-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaMutational Spectrum Drives the Rise of Mutator Bacteria
Understanding how mutator strains emerge in bacterial populations is relevant both to evolutionary theory and to reduce the threat they pose in clinical settings. The rise of mutator alleles is understood as a result of their hitchhiking with linked beneficial mutations, although the factors that govern this process remain unclear. A prominent but underappreciated fact is that each mutator allele increases only a specific spectrum of mutational changes. This spectrum has been speculated to alter the distribution of fitness effects of beneficial mutations, potentially affecting hitchhiking. To study this possibility, we analyzed the fitness distribution of beneficial mutations generated from different mutator and wild-type Escherichia coli strains. Using antibiotic resistance as a model system, we show that mutational spectra can alter these distributions substantially, ultimately determining the competitive ability of each strain across environments. Computer simulation showed that the effect of mutational spectrum on hitchhiking dynamics follows a non-linear function, implying that even slight spectrum-dependent fitness differences are sufficient to alter mutator success frequency by several orders of magnitude. These results indicate an unanticipated central role for the mutational spectrum in the evolution of bacterial mutation rates. At a practical level, this study indicates that knowledge of the molecular details of resistance determinants is crucial for minimizing mutator evolution during antibiotic therapy.
Published in the journal: . PLoS Genet 9(1): e32767. doi:10.1371/journal.pgen.1003167
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003167Summary
Understanding how mutator strains emerge in bacterial populations is relevant both to evolutionary theory and to reduce the threat they pose in clinical settings. The rise of mutator alleles is understood as a result of their hitchhiking with linked beneficial mutations, although the factors that govern this process remain unclear. A prominent but underappreciated fact is that each mutator allele increases only a specific spectrum of mutational changes. This spectrum has been speculated to alter the distribution of fitness effects of beneficial mutations, potentially affecting hitchhiking. To study this possibility, we analyzed the fitness distribution of beneficial mutations generated from different mutator and wild-type Escherichia coli strains. Using antibiotic resistance as a model system, we show that mutational spectra can alter these distributions substantially, ultimately determining the competitive ability of each strain across environments. Computer simulation showed that the effect of mutational spectrum on hitchhiking dynamics follows a non-linear function, implying that even slight spectrum-dependent fitness differences are sufficient to alter mutator success frequency by several orders of magnitude. These results indicate an unanticipated central role for the mutational spectrum in the evolution of bacterial mutation rates. At a practical level, this study indicates that knowledge of the molecular details of resistance determinants is crucial for minimizing mutator evolution during antibiotic therapy.
Introduction
Despite their increased load of deleterious mutations [1], [2], mutator strains of bacteria are isolated routinely in laboratory and clinical settings [3]–[8]. Theory [9]–[11] and experiments [5], [12], [13] explain these observations as a consequence of genetic hitchhiking, whereby mutator alleles reach high frequency by being co-selected with linked beneficial mutations. Mutator evolution is therefore dependent on the absence of horizontal gene transfer [14] and the availability of adaptive mutations with substantial fitness effects [11] – conditions frequently met during adaptation to a host or during antibiotic therapy, which have been invoked to explain the prevalence of mutators among pathogenic bacteria [15]–[17].
Whereas genetic hitchhiking provides a satisfactory mechanism to explain how mutator bacteria can be selected, the precise mechanistic details of this process are still a matter of research. Some authors emphasize that mutator fixation involves many consecutive hitchhiking events, prompted by frequent environmental shifts [9], [18], [19] or by the concurrence of multiple beneficial mutations [10], [11]. Other authors, in contrast, consider mutator success as primarily the result of a single step in which one hitchhiking event takes mutator frequency from rareness to fixation [20]. Despite this theoretical effort, two major observations remain to be accounted for. First, although hitchhiking probability is predicted to increase with the extent of adaptive opportunity offered by the environment (i.e., the number and effects of available beneficial mutations) [14], [20], , selection experiments with comparable adaptive opportunity report contrasting frequencies for mutator emergence [4], [12], [22], [23]. Second, mutators of different strength are predicted to emerge according to the degree of adaptive opportunity [14], [21]; nonetheless, both clinical and laboratory observations show a marked bias towards strong mutators, particularly those caused by defects in the mismatch repair system (MMR) [17], [24]. To explain these deviations, it is argued that mutator alleles of other mutator strengths might be under-represented due to putative fitness disadvantages, and that MMR mutator mutants might be over-represented because they can also be selected for their high recombination rates [24].
An additional, previously unrecognized factor is that each mutator exhibits its own mutational spectrum, that is, mutation rate increase is limited to characteristic types of mutations [25]. This bias stems from the type of mutation avoidance mechanism that is altered in each mutator genotype; depending on which mechanism is affected, its failure will lead to a preferential increase in specific types of transitions, transversions or frameshifts [25]. In line with previous suggestions [12], [26], we hypothesized that if mutators and wild-type strains have differential access to specific beneficial mutations, they might produce mutants with different fitness levels, which could influence their evolutionary dynamics. Mutator alleles that on average generate stronger beneficial mutations will have a better chance to achieve fixation; conversely, those that more often produce weaker adaptive mutations will have limited spread. The fixation probability of a mutator allele would thus depend not only on its mutation rate, but also on its mutational spectrum.
Here we test these predictions by characterizing beneficial mutations from wild-type and knockout strains of the mutT (ΔmutT) and mutY (ΔmutY) antimutator genes of Escherichia coli. mutT-defective strains show a strong mutator phenotype, leading specifically to A·T→C·G transversions [25], whereas mutY defects lead to a moderate mutator phenotype that increases G·C→T·A transversions [25]. Both mutators were reported to arise spontaneously in evolution experiments with E. coli [4], [12]. Since beneficial mutations are exceedingly rare and difficult to detect, our experimental design focused on mutations that confer antibiotic resistance, which are particularly suitable for this kind of study. We show that according to their mutational spectra, wild-type and mutator strains can generate distinct fitness distributions for antibiotic-resistant mutants. Notably, these dissimilarities can significantly alter the competitive abilities of mutators against the wild-type strain, as measured in direct competition experiments. Furthermore, using computer simulation of a simple population genetics model, we show that the hitchhiking dynamics is highly sensitive to average fitness deviations of the beneficial mutations with which mutator alleles hitchhike.
Results/Discussion
Colony size polymorphism of antibiotic-resistant mutants
To test whether mutational spectrum differences translate into significant fitness differences, beneficial mutations were selected by plating cultures of wild-type, ΔmutT and ΔmutY strains in three antibiotics: rifampicin, streptomycin and tetracycline. Rifampicin - and streptomycin-resistant mutants showed marked colony size polymorphism (Figure 1A). Resistance to these antibiotics arises readily through alterations in their targets, the RNA polymerase [27] and the 30S ribosomal subunit [28], respectively. Since many alterations can affect the function of these essential machineries to different degrees, it is unsurprising that resistance mutants show growth differences. Consistent with the predictions of our hypothesis, wild-type and mutator strains displayed distinct colony size polymorphism in each antibiotic (Figure 1A). To test whether access to beneficial mutations with varying selection coefficients was the only factor responsible for differences among strains, we used strains with an insertion bearing a tetracycline-resistance gene and its constitutive repressor [29]. As the probability of achieving resistance to high tetracycline concentrations through point mutation in E. coli is negligible (mutant frequency <10−9), resistance arises here only through loss-of-function mutations, which relieve the repression. All of these loss-of-function mutations can be considered equivalents, and no fitness differences are thus anticipated among tetracycline-resistant mutants. Indeed, these mutants showed little growth polymorphism (Figure 1A). Small differences, apparently independent of the mutator background, probably reflect phenotypic lag or other sources of phenotypic variability.
Fig. 1. Mutational spectrum effects on generation of antibiotic-resistant mutants of wild-type and mutator E. coli. 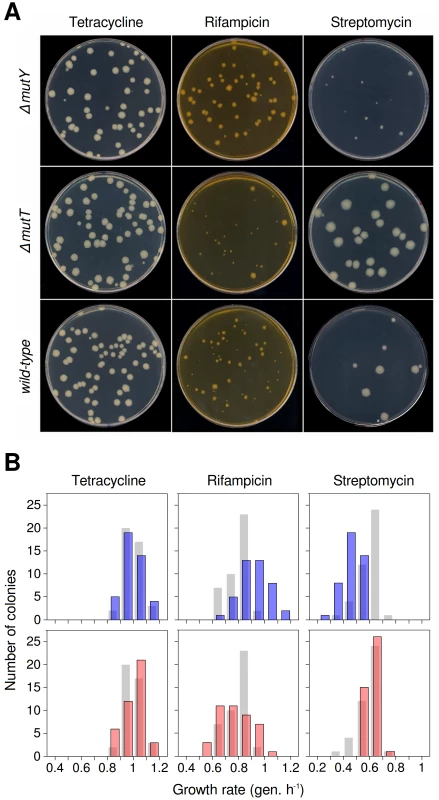
(A) Characteristic colony size polymorphism generated by each strain in tetracycline (left), rifampicin (centre) and streptomycin (right). Resistance mutations to rifampicin and streptomycin impair bacterial growth to varying degrees, and are produced differentially according to mutational spectra. Tetracycline resistance serves here as a control, as resistance mutations in these strains show little variability for fitness. (B) Fitness distributions for antibiotic-resistant mutants generated by each strain. Histograms represent 42 independent growth rate estimations. Blue bars, ΔmutY (top); pink bars, ΔmutT (bottom). Wild-type reference values (grey bars) are placed behind those of each mutator. The greater diversity observed here compared to Figure 1A is a result of the larger number of independent cultures. Distribution of fitness effects on plate of antibiotic-resistant mutants
To quantify the fitness distribution of the mutants generated by each mutator, we randomly selected 42 independent resistant colonies from each combination of genotype and antibiotic, and measured growth rate as a proxy for Darwinian fitness. It is important to remark that we are dealing with the fitness distribution after selection, not the intrinsic fitness distribution; and so it should be understood in what follows. In rifampicin, the distribution of mutants generated by the ΔmutY strain is shifted toward higher growth rates compared with both those of wild-type and ΔmutT strains (Figure 1B, centre), whereas in streptomycin the situation is the reverse (Figure 1B, right) (n = 42, P<0.0001, Kolmogorov-Smirnov's one-sided two-sample test, in all cases). These results suggested that G·C→T·A substitutions in the rpoB gene (the characteristic transversion increased in this mutator) produced alleles encoding a high-fitness rifampicin-resistant RNA polymerase; coincidentally, the same transversion generated rpsL alleles encoding a low-fitness streptomycin-resistant ribosomal protein S12. Similar reasoning could be applied to the ΔmutT strain results, which preferentially raises the A·T→C·G transversion. In the case of tetracycline, no significant differences were found between wild-type and any mutator strains (Figure 1B, left) (n = 42, P>0.18, Kolmogorov-Smirnov's two-sided two-sample test).
Characterization of the genetic basis of antibiotic resistance
To confirm our interpretation, we randomly picked 10 colonies from each combination of antibiotic and strain, sequenced their rpoB and rpsL genes, and measured growth rates (Figure 2). Several G·C→T·A substitutions found in rpoB can explain the higher fitness of rifampicin-resistant ΔmutY mutants, supporting our hypothesis. The highest-fitness class (Figure 1B, centre) is likely to be composed of V146F mutants, the fastest-growing mutant detected (Figure 2A); other high-fitness mutations that resulted from this transversion were H526N and S531Y. The fitness distribution in rifampicin-resistant ΔmutT mutants can be explained, at least in part, by an A·T→C·G substitution that produces the low-fitness mutation Q513P. Among streptomycin-resistant mutants, idiosyncratic transversions in rpsL similarly help to explain the fitness differences. The low fitness of streptomycin-resistant ΔmutY mutants probably reflects predominance of the P90Q mutation (8/10 mutants tested), whereas the high fitness of ΔmutT mutants might be due to prevalence of the K42T mutation (9/10). As a brief remark, 3/10 of the streptomycin-resistant mutants in the wild-type background carry the substitution P90L. This mutation is known to prevent growth in the absence of the antibiotic [28], and offers a suggestive example of how spectra can determine the access to mutations with differences not only in fitness, but also in other related properties.
Fig. 2. Antibiotic resistance mutations generated by wild-type and mutator strains. 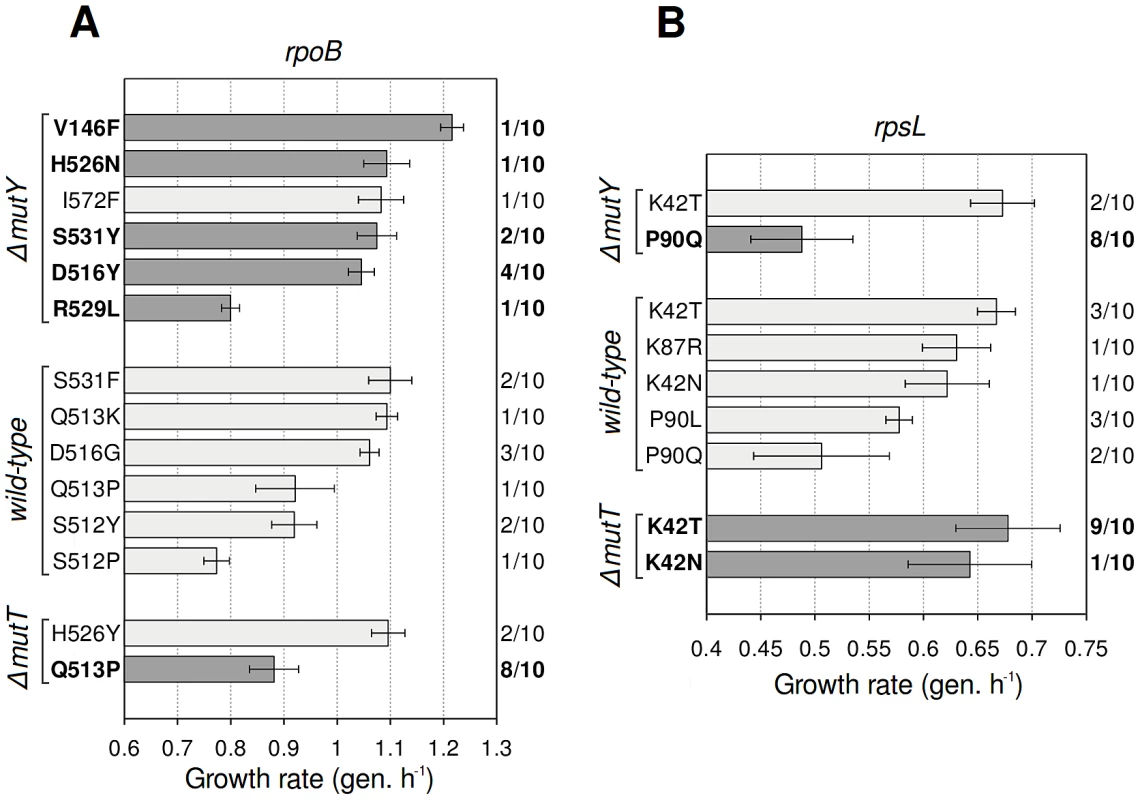
Growth rate (mean ± SD; n = 3) of mutants bearing the specified mutation. Dark grey bars indicate mutations corresponding to the mutational spectrum of each mutator. Frequency of occurrence of each mutation is shown (right axis). All substitutions observed are described to confer antibiotic resistance. (A) Mutations in rpoB, which confer rifampicin resistance. (B) Mutations in rpsL, which confer streptomycin resistance. The biochemical bases of the fitness cost of both rifampicin and streptomycin resistance have been discussed elsewhere. The costs of rpoB mutations are explained by the impairment of the transcription activity of the RNA polymerase [30], [31]. Similarly, it has been long established that rpsL streptomycin resistance mutants exhibit hyperaccurate translation, resulting in a slower rate of protein synthesis and consequently, in a slower growth rate [32].
Average fitness of antibiotic-resistant mutator genotypes
It is tempting to assume that if the fitness distribution of a given mutator is altered compared to that of wild-type, the average fitness of that mutator will change accordingly. These distributions nonetheless have distinct shapes and degrees of overlap (Figure 1B); it is thus of interest to determine the extent to which these differences translate into an overall fitness difference between each mutator and the wild-type genotypes. Direct competition experiments between mutators and wild-type strains in rifampicin and streptomycin showed significant differences in mean fitness (Figure 3) (n = 4, P<0.012, Mann-Whitney U-test, one-sided, all cases), confirming the predictions made by visual examination of Figure 1A.
Fig. 3. Competition experiments for antibiotic-resistant mutants of wild-type and mutator E. coli. 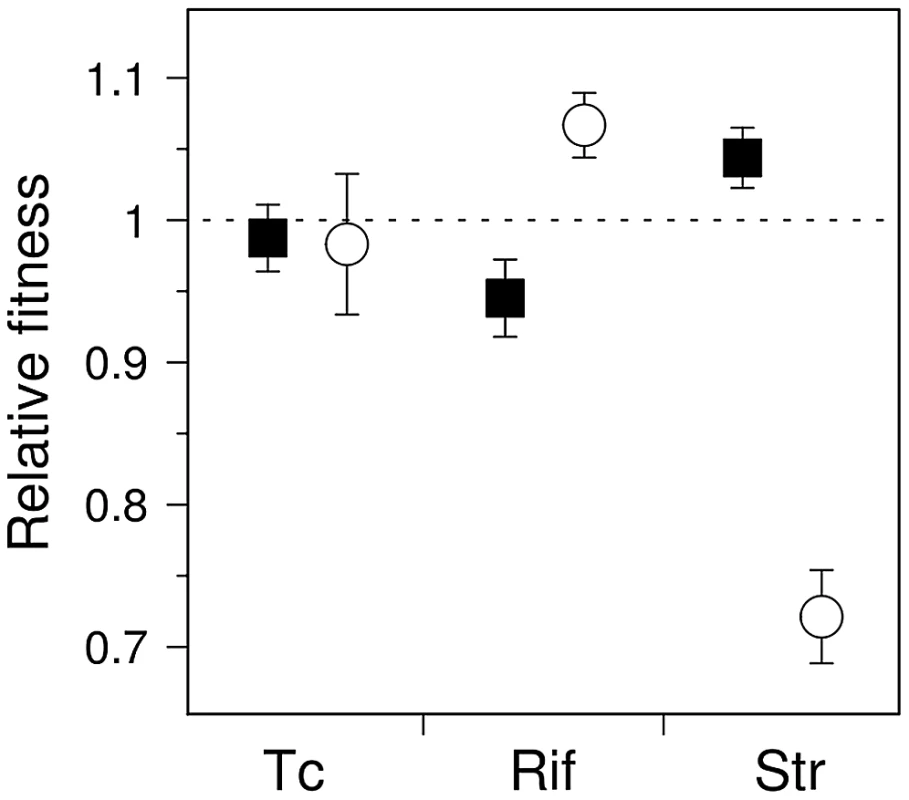
Fitness (mean ± SD; n = 4) of antibiotic-resistant mutants generated by ΔmutY (circles) and ΔmutT (squares). Fitness was calculated relative to the wild-type strain; dashed line indicates equal competitive ability. Note that the relative abundance of competing mutants is anticipated to vary among replications, as mutants were generated stochastically during the growth period prior to competition. Mutational spectrum effect on hitchhiking dynamics
Our data provide evidence that in a specific environment, distinct mutators generate their own fitness distribution among newly-arising mutants, which can influence their competitive ability. Theory predicts the fixation probability of a mutator allele to be dependent on the selection coefficient of the driver allele with which the mutator hitchhikes [11], [20]. According to our results, this coefficient can vary substantially depending on the mutational spectrum; the mutational spectrum should thus have some effect on the hitchhiking dynamics. To estimate how large this effect could be, we used simple computer simulations based on previous studies [10], [11], [20]. Briefly, we simulated the basic scenario of a non-mutator bacteria population growing in batch culture, where only one beneficial mutation is needed to achieve full adaptation. Mutators are generated at a constant rate, and produce the adaptive mutation with a 100-fold higher probability than the wild-type strain. Once the mutation is fixed in either background, the simulation ends. The mutational spectrum effect (σ) was introduced as a multiplicative factor to modify the selection coefficient (s) of the driver allele only on the mutator background (see Material and Methods).
The simulations showed that σ exerts a modest influence on the establishment of mutator genotypes bearing the adaptive mutation (i.e., on the probability that they escape random drift) (Figure 4). This effect is not surprising, as the probability of a beneficial mutation surviving drift is approximately 2s [33]. In contrast, σ had a notable effect on the fate of established genotypes en route to fixation. In the absence of mutational spectrum effects (σ = 1), a mutator genotype bearing the adaptive mutation can only succeed if it reaches fixation before any adapted wild-type bacteria escapes drift; it will otherwise always be outcompeted due to its increased deleterious mutation load (Figure 5, upper row). When the average s of the driver allele is lower on the mutator background (σ<1), there are no qualitative changes. Success is further hindered because drift is more intense and time to fixation is longer, extending the period available for the establishment and subsequent selective sweep of an adapted wild-type strain. In contrast, when σ>1 a threshold appears above which the population dynamics switches. This threshold is determined by the value of σ that offsets the increased deleterious load of mutator genotypes. Above this value, the adapted mutator is fitter than its wild-type counterpart, and therefore needs only to escape drift to reach fixation (Figure 5, lower row); as a consequence, fixation probability rises sharply (Figure 4). It is worth noting that, since the deleterious load is as small as the order of magnitude of the mutation rate [33], only a slight spectrum-dependent fitness advantage is needed to substantially increase mutator success.
Fig. 4. Computer simulation of mutational spectrum effects on hitchhiking dynamics. 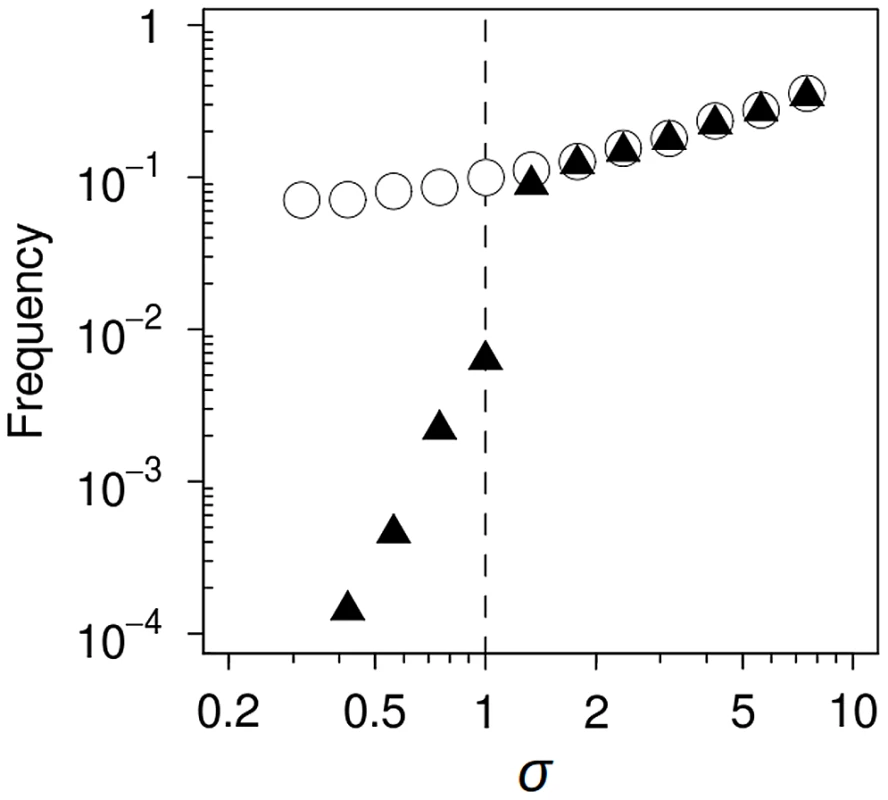
The mutational spectrum can bias the average fitness of mutants produced by a mutator compared to those produced by the wild-type strain. We modelled this bias as a multiplicative factor (σ), such that when σ = 1, there is no difference between the selective advantage conferred by the beneficial mutation on either background. Circles represent the frequency of trials in which a mutator allele escaped drift; triangles show the frequency of trials in which this allele reached fixation. Only slight variations in σ led to a profound effect on hitchhiking dynamics (see text for a definition of σ and a detailed explanation of the dynamics). Fig. 5. Mutational spectrum effect on hitchhiking dynamics. 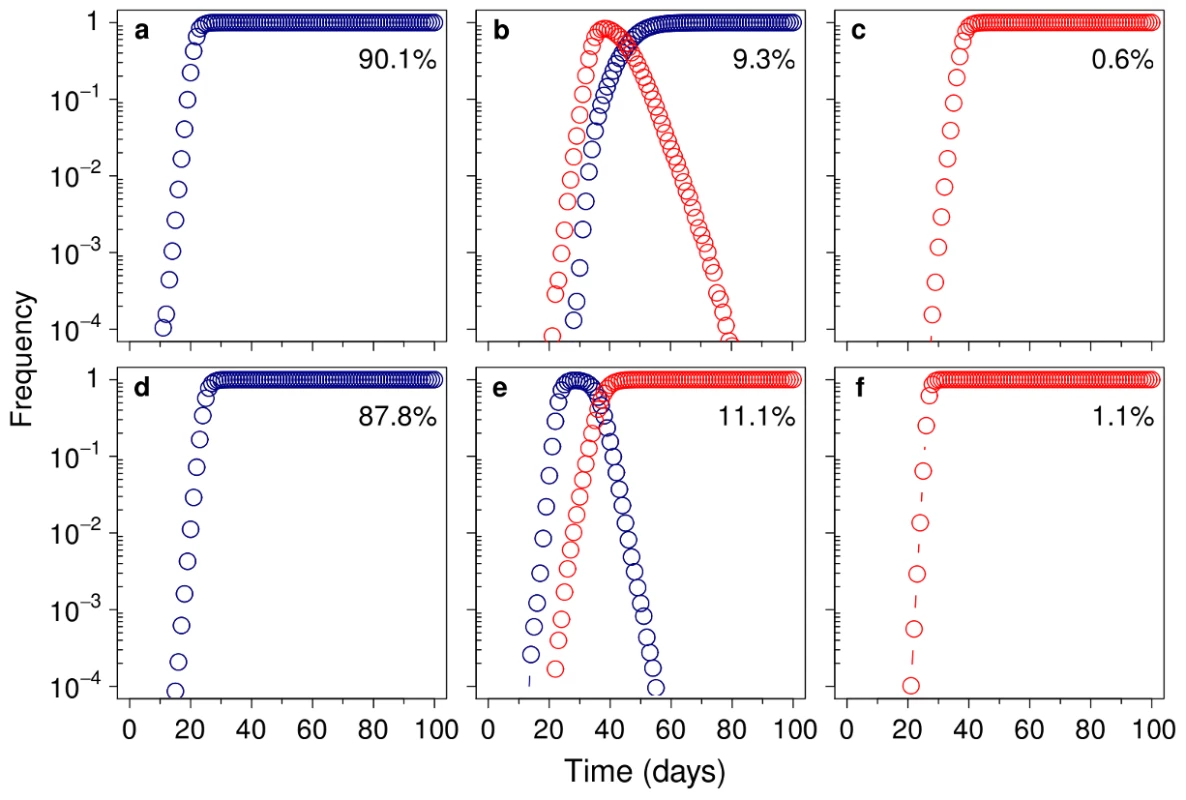
Representative runs showing the change in frequency of the adapted wild-type (100) (blue) and the adapted mutator (101) (red), for σ = 1 (A–C) and σ = 1.8 (D–F). The beneficial mutation confers a 10% increase in relative fitness (sb = 0.2), whereas each deleterious mutation reduces it by 2% (sd = 0.04). All the results can be classified into three main cases: only the adapted wild-type escapes drift (A and D), both genotypes escape drift (B and E), or only the adapted mutator escapes drift (C and F). Numbers are the percentage of runs (of 35,000) that correspond to each case. A–C, Without mutational spectrum effects (σ = 1), in 90.1% of cases the much larger wild-type subpopulation generates an adapted genotype that reaches fixation before any adapted mutator is established (A). In the cases in which both adapted genotypes coexist (9.3% of the total), the wild-type is always fixed, due its lower deleterious load (B). This confines mutator success to the remaining 0.6% of cases, in which they are able to reach fixation before any adapted wild-type genotype escapes drift (C). D–F, When mutational spectrum effects are taken into account (σ = 1.8), mutators not only escape drift more frequently but, due to their higher fitness, they are able to reach fixation even in the presence of an adapted wild-type (E). As a result, there is a marked increase in the percentage of cases in which mutators become fixed (11.1%+1.1% = 12.2% versus 0.6% when σ = 1). Remarkably, the non-linear response of fixation probability to σ implies that previous models could have been underestimating the likelihood of mutator success by several orders of magnitude. This is clearly illustrated in Figure 4 where a change from σ = 0.56 to σ = 1.33, which is equivalent to a change in relative fitness [37] from w = 0.96 to w = 1.03, represents a ∼196-fold increase in fixation probability.
Conclusions
Selection of mutators has been understood exclusively in terms of the increased number of beneficial mutations they generate [5], [10]. Here we show that not only the number but also the type of these linked mutations are relevant. Our results indicate that mutator alleles can bias the average selection coefficient of the beneficial alleles with which they hitchhike. Besides, they suggest that the magnitude of this effect can easily be sufficient to drastically modify their probabilities to reach fixation. The only requirement for this bias is that the locus or loci under selection produce mutants with some variability for fitness. This is a fairly permissive condition, likely to be satisfied in several adaptive scenarios. Examples in which resistant mutants with varying degrees of fitness are commonly found include bacteriophage [34] and antibiotic resistance [35], considered major drivers of mutator evolution [8], [15]. It will be of interest to determine whether the observed relative abundance of each mutator is explained, at least in part, by the effect of its mutational spectrum on successive mutations undergone during adaptation. In the context of clinical infections, future work should address the extent to which antibiotic therapies show different propensities to select for mutator bacteria. Since mutators are recognized as a risk factor for treatment failure [15]–[17], this knowledge could help to improve the design of safer therapeutic strategies.
The fact that even slight mutational spectrum effects markedly alter hitchhiking, together with the apparent commonness of circumstances that potentially allow this to happen, lead us to conclude that the mutational spectrum is a major factor in the evolution of mutators in laboratory and clinical populations of bacteria [3]–[8], as well as in certain cancers [36].
Materials and Methods
Strains and culture conditions
Strains are E. coli MG1655 derivatives, obtained from Dr. I. Matic [29]. Bacteria were grown on Luria broth (LB) or LB agar plates (37°C). Antibiotics used were tetracycline (15 mg/L), rifampicin (100 mg/L) and streptomycin (100 mg/L). Incubation time was 24 h, except for streptomycin plates (42 h).
Growth rate assays
Overnight cultures of each strain were plated on each antibiotic at appropriate dilutions to ensure low colony density (∼50/plate). After incubation, independent colonies were picked at random (by proximity to an arbitrary point) and resuspended in saline solution. Population size (N) was estimated from viable counts by subsequent dilution and plating. Assuming that each colony originated from a single cell, generation number was calculated as log2N and the growth rate expressed as number of generations per hour. Note that this measurement integrates growth rate over all growth phases.
Competition experiments
Overnight cultures of each mutator and wild-type strain were mixed at a ratio based on their mutation rates, plated on antibiotic, and allowed to compete for 24 or 42 h. Initial competitor frequency was calculated by estimating the resistant-mutant frequency of each overnight culture. To distinguish them from the wild-type bacteria, mutators carried antibiotic resistance markers [29], which entailed no significant fitness cost in these conditions (n = 4, P>0.28, Mann-Whitney U-test, two-sided, both cases). After incubation, agar plates were washed in saline solution and the final mutator∶wild-type ratio obtained by plating on LB agar and selective media. Relative fitness was estimated using a standard formula [37].
Computer simulation
(i) General description of the algorithm
We simulated the serial passage of an asexual population, originated from a single wild-type cell, in a laboratory environment in which only one beneficial mutation is available. The overall scheme is the same as in Tenaillon et al. [11], and can be summarized in two phases: (1) population growth, which includes differential reproduction and mutation during a number of generations, and (2) a random sampling process. Programming was carried out using the R programming language [38] (source code available from www.free-bit.org/public/acouce/).
Genotypes are defined by three loci: B, D and M. The first locus codes for the absence (b = 0) or presence (b = 1) of the beneficial mutation, the second locus controls the number of accumulated deleterious mutations (d), and the last encodes the absence (m = 0) or presence (m = 1) of a mutator allele with a 100-fold increase in mutation rate. In this model, the wild-type genotype, for example, will be unequivocally denoted by 000. In each generation, non-mutator individuals reproduce deterministically in accordance with the absolute fitness (rbd0) defined by their genotypes (bd0) as:
where sb and sd are the beneficial and deleterious selection coefficients, respectively. The population grows exponentially until its size exceeds a value of 108 individuals. Then, a random sample of 105 individuals is taken, a bottleneck of moderate strength [39], after which growth is resumed. Simulations end when the beneficial mutation is completely fixed in the wild-type or in the mutator background.(ii) Modeling the mutational spectrum effect
As we showed experimentally, the mutational spectrum can affect the average selection coefficient of the beneficial mutation with which a mutator allele ‘hitches a ride’. To implement this effect, we defined the the absolute fitness of mutators (bd1) as:
where σ represents this mutational spectrum effect.Care has been taken to ensure that equation (2) is only applied to adapted mutator genotypes (1d1) generated from a mutator background (0d1), but not to those generated in the rare but non-negligible case of an already adapted wild-type background (1d0) mutating the M locus.
(iii) Modeling transitions among genotypes
In each generation, after reproduction, all types of mutations are implemented by generating Poisson-distributed pseudorandom numbers [40], according to the population size of each genotype and a defined set of mutation rates (which act as the parameter of the Poisson distribution). Wild-type mutation rates were chosen to be in the range of estimated values for E. coli, 10−7 for beneficial mutations [41], 10−4 for deleterious mutations [42] and 5×10−6 for acquiring a mutator genotype [1]. Lethal mutation was introduced at a rate of 10−5, based on previous reports [10], [11]. Back-mutation was denied. One order of magnitude variation on these parameters showed no qualitative changes relative to the results shown here.
Zdroje
1. BoeL, DanielsenM, KnudsenS, PetersenJB, MaymannJ, et al. (2000) The frequency of mutators in populations of Escherichia coli. Mutat Res 448 : 47–55.
2. FunchainP, YeungA, StewartJL, LinR, SlupskaMM, et al. (2000) The consequences of growth of a mutator strain of Escherichia coli as measured by loss of function among multiple gene targets and loss of fitness. Genetics 154 : 959–970.
3. LeClercJE, LiB, PayneWL, CebulaTA (1996) High mutation frequencies among Escherichia coli and Salmonella pathogens. Science 274 : 1208–1211.
4. SniegowskiPD, GerrishPJ, LenskiRE (1997) Evolution of high mutation rates in experimental populations of E. coli. Nature 387 : 703–705.
5. MaoEF, LaneL, LeeJ, MillerJH (1997) Proliferation of mutators in a cell population. J Bacteriol 179 : 417–422.
6. OliverA, CantónR, CampoP, BaqueroF, BlázquezJ (2000) High frequency of hypermutable Pseudomonas aeruginosa in cystic fibrosis lung infection. Science 288 : 1251–1253.
7. RichardsonAR, YuZ, PopovicT, StojiljkovicI (2002) Mutator clones of Neisseria meningitidis in epidemic serogroup A disease. Proc Natl Acad Sci USA 99 : 6103–6107.
8. PalC, MaciaMD, OliverA, SchacharI, BucklingA (2007) Coevolution with viruses drives the evolution of bacterial mutation rates. Nature 450 : 1079–1081.
9. LeighEG (1970) Natural selection and mutability. Am Nat 104 : 301–305.
10. TaddeiF, RadmanM, Maynard-SmithJ, ToupanceB, GouyonPH, et al. (1997) Role of mutator alleles in adaptive evolution. Nature 387 : 700–702.
11. TenaillonO, ToupanceB, Le NagardH, TaddeiF, GodelleB (1999) Mutators, population size, adaptive landscape and the adaptation of asexual populations of bacteria. Genetics 152 : 485–493.
12. Notley-McRobbL, SeetoS, FerenciT (2002) Enrichment and elimination of mutY mutators in Escherichia coli populations. Genetics 162 : 1055–1062.
13. ShaverAC, DombrowskiPG, SweeneyJY, TreisT, ZappalaRM, et al. (2002) Fitness evolution and the rise of mutator alleles in experimental Escherichia coli populations. Genetics 162 : 557–566.
14. TenaillonO, Le NagardH, GodelleB, TaddeiF (2000) Mutators and sex in bacteria: Conflict between adaptive strategies. Proc Natl Acad Sci USA 97 : 10465–10470.
15. BlázquezJ (2003) Hypermutation as a factor contributing to the acquisition of antimicrobial resistance. Clin Infect Dis 37 : 1201–1209.
16. LabatF, PradillonO, GarryL, PeuchmaurM, FantinB, et al. (2005) Mutator phenotype confers advantage in Escherichia coli chronic urinary tract infection pathogenesis. FEMS Immunol. Med Microbiol 44 : 317–321.
17. OliverA, MenaA (2010) Bacterial hypermutation in cystic fibrosis, not only for antibiotic resistance. Clin Microbiol Infect 16 : 798–808.
18. TravisJMJ, TravisER (2002) Mutator dynamics in fluctuating environments. Proc Biol Sci 269 : 591–597.
19. TanakaMM, BergstromCT, LevinBR (2003) The evolution of mutator genes in bacterial populations: the roles of environmental change and timing. Genetics 164 : 843–854.
20. WylieCS, GhimCM, KesslerD, LevineH (2009) The fixation probability of rare mutators in finite asexual populations. Genetics 181 : 1595–1612.
21. AndreJ, GodelleB (2006) The evolution of mutation rate in finite asexual populations. Genetics 172 : 611–626.
22. MaharjanR, SeetoS, Notley-McRobbL, FerenciT (2006) Clonal adaptive radiation in a constant environment. Science 313 : 514–517.
23. TenaillonO, Rodríguez-VerdugoA, GautRL, McDonaldP, BennettAF, et al. (2012) The molecular diversity of adaptive convergence. Science 335 : 457–461.
24. DenamurE, MaticI (2006) Evolution of mutation rates in bacteria. Mol Microbiol 60 : 820–827.
25. MillerJH (1996) Spontaneous mutators in bacteria: insights into pathways of mutagenesis and repair. Annu Rev Microbiol 50 : 625–643.
26. MacLeanRC, HallAR, PerronGG, BucklingA (2010) The population genetics of antibiotic resistance: integrating molecular mechanisms and treatment contexts. Nat Rev Genet 11 : 405–414.
27. GaribyanL, HuangT, KimM, WolffE, NguyenA, et al. (2003) Use of the rpoB gene to determine the specificity of base substitution mutations on the Escherichia coli chromosome. DNA Repair 2 : 593–608.
28. TimmsAR, SteingrimsdottirH, LehmannAR, BridgesBA (1992) Mutant sequences in the rpsL gene of Escherichia coli B/r: mechanistic implications for spontaneous and ultraviolet light mutagenesis. Mol Gen Genet 232 : 89–96.
29. BjedovI, DasguptaCN, SladeD, Le BlastierS, SelvaM, et al. (2007) Involvement of Escherichia coli DNA polymerase IV in tolerance of cytotoxic alkylating DNA lesions in vivo. Genetics 176 : 1431–1440.
30. HallAR, IlesJC, MacLeanRC (2002) The fitness cost of rifampicin resistance in Pseudomonas aeruginosa depends on demand for RNA polymerase. Genetics 187 : 817–822.
31. ReynoldsMG (2000) Compensatory evolution in rifampin-resistant Escherichia coli. Genetics 156 : 1471–1481.
32. Kurland C, Hughes D, Ehrenberg M (1996) Limitations of translational accuracy. In: Neidhardt F.C III, et al., editors. Escherichia coli and Salmonella: Cellular and molecular biology. ASM Press; Washington, DC: 1996. pp. 979–1004.
33. Kimura M (1994) Population genetics, molecular evolution, and the neutral theory: selected papers (University of Chicago Press)
34. BohannanBJ, LenskiRE (2000) Linking genetic change to community evolution: insights from studies of bacteria and bacteriophage. Ecol Lett 3 : 362–377.
35. AnderssonDI, HughesD (2010) Antibiotic resistance and its cost: is it possible to reverse resistance? Nat Rev Micro 8 : 260–271.
36. PrindleMJ, FoxEJ, LoebLA (2010) The mutator phenotype in cancer: molecular mechanisms and targeting strategies. Curr. Drug Targets 11 : 1296–1303.
37. LenskiRE, RoseMR, SimpsonSC, TadlerSC (1991) Long-term experimental evolution in Escherichia coli I. Adaptation and divergence during 2,000 generations. Am Nat 138 : 1315–1341.
38. R Development Core Team (2011) R: A language and environment for statistical computing, reference index version 2.13.0. (R Foundation for Statistical Computing, Vienna).
39. de VisserJA, ZeylCW, GerrishPJ, BlanchardJL, LenskiRE (1999) Diminishing returns from mutation supply rate in asexual populations. Science 283 : 404–406.
40. AhrensJH, DieterU (1982) Computer generation of Poisson deviates from modified normal distributions. ACM Trans Math Softw 8 : 163–179.
41. RozenDE, de VisserJAGM, GerrishPJ (2002) Fitness effects of fixed beneficial mutations in microbial populations. Curr Biol 12 : 1040–1045.
42. TrindadeS, PerfeitoL, GordoI (2010) Rate and effects of spontaneous mutations that affect fitness in mutator Escherichia coli. Philos Trans R Soc Lond B Biol Sci 365 : 1177–1186.
Štítky
Genetika Reprodukční medicína
Článek Comparative Genome Structure, Secondary Metabolite, and Effector Coding Capacity across PathogensČlánek TATES: Efficient Multivariate Genotype-Phenotype Analysis for Genome-Wide Association StudiesČlánek Secondary Metabolism and Development Is Mediated by LlmF Control of VeA Subcellular Localization inČlánek Human Disease-Associated Genetic Variation Impacts Large Intergenic Non-Coding RNA ExpressionČlánek The Roles of Whole-Genome and Small-Scale Duplications in the Functional Specialization of GenesČlánek The Role of Autophagy in Genome Stability through Suppression of Abnormal Mitosis under Starvation
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2013 Číslo 1
-
Všechny články tohoto čísla
- A Model of High Sugar Diet-Induced Cardiomyopathy
- Comparative Genome Structure, Secondary Metabolite, and Effector Coding Capacity across Pathogens
- Emerging Function of Fat Mass and Obesity-Associated Protein (Fto)
- Positional Cloning Reveals Strain-Dependent Expression of to Alter Susceptibility to Bleomycin-Induced Pulmonary Fibrosis in Mice
- Genetics of Ribosomal Proteins: “Curiouser and Curiouser”
- Transposable Elements Re-Wire and Fine-Tune the Transcriptome
- Function and Regulation of , a Gene Implicated in Autism and Human Evolution
- MAML1 Enhances the Transcriptional Activity of Runx2 and Plays a Role in Bone Development
- Predicting Mendelian Disease-Causing Non-Synonymous Single Nucleotide Variants in Exome Sequencing Studies
- A Systematic Mapping Approach of 16q12.2/ and BMI in More Than 20,000 African Americans Narrows in on the Underlying Functional Variation: Results from the Population Architecture using Genomics and Epidemiology (PAGE) Study
- Transcription of the Major microRNA–Like Small RNAs Relies on RNA Polymerase III
- Histone H3K56 Acetylation, Rad52, and Non-DNA Repair Factors Control Double-Strand Break Repair Choice with the Sister Chromatid
- Genome-Wide Association Study Identifies a Novel Susceptibility Locus at 12q23.1 for Lung Squamous Cell Carcinoma in Han Chinese
- Genetic Disruption of the Copulatory Plug in Mice Leads to Severely Reduced Fertility
- The [] Prion Exists as a Dynamic Cloud of Variants
- Adult Onset Global Loss of the Gene Alters Body Composition and Metabolism in the Mouse
- Fis Protein Insulates the Gene from Uncontrolled Transcription
- The Meiotic Nuclear Lamina Regulates Chromosome Dynamics and Promotes Efficient Homologous Recombination in the Mouse
- Genome-Wide Haplotype Analysis of Expression Quantitative Trait Loci in Monocytes
- TATES: Efficient Multivariate Genotype-Phenotype Analysis for Genome-Wide Association Studies
- Structural Basis of a Histone H3 Lysine 4 Demethylase Required for Stem Elongation in Rice
- The Ecm11-Gmc2 Complex Promotes Synaptonemal Complex Formation through Assembly of Transverse Filaments in Budding Yeast
- MCM8 Is Required for a Pathway of Meiotic Double-Strand Break Repair Independent of DMC1 in
- Comparative Genomic Analysis of the Endosymbionts of Herbivorous Insects Reveals Eco-Environmental Adaptations: Biotechnology Applications
- Integration of Nodal and BMP Signals in the Heart Requires FoxH1 to Create Left–Right Differences in Cell Migration Rates That Direct Cardiac Asymmetry
- Pharmacodynamics, Population Dynamics, and the Evolution of Persistence in
- A Hybrid Likelihood Model for Sequence-Based Disease Association Studies
- Aberration in DNA Methylation in B-Cell Lymphomas Has a Complex Origin and Increases with Disease Severity
- Multiple Opposing Constraints Govern Chromosome Interactions during Meiosis
- Transcriptional Dynamics Elicited by a Short Pulse of Notch Activation Involves Feed-Forward Regulation by Genes
- Dynamic Large-Scale Chromosomal Rearrangements Fuel Rapid Adaptation in Yeast Populations
- Heterologous Gln/Asn-Rich Proteins Impede the Propagation of Yeast Prions by Altering Chaperone Availability
- Gene Copy-Number Polymorphism Caused by Retrotransposition in Humans
- An Incompatibility between a Mitochondrial tRNA and Its Nuclear-Encoded tRNA Synthetase Compromises Development and Fitness in
- Secondary Metabolism and Development Is Mediated by LlmF Control of VeA Subcellular Localization in
- Single-Stranded Annealing Induced by Re-Initiation of Replication Origins Provides a Novel and Efficient Mechanism for Generating Copy Number Expansion via Non-Allelic Homologous Recombination
- Tbx2 Controls Lung Growth by Direct Repression of the Cell Cycle Inhibitor Genes and
- Suv4-20h Histone Methyltransferases Promote Neuroectodermal Differentiation by Silencing the Pluripotency-Associated Oct-25 Gene
- A Conserved Helicase Processivity Factor Is Needed for Conjugation and Replication of an Integrative and Conjugative Element
- Telomerase-Null Survivor Screening Identifies Novel Telomere Recombination Regulators
- Genome-Wide Analysis Reveals Selection for Important Traits in Domestic Horse Breeds
- Coordinated Degradation of Replisome Components Ensures Genome Stability upon Replication Stress in the Absence of the Replication Fork Protection Complex
- Nkx6.1 Controls a Gene Regulatory Network Required for Establishing and Maintaining Pancreatic Beta Cell Identity
- HIF- and Non-HIF-Regulated Hypoxic Responses Require the Estrogen-Related Receptor in
- Delineating a Conserved Genetic Cassette Promoting Outgrowth of Body Appendages
- The Telomere Capping Complex CST Has an Unusual Stoichiometry, Makes Multipartite Interaction with G-Tails, and Unfolds Higher-Order G-Tail Structures
- Comprehensive Methylome Characterization of and at Single-Base Resolution
- Loci Associated with -Glycosylation of Human Immunoglobulin G Show Pleiotropy with Autoimmune Diseases and Haematological Cancers
- Switchgrass Genomic Diversity, Ploidy, and Evolution: Novel Insights from a Network-Based SNP Discovery Protocol
- Centromere-Like Regions in the Budding Yeast Genome
- Sequencing of Loci from the Elephant Shark Reveals a Family of Genes in Vertebrate Genomes, Forged by Ancient Duplications and Divergences
- Mendelian and Non-Mendelian Regulation of Gene Expression in Maize
- Mutational Spectrum Drives the Rise of Mutator Bacteria
- Human Disease-Associated Genetic Variation Impacts Large Intergenic Non-Coding RNA Expression
- The Roles of Whole-Genome and Small-Scale Duplications in the Functional Specialization of Genes
- Sex-Specific Signaling in the Blood–Brain Barrier Is Required for Male Courtship in
- A Newly Uncovered Group of Distantly Related Lysine Methyltransferases Preferentially Interact with Molecular Chaperones to Regulate Their Activity
- Is Required for Leptin-Mediated Depolarization of POMC Neurons in the Hypothalamic Arcuate Nucleus in Mice
- Unlocking the Bottleneck in Forward Genetics Using Whole-Genome Sequencing and Identity by Descent to Isolate Causative Mutations
- The Role of Autophagy in Genome Stability through Suppression of Abnormal Mitosis under Starvation
- MTERF3 Regulates Mitochondrial Ribosome Biogenesis in Invertebrates and Mammals
- Downregulation and Altered Splicing by in a Mouse Model of Facioscapulohumeral Muscular Dystrophy (FSHD)
- NBR1-Mediated Selective Autophagy Targets Insoluble Ubiquitinated Protein Aggregates in Plant Stress Responses
- Retroactive Maintains Cuticle Integrity by Promoting the Trafficking of Knickkopf into the Procuticle of
- Phenome-Wide Association Study (PheWAS) for Detection of Pleiotropy within the Population Architecture using Genomics and Epidemiology (PAGE) Network
- Genetic and Functional Modularity of Activities in the Specification of Limb-Innervating Motor Neurons
- A Population Genetic Model for the Maintenance of R2 Retrotransposons in rRNA Gene Loci
- A Quartet of PIF bHLH Factors Provides a Transcriptionally Centered Signaling Hub That Regulates Seedling Morphogenesis through Differential Expression-Patterning of Shared Target Genes in
- A Genome-Wide Integrative Genomic Study Localizes Genetic Factors Influencing Antibodies against Epstein-Barr Virus Nuclear Antigen 1 (EBNA-1)
- Mutation of the Diamond-Blackfan Anemia Gene in Mouse Results in Morphological and Neuroanatomical Phenotypes
- Life, the Universe, and Everything: An Interview with David Haussler
- Alternative Oxidase Expression in the Mouse Enables Bypassing Cytochrome Oxidase Blockade and Limits Mitochondrial ROS Overproduction
- An Evolutionarily Conserved Synthetic Lethal Interaction Network Identifies FEN1 as a Broad-Spectrum Target for Anticancer Therapeutic Development
- The Flowering Repressor Underlies a Novel QTL Interacting with the Genetic Background
- Telomerase Is Required for Zebrafish Lifespan
- and Diversified Expression of the Gene Family Bolster the Floral Stem Cell Network
- Susceptibility Loci Associated with Specific and Shared Subtypes of Lymphoid Malignancies
- An Insertion in 5′ Flanking Region of Causes Blue Eggshell in the Chicken
- Increased Maternal Genome Dosage Bypasses the Requirement of the FIS Polycomb Repressive Complex 2 in Arabidopsis Seed Development
- WNK1/HSN2 Mutation in Human Peripheral Neuropathy Deregulates Expression and Posterior Lateral Line Development in Zebrafish ()
- Synergistic Interaction of Rnf8 and p53 in the Protection against Genomic Instability and Tumorigenesis
- Dot1-Dependent Histone H3K79 Methylation Promotes Activation of the Mek1 Meiotic Checkpoint Effector Kinase by Regulating the Hop1 Adaptor
- A Heterogeneous Mixture of F-Series Prostaglandins Promotes Sperm Guidance in the Reproductive Tract
- Starvation, Together with the SOS Response, Mediates High Biofilm-Specific Tolerance to the Fluoroquinolone Ofloxacin
- Directed Evolution of a Model Primordial Enzyme Provides Insights into the Development of the Genetic Code
- Genome-Wide Screens for Tinman Binding Sites Identify Cardiac Enhancers with Diverse Functional Architectures
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Function and Regulation of , a Gene Implicated in Autism and Human Evolution
- An Insertion in 5′ Flanking Region of Causes Blue Eggshell in the Chicken
- Comprehensive Methylome Characterization of and at Single-Base Resolution
- Susceptibility Loci Associated with Specific and Shared Subtypes of Lymphoid Malignancies
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání





