-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaEffectiveness of a combination strategy for linkage and retention in adult HIV care in Swaziland: The Link4Health cluster randomized trial
In a cluster-randomized trial done in Swaziland, Margaret McNairy and colleagues test a combined intervention for linking and retaining adults with HIV infection in care.
Published in the journal: . PLoS Med 14(11): e32767. doi:10.1371/journal.pmed.1002420
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1002420Summary
In a cluster-randomized trial done in Swaziland, Margaret McNairy and colleagues test a combined intervention for linking and retaining adults with HIV infection in care.
Introduction
Achieving the desired impact of HIV treatment, as measured by individual health outcomes and reduced transmission to others, is contingent upon completing all steps in the HIV care continuum, from identifying all individuals who are living with HIV and linking those found to be HIV positive to HIV care to retaining them in lifelong care and on antiretroviral therapy (ART) [1]. Over the past decade, the scale-up of HIV programs has been substantial, with over 18 million persons having initiated ART by the end of 2015 in low - and middle-income countries and an associated substantial decrease in HIV-related morbidity and mortality, as well as evidence of a decrease in HIV incidence in many of the most severely affected countries [2]. However, in order to achieve epidemic control, further optimization of the HIV care continuum is needed so as to achieve the Joint United Nations Programme on HIV/AIDS (UNAIDS) 90/90/90 targets, which require that 90% of individuals with HIV are aware of their diagnosis, that 90% of those aware of their HIV infection are initiated on ART, and that 90% of those on treatment achieve and maintain viral suppression [3].
Findings from HIV programs indicate that linkage to and retention in HIV care currently fall far short of the desired goals [4–6]. Linkage of HIV-positive individuals to HIV care varies from less than half of individuals linking to care within 6 months of an HIV-positive test to 72% who ever linked [5,7,8]. Once linked to care, less than half of HIV-positive patients are retained in care prior to initiation of ART, with only two-thirds of ART-eligible individuals initiating ART [5,7]. Lastly, only approximately three-quarters of patients initiated on ART have been noted to be retained in care at 12 months, with retention decreasing over the ensuing follow-up years [4].
Barriers to linkage to and retention in care are multifactorial and include both individual - and health system-level factors such as stigma, fear of disclosure, distance and cost of travel to clinic, attitudes of providers, and cumbersome clinic procedures with long waiting times [9,10]. Previous studies that aimed to overcome such barriers have largely focused on the assessment of 1 intervention primarily targeting a single step in the HIV care continuum [11–14]. We postulated that in order to address the multiple gaps across the care continuum, a multi-component intervention strategy is needed, with each component targeting one or more steps in the HIV care continuum.
The Link4Health study evaluated the effectiveness of a combination intervention strategy (CIS) utilizing 5 evidence-based interventions that address structural, behavioral, and biomedical barriers across the continuum of care, to improve linkage to and retention in care among newly identified HIV-positive adults in Swaziland.
Methods
Ethics
The study was approved by the institutional review boards at Columbia University and the Swaziland Scientific and Ethics Committee.
Study design
A detailed description of the study methods was previously reported [15]. In brief, Link4Health was an implementation science study using a cluster site-randomized trial design. The study unit of randomization consisted of a public secondary-level HIV clinic paired with its largest affiliated public primary-level HIV clinic. Ten study units were selected from a total of 11 existing secondary-level HIV clinics in the country, based on clinic patient volume. Study units were pair matched, first by implementing partner (matching the 2 study units from implementing partner A) and then by location (urban [4] versus rural [4]) and clinic size, based on the estimated number of adults testing HIV positive in the 2 years prior to study implementation (<50 versus >50 per month for rural study units and <100 versus >100 per month for urban study units). Matched study units were randomized by a computerized random number generator to the CIS or standard of care (SOC) study arm. A cluster design was chosen to avoid disruption of service delivery, enable better fit within the routine workings at the clinical site, and allow the clinic staff to more easily implement the study. The study staff and clinic providers at each study unit were not blinded to the assigned arm for the site.
Study setting and population
Swaziland is located in Southern Africa and has the world’s highest HIV prevalence, with HIV as the leading cause of death in the country [16]. The estimated adult (age 18–49 years) HIV prevalence is 31%, and the estimated incidence is 2.4% (95% CI 2.1–2.8) [17, 18]. The country has made impressive strides in responding to the epidemic, with nearly 70% of persons estimated to be living with HIV having initiated ART as of 2015 [19]. Nevertheless, historic rates of linkage to and retention in care at 12 months after ART initiation remain suboptimal [20]. Data available from Swaziland at the time of the initiation of the study showed that among 1,105 adults who tested HIV positive at community testing venues, only 37% linked to HIV care within 12 months of HIV testing [21]. Retention among adults at 36 months after ART start was 68% in 2011 per national estimates [22].
Inclusion criteria were as follows: adults aged ≥18 years, newly tested HIV positive, and willing to receive HIV care at the study unit and consent to study procedures. Exclusion criteria were as follows: planning on leaving the community during the study, prior enrollment in HIV care or initiation of ART in the past 6 months, currently on ART, reports a current pregnancy, or not able to speak English or SiSwati.
Study interventions
All adults who tested HIV positive at participating sites were informed of the study by their providers. Interested individuals were referred to a study nurse who provided further information, confirmed eligibility, and, if eligible, obtained written consent. All consenting participants provided baseline information and thereafter were managed based on the study arm to which the clinic was randomly assigned.
SOC
Participants at study units randomized to the SOC arm were managed according to country guidelines. These guidelines recommend that individuals identified as HIV positive receive post-test counseling and be referred to an HIV clinic using a national referral form [21]. Thereafter, upon presentation at their first HIV clinic visit, such individuals are to present their referral form, receive a clinical assessment, and have blood drawn for a CD4+ count test as well as hematology and chemistry tests and are instructed to return in 1–2 weeks for receipt of their results. Upon return, those eligible for ART according to then prevailing national guidelines (i.e., with a CD4+ count ≤ 350 cells/mm3) are to receive the first of 3 counseling sessions. Patients who are prescribed ART are instructed to return to the clinic every month for 6 months and then every 3 months, if they are stable on treatment. Patients who are ineligible for ART are instructed to return to clinic every 3 months for follow-up. Peer counselors are encouraged to call patients within 7 days of a missed clinic appointment. All patients are prescribed cotrimoxazole prophylaxis, and condoms, and health informational materials are to be made available in the clinics.
CIS
Participants at clinics randomized to the CIS arm received a multicomponent strategy of 5 evidence-based interventions, targeting structural, biomedical, and behavioral barriers, which are described briefly below (Table 1) [15]. All components of the combination strategy utilized in this study were selected based on evidence of their effectiveness, feasibility, and suitability to patients in diverse healthcare settings.
Tab. 1. Comparison of combination intervention strategy (CIS) to standard of care (SOC) procedures. 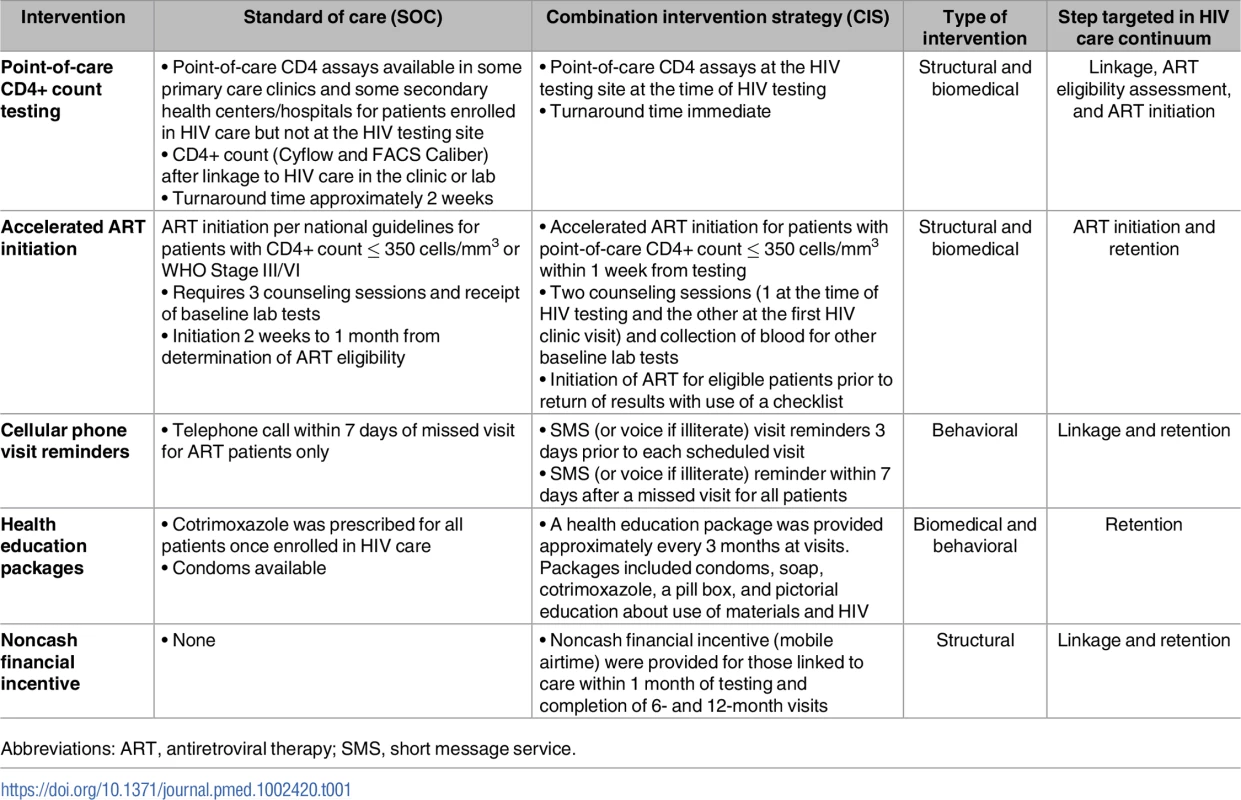
Abbreviations: ART, antiretroviral therapy; SMS, short message service. The first intervention was provision of point-of-care (POC) CD4+ count testing performed immediately after an HIV-positive test, in the same physical location as the HIV testing site, with the aim of improving linkage to care, assessment for ART eligibility, and prompt ART initiation. Several studies have reported higher linkage and ART initiation rates with POC CD4+ count testing as compared to traditional CD4+ count testing [23–26].
The second intervention of accelerated ART initiation for eligible patients (CD4+ count ≤ 350 cells/mm3 or WHO stage III/IV) involved 2 rather than 3 counseling sessions and recommended ART initiation within the first week after linkage to care. Delays in ART initiation among those eligible for treatment have been shown to be associated with increased morbidity and mortality [27]. Late ART initiation is also associated with a longer period of increased infectiousness due to ongoing viral replication [28]. In this study, initiation of ART promptly rather than waiting for the return of baseline safety laboratory test results was strongly encouraged, and a checklist was made available to the providers to assist in identifying those potential participants at risk for renal insufficiency who would require waiting for the serum creatinine results prior to ART initiation.
The third intervention involved use of short-message-service (SMS) appointment reminders, sent from a central server, which aimed at improving linkage to and retention in care among participants. SMS reminders were sent 3 days prior to an appointment and after a missed appointment. The message did not contain any information relating to HIV status. SMS communications have been used in HIV care and other chronic disease management to improve health communication and patient adherence [29–37].
The fourth intervention was a health education package that included health information and supplies such as soap, a toothbrush, and a pill box, which also aimed to improve both linkage to and retention in care. A package of different materials and information was given every 3 months. A similar intervention has been evaluated in Uganda and was associated with high rates of cotrimoxazole use, condom use, and HIV testing of family members [38].
Lastly, financial incentives of modest amount were provided that served to reimburse participants for expenses or lost wages or transport costs for clinic attendance [39]. This intervention was selected because there has been great interest in the use of financial incentives as a structural intervention to achieve positive health behaviors including retention in care [39–45]. A noncash type of financial incentive was provided in the form of a prepaid mobile phone card valued at US$8 and was given to participants upon linkage to care within 1 month of HIV testing and at completion of 6 and 12 months in follow-up care.
Data collection and study measures
All participants completed a baseline questionnaire, at the time of study enrollment, which solicited information on sociodemographic characteristics, HIV disease history, barriers to care, travel time to clinic, depression, social and family support, and HIV-related knowledge. Follow-up questionnaires were conducted at 1 and 12 months after enrollment, at the participant’s home or a prespecified location in the community, to collect information on changes in sociodemographic characteristics, self-reported linkage to care and retention, preferences about the study interventions, and vital status, if the latter was not known. Clinical data including CD4+ count, WHO Stage, date of ART initiation, ART regimen, and clinic and pharmacy visit dates were abstracted from participants’ medical charts—the data source for the primary outcome. These data were collected between 3–6 months after the participant reached the end of the study follow-up period. If the participant’s medical record was missing, he/she was assumed to have not achieved the primary outcome. Death was ascertained via medical record reviews or at the time of the 1 - or 12-month interview. Viral load measurement was done using dried blood samples (DBSs) (HIV-1 RNA Abbott m2000rt system) collected at the time of the 12-month questionnaire at the participant’s home or a prespecified location [46].
Study outcomes
The primary outcome was a combined outcome of linkage to HIV care within 1 month of HIV testing plus retention in care at 12 months from HIV testing among participants at the individual level. Linkage to care was defined by participant attendance of at least 1 visit to an HIV clinic with completion of an intake assessment including medical history and physical exam. Retention in care at 12 months after HIV testing was defined as no documented death and a clinic visit at the study unit within 90 days prior to the end of the study follow-up period. Participants with a missing medical record at the time of medical record abstraction were considered nonretained.
Secondary endpoints included evaluation of the effectiveness of the CIS compared to the SOC with regard to the following: each component of the primary outcome described above, time to linkage, ART eligibility, ART initiation, time to ART initiation, viral suppression defined as HIV-1 RNA < 1,000 copies/mL at 12 months among patients on ART for at least 6 months, and death and loss to follow-up at 12 months after HIV testing. Death and transfer status were ascertained from medical records and through the 12-month follow-up visit questionnaire. Linkage and retention at clinics other than the assigned study unit were assessed in a sensitivity analysis using self-reported linkage and retention data from the 1 - and 12-month questionnaires.
Statistical analysis
The study sample size was calculated by estimating that 35% of the participants in the SOC study arm would achieve the primary outcome (assuming that 50% link to HIV care within 1 month of testing and that 70% of those linking within 1 month are retained at 12 months after testing). We estimated that approximately 2,750 adults would be eligible for study enrollment based on historic HIV testing volume and the proportion of individuals testing HIV positive at the study units in the year prior to the study start. Assuming 80% of eligible individuals would consent to enrollment, we estimated an average enrollment of 220 participants per study unit or 2,200 in total (1,110 per study arm). With this sample size and 5 study units per study arm, we then estimated the minimum difference in the primary outcome we could detect with 80% power, 2-sided alpha of 0.05, assuming an interclass correlation coefficient (ICC) of 0.05. In a post hoc analysis, we estimated the ICC of the primary outcome using the method outlined by Snijders and Bosker for binary outcome data [47].
An intent-to-treat analysis compared the relative risk (RR) of achieving the primary outcome between study arms, with each having 5 clusters per arm. Within study unit clustering was accounted for using random-intercept multilevel models. For dichotomous outcomes, log-Poisson models with robust standard error were used. For continuous outcomes, random-intercept linear regression models were used. Assessment of potential confounding despite cluster randomization was performed by constructing multivariable random-intercept regression models including covariates found statistically different between treatment arms at an alpha of 0.01. Additionally, we conducted a per-protocol analysis comparing the RR of achieving the primary outcome among participants who received the full package of the CIS for the duration of study participation. Sensitivity analysis assessed any changes to the intent-to-treat analysis after including self-reported linkage and retention obtained from follow-up surveys. In post hoc analyses, assessment of the RR for achieving the primary outcome by key subgroups was done using interaction contrast ratios.
Results
Study population
Of the 10 study units included in this study, 6 were located in urban areas, and 4 in rural areas. At study units randomized to the CIS study arm, a total of 1,234 individuals were screened for eligibility, with 1,096 (89%) enrolled in the study from 19 August 2013 to 21 November 2014 (Fig 1). At study units assigned to the SOC study arm, a total of 1,316 were screened, with 1,101 (84%) enrolled. Study refusal differed by study arm, with 23 refusals (1.9%) in the CIS arm and 114 refusals (8.7%) in the SOC arm (p < 0.001). Reasons for ineligibility are shown in Fig 1, with 111 participants ineligible in the CIS arm compared to 101 in the SOC arm.
Fig. 1. Flow diagram of study enrollment. 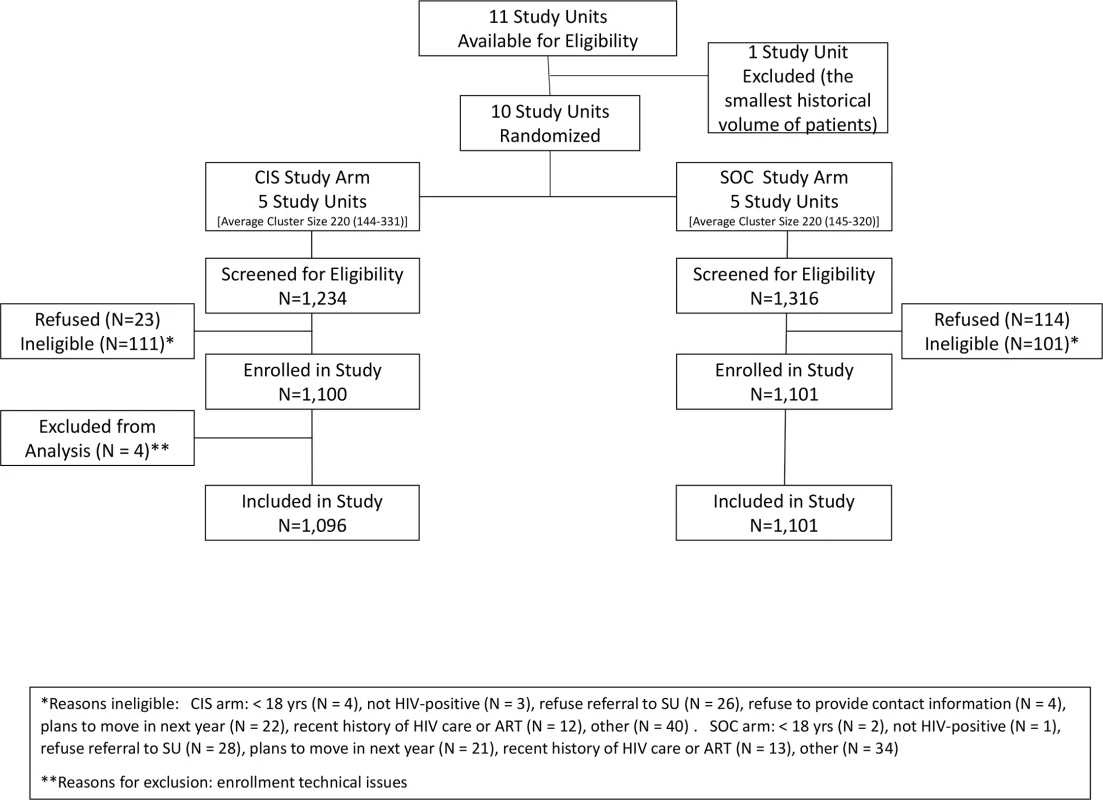
ART, antiretroviral therapy; CIS, combination intervention strategy; SOC, standard of care; SU, study unit. Among 2,197 participants included in this analysis, 1,294 (59%) were female, and the median age was 31 years (IQR 26–39), with 445 (20%) of the participants being young adults aged 18–24 years (Table 2). Forty-five percent reported no education or only primary schooling; approximately half were unemployed. Median individual weekly income was US$9 (IQR US$0–US$37). Eighty-four percent reported living in the current residence for more than 1 year, with 16% reporting travel away from home for over a 1-month duration in the past year. The median travel time from residence to HIV clinic was 30 minutes (IQR 20–50). The majority (80%) were diagnosed with HIV through a voluntary counseling and testing site, with the remainder having received HIV testing through provider-initiated testing and counseling at clinics within the study units. Over half (54%) of the participants reported that this was their first HIV test, while 89% indicated that it was their first positive HIV test.
Tab. 2. Participant characteristics at HIV testing (N = 2,197). 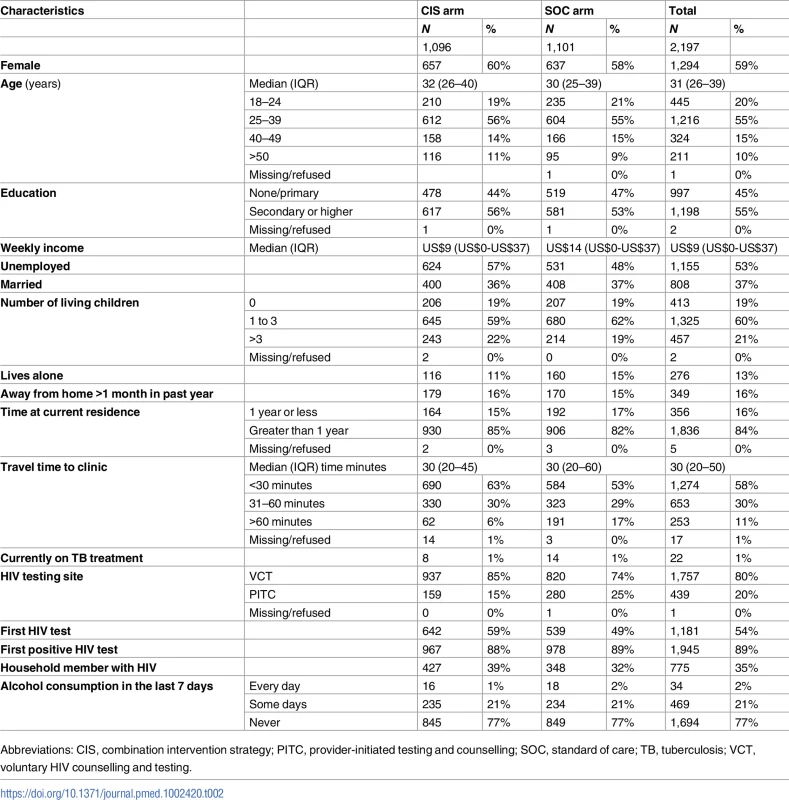
Abbreviations: CIS, combination intervention strategy; PITC, provider-initiated testing and counselling; SOC, standard of care; TB, tuberculosis; VCT, voluntary HIV counselling and testing. Primary outcome
In the intent-to-treat analysis, 705 (64%) participants at sites randomized to the CIS study arm and 477 (43%) participants at sites randomized to the SOC study arm achieved the primary outcome of linkage to HIV care within 1 month of HIV-positive testing plus retention in HIV care at 12 months after HIV testing, for an RR of 1.48 (95% CI 1.37–1.61, p < 0.001). Accounting for clustering within study units, the RR was 1.52 (95% CI 1.19–1.96, p = 0.002) (Fig 2, Table 3). Additionally, adjusting for covariates significant in the bivariate analyses listed in Table 2 did not appreciably change the results. A total of 64 (6%) of participants in the CIS arm and 144 (13%) of participants in the SOC arm did not have a medical record and were classified as “not linked” to HIV care.
Fig. 2. Proportion of participants who achieved the primary outcome of linkage to HIV care within 1 month of HIV testing plus retention in HIV care at 12 months after HIV testing by study arm (combination intervention strategy [CIS] and standard of care [SOC]). ![Proportion of participants who achieved the primary outcome of linkage to HIV care within 1 month of HIV testing plus retention in HIV care at 12 months after HIV testing by study arm (combination intervention strategy [CIS] and standard of care [SOC]).](https://www.prolekarniky.cz/media/cache/resolve/media_object_image_small/media/image/04fbff7181e6478dc5733d84bbb9f0dd.png)
Tab. 3. Primary and secondary outcomes for the combination intervention strategy (CIS) and standard of care (SOC) study arms. 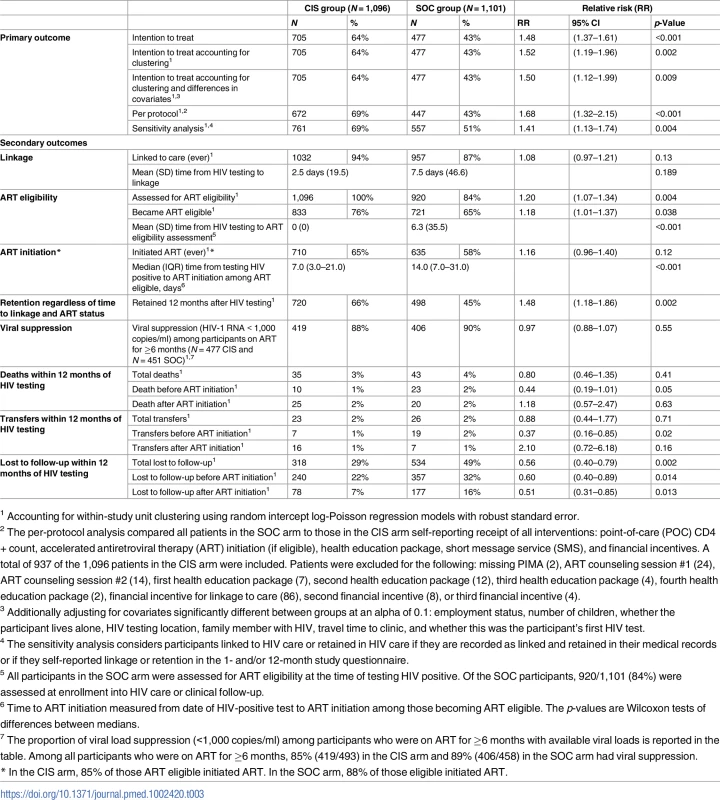
1 Accounting for within-study unit clustering using random intercept log-Poisson regression models with robust standard error. The RR in the per-protocol analysis accounting for clustering for achieving the primary outcome was 1.68 (95% CI 1.32–2.15, p = 0.003) (Table 3). The RR in the sensitivity analysis, accounting for clustering, which included participants who self-reported linkage and retention in the 1 - and 12-month surveys at a clinic other than 1 with their assigned study unit, was 1.41 (95% CI 1.13–1.74, p = 0.004), respectively (Table 3). Using this approach, we calculated an ICC of 0.086, similar to but slightly higher than the assumed ICC used in power and sample size estimation.
The CIS strategy was delivered according to the study protocol to 937 (85%) of the 1,096 participants enrolled in study units assigned to the CIS. Reasons for not receiving all of the CIS strategy intervention components included missing POC CD4+ count testing (<1% CIS participants), missing an ART counseling session per accelerated ART procedures (3%), missing receipt of 1 healthcare bag (2%), and missing receipt of 1 financial incentive (9%). There was heterogeneity in the primary outcome across the 5 pairs of matched study units. The proportion of participants who achieved the primary outcome in study units randomized to the CIS ranged from 49% to 82%, while this ranged from 22% to 57% in the study units randomized to SOC.
Secondary outcomes
A similar proportion of participants linked to care anytime during the study follow-up period in both study arms: 1,032 (94%) in the CIS arm as compared to 957 (87%) in the SOC arm (RR 1.08, 95% CI 0.97–1.21, p = 0.13), with no significant differences in linkage within the same day or 1 month after testing (Table 3). The mean time to linkage to care was shorter in the CIS arm versus the SOC study arm but was not statistically different (2.5 compared to 7.5 days, p = 0.189). However, among those who ever linked to care (1,032 in the CIS study arm and 957 in the SOC study arm), significantly fewer patients (13%) in CIS sites versus SOC sites (18%) did not return for subsequent visits after the first clinic visit (p = 0.008).
Assessment for ART eligibility through either a CD4+ count or WHO staging was done for all participants in the CIS arm as compared to 84% of participants in the SOC arm (RR 1.20, 95% CI 1.07–1.34, p = 0.004). The mean time to ART eligibility assessment was 0 days in the CIS study arm compared to 6.3 days in the SOC arm (p < 0.001). The median CD4+ count among 1,096 participants in the CIS arm who had POC CD4+ count testing done at the time of HIV testing was 311 cells/mm3 (IQR 159–443). Among the 907 (82%) participants in the SOC arm who linked to HIV care and had a CD4+ count done, the median CD4 count was 285 cells/mm3 (155–444) (p = 0.07).
A total of 710 participants (85% of ART-eligible participants) in the CIS arm as compared to 635 (88% among ART-eligible participants) in the SOC arm initiated ART within the study follow-up period (RR 1.16, 95% CI 0.96–1.40, p = 0.12) (Table 3). The median time from HIV testing to ART initiation among eligible patients was 7.0 days (IQR 3.0–21.0) as compared to 14.0 days (IQR 7.0–13.0) in the CIS and SOC study arms, respectively (p < 0.001).
Retention in care, regardless of time to linkage or ART status, at 12 months was significantly greater in participants in the CIS as compared to the SOC study arm, with an RR of 1.48 (95% CI 1.18–1.86, p = 0.002). Loss to follow-up during pre-ART care (RR = 0.60, 95% CI 0.40–0.89, p = 0.014) and after ART initiation (RR = 0.51, 95% CI 0.31–0.85, p = 0.013) was significantly lower in the CIS arm as compared to the SOC arm.
For participants on ART for at least 6 months during follow-up regardless of retention status, viral load data were available for 97% (N = 477/493) of participants in the CIS arm and 98% (N = 451/458) in the SOC arm. Viral suppression among participants on ART ≥6 months with available viral loads was similar by study arm at 88% in CIS and 90% in SOC (RR 0.97, 95% CI 0.88–1.07, p = 0.55).
There were 78 deaths (3.6% of the study population) that occurred during follow-up, and this did not differ by study arm (35 deaths [3%] in the CIS study arm versus 43 deaths [4%] in the SOC arm, with an RR of 0.80, 95% CI 0.46–1.35, p = 0.40) (Table 3). However, there was nonsignificantly lower mortality among participants prior to ART initiation in the CIS arm (10 deaths) compared to the SOC arm (23 deaths), with an RR of 0.44 (95% CI 0.19–1.01, p = 0.05). Fig 3 compares the CIS study arm versus the SOC study arm across the HIV care continuum from linkage to care within 1 month of testing through retention in care at 12 months after testing HIV positive.
Fig. 3. HIV care continuum comparing the combination intervention strategy (CIS) study arm versus the standard of care (SOC) study arm. 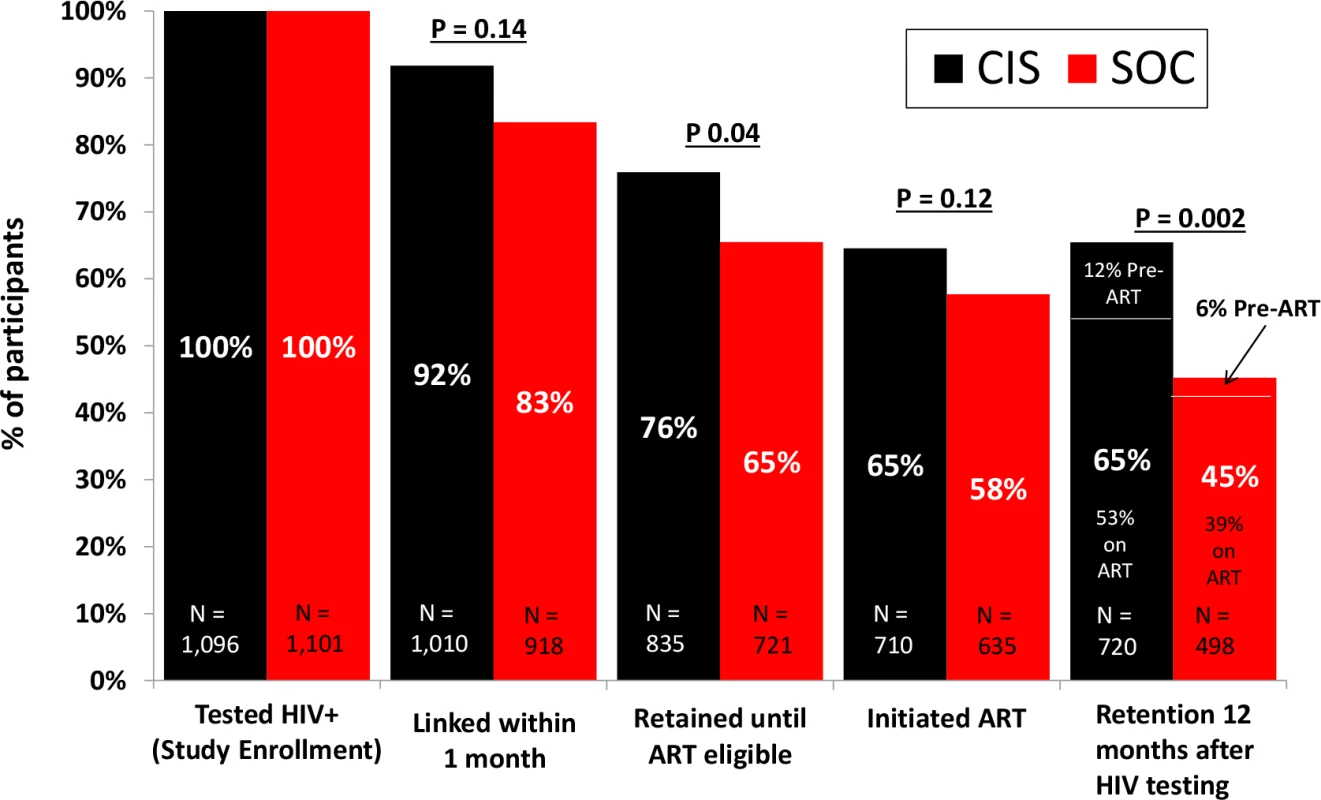
In post hoc analyses, we examined achievement of the primary outcome between study arms by key subgroups. The effect of the CIS, as compared to the SOC, was consistent across all prespecified subgroups, including by age, sex, income, employment, marital status, travel away from home in the past year, travel time to clinic, past HIV testing history, household members with HIV, and type of clinic (Fig 4, S1 Table).
Fig. 4. Primary outcome by subgroups of participants. 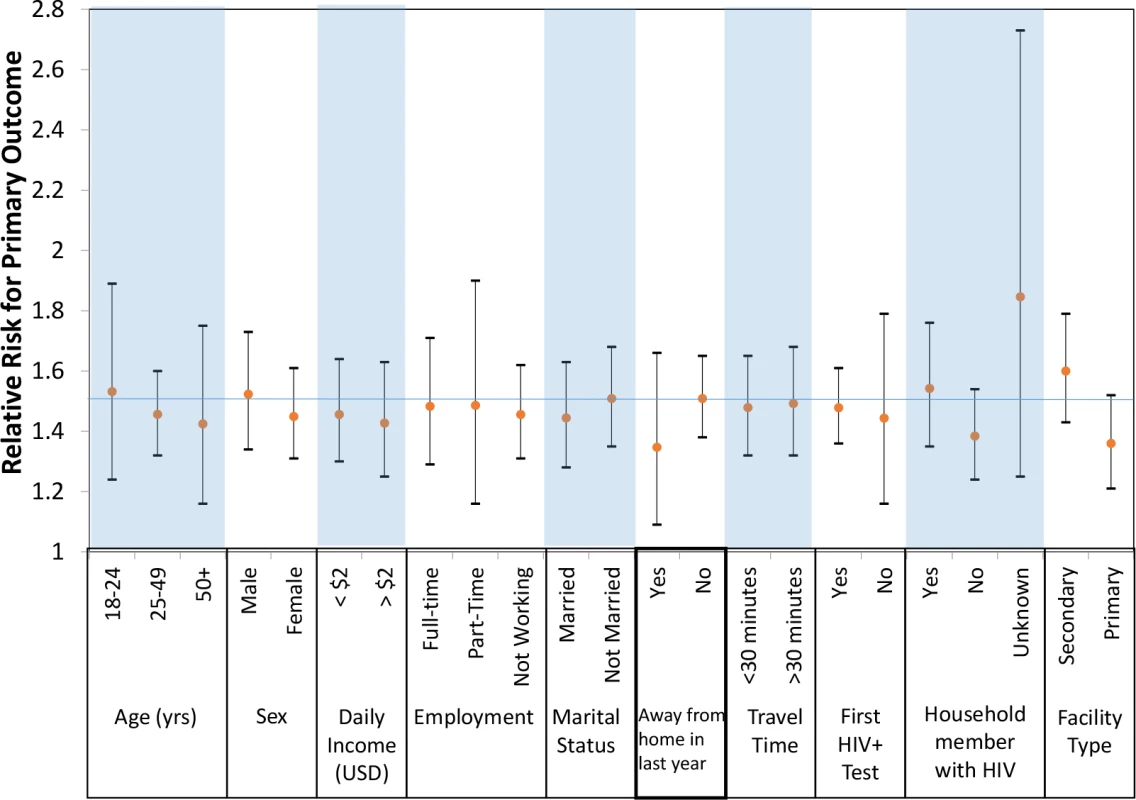
USD, US dollars; yrs, years. Discussion
In this cluster-randomized study, a novel combination strategy, inclusive of 5 evidence-based interventions, was 50% more effective than the SOC in enhancing linkage to care plus retention in care among HIV-positive individuals. The robustness of this outcome is supported by the consistent findings in the per-protocol analysis, in sensitivity analyses, and across subgroups of participants. In addition, the combination strategy was associated with improvements across multiple steps of the care continuum, with an increased proportion of participants who were assessed for ART eligibility, decreased time to ART eligibility assessment, decreased time to ART initiation, increased retention at 12 months after HIV testing regardless of time to linkage and ART status, and decreased mortality among participants prior to ART initiation. However, high rates of viral suppression were similar among ART patients by study arm.
In our study, the effect noted on the primary outcome appeared to be largely driven by enhanced retention rather than by the linkage-to-care component. This finding may be due to the high proportion of participants in both study arms who linked to care within 1 month of HIV testing in both arms of the study (87% in the SOC arm and 92% in the CIS arm), and thus, our sample size was insufficient to show a difference between the arms. The high proportion of participant linkage was likely influenced by a national campaign to improve linkage that was implemented during the study period [21].
The combination strategy significantly reduced loss to follow-up among participants regardless of whether they were in pre-ART care or on treatment. Loss to follow-up, in both study arms, was higher among pre-ART participants, as compared to participants who had initiated ART. This is consistent with findings from other studies, including those from a large study of 390,603 HIV-positive adults in Kenya, Mozambique, Rwanda, and Tanzania, in which 34.8% of all patients who had not initiated ART were lost from care at 12 months, compared to 5.8% among patients on ART [6]. While the pre-ART care phase should largely be minimized with the release of the recent WHO guidelines that recommend offering ART to all HIV-positive individuals irrespective of CD4+ count or WHO disease stage, evidence suggests that retention in care and on ART remains a challenge even in the context of “treatment for all” [48]. For example, while adoption of Option B+, which entails initiation of ART for all HIV-positive pregnant women, has been associated with an increase in the number of pregnant women on ART, loss to follow-up has remained a challenge. Among 21,939 HIV-infected pregnant women who started ART as per Option B+ in Malawi, 17% were lost to follow-up at 6 months after treatment start, with a 5-fold higher loss to follow-up compared to those who initiated ART at a more advanced stage of HIV disease [49]. Thus, the findings from our study remain relevant even though the study was conducted at a time when a CD4+ count threshold was recommended for ART initiation.
In this study, viral suppression was high among all participants on ART for a minimum of 6 months, irrespective of study arm. This confirms the potency of the first-line regimen, consisting of tenofovir, lamivudine, and efavirenz or nevirapine, and suggests that participants were highly adherent to their medications. These findings build upon those from the Population-based HIV Impact Assessment Project surveys that were conducted recently in Malawi, Zambia, Zimbabwe, and Swaziland, which included nationally representative samples of individuals in which 87% of HIV-positive adults who reported being on ART were virally suppressed (HIV-1 RNA < 1,000 copies/ml) [50,51]. Findings from this project survey in Swaziland showed that among adults who were aware of their HIV-positive status and who indicated being on ART, 92% had a suppressed viral load [52]. The finding of similarly high proportions of viral suppression among participants in both arms of our study suggests that the sample size was insufficient to detect a difference. In addition, it is important to note that the interventions used in this study were not designed with a focus specifically on enhancing medication adherence and viral suppression. Design of future combination strategies may prioritize the use of interventions that focus specifically on enhancing adherence to ART, such as the use of financial incentives to improve viral suppression [53].
Every effort was made to ascertain accurate loss to follow-up and mortality outcomes in our study. It should be noted that reporting of accurate loss to follow-up and mortality outcomes by HIV programs has been a controversial topic. This is due to the fact that when tracing was done for individuals reported as lost to follow-up by HIV programs, a substantial proportion were found to have either died or transferred care to another health facility [54]. We feel confident that it is unlikely that such misclassification occurred in our study as home tracing was conducted for all study participants to ascertain their outcomes at the end of the 12-month follow-up period. While the study was not powered to detect a difference between the study arms in terms of mortality, the combination strategy appeared to have a meaningful—albeit not statistically significant—effect, with as much as 50% lower mortality noted among pre-ART patients. This may be due to better retention in care among participants in the intervention arm. Poor retention in care has been demonstrated to be associated with increased mortality, likely due to missed clinic visits that deprive patients of clinical and laboratory assessments for diagnosis of early complications and delay prompt initiation of ART [55].
We observed substantial heterogeneity in the primary outcome across clinics in both the CIS and SOC study arms. This may reflect clinic-level differences such as clinic size and location. For example, the CIS study unit with the lowest achievement of the study’s primary outcome was the largest clinic in urban Swaziland. It is possible that individuals who test HIV positive at such a large clinic may seek ongoing care at clinics closer to their homes. Other reasons could be differences in patient-level factors, such as sex, age, and immunological status, which warrant further analyses.
To date, most intervention studies to address gaps in the HIV care continuum have focused on 1 step in the continuum, largely that of ART initiation. The Rapid Initiation of Treatment trial showed that single-visit ART initiation that included POC CD4+ count testing was associated with significantly higher ART initiation (97%) compared to the standard of care (72%) [56]. The START-ART trial was a stepped-wedge cluster-randomized trial of 20 clinics in Uganda that evaluated an intervention aimed at improving ART initiation among eligible patients; this intervention was associated with a higher proportion of patients initiating ART (80%) within 14 days after determination of ART eligibility compared to 38% in the control group [13]. Finally, the Same Day ART Initiation Study in Haiti, which evaluated the effect of same-day ART initiation on the day of HIV diagnosis among asymptomatic HIV-positive adults with CD4+ count ≤ 500 cells/uL and WHO stage I or II disease, noted that a higher proportion (53%) of participants randomized to same-day ART initiation were retained in care at 12 months with viral suppression compared to those in the standard of care arm (44%) [14].
Our study had several strengths, including the use of a pragmatic approach consistent with implementation science design. Specifically, it utilized broad eligibility criteria, was conducted within established health facilities, tested feasible interventions that were delivered primarily by available staff rather than research staff, and assessed the primary outcome largely through routinely available data. In addition, the study included the majority of clinics in Swaziland and involved cluster-randomized design rather than randomization of individual participants, which allowed for ease of implementation and avoided disruption of services within the clinics. The study also uniquely assessed the effect of the delivery of multiple interventions packaged in 1 strategy aimed at multiple steps in the HIV care continuum. Thus, implementation of the study strategy has the potential to achieve not only prompt ART initiation but also better retention in care and on ART, consequently enhancing individual and society benefits from the “treat all” approach.
The primary limitations of this study included a limited number of clusters, although it was inclusive of all the available clusters in the country. At the time of study initiation, there were only 11 secondary health facilities offering HIV services in Swaziland, and we selected 10 of these for participation in this study. Consequently, it is possible that the cluster randomization did not evenly distribute all determinants of linkage and retention other than the study interventions between treatment arms. While it is encouraging that analyses adjusting for individual-level differences between treatment arms did not appreciably change the results, we cannot definitively rule out residual confounding as a potential explanation of the findings. In addition, the design focused on evaluation of a package of interventions as 1 strategy and, thus, it did not allow for evaluation of the effectiveness of individual components of the combination approach. Another limitation was use of self-reported linkage and retention at other clinics to ascertain undocumented transfers to other clinics outside of the study unit.
Conclusions
The Link4Health study demonstrated that a combination strategy of evidence-based interventions, aimed at gaps in various steps of the HIV care continuum, was highly effective in enhancing linkage of HIV-positive individuals to care plus increasing their retention in care and on ART. The study also showed that once participants initiated ART, viral load suppression was high irrespective of the study arm. Cost effectiveness and qualitative analyses are ongoing in order to inform decision makers considering adoption of this strategy. Our findings offer an effective strategy that can advance the quality of HIV programs in Swaziland and that can be adapted to other similar contexts.
Supporting Information
Zdroje
1. McNairy ML, El-Sadr WM. The HIV care continuum: no partial credit given. AIDS. 2012;26(14):1735–8. doi: 10.1097/QAD.0b013e328355d67b 22614888.
2. UNAIDS. UNAIDS Website. Accessed May 24, 2016 at: http://www.unaids.org/en/resources/presscentre/pressreleaseandstatementarchive/2015/july/20150714_PR_MDG6report.
3. UNAIDS. 90-90-90 An ambitious treatment target to help end the AIDS epidemic. Accessed May 1, 2016 at: http://www.unaids.org/sites/default/files/media_asset/90-90-90_en_0.pdf.
4. Fox MP, Rosen S. Retention of Adult Patients on Antiretroviral Therapy in Low - and Middle-Income Countries: Systematic Review and Meta-analysis 2008–2013. J Acquir Immune Defic Syndr. 2015;69(1):98–108. doi: 10.1097/QAI.0000000000000553 25942461; PubMed Central PMCID: PMC4422218.
5. Mugglin C, Estill J, Wandeler G, Bender N, Egger M, Gsponer T, et al. Loss to programme between HIV diagnosis and initiation of antiretroviral therapy in sub-Saharan Africa: systematic review and meta-analysis. Trop Med Int Health. 2012;17(12):1509–20. Epub 2012/09/22. doi: 10.1111/j.1365-3156.2012.03089.x 22994151; PubMed Central PMCID: PMC3895621.
6. McNairy ML, Lamb MR, Abrams EJ, Elul B, Sahabo R, Hawken MP, et al. Use of a Comprehensive HIV Care Cascade for Evaluating HIV Program Performance: Findings From 4 Sub-Saharan African Countries. J Acquir Immune Defic Syndr. 2015;70(2):e44–51. doi: 10.1097/QAI.0000000000000745 26375466.
7. Fox MP, Shearer K, Maskew M, Meyer-Rath G, Clouse K, Sanne I. Attrition through Multiple Stages of Pre-Treatment and ART HIV Care in South Africa. PLoS ONE. 2014;9(10):e110252. doi: 10.1371/journal.pone.0110252 25330087; PubMed Central PMCID: PMC4203772.
8. Iwuji CC, Orne-Gliemann J, Larmarange J, Okesola N, Tanser F, Thiebaut R, et al. Uptake of Home-Based HIV Testing, Linkage to Care, and Community Attitudes about ART in Rural KwaZulu-Natal, South Africa: Descriptive Results from the First Phase of the ANRS 12249 TasP Cluster-Randomised Trial. PLoS Med. 2016;13(8):e1002107. doi: 10.1371/journal.pmed.1002107 27504637; PubMed Central PMCID: PMC4978506.
9. Govindasamy D, Ford N, Kranzer K. Risk factors, barriers and facilitators for linkage to antiretroviral therapy care: a systematic review. AIDS. 2012;26(16):2059–67. Epub 2012/07/12. doi: 10.1097/QAD.0b013e3283578b9b 22781227.
10. Hall BJ, Sou KL, Beanland R, Lacky M, Tso LS, Ma Q, et al. Barriers and Facilitators to Interventions Improving Retention in HIV Care: A Qualitative Evidence Meta-Synthesis. AIDS Behav. 2016;21(6):1755–67. doi: 10.1007/s10461-016-1537-0 27582088.
11. Fox MP, Rosen S, Geldsetzer P, Barnighausen T, Negussie E, Beanland R. Interventions to improve the rate or timing of initiation of antiretroviral therapy for HIV in sub-Saharan Africa: meta-analyses of effectiveness. J Int AIDS Soc. 2016;19(1):20888. 10.7448/IAS.19.1.20888. 27507249; PubMed Central PMCID: PMC4978859. doi: 10.7448/IAS.19.1.20888 27507249
12. Govindasamy D, Meghij J, Kebede Negussi E, Baggaley RC, Ford N, Kranzer K. Interventions to improve or facilitate linkage to or retention in pre-ART (HIV) care and initiation of ART in low - and middle-income settings—a systematic review. J Int AIDS Soc. 2014;17 : 19032. doi: 10.7448/IAS.17.1.19032 25095831; PubMed Central PMCID: PMC4122816.
13. Amanyire G, Semitala FC, Namusobya J, Katuramu R, Kampiire L, Wallenta J, et al. Effects of a multicomponent intervention to streamline initiation of antiretroviral therapy in Africa: a stepped-wedge cluster-randomised trial. Lancet HIV. 2016;3(11):e539–e48. doi: 10.1016/S2352-3018(16)30090-X 27658873.
14. Koenig SP, Dorvil N, Devieux JG, Hedt-Gauthier BL, Riviere C, Faustin M, et al. Same-day HIV testing with initiation of antiretroviral therapy versus standard care for persons living with HIV: A randomized unblinded trial. PLoS Med. 2017;14(7):e1002357. doi: 10.1371/journal.pmed.1002357 28742880; PubMed Central PMCID: PMC5526526.
15. McNairy ML, Gachuhi AB, Lamb MR, Nuwagaba-Biribonwoha H, Burke S, Ehrenkranz P, et al. The Link4Health study to evaluate the effectiveness of a combination intervention strategy for linkage to and retention in HIV care in Swaziland: protocol for a cluster randomized trial. Implementation science: IS. 2015;10 : 101. doi: 10.1186/s13012-015-0291-4 26189154; PubMed Central PMCID: PMC4506770.
16. Institute for Health Metrics and Evaluation. IHME Health Data Swaziland. Accessed May 30, 2017 at: http://www.healthdata.org/swaziland.
17. Justman J, Reed JB, Bicego G, Donnell D, Li K, Bock N, et al. Swaziland HIV Incidence Measurement Survey (SHIMS): a prospective national cohort study. Lancet HIV. 2016;4(2):e83–e92. doi: 10.1016/S2352-3018(16)30190-4 27863998.
18. CDC In Swaziland. Swaziland Health Factsheet. Accessed November 15 2013 at: https://www.cdc.gov/globalhealth/countries/swaziland/pdf/swaziland_factsheet.pdf.
19. The World Band. Swaziland HIV Data. Accessed December 20 2016 at: http://data.worldbank.org/indicator/SH.HIV.ARTC.ZS.
20. Auld AF, Kamiru H, Azih C, Baughman AL, Nuwagaba-Biribonwoha H, Ehrenkranz P, et al. Implementation and Operational Research: Evaluation of Swaziland's Hub-and-Spoke Model for Decentralizing Access to Antiretroviral Therapy Services. J Acquir Immune Defic Syndr. 2015;69(1):e1–e12. doi: 10.1097/QAI.0000000000000547 25942465.
21. MacKellar DA, Williams D, Storer N, Okello V, Azih C, Drummond J, et al. Enrollment in HIV Care Two Years after HIV Diagnosis in the Kingdom of Swaziland: An Evaluation of a National Program of New Linkage Procedures. PLoS ONE. 2016;11(2):e0150086. doi: 10.1371/journal.pone.0150086 26910847; PubMed Central PMCID: PMC4766101.
22. The Kingdom of Swaziland Ministry of Health. Annual HIV Programs Report 2015. Mbabane, Swaziland: Swaziland Ministry of Health, 2015.
23. Larson BA, Brenna A, McNamara L, Long L, Rosen S, Sanne I, et al. Lost Opportunities to complete CD4+ lymphocyte testing among patients who tested positive for HIV in South Africa. Bull World Health Organ. 2010;88(9):675–80. doi: 10.2471/BLT.09.068981 20865072.
24. Jani IV, Sitoe NE, Alfai ER, Chong PL, Quevedo JI, Rocha BM, et al. Effect of point-of-care CD4 cell count tests on retention of patients and rates of antiretroviral therapy initiation in primary health clinics: an observational cohort study. Lancet. 2011;378(9802):1572–9. doi: 10.1016/S0140-6736(11)61052-0 21951656.
25. Faal M, Naidoo N, Glenncross DK, Venter WD, Osih R. Providing immediate CD4 count results at HIV testing improves ART initiation. J Acquir Immune Defic Syndr. 2011;58(3):e54–9. doi: 10.1097/QAI.0b013e3182303921 21857356.
26. Larson BA, Schnippel K, Ndibongo B, Xulu T, Brennan A, Long L, et al. Rapid point-of-care CD4 testing at mobile HIV testing sites to increase linkage to care: an evaluation of a pilot program in South Africa. J Acquir Immune Defic Syndr. 2012;61(2):e13–7. doi: 10.1097/QAI.0b013e31825eec60 22659650; PubMed Central PMCID: PMC3458178.
27. Lahuerta M, Ue F, Hoffman S, Elul B, Kulkarni SG, Wu Y, et al. The problem of late ART initiation in Sub-Saharan Africa: a transient aspect of scale-up or a long-term phenomenon? J Health Care Poor Underserved. 2013;24(1):359–83. doi: 10.1353/hpu.2013.0014 23377739; PubMed Central PMCID: PMC3655523.
28. Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365(6):493–505. Epub 2011/07/20. doi: 10.1056/NEJMoa1105243 21767103; PubMed Central PMCID: PMC3200068.
29. Chang L, Kagaayi J, Nakigozi G, Packer AH, Serwadda D, Quinn TC, et al. Responding to the human resource crisis: peer health workers, mobile phones, and HIV care in Rakai, Uganda. AIDS Patient Care STDS. 2008;22(3):173–4. doi: 10.1089/apc.2007.0234 PubMed Central PMCID 2674572 18290750
30. Downer SR, Mear JG, Da Costa AC, Sethuraman K. SMS text messaging improves outpatient attendance. Aust Health Rev. Aug 2006;30(3):389–96. 16879098.
31. Fjeldsoe BS, Marshall AL, Miller YD. Behavior change interventions delivered by mobile telephone short-message service. Am J Prev Med. Feb 2009;36(2):165–73. doi: 10.1016/j.amepre.2008.09.040 19135907.
32. Haberer JE, Kiwanuka J, Nansera D, Wilson IB, Bangsberg DR. Challenges in using mobile phones for collection of antiretroviral therapy adherence data in a resource-limited setting. AIDS Behav. Dec 2010;14(6):1294–301. doi: 10.1007/s10461-010-9720-1 20532605.
33. Lester RT, Ritvo P, Mills EJ, Kariri A, Karanja S, Chung MH, et al. Effects of a mobile phone short message service on antiretroviral treatment adherence in Kenya (WelTel Kenya1): a randomised trial. Lancet. Nov 27 2010;376(9755):1838–45. doi: 10.1016/S0140-6736(10)61997-6 21071074.
34. Liew SM, Tong SF, Lee VK, Ng CJ, Leong KC, Teng CL. Text messaging reminders to reduce non-attendance in chronic disease follow-up: a clinical trial. Br J Gen Pract. Dec 2009;59(569):916–20. doi: 10.3399/bjgp09X472250 19712544.
35. Mukund Bahadur KC, Murray PJ. Cell phone short messaging service (SMS) for HIV/AIDS in South Africa: a literature review. Stud Health Technol Inform. 2010;160(Pt 1):530–5. 20841743.
36. Pop-Eleches C, Thirumurthy H, Habyarimana JP, Zivin JG, Goldstein MP, de Walque D, et al. Mobile phone technologies improve adherence to antiretroviral treatment in a resource-limited setting: a randomized controlled trial of text message reminders. AIDS. Mar 27 2011;25(6):825–34. doi: 10.1097/QAD.0b013e32834380c1 21252632.
37. Shet A, de Costa A. India calling: harnessing the promise of mobile phones for HIV healthcare. Trop Med Int Health. 2011;16(2):214–6. doi: 10.1111/j.1365-3156.2010.02678.x 21371214.
38. Colindres P, Mermin J, Ezati E, Kambabazi S, Buyungo P, Sekabembe L, et al. Utilization of a basic care and prevention package by HIV-infected persons in Uganda. AIDS Care. 2008;20(2):139–45. Epub 2007/09/27. doi: 10.1080/09540120701506804 17896196.
39. Giuffrida A, Torgerson DJ. Should we pay the patient? Review of financial incentives to enhance patient compliance. BMJ. 1997;315(7110):703–7. Epub 1997/10/07. 9314754; PubMed Central PMCID: PMC2127496.
40. Volpp KG, John LK, Troxel AB, Norton L, Fassbender J, Loewenstein G. Financial incentive-based approaches for weight loss: a randomized trial. JAMA. 2008;300(22):2631–7. Epub 2008/12/11. doi: 10.1001/jama.2008.804 19066383.
41. Volpp KG, Loewenstein G, Troxel AB, Doshi J, Price M, Laskin M, et al. A test of financial incentives to improve warfarin adherence. BMC Health Serv Res. 2008;8 : 272. Epub 2008/12/24. doi: 10.1186/1472-6963-8-272 19102784; PubMed Central PMCID: PMC2635367.
42. Volpp KG, Troxel AB, Pauly MV, Glick HA, Puig A, Asch DA, et al. A randomized, controlled trial of financial incentives for smoking cessation. N Engl J Med. 2009;360(7):699–709. Epub 2009/02/14. doi: 10.1056/NEJMsa0806819 19213683.
43. Charness G, Gneezy U. Incentives to exercise. Econometrica. 2009;77(3):909–31.
44. Marcus AC, Kaplan CP, Crane LA, Berek JS, Bernstein G, Gunning JE, et al. Reducing loss-to-follow-up among women with abnormal Pap smears. Results from a randomized trial testing an intensive follow-up protocol and economic incentives. Med Care. 1998;36(3):397–410. Epub 1998/04/01. 9520963.
45. Malotte CK, Rhodes F, Mais KE. Tuberculosis screening and compliance with return for skin test reading among active drug users. Am J Public Health. 1998;88(5):792–6. Epub 1998/05/20. 9585747; PubMed Central PMCID: PMC1508952.
46. WHO. Technical and Operational Considerations for Implementing HIV Viral Load Testing. Geneva: WHO, 2014.
47. Snijders TA, Bosker RJ. Multilevel analysis: An introduction to basic and advanced mulitlevel modeling. Thousand Oaks, California: Sage; 1999.
48. WHO. Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV. Geneva: WHO, 2015 September 2015.
49. Tenthani L, Haas AD, Tweya H, Jahn A, van Oosterhout JJ, Chimbwandira F, et al. Retention in care under universal antiretroviral therapy for HIV-infected pregnant and breastfeeding women ('Option B+') in Malawi. AIDS. 2014;28(4):589–98. doi: 10.1097/QAD.0000000000000143 24468999; PubMed Central PMCID: PMC4009400.
50. Columbia University. The Population HIV Impact Assessment (PHIA) Project. Accessed May 31 2017 at: www.phia.icap.columbia.edu.
51. Justman J. Real Progress in the HIV Epidemic: PHIA Findings from Zimbabwe, Malawi, and Zambia. Oral Abstract. Conference of Retroviruses and Opportunistic Infections February 13–15, 2017; Seattle, WA2017.
52. Nkambule R, Nuwagaba-Biribownwoha H, Mnisi Z, Ao T, Duong Y, Patel H, et al. Substantial progress in confronting the HIV epidemic in Swaziland: first evidence of national impact. Abstract 204LB. International AIDS Society 2017; July 24, 2017; Paris, France 2017.
53. El-Sadr WM, Donnell D, Beauchamp G, Hall HI, Torian LV, Zingman B, et al. Financial Incentives for Linkage to Care and Viral Suppression Among HIV-Positive Patients: A Randomized Clinical Trial (HPTN 065). JAMA. 2017;177(8):1083–92. doi: 10.1001/jamainternmed.2017.2158 28628702.
54. Geng EH, Glidden DV, Bwana MB, Musinguzi N, Emenyonu N, Muyindike W, et al. Retention in care and connection to care among HIV-infected patients on antiretroviral therapy in Africa: estimation via a sampling-based approach. PLoS ONE. 2011;6(7):e21797. doi: 10.1371/journal.pone.0021797 21818265; PubMed Central PMCID: PMC3144217.
55. Giordano TP, Gifford AL, White AC Jr., Suarez-Almazor ME, Rabeneck L, Hartman C, et al. Retention in care: a challenge to survival with HIV infection. Clin Infect Dis. 2007;44(11):1493–9. Epub 2007/05/08. doi: 10.1086/516778 17479948.
56. Rosen S, Maskew M, Fox MP, Nyoni C, Mongwenyana C, Malete G, et al. Initiating Antiretroviral Therapy for HIV at a Patient's First Clinic Visit: The RapIT Randomized Controlled Trial. PLoS Med. 2016;13(5):e1002015. doi: 10.1371/journal.pmed.1002015 27163694.
Štítky
Interní lékařství
Článek Contemporary disengagement from antiretroviral therapy in Khayelitsha, South Africa: A cohort studyČlánek Bioequivalence of twice-daily oral tacrolimus in transplant recipients: More evidence for consensus?
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2017 Číslo 11- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Jak postupovat při výběru betablokátoru − doporučení z kardiologické praxe
- Červená fermentovaná rýže účinně snižuje hladinu LDL cholesterolu jako vhodná alternativa ke statinové terapii
-
Všechny články tohoto čísla
- Labour trafficking: Challenges and opportunities from an occupational health perspective
- The end of HIV: Still a very long way to go, but progress continues
- Contemporary disengagement from antiretroviral therapy in Khayelitsha, South Africa: A cohort study
- Bioequivalence of twice-daily oral tacrolimus in transplant recipients: More evidence for consensus?
- Treatment guidelines and early loss from care for people living with HIV in Cape Town, South Africa: A retrospective cohort study
- Perinatal mortality associated with induction of labour versus expectant management in nulliparous women aged 35 years or over: An English national cohort study
- Core Outcome Set-STAndards for Development: The COS-STAD recommendations
- Closing the gaps in the HIV care continuum
- Association between the 2012 Health and Social Care Act and specialist visits and hospitalisations in England: A controlled interrupted time series analysis
- HIV pre-exposure prophylaxis and early antiretroviral treatment among female sex workers in South Africa: Results from a prospective observational demonstration project
- Sexual exploitation of unaccompanied migrant and refugee boys in Greece: Approaches to prevention
- Child sex trafficking in the United States: Challenges for the healthcare provider
- The expanding epidemic of HIV-1 in the Russian Federation
- Cardiovascular disease (CVD) and chronic kidney disease (CKD) event rates in HIV-positive persons at high predicted CVD and CKD risk: A prospective analysis of the D:A:D observational study
- Validity of a minimally invasive autopsy for cause of death determination in maternal deaths in Mozambique: An observational study
- malERA: An updated research agenda for malaria elimination and eradication
- malERA: An updated research agenda for health systems and policy research in malaria elimination and eradication
- A combination intervention strategy to improve linkage to and retention in HIV care following diagnosis in Mozambique: A cluster-randomized study
- Bioequivalence between innovator and generic tacrolimus in liver and kidney transplant recipients: A randomized, crossover clinical trial
- malERA: An updated research agenda for basic science and enabling technologies in malaria elimination and eradication
- Human trafficking and exploitation: A global health concern
- Virological response and resistance among HIV-infected children receiving long-term antiretroviral therapy without virological monitoring in Uganda and Zimbabwe: Observational analyses within the randomised ARROW trial
- Postmenopausal hormone therapy and risk of stroke: A pooled analysis of data from population-based cohort studies
- Lansoprazole use and tuberculosis incidence in the United Kingdom Clinical Practice Research Datalink: A population based cohort
- malERA: An updated research agenda for insecticide and drug resistance in malaria elimination and eradication
- Safety, pharmacokinetics, and immunological activities of multiple intravenous or subcutaneous doses of an anti-HIV monoclonal antibody, VRC01, administered to HIV-uninfected adults: Results of a phase 1 randomized trial
- HIV prevalence and behavioral and psychosocial factors among transgender women and cisgender men who have sex with men in 8 African countries: A cross-sectional analysis
- Treatment eligibility and retention in clinical HIV care: A regression discontinuity study in South Africa
- malERA: An updated research agenda for characterising the reservoir and measuring transmission in malaria elimination and eradication
- Effectiveness of a combination strategy for linkage and retention in adult HIV care in Swaziland: The Link4Health cluster randomized trial
- The value of confirmatory testing in early infant HIV diagnosis programmes in South Africa: A cost-effectiveness analysis
- HIV self-testing among female sex workers in Zambia: A cluster randomized controlled trial
- The US President's Malaria Initiative, transmission and mortality: A modelling study
- Comparison of two cash transfer strategies to prevent catastrophic costs for poor tuberculosis-affected households in low- and middle-income countries: An economic modelling study
- Direct provision versus facility collection of HIV self-tests among female sex workers in Uganda: A cluster-randomized controlled health systems trial
- malERA: An updated research agenda for diagnostics, drugs, vaccines, and vector control in malaria elimination and eradication
- malERA: An updated research agenda for combination interventions and modelling in malaria elimination and eradication
- HIV-1 persistence following extremely early initiation of antiretroviral therapy (ART) during acute HIV-1 infection: An observational study
- Respondent-driven sampling for identification of HIV- and HCV-infected people who inject drugs and men who have sex with men in India: A cross-sectional, community-based analysis
- Extensive virologic and immunologic characterization in an HIV-infected individual following allogeneic stem cell transplant and analytic cessation of antiretroviral therapy: A case study
- Measuring success: The challenge of social protection in helping eliminate tuberculosis
- Prospects for passive immunity to prevent HIV infection
- Reaching global HIV/AIDS goals: What got us here, won't get us there
- Evidence-based restructuring of health and social care
- Extreme exploitation in Southeast Asia waters: Challenges in progressing towards universal health coverage for migrant workers
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Postmenopausal hormone therapy and risk of stroke: A pooled analysis of data from population-based cohort studies
- Bioequivalence between innovator and generic tacrolimus in liver and kidney transplant recipients: A randomized, crossover clinical trial
- HIV pre-exposure prophylaxis and early antiretroviral treatment among female sex workers in South Africa: Results from a prospective observational demonstration project
- Bioequivalence of twice-daily oral tacrolimus in transplant recipients: More evidence for consensus?
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



