-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaPositional Cloning Reveals Strain-Dependent Expression of to Alter Susceptibility to Bleomycin-Induced Pulmonary Fibrosis in Mice
Pulmonary fibrosis is a disease of significant morbidity, with no effective therapeutics and an as yet incompletely defined genetic basis. The chemotherapeutic agent bleomycin induces pulmonary fibrosis in susceptible C57BL/6J mice but not in mice of the C3H/HeJ strain, and this differential strain response has been used in prior studies to map bleomycin-induced pulmonary fibrosis susceptibility loci named Blmpf1 and Blmpf2. In this study we isolated the quantitative trait gene underlying Blmpf2 initially by histologically phenotyping the bleomycin-induced lung disease of sublines of congenic mice to reduce the linkage region to 13 genes. Of these genes, Trim16 was identified to have strain-dependent expression in the lung, which we determined was due to sequence variation in the promoter. Over-expression of Trim16 by plasmid injection increased pulmonary fibrosis, and bronchoalveolar lavage levels of both interleukin 12/23-p40 and neutrophils, in bleomycin treated B6.C3H-Blmpf2 subcongenic mice compared to subcongenic mice treated with bleomycin only, which follows the C57BL/6J versus C3H/HeJ strain difference in these traits. In summary we demonstrate that genetic variation in Trim16 leads to its strain-dependent expression, which alters susceptibility to bleomycin-induced pulmonary fibrosis in mice.
Published in the journal: . PLoS Genet 9(1): e32767. doi:10.1371/journal.pgen.1003203
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003203Summary
Pulmonary fibrosis is a disease of significant morbidity, with no effective therapeutics and an as yet incompletely defined genetic basis. The chemotherapeutic agent bleomycin induces pulmonary fibrosis in susceptible C57BL/6J mice but not in mice of the C3H/HeJ strain, and this differential strain response has been used in prior studies to map bleomycin-induced pulmonary fibrosis susceptibility loci named Blmpf1 and Blmpf2. In this study we isolated the quantitative trait gene underlying Blmpf2 initially by histologically phenotyping the bleomycin-induced lung disease of sublines of congenic mice to reduce the linkage region to 13 genes. Of these genes, Trim16 was identified to have strain-dependent expression in the lung, which we determined was due to sequence variation in the promoter. Over-expression of Trim16 by plasmid injection increased pulmonary fibrosis, and bronchoalveolar lavage levels of both interleukin 12/23-p40 and neutrophils, in bleomycin treated B6.C3H-Blmpf2 subcongenic mice compared to subcongenic mice treated with bleomycin only, which follows the C57BL/6J versus C3H/HeJ strain difference in these traits. In summary we demonstrate that genetic variation in Trim16 leads to its strain-dependent expression, which alters susceptibility to bleomycin-induced pulmonary fibrosis in mice.
Introduction
The pathology of pulmonary fibrosis features excessive deposition of extracellular matrix in the lung interstitium which can occur as the result of known (environmental, therapeutic) exposures, or idiopathically, and can result in impaired lung function and, ultimately, respiratory failure. This devastating disease has an estimated annual incidence of approximately 10 cases per 100000 members of general population [1] and an associated mortality of 50% at 3 years post diagnosis [2]. The mechanisms through which pulmonary fibrosis develops are incompletely understood but likely involve dysregulated repair of alveolar epithelial cell injury [2] which may be influenced by a tissue inflammatory response [3], [4].
Evidence for a genetic component to pulmonary fibrosis development includes heritability of the trait in family studies [2] and recent findings from linkage and association investigations. Regarding the latter, variation in particular genes encoding ELMO domain containing 2 [5], telomerase and surfactant proteins has been associated with the trait [6], and combined investigations of linkage in affected families, and of candidate gene assessment by association and pulmonary expression, have associated variants in the mucin gene MUC5B with pulmonary fibrosis susceptibility [7], [8]. Only a fraction of the genetic variation which contributes to this trait has been accounted for [6], however, and given the limits of case control association studies [9], which can include gene-environment effects, complementary investigations into the genetic basis of this disease in an animal model have been undertaken.
Investigations of mouse models wherein the fibrosis phenotype can be recapitulated in subjects controlled for age, genetic background and environmental exposure, can be completed to isolate candidate genetic variation contributing to the lung response phenotype. Specifically, the lung phenotype of C57BL/6J (B6) mice, following a 7day subcutaneous dose of bleomycin, consists of an alveolar inflammatory cell infiltrate with subpleural regions of fibrosis; a pathology that has been described for clinical cases of idiopathic pulmonary fibrosis [10]. This bleomycin delivery method, developed by Harrison et al. [11], and used by us [12], [13] has also been found to produce more fibrosis in the lung, and a fibrotic phenotype more closely resembling idiopathic pulmonary fibrosis, than the more commonly used experimental method of intratracheal drug delivery [14], [15]. In contrast to B6, mice of the C3Hf/KAM or C3H/HeJ strains develop minimal fibrosis following bleomycin treatment [13], [16] and this phenotypic difference was used to map loci of bleomycin-induced pulmonary fibrosis named Blmpf1 on Chr 17 and Blmpf2 on Chr 11 [17]. From the linkage study it was established that the inheritance of B6 alleles at either of Blmpf1 and Blmpf2 increased the fibrosis phenotype of B6×C3H F2 mice. The protective influence of Chromosome 11 C3H alleles on the fibrosis phenotype has been confirmed as bleomycin-challenged B6.11C3H consomic mice were shown to develop significantly less pulmonary fibrosis than the levels in B6 mice [17].
Herein we generate and phenotype Blmpf2 congenic and subcongenic mice to show Trim16 to be the quantitative trait gene underlying Blmpf2.
Results
Reduction of the Blmpf2 linkage region in subcongenic mice
We initially confirmed Blmpf2 as a locus altering fibrosis susceptibility by generating and phenotyping mice in which a 29.8 Mb fragment of Chromosome 11, encompassed by the D11Mit136 and D11Mit320 markers, from the lung fibrosis-resistant C3H strain, was bred onto the genetic background of lung fibrosis susceptible C57BL/6J mice, by repeated backcrossing. As shown in Figure 1, B6.Blmpf2C3H congenic mice (line 1) developed significantly less fibrosis (p = 0.014) in response to bleomycin treatment than B6 mice, but a level exceeding that of C3H/HeJ mice, (p = 0.002) which is likely due to the presence of B6 alleles at the Blmpf1 locus in the congenic mice.
Fig. 1. Bleomycin-induced lung phenotype of Blmpf2 subcongenic mice. 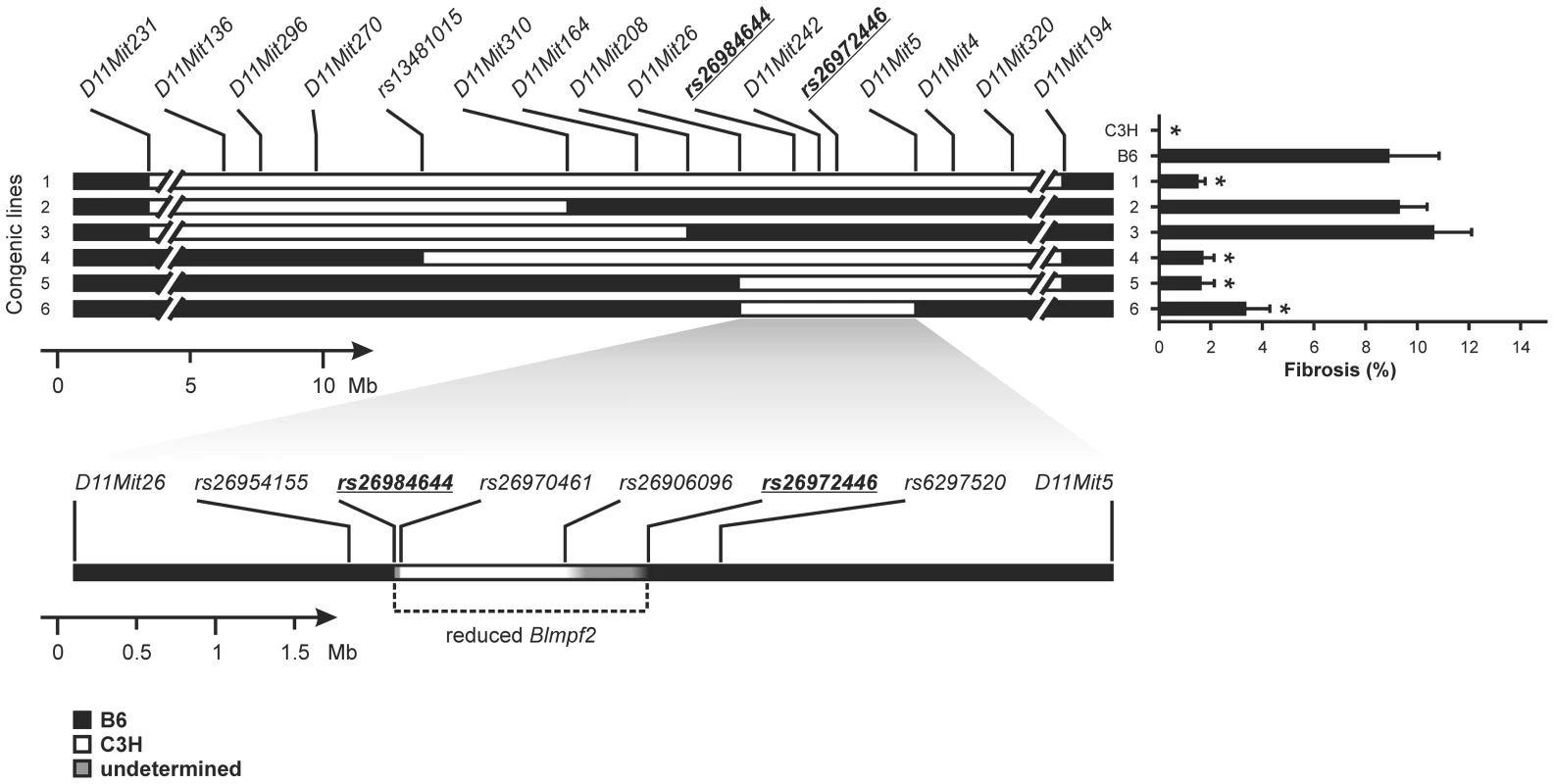
The mice were treated with bleomycin by mini-osmotic pump and euthanized 42 days later. The percentage of the lung with fibrosis was determined from image analysis of histological sections and the mean ± SEM of 10–19 bleomycin-treated mice for each subcongenic line, and for the parental strains, is given. * indicates a significant difference in fibrosis from B6 mice, p<0.05. Genotypes [C3H alleles (white box); B6 alleles (black box)] were determined with microsatellite and SNP markers; genotypes of line 6 are expanded at the bottom of the figure. To narrow the Blmpf2 linkage interval and thus to reduce the number of positional candidate genes contained we created and phenotyped a panel of Blmpf2 subcongenic mice. As shown in Figure 1, mice (named B6.Blmpf2C3H subcongenic mice, line 6 in Figure 1) with C3H alleles from, maximally, marker rs26984644 Chr11 : 62382506 to rs26972446 Chr11 : 64009126 developed the same level of pulmonary fibrosis as the congenic strain (p = 0.95), thus mapping the Blmpf2 fibrosis susceptibility gene to a region of 1.6 Mbp.
Expression and genetic analysis of candidate genes
The reduced 1.6 Mbp region of Blmpf2 contains 13 annotated genes (Genome Reference Consortium: GRCm38; MGI and NCBI gene annotation listed in Table S1). To assess each of the positional candidate genes as potentially contributing to the fibrosis phenotype we measured their expression levels in the lungs of both untreated B6 and C3H mice and following exposure to bleomycin and reviewed documented strain-dependent DNA sequence variation. As shown in Figure 2 one gene, tripartite motif-containing 16 (Trim16), of strain dependent expression, was significantly increased in expression in the lungs of bleomycin-treated B6 mice, compared to the levels in both untreated B6 mice and C3H mice exposed to bleomycin. The gene expression levels agree with data we had previously reported from a microarray study of the B6 and C3H response to bleomycin (GEO record GDS1492; ref 13), for the 7 linkage region genes included in the array. The expression of Cdrt4 was not detected in either strain. We also assessed the level of expression of each of the positional candidate genes, except Cdrt4, in the lungs of B6.Blmpf2C3H subcongenic mice (Figure S1). The majority of genes exhibited expression levels similar to those detected in the lungs from C3H mice, although the expression of Tekt3 was similar to that in the lungs of B6 mice, suggesting trans regulation for this gene. Finally, the expression of Trpv2 and Zfp287 in the lung tissue from B6.Blmpf2C3H subcongenic mice differed from that measured in either inbred strain.
Fig. 2. Pulmonary expression of reduced region Blmpf2 genes. 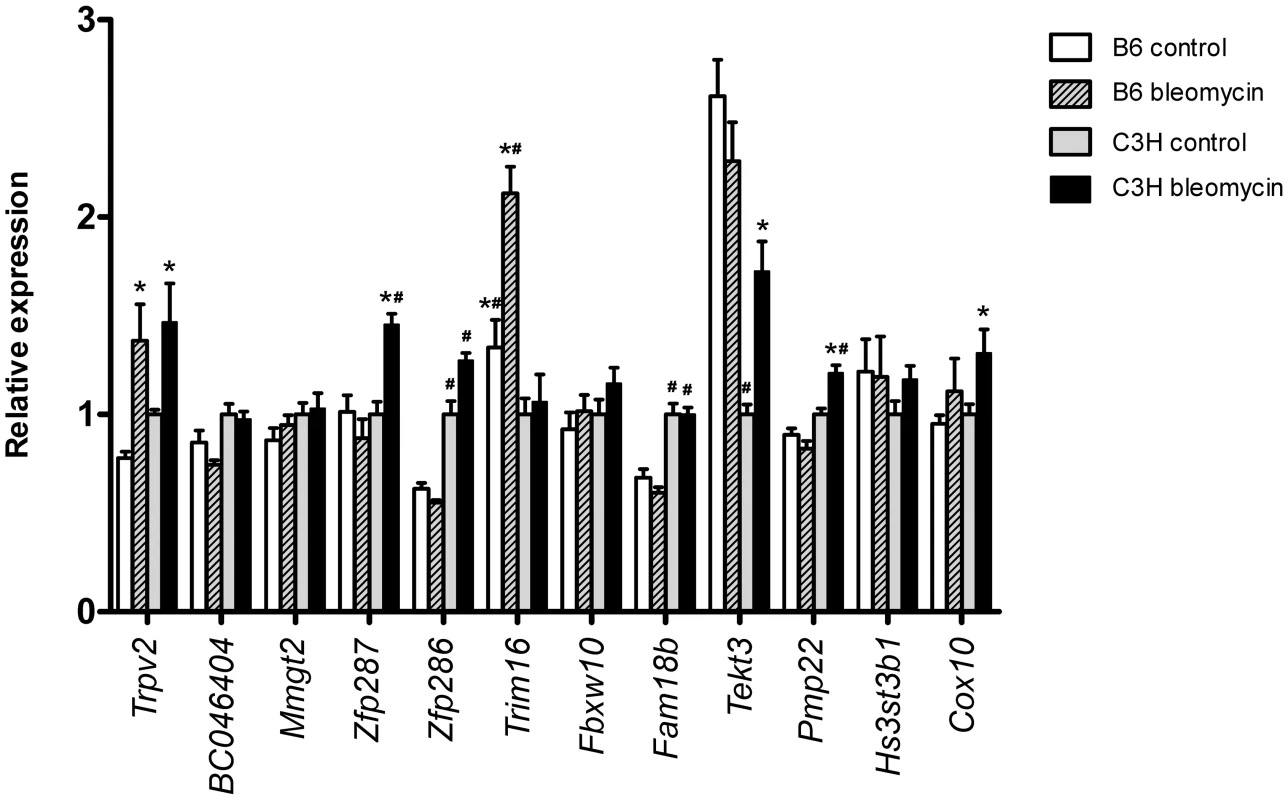
Real-time quantitative PCR of genes mapping to the reduced Blmpf2 region prior to (day 0: non-treated) and following bleomycin treatment (day 42) in the lungs of B6 and C3H mice. Gene expression was normalized to the Ataxin10 reference gene and is presented relative to the level in untreated C3H mice. Mean ±SEM of 5 per group. * indicates a significant difference in expression in lungs of bleomycin-treated mice relative to untreated controls, p<0.05; # indicates a significant difference in expression by strain, p<0.05. Each of the positional candidate genes was reviewed using data from the dbSNP 128 SNP and Sanger databases to identify those genes having coding non synonymous B6/C3H SNP's as these variations could potentially contribute to the fibrosis phenotype of B6 mice and four genes (Zfp286, Zfp287, Trim16 and Fam18b) met this criterion. By in silico analysis [18], [19] the majority of the coding non synonymous B6/C3H variations were deemed tolerated or benign.
From the gene expression and SNP analysis, and based on data demonstrating the encoded protein to affect the secretion of cytokines of relevance of fibrosis [20], Trim16 was further investigated as a fibrosis susceptibility gene.
B6/C3H sequence variation in Trim16
Given the strain dependent expression of Trim16, we investigated the 5′ putative regulatory region for sequence differences which could create this differential expression. As shown in Figure 3 sequence differences in region 5′ to the transcription start site were documented, the majority of which verify data from the Sanger database [21]. Variations which are both evolutionarily conserved (see Figure S2) and which, based on data in PROMO, [22], [23] TRANSFAC [24] and MATCH [25], potentially alter transcription factor binding sites were revealed. To test the influence of this sequence on gene transcription, we completed a promoter assay and as shown in Figure 3, the B6 allele of the region 1.3 kbp upstream of the start site enhanced transcription relative to the C3H allele.
Fig. 3. B6 and C3H Trim16 promoter sequence variation alters transcription. 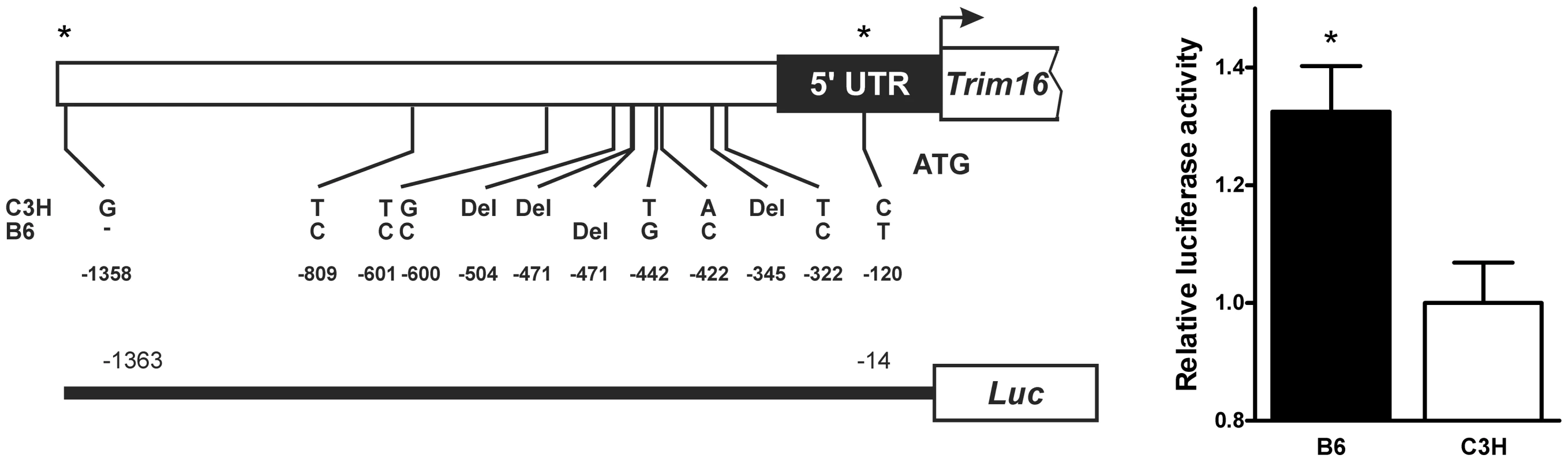
B6/C3H sequence differences in the putative promoter region of Trim16 (* indicates novel to Sanger, MGI). Allele specific promoter sequence alters the expression of a luciferase reporter vector transfected into the RAW 264.7 macrophage cell line. Elevated Trim16 expression increases fibrosis in B6.Blmpf2C3H subcongenic mice
To investigate whether the altered expression of Trim16 influences the fibrosis phenotype we treated B6.Blmpf2C3H subcongenic mice with bleomycin and injected a plasmid of Trim16 in complex with the polyethylenimine (PEI) carrier [26], [27], at day 21 after the initiation of bleomycin treatment. The expression of Trim16 in untreated subcongenic mice was similar to that of untreated C3H mice, (1.09 relative to the C3H level; p = 0.78) which is consistent with cis regulation. Intravenous injection of the Trim16 plasmid increased expression of Trim16 in the lungs of bleomycin-treated subcongenic mice, to B6 levels, as shown in Figure 4A.
Fig. 4. Trim16 over-expression increases pulmonary fibrosis in Blmpf2 subcongenic mice. 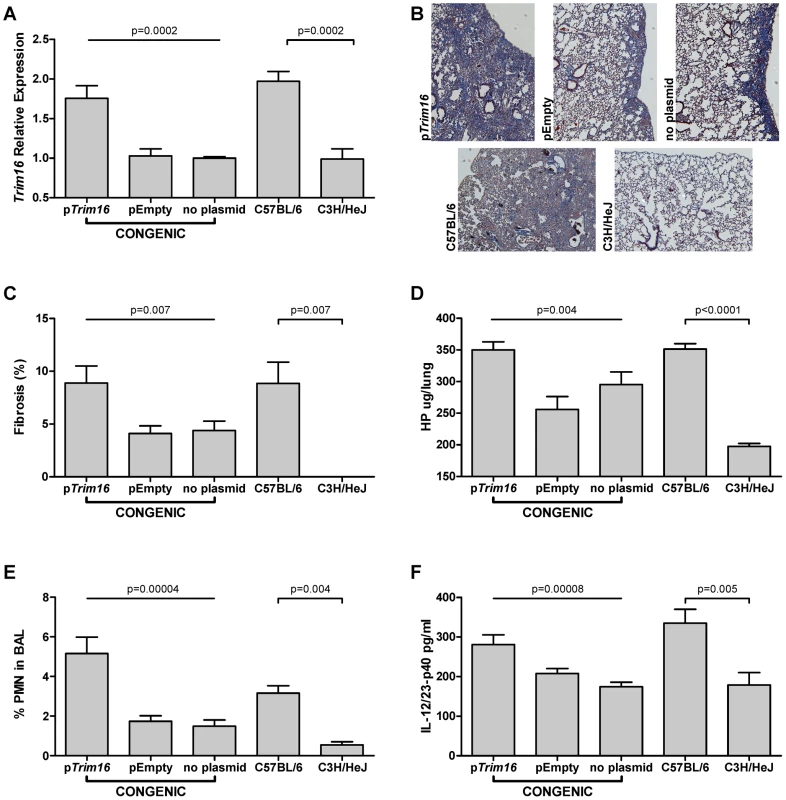
Mice were treated with bleomycin as in Figure 1 and groups of Blmpf2 subcongenic mice were treated with a Trim16 expressing plasmid, an empty plasmid, or received no further treatment, 21 days after bleomycin treatment was initiated. The mice were euthanized at day 42. A. Right lung Trim16 expression, by RT-PCR; n = 4–5 per group. B. histological sections of left lungs of congenic mice, top row and inbred strains, second row, Masson's trichrome stain, magnification of 100×. C. percent fibrosis in the left lung; n = 16–19 per group. D. total right lung hydroxyproline; n = 11–13 per group. E. Bronchoalveolar lavage neutrophil counts; n = 8–13 per group. F. Bronchoalveolar lavage supernatant interleukin-12/23p40 levels n = 8–13 per group. The average of each phenotype ± std. err. is given. B6.Blmpf2C3H subcongenic mice treated with bleomycin and the Trim16 plasmid also developed significantly increased fibrosis, assessed both histologically and with hydroxyproline measures, compared to the levels in subcongenic mice receiving bleomycin alone or bleomycin and a control plasmid (Figure 4B–4D). The Trim16 plasmid treatment resulted in B6.Blmpf2C3H subcongenic mice developing a level of fibrosis similar to that of B6 mice (p = 0.9), indicating a Trim16 deficiency to have reduced the fibrosis development in subcongenic mice.
To elucidate how the fibrosis was enhanced in Trim16 treated mice, the bronchoalveolar lavage fluid from each animal was scored for inflammatory cell types. Subcongenic mice treated with bleomycin and Trim16 plasmid presented with neutrophil levels equal to those in bleomycin treated B6 mice (p = 0.6) and exceeding those of subcongenic mice receiving either of bleomycin alone or bleomycin and an empty vector, and the levels in bleomycin-treated C3H mice, as shown in Figure 4E.
Given that increased levels of the cytokine interleukin 12/23-p40, which is involved in neutrophil recruitment [28], can cause experimental pulmonary fibrosis [29], [30], we therefore assessed whether the increased fibrosis in the subcongenic mice was associated with a Trim16 mediated enhancement of Il12/23-p40 secretion. We initially showed Il12/23-p40 levels to be relevant to fibrosis development in our model by measuring the level of this cytokine in the bronchoalveolar lavage of B6 and C3H mice following exposure to bleomycin. As shown in Figure 4F Il-12/23-p40 was increased to a greater extent in B6 than C3H mice, and in Trim16 plasmid and bleomycin treated subcongenic mice compared to subcongenic challenged with bleomycin alone, thus elevated levels of Trim16 in the bleomycin-treated lung affect the Il12/23-p40 pathway to fibrosis.
Discussion
In previous studies the bleomycin-induced lung response of C57BL/6J mice, an established model of pulmonary fibrosis [31], was contrasted with that of C3H mice and used as the base of a linkage investigation mapping susceptibility to this lung disease to two loci [17]. In this work we moved from the mapped locus to the gene by demonstrating that overexpression of Trim16 can contribute to pulmonary fibrosis development in mice.
Our approach to identify the Blmpf2 quantitative trait gene included experiments in congenic and subcongenic mice, with candidate gene sequence variation and expression analyses, as in other positional cloning studies [32], [33]. Based on this genetic evidence the leading candidate of the genes mapping to the subcongenic interval was tripartite motif-containing 16, or Trim16, which encodes the protein Trim16. As Munding et al. [20] demonstrated that the secretion of the pro inflammatory cytokine interleukin-1β could be augmented in vitro by TRIM16, this gene was also a physiologically relevant candidate for fibrosis susceptibility owing to the inflammatory component of this trait.
To increase the expression of Trim16 in the lungs of B6.Blmpf2C3H subcongenic mice to the levels in the fibrosis prone B6 strain we made use of a plasmid transfection system which had previously been shown to produce a high level of transfected gene expression in the lung without an increase in the tissue inflammatory response [26], [27], [34], [35]. With this system the elevated Trim16 expression significantly increased the pulmonary fibrosis of recipient congenic mice. Further to elevated pulmonary fibrosis the increased Trim16 expression produced higher levels of both Il12/23-p40 and neutrophils in the bronchoalveolar lavage of congenic mice. These findings are relevant as lavage neutrophilia has been associated with pulmonary fibrosis both experimentally [36], and clinically, where Kinder et al. [37] showed greater levels of neutrophils in the lavage to correlate with early mortality from pulmonary fibrosis.
Altered levels of the cytokine interleukin 12/23-p40 have also been implicated in pulmonary fibrosis pathology through studies in animal models. Specifically, Huaux et al. [29] showed the levels of interleukin 12-p40 to increase and to remain elevated in a model of experimental pulmonary fibrosis and for this cytokine subunit to decrease in abundance in resolving lung disease. In a subsequent study, Huaux et al. [30] demonstrated interleukin 12-p40 deficient mice to be protected from fibrosis development in response to challenge and, further, that supplementation with rIL-12p40 both restored the impaired pulmonary fibrotic response in these mice, and augmented the fibrosis in wild-type mice. Finally, Wilson et al. [36] reported bleomycin-induced pulmonary fibrosis and neutrophilia to be decreased in interleukin 12-p40 deficient mice.
Using a positional cloning strategy we have uncovered a novel gene of pulmonary fibrosis susceptibility in the mouse. Elevated levels of this gene, Trim16, increased both the fibrosis and a specific inflammatory phenotype in mice which supports the relevance of tissue inflammation to fibrosis development in this animal model [3]. Mechanistically, although Trim16 can interact with cytokines to increase their expression [20], and a genetic deficiency of related family member Trim21 has been shown to enhance Il12/23p-40 secretion [38], whether Trim16 directly or indirectly results in increased Il12/23-p40 remains to be determined. The finding that allele specific variation in Trim16 can affect both fibrosis susceptibility and lavage neutrophilia could have important translational implications as both the disease phenotype and a subphenotype may be plausibly evaluated for clinical association; which, if confirmed would reveal a specific pathway for the development of this complex disease.
Materials and Methods
Ethics statement
All animals were treated and maintained under a protocol approved by the Animal Care Committee of McGill University, in accordance with guidelines set by the Canadian Council on Animal Use and Care.
Mice
C57BL/6J and C3H/HeJ mice were purchased from Jackson Laboratories (Bar Harbor, ME) and housed in the Meakins Christie's animal facility. Blmpf2 congenic mice were bred by intercrossing the strains, followed by backcrossing successive generations to B6 mice and selecting for C3H alleles in the linkage region of Chromosome 11, followed by intercrossing at the 10th generation. To produce subcongenic mice the congenic mice were crossed to B6 mice, the resultant progeny were intercrossed and their offspring screened for recombination across the Blmpf2 interval using microsatellite markers. Sister/brother progeny segregating the same recombinant intervals were intercrossed to generate strains harbouring homozygous subcongenic intervals. The position of the recombinant events was subsequently refined with SNP genotyping based on the Sanger database (http://www.sanger.ac.uk/resources/mouse/genomes/) and SNP detection performed using Taqman allelic discrimination assays for SNPs rs3023266, rs13481060 and rs6297520 and direct sequencing of amplified PCR products containing SNP sites.
Pulmonary fibrosis phenotyping
Lung damage was elicited by challenging 8 to 10 week old mice with bleomycin sulphate dissolved in sodium chloride (Mayne Pharma, Canada). The mice were treated with 100–125 units/kg of bleomycin through mini-osmotic pumps (Alzet 2001, USA) implanted subcutaneously in the mouse's back as in prior studies [12], [13]. The pump was removed on day 8. The mice were euthanized six weeks later, bronchoalveolar lavage was completed and the lungs were preserved for histology, hydroxyproline measures or gene expression.
For histological analysis the left lungs were perfused with 10% neutral buffered formalin, embedded in paraffin blocks, sectioned and stained with Masson's Trichrome. The fibrosis area was quantified and compared with the surface of the entire lung to yield a percentage of fibrosis using the Image-Pro software (Media Cybernetics) as in prior studies [12], [13], [16]. The hydroxyproline content of the mouse lungs was determined using previously described standard methods [39]. For gene expression lung tissue was immediately homogenized in 2 ml TRI reagent (Sigma-Aldrich, USA) and expression was assessed with Applied Biosystem's assays on demand as in previous studies [40], [41].
Bronchoalveolar lavage fluid analysis
The lungs were lavaged with one mL of PBS, the lavage fluid was centrifuged (302 g for 10 minutes at 4°C) and the supernatant was removed and stored at −85°C. The cellular pellet was resuspended in 0.25 mL PBS. Inflammatory cell counts were performed (400× magnification) on cytocentrifuged cells (214.2 g for three minutes), after staining with a hematoxylin-eosin kit (Hema-3 Stain Set by Protocol).
Sequencing
A 1368 bp region containing the putative Trim16 promoter from strains C57BL/6J and C3H/HeJ was amplified and cloned in plasmid pJet1.2/blunt (Thermo Fisher Scientific). Inserts were sequenced and compared to the mouse genome assembly of the UCSC database (http://genome.ucsc.edu) and to that in the Sanger database to evaluate strain dependent polymorphisms.
Transcription factor binding site analysis
Putative transcription factor binding sites located within the 1.4 kb of the promoter region were identified in silico using the PROMO program [22], [23] and TFsearch (v.1.3) of the TRANSFAC database [24] with a focus on murine specific transcription factors and transcription factor binding sites. The sequence was also analyzed with the MATCH public 1.0 algorithm by Biobase GmbH [24] using vertebrate weight matrices and a minimum false negative profile.
In order to determine evolutionary conservation of promoter region B6/C3H SNPs or deletions we used the Multiz Alignment & Conservation algorithm of the UCSC genome browser (http://genome.ucsc.edu; [42]). The graphic output was created using CodonCode Aligner (v. 3.7.1, Codon Code Corporation).
Promoter cloning and transcription assay
A 1368 bp fragment (−1372 to −4 bp) was amplified from strains C57BL/6J and C3H/HeJ by PCR and cloned into the KpnI/HindIII sites of vector pGL3-Basic (Promega, Madison, WI). Sequencing of the inserts was completed to confirm allelic specificity. RAW246.7 macrophage cells were co-transfected with the reporter vector, or empty pGL3-basic, and pRL-SV40 (Promega), which was used as an internal control for transfection efficiency. Twenty four hours after transfection, cells were assayed for both firefly and renilla luciferase activity using the Dual-Luciferase Reporter Assay System (Promega, Madison, WI).
Plasmid delivery to mouse lung
A plasmid of the full length B6 mouse Trim16 cDNA with the 252 bp 5′ UTR and 3′ UTR (accession NM_053169), cloned into the EcoRI – NotI sites of expression vector pDream2.1/MCS, and an empty modified pDream2.1/MCS vector, were obtained from Genscript (GenScript, USA). Prior to cloning the vector was modified by removing an 18 nt Flag tag sequence to avoid possible interference with the expressed protein structure. Plasmid insert specificity and orientation were confirmed by sequencing. The cationic linear polyethylenimine in vivo-jetPEI (Polyplus-transfection, France; MJS Biolynx, Canada) was used for plasmid transfection to the mouse lung according to the manufacturer's instructions. The PEI (6.5 µl) and Trim16 plasmid (40 µg) complex was resuspended in a sterile 5% glucose solution (400 uL total) and injected into the tail vein of mice.
ELISA
The level of Il-12/23-p40 in the bronchoalveolar lavage supernatant was assayed with a murine Il-12/23(p40) ELISA kit (eBioscience, USA) according to the manufacturer's instructions.
Statistics
Phenotypic differences between groups were assessed with Student's t test and among groups of congenic mice receiving distinct treatments, as in Figure 4, by an analysis of variance followed by Tukey post hoc tests.
Supporting Information
Zdroje
1. RaghuG, CollardHR, EganJJ, MartinezFJ, BehrJ, et al. (2011) An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 183 : 788–824.
2. KingTEJr, PardoA, SelmanM (2011) Idiopathic pulmonary fibrosis. Lancet 378 : 1949–61.
3. WynnTA (2011) Integrating mechanisms of pulmonary fibrosis. J Exp Med 208 : 1339–50.
4. NuovoGJ, HagoodJS, MagroCM, ChinN, KapilR, et al. (2012) The distribution of immunomodulatory cells in the lungs of patients with idiopathic pulmonary fibrosis. Mod Pathol 25 : 416–33.
5. HodgsonU, PulkkinenV, DixonM, Peyrard-JanvidM, RehnM, et al. (2006) ELMOD2 is a candidate gene for familial idiopathic pulmonary fibrosis. Am J Hum Genet 79 : 149–54.
6. GarciaCK (2011) Idiopathic pulmonary fibrosis: update on genetic discoveries. Proc Am Thorac Soc 8 : 158–62.
7. SeiboldMA, WiseAL, SpeerMC, SteeleMP, BrownKK, et al. (2011) A common MUC5B promoter polymorphism and pulmonary fibrosis. N Engl J Med 364 : 1503–12.
8. ZhangY, NothI, GarciaJG, KaminskiN (2011) A variant in the promoter of MUC5B and idiopathic pulmonary fibrosis. N Engl J Med 364 : 1576–7.
9. ToddJL, GoldsteinDB, GeD, ChristieJ, PalmerSM (2011) The State of Genome-Wide Association Studies in Pulmonary Disease: A New Perspective. Am J Respir Crit Care Med 184 : 873–80.
10. GrossTJ, HunninghakeGW (2001) Idiopathic pulmonary fibrosis. N Engl J Med 345 : 517–25.
11. HarrisonJHJr, LazoJS (1987) High dose continuous infusion of bleomycin in mice: a new model for drug-induced pulmonary fibrosis. J Pharmacol Exp Ther 243 : 1185–94.
12. LemayAM, HastonCK (2005) Bleomycin-induced pulmonary fibrosis susceptibility genes in AcB/BcA recombinant congenic mice. Physiol Genomics 23 : 54–61.
13. HastonCK, TomkoTG, GodinN, KerckhoffL, HallettMT (2005) Murine candidate bleomycin induced pulmonary fibrosis susceptibility genes identified by gene expression and sequence analysis of linkage regions. J Med Genet 42 : 464–473.
14. GabazzaEC, TaguchiO, AdachiY (2002) Bleomycin-induced lung fibrosis: the authors should have used another method to induce pulmonary lesions resembling human idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 165 : 845–6.
15. AonoY, LedfordJG, MukherjeeS, OgawaH, NishiokaY, et al. (2012) Surfactant protein-D regulates effector cell function and fibrotic lung remodeling in response to bleomycin injury. Am J Respir Crit Care Med 185 : 525–36.
16. HastonCK, AmosCI, KingTM, TravisEL (1996) Inheritance of susceptibility to bleomycin-induced pulmonary fibrosis in the mouse. Cancer Res 56 : 2596–601.
17. HastonCK, WangM, DejournettRE, ZhouX, NiD, et al. (2002) Bleomycin hydrolase and a genetic locus within the MHC affect risk for pulmonary fibrosis in mice. Hum Mol Genet 11 : 1855–63.
18. KumarP, HenikoffS, NgPC (2009) Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat Protoc 4 : 1073–81.
19. AdzhubeiIA, SchmidtS, PeshkinL, RamenskyVE, GerasimovaA, et al. (2010) A method and server for predicting damaging missense mutations. Nat Methods 7 : 248–9.
20. MundingC, KellerM, NiklausG, PapinS, TschoppJ, et al. (2006) The estrogen-responsive B box protein: a novel enhancer of interleukin-1beta secretion. Cell Death Differ 13 : 1938–49.
21. KeaneTM, GoodstadtL, DanecekP, WhiteMA, WongK, et al. (2011) Mouse genomic variation and its effect on phenotypes and gene regulation. Nature 477 : 289–94.
22. MesseguerX, EscuderoR, FarréD, NúñezO, MartínezJ, et al. (2002) PROMO: detection of known transcription regulatory elements using species-tailored searches. Bioinformatics 18 : 333–4.
23. FarréD, RosetR, HuertaM, AdsuaraJE, RosellóL, et al. (2003) Identification of patterns in biological sequences at the ALGGEN server: PROMO and MALGEN. Nucleic Acids Res 31 : 3651–3.
24. HeinemeyerT, WingenderE, ReuterI, HermjakobH, KelAE, et al. (1998) Databases on transcriptional regulation: TRANSFAC, TRRD and COMPEL. Nucleic Acids Res 26 : 362–7.
25. KelAE, GösslingE, ReuterI, CheremushkinE, Kel-MargoulisOV, et al. (2003) MATCH: A tool for searching transcription factor binding sites in DNA sequences. Nucleic Acids Res 31 : 3576–9.
26. GoulaD, BenoistC, ManteroS, MerloG, LeviG, et al. (1998) Polyethylenimine-based intravenous delivery of transgenes to mouse lung. Gene Ther 5 : 1291–5.
27. GoulaD, BeckerN, LemkineGF, NormandieP, RodriguesJ, et al. (2000) Rapid crossing of the pulmonary endothelial barrier by polyethylenimine/DNA complexes. Gene Ther 7 : 499–504.
28. SmithE, ZarbockA, StarkMA, BurcinTL, BruceAC, et al. (2007) IL-23 is required for neutrophil homeostasis in normal and neutrophilic mice. J Immunol 179 : 8274–9.
29. HuauxF, LardotC, ArrasM, DelosM, ManyMC, et al. (1999) Lung fibrosis induced by silica particles in NMRI mice is associated with an upregulation of the p40 subunit of interleukin-12 and Th-2 manifestations. Am J Respir Cell Mol Biol 20 : 561–72.
30. HuauxF, ArrasM, TomasiD, BarbarinV, DelosM, et al. (2002) A profibrotic function of IL-12p40 in experimental pulmonary fibrosis. J Immunol 169 : 2653–61.
31. MouratisMA, AidinisV (2011) Modeling pulmonary fibrosis with bleomycin. Curr Opin Pulm Med 17 : 355–61.
32. TomidaS, MamiyaT, SakamakiH, MiuraM, AosakiT, et al. (2009) Usp46 is a quantitative trait gene regulating mouse immobile behavior in the tail suspension and forced swimming tests. Nat Genet 41 : 688–95.
33. BhatnagarS, OlerAT, RabagliaME, StapletonDS, SchuelerKL, et al. (2011) Positional cloning of a type 2 diabetes quantitative trait locus; tomosyn-2, a negative regulator of insulin secretion. PLoS Genet 10: e1002323 doi:10.1371/journal.pgen.1002323.
34. OhYK, KimJP, YoonH, KimJM, YangJS, et al. (2001) Prolonged organ retention and safety of plasmid DNA administered in polyethylenimine complexes. Gene Ther 8 : 1587–92.
35. BonnetME, ErbacherP, Bolcato-BelleminAL (2008) Systemic delivery of DNA or siRNA mediated by linear polyethylenimine (L-PEI) does not induce an inflammatory response. Pharm Res 25 : 2972–82.
36. WilsonMS, MadalaSK, RamalingamTR, GochuicoBR, RosasIO, et al. (2010) Bleomycin and IL-1beta-mediated pulmonary fibrosis is IL-17A dependent. J Exp Med 207 : 535–52.
37. KinderBW, BrownKK, SchwarzMI, IxJH, KervitskyA, et al. (2008) Baseline BAL neutrophilia predicts early mortality in idiopathic pulmonary fibrosis. Chest 133 : 226–32.
38. EspinosaA, DardalhonV, BraunerS, AmbrosiA, HiggsR, et al. (2009) Loss of the lupus autoantigen Ro52/Trim21 induces tissue inflammation and systemic autoimmunity by disregulating the IL-23-Th17 pathway. J Exp Med 206 : 1661–71.
39. EdwardsCA, O'BrienWDJr (1980) “Modified assay for determination of hydroxyproline in a tissue hydrolyzate.”. Clin Chim Acta 104 : 161–167.
40. PaunA, FoxJ, BalloyV, ChignardM, QureshiST, et al. (2010) Combined Tlr2 and Tlr4 deficiency increases radiation-induced pulmonary fibrosis in mice. Int J Radiat Oncol Biol Phys 77 : 1198–205.
41. ThomasDM, FoxJ, HastonCK (2010) Imatinib therapy reduces radiation-induced pulmonary mast cell influx and delays lung disease in the mouse. Int J Radiat Biol 86 : 436–44.
42. BlanchetteM, KentWJ, RiemerC, ElnitskiL, SmitAF, et al. (2004) Aligning multiple genomic sequences with the threaded blockset aligner. Genome Res 14 : 708–15.
Štítky
Genetika Reprodukční medicína
Článek Comparative Genome Structure, Secondary Metabolite, and Effector Coding Capacity across PathogensČlánek TATES: Efficient Multivariate Genotype-Phenotype Analysis for Genome-Wide Association StudiesČlánek Secondary Metabolism and Development Is Mediated by LlmF Control of VeA Subcellular Localization inČlánek Human Disease-Associated Genetic Variation Impacts Large Intergenic Non-Coding RNA ExpressionČlánek The Roles of Whole-Genome and Small-Scale Duplications in the Functional Specialization of GenesČlánek The Role of Autophagy in Genome Stability through Suppression of Abnormal Mitosis under Starvation
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2013 Číslo 1
-
Všechny články tohoto čísla
- A Model of High Sugar Diet-Induced Cardiomyopathy
- Comparative Genome Structure, Secondary Metabolite, and Effector Coding Capacity across Pathogens
- Emerging Function of Fat Mass and Obesity-Associated Protein (Fto)
- Positional Cloning Reveals Strain-Dependent Expression of to Alter Susceptibility to Bleomycin-Induced Pulmonary Fibrosis in Mice
- Genetics of Ribosomal Proteins: “Curiouser and Curiouser”
- Transposable Elements Re-Wire and Fine-Tune the Transcriptome
- Function and Regulation of , a Gene Implicated in Autism and Human Evolution
- MAML1 Enhances the Transcriptional Activity of Runx2 and Plays a Role in Bone Development
- Predicting Mendelian Disease-Causing Non-Synonymous Single Nucleotide Variants in Exome Sequencing Studies
- A Systematic Mapping Approach of 16q12.2/ and BMI in More Than 20,000 African Americans Narrows in on the Underlying Functional Variation: Results from the Population Architecture using Genomics and Epidemiology (PAGE) Study
- Transcription of the Major microRNA–Like Small RNAs Relies on RNA Polymerase III
- Histone H3K56 Acetylation, Rad52, and Non-DNA Repair Factors Control Double-Strand Break Repair Choice with the Sister Chromatid
- Genome-Wide Association Study Identifies a Novel Susceptibility Locus at 12q23.1 for Lung Squamous Cell Carcinoma in Han Chinese
- Genetic Disruption of the Copulatory Plug in Mice Leads to Severely Reduced Fertility
- The [] Prion Exists as a Dynamic Cloud of Variants
- Adult Onset Global Loss of the Gene Alters Body Composition and Metabolism in the Mouse
- Fis Protein Insulates the Gene from Uncontrolled Transcription
- The Meiotic Nuclear Lamina Regulates Chromosome Dynamics and Promotes Efficient Homologous Recombination in the Mouse
- Genome-Wide Haplotype Analysis of Expression Quantitative Trait Loci in Monocytes
- TATES: Efficient Multivariate Genotype-Phenotype Analysis for Genome-Wide Association Studies
- Structural Basis of a Histone H3 Lysine 4 Demethylase Required for Stem Elongation in Rice
- The Ecm11-Gmc2 Complex Promotes Synaptonemal Complex Formation through Assembly of Transverse Filaments in Budding Yeast
- MCM8 Is Required for a Pathway of Meiotic Double-Strand Break Repair Independent of DMC1 in
- Comparative Genomic Analysis of the Endosymbionts of Herbivorous Insects Reveals Eco-Environmental Adaptations: Biotechnology Applications
- Integration of Nodal and BMP Signals in the Heart Requires FoxH1 to Create Left–Right Differences in Cell Migration Rates That Direct Cardiac Asymmetry
- Pharmacodynamics, Population Dynamics, and the Evolution of Persistence in
- A Hybrid Likelihood Model for Sequence-Based Disease Association Studies
- Aberration in DNA Methylation in B-Cell Lymphomas Has a Complex Origin and Increases with Disease Severity
- Multiple Opposing Constraints Govern Chromosome Interactions during Meiosis
- Transcriptional Dynamics Elicited by a Short Pulse of Notch Activation Involves Feed-Forward Regulation by Genes
- Dynamic Large-Scale Chromosomal Rearrangements Fuel Rapid Adaptation in Yeast Populations
- Heterologous Gln/Asn-Rich Proteins Impede the Propagation of Yeast Prions by Altering Chaperone Availability
- Gene Copy-Number Polymorphism Caused by Retrotransposition in Humans
- An Incompatibility between a Mitochondrial tRNA and Its Nuclear-Encoded tRNA Synthetase Compromises Development and Fitness in
- Secondary Metabolism and Development Is Mediated by LlmF Control of VeA Subcellular Localization in
- Single-Stranded Annealing Induced by Re-Initiation of Replication Origins Provides a Novel and Efficient Mechanism for Generating Copy Number Expansion via Non-Allelic Homologous Recombination
- Tbx2 Controls Lung Growth by Direct Repression of the Cell Cycle Inhibitor Genes and
- Suv4-20h Histone Methyltransferases Promote Neuroectodermal Differentiation by Silencing the Pluripotency-Associated Oct-25 Gene
- A Conserved Helicase Processivity Factor Is Needed for Conjugation and Replication of an Integrative and Conjugative Element
- Telomerase-Null Survivor Screening Identifies Novel Telomere Recombination Regulators
- Genome-Wide Analysis Reveals Selection for Important Traits in Domestic Horse Breeds
- Coordinated Degradation of Replisome Components Ensures Genome Stability upon Replication Stress in the Absence of the Replication Fork Protection Complex
- Nkx6.1 Controls a Gene Regulatory Network Required for Establishing and Maintaining Pancreatic Beta Cell Identity
- HIF- and Non-HIF-Regulated Hypoxic Responses Require the Estrogen-Related Receptor in
- Delineating a Conserved Genetic Cassette Promoting Outgrowth of Body Appendages
- The Telomere Capping Complex CST Has an Unusual Stoichiometry, Makes Multipartite Interaction with G-Tails, and Unfolds Higher-Order G-Tail Structures
- Comprehensive Methylome Characterization of and at Single-Base Resolution
- Loci Associated with -Glycosylation of Human Immunoglobulin G Show Pleiotropy with Autoimmune Diseases and Haematological Cancers
- Switchgrass Genomic Diversity, Ploidy, and Evolution: Novel Insights from a Network-Based SNP Discovery Protocol
- Centromere-Like Regions in the Budding Yeast Genome
- Sequencing of Loci from the Elephant Shark Reveals a Family of Genes in Vertebrate Genomes, Forged by Ancient Duplications and Divergences
- Mendelian and Non-Mendelian Regulation of Gene Expression in Maize
- Mutational Spectrum Drives the Rise of Mutator Bacteria
- Human Disease-Associated Genetic Variation Impacts Large Intergenic Non-Coding RNA Expression
- The Roles of Whole-Genome and Small-Scale Duplications in the Functional Specialization of Genes
- Sex-Specific Signaling in the Blood–Brain Barrier Is Required for Male Courtship in
- A Newly Uncovered Group of Distantly Related Lysine Methyltransferases Preferentially Interact with Molecular Chaperones to Regulate Their Activity
- Is Required for Leptin-Mediated Depolarization of POMC Neurons in the Hypothalamic Arcuate Nucleus in Mice
- Unlocking the Bottleneck in Forward Genetics Using Whole-Genome Sequencing and Identity by Descent to Isolate Causative Mutations
- The Role of Autophagy in Genome Stability through Suppression of Abnormal Mitosis under Starvation
- MTERF3 Regulates Mitochondrial Ribosome Biogenesis in Invertebrates and Mammals
- Downregulation and Altered Splicing by in a Mouse Model of Facioscapulohumeral Muscular Dystrophy (FSHD)
- NBR1-Mediated Selective Autophagy Targets Insoluble Ubiquitinated Protein Aggregates in Plant Stress Responses
- Retroactive Maintains Cuticle Integrity by Promoting the Trafficking of Knickkopf into the Procuticle of
- Phenome-Wide Association Study (PheWAS) for Detection of Pleiotropy within the Population Architecture using Genomics and Epidemiology (PAGE) Network
- Genetic and Functional Modularity of Activities in the Specification of Limb-Innervating Motor Neurons
- A Population Genetic Model for the Maintenance of R2 Retrotransposons in rRNA Gene Loci
- A Quartet of PIF bHLH Factors Provides a Transcriptionally Centered Signaling Hub That Regulates Seedling Morphogenesis through Differential Expression-Patterning of Shared Target Genes in
- A Genome-Wide Integrative Genomic Study Localizes Genetic Factors Influencing Antibodies against Epstein-Barr Virus Nuclear Antigen 1 (EBNA-1)
- Mutation of the Diamond-Blackfan Anemia Gene in Mouse Results in Morphological and Neuroanatomical Phenotypes
- Life, the Universe, and Everything: An Interview with David Haussler
- Alternative Oxidase Expression in the Mouse Enables Bypassing Cytochrome Oxidase Blockade and Limits Mitochondrial ROS Overproduction
- An Evolutionarily Conserved Synthetic Lethal Interaction Network Identifies FEN1 as a Broad-Spectrum Target for Anticancer Therapeutic Development
- The Flowering Repressor Underlies a Novel QTL Interacting with the Genetic Background
- Telomerase Is Required for Zebrafish Lifespan
- and Diversified Expression of the Gene Family Bolster the Floral Stem Cell Network
- Susceptibility Loci Associated with Specific and Shared Subtypes of Lymphoid Malignancies
- An Insertion in 5′ Flanking Region of Causes Blue Eggshell in the Chicken
- Increased Maternal Genome Dosage Bypasses the Requirement of the FIS Polycomb Repressive Complex 2 in Arabidopsis Seed Development
- WNK1/HSN2 Mutation in Human Peripheral Neuropathy Deregulates Expression and Posterior Lateral Line Development in Zebrafish ()
- Synergistic Interaction of Rnf8 and p53 in the Protection against Genomic Instability and Tumorigenesis
- Dot1-Dependent Histone H3K79 Methylation Promotes Activation of the Mek1 Meiotic Checkpoint Effector Kinase by Regulating the Hop1 Adaptor
- A Heterogeneous Mixture of F-Series Prostaglandins Promotes Sperm Guidance in the Reproductive Tract
- Starvation, Together with the SOS Response, Mediates High Biofilm-Specific Tolerance to the Fluoroquinolone Ofloxacin
- Directed Evolution of a Model Primordial Enzyme Provides Insights into the Development of the Genetic Code
- Genome-Wide Screens for Tinman Binding Sites Identify Cardiac Enhancers with Diverse Functional Architectures
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Function and Regulation of , a Gene Implicated in Autism and Human Evolution
- An Insertion in 5′ Flanking Region of Causes Blue Eggshell in the Chicken
- Comprehensive Methylome Characterization of and at Single-Base Resolution
- Susceptibility Loci Associated with Specific and Shared Subtypes of Lymphoid Malignancies
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



