-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaNovel Loci for Metabolic Networks and Multi-Tissue Expression Studies Reveal Genes for Atherosclerosis
Association testing of multiple correlated phenotypes offers better power than univariate analysis of single traits. We analyzed 6,600 individuals from two population-based cohorts with both genome-wide SNP data and serum metabolomic profiles. From the observed correlation structure of 130 metabolites measured by nuclear magnetic resonance, we identified 11 metabolic networks and performed a multivariate genome-wide association analysis. We identified 34 genomic loci at genome-wide significance, of which 7 are novel. In comparison to univariate tests, multivariate association analysis identified nearly twice as many significant associations in total. Multi-tissue gene expression studies identified variants in our top loci, SERPINA1 and AQP9, as eQTLs and showed that SERPINA1 and AQP9 expression in human blood was associated with metabolites from their corresponding metabolic networks. Finally, liver expression of AQP9 was associated with atherosclerotic lesion area in mice, and in human arterial tissue both SERPINA1 and AQP9 were shown to be upregulated (6.3-fold and 4.6-fold, respectively) in atherosclerotic plaques. Our study illustrates the power of multi-phenotype GWAS and highlights candidate genes for atherosclerosis.
Published in the journal: . PLoS Genet 8(8): e32767. doi:10.1371/journal.pgen.1002907
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002907Summary
Association testing of multiple correlated phenotypes offers better power than univariate analysis of single traits. We analyzed 6,600 individuals from two population-based cohorts with both genome-wide SNP data and serum metabolomic profiles. From the observed correlation structure of 130 metabolites measured by nuclear magnetic resonance, we identified 11 metabolic networks and performed a multivariate genome-wide association analysis. We identified 34 genomic loci at genome-wide significance, of which 7 are novel. In comparison to univariate tests, multivariate association analysis identified nearly twice as many significant associations in total. Multi-tissue gene expression studies identified variants in our top loci, SERPINA1 and AQP9, as eQTLs and showed that SERPINA1 and AQP9 expression in human blood was associated with metabolites from their corresponding metabolic networks. Finally, liver expression of AQP9 was associated with atherosclerotic lesion area in mice, and in human arterial tissue both SERPINA1 and AQP9 were shown to be upregulated (6.3-fold and 4.6-fold, respectively) in atherosclerotic plaques. Our study illustrates the power of multi-phenotype GWAS and highlights candidate genes for atherosclerosis.
Introduction
Five years of genome-wide association studies (GWAS) have successfully identified common variants at >1,000 genomic loci robustly associated with a wide range of human conditions and quantitative traits [1]. Despite this progress, one limitation is that almost all GWAS performed to date have focused on single traits, even in studies involving multiple related phenotypes. Growing evidence for pleiotropy [2], [3], where the same locus is associated with multiple phenotypes, supports the idea that multivariate analysis of multiple phenotypes can provide a substantial boost in power for locus discovery, consistent with simulation studies [4]–[7].
A plethora of metabolites in blood have been described as risk factors for metabolic syndrome, atherosclerosis and coronary artery disease [8], [9]. Using high-throughput nuclear magnetic resonance assays, quantitative profiles of 130 metabolites in two population-based cohorts from Finland, the Cardiovascular Risk in Young Finns Study (YFS) [10] and the Northern Finland Birth Cohort 1966 (NFBC66) [11] have been determined. These metabolites included lipoprotein subclasses of VLDL, LDL, IDL and HDL as well as lipids, amino acids and other small molecules (Materials S1).
Figure 1 illustrates the general flow of our study. We first applied an unsupervised algorithm to identify networks from the observed correlation structure amongst the 130 metabolite measures in 6,600 individuals. For each of these networks, we performed a multivariate test of association for 2.5 million SNPs [4]. Because we also tested all SNPs for association to each metabolite separately, we can assess the relative gain in power of the multivariate approach. To interpret the novel signals, we tested whether the associated SNPs influenced cis-gene expression levels in multiple tissues as well as whether the expression of candidate genes was associated to specific metabolites that drive the initial association. Finally, we analysed arterial tissue from mouse and man to test for a relation between our top candidate genes and atherosclerosis plaques.
Fig. 1. Overview of the study design. 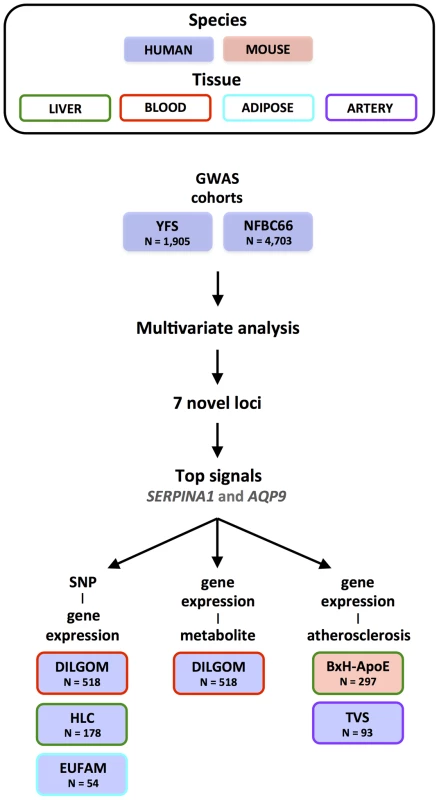
Results
Genotype and phenotype data
We analysed genotype and phenotype data from the YFS (N = 1,905) [10], [12] and the NFBC66 (N = 4,703) [11], [13]. For both YFS and NFBC66, we imputed SNP genotypes using the MACH algorithm [14] and the HapMap Phase 2 reference panel [15]. Serum collected from both cohorts underwent metabolomic profiling on the same proton nuclear magnetic resonance (NMR) platform [16]. The NMR metabolomics platform used here provided absolute quantitative information on 130 distinct metabolic measures [17]. Metabolite levels for both cohorts were normalized and adjusted for age, gender, cohort, and population structure (Materials and Methods).
Identification of metabolic networks
After correcting for cohort effects and pooling the metabolomic data for YFS and NFBC66, we constructed a Pearson correlation matrix that defined the pairwise relationships between all metabolites and applied agglomerative hierarchical clustering in order to identify networks of metabolites (Figure 2). Using a dynamic, data-driven tree-cutting algorithm [18], we identified 11 metabolic networks that represent various metabolic pathways (Materials and Methods and Figure S1). Additional information for each metabolic network, including full descriptions, abbreviations, inter-metabolite correlations, and supporting association analyses, is given in the Materials S1. Briefly, metabolic network 1 comprises multiple measures related mainly to cholesterol metabolism in the apoB-containing lipoproteins. Metabolic network 2 includes branched-chain and aromatic amino acids together with the large TG-rich VLDL particles and serum triglycerides. Metabolic networks 3 and 4 capture the larger and smaller particles of HDL-metabolism, respectively. Metabolic networks 5, 6, 7, and 8 are related to lipid poly-unsaturation, ketone bodies, the glucose-alanine cycle, and renal function, respectively. Metabolic networks 9, 10, and 11 each contain only 2 metabolites and represent measures of fatty acid chain length and composition, mean diameter of LDL and double-bonding of fatty acid chains, urea and acetate, respectively.
Fig. 2. Serum metabolic networks. 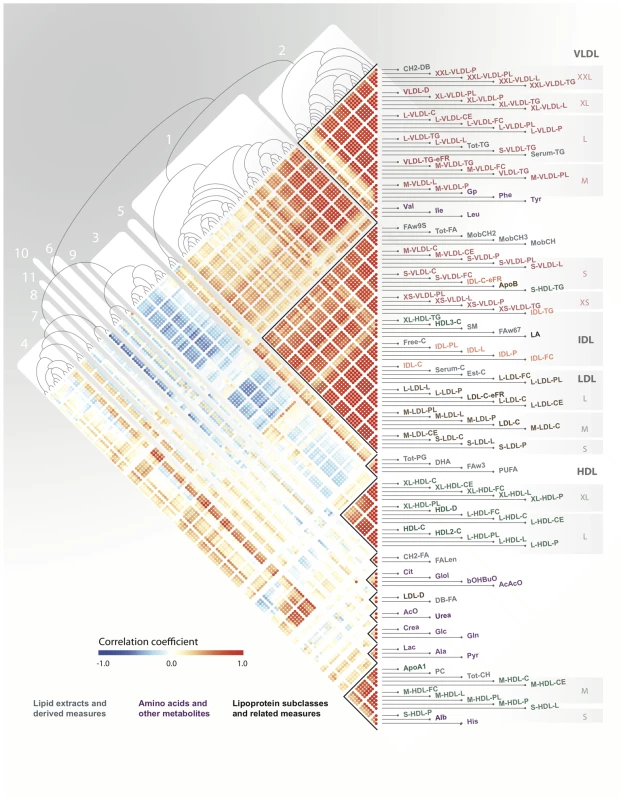
A Pearson correlation matrix of serum metabolites across both YFS and NFBC66 cohorts was hierarchically clustered and the resulting heatmap and dendrogram are presented here with red indicating high positive correlation, blue high negative correlation, and white no correlation. Clusters of tightly correlated metabolites, metabolic networks, are labeled 1–11. Genome-wide association analysis
For each of the 11 metabolic networks, we performed SNP association testing using a multivariate test based on Canonical Correlation Analysis and Wilks' lambda [4]. Each association test yielded an F statistic, corresponding P value, and a loading for each metabolite in the network to indicate the relative contribution of that metabolite to the overall association (Materials and Methods). For univariate analysis, we used standard linear regression where each of the 130 metabolites was regressed onto each SNP.
The implementation of dimensionality reduction and multivariate analysis allowed us to select essentially independent tests based on the correlation structure of the phenotype data. Using multivariate analysis, we tested each SNP only 11 times (one per metabolic network). A Bonferroni correction for testing each SNP to 130 metabolites is overly conservative, since the metabolites are partially correlated, but still common practice [16], [19], [20]. Accordingly, we set genome-wide significance thresholds at P<4.5×10−9 and P<3.8×10−10 for multivariate and univariate analysis, respectively.
To maximize power, we performed a joint analysis of both cohorts, correcting for population structure and cohort-specific effects. We observed little evidence for test statistic inflation, lambda range 1.01–1.06 (Materials and Methods and Table S1). Across all 11 metabolic networks, the joint multivariate analysis yielded 713 SNPs significantly associated with one or more metabolic networks (P<4.5×10−9). This corresponded to 34 distinct loci and 75 significant locus-network associations overall (Table 1 and Materials and Methods). Loci were considered novel if they had not been previously associated at genome-wide significance with a metabolite or other metabolic phenotype in the NHGRI Catalogue of Published GWAS [1] and if they were independent of other proximal signals (Materials and Methods and Table S2). Of the 34 loci detected, 27 were previously identified to be associated with fasting glucose levels [21], [22], total measures of LDL, HDL and triglycerides [23], [24], bradykinin [25], glutamine [16], [25], alanine-valine ratio [16], phenylalanine [16], citrate [16], and sphingolipids [26]. Overall, we found 7 novel loci associated with 12 metabolic networks in total (Table 1).
Tab. 1. Loci detected using joint multivariate association analysis. 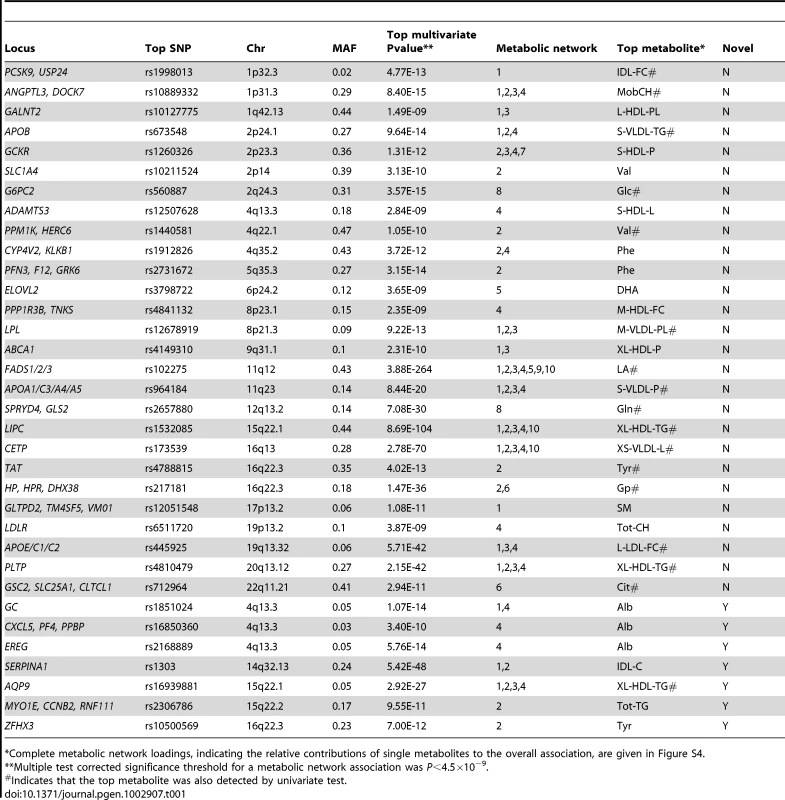
Complete metabolic network loadings, indicating the relative contributions of single metabolites to the overall association, are given in Figure S4. In comparing multivariate and univariate P values for a given SNP, we selected the lowest univariate P value for any single metabolite in a given network. We found that multivariate tests yielded more significant P values, reflecting increased power compared to univariate tests (Figure S2). In terms of number of significant associations, multivariate analysis outperformed univariate in both cohorts. When their respective genome-wide significance thresholds are applied, multivariate analysis uncovered 75 locus-metabolic network associations, whereas univariate analysis found only 40 (almost all of which were detected by multivariate analysis), leading to the detection of 7 novel loci instead of one (Figure 3). This demonstrates the relative gain of multivariate testing compared to univariate testing. Notably, multivariate analysis still uncovered more associations (69% more) than univariate analysis even when applying the more stringent genome-wide threshold for 130 independent metabolites. Multivariate also outperformed univariate when assessing only known loci, i.e. those with prior genome-wide significant association to metabolites in the NHGRI Catalogue of Published GWAS [1] (Figure S3).
Fig. 3. Associations detected between genomic loci and metabolic networks. 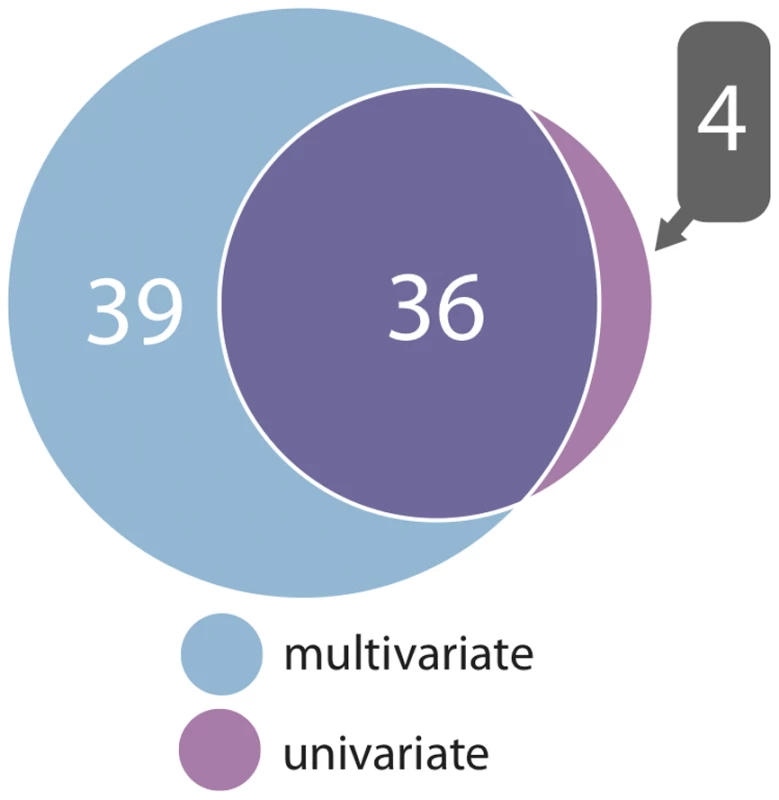
A Venn diagram showing the number of associations between all genomic loci and metabolic networks stratified by joint multivariate and univariate analysis (for univariate, at least one metabolite from a network need be associated). From the multivariate analysis, our strongest association signal overall was due to a nonsynonymous SNP, rs1303 (Glu400Asp) located in the last exon of SERPINA1. This variant was associated with metabolic networks 1 and 2 (P = 5.4×10−48 and P = 7.4×10−22, respectively; Figure S4). To explore the extent to which rs1303 perturbs protein structure, we utilized the PolyPhen2 algorithm [27]. PolyPhen2 predicted the Glu to Asp mutation to be benign (naïve Bayes posterior probability = 0.0 and 0.005 for the HumDiv and HumVar training sets, respectively). The next strongest signal overall was an intronic SNP (rs16939881) at the AQP9 locus, associated with metabolic networks 1, 2, 3, and 4 (P = 2.9×10−27, P = 4.9×10−15, P = 2.3×10−18 and P = 2.0×10−14, respectively; Figure S4). The metabolic network associations at AQP9 remained highly significant after conditioning on the previously identified LIPC locus, 250 Kb downstream (Table S2). We focus on our two top signals for subsequent in-depth analyses. Because our top signals are within the SERPINA1 and AQP9 genes, we assume these to be the most likely candidate genes.
Fine-mapping and conditional analysis of SERPINA1 and AQP9
Using the 1000 Genomes Phase I integrated variant set and the IMPUTE2 algorithm [28], we generated denser maps of genetic variants for the SERPINA1 and AQP9 loci. We then performed a multivariate test for each SNP with metabolic networks 1–4 as well as a conditional analysis to ascertain any independent signals in each region.
After 1000 Genomes imputation, rs1303 remained the top signal at the SERPINA1 locus for metabolic networks 1 and 2. Conditioning on rs1303 revealed an independent association between another nsSNP, rs28929474, and metabolic networks 1 and 2 (P = 1.7×10−19 and P = 3.7×10−13 respectively) (Figure S5). Rs28929474 (Glu366Lys) lies in the last exon of SERPINA1 and, unlike rs1303, it was predicted by PolyPhen2 to be a probable damaging mutation with a naïve Bayes posterior probability = 1.0 for both HumDiv and HumVar.
Imputation of the AQP9 locus with the 1000 Genomes panel yielded less confidently inferred genotypes than the HapMap2 panel at the top SNP rs16939881 (posterior probability >0.90 for a genotype call). Consequently we had less power at rs16939881, however it still remained significantly associated with metabolic networks 1, 2, and 3. Even with reduced power, conditional analysis showed that the signal at AQP9 could be explained by rs16939881 alone (Figure S6).
Novel variants drive expression of SERPINA1 and AQP9 in multiple tissues
We next investigated metabolic network associated variants for eQTL effects on SERPINA1 and AQP9. We used three resources (a) the DILGOM cohort, a Finnish population-based cohort (N = 518) with gene expression data (from whole blood) and serum metabolomic data [29], (b) a subset of the EUFAM study (N = 54) with familial low HDL cholesterol phenotype [30] and subcutaneous adipose tissue gene expression data, and (c) the Human Liver Cohort, a Caucasian cohort (HLC; N = 178) with liver tissue gene expression data [31]–[33]. These three resources also comprise genome-wide SNP data. We summarize the eQTL analyses in Table S3.
In the DILGOM study, the SNP explaining the most variance in SERPINA1 expression (rs11628917; linear regression P = 6.0×10−10; adjusted R2 = 0.07) was also strongly associated with metabolic networks 1 and 2 from the YFS and NFBC66 joint analysis (P = 9.6×10−14 and P = 1.9×10−11) (Figure 4). In our data, there was moderate linkage disequilibrium (LD; r2 = 0.47; D′ = 0.99) between the top SERPINA1 SNP (rs1303) and the blood eQTL (rs11628917). Conditional analysis showed that the association of rs11628917 with both metabolic networks could be explained by rs1303, suggesting non-independence. Rs1303 itself was nominally associated with SERPINA1 expression (P = 0.01). The independent signal at rs28929474 showed no evidence of influencing SERPINA1 expression (and was not in LD with any eQTLs), suggesting that its primary effect may be protein structure destabilization. No blood eQTLs were detected for AQP9.
Fig. 4. Connecting genetic variation, gene expression, metabolites, and atherosclerosis for SERPINA1 and AQP9. 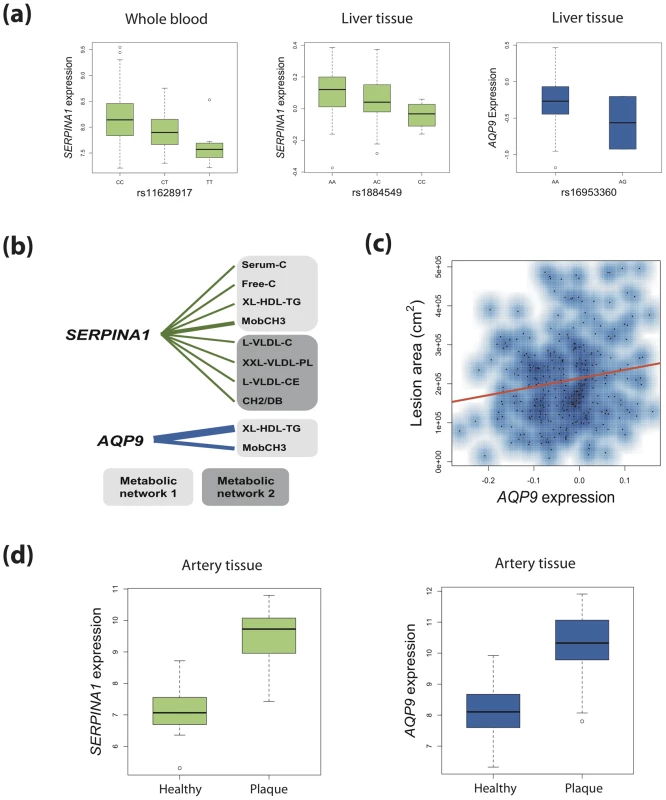
(a) Boxplots show SNPs associated with metabolic networks are also cis eQTLs for SERPINA1 (human blood and liver) and AQP9 (human liver). Boxplots consist of median log2-normalised expression for each genotype with first and third quartiles designated by box edges. Whiskers extend to +/−1.5 times interquartile range. (b) Human blood expression of SERPINA1 and AQP9 was associated with metabolites derived from the same metabolic networks as their corresponding genetic variants. Edge widths are proportional to the strength of association (P value). (c) Liver expression of AQP9 (but not SERPINA1) in mice on a hyperlipidemic APOE−/− background showed significant positive association with aortic lesion area. (d) Boxplots for log2-normalised expression of SERPINA1 and AQP9 in healthy human arterial tissue versus that for atherosclerotic plaques. In the EUFAM study, the top and bottom 10th percentiles of HDL-C concentrations (Finnish population age and sex specific percentiles) were used to define high and low HDL-C groups (N = 19 and N = 35, respectively). First, we tested for differences in AQP9 and SERPINA1 between high and low HDL-C groups. Both AQP9 and SERPINA1 expression were upregulated in adipose tissue of individuals with low HDL-C (fold changes 3.47 and 2.29, P = 9.0×10−4 and P = 0.03, respectively). Analysis of genetic variants did not yield any eQTLs at the AQP9 the locus. Given the independent but proximal signals at AQP9 and LIPC, we did detect an eQTL 210 Kb downstream within the LIPC locus that influenced the adipose expression of AQP9 (rs1825955; P = 4.8×10−3) but not LIPC. There was low LD between rs1825955 and the top multivariate AQP9 SNP rs16939881 (r2 = 0.17; D′ = 0.94). SERPINA1 also did not harbour adipose eQTLs (including rs11628917, P>0.05), indicating either potentially tissue-specific function of the SNP or lack of statistical power.
The HLC allowed for the analysis of gene expression in the human liver. In the HLC, we detected eQTLs for both SERPINA1 and AQP9 (Figure 4). An eQTL in the promoter region of SERPINA1 explained 3.9% of the liver expression of the gene (rs1884549; P = 4.3×10−3). Rs1884549 was also associated with metabolic network 1 (P = 9.6×10−22) and in moderate LD (r2 = 0.38; D′ = 0.99) with rs1303. A variant within AQP9 was associated with its expression in the liver (rs16953360; P = 4.6×10−3; adjusted R2 = 0.04) as well as metabolic networks 1–4 (P = 1.0×10−25; P = 4.7×10−14; P = 8.1×10−18; P = 7.1×10−14 respectively). SNPs rs16953360 and rs16939881 are in very strong LD (r2 = 0.97; D′ = 0.98).
SERPINA1 and AQP9 expression is associated with metabolites
We next investigated whether there was a relationship between SERPINA1 and AQP9 and metabolites levels in the DILGOM cohort. To do this, we considered those metabolic networks associated with SERPINA1 and AQP9 SNPs then regressed individual metabolite levels on gene expression (Table S4). Genetic variation in AQP9 was associated with metabolic networks 1–4 and here we observed significant association between expression of AQP9 and two metabolites from network 1 (XL-HDL-TG: P = 8.5×10−9; MobCH3: P = 7.2×10−5). SERPINA1 harboured genetic variants associated with metabolic networks 1 and 2, and expression of SERPINA1 was associated with eight metabolites, four from metabolic network 1 and four from metabolic network 2 (Table S4).
SERPINA1 and AQP9 expression is associated with atherosclerosis
Since genetic variation and gene expression of SERPINA1 and AQP9 were associated with lipoprotein levels, lipid transporters central to atherosclerosis, we investigated the relationship between these genes and atherosclerosis.
We first investigated a mouse model (BxH-ApoE, N = 298) on a hyperlipidemic apolipoprotein-E (ApoE) null background with liver gene expression profiles and quantified aortic lesions [34]–[36]. BxH-ApoE consisted of an F2 population derived from a backcross of mice highly susceptible to atherosclerosis (C57BL/6J ApoE−/−) and highly resistant (C3H/HeJ ApoE−/−). The F2 population was then fed on a high-fat, western diet for 16 weeks then euthanized at 24 weeks. Using linear regression, we tested for association between liver expression of Serpina1a (the mouse ortholog of SERPINA1) and AQP9 and the area of atherosclerotic lesion in the aorta. Expression of AQP9 showed significant association with atherosclerotic plaque area (P = 5.0×10−3; Figure 4), with samples in the top decile of AQP9 expression having on average 29% larger lesion area than those in the bottom decile. The association remained significant after correction for gender, total cholesterol, triglycerides and HDL. On this background, Serpina1a expression did not show association with lesion area (P = 0.58).
Finally, we utilized the Tampere Vascular Study (TVS) a collection of atherosclerotic plaque samples from patients undergoing peripheral vascular surgery (carotid and femoral endarterectomy and aortic bypass procedures due to atherosclerosis) and control samples from individuals undergoing coronary artery by-pass surgery (Materials and Methods). In TVS, both SERPINA1 and AQP9 showed strong association with lesion status (Figure 4). AQP9 was expressed at a 4.67 fold higher level in lesions compared to controls (Mann Whitney P = 4.64×10−12), and similarly SERPINA1 exhibited 6.33 fold higher expression (Mann Whitney P = 2.49×10−13). The TVS results suggest that both AQP9 and SERPINA1 are candidate genes for atherosclerosis.
Discussion
We have empirically demonstrated the power of multivariate association testing of metabolite networks. We detected 7 novel loci and investigated the gene expression of our top loci, SERPINA1 and AQP9, in multiple human tissues as well as their potential role in atherosclerosis.
SERPINA1 was associated with metabolic networks 1 and 2 (top metabolites: total cholesterol in IDL and mean diameter of VLDL, respectively), which are mainly related to cholesterol and triglyceride pathways of apoB-containing lipoproteins as well as diabetes associated amino acids [37]. SERPINA1 encodes alpha 1-antitrypsin (A1AT), a protease inhibitor that protects surrounding tissues at sites of inflammation, and various studies have suggested A1AT's role in atherosclerosis. A1AT has been detected within HDL particles but not LDL [38], although complexes of A1AT and LDL have been found in the intimal arterial wall and in human atherosclerotic lesions in the coronary artery [39]. Proteolytic degradation of LDL by murine peritoneal macrophages has been shown to be enhanced by A1AT binding, and immunostaining and in situ hybridization have also suggested that A1AT is produced by macrophages in the arterial wall [39].
AQP9 encodes aquaporin 9, a liver glycerol channel [40], and contains variants which showed association with metabolic networks 1 and 2 (top metabolites: triglycerides in very large HDL and mean diameter of VLDL) as well as networks 3 and 4 (top metabolites: mean diameter of HDL and phosphatidylcholine). The proximity of AQP9 to the well-known LIPC gene 250 Kb downstream raises the question of whether the AQP9 and LIPC loci harbour independent effects. Our conditional analyses of metabolic network associated SNPs indicate that these are indeed independent genetic signals. In addition, LIPC expression in whole blood from DILGOM was not associated with metabolites from the relevant networks 1, 2, 3, or 4, and LIPC liver expression in mouse only slightly attenuated the association of AQP9 with atherosclerotic lesion area in a linear model (P = 0.059). In human aorta, LIPC was nominally differentially expressed between healthy and plaque samples (P = 0.01) and did not affect the substantially larger aortic differential expression of AQP9. Previous experiments have shown AQP9's involvement in gluconeogenesis. AQP9 mRNA and protein have been shown to be greater in human obese T2D patients relative to lean normoglycemics in adipose tissue [41]. The opposite is true in liver, suggesting that reduction in glycerol influx in hepatocytes via AQP9 could prevent excessive lipid accumulation and may reduce hyperglycaemia in obesity [41]. Further, AQP9−/− mice have previously been shown to have elevated levels of plasma glycerol and triglycerides, and inhibition of AQP9 by a small molecule inhibitor showed that it is required for glycerol-dependent glucose production in murine hepatocytes [42], [43].
Our findings for SERPINA1 and AQP9 are consistent with the above studies suggesting associations with cardiometabolic risk factors and show that (a) common variants in both are associated with metabolic networks, (b) these variants modulate gene expression and suggest that there may be potential heterogeneous genetic control in different tissues, (c) expression of both genes was associated with metabolites from the relevant networks, and finally (d) gene expression was positively associated with atherosclerotic lesion area in mice (AQP9) and upregulated in atherosclerotic tissue in humans (SERPINA1 and AQP9). We also speculate that the roles of SERPINA1 and AQP9 in atherosclerosis are tissue-specific where AQP9 displays an effect in both liver and arterial tissue and SERPINA1 only in the latter.
Of the five other novel loci, there were variants proximal to ZFHX3 on Chr 16, MYO1E on Chr 15, as well as three independent signals at 4q13. ZFHX3 encodes ATBF1, a transcription factor involved in neuronal differentiation and survival [44], [45] that has also been previously implicated in Kawasaki disease, atrial fibrillation and ischemic stroke [46], [47]. Variants at the ZFHX3 locus were associated with metabolic network 2 where the metabolite with the greatest loading was tyrosine. Little is known about the role of the tyrosine in circulation, however a recent study [37] investigating the predictive ability of five amino acids for type 2 diabetes onset suggested that amino acid metabolism, including tyrosine, plays a role in the pathophysiology of metabolic syndrome, where it is known that individuals with either metabolic syndrome and/or diabetes are at increased risk for stroke. At 4q13, a band that contains the ALB albumin gene, metabolic networks 1 and 4 were associated with variants 30 Kb upstream of group-specific component, a vitamin D binding protein (top metabolites: triglycerides in IDL and albumin, respectively). Metabolic network 4, with albumin as the top metabolite, was also associated with variants 10 Kb upstream of EREG and independent variants 8 Kb upstream of CXCL5. EREG encodes epiregulin, part of a family of epidermal growth factors for which there is evidence that osmotic pressure has a role in signal transduction [48], and CXCL5 encodes a cytokine that has previously been linked with obesity and insulin resistance [49]. Finally, 15q22 harboured intronic variants within the MYO1E gene, a non-muscle class I myosin protein, associated with metabolic network 2 (top metabolite: total triglycerides). Myosin 1E has previously been shown to bind phospholipids [50], regulate podocyte function and glomerular filtration [51], as well as contain nsSNPs which display linkage to kidney disease [52].
This study illustrates the importance of accounting for fine-scale phenotypic structure. Although the current GWAS paradigm is based on the testing of one phenotype and one marker at a time, the quantitative phenotype profiles of individuals and corresponding biological samples are rapidly expanding in scope and depth. We are being faced with more complex multivariate phenotypic information, and biologically heterogeneous phenotypes can now be fine-mapped to reveal more informative patterns of association. Powerful statistical approaches that leverage the network covariance can provide novel insights and link genetics with disease.
Materials and Methods
Cohorts
The Cardiovascular Risk in Young Finns Study (YFS) is a population based prospective cohort study. It was conducted at 5 university departments of medical schools in Finland (i.e. Turku, Helsinki, Kuopio, Tampere and Oulu), with the aim of studying the levels of cardiovascular risk factors in children and adolescents in different parts of the country. The latest follow-up was conducted in 2007. The serum samples for this metabolomics study were collected at the latest follow up. The study and data collection protocols have been described in detail in [10]. The YFS study protocols have been approved by local ethics committees.
The Northern Finland Birth Cohort 1966 (NFBC66) has been described in detail previously [11]. The original study design focused to study factors affecting pre-term birth, low birth weight, and subsequent morbidity and mortality. Mothers living in the two northern-most provinces of Finland were invited to participate if they had expected delivery dates during 1966. Individuals still living in the Helsinki area or Northern Finland (N = 4,703) were asked to participate in a detailed biological and medical examination as well as a questionnaire at the age of 31 years. The NFBC66 study protocols have been approved by local ethics committees.
The subjects used in the adipose tissue eQTL analysis were obtained from the EUFAM study (European Multicenter Study of Familial Dyslipidemias) database [30] including a Finnish cohort with familial low HDL-C phenotype. The Ethical Committee of the Department of Medicine, Helsinki University Central Hospital approved the EUFAM study. Top and bottom 10th percentiles of HDL-C concentrations (Finnish population age and sex specific percentiles) were used to define the high and low HDL-C groups, respectively, and subject who were not matched for BMI were removed. Subcutaneous adipose tissue biopsies were obtained from 54 individuals. Out of these, 35 individuals had low HDL-C and 19 individuals high HDL-C. Individuals in both low and high HDL-C groups were matched by age and gender. Fat biopsies were collected, RNA extracted and quantified as previously described [53]. RNA labeling, array processing and scanning were done according to the standard protocol by Affymetrix using HG-U133 (Plus 2.0) arrays. Pre-processing of the expression data was done using GC-RMA normalization. Genotyping was performed using the HumanCNV370v1_C platform at the Broad Institute. SNPs with genotype rate <90% were excluded from the analyses and samples were removed if fewer than 95% of SNPs could be genotyped in them.
In the Tampere vascular study (TVS), vascular samples were collected from patients undergoing peripheral vascular surgery due to symptomatic atherosclerosis (cerebrovascular disease due to carotid stenosis, peripheral arterial disease). All of these patients had a polyvascular disease which had affected at least two different vascular beds. Control samples were taken from left internal thoracic arteries (LITA) during coronary artery by-pass surgery (n = 25). Atherosclerotic plaques were collected by endarterectomy from the following arterial sites: femoral artery (n = 24) carotid artery (n = 29) and abdominal aorta (n = 15) all together from a total of 93 patients. The vascular samples were classified according to the American Heart Association classification (AHA) [54]. The carotid and femoral artery samples were of type V or VI, aortic samples were type VI and all control vessels were macroscopically and microscopically healthy. The samples were taken from patients subjected to open vascular surgical procedures at the Division of Vascular Surgery, Tampere University Hospital. The study was approved by the Ethics Committee of Tampere University Hospital. All patients gave informed consent.
The HLC and BxH-ApoE data was obtained from the Sage BioNetworks repository. A detailed description of the HLC data can be found here [31], [32]. Detailed information on mouse experiments and sample handling can be obtained here [34], [35]. An outlier with extreme lesion area (Z-score = 4.166, P = 1.5×10−5) was removed from analysis. Inclusion of the outlier did not affect significance.
NMR metabolomics
The samples from the NFBC66, YFS and DILGOM cohorts were analyzed using the same high-throughput serum NMR metabolomics platform [17] providing information on lipoprotein subclass distribution and lipoprotein particle concentrations, low-molecular-weight metabolites such as amino acids, 3-hydroxybutyrate, and creatinine, and detailed molecular information on serum lipids including free and esterified cholesterol, sphingomyelin, saturation and ω-3 fatty acids. Further details of the NMR spectroscopy, data analyses as well as the full metabolite identifications have been described previously [17], [29].
Metabolomic data processing
Individuals known to be on lipid-lowering therapy or pregnant were excluded from analysis. To calculate residuals for all metabolites, each study included the following as covariates: gender, age (only YFS, the NFBC66 is a birth cohort), and loadings of the first 10 principal components from genetic data to correct for cryptic population stratification. Residuals were normalized using an inverse normal transformation to have a mean of zero and a standard deviation of 1. In combining the metabolomic data for the YFS and NFBC66, residuals for all metabolites also included the cohort as a covariate. Processing of metabolites from the DILGOM cohort has been described previously [29].
Genotyping and imputation
The YFS and NFBC66 cohorts studied were genotyped using standard protocols on the Illumina 670 BeadArray and Illumina 370CNVduo (Illumina, Inc. San Diego, CA, USA) respectively. Prior to imputation, stringent quality filtering was employed for each cohort. Quality control was performed independently for each study prior to imputation. Low quality SNPs (>5% missingness) and poor DNA samples (>5% individual missingness) were removed. In addition, individuals with high genomic heterozygosity (indicative of sample contamination), gender discrepancies or closely related individuals were removed from the data.
Genotype imputation was performed using the MACH algorithm [14] and the CEPH reference panel from HapMapII [15]. After filtering, sample numbers were 1,905 and 4,703 for the YFS and NFBC66 cohorts respectively. After imputation, SNPs were filtered within each cohort via the estimated squared correlation between imputed and true genotypes (Rsq<0.30), estimated minor allele frequency (MAF<0.01), and Hardy-Weinberg equilibrium exact test (P<1.0×10−6). After SNP filtering, 2,406,682 and 2,360,512 SNPs in the YFS and NFBC66 cohorts respectively were taken forward for further genome-wide analyses. The intensity cluster plots for the top, directly-genotyped SNPs were visually inspected for failures in genotype assay and calling.
Metabolite clustering
In order to define matrices of related endogenous variates, groupings of metabolites must be defined. Normalized metabolite measurements across the YFS and NFBC66 cohorts were pooled, and the metabolite-metabolite Pearson correlation matrix was hierarchically clustered. From the resulting dendrogram, metabolite cluster detection was done using a dynamic tree cutting algorithm [18] with a minimum cluster membership of one metabolite. We selected the dynamic tree cutting algorithm because it has been shown to outperform other popular methods in simulations as well as give biologically relevant results on real data [29], [55], [56]. In order to maximize power to detect associations, Ferreira and Purcell showed that, within a phenotype set, one should maximize both the number of phenotypes and the level of correlation between phenotypes [4], however in practice these two parameters are inversely related. That is, given a of phenotype measures and individuals, increasing the number of phenotype clusters leads to increasing correlation within clusters and vice versa. For the dynamic tree cut algorithm, we investigated the sensitivity of cluster splitting using the deepSplit parameter. Lower integers values of deepSplit correspond to lower sensitivity for cluster splitting and thus fewer clusters. Both high and low sensitivity (deepSplit = 4 and deepSplit = 0, respectively) for cluster splitting were explored using the YFS discovery cohort. The high setting assigned 11 metabolic networks (Figure S1) whereas the low setting assigned 5 metabolic networks (Figure S7). Both clusterings were empirically assessed using the multivariate test above, and both settings detected the same number of loci at genome-wide significance. This was consistent with the inverse relationship between intra-cluster correlation and number of metabolites per cluster. Given no difference in locus detection, we considered the biological interpretation of the clusters. We noted that the low setting could not differentiate TG-rich VLDL particles nor lipid poly-unsaturation and conflated various energy metabolites with small HDL metabolism. Since it presented more straight-forward biological interpretation, we proceeded to downstream analysis with the high sensitivity, corresponding to 11 metabolic networks.
Statistical analysis
Association testing of SNPs and metabolites was done using two strategies: univariate linear regression and multivariate Canonical Correlation Analysis (CCA). For the former, we used the standard framework where Yi is the normalized metabolite measure for individual i, Xi is the genotype of the individual at a given SNP (encoded as 0, 1, or 2 for the number of minor alleles), and εi is a normally distributed random variable with mean equal to zero and constant variance. To implement linear regression, we used the PLINK analysis software [57]. The reported P values assume a NULL hypothesis of no association, b = 0.
When testing hypotheses that include multiple endogenous variables, the relationships among the endogenous variables must be taken into account in addition to those between the endogenous and exogenous variables. Given these two sets of variables, the aim is to simultaneously find the best predictor of the linear functions of one set as well as the linear function of the other set it best predicts. This yields a pair of variates which are referred to as the first pair of canonical variates. Using the residuals of these linear functions, the process can be repeated to obtain the second pair of canonical variates, and so on. The full sequence of these pairs of variates and their correlations then fully describe the invariant relationships between the endogenous variable set and the exogenous variable set [58].
For multivariate testing, we use the CCA framework implemented in the PLINK.multivariate analysis tool [4] and the R statistical programming language. In this case, the exogenous set consists of only one variable, the SNP, and consequently only one pair of canonical variates is calculated.
Wilks' lambda (λ) is a multivariate analogy of the F-test in one-way analysis of variance. In a genetic setting, λ is a statistic which tests for differences between the means of the three genotype groups (AA, AB, and BB) on a combination of endogenous variates (a network of metabolite variables). In this case, λ = 1−ρ2 where ρ is the canonical correlation coefficient between the SNP and the network of metabolite variables. The calculation of a P-value arises from a transformation of Wilks' lambda into a statistic which is approximately F distributed.
Genomic inflation of test statistics can be an indicator of subtle biases in the data and testing (e.g. cryptic population structure). To assess genomic inflation, we compared our observed distribution of −log10(P) values to that expected in the absence of association. A linear model was then fitted to the lowest 90% of the distribution and genomic inflation was taken as the slope of the fitted line. Table S1 gives genomic inflation values for multivariate testing of YFS, NFBC66 and meta-analysis across all metabolic networks.
A locus was defined as a 200 kb genomic region centered on the top significantly associated SNP. To determine the independence of the locus-network signals, conditional multivariate association analysis was used for signals either at 4q13.3, close to LIPC (i.e. AQP9 variants), or close to FADS1/2/3 (i.e. CD5/CD6 and INCENP/FTH1/BEST1 variants). For the top SNP(s) at a proximal locus (Table S2), each metabolite in a network was regressed onto the proximal SNP(s) and the resulting residuals were used as endogenous variables in the multivariate test of the target locus. An attenuated signal indicates non-independence, e.g. the SNPs tag the same causal variant. As a result, two loci (CD5/CD6 and INCENP/FTH1/BEST1) were largely attenuated and not regarded as independent from the FADS1/2/3 locus.
Due to the different number of statistical tests, genome-wide significance differs between multivariate and univariate testing. Here, as the basis of genome-wide significance, we use the common threshold of 5.0×10−8, derived from the number of independent common haploblocks in genomes of European descent [59]. Univariate testing of all 130 metabolites, implies a Bonferroni corrected significance level of 5.0×10−8/130 = 3.8×10−10. Multivariate testing of the metabolic networks we identify here (N = 11) gives a Bonferroni corrected significance level of 5.0×10−8/11 = 4.5×10−9.
Analysis of the DILGOM cohort considered the bead-weighted and quantile normalized gene expression data from the Illumina HT-12 expression array as described previously [55] and metabolomic measures also described previously [29]. Only those metabolites which were part of the original metabolic network associated with a particular locus were considered. For example, because genetic variation at SERPINA1 was associated with metabolic networks 1 and 2, expression of SERPINA1 was only tested for association with metabolites from networks 1 and 2. Since loci were associated with different metabolic networks, different numbers of tests were performed for each candidate gene. We therefore implemented appropriate multiple testing thresholds for each gene where significance was set at P<(0.05/total_number_metabolites_in_tested_networks).
For the EUFAM study, statistical eQTL analyses were performed using a linear regression model adjusting for the gender, BMI, and low/high HDL-C affection status. Probe intensities were treated as dependent and genotypes as independent variables. The comparison of gene-expression between the low and high HDL-C groups was performed using a linear regression model. Fold change for each probe was calculated by dividing the mean probe intensity in the low HDL-C group by the mean probe intensity in the high HDL-C group.
For the TVS study, all vascular specimens were immediately frozen and RNA was extracted as previously described [60]. RNA was reverse transcribed into cRNA, biotin-UTP labelled using the Illumina TotalPrep RNA Amplification Kit (Ambion) and cRNA hybridized to the Illumina HumanHT-12 v3 Expression BeadChip. BeadChips were scanned with the Illumina iScan system. Data processing was conducted using R language and appropriate Bioconductor modules. Robust multi-array averaging (RMA) [61] was used to correct negative intensity values after background subtraction. Between arrays normalization was done using robust spline normalization (RSN) [61]. Quality control was performed using sample clustering and multi-dimensional scaling. Seven outliers were removed due to low expression profiles, 4 from carotid artery group and 3 from LITA group.
Fold changes (FCs) for differentially expressed genes were calculated from log2-transformed median expression values between case (carotid, abdominal, femoral) and control group (LITA), and the significance of the differences were evaluated with non-parametric Mann-Whitney U test due to non-normal distribution of expression values and relatively small sample sizes of TVS. If there were more than one probe presenting a gene in the expression chip, the probe with highest median expression value was selected for FC calculation.
Supporting Information
Zdroje
1. HindorffLA, SethupathyP, JunkinsHA, RamosEM, MehtaJP, et al. (2009) Potential etiologic and functional implications of genome-wide association loci for human diseases and traits. Proc Natl Acad Sci U S A 106 : 9362–9367.
2. SivakumaranS, AgakovF, TheodoratouE, PrendergastJG, ZgagaL, et al. (2011) Abundant pleiotropy in human complex diseases and traits. Am J Hum Genet 89 : 607–618.
3. CotsapasC, VoightBF, RossinE, LageK, NealeBM, et al. (2011) Pervasive sharing of genetic effects in autoimmune disease. PLoS Genet 7: e1002254 doi:10.1371/journal.pgen.1002254.
4. FerreiraMA, PurcellSM (2009) A multivariate test of association. Bioinformatics 25 : 132–133.
5. AllisonDB, ThielB, St JeanP, ElstonRC, InfanteMC, et al. (1998) Multiple phenotype modeling in gene-mapping studies of quantitative traits: power advantages. Am J Hum Genet 63 : 1190–1201.
6. KimS, XingEP (2009) Statistical estimation of correlated genome associations to a quantitative trait network. PLoS Genet 5: e1000587 doi:10.1371/journal.pgen.1000587.
7. BanerjeeS, YandellBS, YiN (2008) Bayesian quantitative trait loci mapping for multiple traits. Genetics 179 : 2275–2289.
8. KuulasmaaK, Tunstall-PedoeH, DobsonA, FortmannS, SansS, et al. (2000) Estimation of contribution of changes in classic risk factors to trends in coronary-event rates across the WHO MONICA Project populations. Lancet 355 : 675–687.
9. LibbyP, RidkerPM, HanssonGK (2011) Progress and challenges in translating the biology of atherosclerosis. Nature 473 : 317–325.
10. RaitakariOT, JuonalaM, RonnemaaT, Keltikangas-JarvinenL, RasanenL, et al. (2008) Cohort profile: the cardiovascular risk in Young Finns Study. Int J Epidemiol 37 : 1220–1226.
11. RantakallioP (1969) Groups at risk in low birth weight infants and perinatal mortality. Acta Paediatr Scand 193: Suppl 193 : 191+.
12. OkserS, LehtimakiT, EloLL, MononenN, PeltonenN, et al. (2010) Genetic variants and their interactions in the prediction of increased pre-clinical carotid atherosclerosis: the cardiovascular risk in young Finns study. PLoS Genet 6: e1001146 doi:10.1371/journal.pgen.1001146.
13. SabattiC, ServiceSK, HartikainenAL, PoutaA, RipattiS, et al. (2009) Genome-wide association analysis of metabolic traits in a birth cohort from a founder population. Nat Genet 41 : 35–46.
14. LiY, WillerC, SannaS, AbecasisG (2009) Genotype imputation. Annu Rev Genomics Hum Genet 10 : 387–406.
15. FrazerKA, BallingerDG, CoxDR, HindsDA, StuveLL, et al. (2007) A second generation human haplotype map of over 3.1 million SNPs. Nature 449 : 851–861.
16. KettunenJ, TukiainenT, SarinAP, Ortega-AlonsoA, TikkanenE (2012) A genome-wide association study identifies 31 genetic loci associated with human serum metabolites. Nat Genet 44 : 269–276.
17. SoininenP, KangasAJ, WurtzP, TukiainenT, TynkkynenT, et al. (2009) High-throughput serum NMR metabonomics for cost-effective holistic studies on systemic metabolism. Analyst 134 : 1781–1785.
18. LangfelderP, ZhangB, HorvathS (2008) Defining clusters from a hierarchical cluster tree: the Dynamic Tree Cut package for R. Bioinformatics 24 : 719–720.
19. IlligT, GiegerC, ZhaiG, Romisch-MarglW, Wang-SattlerR, et al. (2010) A genome-wide perspective of genetic variation in human metabolism. Nat Genet 42 : 137–141.
20. GiegerC, GeistlingerL, AltmaierE, Hrabe de AngelisM, KronenbergF, et al. (2008) Genetics meets metabolomics: a genome-wide association study of metabolite profiles in human serum. PLoS Genet 4: e1000282 doi:10.1371/journal.pgen.1000282.
21. SaxenaR, VoightBF, LyssenkoV, BurttNP, de BakkerPI, et al. (2007) Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science 316 : 1331–1336.
22. Bouatia-NajiN, RocheleauG, Van LommelL, LemaireK, SchuitF, et al. (2008) A polymorphism within the G6PC2 gene is associated with fasting plasma glucose levels. Science 320 : 1085–1088.
23. TeslovichTM, MusunuruK, SmithAV, EdmondsonAC, StylianouIM, et al. (2010) Biological, clinical and population relevance of 95 loci for blood lipids. Nature 466 : 707–713.
24. WaterworthDM, RickettsSL, SongK, ChenL, ZhaoJH, et al. (2010) Genetic variants influencing circulating lipid levels and risk of coronary artery disease. Arterioscler Thromb Vasc Biol 30 : 2264–2276.
25. SuhreK, ShinSY, PetersenAK, MohneyRP, MeredithD, et al. (2011) Human metabolic individuality in biomedical and pharmaceutical research. Nature 477 : 54–60.
26. DemirkanA, van DuijnCM, UgocsaiP, IsaacsA, PramstallerPP, et al. (2012) Genome-wide association study identifies novel loci associated with circulating phospho - and sphingolipid concentrations. PLoS Genet 8: e1002490 doi:10.1371/journal.pgen.1002490.
27. AdzhubeiIA, SchmidtS, PeshkinL, RamenskyVE, GerasimovaA, et al. (2010) A method and server for predicting damaging missense mutations. Nat Methods 7 : 248–249.
28. HowieBN, DonnellyP, MarchiniJ (2009) A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLoS Genet 5: e1000529 doi:10.1371/journal.pgen.1000529.
29. InouyeM, KettunenJ, SoininenP, SilanderK, RipattiS, et al. (2010) Metabonomic, transcriptomic, and genomic variation of a population cohort. Mol Syst Biol 6 : 441.
30. SoroA, PajukantaP, LiljaHE, YlitaloK, HiekkalinnaT, et al. (2002) Genome scans provide evidence for low-HDL-C loci on chromosomes 8q23, 16q24.1-24.2, and 20q13.11 in Finnish families. Am J Hum Genet 70 : 1333–1340.
31. SchadtEE, MolonyC, ChudinE, HaoK, YangX, et al. (2008) Mapping the genetic architecture of gene expression in human liver. PLoS Biol 6: e107 doi:10.1371/journal.pbio.0060107.
32. YangX, ZhangB, MolonyC, ChudinE, HaoK, et al. (2010) Systematic genetic and genomic analysis of cytochrome P450 enzyme activities in human liver. Genome Res 20 : 1020–1036.
33. The Human Liver cohort was contributed by Merck Pharmaceutical through the Sage Bionetworks Repository. The tissues were provided by Fred Guengerich SS, Erin Schuetz and Merck Research Laboratories
34. YangX, SchadtEE, WangS, WangH, ArnoldAP, et al. (2006) Tissue-specific expression and regulation of sexually dimorphic genes in mice. Genome Res 16 : 995–1004.
35. WangSS, SchadtEE, WangH, WangX, Ingram-DrakeL, et al. (2007) Identification of pathways for atherosclerosis in mice: integration of quantitative trait locus analysis and global gene expression data. Circ Res 101: e11–30.
36. We acknowledge that the Mouse Model of sexually Dimorphic Atherosclerotic traits data was generated and is contributed by Jake Lusis ESaMPttSBR.
37. WangTJ, LarsonMG, VasanRS, ChengS, RheeEP, et al. (2011) Metabolite profiles and the risk of developing diabetes. Nat Med 17 : 448–453.
38. Ortiz-MunozG, HouardX, Martin-VenturaJL, IshidaBY, LoyauS, et al. (2009) HDL antielastase activity prevents smooth muscle cell anoikis, a potential new antiatherogenic property. FASEB J 23 : 3129–3139.
39. MashibaS, WadaY, TakeyaM, SugiyamaA, HamakuboT, et al. (2001) In vivo complex formation of oxidized alpha(1)-antitrypsin and LDL. Arterioscler Thromb Vasc Biol 21 : 1801–1808.
40. MaedaN, FunahashiT, ShimomuraI (2008) Metabolic impact of adipose and hepatic glycerol channels aquaporin 7 and aquaporin 9. Nat Clin Pract Endocrinol Metab 4 : 627–634.
41. RodriguezA, CatalanV, Gomez-AmbrosiJ, Garcia-NavarroS, RotellarF, et al. (2011) Insulin - and leptin-mediated control of aquaglyceroporins in human adipocytes and hepatocytes is mediated via the PI3K/Akt/mTOR signaling cascade. J Clin Endocrinol Metab 96: E586–597.
42. RojekAM, SkowronskiMT, FuchtbauerEM, FuchtbauerAC, FentonRA, et al. (2007) Defective glycerol metabolism in aquaporin 9 (AQP9) knockout mice. Proc Natl Acad Sci U S A 104 : 3609–3614.
43. JelenS, WackerS, Aponte-SantamariaC, SkottM, RojekA, et al. (2011) Aquaporin-9 protein is the primary route of hepatocyte glycerol uptake for glycerol gluconeogenesis in mice. J Biol Chem 286 : 44319–44325.
44. NojiriS, JohT, MiuraY, SakataN, NomuraT, et al. (2004) ATBF1 enhances the suppression of STAT3 signaling by interaction with PIAS3. Biochem Biophys Res Commun 314 : 97–103.
45. KimTS, KawaguchiM, SuzukiM, JungCG, AsaiK, et al. (2010) The ZFHX3 (ATBF1) transcription factor induces PDGFRB, which activates ATM in the cytoplasm to protect cerebellar neurons from oxidative stress. Dis Model Mech 3 : 752–762.
46. BurgnerD, DavilaS, BreunisWB, NgSB, LiY, et al. (2009) A genome-wide association study identifies novel and functionally related susceptibility Loci for Kawasaki disease. PLoS Genet 5: e1000319 doi:10.1371/journal.pgen.1000319.
47. GudbjartssonDF, HolmH, GretarsdottirS, ThorleifssonG, WaltersGB, et al. (2009) A sequence variant in ZFHX3 on 16q22 associates with atrial fibrillation and ischemic stroke. Nat Genet 41 : 876–878.
48. RosetteC, KarinM (1996) Ultraviolet light and osmotic stress: activation of the JNK cascade through multiple growth factor and cytokine receptors. Science 274 : 1194–1197.
49. ChaveyC, LazennecG, LagarrigueS, ClapeC, IankovaI, et al. (2009) CXC ligand 5 is an adipose-tissue derived factor that links obesity to insulin resistance. Cell Metab 9 : 339–349.
50. FeeserEA, IgnacioCM, KrendelM, OstapEM (2010) Myo1e binds anionic phospholipids with high affinity. Biochemistry 49 : 9353–9360.
51. KrendelM, KimSV, WillingerT, WangT, KashgarianM, et al. (2009) Disruption of Myosin 1e promotes podocyte injury. J Am Soc Nephrol 20 : 86–94.
52. MeleC, IatropoulosP, DonadelliR, CalabriaA, MarantaR, et al. (2011) MYO1E mutations and childhood familial focal segmental glomerulosclerosis. N Engl J Med 365 : 295–306.
53. PajukantaP, LiljaHE, SinsheimerJS, CantorRM, LusisAJ, et al. (2004) Familial combined hyperlipidemia is associated with upstream transcription factor 1 (USF1). Nat Genet 36 : 371–376.
54. StaryHC, ChandlerAB, DinsmoreRE, FusterV, GlagovS, et al. (1995) A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Arterioscler Thromb Vasc Biol 15 : 1512–1531.
55. InouyeM, SilanderK, HamalainenE, SalomaaV, HaraldK, et al. (2010) An immune response network associated with blood lipid levels. PLoS Genet 6: e1001113 doi:10.1371/journal.pgen.1001113.
56. TorkamaniA, DeanB, SchorkNJ, ThomasEA (2010) Coexpression network analysis of neural tissue reveals perturbations in developmental processes in schizophrenia. Genome Res 20 : 403–412.
57. PurcellS, NealeB, Todd-BrownK, ThomasL, FerreiraMA, et al. (2007) PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 81 : 559–575.
58. HotellingH (1936) Relations between two sets of variates. Biometrika 28 : 321–377.
59. Pe'erI, YelenskyR, AltshulerD, DalyMJ (2008) Estimation of the multiple testing burden for genomewide association studies of nearly all common variants. Genet Epidemiol 32 : 381–385.
60. OksalaN, LevulaM, AirlaN, Pelto-HuikkoM, OrtizRM, et al. (2009) ADAM-9, ADAM-15, and ADAM-17 are upregulated in macrophages in advanced human atherosclerotic plaques in aorta and carotid and femoral arteries–Tampere vascular study. Ann Med 41 : 279–290.
61. SchmidR, BaumP, IttrichC, Fundel-ClemensK, HuberW, et al. (2010) Comparison of normalization methods for Illumina BeadChip HumanHT-12 v3. BMC Genomics 11 : 349.
Štítky
Genetika Reprodukční medicína
Článek Mutational Signatures of De-Differentiation in Functional Non-Coding Regions of Melanoma GenomesČlánek Rescuing Alu: Recovery of Inserts Shows LINE-1 Preserves Alu Activity through A-Tail ExpansionČlánek Genetics and Regulatory Impact of Alternative Polyadenylation in Human B-Lymphoblastoid CellsČlánek Retrovolution: HIV–Driven Evolution of Cellular Genes and Improvement of Anticancer Drug ActivationČlánek The Mi-2 Chromatin-Remodeling Factor Regulates Higher-Order Chromatin Structure and Cohesin DynamicsČlánek Identification of Human Proteins That Modify Misfolding and Proteotoxicity of Pathogenic Ataxin-1
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 8
-
Všechny články tohoto čísla
- Mutational Signatures of De-Differentiation in Functional Non-Coding Regions of Melanoma Genomes
- Rescuing Alu: Recovery of Inserts Shows LINE-1 Preserves Alu Activity through A-Tail Expansion
- Genetics and Regulatory Impact of Alternative Polyadenylation in Human B-Lymphoblastoid Cells
- Chromosome Territories Meet a Condensin
- It's All in the Timing: Too Much E2F Is a Bad Thing
- Fine-Mapping and Initial Characterization of QT Interval Loci in African Americans
- Genome Patterns of Selection and Introgression of Haplotypes in Natural Populations of the House Mouse ()
- A Combinatorial Amino Acid Code for RNA Recognition by Pentatricopeptide Repeat Proteins
- Advances in Quantitative Trait Analysis in Yeast
- Experimental Evolution of a Novel Sexually Antagonistic Allele
- Variation of Contributes to Dog Breed Skull Diversity
- , a Gene Involved in Axonal Pathfinding, Is Mutated in Patients with Kallmann Syndrome
- A Single Origin for Nymphalid Butterfly Eyespots Followed by Widespread Loss of Associated Gene Expression
- Cryptocephal, the ATF4, Is a Specific Coactivator for Ecdysone Receptor Isoform B2
- Retrovolution: HIV–Driven Evolution of Cellular Genes and Improvement of Anticancer Drug Activation
- The PARN Deadenylase Targets a Discrete Set of mRNAs for Decay and Regulates Cell Motility in Mouse Myoblasts
- A Sexual Ornament in Chickens Is Affected by Pleiotropic Alleles at and , Selected during Domestication
- Use of Allele-Specific FAIRE to Determine Functional Regulatory Polymorphism Using Large-Scale Genotyping Arrays
- Novel Loci for Metabolic Networks and Multi-Tissue Expression Studies Reveal Genes for Atherosclerosis
- The Genetic Basis of Pollinator Adaptation in a Sexually Deceptive Orchid
- Uncovering the Genome-Wide Transcriptional Responses of the Filamentous Fungus to Lignocellulose Using RNA Sequencing
- Inheritance Beyond Plain Heritability: Variance-Controlling Genes in
- The Metabochip, a Custom Genotyping Array for Genetic Studies of Metabolic, Cardiovascular, and Anthropometric Traits
- Reprogramming to Pluripotency Can Conceal Somatic Cell Chromosomal Instability
- Condensin II Promotes the Formation of Chromosome Territories by Inducing Axial Compaction of Polyploid Interphase Chromosomes
- PTEN Negatively Regulates MAPK Signaling during Vulval Development
- A Dynamic Response Regulator Protein Modulates G-Protein–Dependent Polarity in the Bacterium
- Population Genomics of the Facultatively Mutualistic Bacteria and
- Components of a Fanconi-Like Pathway Control Pso2-Independent DNA Interstrand Crosslink Repair in Yeast
- Polysome Profiling in Liver Identifies Dynamic Regulation of Endoplasmic Reticulum Translatome by Obesity and Fasting
- Stromal Liver Kinase B1 [STK11] Signaling Loss Induces Oviductal Adenomas and Endometrial Cancer by Activating Mammalian Target of Rapamycin Complex 1
- Reprogramming of H3K27me3 Is Critical for Acquisition of Pluripotency from Cultured Tissues
- Transgene Induced Co-Suppression during Vegetative Growth in
- Hox and Sex-Determination Genes Control Segment Elimination through EGFR and Activity
- A Quantitative Comparison of the Similarity between Genes and Geography in Worldwide Human Populations
- Minibrain/Dyrk1a Regulates Food Intake through the Sir2-FOXO-sNPF/NPY Pathway in and Mammals
- Comparative Analysis of Regulatory Elements between and by Genome-Wide Transcription Start Site Profiling
- Simple Methods for Generating and Detecting Locus-Specific Mutations Induced with TALENs in the Zebrafish Genome
- S Phase–Coupled E2f1 Destruction Ensures Homeostasis in Proliferating Tissues
- Cell-Nonautonomous Signaling of FOXO/DAF-16 to the Stem Cells of
- The Mi-2 Chromatin-Remodeling Factor Regulates Higher-Order Chromatin Structure and Cohesin Dynamics
- Comparative Analysis of the Genomes of Two Field Isolates of the Rice Blast Fungus
- Role of Mex67-Mtr2 in the Nuclear Export of 40S Pre-Ribosomes
- Genetic Modulation of Lipid Profiles following Lifestyle Modification or Metformin Treatment: The Diabetes Prevention Program
- HAL-2 Promotes Homologous Pairing during Meiosis by Antagonizing Inhibitory Effects of Synaptonemal Complex Precursors
- SLX-1 Is Required for Maintaining Genomic Integrity and Promoting Meiotic Noncrossovers in the Germline
- Phylogenetic and Transcriptomic Analysis of Chemosensory Receptors in a Pair of Divergent Ant Species Reveals Sex-Specific Signatures of Odor Coding
- Reduced Prostasin (CAP1/PRSS8) Activity Eliminates HAI-1 and HAI-2 Deficiency–Associated Developmental Defects by Preventing Matriptase Activation
- Dissecting the Gene Network of Dietary Restriction to Identify Evolutionarily Conserved Pathways and New Functional Genes
- Identification of Human Proteins That Modify Misfolding and Proteotoxicity of Pathogenic Ataxin-1
- and Link Transcription of Phospholipid Biosynthetic Genes to ER Stress and the UPR
- CDK9 and H2B Monoubiquitination: A Well-Choreographed Dance
- Rare Copy Number Variations in Adults with Tetralogy of Fallot Implicate Novel Risk Gene Pathways
- Ccdc94 Protects Cells from Ionizing Radiation by Inhibiting the Expression of
- NOL11, Implicated in the Pathogenesis of North American Indian Childhood Cirrhosis, Is Required for Pre-rRNA Transcription and Processing
- Human Developmental Enhancers Conserved between Deuterostomes and Protostomes
- A Luminal Glycoprotein Drives Dose-Dependent Diameter Expansion of the Hindgut Tube
- Melanophore Migration and Survival during Zebrafish Adult Pigment Stripe Development Require the Immunoglobulin Superfamily Adhesion Molecule Igsf11
- Dynamic Distribution of Linker Histone H1.5 in Cellular Differentiation
- Combining Comparative Proteomics and Molecular Genetics Uncovers Regulators of Synaptic and Axonal Stability and Degeneration
- Chemical Genetics Reveals a Specific Requirement for Cdk2 Activity in the DNA Damage Response and Identifies Nbs1 as a Cdk2 Substrate in Human Cells
- Experimental Relocation of the Mitochondrial Gene to the Nucleus Reveals Forces Underlying Mitochondrial Genome Evolution
- Rates of Gyrase Supercoiling and Transcription Elongation Control Supercoil Density in a Bacterial Chromosome
- Mutations in a P-Type ATPase Gene Cause Axonal Degeneration
- A General G1/S-Phase Cell-Cycle Control Module in the Flowering Plant
- Multiple Roles and Interactions of and in Development of the Respiratory System
- UNC-40/DCC, SAX-3/Robo, and VAB-1/Eph Polarize F-Actin during Embryonic Morphogenesis by Regulating the WAVE/SCAR Actin Nucleation Complex
- Epigenetic Remodeling of Meiotic Crossover Frequency in DNA Methyltransferase Mutants
- Modulating the Strength and Threshold of NOTCH Oncogenic Signals by
- Loss of Axonal Mitochondria Promotes Tau-Mediated Neurodegeneration and Alzheimer's Disease–Related Tau Phosphorylation Via PAR-1
- Acetyl-CoA-Carboxylase Sustains a Fatty Acid–Dependent Remote Signal to Waterproof the Respiratory System
- ATXN2-CAG42 Sequesters PABPC1 into Insolubility and Induces FBXW8 in Cerebellum of Old Ataxic Knock-In Mice
- Cohesin Rings Devoid of Scc3 and Pds5 Maintain Their Stable Association with the DNA
- The MicroRNA Inhibits Calcium Signaling by Targeting the TIR-1/Sarm1 Adaptor Protein to Control Stochastic L/R Neuronal Asymmetry in
- Rapid-Throughput Skeletal Phenotyping of 100 Knockout Mice Identifies 9 New Genes That Determine Bone Strength
- The Genes Define Unique Classes of Two-Partner Secretion and Contact Dependent Growth Inhibition Systems
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Dissecting the Gene Network of Dietary Restriction to Identify Evolutionarily Conserved Pathways and New Functional Genes
- It's All in the Timing: Too Much E2F Is a Bad Thing
- Variation of Contributes to Dog Breed Skull Diversity
- The PARN Deadenylase Targets a Discrete Set of mRNAs for Decay and Regulates Cell Motility in Mouse Myoblasts
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



