-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaThe Genetic Basis of Pollinator Adaptation in a Sexually Deceptive Orchid
In plants, pollinator adaptation is considered to be a major driving force for floral diversification and speciation. However, the genetic basis of pollinator adaptation is poorly understood. The orchid genus Ophrys mimics its pollinators' mating signals and is pollinated by male insects during mating attempts. In many species of this genus, chemical mimicry of the pollinators' pheromones, especially of alkenes with different double-bond positions, plays a key role for specific pollinator attraction. Thus, different alkenes produced in different species are probably a consequence of pollinator adaptation. In this study, we identify genes that are likely involved in alkene biosynthesis, encoding stearoyl-acyl carrier protein (ACP) desaturases (SAD), in three closely related Ophrys species, O. garganica, O. sphegodes, and O. exaltata. Combining floral odor and gene expression analyses, two SAD homologs (SAD1/2) showed significant association with the production of (Z)-9 - and (Z)-12-alkenes that were abundant in O. garganica and O. sphegodes, supporting previous biochemical data. In contrast, two other newly identified homologs (SAD5/6) were significantly associated with (Z)-7-alkenes that were highly abundant only in O. exaltata. Both molecular evolutionary analyses and pollinator preference tests suggest that the alkenes associated with SAD1/2 and SAD5/6 are under pollinator-mediated divergent selection among species. The expression patterns of these genes in F1 hybrids indicate that species-specific expression differences in SAD1/2 are likely due to cis-regulation, while changes in SAD5/6 are likely due to trans-regulation. Taken together, we report a genetic mechanism for pollinator-mediated divergent selection that drives adaptive changes in floral alkene biosynthesis involved in reproductive isolation among Ophrys species.
Published in the journal: . PLoS Genet 8(8): e32767. doi:10.1371/journal.pgen.1002889
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002889Summary
In plants, pollinator adaptation is considered to be a major driving force for floral diversification and speciation. However, the genetic basis of pollinator adaptation is poorly understood. The orchid genus Ophrys mimics its pollinators' mating signals and is pollinated by male insects during mating attempts. In many species of this genus, chemical mimicry of the pollinators' pheromones, especially of alkenes with different double-bond positions, plays a key role for specific pollinator attraction. Thus, different alkenes produced in different species are probably a consequence of pollinator adaptation. In this study, we identify genes that are likely involved in alkene biosynthesis, encoding stearoyl-acyl carrier protein (ACP) desaturases (SAD), in three closely related Ophrys species, O. garganica, O. sphegodes, and O. exaltata. Combining floral odor and gene expression analyses, two SAD homologs (SAD1/2) showed significant association with the production of (Z)-9 - and (Z)-12-alkenes that were abundant in O. garganica and O. sphegodes, supporting previous biochemical data. In contrast, two other newly identified homologs (SAD5/6) were significantly associated with (Z)-7-alkenes that were highly abundant only in O. exaltata. Both molecular evolutionary analyses and pollinator preference tests suggest that the alkenes associated with SAD1/2 and SAD5/6 are under pollinator-mediated divergent selection among species. The expression patterns of these genes in F1 hybrids indicate that species-specific expression differences in SAD1/2 are likely due to cis-regulation, while changes in SAD5/6 are likely due to trans-regulation. Taken together, we report a genetic mechanism for pollinator-mediated divergent selection that drives adaptive changes in floral alkene biosynthesis involved in reproductive isolation among Ophrys species.
Introduction
Understanding the genetic basis of adaptation is of great interest to evolutionary biologists. For over a century, it has been debated whether adaptations are likely caused by a large number of mutations of small phenotypic effect or by very few genetic changes of large effect [1]–[3]. To address this question, it is necessary to identify the genetic basis of adaptive traits and their ecological significance in any given study system [4].
Pollinator-mediated selection on floral traits has been considered to be a major driving force of floral diversification and speciation in plants [4]–[9]. Closely related species featuring distinct floral traits, such as floral color, odor, or spur lengths, are widely thought to be a consequence of pollinator adaptation [4], [6], [8]–[11]. Furthermore, pollinator adaptation often conveys reproductive isolation [12], [13], and thus may directly contribute to the origin of novel species. Therefore, floral traits associated with pollinator adaptation are of special interest for the understanding plant speciation and evolution.
Ophrys orchids mimic their pollinators' mating signals and are pollinated by male insects during mating attempts with the flower. This pollination by so-called sexual deception is very specific, and each orchid species only attracts one or very few insect species [14], [15]. Specific pollinator attraction has been reported to be the main reproductive barrier in Ophrys [15]–[17]. The key to specific pollinator attraction is the chemical mimicry of the insect female's sex pheromone [11], [18]–[22], usually a blend of cuticular hydrocarbons, namely alkanes and alkenes. Among these, alkenes with different double-bond position are particularly important for the specificity of pollinator attraction [11], [20], [22]. Thus, genes specifying alkene double-bond positions may be directly associated with pollinator adaptation.
In plants, alkene double-bonds are likely determined by desaturases such as stearoyl-ACP (acyl carrier protein) desaturases (SADs) [23]–[25]. At the onset of alkene biosynthesis, desaturases can insert a cis-double-bond into a saturated fatty acid (FA) intermediate, such as 16 : 0-ACP (C:D denotes a fatty acyl group of C carbons length with D double-bonds), to produce an unsaturated FA such as 16 : 1ω-7-ACP or 16 : 1ω-9-ACP (double-bond at position ω-7 or ω-9, counting from the aliphatic end). Unsaturated FA intermediates are elongated from the ACP end [26], leading to the production of (Z)-7 - or 9-alkenes from ω-7 or ω-9 FA intermediates, respectively [23], [24] (Since all alkenes in this study are presumed to be in the cis (Z) configuration, only double-bond position will be indicated for alkenes in the following text). Therefore, changes in SAD enzyme activity or SAD gene expression may result in alkene double-bond position differences among species [25].
In this study, we focused on three closely related sympatric species of the genus Ophrys: O. garganica, O. sphegodes, and O. exaltata, among which the phylogenetic relationship could not yet be resolved by neutral sequence markers [27]. These three co-flowering species largely overlap in their geographical distributions and are similar in floral morphology [28], [29], however, they are reproductively isolated from each other due to the attraction of different pollinators [17]. Although the color of floral petals also varies among species, the key factor for differential pollinator attraction is floral scent [30]. Flowers of both O. garganica and O. sphegodes produce a high proportion of 9 - and 12-alkenes (with differences in carbon chain length), whereas O. exaltata produces high amounts of 7-alkenes. These 7-alkenes have previously been shown to be important for attraction of the bee, Colletes cunicularius, the pollinator of O. exaltata [19], [31]. We have previously shown that in O. sphegodes, 9 - and 12-alkene production is associated with the SAD2 gene, which encodes a functional desaturase [24]. However, the genetic basis of 7-alkene biosynthesis in O. exaltata and the driving forces for evolutionary divergence in alkene production among species are unknown. Here we investigate gene expression and evolutionary relationships among SAD gene family members in different natural orchid populations and species. Moreover, we discuss the biosynthetic origin of 7-alkenes as well as the role of pollinator-mediated selection in changing alkene composition among species. Specifically, we address the following questions: (a) Which desaturase is responsible for 7-alkene biosynthesis? (b) What is the role of pollinator-mediated selection in the evolution of alkene production? (c) How is the biosynthesis of different alkenes by different Ophrys species regulated genetically?
Results
Stearoyl-ACP-desaturase homologs in Ophrys
We identified six SAD homologs, which we named SAD1–SAD6. Three of these (SAD1–3) have been described previously [24]; SAD4–6 were identified by homology from high-throughput transcriptome sequencing of O. sphegodes flowers. Phylogenetic analysis of plant SAD genes indicated that all six Ophrys homologs evolved via gene duplication, forming three distinct lineages, SAD1/2, SAD3, and SAD4/5/6. The split of SAD1/2 from SAD4/5/6 was more recent than the split of these groups from SAD3 (Figure 1).
Fig. 1. Bayesian inference phylogenetic tree of monocot SAD homologs and eudicot outgroup. 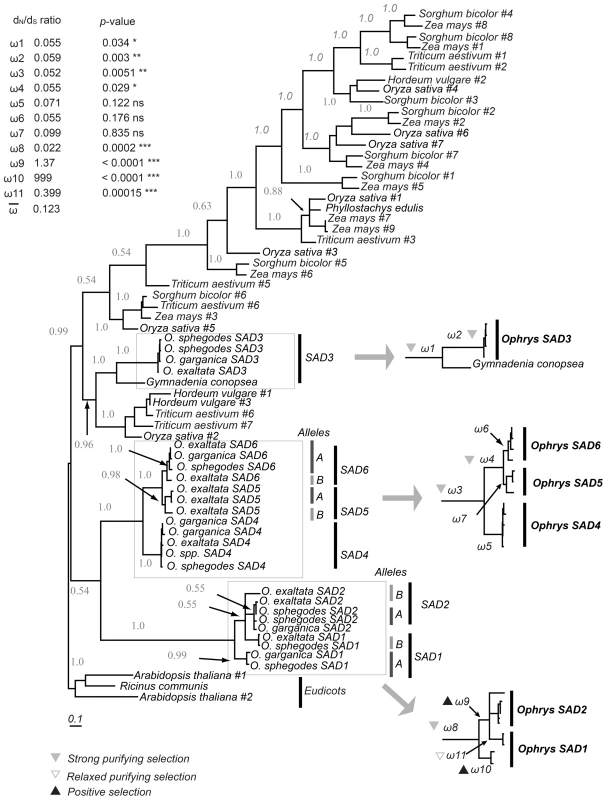
Numbers indicate posterior probabilities (where >0.5) above branches. Ophrys SAD homologs and their assignment to different allele groups are highlighted and ω = dN/dS ratios for branches of interest indicated. A black upward triangle indicates significant positive selection; a gray downward triangle indicates purifying selection, and a non-filled triangle indicates relaxed purifying selection. The inset lists the ω-values, associated p-value, and significance (*, p<0.05; **, p<0.01; ***, p<0.001). To test for the signature of selection, a codon-based analysis comparing the rates of synonymous and non-synonymous mutations was performed. It revealed a significant signal indicative of positive (ω9 and ω10) and relaxed purifying selection (ω11, all p<0.001, Figure 1) after the split of SAD1 and SAD2, concordant with previous findings [24]. However, no indication of positive selection was found for any other SAD locus or clade. Purifying selection significantly stronger than the background rate was found on the SAD3 branch, as well as prior to the split of SAD1/2, and prior to the split of SAD4/5/6.
Expression of certain SAD homologs is correlated with alkene production during development
Among the six SAD homologs investigated for tissue - and floral developmental stage-specific expression, five (SAD1–5) were found to be expressed in the 11 tested greenhouse-grown individuals. Four homologs (all except SAD3) showed flower-specific expression (Figure S1). The expression levels of SAD2 and SAD5 were significantly associated with the presence of alkenes: SAD2 expression was significantly (p<0.001) associated with both 12 - and 9-alkenes, whereas SAD5 expression was significantly (p<0.001) associated with 7-alkenes in O. exaltata across different tissues and floral developmental stages (Figure 2).
Fig. 2. Regression of normalized desaturase gene expression with alkenes. 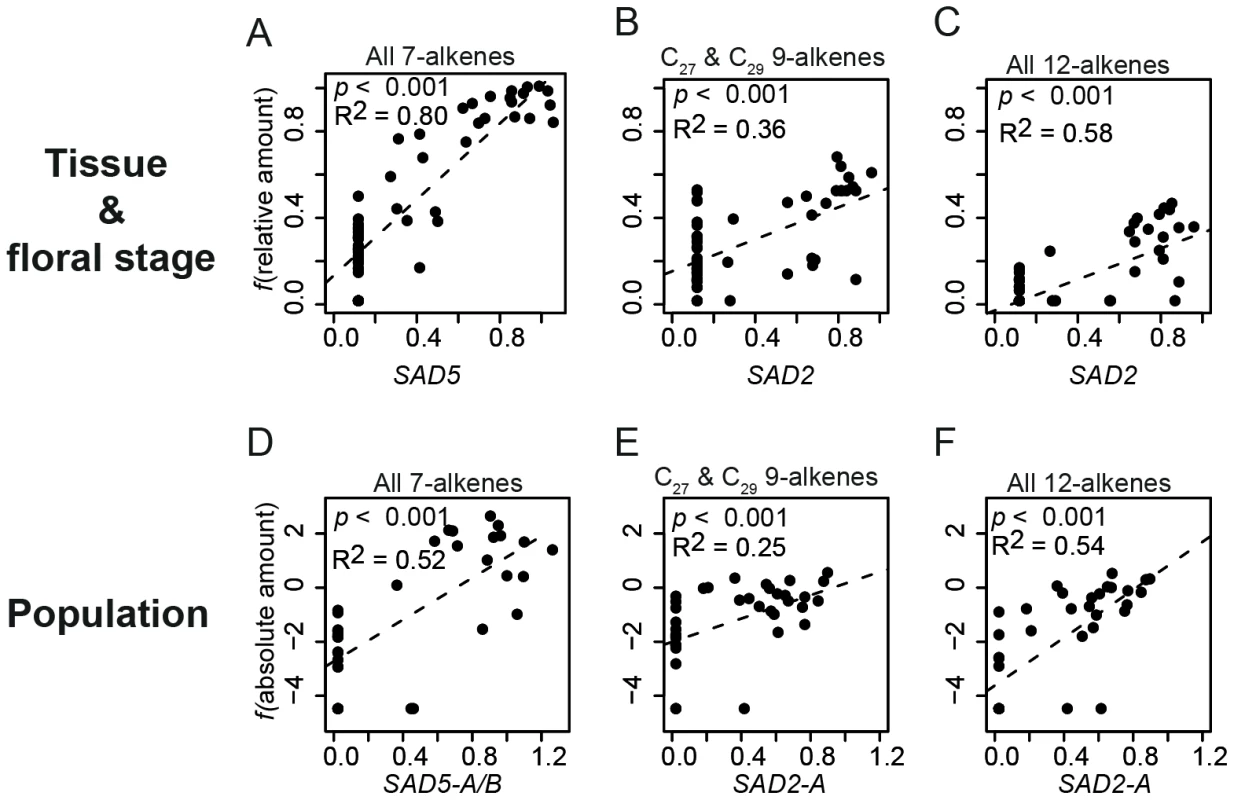
(A–C) Relative amount of alkenes was used after f(x) = arcsin x0.5 transformation for tissues and floral stages of O. exaltata (A) and O. sphegodes (B,C). (D–F) Absolute amount of alkenes (in µg) was used after f(x) = ln (x+0.01) transformation for population data from three species. For both datasets, normalized SAD expression was used after f(x) = x0.5 transformation. Adjusted R2 is indicated in each graph. (A,D) Correlation of all (C21-C29) 7-alkenes with SAD5; (B,E) Correlation of C27+C29 9-alkenes with SAD2 expression; (C,F), Correlation of all (C25-C29) 12-alkenes with SAD2 expression. Allelic variation of SAD homologs among species
All SAD homologs except SAD3 and SAD4 showed species-specific patterns of allelic variation among the three studied orchid species (Figure S2). Two allele groups were found for each SAD1 (SAD1-A/B), SAD5 (SAD5-A/B) and SAD6 (SAD6-A/B), whereas four allele groups were found for SAD2 (SAD2-A/B/C/D). Among these SAD allele groups, biochemical activity assays suggest that SAD1-B and SAD2-C alleles do not encode functional desaturases, whereas one SAD2-A allele has been shown to be functional [24]. However, two further allele groups are unlikely to be functional: one group (SAD2-D) had a repetitive sequence insertion at the start of the coding sequence, and one (SAD6-B) contained significantly more stop and frame-shift mutations than expected by chance (Table S1).
Combining putative coding sequence functionality and biochemical activity data, we classified all alleles into three categories: (a) putatively functional and expressed, (b) putatively nonfunctional and expressed, and (c) non-expressed alleles (Figure S3). For SAD1/2/5/6, the distributions of these allele categories are significantly different between O. exaltata and the other two species (Figure 3). For SAD1/2, functional expressed alleles were significantly more common in O. garganica and O. sphegodes than in O. exaltata. By contrast, SAD5/6 showed the opposite pattern, functional expressed alleles being more common in O. exaltata.
Fig. 3. Distribution of SAD allele occurrence in three species. 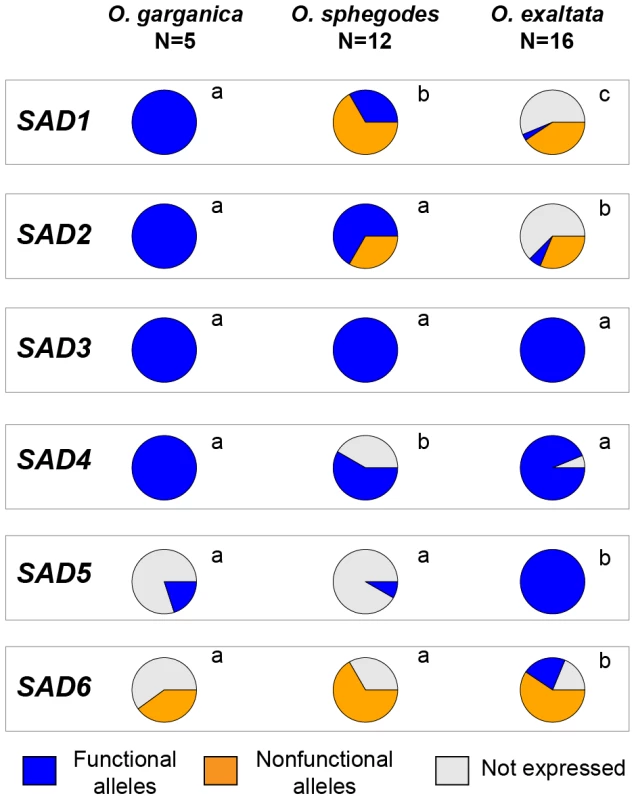
Color indicates different allele groups (blue: putatively functional alleles; orange: putatively nonfunctional alleles; gray: alleles not expressed in a given species). Different letters beside pie charts indicate statistical difference (p<0.05; χ2 test). To estimate FST values for all expressed SAD homologs in all three species, an in silico resampling approach was employed (see Methods), treating all individuals as diploids based on flow cytometry data [17]. O. garganica was not included in this analysis due to the smaller sample size, and FST could not be calculated for SAD5 because it was only observed in O. exaltata. SAD2 and SAD6 showed significantly higher FST, (0.44±0.02 and 0.32±0.006 respectively, mean ± standard error, p<0.01) than SAD1, SAD3 and SAD4 (0.22±0.005, 0.25±0.006 and 0.23±0.01 respectively, mean ± standard error).
Allelic gene expression of SAD is associated with alkene composition differences among species
Five SAD copies (all except SAD3) showed divergent species-specific gene expression (Figure S3). Among the alleles of SAD1, SAD1-A was highly expressed in O. sphegodes and O. garganica but not in O. exaltata (Figure 4), whereas SAD1-B was highly expressed in O. sphegodes and O. exaltata (Figure S3). Among the SAD2 allele groups, SAD2-A/B were highly expressed in both O. sphegodes and O. garganica, whereas the expression of SAD2-C/D was low in all study species (Figure S3). The expression of SAD4 was high in O. garganica and O. exaltata, but low in O. sphegodes. All alleles of SAD5 and SAD6 were highly expressed mostly in O. exaltata.
Fig. 4. Allelic gene expression and alkene production in different species. 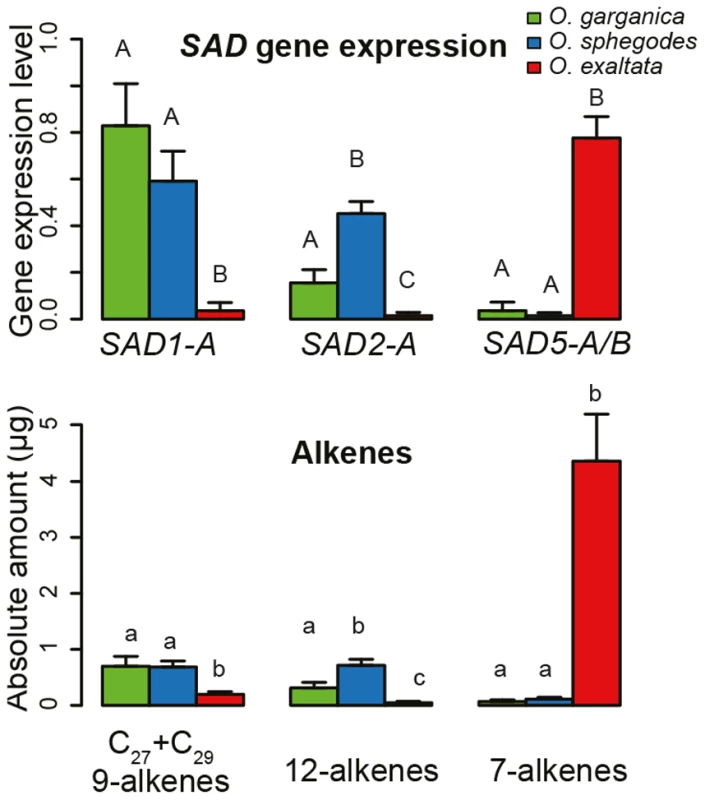
Top, gene expression of different SAD alleles; Bottom, alkene levels. Colors indicate species (red, O. exaltata; green, O. garganica; blue, O. sphegodes). Error bars show standard error. Different letters indicate significant differences among species for each allele (p<0.05; ANOVA). Natural F1 hybrids among O. sphegodes and O. exaltata (identified from AFLP data) showed a similar scent [17] and SAD expression pattern to O. sphegodes (Figure S3). For SAD1 and SAD2, alleles (SAD1-A/B, SAD2-A/B) that were most likely inherited from O. sphegodes were found to be highly expressed in these F1 hybrids, whereas none of the SAD5/6 alleles was expressed (Figure S3).
Statistical analysis of data from natural populations showed a strong correlation between the expression of specific SAD allele groups and alkene production. The expression level of SAD1-A was found to be significantly (p<0.001) positively correlated with 9-C29, 12-C27 and 12-C29 alkenes; SAD2-A was significantly (p<0.001) positively correlated with 9-C27, 9-C29, 12-C25, 12-C27, and 12-C29 alkenes (Figure 2, Figure S4); SAD5-A/B were significantly (p<0.001) correlated with all 7-alkenes (Figure 2, Figure S4).
Alkene composition affects pollinator attraction
To understand the driving force for allelic evolution of SAD homologs in Ophrys, we tested the effects of alkenes with different double-bond position – associated with different SAD homologs – on pollinator behavior. We quantified pollinator responses to control and manipulated flowers and scent extracts of O. exaltata and O. sphegodes. Addition of 9 - and 12-alkenes (associated with SAD2-A) to O. exaltata labella reduced the attractiveness to its pollinator (the masked bee Colletes cunicularius) by about 40% for approach and contact (N = 18, p = 0.023 and p = 0.015 respectively, Wilcoxon signed rank test, Figure 5A). Adding 7-alkenes (associated with SAD5-A/B) to floral scent extracts of O. sphegodes reduced the attractiveness to its pollinator (the mining bee Andrena nigroaenea) by about 30% and 60% for approach and contact respectively (N = 17, p = 0.028 and p = 0.045, Wilcoxon signed rank test, Figure 5B).
Fig. 5. Effects of alkenes with different double-bond position on pollinator behavior. 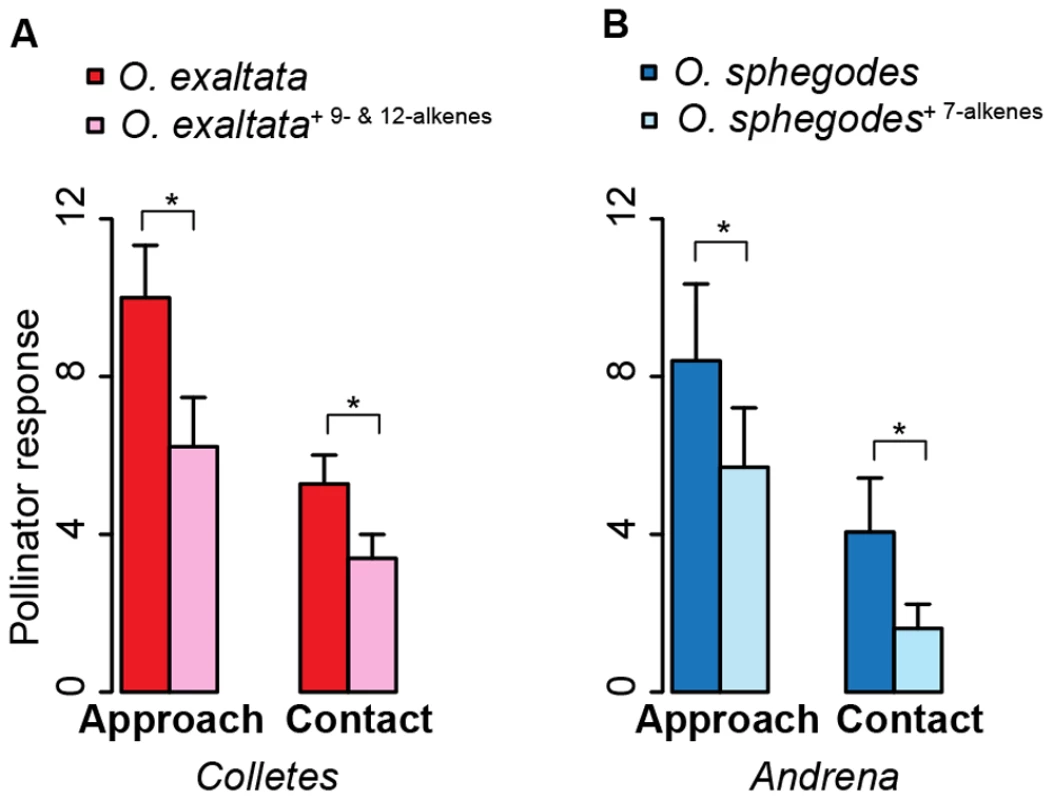
(A) Addition of 9- and 12- alkenes (associated with SAD1/2) onto O. exaltata flowers reduced its pollinator (C. cunicularius) response (N = 18). Red bar, control O. exaltata; pinkish bar, scent manipulated O. exaltata. (B) Adding 7-alkenes (associated with SAD5/6) into O. sphegodes floral scent extract reduced its pollinator (A. nigroaenea) response (N = 17). Blue bar, control floral scent of O. sphegodes; light blue bar, manipulated floral scent of O. sphegodes. Y-axis refers to number of counts. Asterisks indicate statistical significance (pairwise Mann-Whitney U test): *, p<0.05. Discussion
Differences in alkene production are associated with SAD gene expression
Our data indicate that alkene biosynthesis is associated with the expression of certain SAD homologs in Ophrys. SAD2 catalyzes the introduction of a double-bond at position 9 of 18 : 0-ACP and position 4 of 16 : 0-ACP [24], producing 18 : 1ω-9-ACP and 16 : 1ω-12-ACP, which should eventually lead to the production of 9 - and 12-alkenes [23], [24]. The significant correlation of SAD2 expression with the amounts of certain 9 - and 12-alkenes in different plant tissues, floral developmental stages, and natural populations lends further support to this hypothesis. Although biochemical assays suggested that SAD1 (allele group SAD1-B) is not catalytically active [24], this may not be true for other SAD1 alleles (SAD1-A) found in both O. sphegodes and O. garganica, SAD1-A and SAD1-B differing by 14% at the amino acid sequence level. Furthermore, SAD1-A expression was significantly correlated with some 9 - and 12-alkenes (Figure S4). This indicates that, like SAD2, SAD1 may also contribute to 9 - and/or 12-alkene biosynthesis in natural populations.
The significant correlation of SAD5 expression with the amount of 7-alkenes (Figure 2) suggests that SAD5 is involved in 7-alkene biosynthesis. In addition, the high sequence identity (>95% at the amino acid level) of SAD5 to SAD6-A, which was highly expressed in one O. exaltata population (SPF), indicates that both may have the same enzymatic function. We hypothesize that SAD5/6 introduces a double-bond at position 11 into 18 : 0-ACP, producing 18 : 1ω-7-ACP, or at position 9 of 16 : 0-ACP, producing 16 : 1ω-7-ACP. Further biochemical studies are required to test this hypothesis. These unsaturated intermediates could then be elongated to produce 7-alkenes. Therefore, changes in the expression of SAD5/6 could directly lead to different amounts of 7-alkenes in different Ophrys species.
SAD homologs are under pollinator-mediated selection
Our data suggest that SAD homologs evolve under pollinator-mediated selection, considering that genetic drift is a less likely explanation given large effective population sizes in our study species [11]. The expression of SAD1/2 was high in both O. garganica and O. sphegodes, but was very low in most O. exaltata individuals (Figure 4, Figure S3). In those very few individuals of O. exaltata that did highly express SAD2, it either was rendered nonfunctional by a repetitive sequence insertion (SAD2-D; population MDL), or had amino acid substitutions located on the surface of the protein (SAD2-C allele group), which have been suggested to reduce the activity of SAD2 [24]. This indicates that disruptive selection might act on SAD2 (in terms of gene expression or overall enzymatic activity) to maintain (in O. sphegodes and O. garganica) or reduce (in O. exaltata) the production of 9 - and/or 12-alkenes in Ophrys floral odor. Evidence of positive selection on SAD1/2 detected by dN/dS ratio tests is consistent with this hypothesis (Figure 1 and ref. [24]). Indeed, our pollinator-attraction assay suggests disruptive selection on 9 - and/or 12-alkenes among species. This behavioral test demonstrated that adding 9 - and 12-alkenes onto the floral labella of O. exaltata reduced its attractiveness to the pollinator by about 40% (Figure 5A). This indicates that while 9/12-alkenes act as the main attractants of O. sphegodes to its pollinator A. nigroaenea [18], these compounds actually reduce the attractiveness of the odor bouquet of O. exaltata to its pollinator C. cunicularius. Reduced responses of pollinator males to heterospecific odor blends may have evolved under sexual selection for maximum speed and accuracy of finding conspecific females [32]. Therefore, pollinator-imposed disruptive selection acts to change 9/12-alkene composition among these two Ophrys species by changing the expression or enzymatic activity of SAD1/2.
However, for SAD5/6, the opposite pattern was observed. SAD5/6 were highly expressed in O. exaltata, but hardly expressed in O. sphegodes and O. garganica. A significantly higher frequency of frame-shifts or premature stop codons was found in SAD6 of O. sphegodes and O. garganica (Table S1), indicating that SAD6 alleles in these two species may be released from purifying selection such that loss-of-function mutations can accumulate. Neither positive selection nor purifying selection was detected on SAD5/6 using codon-based methods (Figure 1), suggesting that pollinator-mediated selection on 7-alkenes in O. sphegodes and O. garganica primarily acts on the expression level of SAD5/6. Indeed, selection against 7-alkenes in O. sphegodes was confirmed by behavioral tests with its pollinator, since addition of 7-alkenes to the floral scent of O. sphegodes resulted in a significant reduction in pollinator attraction (Figure 5B). Therefore, while 7-alkenes attract O. exaltata's pollinator [19], [31], they reduce the attractiveness of O. sphegodes to its pollinator. Hence, pollinator-mediated disruptive selection may also drive the evolution of 7-alkene quantity in these two Ophrys species by changing the expression of SAD5/6. In contrast to these genes that are associated with alkene production, SAD3 and SAD4, which were not significantly correlated with alkene occurrence, showed no significant sequence divergence among species (Figure 3, Figure S2), as would be expected for genes that are not targets of selection.
Overall, our data suggest that pollinator adaptation in Ophrys is achieved via reciprocal regulation or activity changes of desaturases involved in 7 - and 9/12-alkene biosynthesis in response to disruptive selection by different pollinator preferences.
Both cis - and trans-regulatory elements may be involved in controlling species-specific alkene compositions
The changes in 7 - and 9/12-alkene production that are linked to differences in SAD gene expression may be explained by the action of cis- or trans-acting elements. The expression of SAD1/2, which is associated with 9/12-alkene production, differed among O. exaltata (weak expression) and O. sphegodes (strong expression) (Figure S3). However, two putative F1 hybrids only expressed the alleles expected to be inherited from O. sphegodes, but not O. exaltata (Table S2; Figure S3). This indicates that down-regulation of SAD1/2 expression in O. exaltata might be due to changes in a cis-regulatory element (such as a promoter or enhancer). In contrast, although differences in expression of SAD5/6, which are associated with 7-alkene production, were found between O. sphegodes and O. exaltata, the putative F1 hybrids did not express either allele expected from the parental species (Table S2; Figure S3). This suppression of expression of SAD5/6 in F1 hybrids indicates that – while additional cis-regulatory changes cannot be ruled out – a trans-acting factor is likely involved in the different SAD5/6 gene expression among species. This suggests the presence of a (dominant) suppressor of SAD5/6 expression in O. sphegodes (e.g., a transcriptional repressor or a miRNA reducing SAD5/6 mRNA levels) that is absent or inactive in O. exaltata. However, it is also possible that the dominant expression of SAD5/6 genes in F1 hybrids is due to epigenetic changes upon hybridization [33], [34].
In conclusion, our data based on multiple independent lines of evidence suggest that pollinator adaptation in the three studied Ophrys species is largely due to changes in SAD1/2 and SAD5/6, in terms of gene expression and potentially also in terms of the function of their gene products, and that both cis- and trans-regulation of gene expression contribute to this process. Our data indicate that pollinator adaptation in plants with a specialized pollination system may be due to few changes in the genome, with a large phenotypic effect. Furthermore, because reproductive isolation among closely related Ophrys species is mainly a consequence of specific pollinator attraction, such adaptation to different pollinators can directly prevent gene flow and ultimately lead to speciation.
Materials and Methods
Plant material
Population samples were collected in southern Italy (Table S3), at the same locations as described in Xu et al. [17], with three additional O. exaltata individuals from San Pietro in Fine (SPF) in southern Italy (N41°25′38″, E13°58′04″). Two F1 hybrids between O. exaltata and O. sphegodes were previously identified based on AFLP markers [17]. For each plant individual, one labellum of an unpollinated flower was used for floral odor extraction as described previously [17], and then immediately flash frozen in liquid nitrogen, and stored at −80°C until RNA extraction. For developmental stage - and tissue-specific analysis of hydrocarbons and gene expression, five O. exaltata and six O. sphegodes individuals grown in a greenhouse at the Botanical Garden of the University of Zürich were used and processed as described previously [24].
GC and GC/MS analysis
GC and GC/MS analysis, identification and quantification of compounds were performed as described previously [11], with modifications [17]. Discrimination of 11 - and 12-alkenes was not possible with the GC parameters used, however, 12-alkenes were earlier determined to be the predominant isomers in O. sphegodes [35], [36]. The absolute amounts of alkenes and alkanes with a carbon chain length from 21 to 29 were calculated based on an internal standard. For tissue/stage-specific samples, the relative amount of alkenes was calculated since the use of comparable amounts of tissue could not be ensured.
RNA extraction, cDNA synthesis, RACE, and RT–PCR
Total RNA was extracted using Trizol reagent (Invitrogen, Carlsbad, USA) following the manufacturer's protocol, and RNA quality and quantity were assessed by agarose gel electrophoresis and spectrophotometry on a NanoDrop ND-1000 (Witec AG, Littau, Switzerland). First strand cDNA was synthesized as described in [24]. To obtain the full-length coding sequence of candidate genes, 5′ RACE was performed as described in [37], with minor modifications [24], and 3′ RACE as in [38]. Gene-specific primers used for RACE are listed in Table S4. Advantage GC 2 DNA Polymerase (Clontech Laboratories Inc, Mountain View, USA) was used for RACE PCR with a touchdown program: 96°C 15 s; 3 cycles of [94°C 20 s, 68°C 3 min 30 s]; 7 cycles of [94°C 20 s, 67°C (1°C decrease per cycle) 30 s, 68°C 3 min 30 s]; 30 cycles of [94°C 25 s, 55°C 30 s, 68°C 3 min 30 s]; final extension at 68°C for 10 min. The amplified fragments were cloned into pDRIVE vector (Qiagen, Hilden, Germany), following the provided protocol. Desaturase homologs were amplified for all cDNA samples, using gene-specific primers containing attB adapter sequences (Table S4). RT-PCR was performed in 15 µl reaction volume containing cDNA template equivalent to 15 ng RNA as follows: 95°C 3 min; 33 cycles of [95°C 30 s, 58–60°C 30 s (see Table S4 for annealing temperatures), 72°C 1 min 30 s]; final extension at 72°C for 10 min, using REDTaq ReadyMix (Sigma-Aldrich, St. Louis, USA) mix supplemented with 0.6 units Pfu polymerase (Promega AG, Dübendorf, Switzerland). Three µl PCR product were loaded on an agarose gel to confirm amplification.
Cloning and sequencing
Amplified PCR products from each population of each species were pooled and then purified with Wizard SV Gel and PCR Clean-Up kit (Promega AG, Dübendorf, Switzerland), and recombined into Gateway cloning vector pDONR221 (Invitrogen, Carlsbad, USA) using the manufacturers' protocols. Competent E. coli One Shot TOP10 cells (Invitrogen, Carlsbad, USA) were used for transformation. In order to recover all possible alleles, the number of clones picked and screened by PCR was at least three times the number of possible alleles in diploids, for each cloning library. Clones were PCR amplified, purified and sequenced as previously described [24]. All sequences were deposited in GenBank (accession numbers are listed in Table S5).
Sequence analysis and allele group assignment
Forward and reverse sequences of each clone were assembled and manually edited in SeqMan v7.1.0 (Lasergene DNAstar, Wisconsin, USA). For each SAD homolog, the assembled sequences of each clone were aligned using Clustal W [39]. Sequences with less than two nucleotide differences were considered to be the same allele with PCR or sequencing errors, and were merged into one consensus sequence. The consensus sequences and all singleton sequences, which differed by more than two nucleotides, were used for assignment to allele groups. To do so, given the low sequence divergence, a dendrogram was constructed for each SAD homolog in MEGA 4.0 [40], using a pairwise distance and the UPGMA method, with pairwise deletion of gaps, and a homogeneous substitution pattern among lineages and sites. Bootstrap analysis was conducted using 1000 pseudo-replicates. Allele groups were assigned based on UPGMA tree topology with bootstrap support (Figure S2). The relationship among alleles was also inferred by Bayesian analysis (Figure S5) in MrBayes (v3.2.1; for details see below) [41], which was congruent and largely confirmed the clusters from UPGMA analysis. The Bayesian analysis showed SAD2-C alleles to be nested in SAD2-B, and SAD6-A in SAD6-B, but was in agreement with our primary allele group assignment.
Measuring gene expression by semi-quantitative PCR
SAD gene expression was assessed by semi-quantitative RT-PCR with allele-specific primers (Table S4). PCR was performed in 10 µl reaction volume with cDNA from 12 ng total RNA as a template. Each PCR was performed as: 95°C 3 min; 29 cycles of [95°C 30 s, 58–60°C 30 s (see Table S4 for different primer annealing temperatures), 72°C 1 min 30 s]; final extension at 72°C for 10 min using REDTaq ReadyMix (Sigma-Aldrich, St. Louis, USA). For all RT-PCRs, the putative Ophrys housekeeping gene G3PDH [24] was used as control. Five µl of each PCR product were loaded on 0.8% agarose gel, recorded and quantified using ImageJ (1.42q) [42] as described in [24].
Bioassay for pollinator preferences on floral scents
Bioassays for pollinator preferences were performed between beginning of March to middle April 2011 at Capoiale (Southern Italy) and Charrat (Wallis, Switzerland) for O. exaltata and O. sphegodes, respectively. For both species, pollinator preferences on control and manipulated scent (for O. exaltata, 9/12-alkenes added; for O. sphegodes, 7-alkenes added) were tested (Table S6 and Table S7). The preference of O. exaltata's pollinator, C. cunicularius, was assessed with whole inflorescences (bearing 2–3 flowers) assayed individually. Each inflorescence was placed on bushes where C. cunicularius males were abundant; pollinator responses were recorded for 10 minutes. Afterwards, a 9/12-alkene mixture mimicking natural blends occurring in O. sphegodes was added onto each floral labellum of the same inflorescence (see Table S6 and Table S7) and the pollinator responses monitored for a further 10 min. For each subsequent test, the plants were placed at a different position to avoid habituation effects often found after multiple subsequent testing at one location. In total, 18 inflorescences that had at least 2 flowers were tested. The preference of the pollinator of O. sphegodes, A. nigroaenea, was assessed in choice experiments on floral scent with black plastic beads. This different testing procedure was chosen because no natural plants were available at this testing location. The floral scent of floral labella was first extracted with 500 µl hexane [17]. For each choice experiment, 100 µl of floral extract was tested against 100 µl of floral extract of the same flower plus 7-alkene mixture (see Table S6 and Table S7). The dummy was placed on bushes where A. nigroaenea males were abundant. Pollinator responses were recorded for six minutes. In total, 17 replicates of this experiment were performed. For all pollinator behavioral tests, pollinator responses were classified into: approach (a zig-zagging or undulating approach towards the tested flowers or beads) and contact (either a short punching contact or landing on the tested flowers or beads) [19].
Bioinformatics and statistical analysis
All monocot SAD sequences were taken from the plant SAD homolog data set of [24]. Sequences were re-aligned based on amino acid sequence using Muscle 3.8.31 [43]. Phylogenetic analysis was performed in MrBayes 3.1.2 [41] (burn-in 13 out of 40 million generations) using the GTR+I+G model, which was estimated to be the best model by MrModeltest (2.3; AIC criterion) [44]. All sequence data were partitioned by codon positions, and MrBayes analysis performed using one cold and three heated chains, trees sampled every 1000 generations, and combined into a 50% majority rule consensus tree. The signature of selection on selected branches was tested using PAML 4.4 [45], as previously described [24]. The significance of different amounts of floral odor and gene expression among species was assessed by ANOVA after normality testing of the data distribution by the Shapiro test [46]. Differences in allele distribution among different species were assessed by χ2-testing. To estimate the pattern of divergence for each SAD homolog, we first genotyped each Ophrys individual by allele-specific RT-PCR; then we randomly sampled two sequences from the allele groups based on species and population information. This procedure was repeated 100 times for in silico re-sampling. FST was calculated using Arlsumstat, a modified version of Arlequin (v.3.5.1.3) [47]. The association between floral scent and gene expression in natural populations was assessed using a generalized linear model (GLM) and a linear mixed-effect model (LME) with population as random factor. These models were simplified by stepwise removal of factors using the stepAIC method [48]. For the tissue/stage-specific dataset, the relative amount of each floral scent compound was used (arcsine square-root transformed) as described by Schlüter et al. [24], since the size of floral labella varies in different developmental stages. For the population data set, the absolute amount of each floral scent compound was used. The significance of the presence of nonfunctional alleles in different allele groups was tested using Fisher's exact test. All statistical analyses were performed in R 2.11.0 [49].
Supporting Information
Zdroje
1. OrrHA, CoyneJA (1992) The genetics of adaptation - A reassessment. American Naturalist 140 : 725–742.
2. GillhamNW (2001) Evolution by jumps: Francis Galton and William Bateson and the mechanism of evolutionary change. Genetics 159 : 1383–1392.
3. WidmerA, LexerC, CozzolinoS (2009) Evolution of reproductive isolation in plants. Heredity 102 : 31–38.
4. SchemskeDW, BradshawHDJr (1999) Pollinator preference and the evolution of floral traits in monkeyflowers (Mimulus). Proceedings of the National Academy of Sciences of the United States of America 96 : 11910–11915.
5. GrantV (1994) Modes and origins of mechanical and ethological isolation in angiosperms. Proceedings of the National Academy of Sciences of the United States of America 91 : 3–10.
6. StebbinsGL (1970) Adaptive radiation of reproductive characteristics in angiosperms, I: Pollination mechanisms. Annual Review of Ecology and Systematics 1 : 307–326.
7. KiesterAR, LandeR, SchemskeDW (1984) Models of coevolution and speciation in plants and their pollinators. American Naturalist 124 : 220–243.
8. Johnson SD (2006) Pollinator driven speciation in plants. In: L. D Harder, Barrett SCH, editors. Ecology and Evolution of Flowers. Oxford: Oxford Univ. Press. pp. 295–310.
9. WhittallJB, HodgesSA (2007) Pollinator shifts drive increasingly long nectar spurs in columbine flowers. Nature 447 : 706–U712.
10. Waser NM, Campbell DR (2004) Ecological speciation in flowering plants. In: U. Dieckmann, M. Doebeli, M.J. Metz, D. Tautz, editors. Adaptive Speciation. Cambridge, UK: Cambridge Univ. Press. pp. 264–277.
11. MantJ, PeakallR, SchiestlFP (2005) Does selection on floral odor promote differentiation among populations and species of the sexually deceptive orchid genus Ophrys? Evolution 59 : 1449–1463.
12. SchiestlFP, SchlüterPM (2009) Floral isolation, specialized pollination, and pollinator behavior in orchids. Annual Review of Entomology 54 : 425–446.
13. KayKM, SargentRD (2009) The role of animal pollination in plant speciation: integrating ecology, geography, and genetics. Annual Review of Ecology Evolution and Systematics 40 : 637–656.
14. KullenbergB (1961) Studies in Ophrys pollination. Zoologiska Bidrag Uppsala 34 : 1–340.
15. PaulusHF, GackC (1990) Pollinators as prepollinating isolation factors - evolution and speciation in Ophrys (Orchidaceae). Israel Journal of Botany 39 : 43–79.
16. SchiestlFP, AyasseM (2002) Do changes in floral odor cause speciation in sexually deceptive orchids? Plant Systematics and Evolution 234 : 111–119.
17. XuS, SchlüterPM, ScopeceG, BreitkopfH, GrossK, et al. (2011) Floral isolation is the main reproductive barrier among closely related sexually deceptive orchids. Evolution 65 : 2606–2620.
18. SchiestlFP, AyasseM, PaulusHF, LöfstedtC, HanssonBS, et al. (1999) Orchid pollination by sexual swindle. Nature 399 : 421–422.
19. MantJ, BrändliC, VereeckenNJ, SchulzCM, FranckeW, et al. (2005) Cuticular hydrocarbons as sex pheromone of the bee Colletes cunicularius and the key to its mimicry by the sexually deceptive orchid, Ophrys exaltata. Journal of Chemical Ecology 31 : 1765–1787.
20. StöklJ, SchlüterPM, StuessyTF, PaulusHF, FrabergerR, et al. (2009) Speciation in sexually deceptive orchids: pollinator-driven selection maintains discrete odour phenotypes in hybridizing species. Biological Journal of the Linnean Society 98 : 439–451.
21. StöklJ, TweleR, ErdmannDH, FranckeW, AyasseM (2007) Comparison of the flower scent of the sexually deceptive orchid Ophrys iricolor and the female sex pheromone of its pollinator Andrena morio. Chemoecology 17 : 231–233.
22. StöklJ, PaulusH, DafniA, SchulzC, FranckeW, et al. (2005) Pollinator attracting odour signals in sexually deceptive orchids of the Ophrys fusca group. Plant Systematics and Evolution 254 : 105–120.
23. PereraMADN, QinWM, Yandeau-NelsonM, FanL, DixonP, et al. (2010) Biological origins of normal-chain hydrocarbons: a pathway model based on cuticular wax analyses of maize silks. Plant Journal 64 : 618–632.
24. SchlüterPM, XuS, GagliardiniV, WhittleE, ShanklinJ, et al. (2011) Stearoyl-acyl carrier protein desaturases are associated with floral isolation in sexually deceptive orchids. Proceedings of the National Academy of Sciences of the United States of America 108 : 5696–5701.
25. SchlüterPM, SchiestlFP (2008) Molecular mechanisms of floral mimicry in orchids. Trends in Plant Science 13 : 228–235.
26. Post-BeittenmillerD (1996) Biochemistry and molecular biology of wax production in plants. Annual Review of Plant Physiology and Plant Molecular Biology 47 : 405–430.
27. DeveyDS, BatemanRM, FayMF, HawkinsJA (2008) Friends or relatives? Phylogenetics and species delimitation in the controversial European orchid genus Ophrys. Annals of Botany 101 : 385–402.
28. Delforge P (2006) Orchids of Europe, North Africa, and the Middle East. Penent L, Collin C, translator. London: A&C Black. 640 p.
29. XuS, SchlüterPM, SchiestlFP (2012) Pollinator-driven speciation in sexually deceptive orchids. International Journal of Ecology. Article ID 285081
30. VereeckenNJ, SchiestlFP (2009) On the roles of colour and scent in a specialized floral mimicry system. Annals of Botany 104 : 1077–1084.
31. VereeckenNJ, SchiestlFP (2008) The evolution of imperfect floral mimicry. Proceedings of the National Academy of Sciences of the United States of America 105 : 7484–7488.
32. Wyatt T (2003) Pheromones and Animal Behavior: Communication by Smell and Taste. . Oxford, UK: Oxford Univ. Press.
33. ChenZJ, PikaardCS (1997) Epigenetic silencing of RNA polymerase I transcription: a role for DNA methylation and histone modification in nucleolar dominance. Genes & Development 11 : 2124–2136.
34. LeeHS, ChenZJ (2001) Protein-coding genes are epigenetically regulated in Arabidopsis polyploids. Proceedings of the National Academy of Sciences of the United States of America 98 : 6753–6758.
35. Erdmann HD (1996) Identifizierung und Synthese Flüchtiger Signalstoffe aus Insekten und Ihren Wirtspflanzen [Phd Thesis]. Hamburg: Universität Hamburg.
36. Schulz CM (2005) Chemische Kommunikation bei Insekten: Identifizierung und Synthese flüchtiger Inhaltsstoffe [Phd Thesis]. Hamburg: Universität Hamburg.
37. Scotto-LavinoE, DuG, FrohmanMA (2006) Amplification of 5′ end cDNA with ‘new RACE’. Nature Protocols 1 : 3056–3061.
38. Scotto-LavinoE, DuG, FrohmanMA (2006) 3′ end cDNA amplification using classic RACE. Nature Protocols 1 : 2742–2745.
39. ThompsonJD, HigginsDG, GibsonTJ (1994) Clustal-W - improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Research 22 : 4673–4680.
40. TamuraK, DudleyJ, NeiM, KumarS (2007) MEGA4: Molecular evolutionary genetics analysis (MEGA) software version 4.0. Molecular Biology and Evolution 24 : 1596–1599.
41. RonquistF, HuelsenbeckJP (2003) MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19 : 1572–1574.
42. AbramoffMD, MagelhaesPJ, RamSJ (2004) Image processing with ImageJ. Biophotonics International 11 : 36–42.
43. EdgarRC (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research 32 : 1792–1797.
44. Nylander JAA (2004) MrModeltest v2. Program distributed by the author: Evolutionary Biology Centre, Uppsala University.
45. YangZH (2007) PAML 4: Phylogenetic analysis by maximum likelihood. Molecular Biology and Evolution 24 : 1586–1591.
46. RoystonP (1982) An extension of Shapiro and Wilk's W test for normality to large samples. Applied Statistics 31 : 115–124.
47. ExcoffierL, LischerHEL (2010) Arlequin suite ver 3.5: a new series of programs to perform population genetics analyses under Linux and Windows. Molecular Ecology Resources 10 : 564–567.
48. VenablesWN, RipleyBD (2002) Modern Applied Statistics with S: Springer.
49. R Development Core Team (2010) R: A Language and Environment for Statistical Computing. 2.11.0 ed. Vienna, Austria.
Štítky
Genetika Reprodukční medicína
Článek Mutational Signatures of De-Differentiation in Functional Non-Coding Regions of Melanoma GenomesČlánek Rescuing Alu: Recovery of Inserts Shows LINE-1 Preserves Alu Activity through A-Tail ExpansionČlánek Genetics and Regulatory Impact of Alternative Polyadenylation in Human B-Lymphoblastoid CellsČlánek Retrovolution: HIV–Driven Evolution of Cellular Genes and Improvement of Anticancer Drug ActivationČlánek The Mi-2 Chromatin-Remodeling Factor Regulates Higher-Order Chromatin Structure and Cohesin DynamicsČlánek Identification of Human Proteins That Modify Misfolding and Proteotoxicity of Pathogenic Ataxin-1
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 8
-
Všechny články tohoto čísla
- Mutational Signatures of De-Differentiation in Functional Non-Coding Regions of Melanoma Genomes
- Rescuing Alu: Recovery of Inserts Shows LINE-1 Preserves Alu Activity through A-Tail Expansion
- Genetics and Regulatory Impact of Alternative Polyadenylation in Human B-Lymphoblastoid Cells
- Chromosome Territories Meet a Condensin
- It's All in the Timing: Too Much E2F Is a Bad Thing
- Fine-Mapping and Initial Characterization of QT Interval Loci in African Americans
- Genome Patterns of Selection and Introgression of Haplotypes in Natural Populations of the House Mouse ()
- A Combinatorial Amino Acid Code for RNA Recognition by Pentatricopeptide Repeat Proteins
- Advances in Quantitative Trait Analysis in Yeast
- Experimental Evolution of a Novel Sexually Antagonistic Allele
- Variation of Contributes to Dog Breed Skull Diversity
- , a Gene Involved in Axonal Pathfinding, Is Mutated in Patients with Kallmann Syndrome
- A Single Origin for Nymphalid Butterfly Eyespots Followed by Widespread Loss of Associated Gene Expression
- Cryptocephal, the ATF4, Is a Specific Coactivator for Ecdysone Receptor Isoform B2
- Retrovolution: HIV–Driven Evolution of Cellular Genes and Improvement of Anticancer Drug Activation
- The PARN Deadenylase Targets a Discrete Set of mRNAs for Decay and Regulates Cell Motility in Mouse Myoblasts
- A Sexual Ornament in Chickens Is Affected by Pleiotropic Alleles at and , Selected during Domestication
- Use of Allele-Specific FAIRE to Determine Functional Regulatory Polymorphism Using Large-Scale Genotyping Arrays
- Novel Loci for Metabolic Networks and Multi-Tissue Expression Studies Reveal Genes for Atherosclerosis
- The Genetic Basis of Pollinator Adaptation in a Sexually Deceptive Orchid
- Uncovering the Genome-Wide Transcriptional Responses of the Filamentous Fungus to Lignocellulose Using RNA Sequencing
- Inheritance Beyond Plain Heritability: Variance-Controlling Genes in
- The Metabochip, a Custom Genotyping Array for Genetic Studies of Metabolic, Cardiovascular, and Anthropometric Traits
- Reprogramming to Pluripotency Can Conceal Somatic Cell Chromosomal Instability
- Condensin II Promotes the Formation of Chromosome Territories by Inducing Axial Compaction of Polyploid Interphase Chromosomes
- PTEN Negatively Regulates MAPK Signaling during Vulval Development
- A Dynamic Response Regulator Protein Modulates G-Protein–Dependent Polarity in the Bacterium
- Population Genomics of the Facultatively Mutualistic Bacteria and
- Components of a Fanconi-Like Pathway Control Pso2-Independent DNA Interstrand Crosslink Repair in Yeast
- Polysome Profiling in Liver Identifies Dynamic Regulation of Endoplasmic Reticulum Translatome by Obesity and Fasting
- Stromal Liver Kinase B1 [STK11] Signaling Loss Induces Oviductal Adenomas and Endometrial Cancer by Activating Mammalian Target of Rapamycin Complex 1
- Reprogramming of H3K27me3 Is Critical for Acquisition of Pluripotency from Cultured Tissues
- Transgene Induced Co-Suppression during Vegetative Growth in
- Hox and Sex-Determination Genes Control Segment Elimination through EGFR and Activity
- A Quantitative Comparison of the Similarity between Genes and Geography in Worldwide Human Populations
- Minibrain/Dyrk1a Regulates Food Intake through the Sir2-FOXO-sNPF/NPY Pathway in and Mammals
- Comparative Analysis of Regulatory Elements between and by Genome-Wide Transcription Start Site Profiling
- Simple Methods for Generating and Detecting Locus-Specific Mutations Induced with TALENs in the Zebrafish Genome
- S Phase–Coupled E2f1 Destruction Ensures Homeostasis in Proliferating Tissues
- Cell-Nonautonomous Signaling of FOXO/DAF-16 to the Stem Cells of
- The Mi-2 Chromatin-Remodeling Factor Regulates Higher-Order Chromatin Structure and Cohesin Dynamics
- Comparative Analysis of the Genomes of Two Field Isolates of the Rice Blast Fungus
- Role of Mex67-Mtr2 in the Nuclear Export of 40S Pre-Ribosomes
- Genetic Modulation of Lipid Profiles following Lifestyle Modification or Metformin Treatment: The Diabetes Prevention Program
- HAL-2 Promotes Homologous Pairing during Meiosis by Antagonizing Inhibitory Effects of Synaptonemal Complex Precursors
- SLX-1 Is Required for Maintaining Genomic Integrity and Promoting Meiotic Noncrossovers in the Germline
- Phylogenetic and Transcriptomic Analysis of Chemosensory Receptors in a Pair of Divergent Ant Species Reveals Sex-Specific Signatures of Odor Coding
- Reduced Prostasin (CAP1/PRSS8) Activity Eliminates HAI-1 and HAI-2 Deficiency–Associated Developmental Defects by Preventing Matriptase Activation
- Dissecting the Gene Network of Dietary Restriction to Identify Evolutionarily Conserved Pathways and New Functional Genes
- Identification of Human Proteins That Modify Misfolding and Proteotoxicity of Pathogenic Ataxin-1
- and Link Transcription of Phospholipid Biosynthetic Genes to ER Stress and the UPR
- CDK9 and H2B Monoubiquitination: A Well-Choreographed Dance
- Rare Copy Number Variations in Adults with Tetralogy of Fallot Implicate Novel Risk Gene Pathways
- Ccdc94 Protects Cells from Ionizing Radiation by Inhibiting the Expression of
- NOL11, Implicated in the Pathogenesis of North American Indian Childhood Cirrhosis, Is Required for Pre-rRNA Transcription and Processing
- Human Developmental Enhancers Conserved between Deuterostomes and Protostomes
- A Luminal Glycoprotein Drives Dose-Dependent Diameter Expansion of the Hindgut Tube
- Melanophore Migration and Survival during Zebrafish Adult Pigment Stripe Development Require the Immunoglobulin Superfamily Adhesion Molecule Igsf11
- Dynamic Distribution of Linker Histone H1.5 in Cellular Differentiation
- Combining Comparative Proteomics and Molecular Genetics Uncovers Regulators of Synaptic and Axonal Stability and Degeneration
- Chemical Genetics Reveals a Specific Requirement for Cdk2 Activity in the DNA Damage Response and Identifies Nbs1 as a Cdk2 Substrate in Human Cells
- Experimental Relocation of the Mitochondrial Gene to the Nucleus Reveals Forces Underlying Mitochondrial Genome Evolution
- Rates of Gyrase Supercoiling and Transcription Elongation Control Supercoil Density in a Bacterial Chromosome
- Mutations in a P-Type ATPase Gene Cause Axonal Degeneration
- A General G1/S-Phase Cell-Cycle Control Module in the Flowering Plant
- Multiple Roles and Interactions of and in Development of the Respiratory System
- UNC-40/DCC, SAX-3/Robo, and VAB-1/Eph Polarize F-Actin during Embryonic Morphogenesis by Regulating the WAVE/SCAR Actin Nucleation Complex
- Epigenetic Remodeling of Meiotic Crossover Frequency in DNA Methyltransferase Mutants
- Modulating the Strength and Threshold of NOTCH Oncogenic Signals by
- Loss of Axonal Mitochondria Promotes Tau-Mediated Neurodegeneration and Alzheimer's Disease–Related Tau Phosphorylation Via PAR-1
- Acetyl-CoA-Carboxylase Sustains a Fatty Acid–Dependent Remote Signal to Waterproof the Respiratory System
- ATXN2-CAG42 Sequesters PABPC1 into Insolubility and Induces FBXW8 in Cerebellum of Old Ataxic Knock-In Mice
- Cohesin Rings Devoid of Scc3 and Pds5 Maintain Their Stable Association with the DNA
- The MicroRNA Inhibits Calcium Signaling by Targeting the TIR-1/Sarm1 Adaptor Protein to Control Stochastic L/R Neuronal Asymmetry in
- Rapid-Throughput Skeletal Phenotyping of 100 Knockout Mice Identifies 9 New Genes That Determine Bone Strength
- The Genes Define Unique Classes of Two-Partner Secretion and Contact Dependent Growth Inhibition Systems
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Dissecting the Gene Network of Dietary Restriction to Identify Evolutionarily Conserved Pathways and New Functional Genes
- It's All in the Timing: Too Much E2F Is a Bad Thing
- Variation of Contributes to Dog Breed Skull Diversity
- The PARN Deadenylase Targets a Discrete Set of mRNAs for Decay and Regulates Cell Motility in Mouse Myoblasts
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



