-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaAntagonistic Gene Activities Determine the Formation of Pattern Elements along the Mediolateral Axis of the Fruit
The Arabidopsis fruit mainly consists of a mature ovary that shows three well defined territories that are pattern elements along the mediolateral axis: the replum, located at the medial plane of the flower, and the valve and the valve margin, both of lateral nature. JAG/FIL activity, which includes the combined functions of JAGGED (JAG), FILAMENTOUS FLOWER (FIL), and YABBY3 (YAB3), contributes to the formation of the two lateral pattern elements, whereas the cooperating genes BREVIPEDICELLUS (BP) and REPLUMLESS (RPL) promote replum development. A recent model to explain pattern formation along the mediolateral axis hypothesizes that JAG/FIL activity and BP/RPL function as antagonistic lateral and medial factors, respectively, which tend to repress each other. In this work, we demonstrate the existence of mutual exclusion mechanisms between both kinds of factors, and how this determines the formation and size of the three territories. Medial factors autonomously constrain lateral factors so that they only express outside the replum, and lateral factors negatively regulate the medially expressed BP gene in a non-autonomous fashion to ensure correct replum development. We also have found that ASYMMETRIC LEAVES1 (AS1), previously shown to repress BP both in leaves and ovaries, collaborates with JAG/FIL activity, preventing its repression by BP and showing synergistic interactions with JAG/FIL activity genes. Therefore AS gene function (the function of the interacting genes AS1 and AS2) has been incorporated in the model as a new lateral factor. Our model of antagonistic factors provides explanation for mutant fruit phenotypes in Arabidopsis and also may help to understand natural variation of fruit shape in Brassicaceae and other species, since subtle changes in gene expression may cause conspicuous changes in the size of the different tissue types.
Published in the journal: . PLoS Genet 8(11): e32767. doi:10.1371/journal.pgen.1003020
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003020Summary
The Arabidopsis fruit mainly consists of a mature ovary that shows three well defined territories that are pattern elements along the mediolateral axis: the replum, located at the medial plane of the flower, and the valve and the valve margin, both of lateral nature. JAG/FIL activity, which includes the combined functions of JAGGED (JAG), FILAMENTOUS FLOWER (FIL), and YABBY3 (YAB3), contributes to the formation of the two lateral pattern elements, whereas the cooperating genes BREVIPEDICELLUS (BP) and REPLUMLESS (RPL) promote replum development. A recent model to explain pattern formation along the mediolateral axis hypothesizes that JAG/FIL activity and BP/RPL function as antagonistic lateral and medial factors, respectively, which tend to repress each other. In this work, we demonstrate the existence of mutual exclusion mechanisms between both kinds of factors, and how this determines the formation and size of the three territories. Medial factors autonomously constrain lateral factors so that they only express outside the replum, and lateral factors negatively regulate the medially expressed BP gene in a non-autonomous fashion to ensure correct replum development. We also have found that ASYMMETRIC LEAVES1 (AS1), previously shown to repress BP both in leaves and ovaries, collaborates with JAG/FIL activity, preventing its repression by BP and showing synergistic interactions with JAG/FIL activity genes. Therefore AS gene function (the function of the interacting genes AS1 and AS2) has been incorporated in the model as a new lateral factor. Our model of antagonistic factors provides explanation for mutant fruit phenotypes in Arabidopsis and also may help to understand natural variation of fruit shape in Brassicaceae and other species, since subtle changes in gene expression may cause conspicuous changes in the size of the different tissue types.
Introduction
The fruit, a pivotal structure in angiosperms, is the specialized plant organ that develops from the gynoecium after fertilization of the ovules. The very term angiosperm comes from the Greek and means “seeds enclosed in a vessel” (angion, vessel, and sperma, seed), describing the main functions of this organ: seed protection and dispersal. Our present knowledge on fruit development principally derives from research in the crucifer Arabidopsis thaliana, Arabidopsis hereafter [1]–[7]. All the tissues of the Arabidopsis fruit are already present in the bicarpelate pistil, whose development is initiated as a group of cells that form a dome-shaped primordium. Subsequently, polarity is determined along the main axes of symmetry giving rise to pattern elements with specific tissue types. Thus, for instance, along the apical-basal axis both pistils and fruits show, from bottom to top, the basal gynophore, the ovary, the style and the apical stigma (Figure 1A).
Fig. 1. Main pattern elements along the mediolateral axis of the Arabidopsis wild-type fruit. 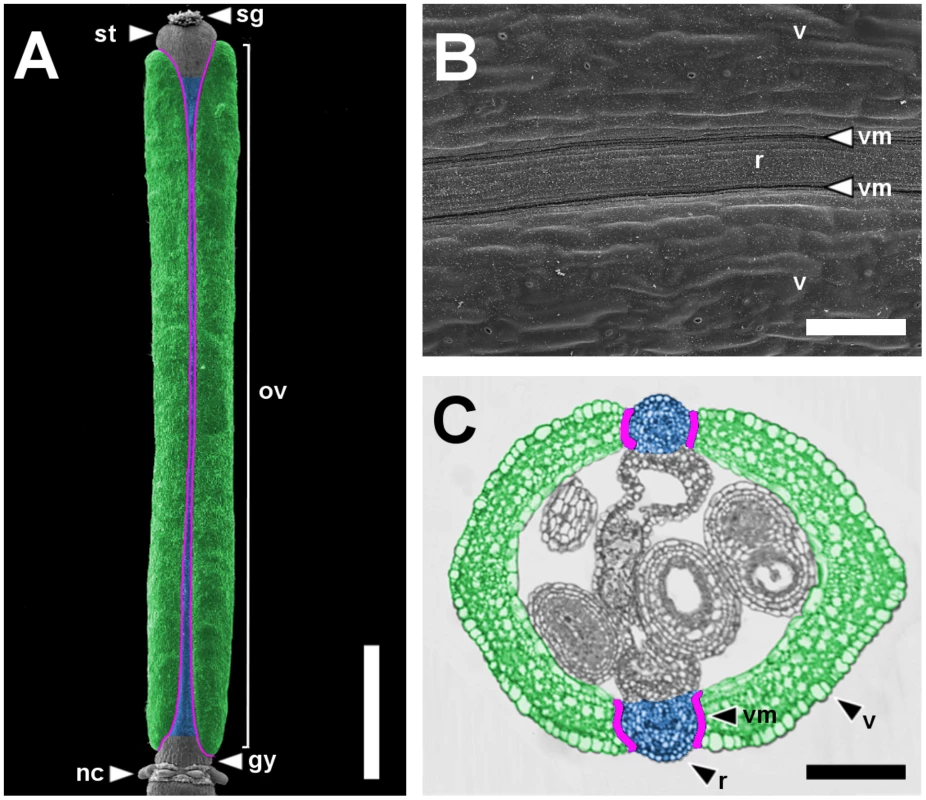
Scanning Electron Microscope (SEM) micrograph of a stage 17 fruit artificially colored to highlight the main pattern elements along the mediolateral axis: valves in green, replum in blue and valve margins in purple (A) and a higher magnification of the same fruit (B). Cross-section of a fruit at the level of the ovary with the same color code as in A (C). gy, gynophore; nc, nectaries; ov, ovary; r, replum; sg, stigma; st, style; v, valve; vm, valve margin. Scale bars: 1 mm (A), 100 µm (B, C). The dehiscent fruit of Arabidopsis is essentially an expanded ovary encompassing the seeds [8], and consists of three different territories that constitute the pattern elements along the mediolateral axis. The replum, located at the medial plane of the flower, is a narrow structure that separates two lateral valves. At the valve-replum boundary, the valve margin, another lateral tissue, comprises a few rows of small and rounded cells (Figure 1A–1C). Ripening of the fruit involves the formation of a dehiscence zone in the valve margin and the consequent detachment of the valves from the replum that precedes seed dispersal [9].
The MADS-box gene FRUITFULL (FUL) [10] and the homeobox gene REPLUMLESS (RPL, aka BELLRINGER, PENNYWISE, LARSON and VAAMANA) [11]–[14] are expressed within the valve and replum tissues, respectively. RPL and FUL, in their corresponding domains of activity, prevent the ectopic expression of the valve margin identity genes SHATTERPROOF1 and 2 (SHP1, SHP2) [15], INDEHISCENT (IND) [16] and ALCATRAZ (ALC) [17]. This regulation ensures the correct formation of valves and replum territories and limits the expression of the valve margin identity genes to the valve-replum boundaries. Thus, in fruits completely lacking both FUL and RPL activities, valves and replum epidermal cells acquire valve margin identity as a consequence of the ectopic expression of valve margin identity genes [13], [16], [18].
Early in pistil development, medial tissues form two internal ridges that fuse to form the septum and the placenta, suggesting that presumptive repla have meristematic properties [5], [19], [20]. Accordingly, they exhibit expression of meristem genes, as RPL [13] and the class I KNOTTED1-like homeobox (KNOX) genes BREVIPEDICELLUS (BP, aka KNAT1) [21]–[24] and SHOOT MERISTEMLESS (STM) [25]. Different from the replum, valves show a more leaf-like nature, and consequently, they express genes with crucial roles in leaf development, as the YABBY1 (YAB1) group genes FILAMENTOUS FLOWER (FIL) and YABBY3 (YAB3) [26]–[30], JAGGED (JAG), which codes for a transcription factor with a single C2H2 zinc-finger domain [31], [32], and the MYB transcription factor-encoding gene ASYMMETRIC LEAVES1 (AS1) [33], [34]. FIL, YAB3 and JAG positively regulate the expression of FUL and valve margin identity genes, so that the cooperating activities of these three genes in ovaries have been called JAG/FIL activity. Valve margin identity genes are activated in places close to the presumptive replum where the levels of this activity are low, whereas higher levels activate FUL expression in valves [35].
Replum and valves apparently mirror the antagonistic relationships between meristem and leaves [19], [20]. Thus, AS1 prevents the ectopic expression of BP in valves while RPL impedes that of JAG/FIL activity genes in the replum [19], [35]. Based on this antagonism, a model has been proposed to account for mediolateral patterning of the ovary, which puts forward that the different tissue types along the mediolateral axis are determined by the opposing activities of two antagonistic factors: valve factors that basically are the genes involved in the JAG/FIL activity, and replum factors that are composed by BP and RPL [19], [36], whose products dimerize to migrate into the nucleus [11], [14], [37]–[42]. In accordance to the model, replum and valves would form in territories with high activity of replum and valve factors, respectively, whereas the valve margin would develop in a narrow ridge in which both activities would show low levels and overlap [19].
Nevertheless, recent research has shown that valve and replum factor activities do not overlap, since BP and RPL are not active in the valve margin [36], [43] (our unpublished results). Therefore, BP and RPL will be hereafter referred to as replum or medial factors, whereas genes involved in JAG/FIL activity (hereafter referred as JAG/FIL activity genes) will be called lateral (valve and valve margin) factors. In this report, we demonstrate that, indeed, both medial and lateral factors are mutually antagonistic, as they repress each other. We have observed that lateral factors negatively regulate in a non-autonomous fashion BP, thus restricting the size of the medial region, an essential condition for proper replum development, whereas medial factors limit in an autonomous way the expression of JAG/FIL activity genes, whose products only are detected outside the replum. Furthermore, we have also found that AS1 collaborates with lateral factors by preventing downregulation of JAG/FIL activity genes by the ectopic expression of BP in lateral regions. Here, we propose a non-overlapping model whereby the opposing activities of medial and lateral factors determine the specification and size of pattern elements along the mediolateral axis of the Arabidopsis fruit. In accordance with this model, an increase in the expression of medial factors and a decrease in lateral factor activities lead to the overproduction of medial tissues along with a large reduction in the size of the lateral domains.
Results
BP is involved in the replum defects of mutants with impaired JAG/FIL activity
We have previously demonstrated that the MYB transcription factor AS1 regulates patterning along the mediolateral axis of the Arabidopsis fruit. When compared to wild type, in as1 fruit, the replum contains more epidermal cells, increasing its width. This phenotype is accompanied with a reduction in the final size of the valves as the valve epidermal layer contains fewer cells [19]. We previously found that the as1 fruit phenotype was largely associated with the misregulation of BP, because: 1) 35S::BP had the same fruit alterations as seen in as1 plants, 2) BP was ectopically expressed in lateral regions of as1 pistils and 3) in as1 bp fruits, replum and valves almost completely recovered the wild-type size [19]. However, the increase in the number of replum cells is not the only alteration observed in as1 (or 35S::BP) repla. Whereas in the wild-type pistils the replum contains long and narrow cells and no stomata structures form (Figure 1B and Figure 2A), a closer inspection of altered repla in as1 and 35S::BP plants revealed, on the contrary, the presence of extra-large cells, as well as a few interspersed stomata (Figure S1A, S1B). These observations indicate that the negative regulation of BP by AS1 is not only essential in regulating the size of pattern elements along the mediolateral axis, but also for the correct specification of replum identity.
Fig. 2. Abnormal repla in mutants affected in JAG/FIL activity. 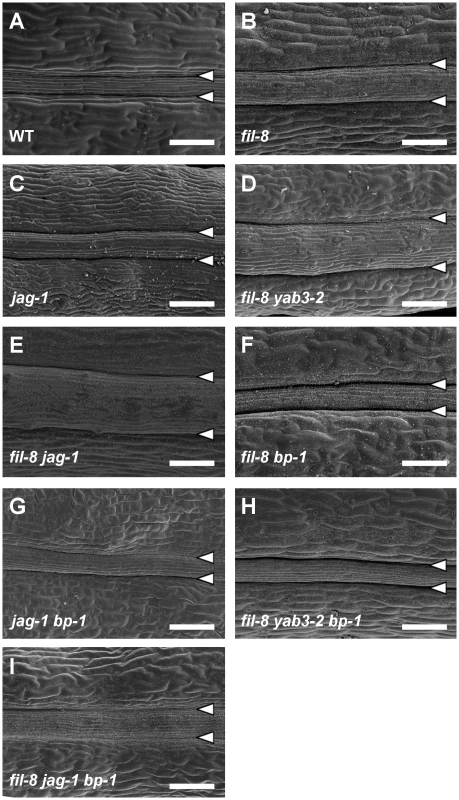
(A–E) SEM micrographs of stage 17 fruits show enlarged repla in single and multiple mutants affected in JAG/FIL activity. The fil-8 (B) and jag-1 (C) single mutants display large repla respect to the wild type (A). The fil-8 yab3-2 (D) and fil-8 jag-1 (E) double mutants exhibit more severe phenotypes. (F–I) SEM micrographs of stage 17 fruits showing the rescue of mutant phenotypes by bp-1 in fil-8 bp-1 (F), jag-1 bp-1 (G), fil-8 yab3-2 bp-1 (H) and fil-8 jag-1 bp-1 (I) plants. Arrowheads indicate the positions of the valve margins. Scale bars: 100 µm. As mentioned in the introduction, JAG/FIL activity genes [35] have been postulated to be the valve functions (that we refer in this work as lateral factors) patterning the mediolateral axis of the fruit in Arabidopsis. Consequently, similar to as1 mutants, a decrease in this activity drastically affects the valves [7], [19], [35]. Furthermore, according to our current model, reduced levels of JAG/FIL activity should not only cause a reduction in the size of the valve territory, but also a mutant replum phenotype consisting in increased width [7], [19]. Fitting with this hypothesis, we observed that fruits in jag and fil plants, besides their defects in lateral regions [35], clearly exhibited oversized repla (Figure 2B, 2C). Moreover, a close inspection of the replum surface by SEM revealed the presence of stomata in both fil and jag repla (Figure S1C, S1D). These abnormalities were even more dramatic when the JAG/FIL activity was further reduced, as for example in fil yab3 or fil jag backgrounds (Figure 2D, 2E and Figure S1E, S1F).
Because of the similarities between these defects and the ones described before for as1 or 35S::BP repla [19], we investigated whether the lack of BP was capable of rescuing the fruit phenotypes of mutants affected in the JAG/FIL activity. Indeed, fil bp, jag bp, fil yab3 bp and fil jag bp fruits showed narrow repla and contained no replum stomata (Figure 2F–2I). These observations suggest that JAG/FIL activity regulates the expression of BP in the Arabidopsis fruit and that misregulation of BP is essential to produce the repla defects seen in mutants affected in this activity.
JAG/FIL activity negatively regulates BP expression in fruits
The phenotypic similarities of fruits in mutants affected in JAG/FIL activity genes to those of as1 and their rescue by bp led us to investigate whether BP was also negatively regulated by JAG/FIL activity in Arabidopsis ovaries as it is by AS1 [19]. Interestingly two members of this activity, the YAB1 group genes FIL and YAB3, have been previously described to repress BP in leaves [44]. But so far no evidence indicates that this control also occurs in fruits. We therefore analyzed the expression of the BP::GUS reporter construct in mutant backgrounds affected in JAG/FIL activity. In wild-type ovaries, BP::GUS expression is primarily detected in the medial region, corresponding to the replum (Figure 3A) [19], [43]. When the JAG/FIL activity was compromised, we observed that the intensity of the BP::GUS signal increased and its expression domain expanded, achieving the widest domain in the fil yab3 jag triple mutant (Figure 3B–3F). The exception was the yab3 single mutant, in which the behavior of the BP::GUS reporter was indistinguishable from that of the wild type (data not shown). Nevertheless, we observed by qRT-PCR (quantitative real-time polymerase chain reaction) a significant increase in the expression levels of BP transcripts in the pistils of all backgrounds affected in JAG/FIL activity, including yab3 (Figure 3G). Therefore, these results and those shown in the previous section indicate that the JAG/FIL activity, functioning in valves and valve margins, negatively regulates BP expression in medial domains and that this repression is required for the correct specification of the replum.
Fig. 3. The Expression of BP increases in mutants with impaired JAG/FIL activity. 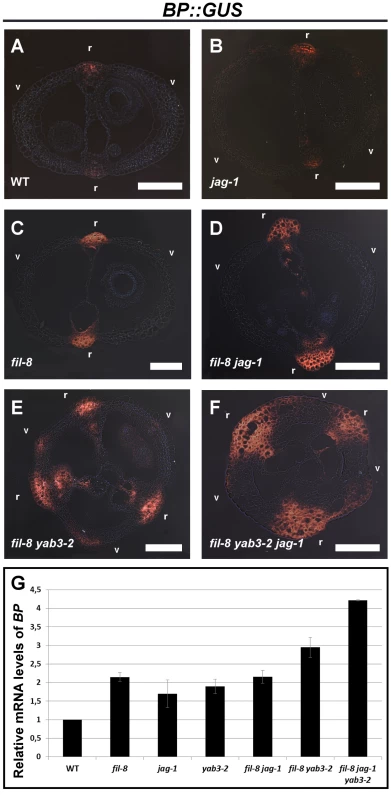
(A–F) Cross-sections of stage 15 fruits showing the expression of the BP::GUS reporter in the wild type (A), jag-1 (B), fil-8 (C), fil-8 jag-1 (D), fil-8 yab3-2 (E), and fil-8 yab3-2 jag-1 (F). (G) Relative mRNA levels of BP in stage 10–13 pistils quantified by qRT-PCR. r, replum; v, valve. Scale bars: 100 µm. However, these data pose the question of whether the increased expression of BP in mutant backgrounds affected in JAG/FIL activity simply reflects the augmented sizes of the corresponding repla. Contrary to this line of reasoning, the GUS signal in repla of such mutants is not only wider than that of wild type but also more intense (Figure 3), suggesting that the increase in replum width is not the only cause of the higher levels of BP expression in mutant pistils. To further address this issue, we tested, by qRT-PCR, the expression levels of another replum gene, RPL, in multiple genetic conditions with impaired JAG/FIL activity and lacking BP function (Figure S2). In the resulting mutants, repla show reduced width as compared to the same backgrounds but with unaltered BP activity (Figure 2). Levels of RPL transcripts in wild-type and bp pistils were quite similar, indicating that loss of BP function has little effect on RPL expression. However, in pistils of fil yab3 bp and fil jag bp, RPL expression was significantly higher than in those of both the wild type and the bp mutant, despite the moderate width of the repla in the two triple mutants (Figure S2). Therefore, enhanced expression of RPL, and most likely of BP, in such mutant backgrounds does not exclusively depend on replum size, supporting again the negative regulation of JAG/FIL activity on replum genes.
BP negatively regulates the expression of JAG/FIL activity genes
The model for mediolateral fruit patterning hypothesizes that lateral factors repress medial factors and vice versa [19]. Fitting with the model, we have found that JAG/FIL activity negatively regulates BP. Therefore we decided to study if there exists such a reciprocal repression. If that were the case, BP would negatively regulate JAG/FIL activity [19]. To test this prediction of the model, we made use of genetic backgrounds in which BP was misregulated. As BP is ectopically active in fruit valves of as1 mutants [19] we therefore first examined the expression of JAG/FIL activity genes in as1 pistils.
We tested by mRNA in situ hybridization the expression pattern of FIL in wild-type and as1 gynoecia. As previously published [28], [29], [35], we found that the FIL mRNA is located in lateral domains of wild-type pistils (Figure 4E). However in as1 pistils the transcript of FIL was detected with less intensity and in a more reduced territory (Figure 4F). This decay in FIL activity was also seen when the FIL::GFP reporter was assayed in as1 pistils (Figure 4I, 4J and Figure S3A, S3B, S3D, S3E, S3G, S3H). A similar behavior was seen when the expression of JAG was monitored using a transgenic GUS-reporter line. In wild-type ovaries JAG::GUS signal is exclusively localized in lateral regions, while in as1, although the signal is detected in the same region, the levels of GUS activity were conspicuously lower (Figure 4L, 4M).
Fig. 4. BP misregulation affects fruit morphology and expression of JAG/FIL activity genes. 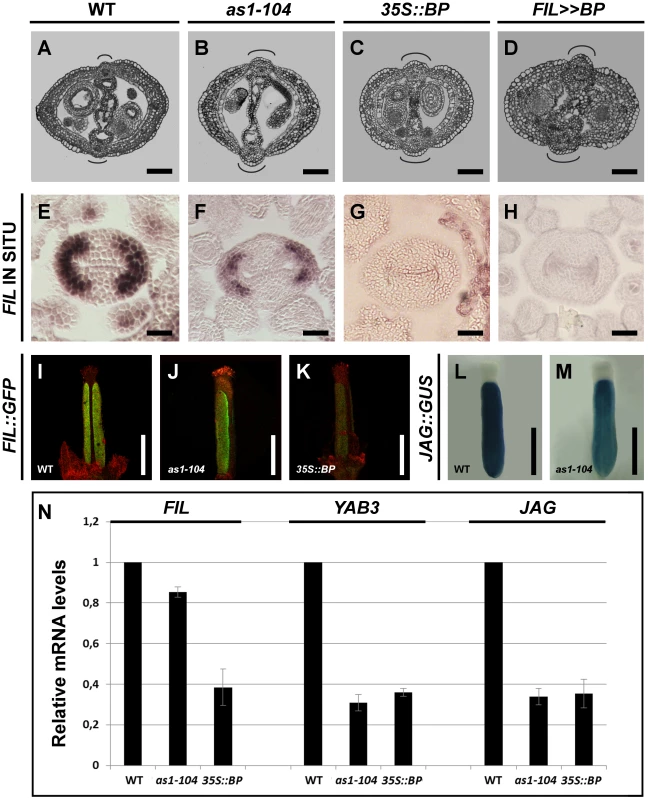
(A–D) Cross-sections of stage 15 fruits show defects in replum and valve formation in mutants misexpressing BP. Wild type (A), as1-104 (B), 35S::BP (C), and FIL>>BP (D). (E–K) BP misexpression produces a reduction in the expression of FIL. In situ hybridization of FIL mRNA in cross-sections of stage 8 pistils of the wild type (E), as1-104 (F), 35S::BP (G), and FIL>>BP (H); and expression of the FIL::GFP reporter in stage 14 gynoecia of wild type (I), as1-104 (J), and 35S::BP (K). Although FIL mRNA is undetectable by in situ hybridization in 35S::BP gynoecia, the FIL::GFP transgene provides a more sensitive detection of FIL promoter expression. (L–M) Whole mount staining of anthesis gynoecia for JAG::GUS, showing higher expression levels in the wild-type (L) than in as1-104 (M). (N) Relative mRNA levels of JAG/FIL activity genes in stage 10–13 pistils quantified by qRT-PCR. In A–D, the curved lines indicate replum size. Scale bars: 100 µm (A–D); 50 µm (E–H); 500 µm (I–M). Interestingly, when compared to wild type, 35S::BP plants produced flowers with fewer and narrower petals (Figure S4A, S4B), virtually phenocopying fil mutants (Figure S4C). These observations suggest that FIL activity might be severely compromised in 35S::BP plants. We, therefore, studied the expression pattern of FIL in 35S::BP pistils by in situ hybridization, being unable to detect any signal of FIL transcripts (Figure 4G). We also analyzed the FIL::GFP reporter in 35S::BP gynoecia and observed a drastic reduction in GFP signal when compared to those of wild-type plants (Figure 4K and Figure S3C, S3F, S3I). Unlike the result of the in situ hybridization, in which no FIL expression was detected in 35S::BP ovaries, the reporter provided a slight but perceptible signal, possibly because of a higher sensitivity in the detection of GFP. All together these data strongly suggest that ectopically expressed BP, directly or indirectly, downregulates JAG/FIL activity genes in ovaries. Despite this result, 35S::BP fruits exhibited normal expression of both the ful-1 enhancer trap (FUL::GUS) and the SHP2::GUS construct (Figure S4D, S4E)
To further investigate how ectopic BP expression affects the fruit, we made use of transgenic FIL>>BP plants, in which the BP coding region is transcribed in the FIL expression domain. For this condition, the model predicts that the expression of BP in lateral domains should counteract the JAG/FIL activity, affecting not only this tissue, but also the replum that would acquire a larger size. As expected, FIL>>BP fruits were strikingly similar to those of 35S::BP and as1 plants, with oversized repla and reduced valves (Figure 4A–4D). Accordingly, in FIL>>BP pistils, we were not able to detect FIL transcripts by in situ hybridization (Figure 4H).
Our qRT-PCR mRNA quantification also showed that in both 35S::BP and as1 pistils JAG/FIL activity genes were downregulated, according to the results presented above (Figure 4N). Remarkably, higher relative levels of FIL messenger were detected in as1 when compared to 35S::BP, which might be explained by the stronger expression of BP in 35S::BP than in as1 background (our unpublished results). Therefore, all the results presented so far indicate that JAG/FIL activity represses BP, which in turn, negatively regulates the JAG/FIL activity genes. These data further confirm that both sets of factors are mutually antagonistic in the mediolateral axis of the Arabidopsis fruit.
AS1 and JAG synergistically interact during mediolateral pattern formation
The strong similarities between AS1 and JAG/FIL activity in negatively controlling BP expression in fruits led us to generate multiple loss-of-function mutant combinations affected in both functions to reveal the contribution of these genes to mediolateral patterning of fruits. Because of the phenotypic similarities between as1 and plants misexpressing BP, we also crossed 35S::BP plants to mutants affected in JAG/FIL activity. These sets of genetic combinations helped us to test whether the presumable fruit defects generated when as1 and mutations in JAG/FIL activity genes combine are exclusively attributable to BP misexpression.
Fruits of 35S::BP jag plants showed a slight increase in replum size and more reduced valves when compared to those of 35S::BP or jag backgrounds (Figure 5A, 5D, 5F). As seen in fil jag fruits, although less frequently, we also found stripes of valve margin tissue at the upper position of the lateral-most region of 35S::BP jag valves (Figure 5D and Figure S5A, S5E). The similarity between 35S::BP jag and fil jag fruit alterations can be explained by the negative regulation of BP on YAB1 group genes.
Fig. 5. Synergistic interaction between loss-of-function alleles of AS1 and JAG. 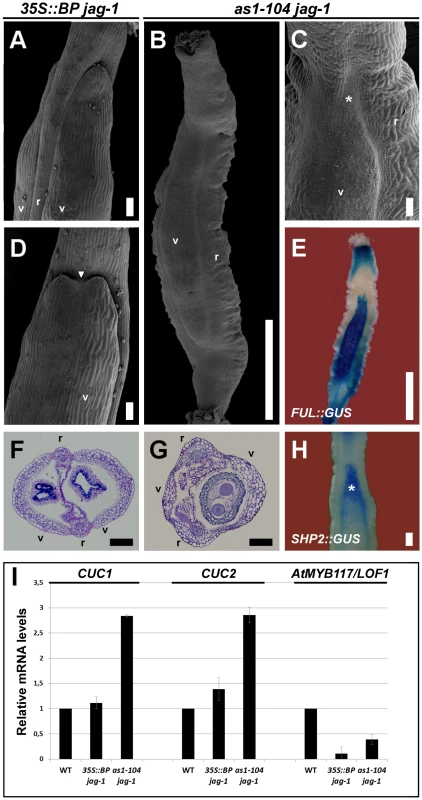
(A, D) SEM micrographs and (F) cross-section of stage 17 fruits of 35S::BP jag-1 plants. These fruits show a moderate mutant phenotype (A, F) with the occasional formation of ectopic valve margin at the apical region of valves (white arrowhead in D). (B, C) SEM images and (G) cross-section of stage 17 fruits of as1-104 jag-1 plants, showing the synergistic interaction between these two mutant alleles. Although these fruits show a similar appearance to those of ful mutants, with large and twisted repla and small valves (B, G), the presence of ectopic valve margin is only restricted to the apical region of the valves (asterisk in C). (E, H) Whole mount staining in stage 15 as1-104 jag-1 fruits for GUS expression driven by the ful-1 enhancer trap (E), which is detected in the small valves, and for SHP2::GUS (H), which is detected in the valve margin and in the apical region of valves where ectopic valve margin forms (asterisk). (I) Relative mRNA levels of CUC1, CUC2 and AtMYB117/LOF1 in stage 10–13 pistils quantified by qRT-PCR. r, replum; v, valve. Scale bars: 100 µm (A, C, D, F–H); 1 mm (B, E). Surprisingly, fruits of as1 jag mutants appeared by far more affected than those of 35S::BP jag plants, showing strong reduction of valve size, as well as enlarged and twisted repla (Figure 5B, 5C, 5G; Figure S6; and Figure S7D, S7H), a phenotype reminiscent to that of ful mutants [10]. However, whereas ful valves show small and rounded epidermal cells, and do not contain any stomata, valves of as1 jag fruits exhibited larger cells and stomata. In line with this phenotype, the activity of FUL::GUS in as1 jag pistils was detected in the reduced valves (Figure 5E), explaining the low levels of FUL expression detected in this background (Figure S8).
Since in ful fruits the valve margin identity genes become ectopically expressed in valve tissue, we studied the activity of the SHP2::GUS reporter in as1 jag fruits. Our previous work showed that this reporter expresses normally in as1 fruits [19]. Whole-mount staining of as1 jag fruits revealed normal expression for the SHP2 reporter in the valve margin, but an expansion of the signal towards the lateral domains was detected at the upper part of the valve (asterisk in Figure 5H), consistent with an enlargement of the valve margin in this area (asterisk in Figure 5C).
The phenotypic difference between 35S::BP jag and as1 jag fruits strongly suggests that, besides BP, AS1 and JAG likely cooperate in negatively regulating other genes for mediolateral fruit patterning. It has been previously established that both AS1 and JAG interact to promote sepal and petal development by downregulating the boundary-specifying genes CUP-SHAPED COTYLEDONS1 (CUC1), CUC2 and PETAL LOSS (PTL) [45]. The LATERAL ORGAN FUSION1 (AtMYB117/LOF1) gene [46] was also considered as an additional candidate, since its ectopic expression results in enlargement of the replum [47], similar to that of as1, 35S::BP or jag fruits. Therefore, we determined by qRT-PCR the transcript levels of these genes in wild-type, as1 jag and 35S::BP jag pistils (Figure 5I). As previously reported, PTL is not expressed in pistil tissues [48] and basically no transcripts were detected in any of the tested backgrounds (data not shown). Transcript levels of AtMYB117/LOF1 were downregulated in both as1 jag and 35S::BP jag pistils (Figure 5I), which ruled out this candidate. But, interestingly, CUC1 and CUC2 transcripts accumulated at much higher levels in as1 jag than in 35S::BP jag pistils (Figure 5I), suggesting that CUC genes might be involved in the strong phenotype found in as1 jag double mutant fruits. Interestingly, Ishida and coworkers showed that CUC2 is involved in fruit development [49] and, in line with our hypothesis, we have observed that the cuc2 gain-of-function allele (cuc2-d) [50] leads to the formation of short fruits that develop enlarged repla (Figure S9).
Synergistic interaction between loss-of-function alleles of YAB1 group genes and as1
We next checked the effect of misregulating BP (as1 and 35S::BP) in mutant backgrounds affected in YAB1 group genes. Fruits of 35S::BP fil and 35S::BP yab3 showed a similar phenotype, exhibiting stripes of valve margin tissue developing ectopically at the basal region of the valves, whereas the apical region of the ovary lacked valve margin (Figure 6A–6D). Because these defects were reminiscent of those seen in fil yab3 double mutants [35] (Figure S5C, S5G), it is likely that the negative effect of BP on JAG/FIL activity genes could account for this phenotype. However, as1 fil and as1 yab3 fruits exhibited moderate phenotypes when compared to those of 35S::BP fil and 35S::BP yab3 (Figure 6E, 6F, 6J), but still showed a conspicuous reduction in valve size concomitant with an increase in replum width (Figure S6). In fact, in as1 fil mutants, the replum epidermis contained larger cells and more stomata than in any of the single mutants (Figure S7C, S7G).
Fig. 6. BP misexpression enhances the fruit defects of mutants with impaired JAG/FIL activity. 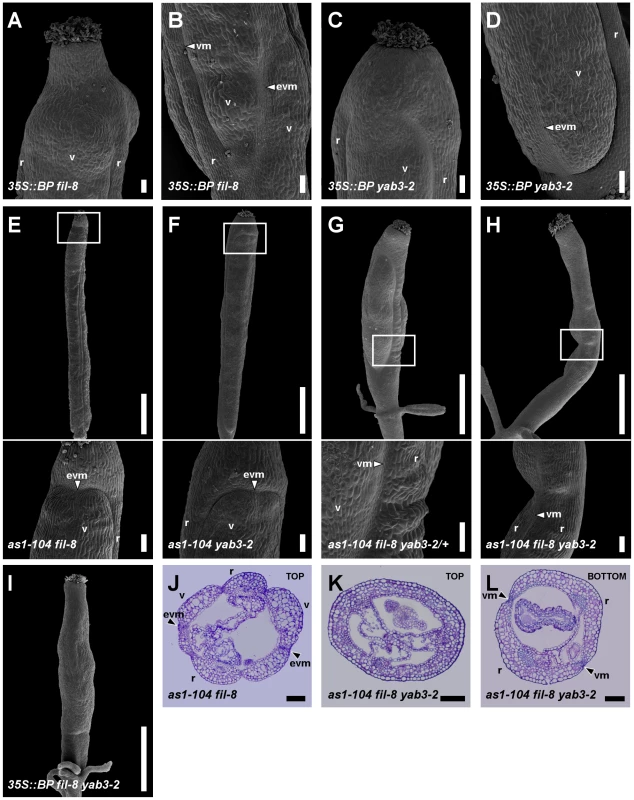
(A–D) SEM micrographs of stage 17 fruits of 35S::BP fil-8 and 35S::BP yab3-2 plants. Similar to fil yab3 fruits, in 35S::BP fil-8 (A, B) and 35S::BP yab3-2 (C, D) fruits, the apical regions lack valve margin whereas the basal regions show ectopic valve margin tissue. (E–I) SEM micrographs and (J–L) cross-sections of stage 17 fruits of several combinations of as1-104 with mutant alleles in YAB1 group genes. In panels E–H, insets indicate the magnified area shown in the image below. The apical regions of the ovaries in as1-104 fil-8 (E, J) and as1-104 yab3-2 (F) fruits show ectopic valve margin, which is reminiscent of fil YAB3/yab3 fruits. The increase in the mutant phenotype is evident in as1-104 fil-8 YAB3/yab3-2 fruits (G), resembling ful mutants, although unlike these the valves of the multiple mutant show a few interspersed stomata. The fruit of the as1-104 fil-8 yab3-2 triple mutant exhibits an extreme phenotype, which implies the complete absence of valves and the presence of two very huge repla separated by valve margin tissue in the basal region of the ovary (H, L), whereas this tissue is absent in its apical region (H, K). Fruits of 35S::BP fil-8 yab3-2 show in all their lengths the same phenotype exhibited in the apical region of triple mutant ovaries (I). evm, ectopic valve margin; r, replum; v, valve; vm, valve margin. Scale bars: 100 µm (A–D, insets in E–H, J–L,); 1 mm (upper images in E–H, I). In fruits of the sesquimutant fil YAB3/yab3, a stripe of valve margin tissue often appears in the apical region of the valves [35] (Figure S5B, S5F). Interestingly, we observed the formation of ectopic valve margin tissue in the valves of both as1 fil and as1 yab3 fruits (Figure 6E, 6F, 6J), although with smaller size and lower frequency (40% and 30% in as1 fil and as1 yab3 fruits, respectively, versus 90% in fil YAB3/yab3 fruits). These observations suggest a further reduction of JAG/FIL activity in both double mutants with respect to fil and yab3 single mutants.
It has been previously shown that low levels of FUL activity in fil YAB3/yab3 fruits lead to the ectopic expression of valve margin identity genes in valves [35]. Therefore, we analyzed the expression of FUL and the valve margin identity gene SHP2 in as1 fil and as1 yab3 pistils. By qRT-PCR assays in pistils, we found that levels of FUL transcripts in both double mutants were significantly reduced comparing to those in wild type or in as1 pistils (Figure S8). In line with the phenotypes above described, FUL::GUS signal in as1 fil fruits was detected at lower levels in the apical regions of valves (Figure 7A), just where SHP2::GUS expresses ectopically (Figure 7D) and ectopic valve margin is produced (Figure 6E, 6J).
Fig. 7. Effect of mutant combinations of loss-of-function alleles of AS1 and YAB1 group genes on gene expression. 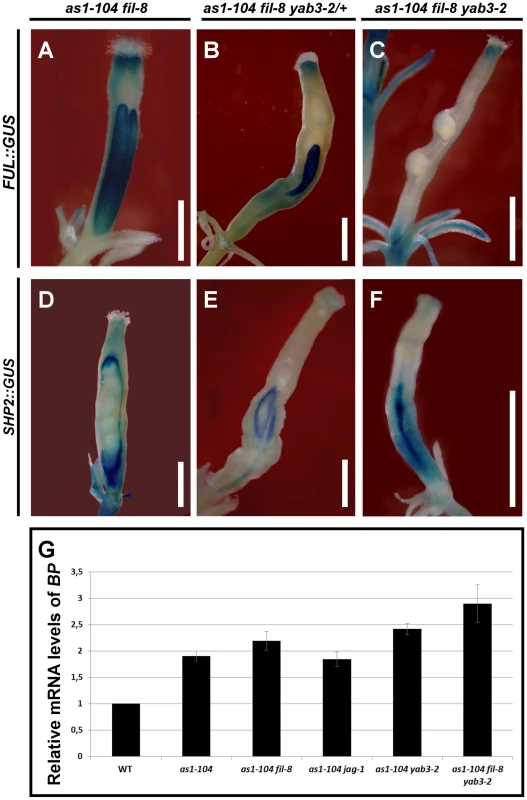
(A–F) Whole mount staining of the GUS reporter under control of the FUL (A–C) or the SHP2 (D–F) promoter in multiple mutant fruits carrying the as1-104 alelle. The expression of the ful-1 enhancer trap (FUL::GUS) and SHP2::GUS in the as1-104 fil-8 mutant appears mostly normal, with exception of some fruits in which the expression of the FUL::GUS declines in both the basal and the apical regions of the ovary (A) where the SHP2::GUS is expressed at higher levels (D). The as1-104 fil-8 YAB3/yab3-2 mutant shows reduced valves which express the FUL::GUS (B), whereas the SHP2::GUS expression is detected encompassing the small valves (E). Most as1-104 fil-8 yab3-2 triple mutant fruits lack FUL::GUS in the valves (C), and exhibit a line of SHP2::GUS expression running along the basal two-thirds of the valve (F). (G) Relative mRNA levels of BP in stage 10–13 pistils quantified by qRT-PCR. Scale bars: 1 mm. When one copy of yab3 was introduced into the as1 fil background (as1 fil YAB3/yab3 plants), the severity of the mutant phenotype was intensified and fruits exhibited smaller valves and larger repla when compared to those of as1 fil and as1 yab3 double mutants (Figure 6G, Figure S6, and Figure S7E and S7I). We also noticed that as1 fil YAB3/yab3 and as1 jag siliques were very similar, although in as1 fil YAB3/yab3 the replum had fewer cells and was less twisted (Figure S7D, S7E, S7H, S7I). Similar to mutant fruits affected in JAG/FIL activity genes, repla of as1 fil YAB3/yab3 fruits were abnormally wider, showing more and larger epidermal cells, and also presented frequent interspersed stomata, being quite difficult to distinguish them from valves (Figure S7E, S7I). In this scenario, levels of FUL mRNA were drastically reduced (Figure S8), and FUL reporter signal was restricted to small areas which correspond to the valves (Figure 7B), while the SHP2::GUS marked the position of the valve margins around the reduced valves (Figure 7E).
The complete loss of both AS1 and YAB1 group genes in the as1 fil yab3 triple mutant produced dramatic and deleterious defects on mediolateral fruit patterning (Figure 6H, 6K, 6L and Figure S6). In the basal region of as1 fil yab3 ovaries, the most prevalent phenotype was the presence of two thin stripes of valve margin located at the lateral-most regions of the ovary, both separating what we called two giant “super-repla” (Figure 6H, 6L). We also found fruits with extremely small valves separated from the oversized repla by valve margin tissue (Figure S10B). The aberrant replum of as1 fil yab3 fruits contained wide and large cells and fully developed stomata, making this tissue to adopt a similar appearance to wild-type valves (Figure S7F). In fact, the phenotype was even stronger in the apical region of the ovary where only these wide and large cells and stomata could be observed, completely lacking valve margin tissue (Figure 6H, 6K). Accordingly, as1 fil yab3 pistils showed very low levels of FUL messenger (Figure S8) and FUL reporter activity was only detected in fruits in which valve tissue developed (Figure 7C and Figure S10A). In line with these observations, the expression of the SHP2::GUS reporter was mostly seen forming a stripe in the lateral-most region of as1 fil yab3 ovaries (Figure 7F). These abnormalities make as1 fil yab3 fruits quite different from those of jag fil yab3 triple mutants, since the former are mainly composed of giant replum, while the latter clearly show valve and replum regions, as the signal for the BP::GUS revealed (Figure S11). In 35S::BP fil yab3 plants, the fruit mutant phenotype was even stronger, and both apical and basal regions of the ovary showed the same aspect as the apical region of ovaries in as1 fil yab3 fruits (Figure 6I).
Our model predicts that an increase in the activity (or misexpression) of replum factors (BP) along with a reduction in the function of lateral factors (JAG/FIL activity) should lead to the formation of fruits with an enormous replum territory and very small valves [19]. The fruit phenotypes described for combinations of as1 and mutant alleles in JAG/FIL activity genes are very much in line with these predictions (Figure 7G and Figure S6). In strong agreement, as1 fil yab3 and 35S::BP fil yab3 plants produced fruits with huge repla that contained abnormal cell types, and an extreme reduction or abolishment of valve development (Figure 6H, 6I, 6K, 6L and Figure S10B). This phenotype is mainly due to ectopic expression of BP in a background with reduced JAG/FIL activity.
Discussion
The current model for mediolateral fruit development in Arabidopsis hypothesizes that the final pattern is established by the concurrence of two opposing and antagonistic activities (lateral and medial factors) [7], [19]. JAG/FIL activity genes are lateral factors responsible for the establishment of the lateral pattern elements: valves and valve margins [35]. In the replum (the medial pattern element), the cooperating medial (or replum) factors BP and RPL [11], [12], [14] are required for replum formation and growth [19], [36]. The results presented in this work show that both lateral and medial factors actually repress each other, and that this mutual antagonism results in proper pattern formation along the mediolateral axis of the Arabidopsis fruit.
AS1 intimately cooperates with lateral factors by negatively regulating BP, which prevents its ectopic expression in valves and secures correct level of JAG/FIL activity. Consequently, strong reduction of JAG/FIL activity in combination with misregulation of BP (by either 35S::BP or as1) leads to the giant “super-replum” phenotype. Therefore, in this developmental program, AS1 and its molecular partner AS2 can be also considered as lateral factors.
JAG/FIL activity specifies replum morphology by negatively regulating the replum factor BP
Besides their activities in fruit patterning, JAG/FIL activity genes have been previously described by their participation in the formation of other lateral organs. Consequently, they all are expressed in lateral organs but not in meristematic tissues. The YAB1 group genes, FIL and YAB3, promote leaf development by repressing the expression of class I KNOX meristematic genes in leaves [44], and specify ventral (abaxial) fate [28], [29], [51]. Nevertheless, although FIL and YAB3 are not expressed in meristems, by means of a non-cell-autonomous mechanism, they contribute to shoot apical meristem (SAM) maintenance by negatively regulating WUSCHEL (WUS) and CLAVATA3 (CLV3) genes, both expressed at the central meristem domain [52]. In fact, this mechanism also affects the floral meristem [52], and fil yab3 mutants exhibit a high frequency of fruits with three valves (Figure 3E, 3F), possibly due to an increase in floral meristem size caused by the expansion of the WUS expression domain.
Similarly, the results presented in this work show that, despite FIL and YAB3, as well as JAG, are active in lateral regions of the ovary and not expressed in the presumptive replum (medial tissue), mutants affected in JAG/FIL activity have oversized repla with extra-large cells and interspersed stomata, indicating that these laterally expressed genes make an important contribution to the correct development of the medial region in the Arabidopsis fruit. Hence, it is most likely that JAG/FIL activity mediate replum development by negatively regulating, also via non-autonomous mechanisms, the expression of meristematic genes, specifically BP, in the replum. This is deduced 1) from the enhanced expression of BP in mutants affected in JAG/FIL activity genes, and 2) from the rescue of the replum phenotype in jag bp, fil bp, fil yab3 bp and fil jag bp fruits. Altogether, these data provide an additional analogy between meristem and replum, as well as between lateral organs and valves.
Nothing is known about how YAB1 group genes control BP expression at the molecular level, and it has been previously shown in vitro that FIL protein binds DNA nonspecifically [53]. In SAM homeostasis, FIL and YAB3 proteins interact with members of the LEUNIG (LUG) and SEUSS-like (SEU-like) families of transcriptional co-repressors, and the resulting multicompetent protein complexes likely recruit additional transcriptional regulators to acquire then DNA sequence specificity [54]. It is likely that a similar mechanism might be operating during mediolateral patterning of the Arabidopsis fruit to prevent misexpression of medial factors such as BP.
The JAG gene, similarly as YAB3 and FIL, controls leaf polarity and, in cooperation with its closest paralog NUBBIN (NUB), inhibits premature tissue differentiation by maintaining cell proliferation [31], [32], [55]. Interestingly, the JAG protein contains an EAR (ERF-associated amphiphilic repression)-motif [56] near the N-terminus [31], [57]. This motif is known to be involved in transcriptional repression and critically intervenes during the molecular interaction between transcriptional regulators and co-repressors [58]–[64]. Therefore, it is possible that FIL/YAB3 and LUG/LUH (LEUNIG HOMOLOG)-SEU-like complexes might recruit JAG, and perhaps other regulatory proteins, to target specific DNA sequences. A detailed analysis of this possibility might be of interest and would corroborate, at the molecular level, the genetic interactions that occur for both SAM homeostasis and mediolateral fruit patterning.
AS1 cooperates with the JAG/FIL activity to repress the replum identity factor BP
The relationship between replum and valves closely mirrors the antagonism that there exists between meristem and lateral organs [4], [19], [20]. One of such antagonistic relationships is established between class I KNOX genes, expressed in meristem, and AS1 expressed in leaves. In the meristem, the class I KNOX gene STM negatively regulates AS1 whereas, in turn, AS1 physically interacts with AS2 to directly repress BP in leaves [33], [65]–[68]. Similarly, AS1 (and AS2) also negatively regulates BP in pistils, and thus, in as1 mutants BP is ectopically expressed in valves and show higher levels of expression in the replum [19].
Interestingly, as1 and 35S::BP pistils show similar replum defects as those described for mutants affected in JAG/FIL activity genes, in which BP expression is also enhanced in its own medial domain, and replum defects increase when as1 alleles or 35S::BP construct are combined with jag and/or mutant alleles in YAB1 group genes [19] (this work). These findings indicate that JAG/FIL activity and AS genes cooperate to repress the expression of the replum factor BP in the medial region of pistils, and that this regulation is critical to achieve proper replum pattern. Furthermore, we have observed that valve alterations are also drastically enhanced in these mutant combinations, and our genetic and molecular analyses also evidenced that ectopic expression of BP downregulates JAG and YAB1 group genes in lateral tissues. Therefore, we can conclude that BP repression in lateral regions by AS1 (and AS2) plays an important role in valve development by maintaining normal levels of JAG/FIL activity.
AS1 and JAG regulate other factors besides BP during fruit patterning
Nevertheless, although most of the as1 fruit phenotype can be explained by misregulation of BP, the lack of AS1 does not justify all the fruit defects observed in the mutants. This is better seen in as1 bp fruits, which nearly had wild-type appearance but still showed some subtle abnormalities [19]. This observation indicates that, besides controlling BP expression, AS1 plays additional roles in fruit.
The existence of such additional AS1 functions is further supported by the stronger phenotype of as1 jag fruits when compared to those of 35S::BP jag plants. Interestingly, AS1 and JAG also interact in the flower to promote petal and sepal development by negatively regulating the boundary-specifying genes CUC1 and CUC2 [45]. In as1 jag flowers, both sepal and petal development is aborted [45]. However, in 35S::BP jag plants these floral organs develop normally (data not shown). In pistils, our qRT-PCR data revealed that both CUC1 and CUC2 are upregulated in as1 jag at much higher levels than in 35S::BP jag. On the other hand, cuc2 gain-of-function allele produced an increase in replum width that resembles that of as1 and jag mutants. All together these data suggest that AS1 and JAG cooperate to negatively regulate CUC function in fruit and that this repression may play an important role in mediolateral patterning.
Antagonistic interactions between medial and lateral factors pattern the Arabidopsis fruit
The basis of the model for mediolateral patterning of the Arabidopsis fruit lies on the antagonistic activities of medial factors (BP and RPL) and lateral factors (JAG/FIL activity genes) [19]. In accordance to the model, the giant “super-replum” phenotype requires both low levels of JAG/FIL activity and ectopic BP expression in valves. This was the case for as1 fil yab3 or 35S::BP fil yab3 siliques (this work). On the other hand, transformation of the replum into a lateral tissue, the valve margin, requires reduction of medial factor activity and increased activity of lateral factors, as in rpl and rpl bp fruits [13], [19], [35]. All together support the idea that BP promotes replum fate [19], [36] and, again, strongly suggest that medial and lateral factors oppose each other to specify pattern elements along the mediolateral axis.
Pattern formation by the contribution of antagonistic activities is not uncommon in plant development. For example, leaf adaxial (dorsal)/abaxial (ventral) polarity is established by antagonistic interactions between genes that specify either abaxial or adaxial identity, such as KANADI and class III HD-Zip genes [69], [70]. During embryo development, the apical/shoot versus basal/root polarity is determined by the antagonistic relationship between class III HD-Zip and PLETHORA (PLT) genes [71].
The model also proposed that lateral and medial factors work through gradients with their minimal activities in the valve margin, where they likely overlap [19]. This easily allowed to explain the low levels of JAG/FIL activity required to produce valve margin [35]. However, recent studies have determined that BP is only expressed and active in the replum, so that it does not overlap in the valve margin with lateral factors [36], [43] (our unpublished results). This favours a non-overlapping model whereby the medial factors are not required to function through a gradient. Nevertheless, the low levels of JAG/FIL activity needed for promoting valve margin identity suggest a gradient in the activity of lateral factors. Above a certain threshold lateral factors specify valve fate and allow other genes to function (such as FUL) and below that threshold valve margin tissue forms [7], [19], [20], [35].
Furthermore, the phenotypes of as1 and 35S::BP also support the existence of such JAG/FIL activity gradient. Misexpression of BP in these backgrounds reduces the expression of lateral factors, shifting to a more lateral position the region of low levels of JAG/FIL activity that produce valve margin. Farther away from the replum, these levels are high enough to activate the expression of FUL and specify valve development. Consequently, when mutations in AS1, JAG and YAB1 group genes combine, the more JAG/FIL activity is eliminated, the more laterally the valve margin is placed. This can be easily observed in the basal region of as1 fil yab3 ovaries that exhibit a stripe of valve margin in their lateral-most position. In the model, AS function (AS1 together with AS2) is integrated as another lateral factor (Figure 8).
Fig. 8. Simplified model for patterning along the mediolateral axis of the Arabidopsis fruit. 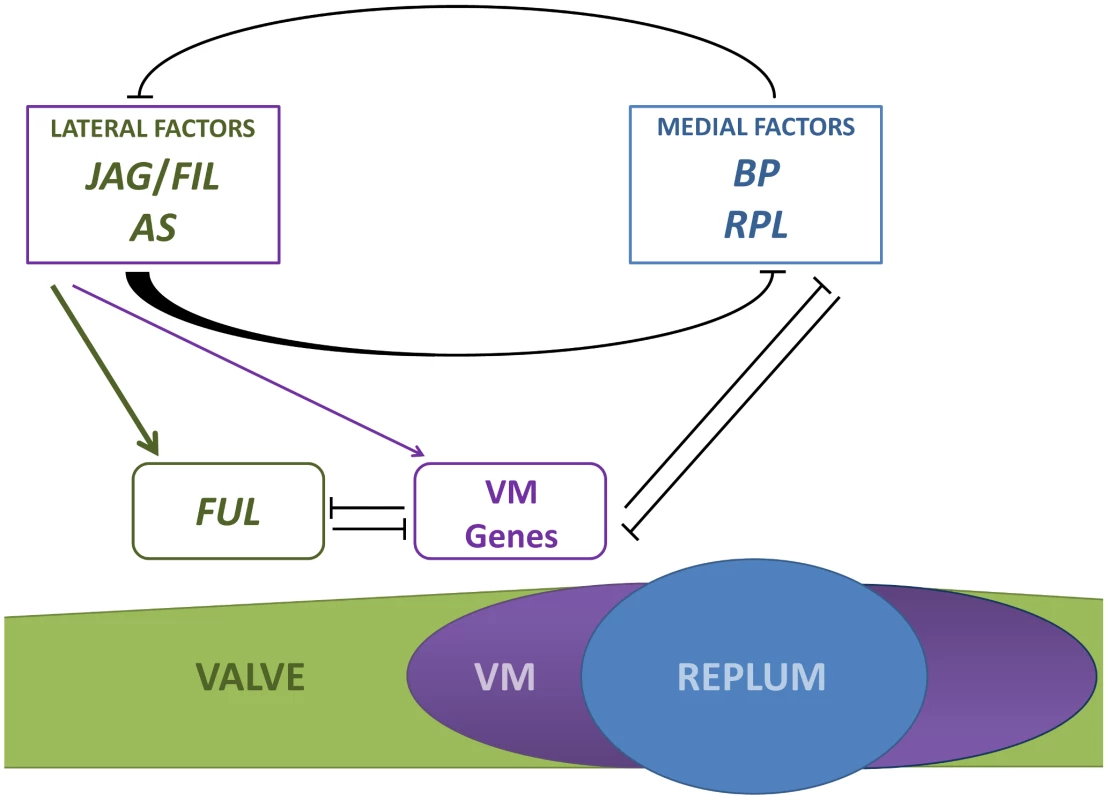
The antagonistic activities of lateral factors (JAG/FIL activity and AS1/2 genes) and medial factors (BP and RPL) are responsible for the formation and size of the three pattern elements in the mediolateral axis: valve, valve margin (VM) and replum. Lateral factors form a gradient of activity to determine valve and valve margin development. High activity of lateral factors promotes FUL expression in valves whereas lower levels of this activity induce the expression of valve margin identity genes (VM genes) [35]. Medial factors specify replum development [19], [36]. Lateral factors negatively regulate the expression of medial factors in a non-autonomous fashion and medial factors impede in an autonomous way the expression of lateral factors in the replum. The whole of genetic interactions along the mediolateral axis indicates that genes in all three tissue types negatively regulate the genes expressed in the other territories [13], [16], [19], [35], [36], [88]. BP overexpression in the repla of ap2 mutants does not affect valve development [36], suggesting that expressions of JAG/FIL activity genes are not affected in these backgrounds and that medial factors work in a cell-autonomous way to prevent the ectopic expression of lateral factors in the presumptive replum. These observations further suggest that medial factors are not required to generate the gradient of lateral factors. On the other hand, lateral factors restrict medial factor expression to a small area that becomes the replum and, in a non-autonomous fashion, limit the expression levels of BP and RPL in medial tissues to ensure proper replum development (Figure 8). In replum tissue, AP2 cooperates with lateral factors to negatively modulate the expression of BP and RPL in the medial domain [19], [36].
Further work will be needed to elucidate how the gradient of lateral factors is generated, although the phytohormone auxin is a possible candidate. In this sense, it has been postulated that a gradient of auxin patterns the apical-basal axis of the Arabidopsis fruit, with the AUXIN RESPONSE FACTOR3 (ARF3; aka ETTIN, ETT) in charge of interpreting intermediate levels of auxin to specify the ovary [72]. Interestingly, mutants affected in JAG/FIL activity genes, both with and without as1, show phenotypic differences along the apical-basal axis, being the phenotype always stronger in the apical region of the ovary [35] (this work), and it has been shown that ETT positively regulates FIL activity during leaf development [73], [74]. Furthermore, a recent research found that IND creates an auxin minimum, by regulating auxin efflux, necessary for the formation of the separation layer of the valve margin [74].
The mechanism we propose for patterning the mediolateral axis of Arabidopsis fruit ensures a high plasticity, and possibly may help to understand, at least in part, the variability of fruit shapes in Brassicaceae and other related species. It might be possible that subtle changes in the expression of the antagonistic factors involved in this process could produce drastic changes in the size of the different tissue types. According to this line of argument, Arnaud and coworkers have recently discovered that the reduced replum of Brassica plants is due to a single nucleotide change in a cis-regulatory element between the RPL orthologs of Brassica and Arabidopsis, which makes the Brassica wild-type allele less functional [75].
Materials and Methods
Plant material, growth conditions, and genetics
The mutant lines used in this work were in Landsberg erecta (Ler) background and this accession was the wild-type reference. The original 35S::BP line, in No-0 background, was introgressed four times into Ler. In experiments involving reporter genes (GUS and GFP), the references were wild-type segregants showing the er phenotype, as previously described [35]. fil-8 and yab3-2 [44], jag-1 [31], [35], ful-1 [10], bp-1 [76], 35S::BP [22], as1-104 [19], FIL>>BP [77], SHP2::GUS [78], KNAT1::GUS-18 (BP::GUS) [65] and FIL::GFP [30] have been described before. JAG::GUS has been generated by J.R. Dinneny. Briefly, to generate the JAG::GUS transgenic line, a JAG promoter fragment was amplified from the T26J14 BAC using the primers oJD196 (5′-AAGCTTCCACTGGGCTTGTATTCCCATCC-3′) and oJD197 (5′-GGATCCAGTGGGAAATGAGAGATTGGCGTGAG-3′), which added HindIII and BamHI restriction sites to the 5′ and 3′ ends, respectively. This fragment was cloned into the pDW294 binary vector to create the construct pJD145, which was transformed after checking its integrity into Col-0 plants.
Plants were grown at 20–22°C with continuous cool-white fluorescent light as previously described [79]. Multiple mutants were identified among the F2 from the characteristic mutant phenotype caused by individual mutations and/or by molecular genotyping. The fil-8, yab3-2 and jag-1 alleles were genotyped using primers previously published [31], [35] (Table S1). Plants with genotypes showing defective development of stamens and poor fertility were hand-pollinated to allow the formation of fruits.
Microscopy
Light microscopy analysis and scanning electron microscopy (SEM) were performed as previously described [79]. GFP signal was examined under a Nikon SMZ1500 stereo microscope equipped with a mercury UV lamp, and the emitted fluorescence was monitored using a filter permeable for wavelengths over 505 nm. For GUS staining, samples were treated as previously described [19], [35].
In situ hybridization was carried out as previously described [19] with the following modifications. The DIG-labeled antisense probe for FIL mRNA was obtained from the original plasmid pY1-Y (provided by J. Bowman), amplifying the insert by PCR with M13 forward and reverse primers. The amplified DNA was used as template to transcribe the probe with a T7 RNA polymerase (Fermentas).
Quantitative real-time polymerase chain reaction (qRT–PCR)
RNA from pistils at stages 10–13 was extracted using the PureLink RNA Mini Kit (Invitrogen), and DNA contamination was removed by treatment with DNaseI (Takara). Reverse-transcription was performed from 1 µg of total RNA using the RevertAid H Minus M-MuLV Reverse Transcriptase (Fermentas). Real-time PCR was carried out using the LightCycler FastStart DNA MasterPLUS SYBR Green I (Roche) in a volume of 20 µl on the LightCycler 1.5 instrument (Roche), as previously published [80] with minor modifications. RNA levels were normalized relative to the constitutive OTC gene [81] and to the wild-type levels, and expression results were calculated by an efficiency correction quantification method [82]. All individual experiments were performed by triplicate, and checked twice using new cDNA every time. The reported values are averages of both biological replicates. Primers for qRT-PCR were as previously published for AtMYB117/LOF1 [47], BP [83], CUC1 [84], CUC2 [85], FUL [86] and OTC [87]. A complete list of primers used in these experiments can be found in Table S1.
Translation
A translation of the title, abstract, and author summary into Spanish is provided in Text S1.
Supporting Information
Zdroje
1. BowmanJL, BaumSF, EshedY, PutterillJ, AlvarezJ (1999) Molecular genetics of gynoecium development in Arabidopsis. Curr Top Dev Biol 45 : 155–205.
2. FerrandizC, PelazS, YanofskyMF (1999) Control of carpel and fruit development in Arabidopsis. Annu Rev Biochem 68 : 321–354.
3. DinnenyJR, YanofskyMF (2005) Drawing lines and borders: how the dehiscent fruit of Arabidopsis is patterned. Bioessays 27 : 42–49.
4. BalanzaV, NavarreteM, TriguerosM, FerrandizC (2006) Patterning the female side of Arabidopsis: the importance of hormones. J Exp Bot 57 : 3457–3469.
5. RoederAH, YanofskyMF (2006) Fruit development in Arabidopsis. Arabidopsis Book 4: e0075.
6. OstergaardL (2009) Don't ‘leaf’ now. The making of a fruit. Curr Opin Plant Biol 12 : 36–41.
7. Martinez-Laborda A, Vera A (2009) Arabidopsis fruit development. In: Ostergaard L, editor. Fruit Development and Seed Dispresal Annual Plant Reviews, Volume 38. Oxford, UK: Wiley-Blackwell.
8. Vivian-SmithA, KoltunowAM (1999) Genetic analysis of growth-regulator-induced parthenocarpy in Arabidopsis. Plant Physiol 121 : 437–451.
9. FerrandizC (2002) Regulation of fruit dehiscence in Arabidopsis. J Exp Bot 53 : 2031–2038.
10. GuQ, FerrandizC, YanofskyMF, MartienssenR (1998) The FRUITFULL MADS-box gene mediates cell differentiation during Arabidopsis fruit development. Development 125 : 1509–1517.
11. ByrneME, GrooverAT, FontanaJR, MartienssenRA (2003) Phyllotactic pattern and stem cell fate are determined by the Arabidopsis homeobox gene BELLRINGER. Development 130 : 3941–3950.
12. SmithHM, HakeS (2003) The interaction of two homeobox genes, BREVIPEDICELLUS and PENNYWISE, regulates internode patterning in the Arabidopsis inflorescence. Plant Cell 15 : 1717–1727.
13. RoederAH, FerrandizC, YanofskyMF (2003) The role of the REPLUMLESS homeodomain protein in patterning the Arabidopsis fruit. Curr Biol 13 : 1630–1635.
14. BhattAM, EtchellsJP, CanalesC, LagodienkoA, DickinsonH (2004) VAAMANA–a BEL1-like homeodomain protein, interacts with KNOX proteins BP and STM and regulates inflorescence stem growth in Arabidopsis. Gene 328 : 103–111.
15. LiljegrenSJ, DittaGS, EshedY, SavidgeB, BowmanJL, et al. (2000) SHATTERPROOF MADS-box genes control seed dispersal in Arabidopsis. Nature 404 : 766–770.
16. LiljegrenSJ, RoederAH, KempinSA, GremskiK, OstergaardL, et al. (2004) Control of fruit patterning in Arabidopsis by INDEHISCENT. Cell 116 : 843–853.
17. RajaniS, SundaresanV (2001) The Arabidopsis myc/bHLH gene ALCATRAZ enables cell separation in fruit dehiscence. Curr Biol 11 : 1914–1922.
18. FerrandizC, LiljegrenSJ, YanofskyMF (2000) Negative regulation of the SHATTERPROOF genes by FRUITFULL during Arabidopsis fruit development. Science 289 : 436–438.
19. Alonso-CantabranaH, RipollJJ, OchandoI, VeraA, FerrandizC, et al. (2007) Common regulatory networks in leaf and fruit patterning revealed by mutations in the Arabidopsis ASYMMETRIC LEAVES1 gene. Development 134 : 2663–2671.
20. GirinT, SorefanK, OstergaardL (2009) Meristematic sculpting in fruit development. J Exp Bot 60 : 1493–1502.
21. LincolnC, LongJ, YamaguchiJ, SerikawaK, HakeS (1994) A knotted1-like homeobox gene in Arabidopsis is expressed in the vegetative meristem and dramatically alters leaf morphology when overexpressed in transgenic plants. Plant Cell 6 : 1859–1876.
22. ChuckG, LincolnC, HakeS (1996) KNAT1 induces lobed leaves with ectopic meristems when overexpressed in Arabidopsis. Plant Cell 8 : 1277–1289.
23. DouglasSJ, ChuckG, DenglerRE, PelecandaL, RiggsCD (2002) KNAT1 and ERECTA regulate inflorescence architecture in Arabidopsis. Plant Cell 14 : 547–558.
24. VenglatSP, DumonceauxT, RozwadowskiK, ParnellL, BabicV, et al. (2002) The homeobox gene BREVIPEDICELLUS is a key regulator of inflorescence architecture in Arabidopsis. Proc Natl Acad Sci U S A 99 : 4730–4735.
25. LongJA, MoanEI, MedfordJI, BartonMK (1996) A member of the KNOTTED class of homeodomain proteins encoded by the STM gene of Arabidopsis. Nature 379 : 66–69.
26. ChenQ, AtkinsonA, OtsugaD, ChristensenT, ReynoldsL, et al. (1999) The Arabidopsis FILAMENTOUS FLOWER gene is required for flower formation. Development 126 : 2715–2726.
27. SawaS, ItoT, ShimuraY, OkadaK (1999) FILAMENTOUS FLOWER controls the formation and development of arabidopsis inflorescences and floral meristems. Plant Cell 11 : 69–86.
28. SawaS, WatanabeK, GotoK, LiuYG, ShibataD, et al. (1999) FILAMENTOUS FLOWER, a meristem and organ identity gene of Arabidopsis, encodes a protein with a zinc finger and HMG-related domains. Genes Dev 13 : 1079–1088.
29. SiegfriedKR, EshedY, BaumSF, OtsugaD, DrewsGN, et al. (1999) Members of the YABBY gene family specify abaxial cell fate in Arabidopsis. Development 126 : 4117–4128.
30. WatanabeK, OkadaK (2003) Two discrete cis elements control the abaxial side-specific expression of the FILAMENTOUS FLOWER gene in Arabidopsis. Plant Cell 15 : 2592–2602.
31. DinnenyJR, YadegariR, FischerRL, YanofskyMF, WeigelD (2004) The role of JAGGED in shaping lateral organs. Development 131 : 1101–1110.
32. OhnoCK, ReddyGV, HeislerMG, MeyerowitzEM (2004) The Arabidopsis JAGGED gene encodes a zinc finger protein that promotes leaf tissue development. Development 131 : 1111–1122.
33. ByrneME, BarleyR, CurtisM, ArroyoJM, DunhamM, et al. (2000) Asymmetric leaves1 mediates leaf patterning and stem cell function in Arabidopsis. Nature 408 : 967–971.
34. ByrneME, SimorowskiJ, MartienssenRA (2002) ASYMMETRIC LEAVES1 reveals knox gene redundancy in Arabidopsis. Development 129 : 1957–1965.
35. DinnenyJR, WeigelD, YanofskyMF (2005) A genetic framework for fruit patterning in Arabidopsis thaliana. Development 132 : 4687–4696.
36. RipollJJ, RoederAH, DittaGS, YanofskyMF (2011) A novel role for the floral homeotic gene APETALA2 during Arabidopsis fruit development. Development 138 : 5167–5176.
37. BellaouiM, PidkowichMS, SamachA, KushalappaK, KohalmiSE, et al. (2001) The Arabidopsis BELL1 and KNOX TALE homeodomain proteins interact through a domain conserved between plants and animals. Plant Cell 13 : 2455–2470.
38. SmithHM, BoschkeI, HakeS (2002) Selective interaction of plant homeodomain proteins mediates high DNA-binding affinity. Proc Natl Acad Sci U S A 99 : 9579–9584.
39. ColeM, NolteC, WerrW (2006) Nuclear import of the transcription factor SHOOT MERISTEMLESS depends on heterodimerization with BLH proteins expressed in discrete sub-domains of the shoot apical meristem of Arabidopsis thaliana. Nucleic Acids Res 34 : 1281–1292.
40. KanrarS, OngukaO, SmithHM (2006) Arabidopsis inflorescence architecture requires the activities of KNOX-BELL homeodomain heterodimers. Planta 224 : 1163–1173.
41. ScofieldS, DewitteW, MurrayJA (2007) The KNOX gene SHOOT MERISTEMLESS is required for the development of reproductive meristematic tissues in Arabidopsis. Plant J 50 : 767–781.
42. RutjensB, BaoD, van Eck-StoutenE, BrandM, SmeekensS, et al. (2009) Shoot apical meristem function in Arabidopsis requires the combined activities of three BEL1-like homeodomain proteins. Plant J 58 : 641–654.
43. RagniL, Belles-BoixE, GunlM, PautotV (2008) Interaction of KNAT6 and KNAT2 with BREVIPEDICELLUS and PENNYWISE in Arabidopsis inflorescences. Plant Cell 20 : 888–900.
44. KumaranMK, BowmanJL, SundaresanV (2002) YABBY polarity genes mediate the repression of KNOX homeobox genes in Arabidopsis. Plant Cell 14 : 2761–2770.
45. XuB, LiZ, ZhuY, WangH, MaH, et al. (2008) Arabidopsis genes AS1, AS2, and JAG negatively regulate boundary-specifying genes to promote sepal and petal development. Plant Physiol 146 : 566–575.
46. LeeDK, GeislerM, SpringerPS (2009) LATERAL ORGAN FUSION1 and LATERAL ORGAN FUSION2 function in lateral organ separation and axillary meristem formation in Arabidopsis. Development 136 : 2423–2432.
47. GomezMD, UrbezC, Perez-AmadorMA, CarbonellJ (2011) Characterization of constricted fruit (ctf) mutant uncovers a role for AtMYB117/LOF1 in ovule and fruit development in Arabidopsis thaliana. PLoS ONE 6: e18760 doi:10.1371/journal.pone.0018760.
48. BrewerPB, HowlesPA, DorianK, GriffithME, IshidaT, et al. (2004) PETAL LOSS, a trihelix transcription factor gene, regulates perianth architecture in the Arabidopsis flower. Development 131 : 4035–4045.
49. IshidaT, AidaM, TakadaS, TasakaM (2000) Involvement of CUP-SHAPED COTYLEDON genes in gynoecium and ovule development in Arabidopsis thaliana. Plant Cell Physiol 41 : 60–67.
50. LarueCT, WenJ, WalkerJC (2009) A microRNA-transcription factor module regulates lateral organ size and patterning in Arabidopsis. Plant J 58 : 450–463.
51. EshedY, IzhakiA, BaumSF, FloydSK, BowmanJL (2004) Asymmetric leaf development and blade expansion in Arabidopsis are mediated by KANADI and YABBY activities. Development 131 : 2997–3006.
52. GoldshmidtA, AlvarezJP, BowmanJL, EshedY (2008) Signals derived from YABBY gene activities in organ primordia regulate growth and partitioning of Arabidopsis shoot apical meristems. Plant Cell 20 : 1217–1230.
53. KanayaE, NakajimaN, OkadaK (2002) Non-sequence-specific DNA binding by the FILAMENTOUS FLOWER protein from Arabidopsis thaliana is reduced by EDTA. J Biol Chem 277 : 11957–11964.
54. StahleMI, KuehlichJ, StaronL, von ArnimAG, GolzJF (2009) YABBYs and the transcriptional corepressors LEUNIG and LEUNIG_HOMOLOG maintain leaf polarity and meristem activity in Arabidopsis. Plant Cell 21 : 3105–3118.
55. DinnenyJR, WeigelD, YanofskyMF (2006) NUBBIN and JAGGED define stamen and carpel shape in Arabidopsis. Development 133 : 1645–1655.
56. OhtaM, MatsuiK, HiratsuK, ShinshiH, Ohme-TakagiM (2001) Repression domains of class II ERF transcriptional repressors share an essential motif for active repression. Plant Cell 13 : 1959–1968.
57. KagaleS, LinksMG, RozwadowskiK (2010) Genome-wide analysis of ethylene-responsive element binding factor-associated amphiphilic repression motif-containing transcriptional regulators in Arabidopsis. Plant Physiol 152 : 1109–1134.
58. HiratsuK, OhtaM, MatsuiK, Ohme-TakagiM (2002) The SUPERMAN protein is an active repressor whose carboxy-terminal repression domain is required for the development of normal flowers. FEBS Lett 514 : 351–354.
59. HiratsuK, MitsudaN, MatsuiK, Ohme-TakagiM (2004) Identification of the minimal repression domain of SUPERMAN shows that the DLELRL hexapeptide is both necessary and sufficient for repression of transcription in Arabidopsis. Biochem Biophys Res Commun 321 : 172–178.
60. TiwariSB, HagenG, GuilfoyleTJ (2004) Aux/IAA proteins contain a potent transcriptional repression domain. Plant Cell 16 : 533–543.
61. LiuZ, KarmarkarV (2008) Groucho/Tup1 family co-repressors in plant development. Trends Plant Sci 13 : 137–144.
62. SzemenyeiH, HannonM, LongJA (2008) TOPLESS mediates auxin-dependent transcriptional repression during Arabidopsis embryogenesis. Science 319 : 1384–1386.
63. GallavottiA, LongJA, StanfieldS, YangX, JacksonD, et al. (2010) The control of axillary meristem fate in the maize ramosa pathway. Development 137 : 2849–2856.
64. PauwelsL, BarberoGF, GeerinckJ, TillemanS, GrunewaldW, et al. (2010) NINJA connects the co-repressor TOPLESS to jasmonate signalling. Nature 464 : 788–791.
65. OriN, EshedY, ChuckG, BowmanJL, HakeS (2000) Mechanisms that control knox gene expression in the Arabidopsis shoot. Development 127 : 5523–5532.
66. SemiartiE, UenoY, TsukayaH, IwakawaH, MachidaC, et al. (2001) The ASYMMETRIC LEAVES2 gene of Arabidopsis thaliana regulates formation of a symmetric lamina, establishment of venation and repression of meristem-related homeobox genes in leaves. Development 128 : 1771–1783.
67. XuL, XuY, DongA, SunY, PiL, et al. (2003) Novel as1 and as2 defects in leaf adaxial-abaxial polarity reveal the requirement for ASYMMETRIC LEAVES1 and 2 and ERECTA functions in specifying leaf adaxial identity. Development 130 : 4097–4107.
68. GuoM, ThomasJ, CollinsG, TimmermansMC (2008) Direct repression of KNOX loci by the ASYMMETRIC LEAVES1 complex of Arabidopsis. Plant Cell 20 : 48–58.
69. BowmanJL, EshedY, BaumSF (2002) Establishment of polarity in angiosperm lateral organs. Trends Genet 18 : 134–141.
70. KidnerCA, TimmermansMC (2007) Mixing and matching pathways in leaf polarity. Curr Opin Plant Biol 10 : 13–20.
71. SmithZR, LongJA (2010) Control of Arabidopsis apical-basal embryo polarity by antagonistic transcription factors. Nature 464 : 423–426.
72. NemhauserJL, FeldmanLJ, ZambryskiPC (2000) Auxin and ETTIN in Arabidopsis gynoecium morphogenesis. Development 127 : 3877–3888.
73. GarciaD, CollierSA, ByrneME, MartienssenRA (2006) Specification of leaf polarity in Arabidopsis via the trans-acting siRNA pathway. Curr Biol 16 : 933–938.
74. SorefanK, GirinT, LiljegrenSJ, LjungK, RoblesP, et al. (2009) A regulated auxin minimum is required for seed dispersal in Arabidopsis. Nature 459 : 583–586.
75. ArnaudN, LawrensonT, OstergaardL, SablowskiR (2011) The same regulatory point mutation changed seed-dispersal structures in evolution and domestication. Curr Biol 21 : 1215–1219.
76. KoornneefM, van EdenJ, HanhartCJ, StamP, BraaksmaFJ, et al. (1983) Linkage map of Arabidopsis thaliana. J Hered 74 : 265–272.
77. HayA, TsiantisM (2006) The genetic basis for differences in leaf form between Arabidopsis thaliana and its wild relative Cardamine hirsuta. Nat Genet 38 : 942–947.
78. SavidgeB, RounsleySD, YanofskyMF (1995) Temporal relationship between the transcription of two Arabidopsis MADS box genes and the floral organ identity genes. Plant Cell 7 : 721–733.
79. RipollJJ, FerrandizC, Martinez-LabordaA, VeraA (2006) PEPPER, a novel K-homology domain gene, regulates vegetative and gynoecium development in Arabidopsis. Dev Biol 289 : 346–359.
80. RipollJJ, Rodriguez-CazorlaE, Gonzalez-ReigS, AndujarA, Alonso-CantabranaH, et al. (2009) Antagonistic interactions between Arabidopsis K-homology domain genes uncover PEPPER as a positive regulator of the central floral repressor FLOWERING LOCUS C. Dev Biol 333 : 251–262.
81. QuesadaV, PonceMR, MicolJL (1999) OTC and AUL1, two convergent and overlapping genes in the nuclear genome of Arabidopsis thaliana. FEBS Lett 461 : 101–106.
82. PfafflMW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29: e45.
83. OchandoI, Jover-GilS, RipollJJ, CandelaH, VeraA, et al. (2006) Mutations in the microRNA complementarity site of the INCURVATA4 gene perturb meristem function and adaxialize lateral organs in Arabidopsis. Plant Physiol 141 : 607–619.
84. KoyamaT, FurutaniM, TasakaM, Ohme-TakagiM (2007) TCP transcription factors control the morphology of shoot lateral organs via negative regulation of the expression of boundary-specific genes in Arabidopsis. Plant Cell 19 : 473–484.
85. LaufsP, PeaucelleA, MorinH, TraasJ (2004) MicroRNA regulation of the CUC genes is required for boundary size control in Arabidopsis meristems. Development 131 : 4311–4322.
86. YantL, MathieuJ, DinhTT, OttF, LanzC, et al. (2010) Orchestration of the floral transition and floral development in Arabidopsis by the bifunctional transcription factor APETALA2. Plant Cell 22 : 2156–2170.
87. HayA, BarkoulasM, TsiantisM (2006) ASYMMETRIC LEAVES1 and auxin activities converge to repress BREVIPEDICELLUS expression and promote leaf development in Arabidopsis. Development 133 : 3955–3961.
88. GirinT, StephensonP, GoldsackCM, KempinSA, PerezA, et al. (2010) Brassicaceae INDEHISCENT genes specify valve margin cell fate and repress replum formation. Plant J 63 : 329–338.
Štítky
Genetika Reprodukční medicína
Článek The Covariate's DilemmaČlánek Plant Vascular Cell Division Is Maintained by an Interaction between PXY and Ethylene SignallingČlánek Lessons from Model Organisms: Phenotypic Robustness and Missing Heritability in Complex Disease
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 11
-
Všechny články tohoto čísla
- Inference of Population Splits and Mixtures from Genome-Wide Allele Frequency Data
- The Covariate's Dilemma
- Plant Vascular Cell Division Is Maintained by an Interaction between PXY and Ethylene Signalling
- Plan B for Stimulating Stem Cell Division
- Discovering Thiamine Transporters as Targets of Chloroquine Using a Novel Functional Genomics Strategy
- Is a Modifier of Mutations in Retinitis Pigmentosa with Incomplete Penetrance
- Evolutionarily Ancient Association of the FoxJ1 Transcription Factor with the Motile Ciliogenic Program
- Genome Instability Caused by a Germline Mutation in the Human DNA Repair Gene
- Transcription Factor Oct1 Is a Somatic and Cancer Stem Cell Determinant
- Controls of Nucleosome Positioning in the Human Genome
- Disruption of Causes Defective Meiotic Recombination in Male Mice
- A Novel Human-Infection-Derived Bacterium Provides Insights into the Evolutionary Origins of Mutualistic Insect–Bacterial Symbioses
- Trps1 and Its Target Gene Regulate Epithelial Proliferation in the Developing Hair Follicle and Are Associated with Hypertrichosis
- Zcchc11 Uridylates Mature miRNAs to Enhance Neonatal IGF-1 Expression, Growth, and Survival
- Population-Based Resequencing of in 10,330 Individuals: Spectrum of Genetic Variation, Phenotype, and Comparison with Extreme Phenotype Approach
- HP1a Recruitment to Promoters Is Independent of H3K9 Methylation in
- Transcription Elongation and Tissue-Specific Somatic CAG Instability
- A Germline Polymorphism of DNA Polymerase Beta Induces Genomic Instability and Cellular Transformation
- Interallelic and Intergenic Incompatibilities of the () Gene in Mouse Hybrid Sterility
- Comparison of Mitochondrial Mutation Spectra in Ageing Human Colonic Epithelium and Disease: Absence of Evidence for Purifying Selection in Somatic Mitochondrial DNA Point Mutations
- Mutations in the Transcription Elongation Factor SPT5 Disrupt a Reporter for Dosage Compensation in Drosophila
- Evolution of Minimal Specificity and Promiscuity in Steroid Hormone Receptors
- Blockade of Pachytene piRNA Biogenesis Reveals a Novel Requirement for Maintaining Post-Meiotic Germline Genome Integrity
- RHOA Is a Modulator of the Cholesterol-Lowering Effects of Statin
- MIG-10 Functions with ABI-1 to Mediate the UNC-6 and SLT-1 Axon Guidance Signaling Pathways
- Loss of the DNA Methyltransferase MET1 Induces H3K9 Hypermethylation at PcG Target Genes and Redistribution of H3K27 Trimethylation to Transposons in
- Genome-Wide Association Studies Reveal a Simple Genetic Basis of Resistance to Naturally Coevolving Viruses in
- The Principal Genetic Determinants for Nasopharyngeal Carcinoma in China Involve the Class I Antigen Recognition Groove
- Molecular, Physiological, and Motor Performance Defects in DMSXL Mice Carrying >1,000 CTG Repeats from the Human DM1 Locus
- Genomic Study of RNA Polymerase II and III SNAP-Bound Promoters Reveals a Gene Transcribed by Both Enzymes and a Broad Use of Common Activators
- Long Telomeres Produced by Telomerase-Resistant Recombination Are Established from a Single Source and Are Subject to Extreme Sequence Scrambling
- The Yeast SR-Like Protein Npl3 Links Chromatin Modification to mRNA Processing
- Deubiquitylation Machinery Is Required for Embryonic Polarity in
- dJun and Vri/dNFIL3 Are Major Regulators of Cardiac Aging in Drosophila
- CtIP Is Required to Initiate Replication-Dependent Interstrand Crosslink Repair
- Notch-Mediated Suppression of TSC2 Expression Regulates Cell Differentiation in the Intestinal Stem Cell Lineage
- A Combination of H2A.Z and H4 Acetylation Recruits Brd2 to Chromatin during Transcriptional Activation
- Network Analysis of a -Mouse Model of Autosomal Dominant Polycystic Kidney Disease Identifies HNF4α as a Disease Modifier
- Mitosis in Neurons: Roughex and APC/C Maintain Cell Cycle Exit to Prevent Cytokinetic and Axonal Defects in Photoreceptor Neurons
- CELF4 Regulates Translation and Local Abundance of a Vast Set of mRNAs, Including Genes Associated with Regulation of Synaptic Function
- Mechanisms Employed by to Prevent Ribonucleotide Incorporation into Genomic DNA by Pol V
- The Genomes of the Fungal Plant Pathogens and Reveal Adaptation to Different Hosts and Lifestyles But Also Signatures of Common Ancestry
- A Genome-Scale RNA–Interference Screen Identifies RRAS Signaling as a Pathologic Feature of Huntington's Disease
- Lessons from Model Organisms: Phenotypic Robustness and Missing Heritability in Complex Disease
- Population Genomic Scan for Candidate Signatures of Balancing Selection to Guide Antigen Characterization in Malaria Parasites
- Tissue-Specific Regulation of Chromatin Insulator Function
- Disruption of Mouse Cenpj, a Regulator of Centriole Biogenesis, Phenocopies Seckel Syndrome
- Genome, Functional Gene Annotation, and Nuclear Transformation of the Heterokont Oleaginous Alga CCMP1779
- Antagonistic Gene Activities Determine the Formation of Pattern Elements along the Mediolateral Axis of the Fruit
- Lung eQTLs to Help Reveal the Molecular Underpinnings of Asthma
- Identification of the First ATRIP–Deficient Patient and Novel Mutations in ATR Define a Clinical Spectrum for ATR–ATRIP Seckel Syndrome
- Cooperativity of , , and in Malignant Breast Cancer Evolution
- Loss of Prohibitin Membrane Scaffolds Impairs Mitochondrial Architecture and Leads to Tau Hyperphosphorylation and Neurodegeneration
- Microhomology Directs Diverse DNA Break Repair Pathways and Chromosomal Translocations
- MicroRNA–Mediated Repression of the Seed Maturation Program during Vegetative Development in
- Selective Pressure Causes an RNA Virus to Trade Reproductive Fitness for Increased Structural and Thermal Stability of a Viral Enzyme
- The Tumor Suppressor Gene Retinoblastoma-1 Is Required for Retinotectal Development and Visual Function in Zebrafish
- Regions of Homozygosity in the Porcine Genome: Consequence of Demography and the Recombination Landscape
- Histone Methyltransferases MES-4 and MET-1 Promote Meiotic Checkpoint Activation in
- Polyadenylation-Dependent Control of Long Noncoding RNA Expression by the Poly(A)-Binding Protein Nuclear 1
- A Unified Method for Detecting Secondary Trait Associations with Rare Variants: Application to Sequence Data
- Genetic and Biochemical Dissection of a HisKA Domain Identifies Residues Required Exclusively for Kinase and Phosphatase Activities
- Informed Conditioning on Clinical Covariates Increases Power in Case-Control Association Studies
- Biochemical Diversification through Foreign Gene Expression in Bdelloid Rotifers
- Genomic Variation and Its Impact on Gene Expression in
- Spastic Paraplegia Mutation N256S in the Neuronal Microtubule Motor KIF5A Disrupts Axonal Transport in a HSP Model
- Lamin B1 Polymorphism Influences Morphology of the Nuclear Envelope, Cell Cycle Progression, and Risk of Neural Tube Defects in Mice
- A Targeted Glycan-Related Gene Screen Reveals Heparan Sulfate Proteoglycan Sulfation Regulates WNT and BMP Trans-Synaptic Signaling
- Dopaminergic D2-Like Receptors Delimit Recurrent Cholinergic-Mediated Motor Programs during a Goal-Oriented Behavior
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Mechanisms Employed by to Prevent Ribonucleotide Incorporation into Genomic DNA by Pol V
- Inference of Population Splits and Mixtures from Genome-Wide Allele Frequency Data
- Zcchc11 Uridylates Mature miRNAs to Enhance Neonatal IGF-1 Expression, Growth, and Survival
- Histone Methyltransferases MES-4 and MET-1 Promote Meiotic Checkpoint Activation in
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



