-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaHP1a Recruitment to Promoters Is Independent of H3K9 Methylation in
Heterochromatin protein 1 (HP1) proteins, recognized readers of the heterochromatin mark methylation of histone H3 lysine 9 (H3K9me), are important regulators of heterochromatin-mediated gene silencing and chromosome structure. In Drosophila melanogaster three histone lysine methyl transferases (HKMTs) are associated with the methylation of H3K9: Su(var)3-9, Setdb1, and G9a. To probe the dependence of HP1a binding on H3K9me, its dependence on these three HKMTs, and the division of labor between the HKMTs, we have examined correlations between HP1a binding and H3K9me patterns in wild type and null mutants of these HKMTs. We show here that Su(var)3-9 controls H3K9me-dependent binding of HP1a in pericentromeric regions, while Setdb1 controls it in cytological region 2L:31 and (together with POF) in chromosome 4. HP1a binds to the promoters and within bodies of active genes in these three regions. More importantly, however, HP1a binding at promoters of active genes is independent of H3K9me and POF. Rather, it is associated with heterochromatin protein 2 (HP2) and open chromatin. Our results support a hypothesis in which HP1a nucleates with high affinity independently of H3K9me in promoters of active genes and then spreads via H3K9 methylation and transient looping contacts with those H3K9me target sites.
Published in the journal: . PLoS Genet 8(11): e32767. doi:10.1371/journal.pgen.1003061
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003061Summary
Heterochromatin protein 1 (HP1) proteins, recognized readers of the heterochromatin mark methylation of histone H3 lysine 9 (H3K9me), are important regulators of heterochromatin-mediated gene silencing and chromosome structure. In Drosophila melanogaster three histone lysine methyl transferases (HKMTs) are associated with the methylation of H3K9: Su(var)3-9, Setdb1, and G9a. To probe the dependence of HP1a binding on H3K9me, its dependence on these three HKMTs, and the division of labor between the HKMTs, we have examined correlations between HP1a binding and H3K9me patterns in wild type and null mutants of these HKMTs. We show here that Su(var)3-9 controls H3K9me-dependent binding of HP1a in pericentromeric regions, while Setdb1 controls it in cytological region 2L:31 and (together with POF) in chromosome 4. HP1a binds to the promoters and within bodies of active genes in these three regions. More importantly, however, HP1a binding at promoters of active genes is independent of H3K9me and POF. Rather, it is associated with heterochromatin protein 2 (HP2) and open chromatin. Our results support a hypothesis in which HP1a nucleates with high affinity independently of H3K9me in promoters of active genes and then spreads via H3K9 methylation and transient looping contacts with those H3K9me target sites.
Introduction
Genomic DNA in eukaryotes is organized into chromatin, which has historically been divided into two distinct forms, euchromatin and heterochromatin, based on histological staining patterns. Euchromatin is defined as being decondensed during interphase while heterochromatin remains condensed throughout the cell-cycle, as reviewed in [1]. Euchromatin and heterochromatin can be distinguished by distinct histone modifications, one of which (H3 lysine 9 methylation, H3K9me) is generally associated with transcription repression and heterochromatin, or “green chromatin” according to recent, more elaborate chromatin structure-based definitions [2] used here. Three H3K9-specific methyl transferases (HKMTs) have been described in Drosophila: Su(var)3-9, Setdb1 and G9a. Setdb1 (also known as eggless) plays an important role in oogenesis and its loss leads to female sterility [3]–[5]. Su(var)3-9 and G9a, in contrast, are not essential for viability and their role during development appears to be less specific [6]–[9]. In addition to playing different roles during development the three HKMTs have been ascribed different regional properties. Chromosome staining experiments have shown that levels of pericentric H3K9me are reduced, but the enrichment of H3K9me on the 4th chromosome remains unaltered in Su(var)3-9 mutants [8], [10], [11]. Similar experiments on Setdb1 mutants have shown that Setdb1 controls H3K9 methylation on the 4th chromosome [12], [13]. However, the roles and specificity of G9a remains unclear, as does the redundancy of the three HKMTs [6], [7], [9].
The H3K9me-enriched heterochromatin, or more specifically the “green chromatin”, is also enriched in a number of specialized proteins [2], [14]. The pivotal protein for defining the “green chromatin” enriched in pericentromeric regions and on the 4th chromosome is HP1a (heterochromatin protein 1, also known as Su(var)2-5). It is generally accepted that H3K9me stabilizes the binding of HP1a to chromatin [15]–[18]. The prevailing model postulates that HP1a forms a dimer through its C-terminal chromo-shadow domain and the two chromo-domains of the dimer link two adjacent nucleosomes through interactions with H3K9me. As HP1a interacts with the HKMTs (Su(var)3-9 or Setdb1), nearby H3K9 becomes methylated and HP1a spreading is propagated [19]. The link between H3K9me and HP1a is the most frequently described HP1a-chromatin interaction, but HP1 has also been shown to bind nucleosomes independent of the H3 tail [20] and with high affinity to the H3 histone-fold although the in vivo relevance of this binding is not known [21]–[24].
HP1a - and H3K9me-enriched chromatin is present in the pericentromeric regions as well as along the entire length of the small fourth chromosome in Drosophila melanogaster. Interestingly, despite the 4th chromosome's repressive nature, with heterochromatic markers and large blocks of repeated sequences and transposed elements, genes on this chromosome are expressed with similar strength, or even more strongly, on average than genes on the other chromosomes [25], [26]. This can be partly explained by the fact that the 4th chromosome has a unique chromosome-specific dosage compensating system mediated by the protein Painting of fourth (POF) [27]–[31]. POF is a protein that specifically targets active genes on the 4th chromosome, binds their nascent RNA and mediates increases in their expression [26], [27], [32], [33]. Flies can survive without one copy of their 4th chromosome or POF. However, haplo-fourth flies die if they lack POF [27]. POF has been shown to counteract the repressive influence on chromosome 4 genes caused by the heterochromatic nature of the chromosome [26], [27], [33]. The 4th chromosome in D. melanogaster is therefore regarded as a good model for studying gene expression in heterochromatic environments [31], [34], [35]. Notably in this context, despite its name HP1a binds to active genes on the 4th chromosome. On this chromosome an RNAi mediated knock-down of HP1a is associated with increased gene expression in adult flies [27], but there are also reported examples of HP1a loss or reduction being associated with decreased transcription [36]–[39].
High-resolution chromosomal distributions of HP1a [2], [14], [32], [35], [40]–[42] and POF [32] have been previously reported, but the dependence of HP1a binding on H3K9me2/3 and the different HKMTs at higher resolution has remained elusive. Therefore, to study the division of labor between the HKMTs and the dependence of HP1a binding on H3K9 methylation and POF in vivo, we have generated and analyzed high resolution H3K9me2, H3K9me3 and HP1a ChIP-chip profiles in wild type flies, null mutants of the three HKMTs and Pof mutants.
Our results show that Su(var)3-9 is mainly responsible for HP1a H3K9me-dependent binding to the pericentromeric regions while Setdb1 is responsible for HP1a H3K9me-dependent binding to cytological region 2L:31 and (together with POF) chromosome 4. HP1a binds to the promoters and within bodies of active genes in these three regions. More importantly, HP1a binding at promoters of active genes is independent of H3K9me and POF. Our results supports a model in which HP1a nucleates with high affinity independently of H3K9me and then spreads via transient looping contacts with H3K9me target sites.
Results
HP1a profiles in HKMT mutants
To study the dependence of HP1a binding on the three HKMTs in Drosophila we performed ChIP-chip analysis on chromatin extracts from salivary glands of 3rd instar larvae, comparing wild type profiles to those of Su(var)3-9, Setdb1 and G9a mutants (Figure 1A). It has previously been documented that HP1a binds to pericentromeric heterochromatin, chromosome 4 and part of cytological section 2L:31 [35], [36], [42]–[44]. Our generated ChIP-chip profiles confirm these observations: HP1a is enriched in region 2L:31, in the pericentromeric regions as exemplified by p2L (proximal 2L), and on chromosome 4 (Figure 1A, Figure S1). In Setdb1 mutants we observed a strong reduction of HP1a on the 4th chromosome and in region 2L:31, but region p2L appeared to be unaffected. In contrast, in Su(var)3-9 mutants we observed a strong reduction of HP1a in p2L, but the 4th chromosome and 2L:31 region were unaffected. In the G9a mutants we observed no regional or chromosome-specific reduction. Note that any potential global increase or decrease in enrichment is difficult to measure in ChIP-chip experiments since the dynamic range of ChIPs typically varies substantially between experiments.
Fig. 1. HP1a profiles in HKMT mutants. 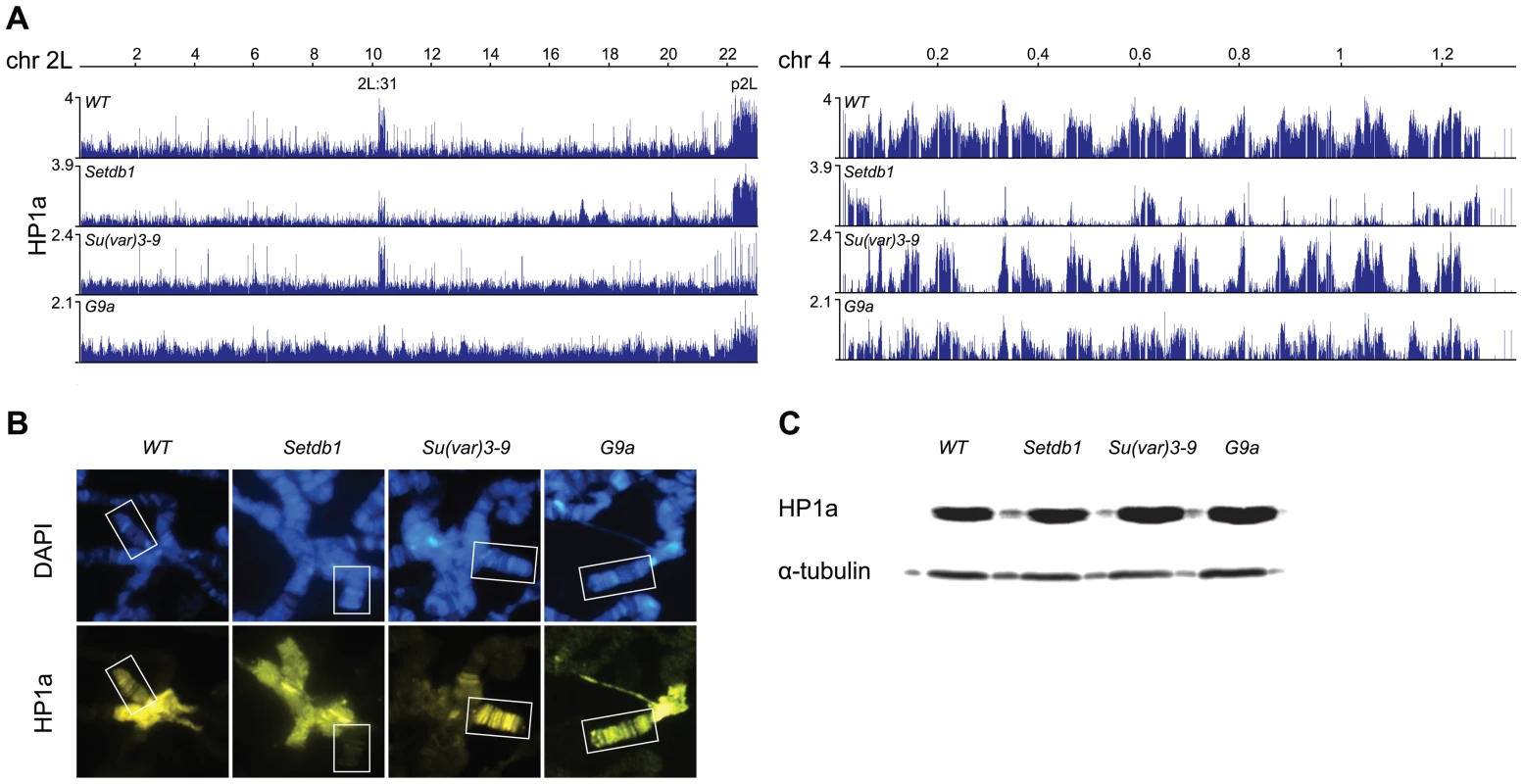
(A) HP1a binding profiles for the entire chromosome arm 2L and the 4th chromosome in salivary gland tissue from wild type and null Su(var)3-9, Setdb1 and G9a mutants. p2L and 2L:31 indicate the centromere proximal heterochromatin in chromosome 2L and the HP1a-enriched cytogenetic region in the middle of the chromosome arm, respectively. Numbers along the x-axis denote chromosomal positions along the chromosomes in Mb. The y-axis shows the ChIP enrichment in log2 ratios. (B) Immunostaining of polytene chromosomes from wild type and Su(var)3-9, Setdb1 and G9a mutants with DAPI (blue) and HP1a (yellow). The fourth chromosomes are indicated by boxes. Note that HP1a is decreased on the 4th chromosome in Setdb1 mutants and in the pericentromeric heterochromatin in Su(var)3-9 mutants. (C) Western blot showing total HP1a and tubulin from dissected salivary glands from wild type and Su(var)3-9, Setdb1 and G9a mutants. Results from polytene chromosome stainings of HP1a in wild type and the three HKMT mutants (performed to confirm the results and look for global changes) were in good agreement with the ChIP-chip profiles. As can be seen in Figure 1B, HP1a is reduced on the 4th chromosome in Setdb1 mutants compared to wild type and in the pericentromeric regions in Su(var)3-9 mutants. No obvious difference in HP1a binding was observed between wild type and G9a mutants (Figure 1B). Using Western blot analysis we observed no clear differences in total amounts of HP1a protein between wild type and the three HKMT mutants (Figure 1C). We conclude that HP1a binding on the 4th chromosome and region 2L:31 depends on Setdb1, HP1a binding in pericentromeric regions depends on Su(var)3-9 while we found no clear dependence of its binding on G9a in our experiments.
Drosophila HKMTs have region-specific functions in generating H3K9me
H3K9me2 and H3K9me3 are documented targets for HP1a binding [15]–[18]. We therefore tested the dependence of these two modifications on each of the three HKMTs. The results show that H3K9me2 was strongly reduced in region 2L:31 and the 4th chromosome, while virtually all H3K9me3 was lost in region 2L:31 and strongly reduced on chromosome 4 in Setdb1 mutants (Figure 2). In Su(var)3-9 mutants both H3K9me2 and H3K9me3 were strongly reduced in the pericentromeric regions. Importantly, in both Su(var)3-9 and Setdb1 mutant backgrounds, the H3K9me3 reductions were more dramatic than the corresponding H3K9me2 reductions, although the affected regions differed between the mutants. In the G9a mutants we detected no specific effects on either H3K9me2 or H3K9me3 (Figure 2). Comparing the HP1a binding and H3K9me profiles on the 4th chromosome we observed regions that were not dependent on Setdb1. These regions will be described in more detail later. Since the overall HP1a, H3K9me2 and H3K9me3 profiles in G9a mutants were not altered compared to wild type we concentrated our analysis on Setdb1 and Su(var)3-9. We conclude that Setdb1 and Su(var)3-9 methylates H3K9 in different regions and loss of Su(var)3-9 or Setdb1 affects both H3K9me2 and H3K9me3, but in different regions of the genome.
Fig. 2. H3K9 methylation profiles in HKMT mutants. 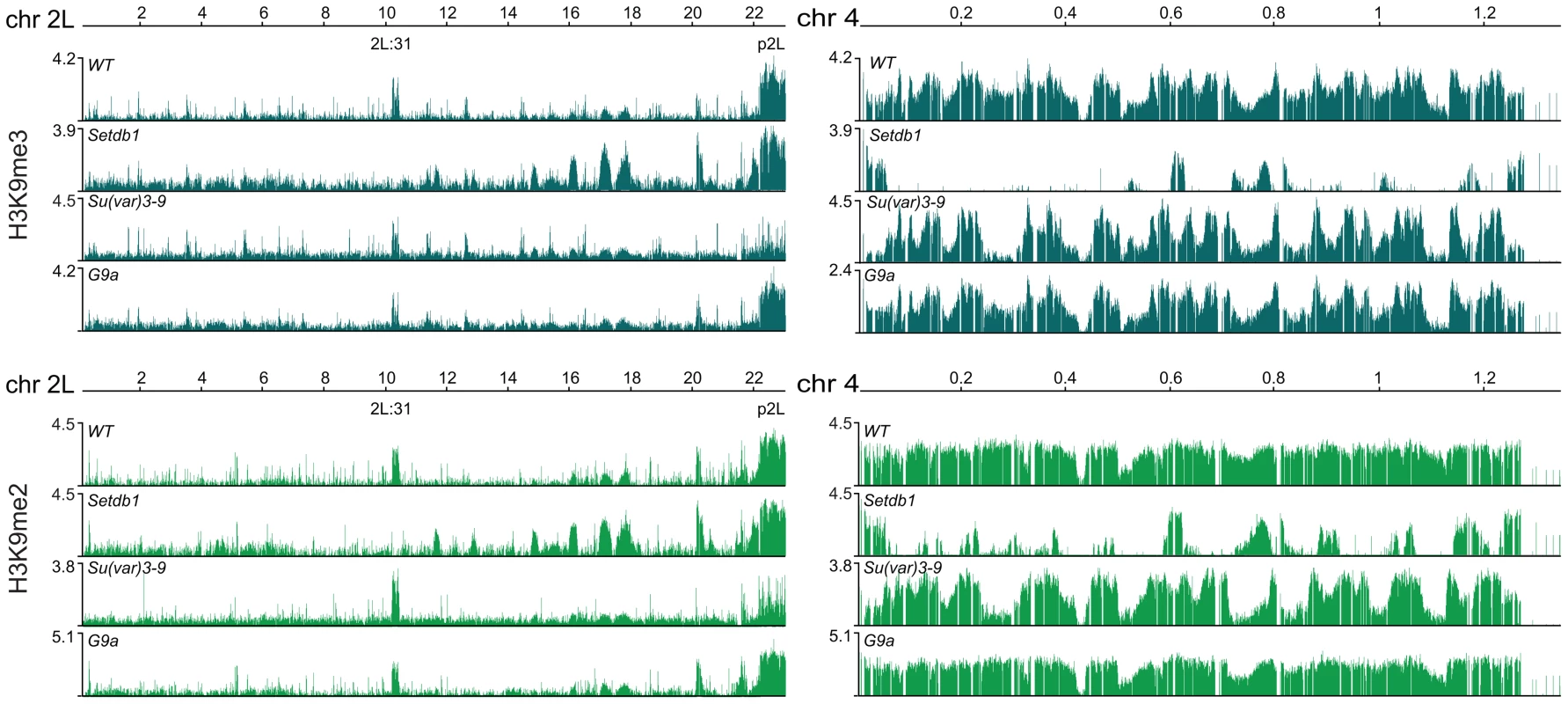
H3K9me3 (gray) and H3K9me2 (green) profiles for the entire chromosome arm 2L and the 4th chromosome in salivary gland tissue from wild type and null Su(var)3-9, Setdb1 and G9a mutants. p2L and 2L:31 indicate the centromere proximal heterochromatin in chromosome 2L and the HP1a-enriched cytogenetic region in the middle of the chromosome arm, respectively. Numbers along the x-axis denote chromosomal positions along the chromosomes in Mb. The y-axis shows the ChIP enrichment in log2 ratios. Note that both H3K9me2 and H3K9me3 are strongly reduced in region 2L:31 and chromosome 4 in Setdb1 mutants, and strongly reduced in p2L in Su(var)3-9 mutants. HP1a binds promoters independently of methylation
We have previously shown that HP1a binds not only within bodies of expressed genes on the 4th chromosome but also in a distinct peak in the promoter of most targeted genes [32]. Interestingly, in the present study we observed several HP1a peaks on the 4th chromosome and region 2L:31 of Setdb1 mutants that did not correspond to H3K9me. This can be readily seen by comparing the HP1a and H3K9me profiles for Setdb1 (Figure 1A and Figure 2, respectively, i.e., regions that lack H3K9me and retain HP1a in the Setdb1 mutant background). We therefore explored the locations of these “methylation-independent” HP1a peaks, and found them to be concentrated in the promoters of genes, which importantly contain no detectable H3K9me (Figure 3A and Figure S2). To analyze the binding further at a gene feature level we generated average gene profiles of all active genes in the four defined regions (chromosome 4, pericentromeric regions, euchromatic regions and 2L:31) as described in Materials and Methods. The average HP1a binding, H3K9me2 and H3K9me3 profiles (Figure 3B) show that HP1a within the gene bodies in specific regions is lost in mutants of the corresponding region-specific HKMTs. However, more importantly, the HP1a peaks in the promoters are resistant to the loss of H3K9me. Interestingly, H3K9me in pericentromeric promoters depends on Setdb1 and not on Su(var)3-9 (Figure 3B and Figure S2). Note that the enrichment of HP1a in the promoter peaks is reduced upon loss of methylation, but can still be readily seen (Figure 3A and 3B). These findings indicate that HP1a may bind to promoters independently of methylation, then further H3K9me-dependent binding occurs through interaction with the corresponding HKMT (Setdb1 for the 4th chromosome and 2L:31 and Su(var)3-9 for the pericentromeric region). This model explains not only the observed promoter peaks but also spreading of the HP1a binding to the gene bodies. The HP1a enrichment within the gene bodies is most pronounced on the 4th chromosome.
Fig. 3. HP1a binds independently of H3K9me to promoters and spreads H3K9me-dependently into gene bodies. 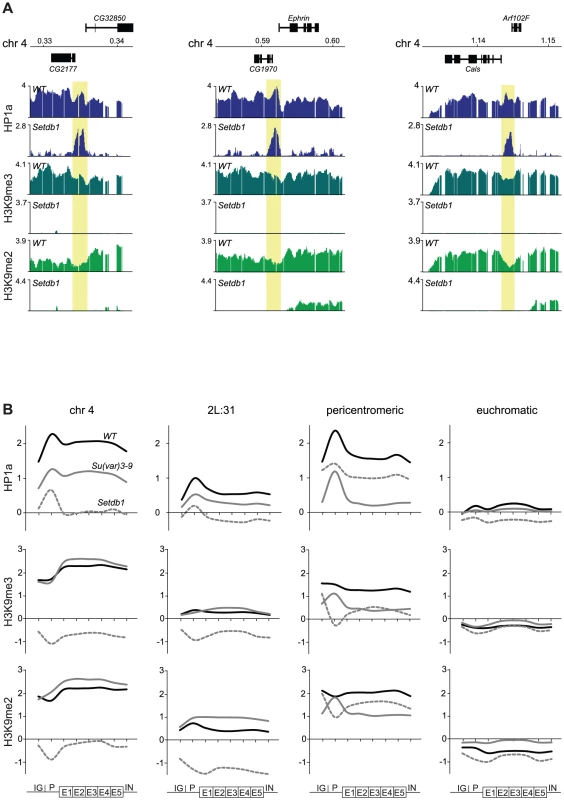
(A) HP1a and H3K9me profiles in three illustrative regions of the 4th chromosome in wild type and Setdb1 mutant backgrounds. Numbers along the x-axis denote chromosomal positions along the chromosomes in Mb. The y-axis shows the ChIP enrichment in log2 ratios. Genes expressed from left to right and vice versa are shown above and below the horizontal lines, respectively. The HP1a methylation-independent promoter peaks are indicated by yellow boxes (B) Average metagene profiles of HP1a, H3K9me2 and H3K9me3 on the 4th chromosome, region 2L:31,the pericentromeric regions and the euchromatic regions, based on eight enrichment values for each active gene in the respective regions (x-axis). The y-axis shows the ChIP enrichment in log2 ratios. The first points (IG) of the curves show average values for the intergenic regions upstream of the designated promoters of the genes. The promoter (P) is defined as the 500 bp region upstream of the TSS. The gene bodies are divided into five bins (E1–E5) and the average enrichment in introns (IN) is indicated by the last point of each curve. The average profiles for wild type (solid black), Su(var)3-9 mutants (solid gray) and Setdb1 mutants (dashed gray) are shown. HP1a-enriched promoters are A/T rich and enriched in HP2
Intrigued by the methylation independence of HP1a in the promoters we further characterized HP1-enriched promoters by exploring possible correlations between them and both sequence composition and protein factors. To test for specific sequence motifs we defined HP1a promoter peaks for genes on the 4th chromosome, region 2L:31 and p2L as 50 bp +/ − each HP1a peak center and used these sequences as input in the CompleteMOTIFS platform. An A/T rich motif was found that was significantly enriched in promoters in chromosome 4, 2L:31 and pericentromeric regions compared to the rest of chromosome 2L (Figure 4A–4B). Motif scores were calculated by scanning the identified motif in promoters (500 bp upstream of TSS) in the 4th chromosome, region 2L:31, p2L, and the remaining part of chromosome 2L as a control. A/T rich motifs have previously been shown to be enriched in HP1a binding sites [45]. Since the HP1a bound promoters were also found to be enriched with an A/T rich motif, we assessed the A/T content in these promoters, and found it to be significantly higher in the three HP1a-enriched regions than in promoters in the control chromosome region (Figure 4C).
Fig. 4. HP1a-enriched promoters are A/T rich, bound by HP2 and DNase sensitive. 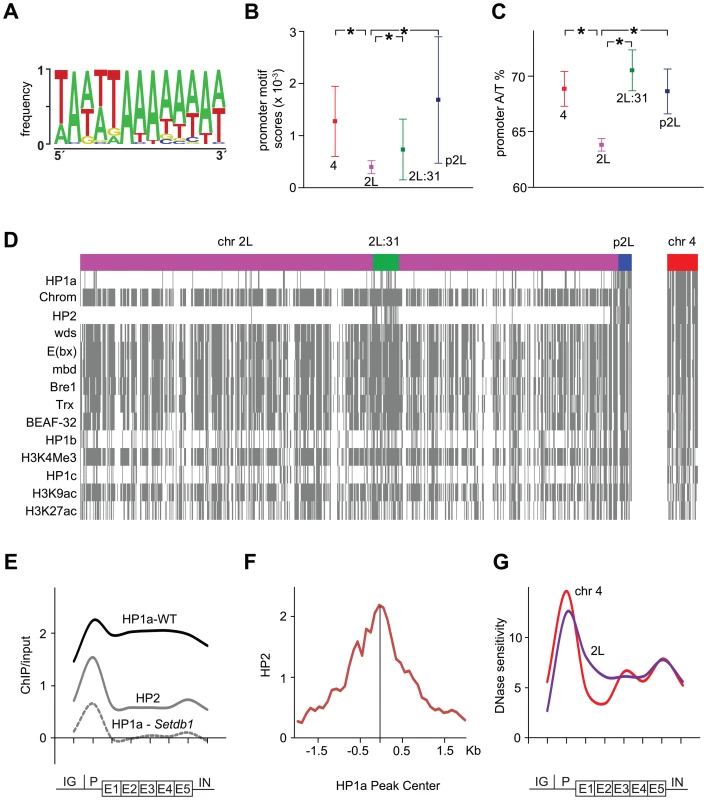
(A) Sequence motif over-represented in HP1a-enriched promoters. (B) Motif scores in promoters from chromosome 4 (red), 2L:31 (green), pericentromeric region (blue) and, as a control, chromosome 2L (pink). (C) A/T content in promoters. In (B) and (C) significant differences are indicated by * (Mann-Whitney U test, p<0.01). (D) Binary heat-map showing significant enrichment of indicated chromatin factors at promoters on chromosomes 2L and 4. Presence of a protein at a promoter is indicated by a gray line. Note the almost perfect correlation between HP1a and HP2. (E) Average metagene profiles showing the ChIP enrichments of HP1a in wild type (black), HP1a in Setdb1 mutants (dashed gray) and HP2 in wild type (gray) on the 4th chromosome (log2 ratios). (F) HP2 enrichment (log2 ratios) plotted around HP1a peak centers of 34 methylation-independent HP1a peaks. (G) Average metagene profiles of DNase sensitivity for the 4th chromosome (red) and chromosome 2L (blue). The y-axis shows the density of mapped DNaseI cleavages in a 150 bp sliding window [82]. Next, to seek protein factors and chromatin modifications that correlate with enriched HP1a levels in promoters we identified chromatin factors that were classified as bound (according to modENCODE classification) to more than 50% of the promoters of active genes on the 4th chromosome. Out of 51 tested chromatin factors 14 were classified as bound to >50% of chromosome 4 promoters including, as expected, HP1a as the protein with highest overlap. A binary heat-map of these factors is shown in Figure 4D. As shown, HP1a is classified as bound to a number of promoters along chromosome 2L and concentrated in region 2L:31 and p2L. The promoters bound by HP1a are targeted by a number of other factors, e.g., Chromator, E(bx), Trx and BEAF-32. Most of these factors are not specific for the promoters bound by HP1a but bind to a large number of promoters (Figure 4D). However, heterochromatin protein 2 (HP2) binding shows a close to perfect correlation with HP1a bound promoters and is almost completely absent outside 2L:31 and p2L on chromosome 2L (Figure 4D and Figure S3). Furthermore, the gene average and enrichment profiles of HP2 show that the correlation between HP1a and HP2 is indeed concentrated in the promoters (Figure 4E and Figure S3). To further test the link between HP2 and the methylation-independent HP1a promoter peaks we manually annotated 34 confidently identified HP1a promoter peaks on the 4th chromosome and plotted the HP2 enrichment in relation to their centers. The results show that the HP2 enrichment is significantly higher in these peaks than in other promoters (Figure 4E and 4F, Table S1) and that HP2 peaks in the annotated center of the HP1a peaks. We conclude that HP2 is strongly associated with the HP1a methylation-independent promoter peaks. Again, this is suggestive of a fundamental difference between HP1a binding to promoters and in gene bodies.
As its name implies, HP1a is thought to cause the formation of inaccessible, condensed chromatin. We therefore plotted average DNase sensitivity profiles, as a marker for accessible chromatin. This revealed that chromosome 4 promoters are more sensitive to DNase than chromosome 2L, despite the enriched HP1a levels in chromosome 4 promoters (Figure 4G). In contrast, within the gene bodies, chromosome 4 genes are slightly less sensitive. Interestingly, on the 4th chromosome HP1a binds both to promoters and gene bodies, although the DNase sensitivity is still slightly lowered in gene bodies but increased in promoters compared to other genomic regions. These correlation-based findings support the hypothesis that HP1a binding to promoters and gene bodies differs in both mechanism and outcome.
The gene body targeting but not the promoter targeting of HP1a depends on POF
Considering the hypotheses that HP1a binds independently of methylation to promoters and this is followed by H3K9me-dependent spreading we were interested to identify factors that may be linked to the spreading. It has been previously shown that binding of HP1a to gene bodies is linked to active genes [32], [35], and we have shown by polytene chromosome stainings that binding of HP1a to the 4th chromosome depends on POF [27]. To test the dependence of HP1a binding to this chromosome on POF at a gene level we performed ChIP-chip analyses of HP1a-DNA interactions in Pof mutants (Figure 5). Strikingly, the HP1a profiles of Pof and Setdb1 mutants were very similar, i.e., the gene binding of HP1a was lost but the prominent HP1a promoter peaks were retained. We conclude that spreading of HP1a to the gene bodies depends on H3K9me generated by Setdb1 and the presence of POF protein. Furthermore, HP1a promoter peaks on the 4th chromosome are independent of H3K9me and are resistant to losses of both Setdb1 and POF.
Fig. 5. HP1a binds independently of POF and Setdb1 to promoters. 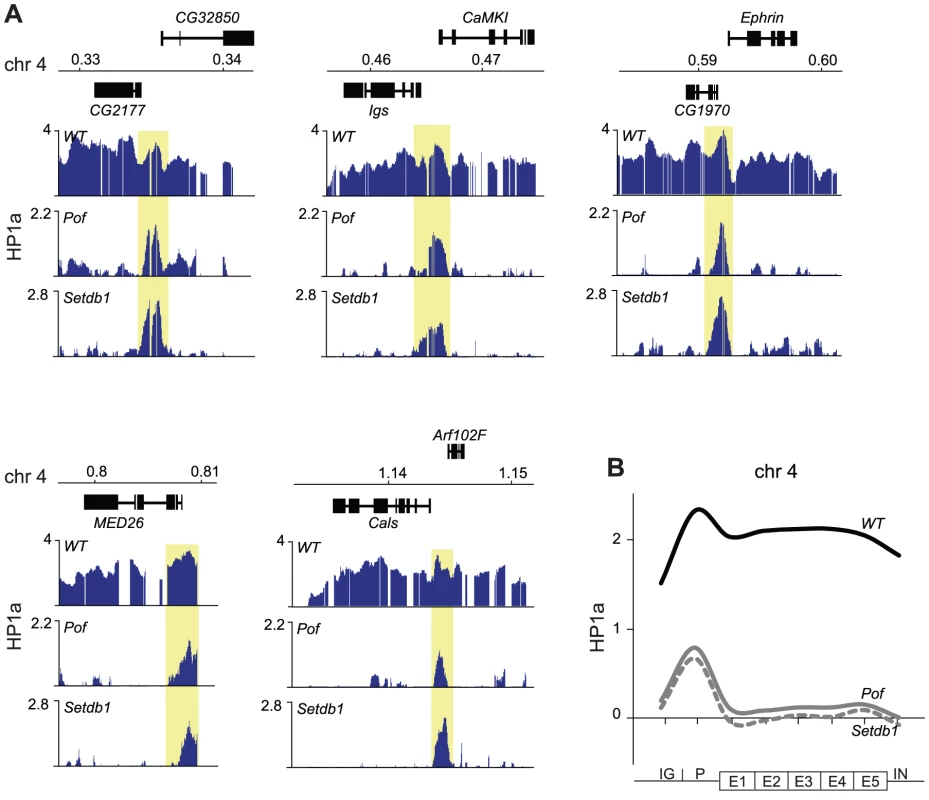
(A) HP1a profiles in five illustrative regions of the 4th chromosome in wild type, Pof and Setdb1 backgrounds. Numbers along the x-axis denote chromosomal positions along the chromosomes in Mb. The y-axis shows the ChIP enrichment in log2 ratios. Genes expressed from left to right and vice versa are shown above and below the horizontal lines, respectively. The HP1a methylation-independent promoter peaks are indicated by yellow boxes. (B) Average metagene profiles of HP1a enrichments on the 4th chromosome in wild type (solid black), Pof (solid gray) and Setdb1 (dashed gray) backgrounds. The 4th chromosome contains domains targeted by HP1a independently of Pof and Setdb1
Analyzing the HP1a profile on the 4th chromosome in wild type and HKMTs mutants we observed three regions that were resistant to the loss of Setdb1 (see Figure 1A). First, there is a region in the proximal part of the 4th chromosome that retains HP1a in Setdb1 mutants, covering the three pseudogenes CR32009, CR320010 and CR320011. This region does not respond to Setdb1 but responds to Su(var)3-9. Thus, the proximal region of the 4th chromosome behaves similarly in this respect to the proximal region of the other chromosome arms, e.g., p2L. Secondly, in the most distal gene on the 4th chromosome, Caps, HP1a binding is independent of Setdb1. Finally, there is a region in the middle of the chromosome (region 600–630 kb with two genes, onecut and CG1909) in which the HP1a binding is independent of Setdb1 and POF (Figure 6). The HP1a enrichment in these two genes is reduced in Su(var)3-9 mutants but not lost. Interestingly, these two genes are not expressed in salivary glands, thus they represent two HP1a targeted, but unexpressed genes.
Fig. 6. HP1a binds two unexpressed genes in the middle of the 4th chromosome independently of Setdb1 and POF. 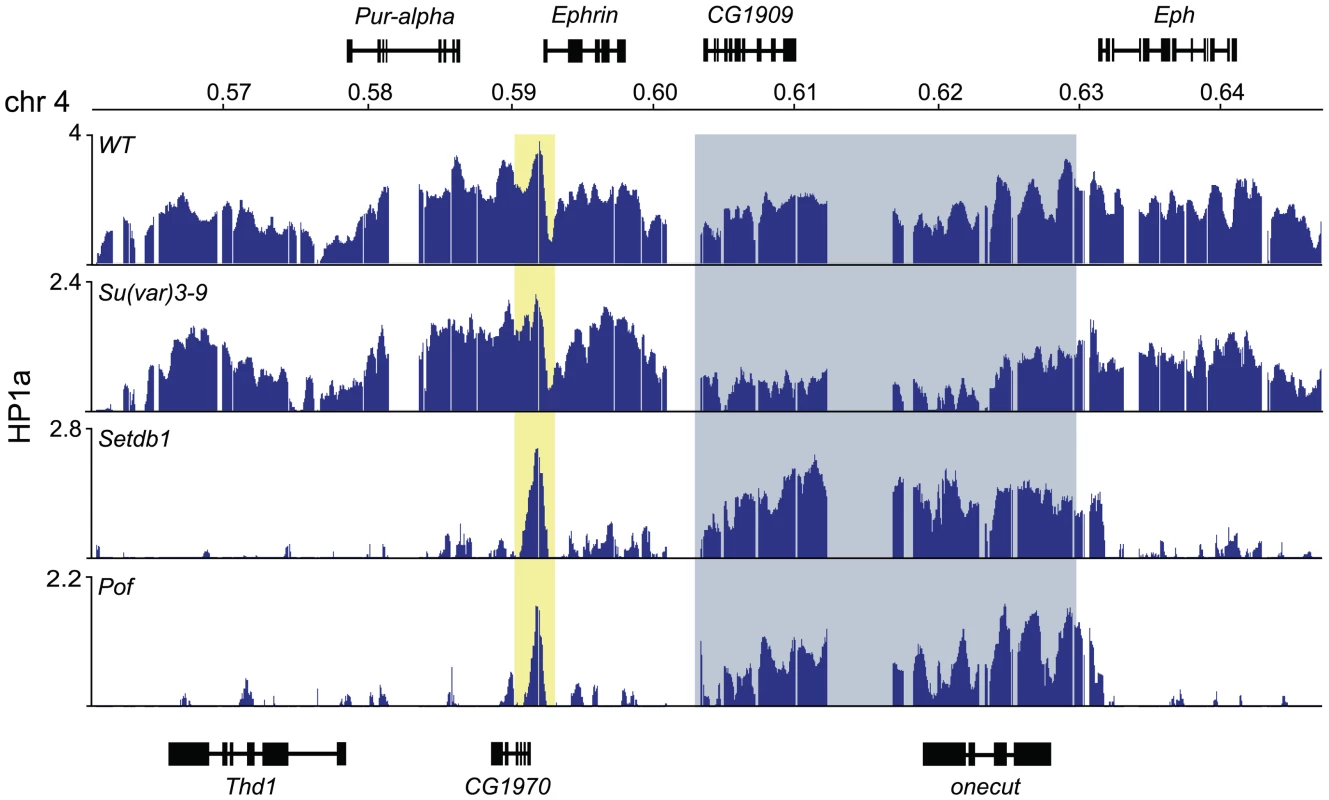
HP1a enrichment profiles at a 90 kb region in the middle of the 4th chromosome in wild type, Pof and Setdb1 backgrounds. Numbers along the x-axis denote chromosomal positions along the chromosomes in Mb. The y-axis shows the ChIP enrichment in log2 ratios. Genes expressed from left to right and vice versa are shown above and below the horizontal lines, respectively. The peak within the yellow box corresponds to the CG1970 Ephrin promoter peak. The two genes CG1909 and onecut within the gray box are unexpressed genes that bind HP1a independently of POF and Setdb1. Discussion
Chromosome 4 is considered to be a repressive environment that is enriched in heterochromatin markers such as HP1a and methylated H3K9 [8], [11], [35], [46]. It contains large blocks of repeated sequences and transposable elements interspersed with the genes [47]–[52], and transgenes inserted on the 4th chromosome often show variegated expression because of partial silencing [53]–[56]. Despite its heterochromatic nature genes located on the 4th chromosome are expressed as strongly on average, or even more strongly, than genes on other chromosomes [25], [26]. Traditionally, the division of genomes into heterochromatic and euchromatic regions was based on cytological characteristics of chromatin in interphase. Today more elaborate definitions of chromatin states are available based on chromatin components, such as the five principal chromatin types defined in [2]. According to these definitions, pericentromeric heterochromatin and the 4th chromosome is highly enriched in “green-chromatin”. Similar definitions have been constructed by the modENCODE project, distinguishing nine chromatin states [14], one of which (chromatin state 7) corresponds to “green-chromatin”. HP1a and H3K9me2/me3 are the key components distinguishing green-chromatin and chromatin state 7. Our maps of HP1a and H3K9me2/me3 in chromatin from dissected salivary gland tissue correlate with previously reported high-resolution ChIP-chip and DamID maps of chromatin from various cell lines, embryos and fly heads [2], [14], [35], [40], [41]. Thus, the main regional chromosome organization into this chromatin type appears to be stable during development.
Our results show that the regional enrichment of HP1a depends on region-specificity of the HKMTs. The regional differences we observed in whole chromosomes confirm previous results based on chromosome stainings, i.e., loss of Su(var)3-9 causes a reduction of HP1a and H3K9me in pericentromeric regions but not the 4th chromosome [8], [10], [11] while loss of Setdb1 causes reductions of HP1a and H3K9me on the 4th chromosome and in region 2L:31 [12], [13]. Loss of G9a results in no difference in H3K9me, in accordance with previous reports [6]. We see no clear indications of redundancy between the different HKMTs.
The most important observations in our study are the fundamental differences between HP1a enrichment responses in gene bodies and promoters to losses of HKMT and H3K9 methylation. Upon loss of the region-specific HKMT, HP1a is strongly reduced or lost in gene bodies but the HP1a promoter peak is retained. These effects were observed in the pericentromeric region in Su(var)3-9 mutants and both the 4th chromosome and region 2L:31 in Setdb1 mutants. The observed HP1a binding in promoters is strongly indicative of H3K9me-independent nucleation sites. Interestingly, although the interaction between HP1a and methylated H3K9 is well documented, and was confirmed in our experiments, HP1 proteins have been shown to bind only weakly to reconstituted methylated nucleosomal arrays and purified native chromatin [22], [57], [58]. For example, H3 peptides containing H3K9me3 bind HP1 with relatively weak (µM) affinity [18], [59]. This is in stark contrast to their nM affinity for unmodified histones [60] and the stable interaction of HP1, probably to the histone fold region of H3, that occurs in S phase when DNA replication disrupts the histone octamers [21], [22]. We conclude that HP1a binds to two distinct targets in chromatin: very stably and methylation-independently to promoters of active genes (probably via interactions within the nucleosomes) and less stably (but with perfect correlation) to methylated H3K9 sites.
Considering the methylation-dependent and -independent binding of HP1a it is interesting to note that HP1a is essential for viability [46], in contrast to the three studied HKMTs. Su(var)3-9 is not required for viability and homozygous null mutant stocks can be kept. The same is true for G9a [6]. Setdb1 is claimed to be essential in Drosophila [12] and is certainly required for female fertility [4]. Nevertheless, in uncrowded conditions Setdb110.1a homozygous flies hatch although they have decreased viability (J. Larsson unpublished results), and pairwise crossings of the HKMT mutants have showed no clear effects in terms of reduced viability [6], [7]. Our findings of H3K9me-independent HP1a binding to promoters tempt speculation that HP1a may be essential for survival due to the methylation-independent promoter binding of HP1a. However, HP1a has also been associated with non-chromatin based functions such as linkage to hnRNP particles, suggesting it may also be involved in RNA compaction [38], although the importance of this function remains elusive.
The characterization of the HP1a bound promoter peaks led to two important findings. Firstly, promoters in the 4th chromosome and region 2L:31 have a significantly higher A/T content compared to promoters at other chromosomal locations. The presence of A/T rich motifs in general HP1a target sites have previously been reported [45] and our results confirm that this is also true for promoter-specific HP1a targets. It has been shown that poly(dA:dT) tracts in promoters disfavours nucleosomes and modulate gene expression levels [61], [62]. In addition, promoters in the 4th chromosome are more DNase sensitive than promoters at other genomic locations, suggesting that the chromatin structure is more open within these promoters. In fact, it has been proposed that HP1a promotes an open chromatin structure at bound promoters [63]. In contrast, gene bodies in the 4th chromosome are slightly less accessible to DNase, which again indicates that the HP1a binding to promoters is fundamentally different to the HP1a targeting in gene bodies. The slightly reduced DNase sensitivity in gene bodies is also consistent with the previously observed reduction of transcription elongation efficiency of genes on the 4th chromosome [26]. Secondly, in our search for chromatin-associated factors that correlate with the HP1a binding promoter peaks we found that HP2 shows a close to perfect correlation. HP2 has previously been shown to interact with HP1a [64]–[66], and it has been suggested that HP2 interaction with the HP1a chromo-shadow domain drives HP1a dimerization [64]. Our results show that the HP1a-HP2 interactions mainly occur at the HP1a bound promoters. This is consistent with previous observations that all mutations affecting HP1 dimerization abolish the interactions between HP1 and non-modified H3 [24], since these interactions should mainly occur at promoters according to our data.
Our results provide strong support for the model proposed by [22] that high affinity HP1a binding to the histone fold provides a nucleation site for HP1a targeting to chromatin. It is interesting to note that this incorporation is suggested to occur when the histone fold region of H3 becomes exposed because of active transcription, histone variant exchange or replication. A link between HP1 and replication has also been demonstrated by observed interactions between HP1 and the origin recognition complex (ORC) [67], [68], and the requirement of human ORC association with HP1 for correct targeting to heterochromatin [69]. In addition, HP1a modulates replication timing in Drosophila and reduced levels of HP1a result in delayed replication of chromosome 4 [39]. We speculate that HP1a binding to promoters avoids delay of this heterochromatic region's replication, that it provides an epigenetic nucleation mark for HP1a, and that the resulting nucleation is followed by a low affinity spreading to gene bodies. We envision a transient looping contact model in which the low affinity between HP1a and H3K9me provides the means for spreading of HP1a, analogous to the proposed model for the interactions of another Drosophila chromodomain protein, Polycomb (Pc). The chromo-domain of Pc interacts with H3K27me, but the nucleation sites for Pc are the Polycomb Response Elements, which have lower levels of H3K27me [70]. Thus, the nucleation appears to be independent of H3K27me and is followed by spreading caused by transient contacts between Pc and H3K27me [71], [72]. Similarly to HP1a and H3K9me, the affinity of the Pc chromo-domain to H3K27me is relatively weak, with a dissociation constant in the µM range [73]. In the case of HP1a the proposed spreading correlates with (and thus presumably depends on) at least three factors: H3K9me, active transcription and POF. The spreading appears to be generally restricted to transcribed genes, although there are two exceptions (onecut and CG1909) on the 4th chromosome. On the 4th chromosome, where POF binds to gene bodies, the HP1a enrichment is much higher than in region 2L:31. It should be stressed that HP1a and POF bindings are interdependent and POF also requires Setdb1 to target the 4th chromosome [13], [27]. Thus, the relationships between these factors remain elusive. Why is the gene body targeting of HP1a on the 4th chromosome Pof-dependent? This cannot be explained by expression differences, because although expression levels drop in Pof mutants the reductions are minor [26], [27], [33]. Our hypothesis is that POF binding to nascent RNA on active chromosome 4 genes may stabilize the interaction between HP1a and H3K9me as an adaptor system linking histone marks to nascent RNA, similar to the chromatin adaptor model for alternative splicing [74], [75].
The enrichment of H3K9me on the 4th chromosome mainly depends on Setdb1, but in the most proximal region of the 4th chromosome the H3K9me is Su(var)3-9 dependent. Thus, the proximal region of chromosome 4 is similar to the proximal region of other chromosome arms in this respect. Position-effect variegation studies have shown that although most variegated (partially silenced) transgenic inserts on the 4th chromosome are suppressed in Setdb1, but not Su(var)3-9 mutants, the reporter insertion 118E-10 is suppressed in Su(var)3-9 mutants [7], [76]. Interestingly, this transgene is inserted in the pericentric region on the 4th chromosome, i.e., the region that according to our study is dependent on Su(var)3-9.
In summary, we report dual binding properties of the HP1a protein: an H3K9me methylation-independent binding at promoters and a methylation-dependent binding within gene bodies suggested to occur by spreading. Like arms of other chromosomes, the proximal region of the 4th chromosome is enriched in HP1a and Su(var)3-9-dependent H3K9me. However, in contrast to other chromosome arms, the gene-rich portion of the 4th chromosome is enriched in HP1a and H3K9me, and here the enrichment within gene bodies depends on Setdb1. The methylation-independent HP1a promoter binding correlates with HP2 and with “open” chromatin structure. We suggest that the methylation-independent and -dependent binding of HP1a are fundamental steps in the transmission, propagation and spreading of this epigenetic mark, hence our observations provide important insights and the basis of a novel model of gene regulation in highly heterochromatic regions.
Materials and Methods
Fly stocks and genetic crosses
Flies were cultivated and crossed in vials with potato mash-yeast-agar medium at 25°C. The genotypes used for the experiments were wild type (Oregon R), PofD119/PofD119 [27], Su(var)3-906/Su(var)3-9evo [77], Setdb110.1a/Setdb110.1a [12] and G9aRG5/G9aRG5 [6]. Homozygous mutant third instar larvae were collected from the fly stocks Df(1)w67c23y w; PofD119, w; Setdb110.1a/CyO GFP and w; G9aRG5. To generate trans-heterozygous Su(var)3-9evo/Su(var)3-906 third instar larvae we crossed wm4; Su(var)3-9evo to wm4; Su(var)3-906/TM6, Tb and selected the non-Tb larvae offspring.
Chromatin immunoprecipitation
For our ChIP-chip experiments we used salivary glands from third instar larvae. The ChIP experiments were done as previously described [27], [32] using 3 µl of anti-HP1a (PRB291C, Covance), 3 µl of anti-H3K9me2 (ab1220, abcam) and 6 µl of anti-H3K9me3 (ab8898, abcam) for precipitations. We generated two biological replicates with each of three antibodies (anti-HP1a, -H3K9me2 and -H3K9me3) for each of four genotypes: wild type, Su(var)3-9evo/06, Setdb110.1a and G9aRG5. For PofD119 we generated two biological replicates with anti-HP1a antibodies. The purified ChIP and input DNA samples were amplified using a WGA2 GenomePlex Complete Whole Genome Amplification kit (Sigma) according to the supplier's recommendations. The amplified DNA was purified with a QIAquick PCR purification kit (Qiagen). To verify that no amplification bias affected the enrichment profiles, we analyzed the ChIP DNA/input DNA ratios before and after the amplifications, using real time PCR as previously described [32].
Microarray analysis
For tiling array analysis, the amplified ChIP DNA samples were fragmented, labeled and hybridized to an Affymetrix Drosophila Genome 2.0 array. In total, 44 DNA samples were hybridized (including ChIP and input samples). The signal intensity data generated were analyzed with Affymetrix Tiling Analysis Software (v. 1.1.02), using 200 bp bandwidth as a parameter only for smoothing and perfect match. The enrichment profiles were produced as ChIP DNA/input DNA ratios expressed in log2 scale. The complete data set is available at http://www.ncbi.nlm.nih.gov/geo/ (accession: GSE38366). The occupancy profiles obtained were visualized and analyzed using Integrated Genome Browser (6.2.2).
Average gene profiles
Average gene profiles were calculated using a custom perl script and D. melanogaster genome sequence annotation 5.43. Profiles of HP1a binding and the H3K9me2 and H3K9me3 occupancy of all active genes were calculated for four defined regions: the 4th chromosome, region 2L:31 (previously defined in [26]), the pericentromeric regions of 2L, 2R and 3L (previously defined in [35]) and euchromatic regions, i.e. chromosome arms 2L, 2R, 3L and 3R excluding 2L:31 and pericentromeric regions. The genes within each region were rescaled to the same relative length and the transcripts were divided into five bins. For each gene only the most strongly expressed transcript (deduced from results of RNA-seq analysis of 3rd instar larvae salivary gland tissue [78]) and the corresponding transcription start site (TSS) and exons were used. The promoter was defined as the region extending 500 bp upstream of each TSS or to the next TSS or transcription stop site (if closer). The intergenic region for each gene was defined as the region upstream of the defined promoter to the next TSS or transcription stop site and for each gene average binding and occupancy values for introns were calculated. The average profiles were plotted as average values for the defined intergenic regions followed by the promoter, the five transcript bins and finally the introns.
Immunostaining of polytene chromosomes
Polytene chromosomes from the salivary glands of wild type, Su(var)3-906/Su(var)3-9evo, Setdb110.1a and G9aRG5 3rd instar larvae were prepared and stained as previously described [79]. For this, we used anti-HP1a (PRB291C, 1∶400 dilution, Covance) primary antibodies, goat anti-rabbit antibodies conjugated with AlexaFluor555 (1∶300, Molecular Probes) as secondary antibodies and counterstained the squashes with DAPI (1 µg/ml). Images of the squashes were acquired using a Zeiss Axiophot microscope equipped with a KAPPA DX20C CCD camera, then assembled and merged electronically using Adobe Photoshop. For quantitative comparisons of stains, preparations and staining were run in parallel. Nuclei with clear cytology were chosen on the basis of DAPI staining and photographed. At least 20 nuclei for each genotype were used in these comparisons, and at least four slides of each genotype were analyzed.
Western blotting
For Western blot analysis we dissected salivary glands from third instar larvae in 1× PBS. The salivary glands were homogenized in 2× sample buffer (126 mM Tris-HCl [pH 6.8], 20% glycerol, 4% SDS, 0.005% bromophenol blue, 2.5% β-mercaptoethanol) and heated at 100°C for 10 minutes. The protein samples (from 15 pairs of salivary glands per lane) were separated by SDS-PAGE and transferred to Amersham Hybond-ECL nitrocellulose membrane (GE Healthcare). The membrane was blocked in 5% dry milk in 1× PBS +0.05% Tween-20 overnight at 4°C and incubated with anti-HP1a antibody (PRB291C, Covance) for 2 h at room temperature. HRP-conjugated α-rabbit (Jackson Laboratories) was used as secondary antibody and incubated for 2 h at room temperature. The signal was developed using Super Signal West Dura Extended Duration Substrate (Thermo Scientific) according to the supplier's instructions, and visualized using an LAS 4000 imaging system (Fujifilm). The membrane was reprobed using an anti-α-tubulin antibody (T5168, Sigma) and an HRP-conjugated α-mouse (GE Healthcare) as secondary antibody.
Sequence motif and overlapping factor searches
To search for enriched motifs in the HP1a promoter peaks of genes active in salivary gland tissue in the 4th chromosome, region 2L:31 and p2L we used the CompleteMOTIFS platform described in [80]. The regions for analysis were defined as 50 bp +/ − the HP1a peak center in the promoters. Motif scores were calculated by scanning the identified motif in promoter regions (500 bp upstream of TSS) for the 4th chromosome, region 2L:31, p2L, and the remaining part of chromosome 2L as a control. To look for factors overlapping the HP1a promoter peaks we used the binding data for chromatin factors in S2 cell lines generated by the modENCODE project [14], [81]. In total, 51 unique chromatin factors were included in the analysis, i.e., all chromatin-associated factors mapped in S2 cells by modENCODE (modMine release v20). Fourteen chromatin factors were identified as bound to >50% of chromosome 4 promoters. A binary heat-map was generated by classing all promoters on chromosome 2L and chromosome 4 as either bound or unbound by these factors. To generate average profiles of DNase sensitivity we used the DNase hypersensitivity (DHS) data obtained for embryos, developmental stage 14, from [82]. For each gene only the most strongly expressed transcript (deduced from embryo 22–24 h RNA-seq results [78]) and the TSS and exons were used. To generate average HP2 profiles we used the data from S2 cells provided by modENCODE and the corresponding RNA-seq results [14], [78].
Supporting Information
Zdroje
1. EissenbergJC, ReuterG (2009) Cellular mechanism for targeting heterochromatin formation in Drosophila. Int Rev Cell Mol Biol 273 : 1–47.
2. FilionGJ, van BemmelJG, BraunschweigU, TalhoutW, KindJ, et al. (2010) Systematic protein location mapping reveals five principal chromatin types in Drosophila cells. Cell 143 : 212–224.
3. YoonJ, LeeKS, ParkJS, YuK, PaikSG, et al. (2008) dSETDB1 and SU(VAR)3-9 sequentially function during germline-stem cell differentiation in Drosophila melanogaster. PLoS ONE 3: e2234 doi:10.1371/journal.pone.0002234
4. CloughE, MoonW, WangS, SmithK, HazelriggT (2007) Histone methylation is required for oogenesis in Drosophila. Development 134 : 157–165.
5. KochCM, Honemann-CapitoM, Egger-AdamD, WodarzA (2009) Windei, the Drosophila homolog of mAM/MCAF1, is an essential cofactor of the H3K9 methyl transferase dSETDB1/Eggless in germ line development. PLoS Genet 5: e1000644 doi:10.1371/journal.pgen.1000644
6. SeumC, BontronS, ReoE, DelattreM, SpiererP (2007) Drosophila G9a is a nonessential gene. Genetics 177 : 1955–1957.
7. Brower-TolandB, RiddleNC, JiangH, HuisingaKL, ElginSC (2009) Multiple SET methyltransferases are required to maintain normal heterochromatin domains in the genome of Drosophila melanogaster. Genetics 181 : 1303–1319.
8. SchottaG, EbertA, KraussV, FischerA, HoffmannJ, et al. (2002) Central role of Drosophila SU(VAR)3-9 in histone H3-K9 methylation and heterochromatic gene silencing. EMBO J 21 : 1121–1131.
9. StabellM, EskelandR, BjørkmoM, LarssonJ, AalenRB, et al. (2006) The Drosophila G9a gene encodes a multi-catalytic histone methyltransferase required for normal development. Nucleic Acids Res 34 : 4609–4621.
10. EbertA, SchottaG, LeinS, KubicekS, KraussV, et al. (2004) Su(var) genes regulate the balance between euchromatin and heterochromatin in Drosophila. Genes Dev 18 : 2973–2983.
11. CzerminB, MelfiR, McCabeD, SeitzV, ImhofA, et al. (2002) Drosophila enhancer of Zeste/ESC complexes have a histone H3 methyltransferase activity that marks chromosomal Polycomb sites. Cell 111 : 185–196.
12. SeumC, ReoE, PengH, RauscherFJ, SpiererP, et al. (2007) Drosophila SETDB1 is required for chromosome 4 silencing. PLoS Genet 3: e76 doi:10.1371/journal.pgen.0030076
13. TzengTY, LeeCH, ChanLW, ShenCK (2007) Epigenetic regulation of the Drosophila chromosome 4 by the histone H3K9 methyltransferase dSETDB1. Proc Natl Acad Sci U S A 104 : 12691–12696.
14. KharchenkoPV, AlekseyenkoAA, SchwartzYB, MinodaA, RiddleNC, et al. (2011) Comprehensive analysis of the chromatin landscape in Drosophila melanogaster. Nature 471 : 480–485.
15. BannisterAJ, ZegermanP, PartridgeJF, MiskaEA, ThomasJO, et al. (2001) Selective recognition of methylated lysine 9 on histone H3 by the HP1 chromo domain. Nature 410 : 120–124.
16. LachnerM, O'CarrollD, ReaS, MechtlerK, JenuweinT (2001) Methylation of histone H3 lysine 9 creates a binding site for HP1 proteins. Nature 410 : 116–120.
17. NakayamaJ, RiceJC, StrahlBD, AllisCD, GrewalSI (2001) Role of histone H3 lysine 9 methylation in epigenetic control of heterochromatin assembly. Science 292 : 110–113.
18. JacobsSA, KhorasanizadehS (2002) Structure of HP1 chromodomain bound to a lysine 9-methylated histone H3 tail. Science 295 : 2080–2083.
19. VermaakD, MalikHS (2009) Multiple roles for heterochromatin protein 1 genes in Drosophila. Annu Rev Genet 43 : 467–492.
20. ZhaoT, HeydukT, AllisCD, EissenbergJC (2000) Heterochromatin protein 1 binds to nucleosomes and DNA in vitro. J Biol Chem 275 : 28332–28338.
21. BillurM, BartunikHD, SinghPB (2010) The essential function of HP1β: a case of the tail wagging the dog? Trends Biochem Sci 35 : 115–123.
22. DialynasGK, MakatsoriD, KourmouliN, TheodoropoulosPA, McLeanK, et al. (2006) Methylation-independent binding to histone H3 and cell cycle-dependent incorporation of HP1β into heterochromatin. J Biol Chem 281 : 14350–14360.
23. NielsenAL, Oulad-AbdelghaniM, OrtizJA, RemboutsikaE, ChambonP, et al. (2001) Heterochromatin formation in mammalian cells: interaction between histones and HP1 proteins. Mol Cell 7 : 729–739.
24. LavigneM, EskelandR, AzebiS, Saint-AndréV, JangSM, et al. (2009) Interaction of HP1 and Brg1/Brm with the globular domain of histone H3 is required for HP1-mediated repression. PLoS Genet 5: e1000769 doi:10.1371/journal.pgen.1000769
25. HaddrillPR, WaldronFM, CharlesworthB (2008) Elevated levels of expression associated with regions of the Drosophila genome that lack crossing over. Biol Lett 4 : 758–761.
26. JohanssonAM, StenbergP, AllgardssonA, LarssonJ (2012) POF regulates the expression of genes on the 4th chromosome in D. melanogaster by binding to nascent RNA. Mol Cell Biol doi:10.1128/MCB.06622–11
27. JohanssonAM, StenbergP, BernhardssonC, LarssonJ (2007) Painting of fourth and chromosome-wide regulation of the 4th chromosome in Drosophila melanogaster. EMBO J 26 : 2307–2316.
28. LarssonJ, ChenJD, RashevaV, Rasmuson LestanderA, PirrottaV (2001) Painting of fourth, a chromosome-specific protein in Drosophila. Proc Natl Acad Sci U S A 98 : 6273–6278.
29. LarssonJ, SvenssonMJ, StenbergP, MäkitaloM (2004) Painting of fourth in genus Drosophila suggests autosome-specific gene regulation. Proc Natl Acad Sci U S A 101 : 9728–9733.
30. LarssonJ, MellerVH (2006) Dosage compensation, the origin and the afterlife of sex chromosomes. Chromosome Res 14 : 417–431.
31. StenbergP, LarssonJ (2011) Buffering and the evolution of chromosome-wide gene regulation. Chromosoma 120 : 213–225.
32. JohanssonAM, StenbergP, PetterssonF, LarssonJ (2007) POF and HP1 bind expressed exons, suggesting a balancing mechanism for gene regulation. PLoS Genet 3: e209 doi:10.1371/journal.pgen.0030209
33. StenbergP, LundbergLE, JohanssonAM, RydénP, SvenssonMJ, et al. (2009) Buffering of segmental and chromosomal aneuploidies in Drosophila melanogaster. PLoS Genet 5: e1000465 doi:10.1371/journal.pgen.1000465
34. RiddleNC, ElginSC (2006) The dot chromosome of Drosophila: insights into chromatin states and their change over evolutionary time. Chromosome Res 14 : 405–416.
35. RiddleNC, MinodaA, KharchenkoPV, AlekseyenkoAA, SchwartzYB, et al. (2011) Plasticity in patterns of histone modifications and chromosomal proteins in Drosophila heterochromatin. Genome Res 21 : 147–163.
36. CrydermanDE, GradeSK, LiY, FantiL, PimpinelliS, et al. (2005) Role of Drosophila HP1 in euchromatic gene expression. Dev Dyn 232 : 767–774.
37. PiacentiniL, FantiL, BerlocoM, PerriniB, PimpinelliS (2003) Heterochromatin protein 1 (HP1) is associated with induced gene expression in Drosophila euchromatin. J Cell Biol 161 : 707–714.
38. PiacentiniL, FantiL, NegriR, Del VescovoV, FaticaA, et al. (2009) Heterochromatin protein 1 (HP1a) positively regulates euchromatic gene expression through RNA transcript association and interaction with hnRNPs in Drosophila. PLoS Genet 5: e1000670 doi:10.1371/journal.pgen.1000670
39. SchwaigerM, KohlerH, OakeleyEJ, StadlerMB, SchübelerD (2010) Heterochromatin protein 1 (HP1) modulates replication timing of the Drosophila genome. Genome Res 20 : 771–780.
40. de WitE, GreilF, van SteenselB (2005) Genome-wide HP1 binding in Drosophila: developmental plasticity and genomic targeting signals. Genome Res 15 : 1265–1273.
41. de WitE, GreilF, van SteenselB (2007) High-resolution mapping reveals links of HP1 with active and inactive chromatin components. PLoS Genet 3: e38 doi:10.1371/journal.pgen.0030038
42. YinH, SweeneyS, RahaD, SnyderM, LinH (2011) A high-resolution whole-genome map of key chromatin modifications in the adult Drosophila melanogaster. PLoS Genet 7: e1002380 doi:10.1371/journal.pgen.1002380
43. FantiL, BerlocoM, PiacentiniL, PimpinelliS (2003) Chromosomal distribution of heterochromatin protein 1 (HP1) in Drosophila: a cytological map of euchromatic HP1 binding sites. Genetica 117 : 135–147.
44. JamesTC, EissenbergJC, CraigC, DietrichV, HobsonA, et al. (1989) Distribution patterns of HP1, a heterochromatin-associated nonhistone chromosomal protein of Drosophila. Eur J Cell Biol 50 : 170–180.
45. GreilF, van der KraanI, DelrowJ, SmothersJF, de WitE, et al. (2003) Distinct HP1 and Su(var)3-9 complexes bind to sets of developmentally coexpressed genes depending on chromosomal location. Genes Dev 17 : 2825–2838.
46. EissenbergJC, MorrisGD, ReuterG, HartnettT (1992) The heterochromatin-associated protein HP1 is an essential protein in Drosophila with dosage-dependent effects on position effect variegation. Genetics 131 : 345–352.
47. LockeJ, PodemskiL, RoyK, PilgrimD, HodgettsR (1999) Analysis of two cosmid clones from chromosome 4 of Drosophila melanogaster reveals two new genes amid an unusual arrangement of repeated sequences. Genome Res 9 : 137–149.
48. LockeJ, Howard LT, AippersbachN, PodemskiL, Hodgetts RB (1999) The characterization of DINE-1, a short, interspersed repetitive element present on chromosome and in the centric heterochromatin of Drosophila melanogaster. Chromosoma 108 : 356–366.
49. MiklosGLG, YamamotoMT, DaviesJ, PirrottaV (1988) Microcloning reveals a high frequency of repetitive sequences characteristic of chromosome four and the β-heterochromatin of Drosophila melanogaster. Proc Natl Acad Sci U S A 85 : 2051–2055.
50. PimpinelliS, BerlocoM, FantiL, DimitriP, BonaccorsiS, et al. (1995) Transposable elements are stable structural components of Drosophila melanogaster heterochromatin. Proc Natl Acad Sci U S A 92 : 3804–3808.
51. KaminkerJS, BergmanCM, KronmillerB, CarlsonJ, SvirskasR, et al. (2002) The transposable elements of the Drosophila melanogaster euchromatin: a genomics perspective. Genome Biol 3: RESEARCH0084.
52. StenbergP, PetterssonF, SauraAO, BerglundA, LarssonJ (2005) Sequence analysis of chromosome identity in three Drosophila species. BMC Bioinformatics 6 : 1–17.
53. SunFL, CuaycongMH, CraigCA, WallrathLL, LockeJ, et al. (2000) The fourth chromosome of Drosophila melanogaster: interspersed euchromatic and heterochromatic domains. Proc Natl Acad Sci U S A 97 : 5340–5345.
54. SunFL, HaynesK, SimpsonCL, LeeSD, CollinsL, et al. (2004) cis-Acting determinants of heterochromatin formation on Drosophila melanogaster chromosome four. Mol Cell Biol 24 : 8210–8220.
55. WallrathLL, ElginSC (1995) Position effect variegation in Drosophila is associated with an altered chromatin structure. Genes Dev 9 : 1263–1277.
56. WallrathLL, GunturVP, RosmanLE, ElginSC (1996) DNA representation of variegating heterochromatic P-element inserts in diploid and polytene tissues of Drosophila melanogaster. Chromosoma 104 : 519–527.
57. EskelandR, EberharterA, ImhofA (2007) HP1 binding to chromatin methylated at H3K9 is enhanced by auxiliary factors. Mol Cell Biol 27 : 453–465.
58. MeehanRR, KaoCF, PenningsS (2003) HP1 binding to native chromatin in vitro is determined by the hinge region and not by the chromodomain. EMBO J 22 : 3164–3174.
59. JacobsSA, TavernaSD, ZhangY, BriggsSD, LiJ, et al. (2001) Specificity of the HP1 chromo domain for the methylated N-terminus of histone H3. EMBO J 20 : 5232–5241.
60. FanJY, RangasamyD, LugerK, TremethickDJ (2004) H2A.Z alters the nucleosome surface to promote HP1α-mediated chromatin fiber folding. Mol Cell 16 : 655–661.
61. Raveh-SadkaT, LevoM, ShabiU, ShanyB, KerenL, et al. (2012) Manipulating nucleosome disfavoring sequences allows fine-tune regulation of gene expression in yeast. Nat Genet 44 : 743–750.
62. SegalE, WidomJ (2009) Poly(dA:dT) tracts: major determinants of nucleosome organization. Curr Opin Struct Biol 19 : 65–71.
63. CrydermanDE, VitaliniMW, WallrathLL (2011) Heterochromatin protein 1a is required for an open chromatin structure. Transcription 2 : 95–99.
64. MendezDL, KimD, ChruszczM, StephensGE, MinorW, et al. (2011) The HP1a disordered C terminus and chromo shadow domain cooperate to select target peptide partners. Chembiochem 12 : 1084–1096.
65. StephensGE, SlawsonEE, CraigCA, ElginSC (2005) Interaction of heterochromatin protein 2 with HP1 defines a novel HP1-binding domain. Biochemistry 44 : 13394–13403.
66. StephensGE, XiaoH, LankenauDH, WuC, ElginSC (2006) Heterochromatin protein 2 interacts with Nap-1 and NURF: a link between heterochromatin-induced gene silencing and the chromatin remodeling machinery in Drosophila. Biochemistry 45 : 14990–14999.
67. PakDT, PflummM, ChesnokovI, HuangDW, KellumR, et al. (1997) Association of the origin recognition complex with heterochromatin and HP1 in higher eukaryotes. Cell 91 : 311–323.
68. AuthT, KunkelE, GrummtF (2006) Interaction between HP1α and replication proteins in mammalian cells. Exp Cell Res 312 : 3349–3359.
69. PrasanthSG, ShenZ, PrasanthKV, StillmanB (2010) Human origin recognition complex is essential for HP1 binding to chromatin and heterochromatin organization. Proc Natl Acad Sci U S A 107 : 15093–15098.
70. SchwartzYB, KahnTG, NixDA, LiXY, BourgonR, et al. (2006) Genome-wide analysis of Polycomb targets in Drosophila melanogaster. Nat Genet 38 : 700–705.
71. SchwartzYB, PirrottaV (2007) Polycomb silencing mechanisms and the management of genomic programmes. Nat Rev Genet 8 : 9–22.
72. KahnTG, SchwartzYB, DellinoGI, PirrottaV (2006) Polycomb complexes and the propagation of the methylation mark at the Drosophila ubx gene. J Biol Chem 281 : 29064–29075.
73. FischleW, WangY, JacobsSA, KimY, AllisCD, et al. (2003) Molecular basis for the discrimination of repressive methyl-lysine marks in histone H3 by Polycomb and HP1 chromodomains. Genes Dev 17 : 1870–1881.
74. LucoRF, PanQ, TominagaK, BlencoweBJ, Pereira-SmithOM, et al. (2010) Regulation of alternative splicing by histone modifications. Science 327 : 996–1000.
75. LucoRF, AlloM, SchorIE, KornblihttAR, MisteliT (2011) Epigenetics in alternative pre-mRNA splicing. Cell 144 : 16–26.
76. HaynesKA, GrachevaE, ElginSC (2007) A distinct type of heterochromatin within Drosophila melanogaster chromosome 4. Genetics 175 : 1539–1542.
77. SwaminathanJ, BaxterEM, CorcesVG (2005) The role of histone H2Av variant replacement and histone H4 acetylation in the establishment of Drosophila heterochromatin. Genes Dev 19 : 65–76.
78. GraveleyBR, BrooksAN, CarlsonJW, DuffMO, LandolinJM, et al. (2011) The developmental transcriptome of Drosophila melanogaster. Nature 471 : 473–479.
79. JohanssonAM, AllgardssonA, StenbergP, LarssonJ (2011) msl2 mRNA is bound by free nuclear MSL complex in Drosophila melanogaster. Nucleic Acids Res 39 : 6428–6439.
80. KuttippurathuL, HsingM, LiuY, SchmidtB, MaskellDL, et al. (2011) CompleteMOTIFs: DNA motif discovery platform for transcription factor binding experiments. Bioinformatics 27 : 715–717.
81. CelnikerSE, DillonLA, GersteinMB, GunsalusKC, HenikoffS, et al. (2009) Unlocking the secrets of the genome. Nature 459 : 927–930.
82. ThomasS, LiXY, SaboPJ, SandstromR, ThurmanRE, et al. (2011) Dynamic reprogramming of chromatin accessibility during Drosophila embryo development. Genome Biol 12: R43.
Štítky
Genetika Reprodukční medicína
Článek The Covariate's DilemmaČlánek Plant Vascular Cell Division Is Maintained by an Interaction between PXY and Ethylene SignallingČlánek Lessons from Model Organisms: Phenotypic Robustness and Missing Heritability in Complex Disease
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 11
-
Všechny články tohoto čísla
- Inference of Population Splits and Mixtures from Genome-Wide Allele Frequency Data
- The Covariate's Dilemma
- Plant Vascular Cell Division Is Maintained by an Interaction between PXY and Ethylene Signalling
- Plan B for Stimulating Stem Cell Division
- Discovering Thiamine Transporters as Targets of Chloroquine Using a Novel Functional Genomics Strategy
- Is a Modifier of Mutations in Retinitis Pigmentosa with Incomplete Penetrance
- Evolutionarily Ancient Association of the FoxJ1 Transcription Factor with the Motile Ciliogenic Program
- Genome Instability Caused by a Germline Mutation in the Human DNA Repair Gene
- Transcription Factor Oct1 Is a Somatic and Cancer Stem Cell Determinant
- Controls of Nucleosome Positioning in the Human Genome
- Disruption of Causes Defective Meiotic Recombination in Male Mice
- A Novel Human-Infection-Derived Bacterium Provides Insights into the Evolutionary Origins of Mutualistic Insect–Bacterial Symbioses
- Trps1 and Its Target Gene Regulate Epithelial Proliferation in the Developing Hair Follicle and Are Associated with Hypertrichosis
- Zcchc11 Uridylates Mature miRNAs to Enhance Neonatal IGF-1 Expression, Growth, and Survival
- Population-Based Resequencing of in 10,330 Individuals: Spectrum of Genetic Variation, Phenotype, and Comparison with Extreme Phenotype Approach
- HP1a Recruitment to Promoters Is Independent of H3K9 Methylation in
- Transcription Elongation and Tissue-Specific Somatic CAG Instability
- A Germline Polymorphism of DNA Polymerase Beta Induces Genomic Instability and Cellular Transformation
- Interallelic and Intergenic Incompatibilities of the () Gene in Mouse Hybrid Sterility
- Comparison of Mitochondrial Mutation Spectra in Ageing Human Colonic Epithelium and Disease: Absence of Evidence for Purifying Selection in Somatic Mitochondrial DNA Point Mutations
- Mutations in the Transcription Elongation Factor SPT5 Disrupt a Reporter for Dosage Compensation in Drosophila
- Evolution of Minimal Specificity and Promiscuity in Steroid Hormone Receptors
- Blockade of Pachytene piRNA Biogenesis Reveals a Novel Requirement for Maintaining Post-Meiotic Germline Genome Integrity
- RHOA Is a Modulator of the Cholesterol-Lowering Effects of Statin
- MIG-10 Functions with ABI-1 to Mediate the UNC-6 and SLT-1 Axon Guidance Signaling Pathways
- Loss of the DNA Methyltransferase MET1 Induces H3K9 Hypermethylation at PcG Target Genes and Redistribution of H3K27 Trimethylation to Transposons in
- Genome-Wide Association Studies Reveal a Simple Genetic Basis of Resistance to Naturally Coevolving Viruses in
- The Principal Genetic Determinants for Nasopharyngeal Carcinoma in China Involve the Class I Antigen Recognition Groove
- Molecular, Physiological, and Motor Performance Defects in DMSXL Mice Carrying >1,000 CTG Repeats from the Human DM1 Locus
- Genomic Study of RNA Polymerase II and III SNAP-Bound Promoters Reveals a Gene Transcribed by Both Enzymes and a Broad Use of Common Activators
- Long Telomeres Produced by Telomerase-Resistant Recombination Are Established from a Single Source and Are Subject to Extreme Sequence Scrambling
- The Yeast SR-Like Protein Npl3 Links Chromatin Modification to mRNA Processing
- Deubiquitylation Machinery Is Required for Embryonic Polarity in
- dJun and Vri/dNFIL3 Are Major Regulators of Cardiac Aging in Drosophila
- CtIP Is Required to Initiate Replication-Dependent Interstrand Crosslink Repair
- Notch-Mediated Suppression of TSC2 Expression Regulates Cell Differentiation in the Intestinal Stem Cell Lineage
- A Combination of H2A.Z and H4 Acetylation Recruits Brd2 to Chromatin during Transcriptional Activation
- Network Analysis of a -Mouse Model of Autosomal Dominant Polycystic Kidney Disease Identifies HNF4α as a Disease Modifier
- Mitosis in Neurons: Roughex and APC/C Maintain Cell Cycle Exit to Prevent Cytokinetic and Axonal Defects in Photoreceptor Neurons
- CELF4 Regulates Translation and Local Abundance of a Vast Set of mRNAs, Including Genes Associated with Regulation of Synaptic Function
- Mechanisms Employed by to Prevent Ribonucleotide Incorporation into Genomic DNA by Pol V
- The Genomes of the Fungal Plant Pathogens and Reveal Adaptation to Different Hosts and Lifestyles But Also Signatures of Common Ancestry
- A Genome-Scale RNA–Interference Screen Identifies RRAS Signaling as a Pathologic Feature of Huntington's Disease
- Lessons from Model Organisms: Phenotypic Robustness and Missing Heritability in Complex Disease
- Population Genomic Scan for Candidate Signatures of Balancing Selection to Guide Antigen Characterization in Malaria Parasites
- Tissue-Specific Regulation of Chromatin Insulator Function
- Disruption of Mouse Cenpj, a Regulator of Centriole Biogenesis, Phenocopies Seckel Syndrome
- Genome, Functional Gene Annotation, and Nuclear Transformation of the Heterokont Oleaginous Alga CCMP1779
- Antagonistic Gene Activities Determine the Formation of Pattern Elements along the Mediolateral Axis of the Fruit
- Lung eQTLs to Help Reveal the Molecular Underpinnings of Asthma
- Identification of the First ATRIP–Deficient Patient and Novel Mutations in ATR Define a Clinical Spectrum for ATR–ATRIP Seckel Syndrome
- Cooperativity of , , and in Malignant Breast Cancer Evolution
- Loss of Prohibitin Membrane Scaffolds Impairs Mitochondrial Architecture and Leads to Tau Hyperphosphorylation and Neurodegeneration
- Microhomology Directs Diverse DNA Break Repair Pathways and Chromosomal Translocations
- MicroRNA–Mediated Repression of the Seed Maturation Program during Vegetative Development in
- Selective Pressure Causes an RNA Virus to Trade Reproductive Fitness for Increased Structural and Thermal Stability of a Viral Enzyme
- The Tumor Suppressor Gene Retinoblastoma-1 Is Required for Retinotectal Development and Visual Function in Zebrafish
- Regions of Homozygosity in the Porcine Genome: Consequence of Demography and the Recombination Landscape
- Histone Methyltransferases MES-4 and MET-1 Promote Meiotic Checkpoint Activation in
- Polyadenylation-Dependent Control of Long Noncoding RNA Expression by the Poly(A)-Binding Protein Nuclear 1
- A Unified Method for Detecting Secondary Trait Associations with Rare Variants: Application to Sequence Data
- Genetic and Biochemical Dissection of a HisKA Domain Identifies Residues Required Exclusively for Kinase and Phosphatase Activities
- Informed Conditioning on Clinical Covariates Increases Power in Case-Control Association Studies
- Biochemical Diversification through Foreign Gene Expression in Bdelloid Rotifers
- Genomic Variation and Its Impact on Gene Expression in
- Spastic Paraplegia Mutation N256S in the Neuronal Microtubule Motor KIF5A Disrupts Axonal Transport in a HSP Model
- Lamin B1 Polymorphism Influences Morphology of the Nuclear Envelope, Cell Cycle Progression, and Risk of Neural Tube Defects in Mice
- A Targeted Glycan-Related Gene Screen Reveals Heparan Sulfate Proteoglycan Sulfation Regulates WNT and BMP Trans-Synaptic Signaling
- Dopaminergic D2-Like Receptors Delimit Recurrent Cholinergic-Mediated Motor Programs during a Goal-Oriented Behavior
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Mechanisms Employed by to Prevent Ribonucleotide Incorporation into Genomic DNA by Pol V
- Inference of Population Splits and Mixtures from Genome-Wide Allele Frequency Data
- Zcchc11 Uridylates Mature miRNAs to Enhance Neonatal IGF-1 Expression, Growth, and Survival
- Histone Methyltransferases MES-4 and MET-1 Promote Meiotic Checkpoint Activation in
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



