-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaA Sexual Ornament in Chickens Is Affected by Pleiotropic Alleles at and , Selected during Domestication
Domestication is one of the strongest forms of short-term, directional selection. Although selection is typically only exerted on one or a few target traits, domestication can lead to numerous changes in many seemingly unrelated phenotypes. It is unknown whether such correlated responses are due to pleiotropy or linkage between separate genetic architectures. Using three separate intercrosses between wild and domestic chickens, a locus affecting comb mass (a sexual ornament in the chicken) and several fitness traits (primarily medullary bone allocation and fecundity) was identified. This locus contains two tightly-linked genes, BMP2 and HAO1, which together produce the range of pleiotropic effects seen. This study demonstrates the importance of pleiotropy (or extremely close linkage) in domestication. The nature of this pleiotropy also provides insights into how this sexual ornament could be maintained in wild populations.
Published in the journal: . PLoS Genet 8(8): e32767. doi:10.1371/journal.pgen.1002914
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002914Summary
Domestication is one of the strongest forms of short-term, directional selection. Although selection is typically only exerted on one or a few target traits, domestication can lead to numerous changes in many seemingly unrelated phenotypes. It is unknown whether such correlated responses are due to pleiotropy or linkage between separate genetic architectures. Using three separate intercrosses between wild and domestic chickens, a locus affecting comb mass (a sexual ornament in the chicken) and several fitness traits (primarily medullary bone allocation and fecundity) was identified. This locus contains two tightly-linked genes, BMP2 and HAO1, which together produce the range of pleiotropic effects seen. This study demonstrates the importance of pleiotropy (or extremely close linkage) in domestication. The nature of this pleiotropy also provides insights into how this sexual ornament could be maintained in wild populations.
Introduction
Domestication is the strongest form of short term, highly directional selection known to man. Darwin himself cited domestication as a model for evolution [1]. The domestication process itself is associated with a whole raft of changes in phenotype, despite intentional selection on only a few traits. For example, simultaneous changes in colour, skull shape, and behaviour [2]–[4] are often observed in domestic populations, and can emerge even when selection is limited to tameness [5], [6]. The genetic mechanisms affecting such correlated changes are largely unknown. Pleiotropy, where one allele affects multiple traits, would be one potential mechanism [5], [6]. Alternatively, traits may be linked at a genetic level, but with separate genetic architectures [7], [8].
Of the relatively few domestic animals, the chicken is one of the most viable as a research animal. A combination of small size, rapid generation times, small genome size (∼1.09 Gb) [9] and high recombination (350 kb/cM on average) [10], along with extreme changes in phenotype (focused on production traits, namely egg production and increased growth and overall size) due to domestication make it particularly amenable as a model organism. As well as an increase in production traits, modern domestic chickens also show a large increase in relative comb size, a sexually selected ornament, and bone production [8].
The comb of the chicken is used to base mating decisions on by both males and females in wild-derived populations [11], [12]. In males it is an indicator of social rank, with females actively soliciting matings from males with larger combs [13], [14], as well as also correlating with bone mass [8]. In females it is indicative of greater reproductive potential, through an increase in egg production [8], [15]. In turn, egg production is highly dependent on bone morphology in the chicken, with one of the principal limitations to egg production being calcium deposition. Calcium is stripped from the hard outer cortical bone and transferred into the soft, inner medullary and trabecular bone and from there mobilised to the egg shell [16]. Similarly, calcium is also mobilised away from the ends of the bones (the metaphyses) and into the central part (the diaphysis) during egg-laying periods, where it is then more easily mobilised into medullary bone (see Figure 1). The genetic architectures for comb mass, egg production and bone allocation (important for egg production) have all been shown to overlap in the chicken [8]. Therefore fine-scale quantitative trait loci (QTL) mapping and expression quantitative trait locus (eQTL) mapping of these separate traits (comb mass, bone allocation and egg production) should allow the assessment of the importance of pleiotropy in both domestication and a sexual ornament. The bi-sexual expression of the comb in both males and females makes the comb somewhat unusual as a sexual ornament, with male-only effects of ornaments more often considered [17], and also makes the genetic analysis of this ornament in particular of greater relevance.
Fig. 1. Cartoon of a female femoral bone. 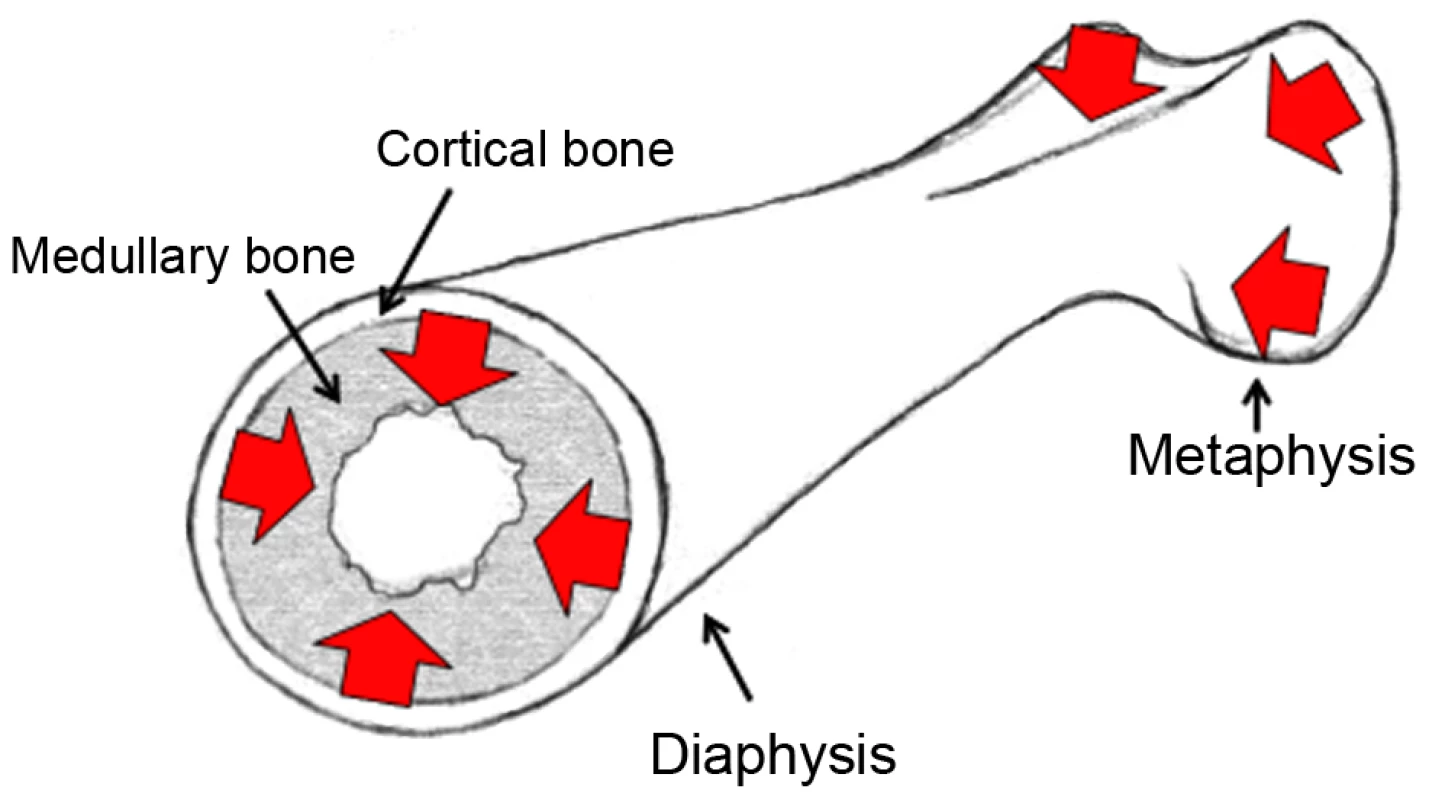
The location of medullary and cortical bone is highlighted, as well as the metaphysis and diaphysis. The flow of calcium in egg-laying females is indicated with red arrows, illustrating the transfer of calcium from the metaphyses to the diaphysis, and from the hard cortical bone to the soft medullary bone. Here we present the identification of a two-gene block controlling multiple phenotypic and fitness traits. This block was identified due to its extreme effect on comb mass, with its effects on bone allocation and fecundity then ascertained. A total of three different intercrosses, based on two different Layer populations and two different Red Junglefowl populations, were used to QTL map these traits. The two Layer populations consisted of two White Leghorn (WL) layer breeds (termed Obese strain (OS) and Line 13 (L13) hereafter), whilst these were crossed with two different populations of Red Junglefowl (RJF) chickens (the wild ancestor to the domestic chicken). Both Layer populations show a marked increase in comb mass and bone allocation, as compared to the RJF populations. The L13 cross was in addition expanded from an F2 population to an F8 advanced intercross. Therefore a F2L13, a F8L13 and a F2OS cross were all utilised. Fine-scale resolution and replication was achieved by over-laying the multiple QTL signals from the different crosses in combination with the small (40 kb) signatures of selected loci that have been previously identified in the domestic chicken using extensive resequencing [18]. Finally, eQTL analysis was performed using specific tissue types from the F8L13 cross to further test the causal association of genes affecting the sexual ornament of comb mass and the fecundity and fitness trait of medullary bone allocation.
Results
Comb Mass Architecture
The genetic architecture for comb mass from all three cross populations was shown to be principally restricted to six distinct regions in the genome (see Table S1), with a strongly significant degree of overlap between the different crosses (clustering test with 10000 permutations, P = 0.0016, see Tables S1 and S2 and Figure S1). In five of the six clusters, all QTL in the cluster had the same direction of effect (i.e. the allele conferring the larger effect was always based in the same population), adding strength that these were independent replicates of the same QTL. All of these alleles that were associated with larger combs derived from the WL strain, with the exception of a locus on chromosome one that showed transgressive segregation. Even here, the same pattern of transgressive segregation was mirrored in all crosses, once again reinforcing the idea of independent replication. The largest effect QTL in the F2L13 and F8L13 crosses was on chromosome three, with an additive effect of 4.3 grams and 10.9 grams, respectively, and LOD scores of 10 and 38 (R2 = 11% in F2L13 males, 30% in F8L13 males and 10% in F8L13 females). The confidence interval (C.I., calculated with a 1.8 LOD drop) for the F2L13 cross was from 6.6 Mb to 15.6 Mb, whereas the C.I. for the F8L13 was from 15.6 Mb to 16.0 Mb. The two intervals therefore only overlap at 15.6 Mb (see Figure 2), with the two markers defining these regions in the F2L13 and F8L13 crosses within 100 bp of one another. Of the other significant QTL identified in the F8L13, the one with the second smallest C.I. was located on chromosome 8, from 19.6–21.6 Mb with a LOD score of 10. In both the case of the chromosome three and eight QTL regions, although the loci were found in the F2OS cross, the size of the overall C.I. was not reduced.
Fig. 2. Chromosome 3 QTL peaks and confidence intervals (C.I.) from the F2-L13 (blue line) and F8-L13 (dark red line) crosses. 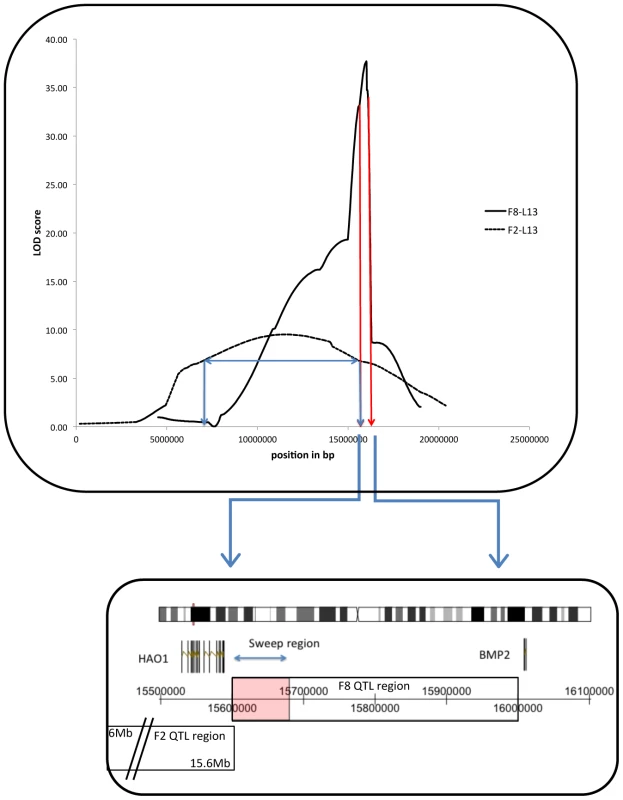
In the case of the F2-L13, the horizontal arrow indicates the 1.8 LOD drop C.I. of the QTL position. The location of the F8-L13 C.I. has been expanded below the graph to show the location of the two candidate genes and the selective sweep, and the overlap with the F2-L13 C.I. Comb QTL and Selective Sweep Overlap
Strong selection has been hypothesised to leave signatures of selection (selective sweeps) identifiable through linkage disequilibrium (LD) blocks present in the genome. The identification of such sweeps is becoming more common, though as yet all the successes of gene identification that have occurred through this approach have been ones of major effect, more akin to a straight Mendelian than a quantitative trait (but see [19]). Indeed, there is a great deal of debate as to whether such sweeps are even relevant for quantitative traits, or whether polygenic adaptation (small changes in gene frequency) is a more viable mechanism [20]. Despite this, resequencing of domestic and wild strains has yielded a number of putative selective sweeps in the chicken [18], each ∼40 Kb in size. These regions were overlaid with the six overlapping C.I. from the comb QTL studies, with five of the six overlap regions containing one or more sweeps, a significant enrichment (clustering test, P = 2×10−4). All of these were either layer-specific (i.e. regions fixed in all layers) or all-domestic (i.e. regions fixed in both layers and broilers), see Table S2. The largest QTL on chromosome 3 contained a selective sweep, located at 15.6 Mb, at precisely the region where the overlap between the F2L13 and F8L13 crosses occurred. The chromosome 8 QTL also contained a selective sweep present at the QTL peak, at 19.7 Mb.
Expression of Candidate Genes in Comb Mass
The chromosome 3 QTL C.I. contains 2 reference genes and a single selective sweep. This sweep contains the gene hydroxyacid oxidase (HAO1), whilst the gene bone morphogenetic protein 2 (BMP2) is adjacent to it (see Figure 2). Gene expression analysis was performed for the two putative causative genes on chromosome 3 using material from the comb base of 41 male F8L13 birds and from the diaphyseal medullary bone of 20 F8L13 female birds. Medullary bone tissue was used as analysis of medullary area showed a suggestive QTL co-localising at the identical location as to the comb mass QTL, with its peak also at 15.6 Mb. Both genes were found to be differentially expressed between RJF and WL alleles in comb base tissue (HAO1 P = 2×10−6, BMP2 P = 0.02), and the expression levels were strongly positively correlated with comb mass in both cases (HAO1 P = 0.0001, BMP2 P = 0.002, see Table 1.
Tab. 1. General Linear Model results for gene expression and QTL effect on comb mass. 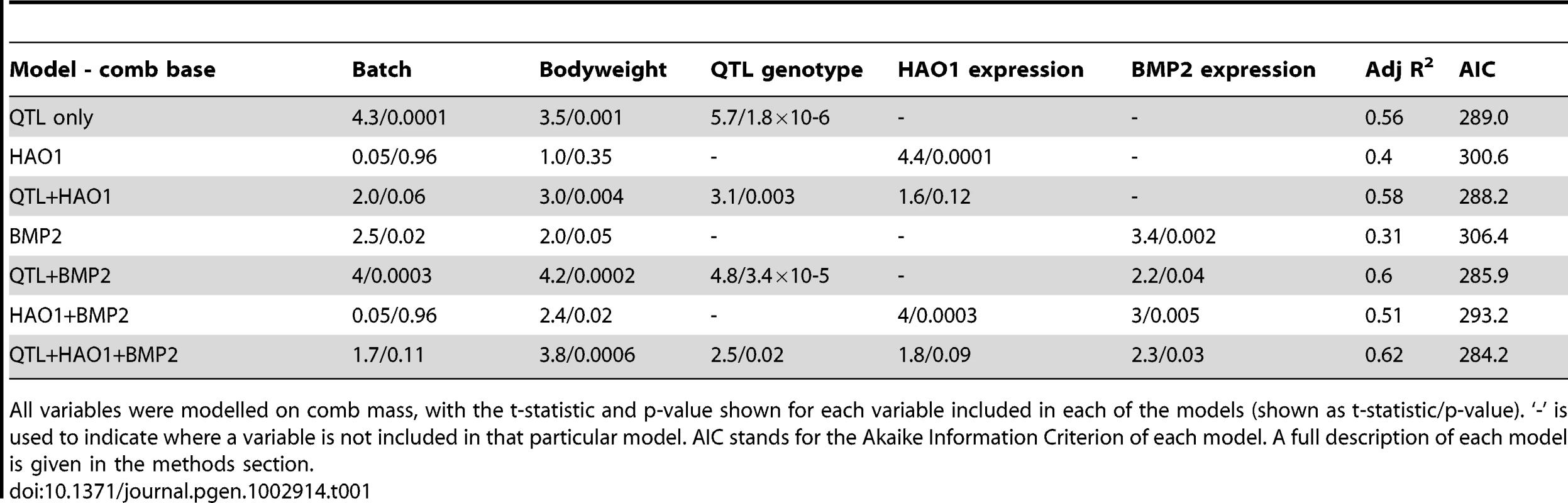
All variables were modelled on comb mass, with the t-statistic and p-value shown for each variable included in each of the models (shown as t-statistic/p-value). ‘-’ is used to indicate where a variable is not included in that particular model. AIC stands for the Akaike Information Criterion of each model. A full description of each model is given in the methods section. A test to distinguish linkage disequilibrium (LD) from true causality was conducted using expression as a covariate in the genotype/phenotype model [21]. Theoretically, if the QTL effect on the phenotype drops in significance when the gene expression covariate is included this should indicate the gene in question is causal to the QTL [21], [22], as the quantitative trait transcript (QTT) will explain more of the variance previously accounted by the genotype factor. In the case of HAO1 this occurs, with the variance explained by genotype (R2) falling from 44% to ∼18% (P = 2×10−6 to P = 0.003) with this covariate inclusion, with the AIC for the model falling from 289 to 288.2, see Table 1. BMP2 is significantly correlated with comb mass when placed as a covariate in a model already containing genotype (P = 0.04), and genotype significance also decreases with this covariate inclusion (P = 1.8×10−6 to P = 3.4×10−5, see Table 1, with the R2 falling from 44% to 29%), whilst the AIC for the model decreases from 289 to 285.9. Although the principal effect on comb mass therefore appears to be coming from HAO1 expression, BMP2 also appears to have an effect. A model including both HAO1 and BMP2 gene expression levels explains more of the variation present in comb mass, the R2 rising from 40% to 51% and the AIC decreasing from 300.6 to 293.2, compared to the model with only HAO1 present, with both HAO1 and BMP2 having significant effects on comb mass in the model (HAO1 P = 0.0003, BMP2 P = 0.005). The model with HAO1, BMP2 and the QTL effect had the lowest AIC, 284.2, and the highest R2, 62%, see Table 1. This demonstrates that both genes are important for determining comb mass, between them explaining the majority of the variation in comb size in the sample used for comb base gene expression.
Associations between a QTL locus, the expression of a gene and a complex trait can be used to try model the path of the QTL effect [23]. Using this idea, both HAO1 and BMP2 expression levels can be modelled on all the QTL for comb mass detected in the F8L13 cross. This analysis can indicate if the other detected QTL are acting through the same HAO1/BMP2 pathway as the chromosome 3 QTL, though with the caveat that such tests are still in essence correlative and not a substitute for functional assays. Three QTL in a combined model explain more of HAO1 expression than the chromosome 3 locus in isolation (chr1@35.5 Mb P = 0.03, chr1@172 Mb P = 0.02, chr3@16 Mb P = 2×10−6, R2 rose from 63% to 71% with all three QTL in the model with the AIC decreasing from −401.1 to −408.8). Similarly, BMP2 expression is correlated with a total of three QTL in a combined model, two of which are interacting (chr1@35.5 Mb P = 0.02, chr1@35.5 Mb×chr5@32.7 Mb P = 0.03, chr3@16 Mb P = 0.03, R2 rose from 32% to 50% with all three QTL in the model, with the AIC decreasing from −126.8 to −134). It therefore appears that both HAO1 and BMP2 are determining comb mass, functioning on two separate pathways. The first one involves HAO1 (and therefore the chr3 QTL), with the QTL on chromosome 1 at 35.5 Mb and 172 Mb are also part of this pathway. The second, involving BMP2, involves the chromosome 3 QTL again, with the QTL on chromosome 1@35.5 Mb and chromosome 5@32.7 Mb also involved in this pathway.
Comb Mass and Bone Allocation
While comb mass is known to be an indicator of reproductive capacity [8], [15], the links between bone allocation and comb mass are less known [8], though given the close relationship between egg production and bone allocation, some correlation may be expected. Therefore we measured metaphyseal and diaphyseal (see Figure 1, Figure 3a and 3b) bone morphometrics in both the F2OS and F8L13 mapping populations, using a Computerised Tomography (pQCT) machine (see Materials and methods), and then modelled these bone characteristics on comb mass. Results of the combined General Linear Models are shown in Table S3 and Figure 3. Females with larger combs deposit more calcium in the diaphysis where it is more easily mobilised into the eggshell. This is shown by diaphyseal cortical bone measures being positively correlated with comb mass in both the F2OS and F8L13, whereas metaphyseal total bone density is negatively correlated in the F2OS, and metaphyseal medullary density is negatively correlated in the F8L13. Females with larger combs are also producing more of the diaphyseal medullary bone required for egg production. This is indicated by diaphyseal medullary area being positively correlated with comb mass in the F2OS (see Figure 3) and diaphyseal medullary density being positively correlated and metaphyseal medullary density negatively correlated with comb mass in the F8L13. In males, metaphyseal medullary area and density and diaphyseal cortical content are also positive predictors of comb size, once again showing that males with larger combs are more likely to have greater overall bone density and strength (see Figure 3).
Fig. 3. Illustration of male and female RJF (lower left) and WL (upper right) comb size. 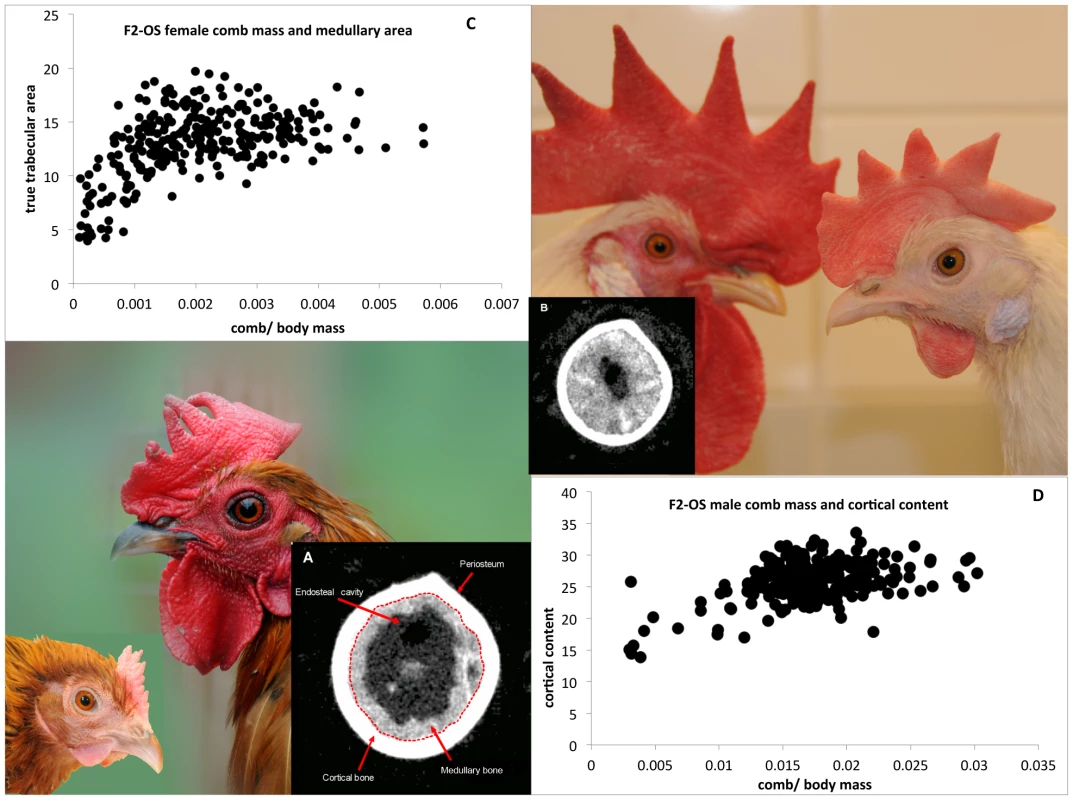
CT pictures of two diaphyseal bone sections are shown in (A) and (B), with the cortical and medullary bone marked. The correlation between female comb mass relative to body mass and diaphyseal trabecular area shown in (C), and the correlation between relative male comb mass and cortical content is shown in (D). A QTL for medullary bone was also identified at the same locus that effected comb mass on chromosome 3. HAO1 and BMP2 were therefore also tested as potentially causative loci for effecting medullary bone. The results for medullary bone similarly indicate a greater effect of HAO1 rather than BMP2 on medullary bone area, though the causal tests themselves are less conclusive. When HAO1 is included in the QTL model as a covariate, the variance explained by QTL genotype increases from R2 = 14% to 27% (P = 0.02 to 0.002), with HAO1 significant in this model (P = 0.03, R2 = 10%), whilst the AIC decreases from 135.5 to 131.4, see Table 2. Comb mass was negatively correlated with medullary area (this mirrors the correlation seen between comb and medullary bone in the F8L13 cross, where medullary density was more strongly and positively correlated with comb mass). Once again, BMP2 expression has less effect on QTL genotype, with the variance explained by genotype unchanging (R2 = 14% to 17%), and the AIC increasing from 135.5 to 136.3, and BMP2 expression itself is also not significant (P = 0.35). Expression levels of BMP2 and HAO1 in bone tissue were found to be correlated with several related relevant bone strength and fecundity phenotypes, see Table 3. HAO1 was positively correlated with an increase in egg production (t = 2.8, P = 0.02), a lack of broodiness (egg incubation behaviour, see Materials and methods, t = −2.4, P = 0.03), and an increase in the number of eggs produced (t = 2.6, P = 0.02). BMP2 expression was correlated with an increase in total density of the diaphyseal endosteal cavity (t = 2.4, P = 0.03) and a decrease in diaphyseal endosteal cavity area (t = −2.2, P = 0.05). It therefore appears that the two linked genes have a pleiotropic effect between them, affecting comb mass, egg production, medullary bone area, and total area and density of the endosteal cavity.
Tab. 2. General Linear Model results for gene expression and QTL effect on bone mass. 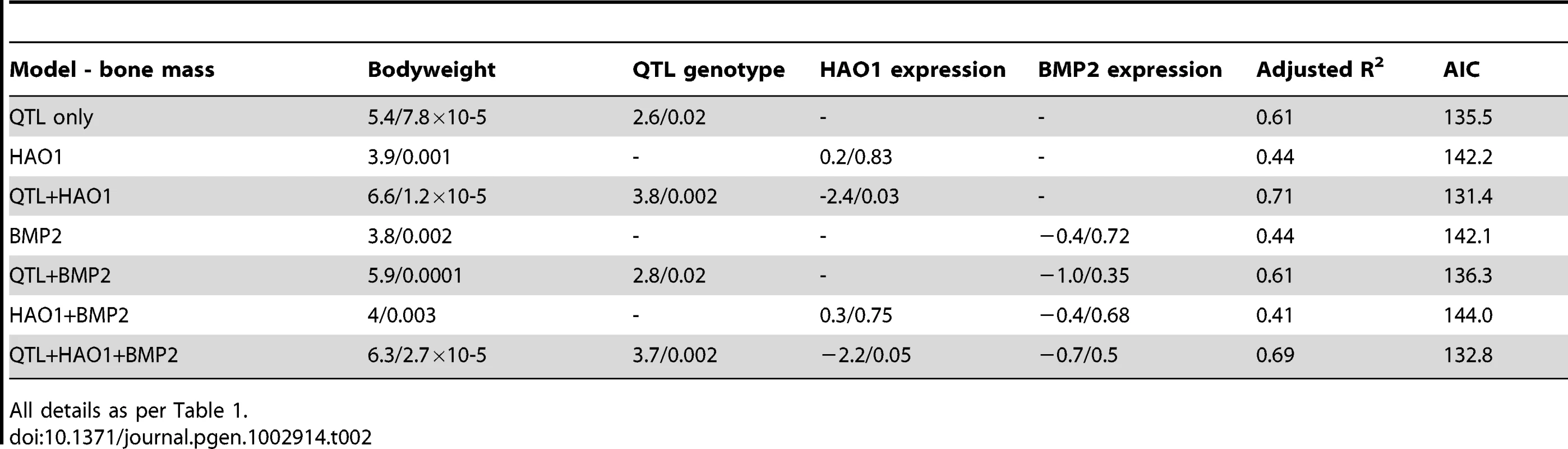
All details as per Table 1. Tab. 3. Additional fecundity and bone morphometric traits correlated with HAO1 and BMP2 expression in bone samples. 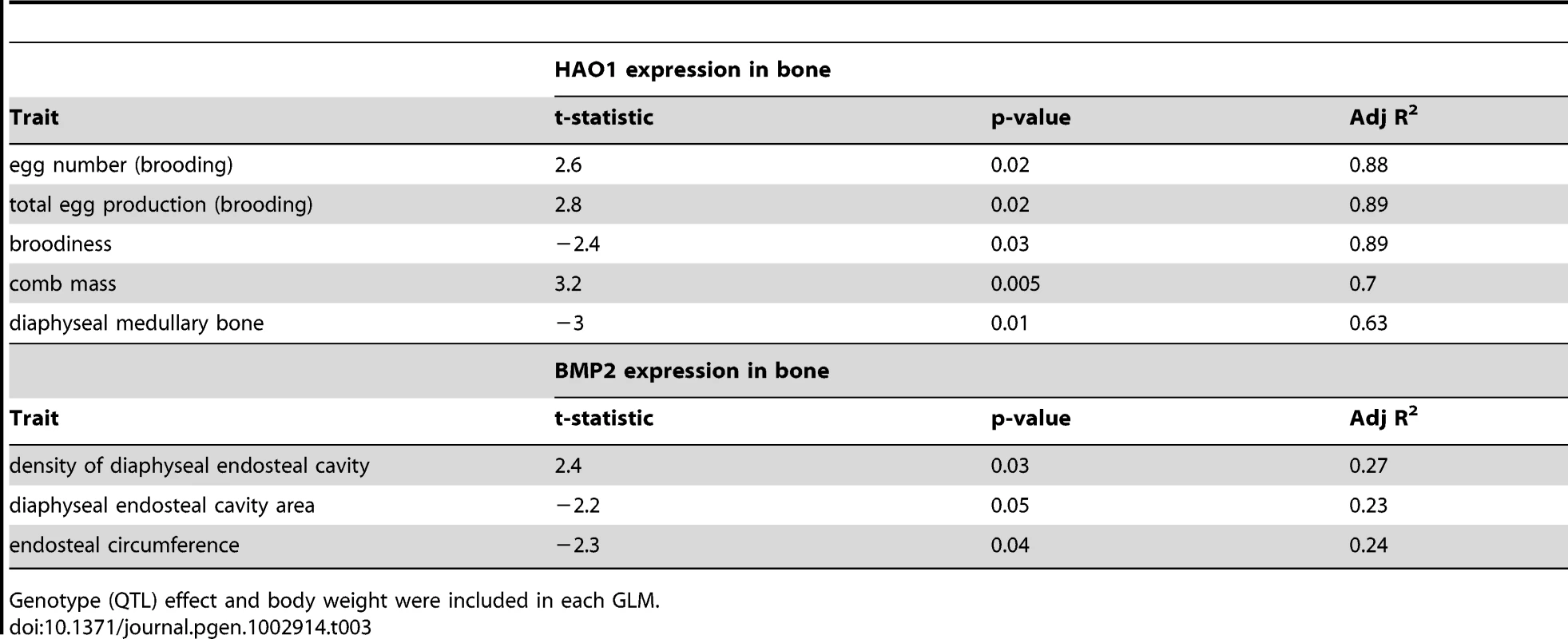
Genotype (QTL) effect and body weight were included in each GLM. Discussion
In wild chicken populations, sexual ornaments are closely related with fitness traits [15]. We demonstrate that a block of two genes is controlling multiple aspects of the comb and bone allocation in the chicken, though the relative effects of both must be verified through functional assays. BMP genes have been shown to have numerous effects on bone physiology and increasing bone deposition [24], stimulating osteoblast proliferation and differentiation and stimulating bone formation. Cartilage is the precursor to all bone formation (the skeleton is first laid down cartilaginously, with this then becoming ossified [25]). This explains the link between the cartilage production required for comb growth and bone production physiology. HAO1 on the other hand is a novel candidate for altering bone and cartilage deposition, to date principally shown to be expressed in the liver. Previous work has also highlighted its role in the liver metabolic pathways as a peroxisomal enzyme [26], and it is also implicated in lymphoblastic leukemia [27]. Peroxisomal enzymes feature heavily in the catabolism of long chain fatty acids [28] and are important for energy metabolism. Peroxisomal oxylate enzymes have also been linked to effects on calcium binding, through links with hereditary calcium oxalate kidney stone diseases (Primary Hyperoxaluria) [29], demonstrating potential interactions with calcium binding which may also affect the cartilage/bone allocation system.
Despite the pleiotropic effects seen between comb mass and bone allocation/egg production the overlap between comb mass QTL and selective sweeps may be due to two potential effects. On the one hand, if all comb mass loci exhibit pleiotropic effects on bone allocation or egg production, the strong selection for egg production will naturally cause this overlap to occur. However, an alternative explanation for this overlap is direct selection for comb mass itself is potentially occurring in these Layer breeds. This may be due to breeders intentionally selecting for large combs, realising they are a good indicator of egg production or indeed are a beneficial trait in of themselves. The comb is involved in heat regulation in the chicken [30], and therefore may also assist in survival in crowded domestic conditions.
The results also show that with one or both genes of this two-gene block, allelic variants can be seen to have effects on bone allocation, fecundity, brooding and comb growth. This highlights the importance of both pleiotropy and linkage in such systems. In this instance the extremely close linkage between the two genes in this block results in essentially a pleiotropic effect occurring from the alleles at these loci. This has important ramifications for understanding multiple complex trait interactions in domestication. Pleiotropy and close-linkage can work together to produce a pleiotropic effect on multiple divergent traits. Even here, the close linkage can be disrupted by recombination, enhancing the flexibility of the system. These results show that the pleiotropic core of domestication ‘modules’ (regions of the genome controlling multiple aspects of the domestication phenotype [7], [31]–[33]) contain both alleles with pleiotropic effect and extremely close linkage between QTL. Such a pattern of linkage and domestication modules are also seen in domesticated plants [31], [32], [34], [35], indicating such a system of loose and tight linkage is responsible for the domestication phenotype in a diverse range of taxa.
This study also potentially sheds light on the genetics of sexual selection. Although in the case of the domestic population it is no longer subject to sexual selection, the current artificial selection will be occurring on the pre-existing genetic architecture of the ancestral Red Junglefowl. In this instance, the natural selection constraints acting in the wild Junglefowl (which will limit the expensive investment of bone into fecundity in females to conserve longevity and fitness) can be ‘decoupled’ from the sexual ornament. The domestic population can potentially therefore show a counterpoint to the wild population in this regard. Interestingly, the observed pleiotropy ties in well with much of the previous work on sexual selection theory, as it has long been considered important in an accurate ornamental indicator signal [36] and in sexual selection theory in general [37]. Pleiotropy may also underlie the genetic covariation between traits that may in turn constrain the individual evolution of each trait independently [38], [39]. Similarly, condition dependence of a sexual ornament may also be due to pleiotropic effects [40]. Examples of the importance of pleiotropy in sexual ornaments can also be found by the overlap of multiple, functionally related, sexually selected traits. In Drosophila cuticular hydrocarbons [41] and wing song components [42], [43] have all been found to exhibit this overlapping architecture.
Pleiotropy can also have large consequences for long term phenotypic evolution and on complex traits in general [44]. Using global expression data, large-scale expression variation in between-sex [45] and breed [46]–[48] comparisons is at odds with the relatively modest QTL genetic architecture which is also detected in these comparisons (although power of detection is always a potential issue with QTL analysis). In these instances modules of genes (either pleiotropic or closely linked) once again appear to underlie the large-scale transcriptional changes. For example in a laboratory×wild cross of S.cerevisiae strains, genetic variation at a single gene was shown to have a major impact on global transcription [49], whilst recombinant inbred line studies using mice [50] and Drosophila [51] have also found modular effects of gene expression.
In the case of Drosophila, wild-derived inbred lines also show a high degree of modularisation in transcription [52]. It is perhaps pertinent that the modular patterns observed in domesticated animals is also seen once again in the wider variety of organisms previously listed. In the case of the chicken model presented here, the effect of domestication has been to decouple a sexual ornament from the limitations of natural selection. In wild populations, natural selection will act to limit the extreme allocation of calcium into medullary bone (which will increase short term reproductive gains at the expense of longer term survival), thereby preventing these alleles from becoming fixed in the population. During domestication this barrier has been removed (in fact this increased short-term fecundity is being actively selected for). However, by revealing the pleiotropic effects acting between the sexual ornament and the fitness trait, this does reveal how the indicator mechanism is maintained in the wild population, albeit not to the extreme levels seen in domestic populations.
Materials and Methods
Study Populations
Two separate crosses were conducted for this analysis, the first consisting of an F2 intercross between Obese Strain White Leghorn (WL-OS) chickens and a Red Junglefowl (RJF) population derived from a Swedish zoo population and maintained in Götala, Sweden (RJF-Götala). The second intercross was an eighth generation intercross between a line of selected White Leghorn (WL-L13) maintained from the 1960s and a population of RJF originally obtained from Thailand (RJF-T). This F8 advanced intercross line (AIL) is based on an F2 intercross that has been measured for comb mass and a variety of bone morphologies, see [8], [33], [53]. The WL-OS and WL-L13 cross have been separated since approximately 1955, so although they come from the same base population of White Leghorn chickens they have now had over 50 years of separation. Hereafter the abbreviation F2-L13 will be used for the F2 RJF-T/WL-L13 cross, F8-L13 for the F8 RJF-T/WL-L13 AIL, and F2-OS for the WL-OS/RJF-Götala F2 cross. In the case of the F2-OS cross, these were raised in a total of 4 batches at Götala research station of the Swedish University of Agricultural Sciences, Skara, Sweden. Chickens were maintained on standardised conditions and fed ad libitum, under a 12∶12 hour light/dark regime. Pens measuring 3 m×3 m were used for housing, with perches also provided. Individuals were culled at 200 days of age, with their combs surgically removed post-mortem and weighed and both femoral bones extracted. The F8-L13 cross used in this study was generated in 5 batches and reared at the research station of Linköping University, Sweden. Pens for these animals were 2 m×2 m and comprised of three separate levels, perches and food were once again supplied ad libitum. Animals were culled at 212 days of age, with the comb surgically removed post-mortem and weighed, and both femoral bones extracted.
WL-OS/RJF-G
A total of 640 F2 individuals were used for the comb mass analysis, consisting of 308 males and 332 females. Of these, 543 of the individuals were also measured for a variety of bone morphology traits (245 males and 298 females). The WL-OS strain was originally isolated as a model of Hashimoto's thyroiditis, as they suffer from hypothyroidism and therefore require thyroxin to be provided in a food supplement (500 µg/kg food) (Levaxin Tablets, Nycomed AB, Sweden, were administered to all but one batch until 180 days of age). Although this strain tends to be slightly smaller than usual WL strains, they are still substantially larger, and with far greater sized combs, as compared to RJF. In addition, the degree of infiltration that had occurred in the F2 animals thyroid was also measured, and correlated with both comb mass and body weight. No significant correlations were found, and additionally the majority of the QTL discovered in this cross (7 of 11) all go in the expected direction, i.e. alleles of greater effect come from the WL, not RJF, line (with one of the three transgressive QTL also corroborated as transgressive in the other crosses as well). If any comb loci were caused by thyroiditis this would be expected to be the reverse, so we have good cause to consider these QTL are due to generic WL-strain based differences. Animals were culled at 200 days of age. The animal experiments were approved by the ethical committee for animal experiments in Göteborg, Sweden, no. 55-2005 and 233-2006, and by the ethical committee for animal experiments in Uppsala no. 2008-12-19, C321/8.
WL-L13/RJF-T F8
A total of 447 F8 individuals were assayed for comb measurements (216 males and 231 females). These individuals were generated from a total of 107 families using 122 F7 individuals (63 females and 59 males). Average family size 4.7+/−3.1 (mean, standard deviation). These were the continuation of an inter-cross initially based on 3 WL females and one RJF male, which were then expanded into 811 F2 progeny and then maintained with at a population size of approximately 100 birds per generation until the F7 generation. Of the F8 individuals, a total of 41 males were used as the basis of a qPCR experiment using comb base tissue to check for candidate genes at the major chromosome 3 QTL locus at 15.7 Mb (12 RJF homozygotes, 15 WL homozygotes, 14 heterozygotes). These individuals came from two of the five batches used to produce the F8. Additionally, 20 females were used as the basis for a qPCR experiment using diaphyseal medullary bone (10 of each homozygote class, though one had no metaphyseal CT bone measurements, reducing the correlation with bone density to 19 individuals). The study was approved by the local Ethical Committee of the Swedish National Board for Laboratory Animals.
WL-L13/RJF-T F2
A total of 352 males and 122 females were used in this analysis, with these QTL already detailed in [8], .
Bone Phenotypic Measurements
The right femoral bone of each F2OS and F8L13 individual was measured using a Peripheral Quantitative Computerised Tomography (pQCT) machine (Stratec XCT – Research SA machine, Stratec Medizintechnik, Germany), with two sections taken at 6% (metaphyseal) and two at 50% (diaphyseal) of the total femoral length. Cortical bone was measured using the CORTMODE1 setting, with a density threshold of >1000 mg/cm3. Cortical measures were cortical area, cortical bone content, cortical thickness and cortical density. Medullary measures were recorded using the PEELMODE2 function, using an inner threshold of 1000 mg/cm3 to separate cortical from medullary bone and gives total density, total bone content and total area of the endosteal cavity measures. An additional inner threshold of 150 mg/cm3 in a combination of two PEELMODE2 gives the medullary area, medullary density and medullary bone content.
Fecundity Phenotypic Measures
Two separate fecundity trials were performed. Initially birds were housed individually and eggs were collected daily over a two-week period. The second trial was performed immediately after the first and was identical except birds were given two dummy eggs to incubate and were allowed to keep all eggs laid over a two-week period. At the end of each trial, number of eggs produced, total weight of eggs produced and mean egg weight were recorded. Chickens which are actively brooding (incubating) their eggs will stop producing eggs when a clutch size of around 6–8 eggs has been produced, whilst domestic layers will continually produce eggs and never go into such brooding behaviour. Therefore one method for ascertaining if an individual is brooding will be to deduct the total number of eggs produced in the first trial from the number of eggs produced in the second trial. Negative values therefore indicate that an individual is decreasing egg production when allowed to build up a clutch.
Phenotypic Correlations
To analyse phenotypic correlations between comb mass and bone characteristics in the F2OS and F8L13 crosses, a GLM was fitted for each individual bone trait measured and included batch and sex fixed effects and weight at slaughter as a covariate. All significantly correlated bone measures were then combined into a global model. The significance values of each measure was then ascertained in this global model.
Clustering Analysis
The clustering test was performed using a permutation test based on the total length of the chicken genome (1.09 Gb), which then had a number of regions equal to the number of QTL detected in the F2OS and F8L13 cross randomly distributed along it. The size of these regions was equal to the average C.I. of QTL from the F2-OS cross (15 Mb) and the F8L13 cross (5 Mb), and tested against the observed number of overlaps between the F2OS and F8L13 (6). The F2L13 cross was not used in this analysis, as 4 of the 5 QTL detected in this cross were strongly replicated in the F8L13 and their inclusion could artificially inflate the degree of replication observed between the two different cross populations. This was repeated 1000 times, with the number of overlaps recorded each time used to generate a significance value. A similar procedure was used to predict the probability of selective sweeps occurring within the overlap regions. In this instance the total number of sweeps detected (133) and the six overlap regions were used, based against the observation that 13 sweeps were observed within the six 6 Mb overlap intervals. When calculating the overlap regions, an extra 1 Mb was added to the region size upstream and downstream, in case using only the overlap between the OS and L13 crosses gave an artificially small region.
Genotyping and QTL Analysis
DNA preparation for both crosses was performed by Agowa GmbH (Berlin, Germany), using standard salt extraction. A total number of 347 SNPs and 20 microsatellite markers were used for the OS cross, with the marker map generated using Crimap [54], covering ∼2535 cM, with an average marker spacing of ∼7.5 cM. For the F8L13 cross, 652 SNP markers were used to generate a map of length ∼8760 cM, with an average marker spacing of ∼15 cM. QTL analysis was performed using QTL Express (http://qtlcap.ed.ac.uk), qxpak v2.16 [55] and R/Qtl [56] for the standard interval mapping and epistatic analyses. Interval mapping was performed using additive and additive+dominance models. Batch and sex were always included in the model as a fixed effect, whilst bodyweight at slaughter was included as a covariate. To account for a particular QTL varying between the sexes, a sex-interaction analysis was also performed.
Significance Thresholds
Significance thresholds for both crosses were calculated using permutation tests [57], [58], however these values differed greatly between the two study populations due to the massively increased number of recombinations in the F8L13 population (as reflected in the inflated map size) and also to account for possible family effects in this population. A suggestive significance level of a genome-wide 20% threshold was used (mainly due to being more conservative than the standard suggestive threshold suggested by Lander and Kruglyak [59]). For the F2OS cross, additive+dominance effects had a suggestive threshold of around ∼3.0 and a significant threshold of ∼3.9, depending on the trait, whilst additive+dominance sex interaction models had a suggestive threshold of ∼3.8 LOD and a significant threshold of ∼4.6 LOD. In the case of the comb analysis, a single point threshold was used where a QTL had been indicated in the F8L13 or F2L13 mapping populations at a locus, but where a significant or suggestive QTL in the F2OS population was not immediately apparent. 5% single point threshold for additive only model was LOD ∼1.4, whilst for an additive+dominance model this was LOD ∼2.0. Confidence intervals (C.I.) for QTL were calculated using a 1.8 LOD drop method (i.e. where the LOD score on either side of the peak decreases by 1.8 LOD). The nearest marker to this 1.8 LOD decrease is then used to give the C.I. in megabases (the use of the physical location of markers allows a direct comparison between the different crosses). The use of a 1.8 LOD drop has been shown to most reliably give an accurate 95% confidence interval for an intercross type population [60].
Thresholds for an AIL are potentially problematic, as the family structure can cause inflated LOD scores, resulting in false positive results. To avoid this initially, we used a large number of families (107) to generate the total number of individuals, to break down this sub-structure as much as possible. For instance, if only one offspring were used per family, there would be no structure and the population would function exactly as recombinant inbred lines [61]. To check if family was a significant factor on the comb trait, a GLM consisting of sex, batch, family and weight was used to predict comb weight. In this instance, only one family out of 107 was significant (p = 10−6), and consisted of two large males. Furthermore to generate the significance thresholds we also used a family factor included in the permutation model, which resulted in greater thresholds. The threshold without family as a factor was LOD 3.4 for a suggestive threshold and LOD 4.15 for a significant threshold. With family included as a cofactor, a suggestive threshold was 4.55 and a significant threshold was 5.4 was obtained.
Gene Expression Measurements
Comb base RNA was isolated with Ambion TRI reagent (Applied Biosystems, Carlsbad, CA, USA), following the manufacturer's protocol. After removal of the comb, a part of the forehead was frozen in liquid nitrogen. Frozen comb base tissue was removed with a razor and disrupted with a hammer and bag. Samples were homogenized on a FastPrep-24 instrument (MP Biomedicals, Solon, OH, USA) in tubes with TRI and ceramic beads (Lysing matrix D, MP Biomedicals).
First strand cDNA for qPCR was made with Fermentas (St. Leon-Rot, Germany) RevertAid Reverse Transcriptase, using 10 mM dNTPs, RiboLock nuclease inhibitor, and oligo(dT)18 primer (Thermo Fisher Scientific, Freemont, CA, USA), according to the manufacturer's protocol. qPCR was performed with Maxima SYBR Green qPCR mastermix (Thermo Fischer Scientific) in 15 µl reactions with 0.3 M of each oligonucleotide primer on a Rotor-Gene 6000 real-time cycler (Corbett Research, Cambridge, UK). The PCR program consised of a 10 min activation step at 95°C, followed by 40 cycles of 15 s at 95°C, and 1 minute at 60°C. After cycling, products were melted by ramping the temperature from 72°C to 95°C. qPCR data was analysed with the comparative ΔΔCt method [62]. Target gene threshold cycle values were normalized by subtracting the geometric mean value of three reference genes, β2 microglobulin, TATA box binding protein, and RNA polymerase II subunit C1. A batch effect (due to individuals coming from two of the five batches comprising the cross) was included in the General Linear Model analysis, as well as weight at slaughter for all individuals, when comb mass was included in the model.
Linear Models and Expression
To ascertain the effects of differential gene expression from the two candidate genes in the QTL interval on the phenotypic trait, the gene expression levels (described above) were used in a linear model. The initial model to test gene expression was y (comb mass) = mean + batch + weight at slaughter + expression level (called either HAO1 or BMP2 in the model column in Tables 1 and 2). To test the effect of this on genotype an additional QTL genotype factor was added (model HAO1 + QTL and BMP2 + QTL in Tables 1 and 2). One measure of causality is that the additional of a candidate gene expression covariate should decrease the QTL genotype effect in this model, therefore these models were then checked against the model y = mean + batch + weight at slaughter + QTL genotype (QTL only model in Tables 1 and 2), to observe the drop in QTL significance. Additionally, two further models were also checked. These were y = mean + batch + weight at slaughter + BMP2 expression + HAO1 expression, and y = mean + batch + weight at slaughter + BMP2 expression + HAO1 expression+QTL genotype. These were fitted to check for the potential of gene interactions between both candidates affecting the trait.
Linear models were also fitted to test the association between HAO1 and BMP2 levels with the other comb mass QTL identified. Initially, a basic model of y (gene expression level) = mean + batch + weight at slaughter + QTL genotype was fitted for each QTL separately. All significant QTL were then combined into a single model (y = mean + batch + QTL1 + QTL2, etc) to test for the global combined significance. This global model was then compared to the base model with only the chromosome 3 QTL genotype included.
Linear models were also used to test the effects of HAO1 and BMP2 expression levels on other related fecundity and bone morphometric traits. In this case, a model of y (trait of interest) = mean + weight at slaughter + QTL genotype (chromosome 3) + HAO1/BMP2 expression level was fitted.
Supporting Information
Zdroje
1. Darwin C (1868) The Variation of Animals and Plants under Domestication. London: John Murray.
2. AnderssonL, GeorgesM (2004) Domestic anmial genomics: deciphering the genetics of complex traits. Nature Reviews Genetics 5 : 202–212.
3. JensenP, AnderssonL (2005) Genomics Meets Ethology: A New Route to Understanding Domestication, Behavior, and Sustainability in Animal Breeding. AMBIO: A Journal of the Human Environment 34 : 320–324.
4. Price EO (2002) Animal domestication and behaviour. Wallingford: CABI Publishing. 297 p.
5. BelyaevDK (1979) Destabilizing selection as a factor of domestication. Journal of Heredity 70 : 301–308.
6. BelyaevDK, RuvinskyAO, TrutLN (1981) Inherited activation-inactivation of the star gene in foxes: its bearing on the problem of domestication. Journal of Heredity 72 : 267–274.
7. AlbertFW, CarlborgO, PlyusninaIZ, BesnierF, HedwigD, et al. (2009) Genetic Architecture of Tameness in a Rat Model of Animal Domestication. Genetics genetics.109.102186.
8. WrightD, KerjeS, BrändströmH, SchützK, KindmarkA, et al. (2008) The genetic architecture of a female sexual ornament. Evolution 62 : 86–98.
9. Consortium ICGS (2004) Sequence and comparative analysis of the chicken genome provide unique perspectives on vertebrate evolution. Nature 432 : 695–716.
10. GroenenMAM, WahlbergP, FoglioM, ChengHH, MegensH-J, et al. (2009) A high-density SNP-based linkage map of the chicken genome reveals sequence features correlated with recombination rate. Genome Research 19 : 510–519.
11. PizzariT, BirkheadTR (2000) Female feral fowl eject sperm of subordinate males. Nature 405 : 787.
12. PizzariT, CornwallisCK, LovlieH, JakobssonS, BirkheadTR (2003) Sophisticated sperm allocation in male fowl. Nature 426 : 70–73.
13. ParkerT, LigonJ (2003) Female mating preferences in red junglefowl: a meta-analysis. Ethology Ecology and Evolution 15 : 63–72.
14. ZukM, ThornhillR, LigonJD, JohnsonK, AustadS, et al. (1990) The role of male ornaments and courtship behaviour in female mate choice of red junglefowl. American Naturalist 136 : 459–473.
15. CornwallisCK, BirkheadTR (2007) Experimental evidence that female ornamentation increases the acquisition of sperm and signals fecundity. Proceedings of the Royal Society B: Biological Sciences 274 : 583–590.
16. CransbergPH, ParkinsonGB, WilsonS, ThorpBH (2001) Sequential studies of skeletal calcium reserves and structural bone volumes in a commercial layer flock. Br Poultry Sci 260–265.
17. AmundsenT (2000) Why are female birds ornamented? Trends in Ecology and Evolution 15 : 149–155.
18. RubinC-J, ZodyMC, ErikssonJ, MeadowsJRS, SherwoodE, et al. (2010) Whole-genome resequencing reveals loci under selection during chicken domestication. Nature 464 : 587–591.
19. Van LaereAS, NguyenM, BraunschweigM, NezerC, ColletteC, et al. (2003) A regulatory mutation in IGF2 causes a major QTL effect on muscle growth in pigs. Nature 425 : 832–836.
20. PritchardJK, Di RienzoA (2010) Adaptation - not by sweeps alone. Nat Rev Genet 11 : 665–667.
21. Le Bihan-DuvalE, NadafJ, BerriCc, PitelFdr, GrauletBÆ, et al. (2011) Detection of a Cis eQTL Controlling BMCO1 Gene Expression Leads to the Identification of a QTG for Chicken Breast Meat Color. PLoS ONE 6: e14825 doi:10.1371/journal.pone.0014825.
22. LeducMS, HagemanRS, VerdugoRA, TsaihS-W, WalshK, et al. (2011) Integration of QTL and bioinformatic tools to identify candidate genes for triglycerides in mice. Journal of Lipid Research 52 : 1672–1682.
23. SchadtEE, LambJ, YangX, ZhuJ, EdwardsS, et al. (2005) An integrative genomics approach to infer causal associations between gene expression and disease. Nat Genet 37 : 710–717.
24. LinkhartTA, MohanS, BaylinkDJ (1996) Growth factors for bone growth and repair: IGF, TGF[beta] and BMP. Bone 19: S1–S12.
25. de CrombruggheB, LefebvreV, NakashimaK (2001) Regulatory mechanisms in the pathways of cartilage and bone formation. Current Opinion in Cell Biology 13 : 721–728.
26. RecalcatiS, TacchiniL, AlberghiniA, ConteD, CairoG (2003) Oxidative stress-mediated down-regulation of rat hydroxyacid oxidase 1, a liver-specific peroxisomal enzyme. Hepatology 38 : 1159–1166.
27. HanS, LeeK-M, ParkSK, LeeJE, AhnHS, et al. (2010) Genome-wide association study of childhood acute lymphoblastic leukemia in Korea. Leukemia Research 34 : 1271–1274.
28. WandersRJA, WaterhamHR (2006) Biochemistry of Mammalian Peroxisomes Revisited. Annual Review of Biochemistry 75 : 295–332.
29. BehnamJT, WilliamsEL, BrinkS, RumsbyG, DanpureCJ (2006) Reconstruction of human hepatocyte glyoxylate metabolic pathways in stably transformed Chinese-hamster ovary cells. Biochem J 394 : 409–416.
30. van KampenM (1971) Some aspects of thermoregulation in the white leghorn fowl. International Journal of Biometeorology 15 : 244–246.
31. KoinangeEMK, SinghSP, GeptsP (1996) Genetic control of the domestication syndrome in common bean. Crop Science 36 : 1282–1291.
32. Pérez-VegaE, PañedaA, Rodríguez-SuárezC, CampaA, GiraldezR, et al. (2010) Mapping of QTLs for morpho-agronomic and seed quality traits in a RIL population of common bean (Phaseolus vulgaris L.). TAG Theoretical and Applied Genetics 2010 online first.
33. WrightD, RubinCJ, Martinez BarrioA, SchützK, KerjeS, et al. (2010) The genetic architecture of domestication in the chicken: effects of pleiotropy and linkage. Molecular Ecology 19 : 5140–5156.
34. BurkeJM, TangS, KnappSJ, RiesebergLH (2002) Genetic Analysis of Sunflower Domestication. Genetics 161 : 1257–1267.
35. CaiHW, MorishimaH (2002) QTL clusters reflect character associations in wild and cultivated rice. TAG Theoretical and Applied Genetics 104 : 1217–1228.
36. Andersson M (1994) Sexual Selection: Princeton University Press.
37. ChenowethS, McGuiganK (2010) The genetic basis of sexually selected variation. Annual Review of Ecology, Evolution, and Systematics 41 : 81–101.
38. LandeR (1979) Quantitative genetic analysis of multivariate evolution, applied to brain-body size allometry. Evolution 33 : 402–416.
39. WalshB, BlowsM (2009) Abundant genetic variation+strong selection = multivariate genetic constraints: a geometric view of adaptation. Annual Reviews in Ecology and Evolution Systems 40 : 41–59.
40. RoweL, HouleD (1996) The Lek Paradox and the Capture of Genetic Variance by Condition Dependent Traits. Proceedings of the Royal Society of London Series B: Biological Sciences 263 : 1415–1421.
41. FoleyB, ChenowethS, NuzhdinS, BlowsM (2007) Natural genetic variation in cuticular hydrocarbon expression in male and female Drosophila melanogaster. Genetics 175 : 1465–1477.
42. EtgesWJ, De OliveiraCC, GraggE, Ortíz-BarrientosD, NoorMAF, et al. (2007) Evolution 61 : 1106–1119.
43. EtgesWJ, De OliveiraCC, RitchieMG, NoorMAF (2009) Genetics of Incipient Speciation in Drosophilia mojavensis: II. Host Plants and Mating Status Influence Cuticular Hydro-Carbon QTL Expression and GxE Interactions. Evolution 63 : 1712–1730.
44. KokkoH, JennionsMD, BrooksR (2006) Unifying and Testing Models of Sexual Selection. Annual Review of Ecology, Evolution, and Systematics 37 : 43–66.
45. RanzJM, Castillo-DavisCI, MeiklejohnCD, HartlDL (2003) Sex-Dependent Gene Expression and Evolution of the Drosophila Transcriptome. Science 300 : 1742–1745.
46. LaiZ, KaneNC, ZouY, RiesebergLH (2008) Natural Variation in Gene Expression Between Wild and Weedy Populations of Helianthus annuus. Genetics 179 : 1881–1890.
47. RubinC-J, LindbergJ, FitzsimmonsC, SavolainenP, JensenP, et al. (2007) Differential gene expression in femoral bone from red junglefowl and domestic chicken, differing for bone phenotypic traits. BMC Genomics 8 : 208.
48. NattD, RubinC-J, WrightD, JohnssonM, BeltekyJ, et al. (2012) Heritable genome-wide variation of gene expression and promoter methylation between wild and domesticated chickens. BMC Genomics 13 : 59.
49. LitvinO, CaustonHC, ChenB-J, Pe'erD (2009) Modularity and interactions in the genetics of gene expression. Proceedings of the National Academy of Sciences 106 : 6441–6446.
50. CheslerEJ, LuL, ShouS, QuY, GuJ, et al. (2005) Complex trait analysis of gene expression uncovers polygenic and pleiotropic networks that modulate nervous system function. Nat Genet 37 : 233–242.
51. McGrawEA, YeYH, FoleyB, ChenowethSF, HiggieM, et al. (2011) High-Dimensional Variance Partitioning Reveals the Modular Genetic Basis of Adaptive Divergence in Gene Expression during Reproductive Character Displacement. Evolution 65 : 3126–3137.
52. AyrolesJF, CarboneMA, StoneEA, JordanKW, LymanRF, et al. (2009) Systems genetics of complex traits in Drosophila melanogaster. Nat Genet 41 : 299–307.
53. RubinC-J, BrändströmH, WrightD, KerjeS, GunnarssonU, et al. (2007) Quantitative trait loci for BMD and bone strength in an intercross between domestic and wildtype chickens. Journal of Bone and Mineral Research 22 : 375–384.
54. Green P, Falls K, Crooks S (1990) Cri-Map. 2.4 ed. St Louis: Washington University School of Medicine.
55. Perez-EncisoM, MisztalI (2004) Qxpak: a versatile mixed model application for genetical genomics and QTL analyses. Bioinformatics 20 : 2792–2798.
56. BromanKW, WuH, SenS, ChurchillGA (2003) R/qtl: QTL maping in experimental crosses. Bioinformatics 19 : 889–890.
57. ChurchillGA, DoergeRW (1994) Empirical Threshold values for Quantitative Trait Mapping. Genetics 138 : 964–971.
58. DoergeRW, ChurchillGA (1996) Permutation Tests for Multiple Loci Affecting a Quantitative Character. Genetics 142 : 285–294.
59. LanderES, KruglyakL (1995) Genetic Dissection of Complex Traits: Guidelines for Interpreting and Reporting Linkage Results. Nature Genetics 11 : 241–247.
60. ManichaikulA, DupuisJ, SenS, BromanKW (2006) Poor Performance of Bootstrap Confidence Intervals for the Location of a Quantitative Trait Locus. Genetics 174 : 481–489.
61. PeirceJL, BromanKW, LuL, CheslerEJ, ZhouG, et al. (2008) Genome Reshuffling for Advanced Intercross Permutation (GRAIP): Simulation and Permutation for Advanced Intercross Population Analysis. PLoS ONE 3: e1977 doi:10.1371/journal.pone.0001977.
62. LivakJ, SchmittgenT (2001) Analysis of relative gene expression data using real-time quantitativ PCR and the 2−ΔΔCT Method. Methods 25 : 402–408.
Štítky
Genetika Reprodukční medicína
Článek Mutational Signatures of De-Differentiation in Functional Non-Coding Regions of Melanoma GenomesČlánek Rescuing Alu: Recovery of Inserts Shows LINE-1 Preserves Alu Activity through A-Tail ExpansionČlánek Genetics and Regulatory Impact of Alternative Polyadenylation in Human B-Lymphoblastoid CellsČlánek Retrovolution: HIV–Driven Evolution of Cellular Genes and Improvement of Anticancer Drug ActivationČlánek The Mi-2 Chromatin-Remodeling Factor Regulates Higher-Order Chromatin Structure and Cohesin DynamicsČlánek Identification of Human Proteins That Modify Misfolding and Proteotoxicity of Pathogenic Ataxin-1
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 8
-
Všechny články tohoto čísla
- Mutational Signatures of De-Differentiation in Functional Non-Coding Regions of Melanoma Genomes
- Rescuing Alu: Recovery of Inserts Shows LINE-1 Preserves Alu Activity through A-Tail Expansion
- Genetics and Regulatory Impact of Alternative Polyadenylation in Human B-Lymphoblastoid Cells
- Chromosome Territories Meet a Condensin
- It's All in the Timing: Too Much E2F Is a Bad Thing
- Fine-Mapping and Initial Characterization of QT Interval Loci in African Americans
- Genome Patterns of Selection and Introgression of Haplotypes in Natural Populations of the House Mouse ()
- A Combinatorial Amino Acid Code for RNA Recognition by Pentatricopeptide Repeat Proteins
- Advances in Quantitative Trait Analysis in Yeast
- Experimental Evolution of a Novel Sexually Antagonistic Allele
- Variation of Contributes to Dog Breed Skull Diversity
- , a Gene Involved in Axonal Pathfinding, Is Mutated in Patients with Kallmann Syndrome
- A Single Origin for Nymphalid Butterfly Eyespots Followed by Widespread Loss of Associated Gene Expression
- Cryptocephal, the ATF4, Is a Specific Coactivator for Ecdysone Receptor Isoform B2
- Retrovolution: HIV–Driven Evolution of Cellular Genes and Improvement of Anticancer Drug Activation
- The PARN Deadenylase Targets a Discrete Set of mRNAs for Decay and Regulates Cell Motility in Mouse Myoblasts
- A Sexual Ornament in Chickens Is Affected by Pleiotropic Alleles at and , Selected during Domestication
- Use of Allele-Specific FAIRE to Determine Functional Regulatory Polymorphism Using Large-Scale Genotyping Arrays
- Novel Loci for Metabolic Networks and Multi-Tissue Expression Studies Reveal Genes for Atherosclerosis
- The Genetic Basis of Pollinator Adaptation in a Sexually Deceptive Orchid
- Uncovering the Genome-Wide Transcriptional Responses of the Filamentous Fungus to Lignocellulose Using RNA Sequencing
- Inheritance Beyond Plain Heritability: Variance-Controlling Genes in
- The Metabochip, a Custom Genotyping Array for Genetic Studies of Metabolic, Cardiovascular, and Anthropometric Traits
- Reprogramming to Pluripotency Can Conceal Somatic Cell Chromosomal Instability
- Condensin II Promotes the Formation of Chromosome Territories by Inducing Axial Compaction of Polyploid Interphase Chromosomes
- PTEN Negatively Regulates MAPK Signaling during Vulval Development
- A Dynamic Response Regulator Protein Modulates G-Protein–Dependent Polarity in the Bacterium
- Population Genomics of the Facultatively Mutualistic Bacteria and
- Components of a Fanconi-Like Pathway Control Pso2-Independent DNA Interstrand Crosslink Repair in Yeast
- Polysome Profiling in Liver Identifies Dynamic Regulation of Endoplasmic Reticulum Translatome by Obesity and Fasting
- Stromal Liver Kinase B1 [STK11] Signaling Loss Induces Oviductal Adenomas and Endometrial Cancer by Activating Mammalian Target of Rapamycin Complex 1
- Reprogramming of H3K27me3 Is Critical for Acquisition of Pluripotency from Cultured Tissues
- Transgene Induced Co-Suppression during Vegetative Growth in
- Hox and Sex-Determination Genes Control Segment Elimination through EGFR and Activity
- A Quantitative Comparison of the Similarity between Genes and Geography in Worldwide Human Populations
- Minibrain/Dyrk1a Regulates Food Intake through the Sir2-FOXO-sNPF/NPY Pathway in and Mammals
- Comparative Analysis of Regulatory Elements between and by Genome-Wide Transcription Start Site Profiling
- Simple Methods for Generating and Detecting Locus-Specific Mutations Induced with TALENs in the Zebrafish Genome
- S Phase–Coupled E2f1 Destruction Ensures Homeostasis in Proliferating Tissues
- Cell-Nonautonomous Signaling of FOXO/DAF-16 to the Stem Cells of
- The Mi-2 Chromatin-Remodeling Factor Regulates Higher-Order Chromatin Structure and Cohesin Dynamics
- Comparative Analysis of the Genomes of Two Field Isolates of the Rice Blast Fungus
- Role of Mex67-Mtr2 in the Nuclear Export of 40S Pre-Ribosomes
- Genetic Modulation of Lipid Profiles following Lifestyle Modification or Metformin Treatment: The Diabetes Prevention Program
- HAL-2 Promotes Homologous Pairing during Meiosis by Antagonizing Inhibitory Effects of Synaptonemal Complex Precursors
- SLX-1 Is Required for Maintaining Genomic Integrity and Promoting Meiotic Noncrossovers in the Germline
- Phylogenetic and Transcriptomic Analysis of Chemosensory Receptors in a Pair of Divergent Ant Species Reveals Sex-Specific Signatures of Odor Coding
- Reduced Prostasin (CAP1/PRSS8) Activity Eliminates HAI-1 and HAI-2 Deficiency–Associated Developmental Defects by Preventing Matriptase Activation
- Dissecting the Gene Network of Dietary Restriction to Identify Evolutionarily Conserved Pathways and New Functional Genes
- Identification of Human Proteins That Modify Misfolding and Proteotoxicity of Pathogenic Ataxin-1
- and Link Transcription of Phospholipid Biosynthetic Genes to ER Stress and the UPR
- CDK9 and H2B Monoubiquitination: A Well-Choreographed Dance
- Rare Copy Number Variations in Adults with Tetralogy of Fallot Implicate Novel Risk Gene Pathways
- Ccdc94 Protects Cells from Ionizing Radiation by Inhibiting the Expression of
- NOL11, Implicated in the Pathogenesis of North American Indian Childhood Cirrhosis, Is Required for Pre-rRNA Transcription and Processing
- Human Developmental Enhancers Conserved between Deuterostomes and Protostomes
- A Luminal Glycoprotein Drives Dose-Dependent Diameter Expansion of the Hindgut Tube
- Melanophore Migration and Survival during Zebrafish Adult Pigment Stripe Development Require the Immunoglobulin Superfamily Adhesion Molecule Igsf11
- Dynamic Distribution of Linker Histone H1.5 in Cellular Differentiation
- Combining Comparative Proteomics and Molecular Genetics Uncovers Regulators of Synaptic and Axonal Stability and Degeneration
- Chemical Genetics Reveals a Specific Requirement for Cdk2 Activity in the DNA Damage Response and Identifies Nbs1 as a Cdk2 Substrate in Human Cells
- Experimental Relocation of the Mitochondrial Gene to the Nucleus Reveals Forces Underlying Mitochondrial Genome Evolution
- Rates of Gyrase Supercoiling and Transcription Elongation Control Supercoil Density in a Bacterial Chromosome
- Mutations in a P-Type ATPase Gene Cause Axonal Degeneration
- A General G1/S-Phase Cell-Cycle Control Module in the Flowering Plant
- Multiple Roles and Interactions of and in Development of the Respiratory System
- UNC-40/DCC, SAX-3/Robo, and VAB-1/Eph Polarize F-Actin during Embryonic Morphogenesis by Regulating the WAVE/SCAR Actin Nucleation Complex
- Epigenetic Remodeling of Meiotic Crossover Frequency in DNA Methyltransferase Mutants
- Modulating the Strength and Threshold of NOTCH Oncogenic Signals by
- Loss of Axonal Mitochondria Promotes Tau-Mediated Neurodegeneration and Alzheimer's Disease–Related Tau Phosphorylation Via PAR-1
- Acetyl-CoA-Carboxylase Sustains a Fatty Acid–Dependent Remote Signal to Waterproof the Respiratory System
- ATXN2-CAG42 Sequesters PABPC1 into Insolubility and Induces FBXW8 in Cerebellum of Old Ataxic Knock-In Mice
- Cohesin Rings Devoid of Scc3 and Pds5 Maintain Their Stable Association with the DNA
- The MicroRNA Inhibits Calcium Signaling by Targeting the TIR-1/Sarm1 Adaptor Protein to Control Stochastic L/R Neuronal Asymmetry in
- Rapid-Throughput Skeletal Phenotyping of 100 Knockout Mice Identifies 9 New Genes That Determine Bone Strength
- The Genes Define Unique Classes of Two-Partner Secretion and Contact Dependent Growth Inhibition Systems
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Dissecting the Gene Network of Dietary Restriction to Identify Evolutionarily Conserved Pathways and New Functional Genes
- It's All in the Timing: Too Much E2F Is a Bad Thing
- Variation of Contributes to Dog Breed Skull Diversity
- The PARN Deadenylase Targets a Discrete Set of mRNAs for Decay and Regulates Cell Motility in Mouse Myoblasts
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



