-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaA Model of High Sugar Diet-Induced Cardiomyopathy
Diets high in carbohydrates have long been linked to progressive heart dysfunction, yet the mechanisms by which chronic high sugar leads to heart failure remain poorly understood. Here we combine diet, genetics, and physiology to establish an adult Drosophila melanogaster model of chronic high sugar-induced heart disease. We demonstrate deterioration of heart function accompanied by fibrosis-like collagen accumulation, insulin signaling defects, and fat accumulation. The result was a shorter life span that was more severe in the presence of reduced insulin and P38 signaling. We provide evidence of a role for hexosamine flux, a metabolic pathway accessed by glucose. Increased hexosamine flux led to heart function defects and structural damage; conversely, cardiac-specific reduction of pathway activity prevented sugar-induced heart dysfunction. Our data establish Drosophila as a useful system for exploring specific aspects of diet-induced heart dysfunction and emphasize enzymes within the hexosamine biosynthetic pathway as candidate therapeutic targets.
Published in the journal: . PLoS Genet 9(1): e32767. doi:10.1371/journal.pgen.1003175
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003175Summary
Diets high in carbohydrates have long been linked to progressive heart dysfunction, yet the mechanisms by which chronic high sugar leads to heart failure remain poorly understood. Here we combine diet, genetics, and physiology to establish an adult Drosophila melanogaster model of chronic high sugar-induced heart disease. We demonstrate deterioration of heart function accompanied by fibrosis-like collagen accumulation, insulin signaling defects, and fat accumulation. The result was a shorter life span that was more severe in the presence of reduced insulin and P38 signaling. We provide evidence of a role for hexosamine flux, a metabolic pathway accessed by glucose. Increased hexosamine flux led to heart function defects and structural damage; conversely, cardiac-specific reduction of pathway activity prevented sugar-induced heart dysfunction. Our data establish Drosophila as a useful system for exploring specific aspects of diet-induced heart dysfunction and emphasize enzymes within the hexosamine biosynthetic pathway as candidate therapeutic targets.
Introduction
Diet-mediated diseases represent an increasing challenge in Western society. Particular attention has focused recently on carbohydrate consumption, which has increased as much as 41% in the past three decades [1]. Dietary sugars have in turn been linked a variety of metabolism-related problems including obesity, insulin resistance, metabolic syndrome, and type 2 diabetes mellitus (T2DM). Heart tissue is thought to be especially sensitive to changes in sugar and insulin flux [2]. Elevated levels of hemoglobin A1c—a measure of long-term blood glucose levels—is an independent risk factor for heart disease in both diabetics and non-diabetics [3]. Regular consumption of sugar-sweetened beverages is associated with a higher risk of coronary heart disease [4]. In addition to coronary heart disease progression of T2DM can lead to diabetic cardiomyopathy, defined as functional or structural defects of myocardial structures in the absence of coronary artery disease or hypertension [5]. As a result, the American Heart Association has recently recommended limiting sources of sugar in the diet [6]. Despite important advances in our understanding of the effects of dietary sugars, our knowledge of the mechanisms that direct sugar-induced heart disease remains incomplete.
Drosophila melanogaster provides a useful complement to mammalian models. Their short life span and powerful genetic tools permit detailed in situ organ analysis. While flies show important differences from their mammalian counterparts, they also show marked similarities. For example, the fly genome encodes seven insulin-like peptide genes (Dilp 1–7) that activate classical insulin pathway signaling [7], [8]. Ablation of insulin producing cells phenocopied several aspects of type 1 diabetes [9]. Further, recent data have indicated that Drosophila is susceptible to diet-mediated metabolic and cardiac organ dysfunctions that are reminiscent of those reported in mammals [10]–[14].
The Drosophila heart shows both similarities and differences to the mammalian heart. It is a linear heart tube that is divided into four chambers by rudimentary valve-like structures. Drosophila have an open circulatory system with a separate tracheal system used for oxygen transport and their hearts are devoid of coronary arteries [15]–[17]. This separation of oxygen delivery from cardiac pumping function has the advantage that alterations in heart function do not immediately affect viability. Figure 1A demonstrates the ventral view of the heart, showing the longitudinal and alary muscles that cover and stabilize the heart tube, respectively [18]. Figure 1B provides a dorsal view of the heart, showing the myocardial cells that form the heart tube and ostia that provide an entry point for hemolymph. Conserved mechanisms of heart development and function are shared between flies and vertebrates [19]–[22]. Recently, increasingly robust tools have been developed to image the fly heart and to characterize its physiological function [15], [23]–[25]. A Drosophila age-related heart disease model has been established and several genes that regulate age-mediated damage have been identified [17], [22], [25]–[28]. Recently, a high fat diet-induced obesity model has been developed in Drosophila that leads to severe cardiac malfunction, demonstrating the utility of this genetic system for studying fundamental aspects of organismal metabolic disorders [29]. Drosophila is limited as a model for particular aspects of diabetes and diabetic cardiomyopathy including hypertension and vascular defects. Nevertheless, it provides an opportunity to explore specific aspects of metabolic dysfunction and heart function.
Fig. 1. Drosophila model of diabetic cardiomyopathy. 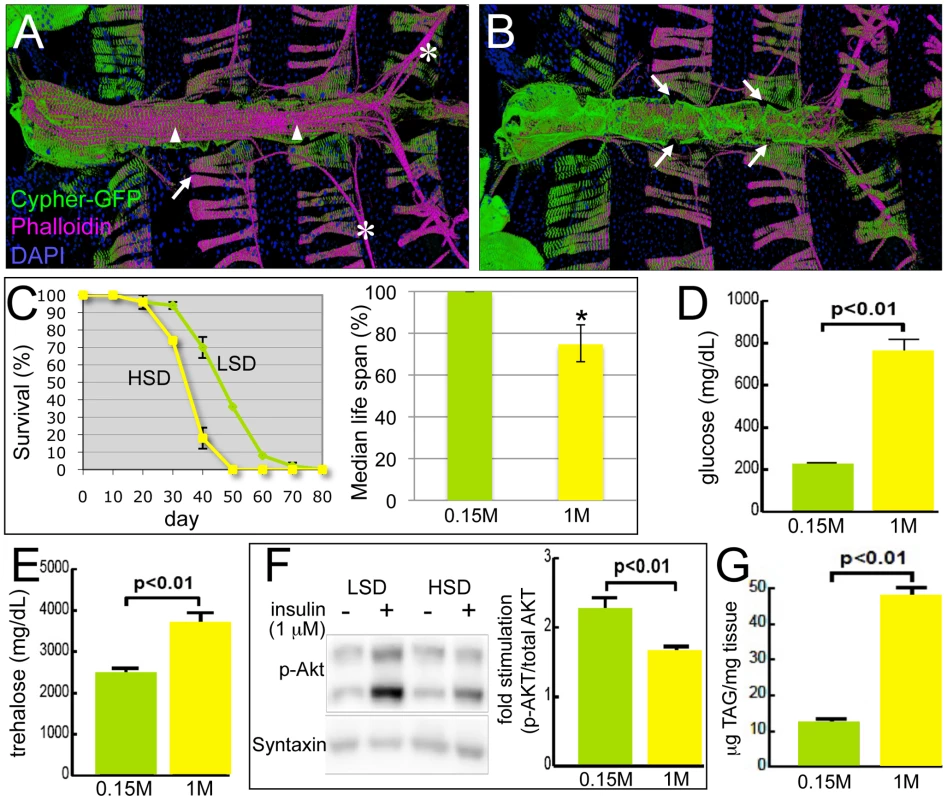
(A) Ventral view of the heart tube. 3D structures are shown. The heart was stained for F-Actin with phalloidin (magenta) and for nuclei with DAPI; Cypher-GFP (green) labeled Z-lines of myofibers within cardiomyocytes. Arrowheads indicate the non-myocardial longitudinal muscle fibers, asterisks the alary muscles that support the heart, and arrows the abdominal muscles. (B) Dorsal view of the heart tube. Myocardial cells wrap in a circular fashion around the central cavity. Arrows show the ostia through which hemolymph from the abdomen enters into the heart tube and circulates. (C) HSD significantly reduced life span. w1118 male flies were raised in 0.15 M or 1.0 M sucrose diet, food was changed every 2–3 days, and flies were counted every 10 days. HSD-fed flies displayed decreased median life span, 10 days shorter than flies fed an LSD. Mean ± SE are shown; n = 50 total flies in two separate experiments. The estimated median life span of HSD flies was also expressed as the percentage of LSD flies (*p = 7.61E-08 by log rank test). See also Figure S1. (D) Hemolymph glucose concentrations in 3-week-old, control and HSD-fed w1118 adult flies. n≥6. (E) Hemolymph trehalose concentrations in 3-week-old, control and high sucrose-fed w1118 adult flies. n≥6. (F) Bodies from w1118 adults fed LSD or HSD for 3 weeks were treated with insulin (1 µM) or vehicle and visualized using antibodies against Drosophila PO4-Akt or Syntaxin. n = 10. Bands from Western blot experiments were quantified, and PO4-Akt was normalized to Syntaxin as a loading control. (G) Total triglycerides (TAG) were assayed enzymatically in 3-week-old control and high sugar-fed w1118 adult flies, and normalized to weight. n≥12. Mean ± SE are shown. An unpaired, two-tailed t-test was used to derive p-values. Here we develop the Drosophila heart as a new model for the study of diet-induced heart dysfunction. Flies were raised on a high-sucrose diet (HSD) to provoke specific aspects of diet-induced metabolic dysfunction including aspects of T2DM. We demonstrate progressive and specific dysfunctions emerging in the hearts of adult flies fed an HSD. We further validate our model by demonstrating that two pathways previously shown to mediate heart dysfunction in mammals — the insulin and P38 MAPK pathways — modulate HSD-induced heart defects in Drosophila as well. Finally, we present evidence that dietary sucrose directs heart damage in part by its flux through the hexosamine biosynthetic pathway. Increasing hexosamine flux phenocopied sugar-mediated heart dysfunction and also led to structural damage. Importantly, decreasing pathway activity led to a significant reduction in sucrose-mediated heart damage, suggesting specific enzyme targets that may prove useful for reducing the effects of high dietary sugars on heart function.
Results
High dietary sugar shortened Drosophila life span and was associated with increased cardiac arrhythmia and heart deterioration
To explore the effects of high carbohydrate feeding and to determine whether Drosophila can show diet-mediated heart dysfunction, we compared adults fed an HSD (standard media brought to a final concentration of 1.0 M sucrose) vs. control low sucrose diet (LSD, 0.15 M sucrose). One characteristic aspect of metabolic dysfunctions including obesity, T2DM, and heart disease is shortened average life expectancy [30]. Adult flies fed an HSD after eclosion exhibited an average life span reduction of approximately ten days compared to those fed an LSD (Figure 1C and Figure S1; [31]). In addition, they exhibited phenotypes typical of metabolic syndrome and T2DM patients including hyperglycemia, hypertrehalosemia, peripheral resistance to exogenous insulin, and accumulation of triglyceride (Figure 1D–1G). These are similar to HSD effects reported for Drosophila larvae [11].
No significant difference was observed in the survival of high sucrose - and low sucrose-fed flies in the first three weeks (Figure 1C) permitting us to use this period to explore the effects of diet on the heart function of fully viable animals. Figure 2A presents an M-mode for flies raised on LSD. By contrast, flies raised on HSD exhibited a deteriorating heartbeat as manifested by the irregular beating patterns (Figure 2A; Videos S1, S2). Arrhythmias observed by M-mode analysis can be quantified as the arrhythmia index [25]. High dietary sucrose significantly increased the arrhythmia index (Figure 2B; 0.16 LSD vs. 0.44 HSD, *P = 1.54E-17 by F-test). However heart period and fractional shortening, a measure of heart contractility, were normal at this age (Figure 2C, 2D).
Fig. 2. High sucrose shortened Drosophila life span and was associated with increased cardiac arrhythmia and heart deterioration. 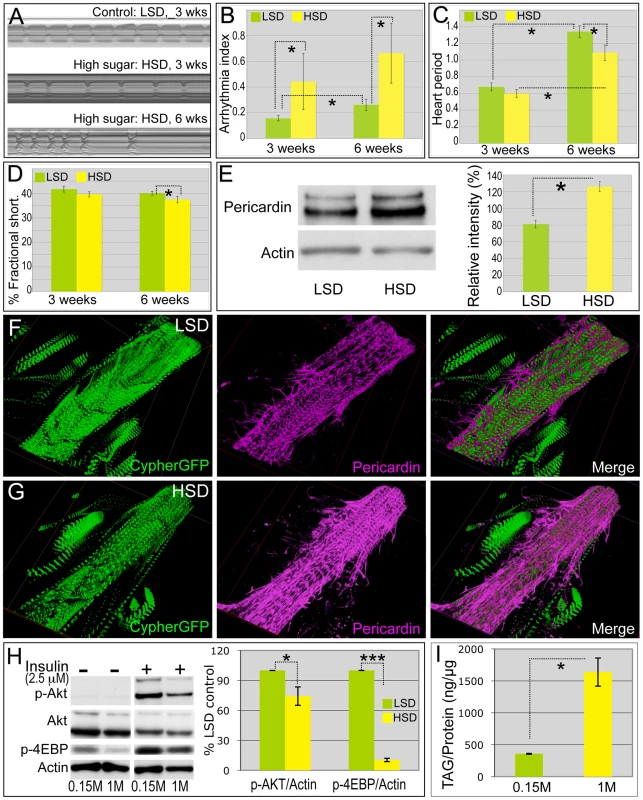
(A) Representative M-mode (5 seconds) from flies fed LSD and HSD. Three-week-old adults fed an HSD showed moderate cardiac arrhythmia; at six weeks arrhythmicity was increased. (B) Arrhythmia index obtained from w1118 flies fed LSD and HSD. Arrhythmias observed in M-mode can be quantified as arrhythmia index, which is the standard deviation of all heart periods in each record normalized to the median heart period for each fly. Mean ± SE are shown. At week three, a significant increase in arrhythmia index was observed in HSD fed flies (0.44) compared to low sucrose fed flies (0.16) (*P = 1.54E-17 by F-test). Arrhythmia index of six-week-old flies increased to 0.66 in HSD and 0.26 in low sucrose diet, respectively (*P = 6.01E-12 by F-test). Data are means ± SE. (C) Heart period of adult files fed low vs. high dietary sucrose. At three weeks of age, no difference was observed between HSD- and LSD-fed flies. Heart period was significantly increased at six weeks of age in both HSD- and LSD-fed flies (*P = 9.64E-06 and 1.44E-09, respectively, by t-test). Interestingly at six weeks of age, heart period of HSD-fed flies was shorter than that of low sucrose fed flies (*P = 0.015 by t-test). Data are means ± SE. (D) Fractional shortening of adult flies fed low vs. high dietary sucrose. At three weeks of age, no difference was observed between HSD- and LSD-fed flies; however, fractional shortening was significantly decreased in flies fed HSD- vs. LSD-fed (*P = 0.043 by t-test). Data are means ± SE. (E) Quantification of Pericardin level of adult heart by Western blot. Eight hearts from three-week-old LSD- and HSD-fed flies, respectively, were loaded. Pericardin level was detected by a monoclonal antibody against Pericardin, and normalized to Actin level. (F,G) Representative confocal images of three-week-old adult fly hearts expressing Cypher-GFP (posterior A2/anterior A3 segment) and stained with anti-Pericardin (magenta) antibody. Pericardin levels in hearts of flies fed an HSD were increased compared to those fed low sucrose. Note that Pericardin was detected in heart tissue but not abdominal muscles. See also Figure S4. (H) Fly hearts from w1118 adults fed LSD or HSD were treated with insulin (2.5 µM) or vehicle and visualized using antibodies against Drosophila PO4-Akt, PO4-4EBP or Actin, showing the response of the heart to exogenous insulin challenge. n = 3. Bands from Western blot experiments were quantified, and PO4-Akt and PO4-4EBP were normalized to Actin as a loading control. The ratio of HSD fed flies was then normalized to that of LSD fed flies. PO4-Akt and PO4-4EBP level were 74.3% and 13.9%, respectively, in HSD-fed flies compared to LSD-fed flies (P = 0.049 and 0.0003, respectively, by t-test). (I) Heart accumulated triglycerides (TAG) were assayed enzymatically in 15 hearts from 3-week-old LSD- and HSD-fed w1118 adult flies, and normalized to protein level. n = 2. (P = 0.028 by t-test). See also Figure S4 and Videos S1, S2, S3. Sucrose-mediated mortality and heart defects increased significantly as the flies were further aged to six weeks. Less than 20% of adult flies survived past 40 days on an HSD compared to 70% fed an LSD (Figure 1C). Consistent with previous findings [25], flies fed an LSD displayed a moderate increase in their arrhythmia index and heart period as they aged (Figure 2B, 2C). Six-week-old animals exhibited more severe heart phenotypes including fibrillations, asystolic periods and arrhythmia; these defects were significantly enhanced in animals fed an HSD, which also exhibited moderate but consistent defects in fractional shortening (Figure 2A–2D, Video S3). We observed similar effects with a 1.0 M sucrose corn meal-based food (Figure S2), further indicating that increased arrhythmias and decreased fractional shortening are due specifically to dietary sugar.
These defects indicated a potential for emergent structural defects and/or metabolic defects. Cardiac fibrosis is a prominent aspect of heart damage that commonly emerges in diabetic patients [32], [33]. To explore this in flies, we examined collagen levels within the fly heart with an antibody against Pericardin, a Drosophila type IV collagen-like protein. Notably, by three weeks of age Pericardin levels and the fibrous extracellular meshwork were significantly and consistently increased within the heart tissue of adult flies fed an HSD, compared to those fed an LSD (Figure 2E–2G).
In addition, the response of the heart to insulin stimulation was defective in HSD-raised flies as manifested by diminished phosphorylation of AKT and 4EBP (Figure 2H and Figure S3). These hearts also accumulated excessive triglyceride (Figure 2I). The observed shortened life span, progressive deterioration of specific heart functions, accumulation of collagen and triglycerides, and aspects of insulin resistance indicate that our HSD feeding regimen represents a useful Drosophila model of diet-mediated heart disease. We next explored the molecular mechanisms by which high dietary sucrose led to heart dysfunction.
P38 and insulin pathways modulate sucrose-induced heart deterioration
P38 is a stress-activated kinase of the mitogen-activated protein kinase (MAPK) superfamily that is activated in models of T2DM. Activation is thought to be related to oxidative stress: for example, the antioxidant lipoic acid increases P38 activation [34] and leads to improved insulin sensitivity in diabetic patients [35], [36]. The Drosophila P38 paralog P38A acts as a prototypical stress kinase, and its impairment leads to dysfunction in the presence of various environmental insults [37]. In larvae, metabolic dysfunction can be assayed by assessing the rate of development to pupariation [11]. Larvae homozygous for a null mutation of p38A exhibited a slight developmental delay when fed an LSD, requiring an extra day to develop to pupariation; this difference was strongly enhanced when animals were raised on an HSD as larvae, resulting in a four day delay (Figure 3A).
Fig. 3. Mutations in p38A and chico attenuate high sucrose-induced heart deterioration. 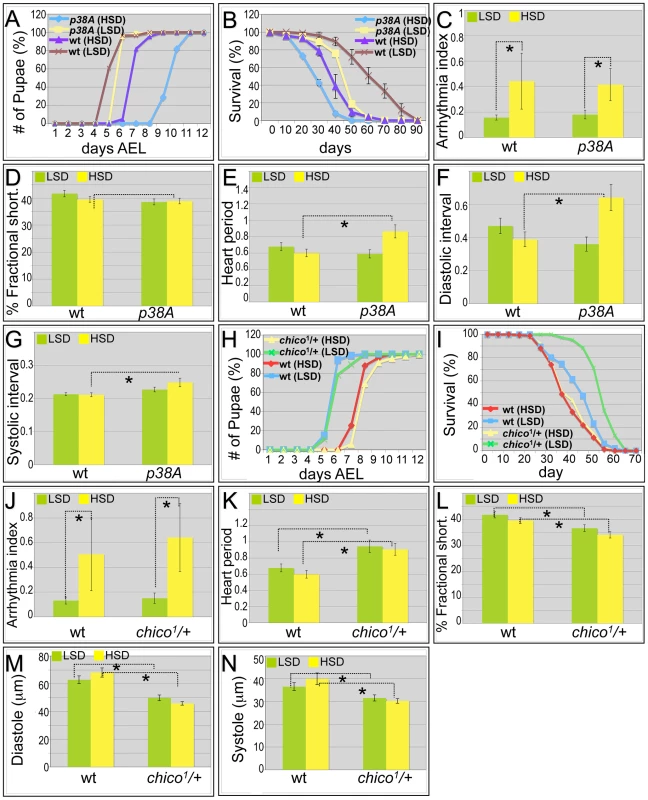
(A) Mutations in p38A enhanced developmental delay of larvae fed an HSD. Eggs were collected onto LSD or HSD food for 16 hours, then permitted to mature at 25°C. Time to pupariation was scored daily. HSD feeding resulted in two days' development delay for w1118 flies. On low sucrose food, p38A mutations exhibited a slight delay compared to w1118 controls; an HSD led to a four-day delay to pupariation. (B) Mutant p38A flies fed high dietary sucrose exhibited reduced life span. Experiments for both p38A mutants and w1118 controls were performed at 22°C due to poor viability of developing p38A mutant flies grown at 25°C on an HSD. The average life span of p38A flies was ten days shorter than w1118 controls fed an HSD. (C) Loss of p38A activity did not alter high dietary sucrose-induced cardiac arrhythmia. Arrhythmia index obtained from w1118 controls and p38A mutants. The differences between LSD and HSD both in wild type controls and p38A mutants were significant (*P = 1.54E-17, and 1.20E-08, respectively, by F-test). However, there was no difference between w1118 controls and p38A mutants. Data are means ± SE. (D) No difference in fractional shortening was observed between w1118 controls and p38A mutants grown in either an LSD or an HSD. Data indicate mean ± SE. (E) Heart period was increased in p38A mutants fed an HSD. The heart period of p38A mutants fed on HSD was 0.86 second, compared to 0.60 second in w1118 controls (*P = 0.008 by t-test). Data indicate mean ± SE. (F) Diastolic interval was increased in p38A mutants fed an HSD. Diastolic interval of p38A mutants fed an HSD was 0.64 second, compared to 0.39 second in w1118 controls (*P = 0.01 by t-test). Data indicate mean ± SE. (G) Systolic interval was increased in in p38A mutants fed an HSD. Systolic interval of p38A mutants fed an HSD was 0.25 second, compared to 0.21 second in w1118 controls (*P = 0.02 by t-test). Data indicate mean ± SE. (H) No difference was observed in developmental rates between w1118 controls and chico1/+ adults fed either an LSD or HSD. Data indicate mean ± SE. (I) Increased life span was observed in chico1/+ adults compared to w1118 controls when both were fed an LSD. However, the observed average life span in HSD is 35 days in chico1/+ mutants, the same as w1118 controls. Data indicate mean ± SE. (J) chico1 mutation did not alter high dietary sucrose-induced cardiac arrhythmia. Arrhythmia index obtained from w1118 controls and chico1/+ mutants. The differences between LSD and HSD both in wild type controls and chico1/+ mutants were significant (*P = 1.54E-17, and 2.10E-10, respectively, by F-test). However, there was no difference between w1118 controls and chico1/+ mutants (P = 0.20 by F-test). Data indicate mean ± SE. (K) Heart period was increased in chico1/+ mutants fed with an LSD or HSD. Heart period of chico1/+ mutants raised on low sucrose was 0.94 second, compared to 0.68 second of w1118 controls (*P = 0.004 by t-test). Heart period of chico1/+ mutants raised on an HSD was 0.91 second, compared to 0.60 second of w1118 controls (*P = 0.0008 by t-test). Data indicate mean ± SE. (L) chico1/+ flies exhibited significantly reduced fractional shortening in both LSD and HSD. Fractional shortening of chico1/+ mutants raised on low sucrose was 37%, compared to 42% of w1118 controls (*P = 0.006 by t-test). Fractional shortening of chico1/+ mutants raised on an HSD was 34%, compared to 40% of w1118 controls (*P = 0.001 by t-test). Data are means ± SE. (M) chico1/+ flies exhibited significantly reduced diastole on both LSD and HSD. Diastole of chico1/+ mutants raised on low sucrose was 49.9 microns, compared to 62.9 microns of w1118 controls (*P = 0.0009 by t-test). Diastole of chico1/+ mutants raised on an HSD was 45.8 microns, compared to 68.1 microns of w1118 controls (*P = 2.14E-08 by t-test). Data are means ± SE. (N) Significantly reduced systole of chico1/+ flies was observed in both LSD and HSD. Systole of chico1/+ mutants raised on low sucrose was 31.6 microns, compared to 36.6 microns of w1118 controls (*P = 0.03 by t-test). Systole of chico1/+ mutants raised on an HSD was 30.2 microns, compared to 40.0 microns of w1118 controls (*P = 0.0002 by t-test). Data are means ± SE. See also Videos S4, S5. Similarly, the shortened life span exhibited by p38A mutant adults was strongly enhanced in the presence of high sucrose, reducing median lifespan by an additional ten days (Figure 3B and Figure S1). The hearts of p38A(−/−) flies fed a control LSD exhibited little deviation from wild type (w1118). However, an HSD led specifically to a strongly increased heart period (decreased heart rate); heart arrhythmia and fractional shortening were unchanged compared to LSD (Figure 3C–3E; Video S4). The HSD-induced increase in heart period was due to significant increases in both the diastolic and systolic intervals (Figure 3F, 3G). The increase in systolic interval length is especially notable in that this parameter is tightly controlled in w1118 flies. These data are consistent with the view that sucrose-mediated deficits in development, longevity, and heart function are normally opposed by P38A activity.
Insulin signaling is a primary regulator of glucose homeostasis. Mis-regulation of pathway components leads to insulin resistance and aspects of metabolic syndrome and diabetes. Reduced insulin signaling can extend life span in worms, flies, and mammals [38]–[40] as well as improve heart function in aging flies [22]. Drosophila fed an HSD became progressively insulin-resistant (Figure 1F, Figure 2H), raising the question of whether systemic reduction of insulin signaling would protect heart function damaged by HSD.
To address these questions we tested the effects of reducing the functional copy number of the insulin receptor substrate (IRS) ortholog chico in the presence of high sucrose feeding. Utilizing a null chico allele, genotypically chico1/+ heterozygous adults showed the same developmental delay in LSD versus HSD as did w1118 (Figure 3H). As anticipated, chico1/+ adults lived 13 days longer on an LSD (Figure 3I and Figure S1). By contrast, removing one functional copy of chico did not extend life span on an HSD: half of the animals died at approximately day 35, similar to w1118 controls fed an HSD (Figure 3I and Figure S1). Metabolic assays indicated that, compared to w1118 controls, chico1/+ heterozygous adults had similar triglyceride levels but higher hemolymph glucose levels (Figure S5).
When examining the heart function of chico1/+ heterozygotes fed an LSD, we observed a slight increase in heartbeat arrhythmicity compared to wild type controls although the difference did not reach statistical significance (Figure 3J; Video S5). By contrast, the heart period of chico1/+ heterozygotes displayed significant differences on both diets when compared to controls: 0.94 seconds when fed LSD and 0.91 seconds in HSD compared to 0.67 and 0.60 seconds, respectively, observed in w1118 flies (Figure 3K; Video S5). Other heart parameters were affected as well. Fractional shortening of chico1/+ hearts was significantly decreased both in high sugar food and low sugar food (Figure 3L), indicating that the pumping ability of the chico1/+ heart was compromised. The heart size of chico1/+ mutant flies was dramatically decreased as demonstrated by decreased diastolic and systolic diameter (Figure 3M, 3N). We validated previous studies that found no reduction in body size of chico1/+ mutants ([41]; not shown), indicating that the decrease in heart size is likely due to a heart-specific effect reduced chico activity. We conclude that high sucrose feeding of chico1/+ heterozygotes leads to compromised heart performance by strongly reducing heart period and fractional shortening, which may contribute to an overall reduction in life span. Thus, the beneficial effects of reduced insulin signaling (e.g., to extend lifespan) strongly depend on the dietary composition [14] and are lost when the animal consumes high dietary sugar.
Activation of the hexosamine pathway led to heart dysfunction
Several metabolic pathways are thought to mediate glucose's ability to provoke metabolic dysfunction, insulin resistance, and T2DM including the polyol, AGE, PKC, and hexosamine biosynthetic pathways [42]. The outcome of excess flux through the hexosamine biosynthetic pathway remains unclear: high levels are thought to damage pancreatic beta cells through O-linked glycosylation [43], but the physiologic significance of hexosamine flux vs. oxidative stress has been questioned [44]. Glucose flux through the hexosamine pathway utilizes the key enzymes glutamine-fructose-6-phosphate transaminase (GFAT) and O-linked beta-N-acetylglucosamine transferase (OGT), yielding the sugar modification UDP-GlcNAc that regulates a broad range of target proteins (Figure 4A). Increased hexosamine flux and O-GlcNAc levels have been implicated in the impaired relaxation of isolated cardiomyocytes, blunted response to angiotensin II and phenylephrine, hyperglycemia-induced cardiomyocyte apoptosis, and endothelial and vascular cell dysfunction [45]. Targeting OGT to the mouse liver led to significant insulin resistance and related metabolic defects through its interaction with phosphoinositides [46].
Fig. 4. Dietary glucosamine shortened life span and induced cardiac dysfunction. 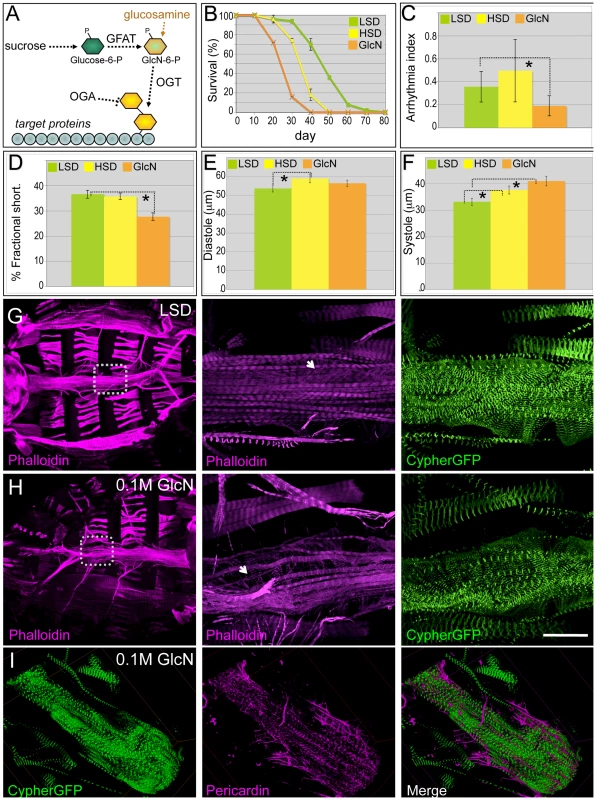
(A) Hexosamine biosynthesis pathway (HBP). The two rate limiting enzymes GFAT and OGT convert glucose to a O-GlcNAc residue that is then targeted to protein substrates; this residue is removed by β-N-acetylglucosaminidase (OGA). Glucosamine bypasses GFAT and increases HBP flux. (B) Dietary glucosamine significantly reduced life span: the average life span of flies fed 0.1 M glucosamine was 25 days compared to 35 days on an HSD and 48 days on a (control) LSD. Data are means ± SE (n = 2 experiments, 25 flies per experiment). (C) Glucosamine-fed flies displayed decreased arrhythmia. Arrhythmia index was obtained from wild type (Canton S) in LSD, HSD, and glucosamine diet. Glucosamine diet significantly reduced arrhythmia index (*P = 0.006 by F-test). Note Canton S flies fed with low sucrose showed slightly higher arrhythmia than w1118 flies fed with low sucrose. Data are means ± SE. (D) Glucosamine reduced fractional shortening in three-week-old adult flies (*P = 0.001 by t-test). Data are means ± SE. (E) Unlike high dietary sucrose (*P = 0.042 by t-test), dietary glucosamine did not change the diastolic diameter (P = 0.32 by t-test). Data are means ± SE. (F) Diets supplemented with glucosamine (*P = 0.0009 by t-test) or HSD (*P = 0.04 by t-test) increased systolic diameter, indicating that changes in fractional shortening are due to changes in systolic diameter. Data are means ± SE. (G,H) Dietary glucosamine led to heart structure defects. F-Actin in the heart was visualized with phalloidin (red) and Cypher-GFP (green) to label the Z-lines of myofibers within cardiomyocytes (magnification = 10×). Insets magnify the boxed regions to show the myofibers at higher magnification (63×). Size bar = 250 µm. (I) Representative confocal images of a three-week old Cypher-GFP fly heart from an animal fed 0.1 M glucosamine. Visualization with anti-Pericardin antibody (magenta) indicated decreased Pericardin levels. Compare with the control shown in Figure 2E. See also Video S6. To specifically access hexosamine pathway activity we fed flies the hexose sugar glucosamine (Figure 4A). Knockdown of GFAT in a subset of tissues (dot>gfat-IR) led to early larval lethality; this lethality was rescued by low levels (0.02–0.04 M) of glucosamine, validating its targeting of hexosamine flux (Figure S6). Diets supplemented with moderate levels of glucosamine (0.1 M) significantly shortened life span: the median life span for glucosamine-fed flies was 25 days compared to animals fed HSD (35 days) or LSD (48 days; Figure 4B). Hearts of flies fed 0.1 M glucosamine did not exhibit elevated arrhythmia; rather, heart rhythmicity was more highly ordered than flies fed an HSD or even, surprisingly, an LSD (Figure 4C; Video S6). However, fractional shortening was significantly decreased to 28%, compared to 37% in flies grown in control food (Figure 4D). The decrease in fractional shortening is likely due to systolic dysfunction: the systolic diameter increased significantly while the diastolic diameter did not change relative to LSD controls (Figure 4E, 4F). Meanwhile, the heart response to exogenous insulin was diminished in glucosamine-fed flies compared to controls (Figure S7).
The impaired contractile ability of the heart observed in glucosamine-fed flies was reminiscent of the heart dilation phenotype reported in mice with cardiomyocyte-restricted knockout of the insulin receptor [47]. In these mice heart dilation was coupled with structural defects, prompting us to examine the heart structures of flies fed control, high sucrose, and glucosamine-supplemented diets. Phalloidin was used to visualize integrity of the cytoskeleton and Cypher-GFP was used to visualize the sarcomeric Z-line patterns within the myofibrils of the contractile myocardium. Adult flies fed an LSD for three weeks retained correctly aligned and organized myofibril organization (Figure 4G). However, three-week old adults fed 0.1 M glucosamine exhibited disorganized longitudinal myofibrillar structures as well as disorganized transverse myofibrils of the underlying myocardium (Figure 4H). The fibers themselves often displayed signs of separation and gaps between them and the myofibrils were thinner than controls. We observed similar but milder defects in three-week adult flies fed 1.0 M sucrose; phenotype penetrance was also lower (data not shown). This data suggests that at least some of the functional defects we observed in hearts of adult flies fed glucosamine can be traced to emergent structural defects.
We did note a consistent difference between high sugar and glucosamine-supplemented diets. Unlike HSD-fed flies, which exhibited increased Pericardin accumulation (Figure 2G), flies fed a glucosamine-supplemented diet consistently exhibited decreased Pericardin levels within the heart (Figure 4I), compared to LSD-fed flies (Figure 2F).
Knockdown of hexosamine pathway components led to improved heart function in adults fed a high sucrose diet
The role of the hexosamine biosynthetic pathway on heart homeostasis is poorly understood in the context of the whole animal. In mice, knockout of OGT resulted in embryonic lethality indicating that hexosamine flux is required for normal development [48]. However, reducing hexosamine pathway-mediated O-GlcNAc protein modification did not prevent insulin resistance in 3T3-L1 adipocytes [49]. Whole animal data in C. elegans has not fully illuminated these issues: reduced ogt activity lowered dauer formation induced by a temperature sensitive insulin-like receptor (daf-2) mutant, while reduced activity of the negative regulator oga augmented dauer formation. However, both ogt and oga mutants showed carbohydrate and lipid metabolism alterations that were similar to changes associated with mammalian insulin resistance [50], [51].
Our data indicate that increased activation of the hexosamine biosynthetic pathway leads to an adverse impact on adult Drosophila cardiac function. We therefore examined whether dietary sucrose does indeed act at least in part through hexosamine flux. Removing one genomic copy of OGT (sxc/+) led to increased hyperglycemia and significant heart dysfunction including increased arrhythmia and decreased fractional shortening in animals fed an LSD or HSD diet (Figure S8). Global knockdown of Drosophila gfat utilizing inverted-repeat (IR) mediated RNA-interference proved lethal early in development (data not shown). Knockdown of ogt with two separate ogt-IR lines—using a strong ubiquitous driver (tub>ogt-IR) or weaker cardiac-specific driver (gmh5>ogt-IR)—permitted viability though we observed a developmental delay that was enhanced in the presence of high dietary sucrose (Figure 5A–5D).
Fig. 5. Genetic manipulation of the hexosamine biosynthetic pathway. 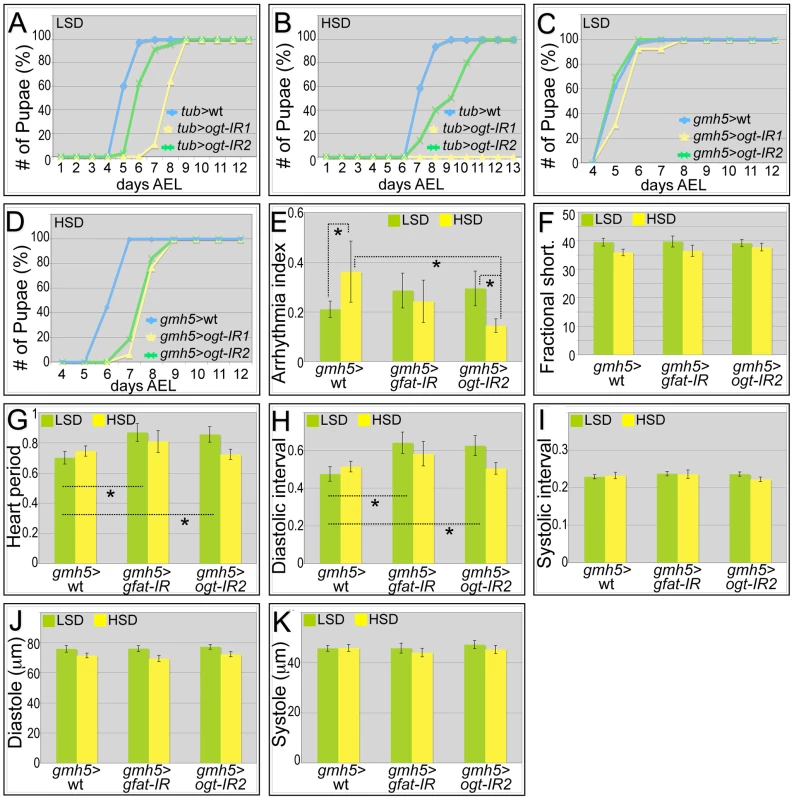
(A) Global knockdown of OGT with two separate ogt-IR lines by tub-gal4 led to a delay in development to pupariation in larvae fed an LSD. (B) This delay was further enhanced in the presence of an HSD: in particular, knockdown of OGT by tub>ogt-IR1 led to lethality. (C) Heart-specific knockdown of OGT (gmh5-gal4) led to little or no effect on development rate in the presence of control food. (D) By contrast, heart-specific knockdown of OGT (gmh5>ogt-IR) led to a 1.5 day developmental delay in the presence of high dietary sucrose. (E) Heart-specific knockdown of OGT or GFAT decreased high sugar induced arrhythmia. Arrhythmia index was somewhat increased—though not statistically significantly— in flies with a heart-specific knockdown of OGT or GFAT flies raised in low sucrose diet compared to wild type controls. However, when raised on an HSD, heart-specific knockdown of OGT or GFAT led to decreased arrhythmia compared to wild type controls; especially for OGT knockdown flies, the decrease in arrhythmia index was significant (*P = 5.57E-08 by F-test). (F) Heart specific knockdown of GFAT or OGT did not alter fractional shortening. Fractional shortening of wild type controls, gmh5>gfat-IR flies and gmh5>ogt-IR2 flies are shown. Data represent means ± SE. (G) Heart period did not change in heart specific knockdown of GFAT or OGT flies raised on an HSD. However, when raised in low sucrose diet, they displayed increased heart period (*P = 0.02 for both GFAT or OGT knockdown by t-test). (H) Diastolic interval did not change in heart specific knockdown of GFAT or OGT flies raised on an HSD. However, when raised in low sucrose diet, they showed increase of diastolic interval (*P = 0.03 for GFAT knockdown and 0.04 for OGT knockdown by t-test). (I) Systolic interval did not change in heart specific knockdown of GFAT or OGT flies raised on an LSD or HSD. (J) Diastole did not change in heart specific knockdown of GFAT or OGT flies raised on an LSD or HSD. (K) Systole did not change in heart specific knockdown of GFAT or OGT flies raised on an LSD or HSD. See also Videos S7, S8, S9. Heart specific knockdown of OGT (gmh5>ogt-IR) or GFAT (gmh5>gfat-IR) displayed increased arrhythmicity in flies fed a low sucrose diet (Figure 5E), though Pericardin levels were unaffected (Figure S9). Importantly, this arrhythmicity was decreased in gmh5>gfat-IR and more strongly decreased in gmh5>ogt-IR flies fed an HSD (Figure 5E, and Videos S7, S8, S9). This result indicates that the effects of dietary sucrose on arrhythmia are mediated at least in part through GFAT and OGT. Although the heart period and diastolic interval of gmh5>ogt-IR and gmh5>gfat-IR flies were increased in control food, they were not significantly different from wild type controls fed an HSD: fractional shortening, systolic interval, diastole and systole remained unchanged (Figure 5F–5K). Of note, we failed to observe a significant diet-induced size change in gmh5-GAL4 control hearts, suggesting GAL4 itself may have a subtle effect on heart size (Figure 5J–5K).
Together, our data suggest that the hexosamine biosynthetic pathway plays a significant role in the development of multiple aspects of diet-mediated heart dysfunction in Drosophila: increased pathway activity led to heart damage while decreased function was protective for flies raised on high dietary sucrose.
Discussion
Heart function in a Drosophila T2DM model
Several mechanisms have been proposed to account for the pathogenesis of diet-induced heart dysfunction. A primary injury may reflect alterations in energy substrate supply and utilization. For example, a mammalian diabetic cardiomyocyte model exhibited defects in glycolysis and glucose oxidation [52] and shifts to β-oxidation of free fatty acids that led to metabolism-based dysfunction of the heart [53]–[58]. Further structural changes arose from myocyte cell death, accumulation of collagen and fibrosis [59].
In our study we observed a subset of these phenotypes. By three weeks, adult flies fed an HSD exhibited detectable heart arrhythmia, a phenotype that increased with age. The contractility of the heart was not detectably impaired by three weeks, as fractional shortening was not decreased. However the size of the heart—as assessed by both diastole and systole — was increased (Figure 4E, 4F), reminiscent of the dilated cardiomyopathy reported for diabetic patients [60]. In addition, contractility decreased with age, phenocopying more advanced aspects of heart disease including diabetic cardiomyopathy. Importantly we also observed progressive fibrosis-like collagen accumulation within the heart, a central aspect of progressive heart disease in mammals (Figure 2E–2G).
Chico and P38 effects on heart function were diet-dependent
Previous work in mammals has demonstrated that insulin signaling is important for heart function. It regulates cardiac size, metabolism, and contractile protein expression [61], and cardiomyocyte-specific insulin receptor knockout mice exhibited impaired heart function [62]. Signaling through the IRS adaptor protein regulates mitochondrial gene expression, mitochondrial function and cardiomyocyte survival [63]. In this work, we demonstrate a similar requirement for the insulin pathway: in the presence of high dietary sugar, reduced activity of the IRS ortholog chico led to a decrease in fractional shortening that in turn contributed to progressive heart failure. This may help explain why reduced insulin pathway activity—which acts to extend lifespan on a normal diet—leads to shorter lifespan in the presence of high dietary sugar: reduced insulin signaling diminishes the ability of the animal to accommodate non-optimal diets.
P38 interacts with the insulin signaling pathway in specific contexts. Phosphorylation of P38 was responsive to insulin stimulation in the retina but not the liver in a mouse model of diabetes [64]. In rat aortic vascular smooth muscle cells, high glucose and chronic insulin treatment that mimicked hyperglycemia and hyperinsulinemia impaired iNOS induction by acute insulin treatment and was associated with sustained P38 activation. Blocking P38 pathway with the chemical inhibitor SB-203580 restored iNOS induction [65]. In contrast, in rat hearts insulin has been shown to protect myocardial contractility [66]; this protection requires P38 MAPK phosphorylation of Hsp27 [67]. In our experimental paradigm P38 proved to be required for full protection from high dietary sugar. This may reflect its role in protecting from cellular stress: Drosophila lacking P38A function are vulnerable to specific environmental stresses including heat shock, oxidative stress and starvation [37].
Hexosamine biosynthetic pathway is a candidate therapeutic target
The hexosamine biosynthetic pathway is critical for cell function and loss of the pathway effector enzyme OGT in mice led to embryonic lethality [68]. We found that reducing activity of either OGT or GFAT specifically in the adult Drosophila heart led to improved heart function as demonstrated by reduced incidence of arrhythmias in the presence of an HSD.
Our data are mostly consistent with results in other model systems. Elevated glucose, glucosamine, or over-expressed OGT led to increased O-linked glycosylation of nuclear proteins and loss of OGT-impaired cardiomyocyte calcium cycling. In contrast, over-expressing the pathway inhibitor OGA reversed sugar-induced heart defects including calcium transients [69]. O-linked glycosylation of mitochondrial proteins impaired mitochondrial function in cardiac myocytes exposed to high glucose, an effect that was reversed by over-expression of OGA [70]. Our data are not consistent with recent observations from rat cardiac fibroblasts, which demonstrated that flux through the hexosamine biosynthetic pathway induced collagen expression [71]: in our whole animal paradigm, glucosamine decreased Pericardin levels (Figure 4I).
Deleting OGT function specifically within the hearts of infarcted mice significantly exacerbated cardiac dysfunction [72]. We also observed moderately impaired heart function in cardiac knockdown of OGT and GFAT of flies fed an LSD. In contrast, cardiac knockdown of OGT and GFAT of flies fed an HSD was strongly protective of heart dysfunction compared to control flies on an HSD (Figure 5E–5H). Reducing GFAT or OGT activity did not detectably alter Pericardin levels (Figure S8) suggesting that GFAT or OGT acts on other components to improve the heart function of HSD-fed flies. The heart dysfunction observed in both reduced and increased hexosamine flux suggests that a correct balance is required to maintain normal heart function. Based on the observation that reducing hexosamine biosynthetic pathway activity rescued several aspects of heart dysfunction in our Drosophila model, our work supports GFAT, OGT and OGA as candidate therapeutic targets.
Materials and Methods
Fly stocks
cn1 P{ry11}chico1/CyO; ry506, P38A and P{w[+mC] = Dot-GAL4.K}43A, y[1] w[*] ([73], cat. #6903) flies were obtained from the Bloomington Drosophila Stock Center. w1118 control and transposable P-element insertions w1118; P{UAS-OGT-IR,w[+]} and w1118; P{UAS-GFAT-IR,w[+]} transgenic flies were obtained from the Vienna Drosophila RNAi Center [74]. gmh5-GAL4 was obtained from Sanford-Burnham Institute [22].
Fly food recipes
All flies, from embryo stage, were raised on Bloomington standard cornmeal food [75]. After eclosion, adults were transferred to LSD or HSD food, which were made based on Bloomington semi-defined medium [76], with adjustment of sugar concentration. 100 ml food contains agar (1 g), yeast (8 g), yeast extract (2 g), peptone (2 g), MgSO4 (200 µl of 1 M solution), CaCl2 (340 µl of 1 M solution), propionic acid (600 µl), mold inhibitor (1000 µl), and sucrose (5.13 g = 0.15 M for LSD, 34.2 g = 1.0 M for HSD). 0.1 M glucosamine was supplemented to the LSD food to make the glucosamine food.
Hemolymph glucose and trehalose measurements
Hemolymph was pooled from 35–45 adult flies to obtain 1 µl for assay. Hemolymph was diluted 1∶10 before assay. Glucose was measured by adding to 99 µl of Thermo Infinity Glucose Reagent (TR15321) in a 96 well plate, then incubated for 3 minutes at 37°C and read immediately at 340 nm. Trehalose was measured using the same reagent after 24 hours of incubation, at which point endogenous trehalase has completely digested the trehalose to produce free glucose. Glucose standards were treated simultaneously and used to quantify the sugar levels in hemolymph.
Western blotting
For insulin sensitivity assays, 3-week-old LSD - or HSD-fed adults were dissected open to expose the body cavity to treatments. Recombinant human insulin (Sigma I0259) or dilution buffer (10 mM HEPES) were added to Schneider's medium and incubated at room temperature for 15 minutes. Heads were removed and bodies were homogenized, sample buffer added, and tissue used to generate Western blots. For heart insulin sensitivity assays, same challenging procedure was applied, and followed by harvesting the heart tissue and directly lysed for Western blotting. Cell Signaling antibodies against Drosophila PO4-Akt (#4054) were used to detect Akt phosphorylation, anti-pan-Akt (#4691) for pan-Akt, anti-PO4-4EBP (#2855) for 4EBP phosphorylation, and anti-Pericardin (DSHB EC11) for Pericardin. Anti-actin (DSHB JLA20) or anti-syntaxin (DSHB 8C-3) was used as a loading control. Secondary antibodies were from Santa Cruz.
Lipid measurements
For whole body TAG, 6 animals were homogenized in PBS+0.1% Tween and heated for 5 minutes at 65°C to inactivate lipases. 2 µl of this homogenate was mixed with 198 µl of Thermo Infinity Triglyceride reagent and analyzed per the manufacturer's instructions. Triglyceride standards were treated simultaneously and used to quantify TAG levels. The heart TAG was measured according to [29].
Optical heartbeat analysis of adult Drosophila hearts
Heart parameters were analyzed from high speed optical recordings of exposed hearts from adult Drosophila (Ocorr et al, PNAS 2007; Ocorr and Vogler, JoVE 2009). High speed digital movies of beating hearts were taken using a Hamamatsu EMCCD 9300 camera (Hamamatsu, Inc.; 100–140 frames per second) and Simple PCI image capture software (Hamamatsu, Inc.).
Heart period, heart diameters, percent fractional shortening and M-modes were determined from the movies using a previously described computer algorithm [24]. Heart periods were defined as the time between the ends of two consecutive diastolic intervals. Regarding M-modes, a single line of pixels bisecting the ventricle was identified in one frame of the movie and the same line of pixels was electronically excised from each subsequent frame and aligned horizontally to generate a snapshot of the movement of the heart edges (y axis) over time (x axis). Diastolic and systolic diameters were obtained as output from the MatLab-based program (Mathworks, Natick, MA). Arrhythmias observed in M-mode can be quantified as an arrhythmia index, which is the standard deviation of all heart periods in each record normalized to the median heart period for each fly.
Fluorescent staining and imaging
Adult hearts were dissected in artificial Drosophila hemolymph (ADH) and rhythmic beating was confirmed in oxygenated ADH. Hearts were fixed with 4% formaldehyde in PBS for 30 minutes on ice, and washed 2×15 minutes with PBSTx at room temperature. Abdominal cuticle and fat body were removed and specimens were incubated with 10 µl primary antibody (Anti-Pericardin 1∶1000, Developmental Studies Hybridoma Bank) diluted in 1× PAXDG (1× PBS with 1% BSA, 0.3% Triton-X, 0.3% sodium deoxycholate, and 5% normal goat serum) overnight at 4°C, washed 3×15 minutes with 10 µl PBSTx at room temperature, incubated in secondary antibody in 1× PAXDG for 2 hrs (in certain cases with Alexa594-phalloidin (1∶200, Molecular Probes)), washed, and mounted. Confocal images were obtained with a Leica DM5500. Experimental and controls were imaged using identical microscope settings. 3D structures were reconstituted from confocal stacks using Amira software.
Developmental delay
Eggs were collected onto low or high sucrose food for 16 hours, then permitted to mature at 25°C. Time to pupariation was scored daily.
Survival
Male flies were raised in control and experimental diet that was changed every 2–3 days, and flies were counted every 5 or 10 days. n = 50 total flies in two separate experiments. Experiments for both p38A mutants and w1118 controls were performed at 22°C due to poor viability of developing p38A mutant flies grown at 25°C on an HSD. Median lifespans were estimated using linear interpolation over scoring intervals. Confidence intervals for estimated medians were computed numerically using the bias-corrected and accelerated bootstrap method with 100000 bootstrap replicates. Pairwise equality of survival distributions was tested by a log-rank test, specifically by an asymptotic permutation test on Sun's log-rank scores for the interval-censored data. Computations were carried out in R (version 2.15.1), a language and environment for statistical computing, using the interval and boot packages.
Glucosamine rescues lethality due to GFAT1 knockdown
To prepare Dot-Gal4/+; UAS-GFATRNAi/+ eggs, unmated females homozygous for Dot-Gal4 were crossed with males homozygous for UAS - GFATRNAi. Dot-Gal4 expresses in a range of tissues including hemocytes, pericardial cells, the gut, and the salivary glands.
Supporting Information
Zdroje
1. MarriottBP, ColeN, LeeE (2009) National Estimates of Dietary Fructose Intake Increased from 1977 to 2004 in the United States. The Journal of Nutrition 139 : 1228S–1235S.
2. MellorKM, RitchieRH, DavidoffAJ, DelbridgeLMD (2010) Elevated dietary sugar and the heart: experimental models and myocardial remodeling. Canadian Journal of Physiology and Pharmacology 88 : 525–540.
3. SelvinE, CoreshJ, GoldenSH, BrancatiFL, FolsomAR, et al. (2005) Glycemic Control and Coronary Heart Disease Risk in Persons With and Without Diabetes: The Atherosclerosis Risk in Communities Study. Arch Intern Med 165 : 1910–1916.
4. FungTT, MalikV, RexrodeKM, MansonJE, WillettWC, et al. (2009) Sweetened beverage consumption and risk of coronary heart disease in women. The American Journal of Clinical Nutrition 89 : 1037–1042.
5. RublerS, DlugashJ, YuceogluYZ, KumralT, BranwoodAW, et al. (1972) New type of cardiomyopathy associated with diabetic glomerulosclerosis. The American Journal of Cardiology 30 : 595–602.
6. Van HornL, JohnsonRK, FlickingerBD, VafiadisDK, Yin-PiazzaS (2010) Translation and Implementation of Added Sugars Consumption Recommendations. Circulation 122 : 2470–2490.
7. BrogioloW, StockerH, IkeyaT, RintelenF, FernandezR, et al. (2001) An evolutionarily conserved function of the Drosophila insulin receptor and insulin-like peptides in growth control. Current Biology 11 : 213–221.
8. GarofaloRS (2002) Genetic analysis of insulin signaling in Drosophila. Trends in Endocrinology and Metabolism 13 : 156–162.
9. RulifsonEJ, KimSK, NusseR (2002) Ablation of Insulin-Producing Neurons in Flies: Growth and Diabetic Phenotypes. Science 296 : 1118–1120.
10. BuchS, MelcherC, BauerM, KatzenbergerJ, PankratzMJ (2008) Opposing Effects of Dietary Protein and Sugar Regulate a Transcriptional Target of Drosophila Insulin-like Peptide Signaling. Cell Metabolism 7 : 321–332.
11. MusselmanLP, FinkJL, NarzinskiK, RamachandranPV, HathiramaniSS, et al. (2011) A high-sugar diet produces obesity and insulin resistance in wild-type Drosophila. Disease Models & Mechanisms
12. SelmanC, TulletJMA, WieserD, IrvineE, LingardSJ, et al. (2009) Ribosomal Protein S6 Kinase 1 Signaling Regulates Mammalian Life Span. Science 326 : 140–144.
13. ReilingJH, DoepfnerKT, HafenE, StockerH (2005) Diet-Dependent Effects of the Drosophila Mnk1/Mnk2 Homolog Lk6 on Growth via eIF4E. 15 : 24–30.
14. GrandisonRC, PiperMDW, PartridgeL (2009) Amino-acid imbalance explains extension of lifespan by dietary restriction in Drosophila. Nature 462 : 1061–1064.
15. WolfMJ, RockmanHA (2008) Drosophila melanogaster as a model system for the genetics of postnatal cardiac function. Drug Discovery Today: Disease Models 5 : 117–123.
16. Miller A (1950) The internal anatomy and histology of the imago of Drosophila melanogaster. . Biology of Drosophila, ed M Demerec Wiley, New York, 1950.
17. Taghli-LamallemO, AkasakaT, HoggG, NudelU, YaffeD, et al. (2008) Dystrophin deficiency in Drosophila reduces lifespan and causes a dilated cardiomyopathy phenotype. Aging Cell 7 : 237–249.
18. MolinaMR, CrippsRM (2001) Ostia, the inflow tracts of the Drosophila heart, develop from a genetically distinct subset of cardial cells. Mechanisms of Development 109 : 51–59.
19. BodmerR (1995) Heart development in Drosophila and its relationship to vertebrates. Trends in Cardiovascular Medicine 5 : 21–28.
20. Bodmer R, Frasch M (2010) Development and Aging of the Drosophila Heart. In: Rosenthal N, Harvey R, editors. Heart Development and Regeneration Amsterdam: Elsevier. pp. 47–86.
21. CrippsRM, OlsonEN (2002) Control of Cardiac Development by an Evolutionarily Conserved Transcriptional Network. Developmental Biology 246 : 14–28.
22. WessellsRJ, FitzgeraldE, CypserJR, TatarM, BodmerR (2004) Insulin regulation of heart function in aging fruit flies. Nat Genet 36 : 1275–1281.
23. OcorrKFM, CammaratoA, BernsteinSI, BodmerR (2009) Semi-automated Optical Heartbeat Analysis of Small Hearts. J Vis Exp 16.
24. FinkM, Callol-MassotC, ChuA, Ruiz-LozanoP, Izpisua BelmonteJC, et al. (2009) A new method for detection and quantification of heartbeat parameters in Drosophila, zebrafish, and embryonic mouse hearts. Biotechniques 46 : 101–113.
25. OcorrK, ReevesNL, WessellsRJ, FinkM, ChenHSV, et al. (2007) KCNQ potassium channel mutations cause cardiac arrhythmias in Drosophila that mimic the effects of aging. Proceedings of the National Academy of Sciences 104 : 3943–3948.
26. OcorrK, AkasakaT, BodmerR (2007) Age-related cardiac disease model of Drosophila. Mechanisms of Ageing and Development 128 : 112–116.
27. WessellsR, ErinF, NicoleP, KarenO, SamanthaM, et al. (2009) d4eBP acts downstream of both dTOR and dFoxo to modulate cardiac functional aging in Drosophila. Aging Cell 8 : 542–552.
28. CammaratoA, DambacherCM, KnowlesAF, KronertWA, BodmerR, et al. (2008) Myosin Transducer Mutations Differentially Affect Motor Function, Myofibril Structure, and the Performance of Skeletal and Cardiac Muscles. Mol Biol Cell 19 : 553–562.
29. BirseRT, ChoiJ, ReardonK, RodriguezJ, GrahamS, et al. (2010) High-Fat-Diet-Induced Obesity and Heart Dysfunction Are Regulated by the TOR Pathway in Drosophila. Cell metabolism 12 : 533–544.
30. TaguchiA, WhiteMF (2008) Insulin-Like Signaling, Nutrient Homeostasis, and Life Span. Annual Review of Physiology 70 : 191–212.
31. SkorupaDA, DervisefendicA, ZwienerJ, PletcherSD (2008) Dietary composition specifies consumption, obesity, and lifespan in Drosophila melanogaster. Aging Cell 7 : 478–490.
32. GiacomelliF, WienerJ (1979) Primary myocardial disease in the diabetic mouse. An ultrastructural study. Lab Invest 40 : 14.
33. WayKJ, IsshikiK, SuzumaK, YokotaT, ZvagelskyD, et al. (2002) Expression of Connective Tissue Growth Factor Is Increased in Injured Myocardium Associated With Protein Kinase C Œ≤2 Activation and Diabetes. Diabetes 51 : 2709–2718.
34. KonradD, SomwarR, SweeneyG, YaworskyK, HayashiM, et al. (2001) The Antihyperglycemic Drug Œ±-Lipoic Acid Stimulates Glucose Uptake via Both GLUT4 Translocation and GLUT4 Activation. Diabetes 50 : 1464–1471.
35. JacobS, RuusP, HermannR, TritschlerHJ, MaerkerE, et al. (1999) Oral administration of rac-[alpha]-lipoic acid modulates insulin sensitivity in patients with type-2 diabetes mellitus: a placebo-controlled pilot trial. Free Radical Biology and Medicine 27 : 309–314.
36. VinayagamoorthiR, BobbyZ, SridharMG (2008) Antioxidants preserve redox balance and inhibit c-Jun-N-terminal kinase pathway while improving insulin signaling in fat-fed rats: evidence for the role of oxidative stress on IRS-1 serine phosphorylation and insulin resistance. J Endocrinol 197 : 287–296.
37. CraigCR, FinkJL, YagiY, IpYT, CaganRL (2004) A Drosophila p38 orthologue is required for environmental stress responses. EMBO reports 5 : 6.
38. GuarenteL, KenyonC (2000) Genetic pathways that regulate ageing in model organisms. Nature 408 : 255–262.
39. TatarM, BartkeA, AntebiA (2003) The Endocrine Regulation of Aging by Insulin-like Signals. Science 299 : 1346–1351.
40. StephenL, HelfandBR (2003) Molecular genetics of aging in the fly: Is this the end of the beginning? Bio Essays 25 : 134–141.
41. BohniR, Riesgo-EscovarJ, OldhamS, BrogioloW, StockerH, et al. (1999) Autonomous Control of Cell and Organ Size by CHICO, a Drosophila Homolog of Vertebrate IRS1 4. Cell 97 : 865–875.
42. BrownleeM (2005) The Pathobiology of Diabetic Complications: A Unifying Mechanism. Diabetes 54 : 1615–1625.
43. LiuK, PatersonAJ, ChinE, KudlowJE (2000) Glucose stimulates protein modification by O-linked GlcNAc in pancreatic β cells: Linkage of O-linked GlcNAc to β cell death. Proceedings of the National Academy of Sciences of the United States of America 97 : 2820–2825.
44. KanetoH, XuG, SongK-H, SuzumaK, Bonner-WeirS, et al. (2001) Activation of the Hexosamine Pathway Leads to Deterioration of Pancreatic beta -Cell Function through the Induction of Oxidative Stress. J Biol Chem 276 : 31099–31104.
45. FrustaciA, KajsturaJ, ChimentiC, JakoniukI, LeriA, et al. (2000) Myocardial Cell Death in Human Diabetes. Circ Res 87 : 1123–1132.
46. YangX, OngusahaPP, MilesPD, HavstadJC, ZhangF, et al. (2008) Phosphoinositide signalling links O-GlcNAc transferase to insulin resistance. Nature 451 : 964–969.
47. HuP, ZhangD, SwensonL, ChakrabartiG, AbelED, et al. (2003) Minimally invasive aortic banding in mice: effects of altered cardiomyocyte insulin signaling during pressure overload. Am J Physiol Heart Circ Physiol 285: H1261–1269.
48. O'DonnellN, ZacharaNE, HartGW, MarthJD (2004) Ogt-Dependent X-Chromosome-Linked Protein Glycosylation Is a Requisite Modification in Somatic Cell Function and Embryo Viability. Mol Cell Biol 24 : 1680–1690.
49. RobinsonKA, BallLE, BuseMG (2007) Reduction of O-GlcNAc protein modification does not prevent insulin resistance in 3T3-L1 adipocytes. Am J Physiol Endocrinol Metab 292: E884–890.
50. HanoverJA, ForsytheME, HennesseyPT, BrodiganTM, LoveDC, et al. (2005) A Caenorhabditis elegans model of insulin resistance: Altered macronutrient storage and dauer formation in an OGT-1 knockout. Proceedings of the National Academy of Sciences of the United States of America 102 : 11266–11271.
51. ForsytheME, LoveDC, LazarusBD, KimEJ, PrinzWA, et al. (2006) Caenorhabditis elegans ortholog of a diabetes susceptibility locus: oga-1 (O-GlcNAcase) knockout impacts O-GlcNAc cycling, metabolism, and dauer. Proceedings of the National Academy of Sciences 103 : 11952–11957.
52. MokudaO, SakamotoY, IkedaT, MashibaH (1990) Effects of Anoxia and Low Free Fatty Acid on Myocardial Energy Metabolism in Streptozotocin-Diabetic Rats. Annals of Nutrition and Metabolism 34 : 259–265.
53. RodriguesB, CamMC, McNeillJH (1998) Metabolic disturbances in diabetic cardiomyopathy. Molecular and Cellular Biochemistry 180 : 53–57.
54. HussJM, KellyDP (2005) Mitochondrial energy metabolism in heart failure: a question of balance. The Journal of Clinical Investigation 115 : 547–555.
55. StanleyWC, ChandlerMP (2002) Energy Metabolism in the Normal and Failing Heart: Potential for Therapeutic Interventions. Heart Failure Reviews 7 : 115–130.
56. ChathamJC, SeymourA-ML (2002) Cardiac carbohydrate metabolism in Zucker diabetic fatty rats. Cardiovasc Res 55 : 104–112.
57. WisneskiJA, GertzEW, NeeseRA, MayrM (1987) Myocardial metabolism of free fatty acids. Studies with 14C-labeled substrates in humans. The Journal of Clinical Investigation 79 : 359–366.
58. ShippJC, OpieLH, ChallonerD (1961) Fatty Acid and Glucose Metabolism in the Perfused Heart. Nature 189 : 1018–1019.
59. ShimizuM, UmedaK, SugiharaN, YoshioH, InoH, et al. (1993) Collagen remodelling in myocardia of patients with diabetes. J Clin Pathol 46 : 32–36.
60. HambyRI, ZoneraichS, ShermanL (1974) Diabetic Cardiomyopathy. JAMA 229 : 1749–1754.
61. BelkeDD, BetuingS, TuttleMJ, GraveleauC, YoungME, et al. (2002) Insulin signaling coordinately regulates cardiac size, metabolism, and contractile protein isoform expression. The Journal of Clinical Investigation 109 : 629–639.
62. KimJ, WendeAR, SenaS, TheobaldHA, SotoJ, et al. (2008) Insulin-Like Growth Factor I Receptor Signaling Is Required for Exercise-Induced Cardiac Hypertrophy. Mol Endocrinol 22 : 2531–2543.
63. RiehleC, WaymentB, PiresKM, MoreiraAB, TheobaldH, et al. (2008) Abstract 3559: Insulin Receptor Substrates (IRS) Signaling are Essential Regulators of Mitochondrial Function and Cardiomyocyte Survival. Circulation 118: S_444-b-.
64. KondoT, KahnCR (2004) Altered Insulin Signaling in Retinal Tissue in Diabetic States. J Biol Chem 279 : 37997–38006.
65. BegumN, RagoliaL (2000) High glucose and insulin inhibit VSMC MKP-1 expression by blocking iNOS via p38 MAPK activation. Am J Physiol Cell Physiol 278: C81–91.
66. LiG, AliIS, CurrieRW (2006) Insulin induces myocardial protection and Hsp70 localization to plasma membranes in rat hearts. Am J Physiol Heart Circ Physiol 291: H1709–1721.
67. LiG, AliIS, CurrieRW (2008) Insulin-induced myocardial protection in isolated ischemic rat hearts requires p38 MAPK phosphorylation of Hsp27. Am J Physiol Heart Circ Physiol 294: H74–87.
68. ShafiR, IyerSPN, ElliesLG, O'DonnellN, MarekKW, et al. (2000) The O-GlcNAc transferase gene resides on the X chromosome and is essential for embryonic stem cell viability and mouse ontogeny. Proceedings of the National Academy of Sciences of the United States of America 97 : 5735–5739.
69. ClarkRJ, McDonoughPM, SwansonE, TrostSU, SuzukiM, et al. (2003) Diabetes and the Accompanying Hyperglycemia Impairs Cardiomyocyte Calcium Cycling through Increased Nuclear O-GlcNAcylation. J Biol Chem 278 : 44230–44237.
70. HuY, SuarezJ, FricovskyE, WangH, ScottBT, et al. (2009) Increased Enzymatic O-GlcNAcylation of Mitochondrial Proteins Impairs Mitochondrial Function in Cardiac Myocytes Exposed to High Glucose. J Biol Chem 284 : 547–555.
71. KohdaY, KanematsuM, KonoT, TerasakiF, TanakaT (2009) Protein O-Glycosylation Induces Collagen Expression and Contributes to Diabetic Cardiomyopathy in Rat Cardiac Fibroblasts. Journal of Pharmacological Sciences 111 : 446–450.
72. WatsonLJ, FacundoHT, NgohGA, AmeenM, BrainardRE, et al. (2010) O-linked β-N-acetylglucosamine transferase is indispensable in the failing heart. Proceedings of the National Academy of Sciences 107 : 17797–17802.
73. KimbrellDA, HiceC, BolducC, KleinhesselinkK, BeckinghamK (2002) The Dorothy enhancer has tinman binding sites and drives hopscotch-induced tumor formation. genesis 34 : 23–28.
74. DietzlG, ChenD, SchnorrerF, SuK-C, BarinovaY, et al. (2007) A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature 448 : 151–156.
75. LakovaaraS (1969) Malt as a culture medium for Drosophila species. Drosophila Information Service 44 : 128.
76. BackhausB, SulkowskiE, SchloteFW (1984) A semi-synthetic, general-purpose medium for Drosophila melanogaster. Drosophila Information Service 60 : 210–212.
Štítky
Genetika Reprodukční medicína
Článek Comparative Genome Structure, Secondary Metabolite, and Effector Coding Capacity across PathogensČlánek TATES: Efficient Multivariate Genotype-Phenotype Analysis for Genome-Wide Association StudiesČlánek Secondary Metabolism and Development Is Mediated by LlmF Control of VeA Subcellular Localization inČlánek Human Disease-Associated Genetic Variation Impacts Large Intergenic Non-Coding RNA ExpressionČlánek The Roles of Whole-Genome and Small-Scale Duplications in the Functional Specialization of GenesČlánek The Role of Autophagy in Genome Stability through Suppression of Abnormal Mitosis under Starvation
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2013 Číslo 1
-
Všechny články tohoto čísla
- A Model of High Sugar Diet-Induced Cardiomyopathy
- Comparative Genome Structure, Secondary Metabolite, and Effector Coding Capacity across Pathogens
- Emerging Function of Fat Mass and Obesity-Associated Protein (Fto)
- Positional Cloning Reveals Strain-Dependent Expression of to Alter Susceptibility to Bleomycin-Induced Pulmonary Fibrosis in Mice
- Genetics of Ribosomal Proteins: “Curiouser and Curiouser”
- Transposable Elements Re-Wire and Fine-Tune the Transcriptome
- Function and Regulation of , a Gene Implicated in Autism and Human Evolution
- MAML1 Enhances the Transcriptional Activity of Runx2 and Plays a Role in Bone Development
- Predicting Mendelian Disease-Causing Non-Synonymous Single Nucleotide Variants in Exome Sequencing Studies
- A Systematic Mapping Approach of 16q12.2/ and BMI in More Than 20,000 African Americans Narrows in on the Underlying Functional Variation: Results from the Population Architecture using Genomics and Epidemiology (PAGE) Study
- Transcription of the Major microRNA–Like Small RNAs Relies on RNA Polymerase III
- Histone H3K56 Acetylation, Rad52, and Non-DNA Repair Factors Control Double-Strand Break Repair Choice with the Sister Chromatid
- Genome-Wide Association Study Identifies a Novel Susceptibility Locus at 12q23.1 for Lung Squamous Cell Carcinoma in Han Chinese
- Genetic Disruption of the Copulatory Plug in Mice Leads to Severely Reduced Fertility
- The [] Prion Exists as a Dynamic Cloud of Variants
- Adult Onset Global Loss of the Gene Alters Body Composition and Metabolism in the Mouse
- Fis Protein Insulates the Gene from Uncontrolled Transcription
- The Meiotic Nuclear Lamina Regulates Chromosome Dynamics and Promotes Efficient Homologous Recombination in the Mouse
- Genome-Wide Haplotype Analysis of Expression Quantitative Trait Loci in Monocytes
- TATES: Efficient Multivariate Genotype-Phenotype Analysis for Genome-Wide Association Studies
- Structural Basis of a Histone H3 Lysine 4 Demethylase Required for Stem Elongation in Rice
- The Ecm11-Gmc2 Complex Promotes Synaptonemal Complex Formation through Assembly of Transverse Filaments in Budding Yeast
- MCM8 Is Required for a Pathway of Meiotic Double-Strand Break Repair Independent of DMC1 in
- Comparative Genomic Analysis of the Endosymbionts of Herbivorous Insects Reveals Eco-Environmental Adaptations: Biotechnology Applications
- Integration of Nodal and BMP Signals in the Heart Requires FoxH1 to Create Left–Right Differences in Cell Migration Rates That Direct Cardiac Asymmetry
- Pharmacodynamics, Population Dynamics, and the Evolution of Persistence in
- A Hybrid Likelihood Model for Sequence-Based Disease Association Studies
- Aberration in DNA Methylation in B-Cell Lymphomas Has a Complex Origin and Increases with Disease Severity
- Multiple Opposing Constraints Govern Chromosome Interactions during Meiosis
- Transcriptional Dynamics Elicited by a Short Pulse of Notch Activation Involves Feed-Forward Regulation by Genes
- Dynamic Large-Scale Chromosomal Rearrangements Fuel Rapid Adaptation in Yeast Populations
- Heterologous Gln/Asn-Rich Proteins Impede the Propagation of Yeast Prions by Altering Chaperone Availability
- Gene Copy-Number Polymorphism Caused by Retrotransposition in Humans
- An Incompatibility between a Mitochondrial tRNA and Its Nuclear-Encoded tRNA Synthetase Compromises Development and Fitness in
- Secondary Metabolism and Development Is Mediated by LlmF Control of VeA Subcellular Localization in
- Single-Stranded Annealing Induced by Re-Initiation of Replication Origins Provides a Novel and Efficient Mechanism for Generating Copy Number Expansion via Non-Allelic Homologous Recombination
- Tbx2 Controls Lung Growth by Direct Repression of the Cell Cycle Inhibitor Genes and
- Suv4-20h Histone Methyltransferases Promote Neuroectodermal Differentiation by Silencing the Pluripotency-Associated Oct-25 Gene
- A Conserved Helicase Processivity Factor Is Needed for Conjugation and Replication of an Integrative and Conjugative Element
- Telomerase-Null Survivor Screening Identifies Novel Telomere Recombination Regulators
- Genome-Wide Analysis Reveals Selection for Important Traits in Domestic Horse Breeds
- Coordinated Degradation of Replisome Components Ensures Genome Stability upon Replication Stress in the Absence of the Replication Fork Protection Complex
- Nkx6.1 Controls a Gene Regulatory Network Required for Establishing and Maintaining Pancreatic Beta Cell Identity
- HIF- and Non-HIF-Regulated Hypoxic Responses Require the Estrogen-Related Receptor in
- Delineating a Conserved Genetic Cassette Promoting Outgrowth of Body Appendages
- The Telomere Capping Complex CST Has an Unusual Stoichiometry, Makes Multipartite Interaction with G-Tails, and Unfolds Higher-Order G-Tail Structures
- Comprehensive Methylome Characterization of and at Single-Base Resolution
- Loci Associated with -Glycosylation of Human Immunoglobulin G Show Pleiotropy with Autoimmune Diseases and Haematological Cancers
- Switchgrass Genomic Diversity, Ploidy, and Evolution: Novel Insights from a Network-Based SNP Discovery Protocol
- Centromere-Like Regions in the Budding Yeast Genome
- Sequencing of Loci from the Elephant Shark Reveals a Family of Genes in Vertebrate Genomes, Forged by Ancient Duplications and Divergences
- Mendelian and Non-Mendelian Regulation of Gene Expression in Maize
- Mutational Spectrum Drives the Rise of Mutator Bacteria
- Human Disease-Associated Genetic Variation Impacts Large Intergenic Non-Coding RNA Expression
- The Roles of Whole-Genome and Small-Scale Duplications in the Functional Specialization of Genes
- Sex-Specific Signaling in the Blood–Brain Barrier Is Required for Male Courtship in
- A Newly Uncovered Group of Distantly Related Lysine Methyltransferases Preferentially Interact with Molecular Chaperones to Regulate Their Activity
- Is Required for Leptin-Mediated Depolarization of POMC Neurons in the Hypothalamic Arcuate Nucleus in Mice
- Unlocking the Bottleneck in Forward Genetics Using Whole-Genome Sequencing and Identity by Descent to Isolate Causative Mutations
- The Role of Autophagy in Genome Stability through Suppression of Abnormal Mitosis under Starvation
- MTERF3 Regulates Mitochondrial Ribosome Biogenesis in Invertebrates and Mammals
- Downregulation and Altered Splicing by in a Mouse Model of Facioscapulohumeral Muscular Dystrophy (FSHD)
- NBR1-Mediated Selective Autophagy Targets Insoluble Ubiquitinated Protein Aggregates in Plant Stress Responses
- Retroactive Maintains Cuticle Integrity by Promoting the Trafficking of Knickkopf into the Procuticle of
- Phenome-Wide Association Study (PheWAS) for Detection of Pleiotropy within the Population Architecture using Genomics and Epidemiology (PAGE) Network
- Genetic and Functional Modularity of Activities in the Specification of Limb-Innervating Motor Neurons
- A Population Genetic Model for the Maintenance of R2 Retrotransposons in rRNA Gene Loci
- A Quartet of PIF bHLH Factors Provides a Transcriptionally Centered Signaling Hub That Regulates Seedling Morphogenesis through Differential Expression-Patterning of Shared Target Genes in
- A Genome-Wide Integrative Genomic Study Localizes Genetic Factors Influencing Antibodies against Epstein-Barr Virus Nuclear Antigen 1 (EBNA-1)
- Mutation of the Diamond-Blackfan Anemia Gene in Mouse Results in Morphological and Neuroanatomical Phenotypes
- Life, the Universe, and Everything: An Interview with David Haussler
- Alternative Oxidase Expression in the Mouse Enables Bypassing Cytochrome Oxidase Blockade and Limits Mitochondrial ROS Overproduction
- An Evolutionarily Conserved Synthetic Lethal Interaction Network Identifies FEN1 as a Broad-Spectrum Target for Anticancer Therapeutic Development
- The Flowering Repressor Underlies a Novel QTL Interacting with the Genetic Background
- Telomerase Is Required for Zebrafish Lifespan
- and Diversified Expression of the Gene Family Bolster the Floral Stem Cell Network
- Susceptibility Loci Associated with Specific and Shared Subtypes of Lymphoid Malignancies
- An Insertion in 5′ Flanking Region of Causes Blue Eggshell in the Chicken
- Increased Maternal Genome Dosage Bypasses the Requirement of the FIS Polycomb Repressive Complex 2 in Arabidopsis Seed Development
- WNK1/HSN2 Mutation in Human Peripheral Neuropathy Deregulates Expression and Posterior Lateral Line Development in Zebrafish ()
- Synergistic Interaction of Rnf8 and p53 in the Protection against Genomic Instability and Tumorigenesis
- Dot1-Dependent Histone H3K79 Methylation Promotes Activation of the Mek1 Meiotic Checkpoint Effector Kinase by Regulating the Hop1 Adaptor
- A Heterogeneous Mixture of F-Series Prostaglandins Promotes Sperm Guidance in the Reproductive Tract
- Starvation, Together with the SOS Response, Mediates High Biofilm-Specific Tolerance to the Fluoroquinolone Ofloxacin
- Directed Evolution of a Model Primordial Enzyme Provides Insights into the Development of the Genetic Code
- Genome-Wide Screens for Tinman Binding Sites Identify Cardiac Enhancers with Diverse Functional Architectures
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Function and Regulation of , a Gene Implicated in Autism and Human Evolution
- An Insertion in 5′ Flanking Region of Causes Blue Eggshell in the Chicken
- Comprehensive Methylome Characterization of and at Single-Base Resolution
- Susceptibility Loci Associated with Specific and Shared Subtypes of Lymphoid Malignancies
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



