-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaComparative Analysis of Regulatory Elements between and by Genome-Wide Transcription Start Site Profiling
Genome-wide transcription start site (TSS) profiles of the enterobacteria Escherichia coli and Klebsiella pneumoniae were experimentally determined through modified 5′ RACE followed by deep sequencing of intact primary mRNA. This identified 3,746 and 3,143 TSSs for E. coli and K. pneumoniae, respectively. Experimentally determined TSSs were then used to define promoter regions and 5′ UTRs upstream of coding genes. Comparative analysis of these regulatory elements revealed the use of multiple TSSs, identical sequence motifs of promoter and Shine-Dalgarno sequence, reflecting conserved gene expression apparatuses between the two species. In both species, over 70% of primary transcripts were expressed from operons having orthologous genes during exponential growth. However, expressed orthologous genes in E. coli and K. pneumoniae showed a strikingly different organization of upstream regulatory regions with only 20% identical promoters with TSSs in both species. Over 40% of promoters had TSSs identified in only one species, despite conserved promoter sequences existing in the other species. 662 conserved promoters having TSSs in both species resulted in the same number of comparable 5′ UTR pairs, and that regulatory element was found to be the most variant region in sequence among promoter, 5′ UTR, and ORF. In K. pneumoniae, 48 sRNAs were predicted and 36 of them were expressed during exponential growth. Among them, 34 orthologous sRNAs between two species were analyzed in depth, and the analysis showed that many sRNAs of K. pneumoniae, including pleiotropic sRNAs such as rprA, arcZ, and sgrS, may work in the same way as in E. coli. These results reveal a new dimension of comparative genomics such that a comparison of two genomes needs to be comprehensive over all levels of genome organization.
Published in the journal: . PLoS Genet 8(8): e32767. doi:10.1371/journal.pgen.1002867
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002867Summary
Genome-wide transcription start site (TSS) profiles of the enterobacteria Escherichia coli and Klebsiella pneumoniae were experimentally determined through modified 5′ RACE followed by deep sequencing of intact primary mRNA. This identified 3,746 and 3,143 TSSs for E. coli and K. pneumoniae, respectively. Experimentally determined TSSs were then used to define promoter regions and 5′ UTRs upstream of coding genes. Comparative analysis of these regulatory elements revealed the use of multiple TSSs, identical sequence motifs of promoter and Shine-Dalgarno sequence, reflecting conserved gene expression apparatuses between the two species. In both species, over 70% of primary transcripts were expressed from operons having orthologous genes during exponential growth. However, expressed orthologous genes in E. coli and K. pneumoniae showed a strikingly different organization of upstream regulatory regions with only 20% identical promoters with TSSs in both species. Over 40% of promoters had TSSs identified in only one species, despite conserved promoter sequences existing in the other species. 662 conserved promoters having TSSs in both species resulted in the same number of comparable 5′ UTR pairs, and that regulatory element was found to be the most variant region in sequence among promoter, 5′ UTR, and ORF. In K. pneumoniae, 48 sRNAs were predicted and 36 of them were expressed during exponential growth. Among them, 34 orthologous sRNAs between two species were analyzed in depth, and the analysis showed that many sRNAs of K. pneumoniae, including pleiotropic sRNAs such as rprA, arcZ, and sgrS, may work in the same way as in E. coli. These results reveal a new dimension of comparative genomics such that a comparison of two genomes needs to be comprehensive over all levels of genome organization.
Introduction
Escherichia coli K-12 MG1655 and Klebsiella pneumoniae MGH78578 belong to the same enteric family of bacteria of the class gammaproteobacteria. While E. coli K-12 MG1655 represents an extensively studied laboratory strain that is not known to be pathogenic, K. pneumoniae MGH78578 is a well-known pathogenic strain isolated from a patient with pneumonia [1]. There have been many comparative genomics approaches used to understand the similarities of closely related species in a wide range of genera such as Escherichia, Klebsiella, Salmonella, and Listeria [2], [3], [4], [5], [6]. These comparative genomics studies have mostly focused on comparing the gene contents, either shared or specific for each genome. However, it is also important to investigate the similarities and differences in non-coding regulatory elements including promoter, 5′ untranslated region (5′ UTR), and small RNA (sRNA), due to their influence on transcriptional and post-transcriptional processes.
The transcription start site (TSS) is where transcription begins and is the +1 position of the 5′ untranslated region (5′ UTR) of mRNA. The promoter, which governs the ability to initiate transcription and control the expression of genes, is directly upstream of the TSS. The identification of promoter elements in DNA by computational methods depends on the statistical analysis of consensus sequences as overrepresented regions [7], [8]. Regulatory sequence elements have been studied by computational methods based on the genomic sequence of the non-coding upstream region [9], [10], [11], however those sequence elements in promoters are short and not fully conserved in the sequence. Thus, there is a high probability of finding similar sequence elements outside the promoter regions. In the case of the TSS, the region is not overrepresented enough by any consensus sequences and is thus difficult to predict by computational efforts. However, when the TSS is known, the DNA region most likely to contain regulatory binding sites is circumscribed, and the effectiveness of searching sequence motifs of interest is greatly enhanced [12]. Thus, determining the precise locations of TSSs by experimental methods is necessary to accurately annotate the promoter region and the untranslated region. Knowledge of the 5′ UTR region is important for studying the sequence and structure of the 5′ end of mRNA (which is associated with transcription regulation, mRNA transcript stability, and translational efficiency) because translational efficiency in bacteria is often controlled by RNA-binding proteins, noncoding regulatory RNAs, endoRNases, the 30S subunit of ribosome, and structural rearrangements within 5′ UTR [13].
Genome-wide identification of TSSs with the aid of deep sequencing has allowed researchers to reveal a landscape of TSSs across the whole genome in many microorganisms, including E. coli [14], [15], [16], H. pylori [17], G. sulfurreducens [18], and other species [12], [19]. In these studies, experimental TSS datasets were used to understand the transcription architecture, to appreciate the complexity of genomic structure, and to analyze regulatory elements for each species. Comparison of regulatory elements, which can be addressed by experimentally determined TSSs under the same growth condition, is expected to elucidate any regulatory similarities or differences, based not only on the genomic sequence, but also on the transcriptional context of compared species as well.
Here, we carried out the genome-wide TSS profiling experiments for E. coli K-12 MG1655 and K. pneumoniae MGH78578 to accurately determine the boundaries in the regulatory regions between the promoter region and the 5′ UTR. The upstream regulatory regions between those two closely related species were then compared to investigate whether those regions are conserved and organized in similar manners. In addition, we used the TSS dataset to identify sRNAs in K. pneumoniae, because very little is known about them. We then compared the K. pneumoniae sRNAs to orthologous sRNAs in E. coli, in terms of sequence conservation and their target sites. The range of sequence conservation or diversion between non-coding regulatory elements in interspecies microorganisms could lead to insights about regulatory features that may also play similar roles in the respective species.
Results
Experimental identification of TSSs in E. coli and K. pneumoniae
Primary mRNA transcripts in prokaryotes are triphosphorylated at the 5′ ends. We isolated total RNA from E. coli and K. pneumoniae cells growing in mid-exponential phase, and enriched primary mRNAs by removing any monophosphorylated ribosomal 23S, 16S rRNA, tRNA, and any degraded mRNAs by treatment with terminator exonuclease [17], [18]. By using a modified 5′RACE (rapid amplification of cDNA ends) followed by deep sequencing as described in [18], libraries were prepared and sequenced to determine potential TSSs for each strain of E. coli K-12 MG1655 and K. pneumoniae MGH78578. These TSS libraries yielded >11.6 million and >2.4 million sequence reads for E. coli and K. pneumoniae, respectively. 15.70% and 19.60% of those sequence reads were uniquely mapped with 36 bp read length onto the E. coli and K. pneumoniae reference genomes respectively. Unique sequence reads that perfectly matched the respective genome sequence were mapped to annotate a total of 3,746 and 3,143 TSSs for the E. coli K-12 MG1655 and K. pneumoniae MGH78578 genome, respectively (Table S1). The average number of TSS reads of E. coli and K. pneumoniae TSSs was 107.8 and 78.5, respectively. The lower number of identified TSSs for K. pneumoniae could be due to a lesser number of sequence reads, and this factor was taken into account in further analysis.
To verify the quality of the TSS data, we compared our experimental E. coli TSS data with previously published E. coli TSS datasets [15], [16]. There is no public genome-wide TSS dataset available for K. pneumoniae, which is why only TSS data for E. coli was used for this analysis. In RegulonDB, there are 1258 upstream sense TSSs annotated for E. coli, generated by 5′ triphosphate enrichment method. 624 (49.6%) TSSs out of 1258 matched exactly with TSSs of this study, and 257 (20.4%) TSSs matched within 3 bp tolerance. Thus, 70.0% of known TSSs from RegulonDB agreed with the TSSs from our study. From the TSS dataset generated without 5′ triphosphate enrichment method, 3661 TSSs were reported for the exponential growth condition. 1603 (43.8%) TSSs matched exactly with TSSs of this study, and 527 (14.4%) TSSs matched within 3 bp tolerance. In sum, 58.2% of TSSs were found in TSSs of this study. A comparison of our TSS dataset with two other datasets suggested TSS datasets generated by a similar method were in better agreement, and E. coli TSSs determined by an independent experiment were matched by TSSs used in this study.
A genome-wide TSS landscape of E. coli and K. pneumoniae was built by assigning TSSs to the nearest downstream gene including ORFs and sRNAs, but excluding TSSs located beyond 700 bp from the translation start site of the closest ORF in a strand specific manner (Fig. 1A). In E. coli, TSSs were assigned to 2654 genes, while TSSs in K. pneumoniae were assigned to 2301 genes (2175 genes in the main chromosome, and 126 genes in the plasmids).
Fig. 1. Experimentally determined TSSs and their association with annotated genes. 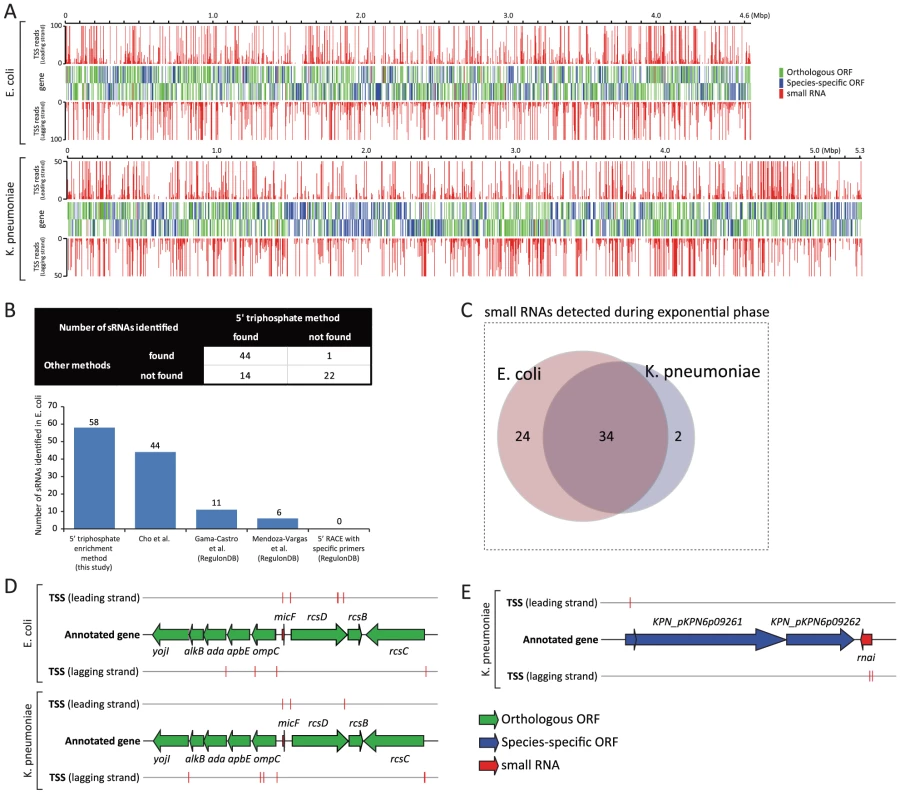
(A) Genome-wide TSS mapped onto E. coli and K. pneumoniae genome annotation. (B) Number of E. coli sRNAs detected with 5 TSS datasets generated by different methods. (C) Number of sRNAs detected from E. coli and K. pneumoniae during the exponential growth. (D) Schematic drawing of annotated TSSs assigned to orthologous micF sRNA and coding genes surrounding micF in E. coli and K. pneumoniae. (E) Schematic drawing of annotated TSSs assigned to K. pneumoniae sRNA, rnai, and coding genes near rnai. Identification of small RNAs in K. pneumoniae
While over 80 sRNAs have been identified and experimentally verified in E. coli, very little is known about K. pneumoniae sRNAs. Identifying the occurrence of sRNAs and determining their boundaries in a genome-wide manner is challenging, especially for less studied organisms, because sRNAs generally have no clear-cut signatures unlike protein-coding genes, which are specified by a genetic code. In order to overcome limitations of previous experimental approaches, and to interrogate sRNAs in a genome-wide manner, a deep-sequencing approach was applied and proved successful [20]. Before investigating the possible presence of sRNAs in K. pneumoniae, E. coli TSS datasets were analyzed to assess how many currently annotated sRNAs in E. coli could be identified under the experimented condition, and how well TSS signals were matched with 5′ ends of those sRNAs. In addition, TSS datasets generated with the 5′ triphosphate enrichment method in this study were compared to four other TSS datasets generated by different methods [14], [15], [16] in the light of using 5′ triphosphate enrichment. Many sRNAs are subjected to post-transcriptional processing, however, which results in an accumulation of shorter products with 5′ monophosphate [21], [22], [23]. Therefore, only unprocessed sRNAs or precursor transcripts of sRNAs, which have 5′ triphosphate and can be detected by this method, were analyzed.
Of 81 annotated sRNAs in E. coli, 58 (71.6%) had corresponding TSSs, and were thus considered to be expressed during exponential growth. Expression profiling data taken from [16] also supported the expression of those sRNAs under the experimented condition, although rprA showed no significant expression according to that data (Figure S1). This could be because E. coli RprA transcript is subject to specific endoribonuclease cleavage [21], resulting in the accumulation of processed shorter form, which is not long enough to be detected by the tiling array. TSS signals were well matched with the 5′ ends of unprocessed or precursor transcripts of 58 sRNAs including rprA (Fig. 1B, Table S2). In comparison, TSS datasets generated by deep-sequencing without 5′ triphosphate enrichment [16] presented TSS signals for 44 sRNAs (54.3%). Three other TSS datasets, generated by other methods [14], [15], were obtained from RegulonDB database (http://regulondb.ccg.unam.mx/). They showed TSSs assigned to 11 (13.6%), 6 (7.4%), and 0 (0%) sRNAs for each method (Fig. 1B). Thus, experimental TSS generated by deep sequencing is a practical indicator that shows the occurrences of sRNAs in E. coli and determines the genomic positions of their 5′ ends. Additionally, our TSS dataset detected the largest number of annotated sRNAs in E. coli, compared to previous methods. We believe this result shows that the TSS dataset for K. pneumoniae, generated with the same method, can be used to detect possible sRNAs in that species and to determine the 5′ ends of those sRNA candidates.
In order to identify and confirm the occurrence of sRNAs in K. pneumoniae by experimentally determined TSSs, tentative sRNA candidates should first be predicted by computational methods. A number of computational algorithms have been developed over the last decade for the purpose of predicting sRNAs in bacterial genomes, and primary sequence conservation in closely related species is one of the most useful data types for predicting whether a genomic sequence corresponds to an sRNA [24]. Since a majority of E. coli annotated coding genes (63.7%) have homologs in the K. pneumoniae genome, and conserved sRNAs are frequently identified adjacent to conserved coding genes in other organisms, we looked up the closest orthologous ORFs to annotated sRNAs of E. coli, and then searched tentative sRNA sequences in K. pneumoniae genomic sequences bound to those neighboring orthologous genes. For example, in E. coli, micF sRNA is surrounded by ompC and rcsD, both of which are conserved coding genes between the two species. The K. pneumoniae genomic sequence bound to ompC and rcsD orthologous ORFs was used for searching the genomic sequence of micF by sequence alignment (Fig. 1D, detailed method described in Materials and Methods section). This approach was supplemented by running Infernal algorithm [25] with sRNA models from the Rfam database 10.1 (http://rfam.sanger.ac.uk/). Using this combined approach, we identified 48 tentative sRNAs in the K. pneumoniae genome, and 36 of them were expressed by associated TSSs (Fig. 1C, Table S3). Expression of those sRNAs was also supported with expression profiling data, with the one exception being rprA (Figure S1). rprA of K. pneumoniae showed no significant level of transcription according to the expression profiling data, however rprA had an assigned TSS with 1865 reads, which was also observed in E. coli rprA with an assigned TSS of 3012 reads. This indicates a possibility of post-transcriptional processing of K. pneumoniae RprA transcript as is the case in E. coli. 47 of 48 putative sRNAs were located in the main chromosome (NC_009648) of K. pneumoniae, while one sRNA, rnai, was found in the plasmid (NC_009652) (Fig. 1E).
Of 36 small RNAs detected during the exponential phase in K. pneumoniae, 34 had orthologous sRNAs in E. coli, leaving 2 non-orthologous sRNAs, rnai, and ryhB-2 (Figure S1, Figure S2 and Figure S3A). Their expression was supported by TSS and expression profiling (Figure S3A). ryhB-2 was so-named because another orthologous ryhB sRNA was identified in a position between orthologous ORFs yhhX and yhhY. rnai non-coding RNA is an antisense repressor of the replication of some E. coli plasmids [26]. While E. coli K-12 MG1655 does not have any plasmid, K. pneumoniae MGH78578 has 5 plasmids, one of which (NC_009652) contains rnai sRNA.
Similar usage of regulatory features
The majority of E. coli annotated genes, 1945 (73.5%), were annotated with a single TSS, and the remaining 26.5% had multiple TSSs mainly ranging from 2 to 7, allowing alternative transcripts (Fig. 2A). Similar to the complex organization of promoter regions and usage of multiple TSSs shown in E. coli, 534 (22.8%) of K. pneumoniae annotated genes had multiple TSSs, leaving a large fraction of genes, 1802 (77.2%), which were assigned to a single TSS (Fig. 2A).
Fig. 2. TSS annotation and structure of promoter region and 5′ UTR. 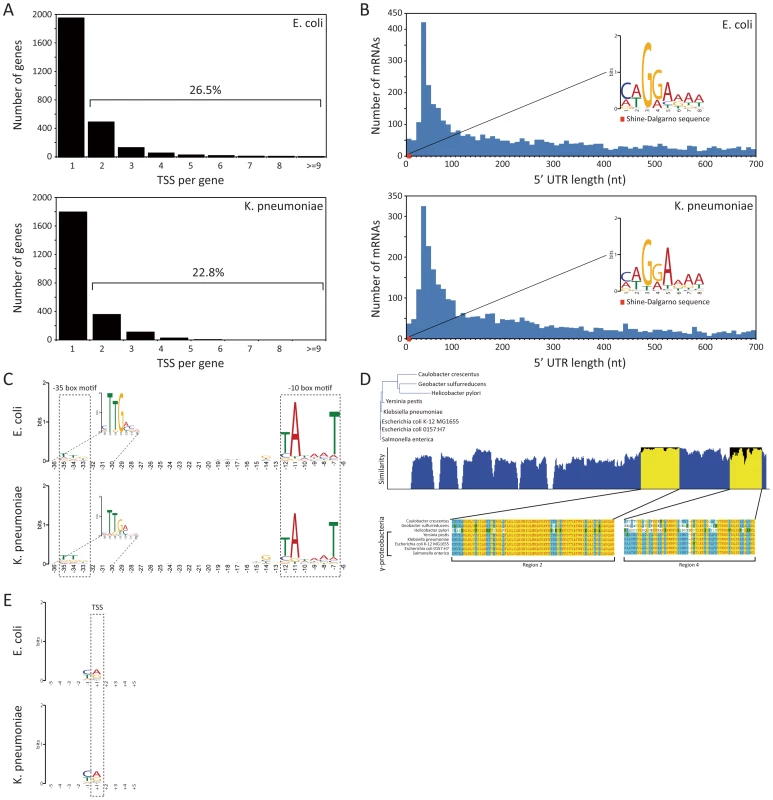
(A) Number of TSSs assigned per annotated genes. (B) Distribution of 5′ UTR lengths for E. coli and K. pneumoniae, and the Shine-Dalgarno sequence motif. (C) Sequence motif of promoter region containing −10 and −35 boxes. (D) Conservation of RpoD amino acid sequences of 5 species in gammaproteobacteria and 3 other species belonging to proteobacteria. (E) Di-nucleotide preference near the TSS site. In order to investigate other regulatory features shared by E. coli and K. pneumoniae, the length distribution of the 5′ UTR bounded by experimental TSS and translational start site was calculated, and possible sequence motifs were examined with the MEME motif search algorithm [27]. The length of the 5′ UTR ranged from 0 to 700 nucleotides, with the most abundant length found to be between 25 to 35 bp for both bacterial species (Fig. 2B). For 18 genes from E. coli and 10 genes from K. pneumoniae, leaderless mRNAs with the TSSs corresponding exactly to the start codon were found. The leaderless mRNAs encoded proteins of various functions (Table S4).
Experimentally determined TSSs in E. coli and K. pneumoniae were used to detect the Shine-Dalgarno (SD) sequence of the ribosome binding site (RBS). Expecting to find that motif within the boundaries of the 5′ UTR, which are defined by the TSS and translation start site of the downstream ORF, we took sequences from 5′ UTR regions in E. coli and K. pneumoniae and searched for consensus motifs. A conserved caGGaaaa sequence motif (lower-case characters indicate an information content <1 bit) was found in E. coli, and an identical conserved caGGaaaa motif was also found in the 5′ UTR of K. pneumoniae. The most dominant distance between the SD sequence motif and translational start site was 6 nucleotides in both species. Motif logos for both species are illustrated in Fig. 2B.
Bacterial promoters usually contain specific sequences, which RNA polymerase-associated sigma factors can recognize and to which they can bind. For example, the E. coli housekeeping sigma factor σ70 (rpoD, b3067) is known to recognize −10 (TATAAT) and −35 (TTGACA) boxes [28]. Although sequence motifs of major E. coli sigma factors have been investigated by experimental and computational approaches, less is known for K. pneumoniae sigma factors and their binding motifs. E. coli and K. pneumoniae are closely related, and they share major sigma factors, such as rpoD, rpoS, rpoH, rpoN, and rpoE with a high level of amino acid sequence conservation over 95%, with the exception of rpoN that has 89.8% amino acid sequence similarity (Table S5). Since sigma factor σ70 is housekeeping during exponential growth in E. coli and presumably in other gammaproteobacteria including K. pneumoniae as well, conservation of subregions 2 and 4 of bacterial sigma factor σ70, which are known to recognize the −10 and −35 boxes, can give insights toward understanding the promoter structure of K. pneumoniae. Thus, amino acid sequences of rpoD of 5 strains belonging to gammaproteobacteria and 3 strains belonging to other classes were aligned and analyzed (Fig. 2D). Notably, region 2, which recognizes the −10 box, was perfectly conserved among species in gammaproteobacteria, and region 4, which recognizes the −35 box, was almost conserved as well. Since the conservation of sigma factor σ70 subregions recognizing sequence motifs in the promoter and the expression of housekeeping rpoD in E. coli and K. pneumoniae was confirmed with the TSS dataset and expression profile, it is likely that the promoter structure of those species are identical. Thus, TSSs in E. coli and K. pneumoniae identified in this study were used to find sequence motifs of the promoter region, which includes the −10 and −35 boxes, in order to see whether two closely related bacteria share similar or identical promoter sequence motifs. We extracted 50 bp long sequences directly upstream of the TSSs, which are long enough to cover the −10 and −35 boxes, and ran the MEME motif search algorithm. As a result, the consensus sequence of the extended Pribnow box motif (tgnTAtaaT) including the −10 box was obtained, and the −35 box sequence motif (cTTgaca) was also found, as expected (Fig. 2C). Moreover, the most dominant distances between the −10 box and TSS and between the −10 and −35 boxes were also the same in both bacteria. Although the sequence motif obtained herein is based on genome-wide TSS profiles generated only under exponential growth and other sigma factors having different binding sequence motifs may play a minor role in transcription regulation under the experimented condition, overrepresented sequence motifs of promoter regions in E. coli are in accordance with prior knowledge, and the two species in this study showed identical sequence motifs of the promoter. Thus, these closely related species seem to share identical promoter structures, reflecting a high conservation of major sigma factors.
Previous studies have shown evidence of a purine (A/G) preference at the TSS in E. coli [29]. Here, we investigated if the experimentally derived TSS data provide insights into any such nucleotide preference at the TSS. Thus, nucleotide preferences from −5 to +5 sites surrounding the TSSs for E. coli and K. pneumoniae were calculated. The current experimentally derived TSSs in both species also showed a significant dinucleotide preference at the +1 TSS and −1 site (Fig. 2E). In E. coli, 78.6% of the TSSs were represented by purine base (45.2% A and 33.4% G) at the TSS. Similarly, 79.4% of K. pneumoniae TSSs presented the purine base (48.0% A and 31.4% G) at that site. Interestingly, another nucleotide preference at the −1 site, the nucleotide before the TSS and the last nucleotide that is not transcribed, was observed in both species. In E. coli, 80.2% showed the pyrimidine base (35.4% T and 44.8% C) preference at the −1 site. Likewise, in K. pneumoniae, 81.5% of cases also showed the pyrimidine base (31.0% T and 50.5% C) at the −1 site. Flanking regions ranging from +2 to +5 sites and −2 to −5 sites showed no significant nucleotide preference (Fig. 2E). Thus, both species showed the purine preference at the +1 TSS and the pyrimidine preference at the −1 site. In accordance with this observation, H. pylori, which belongs to a different class of alphaproteobacteria, also showed purine preference at the TSS (66.0% A or G) and pyrimidine preference at the −1 site (68.3% T or C) [17]. Similar to the dinucleotide sequence preference at +1 and −1 sites found in bacteria, transcription from the S. cerevisiae promoter [30] and the mammalian [31] promoter preferentially starts with a purine at position +1, having a preference for pyrimidine at position −1.
These results suggest that E. coli and K. pneumoniae share many regulatory features at the transcriptional and translational level. They have a conserved promoter structure reflecting preserved sigma factors, use multiple TSSs that extensively increase transcriptome complexity by resulting in alternative transcripts, and show dinucleotide preference near the TSS position. In addition to this similarity in transcriptional features, E. coli and K. pneumoniae exhibit conserved Shine-Dalgarno sequence motifs, the same distance from Shine-Dalgarno motif to translation start site, and 5′ UTR length distribution, suggesting similarity in regulatory features of translation.
Different organization of the upstream regulatory region
While E. coli and K. pneumoniae share several regulatory features, it is still unknown whether the two species use them to regulate gene expression in the same manner. Thus, we analyzed the usage of regulatory elements upstream of orthologous genes between two strains in order to investigate whether those conserved genes are regulated in a similar or different manner. The orthologous genes present in E. coli and K. pneumoniae were selected by reciprocal alignments using a threshold of 50% amino acid sequence similarity and 50% alignment length between the encoded proteins, resulting in a set of 2,876 orthologs (Fig. 3A, Table S5). 2962 (79.1%) E. coli TSSs were assigned to orthologous genes defined herein, and in K. pneumoniae, 2317 (73.1%) of TSSs were assigned to orthologous genes. Considering 63.7% (2876 out of 4513) of genes in E. coli and 54.2% (2876 out of 5305) of genes in K. pneumoniae were orthologous, detection of over 79.1% of TSSs in E. coli and 73.1% in K. pneumoniae assigned to orthologous coding genes implies over 73% of primary transcripts were expressed from operons or transcription units having orthologous genes at the first position. In E. coli, the average number of genes in an operon is about 1.5 [16], and operons containing orthologous genes in E. coli have a tendency to keep their sequential position in K. pneumoniae, suggesting possible conservation of operon structures. This result suggests that the majority of primary transcripts were expressed from operons containing conserved orthologous genes during exponential growth in both species. Thus, further analysis of regulatory regions upstream of orthologous genes with genome-wide TSSs covers a majority of expressed gene contents under the experimented condition.
Fig. 3. Different organization of upstream regulatory region between E. coli and K. pneumoniae. 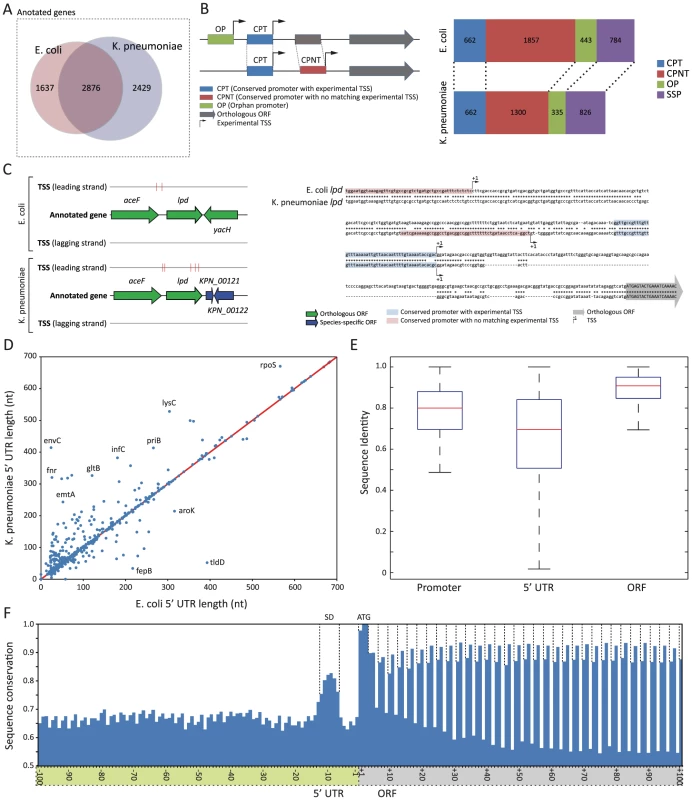
(A) Venn diagram showing orthologous genes and species-specific genes between E. coli and K. pneumoniae. (B) 4 different types of promoter regions, and their numbers identified in two species. (C) Schematic drawing of annotated TSSs and sequence comparison of regulatory region upstream of lpd. (D) Length difference between the pairs of comparable 5′ UTR. (E) Comparison of sequence conservation of promoter, 5′ UTR, and ORF regions. (F) Sequence conservation of genomic regions surrounding translation start sites. Despite the fact that orthologous genes were used to express the majority of primary transcripts during exponential growth, regulatory regions upstream of those conserved coding genes were organized in a different manner with multiple TSSs (Fig. 3B). In order to perform a detailed investigation comparing promoter regions between two species, each TSS was used to define a promoter region. A promoter region was defined as 50 bp long nucleotide sequences upstream of each TSS, which was long enough to include most of the regulatory elements identified, including the −1 site, −10 box, and −35 box, but not too long to exclude unnecessary sequences. Then, the promoter region was categorized into one of four groups, based on sequence conservation of the promoter region and presence of an experimental TSS: conserved promoter region with TSS (CPT), conserved promoter region with no matching TSS (CPNT), orphan promoter region (OP), or species-specific promoter (SSP). CPT was defined as a promoter region with a conserved sequence in both strains with a matching experimental TSS, and was used to define the promoter region and 5′ UTR, which were comparable between the two species. CPNT was defined as a promoter region with a conserved sequence in both strains, however with experimentally determined TSSs in only one species. Similarly, OP was defined as a promoter region with no conserved sequence between E. coli and K. pneumoniae, and with experimental TSSs in only one species. SSP was defined as a promoter region upstream of non-orthologous genes. (More details described in Materials and Methods section)
If the sequence of regulatory regions upstream of orthologous coding genes is also conserved, then conserved promoter (CPT) should be the most frequent type of promoter region. However, an exhaustive comparison of promoter regions resulted in only 662 conserved promoters (CPT) between E. coli and K. pneumoniae, which covered 17.7% of TSSs and corresponding promoter regions in E. coli and 21.1% in K. pneumoniae. An unexpectedly small portion of conserved promoter regions with matching TSSs in two species under the exponential growth supports a different organization of regulatory regions containing multiple TSSs and their associated promoters between those two closely related species. Interestingly, in both species, the promoter type with the largest number was the conserved promoter with no matching TSS (CNPT). In E. coli, 49.6% of TSSs were associated with promoters with conserved sequence, and no matching TSSs were found upstream of corresponding orthologous genes in K. pneumoniae. Similarly, 41.3% of TSSs of K. pneumoniae were associated with that type of promoter. A smaller number of TSSs was detected in K. pneumoniae versus E. coli, despite K. pneumoniae having the larger genome. This was possibly due to fewer raw reads being obtained from the K. pneumoniae TSS library. Thus, it is arguable that the portion of conserved promoters with matching TSSs could increase as the coverage of TSS reads goes up. However, over 40% of promoters with conserved sequences had TSSs in one species, but had no matching TSS in the other species. Thus, the regulatory regions upstream of orthologous genes are organized in a different manner, despite a large portion of promoters having conserved sequences between two species. This suggests different sets of TSSs are used to express those orthologous genes. For example, lpd had two experimental TSSs in E. coli and K. pneumoniae (Fig. 3C). Proximal TSSs were matching, and had a highly conserved promoter sequence. Distal TSSs of lpd had conserved sequences, but were in different locations. Moreover, promoters with no conserved sequence and TSS in one species (OP, orphan promoter) also support that interpretation. Thus, while two closely related species may share identical transcriptional machineries including sigma factors and RNA polymerase, upstream regulatory regions are organized differently, so that even conserved genes can be regulated differently, and in many cases mRNA transcripts from orthologous genes can have different 5′ UTRs, which may have disparate regulatory elements in that region.
To investigate similarities and differences in 5′ UTR regions, their length and sequences were defined by 662 comparable conserved promoter regions with TSSs in both species, as shown in Fig. 3D and Fig. 3E. The length comparison of the 5′ UTR between E. coli and K. pneumoniae showed a strong correlation (R2 value of 0.877), and 169 (25.5%) 5′ UTR regions had exactly the same length. However, in general, the K. pneumoniae 5′ UTR was longer than that of E. coli, reflecting the bigger size of the genome (Fig. 3D). For example, the 5′ UTR length of rpoS, which is one of the orthologous genes, was 566 in E. coli, while the length of the K. pneumoniae rpoS was 670. To investigate the sequence conservation between those comparable 5′ UTR regions, sequences of 5′ UTR regions from two species were aligned and percentage sequence identity for each 5′ UTR pair was calculated. The sequence variation of the 5′ UTR region along with the percentage identity of corresponding promoter and ORF is shown in Fig. 3E. Consequently, ORF sequence was found to be the most conserved element, followed by sequence of promoter regions and sequence of the 5′ UTR region as the most diverse regulatory element among them. The averages of sequence identity of orthologous ORFs, comparable conserved promoters, and their 5′ UTR were 88.9%, 79.0%, and 66.0%, respectively. In order to calculate the level of conservation of the regions surrounding translation start site of orthologous genes, sequences of 200 bp long regions around translation start sites were aligned for orthologous genes having clearly aligned translation start sites between E. coli and K. pneumoniae (Fig. 3F). In the 5′ UTR, there was a relatively more conserved regions 6 bp upstream of the translation start site. This region was considered to be the Shine-Dalgarno sequence of the ribosome binding site because in both species the most dominant distance between the Shine-Dalgarno sequence motif and translation start site was 6 nucleotides. In the coding region, the first codon, frequently ATG, was most conserved with slightly less conservation of the first nucleotide of the first codon. This was because the start codon, ATG, was replaced with GTG or TTG in some orthologs. In agreement with the wobble theory [32], the third nucleotide of each codon was least conserved. Interestingly, however, the second nucleotide was more conserved than the first in every codon analyzed. This might be because conservation of the second nucleotide can contribute to preserving the same amino acids like leucine, or amino acids with a similar property. Accordingly, codon analysis of the coding sequence between orthologous genes of the two species suggested that the majority of substitutions in the first nucleotide of the codon resulted in either keeping leucine or changing amino acids having similar properties, such as leucine/isoleucine, leucine/valine, valine/isoleucine, serine/threonine, glutamine/glutamic acid, or asparagine/aspartic acid.
In addition to species-specific gene content, E. coli and K. pneumoniae also exhibited differences in the organization of regulatory regions upstream of conserved orthologous genes. Different usage of TSSs and their promoter regions can contribute to varied regulation of genes downstream of those promoters, resulting in transcripts with different 5′ UTR. Moreover, both species extensively use multiple TSSs, which increase the complexity and diverse nature of regulatory regions. Thus, E. coli and K. pneumoniae, which are closely related, have regulatory regions of orthologous genes organized in a different manner.
Comparison of regulatory non-coding small RNAs
The investigation of regulatory features of coding genes based on genome-wide TSSs and their comparison between two closely related enterobacteria showed that the two species share almost identical regulatory features. However, they deploy those regulatory features upstream of conserved or orthologous coding genes in a different manner, suggesting a variation of transcriptional regulation by using multiple TSSs and post-transcriptional regulation by having different 5′ UTRs, generated from a different set of TSSs. Since small regulatory RNAs can function in post-transcriptional control of gene expression in many processes including stress responses, metabolic reactions, and pathogenesis [33], [34], and identification of sRNAs in K. pneumoniae resulted in 34 orthologous sRNA pairs between two species, we compared sequences of those conserved sRNAs and investigated whether they would regulate their target genes in the same manner. This was done, similarly in previous studies [23], [35], [36].
The conserved RNA-binding protein Hfq, first discovered in E. coli, is a pleiotropic regulator that modulates the stability or the translation of an increasing number of mRNAs [37], [38]. Thus, knowledge of hfq in K. pneumoniae is preliminary in terms of analyzing and comparing sRNAs between two species. Similar to E. coli and other K. pneumoniae strains [39], [40], hfq of K. pneumoniae MGH78578 (KPN_04570), existed between conserved miaA and hflX in the genome. E. coli K-12 MG1655 hfq (b4172) and K. pneumoniae MGH78578 hfq (KPN_04570) had one TSS detected upstream of hfq and in the coding region of miaA, with the genomic position of 4,397,824 and 5,000,510, respectively (Fig. 4A). Similar to the high level of sequence conservation of the hfq ORF, sequences of promoter regions defined by experimental TSSs were perfectly conserved. 5′ UTR sequences were also highly conserved; preserving sequences for the Shine-Dalgarno sequence of the ribosome binding site 6 bp upstream of translation start sites in both species (Fig. 4B). This result supports the existence of a sequence of K. pneumoniae hfq ORF in the genome and is expressed with matching TSSs. Furthermore, sequence conservation of the promoter region and 5′ UTR indicates they could be regulated in a similar way, at least during the experimented condition.
Fig. 4. Comparison analysis of orthologous sRNAs. 
(A) Expression of RNA-binding protein hfq (B) Sequence conservation of regulatory region upstream of hfq ORF, including promoter, TSS and 5′ UTR. (C) Conservation and expression of non-coding regulatory sRNAs, rprA, arcZ and sgrS. (D) Sequence comparison analysis of rprA and arcZ regulating translation of rpoS. (E) Sequence comparison analysis of sgrS regulating translation of ptsG and manX. With the occurrence of hfq in both species, we further investigated expression of orthologous sRNAs, compared their sequence, and analyzed possible working mechanisms in K. pneumoniae with prior knowledge of those sRNAs from E. coli. 34 orthologous sRNA candidates were confirmed to be expressed during exponential phase by TSS signals matching their 5′ ends. Their expression was also supported by expression profiling data (Figure S1 and Figure S2). However, those 34 expressed orthologous sRNAs showed different degrees of sequence conservation levels, ranging from 47.3% to 98.8% with an average of 83.1%. rybB has the most conserved sequence, whereas sroH has the least. Essentially, no sRNAs of K. pneumoniae had perfect sequence conservation compared to those of E. coli, which raised the question as to whether K. pneumoniae sRNA would work in a similar way as the E. coli sRNA. Thus, we compiled known target sites of E. coli sRNAs from the EcoCyc database [41], and mapped them onto the corresponding genomic sequence of K. pneumoniae (Table S6, Fig. 4D, Fig. 4E).
3 sRNAs, rprA, arcZ, and dsrA were known to regulate the expression of rpoS by making base paring in the middle region of 5′ UTR of rpoS mRNA with the aid of Hfq protein [42], [43], [44]. rprA and arcZ in E. coli target and bind to the same region of 5′ UTR of rpoS. Thus, based on the fact that those two sRNAs are expressed in K. pneumoniae, if the sequence of the target site in rpoS mRNA and the sequence of the corresponding region of sRNA which binds to that target site are conserved, then one can hypothesize that rprA and arcZ sRNAs of K. pneumoniae would regulate the expression of rpoS in a similar manner as in E. coli. As expected, rprA and arcZ of K. pneumoniae were expressed during exponential growth with TSSs, which match the TSSs of E. coli's rprA and arcZ (Fig. 4C). Furthermore, regions that bind to the target site of rpoS were also conserved (Fig. 4D). Analogously, rpoS of E. coli and K. pneumoniae was expressed with TSS at 2,866,139 in E. coli and 3,401,901 in K. pneumoniae, and the sequence of the promoter region of rpoS was highly conserved between the two species, although the 5′ UTR defined by those TSSs showed significantly different lengths with a long nucleotide addition in the 5′ UTR of K. pneumoniae rpoS. However, the sequence of the target site of rprA and arcZ was almost perfectly conserved with one nucleotide replacement (Fig. 4D). Considering that rpoS was expressed from conserved promoters in both species, and rprA and arcZ targeting the conserved regions of the 5′ UTR of rpoS transcript were also conserved and expressed, it is quite likely those sRNAs in K. pneumoniae regulate the expression of rpoS in the same manner as in E. coli.
Furthering the analysis of rpoS-targeting rprA and arcZ, another sRNA sgrS was similarly analyzed. In E. coli, sgrS was shown to regulate expression of two metabolic transporters, ptsG and manX, by base-pairing dependent manner [45], [46]. Although sgrS sRNA of E. coli and K. pneumoniae was expressed during exponential phase (Fig. 4C), the sequence conservation was quite low at 56.3%. ptsG and manX, which are targeted and regulated by sgrS in E. coli, were also expressed in K. pneumoniae. However, E. coli and K. pneumoniae ptsG was expressed by different promoters, resulting in ptsG transcripts with different 5′ UTR, while manX was expressed by a conserved promoter with matching TSSs. Although the overall conservation level of sgrS between E. coli and K. pneumoniae was quite low, the region, which is known to bind to ptsG and manX in E. coli, was highly conserved (Fig. 4E). Besides its target sites in ptsG and manX transcripts were also highly conserved, which suggests sgrS would regulate the expression of ptsG and manX in a similar way as in E. coli.
Comparisons of sRNAs and their working mechanisms should be performed not only with just the sequence of sRNAs and their target sites, but also with the working context, including expression of those sRNAs, transcripts of genes containing target sites, and occurrence of Hfq, if the sRNA requires the protein. In depth comparisons of sRNAs and their working context suggest that many of the orthologous sRNAs identified with the TSS dataset of this study could work in a similar way as in E. coli since target sequences of those sRNAs and sequences of sRNAs known to bind to the target sites are conserved and exist in the primary transcript of target genes under the given condition. Moreover, high conservation of regions that bind to the target sites despite poor conservation of whole sRNA sequences suggests that sequence comparison and conservation can determine which region may be more important in terms of regulation and their working context.
Discussion
E. coli K-12 MG1655 is an extensively studied laboratory strain with a wealth of genome-wide studies. As such, genome-wide TSS determination of the E. coli genome with single base pair resolution by deep sequencing has been performed by a number of studies [14], [15], [16]. However, K. pneumoniae has only recently been studied using genome-wide approaches [3]. A number of TSSs have been reported and investigated with specific focus on genes involved mostly in virulence [47], [48], [49], [50], [51], [52], [53], [54] and nitrogen metabolism [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65] in other strains of K. pneumoniae. In addition to previously known TSSs of K. pneumoniae, this study extended the scope of knowledge by adding over 3,000 experimental TSSs for that species and by performing an in-depth look at the intergenic region of the K. pneumoniae genome.
Regulatory features discussed herein with E. coli and K. pneumoniae are not limited to the class of gammaproteobacteria. G. sulfurreducens in deltaproteobacteria [18], H. pylori in epsilonproteobacteria [17], C. crescentus in alphaproteobacteria [12] and methanogenic archaea Methanosarcina mazei [19] were also shown to have a significant amount of multiple TSS usage. Similarly, a mammalian promoter was also reported to have multiple TSSs [66], [67]. Thus, extensive use of multiple TSSs is a common strategy in a wide range of living organisms, exploiting alternative transcripts and providing complexity in gene expression and regulation. The level of multiple TSS usage differs by organism, however. E. coli, a generalist which can adjust and live in a wider range of environments, showed extensive usage of multiple TSSs, suggesting more complicated transcription regulation. On the other hand, G. sulfurreducens or M. mazei, a specialist that thrives in a more specific niche, showed lesser multiple TSSs. A significant fraction of operons had multiple TSSs in both E. coli and K. pneumoniae and encoded genes with essential functions, e.g., genes involved in amino acid biosynthesis, central metabolism, and transport, similar to G. sulfurreducens [18]. In addition to the usage of multiple TSSs, bacterial strains in different classes of proteobacteria show a similar distribution of 5′ UTR length. Like E. coli and K. pneumoniae, the preferred length of the 5′ UTR of H. pylori and G. sulfurreducens is 20–40 nucleotides in length. A distinctive regulatory function of the 5′ UTR was reported in yeast [68], however no correlation between the 5′ UTR length and function was found in E. coli or K. pneumoniae. However, when we compared the distribution of the 5′ UTR length between E. coli and K. pneumoniae belonging to the same COG functional group, most of the COG groups showed similar preferences for 5′ UTR length (Figure S5). Only the “Transcription” group showed significant differences (p-value of Wilcoxon rank sum test was 1.76×10−5).
Multiple promoters upstream of a gene can be regulated by transcription factors in different ways. For example, rpoD of E. coli which encodes sigma factor σ70 has multiple TSSs, and each promoter is recognized by σ70, σ32, or σ24, enabling expression of rpoD under conditions including exponential growth, heat shock, or other stresses [69], [70], [71]. Other transcription factors also contribute to the increasing complexity of promoter region structure. Transcription of an essential cell division protein operon of E. coli, ftsQAZ, is under the control of the two core promoters with two TSSs which are separated by 125 bp. Binding of the quorum sensing regulator, SdiA, activates the distal core promoter while it represses the proximal one [72]. K. pneumoniae also has that conserved operon ftsQAZ, and the TSSs of that particular operon were observed in both species and a similar regulation on expression of the ftsQAZ operon may be happening in K. pneumoniae. Another example is the ure operon (ureDABCEFG) in K. pneumoniae, which has two core promoters with distinct TSSs. One core promoter is NAC (nitrogen assimilation control protein) dependent, and the other is not [57]. For this operon, one TSS at the genomic position of 3,790,095 was identified during the mid-exponential growth in K. pneumoniae from this study. Thus, two closely related species in gammaproteobacteria, E. coli and K. pneumoniae, showed extensive usage of multiple TSSs, however they exhibited diverse organization of the regulatory region with different sets of promoters and associated TSSs. In addition to the presence of species-specific genes, this usage of multiple TSSs could potentially confer divergent regulation of orthologous genes, thereby contributing to phenotypic differences between two closely related species
Another advantage of having multiple TSSs is a transcript from each TSS has the different 5′ UTR upstream of coding region. Comparative analysis of 5′ UTRs between two species may provide insight into understanding similar or different roles of the 5′ UTR in the regulation of gene expression. One good example is a comparison of orthologous sRNAs and their binding onto the 5′ UTR region of target genes. Many orthologous sRNAs, including rprA, arcZ, and sgrS, showed enough evidence to postulate that their regulatory mechanism by the base pair dependent manner proven in E. coli may work similarly in K. pneumoniae. This conclusion is further supported by phylogenetic analysis of sRNA evolution in E. coli and Shigella genomes [73]. In the previous study, it is claimed that core or conserved sRNAs are more tightly integrated into cellular genetic regulatory networks, and over 80% of genes targeted by Hfq-associated core sRNAs have been transferred intact. 90% of orthologous sRNAs identified in our study were also categorized as core or conserved sRNAs in [73], supporting conserved regulatory mechanisms of those orthologous sRNAs.
An interesting additional attribute of the 5′ UTR sequence is that it can potentially serve as a transcription factor binding site, thereby contributing to the transcriptional regulation of a downstream gene or operon. For example, the ArgR (b3237) transcription regulator is known to bind to the promoter region and 5′ UTR of argC in E. coli [74], [75] (Figure S6A). The conserved promoter regions and TSSs were identified from the analysis of this study, and the sequence alignment of the upstream regulatory region and ORF suggests the binding regions of ArgR upstream of argC were highly conserved. Similarly, OxyR (b3961) transcription regulator, which auto-regulates its transcript by binding the promoter region and upstream regulatory region [76], also had conserved promoter regions and 5′ UTR defined by experimental TSSs identified in this study (Figure S6B). The binding regions of OxyR upstream of oxyR were also highly conserved. Considering those two transcription regulators, ArgR and OxyR, had a high level of amino acid sequence similarity of 94.2% and 96.1% respectively, and their target genes were expressed with conserved promoters and matching TSSs, it is likely that K. pneumoniae, ArgR, and OxyR may regulate argC and oxyR in a similar manner as in E. coli. However, unlike ArgR and OxyR regulation, NarL and AlaS transcription regulators showed opposite tendencies. In E. coli, NarL (b1221) regulates ogt by binding the upstream region of ogt [77] (Figure S6C). AlaS (b2697) of E. coli auto-regulates transcription of alaS by binding to the region covering parts of promoter region and the 5′ UTR [78] (Figure S6D). A high conservation level of those transcription factors (94.9% amino acid sequence similarity for NarL and 91.1% for AlaS) suggests the sequence motifs of their binding sites would be similar between E. coli and K. pneumoniae. However, sequence alignments of upstream regulatory regions of ogt and alaS between two species showed NarL and AlaS of K. pneumoniae may not regulate ogt and alaS by binding the same regions of binding sites. Thus, comparative analysis of upstream regions including the promoter region and the 5′ UTR of two closely related species can hint that the possibility of regulatory mechanisms of a lesser-studied microorganism, K. pneumoniae, by transferring ample knowledge from a well-studied microorganism such as E. coli.
Analysis of upstream regulatory regions performed in this study was based on the assumption that the current gene annotation of analyzed species is correct. Unlike the current gene annotation of E. coli, which is the most well-studied microorganism, the current gene annotation of K. pneumoniae was built by the computational methods and has not been fully confirmed with proteomic data, leaving the possibility of incorrect annotation of protein coding genes. Analyzing the sequence of coding regions and upstream regions of orthologous coding genes by sequence alignment suggested that many K. pneumoniae orthologous genes were longer than E. coli orthologous genes. The longer length of K. pneumoniae genes was mostly due to the fact that the current annotation of those genes had longer sequences at the N-terminus side (Figure S4A). When the genomic position of translation start sites were changed based on sequence alignment analysis of the coding region and upstream flanking region, 8 TSSs were found upstream of those changed translation start sites of K. pneumoniae orthologous coding genes (Table S7, Figure S4B).
TSS profiling through high-throughput sequencing techniques provides a comprehensive source of experimentally derived information related to the initiation of transcription. Annotation of non-coding regions in bacterial genomes, including the promoter regions and untranslated regions of transcripts, allows for the comparison of regulatory elements of transcription and translation between closely related species, and the identification of a spectrum from highly conserved to diverse regulatory elements. Direct comparison between cross species of bacteria also assists in transferring regulatory information of lesser-studied bacterial species and significantly improves annotation of regulatory regions. Thus, the comparative approach of this study provides a starting point for the determination of conserved and specific features of the transcriptional output of closely related bacteria at single nucleotide resolution.
Materials and Methods
Bacterial strains, media, and growth conditions
Escherichia coli K-12 MG1655 and Klebsiella pneumoniae subsp. pneumoniae MGH78578 were grown in glucose (2 g/L) minimal M9 medium containing 2 ml/L 1 M MgSO4, 50 µl/L 1 M CaCl2, 12.8 g/L Na2HPO4.7H2O, 3 g/L KH2PO4, 0.5 g/L NaCl, 1 g/L NH4Cl and 1 ml trace element solution (100×) containing 1 g EDTA, 29 mg ZnSO4.7H2O, 198 mg MnCl2.4H2O, 254 mg CoCl2.6H2O, 13.4 mg CuCl2, and 147 mg CaCl2. Glycerol stocks of the E. coli and K. pneumoniae strains were inoculated into the minimal medium supplemented with glucose and cultured at 37°C with constant agitation overnight. The cultures were diluted 1∶100 into 50 mL of the fresh minimal medium and then cultured at 37°C to an appropriate cell density.
Total RNA isolation
Three milliliters of cells from mid-log (OD = 0.6) phase culture were mixed with 6 ml RNAprotect Bacteria Reagent (Qiagen). Samples were mixed immediately by vortexing for 5 seconds, incubated for 5 minutes at room temperature, and then centrifuged at 5000×g for 10 minutes. The supernatant was decanted and any residual supernatant was removed by inverting the tube once onto a paper towel. Total RNA samples were then isolated using RNeasy Plus Mini kit (Qiagen) in accordance with the manufacturer's instruction. Samples were then quantified using a NanoDrop 1000 spectrophotometer (Thermo Scientific) and quality of the isolated RNA was checked by visualization on agarose gels and by measuring the sample's A260/A280 ratio (>1.8).
Modified 5′RACE for 5′tri-phosphorylated mRNA profiling
TSS determination protocol previously described [18] was adapted for the bacteria strains in the current study. To enrich intact 5′ tri-phosphorylated mRNAs from the total RNA, 5′ mono-phosphorylated ribosomal RNA (rRNA) and any degraded mRNA were removed by treatment with a Terminator 5′-Phosphate Dependent Exonuclease (Epicentre) at 30°C for 1 hr. The reaction mixture consisted of 10 µg purified total RNA, 1 µL terminator exonuclease, reaction buffer and RNase-free water up to total 20 µL. The reaction was terminated by adding 1 µL of 100 mM EDTA (pH 8.0). Intact tri-phosphorylated RNAs were precipitated by adding 1/10 volume of 3 M sodium acetate (pH 5.2), 3 volume of ethanol and 2 µL of 20 mg/mL glycogen. RNA was precipitated at −80°C for 20 min and pelleted, washed with 70% ethanol, dried in Speed-Vac for 7 minutes without heat and resuspended in 20 µL nuclease free water. The tri-phosphorylated RNA was then treated with RNA 5′-Polyphosphatase (Epicentre) to generate 5′-end mono-phosphorylated RNA for ligation to adaptors. The RNA sample from the previous step was mixed with 2 µL 10× reaction buffer, 0.5 µL SUPERase-In (Ambion), 1 µL RNA 5′-Polyphosphatase and RNase-free water up to 20 µL. The mixture was incubated at 37°C for 30 minutes and reaction was stopped by phenol-chloroform extraction. Ethanol precipitation was carried out for isolating the RNA as described above. To ligate 5′ small RNA adaptor (5′ GUUCAGAGUUCUACAGUCCGACGAUC 3′) to the 5′-end of the mono-phosphorylated RNA, the enriched RNA samples were incubated with 100 µM of the adaptor and 2.5 U of T4 RNA ligase (New England Biolabs). cDNAs were synthesized using the adaptor-ligated mRNAs as template using a modified small RNA RT primer from Illumina (5′ CAAGCAGAA GACGGCATACGANNNNNNNNN 3′′) and Superscript II Reverse Transcriptase (Invitrogen). The RNA was mixed with 25 µM modified small RNA RT primer and incubated at 70°C for 10 min and then at 25°C for 10 min. Reverse transcription was carried out at 25°C for 10 min, 37°C for 60 min, 42°C for 60 min and followed by incubation at 70°C for 10 min. A reaction mixture for reverse transcription consisted of the following components: 5×1st strand buffer; 0.01 M DTT; 10 mM dNTP mix; 30 U SUPERase•In™ (Ambion); and 1500 U SuperScript™ II (Invitrogen). After the reaction, RNA was hydrolysed by adding 20 µL of 1 N NaOH and incubation at 65°C for 30 min. The reaction mixture was neutralized by adding 20 µL of 1 N HCl. The cDNA samples were amplified using a mixture of 1 µL of the cDNA, 10 µL of Phusion HF buffer (NEB), 1 µL of dNTPs (10 mM), 1 µL SYBR green (Qiagen), 0.5 µL of HotStart Phusion (NEB), and 5 pmole of small RNA PCR primer mix. The amplification primers used were 5′AATGATACGGCGACCACCGACAGGTTCAGAGTTCTACAGTCCGA3′ and 5′ CAA GCA GAA GAC GGC ATA CGA 3′. The PCR mixture was denatured at 98°C for 30 s and cycled to 98°C for 10 s, 57°C for 20 s and 72°C for 20 s. Amplification was monitored by a LightCycler (Bio-Rad) and stopped at the beginning of the saturation point. Amplified DNA was run on a 6% TBE gel (Invitrogen) by electrophoresis and DNA of size ranging from 100 to 300 bp were size fractionated. Gel slices were dissolved in two volumes of EB buffer (Qiagen) and 1/10 volume of 3 M sodium acetate (pH 5.2). The amplified DNA was ethanol-precipitated and resuspended in 15 µL DNase-free water (USB). The final samples were then quantified using a NanoDrop 1000 spectrophotometer (Thermo Scientific).
Sequencing, data processing, and mapping
The amplified cDNA libraries from two biological replicates for each E. coli and K. pneumoniae were sequenced on an Illumina Genome Analyzer. Sequence reads for cDNA libraries for E. coli and K. pneumoniae were aligned onto the E. coli K-12 MG1655 genome (NC_000913) and K. pneumoniae subsp. pneumoniae MGH78578 genome with 5 plasmids (NC_009648, NC_009649, NC_009650, NC_009651, NC_009652, NC_009653), respectively, using Mosaik (http://code.google.com/p/mosaik-aligner) with the following arguments: hash size = 10, mismatach = 0, and alignment candidate threshold = 30 bp. Only reads that aligned to unique genomic location were retained. Two biological replicates were processed separately, and only sequence reads presented in both biological replicates were considered for further process. The genome coordinates of the 5′-end of these uniquely aligned reads were defined as potential TSSs. Among potential TSSs, only TSSs with the strongest signal within 10 bp window were kept to remove possible noise signals, and. TSSs with greater than or equal to 50% of the strongest signal upstream of an annotated gene were considered as multiple TSSs.
Transcriptome analysis
Transcriptome dataset with oligonucleotide tiling microarrays for E. coli grown in glucose minimal media to the mid-exponential phase was taken from [16]. In order to get the transcriptome dataset for K. pneumoniae, the protocol previously described [18] was adapted for the K. pneumoniae in the current study. Briefly, 10 g of purified total RNA sample was reverse transcribed to cDNA with amino-allyl dUTP. The amino-allyl labeled cDNA samples were then coupled with Cy3 Monoreactive dyes (Amersham). Cy3 labeled cDNAs were fragmented to 50∼300 bp range with DNase I (Epicentre). High-density oligonucleotide tiling arrays consisting of 379,528 50-mer probes spaced 30 bp apart across the whole K. pneumoniae genome and 5 plasmids were used (Roche Nimblegen). Hybridization, wash and scan were performed in accordance with manufacturer's instruction. Two biological replicates were utilized for mid exponential growth under glucose minimal media. Probe level data were normalized with RMA (Robust Multiarray Analysis) algorithm [79] without background correction, as implemented in NimbleScan 2.4 software.
Defining orthologous ORF in E. coli and K. pneumoniae
Genome annotation for E. coli K-12 MG1655 and K. pneumoniae MGH 78578 were obtained from the NCBI Genome Database. The refseq ID of E. coli K-12 MG1655 is NC_000913, and the refseq IDs of K. pneumoniae MGH 78578 genome and 5 plasmids are NC_009648, NC_009649, NC_009650, NC_009651, NC_009652 and NC_009653. In order to define orthologous ORFs between E. coli and K. pneumoniae, we performed reciprocal alignment for exhaustive pairs of amino acid sequences of ORFs in both strains, by using ClustalW2 software [80]. From the reciprocal alignment, we calculated percentage identity and percentage aligned scores, and used 50% as a cutoff for both percentage identity and percentage aligned scores of amino acid sequence alignment (Table S5).
Data processing, visualization, and availability
Graphs representing the number of uniquely mapped reads per nucleotide were stored in GFF (generalized feature format) format files and visualized using MetaScope (http://sbrg.ucsd.edu/Downloads/MetaScope) and SignalMap software from Nimblegen (http://www.nimblegen.com/products/software/). Motif logos were calculated and drawn by MEME [27] and Venn diagrams and histograms were prepared by Microsoft Excel software. Experimental data were formatted to GFF format and visualized in MetaScope. The raw TSS reads for E. coli and K. pneumoniae and expression profiling dataset for K. pneumoniae have been deposited in the Gene Expression Omnibus (GEO) database (http://www.ncbi.nlm.nih.gov/geo/), GSE35822. Processed experimental data in this study are available at http://www.sbrg.ucsd.edu.
Identification of potential sRNA
Potential sRNAs in K. pneumoniae were predicted by two methods. The first method is sRNA sequence search in the target region bound by neighboring orthologous genes. With the list of orthologous genes between E. coli and K. pneumoniae, closest orthologous genes neighboring each sRNA in E. coli were searched. Then, target region in K. pneumoniae genome was decided by neighboring orthologous genes. The sequence of E. coli sRNA was used to search conserved sequence in the target region in K. pneumoniae genome. For example, E. coli glmY is surrounded by glrK (b2556) and purL (b2557) orthologous genes. Thus, the target region in K. pneumoniae was chosen with the boundaries by glrK (KPN_02881) and purl (KPN_02882). Then, the sequence of E. coli glmY was searched in the target region, by sequence alignment between glmY and the target region with ClustalW2. This approach resulted in 48 putative sRNA candidates. The potential sRNA candidates were supplemented with prediction with Infernal (http://infernal.janelia.org) [25]. Rfam database 10.1 was used as model for sRNA prediction. Hits with E-value less than 10−5 were mapped to TSS dataset previously identified, and hits with the 5′ end matching to experimental TSSs were considered as potential sRNAs. The Infernal and Rfam approach resulted in 41 sRNA candidates. In sum, a total 50 number of sRNA candidates were prediction in a combination of two approaches.
Categorization of promoter region based on conservation and presence of TSS
Each TSS experimentally identified was considered to be associated with one promoter region, so 50 bp long genomic region directly upstream of TSS was defined as promoter region. With the list of orthologous genes between E. coli and K. pneumoniae, TSS and its associated promoter region was categorized as species-specific promoter region (SSP), if that TSS was not assigned to any of orthologous genes. TSSs assigned to orthologous genes were categorized as one of three groups: conserved promoter region with TSS (CPT), conserved promoter region with no matching TSS (CPNT) or orphan promoter region (OP). For each orthologous gene, all TSSs assigned to that gene in both species were used to define promoter regions. Then the sequence of each promoter region of one species was aligned onto the sequence of 800 bp long genomic region upstream of the orthologous gene in the other species, in order to see the 3′ end of the alignment match with any TSS of the other species with 2 bp tolerance. If there is a matching TSS, the promoter region of that TSS was aligned again back onto the 800 bp upstream region of the first species, and if the 3′ end of the second alignment matched with the first TSS, then those two TSS in both species were categorized as CPT. If the 3′ end of the first alignment didn't match any TSS of the other species, then the sequence of alignment of the other species was aligned back onto the upstream region of the first species. If the 3′ end of the second alignment matched with the first TSS, then the promoter region defined by that TSS was categorized as CPNT. If the 3′ end of the second alignment did not match with the first TSS, then the promoter region was categorized as OP.
Supporting Information
Zdroje
1. OgawaW, LiDW, YuP, BegumA, MizushimaT, et al. (2005) Multidrug resistance in Klebsiella pneumoniae MGH78578 and cloning of genes responsible for the resistance. Biol Pharm Bull 28 : 1505–1508.
2. McClellandM, FloreaL, SandersonK, CliftonSW, ParkhillJ, et al. (2000) Comparison of the Escherichia coli K-12 genome with sampled genomes of a Klebsiella pneumoniae and three salmonella enterica serovars, Typhimurium, Typhi and Paratyphi. Nucleic Acids Res 28 : 4974–4986.
3. FoutsDE, TylerHL, DeBoyRT, DaughertyS, RenQ, et al. (2008) Complete genome sequence of the N2-fixing broad host range endophyte Klebsiella pneumoniae 342 and virulence predictions verified in mice. PLoS Genet 4: e1000141 doi:10.1371/journal.pgen.1000141.
4. DieterichG, KarstU, FischerE, WehlandJ, JanschL (2006) LEGER: knowledge database and visualization tool for comparative genomics of pathogenic and non-pathogenic Listeria species. Nucleic Acids Res 34: D402–406.
5. EdwardsRA, OlsenGJ, MaloySR (2002) Comparative genomics of closely related salmonellae. Trends Microbiol 10 : 94–99.
6. GlaserP, FrangeulL, BuchrieserC, RusniokC, AmendA, et al. (2001) Comparative genomics of Listeria species. Science 294 : 849–852.
7. HertzGZ, StormoGD (1996) Escherichia coli promoter sequences: analysis and prediction. Methods Enzymol 273 : 30–42.
8. HarleyCB, ReynoldsRP (1987) Analysis of E. coli promoter sequences. Nucleic Acids Res 15 : 2343–2361.
9. RajewskyN, SocciND, ZapotockyM, SiggiaED (2002) The evolution of DNA regulatory regions for proteo-gamma bacteria by interspecies comparisons. Genome Res 12 : 298–308.
10. DieterichC, WangH, RateitschakK, LuzH, VingronM (2003) CORG: a database for COmparative Regulatory Genomics. Nucleic Acids Res 31 : 55–57.
11. GelfandMS, KooninEV, MironovAA (2000) Prediction of transcription regulatory sites in Archaea by a comparative genomic approach. Nucleic Acids Res 28 : 695–705.
12. McGrathPT, LeeH, ZhangL, IniestaAA, HottesAK, et al. (2007) High-throughput identification of transcription start sites, conserved promoter motifs and predicted regulons. Nat Biotechnol 25 : 584–592.
13. KaberdinVR, BlasiU (2006) Translation initiation and the fate of bacterial mRNAs. FEMS Microbiol Rev 30 : 967–979.
14. Mendoza-VargasA, OlveraL, OlveraM, GrandeR, Vega-AlvaradoL, et al. (2009) Genome-wide identification of transcription start sites, promoters and transcription factor binding sites in E. coli. PLoS ONE 4: e7526 doi:10.1371/journal.pone.0007526.
15. Gama-CastroS, SalgadoH, Peralta-GilM, Santos-ZavaletaA, Muniz-RascadoL, et al. (2011) RegulonDB version 7.0: transcriptional regulation of Escherichia coli K-12 integrated within genetic sensory response units (Gensor Units). Nucleic Acids Res 39: D98–105.
16. ChoBK, ZenglerK, QiuY, ParkYS, KnightEM, et al. (2009) The transcription unit architecture of the Escherichia coli genome. Nat Biotechnol 27 : 1043–1049.
17. SharmaCM, HoffmannS, DarfeuilleF, ReignierJ, FindeissS, et al. (2010) The primary transcriptome of the major human pathogen Helicobacter pylori. Nature 464 : 250–255.
18. QiuY, ChoBK, ParkYS, LovleyD, PalssonBO, et al. (2010) Structural and operational complexity of the Geobacter sulfurreducens genome. Genome Res 20 : 1304–1311.
19. JagerD, SharmaCM, ThomsenJ, EhlersC, VogelJ, et al. (2009) Deep sequencing analysis of the Methanosarcina mazei Go1 transcriptome in response to nitrogen availability. Proc Natl Acad Sci U S A 106 : 21878–21882.
20. RaghavanR, GroismanEA, OchmanH (2011) Genome-wide detection of novel regulatory RNAs in E. coli. Genome Res 21 : 1487–1497.
21. ArgamanL, HershbergR, VogelJ, BejeranoG, WagnerEG, et al. (2001) Novel small RNA-encoding genes in the intergenic regions of Escherichia coli. Curr Biol 11 : 941–950.
22. VogelJ, BartelsV, TangTH, ChurakovG, Slagter-JagerJG, et al. (2003) RNomics in Escherichia coli detects new sRNA species and indicates parallel transcriptional output in bacteria. Nucleic Acids Res 31 : 6435–6443.
23. PapenfortK, SaidN, WelsinkT, LucchiniS, HintonJC, et al. (2009) Specific and pleiotropic patterns of mRNA regulation by ArcZ, a conserved, Hfq-dependent small RNA. Mol Microbiol 74 : 139–158.
24. LuX, Goodrich-BlairH, TjadenB (2011) Assessing computational tools for the discovery of small RNA genes in bacteria. RNA 17 : 1635–1647.
25. NawrockiEP, KolbeDL, EddySR (2009) Infernal 1.0: inference of RNA alignments. Bioinformatics 25 : 1335–1337.
26. MasukataH, TomizawaJ (1986) Control of primer formation for ColE1 plasmid replication: conformational change of the primer transcript. Cell 44 : 125–136.
27. BaileyTL, ElkanC (1995) The value of prior knowledge in discovering motifs with MEME. Proc Int Conf Intell Syst Mol Biol 3 : 21–29.
28. BurgessRR, AnthonyL (2001) How sigma docks to RNA polymerase and what sigma does. Curr Opin Microbiol 4 : 126–131.
29. HawleyDK, McClureWR (1983) Compilation and analysis of Escherichia coli promoter DNA sequences. Nucleic Acids Res 11 : 2237–2255.
30. ZhangZ, DietrichFS (2005) Mapping of transcription start sites in Saccharomyces cerevisiae using 5′ SAGE. Nucleic Acids Res 33 : 2838–2851.
31. CarninciP, SandelinA, LenhardB, KatayamaS, ShimokawaK, et al. (2006) Genome-wide analysis of mammalian promoter architecture and evolution. Nat Genet 38 : 626–635.
32. CrickFH (1966) Codon–anticodon pairing: the wobble hypothesis. J Mol Biol 19 : 548–555.
33. RombyP, VandeneschF, WagnerEG (2006) The role of RNAs in the regulation of virulence-gene expression. Curr Opin Microbiol 9 : 229–236.
34. ParkSY, CromieMJ, LeeEJ, GroismanEA (2010) A bacterial mRNA leader that employs different mechanisms to sense disparate intracellular signals. Cell 142 : 737–748.
35. PeerA, MargalitH (2011) Accessibility and evolutionary conservation mark bacterial small-rna target-binding regions. J Bacteriol 193 : 1690–1701.
36. GogolEB, RhodiusVA, PapenfortK, VogelJ, GrossCA (2011) Small RNAs endow a transcriptional activator with essential repressor functions for single-tier control of a global stress regulon. Proc Natl Acad Sci U S A 108 : 12875–12880.
37. MollerT, FranchT, HojrupP, KeeneDR, BachingerHP, et al. (2002) Hfq: a bacterial Sm-like protein that mediates RNA-RNA interaction. Mol Cell 9 : 23–30.
38. Valentin-HansenP, EriksenM, UdesenC (2004) The bacterial Sm-like protein Hfq: a key player in RNA transactions. Mol Microbiol 51 : 1525–1533.
39. ChiangMK, LuMC, LiuLC, LinCT, LaiYC (2011) Impact of Hfq on global gene expression and virulence in Klebsiella pneumoniae. PLoS ONE 6: e22248 doi:10.1371/journal.pone.0022248.
40. WuKM, LiLH, YanJJ, TsaoN, LiaoTL, et al. (2009) Genome sequencing and comparative analysis of Klebsiella pneumoniae NTUH-K2044, a strain causing liver abscess and meningitis. J Bacteriol 191 : 4492–4501.
41. KeselerIM, Bonavides-MartinezC, Collado-VidesJ, Gama-CastroS, GunsalusRP, et al. (2009) EcoCyc: a comprehensive view of Escherichia coli biology. Nucleic Acids Res 37: D464–470.
42. SoperT, MandinP, MajdalaniN, GottesmanS, WoodsonSA (2010) Positive regulation by small RNAs and the role of Hfq. Proc Natl Acad Sci U S A 107 : 9602–9607.
43. MajdalaniN, HernandezD, GottesmanS (2002) Regulation and mode of action of the second small RNA activator of RpoS translation, RprA. Mol Microbiol 46 : 813–826.
44. MandinP, GottesmanS (2010) Integrating anaerobic/aerobic sensing and the general stress response through the ArcZ small RNA. Embo Journal 29 : 3094–3107.
45. KawamotoH, KoideY, MoritaT, AibaH (2006) Base-pairing requirement for RNA silencing by a bacterial small RNA and acceleration of duplex formation by Hfq. Mol Microbiol 61 : 1013–1022.
46. HorlerRS, VanderpoolCK (2009) Homologs of the small RNA SgrS are broadly distributed in enteric bacteria but have diverged in size and sequence. Nucleic Acids Res 37 : 5465–5476.
47. WuCC, HuangYJ, FungCP, PengHL (2010) Regulation of the Klebsiella pneumoniae Kpc fimbriae by the site-specific recombinase KpcI. Microbiology 156 : 1983–1992.
48. WuCC, LinCT, ChengWY, HuangCJ, WangZC, et al. (2012) Fur-dependent MrkHI regulation of type 3 fimbriae in Klebsiella pneumoniae CG43. Microbiology 158 : 1045–1056.
49. WilkschJJ, YangJ, ClementsA, GabbeJL, ShortKR, et al. (2011) MrkH, a novel c-di-GMP-dependent transcriptional activator, controls Klebsiella pneumoniae biofilm formation by regulating type 3 fimbriae expression. PLoS Pathog 7: e1002204 doi:10.1371/journal.ppat.1002204.
50. RosenblumR, KhanE, GonzalezG, HasanR, SchneidersT (2011) Genetic regulation of the ramA locus and its expression in clinical isolates of Klebsiella pneumoniae. Int J Antimicrob Agents 38 : 39–45.
51. LinCT, PengHL (2006) Regulation of the homologous two-component systems KvgAS and KvhAS in Klebsiella pneumoniae CG43. J Biochem 140 : 639–648.
52. CaoV, LambertT, CourvalinP (2002) ColE1-like plasmid pIP843 of Klebsiella pneumoniae encoding extended-spectrum beta-lactamase CTX-M-17. Antimicrob Agents Chemother 46 : 1212–1217.
53. RiceLB, CariasLL, HujerAM, BonafedeM, HuttonR, et al. (2000) High-level expression of chromosomally encoded SHV-1 beta-lactamase and an outer membrane protein change confer resistance to ceftazidime and piperacillin-tazobactam in a clinical isolate of Klebsiella pneumoniae. Antimicrob Agents Chemother 44 : 362–367.
54. AchenbachLA, YangW (1997) The fur gene from Klebsiella pneumoniae: characterization, genomic organization and phylogenetic analysis. Gene 185 : 201–207.
55. de la RivaL, BadiaJ, AguilarJ, BenderRA, BaldomaL (2008) The hpx genetic system for hypoxanthine assimilation as a nitrogen source in Klebsiella pneumoniae: gene organization and transcriptional regulation. J Bacteriol 190 : 7892–7903.
56. GossTJ (2008) The ArgP protein stimulates the Klebsiella pneumoniae gdhA promoter in a lysine-sensitive manner. J Bacteriol 190 : 4351–4359.
57. LiuQ, BenderRA (2007) Complex regulation of urease formation from the two promoters of the ure operon of Klebsiella pneumoniae. J Bacteriol 189 : 7593–7599.
58. RosarioCJ, BenderRA (2005) Importance of tetramer formation by the nitrogen assimilation control protein for strong repression of glutamate dehydrogenase formation in Klebsiella pneumoniae. J Bacteriol 187 : 8291–8299.
59. GrandeRA, ValderramaB, MorettE (1999) Suppression analysis of positive control mutants of NifA reveals two overlapping promoters for Klebsiella pneumoniae rpoN. J Mol Biol 294 : 291–298.
60. CheemaAK, ChoudhuryNR, DasHK (1999) A - and T-tract-mediated intrinsic curvature in native DNA between the binding site of the upstream activator NtrC and the nifLA promoter of Klebsiella pneumoniae facilitates transcription. J Bacteriol 181 : 5296–5302.
61. AchenbachLA, GenovaEG (1997) Transcriptional regulation of a second flavodoxin gene from Klebsiella pneumoniae. Gene 194 : 235–240.
62. LinJT, StewartV (1996) Nitrate and nitrite-mediated transcription antitermination control of nasF (nitrate assimilation) operon expression in Klebsiella pheumoniae M5al. J Mol Biol 256 : 423–435.
63. CollinsCM, GutmanDM, LamanH (1993) Identification of a nitrogen-regulated promoter controlling expression of Klebsiella pneumoniae urease genes. Mol Microbiol 8 : 187–198.
64. CharltonW, CannonW, BuckM (1993) The Klebsiella pneumoniae nifJ promoter: analysis of promoter elements regulating activation by the NifA promoter. Mol Microbiol 7 : 1007–1021.
65. BuckM, CannonW (1989) Mutations in the RNA polymerase recognition sequence of the Klebsiella pneumoniae nifH promoter permitting transcriptional activation in the absence of NifA binding to upstream activator sequences. Nucleic Acids Res 17 : 2597–2612.
66. KawajiH, FrithMC, KatayamaS, SandelinA, KaiC, et al. (2006) Dynamic usage of transcription start sites within core promoters. Genome Biol 7: R118.
67. SuzukiY, YamashitaR, SuganoS, NakaiK (2004) DBTSS, DataBase of Transcriptional Start Sites: progress report 2004. Nucleic Acids Res 32: D78–81.
68. DavidL, HuberW, GranovskaiaM, ToedlingJ, PalmCJ, et al. (2006) A high-resolution map of transcription in the yeast genome. Proc Natl Acad Sci U S A 103 : 5320–5325.
69. CowingDW, GrossCA (1989) Interaction of Escherichia coli RNA polymerase holoenzyme containing sigma 32 with heat shock promoters. DNase I footprinting and methylation protection. J Mol Biol 210 : 513–520.
70. LupskiJR, SmileyBL, GodsonGN (1983) Regulation of the rpsU-dnaG-rpoD macromolecular synthesis operon and the initiation of DNA replication in Escherichia coli K-12. Mol Gen Genet 189 : 48–57.
71. BurtonZF, GrossCA, WatanabeKK, BurgessRR (1983) The operon that encodes the sigma subunit of RNA polymerase also encodes ribosomal protein S21 and DNA primase in E. coli K12. Cell 32 : 335–349.
72. YamamotoK, YataK, FujitaN, IshihamaA (2001) Novel mode of transcription regulation by SdiA, an Escherichia coli homologue of the quorum-sensing regulator. Mol Microbiol 41 : 1187–1198.
73. SkippingtonE, RaganMA (2012) Evolutionary Dynamics of Small RNAs in 27 Escherichia coli and Shigella Genomes. Genome Biol Evol 4 : 330–345.
74. CaldaraM, CharlierD, CuninR (2006) The arginine regulon of Escherichia coli: whole-system transcriptome analysis discovers new genes and provides an integrated view of arginine regulation. Microbiology 152 : 3343–3354.
75. CharlierD, RooversM, Van VlietF, BoyenA, CuninR, et al. (1992) Arginine regulon of Escherichia coli K-12. A study of repressor-operator interactions and of in vitro binding affinities versus in vivo repression. J Mol Biol 226 : 367–386.
76. ToledanoMB, KullikI, TrinhF, BairdPT, SchneiderTD, et al. (1994) Redox-dependent shift of OxyR-DNA contacts along an extended DNA-binding site: a mechanism for differential promoter selection. Cell 78 : 897–909.
77. SquireDJ, XuM, ColeJA, BusbySJ, BrowningDF (2009) Competition between NarL-dependent activation and Fis-dependent repression controls expression from the Escherichia coli yeaR and ogt promoters. Biochem J 420 : 249–257.
78. PutneySD, SchimmelP (1981) An aminoacyl tRNA synthetase binds to a specific DNA sequence and regulates its gene transcription. Nature 291 : 632–635.
79. IrizarryRA, HobbsB, CollinF, Beazer-BarclayYD, AntonellisKJ, et al. (2003) Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 4 : 249–264.
80. ChennaR, SugawaraH, KoikeT, LopezR, GibsonTJ, et al. (2003) Multiple sequence alignment with the Clustal series of programs. Nucleic Acids Res 31 : 3497–3500.
Štítky
Genetika Reprodukční medicína
Článek Mutational Signatures of De-Differentiation in Functional Non-Coding Regions of Melanoma GenomesČlánek Rescuing Alu: Recovery of Inserts Shows LINE-1 Preserves Alu Activity through A-Tail ExpansionČlánek Genetics and Regulatory Impact of Alternative Polyadenylation in Human B-Lymphoblastoid CellsČlánek Retrovolution: HIV–Driven Evolution of Cellular Genes and Improvement of Anticancer Drug ActivationČlánek The Mi-2 Chromatin-Remodeling Factor Regulates Higher-Order Chromatin Structure and Cohesin DynamicsČlánek Identification of Human Proteins That Modify Misfolding and Proteotoxicity of Pathogenic Ataxin-1
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 8
-
Všechny články tohoto čísla
- Mutational Signatures of De-Differentiation in Functional Non-Coding Regions of Melanoma Genomes
- Rescuing Alu: Recovery of Inserts Shows LINE-1 Preserves Alu Activity through A-Tail Expansion
- Genetics and Regulatory Impact of Alternative Polyadenylation in Human B-Lymphoblastoid Cells
- Chromosome Territories Meet a Condensin
- It's All in the Timing: Too Much E2F Is a Bad Thing
- Fine-Mapping and Initial Characterization of QT Interval Loci in African Americans
- Genome Patterns of Selection and Introgression of Haplotypes in Natural Populations of the House Mouse ()
- A Combinatorial Amino Acid Code for RNA Recognition by Pentatricopeptide Repeat Proteins
- Advances in Quantitative Trait Analysis in Yeast
- Experimental Evolution of a Novel Sexually Antagonistic Allele
- Variation of Contributes to Dog Breed Skull Diversity
- , a Gene Involved in Axonal Pathfinding, Is Mutated in Patients with Kallmann Syndrome
- A Single Origin for Nymphalid Butterfly Eyespots Followed by Widespread Loss of Associated Gene Expression
- Cryptocephal, the ATF4, Is a Specific Coactivator for Ecdysone Receptor Isoform B2
- Retrovolution: HIV–Driven Evolution of Cellular Genes and Improvement of Anticancer Drug Activation
- The PARN Deadenylase Targets a Discrete Set of mRNAs for Decay and Regulates Cell Motility in Mouse Myoblasts
- A Sexual Ornament in Chickens Is Affected by Pleiotropic Alleles at and , Selected during Domestication
- Use of Allele-Specific FAIRE to Determine Functional Regulatory Polymorphism Using Large-Scale Genotyping Arrays
- Novel Loci for Metabolic Networks and Multi-Tissue Expression Studies Reveal Genes for Atherosclerosis
- The Genetic Basis of Pollinator Adaptation in a Sexually Deceptive Orchid
- Uncovering the Genome-Wide Transcriptional Responses of the Filamentous Fungus to Lignocellulose Using RNA Sequencing
- Inheritance Beyond Plain Heritability: Variance-Controlling Genes in
- The Metabochip, a Custom Genotyping Array for Genetic Studies of Metabolic, Cardiovascular, and Anthropometric Traits
- Reprogramming to Pluripotency Can Conceal Somatic Cell Chromosomal Instability
- Condensin II Promotes the Formation of Chromosome Territories by Inducing Axial Compaction of Polyploid Interphase Chromosomes
- PTEN Negatively Regulates MAPK Signaling during Vulval Development
- A Dynamic Response Regulator Protein Modulates G-Protein–Dependent Polarity in the Bacterium
- Population Genomics of the Facultatively Mutualistic Bacteria and
- Components of a Fanconi-Like Pathway Control Pso2-Independent DNA Interstrand Crosslink Repair in Yeast
- Polysome Profiling in Liver Identifies Dynamic Regulation of Endoplasmic Reticulum Translatome by Obesity and Fasting
- Stromal Liver Kinase B1 [STK11] Signaling Loss Induces Oviductal Adenomas and Endometrial Cancer by Activating Mammalian Target of Rapamycin Complex 1
- Reprogramming of H3K27me3 Is Critical for Acquisition of Pluripotency from Cultured Tissues
- Transgene Induced Co-Suppression during Vegetative Growth in
- Hox and Sex-Determination Genes Control Segment Elimination through EGFR and Activity
- A Quantitative Comparison of the Similarity between Genes and Geography in Worldwide Human Populations
- Minibrain/Dyrk1a Regulates Food Intake through the Sir2-FOXO-sNPF/NPY Pathway in and Mammals
- Comparative Analysis of Regulatory Elements between and by Genome-Wide Transcription Start Site Profiling
- Simple Methods for Generating and Detecting Locus-Specific Mutations Induced with TALENs in the Zebrafish Genome
- S Phase–Coupled E2f1 Destruction Ensures Homeostasis in Proliferating Tissues
- Cell-Nonautonomous Signaling of FOXO/DAF-16 to the Stem Cells of
- The Mi-2 Chromatin-Remodeling Factor Regulates Higher-Order Chromatin Structure and Cohesin Dynamics
- Comparative Analysis of the Genomes of Two Field Isolates of the Rice Blast Fungus
- Role of Mex67-Mtr2 in the Nuclear Export of 40S Pre-Ribosomes
- Genetic Modulation of Lipid Profiles following Lifestyle Modification or Metformin Treatment: The Diabetes Prevention Program
- HAL-2 Promotes Homologous Pairing during Meiosis by Antagonizing Inhibitory Effects of Synaptonemal Complex Precursors
- SLX-1 Is Required for Maintaining Genomic Integrity and Promoting Meiotic Noncrossovers in the Germline
- Phylogenetic and Transcriptomic Analysis of Chemosensory Receptors in a Pair of Divergent Ant Species Reveals Sex-Specific Signatures of Odor Coding
- Reduced Prostasin (CAP1/PRSS8) Activity Eliminates HAI-1 and HAI-2 Deficiency–Associated Developmental Defects by Preventing Matriptase Activation
- Dissecting the Gene Network of Dietary Restriction to Identify Evolutionarily Conserved Pathways and New Functional Genes
- Identification of Human Proteins That Modify Misfolding and Proteotoxicity of Pathogenic Ataxin-1
- and Link Transcription of Phospholipid Biosynthetic Genes to ER Stress and the UPR
- CDK9 and H2B Monoubiquitination: A Well-Choreographed Dance
- Rare Copy Number Variations in Adults with Tetralogy of Fallot Implicate Novel Risk Gene Pathways
- Ccdc94 Protects Cells from Ionizing Radiation by Inhibiting the Expression of
- NOL11, Implicated in the Pathogenesis of North American Indian Childhood Cirrhosis, Is Required for Pre-rRNA Transcription and Processing
- Human Developmental Enhancers Conserved between Deuterostomes and Protostomes
- A Luminal Glycoprotein Drives Dose-Dependent Diameter Expansion of the Hindgut Tube
- Melanophore Migration and Survival during Zebrafish Adult Pigment Stripe Development Require the Immunoglobulin Superfamily Adhesion Molecule Igsf11
- Dynamic Distribution of Linker Histone H1.5 in Cellular Differentiation
- Combining Comparative Proteomics and Molecular Genetics Uncovers Regulators of Synaptic and Axonal Stability and Degeneration
- Chemical Genetics Reveals a Specific Requirement for Cdk2 Activity in the DNA Damage Response and Identifies Nbs1 as a Cdk2 Substrate in Human Cells
- Experimental Relocation of the Mitochondrial Gene to the Nucleus Reveals Forces Underlying Mitochondrial Genome Evolution
- Rates of Gyrase Supercoiling and Transcription Elongation Control Supercoil Density in a Bacterial Chromosome
- Mutations in a P-Type ATPase Gene Cause Axonal Degeneration
- A General G1/S-Phase Cell-Cycle Control Module in the Flowering Plant
- Multiple Roles and Interactions of and in Development of the Respiratory System
- UNC-40/DCC, SAX-3/Robo, and VAB-1/Eph Polarize F-Actin during Embryonic Morphogenesis by Regulating the WAVE/SCAR Actin Nucleation Complex
- Epigenetic Remodeling of Meiotic Crossover Frequency in DNA Methyltransferase Mutants
- Modulating the Strength and Threshold of NOTCH Oncogenic Signals by
- Loss of Axonal Mitochondria Promotes Tau-Mediated Neurodegeneration and Alzheimer's Disease–Related Tau Phosphorylation Via PAR-1
- Acetyl-CoA-Carboxylase Sustains a Fatty Acid–Dependent Remote Signal to Waterproof the Respiratory System
- ATXN2-CAG42 Sequesters PABPC1 into Insolubility and Induces FBXW8 in Cerebellum of Old Ataxic Knock-In Mice
- Cohesin Rings Devoid of Scc3 and Pds5 Maintain Their Stable Association with the DNA
- The MicroRNA Inhibits Calcium Signaling by Targeting the TIR-1/Sarm1 Adaptor Protein to Control Stochastic L/R Neuronal Asymmetry in
- Rapid-Throughput Skeletal Phenotyping of 100 Knockout Mice Identifies 9 New Genes That Determine Bone Strength
- The Genes Define Unique Classes of Two-Partner Secretion and Contact Dependent Growth Inhibition Systems
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Dissecting the Gene Network of Dietary Restriction to Identify Evolutionarily Conserved Pathways and New Functional Genes
- It's All in the Timing: Too Much E2F Is a Bad Thing
- Variation of Contributes to Dog Breed Skull Diversity
- The PARN Deadenylase Targets a Discrete Set of mRNAs for Decay and Regulates Cell Motility in Mouse Myoblasts
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



