-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaHIV self-testing among female sex workers in Zambia: A cluster randomized controlled trial
In a cluster-randomized trial done in Zambia, Catherine Oldenburg and colleagues study HIV self-testing for female sex workers.
Published in the journal: . PLoS Med 14(11): e32767. doi:10.1371/journal.pmed.1002442
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1002442Summary
In a cluster-randomized trial done in Zambia, Catherine Oldenburg and colleagues study HIV self-testing for female sex workers.
Introduction
Achieving high HIV testing coverage is essential for realizing the first step of the Joint United Nations Programme on HIV/AIDS (UNAIDS) 90-90-90 target of diagnosing 90% of all people living with HIV by 2020 [1]. In December 2016, the World Health Organization (WHO) released guidelines related to HIV self-testing (HIVST) [2,3], recommending that HIVST be offered in addition to standard HIV testing services to help achieve realization of this target and as an entry point into HIV prevention services for those testing negative. In particular, the guidelines recognize the importance of the development of new approaches such as HIVST for members of key populations that frequently have lower uptake of HIV testing services due to multilevel factors such as healthcare provider stigma [4,5] and lack of legal protection [6].
Oral HIVST has been shown to be acceptable in diverse populations globally, and provision of HIV self-tests has been shown to increase HIV testing compared to standard testing services in some populations [7–10]. Currently, limited data exist regarding HIVST among female sex workers (FSWs). A concurrent randomized controlled trial of HIVST among FSWs in Kampala, Uganda, found that HIVST increased recent and repeat HIV testing compared to referral to standard testing services [11]. A cohort study among FSWs in Kenya found that 71% of participants used an HIV self-test after it was made available to them, but this study did not include a comparison group for standard testing services [12]. WHO recommends frequent retesting for members of key populations, including FSWs [3]. Although there are limited data on the HIV care continuum for FSWs, available estimates suggest that all indicators are far behind the 90-90-90 targets [13–15]. Alternative testing strategies, such as HIVST, may help close the gap between current HIV testing coverage among FSWs and achieving UNAIDS’s first 90% target of diagnosing 90% of all people living with HIV by 2020.
Even though HIVST may reduce some barriers to HIV testing, low access to or uptake of HIVST would limit its ability to improve HIV testing coverage. Here, we test 2 HIVST delivery mechanisms—direct delivery of an HIV self-test and facility-based distribution—compared to standard-of-care HIV testing among transit-town-based FSWs in Zambia. The 2 HIVST arms were designed to evaluate whether HIVST is acceptable (by measuring whether individuals who are directly given the test use it) and whether participants will access HIVST via the existing health system (by measuring whether participants collect and use HIV self-tests from existing facilities) [16]. The second arm is important because it mirrors the likely approach countries will take in providing routine access to HIVST. We hypothesized that the active approach of peer-based HIV self-test delivery would perform better in terms of HIV testing and HIV status knowledge than the more passive facility-based approach. We further hypothesized that both types of HIV self-test kit provision would lead to significantly improved recent HIV testing and better HIV status knowledge compared to standard testing.
Methods
Study design
The Zambian Peer Educators for HIV Self-Testing (ZEST) study was a 3-arm 1 : 1:1 cluster randomized trial evaluating the effect of 2 different health system mechanisms for HIV self-test delivery compared to referral to standard HIV testing. Clusters were defined as groups of FSW participants and a peer educator who recruited them and facilitated interventions. Complete methods for the ZEST study have been previously reported [16]. The full trial protocol is available online (S1 Protocol). Institutional review board approval was obtained from the Harvard T.H. Chan School of Public Health in Boston, MA, and the ERES Converge institutional review board in Lusaka, Zambia. Written informed consent was obtained from all participants.
Peer educators
Participants were recruited in 3 Zambian transit towns (Kapiri Mposhi, Chirundu, and Livingstone) by peer educators. Peer educators were current or former FSWs who had been recruited and trained by study staff prior to study initiation; many had formally worked as peer educators for previous FSW implementation projects in their region. Peer educators were recruited via contacts with current or former FSW organizations in each study town by study staff members. Peer educators were hired based on their willingness to participate for the duration of the study and their reliability. All peer educators were 18 years of age or older and self-reported being current or former sex workers [17]. There was no enumeration list or sampling frame of FSWs in the study community. Thus, peer educators recruited members of their social network via direct contact, and referred interested individuals to study staff for eligibility assessment and enrollment. We purposefully chose this sampling approach because we wanted to carry out our test of the effect of alternative delivery strategies for HIVST among FSWs who could be easily reached through peer networks. This sampling approach allows similar HIVST interventions to be carried out in other settings.
Participants and procedures
Potential participants contacted a research assistant by phone for preliminary eligibility screening and then, if eligible, were formally screened and enrolled in person. Eligible participants were 18 years of age or older at the time of enrollment, had exchanged sex (vaginal, oral, and/or anal) for money or goods at least once in the past month, self-reported an HIV-uninfected status and had not had an HIV test in the previous 3 months or self-reported that their HIV status was unknown, and were permanent residents of their study town of enrollment. The target enrollment was 6 study participants per peer educator.
Randomization
Peer educator–participant groups were randomized as clusters in a 1 : 1:1 fashion to 1 of the 3 study arms: (1) direct delivery of the HIV self-test from the peer educator to the participant (henceforth, delivery), (2) distribution of a coupon from the peer educator to the participant that could be used for collection of an HIV self-test from a fixed distribution point (henceforth, coupon), or (3) referral to standard testing (henceforth, standard of care). Group randomization occurred after all of the participants in a group had completed their baseline study assessment. The randomization list was generated in R (version 3.3.1, R Foundation for Statistical Computing, Vienna, Austria) in random blocks of size 3, 6, and 9 and stratified by study site (Kapiri Mposhi, Chirundu, or Livingstone) to ensure balance in study arms by site. Because of the nature of the intervention, the study was not masked; however, the peer educator’s study arm assignment was concealed until all participants in her group had been enrolled.
Interventions
In all study arms after randomization, participants completed 4 peer educator intervention visits at weeks 0, 2, 6, and 10 that consisted of HIV risk reduction counseling, condom distribution, and information on where to get HIV testing. The first intervention visit was conducted in a group, and all subsequent interventions were one-on-one visits between the peer educator and participant. To emulate real-life peer educator interventions and improve the generalizability of our results, study staff were not present at peer educator visits. Participants reported how many times they met with their peer educator in the past month at each study assessment.
In the delivery arm, peer educators distributed 2 HIV self-test kits (OraQuick ADVANCE Rapid HIV-1/2 Antibody Test, OraSure Technologies, Bethlehem, PA): one at the first (week 0) peer educator intervention visit, and a second one at the fourth (week 10) peer educator intervention visit. The kits included the manufacturer’s pictorial and written instructions in English, Nyanja, Bemba, and Tonga. Peer educators were trained on use of the oral HIV self-test and shared this information with participants. All participants in the HIVST arms were offered a second HIV self-test kit, regardless of HIV status. To preserve participant confidentiality, peer educators did not ask participants their HIV status. The second HIV self-test kit distribution was timed to measure repeat use of HIVST by participants. We distributed the second test kit at an interval approximating the manufacturer’s recommended testing interval for repeat HIVST.
In the coupon arm, peer educators distributed coupons that participants could use to collect an OraQuick HIV self-test at a distribution site, which was an existing health facility (health clinic or pharmacy). HIV self-test kits were distributed free of charge to participants in exchange for the coupon. HIV self-tests were not available for purchase to participants in other study arms. There was no change in the health facilities with regards to hours of operation or staffing. Existing staff were briefly trained on study procedures and the use of the HIV self-test. As with the delivery arm, peer educators distributed 1 coupon at the first (week 0) and fourth (week 10) peer educator intervention visits. The content of the test and instructions provided to participants were identical to those in the delivery arm. As with the delivery arm, there was no HIV status requirement for distribution of the second coupon. To minimize contamination, only participants who had the study coupon received an HIV self-test kit at the facility.
In the standard-of-care arm, peer educators only provided information about existing HIV testing services, including the locations and working hours, where participants could obtain an HIV test. Identical information was provided to participants in the delivery and coupon arms. HIV care and antiretroviral therapy (ART) were available at some, but not all, of the existing HIV testing services. Participants in any arm who tested positive at a facility that did not provide ART were referred to facilities that provided ART.
A 24-hour hotline was made available to participants in all arms. Participants were instructed to call the hotline if they needed help with HIV testing (including using the HIV self-test), experienced any adverse events, and/or needed other assistance.
Assessments
Assessments occurred at baseline prior to randomization and at 1 and 4 months after the first peer educator visit. All assessments were conducted by a research assistant using computer-assisted personal interviewing.
At baseline, participants were asked about sociodemographic characteristics (age, literacy, educational attainment, mobile phone ownership, monthly income, and if they had a primary partner—defined as a stable, non-commercial partner, such as a husband or a boyfriend). Participants were asked about their sex work history, including the age at which they started exchanging sex for money, average number of clients per night, and condom use with these clients. Inconsistent condom use with clients was defined as reporting non-condom use with any client. Participants were asked if they had ever had an HIV test, and if they had, the number of months since their last test. Finally, participants were asked if any sexual partner (including both commercial and non-commercial partners) had physically (hit, slapped, punched, pushed, shoved, or done something else to physically harm) or sexually (physically forced to have sex) hurt them in the previous 12 months.
The prespecified primary outcome was past 1-month HIV testing at the 1-month and 4-month study assessments. This outcome was chosen as it is applicable to all study arms (i.e., there was no HIV self-test use in the standard-of-care arm), and to measure the overall impact of HIV self-test access on all types of HIV testing (e.g., there could be changes in clinic-based testing as a result of having an HIVST coupon). Data on past 1-month HIV testing at the 4-month visit was collected to measure repeat HIV testing. Participants who reported testing within the study period at the 1 - or 4-month visit were considered to have tested at least once over the course of the study. Participants were asked about recent HIV testing history, including when their last HIV test was, where they received the HIV test (facility versus self-test), their HIV status at their last test, and, among those who reported a positive test, if they sought medical care following their positive test and if they initiated ART. In the delivery and coupon arms, participants were asked if they were offered a test/coupon by the peer educator, if they took the test/coupon, if they collected the kit (in the coupon arm), and if they used the HIV self-test. Whereas we assessed HIV testing specifically in the past month for the overall HIV testing outcome, we measured if participants used an HIV self-test independently of when they used it. At 4 months, participants were additionally asked how many kits in total they used during the study period.
To measure HIV status knowledge, research assistants asked participants to self-report their HIV status at the 4-month visit and then take a rapid test to confirm their status. Participants were told that they would receive a small gift (worth approximately US$1) for correctly self-reporting their best guess of their HIV status; all participants received the gift regardless of whether their reported HIV status matched the self-report. Pre-test and post-test counseling by a research assistant was available to all participants who chose to participate in the HIV status knowledge assessment.
To measure actual use of the HIV self-tests, at the end of the study participants were asked to return any unused HIV self-test kits in exchange for approximately US$1. Participants were not told of this offer prior to their 4-month visit to avoid biasing the study. The first buy-back offer was not made until at least 1 month after the last test kit was distributed in the study, to avoid the possibility that rumors of the study buying back kits could lead to participants choosing not to use them.
Adverse events
Adverse events were monitored in all study arms throughout the course of the study by research assistants and peer educators and via the study hotline. At each peer educator intervention visit and during study assessments, participants were screened for physical, sexual, or verbal intimate partner violence, unintentional disclosure of HIV status, and self-harm, and were given an opportunity to report any other events.
Sample size determination
Sample size determination was based on the primary outcome: testing for HIV in the past month at the 1-month visit. Power calculations were performed using methods for cluster randomized trials, with the peer educator–participant group as the randomization unit. Based on previous data from FSWs in Livingstone and Chirundu [18,19], we assumed that 50% of participants would have tested in the previous month in the standard-of-care arm, and assumed 20% loss to follow-up. We estimated that 50 peer educators per arm (150 total) and 6 participants per peer educator (900 total) would yield 89% power to detect a risk ratio (RR) of 1.3 for recent testing, assuming a type I error probability of 0.05 and an intracluster correlation of 0.03. During enrollment, 10 additional peer educators were recruited, yielding a total of 160 peer educators and 965 participants.
Statistical methods
Our prespecified primary outcome was the proportion of participants reporting testing for HIV in the previous 1 month as measured at the 1-month and 4-month assessments. Models of HIV testing at both time points included all participants. All pairwise comparisons for the 3 study arms were prespecified. Our prespecified analysis was a multilevel mixed-effects logistic regression model to account for clustering by peer educator group and study site. To estimate RRs and accommodate how common most outcomes were, we used a mixed-effects generalized linear model with a Poisson distribution, log link, and robust error term [20], with fixed effects for randomization arm and study site and a random effect for peer educator group. Modeling with a binomial distribution and logit link did not change conclusions (S8 Table). Each time point was modeled separately.
Secondary outcomes were analyzed with an identical model. Analyses of seeking medical care for HIV and ART use were restricted to individuals who reported that their most recent HIV test was positive, a post-randomization characteristic. Use of the HIV self-test kit was compared between the 2 HIVST arms (delivery and coupon). This model was identical to that used for the primary outcome, with the exception that the term for study arm contained only 2 levels (delivery or coupon). A similar model was used for being offered the test kit or coupon and taking the test kit or coupon.
Sensitivity analyses
As a sensitivity analysis, we calculated the proportion of participants within each peer educator group reporting each outcome, and compared the proportions across study arms using a linear regression model with a term for study arm and for site. This model avoids the need to model the covariance structure by analyzing at the unit of randomization (the peer educator group). We also compared the effect of HIVST either via delivery or coupon versus standard testing on HIV testing and linkage to care outcomes by pooling participants in the delivery and coupon arms in a non-prespecified secondary analysis.
Data collection for the 1-month study assessments was interrupted after approximately 85% of participants had completed their assessment, and was delayed for approximately 1 month due to logistical issues with study operations. Participants who were interviewed late who tested during the first month of the study therefore would have responded that their most recent test was more than 1 month ago. All participants were included in our primary analysis per our prespecified analysis plan. As a non-prespecified sensitivity analysis, we assessed HIV testing in the previous 3 months as measured at the 1-month visit. Given that participants were not eligible to participate if they had tested in the 3 months prior to enrollment, past 3-month testing captures recent testing during the study for all participants. An additional sensitivity analysis was run for the primary analysis (HIV testing within the past month) with a term for whether the participant was assessed before or after the delay.
Our prespecified primary analysis was a complete-case analysis. Analyses were intention-to-treat, with the exception of linkage to care and ART initiation outcomes, which were conditioned on self-reported HIV status. All tests were 2-sided with no adjustments for multiple comparisons. All analyses were conducted in Stata 14.1 (StataCorp, College Station, TX).
Results
Between September 16 and October 12, 2016, 1,280 potential participants were screened and 965 were eligible and enrolled in the study (Fig 1); 160 peer educator–participant groups were randomized to 1 of 3 study arms (mean 6.0 participants per peer educator group, range 4 to 8). Baseline characteristics were similar between the 3 groups (Table 1). A total of 885 (91.7%) of participants returned for follow-up at 1 month, and 898 (93.1%) returned at 4 months, which comprised the analytic population. There was no difference by study arm in follow-up at 1 month (P = 0.35) or 4 months (P = 0.65).
Fig. 1. CONSORT flow diagram of screened, randomized, and analyzed participants. 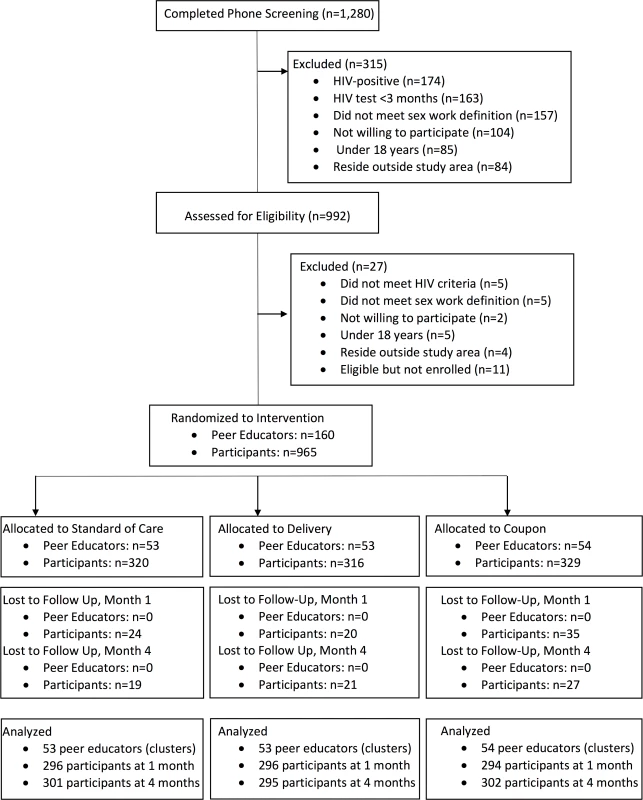
Tab. 1. Baseline descriptive characteristics by randomization arm. 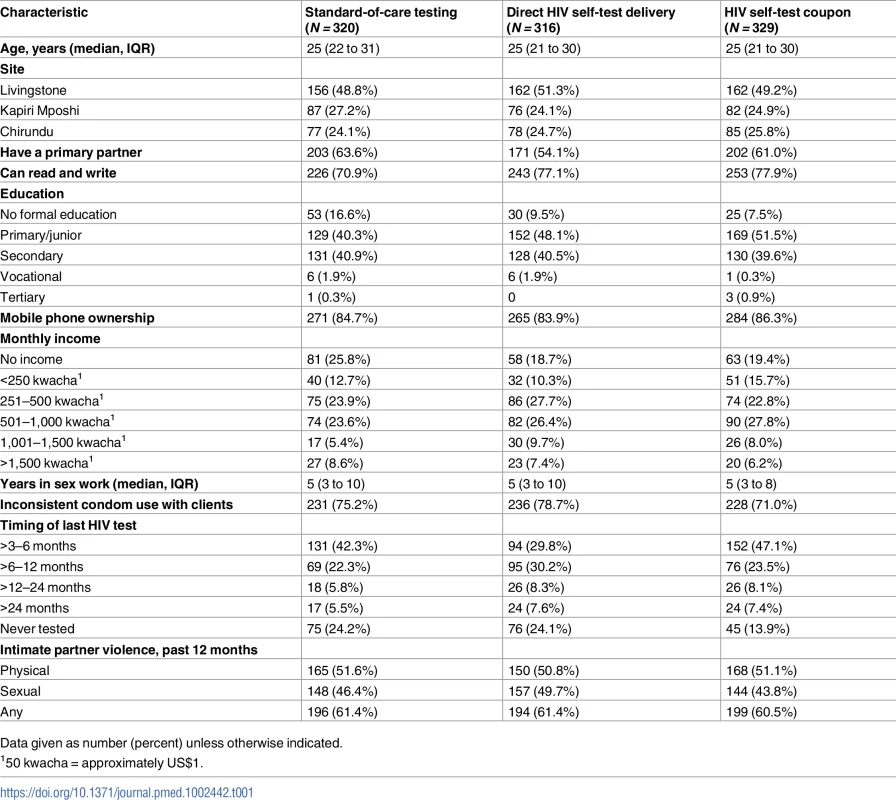
Data given as number (percent) unless otherwise indicated. One-month HIV testing
Overall, 89.3% and 79.6% of participants reported testing for HIV (all types of testing) in the previous 1 month at the 1 - and 4-month assessment, respectively. At 1 month, 94.9% and 84.4% of participants in the delivery and coupon arms reported testing in the past month, compared to 88.5% in the standard-of-care arm (Table 2). The differences between the HIVST arms and the standard of care arm were not statistically significant (RR delivery versus standard of care 1.07, 95% CI 0.99–1.15, P = 0.10; RR coupon versus standard of care 0.95, 95% CI 0.86–1.05, P = 0.29). Participants in the delivery arm were statistically significantly more likely to report testing in the past 1 month than participants in the coupon arm (RR 1.13, 95% CI 1.04–1.22, P = 0.005; S3 Table). The observed intracluster correlation coefficient for the 1-month primary outcome was 0.58 (95% CI 0.42–0.72). The results for past 1-month HIV testing at the 1-month visit were robust to a sensitivity analysis including a term for whether the participant was assessed before or after the delay in outcome assessment (RR delivery versus standard of care 1.05, 95% CI 0.98–1.13, P = 0.19; RR coupon versus standard of care 0.97, 95% CI 0.88–1.06, P = 0.44; RR delivery versus coupon 1.09, 95% CI 1.00–1.18, P = 0.045).
Tab. 2. HIV testing and linkage to care at 1 and 4 months by study arm. 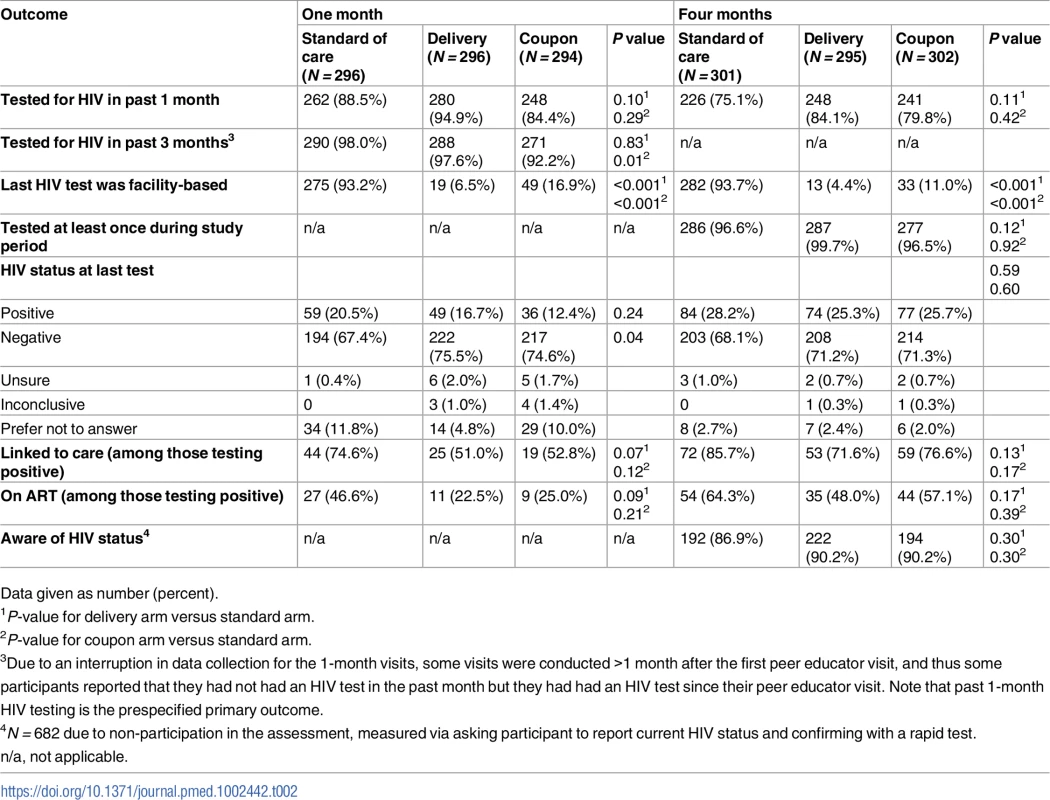
Data given as number (percent). Four-month HIV testing
At 4 months, 84.1% of participants in the delivery arm and 79.8% in the coupon arm reported past 1-month testing (all types of tests), compared to 75.1% in the standard-of-care arm (Table 2). There was no significant difference in past-month HIV testing between the delivery (RR 1.11, 95% CI 0.98–1.27, P = 0.11) or coupon (RR 1.06, 95% CI 0.92–1.22, P = 0.42) arm compared to the standard-of-care arm. There was also no difference in past-month HIV testing at 4 months between the delivery and coupon arms (RR 1.05, 95% CI 0.94–1.18, P = 0.40; S3 Table). In all, 80.4% of women who reported at the 1-month visit that their most recent HIV test was positive reported testing for HIV at 4 months compared to 84.9% of women who reported at 1 month that their most recent HIV test was negative.
Participants at both 1 and 4 months in the HIVST arms were significantly less likely to test for HIV in a facility than participants in the standard-of-care arm (Table 2). There was no difference across study arms in testing at least once during the study period (Table 2).
Linkage to care
At 1 month, 144 (16.3%) participants reported that their most recent HIV test was positive, which increased to 235 (26.2%) at 4 months. At 1 month, among women reporting that their most recent HIV test was positive, 61.1% reported that they had sought medical care. Nearly three-quarters (74.6%) of participants in the standard-of-care arm reported they had sought care following their positive test, compared to 51.0% in the delivery arm and 52.8% in the coupon arm (Table 2). These differences were not statistically significant, although in a non-prespecified analysis pooling the HIVST arms, participants testing positive in the self-testing arms were 27% less likely to seek care for their HIV than participants in the standard-of-care arm (RR 0.73, 95% CI 0.56–0.96, P = 0.03). At 1 month, 46.6% of participants who had tested positive in the standard-of-care arm reported initiating ART, compared to 22.5% and 25.0% in the delivery and coupon arms, respectively. These differences were not statistically significant, although there was a non-significant 42% lower proportion of ART initiation in the HIVST arms compared to the standard-of-care arm (RR 0.58, 95% CI 0.33–1.00, P = 0.05).
Linkage to care and ART initiation increased in all arms by 4 months (Fig 2). Of those who had tested positive by 4 months, 85.7% in the standard-of-care arm, 71.6% in the delivery arm, and 76.6% in the coupon arm reported linking to care. Approximately half of participants in the delivery and coupon arms (48.0% and 57.1%, respectively) reported initiating ART by 4 months, compared to 64.3% in the standard-of-care arm. There were no significant differences in linkage to care or ART initiation at 4 months by study arm.
Fig. 2. HIV care cascade among women who self-reported a positive HIV status at 1 and 4 months after randomization. 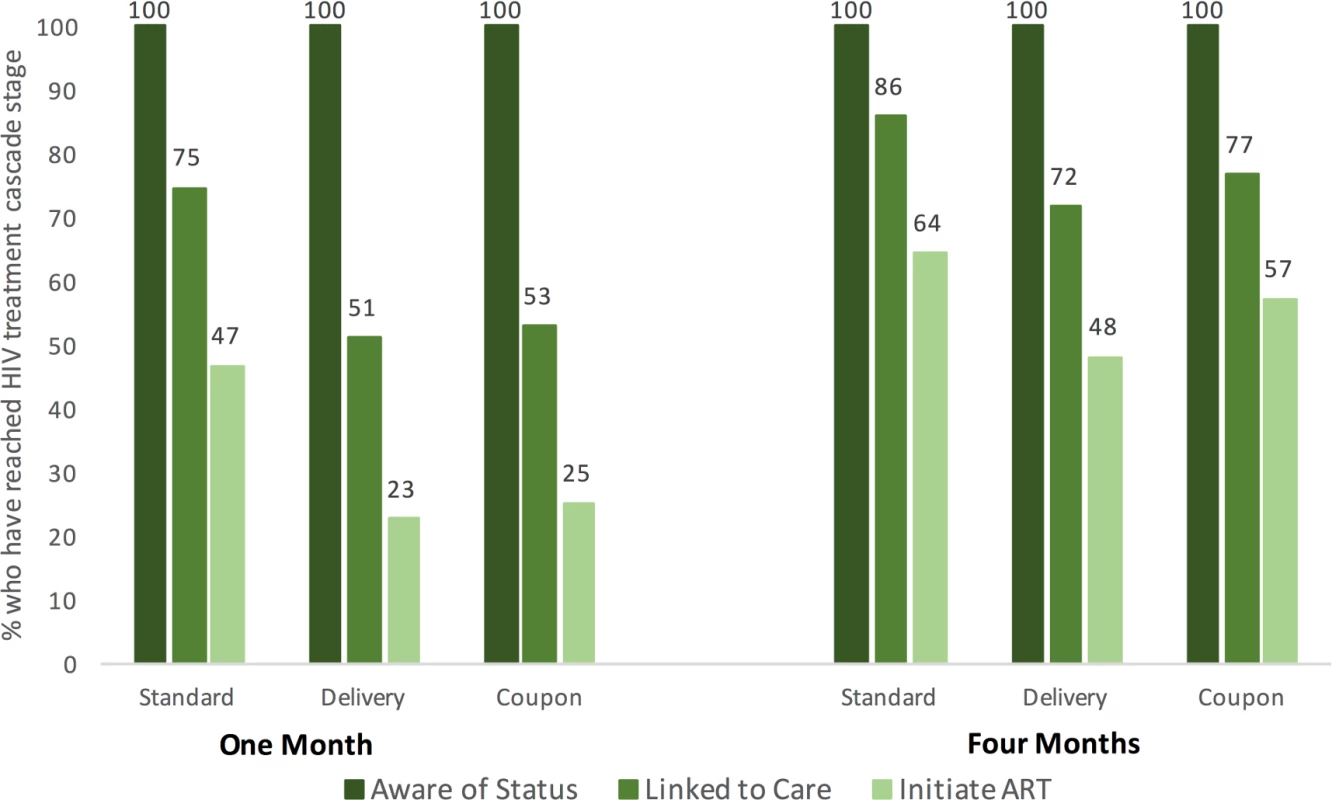
Note that by definition all women are aware of their status, as this figure is restricted to women who self-reported having tested positive for HIV. HIV status knowledge
A total of 682 women (75.9% of those returning for 4-month follow-up) participated in the HIV status knowledge assessment. Of these women, 223 (32.7%) tested positive with a rapid test. Of the women testing positive, 156 (70.0%) self-reported that they were positive. Of 459 women testing negative, 452 (98.5%) reported a negative status. Overall, 89.2% of individuals correctly identified their status. There was no difference in HIV status knowledge between the 3 arms (Table 2).
HIV self-test use
In the HIVST arms, 92.3% and 89.5% of participants reported using the HIV self-test at 1 and 4 months, respectively. At 1 month, participants in the delivery arm were more likely to report using the HIV self-test compared to participants in the coupon arm (RR delivery versus coupon 1.14, 95% CI 1.05–1.23, P = 0.001). There was no significant difference in HIV self-test use at 4 months (RR delivery versus coupon 1.01, 95% CI 0.93–1.09, P = 0.88; Table 3). At 4 months, 84.3% of participants in the delivery arm reported using 2 self-test kits, and 83.2% in the coupon arm reported using 2 kits (RR delivery versus coupon 1.02, 95% CI 0.92–1.12, P = 0.75). Overall, at least 1 HIV self-test was collected from 14.2% of participants, 17.7% in the delivery arm and 12.2% in the coupon arm (RR delivery versus coupon 1.58, 95% CI 0.56–4.45, P = 0.38). One self-test was collected from 47.7% of participants who reported using 1 test kit over the course of the study. No test kits were collected from participants who reported using no test kits over the course of the study, and at least 1 test kit was collected from 9.2% of participants who reported using 2 test kits over the course of the study.
Tab. 3. HIV self-test kit distribution and use at 1 and 4 months by study arm. 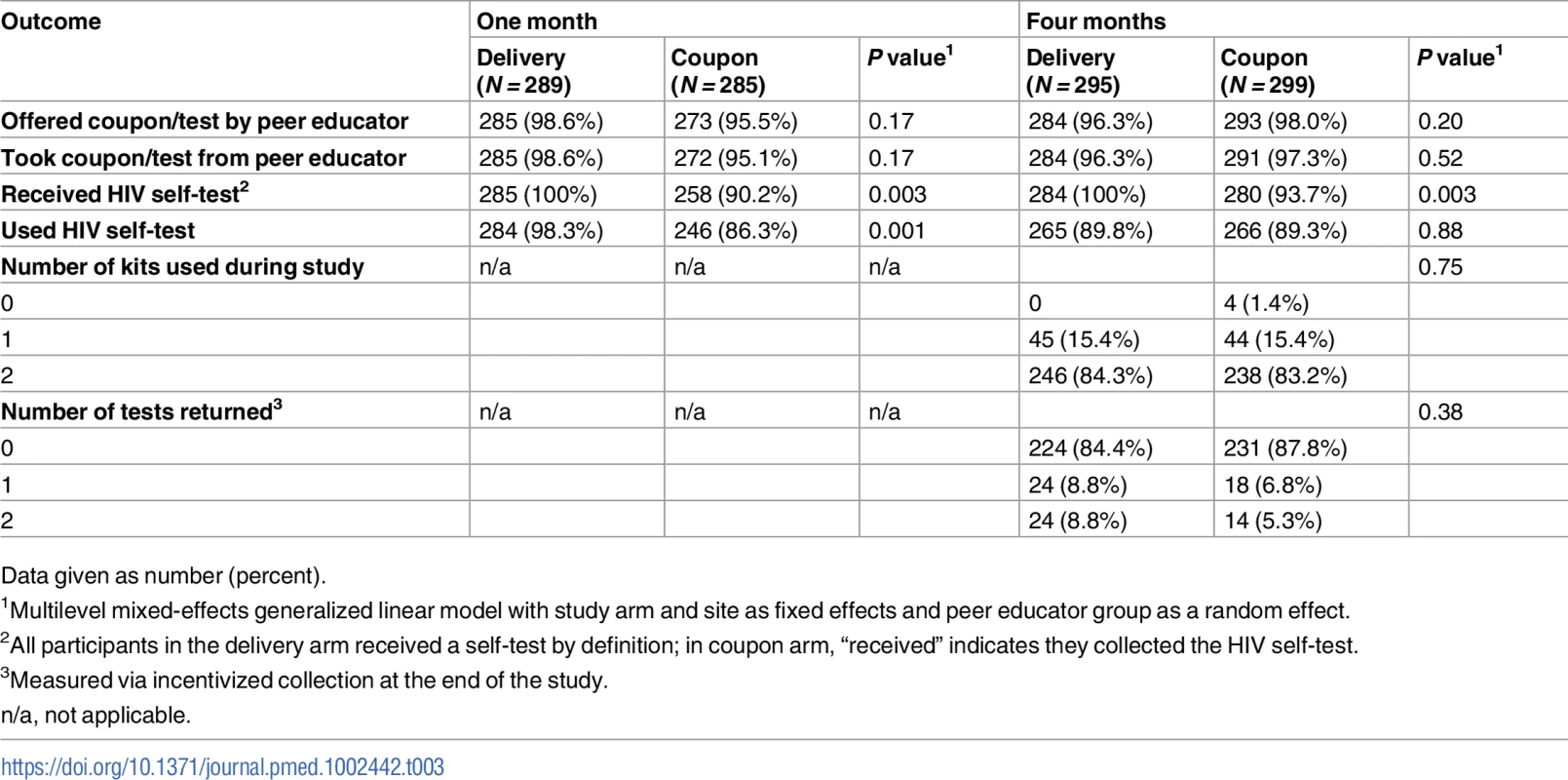
Data given as number (percent). Adverse events
Four instances of intimate partner violence related to study participation were reported, 2 in the delivery arm and 2 in the coupon arm. Three participants reported physical violence following their primary partner learning of their HIV self-test use, and 1 reported physical and sexual violence following her primary partner learning about her engagement in sex work. One death was reported in the delivery arm, which was not related to study participation. No other adverse events were reported.
Discussion
In this study of FSWs in Zambia, a majority of participants at 1 - and 4-month assessments reported use of HIV self-tests that were provided either directly via a peer educator or via health clinics or pharmacies. At the 1-month visit, more participants who directly received an HIV self-test reported using the self-test compared to those who had to collect the test from a health facility, and more participants in the delivery arm reported any HIV testing in the previous month compared to the coupon arm. However, there was no difference between the HIVST arms in either measure by the 4-month time point. In the short term, direct delivery of the HIV self-test may be more effective because it removes some barriers to using the self-test—such as concerns related to confidentiality or logistical barriers—that are mitigated over time. In many health systems, the most realistic distribution mechanism for HIV self-tests will be via existing health clinics and pharmacies. Furthermore, in the delivery arm, the test is immediately available, whereas in the coupon arm, there is necessarily a delay in collecting and using the test since the coupon requires participants to take time to visit a health facility and collect the HIV self-test. The results of this study indicate that facility provision is acceptable to FSWs and can lead to uptake of HIVST just as high as through direct delivery within a few months.
There was no difference in recent HIV testing coverage among women with access to HIV self-tests compared to women who were referred to existing standard HIV testing facilities, and past 1-month HIV testing coverage exceeded 75% in all study arms. Participants in all arms had access to a peer educator, with whom they met a minimum of 4 times over the course of the study. Previous peer-educator-based interventions in diverse settings have generally led to increased engagement in HIV prevention and care [21–23]. Data from pre-study focus groups with peer educators indicated that stigma at multiple levels is a key barrier to HIV testing among FSWs in Zambian transit towns [17]. The provision of peer educator support in all study arms may have mitigated some concerns related to stigma and may have facilitated HIV testing [21,23–27]. Thus, it is possible that our control arm represented an “augmented” standard of care, leading to HIV testing being higher than we would have seen if we had not worked with peer educators. Nevertheless, peer-educator-based interventions are common among organizations working with FSW populations globally [28,29]. This study indicates that HIV testing interventions (including self-testing) that are delivered via peer educators may have a large effect on HIV testing, supporting their use. Furthermore, the use of an “augmented” standard of care in this study means that the effects shown are likely to be conservative, and may represent an underestimate of the effect of self-test provision.
ART initiation was lower in the HIVST arms compared to the standard-of-care arm, although it approximately doubled between the 1 - and 4-month visits, from 25% to 50%. Both linkage to care and ART initiation increased rapidly in the HIVST arms, approaching the standard-of-care arm by 4 months, supporting the hypothesis that linkage to care and ART initiation take longer with HIVST, and mitigating some concern that individuals who self-test will not link to care [30]. By 4 months, ART initiation in the standard testing arm approached previously described estimates of ART coverage among FSWs in Zimbabwe [14] and exceeded a previous global estimate of 36% among FSWs in low - and middle-income countries [31]. In the general population in Zambia, 65% of people living with HIV are on ART [1]. In this study, where participants had access to a peer educator, linkage to care and ART initiation following a positive HIV self-test was high and increased over time. Future studies should consider linkage to care and ART interventions following HIVST, including the role of peer educators for facilitating HIV care cascade progression.
There were 3 cases of intimate partner violence related to HIVST in this study. We monitored intimate partner violence throughout the study as a potential adverse event related to HIVST. Intimate partner violence is a concern in HIVST interventions where participants test with or distribute kits to partners [12,32]. In this study, participants were only given HIV self-test kits for their own use. However, intimate partner violence is common among FSWs, and it is possible that personal use of HIVST could lead to intimate partner violence if, for example, a partner found the HIV self-test kit. We noted a lower rate of intimate partner violence related to HIVST in this study compared to a previous study of HIV self-test provision for partner testing among FSWs in Kenya (0.3% in the current study compared to 2% in the Kenya study) [12]. This lower rate may be at least in part due to the fact that we did not include partner testing in this study. These results indicate that HIVST among FSWs is safe when provided for their own use, although the potential for intimate partner violence following HIVST should be considered, and resources for women experiencing violence made available.
The results of this study must be considered in the context of several limitations. The majority of outcomes in this study relied on self-report, including HIV testing, linkage to care, and ART initiation outcomes. It is possible that participants were affected by social desirability bias, which likely would have led to overestimation of HIV testing and underestimation of positive HIV test results, which could affect answers to questions related to progression in the HIV care cascade. Although we attempted to improve measurement of HIV self-test kit use by buying kits back at the end of the study, this occurred only 1 month after the last kit was distributed, and it is possible that not all kits that would have been used eventually had been used by then. Participants in all 3 arms were recruited in the same study communities, and thus there could have been contamination between arms. We attempted to minimize this by distributing only a single HIV self-test kit at a time and by requiring a study coupon in exchange for the HIV self-test kit in the coupon arm. Participants who lost their coupon would not be able to collect the test kit; however, report of collection and use at both time points was high. Analyses of linkage to care and ART initiation were restricted to individuals who reported a positive HIV test, and thus do not represent a randomized comparison. In addition, with only 4 months of follow-up, the time frame for linkage to care and ART initiation was short. It is possible that over a longer time period the percentage of participants seeking care and initiating ART in the HIVST arms would approach that of the standard-of-care arm. However, to our knowledge this study represents one of the largest samples of individuals reporting positive HIV status following HIVST reported in the literature, and thus provides important data on HIV care cascade progression following self-testing. The power calculation for the study was based on an estimate that 50% of participants in the standard-of-care arm would test for HIV. Actual HIV testing coverage was much higher, and as such the trial may have been underpowered. However, loss to follow-up was lower than the anticipated 20%. During the 1-month study assessment, there was an unavoidable delay in data collection that may have resulted in an underestimate of past-month HIV testing. This study was conducted among Zambian FSWs in transit towns with relatively little involvement in HIV research. The results of this study may not be generalizable to populations of FSWs that are more engaged in research or in higher socioeconomic brackets. This sample was drawn from the social networks of peer educators, which could further affect generalizability. However, the age, educational attainment, and HIV testing history of our sample were very similar to those of a previous survey of FSWs in the same towns conducted via time-location sampling [18]. These results are therefore likely generalizable to FSWs working in similar contexts.
We demonstrate high uptake of HIVST in a sample of FSWs living in Zambian transit towns. Although women appeared to adopt the tests more quickly when they were directly distributed, by 4 months women who had access to HIV self-tests via a coupon that could be used to collect them from a health clinic or pharmacy used the test kits at a rate similar to that of women who received them directly. These findings suggest that distribution of HIV self-tests via existing health infrastructure, such as pharmacies and clinics, will be acceptable in populations to which this study is generalizable. Linkage to care and ART initiation were relatively high in the HIVST arms, although additional research and monitoring is needed to ensure that the HIV care cascade targets are met following HIVST. Or findings indicate that HIVST is acceptable, accessible, and safe for FSWs in Zambia, and should be considered as part of a national HIV testing strategy.
Supporting Information
Zdroje
1. Joint United Nations Programme on HIV/AIDS. 90-90-90: an ambitious treatment target to help end the AIDS epidemic. Geneva: Joint United Nations Programme on HIV/AIDS; 2014 Oct [cited 2017 Oct 17]. Available from: http://www.unaids.org/en/resources/documents/2017/90-90-90.
2. World Health Organization. Guidelines on HIV self-testing and partner notification: supplement to consolidated guidelines on HIV testing services. Geneva: World Health Organization; 2016 Dec [cited 2017 Oct 17]. Available from: http://www.who.int/hiv/pub/vct/hiv-self-testing-guidelines/en/.
3. World Health Organization. Consolidated guidelines on HIV testing. Geneva: World Health Organization; 2015 Jul [cited 2017 Oct 17]. Available from: http://who.int/hiv/pub/guidelines/hiv-testing-services/en/.
4. Bodkin K, Delahunty-Pike A, O’Shea T. Reducing stigma in healthcare and law enforcement: a novel approach to service provision for street level sex workers. Int J Equity Health. 2015;14 : 322–7. doi: 10.1186/s12939-015-0156-0 25879639
5. King EJ, Maman S, Bowling JM, Moracco KE, Dudina V. The influence of stigma and discrimination on female sex workers’ access to HIV services in St. Petersburg, Russia. AIDS Behav. 2013;17 : 2597–603. doi: 10.1007/s10461-013-0447-7 23525789
6. Oldenburg CE, Reisner SL, Mayer KH, Mimiaga MJ, Barnighausen T, Perez-Brumer AG, et al. Human rights protections and HIV prevalence among MSM who sell sex: cross-country comparisons from a systematic review and meta - analysis. Glob Public Health. 2016 Mar 15. doi: 10.1080/17441692.2016.1149598 26979302
7. Stevens DR, Vrana CJ, Dlin RE, Korte JE. A global review of HIV self-testing: themes and implications. AIDS Behav. 2017 Feb 2. doi: 10.1007/s10461-017-1707-8 28155039
8. Jamil MS, Prestage G, Fairley CK, Grulich AE, Smith KS, Chen M, et al. Effect of availability of HIV self-testing on HIV testing frequency in gay and bisexual men at high risk of infection (FORTH): a waiting-list randomised controlled trial. Lancet HIV. 2017;4:e241–50. doi: 10.1016/S2352-3018(17)30023-1 28219619
9. Masters SH, Agot K, Obonyo B, Napierala-Mavedzenge S, Maman S, Thirumurthy H. Promoting Partner testing and couples testing through secondary distribution of HIV self-tests: a randomized clinical trial. PLoS Med. 2016;13:e1002166. doi: 10.1371/journal.pmed.1002166 27824882
10. Johnson CC, Kennedy C, Fonner V, Siegfried N, Figueroa C, Dalal S, et al. Examining the effects of HIV self-testing compared to standard HIV testing services: a systematic review and meta-analysis. J Int AIDS Soc. 2017;20 : 21594. doi: 10.7448/IAS.20.1.21594 28530049
11. Ortblad K, Kibuuka Musoke D, Ngabirano T, Nakitende A, Magoola J, Kayiira P, et al. Direct provision versus facility collection of HIV self-tests among female sex workers in Uganda: a cluster-randomized controlled health systems trial. PLoS Med. In press. doi: 10.1371/journal.pmed.1002458
12. Thirumurthy H, Masters SH, Mavedzenge SN, Maman S, Omanga E, Agot K. Promoting male partner HIV testing and safer sexual decision making through secondary distribution of self-tests by HIV-negative female sex workers and women receiving antenatal and post-partum care in Kenya: a cohort study. Lancet HIV. 2016;3:e266–74. doi: 10.1016/S2352-3018(16)00041-2 27240789
13. Gupta S, Granich R. National HIV care continua for key populations. J Int Assoc Provid AIDS Care. 2017;16 : 125–32. doi: 10.1177/2325957416686195 28090799
14. Cowan FM, Davey C, Fearon E, Mushati P, Mushati, Dirawo J, et al. The HIV care cascade among female sex workers in Zimbabwe: results of a population-based survey from the Sisters Antiretroviral Therapy Programme for Prevention of HIV, an Integrated Response (SAPPH-IRe) Trial. J Acquir Immune Defic Syndr. 2017;74 : 375–82. doi: 10.1097/QAI.0000000000001255 27930599
15. Schwartz S, Lambert A, Phaswana-Mafuya N, Kose Z, Mcingana M, Holland C, et al. Engagement in the HIV care cascade and barriers to antiretroviral therapy uptake among female sex workers in Port Elizabeth, South Africa: findings from a respondent-driven sampling study. Sex Transm Infect. 2017;93 : 290–6. doi: 10.1136/sextrans-2016-052773 27888205
16. Oldenburg CE, Ortblad KF, Chanda M, Mwanda K, Nicodemus W, Sikaundi R, et al. Zambian Peer Educators for HIV Self-Testing (ZEST) study: rationale and design of a cluster randomized trial of HIV self-testing among female sex workers in Zambia. BMJ Open. 2017;20:e014780.
17. Chanda MM, Perez-Brumer AG, Ortblad KF, Mwale M, Chongo S, Kamungoma N, et al. Barriers and facilitators to HIV testing among Zambian female sex workers in three transit hubs. AIDS Patient Care STDs. 2017;31 : 290–6. doi: 10.1089/apc.2017.0016 28581820
18. Family Health International. Round 3: behavioural surveillance survey—Zambia, 2006. Female sex workers in border and transportation routes: with trend analysis 2000–2006. Durham (North Carolina): Family Health International; 2006Jun [cited 2017 Oct 17]. Available from: https://www.fhi360.org/sites/default/files/media/documents/Round%203%20BSS%20Female%20Sex%20Workers%20in%20Border%20and%20Transportation%20Routes_0.pdf.
19. Wilson D. Corridors of hope southern Africa: HIV prevention needs and opportunities in four border towns. Durham (North Carolina): Family Health International; 2005.
20. Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159 : 702–6. doi: 10.1093/aje/kwh090 15033648
21. Krishnamurthy P, Hui SK, Shivkumar N, Gowda C, Pushpalatha R. Assessing the impact of peer educator outreach on the likelihood and acceleration of clinic utilization among sex workers. PLoS ONE. 2016;11:e0159656. doi: 10.1371/journal.pone.0159656 27467124
22. Oldenburg CE, Biello KB, Colby D, Closson EF, Nguyen T, Trang NNN, et al. Engagement with peer health educators is associated with willingness to use pre-exposure prophylaxis among male sex workers in Ho Chi Minh City, Vietnam. AIDS Patient Care STDs. 2014;28 : 109–12. doi: 10.1089/apc.2013.0372 24601733
23. Sarafian I. Process assessment of a peer education programme for HIV prevention among sex workers in Dhaka, Bangladesh: a social support framework. Soc Sci Med. 2012;75 : 668–75. doi: 10.1016/j.socscimed.2011.09.003 22056497
24. Onyango MA, Adu-Sarkodie Y, Agyarko-Poku T, Asafo MK, Sylvester J, Wondergem P, et al. “It’s all about making a life”: poverty, HIV, violence, and other vulnerabilities faced by young female sex workers in Kumasi, Ghana. J Acquir Immune Defic Syndr. 2016;68:S131–7.
25. Hoffman IF, Latkin CA, Kukhareva PV, Malov SV, Batluk JV, Shaboltas AV, et al. A peer-educator network HIV prevention intervention among injection drug users: results of a randomized controlled trial in St. Petersburg, Russia. AIDS Behav. 2013;17 : 2510–20. doi: 10.1007/s10461-013-0563-4 23881187
26. Geibel S, King’ola N, Temmerman M, Luchters S. The impact of peer outreach on HIV knowledge and prevention behaviours of male sex workers in Mombasa, Kenya. Sex Transm Infect. 2012;88 : 357–62. doi: 10.1136/sextrans-2011-050224 22332149
27. Morisky DE, Malow RM, Tiglao TV, Lyu S - Y, Vissman AT, Rhodes SD. Reducing sexual risk among Filipina female bar workers: effects of a CBPR-developed structural and network intervention. AIDS Educ Prev. 2010;22 : 371–85. doi: 10.1521/aeap.2010.22.4.371 20707696
28. Medley A, Kennedy C, O’Reilly K, Sweat M. Effectiveness of peer education interventions for HIV prevention in developing countries: a systematic review and meta-analysis. AIDS Educ Prev. 2009;21 : 181–206. doi: 10.1521/aeap.2009.21.3.181 19519235
29. Shahmanesh M, Patel V, Mabey D, Cowan F. Effectiveness of interventions for the prevention of HIV and other sexually transmitted infections in female sex workers in resource poor setting: a systematic review. Trop Med Int Health. 2008;13 : 659–79. doi: 10.1111/j.1365-3156.2008.02040.x 18266784
30. Bain LE, Ditah CM, Awah PK, Ekukwe NC. Ethical implications of HIV self-testing: the game is far from being over. Pan Afr Med J. 2016;25 : 114. doi: 10.11604/pamj.2016.25.114.8303 28292077
31. Mountain E, Mishra S, Vickerman P, Pickles M, Gilks C, Boily M-C. Antiretroviral therapy uptake, attrition, adherence and outcomes among HIV-infected female sex workers: a systematic review and meta-analysis. PLoS ONE. 2014;9:e105645. doi: 10.1371/journal.pone.0105645 25265158
32. Choko AT, Kumwenda MK, Johnson CC, Sakala DW, Chikalipo MC, Fielding K, et al. Acceptability of woman-delivered HIV self-testing to the male partner, and additional interventions: a qualitative study of antenatal care participants in Malawi. J Int AIDS Soc. 2017;20 : 21610. doi: 10.7448/IAS.20.1.21610 28691442
Štítky
Interní lékařství
Článek Contemporary disengagement from antiretroviral therapy in Khayelitsha, South Africa: A cohort studyČlánek Bioequivalence of twice-daily oral tacrolimus in transplant recipients: More evidence for consensus?
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2017 Číslo 11- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Červená fermentovaná rýže účinně snižuje hladinu LDL cholesterolu jako vhodná alternativa ke statinové terapii
- Jak postupovat při výběru betablokátoru − doporučení z kardiologické praxe
-
Všechny články tohoto čísla
- Labour trafficking: Challenges and opportunities from an occupational health perspective
- The end of HIV: Still a very long way to go, but progress continues
- Contemporary disengagement from antiretroviral therapy in Khayelitsha, South Africa: A cohort study
- Bioequivalence of twice-daily oral tacrolimus in transplant recipients: More evidence for consensus?
- Treatment guidelines and early loss from care for people living with HIV in Cape Town, South Africa: A retrospective cohort study
- Perinatal mortality associated with induction of labour versus expectant management in nulliparous women aged 35 years or over: An English national cohort study
- Core Outcome Set-STAndards for Development: The COS-STAD recommendations
- Closing the gaps in the HIV care continuum
- Association between the 2012 Health and Social Care Act and specialist visits and hospitalisations in England: A controlled interrupted time series analysis
- HIV pre-exposure prophylaxis and early antiretroviral treatment among female sex workers in South Africa: Results from a prospective observational demonstration project
- Sexual exploitation of unaccompanied migrant and refugee boys in Greece: Approaches to prevention
- Child sex trafficking in the United States: Challenges for the healthcare provider
- The expanding epidemic of HIV-1 in the Russian Federation
- Cardiovascular disease (CVD) and chronic kidney disease (CKD) event rates in HIV-positive persons at high predicted CVD and CKD risk: A prospective analysis of the D:A:D observational study
- Validity of a minimally invasive autopsy for cause of death determination in maternal deaths in Mozambique: An observational study
- malERA: An updated research agenda for malaria elimination and eradication
- malERA: An updated research agenda for health systems and policy research in malaria elimination and eradication
- A combination intervention strategy to improve linkage to and retention in HIV care following diagnosis in Mozambique: A cluster-randomized study
- Bioequivalence between innovator and generic tacrolimus in liver and kidney transplant recipients: A randomized, crossover clinical trial
- malERA: An updated research agenda for basic science and enabling technologies in malaria elimination and eradication
- Human trafficking and exploitation: A global health concern
- Virological response and resistance among HIV-infected children receiving long-term antiretroviral therapy without virological monitoring in Uganda and Zimbabwe: Observational analyses within the randomised ARROW trial
- Postmenopausal hormone therapy and risk of stroke: A pooled analysis of data from population-based cohort studies
- Lansoprazole use and tuberculosis incidence in the United Kingdom Clinical Practice Research Datalink: A population based cohort
- malERA: An updated research agenda for insecticide and drug resistance in malaria elimination and eradication
- Safety, pharmacokinetics, and immunological activities of multiple intravenous or subcutaneous doses of an anti-HIV monoclonal antibody, VRC01, administered to HIV-uninfected adults: Results of a phase 1 randomized trial
- HIV prevalence and behavioral and psychosocial factors among transgender women and cisgender men who have sex with men in 8 African countries: A cross-sectional analysis
- Treatment eligibility and retention in clinical HIV care: A regression discontinuity study in South Africa
- malERA: An updated research agenda for characterising the reservoir and measuring transmission in malaria elimination and eradication
- Effectiveness of a combination strategy for linkage and retention in adult HIV care in Swaziland: The Link4Health cluster randomized trial
- The value of confirmatory testing in early infant HIV diagnosis programmes in South Africa: A cost-effectiveness analysis
- HIV self-testing among female sex workers in Zambia: A cluster randomized controlled trial
- The US President's Malaria Initiative, transmission and mortality: A modelling study
- Comparison of two cash transfer strategies to prevent catastrophic costs for poor tuberculosis-affected households in low- and middle-income countries: An economic modelling study
- Direct provision versus facility collection of HIV self-tests among female sex workers in Uganda: A cluster-randomized controlled health systems trial
- malERA: An updated research agenda for diagnostics, drugs, vaccines, and vector control in malaria elimination and eradication
- malERA: An updated research agenda for combination interventions and modelling in malaria elimination and eradication
- HIV-1 persistence following extremely early initiation of antiretroviral therapy (ART) during acute HIV-1 infection: An observational study
- Respondent-driven sampling for identification of HIV- and HCV-infected people who inject drugs and men who have sex with men in India: A cross-sectional, community-based analysis
- Extensive virologic and immunologic characterization in an HIV-infected individual following allogeneic stem cell transplant and analytic cessation of antiretroviral therapy: A case study
- Measuring success: The challenge of social protection in helping eliminate tuberculosis
- Prospects for passive immunity to prevent HIV infection
- Reaching global HIV/AIDS goals: What got us here, won't get us there
- Evidence-based restructuring of health and social care
- Extreme exploitation in Southeast Asia waters: Challenges in progressing towards universal health coverage for migrant workers
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Postmenopausal hormone therapy and risk of stroke: A pooled analysis of data from population-based cohort studies
- Bioequivalence between innovator and generic tacrolimus in liver and kidney transplant recipients: A randomized, crossover clinical trial
- HIV pre-exposure prophylaxis and early antiretroviral treatment among female sex workers in South Africa: Results from a prospective observational demonstration project
- Bioequivalence of twice-daily oral tacrolimus in transplant recipients: More evidence for consensus?
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



