-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaPlan B for Stimulating Stem Cell Division
article has not abstract
Published in the journal: . PLoS Genet 8(11): e32767. doi:10.1371/journal.pgen.1003117
Category: Perspective
doi: https://doi.org/10.1371/journal.pgen.1003117Summary
article has not abstract
Plant development relies on two kinds of coordinated regulatory inputs to generate an optimal plant body. First are inputs regulating the spatial organization of cells in the plant. These “hardwired” inputs are invariant between individuals and their actions are buffered from the environment. Second are variable inputs that modify the development of tissues to optimize growth for given conditions of water, gravity, nutrients, and light. Defining these pathways and understanding how they work together is a major challenge for plant biologists. Work by Turner and colleagues in this issue of PLOS Genetics [1] moves us a step closer by elucidating a link between two pathways that control proliferation of a stem cell population that produces vascular cells. These two pathways are a receptor–ligand pathway, which represents the first type of hardwired machinery, and the ethylene signaling pathway, which traditionally has been considered an environmentally dependent pathway.
In growing plants, stem cells at the tips of roots and shoots add new cells to the plant body. In shoots these cells generate new organs, leaves, and stem sections, each segment of leaf and stem adding additional length to the plant body. This can lead to very long branches. However, in order for plants to reach a significant size, they must add cells to their girth as well. Adding girth allows the plant to support branches and lets these expand the canopy where newly made leaves can compete for sunlight.
Girth is added through the action of a second source of stem cells. These stem cells are located in a ring within the stem or trunk of a tree, where they generate new vascular cells (Figure 1A). In the stem of Arabidopsis, these are called procambial cells. The procambial stem cells are sandwiched between the two vascular cell types they give rise to: phloem cells (toward the outside of the plant), the carriers of sugars from the leaves to roots, fruits, and other “sink” organs; and xylem cells (toward the inside of the plant), the carriers of water and minerals. Procambium is used to denote the stem cells in vascular bundles of newly formed organs. In plants such as trees that show persistent lateral growth, the procambium gives rise to a more substantial, continuous ring of stem cells called a cambium. Arabidopsis, while not a perennial, and certainly not a tree, nevertheless exhibits secondary growth and a well-developed cambial stem cell population in the hypocotyl, the short stem below the rosette.
Fig. 1. Role of the TDIF/PXY ligand receptor and ethylene signaling pathways in the promotion of cell division in the cambial stem cells of Arabidopsis. 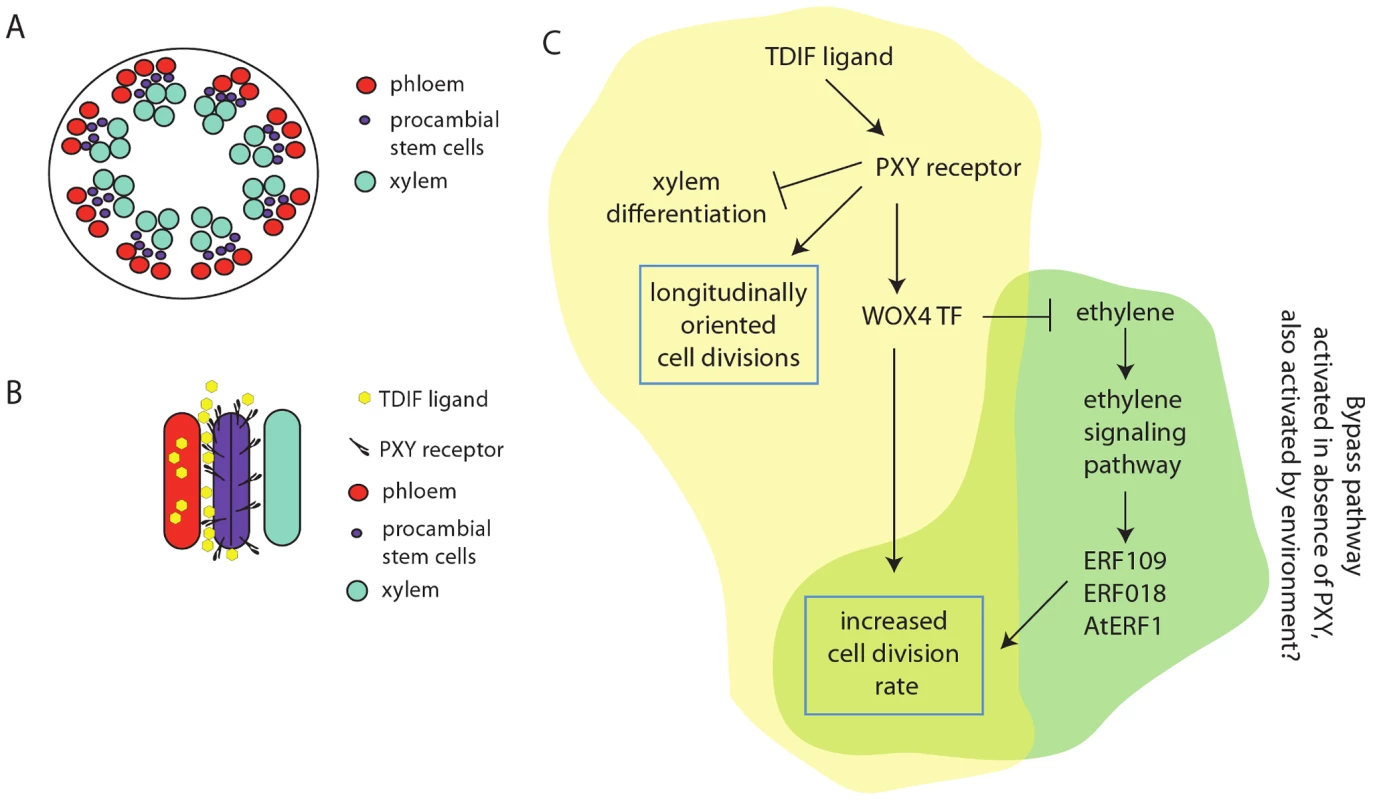
(A) Schematic of stem cross section. In dicots such as Arabidopsis the phloem (red) and xylem (blue) form concentric rings near the surface of the plant. Procambial stem cells (purple) are located between them. (B) Longitudinal schematic of phloem, procambial, and xylem cells. The TDIF peptide is made in the phloem and acts non–cell autonomously on the PXY receptor-expressing procambial stem cells. The asymmetric signal from TDIF on PXY acts to orient cell division longitudinally such that daughter cells are long and slender and such that stem cell descendants are pushed either outward toward phloem or inward toward the xylem, thus maintaining the organization of these tissues. TDIF, acting through PXY, suppresses xylem differentiation and promotes cell division. Increasing the rate of cell division requires the WOX4 transcription factor. (C) The TDIF signal acts on PXY to activate WOX4, to promote cell division. The TDIF effects on the orientation of cell division and on the suppression of xylem differentiation do not go through WOX4. In this model, TDIF/PXY also represses ethylene production and thus the alternative branch of the pathway. In the absence of the PXY protein, ethylene levels are increased resulting in an increase in ERF109, ERF018, and AtERF1 levels and stimulation of cell division. The position of the procambium between the two types of descendant cells is critical to its production of organized phloem and xylem strands. Regulated orientation of cell divisions within the procambium maintains this organization as newly generated cells are fed into the differentiation pathways. Cell divisions in the long, narrow progenitor cells are oriented along the long axis of the stem (Figure 1B). Since most plant cell division planes cut across the narrowest dimension of the cell, orienting new walls such that they span the longest dimension likely requires specialized machinery controlled by specialized regulators.
Environmental cues affect the activity of cambial stem cells. In trees, the vascular cambium goes through cycles of activity and inactivity with seasons. In winter the cambium is dormant, but it becomes active again during summer, resulting in the characteristic annular rings of wood. Gravity also regulates cambial growth: when trees lean, the cambium on the upper side of the trunk grows at a different rate from the lower side to generate structural support (i.e., “tension wood”) [2].
In Arabidopsis, the tracheary element differentiation inhibition factor/CLE41 (TDIF/CLE41) peptide ligand is secreted from the phloem and interacts with the TDIF RECEPTOR/PHLOEM INTERCALATED WITH XYLEM (TDR/PXY) membrane receptor kinase expressed in adjacent cambial stem cells (Figure 1B) [3]. This signal accomplishes three things. First, it stimulates cell division within the cambium. To do this, it requires the downstream transcription factor WOX4. Second, it prevents stem cells in the cambium from becoming xylem cells. Third, it regulates the orientation of cell divisions. The latter two steps do not require WOX4 action [4], [5].
Regulation of cell division orientation in the procambium requires the polar production of TDIF peptide [4]. If TDIF peptide is produced on both sides of the cambium, or only on the xylem side, cell division planes in the procambial cells become highly irregular. Thus, the tissue-specific synthesis of TDIF (in the phloem) and the detection of its asymmetric distribution (in the procambium) are part of an invariant developmental pathway that produces spatially organized vascular strands.
The phenotypes caused by overexpressed TDIF peptide were eliminated in pxy mutants, indicating that the TDIF signal requires the PXY receptor to act. However, surprisingly, loss-of-function pxy mutants exhibited only a mild decrease in procambial cell numbers. This suggests that the plant possesses a “plan B” to stimulate procambial stem cell division. In their new work, Turner and colleagues [1] identify the bypass mechanism as signaling through the gaseous hormone ethylene.
The first clue that ethylene mediates the bypass pathway came when Etchells et al. found increases in mRNA abundance for some members of the APETALA2/ETHYLENE RESPONSE FACTOR (AP2/ERF) family of transcription factors in pxy mutants. Since AP2/ERF gene family expression is elevated in response to ethylene and mediates ethylene response, Etchells et al. reasoned that ERFs might compensate for the absence of PXY. Indeed, when loss-of-function mutations in the AP2/ERF factor genes ERF109, ERF018, and At1ERF were combined with mutations at the PXY locus, the resulting pxy erf double mutants had significantly reduced numbers of cambial stem cells. This demonstrates that the AP2/ERF genes act in a pathway that is functionally redundant to the PXY pathway.
Etchells et al. also show that an increase in ethylene stimulates cell division in procambial stem cells. Moreover, ethylene upregulates these particular AP2/ERF genes. Finally, when pxy mutations are combined with mutations that disrupt the ethylene signaling pathway upstream of the ERF factors, procambial cell numbers are significantly decreased. Thus, the bypass pathway requires ethylene to function.
Yet another link to the ethylene pathway exists in this system: ACS6 mRNA levels (ACS is an enzyme that catalyzes ethylene biosynthesis) are upregulated in pxy mutants. These findings suggest the existence of an ethylene-based bypass pathway that is normally off but becomes activated when PXY activity is low or missing (Figure 1C). The TDIF–PXY–WOX4 pathway normally keeps ethylene levels low and, through an as yet unknown pathway, stimulates cell division in procambial stem cells. In the absence of PXY, ethylene increases and stimulates the production of the ERF109, ERF018, and AtERF1 transcription factors, which in turn activate procambial stem cell division.
These findings are consistent with, and to some degree inspired by, earlier findings on the role of ethylene in promoting cell division in poplar trees [6]. Blocking ethylene perception, either chemically with an ethylene antagonist or genetically by introducing a dominant negative ethylene receptor mutation, blocked cell division in the cambium. Moreover, excess ethylene increased the number of cell divisions in the cambium. Significantly, in woody plants that lean to one side, blocking ethylene action resulted in a failure to form tension wood, indicating that the mechanical/gravitational changes sensed by the cambium in poplar require ethylene signaling.
In summary, a spatially organized, tissue-specific program is established by TDIF ligand synthesis in one pole of the vascular strand and asymmetric TDIF sensing by the corresponding PXY receptor present in the procambial stem cells. It is likely that this pathway (highlighted in yellow in Figure 1C) is responsible for directing the pattern of early cell divisions in the procambial stem cells of the vascular bundles. A second pathway, the ethylene signaling pathway (highlighted in green in Figure 1C) can also stimulate procambial cell divisions. This pathway is normally “OFF” when PXY functions; this “OFF” state may limit procambial cell divisions during critical developmental stages when new vascular strands are established and form connections with established veins. It remains to be seen under what conditions the alternative ethylene branch of the pathway is activated. Given the ethylene-dependent stimulation of tension wood formation by gravity in poplar, it is tempting to speculate that there are as yet undiscovered environmental inputs for this bypass pathway. If this is the case, the two pathways may provide a prototypic example of how invariant pathways that specify the spatial organization and division activity of cells are integrated with environmental cues that further tune the plant body to its environment.
Zdroje
1. EtchellsJP, ProvostCM, TurnerSR (2012) Plant vascular cell division is maintained by an interaction between PXY and ethylene signalling. PLoS Genet 8: e1002997 doi:10.1371/journal.pgen.1002997
2. TelewskiFW (2006) A unified hypothesis of mechanoperception in plants. Am J Bot 93 : 1466–1476.
3. HirakawaY, ShinoharaH, KondoY, InoueA, NakanomyoI, et al. (2008) Non-cell-autonomous control of vascular stem cell fate by a CLE peptide/receptor system. Proc Natl Acad Sci U S A 105 : 15208–15213.
4. EtchellsJP, TurnerSR (2010) The PXY-CLE41 receptor ligand pair defines a multifunctional pathway that controls the rate and orientation of cell division. Development 137 : 767–774.
5. HirakawaY, KondoY, FukudaH (2010) TDIF peptide signaling regulates vascular stem cell proliferation via the WOX4 homeobox gene in Arabidopsis. Plant Cell 22 : 2618–2629.
6. LoveJ, BjörklundS, VahalaJ, HertzbergM, KanagasjärviJ, et al. (2009) Ethylene is an endogenous stimulator of cell division in the cambial meristem of Populus. Proc Natl Acad Sci U S A 106 : 5984–5989.
Štítky
Genetika Reprodukční medicína
Článek The Covariate's DilemmaČlánek Plant Vascular Cell Division Is Maintained by an Interaction between PXY and Ethylene SignallingČlánek Lessons from Model Organisms: Phenotypic Robustness and Missing Heritability in Complex Disease
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 11
-
Všechny články tohoto čísla
- Inference of Population Splits and Mixtures from Genome-Wide Allele Frequency Data
- The Covariate's Dilemma
- Plant Vascular Cell Division Is Maintained by an Interaction between PXY and Ethylene Signalling
- Plan B for Stimulating Stem Cell Division
- Discovering Thiamine Transporters as Targets of Chloroquine Using a Novel Functional Genomics Strategy
- Is a Modifier of Mutations in Retinitis Pigmentosa with Incomplete Penetrance
- Evolutionarily Ancient Association of the FoxJ1 Transcription Factor with the Motile Ciliogenic Program
- Genome Instability Caused by a Germline Mutation in the Human DNA Repair Gene
- Transcription Factor Oct1 Is a Somatic and Cancer Stem Cell Determinant
- Controls of Nucleosome Positioning in the Human Genome
- Disruption of Causes Defective Meiotic Recombination in Male Mice
- A Novel Human-Infection-Derived Bacterium Provides Insights into the Evolutionary Origins of Mutualistic Insect–Bacterial Symbioses
- Trps1 and Its Target Gene Regulate Epithelial Proliferation in the Developing Hair Follicle and Are Associated with Hypertrichosis
- Zcchc11 Uridylates Mature miRNAs to Enhance Neonatal IGF-1 Expression, Growth, and Survival
- Population-Based Resequencing of in 10,330 Individuals: Spectrum of Genetic Variation, Phenotype, and Comparison with Extreme Phenotype Approach
- HP1a Recruitment to Promoters Is Independent of H3K9 Methylation in
- Transcription Elongation and Tissue-Specific Somatic CAG Instability
- A Germline Polymorphism of DNA Polymerase Beta Induces Genomic Instability and Cellular Transformation
- Interallelic and Intergenic Incompatibilities of the () Gene in Mouse Hybrid Sterility
- Comparison of Mitochondrial Mutation Spectra in Ageing Human Colonic Epithelium and Disease: Absence of Evidence for Purifying Selection in Somatic Mitochondrial DNA Point Mutations
- Mutations in the Transcription Elongation Factor SPT5 Disrupt a Reporter for Dosage Compensation in Drosophila
- Evolution of Minimal Specificity and Promiscuity in Steroid Hormone Receptors
- Blockade of Pachytene piRNA Biogenesis Reveals a Novel Requirement for Maintaining Post-Meiotic Germline Genome Integrity
- RHOA Is a Modulator of the Cholesterol-Lowering Effects of Statin
- MIG-10 Functions with ABI-1 to Mediate the UNC-6 and SLT-1 Axon Guidance Signaling Pathways
- Loss of the DNA Methyltransferase MET1 Induces H3K9 Hypermethylation at PcG Target Genes and Redistribution of H3K27 Trimethylation to Transposons in
- Genome-Wide Association Studies Reveal a Simple Genetic Basis of Resistance to Naturally Coevolving Viruses in
- The Principal Genetic Determinants for Nasopharyngeal Carcinoma in China Involve the Class I Antigen Recognition Groove
- Molecular, Physiological, and Motor Performance Defects in DMSXL Mice Carrying >1,000 CTG Repeats from the Human DM1 Locus
- Genomic Study of RNA Polymerase II and III SNAP-Bound Promoters Reveals a Gene Transcribed by Both Enzymes and a Broad Use of Common Activators
- Long Telomeres Produced by Telomerase-Resistant Recombination Are Established from a Single Source and Are Subject to Extreme Sequence Scrambling
- The Yeast SR-Like Protein Npl3 Links Chromatin Modification to mRNA Processing
- Deubiquitylation Machinery Is Required for Embryonic Polarity in
- dJun and Vri/dNFIL3 Are Major Regulators of Cardiac Aging in Drosophila
- CtIP Is Required to Initiate Replication-Dependent Interstrand Crosslink Repair
- Notch-Mediated Suppression of TSC2 Expression Regulates Cell Differentiation in the Intestinal Stem Cell Lineage
- A Combination of H2A.Z and H4 Acetylation Recruits Brd2 to Chromatin during Transcriptional Activation
- Network Analysis of a -Mouse Model of Autosomal Dominant Polycystic Kidney Disease Identifies HNF4α as a Disease Modifier
- Mitosis in Neurons: Roughex and APC/C Maintain Cell Cycle Exit to Prevent Cytokinetic and Axonal Defects in Photoreceptor Neurons
- CELF4 Regulates Translation and Local Abundance of a Vast Set of mRNAs, Including Genes Associated with Regulation of Synaptic Function
- Mechanisms Employed by to Prevent Ribonucleotide Incorporation into Genomic DNA by Pol V
- The Genomes of the Fungal Plant Pathogens and Reveal Adaptation to Different Hosts and Lifestyles But Also Signatures of Common Ancestry
- A Genome-Scale RNA–Interference Screen Identifies RRAS Signaling as a Pathologic Feature of Huntington's Disease
- Lessons from Model Organisms: Phenotypic Robustness and Missing Heritability in Complex Disease
- Population Genomic Scan for Candidate Signatures of Balancing Selection to Guide Antigen Characterization in Malaria Parasites
- Tissue-Specific Regulation of Chromatin Insulator Function
- Disruption of Mouse Cenpj, a Regulator of Centriole Biogenesis, Phenocopies Seckel Syndrome
- Genome, Functional Gene Annotation, and Nuclear Transformation of the Heterokont Oleaginous Alga CCMP1779
- Antagonistic Gene Activities Determine the Formation of Pattern Elements along the Mediolateral Axis of the Fruit
- Lung eQTLs to Help Reveal the Molecular Underpinnings of Asthma
- Identification of the First ATRIP–Deficient Patient and Novel Mutations in ATR Define a Clinical Spectrum for ATR–ATRIP Seckel Syndrome
- Cooperativity of , , and in Malignant Breast Cancer Evolution
- Loss of Prohibitin Membrane Scaffolds Impairs Mitochondrial Architecture and Leads to Tau Hyperphosphorylation and Neurodegeneration
- Microhomology Directs Diverse DNA Break Repair Pathways and Chromosomal Translocations
- MicroRNA–Mediated Repression of the Seed Maturation Program during Vegetative Development in
- Selective Pressure Causes an RNA Virus to Trade Reproductive Fitness for Increased Structural and Thermal Stability of a Viral Enzyme
- The Tumor Suppressor Gene Retinoblastoma-1 Is Required for Retinotectal Development and Visual Function in Zebrafish
- Regions of Homozygosity in the Porcine Genome: Consequence of Demography and the Recombination Landscape
- Histone Methyltransferases MES-4 and MET-1 Promote Meiotic Checkpoint Activation in
- Polyadenylation-Dependent Control of Long Noncoding RNA Expression by the Poly(A)-Binding Protein Nuclear 1
- A Unified Method for Detecting Secondary Trait Associations with Rare Variants: Application to Sequence Data
- Genetic and Biochemical Dissection of a HisKA Domain Identifies Residues Required Exclusively for Kinase and Phosphatase Activities
- Informed Conditioning on Clinical Covariates Increases Power in Case-Control Association Studies
- Biochemical Diversification through Foreign Gene Expression in Bdelloid Rotifers
- Genomic Variation and Its Impact on Gene Expression in
- Spastic Paraplegia Mutation N256S in the Neuronal Microtubule Motor KIF5A Disrupts Axonal Transport in a HSP Model
- Lamin B1 Polymorphism Influences Morphology of the Nuclear Envelope, Cell Cycle Progression, and Risk of Neural Tube Defects in Mice
- A Targeted Glycan-Related Gene Screen Reveals Heparan Sulfate Proteoglycan Sulfation Regulates WNT and BMP Trans-Synaptic Signaling
- Dopaminergic D2-Like Receptors Delimit Recurrent Cholinergic-Mediated Motor Programs during a Goal-Oriented Behavior
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Mechanisms Employed by to Prevent Ribonucleotide Incorporation into Genomic DNA by Pol V
- Inference of Population Splits and Mixtures from Genome-Wide Allele Frequency Data
- Zcchc11 Uridylates Mature miRNAs to Enhance Neonatal IGF-1 Expression, Growth, and Survival
- Histone Methyltransferases MES-4 and MET-1 Promote Meiotic Checkpoint Activation in
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



