-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaThe value of confirmatory testing in early infant HIV diagnosis programmes in South Africa: A cost-effectiveness analysis
Lorna Dunning examine the cost-effectiveness of confirmatory testing in early infant HIV diagnosis programmes in South Africa.
Published in the journal: . PLoS Med 14(11): e32767. doi:10.1371/journal.pmed.1002446
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1002446Summary
Lorna Dunning examine the cost-effectiveness of confirmatory testing in early infant HIV diagnosis programmes in South Africa.
Introduction
Despite the success of programmes to prevent mother-to-child transmission (MTCT) of HIV, paediatric HIV remains a substantial burden in sub-Saharan Africa, with 170,000 infants infected with HIV in 2015 [1]. Perinatally infected, untreated infants are at highest risk for rapid disease progression and mortality, with 1 in 2 untreated HIV-infected infants dying before their second birthday [2–5]. The World Health Organization (WHO) recommends testing HIV-exposed infants by 6 weeks of life and immediately referring those who test positive for initiation of HIV care and antiretroviral therapy (ART) [6].
Inexpensive serological antibody assays routinely used for diagnosis in adults cannot be easily interpreted for HIV-exposed infants (those born to HIV-infected women), because transplacental transfer of maternal antibodies leads infants to be seropositive for as long as 18 months [7,8]. Infants therefore require virological tests (nucleic acid amplification tests [NAATs] detecting HIV RNA or DNA) to diagnose HIV infection [9]. Despite reported specificities of >99%, NAATs still have the possibility for false-positive diagnoses [10,11]. As the incidence of paediatric HIV falls with improved access to ART for pregnant/breastfeeding women, the positive predictive value (PPV) of diagnostic assays also decreases, leading to a greater proportion of uninfected infants receiving false-positive diagnoses and starting ART. After ART is initiated, it may be impossible to distinguish truly infected infants from uninfected infants, because effective ART may lead to undetectable HIV RNA in HIV-infected infants whilst also preventing the development of endogenous anti-HIV antibody after maternal antibody fades from the circulation. Because truly uninfected infants may therefore have identical laboratory results to treated infected infants, they may face many years or even a lifetime of incorrect diagnosis and ART [7,12,13].
Current WHO and South African guidelines strongly recommend the use of confirmatory NAAT testing with ART initiation at the first positive result as part of early infant diagnosis (EID) programmes [6,14]. However, a recent policy survey by WHO demonstrated that despite confirmatory testing being used routinely for diagnosis of HIV in adults and across most domains of adult and paediatric medicine, the uptake of confirmatory testing within EID programmes remains limited, with 38% (8/21) of high-burden countries not including confirmatory testing for infants in their guidelines [15–17]. Even where guidelines recommend confirmatory testing, it is rarely implemented [18]. Cost is often cited as a key barrier; many low-resource countries struggle to implement EID programmes due to the high costs of the NAATs required for infant diagnosis. We used a computer simulation model of paediatric HIV infection, diagnosis, and treatment to examine the clinical and economic outcomes of EID programmes without and with confirmatory testing in South Africa.
Methods
Overview
We used the Cost-Effectiveness of Preventing AIDS Complications (CEPAC)–Pediatric model to simulate a cohort of HIV-exposed infants in South Africa undergoing 2 EID algorithms: 6-week EID testing without confirmatory testing and 6-week EID testing with confirmatory testing. The CEPAC–Pediatric model is a first-order Monte Carlo simulation model of paediatric HIV infection, disease progression, diagnosis, and treatment [19–21]. For this analysis, we simulated HIV-exposed infants (born to women living with HIV) from birth through death. Risk of intrauterine or intrapartum HIV infection was modelled as a 1-time risk, based on 3 key maternal characteristics: the probability a mother was aware of her HIV diagnosis during pregnancy; the probability that she received ART during pregnancy, reflecting prevention of MTCT (PMTCT) coverage; and maternal CD4 count for women not receiving ART, reflecting disease stage. Uninfected infants faced a monthly risk of postpartum transmission based on these same characteristics until complete cessation of breastfeeding. All simulated patients faced monthly risks of non-HIV-related mortality. After HIV infection occurred, patients faced additional risks of opportunistic infections (OIs) and HIV-related mortality based on their age, CD4 percent (age < 5 years) or CD4 count (age ≥ 5 years), retention in care, and ART use. Full details of the CEPAC–Pediatric model structure are available in S1 Text and S2 Table and at http://web2.research.partners.org/cepac/model.html.
This work was approved by the Partners Human Research Committee, Boston, MA, US.
Modelled population
In the base-case analysis, we simulated HIV-exposed South African children presenting to care at 6 weeks of age. EID in South Africa is currently directed at infants with known HIV exposure [14]; we therefore included only infants born to women identified as living with HIV during pregnancy. South African guidelines currently recommend ‘Option B+’, or lifelong ART for all pregnant/breastfeeding women identified as living with HIV [14]. We assumed that 90% of women had access to ART during pregnancy and breastfeeding [1]. Based on early data after the release of new infant feeding guidelines in South Africa, we assumed 80% of the cohort was breastfed, for a mean duration of 12 months [6,14,22].
Model outcomes
The model records true infection status for all infants, as well as the results of each administered assay, allowing the direct reporting of true-positive, true-negative, false-positive, and false-negative diagnoses. The primary model outcomes were the number of infants with false-positive diagnoses linked to care as a proportion of total ART initiations, PPV (defined as the proportion of positive test results due to truly HIV-infected infants), life expectancy (LE, in years), and average per-person lifetime HIV-related healthcare costs, from the perspective of the healthcare provider. We projected survival, LE, and costs separately for HIV-infected infants and for the complete birth cohort of HIV-exposed infants, which included both HIV-infected and HIV-uninfected infants. Costs are presented in 2013 US dollars; costs and life expectancies were modelled both undiscounted and discounted at a rate of 3%/year [23]. We first calculated per-person outcomes, then translated these to population outcomes for all 350,000 HIV-exposed infants born in South Africa in 1 year [24,25]. Where clinical outcomes were equal for both strategies, calculation of incremental cost-effectiveness was not necessary; we considered alternative strategies as either cost-saving or more costly in these cases.
Modelled strategies
The strategy ‘without confirmatory testing’ simulated all HIV-exposed infants receiving a NAAT at 6 weeks of age; in this strategy, all infants who received positive results initiated ART upon result return (mean turnaround time: 1 month). The strategy ‘with confirmatory testing’ simulated all HIV-exposed infants receiving a NAAT at 6 weeks of age; all infants who received positive results on the first test initiated ART at result return, but a second blood sample was drawn before ART initiation and sent for a confirmatory NAAT [6,14]. We assumed conditional independence of the primary and confirmatory NAAT results, because WHO recommends that a second specimen be used for confirmatory testing, and most false-positive NAAT results are likely consequences of specimen handling error rather than biological phenomena [6,26,27]. Infants with negative confirmatory tests underwent a third test per WHO guidelines before HIV infection was ruled out and ART stopped. For infants who were truly uninfected but initiated ART and therefore entered HIV care after a false-positive diagnosis (both strategies), we assigned costs for 10 years of routine HIV care, ART, and laboratory monitoring. We varied this duration widely in sensitivity analysis. For truly infected infants diagnosed and linked to care, the model includes lifetime costs for routine care, ART, and laboratory monitoring, as well as care for OIs. We conservatively excluded clinical harms from incorrect ART initiations, such as medication toxicity and stigma.
Input data
We modelled cohort characteristics, PMTCT coverage and MTCT risks, disease progression, and ART outcomes using data from published trials and cohort studies in sub-Saharan Africa (Table 1; Table A in S1 Text; S2 Table) [28–32]. The specificity of NAATs was modelled as 99.6%, from a 2015 WHO meta-analysis [33]. We modelled NAAT sensitivity as a function of time since infection, reaching 100% among infants infected during pregnancy or at least 4 weeks prior to testing (Table 1) [34]. In the base case, laboratory-based NAAT cost was US$25, which included assays, reagents, and human personnel resources associated with specimen processing and result return. To describe the full potential impact of each strategy, in the base-case analysis we modelled guideline-concordant (100%) probabilities of presentation to testing, result return (first NAAT: 1 month), and ART initiation after an HIV diagnosis; we varied these widely in sensitivity and scenario analyses to reflect implementation across a range of settings [1,35–37].
Tab. 1. Selected data parameters for CEPAC–Pediatric model analysis of early infant HIV diagnosis testing in South Africa. 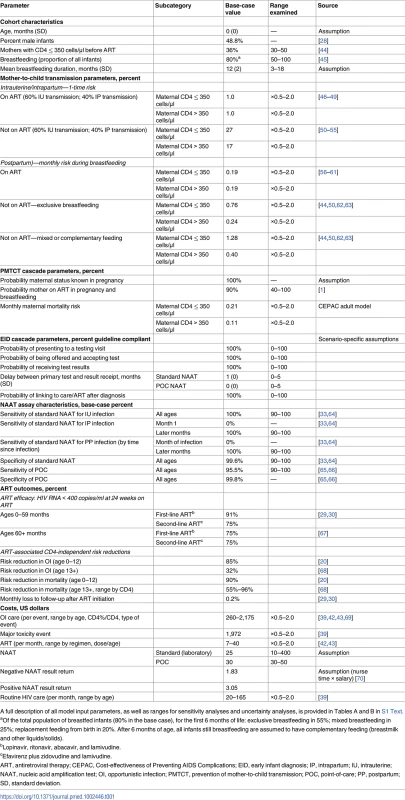
A full description of all model input parameters, as well as ranges for sensitivity analyses and uncertainty analyses, is provided in Tables A and B in S1 Text. We incorporated published South African healthcare costs for HIV-infected children less than 5 years of age. Costs associated with OI care for children 5 years of age and older were calculated from South African adult resource utilisation (outpatient visits, inpatient days, and laboratory testing) multiplied by South African unit costs [38,39]. Routine HIV care costs (all ages) ranged by age from US$20 to US$165 per month [40,41]. First-line ART costs were from Clinton Health Access Initiative price lists and WHO weight-based dosing recommendations, ranging by age and weight from US$7 to US$40 per month (Table 1) [42,43].
Sensitivity analyses
Following ISPOR–SMDM Good Research Practices Task Force (S1 Table) guidelines on uncertainty in model-based analyses, we conducted extensive univariate and multivariate sensitivity analyses (Tables B and D in S1 Text) [71]. We first conducted univariate sensitivity analyses, in which we varied NAAT specificity and sensitivity, infant HIV prevalence via PMTCT coverage and MTCT risks, the probability of initiating ART after a positive NAAT result, the amount of time infants with a false-positive diagnosis spent on ART, the costs of NAATs, routine HIV care and OI care costs, and ART treatment costs (S1 Table; Task Force recommendation VI-9). Holding all other parameters at their base-case values, we identified the threshold value for each parameter at which the comparison between with and without confirmatory testing would change (S1 Table; Task Force recommendation VI-12). We next performed multivariate sensitivity analyses to evaluate the impact of simultaneous variation in multiple parameters (S1 Table; Task Force recommendation VI-10). We also examined the assumption of conditional independence of the first and confirmatory assays by varying their specificities simultaneously, and assigned a combination of assay cost, sensitivity, specificity, result return time, and result return probability to reflect emerging point-of-care (POC) EID assays (Table 1).
Implementation scenario analyses
We conducted 3 scenario analyses to examine important issues in EID implementation (S1 Table; Task Force recommendation VI-10). First, we simulated lower rates of implementation of 6-week EID testing incorporating input parameters from current testing and treatment cascades in resource-limited countries. We modelled (a) probability of presenting to testing as 73% and probability of ART initiation as 71% of infants with a positive test result and (b) probability of presenting to testing as 95% (2016 UNAIDS estimates for EID coverage in South Africa), probability of result return to caregiver as 80%, and probability of ART initiation as 71% [1,35–37]. Second, we examined programmes offering routine EID testing at both birth and 6 weeks of age to all HIV-exposed infants. Birth tests were modelled as standard laboratory-based NAAT tests with a 1-month result return time (Table 1). Third, WHO strongly recommends prompt ART initiation after a first positive test result (as in our base-case analysis) to avoid delays during a period of high mortality without treatment, but that NAAT testing be repeated on a second specimen drawn prior to ART initiation, to confirm the initial diagnosis [6,7]. Because some providers may be reluctant to initiate ART before receiving a confirmatory result, we also examined the impact of postponing ART until return of the confirmatory test result (1–2 months).
Uncertainty analyses
The univariate sensitivity analyses described above reveal the sensitivity of policy conclusions to variations in key parameters through a wide range of plausible values. To reflect the uncertainty in the primary data estimates, we also varied key parameters through reported 95% confidence intervals (or range or interquartile ranges, if 95% confidence intervals were not available; S1 Table; Task Force recommendations VI-6 and VI-7). For most parameters, this interval fell well within the range examined in sensitivity analyses. We used the results of previously reported model calibration and validation analyses [19,20] to examine the impact of parameter and model structural uncertainty on the comparison between without and with confirmatory testing (S1 Table; Task Force recommendation VI-14).
Results
Base-case results: Clinical outcomes
In the base-case analysis, the birth cohort was projected to include 1.8% infants with intrauterine HIV infection, 1.2% with intrapartum infection, and 1.9% with postpartum infection (cumulative MTCT risk 4.9%), with 95.1% of the cohort HIV-exposed but uninfected (Table 2). In both 6-week EID algorithms (without and with confirmatory testing), HIV-infected infants had a projected 1-year survival of 75.7% and LE of 26.2 years; there was also no clinical difference between the 2 strategies for the entire birth cohort (1-year survival 93.3%, LE 61.4 years). Without confirmatory testing, 128 infants of every 1,000 who initiated ART were truly uninfected, reflecting false-positive diagnosis; this led to a PPV of 87.2%. With confirmatory testing, this proportion fell to only 1 in 1,000 ART initiations, for a PPV of 99.9%.
Tab. 2. Base-case model results: Early infant HIV diagnosis testing at 6 weeks in South Africa with and without confirmatory testing. 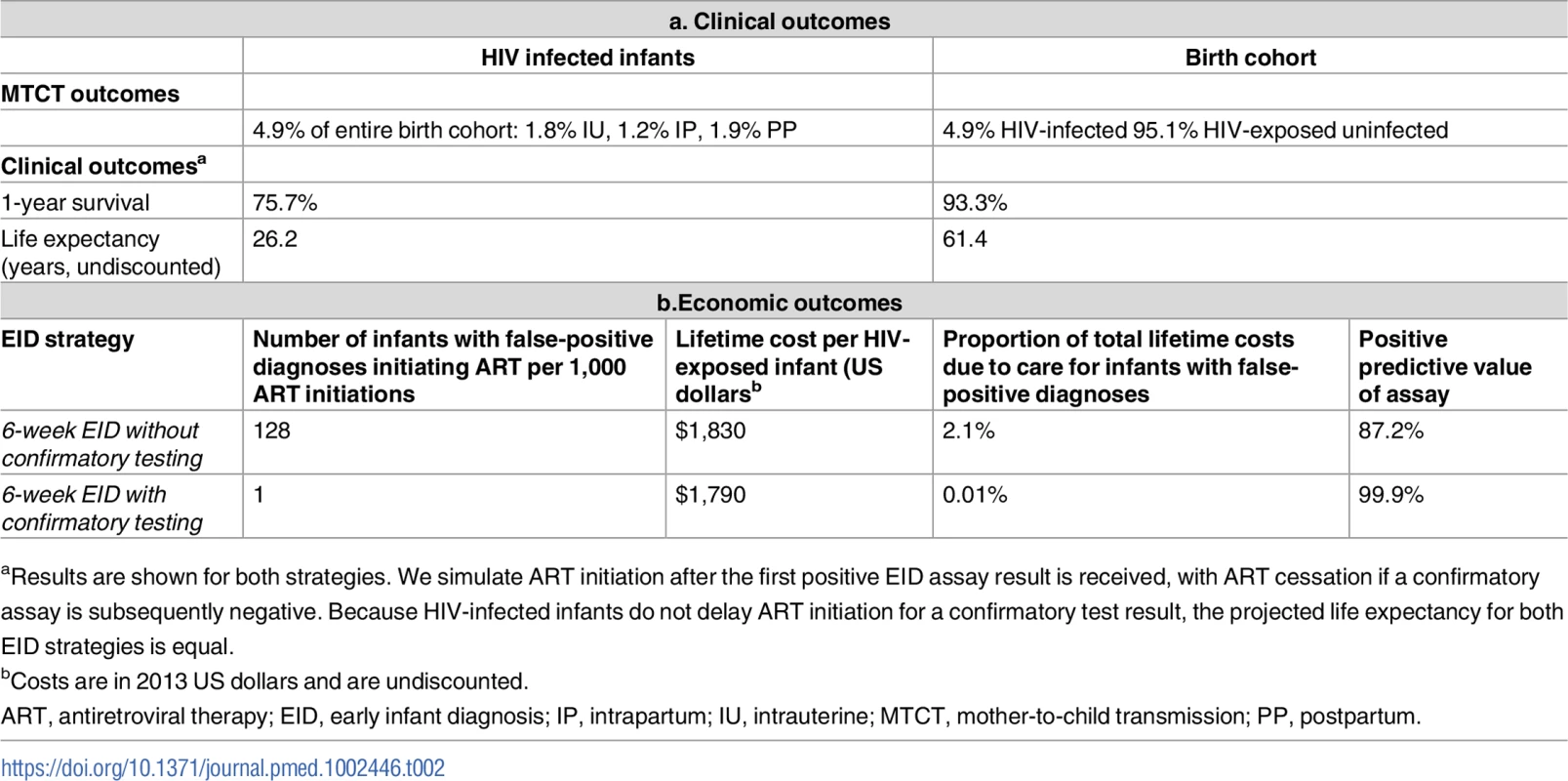
aResults are shown for both strategies. We simulate ART initiation after the first positive EID assay result is received, with ART cessation if a confirmatory assay is subsequently negative. Because HIV-infected infants do not delay ART initiation for a confirmatory test result, the projected life expectancy for both EID strategies is equal. Base-case results: Costs and cost-savings
Lifetime costs of HIV-exposed infants were US$1,830/infant without confirmatory testing and US$1,790/infant with confirmatory testing (Table 2; Fig 1). The approach of using confirmatory testing was therefore equally effective and was cost-saving; it became cost-saving 3 months after ART initiation (Fig A in S1 Text). Without confirmatory testing, 2.1% of total lifetime HIV-related costs for the birth cohort were accrued by truly uninfected infants after false-positive diagnoses, compared to only 0.01% of lifetime costs with confirmatory testing (Fig 1, orange). If 6-week EID programmes were available for the 350,000 HIV-exposed infants born annually in South Africa, 11,000 infants would require a second NAAT to confirm a first positive result. The cost of these confirmatory NAATs would be approximately US$260,000, but they would avert unnecessary HIV care and ART for 1,400 infants. By averting unnecessary ART and HIV costs for uninfected infants, confirmatory testing would save over US$1,050,000 in the first year and US$13,860,000 in lifetime costs for a South African birth cohort, compared to the approach of not using confirmatory testing [24,25].
Fig. 1. Total lifetime costs per HIV-exposed infant by EID strategy. 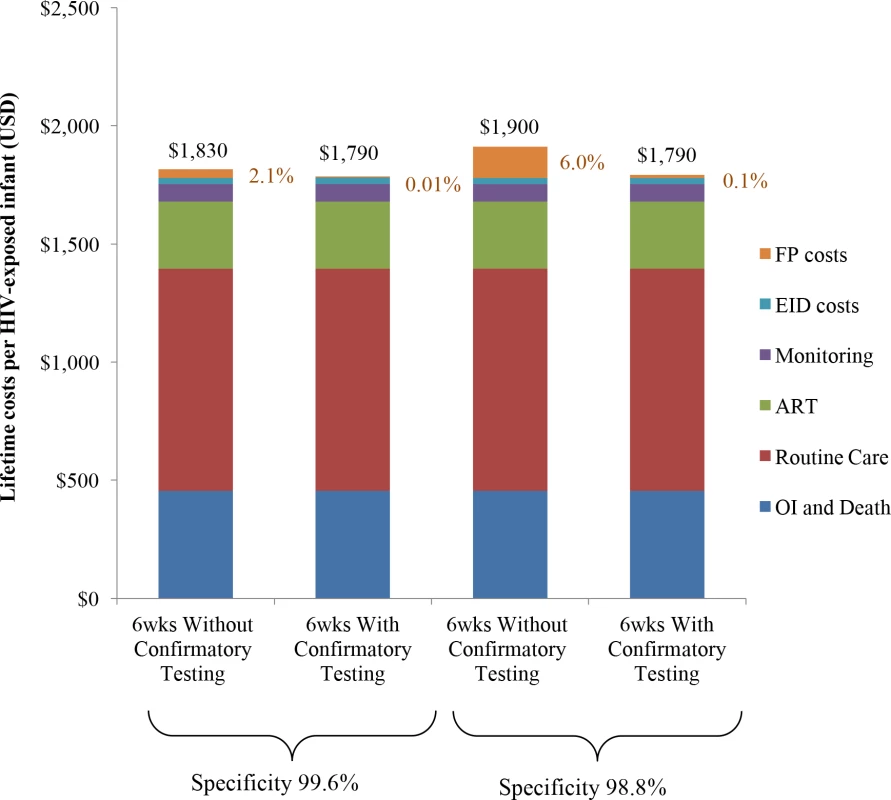
Columns include components of lifetime total costs per HIV-exposed infant tested: routine HIV care, CD4 and HIV viral load monitoring, OIs and end-of-life care, ART, EID costs, and false-positive costs. EID programme costs are shown in blue and comprise 2%–3% of lifetime costs, as shown previously [21]; false-positive costs are shown in orange and are made up of all component costs acquired for HIV-infected infants other than OI costs. ART, antiretroviral therapy; EID, early infant diagnosis; FP, false-positive; OI, opportunistic infection. Univariate sensitivity analyses: Costs
The use of confirmatory testing remained cost-saving even with wide variations in model parameters, including the cost, specificity, and sensitivity of the NAAT; the probability of presentation at each step in the EID cascade; and the costs of routine care and OI care. Robustness of model results to these key parameters is shown in Fig 2. However, there were 3 key exceptions: confirmatory testing was no longer cost-saving if the duration spent by HIV-uninfected infants in care and on ART after false-positive diagnosis was <3 months (Fig A in S1 Text), if the first and confirmatory assays were no longer considered to be independent (specificity of confirmatory assay <15%; Tables D and E in S1 Text), or if the cost of the NAAT was 16-fold higher than in the base case (>US$400).
Fig. 2. Univariate sensitivity analyses examining the impact of variation in individual input parameters on the difference in cost per HIV-exposed infant between the without and with confirmatory testing strategies. 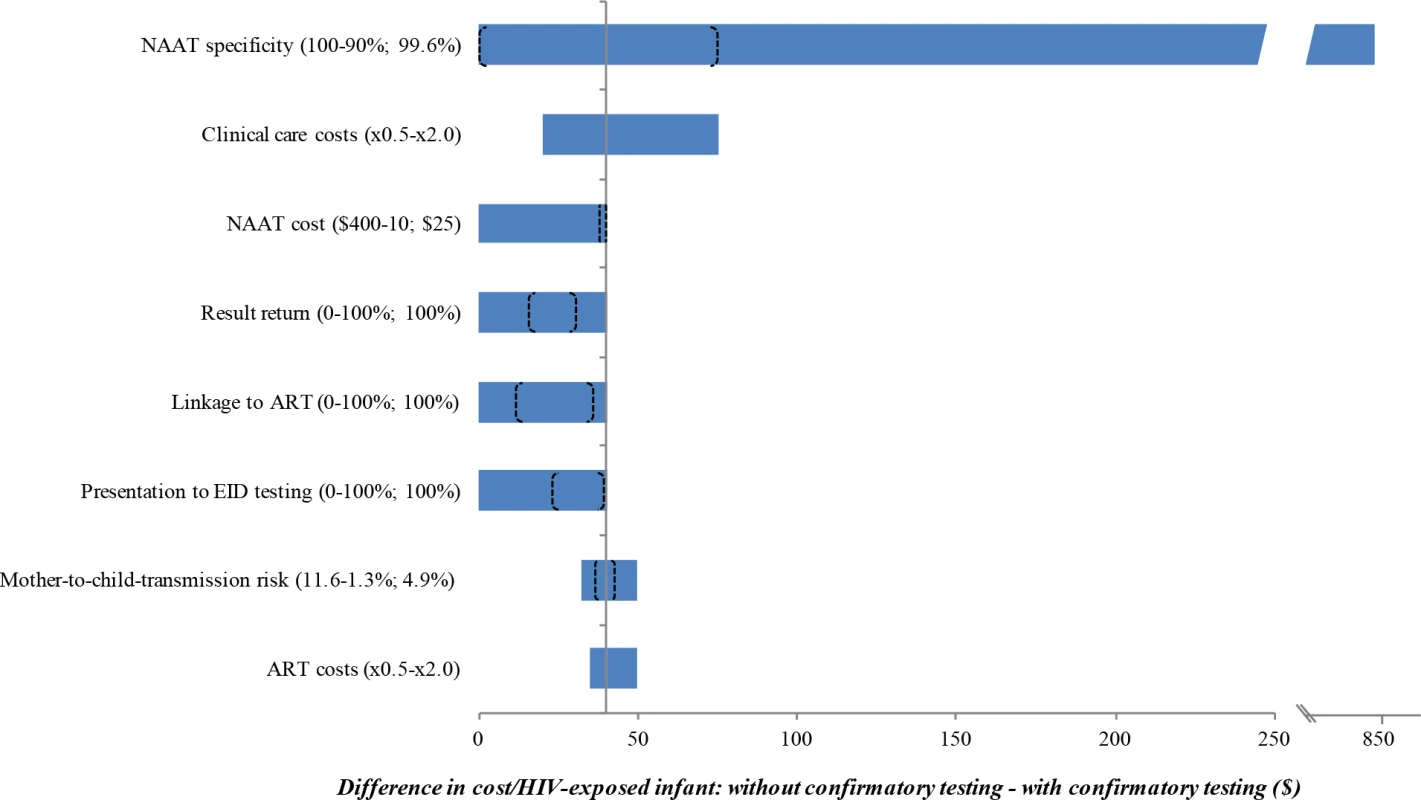
Key parameters varied in sensitivity analyses are shown on the left. Values in parentheses indicate the range examined (from the value leading to the lowest difference in cost to the value leading to the greatest difference, with base-case values after the semicolon). The horizontal axis shows the difference in cost between the 2 strategies: without confirmatory testing minus with confirmatory testing. The bounds of the blue bar indicate the cost differences at the extreme parameter values; longer bars therefore indicate parameters to which the model results were more sensitive. Where confidence intervals were available for the primary data estimates used in the base case, we indicate the bounds of these confidence intervals with brackets overlying the blue bars; the distance between brackets therefore indicates the degree to which the base-case estimates are affected by parameter uncertainty. The blue bar reaches the far left axis (indicating a cost difference of 0) at the threshold value for each parameter where confirmatory testing is no longer cost-saving compared to without confirmatory testing. The grey vertical line indicates the value for each parameter at the base-case result: a savings of US per infant with confirmatory testing. ART, antiretroviral therapy; EID, early infant diagnosis; NAAT, nucleic acid amplification test. We repeated the base-case analysis using a NAAT specificity of 98.8%, as reported by WHO in a previous meta-analysis [33]. The cost-savings associated with confirmatory testing were greater than in the base case (US$1,900/infant without confirmatory testing; US$1,790/infant with confirmatory testing; Fig 1; Table C in S1 Text). With this lower specificity, false-positive diagnoses accounted for 6.0% of total lifetime costs without confirmatory testing, compared to 0.1% with confirmatory testing. After false-positive diagnosis in this scenario, if the HIV status of infants incorrectly identified as infected could be ascertained and their ART interrupted within 2 months of starting ART, confirmatory testing would no longer be cost-saving.
Univariate sensitivity analyses: Clinical outcomes
Several variations in model parameters changed the proportion of ART initiations due to false-positive diagnosis (Table D in S1 Text). Increases in specificity, such as those recently described for novel POC EID assays, reduced the number of false-positive diagnoses in the approach without confirmatory testing to 69/1,000 ART initiations (Table D in S1 Text; Fig 3) [65,66]. A reduction in NAAT sensitivity of 5% only minimally increased the number of infants initiating ART after false-positive diagnosis without confirmatory testing (from 128/1,000 ART initiations in the base case to 133/1,000), but LE for HIV-infected infants was reduced to 25.9 years without confirmatory testing and to 25.7 years with confirmatory testing (Table D footnote in S1 Text). Reductions in the number of infected infants successfully undergoing EID testing, receiving test results, and initiating ART had minimal effects on the proportion of ART initiations due to false-positive diagnoses, but reduced the LE for HIV-infected infants: if only 50% of infected infants completed the EID cascade, LE for HIV-infected infants was reduced to 23.6 years (Table D in S1 Text).
Fig. 3. Number of infants linked to ART after false-positive diagnosis, per 1,000 ART initiations, by assay specificity. 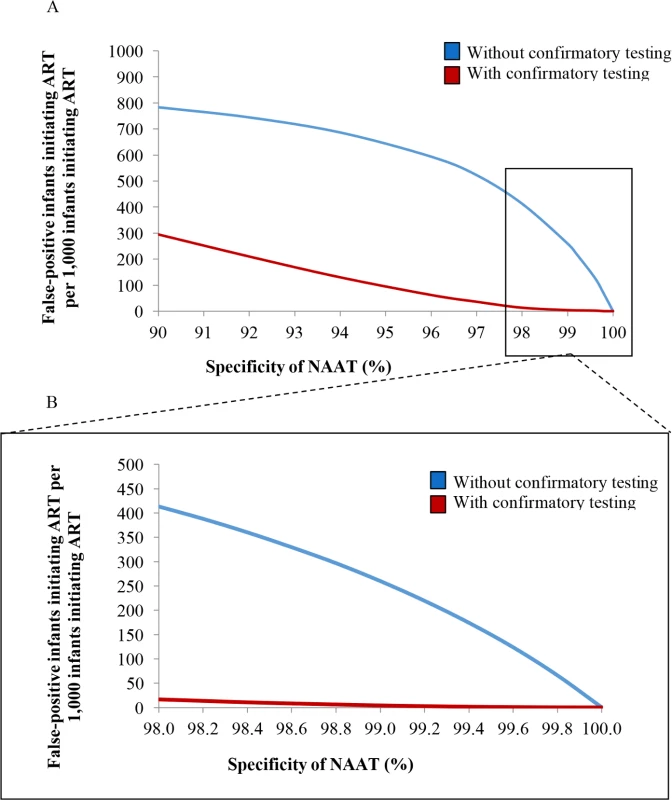
(A) Univariate sensitivity analysis varying NAAT specificity without and with confirmatory testing for 6-week EID testing. The vertical axis depicts the number of infants with a false-positive diagnosis who initiate ART, per 1,000 ART initiations. The horizontal axis depicts the specificity of the NAAT. (B) The inset panel depicts results at higher specificity values, as reported for most NAATs (Table 1). ART, antiretroviral therapy; EID, early infant diagnosis; NAAT, nucleic acid amplification test. Multivariate sensitivity analyses
In multivariate sensitivity analyses, results were also sensitive to reductions in NAAT specificity and infant HIV prevalence (lower MTCT risks), as depicted in Fig 4. When MTCT risks were <1.3% and NAAT specificity was 98.0%, 749/1,000 infants initiating ART without confirmatory testing were truly uninfected (Fig 4; Table F in S1 Text). With a very high MTCT risk of 9.6% and NAAT specificity of 99.8%, this fell to 34/1,000 ART initiations without confirmatory testing. In all multivariate sensitivity analyses varying specificity (<100%) and infant HIV prevalence, confirmatory testing remained cost-saving (Table F in S1 Text).
Fig. 4. Number of infants linked to ART after false-positive diagnosis, per 1,000 ART initiations, by assay specificity and MTCT risk. 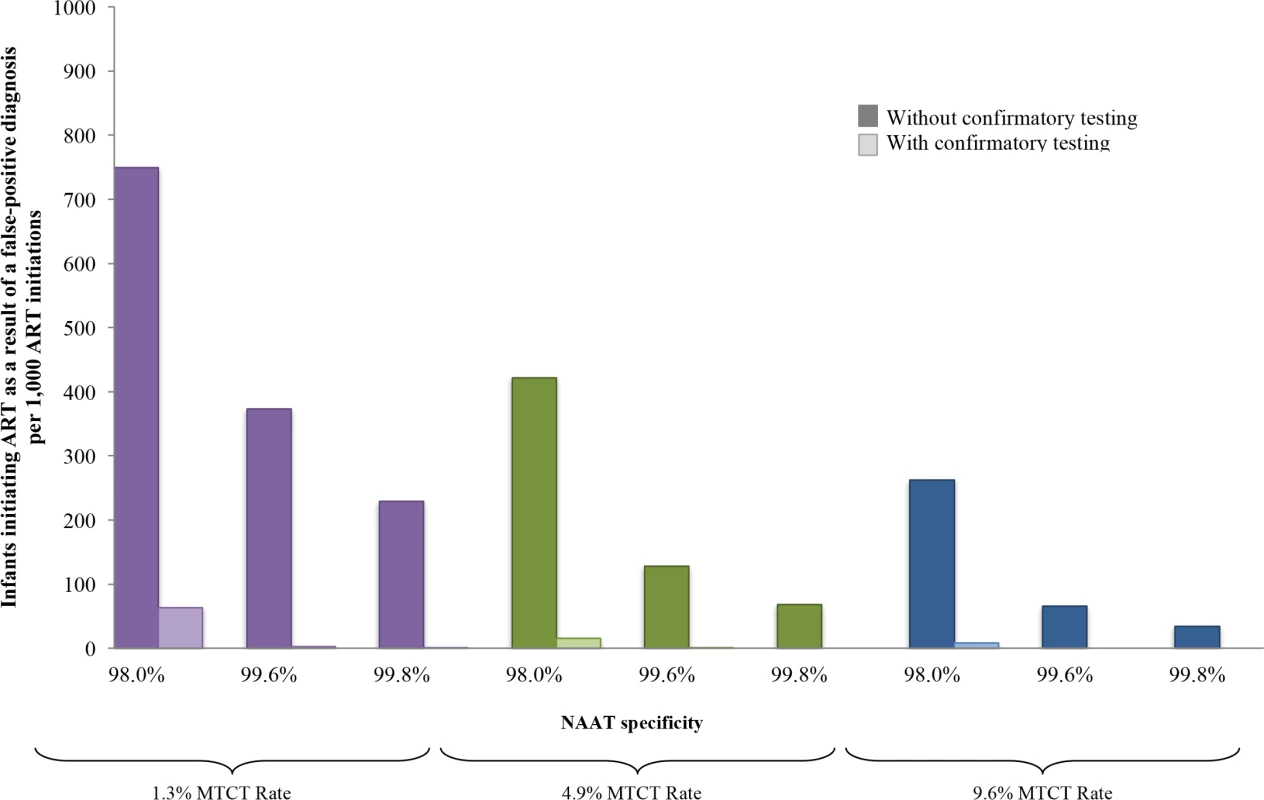
Multivariate sensitivity analysis varying specificity of the NAAT and infant HIV prevalence modelled by increasing MTCT risk. The vertical axis shows the number of infants with false-positive diagnosis initiating ART, per 1,000 ART initiations. Groups of coloured bars indicate 3 values for infant HIV prevalence at weaning (12 months of age): purple indicates a low MTCT risk scenario, with 12-month risk of 1.3%; green indicates the base-case value of 4.9%; and blue indicates a high MTCT risk scenario, with risk of 9.6%. Three values of NAAT specificity are shown within each MTCT risk scenario. For each combination of MTCT risk and NAAT specificity, bars indicate those who are truly HIV-uninfected (false-positive diagnosis). The left, dark-coloured bar in each pair reflects the outcome without confirmatory testing, and the right, light-coloured bar reflects the outcome with confirmatory testing. ART, antiretroviral therapy; EID, early infant diagnosis; MTCT, mother-to-child transmission; NAAT, nucleic acid amplification test. Implementation scenario analyses
Incorporating input parameters from current testing and treatment cascades reduced the LE of HIV-infected infants, but the number of infants with a false-positive diagnosis initiating ART remained 128/1,000 ART initiations (Table 3). For programmes offering EID at both birth and 6 weeks of age, the proportion of infants initiating ART after false-positive diagnosis without confirmatory testing increased to 213/1,000 ART initiations, accounting for 4.0% of total costs (Table D and Fig B in S1 Text). When ART was not initiated until the return of the confirmatory NAAT result, mortality during the delay to ART initiation reduced the projected 1-year survival (74.8%) and LE (25.9 years) for HIV-infected infants with confirmatory testing; results for the approach without confirmatory testing (1-year survival 75.7%; LE 26.2 years) were unchanged (Table D in S1 Text). HIV-related healthcare costs remained lower with confirmatory testing than without confirmatory testing in all 3 scenarios.
Tab. 3. Implementation scenario model results: Early infant HIV diagnosis testing at 6 weeks in South Africa with and without confirmatory testing. 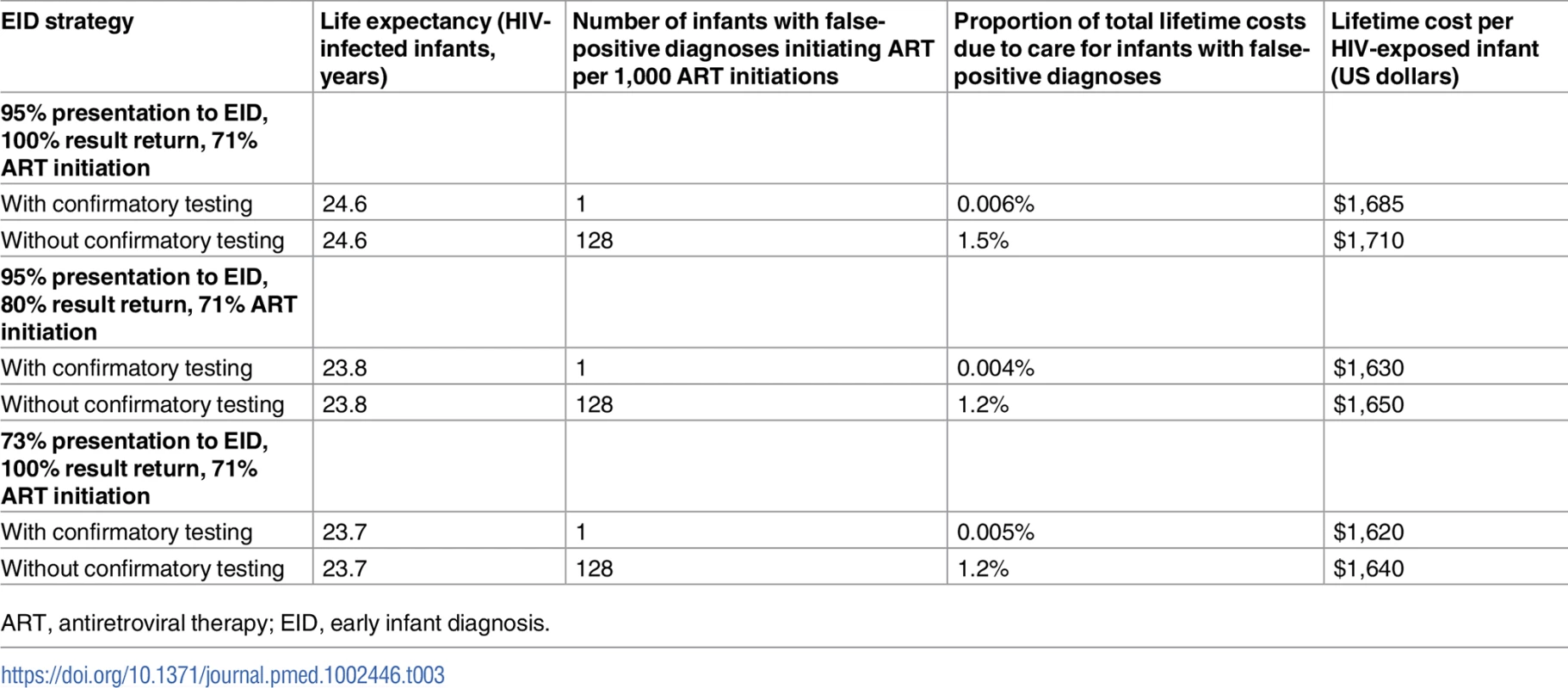
ART, antiretroviral therapy; EID, early infant diagnosis. Uncertainty analyses
The impact of varying key parameters through the confidence intervals around base-case estimates is shown in Fig 2. In all parameter sets derived from formal calibration analyses, confirmatory testing remained cost-saving (Fig C in S1 Text).
Discussion
We simulated EID strategies without and with confirmatory testing for HIV-exposed infants in South Africa. The primary finding was that, when confirmatory testing was not used in the model, more than 10% of infants who initiated ART reflected false-positive diagnoses. These model results are comparable to empirical data from Africa: when records of infants receiving positive EID results and/or initiating ART were reviewed in detail, the proportion found to be truly HIV-uninfected was 2.5% in Kenya, 6.3% in South Africa, 14.6% in Malawi, and 16% in Côte d’Ivoire and Burkina Faso [72–75]. HIV-uninfected infants incorrectly initiating ART not only receive unnecessary medication exposure and treatment costs, but may also experience long-term medication toxicities and the substantial stigma associated with HIV diagnosis [7]. To remain conservative, we did not include these detriments in our analysis. Confirmatory testing would therefore prevent a broader set of adverse outcomes than those described here in a substantial proportion of HIV-uninfected infants.
False-positive results occur for a variety of reasons but are likely the result of stretched human resources compromising test specificity and sensitivity via specimen handling errors such as mislabelling of specimens, incorrect specimen placement in the device, or inadequate quality control resulting in contamination between specimens during PCR amplification [27,76]. WHO recommends the use of a second specimen for a confirmatory test, ensuring the independence of the 2 assays and reducing the likelihood of a second false-positive result. Ensuring that an initial HIV diagnosis is correct is essential because identifying a misdiagnosed, truly HIV-uninfected infant remains extremely difficult after ART is initiated [6]. Lack of detectable anti-HIV antibody, HIV RNA, or HIV DNA may reflect either absence of true infection or the impact of effective ART, which makes withdrawal of treatment the only mechanism available for clinicians wishing to determine the true HIV status of an infant on ART. However, treatment interruptions are not currently recommended, due to concerns about viral rebound and disease progression [77,78]. Unconfirmed EID test results therefore cause diagnostic dilemmas for the provider, whilst families may experience confusion and uncertainty about the health system, discouraging future engagement in care [79].
The second key finding from this analysis is that including confirmatory testing in EID programmes is cost-saving. We found that confirmatory testing reduced the cost per HIV-exposed infant tested from US$1,830 to US$1,790. Although concerns have been raised about the capacity of laboratories to conduct the additional assays needed for confirmatory testing, we found that the number of additional tests was relatively small, as they are required by only 3% of HIV-exposed infants [9]. The costs of these additional tests to confirm HIV infection would be offset by the reduction in false-positive diagnoses and their associated unnecessary HIV care, ART, and ART toxicity costs. Infants with a false-positive diagnosis only had to remain in care and on ART for longer than 3 months for confirmatory testing to become cost-saving; a shorter duration on ART would be extremely unlikely due to the difficulty of identifying uninfected infants after ART initiation. Confirmatory testing remained cost-saving despite wide variations in the costs of clinical care and ART, and at any plausible NAAT cost. In addition, our findings demonstrate that confirmatory testing is cost-saving even with increases in the probability of becoming lost to follow-up between presenting to EID testing, receiving test results, and initiating ART, but lowering the number of HIV-infected infants initiating ART reduces LE for truly infected infants.
A third key finding of this analysis is that when confirmatory testing is done, ART should be initiated after the first positive result, as WHO recommends. If ART was delayed until the result of the confirmatory assay was available, short-term survival and LE were markedly reduced. Waiting even 1 month to initiate ART until the return of the confirmatory result can reduce 1-year survival for HIV-infected infants substantially (from 75.8% to 74.5%) and overall LE for HIV-infected infants (from 26.2 years to 25.9 years). Novel POC EID assays, which offer in-clinic testing and same-day result return, have been proposed as an approach to improving timely ART initiation. Based on preliminary published values for POC NAAT sensitivity (95.5%), specificity (99.8%), and cost (US$30), our analyses suggest that confirmatory testing would likely remain cost-saving in EID programmes using POC assays in place of traditional laboratory assays [65,66].
In South Africa, access to ART during pregnancy has steadily increased, leading to a 76% reduction in new HIV infections among children [1]. If PMTCT programmes continue their success, MTCT rates will fall below 2%, as they have in countries such as Thailand and Cuba [80,81]. As expected, we found that confirmatory testing becomes increasingly critical to reduce false-positive diagnoses when MTCT risks are very low, due to the reduced PPV of diagnostic assays at low disease prevalence (Fig 3). This remained true even when NAAT specificity was extremely high (99.9%). In addition to disease prevalence, assay sensitivity also contributes to PPV. We found that small reductions in NAAT sensitivity, a theoretical result of maternal and infant ART for treatment and MTCT prevention [82], would only minimally increase the number of infants initiating ART after false-positive diagnosis, although lower sensitivity would lead to fewer infected infants being identified and would reduce LE for HIV-infected infants. Finally, opportunities for false-positive results increase when testing is done twice; the inclusion of confirmatory testing within the EID cascade is even more important when programmes consider the addition of birth testing to a 6-week testing programme.
There were 3 main limitations of the analysis. First, uncertainty exists in all long-term projections; although our model was calibrated to ensure results match current survival and OI data, developments in treatment and technology will undoubtedly occur in the coming years [19,20]. Our model structure does not currently permit formal probabilistic sensitivity analyses; however, we performed extensive univariate and multivariate sensitivity analyses to assess the impact of uncertainty around all key input parameters, in keeping with international guidelines [71] (Table G in S1 Text). Confirmatory testing remained cost-saving in a wide range of evaluated scenarios, unless the unlikely thresholds shown in Fig 2 were met or surpassed. Second, while we included all relevant costs for HIV-uninfected infants on ART, we did not include negative clinical impacts for these patients, such as stigma, morbidity or mortality related to ART toxicity, or reduced quality of life after false-positive diagnosis. Including such harms from false-positive diagnosis would further improve the value of confirmatory testing. Finally, this work primarily addresses HIV-exposed infants in South Africa, although our findings may be generalisable to a range of settings: sensitivity analyses demonstrated that confirmatory testing remained cost-saving even with wide variations in MTCT risk, NAAT specificity and sensitivity, NAAT costs, and costs for HIV care and ART.
In summary, we find that use of a second NAAT for confirmatory testing in EID programmes substantially reduces the proportion of infants incorrectly diagnosed as HIV-infected and initiated on ART. While projected cost differences are small, confirmatory testing is cost-saving under a wide range of scenarios in South Africa. Confirmatory testing with ART initiation at the first positive result, as recommended by WHO for EID, should be implemented in settings using NAAT for EID.
Supporting Information
Zdroje
1. World Health Organization. 2015 progress report on the global plan towards the elimination of new HIV infections among children and keeping their mothers alive. Geneva: World Health Organization; 2015 [cited 2017 Oct 20]. Available from: http://www.unaids.org/sites/default/files/media_asset/JC2774_2015ProgressReport_GlobalPlan_en.pdf.
2. Newell ML, Coovadia H, Cortina-Borja M, Rollins N, Gaillard P, Dabis F. Mortality of infected and uninfected infants born to HIV-infected mothers in Africa: a pooled analysis. Lancet. 2004;364(9441):1236–43. doi: 10.1016/S0140-6736(04)17140-7 15464184
3. Ghadrshenas A, Ben Amor Y, Chang J, Dale H, Sherman G, Vojnov L. Improved access to early infant diagnosis is a critical part of a child-centric prevention of mother-to-child transmission agenda. AIDS. 2013;27(Supp 2):S197–205.
4. Mayaux M-J, Burgard M, Teglas J, Cottalorda J, Krivine A, Puel J, et al. Neonatal characteristics in rapidly progressive perinatally acquired HIV-1 disease. JAMA. 1996;275(8):606–10. 8594241
5. Violari A, Cotton MF, Gibb DM, Babiker AG, Steyn J, Madhi SA, et al. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med. 2008;359(21):2233–44. doi: 10.1056/NEJMoa0800971 19020325
6. World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. Geneva: World Health Organization; 2013 [cited 2017 Oct 20]. Available from: http://www.who.int/hiv/pub/guidelines/arv2013/download/en/index.html.
7. World Health Organization. Consolidated guidelines on HIV testing services. 5Cs: consent, confidentiality, counselling, correct results, and connection. Geneva: World Health Organization; 2015.
8. Simpson BJ, Andiman WA. Difficulties in assigning human immunodeficiency virus-1 infection and seroreversion status in a cohort of HIV-exposed in children using serologic criteria established by the Centers for Disease Control and Prevention.Pediatrics. 1994;93(5):840–2. 8165094
9. World Health Organization. WHO recommendations on the diagnosis of HIV infection in infants and children. Geneva: World Health Organization; 2010 [cited 2017 Oct 20]. Available from: http://whqlibdoc.who.int/publications/2010/9789241599085_eng.pdf.
10. Burgard M, Blanche S, Jasseron C, Descamps P, Allemon MC, Ciraru-Vigneron N, et al. Performance of HIV-1 DNA or HIV-1 RNA tests for early diagnosis of perinatal HIV-1 infection during anti-retroviral prophylaxis. J Pediatr. 2012;160(1):60–7. doi: 10.1016/j.jpeds.2011.06.053 21868029
11. Sherman GG, Cooper PA, Coovadia AH, Puren AJ, Jones SA, Mokhachane M, et al. Polymerase chain reaction for diagnosis of human immunodeficiency virus infection in infancy in low resource settings. Pediatr Infect Dis J. 2005;24(11):993–7. 16282936
12. Kuhn L, Schramm D, Shiau S, Strehlau R, Pinillos F, Technau K, et al. Young age at start of antiretroviral therapy and negative HIV antibody results in HIV-infected children when suppressed. AIDS. 29(9):1053–60. doi: 10.1097/QAD.0000000000000677 25870988
13. Payne H, Mkhize N, Otwombe K, Lewis J, Panchia R, Callard R, et al. Reactivity of routine HIV antibody tests in children who initiated antiretroviral therapy in early infancy as part of the Children with HIV Early Antiretroviral Therapy (CHER) trial. Lancet Infect Dis. 2015;15(7):803–9. doi: 10.1016/S1473-3099(15)00087-0 26043884
14. South Africa National Department of Health. National consolidated guidelines for the prevention of mother-to-child transmission of HIV (PMTCT) and the management of HIV in children, adolescents and adults. Pretoria: South Africa National Department of Health; 2015 [cited 2017 Oct 20]. Available from: https://aidsfree.usaid.gov/sites/default/files/tx_south-africa_pmtct_2015.pdf.
15. World Health Organization. WHO recommendations on the diagnosis of HIV infection in infants and children: annexes. Geneva: World Health Organization; 2010 [cited 2017 Nov 7]. Available from: http://apps.who.int/iris/bitstream/10665/44275/2/9789241599085_eng_Annexes.pdf.
16. World Health Organization. March 2014 supplement to the 2013 consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. Geneva: World Health Organization; 2014 [cited 2017 Oct 20]. Available from: http://apps.who.int/iris/bitstream/10665/104264/1/9789241506830_eng.pdf?ua=1.
17. World Health Organization. Social, public health, human rights, ethical and legal implications of misdiagnosis of HIV status. Geneva: World Health Organization; 2016 [cited 2017 Nov 7]. Available from: http://www.who.int/hiv/pub/meetingreports/hiv-misdiagnosis-report/en/.
18. Shanks L, Klarkowski D, O’Brien DP. False positive HIV diagnoses in resource limited settings: operational lessons learned for HIV programmes. PLoS ONE. 2013;8(3):e59906. doi: 10.1371/journal.pone.0059906 23527284
19. Ciaranello AL, Morris BL, Walensky RP, Weinstein MC, Ayaya S, Doherty K, et al. Validation and calibration of a computer simulation model of pediatric HIV infection. PLoS ONE. 2013;8(12):e83389. doi: 10.1371/journal.pone.0083389 24349503
20. Ciaranello AL, Doherty K, Penazzato M, Lindsey JC, Harrison L, Kelly K, et al. Cost-effectiveness of first-line antiretroviral therapy for HIV-infected African children less than 3 years of age. AIDS. 2015;29(10):1247–59. doi: 10.1097/QAD.0000000000000672 25870982
21. Francke JA, Penazzato M, Hou T, Abrams EJ, MacLean RL, Myer L, et al. Clinical impact and cost-effectiveness of diagnosing HIV infection during early infancy in South Africa: test timing and frequency. J Infect Dis. 2016;214(9):1319–28. doi: 10.1093/infdis/jiw379 27540110
22. Kuhn L, Kroon M. Breastfeeding and the 2015 South African guidelines for prevention of mother-to-child transmission of HIV. S Afr J HIV Med. 2015;16(1):377.
23. Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D. Consolidated Health Economic Evaluation Reporting Standards (CHEERS)—explanation and elaboration: a report of the ISPOR Health Economic Evaluations Publication Guidelines Task Force. Value Health. 2013;16 : 231–50. doi: 10.1016/j.jval.2013.02.002 23538175
24. Statistics South Africa. Recorded live births: 2013. Statistical release P0305. Pretoria: Statistics South Africa; 2016 [cited 2017 Oct 23]. Available from: http://www.statssa.gov.za/publications/P0305/P03052013.pdf.
25. South Africa National Department of Health. The 2013 national antenatal sentinel HIV prevalence survey South Africa. Pretoria: South Africa National Department of Health; 2015 [cited 2017 Oct 23]. Available from: https://www.health-e.org.za/wp-content/uploads/2016/03/Dept-Health-HIV-High-Res-7102015.pdf.
26. World Health Organization. Early detection of HIV infection in infants and children: guidance note on the selection of technology for the early diagnosis of HIV in infants and children. Geneva: World Health Organization; 2007.
27. Feucht UD, Forsyth B, Kruger M. False-positive HIV DNA PCR testing of infants: implications in a changing epidemic. S Afr Med J. 2012;102(3 Pt 1):149–52.
28. Ciaranello AL, Lu Z, Ayaya S, Losina E, Musick B, Vreeman R, et al. Incidence of WHO stage 3 and 4 events, tuberculosis, and mortality in untreated, HIV-infected children enrolling in care before 1 year of age: an IeDEA (International Epidemiologic Databases To Evaluate AIDS) East Africa regional analysis. Pediatr Infect Dis J. 2014;33(6):623–9. doi: 10.1097/INF.0000000000000223 24378935
29. Violari A, Lindsey JC, Hughes MD, Mujuru HA, Barlow-Mosha L, Kamthunzi P, et al. Nevirapine versus ritonavir-boosted lopinavir for HIV-infected children. N Engl J Med. 2012;366(25):2380–9. doi: 10.1056/NEJMoa1113249 22716976
30. Palumbo P, Lindsey J, Hughes M. Antiretroviral treatment for children with peripartum nevirapine exposure. N Engl J Med. 2010;363 : 1510–20. doi: 10.1056/NEJMoa1000931 20942667
31. Becquet R, Marston M, Dabis F, Moulton LH, Gray G, Coovadia HM, et al. Children who acquire HIV infection perinatally are at higher risk of early death than those acquiring infection through breastmilk: a meta-analysis. PLoS ONE. 2012;7(2):e28510. doi: 10.1371/journal.pone.0028510 22383946
32. Ciaranello AL, Perez F, Keatinge J, Park JE, Engelsmann B, Maruva M, et al. What will it take to eliminate pediatric HIV? Reaching WHO target rates of mother-to-child HIV transmission in Zimbabwe: a model-based analysis. PLoS Med. 2012;9(1):e1001156. doi: 10.1371/journal.pmed.1001156 22253579
33. Mallampati D, Ford N, Hanaford A, Sugandhi N, Penazzato M. Performance of virological testing for early infant diagnosis: a systematic review. J Acquir Immune Defic Syndr. 2017;75(3):308–14. doi: 10.1097/QAI.0000000000001387 28418986
34. Lilian RR, Kalk E, Bhowan K, Berrie L, Carmona S, Technau K, et al. Early diagnosis of in utero and intrapartum HIV infection in infants prior to 6 weeks of age. J Clin Microbiol. 2012;50(7):2373–7. doi: 10.1128/JCM.00431-12 22518871
35. Ciaranello AL, Park J-E, Ramirez-Avila L, Freedberg KA, Walensky RP, Leroy V. Early infant HIV-1 diagnosis programs in resource-limited settings: opportunities for improved outcomes and more cost-effective interventions. BMC Med. 2011;9 : 59. doi: 10.1186/1741-7015-9-59 21599888
36. Hsiao N-Y, Stinson K, Myer L. Linkage of HIV-infected infants from diagnosis to antiretroviral therapy services across the Western Cape, South Africa. PLoS ONE. 2013;8(2):e55308. doi: 10.1371/journal.pone.0055308 23405133
37. Sherman GG, Lilian RR, Bhardwaj S, Candy S, Barron P. Laboratory information system data demonstrate successful implementation of the prevention of mother-to-child transmission programme in South Africa. S Afr Med J. 2014;104(3):235–8.
38. Holmes C, Wood R, Badri M. CD4 decline and incidence of opportunistic infections in Cape Town, South Africa: implications for prophylaxis and treatment. J Acquir Immune Defic Syndr. 2006;42 : 464–9. doi: 10.1097/01.qai.0000225729.79610.b7 16810113
39. Cleary S, Okorafor OA, Chitha W, Boulle A, Jikwana S. Financing antiretroviral treatment and primary health care services. S Afr Health Rev. 2005;58–74.
40. World Health Organization. Cost effectiveness and strategic planning (WHO-CHOICE). Geneva: World Health Organization; 2017 [cited 2017 Oct 31]. Available from: http://www.who.int/choice/en/.
41. World Health Organization. Tables of costs and prices used in WHO-CHOICE analysis. Geneva: World Health Organization; 2017 [cited 2017 Oct 31]. Available from: http://www.who.int/choice/costs/en/.
42. Clinton Health Access Initiative. 2015 antiretroviral (ARV) CHAI reference price list. Boston: Clinton Health Access Initiative; 2015 [cited 2017 Nov 7]. Available at: http://www.clintonhealthaccess.org/content/uploads/2016/01/2015-CHAI-ARV-Reference-Price-List.pdf.
43. Doherty K, Essajee S, Penazzato M, Holmes C, Resch S, Ciaranello A. Estimating age-based antiretroviral therapy costs for HIV-infected children in resource-limited settings based on World Health Organization weight-based dosing recommendations. BMC Health Serv Res. 2014;14(1):201.
44. Iliff PJ, Piwoz EG, Tavengwa NV, Zunguza CD, Marinda ET, Nathoo KJ, et al. Early exclusive breastfeeding reduces the risk of postnatal HIV-1 transmission and increases HIV-free survival. AIDS. 2005;19(7):699–708. 15821396
45. Lilian RR, Johnson LF, Moolla H, Sherman GG. A mathematical model evaluating the timing of early diagnostic testing in HIV-exposed infants in South Africa. J Acquir Immune Defic Syndr. 2014;67(3):341–8. doi: 10.1097/QAI.0000000000000307 25118910
46. Kilewo C, Karlsson K, Ngarina M, Massawe A, Lyamuya E, Swai A, et al. Prevention of mother-to-child transmission of HIV-1 through breastfeeding by treating mothers with triple antiretroviral therapy in Dar es Salaam, Tanzania: the Mitra Plus study. J Acquir Immune Defic Syndr. 2009;52(3):406–16. doi: 10.1097/QAI.0b013e3181b323ff 19730269
47. Shapiro RL, Hughes MD, Ogwu A, Kitch D, Lockman S, Moffat C, et al. Antiretroviral regimens in pregnancy and breast-feeding in Botswana. N Engl J Med. 2010;362(24):2282–94. doi: 10.1056/NEJMoa0907736 20554983
48. Kesho Bora Study Group, de Vincenzi I. Triple antiretroviral compared with zidovudine and single-dose nevirapine prophylaxis during pregnancy and breastfeeding for prevention of mother-to-child transmission of HIV-1 (Kesho Bora study): a randomised controlled trial. Lancet Infect Dis. 2011;11(3):171–80. doi: 10.1016/S1473-3099(10)70288-7 21237718
49. Tonwe-Gold B, Ekouevi DK, Viho I, Amani-Bosse C, Toure S, Coffie PA, et al. Antiretroviral treatment and prevention of peripartum and postnatal HIV transmission in West Africa: evaluation of a two-tiered approach. PLoS Med. 2007;4(8):e257. doi: 10.1371/journal.pmed.0040257 17713983
50. Fawzi W, Msamanga G, Hunter D, Renjifo B, Antelman G, Bang H. Randomized trial of vitamin supplements in relation to transmission of HIV-1 through breastfeeding and early child mortality. AIDS. 16(14):1935–44. 12351954
51. Petra Study Team. Efficacy of three short-course regimens of zidovudine and lamivudine in preventing early and late transmission of HIV-1 from mother to child in Tanzania, South Africa, and Uganda (Petra study): a randomised, double-blind, placebo-controlled trial. Lancet. 2002;359(9313):1178–86. doi: 10.1016/S0140-6736(02)08214-4 11955535
52. Leroy V, Karon J, Alioum A, Ekpini E, Meda N, Greenberg A. Twenty-four month efficacy of a maternal short-course zidovudine regimen to prevent mother-to-child transmission of HIV-1 in West Africa. AIDS. 2002;16(4):631–41. 11873008
53. Chigwedere P, Seage G, Lee T, Essex M. Efficacy of antiretroviral drugs in reducing mother-to-child transmission of HIV in Africa: a meta-analysis of published clinical trials. AIDS Res Hum Retroviruses. 2008;24(6):827–37. doi: 10.1089/aid.2007.0291 18544018
54. Scott GB, Brogly SB, Muenz D, Stek AM, Read JS, International Maternal Pediatric Adolescent AIDS Clinical Trials Group (IMPAACT) P1025 Study Team. Missed opportunities for prevention of mother-to-child transmission of human immunodeficiency virus. Obstet Gynecol. 2017;129(4):621–8. doi: 10.1097/AOG.0000000000001929 28277349
55. Dabis F, Bequet L, Ekouevi DK, Viho I, Rouet F, Horo A, et al. Field efficacy of zidovudine, lamivudine and single-dose nevirapine to prevent peripartum HIV transmission. AIDS. 2005;19(3):309–18. 15718842
56. Thior I, Lockman S, Smeaton LM, Shapiro RL, Wester C, Heymann SJ, et al. Breastfeeding plus infant zidovudine prophylaxis for 6 months vs formula feeding plus infant zidovudine for 1 month to reduce mother-to-child HIV transmission in Botswana. JAMA. 2006;296(7):794. doi: 10.1001/jama.296.7.794 16905785
57. Peltier CA, Ndayisaba GF, Lepage P, van Griensven J, Leroy V, Pharm CO, et al. Breastfeeding with maternal antiretroviral therapy or formula feeding to prevent HIV postnatal mother-to-child transmission in Rwanda. AIDS. 2009;23 : 2415–23. doi: 10.1097/QAD.0b013e32832ec20d 19730349
58. Palombi L, Marazzi MC, Voetberg A, Magid NA. Treatment acceleration program and the experience of the DREAM program in prevention of mother-to-child transmission of HIV. AIDS. 2007;21(Suppl 4):S65–71.
59. Thomas T, Masaba R, Borkowf C, Ndivo R, Zeh C, Misore A. Triple-antiretroviral prophylaxis to prevent mother-to-child HIV transmission through breastfeeding—the Kisumu Breastfeeding Study, Kenya: a clinical trial. PLoS Med. 2011;8(3):e1001015. doi: 10.1371/journal.pmed.1001015 21468300
60. Vyankandondera J, Luchters S, Hassink E. Reducing risk of HIV-1 transmission from mother to infant through breastfeeding using antiretroviral prophylaxis in infants (SIMBA study). Abstract number LB7. 2nd International AIDS Society Conference on HIV Pathogenesis and Treatment; 2003 Jul 13–17; Paris, France.
61. Chasela C, Hudgens M, Jamieson D, Kayira D, Hosseinipour M, Kourtis A. Maternal or infant antiretroviral drugs to reduce HIV-1 transmission. N Engl J Med. 2010;362(24):2271–81. doi: 10.1056/NEJMoa0911486 20554982
62. Kuhn L, Aldrovandi GM, Sinkala M, Kankasa C, Semrau K, Mwiya M, et al. Effects of early, abrupt weaning on HIV-free survival of children in Zambia. N Engl J Med. 2008;359(2):130–41. doi: 10.1056/NEJMoa073788 18525036
63. Kuhn L, Aldrovandi G, Sinkala M, Kankasa C, Mwiya M, Thea D. Potential impact of new WHO criteria for antiretroviral treatment for prevention of mother-to-child HIV transmission. AIDS. 2010;24(9):1374–7. 20568677
64. World Health Organization. New strategies for infant HIV diagnosis: expert review meeting. Geneva: World Health Organization; 2013 Sep 18–19.
65. Hsiao N-Y, Dunning L, Kroon M, Myer L. Laboratory evaluation of the Alere q point-of-care system for early infant HIV diagnosis. PLoS ONE. 2016;11(3):e0152672. doi: 10.1371/journal.pone.0152672 27032094
66. Jani IV, Meggi B, Mabunda N, Vubil A, Sitoe NE, Tobaiwa O, et al. Accurate early infant HIV diagnosis in primary health clinics using a point-of-care nucleic acid test. J Acquir Immune Defic Syndr. 2014;67(1):e1–4. doi: 10.1097/QAI.0000000000000250 24933096
67. Babiker A, Castro nee Green H, Compagnucci A, Fiscus S, Giaquinto C, Gibb D. First-line antiretroviral therapy with a protease inhibitor versus non-nucleoside reverse transcriptase inhibitor and switch at higher versus low viral load in HIV-infected children: an open-label, randomised phase 2/3 trial. Lancet Infect Dis. 2011;11(4):273–83. doi: 10.1016/S1473-3099(10)70313-3 21288774
68. Losina E, Yazdanpanah Y, Deuffic-Burban S, Wang B, Wolf LL, Messou E, et al. The independent effect of highly active antiretroviral therapy on severe opportunistic disease incidence and mortality in HIV-infected adults in Cote d’Ivoire. Antivir Ther. 2007;12(4):543–51. 17668563
69. Thomas LS. Costing of HIV/AIDS services at a tertiary level hospital in Gauteng Province [thesis]. Witwatersrand: University of Witwatersrand; 2006 [cited 2017 Oct 23]. Available from: http://wiredspace.wits.ac.za/handle/10539/2008.
70. Bassett I V, Giddy J, Nkera J, Wang B, Losina E, Lu Z, et al. Routine voluntary HIV testing in Durban, South Africa: the experience from an outpatient department. J Acquir Immune Defic Syndr. 2007;46(2):181–6. doi: 10.1097/QAI.0b013e31814277c8 17667332
71. Briggs A, Weinstein M, Fenwick E. Model parameter estimation and uncertainty: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force–6. Value Health. 2012;15 : 835–42. doi: 10.1016/j.jval.2012.04.014 22999133
72. Feucht U, Thomas W, Forsyth B, Kruger M. Incorrectly diagnosing children as HIV-infected: experiences from a large paediatric antiretroviral therapy site in South Africa. SAJCH. 2012;6(3):72–5.
73. Kachega S, Okoth V, Kadima S, Vihenda S, Okapesi E, Nyambura E, et al. Discrepant test findings in early infant diagnosis of HIV in a national reference laboratory in Kenya: challenges and opportunities for programs. J Trop Pediatr. 2012;58(4):247–52. doi: 10.1093/tropej/fmr076 22052701
74. Dube Q, Dow A, Chirambo C, Lebov J, Tenthani L, Moore M, et al. Implementing early infant diagnosis of HIV infection at the primary care level: experiences and challenges in Malawi. Bull World Health Organ. 2012;90(9):699–704. doi: 10.2471/BLT.11.100776 22984315
75. Dahourou D, Amorissani-Folquet M, Coulibaly M, Avit-Edi D, Meda N, Timite-Konan M. Missed opportunities of inclusion in a cohort of HIV-infected children to initiate antiretroviral treatment before the age of two in West Africa, 2011 to 2013. J Int AIDS Soc. 2016;19(1):20601. doi: 10.7448/IAS.19.1.20601 27015798
76. Feucht UD, Meyer A, Thomas WN, Forsyth BWC, Kruger M. Early diagnosis is critical to ensure good outcomes in HIV-infected children: outlining barriers to care. AIDS Care. 2016;28(1):32–42. doi: 10.1080/09540121.2015.1066748 26273853
77. Gibb D, Duong T, Leclezio VA, Walker AS, Verweel G, Dunn DT, et al. Immunologic changes during unplanned treatment interruptions of highly active antiretroviral therapy in children with human immunodeficiency virus type 1 infection. Pediatr Infect Dis J. 2004;23(5):446–50. 15131469
78. Wamalwa D, Benki-nugent S, Langat A, Tapia K, Ngugi E, Moraa H, et al. Treatment interruption after 2-year antiretroviral treatment (ART) initiated during acute/early HIV in infancy: a randomized trial. AIDS. 2016;30(15):2303–13. doi: 10.1097/QAD.0000000000001158 27177316
79. Bhattacharya R, Barton S, Catalan J. When good news is bad news: psychological impact of false positive diagnosis of HIV. AIDS Care. 2008;20(5):560–4. doi: 10.1080/09540120701867206 18484325
80. World Health Organization. WHO validates countries’ elimination of mother-to-child transmission of HIV and syphilis. Geneva: World Health Organization; 2016 [cited 2017 Nov 7]. Available from: http://www.who.int/mediacentre/news/statements/2016/mother-child-hiv-syphilis/en/.
81. World Health Organization. WHO validates elimination of mother-to-child transmission in Cuba. Geneva: World Health Organization; 2015 [cited 2017 Nov 7]. Available from: http://www.who.int/mediacentre/news/releases/2015/mtct-hiv-cuba/en/.
82. Sherman G. HIV testing during the neonatal period. S Afr J HIV Med. 2015;16(1):2–4.
Štítky
Interní lékařství
Článek Contemporary disengagement from antiretroviral therapy in Khayelitsha, South Africa: A cohort studyČlánek Bioequivalence of twice-daily oral tacrolimus in transplant recipients: More evidence for consensus?
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2017 Číslo 11- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Příznivý vliv Armolipidu Plus na hladinu cholesterolu a zánětlivé parametry u pacientů s chronickým subklinickým zánětem
- Jak postupovat při výběru betablokátoru − doporučení z kardiologické praxe
- Červená fermentovaná rýže účinně snižuje hladinu LDL cholesterolu jako vhodná alternativa ke statinové terapii
-
Všechny články tohoto čísla
- Labour trafficking: Challenges and opportunities from an occupational health perspective
- The end of HIV: Still a very long way to go, but progress continues
- Contemporary disengagement from antiretroviral therapy in Khayelitsha, South Africa: A cohort study
- Bioequivalence of twice-daily oral tacrolimus in transplant recipients: More evidence for consensus?
- Treatment guidelines and early loss from care for people living with HIV in Cape Town, South Africa: A retrospective cohort study
- Perinatal mortality associated with induction of labour versus expectant management in nulliparous women aged 35 years or over: An English national cohort study
- Core Outcome Set-STAndards for Development: The COS-STAD recommendations
- Closing the gaps in the HIV care continuum
- Association between the 2012 Health and Social Care Act and specialist visits and hospitalisations in England: A controlled interrupted time series analysis
- HIV pre-exposure prophylaxis and early antiretroviral treatment among female sex workers in South Africa: Results from a prospective observational demonstration project
- Sexual exploitation of unaccompanied migrant and refugee boys in Greece: Approaches to prevention
- Child sex trafficking in the United States: Challenges for the healthcare provider
- The expanding epidemic of HIV-1 in the Russian Federation
- Cardiovascular disease (CVD) and chronic kidney disease (CKD) event rates in HIV-positive persons at high predicted CVD and CKD risk: A prospective analysis of the D:A:D observational study
- Validity of a minimally invasive autopsy for cause of death determination in maternal deaths in Mozambique: An observational study
- malERA: An updated research agenda for malaria elimination and eradication
- malERA: An updated research agenda for health systems and policy research in malaria elimination and eradication
- A combination intervention strategy to improve linkage to and retention in HIV care following diagnosis in Mozambique: A cluster-randomized study
- Bioequivalence between innovator and generic tacrolimus in liver and kidney transplant recipients: A randomized, crossover clinical trial
- malERA: An updated research agenda for basic science and enabling technologies in malaria elimination and eradication
- Human trafficking and exploitation: A global health concern
- Virological response and resistance among HIV-infected children receiving long-term antiretroviral therapy without virological monitoring in Uganda and Zimbabwe: Observational analyses within the randomised ARROW trial
- Postmenopausal hormone therapy and risk of stroke: A pooled analysis of data from population-based cohort studies
- Lansoprazole use and tuberculosis incidence in the United Kingdom Clinical Practice Research Datalink: A population based cohort
- malERA: An updated research agenda for insecticide and drug resistance in malaria elimination and eradication
- Safety, pharmacokinetics, and immunological activities of multiple intravenous or subcutaneous doses of an anti-HIV monoclonal antibody, VRC01, administered to HIV-uninfected adults: Results of a phase 1 randomized trial
- HIV prevalence and behavioral and psychosocial factors among transgender women and cisgender men who have sex with men in 8 African countries: A cross-sectional analysis
- Treatment eligibility and retention in clinical HIV care: A regression discontinuity study in South Africa
- malERA: An updated research agenda for characterising the reservoir and measuring transmission in malaria elimination and eradication
- Effectiveness of a combination strategy for linkage and retention in adult HIV care in Swaziland: The Link4Health cluster randomized trial
- The value of confirmatory testing in early infant HIV diagnosis programmes in South Africa: A cost-effectiveness analysis
- HIV self-testing among female sex workers in Zambia: A cluster randomized controlled trial
- The US President's Malaria Initiative, transmission and mortality: A modelling study
- Comparison of two cash transfer strategies to prevent catastrophic costs for poor tuberculosis-affected households in low- and middle-income countries: An economic modelling study
- Direct provision versus facility collection of HIV self-tests among female sex workers in Uganda: A cluster-randomized controlled health systems trial
- malERA: An updated research agenda for diagnostics, drugs, vaccines, and vector control in malaria elimination and eradication
- malERA: An updated research agenda for combination interventions and modelling in malaria elimination and eradication
- HIV-1 persistence following extremely early initiation of antiretroviral therapy (ART) during acute HIV-1 infection: An observational study
- Respondent-driven sampling for identification of HIV- and HCV-infected people who inject drugs and men who have sex with men in India: A cross-sectional, community-based analysis
- Extensive virologic and immunologic characterization in an HIV-infected individual following allogeneic stem cell transplant and analytic cessation of antiretroviral therapy: A case study
- Measuring success: The challenge of social protection in helping eliminate tuberculosis
- Prospects for passive immunity to prevent HIV infection
- Reaching global HIV/AIDS goals: What got us here, won't get us there
- Evidence-based restructuring of health and social care
- Extreme exploitation in Southeast Asia waters: Challenges in progressing towards universal health coverage for migrant workers
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Postmenopausal hormone therapy and risk of stroke: A pooled analysis of data from population-based cohort studies
- Bioequivalence between innovator and generic tacrolimus in liver and kidney transplant recipients: A randomized, crossover clinical trial
- HIV pre-exposure prophylaxis and early antiretroviral treatment among female sex workers in South Africa: Results from a prospective observational demonstration project
- Bioequivalence of twice-daily oral tacrolimus in transplant recipients: More evidence for consensus?
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



