-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaVariation of Contributes to Dog Breed Skull Diversity
Since the beginnings of domestication, the craniofacial architecture of the domestic dog has morphed and radiated to human whims. By beginning to define the genetic underpinnings of breed skull shapes, we can elucidate mechanisms of morphological diversification while presenting a framework for understanding human cephalic disorders. Using intrabreed association mapping with museum specimen measurements, we show that skull shape is regulated by at least five quantitative trait loci (QTLs). Our detailed analysis using whole-genome sequencing uncovers a missense mutation in BMP3. Validation studies in zebrafish show that Bmp3 function in cranial development is ancient. Our study reveals the causal variant for a canine QTL contributing to a major morphologic trait.
Published in the journal: . PLoS Genet 8(8): e32767. doi:10.1371/journal.pgen.1002849
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002849Summary
Since the beginnings of domestication, the craniofacial architecture of the domestic dog has morphed and radiated to human whims. By beginning to define the genetic underpinnings of breed skull shapes, we can elucidate mechanisms of morphological diversification while presenting a framework for understanding human cephalic disorders. Using intrabreed association mapping with museum specimen measurements, we show that skull shape is regulated by at least five quantitative trait loci (QTLs). Our detailed analysis using whole-genome sequencing uncovers a missense mutation in BMP3. Validation studies in zebrafish show that Bmp3 function in cranial development is ancient. Our study reveals the causal variant for a canine QTL contributing to a major morphologic trait.
Introduction
Canine skull shape variation among dog breeds is in large part a human-created phenomenon, occurring through artificial selection and consolidation of desired traits. Morphological distinction between wolves and dogs dates as far back as 31,000 years ago [1], [2]. Changes in skull shape are a key feature of dog domestication, foreshadowing the wide variety of shapes displayed by modern dog breeds.
Skull shapes differ tremendously from one another, so much so that such differences are breed-defining. Two such skull shapes are brachycephaly (“shortened head”, e.g. Bulldog, Pug, Boxer) and dolichocephaly (“elongated head”, e.g. Greyhound, Saluki, Collie), which are named after their resemblance to human cephalic disorders. Although canine cranial shape is subject to multigenic control [3]–[5], the molecular underpinnings of this variation remain poorly defined. Candidate gene studies failed to uncover compelling causal variants of canine brachycephaly [6]–[8]. Airorhynchy (dorsal bending of the snout; a feature common to brachycephalic breeds) and midface length was previously correlated with polyglutamine and polyalanine repeat length of the transcription factor RUNX2 [4]. More recently, genome wide association scans (GWAS) and homozygosity mapping have converged on chromosome 1 (CFA1) as a locus that is highly associated with brachycephaly, implicating a 296 kb haplotype that spans THSB2 and intergenic sequence proximal to SMOC2 [3], [9], [10].
Here we present data indicating that at least five genetic loci are responsible for the cranioskeletal differences that differentiate dolichocephalic and brachycephalic dog breeds. Our conclusions are based on a GWAS that coupled craniometric breed-sex averages collected from 533 modern specimens from museum and private collections with the genetic profiles of 576 purebred dogs (62 breeds) assayed via single nucleotide polymorphism (SNP) chips. To identify candidates of phenotype causality, we filtered genetic variants derived from whole genome sequencing of eleven different breeds. This led to discovery of a compelling candidate for causality at the CFA32 QTL: a derived missense mutation in BMP3 that is nearly fixed among small, brachycephalic dog breeds. To evaluate the functional potential of this variant in vivo, we turned to zebrafish. We show that Bmp3 is indispensable for normal craniofacial development in zebrafish, and comparison of missexpression assays using BMP3 and its canine variant suggests enhanced activity in the latter. Together, our data reveal for the first time the molecular underpinnings of a quantitative trait, selected by dog fanciers to modulate a prominent morphological trait in domestic dogs.
Results
To capture the three-dimensional morphological complexities present among modern dogs, we digitized 51 stereotyped landmarks from 533 skulls representing 120 breeds and four gray wolf subspecies (Figure 1A–1D, Figure S1A–S1E, Tables S1 and S2). As most skulls used in our study originated from museums, we selected only those specimens with unambiguous breed designations, sex status, and recent time of death (within the past 40 years) for use in this study. Using MorphoJ software [11], we identified four principal components that accounted for nearly 75.5% of shape variance, with the majority of variance explained by the first component (PC1 = 59.4%, PC2 = 8.2%, PC3 = 4.2%, PC4 = 3.8%). PC1 describes profound changes in rostrum length and angle, palate and zygomatic arch width, and depth of the neurocranium: essentially the continuum of cranioskeletal features that extend between dolichocephalic and brachycephalic breeds (Figure 1E, 1F).
Fig. 1. Quantitative and qualitative assessments of PC1 on canine cranioskeletal shape. 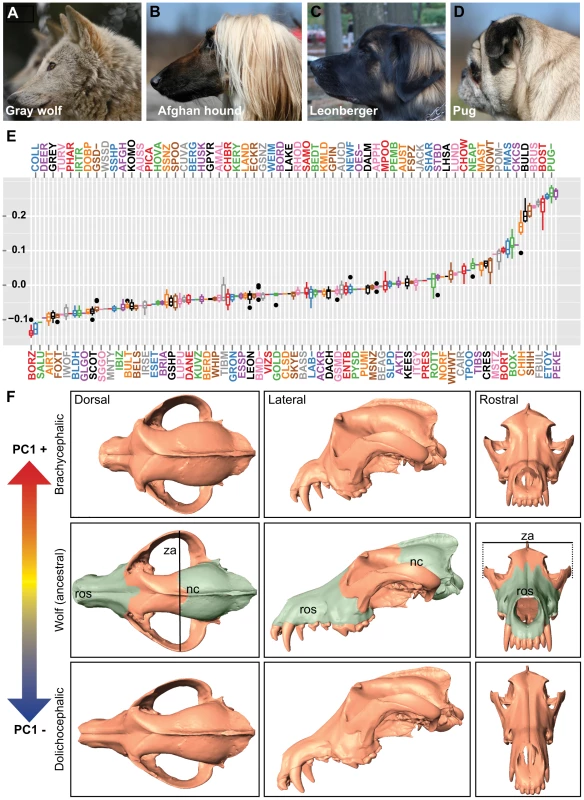
(A) Gray wolf (mesocephalic, ancestor to dogs) (B) Afghan hound (dolichocephalic), (C) Leonberger (mesocephalic), (D) Pug (brachycephalic). (E) Boxplots of PC1 (corresponding breed names are listed in Table S2). (F) Surface scans of a gray wolf skull illustrate morphological changes associated with PC1. Columns (left to right) are dorsal, lateral, and rostral views. Top row: a gray wolf skull morphed by positive PC1. Middle row: a gray wolf skull (no morphing). Bottom row: a gray wolf skull morphed by negative PC1. Pseudocoloring of the gray wolf skull indicates rostrum (ros) and neurocranium (nc). Line indicates width of the zygomatic arches (za). Since purebred dogs must conform to specific morphological standards [12], morphological traits like skull shape became highly uniform by breed, permitting association studies using one set of samples for genotyping and others for phenotyping. This strategy of using breed stereotypes has proven successful in mapping a number of canine morphologic traits by independent groups [3], [13], [14]. Using breed allele frequencies collected by the CanMap project [3], we conducted genome-wide scans of QTLs associated with breed-sex averages for PC1 (1–10 specimen(s)/breed/sex, mean n = 3, Table S3).
Initially, we scanned for PC1 associations using an additive linear regression model (Figure S2A, Table S4) [15]. Size correction in the regression suggested potential confounders (compare Figure S2A and S2B) on CFA10 and 15, which were previously associated with body size [3], [13], [14], [16]. As expected, addition of log(neurocranium centroid) breed-sex values as a covariate removed those associations (Figure S2B, see Materials and Methods for more details).
False associations derived from breed relatedness were excluded using GEMMA [17]. Discounting associations on CFA10 and 15, we identified six PC1-associated regions of interest indicated by SNPs at CFA1.59832965, CFA5.32359028, CFA24.26359293, CFA30.35656568, CFA32.8384767, and CFAX.44401786 (−log10(P) = 6.13–17.9, Figure 2A, Table S4). Of note, a suggestive association on CFAX was also observed, marked by SNP CFAX.104724717. Including a neurocranium centroid size covariate in the mixed-model removed associations at CFA10 and 15, as well as those on CFA30, 32, and X.44401786 and enhanced the association on CFAX.104724717 to significance (Figure 2B, Table S4). Since nearly all extreme brachycephalic breeds used in our study are also small breeds, and therefore substantially related to small, non-brachycephalic breeds, we reasoned that use of a size covariate in the mixed-model was overcorrecting associations that could be driven by diminutive breeds [3], [18], [19]. To reduce the contrast in relatedness among our study population, we reran the mixed-model using only breeds with a log(neurocranium centroid) below the 50th percentile. This resulted in recovery of the CFA32 QTL, as well as new associations marked by SNPs at CFA9.50988217 and CFA13.26492600. Although the association on CFA30 remained below threshold for statistical significance, its association markedly improved (Figure 2C). When brachycephalic breeds were removed from the mixed-model, all aforementioned markers dropped below significance except for CFA5.36476657 (Figure 2D). Summarizing these findings, QTLs on CFA1, 5, 24, 32, and X (X:104724717) account for skull shape changes that occur along the continuum of canine brachycephaly-dolichocephaly. Additional associations reside on CFA9, CFA13, CFA30, and CFAX (X: 44401786), though their instability across mixed-model scans suggest they are either allometric in nature, driven by variation that is marginally represented by the breed composition present in our GWAS, or possibly false positives (Figure 2A–2D, Table S4).
Fig. 2. PC1 GWAS and fine mapping at CFA32. 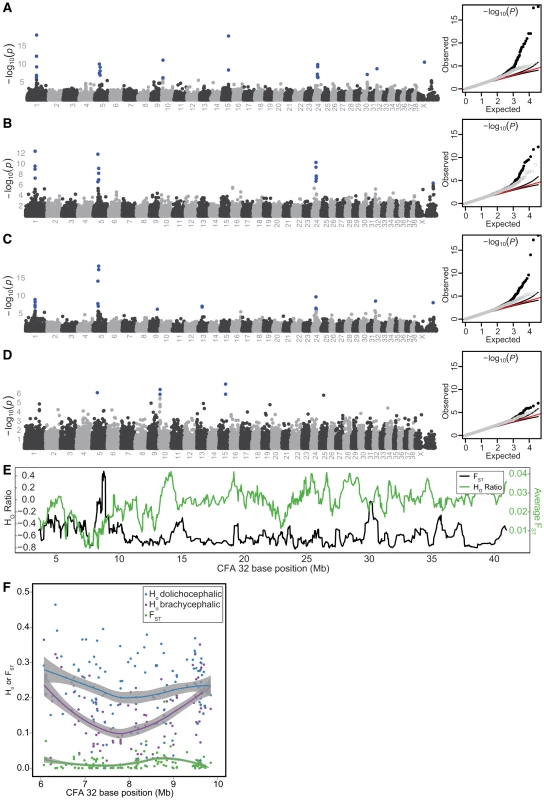
All GWAS used the mixed-model GEMMA. Chromosomes listed on the x-axis, −log10(P) on the y-axis. SNPs remaining significant following Bonferroni correction are colored blue. Q-Q plots of observed versus expected −log10(P) are depicted on right, with full SNP dataset (black circles), pruned dataset (grey circles), expected values (red lines), and 95% confidence intervals (black lines). Scan results using breed-sex averages of PC1 without (A) and with a breed-sex average size covariate (B). Including a size covariate in the mixed-model overcorrects, leading to loss of associations on CFA 30 and 32.(C) Scan results using PC1 breed-sex averages and breed-sex size covariates. In this scan, only breeds whose neurocranium size ranked within the smallest 50% of our dataset where analyzed. By reducing relatedness disparity in our study population, the association on CFA32 remains significant despite size correction. (D) Scan results using all breed-sex averages of PC1, but excluding extreme brachycephalic breeds (Pug, Pekingese, Boston Terrier, Shih Tzu, Brussels Griffon, French Bulldog, Bulldog, Boxer, Cavalier King Charles Spaniel, Chihuahua). (E) Average log(HO ratios) or FST from ten-SNP sliding windows. (F) Regional HO or FST values, and their respective Lowess best fit curves. Because shape variation is the result of artificial selection, we expected critical loci to be marked by reduction of observed heterozygosity (Ho) and elevated genetic differentiation (FST), hallmarks of selective sweeps [20], [21]. Among autosomes, QTLs on CFA1, 5, 30, and 32 displayed particularly strong reductions in HO among brachycephalic breeds, relative to dolichocephalic breeds (HR, see Materials and Methods). Sliding HR windows corresponding to these QTLs placed with the smallest <0.2% of the distribution. Among sliding window FST averages, windows corresponding to CFA1, 5, 24, 30, and 32 placed within the top 99.6% of the distribution (Figure 2E–2F, Figure S3A–S3I, and Table S5).
We focused on the CFA32 QTL because it was the second most highly associated, non-allometric locus in our initial analysis (Figure S2A), it showed compelling evidence of selection, and unlike the CFA1 QTL, it was previously unexplored [9]. Haplotype sharing at this locus among six of the seven extreme brachycephalic breeds, including the French Bulldog, Bulldog, Boston Terrier, Pekingese, Pug, and Brussels Griffon defined a critical interval spanning 190 kb (8,152,258–8,342,370, Table S6). Although this region included just two genes, both were excellent candidates: cGMP-dependent protein kinase 2 (PRKG2) and bone morphogenetic protein 3 (BMP3) [22]–[27].
To identify variants within the critical interval, we used whole-genome sequence analysis from eleven dog breeds of widely varying skull shapes (unpublished data). Notably, brachycephalic breeds including a Pekingese and a Bulldog were among the eleven, enabling the evaluation of phenotype association with genotype at nearly every position. Initial examination of variant calls in the 190 kb critical interval revealed over 2,000 polymorphisms (Table S7). Of particular interest, allelic differences between the Bulldog and Pekingese extended downstream of 8,237,936, suggesting a recombination breakpoint in the Pekingese. Confirmation of this breakpoint among 25 additional Pekingese reduced the critical interval to 85 kb (8,152,258–8,237,937 kb) (Figure 3A, Table S8).
Fig. 3. Genetic variation at the CFA32 QTL includes a brachycephaly-associated missense mutation within BMP3. 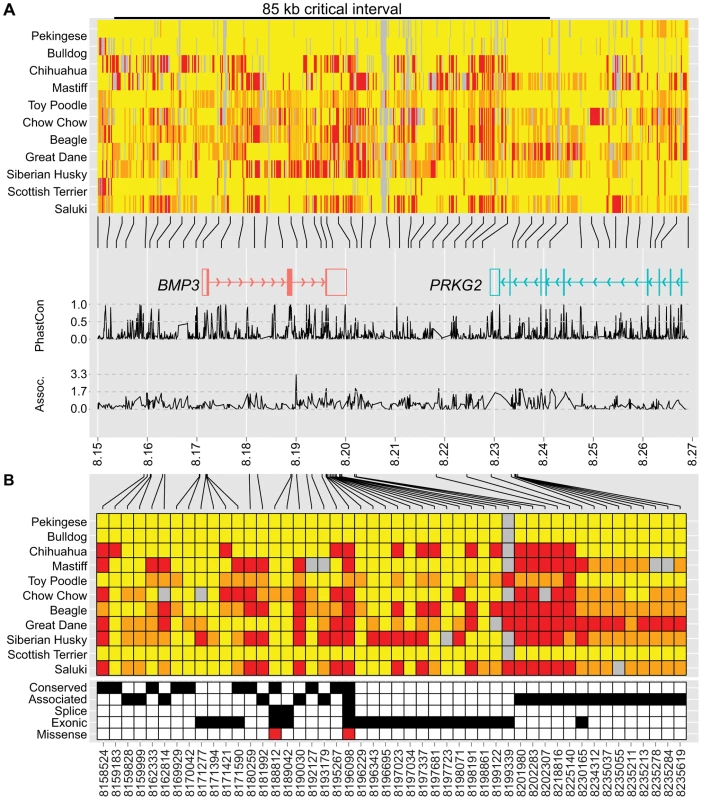
For display purposes, we set the reference sequence to be the allele most common to Pekingese and Bulldog. Variants located within 8.15–8.27 Mb (A) or the 85 kb critical interval (B) are illustrated (homozygous reference = yellow, heterozygous = orange, homozygous variant = red). (A) Pekingese and Bulldog agree across an 85 kb interval (black bar) including BMP3 (red) and a portion of PRKG2 (aqua). Line graphs below genes plot conservation (phastCons4way) and association (−log10(P)) with respect to variant position (28). (B) Variants of interest met one or more of the following criteria: conserved (phastCons4way score ≥0.7), associated (an association P-value among the smallest 5% of P-values, see Materials and Methods), exonic (untranslated regions and coding), or splice (located within 20 bp of an exon boundary). Forty-eight variants of interest remained after applying filtering criteria, including a F452L mutation in BMP3 at position 8,196,098. Within the 85 kb interval, 48 variants that met one or more standard criteria were retained for further evaluation (Figure 3B). Only one variant remained a compelling candidate for causality: a SNP at 8,196,098 that encodes a missense mutation in BMP3, changing a phenylalanine to a leucine (BMP3F452L or F452L). The Protein Specific Scoring Matrix (PSSM) for TGF-β superfamily members indicates that position 452 is nearly invariably occupied by an aromatic amino acid such as tyrosine or phenylalanine (PSSM raw frequency = 0.84) and PolyPhen-2 substitution modeling predicted that the F452L substitution is likely damaging (HumDiv = 1.0, HumVar = 0.97) [28]. Moreover, F452 flanks highly conserved residues predicted to reside at the receptor-ligand interface [29]. Finally, expanded genotyping among 842 dogs from 113 breeds revealed that the BMP3F452L mutation is nearly fixed among extreme brachycephalic breeds. Furthermore, the PC1 scores of most carrier breeds fall between wolves (ancestral) and extreme brachycephalic breeds (Table S9).
BMP3's role in cranioskeletal development is enigmatic in terms of molecular interactions and function. BMP3 antagonizes other BMPs and Activins through binding the ActRIIb receptor, and in vivo, BMP3 appears to restrict bone growth [23], [30], [31]. However, the absence of a knockout mouse craniofacial phenotype suggested that BMP3 function might be subtle, dispensable, or divergent to other mammals. We therefore assayed BMP3 function using the zebrafish model. Based on peptide similarity and synteny to CFA32 (96.4% identical within mature protein, 60.5% overall), the BMP3 ortholog was identified on zebrafish chromosome 5. Endogenous expression of zebrafish bmp3 is highly dynamic, first appearing during mid-somitogenesis as ubiquitous expression throughout the head, brain ventricles, and as was shown previously, the posterior somites (data not shown) [32]. After 48 hours post fertilization (hpf), bmp3 expression emerges in pectoral fins, the pharyngeal arch region, heart, and jaw structures (Figure 4A–4D, data not shown). Prechondrogenic expression of bmp3 among cranial structures suggests a role for Bmp3 in cranioskeletal development. To formally test this hypothesis, we knocked down endogenous Bmp3 activity via injection of translation-blocking antisense morpholino oligonucleotides (MO). Strikingly, MO-injected embryos demonstrated severe deficiencies in jaw development (Figure 4E, 4H, 4K). Alcian blue staining revealed loss or hypoplasia of multiple cartilage elements that form the viscerocranium and neurocranium (Figure 4F, 4G, 4I, 4J, 4L, 4M). Cartilage defects are specific to loss of Bmp3 activity since injection of two non-overlapping MOs produced identical craniofacial phenotypes, as did co-injection of both MOs at concentrations insufficient to cause phenotypes when injected alone (data not shown). These results indicate that Bmp3 is required for zebrafish craniofacial development, and indicate that Bmp3's role in craniofacial development is ancient. Furthermore, overexpression assays using BMP3, as well as other TGFβs, indicate that variation at the F452L residue has context-dependent effects on these molecules' activities (Figures S4, S5).
Fig. 4. Zebrafish cranioskeletal development requires Bmp3 function. 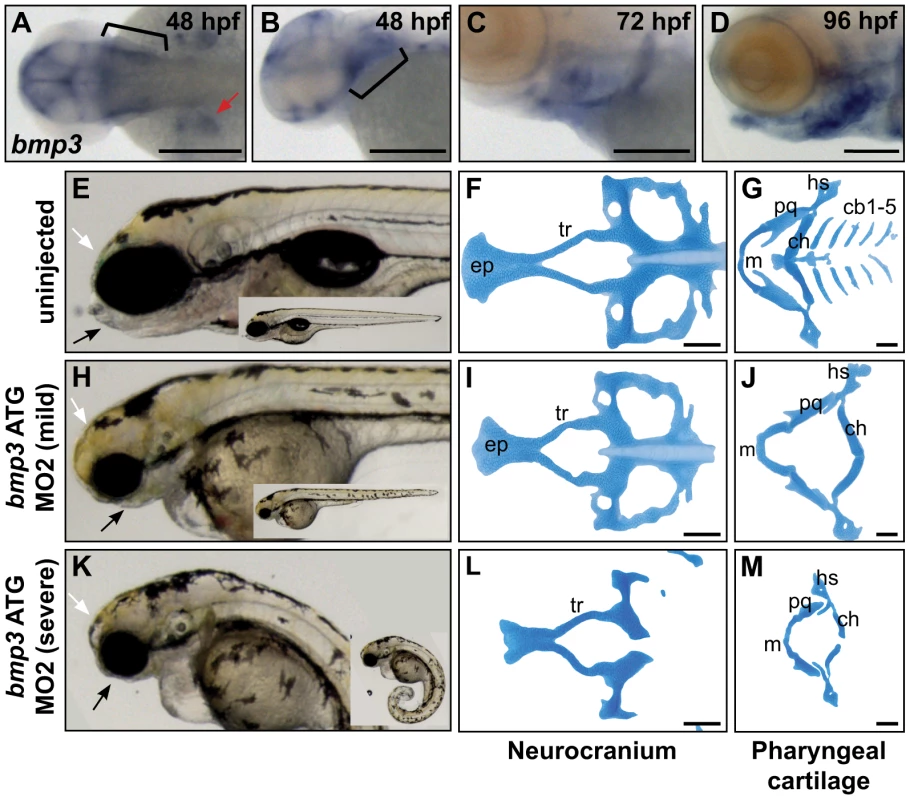
(A–H) Wholemount RNA in situ hybridization of bmp3 expression at 48 hpf (A,B), 72 hpf (C), and 96 hpf (D) stages. Anterior to the left. (A) Dorsal view, (B–D) lateral view. Pharyngeal arches indicated by brackets, pectoral fins by red arrowheads. Wholemounts (E,H,K) and alcian blue cartilage stains (F,G,I,J,L,M) of 96 hpf embryos from uninjected (E–G) and morpholino-injected embryos (h–j, mild phenotype, n = 72/177; k–m, severe phenotype, n = 83/177). Phenotypic severity is distinguished by tail curling (compare insets). Loss of jaw structures (black arrows) and frontal bossing (white arrowheads) is apparent in both classes of morphants. Cartilage is severely dysmorphic, hypoplastic, or absent following Bmp3 knockdown. Abbreviations correspond to ceratobranchial (cb), ceratohyal (ch), eythmoid plate (ep), hyosymplectic (hs), Meckel's (m), palatoquadrate (pq), and trabeculae (tr) cartilages. Discussion
Distortion of the skull, as observed among brachycephalic and dolichocephalic dog breeds, affects bones presumably derived from endochondral and intramembraneous ossification. We show that the genetic basis of this distortion is complex, relying on the contributions of at least five QTLs. We propose that the BMP3F452L variant was selected by dog fanciers for its influence on skull shape, but the specific aspects of cranioskeletal development that the F452L variant affects within the brachycephalic skull remain unclear.
Previous studies, as well as ours, indicate that the CFA1 QTL is highly associated with canine brachycephaly and is robust to size-stratified GWAS (Figure 2A and 2C, data not shown), suggesting that the underlying causal variant at this locus is shared by both large and small brachycephalic breeds [3], [9]. Homozygosity mapping also implicated selective sweeps on CFA1, as well as CFA26, among Boxers, French Bulldogs, and Bulldogs [10]. Despite different morphometric approaches, skulls specimens, and utilization of CanMap genotypes profiles, our QTLs overlap with those reported by Boyko et al. for snout length (CFA1, 5, 32, X), cranial vault depth (CFAX), palate width (CFA30), and zygomatic arch width (CFA24) [3]. The associations that we report on CFA9 and 13 were revealed following size-stratified scans, raising caution regarding the implementation of mixed-model scans among domesticated populations whose traits and relatedness are difficult to disentangle. Notably, a snout ratio QTL on CFA9 was previously reported by Jones et al. in a study that also used breed stereotypes as phenotypes; our data independently replicates their finding [14].
We chose the zebrafish model to validate our GWAS results based on its rapid development, gene conservation, and flexibility for rapidly knocking down and overexpressing gene products of interest. Though loss-of-function using zebrafish indicates an ancient role for Bmp3 during craniofacial development, ontogenetic differences between teleost and amniote cranial development limit the extent to which specific phenotypic features can be recapitulated in both zebrafish and dogs.
Bmp3−/− mice described by Daluiski et al. have excessive trabeculation of the long bones, but defects in the cranial bones were not reported [23]. Interestingly, when authors of this study moved the Bmp3 null allele to an inbred background, Bmp3−/ − mutants died perinatally due to lung defects. A preliminary craniofacial analysis of E18.5 embryos suggests that a number of morphogenesis defects occur in the mutants (unpublished data, JJS and KLM; personal communication with KLM). In dogs, the BMP3 mutation is but one of at least five QTLs that modulate canine skull shape variation. Thus, it is possible that genetic interactions with other QTLs enhance or act permissively to BMP3F452L's effects on cranioskeletal development.
Microdeletions that include or flank BMP3 are described in humans [33]. Although craniofacial abnormalities associated with these microdeletions were attributed to loss of PRKG2, our results suggest that haploinsufficiency for BMP3 might also contribute to the clinical features of 4q21 syndrome. Furthermore, isolated BMP3 dysfunction could be the basis of human cephalic conditions whose genetic etiologies remain unknown.
The development of modern dog breeds is one of the most extensive genetic experiments ever conducted. Their existence allows us to exploit breed-average phenotypes for genetic analysis. In the past, the extensive linkage disequilibrium inherent to artificial selection often hindered the process of fine mapping causal variants in the dog [16]. We overcame this limitation using whole-genome sequencing to comprehensively evaluate candidate variants. Combining the resulting insights with the functional utility of zebrafish, we identified a causal mutation underlying a quantitative trait in the dog. Together these approaches have allowed us to extend the paradigm of leveraging breed-average phenotypes to include the identification of causal mutations. We can now work towards assembling the full inventory of genes associated with vertebrate cranioskeletal shape, in turn illuminating evolutionarily conserved mechanisms of cranioskeletal development in our own species.
Materials and Methods
Morphometrics and CanMap phenotype assignments
Fifty-one measurements were captured using an Immersion MicroScribe Digitizer G2X running Microscribe Utility Software and Diagnostics (v5.0.0.2). In total, 533 canid skulls representative of 120 breeds and 4 gray wolf subspecies located in museums and private collections were documented. Dorsal and ventral landmark datasets were captured separately and merged based on landmarks in common between datasets (landmarks 1, 2, 28, and 29) using File Converter software (Klingenberg lab). Procrustes fit, PCA, and residuals were generated using MorphoJ [11]. Residuals of nonallometric shape were calculated as implemented in MorphoJ (v1.03a) using linear regression (pooled by sex and breed), with symmetric component and log(neurocranium centroid) corresponding to dependent and independent variables, respectively. Ten thousand permutations were performed. Refer to Figure S1 to see landmarks used by MorphoJ to calculate neurocranium centroid.
A covariance matrix based on residuals was analyzed by PCA. GWAS was performed using a subset of the CanMap dataset of genotypes [3]. In total, 72 breed-sex averages of PC1 were assigned to CanMap breeds. In 30 instances, only one skull per breed-sex was measured. In such cases, the actual PC1 score was used for CanMap phenotype assignments. Log(neurocranium centroid) values were similarly assigned and used in subsequent analyses as a size covariate for PLINK and GEMMA association analyses (see next section).
Skull surface scans (1 Pug, 1 gray wolf) were done by Konica Minolta (3D Sensing Labs, Ramsey, NJ). Decimated scans were loaded into Landmark Editor (v3.6) [34]. Skull morphing was done using PC1 landmark coordinates exported from MorphoJ. Coordinate files used for morphing were generated from representatives of dolichocephalic and brachycephalic breeds (a Collie and Pug).
Genomic analyses
Base pair positions stated throughout refer to CanFam2 (Broad/May 2005) coordinates. Single marker and haplotype association analyses were done using PLINK (v1.07) [15] or mixed model GEMMA (v0.91) [17] where specified. CanMap markers used in the analysis included SNPs with missingness <0.10 and minor allele frequency >0.01. In the full dataset (all breeds with breed-sex PC1 averages), 61,270 SNPs were analyzed by PLINK from 576 dogs representing 62 American Kennel Club-recognized breeds. In the mixed-model, ∼36,685 SNPs were analyzed. Breeds used in size-stratified analyses are listed in Table S2. Significantly associated SNPs surpassed Bonferroni correction at the 0.05 level (−log10(P)> = 5.86). HO was calculated by treating CanMap breeds at the polar extremes of PC1 as two comparisons populations (Pug, Pekingese, Boston Terrier, Shih Tzu, Brussels Griffon, French Bulldog, Bulldog, Boxer, Cavalier King Charles Spaniel, Chihuahua versus Collie, Borzoi, Saluki, Scottish Deerhound, Bloodhound, Greyhound, Scottish Terrier, Doberman Pinscher, and Irish Wolfhound). FST was calculated treating brachycephalic breeds (listed above) as a single subpopulation. HO, HR (the ratio of dolicho - and brachycephalic HO), and FST values were calculated using custom R scripts. fastPHASE was used to generate haplotype frequencies by breed, using CanMap genotypes using the clustering parameter k = 15 [35]. “Extreme brachycephalic breeds” were designated as such if both PC1 breed-sex averages exceeded 0.15. This cutoff was chosen based on the obvious jump in magnitude of PC1 values (see Figure 1E, Figure S6). Breeds that meet this classification include the Pug, Pekingese, Boston Terrier, French Bulldog, Bulldog, Brussels Griffon, and Shih Tzu.
Sample collection, Sanger sequencing, and genotyping
DNA used in our study was extracted from blood samples as previously described [16]. In addition to whole-genome sequencing (see below), BMP3 and PRKG2 were Sanger sequenced using six brachycephalic and six dolichocephalic breeds (data not shown). The BMP3 8,196,098 C/A transversion was sequenced in an expanded panel composed of 847 dogs from 113 breeds. Primers were designed with a melting temperature (Tm) ranging between 68–72°C, GC content ranging between 20–80%, length ranging between 18–32 nucleotides, and included 5′ M13 tags (Table S10). PCR products for sequencing were generated with a 2-step thermocycler program:
-
Initial Denaturation: 1×—95°C, 5 minutes
-
Two-step Cycles: 35×—95°C, 30 seconds; 68°C, 2 minutes
-
Extension: 1×—72°C, 10 minutes
PCR products were sequenced using a standard protocol [16]. During the course of SNP discovery, we discovered errors in the reference genome sequence for canine BMP3, producing two early stop codons in the first exon. Sequencing of 13 dogs, including the individual from which the reference genome sequence was derived, indicates these stop codons are the results of errors in the reference sequence.
Whole-genome sequencing and variant filtering
Paired-end libraries were prepared from DNA from eleven dogs of breeds with widely varying skull shapes. Sequencing was conducted on an Illumina HiSeq 2000 sequencer to a depth of 5.6–8.5× per dog using manufacturer protocols. The resulting 101-base paired-end sequences were mapped to the genome (CanFam2 release May 2005) with bwa version 0.5.9-r16 with read trimming set to 15. SNPs were called with samtools mpileup version 0.1.18 and custom R scripts [36]–[39]. Thirteen SNPs in the PC1-associated region overlap with the CanineHD Genotyping BeadChip (Illumina cat. no. WG-440). DNA from four dogs was assayed with the chip; all resulting genotypes were identical in the deep sequencing and chip results. Four hundred and fifty-two SNPs were identified in the critical interval (85.7 kb between 8,152,258 and 8,237,937), and subjected to further filters. Genotypes with a genotype quality score below 8 were reset to “unknown.” We performed association analysis using PLINK with options specifying an additive model omitting the Scottish Terrier, a dolichocephalic breed that appears to be an outlier [15]. After correcting for multiple testing, no SNPs were significantly associated due to limited statistical strength of the test. SNPs in the 5th percentile for association scores were retained. Cross-species conservation was assessed by the UCSC phastCons4way calculations [40] downloaded November 30, 2011, which is generated by using the phastCons program to score the extent of conservation between dog, human, mouse and rat. SNPs with a phastCons4way score above 0.7 were retained. SNPs in an exon or within 20 bases of a splice junction were retained.
Morpholino injections
Morpholino knockdown experiments of bmp3 used two translation blockers (MO1 : 5′-TGACAGCGATCCATGCTGGAGGTGC-3′, MO2 5′-CGGGACTATGGAAGCTGATCTA-3′), which overlapped by one nucleotide. Morpholino injections used 5.1 ng (MO1) or 7.5 ng (MO2), as determined by titrations.
RNA synthesis and injections
Zebrafish bmp3 (IMAGE Id 7052011) and human BMP3 (Origene clone SC302990) cDNAs were sequenced and determined to be full length. Missense F→L mutations for mouse GDF1 [41], human Bmp3, and zebrafish bmp2b [42] were introduced using site-directed mutagenesis and confirmed by sequencing. Zebrafish bmp3 wt and F→L cDNAs were PCR-amplified using gene-specific primers with attB sites. PCR products were subcloned into entry and destination vectors (pCSDest) using Gateway recombination, as previously described [43], [44]. To construct the human BMP3 expression vector, we PCR-amplified the TGF-β signaling domain using primers with XbaI and XhoI restriction sites. PCR products were ligated into an expression vector bearing the Xenopus BMP2 prodomain, as such heterologous fusion constructs were previously shown to enhance propeptide cleavage and biological activity [41]. mRNA was synthesized using Ambion's SP6 mMessage kit from plasmid that was linearized with Not I. Embryo analyses of RNA injections were done based on injections of the following amounts: 25–300 pg human BMP3 mRNA, 25–300 pg mouse Gdf1 mRNA, 1–100 pg zebrafish bmp2b. mRNA overexpression assays were repeated three or more times at each stated concentration, unless stated otherwise.
In Situ and Alcian blue stains
In situ hybridization was completed as described in Thisse and Thisse 2008 [45], except probes were hydrolyzed for 2 minutes at 65°C, the hybridization solution contained 5% dextran sulfate, and the anti-DIG-AP incubation and subsequent washes were performed in Malic Acid Buffer rather than PBST. Alcian blue stains were done as previously described by Schilling et al. [46], except that staining solution was composed of 0.15% Alcian blue, 50% EtOH, and 0.1 M HCl (pH = ∼1).
Plots and images
Embryos were imaged using Zeiss Axio Imager.M1, Zeiss SteREO Lumar v12, or Leica M216F compound microscope. Zeiss Axiovision v4.8.1 software was used for image capture. Nonspecific background and dissection debris were removed from images of Alcian blue cartilage dissections using Adobe Photoshop CS3. All plots were generated using custom scripts, in conjunction with R Cran packages ggplot2 [38], reshape2 [39], and RColorbrewer [47]. Manhattan plot and Q-Q plot scripts were adapted from examples posted on the blog “Getting Genetics Done” [48]. Post-processing of plots was done using Adobe CS4 Creative Suite softwares Photoshop, InDesign, and Illustrator.
Ethics statement
Informed consent was obtained for all collected dog samples. All animal protocols (dog and zebrafish) were approved by the Animal Care and Use Committees of the Intramural Program of the National Human Genome Research Institute at the National Institutes of Health or by Animal Care Committee of the Hospital for Sick Children Research Institute. Wild canids samples were graciously provided by Dr. Robert Wayne, in accordance with UCLA Approved Animal Care and Use Committee Policies.
Supporting Information
Zdroje
1. SablinMVKhlopachevGA 2002 The Earliest Ice Age Dogs: Evidence from Eliseevichi 1. Current Anthropology 43 795 799
2. GermonpréMSablinMVStevensREHedgesREHofreiterM 2009 Fossil dogs and wolves from Palaeolithic sites in Belgium, the Ukraine and Russia: osteometry, ancient DNA and stable isotopes. Journal of Archaeological Science 36 473 490
3. BoykoARQuignonPLiLSchoenebeckJJDegenhardtJD 2010 A simple genetic architecture underlies morphological variation in dogs. PLoS Biol 8 e1000451 doi:10.1371/journal.pbio.1000451
4. FondonJWGarnerHR 2004 Molecular origins of rapid and continuous morphological evolution. Proc Natl Acad Sci USA 101 18058 18063
5. StockardCRAndersonODJamesWT Wistar Institute of Anatomy and Biology 1941 The Genetic and Endocrinic Basis for Differences in Form and Behavior Philadelphia The Wistar Institute of Anatomy and Biology
6. HaworthKEIslamIBreenMPuttWMakrinouE 2001 Canine TCOF1; cloning, chromosome assignment and genetic analysis in dogs with different head types. Mammalian Genome 12 622 629
7. HunemeierTSalzanoFMBortoliniMC 2009 TCOF1 T/Servariant and brachycephaly in dogs. Animal Genetics 40 357 358
8. HaworthKBreenMBinnsMHopkinsonDAEdwardsYH 2001 The canine homeobox gene MSX2: sequence, chromosome assignment and genetic analysis in dogs of different breeds. Animal Genetics 32 32 36
9. BannaschDYoungAMyersJTruvéKDickinsonP 2010 Localization of canine brachycephaly using an across breed mapping approach. PLoS ONE 5 e9632 doi:10.1371/journal.pone.0009632
10. QuilezJShortADMartínezVKennedyLJOllierW 2011 A selective sweep of >8 Mb on chromosome 26 in the Boxer genome. BMC Genomics 12 339
11. KlingenbergCP 2011 MorphoJ: an integrated software package for geometric morphometrics. Molecular Ecology Resources 11 353 357
12. The Complete Dog Book 1998 The Complete Dog Book. 19th ed New York John Wiley & Sons
13. VaysseARatnakumarADerrienTAxelssonEPielbergGR 2011 Identification of Genomic Regions Associated with Phenotypic Variation between Dog Breeds using Selection Mapping. PLoS Genet 7 e1002316 doi:10.1371/journal.pgen.1002316
14. JonesPChaseKMartinADavernPOstranderEA 2008 Single-nucleotide-polymorphism-based association mapping of dog stereotypes. Genetics 179 1033 1044
15. PurcellSNealeBTodd-BrownKThomasLFerreiraMAR 2007 PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 81 559 575
16. SutterNBBustamanteCDChaseKGrayMMZhaoK 2007 A Single IGF1 Allele Is a Major Determinant of Small Size in Dogs. Science 316 112 115
17. ZhouXStephensM 2012 Genome-wide Efficient Mixed Model Analysis for Association Studies. Nature Genetics doi:10.1038/ng.2310
18. ParkerHGKukekovaAVAkeyDTGoldsteinOKirknessEF 2007 Breed relationships facilitate fine-mapping studies: A 7.8-kb deletion cosegregates with Collie eye anomaly across multiple dog breeds. Genome Research 17 1562 1571
19. ParkerHG 2004 Genetic Structure of the Purebred Domestic Dog. Science 304 1160 1164
20. AkeyJMZhangGZhangKJinLShriverMD 2002 Interrogating a high-density SNP map for signatures of natural selection. Genome Research 12 1805 1814
21. PollingerJPBustamanteCDFledel-AlonASchmutzSGrayMM 2005 Selective sweep mapping of genes with large phenotypic effects. Genome Research 15 1809 1819
22. ChikudaHKugimiyaFHoshiKIkedaTOgasawaraT 2004 Cyclic GMP-dependent protein kinase II is a molecular switch from proliferation to hypertrophic differentiation of chondrocytes. Genes & Development 18 2418 2429
23. DaluiskiAEngstrandTBahamondeMEGamerLWAgiusE 2001 Bone morphogenetic protein-3 is a negative regulator of bone density. Nature Genetics 27 84 88
24. PfeiferAAszódiASeidlerURuthPHofmannF 1996 Intestinal secretory defects and dwarfism in mice lacking cGMP-dependent protein kinase II. Science 274 2082 2086
25. SunYZhangQ-JZhongJWangY-Q 2010 Characterization and expression of AmphiBMP3/3b gene in amphioxus Branchiostoma japonicum. Development, Growth & Differentiation 52 157 167
26. TakaoMHinoJTakeshitaNKonnoYNishizawaT 1996 Identification of rat bone morphogenetic protein-3b (BMP-3b), a new member of BMP-3. Biochem Biophys Res Commun 219 656 662
27. KettunenPNieXKvinnslandIHLuukkoK 2006 Histological development and dynamic expression of Bmp2-6 mRNAs in the embryonic and postnatal mouse cranial base. Anat Rec 288 1250 1258
28. AdzhubeiIASchmidtSPeshkinLRamenskyVEGerasimovaA 2010 A method and server for predicting damaging missense mutations. Nat Methods 7 248 249
29. AllendorphGPIsaacsMJKawakamiYIzpisua BelmonteJCChoeS 2007 BMP-3 and BMP-6 structures illuminate the nature of binding specificity with receptors. Biochemistry 2007 12238 12247
30. GamerLWNoveJLevinMRosenV 2005 BMP-3 is a novel inhibitor of both activin and BMP-4 signaling in Xenopus embryos. Developmental Biology 285 156 168
31. KokabuSGamerLCoxKLoweryJTsujiK 2011 BMP3 Suppresses Osteoblast Differentiation of Bone Marrow Stromal Cells via Interaction with Acvr2b. Mol Endocrinol 26 87 94
32. MuellerRLHuangCHoRK 2010 Spatio-temporal regulation of Wnt and retinoic acid signaling by tbx16/spadetail during zebrafish mesoderm differentiation. BMC Genomics 11 492 492
33. BonnetCAndrieuxJBeri-DexheimerMLeheupBBouteO 2010 Microdeletion at chromosome 4q21 defines a new emerging syndrome with marked growth restriction, mental retardation and absent or severely delayed speech. Journal of Medical Genetics 47 377 384
34. WileyDAmentaNAlcantaraDGhoshDKilYJ 2005 Evolutionary morphing. 431 438 Visualization, 2005 VIS 05 IEEE
35. ScheetPStephensM 2006 A Fast and Flexible Statistical Model for Large-Scale Population Genotype Data: Applications to Inferring Missing Genotypes and Haplotypic Phase. The American Journal of Human Genetics 78 629 644
36. IhakaRGentlemanR 1996 R: A Language for Data Analysis and Graphics. Journal of Computational and Graphical Statistics 5 299 314
37. LiHHandsakerBWysokerAFennellTRuanJ 2009 The Sequence Alignment/Map format and SAMtools. Bioinformatics 25 2078 2079
38. WickhamH 2009 ggplot2: Elegant Graphics for Data Analysis (Use R). 2nd ed Springer
39. WickhamH 2007 Reshaping data with the reshape package. J Stat Softw 21 1 20
40. SiepelABejeranoGPedersenJSHinrichsASHouM 2005 Evolutionarily conserved elements in vertebrate, insect, worm, and yeast genomes. Genome Research 15 1034 1050
41. WallNACraigEJLaboskyPAKesslerDS 2000 Mesendoderm induction and reversal of left-right pattern by mouse Gdf1, a Vg1-related gene. Developmental Biology 227 495 509
42. NikaidoMTadaMSajiTUenoN 1997 Conservation of BMP signaling in zebrafish mesoderm patterning. Mechanisms of Development 61 75 88
43. VillefrancJAAmigoJLawsonND 2007 Gateway compatible vectors for analysis of gene function in the zebrafish. Dev Dyn 236 3077 3087
44. KwanKMFujimotoEGrabherCMangumBDHardyME 2007 The Tol2kit: a multisite gateway-based construction kit for Tol2 transposon transgenesis constructs. Dev Dyn 236 3088 3099
45. ThisseCThisseB 2008 High-resolution in situ hybridization to whole-mount zebrafish embryos. Nat Protoc 3 59 69
46. SchillingTFPiotrowskiTGrandelHBrandMHeisenbergCP 1996 Jaw and branchial arch mutants in zebrafish I: branchial arches. Development 123 329 344
47. NeuwirthE 2007 RColorBrewer: ColorBrewer palettes. R package version
48. TurnerSBushW 2010 Getting Genetics Done. gettinggeneticsdone.blogspot.com/
Štítky
Genetika Reprodukční medicína
Článek Mutational Signatures of De-Differentiation in Functional Non-Coding Regions of Melanoma GenomesČlánek Rescuing Alu: Recovery of Inserts Shows LINE-1 Preserves Alu Activity through A-Tail ExpansionČlánek Genetics and Regulatory Impact of Alternative Polyadenylation in Human B-Lymphoblastoid CellsČlánek Retrovolution: HIV–Driven Evolution of Cellular Genes and Improvement of Anticancer Drug ActivationČlánek The Mi-2 Chromatin-Remodeling Factor Regulates Higher-Order Chromatin Structure and Cohesin DynamicsČlánek Identification of Human Proteins That Modify Misfolding and Proteotoxicity of Pathogenic Ataxin-1
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 8
-
Všechny články tohoto čísla
- Mutational Signatures of De-Differentiation in Functional Non-Coding Regions of Melanoma Genomes
- Rescuing Alu: Recovery of Inserts Shows LINE-1 Preserves Alu Activity through A-Tail Expansion
- Genetics and Regulatory Impact of Alternative Polyadenylation in Human B-Lymphoblastoid Cells
- Chromosome Territories Meet a Condensin
- It's All in the Timing: Too Much E2F Is a Bad Thing
- Fine-Mapping and Initial Characterization of QT Interval Loci in African Americans
- Genome Patterns of Selection and Introgression of Haplotypes in Natural Populations of the House Mouse ()
- A Combinatorial Amino Acid Code for RNA Recognition by Pentatricopeptide Repeat Proteins
- Advances in Quantitative Trait Analysis in Yeast
- Experimental Evolution of a Novel Sexually Antagonistic Allele
- Variation of Contributes to Dog Breed Skull Diversity
- , a Gene Involved in Axonal Pathfinding, Is Mutated in Patients with Kallmann Syndrome
- A Single Origin for Nymphalid Butterfly Eyespots Followed by Widespread Loss of Associated Gene Expression
- Cryptocephal, the ATF4, Is a Specific Coactivator for Ecdysone Receptor Isoform B2
- Retrovolution: HIV–Driven Evolution of Cellular Genes and Improvement of Anticancer Drug Activation
- The PARN Deadenylase Targets a Discrete Set of mRNAs for Decay and Regulates Cell Motility in Mouse Myoblasts
- A Sexual Ornament in Chickens Is Affected by Pleiotropic Alleles at and , Selected during Domestication
- Use of Allele-Specific FAIRE to Determine Functional Regulatory Polymorphism Using Large-Scale Genotyping Arrays
- Novel Loci for Metabolic Networks and Multi-Tissue Expression Studies Reveal Genes for Atherosclerosis
- The Genetic Basis of Pollinator Adaptation in a Sexually Deceptive Orchid
- Uncovering the Genome-Wide Transcriptional Responses of the Filamentous Fungus to Lignocellulose Using RNA Sequencing
- Inheritance Beyond Plain Heritability: Variance-Controlling Genes in
- The Metabochip, a Custom Genotyping Array for Genetic Studies of Metabolic, Cardiovascular, and Anthropometric Traits
- Reprogramming to Pluripotency Can Conceal Somatic Cell Chromosomal Instability
- Condensin II Promotes the Formation of Chromosome Territories by Inducing Axial Compaction of Polyploid Interphase Chromosomes
- PTEN Negatively Regulates MAPK Signaling during Vulval Development
- A Dynamic Response Regulator Protein Modulates G-Protein–Dependent Polarity in the Bacterium
- Population Genomics of the Facultatively Mutualistic Bacteria and
- Components of a Fanconi-Like Pathway Control Pso2-Independent DNA Interstrand Crosslink Repair in Yeast
- Polysome Profiling in Liver Identifies Dynamic Regulation of Endoplasmic Reticulum Translatome by Obesity and Fasting
- Stromal Liver Kinase B1 [STK11] Signaling Loss Induces Oviductal Adenomas and Endometrial Cancer by Activating Mammalian Target of Rapamycin Complex 1
- Reprogramming of H3K27me3 Is Critical for Acquisition of Pluripotency from Cultured Tissues
- Transgene Induced Co-Suppression during Vegetative Growth in
- Hox and Sex-Determination Genes Control Segment Elimination through EGFR and Activity
- A Quantitative Comparison of the Similarity between Genes and Geography in Worldwide Human Populations
- Minibrain/Dyrk1a Regulates Food Intake through the Sir2-FOXO-sNPF/NPY Pathway in and Mammals
- Comparative Analysis of Regulatory Elements between and by Genome-Wide Transcription Start Site Profiling
- Simple Methods for Generating and Detecting Locus-Specific Mutations Induced with TALENs in the Zebrafish Genome
- S Phase–Coupled E2f1 Destruction Ensures Homeostasis in Proliferating Tissues
- Cell-Nonautonomous Signaling of FOXO/DAF-16 to the Stem Cells of
- The Mi-2 Chromatin-Remodeling Factor Regulates Higher-Order Chromatin Structure and Cohesin Dynamics
- Comparative Analysis of the Genomes of Two Field Isolates of the Rice Blast Fungus
- Role of Mex67-Mtr2 in the Nuclear Export of 40S Pre-Ribosomes
- Genetic Modulation of Lipid Profiles following Lifestyle Modification or Metformin Treatment: The Diabetes Prevention Program
- HAL-2 Promotes Homologous Pairing during Meiosis by Antagonizing Inhibitory Effects of Synaptonemal Complex Precursors
- SLX-1 Is Required for Maintaining Genomic Integrity and Promoting Meiotic Noncrossovers in the Germline
- Phylogenetic and Transcriptomic Analysis of Chemosensory Receptors in a Pair of Divergent Ant Species Reveals Sex-Specific Signatures of Odor Coding
- Reduced Prostasin (CAP1/PRSS8) Activity Eliminates HAI-1 and HAI-2 Deficiency–Associated Developmental Defects by Preventing Matriptase Activation
- Dissecting the Gene Network of Dietary Restriction to Identify Evolutionarily Conserved Pathways and New Functional Genes
- Identification of Human Proteins That Modify Misfolding and Proteotoxicity of Pathogenic Ataxin-1
- and Link Transcription of Phospholipid Biosynthetic Genes to ER Stress and the UPR
- CDK9 and H2B Monoubiquitination: A Well-Choreographed Dance
- Rare Copy Number Variations in Adults with Tetralogy of Fallot Implicate Novel Risk Gene Pathways
- Ccdc94 Protects Cells from Ionizing Radiation by Inhibiting the Expression of
- NOL11, Implicated in the Pathogenesis of North American Indian Childhood Cirrhosis, Is Required for Pre-rRNA Transcription and Processing
- Human Developmental Enhancers Conserved between Deuterostomes and Protostomes
- A Luminal Glycoprotein Drives Dose-Dependent Diameter Expansion of the Hindgut Tube
- Melanophore Migration and Survival during Zebrafish Adult Pigment Stripe Development Require the Immunoglobulin Superfamily Adhesion Molecule Igsf11
- Dynamic Distribution of Linker Histone H1.5 in Cellular Differentiation
- Combining Comparative Proteomics and Molecular Genetics Uncovers Regulators of Synaptic and Axonal Stability and Degeneration
- Chemical Genetics Reveals a Specific Requirement for Cdk2 Activity in the DNA Damage Response and Identifies Nbs1 as a Cdk2 Substrate in Human Cells
- Experimental Relocation of the Mitochondrial Gene to the Nucleus Reveals Forces Underlying Mitochondrial Genome Evolution
- Rates of Gyrase Supercoiling and Transcription Elongation Control Supercoil Density in a Bacterial Chromosome
- Mutations in a P-Type ATPase Gene Cause Axonal Degeneration
- A General G1/S-Phase Cell-Cycle Control Module in the Flowering Plant
- Multiple Roles and Interactions of and in Development of the Respiratory System
- UNC-40/DCC, SAX-3/Robo, and VAB-1/Eph Polarize F-Actin during Embryonic Morphogenesis by Regulating the WAVE/SCAR Actin Nucleation Complex
- Epigenetic Remodeling of Meiotic Crossover Frequency in DNA Methyltransferase Mutants
- Modulating the Strength and Threshold of NOTCH Oncogenic Signals by
- Loss of Axonal Mitochondria Promotes Tau-Mediated Neurodegeneration and Alzheimer's Disease–Related Tau Phosphorylation Via PAR-1
- Acetyl-CoA-Carboxylase Sustains a Fatty Acid–Dependent Remote Signal to Waterproof the Respiratory System
- ATXN2-CAG42 Sequesters PABPC1 into Insolubility and Induces FBXW8 in Cerebellum of Old Ataxic Knock-In Mice
- Cohesin Rings Devoid of Scc3 and Pds5 Maintain Their Stable Association with the DNA
- The MicroRNA Inhibits Calcium Signaling by Targeting the TIR-1/Sarm1 Adaptor Protein to Control Stochastic L/R Neuronal Asymmetry in
- Rapid-Throughput Skeletal Phenotyping of 100 Knockout Mice Identifies 9 New Genes That Determine Bone Strength
- The Genes Define Unique Classes of Two-Partner Secretion and Contact Dependent Growth Inhibition Systems
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Dissecting the Gene Network of Dietary Restriction to Identify Evolutionarily Conserved Pathways and New Functional Genes
- It's All in the Timing: Too Much E2F Is a Bad Thing
- Variation of Contributes to Dog Breed Skull Diversity
- The PARN Deadenylase Targets a Discrete Set of mRNAs for Decay and Regulates Cell Motility in Mouse Myoblasts
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



