-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaTranscription Factor Oct1 Is a Somatic and Cancer Stem Cell Determinant
Defining master transcription factors governing somatic and cancer stem cell identity is an important goal. Here we show that the Oct4 paralog Oct1, a transcription factor implicated in stress responses, metabolic control, and poised transcription states, regulates normal and pathologic stem cell function. Oct1HI cells in the colon and small intestine co-express known stem cell markers. In primary malignant tissue, high Oct1 protein but not mRNA levels strongly correlate with the frequency of CD24LOCD44HI cancer-initiating cells. Reducing Oct1 expression via RNAi reduces the proportion of ALDHHI and dye effluxHI cells, and increasing Oct1 increases the proportion of ALDHHI cells. Normal ALDHHI cells harbor elevated Oct1 protein but not mRNA levels. Functionally, we show that Oct1 promotes tumor engraftment frequency and promotes hematopoietic stem cell engraftment potential in competitive and serial transplants. In addition to previously described Oct1 transcriptional targets, we identify four Oct1 targets associated with the stem cell phenotype. Cumulatively, the data indicate that Oct1 regulates normal and cancer stem cell function.
Published in the journal: . PLoS Genet 8(11): e32767. doi:10.1371/journal.pgen.1003048
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003048Summary
Defining master transcription factors governing somatic and cancer stem cell identity is an important goal. Here we show that the Oct4 paralog Oct1, a transcription factor implicated in stress responses, metabolic control, and poised transcription states, regulates normal and pathologic stem cell function. Oct1HI cells in the colon and small intestine co-express known stem cell markers. In primary malignant tissue, high Oct1 protein but not mRNA levels strongly correlate with the frequency of CD24LOCD44HI cancer-initiating cells. Reducing Oct1 expression via RNAi reduces the proportion of ALDHHI and dye effluxHI cells, and increasing Oct1 increases the proportion of ALDHHI cells. Normal ALDHHI cells harbor elevated Oct1 protein but not mRNA levels. Functionally, we show that Oct1 promotes tumor engraftment frequency and promotes hematopoietic stem cell engraftment potential in competitive and serial transplants. In addition to previously described Oct1 transcriptional targets, we identify four Oct1 targets associated with the stem cell phenotype. Cumulatively, the data indicate that Oct1 regulates normal and cancer stem cell function.
Introduction
Mammalian somatic stem cells have been identified in blood, lung, intestine, breast, epidermis and other tissues [1]–[6]. Cancer stem cells (or cancer-initiating cells) have been defined in a variety of developmentally heterogeneous neoplasms [7]–[11]. Two functional properties are consistently used to define both normal stem cells and cancer stem cells (CSCs): the ability to self-renew and the ability to generate progeny cells with more differentiated phenotypes [12]. Because CSCs may have metastatic ability, and are thought to be chemo - and radio-resistant and thus provide a reservoir for replenishing tumor mass [13]–[15], there is interest in identifying cellular activities that regulate CSC populations. Similarly, the central role of somatic stem cells in maintaining tissue homeostasis places priority on identifying cellular activities governing their function. Wnt-, Notch - and Hedgehog-mediated signaling contributes to the maintenance of certain adult somatic and CSC populations [16]–[18]. Identifying additional regulators would allow for robust stem cell identification and provide possible therapeutic targets.
The Oct1 transcription factor is widely expressed in adult tissues. It is related to Oct4, a regulator of embryonic stem (ES) cell pluripotency, and has similar in vitro DNA binding specificity [19]. Oct1 enforces poised transcriptional states [20] and promotes a glycolytic metabolic profile associated with dampened mitochondrial function and reactive oxygen species (ROS) levels [21]. This metabolic state is emblematic of both tumor cells and stem cells [13], [22]–[25]. Loss of Oct1 has little impact on cell growth and viability in culture, or on immortalization by serial passage, but antagonizes oncogenic transformation in vitro and tumorigenicity in vivo [21]. Oct1 also promotes resistance to genotoxic and oxidative stresses, a phenotype observed in stem cells [26]. Oct1 message levels are increased in some forms of gastric cancer [27], however consistent changes in Oct1 gene expression have not been noted for most malignancies. Oct1 protein levels and post-translational modification states in stem cell compartments and malignancy have not been studied.
Here we show that Oct1 controls multiple stem cell phenotypes in normal and tumor cells. In epithelial cells, strong Oct1 protein expression spatially correlates with stem cell niches and high levels of ALDH1, Lrig1 and Lgr5, known stem cell markers. Elevated Oct1 protein expression also correlates with elevated ALDHHI and CD24LOCD44HI stem cell-like populations in tumor cell lines and primary breast cancer samples, respectively. In contrast, the correlation with Oct1 mRNA expression is poor. Using ALDH and dye efflux activity as readouts, we demonstrate that Oct1 ablation selectively depletes stem-like populations in multiple human tumor cell lines. Abcg2, Abcb1, Abcb4 and Aldh1a1, genes associated with dye efflux and ALDH activity, are direct Oct1 targets. We show that stable Oct1 knockdown in two different tumor cell lines reduces tumor-initiating frequency, while Oct1 ectopic expression increases tumor initiation. Finally, we show that Oct1 deficient fetal liver hematopoietic progenitors manifest engraftment defects in competitive and serial transplantation situations. The results indicate that Oct1 protein can be used as a stem cell marker, and that Oct1 is a normal and malignant stem cell determinant.
Results
Elevated Oct1 protein expression is associated with epithelial stem cells
We examined Oct1 expression in frozen human colon sections using indirect immunofluorescence (IF). The Oct1 antibody (Millipore) did not cross-react to human Oct2, Oct4 or Oct6 in control experiments (Figure S1). IF revealed a sub-population of intensely staining Oct1HI cells, one cell removed from the lumen but clearly within the basement membrane that defines the crypt (Figure 1A, arrows). Similar findings were made using immunohistochemistry (Figure S2). To examine the location and identity of these cells more closely, we used paraffin-embedded normal human colon sections with antibodies to Oct1 and ALDH1, which has been shown to mark somatic stem cells in multiple compartments, including the colon [9], [28], [29]. Oct1 and ALDH1 staining were widely detectable at low levels relative to controls lacking primary antibodies (not shown), consistent with the wide expression of these proteins. However, in a subset of cells, including within gut crypts, more intense co-staining was evident (Figure 1B). Merging the fields confirmed that cells at the crypt base stained strongly for both proteins (arrows). A few cells (not in the crypt base) displayed strong ALDH1 expression only (Figure 1B, asterisk). We identified multiple additional examples of staining with only one antibody (not shown), indicating that the results are not due to spectral overlap. We analyzed six independent tissue sections corresponding to 117 crypts, identifying 77% of cells with both Oct1HI and ALDH1HI expression. 19% of cells stained strongly with ALDH1 only and 4% with Oct1 only. These findings identified a high concordance between cells with high Oct1 and ALDH1 protein levels.
Fig. 1. Association of Oct1 with normal somatic stem cells. 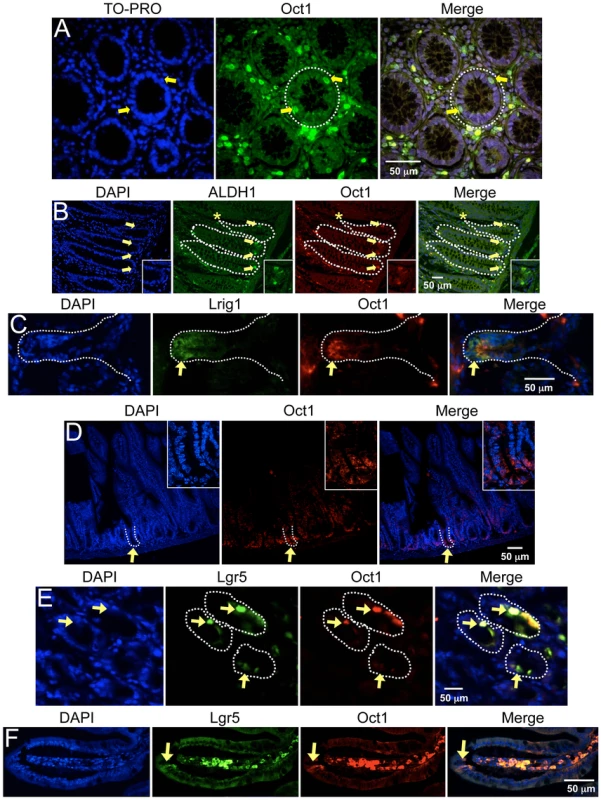
A. IF images of cross-sections from grossly uninvolved colon margins of a male familial adenomatous polyposis patient. Frozen sections were stained with mouse anti-Oct1 antibodies (Millipore MAB5434) and co-stained with TO-PRO. Crypts are shown in cross-section. White dashed circle highlights a crypt. Arrows indicate cells staining strongly for Oct1. B. IF images of colon crypt sections from a normal male individual. Sections were stained with DAPI, and anti-Oct1 and anti-ALDH1a1 antibodies. Merged images are shown at right. White dashed lines highlight crypts. Examples of cells co-staining with Oct1 and ALDH1a1 are highlighted with yellow arrows. An example cell staining with ALDH1a1 only is highlighted with an asterisk. Inset at lower right-hand corner is a digital magnification of the central portion of the image. Sections were formalin-fixed and paraffin-embedded. C. Frozen mouse colon tissue sections were stained with DAPI, and anti-Lrig1 and anti-Oct1 antibodies. IF images of longitudinal sections are shown. White dashed lines highlight the crypt. D. IF images of mouse small intestine sections. Sections were stained with DAPI and anti-Oct1 antibodies. Merged images are shown at right. White dashed lines highlight a crypt. Inset at upper right-hand corner is a digital magnification of the central portion of the image. Sections were formalin-fixed and paraffin-embedded. E. IF images of cross-sectional duodenum sections from a normal C57BL/6 mouse. Frozen sections were stained with DAPI and anti-Oct1 and anti-Lgr5 antibodies. Merged images are shown at right. Examples of co-staining cells are highlighted with yellow arrows. White dashed lines highlights crypts. F. Longitudinal sections. To extend these findings we also stained frozen mouse colon tissue sections with antibodies against Lrig1, which is highly expressed in stem cells at the crypt base [30], [31]. We identified general co-localization using anti-Oct1 and anti-Lrig1 antibodies, though the Oct1 staining was somewhat more restricted compared to Lrig1 (Figure 1C, arrows). Analysis of Lrig1-positive crypts indicated that 19/30 co-stained with high Oct1 expression. No crypts stained with Oct1 only but not Lrig1.
The small intestine crypt is one of the best-characterized stem cell niches. We examined Oct1 expression in formalin-fixed paraffin-embedded mouse small intestinal (duodenum) tissue. In this case we used different anti-Oct1 antibodies (from Bethyl). As with colon, IF revealed a sub-population of intense-staining Oct1HI cells at the crypt base (Figure 1D, arrows). To examine these cells more closely, we performed co-localization studies using leucine-rich repeat-containing G protein-coupled receptor-5 (Lgr5). Lgr5 marks stem cells in the crypt [32], where it helps transduce Wnt signals [33], [34]. We used frozen normal mouse small intestine (duodenum) sections together with anti-Oct1 and anti-Lgr5 antibodies in IF. Oct1/Lgr5 co-staining was observed (Figure 1E and F, arrows). The signal was not due to nonspecific secondary antibody binding or autofluorescence, as removing either primary antibody eliminated signal only in the appropriate channel (Figure S3). Analysis of Oct1/Lgr5 co-localization using tissue from a green fluorescent protein-Lgr5 knock-in mouse [32] (a gift of H. Clevers) indicated that 27/30 Lgr5-positive crypts co-localized with Oct1 (90%). For Figure 1, longer exposure in the Oct1 channel and comparison to samples processed without the Oct1 antibody indicated that Oct1 was expressed in all cells, but much more strongly expressed in stem cells. Another protein, B lymphoma Moloney murine leukemia virus insertion region homolog-1 (Bmi1), is known to mark a different group of stem cells at the “+4” position in the small intestinal crypt [35]. Using duodenum from a tamoxifen-injected Bmi1-Cre-ER;Rosa26-Cre reporter mouse (a gift of M. Capecchi), we did not observe significant co-localization (0/27 Bmi1-positive crypts, data not shown). Cumulatively these data indicate that high Oct1 protein levels mark a specific population of normal stem cells in both colon and small intestine. The high Oct1 signal in stem cell compartments was not due to peculiarities with one particular antibody, autofluorescence or spectral overlap.
Oct1 correlates with stem cell markers in primary malignant cells
We performed Oct1/ALDH1 IF on malignant human breast carcinoma sections (estrogen receptorPOS, progesterone receptorPOS, Her2NEG). In addition to somatic stem cells, ALDH1 expression marks CSCs, including in breast and colon [9], [28], [36]. Dual staining was evident (Figure 2A, arrows) though again we observed examples of cells that stained with Oct1 and not ALDH1 and vice-versa (asterisks). These results indicate that Oct1 levels are elevated in a subset of breast cancer cells that also express high levels of ALDH1.
Fig. 2. Oct1 protein levels correlate with a stem cell phenotype in primary human malignancy. 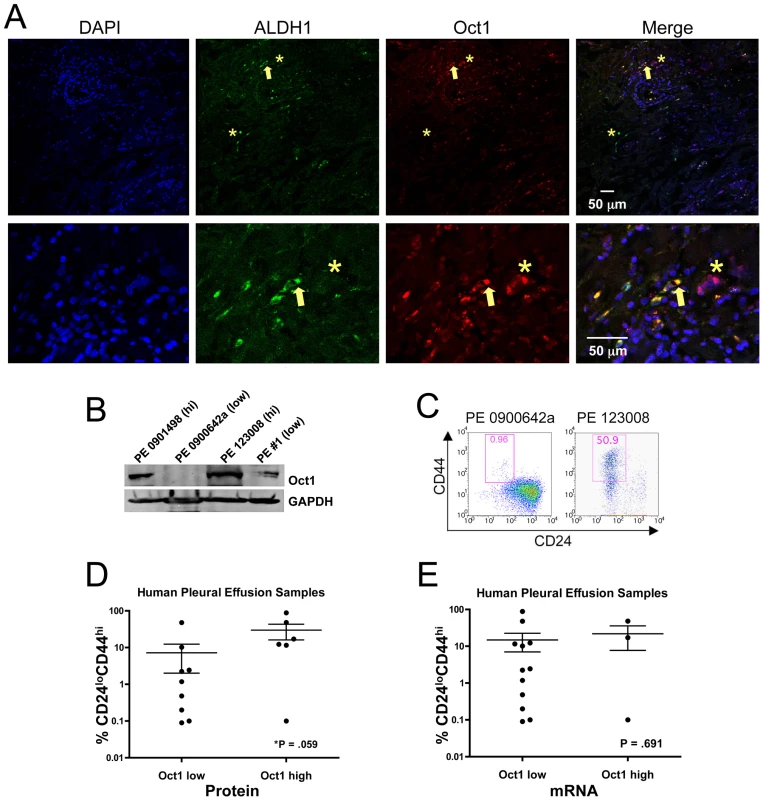
A. IF images are shown. Frozen malignant breast carcinoma sections (non-familial stage IIIA node-positive infiltrating ductal carcinoma, ER+PR+HER2−) were stained with mouse anti-Oct1 antibodies (Millipore MAB5434) and rabbit anti-ALDH1 antibodies (Abcam ab52492). Arrow indicates example double-positive cell. Asterisks show examples of an Oct1HI cell with low ALDH1 and ALDH1HI with low Oct1. Detail is shown in images below. B. Western blot depicting Oct1 levels in a panel of malignant epithelial cells isolated from pleural effusions (human breast carcinoma lung metastases). GAPDH is shown as a loading control. C. Examples of the CD24/44 profile from pleural effusions with highest and lowest Oct1 protein levels. D. Correlation of Oct1 protein and CD24LOCD44HI stem content in 15 individual patient samples (7 ER+PR+HER2−, 5 ER−PR−HER2−, 3 ER−PR−HER2+) collected from 14 different patients. One patient had tumor cells collected twice 22 months apart. Samples were placed into Oct1LO and Oct1HI categories based on Oct1 Western blot signal intensity relative to GAPDH staining. Cells were tested for percentage CD24LOCD44HI stem cell content and plotted. P-value was calculated using the two-tailed student T-test. E. The same analysis for Oct1 mRNA as measured by qRT-PCR. To corroborate these findings, we performed Oct1 Western blotting using primary human metastatic pleural effusion breast carcinoma cells [37]. A variety of subtypes were tested (see figure legend). Unexpectedly, Oct1 protein expression was highly variable. Samples naturally partitioned into Oct1-high and -low categories (e.g., Figure 2B). We then determined whether Oct1 levels correlated with cancer–initiating cell frequency using CD24/44 as a measure of mammary tumor-initiating cells [6]. Pleural effusions with low Oct1 protein displayed low frequencies of CD24LOCD44HILinNEG cells, whereas those with high Oct1 expression displayed a greater proportion. Examples from each category are shown in Figure 2C. Quantification from 15 samples is shown in Figure 2D. The observed differences were significant (P = 0.059). For the samples shown in Figure 2A, Oct1 mRNA levels were modulated in a similar manner to protein, however for other tested samples, changes in Oct1 message levels were insignificant (not shown). Performing the same analysis comparing CD24/44 with Oct1 mRNA yielded an insignificant P-value (Figure 2E). These results suggest that a combination of transcriptional/RNA regulation, but mostly regulation at the level of protein production or stability, underlies Oct1 variation in human breast cancer tissue.
Oct1 controls stem cell markers in multiple tumor cell lines
We used human epithelial tumor cell lines in which we could manipulate Oct1 levels by RNAi and retroviral overexpression. High ALDH activity, as measured by Aldefluor, is a marker of CSCs and tumor cell line populations with stem-like properties [9], [36]. We evaluated ALDH activity following Oct1 RNAi in A549 lung alveolar adenocarcinoma cells infected with pools of lentiviruses expressing scrambled or Oct1-specific shRNAs. Oct1-specific RNAi reduced activity in the main population by less than two-fold as measured by mean fluorescence. However, Oct1-specific RNAi more significantly impacted the number of ALDHHI cells such that the AldefluorHI “tail” collapsed into a more symmetric distribution after Oct1-specific shRNA expression (Figure 3A). Effective RNAi was confirmed by Western blot (Figure 3B). We also studied ALDH activity in two breast cancer cell lines, MDA-MB-231 and MCF-7, using transiently transfected siRNA pools. Oct1 RNAi again minimally affected activity in the main population while significantly reducing the number of ALDHHI cells (Figure S4). Therefore, in three tumor cell lines Oct1 ablation reduces ALDH activity most significantly in ALDHHI cells.
Fig. 3. Oct1 controls the AldefluorHI population in human tumor cell lines. 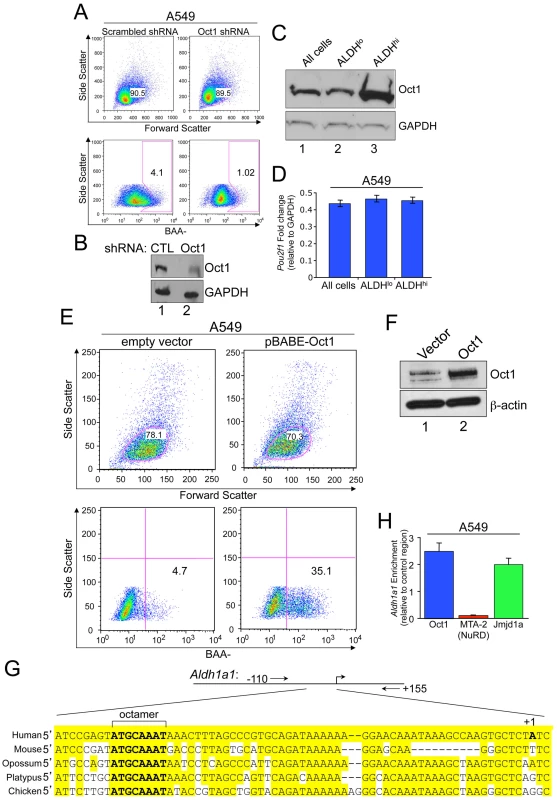
A. Aldefluor staining profile of A549 cells infected with a doxycycline-inducible lentiviral shRNA. MFI was 169.0 for scrambled and 91.2 for Oct1-specific shRNA. A549 cells were infected with control or Oct1-specific lentiviral particles (Santa Cruz), selected with puromycin, and subjected to analysis after 48 hr. B. Efficacy of the A549 knockdown as assessed by Western blotting using anti-Oct1 antibodies and an anti-GAPDH loading control. C. Oct1 Western blot of unsorted normal A549 cells cultured under normal conditions, and sorted AldefluorHI and AldefluorLO populations is shown. GAPDH is used as a loading control. D. The same sorted cells or unsorted cells were subjected to qRT-PCR to determine Oct1 mRNA levels. Levels are show relative to GAPDH. Error bars depict standard deviations. E. Oct1 was ectopically expressed in A549 cells using a retrovirus (pBabe-Oct1) or empty vector. The mixed population of cells was subjected to selection with puromycin, and ALDH activity determined. F. Western blot using anti-Oct1 antibodies of the same cells shown in (E). ß-actin is shown as a loading control. G. Alignment of the Aldh1a1 promoter regions in several example vertebrate species. The conserved perfect octamer sequence centered at approximately −55 bp relative to the transcription start site is highlighted. Alignments were generated using a Clustal W-based algorithm within the Vector NTI software package (Invitrogen). Positions of PCR primer pairs for ChIP amplification are also shown. H. Quantification of ChIP enrichment using A549 cells and specific antibodies directed against Oct1, Mta2 (a component of the NuRD complex) and Jmjd1a. The PCR primer pair spanned the human Aldh1a1 octamer site. ChIP enrichment was quantified relative to isotype control anti-C/EBPß antibodies and relative to a control region as described in the methods section. Values are the average of four independent experiments. Error bars represent standard deviations. Because an increase in Oct1 protein levels in stem-like AldefluorHI cells may underlie the selective effects of Oct1 ablation, we used fluorescence-activated cell sorting (FACS) to isolate normal A549 cells on the basis of Aldefluor activity and compared endogenous Oct1 protein levels. Oct1 levels were significantly increased in the AldefluorHI population relative to unsorted cells (Figure 3C). In contrast, no difference in Oct1 (Pou2f1) mRNA was observed (Figure 3D). If elevated Oct1 protein is responsible for conferring an AldefluorHI phenotype, elevation of Oct1 protein levels should increase the population of AldefluorHI cells. A549 cells were infected with retroviruses encoding human Oct1 or empty vector controls. Oct1 overexpression did not grossly effect cells as measured by forward/side scatter (Figure 3E, top panels) or cell growth or viability [21], but did increase the proportion of AldefluorHI cells (Figure 3E, bottom panels). We confirmed Oct1 overexpression by Western blot (Figure 3F). These findings indicate that Oct1 controls the setpoint of AldefluorHI vs. AldefluorLO A549 cells.
An Oct1 binding site has been identified in the Aldh1a1 immediate promoter region [38]. This site is highly conserved (Figure 3G). We conducted ChIP using normal A549 cells and the Aldh1a1 promoter-proximal region to confirm Oct1 binding. A robust signal was observed using anti-Oct1 antibodies relative to an intergenic region and to an isotype control antibody (Figure 3H). Oct1 has been associated with two transcription cofactors, NuRD (in a negative regulatory capacity) and Jmjd1a (in a positive capacity), in different conditions [20]. ChIP using anti-Jmjd1a or anti-NuRD (Mta2) antibodies resulted in strong enrichment of Jmjd1a but not NuRD (Figure 3H), consistent with Oct1 mediating an activation function at Aldh1a1 in A549 cells.
To further buttress these findings we studied an independent stem cell marker. Normal and cancer stem cells are frequently dye effluxHI such that incubation with Hoechst results in a fraction of cells (the side population, SP) that can be identified by low fluorescence [39]–[41]. Adenosine triphosphate (ATP)-binding cassette (ABC) multidrug transporters mediate this activity and contribute to the relative resistance to cytotoxic compounds associated with a stem cell phenotype [39], [42]. A549 cells contain a robust SP enriched in tumor-initiating cells [43]. To determine whether stable Oct1 knockdown selectively alters the SP, we used a previously established A549 inducible shRNA system [21]. A separate A549 clone inducibly expressing scrambled shRNAs was also used. Cells were stained with Hoechst Red, Hoechst Blue and propidium iodide. Dead cells, which were gated out, did not change significantly in the Oct1 depleted condition (not shown). Although the percentage of cells in the SP varied three-fold from experiment to experiment (and between A549 clones), induction of Oct1 shRNA by the addition of doxycycline uniformly and significantly reduced the SP, while minimally affecting the main population (Figure 4A, bottom panels). In contrast, little effect was observed upon doxycycline treatment of cells stably transduced with scrambled shRNAs (top panels). As expected, the SP was also reduced using the efflux transport inhibitor verapamil (not shown). Oct1 knockdown under these conditions was robust (Figure 4B). Averaged data from three independent experiments is shown in Figure 4C. These data show that Oct1 RNAi specifically decreases the SP in A549 cells.
Fig. 4. Oct1 RNAi diminishes the side population in A549 cells. 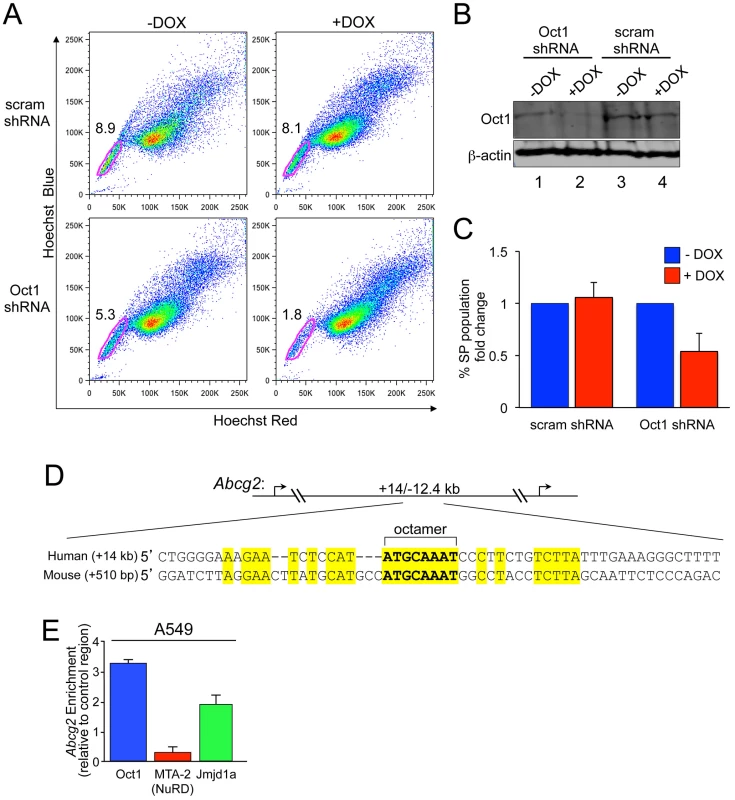
A. SP assay of luciferase-positive A549 cells carrying an inducible scrambled or Oct1-specific shRNA [21]. Cells were treated with doxycycline for 4 days. Side population assays were conducted as described in the methods section. B. Western blot showing Oct1 protein levels in the two cell lines from (A) with and without 4-day culture in doxycycline. ß-actin is shown as a loading control. C. Quantification of the changed in SP percentage from three independent trials. Error bars denote standard deviations. D. Alignment of Abcg2 first intron regions in human and mouse. The perfect octamer sequence in both species is highlighted. Human Abcg2 has two annotated transcription start sites, so the element is located at both +14 kb and −12.4 kb relative to the transcription start sites. E. Quantification of ChIP enrichment using A549 cells and specific antibodies directed against Oct1, Mta2 and Jmjd1a. The PCR primer pair spanned the human Oct1 binding site in Abcg2. ChIP enrichment was quantified relative to isotype control anti-C/EBPß antibodies and relative to a control region as described in the methods section. Values are the average of four independent experiments. Error bars represent standard deviations. ABC transporter G2 (ABCG2, also known as Bcrp1) regulates dye efflux activity, including in A549 cells [43], and stem cell chemoresistance [42]. We identified a consensus Oct1 binding element in the human Abcg2 first intron (Figure 4D). ENCODE consortium data [44] indicates that the related Oct4 transcription factor interacts with this region in human ES cells. There is also a perfect octamer in the mouse Abcg2 first intron (Figure 4D). FASTA alignment of the two sequences indicated that the homology is largely limited to the octamer element. ChIP indicated that Oct1 binds the Abcg2 octamer element-containing region in A549 cells (Figure 4E). As with Aldh1a1, strong enrichment of Jmjd1a but not NuRD was observed (Figure 4E), consistent with Oct1 mediating an activation function at Abcg2.
Previous work identified an oxidative stress response mechanism in which Oct1 phosphorylation alters DNA binding specificity, causing induced Oct1 binding to DNA binding sites more complex than the canonical octamer element [19]. One such site is known as the MORE (More palindromic Octamer Related Element). Using H2O2-treated HeLa cells and ChIPseq, induced Oct1 binding was observed at MORE-containing targets such as Hmgb3, Blcap, Rras and Rras2. We identified another MORE sequence in the ABC transporter Abcb1, at position +250, and strong Oct1 ChIP enrichment at Abcb1 in HeLa and A549 cells exposed to 1 mM H2O2 (Figure S5). Another transporter, Abcb4, is adjacent to Abcb1 on human chromosome 7. ChIPseq previously identified inducible Oct1 binding to Abcb4 following H2O2 exposure [19]. Inspection of this region revealed a MORE (not shown). We confirmed inducible binding in A549 cells (Figure S5).
Oct1 controls tumor engraftment frequency
The above findings indicate that Oct1 controls multiple markers and activities associated with stem cells, but do not address whether Oct1 controls the stem cell phenotype itself. We previously showed that stable Oct1 RNAi in luciferase-expressing A549 cells reduces tumorigenicity in xenograft assays without affecting growth rates in culture [21]. In these experiments, 2×106 cells were transplanted and tumor mass was partially reduced (by approximately 60%). We hypothesized that differences in tumor initiating frequency underlie this effect. We injected reduced numbers of cells harboring scrambled or Oct1-specific shRNAs into opposite flanks of nude mice. Cells were pre-treated with doxycycline for 48 hr and injected into immunocompromised mice maintained on doxycycline. Using 1×106 cells, both the scrambled and Oct1-specific shRNA-expressing cells engrafted 15/15 recipient mice. A further tenfold reduction resulted in 12/13 mice engrafted using cells expressing scrambled shRNAs while cells expressing Oct1-specific shRNAs engrafted 4/13 mice in the contralateral flank (Figure 5A, 5B). The remaining six mice showed no evidence of engraftment as assessed by visual inspection, palpation or bioluminescence. 50,000 cells engrafted poorly (4/15) using scrambled shRNAs and not at all with Oct1-specific shRNAs. Using even fewer cells, 0/15 mice engrafted regardless of Oct1 status (Figure 5B). These findings allowed us to calculate that Oct1 shRNA reduces the frequency of initiating cells from ∼1/96,000 to ∼1/350,000 (Figure 5A). We also over-expressed Oct1 in luciferase-expressing A549 cells. In this case no pre-treatment took place and the mice were not administered doxycycline. Oct1 overexpression was moderate (Figure 3F). This level of Oct1 expression leads to a >2-fold increase in TIC frequency (Figure 5C). Similar results were obtained with MDA-MB-231 human breast adenocarcinoma cells and mammary fat pad engraftment. Using Oct1 lentiviral knockdown, TIC frequency shifted downwards from ∼1/50,000 to ∼1/175,000 (Figure 5D–5F). Oct1 overexpression increased TIC frequency ∼3-fold from ∼1/50,000 to ∼1/17,000 (Figure 5G–5H).
Fig. 5. Oct1 controls tumor initiation frequency in A549 and MDA-MB-231 cells. 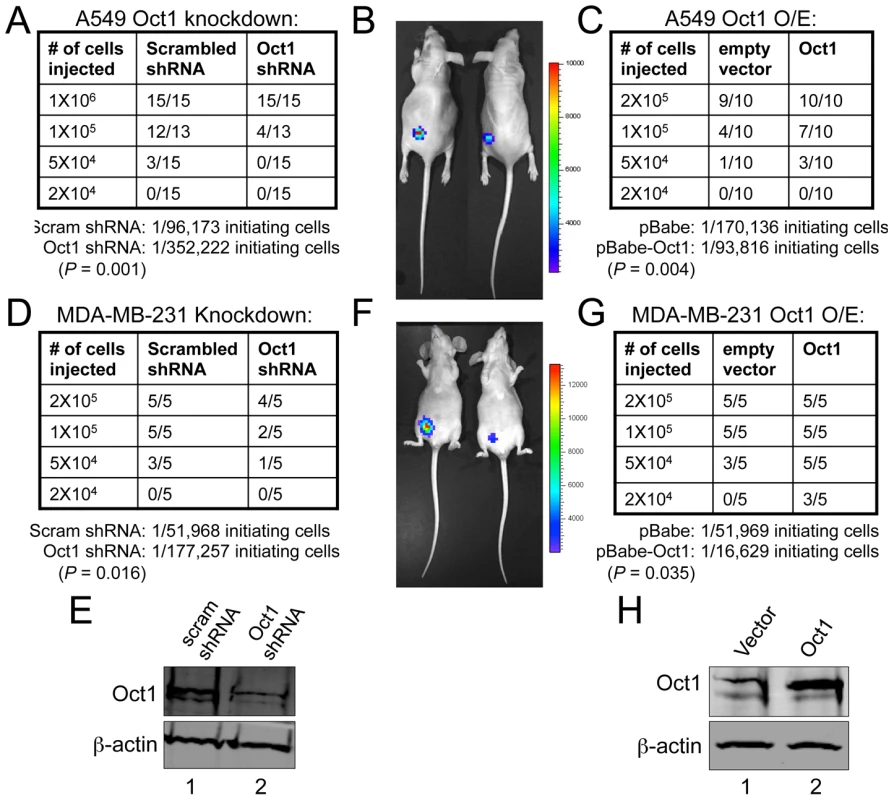
A. Numbers of nude mice engrafted in the left flank with A549 cells expressing the same Oct1-specific shRNA as shown in Figure 4. Scrambled shRNAs in the contralateral flank were used as controls. The fraction of mice successfully engrafted is shown in tabular format. For the Oct1-specific and control shRNA lines, calculation of TIC frequency is shown at bottom. B. Images of engrafted tumors from animals receiving 1×105 cells. Left flank: scrambled shRNA. Right flank: Oct1-specific shRNA. C. Similar to (A), except human Oct1 was over-expressed in luciferase-expressing A549 cells using retroviral gene transduction. D. 4th inguinal mammary fat pads of nude mice were engrafted with the indicated number of luciferase-expressing MDA-MB-231 cells expressing scrambled or Oct1-specific shRNAs. The fraction of engrafted animals is shown in tabular format. E. Western blot showing efficacy of lentiviral knockdown. ß-actin is shown as a loading control. F. Images of engrafted tumors from animals receiving 2×105 cells. Ventral view is shown. Right side: scrambled shRNAs. Left side: Oct1-specific shRNAs. G. Similar to (D) except human Oct1 was overexpressed using retroviral gene transduction. H. Western blot showing degree of Oct1 overexpression in cells used in (G). ß-actin is shown as a loading control. Oct1 regulates hematopoietic transplantation potential
Germline Oct1 deletion results in early embryonic lethality due to defects in extra-embryonic lineages, in particular trophoblast stem cells [45]. A slightly less severe Oct1 deficient allele dies over a wider developmental window beginning at mid-gestation (E11.5) and exhibits pale fetal liver, reduced ß-globin gene expression and reduced Ter119-positive cells [46]. Although these phenotypes are consistent with impaired hematopoietic stem cell (HSC) function, using this allele it was found that Oct1 deficient fetal livers reconstitute long-term B and T lymphopoiesis in adult recipients [47] and erythropoiesis in lethally irradiated hosts (not shown). To more carefully assess a cell-intrinsic role of Oct1 in hematopoiesis, we performed serial transplants, and competitive transplants using congenic markers.
In primary transplants Oct1 deficient fetal liver engrafted sublethally irradiated Rag1−/− primary recipients as evidenced by the presence of B220+ and Thy1.2+ Oct1 deficient donor B and T cells in peripheral blood (Figure 6A, primary transfer), consistent with prior findings in which engraftment is stable beyond 16 weeks [47]. Wild-type (WT) and Oct1−/− bone marrow from primary recipients also engrafted secondary recipients comparably at 5 weeks (not shown). However at later time points Oct1 deficient cells reproducibly showed nearly complete failure (Figure 6A, secondary transfer). Combined results from 5 independent trials are shown in Figure 6B.
Fig. 6. Loss of Oct1 interferes with hematopoietic engraftment. 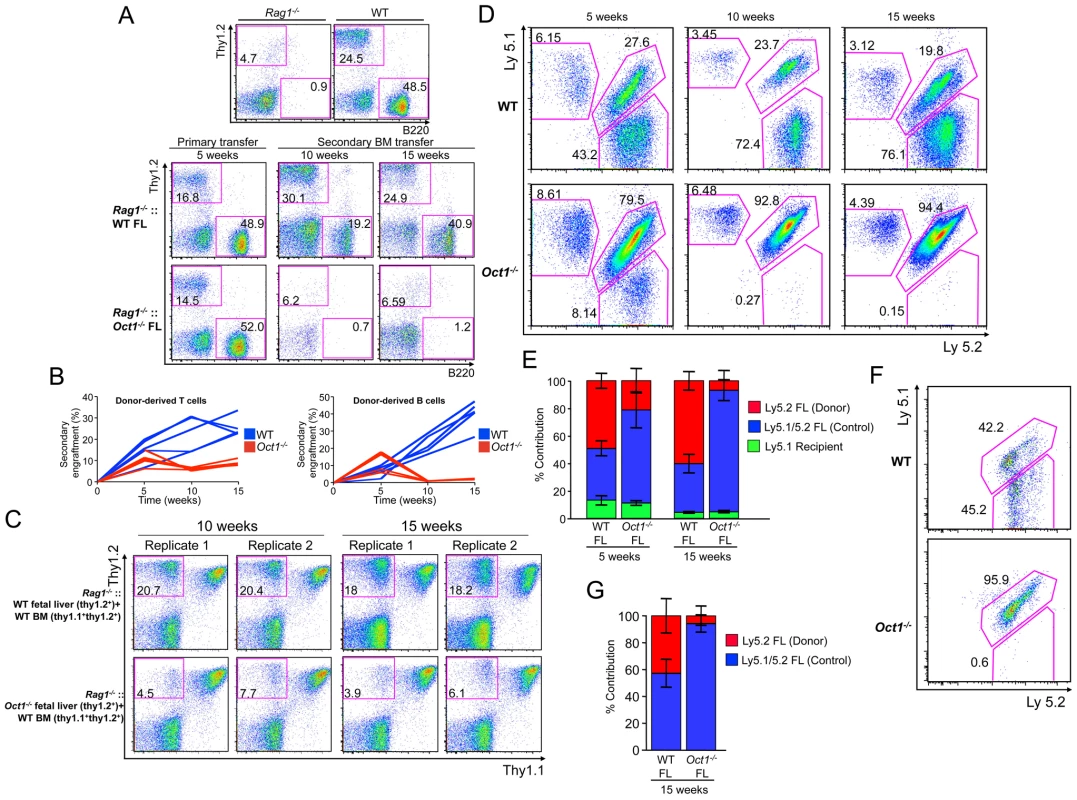
A. Peripheral blood leukocyte B and T cell profiles from primary and secondary animals transplanted with Oct1 deficient and WT littermate control fetal liver (FL). Cells were stained with anti-Thy1.2 and anti-B220 antibodies to reveal T and B cells. Control Rag1−/− and WT animals are shown at the top for comparison. B. B and T cell repopulation is shown as a percentage of total cells for 5 independent experiments. C. Flow cytometry plots showing degree of T cell reconstitution in peripheral blood leukocytes in four Rag1−/− recipient animals. For each animal, WT (top panels) or littermate Oct1 deficient (bottom panels) fetal livers were mixed with Thy1.1+/Thy1.2+ WT bone marrow (BM) and injected retro-orbitally. Peripheral blood repopulation was analyzed at 10 and 15 weeks. D. The same peripheral blood analysis as in (C) was performed using Ly5.1/2 instead of Thy1.1/2 as a congenic marker. A single mouse for each condition is shown. E. Averages of 4 WT and 4 Oct1 deficient competitive repopulations as performed in (D) are shown. Peripheral blood was analyzed at 5 and 15 weeks. Error bars depict +/− standard deviation. F. Bone marrow from the mice in (D) were gated on LSK and analyzed for Ly5 expression. G. Averages of the 4 WT and 4 Oct1 deficient competitive repopulations at the level of LSK bone marrow precursors. Thy1.2+ WT or Oct1 deficient fetal liver cells were combined 1∶1 with Thy1.1+/Thy1.2+ WT bone marrow for competitive reconstitutions. Unlike WT littermate controls, Oct1−/− fetal liver cells engrafted poorly in the presence of WT bone marrow (Figure 6C). Similar results were obtained using the Ly5 marker, which can be used to detect a broader array of blood cells. Ly5.1+ C57BL/6 recipient mice were engrafted with WT or Oct1 deficient Ly5.2+ fetal liver cells combined 1∶1 with WT Ly5.1+/Ly5.2+ fetal liver cells. Again, Oct1 deficient cells were found in peripheral blood at 5 weeks, however large defects were observed, specifically using Oct1 deficient fetal liver, at 15 weeks (Figure 6D). Quantified results from four WT and four Oct1 deficient competitive situations is shown in Figure 6E.
To test whether the engraftment defect arises from a hematopoietic stem/progenitor cell defect, we analyzed LinNEGSca1POSc-kitPOS (LSK) bone marrow hematopoietic precursor cells in the above mice. Defects at least as robust as those seen in peripheral blood were observed in the Oct1 deficient fetal liver cell-derived bone marrow LSK compartment (two mice in Figure 6F, see Figure 6G for the whole cohort). These findings strongly suggest that Oct1 deficient LSK cells are less robust than their normal counterparts in competitive engraftment assays.
We also performed primary transplants using freshly isolated fetal liver cells from Oct1 deficient animals or wild-type littermate controls, or the same cells cultured for two days. Freshly isolated Oct1 deficient cells engrafted lethally irradiated recipient animals as before. Culture of the cells in media containing IL-3, IL-6 and SCF was sufficient to maintain engraftment potential in wild-type cells, but caused complete engraftment failure in Oct1 deficient cells (Figure S6). Oct1 regulates intracellular redox levels [21], [26]. Because culture with antioxidants had been found to correct a similar engraftment defect due to ATM deficiency [48], we incubated the fetal liver cells in cytokine-supplemented media in low-oxygen conditions or in the presence of N-acetylcysteine. Neither treatment restored engraftment potential (Figure S6).
Discussion
Here we show that the Oct1 transcription factor (gene symbol Pou2f1, not to be confused with the organic cation transporter, Oct1) regulates the stem cell phenotype. The expression of Oct1 in multiple tissues, coupled with our findings in both epithelial and hematopoietic cells, indicates that it may control stem cell function in multiple compartments. Epithelial cells in colon and small intestine crypts show variegated Oct1 expression. The observation of variegated Oct1 expression is consistent with work in the developing eye [49]. Cells with high Oct1 protein expression also strongly express known stem cell markers, including Lgr5. These findings are consistent with a recent study [50], identifying Oct1 as one of a select group of factors whose protein but not mRNA levels are increased in isolated Lgr5-positive stem cells.
We examined four parameters of CSC phenotype and function: CD24/44 levels, dye efflux, ALDH activity and tumor initiating frequency in xenograft models. Elevated Oct1 expression correlates with ALDH1HI cells in tumor sections, and with the contribution of CD24LOCD44HI cells in breast tumor samples. Oct1 loss of function reduces dye efflux, ALDH activity and tumor initiating frequency in tumor cell lines. Oct1 protein levels are elevated in sorted ALDH1HI populations, and forced Oct1 overexpression increases ALDH1HI cells. Although these assays have their individual limitations [e.g.], [ 51,52], the common finding of an underlying role for Oct1 suggests that it is a controller of the CSC phenotype.
In contrast to Oct1 protein, the association between Oct1 mRNA levels and stem cell phenotypes is poor. This observation is consistent with findings that Oct1 target sites are highly enriched in the promoters of significantly up-regulated genes in lung and breast adenocarcinoma, leukemia, and myeloid leukemia stem cells without concomitant increases in Oct1 mRNA levels [53]–[57]. Elevated expression of Oct1 protein, but not mRNA, in stem cells may be due to increased rates protein synthesis, decreased rates of destruction, or both. Oct1 is known to be ubiquitinated [58] suggesting that regulated protein stability may be important, but the mechanism is unknown. Much of the increased Oct1 protein in stem cells appears to be cytoplasmic (Figure 1). The role of this cytoplasmic Oct1 is currently unknown, however Oct1 can be regulated at the level of nuclear/cytoplasmic localization [59], [60]. In addition, transcriptionally active Oct1 residing in the nucleus may be post-translationally modified in a way that alters its activity. Oct1 activity is regulated by cyclic AMP [60], cellular stress signals [61] and MAP kinase activity [20], however Oct1 post-translational modification states in stem cells and malignancy have not been carefully studied.
Oct1 may control stem cell phenotypes, in part, through its ability to regulate metabolism. Oct1 controls the expression of metabolic genes such as Pcx and Pdk4 and dampens ROS levels [21], [26]. Reactive oxygen species (ROS) negatively modulate stem cell maintenance and self-renewal [62]. Stem cells are frequently characterized by glycolytic metabolic states and low ROS [13], [24]. Other relevant Oct1 targets include Hmgb3 [61], with controls HSC function [63]. Here we show that Oct1 also associates with sites in the Abcg2, Aldh1a1, Abcb1 and Abcb4 target genes.
Loss of Oct1 reduces engraftment potential in competitive and serial hematopoietic repopulation assays, and compromises the LSK stem/progenitor cell compartment in competitive transplants. These results are consistent with fetal HSC deficiency, though because LSK is an impure population it is formally possible that Oct1 deficient HSCs engraft but function poorly. Bmi1 loss of function also results in hematopoietic failure [64]. As with Bmi1 [24], Oct1 is linked to mitochondrial function and the DNA damage response [21], [26], [61]. Hematopoietic defects are less readily apparent with Oct1 as compared to Bmi1. Both Dnmt1 deficiency and combined FOXO1/3/4 deficiency manifest milder hematopoietic defects similar to Oct1 [65], [66].
Previous studies identified embryonic pluripotency gene expression signatures in aggressive human breast carcinomas and in myeloid leukemia stem cells without observed Oct4 expression, suggesting a potential role for Oct4 paralogs [55], [56]. Oct1 and Oct4 share numerous common targets [19] and common modes of upstream regulation [61]. Therefore, an attractive model is that in those phenotypes common to ES cell pluripotency and somatic/cancer stem cells, Oct1 expressed at high levels mediates a subset of Oct4 functions. This may be particularly true if, in response to signals, Oct1 assumes additional or augmented functionalities.
Materials and Methods
Indirect immunofluorescence
For Figure 1A, frozen sections were fixed using 3.7% paraformaldehyde in phosphate buffered saline (PBS), and permeabilized using PBS with 0.05% Tween 20 (Sigma). Cells were stained with a mouse anti-Oct1 antibody (Milipore MAB5434), counter-stained using TO-PRO. Formalin-fixed paraffin-embedded human tissue microarrays (Imgenex) were used for images in Figure 1B and Figure 2A. Formalin-fixed paraffin-embedded mouse small intestine tissue blocks were sectioned and used for Figure 1D. Deparaffinization, hydration and antigen retrieval of human formalin-fixed paraffin-embedded tissue sections was performed as follows: Slides were incubated in a dry oven at 62°C for 1 hour, then dewaxed in xylene for 5×4 minutes, and hydrated in 100%, 95% and 75% ethanol for 2×3 minutes each. Slides were immersed in tap water for 5 minutes, then immersed in citrate buffer (0.01 M, pH 6.0), and microwaved on medium power for 5 min, then on low power for 5 min, and immersed in cold PBS. Non-malignant sections were blocked with 10% horse serum for 30 minutes in a humidified chamber, and incubated with rabbit anti-ALDH1a1 (Abcam ab52492) in 1% horse serum for 2 hours at room temperature. Sections were incubated with goat anti-rabbit Alexa488 (Invitrogen) for 1 hr at room temperature. Sections were blocked a second time with 1% BSA for 30 minutes at room temperature, then incubated with mouse anti-Oct1 (Millipore MAB5434) in 1% BSA overnight at 4°C. Sections were then incubated with goat anti-mouse Alexa568 (Invitrogen) for 1 hr at room temperature. Cells were mounted using media containing DAPI (Vector). For panels 1D and 2A, two mixed rabbit anti-Oct1 antibodies (Bethyl, A301-716A, A301-717A) were used. Panel 2A additionally used a mouse anti-ALDH1 antibody (Becton-Dickinson, BD 611194). Frozen mouse small intestine sections were used in panels 1C, 1E and 1F and were fixed in 1% paraformaldehyde then washed in PBS. Antigen retrieval was performed as published [67]. Mouse anti-Oct1 (Millipore MAB5434), goat anti-Lrig1 (R&D Systems AF3688) and rabbit anti-Lgr5 (Abgent AP2745d) were used with the M.O.M. kit (Vector labs) following the vendor protocol. Biotinylated mouse, goat and rabbit secondary antibodies (1∶50 dilution) were added, followed by streptavidin–horseradish peroxidase (Vector Vectastain Elite ABC kit). The signals were enhanced with the TSA kits NEL 741/744 (Perkin Elmer) according to the manufacturer's protocol, with fluorescein and Cy3 as the fluorophores.
Cell culture
A549 and HeLa cells were maintained in DMEM (Invitrogen) supplemented with 10% serum (1∶1 calf∶fetal calf, Atlanta Biologicals), 6 mM L-glutamine/50 U/ml penicillin/50 µg/ml streptomycin (Invitrogen), and 50 µM ß-mercaptoethanol (Sigma). A549 cells expressing constitutive firefly luciferase and tet-inducible Oct1 shRNA were cultured and maintained as described previously [21]. MDA-MB-231 cells were engineered to express luciferase using a hygromycin-selectable cassette as for A549 cells [21]. Pleural effusions (PEs) from breast cancer patients were initially pelleted, and red blood cells were lysed by resuspending the pellet in ACK lysis buffer (150 mM NH4Cl, 10 mM KHCO3, 0.1 mM Na2EDTA) and incubating at room temperature for 10 min. The cells were re-pelleted and washed with DMEM/F-12 medium three times. During these washes, cancer cells were collected with rapid (1 minute) spins to enrich the pellet with tumor organoids. These cells were either immediately frozen in a solution of 10% DMSO/90% FBS, or maintained in DMEM/F12 1∶1 supplemented with 10 mM Hepes, 5% fetal bovine serum, 1 mg/ml bovine serum albumin, 1 µg/ml insulin with transferrin/selenium (Inivtrogen), 0.5 µg/ml hydrocortisone and 50 µg/ml gentamycin. All cells were maintained in 5% CO2 and air in a humidified 37°C incubator.
Hematopoietic transplantations
Fetal liver cells were genotyped and transplanted, and Rag1−/− recipients analyzed as previously described [47]. Bone marrow cells (from femur and tibia) were depleted of red blood cells using ACK lysis buffer for 1 minute at room temperature. T cells were depleted using a biotinylated CD3 antibody (eBioscience) and anti-biotin microbeads (Miltenyi). For competitive transplants, 1.5×106 Thy1.2+ WT or Oct1 deficient fetal liver cells were combined 1∶1 with Thy1.1+/Thy1.2+ WT bone marrow depleted of CD3+ T cells, and transplanted into Rag1−/− recipients via retro-orbital injection. For serial transplants, 1.5×106 bone marrow cells from primary Rag1−/− recipient mice were used in the secondary transplant. Lethal radiation of WT C57BL/6 recipient animals was achieved with a split dose of 2×4.5 cGy, spaced 1 hr apart.
Flow cytometry
For CD24/44 staining, PE cells were cultured overnight to allow the epithelial cells to adhere. Cells were then washed with PBS to deplete dead and hematopoietic cells. Epithelial cells were removed with trypsin-EDTA. Cells were stained with 7-AAD, anti-CD24, anti-CD44 and antibodies against lineage markers (CD2, CD3, CD10, CD16, CD18, CD31, CD64, CD140b) as published [6]. Non-viable and lineage-positive cells were gated out. CD24LOCD44HI cell quantification was determined without prior knowledge of Oct1 expression levels. Aldehyde dehydrogenase activity was measured in cells as described [36] using the Aldefluor kit (Stem Cell Technologies) with 125 ng ALDH substrate and 100 mM DEAB (Sigma-Aldrich). Hoechst side population assays were performed as described [68], with the following modifications: dye incubation was performed in DMEM with 10% FBS, and the buffer for flow cytometry was PBS with 1 mM EDTA/0.5 mM EGTA. Cells were cultured in doxycycline (2 µg/ml) four days prior to analysis. Cells were co-stained with propidium iodide and dead cells were gated out from the analysis. Bone marrow LSK precursor cells were identified as described [69].
qRT–PCR
RNA was isolated using Trizol (Invitrogen), followed by RNAeasy cleanup (Qiagen). cDNA was synthesized using Superscript III and random hexamers (Invitrogen). For Pou2f1 RT-PCR, 100 ng of cDNA was used for quantitative RT-PCR using a LightCycler 480 (Roche). ΔCt values were determined by subtracting input DNA, and ΔΔCt was determined by subtracting the ΔCt value for control primers. The ΔΔCt were converted to fold change using the formula fold change = 2eΔΔCt and were averaged. Sequences for Pou2f1 qRT-PCR were: Pou2f1 forward, 5′ AAAAGAAATCAACCCACCAAGC; Pou2f1 reverse, 3′ GCTAGTCACAAGGCTTGGTGT. Sequences for Gapdh qRT-PCR were: Gapdh forward, 5′ GGCCAAGGTCATCCATGACAA; Gapdh reverse, 3′AGGGGCCATCCACAGTCTTCT.
RNAi
A549 cells were infected with lentiviral particles containing scrambled or pooled Oct1-specific shRNAs (Santa Cruz), and selected using puromycin. Inducible shRNA knockdown of Oct1 using A549 cells transduced with lentiviruses encoding three different shRNAs was described previously [21].
Chromatin immunoprecipitation
A combination of two rabbit anti-Oct1 antibodies (Bethyl, A301-716A, A301-717A) were mixed and used for Oct1 immunoprecipitation. Anti-Jmjd1a and anti-NuRD (Mta2) antibodies were purchased from Abcam. ChIP conditions for the Aldh1a1 and Abcg2 regulatory regions were described previously [20]. Primer sequences for enrichment at Aldh1a1 were: For, 5′ TTGAATCTTCAAATCGGTGAGTAGG; Rev, 5′ AAGTTTAAAGTCAAAGGCTTCCTGC. Primer sequences for enrichment at Abcg2 were: For, 5′ ATGGCTTTACACTTTACCTGATCCC; Rev, 5′ TGAATGACATAGGTAGACCAGCACG. Intergenic primers were from an intergenic region of human chromosome 19 between the Gadd45b and Lmnb2 loci. The sequences were: F2395, 5′ TTCTATGCCAAGCCCATTCTAGGTC; F2396 5′ GAGAGGCTCTGTCTGAGGTCACG. ChIP grade rabbit control IgG was purchased from Abcam (ab46540).
Tumor xenograft
Luciferase-expressing A549 cells with inducible Oct1 shRNA knockdown [21] were cultured in the presence of doxycycline (2 µg/ml) for 48 hr and the indicated number injected subcutaneously into NCr nude mice (Taconic) provided with 2 mg/ml doxycycline in the drinking water two days prior and throughout the assay. Luciferase-expressing MDA-MB-231 cells were infected with Oct1-specific or control lentiviral knockdown constructs and implanted into the 4th inguinal mammary fat pads of nude mice. For both cell lines, tumor engraftment was calculated at 8 weeks. Analyses were computed as previously described [70]. Briefly, analyses used the ‘statmod’ software package for the R computing environment (http://www.R-project.org). Tumor initiating cell frequencies were estimated using a complementary log-log generalized linear model. Two-sided 95% Wald confidence intervals were computed, except in the case of zero outgrowths, when one-sided 95% Clopper-Pearson intervals were used instead. The single-hit assumption was tested as recommended and was not rejected for any dilution series (P>0.05).
Ethics statement
The study makes use of laboratory mouse models and primary human tissue. The latter were supplied commercially and from institutional samples. In those cases where institutional samples were used, institutional review board approval covering their use is on file at the University of Utah under the authors' names. Similarly, institutional animal care and use committee approval is present for all mouse procedures, and is on file under the authors' names. All procedures conformed to relevant regulatory standards.
Supporting Information
Zdroje
1. KimCF, JacksonEL, WoolfendenAE, LawrenceS, BabarI, et al. (2005) Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell 121 : 823–835.
2. EmaH, TakanoH, SudoK, NakauchiH (2000) In vitro self-renewal division of hematopoietic stem cells. J Exp Med 192 : 1281–1288.
3. ClaytonE, DoupeDP, KleinAM, WintonDJ, SimonsBD, et al. (2007) A single type of progenitor cell maintains normal epidermis. Nature 446 : 185–189.
4. HeXC, YinT, GrindleyJC, TianQ, SatoT, et al. (2007) PTEN-deficient intestinal stem cells initiate intestinal polyposis. Nat Genet 39 : 189–198.
5. PottenCS, BoothC, TudorGL, BoothD, BradyG, et al. (2003) Identification of a putative intestinal stem cell and early lineage marker; musashi-1. Differentiation 71 : 28–41.
6. Al-HajjM, WichaMS, Benito-HernandezA, MorrisonSJ, ClarkeMF (2003) Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A 100 : 3983–3988.
7. BonnetD, DickJE (1997) Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med 3 : 730–737.
8. YilmazOH, ValdezR, TheisenBK, GuoW, FergusonDO, et al. (2006) Pten dependence distinguishes haematopoietic stem cells from leukaemia-initiating cells. Nature 441 : 475–482.
9. GinestierC, HurMH, Charafe-JauffretE, MonvilleF, DutcherJ, et al. (2007) ALDH1 Is a Marker of Normal and Malignant Human Mammary Stem Cells and a Predictor of Poor Clinical Outcome. Cell Stem Cell 1 : 555–567.
10. LiuS, GinestierC, Charafe-JauffretE, FocoH, KleerCG, et al. (2008) BRCA1 regulates human mammary stem/progenitor cell fate. Proc Natl Acad Sci U S A 105 : 1680–1685.
11. BoikoAD, RazorenovaOV, van de RijnM, SwetterSM, JohnsonDL, et al. (2010) Human melanoma-initiating cells express neural crest nerve growth factor receptor CD271. Nature 466 : 133–137.
12. ReyaT, MorrisonSJ, ClarkeMF, WeissmanIL (2001) Stem cells, cancer, and cancer stem cells. Nature 414 : 105–111.
13. DiehnM, ChoRW, LoboNA, KaliskyT, DorieMJ, et al. (2009) Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature 458 : 780–783.
14. PhillipsTM, McBrideWH, PajonkF (2006) The response of CD24(-/low)/CD44+ breast cancer-initiating cells to radiation. J Natl Cancer Inst 98 : 1777–1785.
15. BaoS, WuQ, McLendonRE, HaoY, ShiQ, et al. (2006) Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 444 : 756–760.
16. DierksC, BeigiR, GuoGR, ZirlikK, StegertMR, et al. (2008) Expansion of Bcr-Abl-positive leukemic stem cells is dependent on Hedgehog pathway activation. Cancer Cell 14 : 238–249.
17. FarnieG, ClarkeRB (2007) Mammary stem cells and breast cancer-role of notch signalling. Stem Cell Rev 3 : 169–175.
18. SanchoE, BatlleE, CleversH (2004) Signaling pathways in intestinal development and cancer. Annu Rev Cell Dev Biol 20 : 695–723.
19. KangJ, ShakyaA, TantinD (2009) Stem cells, stress, metabolism and cancer: a drama in two Octs. Trends Biochem Sci 34 : 491–499.
20. ShakyaA, KangJ, ChumleyJ, WilliamsMA, TantinD (2011) Oct1 is a switchable, bipotential stabilizer of repressed and inducible transcriptional states. J Biol Chem 286 : 450–459.
21. ShakyaA, CookseyR, CoxJE, WangV, McClainDA, et al. (2009) Oct1 loss of function induces a coordinate metabolic shift that opposes tumorigenicity. Nat Cell Biol 11 : 320–327.
22. TsatmaliM, WalcottEC, CrossinKL (2005) Newborn neurons acquire high levels of reactive oxygen species and increased mitochondrial proteins upon differentiation from progenitors. Brain Res 1040 : 137–150.
23. SmithJ, LadiE, Mayer-ProschelM, NobleM (2000) Redox state is a central modulator of the balance between self-renewal and differentiation in a dividing glial precursor cell. Proc Natl Acad Sci U S A 97 : 10032–10037.
24. LiuJ, CaoL, ChenJ, SongS, LeeIH, et al. (2009) Bmi1 regulates mitochondrial function and the DNA damage response pathway. Nature 459 : 387–392.
25. MohyeldinA, Garzon-MuvdiT, Quinones-HinojosaA (2010) Oxygen in Stem Cell Biology: A Critical Component of the Stem Cell Niche. Cell Stem Cell 7 : 150–161.
26. TantinD, Schild-PoulterC, WangV, HacheRJ, SharpPA (2005) The octamer binding transcription factor Oct-1 is a stress sensor. Cancer Res 65 : 10750–10758.
27. AlmeidaR, AlmeidaJ, ShoshkesM, MendesN, MesquitaP, et al. (2005) OCT-1 is over-expressed in intestinal metaplasia and intestinal gastric carcinomas and binds to, but does not transactivate, CDX2 in gastric cells. J Pathol 207 : 396–401.
28. HuangEH, HynesMJ, ZhangT, GinestierC, DontuG, et al. (2009) Aldehyde dehydrogenase 1 is a marker for normal and malignant human colonic stem cells (SC) and tracks SC overpopulation during colon tumorigenesis. Cancer Res 69 : 3382–3389.
29. HessDA, WirthlinL, CraftTP, HerrbrichPE, HohmSA, et al. (2006) Selection based on CD133 and high aldehyde dehydrogenase activity isolates long-term reconstituting human hematopoietic stem cells. Blood 107 : 2162–2169.
30. WongVW, StangeDE, PageME, BuczackiS, WabikA, et al. (2012) Lrig1 controls intestinal stem-cell homeostasis by negative regulation of ErbB signalling. Nat Cell Biol 14 : 401–408.
31. PowellAE, WangY, LiY, PoulinEJ, MeansAL, et al. (2012) The pan-ErbB negative regulator Lrig1 is an intestinal stem cell marker that functions as a tumor suppressor. Cell 149 : 146–158.
32. BarkerN, van EsJH, KuipersJ, KujalaP, van den BornM, et al. (2007) Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449 : 1003–1007.
33. de LauW, BarkerN, LowTY, KooBK, LiVS, et al. (2011) Lgr5 homologues associate with Wnt receptors and mediate R-spondin signalling. Nature 476 : 293–297.
34. CarmonKS, GongX, LinQ, ThomasA, LiuQ (2011) R-spondins function as ligands of the orphan receptors LGR4 and LGR5 to regulate Wnt/beta-catenin signaling. Proc Natl Acad Sci U S A 108 : 11452–11457.
35. SangiorgiE, CapecchiMR (2008) Bmi1 is expressed in vivo in intestinal stem cells. Nat Genet 40 : 915–920.
36. Charafe-JauffretE, GinestierC, IovinoF, WicinskiJ, CerveraN, et al. (2009) Breast cancer cell lines contain functional cancer stem cells with metastatic capacity and a distinct molecular signature. Cancer Res 69 : 1302–1313.
37. DeRoseYS, WangG, LinYC, BernardPS, BuysSS, et al. (2011) Tumor grafts derived from women with breast cancer authentically reflect tumor pathology, growth, metastasis and disease outcomes. Nat Med 17 : 1514–1520.
38. YanagawaY, ChenJC, HsuLC, YoshidaA (1995) The transcriptional regulation of human aldehyde dehydrogenase I gene. The structural and functional analysis of the promoter. J Biol Chem 270 : 17521–17527.
39. WuC, AlmanBA (2008) Side population cells in human cancers. Cancer Lett 268 : 1–9.
40. SummerR, KottonDN, SunX, MaB, FitzsimmonsK, et al. (2003) Side population cells and Bcrp1 expression in lung. Am J Physiol Lung Cell Mol Physiol 285: L97–104.
41. GoodellMA, BroseK, ParadisG, ConnerAS, MulliganRC (1996) Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med 183 : 1797–1806.
42. ZhouS, SchuetzJD, BuntingKD, ColapietroAM, SampathJ, et al. (2001) The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat Med 7 : 1028–1034.
43. ScharenbergCW, HarkeyMA, Torok-StorbB (2002) The ABCG2 transporter is an efficient Hoechst 33342 efflux pump and is preferentially expressed by immature human hematopoietic progenitors. Blood 99 : 507–512.
44. BirneyE, StamatoyannopoulosJA, DuttaA, GuigoR, GingerasTR, et al. (2007) Identification and analysis of functional elements in 1% of the human genome by the ENCODE pilot project. Nature 447 : 799–816.
45. SebastianoV, DalvaiM, GentileL, SchubartK, SutterJ, et al. (2010) Oct1 regulates trophoblast development during early mouse embryogenesis. Development 137 : 3551–3560.
46. WangVEH, SchmidtT, ChenJ, SharpPA, TantinD (2004) Embryonic Lethality, Decreased Erythopoiesis, and Defective Octamer-Dependent Promoter Activation in Oct-1-Deficient Mice. Mol Cell Biol 24 : 1022–1032.
47. WangVE, TantinD, ChenJ, SharpPA (2004) B cell development and immunoglobulin transcription in Oct-1-deficient mice. Proc Natl Acad Sci U S A 101 : 2005–2010.
48. ItoK, HiraoA, AraiF, MatsuokaS, TakuboK, et al. (2004) Regulation of oxidative stress by ATM is required for self-renewal of haematopoietic stem cells. Nature 431 : 997–1002.
49. DonnerAL, EpiskopouV, MaasRL (2007) Sox2 and Pou2f1 interact to control lens and olfactory placode development. Dev Biol 303 : 784–799.
50. MunozJ, StangeDE, SchepersAG, van de WeteringM, KooBK, et al. (2012) The Lgr5 intestinal stem cell signature: robust expression of proposed quiescent ‘+4’ cell markers. Embo J 31 : 3079–3091.
51. BroadleyKW, HunnMK, FarrandKJ, PriceKM, GrassoC, et al. (2011) Side population is not necessary or sufficient for a cancer stem cell phenotype in glioblastoma multiforme. Stem Cells 29 : 452–461.
52. QuintanaE, ShackletonM, SabelMS, FullenDR, JohnsonTM, et al. (2008) Efficient tumour formation by single human melanoma cells. Nature 456 : 593–598.
53. LiL, LiM, SunC, FranciscoL, ChakrabortyS, et al. (2011) Altered hematopoietic cell gene expression precedes development of therapy-related myelodysplasia/acute myeloid leukemia and identifies patients at risk. Cancer Cell 20 : 591–605.
54. ReymannS, BorlakJ (2008) Transcription profiling of lung adenocarcinomas of c-myc-transgenic mice: identification of the c-myc regulatory gene network. BMC Syst Biol 2 : 46.
55. Ben-PorathI, ThomsonMW, CareyVJ, GeR, BellGW, et al. (2008) An embryonic stem cell-like gene expression signature in poorly differentiated aggressive human tumors. Nat Genet 40 : 499–507.
56. SomervailleTC, MathenyCJ, SpencerGJ, IwasakiM, RinnJL, et al. (2009) Hierarchical maintenance of MLL myeloid leukemia stem cells employs a transcriptional program shared with embryonic rather than adult stem cells. Cell Stem Cell 4 : 129–140.
57. MattisonJ, KoolJ, UrenAG, de RidderJ, WesselsL, et al. (2010) Novel Candidate Cancer Genes Identified by a Large-Scale Cross-Species Comparative Oncogenomics Approach. Cancer Res 70 : 883–895.
58. KangJ, GoodmanB, ZhengY, TantinD (2011) Dynamic Regulation of Oct1 during Mitosis by Phosphorylation and Ubiquitination. PLoS ONE 6: e23872 doi:10.1371/journal.pone.0023872
59. WangP, JinT (2010) Hydrogen peroxide stimulates nuclear import of the POU homeodomain protein Oct-1 and its repressive effect on the expression of Cdx-2. BMC Cell Biol 11 : 56.
60. WangP, WangQ, SunJ, WuJ, LiH, et al. (2009) POU homeodomain protein Oct-1 functions as a sensor for cyclic AMP. J Biol Chem 284 : 26456–26465.
61. KangJ, GemberlingM, NakamuraM, WhitbyFG, HandaH, et al. (2009) A general mechanism for transcription regulation by Oct1 and Oct4 in response to genotoxic and oxidative stress. Genes Dev 23 : 208–222.
62. ItoK, HiraoA, AraiF, TakuboK, MatsuokaS, et al. (2006) Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nat Med 12 : 446–451.
63. NemethMJ, KirbyMR, BodineDM (2006) Hmgb3 regulates the balance between hematopoietic stem cell self-renewal and differentiation. Proc Natl Acad Sci U S A 103 : 13783–13788.
64. ParkIK, QianD, KielM, BeckerMW, PihaljaM, et al. (2003) Bmi-1 is required for maintenance of adult self-renewing haematopoietic stem cells. Nature 423 : 302–305.
65. TothovaZ, KolliparaR, HuntlyBJ, LeeBH, CastrillonDH, et al. (2007) FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell 128 : 325–339.
66. TrowbridgeJJ, SnowJW, KimJ, OrkinSH (2009) DNA methyltransferase 1 is essential for and uniquely regulates hematopoietic stem and progenitor cells. Cell Stem Cell 5 : 442–449.
67. PhelpsRA, ChidesterS, DehghanizadehS, PhelpsJ, SandovalIT, et al. (2009) A two-step model for colon adenoma initiation and progression caused by APC loss. Cell 137 : 623–634.
68. PatrawalaL, CalhounT, Schneider-BroussardR, ZhouJ, ClaypoolK, et al. (2005) Side population is enriched in tumorigenic, stem-like cancer cells, whereas ABCG2+ and ABCG2 − cancer cells are similarly tumorigenic. Cancer Res 65 : 6207–6219.
69. ChoS, SpangrudeGS (2011) Enrichment of Functionally Distinct Mouse Hematopoietic Progenitor Cell Populations using CD62L. J Immunol 187 : 5203–5210.
70. ShackletonM, VaillantF, SimpsonKJ, StinglJ, SmythGK, et al. (2006) Generation of a functional mammary gland from a single stem cell. Nature 439 : 84–88.
Štítky
Genetika Reprodukční medicína
Článek The Covariate's DilemmaČlánek Plant Vascular Cell Division Is Maintained by an Interaction between PXY and Ethylene SignallingČlánek Lessons from Model Organisms: Phenotypic Robustness and Missing Heritability in Complex Disease
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 11
-
Všechny články tohoto čísla
- Inference of Population Splits and Mixtures from Genome-Wide Allele Frequency Data
- The Covariate's Dilemma
- Plant Vascular Cell Division Is Maintained by an Interaction between PXY and Ethylene Signalling
- Plan B for Stimulating Stem Cell Division
- Discovering Thiamine Transporters as Targets of Chloroquine Using a Novel Functional Genomics Strategy
- Is a Modifier of Mutations in Retinitis Pigmentosa with Incomplete Penetrance
- Evolutionarily Ancient Association of the FoxJ1 Transcription Factor with the Motile Ciliogenic Program
- Genome Instability Caused by a Germline Mutation in the Human DNA Repair Gene
- Transcription Factor Oct1 Is a Somatic and Cancer Stem Cell Determinant
- Controls of Nucleosome Positioning in the Human Genome
- Disruption of Causes Defective Meiotic Recombination in Male Mice
- A Novel Human-Infection-Derived Bacterium Provides Insights into the Evolutionary Origins of Mutualistic Insect–Bacterial Symbioses
- Trps1 and Its Target Gene Regulate Epithelial Proliferation in the Developing Hair Follicle and Are Associated with Hypertrichosis
- Zcchc11 Uridylates Mature miRNAs to Enhance Neonatal IGF-1 Expression, Growth, and Survival
- Population-Based Resequencing of in 10,330 Individuals: Spectrum of Genetic Variation, Phenotype, and Comparison with Extreme Phenotype Approach
- HP1a Recruitment to Promoters Is Independent of H3K9 Methylation in
- Transcription Elongation and Tissue-Specific Somatic CAG Instability
- A Germline Polymorphism of DNA Polymerase Beta Induces Genomic Instability and Cellular Transformation
- Interallelic and Intergenic Incompatibilities of the () Gene in Mouse Hybrid Sterility
- Comparison of Mitochondrial Mutation Spectra in Ageing Human Colonic Epithelium and Disease: Absence of Evidence for Purifying Selection in Somatic Mitochondrial DNA Point Mutations
- Mutations in the Transcription Elongation Factor SPT5 Disrupt a Reporter for Dosage Compensation in Drosophila
- Evolution of Minimal Specificity and Promiscuity in Steroid Hormone Receptors
- Blockade of Pachytene piRNA Biogenesis Reveals a Novel Requirement for Maintaining Post-Meiotic Germline Genome Integrity
- RHOA Is a Modulator of the Cholesterol-Lowering Effects of Statin
- MIG-10 Functions with ABI-1 to Mediate the UNC-6 and SLT-1 Axon Guidance Signaling Pathways
- Loss of the DNA Methyltransferase MET1 Induces H3K9 Hypermethylation at PcG Target Genes and Redistribution of H3K27 Trimethylation to Transposons in
- Genome-Wide Association Studies Reveal a Simple Genetic Basis of Resistance to Naturally Coevolving Viruses in
- The Principal Genetic Determinants for Nasopharyngeal Carcinoma in China Involve the Class I Antigen Recognition Groove
- Molecular, Physiological, and Motor Performance Defects in DMSXL Mice Carrying >1,000 CTG Repeats from the Human DM1 Locus
- Genomic Study of RNA Polymerase II and III SNAP-Bound Promoters Reveals a Gene Transcribed by Both Enzymes and a Broad Use of Common Activators
- Long Telomeres Produced by Telomerase-Resistant Recombination Are Established from a Single Source and Are Subject to Extreme Sequence Scrambling
- The Yeast SR-Like Protein Npl3 Links Chromatin Modification to mRNA Processing
- Deubiquitylation Machinery Is Required for Embryonic Polarity in
- dJun and Vri/dNFIL3 Are Major Regulators of Cardiac Aging in Drosophila
- CtIP Is Required to Initiate Replication-Dependent Interstrand Crosslink Repair
- Notch-Mediated Suppression of TSC2 Expression Regulates Cell Differentiation in the Intestinal Stem Cell Lineage
- A Combination of H2A.Z and H4 Acetylation Recruits Brd2 to Chromatin during Transcriptional Activation
- Network Analysis of a -Mouse Model of Autosomal Dominant Polycystic Kidney Disease Identifies HNF4α as a Disease Modifier
- Mitosis in Neurons: Roughex and APC/C Maintain Cell Cycle Exit to Prevent Cytokinetic and Axonal Defects in Photoreceptor Neurons
- CELF4 Regulates Translation and Local Abundance of a Vast Set of mRNAs, Including Genes Associated with Regulation of Synaptic Function
- Mechanisms Employed by to Prevent Ribonucleotide Incorporation into Genomic DNA by Pol V
- The Genomes of the Fungal Plant Pathogens and Reveal Adaptation to Different Hosts and Lifestyles But Also Signatures of Common Ancestry
- A Genome-Scale RNA–Interference Screen Identifies RRAS Signaling as a Pathologic Feature of Huntington's Disease
- Lessons from Model Organisms: Phenotypic Robustness and Missing Heritability in Complex Disease
- Population Genomic Scan for Candidate Signatures of Balancing Selection to Guide Antigen Characterization in Malaria Parasites
- Tissue-Specific Regulation of Chromatin Insulator Function
- Disruption of Mouse Cenpj, a Regulator of Centriole Biogenesis, Phenocopies Seckel Syndrome
- Genome, Functional Gene Annotation, and Nuclear Transformation of the Heterokont Oleaginous Alga CCMP1779
- Antagonistic Gene Activities Determine the Formation of Pattern Elements along the Mediolateral Axis of the Fruit
- Lung eQTLs to Help Reveal the Molecular Underpinnings of Asthma
- Identification of the First ATRIP–Deficient Patient and Novel Mutations in ATR Define a Clinical Spectrum for ATR–ATRIP Seckel Syndrome
- Cooperativity of , , and in Malignant Breast Cancer Evolution
- Loss of Prohibitin Membrane Scaffolds Impairs Mitochondrial Architecture and Leads to Tau Hyperphosphorylation and Neurodegeneration
- Microhomology Directs Diverse DNA Break Repair Pathways and Chromosomal Translocations
- MicroRNA–Mediated Repression of the Seed Maturation Program during Vegetative Development in
- Selective Pressure Causes an RNA Virus to Trade Reproductive Fitness for Increased Structural and Thermal Stability of a Viral Enzyme
- The Tumor Suppressor Gene Retinoblastoma-1 Is Required for Retinotectal Development and Visual Function in Zebrafish
- Regions of Homozygosity in the Porcine Genome: Consequence of Demography and the Recombination Landscape
- Histone Methyltransferases MES-4 and MET-1 Promote Meiotic Checkpoint Activation in
- Polyadenylation-Dependent Control of Long Noncoding RNA Expression by the Poly(A)-Binding Protein Nuclear 1
- A Unified Method for Detecting Secondary Trait Associations with Rare Variants: Application to Sequence Data
- Genetic and Biochemical Dissection of a HisKA Domain Identifies Residues Required Exclusively for Kinase and Phosphatase Activities
- Informed Conditioning on Clinical Covariates Increases Power in Case-Control Association Studies
- Biochemical Diversification through Foreign Gene Expression in Bdelloid Rotifers
- Genomic Variation and Its Impact on Gene Expression in
- Spastic Paraplegia Mutation N256S in the Neuronal Microtubule Motor KIF5A Disrupts Axonal Transport in a HSP Model
- Lamin B1 Polymorphism Influences Morphology of the Nuclear Envelope, Cell Cycle Progression, and Risk of Neural Tube Defects in Mice
- A Targeted Glycan-Related Gene Screen Reveals Heparan Sulfate Proteoglycan Sulfation Regulates WNT and BMP Trans-Synaptic Signaling
- Dopaminergic D2-Like Receptors Delimit Recurrent Cholinergic-Mediated Motor Programs during a Goal-Oriented Behavior
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Mechanisms Employed by to Prevent Ribonucleotide Incorporation into Genomic DNA by Pol V
- Inference of Population Splits and Mixtures from Genome-Wide Allele Frequency Data
- Zcchc11 Uridylates Mature miRNAs to Enhance Neonatal IGF-1 Expression, Growth, and Survival
- Histone Methyltransferases MES-4 and MET-1 Promote Meiotic Checkpoint Activation in
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



