-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaA Quantitative Comparison of the Similarity between Genes and Geography in Worldwide Human Populations
Multivariate statistical techniques such as principal components analysis (PCA) and multidimensional scaling (MDS) have been widely used to summarize the structure of human genetic variation, often in easily visualized two-dimensional maps. Many recent studies have reported similarity between geographic maps of population locations and MDS or PCA maps of genetic variation inferred from single-nucleotide polymorphisms (SNPs). However, this similarity has been evident primarily in a qualitative sense; and, because different multivariate techniques and marker sets have been used in different studies, it has not been possible to formally compare genetic variation datasets in terms of their levels of similarity with geography. In this study, using genome-wide SNP data from 128 populations worldwide, we perform a systematic analysis to quantitatively evaluate the similarity of genes and geography in different geographic regions. For each of a series of regions, we apply a Procrustes analysis approach to find an optimal transformation that maximizes the similarity between PCA maps of genetic variation and geographic maps of population locations. We consider examples in Europe, Sub-Saharan Africa, Asia, East Asia, and Central/South Asia, as well as in a worldwide sample, finding that significant similarity between genes and geography exists in general at different geographic levels. The similarity is highest in our examples for Asia and, once highly distinctive populations have been removed, Sub-Saharan Africa. Our results provide a quantitative assessment of the geographic structure of human genetic variation worldwide, supporting the view that geography plays a strong role in giving rise to human population structure.
Published in the journal: . PLoS Genet 8(8): e32767. doi:10.1371/journal.pgen.1002886
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002886Summary
Multivariate statistical techniques such as principal components analysis (PCA) and multidimensional scaling (MDS) have been widely used to summarize the structure of human genetic variation, often in easily visualized two-dimensional maps. Many recent studies have reported similarity between geographic maps of population locations and MDS or PCA maps of genetic variation inferred from single-nucleotide polymorphisms (SNPs). However, this similarity has been evident primarily in a qualitative sense; and, because different multivariate techniques and marker sets have been used in different studies, it has not been possible to formally compare genetic variation datasets in terms of their levels of similarity with geography. In this study, using genome-wide SNP data from 128 populations worldwide, we perform a systematic analysis to quantitatively evaluate the similarity of genes and geography in different geographic regions. For each of a series of regions, we apply a Procrustes analysis approach to find an optimal transformation that maximizes the similarity between PCA maps of genetic variation and geographic maps of population locations. We consider examples in Europe, Sub-Saharan Africa, Asia, East Asia, and Central/South Asia, as well as in a worldwide sample, finding that significant similarity between genes and geography exists in general at different geographic levels. The similarity is highest in our examples for Asia and, once highly distinctive populations have been removed, Sub-Saharan Africa. Our results provide a quantitative assessment of the geographic structure of human genetic variation worldwide, supporting the view that geography plays a strong role in giving rise to human population structure.
Introduction
The geographic structure of human genetic variation has long been of interest for its implications for studying human evolutionary history [1], [2], [3], [4], [5]. In recent years, the expansion of population-genetic datasets has contributed to an increase in geographic investigations of human genetic variation, often on the basis of classic multivariate statistical techniques such as PCA and MDS [6], [7], [8], [9], [10]. In PCA, samples are projected onto a series of orthogonal axes (principal components or PCs) that are constructed from a linear combination of genotypic values across genetic markers, such that each PC sequentially maximizes the variance among samples projected on it [11], [12]. Classic MDS analyzes a genetic distance matrix between pairs of samples and places the samples in a low-dimensional space in such a way that pairwise Euclidean distances among samples in the low-dimensional space approximate their relative genetic distances [13]. The population structure of genetic variation is often summarized in easily visualized two-dimensional statistical maps obtained from the first two components of PCA or MDS. Especially for large-scale single-nucleotide polymorphism (SNP) data, PCA and MDS are popular because of their computational efficiency and high level of resolution in decomposing the complex structure of human genetic variation [12], [14]. Generally, results produced by PCA and MDS are very similar to each other [15].
Several recent studies have reported detectable similarity between statistical maps of genetic variation and geographic maps of population locations. Such observations are particularly prominent within Europe, where striking similarity between genes and geography is observed both at a continental level [9], [16], [17] and in more localized studies such as in Finland [18], [19], Iceland [20], and Sweden [21]. Analogous but visually less striking observations have also been reported in studies of other geographic regions, including in worldwide samples [6], [7], [8], [10], [22], [23] and in samples from Asia [23], [24], [25], Africa [26], [27], China [28], [29], and Japan [30]. However, this similarity of genes and geography is in many cases reported in a qualitative sense and has not been assessed systematically across different studies, so that it has been difficult to compare levels of agreement between genes and geography in different regions. Further, different studies have used different sets of genetic markers and different statistical techniques (e.g. PCA and MDS), further complicating comparisons across datasets. Even for studies that used PCA, several versions of this technique have been employed in different studies. For example, some studies have performed PCA on genotypic matrices [9], [10], [12], [20], whereas others have applied PCA on pairwise genetic distance matrices [7], [22], [23].
A formal comparison of genes and geography in different regions using a single technique and a common marker set can provide a systematic basis for evaluating the role of geography in explaining the genetic similarity of individuals or populations in different locations. We have previously developed a Procrustes analysis approach to quantify the similarity between statistical maps of genetic variation and geographic maps [15]. This approach identifies data transformations that minimize the sum of squared Euclidean distances between two sets of coordinates while preserving relative pairwise distances among points within each set. The statistical significance of the similarity between genetic coordinates and geographic coordinates is then examined using a permutation test.
In this study, we apply the Procrustes approach together with PCA to systematically study the geographic structure of human genetic variation across different geographic regions. By compiling data from a variety of published sources [9], [23], [26], [31], [32], we have assembled genome-wide SNP data and geographic coordinates for 149 populations worldwide. Based on a common set of autosomal SNP markers shared by datasets collected from different studies, we evaluate the similarity between genes and geography in examples from Europe, Sub-Saharan Africa, Asia, East Asia, and Central/South Asia, as well as in a worldwide sample. We compare the level of similarity across the various datasets, finding that all show a high level of similarity, and that the highest similarity score appears in Asia. We also examine the dependence of the similarity on the choice of populations included in the analysis and on the number of markers studied. Our results provide information about the importance of geography in human evolutionary history, and can facilitate statistical methods for inferring the ancestral origin of human individuals from their genotypes.
Results
We integrated published genome-wide SNP data on 4,257 individuals from 149 worldwide populations, taking data from the Human Genome Diversity Project (HGDP) [7], 31, International Haplotype Map Project Phase III (HapMap Phase III) [31], [33], and POPRES [9] samples, as well as from several other publications [23], [26], [32]. In our analyses, we focused on the data from 128 populations (Tables S1, S2, S3). We constructed six datasets for evaluating the geographic structure of genetic variation in different geographic regions: a worldwide sample, continental samples from Europe, Sub-Saharan Africa, and Asia, and subcontinental samples from East Asia and Central/South Asia (Table 1).
Tab. 1. SNP datasets for different geographic regions. 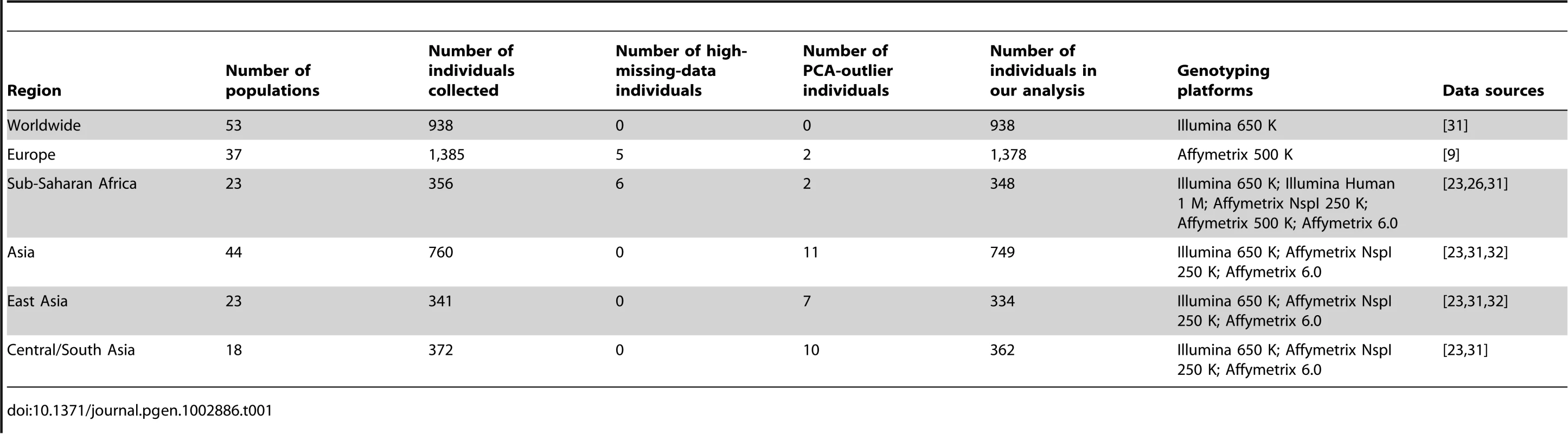
Our analyses were based on 32,991 autosomal SNP markers that were shared among datasets obtained from different genotyping platforms. We applied PCA on datasets after quality control and removal of PCA outliers (see Materials and Methods), and we then used Procrustes analysis to compute the similarity score, denoted as , between the first two PCs of genetic variation and the geographic coordinates of the populations.
We evaluated the statistical significance of the similarity score by permutation. We further examined the robustness of our results using a leave-one-out approach, in which we repeated PCA and Procrustes analysis on datasets with a single population excluded. PCA coordinates obtained from these new datasets were compared to the original PCA coordinates obtained from the whole dataset and to the geographic coordinates, with the respective Procrustes similarity scores denoted as and (see Materials and Methods). These analyses were applied systematically on all datasets.
Worldwide sample
Our worldwide example was based on 938 unrelated individuals from 53 worldwide populations (Figure 1A), taken from the study of Li et al. [7]. None of these individuals was found to have 5% missing data or to appear as a PCA outlier.
Fig. 1. Procrustes analysis of genetic and geographic coordinates of worldwide populations. 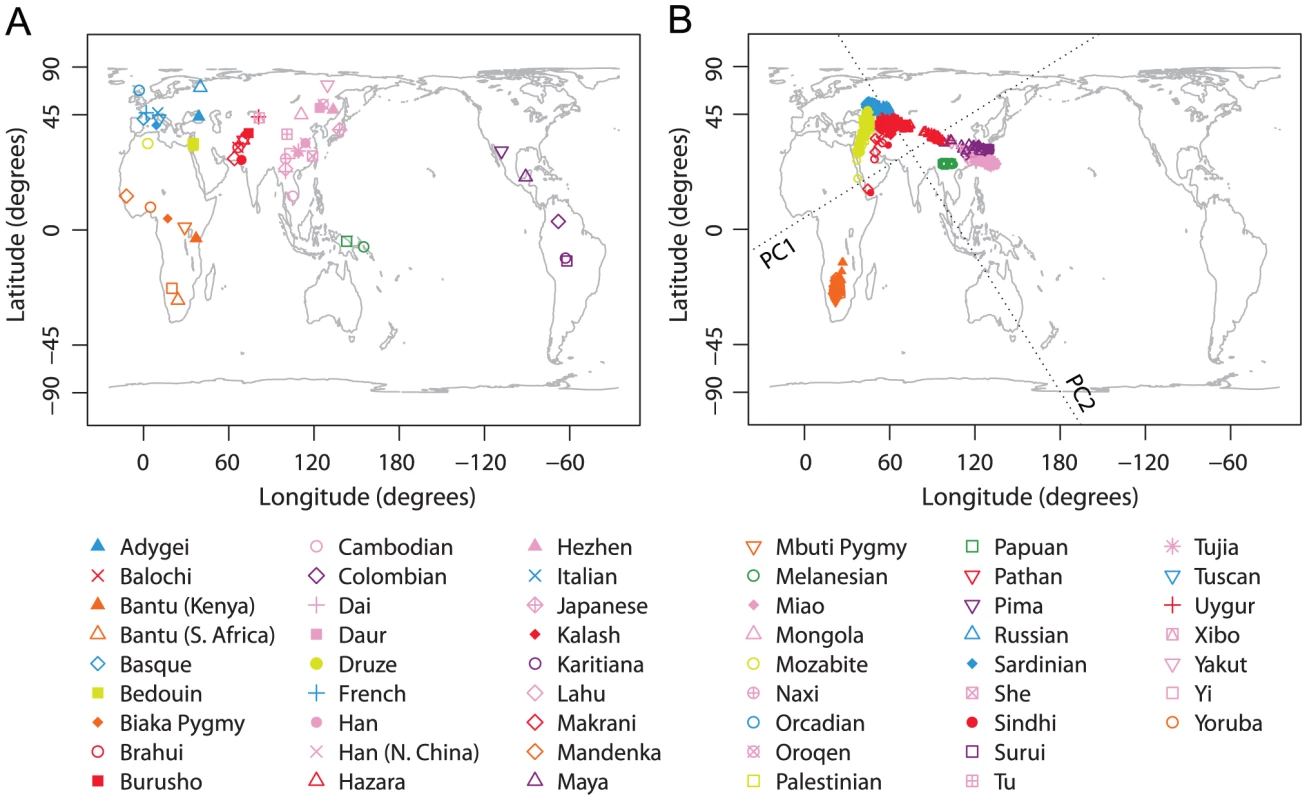
(A) Geographic coordinates of 53 populations. (B) Procrustes-transformed PCA plot of genetic variation. The Procrustes analysis is based on the Gall-Peters projected coordinates of geographic locations and PC1-PC2 coordinates of 938 individuals. The figures are plotted according to the Gall-Peters projection. PC1 and PC2 are indicated by dotted lines, crossing over the centroid of all individuals. PC1 and PC2 account for 6.22% and 4.72% of the total variance, respectively. The Procrustes similarity is (). The rotation angle of the PCA map is . A PCA plot finds that as in previous studies [7], [8], [10], samples from the same geographic region (indicated by colors in Figure 1) generally cluster together, and that different clusters align on the PCA plot in a way that qualitatively resembles the geographic map of sampling locations. The first two PCs of our PCA explain 6.22% and 4.72% of the total genetic variation, respectively. These values are considerably less than the values reported by Li et al. [7] in their Figure S3B, which were 52.3% for PC1 and 27.8% for PC2. The difference can be attributed primarily to the different versions of PCA used in the analyses. We applied PCA on the genotypic matrix for individuals and loci, whereas Li et al. applied PCA on an matrix recording levels of identity-by-state for pairs of individuals [7]. Although the two approaches provide visually similar PCA plots, the values and the interpretation of the proportions of variance explained by each PC differ, as they are based on quite distinct computations.
Using Procrustes analysis, we identified an optimal alignment of the genetic coordinates to the (Gall-Peters-projected) geographic coordinates that involved a rotation of the PCA plot by 31.91 counterclockwise. The genetic coordinates were then superimposed on the geographic map by applying the optimal transformation, thereby highlighting the similarity between genes and geography (Figure 1). This qualitative resemblance is demonstrated by the Procrustes similarity score of , which is highly significant in 100,000 permutations (). Applying the leave-one-out approach with populations excluded individually, the similarity score between genes and geography ranges from 0.697 to 0.715, with mean 0.705 and standard deviation 0.003 (Table S4). Some populations, such as Native American and Oceanian populations, align in Figure 1B distantly from their geographic locations. In most but not all cases, excluding one of these populations leads to an increase in the Procrustes similarity score.
Europe
Visually striking similarity betweeen PCA plots of genetic variation and a geographic map of Europe has been reported by several studies [9], [16], [17]. Our analysis was based on nearly the same sample studied by Novembre et al. [9], containing 1,385 individuals from 37 populations widely spread across Europe (Figure 2A). After excluding five individuals with 5% missing data and two PCA outliers, our final analysis examined 1,378 individuals.
Fig. 2. Procrustes analysis of genetic and geographic coordinates of European populations. 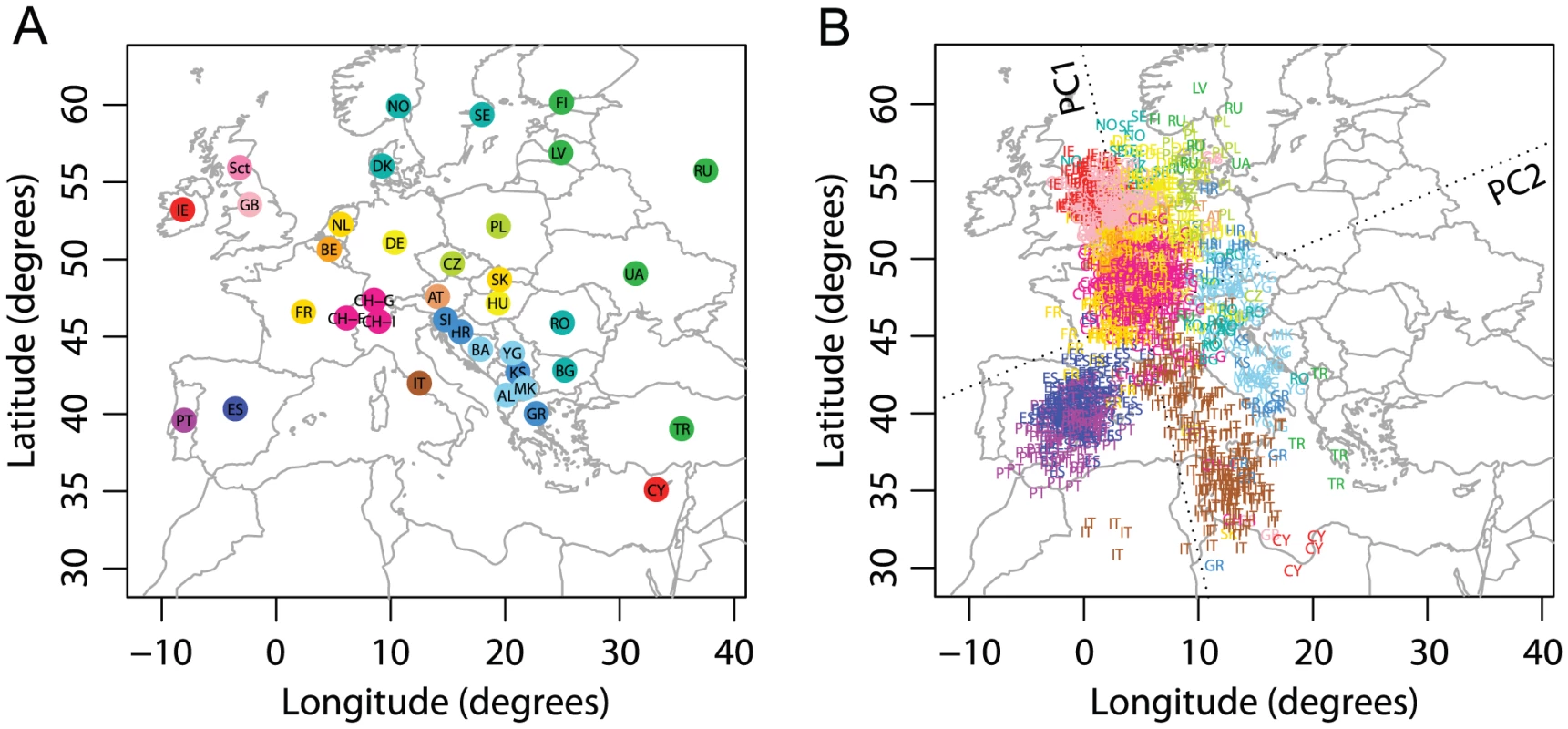
(A) Geographic coordinates of 37 populations. (B) Procrustes-transformed PCA plot of genetic variation. The Procrustes analysis is based on the unprojected latitude-longitude coordinates and PC1-PC2 coordinates of 1378 individuals. PC1 and PC2 are indicated by dotted lines, crossing over the centroid of all individuals. Abbreviations are as follows: AL, Albania; AT, Austria; BA, Bosnia-Herzegovina; BE, Belgium; BG, Bulgaria; CH-F, Swiss-French; CH-G, Swiss-German; CH-I, Swiss-Italian; CY, Cyprus; CZ, Czech Republic; DE, Germany; DK, Denmark; ES, Spain; FI, Finland; FR, France; GB, United Kingdom; GR, Greece; HR, Croatia; HU, Hungary; IE, Ireland; IT, Italy; KS, Kosovo; LV, Latvia; MK, Macedonia; NL, Netherlands; NO, Norway; PL, Poland; PT, Portugal; RO, Romania; RU, Russia; Sct, Scotland; SE, Sweden; SI, Slovenia; TR, Turkey; UA, Ukraine; YG, Serbia and Montenegro. Population labels follow the color scheme of Novembre et al. [9]. PC1 and PC2 account for 0.30% and 0.16% of the total variance, respectively. The Procrustes similarity is (). The rotation angle of the PCA map is . Our PCA plot is very similar to the plot of Novembre et al. [9], with a close correspondence of genes and geography (Figure 2B). One difference is that in the PCA plot of Novembre et al. [9], individuals are more widely spread along PC2 than in our plot. As we applied PCA in the same way as Novembre et al. [9], the difference arises primarily because they employed coordinates given directly by the eigenvectors in PCA, such that PC1 and PC2 were scaled to have the same variance (J. Novembre, personal communication). To simplify the standardization of analyses across datasets, we chose not to scale the PC axes in our analyses, so that the relative amounts of genetic variation explained by each PC are reflected in the PCA plot (see Materials and Methods). Our PC1 and PC2 explain 0.30% and 0.16% of the total genetic variation respectively, in close agreement with the values of 0.30% and 0.15% reported by Novembre et al. [9].
We used Procrustes analysis to superimpose the PCA plot on the geographic map, rotating the PCA coordinates 72.66 clockwise (Figure 2). The rotated genetic coordinates of the European samples are spread over a larger distance along longitudinal lines than along latitudinal lines, although the geographic locations of the samples are distributed in the opposite way. This observation reflects the result that the genetic differentiation among Europeans is larger in a north-south direction than in an east-west direction [34]. The Procrustes similarity between the genetic coordinates and the geographic coordinates is (). Excluding populations from the analysis individually, the Procrustes similarity between genes and geography ranges from 0.764 for the analysis without the United Kingdom to 0.810 for the analysis without Italy, with a mean of 0.780 across populations and a standard deviation of 0.007 (Table S5). Populations that have a relatively large effect on the similarity score are mostly those with large sample sizes (e.g., Italy, Portugal, Spain and United Kingdom). The Russian population is an exception; its sample size is small (), but the genetic coordinates of the Russian sample align poorly with the geographic coordinates [9] (Figure 2). Thus, this population has a relatively large effect on the similarity with geography ( when excluding Russians, Table S5). Excluding Russians has minimal effect on the PCA coordinates for the remaining samples, however, as reflected in the high similarity score between the PCA coordinates before and after excluding the Russian sample (, Table S5). Reducing the sizes of large samples also has a relatively small impact; when repeating our analyses on a subset of the data in which 50 individuals are selected randomly from populations with larger samples, changes slightly to 0.777, and both and the proportions of variance explained by PC1 and PC2 undergo slight increases (Figure S1).
Sub-Saharan Africa
Sub-Saharan Africa is the location of the origin of modern humans and has the highest genetic variation among all continents [7], [22], [35], [36], [37]. Previous studies have found that when isolated hunter-gatherer populations are included in the analysis, PCA plots of genetic variation in Sub-Saharan Africa display low qualitative similarity to the geographic map of sampling locations [7], [22], [38]. Bryc et al. recently studied 12 populations in West Africa, and revealed a high similarity between a SNP-based PCA map and the corresponding geographic map, when Mbororo Fulani, a nomadic pastoralist population, was excluded from the analysis [26]. By integrating SNP data from multiple sources [23], [26], [31], we investigated Sub-Saharan African populations in a broader region than in the analysis of Bryc et al. [26]. We first excluded four hunter-gatherer populations (!Kung, San, Biaka Pygmy, and Mbuti Pygmy) and Mbororo Fulani. After further excluding six individuals with 5% missing data and two PCA outliers, our analyses examined 348 individuals from 23 populations in Sub-Saharan Africa (Figure 3A).
Fig. 3. Procrustes analysis of genetic and geographic coordinates of Sub-Saharan African populations, excluding hunter-gatherer populations and Mbororo Fulani. 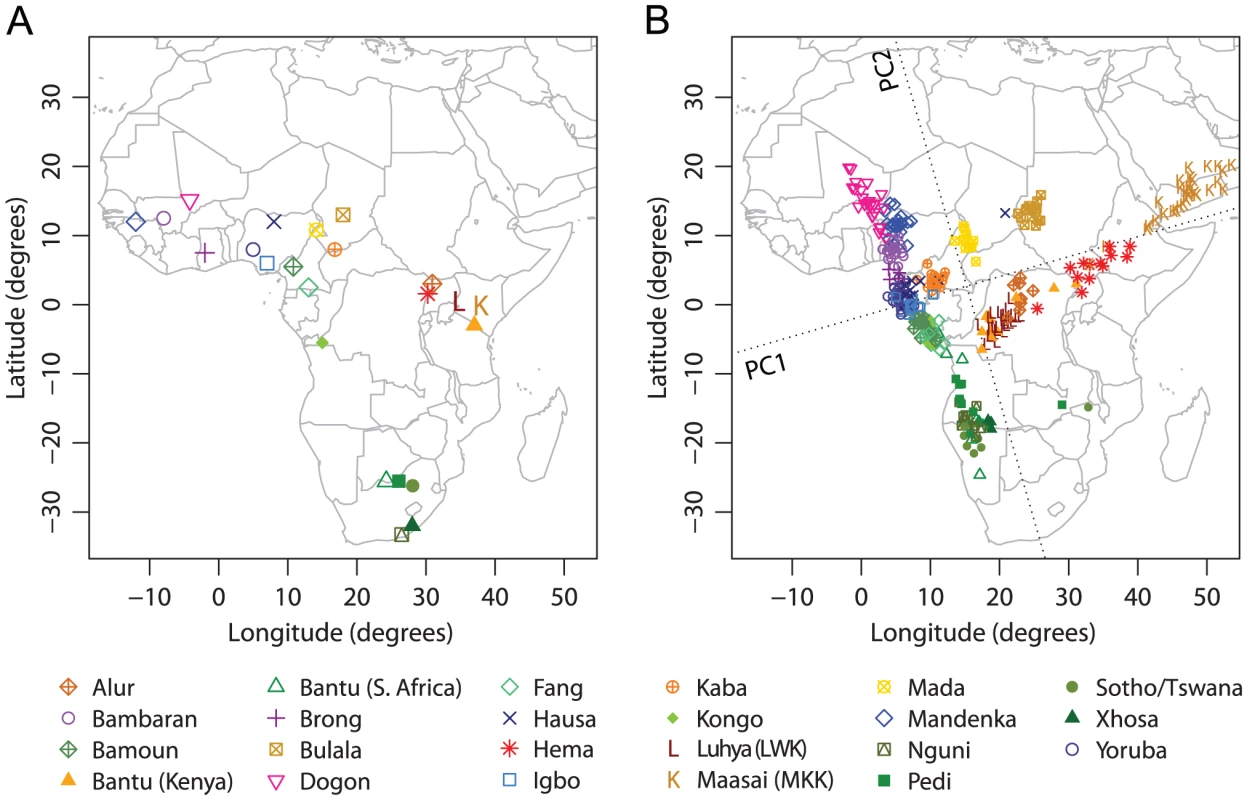
(A) Geographic coordinates of 23 populations. (B) Procrustes-transformed PCA plot of genetic variation. The Procrustes analysis is based on the unprojected latitude-longitude coordinates and PC1-PC2 coordinates of 348 individuals. PC1 and PC2 are indicated by dotted lines, crossing over the centroid of all individuals. PC1 and PC2 account for 1.34% and 0.69% of the total variance, respectively. The Procrustes similarity is (). The rotation angle of the PCA map is . Applying PCA on this combined Sub-Saharan African dataset, we found that PC1 accounts for 1.34% of the total genetic variation, largely separating populations from west to east. PC2 accounts for 0.69% of the total genetic variation and largely separates populations from north to south (Figure 3B). Generally, populations along the west coast of Africa cluster closely with each other, while interior populations form relatively isolated clusters. Bantu-speaking populations tend to cluster with each other, and can be divided into three groups according to their geographic locations: two populations in the west (Fang and Kongo), two in the east (Kenyan Bantus from the HGDP and Luhya), and five in the south (Southern African Bantus from the HGDP, Nguni, Pedi, Sotho/Tswana, and Xhosa). Despite the large geographic separation among these three groups, their genetic separation in the PCA plot is relatively small (Figure 3B). In particular, Luhya and Kenyan Bantus from the HGDP align between the western Bantu populations and the eastern non-Bantu populations such as Alur and Hema. The Maasai sample, consisting of 30 unrelated individuals randomly selected from the HapMap Phase III [31], [33], forms a cluster distant from the other populations along PC1 (and PC3, results not shown).
Procrustes analysis identifies a rotation angle of 16.11 counterclockwise for the genetic coordinates (Figure 3B), and the similarity score between genes and geography is (). Among all populations, Maasai has the largest impact on both the PCA and Procrustes analysis (Table S6); as shown in Figure S2, when analyzed without Maasai, the other 22 populations align more closely with geography, and the Procrustes similarity score increases to 0.832 (). Excluding any of the populations in South Africa leads to a decrease of the similarity between genes and geography, and the lowest similarity is obtained when excluding the combined Sotho/Tswana sample (, Table S6). This result suggests that the genetic map of Sub-Saharan Africans might look more similar to the geographic map if additional populations from the undersampled southern region of Africa were included.
When hunter-gatherer populations (!Kung, San, Biaka Pygmy, and Mbuti Pygmy) and Mbororo Fulani were included in the analysis, they appeared as isolated clusters on the PCA plots and greatly reduced the similarity between PCA maps and geographic maps (Figure S3, Table S7). The similarity score decreased from 0.790 to 0.548 after including all five of these populations in the analysis. This value, however, is still statistically significant, with a -value of ; further, if we disregard the hunter-gatherer populations and Mbororo Fulani in Figure S3B and only examine the relative locations of the original 23 populations, we can still find a clear resemblance between genetic and geographic coordinates. Compared to the other 23 populations, the four hunter-gatherer populations appear as isolated groups at the south, and Mbororo Fulani appears at the north. These observations are clearer in plots with only one among the five outlier populations included at a time (Figure S3C–S3G), each of which also produces significant similarity scores between genetic and geographic coordinates (Figure S4, Table S7).
Asia
Our Asian example included 760 individuals from 44 populations distributed widely across Asia (Figure 4A). Previous studies based on largely overlapping datasets have reported correlations between genetic and geographic distances across Eurasia [22], [23]. Our dataset combined data from these studies as well as from Li et al. [7] and Simonson et al. [32], and after excluding 11 PCA outliers, our final dataset for Asia contains 749 individuals.
Fig. 4. Procrustes analysis of genetic and geographic coordinates of Asian populations. 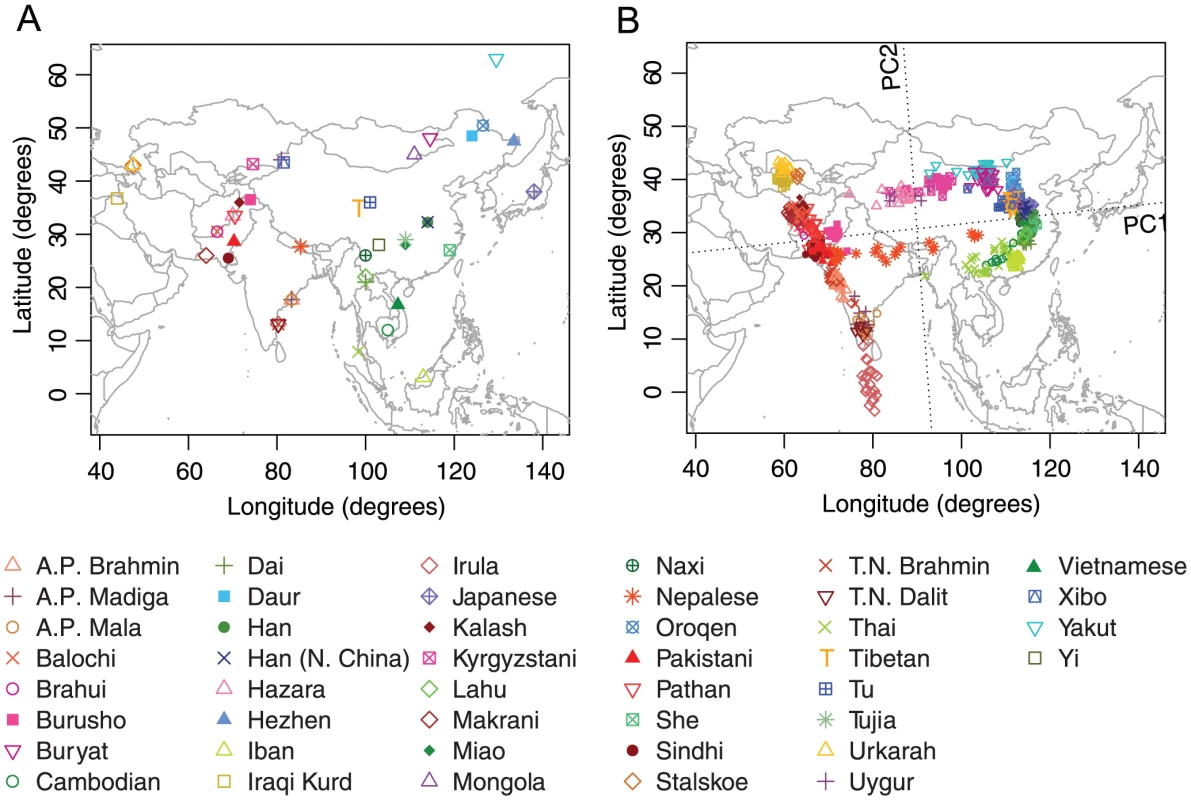
(A) Geographic coordinates of 44 populations. (B) Procrustes-transformed PCA plot of genetic variation. The Procrustes analysis is based on the unprojected latitude-longitude coordinates and PC1-PC2 coordinates of 749 individuals. PC1 and PC2 are indicated by dotted lines, crossing over the centroid of all individuals. PC1 and PC2 account for 5.42% and 0.85% of the total variance, respectively. The Procrustes similarity is (). The rotation angle of the PCA map is . In our PCA plot (Figure 4B), PC1 largely separates populations on different sides of the Himalayas, accounting for genetic variation in an east-west direction. PC2, on the other hand, distinguishes northern and southern populations. PC1 accounts for 5.42% of the total genetic variation, a much larger value than the 0.85% captured by PC2, reflecting large genetic distances between populations separated by the Himalayas. Interestingly, populations around the Himalayas form a ring shape on the PCA plot, with the Nepalese population from the Himalaya region aligning in the middle. As noted by Xing et al. [23], the Nepalese samples were collected from different subgroups that have different levels of ancestry shared with Central/South Asians and East Asians, and the dispersion of the Nepalese sample is therefore not unexpected. Tibetans, on the northern side of the Himalayas, do not spread over a large area in the plot and are well clustered with other East Asian populations.
One interesting result concerns the Uygur and Kyrgyzstani populations, both of which lie along ancient trade routes between Europe and East Asia. Compared to the Uygur population, which lies farther to the east, the Kyrgyzstani population clusters closer to East Asian populations, especially to the Yakut and Buryat populations, supporting a view that the Kyrgyzstani group has a proportion of its ancestry in Siberia [39]. A third population sampled from near the Uygur and Kyrgyzstani populations is the Xibo population, which clusters clearly with East Asians from northeastern China. This pattern matches the expectation given documentation that this Xibo group moved in 1764 from northeastern China to Xinjiang province [40], [41].
The PCA map of genetic variation in Asia is rotated 5.05 counterclockwise in the Procrustes superposition on the geographic map (Figure 4B). Despite the discontinuity caused by the Himalayas, most populations align in a way that is highly concordant with their geographic locations. This observation is confirmed by a Procrustes similarity score of (). Among all populations, the tribal population Irula, which appears south of India as an isolated cluster in Figure 4B, has the largest impact among all populations on the Procrustes similarity with geography (Table S8). When excluding Irula, the PCA map aligns more closely with geography, with the Procrustes similarity increasing to 0.871 (, Figure S5). This exclusion generates increased separation on the PCA map for some populations. For example, in Figure S5, Iban from Sarawak is more clearly distinguished from other Southeast Asian populations. Overall, the similarity score between genes and geography in Asia is robust to the exclusion of any one population, with the lowest Procrustes similarity score of occurring when the Buryat population is excluded (Table S8).
East Asia
To further examine populations on either side of the Himalaya Moutains, we performed additional analyses of East Asia and Central/South Asia. We first considered the East Asian populations in our Asian example. This dataset consists of 341 individuals from 23 populations. After excluding seven PCA outliers, our analyses were based on 334 individuals from 23 East Asian populations (Figure 5A).
Fig. 5. Procrustes analysis of genetic and geographic coordinates of East Asian populations. 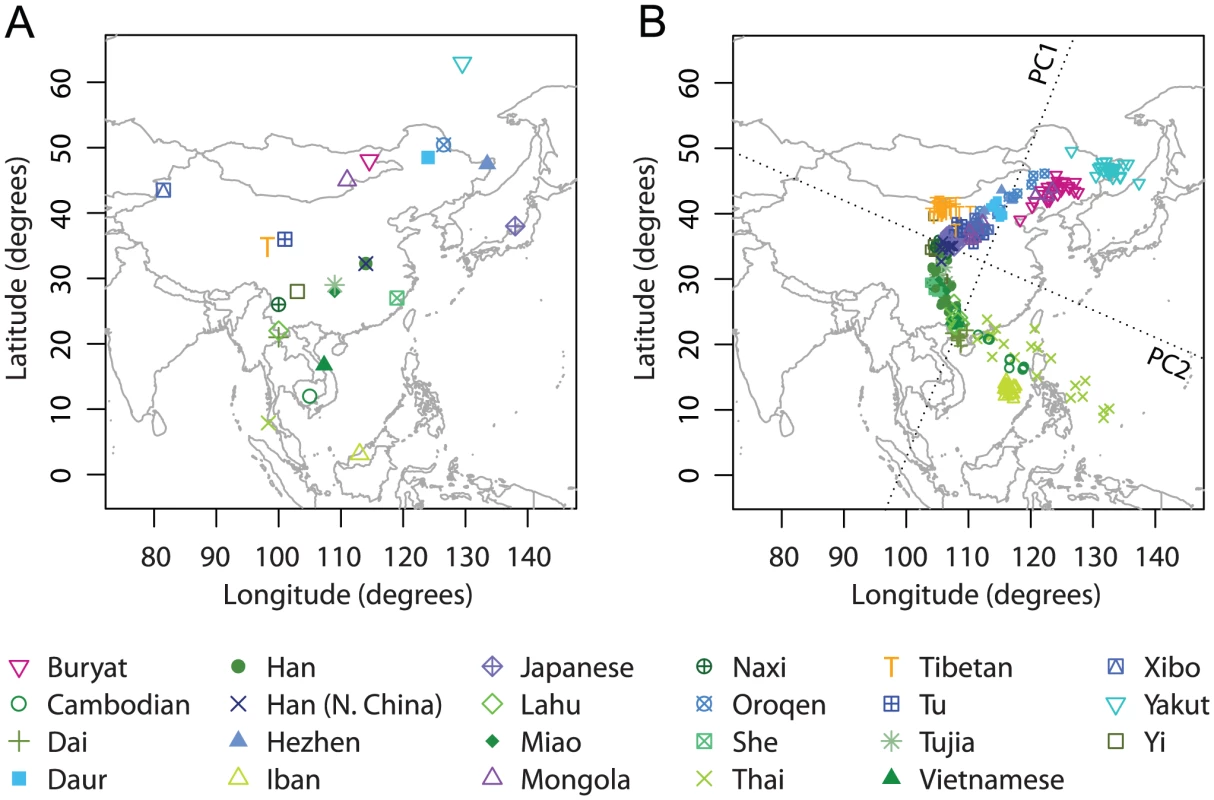
(A) Geographic coordinates of 23 populations. (B) Procrustes-transformed PCA plot of genetic variation. The Procrustes analysis is based on the unprojected latitude-longitude coordinates and PC1-PC2 coordinates of 334 individuals. PC1 and PC2 are indicated by dotted lines, crossing over the centroid of all individuals. PC1 and PC2 account for 1.58% and 0.98% of the total variance, respectively. The Procrustes similarity statistic is (). The rotation angle of the PCA map is . Individuals in this East Asian dataset generally align along a curve on the PCA plot. PC1 explains 1.58% of the total genetic variation and largely accounts for a north-south genetic gradient; PC2 explains 0.98% of the genetic variation and mainly separates two Siberian populations (Buryat and Yakut) and three Southeast Asian populations (Cambodians, Iban, and Thai) from the other East Asian populations (Figure 5B). The Tibetan population is also separated by PC2, but on the opposite side to the Siberians and Southeast Asians. Overall, PC1 largely matches geography in the north-south direction, and PC2 shows only a partial similarity to the east-west direction.
The imperfect match between PCA coordinates and geography is reflected by a relatively low Procrustes similarity score of , which, however, is still statistically significant with . The optimal transformation rotates the PCA map 67.27 counterclockwise prior to superposition on the geographic map (Figure 5B). Interestingly, excluding populations one at a time, we found that the PCA coordinates were reflected over PC1 when Procrustes-transformed to match the geographic coordinates if either the Iban, Tibetan, or Yakut population was excluded (Figure S6). Such abrupt changes of the Procrustes transformation are consistent with the fact that PC2 matches less closely with geography; a reflection over PC1 has a small effect on the similarity score. The Procrustes similarity score with geography can be substantially increased by excluding Japanese (, ); other than the Japanese population, Iban, Thai, and Yakut have the largest effect on the similarity scores both with geography and with the original PCA (Table S9).
Central/South Asia
Our last example focused on Central/South Asia, using an initial sample of 372 individuals from 18 populations. Ten individuals were excluded as PCA outliers, leaving 362 individuals from 18 populations for the final analysis (Figure 6A).
Fig. 6. Procrustes analysis of genetic and geographic coordinates of Central/South Asian populations. 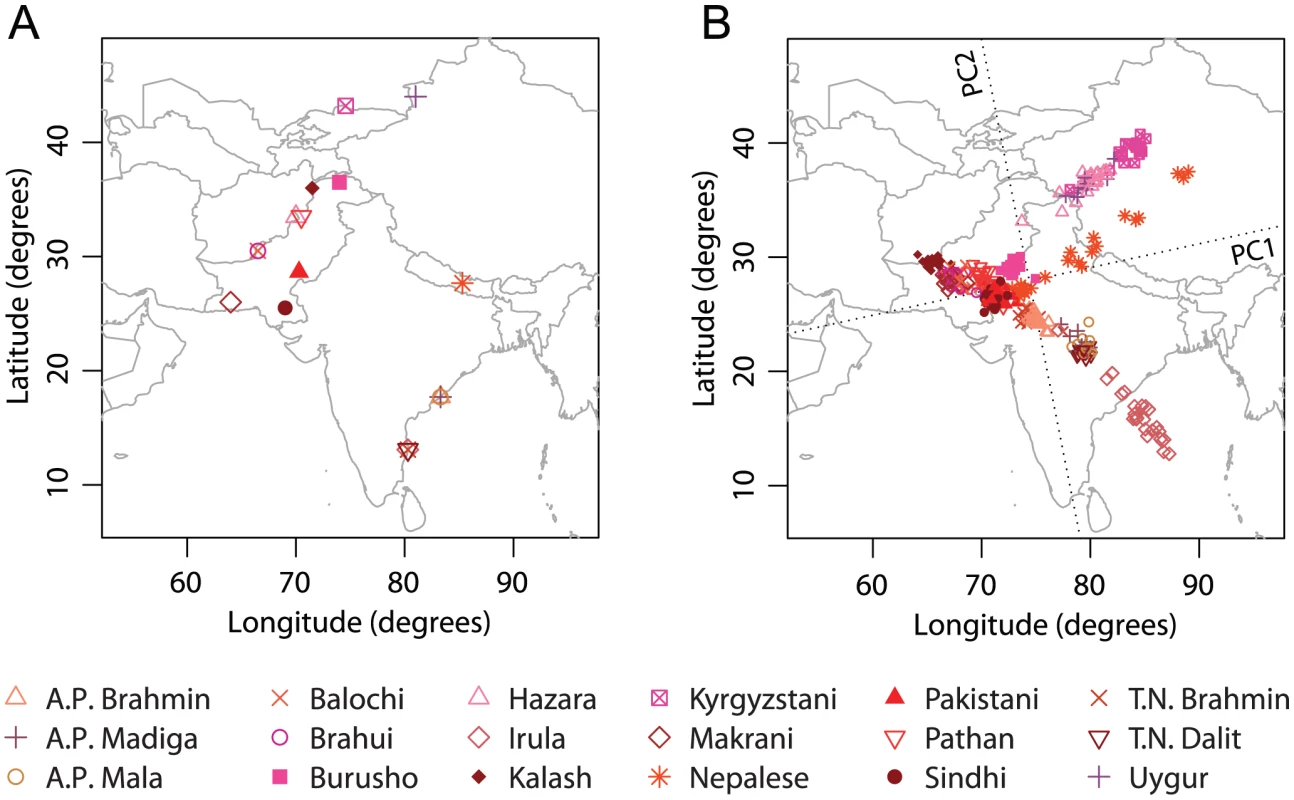
(A) Geographic coordinates of 18 populations. (B) Procrustes-transformed PCA plot of genetic variation. The Procrustes analysis is based on the unprojected latitude-longitude coordinates and PC1-PC2 coordinates of 362 individuals. PC1 and PC2 are indicated by dotted lines, crossing over the centroid of all individuals. PC1 and PC2 account for 1.59% and 1.31% of the total variance, respectively. The Procrustes similarity statistic is (). The rotation angle of the PCA map is . The first two components of the PCA anlaysis account for 1.59% and 1.31% of the total genetic variation, respectively. Overall, the PCA pattern for the separate anlaysis of Central/South Asian populations is similar to the pattern for the same set of populations in our analysis of all of Asia (Figure 4). After rotating the PCA coordinates 11.78 counterclockwise, we obtained a Procrustes similarity score of 0.737 () when comparing PCA coordinates to geography (Figure 6B). Most populations from Pakistan cluster closely on the first two PCs except for the Hazara population, which clusters with the Uygur population and aligns distantly from its sampling location. When excluding Hazara, the Procrustes similarity score to geography increases from 0.737 to , larger than for any other exclusion (Table S10). Excluding Irula has the second largest effect on the similarity score to geography, but more interestingly, this exclusion has the largest effect on the PCA coordinates (smallest value for in Table S10). A closer examination of the PCA results reveals that when Irula is excluded, the Kalash population in Pakistan is separated from the other Pakistani populations and appears as an isolated group in the north (results not shown). This result accords with the identification of this isolated group as distinct in previous studies [8], [36].
Comparison across geographic regions
We have found that significant similarity between genes and geography exists in general at different geographic levels (Table 2). The highest similarity score was found in the data from Asia, followed by Sub-Saharan Africa when five outlier populations were excluded, and by Europe. Five of the six analyses had -values smaller than , and only the data from East Asia had a nonzero -value in 100,000 permutations. When comparing the permutation distributions of the similarity score (Figure 7), however, a difference in the significance levels is evident for the five examples with . The worldwide and Asian datasets have similarity scores considerably exceeding the similarity scores from all 100,000 permutations (Figure 7A and 7D). By contrast, although the European, Sub-Saharan African, and Central/South Asian datasets have similarity scores higher than that of the worldwide dataset, their similarity scores are closer to the corresponding permutation distributions (Figure 7B, 7C, and 7F), indicating relatively high -values compared to the worldwide data.
Fig. 7. Histograms of the Procrustes similarity of 100,000 permutations for analyses in Figure 1, Figure 2, Figure 3, Figure 4, Figure 5, and Figure 6. 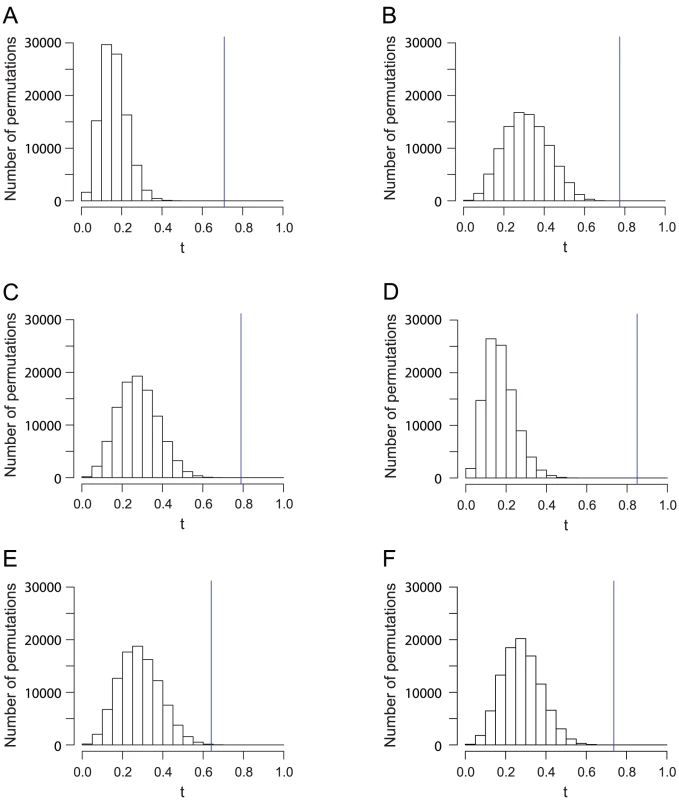
The blue vertical lines indicate the value of . (A) The worldwide dataset in Figure 1 (, ). (B) The European dataset in Figure 2 (, ). (C) The Sub-Saharan African dataset in Figure 3 (, ). (D) The Asian dataset in Figure 4 (, ). (E) The East Asian dataset in Figure 5 (, ). (F) The Central/South dataset in Figure 6 (, ). Tab. 2. Summary of the results for datasets from different geographic regions. 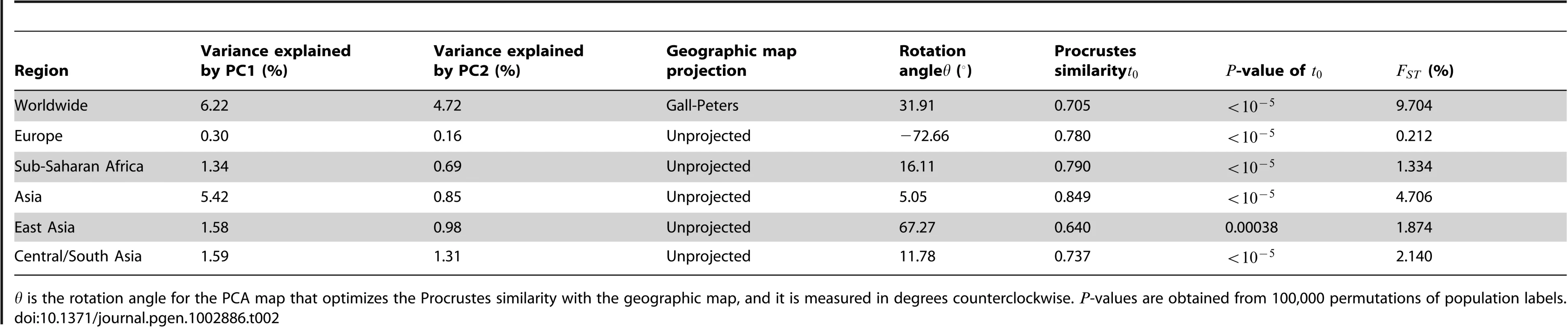
is the rotation angle for the PCA map that optimizes the Procrustes similarity with the geographic map, and it is measured in degrees counterclockwise. -values are obtained from 100,000 permutations of population labels. To examine the robustness of our results to the number of SNPs analyzed, we repeated our analyses with subsets of randomly selected loci. We found that our Procrustes similarity scores between genes and geography are quite robust as long as enough SNPs (10,000) are used (Figure 8). Indeed, for the worldwide and Asian datasets, 1,000 SNPs are sufficient to obtain a similarity score between genes and geography close to the score obtained using all 32,991 SNPs. For the African, East Asian, and Central/South Asian datasets, the number of SNPs needed increases to 4,000. Interestingly, many more SNPs are required for the European dataset to reach a high similarity score between genes and geography. Although the increase of the similarity score for the European dataset becomes slow when the number of SNPs exceeds 10,000, it continues even when the number of SNPs is as high as 30,000 (Figure 8). If we use the same 197,146 SNPs as used by Novembre et al. [9], the similarity score between genes and geography for the European example would become 0.799, slightly higher than the value for our Sub-Saharan African example based on 32,991 SNPs. This larger number of SNPs required might reflect a relatively homogeneous population structure in Europe that requires more genetic markers to characterize subtle differentiation.
Fig. 8. Procrustes analyses of genetic and geographic coordinates based on different numbers of loci. 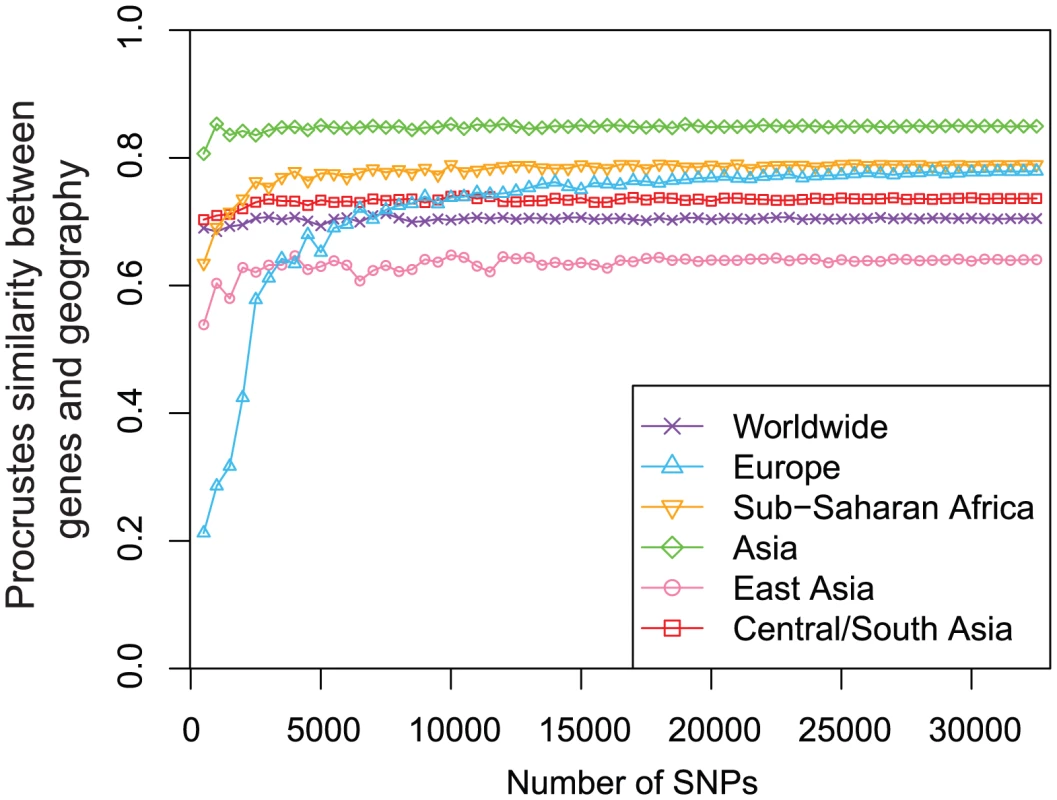
The same sets of randomly selected markers were used to generate PCA maps of genetic variation to compare with geographic maps for different regions. . To explore the relationship between genetic differentiation and the number of SNPs required to produce convergence in the Procrustes similarity, we computed across populations, a measurement of population differentiation, for all of our datasets, on the basis of the 32,991 autosomal SNP markers. We found for the European dataset, much smaller than the values of 9.704% and 4.706% for the worldwide and Asian datasets. The values of for the Sub-Saharan Africans (without outlier populations), the East Asians, and the Central/South Asians are 1.334%, 1.874% and 2.140%, respectively. As expected, datasets that have less population differentiation, as indicated by smaller values, need more markers to reveal geographic structure in the PCA plot, consistent with a previous finding that the dataset size required for the population structure to be evident in PCA is inversely related to [12]. Further, we found and the sum of the proportions of variance explained by PC1 and PC2 to be positively correlated (Pearson correlation , Figure 9). This strong linear correlation is not surprising because of the connection between and the proportions of variance: can be computed as the proportion of the variance in an allelic indicator variable contributed by between-population differences [42]. It has been shown under a two-population model that the proportion of the total variance explained by PC1 is approximately equal to [43]. Here, we have observed a qualitatively similar relationship.
Fig. 9. Relationship between and the proportion of genetic variation explained by the first two components of the PCA. 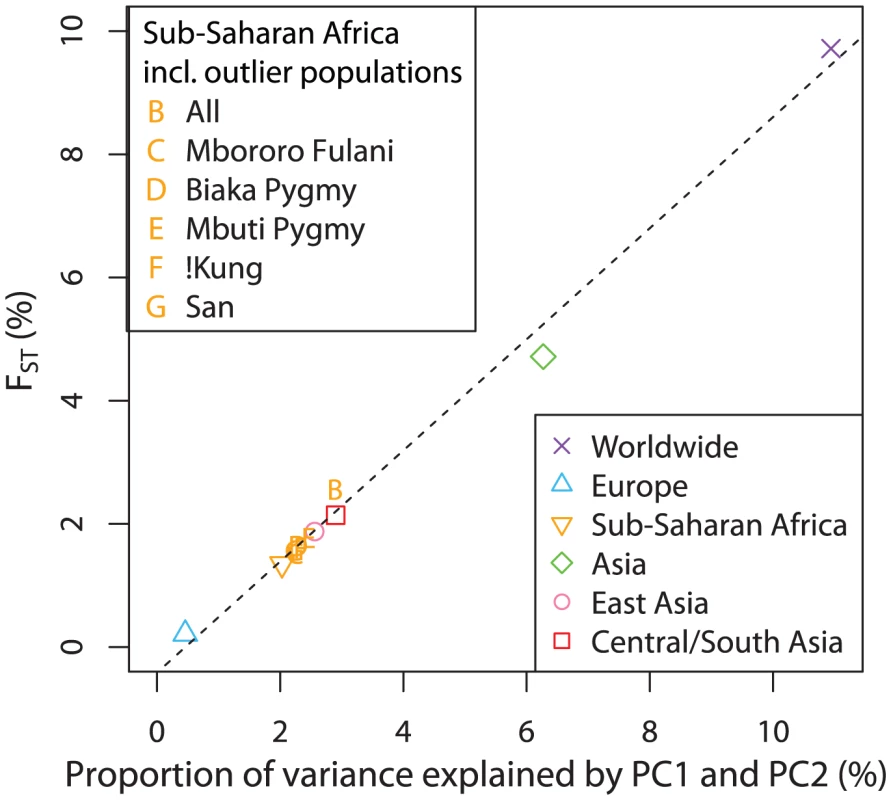
Both the main analyses of the paper in Table 2 and the supplementary analyses of Sub-Saharan Africa, in which certain populations excluded from the main analysis are included, are considered in obtaining the regression line. The values on the x-axis were obtained by summing the proportions of variance explained by PC1 and PC2 (columns 2 and 3 in Table 2, columns 6 and 7 in Table S7). values were estimated from the same datasets as used in the PCA (column 7 in Table 2, column 11 in Table S7). The dashed line indicates the linear least squares fit of . The Pearson correlation is . Discussion
Both simulation-based and theoretical studies have shown that under spatial models in which migration and gene flow occur in a homogeneous manner over short distances, a similarity between PCA maps of genetic variation and geography is predicted [43], [44]. In this study, we have systematically assessed this similarity in different geographic regions using a shared set of autosomal SNPs and a shared statistical approach. We have found that although they generally explain a relatively small proportion of the total genetic variation, the first two principal components in PCA often produce a map that resembles the geographic distribution of sampling locations. Our results quantitatively demonstrate the general existence in different geographic regions of a considerable similarity between genes and geography, supporting the view that geography, in the form of incremental migration and gene flow primarily with nearby neighbors, plays a strong role in producing human population structure.
One particularly interesting observation concerns our analysis of the Asian dataset. Asia contains the Himalaya region, a strong geographic barrier to gene flow that has generated noticeable genetic differentiation between populations on opposite sides [45]. Such barrier effects can produce a distortion of PCA maps from those expected under homogeneous isolation-by-distance models [43], [44], leading to a decrease in the similarity to geography. However, although the concordance of a PCA plot with geography is perhaps best known for Europe — which does not have a barrier of comparable importance to the Himalayas — we obtained the unexpected result that in spite of the Himalaya barrier, the Procrustes similarity score was actually highest in Asia. When further examining the population structure on separate sides of the Himalayas, we found lower similarity scores between genes and geography in our East Asian and Central/South Asian samples. Especially for the East Asian sample, our results indicate weaker correlation between genes and geography in the east-west direction.
To make the similarity scores between genes and geography commensurable for different datasets, we performed our analyses with the same markers and the same statistical approach. However, one aspect of the analysis that is not homogeneous across datasets is the nature of the geographic coordinates. For example, while most of the analyses employed population sampling locations, for the European dataset, coordinates did not necessarily represent sampling locations. Sampling locations may also vary in the extent to which they represent long-term locations where groups have resided. One example that highlights this issue is the Xibo population, which was sampled in northwestern China, but which clusters genetically with populations in northeastern China (Figure 5). This group is known to have migrated westward from near Shenyang in northeastern China about 250 years ago [40], [41], and if we were to use the coordinates of Shenyang (N, E) for Xibo rather than the sampling location, would increase from 0.640 to 0.654 for the East Asian dataset, from 0.849 to 0.859 for the Asian dataset, and from 0.705 to 0.709 for the worldwide dataset.
Additional limitations apply to our geographic analysis. In all of the datasets, population-level rather than individual-level coordinates were used, so that all individuals from the same population were assigned to a single geographic location. This approach can potentially obscure substructure within populations. For example, although both the northern and southern Han Chinese groups from the HGDP dataset were assigned to the same location, they can be genetically distinguished from each other, with the northern group clustering closer to the northern populations in China (Figure 5). Use of individual-level coordinates might lead to higher values of the similarity score . Another concern is that the choice of a map projection (including the projection that consists of using unprojected latitudes and longitudes as a rectangular coordinate system) can have different effects in geographic regions at different distances from the equator, as the level of distortion of the surface of the earth varies with the choice of projection. This issue is expected to be of greatest concern in analyses at high latitudes or in datasets with a wide range of latitudes.
We note that theoretical work and simulation studies have found that results from the PCA approach can be sensitive to the sample size distribution over geographic space [43], [44], [46]. In most of our analyses excluding one population at a time, patterns in PC1 and PC2 did not differ greatly from analyses in which all populations were included. However, exclusions of genetically distinctive populations, populations that were geographically distant from the center of a dataset, or populations with large sample sizes sometimes had sizeable effects on . In some analyses, particularly in considering the Luhya and Maasai populations from the HapMap, we therefore included only a subset of available individuals in order to reduce the influence of the large sample sizes for these populations. More generally, an analysis of the role of the geographic distribution of the sample can be performed by analysis of subsamples of a full dataset with different levels of geographic unevenness. A previous analysis of population structure inference using STRUCTURE for a variety of samples with different geographic distributions did not find a particularly strong role for the geographic dispersion of the sample [47], but the issue has not yet been systematically investigated with PCA.
Through a combination of PCA and Procrustes analysis, we have investigated genes and geography using the same standardized approach in different regions. The general observation of a concordance of genes and geography in different regions and at different geographic levels can provide a foundation for refinement of methods for inferring local geographic origin of human individuals from their genotypes [e.g. 9], [19], [48]. In addition, our computations illustrate the use of Procrustes analysis in assisting the interpretation of PCA, such as in comparing PCA maps to different types of spatial maps and in assessing the impact of certain populations or individuals on PCA results. Similar applications of PCA and Procrustes approaches can be used to evaluate evolutionary models by comparing PCA maps obtained from observed data to those obtained from simulated data generated by these models. With the incorporation of the Procrustes similarity score for quantifying patterns in PCA, results from PCA can potentially find new uses in additional applications in population-genetic studies.
Materials and Methods
Genotype data
We examined genome-wide SNP datasets previously reported in several studies [9], [23], [26], [31], [32]. The data of Pemberton et al. [31] merged unrelated samples from earlier datasets obtained from the HGDP [7] and HapMap Phase III [33], [49]. Some of the data of Xing et al. [23] were previously reported in an earlier paper of Xing et al. [22].
Because the datasets were genotyped on different genotyping platforms, including Illumina 650 K [31], Illumina Human 1 M [31], Affymetrix 500 K [9], [26], Affymetrix NspI 250 K [23], and Affymetrix 6.0 [23], [31], [32], we identified a shared set of 32,991 autosomal SNPs included in all five datasets [9], [23], [26], [31], [32]. This number was smaller than the maximum possible set of overlapping SNPs shared among these genotyping platforms, because some SNPs were excluded during the quality control procedures of the studies that originally published the data [9], [23], [26], [31], [32]. At 6,549 among these 32,991 markers, the datasets from Novembre et al. [9] and Bryc et al. [26] had genotypes given for opposite strands when compared to the datasets of Xing et al. [23], Pemberton et al. [31], and Simonson et al. [32]. In these instances, we converted the genotypes from Novembre et al. [9] and Bryc et al. [26] to the opposite strand, so that genotypes were consistent across datasets from different sources. In total, we obtained genotype data on 32,991 autosomal SNPs for 4,257 samples from 149 populations worldwide, with dense sampling from Asia, Europe, and Sub-Saharan Africa. In our final dataset, the physical distance between pairs of nearby SNPs has mean 84 kb (median 45 kb).
We next created six datasets at different geographic scales, including a worldwide sample, continental samples for Europe, Sub-Saharan Africa, and Asia, and subcontinental samples from East Asia and Central/South Asia (Figure S7, Table 1). For the worldwide example, we included 938 unrelated individuals from 53 populations in the HGDP [7], [31]. For the European sample, we used a set of individuals that was nearly identical to that analyzed by Novembre et al. [9], containing 1,385 individuals from 37 populations defined by ancestral origins. We did not include two French individuals (sample ID 31645 and 32480) that were included by Novembre et al. [9] but that are not found in the release we obtained of the POPRES dataset in the NCBI dbGaP database [50], [51]. For Sub-Saharan Africa, we integrated data on African populations from three sources [23], [26], [31], including 30 unrelated Luhya (LWK) individuals and 30 unrelated Maasai (MKK) individuals, both randomly selected from the HapMap Phase III [31]. Because some populations in Sub-Saharan Africa are known to be genetically distinctive when compared to most other Sub-Saharan Africans [7], [8], [23], [26], [36], [37], we created two datasets for Sub-Saharan Africa, one including and the other excluding these distinctive populations (!Kung, San, Biaka Pygmy, Mbuti Pygmy, and Mbororo Fulani). When excluding all five of these populations, we have 356 individuals from 23 Sub-Saharan African populations. Including them, we have 422 individuals from 28 groups. Note that both Pygmy populations that we examined are from the HGDP [7], [31], and we did not include the Mbuti Pygmy data from Xing et al. [23]. Further, we also did not include the Luhya individuals from Xing et al. [23]; these individuals are a subset of those of the HapMap [31], [33]. As in Xing et al. [23], we analyzed three Sotho samples and five Tswana samples together as a single population, labeled as “Sotho/Tswana.”
Our sample from Asia has 760 individuals from 44 populations with sampling locations distributed widely across Asia. These data include 27 populations from the HGDP dataset [7], [31], 16 populations from Xing et al. [23], and one population (Tibetan) from Simonson et al. [32]. For populations studied by both Pemberton et al. [31] and Xing et al. [23] (Cambodian, Han Chinese, and Japanese), we only included the HGDP samples from Pemberton et al. [31]. Samples for East Asia and Central/South Asia are subsets of the Asian sample. The East Asian sample consists of 341 individuals from 23 populations: 18 populations from the HGDP dataset [7], [31], 4 populations from Xing et al. [23], and the Tibetan population from Simonson et al. [32]. The Central/South Asian sample has 372 individuals from 18 populations in total, including 9 populations each from the HGDP dataset [7], [31] and the Xing et al. dataset [23].
We applied two additional processing steps on each dataset to remove samples with high missing data rates and samples that appear to be outliers. First, we removed individuals with more than 5% missing data in the 32,991 SNPs. Next, in each analysis, we used an iterative PCA approach to identify and remove outlier individuals, as outliers can potentially distort PCA maps of genetic variation [52]. After applying PCA on a dataset, individuals greater than 10 standard deviations from the mean PC position on at least one of the top 10 PCs were considered outliers and were removed from the dataset. This procedure was repeated iteratively until no more outliers were detected. For all datasets, only a small proportion of samples were identified as outliers and removed by this procedure (Table 1). The data processing procedures are illustrated in Figures S7, S8, S9, and are summarized in Table 1. Individuals that were identified as PCA outliers are listed in Table S11.
Geographic coordinates
We assigned all individuals from the same population to a single geographic location, as listed in Tables S1, S2, S3. For the HGDP samples [31], we used previously reported coordinates as the geographic locations for all populations (Table 1 in [45]). The geographic locations for the European dataset were reported in Table S3 of Novembre et al. [9], and represent countries of origin. The geographic coordinates for the African populations from Bryc et al. [26] are sampling locations, and we used the values reported by Tishkoff et al. [37] in their Table S1. Geographic coordinates for populations from Xing et al. [23] were kindly provided by J. Xing. For the Tibetan samples, we used the sampling location reported by Simonson et al. [32]. For the two HapMap populations included in this study (Luhya and Maasai), we used the sampling locations reported by HapMap [33].
We used longitude and latitude measured in degrees as our geographic coordinates for all datasets except the worldwide dataset. Latitudes in the southern hemisphere and longitudes in the western hemisphere were denoted by negative values. For the worldwide dataset, we shifted the Americas by adding to longitudes smaller than . We then used the Gall-Peters projection, an equal-area projection that preserves distance along the N parallel, to obtain rectangular coordinates as our geographic coordinates. For other datasets, we used unprojected longitude-latitude coordinates.
Principal components analysis
We coded the genotype data for each dataset by an matrix , in which counts the number of copies of a reference allele at locus of individual , is the number of individuals, and is the number of loci. For autosomal SNPs, is 0, 1, 2, or missing. We first ignored missing data and estimated the reference allele frequency among nonmissing genotypes, or . Following the smartpca program [12], we standardized the nonmissing entries in by
where is a matrix with the same dimensions as . If a locus was monomorphic in a dataset ( or 1), eq. 1 is undefined, and we set all entries in the column of for this locus to zero. Entries representing missing data were set to zero in as well.We performed PCA by applying the function eigen in R (www.r-project.org) to the matrix [43]. The coordinates of the individuals on the th PC are given by , where is the th eigenvalue of , sorted in decreasing order, and is the corresponding eigenvector. The proportion of variance explained by the th PC is calculated as , where is the total number of eigenvectors of . This quantity measures the variation among individuals along the th PC direction, relative to the total variance in the standardized genotypic matrix . In our examples, , and because has rank after standardization (eq. 1).
We note that some studies have used the eigenvectors directly as PCs, so that all PCs have equal variance. We follow an alternative convention [43], [53], reporting PCs using , so that the proportions of variance explained by each PC are reflected on the PCA plot. In PCA plots superimposed on geographic maps, because horizontal and vertical axes are plotted on different scales, PC1 and PC2 can appear to not be perpendicular.
Procrustes analysis and permutation test
We applied Procrustes analysis [13], [15] to compare the individual-level coordinates of the first two components (PC1 and PC2) in the PCA performed on the SNP data to the geographic coordinates. Procrustes analysis minimizes the sum of squared Euclidean distances between two sets of points (two “maps”) by transforming one set of points to optimally match the other set, while preserving the relative pairwise distances among all points within maps. Possible transformations include translation, scaling, rotation, and reflection. The similarity between two maps is then quantified by a Procrustes similarity statistic , in which is the minimum sum of squared Euclidean distances between the two maps across all possible transformations. , which is given by equation 6 in Wang et al. [15], has been scaled to have minimum 0 and maximum 1. The similarity statistic therefore also ranges from 0 to 1. In our analyses, we fixed the geographic coordinates and Procrustes-transformed the PCA coordinates in order to superimpose the PCA maps on the geographic maps. In addition to , we also report the rotation angle of the PCA map as given by the Procrustes analysis, measured in degrees counterclockwise.
To test the statistical significance of , we used a permutation test. In each permutation, we randomly permuted the population geographic locations, assigning all individuals from the same population to a single geographic location in the permuted dataset. We then applied Procrustes analysis to compute the similarity score between the PCA coordinates and the randomly permuted geographic coordinates. We calculated the -value as , representing the probability of observing a similarity statistic higher than under the null hypothesis that no geographic pattern exists in the population structure. For each dataset, we employed 100,000 permutations for the permutation test.
Analyses with populations excluded individually
We investigated the effect of each population on our PCA and Procrustes analysis using a leave-one-out approach. For each dataset, we excluded one population at a time and repeated PCA to obtain a new set of genetic coordinates (for each population excluded, this PCA started from the same final set of individuals after exclusions owing to missing data and PCA outliers, and we did not repeat the search for outliers). We then performed two Procrustes analyses. In the first one, we compared the new PCA coordinates and the original PCA coordinates obtained before removing any population. This comparison was based on the common set of individuals included in both analyses, and its similarity score was denoted . In the second Procrustes analysis, we computed the similarity between the new set of PCA coordinates and the corresponding geographic coordinates, denoting the similarity score by .
Subsets of loci
To investigate the effect of the number of markers on our results, we created a series of marker lists by randomly selecting loci from the 32,991 total loci. These marker lists were selected independently of each other and had . We then repeated PCA and Procrustes analysis for each geographic region using genotypes at the loci in each of our marker lists. For Sub-Saharan Africa, we used the dataset that excludes hunter-gatherer populations and the Mbororo Fulani. Given , the analyses for different geographic regions are based on the same set of markers, so that their results are comparable.
estimation
We calculated in each dataset using Weir and Cockerham's estimator (eq. 10 in [54]) based on all 32,991 loci.
Supporting Information
Zdroje
1. SokalRR, OdenNL, WilsonC (1991) Genetic evidence for the spread of agriculture in Europe by demic diffusion. Nature 351 : 143–145.
2. Cavalli-Sforza LL, Menozzi P, Piazza A (1994) The History and Geography of Human Genes. Princeton: Princeton University Press.
3. BarbujaniG (2000) Geographic patterns: how to identify them and why. Hum Biol 72 : 133–153.
4. Cavalli-SforzaLL, FeldmanMW (2003) The application of molecular genetic approaches to the study of human evolution. Nat Genet 33(Suppl):266–275.
5. NovembreJ, RamachandranS (2011) Perspectives on human population structure at the cusp of the sequencing era. Annu Rev Genomics Hum Genet 12 : 245–274.
6. RamachandranS, DeshpandeO, RosemanCC, RosenbergNA, FeldmanMW, et al. (2005) Support from the relationship of genetic and geographic distance in human populations for a serial founder effect originating in Africa. Proc Natl Acad Sci USA 102 : 15942–15947.
7. LiJZ, AbsherDM, TangH, SouthwickAM, CastoAM, et al. (2008) Worldwide human relationships inferred from genome-wide patterns of variation. Science 319 : 1100–1104.
8. JakobssonM, ScholzSW, ScheetP, GibbsJR, VanLiereJM, et al. (2008) Genotype, haplotype and copy-number variation in worldwide human populations. Nature 451 : 998–1003.
9. NovembreJ, JohnsonT, BrycK, KutalikZ, BoykoAR, et al. (2008) Genes mirror geography within Europe. Nature 456 : 98–101.
10. BiswasS, ScheinfeldtLB, AkeyJM (2009) Genome-wide insights into the patterns and determinants of fine-scale population structure in humans. Am J Hum Genet 84 : 641–650.
11. MenozziP, PiazzaA, Cavalli-SforzaL (1978) Synthetic maps of human gene frequencies in Europeans. Science 201 : 786–792.
12. PattersonN, PriceAL, ReichD (2006) Population structure and eigenanalysis. PLoS Genet 2: e190 doi:10.1371/journal.pgen.0020190.
13. Cox TF, Cox MAA (2001) Multidimensional Scaling. Boca Raton: Chapman & Hall, 2nd edition.
14. PaschouP, ZivE, BurchardEG, ChoudhryS, Rodriguez-CintronW, et al. (2007) PCA-correlated SNPs for structure identification in worldwide human populations. PLoS Genet 3: e160 doi:10.1371/journal.pgen.0030160.
15. WangC, SzpiechZA, DegnanJH, JakobssonM, PembertonTJ, et al. (2010) Comparing spatial maps of human population-genetic variation using Procrustes analysis. Stat Appl Genet Mol Biol 9: Article 13.
16. LaoO, LuTT, NothnagelM, JungeO, Freitag-WolfS, et al. (2008) Correlation between genetic and geographic structure in Europe. Curr Biol 18 : 1241–1248.
17. HeathSC, GutIG, BrennanP, McKayJD, BenckoV, et al. (2008) Investigation of the fine structure of European populations with applications to disease association studies. Eur J Hum Genet 16 : 1413–1429.
18. JakkulaE, RehnströmK, VariloT, PietiläinenOPH, PaunioT, et al. (2008) The genome-wide patterns of variation expose significant substructure in a founder population. Am J Hum Genet 83 : 787–794.
19. HoggartCJ, O'ReillyPF, KaakinenM, ZhangW, ChambersJC, et al. (2012) Fine-scale estimation of location of birth from genome-wide single-nucleotide polymorphism data. Genetics 190 : 669–677.
20. PriceAL, HelgasonA, PalssonS, StefanssonH, St ClairD, et al. (2009) The impact of divergence time on the nature of population structure: an example from Iceland. PLoS Genet 5: e1000505 doi:10.1371/journal.pgen.1000505.
21. SalmelaE, LappalainenT, LiuJ, SistonenP, AndersenPM, et al. (2011) Swedish population substructure revealed by genome-wide single nucleotide polymorphism data. PLoS ONE 6: e16747 doi:10.1371/journal.pone.0016747.
22. XingJ, WatkinsWS, WitherspoonDJ, ZhangY, GutherySL, et al. (2009) Fine-scaled human genetic structure revealed by SNP microarrays. Genome Res 19 : 815–825.
23. XingJ, WatkinsWS, ShlienA, WalkerE, HuffCD, et al. (2010) Toward a more uniform sampling of human genetic diversity: a survey of worldwide populations by high-density genotyping. Genomics 96 : 199–210.
24. The HUGO Pan-Asian SNP Consortium (2009) Mapping human genetic diversity in Asia. Science 326 : 1541–1545.
25. TianC, KosoyR, LeeA, RansomM, BelmontJW, et al. (2008) Analysis of East Asia genetic substructure using genome-wide SNP arrays. PLoS ONE 3: e3862 doi:10.1371/journal.pone.0003862.
26. BrycK, AutonA, NelsonMR, OksenbergJR, HauserSL, et al. (2010) Genome-wide patterns of population structure and admixture in West Africans and African Americans. Proc Natl Acad Sci USA 107 : 786–791.
27. SikoraM, LaayouniH, CalafellF, ComasD, BertranpetitJ (2011) A genomic analysis identifies a novel component in the genetic structure of sub-Saharan African populations. Eur J Hum Genet 19 : 84–88.
28. ChenJ, ZhengH, BeiJX, SunL, JiaWH, et al. (2009) Genetic structure of the Han Chinese population revealed by genome-wide SNP variation. Am J Hum Genet 85 : 775–785.
29. XuS, YinX, LiS, JinW, LouH, et al. (2009) Genomic dissection of population substructure of Han Chinese and its implication in association studies. Am J Hum Genet 85 : 762–774.
30. Yamaguchi-KabataY, NakazonoK, TakahashiA, SaitoS, HosonoN, et al. (2008) Japanese population structure, based on SNP genotypes from 7003 individuals compared to other ethnic groups: effects on population-based association studies. Am J Hum Genet 83 : 445–456.
31. PembertonTJ, AbsherD, FeldmanMW, MyersRM, RosenbergNA, et al. Genomic patterns of homozygosity in worldwide human populations. Am J Hum Genet (in press).
32. SimonsonT, YangY, HuffCD, YunH, QinG, et al. (2010) Genetic evidence for high-altitude adaptation in Tibet. Science 329 : 72–75.
33. The International HapMap 3 Consortium (2010) Integrating common and rare genetic variation in diverse human populations. Nature 467 : 52–58.
34. AutonA, BrycK, BoykoAR, LohmuellerKE, NovembreJ, et al. (2009) Global distribution of genomic diversity underscores rich complex history of continental human populations. Genome Res 19 : 795–803.
35. BowcockAM, Ruiz-LinaresA, TomfohrdeJ, MinchE, KiddJR, et al. (1994) High resolution of human evolutionary trees with polymorphic microsatellites. Nature 368 : 455–457.
36. RosenbergNA, PritchardJK, WeberJL, CannHM, KiddKK, et al. (2002) Genetic structure of human populations. Science 298 : 2381–2385.
37. TishkoffSA, ReedFA, FriedlaenderFR, EhretC, RanciaroA, et al. (2009) The genetic structure and history of Africans and African Americans. Science 324 : 1035–1044.
38. HennBM, GignouxCR, JobinM, GrankaJM, MacphersonJM, et al. (2011) Hunter-gatherer genomic diversity suggests a southern African origin for modern humans. Proc Natl Acad Sci USA 108 : 5154–5162.
39. Bregel Y (2003) An Historical Atlas of Central Asia. Boston: Brill.
40. Du R, Yip VF (1993) Ethnic Groups in China. Beijing: Science Press.
41. PowellGT, YangH, Tyler-SmithC, XueY (2007) The population history of the Xibe in northern China: a comparison of autosomal, mtDNA and Y-chromosomal analyses of migration and gene ow. Forensic Sci Int Genet 1 : 115–119.
42. Weir BS (1996) Genetic Data Analysis II. Sunderland, MA: Sinauer.
43. McVeanG (2009) A genealogical interpretation of principal components analysis. PLoS Genet 5: e1000686 doi:10.1371/journal.pgen.1000686.
44. NovembreJ, StephensM (2008) Interpreting principal component analyses of spatial population genetic variation. Nature Genet 40 : 646–649.
45. RosenbergNA (2011) A population-genetic perspective on the similarities and differences among worldwide human populations. Hum Biol 83 : 659–684.
46. EngelhardtBE, StephensM (2010) Analysis of population structure: a unifying framework and novel methods based on sparse factor analysis. PLoS Genet 6: e1001117 doi:10.1371/journal.pgen.1001117.
47. RosenbergNA, MahajanS, RamachandranS, ZhaoC, PritchardJK, et al. (2005) Clines, clusters, and the effect of study design on the inference of human population structure. PLoS Genet 1 doi:10.1371/journal.pgen.0010070.
48. YangWY, NovembreJ, EskinE, HalperinE (2012) A model-based approach for analysis of spatial structure in genetic data. Nat Genet 44 : 725–731.
49. PembertonTJ, WangC, LiJZ, RosenbergNA (2010) Inference of unexpected genetic relatedness among individuals in HapMap Phase III. Am J Hum Genet 87 : 457–464.
50. NelsonMR, BrycK, KingKS, IndapA, BoykoAR, et al. (2008) The Population Reference Sample, POPRES: a resource for population, disease, and pharmacological genetics research. Am J Hum Genet 83 : 347–358.
51. MailmanMD, FeoloM, JinY, KimuraM, TrykaK, et al. (2007) The NCBI dbGaP database of genotypes and phenotypes. Nat Genet 39 : 1181–1186.
52. PriceAL, PattersonNJ, PlengeRM, WeinblattME, ShadickNA, et al. (2006) Principal components analysis corrects for stratification in genome-wide association studies. Nature Genet 38 : 904–909.
53. Hastie T, Tibshirani R, Friedman J (2009) The Elements of Statistical Learning: Data Mining, Inference, and Prediction. New York: Springer, 2nd edition.
54. WeirBS, CockerhamCC (1984) Estimating F-statistics for the analysis of population structure. Evolution 38 : 1358–1370.
Štítky
Genetika Reprodukční medicína
Článek Mutational Signatures of De-Differentiation in Functional Non-Coding Regions of Melanoma GenomesČlánek Rescuing Alu: Recovery of Inserts Shows LINE-1 Preserves Alu Activity through A-Tail ExpansionČlánek Genetics and Regulatory Impact of Alternative Polyadenylation in Human B-Lymphoblastoid CellsČlánek Retrovolution: HIV–Driven Evolution of Cellular Genes and Improvement of Anticancer Drug ActivationČlánek The Mi-2 Chromatin-Remodeling Factor Regulates Higher-Order Chromatin Structure and Cohesin DynamicsČlánek Identification of Human Proteins That Modify Misfolding and Proteotoxicity of Pathogenic Ataxin-1
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 8
-
Všechny články tohoto čísla
- Mutational Signatures of De-Differentiation in Functional Non-Coding Regions of Melanoma Genomes
- Rescuing Alu: Recovery of Inserts Shows LINE-1 Preserves Alu Activity through A-Tail Expansion
- Genetics and Regulatory Impact of Alternative Polyadenylation in Human B-Lymphoblastoid Cells
- Chromosome Territories Meet a Condensin
- It's All in the Timing: Too Much E2F Is a Bad Thing
- Fine-Mapping and Initial Characterization of QT Interval Loci in African Americans
- Genome Patterns of Selection and Introgression of Haplotypes in Natural Populations of the House Mouse ()
- A Combinatorial Amino Acid Code for RNA Recognition by Pentatricopeptide Repeat Proteins
- Advances in Quantitative Trait Analysis in Yeast
- Experimental Evolution of a Novel Sexually Antagonistic Allele
- Variation of Contributes to Dog Breed Skull Diversity
- , a Gene Involved in Axonal Pathfinding, Is Mutated in Patients with Kallmann Syndrome
- A Single Origin for Nymphalid Butterfly Eyespots Followed by Widespread Loss of Associated Gene Expression
- Cryptocephal, the ATF4, Is a Specific Coactivator for Ecdysone Receptor Isoform B2
- Retrovolution: HIV–Driven Evolution of Cellular Genes and Improvement of Anticancer Drug Activation
- The PARN Deadenylase Targets a Discrete Set of mRNAs for Decay and Regulates Cell Motility in Mouse Myoblasts
- A Sexual Ornament in Chickens Is Affected by Pleiotropic Alleles at and , Selected during Domestication
- Use of Allele-Specific FAIRE to Determine Functional Regulatory Polymorphism Using Large-Scale Genotyping Arrays
- Novel Loci for Metabolic Networks and Multi-Tissue Expression Studies Reveal Genes for Atherosclerosis
- The Genetic Basis of Pollinator Adaptation in a Sexually Deceptive Orchid
- Uncovering the Genome-Wide Transcriptional Responses of the Filamentous Fungus to Lignocellulose Using RNA Sequencing
- Inheritance Beyond Plain Heritability: Variance-Controlling Genes in
- The Metabochip, a Custom Genotyping Array for Genetic Studies of Metabolic, Cardiovascular, and Anthropometric Traits
- Reprogramming to Pluripotency Can Conceal Somatic Cell Chromosomal Instability
- Condensin II Promotes the Formation of Chromosome Territories by Inducing Axial Compaction of Polyploid Interphase Chromosomes
- PTEN Negatively Regulates MAPK Signaling during Vulval Development
- A Dynamic Response Regulator Protein Modulates G-Protein–Dependent Polarity in the Bacterium
- Population Genomics of the Facultatively Mutualistic Bacteria and
- Components of a Fanconi-Like Pathway Control Pso2-Independent DNA Interstrand Crosslink Repair in Yeast
- Polysome Profiling in Liver Identifies Dynamic Regulation of Endoplasmic Reticulum Translatome by Obesity and Fasting
- Stromal Liver Kinase B1 [STK11] Signaling Loss Induces Oviductal Adenomas and Endometrial Cancer by Activating Mammalian Target of Rapamycin Complex 1
- Reprogramming of H3K27me3 Is Critical for Acquisition of Pluripotency from Cultured Tissues
- Transgene Induced Co-Suppression during Vegetative Growth in
- Hox and Sex-Determination Genes Control Segment Elimination through EGFR and Activity
- A Quantitative Comparison of the Similarity between Genes and Geography in Worldwide Human Populations
- Minibrain/Dyrk1a Regulates Food Intake through the Sir2-FOXO-sNPF/NPY Pathway in and Mammals
- Comparative Analysis of Regulatory Elements between and by Genome-Wide Transcription Start Site Profiling
- Simple Methods for Generating and Detecting Locus-Specific Mutations Induced with TALENs in the Zebrafish Genome
- S Phase–Coupled E2f1 Destruction Ensures Homeostasis in Proliferating Tissues
- Cell-Nonautonomous Signaling of FOXO/DAF-16 to the Stem Cells of
- The Mi-2 Chromatin-Remodeling Factor Regulates Higher-Order Chromatin Structure and Cohesin Dynamics
- Comparative Analysis of the Genomes of Two Field Isolates of the Rice Blast Fungus
- Role of Mex67-Mtr2 in the Nuclear Export of 40S Pre-Ribosomes
- Genetic Modulation of Lipid Profiles following Lifestyle Modification or Metformin Treatment: The Diabetes Prevention Program
- HAL-2 Promotes Homologous Pairing during Meiosis by Antagonizing Inhibitory Effects of Synaptonemal Complex Precursors
- SLX-1 Is Required for Maintaining Genomic Integrity and Promoting Meiotic Noncrossovers in the Germline
- Phylogenetic and Transcriptomic Analysis of Chemosensory Receptors in a Pair of Divergent Ant Species Reveals Sex-Specific Signatures of Odor Coding
- Reduced Prostasin (CAP1/PRSS8) Activity Eliminates HAI-1 and HAI-2 Deficiency–Associated Developmental Defects by Preventing Matriptase Activation
- Dissecting the Gene Network of Dietary Restriction to Identify Evolutionarily Conserved Pathways and New Functional Genes
- Identification of Human Proteins That Modify Misfolding and Proteotoxicity of Pathogenic Ataxin-1
- and Link Transcription of Phospholipid Biosynthetic Genes to ER Stress and the UPR
- CDK9 and H2B Monoubiquitination: A Well-Choreographed Dance
- Rare Copy Number Variations in Adults with Tetralogy of Fallot Implicate Novel Risk Gene Pathways
- Ccdc94 Protects Cells from Ionizing Radiation by Inhibiting the Expression of
- NOL11, Implicated in the Pathogenesis of North American Indian Childhood Cirrhosis, Is Required for Pre-rRNA Transcription and Processing
- Human Developmental Enhancers Conserved between Deuterostomes and Protostomes
- A Luminal Glycoprotein Drives Dose-Dependent Diameter Expansion of the Hindgut Tube
- Melanophore Migration and Survival during Zebrafish Adult Pigment Stripe Development Require the Immunoglobulin Superfamily Adhesion Molecule Igsf11
- Dynamic Distribution of Linker Histone H1.5 in Cellular Differentiation
- Combining Comparative Proteomics and Molecular Genetics Uncovers Regulators of Synaptic and Axonal Stability and Degeneration
- Chemical Genetics Reveals a Specific Requirement for Cdk2 Activity in the DNA Damage Response and Identifies Nbs1 as a Cdk2 Substrate in Human Cells
- Experimental Relocation of the Mitochondrial Gene to the Nucleus Reveals Forces Underlying Mitochondrial Genome Evolution
- Rates of Gyrase Supercoiling and Transcription Elongation Control Supercoil Density in a Bacterial Chromosome
- Mutations in a P-Type ATPase Gene Cause Axonal Degeneration
- A General G1/S-Phase Cell-Cycle Control Module in the Flowering Plant
- Multiple Roles and Interactions of and in Development of the Respiratory System
- UNC-40/DCC, SAX-3/Robo, and VAB-1/Eph Polarize F-Actin during Embryonic Morphogenesis by Regulating the WAVE/SCAR Actin Nucleation Complex
- Epigenetic Remodeling of Meiotic Crossover Frequency in DNA Methyltransferase Mutants
- Modulating the Strength and Threshold of NOTCH Oncogenic Signals by
- Loss of Axonal Mitochondria Promotes Tau-Mediated Neurodegeneration and Alzheimer's Disease–Related Tau Phosphorylation Via PAR-1
- Acetyl-CoA-Carboxylase Sustains a Fatty Acid–Dependent Remote Signal to Waterproof the Respiratory System
- ATXN2-CAG42 Sequesters PABPC1 into Insolubility and Induces FBXW8 in Cerebellum of Old Ataxic Knock-In Mice
- Cohesin Rings Devoid of Scc3 and Pds5 Maintain Their Stable Association with the DNA
- The MicroRNA Inhibits Calcium Signaling by Targeting the TIR-1/Sarm1 Adaptor Protein to Control Stochastic L/R Neuronal Asymmetry in
- Rapid-Throughput Skeletal Phenotyping of 100 Knockout Mice Identifies 9 New Genes That Determine Bone Strength
- The Genes Define Unique Classes of Two-Partner Secretion and Contact Dependent Growth Inhibition Systems
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Dissecting the Gene Network of Dietary Restriction to Identify Evolutionarily Conserved Pathways and New Functional Genes
- It's All in the Timing: Too Much E2F Is a Bad Thing
- Variation of Contributes to Dog Breed Skull Diversity
- The PARN Deadenylase Targets a Discrete Set of mRNAs for Decay and Regulates Cell Motility in Mouse Myoblasts
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání




