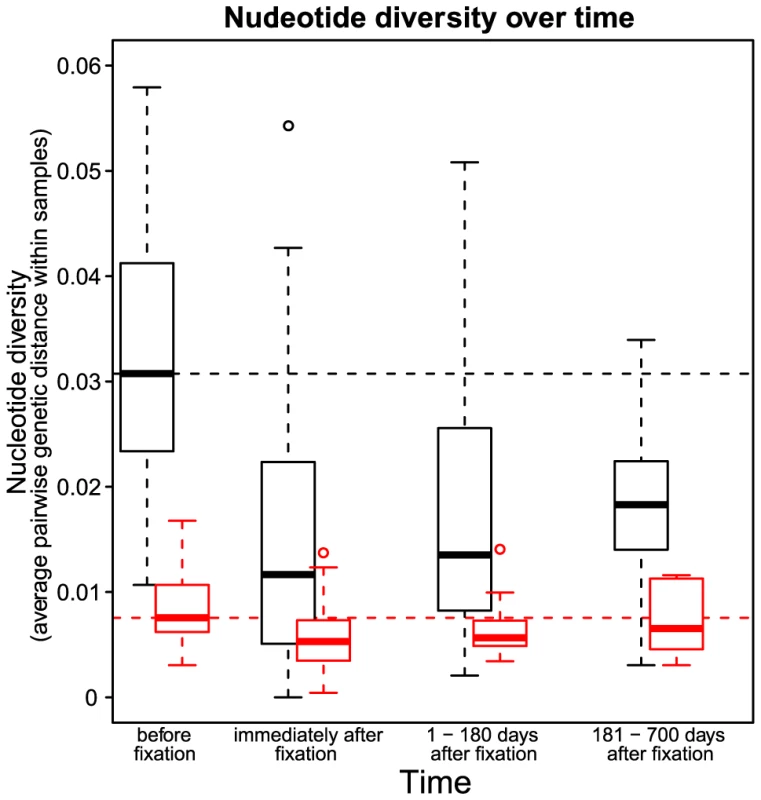
Nucleotide diversity at synonymous (black) and non-synonymous (red) sites. The time point “before fixation” is the last sample in each patient taken before the observed fixation of a resistance mutation. The time point “immediately after fixation” is the first sample in each patient in which the drug resistance mutation is observed to be fixed. The third and the fourth time points denote samples in each patient taken 1–180 days and 181–700 days after the observed fixation of the resistance mutation respectively. Dashed horizontal lines show the nucleotide diversity at synonymous (black) and non-synonymous (red) sites at the time point “before fixation” to provide a reference for subsequent time points. The loss of diversity that we observe is a result of hitchhiking: when an adaptive mutation rapidly increases in frequency, it takes with it the genetic background on which it arose [3]. Three factors can attenuate the observed loss of diversity after a sweep. First, recombination can preserve some diversity by allowing sites at some distance from the selected site to escape the effects of the sweep [16]. Recombinational escape does not appear to be a factor in these data however because we do not observe a correlation between post-sweep heterozygosity and distance from the selected site (see Recombination). This is consistent with previously reported observations in HIV [17].
Second, the amount of diversity lost depends on the origin of the adaptive allele. Diversity may survive the fixation of an adaptive allele even in the absence of recombination if the selective sweep is “soft”, i.e., if the same adaptive allele arises via multiple mutations on different genetic backgrounds [18]. On the other hand, genetic diversity at neighboring sites is reduced dramatically if the selective sweep is “hard”, i.e., if the adaptive allele originates through a single mutation on one genetic background [3]. We demonstrate below that both soft and hard sweeps occurred in these patients. Importantly, whether soft or hard sweeps predominate in a population depends on the supply of adaptive mutations which is determined by the product of the beneficial mutation rate and the current effective population size. If adaptive mutations appear in the population on average more than once per viral generation, they are likely to fix via soft sweeps. If adaptive mutations appear on average less than once per generation, they typically fix via hard sweeps [4]. Thus, we can assess the supply rate of resistance mutations by determining whether the observed selective sweeps are soft or hard.
Third, the observed loss of diversity depends on whether new mutations occur between the fixation of a resistance allele and the time when samples are taken and the sweep is observed. Since we do not know the actual time of fixation, we do not know the absolute amount of diversity loss due to the sweep. However, this fact does not obscure informative patterns of relative loss of diversity, for synonymous versus non-synonymous mutations and for soft versus hard sweeps.
Estimating from the frequency of soft sweeps
In 17 of the 30 HIV populations (), we observe the fixation of just one, well-known drug resistance mutation, K103N (lysine to asparagine) in RT. This mutation confers high-level resistance to the non-nucleoside RT inhibitor used by the patients [12]. Two different nucleotide changes produce the same K103N amino acid change. The wildtype codon AAA encoding lysine can mutate to either codon AAC or codon AAT both encoding asparagine which confers resistance. This property is unique to the K103N mutation among all resistance mutations observed in these patients (see Suppl. Table S1) and can be used to distinguish soft and potentially hard sweeps.
In 7 of these 17 populations () both codons (AAC and AAT) that code for asparagine are present in the population in the first sample after the sweep (Figure 1). These are certain to be soft sweeps. As expected, the median reduction of diversity in these populations is only and not significantly different from 0. In the remaining 10 patients () only one codon is present after the sweep (AAC in 6 and AAT in 4 patients; Figure 2). These sweeps could be either soft or hard. If in these 10 “one-codon” patients the resistance allele typically arose via the same number of unique mutation events as in the 7 “two-codon” patients, we would expect to see a similarly small reduction in diversity at linked sites in these two groups of patients. In contrast, we find that in the 10 “one-codon” viral populations, genetic diversity is reduced by (), and the difference between the “two-codon” and “one-codon” populations is significant (). It is possible that two codons initially fixed in the “one-codon” patients but one of the codons was lost (for example, due to another sweep) before the sample was taken. This scenario would have been plausible if the time intervals between samples were, for some reason, longer in the “one-codon” than in the “two-codon” patients. However, we observe no statistical difference in the inter-sample time intervals between the “two-codon” and “one-codon” patients (). We therefore conclude that the selective sweeps in the “one-codon” patients are due to fewer mutational origins than in the “two-codon” patients, and some are likely hard sweeps (see Figure 2).
The fact that we observe both soft and hard sweeps implies that the K103N mutation appears roughly once per generation [4]. A more accurate estimate of the mutation supply rate based on the frequencies of AAC and AAT alleles in the post-sweep samples yields new K103N mutations per generation ( confidence interval ). Assuming that we sample 6 viral isolates per patient (which is the median in our dataset), theory predicts that, if , of populations would show a hard sweep signature (i.e., the sweep would be due to a single origin), which is consistent with our observations. Assuming the point mutation rate of per generation for transversions [19], we estimate the effective population size of HIV populations to be (see Estimating the supply rate of resistance mutations for details).
We estimated based on data from the 17 patients in which the K103N mutation fixed. Although we cannot do the same analysis with the other resistance mutations in the dataset, we find that the median reduction in diversity in the other 13 patients is intermediate between the “one-codon” and “two-codon” K103N patients (see Suppl. Figure S1), which suggests that K103N mutation is not special and that similar processes are taking place at the other resistance sites. Further, the haplotype structures in two patients with amino-acid changes in the 190th codon position suggest a soft sweep in one and a hard sweep in the other (see Suppl. Figure S2). Thus adaptation at the 103rd codon position appears to be representative of adaptation at other codon positions.
There are other amino acid changes that are similar to the lysine to asparagine (K to N) change in that they can be achieved by two different nucleotide changes. We provide a list of these amino acid changes (limited to those changes that are one mutational step away) in Suppl. Table S2. One of the changes on this list is the methionine to leucine (M to L) change, which is relevant for drug resistance in HIV. The M46L change in the Protease gene leads to resistance to certain Protease Inhibitors. A six-patient dataset from 1997 [20] provides two examples of the fixation of the M46L change from wildtype (ATG) to resistant (TTG or CTG). In one of these two patients, we observe a mixture of the TTG and CTG codons (thus a soft sweep) whereas in the other patient we only observe the TTG codon (potentially a hard sweep, see Suppl. Figure S3). Of the other four patients, two did not fix the M46L mutation and two fixed it at the same time as another resistance mutation. It is difficult to draw conclusions based solely on the data from this study because it consists of only 6 patients [20], but they do provide independent support for our observation that drug resistance mutations in HIV fix by means of both soft and hard sweeps.
An interesting question is whether the resistance mutations that fix stem from standing genetic variation or are due to de novo mutations during treatment. If the resistance mutations stem from standing genetic variation, then our estimate of would reflect the pre-treatment effective population size, whereas, if the mutations would be due to de novo mutation, our estimate would reflect the effective population size during treatment. We compared the times of occurrence of soft and hard sweeps relative to the onset of treatment and found that these were statistically indistinguishable. There are three possible explanations for this observation. First, almost all observed sweeps are due to standing genetic variation, and our estimate of reflects the pre-treatment population. Second, almost all sweeps are due to new mutations and our estimate reflects only the on-treatment . Third, sweeps we observe originated from both standing genetic variation and de novo mutation, but the start of the treatment had no discernible effect on the effective population size, and our estimate reflects both the pre-treatment and on-treatment population size.
We currently have no statistical power to distinguish between these three possibilities, and so it is possible that, if the second explanation is true, the pre-treatment population size is much larger than our estimate. However, some of the sweeps we observed occurred very early during treatment (within one or two months), and it seems likely that at least these early sweeps are due to standing genetic variation (see also [21]). Note that, counter to intuition, whether mutations stem from the standing genetic variation or not does not affect the expected number of origins in a sample (see Suppl. Figure S4 and [4]).
Our estimate of is similar to earlier estimates that were also based on resistance mutations [9] and other beneficial mutations [10]. Our approach may be viewed as a combination of these two previous approaches. Rouzine and Coffin [10] tested whether a recombinant or double mutant genotype is present, given that two beneficial mutations are sweeping through the population at the same time. Similar to a second mutation that leads to a soft sweep, a double mutant or recombinant can only occur in a population with sufficiently high mutational or recombinational input, so that the frequency of such a genotype in a sample permits the estimation of the mutational or recombinational input. Frost et al [9] used inferred pre-treatment frequencies of two resistance mutations at the same codon (RT M184I and M184V) to estimate the effective population size. They found that the two mutations were present at different pre-treatment frequencies in seven patients. In a very large population, the frequency of such mutations would be close to the expected frequency () in every patient, but in smaller populations frequencies vary stochastically, and the spread in the observed frequencies can be used to obtain an upper bound for the population size.
Our estimate of the supply rate of resistance mutations is much lower than would be expected if the effective population size were equal to the number of virus-infected cells in the body, which is estimated to be [7], [8]. At the same time, it is much higher than estimates of the effective population size based on the level of diversity at synonymous sites (assuming neutrality of synonymous variation) which would be around [6]. Next we discuss possible reasons for these discrepancies.
Why is our estimate higher than from synonymous diversity?
We propose three possible explanations for the large difference between our estimate based on the frequency of soft sweeps and the estimate based on the traditional analyses of synonymous diversity. All three explanations may be contributing to this difference. First, some synonymous mutations may be deleterious [22]. Second, positive and negative selection on linked sites (draft and background selection) reduce diversity at synonymous sites, but may have a weaker effect on beneficial mutations such as K103N. Our observation that the fixation of drug resistance mutations is accompanied by a reduction in diversity provides evidence for this explanation. Third, synonymous diversity may take a long time to recover after a sweep or a bottleneck. We discuss the third option in more detail and provide evidence from the data for this explanation.
We observe that diversity at both synonymous and non-synonymous sites steadily recovers after the sweep (Figure 3). However, even after 6 to 18 months, synonymous diversity remains significantly depressed, with a median reduction of () compared to pre-sweep diversity, up from () directly after the sweep. In contrast, after 6 to 18 months after the sweep, non-synonymous diversity is fully recovered, with the median amount of diversity increased by (n.s.) compared to pre-sweep, up from () directly after the sweep. Hence, even relatively infrequent selective sweeps can keep synonymous diversity at a level much below the equilibrium.
The observation that stronger negative selection leads to faster recovery is consistent with recent predictions about the approach of the distribution of allele frequencies to stationarity at a single locus [23]. This effect is well known in systems biology: stronger negative feedback leads to a faster the recovery time [24]. A heuristic analysis (Text S1), similar to analyses in [25]–[27], shows that neutral sites recover half of their diversity in roughly generations while deleterious sites recover half of their diversity in roughly generations, where is the strength of selection against deleterious mutations. Interestingly, for realistic values of the mutation rate, the recovery time is independent of the mutation rate (see Text S1 and Suppl. Figure S5).
The dynamics of how diversity recovers are likely not specific to HIV but typical to any large population. In humans, for example, Europeans have reduced synonymous diversity, but a similar amount of non-synonymous diversity, compared to Africans [28], [29]. This is consistent with the explanation that non-synonymous diversity has had enough time to recover since the out-of-Africa bottleneck, but synonymous diversity has not. This observation leads to a counterintuitive practical implication. Since non-synonymous diversity recovers faster, it is usually closer to its equilibrium than synonymous diversity. Thus non-synonymous sites may in fact be more useful for classical population genetics inference than synonymous sites.
Why is our estimate lower than the number of infected cells?
HIV populations consist of a heterogeneous collection of genotypes, many of which may carry deleterious and advantageous mutations. A new mutation arises in one such genotype and is therefore linked, at least temporarily, to other mutations already present in it. The probability that a new mutation eventually fixes or goes extinct thus depends not only on its own selective effect but also on the combined fitness of the genetic background in which it arose [30], [31]. The effects of linkage on the fates of new mutations are complex and comprise an active area of research [30]–[39], but the qualitative picture is straightforward. The fates of neutral, deleterious and weakly beneficial mutations are entirely determined by the background in which they arise [31]: only mutations that arise on high-fitness genotypes have a chance to persist in the population (Figure 4a, b). The number of segregating neutral mutations in such a population will be small because high-fitness individuals comprise only a small fraction of the population [30], [33], [34]. At the other extreme, adaptive mutations with very large selective effects survive and spread irrespective of the genetic background in which they arise (Figure 4c). The effective population size for such mutations may be close to the census population size [13]. The effective population size for mutations with intermediate effects is somewhere in between (Figure 4d).
Fig. 4. Fates of mutations in a heterogeneous population. 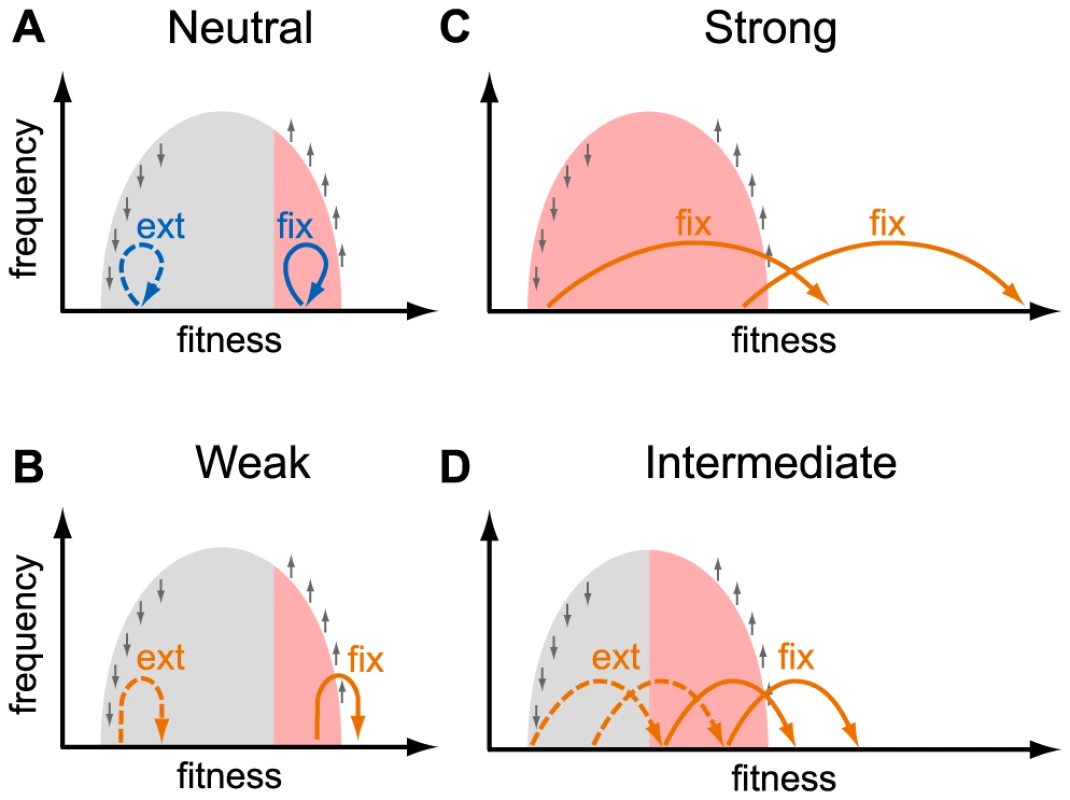
This schematic shows the fates (“fix”, fixation; “ext” extinction) of mutations with different selective effects, as indicated in each panel, depending on the fitness of the genotype they occur in. The light gray area shows the distribution of fitnesses in the population. Small gray arrows show how natural selection changes the distribution of genotypes: fitter than average genotypes increase in frequency, less fit than average genotypes decrease in frequency. Colored arrows show the effect size of new mutations: neutral mutations (effect size ) are shown in blue (panel a.), adaptive mutations (effect size ) are shown in orange (panels b. weakly beneficial, panel c. strongly beneficial, panel d. intermediate beneficial effect). Mutations that are destined for fixation (extinction) are shown with solid (dashed) arrows. Mutations with a large beneficial effect can fix if they arise in any background (panel c), whereas mutations with a small beneficial effect can fix only if they arise in a very fit background (panels a., b., and d.). The resulting effective population size for mutations with a given effect size is shown as red shading. The effective size we estimate from the K103N mutation is several orders of magnitude smaller than the estimated census size. This could mean that the distribution of fitnesses in the viral population due to ongoing positive and negative selection is wide, so that even a highly beneficial mutation such as K103N needs to appear on a relatively fit genetic background to have a good chance of survival. The width of the distribution of fitnesses in within-patient HIV populations is currently unknown. Theory shows that the width of the fitness distribution depends on the rates and typical effects of beneficial and deleterious mutations and on the viral census size [30], [39], [40], quantities that are difficult to estimate. Neher and Leitner recently estimated that at least of non-synonymous mutations have a selection coefficient exceeding [41]. In another study, Batorsky et al concluded that the rate of adaptation of HIV rate is consistent with an exponential distribution of fitness effects of adaptive mutations with mean [42]. If these estimates are accurate, they could imply that the distributions of fitnesses in within-patient HIV populations are narrow so that fixation of highly beneficial mutations like K103N should not be affected by linked selection.
Other effects may help explain why the effective size of HIV is smaller than the census size. For example, the treatment itself may reduce the population size of the virus before resistance evolves. If this is true, then soft sweeps must be especially common in patients who were treated with inferior drugs in the 1980s and 1990s, because early treatments were not as successful in reducing the population size of the virus. If treatment reduces the effective population size, then this would also mean that soft sweeps should be more common in patients who fail early versus patients who fail later (after more time on treatment). We find no evidence for such an effect in the current data set (results not shown). Alternatively, spatial population structure and epistatic effects may reduce the effective population size for resistance mutations.
We obtained our population size estimate under the assumption—most certainly violated in reality at least to some extent—that all patients have the same constant effective population size. Unfortunately, we do not have sufficient statistical power to relax this assumption. Longitudinal data with denser and deeper sampling is required to study inter-patient and temporal heterogeneity in viral adaptation.
Conclusions
We observe soft and hard sweeps in HIV, which leads to an estimated effective population size relevant for adaptation of around . This number is much higher than what the observed level of synonymous diversity suggests. An important caveat of our approach is that we assume that the supply rate of beneficial mutations is similar in all patients, which may not be true. Larger samples are needed to estimate the patient-specific supply rate of adaptive mutations. Our results show that neither synonymous diversity, nor the census population size provide sufficient information to infer a population's adaptive potential, which explains, in part, why it is hard to predict whether and when adaptive evolution will occur. Our finding that adaptive evolution in HIV is limited by the supply rate of beneficial mutations suggests that a relatively modest reduction of the effective population size may help prevent the evolution of drug resistance, and it is possible that the reduction of that results from modern combination therapy, does just that [21]. In addition, if the adaptive potential of the virus is limited because a large proportion of the beneficial mutations land on inferior genetic backgrounds, then the use of mutagenic drugs, in combination with standard antiretroviral drugs, should reduce the adaptive potential of the virus [43]. Finally, our results confirm what has already been predicted theoretically, that the idea of a single effective population that can be used to describe different aspects of the behavior of a population does not work [13], [44].
Materials and Methods
Loss of diversity
Out of a larger dataset [12], we used viral sequences from patients for which we had samples at two consecutive time-points that satisfy the following two criteria. (1) At the first time-point, no known resistance mutations to any of the drugs used in the trial was present at more than frequency in the sample. See below for the list of drugs and mutations. (2) At the next time-point, at least one drug resistant allele increases in frequency by at least . There were 30 such patients. In most cases (26 out of 30) the frequency of the mutations changes from 0 to . The four exceptions are patient 89 (mutation G190S changed from to ), patient 168 (mutation K103N changed from to ), patient 22 (mutation K103N changed from to , while mutation V82A changed from to ), and patient 81 (mutation K103N changed from to ). In most cases (24 out of 30) only a single drug-resistance mutation went to fixation (2 mutations in patients 22, 56, 87, 91, 154 and 166). For details per patient, see supplementary table S1.
The patients were treated with Zidovudine, Lamivudine, Efavirenz and Indinavir [12]. There are many known mutations that confer resistance to one or more of these drugs. We are interested in the fixation of the first resistance mutation in a viral population. We used the following list of major drug-resistance mutations (the number is the codon and the letter is the amino-acid that confers resistance). Protease: 46IL, 82AFT, 84V; Reverse Transcriptase: 41L, 62V, 65R, 67N, 70R, 75I, 77L, 100I, 101P, 103N, 106MA, 108I, 116Y, 151M, 181CI, 184VI, 188LCH, 190SA, 210W, 215YF, 219QE, 225H [45].
Our dataset consists of coding sequences of the regions of the Pol gene that encode for protease (all 297 basepairs) and reverse transcriptase (first 689 basepairs). We analyze separately all third codon position sites and all first and second codon position sites. Most observed mutations at third codon position sites have no effect on the amino acid, and we expect these synonymous mutations to be neutral or nearly neutral. Throughout the paper, we refer to mutations at first and second position sites as non-synonymous and mutations at third position sites as synonymous. Most mutations at the first or second codon positions change the amino acid, and we expect that most such non-synonymous mutations are selected against.
No change in genetic diversity was observed in 27 control intervals that also started with drug susceptible virus but in which no fixation of drug resistant amino-acids occurred (largest frequency change of drug resistant amino-acid in these intervals was ).
We found no difference in pre-sweep diversity at third codon position sites between hard sweep and soft sweep patients.
Recombination
To determine whether recombination affects the reduction of diversity, we split the sequences into a part close to the selected site (less than 50, 100, or 200 basepairs distance from the selected site) and the complementary part far from the selected site. We found no difference in the amount of diversity loss in the close versus far sites. Earlier work suggests that selection may be so strong, relative to the recombination rate, that the sweeps can affect the entire genome [17], [41].
Estimating the supply rate of resistance mutations
We can use the counts of the two beneficial alleles (AAT and AAC at the 103rd amino acid of RT) in the post-sweep samples to estimate the the supply rate of resistance mutations. We assume that the mutation rate to the AAT codon and the mutation rate to the AAC codon are equal to each other, . If is the effective population size, then , and is the total population-wide beneficial mutation rate, which we also call “the supply rate of resistance mutations”.
Theory predicts that the population frequency of the AAC allele follows a beta-distribution with parameters (see [4]). The number of AAC alleles in a sample of size follows the binomial distribution with parameters and . Thus, the likelihood of observing AAC alleles in samples of size in patients is given by the product of beta-binomial distributions,
where is the beta-function. By maximizing expression (1), we obtain the maximum likelihood estimate . The estimated supply rate of K103N mutations is . The bootstrap confidence interval for the supply rate obtained by resampling patients with replacement is .We estimate the effective population size as . Because we only consider the K103N mutation, [19], [46]. Note that this number is lower than the typical per site mutation rate, because most () mutations in HIV are transitions, whereas the A to C and A to T mutations that create the K103N change, are both transversions. We thus estimate the effective population size to be ( confidence interval ).
For a given sample size and a given supply rate of beneficial mutations, we can also predict the probability that the beneficial mutation originates from 1, 2 or more mutational origins following [4]. For a sample of size 6 (the median in the dataset) and the estimated supply rate of beneficial mutations of , the predicted probability that all observed beneficial alleles in the sample stem from a single origin is , the probability that they stem from 2 origins is , 3 origins: and 4, 5 or 6 origins: . This shows that even if the supply rate is exactly the same in all populations, the observed sweep signature can vary widely.
Supporting Information
Zdroje
1. HaydenEJ, FerradaE, WagnerA (2011) Cryptic genetic variation promotes rapid evolutionary adaptation in an RNA enzyme. Nature 474 : 92–95.
2. LauringAS, FrydmanJ, AndinoR (2013) The role of mutational robustness in RNA virus evolution. Nat Rev Microbiol 11 : 327–336.
3. Maynard SmithJ, HaighJ (1974) The hitchhiking effect of a favorable gene. Genet Res 23 : 23–35.
4. PenningsP, HermissonJ (2006) Soft sweeps II: Molecular population genetics of adaptation from recurrent mutation or migration. Molecular Biology and Evolution 23 : 1076–1084.
5. KouyosRD, AlthausCL, BonhoefferS (2006) Stochastic or deterministic: what is the effective population size of HIV-1? Trends Microbiol 14 : 507–511.
6. Leigh-BrownAJ (1997) Analysis of hiv-1 env gene sequences reveals evidence for a low effective number in the viral population. Proceedings of the National Academy of Sciences of the United States of America 94 : 1862–1865.
7. HaaseAT (1994) The role of active and covert infections in lentivirus pathogenesis. Annals of the New York Academy of Sciences 724 : 75–86.
8. CoffinJM (1995) HIV population dynamics in vivo - implications for genetic variation, pathogenesis, and therapy. Science 267 : 483–489.
9. FrostSDW, NijhuisM, SchuurmanR, BoucherCAB, BrownAJL (2000) Evolution of lamivudine resistance in human immunodeficiency virus type 1-infected individuals: the relative roles of drift and selection. Journal of Virology 74 : 6262–6268.
10. RouzineIM, CoffinJM (1999) Linkage disequilibrium test implies a large effective population number for HIV in vivo. Proceedings of the National Academy of Sciences 96 : 10758–10763.
11. Maldarelli (2013) HIV populations are large and accumulate high genetic diversity in nonlinear fashion. J Virol 87 : 10313–23 doi: 10.1128/JVI.01225-12
12. BachelerLT, AntonED, KudishP, BakerD, BunvilleJ, et al. (2000) Human immunodeficiency virus type 1 mutations selected in patients failing efavirenz combination therapy. Antimicrobial Agents and Chemotherapy 44 : 2475–2484.
13. KarasovT, MesserP, PetrovD (2010) Evidence that adaptation in drosophila is not limited by mutation at single sites. PLoS Genetics 6: e1000924.
14. NairS, NashD, SudimackD, JaideeA, BarendsM, et al. (2007) Recurrent gene amplification and soft selective sweeps during evolution of multidrug resistance in malaria parasites. Molecular Biology and Evolution 24 : 562–573.
15. BoltzVF, AmbroseZ, KearneyMF, ShaoW, KewalramaniVN, et al. (2012) Ultrasensitive allele-specific PCR reveals rare preexisting drugresistant variants and a large replicating virus population in macaques infected with a simian immunodeficiency virus containing human immunodeficiency virus reverse transcriptase. Journal of Virology 86 : 12525–30.
16. KaplanNL, HudsonRR, LangleyCH (1989) The “hitchhiking effect” revisited. Genetics 123 : 887–899.
17. NijhuisM, BoucherCa, SchipperP, LeitnerT, SchuurmanR, et al. (1998) Stochastic processes strongly inuence HIV-1 evolution during suboptimal protease-inhibitor therapy. Proceedings of the National Academy of Sciences of the United States of America 95 : 14441–6.
18. PenningsP, HermissonJ (2006) Soft sweeps III: The signature of positive selection from recurrent mutation. PLoS Genetics 2: e186.
19. AbramME, FerrisAL, ShaoW, AlvordWG, HughesSH (2010) Nature, position, and frequency of mutations made in a single cycle of HIV-1 replication. Journal of Virology 84 : 9864–78.
20. ZhangY, ImamichiH, ImamichiT, LaneC, JF, et al. (1997) Drug resistance during indinavir therapy is caused by mutations in the protease gene and in its gag substrate cleavage sites. Journal of Virology 71 : 6662–6670.
21. PenningsPS (2012) Standing genetic variation and the evolution of drug resistance in HIV. PLoS Comput Biol 8: e1002527.
22. SanjuánR, BorderíaAV (2011) Interplay between RNA structure and protein evolution in HIV-1. Molecular Biology and Evolution 28 : 1333–8.
23. SongYS, SteinrückenM (2012) A simple method for finding explicit analytic transition densities of diffusion processes with general diploid selection. Genetics 190 : 1117–1129.
24. Alon U (2006) An Introduction to Systems Biology: Design Principles of Biological Circuits. Chapman and Hall/CRC, 1 edition.
25. Malecot G (1969). The Mathematics of Heredity. Revised, edited and translated by D.M. Yermanos.
26. NeiM, MaruyamaT, ChakrabortyR (1975) The bottleneck effect and genetic variability in populations. Evolution 29 : 1–10.
27. GordoI, DionisioF (2005) Nonequilibrium model for estimating parameters of deleterious mutations. Physical Review E 71 : 031907.
28. LohmuellerKE, IndapAR, SchmidtS, BoykoAR, HernandezRD, et al. (2008) Proportionally more deleterious genetic variation in European than in African populations. Nature 451 : 994–U5.
29. KiezunA, PulitSL, FrancioliLC, van DijkF, SwertzM, et al. (2013) Deleterious alleles in the human genome are on average younger than neutral alleles of the same frequency. PLoS Genetics 9: e1003301.
30. DesaiMM, FisherDS (2007) Beneficial mutation-selection balance and the effect of linkage on positive selection. Genetics 176 : 1759–1798.
31. SchiffelsS, SzöllősiGJ, MustonenV, LässigM (2011) Emergent neutrality in adaptive asexual evolution. Genetics 189 : 1361–1375.
32. NeherRA, ShraimanBI (2011) Genetic draft and quasi-neutrality in large facultatively sexual populations. Genetics 188 : 975–96.
33. WalczakAM, NicolaisenLE, PlotkinJB, DesaiMM (2012) The structure of genealogies in the presence of purifying selection: A fitness-class coalescent. Genetics 190 : 753–779.
34. DesaiMM, WalczakAM, FisherDS (2012) Genetic diversity and the structure of genealogies in rapidly adapting populations. Genetics 193 : 565–585.
35. RouzineIM, CoffinJM (2010) Multi-site adaptation in the presence of infrequent recombination. Theoretical Population Biology 77 : 189–204.
36. RouzineIM, WakeleyJ, CoffinJM (2003) The solitary wave of asexual evolution. Proceedings of the National Academy of Sciences of the United States of America 100 : 587–592.
37. RouzineIM, BrunetE, WilkeCO (2008) The traveling-wave approach to asexual evolution: Muller's ratchet and speed of adaptation. Theoretical Population Biology 73 : 24–46.
38. NeherRA, ShraimanBI, FisherDS (2010) Rate of adaptation in large sexual populations. Genetics 184 : 467–81.
39. GoodBH, RouzineIM, BalickDJ, HallatschekO, DesaiMM (2012) Distribution of fixed beneficial mutations and the rate of adaptation in asexual populations. Proceedings of the National Academy of Sciences of the United States of America 109 : 4950–5.
40. GoyalS, BalickDJ, JerisonER, NeherRA, ShraimanBI, et al. (2012) Dynamic mutation-selection balance as an evolutionary attractor. Genetics 191 : 1309–1319.
41. NeherRA, LeitnerT (2010) Recombination rate and selection strength in HIV intra-patient evolution. PLoS Computational Biology 6: e1000660.
42. BatorskyR, KearneyMF, PalmerSE, MaldarelliF, RouzineIM, et al. (2011) Estimate of effective recombination rate and average selection coef - ficient for HIV in chronic infection. Proceedings of the National Academy of Sciences of the United States of America 108 : 5661–5666.
43. MullinsJI, HeathL, HughesJP, KichaJ, StyrchakS, et al. (2011) Mutation of HIV-1 genomes in a clinical population treated with the mutagenic nucleoside KP1461. PLoS One 6: e15135.
44. WeissmanDB, BartonNH (2012) Limits to the rate of adaptive substitution in sexual populations. PLoS Genetics 8: e1002740.
45. JohnsonV, Brun-VézinetF, ClotetB, GünthardH, KuritzkesD, et al. (2010) Update of the drug resistance mutations in HIV-1: December 2010. Top HIV Med 18 : 156–163.
46. ManskyLM, TeminHM (1995) Lower in vivo mutation rate of human immunodeficiency virus type 1 than that predicted from the fidelity of purified reverse transcriptase. Journal of Virology 69 : 5087–94.
Štítky
Genetika Reprodukční medicína
Článek Unwrapping BacteriaČlánek A Chaperone-Assisted Degradation Pathway Targets Kinetochore Proteins to Ensure Genome StabilityČlánek The Candidate Splicing Factor Sfswap Regulates Growth and Patterning of Inner Ear Sensory OrgansČlánek The SPF27 Homologue Num1 Connects Splicing and Kinesin 1-Dependent Cytoplasmic Trafficking inČlánek Down-Regulation of eIF4GII by miR-520c-3p Represses Diffuse Large B Cell Lymphoma DevelopmentČlánek Meta-Analysis Identifies Gene-by-Environment Interactions as Demonstrated in a Study of 4,965 MiceČlánek High Risk Population Isolate Reveals Low Frequency Variants Predisposing to Intracranial Aneurysms
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2014 Číslo 1
-
Všechny články tohoto čísla
- How Much Is That in Dog Years? The Advent of Canine Population Genomics
- The Sense and Sensibility of Strand Exchange in Recombination Homeostasis
- Unwrapping Bacteria
- DNA Methylation Changes Separate Allergic Patients from Healthy Controls and May Reflect Altered CD4 T-Cell Population Structure
- Evidence for Mito-Nuclear and Sex-Linked Reproductive Barriers between the Hybrid Italian Sparrow and Its Parent Species
- Translation Enhancing ACA Motifs and Their Silencing by a Bacterial Small Regulatory RNA
- Relationship Estimation from Whole-Genome Sequence Data
- Genetic Models of Apoptosis-Induced Proliferation Decipher Activation of JNK and Identify a Requirement of EGFR Signaling for Tissue Regenerative Responses in
- ComEA Is Essential for the Transfer of External DNA into the Periplasm in Naturally Transformable Cells
- Loss and Recovery of Genetic Diversity in Adapting Populations of HIV
- Bioelectric Signaling Regulates Size in Zebrafish Fins
- Defining NELF-E RNA Binding in HIV-1 and Promoter-Proximal Pause Regions
- Loss of Histone H3 Methylation at Lysine 4 Triggers Apoptosis in
- Cell-Cycle Dependent Expression of a Translocation-Mediated Fusion Oncogene Mediates Checkpoint Adaptation in Rhabdomyosarcoma
- How a Retrotransposon Exploits the Plant's Heat Stress Response for Its Activation
- A Nonsense Mutation in Encoding a Nondescript Transmembrane Protein Causes Idiopathic Male Subfertility in Cattle
- Deletion of a Conserved -Element in the Locus Highlights the Role of Acute Histone Acetylation in Modulating Inducible Gene Transcription
- Developmental Link between Sex and Nutrition; Regulates Sex-Specific Mandible Growth via Juvenile Hormone Signaling in Stag Beetles
- PP2A/B55 and Fcp1 Regulate Greatwall and Ensa Dephosphorylation during Mitotic Exit
- Differential Effects of Collagen Prolyl 3-Hydroxylation on Skeletal Tissues
- Comprehensive Functional Annotation of 77 Prostate Cancer Risk Loci
- Evolution of Chloroplast Transcript Processing in and Its Chromerid Algal Relatives
- A Chaperone-Assisted Degradation Pathway Targets Kinetochore Proteins to Ensure Genome Stability
- New MicroRNAs in —Birth, Death and Cycles of Adaptive Evolution
- A Genome-Wide Screen for Bacterial Envelope Biogenesis Mutants Identifies a Novel Factor Involved in Cell Wall Precursor Metabolism
- FGFR1-Frs2/3 Signalling Maintains Sensory Progenitors during Inner Ear Hair Cell Formation
- Regulation of Synaptic /Neuroligin Abundance by the /Nrf Stress Response Pathway Protects against Oxidative Stress
- Intrasubtype Reassortments Cause Adaptive Amino Acid Replacements in H3N2 Influenza Genes
- Molecular Specificity, Convergence and Constraint Shape Adaptive Evolution in Nutrient-Poor Environments
- WNT7B Promotes Bone Formation in part through mTORC1
- Natural Selection Reduced Diversity on Human Y Chromosomes
- In-Vivo Quantitative Proteomics Reveals a Key Contribution of Post-Transcriptional Mechanisms to the Circadian Regulation of Liver Metabolism
- The Candidate Splicing Factor Sfswap Regulates Growth and Patterning of Inner Ear Sensory Organs
- The Acid Phosphatase-Encoding Gene Contributes to Soybean Tolerance to Low-Phosphorus Stress
- p53 and TAp63 Promote Keratinocyte Proliferation and Differentiation in Breeding Tubercles of the Zebrafish
- Affects Plant Architecture by Regulating Local Auxin Biosynthesis
- The SET Domain Proteins SUVH2 and SUVH9 Are Required for Pol V Occupancy at RNA-Directed DNA Methylation Loci
- Down-Regulation of Rad51 Activity during Meiosis in Yeast Prevents Competition with Dmc1 for Repair of Double-Strand Breaks
- Multi-tissue Analysis of Co-expression Networks by Higher-Order Generalized Singular Value Decomposition Identifies Functionally Coherent Transcriptional Modules
- A Neurotoxic Glycerophosphocholine Impacts PtdIns-4, 5-Bisphosphate and TORC2 Signaling by Altering Ceramide Biosynthesis in Yeast
- Subtle Changes in Motif Positioning Cause Tissue-Specific Effects on Robustness of an Enhancer's Activity
- C/EBPα Is Required for Long-Term Self-Renewal and Lineage Priming of Hematopoietic Stem Cells and for the Maintenance of Epigenetic Configurations in Multipotent Progenitors
- The SPF27 Homologue Num1 Connects Splicing and Kinesin 1-Dependent Cytoplasmic Trafficking in
- Down-Regulation of eIF4GII by miR-520c-3p Represses Diffuse Large B Cell Lymphoma Development
- Genome Sequencing Highlights the Dynamic Early History of Dogs
- Re-sequencing Expands Our Understanding of the Phenotypic Impact of Variants at GWAS Loci
- Meta-Analysis Identifies Gene-by-Environment Interactions as Demonstrated in a Study of 4,965 Mice
- , a -Antisense Gene of , Encodes a Evolved Protein That Inhibits GSK3β Resulting in the Stabilization of MYCN in Human Neuroblastomas
- A Transcription Factor Is Wound-Induced at the Planarian Midline and Required for Anterior Pole Regeneration
- A Comprehensive tRNA Deletion Library Unravels the Genetic Architecture of the tRNA Pool
- A PNPase Dependent CRISPR System in
- Genomic Confirmation of Hybridisation and Recent Inbreeding in a Vector-Isolated Population
- Zinc Finger Transcription Factors Displaced SREBP Proteins as the Major Sterol Regulators during Saccharomycotina Evolution
- GATA6 Is a Crucial Regulator of Shh in the Limb Bud
- Tissue Specific Roles for the Ribosome Biogenesis Factor Wdr43 in Zebrafish Development
- A Cell Cycle and Nutritional Checkpoint Controlling Bacterial Surface Adhesion
- High Risk Population Isolate Reveals Low Frequency Variants Predisposing to Intracranial Aneurysms
- E3 Ubiquitin Ligase CHIP and NBR1-Mediated Selective Autophagy Protect Additively against Proteotoxicity in Plant Stress Responses
- Evolutionary Rate Covariation Identifies New Members of a Protein Network Required for Female Post-Mating Responses
- 3′ Untranslated Regions Mediate Transcriptional Interference between Convergent Genes Both Locally and Ectopically in
- Single Nucleus Genome Sequencing Reveals High Similarity among Nuclei of an Endomycorrhizal Fungus
- Metabolic QTL Analysis Links Chloroquine Resistance in to Impaired Hemoglobin Catabolism
- Notch Controls Cell Adhesion in the Drosophila Eye
- AL PHD-PRC1 Complexes Promote Seed Germination through H3K4me3-to-H3K27me3 Chromatin State Switch in Repression of Seed Developmental Genes
- Genomes Reveal Evolution of Microalgal Oleaginous Traits
- Large Inverted Duplications in the Human Genome Form via a Fold-Back Mechanism
- Variation in Genome-Wide Levels of Meiotic Recombination Is Established at the Onset of Prophase in Mammalian Males
- Age, Gender, and Cancer but Not Neurodegenerative and Cardiovascular Diseases Strongly Modulate Systemic Effect of the Apolipoprotein E4 Allele on Lifespan
- Lifespan Extension Conferred by Endoplasmic Reticulum Secretory Pathway Deficiency Requires Induction of the Unfolded Protein Response
- Is Non-Homologous End-Joining Really an Inherently Error-Prone Process?
- Vestigialization of an Allosteric Switch: Genetic and Structural Mechanisms for the Evolution of Constitutive Activity in a Steroid Hormone Receptor
- Functional Divergence and Evolutionary Turnover in Mammalian Phosphoproteomes
- A 660-Kb Deletion with Antagonistic Effects on Fertility and Milk Production Segregates at High Frequency in Nordic Red Cattle: Additional Evidence for the Common Occurrence of Balancing Selection in Livestock
- Comparative Evolutionary and Developmental Dynamics of the Cotton () Fiber Transcriptome
- The Transcription Factor BcLTF1 Regulates Virulence and Light Responses in the Necrotrophic Plant Pathogen
- Crossover Patterning by the Beam-Film Model: Analysis and Implications
- Single Cell Genomics: Advances and Future Perspectives
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- GATA6 Is a Crucial Regulator of Shh in the Limb Bud
- Large Inverted Duplications in the Human Genome Form via a Fold-Back Mechanism
- Differential Effects of Collagen Prolyl 3-Hydroxylation on Skeletal Tissues
- Affects Plant Architecture by Regulating Local Auxin Biosynthesis
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání




