-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaLoss of Axonal Mitochondria Promotes Tau-Mediated Neurodegeneration and Alzheimer's Disease–Related Tau Phosphorylation Via PAR-1
Abnormal phosphorylation and toxicity of a microtubule-associated protein tau are involved in the pathogenesis of Alzheimer's disease (AD); however, what pathological conditions trigger tau abnormality in AD is not fully understood. A reduction in the number of mitochondria in the axon has been implicated in AD. In this study, we investigated whether and how loss of axonal mitochondria promotes tau phosphorylation and toxicity in vivo. Using transgenic Drosophila expressing human tau, we found that RNAi–mediated knockdown of milton or Miro, an adaptor protein essential for axonal transport of mitochondria, enhanced human tau-induced neurodegeneration. Tau phosphorylation at an AD–related site Ser262 increased with knockdown of milton or Miro; and partitioning defective-1 (PAR-1), the Drosophila homolog of mammalian microtubule affinity-regulating kinase, mediated this increase of tau phosphorylation. Tau phosphorylation at Ser262 has been reported to promote tau detachment from microtubules, and we found that the levels of microtubule-unbound free tau increased by milton knockdown. Blocking tau phosphorylation at Ser262 site by PAR-1 knockdown or by mutating the Ser262 site to unphosphorylatable alanine suppressed the enhancement of tau-induced neurodegeneration caused by milton knockdown. Furthermore, knockdown of milton or Miro increased the levels of active PAR-1. These results suggest that an increase in tau phosphorylation at Ser262 through PAR-1 contributes to tau-mediated neurodegeneration under a pathological condition in which axonal mitochondria is depleted. Intriguingly, we found that knockdown of milton or Miro alone caused late-onset neurodegeneration in the fly brain, and this neurodegeneration could be suppressed by knockdown of Drosophila tau or PAR-1. Our results suggest that loss of axonal mitochondria may play an important role in tau phosphorylation and toxicity in the pathogenesis of AD.
Published in the journal: . PLoS Genet 8(8): e32767. doi:10.1371/journal.pgen.1002918
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002918Summary
Abnormal phosphorylation and toxicity of a microtubule-associated protein tau are involved in the pathogenesis of Alzheimer's disease (AD); however, what pathological conditions trigger tau abnormality in AD is not fully understood. A reduction in the number of mitochondria in the axon has been implicated in AD. In this study, we investigated whether and how loss of axonal mitochondria promotes tau phosphorylation and toxicity in vivo. Using transgenic Drosophila expressing human tau, we found that RNAi–mediated knockdown of milton or Miro, an adaptor protein essential for axonal transport of mitochondria, enhanced human tau-induced neurodegeneration. Tau phosphorylation at an AD–related site Ser262 increased with knockdown of milton or Miro; and partitioning defective-1 (PAR-1), the Drosophila homolog of mammalian microtubule affinity-regulating kinase, mediated this increase of tau phosphorylation. Tau phosphorylation at Ser262 has been reported to promote tau detachment from microtubules, and we found that the levels of microtubule-unbound free tau increased by milton knockdown. Blocking tau phosphorylation at Ser262 site by PAR-1 knockdown or by mutating the Ser262 site to unphosphorylatable alanine suppressed the enhancement of tau-induced neurodegeneration caused by milton knockdown. Furthermore, knockdown of milton or Miro increased the levels of active PAR-1. These results suggest that an increase in tau phosphorylation at Ser262 through PAR-1 contributes to tau-mediated neurodegeneration under a pathological condition in which axonal mitochondria is depleted. Intriguingly, we found that knockdown of milton or Miro alone caused late-onset neurodegeneration in the fly brain, and this neurodegeneration could be suppressed by knockdown of Drosophila tau or PAR-1. Our results suggest that loss of axonal mitochondria may play an important role in tau phosphorylation and toxicity in the pathogenesis of AD.
Introduction
Mitochondria are principal mediators of local ATP supply and Ca2+ buffering. In neuronal axons, these requirements need to be addressed locally, and the proper distribution of mitochondria is essential for neuronal functions and survival [1]. Defects in mitochondrial distribution have been observed in the brains of patients suffering from several neurodegenerative diseases including Alzheimer's disease (AD) [2]. Recent studies have shown that the localization of mitochondria to the axon is reduced in neurons in the AD brain, as well as in cellular and animal models of AD [3]–[14]. The reduction in mitochondria in the axon may be due to alterations in mitochondrial fission/fusion [3], [5], [6] and/or due to defects in the axonal transport of mitochondria [4], [6], [8], [10]–[12]. However, how it contributes to the pathogenesis of AD remains elusive.
Tau is a microtubule-associated protein that is expressed in neurons and localizes predominantly in the axons, where it regulates microtubule dynamics. Tau is phosphorylated at a number of sites, and a fine-tuned balance between phosphorylation and dephosphorylation of tau is critical for its physiological functions, such as microtubule stabilization, in the axons [15].
Hyperphosphorylated tau is found in neurofibrillary tangles, the intracellular protein inclusions that are associated with a range of neurodegenerative diseases including AD [15]. In AD brains, tau phosphorylation is abnormally increased at several specific sites, and these changes are associated with tau toxicity [15], [16]. However, the effects of loss of axonal mitochondria on abnormal phosphorylation and toxicity of tau has not been fully elucidated.
Mitochondrial transport is regulated by a series of molecular adaptors that mediate the attachment of mitochondria to molecular motors [17]. In Drosophila, mitochondrial transport is facilitated by milton and Miro, which regulate mitochondrial attachment to microtubules via kinesin heavy chain [18], [19]. In mammals, two isoforms of milton (OIP106 and GRIF1) and Miro (Miro1 and Miro2) have been identified and are proposed to act in a similar manner [20]. In Drosophila, in the absence of milton or Miro, synaptic terminals and axons lack mitochondria, although mitochondria are numerous in the neuronal cell body [18], [21].
In this study, using Drosophila as a model system, we investigated the effects of knockdown of milton or Miro, an adaptor protein essential for axonal transport of mitochondria, on tau phosphorylation and toxicity. We demonstrate that loss of axonal mitochondria caused by milton knockdown increases tau phosphorylation at Ser262 through PAR-1, promotes detachment of tau from microtubules, and enhances tau-mediated neurodegeneration.
Results
Knockdown of milton or Miro significantly enhances human tau-mediated neurodegeneration
To test whether loss of axonal mitochondria enhances human tau toxicity in vivo, we used transgenic Drosophila expressing human tau [22]. Wild-type human 0N4R tau, which has four tubulin-binding domains (R) and no N-terminal insert (N), was expressed in fly eyes using the GAL4/UAS system [23] with the pan-retinal gmr-GAL4 driver. Expression of human tau causes age-dependent and progressive neurodegeneration in the lamina, the first synaptic neuropil of the optic lobe containing photoreceptor axons: degeneration in the lamina is undetectable or very mild in flies at 3-day-old, while it is prominent at 10-day-old (Figure S1A and S1B; S1D, quantification).
It has been reported that overexpression of tau alone can reduce anterograde transport of a variety of kinesin cargos, including mitochondria [4], [9], [11], [12], [24]. We examined whether tau expression alone causes the loss of mitochondria at the synaptic terminals of young tau flies by electron microscopy. Mitochondria were observed in the synaptic terminals of photoreceptor neurons expressing tau at 3-day-old (Figure S2), suggesting that, under our experimental conditions, severe defects in microtubule-dependent transport are not occurred in the young flies expressing human tau.
Milton is a component of an adaptor complex that links mitochondria to kinesin heavy chain and is essential for axonal transport of mitochondria (Figure 1A) [19]. Previously, we have shown that milton RNAi expression effectively reduces milton protein levels, reduces the axonal distribution of mitochondria and increases the mitochondrial localization to the cell body in the fly brain [7]. We confirmed that expression of milton RNAi in fly eyes caused loss of mitochondria in the synaptic terminals of the photoreceptor neurons by electron microscopy analysis. Mitochondria were seldom observed in the synaptic terminals of photoreceptor neurons expressing milton RNAi, while mitochondria were abundant in the synaptic terminals of control flies (compare Figure 1B and 1C). In addition, the presynaptic terminals contained vesicles with a wider range of sizes in the milton knockdown flies than in controls (Figure S3), as previously observed in milton mutant flies [25].
Fig. 1. RNAi–mediated knockdown of milton or Miro enhances human tau-induced neurodegeneration. 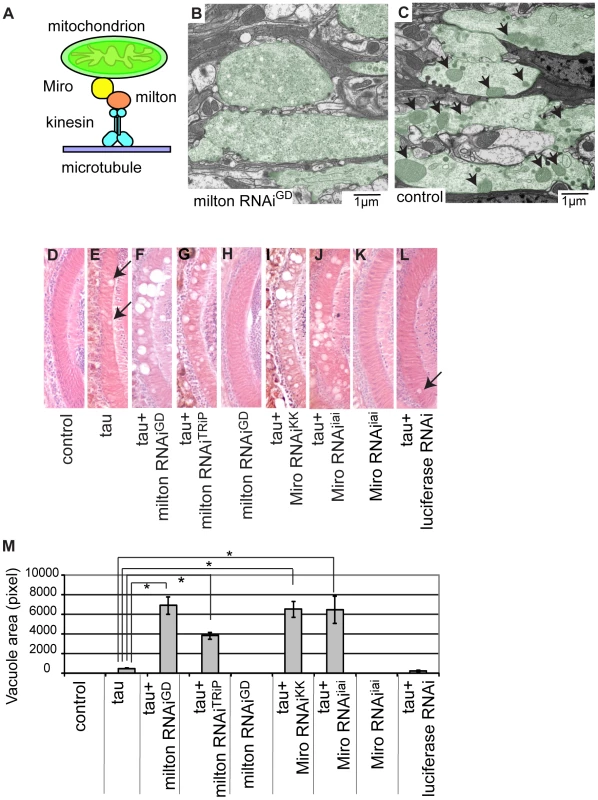
(A) The mitochondrial transport machinery. (B–C) Transmission electron micrographs of presynaptic terminals in the lamina of flies expressing milton RNAi with the gmr-GAL4 driver (B) and control flies bearing the gmr-GAL4 driver only (C). Presynaptic terminals in B and C are colored green to accentuate the structures. Arrows in C indicate mitochondria in synaptic terminals. Note that synaptic terminals in milton knockdown flies (B) lack mitochondria, while the synaptic terminals of control flies (C) contain mitochondria. Flies are 3 days-after-eclosion (day-old). (D–M) The lamina of control flies bearing the driver only (D), flies expressing tau alone (E), co-expressing tau and milton RNAiGD (F), co-expressing tau and milton RNAiTRiP (G), expressing milton RNAiGD alone (H), co-expressing tau and Miro RNAiKK (I), co-expressing tau and Miro RNAiiai (J), expressing Miro RNAiiai alone (K), or co-expressing tau and luciferase RNAi (L). In E and L, neurodegeneration is indicated by arrows. (M) Quantification of neurodegeneration, mean ± SEM, n = 10–33. *, p<0.05, Student's t-test. Flies were 3 days-after-eclosion (day-old). To investigate tau toxicity under the condition in which mitochondria are chronically depleted from the axon, we co-expressed milton RNAi with human tau. We confirmed that milton knockdown caused loss of axonal mitochondria in the neurons expressing tau by electron microscopy (Figure S4). Co-expression of tau with milton RNAi (milton RNAiGD) dramatically enhanced neurodegeneration in the lamina at 3-day-old compared to fly eyes expressing tau alone (Figure 1E and 1F; 1M, quantification). In 3-day-old flies, knockdown of milton alone did not cause neurodegeneration (Figure 1H) [21]. To limit the possibility of off-target effects of RNAi, another independent transgenic fly line carrying a milton RNAi that targets a different region of milton (milton RNAiTRiP) was used. Expression of this RNAi in neurons reduced milton mRNA levels in the fly brain (Figure S5A) as well as the axonal distribution of mitochondria (Figure S5B). The enhancement of tau-induced neurodegeneration was also observed with milton RNAiTRiP (Figure 1G; 1M, quantification).
We also tested the effect of knockdown of Miro, which is another critical component of the adaptor complex that controls mitochondrial trafficking in the axons [18], [19] (Figure 1A), on tau-mediated neurodegeneration. Expression of Miro RNAi (Miro RNAiKK) [26] reduced the axonal distribution of mitochondria in the fly brain (Figure S6) and significantly enhanced tau-induced neurodegeneration (Figure 1I; 1M, quantification). To limit the possibility of off-target effects of RNAi, we generated another independent transgenic fly line carrying Miro RNAi (Miro RNAiiai) that targets a different region of Miro. Expression of Miro RNAiiai reduced Miro mRNA levels (Figure S7) and significantly enhanced tau-induced neurodegeneration (Figure 1J; 1M, quantification). Similar to a previous report [21], knockdown of Miro alone did not cause neurodegeneration in 3-day-old flies (Figure 1K).
The enhancement of tau-induced neurodegeneration by milton RNAi or Miro RNAi is not due to non-specific effects of RNAi overexpression, since the expression of an RNAi against firefly luciferase (Figure 1L), as well as the expression of many other RNAis (Figure S8), did not enhance tau-induced neurodegeneration.
Expression of human tau in Drosophila eyes reduces the external eye size (Figure S9B), which is due to apoptosis during the larval stage [27]. Genetic screens assessing changes in this phenotype have identified a number of modifiers of tau toxicity [28]–[30]. However, neither milton nor Miro was identified in the previous screens [28]–[30]. We found that knockdown of either milton or Miro did not enhance the tau-induced reduction in external eye size (Figure S9C and S9D). These results indicate that the modifier screen using tau-induced lamina degeneration as a read-out phenotype yields new genes involved in tau-induced neurodegeneration.
Taken together, these results demonstrate that the knockdown of milton or Miro enhances human tau-mediated neurodegeneration.
Milton knockdown enhances tau-induced axon degeneration without neurofibrillary tangle formation
Neurodegeneration in the lamina in flies expressing tau alone (Figure 2A) or expressing tau and milton RNAi (Figure 2B, 2E, 2F and 2G) at 3-day-old was examined at the ultrastructural level. Axon pathology, including the formation of vacuoles in the axons (asterisks in Figure 2A, 2B and 2E) and swollen axons (arrows in Figure 2E), were observed. In the presynaptic terminals, vacuoles (Figure 2F, asterisks) and the accumulation of autophagic bodies and multivesicular bodies (Figure 2F and 2G, arrows) were observed. These pathological changes were more severe and prominent in the lamina of flies expressing tau and milton RNAi than in flies expressing tau alone. Neurofibrillary tangles were not detected in flies expressing human tau and milton RNAi, indicating that milton knockdown enhances the tau-induced axonopathy without the formation of large tau aggregates. Axonal or presynaptic degeneration was not observed in the control flies (Figure 2C and 2H) or in flies with milton knockdown alone at 3-day-old (Figure 2D and 2I).
Fig. 2. Milton or Miro knockdown enhances tau-induced axon degeneration. 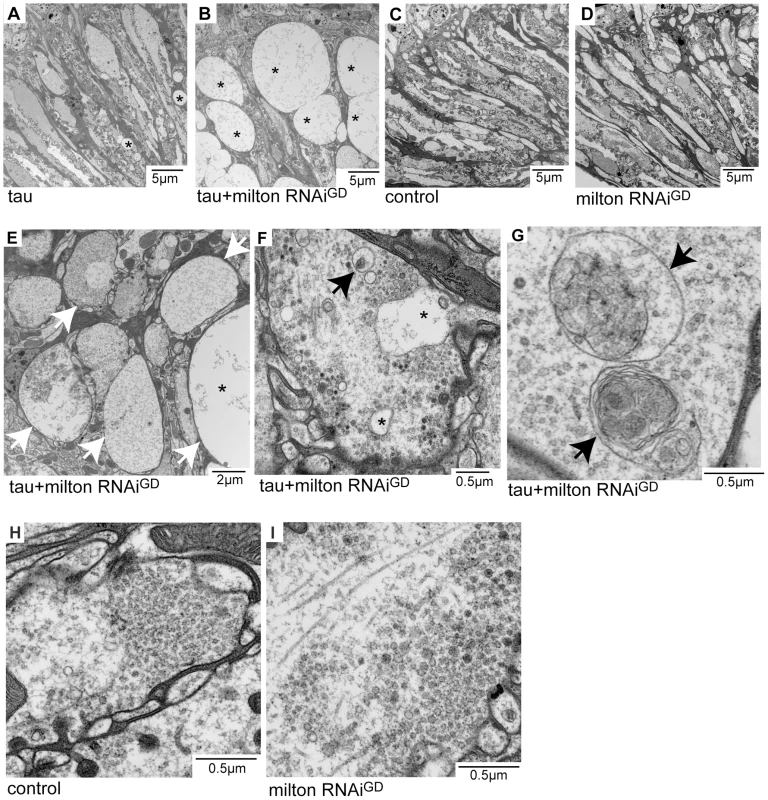
Transmission electron micrographs of lamina areas (A–E) and presynaptic terminals of photoreceptor neurons in the lamina (F–I) in flies expressing tau (A), flies co-expressing tau and milton RNAi (B, E, F and G), control flies bearing the gmr-GAL4 driver only (C and H), or flies expressing milton RNAi alone (D and I). Asterisks in A, B, E and F indicate vacuoles. Arrows in E indicate swollen axons. Arrows in F and G indicate autophagic and multivesicular bodies. All flies were 3 days-after-eclosion (day-old). Milton knockdown increases tau phosphorylation levels at AD–related Ser262
A group of Ser/Thr phosphorylation sites in tau is abnormally phosphorylated in the AD brain [31]. Using well-characterized phospho-tau-specific antibodies, we examined whether milton knockdown affects tau phosphorylation at AD-related sites by Western blotting. The level of tau phosphorylated at Ser262 was significantly increased by milton knockdown (Figure 3A). Knockdown of Miro also increased the levels of tau phosphorylated at Ser262 (Figure 3B). In contrast, tau phosphorylation at the AT8 epitope (phospho-Ser202) or the AT180 epitope (phospho-Thr231) was not significantly altered by milton knockdown (Figure 3A). The levels of total tau were not significantly changed (Figure 3A), indicating that milton knockdown does not cause tau accumulation.
Fig. 3. Knockdown of milton or Miro increases tau phosphorylation levels at AD–related Ser262. 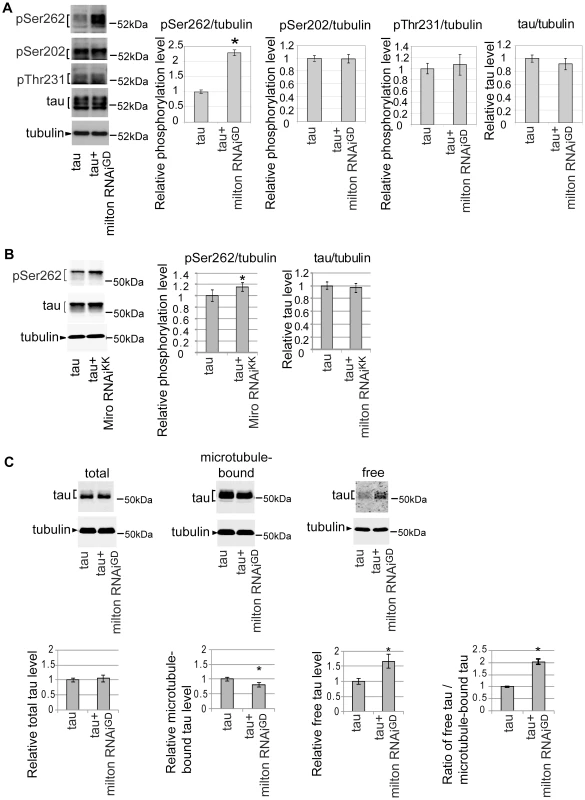
(A) Milton knockdown increases tau phosphorylation levels at Ser262. Western blots of eyes from flies expressing tau alone (tau) or co-expressing tau and milton RNAi (tau+milton RNAiGD). Blots were probed with anti-phospho-Ser262 tau, anti-phospho-Ser202 tau, anti-phospho-Thr231 tau, anti-tau, or anti-tubulin. Tubulin was used as a loading control. Mean ± SD, n = 5; *, p<0.05, Student's t-test. Representative blots are shown. (B) Miro knockdown increases tau phosphorylation levels at Ser262. Western blots of eyes from flies expressing tau alone (tau) or co-expressing tau and Miro RNAiKK (tau+Miro RNAiKK). Blots were probed with anti-phospho-Ser262 tau, anti-tau, or anti-tubulin. Tubulin was used as a loading control. Mean ± SD, n = 5; *, p<0.05, Student's t-test. Representative blots are shown. (C) Microtubule binding assay of tau. Proteins were extracted from heads of flies expressing tau alone (tau) or co-expressing tau and milton RNAi (tau+milton RNAiGD). Tau or tubulin in the lysate before fractionation (total), the fraction free from microtubules (free) or the fraction containing microtubules (microtubule-bound) were analyzed with western blotting using anti-tau or anti-tubulin. The same amount of protein was loaded to each lane. Each graph displays tau levels relative to control, or the ratio of free tau relative to microtubule-bound tau (Mean ± SD, n = 6; *, p<0.05, Student's t-test). Representative blots are shown. All flies were 3 days-after-eclosion (day-old). Tau phosphorylation at Ser262 has been reported to reduce tau binding to microtubules [32], [33]. We tested whether milton knockdown alters the binding of tau to microtubules by using microtubule binding assay. Microtubules and microtubule-bound proteins were recovered as the pellet by centrifugation from brains of flies expressing human tau alone or co-expressing human tau and milton RNAi. The pellet (microtubule-bound fraction) and supernatant (microtubule-free fraction) were separated by SDS-PAGE, and tau levels in these fractions were analyzed by Western blotting. Milton knockdown caused a significant reduction in the amount of tau in the pellet and an increase in tau in the supernatant (Figure 3C). This result indicates that milton knockdown reduces tau binding to microtubules and increases the levels of microtubule-unbound, free tau in the fly brain.
PAR-1 mediates the increase in tau phosphorylation at Ser262 caused by milton knockdown
Drosophila partitioning defective-1 (PAR-1) and the mammalian homolog of PAR-1, microtubule affinity-regulating kinase (MARK), are reported to phosphorylate tau at Ser262 in vivo [34], [35]. RNAi-mediated knockdown of PAR-1 in fly eyes caused a significant reduction in tau phosphorylation at Ser262, suggesting that PAR-1 is the major Ser262 kinase in the fly eye (Figure 4A) [35]. We examined whether blocking PAR-1 activity suppresses the increase in tau phosphorylation at Ser262 caused by milton knockdown. In the PAR-1 knockdown background, milton knockdown did not increase tau phosphorylation levels at Ser262 (Figure 4B), indicating that PAR-1 mediates the increase in tau phosphorylation at Ser262 caused by milton knockdown.
Fig. 4. PAR-1 mediates the increase in tau phosphorylation at Ser262 caused by milton knockdown. 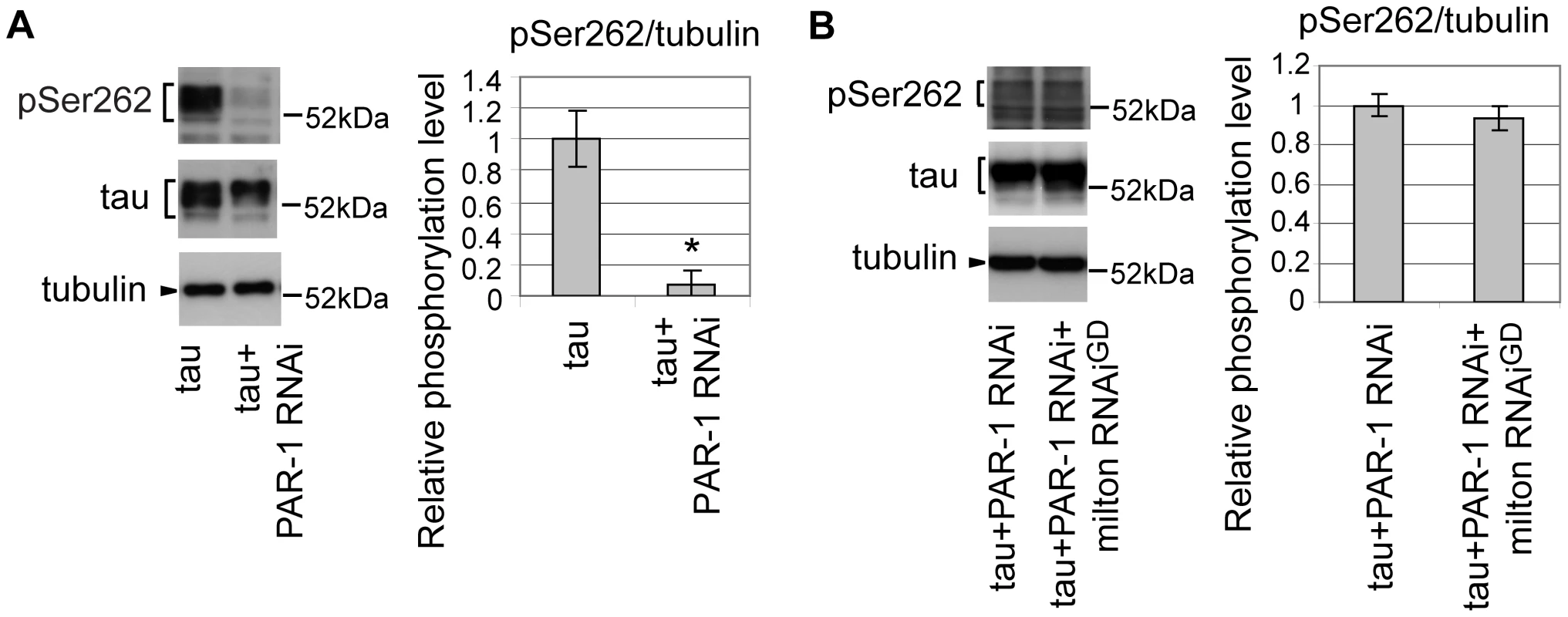
(A) Western blots of eyes from flies expressing tau alone or co-expressing tau and PAR-1 RNAi. Blots were probed with anti-phospho-Ser262 tau, anti-tau, or anti-tubulin. Mean ± SD, n = 5; *, p<0.05, Student's t-test. (B) Western blots of eyes from flies co-expressing tau and PAR-1 RNAi, or co-expressing tau, PAR-1 RNAi and milton RNAi. Blots were probed with anti-phospho-Ser262, anti-tau, or anti-tubulin. No significant difference was found (mean ± SD, n = 5; p>0.05, Student's t-test). Representative blots are shown. All flies were 3 days-after-eclosion (day-old). PAR-1 and tau phosphorylation site Ser262 are critical for the enhancement of tau-induced axon degeneration caused by milton knockdown
We investigated the role of tau phosphorylation at Ser262 in the enhancement of tau-induced axon degeneration caused by milton knockdown. We first examined whether PAR-1 knockdown enhances or suppresses tau-induced axon degeneration in the milton knockdown background. RNAi-mediated knockdown of PAR-1 significantly suppressed neurodegeneration in the lamina of flies expressing human tau and milton RNAi (Figure 5A and 5B; 5D, quantification). This effect is not due to titration of the effectiveness of RNAi, since the expression of an RNAi against firefly luciferase did not significantly suppress tau-induced neurodegeneration in the milton knockdown background (Figure 5A and 5C; 5D, quantification).
Fig. 5. PAR-1 and tau phosphorylation site Ser262 are critical for the enhancement of tau-induced neurodegeneration caused by milton knockdown. 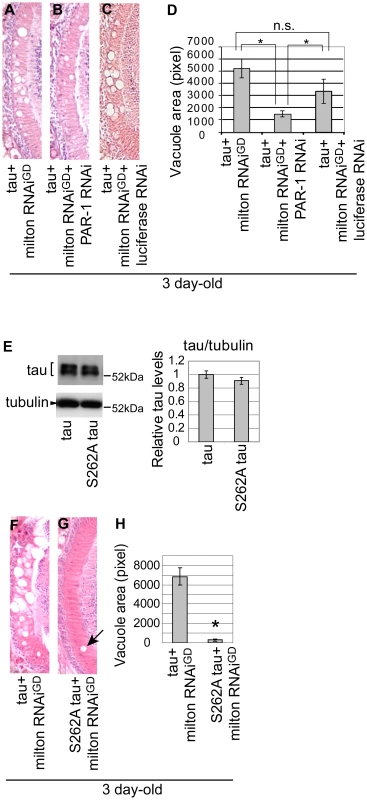
(A–C) PAR-1 knockdown suppresses the enhancement of tau-induced neurodegeneration caused by milton knockdown. (A–C) The lamina co-expressing tau and milton RNAi (A), co-expressing tau, milton RNAi and PAR-1 RNAi (B), and co-expressing tau, milton RNAi and luciferase RNAi (C). (D) Quantification of neurodegeneration, mean ± SEM, n = 12. Significant difference was found between tau+milton RNAiGD and tau+ milton RNAiGD+PAR-1 RNAi or between tau+milton RNAiGD +luciferase RNAi and tau+ milton RNAiGD+PAR-1 RNAi (*, p<0.05, Student's t-test), but not between tau+milton RNAiGD and tau+ milton RNAiGD+luciferase RNAi. All flies were 3 days-after-eclosion (day-old). (E–H) The Ser262 phosphorylation site in tau is necessary for the enhancement of tau-induced neurodegeneration caused by milton knockdown. (E) Western blots of the eyes from flies expressing wild-type tau or S262A mutant tau. Blots were probed with anti-tau or anti-tubulin (loading control). Expression levels were similar (mean ± SD, n = 5; p>0.05, Student's t-test). (F–H) The lamina co-expressing wild-type tau and milton RNAi (F), or co-expressing S262A tau and milton RNAi (G). (H) Quantification of neurodegeneration, presented as mean ± SEM, n = 9–12. *, p<0.05, Student's t-test. All flies were 3 days-after-eclosion (day-old). Next, to determine whether the Ser262 site is required for the knockdown of milton to enhance tau-induced axon degeneration, transgenic flies carrying human tau with the S262A mutation (S262A tau) expressed at the levels similar to the expression of wild-type tau ([36] and Figure 5E) were used. It has been reported that introduction of the S262A mutation dramatically rescues tau-induced reduction in external eye size [35], [36], which is due to apoptosis during the larval stage [27]. Interestingly, we found that expression of S262A tau caused age-dependent neurodegeneration in the lamina similar to that caused by wild type tau: in the flies expressing S262A tau, degeneration in the lamina was undetectable or very mild in flies at 3-day-old, while it was prominent at 10-day-old (Figure S10). Using S262A tau flies, we found that the introduction of the S262A mutation suppressed the enhancement of tau-induced axon degeneration caused by milton knockdown (Figure 5F and 5G; 5H, quantification). Taken together, these results suggest that tau phosphorylation at Ser262 and PAR-1 play a critical role in the enhancement of tau-induced neurodegeneration caused by milton knockdown.
Knockdown of milton or Miro increases the levels of active PAR-1
Our results demonstrate that knockdown of milton or Miro enhances tau-induced neurodegeneration and increases tau phosphorylation at Ser262. PAR-1 mediates the increase in tau phosphorylation at Ser262, and tau phosphorylation site Ser262 and PAR-1 are critical for the enhancement of tau-induced neurodegeneration caused by milton knockdown. To further investigate the relationship between loss of axonal mitochondria and PAR-1, the effect of knockdown of milton or Miro on PAR-1 activity was examined.
To detect active PAR-1, a phospho-specific antibody that recognizes phosphorylated Thr408 of PAR-1, which is important for PAR-1 activity [37], was used. The titer of the antibody is not sufficient to detect endogenous PAR-1 in fly eyes [37], but the antibody recognizes the active form of PAR-1 when PAR-1 is overexpressed [37]. Co-expression of milton RNAi increases the levels of Thr408-phosphorylated PAR-1 in the fly eye (Figure 6A). In addition to the levels of Thr408-phosphorylated PAR-1, we observed an increase in total PAR-1 levels with knockdown of milton (Figure 6A). Similar results were obtained with another milton RNAi line (milton RNAiTRiP) (Figure 6B). Furthermore, expression of Miro RNAi also caused an increase in the levels of Thr408-phosphorylated PAR-1 as well as total PAR-1 in the fly eyes (Figure 6C). These effects are not due to non-specific effect of RNAi expression, since the expression of an RNAi against firefly luciferase did not increase either the levels of Thr408-phosphorylated PAR-1 or total PAR-1 (Figure 6D).
Fig. 6. Knockdown of milton or Miro increases the levels of active PAR-1. 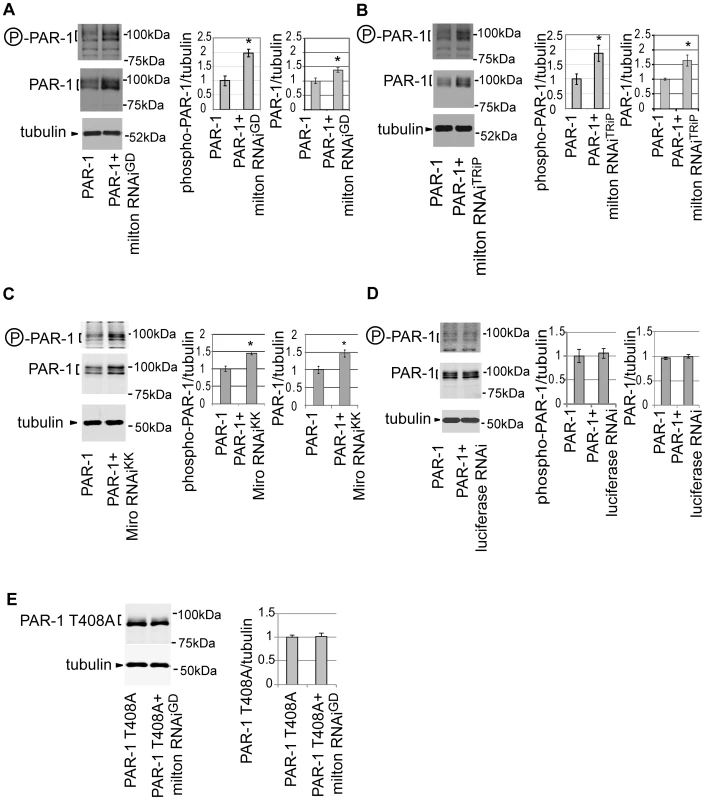
(A) Western blots of eyes from flies expressing myc-tagged PAR-1 alone or flies co-expressing PAR-1 and milton RNAiGD. Blots were probed with anti-phospho-T408 PAR-1 (P-PAR-1), anti-myc (PAR-1), or anti-tubulin. Tubulin was used as loading control. Mean ± SD, n = 5; *, p<0.05, Student's t-test. (B) Western blots of eyes from flies expressing myc-tagged PAR-1 or co-expressing PAR-1 and milton RNAiTRiP. Blots were probed with anti-phospho-T408 PAR-1 (P-PAR-1), anti-myc (PAR-1), or anti-tubulin. Mean ± SD, n = 5; *, p<0.05, Student's t-test. (C) Western blots of eyes from flies expressing myc-tagged PAR-1 alone or flies co-expressing PAR-1 and Miro RNAiKK. Blots were probed with anti-phospho-T408 PAR-1 (P-PAR-1), anti-myc (PAR-1), or anti-tubulin. Mean ± SD, n = 5; *, p<0.05, Student's t-test. (D) Western blots of eyes from flies expressing myc-tagged PAR-1 alone or flies co-expressing PAR-1 and luciferase RNAi. Blots were probed with anti-phospho-T408 PAR-1 (P-PAR-1), anti-myc (PAR-1), or anti-tubulin. No significant difference was found (mean ± SD, n = 5; p>0.05, Student's t-test). (E) Western blots of eyes from flies expressing myc-tagged PAR-1 T408A alone or flies co-expressing PAR-1 T408A and milton RNAiGD. Blots were probed with anti-myc (PAR-1 T408A) or anti-tubulin. No significant difference was found (mean ± SD, n = 5; p>0.05, Student's t-test). Flies were 3 days-after-eclosion (day-old). A previous report showed that total PAR-1 level increased when it was phosphorylated at Thr408 in Drosophila [37]. To examine whether the increase in PAR-1 levels with milton knockdown is Thr408-dependent, transgenic flies carrying PAR-1 with unphosphorylatable alanine mutation at Thr408 (PAR-1 T408A) [37] were used. Milton RNAi coexpression did not increase PAR-1 T408A protein levels (Figure 6E), indicating that Thr408 is important for the increase in PAR-1 levels caused by milton knockdown.
Milton knockdown did not cause non-specific activation of kinases, since it did not increase the level of phosphorylated, active p44mapk in fly eyes (Figure S11). Moreover, milton knockdown did not cause non-specific accumulation of overexpressed proteins, or an increase in the expression of genes under the control of GAL4/UAS system, since it did not increase the levels of total p44mapk, GFP, or amyloid precursor protein expressed via the GAL4/UAS system (Figure S11). Taken together, these results demonstrate that milton knockdown specifically increases the level of active PAR-1.
We also observed that the phenotype induced by PAR-1 overexpression was enhanced by milton knockdown. Overexpression of PAR-1 in fly eyes has been reported to cause eye degeneration [35], and we found that overexpression of PAR-1 caused age-dependent, mild neurodegeneration in the lamina: neurodegeneration in the lamina is undetectable in flies overexpressing PAR-1 at 3-day-old, while it is detectable at 10-day-old (Figure S12A–S12C, S12D, quantification). Co-expression of PAR-1 and milton RNAi caused prominent neurodegeneration in the lamina at 3-day-old, when neither PAR-1 overexpression alone or knockdown of milton alone caused neurodegeneration (Figure S12, S12E–S12G; S12H, quantification). These phenotypic analyses further suggest that milton knockdown increases PAR-1 activity in the eye.
RNAi–mediated knockdown of milton or Miro alone in neurons causes age-dependent neurodegeneration in the fly brain
Although knockdown of milton or Miro without human tau overexpression did not cause neurodegeneration in the young flies (Figure 1H and 1K), we found that knockdown of milton or Miro alone caused late-onset neurodegeneration in the fly brain. Expression of milton RNAi in the fly eyes and brains with a combination of two GAL4 drivers, the pan-retinal gmr-GAL4 driver and pan-neuronal elav-GAL4 driver, caused age-dependent neurodegeneration (Figure 7A, 21 days-after-eclosion (day-old)). Interestingly, although milton RNAi was expressed in the all neurons in the eye and brain, degeneration was the most prominent in the optic lobe (Figure 7A). No degeneration was observed in the brain in flies expressing an RNAi against firefly luciferase at the same age (Figure 7B, control).
Fig. 7. RNAi–mediated knockdown of milton or Miro in neurons causes age-dependent neurodegeneration in the fly brain. 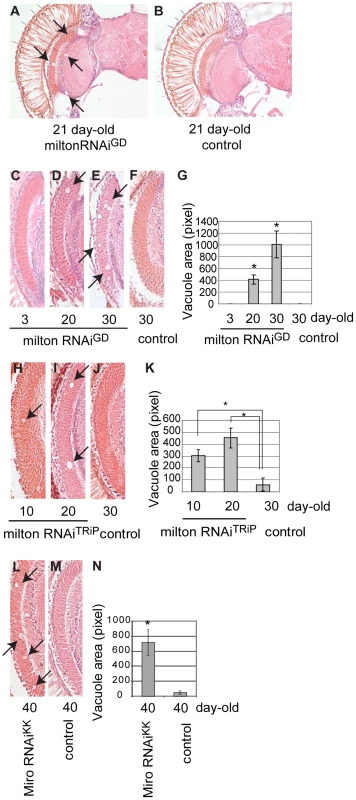
(A–B) The brain of flies expressing milton RNAi with a combination of two drivers, the pan-neuronal elav-GAL4 driver and pan-retinal gmr-GAL4 driver (A) and control (luciferase RNAi) (B). Flies are at 21-day-old. Neurodegeneration is indicated by arrows. (C–G) The lamina in flies expressing milton RNAi at 3-day-old (C), 20-day-old (D) or 30-day-old (E), or the lamina of control flies (gmr-GAL4 driver only) at 30-day-old (F). (G) Quantification of neurodegeneration (indicated by arrows in D and E), mean ± SEM, n = 10–12. *, p<0.05, Student's t-test. (H-K) Quantification of neurodegeneration in the lamina in the flies expressing milton RNAiTRiP with a combination of the elav-GAL4 driver and gmr-GAL4 driver at 10-day-old (H) and 20-day-old (I), or neurodegeneration in the lamina of control flies (drivers only) at 30-day-old (J). (K) Quantification of neurodegeneration (arrows in H and I). Mean ± SEM, n = 10–12. *, p<0.05, Student's t-test. (L–N) Quantification of neurodegeneration in the lamina in the flies expressing Miro RNAiKK with the gmr-GAL4 driver (L) or in the lamina of control flies (drivers only) (M) at 40-day-old. (N) Quantification of neurodegeneration (arrows in L). Mean ± SEM, n = 10–12. *, p<0.05, Student's t-test. We quantified age-dependent progression of neurodegeneration caused by milton knockdown in the lamina, where neurodegeneration was the most prominent. Degeneration in the lamina is undetectable at 3-day-old, which is in line with the previous observation in milton mutant flies [25]. In contrast, degeneration was observed at 20-day-old and was more prominent at 30-day-old, indicating that milton knockdown causes late-onset and progressive neurodegeneration (Figure 7, compare 7C (3-day-old), 7D (20-day-old) and 7E (30-day-old); G, quantification of vacuole areas). No degeneration was observed in the lamina in control flies at 30-day-old (Figure 7F).
To limit the possibility of off-target effects of RNAi, another independent transgenic fly line carrying a milton RNAi that targets a different region of milton (milton RNAiTRiP) was used. Expression of milton RNAiTRiP also caused age-dependent neurodegeneration in the lamina (Figure 7H–7J; 7K, quantification of vacuole areas). Knockdown of Miro in the fly brains also caused neurodegeneration in the lamina in aged flies (Figure 7L–7M; 7N, quantification of vacuole areas). Collectively, these results suggest that loss of axonal mitochondria is sufficient to cause late-onset neurodegeneration.
While our paper is under review, it was reported that knockdown of milton led to progressive axon degeneration in the Drosophila wing neurons [38].
RNAi–mediated knockdown of Drosophila tau or PAR-1 suppresses milton knockdown-induced neurodegeneration
The Drosophila tau exhibits a high degree of similarity with the human tau protein and shares a numbers of important features such as the microtubule-binding domains [39] and several phosphorylation sites critical for its functions and toxicity [40]. Overexpression of Drosophila tau has been reported to be capable of inducing neuronal dysfunction and neurodegeneration [41]–[43]. We examined whether Drosophila tau is involved in neurodegeneration caused by milton knockdown. We found that expression of RNAi targeting Drosophila tau reduced tau mRNA levels in the fly brain (Figure S13) and significantly suppressed neurodegeneration in the lamina caused by milton knockdown (Figure 8A and 8B; 8D, quantification). This effect is not due to titration of the effectiveness of RNAi, since the expression of an RNAi against firefly luciferase did not suppress neurodegeneration caused by milton knockdown (Compare Figure 8A and 8C; 8D, quantification). We further examined whether PAR-1 is involved in neurodegeneration caused by milton knockdown. RNAi-mediated knockdown of PAR-1 significantly suppressed neurodegeneration in the lamina of flies expressing milton RNAi (Figure 8E and 8F; 8G, quantification). These results suggest that Drosophila endogenous tau and PAR-1 contribute to milton knockdown-induced neurodegeneration and further support the conclusions of this study.
Fig. 8. RNAi–mediated knockdown of Drosophila tau or PAR-1 suppresses milton knockdown-induced neurodegeneration. 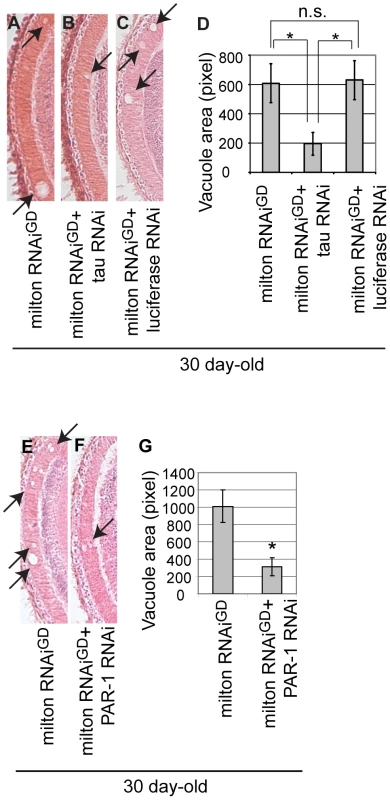
(A–D) The lamina of flies expressing milton RNAi alone (A), co-expressing milton RNAi and Drosophila tau RNAi (B), and co-expressing milton RNAi and luciferase RNAi (C). (D) Quantification of neurodegeneration (indicated by arrows), mean ± SEM, n = 12. *, p<0.05, Student's t-test. Flies were 30-day-old. (E–G) The lamina of flies expressing milton RNAi alone (E) and co-expressing milton RNAi and PAR-1 RNAi (F). (G) Quantification of neurodegeneration (indicated by arrows), mean ± SEM, n = 10–12. *, p<0.05, Student's t-test. Flies were 30-day-old. Discussion
Abnormal phosphorylation and toxicity of tau are thought to play a critical role in the pathogenesis of Alzheimer's disease (AD). Accumulation of amyloid-β peptides is thought to be causative for AD and has been suggested to cause tau abnormality [44]–[53], however, the underlying mechanisms are not clear. A reduction in the number of mitochondria in the axon is observed in the brains of AD patients [3], and we and others previously reported that amyloid-β peptides reduce the number of mitochondria in the axons [3]–[11]. In this study, we examined whether and how loss of axonal mitochondria increases phosphorylation of human tau at AD-related sites and enhances tau toxicity. Our data demonstrate that loss of axonal mitochondria caused by knockdown of milton or Miro increases tau phosphorylation at an AD-related site Ser262 through PAR-1, promotes detachment of tau from microtubules, and enhances tau-mediated neurodegeneration. These results suggest that loss of axonal mitochondria may play an important role in tau phosphorylation and toxicity in the pathogenesis of AD.
It has been reported that, in non-neuronal cultured cells or primary-cultured hippocampal neurons with virus-mediated overexpression of human tau, an excess of microtubule-bound tau blocks microtubule-dependent transport of vesicles and organelle including mitochondria and causes synaptic degeneration [54], [55]. These works have demonstrated that tau phosphorylation at Ser262 by a PAR-1 homolog MARK2 removes tau from the microtubule tracks, which restores microtubule-dependent transport of vesicles and organelle, and rescues accompanied synaptic degeneration [54]. Thus, tau phosphorylation at Ser262 plays a protective role against tau-induced toxicity in their models in which an excess of microtubule-bound tau blocks traffic of vesicles and organelle.
In contrast, this study examined whether and how specific loss of axonal mitochondria promotes tau phosphorylation and toxicity. To address this question in vivo, we used a Drosophila model of human tau toxicity [22]. In this model, we did not observe severe defects in microtubule-dependent transport under our experimental condition, since mitochondria are present at the synaptic terminals of neurons expressing human tau in the young flies (Figure S2). To chronically deplete mitochondria from the axon, we used knockdown of milton or Miro. The most critical difference between the models used in Thies and Mandelkow [54] and our model is that, in the models of Thies and Mandelkow, mitochondrial transport defects were depending on tau binding to microtubules, while in our model, mitochondria were chronically depleted from the axon by milton knockdown.
Using this model system, we found that milton knockdown significantly enhanced tau-mediated neurodegeneration. Milton knockdown increased the levels of active PAR-1 and tau phosphorylation at Ser262, and promoted detachment of tau from microtubules. If the enhancement of tau toxicity caused by milton knockdown in our model is due to an additive reduction in the number of axonal mitochondria, blocking tau phosphorylation at Ser262, which increases tau binding to microtubules and blocks microtubule-dependent transport, would enhance neurodegeneration. However, our results have shown that blocking tau phosphorylation at Ser262 by PAR-1 knockdown rescues the enhancement of tau-mediated neurodegeneration in the milton knockdown background. These results suggest that the enhancement of tau toxicity in the milton knockdown background is not likely to be due to an additive reduction of axonal transport of mitochondria caused by an excess of microtubule-bound tau. Rather, this study suggests that, when axonal mitochondria are chronically depleted, increased free, microtubule-unbound, Ser262-phosphorylated tau promotes neurodegeneration.
A fine-tuned balance of microtubule-binding of tau is critical for its physiological functions. It has been suggested that both an excess of microtubule-bound tau and an excess of free, microtubule-unbound tau can cause toxicity [56]. Since tau phosphorylation at Ser262 promotes tau detachment from microtubules [32], [33], Ser262 phosphorylation by MARK/PAR-1 plays critical roles under both physiological and pathological conditions. Thus, when axonal distribution of vesicles and organelle are reduced by tau, detachment of tau can play a protective role by temporarily enhancing microtubule-dependent transport [54]. However, our results suggest that, under pathological environments in which axonal mitochondria are chronically depleted, microtubule-unbound, free, Ser262-phosphorylated tau in the axons may become toxic and cause neurodegeneration.
We found that the levels of active PAR-1 are increased by milton knockdown (Figure 6). PAR-1 is activated by various stress stimuli such as high osmolarity and amyloid precursor protein accumulation in Drosophila [37]. Our results suggest that loss of axonal mitochondria may trigger a process that increases the levels of active PAR-1. However, the detailed mechanisms by which milton knockdown increases the levels of active PAR-1 require further investigations. PAR-1 activity is regulated by various kinases including LKB1, aPKC, and Death-associated protein kinase (DAPK) [37], [57], [58]. A recent report demonstrated that PAR-1 protein levels were regulated by the Drosophila homolog of adducin, a cytoskeletal protein involved in regulating actin filament dynamics [59]. Milton knockdown may act through one or a combination of the mechanisms to increase the level of active PAR-1.
Detachment of tau from microtubules has been suggested to initiate its abnormal metabolism and toxicity of tau in AD pathogenesis [56], however, the underlying mechanisms are not fully understood. This study shows that loss of axonal mitochondria promotes detachment tau from microtubules and enhances tau-mediated neurodegeneration, and tau phosphorylation at AD-related Ser262 by PAR-1 plays a critical role in this process. Our results also suggest that an increase in Ser262-phosphorylated, microtubule-unbound tau may contribute to neurodegeneration under pathological conditions in which axonal mitochondria is depleted. An important question is how free, microtubule-unbound, Ser262-phosphorylated tau causes neurodegeneration under such pathological conditions. Loss of axonal mitochondria would disrupt multiple signaling pathways in the axon, and those changes may further enhance toxicity of tau. Elucidation of such mechanisms will further our understanding of tau-mediated neurodegeneration in the pathogenesis of AD.
In summary, this study highlights a potential role of loss of axonal mitochondria in tau phosphorylation and toxicity in AD pathogenesis. Reductions in the function and number of mitochondria in the axon have also been implicated in several neurodegenerative diseases such as Parkinson's disease, Huntington's disease, and amyotrophic lateral sclerosis [60]. Our study raises an interesting possibility that mitochondrial mislocalization may cause abnormal metabolism and toxicity of other disease-related, aggregation-prone proteins.
Materials and Methods
Fly stocks
Transgenic fly lines carrying UAS-luciferase RNAi were established following the method described previously [61]. Target sequences were amplified by PCR from luciferase cDNA using primers (for, 5′-CCGGAATTCGATATGGGCTGAATACAAATCACAGAATCG-3′, rev, 5′-CTAGTCTAGATTCATTAAAACCGGGAGGTAGATGAGATGT-3′ ), and the resulting constructs were subcloned into the pUAST Drosophila transformation vector and microinjected into fly embryos of the w1118 genotype. Transgenic fly lines carrying UAS-Miro RNAi were established by microinjecting the Miro RNAi construct (a kind gift from Dr. Barry Dickson (Research Institute of Molecular Pathology, Austria)) into fly embryos of the w1118 genotype. The transgenic fly lines carrying S262A mutant tau was described previously [36]. Other fly stocks are listed in Table S1. Fly genotypes used in each experiment are listed in Table S2.
Electron microscopy
Probosces were removed from decapitated heads, which were then immersion-fixed overnight in 2.5% glutaraldehyde and 2% paraformaldehyde in 0.1 M sodium cacodylate buffer at 4°C. Samples were post-fixed 1 hr in 1% osmium tetroxide in 0.1 M sodium cacodylate buffer on ice. After washing, samples were stained en bloc with 0.5% aqueous uranyl acetate for 1 hr, dehydrated with ethanol and embedded in Epon. Thin-sections (70 nm) of laminas, in which photoreceptor axons were cut longitudinally, were collected on copper grids. The sections were stained with 2% uranyl acetate in 70% ethanol and Reynolds' lead citrate solution. Electron micrographs were obtained with a VELETA CCD Camera (Olympus Soft Imaging Solutions GMBH) mounted on a JEM-1010 electron microscope (Jeol Ltd.).
Histological analysis
Preparation of paraffin sections, hematoxylin and eosin staining, and analysis of neurodegeneration were described previously [53]. To analyze internal eye structure, heads of female flies were fixed in Bouin's fixative (EMS) for 48 hr at room temperature, incubated 24 hr in 50 mM Tris/150 mM NaCl, and embedded in paraffin. Serial sections (6 µm thickness) through the entire heads were prepared, stained with hematoxylin and eosin (Vector), and examined by bright-field microscopy. Images of the sections that include the lamina were captured with Insight 2 CCD Camera (SPOT), and vacuole area was measured using Image J (NIH). Heads from more than five flies (more than 10 hemispheres) were analyzed for each genotype.
Western blotting
Twenty fly heads for each genotype were homogenized in SDS-Tris-Glycine sample buffer, and the same amount of the lysate was loaded to each lane of multiple 10% Tris-Glycine gels and transferred to nitrocellulose membrane. The membranes were blocked with 5% milk (Nestle), blotted with the antibodies described below, incubated with appropriate secondary antibody and developed using ECL plus Western Blotting Detection Reagents (GE Healthcare) or imaging with an Odyssey system. One of the membranes was probed with anti-tubulin, and used as the loading control for other blots in each experiment. Anti-tau monoclonal antibody (Tau46, Zymed), and anti-tau pSer262 (Biosource and Calbiochem), phospho-Thr231 (AT180, Thermo and Endogen), anti-HA (Santa Cruz), anti-myc (Millipore), anti-active p44mapk (Promega), anti-tubulin (Sigma), anti-GFP (Clontech) were purchased. Anti-tau pS202 (CP13) was a kind gift from Dr. Peter Davis (Albert Einstein College of Medicine). Anti-PAR-1 pT408 was described previously [37]. The signal intensity was quantified using ImageJ (NIH) or an Odyssey system. Western blots were repeated a minimum of three times with different animals and representative blots are shown. Flies used for Western blotting were 3–5 day-old after eclosion.
RNA extraction and quantitative real-time PCR analysis
Quantitative real-time PCR analysis was performed as described previously [53]. More than thirty flies for each genotype were collected and frozen. Heads were mechanically isolated, and total RNA was extracted using TRIzol (Invitrogen) according to the manufacturer's protocol with an additional centrifugation step (11,000× g for 10 min) to remove cuticle membranes prior to the addition of chloroform. Total RNA was reverse-transcribed using Superscript II reverse transcriptase (Invitrogen), and the resulting cDNA was used as a template for PCR on a 7500 fast real time PCR system (Applied Biosystems). The average threshold cycle value (Ct) was calculated from five replicates per sample. Expression of genes of interest was standardized relative to actin, rp49 or TBP. Relative expression values were determined by the deltaCt method according to quantitative PCR Analysis User Bulletin (Applied Biosystems). Primers were designed using Primer-Blast (NIH).
milton for 5′-GGCTTCAGGGCCAGGTATCT-3′
milton rev 5′-GCCGAACTTGGCTGACTTTG-3′
Miro for 5′-AAAAGCACCTCATTCTGCGT-3′
Miro rev 5′-CCTCAGGTGAGGAAACGCT-3′
dTau for 5′-AAGCCCGGTGGCGGTGAGAA-3′
dTau rev 5′-GCGCCAGAAGCCGTCATGGA-3′
Actin for 5′-TGCACCGCAAGTGCTTCTAA-3′
Actin rev 5′-TGCTGCACTCCAAACTTCCA-3′
rp49 for 5′-GCTAAGCTGTCGCACAAATG-3′
rp49 rev 5′ - GTTCGATCCGTAACCGATGT-3′
TBP for 5′ - GCGGCTGTGATTATGCGAAT-3′
TBP rev 5′-AGGGAAACCGAGCTTTTGGA-3′
Microtubule binding assay
Microtubule binding assay was performed using a previously reported procedure with a modification [62]. Fifty heads from adult flies expressing the human tau protein with the gmr-GAL4 driver were collected and homogenized in 150 µl of Buffer-C+ [50 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid pH 7.1, 1 mM MgCl2, 1 mM ethylene glycol tetraacetic acid, protease inhibitor cocktail (Roche), and phosphatase inhibitor cocktail (Sigma-Aldrich)] in the presence of taxol 20 µM (Sigma-Aldrich) diluted in dimethylsulfoxide. After centrifugation at 1,000× g for 10 min, aliquot of supernatant was subjected to Western blotting as the “total fraction”. The remaining supernatant was layered onto a 2 volume cushion of buffer-C+ with 50% sucrose. After centrifugation at 100, 000× g for 30 min, the upper fraction containing soluble tubulin was collected as the microtubule-free fraction and the pellet containing microtubule polymers and proteins bound to microtubules was resuspended in 150 µl of SDS-Tris-Glycine sample buffer. Protein concentration in each fraction was measured using the BCA Protein Assay Kit (Pierce). The same amount of protein was loaded to each lane of Tris-Glycine gels and analyzed by western blotting using anti-tau antibody (Tau46, Zymed) or anti-tubulin (Sigma). For quantification, the signal intensity in each lane was quantified with an Odyssey system.
Statistics
Statistics was done with the JMP software (SAS) with Student's t or Tukey-Kramer HSD. Values are given as mean ± standard deviation or standard error.
Supporting Information
Zdroje
1. WangX, SchwarzTL (2009) The mechanism of Ca2+ -dependent regulation of kinesin-mediated mitochondrial motility. Cell 136 : 163–174.
2. DuncanJE, GoldsteinLS (2006) The genetics of axonal transport and axonal transport disorders. PLoS Genet 2: e124 doi:10.1371/journal.pgen.0020124.
3. WangX, SuB, LeeHG, LiX, PerryG, et al. (2009) Impaired balance of mitochondrial fission and fusion in Alzheimer's disease. J Neurosci 29 : 9090–9103.
4. StokinGB, LilloC, FalzoneTL, BruschRG, RockensteinE, et al. (2005) Axonopathy and transport deficits early in the pathogenesis of Alzheimer's disease. Science 307 : 1282–1288.
5. ChoDH, NakamuraT, FangJ, CieplakP, GodzikA, et al. (2009) S-nitrosylation of Drp1 mediates beta-amyloid-related mitochondrial fission and neuronal injury. Science 324 : 102–105.
6. ZhaoXL, WangWA, TanJX, HuangJK, ZhangX, et al. (2010) Expression of beta-amyloid Induced age-dependent presynaptic and axonal changes in Drosophila. J Neurosci 30 : 1512–1522.
7. Iijima-AndoK, HearnSA, ShentonC, GattA, ZhaoL, et al. (2009) Mitochondrial Mislocalization Underlies Aβ42-Induced Neuronal Dysfunction in a Drosophila Model of Alzheimer's Disease. PLoS ONE 4: e8310 doi:10.1371/journal.pone.0008310.
8. RuiY, TiwariP, XieZ, ZhengJQ (2006) Acute impairment of mitochondrial trafficking by beta-amyloid peptides in hippocampal neurons. J Neurosci 26 : 10480–10487.
9. VosselKA, ZhangK, BrodbeckJ, DaubAC, SharmaP, et al. (2010) Tau reduction prevents Abeta-induced defects in axonal transport. Science 330 : 198.
10. ZempelH, ThiesE, MandelkowE, MandelkowEM (2010) Abeta oligomers cause localized Ca(2+) elevation, missorting of endogenous Tau into dendrites, Tau phosphorylation, and destruction of microtubules and spines. J Neurosci 30 : 11938–11950.
11. StoothoffW, JonesPB, Spires-JonesTL, JoynerD, ChhabraE, et al. (2009) Differential effect of three-repeat and four-repeat tau on mitochondrial axonal transport. J Neurochem 111 : 417–427.
12. CheeFC, MudherA, CuttleMF, NewmanTA, MacKayD, et al. (2005) Over-expression of tau results in defective synaptic transmission in Drosophila neuromuscular junctions. Neurobiol Dis 20 : 918–928.
13. DubeyM, ChaudhuryP, KabiruH, SheaTB (2008) Tau inhibits anterograde axonal transport and perturbs stability in growing axonal neurites in part by displacing kinesin cargo: neurofilaments attenuate tau-mediated neurite instability. Cell Motil Cytoskeleton 65 : 89–99.
14. QuintanillaRA, Matthews-RobersonTA, DolanPJ, JohnsonGV (2009) Caspase-cleaved tau expression induces mitochondrial dysfunction in immortalized cortical neurons: implications for the pathogenesis of Alzheimer disease. J Biol Chem 284 : 18754–18766.
15. LeeVM, GoedertM, TrojanowskiJQ (2001) Neurodegenerative tauopathies. Annu Rev Neurosci 24 : 1121–1159.
16. AugustinackJC, SchneiderA, MandelkowEM, HymanBT (2002) Specific tau phosphorylation sites correlate with severity of neuronal cytopathology in Alzheimer's disease. Acta Neuropathol 103 : 26–35.
17. HollenbeckPJ, SaxtonWM (2005) The axonal transport of mitochondria. J Cell Sci 118 : 5411–5419.
18. GuoX, MacleodGT, WellingtonA, HuF, PanchumarthiS, et al. (2005) The GTPase dMiro is required for axonal transport of mitochondria to Drosophila synapses. Neuron 47 : 379–393.
19. GlaterEE, MegeathLJ, StowersRS, SchwarzTL (2006) Axonal transport of mitochondria requires milton to recruit kinesin heavy chain and is light chain independent. J Cell Biol 173 : 545–557.
20. FranssonS, RuusalaA, AspenstromP (2006) The atypical Rho GTPases Miro-1 and Miro-2 have essential roles in mitochondrial trafficking. Biochem Biophys Res Commun 344 : 500–510.
21. StowersRS, MegeathLJ, Gorska-AndrzejakJ, MeinertzhagenIA, SchwarzTL (2002) Axonal transport of mitochondria to synapses depends on milton, a novel Drosophila protein. Neuron 36 : 1063–1077.
22. WittmannCW, WszolekMF, ShulmanJM, SalvaterraPM, LewisJ, et al. (2001) Tauopathy in Drosophila: neurodegeneration without neurofibrillary tangles. Science 293 : 711–714.
23. BrandAH, PerrimonN (1993) Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118 : 401–415.
24. StamerK, VogelR, ThiesE, MandelkowE, MandelkowEM (2002) Tau blocks traffic of organelles, neurofilaments, and APP vesicles in neurons and enhances oxidative stress. J Cell Biol 156 : 1051–1063.
25. Gorska-AndrzejakJ, StowersRS, BoryczJ, KostylevaR, SchwarzTL, et al. (2003) Mitochondria are redistributed in Drosophila photoreceptors lacking milton, a kinesin-associated protein. J Comp Neurol 463 : 372–388.
26. LiuS, SawadaT, LeeS, YuW, SilverioG, et al. (2012) Parkinson's Disease-Associated Kinase PINK1 Regulates Miro Protein Level and Axonal Transport of Mitochondria. PLoS Genet 8: e1002537 doi:10.1371/journal.pgen.1002537.
27. JacksonGR, Wiedau-PazosM, SangT-K, WagleN, BrownCA, et al. (2002) Human Wild-Type Tau Interacts with wingless Pathway Components and Produces Neurofibrillary Pathology in Drosophila. Neuron 34 : 509–519.
28. ShulmanJM, FeanyMB (2003) Genetic modifiers of tauopathy in Drosophila. Genetics 165 : 1233–1242.
29. BlardO, FeuilletteS, BouJ, ChaumetteB, FrebourgT, et al. (2007) Cytoskeleton proteins are modulators of mutant tau-induced neurodegeneration in Drosophila. Hum Mol Genet 16 : 555–566.
30. AmbegaokarSS, JacksonGR (2011) Functional Genomic Screen and Network Analysis Reveal Novel Modifiers of Tauopathy Dissociated from Tau Phosphorylation. Hum Mol Genet 20 : 4947–4977.
31. GotzJ, GladbachA, PennanenL, van EerselJ, SchildA, et al. (2010) Animal models reveal role for tau phosphorylation in human disease. Biochim Biophys Acta 1802 : 860–871.
32. BiernatJ, GustkeN, DrewesG, MandelkowEM, MandelkowE (1993) Phosphorylation of Ser262 strongly reduces binding of tau to microtubules: distinction between PHF-like immunoreactivity and microtubule binding. Neuron 11 : 153–163.
33. DrewesG, TrinczekB, IllenbergerS, BiernatJ, Schmitt-UlmsG, et al. (1995) Microtubule-associated protein/microtubule affinity-regulating kinase (p110mark). A novel protein kinase that regulates tau-microtubule interactions and dynamic instability by phosphorylation at the Alzheimer-specific site serine 262. J Biol Chem 270 : 7679–7688.
34. MarxA, NugoorC, PanneerselvamS, MandelkowE (2010) Structure and function of polarity-inducing kinase family MARK/Par-1 within the branch of AMPK/Snf1-related kinases. FASEB J 24 : 1637–1648.
35. NishimuraI, YangY, LuB (2004) PAR-1 kinase plays an initiator role in a temporally ordered phosphorylation process that confers tau toxicity in Drosophila. Cell 116 : 671–682.
36. Iijima-AndoK, ZhaoL, GattA, ShentonC, IijimaK (2010) A DNA damage-activated checkpoint kinase phosphorylates tau and enhances tau-induced neurodegeneration. Hum Mol Genet 19 : 1930–1938.
37. WangJW, ImaiY, LuB (2007) Activation of PAR-1 kinase and stimulation of tau phosphorylation by diverse signals require the tumor suppressor protein LKB1. J Neurosci 27 : 574–581.
38. FangY, SoaresL, TengX, GearyM, BoniniNM (2012) A Novel Drosophila Model of Nerve Injury Reveals an Essential Role of Nmnat in Maintaining Axonal Integrity. Curr Biol 10.1016/j.cub.2012.01.065.
39. HeidaryG, FortiniME (2001) Identification and characterization of the Drosophila tau homolog. Mech Dev 108 : 171–178.
40. SofolaO, KerrF, RogersI, KillickR, AugustinH, et al. (2010) Inhibition of GSK-3 ameliorates Abeta pathology in an adult-onset Drosophila model of Alzheimer's disease. PLoS Genet 6: e1001087 doi:10.1371/journal.pgen.1001087.
41. MershinA, PavlopoulosE, FitchO, BradenBC, NanopoulosDV, et al. (2004) Learning and memory deficits upon TAU accumulation in Drosophila mushroom body neurons. Learn Mem 11 : 277–287.
42. UbhiKK, ShaibahH, NewmanTA, ShepherdD, MudherA (2007) A comparison of the neuronal dysfunction caused by Drosophila tau and human tau in a Drosophila model of tauopathies. Invert Neurosci 7 : 165–171.
43. ChenX, LiY, HuangJ, CaoD, YangG, et al. (2007) Study of tauopathies by comparing Drosophila and human tau in Drosophila. Cell Tissue Res 329 : 169–178.
44. NicollJA, WilkinsonD, HolmesC, SteartP, MarkhamH, et al. (2003) Neuropathology of human Alzheimer disease after immunization with amyloid-beta peptide: a case report. Nat Med 9 : 448–452.
45. LewisJ, DicksonDW, LinWL, ChisholmL, CorralA, et al. (2001) Enhanced neurofibrillary degeneration in transgenic mice expressing mutant tau and APP. Science 293 : 1487–1491.
46. GotzJ, ChenF, van DorpeJ, NitschRM (2001) Formation of neurofibrillary tangles in P301l tau transgenic mice induced by Abeta 42 fibrils. Science 293 : 1491–1495.
47. OddoS, CaccamoA, ShepherdJD, MurphyMP, GoldeTE, et al. (2003) Triple-transgenic model of Alzheimer's disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron 39 : 409–421.
48. OddoS, BillingsL, KesslakJP, CribbsDH, LaFerlaFM (2004) Abeta immunotherapy leads to clearance of early, but not late, hyperphosphorylated tau aggregates via the proteasome. Neuron 43 : 321–332.
49. RapoportM, DawsonHN, BinderLI, VitekMP, FerreiraA (2002) Tau is essential to beta -amyloid-induced neurotoxicity. Proc Natl Acad Sci U S A 99 : 6364–6369.
50. KingME, KanHM, BaasPW, ErisirA, GlabeCG, et al. (2006) Tau-dependent microtubule disassembly initiated by prefibrillar beta-amyloid. J Cell Biol 175 : 541–546.
51. RobersonED, Scearce-LevieK, PalopJJ, YanF, ChengIH, et al. (2007) Reducing endogenous tau ameliorates amyloid beta-induced deficits in an Alzheimer's disease mouse model. Science 316 : 750–754.
52. FulgaTA, Elson-SchwabI, KhuranaV, SteinhilbML, SpiresTL, et al. (2007) Abnormal bundling and accumulation of F-actin mediates tau-induced neuronal degeneration in vivo. Nat Cell Biol 9 : 139–148.
53. IijimaK, GattA, Iijima-AndoK (2010) Tau Ser262 phosphorylation is critical for Abeta42-induced tau toxicity in a transgenic Drosophila model of Alzheimer's disease. Hum Mol Genet 19 : 2947–2957.
54. ThiesE, MandelkowEM (2007) Missorting of tau in neurons causes degeneration of synapses that can be rescued by the kinase MARK2/Par-1. J Neurosci 27 : 2896–2907.
55. MandelkowEM, ThiesE, TrinczekB, BiernatJ, MandelkowE (2004) MARK/PAR1 kinase is a regulator of microtubule-dependent transport in axons. J Cell Biol 167 : 99–110.
56. BallatoreC, LeeVM, TrojanowskiJQ (2007) Tau-mediated neurodegeneration in Alzheimer's disease and related disorders. Nat Rev Neurosci 8 : 663–672.
57. DoerflingerH, VogtN, TorresIL, MirouseV, KochI, et al. (2003) Bazooka is required for polarisation of the Drosophila anterior-posterior axis. Development 137 : 1765–1773.
58. WuPR, TsaiPI, ChenGC, ChouHJ, HuangYP, et al. (2011) DAPK activates MARK1/2 to regulate microtubule assembly, neuronal differentiation, and tau toxicity. Cell Death Differ 18 : 1507–1520.
59. WangS, YangJ, TsaiA, KucaT, SannyJ, et al. (2011) Drosophila adducin regulates Dlg phosphorylation and targeting of Dlg to the synapse and epithelial membrane. Dev Biol 357 : 392–403.
60. SchonEA, PrzedborskiS (2011) Mitochondria: the next (neurode)generation. Neuron 70 : 1033–1053.
61. DietzlG, ChenD, SchnorrerF, SuKC, BarinovaY, et al. (2007) A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature 448 : 151–156.
62. FeuilletteS, MiguelL, FrebourgT, CampionD, LecourtoisM (2010) Drosophila models of human tauopathies indicate that Tau protein toxicity in vivo is mediated by soluble cytosolic phosphorylated forms of the protein. J Neurochem 113 : 895–903.
Štítky
Genetika Reprodukční medicína
Článek Mutational Signatures of De-Differentiation in Functional Non-Coding Regions of Melanoma GenomesČlánek Rescuing Alu: Recovery of Inserts Shows LINE-1 Preserves Alu Activity through A-Tail ExpansionČlánek Genetics and Regulatory Impact of Alternative Polyadenylation in Human B-Lymphoblastoid CellsČlánek Retrovolution: HIV–Driven Evolution of Cellular Genes and Improvement of Anticancer Drug ActivationČlánek The Mi-2 Chromatin-Remodeling Factor Regulates Higher-Order Chromatin Structure and Cohesin DynamicsČlánek Identification of Human Proteins That Modify Misfolding and Proteotoxicity of Pathogenic Ataxin-1
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 8
-
Všechny články tohoto čísla
- Mutational Signatures of De-Differentiation in Functional Non-Coding Regions of Melanoma Genomes
- Rescuing Alu: Recovery of Inserts Shows LINE-1 Preserves Alu Activity through A-Tail Expansion
- Genetics and Regulatory Impact of Alternative Polyadenylation in Human B-Lymphoblastoid Cells
- Chromosome Territories Meet a Condensin
- It's All in the Timing: Too Much E2F Is a Bad Thing
- Fine-Mapping and Initial Characterization of QT Interval Loci in African Americans
- Genome Patterns of Selection and Introgression of Haplotypes in Natural Populations of the House Mouse ()
- A Combinatorial Amino Acid Code for RNA Recognition by Pentatricopeptide Repeat Proteins
- Advances in Quantitative Trait Analysis in Yeast
- Experimental Evolution of a Novel Sexually Antagonistic Allele
- Variation of Contributes to Dog Breed Skull Diversity
- , a Gene Involved in Axonal Pathfinding, Is Mutated in Patients with Kallmann Syndrome
- A Single Origin for Nymphalid Butterfly Eyespots Followed by Widespread Loss of Associated Gene Expression
- Cryptocephal, the ATF4, Is a Specific Coactivator for Ecdysone Receptor Isoform B2
- Retrovolution: HIV–Driven Evolution of Cellular Genes and Improvement of Anticancer Drug Activation
- The PARN Deadenylase Targets a Discrete Set of mRNAs for Decay and Regulates Cell Motility in Mouse Myoblasts
- A Sexual Ornament in Chickens Is Affected by Pleiotropic Alleles at and , Selected during Domestication
- Use of Allele-Specific FAIRE to Determine Functional Regulatory Polymorphism Using Large-Scale Genotyping Arrays
- Novel Loci for Metabolic Networks and Multi-Tissue Expression Studies Reveal Genes for Atherosclerosis
- The Genetic Basis of Pollinator Adaptation in a Sexually Deceptive Orchid
- Uncovering the Genome-Wide Transcriptional Responses of the Filamentous Fungus to Lignocellulose Using RNA Sequencing
- Inheritance Beyond Plain Heritability: Variance-Controlling Genes in
- The Metabochip, a Custom Genotyping Array for Genetic Studies of Metabolic, Cardiovascular, and Anthropometric Traits
- Reprogramming to Pluripotency Can Conceal Somatic Cell Chromosomal Instability
- Condensin II Promotes the Formation of Chromosome Territories by Inducing Axial Compaction of Polyploid Interphase Chromosomes
- PTEN Negatively Regulates MAPK Signaling during Vulval Development
- A Dynamic Response Regulator Protein Modulates G-Protein–Dependent Polarity in the Bacterium
- Population Genomics of the Facultatively Mutualistic Bacteria and
- Components of a Fanconi-Like Pathway Control Pso2-Independent DNA Interstrand Crosslink Repair in Yeast
- Polysome Profiling in Liver Identifies Dynamic Regulation of Endoplasmic Reticulum Translatome by Obesity and Fasting
- Stromal Liver Kinase B1 [STK11] Signaling Loss Induces Oviductal Adenomas and Endometrial Cancer by Activating Mammalian Target of Rapamycin Complex 1
- Reprogramming of H3K27me3 Is Critical for Acquisition of Pluripotency from Cultured Tissues
- Transgene Induced Co-Suppression during Vegetative Growth in
- Hox and Sex-Determination Genes Control Segment Elimination through EGFR and Activity
- A Quantitative Comparison of the Similarity between Genes and Geography in Worldwide Human Populations
- Minibrain/Dyrk1a Regulates Food Intake through the Sir2-FOXO-sNPF/NPY Pathway in and Mammals
- Comparative Analysis of Regulatory Elements between and by Genome-Wide Transcription Start Site Profiling
- Simple Methods for Generating and Detecting Locus-Specific Mutations Induced with TALENs in the Zebrafish Genome
- S Phase–Coupled E2f1 Destruction Ensures Homeostasis in Proliferating Tissues
- Cell-Nonautonomous Signaling of FOXO/DAF-16 to the Stem Cells of
- The Mi-2 Chromatin-Remodeling Factor Regulates Higher-Order Chromatin Structure and Cohesin Dynamics
- Comparative Analysis of the Genomes of Two Field Isolates of the Rice Blast Fungus
- Role of Mex67-Mtr2 in the Nuclear Export of 40S Pre-Ribosomes
- Genetic Modulation of Lipid Profiles following Lifestyle Modification or Metformin Treatment: The Diabetes Prevention Program
- HAL-2 Promotes Homologous Pairing during Meiosis by Antagonizing Inhibitory Effects of Synaptonemal Complex Precursors
- SLX-1 Is Required for Maintaining Genomic Integrity and Promoting Meiotic Noncrossovers in the Germline
- Phylogenetic and Transcriptomic Analysis of Chemosensory Receptors in a Pair of Divergent Ant Species Reveals Sex-Specific Signatures of Odor Coding
- Reduced Prostasin (CAP1/PRSS8) Activity Eliminates HAI-1 and HAI-2 Deficiency–Associated Developmental Defects by Preventing Matriptase Activation
- Dissecting the Gene Network of Dietary Restriction to Identify Evolutionarily Conserved Pathways and New Functional Genes
- Identification of Human Proteins That Modify Misfolding and Proteotoxicity of Pathogenic Ataxin-1
- and Link Transcription of Phospholipid Biosynthetic Genes to ER Stress and the UPR
- CDK9 and H2B Monoubiquitination: A Well-Choreographed Dance
- Rare Copy Number Variations in Adults with Tetralogy of Fallot Implicate Novel Risk Gene Pathways
- Ccdc94 Protects Cells from Ionizing Radiation by Inhibiting the Expression of
- NOL11, Implicated in the Pathogenesis of North American Indian Childhood Cirrhosis, Is Required for Pre-rRNA Transcription and Processing
- Human Developmental Enhancers Conserved between Deuterostomes and Protostomes
- A Luminal Glycoprotein Drives Dose-Dependent Diameter Expansion of the Hindgut Tube
- Melanophore Migration and Survival during Zebrafish Adult Pigment Stripe Development Require the Immunoglobulin Superfamily Adhesion Molecule Igsf11
- Dynamic Distribution of Linker Histone H1.5 in Cellular Differentiation
- Combining Comparative Proteomics and Molecular Genetics Uncovers Regulators of Synaptic and Axonal Stability and Degeneration
- Chemical Genetics Reveals a Specific Requirement for Cdk2 Activity in the DNA Damage Response and Identifies Nbs1 as a Cdk2 Substrate in Human Cells
- Experimental Relocation of the Mitochondrial Gene to the Nucleus Reveals Forces Underlying Mitochondrial Genome Evolution
- Rates of Gyrase Supercoiling and Transcription Elongation Control Supercoil Density in a Bacterial Chromosome
- Mutations in a P-Type ATPase Gene Cause Axonal Degeneration
- A General G1/S-Phase Cell-Cycle Control Module in the Flowering Plant
- Multiple Roles and Interactions of and in Development of the Respiratory System
- UNC-40/DCC, SAX-3/Robo, and VAB-1/Eph Polarize F-Actin during Embryonic Morphogenesis by Regulating the WAVE/SCAR Actin Nucleation Complex
- Epigenetic Remodeling of Meiotic Crossover Frequency in DNA Methyltransferase Mutants
- Modulating the Strength and Threshold of NOTCH Oncogenic Signals by
- Loss of Axonal Mitochondria Promotes Tau-Mediated Neurodegeneration and Alzheimer's Disease–Related Tau Phosphorylation Via PAR-1
- Acetyl-CoA-Carboxylase Sustains a Fatty Acid–Dependent Remote Signal to Waterproof the Respiratory System
- ATXN2-CAG42 Sequesters PABPC1 into Insolubility and Induces FBXW8 in Cerebellum of Old Ataxic Knock-In Mice
- Cohesin Rings Devoid of Scc3 and Pds5 Maintain Their Stable Association with the DNA
- The MicroRNA Inhibits Calcium Signaling by Targeting the TIR-1/Sarm1 Adaptor Protein to Control Stochastic L/R Neuronal Asymmetry in
- Rapid-Throughput Skeletal Phenotyping of 100 Knockout Mice Identifies 9 New Genes That Determine Bone Strength
- The Genes Define Unique Classes of Two-Partner Secretion and Contact Dependent Growth Inhibition Systems
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Dissecting the Gene Network of Dietary Restriction to Identify Evolutionarily Conserved Pathways and New Functional Genes
- It's All in the Timing: Too Much E2F Is a Bad Thing
- Variation of Contributes to Dog Breed Skull Diversity
- The PARN Deadenylase Targets a Discrete Set of mRNAs for Decay and Regulates Cell Motility in Mouse Myoblasts
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



