-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaDisruption of Mouse Cenpj, a Regulator of Centriole Biogenesis, Phenocopies Seckel Syndrome
Disruption of the centromere protein J gene, CENPJ (CPAP, MCPH6, SCKL4), which is a highly conserved and ubiquitiously expressed centrosomal protein, has been associated with primary microcephaly and the microcephalic primordial dwarfism disorder Seckel syndrome. The mechanism by which disruption of CENPJ causes the proportionate, primordial growth failure that is characteristic of Seckel syndrome is unknown. By generating a hypomorphic allele of Cenpj, we have developed a mouse (Cenpjtm/tm) that recapitulates many of the clinical features of Seckel syndrome, including intrauterine dwarfism, microcephaly with memory impairment, ossification defects, and ocular and skeletal abnormalities, thus providing clear confirmation that specific mutations of CENPJ can cause Seckel syndrome. Immunohistochemistry revealed increased levels of DNA damage and apoptosis throughout Cenpjtm/tm embryos and adult mice showed an elevated frequency of micronucleus induction, suggesting that Cenpj-deficiency results in genomic instability. Notably, however, genomic instability was not the result of defective ATR-dependent DNA damage signaling, as is the case for the majority of genes associated with Seckel syndrome. Instead, Cenpjtm/tm embryonic fibroblasts exhibited irregular centriole and centrosome numbers and mono - and multipolar spindles, and many were near-tetraploid with numerical and structural chromosomal abnormalities when compared to passage-matched wild-type cells. Increased cell death due to mitotic failure during embryonic development is likely to contribute to the proportionate dwarfism that is associated with CENPJ-Seckel syndrome.
Published in the journal: . PLoS Genet 8(11): e32767. doi:10.1371/journal.pgen.1003022
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003022Summary
Disruption of the centromere protein J gene, CENPJ (CPAP, MCPH6, SCKL4), which is a highly conserved and ubiquitiously expressed centrosomal protein, has been associated with primary microcephaly and the microcephalic primordial dwarfism disorder Seckel syndrome. The mechanism by which disruption of CENPJ causes the proportionate, primordial growth failure that is characteristic of Seckel syndrome is unknown. By generating a hypomorphic allele of Cenpj, we have developed a mouse (Cenpjtm/tm) that recapitulates many of the clinical features of Seckel syndrome, including intrauterine dwarfism, microcephaly with memory impairment, ossification defects, and ocular and skeletal abnormalities, thus providing clear confirmation that specific mutations of CENPJ can cause Seckel syndrome. Immunohistochemistry revealed increased levels of DNA damage and apoptosis throughout Cenpjtm/tm embryos and adult mice showed an elevated frequency of micronucleus induction, suggesting that Cenpj-deficiency results in genomic instability. Notably, however, genomic instability was not the result of defective ATR-dependent DNA damage signaling, as is the case for the majority of genes associated with Seckel syndrome. Instead, Cenpjtm/tm embryonic fibroblasts exhibited irregular centriole and centrosome numbers and mono - and multipolar spindles, and many were near-tetraploid with numerical and structural chromosomal abnormalities when compared to passage-matched wild-type cells. Increased cell death due to mitotic failure during embryonic development is likely to contribute to the proportionate dwarfism that is associated with CENPJ-Seckel syndrome.
Introduction
Seckel syndrome is a clinically and genetically heterogeneous primordial dwarfism disorder that is characterised by intrauterine growth retardation, postnatal dwarfism, severe microcephaly, mental retardation, a prominent curved nose and receding chin, together with other clinical abnormalities [1], [2], [3]. Mutations in five loci have been linked with Seckel syndrome: SCKL1 and SCKL2 are due to mutation of the genes for the DNA damage response proteins ATR and CtIP (RBBP8), respectively; SCKL4 and SCKL5 are due to mutation of the genes for the centrosomal proteins CENPJ (Centromere protein J, or centrosomal P4.1-associated protein, CPAP; Figure 1A) and CEP152; while the gene responsible for SCKL3 is currently unknown [4], [5], [6], [7]. Mutations in PCNT (pericentrin), another centrosomal protein, have been associated with both Seckel syndrome and the overlapping dwarfism disorder, microcephalic osteodysplastic primordial dwarfism type II (MOPDII) [8], [9], [10]. Interestingly, mutations in the centrosomal proteins CEP152 (MCPH4) and CENPJ (MCPH6), which are thought to interact with each other during centriole biogenesis [11], [12], have also been associated with primary autosomal recessive microcephaly, a genetically heterogeneous condition caused by mutation of one of eight loci (MCPH1–8; [13], [14], [15]) result in clinically indistinguishable features that include mental retardation and a severely reduced brain size of greater than two standard deviations below the average. Primary autosomal recessive microcephaly presents at birth or becomes apparent within the first few years of life [16]. Primary microcephaly is thought to be caused by a reduction in neurogenesis while the proportionate dwarfism of Seckel syndrome is thought to be the result of premature death of proliferating cells; it is not clear why different mutations in centrosomal proteins cause Seckel syndrome, MOPDII or primary microcephaly.
Fig. 1. Generation of a mouse model of CENPJ-Seckel syndrome. 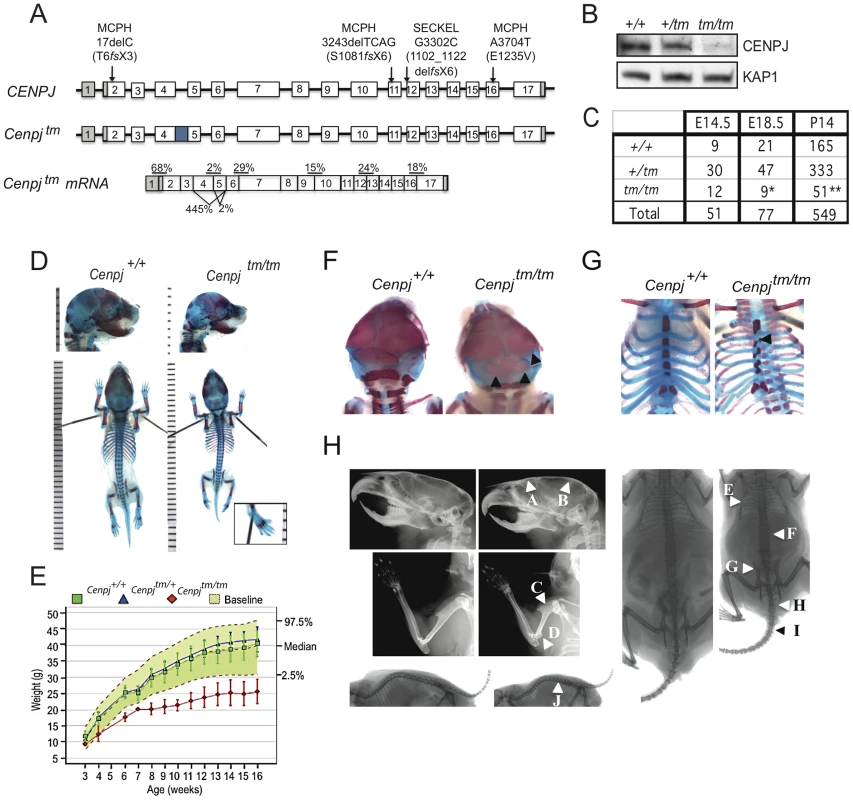
A. The CENPJ gene spans 40 kb and comprises 17 exons. The 3′ and 5′ untranslated regions are depicted in grey. Mutations in CENPJ have been associated with either primary microcephaly (MCPH) or Seckel syndrome (SECKEL). The mutation in intron 11 that has been associated with Seckel syndrome results in the generation of three transcripts: one lacking exon 12, one lacking 11 and 12 and one lacking exons 11,12 and 13. Disruption of mouse Cenpj by insertion of a cassette (depicted by the blue square) between exons 4 and 5 results in low levels of splicing over the cassette and cryptic splicing between exons 3 and 6 or 4 and 6; the latter two transcripts are predicted to result in truncated proteins. The allele was designated Cenpjtm1a(EUCOMM)Wtsi and abbreviated to Cenpjtm. Percentages show mean expression of Cenpj across exon boundaries as determined by quantitative RT-PCR relative to Gapdh for Cenpjtm/tm relative to Cenpj+/+ for RNA extracted from n = 3 murine embryonic fibroblast (MEF) lines. B. Immunoblot to show reduction in Cenpj levels in protein extracted from Cenpjtm/tm (tm/tm), Cenpj+/tm (+/tm), and Cenpj+/+(+/+) MEFs. KAP1 was used as a loading control. C. Table shows frequency of Cenpjtm/tm mice born from heterozygote intercrosses. Cenpjtm/tm showed partial embryonic lethality as shown by their reduced frequency at E18.5 and P14 (25% expected, *P = 0.02, **P = 0.0001, χ2 test). D. Representative images of E18.5 skeletal preparations of Cenpj+/+ and Cenpjtm/tm embryos. Staining with alcian blue (cartilage) and alizarin red (bone). Cenpjtm/tm embryo with a sloping forehead and polysyndactylism of digit one of the left hindpaw (inset). E. Bodyweights of male Cenpjtm/tm (n = 8), Cenpj+/tm (n = 7), Cenpj+/+(n = 40) and baseline wild-type controls (n = 912) from 3–16 weeks of age. Data show that Cenpjtm/tm are significantly smaller than Cenpj+/+ mice at all ages (P = 2.2×10−16, Mann-Whitney-Wilcoxon test). F. Skeletal preparations of E18.5 Cenpjtm/tm embryos showed irregular ossification of the cranium and G. sternum. H. X-Rays show that adult Cenpjtm/tm mice may present with a flatter, sloping forehead (A), mild elevation of the parietal bone (B), a short humerus with a prominent deltoid tuberosity (C), prominent medial epicondyle (D), an irregular ribcage (E), short lumbar and sacral vertebrae (F), an abnormal pelvis (G), extra sacrocaudal transitional vertebrae (H), short, abnormal/fused caudal vertebrae 2/3 – caudal vertebrae 7/8 (I) and reduced intervertebral joint space (J). CENPJ is a conserved, ubiquitously expressed centrosomal protein with a key role in centriole biogenesis [17], [18], [19], [20], [21]. The centrosome is a major microtubule organizing centre in somatic cells that undergoes a duplication cycle that is tightly coupled with DNA replication (reviewed by [22] and [23]). Briefly, in G1 the centrosome is composed of a pair of loosely connected centrioles that are embedded in a proteinaceous matrix. In concert with DNA replication, a single procentriole forms next to each parental centriole during S-phase. The procentrioles continue to elongate and by the onset of mitosis two centrosomes are present, each comprising an older and younger centriole. The two centrosomes aid the formation of the poles of the bipolar spindle, the molecular machinery responsible for correct segregation of sister chromatids into daughter cells. Centrosome attachment to the poles also ensures that each daughter cell inherits a single centrosome, thus tightly regulating ploidy and centrosome numbers. Impaired centrosome duplication cycles or a failure of centrosome segregation result in abnormal centrosome numbers that in turn perturb bipolar spindle assembly and chromosome segregation [24].
CENPJ contains 17 exons and encodes a 1338 amino acid residue protein with a chromosomal segregation ATPase domain and a T-complex protein 10 (TCP10)-like C-terminal domain. Seckel-syndrome of a consanguineous Saudi Arabian family has been associated with a homozygous splice acceptor mutation in the last nucleotide of CENPJ intron 11 (Figure 1A) that results in the production of three transcripts lacking either exon 12, exons 11 and 12 or exons 11, 12 and 13 [4]. Three CENPJ-microcephaly mutations in three consanguineous Pakistani families have been reported to date and all are predicted to cause a truncating stop codon (Figure 1A; [14], [15]).
ATR, RBBP and CEP152 have been shown to play a role in maintaining genomic stability through regulation of the DNA damage response [5], [6], [25], however such a role has not yet been defined for CENPJ. We set out to develop a mouse model of CENPJ-Seckel syndrome in order to establish the mechanism by which mutation of CENPJ results in this subtype of primordial dwarfism. We show that the Cenpj hypomorphic mouse that we created recapitulates many key features of Seckel syndrome, including microcephaly with memory impairment, dwarfism from birth, and skeletal abnormalities. We further establish that wide-scale genomic instability is the likely cause of cell death within Cenpjtm/tm embryos and suggest that this contributes to the developmental phenotypes observed in CENPJ-Seckel patients.
Results
Generation and phenotyping of a Cenpj hypomorphic mouse
Knockout mice carrying the Cenpjtm1a(EUCOMM)Wtsi allele (Figure 1A and Figure S1A) were generated on a C57BL/6NTac; C57BL/6-Tyrc-Brd background by the Sanger Mouse Genetics Project as part of the European Conditional Mouse Mutagenesis Program (EUCOMM; [26]). Correct gene targeting in founder mice was determined by a combination of standard and quantitative PCR (Figure S1). LacZ staining was detected in the brain and kidneys, while strong staining was present in the testes of mice heterozygous for the Cenpjtm1a(EUCOMM)Wtsi allele (Figure S2A).
The tm1a(EUCOMM)Wtsi gene-trap cassette that was introduced into the Cenpj locus is designed to truncate mRNA expression and to generate out-of-frame products following the deletion of a critical exon. Previous studies have indicated that mRNAs of certain microcephaly-associated genes are very stable [27] prompting us to perform a detailed analysis of expression and splicing at the Cenpjtm1a(EUCOMM)Wtsi locus. We generated Cenpjtm1a(EUCOMM)Wtsi/tm1a(EUCOMM)Wtsi (Cenpjtm/tm) mouse embryonic fibroblasts (MEFs; 13.5 d.p.c.) and performed SYBR Green qPCR on cDNA using primers spanning the boundaries between different exons (Figure 1A). We observed a low but detectable amount of splicing over the gene-trap cassette in Cenpjtm/tm MEFs (2.1±0.5% of wildtype exon 4–5 levels) and immunoblotting (Figure 1B) confirmed the production of low levels of apparently full-length Cenpj protein [27]. Splicing from exons 3 to 6 and 4 to 6 was detected in both Cenpjtm/tm and wildtype MEFs (Figure S2B). Between exons 3 and 6 the level of splicing detected in Cenpjtm/tm MEFs was increased relative to the levels in control MEFs (444±95%), while decreased levels of splicing were observed between exons 4 and 6 (2.1±0.5%). Using the web-based ExPASy translation tool (http://web.expasy.org/translate/) we predict that mRNAs that are spliced between exons 3–6 and exons 4–6 lead to the production of proteins truncated in exon 6 (Figure S2C). Upstream of the tm1a(EUCOMM)Wtsi cassette (exons 1–2) Cenpj mRNA levels were 68±19% of wildtype levels. Downstream (from exon 6 to 17) of the tm1a(EUCOMM)Wtsi cassette Cenpj mRNA levels were approximately 20% of the levels observed in MEFs from wildtype littermates (mean±SEM, n = 3). In summary, Cenpjtm/tm MEFs are able to produce small amounts of full-length Cenpj protein due to splicing over the tm1a(EUCOMM)Wtsi gene-trap cassette (exons 4–5) and we predict that small amounts of truncated, N-terminal Cenpj protein (corresponding to exons 1 to 3 or 1 to 4) will also be produced.
Phenotyping of mice was performed at the Wellcome Trust Sanger Institute (http://www.sanger.ac.uk/mouseportal/search?query=cenpj). Cenpjtm1a(EUCOMM)Wtsi/+ intercrosses gave close to the expected Mendelian frequency (25%) of homozygote embryos at E14.5 (23.5%); however, by E18.5 this had reduced to 11.6%, suggesting that disruption of Cenpj causes partial embryonic lethality between E14.5 and E18.5 (χ2 test, P = 0.02; Figure 1C). The majority of runted pups identified between P0 and P21 were Cenpjtm/tm (22% of Cenpjtm/tm offspring vs. 4.7% for Cenpj+/tm, and 2.5% for Cenpj+/+). Stunted growth was unlikely to be the result of a major feeding problem, since milk spots were observed in the stomachs of pups of all genotypes at P0. At P14, the frequency of Cenpjtm/tm mice was not significantly different to that found at E18.5 (P14 : 9.2% vs. E18.5 : 11.6%; Figure 1C), suggesting that although dwarfed, Cenpjtm/tm mice are not postnatally sub-viable.
Cenpj-deficiency causes intrauterine and postnatal growth retardation
One of the defining characteristics of primordial dwarfism disorders, such as Seckel syndrome, is a fetus that is small for its gestational age with postnatal growth retardation [8], [28], [29]; specifically, the Saudi Arabian CENPJ-Seckel kindred all have anthropometric values at least seven standard deviations below the mean [4]. Cenpjtm/tm mice showed intrauterine growth retardation (Figure 1D; mean ± SEM bodyweight at E18.5, Cenpj+/+ 1.12±0.03 g, Cenpjtm/tm 0.8±0.04 g; P = 0.0001, t-test; crown-rump length at E18.5, Cenpj+/+ 23.5±0.26 mm, Cenpjtm/tm 20.7±0.54 mm; P = 0.0001, t-test). From 3–16 weeks, Cenpjtm/tm mice were significantly smaller than wild-type controls (P = 2.2×10−16, Mann-Whitney-Wilcoxon test; Figure 1E). The body weight of adult Cenpjtm/tm animals was 64% of wild-type controls (mean ± SEM bodyweight at 16 weeks, Cenpj+/+ 39.1±1.35 g, Cenpjtm/tm 25.4±1.34 g; P = 9.3×10−11, t-test; Figure 1E) and length was 76% of controls (mean ± SEM nose-to-tail base length at 14 weeks, Cenpj+/+10.4±0.05 cm, Cenpjtm/tm 7.9±0.07; P = 2.2×10−16, t-test; Figure S2D).
Skeletal abnormalities and abnormal ossification of bone from Cenpjtm/tm mice
The skeletal abnormalities of Seckel syndrome associated with an intron 11 mutation in CENPJ include a receding chin, high forehead and prominent nasal spine [4]. Although the facial features of the CENPJ-Seckel kindred (two siblings and three cousins) were strikingly similar, the skeletal survey of sibling one was largely normal and that of sibling two revealed 11 ribs instead of 12 and a steep acetabular roof [4]. These findings highlight the fact that the same mutation results in clinical heterogeneity and prompted us to perform a thorough skeletal analysis of Cenpjtm/tm embryos and adult mice; Cenpjtm/tm embryos had significantly smaller skulls (Figure 1D and 1H; mean ± SEM at E18.5: skull length, Cenpj+/+ 9.8±0.12 mm, Cenpjtm/tm 9.3±0.17 mm, P = 0.0379; inner canthal distance Cenpj+/+ 3.22±0.05 mm, Cenpjtm/tm 2.96±0.09 mm, P = 0.0198) and adult mice presented with a flatter, sloping forehead and mild elevation of the parietal bone compared to controls (Figure 1H; 16 weeks of age, n = 8 males and n = 7 females). Although the Saudi Arabian CENPJ-Seckel kindred do not have clinodactyly (curvature of the fifth finger), it is a frequently reported characteristic of Seckel patients [1], [4], [29], [30]. We did not observe clinodactyly in Cenpjtm/tm mice, however we noted polysyndactylism of the first digit of the left hind paw in 2/9 Cenpjtm/tm embryos (Figure 1D, inset), which has also been reported in mutant Pcnt (pericentrin) mice [31]. Furthermore, retarded ossification and decreased bone age is reported in the majority of cases of Seckel syndrome, although this clinical abnormality was not specifically addressed for the CENPJ-Seckel kindred [1]. A higher proportion of Cenpjtm/tm embryos showed incomplete or irregular ossification of the parietal and occipital bones when compared to controls (Figure 1F; 3/5 Cenpjtm/tm, 1/38 Cenpj+/tm, 1/13 Cenpj+/+). In addition, a subset of Seckel patients, including a CENPJ-Seckel patient, have 11 ribs instead of the usual 12 [4], [30], [32]. Although all ribs were present in Cenpjtm/tm embryos, we noted that the attachment of the ribs to the sternum followed an irregular pattern that closely corresponded to the asymmetrical distribution of ossification centers along the sternum (Figure 1G; 3/5 Cenpjtm/tm). Adult Cenpjtm/tm mice displayed an irregular ribcage, with crowding of the ribs (Figure 1H; 9/15). Moreover, a subset of Seckel patients have been reported to have bilateral dislocation of the hips and elbows, with a decreased range of motion at the elbows [1]. While we did not find any evidence of dislocation we noted that the humeri of adult Cenpjtm/tm mice were anatomically disproportionate when compared with those of wild-type mice; the deltoid tuberosities were closer to the greater tubercle when normalized to humeri length (mean±SEM.: right humeri, Cenpj+/+ 49.6±0.28%, Cenpjtm/tm 47.1±0.93%; P = 0.02, t-test; Figure 1H), and humeri were sometimes bowed (6/15) with a very prominent medial epicondyle (11/15; Figure 1H). Furthermore, all adult Cenpjtm/tm mice (15/15) displayed an abnormal pelvis that was wider at the iliac crests (Figure 1H, iliac crest normalised to ischiac, mean±S.E.M.: Cenpj+/+ 69.7±1.18%, Cenpjtm/tm 60.8±1.72%; P = 0.0003) and sometimes asymmetrical. Finally, we observed that all Cenpjtm/tm mice had a reduced intervertebral joint space in the lumbar and caudal regions (Figure 1H). In general, lumbar and sacral vertebrae were shorter and Cenpjtm/tm mice had one to two extra sacrocaudal transitional vertebrae as a result. In 13/15 Cenpjtm/tm mice, Caudal 2/3 – Caudal 7/8 were abnormal in morphology and fused (Figure 1H).
Neuropathological abnormalities and memory impairment
Microcephaly is one of the defining characteristics of Seckel syndrome [1]. Microcephaly has been clinically defined as a head circumference of at least two standard deviations below the normal range; and in the case of Seckel syndrome associated with mutations in intron 11 of CENPJ, head circumference is seven standard deviations below the mean [4], [33]. The average Cenpjtm/tm mouse brain weight was two standard deviations below that of control mice (Figure 2A; P = 0.0002, t-test). Although the two and four year-old siblings with CENPJ-Seckel syndrome described to date had relatively normal magnetic resonance imaging (MRI), cranial MRI of adult patients with Seckel syndrome has revealed several neuroanatomical abnormalities aside from a reduction in brain volume [4], [32], [34], [35]. We therefore assessed the area of brain regions and the thickness of the neuronal layers of the adult mouse brain (16 weeks; Figure S3). Although the patterning of the hippocampal layers appeared normal, the length of the dentate gyrus was significantly reduced in Cenpjtm/tm when compared to Cenpj+/+ control mice (mean±SEM.: Cenpj+/+ 4380±64 µm, Cenpjtm/tm 3797±181 µm, P = 0.01, t-test; Figure 2B). The average thickness of the cortex, which is often reduced with mutation of microcephaly genes in mice [36], [37], and of the molecular, striatum radiatum and oriens layers of the hippocampus were not significantly different to wild-type controls. Similarly, the total areas of the hippocampus, corpus callosum and dorsal third ventricle were unchanged as was the total internal length of the pyramidal cell layer.
Fig. 2. Neuropathological abnormalities. 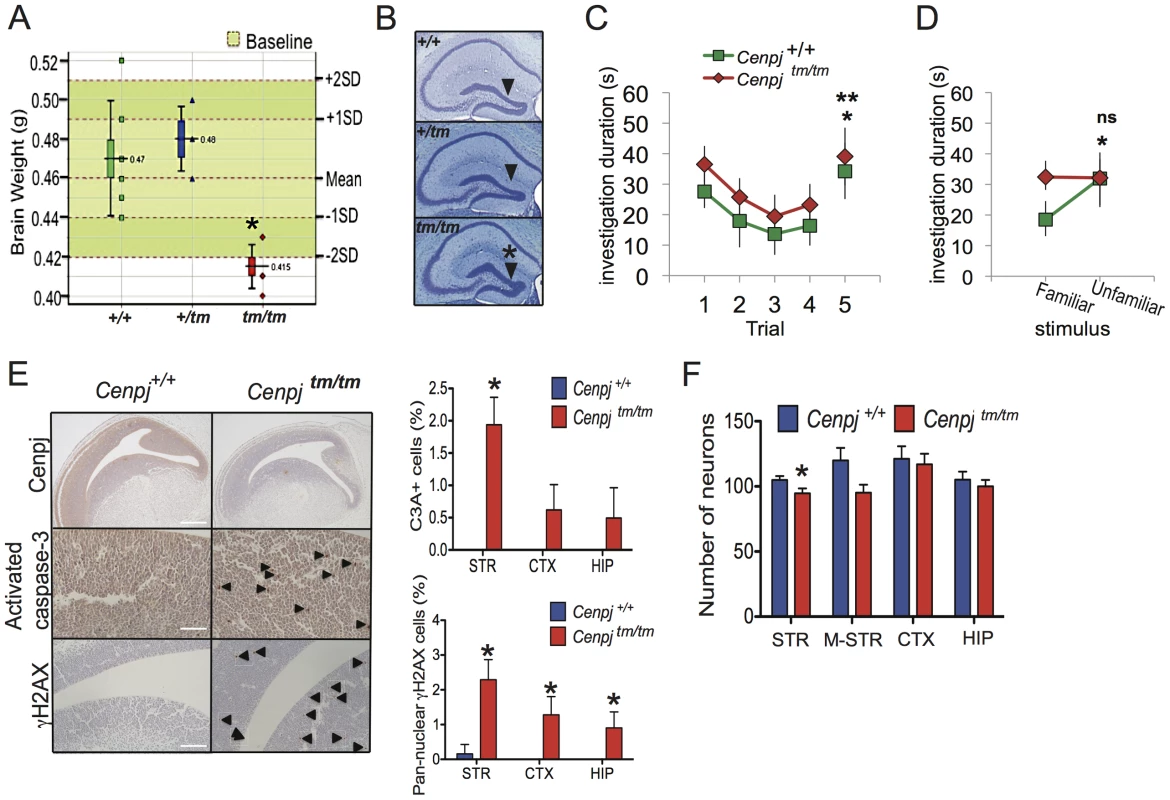
A. Cenpjtm/tm mouse brain weights were two standard deviations below that of control mice (n = 144 baseline control mice). *P = 0.0002, t-test, Cenpjtm/tm, n = 6 and Cenpj+/+ n = 10. The lower whisker extends to the lowest datum still within 1.5 Inter-quartile range (IQR) of the lower quartile. The upper whisker extends to the highest datum still within 1.5 IQR of the upper quartile B. The dentate gyrus was significantly shorter in Cenpjtm/tm mice (n = 3) when compared to Cenpj+/+ control mice (n = 30), *P = 0.01, t-test. Scale bar 1 mm C. Social recognition test. When tested for habituation-dishabituation, both Cenpjtm/tm (n = 7) and Cenpj+/+ (n = 7) mice recognized a novel stimulus mouse as shown by a decline in investigation time over the first four trials that was recovered on trial five, when they were exposed to a novel mouse (trial four vs. trial five, * P = 0.0033 and ** P = 0.0014, two-way ANOVA followed by post-hoc t-test). D. A discrimination test was performed 24 h later the habituation-dishabituation test. When given a choice between the familiar (same stimulus animal used for trials one to four) and a new unfamiliar mouse 24 h later, Cenpjtm/tm mice could not discriminate as shown by the similar investigation time for both stimulus animals (Cenpj+/+ P = 0.0326, Cenpjtm/tm P = 0.957, t-test). E. Representative images of immunohistochemical stainings of E14.5 embryo sections. Cenpj was highly expressed in areas of active neurogenesis within the telencephalon. Scale bar 400 µm. There was a generalized increase in cleaved (activated) caspase-3-positive (scale bar 100 µm) and Ser139-phosphorylated H2AX (γH2AX; scale bar 200 µm) cells throughout embryos, images of striatum are shown.. The number of cells positive (as a percentage of total in two different 75 µm2 areas) for cleaved (activated) caspase-3 (C3A+) and pan-nuclear Ser139-phosphorylated H2AX (γH2AX) was increased in areas of active neurogenesis within the striatum and cortex. *P<0.05; Mann-Whitney with continuity correction, Cenpjtm/tm n = 3 and Cenpj+/+ n = 3. Data shows mean and SEM. F. Neuron densities were counted in three different areas (75 µm2) of active neurogenesis for each of the striatum (STR), cortex (CTX) and pro-hippocampus (HIP) and three areas of 150 µm2 in the mid-striatum (M-STR) of E14.5 embryos, *P = 0.0008, t-test, Cenpjtm/tm n = 3 and Cenpj+/+ n = 3. Data shows mean and SEM. History was suggestive of normal cognitive and motor development for four of the five cases within the CENPJ-Seckel kindred while one patient clearly had intellectual impairment (IQ 60; MRI not performed [4]). Since the hippocampus is involved in learning and memory formation and since Seckel patients generally display learning impairments [3], [34], we performed a social recognition test with Cenpjtm/tm and control animals [38], [39], [40]; Figure 2C and 2D). Thus, on day one, mice were tested for habituation-dishabituation: male mice were presented with a novel, anaesthetized stimulus mouse and the time of investigation was recorded. Mice were then given a 10 minute resting period before this was repeated a further three times with the same stimulus mouse. On the fifth trial, mice were presented with an unfamiliar stimulus mouse (Figure 2C). Both Cenpjtm/tm (n = 7) and Cenpj+/+ (n = 7) mice recognized and habituated to the novel stimulus mouse, as there was a decline in investigation time over the first four trials that was recovered on trial five (Figure 2C, two-way ANOVA, repeated measures for trial F4,48, = P<0.001, effect for genotype F1,48 = 0.5482, P = 0.433, interaction F4,48 = 0.09258, P = 0.9844), when they were exposed to a novel mouse (Figure 2C. Trial four vs. trial five, P = 0.0033 and P = 0.0074, post-hoc t-test). These data suggest that olfaction in Cenpjtm/tm mice is not markedly affected. Twenty-four hours after the habituation-dishabituation test, a discrimination-based olfactory memory test was performed. When given a choice between the familiar (same stimulus animal used for trials one to four) and a new unfamiliar mouse, Cenpj+/+ animals spent less time investigating the familiar mouse than the unfamiliar one (Figure 2D. P = 0.0326, t-test). However, Cenpjtm/tm mice were less able to recognize the familiar from the unfamiliar animal as shown by the similar investigation time for both stimulus animals (Figure 2D. P = 0.957, t-test; Normalized discrimination Cenpj+/+ vs Cenpjtm/tm, P = 0.0417). In summary, short-term memory and olfaction appear to be unaffected in Cenpjtm/tm mice, however long-term memory was significantly impaired. All other tests of neurological function were normal, including open field, grip strength, modified SHIRPA (the SmithKline Beecham, Harwell, Imperial College, Royal London Hospital, phenotype assessment is a set of behavioural tests designed to test muscle, cerebellar, sensory and neuropsychiatric function), auditory brainstem response and hot plate assessment.
Increased DNA damage, apoptosis, and reduced neuron density in Cenpjtm/tm mice
All of the genes associated with Seckel syndrome have so far been shown to result in defective DNA damage responses and a lowered apoptotic threshold [5], [6], [7], [25], [41]. To test whether Cenpj-deficiency is associated with elevated levels of DNA strand breaks and/or apoptosis, we performed immunohistochemical staining of E14.5 embryos. Phosphorylation of histone H2AX on serine 139 (γH2AX) by the ATR, DNA-PK or ATM kinases occurs at sites flanking DNA strand breaks and enhances the recruitment of DNA repair proteins to sites of damage [42]; if the damage is irreparable then the cell death cascade is normally activated [43]. Compared to controls, there was a general increase in the number of γH2AX-positive cells throughout Cenpjtm/tm embryos (Figure S4) and this was most pronounced in the developing telencephalon (Figure 2E; mean±SEM. γH2AX-positive cells as a percentage of total: striatum Cenpj+/+ 0.47±0.2%, Cenpjtm/tm 2.3±0.3%, P = 0.004; cortex Cenpj+/+ 0.0%, Cenpjtm/tm 1.3±0.3%, P = 0.01; pro-hippocampus Cenpj+/+ 0.0%, Cenpjtm/tm 0.9±0.3%, P = 0.03. Mann-Whitney with continuity correction). Similarly, there was an increase in the number of cleaved caspase-3-positive cells throughout Cenpjtm/tm embryos (Figure S4), although this was most pronounced in areas of active neurogenesis (as determined by Ki67 staining; Figure S4) within the telencephalon, where Cenpj was most highly expressed (Figure 2E; mean±S.E.M. cleaved caspase-3-positive cells as a percentage of total cells: striatum Cenpj+/+ 0.0%, Cenpjtm/tm 1.9±0.2%, P = 0.001; cortex Cenpj+/+ 0.0%, Cenpjtm/tm 0.6±0.2%, P = 0.05; pro-hippocampus Cenpj+/+ 0.0%, Cenpjtm/tm 0.5±0.3%, P = 0.14, t-test). There was no detectable difference in the patterns of cellular proliferation between Cenpjtm/tm and Cenpj+/+ embryos when examined using the marker Ki67 (Figure S4).
Consistent with increased levels of apoptosis in the developing telencephalon of Cenpjtm/tm embryos, reports of fetal stage Seckel syndrome (loci responsible unknown) have shown reduced neuron density and disorganization of cortical layers at 30 weeks gestation [28], [29]. We therefore quantified neuron densities in areas of active neurogenesis within the telencephalon (areas of Ki67-positive staining; Figure S4) and in the mid-striatum of embryos during mid-neurogenesis (E14.5) and found that, in general, the number of neurons were decreased in Cenpjtm/tm embryos and the reduction was significant for the striatum (Figure 2F; mean±SEM. for n = 3 (average of two different 75 µm2 areas) in striatum: Cenpj+/+ 104.9±3.1, Cenpjtm/tm 94.7±3.8, P = 0.0008, t-test).
Delayed puberty of female Cenpjtm/tm mice
Several clinical reports of patients with Seckel syndrome have described precocious puberty or premature thelarche [44], [45]. It is not yet known whether the sexual development of patients with Seckel syndrome associated with CENPJ mutations is normal since the cases described so far report the phenotype of infants [4]. A thorough histopathological analysis of adult male and female Cenpjtm/tm and Cenpj+/tm mice (n = 3 of each gender and genotype at 16 wks) revealed several anomalies, including corticomedullary pigmentation in the adrenals of female Cenpjtm/tm mice (Figure 3A). Corticomedullary pigmentation is associated with ‘X-zone’ degeneration in female mice, a sex hormone-dependent change that occurs during puberty in virgin females and is often complete by 16 weeks in C57BL/6 mice, or earlier in pregnant females [46]. By using cleaved-caspase 3 as a marker of apoptosis, we found that the adrenals of virgin Cenpjtm/tm female mice have pronounced and ongoing X-zone degeneration at 16 weeks when compared to virgin Cenpj+/+ females (Figure 3A). Wild-type C57BL/6 female mice reach sexual maturity at around 6–7 weeks of age. These findings suggest that, in contrast to Seckel syndrome patients, puberty is delayed in Cenpjtm/tm female mice. In support of this, breeding records of females set up with Cenpjtm/tm males at 6–7 weeks of age showed that Cenpjtm/tm females produce their first litter around four weeks later than Cenpj+/+ females (P = 0.012, t-test; Figure 3B). There were no morphological differences in the reproductive tract of male or female Cenpjtm/tm animals when examined at 16 weeks of age (data not shown).
Fig. 3. Delayed onset to puberty and ocular, endocrine, haematological, and plasma abnormalities. 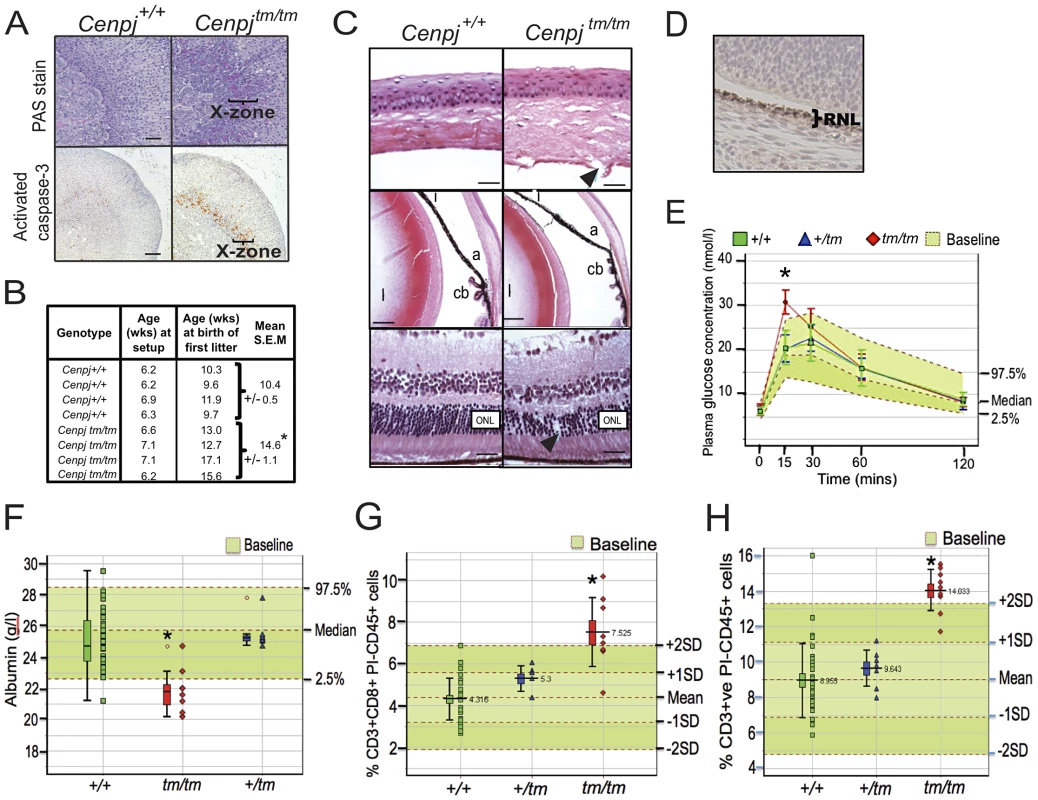
A. Periodic acid-Schiff (PAS) staining and cleaved (activated) caspase-3 immunostaining of adrenal sections from 16 week-old virgin female Cenpjtm/tm mice (n = 3) confirmed corticomedullary pigmentation and ongoing apoptosis in the X-zone, respectively (representative images, scale bars 100 µm). B. Breeding records of Cenpjtm/tm females set up with Cenpjtm/tm males at 6–7 weeks of age showed that Cenpjtm/tm females produce their first litter around four weeks later than Cenpj+/+ females. *P = 0.012, t-test. C. Top panel shows normal cornea from a Cenpj+/+ mouse. Cenpjtm/tm mice had disruption of the Descemet's membrane and corneal endothelium (arrow). Middle panel shows normal anterior segment from a Cenpj+/+ mouse. The angle was displaced anteriorly in eyes from Cenpjtm/tm mice and ciliary process morphology was abnormal. (a, angle; i, iris; cb, ciliary body; l, lens). Bottom panel shows normal retina from a Cenpj+/+ mouse eye. The retina photoreceptor cells of Cenpjtm/tm mice were reduced in number and showed columnar disorganized (arrow). (ONL, outer nuclear layer). D. Immunohistochemical staining for Cenpj in Cenpj+/+ embryo eye (E14.5; RNL retinal neuroblast layer). E. Intra-peritoneal glucose tolerance test to show that female Cenpjtm/tm mice have a 15 minute delay in response to glucose challenge (n = 4 Cenpjtm/tm vs. n = 32 Cenpj+/+, *P = 2×10−5, t-test). Graph also shows n = 9 Cenpjtm/+ and n = 670 baseline wildtype controls. F. Plasma albumin levels were decreased in Cenpjtm/tm males (n = 8 Cenpjtm/tm vs. n = 35 Cenpj+/+, *P = 4.9×10−5, t-test). Graph also shows n = 7 Cenpjtm/+ and n = 768 baseline wildtype controls. G. Flow cytometric analysis of peripheral blood leukocytes in Cenpjtm/tm mice revealed an increase in the number of CD8+CD3+ and H. total CD3+ cells. Data shows total counts per 30 000 propidium-iodide (PI) negative, CD45-positive cells from male mice. For n = 9 Cenpjtm/tm vs. n = 30 Cenpj+/+: CD3+CD8+ *P = 0.0002 and CD3 *P = 2.9×10−5, Mann-Whitney-Wilcoxon test. Graphs also show n = 7 Cenpj+/tm and n = 356 baseline wildtype controls. For all ‘Box and Whisker’ plots, the lower whisker extends to the lowest datum still within 1.5 Inter-quartile range (IQR) of the lower quartile. The upper whisker extends to the highest datum still within 1.5 IQR of the upper quartile. Abnormal development and structural abnormalities of the eye in Cenpjtm/tm mice
Although not reported for the CENPJ-Seckel kindred [4], several individuals that have been clinically diagnosed with Seckel syndrome have ocular defects, such as spontaneous lens dislocation, myopia, astigmatism, and retinal degeneration [47] [48]. A higher proportion of Cenpjtm/tm embryos had secondary anophthalmia (E18.5, 0/13 Cenpj+/+, 1/38 Cenpj+/tm and 1/5 Cenpjtm/tm) and a higher proportion of Cenpjtm/tm pups still had their eyes closed at P14 (5/28 Cenpjtm/tm vs. 1/46 wild-type). At 16 weeks of age, histological analysis of the eyes from Cenpjtm/tm mice showed various structural abnormalities. In the anterior segment, the corneal endothelium and Descemet's membrane was occasionally broken (Figure 3C). The anterior chamber was of normal depth but the angle was anteriorly displaced in some cases (Figure 3C). Significant cataracts were not observed in Cenpjtm/tm mice but the iris showed adhesions to the lens and its base was anteriorly shifted in relation to the ciliary body (Figure 3C). Also, the ciliary body processes were spaced far apart or blunted in Cenpjtm/tm animals, and in some cases, ciliary process morphology was abnormal (Figure 3C). In the retina, the photoreceptor nuclei were variably reduced in number and columns were loosely packed or disorganized in Cenpjtm/tm animals (Figure 3C). Other cell layers, including the retinal ganglion, inner nuclear, and retinal pigment epithelium appeared normal, and the optic nerve did not show thinning. At E14.5, Cenpj was highly expressed in the retina neuroblast layer, where cells are rapidly differentiating and proliferating, but not in the inner retinal ganglion cell progenitor layer (Figure 3D).
Delayed response to glucose challenge in Cenpjtm/tm mice
During routine phenotyping, mice were subject to an intra-peritoneal glucose tolerance test, in which mice were fasted for 16 hours, a bolus of glucose was administered intraperitoneally and blood glucose concentration was monitored for 2 hours. Fifteen minutes after administration of glucose, 4/4 female Cenpjtm/tm mice (Cenpj+/+ 20.5±0.7 mmol/l, Cenpjtm/tm 30.7±1.60 mmol/l, P = 2×10−5, t-test) and 2/5 male Cenpjtm/tm had blood glucose levels greater than or equal to the 97.5th centile of baseline controls (n = 670 females, n = 669 males), although this had returned to normal by 30 minutes (Figure 3E).
Cenpj-deficiency is associated with karyomegaly of cardiomyocytes in young mice
Despite being one of the less frequently reported characteristics of Seckel syndrome, there are numerous case-reports of severe cardiac anomalies in Seckel syndrome patients [49], [50], [51], [52], [53]. Strikingly, the majority of 16-week old Cenpjtm/tm mice (5/6) and only 1/6 Cenpj+/tm and 0/4 wildtype mice showed disorganization of cardiomyocytes with an increased incidence of karyomegaly and multinucleate cells, predominantly within the interventricular septum, papillary muscle and inner myocardium (Figure S2E). Cardiomyocyte karyomegaly has previously been observed in wild-type mice [54] where it may be associated with reparative processes [55] and may represent polyploidy [56]. Although the incidence and extent of karyomegaly was noticeably increased in hearts from Cenpjtm/tm mice compared to wildtype animals in this study, there was no evidence of fibrosis (consistent with previous cardiac damage) based on trichrome staining or alterations in apoptosis or proliferation (cleaved caspase-3 and Ki67, respectively; data not shown). Interestingly, the preponderance of karyomegaly in cardiomyocytes, hepatocytes and cells of the Harderian glands was increased in aged Cenpjtm/tm mice (13-month old) when compared to age-matched control (Figure S2E).
Hypoalbuminemia of Cenpjtm/tm mice
Clinical chemistry was performed on animals at 16 weeks of age. Albumin levels were generally decreased in Cenpjtm/tm mice of both genders compared to controls, and this was statistically significant for males (mean±S.E.M, Cenpj+/+ 25.1±0.31, Cenpjtm/tm 21.9±0.52, P = 4.9×10−5, t-test; Figure 3F).
Increased levels of CD3+CD8+ T cells in Cenpjtm/tm mice
Flow cytometric analysis of peripheral blood leukocytes at 16 weeks of age revealed a marked increase in the frequency of the CD8 T cell subset (CD3+ CD8+) in both genders of Cenpjtm/tm mice compared to wild-type controls (mean±S.E.M Males: CD8+CD3+ gated on PI− CD45+: Cenpj+/+ 4.3%±0.18, Cenpjtm/tm 7.5%±0.55, P = 0.0002 Mann-Whitney-Wilcoxon test; Figure 3G). Furthermore, the increase in the proportion of the peripheral blood CD8 T cell population was reflected in a concomitant increase in the frequency of total T cells in Cenpjtm/tm mice (mean±S.E.M, CD3+ gated on PI− CD45+: Cenpj+/+ 9%±0.39, Cenpjtm/tm 14%±0.41, P = 2.9×10−5, Mann-Whitney-Wilcoxon test; Figure 3H). The frequency of CD4 T cells in the peripheral blood was unaffected (data not shown).
Increased genomic instability in embryonic fibroblasts from Cenpj-deficient mice
CENPJ depletion in cultured cells has been reported to impair centriole assembly, disrupt centrosome integrity and lead to the formation of monopolar and multipolar spindles instead of bipolar spindles [19], [20], [21], [24], [57]. To assess the cellular phenotype of Cenpjtm/tm mice, MEFs were derived from Cenpj+/+, Cenpj+/tm and Cenpjtm/tm littermates. For all experiments, MEFs were of early passage (P<5) and were passage-matched. Cenpj protein levels were reduced in the centrosomes of Cenpjtm/tm MEFs, but residual protein remained detectable in most cells (Figure 4A). Consistent with previous findings, frequencies of both monopolar and multipolar spindles were elevated in two independently derived Cenpjtm/tm MEF lines (Figure 4B; Cenpj+/+: 2.1% monopolar and 8% multipolar; Cenpjtm/tm (1): 10.6% monopolar and 20.8% multipolar; Cenpjtm/tm (2): 8.9% monopolar and 18.9% multipolar). Distribution and intensities of the centrosomal proteins γ-tubulin (Figure 4A), CDK5RAP2 (Figure 4B), and pericentrin (data not shown) were unaffected in the mutant. We next asked whether spindle abnormalities are accompanied by aberrant centrosome and centriole numbers in Cenpjtm/tm MEFs. A normal mitotic cell contains two centrosomes, each containing a pair of centrioles. Supernumerary centrioles were visible in mitotic Cenpjtm/tm cells (Figure S5). To facilitate counting of centrioles, cells were arrested in mitosis using monastrol, a microtubule motor poison that prevents separation of spindle poles and thereby generates monopoles [58]. Cells with three or four centrioles were considered normal, since it is not always possible to resolve centrioles within a pair. We observed an increase in cells containing both too few (≤2) and too many centrioles (≥5) in the mutant (Figure 4C). To survive, cells with supernumerary centrosomes must either inactivate these or cluster active centrosomes into two poles, a process that ensures bipolar division [23], [59]. Clustered centrosomes were indeed observed in Cenpjtm/tm MEFs (Figure 4B). Centrosome clustering however does not prevent unequal partitioning of centrosomes into daughter cells (Figure S5), which ultimately causes a disassociation between centrosome numbers and DNA ploidy.
Fig. 4. Centrosome and mitotic spindle abnormalities are elevated in Cenpj-deficient cells. 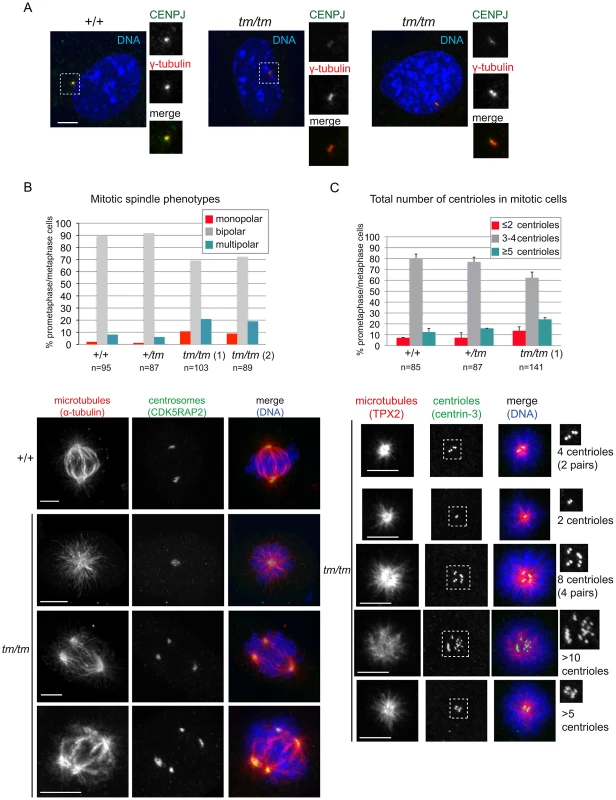
A. Images show examples of Cenpj staining in centrosomes of Cenpj+/+ and Cenpjtm/tm mouse embryonic fibroblasts (MEFs). Cells were stained with antibodies against Cenpj (green in merge) and the centrosomal protein γ-tubulin (red in merge). Framed areas are shown at higher magnification. B. Graph shows mitotic spindle phenotypes in MEFs derived from Cenpj+/+, Cenpj+/tm and two independent Cenpjtm/tm embryos (littermates, +/+ MEFs passage 4, +/tm and tm/tm MEFs passage 3): tm/tm (1) and tm/tm (2). Number of mitotic cells scored are shown for each genotype. Examples for monopolar and multipolar spindle are shown. Note cell on bottom panels forming a bipolar spindle by clustering supernumerary centrosomes. Cells were stained with antibodies against α-tubulin (green in merge) and the centrosomal protein, Cdk5RAP2 (red in merge). C. Graph shows centriole numbers in mitotic MEFs of indicated genotypes (littermates, +/+ MEFs passage 4, +/tm and tm/tm MEFs passage 3). Cells were arrested in mitosis with monastrol that caused monopolar spindle formation and facilitated visualization of centrioles. Note that mitotic cells should normally contain a total of 4 centrioles, but even in wild-type cells we occasionally detect 3 centrioles probably due to insufficient spatial resolution, so 3 or 4 centrioles were considered a single class. Data were collected from two independent experiments; bars show mean ±SD, number of mitotic cells scored are shown for each genotype. Images below depict examples for cells with different centriole numbers (top cell with 4 centrioles is normal, all other cells have too few or too many centrioles). Cells were stained with antibodies against the microtubule-binding protein Tpx2 (green in merge) and the centriolar protein, centrin-3 (red in merge). Framed areas are shown at higher magnification. Scale bars = 5 µm. Cell-cycle analysis of Cenpjtm/tm MEFs revealed a significant increase in the number of 4C and elevated levels of >4C cells (Figure 5A), indicative of polyploidy. We examined the ploidy of fifty metaphase spreads of Cenpjtm/tm and Cenpj+/+ MEFs and found that a remarkably high percentage of Cenpjtm/tm cells were near tetraploid (Cenpj+/+ 11% vs. Cenpjtm/tm 41%). Twenty metaphase spreads with good fluorescent in situ hybridization (FISH) signals were selected for multiplex-FISH karyotyping which confirmed that many cells were near tetraploid and revealed additional defects such as aneuploidy, centromere loss, centric fusions (Figure 5B) and translocations (Figure 5C; for a breakdown of anomalies see Figure S2F). Consistently, we found evidence of lagging chromosomes in anaphase Cenpjtm/tm MEFs (Figure S5). Furthermore we show that adult Cenpjtm/tm mice have an increased prevalence of micronucleated normochromatic erythrocytes (P = 0.000004, t-test; Figure 5D), thus confirming that these mutants have spontaneous genomic instability.
Fig. 5. Genomic instability is associated with abnormal ploidy of Cenpjtm/tm cells rather than an impaired DNA damage response. 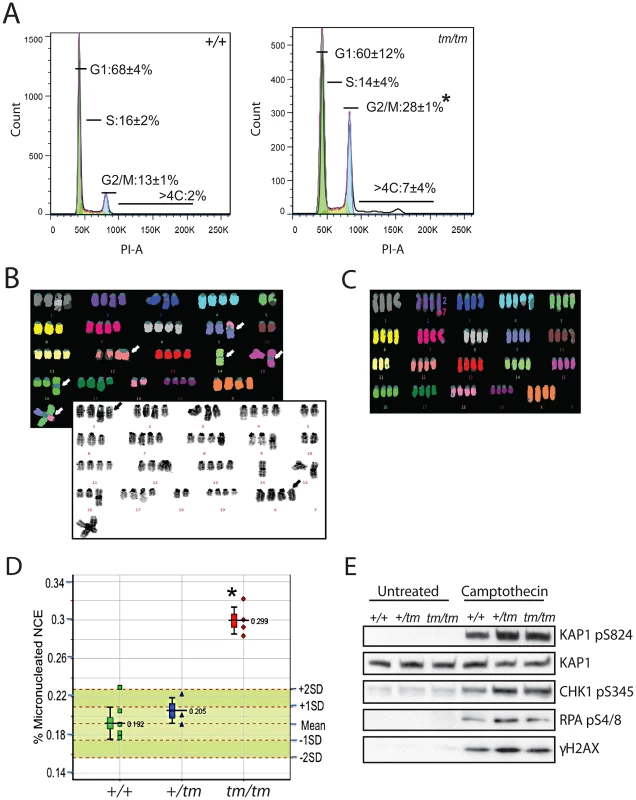
A. Cell cycle analysis of Cenpjtm/tm mouse embryonic fibroblasts (MEFs) by flow cytometry showed an increase in the percentage of cells in G2 (4C) and cells containing >4C DNA content when compared to Cenpj+/+ cells. Percentages represent means of n = 3 independent MEF lines per genotype (each pair of +/+ and tm/tm cells were passage-matched (passage<5) and derived from littermates), *P<0.05, t-test. PI, propidium iodide. B. Example multiplex fluorescent in situ hybridization (M-FISH; top) and DAPI banded (bottom) karyotype of a Cenpjtm/tm MEF metaphase (passage 4). The karyotype is near tetraploid, with centric fusions (white arrows) and chromosomes that have apparently lost their centromeres (black arrows). C. Example M-FISH of a Cenpjtm/tm MEF metaphase (passage 4) showing near tetraploid karyotype with a translocation (t(2;7)). D. Adult Cenpjtm/tm (n = 4) mice showed increased genomic instability when compared to Cenpj+/+ mice (n = 6) as determined by the increased prevalence of micronucleated normochromatic erythrocytes using a flow cytometric assay of micronucleus formation. *P = 0.000004, t-test. The lower whisker extends to the lowest datum still within 1.5 Inter-quartile range (IQR) of the lower quartile. The upper whisker extends to the highest datum still within 1.5 IQR of the upper quartile. E. Immunoblots show normal activation of DNA damage response markers in Cenpj-deficient MEFs (passage 2) before and after treatment with the DNA damaging agent camptothecin (1 µM for 1 h). KAP1 was used as a loading control. The genomic instability observed in Cenpjtm/tm mice does not reflect defective ATR - or ATM-dependent DNA–damage signaling
Seckel syndrome belongs to a group of genome instability disorders collectively referred to as DNA-damage response and repair-defective syndromes [60]. So far, all cells derived from Seckel patients have been found to be impaired in signaling mediated by the DNA-damage responsive protein kinase ATR, and therefore display reduced phosphorylation of downstream ATR substrates such as the checkpoint kinase Chk1, and have impaired G2/M cell-cycle checkpoint arrest upon treatment with DNA-damaging agents [60], [61]. We therefore treated fibroblasts from Cenpjtm/tm embryos (13.5 d.p.c) with the DNA damaging agent camptothecin, a DNA topoisomerase I inhibitor that causes DNA double-strand breaks specifically in S-phase [62]. Analyses revealed that Cenpjtm/tm fibroblasts were proficient for ATR-dependent and ATM-dependent phosphorylation of Chk1 (pS345) and KAP1 (pS824), respectively, and showed normal activation of γH2AX (Figure 5E). We found no evidence of an impaired G2/M DNA damage checkpoint as determined by the percentage of MPM2-positive cells following irradiation (Figure S2G). Furthermore, CtIP-Seckel cells show defective phosphorylation of replication protein A (RPA) after camptothecin treatment, a phenotype associated with impaired DNA-end resection and homologous recombination [6]. However, we found no evidence of this in Cenpjtm/tm MEFs (Figure 5E).
Discussion
The Cenpj hypomorphic mouse (Cenpjtm/tm) that we have created displays many of the classical clinical features of Seckel syndrome, including intrauterine and postnatal dwarfism, microcephaly, a sloping forehead, neuropathogical abnormalities, memory impairment and genomic instability [1], [4], [7], [15], [28], [63]. In addition, we have shown that Cenpjtm/tm mice display some of the less frequently reported characteristics of the syndrome, including retarded bone ossification [1], [32], [64], as well as vertebral abnormalities and several other interesting histopathological and hematological abnormalities that have not previously been reported in patients.
Microcephaly versus dwarfism of Cenpjtm/tm mice
Neuroepithelial cells have apical-basal polarity, and the switch from proliferative, symmetric to neurogenic, asymmetric division is controlled by the orientation of the spindle pole during mitotic division [65]. Primary microcephaly is caused by mutations of centrosomal proteins and is thought to arise from an increase in asymmetric divisions that reduces the size of the neural progenitor pool available for future brain growth, hence the growth deficit is restricted to the brain [66]. Seckel syndrome is characterized by microcephaly and a small body size (proportionate dwarfism). Interestingly, different mutations in the centrosomal proteins CENPJ or CEP152 can cause microcephaly or Seckel syndrome [4], [14], [15]. The CENPJ-microcephaly mutations reported to date affect exons 2 (17delC), 11 (3243–3246delTCAG) and 16 (A3704T) [14], [15]; these mutations are predicted, but not proven, to cause defects in spindle pole orientation and proliferation of neural progenitors in a similar manner to other microcephaly genes. CENPJ-Seckel syndrome has been associated with a homozygous splice acceptor mutation in the last nucleotide of CENPJ intron 11 that results in the skipping of either exon 12, exons 12 and 13 or exons 11,12 and 13 during transcription [4]. This may represent a cellular attempt to salvage this important protein since the latter two transcripts are predicted to result in in-frame deletion and preservation of the C-terminus [4].
Notably, we found that insertion of a cassette between exons 4 and 5 of Cenpj resulted in splicing over the cassette and cryptic splicing, such that three different Cenpj mRNAs were expressed at very low levels: full length, one lacking exons 4 and 5 and one lacking exon 5. Skipping of exons 4 and 5 or exon 5 is predicted to result in a premature stop codon and protein products that are truncated after translation of exon 3 or 4, respectively. Immunoblotting and immunofluorescence revealed higher than expected levels of apparently full-length Cenpj protein were present in Cenpjtm/tm MEFs, which may be the result of post-transcriptional or translational regulation since the level of full-length Cenpj mRNA in Cenpjtm/tm MEFs was only 2% of wild-type levels. Furthermore, mRNA levels varied greatly between Cenpjtm/tm mouse embryonic fibroblasts, which may be due to genetic modifiers.
Together with studies of CENPJ-Seckel cells, which have shown that mutations in CENPJ may result in exon skipping and the generation of multiple transcripts that may generate in-frame protein products [4], these data suggest that expression of this critical protein may be rescued to some extent by cryptic splicing over deleterious mutations. These produce alternatively spliced mRNAs and thus the same mutation might not result in the same mRNA or protein levels in each individual. Moreover, cryptic splicing may also differ between tissues. Without a complete examination of the effects of different CENPJ-mutations on mRNA levels and splicing, and CENPJ protein levels, it is difficult to say why CENPJ mutations can either result in primary microcephaly or Seckel syndrome, or why the Cenpjtm1a(EUCOMM)Wtsi allele results in a mouse with a Seckel syndrome-like phenotype. However, we propose that Cenpjtm/tm mice display a Seckel syndrome-like phenotype, rather than primary microcephaly, due to a major reduction in full length Cenpj protein and therefore a lack of the protein domain(s) encoded by exons 11, 12 and/or 13.
The microcephaly of Cenpjtm/tm mice (brain weight two standard deviations below the mean) was not as severe as CENPJ-Seckel syndrome patients, who display anthropometric values that are all at least seven standard deviations below the mean [4]. The evolutionary lineage leading to humans is marked by a dramatic increase in brain size, suggesting that disruption of genes involved in neurogenesis will have a less profound effect in mice than in humans [67]. However, this is confounded by the fact that there are several mouse models of microcephaly, such as the humanized ATR-Seckel mouse and the Cdk5rap2 mutant mouse, which display severe reductions in brain size [25], [37]. The discrepancy between microcephaly of Cenpjtm/tm mice and CENPJ-Seckel patients may instead be due to the hypomorphic nature of the Cenpjtm1a(EUCOMM)Wtsi allele or by the rapid evolution of the Cenpj gene between mice (80% sequence identity) and humans; the human CENPJ protein may be more efficient at regulating neurogenesis than that of the mouse [67].
The dwarfism and microcephaly of Cenpjtm/tm mice appeared to be the result of widespread DNA damage and apoptosis in embryos, rather than a reduction in cell proliferation. The level of cell death within the forebrain of the Cenpj-hypomorph embryonic mouse brain was comparable with that of the humanized ATR-Seckel mouse (approximately 1.5%; [25]). Similarly, ATR-, CtIP-, CEP152 - and PCNT-Seckel cells have increased levels of DNA damage and a lowered apoptotic threshold with no change in the rate of proliferation [5], [6], [25]. In contrast to cells from ATR-, CtIP-, CEP152 - and PCNT-Seckel syndrome patients, we have shown that MEFs from Cenpj-deficient mice are not impaired in ATR-dependent DNA damage signaling but instead show an elevated frequency of extra centrioles, multipolar spindles, and near tetraploid karyotypes. We suspect that the embryonic fibroblast line showing 41% near tetraploid cells could come from an embryo that would not have survived to term, indicating that genomic instability may also explain the sub-Mendelian birth ratio of Cenpjtm/tm mice. We also found evidence of chromosome missegregation, chromosomal translocations and centric fusions in Cenpjtm/tm MEFs. Increased levels of pan-nuclear γH2AX in embryos may be the result of chromosome breakage, micronucleus formation or missegregation [68], however it is possible that this reflects phosphorylation of H2AX during apoptosis-driven fragmentation of DNA [69].
Cenpj is required for normal neuronal density and long-term memory
The neuropathological features of Cenpjtm/tm E14.5 embryos were remarkably similar to fetal stage Seckel syndrome. At E14.5, we found there was a reduction in neuron density within the developing telencephalon of Cenpjtm/tm mice. There are only two neuropathological reports of fetal stage Seckel syndrome (30 weeks gestation), although both showed that the cortical layers of the telencephalon were thin and that neuronal populations were less dense and less organized than age - or length-matched controls [28], [29]. As with Cenpjtm/tm mice, the hippocampal formation was short in one fetus, but displayed normal cytoarchitectural progression [28], [29]. Both reports indicated that the major nuclear groups of the basal ganglia, thalamus, cerebellum and brainstem showed no abnormalities in fetal stage Seckel syndrome [28], [29]. Interestingly, we saw a >50% reduction in the number of Cenpjtm/tm embryos between mid neurogenesis (E14.5) and the completion of neurogenesis (E18.5), when Cenpj is strongly expressed in the ventricular layers of the diencephalon, telencephalon, midbrain and cerebellum (www.emouseatlas.org, www.eurexpress.org), suggesting that Cenpj-deficiency during this critical period of neurogenesis causes partial lethality.
The majority of patients with Seckel syndrome are reported to have an IQ of <50 and are delayed in speech and reaching motor milestones, as well as displaying pyramidal signs, hyperactivity and an attention deficit [3], [34]. Cranial MRI of adult patients with Seckel syndrome has shown a reduction in brain volume, especially the cerebral cortex, a simplified gyral pattern (number of gyri reduced and shallow sulci), poorly developed frontal lobes, agenesis of the corpus callosum, reduction of white matter, brainstem and cerebellar hypoplasia, and dysmorphic or enlarged lateral ventricles [32], [34], [35]. A relatively normal MRI was reported for two siblings (aged two and four years-old) of the CENPJ-Seckel kindred and together with two cousins (aged five and six years-old), all had a history of normal cognitive and motor development [4]. The third cousin (MRI not performed, aged 16 years-old) had an IQ<60. Similarly, the brain regions of adult Cenpjtm/tm mice appeared anatomically proportionate, although these mice had a significantly shorter dentate gyrus than controls and this was accompanied by cognitive impairments reminiscent of Seckel syndrome patients.
Centrioles, mitotic spindles, and ploidy
dSas-4 is the Drosophila homologue of CENPJ. Unlike dSas-4-depleted cells or dSas-4 mutant flies that progressively lose centrioles, Cenpjtm/tm MEFs contain centrioles even after several passages [70], [71]. While the increase in Cenpjtm/tm cells with two or fewer centrioles is consistent with an impairment of centriole assembly, this effect is relatively mild, and therefore suggests that the mutant expresses residual, functional Cenpj protein. Ciliogenesis requires centriole biogenesis and therefore dSas-4 mutants lack both primary and motile cilia [70]. The role of CENPJ in ciliogenesis has not been extensively explored in mammals, but depletion of CENPJ in cultured cells is reported to impair primary cilium formation [72]. Cenpjtm/tm mice (16 weeks old) did not display phenotypes normally associated with ciliopathies such as situs inversus or renal cystic disease, suggesting that sufficient amounts of Cenpj are available in the mutant for cilia formation in the majority of cells. However, the abnormalities in ciliary processes and photoreceptor nuclei within the eye may be attributed to ciliary defects. Moreover, unlike dSas-4 mutant males that display loss of flagella and sperm motility, Cenpjtm/tm male mice are fertile [70], which could again be due to residual expression of Cenpj.
While Cenpjtm/tm MEFs displayed irregular centriole numbers and mono - and multipolar spindles, they also showed extensive polyploidy and aneuploidy. Thus, we cannot conclude whether abnormal centriole and centrosome numbers are the cause or consequence of aberrant ploidy. Figure S6A shows the possible sequence of events that may lead to the abnormal ploidy of CENPJ-Seckel cells. Aberrant centrosome numbers are known to cause mitotic spindle abnormalities, culminating in mitotic delay, chromosome missegegration, cytokinetic failure and polyploidy. Prolonged mitotic delay can cause DNA damage, cell cycle arrest and apoptosis [73], [74]. Chromosome missegregation can also damage chromosomes, hence triggering activation of DNA damage checkpoints [68], [75]. Chromosome instability could therefore explain the increase in γH2AX levels and potentially, the increase in apoptosis in the mutant embryonic brain. Of all chromosome aberrations detected in the mutant MEFs, tetraploidy was the most prominent. A common cause of tetraploidy is an abortive mitotic cell cycle whereby cells enter but fail to complete mitosis [76]. Mitotic spindle abnormalities in Cenpjtm/tm cells could trigger extended mitotic arrest followed by mitotic slippage producing a tetraploid cell (Figure S6A). Tetraploid Cenpjtm/tm MEFs seem to be able to proliferate, since they represented almost 40% of the metaphase cells obtained for karyotyping. Interestingly, dSas-4 mutant flies show only a small increase in the proportion of aneuploid cells (1% in wild-type vs. 3% in mutants) and no polyploidy [70], whereas the proportion of near tetraploid Cenpjtm/tm embryonic fibroblasts was surprisingly high (∼10% in wildtype vs ∼40% in Cenpjtm/tm MEFs). We suspect that Cenpj-deficiency exacerbates tetraploidy in MEFs, which are particularly susceptible to tetraploidy with passaging [77]. Nonetheless, adult Cenpjtm/tm mice show increased micronucleus induction, which is likely the result of lagging chromosomes and chromosome breakage.
Polyploidy as a potential cause of karyomegaly in Cenpjtm/tm tissues
Cenpjtm/tm mice of both genders showed an increased incidence of hypertrophic, disorganized cardiomyoctes with karyomegaly in the endocarium and interventricular septum when compared to wildtype mice. The areas showed no evidence of degeneration or repair, however since a high proportion of Cenpjtm/tm MEFs are polyploid, this is likely to be the cause of the karyomegaly. Although one of the less frequently reported characteristics of Seckel syndrome, there are numerous case-reports of severe cardiac anomalies in Seckel syndrome patients, including atrial and ventricular septal defects, pulmonary atresia, patent ductus arteriosus and congenital heart disease [49], [50], [51], [52], [53]. It will be interesting to see whether CENPJ-Seckel patients develop cardiac defects as they age. At 16 weeks of age Cenpjtm/tm mice showed hypoalbuminemia, which is associated with chronic liver and kidney diseases, although histopathological analysis of their livers and kidneys did not reveal any abnormalities. However, the preponderance of karyomegaly in the liver and Harderian glands was increased in aged Cenpjtm/tm mice. Cenpj-deficiency may exacerbate this phenomenon in the cells of both of these tissues, which are prone to karyomegaly [78].
Susceptibility to malignancy
Familial syndromes associated with genomic instability often predispose to cancer formation since DNA damage is the source of mutations that drive malignant transformation. However only a few cancers have been reported for Seckel syndrome patients, possibly due to the shorter life-expectancy of patients with primordial dwarfism. Furthermore, since mutation of each of the five known Seckel genes, ATR, PCNT, CENPJ, CEP152 and RBBP8 (CtIP), cause genomic instability that is associated with apoptosis, it is possible that Seckel cells may not have the opportunity to accumulate cancer-causing mutations. The chromosomal instability that is associated with Cenpj-deficiency could result in aneuploidy or translocations that cause loss of tumour suppressors or the formation of oncogenic fusion proteins, respectively [79]. We are currently ageing a cohort of Cenpjtm/tm mice (currently 7–14 months old) to determine whether Cenpj-deficiency alters the frequency of malignancy or shortens life-expectancy.
Cenpj-deficient mouse phenotypes for which there are currently no clinical correlates
We noted a small number of abnormalities in Cenpjtm/tm mice that have not been previously reported for Seckel syndrome patients or mouse models. Seckel syndrome is associated with ocular defects in humans, including spontaneous lens dislocation, myopia, astigmatism, and retinal degeneration. Ocular examination of CENPJ-Seckel patients has not yet been reported [47], [48], however Cenpj was highly expressed in the rapidly proliferating retinal neuroblast layer in the 14.5 d.p.c. mouse embryo and Cenpj-deficient mice presented with a number of ocular abnormalities. Furthermore, a small number of reports suggest that Seckel-like syndromes are associated with precocious puberty or premature thelarche [44], [45]. In contrast, female Cenpjtm/tm mice showed signs of delayed puberty, although the reproductive tract appeared normal at 16 weeks. We built a protein-protein interaction network using all known Seckel Syndrome associated genes as query (Figure S6B). By using gene ontology enrichment analysis we showed that 265 biological processes (level 3 classification) are significantly over-represented in the Seckel syndrome network (Table S1). As expected, many of the processes were involved in the regulation of cell cycle, cell growth and cell death. Interestingly, the network was also enriched for genes involved in the ‘response to hormone stimulus’ ‘ovulation cycle process’ and ‘sex differentiation’ (Table S1). Transient insulin resistance during puberty is a well documented phenomenon [80]. Cenpjtm/tm mice of both genders had a delayed response to glucose challenge although this was more marked in 16 week-old female mice, which may be explained by delayed puberty in female Cenpjtm/tm mice. While there are no reports of an association between abnormal glucose homeostasis and Seckel syndrome, interestingly, most individuals with MOPDII, including PCNT-MOPDII, develop insulin resistance and diabetes during childhood [81], [82]. Aside from centrosome-mediated regulation of the cell-cycle, PCNT is thought to regulate insulin secretory vesicle docking in mouse pancreatic β-cells [83]. Whether CENPJ plays a role in glucose homeostasis remains to be determined. Finally, the proportion of CD8+CD3+ T cells were elevated in Cenpjtm/tm mice, although this was more pronounced in males. The Seckel syndrome protein-protein interaction network that we generated was significantly enriched for genes involved in ‘leukocyte mediated immunity’, ‘leukocyte mediated cytotoxicity’, ‘leukocyte activation’ and ‘interleukin-2 production’ (Table S1). While we are uncertain of the biological basis for these relationships, it will be interesting to see whether there are clinical correlates for these abnormalities and whether other mouse models of Seckel syndrome or primordial dwarfism share these anomalies.
Summary
Mouse models of Seckel syndrome may go some way towards the molecular genetic delineation of this heterogeneous condition. The generalized activation of apoptosis as a result of genomic instability in ATR-Seckel and Cenpjtm/tm mouse embryos provides one explanation for the proportionate dwarfism of Seckel syndrome patients. In agreement with the intron 11 CENPJ-Seckel mutation, which results in the formation of three transcripts, we showed that Cenpj expression is rescued to some extent by cryptic splicing over the cassette to produce a variety of truncated mRNAs, and that there is a moderate degree of individual variation in the ability of an organism to perform this rescue. These data highlight the need for detailed mRNA expression, splicing studies, and protein analysis to establish how individual mutations affect the normal and cryptic splicing of CENPJ mRNAs for each patient directly, and not with prediction analysis tools, so as to understand why some CENPJ mutations cause microcephaly and others Seckel syndrome and how the same CENPJ mutation can cause clinical heterogeneity [4].
Materials and Methods
Confirmation of correct targeting and animal husbandry
Mutant mice carrying the Cenpjtm1a(EUCOMM)Wtsi allele were generated on a C57BL/6NTac; C57BL/6-Tyrc-Brd background (clone EPD0028_7_G05) by the Sanger Mouse Genetics Project as part of the European Conditional Mouse Mutagenesis Program (EUCOMM; [26]). Correct gene targeting in founder mice was determined by a combination of standard PCR and quantitative PCR (qPCR; see Figure S1 for more details). Following confirmation of correct targeting, mice were genotyped for the Cenpjtm1a(EUCOMM)Wtsi allele by PCR using primers specific to the wildtype and mutant Cenpj alleles and to LacZ (Figure S1D). In individual experiments, all mice were matched for age and gender. A cumulative baseline was generated from data arising from controls from the same genetic background, age and gender. The care and use of all mice in this study was carried out in accordance with UK Home Office regulations, UK Animals (Scientific Procedures) Act of 1986. Mice were maintained in a specific pathogen free unit on a 12 hr light: 12 hr dark cycle with lights off at 7 : 30pm and no twilight period. The ambient temperature was 21±2°C and the humidity was 55±10%. Mice were housed using a stocking density of 3–5 mice per cage (overall dimensions of caging: (L×W×H) 365×207×140 mm, floor area 530 cm2) in individually ventilated caging (Tecniplast Seal Safe1284L) receiving 60 air changes per hour. In addition to Aspen bedding substrate, standard environmental enrichment of two nestlets, a cardboard Fun Tunnel and three wooden chew blocks was provided. Mice were given water and diet ad libitum. At 4 weeks of age, mice were transferred from Mouse Breeders Diet (Lab Diets, 5021–3) to a high fat (21.4% fat by crude content) dietary challenge (Special Diet Services, Western RD 829100).
Quantitative real-time PCR
To test for expression of Cenpj and cryptic splicing, total RNA was isolated from mouse embryonic fibroblasts (13.5 days post coitum (d.p.c.), n = 3) using the RNeasy minikit (Qiagen). RT-PCR was performed using the BD Sprint kit containing random hexamers (BD Clontech, CA, USA). Primers were designed to exon boundaries and sequences are available on request. cDNA was quantified using SYBR Green on an ABI7900HT (ABI, CA, USA). Gene expression was normalised to Gapdh and to wild-type control.
Immunoblotting
Protein extracts were prepared from mouse embryonic fibroblasts (13.5 d.p.c.) by directly harvesting cells in Laemmli buffer. Proteins were separated by SDS-PAGE, and membranes were incubated with primary antibodies to CENPJ (Stratech, Newmarket, UK. Rabbit 1∶500), KAP1 (Abcam, Cambridge, UK. Rabbit 1∶10 000), KAP1 phospho-Ser-824 (Bethyl. Texas, US. Rabbit 1∶1000), Chk1 phospho-Ser-345 (Cell Signaling, Boston, US. Rabbit 1∶5000), RPA phospho-Ser-4/8 (Bethyl, Texas, US. Rabbit 1∶10 000), H2AX phospho-Ser-139 (γH2AX; Millipore, Billerica, US. Mouse 1∶1000). Camptothecin was from Sigma (Poole, UK).
LacZ staining
LacZ staining was performed on tissues perfused, removed and fixed (30 min) with 4% paraformaldehyde (pH8). Tissues were then washed three times in PBS. Tissues were then incubated at 4°C for 48 h in staining buffer (5 mM K3Fe(CN)6, 5 mM K4Fe(CN)6, 0.02% IGEPAL CA-630, 0.01% deoxycholate, 2 mM MgCl2, 1 mg/ml bromo-chloro-indolyl-galactopyranoside in the dark. Tissues were fixed again with 4% paraformaldehyde overnight at 4°C before being transferred through increasing glycerol concentrations and archiving in 70% glycerol+0.01% sodium azide.
E18.5 measurements and skeletal preparations
At 18.5 d.p.c. litters were harvested and euthanized. The crown-rump length was measured and measurements of skulls were adapted from published methods used to assess adult mouse skulls [84]. Embryos were scalded at 67°C for 30 seconds to facilitate removal of skin, muscle and fat. Dissected embryos were then fixed in 100% ethanol for 48 h and transferred to acetone for 48 h to further de-fat the skeletons. Skeletal embryos were then stained with 0.015% Alcian Blue (Sigma-Aldrich, UK; in Ethanol/Glacial Acetic Acid) for 24 h, washed five times in 100% ethanol and transferred to 0.1% potassium hydroxide overnight. Skeletons were then stained with 0.005% Alizarin Red (Merck, UK; in 1% KOH) for 3 h, washed with 1% KOH three times and transferred to 20% Glycerol/1% KOH for clearing for ∼3 days. Skeletons were then transferred through increasing glycerol concentrations and archived in 70% glycerol +0.01% sodium azide.
X-ray imaging
X-ray imaging was performed at 14 weeks of age using a Faxitron MX-20 cabinet (Faxitron Bioptics, IL, USA). Mice were anesthetized with a preparation of 100 mg/kg Ketamine/10 mg/kg Xylazine. Weight and body length were measured prior to the scan. Five images were acquired: dorsoventral and lateral images of the whole body at ×1 magnification, dorsoventral and lateral images of the head at ×4 magnification and a dorsoventral image of the left forepaw at ×5 magnification. Images were acquired using an energy of 23 kV for 10 seconds per image. Images were studied visually to assess abnormalities within 41 standard parameters. To show that humeri were anatomically disproportionate the right greater tubercle - deltoid tuberosity length (mm) was normalized to the right greater tubercle – trochlea length (mm) and data were represented as a percentage.
Histology
Adult tissue samples were fixed in 10% neutral buffered formalin and E14.5 embryos were fixed in 4% PFA. Samples were dehydrated, paraffin embedded and 4 µm sections were cut before hematoxylin and eosin staining. Neuron densities of E14.5 embryo brains were determined by counting the number of nuclei in three different areas that were 75 µm2 for the densely populated ventricular zones and 150 µm2 for the less densely populated mid-striatum. Adult neuroanatomical measurements (Figure S3) were carried out on 40 µm thick Nissl stained sections using ImageJ freeware (NIH). Heart fibrosis was assessed by Masson's Trichrome and X-zone pigmentation of the adrenals was confirmed by Periodic Acid-Schiff's staining using standard methods. Whole mouse eyes were enucleated, fixed, and sectioned for histological studies as previously described [85].
Immunohistochemistry
Sections of paraffin embedded tissues were dewaxed and antigen retrieval was performed in boiling 10 mM citrate buffer pH6. Endogenous peroxidases were quenched in 3% hydrogen peroxide (Sigma, UK) before blocking in normal serum (VectorLabs, UK). Sections were incubated in primary antibodies to Cenpj (Stratech), cleaved caspase-3 (Cell Signaling Technologies, CA, USA), Ki67 (DAKO Ltd, UK) and phospho-Ser 139 H2AX (γH2AX, Cell Signaling Technologies, CA, USA). Vectorstain ABC kit and DAB (VectorLabs, UK) were used according to the manufacturer's instructions. Sections were counterstained with haematoxylin before clearing and mounting. The number of cells positive for cleaved-caspase 3 or γH2AX were counted in two areas of 75 µm2 the striatum, cortex and pro-hippocampus and data shown are the percentage of total cells in the area. Counts were performed independently by two individuals to confirm reproducibility.
Neurobehavioral and sensory assessment
At nine weeks of age, open field, grip strength and modified SHIRPA were performed as described previously [86], [87]. Hot plate assessment was performed at 10 weeks of age using standard techniques. Tests for social recognition and olfaction (Cenpjtm/tm n = 9 and Cenpj+/+ n = 8) were performed on male mice at 3–6 months of age. Mice were habituated to a test arena identical to their home cage for 10 min. For social recognition, a stimulus mouse was placed into the test arena for 1 min, repeated four times at 10 min intervals. In the fifth trial, a second stimulus mouse was presented. 24 h later the test animals were presented with the familiar animal from trials 1–4 and a new unfamiliar animal, for 2 min. Trials were performed under red light and recorded with an overhead camera and the videos scored blind of genotype. The amount of time the test animal spent investigating, by oronasal contact or approaching within 1–2 cm, was recorded. Stimulus animals, 2–4 months old, were weight-matched to Cenpjtm/tm mice and sedated with ketamine/xylazine (i.p. 1 g/0.1 g per kg of body weight). C57BL/6NTac mice were used for trials 1–4 and for the 24 h discrimination test, 129P2/OlaHsd mice were used for trial 5. Three animals (two Cenpjtm/tm and one Cenpj+/+) were taken out of the analysis because of their low investigation times (less than 10 s on trial one or during the discrimination test).
Glucose tolerance
We assessed glucose tolerance in mice fed on a high-fat diet (Western RD, 829100, Special Diets Services) from 4 weeks of age until 13 weeks of age. At 13 weeks, mice were fasted overnight before a blood sample was taken and glucose was measured using an Accu-Chek Aviva (Roche). To perform an intra-peritoneal glucose tolerance test (IP-GTT), mice were fasted for 16 h, a bolus of glucose was administered intraperitoneally and blood glucose concentration from the tail vein was measured using Accu-Chek Aviva (Roche) after 15, 30, 60 and 120 min.
Clinical chemistry
We performed clinical chemistry on 16-week-old mice. Mice were terminally anaesthetized and blood was collected from the retro-orbital sinus into lithium-heparin tubes. The plasma was immediately analyzed on an Olympus AU400 Analyzer.
Flow cytometry
At 16 weeks of age blood samples were collected into heparin-coated tubes. The main leukocyte populations in peripheral blood were characterized by 8-colour flow cytometry with an LSRII (BD Bioscience, UK Biosciences, UK) and associated software. Briefly, samples were centrifuged at 5000 g for 10 min at 8°C to remove the plasma layer. Red blood cells were lysed (Pharmalyse, BD Bioscience, UK Biosciences, UK) and samples were centrifuged at 400 g for 3 min to pellet the white cells. White cells were resuspended in buffer (PBS pH 7.45 containing 0.5% BSA), transferred to 96-well plate and washed in buffer several times before incubation in 50 µl 10 µg/ml Mouse FcBlock (BD Bioscience, UK Biosciences, UK) for 15 min on ice. Cells were washed in buffer and incubated with 50 µl antibody mix from two staining panels (Tables S2 and S3) for 15 min on ice. Propidium iodide (2.5 mg/ml; Sigma, UK) was added to each well and cells were incubated for a further 5 min. Cells were washed in buffer several times before 30 000 propidium iodide-negative, CD45-positive events were collected. Data were interpreted using FlowJo (v7.6, Tree Star, Inc., OR, USA). The frequency of micronucleated normochromatic erythrocytes was determined by flow cytometry (FC500, Beckman Coulter, USA) as described previously [88] and data were interpreted using FlowJo (v9.3.1, Tree Star, Inc., OR, USA). Cell cycle analysis was performed on mouse embryonic fibroblasts (13.5 d.p.c.). Briefly, cells were fixed in 70% ethanol overnight and stained with PI solution before analyzing on a flow cytometer (LSR Fortessa, BD, USA). A total of 10,000 events were acquired per sample. The cells were gated on PI fluorescence area versus PI fluorescence width to discriminate any doublets and clumps. The gated events were displayed on a histogram plot of PI fluorescence area. Data were analyzed using FlowJo (v9.3.1, Tree Star, Inc., OR, USA).
Immunofluorescence
Mouse embryonic fibroblasts (13.5 d.p.c.) were collected and cultured using standard methods. Primary antibodies used in this study were CDK5RAP2 (Bethyl), centrin-3 (Abnova), TPX2 (Abnova), gamma-tubulin (GTU88; Sigma-Aldrich), alpha-tubulin (DM1A; Sigma-Aldrich). Secondary antibodies conjugated to Alexa Fluor 488 and 555 (Invitrogen) were used. DNA was stained with Hoescht (Sigma-Aldrich). To detect centrosomal markers, cells were fixed in −20°C methanol for 5 min. After fixation, cells were processed for immunofluorescence and microscopy as described in [89]. For centriole counts in Figure 4C cells were treated with 100 µM monastrol for 16 h before fixation.
G2 checkpoint assay
Cells were irradiated with 3 Gy ionizing radiation using a Faxitron cabinet X-ray system and left recover for 8 h in the presence of 1 µg/ml of nocodazole (Sigma, Poole, UK) to trap mitotic cells. Cells were trypsinized and fixed with 4% paraformaldehyde, permeabilized with 1× phosphate buffered saline containing 0.2% Triton X-100 for 30 min on ice, and incubated in the presence of MPM2 primary antibodies (Millipore, Billerica, US. Mouse 1∶100) and then Alexa-Fluor-488 secondary antibodies (Life Technologies, Paisley, Scotland. Goat anti-mouse 1∶200) to detect mitotic cells.
Multiplex-fluorescence in situ hybridization (FISH)
Metaphase spreads from MEFs (Cenpjtm/tm and Cenpj+/+ littermates) were prepared as for metaphase FISH and multiplex-FISH was carried out as previously described [90].
Network generation and analysis
A network of Protein-Protein interactions for Seckel syndrome genes was generated using the Cytoscape 2.8.1 [91] plug-in BisoGenet 1.41.00 [92]. The Entrez Gene symbols of the Seckel syndrome associated genes (CENPJ, PCNT, CEP152, ATR, RBBP8 (CtIP), SCKL3, Entrez Gene IDs = 55835, 5116, 22995, 545, 5932 and 386616 respectively) [4], [5], [6], [41] were used as query to build a network of experimentally validated Protein-Protein interactions, by adding neighbours to the input nodes up to a distance of one. All the interactions are downloaded by BisoGenet from its own database SysBiomics and have been validated by one or different experimental methodologies such as X-ray crystallography, surface plasmon resonance, two hybrid systems, three hybrid systems and Western blot. The network's characteristic path length was calculated using Cytoscape's built-in plug-in NetworkAnalyzer [93].
Gene Ontology (GO) over-representation analysis
The GOs over-representation analysis was performed using the Over-representation analysis tool of the Consensus Path Database website [94]. Statistical significance of the different GOs represented in our network was calculated by the website's tool using a Hypergeometric test. After calculating the p-value, the tool corrects it for the false discovery rate generating the corrected q-value. A q-value <0.01 was used as threshold for all significant results.
Statistical analyses
A Shapiro-Wilk normality test was performed to assess whether data were normally distributed followed by an F-Test to assess whether equality of variances could be assumed. The significance of the difference between the means of both data sets was tested by applying a two-sided T-Test, assuming variance equality whenever the F-Test was positive (P>0.05). For all cases where the Shapiro-Wilk normality test was negative (P>0.05) a Mann-Whitney non-parametric test was applied to assess the significance. Statistical analyses were performed using R-2.13.0 [95].
Supporting Information
Zdroje
1. MajewskiF, GoeckeT (1982) Studies of microcephalic primordial dwarfism I: approach to a delineation of the Seckel syndrome. American journal of medical genetics 12 : 7–21.
2. FaivreL, Le MerrerM, LyonnetS, PlauchuH, DagoneauN, et al. (2002) Clinical and genetic heterogeneity of Seckel syndrome. American journal of medical genetics 112 : 379–383.
3. Harsha VardhanBG, MuthuMS, SaraswathiK, KoteeswaranD (2007) Bird-headed dwarf of Seckel. Journal of the Indian Society of Pedodontics and Preventive Dentistry 25Suppl: S8–9.
4. Al-DosariMS, ShaheenR, ColakD, AlkurayaFS (2010) Novel CENPJ mutation causes Seckel syndrome. J Med Genet 47 : 411–414.
5. KalayE, YigitG, AslanY, BrownKE, PohlE, et al. (2011) CEP152 is a genome maintenance protein disrupted in Seckel syndrome. Nature genetics 43 : 23–26.
6. QvistP, HuertasP, JimenoS, NyegaardM, HassanMJ, et al. (2011) CtIP Mutations Cause Seckel and Jawad Syndromes. PLoS genetics 7: e1002310.
7. O'DriscollM, Ruiz-PerezVL, WoodsCG, JeggoPA, GoodshipJA (2003) A splicing mutation affecting expression of ataxia-telangiectasia and Rad3-related protein (ATR) results in Seckel syndrome. Nature genetics 33 : 497–501.
8. MajewskiF, RankeM, SchinzelA (1982) Studies of microcephalic primordial dwarfism II: the osteodysplastic type II of primordial dwarfism. American journal of medical genetics 12 : 23–35.
9. RauchA, ThielCT, SchindlerD, WickU, CrowYJ, et al. (2008) Mutations in the pericentrin (PCNT) gene cause primordial dwarfism. Science 319 : 816–819.
10. WillemsM, GenevieveD, BorckG, BaumannC, BaujatG, et al. (2010) Molecular analysis of pericentrin gene (PCNT) in a series of 24 Seckel/microcephalic osteodysplastic primordial dwarfism type II (MOPD II) families. Journal of medical genetics 47 : 797–802.
11. HatchEM, KulukianA, HollandAJ, ClevelandDW, StearnsT (2010) Cep152 interacts with Plk4 and is required for centriole duplication. The Journal of cell biology 191 : 721–729.
12. CizmeciogluO, ArnoldM, BahtzR, SetteleF, EhretL, et al. (2010) Cep152 acts as a scaffold for recruitment of Plk4 and CPAP to the centrosome. The Journal of cell biology 191 : 731–739.
13. LealGF, RobertsE, SilvaEO, CostaSM, HampshireDJ, et al. (2003) A novel locus for autosomal recessive primary microcephaly (MCPH6) maps to 13q12.2. J Med Genet 40 : 540–542.
14. GulA, HassanMJ, HussainS, RazaSI, ChishtiMS, et al. (2006) A novel deletion mutation in CENPJ gene in a Pakistani family with autosomal recessive primary microcephaly. J Hum Genet 51 : 760–764.
15. BondJ, RobertsE, SpringellK, LizarragaSB, ScottS, et al. (2005) A centrosomal mechanism involving CDK5RAP2 and CENPJ controls brain size. Nat Genet 37 : 353–355.
16. KaindlAM, PassemardS, KumarP, KraemerN, IssaL, et al. (2010) Many roads lead to primary autosomal recessive microcephaly. Progress in neurobiology 90 : 363–383.
17. DelavalB, DoxseySJ (2010) Pericentrin in cellular function and disease. The Journal of cell biology 188 : 181–190.
18. HungLY, TangCJ, TangTK (2000) Protein 4.1 R-135 interacts with a novel centrosomal protein (CPAP) which is associated with the gamma-tubulin complex. Mol Cell Biol 20 : 7813–7825.
19. KohlmaierG, LoncarekJ, MengX, McEwenBF, MogensenMM, et al. (2009) Overly long centrioles and defective cell division upon excess of the SAS-4-related protein CPAP. Current biology: CB 19 : 1012–1018.
20. SchmidtTI, Kleylein-SohnJ, WestendorfJ, Le ClechM, LavoieSB, et al. (2009) Control of centriole length by CPAP and CP110. Current biology: CB 19 : 1005–1011.
21. TangCJ, FuRH, WuKS, HsuWB, TangTK (2009) CPAP is a cell-cycle regulated protein that controls centriole length. Nat Cell Biol 11 : 825–831.
22. NiggEA, RaffJW (2009) Centrioles, centrosomes, and cilia in health and disease. Cell 139 : 663–678.
23. ZyssD, GergelyF (2009) Centrosome function in cancer: guilty or innocent? Trends in cell biology 19 : 334–346.
24. GanemNJ, GodinhoSA, PellmanD (2009) A mechanism linking extra centrosomes to chromosomal instability. Nature 460 : 278–282.
25. MurgaM, BuntingS, MontanaMF, SoriaR, MuleroF, et al. (2009) A mouse model of ATR-Seckel shows embryonic replicative stress and accelerated aging. Nature genetics 41 : 891–898.
26. SkarnesWC, RosenB, WestAP, KoutsourakisM, BushellW, et al. (2011) A conditional knockout resource for the genome-wide study of mouse gene function. Nature 474 : 337–342.
27. SirJH, BarrAR, NicholasAK, CarvalhoOP, KhurshidM, et al. (2011) A primary microcephaly protein complex forms a ring around parental centrioles. Nature genetics 43 : 1147–1153.
28. FitzgeraldB, O'DriscollM, ChongK, KeatingS, ShannonP (2012) Neuropathology of fetal stage Seckel syndrome: a case report providing a morphological correlate for the emerging molecular mechanisms. Brain & development 34 : 238–243.
29. HoriA, TamagawaK, EberSW, WestmeierM, HansmannI (1987) Neuropathology of Seckel syndrome in fetal stage with evidence of intrauterine developmental retardation. Acta neuropathologica 74 : 397–401.
30. ArnoldSR, SpicerD, KouseffB, LacsonA, Gilbert-BarnessE (1999) Seckel-like syndrome in three siblings. Pediatric and developmental pathology: the official journal of the Society for Pediatric Pathology and the Paediatric Pathology Society 2 : 180–187.
31. Endoh-YamagamiS, KarkarKM, MaySR, CobosI, ThwinMT, et al. (2010) A mutation in the pericentrin gene causes abnormal interneuron migration to the olfactory bulb in mice. Developmental biology 340 : 41–53.
32. CarfagniniF, TaniG, AmbrosettoP (1999) MR findings in Seckel's syndrome: report of a case. Pediatric radiology 29 : 849–850.
33. AbueloD (2007) Microcephaly syndromes. Seminars in pediatric neurology 14 : 118–127.
34. CapovillaG, LorenzettiME, MontagniniA, BorgattiR, PiccinelliP, et al. (2001) Seckel's syndrome and malformations of cortical development: report of three new cases and review of the literature. Journal of child neurology 16 : 382–386.
35. ShanskeA, CarideDG, Menasse-PalmerL, BogdanowA, MarionRW (1997) Central nervous system anomalies in Seckel syndrome: report of a new family and review of the literature. American journal of medical genetics 70 : 155–158.
36. GruberR, ZhouZ, SukchevM, JoerssT, FrappartPO, et al. (2011) MCPH1 regulates the neuroprogenitor division mode by coupling the centrosomal cycle with mitotic entry through the Chk1-Cdc25 pathway. Nature cell biology 13 : 1325–1334.
37. LizarragaSB, MargossianSP, HarrisMH, CampagnaDR, HanAP, et al. (2010) Cdk5rap2 regulates centrosome function and chromosome segregation in neuronal progenitors. Development 137 : 1907–1917.
38. Sanchez-AndradeG, KendrickKM (2011) Roles of alpha - and beta-estrogen receptors in mouse social recognition memory: effects of gender and the estrous cycle. Hormones and behavior 59 : 114–122.
39. KoganJH, FranklandPW, SilvaAJ (2000) Long-term memory underlying hippocampus-dependent social recognition in mice. Hippocampus 10 : 47–56.
40. EngelmannM, HadickeJ, NoackJ (2011) Testing declarative memory in laboratory rats and mice using the nonconditioned social discrimination procedure. Nature protocols 6 : 1152–1162.
41. GriffithE, WalkerS, MartinCA, VagnarelliP, StiffT, et al. (2008) Mutations in pericentrin cause Seckel syndrome with defective ATR-dependent DNA damage signaling. Nature genetics 40 : 232–236.
42. PoloSE, JacksonSP (2011) Dynamics of DNA damage response proteins at DNA breaks: a focus on protein modifications. Genes & development 25 : 409–433.
43. ZhivotovskyB, KroemerG (2004) Apoptosis and genomic instability. Nature reviews Molecular cell biology 5 : 752–762.
44. AdiyamanP, BerberogluM, AycanZ, EvliyaogluO, OcalG (2004) Seckel-like syndrome: a patient with precocious puberty associated with nonclassical congenital adrenal hyperplasia. Journal of pediatric endocrinology & metabolism: JPEM 17 : 105–110.
45. StoppoloniG, StabileM, RinaldiMM, PriscoF, RabuanoRG, et al. (1992) Seckel syndrome: report of three sibships with the type I primordial dwarfism. Possible linkage with HLA locus. Annales de genetique 35 : 213–216.
46. DaughadayW (1941) A comparison of the X-zone of the adrenal cortex in two inbred strains of mice. Cancer Research 1 : 883–885.
47. ReddyS, StarrC (2007) Seckel syndrome and spontaneously dislocated lenses. Journal of cataract and refractive surgery 33 : 910–912.
48. GuirgisMF, LamBL, HowardCW (2001) Ocular manifestations of Seckel syndrome. American journal of ophthalmology 132 : 596–597.
49. CanE, BulbulA, UsluS, DemirinH, ComertS, et al. (2010) A case of Seckel syndrome with Tetralogy of Fallot. Genetic counseling 21 : 49–51.
50. UcarB, KilicZ, DinleyiciEC, YakutA, DogruelN (2004) Seckel syndrome associated with atrioventricular canal defect: a case report. Clinical dysmorphology 13 : 53–55.
51. RappenU, von BrenndorffAI (1993) [Cardiac symptoms in 2 patients with Seckel syndrome]. Monatsschrift Kinderheilkunde: Organ der Deutschen Gesellschaft fur Kinderheilkunde 141 : 584–586.
52. HowanietzH, FrischH, Jedlicka-KohlerI, StegerH (1989) [Seckel dwarfism based on a personal case]. Klinische Padiatrie 201 : 139–141.
53. FukudaS, MorishitaY, HashiguchiM, TairaA (1991) [Seckel's syndrome associated with atrial septal defect: a case report and review of the literature in Japan]. Kyobu geka The Japanese journal of thoracic surgery 44 : 411–413.
54. Elwell M, Mahler J (1999) Pathology of the Mouse; Maronpot R, editor: Cache River Press.
55. LiuZ, YueS, ChenX, KubinT, BraunT (2010) Regulation of cardiomyocyte polyploidy and multinucleation by CyclinG1. Circulation research 106 : 1498–1506.
56. KeenanCM, VidalJD (2006) Standard morphologic evaluation of the heart in the laboratory dog and monkey. Toxicologic pathology 34 : 67–74.
57. ChoJH, ChangCJ, ChenCY, TangTK (2006) Depletion of CPAP by RNAi disrupts centrosome integrity and induces multipolar spindles. Biochem Biophys Res Commun 339 : 742–747.
58. MayerTU, KapoorTM, HaggartySJ, KingRW, SchreiberSL, et al. (1999) Small molecule inhibitor of mitotic spindle bipolarity identified in a phenotype-based screen. Science 286 : 971–974.
59. GergelyF, BastoR (2008) Multiple centrosomes: together they stand, divided they fall. Genes & development 22 : 2291–2296.
60. AldertonGK, JoenjeH, VaronR, BorglumAD, JeggoPA, et al. (2004) Seckel syndrome exhibits cellular features demonstrating defects in the ATR-signalling pathway. Human molecular genetics 13 : 3127–3138.
61. CimprichKA, CortezD (2008) ATR: an essential regulator of genome integrity. Nature reviews Molecular cell biology 9 : 616–627.
62. PommierY (2006) Topoisomerase I inhibitors: camptothecins and beyond. Nature reviews Cancer 6 : 789–802.
63. O'DriscollM, GenneryAR, SeidelJ, ConcannonP, JeggoPA (2004) An overview of three new disorders associated with genetic instability: LIG4 syndrome, RS-SCID and ATR-Seckel syndrome. DNA repair 3 : 1227–1235.
64. KjaerI, HansenN, BecktorKB, BirkebaekN, BalslevT (2001) Craniofacial morphology, dentition, and skeletal maturity in four siblings with Seckel syndrome. The Cleft palate-craniofacial journal: official publication of the American Cleft Palate-Craniofacial Association 38 : 645–651.
65. GotzM, HuttnerWB (2005) The cell biology of neurogenesis. Nature reviews Molecular cell biology 6 : 777–788.
66. LuB, JanL, JanYN (2000) Control of cell divisions in the nervous system: symmetry and asymmetry. Annual review of neuroscience 23 : 531–556.
67. EvansPD, VallenderEJ, LahnBT (2006) Molecular evolution of the brain size regulator genes CDK5RAP2 and CENPJ. Gene 375 : 75–79.
68. CrastaK, GanemNJ, DagherR, LantermannAB, IvanovaEV, et al. (2012) DNA breaks and chromosome pulverization from errors in mitosis. Nature 482 : 53–58.
69. RogakouEP, Nieves-NeiraW, BoonC, PommierY, BonnerWM (2000) Initiation of DNA fragmentation during apoptosis induces phosphorylation of H2AX histone at serine 139. The Journal of biological chemistry 275 : 9390–9395.
70. BastoR, LauJ, VinogradovaT, GardiolA, WoodsCG, et al. (2006) Flies without centrioles. Cell 125 : 1375–1386.
71. DobbelaereJ, JosueF, SuijkerbuijkS, BaumB, TaponN, et al. (2008) A genome-wide RNAi screen to dissect centriole duplication and centrosome maturation in Drosophila. PLoS biology 6: e224.
72. GraserS, StierhofYD, LavoieSB, GassnerOS, LamlaS, et al. (2007) Cep164, a novel centriole appendage protein required for primary cilium formation. The Journal of cell biology 179 : 321–330.
73. UetakeY, SluderG (2010) Prolonged prometaphase blocks daughter cell proliferation despite normal completion of mitosis. Current biology: CB 20 : 1666–1671.
74. OrthJD, LoewerA, LahavG, MitchisonTJ (2012) Prolonged mitotic arrest triggers partial activation of apoptosis, resulting in DNA damage and p53 induction. Molecular biology of the cell 23 : 567–576.
75. JanssenA, van der BurgM, SzuhaiK, KopsGJ, MedemaRH (2011) Chromosome segregation errors as a cause of DNA damage and structural chromosome aberrations. Science 333 : 1895–1898.
76. StorchovaZ, PellmanD (2004) From polyploidy to aneuploidy, genome instability and cancer. Nature reviews Molecular cell biology 5 : 45–54.
77. BorelF, LohezOD, LacroixFB, MargolisRL (2002) Multiple centrosomes arise from tetraploidy checkpoint failure and mitotic centrosome clusters in p53 and RB pocket protein-compromised cells. Proceedings of the National Academy of Sciences of the United States of America 99 : 9819–9824.
78. ThoolenB, MaronpotRR, HaradaT, NyskaA, RousseauxC, et al. (2010) Proliferative and nonproliferative lesions of the rat and mouse hepatobiliary system. Toxicologic pathology 38 : 5S–81S.
79. HoeijmakersJH (2009) DNA damage, aging, and cancer. The New England journal of medicine 361 : 1475–1485.
80. JefferyAN, MetcalfBS, HoskingJ, StreeterAJ, VossLD, et al. (2012) Age before stage: insulin resistance rises before the onset of puberty: a 9-year longitudinal study (EarlyBird 26). Diabetes care 35 : 536–541.
81. Huang-DoranI, BicknellLS, FinucaneFM, RochaN, PorterKM, et al. (2011) Genetic defects in human pericentrin are associated with severe insulin resistance and diabetes. Diabetes 60 : 925–935.
82. KlingseisenA, JacksonAP (2011) Mechanisms and pathways of growth failure in primordial dwarfism. Genes & development 25 : 2011–2024.
83. JurczykA, PinoSC, O'Sullivan-MurphyB, AddorioM, LidstoneEA, et al. (2010) A novel role for the centrosomal protein, pericentrin, in regulation of insulin secretory vesicle docking in mouse pancreatic beta-cells. PloS one 5: e11812.
84. RichtsmeierJT, BaxterLL, ReevesRH (2000) Parallels of craniofacial maldevelopment in Down syndrome and Ts65Dn mice. Developmental dynamics: an official publication of the American Association of Anatomists 217 : 137–145.
85. MahajanVB, SkeieJM, AssefniaAH, MahajanM, TsangSH (2011) Mouse eye enucleation for remote high-throughput phenotyping. Journal of visualized experiments: JoVE 57: e3184.
86. HancockJM, AdamsNC, AidinisV, BlakeA, BogueM, et al. (2007) Mouse Phenotype Database Integration Consortium: integration [corrected] of mouse phenome data resources. Mammalian genome: official journal of the International Mammalian Genome Society 18 : 157–163.
87. MasuyaH, InoueM, WadaY, ShimizuA, NaganoJ, et al. (2005) Implementation of the modified-SHIRPA protocol for screening of dominant phenotypes in a large-scale ENU mutagenesis program. Mammalian genome: official journal of the International Mammalian Genome Society 16 : 829–837.
88. DertingerSD, TorousDK, TometskoKR (1996) Simple and reliable enumeration of micronucleated reticulocytes with a single-laser flow cytometer. Mutation research 371 : 283–292.
89. PierceMJ, MorseRP (2012) The neurologic findings in Taybi-Linder syndrome (MOPD I/III): case report and review of the literature. American journal of medical genetics Part A 158A: 606–610.
90. LoizouJI, SanchoR, KanuN, BollandDJ, YangF, et al. (2011) ATMIN is required for maintenance of genomic stability and suppression of B cell lymphoma. Cancer Cell 19 : 587–600.
91. ShannonP, MarkielA, OzierO, BaligaNS, WangJT, et al. (2003) Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome research 13 : 2498–2504.
92. MartinA, OchagaviaME, RabasaLC, MirandaJ, Fernandez-de-CossioJ, et al. (2010) BisoGenet: a new tool for gene network building, visualization and analysis. BMC bioinformatics 11 : 91.
93. AssenovY, RamirezF, SchelhornSE, LengauerT, AlbrechtM (2008) Computing topological parameters of biological networks. Bioinformatics 24 : 282–284.
94. KamburovA, WierlingC, LehrachH, HerwigR (2009) ConsensusPathDB–a database for integrating human functional interaction networks. Nucleic acids research 37: D623–628.
95. Team RDC (2011) R: A language and environment for statistical computing. Vienna, Austria.: R Foundation for Statistical Computing.
Štítky
Genetika Reprodukční medicína
Článek The Covariate's DilemmaČlánek Plant Vascular Cell Division Is Maintained by an Interaction between PXY and Ethylene SignallingČlánek Lessons from Model Organisms: Phenotypic Robustness and Missing Heritability in Complex Disease
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 11
-
Všechny články tohoto čísla
- Inference of Population Splits and Mixtures from Genome-Wide Allele Frequency Data
- The Covariate's Dilemma
- Plant Vascular Cell Division Is Maintained by an Interaction between PXY and Ethylene Signalling
- Plan B for Stimulating Stem Cell Division
- Discovering Thiamine Transporters as Targets of Chloroquine Using a Novel Functional Genomics Strategy
- Is a Modifier of Mutations in Retinitis Pigmentosa with Incomplete Penetrance
- Evolutionarily Ancient Association of the FoxJ1 Transcription Factor with the Motile Ciliogenic Program
- Genome Instability Caused by a Germline Mutation in the Human DNA Repair Gene
- Transcription Factor Oct1 Is a Somatic and Cancer Stem Cell Determinant
- Controls of Nucleosome Positioning in the Human Genome
- Disruption of Causes Defective Meiotic Recombination in Male Mice
- A Novel Human-Infection-Derived Bacterium Provides Insights into the Evolutionary Origins of Mutualistic Insect–Bacterial Symbioses
- Trps1 and Its Target Gene Regulate Epithelial Proliferation in the Developing Hair Follicle and Are Associated with Hypertrichosis
- Zcchc11 Uridylates Mature miRNAs to Enhance Neonatal IGF-1 Expression, Growth, and Survival
- Population-Based Resequencing of in 10,330 Individuals: Spectrum of Genetic Variation, Phenotype, and Comparison with Extreme Phenotype Approach
- HP1a Recruitment to Promoters Is Independent of H3K9 Methylation in
- Transcription Elongation and Tissue-Specific Somatic CAG Instability
- A Germline Polymorphism of DNA Polymerase Beta Induces Genomic Instability and Cellular Transformation
- Interallelic and Intergenic Incompatibilities of the () Gene in Mouse Hybrid Sterility
- Comparison of Mitochondrial Mutation Spectra in Ageing Human Colonic Epithelium and Disease: Absence of Evidence for Purifying Selection in Somatic Mitochondrial DNA Point Mutations
- Mutations in the Transcription Elongation Factor SPT5 Disrupt a Reporter for Dosage Compensation in Drosophila
- Evolution of Minimal Specificity and Promiscuity in Steroid Hormone Receptors
- Blockade of Pachytene piRNA Biogenesis Reveals a Novel Requirement for Maintaining Post-Meiotic Germline Genome Integrity
- RHOA Is a Modulator of the Cholesterol-Lowering Effects of Statin
- MIG-10 Functions with ABI-1 to Mediate the UNC-6 and SLT-1 Axon Guidance Signaling Pathways
- Loss of the DNA Methyltransferase MET1 Induces H3K9 Hypermethylation at PcG Target Genes and Redistribution of H3K27 Trimethylation to Transposons in
- Genome-Wide Association Studies Reveal a Simple Genetic Basis of Resistance to Naturally Coevolving Viruses in
- The Principal Genetic Determinants for Nasopharyngeal Carcinoma in China Involve the Class I Antigen Recognition Groove
- Molecular, Physiological, and Motor Performance Defects in DMSXL Mice Carrying >1,000 CTG Repeats from the Human DM1 Locus
- Genomic Study of RNA Polymerase II and III SNAP-Bound Promoters Reveals a Gene Transcribed by Both Enzymes and a Broad Use of Common Activators
- Long Telomeres Produced by Telomerase-Resistant Recombination Are Established from a Single Source and Are Subject to Extreme Sequence Scrambling
- The Yeast SR-Like Protein Npl3 Links Chromatin Modification to mRNA Processing
- Deubiquitylation Machinery Is Required for Embryonic Polarity in
- dJun and Vri/dNFIL3 Are Major Regulators of Cardiac Aging in Drosophila
- CtIP Is Required to Initiate Replication-Dependent Interstrand Crosslink Repair
- Notch-Mediated Suppression of TSC2 Expression Regulates Cell Differentiation in the Intestinal Stem Cell Lineage
- A Combination of H2A.Z and H4 Acetylation Recruits Brd2 to Chromatin during Transcriptional Activation
- Network Analysis of a -Mouse Model of Autosomal Dominant Polycystic Kidney Disease Identifies HNF4α as a Disease Modifier
- Mitosis in Neurons: Roughex and APC/C Maintain Cell Cycle Exit to Prevent Cytokinetic and Axonal Defects in Photoreceptor Neurons
- CELF4 Regulates Translation and Local Abundance of a Vast Set of mRNAs, Including Genes Associated with Regulation of Synaptic Function
- Mechanisms Employed by to Prevent Ribonucleotide Incorporation into Genomic DNA by Pol V
- The Genomes of the Fungal Plant Pathogens and Reveal Adaptation to Different Hosts and Lifestyles But Also Signatures of Common Ancestry
- A Genome-Scale RNA–Interference Screen Identifies RRAS Signaling as a Pathologic Feature of Huntington's Disease
- Lessons from Model Organisms: Phenotypic Robustness and Missing Heritability in Complex Disease
- Population Genomic Scan for Candidate Signatures of Balancing Selection to Guide Antigen Characterization in Malaria Parasites
- Tissue-Specific Regulation of Chromatin Insulator Function
- Disruption of Mouse Cenpj, a Regulator of Centriole Biogenesis, Phenocopies Seckel Syndrome
- Genome, Functional Gene Annotation, and Nuclear Transformation of the Heterokont Oleaginous Alga CCMP1779
- Antagonistic Gene Activities Determine the Formation of Pattern Elements along the Mediolateral Axis of the Fruit
- Lung eQTLs to Help Reveal the Molecular Underpinnings of Asthma
- Identification of the First ATRIP–Deficient Patient and Novel Mutations in ATR Define a Clinical Spectrum for ATR–ATRIP Seckel Syndrome
- Cooperativity of , , and in Malignant Breast Cancer Evolution
- Loss of Prohibitin Membrane Scaffolds Impairs Mitochondrial Architecture and Leads to Tau Hyperphosphorylation and Neurodegeneration
- Microhomology Directs Diverse DNA Break Repair Pathways and Chromosomal Translocations
- MicroRNA–Mediated Repression of the Seed Maturation Program during Vegetative Development in
- Selective Pressure Causes an RNA Virus to Trade Reproductive Fitness for Increased Structural and Thermal Stability of a Viral Enzyme
- The Tumor Suppressor Gene Retinoblastoma-1 Is Required for Retinotectal Development and Visual Function in Zebrafish
- Regions of Homozygosity in the Porcine Genome: Consequence of Demography and the Recombination Landscape
- Histone Methyltransferases MES-4 and MET-1 Promote Meiotic Checkpoint Activation in
- Polyadenylation-Dependent Control of Long Noncoding RNA Expression by the Poly(A)-Binding Protein Nuclear 1
- A Unified Method for Detecting Secondary Trait Associations with Rare Variants: Application to Sequence Data
- Genetic and Biochemical Dissection of a HisKA Domain Identifies Residues Required Exclusively for Kinase and Phosphatase Activities
- Informed Conditioning on Clinical Covariates Increases Power in Case-Control Association Studies
- Biochemical Diversification through Foreign Gene Expression in Bdelloid Rotifers
- Genomic Variation and Its Impact on Gene Expression in
- Spastic Paraplegia Mutation N256S in the Neuronal Microtubule Motor KIF5A Disrupts Axonal Transport in a HSP Model
- Lamin B1 Polymorphism Influences Morphology of the Nuclear Envelope, Cell Cycle Progression, and Risk of Neural Tube Defects in Mice
- A Targeted Glycan-Related Gene Screen Reveals Heparan Sulfate Proteoglycan Sulfation Regulates WNT and BMP Trans-Synaptic Signaling
- Dopaminergic D2-Like Receptors Delimit Recurrent Cholinergic-Mediated Motor Programs during a Goal-Oriented Behavior
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Mechanisms Employed by to Prevent Ribonucleotide Incorporation into Genomic DNA by Pol V
- Inference of Population Splits and Mixtures from Genome-Wide Allele Frequency Data
- Zcchc11 Uridylates Mature miRNAs to Enhance Neonatal IGF-1 Expression, Growth, and Survival
- Histone Methyltransferases MES-4 and MET-1 Promote Meiotic Checkpoint Activation in
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



