-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaHIV pre-exposure prophylaxis and early antiretroviral treatment among female sex workers in South Africa: Results from a prospective observational demonstration project
In an observational study, Gabriela Gomez and colleagues report on provision of pre-exposure prophylaxis and antiretroviral treatment for female sex workers in South Africa.
Published in the journal: . PLoS Med 14(11): e32767. doi:10.1371/journal.pmed.1002444
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1002444Summary
In an observational study, Gabriela Gomez and colleagues report on provision of pre-exposure prophylaxis and antiretroviral treatment for female sex workers in South Africa.
Introduction
Rates of new HIV infections remain high, especially in key populations in sub-Saharan Africa, necessitating new options for HIV prevention and treatment [1,2]. Mathematical and clinical studies suggest that a programme combining current HIV prevention options with oral pre-exposure prophylaxis (PrEP), i.e., giving antiretrovirals to uninfected individuals, and early antiretroviral treatment (ART), i.e., giving antiretrovirals to HIV-infected individuals irrespective of CD4 count, could make significant inroads on reducing new infections in key and general populations [3–7].
Following the transition of WHO treatment guidelines to recommending both PrEP and early ART, research has shifted focus to answering questions about implementation [5], including uptake, adherence, and retention in existing programmes [8]. Demonstration projects, open-label extension studies, and population-based implementation trials testing feasibility in ‘real world’ settings are ongoing or have been recently completed [9,10]. Most of the completed demonstration projects have focused on men who have sex with men (MSM) populations throughout the world (though primarily in the United States, South America, and Europe) [10]. One or two other projects have been completed among serodiscordant couples [11], and only one randomised control trial implementation study had been completed among female sex workers (FSWs) at the time of the writing of this report [12].
In South Africa, although sex work is still criminalised, FSWs have been prioritised for focused, tailored services in the previous and new National Department of Health (NDoH) national strategic plans for HIV, as well as a specialised plan for sex workers, as the population with the highest incidence and prevalence of HIV, especially in urban areas [13–15]. The national sex worker HIV programme includes support for demonstration projects to further the development of specialised services including PrEP and early ART.
The TAPS (Treatment And Prevention for female Sex workers) Demonstration Project, nested within the long-standing Sex Worker Programme (SWP) [16], was designed to support integration of oral PrEP, as part of a combination prevention approach, and early ART into existing HIV services in 2 urban settings, with specific aims to assess uptake, retention, and adherence among FSWs and to estimate the cost of this strategy. TAPS was the first demonstration project in South Africa to include PrEP and early ART for FSWs, and among the first few in sub-Saharan Africa as well. This paper presents the primary results.
Methods
The TAPS Demonstration Project was a prospective observational cohort designed as a real-world implementation study to integrate PrEP, as part of a combination prevention approach, and early ART into existing services for evaluation. The protocol has been described in detail in a previous publication [17]. The TAPS Demonstration Project protocol was approved by the University of the Witwatersrand Human Research Ethics Committee (reference number: 140502) and the South African Medicines Control Council (reference number: 20140740). All participants completed a written informed consent process for the study consisting of a comprehensive consent form for the main study, as well as additional forms for laboratory sample storage and the qualitative study components. Costing studies were also performed after securing consent from clinic staff.
Setting and intervention
TAPS was embedded within the SWP, operated by Wits Reproductive Health and HIV Institute, which is integrated into the South African public health clinical service. The SWP has been in existence since 1996 and is run by nurses, community health workers, and peer educators [16]. Systematic formative research conducted to support the design and in preparation for TAPS is presented elsewhere [18].
The 2 clinic sites were situated in inner-city Johannesburg and Pretoria. These clinics cater to an urban population of sex workers working in hotel brothels, on streets, and in other informal environments. The clinics provide standard-of-care primary healthcare including HIV testing services, male and female condom distribution, nurse-initiated and managed ART, tuberculosis (TB) screening, contraceptive provision, cervical cancer screening, clinical services for sexually transmitted infections (STIs) and minor ailments, psychosocial support, and referrals for pregnancy and other clinical and legal services.
Recruitment of FSWs took place in the clinics and in surrounding brothels, bars, and streets, relying on existing peer educator outreach services. Screening for participation was conducted in 2 steps. First, women were tested for HIV using a standard-of-care rapid test. This step included obtaining NDoH HIV testing service written consent and asking brief questions as to current pregnancy and whether they were a sex worker (e.g., they were asked to self-identify after peer recruitment in their places of work). Women were then either referred to other relevant care (if they were pregnant, were not a sex worker, or decided not to proceed) or they continued on to clinical screening for the TAPS study. This process always occurred on the same day to ensure that HIV testing and clinical assessments were aligned. The next phase of the screening process began by obtaining study-specific written consent, then continued with the assessment of clinical history, administration of demographic and behavioural questionnaires, TB and pregnancy screening, and blood draws for creatinine measurement, hepatitis B screening, and syphilis testing. HIV status confirmation with ELISA was done for HIV-positive participants at a private laboratory, where all the other samples were also analysed. Women were offered contraception, as well as advised of the need to use condoms to prevent HIV, STIs, and unwanted pregnancies.
After screening, participants were asked to return a week later, to allow for laboratory analyses to be completed, for potential enrolment into either PrEP for HIV-negative FSWs or early ART for HIV-positive FSWs. The definition of early ART has evolved in line with national guidelines. At the start of the study, the CD4 threshold for treatment initiation was 350 cells/ml; the threshold changed to 500 cells/ml, and then treatment initiation became independent of CD4 count during the course of the study. Since our purpose was to offer treatment to those not eligible for ART under current guidelines, TAPS transitioned with the national treatment initiation definition. Project milestones are provided in S1 Fig. Women were eligible for the study if they were age 18 years or over, were not pregnant at enrolment, did not have multidrug-resistant TB, were not participating in another clinical study, and had finished their regimen for post-exposure prophylaxis if they were taking it at the time of assessment. Those who became pregnant through the course of the study were given the option to remain in the study on current medication, discontinue use during the period of pregnancy and remain in the study, or transfer out completely to antenatal care. The first 2 options also included referrals to antenatal care.
Once initiated on PrEP or early ART, participants were scheduled for an initial 1-month check-in to assess safety and/or adherence issues, and then were scheduled for quarterly clinical testing and safety monitoring visits thereafter. All clinical monitoring followed South African national guidelines throughout the study (also detailed in the published protocol) [17], and all services were provided free of charge as per the public health clinic standard, including PrEP and early ART. Participants were given refills of 1–3 months depending on ability to safely store the products and desire to come to the clinic for refills. The importance of consistent and high adherence was emphasised for PrEP use; however, participants could cycle on and off medication during periods of lower risk as desired and remain in the study. Indeed, the PrEP intervention for HIV-negative women included not only the use of PrEP but also other available prevention options (such as condoms, continuous testing and counselling, and STI screening). Participants were offered short message service (SMS) reminders for clinic visits and positive messaging around adherence, health, and psychosocial issues.
For both groups, participants were asked about pill-taking habits using a motivational technique at each staff touch point: counselling, clinical, and pharmacy. Counselling followed the current standard of care for ART provision. Blood samples were taken for analysis of drug levels in PrEP participants, the results of which will be presented at a later date as they are not currently available. Adverse events were recorded per Good Clinical Practice guidelines and WHO recommended grading [19,20].
Evaluation
Programmatic data to describe eligibility and uptake were collected in activity reports including the number of outreach contacts and appointments booked. Routinely collected demographic, behavioural, and clinical data were also analysed. Retention in care at 12 months was the primary outcome. Participants exited the analysis if they withdrew from the study or were lost to follow-up, defined as no contact over 2 consecutive quarterly clinic visits or not completing an exit visit at 12 months. Participants were considered retained in the programme if they had not withdrawn or been lost to follow-up during the 12-month period. We used an adapted cascade approach to measure uptake and retention through a series of touch points with participants. These points started during outreach and the enrolment process. Retention data are reported at each of the time points (3, 6, 9 and 12 months). Women who missed visits and returned later were included in the analysis. Data for those women who completed more than 12 months of follow-up are included in S3 Text.
Data were analysed on self-reported adherence and sexual behaviour, STI diagnoses, and occurrence of side effects. We also monitored any possible seroconversions on PrEP and virological failures on early ART. Qualitative research including in-depth interviews (with a selection of up to 10% of study participants), clinic observations, and group discussions with providers was also conducted and will be presented separately.
Costs were estimated from a healthcare provider perspective for each clinic using an ingredient approach. We included capital costs (equipment, buildings, non-recurrent training) as well as recurrent costs (personnel, supplies, management, and maintenance of buildings). Capital costs were annualised. Data collection activities included measurement of clinic space, completion of weekly timesheets by staff involved in the programme that detailed time per programme activity versus research activities, observations of practice detailing all clinical activities during participant visits, interviews with staff exploring time shared with other programmes, and review of expenditure and utilisation data with regards to the programme. Sources used in the valuation of all resources, and methods for allocation of shared resources, are detailed in Table A of S2 Text. We used both bottom-up and top-down approaches to calculate unit cost per visit. Economic costs were assigned to drugs as per procurement prices from NDoH for generics (US$4.8/month for PrEP; US$8.3/month for early ART); quantities of drugs dispensed per patient were sourced from clinical and pharmacy records. Services, activities, and healthcare providers involved in each type of visit are listed in Table B of S2 Text. We present unit costs for the different types of visits (outreach contact, testing session, enrolment, follow-up, and refill visits). Our unit costs per person-year for PrEP and early ART include enrolment, follow-up, and refill visits taking place during the first year of services and reflect the attendance patterns observed during the TAPS Demonstration Project. All prices were collected in local currency and are reported in 2015 US dollars. The average exchange rate used was US$1 = R 11.6. All data were analysed in STATA 12 (StataCorp) and EXCEL 2016 (Microsoft).
A national programme including PrEP and early ART for FSWs was launched in June 2016, during the study, and may have influenced enrolment and retention. To test this, a stratified analysis of uptake and retention indicators is provided in S3 Text, comparing the cascade results among TAPS cohorts disaggregated by time of enrolment and follow-up period. We tested the null hypothesis that the 2 proportions are the same at each step of the cascade and report p-values for this testing. This analysis was the only additional analysis not prospectively defined.
Results
Outreach and uptake
Enrolment for the TAPS study occurred between 30 March 2015 and 31 July 2016. The process began with outreach and recruitment, by which 7,140 FSWs were contacted of the 10,548 names gathered across the 2 sites, after excluding duplicates and inaccurate phone numbers (Fig 1). Contacts included women recorded on waiting lists leading up to the launch of the study that were compiled during pre-study outreach and community education activities. After making contact, a total of 947 FSWs were seen in the clinic, of which 692 were HIV tested (73%) as per standard of care. At this point, 21% (n = 197) of the women were found to be already taking ART, to be pregnant, or to not consider themselves to be sex workers, making them ineligible for enrolment. These women were referred to relevant care options, and therefore did not complete the next phase of study screening, which occurred in the same visit as the HIV testing. Among those tested for HIV, there was a prevalence of 49% (n = 341/692). Following HIV testing, 79% (n = 241/351) and 69% (n = 270/341) participants returned for enrolment for PrEP and early ART, respectively. Among the HIV-negative participants who returned for eligibility assessment, 93% were assessed as clinically eligible for PrEP (n = 224/241). Among the HIV-positive participants who returned for eligibility assessment, 41% (n = 110/270) had CD4 counts within NDoH ART initiation guidelines at assessment, and 93% of those remaining were eligible for early ART (n = 148/160). Reasons for non-eligibility are presented in Fig 1. Uptake among those eligible was high: 98% (n = 219/224) of FSWs offered PrEP accepted, and 94% (n = 139/148) of FSWs offered early ART accepted. At the end of this process, 219 HIV-negative and 139 HIV-positive FSWs were enrolled. The detailed numbers of participants at each step of enrolment and follow-up are presented in Table A of S3 Text.
Fig. 1. Flowchart of participant enrolment and follow-up. 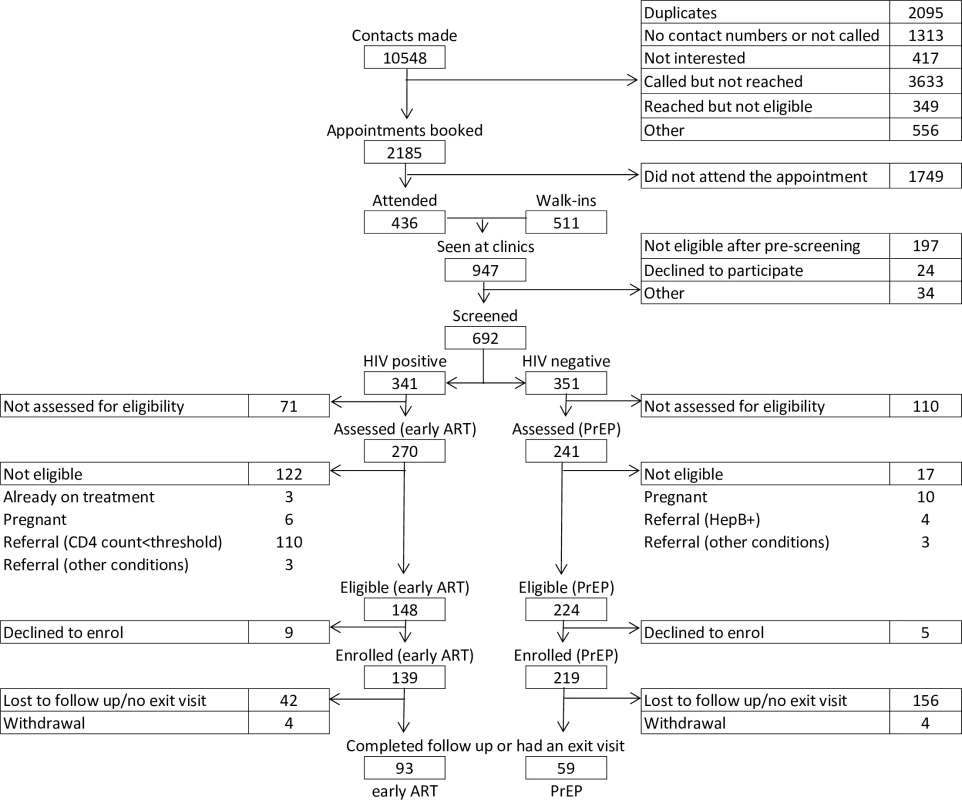
ART, antiretroviral treatment; HepB+, hepatitis B positive; CD4 count<threshold, CD4 count results below the threshold for ART initiation at the time of assessment; PrEP, pre-exposure prophylaxis. The comparison of TAPS cohorts, both PrEP and early ART, disaggregated by time of enrolment (e.g., before or after the launch of the national sex worker HIV programme) revealed no statistically significant differences throughout the enrolment and retention cascade, aside from 2 points: number of appointments booked (20% of contacts made resulted in an appointment being booked in the cohort enrolled before the national programme as opposed to 26% among those enrolled after the programme launch) and number eligible for early ART (more HIV-positive participants were eligible for early ART in the cohort recruited after the programme launch, 95%, compared to the cohort enrolled before the programme launch, 51%). In particular, the higher proportion of appointments booked from contacts made among those enrolled in the cohort after the launch of the programme compared to those enrolled before the programme could be related to larger recruitment and education efforts at the sites. The higher proportion of participants eligible for early ART in the cohort recruited after the programme started could be an indication of earlier presentation. The comparison of TAPS cohorts, both PrEP and early ART, disaggregated by completion of follow-up period (e.g., 12-month follow-up completed before or after the launch of the national programme) revealed no statistically significant differences throughout the enrolment and retention cascade. These results are presented in Table B of S3 Text. Further details regarding enrolment statistics over time can be found in Fig C of S3 Text.
Baseline characteristics
In Table 1, selected baseline characteristics of enrolled participants are presented. Women enrolled in the PrEP group were significantly younger than those enrolled in the early ART group. In both groups, a substantial fraction of FSWs were currently in a steady partnership (48% on early ART and 53% on PrEP), were born in Zimbabwe (48% on early ART and 67% on PrEP), and had a secondary education (86% on early ART and 87% on PrEP). A substantial majority of the participants worked in brothels: 62% in the early ART group and 77% in the PrEP group.
Tab. 1. Baseline characteristics of female sex worker participants enrolled (total n = 358). 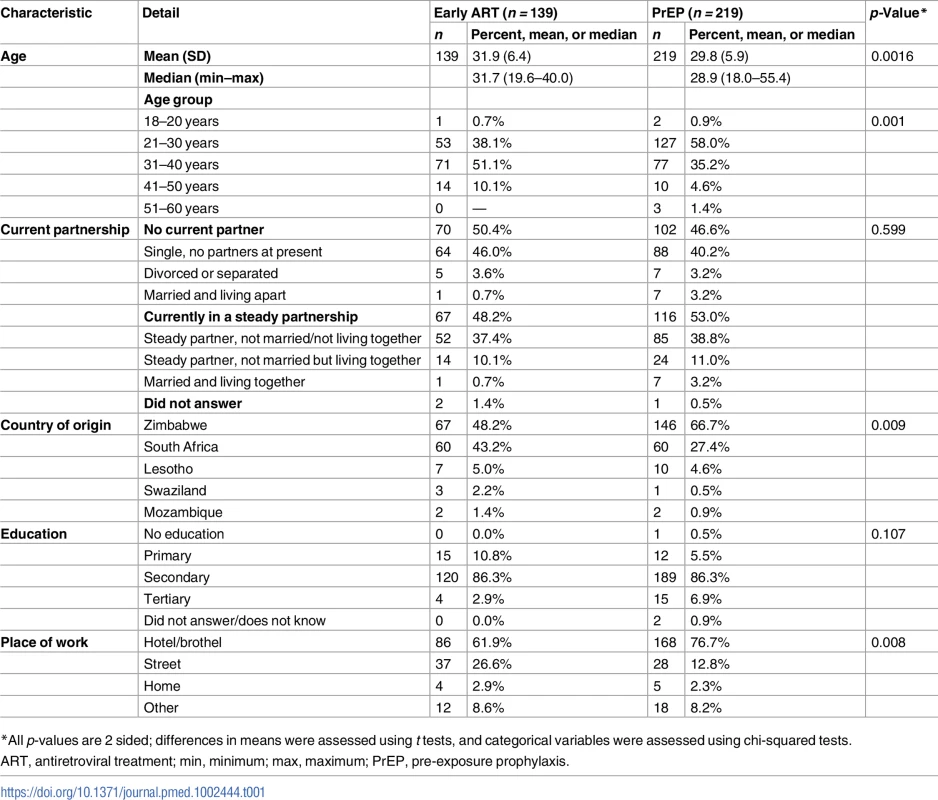
*All p-values are 2 sided; differences in means were assessed using t tests, and categorical variables were assessed using chi-squared tests. Visit attendance and retention
During the first 12 months of follow-up, 156 and 42 participants (71% and 30%) were lost to follow-up (missed 2 clinical monitoring visits or did not complete an exit visit at 12 months) from the PrEP and early ART groups, respectively. The prevention and care cascades are shown in Fig 2.
Fig. 2. HIV prevention and care cascades. 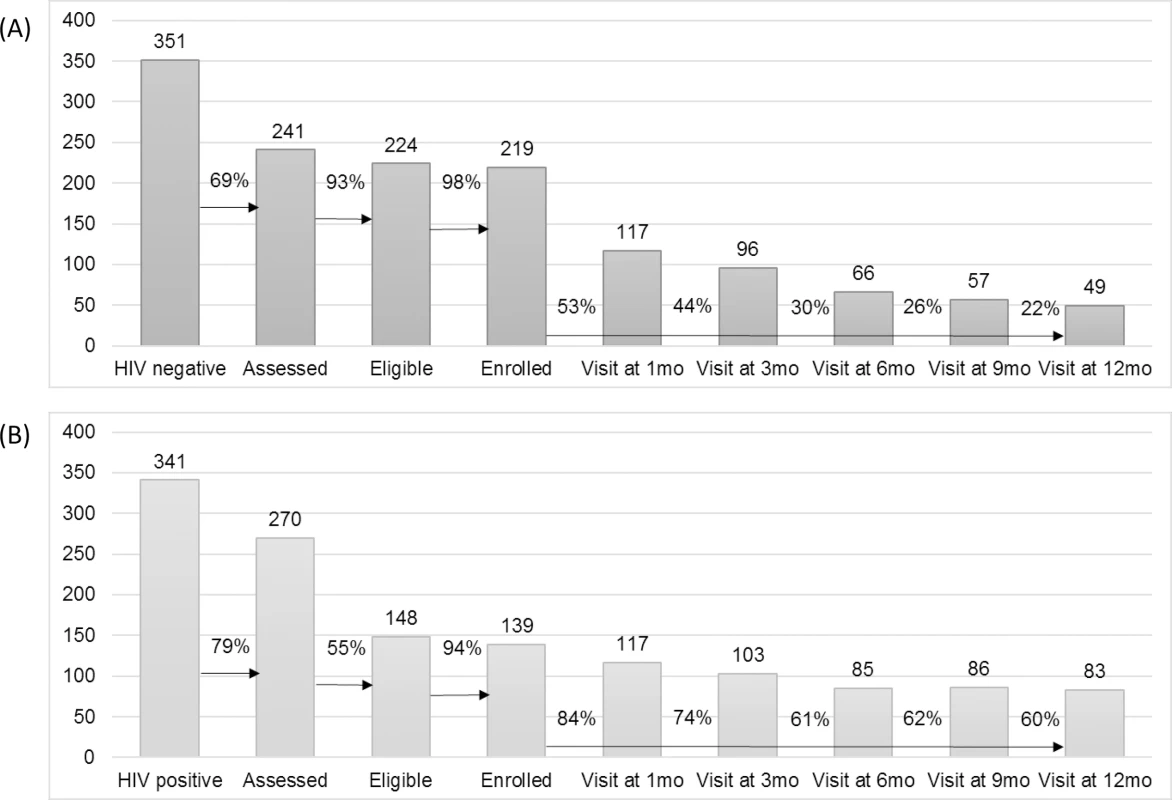
(A) HIV prevention cascade: pre-exposure prophylaxis cohort. (B) HIV treatment cascade: early antiretroviral treatment cohort. The study follow-up period closed in 30 June 2017. In all, 22% and 60% of the enrolled PrEP and early ART cohorts, respectively, attended the 12-month visit. Overall, programme adherence was higher in the early ART group compared to the PrEP group, where participants tended to be less regular in their attendance. This is illustrated graphically in Fig D of S3 Text. Eight women withdrew, 4 from each group. Reasons for withdrawal were either side effects or moving to another location. Extended cascade results for those women who attended the study for a period of time longer than 12 months are presented in Table E of S3 Text. When examining loss to follow-up over time, as shown in Fig F of S3 Text, we observed that most women dropped off earlier in the schedule, in particular in the PrEP group. Denominators for each point in the cascade are also shown in Table E of S3 Text and should be taken into account for the results regarding loss to follow-up over time shown in Fig F of S3 Text.
Secondary outcomes
In Fig 3, self-reported consistent condom use by partner type and study group is shown over time. Higher condom use was reported with clients than with partners, especially main partners (steady partners). Women on early ART appeared to use condoms slightly more with casual and main partners than those on PrEP. Little change in reported condom use over time by type of partner was observed. Additionally, there was no apparent increase in number of partners over time. Further details on these data are presented in Table G in S3 Text. Number of STI episodes at baseline and every 3 months are presented in Table H in S3 Text. As compared with the number of episodes at study baseline, FSWs had significantly fewer STI episodes while participating in the study in both groups. These data are presented with the caveat that dropout over time may contribute to bias in the numbers at later time points.
Fig. 3. Sexual behaviour over time by partner type: consistent condom use and number of partners in last 7 days. 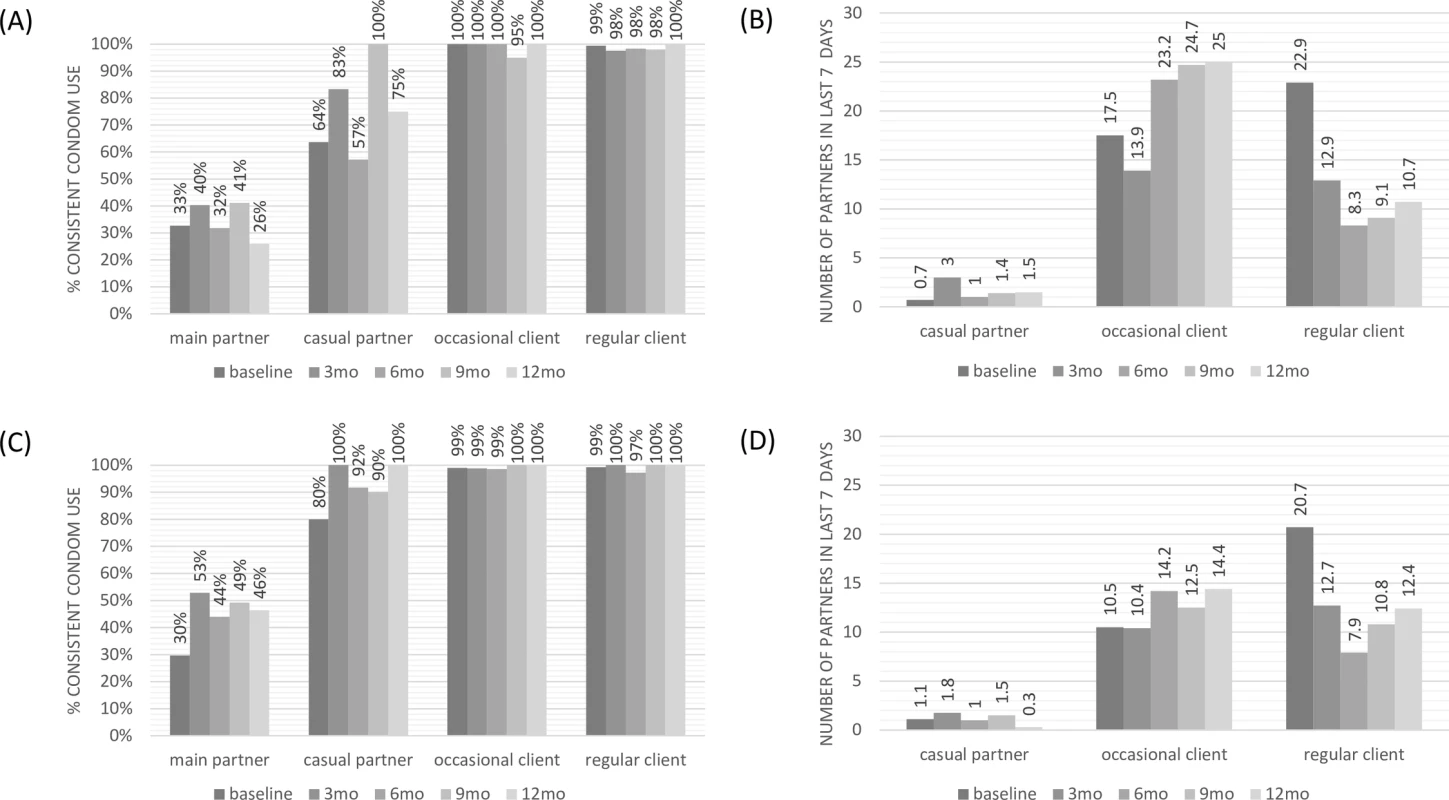
(A) Consistent condom use: pre-exposure prophylaxis cohort. (B) Number of partners in the last 7 days: pre-exposure prophylaxis cohort. (C) Consistent condom use: early antiretroviral treatment cohort. (D) Number of partners in the last 7 days: early antiretroviral treatment cohort. The proportion of women reporting taking PrEP pills daily varied over time between 70% and 85%, whereas over 90% of participants reported taking pills daily while on early ART during the first 12 months of follow-up (Table I in S3 Text).
During the first 12 months of follow-up, there were 7 pregnancies in the PrEP group: Three women chose to terminate the pregnancy and continue on PrEP in the study, 3 were lost to follow-up, and 1 chose to continue on PrEP in the study while pregnant. There were 8 pregnancies in the early ART group, and an additional 4 in the extended follow-up period, 2 of which were identified at study exit. All early ART participants chose to continue with the study and their pregnancies.
One participant from the PrEP group who had previously decided to leave the study returned after she became HIV-positive and was referred for ART initiation. In the early ART group, there were 12 confirmed cases of virological failure—11 of which resulted in high-level virological resistance to at least 1 drug (11 with resistance to efavirenz, 5 to emtricitabine, and 2 to tenofovir)—among the women retained in the study. One patient had fully susceptible virus at resistance testing.
Of all 692 women who completed the screening process, only 1 participant presented with serious complicating health issues, including suspected TB and a creatinine clearance of 50 ml/min. There were no other cases presenting with creatinine clearance values outside of the threshold for initiation of tenofovir.
PrEP and treatment drugs were well tolerated, with the majority of adverse events being reported as mild for both groups, and few moderate. Of the 306 total adverse events recorded, only 17 were assessed to be drug related: 8 women on PrEP reported mild headaches, nausea, drowsiness, dizziness, or diarrhoea, and 9 women on early ART reported mild nausea, diarrhoea, dizziness, or drowsiness. One participant developed abnormal liver function tests, presenting ill after taking early ART for 4 months. Liver function tests revealed severely abnormal liver function, and the woman was referred to a specialist, where the diagnosis was HIV-induced sclerosing cholangitis.
Finally, in Table 2, both unit cost per visit type and overall cost of a person-year on early ART and PrEP are shown. We found lower unit costs for the visit types involving low overheads, few drugs or tests (i.e., outreach visits), or less staff time (i.e., refill visits). The distribution of costs by input is presented in Fig J of S3 Text.
Tab. 2. Unit costs per visit and per person-year for PrEP and early ART among female sex workers (2015 US dollars). 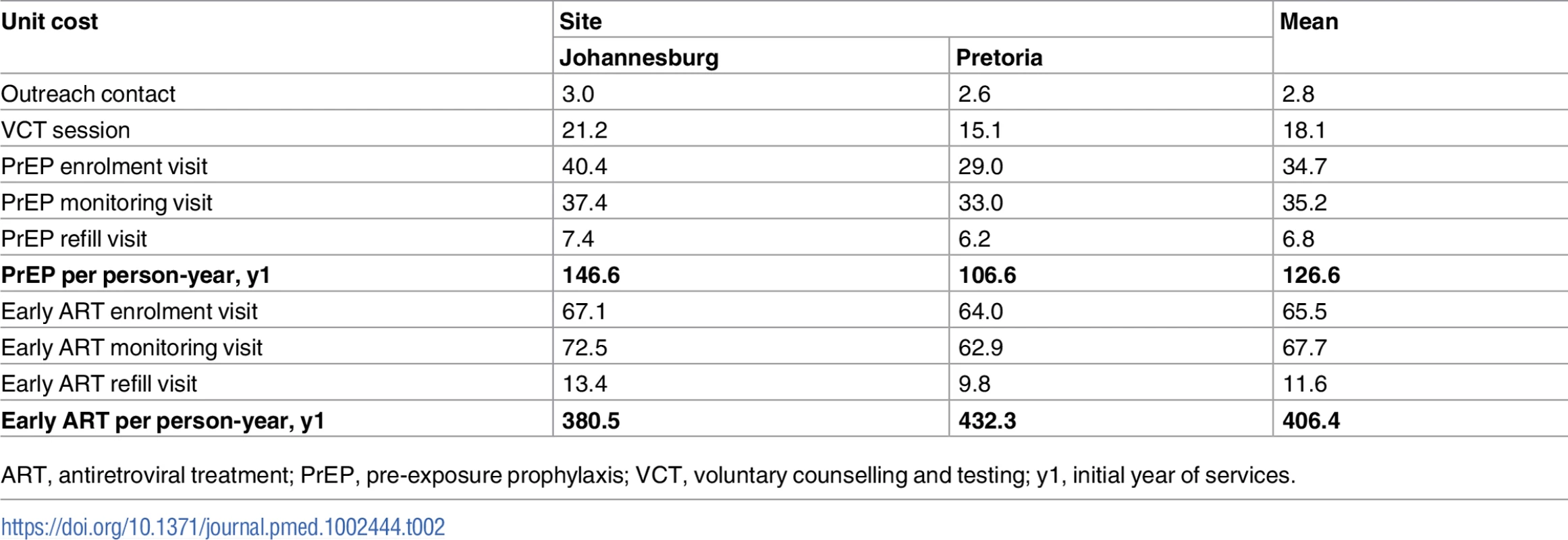
ART, antiretroviral treatment; PrEP, pre-exposure prophylaxis; VCT, voluntary counselling and testing; y1, initial year of services. Discussion
In this prospective observational demonstration project, we observed high uptake of the PrEP and early ART interventions among eligible FSWs. Retention in the early ART group was consistent with the national programme [21], while retention in the PrEP group was lower at the 12-month assessment. There were few virological failures in the early ART group and no seroconversions in the PrEP group. However, given the low rate of retention at the end of the study in the PrEP group, it is possible that women who dropped out and were not tracked became HIV-positive. To our knowledge, this is the first demonstration project to present results following the integration of a combined PrEP/early ART intervention into an existing sex worker health programme. The results of this project supported the establishment of the first official combined guidelines for oral PrEP and early ART in Africa, as well as the first specialised national programming including these interventions for sex workers in Africa [22,23]. The South African PrEP and early ART (or test and treat) guidelines were launched initially for sex workers in June 2016, at which point PrEP and initiation of ART irrespective of CD4 count became standard of care for sex workers. Test and treat was then extended to all HIV-positive people in South Africa in September 2016.
Additionally, TAPS has produced 2 important programmatic findings. First, the high number of women informed about the study translated into a much smaller number actually presenting to take up the interventions. Since both PrEP and early ART are novel interventions in this setting and for this population group, it is possible that as knowledge and use spread in this community, overall uptake might increase, as seen with the introduction of contraception [24]. Normalisation of new interventions may be key to their successful implementation [25], and this will be explored further in the analysis of qualitative data. Second, among those who presented at the clinic, there were high levels of intervention uptake for both PrEP and early ART.
The early ART group had a higher level of retention than the PrEP group. It is important to note that retention in care refers to 2 different constructs depending on whether participants are being retained in a prevention or treatment intervention. For the PrEP group, we formulated our definition of retention to reflect maintenance of continuous contact with healthcare services and access to prevention packages, even though the women were allowed to cycle in and out of PrEP medication use. For the early ART group, we valued retention in care as an essential component of a successful treatment programme. Interestingly, women who enrolled on PrEP earlier in the study tended to be the ones who continued the longest, suggesting that those most motivated, interested, and/or able to take up the interventions came earlier. It is important to note that participants were not reimbursed for their participation, suggesting that dedicated sex worker services can result in high levels of engagement in care among FSWs.
We observed a significant participant drop-off between screening and assessment for eligibility. Much of the loss may be directly related to the waiting period between screening and enrolment required for research purposes and to assess laboratory results. This may not reflect service delivery in future programmes where same-day initiation is being considered for both PrEP and early ART. We had only a single creatinine case at screening meriting concern, and immediate initiation of PrEP, with follow-up and potential discontinuation for those with concerning results, may be possible.
Women who became pregnant in either study group were given options regarding maintaining PrEP/ART use and staying in the study or transferring into other care. Of those who continued on PrEP in the study, 1 woman continued her pregnancy, while 3 terminated their pregnancies. It is possible that those who were lost to follow-up decided to see their pregnancies through, but this is unconfirmed. It is also possible that by excluding those who were pregnant at enrolment, women highly motivated to take PrEP were not given the opportunity. Given the heightened risk of HIV infection in women who become pregnant, being able to continue PrEP use during pregnancy may be a critical programming consideration.
At baseline, the TAPS cohorts had similar demographic characteristics (age, education, and place of origin) to the recently described local FSW population in Johannesburg [26]. Yet there was a higher rate of FSWs in stable relationships in our sample. This is likely a reflection of the group of FSWs accessing SWP clinics in general, as a similar profile was recently reported among SWP attendees not enrolled in the study [27].
The patterns of visits to the clinic for PrEP combined with the reported adherence would suggest intermittent use of PrEP while maintaining an overall engagement with services. Although we only analysed self-reported adherence data in this paper, the data were collected at every clinic and pill collection visit, and women did not report perfect adherence and usually reported when they took ‘PrEP breaks’, suggesting a low level of social desirability bias. Additionally, with no seroconversions among the PrEP users, intermittent PrEP use does not appear to be an issue in maintaining negative HIV status, noting also the high reported use of condoms with clients. Reassuringly, there was no observed change over time in the study in reported consistent condom use or number of partners in the last 7 days. There were fewer STI episodes during the study than at baseline for both groups. This finding, combined with the results of rigorous and consistent data collection on condom use over time with all sexual partners, suggests that the women who remained in the study may have been improving their attention to prevention through engagement in the study. This finding makes for an interesting comparison with another completed demonstration project study among MSM, where moderate increases in STIs during the study suggested slight reductions in condom use [28].
Finally, we estimated the total costs for PrEP and early ART per person-year to be in the same order of magnitude as recently published estimates [29,30]. The higher TAPS costs for early ART compared to the costs included in the South African HIV and TB Investment Case reflect the TAPS programme procedures, service utilisation, and staff costs, which are likely higher than the costs of routine services [30].
Limitations
One limitation of the study is that no comparison group was included for either intervention, limiting the ability to assess the effectiveness of integrated services, as it would not have been ethical to maintain comparison groups with no or delayed access to interventions shown to be effective [31]. A second limitation is that efforts to replicate healthcare delivery through an integrated service were affected both by regulatory (dispensing from a pharmacy and medical officer assessment of adverse events) and ethics committee (informed consent administration) requirements. However, we expect that the removal of these requirements would improve rates of uptake and retention.
In addition, a national PrEP and early ART programme for FSWs in South Africa started 1 June 2016. After its initiation, this programme could have influenced the uptake of the interventions for those FSWs approached for enrolment in TAPS. Conversely, it could have influenced retention within the programme for those FSWs for whom the follow-up period overlapped with the provision of the national programme. The TAPS clinics were the only providers of PrEP free of charge to FSWs in the public setting and required informed consent to access the interventions as part of a research study, until the rollout of the national programme, which also provided these services at no cost to clients. Furthermore, the implementation of PrEP as part of the national programme meant that PrEP could be viewed as the standard of care and was therefore more normalised from the population’s perspective. Since PrEP was no longer considered experimental after 31 May 2016, we expected that women would think differently about PrEP, which could have affected uptake and retention in TAPS. For example, as the programme was scaled up in the initial 11 sites (including in the 2 TAPS sites), we became aware that some of the TAPS participants opted to collect their PrEP from the mobile clinics in Pretoria, rather than coming to the fixed clinic where TAPS was located.
Finally, we do not have data on the women who did not come back to the study, and the adherence and sexual behaviour data are self-reported, increasing the possibility of bias. Unfortunately, drug level analyses in the PrEP group were not available at the time of writing. These analyses will be presented in a more in-depth analysis in a subsequent publication. Additionally, the data reported are based on populations of FSWs in 2 specific urban settings, possibly limiting generalisability.
Conclusion
The findings from TAPS informed the setup of the national programme of PrEP and test and treat for sex workers. This analysis shows that PrEP and early ART can be aligned with existing health service programming for FSWs safely, without significant behaviour change, with high rates of uptake for both interventions, and with expected cost reduction in routine settings at scale.
Supporting Information
Zdroje
1. GBD 2015 HIV Collaborators. Estimates of global, regional, and national incidence, prevalence, and mortality of HIV, 1980–2015: the Global Burden of Disease Study 2015. Lancet HIV. 2016;3:e361–87. doi: 10.1016/S2352-3018(16)30087-X 27470028
2. Joint United Nations Programme on HIV and AIDS. Prevention gap report. Geneva: Joint United Nations Programme on HIV and AIDS; 2016 [cited 2017 Oct 20]. Available from: http://www.unaids.org/sites/default/files/media_asset/2016-prevention-gap-report_en.pdf.
3. Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Antiretroviral therapy for the prevention of HIV-1 transmission. N Engl J Med. 2016;375 : 830–9. doi: 10.1056/NEJMoa1600693 27424812
4. Iwuji C, Orne-Gliemann J, Balestre E, Larmarange J, Thiebaut R, Tanser F, et al. FRAC0105LB: the impact of universal test and treat on HIV incidence in a rural South African population: ANRS 12249 TasP trial, 2012–2016. 21st International AIDS Conference; 2016 Jul 18–22; Durban, South Africa.
5. Fonner VA, Dalglish SL, Kennedy CE, Baggaley R, O’Reilly KR, Koechlin FM, et al. Effectiveness and safety of oral HIV preexposure prophylaxis for all populations. AIDS. 2016;30 : 1973–83. doi: 10.1097/QAD.0000000000001145 27149090
6. Eaton JW, Johnson LF, Salomon JA, Bärnighausen T, Bendavid E, Bershteyn A, et al. HIV treatment as prevention: systematic comparison of mathematical models of the potential impact of antiretroviral therapy on HIV incidence in South Africa. PLoS Med. 2012;9(7):e1001245. doi: 10.1371/journal.pmed.1001245 22802730
7. Hallett TB, Baeten JM, Heffron R, Barnabas R, de Bruyn G, Cremin Í, et al. Optimal uses of antiretrovirals for prevention in HIV-1 serodiscordant heterosexual couples in South Africa: a modelling study. PLoS Med. 2011;8(11):e1001123. doi: 10.1371/journal.pmed.1001123 22110407
8. Rosen S, Fox MP. Retention in HIV care between testing and treatment in sub-Saharan Africa: a systematic review. PLoS Med. 2011;8(7):e1001056. doi: 10.1371/journal.pmed.1001056 21811403
9. Reynolds LJ, Camlin CS, Ware NC, Seeley J. Exploring critical questions for the implementation of “universal test and treat” approaches to HIV prevention and care. AIDS Care. 2016;28(Suppl 3):1–6. doi: 10.1080/09540121.2016.1178960 27421046
10. AVAC: Global Advocacy for HIV Prevention. Ongoing and planned PrEP demonstration and implementation studies. New York: AVAC: Global Advocacy for HIV Prevention; 2017 October [cited 2017 Nov 1]. Available from: http://www.avac.org/resource/ongoing-and-planned-prep-demonstration-and-implementation-studies.
11. Baeten JM, Heffron R, Kidoguchi L, Mugo NR, Katabira E, Bukusi EA, et al. Integrated delivery of antiretroviral treatment and pre-exposure prophylaxis to HIV-1–serodiscordant couples: a prospective implementation study in Kenya and Uganda. PLOS Med. 2016;13(8):e1002099. doi: 10.1371/journal.pmed.1002099 27552090
12. Cowan F. Results of the SAPPH-IRe Trial: a cluster randomised trial of a combination intervention to empower female sex workers in Zimbabwe to link and adhere to antiretrovirals for treatment and prevention. 21st International AIDS Conference; 2016 Jul 18–22; Durban, South Africa. Available from: http://programme.aids2016.org/Abstract/Abstract/10619.
13. South African National AIDS Council. National strategic plan on HIV, STIs and TB: 2012–2016. Pretoria: South African National AIDS Council; 2012.
14. South African National AIDS Programme. Let our actions count: South Africa’s national strategic plan for HIV, TB and STIs 2017–2022. Pretoria: South African National AIDS Council; 2017 [cited 2017 Oct 20]. Available from: http://sanac.org.za/wp-content/uploads/2017/05/NSP_FullDocument_FINAL.pdf.
15. South African National AIDS Council. National strategic plan for HIV prevention, care and treatment for sex workers. Pretoria: South African National AIDS Council; 2013.
16. Sibanyoni M. Sex in the cities: comprehensive healthcare for sex workers. 7th SA AIDS Conference; 2015 Jun 9–12; Durban, South Africa.
17. Gomez GB, Eakle R, Mbogua J, Akpomiemie G, Venter WDF, Rees H. Treatment And Prevention for female Sex workers in South Africa: protocol for the TAPS Demonstration Project. BMJ Open. 2016;6:e011595. doi: 10.1136/bmjopen-2016-011595 27678533
18. Eakle R, Manthata G, Stadler J, Mbogua J, Sibanyoni M, Venter WDF, et al. Preparing for PrEP & immediate treatment: focus group discussions in advance of a demonstration project in South Africa. AIDS Res Hum Retroviruses. 2014;30(S1):A269–70. doi: 10.1089/aid.2014.5606.abstract
19. US Department of Health and Human Services, US Food and Drug Administration, Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research. Guidance for industry E6 good clinical practice: consolidated guidance. Silver Spring (Maryland): US Food and Drug Administration; 1996 [cited 2017 Jul 23]. Available from: https://www.fda.gov/downloads/drugs/guidances/ucm073122.pdf.
20. World Health Organization. Adverse drug reactions monitoring. Geneva: World Health Organization; 2017 [cited 2017 Jul 23]. Available from: http://www.who.int/medicines/areas/quality_safety/safety_efficacy/advdrugreactions/en/
21. Fox MP, Rosen R. Retention of adult patients on antiretroviral therapy in low - and middle-income countries: systematic review and meta-analysis 2008–2013. J Acquir Immune Defic Syndr. 2015;69 : 98–108. doi: 10.1097/QAI.0000000000000553 25942461
22. South African National AIDS Council. The South African national sex worker HIV plan, 2016–2019. Pretoria: South African National AIDS Council; 2016 Mar [cited 2017 Oct 20]. Available from: http://southafrica.unfpa.org/publications/south-african-national-sex-worker-hiv-plan-2016-2019.
23. South Africa National Department of Health. Guidelines for expanding combination prevention and treatment options for sex workers: oral pre-exposure prophylaxis (PrEP) and test and treat (T&T). Pretoria: South Africa National Department of Health; 2016 May [cited 2017 Oct 20]. Available from: http://www.sahivsoc.org/Files/PrEP%20and%20TT%20Guidelines%20-%20Final%20Draft%20-%2011%20May%202016.pdf.
24. Myers JE, Sepkowitz KA. A pill for HIV prevention: déjà vu all over again? Clin Infect Dis. 2013;56 : 1604–12. doi: 10.1093/cid/cit085 23408681
25. May C, Finch T, Mair F, Ballini L, Dowrick C, Eccles M, et al. Understanding the implementation of complex interventions in health care: the normalization process model. BMC Health Serv Res. 2007;7 : 148. doi: 10.1186/1472-6963-7-148 17880693
26. University of California, San Francisco, Anova Health Institute, Wits Reproductive Health and HIV Institute. South African Health Monitoring Survey (SAHMS): an integrated biological and behavioural survey among female sex workers, South Africa 2013–2014. San Francisco: University of California, San Francisco; 2015.
27. Slabbert M, Venter F, Gay C, Roelofsen C, Lalla-Edward S, Rees H. Sexual and reproductive health outcomes among female sex workers in Johannesburg and Pretoria, South Africa: recommendations for public health programmes. BMC Public Health. 2017;17 : 17–27. doi: 10.1186/s12889-017-4346-0
28. PrEP: demonstration for implementation. Oral Abstract Session Track C: TUAC02. 8th IAS Conference on HIV Pathogenesis, Treatment and Prevention; 2015 Jul 19–22; Vancouver, Canada.
29. Smith JA, Anderson S-J, Harris KL, McGillen JB, Lee E, Garnett GP, et al. Maximising HIV prevention by balancing the opportunities of today with the promises of tomorrow: a modelling study. Lancet HIV. 2016;3:e289–96. doi: 10.1016/S2352-3018(16)30036-4 27365203
30. South Africa National Department of Health, South African National AIDS Council. South African HIV and TB investment case: summary report—phase 1. Pretoria: South African National AIDS Council; 2016 Mar [cited 2017 Oct 20]. Available from: http://sanac.org.za/wp-content/uploads/2016/03/1603-Summary-Report-LowRes-18-Mar.pdf.
31. McCormack S, Dunn DT, Desai M, Dolling DI, Gafos M, Gilson R, et al. Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet. 2016;387 : 53–60. doi: 10.1016/S0140-6736(15)00056-2 26364263
Štítky
Interní lékařství
Článek Contemporary disengagement from antiretroviral therapy in Khayelitsha, South Africa: A cohort studyČlánek Bioequivalence of twice-daily oral tacrolimus in transplant recipients: More evidence for consensus?
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2017 Číslo 11- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Červená fermentovaná rýže účinně snižuje hladinu LDL cholesterolu jako vhodná alternativa ke statinové terapii
- Jak postupovat při výběru betablokátoru − doporučení z kardiologické praxe
-
Všechny články tohoto čísla
- Labour trafficking: Challenges and opportunities from an occupational health perspective
- The end of HIV: Still a very long way to go, but progress continues
- Contemporary disengagement from antiretroviral therapy in Khayelitsha, South Africa: A cohort study
- Bioequivalence of twice-daily oral tacrolimus in transplant recipients: More evidence for consensus?
- Treatment guidelines and early loss from care for people living with HIV in Cape Town, South Africa: A retrospective cohort study
- Perinatal mortality associated with induction of labour versus expectant management in nulliparous women aged 35 years or over: An English national cohort study
- Core Outcome Set-STAndards for Development: The COS-STAD recommendations
- Closing the gaps in the HIV care continuum
- Association between the 2012 Health and Social Care Act and specialist visits and hospitalisations in England: A controlled interrupted time series analysis
- HIV pre-exposure prophylaxis and early antiretroviral treatment among female sex workers in South Africa: Results from a prospective observational demonstration project
- Sexual exploitation of unaccompanied migrant and refugee boys in Greece: Approaches to prevention
- Child sex trafficking in the United States: Challenges for the healthcare provider
- The expanding epidemic of HIV-1 in the Russian Federation
- Cardiovascular disease (CVD) and chronic kidney disease (CKD) event rates in HIV-positive persons at high predicted CVD and CKD risk: A prospective analysis of the D:A:D observational study
- Validity of a minimally invasive autopsy for cause of death determination in maternal deaths in Mozambique: An observational study
- malERA: An updated research agenda for malaria elimination and eradication
- malERA: An updated research agenda for health systems and policy research in malaria elimination and eradication
- A combination intervention strategy to improve linkage to and retention in HIV care following diagnosis in Mozambique: A cluster-randomized study
- Bioequivalence between innovator and generic tacrolimus in liver and kidney transplant recipients: A randomized, crossover clinical trial
- malERA: An updated research agenda for basic science and enabling technologies in malaria elimination and eradication
- Human trafficking and exploitation: A global health concern
- Virological response and resistance among HIV-infected children receiving long-term antiretroviral therapy without virological monitoring in Uganda and Zimbabwe: Observational analyses within the randomised ARROW trial
- Postmenopausal hormone therapy and risk of stroke: A pooled analysis of data from population-based cohort studies
- Lansoprazole use and tuberculosis incidence in the United Kingdom Clinical Practice Research Datalink: A population based cohort
- malERA: An updated research agenda for insecticide and drug resistance in malaria elimination and eradication
- Safety, pharmacokinetics, and immunological activities of multiple intravenous or subcutaneous doses of an anti-HIV monoclonal antibody, VRC01, administered to HIV-uninfected adults: Results of a phase 1 randomized trial
- HIV prevalence and behavioral and psychosocial factors among transgender women and cisgender men who have sex with men in 8 African countries: A cross-sectional analysis
- Treatment eligibility and retention in clinical HIV care: A regression discontinuity study in South Africa
- malERA: An updated research agenda for characterising the reservoir and measuring transmission in malaria elimination and eradication
- Effectiveness of a combination strategy for linkage and retention in adult HIV care in Swaziland: The Link4Health cluster randomized trial
- The value of confirmatory testing in early infant HIV diagnosis programmes in South Africa: A cost-effectiveness analysis
- HIV self-testing among female sex workers in Zambia: A cluster randomized controlled trial
- The US President's Malaria Initiative, transmission and mortality: A modelling study
- Comparison of two cash transfer strategies to prevent catastrophic costs for poor tuberculosis-affected households in low- and middle-income countries: An economic modelling study
- Direct provision versus facility collection of HIV self-tests among female sex workers in Uganda: A cluster-randomized controlled health systems trial
- malERA: An updated research agenda for diagnostics, drugs, vaccines, and vector control in malaria elimination and eradication
- malERA: An updated research agenda for combination interventions and modelling in malaria elimination and eradication
- HIV-1 persistence following extremely early initiation of antiretroviral therapy (ART) during acute HIV-1 infection: An observational study
- Respondent-driven sampling for identification of HIV- and HCV-infected people who inject drugs and men who have sex with men in India: A cross-sectional, community-based analysis
- Extensive virologic and immunologic characterization in an HIV-infected individual following allogeneic stem cell transplant and analytic cessation of antiretroviral therapy: A case study
- Measuring success: The challenge of social protection in helping eliminate tuberculosis
- Prospects for passive immunity to prevent HIV infection
- Reaching global HIV/AIDS goals: What got us here, won't get us there
- Evidence-based restructuring of health and social care
- Extreme exploitation in Southeast Asia waters: Challenges in progressing towards universal health coverage for migrant workers
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Postmenopausal hormone therapy and risk of stroke: A pooled analysis of data from population-based cohort studies
- Bioequivalence between innovator and generic tacrolimus in liver and kidney transplant recipients: A randomized, crossover clinical trial
- HIV pre-exposure prophylaxis and early antiretroviral treatment among female sex workers in South Africa: Results from a prospective observational demonstration project
- Bioequivalence of twice-daily oral tacrolimus in transplant recipients: More evidence for consensus?
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



