-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaContemporary disengagement from antiretroviral therapy in Khayelitsha, South Africa: A cohort study
In a retrospective study, Samantha Kaplan and colleagues document the continuity of antiretroviral treatment and care in Khayelitsha, South Africa.
Published in the journal: . PLoS Med 14(11): e32767. doi:10.1371/journal.pmed.1002407
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1002407Summary
In a retrospective study, Samantha Kaplan and colleagues document the continuity of antiretroviral treatment and care in Khayelitsha, South Africa.
Introduction
With the 2015 World Health Organization (WHO) guidelines recommending treatment for all HIV-infected individuals regardless of CD4 status and the continued high HIV incidence rates in endemic areas, there are increasing numbers of patients eligible for and starting lifelong antiretroviral therapy (ART). In order for health systems to meet the UNAIDS 90-90-90 treatment targets of patients receiving sustained ART and maintaining viral suppression, retention in care is an essential focus [1]. Viral suppression reduces HIV transmission [2], and in a modeling study has been shown to contribute to the public health goal of ending the HIV/AIDS epidemic [3]. Patients who disengage from care have an increased risk of poor health outcomes, transmitting HIV to others, and developing drug resistance, thereby undermining overall program impact as well as the global public health goal of ending the HIV epidemic. In Southern Africa, as of 2014, WHO-estimated ART retention rates after 5 years to be less than 50%, and the United States President’s Emergency Plan for AIDS Relief (PEPFAR) countries in this region reported 77% retention at 12 months in 2015 [4,5].
In Khayelitsha township (population approximately 500,000) in Cape Town, South Africa, an HIV treatment program was established in 2001 as a partnership between Médecins sans Frontières and the provincial government at 3 public-sector primary care clinics. This represented the first initiative to provide ART in the South African public sector [6]. As this program has matured and grown, disengagement from ART care has become a great challenge: cumulative loss to follow-up (LTFU) at 1 year was shown to have increased from 0% in 2001 to 7.6% in 2007, and a third of patients who were LTFU by 2008 were found to have died [7,8].
Over the 15 years since the Khayelitsha ART program’s inception, clinics have grown in size considerably [9], due to the high HIV prevalence and updating of the South African National ART guidelines, which have progressively expanded the eligibility criteria for ART initiation to higher CD4 count thresholds [10]. This has resulted in a massive increase of patients eligible for ART and an increase in the proportion of patients who are starting ART when asymptomatic. ART medication regimens have also become more tolerable, and fixed-dose combination formulations have improved convenience for patients. ART adherence clubs have been established for stable ART patients on treatment for >12 months who are virally suppressed. These clubs are managed by lay health workers and meet 5 times a year, allowing ease of medication refills and communal peer support [11,12]. These changes in the ART guidelines and service provision may alter the rates of disengagement reported previously [7,8]. In the current analysis, we merged different data sources to create a unique dataset of all Khayelitsha ART patients from provincial and municipal clinics. Using these routinely collected data, we sought to quantify disengagement from care and identify risk factors and outcomes for those patients who disengaged using electronic tracing methods.
Methods
Ethics statement
This study was approved by the Yale University Human Investigation Committee (Protocol # 1504015732) and the University of Cape Town Human Research Ethics Committee (HREC REF: 568/2015) per the protocol “Sub-study of protocol ‘Enhanced routine surveillance of patients in HIV care in Khayelitsha’ (HREC 395/2005)” (S1 Protocol Original). All data were extracted from databases containing patient data collected during routine ART or healthcare visits, and therefore per the approved protocol, informed consent was not obtained from individual patients. Patient data were de-identified prior to analysis. The original plan was to conduct a nested case-control study to determine risk factors for disengagement. However, early during data collection, the decision was taken to analyze risk factors on the whole cohort using Cox proportional hazards models rather than restrict to selected controls, as we determined that we would not be able to reliably abstract additional data on potential associations and confounders that were not already routinely available (S1 Protocol Amendment).
Setting and inclusion criteria
A cohort study was conducted using data from all provincial and municipal public-sector ART clinics (n = 13) in Khayelitsha, Cape Town, South Africa. These clinics have provided ART to >50,000 patients since the program’s inception, with >30,000 patients receiving ART in 2015. The cohort is described in detail elsewhere [9]. Patients on ART in Khayelitsha constitute 17.5% of the total number on ART in the Western Cape province where treatment is provided across 250 clinics and roughly 1% of the national number of patients on ART across approximately 3,800 clinics.
The study included all patients who had at least 1 visit at a Khayelitsha ART clinic between 1 January 2013 and 31 December 2014 regardless of the date they initiated ART, provided it was prior to 31 December 2014 (Fig 1). Patients not started on ART were excluded. This date range was selected to examine a contemporary cohort, but data included historical ART data prior to 2013 for patients selected for the cohort. Adults and adolescents age ≥10 years of age by 1 January 2013 were included, as we wanted to include adolescents in our analysis of risk factors for disengagement and age 10 is the start of adolescence as defined by WHO [13].
Fig. 1. Flow diagram of study cohort. 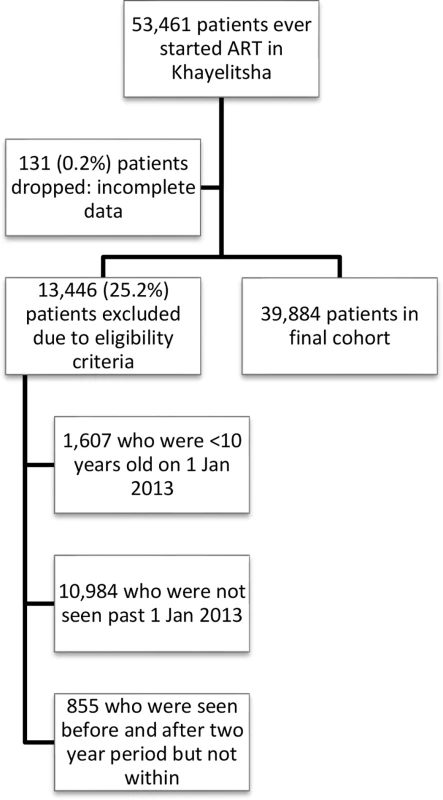
ART, antiretroviral therapy. ART eligibility, monitoring, and treatment regimens
ART eligibility criteria, patient monitoring, and treatment regimens have progressively changed since program initiation in 2001 (S1 Table). CD4 count and HIV viral load measurements were conducted at accredited National Health Laboratory Service (NHLS) laboratories.
Data collection and management
In all Khayelitsha clinics, doctors and nurses who see patients enter visit data onto structured paper clinical records, which are subsequently captured on site into an electronic patient information system by data capturers. The municipal and provincial sites use different electronic patient information systems, but data are formatted and then exported by clinics based on an internationally implemented data exchange standard for HIV treatment data [14]. All record keeping and data capture are part of routine patient management, per provincial guidelines.
Civil identification numbers, when available, were used to ascertain or confirm dates of death up to 30 June 2015. Death dates were also ascertained from clinic records, if available. Western Cape province unique health identifiers were linked with the province-wide laboratory, pharmacy, and health facility visit databases to determine outcomes for those who disengaged, if available, up to 30 June 2015, and to supplement laboratory data where these were missing until 31 December 2014.
Key definitions
Key study terms are defined below. Additional definitions can be found in S2 Table.
Disengagement: a patient not seen in an ART clinic nor having an ART-related laboratory test or ART prescription in Khayelitsha for >180 days after his or her last visit date. This definition was chosen because patients may have up to 120-day intervals between scheduled visits and to align with previous studies [15,16]. It excludes “silent transfers.”
Date of disengagement: start date of the 180-day period for which a patient was defined as disengaged.
Silent transfer: patients who met the definition for disengagement but returned to an ART or primary care clinic somewhere else in the Western Cape province without formal transfer in ≤180 days.
Origin date: The patient’s ART start date
Cohort entry date: Either 1 January 2013 or the date of ART start or reentry into care if after 1 January 2013
Data analysis
Data were analyzed using STATA/SE version 14.0 (StataCorp, College Station, TX, USA).
The cohort analysis was conducted in two parts: 1) analysis of time to disengagement from care in the cohort and analysis of risk factors for disengagement, using cumulative incidence curves and Cox proportional hazards models; and 2) for patients who disengaged from care, a description of outcomes after disengagement and times to these outcomes.
1) Cumulative incidence estimates and Cox proportional hazards model for disengagement
Entry into the study was at the beginning of 2013 for those who started ART prior to the analysis window, or at ART initiation if after 1 Jan 2013. Time to disengagement (failure) was defined as time from date of study entry to date of first disengagement within the window of 1 January 2013–31 December 2014 (Fig 2). Time origin was study entry and, in a second analysis, first ART start date, allowing for delayed entry into the survival analysis (Fig 3A). Date of disengagement was defined as the first date of the ≥180-day period that the patient was not in care. While survival time (until disengagement) was only calculated through 31 December 2014, the database was closed at 30 June 2015 to allow disengagement ascertainment (i.e., the opportunity to meet the disengagement definition) for those whose last visit was in late 2014. Therefore, if a patient disengaged prior to 1 January 2013, but returned to care prior to 1 January 2013, time to disengagement was calculated from their cohort entry date (or ART start date, depending on the analysis) until a date of disengagement within the cohort window if this occurred. Such a patient was also classified as “previously disengaged” at study entry. If a patient disengaged prior to 1 January 2013 but returned to care within the study window, the entry date was indicated as the date they reentered care within the study window and they were also classified as a patient who “previously disengaged.” For transferring patients (identified from clinical records, or by data clerks at the receiving clinics), if they transferred out prior to the study window but reentered within the study window, their entry date was their date of reentry but their origin date remained their ART start date. S1 Fig illustrates several examples of patients’ contribution to survival time. Possible outcomes for patients as of 31 December 2014 were 1) alive and in care; 2) dead; 3) transferred out; or 4) disengaged. The primary outcome was time to disengagement during the 2-year window of analysis. Secondary analyses focused on risk factors associated with disengagement.
Fig. 2. Cumulative incidence (competing risks analysis) of disengagement, transfer (including silent transfers), and mortality, as estimated by a flexible parametric survival model based on time to disengagement from cohort entry date to 31 December 2014. 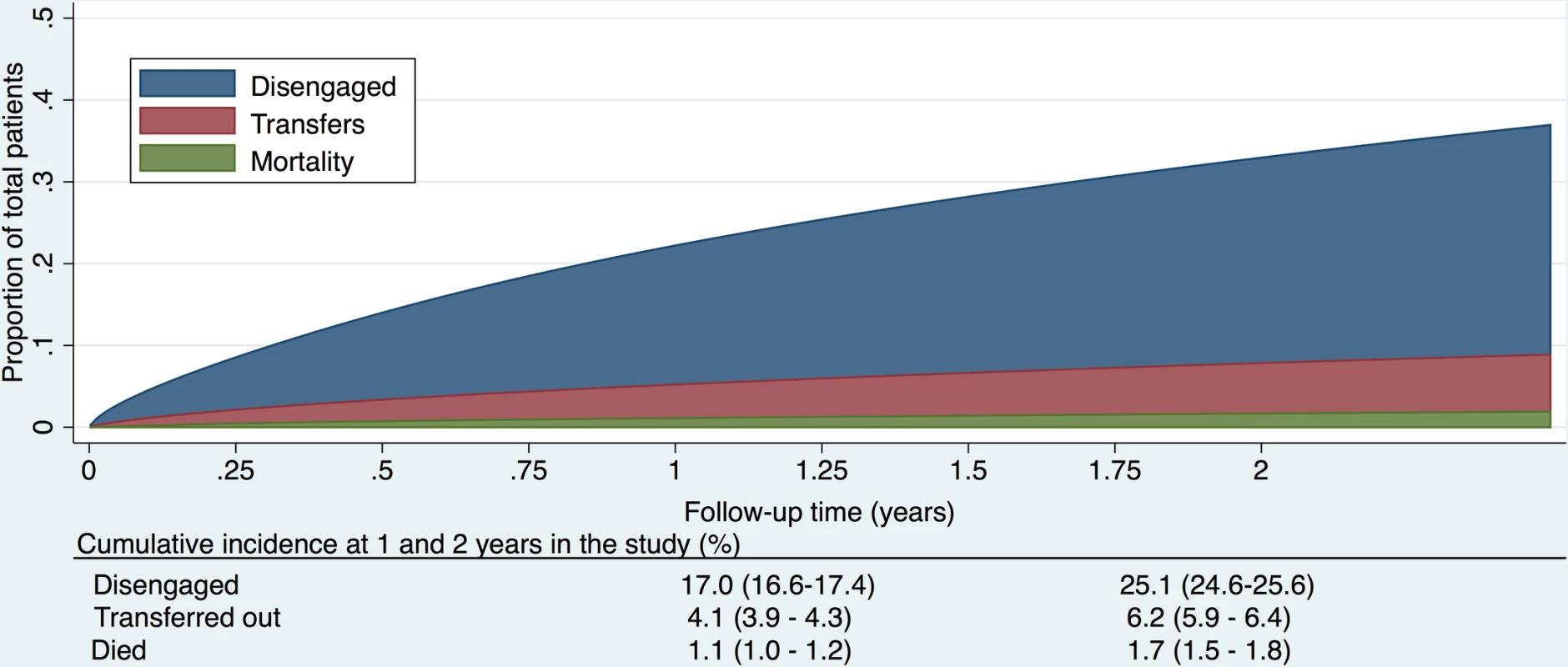
Fig. 3. 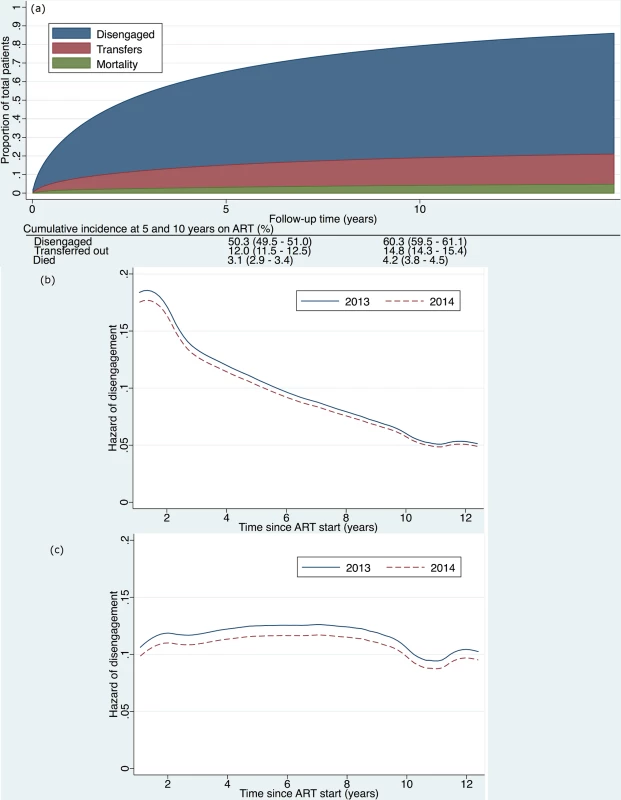
<b>(a) Cumulative incidence (competing risks analysis) of disengagement, transfer (including silent transfers), and mortality, as estimated by a flexible parametric survival model based on time contributed during a 2-year period, but analyzed relative to ART initiation date, allowing for delayed entry into the analysis. (b) Hazard of disengagement by duration since ART start, with calendar year (2013 versus 2014) fitted as a time-varying covariate in a Cox proportional hazards model. (c) Hazard of disengagement by duration since ART start, with calendar year (2013 versus 2014) fitted as a time-varying covariate in a Cox proportional hazards model adjusted for the patient characteristics listed in <em class="ref">Table 2</em>.</b> ART, antiretroviral therapy. For patients who officially transferred out within the study window, if they returned to care in Khayelitsha ≤180 days later, we ignored the transfer outcome and followed them until their next outcome (disengagement, transfer, death, or alive and in care). If the gap was >180 days (or they returned to care outside of Khayelitsha at any point), we classified them as transfer out and censored them at the transfer date. Silent transfers were censored on their last visit date in Khayelitsha. They were then reclassified as not disengaged. If their gap in care was >180 days, the patient remained classified as disengaged.
A patient was censored if they a) died (censored on death date); b) transferred (censored on transfer date); or c) were alive without disengagement at study end (administrative censor, on 31 December 2014). If a patient appeared in the National Death Registry within 90 days of a presumed date of disengagement, then they were reclassified to a death and not a patient who had disengaged. If a patient was seen in the cohort window for only 1 visit, we added 1 day to their outcome date so that they were included in the survival analysis [16].
Descriptive analysis of cumulative incidence (competing risks analysis). Cumulative incidence curves were estimated for each of disengagement, transfer, and death, with the other events treated as competing events based on person time contributed during the analysis window, but analyzed from time since 1) entry into the study or 2) ART initiation. Death was a competing risk if it occurred ≤90 days after a date of disengagement or was the last outcome for a patient who had not disengaged prior to 31 December 2014. Transfer was a competing risk if it was the last outcome for a patient who had not disengaged prior to 31 December 2014, and included silent transfers. To estimate the hazard of disengagement over time, calendar time captured as year (2013 or 2014) was included as a time-varying covariate in the survival analysis. Follow-up time was split into 2 records for patients with follow-up time in both years. The underlying hazard was estimated following the fitting of the Cox models with and without adjustment for covariates.
Model creation and testing. Variables (decided upon a priori) were entered into a multivariable Cox proportional hazards model to determine risk factors for the hazard of disengagement. Cox proportional hazards assumptions (PHAs) were tested graphically for each variable. If the PHA was not met, the model was stratified with respect to the respective variable.
Missing data. We used imputation by chained equations [17] to impute missing data for TB, weight, club participation, CD4 count, HIV viral load, and drug regimens 5 times. The imputation model included all measured variables, used predictive mean matching for variables with skewed distributions (CD4 count, log HIV viral load), and used multinomial logistic regression for binary and categorical variables. The primary modeling results are based on the imputed data. In secondary analyses, we report results for a complete case analysis excluding missing data, as well as an imputed model restricted to patients with national identification numbers, which permitted linking to the National Death Registry.
2) Disengagement outcome analysis
This analysis included only those who disengaged, and analyzed their subsequent outcomes after the first date of disengagement. For purposes of determining outcomes, the date of administrative censoring was extended to 30 June 2015. All outcomes after disengagement were ascertained from Western Cape province electronic data systems and the National Death Registry. Possible primary outcomes for those who disengaged were: 1) death; 2) return to care at a different facility within 180 days (silent transfer); 3) return to care after 180 days; 4) hospital admission; 5) other hospital contact (outpatient or emergency visit); 6) alive on 30 June 2015 if they had a national identification number but no death date was found in the National Death Registry; or 7) no information, still disengaged. Primary outcomes were identified as the first of these outcomes after the date of disengagement. Secondary outcomes were time to return to care and time to death after disengagement. Overall deaths and hospitalizations at any point post disengagement until 30 June 2015 were also calculated. We conducted a sensitivity analysis of disengagement outcomes for those with national identification numbers to restrict analyses to only those with reliable vital status ascertainment.
Geographic Information System (GIS) analysis. GIS mapping and analysis were conducted using ArcGIS Online based on official facility coordinates in order to map the locations at which silent transfers and those who disengaged returned to care.
Results
Cohort characteristics
A total of 53,461 patients initiated ART at any Khayelitsha site since program inception through 31 December 2014. For the cohort study, we excluded 11,839 patients who did not have a visit in the time period between 1 January 2013 and 31 December 2014, and 1,607 who were less than 10 years old at 1 January 2013. An additional 131 were excluded due to incomplete data (Fig 1). Of the 39,884 patients remaining (Table 1), the median follow-up from ART start date to 31 December 2014 was 33.6 months (IQR 12.4–63.2). A total of 25,864 patients started ART prior to 1 January 2013 (contrasted with those who started after this date in S3 Table). We also indicate the proportion of patients with missing data for each variable (Table 1, S3 Table).
Tab. 1. Characteristics of patients who did and did not disengage from care. 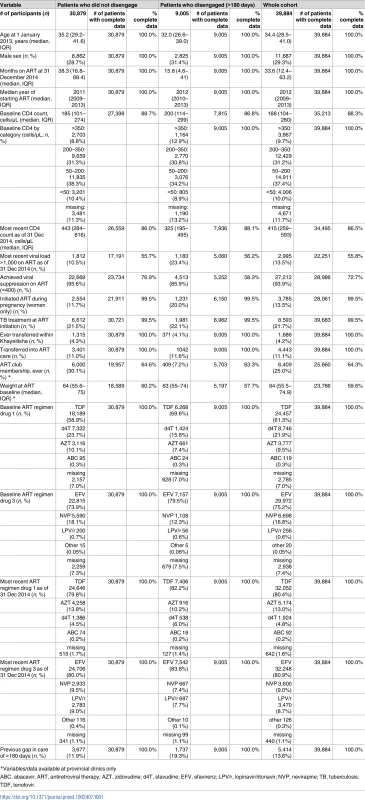
*Variables/data available at provincial clinics only Disengagement from care and other outcomes during the two-year study period
From the perspective of the Khayelitsha clinic system, a total of 9,992 (25.1%) patients disengaged from care. However, after linkage of these patients to Western Cape data systems, we found that patients who disengaged (excluding those who silently transferred) numbered 9,005 (22.6%). From this point on in the manuscript, “patients who disengaged” refers to those who disengaged, excluding silent transfers.
As of 31 December 2014, of the total cohort, 592 (1.5%) died, 1,231 (3.1%) transferred out, 987 (2.5%) were silent transfers and visited another ART or primary care clinic in the same province (Western Cape) within 180 days of their last visit date, 9,005 (22.6%) disengaged, and 28,069 (70.4%) were in care. Of those in our study cohort, 1,179 (3.0%) patients disengaged prior to 2013 but returned to care prior to 1 January 2013. 4,156 (10.4%) disengaged prior to 2013 but returned within the study window.
Of the patients who disengaged, 5,463 (60.7%) had South African national identification numbers and could be linked to the National Death Registry. Total mortality for the entire cohort as of 30 June 2015 was 2.4% (n = 939), and 3.9% (n = 822) when restricted to those with national identification numbers.
The cumulative incidence of disengagement from care, before any other event could occur, was estimated to be 25.1% at 2 years, analyzed by time in the study (Fig 2). The cumulative incidence of disengagement was 50.3% and 60.3% at 5 and 10 years on ART, respectively (Fig 3A), based on the person time contributed during the analysis window but analyzed relative to ART initiation date. The higher hazard of disengagement soon after starting ART was largely attenuated after adjusting for the patient characteristics included in Table 2, without evidence of a temporal effect comparing 2013 to 2014 (Fig 3B and 3C).
Factors associated with disengagement
The strongest adjusted associations with disengagement were most recent CD4 count <350 cells/μl (CD4 200–350 hazard ratio (HR) 2.03; 95% CI 1.91–2.15; CD4 50–200 HR 3.07; 95% CI 2.84–3.31; CD4 <50 HR 3.34; 95% CI 2.92–3.83, all relative to CD4 > 350), use of d4T (stavudine) at last visit (HR 1.72; 95% CI 1.57–1.89), and pregnancy at ART start (HR 1.58; 95% CI 1.47–1.69) (Table 2).
Tab. 2. Cox proportional hazards model for risk of disengagement, univariate and multivariable, imputed. 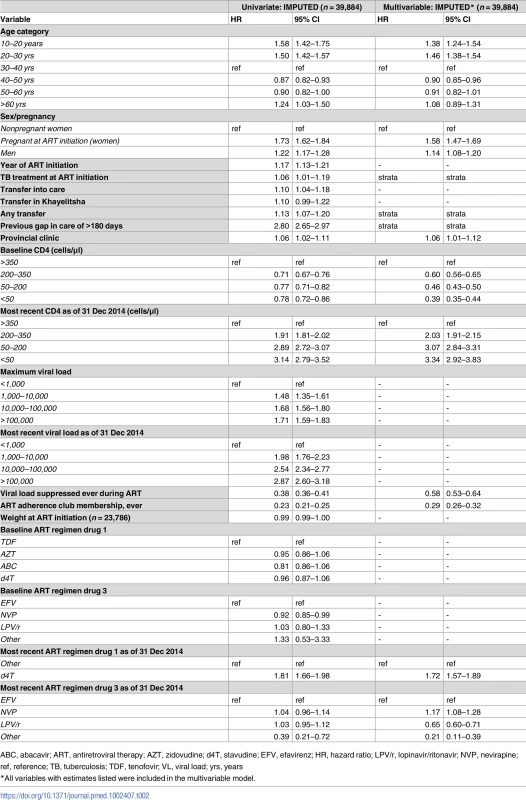
ABC, abacavir; ART, antiretroviral therapy; AZT, zidovudine; d4T, stavudine; EFV, efavirenz; HR, hazard ratio; LPV/r, lopinavir/ritonavir; NVP, nevirapine; ref, reference; TB, tuberculosis; TDF, tenofovir; VL, viral load; yrs, years Other associations included younger age group (<30 years) and male sex. The factors associated with retention were ART adherence club membership (HR 0.29; 95% CI 0.26–0.32), a suppressed HIV viral load at any point during ART (HR 0.58; 95% CI 0.53–0.64), and a baseline CD4 count <350 cells/μl (CD4 200–350 HR 0.60; 95% CI 0.56–0.65; CD4 50–200 HR 0.46; 95% CI 0.43–0.50; CD4<50 HR 0.39; 95% CI 0.35–0.44, all relative to CD4 > 350) (Table 2). Post-imputation distributions for variables with skewed distributions were compared with observed distributions and were acceptable (S2 Fig). Proportional hazard assumptions for imputed variables were also met, with only a small deviation for long follow-up for ART regimen drug 3 (S3 Fig). Sensitivity analyses restricted to complete data or to patients with national identification numbers did not materially alter these associations (S4 Table).
Outcomes after disengagement
Of those who disengaged (n = 9,005), the median length of follow-up from date of disengagement to 30 June 2015 was 16.7 months (IQR 11–22.4). 7,061 (78.4%) of those who disengaged could be linked using a national identification number or medical record number for outcomes analysis. The 2 most common first outcomes were return to ART care after 180 days (33%; n = 2,976), followed by alive but not in care (25%; n = 2,255; valid national identification number allowing ascertainment of vital status, but no other information) (Table 3). Because only 60.7% (n = 5,463) of those who disengaged had valid national identification numbers that could be linked to the National Death Registry, we conducted a sensitivity analysis of only these patients (Table 3). In this analysis, 75% (n = 4,125) had either returned to care (34%; n = 1,877) or remained disengaged but were known to be alive (41%; n = 2,248), with the remainder having been admitted or seen as an outpatient or emergency visit at a hospital (24%; n = 1,280) or having died (1%; n = 58) as their first outcome. Not restricting to the first outcome, 3% (n = 237) of those who disengaged died at any point during subsequent follow-up, and 16% (n = 1,459) were admitted to the hospital (S5 Table).
Tab. 3. Initial outcomes for patients who disengaged, as ascertained from Western Cape province data systems until 30 June 2015. 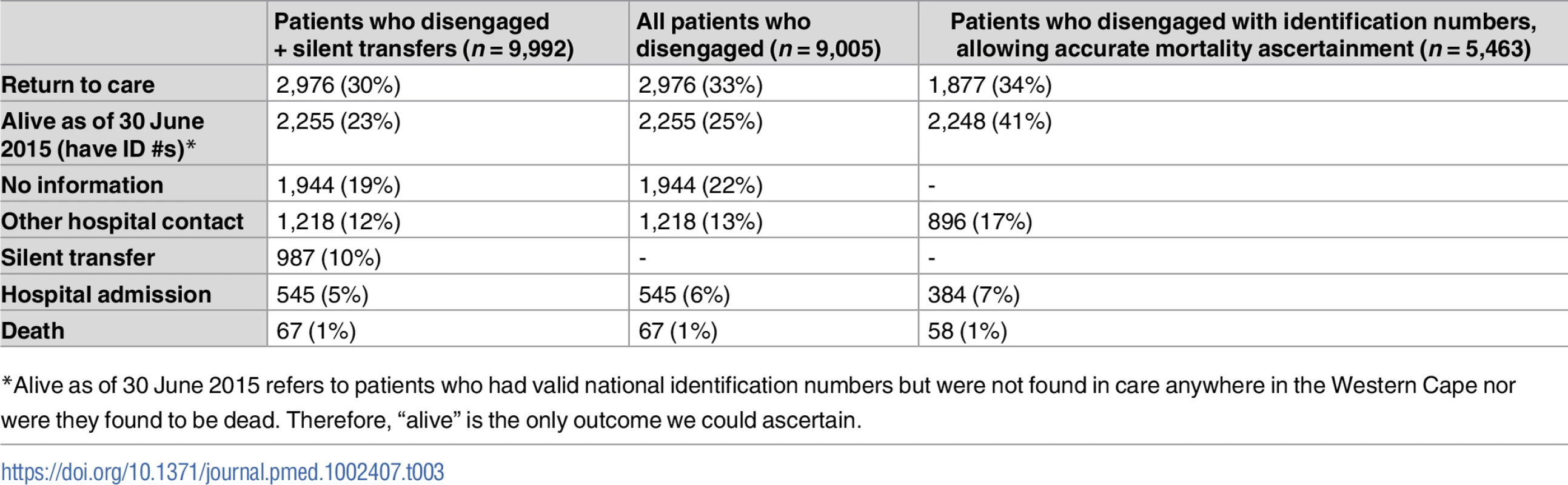
*Alive as of 30 June 2015 refers to patients who had valid national identification numbers but were not found in care anywhere in the Western Cape nor were they found to be dead. Therefore, “alive” is the only outcome we could ascertain. A Kaplan-Meier analysis of time to returning to care for patients who disengaged estimated that approximately 50% of patients who disengage will return to care by 2.5 years (S4A Fig). A Kaplan-Meier analysis for time to death after disengagement, restricted to patients with valid identification numbers, estimated that >90% of patients were alive at 2.5 years post-disengagement (S4B Fig). For those patients who silently transferred or disengaged and then returned to care >180 days later, GIS mapping indicated that most of these patients returned to care very close to Khayelitsha (some at the same clinic), but many also returned to a variety of locations throughout the Western Cape province (Fig 4). Median distance from Khayelitsha to clinic of return was 3.8 km (IQR 2.7–9.6, range 0.3–434), an estimate that includes patients who returned to the same clinic after 180 days. Seven percent (n = 207) returned >50km from Khayelitsha, but within the Western Cape province. In a logistic regression model, some of the associations with failure to re-engage in care after disengagement were the same as those associated with disengagement: male sex (OR 1.16; 95% CI 1.04–1.29) and pregnancy at ART start (OR 1.36; 95% CI 1.15–1.61). Older age was associated with failure to return (>60 years; OR 1.76; 95% CI 1.11–2.78). However, younger age and CD4 count were not associated with failure to return to care (S6 and S7 Tables).
Fig. 4. Map of Western Cape province indicating clinics where silent transfers and patients who disengaged returned to care until 30 June 2015. 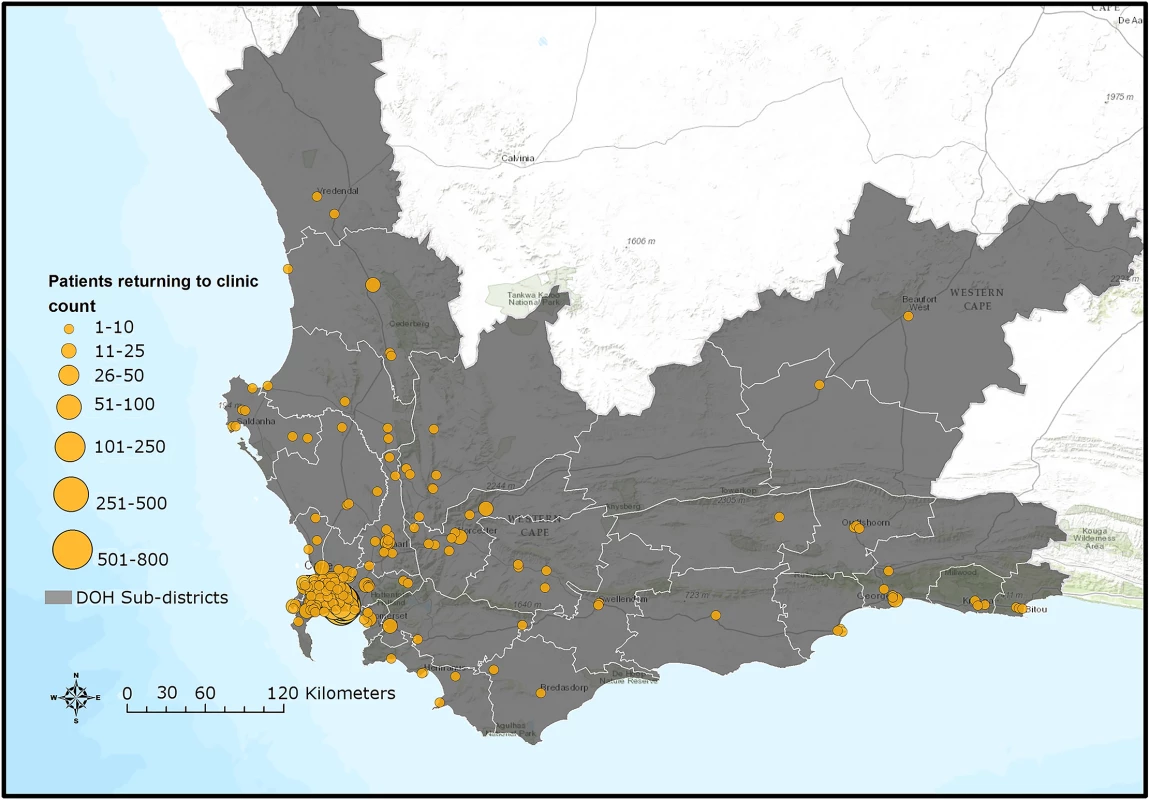
DOH, Department of Health. Sources: Basemap: Used with permission. Copyright 2017 Esri, ArcGIS Desktop, Western Cape Department of Health, and the GIS User Community. CC BY 4.0. South African Department of Health Layer: Boundaries of the Department of Health Sub-districts falling under the authority of the Western Cape Government and the City of Cape Town municipality (data provided June 2016 by Deputy Director, Information Management Health, Western Cape Government, South Africa). South African public health facilities layer: Locations of facilities falling under the authority of the Western Cape Government and the City of Cape Town. Includes some Inactive facilities as listed in the operational Sinjani system (data provided June 2016 by Deputy Director, Information Management Health, Western Cape Government, South Africa). Software: ESRI 2016. ArcGIS Desktop: version 10.4. Redlands, CA: Environmental Systems Research Institute. Discussion
In this study, we examined disengagement from ART care during 2013–2014 among patients of the large, peri-urban cohort in Khayelitsha—one of the oldest public-sector ART cohorts in South Africa. Roughly 1 in 5 patients disengaged from care, demonstrating a high rate of disengagement and a key challenge to reaching the UNAIDS 90-90-90 treatment targets. Factors associated with disengagement were age <30 years, male sex, pregnancy at ART initiation, and last CD4 count <350 cells/μl. Factors associated with retention were ART adherence club membership and baseline CD4 <350 cells/μl. However, despite the high incidence of disengagement, many of those who disengaged did not do so permanently. While 48% (n = 4,199) of patients could not be traced (either did not have a national identification number or had an ID number and/or medical record number but no additional data were found), and 16% (n = 1,459) were admitted to the hospital at some point after disengagement, roughly 1 in 3 patients returned to care within the province during the study period, and half were estimated to return to care within 2.5 years. Additionally, not included in the overall estimate of disengagement are the 2.5% of silent transfers who appeared to disengage from a clinic perspective but were actually in care elsewhere in the Western Cape province when province-wide data linkage was performed. These data indicate that a substantial proportion of patients are cycling in and out of care as well as transferring elsewhere in the province (often “silently”), and potentially to facilities outside of the Western Cape province (something that our study could not ascertain).
Disengagement in Khayelitsha
Previous studies of the Khayelitsha cohort have reported on disengagement (termed “lost to follow-up, LTFU”, in those studies) and mortality up until 2009 [7,8,18]. These studies are not directly comparable, as they calculated LTFU at the end of a defined period allowing for prior return to care, as opposed to the current study, in which we defined disengagement as the first episode in a 2-year period. In doing so, we report a contemporary cross-sectional perspective of disengagement rather than a cumulative snapshot. Temporal trends based on the former approach have further been shown to be biased [19]. Further, the current study accounted for silent transfers and mortality through data linkage, whereas the former studies only accounted for mortality. Nevertheless, the most recent of the previous studies estimated cumulative LTFU of 23% at 5 years on ART in 2008 [8], compared to the current contemporary estimate of 25% meeting the definition of LTFU at least once in a 2-year period, irrespective of duration on ART or previous disengagement. When extrapolated, and assuming that this incidence is unchanged over time and is homogeneously distributed, this would mean that 50% of patients would meet this definition by 5 years on ART. In spite of improved ascertainment of patients transferring their care, the study provides continued and robust evidence of high rates of disengagement, underscoring the importance of interventions targeted primarily at retention in continuous ART care across a platform or jurisdiction.
Risk factors for disengagement
Our assessment of risk factors associated with disengagement was limited to those in the Khayelitsha database. Male sex and pregnancy at ART initiation were strongly associated with disengagement, consistent with previous studies [7,20–25]. While d4T—with its high risk for drug toxicity [26,27]—was associated with increased risk of disengagement in our study, the effect is potentially confounded, as d4T was only prescribed earlier in the cohort period. Nonetheless, associations with ART toxicity and disengagement have been reported, indicating that ART drugs that minimize toxicities may decrease the risk of disengagement [28].
Our results indicate that a baseline CD4 count <350 cells/μl was associated with retention, and that a most recent CD4 count <350 cells/μl was predictive of disengagement. While these findings could suggest that those who are less sick early in treatment are more likely to disengage in line with previous studies [22,29,30], this might be confounded by non-CD4-based clinical indications for ART initiation, as clinical guidelines at the time did not provide for universal eligibility of patients with CD4 counts in this range.
We also found an association with retention for adherence club membership in Khayelitsha—which is confounded by indication—as more stable, engaged patients (on ART for >12 months, virally suppressed) are referred to adherence clubs. Twenty-six percent of patients in the entire cohort were in clubs by June 2014, and recent data have shown that adherence club membership is associated with a 67% reduction in LTFU [9,31].
We found that the higher hazard of disengagement soon after starting ART seemed to be accounted for by other risk factors in that this was no longer present in adjusted analyses (Fig 3C). Other studies have suggested that there is a higher risk of disengagement early in ART treatment [24,32], but they did not adjust for other risk factors as we did in our analysis. This lends support to the conclusion that the high early loss to ART care may be accounted for by key demographic and clinical patient characteristics such as age, sex, pregnancy, and CD4 count.
Silent transfers and return to care: Cyclical engagement
The combination of high proportions of patients being silent transfers or returning to care after disengagement in this setting casts the challenge as cycling in and out of care and between facilities, rather than as definitive losses to care. This represents a major shift in the way ART care is now being delivered. Many of these patients spent substantial time out of care and were likely viremic. This cyclical engagement undermines the ultimate goals of the UNAIDS 90-90-90 agenda because during these interruptions in care, HIV may progress in individuals, and transmission of HIV to others is more likely.
In the current analysis, the 10% of disengagements that were in fact silent transfers underscore the relative importance of undocumented transfer being a reason for apparent disengagement. While the estimate may sound lower than previous findings of this figure, which is closer to 20% in the Western Cape [33] and globally [34], we required that the patient transfer be to a site outside of Khayelitsha in order to exclude transfers within the subdistrict and that the transfer be within 6 months of the last visit.
In terms of return to care, one study in the Cape Town area found that 33% of those who disengaged returned to care with the probability of resuming within 3 years of 42%, a median of 228 days after disengagement [22], very similar to the findings in the current study. In terms of mobility issues, a rural South African cohort study found that 32% of those LTFU had migrated outside of the area after disengagement [29]. Indeed, data from Khayelitsha and the neighboring Mitchell’s Plain district have indicated that approximately 84% of black South African adults were born outside of Cape Town, the majority in the Eastern Cape, and these people frequently travel to visit their families in that province or travel between the provinces for employment or annual vacation [35–37].
However, half of patients who disengaged in this study did not return to care in the Western Cape province. Over 15% of the patients in this study were admitted to a hospital after disengagement, and 3% died. These adverse events could illustrate the potential clinical consequences of disengagement with associated healthcare costs. Other studies have illustrated such problems that arise when patients disengage from ART care: one study indicated that HIV contributed to over 60% of medical admissions to a South African district hospital in Cape Town from 2012–2013, and 19.3% of these HIV-infected patients had interrupted ART therapy [38]. Another study from Johannesburg indicated that 10.4% of patients LTFU were hospitalized [28].
Strengths and study implications
The Khayelitsha ART program is one of the largest and oldest public-sector ART programs in South Africa, lending credibility to our findings and conclusions. Additionally, we focused on a contemporary cohort, which allows us to draw conclusions that are applicable to current ART programs across the country, as well as in the region. The ability to track patients using a unique identifier across different health services and laboratory data, and link patients to the National Death Registry for mortality ascertainment [39], are distinctive within sub-Saharan Africa. This enabled us to provide robust estimates of true disengagement and reassurance that almost half of patients originally seen by the clinic as lost to follow-up are indeed either retained in care or returned to care. A study of the National Death Registry indicated that 94% of deaths were recorded by civil registration, lending support to the notion that those who we reported as truly disengaged from care and not registered as dead are most likely alive [8,39].
Potential interventions to improve patient tracking and retention, as well as provide a greater degree of flexibility in the system may include: rational use of patient held cards/records (which exist in Khayelitsha but are not always utilized), nationally accessible electronic health records, increasing the period for ART prescription to allow patient travel, editing clinical management protocols to include sections on transferring and receiving patients, improving the ability of clinics to communicate with each other and, perhaps most importantly, accepting that patients cycle through care and promoting healthcare worker understanding of the need to adapt care around patients’ lifestyles and mobility through health worker training [37].
Limitations
We likely underestimate true silent transfer and return to care, as we only tracked patients in the Western Cape province. Because of the mobility of this population, it is likely that additional patients returned to care in other provinces, particularly the Eastern Cape. If person-level data were cascaded up to the national level in line with recommendations [14], and a unique health identifier was used nationally, it may be possible to track patient movements country-wide. Implementation of such a system has already been proposed by the South African National Department of Health [40]. We do recognize that patients who migrated from other countries may have poorer treatment outcomes due to mobility and legal status issues and would be less likely to have a national identification number and be in the death registry. Therefore, we may have underestimated mortality in this group.
We had a short period for follow-up. We were also unable to ascertain causes of death. As we included only patient follow-up from 2013–2014 by design in order to maximize the generalizability of our findings to current programs, this limited our ability to more fully describe temporal trends in first disengagement. Additionally, we recognize the nonuniform collection of data, which necessitated dropping particular variables from our analyses and performing multiple imputation for missing data. It is also possible that some clinic and/or lab visits were not captured in the database. This is somewhat mitigated by the large size of the dataset and power of the analysis.
Finally, the legacy of South African apartheid has contributed to specific mobility issues and social problems in communities such as Khayelitsha that may not be generalizable to other urban settings in sub-Saharan Africa.
Conclusions
The Khayelitsha ART program is one of the oldest in South Africa and has grown greatly in size. The merging of all provincial and municipal clinic data in this study is unique and lends completeness to the cohort data as representative of an entire community. The ability to electronically trace patients throughout the Western Cape province and link to the National Death Registry allowed us to correct misclassified patient outcomes. For these reasons, the findings from this study are of value in directing appropriate patient and clinic-based interventions to improve long-term retention and meet the 90-90-90 HIV treatment targets in high prevalence urban settings such as this one throughout sub-Saharan Africa.
Our finding that many patients who disengaged return to care suggests that many patients in ART programs in Africa, particularly in urban settings, cycle in and out of care. This suggests a shift in the provision of ART care: that the linear UNAIDS model of HIV diagnosis, ART initiation, and viral suppression may need to be reconceptualized to account for this cycling in and out of care and the mobile populations served by ART programs in many sub-Saharan African countries. As “Universal Test and Treat” is implemented in both South Africa and the rest of sub-Saharan Africa, more patients will enroll in ART care with higher CD4 counts and need to be retained. It will be important to find ways to adapt services to accommodate mobile populations to retain patients in care and prevent morbidity, mortality, and HIV transmission. Even if these systems are slow to improve, clinics across sub-Saharan Africa that are familiar with and accommodating of the mobility of their patients will be able to better care and advocate for them.
Supporting Information
Zdroje
1. UNAIDS. 90-90-90: An ambitious target to help end the AIDS epidemic 2014. Available from: http://www.unaids.org/en/resources/documents/2014/90-90-90.
2. Rodger AJ, Cambiano V, Bruun T, Vernazza P, Collins S, van Lunzen J, et al. Sexual activity without condoms and risk of HIV transmission in serodifferent couples when the HIV-positive partner is using suppressive antiretroviral therapy. JAMA. 2016;316(2):171–81. doi: 10.1001/jama.2016.5148 27404185
3. Stover J, Bollinger L, Izazola JA, Loures L, DeLay P, Ghys PD, et al. What Is Required to End the AIDS Epidemic as a Public Health Threat by 2030? The Cost and Impact of the Fast-Track Approach. PLoS One. 2016;11(5):e0154893. doi: 10.1371/journal.pone.0154893 27159260
4. World Health Organization. Global Health Sector Response to HIV, 2000–2015: Focus on Innovations in Africa: Progress report 2015 [updated 2015]. Available from: http://www.who.int/hiv/pub/progressreports/2015-progress-report/en/.
5. PEPFAR. PEPFAR 2016 Annual Report to Congress 2016. Available from: https://www.pepfar.gov/documents/organization/253940.pdf.
6. Garone D, Hilderbrand K, Boulle A, Coetzee D, Goemaere E, Van Cutsem G, et al. Khayelitsha 2001–2011 : 10 years of primary care HIV and TB programmes. South Afr J HIV Med. 2011;12(4):33. doi: 10.4102/hivmed.v12i4.170
7. Van Cutsem G, Ford N, Hildebrand K, Goemaere E, Mathee S, Abrahams M, et al. Correcting for mortality among patients lost to follow up on antiretroviral therapy in South Africa: a cohort analysis. PLoS One. 2011;6(2):e14684. doi: 10.1371/journal.pone.0014684 21379378
8. Boulle A, Van Cutsem G, Hilderbrand K, Cragg C, Abrahams M, Mathee S, et al. Seven-year experience of a primary care antiretroviral treatment programme in Khayelitsha, South Africa. AIDS. 2010;24(4):563–72. doi: 10.1097/QAD.0b013e328333bfb7 20057311
9. Stinson K, Goemaere E, Coetzee D, van Cutsem G, Hilderbrand K, Osler M, et al. Cohort Profile: The Khayelitsha antiretroviral programme, Cape Town, South Africa. Int J Epidemiol. 2016;2016 : 1–9. doi: 10.1093/ije/dyw057 27208042
10. National Department of Health RoSA. National Consolidated Guidelines for the Prevention of Mother-To-Child Transmission of HIV (PMTCT) and the Management of HIV in Children, Adolescents, and Adults. 2015. Available from: https://aidsfree.usaid.gov/sites/default/files/tx_south-africa_pmtct_2015.pdf
11. Luque-Fernandez MA, Van Cutsem G, Goemaere E, Hilderbrand K, Schomaker M, Mantangana N, et al. Effectiveness of patient adherence groups as a model of care for stable patients on antiretroviral therapy in Khayelitsha, Cape Town, South Africa. PLoS One. 2013;8(2):e56088. doi: 10.1371/journal.pone.0056088 23418518
12. Wilkinson L, Harley B, Sharp J, Solomon S, Jacobs S, Cragg C, et al. Expansion of the Adherence Club model for stable antiretroviral therapy patients in the Cape Metro, South Africa 2011–2015. Trop Med Int Health. 2016;21(6):743–9. doi: 10.1111/tmi.12699 27097834
13. World Health Organization. Adolescent health 2017 [cited 2017 5 May]. Available from: http://www.who.int/topics/adolescent_health/en/.
14. Osler M, Hilderbrand K, Hennessey C, Arendse J, Goemaere E, Ford N, et al. A three-tier framework for monitoring antiretroviral therapy in high HIV burden settings. JIAS. 2014;17 : 18908. doi: 10.7448/ias.17.1.18908 24780511
15. Chi BH, Yiannoutsos CT, Westfall AO, Newman JE, Zhou J, Cesar C, et al. Universal definition of loss to follow-up in HIV treatment programs: a statistical analysis of 111 facilities in Africa, Asia, and Latin America. PLoS Med. 2011;8(10):e1001111. doi: 10.1371/journal.pmed.1001111 22039357
16. Grimsrud AT, Cornell M, Egger M, Boulle A, Myer L. Impact of definitions of loss to follow-up (LTFU) in antiretroviral therapy program evaluation: variation in the definition can have an appreciable impact on estimated proportions of LTFU. J Clin Epidemiol. 2013;66(9):1006–13. doi: 10.1016/j.jclinepi.2013.03.013 23774112
17. White IR, Royston P, Wood AM. Multiple imputation using chained equations. Stat Med. 2011;30 : 377–99. doi: 10.1002/sim.4067 21225900
18. Medicines Sans Frontieres, Western Cape Province Department of Health, City of Cape Town Department of Health, University of Cape Town, Centre for Infectious Disease Epidemiology and Research. Khayelitsha 2001–2011 Activity Report: 10 Years of HIV/TB Care at Primary Health Care Level. South Africa: 2011. Available from: http://www.msf.org/en/article/khayelitsha-activity-report-2001-2011-10-years-hivtb-care-primary-health-care-level
19. Johnson LF, Estill J, Keiser O, Cornell M, Moolla H, Schomaker M, et al. Do Increasing Rates of Loss to Follow-up in Antiretroviral Treatment Programs Imply Deteriorating Patient Retention? Am J Epidemiol. 2014;180(12):1208–12. doi: 10.1093/aje/kwu295 25399412
20. Tenthani L, Haas AD, Tweya H, Jahn A, van Oosterhout JJ, Chimbwandira F, et al. Retention in care under universal antiretroviral therapy for HIV-infected pregnant and breastfeeding women ('Option B+') in Malawi. AIDS. 2014;28(4):589–98. doi: 10.1097/QAD.0000000000000143 24468999
21. Wang B, Losina E, Stark R, Munro A, Walensky RP, Wilke M, et al. Loss to follow-up in a community clinic in South Africa—roles of gender, pregnancy and CD4 count. S Afr Med J. 2011;101(4):253–7. 21786730
22. Kranzer K, Lewis JJ, Ford N, Zeinecker J, Orrell C, Lawn SD, et al. Treatment interruption in a primary care antiretroviral therapy program in South Africa: cohort analysis of trends and risk factors. JAIDS. 2010;55(3):e17–23. doi: 10.1097/QAI.0b013e3181f275fd 20827216
23. Kaplan R, Orrell C, Zwane E, Bekker LG, Wood R. Loss to follow-up and mortality among pregnant women referred to a community clinic for antiretroviral treatment. AIDS. 2008;22(13):1679–81. doi: 10.1097/QAD.0b013e32830ebcee 18670232
24. Boyles TH, Wilkinson LS, Leisegang R, Maartens G. Factors influencing retention in care after starting antiretroviral therapy in a rural South African programme. PLoS One. 2011;6(5):e19201. doi: 10.1371/journal.pone.0019201 21559280
25. Myer L, Zulliger R, Bekker L-G, Abrams E. Systemic delays in the initiation of antiretroviral therapy during pregnancy do not improve outcomes of HIV-positive mothers: a cohort study. BMC Pregnancy Childbirth. 2012;12.
26. Magula N, Dedicoat M. Low dose versus high dose stavudine for treating people with HIV infection. Cochrane Database Syst Rev. 2015;1:Cd007497. doi: 10.1002/14651858.CD007497.pub2 25627012
27. van Oosterhout JJ, Mallewa J, Kaunda S, Chagoma N, Njalale Y, Kampira E, et al. Stavudine toxicity in adult longer-term ART patients in Blantyre, Malawi. PLoS One. 2012;7(7):e42029. doi: 10.1371/journal.pone.0042029 22848696
28. Dalal RP, Macphail C, Mqhayi M, Wing J, Feldman C, Chersich MF, et al. Characteristics and outcomes of adult patients lost to follow-up at an antiretroviral treatment clinic in Johannesburg, South Africa. JAIDS. 2008;47(1):101–7. doi: 10.1097/QAI.0b013e31815b833a 17971708
29. Mutevedzi PC, Lessells RJ, Newell M-L. Disengagement from care in a decentralised primary health care antiretroviral treatment programme: cohort study in rural South Africa. Trop Med Int Health. 2013;18(8):934–41. doi: 10.1111/tmi.12135 23731253
30. Cornell M, Grimsrud A, Fairall L, Fox MP, van Cutsem G, Giddy J, et al. Temporal changes in programme outcomes among adult patients initiating antiretroviral therapy across South Africa, 2002–2007. AIDS. 2010;24(14):2263–70. doi: 10.1097/QAD.0b013e32833d45c5 20683318
31. Grimsrud A, Lesosky M, Kalombo C, Bekker LG, Myer L. Implementation and Operational Research: Community-Based Adherence Clubs for the Management of Stable Antiretroviral Therapy Patients in Cape Town, South Africa: A Cohort Study. JAIDS. 2016;71(1):e16–23. doi: 10.1097/QAI.0000000000000863 26473798
32. Brinkhof MW, Dabis F, Myer L, Bangsberg DR, Boulle A, Nash D, et al. Early loss of HIV-infected patients on potent antiretroviral therapy programmes in lower-income countries. Bull World Health Organ. 2008;86(7):559–67. doi: 10.2471/BLT.07.044248 18670668
33. Nglazi MD, Lawn SD, Kaplan R, Kranzer K, Orrell C, Wood R, et al. Changes in programmatic outcomes during 7 years of scale-up at a community-based antiretroviral treatment service in South Africa. JAIDS. 2011;56(1):e1–e8. doi: 10.1097/QAI.0b013e3181ff0bdc 21084996
34. Wilkinson LS, Skordis-Worrall J, Ajose O, Ford N. Self-transfer and mortality amongst adults lost to follow-up in ART programmes in low - and middle-income countries: systematic review and meta-analysis. Trop Med Int Health. 2015;20(3):365–79. doi: 10.1111/tmi.12434 25418366
35. Yu D, Nieftagodien S. Poverty and Migration: Evidence from the Khayelitsha/Mitchell’s Plain Area. A Southern Africa Labour and Development Research Unit Working Paper Number 11. Cape Town: SALDRU, University of Cape Town, 2008. Available from: https://core.ac.uk/download/pdf/7003867.pdf
36. Ndegwa D, Horner D, Esau F. The Links between Migration, Poverty and Health: Evidence from Khayelitsha and Mitchell’s Plain. Social Indicators Research. 2006;81(2):223–34. doi: 10.1007/s11205-006-9008-z
37. Hennessey C. From Lost to Found: the Silent Transfer of Patients on Antiretroviral Therapy in Khayelitsha, South Africa. M.P.H Thesis, University of Cape Town. 2015.
38. Meintjes G, Kerkhoff AD, Burton R, Schutz C, Boulle A, Van Wyk G, et al. HIV-Related Medical Admissions to a South African District Hospital Remain Frequent Despite Effective Antiretroviral Therapy Scale-Up. Medicine. 2015;94(50):e2269. doi: 10.1097/MD.0000000000002269 26683950
39. Johnson LF, Dorrington RE, Laubscher R, Hoffmann CJ, Wood R, Fox MP, et al. A comparison of death recording by health centres and civil registration in South Africans receiving antiretroviral treatment. JIAS. 2015;18 : 20628. doi: 10.7448/ias.18.1.20628 26685125
40. Geldenhuys P, Botha A, editors. On the design of an electronic health patient registration system for South Africa. 9th IDIA Conference, IDIA 2015; 2015; Nungwi, Zanzibar.
41. National Department of Health RoSA. National Antiretroviral Treatment Guidelines. In: Do Health, editor. First Edition ed: Jacana; 2004. Available from: http://apps.who.int/medicinedocs/documents/s17758en/s17758en.pdf.
42. National Department of Health RoSA. Clinical Guidelines for the Management of HIV & AIDS in Adults and Adolescents. In: Health NDo, editor. 2010. Available from: http://apps.who.int/medicinedocs/documents/s19153en/s19153en.pdf.
43. National Department of Health RoSA. The South African Antiretroviral Treatment Guidelines. In: Health Do, editor. 2013. Available from: http://www.sahivsoc.org/Files/2013%20ART%20Treatment%20Guidelines%20Final%2025%20March%202013%20corrected.pdf.
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2017 Číslo 11- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Jak postupovat při výběru betablokátoru − doporučení z kardiologické praxe
- Červená fermentovaná rýže účinně snižuje hladinu LDL cholesterolu jako vhodná alternativa ke statinové terapii
-
Všechny články tohoto čísla
- Labour trafficking: Challenges and opportunities from an occupational health perspective
- The end of HIV: Still a very long way to go, but progress continues
- Contemporary disengagement from antiretroviral therapy in Khayelitsha, South Africa: A cohort study
- Bioequivalence of twice-daily oral tacrolimus in transplant recipients: More evidence for consensus?
- Treatment guidelines and early loss from care for people living with HIV in Cape Town, South Africa: A retrospective cohort study
- Perinatal mortality associated with induction of labour versus expectant management in nulliparous women aged 35 years or over: An English national cohort study
- Core Outcome Set-STAndards for Development: The COS-STAD recommendations
- Closing the gaps in the HIV care continuum
- Association between the 2012 Health and Social Care Act and specialist visits and hospitalisations in England: A controlled interrupted time series analysis
- HIV pre-exposure prophylaxis and early antiretroviral treatment among female sex workers in South Africa: Results from a prospective observational demonstration project
- Sexual exploitation of unaccompanied migrant and refugee boys in Greece: Approaches to prevention
- Child sex trafficking in the United States: Challenges for the healthcare provider
- The expanding epidemic of HIV-1 in the Russian Federation
- Cardiovascular disease (CVD) and chronic kidney disease (CKD) event rates in HIV-positive persons at high predicted CVD and CKD risk: A prospective analysis of the D:A:D observational study
- Validity of a minimally invasive autopsy for cause of death determination in maternal deaths in Mozambique: An observational study
- malERA: An updated research agenda for malaria elimination and eradication
- malERA: An updated research agenda for health systems and policy research in malaria elimination and eradication
- A combination intervention strategy to improve linkage to and retention in HIV care following diagnosis in Mozambique: A cluster-randomized study
- Bioequivalence between innovator and generic tacrolimus in liver and kidney transplant recipients: A randomized, crossover clinical trial
- malERA: An updated research agenda for basic science and enabling technologies in malaria elimination and eradication
- Human trafficking and exploitation: A global health concern
- Virological response and resistance among HIV-infected children receiving long-term antiretroviral therapy without virological monitoring in Uganda and Zimbabwe: Observational analyses within the randomised ARROW trial
- Postmenopausal hormone therapy and risk of stroke: A pooled analysis of data from population-based cohort studies
- Lansoprazole use and tuberculosis incidence in the United Kingdom Clinical Practice Research Datalink: A population based cohort
- malERA: An updated research agenda for insecticide and drug resistance in malaria elimination and eradication
- Safety, pharmacokinetics, and immunological activities of multiple intravenous or subcutaneous doses of an anti-HIV monoclonal antibody, VRC01, administered to HIV-uninfected adults: Results of a phase 1 randomized trial
- HIV prevalence and behavioral and psychosocial factors among transgender women and cisgender men who have sex with men in 8 African countries: A cross-sectional analysis
- Treatment eligibility and retention in clinical HIV care: A regression discontinuity study in South Africa
- malERA: An updated research agenda for characterising the reservoir and measuring transmission in malaria elimination and eradication
- Effectiveness of a combination strategy for linkage and retention in adult HIV care in Swaziland: The Link4Health cluster randomized trial
- The value of confirmatory testing in early infant HIV diagnosis programmes in South Africa: A cost-effectiveness analysis
- HIV self-testing among female sex workers in Zambia: A cluster randomized controlled trial
- The US President's Malaria Initiative, transmission and mortality: A modelling study
- Comparison of two cash transfer strategies to prevent catastrophic costs for poor tuberculosis-affected households in low- and middle-income countries: An economic modelling study
- Direct provision versus facility collection of HIV self-tests among female sex workers in Uganda: A cluster-randomized controlled health systems trial
- malERA: An updated research agenda for diagnostics, drugs, vaccines, and vector control in malaria elimination and eradication
- malERA: An updated research agenda for combination interventions and modelling in malaria elimination and eradication
- HIV-1 persistence following extremely early initiation of antiretroviral therapy (ART) during acute HIV-1 infection: An observational study
- Respondent-driven sampling for identification of HIV- and HCV-infected people who inject drugs and men who have sex with men in India: A cross-sectional, community-based analysis
- Extensive virologic and immunologic characterization in an HIV-infected individual following allogeneic stem cell transplant and analytic cessation of antiretroviral therapy: A case study
- Measuring success: The challenge of social protection in helping eliminate tuberculosis
- Prospects for passive immunity to prevent HIV infection
- Reaching global HIV/AIDS goals: What got us here, won't get us there
- Evidence-based restructuring of health and social care
- Extreme exploitation in Southeast Asia waters: Challenges in progressing towards universal health coverage for migrant workers
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Postmenopausal hormone therapy and risk of stroke: A pooled analysis of data from population-based cohort studies
- Bioequivalence between innovator and generic tacrolimus in liver and kidney transplant recipients: A randomized, crossover clinical trial
- HIV pre-exposure prophylaxis and early antiretroviral treatment among female sex workers in South Africa: Results from a prospective observational demonstration project
- Bioequivalence of twice-daily oral tacrolimus in transplant recipients: More evidence for consensus?
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



