-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamamalERA: An updated research agenda for combination interventions and modelling in malaria elimination and eradication
Richard Steketee and colleagues propose an updated research agenda for combination interventions and modelling in malaria elimination and eradication.
Published in the journal: . PLoS Med 14(11): e32767. doi:10.1371/journal.pmed.1002453
Category: Collection Review
doi: https://doi.org/10.1371/journal.pmed.1002453Summary
Richard Steketee and colleagues propose an updated research agenda for combination interventions and modelling in malaria elimination and eradication.
Summary points
Since 2011, there have been significant improvements in the development, organisation, and infrastructure of country programmes for malaria control and elimination globally. This has included the increasing use of combinations of interventions against the mosquito vector and the parasite in humans to reduce transmission in large and expanding geographies and populations and an adaptation of these interventions as transmission is progressively reduced.
Similarly, there has been substantial improvement in the sophistication and field validation of malaria transmission models and their ability to describe and predict the effects of ecologic changes and the impact of specific interventions. These advances permit the investigation and comparison of multiple complementary interventions in elimination settings.
There is an increasing need to combine interventions into ‘packages’ that can be tailored to specific settings based on the characteristics of their transmission dynamics and epidemiology (landscape stratification). The challenge is to identify the complementary components of each intervention package and establish the triggers and thresholds for their deployment (or withdrawal) throughout the elimination process, including maintaining elimination once transmission has been interrupted.
Introduction
In 2011, the Malaria Elimination Research Agenda (malERA) made recommendations for how mathematical modelling efforts could best inform policy and guide research for specific intervention tools for elimination—diagnostics, drugs, vector control, and vaccines [1]. Since then, experience with malaria intervention tools has grown, and the toolbox has expanded with new drugs, new insecticides, better diagnostics, and a first vaccine [2]. As more countries seek elimination, grouping tools to best address diverse and changing transmission intensity has become a central issue. Some tools are oriented primarily towards reducing disease burden, e.g., seasonal malaria chemoprevention; others are dedicated to reducing transmission, e.g., drug-based population-wide parasite clearance; and some meet both of these objectives, e.g., vector control. Thus, not all tools will contribute equally to malaria elimination, and the timing and duration of their use must adapt as programmes progress.
This paper summarises progress since the initial malERA publication regarding transmission-aligned ‘elimination tool packages’ and deployment strategies and opportunities for models to help inform and prioritise intervention choices. The findings come from an extensive literature review of published and unpublished materials and the deliberations of the 2015 malERA Refresh Consultative Panel on Combination Interventions and Modelling, which includes specialists from malaria modelling, field researchers, and National Malarial Control Programme (NMCP) representatives [3].
Methods
The findings presented in this paper result from an extensive literature review of published and unpublished materials and the deliberations of the 2015 malERA Refresh Consultative Panel on Combination Interventions and Modelling. Electronic databases were systematically searched for published literature from 1 January 2010 until 1 August 2015, without language limitations. The websites of the institutions that apply modelling techniques to malaria research questions and the MESA Track database of current research projects relevant to malaria elimination were systematically searched to identify pertinent ongoing research. Panellists were invited to recommend additional literature and additional ongoing research projects. The comprehensive search for literature and ongoing research provided the basis for launching the second step.
A 2-day workshop was held with the majority of the panel members, including specialists from malaria modelling, field researchers, and NMCP representatives. The panel broke into 2 working groups to identify the issues in combining interventions and how mathematical modelling could be applied to these problems. Each group fed back to a plenary session in which further robust discussions and input occurred. This helped refine the opportunities and gap areas in which research is needed. The final findings were arrived at with inputs from all panellists and several iterations of the manuscript.
Intervention packages to achieve elimination
Over the past 5 years, regardless of initial local transmission levels, most countries have continued to reduce the clinical burden of malaria and transmission [4]. The World Health Organization (WHO) recently published its Global Technical Strategy (GTS) for Malaria 2016–2030 (Fig 1) [5]. This builds on the core activities of vector control, case management, and surveillance, with additional interventions to accelerate progress to elimination. In the GTS, for the first time, modelling studies were used to support goal setting [5].
Fig. 1. Schematic of the pillars and supporting elements of the World Health Organization (WHO) Global Technical Strategy for Malaria 2016–2030 (source: WHO, 2015) [<em class="ref">5</em>]. ![Schematic of the pillars and supporting elements of the World Health Organization (WHO) Global Technical Strategy for Malaria 2016–2030 (source: WHO, 2015) [<em class="ref">5</em>].](https://www.prolekarniky.cz/media/cache/resolve/media_object_image_small/media/image/d674ea6b56f101dc4c97e086246e277e.png)
The malERA Refresh Consultative Panel on Combination Interventions and Modelling approach encompassed the full spectrum of malaria transmission—addressing emerging programmatic aims and combining into ‘packages’ the available tools and strategies directed towards malaria elimination (Fig 2). As transmission is reduced to very low levels, the intervention packages must adapt to increasingly focal and heterogeneous populations, in which infections are rare. Given the extensive range of available tools and the diversity/heterogeneity of transmission settings, it becomes difficult to field test all possible intervention packages. Models can assist the prioritisation and design of clinical trials and in the choice of an intervention package to achieve their desired goals.
Fig. 2. An example of the role of modelling across the spectrum of malaria elimination. 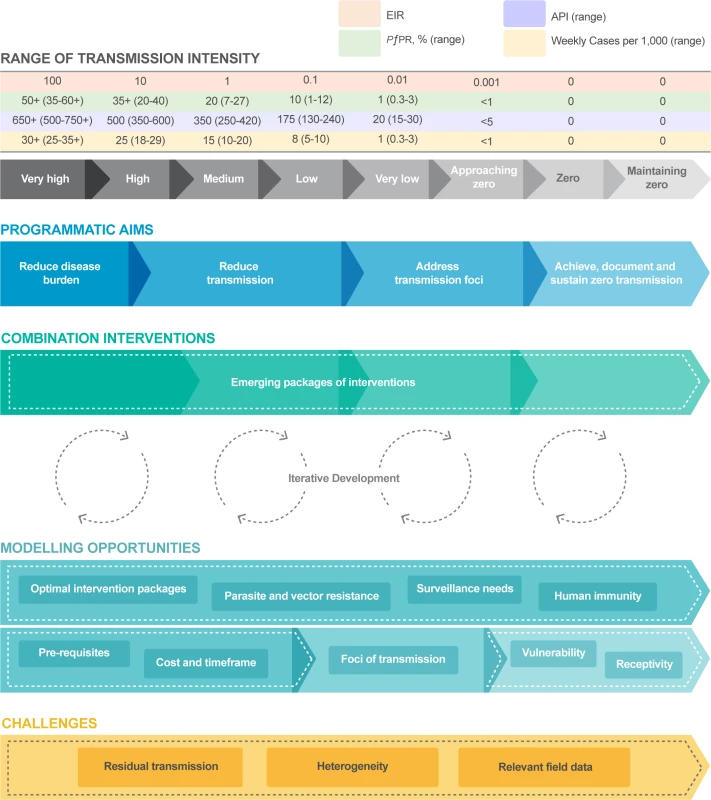
Note that the measures of transmission are based on sub-Saharan Africa, and other constructs and transmission levels may be relevant in different geographical areas. Malaria transmission intensity measures and the relationship entomologic inoculation rate for Plasmodium falciparum from very high to zero transmission are adapted from data presented in [6]; personal communication from D. Smith and P. Gething. Zero refers to no locally transmitted cases of malaria infection; imported infections may be identified. Intervention package components and sequencing will depend on transmission intensity at the start of the elimination programme, the speed at which transmission declines, and the underlying typology (i.e., malaria epidemiology, species, vector ecology, and health system factors). EIR, entomologic inoculation rate: average number of infectious mosquito bites per person per year; N.B. the table is organised by log differences in the EIR, and other measures are aligned (approximated) based on these entomologic measures. PfPR, P. falciparum parasite rate: proportion of people with a current infection with P. falciparum—typically determined by a population-based survey and often timed to a specific interval of the transmission season. API, annual parasite index: number of confirmed malaria cases per 1,000 population per year. Cases, cases per health facility per week: average number of confirmed malaria cases expected to present on an average week to a health facility serving a population of 5,000 people. Because many infections can be asymptomatic at any point in time (and thus not present to health services), the proportion of asymptomatic individuals varies with transmission intensity, and because most transmission is seasonal, these average estimates may vary substantially by location and season. Progress in combination interventions and modelling
Initial malERA recommendations for a research and development agenda in mathematical modelling are shown in Box 1 [1]. Subsequently, the scope and depth of research has expanded to include diverse vector control strategies, complex diagnostics, drug and vaccine dynamics, and deployment strategies. Additionally, infection models have advanced following incorporation of new field trial data, particularly regarding mass drug administration (MDA) and specific aspects of vector control, providing greater plausibility to model predictions.
Box 1. 2011 malERA research agenda for modelling to support malaria elimination.
Further development of models and model systems:
Within-host dynamics of Plasmodium infections
The human infectious reservoir
Bionomics and ecology of the vectors
Dynamics of the stimulation and decay of human immunity across a range of transmission settings
Heterogeneities in host, vector, and parasite dynamics
Heterogeneities in host and vector movements
Drug pharmacokinetics/pharmacodynamics
Vaccines that interrupt malaria transmission
Ecology of genetically modified mosquitoes
Development and impact of drug and pesticide resistance
Integration of health system attributes and linking to microeconomic outputs
The interface between modelling and implementation has not developed as was perhaps envisaged, in terms of appropriate portals to allow 'end users' access to relevant software and explore the effect of varying conditions on the ideal choice of control measures. However, the development, organisation, and infrastructure of malaria modelling has improved (Box 2), and recent efforts include an expansion of open-access data and software [6–13]. Also, modelling has been incorporated at the policy level within WHO [5] and included in planning tools for malaria elimination [14]. Wider implementation is possibly now dependent upon the development of next-generation models that sufficiently address combination interventions against a background of heterogeneity and low transmission as more countries move towards elimination.
Box 2. Recent advances in malaria modelling.
Communications:
A growing number of modelling groups are working in a collaborative fashion
Greater engagement between modellers, country programmes, and operational research partners has helped refine the paramount research questions
Models:
The development of model systems that are diverse but much improved in terms of their incorporation of malaria biology and natural history, as well as validated estimates for intervention effects, drug pharmacokinetics/ pharmacodynamics, and vaccine dynamics
The development of models that allow the investigation of target product profiles for new tools—for example, diagnostics, surveillance systems, and drugs
Infrastructure:
Greater dissemination of malaria models at different levels of user-interface complexity, through online hosting and open-source code repositories leading to wider access to modelling information for programme implementers, planners, and policy decision makers
Improved means of compiling data and using common ontologies, frameworks, and metadata standards with growing international databases of some measures of malaria transmission, e.g., parasite rate surveys
These advances are complemented by discoveries in basic science, large field trials of new and existing interventions, and substantial data gathering efforts that provide the raw evidence to further validate models. A number of recent reports used models to address the role of multiple complementary interventions (Table 1) [15–35], and additional field trials are ongoing (Table 2) [29].
Tab. 1. Key modelling studies on combination interventions quarter 4 2010–quarter 1 2016, with the main outcome indicated. 
ITN, insecticide-treated bed net; LLIN, long-lasting insecticidal bed net; MSAT, mass screening and treatment. Tab. 2. Ongoing field studies in combination interventions as reported on the MESA Track database [29]. ![Ongoing field studies in combination interventions as reported on the MESA Track database [<em class="ref">29</em>].](https://www.prolekarniky.cz/media/cache/resolve/media_object_image_small/media/image/4640fe89d03507233b8486c689f12446.png)
IRS, indoor residual spraying; LLIN, long-lasting insecticidal net; SE, Southeast. Consensus modelling
In consensus modelling, independent modelling groups examine the same research question, sometimes using the same source dataset to parameterise their model. Through objective comparison and critique, modelling groups have reached a degree of consensus on important issues, such as the relationship between health burden and transmission intensity [6], and have undertaken an in-depth analysis for the RTS,S vaccine [36]. Such efforts are resource intensive but may give robust answers incorporating the breadth of uncertainty in our understanding. There is also value in less intensive forms of model comparison in which common findings from work conducted independently are assessed (Table 3) [9,18,21,23,24,28,30–32,35,37–58]. This approach can also be particularly useful for identifying areas in which there is a lack of consensus, as this can focus efforts on further model development, basic science, and field data collection needs.
Tab. 3. Consensus across multiple groups from modelling analyses conducted by each of the Malaria Modelling Consortiuma groups, which assessed impact on malaria transmission of combining multiple interventions or multiple methods of using a single interventionb. 
a Imperial College, London, United Kingdom; Institute for Disease Modelling, Seattle, Washington, US; Mahidol Oxford Tropical Medicine Research Unit, Bangkok, Thailand; Swiss Tropical and Public Health Institute, Basel, Switzerland; and University of Oxford, Oxford, UK. Next steps for combination interventions and modelling in malaria elimination
Fig 2 provides an example of how transmission strata, programmatic aims, the choices of intervention packages, and the iterative development between modelling and programme choices change together as malaria transmission intensity is progressively reduced towards zero, summarizing key opportunities and identifying challenges. Note that not all countries will start from high transmission levels and that the measures of transmission used in Fig 2 are based on sub-Saharan Africa. Thus, other constructs and transmission levels may be relevant in different geographical areas.
Opportunities
Combination intervention modelling
There has been considerable progress in modelling combination interventions. Models have been developed to examine the overall expected impact of diagnostic, drug, vaccine, and vector control intervention combinations, including cost-effectiveness [16,18–20,24,48,57,59–62], and comparing interventions added to the backbone of standard measures [21–23,25–27,30,36,63,64]. Modelling studies have investigated the applications of several new potential interventions such as the RTS,S vaccine [36], ivermectin [19,54], mosquito traps [17], and next-generation diagnostics [25,33,65,66] and have highlighted critical attributes of new products, such as a preerythrocytic vaccine [20,67–69], genetically modified mosquitos [70–72], and combinations of future interventions [73].
Models are designed to allow scale-up and scale-down of interventions over time. The next step is to define the epidemiological information that would be most informative for making such dynamic changes and the triggers for switching or scaling. The aim is to develop a set of rules that define the characteristics of transmission that can direct specific changes in the composition and phasing of intervention packages and their targeting to specific locations and populations. These predictions can then be evaluated with further evidence from specific field trials. If reliable, such measures could be used in the subnational stratification of intervention packages.
Accelerating community clearance of malaria parasites
One hypothesis being tested in various settings is the potential to accelerate elimination by targeting the human parasite reservoir (symptomatic and asymptomatic) with time-limited deployment of community-based interventions such as MDA or mass screening and treatment (MSAT) [74]. If the intervention is justified, a wealth of modelling studies provides guidance on optimizing its deployment [15,18,28,32,33,41,53–55,57,75–79]. However, estimating the level of coverage required for successful MDA is critical [53], and for MSAT, the sensitivity of the diagnostic tool is an additional key determinant of efficacy as the current tests may fail to detect low-level infections [16,25].
Current models of MDA all include the parameters whereby immediately following MDA, there is a dramatic drop in malaria prevalence, but in the absence of elimination, prevalence returns to preintervention levels (albeit at different rates depending on the model) [53]. Country malaria programs are increasingly aware of this potential and have learned not to rely solely on MDA to eliminate transmission; thus, MDA is an accelerator used to move to a next set of interventions and strategies to find and clear the remaining transmission foci. The models must now be adapted to include a next set of actions with the potential to end transmission, i.e., MDA moving to focal MDA (fMDA) and other reactive strategies in households and neighbourhoods with rare but remaining transmission [33,79,80]. In the field, these increasingly infrequent actions will require robust local information systems as part of the intervention, rather than models.
Non-falciparum species
Recent progress has been made in models considering non-falciparum parasites and vectors, though further work is needed [76,81–95]. To address the public health and public engagement challenge of eliminating all human malaria species, multispecies mathematical models that consider unified strategies and exploit the interactions between the species for improved cost-effectiveness should be used [96]. Notably, where P. vivax is present, the malaria programme might be sustained even as P. falciparum becomes rare and is eliminated. However, different approaches to both surveillance and malaria interventions would be required to reduce the P. vivax burden while detecting P. falciparum cases and preventing the reestablishment of P. falciparum transmission.
Surveillance as an intervention
Surveillance is an intervention tool. When honed for elimination purposes, surveillance must evolve to be able to discover evidence of transmission; establish its location, timing, nature, and causes; identify and eliminate residual foci; prevent, detect, and contain imported malaria; and demonstrate the attainment and maintenance of zero malaria transmission [97]. As transmission declines, modification of data collection and reporting systems requires substantial investment and coordination across the malaria programmes and the surveillance management unit. Designing the necessary flexibility into a surveillance system to allow for adaptation to an elimination context will be critical.
There is an opportunity to use modelling to define the required components of surveillance systems depending on the stage of the elimination programme. This requires quantification of the detrimental effects of inaccurate, insufficient, or untimely surveillance and the beneficial effects of adding new measures to the surveillance system [25]. Modelling could also be used to assess the level of hidden/unidentifiable cases/infections that would hinder (or would not hinder) elimination (e.g., asymptomatic or individuals with minor symptomology who would not seek treatment). As transmission declines, the addition of serological measures of past exposure [65,98–102] or active community-based transmission measurements and reactive case management [103–107] may be considered. Modelling can estimate the incremental benefit of adding specific surveillance activities to an already established surveillance system and could examine cost-effectiveness issues [48,108], specific epidemiologic aspects of contract tracing [109], and the target product profile of diagnostics [25,65,66] in case-investigation or foci-investigation settings.
Parasite and vector resistance
As efforts to reduce transmission are intensified, the risk and impact of parasite drug resistance and vector insecticide resistance becomes a key concern [110–112]. Modelling has been used to investigate the effects of resistance [25,26,30,32,113–116], and there have been some studies examining risk factors for resistance and drug failure [114,117–119]. Geostatistical models are also being developed to predict localities where resistance might be present in order to target surveillance activities, for example, mapping artemisinin-resistance in Southeast Asia [120]. The biology and natural history of mosquito vectors and malaria parasites tells us that the development and evolution of resistance will continue, given the pressure of insecticides and drugs. In terms of drug treatments, with artemisinin-based combination therapies (ACTs) globally recommended for malaria treatment, the focus must be on investigation of artemisinin and partner drug resistance, in terms of how this can be contained within the Greater Mekong subregion [111], and how its emergence or importation can be avoided in other regions [25,115]. Note that as transmission declines, the remaining parasites are those most likely to harbour resistance. Thus, even as malaria cases decline, continued field studies and modelling must be supported to address the efficacy and effectiveness of intervention tools critical for elimination programming. The next steps are to investigate how packages of interventions can be modified to mitigate the effects of resistance on existing interventions [30,121–123], how resistance can be contained [32], and how resistance can be avoided, particularly for new drugs and insecticides [124,125].
Human immunity
A gradual decline in human immunity to malaria across the population is an inevitable consequence of reducing malaria transmission and contracting parasite diversity [126,127]. The resulting delay in acquiring immunity likely will alter the age distribution and severity of malaria infections [126,128,129]. Understanding these changes is necessary to identify the most vulnerable populations or those most likely to need an intervention [128,130]. Models already include age-dependent immune factors and have dynamic modulation of immunity as a function of entomological inoculation rate [128,131], though additional temporal data could help reduce the uncertainty surrounding these functions. Gaps remain in our understanding of immunity in areas of long-standing low transmission (e.g., Haiti), where the level of asymptomatic infections is much higher than previously thought [132].
Modelling to inform policy
Strategic decisions are already being taken as part of elimination planning in a number of countries. There are numerous opportunities for modelling to inform these decisions—for example, scenario planning. An Elimination Scenario Planning (ESP) toolkit was published by WHO in 2014 following field testing using data from The Gambia and Senegal [14]. The manual is linked to software that models malaria transmission (currently limited to P. falciparum in Africa), which allows users to explore the effect of a range of combinations of malaria control interventions in order to achieve elimination. Such an approach has wide application and could be extended to P. falciparum outside Africa or P. vivax settings in the future. A key consideration is that malaria policy will need to respond to climate change. Historical data may become less reliable as seasonal patterns of rainfall and land use alter. Mapping climate change effects and possible scenarios following the varied consequences of climate change for human and vector population distributions has been investigated at continental and national levels, but incorporating this into policy is more challenging [133–151].
Mathematical models can provide a framework for exploring the relationship between population movement, heterogeneous transmission, and the deployment logistics of a national or regional elimination strategy. To carry out such analyses, new model frameworks should be developed that benefit from new field and genetic data characterising and measuring spatially and temporally dynamic transmission routes.
There is an increasing demand from NMCPs for pertinent and prompt mathematical modelling analyses to support their malaria elimination strategies. Established modelling groups have engaged in local capacity building. Also, malaria modelling research is being published by research groups from malaria-endemic countries [33,34,62,89,152–154], and this trend could be supported to the benefit of NMCPs.
Modelling to maintain zero
As noted above, when transmission becomes rare, models are increasingly challenged in informing policy and intervention choices; similarly, when there is no transmission, the evaluation of risk for the reintroduction of infection (vulnerability) and the risk of propagating local transmission given its reintroduction (receptivity) can present challenges to models designed to answer questions at high endemicity levels. A new class of highly heterogeneous, stochastic malaria models is being developed to inform the design of an elimination surveillance system.
Vulnerability (risk of introduction or reintroduction)
Measuring vulnerability to malaria reintroduction requires pairing up-to-date maps of national and international parasite prevalence with human movement models. Both of these fields have advanced in recent years [33,34,117,155–160]. Human movement models, paired with travel survey and microcensus data, have improved their description of routine human movement (e.g., holiday season travel) [159,161]. Increasing use of mobile phones has enabled the tracking of human movement and permitted distribution advice on infection avoidance [159,162–164]. However, many national and international seasonal migrations remain difficult to predict, and their direct relationship to moving malaria infections requires additional investigation.
Receptivity (risk of transmission given introduction)
In order to direct interventions, models must incorporate both the risk of importation and the risk for the reestablishment of local transmission [165–173]. The risk of malaria transmission reestablishment can be measured as a function of selected host, vector, and environmental data [156,170,171, 174]. For example, measures might include human use of insecticide-treated bed nets or indoor residual spraying, mosquito habitat suitability and its link to abundance, and climatic conditions (e.g., temperature, rainfall, and vegetation index measures) that support or accelerate vector and parasite development. If such data are collected widely enough, models can be validated using the occasional areas that do experience local transmission. Deciding which environmental and entomological data would be most valuable to collect could be iteratively informed by testing hypotheses based on longitudinal data from areas that have recently eliminated malaria, for example, Sri Lanka. The next step is to translate risk mapping into programmatic actions, such as better allocation of human resources, and maintenance and targeting of vector control [50,175]. This will become increasingly important as more countries reach elimination.
Challenges
Residual transmission
Variable human and vector behaviours may enable sustained transmission in highly seasonal, heterogeneous environments, despite high intervention coverage [176]. The magnitude and importance of residual transmission in different settings require further field studies. In particular, human sociobehavioural data including human behaviour’s relevance for compliance and entomological data investigating the contribution of outdoor transmission are needed to develop models testing novel strategies and tools [102].
Low transmission and incorporating heterogeneity
Models have mostly been used to examine sub-Saharan Africa high transmission contexts with P. falciparum and relevant vector species, though they may be parameterised across the full spectrum of transmission. When modelling an isolated homogeneous population, it can be difficult to sustain transmission much below the 1% parasite prevalence level (though the precise level depends on the model), with the model becoming unstable, leading to ‘stochastic extinction’, i.e., the extinction of parasites based on random effects within the model, an effect that is compounded with increasing heterogeneity [177]. This suggests that importation of infections and local heterogeneities in host, vector, and parasite dynamics and in health service delivery systems are likely to play an important role in sustaining malaria in low transmission settings [178].
As a country progresses to very low levels of malaria transmission, the spatial and temporal heterogeneity of transmission increases in importance. In these contexts of varying historical transmission intensity, intervention coverage, human movement, and access to health system resources, malaria will tend to persist in the most remote regions and the poorest and most vulnerable populations [179,180]. While this issue may not require new models per se, heterogeneity will need to be better captured as transmission declines. Spatial heterogeneity is probably least well developed, and the required level of spatial granularity and relevant metrics for answering specific questions in low transmission settings requires definition [181,182]. However, at some point heterogeneity will exceed the ability of models to establish granularity, and decision making will require local health system and entomological data.
Modelling malaria at borders
When malaria transmission is moderate to high and similar on both sides of a border, often little attention is paid to border areas for specific disease interventions; however, this changes when one nation may be markedly reducing transmission and the other is not. Border areas present particular difficulties for malaria control and elimination efforts [183–187]. The complexity of human movements for trade, business, and visiting family, sometimes including vulnerable populations [188], and the coordination of efforts between different political and organisational frameworks increase the complexity of malaria control [184]. Some of the issues relate to spatial and temporal heterogeneity and could possibly be addressed with greater data on human cross-border movement and parasite genetics [189–191]. However, human factors, such as local conflicts, poverty, and the disenfranchisement of particular ethnic groups, can be highly variable in time and place and are more challenging to incorporate into transmission models [192,193]. Alternative complementary approaches include mapping malaria risk, for better targeting of resources, plus goal setting by modelling what could potentially be achieved with coordinated versus independent elimination campaigns [33,185,194,195]. Once the potential benefits are understood, the barriers to reaching these goals can be researched and the feasibility of overcoming them explored.
Iteration and validation
Finally, models directed at assessing combination interventions must embrace a process of iteration with field data. In particular, data are needed from low to near-zero transmission settings. Such data needs might include high-resolution geographic information on cases, frequency and location of associated secondary cases, travel history identifying infection sources, vector-associated data, climate, and environmental parameters [109]. The requirement for field data to validate models remains problematic, as field data on intervention efficacy and the diverse parameters noted above can be difficult to assemble. When developing models, validation requirements should be clearly defined and data should be feasible to obtain. Amidst these challenges, modellers then need to consider how to best contribute to and bear responsibility for the assembly of required field data. Although capacity building and integration of modellers into NMCPs may address this at a local scale, there is a need for innovative mechanisms to allow increased exchanges in malaria elimination research, to allow better access to field empirical data for modellers.
Conclusions
Given the ongoing social and economic impact of malaria-related mortality and morbidity and the inevitable resource constraints for national malaria programmes, identifying the most timely and most cost-effective path to malaria elimination is a priority. Box 3 presents a research and development agenda for combination interventions and modelling in malaria elimination. Modelling affords a feasible and practical means of investigating rational combinations of interventions and the most appropriate setting for their deployment. Nevertheless, without a substantive dataset from operations research, the construction of meaningful models is not possible. Models must also be continuously validated against field data, through programmatic experience and against clinical trials, with measures and outcomes data relevant to the transmission setting identified and collected for use in further model refinement. This is especially the case as we increasingly encounter transmission settings that are shrinking in size and number and becoming more focal and heterogeneous and for which there are fewer field data. Thus, there is a codependency between modelling and field data, and the quality of both must be assured for findings to be valid and impactful. Since malERA 2011, there has been significant progress in aligning modelling with programmatic requirements and more effective communication with policy makers. This ongoing dialogue will ultimately determine the relevance of modelling to policy decision and its contribution towards achieving and maintaining malaria elimination.
Box 3. Research and development agenda for combination interventions and modelling.
Determine which combinations of interventions to use in which sequence and in response to which triggers throughout elimination
Identify the circumstances in which time-limited elimination acceleration interventions, such as mass drug administration (MDA), are appropriate and what needs to be done to retain the gains in transmission reduction following their withdrawal
Model the effect of parasite drug and vector insecticide resistance on combination interventions and how resistance might be avoided or contained
Understand human immunity in areas where transmission has always been low and parasite diversity very low and modelling the effect of changes in human immunity as transmission declines
Identify which additional data would be most useful for validating or changing model predictions in order to drive iterative development and decision making
Surveillance as an intervention
Model the target product profile of an elimination-specific surveillance system
Determine the threshold at which reactive case strategies become feasible
Strategic modelling
Estimate the long-term costs of elimination in different settings and with different intervention packages
Assess the potential duration of an elimination campaign in various settings to help define the investment case and financing needs for elimination
Estimate the maximal impact of currently available tools on elimination in various settings
Determine the counterfactual to elimination, i.e., the effect of continuing current interventions in various settings
Support capacity building of modellers embedded in National Malaria Control Programmes (NMCPs)
Modelling to maintain zero
Investigate how vulnerability and receptivity measures can be translated into specific programme actions
Addressing transmission
Apply models to low transmission settings, incorporating all relevant parasites/vectors
Investigate the importance of residual transmission in different settings and what new strategies or novel tools are needed to overcome it
Incorporating heterogeneity
Determine the relevance of spatial and temporal heterogeneity in transmission in different settings
Investigate how much heterogeneity in transmission needs to be captured by models to make predictions in elimination settings
Iteration and validation
Determine which measures of transmission or other metrics are most appropriate for guiding programmatic decisions in low transmission to maintaining-zero settings
Define which new data need to be collected from low transmission to maintaining-zero settings in order to increase confidence in model predictions
Zdroje
1. malERA Consultative Group on Modeling. A research agenda for malaria eradication: modeling. PLoS Med. 2011;8(1):e1000403. doi: 10.1371/journal.pmed.1000403 21283605
2. The malERA Refresh Consultative Panel on Tools for Malaria Elimination. malERA: An updated research agenda for diagnostics, drugs, vaccines, and vector control in malaria elimination and eradication PLoS Med. 2017;14(11):e1002455. doi: 10.1371/journal.pmed.1002455
3. Rabinovich RN, Drakeley C, Djimde AA, Hall BF, Hay SI, Hemingway J, et al. malERA: An updated research agenda for malaria elimination and eradication. PLoS Med. 2017;14(11):e1002456. doi: 10.1371/journal.pmed.1002456
4. World Health Organization. World malaria report 2015 Geneva: WHO; 2015. Available from: http://www.who.int/malaria/publications/world-malaria-report-2015/report/en/.
5. World Health Organization. Global technical strategy for malaria 2016–2030 Geneva: WHO; 2015. Available from: http://apps.who.int/iris/bitstream/10665/176712/1/9789241564991_eng.pdf
6. Gething PW, Battle KE, Bhatt S, Smith DL, Eisele TP, Cibulskis RE, et al. Declining malaria in Africa: improving the measurement of progress. Malar J. 2014;13 : 39. doi: 10.1186/1475-2875-13-39 24479555
7. Battle KE, Guerra CA, Golding N, Duda KA, Cameron E, Howes RE, et al. Global database of matched Plasmodium falciparum and P. vivax incidence and prevalence records from 1985–2013. Sci Data. 2015;2 : 150012. doi: 10.1038/sdata.2015.12 26306203
8. Bejon P, White MT, Olotu A, Bojang K, Lusingu JP, Salim N, et al. Efficacy of RTS,S malaria vaccines: individual-participant pooled analysis of phase 2 data. Lancet Infect Dis. 2013;13(4):319–27. doi: 10.1016/S1473-3099(13)70005-7 23454164
9. Bhatt S, Weiss DJ, Cameron E, Bisanzio D, Mappin B, Dalrymple U, et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature. 2015;526(7572):207–11. doi: 10.1038/nature15535 26375008
10. Blagborough AM, Churcher TS, Upton LM, Ghani AC, Gething PW, Sinden RE. Transmission-blocking interventions eliminate malaria from laboratory populations. Nat Commun. 2013;4 : 1812. doi: 10.1038/ncomms2840 23652000
11. Cameron E, Battle KE, Bhatt S, Weiss DJ, Bisanzio D, Mappin B, et al. Defining the relationship between infection prevalence and clinical incidence of Plasmodium falciparum malaria. Nat Commun. 2015;6 : 8170. doi: 10.1038/ncomms9170 26348689
12. Moyes CL, Temperley WH, Henry AJ, Burgert CR, Hay SI. Providing open access data online to advance malaria research and control. Malar J. 2013;12 : 161. doi: 10.1186/1475-2875-12-161 23680401
13. Wu L, van den Hoogen LL, Slater H, Walker PG, Ghani AC, Drakeley CJ, et al. Comparison of diagnostics for the detection of asymptomatic Plasmodium falciparum infections to inform control and elimination strategies. Nature. 2015;528(7580):S86–93. doi: 10.1038/nature16039 26633770
14. World Health Organization. From malaria control to malaria elimination: a manual for elimination scenario planning Geneva: WHO; 2014. Available from: http://apps.who.int/iris/bitstream/10665/112485/1/9789241507028_eng.pdf
15. Gerardin J, Ouedraogo AL, McCarthy KA, Eckhoff PA, Wenger EA. Characterization of the infectious reservoir of malaria with an agent-based model calibrated to age-stratified parasite densities and infectiousness. Malar J. 2015;14 : 231. doi: 10.1186/s12936-015-0751-y 26037226
16. Gerardin J, Bever CA, Hamainza B, Miller JM, Eckhoff PA, Wenger EA. Optimal population-level infection detection strategies for malaria control and elimination in a spatial model of malaria transmission. PLoS Comput Biol. 2016;12(1):e1004707. doi: 10.1371/journal.pcbi.1004707 26764905
17. Marshall JM, White MT, Ghani AC, Schlein Y, Muller GC, Beier JC. Quantifying the mosquito's sweet tooth: modelling the effectiveness of attractive toxic sugar baits (ATSB) for malaria vector control. Malar J. 2013;12 : 291. doi: 10.1186/1475-2875-12-291 23968494
18. Okell LC, Griffin JT, Kleinschmidt I, Hollingsworth TD, Churcher TS, White MJ, et al. The potential contribution of mass treatment to the control of Plasmodium falciparum malaria. PLoS ONE. 2011;6(5):e20179. doi: 10.1371/journal.pone.0020179 21629651
19. Slater HC, Walker PG, Bousema T, Okell LC, Ghani AC. The potential impact of adding ivermectin to a mass treatment intervention to reduce malaria transmission: a modelling study. J Infect Dis. 2014;210(12):1972–80. doi: 10.1093/infdis/jiu351 24951826
20. Wenger EA, Eckhoff PA. A mathematical model of the impact of present and future malaria vaccines. Malar J. 2013;12 : 126. doi: 10.1186/1475-2875-12-126 23587051
21. Brady OJ, Godfray HC, Tatem AJ, Gething PW, Cohen JM, McKenzie FE, et al. Vectorial capacity and vector control: reconsidering sensitivity to parameters for malaria elimination. Trans R Soc Trop Med Hyg. 2016;110(2):107–17. doi: 10.1093/trstmh/trv113 26822603
22. Eckhoff P. Mathematical models of within-host and transmission dynamics to determine effects of malaria interventions in a variety of transmission settings. Am J Trop Med Hyg. 2013;88(5):817–27. doi: 10.4269/ajtmh.12-0007 23589530
23. Griffin JT, Bhatt S, Sinka ME, Gething PW, Lynch M, Patouillard E, et al. Potential for reduction of burden and local elimination of malaria by reducing Plasmodium falciparum malaria transmission: a mathematical modelling study. Lancet Infect Dis. 2016;16(4):465–72. doi: 10.1016/S1473-3099(15)00423-5 26809816
24. Lutambi AM, Chitnis N, Briet OJ, Smith TA, Penny MA. Clustering of vector control interventions has important consequences for their effectiveness: a modelling study. PLoS ONE. 2014;9(5):e97065. doi: 10.1371/journal.pone.0097065 24823656
25. Slater HC, Ross A, Ouedraogo AL, White LJ, Nguon C, Walker PG, et al. Assessing the impact of next-generation rapid diagnostic tests on Plasmodium falciparum malaria elimination strategies. Nature. 2015;528(7580):S94–101. doi: 10.1038/nature16040 26633771
26. Slater HC, Griffin JT, Ghani AC, Okell LC. Assessing the potential impact of artemisinin and partner drug resistance in sub-Saharan Africa. Malar J. 2016;15(1):10.
27. White MT, Griffin JT, Churcher TS, Ferguson NM, Basanez MG, Ghani AC. Modelling the impact of vector control interventions on Anopheles gambiae population dynamics. Parasit Vectors. 2011;4 : 153. doi: 10.1186/1756-3305-4-153 21798055
28. Crowell V, Briet OJ, Hardy D, Chitnis N, Maire N, Di Pasquale A, et al. Modelling the cost-effectiveness of mass screening and treatment for reducing Plasmodium falciparum malaria burden. Malar J. 2013;12 : 4. doi: 10.1186/1475-2875-12-4 23286228
29. MESA Track: Malaria Eradication Scientific Alliance; [updated 15 March 2016. Available from: http://www.malariaeradication.org/mesa-track
30. Maude RJ, Nguon C, Dondorp AM, White LJ, White NJ. The diminishing returns of atovaquone-proguanil for elimination of Plasmodium falciparum malaria: modelling mass drug administration and treatment. Malar J. 2014;13 : 380. doi: 10.1186/1475-2875-13-380 25249272
31. Maude RJ, Pontavornpinyo W, Saralamba S, Aguas R, Yeung S, Dondorp AM, et al. The last man standing is the most resistant: eliminating artemisinin-resistant malaria in Cambodia. Malaria Journal. 2009;8(1):31.
32. Maude RJ, Socheat D, Nguon C, Saroth P, Dara P, Li G, et al. Optimising strategies for Plasmodium falciparum malaria elimination in Cambodia: primaquine, mass drug administration and artemisinin resistance. PLoS ONE. 2012;7(5):e37166. doi: 10.1371/journal.pone.0037166 22662135
33. Silal SP, Little F, Barnes KI, White LJ. Predicting the impact of border control on malaria transmission: a simulated focal screen and treat campaign. Malar J. 2015;14 : 268. doi: 10.1186/s12936-015-0776-2 26164675
34. Silal SP, Little F, Barnes KI, White LJ. Hitting a moving target: A model for malaria elimination in the presence of population movement. PLoS ONE. 2015;10(12):e0144990. doi: 10.1371/journal.pone.0144990 26689547
35. White LJ, Maude RJ, Pongtavornpinyo W, Saralamba S, Aguas R, Van Effelterre T, et al. The role of simple mathematical models in malaria elimination strategy design. Malar J. 2009;8 : 212. doi: 10.1186/1475-2875-8-212 19747403
36. Penny MA, Verity R, Bever CA, Sauboin C, Galactionova K, Flasche S, et al. Public health impact and cost-effectiveness of the RTS,S/AS01 malaria vaccine: a systematic comparison of predictions from four mathematical models. Lancet. 2016;387(10016):367–75. doi: 10.1016/S0140-6736(15)00725-4 26549466
37. Bhatt S, Weiss DJ, Mappin B, Dalrymple U, Cameron E, Bisanzio D, et al. Coverage and system efficiencies of insecticide-treated nets in Africa from 2000 to 2017. Elife. 2015;4:e09672. doi: 10.7554/eLife.09672 26714109
38. Briet O, Hardy D, Smith TA. Importance of factors determining the effective lifetime of a mass, long-lasting, insecticidal net distribution: a sensitivity analysis. Malaria Journal. 2012;11(1):20.
39. Chitnis N, Schapira A, Smith T, Steketee R. Comparing the effectiveness of malaria vector-control interventions through a mathematical model. Am J Trop Med Hyg. 2010;83(2):230–40. doi: 10.4269/ajtmh.2010.09-0179 20682861
40. Griffin JT. The interaction between seasonality and pulsed interventions against malaria in their effects on the reproduction number. PLoS Comput Biol. 2015;11(1):e1004057. doi: 10.1371/journal.pcbi.1004057 25590612
41. Griffin JT, Hollingsworth TD, Okell LC, Churcher TS, White M, Hinsley W, et al. Reducing Plasmodium falciparum malaria transmission in Africa: a model-based evaluation of intervention strategies. PLoS Med. 2010;7(8):e1000324. doi: 10.1371/journal.pmed.1000324 20711482
42. Walker PG, Griffin JT, Ferguson NM, Ghani AC. Estimating the most efficient allocation of interventions to achieve reductions in Plasmodium falciparum malaria burden and transmission in Africa: a modelling study. Lancet Glob Health. 2016;4(7):e474–84. doi: 10.1016/S2214-109X(16)30073-0 27269393
43. Briet OJ, Penny MA. Repeated mass distributions and continuous distribution of long-lasting insecticidal nets: modelling sustainability of health benefits from mosquito nets, depending on case management. Malar J. 2013;12 : 401. doi: 10.1186/1475-2875-12-401 24200296
44. Brady OJ, Godfray HC, Tatem AJ, Gething PW, Cohen JM, McKenzie FE, et al. Adult vector control, mosquito ecology and malaria transmission. Int Health. 2015;7(2):121–9. doi: 10.1093/inthealth/ihv010 25733562
45. Eckhoff PA. A malaria transmission-directed model of mosquito life cycle and ecology. Malar J. 2011;10 : 303. doi: 10.1186/1475-2875-10-303 21999664
46. Killeen GF, Seyoum A, Gimnig JE, Stevenson JC, Drakeley CJ, Chitnis N. Made-to-measure malaria vector control strategies: rational design based on insecticide properties and coverage of blood resources for mosquitoes. Malaria Journal. 2014;13 : 146. doi: 10.1186/1475-2875-13-146 24739261
47. Okell LC, Drakeley CJ, Bousema T, Whitty CJ, Ghani AC. Modelling the impact of artemisinin combination therapy and long-acting treatments on malaria transmission intensity. PLoS Med. 2008;5(11):e226. doi: 10.1371/journal.pmed.0050226 19067479
48. Ross A, Maire N, Sicuri E, Smith T, Conteh L. Determinants of the cost-effectiveness of intermittent preventive treatment for malaria in infants and children. PLoS ONE. 2011;6(4):e18391. doi: 10.1371/journal.pone.0018391 21490967
49. Ross A, Penny M, Maire N, Studer A, Carneiro I, Schellenberg D, et al. Modelling the epidemiological impact of intermittent preventive treatment against malaria in infants. PLoS ONE. 2008;3(7):e2661. doi: 10.1371/journal.pone.0002661 18628828
50. Crowell V, Hardy D, Briet O, Chitnis N, Maire N, Smith T. Can we depend on case management to prevent re-establishment of P. falciparum malaria, after local interruption of transmission? Epidemics. 2012;4(1):1–8. doi: 10.1016/j.epidem.2011.10.003 22325009
51. Smith DL, Cohen JM, Chiyaka C, Johnston G, Gething PW, Gosling R, et al. A sticky situation: the unexpected stability of malaria elimination. Philos Trans R Soc Lond B Biol Sci. 2013;368(1623):20120145. doi: 10.1098/rstb.2012.0145 23798693
52. Cairns M, Ghani A, Okell L, Gosling R, Carneiro I, Anto F, et al. Modelling the protective efficacy of alternative delivery schedules for intermittent preventive treatment of malaria in infants and children. PLoS ONE. 2011;6(4):e18947. doi: 10.1371/journal.pone.0018947 21533088
53. Okell L, Slater H, Ghani A, P P-R, Smith TA, Chitnis N, et al. Consensus modelling evidence to support the design of mass drug administration programmes Geneva: WHO; 2015 [updated 14th March 2016. Available from: http://www.who.int/malaria/mpac/mpac-sept2015-consensus-modelling-mda.pdf
54. Stuckey EM, Miller JM, Littrell M, Chitnis N, Steketee R. Operational strategies of anti-malarial drug campaigns for malaria elimination in Zambia's southern province: a simulation study. Malar J. 2016;15(1):148.
55. Stuckey EM, Stevenson J, Galactionova K, Baidjoe AY, Bousema T, Odongo W, et al. Modeling the cost effectiveness of malaria control interventions in the highlands of western Kenya. PLoS ONE. 2014;9(10):e107700. doi: 10.1371/journal.pone.0107700 25290939
56. Eckhoff PA, Bever CA, Gerardin J, Wenger EA. Fun with maths: exploring implications of mathematical models for malaria eradication. Malar J. 2014;13 : 486. doi: 10.1186/1475-2875-13-486 25495423
57. Gerardin J, Eckhoff P, Wenger EA. Mass campaigns with antimalarial drugs: a modelling comparison of artemether-lumefantrine and DHA-piperaquine with and without primaquine as tools for malaria control and elimination. BMC Infect Dis. 2015;15 : 144. doi: 10.1186/s12879-015-0887-y 25887935
58. Global Health Group at the University of California, Malaria Centre at the London School of Hygiene & Tropical Medicine. Single low-dose primaquine to interrupt P. falciparum transmission in Africa: a roadmap update and meeting summary 2014. Available from: http://www.shrinkingthemalariamap.org/sites/www.shrinkingthemalariamap.org/files/content/resource/attachment/London%20PQ%202016%20summary%20final%20for%20internet%209-12-16%20%25282%2529.pdf
59. Rao VB, Schellenberg D, Ghani AC. The potential impact of improving appropriate treatment for fever on malaria and non-malarial febrile illness management in under-5s: a decision-tree modelling approach. PLoS ONE. 2013;8(7):e69654. doi: 10.1371/journal.pone.0069654 23922770
60. Drake TL, Kyaw SS, Kyaw MP, Smithuis FM, Day NP, White LJ, et al. Cost effectiveness and resource allocation of Plasmodium falciparum malaria control in Myanmar: a modelling analysis of bed nets and community health workers. Malar J. 2015;14 : 376. doi: 10.1186/s12936-015-0886-x 26416075
61. Drake TL, Devine A, Yeung S, Day NP, White LJ, Lubell Y. Dynamic transmission economic evaluation of infectious disease interventions in low - and middle-income countries: A systematic literature review. Health Econ. 2016;25 Suppl 1 : 124–39.
62. Kyaw SS, Drake T, Thi A, Kyaw MP, Hlaing T, Smithuis FM, et al. Malaria community health workers in Myanmar: a cost analysis. Malar J. 2016;15(1):41.
63. Okell LC, Cairns M, Griffin JT, Ferguson NM, Tarning J, Jagoe G, et al. Contrasting benefits of different artemisinin combination therapies as first-line malaria treatments using model-based cost-effectiveness analysis. Nat Commun. 2014;5 : 5606. doi: 10.1038/ncomms6606 25425081
64. Hodel EM, Kay K, Hayes DJ, Terlouw DJ, Hastings IM. Optimizing the programmatic deployment of the anti-malarials artemether-lumefantrine and dihydroartemisinin-piperaquine using pharmacological modelling. Malar J. 2014;13 : 138. doi: 10.1186/1475-2875-13-138 24708571
65. Fernando SD, Navaratne CJ, Galappaththy GN, Abeyasinghe RR, Silva N, Wickermasinghe R. The importance of accuracy in diagnosis of positive malaria cases in a country progressing towards malaria elimination. J Glob Infect Dis. 2013;5(4):127–30. doi: 10.4103/0974-777X.121992 24672172
66. Mavandadi S, Feng S, Yu F, Dimitrov S, Nielsen-Saines K, Prescott WR, et al. A mathematical framework for combining decisions of multiple experts toward accurate and remote diagnosis of malaria using tele-microscopy. PLoS ONE. 2012;7(10):e46192. doi: 10.1371/journal.pone.0046192 23071544
67. McCarthy KA, Wenger EA, Huynh GH, Eckhoff PA. Calibration of an intrahost malaria model and parameter ensemble evaluation of a pre-erythrocytic vaccine. Malar J. 2015;14 : 6. doi: 10.1186/1475-2875-14-6 25563798
68. Smith T, Ross A, Maire N, Chitnis N, Studer A, Hardy D, et al. Ensemble modeling of the likely public health impact of a pre-erythrocytic malaria vaccine. PLoS Med. 2012;9(1):e1001157. doi: 10.1371/journal.pmed.1001157 22272189
69. White MT, Griffin JT, Riley EM, Drakeley CJ, Moorman AM, Sumba PO, et al. Efficacy model for antibody-mediated pre-erythrocytic malaria vaccines. Proc Biol Sci. 2011;278(1710):1298–305. doi: 10.1098/rspb.2010.1697 20943696
70. Diaz H, Ramirez AA, Olarte A, Clavijo C. A model for the control of malaria using genetically modified vectors. J Theor Biol. 2011;276(1):57–66. doi: 10.1016/j.jtbi.2011.01.053 21300074
71. Li J. Discrete-time models with mosquitoes carrying genetically-modified bacteria. Math Biosci. 2012;240(1):35–44. doi: 10.1016/j.mbs.2012.05.012 22771952
72. Legros M, Xu C, Okamoto K, Scott TW, Morrison AC, Lloyd AL, et al. Assessing the feasibility of controlling Aedes aegypti with transgenic methods: a model-based evaluation. PLoS ONE. 2012;7(12):e52235. doi: 10.1371/journal.pone.0052235 23284949
73. White MT, Smith DL. Synergism from combinations of infection-blocking malaria vaccines. Malar J. 2013;12 : 280. doi: 10.1186/1475-2875-12-280 23927630
74. WHO Malaria Policy Advisory Committee Secretariat. Malaria Policy Advisory Committee to the WHO: conclusions and recommendations of eighth biannual meeting (September 2015). Malar J. 2016;15(1):117.
75. Guyant P, Corbel V, Guerin PJ, Lautissier A, Nosten F, Boyer S, et al. Past and new challenges for malaria control and elimination: the role of operational research for innovation in designing interventions. Malar J. 2015;14 : 279. doi: 10.1186/s12936-015-0802-4 26185098
76. Hsiang MS, Hwang J, Tao AR, Liu Y, Bennett A, Shanks GD, et al. Mass drug administration for the control and elimination of Plasmodium vivax malaria: an ecological study from Jiangsu province, China. Malar J. 2013;12 : 383. doi: 10.1186/1475-2875-12-383 24175930
77. Kiware SS, Chitnis N, Devine GJ, Moore SJ, Majambere S, Killeen GF. Biologically meaningful coverage indicators for eliminating malaria transmission. Biol Lett. 2012;8(5):874–7. doi: 10.1098/rsbl.2012.0352 22647930
78. Poirot E, Skarbinski J, Sinclair D, Kachur SP, Slutsker L, Hwang J. Mass drug administration for malaria. Cochrane Database Syst Rev. 2013;12:CD008846.
79. Rosas-Aguirre A, Erhart A, Llanos-Cuentas A, Branch O, Berkvens D, Abatih E, et al. Modelling the potential of focal screening and treatment as elimination strategy for Plasmodium falciparum malaria in the Peruvian Amazon Region. Parasit Vectors. 2015;8 : 261. doi: 10.1186/s13071-015-0868-4 25948081
80. Eisele TP, Silumbe K, Finn T, Chalwe V, Kamuliwo M, Hamainza B, et al. Assessing the effectiveness of household-level focal mass drug administration and community-wide mass drug administration for reducing malaria parasite infection prevalence and incidence in Southern Province, Zambia: study protocol for a community randomized controlled trial. Trials. 2015;16 : 347. doi: 10.1186/s13063-015-0862-3 26268804
81. Alegana VA, Wright JA, Nahzat SM, Butt W, Sediqi AW, Habib N, et al. Modelling the incidence of Plasmodium vivax and Plasmodium falciparum malaria in Afghanistan 2006–2009. PLoS ONE. 2014;9(7).
82. Chen Z, Shi L, Zhou XN, Xia ZG, Bergquist R, Jiang QW. Elimination of malaria due to Plasmodium vivax in central part of the People's Republic of China: analysis and prediction based on modelling. Geospatial Health. 2014;9(1):169–77. doi: 10.4081/gh.2014.14 25545934
83. Galappaththy Gawrie NL, Tharyan P, Kirubakaran R. Primaquine for preventing relapse in people with Plasmodium vivax malaria treated with chloroquine. Cochrane Database of Systematic Reviews [Internet]. 2013; (10). Available from: http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD004389.pub3/abstract
84. Lindsay SW, Hole DG, Hutchinson RA, Richards SA, Willis SG. Assessing the future threat from vivax malaria in the United Kingdom using two markedly different modelling approaches. Malaria Journal. 2010;9.
85. Roy M, Bouma MJ, Ionides EL, Dhiman RC, Pascual M. The potential elimination of Plasmodium vivax malaria by relapse treatment: insights from a transmission model and surveillance data from NW India. PLoS Negl Trop Dis. 2013;7(1):e1979. doi: 10.1371/journal.pntd.0001979 23326611
86. Shi B, Liu J, Zhou XN, Yang GJ. Inferring Plasmodium vivax transmission networks from tempo-spatial surveillance data. PLoS Negl Trop Dis. 2014;8(2):e2682. doi: 10.1371/journal.pntd.0002682 24516684
87. Robinson LJ, Wampfler R, Betuela I, Karl S, White MT, Li Wai Suen CS, et al. Strategies for understanding and reducing the Plasmodium vivax and Plasmodium ovale hypnozoite reservoir in Papua New Guinean children: a randomised placebo-controlled trial and mathematical model. PLoS Med. 2015;12(10):e1001891. doi: 10.1371/journal.pmed.1001891 26505753
88. White MT, Karl S, Battle KE, Hay SI, Mueller I, Ghani AC. Modelling the contribution of the hypnozoite reservoir to Plasmodium vivax transmission. Elife. 2014;3:e04692.
89. Abdullahi MB, Hasan YA, Abdullah FA. Optimal control of Plasmodium knowlesi malaria in human and macaques. British Journal of Mathematics & Computer Science. 2014;4(2):271–87.
90. Abdullahi MB, Hasan YA, Abdullah FA. Optimal strategy for controlling the spread of Plasmodium knowlesi malaria: Treatment and culling. AIP Conference Proceedings. 2015;1660(1):050005.
91. Adekunle AI, Pinkevych M, McGready R, Luxemburger C, White LJ, Nosten F, et al. Modeling the dynamics of Plasmodium vivax infection and hypnozoite reactivation in vivo. PLoS Negl Trop Dis. 2015;9(3):e0003595. doi: 10.1371/journal.pntd.0003595 25780913
92. Qi Q, Guerra CA, Moyes CL, Elyazar IR, Gething PW, Hay SI, et al. The effects of urbanization on global Plasmodium vivax malaria transmission. Malar J. 2012;11 : 403. doi: 10.1186/1475-2875-11-403 23217010
93. White MT, Shirreff G, Karl S, Ghani AC, Mueller I. Variation in relapse frequency and the transmission potential of Plasmodium vivax malaria. Proc Biol Sci. 2016;283(1827).
94. Ross A, Koepfli C, Schoepflin S, Timinao L, Siba P, Smith T, et al. The incidence and differential seasonal patterns of Plasmodium vivax primary infections and relapses in a cohort of children in Papua New Guinea. PLoS Negl Trop Dis. 2016;10(5):e0004582. doi: 10.1371/journal.pntd.0004582 27144482
95. Imai N, White MT, Ghani AC, Drakeley CJ. Transmission and control of Plasmodium knowlesi: a mathematical modelling study. PLoS Negl Trop Dis. 2014;8(7):e2978. doi: 10.1371/journal.pntd.0002978 25058400
96. Aguas R, Ferreira MU, Gomes MG. Modeling the effects of relapse in the transmission dynamics of malaria parasites. J Parasitol Res. 2012;2012 : 921715. doi: 10.1155/2012/921715 21966590
97. Pampana E. Textbook of Malaria Eradication. Oxford: Oxford University Press; 1969.
98. Ashton RA, Kefyalew T, Rand A, Sime H, Assefa A, Mekasha A, et al. Geostatistical modeling of malaria endemicity using serological indicators of exposure collected through school surveys. American Journal of Tropical Medicine and Hygiene. 2015;93 (1):168–77. doi: 10.4269/ajtmh.14-0620 25962770
99. Bousema T, Drakeley C, Gesase S, Hashim R, Magesa S, Mosha F, et al. Identification of hot spots of malaria transmission for targeted malaria control. J Infect Dis. 2010;201(11):1764–74. doi: 10.1086/652456 20415536
100. Mosha JF, Sturrock HJ, Greenwood B, Sutherland CJ, Gadalla NB, Atwal S, et al. Hot spot or not: a comparison of spatial statistical methods to predict prospective malaria infections. Malar J. 2014;13 : 53. doi: 10.1186/1475-2875-13-53 24517452
101. Pothin E, Ferguson NM, Drakeley CJ, Ghani AC. Estimating malaria transmission intensity from Plasmodium falciparum serological data using antibody density models. Malar J. 2016;15(1):79.
102. malERA Refresh Consultative Panel on Characterising the Reservoir and Measuring Transmission. malERA: An updated research agenda for characterising the reservoir and measuring transmission in malaria elimination and eradication. PLoS Med. 2017;14(11):e1002452. doi: 10.1371/journal.pmed.1002452
103. Hustedt J, Canavati SE, Rang C, Ashton RA, Khim N, Berne L, et al. Reactive case-detection of malaria in Pailin Province, Western Cambodia: lessons from a year-long evaluation in a pre-elimination setting. Malar J. 2016;15(1):132.
104. Larsen DA, Chisha Z, Winters B, Mwanza M, Kamuliwo M, Mbwili C, et al. Malaria surveillance in low-transmission areas of Zambia using reactive case detection. Malar J. 2015;14(1):465.
105. Littrell M, Sow GD, Ngom A, Ba M, Mboup BM, Dieye Y, et al. Case investigation and reactive case detection for malaria elimination in northern Senegal. Malar J. 2013;12 : 331. doi: 10.1186/1475-2875-12-331 24044506
106. van Eijk AM, Ramanathapuram L, Sutton PL, Kanagaraj D, Sri Lakshmi Priya G, Ravishankaran S, et al. What is the value of reactive case detection in malaria control? A case-study in India and a systematic review. Malar J. 2016;15(1):67.
107. Zhou SS, Zhang SS, Zhang L, Rietveld AE, Ramsay AR, Zachariah R, et al. China's 1-3-7 surveillance and response strategy for malaria elimination: Is case reporting, investigation and foci response happening according to plan? Infect Dis Poverty. 2015;4 : 55. doi: 10.1186/s40249-015-0089-2 26654106
108. Cohen JM, Smith DL, Cotter C, Ward A, Yamey G, Sabot OJ, et al. Malaria resurgence: a systematic review and assessment of its causes. Malar J. 2012;11 : 122. doi: 10.1186/1475-2875-11-122 22531245
109. Reiner RC, Le Menach A, Kunene S, Ntshalintshali N, Hsiang MS, Perkins TA, et al. Mapping residual transmission for malaria elimination. Elife. 2015;4:e09520. doi: 10.7554/eLife.09520 26714110
110. Hemingway J, Ranson H, Magill A, Kolaczinski J, Fornadel C, Gimnig J, et al. Averting a malaria disaster: will insecticide resistance derail malaria control? Lancet. 2016;387 : 1785–8. doi: 10.1016/S0140-6736(15)00417-1 26880124
111. World Health Organization. Emergency response to artemisinin resistance in the Greater Mekong subregion. Regional framework for action 2013–2015 Geneva: WHO; 2013. Available from: http://www.who.int/malaria/publications/atoz/9789241505321/en/.
112. malERA Refresh Consultative Panel on Insecticide and Drug Resistance. malERA: An updated research agenda for insecticide and drug resistance in malaria elimination and eradication. PLoS Med. 2017;14(11):e1002450. doi: 10.1371/journal.pmed.1002450
113. Briet OJ, Penny MA, Hardy D, Awolola TS, Van Bortel W, Corbel V, et al. Effects of pyrethroid resistance on the cost effectiveness of a mass distribution of long-lasting insecticidal nets: a modelling study. Malar J. 2013;12 : 77. doi: 10.1186/1475-2875-12-77 23442575
114. Killeen GF, Chitnis N. Potential causes and consequences of behavioural resilience and resistance in malaria vector populations: a mathematical modelling analysis. Malar J. 2014;13 : 97. doi: 10.1186/1475-2875-13-97 24629066
115. Lubell Y, Dondorp A, Guerin PJ, Drake T, Meek S, Ashley E, et al. Artemisinin resistance—modelling the potential human and economic costs. Malar J. 2014;13 : 452. doi: 10.1186/1475-2875-13-452 25418416
116. Griffin JT, Cairns M, Ghani AC, Roper C, Schellenberg D, Carneiro I, et al. Protective efficacy of intermittent preventive treatment of malaria in infants (IPTi) using sulfadoxine-pyrimethamine and parasite resistance. PLoS ONE. 2010;5(9):e12618. doi: 10.1371/journal.pone.0012618 20838642
117. Hlaing T, Wai KT, Oo T, Sint N, Min T, Myar S, et al. Mobility dynamics of migrant workers and their socio-behavioral parameters related to malaria in Tier II, Artemisinin Resistance Containment Zone, Myanmar. BMC Public Health. 2015;15 : 886. doi: 10.1186/s12889-015-2241-0 26370297
118. Malisa AL, Pearce RJ, Abdulla S, Mshinda H, Kachur PS, Bloland P, et al. Drug coverage in treatment of malaria and the consequences for resistance evolution—evidence from the use of sulphadoxine/pyrimethamine. Malar J. 2010;9 : 190. doi: 10.1186/1475-2875-9-190 20602754
119. Winter K, Hastings IM. Development, evaluation, and application of an in silico model for antimalarial drug treatment and failure. Antimicrob Agents Chemother. 2011;55(7):3380–92. doi: 10.1128/AAC.01712-10 21537019
120. Grist EP, Flegg JA, Humphreys G, Mas IS, Anderson TJ, Ashley EA, et al. Optimal health and disease management using spatial uncertainty: a geographic characterization of emergent artemisinin-resistant Plasmodium falciparum distributions in Southeast Asia. Int J Health Geogr. 2016;15(1):37. doi: 10.1186/s12942-016-0064-6 27776514
121. Corbel V, Akogbeto M, Damien GB, Djenontin A, Chandre F, Rogier C, et al. Combination of malaria vector control interventions in pyrethroid resistance area in Benin: a cluster randomised controlled trial. Lancet Infect Dis. 2012;12(8):617–26. doi: 10.1016/S1473-3099(12)70081-6 22682536
122. Menger DJ, Omusula P, Holdinga M, Homan T, Carreira AS, Vandendaele P, et al. Field evaluation of a push-pull system to reduce malaria transmission. PLoS ONE. 2015;10(4):e0123415. doi: 10.1371/journal.pone.0123415 25923114
123. Tchuenche JM, Chiyaka C, Chan D, Matthews A, Mayer G. A mathematical model for antimalarial drug resistance. Math Med Biol. 2011;28(4):335–55. doi: 10.1093/imammb/dqq017 20884768
124. Kunkel A, Colijn C, Lipsitch M, Cohen T. How could preventive therapy affect the prevalence of drug resistance? Causes and consequences. Philos Trans R Soc Lond B Biol Sci. 2015;370(1670):20140306. doi: 10.1098/rstb.2014.0306 25918446
125. Barbosa S, Hastings IM. The importance of modelling the spread of insecticide resistance in a heterogeneous environment: the example of adding synergists to bed nets. Malar J. 2012;11 : 258. doi: 10.1186/1475-2875-11-258 22856525
126. Eckhoff PA. Malaria parasite diversity and transmission intensity affect development of parasitological immunity in a mathematical model. Malar J. 2012;11 : 419. doi: 10.1186/1475-2875-11-419 23241282
127. Fowkes FJ, Boeuf P, Beeson JG. Immunity to malaria in an era of declining malaria transmission. Parasitology. 2016;143(2):139–53. doi: 10.1017/S0031182015001249 26741253
128. Griffin JT, Ferguson NM, Ghani AC. Estimates of the changing age-burden of Plasmodium falciparum malaria disease in sub-Saharan Africa. Nat Commun. 2014;5 : 3136. doi: 10.1038/ncomms4136 24518518
129. Griffin JT, Hollingsworth TD, Reyburn H, Drakeley CJ, Riley EM, Ghani AC. Gradual acquisition of immunity to severe malaria with increasing exposure. Proc Biol Sci. 2015;282(1801):20142657. doi: 10.1098/rspb.2014.2657 25567652
130. Eckhoff P. P. falciparum infection durations and infectiousness are shaped by antigenic variation and innate and adaptive host immunity in a mathematical model. PLoS ONE. 2012;7(9):e44950. doi: 10.1371/journal.pone.0044950 23028698
131. Smith T, Ross A, Maire N, Rogier C, Trape JF, Molineaux L. An epidemiologic model of the incidence of acute illness in Plasmodium falciparum malaria. Am J Trop Med Hyg. 2006;75(2 Suppl):56–62.
132. Elbadry MA, Al-Khedery B, Tagliamonte MS, Yowell CA, Raccurt CP, Existe A, et al. High prevalence of asymptomatic malaria infections: a cross-sectional study in rural areas in six departments in Haiti. Malar J. 2015;14 : 510. doi: 10.1186/s12936-015-1051-2 26689195
133. Alimi TO, Fuller DO, Qualls WA, Herrera SV, Arevalo-Herrera M, Quinones ML, et al. Predicting potential ranges of primary malaria vectors and malaria in northern South America based on projected changes in climate, land cover and human population. Parasit Vectors. 2015;8 : 431. doi: 10.1186/s13071-015-1033-9 26289677
134. Baeza A, Bouma MJ, Dhiman R, Pascual M. Malaria control under unstable dynamics: reactive vs. climate-based strategies. Acta Trop. 2014;129 : 42–51. doi: 10.1016/j.actatropica.2013.04.001 23567551
135. Caminade C, Kovats S, Rocklov J, Tompkins AM, Morse AP, Colon-Gonzalez FJ, et al. Impact of climate change on global malaria distribution. Proc Natl Acad Sci U S A. 2014;111(9):3286–91. doi: 10.1073/pnas.1302089111 24596427
136. Caruana CM. A new breed of model: estimating the impact of climate change on malaria transmission. Environ Health Perspect. 2013;121(10):A310. doi: 10.1289/ehp.121-A310 24218662
137. Christiansen-Jucht C, Erguler K, Shek CY, Basanez MG, Parham PE. Modelling Anopheles gambiae s.s. population dynamics with temperature - and age-dependent survival. Int J Environ Res Public Health. 2015;12(6):5975–6005. doi: 10.3390/ijerph120605975 26030468
138. Khormi HM, Kumar L. Future malaria spatial pattern based on the potential global warming impact in South and Southeast Asia. Geospat Health. 2016;11(3):416. doi: 10.4081/gh.2016.416 27903054
139. Laporta GZ, Linton YM, Wilkerson RC, Bergo ES, Nagaki SS, Sant'Ana DC, et al. Malaria vectors in South America: current and future scenarios. Parasit Vectors. 2015;8 : 426. doi: 10.1186/s13071-015-1038-4 26283539
140. Leedale J, Tompkins AM, Caminade C, Jones AE, Nikulin G, Morse AP. Projecting malaria hazard from climate change in eastern Africa using large ensembles to estimate uncertainty. Geospat Health. 2016;11(1 Suppl):393.
141. Mweya CN, Kimera SI, Stanley G, Misinzo G, Mboera LE. Climate change influences potential distribution of infected Aedes aegypti co-occurrence with dengue epidemics risk areas in tanzania. PLoS ONE. 2016;11(9):e0162649. doi: 10.1371/journal.pone.0162649 27681327
142. Ngarakana-Gwasira ET, Bhunu CP, Masocha M, Mashonjowa E. Assessing the role of climate change in malaria transmission in Africa. Malar Res Treat. 2016;2016 : 7104291. doi: 10.1155/2016/7104291 27066290
143. Onyango EA, Sahin O, Awiti A, Chu C, Mackey B. An integrated risk and vulnerability assessment framework for climate change and malaria transmission in East Africa. Malar J. 2016;15(1):551. doi: 10.1186/s12936-016-1600-3 27835976
144. Pascual M. Climate and population immunity in malaria dynamics: Harnessing information from endemicity gradients. Trends Parasitol. 2015;31(11):532–4. doi: 10.1016/j.pt.2015.08.009 26422773
145. Ryan SJ, McNally A, Johnson LR, Mordecai EA, Ben-Horin T, Paaijmans K, et al. Mapping physiological suitability limits for malaria in Africa under climate change. Vector Borne Zoonotic Dis. 2015;15(12):718–25. doi: 10.1089/vbz.2015.1822 26579951
146. Salahi-Moghaddam A, Khoshdel A, Dalaei H, Pakdad K, Nutifafa GG, Sedaghat MM. Spatial changes in the distribution of malaria vectors during the past 5 decades in Iran. Acta Trop. 2017;166 : 45–53. doi: 10.1016/j.actatropica.2016.11.001 27826012
147. Song Y, Ge Y, Wang J, Ren Z, Liao Y, Peng J. Spatial distribution estimation of malaria in northern China and its scenarios in 2020, 2030, 2040 and 2050. Malar J. 2016;15(1):345. doi: 10.1186/s12936-016-1395-2 27387921
148. Tompkins AM, Caporaso L. Assessment of malaria transmission changes in Africa, due to the climate impact of land use change using Coupled Model Intercomparison Project Phase 5 earth system models. Geospat Health. 2016;11(1 Suppl):380.
149. Tonnang HE, Tchouassi DP, Juarez HS, Igweta LK, Djouaka RF. Zoom in at African country level: potential climate induced changes in areas of suitability for survival of malaria vectors. Int J Health Geogr. 2014;13 : 12. doi: 10.1186/1476-072X-13-12 24885061
150. Warszawski L, Frieler K, Huber V, Piontek F, Serdeczny O, Schewe J. The Inter-Sectoral Impact Model Intercomparison Project (ISI-MIP): project framework. Proc Natl Acad Sci U S A. 2014;111(9):3228–32. doi: 10.1073/pnas.1312330110 24344316
151. Yamana TK, Eltahir EA. Projected impacts of climate change on environmental suitability for malaria transmission in West Africa. Environ Health Perspect. 2013;121(10):1179–86. doi: 10.1289/ehp.1206174 24043443
152. Bakare E, Nwozo C. On the mathematical analysis of the influence of chemoprophylaxis on the malaria epidemic model. International Journal of Contemporary Mathematical Sciences. 2016;11 : 45–63.
153. Kyaw SS, Drake T, Ruangveerayuth R, Chierakul W, White NJ, Newton PN, et al. Cost of treating inpatient falciparum malaria on the Thai-Myanmar border. Malar J. 2014;13 : 416. doi: 10.1186/1475-2875-13-416 25351915
154. Labadin J, Kon M, Juan S. Deterministic malaria transmission model with acquired immunity WCECS 2009, October 20–22, 2009, San Francisco, USA2009. Available from: http://www.iaeng.org/publication/WCECS2009/WCECS2009_pp779-784.pdf
155. Hagenlocher M, Castro MC. Mapping malaria risk and vulnerability in the United Republic of Tanzania: a spatial explicit model. Popul Health Metr. 2015;13(1):2. doi: 10.1186/s12963-015-0036-2 25674040
156. Ranjbar M, Shoghli A, Kolifarhood G, Tabatabaei SM, Amlashi M, Mohammadi M. Predicting factors for malaria re-introduction: an applied model in an elimination setting to prevent malaria outbreaks. Malar J. 2016;15(1):138.
157. Acevedo MA, Prosper O, Lopiano K, Ruktanonchai N, Caughlin TT, Martcheva M, et al. Spatial heterogeneity, host movement and mosquito-borne disease transmission. PLoS ONE. 2015;10(6):e0127552. doi: 10.1371/journal.pone.0127552 26030769
158. Bomblies A. Agent-based modeling of malaria vectors: the importance of spatial simulation. Parasit Vectors. 2014;7 : 308. doi: 10.1186/1756-3305-7-308 24992942
159. Pindolia DK, Garcia AJ, Huang Z, Fik T, Smith DL, Tatem AJ. Quantifying cross-border movements and migrations for guiding the strategic planning of malaria control and elimination. Malar J. 2014;13 : 169. doi: 10.1186/1475-2875-13-169 24886389
160. Smith DL, Perkins TA, Reiner RC Jr., Barker CM, Niu T, Chaves LF, et al. Recasting the theory of mosquito-borne pathogen transmission dynamics and control. Trans R Soc Trop Med Hyg. 2014;108(4):185–97. doi: 10.1093/trstmh/tru026 24591453
161. Tatem AJ, Huang Z, Narib C, Kumar U, Kandula D, Pindolia DK, et al. Integrating rapid risk mapping and mobile phone call record data for strategic malaria elimination planning. Malar J. 2014;13 : 52. doi: 10.1186/1475-2875-13-52 24512144
162. Blanas DA, Ndiaye Y, MacFarlane M, Manga I, Siddiqui A, Velez O, et al. Health worker perceptions of integrating mobile phones into community case management of malaria in Saraya, Senegal. Int Health. 2015;7(3):176–82. doi: 10.1093/inthealth/ihu075 25316707
163. Ruktanonchai NW, Bhavnani D, Sorichetta A, Bengtsson L, Carter KH, Cordoba RC, et al. Census-derived migration data as a tool for informing malaria elimination policy. Malar J. 2016;15(1):273. doi: 10.1186/s12936-016-1315-5 27169470
164. Tompkins AM, McCreesh N. Migration statistics relevant for malaria transmission in Senegal derived from mobile phone data and used in an agent-based migration model. Geospat Health. 2016;11(1 Suppl):408.
165. Danis K, Lenglet A, Tseroni M, Baka A, Tsiodras S, Bonovas S. Malaria in Greece: historical and current reflections on a re-emerging vector borne disease. Travel Med Infect Dis. 2013;11(1):8–14. doi: 10.1016/j.tmaid.2013.01.001 23434287
166. Miguel RB, Peiter PC, de Albuquerque H, Coura JR, Moza PG, Costa Ade P, et al. Malaria in the state of Rio de Janeiro, Brazil, an Atlantic Forest area: an assessment using the health surveillance service. Mem Inst Oswaldo Cruz. 2014;109(5):634–40. doi: 10.1590/0074-0276130558 25185004
167. Dharmawardena P, Premaratne RG, Gunasekera WM, Hewawitarane M, Mendis K, Fernando D. Characterization of imported malaria, the largest threat to sustained malaria elimination from Sri Lanka. Malar J. 2015;14 : 177. doi: 10.1186/s12936-015-0697-0 25902716
168. Wang D, Li S, Cheng Z, Xiao N, Cotter C, Hwang J, et al. Transmission risk from imported Plasmodium vivax malaria in the China-Myanmar border region. Emerg Infect Dis. 2015;21(10):1861–4. doi: 10.3201/eid2110.150679 26401843
169. Ren Z, Wang D, Ma A, Hwang J, Bennett A, Sturrock HJ, et al. Predicting malaria vector distribution under climate change scenarios in China: Challenges for malaria elimination. Sci Rep. 2016;6 : 20604. doi: 10.1038/srep20604 26868185
170. Noor AM, Uusiku P, Kamwi RN, Katokele S, Ntomwa B, Alegana VA, et al. The receptive versus current risks of Plasmodium falciparum transmission in northern Namibia: implications for elimination. BMC Infect Dis. 2013;13 : 184. doi: 10.1186/1471-2334-13-184 23617955
171. Shahandeh K, Basseri HR, Sharifzadeh Y. An application of cultural model to assess and compare malaria prevention among Afghani migrant and Baluchi resident in the endemic area, southeastern Iran. J Immigr Minor Health. 2014;16(1):102–10. doi: 10.1007/s10903-013-9850-4 23775110
172. Gomes E, Capinha C, Rocha J, Sousa C. Mapping risk of malaria transmission in mainland Portugal using a mathematical modelling approach. PLoS ONE. 2016;11(11):e0164788. doi: 10.1371/journal.pone.0164788 27814371
173. Ranjbar M, Shoghli A, Kolifarhood G, Tabatabaei SM, Amlashi M, Mohammadi M. Predicting factors for malaria re-introduction: an applied model in an elimination setting to prevent malaria outbreaks. Malar J. 2016;15 : 138. doi: 10.1186/s12936-016-1192-y 26935846
174. Cohen JM, Ernst KC, Lindblade KA, Vulule JM, John CC, Wilson ML. Local topographic wetness indices predict household malaria risk better than land-use and land-cover in the western Kenya highlands. Malar J. 2010;9 : 328. doi: 10.1186/1475-2875-9-328 21080943
175. Tatarsky A, Aboobakar S, Cohen JM, Gopee N, Bheecarry A, Moonasar D, et al. Preventing the reintroduction of malaria in Mauritius: a programmatic and financial assessment. PLoS ONE. 2011;6(9):e23832. doi: 10.1371/journal.pone.0023832 21912645
176. Durnez L, Coosemans M. Residual transmission of malaria: an old issue for new approaches: InTech; 2013. Available from: http://www.intechopen.com/books/anopheles-mosquitoes-new-insights-into-malaria-vectors/residual-transmission-of-malaria-an-old-issue-for-new-approaches
177. Lloyd AL, Zhang J, Root AM. Stochasticity and heterogeneity in host-vector models. J R Soc Interface. 2007;4(16):851–63. doi: 10.1098/rsif.2007.1064 17580290
178. Vazquez-Prokopec GM, Perkins TA, Waller LA, Lloyd AL, Reiner RC Jr., Scott TW, et al. Coupled heterogeneities and their impact on parasite transmission and control. Trends Parasitol. 2016;32(5):356–67. doi: 10.1016/j.pt.2016.01.001 26850821
179. Rao VB, Schellenberg D, Ghani AC. Overcoming health systems barriers to successful malaria treatment. Trends Parasitol. 2013;29(4):164–80. doi: 10.1016/j.pt.2013.01.005 23415933
180. Cohen JM, Sabot O, Sabot K, Gordon M, Gross I, Bishop D, et al. A pharmacy too far? Equity and spatial distribution of outcomes in the delivery of subsidized artemisinin-based combination therapies through private drug shops. BMC Health Serv Res. 2010;10 Suppl 1:S6.
181. Perkins TA, Scott TW, Le Menach A, Smith DL. Heterogeneity, mixing, and the spatial scales of mosquito-borne pathogen transmission. PLoS Comput Biol. 2013;9(12):e1003327. doi: 10.1371/journal.pcbi.1003327 24348223
182. Eckhoff P, Bever CA, Gerardin J, Wenger E, Smith D. From puddles to planet: Modeling approaches to vector-borne diseases at varying resolution and scale. Current Opinion in Insect Science. 2015;10 : 118–23.
183. Moonasar D, Maharaj R, Kunene S, Candrinho B, Saute F, Ntshalintshali N, et al. Towards malaria elimination in the MOSASWA (Mozambique, South Africa and Swaziland) region. Malar J. 2016;15(1):419. doi: 10.1186/s12936-016-1470-8 27538990
184. Raman J, Morris N, Frean J, Brooke B, Blumberg L, Kruger P, et al. Reviewing South Africa's malaria elimination strategy (2012–2018): progress, challenges and priorities. Malar J. 2016;15(1):438. doi: 10.1186/s12936-016-1497-x 27567642
185. Sturrock HJ, Roberts KW, Wegbreit J, Ohrt C, Gosling RD. Tackling imported malaria: an elimination endgame. Am J Trop Med Hyg. 2015;93(1):139–44. doi: 10.4269/ajtmh.14-0256 26013369
186. Wangdi K, Gatton ML, Kelly GC, Clements AC. Cross-border malaria: a major obstacle for malaria elimination. Adv Parasitol. 2015;89 : 79–107. doi: 10.1016/bs.apar.2015.04.002 26003036
187. Ferreira MU, Castro MC. Challenges for malaria elimination in Brazil. Malar J. 2016;15(1):284. doi: 10.1186/s12936-016-1335-1 27206924
188. Gryseels C, Peeters Grietens K, Dierickx S, Xuan XN, Uk S, Bannister-Tyrrell M, et al. High mobility and low use of malaria preventive measures among the Jarai male youth along the Cambodia-Vietnam border. Am J Trop Med Hyg. 2015;93(4):810–8. doi: 10.4269/ajtmh.15-0259 26283747
189. Hu Y, Zhou G, Ruan Y, Lee MC, Xu X, Deng S, et al. Seasonal dynamics and microgeographical spatial heterogeneity of malaria along the China-Myanmar border. Acta Trop. 2016;157 : 12–9. doi: 10.1016/j.actatropica.2016.01.022 26812008
190. Wangdi K, Banwell C, Gatton ML, Kelly GC, Namgay R, Clements AC. Development and evaluation of a spatial decision support system for malaria elimination in Bhutan. Malar J. 2016;15(1):180.
191. Bi Y, Hu W, Yang H, Zhou XN, Yu W, Guo Y, et al. Spatial patterns of malaria reported deaths in Yunnan Province, China. Am J Trop Med Hyg. 2013;88(3):526–35. doi: 10.4269/ajtmh.2012.12-0217 23269660
192. Lyttleton C. Deviance and resistance: Malaria elimination in the greater Mekong subregion. Soc Sci Med. 2016;150 : 144–52. doi: 10.1016/j.socscimed.2015.12.033 26751710
193. Wang RB, Dong JQ, Xia ZG, Cai T, Zhang QF, Zhang Y, et al. Lessons on malaria control in the ethnic minority regions in Northern Myanmar along the China border, 2007–2014. Infect Dis Poverty. 2016;5(1):95. doi: 10.1186/s40249-016-0191-0 27716435
194. Kanyangarara M, Mamini E, Mharakurwa S, Munyati S, Gwanzura L, Kobayashi T, et al. Individual - and household-level risk factors associated with malaria in Mutasa district, Zimbabwe: a serial cross-sectional study. Am J Trop Med Hyg. 2016;95(1):133–40. doi: 10.4269/ajtmh.15-0847 27114289
195. Zhang Q, Sun J, Zhang Z, Geng Q, Lai S, Hu W, et al. Risk assessment of malaria in land border regions of China in the context of malaria elimination. Malar J. 2016;15(1):546. doi: 10.1186/s12936-016-1590-1 27825379
Štítky
Interní lékařství
Článek Contemporary disengagement from antiretroviral therapy in Khayelitsha, South Africa: A cohort studyČlánek Bioequivalence of twice-daily oral tacrolimus in transplant recipients: More evidence for consensus?
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2017 Číslo 11- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Jak postupovat při výběru betablokátoru − doporučení z kardiologické praxe
- Červená fermentovaná rýže účinně snižuje hladinu LDL cholesterolu jako vhodná alternativa ke statinové terapii
-
Všechny články tohoto čísla
- Labour trafficking: Challenges and opportunities from an occupational health perspective
- The end of HIV: Still a very long way to go, but progress continues
- Contemporary disengagement from antiretroviral therapy in Khayelitsha, South Africa: A cohort study
- Bioequivalence of twice-daily oral tacrolimus in transplant recipients: More evidence for consensus?
- Treatment guidelines and early loss from care for people living with HIV in Cape Town, South Africa: A retrospective cohort study
- Perinatal mortality associated with induction of labour versus expectant management in nulliparous women aged 35 years or over: An English national cohort study
- Core Outcome Set-STAndards for Development: The COS-STAD recommendations
- Closing the gaps in the HIV care continuum
- Association between the 2012 Health and Social Care Act and specialist visits and hospitalisations in England: A controlled interrupted time series analysis
- HIV pre-exposure prophylaxis and early antiretroviral treatment among female sex workers in South Africa: Results from a prospective observational demonstration project
- Sexual exploitation of unaccompanied migrant and refugee boys in Greece: Approaches to prevention
- Child sex trafficking in the United States: Challenges for the healthcare provider
- The expanding epidemic of HIV-1 in the Russian Federation
- Cardiovascular disease (CVD) and chronic kidney disease (CKD) event rates in HIV-positive persons at high predicted CVD and CKD risk: A prospective analysis of the D:A:D observational study
- Validity of a minimally invasive autopsy for cause of death determination in maternal deaths in Mozambique: An observational study
- malERA: An updated research agenda for malaria elimination and eradication
- malERA: An updated research agenda for health systems and policy research in malaria elimination and eradication
- A combination intervention strategy to improve linkage to and retention in HIV care following diagnosis in Mozambique: A cluster-randomized study
- Bioequivalence between innovator and generic tacrolimus in liver and kidney transplant recipients: A randomized, crossover clinical trial
- malERA: An updated research agenda for basic science and enabling technologies in malaria elimination and eradication
- Human trafficking and exploitation: A global health concern
- Virological response and resistance among HIV-infected children receiving long-term antiretroviral therapy without virological monitoring in Uganda and Zimbabwe: Observational analyses within the randomised ARROW trial
- Postmenopausal hormone therapy and risk of stroke: A pooled analysis of data from population-based cohort studies
- Lansoprazole use and tuberculosis incidence in the United Kingdom Clinical Practice Research Datalink: A population based cohort
- malERA: An updated research agenda for insecticide and drug resistance in malaria elimination and eradication
- Safety, pharmacokinetics, and immunological activities of multiple intravenous or subcutaneous doses of an anti-HIV monoclonal antibody, VRC01, administered to HIV-uninfected adults: Results of a phase 1 randomized trial
- HIV prevalence and behavioral and psychosocial factors among transgender women and cisgender men who have sex with men in 8 African countries: A cross-sectional analysis
- Treatment eligibility and retention in clinical HIV care: A regression discontinuity study in South Africa
- malERA: An updated research agenda for characterising the reservoir and measuring transmission in malaria elimination and eradication
- Effectiveness of a combination strategy for linkage and retention in adult HIV care in Swaziland: The Link4Health cluster randomized trial
- The value of confirmatory testing in early infant HIV diagnosis programmes in South Africa: A cost-effectiveness analysis
- HIV self-testing among female sex workers in Zambia: A cluster randomized controlled trial
- The US President's Malaria Initiative, transmission and mortality: A modelling study
- Comparison of two cash transfer strategies to prevent catastrophic costs for poor tuberculosis-affected households in low- and middle-income countries: An economic modelling study
- Direct provision versus facility collection of HIV self-tests among female sex workers in Uganda: A cluster-randomized controlled health systems trial
- malERA: An updated research agenda for diagnostics, drugs, vaccines, and vector control in malaria elimination and eradication
- malERA: An updated research agenda for combination interventions and modelling in malaria elimination and eradication
- HIV-1 persistence following extremely early initiation of antiretroviral therapy (ART) during acute HIV-1 infection: An observational study
- Respondent-driven sampling for identification of HIV- and HCV-infected people who inject drugs and men who have sex with men in India: A cross-sectional, community-based analysis
- Extensive virologic and immunologic characterization in an HIV-infected individual following allogeneic stem cell transplant and analytic cessation of antiretroviral therapy: A case study
- Measuring success: The challenge of social protection in helping eliminate tuberculosis
- Prospects for passive immunity to prevent HIV infection
- Reaching global HIV/AIDS goals: What got us here, won't get us there
- Evidence-based restructuring of health and social care
- Extreme exploitation in Southeast Asia waters: Challenges in progressing towards universal health coverage for migrant workers
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Postmenopausal hormone therapy and risk of stroke: A pooled analysis of data from population-based cohort studies
- Bioequivalence between innovator and generic tacrolimus in liver and kidney transplant recipients: A randomized, crossover clinical trial
- HIV pre-exposure prophylaxis and early antiretroviral treatment among female sex workers in South Africa: Results from a prospective observational demonstration project
- Bioequivalence of twice-daily oral tacrolimus in transplant recipients: More evidence for consensus?
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



