-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaDisruption of Causes Defective Meiotic Recombination in Male Mice
CHTF18 (chromosome transmission fidelity factor 18) is an evolutionarily conserved subunit of the Replication Factor C-like complex, CTF18-RLC. CHTF18 is necessary for the faithful passage of chromosomes from one daughter cell to the next during mitosis in yeast, and it is crucial for germline development in the fruitfly. Previously, we showed that mouse Chtf18 is expressed throughout the germline, suggesting a role for CHTF18 in mammalian gametogenesis. To determine the role of CHTF18 in mammalian germ cell development, we derived mice carrying null and conditional mutations in the Chtf18 gene. Chtf18-null males exhibit 5-fold decreased sperm concentrations compared to wild-type controls, resulting in subfertility. Loss of Chtf18 results in impaired spermatogenesis; spermatogenic cells display abnormal morphology, and the stereotypical arrangement of cells within seminiferous tubules is perturbed. Meiotic recombination is defective and homologous chromosomes separate prematurely during prophase I. Repair of DNA double-strand breaks is delayed and incomplete; both RAD51 and γH2AX persist in prophase I. In addition, MLH1 foci are decreased in pachynema. These findings demonstrate essential roles for CHTF18 in mammalian spermatogenesis and meiosis, and suggest that CHTF18 may function during the double-strand break repair pathway to promote the formation of crossovers.
Published in the journal: . PLoS Genet 8(11): e32767. doi:10.1371/journal.pgen.1002996
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002996Summary
CHTF18 (chromosome transmission fidelity factor 18) is an evolutionarily conserved subunit of the Replication Factor C-like complex, CTF18-RLC. CHTF18 is necessary for the faithful passage of chromosomes from one daughter cell to the next during mitosis in yeast, and it is crucial for germline development in the fruitfly. Previously, we showed that mouse Chtf18 is expressed throughout the germline, suggesting a role for CHTF18 in mammalian gametogenesis. To determine the role of CHTF18 in mammalian germ cell development, we derived mice carrying null and conditional mutations in the Chtf18 gene. Chtf18-null males exhibit 5-fold decreased sperm concentrations compared to wild-type controls, resulting in subfertility. Loss of Chtf18 results in impaired spermatogenesis; spermatogenic cells display abnormal morphology, and the stereotypical arrangement of cells within seminiferous tubules is perturbed. Meiotic recombination is defective and homologous chromosomes separate prematurely during prophase I. Repair of DNA double-strand breaks is delayed and incomplete; both RAD51 and γH2AX persist in prophase I. In addition, MLH1 foci are decreased in pachynema. These findings demonstrate essential roles for CHTF18 in mammalian spermatogenesis and meiosis, and suggest that CHTF18 may function during the double-strand break repair pathway to promote the formation of crossovers.
Introduction
Precise chromosome segregation is crucial to ensure that germ cell development proceeds normally during meiosis, and that genetic information is accurately transmitted to the gametes. For chromosome segregation to proceed flawlessly during meiosis, homologous chromosomes must undergo several processes that allow them to pair and remain physically joined until anaphase I. The physical connections between homologous chromosomes are established by at least three different mechanisms. Sister chromatids are connected between arms and at centromeres by cohesion, a process mediated by cohesins, i.e. multiprotein complexes that are established during S-phase [1]–[6]. Different cohesin complexes exist depending on the cell type and stage, and each consists of at least four subunits, some of which are specific to meiosis [7], [8]. The physical connection between homologues also occurs by synapsis during meiotic prophase I, when homologous chromosomes pair through formation of a tripartite protein structure: the synaptonemal complex (for a review see [9]). Meiosis-specific cohesin complexes are believed to form a scaffold to which components of the synaptonemal complex can attach [10]. During synapsis, additional physical contacts occur at points of DNA crossover (chiasmata) through reciprocal recombination between nonsister chromatids (reviewed in [11], [12]). At the end of prophase I, the synaptonemal complex disassembles, but homologous chromosomes remain joined across sister chromatid arms and at centromeres. At anaphase I, dissolution of cohesion between sister chromatid arms and resolution of chiasmata allow homologous chromosomes to migrate away from the metaphase plate [13]. Although cohesion between sister chromatid arms is dissolved, cohesion at centromeres is preserved to keep sister chromatids connected until they segregate in anaphase II following attachment to the spindle [14]. Thus, cohesion and chiasmata between sister chromatid arms prevent homologues from separating prematurely [10], [11].
Maintenance of genome integrity is mediated in part by Replication Factor C - like complexes (RLCs) which function in DNA replication, chromosome cohesion, and the DNA damage checkpoint [15]. CTF18, a component of RLC-CTF18, was initially discovered in Saccharomyces cerevisiae [16]. In yeast, RLC-CTF18 is essential for establishment of sister chromatid cohesion and genome stability [15], [17], [18]. CTF18 is also crucial for germline development in the fruitfly. In CTF18 mutant flies, termed Cutlet, a loss-of-function mutation causes failure of germline stem cells to proliferate normally, resulting in sterility [19]. Recently, studies of the human RLC-CTF18 (termed RLC-CHTF18) complex in vitro and in immortalized cell lines have demonstrated a role for CHTF18 in mammalian DNA replication [20]–[22]. Previously, we cloned and characterized Chtf18, the murine orthologue of CTF18. We showed that CHTF18 is expressed throughout the male and female germline of the mouse, suggesting a role for it in gametogenesis [23]. However, the role of CHTF18 in mammals has not been fully elucidated.
Here we report the crucial roles CHTF18 plays during male meiosis in vivo. Our data demonstrate that CHTF18 functions in mammalian spermatogenesis to ensure fertility in males. Our results also suggest a role for CHTF18 in male meiotic recombination, and that it may function in maintaining the linkage of homologous chromosomes during meiotic prophase I.
Results
Derivation of Chtf18−/− and Chtf18flox/flox mice
CHTF18 is expressed throughout the male germline of the mouse, suggesting a role for it in spermatogenesis [23]. In order to study CHTF18 function in vivo, we employed gene targeting to derive Chtf18 mutant mice. We constructed Chtf18-null and conditional alleles by homologous recombination in embryonic stem (ES) cells (Figure 1A). The mouse Chtf18 gene consists of 22 exons spanning 8 kb of genomic DNA [23]. We chose to target exons 7–10, because these exons encode sequence motifs with high sequence similarity to Replication Factor C (RFC) (Figure 1A). These sequence motifs are called RFC boxes and are required for the function of RFC in yeast and in human cells [24]–[26]. Following electroporation and screening of 300 ES cell clones, five correctly targeted clones containing the Chtf18loxP-flanked (Chtf18flox) allele were identified (three are shown in Figure 1B). Cells from each of three clones were injected into mouse blastocysts and yielded 19 highly chimeric (>90%) male mice, which resulted in germline transmission of the targeted allele. Mice carrying this allele were then mated with transgenic Cre mice under the control of the E2A promoter [27]. The resulting heterozygotes were bred to homozygosity to generate Chtf18−/− mice. Absence of CHTF18 protein in Chtf18−/− testes was confirmed by Western blot analysis, indicating that this is a null allele (Figure 1C). To derive Chtf18flox/− TNAP-Cre mice, mice homozygous for the Chtf18flox allele were bred with transgenic Cre mice under the control of the germ cell-specific promoter of the tissue non-specific alkaline phosphatase (TNAP) gene [28], following FLP-mediated excision of the neomycin resistance cassette in vivo (Figure 1 and Figure S1).
Fig. 1. Derivation of Chtf18−/− and Chtf18flox/flox mice. 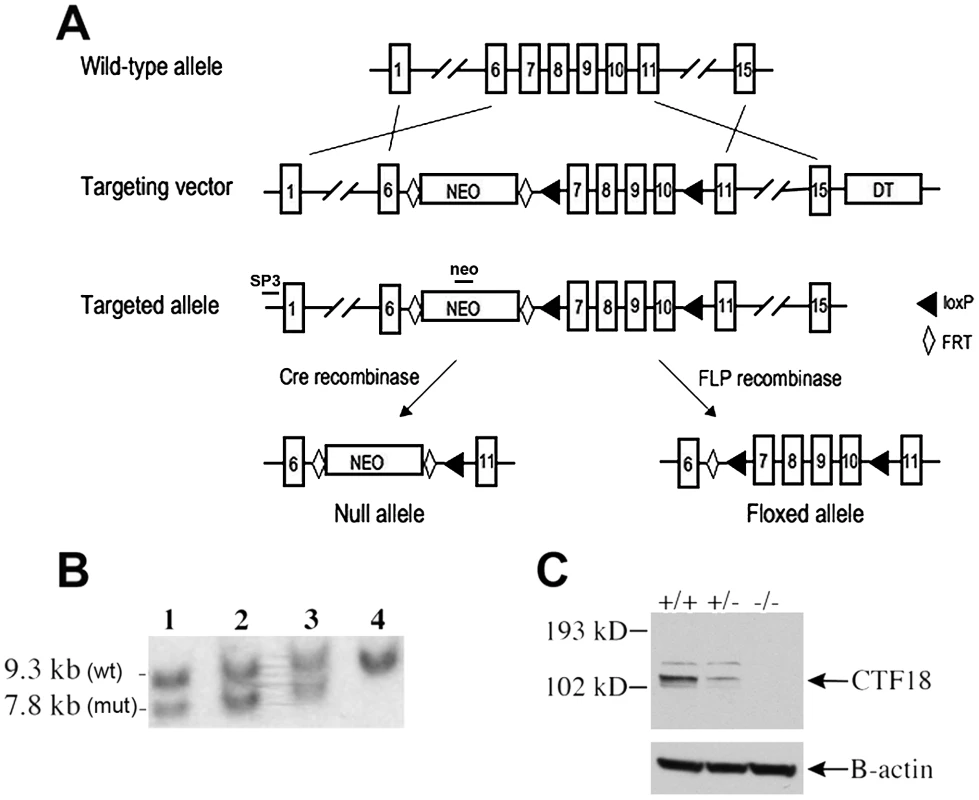
(A) Gene targeting strategy (exons are not drawn to scale). LoxP sites are represented by triangles. Diamonds represent FRT sites. (B) Southern blot analysis of targeted ES cells (lanes 1, 2, and 3) and wild-type ES cells (lane 4). The probes used for Southern blot analysis are depicted as SP3 and neo. (C) Western blot analysis of testis protein extracts from Chtf18-null mice with CHTF18 antibody. A ß-actin specific antibody was used as a control. Chtf18-null mice are viable but smaller in body weight
Although Chtf18−/− mice were viable with no overt defects, they were smaller in body weight (Chtf18−/− adult mean weight about 15% less than adult mean weight of wild-type), and were born at submendelian ratios (mean ratio of expected/observed embryos, Chtf18−/− 0.6, Chtf18+/+ 1.2, Chtf18+/− 1.1, p<0.007 using ANOVA, Figure 2C). Data collected from Chtf18+/− intercross matings revealed approximately 50% of the expected number of Chtf18−/− offspring, compared to Chtf18+/+ and Chtf18+/− offspring (Figure 2A). Data collected postnatally and from embryos at 14.5–18.5 dpc (Figure 2B and 2C) revealed virtually the same ratios of observed/expected for each genotype, and confirmed that these numbers were due to higher rates of death among Chtf18−/− mice during embryonic development.
Fig. 2. Chtf18−/− mice are born at submendelian ratios. 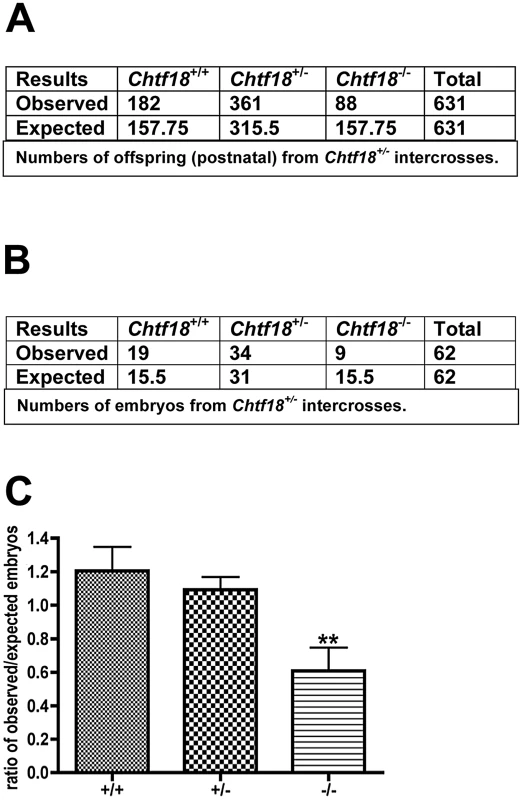
Data collected from Chtf18+/− intercross matings. (A and B) Numbers of expected and observed Chtf18+/+, Chtf18+/−, and Chtf18−/− offspring and embryos (14.5–18.5 dpc), respectively. (C) Histograms showing observed/expected ratios of Chtf18+/+, Chtf18+/−, and Chtf18−/− embryos at 14.5–18.5 dpc. Values are the mean ratio ± SEM; p≤0.007 using ANOVA. Impaired spermatogenesis, oligospermia, and decreased fertility in Chtf18-null males
Although Chtf18−/− mice appeared grossly normal but smaller in body weight, testes of Chtf18−/− males were significantly smaller (testis weight per body weight for Chtf18+/+ and Chtf18−/− mice mg/g, means ± SEM, Chtf18+/+ 6.98±0.30, n = 9 males; Chtf18−/− 3.03±0.32, n = 8 males, P<0.0001 using the Student's t-test, Figure 3A and 3B) and morphologically abnormal compared to those of control littermates (Figure 4). Although seminiferous tubules of Chtf18−/− and wild-type testes contained the complete spectrum of spermatogenic cells, including spermatogonia, spermatocytes, spermatids, and spermatozoa, indicating that there is not a block in spermatogenesis at any specific stage, the cells demonstrated a range of abnormalities. Chtf18−/− seminiferous tubules contained large multinucleated and aberrant-appearing spermatogenic cells, while others were almost devoid of spermatogenic cells (Figure 4B), although a few tubules showed almost normal morphology (Figure 4D). In addition, the stereotypical arrangement of spermatogenic cells demonstrating the orderly progression of spermatogenesis within seminiferous tubules appeared absent in most Chtf18−/− tubules, suggesting that spermatogenesis is impeded or disrupted (Figure 4E and 4F).
Fig. 3. Chtf18−/− testes are significantly smaller than those of wild-type mice. 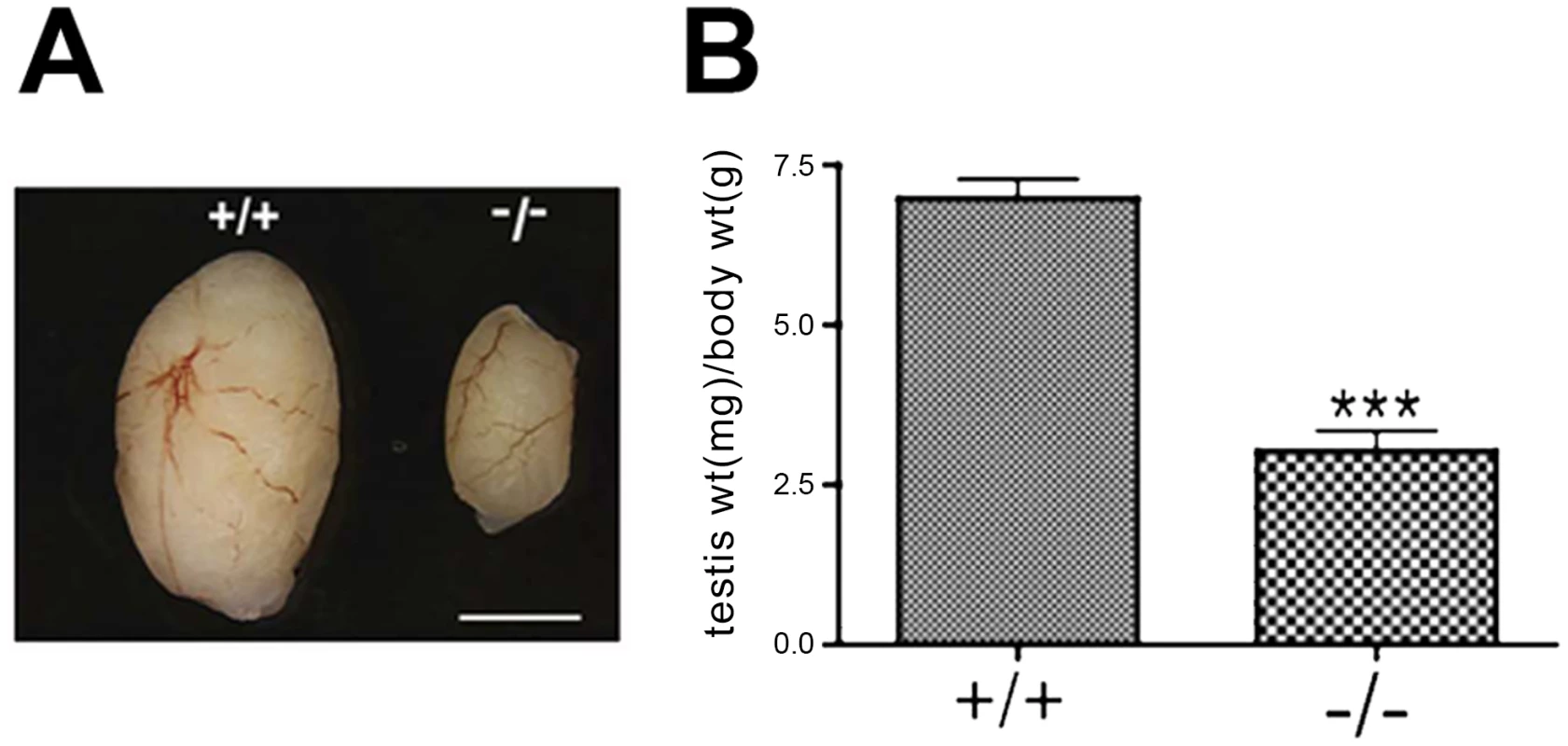
(A) Dramatic size reduction in Chtf18+/+ and Chtf18−/− adult testes. Scale bar represents 2 mm. (B) Histograms showing testis weight per body weight for Chtf18+/+ and Chtf18−/− mice (mg/g, means ± SEM) Chtf18+/+ 6.98±0.30, n = 9 mice; Chtf18−/− testes 3.03±0.32, n = 8 mice, P<0.0001 using the Student's t-test. Fig. 4. Impaired spermatogenesis in Chtf18−/− mice. 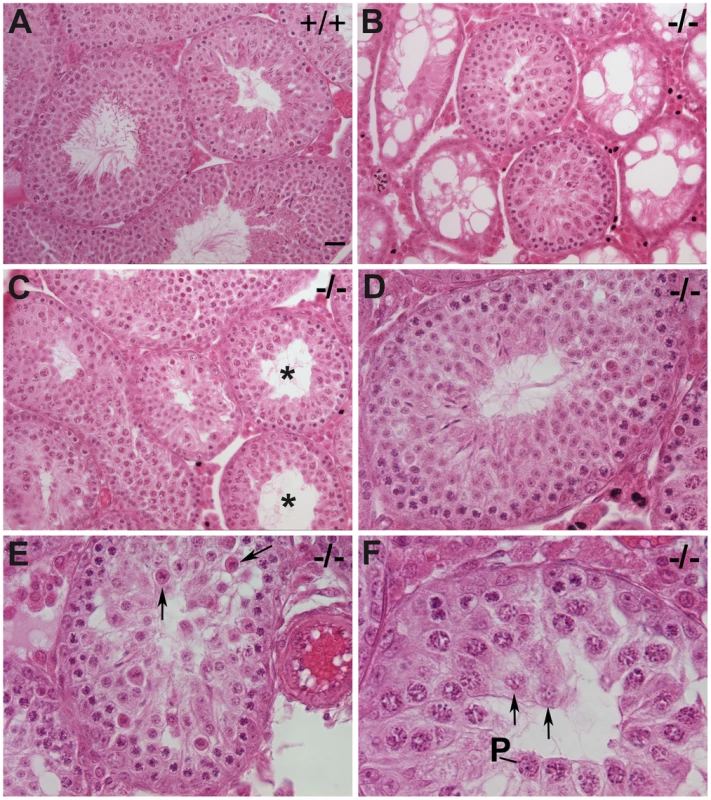
Seminiferous tubules stained with hematoxylin and eosin from 6–12 week old wild-type (A) and Chtf18−/− testes (B–F) are shown. Chtf18−/− tubules show a range of abnormalities. A paucity of spermatids and spermatozoa (asterisks in C) or almost complete absence of spermatogenic cells and large vacuoles (B) are seen, although a few Chtf18−/− tubules demonstrate almost normal morphology (D). Disorganized testes morphology and aberrant spermatogenic cells (E and F) are also seen. Spermatogenic cells appear to be in pachynema, P, are located at the lumen, and arrows in E and F indicate aberrant cells in Chtf18−/− seminiferous tubules. Scale bar is 25 µm. To determine the physiologic consequences of the disruption seen in seminiferous tubules of Chtf18−/− mice, we quantified the number of sperm recovered from the caudal region of the epididymis. We found that the sperm counts of Chtf18−/− mice were reduced more than 5-fold compared to those of wild-type males (Figure 5A). To evaluate the impact of this severe oligospermia on fertility of Chtf18−/− males, we mated Chtf18−/− or wild-type males with pairs of wild-type females over a period of five months. As expected from the low sperm counts, we found that Chtf18−/− males are subfertile compared to wild-type controls (Figure 5B). Loss of Chtf18 leads to oligospermia and not meiotic arrest, since some mature spermatids are indeed produced. Thus, the phenotype results in subfertility and not sterility in males. To assess whether apoptosis was an underlying cause of the paucity of spermatogenic cells in Chtf18−/− seminiferous tubules, we performed TUNEL assays. We found an increased number of apoptotic cells in Chtf18−/− tubules (mean number of apoptotic cells per seminiferous tubule, 7.66 and 3.08 for three Chtf18−/− and three wild-type adult males, respectively, p<0.0001, Figure 5C and 5D).
Fig. 5. Oligospermia and decreased fertility in Chtf18-null mice. 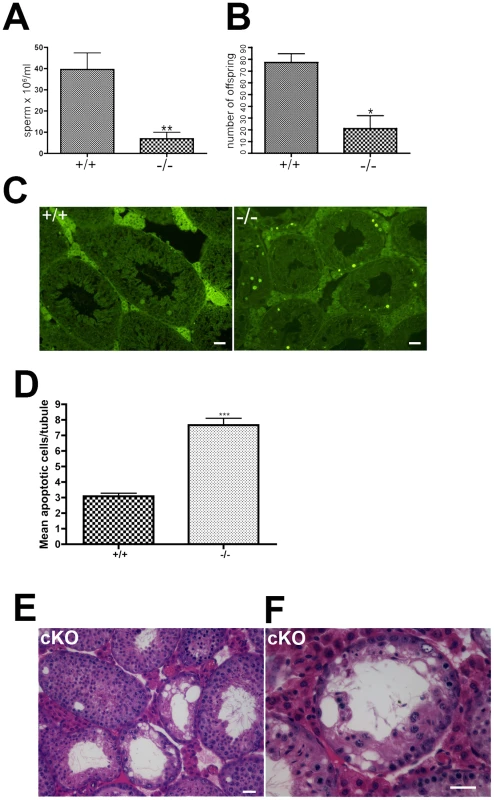
(A) Histograms showing caudal epididymal sperm concentrations from 10–12 week old Chtf18−/− mice and Chtf18+/+ controls. Values are the number of sperm ×106 per ml, means ± SEM, Chtf18+/+ 39.60±7.71, n = 9 mice; Chtf18−/− 6.90±3.04, n = 8 mice, P≤0.002 using Student's t-test (B) Histograms showing the number of offspring from wild-type females bred with Chtf18−/− and Chtf18+/+ male mice over a 5 month period. Each male was paired with two wild-type females (number of offspring from 12 pairs of wild-type females mated with Chtf18+/+ and Chtf18−/− males, means ± SEM, Chtf18+/+ 77.33±7.36, n = 3 males; Chtf18−/− 21.00±10.97, n = 3 males, P≤0.0130 using Student's t-test) (C) TUNEL staining of seminiferous tubule sections from three Chtf18−/− and three wild-type 7 week old mice demonstrates that apoptotic cells are increased in Chtf18−/− tubules. Scale bar is 25 µm. (D) Histograms showing mean apoptotic cells per tubule ± SEM; wild-type (+/+) 3.083±0.197; N = 145 vs. Chtf18−/− (−/−) 7.658±0.477; N = 155; p<0.0001 (E and F) Tubules from 13–14 week old Chtf18flox/−; TNAP Cre (cKO) mice stained with hematoxylin and eosin demonstrate a morphological phenotype that is indistinguishable from Chtf18−/− tubules (Figure 4). Scale bars are 25 µm and 50 µm for E and F, respectively. Because CHTF18 is expressed in both the somatic and germ cell lineages in the testes, we wanted to determine whether Chtf18 is required specifically in germ cells. To this end, we derived Chtf18flox/− TNAP Cre mice (cKO), in which Chtf18 is deleted only in germ cells (Figure S1). Several studies have demonstrated the use of the TNAP Cre transgenic mouse to effect highly specific and efficient germ cell-specific deletion [28]–[32]. As shown in Figure 5E and 5F, the morphological phenotype of affected cKO spermatogenic cells is indistinguishable from those seen in Chtf18−/− testes, suggesting that Chtf18 is required cell-autonomously in germ cells. While pre-meiotic effects cannot be ruled out, somatic effects of the testes can be excluded since TNAP is not expressed in these cells.
CTF18 mutant flies (called Cutlet) exhibit cessation of germline stem cell proliferation in mitotic stages of amplification [19]. Therefore, we speculated that a defect in establishment or maintenance of the early spermatogonial pool (prospermatogonia) might contribute to the paucity of germ cells seen in Chtf18−/− tubules. To evaluate this population of cells we stained postnatal day 3 (P3) testis sections from Chtf18−/− compared to wild-type tubules with an antibody to mouse vasa homolog (MVH), a germ cell-specific marker. Although we did not see a progressive loss of spermatogonial cells in Chtf18−/− tubules, we found that the number of prospermatogonia was significantly decreased in seminiferous tubules of Chtf18−/− compared to wild-type mice (mean number of germ cells per seminiferous tubule, 2.399 and 1.750 for four males each, respectively, p<0.0001, Student's t-test, Figure 6A–6C). In addition, the number of tubules completely lacking prospermatgonia was significantly greater in Chtf18−/− than wild-type testes (61, N = 280 tubules and 30, N = 278 tubules for four males each, respectively, p = 0.0005, Fisher's exact test, Figure 6D). These data suggest that that there is a defect in the early germ cell lineage of Chtf18−/− males.
Fig. 6. The number of prospermatogonia is decreased in Chtf18−/− neonatal testes. 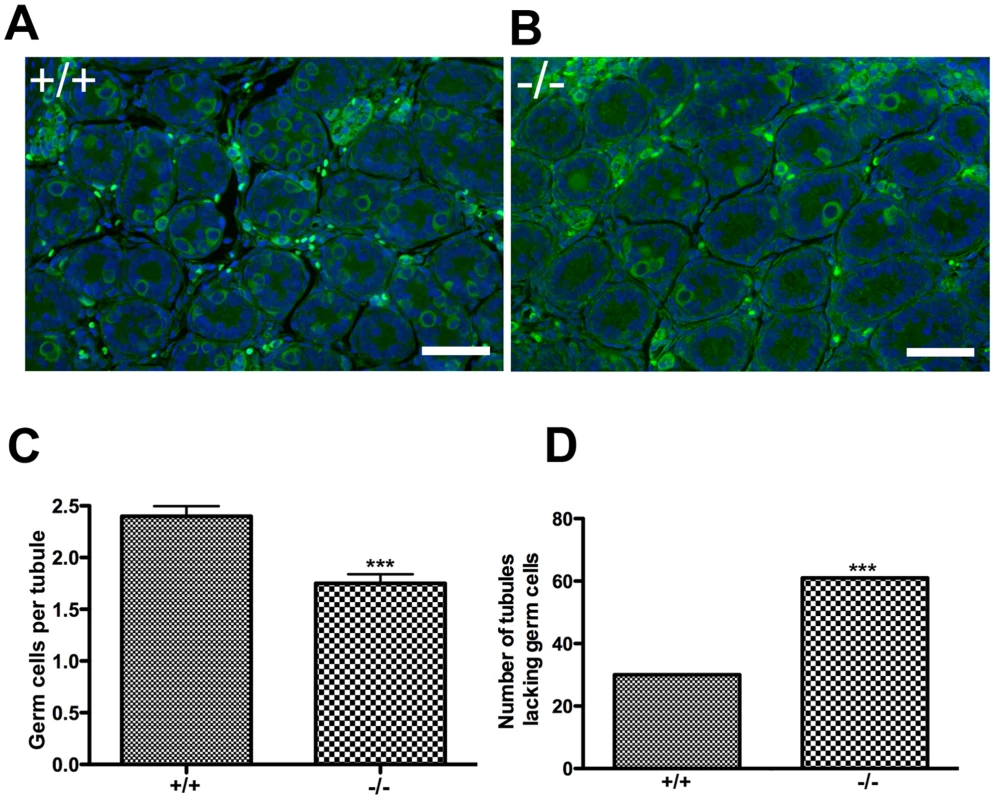
(A and B) Testis sections from postnatal day 3 Chtf18-null and wild-type males were stained with anti-MVH (green) and DAPI (blue). Scale bars are 50 µm. (C) Histogram showing the mean number of MVH-positive cells per seminiferous tubule ± SEM; wildtype (+/+) 2.399±0.097; N = 278 tubules and Chtf18−/− (−/−) 1.750±0.090; N = 280 tubules; p<0.0001 using Student's t-test (D) Histogram showing the number of seminiferous tubules completely lacking prospermatgonia in Chtf18−/− and wild-type testes; wild-type (+/+) 30, N = 278 and Chtf18−/− 61, N = 280, p = 0.0005 using Fisher's exact test. Homologous chromosomes separate prematurely in Chtf18-null mice
To begin to identify the molecular basis underlying the observed defects in Chtf18−/− mice, we examined meiosis. Surface spread analysis of Chtf18−/− spermatocytes, immunostained with anti-SYCP1 and anti-SYCP2 antibodies which label central and axial/lateral elements of the synaptonemal complex, were used to evaluate the progression of meiotic prophase I. We found that the leptotene and zygotene stages of prophase I progressed normally in Chtf18−/− spermatocytes as demonstrated by accumulation of SYCP1 and SYCP2 on homologues (Figure 7A and 7B). Synapsis of homologous chromosomes during the pachytene stage was also normal as demonstrated by the complete co-localization of SYCP1 and SYCP2 on autosomes of 105 Chtf18−/− pachytene spermatocytes compared to 100 pachytene wild-type cells (Figure 7C). However, examination of the diplotene stage of prophase I revealed the presence of separated homologues, consistent with univalent chromosomes (Figure 7D). To confirm the presence of univalent chromosomes we used CREST autoimmune serum, which stains centromeres, and anti-SYCP3, which stains the axial/lateral elements of the synaptonemal complex (Figure 7E and 7F). We quantified the number of CREST foci on homologues in Chtf18-null compared to wild-type spermatocytes. Univalent chromosomes were counted in diplotene spermatocytes containing greater than 21 CREST foci on separated homologues. We found that 42% of Chtf18−/− diplotene spermatocytes (66 cells counted in four males) contained univalent chromosomes (Figure 7F), while no univalent chromosomes were seen in wild-type diplotene spermatocytes (70 cells counted in three males, Figure 7E). In affected Chtf18−/− diplotene spermatocytes, we found two or more univalent chromosomes. These data are consistent with premature separation of homologous chromosomes during prophase I and not asynapsis because pairing of homologues and chromosomal synapsis through the pachytene stage in Chtf18−/− mice were normal.
Fig. 7. Formation of univalent chromosomes in Chtf18−/− spermatocytes. 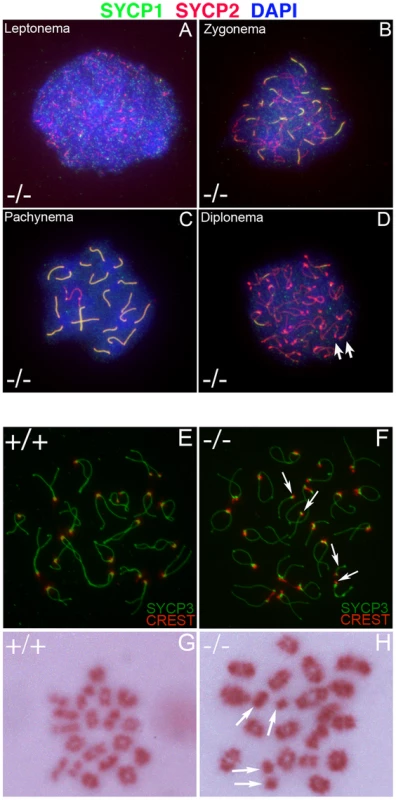
Meiotic chromosome spreads from Chtf18−/− juvenile males at postnatal day 21 were stained by immunofluorescence with anti-SYCP1 serum (green), anti-SYCP2 serum (red), and DAPI (blue) (A–D). SYCP1 localizes to central elements of the synaptonemal complex and SYCP2 localizes to the axial/lateral elements of the synaptonemal complex. The leptone, zygotene, pachytene, and diplotene stages of prophase I Chtf18−/− spermatocyte (−/−) are shown (A, B, C, and D respectively). Arrows in D reveal the presence of univalent chromosomes in the diplotene stage of prophase I. Wild-type and Chtf18−/− spermatocytes from juvenile males at postnatal day 21 (E and F, respectively) were stained with CREST autoimmune serum, which stains centromeres, and anti-SYCP3, which stains the axial/lateral elements of the synaptonemal complex. Arrows in F indicate univalent chromosomes in a Chtf18−/− diplotene spermatocyte. Metaphase I spread analysis of spermatocyte nuclei from juvenile males at postnatal day 21 stained with Giemsa (G and H). Twenty bivalent chromosomes are present in the wild-type metaphase I spermatocyte (G). A Chtf18−/− metaphase I spermatocyte (H) demonstrates the presence of four univalent chromosomes (arrows in H). Early disjunction of Chtf18-null homologues persists into metaphase I
Next we performed metaphase I spread analysis of spermatocytes to determine whether univalent chromosomes persist after synaptonemal complex dissolution at the end of prophase I (Figure 7G and 7H). Univalent chromosomes (as many as six) were present in 20% (35 cells counted) of Chtf18−/− metaphase I spermatocytes (Figure 7H), a number that is highly significant in light of the fact that such chromosomes were not observed in spermatocytes of wild-type males (50 cells counted). These findings reveal that homologues in Chtf18−/− spermatocytes separate prematurely during meiotic prophase I and that the defect persists through metaphase I, resulting in formation of univalent chromosomes.
Meiotic recombination is defective in Chtf18-null spermatocytes
In order to evaluate progression of meiotic recombination and DNA double-strand break (DSB) repair, we performed immunostaining with antibodies to γH2AX and RAD51, markers of DSB repair. Following formation of DSBs during leptonema, γH2AX is found along chromatin of both autosomes and sex chromosomes during normal meiotic progression [33]. γH2AX staining decreases during prophase I as DSBs are repaired until it is confined to the sex body, a region containing both the X and Y chromosomes, during pachynema. RAD51 foci appear as early meiotic recombination nodules, and they are abundant throughout prophase I. The number of RAD51 foci peaks in leptotema and early zygonema, and decreases in late pachynema as DSBs are repaired [34]–[36].
In both wild-type and Chtf18-null spermatocytes meiotic recombination initiated normally as demonstrated by the appearance of γH2AX during leptonema (Figure 8A and 8E). Although γH2AX staining decreased similarly in both wild-type and Chtf18-null spermatocytes during zygonema (Figure 8B and 8F) and became restricted to the sex body in wild type spermatocytes in pachynema and diplonema (Figure 8C and 8D), it persisted on the autosomes of Chtf18−/− spermatocytes into pachynema and diplonema (Figure 8G and 8H). This suggests that DSBs are formed but not repaired efficiently in the absence of CHTF18. Immunostaining with anti-RAD51 showed normal deposition of RAD51 on wild-type homologues in zygonema and pachynema (Figure 8I and 8L), but revealed the persistence of meiotic recombination nodules in Chtf18-null spermatocytes into zygonema and pachynema (Figure 8J and 8M). While the number of RAD51 foci decreased in wild-type spermatocytes by pachynema, Chtf18-null spermatocytes maintained a significantly greater number (mean number of RAD51 foci per nucleus, 10.27 and 19.95, in four control and four mutant males, respectively, p<0.0001, Figure 8N and 8O). DSB repair also appeared to be delayed as suggested by a significantly greater number of RAD51 foci detected on Chtf18-null compared to wild-type homologues during zygonema (mean number of RAD51 foci per nucleus, 195.2 and 166.2 for four Chtf18−/− and four wild-type males, respectively, p<0.0001, Figure 8K and 8O), indicating that the early stages of meiotic recombination are affected by loss of Chtf18.
Fig. 8. Meiotic recombination is defective in Chtf18−/− spermatocytes. 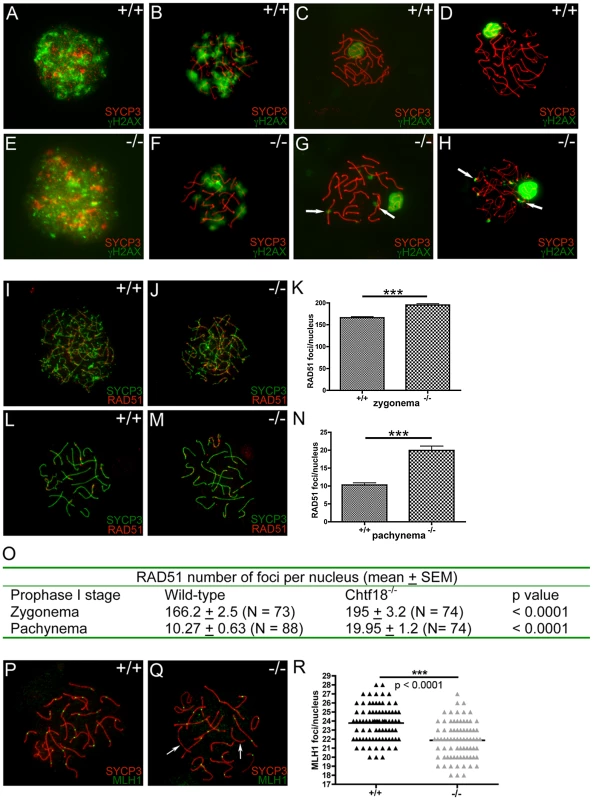
Meiotic chromosome spreads from wild-type (A–D) and Chtf18−/− (E–H) juvenile males at postnatal day 14 or 21 were stained with anti-SYCP3 (red) and γH2AX (green). A & E represent leptonema; B & F represent zygonema; C & G represent pachynema; D & H represent diplonema. Arrows in G and H show persistence of γH2AX in Chtf18−/− spermatocytes. Meiotic chromosome spreads from wild-type (I and L) and Chtf18−/− (J and M) males were stained with anti-SYCP3 (green) and anti-RAD51 (red). I and J represent zygonema; L and M represent pachynema. RAD51 focus counts per nucleus in zygonema (K and O) and pachynema (N and O) are shown. Meiotic chromosome spreads from wild-type and Chtf18−/− spermatocytes were stained with anti-SYCP3 (red) and anti-MLH1 (green) (P and Q). Arrows indicate homologues without MLH1 foci. MLH1 focus counts per nucleus are shown in R. Next we used an antibody to the mismatch repair protein, MLH1, to evaluate DSB repair and formation of meiotic crossovers in Chtf18−/− spermatocytes. MLH1 localizes to meiotic nodules that are believed to be the sites where chiasmata form [37], [38], and resolution of chiasmata is necessary for homologue disjunction. While each homologue should have at least one crossover (MLH focus), we found a slight but statistically significant decrease in the number of MLH1 foci in Chtf18−/− compared to wild-type spermatocytes (21.87 and 23.77 for four Chtf18−/− and four wild-type males, respectively, p<0.0001, Figure 8Q and 8R). In addition, 16.9% of Chtf18−/− spermatocytes contained at least one autosome that completely lacked a MLH1 focus (N = 83 late pachytene cells) compared to 3.4% of wild-type spermatocytes (N = 87 late pachytene cells). Analysis excluding these cells revealed that the average number of MLH1 foci was still significantly decreased in Chtf18−/− spermatocytes (that did not lack foci) compared to wild-type spermatocytes (22.17 and 23.82 for four Chtf18−/− and four wild-type males respectively, p<0.0001, Figure S2). Since each homologue normally forms at least one crossover (the obligate CO), these data are consistent with fewer Chtf18−/− spermatocytes (i.e. those not lacking MLH1 foci) containing autosomes with two or more MLH1 foci (instead of one) compared to wild-type spermatocytes. These findings indicate that the meiotic recombination defects seen in Chtf18−/− spermatocytes persist well into the late stages of meiotic recombination, and suggest a role for Chtf18 in facilitating normal rates of crossover during prophase I. In addition, the presence of univalent chromosomes in Chtf18-null diplotene and metaphase I spermatocytes (Figure 7) is due, at least in part to defective crossover formation at the pachytene stage.
Discussion
We derived mice lacking CHTF18, an evolutionarily conserved protein that is crucial for fertility in the fruitfly, and essential for accurate chromosome segregation in yeast. We demonstrated that spermatogenesis is severely disrupted and fertility is significantly impaired in Chtf18-null males.
Chtf18 is the murine orthologue of CTF18, a subunit of the replication factor C-like complex (RLC), RLC-CTF18, which consists of seven subunits (CTF18-CTF8-DCC1-RFC2-RFC3-RFC4-RFC5), and was initially discovered in Saccharomyces cerevisiae [16]. During DNA replication CHTF18 protein forms a complex with other RFC components to load PCNA (proliferating cell nuclear antigen; a replication fork protein essential for DNA replication) onto DNA. Studies in budding yeast have shown that CTF18 also functions in homologous recombination and DSB repair [39]. Loss of CTF18 in yeast results in improper establishment of sister chromatid cohesion, genetic instability, and aneuploidy [15], [17], [18]. RLC-CTF18 seems to couple DNA replication with sister chromatid cohesion because it is recruited to the replication fork in response to replication arrest [40], but the way in which this occurs is unknown. Consistently, RLC-CTF18 is implicated in the replication checkpoint and functions as an efficient unloader of PCNA in S. cerevisiae [41], [42]. Moreover, CTF18 stabilizes replication forks to facilitate sister chromatid cohesion in Schizosaccharomyces pombe [43]. As mentioned above in the fruit fly, CTF18 (called Cutlet) is necessary for fertility [19]. In humans, formation of the RLC-CHTF18 complex in vitro and in cell lines suggests a role for CHTF18 in mammalian DNA replication; human RLC-CHTF18 interacts with proliferating cell nuclear antigen and bound chromatin preferentially during S phase [20]–[22]. Recently, RLC-CHTF18 was shown to be necessary for the speed of DNA replication fork progression and efficient acetylation of cohesin in human epithelial cells, important processes that are necessary for continued advancement of DNA synthesis [44]. Thus, the functions of RLC-CTF18 appear to be conserved among eukaryotes.
Our data reveal that homologous chromosomes separate prematurely during meiosis I in Chtf18−/− spermatocytes. While homologous chromosomal pairing and synapsis are complete during pachynema, as revealed by deposition and co-localization of SYCP1 and SYCP2 (Figure 7C), univalent chromosomes are detected in diplonema of prophase I and in metaphase I in mutant spermatocytes (Figure 7F and 7H). Our findings suggest that the pachytene checkpoint is not activated in Chtf18−/− spermatocytes, since spermatocytes progress beyond pachynema. Meiotic recombination is initiated and the early steps appear to proceed normally in Chtf18-null homologues, but DNA double-strand break repair (DSB) repair and crossover formation are defective. This is indicated by persistence of both γH2AX and RAD51, and decreased MLH1 foci number during prophase I. In addition, DSB repair appears to be delayed as suggested by a significantly greater number of RAD51 foci detected on Chtf18-null homologous chromosomes during zygonema (Figure 8J, 8K, and 8O). These data suggest that CHTF18 plays crucial roles in mammalian meiosis and that CHTF18 may function in preventing early homologue disjunction. These observations are consistent with a role for CHTF18 in maintaining homologue linkage and possibly in DSB repair. CHTF18 may prevent homologue disjunction through a mechanism that affects DSB repair and/or crossover formation. Homologue disjunction during anaphase I normally occurs with resolution of chiasmata, which necessitates removal of cohesin from chromosome arms distal to chiasmata but not at centromeres [45]–[47]. Persistence of DSBs seen in pachynema and diplonema may arise due to DSBs occurring before meiosis and not as a result of SPO-11 action during early prophase I. However, it is not likely a major contributing defect since synapsis of homologues occurs normally in Chtf18-null spermatocytes. The possibility of pre-meiotic defects during the spermatogonial stages of spermatogenesis cannot be excluded. CHTF18 protein is expressed in all stages of developing germ cells in adult males, and in fetal male germ cells from 13.5 through 15.5 dpc [23], and the number of prospermatogonia is significantly decreased in Chtf18-null compared to wild-type neonatal seminiferous tubules. Although the decreased number of prospermatogonia must originate during establishment or proliferation of the primordial germ cell population, there does not appear to be a defect in spermatogonial stem cell renewal in adult mutant mice. Adult Chtf18-null seminiferous tubules do not progressively lose spermatogonia as seen in the Plzf mutant mouse [48]. The defect in establishment or proliferation of prospermatgonia in Chtf18-null males is not as severe as that observed in the germline of Drosophilia CTF18 mutants (termed Cutlet). In Cutlet mutant ovaries there is cessation of germline stem cell proliferation, and few if any egg chambers are formed, leading to sterility [20]. Cutlet mutant flies also exhibit eye and wing defects, but these defects are relatively mild and the adult organs appear to function normally. Cutlet mutations result in both decreased cellular proliferation and increased apoptosis in affected tissues, and studies implicate Cutlet as an accessory factor for DNA replication [19]. Similarly, Chtf18−/− mice are healthy but smaller in size than wild-type controls, suggesting a role for CHTF18 in cellular proliferation and DNA replication of somatic cells in mammals. The milder phenotype of early germ cell defects observed in Chtf18 mutant mice compared to Cutlet mutant flies suggests a non-essential but more specialized role for CHTF18 in mammalian gametogenesis.
Premature homologue disjunction and a defect in DSB repair in Chtf18−/− spermatocytes are consistent with cohesion-dependent and cohesion-independent mechanisms. Cohesion is mediated by cohesin complexes, which form a ring-like structure and embrace chromatin fibers of sister chromatids from DNA replication until their separation during anaphase [5]. Segregation of homologues during meiosis I is elicited by loss of cohesin complexes along chromosome arms distal to chiasmata [49]. During meiosis, cohesin complexes are necessary for establishing and maintaining cohesion between sister chromatids, and for synapsis and recombination between homologous chromosomes [50]. DSB repair during meiosis also requires cohesion between sister chromatid arms to be maintained [45]. Although the exact role cohesion plays in homologous recombination and DSB repair during meiosis is not known, a recent study in Caenorhabditis elegans reveals that meiotic cohesin promotes DSB processing and recruitment of DNA damage checkpoint proteins early in the DNA damage response. Absence of cohesin from meiotic chromosomes causes loss of chiasmata and the persistence of DSBs with accumulation of recombination intermediates [51]. Therefore, it is possible that CHTF18 is involved in both cohesion action and its effects on DSB repair during meiosis. However, a role for CHTF18 in maintenance of homologous chromosome linkage in mammals has not been shown previously. In both budding yeast and vertebrates efficient repair of DSBs by homologous recombination relies on the ability of cohesin complexes to mediate sister chromatid cohesion, and cohesin complexes accumulate on chromatin at DSBs [52]–[56]. While it has been shown that cohesin accumulates at sites of DSBs in mitotically dividing mammalian cells [57], it is not known whether cohesin complexes can be loaded onto chromosomes of meiotic cells after S phase [8] in mammals. CHTF18 has been shown to interact with the cohesin complexes in human immortalized cells [58], [59]. Thus, CHTF18 may preserve homologue linkage by maintaining cohesion between sister chromatid arms that was established prior to S phase; this could occur by interaction of CHTF18 with cohesin complexes loaded at sites of DSBs during meiosis, by a crossover mechanism, or by a combination of both these mechanisms.
Studies have provided evidence that cohesion is directly coupled to DNA replication by physical interaction between cohesin proteins and proteins involved in DNA replication at the replication fork [60]. Possible mechanisms of replication-dependent sister chromatid cohesion have been provided by studies in yeast and human cell lines [20]–[22], [40]–[43]. Recent studies in yeast have led to a model in which RLC-CTF18 associates with chromosomes to regulate PCNA and establish sister chromatid cohesion at the replication forks [40]–[43]. Thus, CHTF18 may interact with cohesin complexes at the replication fork. While prior studies have demonstrated a clear role for CHTF18 in DNA replication and establishment of sister chromatid arm cohesion, our data suggest a role for CHTF18 in promoting crossover formation through a possible interaction with cohesin complexes. Our data also suggest a role for CHTF18 in DSB repair and ensuring a wild-type number of crossovers in spermatocytes. Interestingly, approximately 17% of Chtf18-null spermatocytes in late pachynema contain at least one autosome that completely lacks a MLH1 focus (i.e. the majority of Chtf18-null spermatocytes have autosomes that each contain at least one MLH1 focus), yet homologues separate prematurely in 42% of Chtf18-null spermatocytes during diplonema. A possible explanation is that one crossover per homologue in the presence of impaired sister chromatid cohesion does not provide enough stability to prevent premature separation of homologues in Chtf18-null spermatocytes. This would suggest that in addition to its canonical function during DNA replication, CHTF18 acts as a cohesion factor to facilitate crossover formation during meiotic recombination. It is unclear exactly how CHTF18 might interact with cohesion factors. It is possible that CHTF18 affects this process during pre-S phase and/or during the DSB repair pathway in meiosis. Both chiasmata and cohesion between sister chromatid arms distal to chiasmata prevent homologues from separating prematurely [10], [11] and Chtf18-null male mice exhibit both premature homologue disjunction and decreased DNA crossovers. Thus, involvement of CHTF18 in chromosome cohesion during meiosis is biologically plausible. Loss-of-function mutations in cohesin genes have been described in mice. The phenotype of Chtf18-null males is not as severe as those for the SMC1β or REC8 cohesin mouse mutants. Both SMC1β mutant males and females are sterile; while SMC1β-deficient spermatocytes exhibit pachytene arrest, oocytes from SMC1β-deficient females show loss of sister chromatid cohesion in metaphase II [61]. REC8 mutant male and female mice are also sterile and show severe defects in synapsis, sister chromatid cohesion, and meiotic recombination [50], [62]. Although CHTF18 is not absolutely required for meiotic recombination, it may serve as functional link between DSB repair and crossover formation in mammals. We propose a model whereby CHTF18 associates or interacts with cohesin proteins to facilitate and maintain linkage of homologues during meiotic prophase I. Our data support a function for CHTF18 downstream of SPO-11 mediated DSB formation, during early stages of RAD51-mediated DSB repair and upstream of MLH1 - mediated DSB repair and crossover formation.
In summary, we derived Chtf18-null mice and demonstrated that the gene is essential for male meiosis. Our work reveals important new functions of CHTF18 in mammals, and suggests compelling roles for CHTF18 in male fertility and meiosis. Deletion of Chtf18 leads to a phenotype in which there is significant impairment of spermatogenesis, meiotic defects, and subfertility. These findings closely resemble those found in humans, where the majority of infertile men present with defects more subtle than complete spermatogenic failure. The requirements for Chtf18 in mammalian spermatogenesis demonstrated above suggest that malfunctioning of CHTF18 may be a cause of oligospermia and infertility in men. Hence, this work provides an important framework for future studies, which may elucidate the functions of CHTF18 in mammalian meiosis and fertility, and may ultimately shed more light on the processes of DSB repair and chromosome cohesion.
Materials and Methods
Derivation of Chtf18−/− mice and Chtf18flox/−; TNAP Cre mice
A 129SV mouse BAC library was screened by PCR and colony hybridization to obtain the Chtf18 genomic clone. Fragments of genomic DNA were then amplified by PCR from the Chtf18 clone and subcloned into the PND1 plasmid (given by G. Radice, Jefferson Medical College) to construct the PND1-Chtf18 targeting vector. Following electroporation and selection of cells, targeted clones were enriched by culture in G418. Approximately 300 surviving colonies were isolated, expanded, and screened for homologous recombinants by Southern blot analysis and PCR. Cells from three correctly targeted clones were expanded further, analyzed for a normal karyotype, and injected into C57BL/6 blastocysts, yielding 19 highly chimeric (≥90%) male mice. The male chimeric mice were mated to C57BL/6 female mice, resulting in successful germline transmission of the Chtf18flox allele. Mice carrying this allele were then mated with transgenic Cre mice under the control of the E2A promoter [27]. The resulting heterozygotes were bred to homozygosity to generate Chtf18-null mice. To derive Chtf18flox/−; TNAP Cre mice, mice heterozygous for the Chtf18flox allele were bred with transgenic Cre mice under the control of the germ-cell specific promoter tissue non-specific alkaline phosphatase (TNAP) [28] following FLP-mediated excision of the neomycin resistance cassette in vivo.
Western blot analysis
For protein analyses of mouse testes, 50 mg of total protein were electrophoretically separated by 4–12% SDS-PAGE, and transferred to polyvinylidene difluoride membranes (Millipore Co., Bedford, MA). Membranes were blocked (Tris-buffered saline solution containing 5% nonfat dry milk and 0.1% Tween 20 [TBST]), and then incubated with IgG-purified mouse CHTF18 antibody (0.31 mg/ml) [23] at 4°C overnight. The blots were washed in TBST and incubated with a goat anti-rabbit immunoglobulin conjugated to horseradish peroxidase (0.2 mg/ml, Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) for 1 h at room temperature. After washing, the CHTF18 protein was detected with Super Signal chemiluminescent substrate (Pierce, Rockford, IL).
Sperm concentration
The caudal epididymides of wild-type and Chtf18−/− adult mice were dissected, and their sperm content was released into PBS. Sperm number and concentration were determined using a hemocytometer. Statistical analysis was performed using the Student's t-test.
Fertility assessment
The number of offspring from wild-type females bred with 3 Chtf18−/− and 3 wild-type male mice over a five month period was documented. Each male was paired with two wild-type females, and the total number of pups from 12 pairs of wild-type females was recorded. Statistical analysis was performed using the Student's t-test.
TUNEL assay
Testes from wild-type and Chtf18−/− mice were fixed in 4% paraformaldehyde and embedded in paraffin. TUNEL assays were performed with the In Situ Cell Death Detection Kit, Fluorescein (Roche Applied Science, Indianapolis, IL) according to the manufacturer's instructions. Two hundred seminiferous tubules from 3 mice of each genotype were counted. Only cross sections and tubules containing at least one apoptotic cell were counted.
Histology, surface spread nuclei, and immunofluorescence
For histology, testes from adult male mice were fixed in Bouin's solution, embedded in paraffin, sectioned, and stained with hematoxylin and eosin. For germ cell counts, testes from postnatal day 3 mice were fixed in 2% paraformaldehyde, embedded in paraffin, sectioned, and stained with anti-DDX4/MVH antibody (rabbit, Abcam, 1∶250) and DAPI. MVH-positive cells were counted in at least 250 tubules from four different mice per genotype. Surface spreads of spermatocyte nuclei were prepared as previously described [63], [64]. Briefly, mouse testes were removed, seminiferous tubules gently minced with tweezers in DMEM, and cells mechanically separated. The cellular suspension was then spun to pellet cellular debris, and the nuclear suspension was pipetted onto slides. Slides were then fixed for 3 minutes each in freshly prepared 2% paraformaldehyde in PBS containing 0.03% SDS, and in 2% paraformaldehyde alone. Slides were rinsed three times for 1 minute each in 0.4% PHOTO-FLO 200 solution (Eastman Kodak Company, Rochester, NY), dried, then blocked in TBST containing 10% goat serum. Slides were then incubated with primary antibodies for 1 hour at 37°C or overnight at 4°C. Primary antibodies used for immunofluorescence were as follows: anti-SYCP2 (guinea pig, 1∶100), anti-SYCP1 serum 458 (rabbit, 1∶500), anti-SYCP3 (rabbit, Abcam, 1∶200) or anti-SYCP3 (mouse, 1∶1 provided by R. Jessberger, Dresden University of Technology, Dresden, Germany), anti-γH2AX (rabbit, Millipore, 1∶500), anti-RAD51 (rabbit, Calbiochem, 1∶400), anti-CREST (human Immunovision, 1∶100), anti-MLH1 (mouse, BD Pharmingen, 1∶50). Metaphase spreads were stained with Giemsa.
Mice
All experiments involving mice were approved by the Institutional Animal Care and Use Committees at the University of Pennsylvania and Drexel University College of Medicine.
Statistical analysis
The data comparing testis size, caudal epididymal sperm concentration, and number of offspring for Chtf18−/− mice and Chtf18+/+ controls were subjected to the Student's t-test. Results from expected/observed ratios of Chtf18+/+, Chtf18+/−, and Chtf18−/− embryos were analyzed by analysis of variance (ANOVA). Germ cell and immunofluorescence focus counts were analyzed using the Chi square test, Fisher's exact test or Student's t-test. All data were expressed as mean ± standard error of the mean (SEM), and p values<0.05 were considered statistically significant. Values were calculated using Prism 4.0 for Macintosh (GraphPad Software, Inc., La Jolla, CA).
Supporting Information
Zdroje
1. GuacciV, KoshlandD, StrunnikovA (1997) A direct link between sister chromatid cohesion and chromosome condensation revealed through the analysis of MCD1 in S. cerevisiae. Cell 91 : 47–57.
2. LosadaA, HiranoM, HiranoT (1998) Identification of Xenopus SMC protein complexes required for sister chromatid cohesion. Genes Dev 12 : 1986–1997.
3. MichaelisC, CioskR, NasmythK (1997) Cohesins: chromosomal proteins that prevent premature separation of sister chromatids. Cell 91 : 35–45.
4. DarwicheN, FreemanLA, StrunnikovA (1999) Characterization of the components of the putative mammalian sister chromatid cohesion complex. Gene 233 : 39–47.
5. GruberS, HaeringCH, NasmythK (2003) Chromosomal cohesin forms a ring. Cell 112 : 765–777.
6. UhlmannF (2004) The mechanism of sister chromatid cohesion. Exp Cell Res 296 : 80–85.
7. EijpeM, HeytingC, GrossB, JessbergerR (2000) Association of mammalian SMC1 and SMC3 proteins with meiotic chromosomes and synaptonemal complexes. J Cell Sci 113(Pt 4): 673–682.
8. RevenkovaE, JessbergerR (2006) Shaping meiotic prophase chromosomes: cohesins and synaptonemal complex proteins. Chromosoma 115 : 235–240.
9. YangF, WangPJ (2009) The Mammalian synaptonemal complex: a scaffold and beyond. Genome Dyn 5 : 69–80.
10. SujaJA, BarberoJL (2009) Cohesin complexes and sister chromatid cohesion in mammalian meiosis. Genome Dyn 5 : 94–116.
11. HandelMA, SchimentiJC (2010) Genetics of mammalian meiosis: regulation, dynamics and impact on fertility. Nat Rev Genet 11 : 124–136.
12. CohenPE, PollackSE, PollardJW (2006) Genetic analysis of chromosome pairing, recombination, and cell cycle control during first meiotic prophase in mammals. Endocr Rev 27 : 398–426.
13. LeeJY, Orr-WeaverTL (2001) The molecular basis of sister-chromatid cohesion. Annu Rev Cell Dev Biol 17 : 753–777.
14. RevenkovaE, JessbergerR (2005) Keeping sister chromatids together: cohesins in meiosis. Reproduction 130 : 783–790.
15. KimJ, MacNeillSA (2003) Genome stability: a new member of the RFC family. Curr Biol 13: R873–875.
16. KouprinaN, KrollE, KirillovA, BannikovV, ZakharyevV, et al. (1994) CHL12, a gene essential for the fidelity of chromosome transmission in the yeast Saccharomyces cerevisiae. Genetics 138 : 1067–1079.
17. HannaJS, KrollES, LundbladV, SpencerFA (2001) Saccharomyces cerevisiae CTF18 and CTF4 are required for sister chromatid cohesion. Mol Cell Biol 21 : 3144–3158.
18. MayerML, GygiSP, AebersoldR, HieterP (2001) Identification of RFC(Ctf18p, Ctf8p, Dcc1p): an alternative RFC complex required for sister chromatid cohesion in S. cerevisiae. Mol Cell 7 : 959–970.
19. JaffeAB, JongensTA (2001) Structure-specific abnormalities associated with mutations in a DNA replication accessory factor in Drosophila. Dev Biol 230 : 161–176.
20. MerkleCJ, KarnitzLM, Henry-SanchezJT, ChenJ (2003) Cloning and characterization of hCTF18, hCTF8, and hDCC1. Human homologs of a Saccharomyces cerevisiae complex involved in sister chromatid cohesion establishment. J Biol Chem 278 : 30051–30056.
21. OhtaS, ShiomiY, SugimotoK, ObuseC, TsurimotoT (2002) A proteomics approach to identify proliferating cell nuclear antigen (PCNA)-binding proteins in human cell lysates. Identification of the human CHL12/RFCs2-5 complex as a novel PCNA-binding protein. J Biol Chem 277 : 40362–40367.
22. ShiomiY, ShinozakiA, SugimotoK, UsukuraJ, ObuseC, et al. (2004) The reconstituted human Chl12-RFC complex functions as a second PCNA loader. Genes Cells 9 : 279–290.
23. BerkowitzKM, KaestnerKH, JongensTA (2008) Germline expression of mammalian CTF18, an evolutionarily conserved protein required for germ cell proliferation in the fly and sister chromatid cohesion in yeast. Mol Hum Reprod 14 : 143–150.
24. CullmannG, FienK, KobayashiR, StillmanB (1995) Characterization of the five replication factor C genes of Saccharomyces cerevisiae. Mol Cell Biol 15 : 4661–4671.
25. UhlmannF, CaiJ, GibbsE, O'DonnellM, HurwitzJ (1997) Deletion analysis of the large subunit p140 in human replication factor C reveals regions required for complex formation and replication activities. J Biol Chem 272 : 10058–10064.
26. UhlmannF, GibbsE, CaiJ, O'DonnellM, HurwitzJ (1997) Identification of regions within the four small subunits of human replication factor C required for complex formation and DNA replication. J Biol Chem 272 : 10065–10071.
27. LaksoM, PichelJG, GormanJR, SauerB, OkamotoY, et al. (1996) Efficient in vivo manipulation of mouse genomic sequences at the zygote stage. Proc Natl Acad Sci U S A 93 : 5860–5865.
28. LomeliH, Ramos-MejiaV, GertsensteinM, LobeCG, NagyA (2000) Targeted insertion of Cre recombinase into the TNAP gene: excision in primordial germ cells. Genesis 26 : 116–117.
29. KanedaM, HirasawaR, ChibaH, OkanoM, LiE, et al. (2010) Genetic evidence for Dnmt3a-dependent imprinting during oocyte growth obtained by conditional knockout with Zp3-Cre and complete exclusion of Dnmt3b by chimera formation. Genes Cells
30. QiuMR, JiangL, MatthaeiKI, SchoenwaelderSM, KuffnerT, et al. (2010) Generation and characterization of mice with null mutation of the chloride intracellular channel 1 gene. Genesis 48 : 127–136.
31. YamaguchiS, KurimotoK, YabutaY, SasakiH, NakatsujiN, et al. (2009) Conditional knockdown of Nanog induces apoptotic cell death in mouse migrating primordial germ cells. Development 136 : 4011–4020.
32. MaatoukDM, LovelandKL, McManusMT, MooreK, HarfeBD (2008) Dicer1 is required for differentiation of the mouse male germline. Biol Reprod 79 : 696–703.
33. MahadevaiahSK, TurnerJM, BaudatF, RogakouEP, de BoerP, et al. (2001) Recombinational DNA double-strand breaks in mice precede synapsis. Nat Genet 27 : 271–276.
34. AshleyT, PlugAW, XuJ, SolariAJ, ReddyG, et al. (1995) Dynamic changes in Rad51 distribution on chromatin during meiosis in male and female vertebrates. Chromosoma 104 : 19–28.
35. MoensPB, KolasNK, TarsounasM, MarconE, CohenPE, et al. (2002) The time course and chromosomal localization of recombination-related proteins at meiosis in the mouse are compatible with models that can resolve the early DNA-DNA interactions without reciprocal recombination. J Cell Sci 115 : 1611–1622.
36. PlugAW, PetersAH, KeeganKS, HoekstraMF, de BoerP, et al. (1998) Changes in protein composition of meiotic nodules during mammalian meiosis. J Cell Sci 111(Pt 4): 413–423.
37. BakerSM, PlugAW, ProllaTA, BronnerCE, HarrisAC, et al. (1996) Involvement of mouse Mlh1 in DNA mismatch repair and meiotic crossing over. Nat Genet 13 : 336–342.
38. EdelmannW, CohenPE, KaneM, LauK, MorrowB, et al. (1996) Meiotic pachytene arrest in MLH1-deficient mice. Cell 85 : 1125–1134.
39. OgiwaraH, OhuchiT, UiA, TadaS, EnomotoT, et al. (2007) Ctf18 is required for homologous recombination-mediated double-strand break repair. Nucl Acids Res 35 : 4989–5000.
40. LengronneA, McIntyreJ, KatouY, KanohY, HopfnerKP, et al. (2006) Establishment of sister chromatid cohesion at the S. cerevisiae replication fork. Mol Cell 23 : 787–799.
41. BylundGO, BurgersPM (2005) Replication protein A-directed unloading of PCNA by the Ctf18 cohesion establishment complex. Mol Cell Biol 25 : 5445–5455.
42. NaikiT, KondoT, NakadaD, MatsumotoK, SugimotoK (2001) Chl12 (Ctf18) forms a novel replication factor C-related complex and functions redundantly with Rad24 in the DNA replication checkpoint pathway. Mol Cell Biol 21 : 5838–5845.
43. AnsbachAB, NoguchiC, KlansekIW, HeidlebaughM, NakamuraTM, et al. (2008) RFCCtf18 and the Swi1–Swi3 complex function in separate and redundant pathways required for the stabilization of replication forks to facilitate sister chromatid cohesion in Schizosaccharomyces pombe. Mol Biol Cell 19 : 595–607.
44. JessbergerR, ChuiG, LinnS, KemperB (1996) Analysis of the mammalian recombination protein complex RC-1. Mutat Res 350 : 217–227.
45. BuonomoSB, ClyneRK, FuchsJ, LoidlJ, UhlmannF, et al. (2000) Disjunction of homologous chromosomes in meiosis I depends on proteolytic cleavage of the meiotic cohesin Rec8 by separin. Cell 103 : 387–398.
46. LeeJ, IwaiT, YokotaT, YamashitaM (2003) Temporally and spatially selective loss of Rec8 protein from meiotic chromosomes during mammalian meiosis. J Cell Sci 116 : 2781–2790.
47. LeeJ, OkadaK, OgushiS, MiyanoT, MiyakeM, et al. (2006) Loss of Rec8 from chromosome arm and centromere region is required for homologous chromosome separation and sister chromatid separation, respectively, in mammalian meiosis. Cell Cycle 5 : 1448–1455.
48. BuaasFW, KirshAL, SharmaM, McLeanDJ, MorrisJL, et al. (2004) Plzf is required in adult male germ cells for stem cell self-renewal. Nat Genet 36 : 647–652.
49. PetronczkiM, SiomosMF, NasmythK (2003) Un menage a quatre: the molecular biology of chromosome segregation in meiosis. Cell 112 : 423–440.
50. XuH, BeasleyMD, WarrenWD, van der HorstGT, McKayMJ (2005) Absence of mouse REC8 cohesin promotes synapsis of sister chromatids in meiosis. Dev Cell 8 : 949–961.
51. LightfootJ, TestoriS, BarrosoC, Martinez-PerezE (2011) Loading of meiotic cohesin by SCC-2 is required for early processing of DSBs and for the DNA damage checkpoint. Curr Biol 21 : 1421–1430.
52. SonodaE, MatsusakaT, MorrisonC, VagnarelliP, HoshiO, et al. (2001) Scc1/Rad21/Mcd1 is required for sister chromatid cohesion and kinetochore function in vertebrate cells. Dev Cell 1 : 759–770.
53. PottsPR, PorteusMH, YuH (2006) Human SMC5/6 complex promotes sister chromatid homologous recombination by recruiting the SMC1/3 cohesin complex to double-strand breaks. Embo J 25 : 3377–3388.
54. SchmitzJ, WatrinE, LenartP, MechtlerK, PetersJM (2007) Sororin is required for stable binding of cohesin to chromatin and for sister chromatid cohesion in interphase. Curr Biol 17 : 630–636.
55. Bekker-JensenS, LukasC, KitagawaR, MelanderF, KastanMB, et al. (2006) Spatial organization of the mammalian genome surveillance machinery in response to DNA strand breaks. J Cell Biol 173 : 195–206.
56. WatrinE, PetersJM (2009) The cohesin complex is required for the DNA damage-induced G2/M checkpoint in mammalian cells. Embo J 28 : 2625–2635.
57. KimJS, KrasievaTB, LaMorteV, TaylorAM, YokomoriK (2002) Specific recruitment of human cohesin to laser-induced DNA damage. J Biol Chem 277 : 45149–45153.
58. BermudezVP, ManiwaY, TappinI, OzatoK, YokomoriK, et al. (2003) The alternative Ctf18-Dcc1-Ctf8-replication factor C complex required for sister chromatid cohesion loads proliferating cell nuclear antigen onto DNA. Proc Natl Acad Sci U S A 100 : 10237–10242.
59. TerretME, SherwoodR, RahmanS, QinJ, JallepalliPV (2009) Cohesin acetylation speeds the replication fork. Nature 462 : 231–234.
60. RyuMJ, KimBJ, LeeJW, LeeMW, ChoiHK, et al. (2006) Direct interaction between cohesin complex and DNA replication machinery. Biochem Biophys Res Commun 341 : 770–775.
61. RevenkovaE, EijpeM, HeytingC, HodgesCA, HuntPA, et al. (2004) Cohesin SMC1 beta is required for meiotic chromosome dynamics, sister chromatid cohesion and DNA recombination. Nat Cell Biol 6 : 555–562.
62. BannisterLA, ReinholdtLG, MunroeRJ, SchimentiJC (2004) Positional cloning and characterization of mouse mei8, a disrupted allelle of the meiotic cohesin Rec8. Genesis 40 : 184–194.
63. KolasNK, MarconE, CrackowerMA, HoogC, PenningerJM, et al. (2005) Mutant meiotic chromosome core components in mice can cause apparent sexual dimorphic endpoints at prophase or X-Y defective male-specific sterility. Chromosoma 114 : 92–102.
64. PetersAH, PlugAW, van VugtMJ, de BoerP (1997) A drying-down technique for the spreading of mammalian meiocytes from the male and female germline. Chromosome Res 5 : 66–68.
Štítky
Genetika Reprodukční medicína
Článek The Covariate's DilemmaČlánek Plant Vascular Cell Division Is Maintained by an Interaction between PXY and Ethylene SignallingČlánek Lessons from Model Organisms: Phenotypic Robustness and Missing Heritability in Complex Disease
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 11
-
Všechny články tohoto čísla
- Inference of Population Splits and Mixtures from Genome-Wide Allele Frequency Data
- The Covariate's Dilemma
- Plant Vascular Cell Division Is Maintained by an Interaction between PXY and Ethylene Signalling
- Plan B for Stimulating Stem Cell Division
- Discovering Thiamine Transporters as Targets of Chloroquine Using a Novel Functional Genomics Strategy
- Is a Modifier of Mutations in Retinitis Pigmentosa with Incomplete Penetrance
- Evolutionarily Ancient Association of the FoxJ1 Transcription Factor with the Motile Ciliogenic Program
- Genome Instability Caused by a Germline Mutation in the Human DNA Repair Gene
- Transcription Factor Oct1 Is a Somatic and Cancer Stem Cell Determinant
- Controls of Nucleosome Positioning in the Human Genome
- Disruption of Causes Defective Meiotic Recombination in Male Mice
- A Novel Human-Infection-Derived Bacterium Provides Insights into the Evolutionary Origins of Mutualistic Insect–Bacterial Symbioses
- Trps1 and Its Target Gene Regulate Epithelial Proliferation in the Developing Hair Follicle and Are Associated with Hypertrichosis
- Zcchc11 Uridylates Mature miRNAs to Enhance Neonatal IGF-1 Expression, Growth, and Survival
- Population-Based Resequencing of in 10,330 Individuals: Spectrum of Genetic Variation, Phenotype, and Comparison with Extreme Phenotype Approach
- HP1a Recruitment to Promoters Is Independent of H3K9 Methylation in
- Transcription Elongation and Tissue-Specific Somatic CAG Instability
- A Germline Polymorphism of DNA Polymerase Beta Induces Genomic Instability and Cellular Transformation
- Interallelic and Intergenic Incompatibilities of the () Gene in Mouse Hybrid Sterility
- Comparison of Mitochondrial Mutation Spectra in Ageing Human Colonic Epithelium and Disease: Absence of Evidence for Purifying Selection in Somatic Mitochondrial DNA Point Mutations
- Mutations in the Transcription Elongation Factor SPT5 Disrupt a Reporter for Dosage Compensation in Drosophila
- Evolution of Minimal Specificity and Promiscuity in Steroid Hormone Receptors
- Blockade of Pachytene piRNA Biogenesis Reveals a Novel Requirement for Maintaining Post-Meiotic Germline Genome Integrity
- RHOA Is a Modulator of the Cholesterol-Lowering Effects of Statin
- MIG-10 Functions with ABI-1 to Mediate the UNC-6 and SLT-1 Axon Guidance Signaling Pathways
- Loss of the DNA Methyltransferase MET1 Induces H3K9 Hypermethylation at PcG Target Genes and Redistribution of H3K27 Trimethylation to Transposons in
- Genome-Wide Association Studies Reveal a Simple Genetic Basis of Resistance to Naturally Coevolving Viruses in
- The Principal Genetic Determinants for Nasopharyngeal Carcinoma in China Involve the Class I Antigen Recognition Groove
- Molecular, Physiological, and Motor Performance Defects in DMSXL Mice Carrying >1,000 CTG Repeats from the Human DM1 Locus
- Genomic Study of RNA Polymerase II and III SNAP-Bound Promoters Reveals a Gene Transcribed by Both Enzymes and a Broad Use of Common Activators
- Long Telomeres Produced by Telomerase-Resistant Recombination Are Established from a Single Source and Are Subject to Extreme Sequence Scrambling
- The Yeast SR-Like Protein Npl3 Links Chromatin Modification to mRNA Processing
- Deubiquitylation Machinery Is Required for Embryonic Polarity in
- dJun and Vri/dNFIL3 Are Major Regulators of Cardiac Aging in Drosophila
- CtIP Is Required to Initiate Replication-Dependent Interstrand Crosslink Repair
- Notch-Mediated Suppression of TSC2 Expression Regulates Cell Differentiation in the Intestinal Stem Cell Lineage
- A Combination of H2A.Z and H4 Acetylation Recruits Brd2 to Chromatin during Transcriptional Activation
- Network Analysis of a -Mouse Model of Autosomal Dominant Polycystic Kidney Disease Identifies HNF4α as a Disease Modifier
- Mitosis in Neurons: Roughex and APC/C Maintain Cell Cycle Exit to Prevent Cytokinetic and Axonal Defects in Photoreceptor Neurons
- CELF4 Regulates Translation and Local Abundance of a Vast Set of mRNAs, Including Genes Associated with Regulation of Synaptic Function
- Mechanisms Employed by to Prevent Ribonucleotide Incorporation into Genomic DNA by Pol V
- The Genomes of the Fungal Plant Pathogens and Reveal Adaptation to Different Hosts and Lifestyles But Also Signatures of Common Ancestry
- A Genome-Scale RNA–Interference Screen Identifies RRAS Signaling as a Pathologic Feature of Huntington's Disease
- Lessons from Model Organisms: Phenotypic Robustness and Missing Heritability in Complex Disease
- Population Genomic Scan for Candidate Signatures of Balancing Selection to Guide Antigen Characterization in Malaria Parasites
- Tissue-Specific Regulation of Chromatin Insulator Function
- Disruption of Mouse Cenpj, a Regulator of Centriole Biogenesis, Phenocopies Seckel Syndrome
- Genome, Functional Gene Annotation, and Nuclear Transformation of the Heterokont Oleaginous Alga CCMP1779
- Antagonistic Gene Activities Determine the Formation of Pattern Elements along the Mediolateral Axis of the Fruit
- Lung eQTLs to Help Reveal the Molecular Underpinnings of Asthma
- Identification of the First ATRIP–Deficient Patient and Novel Mutations in ATR Define a Clinical Spectrum for ATR–ATRIP Seckel Syndrome
- Cooperativity of , , and in Malignant Breast Cancer Evolution
- Loss of Prohibitin Membrane Scaffolds Impairs Mitochondrial Architecture and Leads to Tau Hyperphosphorylation and Neurodegeneration
- Microhomology Directs Diverse DNA Break Repair Pathways and Chromosomal Translocations
- MicroRNA–Mediated Repression of the Seed Maturation Program during Vegetative Development in
- Selective Pressure Causes an RNA Virus to Trade Reproductive Fitness for Increased Structural and Thermal Stability of a Viral Enzyme
- The Tumor Suppressor Gene Retinoblastoma-1 Is Required for Retinotectal Development and Visual Function in Zebrafish
- Regions of Homozygosity in the Porcine Genome: Consequence of Demography and the Recombination Landscape
- Histone Methyltransferases MES-4 and MET-1 Promote Meiotic Checkpoint Activation in
- Polyadenylation-Dependent Control of Long Noncoding RNA Expression by the Poly(A)-Binding Protein Nuclear 1
- A Unified Method for Detecting Secondary Trait Associations with Rare Variants: Application to Sequence Data
- Genetic and Biochemical Dissection of a HisKA Domain Identifies Residues Required Exclusively for Kinase and Phosphatase Activities
- Informed Conditioning on Clinical Covariates Increases Power in Case-Control Association Studies
- Biochemical Diversification through Foreign Gene Expression in Bdelloid Rotifers
- Genomic Variation and Its Impact on Gene Expression in
- Spastic Paraplegia Mutation N256S in the Neuronal Microtubule Motor KIF5A Disrupts Axonal Transport in a HSP Model
- Lamin B1 Polymorphism Influences Morphology of the Nuclear Envelope, Cell Cycle Progression, and Risk of Neural Tube Defects in Mice
- A Targeted Glycan-Related Gene Screen Reveals Heparan Sulfate Proteoglycan Sulfation Regulates WNT and BMP Trans-Synaptic Signaling
- Dopaminergic D2-Like Receptors Delimit Recurrent Cholinergic-Mediated Motor Programs during a Goal-Oriented Behavior
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Mechanisms Employed by to Prevent Ribonucleotide Incorporation into Genomic DNA by Pol V
- Inference of Population Splits and Mixtures from Genome-Wide Allele Frequency Data
- Zcchc11 Uridylates Mature miRNAs to Enhance Neonatal IGF-1 Expression, Growth, and Survival
- Histone Methyltransferases MES-4 and MET-1 Promote Meiotic Checkpoint Activation in
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



