-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamamalERA: An updated research agenda for characterising the reservoir and measuring transmission in malaria elimination and eradication
Christopher Drakeley and colleagues propose an updated research agenda for characterizing the reservoir and measuring transmission in malaria elimination and eradication.
Published in the journal: . PLoS Med 14(11): e32767. doi:10.1371/journal.pmed.1002452
Category: Collection Review
doi: https://doi.org/10.1371/journal.pmed.1002452Summary
Christopher Drakeley and colleagues propose an updated research agenda for characterizing the reservoir and measuring transmission in malaria elimination and eradication.
Summary points
Understanding the sources of transmission (the infectious reservoir) and those at risk of infection at the population level in order to inform programmatic decision-making can progress malaria elimination.
There is considerable evidence for malaria infections at densities beneath the limit of conventional diagnostics. However, the contribution of these low-density infections to malaria transmission in different settings is not known.
Characterising the spatial and temporal heterogeneity of the infectious reservoir becomes increasingly important as transmission declines if interventions are to be efficiently implemented to accelerate malaria elimination.
The proportional contributions of low-density, asymptomatic, and symptomatic infections will differ by malaria typology and will determine the programmatic approach required to reduce transmission.
Plasmodium vivax hypnozoites are undetectable with currently available diagnostics, representing a major barrier to both understanding the transmission reservoir for this parasite and its elimination.
There is a need to standardise both existing transmission metrics and new metrics with greater sensitivity, particularly for their use in low-transmission settings.
Introduction
Transmission of malaria requires sexual-stage parasites, gametocytes, in humans to be taken up by female Anopheles mosquitoes when they feed. After a period of parasite development, mosquitoes can then infect humans. A break in this cycle at any point interrupts malaria transmission. Malaria control has historically focussed on the reduction of morbidity and mortality of the human host rather than on the interruption of transmission from human to mosquito. Understanding the variation in the relationship between infection (the presence of parasites in an individual or mosquito) and infectiousness (the ability to transmit parasites to a mosquito or human) at different transmission intensities and with different levels of intervention coverage is increasingly recognised as critical in the pursuit of malaria elimination.
In 2011, one of the main conclusions of the Malaria Eradication Research Agenda (malERA) process was the need to develop tools to measure transmission at low levels in elimination contexts. This article summarizes progress made since 2011 and for the first time develops a research agenda addressing the reservoir of transmissible parasites and measuring transmission [1,2]. Findings and recommendations presented here result from a systematic search of the literature and the deliberations of the 2015 malERA Refresh Consultative Panel on characterising the reservoir and measuring transmission, including specialists from field and implementation science, entomology, epidemiology, and basic science.
Since the 2011 malERA process, research has ranged from illuminating the basic biology of the development of sexual-stage parasites in humans and mosquitoes to evaluating operational approaches targeting infectious individuals in endemic communities. Additionally, a harmonised set of definitions relevant to malaria transmission and elimination has been developed (Box 1) [3]. However, there remains a need to further validate a ‘toolkit’ of metrics and associated surveillance activities to characterise the infectious reservoir and measure malaria transmission that can be applied programmatically to direct and evaluate interventions and to quantify progress towards malaria elimination. There are multiple factors that contribute to malaria epidemiology including ecology, vectors, parasites, human biology and behaviour, and economic and health-system factors (see Box 1), and these collectively make up a given ‘typology’ of malaria. The selection of appropriate surveillance activities and metrics from this toolkit will not only need to reflect variations in malaria ‘typology’ (Box 1) [3], but will need to be adapted as malaria transmission declines (Fig 1).
Box 1. Terminology
Malaria typologies
Malaria typology is the characterisation of malaria epidemiology according to ecology (climate and environment) and other determinants of transmission for the purpose of guiding malaria interventions. Relevant ecologies include (but are not limited to) savannah, lowland plains and valleys, highlands, desert and oasis, forest and jungle, coastal and marshland, and urban or peri-urban. The unique features of malaria transmission in each ecological area are also strongly driven by region-specific vectors and parasites (species, biology, behaviour, insecticide and antimalarial drug susceptibility), human biology and behaviour, and economic and health-system factors. These are discussed more comprehensively in [4] and [5].
Fig. 1. Research needs and programmatic applications in measuring malaria transmission across the transmission spectrum. 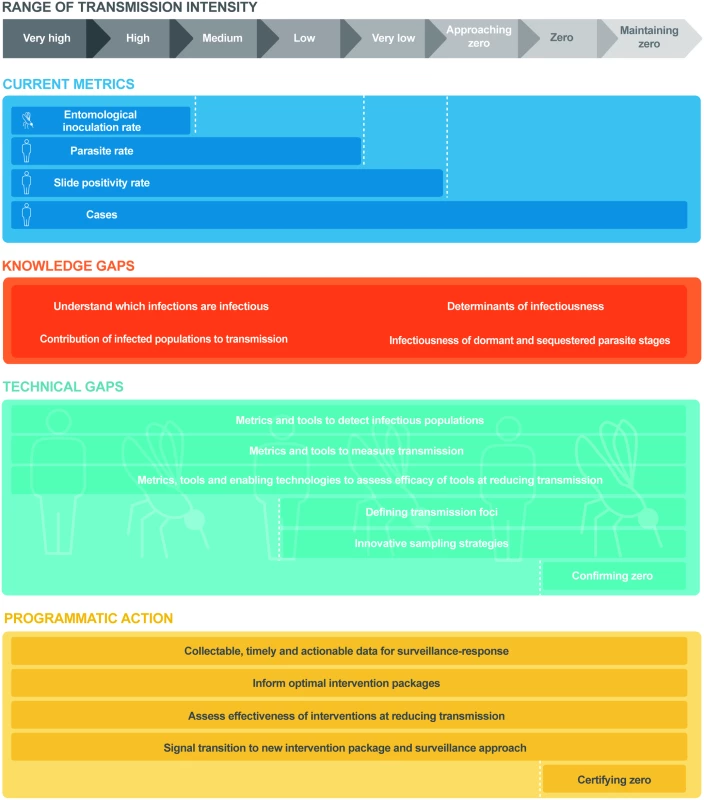
Range of malaria transmission intensity (grey line) from very high intensity to postelimination settings. Current metrics (navy blue line) used for routine measurement of malaria transmission at each level of transmission intensity. Knowledge gaps (orange line) in understanding the biology and epidemiology of malaria transmission and the infectious reservoir at all levels of transmission intensity. Technical gaps (light blue line) in the accurate measurement of transmission at each level of transmission intensity. Programmatic actions (yellow line) required for the interruption of transmission and the prevention of reintroduction at each level of transmission intensity. This paper discusses progress in the measurement and understanding of malaria transmission, highlighting the different malaria typologies in which transmission occurs (Box 1). This differentiation between typologies is needed to determine where existing strategies and systems can sufficiently achieve malaria elimination versus those where additional approaches or tools are required.
Research agenda for characterising the reservoir of infection
Detecting malaria: Infection versus transmission
Malaria infection and transmission can be detected and measured with a variety of metrics (Tables 1 and 2). Their suitability and discriminatory power, however, can vary widely across settings and populations. To reliably confirm clinical malaria, a minimum diagnostic sensitivity of 200 parasites/μL blood is required [6]. Microscopy and some rapid diagnostic tests (RDTs) meet this threshold [6]. In the absence of fever, some individuals will have parasitaemia levels detectable by microscopy and RDTs. These asymptomatic infections are particularly common in areas of high transmission (i.e., above 25 clinical cases per week per 1,000 persons) [7], where high levels of human immunity allow older individuals to carry relatively large parasite burdens chronically [8]. Such individuals would be detected within mass screen and treat (MSAT) programmes using currently available diagnostics. However, through the use of molecular amplification methods, it is now clear that many individuals harbour low-density malaria infections beneath the limit of detection of both microscopy and RDTs [9]. Meta-analyses indicate that molecular methods detect up to twice as many P. falciparum infections as RDT or microscopy [10], and approximately 5 times as many P. vivax infections [11,12]. This gap in sensitivity may be more pronounced when compared against ultra-sensitive molecular methods [13]. Lack of sensitivity of diagnostic detection is more acute for P. vivax infections, which circulate at lower parasite densities hampering accurate estimates of true prevalence. There are also other unique challenges presented by P. vivax that make characterising its transmission reservoir problematic (Box 2) [14–18].
Tab. 1. Summary of currently available entomological malaria transmission metrics. 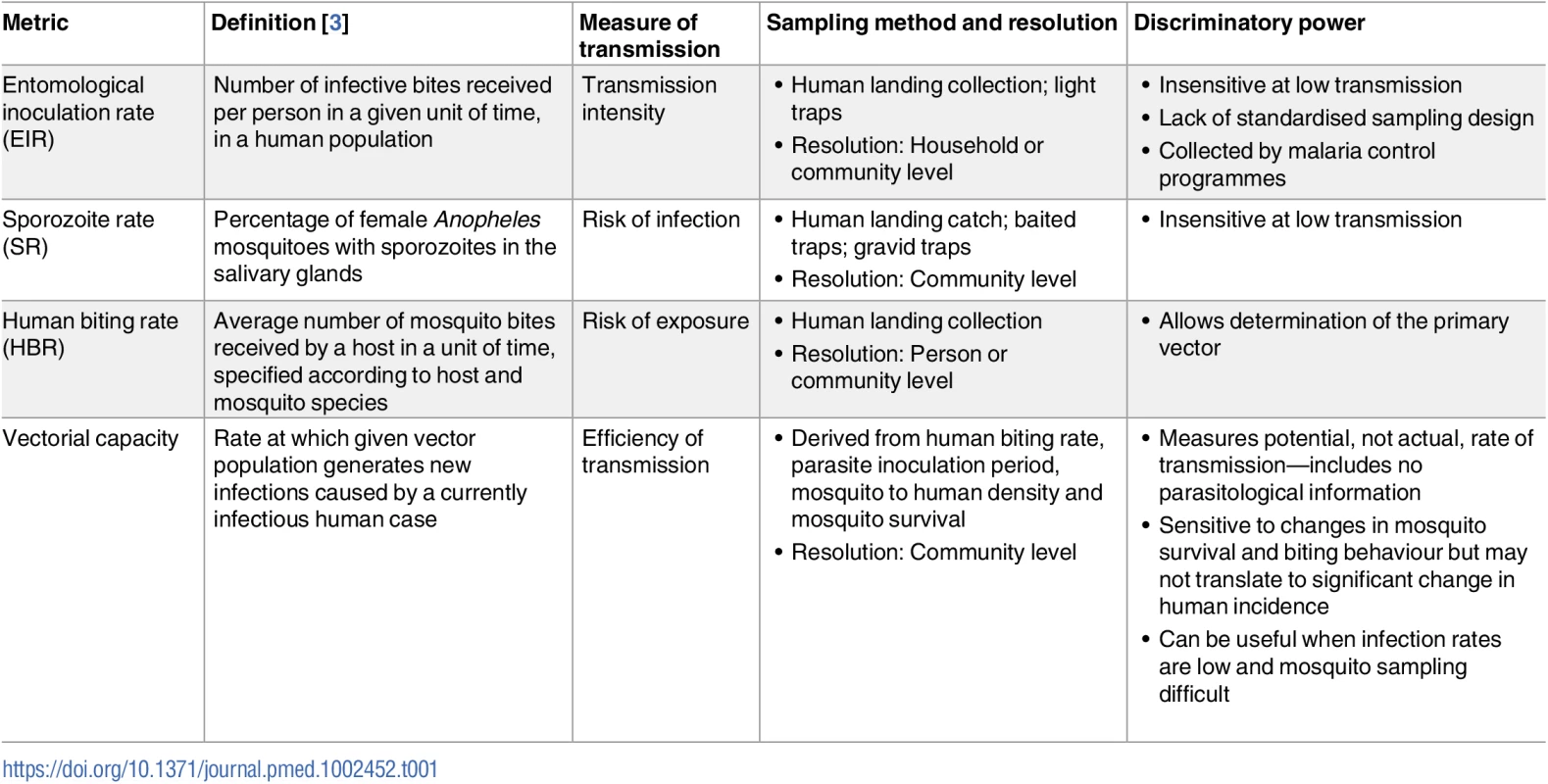
Human landing collection; light traps Tab. 2. Summary of currently available malaria transmission metrics in humans. 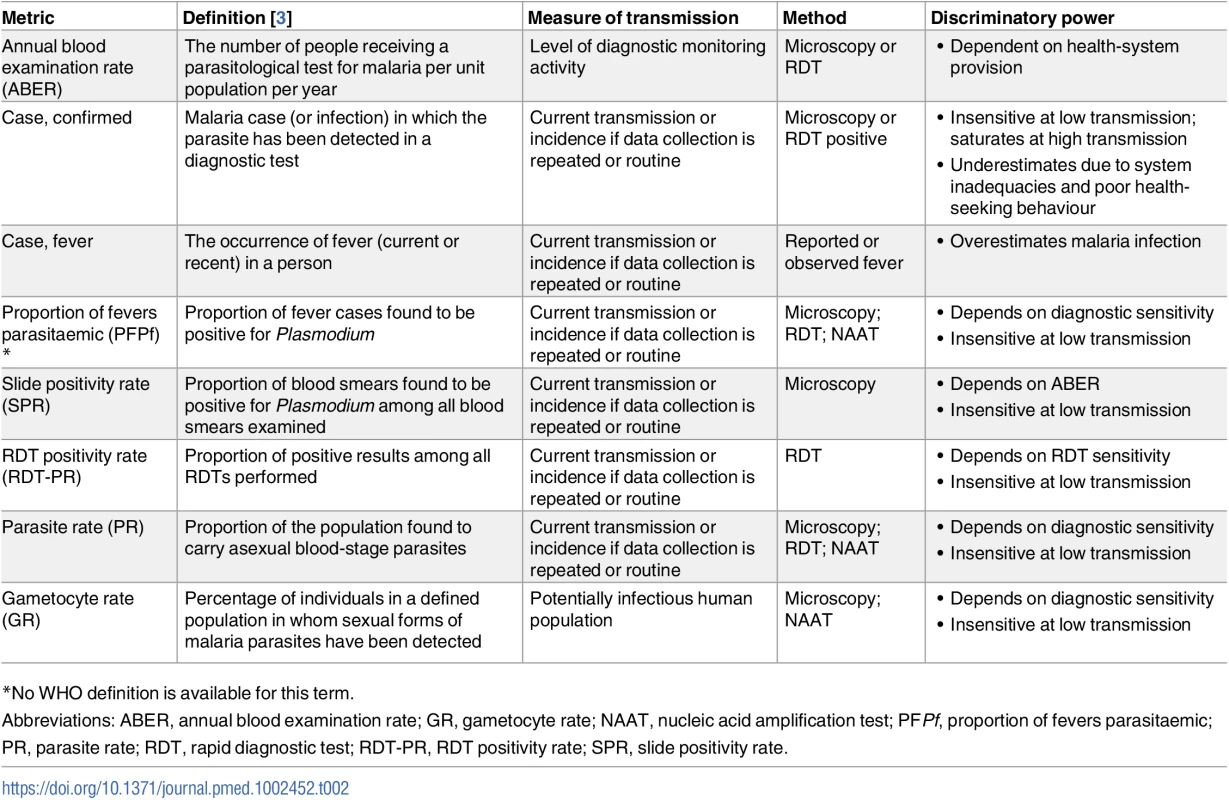
Dependent on health-system provision Box 2. P. vivax and P. ovale
P. vivax and P. ovale have a dormant liver stage, the hypnozoite, which is undetectable by currently available diagnostic methods. Periodic reactivation of hypnozoites results in repeated blood-stage infection (relapses) occurring weeks, or even years, following the initial infection. As control efforts reduce the incidence of P. falciparum cases, P. vivax cases can remain relatively stable and become a greater proportion of malaria cases overall [16]. P. vivax is refractory to traditional vector control methods: hypnozoites enable the parasite to evade conditions unfavourable to transmission and will survive in the host following schizonticidal anti-malarial therapy. Without new anti-hypnozoite drugs or vaccines that could be used safely across entire populations, the P. vivax/ovale transmission reservoir cannot be targeted, making elimination of these parasites challenging in any setting.
Key advances
Relapses drive transmission
In children in Papua New Guinea, 4 of every 5 P. vivax infections and 3 of every 5 P. ovale infections were caused by relapses [14].
Both primary and relapse P. vivax infections generate gametocytes, which typically appear before clinical symptoms, and promote onward ‘silent’ transmission of the parasite [15].
Estimating transmission using the typical entomological measures is of limited relevance when clinical disease can emerge from an individual not recently infected by a mosquito bite.
Research needs
Detection of hypnozoites to inform targeted drug or vaccination strategies
Access to existing anti-hypnozoite therapy needs to be expanded where possible in order to reduce the burden of disease and minimise the risk of human-to-mosquito transmission via relapse.
However, several barriers to mass drug administration (MDA) for P. vivax exist. The 8-aminoquinolines primaquine and tafenoquine are the only known anti-hypnozoite drugs. Both drugs are contraindicated in pregnancy and individuals with glucose-6-phosphate dehydrogenase deficiency [17,18]. Even if rapid, accurate point-of-care tests were available to exclude these individuals from treatment, a significant proportion of the population (typically >10%) will remain untreated.
Without being able to identify hypnozoites, MSAT is of no practical value in reducing P. vivax or P. ovale transmission [14].
Compared to P. falciparum, P. vivax and P. ovale present as much lower parasite densities; therefore, determining the appropriate limit of detection for new diagnostics will be a major challenge.
Improve understanding of parasite-vector bionomics
Parasites can be transported undetected into areas where malaria has been eliminated, leading to outbreaks and the reestablishment of transmission where conditions are receptive. More effort needs to be directed at understanding specific parasite vector interactions to develop targeted vector control strategies for P. vivax/ovale to reduce the risk of mosquito-to-human transmission.
Diagnosis and treatment of clinical malaria is vital for disease control, particularly if this can be rapidly implemented to reduce the likelihood of gametocyte production. There is also a good public health rationale for identifying and treating ‘asymptomatic’ malaria detectable with microscopy or RDTs, as it is increasingly recognised that this is associated with ongoing morbidity (e.g., anaemia, increased susceptibility to bacterial infections, and cognitive function; reviewed in [8]). If the aim is malaria elimination, the contribution of low-density infections to transmission needs to be considered given that, where data are available, low-density infections represent a significant proportion of malaria infections and can be the majority in low-endemic areas [9,10,19,20].
While the countries that have achieved malaria elimination to date have done so largely without specific attempts to detect and treat low-density parasitaemia, these may not be representative of malaria typologies in higher-transmission settings. In many areas, the persistence of malaria can occur despite high coverage of vector control measures and the availability of effective treatment, suggesting that novel approaches are needed for both surveillance and interventions that will accelerate the elimination process [19,21]. Furthermore, studies have documented the failure of strategies to reduce clinical malaria incidence and transmission, such as MSAT, when the transmission reservoir is not adequately identified and targeted with the currently available field diagnostics [22].
It follows that the cost-effectiveness of existing or novel surveillance methods and interventions in reducing malaria transmission cannot be predicted or evaluated unless the relative contribution to transmission of (1) clinical/symptomatic malaria, (2) asymptomatic parasitaemia (detectable by microscopy or RDT), and (3) low-density parasitaemia (not detectable by microscopy or RDT) are estimated for a particular setting. With an increasingly diverse array of potential approaches for malaria elimination [18], but with limited human and financial resources [23], characterising the contribution of low-density parasitaemia to transmission will help to focus elimination efforts.
Low-density parasitaemia and transmission
There are currently no field diagnostics with sufficient sensitivity to identify low-density submicroscopic parasitaemia, though various approaches are under evaluation for performance and scalability (discussed in the malERA Refresh ‘Tools’ paper) [18]. However, even if all infected individuals could be identified, there is a need to understand who is infectious to mosquitoes and for how long.
Understanding the contribution of low-density parasitaemia to the infectious reservoir for a given malaria typology is critical to determine the diagnostic sensitivity required. It will also affect how much effort a programme should commit to detecting and treating these infections and when and where this effort is best deployed. As noted above, the proportion of low-density parasitaemia increases as transmission declines [9,10,19,20,24]. Recent findings from Senegal also suggest that the efficiency of human-to-mosquito transmission increases with decreasing transmission intensity [25].
Currently, the only way to measure human infectiousness is by feeding colony-reared mosquitoes either on humans directly (direct feeding assay [DFA] [26,27]) or on infected human blood via a membrane (direct membrane feeding assay [DMFA] [28]). A number of studies have used these methods to estimate the contribution of low-density infections to malaria transmission [29–34]. For example, studies in Burkina Faso using DMFA found that 28.7% (25 out of 87) of infectious individuals were microscopy negative, causing 17.0% of mosquito infections [29]. Similarly, in Thailand, DFA studies found that 21% (13 out of 62) individuals submicroscopic for either P. falciparum or P. vivax were able to infect mosquitoes [34]. These preliminary studies suggest that surveillance systems could be modified in the future to detect submicroscopic infections and direct transmission reduction efforts. However, understanding the relationship between infectivity as measured in feeding assays and the infectivity in natural transmission settings to local mosquitoes is still a major research challenge. Furthermore, few empirical studies have quantified the proportion of the overall population that is both submicroscopic and infectious, particularly in low-transmission settings (i.e., less than 8 clinical cases per week per 1,000 persons) [7]. This is needed to determine when and where treating low-density parasitaemia is critical for interrupting transmission and the diagnostic sensitivity required to target them. Mathematical models suggest that conventional diagnostics can detect 55% of the infectious reservoir, but with a 100-fold increase in sensitivity of detection level, i.e., from 200 to 2 parasites/μL of blood, up to 95% of infectious individuals could be identified [35]. This level of diagnostic sensitivity could transform our understanding of the malaria transmission reservoir, allowing the development and delivery of better strategies to disrupt transmission toward malaria elimination.
Detecting gametocytes
All malaria infections have the capacity to produce gametocytes. Therefore, in the context of community chemotherapy programmes, treating any individuals who test positive for asexual parasites is a realistic programme aim. However, research tools that measure gametocytaemia are essential to further our understanding of transmission biology and to define the populations and individuals that drive transmission. Some studies have suggested that transmission efficiency may increase as malaria prevalence falls due to higher gametocyte densities. As the development of new transmission-blocking drugs and vaccines advances, understanding the factors that drive this transmission efficiency will be needed to determine in which settings interventions can be successfully trialled and/or implemented [25]. Although gametocytes can be identified using microscopy, they often exist at low densities and may circulate only transiently in the blood. RDTs do not differentiate between gametocytes and asexual parasites. The limit of detection of microscopy is 8–16 gametocytes/μL of blood [30,31]. Predictably, molecular methods are more sensitive, with 0.3 mature females/μL of blood detected with Pfs25 reverse transcription qPCR (RT-qPCR) and 1.8 mature males/μL of blood with Pfs230p RT-qPCR [36]. As gametocyte densities are low, the increased sensitivity of molecular methods considerably increases gametocyte detection rates. For example, a recent study in Kenya found that Pfs25 RT-qPCR detected gametocytes in 44% of the population compared with only 2.6% detected by microscopy [37].
While there is an overall positive association between mosquito infection rates and gametocyte density, there is also evidence of infectiousness for individuals with very low gametocyte densities [27,38]. As the majority of malaria infections are submicroscopic, even if only a small proportion of these individuals are infectious, the contribution to the transmission reservoir is potentially significant enough to impact elimination programmes.
Where data are available, they suggest differences between high - and low-transmission settings in the gametocyte density needed for human infectivity to mosquitoes. In African populations, submicroscopic P. falciparum gametocytaemia is common, and studies in Kenya have found that the majority of infectious children (43 out of 62) had submicroscopic gametocytaemia [30,31]. In contrast, in Cambodia, falciparum-infected subjects with detectable gametocytes by microscopy were significantly more likely than gametocyte-negative individuals to infect mosquitoes, and those with microscopy-detectable gametocytaemia were the source of the majority of all mosquito infections [39].
Heterogeneity in the transmission reservoir
While data demonstrate an advance in our understanding of malaria transmission, they are limited and suggest the infectious reservoir differs across malaria typologies [24]. Most studies investigating human infectiousness have been conducted in high-transmission settings. There is a particular need for data from low-transmission and near-elimination settings, where temporal, spatial, and demographic heterogeneity in transmission can often be more pronounced. Longitudinal data characterising the transmission reservoir are also needed. These would not only allow more accurate assessments of the contributions of the different density infections but could also inform the sequence of intervention delivery needed to reduce transmission. Similarly, these data would inform the necessary intervention changes to most effectively transition countries from high to low transmission and ultimately elimination [40]. A key consideration is to advise when malaria control measures should be reoriented following elimination without the risk of reintroduction, particularly in the context of declining human immunity to malaria and the potential for outbreaks.
As transmission declines and heterogeneity increases, programmes need to adjust in order to respond to increasingly rare clinical cases. The persistence of residual transmission requires more aggressive and/or novel strategies, and targeting these areas will be key to local elimination. Significant progress has been made in approaches to identify transmission foci using a number of field-based, geo-spatial, and modelling approaches [41–53]. However, even where hotspots of malaria transmission can be identified, attempts to target these foci may fail against a background of low-level but widespread transmission [54]. Local implementation and high-coverage control interventions linked to surveillance information will be needed to adequately clear the reservoir at all levels of transmission.
Surveillance systems at low-transmission settings will also need to be equipped to monitor emerging insecticide and drug resistance [55,56] that may threaten the success of existing interventions [56]. Longitudinal monitoring of resistance markers via sentinel surveillance sites could prove invaluable for tracking risk of rebound or reintroduction. However, there are currently no field-based diagnostic tests for drug resistance, and more detailed information may be needed on local drug-resistance patterns in asymptomatic/low-density infections, particularly related to any changed infectiousness to mosquitoes.
Research agenda for measuring transmission
Improved and validated metrics of transmission would enable the optimal design of control programmes and surveillance systems needed for malaria elimination [23]. This would include the ability to better track progress, confirm cases and foci, and identify and contain reintroduction of transmission, should it occur. Validated transmission metrics are also the key outcome to be measured in field trials evaluating the effectiveness of transmission-blocking interventions [18] and can be used to improve mathematical models assessing potential intervention combinations [7].
Measures of malaria transmission can be defined at different points in the transmission cycle (Fig 2). Since 2011, progress has been made in understanding the advantages and limitations of transmission metrics across epidemiological settings [57,58]. Further work is needed to better quantify the correlations between metrics, standardise their application for use in programmatic surveillance activities, and develop and validate new metrics. However, it is necessary that transmission metrics are reliable and reproducible on a consistent basis and can be assembled through existing national systems.
Fig. 2. Key programmatic and research metrics across the malaria parasite transmission cycle. 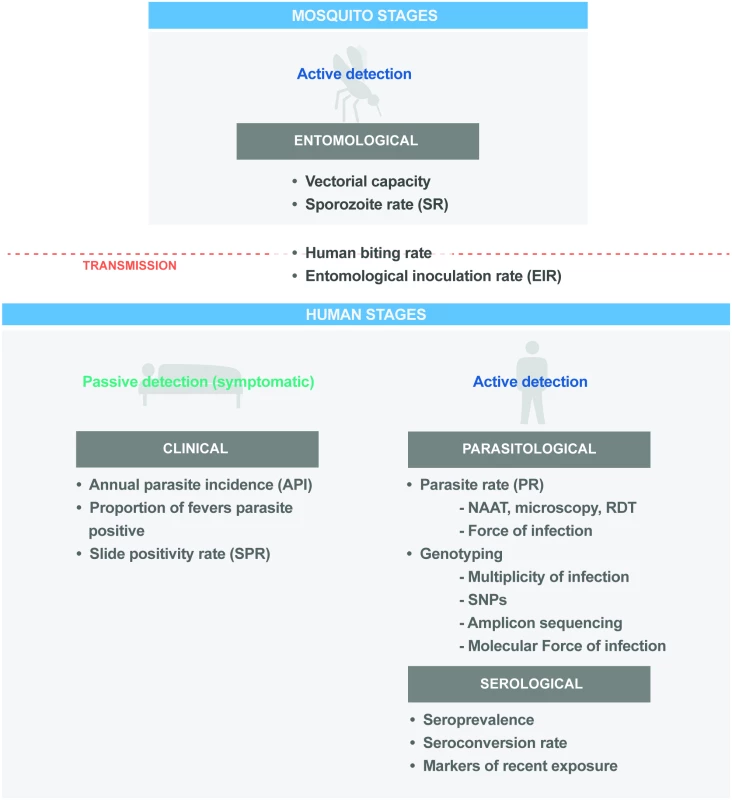
NAAT, nucleic acid amplification test; RDT, rapid diagnostic test. Entomological metrics
Between 30–40 species of Anopheles have been identified as vectors of human malaria, exhibiting varying feeding behaviours and preferences, habitats, and ecologies. Within this complexity, there is a need to standardise current metrics and develop more efficient sampling techniques [57] (Table 1). Whilst developments in sampling methods have been made to evaluate biting densities and infection rates [59–63], human landing collection (HLC) sampling remains the gold standard for providing epidemiologically relevant mosquito-to-human transmission metrics, despite inherent risks [64,65]. Alternative technologies to HLC are being tested that limit human exposure [66,67] and include traps with attractants that mimic a human host [68,69].
New approaches are particularly needed in settings where vector densities are low or heterogeneous. For example, reexamination of vectorial capacity using mathematical modelling to simulate settings with different baseline epidemiological and entomological characteristics has led to new insights into the effective deployment of vector control measures [70]. Technological advances in geolocation and mapping can precisely identify vector habitats that coincide with human activity and movement [71]. This information can be used to determine potential exposure points, enabling targeted sampling in these foci of transmission risk. Other innovative technologies include high-throughput technology, such as infrared spectrometry, to evaluate large samples of mosquitoes for vector age, species, and infection status [72–74], thus providing a measure of vector density and indicating the risk of malaria reintroduction. In this regard, as with parasite drug resistance, longitudinal monitoring of insecticide resistance via sentinel surveillance could prove invaluable.
Human metrics
Current epidemiological metrics of malaria transmission in humans, diagnosed via passive and active systems, microscopy and RDTs, remain key for national malaria control programmes in tracking progress in the reduction of malaria cases and identifying outbreaks and epidemics (Table 2). These data are complemented with large-scale surveys, such as the Demographic and Health Surveys (DHS), the Malaria Indicator Surveys (MIS) and UNICEF Multiple Indicator Cluster Surveys (MICS). However, as transmission declines to low intensity, clinical cases become rare, slide and RDT positivity rates low, and transmission patterns increasingly heterogeneous.
To generate practical estimates of infection without excessive sampling, more sensitive diagnostics and/or combinations of diagnostic approaches are needed. While the utility of RDTs will need to be monitored in regions where deletions in the gene encoding HRP2 have been detected in the parasite population [75,76], research is currently underway to develop RDTs with detection thresholds corresponding to 10–20 parasites/μL or lower [77]. The development of highly sensitive nucleic acid–based tests for parasite detection [78,79], and hemozoin detection using nuclear magnetic resonance [80,81], is also ongoing and may be promising. While tests using molecular methods would increase the number of infections identified, their widespread deployment in low-transmission settings is probably not currently cost-effective for the identification of incident infections. Additionally, in recognition of heterogeneity, approaches should shift from tracking national or regional progress in malaria control towards targeted sampling and community-based surveys characterising transmission risk in key population groups. Once elimination has been achieved, maintaining ‘zero’ transmission will depend on the health system’s ability to identify any emergent malaria cases, triggering case-based investigation to determine the origin (local or imported) and prevent onward transmission.
Metrics to understand transmission
Recent technical advances have produced a number of transmission metrics that are suitable for low-transmission settings (Table 3). Molecular force of infection (mFOI) and multiplicity of infection (MOI) both use parasite genotyping methods to assess the complexity of parasite infections [82]. mFOI can identify superinfected individuals that carry parasites from more than 1 infection, providing a more detailed measure of transmission compared to force of infection based on less sensitive methods (Table 2). Sequencing to determine parasite population structure can also be used to characterise transmission by measuring the genetic relatedness between infections in space and time. Other measures, such as allelic richness, can indicate the level of genetic diversity, which is expected to decline as transmission declines [83,84]. Even more refined sequencing approaches might be capable of assigning parasites as imported or local for monitoring the origin of infections.
Tab. 3. Advances in the development of metrics for measuring malaria transmission. 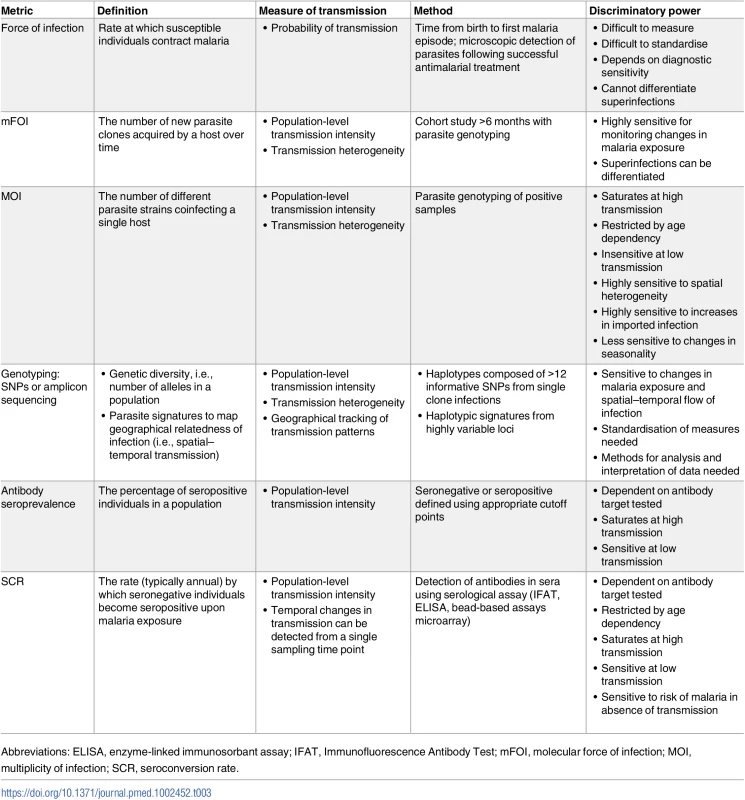
Probability of transmission Antibody seroprevalence and the seroconversion rate (SCR) exploit human antibody responses to characterise previous parasite exposure and are specific to a particular antigen or combination of antigens [85]. Studies using enzyme-linked immunosorbant assays (ELISAs) have shown serological measures correlate well with parasitological and entomological measures in describing transmission levels and spatial and demographic risk [86,87]. Uniquely, serology, when combined with age, allows retrospective examination of exposure history, including the effects of interventions and the absence of recent exposure in elimination settings. High-throughput platforms, such as microarray and bead-based multiplex assays, allow screening of large numbers of potential antigenic targets with specific characteristics [87,88–91]. Targets of interest include stage - or species-specific biomarkers, particularly for P. vivax [88], serological signatures of hypnozoite carriage [92], and vector-specific antigenic targets in mosquito saliva [93,94]. The programmatic applications of serology have yet to be fully tested, though various approaches are being evaluated, including serological markers of incident infections [89,95–109]. Research is currently underway to identify a variety of biomarkers indicative of recent infection that are detectable for different durations following parasite infection, allowing finer-scale estimation of time since infection.
For all these metrics, however, standardisation of methods is necessary, as well as a quantitative comparison to understand the relationship with existing and other new metrics. The development of operationally suitable platforms will ultimately be required to inform real-time or rapid response in programmatic settings. In relation to this, there needs to be a clearer understanding of what measures are needed to better define and monitor transmission, and what measures are useful for control programmes. New approaches to analyse metrics from different sources to improve estimates of transmission, or confirm its interruption, are needed. Looking to the veterinary world could be informative, where probability-based survey methods such as “freedom from infection” are used for animal disease surveillance in the food and agriculture industry [110]. These methods are based on defining the probability that a population is free of infection, allowing operational surveillance thresholds to be set based on the chosen sampling frame and the sensitivity of available diagnostics. Adapting these strategies for use in malaria surveillance will require tailoring the methods for specific malaria transmission measures.
Multimetrics to characterise transmission in time and space
The increasing availability of spatial databases on parasite rate [111,112], serology, vectors [113], malaria genetic epidemiology [114], and human population movements [115–118], together with the increased flexibility and computational efficiency of mathematical and statistical modelling methods [119,120], have led to substantial advances in the spatial–temporal characterisation of malaria transmission intensity. To date, most of these methods have focused on a single metric of endemicity or have relied on parameters derived from small studies. However, dynamic models are being developed that will capture the effect of human population movements, and could incorporate multimetric ensembles to allow self-consistent mapping across the entire spectrum of transmission settings [7]. For these technologies to achieve the greatest impact, they will need to be linked to and used by control programmes to inform operational decision-making in real time.
Summary
Considerable progress has been made not only in understanding the biology and epidemiology of malaria transmission but also in the development of new tools to more accurately quantify transmission; however, challenges remain and Box 3 summarises this Panel’s research and development agenda. The foremost of these is an incomplete understanding of the infectious reservoir in low-transmission and elimination settings, particularly the relative infectiousness of (1) asymptomatic individuals and (2) susceptible vector species across a variety of malaria typologies. The spatial and temporal heterogeneity at which these factors interact will change as countries transition to lower transmission intensity.
Box 3. Research and development agenda
Characterising the reservoir
Objective: Determine the relative contribution to transmission of symptomatic malaria, asymptomatic malaria detectable with microscopy or RDTs, and low-density infections detectable by molecular methods across different malaria typologies; data from low-transmission settings are particularly required.
Research goals
Determine the kinetics of infectiousness of low-density parasitaemia.
Determine the infectiousness of low-density gametocytaemia.
Refine mosquito feeding assays (DMFA or DFA) of human infectivity to mosquitoes and validate these against natural infectivity to local vector species.
Determine the required sensitivity of field-based diagnostics to identify malaria infections contributing to transmission.
Continue to develop field-based molecular and serological diagnostics with sensitivities relevant for evaluation of infectious low-density parasitaemia and gametocytaemia.
Investigate non-invasive diagnostics of malaria infection and infectivity.
Develop hypnozoite diagnostics predictive of P. vivax/P. ovale relapse and subsequent infectivity.
Develop cost-effective programmatic triggers and protocols for the optimal deployment of transmission-based diagnostic tests and their incorporation within surveillance systems.
Evaluate the cost-effectiveness of programmatic actions and interventions directed by transmission-based diagnostics.
Characterise changes in the transmission reservoir as transmission declines.
Conduct longitudinal studies in areas of declining transmission to investigate changes in the nature and distribution of the transmission reservoir.
Evaluate which surveillance activities and metrics are most informative and cost-effective for programmatic goals.
Develop operational methods to rapidly identify antimalarial drug-resistant parasites and insecticide-resistant vectors.
Determine the relevance of spatial–temporal heterogeneity in the transmission reservoir to the acceleration of elimination.
Identify foci of residual transmission.
Identify areas at risk for outbreaks and the reestablishment of malaria transmission following local elimination.
Measuring transmission
Objective: To develop a standardised and validated ‘toolkit’ of metrics and surveillance activities for characterising the infectious reservoir and measuring malaria transmission, which can be applied programmatically to direct interventions, evaluate interventions, and quantify progress towards malaria elimination.
Research goals
Development of entomological as well as human measures and surveillance of transmission.
Continue to develop alternatives to HLC sampling for entomological measures of transmission risk.
Continued quantification of the relationships between different metrics of transmission.
Develop validated metrics for use in low-transmission settings and in the absence of transmission.
Continue to develop methods for evaluating transmission risk in low-transmission settings or in the absence of transmission.
Evaluate multimetric combinations for the efficient integration and analysis of low-intensity and/or heterogeneous transmission.
Evaluate the most cost-effective and informative metrics aligned to programmatic goals as transmission declines.
Develop validated metrics for the evaluation of new tools directed at transmission interruption.
The absolute and relative incidence of clinical and asymptomatic infections can vary widely between different low-transmission settings. Transmission can occur as focal outbreaks caused by human and vector migration. It can also persist for long periods despite aggressive control strategies or quickly rebound after reaching zero. These scenarios are caused by varying patterns of malaria risk across demographic groups, vectors, and parasite species in different ecological settings, which may not be easily captured by simple incidence and prevalence measures.
The application of new and/or refined metrics for routine surveillance activities or research-specific contexts requires investigation. This needs to be done in the context of existing standard measures and the newer data collection platforms to understand the true utility. Metrics will also need to be optimised for the quality of the healthcare system in which they will be implemented. The same applies to the infectious reservoir. Whilst its characterisation across different transmission settings is important, translating this information into actionable programmatic decisions will be key to achieving zero malaria transmission.
Zdroje
1. malERA Consultative Group on Integration Strategies. A research agenda for malaria eradication: cross-cutting issues for eradication. PLoS Med. 2011;8(1):e1000404. doi: 10.1371/journal.pmed.1000404 21283603
2. malERA Consultative Group on Monitoring ES. A research agenda for malaria eradication: monitoring, evaluation, and surveillance. PLoS Med. 2011;8(1):e1000400. doi: 10.1371/journal.pmed.1000400 21311581
3. World Health Organization. WHO malaria terminology Geneva: WHO; 2016. http://apps.who.int/iris/bitstream/10665/208815/1/WHO_HTM_GMP_2016.6_eng.pdf?ua=1.
4. Schapira A, Boutsika K. Malaria ecotypes and stratification. Adv Parasitol. 2012;78 : 97–167. doi: 10.1016/B978-0-12-394303-3.00001-3 22520442
5. Nájera J, Liese B, Hammer J. Malaria: new patterns and perspectives Washington, DC: The World Bank; 1992. http://documents.worldbank.org/curated/en/933151468740676854/pdf/multi-page.pdf.
6. World Health Organization. Malaria rapid diagnostic test performance Geneva: WHO; 2015. http://apps.who.int/iris/bitstream/10665/204118/1/9789241510035_eng.pdf?ua=1.
7. The malERA Refresh Consultative Panel on Combination Interventions and Modelling. malERA: An updated research agenda for combination interventions and modelling in malaria elimination and eradication. PLoS Med. 2017;14(11):e1002453. https://doi.org/10.1371/journal.pmed.1002453
8. Chen I, Clarke SE, Gosling R, Hamainza B, Killeen G, Magill A, et al. "Asymptomatic" malaria: A chronic and debilitating infection that should be treated. PLoS Med. 2016;13(1):e1001942. doi: 10.1371/journal.pmed.1001942 26783752
9. Bousema T, Okell L, Felger I, Drakeley C. Asymptomatic malaria infections: detectability, transmissibility and public health relevance. Nat Rev Microbiol. 2014;12(12):833–40. doi: 10.1038/nrmicro3364 25329408
10. Wu L, van den Hoogen LL, Slater H, Walker PG, Ghani AC, Drakeley CJ, et al. Comparison of diagnostics for the detection of asymptomatic Plasmodium falciparum infections to inform control and elimination strategies. Nature. 2015;528(7580):S86–93. doi: 10.1038/nature16039 26633770
11. Cheng Q, Cunningham J, Gatton ML. Systematic review of sub-microscopic P. vivax infections: prevalence and determining factors. PLoS Negl Trop Dis. 2015;9(1):e3413. doi: 10.1371/journal.pntd.0003413 25569135
12. Moreira CM, Abo-Shehada M, Price RN, Drakeley CJ. A systematic review of sub-microscopic Plasmodium vivax infection. Malar J. 2015;14 : 360. doi: 10.1186/s12936-015-0884-z 26390924
13. Imwong M, Hanchana S, Malleret B, Renia L, Day NP, Dondorp A, et al. High-throughput ultrasensitive molecular techniques for quantifying low-density malaria parasitemias. J Clin Microbiol. 2014;52(9):3303–9. doi: 10.1128/JCM.01057-14 24989601
14. Robinson LJ, Wampfler R, Betuela I, Karl S, White MT, Li Wai Suen CS, et al. Strategies for understanding and reducing the Plasmodium vivax and Plasmodium ovale hypnozoite reservoir in Papua New Guinean children: a randomised placebo-controlled trial and mathematical model. PLoS Med. 2015;12(10):e1001891. doi: 10.1371/journal.pmed.1001891 26505753
15. Douglas NM, Simpson JA, Phyo AP, Siswantoro H, Hasugian AR, Kenangalem E, et al. Gametocyte dynamics and the role of drugs in reducing the transmission potential of Plasmodium vivax. J Infect Dis. 2013;208(5):801–12. doi: 10.1093/infdis/jit261 23766527
16. Anvikar AR, Shah N, Dhariwal AC, Sonal GS, Pradhan MM, Ghosh SK, et al. Epidemiology of Plasmodium vivax malaria in India. Am J Trop Med Hyg. 2016.
17. Llanos-Cuentas A, Lacerda MV, Rueangweerayut R, Krudsood S, Gupta SK, Kochar SK, et al. Tafenoquine plus chloroquine for the treatment and relapse prevention of Plasmodium vivax malaria (DETECTIVE): a multicentre, double-blind, randomised, phase 2b dose-selection study. Lancet. 2014;383(9922):1049–58. doi: 10.1016/S0140-6736(13)62568-4 24360369
18. The malERA Refresh Consultative Panel on Tools for Malaria Elimination. malERA: An updated research agenda for diagnostics, drugs, vaccines, and vector control in malaria elimination and eradication. PLoS Med. 2017;14(11):e1002455. https://doi.org/10.1371/journal.pmed.1002455
19. Lindblade KA, Steinhardt L, Samuels A, Kachur SP, Slutsker L. The silent threat: asymptomatic parasitemia and malaria transmission. Expert Rev Anti Infect Ther. 2013;11(6):623–39. doi: 10.1586/eri.13.45 23750733
20. Greenwood BM. Asymptomatic malaria infections—do they matter? Parasitol Today. 1987;3(7):206–14. 15462957
21. World Health Organization. Global technical strategy for malaria 2016–2030 Geneva: WHO; 2015. http://apps.who.int/iris/bitstream/10665/176712/1/9789241564991_eng.pdf.
22. Tiono AB, Ouedraogo A, Ogutu B, Diarra A, Coulibaly S, Gansane A, et al. A controlled, parallel, cluster-randomized trial of community-wide screening and treatment of asymptomatic carriers of Plasmodium falciparum in Burkina Faso. Malar J. 2013;12 : 79. doi: 10.1186/1475-2875-12-79 23442748
23. The malERA Refresh Consultative Panel on Health Systems and Policy Research. malERA: An updated research agenda for health systems and policy research in malaria elimination and eradication. PLoS Med. 2017;14(11):e1002454. https://doi.org/10.1371/journal.pmed.1002454
24. Lin JT, Saunders DL, Meshnick SR. The role of submicroscopic parasitemia in malaria transmission: what is the evidence? Trends Parasitol. 2014;30(4):183–90. doi: 10.1016/j.pt.2014.02.004 24642035
25. Churcher TS, Trape JF, Cohuet A. Human-to-mosquito transmission efficiency increases as malaria is controlled. Nat Commun. 2015;6 : 6054. doi: 10.1038/ncomms7054 25597498
26. Bousema T, Dinglasan RR, Morlais I, Gouagna LC, van Warmerdam T, Awono-Ambene PH, et al. Mosquito feeding assays to determine the infectiousness of naturally infected Plasmodium falciparum gametocyte carriers. PLoS ONE. 2012;7(8):e42821. doi: 10.1371/journal.pone.0042821 22936993
27. Churcher TS, Bousema T, Walker M, Drakeley C, Schneider P, Ouedraogo AL, et al. Predicting mosquito infection from Plasmodium falciparum gametocyte density and estimating the reservoir of infection. Elife. 2013;2:e00626. doi: 10.7554/eLife.00626 23705071
28. Ouédraogo AL, Guelbéogo WM, Cohuet A, Morlais I, King JG, Gonçalves BP, et al. A protocol for membrane feeding assays to determine the infectiousness of P. falciparum naturally infected individuals to Anopheles gambiae. MWJ. 2013;4(16):1–4.
29. Ouedraogo AL, Goncalves BP, Gneme A, Wenger EA, Guelbeogo MW, Ouedraogo A, et al. Dynamics of the human infectious reservoir for malaria determined by mosquito feeding assays and ultrasensitive malaria diagnosis in Burkina Faso. J Infect Dis. 2016;213(1):90–9. doi: 10.1093/infdis/jiv370 26142435
30. Drakeley C, Sutherland C, Bousema JT, Sauerwein RW, Targett GA. The epidemiology of Plasmodium falciparum gametocytes: weapons of mass dispersion. Trends Parasitol. 2006;22(9):424–30. doi: 10.1016/j.pt.2006.07.001 16846756
31. Schneider P, Bousema JT, Gouagna LC, Otieno S, van de Vegte-Bolmer M, Omar SA, et al. Submicroscopic Plasmodium falciparum gametocyte densities frequently result in mosquito infection. Am J Trop Med Hyg. 2007;76(3):470–4. 17360869
32. Graves PM, Burkot TR, Carter R, Cattani JA, Lagog M, Parker J, et al. Measurement of malarial infectivity of human populations to mosquitoes in the Madang area, Papua, New Guinea. Parasitology. 1988;96 (Pt 2):251–63.
33. Ouedraogo AL, Bousema T, Schneider P, de Vlas SJ, Ilboudo-Sanogo E, Cuzin-Ouattara N, et al. Substantial contribution of submicroscopical Plasmodium falciparum gametocyte carriage to the infectious reservoir in an area of seasonal transmission. PLoS ONE. 2009;4(12):e8410. doi: 10.1371/journal.pone.0008410 20027314
34. Pethleart A, Prajakwong S, Suwonkerd W, Corthong B, Webber R, Curtis C. Infectious reservoir of Plasmodium infection in Mae Hong Son Province, north-west Thailand. Malar J. 2004;3 : 34. doi: 10.1186/1475-2875-3-34 15385050
35. Slater HC, Ross A, Ouedraogo AL, White LJ, Nguon C, Walker PG, et al. Assessing the impact of next-generation rapid diagnostic tests on Plasmodium falciparum malaria elimination strategies. Nature. 2015;528(7580):S94–101. doi: 10.1038/nature16040 26633771
36. Schneider P, Reece SE, van Schaijk BC, Bousema T, Lanke KH, Meaden CS, et al. Quantification of female and male Plasmodium falciparum gametocytes by reverse transcriptase quantitative PCR. Mol Biochem Parasitol. 2015;199(1–2):29–33. doi: 10.1016/j.molbiopara.2015.03.006 25827756
37. Zhou Z, Mitchell RM, Kariuki S, Odero C, Otieno P, Otieno K, et al. Assessment of submicroscopic infections and gametocyte carriage of Plasmodium falciparum during peak malaria transmission season in a community-based cross-sectional survey in western Kenya, 2012. Malar J. 2016;15(1):421. doi: 10.1186/s12936-016-1482-4 27543112
38. Bousema T, Drakeley C. Epidemiology and infectivity of Plasmodium falciparum and Plasmodium vivax gametocytes in relation to malaria control and elimination. Clin Microbiol Rev. 2011;24(2):377–410. doi: 10.1128/CMR.00051-10 21482730
39. Lin JT, Ubalee R, Lon C, Balasubramanian S, Kuntawunginn W, Rahman R, et al. Microscopic Plasmodium falciparum gametocytemia and infectivity to mosquitoes in Cambodia. J Infect Dis. 2016;213(9):1491–4. doi: 10.1093/infdis/jiv599 26667316
40. Trape JF, Tall A, Sokhna C, Ly AB, Diagne N, Ndiath O, et al. The rise and fall of malaria in a West African rural community, Dielmo, Senegal, from 1990 to 2012: a 22 year longitudinal study. Lancet Infect Dis. 2014;14(6):476–88. doi: 10.1016/S1473-3099(14)70712-1 24813159
41. Kangoye DT, Noor A, Midega J, Mwongeli J, Mkabili D, Mogeni P, et al. Malaria hotspots defined by clinical malaria, asymptomatic carriage, PCR and vector numbers in a low transmission area on the Kenyan Coast. Malar J. 2016;15 : 213. doi: 10.1186/s12936-016-1260-3 27075879
42. Mwakalinga VM, Sartorius BK, Mlacha YP, Msellemu DF, Limwagu AJ, Mageni ZD, et al. Spatially aggregated clusters and scattered smaller loci of elevated malaria vector density and human infection prevalence in urban Dar es Salaam, Tanzania. Malar J. 2016;15 : 135. doi: 10.1186/s12936-016-1186-9 26931372
43. Stresman GH, Baidjoe AY, Stevenson J, Grignard L, Odongo W, Owaga C, et al. Focal screening to identify the subpatent parasite reservoir in an area of low and heterogeneous transmission in the Kenya highlands. J Infect Dis. 2015;212(11):1768–77. doi: 10.1093/infdis/jiv302 26019285
44. Bisanzio D, Mutuku F, LaBeaud AD, Mungai PL, Muinde J, Busaidy H, et al. Use of prospective hospital surveillance data to define spatiotemporal heterogeneity of malaria risk in coastal Kenya. Malar J. 2015;14 : 482. doi: 10.1186/s12936-015-1006-7 26625721
45. Ndiath MM, Cisse B, Ndiaye JL, Gomis JF, Bathiery O, Dia AT, et al. Application of geographically-weighted regression analysis to assess risk factors for malaria hotspots in Keur Soce health and demographic surveillance site. Malar J. 2015;14 : 463. doi: 10.1186/s12936-015-0976-9 26581562
46. Zhou G, Afrane YA, Malla S, Githeko AK, Yan G. Active case surveillance, passive case surveillance and asymptomatic malaria parasite screening illustrate different age distribution, spatial clustering and seasonality in western Kenya. Malar J. 2015;14 : 41. doi: 10.1186/s12936-015-0551-4 25627802
47. Espie E, Diene Sarr F, Diop F, Faye J, Richard V, Tall A, et al. Spatio-temporal variations in malaria incidence in children less than 10 years old, health district of Sokone, Senegal, 2010–2013. PLoS ONE. 2015;10(9):e0137737. doi: 10.1371/journal.pone.0137737 26381623
48. Ndiath M, Faye B, Cisse B, Ndiaye JL, Gomis JF, Dia AT, et al. Identifying malaria hotspots in Keur Soce health and demographic surveillance site in context of low transmission. Malar J. 2014;13 : 453. doi: 10.1186/1475-2875-13-453 25418476
49. Bejon P, Williams TN, Nyundo C, Hay SI, Benz D, Gething PW, et al. A micro-epidemiological analysis of febrile malaria in Coastal Kenya showing hotspots within hotspots. Elife. 2014;3:e02130. doi: 10.7554/eLife.02130 24843017
50. Mosha JF, Sturrock HJ, Greenwood B, Sutherland CJ, Gadalla NB, Atwal S, et al. Hot spot or not: a comparison of spatial statistical methods to predict prospective malaria infections. Malar J. 2014;13 : 53. doi: 10.1186/1475-2875-13-53 24517452
51. Davis M, von Cavallar S, Wyres KL, Reumann M, Sepulveda MJ, Rogers P. Spatio-temporal information and knowledge representation of disease incidence and respective intervention strategies. Stud Health Technol Inform. 2014;205 : 1173–7. 25160374
52. Yamana TK, Bomblies A, Laminou IM, Duchemin JB, Eltahir EA. Linking environmental variability to village-scale malaria transmission using a simple immunity model. Parasit Vectors. 2013;6 : 226. doi: 10.1186/1756-3305-6-226 23919581
53. Valle D, Lima JM. Large-scale drivers of malaria and priority areas for prevention and control in the Brazilian Amazon region using a novel multi-pathogen geospatial model. Malar J. 2014;13 : 443. doi: 10.1186/1475-2875-13-443 25412882
54. Bousema T, Stresman G, Baidjoe AY, Bradley J, Knight P, Stone W, et al. The impact of hotspot-targeted interventions on malaria transmission in rachuonyo south district in the estern Kenyan highlands: A cluster-randomized controlled trial. PLoS Med. 2016;13(4):e1001993. doi: 10.1371/journal.pmed.1001993 27071072
55. Maude RJ, Pontavornpinyo W, Saralamba S, Aguas R, Yeung S, Dondorp AM, et al. The last man standing is the most resistant: eliminating artemisinin-resistant malaria in Cambodia. Malar J. 2009;8 : 31. doi: 10.1186/1475-2875-8-31 19228438
56. The malERA Refresh Consultative Panel on Insecticide and Drug Resistance. malERA: An updated research agenda for insecticide and drug resistance in malaria elimination and eradication. PLoS Med. 2017;14(11):e1002450. https://doi.org/10.1371/journal.pmed.1002450
57. Pinder M, Moorthy V, Mendis K, Brown G, on behalf of the WHO MALVAC committee. MALVAC 2010: Measures of efficacy of anti-malarial interventions against malaria transmission, 15–16 November 2010 in Geneva, Switzerland Geneva: WHO; 2010. http://www.who.int/immunization/research/meetings_workshops/MALVAC_2010_meeting_report.pdf.
58. Tusting LS, Bousema T, Smith DL, Drakeley C. Measuring changes in Plasmodium falciparum transmission: precision, accuracy and costs of metrics. Adv Parasitol. 2014;84 : 151–208. doi: 10.1016/B978-0-12-800099-1.00003-X 24480314
59. Briet OJ, Huho BJ, Gimnig JE, Bayoh N, Seyoum A, Sikaala CH, et al. Applications and limitations of Centers for Disease Control and Prevention miniature light traps for measuring biting densities of African malaria vector populations: a pooled-analysis of 13 comparisons with human landing catches. Malar J. 2015;14 : 247. doi: 10.1186/s12936-015-0761-9 26082036
60. Chaki PP, Mlacha Y, Msellemu D, Muhili A, Malishee AD, Mtema ZJ, et al. An affordable, quality-assured community-based system for high-resolution entomological surveillance of vector mosquitoes that reflects human malaria infection risk patterns. Malar J. 2012;11 : 172. doi: 10.1186/1475-2875-11-172 22624853
61. Mathenge EM, Omweri GO, Irungu LW, Ndegwa PN, Walczak E, Smith TA, et al. Comparative field evaluation of the Mbita trap, the Centers for Disease Control light trap, and the human landing catch for sampling of malaria vectors in western Kenya. Am J Trop Med Hyg. 2004;70(1):33–7. 14971695
62. Kilama M, Smith DL, Hutchinson R, Kigozi R, Yeka A, Lavoy G, et al. Estimating the annual entomological inoculation rate for Plasmodium falciparum transmitted by Anopheles gambiae s.l. using three sampling methods in three sites in Uganda. Malar J. 2014;13 : 111. doi: 10.1186/1475-2875-13-111 24656206
63. Wong J, Bayoh N, Olang G, Killeen GF, Hamel MJ, Vulule JM, et al. Standardizing operational vector sampling techniques for measuring malaria transmission intensity: evaluation of six mosquito collection methods in western Kenya. Malar J. 2013;12 : 143. doi: 10.1186/1475-2875-12-143 23631641
64. Sikaala CH, Killeen GF, Chanda J, Chinula D, Miller JM, Russell TL, et al. Evaluation of alternative mosquito sampling methods for malaria vectors in Lowland South-East Zambia. Parasit Vectors. 2013;6 : 91. doi: 10.1186/1756-3305-6-91 23570257
65. Lima JB, Rosa-Freitas MG, Rodovalho CM, Santos F, Lourenco-de-Oliveira R. Is there an efficient trap or collection method for sampling Anopheles darlingi and other malaria vectors that can describe the essential parameters affecting transmission dynamics as effectively as human landing catches? A Review. Mem Inst Oswaldo Cruz. 2014;109(5):685–705. doi: 10.1590/0074-0276140134 25185008
66. Govella NJ, Maliti DF, Mlwale AT, Masallu JP, Mirzai N, Johnson PC, et al. An improved mosquito electrocuting trap that safely reproduces epidemiologically relevant metrics of mosquito human-feeding behaviours as determined by human landing catch. Malar J. 2016;15 : 465. doi: 10.1186/s12936-016-1513-1 27618941
67. Maliti DV, Govella NJ, Killeen GF, Mirzai N, Johnson PC, Kreppel K, et al. Development and evaluation of mosquito-electrocuting traps as alternatives to the human landing catch technique for sampling host-seeking malaria vectors. Malar J. 2015;14 : 502. doi: 10.1186/s12936-015-1025-4 26670881
68. Mweresa CK, Mukabana WR, Omusula P, Otieno B, Van Loon JJ, Takken W. Enhancing attraction of african malaria vectors to a synthetic odor blend. J Chem Ecol. 2016;42(6):508–16. doi: 10.1007/s10886-016-0711-1 27349651
69. Pombi M, Jacobs F, Verhulst NO, Caputo B, della Torre A, Takken W. Field evaluation of a novel synthetic odour blend and of the synergistic role of carbon dioxide for sampling host-seeking Aedes albopictus adults in Rome, Italy. Parasit Vectors. 2014;7 : 580. doi: 10.1186/s13071-014-0580-9 25499569
70. Brady OJ, Godfray HC, Tatem AJ, Gething PW, Cohen JM, McKenzie FE, et al. Vectorial capacity and vector control: reconsidering sensitivity to parameters for malaria elimination. Trans R Soc Trop Med Hyg. 2016;110(2):107–17. doi: 10.1093/trstmh/trv113 26822603
71. Dewald JR, Fuller DO, Muller GC, Beier JC. A novel method for mapping village-scale outdoor resting microhabitats of the primary African malaria vector, Anopheles gambiae. Malar J. 2016;15(1):489. doi: 10.1186/s12936-016-1534-9 27659918
72. Sikulu M, Dowell KM, Hugo LE, Wirtz RA, Michel K, Peiris KH, et al. Evaluating RNAlater(R) as a preservative for using near-infrared spectroscopy to predict Anopheles gambiae age and species. Malar J. 2011;10 : 186. doi: 10.1186/1475-2875-10-186 21740582
73. Mayagaya VS, Michel K, Benedict MQ, Killeen GF, Wirtz RA, Ferguson HM, et al. Non-destructive determination of age and species of Anopheles gambiae s.l. using near-infrared spectroscopy. Am J Trop Med Hyg. 2009;81(4):622–30. doi: 10.4269/ajtmh.2009.09-0192 19815877
74. Ntamatungiro AJ, Mayagaya VS, Rieben S, Moore SJ, Dowell FE, Maia MF. The influence of physiological status on age prediction of Anopheles arabiensis using near infra-red spectroscopy. Parasit Vectors. 2013;6(1):298. doi: 10.1186/1756-3305-6-298 24499515
75. Amoah LE, Abankwa J, Oppong A. Plasmodium falciparum histidine rich protein-2 diversity and the implications for PfHRP 2: based malaria rapid diagnostic tests in Ghana. Malar J. 2016;15 : 101. doi: 10.1186/s12936-016-1159-z 26891848
76. Murillo Solano C, Akinyi Okoth S, Abdallah JF, Pava Z, Dorado E, Incardona S, et al. Deletion of Plasmodium falciparum histidine-rich protein 2 (pfhrp2) and histidine-rich protein 3 (pfhrp3) genes in Colombian parasites. PLoS ONE. 2015;10(7):e0131576. doi: 10.1371/journal.pone.0131576 26151448
77. Tietje K, Hawkins K, Clerk C, Ebels K, McGray S, Crudder C, et al. The essential role of infection-detection technologies for malaria elimination and eradication. Trends Parasitol. 2014;30(5):259–66. doi: 10.1016/j.pt.2014.03.003 24726857
78. Cordray MS, Richards-Kortum RR. Emerging nucleic acid-based tests for point-of-care detection of malaria. Am J Trop Med Hyg. 2012;87(2):223–30. doi: 10.4269/ajtmh.2012.11-0685 22855751
79. Hofmann N, Mwingira F, Shekalaghe S, Robinson LJ, Mueller I, Felger I. Ultra-sensitive detection of Plasmodium falciparum by amplification of multi-copy subtelomeric targets. PLoS Med. 2015;12(3):e1001788. doi: 10.1371/journal.pmed.1001788 25734259
80. Kong TF, Ye W, Peng WK, Hou HW, Marcos, Preiser PR, et al. Enhancing malaria diagnosis through microfluidic cell enrichment and magnetic resonance relaxometry detection. Sci Rep. 2015;5 : 11425. doi: 10.1038/srep11425 26081638
81. Peng WK, Kong TF, Ng CS, Chen L, Huang Y, Bhagat AA, et al. Micromagnetic resonance relaxometry for rapid label-free malaria diagnosis. Nat Med. 2014;20(9):1069–73. doi: 10.1038/nm.3622 25173428
82. Mueller I, Schoepflin S, Smith TA, Benton KL, Bretscher MT, Lin E, et al. Force of infection is key to understanding the epidemiology of Plasmodium falciparum malaria in Papua New Guinean children. Proc Natl Acad Sci U S A. 2012;109(25):10030–5. doi: 10.1073/pnas.1200841109 22665809
83. Nkhoma SC, Nair S, Al-Saai S, Ashley E, McGready R, Phyo AP, et al. Population genetic correlates of declining transmission in a human pathogen. Mol Ecol. 2013;22(2):273–85. doi: 10.1111/mec.12099 23121253
84. Daniels RF, Schaffner SF, Wenger EA, Proctor JL, Chang HH, Wong W, et al. Modeling malaria genomics reveals transmission decline and rebound in Senegal. Proc Natl Acad Sci U S A. 2015;112(22):7067–72. doi: 10.1073/pnas.1505691112 25941365
85. Ondigo BN, Hodges JS, Ireland KF, Magak NG, Lanar DE, Dutta S, et al. Estimation of recent and long-term malaria transmission in a population by antibody testing to multiple Plasmodium falciparum antigens. J Infect Dis. 2014;210(7):1123–32. doi: 10.1093/infdis/jiu225 24737801
86. Drakeley CJ, Corran PH, Coleman PG, Tongren JE, McDonald SL, Carneiro I, et al. Estimating medium - and long-term trends in malaria transmission by using serological markers of malaria exposure. Proc Natl Acad Sci U S A. 2005;102(14):5108–13. doi: 10.1073/pnas.0408725102 15792998
87. Stewart L, Gosling R, Griffin J, Gesase S, Campo J, Hashim R, et al. Rapid assessment of malaria transmission using age-specific sero-conversion rates. PLoS ONE. 2009;4(6):e6083. doi: 10.1371/journal.pone.0006083 19562032
88. Franca CT, Hostetler JB, Sharma S, White MT, Lin E, Kiniboro B, et al. An antibody screen of a Plasmodium vivax antigen library identifies novel merozoite proteins associated with clinical protection. PLoS Negl Trop Dis. 2016;10(5):e0004639. doi: 10.1371/journal.pntd.0004639 27182597
89. Sarr JB, Orlandi-Pradines E, Fortin S, Sow C, Cornelie S, Rogerie F, et al. Assessment of exposure to Plasmodium falciparum transmission in a low endemicity area by using multiplex fluorescent microsphere-based serological assays. Parasit Vectors. 2011;4 : 212. doi: 10.1186/1756-3305-4-212 22059951
90. Helb DA, Tetteh KK, Felgner PL, Skinner J, Hubbard A, Arinaitwe E, et al. Novel serologic biomarkers provide accurate estimates of recent Plasmodium falciparum exposure for individuals and communities. Proc Natl Acad Sci U S A. 2015;112(32):E4438–47. doi: 10.1073/pnas.1501705112 26216993
91. Crompton PD, Kayala MA, Traore B, Kayentao K, Ongoiba A, Weiss GE, et al. A prospective analysis of the Ab response to Plasmodium falciparum before and after a malaria season by protein microarray. Proc Natl Acad Sci U S A. 2010;107(15):6958–63. doi: 10.1073/pnas.1001323107 20351286
92. Goo YK, Seo EJ, Choi YK, Shin HI, Sattabongkot J, Ji SY, et al. First characterization of Plasmodium vivax liver stage antigen (PvLSA) using synthetic peptides. Parasit Vectors. 2014;7 : 64. doi: 10.1186/1756-3305-7-64 24520895
93. Armiyanti Y, Nuryady MM, Arifianto RP, Nurmariana E, Senjarini K, Fitri LE, et al. Detection of immunogenic proteins from Anopheles sundaicus salivary glands in the human serum. Rev Soc Bras Med Trop. 2015;48(4):410–6. doi: 10.1590/0037-8682-0185-2015 26312930
94. Londono-Renteria B, Drame PM, Weitzel T, Rosas R, Gripping C, Cardenas JC, et al. An. gambiae gSG6-P1 evaluation as a proxy for human-vector contact in the Americas: a pilot study. Parasit Vectors. 2015;8 : 533. doi: 10.1186/s13071-015-1160-3 26464073
95. Yao MX, Sun XD, Gao YH, Cheng ZB, Deng WW, Zhang JJ, et al. Multi-epitope chimeric antigen used as a serological marker to estimate Plasmodium falciparum transmission intensity in the border area of China-Myanmar. Infect Dis Poverty. 2016;5(1):98. doi: 10.1186/s40249-016-0194-x 27604628
96. Yman V, White MT, Rono J, Arca B, Osier FH, Troye-Blomberg M, et al. Antibody acquisition models: A new tool for serological surveillance of malaria transmission intensity. Sci Rep. 2016;6 : 19472. doi: 10.1038/srep19472 26846726
97. Baum E, Sattabongkot J, Sirichaisinthop J, Kiattibutr K, Jain A, Taghavian O, et al. Common asymptomatic and submicroscopic malaria infections in Western Thailand revealed in longitudinal molecular and serological studies: a challenge to malaria elimination. Malar J. 2016;15 : 333. doi: 10.1186/s12936-016-1393-4 27333893
98. Baidjoe AY, Stevenson J, Knight P, Stone W, Stresman G, Osoti V, et al. Factors associated with high heterogeneity of malaria at fine spatial scale in the Western Kenyan highlands. Malar J. 2016;15 : 307. doi: 10.1186/s12936-016-1362-y 27259286
99. Yeka A, Nankabirwa J, Mpimbaza A, Kigozi R, Arinaitwe E, Drakeley C, et al. Factors associated with malaria parasitemia, anemia and serological responses in a spectrum of epidemiological settings in Uganda. PLoS ONE. 2015;10(3):e0118901. doi: 10.1371/journal.pone.0118901 25768015
100. Cunha MG, Silva ES, Sepulveda N, Costa SP, Saboia TC, Guerreiro JF, et al. Serologically defined variations in malaria endemicity in Para state, Brazil. PLoS ONE. 2014;9(11):e113357. doi: 10.1371/journal.pone.0113357 25419900
101. Hsiang MS, Hwang J, Kunene S, Drakeley C, Kandula D, Novotny J, et al. Surveillance for malaria elimination in Swaziland: a national cross-sectional study using pooled PCR and serology. PLoS ONE. 2012;7(1):e29550. doi: 10.1371/journal.pone.0029550 22238621
102. Kobayashi T, Chishimba S, Shields T, Hamapumbu H, Mharakurwa S, Thuma PE, et al. Temporal and spatial patterns of serologic responses to Plasmodium falciparum antigens in a region of declining malaria transmission in southern Zambia. Malar J. 2012;11 : 438. doi: 10.1186/1475-2875-11-438 23276228
103. Rosas-Aguirre A, Speybroeck N, Llanos-Cuentas A, Rosanas-Urgell A, Carrasco-Escobar G, Rodriguez H, et al. Hotspots of malaria transmission in the Peruvian Amazon: Rapid assessment through a parasitological and serological survey. PLoS ONE. 2015;10(9):e0137458. doi: 10.1371/journal.pone.0137458 26356311
104. Sepulveda N, Paulino CD, Drakeley C. Sample size and power calculations for detecting changes in malaria transmission using antibody seroconversion rate. Malar J. 2015;14 : 529. doi: 10.1186/s12936-015-1050-3 26715538
105. Stevenson JC, Stresman GH, Baidjoe A, Okoth A, Oriango R, Owaga C, et al. Use of different transmission metrics to describe malaria epidemiology in the highlands of western Kenya. Malar J. 2015;14 : 418. doi: 10.1186/s12936-015-0944-4 26502920
106. van den Hoogen LL, Griffin JT, Cook J, Sepulveda N, Corran P, Conway DJ, et al. Serology describes a profile of declining malaria transmission in Farafenni, The Gambia. Malar J. 2015;14 : 416. doi: 10.1186/s12936-015-0939-1 26492873
107. Zakeri S, van den Hoogen LL, Mehrizi AA, Karimi F, Raeisi A, Drakeley C. Anti-malarial seroprevalence assessment during an elimination programme in Chabahar District, south-eastern Iran. Malar J. 2016;15(1):382. doi: 10.1186/s12936-016-1432-1 27448606
108. Weppelmann TA, von Fricken ME, Lam B, Telisma T, Existe A, Lemoine JF, et al. Sparse serological evidence of Plasmodium vivax transmission in the Ouest and Sud-Est departments of Haiti. Acta Trop. 2016;162 : 27–34. doi: 10.1016/j.actatropica.2016.05.011 27230796
109. Cook J, Speybroeck N, Sochanta T, Somony H, Sokny M, Claes F, et al. Sero-epidemiological evaluation of changes in Plasmodium falciparum and Plasmodium vivax transmission patterns over the rainy season in Cambodia. Malar J. 2012;11 : 86. doi: 10.1186/1475-2875-11-86 22443375
110. Cameron A, Njeumi F, Chibeu D, Martin T. Risk-based disease surveillance: A manual for vetinarians on the deisgn and analysis of surveillance for demonstration of freedom from disease Rome: Food and Agriculture Organization of the United Nations; 2014. http://www.fao.org/3/a-i4205e.pdf.
111. Bhatt S, Weiss DJ, Cameron E, Bisanzio D, Mappin B, Dalrymple U, et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature. 2015;526(7572):207–11. doi: 10.1038/nature15535 26375008
112. Noor AM, Kinyoki DK, Mundia CW, Kabaria CW, Mutua JW, Alegana VA, et al. The changing risk of Plasmodium falciparum malaria infection in Africa: 2000–10: a spatial and temporal analysis of transmission intensity. Lancet. 2014;383(9930):1739–47. doi: 10.1016/S0140-6736(13)62566-0 24559537
113. Tatem AJ. Mapping population and pathogen movements. Int Health. 2014;6(1):5–11. doi: 10.1093/inthealth/ihu006 24480992
114. MalariaGEN. 2016. Cited 05 Sept 2016. https://www.malariagen.net/data.
115. Worldpop. 2016. Cited 05 Sept 2016. http://www.worldpop.org.uk/.
116. Pindolia DK, Garcia AJ, Wesolowski A, Smith DL, Buckee CO, Noor AM, et al. Human movement data for malaria control and elimination strategic planning. Malar J. 2012;11 : 205. doi: 10.1186/1475-2875-11-205 22703541
117. Wesolowski A, Buckee CO, Pindolia DK, Eagle N, Smith DL, Garcia AJ, et al. The use of census migration data to approximate human movement patterns across temporal scales. PLoS ONE. 2013;8(1):e52971. doi: 10.1371/journal.pone.0052971 23326367
118. Marshall JM, Toure M, Ouedraogo AL, Ndhlovu M, Kiware SS, Rezai A, et al. Key traveller groups of relevance to spatial malaria transmission: a survey of movement patterns in four sub-Saharan African countries. Malar J. 2016;15 : 200. doi: 10.1186/s12936-016-1252-3 27068686
119. Blangiardo M, Cameletti M, Baio G, Rue H. Spatial and spatio-temporal models with R-INLA. Spat Spatiotemporal Epidemiol. 2013;4 : 33–49. doi: 10.1016/j.sste.2012.12.001 23481252
120. Ruktanonchai NW, DeLeenheer P, Tatem AJ, Alegana VA, Caughlin TT, Zu Erbach-Schoenberg E, et al. Identifying Malaria Transmission Foci for Elimination Using Human Mobility Data. PLoS Comput Biol. 2016;12(4):e1004846. doi: 10.1371/journal.pcbi.1004846 27043913
Štítky
Interní lékařství
Článek Contemporary disengagement from antiretroviral therapy in Khayelitsha, South Africa: A cohort studyČlánek Bioequivalence of twice-daily oral tacrolimus in transplant recipients: More evidence for consensus?
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2017 Číslo 11- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Jak postupovat při výběru betablokátoru − doporučení z kardiologické praxe
- Červená fermentovaná rýže účinně snižuje hladinu LDL cholesterolu jako vhodná alternativa ke statinové terapii
-
Všechny články tohoto čísla
- Labour trafficking: Challenges and opportunities from an occupational health perspective
- The end of HIV: Still a very long way to go, but progress continues
- Contemporary disengagement from antiretroviral therapy in Khayelitsha, South Africa: A cohort study
- Bioequivalence of twice-daily oral tacrolimus in transplant recipients: More evidence for consensus?
- Treatment guidelines and early loss from care for people living with HIV in Cape Town, South Africa: A retrospective cohort study
- Perinatal mortality associated with induction of labour versus expectant management in nulliparous women aged 35 years or over: An English national cohort study
- Core Outcome Set-STAndards for Development: The COS-STAD recommendations
- Closing the gaps in the HIV care continuum
- Association between the 2012 Health and Social Care Act and specialist visits and hospitalisations in England: A controlled interrupted time series analysis
- HIV pre-exposure prophylaxis and early antiretroviral treatment among female sex workers in South Africa: Results from a prospective observational demonstration project
- Sexual exploitation of unaccompanied migrant and refugee boys in Greece: Approaches to prevention
- Child sex trafficking in the United States: Challenges for the healthcare provider
- The expanding epidemic of HIV-1 in the Russian Federation
- Cardiovascular disease (CVD) and chronic kidney disease (CKD) event rates in HIV-positive persons at high predicted CVD and CKD risk: A prospective analysis of the D:A:D observational study
- Validity of a minimally invasive autopsy for cause of death determination in maternal deaths in Mozambique: An observational study
- malERA: An updated research agenda for malaria elimination and eradication
- malERA: An updated research agenda for health systems and policy research in malaria elimination and eradication
- A combination intervention strategy to improve linkage to and retention in HIV care following diagnosis in Mozambique: A cluster-randomized study
- Bioequivalence between innovator and generic tacrolimus in liver and kidney transplant recipients: A randomized, crossover clinical trial
- malERA: An updated research agenda for basic science and enabling technologies in malaria elimination and eradication
- Human trafficking and exploitation: A global health concern
- Virological response and resistance among HIV-infected children receiving long-term antiretroviral therapy without virological monitoring in Uganda and Zimbabwe: Observational analyses within the randomised ARROW trial
- Postmenopausal hormone therapy and risk of stroke: A pooled analysis of data from population-based cohort studies
- Lansoprazole use and tuberculosis incidence in the United Kingdom Clinical Practice Research Datalink: A population based cohort
- malERA: An updated research agenda for insecticide and drug resistance in malaria elimination and eradication
- Safety, pharmacokinetics, and immunological activities of multiple intravenous or subcutaneous doses of an anti-HIV monoclonal antibody, VRC01, administered to HIV-uninfected adults: Results of a phase 1 randomized trial
- HIV prevalence and behavioral and psychosocial factors among transgender women and cisgender men who have sex with men in 8 African countries: A cross-sectional analysis
- Treatment eligibility and retention in clinical HIV care: A regression discontinuity study in South Africa
- malERA: An updated research agenda for characterising the reservoir and measuring transmission in malaria elimination and eradication
- Effectiveness of a combination strategy for linkage and retention in adult HIV care in Swaziland: The Link4Health cluster randomized trial
- The value of confirmatory testing in early infant HIV diagnosis programmes in South Africa: A cost-effectiveness analysis
- HIV self-testing among female sex workers in Zambia: A cluster randomized controlled trial
- The US President's Malaria Initiative, transmission and mortality: A modelling study
- Comparison of two cash transfer strategies to prevent catastrophic costs for poor tuberculosis-affected households in low- and middle-income countries: An economic modelling study
- Direct provision versus facility collection of HIV self-tests among female sex workers in Uganda: A cluster-randomized controlled health systems trial
- malERA: An updated research agenda for diagnostics, drugs, vaccines, and vector control in malaria elimination and eradication
- malERA: An updated research agenda for combination interventions and modelling in malaria elimination and eradication
- HIV-1 persistence following extremely early initiation of antiretroviral therapy (ART) during acute HIV-1 infection: An observational study
- Respondent-driven sampling for identification of HIV- and HCV-infected people who inject drugs and men who have sex with men in India: A cross-sectional, community-based analysis
- Extensive virologic and immunologic characterization in an HIV-infected individual following allogeneic stem cell transplant and analytic cessation of antiretroviral therapy: A case study
- Measuring success: The challenge of social protection in helping eliminate tuberculosis
- Prospects for passive immunity to prevent HIV infection
- Reaching global HIV/AIDS goals: What got us here, won't get us there
- Evidence-based restructuring of health and social care
- Extreme exploitation in Southeast Asia waters: Challenges in progressing towards universal health coverage for migrant workers
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Postmenopausal hormone therapy and risk of stroke: A pooled analysis of data from population-based cohort studies
- Bioequivalence between innovator and generic tacrolimus in liver and kidney transplant recipients: A randomized, crossover clinical trial
- HIV pre-exposure prophylaxis and early antiretroviral treatment among female sex workers in South Africa: Results from a prospective observational demonstration project
- Bioequivalence of twice-daily oral tacrolimus in transplant recipients: More evidence for consensus?
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



