-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaThe Nuclear Receptor DAF-12 Regulates Nutrient Metabolism and Reproductive Growth in Nematodes
Animals adjust their internal biological processes in response to their environments. In this study, we report that in a nutrient rich environment the free-living nematode, Caenorhabditis elegans, induces an energy-generating metabolic pathway to govern its reproductive growth by activating the nuclear receptor, DAF-12. By responding to its endogenous ligands, called dafachronic acids, DAF-12 induces oxidation of lipids to produce the energy necessary to support growth and reproduction; and likewise, in the absence of dafachronic acids, DAF-12 prevents activation of this pathway. Through gene expression analysis, we show that DAF-12 regulates a network of genes involved in energy homeostasis and lipid metabolism. Given that dafachronic acids are produced only in well-fed worms, we conclude that DAF-12 functions as an environmental sensor that coordinately governs energy homeostasis. Through analogous studies in the incurable human parasite, Strongyloides stercoralis, we demonstrate that this pathway is conserved and that blocking it compromises the viability of the parasites. These findings elucidate a molecular mechanism for how nematodes govern their energy needs in response to the environment, and provide a potential new strategy for treating nematode parasitic diseases.
Published in the journal: . PLoS Genet 11(3): e32767. doi:10.1371/journal.pgen.1005027
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1005027Summary
Animals adjust their internal biological processes in response to their environments. In this study, we report that in a nutrient rich environment the free-living nematode, Caenorhabditis elegans, induces an energy-generating metabolic pathway to govern its reproductive growth by activating the nuclear receptor, DAF-12. By responding to its endogenous ligands, called dafachronic acids, DAF-12 induces oxidation of lipids to produce the energy necessary to support growth and reproduction; and likewise, in the absence of dafachronic acids, DAF-12 prevents activation of this pathway. Through gene expression analysis, we show that DAF-12 regulates a network of genes involved in energy homeostasis and lipid metabolism. Given that dafachronic acids are produced only in well-fed worms, we conclude that DAF-12 functions as an environmental sensor that coordinately governs energy homeostasis. Through analogous studies in the incurable human parasite, Strongyloides stercoralis, we demonstrate that this pathway is conserved and that blocking it compromises the viability of the parasites. These findings elucidate a molecular mechanism for how nematodes govern their energy needs in response to the environment, and provide a potential new strategy for treating nematode parasitic diseases.
Introduction
The evolutionary success of nematodes is derived from their ability to adapt different developmental pathways depending on environmental conditions. The best studied of these pathways exists in the free-living nematode Caenorhabditis elegans (C. elegans). After hatching from eggs when enviromental conditions are favorable, C. elegans larvae continually develop through four stages (L1–L4), which eventually mature into reproductive adults. In contrast, in unfavorable environments, C. elegans larvae interrupt their reproductive growth by arresting at an alternative L3 stage termed dauer, which is characterized by developmental quiescence, stress resistance and a substantial extension of lifespan. Once conditions become favorable, the L3-dauers exit the developmental diapause and rapidly progress into the L4 stage through a series of metabolic and developmental changes that are governed by a coordinated transcriptional network [1]. Through this developmental adjustment, C. elegans is able to maximize its reproductive advantage under diverse environmental conditions [2]. Similar to free-living species like C. elegans, parasitic nematodes also alter their larval development based on environmental conditions [3,4]. Before host infection occurs, larvae of developing parasitic nematodes, such as hookworms and Strongyloides stercoralis (S. stercoralis), arrest their reproductive growth at a dauer-like stage called infectious L3 (iL3). Then, upon infection of their hosts where environmental conditions favor the completion of the parasite’s life cycle, the arrested iL3 larvae resume reproductive growth and develop into fertile egg-laying adults.
At the molecular level, the nematode development program is controlled by a hormonal signaling pathway initiated by insulin/IGF-I and TGF-β, which eventually converges in the activation of a nuclear receptor called DAF-12 [2,5,6]. In C. elegans, favorable environments stimulate insulin/IGF-I and TGF-β pathways that induce expression of DAF-9, a cytochrome P450 enzyme that catalyzes the synthesis of steroid-like hormones, called dafachronic acids [2,5,6]. Dafachronic acids serve as ligands that bind and activate DAF-12 [5–7], which in turn commit the nematode to reproductive growth. Conversely, in unfavorable environments, the insulin/IGF-I and TGF-β pathways remain inactive, preventing dafachronic acid synthesis, which in turn allows DAF-12 to interact with DIN-1, a strong co-repressor that is required for dauer formation [5,6,8]. In C. elegans larvae lacking DAF-12, this repressor activity is absent, causing a dauer-defective phenotype that would be expected to decrease viability in an unfavorable environment [2,9]. In parasitic nematodes, the insulin/IGF-I/DAF-12 signaling pathway controlling development appears to be conserved [10–15]. Similar to C. elegans, in parasitic nematodes ligand-free DAF-12 is required for formation of the dauer-like iL3, whereas ligand-activated DAF-12 is required for reproductive growth [15].
In C. elegans, larvae undergoing reproductive growth or dauer diapause display distinct patterns of energy metabolism. L2–L4 larvae in reproductive growth exhibit aerobic energy metabolism by converting dietary energy sources (carbohydrates and lipids) into acetyl-CoA, which is then fed into the TCA cycle and oxidative phosphorylation [2,16,17]. This aerobic metabolism produces sufficient energy to support rapid, energy-demanding reproductive growth. In contrast, aerobic energy metabolism is greatly reduced in dauer larvae, which instead exhibit a slower rate of anaerobic energy metabolism. Paradoxically, however, anaerobic metabolism also utilizes fat metabolism to meet the nematode’s energy needs for survival during privation [18–21]. The pathways involved in anaerobic energy metabolism are the glyoxylate cycle, a variant of the TCA cycle that converts acetyl-CoA to malate, and malate dismutation, a fermentation pathway that metabolizes malate for energy production [2,16,17]. The tendency towards the lower rate of anaerobic metabolism in dauer larvae prevents premature depletion of energy reserves and facilitates extended lifespan [22]. Thus, two distinct types of metabolism are employed to produce energy during reproductive growth and diapause stages, raising the question as to how the two pathways are differentially regulated.
Although the study of metabolism in parasitic nematodes is hampered by the difficulty in obtaining sufficient numbers, it is known that during reproductive growth in their hosts certain species of parasitic larvae migrate through the circulatory system, lungs and trachea, where aerobic conditions are high [3,4]. Furthermore, iL3 larvae of the hookworm Ancylostoma caninum are suggested to use fat reserves as an energy source [23], and there is an inverse correlation between oxygen consumption and iL3 longevity in parasitic nematodes [24]. These findings suggest that similar mechanisms control developmental energy metabolism in free-living and parasitic nematodes.
In the present study, we show that in addition to governing expression of developmental genes required for entry and exit from dauer diapause, DAF-12 is required for activating a metabolic network that is required for the normal progression to reproductive maturity. Efforts to elucidate the molecular targets of DAF-12 have focused mainly on the identification of heterochronic and microRNA genes that ensure the correct developmental decision is made during entrance and exit from dauer [25–28], and on longevity genes that are repressed in long-lived mutant worms [29–31]. Notably, however, a role for DAF-12 in energy homeostasis has not been well documented. Utilizing a combination of biochemical and genetic approaches, we show that DAF-12 is a key transcriptional regulator of developmental energy metabolism. In C. elegans, DAF-12 induces expression of a gene network that is responsible for aerobic fat utilization during reproductive growth. Further, this DAF-12-dependent metabolic network is conserved in the parasitic nematode, S. stercoralis. This work provides a molecular understanding of how nematodes adjust energy metabolism to assure successful reproduction in wide-ranging environments, and it suggests a therapeutic strategy for treating parasitic diseases by inhibiting fat utilization.
Results
DAF-12 stimulates aerobic fatty acid metabolism and reproductive growth
To investigate the potential role of DAF-12 in regulating energy metabolism in C. elegans, we used a dauer defective double-null mutant lacking both the DAF-12 co-repressor din-1 and the dafachronic acid-synthesizing enzyme daf-9 [8]. Employing a mutant that lacks both din-1 and daf-9 commits C. elegans to constitutive reproductive growth even in the absence of dafachronic acids. The advantage of the din-1;daf-9 mutant is that it permits evaluation of the direct effects of DAF-12 activation on metabolism while at the same time minimizing effects due to the developmental switching that would otherwise occur in the single null mutant of daf-9. We found that treating din-1;daf-9 larvae with the high affinity endogenous ligand, Δ7-dafachronic acid (DA) decreased triglyceride levels in a dose dependent manner (Fig. 1A). This decrease was not due to reduced dietary nutrient uptake, since DA treatment had no effect on pharyngeal pumping rates of the larvae (Fig. 1B) but rather slightly increased dietary fatty acid uptake (Fig. 1C). In contrast, DA treatment increased the fatty acid oxidation and oxygen consumption in din-1;daf-9 larvae (Fig. 1D, E). At the same time, DA treatment did not significantly change either triglyceride levels, fatty acid oxidation, or oxygen consumption in mutants that lack DAF-12 (din-1;daf-12) or in wild type N2 larvae (Fig. 1A, D, E), indicating that the effects of DA on metabolism were DAF-12 dependent.
Fig. 1. DAF-12 activation promotes aerobic lipid metabolism and reproductive growth in C. elegans. 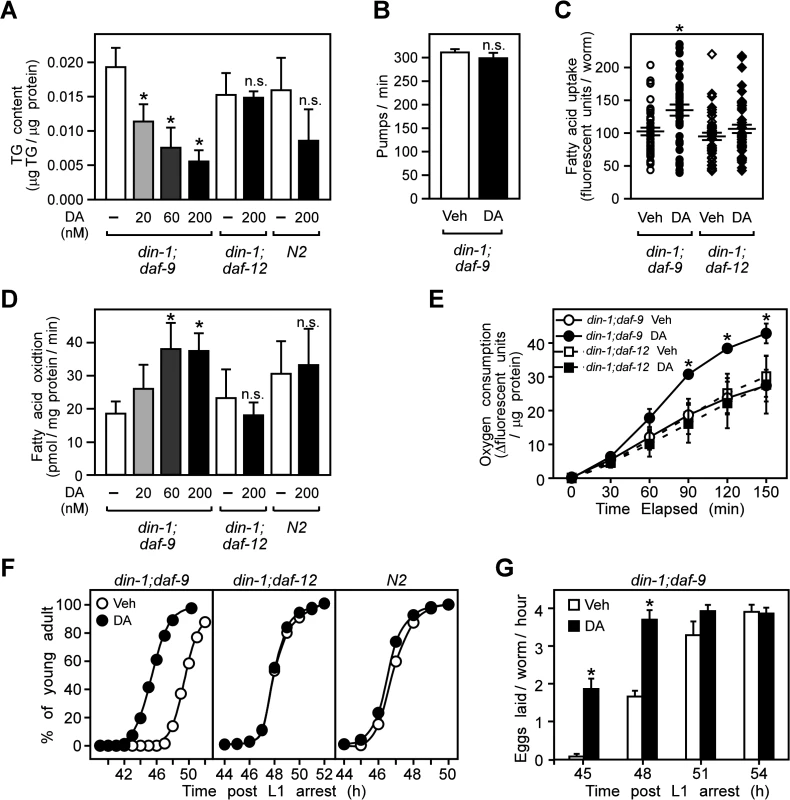
(A) Triglyceride (TG) content, (B) pharyngeal pumping rates, (C) dietary fatty acid uptake, (D) fatty acid oxidation and (E) oxygen consumption in L3 larvae that were synchronized and treated from the L1 stage for 22.5 h at 25°C with vehicle or DA at the indicated concentration (A, D), or 200 nM (B, C, E). (F,) Stimulation of reproductive growth as evaluated by the percentage of L4 larvae that become adults after treatment of synchronized L1 larvae with vehicle or 200 nM DA. (G) Time to egg-laying after treatment of synchronized L1 larvae with vehicle or 200 nM DA. N = 3 ± S.D.; * P < 0.05 comparing vehicle to DA treatment by Student’s t-test in (A–E, G); n.s., not significant. In (C), error bars = S.E.M. Vehicle, ethanol; DA, Δ7-dafachronic acid. We also examined whether DAF-12 activation affects reproduction. As shown in Fig. 1F, progression from L4 to the young adult stage, when reproductive organs become well-developed [32], occurred earlier in din-1;daf-9 larvae treated with DA compared to vehicle in a DAF-12 specific manner. DA treatment also advanced the onset of egg laying, another marker of reproductive maturity (Fig. 1G). Together, these findings demonstrate that DAF-12 activation induces aerobic energy metabolism and accelerates larval reproductive growth.
DAF-12 regulates genes involved in fatty acid metabolism
To gain insight into the molecular mechanism underling the DAF-12-regulated fatty acid metabolism, we evaluated global changes in C. elegans gene expression by comparing vehicle and DA treated L3 larvae. Microarray analysis identified 796 genes that were up-regulated and 985 genes that were down-regulated (>2-fold change and FDR<5%) in response to DAF-12 activation (S1 Table). The DA-regulated transcriptome was then grouped into several functional categories based on gene ontology (DAVID, ref. [33], S1 Table). In addition to the expected changes in expression of heterochronic and molting genes (e.g., dre-1) that coordinately regulate developmental and reproductive pathways [26], DA governed expression of a distinct cadre of genes involved in the metabolism of lipids (S1 Table). In contrast, no changes were observed in the expression of genes required for metabolizing glucose.
We then compared the microarray data from our DA responsive genes with that of genes that have been shown to be regulated during the exit from dauer [1], which is another process where DAF-12 is activated. As expected, the transcriptome of DA up-regulated genes significantly overlapped the trancriptome of genes up-regulated during dauer recovery (S1A Fig.). These data indicate that DAF-12 engages a gene network that governs metabolism and growth during both reproductive development and dauer recovery. We also compared the DAF-12 transcriptome with genes that are regulated by the transcription factor DAF-16 [34]. Whereas DAF-12 activation suppresses dauer, activation of DAF-16 is known to promote dauer [2]. As expected by this reciprocal regulation of dauer, there was no statistically significant overlap between genes regulated by DAF-12 and DAF-16 (S1B Fig. and S1C Fig.). Although this comparison was between the transcriptomes from different stages of worms (L3 for DAF-12 vs. adult for DAF-16), these results suggest that DAF-12 and DAF-16 regulate distinct gene networks to coordinate entry and exit from dauer diapause, and the initiation of metabolic pathways that promote reproductive development.
To further investigate the metabolic network governed by DAF-12, we used qPCR to confirm changes in expression of fatty acid metabolic genes identified in our microarray study, as well as other candidate genes known to be involved in fatty acid metabolism [19,20]. A total of 69 genes were evaluated (S2 Table). A hallmark of the DA-regulated metabolic pathway that correlates to reproductive growth is the induction of aerobic fatty acid oxidation (Fig. 1). DA increased expression of genes involved in every aspect of aerobic fatty acid utilization, including lipolysis, transport, esterification, and oxidation in both peroxisomes and mitochondria (Fig. 2A-E; S2 Table).
Fig. 2. Regulation of fat utilization genes by DAF-12 in C. elegans. 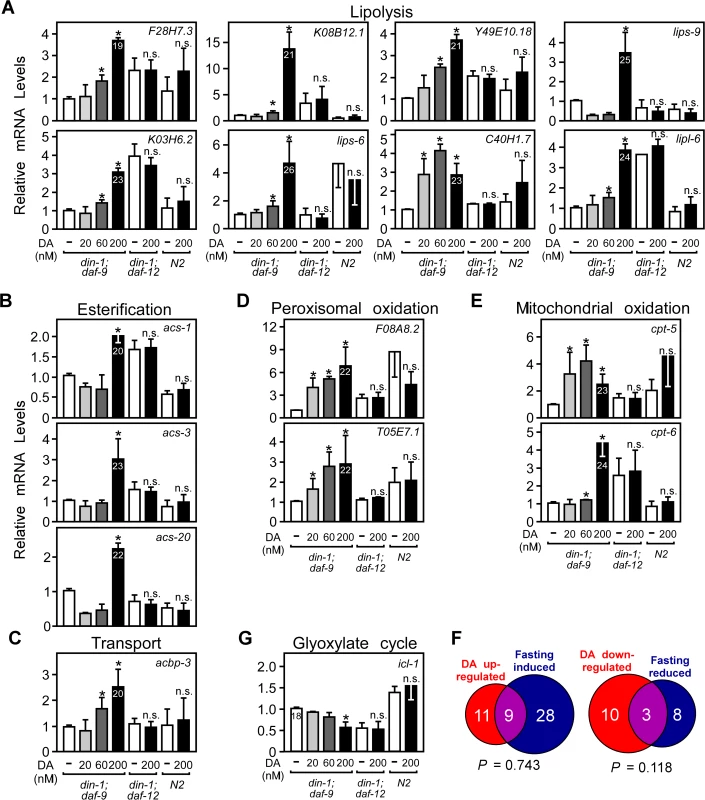
(A–E, G) Fatty acid metabolic gene expression in L3 worms. Synchronized L1 larvae were grown in the presence of vehicle or the indicated concentrations of DA for 22.5 hrs and mRNA expression was quantitated by qPCR. Numbers in bars refer to Ct values. (F) Comparison of DAF-12 and fasting regulated metabolic genes. *P< 0.05 by Student’s t-test comparing vehicle to DA treatment (n = 4 ± S.D.), n.s., not significant. Vehicle, ethanol; DA, Δ7-dafachronic acid. To provide an additional objective assessment of DAF-12’s role in regulating energy metabolism, we compared the DA-regulated lipid metabolic gene profile to changes observed in response to fasting. In addition to reproductive growth, fasting is another physiological process that mobilizes and utilizes fatty acids [19,20]. However, in contrast to reproductive growth, fasting utilizes anaerobic metabolism marked by reduced metabolic rates [21] and activation of the glyoxylate cycle (through icl-1 expression) [19,20]. Of the 69 fatty acid metabolic genes tested above (S2 Table), 20 were increased by DAF-12 and 37 were increased by fasting (Fig. 2F, S2 Table). Importantly, there was no significant overlap (based on hypergeometric distribution) in the number of genes that were either up - or down-regulated under both conditions, demonstrating that DAF-12 and fasting engage distinct gene networks for fatty acid utilization. DA decreased the mRNA levels of icl-1 (Fig. 2G), the bi-functional enzyme with isocitrate lyase and malate synthase activities that is unique to the glyoxlate cycle and thus serves for an indicator of anaerobic fatty acid utilization. Taken together with the biochemical measurements of fatty acid oxidation (Fig. 1), these results suggest that DAF-12 activation selectively governs the pathways that lead to energy mobilization and utilization by increasing expression of genes involved in aerobic lipid metabolism.
We also investigated the signaling pathways that govern metabolism of DA. Interestingly, expression of daf-28 (insulin/IGF-I-like), daf-7 (TGF-β-like) and daf-9 (the DA biosynthesis enzyme) were specifically suppressed by exogenous DA treatment, while expression of strm-1, which quenches DAF-12 ligand synthesis [35], was induced (S2 Fig.). The ability of DAF-12 to repress its own activity is reminiscent of a classic endocrine feedback circuit that functions to maintain homeostasis.
To determine whether the up-regulation of gene transcription by DA was through the direct action of DAF-12, we analyzed the promoters of several of the DA-induced genes that were confirmed by qPCR. In the absence of a DAF-12 antibody to perform chromatin immunoprecipitation experiments, we used bioinformatics to search for the consensus DAF-12 DNA binding element [36] in the promoters/introns of five representative DAF-12-induced genes. Selection of these genes was based on their representation of different metabolic processes, their high levels of expression, their response to DA, and their distinct chromosomal locations (i.e., genes not likely to be in a gene cluster sharing a common promoter). Our analysis revealed 33 putative DAF-12 response elements (S3 Table). We found that DAF-12 bound efficiently to 13 of these elements (Fig. 3A, B; S3 Table) and activated transcription in a standard cell-based reporter assay through four of them (Fig. 3C; S3 Table). These four DAF-12 response elements corresponded to three (K08B12.1, acs-1 and acs-3) of the five genes originally selected for analysis. Consistent with these genes being direct transcriptional targets of DAF-12, K08B12.1 and acs-3 expression was induced rapidly within 30 to 60 minutes after treatment of din-1;daf-9 larvae with DA (Fig. 3D). These data suggest that at least a portion of the genes regulated by DAF-12 are likely to be direct targets.
Fig. 3. Fat utilization genes are direct targets of C. elegans DAF-12. 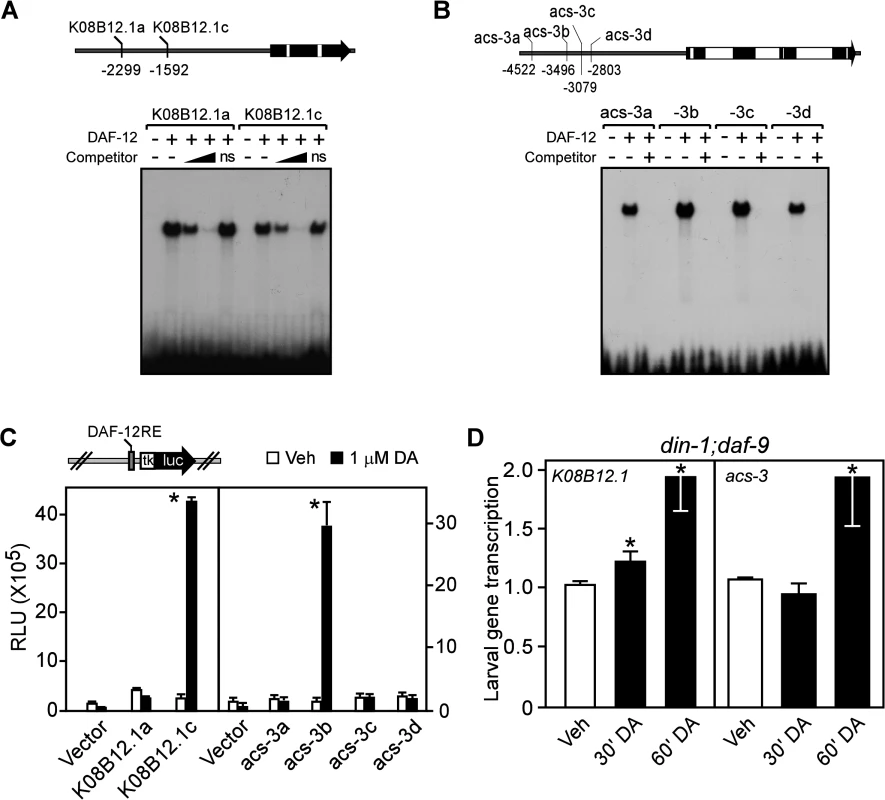
(A, B) Gel electrophoresis mobility shift assays with [32P]-labelled oligonucleotides corresponding to each of the candidate DAF-12 response elements (shown in the schematics of the promoters above the panels) for TG lipase (K08B12.1) and acyl-CoA synthase (acs-3). Gel shifts were performed in presence or absence of in vitro translated DAF-12 protein ± the presence of 20-fold and 200-fold (in A) or 200-fold (in B) excess unlabeled competitor oligonucleotides. ns, non-specific oligo at 200-fold excess. (C) Luciferase reporter assays with candidate response elements shown in (A,B) from HEK293 cells cotransfected with reporter and DAF-12 expression plasmids and treated with vehicle or 1 μM DA (n = 3 ± S.D.). (D) Nuclear run-on assays of K08B12.1 and acs-3 transcription in L2 larvae of din-1;daf-9(dh127;dh6) worms transiently treated by vehicle or 500 nM DA (n = 3 ± S.D.). *P< 0.05 comparing vehicle to DA treatment by Student’s t-test. Vehicle, ethanol; DA, Δ7-dafachronic acid. Normal reproductive growth requires DAF-12 to regulate fat utilization
We next asked whether aerobic fatty acid utilization is required for DAF-12 to promote reproductive growth by inhibiting aerobic fatty acid utilization with etomoxir, a specific inhibitor of the carnitine palmitoyltransferases that mediate fatty acid transport into mitochondria [37]. Etomoxir treatment significantly decreased fatty acid oxidation and increased fat storage in C. elegans (S3 Fig.), demonstrating the effectiveness of the drug in inhibiting this pathway in nematodes. A further consequence of etomoxir treatment was that it completely blocked the earlier onset of egg laying that is dependent on DA, which is a marker for reproductive growth (Fig. 4A). Etomoxir treatment also prevented DA-mediated rescue of reproductive growth in the daf-7 and daf-9 mutants (Fig. 4B, C) and delayed the rescued growth in the daf-2 mutant (Fig. 4D). In this latter mutant, the inability of etomoxir to inhibit growth completely is likely due to compensatory anaerobic fatty acid utilization that is known to occur in the daf-2 mutants [34,38]. Consistent with the data shown in Fig. 1F, DA treatment did not affect reproductive capacity in wild type N2 worms or in mutants lacking DAF-12 expression (din-1;daf-12, daf-9;daf-12, and daf-7;daf-12), regardless of the absence or presence of etomoxir (S4 Fig.). In sum, these data demonstrate that aerobic fatty acid metabolism is required for DAF-12 to promote growth from larvae to reproductive adults in C. elegans.
Fig. 4. Aerobic lipid metabolism is required for DAF-12 to promote reproductive growth in C. elegans. 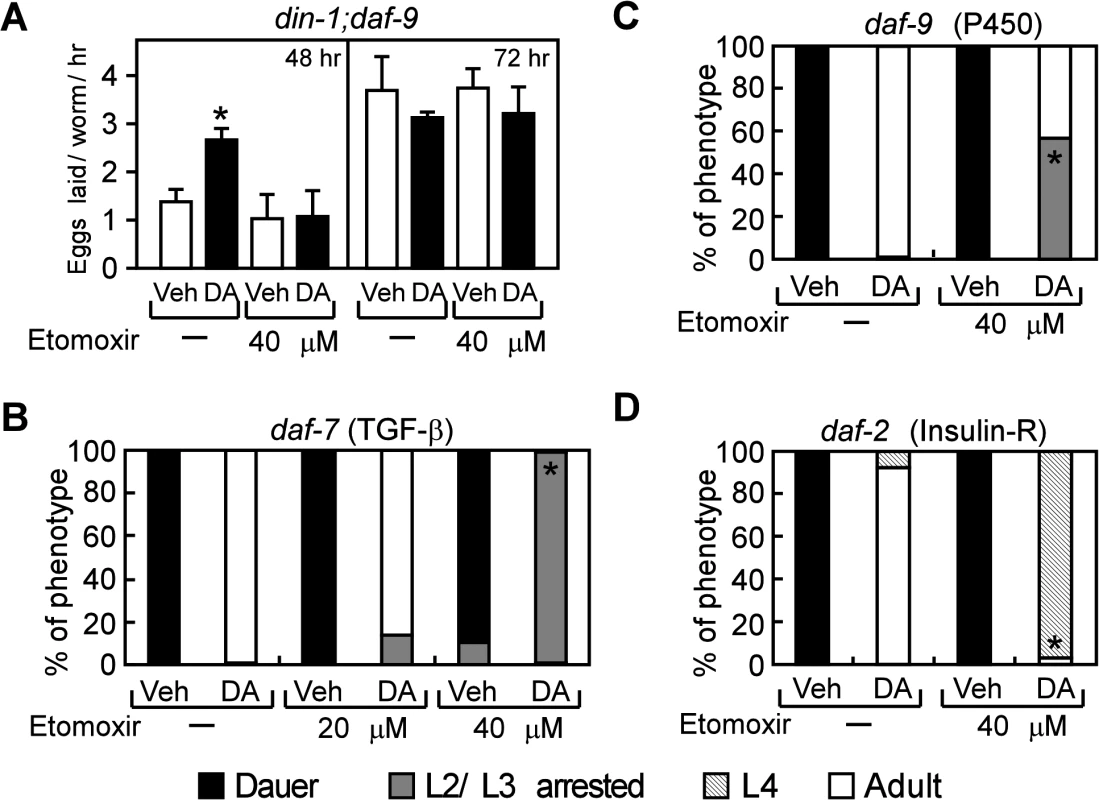
(A) Inhibition of fatty acid oxidation by etomoxir delays the onsets of egg laying in din-1;daf-9 mutants (n = 4), and (B–D) decreases DA-induced reproductive growth in daf-7(e1372), daf-9(dh6), and daf-2(e1368) mutants (n = 3). Synchronized L1 larvae were grown with vehicle or 200 nM DA treatment in the presence or absence of etomoxir at 25°C for 48 h or 72 h (in A) or for 60 h (in B–D) and then phenotyped. *P < 0.05 by Student’s t-test comparing vehicle to DA-treated worms (A) or by Fisher’s exact test comparing DA-treated worms ± etomoxir (B–D). Vehicle, ethanol; DA, Δ7-dafachronic acid. DAF-12 regulates fat utilization and reproductive growth in S. stercoralis
Like C. elegans, many species of parasitic nematodes such as S. stercoralis also use the conserved insulin/IGF-I and DAF-12 signaling pathways to regulate their development [10–15]. We therefore asked whether the role of DAF-12 in promoting fatty acid utilization is conserved during reproductive growth of S. stercoralis. To test this, we confirmed the presence of fat utilization genes in S. stercoralis and then examined whether they are regulated by DA (Fig. 5A, S4 Table). As in C. elegans, expression of genes encoding a lipase (Ss_F28H7.3), acyl-CoA synthase (Ss_acs-1) and a gene involved in acyl-CoA transport (Ss_acbp-3) was induced, while the key glyoxylate cycle gene (Ss_icl-1) was repressed by DA treatment. Expression of the carnitine palmitoyltransferase gene, Ss_W03F9.4, required for mitochondrial β-oxidation, was also increased by DA (Fig. 5A; S4 Table). In contrast, expression of genes involved in peroxisomal β-oxidation (Ss_acox-2, Ss_acox-3 and Ss_ech-8) was repressed by DA (Fig. 5A; S4 Table). This profile of gene regulation supports the notion that DAF-12 activation induces aerobic fat utilization in S. stercoralis similar to that observed for C. elegans (Fig. 2).
Fig. 5. DAF-12-dependent regulation of lipid metabolism is conserved in S. stercoralis. 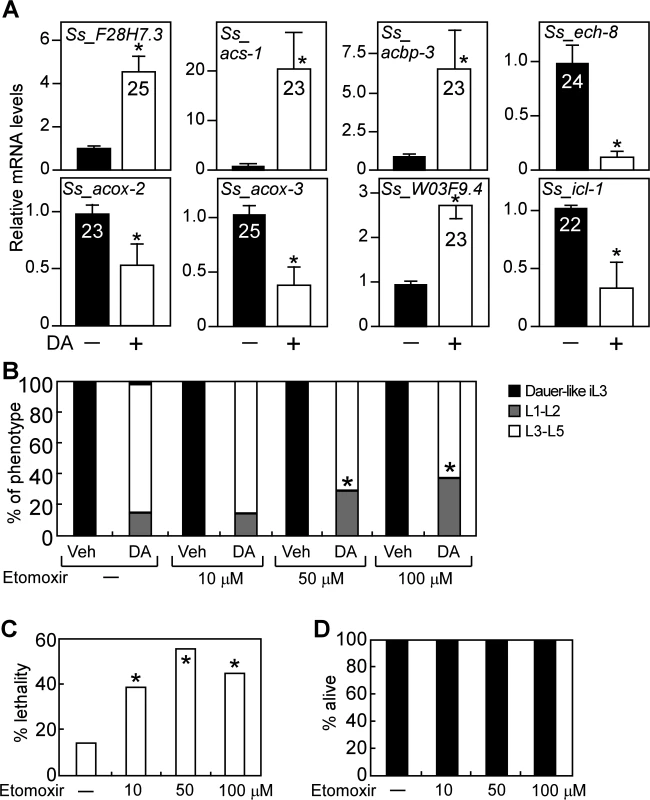
(A) F28H7.3 (lipase), acs-1 (synthase), acbp-3 (transport), W03F9.4 (mitochondrial β-oxidation), ech-8, acox-2 and acox-3 (perioxisomal β-oxidation), and icl-1 (glyoxylate cycle) mRNAs were determined by qPCR from S. stercoralis iL3 larvae treated with or without 400 nM DA. Numbers in bars refer to Ct values. *P-value < 0.05 by Student’s t-test comparing DA to vehicle (n = 3 ± S.D.). (B, C) Effect of the mitochondrial fatty acid oxidation inhibitor, etomoxir, on reproductive development (B) and viability (C) in S. stercoralis post-free-living larvae treated with DA. *P < 0.05 by Fisher’s exact test comparing larvae treated with 300 nM DA ± etomoxir at the indicated concentrations (n = 3). (D) Etomoxir treatment does not cause lethality in developmentally arrested S. stercoralis iL3 larvae (n = 3). Vehicle, ethanol; DA, Δ7-dafachronic acid. We also examined the effect of etomoxir on DA-induced reproductive growth in the post-free-living larvae of S. stercoralis, which typically arrest at the dauer-like iL3 stage. Although DA is less potent as an agonist for DAF-12 in S. stercoralis compared to C. elegans [15], DA treatment was able to induce >85% maturation of S. stercoralis larvae to the L3-L5 stages (Fig. 5B). Notably, co-treatment of etomoxir with DA resulted in a significant decrease in the number of L3-L5 larvae (Fig. 5B). Administration of etomoxir to DA-treated worms also led to a marked increase in lethality of these L3-L5 larvae (Fig. 5C). Importantly, treatment with etomoxir alone did not kill S. stercoralis iL3 larvae (which are in an arrested developmental stage), demonstrating that the lethality observed in L3-L5 worms is due to the specific effects of etomoxir on the reproductive developmental program induced by DA (Fig. 5D).
These results reveal that aerobic fatty acid metabolism is required for DAF-12-dependent reproductive growth of S. stercoralis and suggest that control of this metabolic pathway by DAF-12 might be a promising strategy for regulating development in these important pathogens.
Discussion
The nuclear receptor DAF-12 plays an essential role in C. elegans in linking nutritional status to developmental programs, including the transition from second - to third-stage larval development and the progression into and out of dauer diapause. In this report, we show that activation of DAF-12 by its hormonal ligand DA directly regulates energy homeostasis by inducing aerobic fatty acid utilization, which is required for reproductive growth. DAF-12 was shown previously to regulate heterochronic genes, which determine the stage-specific timing of cell type differentiation [25–27]. Interestingly, the DAF-12 gene regulatory network is distinct from that of DAF-16, which is active in the absence of DA signaling to promote dauer and inhibit reproductive growth. Thus, DAF-12 controls the balance between reproductive growth and dauer both by controlling the expression of genes directing development, and by regulating the flux of nutrients required to fuel the developmental program. Our findings provide a molecular explanation for the longstanding observation that C. elegans switches to aerobic metabolism once reproductive growth has been initiated [2,16].
Under favorable environmental conditions, which promote the biosynthesis of DA, we showed that DAF-12 stimulates aerobic fatty acid utilization in larvae as evidenced by decreased storage of triglycerides and increased oxygen consumption and fatty acid oxidation (Fig. 1A, D, E). Although a previous study originally suggested DAF-12 might increase rather than decrease fat storage [8], it has been shown since that the lipid staining assay used in this previous study is non-specific and ineffective for determining fat content [39]. DA treatment also stimulates progression from L4 to the young adult stages and advances the onset of egg-laying activity (Fig. 1F, G). Thus, DAF-12 coordinates the release of energy required to support the rapid, energy-intensive growth of larvae to reproductive maturity. At the molecular level, DA induces metabolic genes that regulate fatty acid utilization at multiple steps, including fatty acid mobilization, esterification, and peroxisomal and mitochondrial β-oxidation. The rate-limiting step enzyme in fatty acid oxidation, carnitine palmitoyltransferase, is encoded by several homologs in C. elegans, two of which are DAF-12 targets (Fig. 2E) and inhibition of the enzyme’s activity blocks DA-stimulated reproductive growth (Fig. 4). Concomitant with its regulation of genes involved in oxidative metabolism, DA represses the expression of icl-1, which encodes a bifunctional enzyme in the glyoxylate cycle that is essential for anaerobic fatty acid catabolism (Fig. 2G). These results support the role of DAF-12 in promoting reproductive growth of C. elegans by acting as a key controller of energy homeostasis in response to nutrient supply.
The adaptive response to fasting is a process that also mobilizes fat storage to maintain energy homeostasis. However, in contrast to the metabolic pathway involved in reproductive growth, fasting results in an increase in anaerobic metabolism. Fasting decreases metabolic rate [21] and utilizes the glyoxylate cycle to provide energy from fatty acids [18–20], which is diametric to the action of DA. The alternate role of DA in aerobic metabolism is supported by the finding that the metabolic gene networks induced by DAF-12 and by the fasting response are distinct (Fig. 2G). Interestingly, the metabolic response to fasting is mediated by another nematode nuclear receptor, NHR-49 [19,20]. Analogous to the role of DAF-12 in the dauer diapause, NHR-49 is required for the entry and exit of adult reproductive diapause, a process that preserves reproduction in C. elegans during starvation [40]. Thus, DAF-12 and NHR-49 appear to control two separate gene networks that alternatively use aerobic and anaerobic fatty acid utilization to ensure successful reproduction under varying environmental conditions.
Another key finding of the current study is that DAF-12-mediated regulation of energy homeostasis is conserved in the human parasite, S. stercoralis. As in C. elegans, DAF-12 activation stimulates the expression of genes involved in aerobic fatty acid utilization (Fig. 5A). While genes involved in peroxisomal β-oxidation were induced in C. elegans and repressed in S. stercoralis, we note that peroxisomal β-oxidation can support either aerobic or anaerobic fatty acid catabolism. Importantly, however, blocking aerobic fatty acid utilization with etomoxir inhibited DAF-12-induced reproductive growth in S. stercoralis (Fig. 5B-D). Inhibiting this metabolic pathway in other parasitic species using this type of strategy has recently been suggested [41]. To date, there is a very limited armamentarium of anthelmintic drugs that is effective against S. stercoralis, which can cause disseminated strongyloidiasis and multi-organ failure in infected humans. Although further studies in relevant host species are needed, our results suggest targeting metabolic enzymes may lead to a therapeutic approach for treating diseases caused by S. stercoralis and possibly other parasites [42]. To that end, it is interesting that etomoxir and other drugs that were originally developed to regulate fatty acid metabolism [37] as a means for treating diabetes and metabolic disease might be repurposed for treating parasitism.
In summary, our studies reveal a novel facet of DAF-12 activity in both C. elegans and parasitic nematodes, namely the regulation of fatty acid catabolism and energy homeostasis. In this regard, DAF-12 is similar to the PPAR subfamily of nuclear receptors, which coordinately regulate fatty acid homeostasis and energy balance in vertebrates in response to nutrient availability. Our results provide a molecular explanation for how nematodes adjust energy homeostasis in response to changes in environmental conditions for reproduction. Moreover, they suggest a new strategy for developing new classes of anthelmintic drugs.
Materials and Methods
Reagents and nematode strains
Δ7-DA was synthesized as described [6]. C. elegans strains din-1;daf-9(dh127;dh6), din-1;daf-12(dh127;rh61rh411) and daf-9(dh6) were from Dr. Adam Antebi (Max-Planck Institute for Aging); wild type (N2 strain), daf-2(e1368) and daf-7(e1372) worms were from C. elegans Genome Center (University of Minnesota). daf-7;daf-12(e1372;rh61h411) mutant was made by crossing daf-7 hermaphrodites with hemizygous daf-12 males and was screened for dauer defective F2 progenies. The wild type (UPD) strain of S. stercoralis, used for developmental switching studies, and an iso-female line (PV001) of this parasite, used for qPCR studies, were maintained as described [43].
Lipid metabolism
Vehicle or Δ7-DA was mixed with 5× concentrated OP50 bacteria culture and loaded on NGM-agar plates. L1 larvae prepared by egg synchronization were cultured on these plates at 25°C for 22.5 h and the resulting L3 larvae were collected and washed in M9 buffer for the indicated assays. For triglyceride (TG) content, worms were sonicated and the resulting lysates were centrifuged at 13000×g at 4°C. From supernatants, total glyceride (TGs plus free glycerol) and free glycerol were measured by Infinity TG Reagent (Thermo Sci) and Free Glycerol Reagent (Sigma), respectively. TG levels were calculated by subtracting free glycerol from total and were normalized to protein amounts in the lysates.
Fatty acid oxidation was measured as described by the production of H2O from fatty acid [44]. Briefly, the L3 larvae from different treatment groups were incubated with a mix of cold and [3H]-palmitic acid (Perkin Elmer) complexed with fatty acid-free BSA (Sigma), and incubated in a shaker at 25°C for 1 h. The reaction was terminated by adding 10% TCA followed by centrifugation at 13000×g for 5 min to obtain supernatant. Remaining [3H]-palmitic acid was deprotonated by adding 5N NaOH and PBS and removed by ion exchange column (Dowex 1×8 200–400 mesh Cl, strongly basic, Sigma). [3H]2O left in the supernatant was measured by scintillation counting.
For oxygen consumption, worms were mixed with antibiotic-killed OP50 bacteria, and then transferred to Oxygen Biosensor Plates (BD Bioscience) for oxygen measurement. Oxygen consumption was expressed as the increase of fluorescence units (ΔFU) and was normalized by protein amounts of the worms. For fatty acid uptake, worms were mixed with OP50 bacteria and 250 nM of fluorescent tracer (C1-BODIPY-C12 fatty acid, Invitrogen). Following 1 h incubation at 25°C, worms were washed, mounted, and photographed under fluorescence microscopy. Fluorescence density units (FU) of each worm were quantified by the software Image-J.
Measurement of pharyngeal pumping rates
Pharyngeal pumping rates were measured as described [45]. Briefly, din-1;daf-9 L3 larvae were transferred to a fresh NGM plate with OP50 bacteria lawn and were videotaped through a stereomicroscope. Pumping rates were measured by counting the grinder movements and presented as pumps per minute. For each treatment, 10 L3s were assayed.
Reproductive growth assays
Reproductive growth of C. elegans was measured by L4-young adult transition or by egg laying assays. For L4-YA transition assay, synchronized L1 larvae from were cultured on NGM-agar plates pre-loaded with 5× concentrated HT115 bacteria culture. Worms were grown at 20°C and young adults were counted at indicated time points. Data were presented as the percentage of young adults in whole populations. For the egg-laying assay, synchronized din-1;daf-9 L1 larvae were grown at 25°C on NGM-agar plates pre-loaded with 5× concentrated OP50 bacteria culture. At the indicated time points, 10~15 worms were transferred to fresh plates with bacterial lawns for 2.5 h and laid eggs were counted. Data were presented as numbers of the eggs laid by each worm per hour.
Quantitative RT-PCR (qPCR) and microarray analysis
For C. elegans experiments, synchronized L1 larvae were treated with vehicle or Δ7-DA for 22.5 h at 25°C, or synchronized wild type L1 larvae were cultured to L4 stage and then harvested as fed worms or were deprived of food for an additional 12 h to obtain fasted worms. For S. stercoralis experiments, iL3s worms were treated with or without Δ7-DA in M9 buffer at 37°C and 5% CO2 in air for 24 h. Total RNA from worms was extracted with TRIzol Reagent (Life Technologies), and analyzed by qPCR. Relative mRNA levels were normalized to expression of reference genes inf-1 or ama-1 (C. elegans) or 18S ribosomal RNA (S. stercoralis). Data were presented as fold changes of relative mRNA levels in DA versus vehicle treated worms or in fasted versus fed worms.
Total RNA was also subjected to the C. elegans Genome Array (Affymetrix) for whole genome gene expression analysis. Briefly, gene expression values were log2 transformed and genes with >10-fold difference between replicates in either of the treatments were removed from our analysis. To identify the differentially expressed genes, we applied Significance Analysis of Microarrays (SAM) analysis using the R package samr [46]. Genes with median false discovery <5% and fold changes >2.0 were considered differentially expressed.
Electrophoretic mobility shift assay
DAF-12 proteins were prepared with TNT Quick-Coupled Transcription/Translation System (Promega) and blocked with poly-[dI-dC] and non-specific single-stranded oligos. The DAF-12 proteins were then incubated with [32P]-end-labeled dsDNA probes (S3 Table) at room temperature for 30 min and binding to DAF-12 was analyzed by 5% PAGE followed by autography. For competitive binding experiments, 20 - or 200-fold excesses of unlabeled DNA probes were also included in the binding reaction.
Mammalian cell-based reporter assay
Co-transfection and luciferase reporter assays were performed as described in HEK 293 cells [15]. Eight hours post-transfection, cells were treated with vehicle or 1 μM Δ7-DA, and luciferase and β-galactosidase activities were then measured 16 h later. Relative luciferase units (RLU) were normalized to β-galactosidase activity. Reporter plasmids were constructed by inserting DAF-12REs and their 10-bp genomic flanking sequences into a TK-luc reporter plasmid.
Nuclear run-on assay
Synchronized din-1;daf-9 L1 larvae were grown in 5×concentrated OP50 in liquid suspension and shaken at 25°C for 15 h. Resulting L2 larvae were treated transiently with vehicle or 500 nM Δ7-DA and harvested in ice-cold M9 buffer. Cell nuclei were extracted and incubated with ATP, CTP, GTP and 5’-Bromo-UTP (BrUTP) at 30°C for labeling of nascent RNAs with BrUTP (BrUTP-RNAs). The BrUTP-RNAs were then enriched with anti-BrUTP agorase beads (Santa Cruz) and quantified by qPCR.
Development switching assays
Development switching assays were performed as described [5,15]. Briefly, synchronized L1 larvae (C. elegans) or eggs (S. stercoralis) were grown on NGM-plates pre-loaded with etomoxir and phenotypes were observed after 60 h incubation at 25°C. Data from three independent experiments were pooled and significance was determined by Fisher’s exact test.
S. stercoralis gene homolog identification
S. stercoralis homologs were identified as reported [43] by a TBLASTN (NCBI) search of C. elegans versus S. stercoralis (6 December 2011 draft; ftp://ftp.sanger.ac.uk/pub/pathogens/HGI/) databases, followed by annotation to RNA-seq data (ArrayExpress accession number E-MTAB-1164). Phylogenetic tree analyses were constructed to resolve gene homology. S. stercoralis genes with 1 : 1 homology to C. elegans genes were identified as homologous genes.
Statistical analysis
Unless otherwise stated, data were expressed as mean ± SD or SEM and significance tests between vehicle - or DA-treated groups were performed by Student’s t-test. The statistic tests of overlap between two gene sets were based on hypergeometric distribution and calculated by the R function “phyper ()” (https://stat.ethz.ch/R-manual/R-patched/library/stats/html/Hypergeometric.html).
Supporting Information
Zdroje
1. Wang J, Kim SK (2003) Global analysis of dauer gene expression in Caenorhabditis elegans. Development 130 : 1621–1634. 12620986
2. Riddle DL, Albert PS (1997) Genetic and Environmental Regulation of Dauer Larva Development. In: Riddle DL, Blumenthal T, Meyer BJ, Priess JR, editors. C. ELEGANS II. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press pp. 739–768.
3. Hotez PJ, Brooker S, Bethony JM, Bottazzi ME, Loukas A, Xiao S (2004) Hookworm infection. N Engl J Med 351 : 799–807. 15317893
4. Viney ME, Lok JB. Strongyloides spp. (May 23, 2007), WormBook, ed. The C. elegans Research Community, doi/10.1895/wormbook.1.141.1.
5. Motola DL, Cummins CL, Rottiers V, Sharma KK, Li T, Li Y, et al. (2006) Identification of ligands for DAF-12 that govern dauer formation and reproduction in C. elegans. Cell 124 : 1209–1223. 16529801
6. Sharma KK, Wang Z, Motola DL, Cummins CL, Mangelsdorf DJ, Auchus RJ (2009) Synthesis and activity of dafachronic acid ligands for the C. elegans DAF-12 nuclear hormone receptor. Mol Endocrinol 23 : 640–648. doi: 10.1210/me.2008-0415 19196833
7. Mahanti P, Bose N, Bethke A, Judkins JC, Wollam J, Dumas KJ, et al. (2014) Comparative metabolomics reveals endogenous ligands of DAF-12, a nuclear hormone receptor, regulating C. elegans development and lifespan. Cell Metab 19 : 73–83. doi: 10.1016/j.cmet.2013.11.024 24411940
8. Ludewig AH, Kober-Eisermann C, Weitzel C, Bethke A, Neubert K, Gerisch B, et al. (2004) A novel nuclear receptor/coregulator complex controls C. elegans lipid metabolism, larval development, and aging. Genes Dev 18 : 2120–2133. 15314028
9. Antebi A, Yeh WH, Tait D, Hedgecock EM, Riddle DL (2000) daf-12 encodes a nuclear receptor that regulates the dauer diapause and developmental age in C. elegans. Genes Dev 14 : 1512–1527. 10859169
10. Brand A, Hawdon JM (2004) Phosphoinositide-3-OH-kinase inhibitor LY294002 prevents activation of Ancylostoma caninum and Ancylostoma ceylanicum third-stage infective larvae. Int J Parasitol 34 : 909–914. 15217729
11. Castelletto ML, Massey HC Jr., Lok JB (2009) Morphogenesis of Strongyloides stercoralis infective larvae requires the DAF-16 ortholog FKTF-1. PLoS Pathog 5: e1000370. doi: 10.1371/journal.ppat.1000370 19360119
12. Ogawa A, Streit A, Antebi A, Sommer RJ (2009) A conserved endocrine mechanism controls the formation of dauer and infective larvae in nematodes. Curr Biol 19 : 67–71. doi: 10.1016/j.cub.2008.11.063 19110431
13. Stoltzfus JD, Massey HC Jr., Nolan TJ, Griffith SD, Lok JB (2012) Strongyloides stercoralis age-1: a potential regulator of infective larval development in a parasitic nematode. PLoS One 7: e38587. doi: 10.1371/journal.pone.0038587 22701676
14. Tissenbaum HA, Hawdon J, Perregaux M, Hotez P, Guarente L, Ruvkun G (2000) A common muscarinic pathway for diapause recovery in the distantly related nematode species Caenorhabditis elegans and Ancylostoma caninum. Proc Natl Acad Sci U S A 97 : 460–465. 10618440
15. Wang Z, Zhou XE, Motola DL, Gao X, Suino-Powell K, Conneely A, et al. (2009) Identification of the nuclear receptor DAF-12 as a therapeutic target in parasitic nematodes. Proc Natl Acad Sci U S A 106 : 9138–9143. doi: 10.1073/pnas.0904064106 19497877
16. Braeckman BP, Houthoofd K, Vanfleteren JR. Intermediary metabolism (February 16, 2009), WormBook, ed. The C. elegans Research Community, doi/10.1895/wormbook.1.146.1.
17. Burnell AM, Houthoofd K, O'Hanlon K, Vanfleteren JR (2005) Alternate metabolism during the dauer stage of the nematode Caenorhabditis elegans. Exp Gerontol 40 : 850–856. 16221538
18. Liu F, Thatcher JD, Epstein HF (1997) Induction of glyoxylate cycle expression in Caenorhabditis elegans: a fasting response throughout larval development. Biochemistry 36 : 255–260. 8993341
19. Van Gilst MR, Hadjivassiliou H, Jolly A, Yamamoto KR (2005) Nuclear hormone receptor NHR-49 controls fat consumption and fatty acid composition in C. elegans. PLoS Biol 3: e53. 15719061
20. Van Gilst MR, Hadjivassiliou H, Yamamoto KR (2005) A Caenorhabditis elegans nutrient response system partially dependent on nuclear receptor NHR-49. Proc Natl Acad Sci U S A 102 : 13496–13501. 16157872
21. Van Voorhies WA (2002) The influence of metabolic rate on longevity in the nematode Caenorhabditis elegans. Aging Cell 1 : 91–101. 12882338
22. Narbonne P, Roy R (2009) Caenorhabditis elegans dauers need LKB1/AMPK to ration lipid reserves and ensure long-term survival. Nature 457 : 210–214. doi: 10.1038/nature07536 19052547
23. Clark FE (1969) Ancylostoma caninum: food reserves and changes in chemical composition with age in third stage larvae. Exp Parasitol 24 : 1–8. 5774099
24. Costello LC, Grollman S (1958) Oxygen requirements of Strongyloides papillosus infective larvae. Exp Parasitol 7 : 319–327. 13537941
25. Bethke A, Fielenbach N, Wang Z, Mangelsdorf DJ, Antebi A (2009) Nuclear hormone receptor regulation of microRNAs controls developmental progression. Science 324 : 95–98. doi: 10.1126/science.1164899 19342589
26. Fielenbach N, Guardavaccaro D, Neubert K, Chan T, Li D, Feng Q, et al. (2007) DRE-1: an evolutionarily conserved F box protein that regulates C. elegans developmental age. Dev Cell 12 : 443–455. 17336909
27. Hochbaum D, Zhang Y, Stuckenholz C, Labhart P, Alexiadis V, Martin R, et al. (2011) DAF-12 regulates a connected network of genes to ensure robust developmental decisions. PLoS Genet 7: e1002179. doi: 10.1371/journal.pgen.1002179 21814518
28. Hammell CM, Karp X, Ambros V (2009) A feedback circuit involving let-7-family miRNAs and DAF-12 integrates environmental signals and developmental timing in Caenorhabditis elegans. Proc Natl Acad Sci U S A 106 : 18668–18673. doi: 10.1073/pnas.0908131106 19828440
29. Fisher AL, Lithgow GJ (2006) The nuclear hormone receptor DAF-12 has opposing effects on Caenorhabditis elegans lifespan and regulates genes repressed in multiple long-lived worms. Aging Cell 5 : 127–138. 16626392
30. McCormick M, Chen K, Ramaswamy P, Kenyon C (2012) New genes that extend Caenorhabditis elegans' lifespan in response to reproductive signals. Aging Cell 11 : 192–202. doi: 10.1111/j.1474-9726.2011.00768.x 22081913
31. Shen Y, Wollam J, Magner D, Karalay O, Antebi A (2012) A steroid receptor-microRNA switch regulates life span in response to signals from the gonad. Science 338 : 1472–1476. doi: 10.1126/science.1228967 23239738
32. Schedl T (1997) Developmental Genetics of the Germ Line. In: Riddle DL, Blumenthal T, Meyer BJ, Priess JR, editors. C. ELEGANS II. 2nd ed. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press. pp. 241–269.
33. Huang DW, Sherman BT, Lempicki RA (2009) Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4 : 44–57. doi: 10.1038/nprot.2008.211 19131956
34. Tepper RG, Ashraf J, Kaletsky R, Kleemann G, Murphy CT, Bussemaker HJ (2013) PQM-1 complements DAF-16 as a key transcriptional regulator of DAF-2-mediated development and longevity. Cell 154 : 676–690. doi: 10.1016/j.cell.2013.07.006 23911329
35. Hannich JT, Entchev EV, Mende F, Boytchev H, Martin R, Zagoriy V, et al. (2009) Methylation of the sterol nucleus by STRM-1 regulates dauer larva formation in Caenorhabditis elegans. Dev Cell 16 : 833–843. doi: 10.1016/j.devcel.2009.04.012 19531354
36. Shostak Y, Van Gilst MR, Antebi A, Yamamoto KR (2004) Identification of C. elegans DAF-12-binding sites, response elements, and target genes. Genes Dev 18 : 2529–2544. 15489294
37. Shi Y, Burn P (2004) Lipid metabolic enzymes: emerging drug targets for the treatment of obesity. Nat Rev Drug Discov 3 : 695–710. 15286736
38. Murphy CT, McCarroll SA, Bargmann CI, Fraser A, Kamath RS, Ahringer J, et al. (2003) Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature 424 : 277–283. 12845331
39. O'Rourke EJ, Soukas AA, Carr CE, Ruvkun G (2009) C. elegans major fats are stored in vesicles distinct from lysosome-related organelles. Cell Metab 10 : 430–435. doi: 10.1016/j.cmet.2009.10.002 19883620
40. Angelo G, Van Gilst MR (2009) Starvation protects germline stem cells and extends reproductive longevity in C. elegans. Science 326 : 954–958. doi: 10.1126/science.1178343 19713489
41. Taylor CM, Wang Q, Rosa BA, Huang SC, Powell K, Schedl T, et al. (2013) Discovery of anthelmintic drug targets and drugs using chokepoints in nematode metabolic pathways. PLoS Pathog 9: e1003505. doi: 10.1371/journal.ppat.1003505 23935495
42. Huang SC, Freitas TC, Amiel E, Everts B, Pearce EL, Lok JB, et al. (2012) Fatty acid oxidation is essential for egg production by the parasitic flatworm Schistosoma mansoni. PLoS Pathog 8: e1002996. doi: 10.1371/journal.ppat.1002996 23133378
43. Stoltzfus JD, Minot S, Berriman M, Nolan TJ, Lok JB (2012) RNAseq analysis of the parasitic nematode Strongyloides stercoralis reveals divergent regulation of canonical dauer pathways. PLoS Negl Trop Dis 6: e1854. doi: 10.1371/journal.pntd.0001854 23145190
44. Elle IC, Rodkaer SV, Fredens J, Faergeman NJ (2012) A method for measuring fatty acid oxidation in C. elegans. Worm 1 : 26–30. doi: 10.4161/worm.19564 24058820
45. Raizen D, Song BM, Trojanowski N, You YJ. Methods for measuring pharyngeal behaviors WormBook, ed. The C. elegans Research Community, doi/10.1895/wormbook.1.154.1.
46. Tibshirani R, Chu G, Narasimhan B, Li J (2011) samr: SAM: Significance Analysis of Microarrays. R package version 2.0. http://CRANR-projectorg/package=samr.
Štítky
Genetika Reprodukční medicína
Článek NLRC5 Exclusively Transactivates MHC Class I and Related Genes through a Distinctive SXY ModuleČlánek Inhibition of Telomere Recombination by Inactivation of KEOPS Subunit Cgi121 Promotes Cell LongevityČlánek HOMER2, a Stereociliary Scaffolding Protein, Is Essential for Normal Hearing in Humans and MiceČlánek LRGUK-1 Is Required for Basal Body and Manchette Function during Spermatogenesis and Male FertilityČlánek The GATA Factor Regulates . Developmental Timing by Promoting Expression of the Family MicroRNAsČlánek Systems Biology of Tissue-Specific Response to Reveals Differentiated Apoptosis in the Tick VectorČlánek Phenotype Specific Analyses Reveal Distinct Regulatory Mechanism for Chronically Activated p53Článek The ATM Signaling Cascade Promotes Recombination-Dependent Pachytene Arrest in Mouse SpermatocytesČlánek The Small Protein MntS and Exporter MntP Optimize the Intracellular Concentration of Manganese
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2015 Číslo 3
-
Všechny články tohoto čísla
- NLRC5 Exclusively Transactivates MHC Class I and Related Genes through a Distinctive SXY Module
- Licensing of Primordial Germ Cells for Gametogenesis Depends on Genital Ridge Signaling
- A Genomic Duplication is Associated with Ectopic Eomesodermin Expression in the Embryonic Chicken Comb and Two Duplex-comb Phenotypes
- Genome-wide Association Study and Meta-Analysis Identify as Genome-wide Significant Susceptibility Gene for Bladder Exstrophy
- Mutations of Human , Encoding the Mitochondrial Asparaginyl-tRNA Synthetase, Cause Nonsyndromic Deafness and Leigh Syndrome
- Exome Sequencing in an Admixed Isolated Population Indicates Variants Confer a Risk for Specific Language Impairment
- Genome-Wide Association Studies in Dogs and Humans Identify as a Risk Variant for Cleft Lip and Palate
- Rapid Evolution of Recombinant for Xylose Fermentation through Formation of Extra-chromosomal Circular DNA
- The Ribosome Biogenesis Factor Nol11 Is Required for Optimal rDNA Transcription and Craniofacial Development in
- Methyl Farnesoate Plays a Dual Role in Regulating Metamorphosis
- Maternal Co-ordinate Gene Regulation and Axis Polarity in the Scuttle Fly
- Maternal Filaggrin Mutations Increase the Risk of Atopic Dermatitis in Children: An Effect Independent of Mutation Inheritance
- Inhibition of Telomere Recombination by Inactivation of KEOPS Subunit Cgi121 Promotes Cell Longevity
- Clonality and Evolutionary History of Rhabdomyosarcoma
- HOMER2, a Stereociliary Scaffolding Protein, Is Essential for Normal Hearing in Humans and Mice
- Methylation-Sensitive Expression of a DNA Demethylase Gene Serves As an Epigenetic Rheostat
- BREVIPEDICELLUS Interacts with the SWI2/SNF2 Chromatin Remodeling ATPase BRAHMA to Regulate and Expression in Control of Inflorescence Architecture
- Seizures Are Regulated by Ubiquitin-specific Peptidase 9 X-linked (USP9X), a De-Ubiquitinase
- The Fun30 Chromatin Remodeler Fft3 Controls Nuclear Organization and Chromatin Structure of Insulators and Subtelomeres in Fission Yeast
- A Cascade of Iron-Containing Proteins Governs the Genetic Iron Starvation Response to Promote Iron Uptake and Inhibit Iron Storage in Fission Yeast
- Mutation in MRPS34 Compromises Protein Synthesis and Causes Mitochondrial Dysfunction
- LRGUK-1 Is Required for Basal Body and Manchette Function during Spermatogenesis and Male Fertility
- Cis-Regulatory Mechanisms for Robust Olfactory Sensory Neuron Class-restricted Odorant Receptor Gene Expression in
- Effects on Murine Behavior and Lifespan of Selectively Decreasing Expression of Mutant Huntingtin Allele by Supt4h Knockdown
- HDAC4-Myogenin Axis As an Important Marker of HD-Related Skeletal Muscle Atrophy
- A Conserved Domain in the Scc3 Subunit of Cohesin Mediates the Interaction with Both Mcd1 and the Cohesin Loader Complex
- Selective and Genetic Constraints on Pneumococcal Serotype Switching
- Bacterial Infection Drives the Expression Dynamics of microRNAs and Their isomiRs
- The GATA Factor Regulates . Developmental Timing by Promoting Expression of the Family MicroRNAs
- Accumulation of Glucosylceramide in the Absence of the Beta-Glucosidase GBA2 Alters Cytoskeletal Dynamics
- Reproductive Isolation of Hybrid Populations Driven by Genetic Incompatibilities
- The Contribution of Alu Elements to Mutagenic DNA Double-Strand Break Repair
- Systems Biology of Tissue-Specific Response to Reveals Differentiated Apoptosis in the Tick Vector
- Tfap2a Promotes Specification and Maturation of Neurons in the Inner Ear through Modulation of Bmp, Fgf and Notch Signaling
- The Lysine Acetyltransferase Activator Brpf1 Governs Dentate Gyrus Development through Neural Stem Cells and Progenitors
- PHABULOSA Controls the Quiescent Center-Independent Root Meristem Activities in
- DNA Polymerase ζ-Dependent Lesion Bypass in Is Accompanied by Error-Prone Copying of Long Stretches of Adjacent DNA
- Examining the Evolution of the Regulatory Circuit Controlling Secondary Metabolism and Development in the Fungal Genus
- Zinc Finger Independent Genome-Wide Binding of Sp2 Potentiates Recruitment of Histone-Fold Protein Nf-y Distinguishing It from Sp1 and Sp3
- GAGA Factor Maintains Nucleosome-Free Regions and Has a Role in RNA Polymerase II Recruitment to Promoters
- Neurospora Importin α Is Required for Normal Heterochromatic Formation and DNA Methylation
- Ccr4-Not Regulates RNA Polymerase I Transcription and Couples Nutrient Signaling to the Control of Ribosomal RNA Biogenesis
- Phenotype Specific Analyses Reveal Distinct Regulatory Mechanism for Chronically Activated p53
- A Systems-Level Interrogation Identifies Regulators of Blood Cell Number and Survival
- Morphological Mutations: Lessons from the Cockscomb
- Genetic Interaction Mapping Reveals a Role for the SWI/SNF Nucleosome Remodeler in Spliceosome Activation in Fission Yeast
- The Role of China in the Global Spread of the Current Cholera Pandemic
- The Nuclear Receptor DAF-12 Regulates Nutrient Metabolism and Reproductive Growth in Nematodes
- A Zinc Finger Motif-Containing Protein Is Essential for Chloroplast RNA Editing
- Resistance to Gray Leaf Spot of Maize: Genetic Architecture and Mechanisms Elucidated through Nested Association Mapping and Near-Isogenic Line Analysis
- Small Regulatory RNA-Induced Growth Rate Heterogeneity of
- Mitochondrial Dysfunction Reveals the Role of mRNA Poly(A) Tail Regulation in Oculopharyngeal Muscular Dystrophy Pathogenesis
- Complex Genomic Rearrangements at the Locus Include Triplication and Quadruplication
- Male-Biased Aganglionic Megacolon in the TashT Mouse Line Due to Perturbation of Silencer Elements in a Large Gene Desert of Chromosome 10
- Sex Ratio Meiotic Drive as a Plausible Evolutionary Mechanism for Hybrid Male Sterility
- Tertiary siRNAs Mediate Paramutation in .
- RECG Maintains Plastid and Mitochondrial Genome Stability by Suppressing Extensive Recombination between Short Dispersed Repeats
- Escape from X Inactivation Varies in Mouse Tissues
- Opposite Phenotypes of Muscle Strength and Locomotor Function in Mouse Models of Partial Trisomy and Monosomy 21 for the Proximal Region
- Glycosyl Phosphatidylinositol Anchor Biosynthesis Is Essential for Maintaining Epithelial Integrity during Embryogenesis
- Hyperdiverse Gene Cluster in Snail Host Conveys Resistance to Human Schistosome Parasites
- The Class Homeodomain Factors and Cooperate in . Embryonic Progenitor Cells to Regulate Robust Development
- Recombination between Homologous Chromosomes Induced by Unrepaired UV-Generated DNA Damage Requires Mus81p and Is Suppressed by Mms2p
- Synergistic Interactions between Orthologues of Genes Spanned by Human CNVs Support Multiple-Hit Models of Autism
- Gene Networks Underlying Convergent and Pleiotropic Phenotypes in a Large and Systematically-Phenotyped Cohort with Heterogeneous Developmental Disorders
- The ATM Signaling Cascade Promotes Recombination-Dependent Pachytene Arrest in Mouse Spermatocytes
- Combinatorial Control of Light Induced Chromatin Remodeling and Gene Activation in
- Linking Aβ42-Induced Hyperexcitability to Neurodegeneration, Learning and Motor Deficits, and a Shorter Lifespan in an Alzheimer’s Model
- The Complex Contributions of Genetics and Nutrition to Immunity in
- NatB Domain-Containing CRA-1 Antagonizes Hydrolase ACER-1 Linking Acetyl-CoA Metabolism to the Initiation of Recombination during . Meiosis
- Transcriptomic Profiling of Reveals Reprogramming of the Crp Regulon by Temperature and Uncovers Crp as a Master Regulator of Small RNAs
- Osteopetrorickets due to Snx10 Deficiency in Mice Results from Both Failed Osteoclast Activity and Loss of Gastric Acid-Dependent Calcium Absorption
- A Genomic Portrait of Haplotype Diversity and Signatures of Selection in Indigenous Southern African Populations
- Sequence Features and Transcriptional Stalling within Centromere DNA Promote Establishment of CENP-A Chromatin
- Inhibits Neuromuscular Junction Growth by Downregulating the BMP Receptor Thickveins
- Replicative DNA Polymerase δ but Not ε Proofreads Errors in and in
- Unsaturation of Very-Long-Chain Ceramides Protects Plant from Hypoxia-Induced Damages by Modulating Ethylene Signaling in
- The Small Protein MntS and Exporter MntP Optimize the Intracellular Concentration of Manganese
- A Meta-analysis of Gene Expression Signatures of Blood Pressure and Hypertension
- Pervasive Variation of Transcription Factor Orthologs Contributes to Regulatory Network Evolution
- Network Analyses Reveal Novel Aspects of ALS Pathogenesis
- A Role for the Budding Yeast Separase, Esp1, in Ty1 Element Retrotransposition
- Nab3 Facilitates the Function of the TRAMP Complex in RNA Processing via Recruitment of Rrp6 Independent of Nrd1
- A RecA Protein Surface Required for Activation of DNA Polymerase V
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Clonality and Evolutionary History of Rhabdomyosarcoma
- Morphological Mutations: Lessons from the Cockscomb
- Maternal Filaggrin Mutations Increase the Risk of Atopic Dermatitis in Children: An Effect Independent of Mutation Inheritance
- Transcriptomic Profiling of Reveals Reprogramming of the Crp Regulon by Temperature and Uncovers Crp as a Master Regulator of Small RNAs
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



